- 1Sanya Key Laboratory of Marine Mammal and Marine Bioacoustics, Institute of Deep-sea Science and Engineering, Chinese Academy of Sciences, Sanya, China
- 2Dalian Key Laboratory of Conservation Biology for Endangered Marine Mammals, Liaoning Ocean and Fisheries Science Research Institute, Dalian, China
- 3Guangdong Provincial Key Laboratory of Marine Disaster Prediction and Prevention, Shantou University, Shantou, China
- 4Technique Center, Dalian Sun Asia Tourism Holding Co., Ltd, Dalian, China
Acoustic parameters of spotted seals (Phoca largha), including the duration, peak frequency, and peak-to-peak source level, are reported to vary between different ages and sexes; however, to our knowledge, the vocal ontogeny of the spotted seal from newborn to 1 year old is yet to be studied. In the present study, we recorded and analyzed vocalizations of human-cared spotted seals from the Liaodong Bay colony from newborn to 1 year old, aiming to document the first-year development of seal vocalizations. We divided the spotted seal pups into four age groups (i.e., 1–3-month, 4–6-month, 7–9-month, and 10–12-month groups) for both sexes. The results show significant differences in sex patterns in sound parameters in terms of duration, peak frequency, and peak-to-peak source level. The vocalizations of female seal pups were longer in duration but lower in peak frequency and peak-to-peak source level than those of male pups. All three sound parameters were significantly different across different age groups. Specifically, the 1–3-month group had significantly lower values in duration, peak frequency, and peak-to-peak source level than the three other age groups. The 10–12-month group had significantly higher values in duration and peak-to-peak source level than the three other age groups. Our results also indicate a sex-specific development pattern of seal vocalizations from 1 year old until sexual maturity. Our findings will benefit the evaluation of anthropogenic noise impacts on spotted seal pups and further conservation of the seal population.
Introduction
The spotted seal (Phoca largha) is a small polar phocid that breeds on pack ice. It adopts the maternal strategy of a typical phocid, with mothers fasting during lactation time in winter (Atkinson, 1997; Jefferson et al., 2015). Spotted seals reach their sexual maturity at 4 years old; breeding takes place from January to mid-April, with pups being weaned in about 3–4 weeks (Zhang et al., 2014; Jefferson et al., 2015). Spotted seals inhabit eight separate colonies worldwide, with the Liaodong Bay colony in China being the southernmost one (38°43′–40°58′N, 119°50′–122°18′E) (Rugh et al., 1997). Liaodong Bay has recently become an important ocean shipping access to the Northeast Asian Economic Zone in China due to huge strides in the Chinese economy (Wang and Yu, 1997; Gao and Zhang, 2012). Thus, disturbance caused by the potential overlap of spotted seal habitats with human activities, including heavy ocean traffic, coastal development, raw oil exploitation, and aquaculture, is thought to be one of the most important risk factors for the wild seal populations in Liaodong Bay (Xu et al., 2019; Liu et al., 2021; Yang et al., 2022). Noise pollution—a potential environmental factor affecting the survival of spotted seals, especially those who are growing up—in Liaodong Bay has become an important concern.
Acoustic communications are widely used in pinnipeds and play important roles in various aspects of their behaviors, especially for reproduction purposes (Van Opzeeland et al., 2008). For spotted seals, their acoustic behaviors are reported to play an important role in mating (Beier and Wartzok, 1979; Gailey-Phipps, 1984). A recent study also shows that vocalizations of spotted seals are produced predominantly during breeding time, both underwater and in the air, and seals at different ages emitted significantly different sounds (Zhang et al., 2016; Yang et al., 2017). For example, in-air vocalizations from pre-weaning pups are defined as pup calls, and the acoustic parameters are high in frequency and medial in duration; in-air vocalizations from yearlings (ca. 1 year old) are defined as yearling calls and are high in frequency and long. Adult seals have several kinds of call types; the overall acoustic parameters for males are low in frequency and short, whereas those for females are medial in both frequency and duration (Zhang et al., 2016). Therefore, the acoustic parameters of spotted seal vocalizations change alongside their body development. However, to our knowledge, the vocal ontogeny of the spotted seal from newborn to 1 year old has not yet been studied. This research gap directly limits the evaluation of anthropogenic noise impacts on spotted seal pups and further conservation of the seal population in the Liaodong Bay colony.
Dalian Sun Asia Aquarium (DSAA; 38°52′N, 121°34′E), located beside the Liaodong Bay colony, is an important rescue center for wild spotted seals in China. There is a fairly large population of seals rescued from the Liaodong Bay colony in DSAA, and approximately 10 pups are born and subsequently grow up under human care every year. In the present study, we recorded the sounds of spotted seal pups in DSAA consecutively from birth to 1 year old and documented how their acoustic characteristics varied with age and sex factors to reveal the vocal repertoire ontogeny of seal pups.
Materials and methods
Animals and housing condition
Nine spotted seal pups at DSAA were investigated in this study. Four males and four females were born under human care and nursed by their respective mothers. The remaining male was rescued from the wild just after weaning with a body mass of 19.5 kg. All seals were first kept in a 400-m2 indoor enclosure for nursing. After lactation, pups were separated from their mothers and transferred to another 100-m2 indoor enclosure. Harmless microchip bio-tags were subdermally injected for individual identification when they were 1 month old.
Data collection
Recordings of in-air sounds were collected every 15–35 days post-parturition, from 4 February 2013 to 28 February 2014. Sounds were recorded for approximately 3 h between 1900 and 2200 h when there were no public visitors. The in-air sounds were recorded using a self-designed sound collection platform, mainly including a USB-4431 data acquisition card (National Instruments, Austin, TX, USA), 46AE 1/2″ Free-field Microphone Set (GRAS, Holte, Denmark; frequency response of 3.5 Hz–20 kHz, within ±1 dB at 5 Hz–10 kHz) and laptop. The sampling rate for sound recording was 16 kHz, which covered the frequencies of spotted seal in-air vocalizations (Zhang et al., 2016). The distance between the microphone and vocalizing seal pups was 3 to 5 m. The precise distances were marked before sound recordings.
Data analysis
All sound data were first scanned using spectrograms (Hanning Windows, FFT size 1,024 points and 50% overlap for a spectral resolution of 16 Hz and a time resolution of 64 ms) in Adobe Audition (version 3.0, Adobe Systems Inc., San Jose, CA, USA). Sound files were filtered in Audition with a 10-pole Butterworth high-pass filter with a cutoff frequency of 50 Hz. Only the sound signals with distinct and clear vocal contours, which typically had a signal-to-noise ratio of more than 20 dB, were extracted for further analysis. The selected vocalizations for each individual were separated by at least 5-min intervals to minimize data non-independence.
Three sound parameters, i.e., duration (DUR), peak frequency (FP), and peak-to-peak source level (SLPP), were measured from each vocalization using custom-written codes in MATLAB (version 2012b, MathWorks, Natick, MA, USA). The DUR (ms) was determined by the length of a window containing 95% of the total signal energy (Madsen and Wahlberg, 2007). The FP (Hz) was defined as the center frequency of the band with the highest amplitude of the spectrum, which was calculated from a 1,024-point FFT on Hanning windowed data symmetrical around the envelope peak (Madsen and Wahlberg, 2007). The received level was calculated from the peak-to-peak sound pressure values on a dB scale over a 95% energy window and the sensitivity of the microphone. The SLPP (dB re 20 μPa at 1 m) of each vocalization was calculated from the sum of the received level and spherical spreading, i.e., 20log10(DISTANCEseal-microphone) (Rogers, 2014). The three sound parameters were chosen for consistency with a previous study (Zhang et al., 2016).
Statistical analyses were performed using IBM SPSS Statistics 22. To examine age differences in seal pup call characteristics, four age groups were created: 1–3, 4–6, 7–9, and 10–12 months old. Data normality and homogeneity for each age and sex group were examined using the Kolmogorov–Smirnov and Levene’s tests, respectively. Given that not all data satisfied equal variance or normality assumptions, a series of generalized linear models (GLMs) were conducted to examine the age and sex effects on the three sound parameters of seal pup in-air vocalizations. A p-value < 0.05 was considered statistically significant.
Results
In total, 1,115 high-quality vocalizations from nine spotted seal pups were used in the analyses. Sample sizes of seal pup vocalizations for different sex and age groups are summarized in Table 1. Amplitude charts and spectrograms of the vocalizations for each age group are shown in Supplementary Figures 1, 2. Overall, the in-air vocalizations of the nine spotted seal pups had a DUR of 858 ± 361 ms (mean ± SD), an FP of 991 ± 368 Hz, and an SLPP of 103 ± 8 dB re 20 μPa.
The results of the GLMs indicated significant differences in sex patterns in all three sound parameters, i.e., DUR, FP, and SLPP values (Table 2). Specifically, the vocalizations of female pups were longer in DUR (Figure 1A) but lower in FP (Figure 1B) and SLPP (Figure 1C) compared to those of male pups.
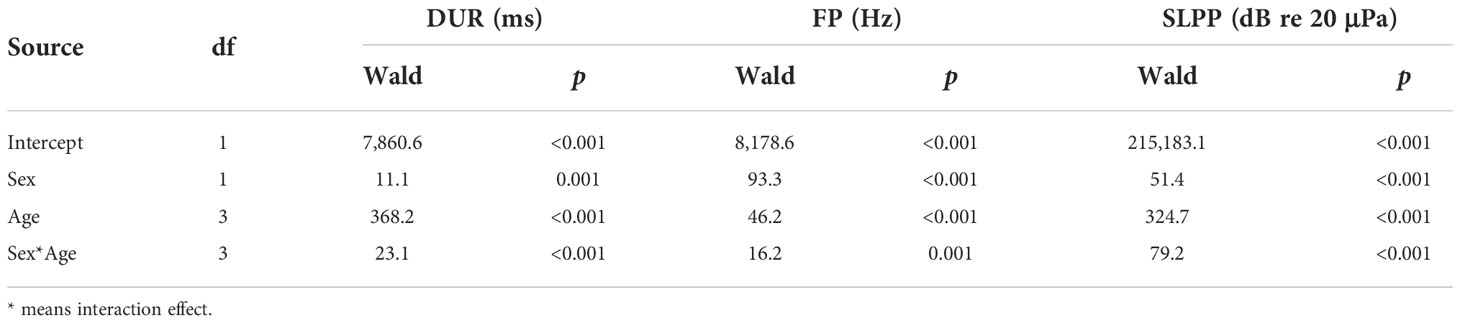
Table 2 Results of a generalized linear model analysis on the sound parameters of duration (DUR), peak frequency (FP), and peak-to-peak source level (SLPP) for different spotted seal pup groups.
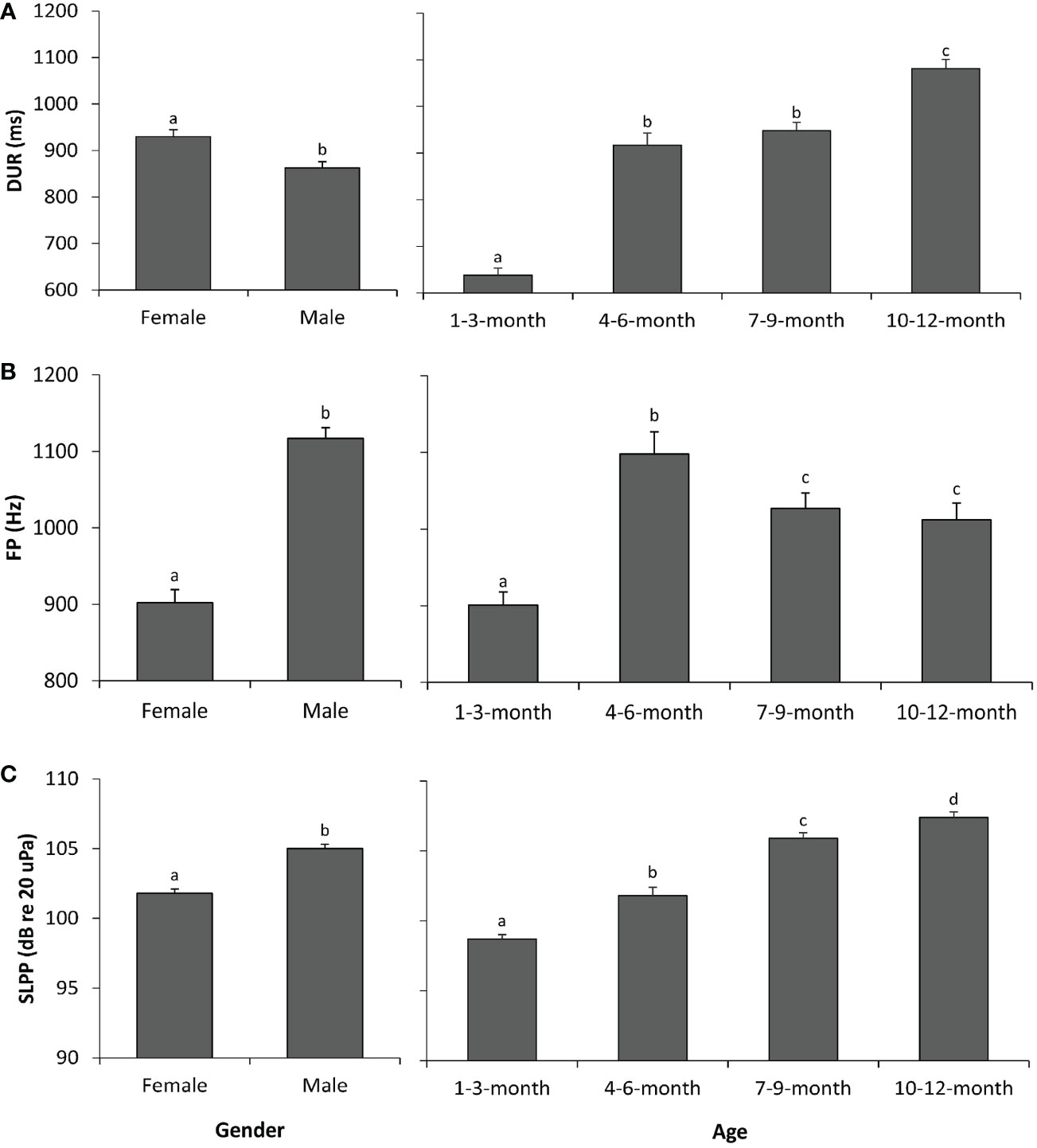
Figure 1 Sex and age effects on the sound parameters of spotted seal pups. (A) Duration (DUR). (B) Peak frequency (FP). (C) Peak-to-peak source level (SLPP). Different lowercase letters (i.e., a, b, c, and d) on each bar indicate significant statistical differences between groups, whereas groups with the same letter indicate no statistical difference. A p-value < 0.05 is considered statistically significant.
Similarly, the results of the GLMs showed that all three sound parameters were significantly different across the four age groups (Table 2). Specifically, the 1–3-month group had significantly lower values in all three sound parameters than the three other age groups (Figure 1). The 10–12-month group had significantly higher values in DUR (Figure 1A) and SLPP (Figure 1C) than the remaining three age groups. No significant differences existed in DUR (Figure 1A) between the 4–6-month and 7–9-month groups; however, the 7–9-month group had significantly higher values in SLPP (Figure 1C) as compared to the 4–6-month groups. The highest FP was observed in the 4–6-month group among the four age groups (Figure 1B). There were no significant differences in FP between the 7–9-month and 10–12-month groups (Figure 1B).
The interaction effect results of GLMs are shown in Figure 2. The vocalizations of female pups were significantly longer in DUR than those of male pups 10–12 months old. However, no significant difference in DUR was observed between female and male pups between 1–3 and 7–9 months old (Figure 2A). Regarding the FP (Figure 2B) and SLPP (Figure 2C) parameters, the males had significantly higher values in all the age groups as compared to the females, except that in the 1–3-month group, the SLPP values of males were significantly lower than those of females (Figure 2C). Additionally, the DUR (Figure 2A) and SLPP (Figure 2C) values of both female and male vocalizations gradually increased from newborn to 1 year old. This was also the case for the FP values of male vocalizations (Figure 2B). However, this was not the case for female vocalizations in FP values. The FP for female vocalizations first increased at 1–3 and 4–6 months old and then decreased at 7–9 and 10–12 months old (Figure 2B).
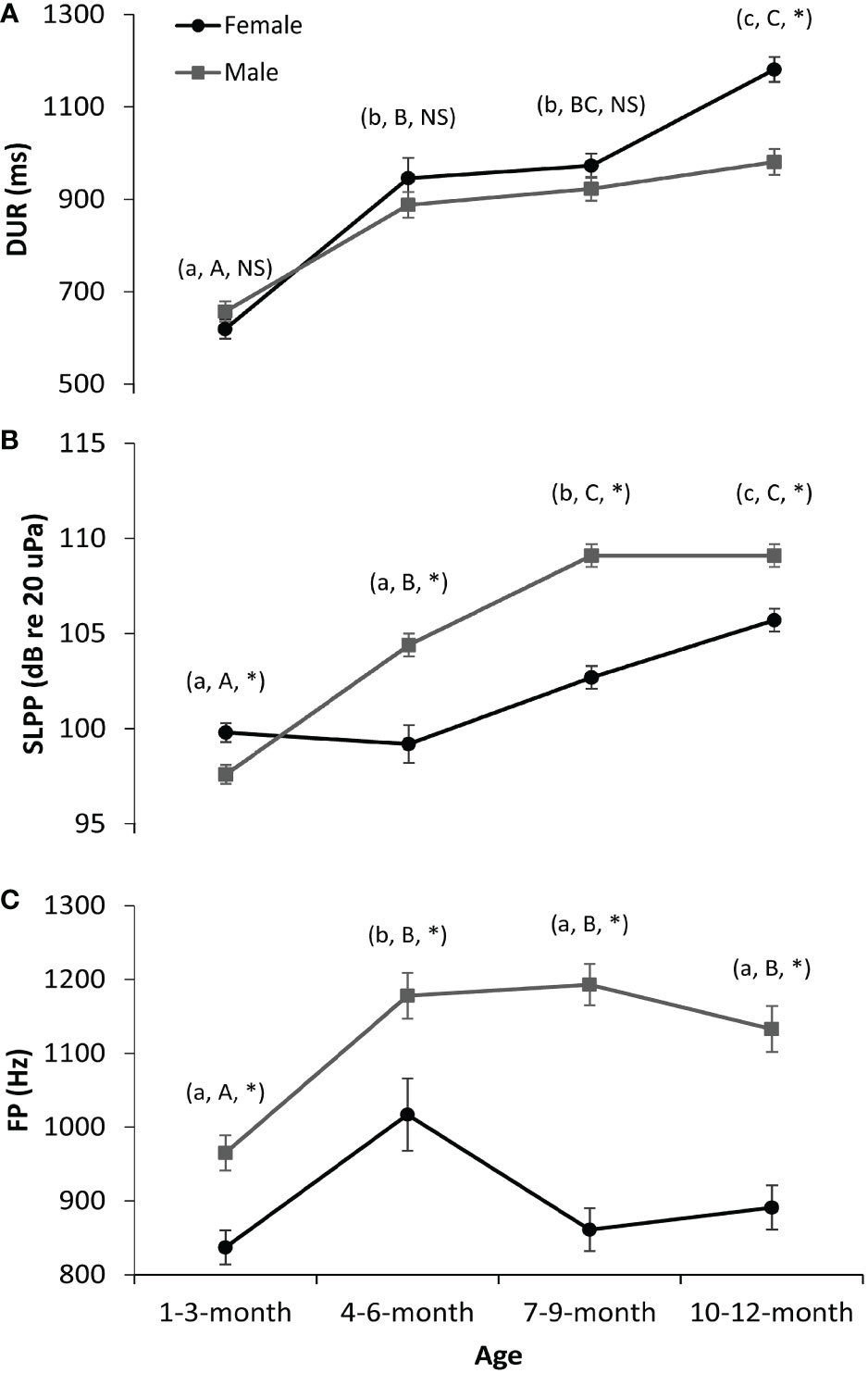
Figure 2 Interaction effects on the sound parameters of spotted seal pups. (A) Duration (DUR). (B) Peak frequency (FP). (C) Peak-to-peak source level (SLPP). The dark lines and circles represent the results for females, while the gray lines and squares represent the results for males. Lowercase letters in the parentheses indicate the comparison results of each sound parameter of female seals among four age groups (i.e., 1–3, 4–6, 7–9, and 10–12 months old). Groups with different lowercase letters indicate a significant statistical difference between them, while those with the same letter indicate no statistical difference. Similarly, uppercase letters in the parentheses indicate the comparison results for male seals. NS denotes no significance between sexes in that age group, while * denotes significance. A p-value < 0.05 is considered statistically significant.
Discussion
Our study, for the first time, documents the detailed process of the ontogeny of spotted seal vocalizations from newborn to 1 year old. The results reveal that the spotted seal pups significantly increased the FP of their vocalizations at first (from 1 to 6 months old) for both sexes. Additionally, the FP for female pups decreased to the same level at 1–3 months, whereas male pups retained their FP at about 1,200 Hz. As to DUR and SLPP, spotted seal pups increased both values with their ages regardless of sex.
Based on our age-stage classifications, the results of our study can probably be explained when considering the development of pups’ ecological behaviors in the wild. In late spring, when spotted seals in the Liaodong Bay colony finish their annual nursing and mating before sea ice melting, they haul themselves out on the shores of the mainland and islands and spend a significant part of the open-water period on their annual bask and molt cycle and then begin their forage migration after the middle of May (Beier and Wartzok, 1979; Won and Yoo, 2004; Han et al., 2005; Boveng et al., 2009; Ma et al., 2018) Seafood in the Bohai Sea is normally poor in winter and spring (Barman et al., 2020), and hence pups have a limited food supply between weaning and first forage migration (March to May), which constrains their body development. We supposed that acoustic characteristics in the 1–3-month seals reflect an innate physiological difference between male and female neonates. We also examined the body mass of male and female pups when they were approximately 1 month old and found that the body mass of female pups (21.1 ± 1.7 kg) was slightly lower than that of males (26.2 ± 6.8 kg), but with no statistical difference (Student’s t-test, p > 0.05), implying that body mass was not a potential factor affecting the intersex vocalization variation at that time.
Stages of 4–6- and 7–9-month-old pups correspond to the summer and autumn, when fishery resources are high in the wild (Barman et al., 2020) and when pups are supposed to be in their foraging ground (Trukhin et al., 2021). Fast body growth may also happen at that time, which explains the fast development of the vocal characteristics of seals at those stages. The 10–12-month group corresponds to seals’ breeding-ground migration in winter. In the northern Yellow Sea, spotted seals finish their sea-ice habitat occupancy in Liaodong Bay in December, i.e., roughly 10 months old for newborn seals (Won and Yoo, 2004; Trukhin et al., 2021). When comparing sound parameters between yearlings (i.e., 10–12-month group) and adults, we can see that there are significant differences in FP and DUR between them (Zhang et al., 2016), implying still inadequate vocal development in yearlings (Figure 3). As we currently have little or no vocal information from elder juvenile seals (i.e., 1–3 years old), more research on the vocalizations of seals of those ages should be conducted in the future.
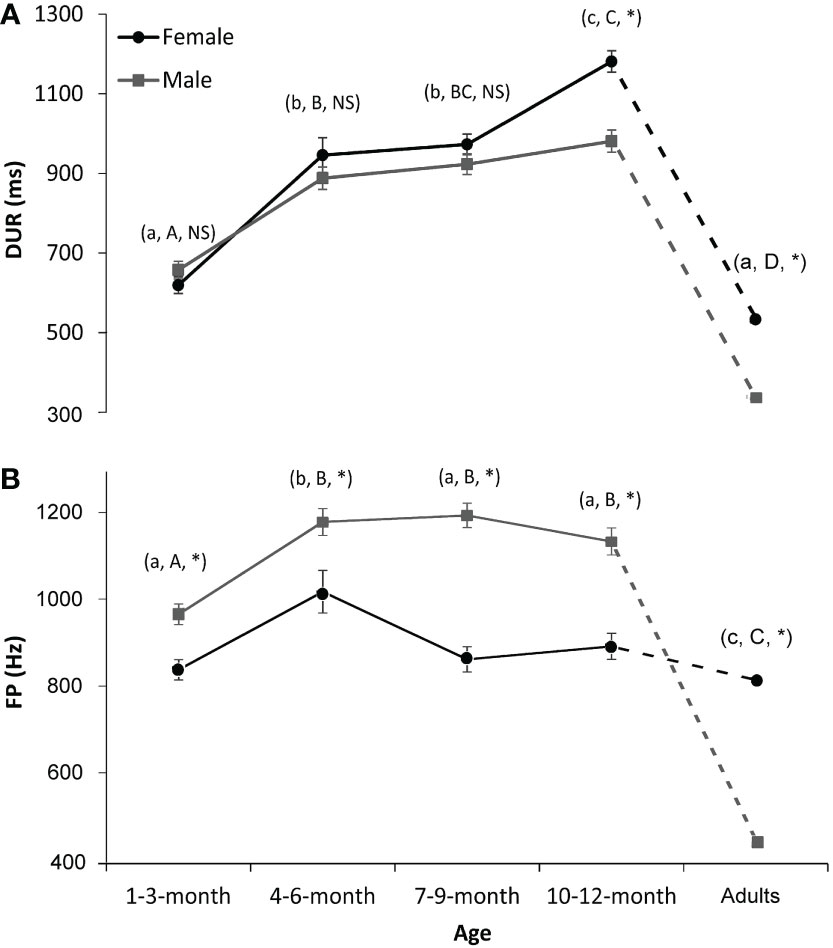
Figure 3 Interaction effects on the sound parameters of spotted seals. (A) Duration (DUR). (B) Peak frequency (FP). The dark lines/dotted lines and circles represent the results for females, while the gray lines/dotted lines and squares represent the results for males. Lowercase letters in the parentheses indicate the comparison results of each sound parameter of female seals among five age groups (i.e., 1–3, 4–6, 7–9, and 10–12 months old and adults). Groups with different lowercase letters indicate a significant statistical difference between them, while those with the same letter indicate no statistical difference. Similarly, uppercase letters in the parentheses indicate the comparison results for male seals. NS denotes no significance between sexes in that age group, while * denotes significance. A p-value < 0.05 is considered statistically significant.
In the present 1-year recording, the sampling sizes for 4–6-month-old pups for both sexes were relatively small as compared to those of the other three age groups. The seal pups were generally quiet at 4–6 months, resulting in a limited sample size. The wild seal pups at the age of 4–6 months migrate out of Liaodong Bay for foraging (Han et al., 2005; Ma et al., 2018). It is possible that they keep silent to save energy for swimming and to reduce potential detection by prey and predators.
A previous study found that vocalizations between pups and yearling spotted seals were significantly different (Zhang et al., 2016), and the present study further provided strong evidence that pups of different ages showed different acoustic parameters. Specifically, the suckling pups had shorter vocalizations than yearling pups (Zhang et al., 2016); the present study further confirmed that the age-based variation in DUR occurred at 4 months old. We also confirmed that sex-based DUR variation occurred at 10 months old.
Regarding the FP, a significant difference was found between the male and female pups, with the FP of male pups constantly higher than in females, from neonates to 1 year old. This result was surprising because this was not the case for yearling and adult spotted seals, with a lower FP for males than females (Zhang et al., 2016). This indicated a continued large change in the FP in seals from 1 year old onward. Overall, our results indicated a clear intersex divergence in sound parameters in spotted seals from 1 year old until sexual maturity (i.e., 4 years old).
The spotted seal is a sibling species of the harbor seal but adopts quite a different nursing strategy. Spotted seals fast completely during nursing, whereas harbor seals forage, similar to otariids (Atkinson, 1997; Jefferson et al., 2015). Acoustic studies on pinnipeds are popular, with a focus on nursing strategy and mother–pup recognition, as a species (i.e., otariids) that can achieve individual vocal recognition is more likely to be studied (e.g., Insley, 1992; Insley, 2000; Insley et al., 2003; Tripovich et al., 2005; Collins et al., 2006; Pitcher et al., 2009; Opzeeland et al., 2012; Reichmuth and Casey, 2014; Sauve et al., 2015), and the same is true for harbor seals (Van Parijs and Kovacs, 2002; Khan et al., 2006; Sauve et al., 2015). Seals that cannot achieve individual vocal recognition, such as spotted seals, have received little attention. The current study adds to the body of knowledge about the vocal development of completely fasting seals during nursing.
There are also some limitations to our study. Firstly, the studied seals were mostly born under human care and originated from the Liaodong Bay colony. Our results reflect the case for captive-born Liaodong Bay seals; further studies are warranted to determine if there is variation between captive-born and wild-born seals or between seals from different colonies. Secondly, after lactation, in the 4–6-month group, pups gradually became silent; as a result, sounds from this group were mainly recorded during feeding and enclosure-cleaning times, when the seals were more vocal due to external disturbance. Regardless, we still collected relatively few sound signals, which had the potential to affect the analysis results. For example, the male pups first increased their sound frequency and then remained steady; but this was not the case for females, possibly due to the sample size limitation. Nonetheless, our findings contribute to our understanding of the vocal development of spotted seal pups in the wild, but they should be interpreted with caution.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by the Ethics Committee for Laboratory Animals of the Institute of Deep-sea Science and Engineering, CAS, No. IDSSE-SYLL-MMMBL-01, and conducted under a permit issued by the Liaoning Fisheries Administration Bureau, Liaoning Province, China (No. LSYXFZ20111105). Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
PZ and ZL designed the study. PZ, JH, and YY performed the experiment. PZ and LY conducted the data analysis and wrote the draft manuscript. SL and ZL edited the manuscript. All authors approved the final manuscript.
Funding
This project was supported by grants from the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2020363), National Natural Science Foundation of China (NSFC) (42276141) and the Ocean Park Conservation Foundation Hong Kong (MM09_1213).
Acknowledgments
We thank the staff from DSAA for their assistance in monitoring the vocal behavior of spotted seals and collecting sound signals.
Conflict of interest
Author YY was employed by Dalian Sun Asia Tourism Holding Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.943030/full#supplementary-material
References
Atkinson S. (1997). Reproductive biology of seals. Rev. Reprod. 2, 175–194. doi: 10.1530/revreprod/2.3.175
Barman P. P., Yanan H., Liu Q. (2020). Dynamic characteristics of community structure and seasonal variation of fishery species in the bohai Sea, China. Appl. Ecol. Env. Res. 18, 817–837. doi: 10.15666/aeer/1801_817837
Beier J. C., Wartzok D. (1979). Mating behaviour of captive spotted seals (Phoca largha). Anim. Behav. 27, 772–781. doi: 10.1016/0003-3472(79)90013-7
Boveng P. L., Bengtson J. L., Buckley T. W., Cameron M. F., Dahle S. P., Kelly B. P., et al. (2009). Status review of the spotted seal (Phoca largha) (Springfield: U.S. Dep. Commer., NOAA Tech. Memo. NMFS-AFSC-200).
Collins K. T., Terhune J. M., Rogers T. L., Wheatley K. E., Harcourt R. G. (2006). Vocal individuality of in-air weddell seal (Leptonychotes weddellii) pup “primary” calls. Mar. Mammal Sci. 22, 933–951. doi: 10.1111/j.1748-7692.2006.00074.x
Gailey-Phipps J. J. (1984) Acoustic communication and behavior of the spotted seal (Phoca largha) (Phocidae, Pinnipedia). [dissertation/doctor’s thesis]. (Baltimore MD: The Johns Hopkins University).
Gao W., Zhang Y. A. (2012). “Study of port-vicinity industry cluster development of liaoning coastal economic belt,” in Proceedings of the 2012 2nd International Conference on Consumer Electronics, Communications and Networks (CECNet), (New York, NYS: IEEE), 1590–1593. doi: 10.1109/CECNet.2012.6201978
Han J. B., Wang W., Ma Z. Q. (2005). Spotted seals in the estuary of shuangtaizi river of Liaodong Bay. Mar. Environ. Sci. 24, 51–53. doi: 10.3969/j.issn.1007-6336.2005.01.015
Insley S. J. (1992). Mother-offspring separation and acoustic stereotypy - a comparison of call morphology in 2 species of pinnipeds. Behaviour 120, 103–122. doi: 10.1163/156853992X00237
Insley S. J. (2000). Long-term vocal recognition in the northern fur seal. Nature 406, 404–404. doi: 10.1038/35019064
Insley S., Phillips A. V., Charrier I. (2003). A review of social recognition in pinnipeds. Aquat. Mammals 29, 181–201. doi: 10.1578/016754203101024149
Jefferson T. A., Webber M. A., Pitman R. L. (2015). Marine mammals of the world: a comprehensive guide to their identification. 2 edn (Pittsburgh: Academic Press).
Khan C. B., Markowitz H., McCowan B. (2006). Vocal development in captive harbor seal pups, Phoca vitulina richardii: Age, sex, and individual differences. J. Acoust. Soc Am. 120, 1684–1694. doi: 10.1121/1.2226530
Liu B., Wu X., Liu X., Gong M. (2021). Assessment of ecological stress caused by maritime vessels based on a comprehensive model using AIS data: case study of the bohai Sea, China. Ecol. Indic. 126, 107592. doi: 10.1016/j.ecolind.2021.107592
Madsen P. T., Wahlberg M. (2007). Recording and quantification of ultrasonic echolocation clicks from free-ranging toothed whales. Deep-sea Res. Pt. I 54, 1421–1444. doi: 10.1016/j.dsr.2007.04.020
Ma Z. Q., Han J. B., Lu Z. C., Tian J. S., Wu J. (2018). Quantitative statistics and behavioral observation of spotted seal in waters around shixian island of jinzhou bay in bohai Sea. Fish. Sci. 37, 684–688. doi: 10.16378/j.cnki.1003-1111.2018.05.017
Opzeeland I. C. V., Parijs S. M. V., Frickenhaus S., Kreiss C. M., Boebel O. (2012). Individual variation in pup vocalizations and absence of behavioral signs of maternal vocal recognition in weddell seals (Leptonychotes weddellii). Mar. Mammal Sci. 28, E158–EE72. doi: 10.1111/j.1748-7692.2011.00505.x
Pitcher B. J., Ahonen H., Harcourt R. G., Charrier I. (2009). Delayed onset of vocal recognition in Australian sea lion pups (Neophoca cinerea). Naturwissenschaften 96, 901–909. doi: 10.1007/s00114-009-0546-5
Reichmuth C., Casey C. (2014). Vocal learning in seals, sea lions, and walruses. Curr. Opin. Neurobiol. 28, 66–71. doi: 10.1016/j.conb.2014.06.011
Rogers T. L. (2014). Source levels of the underwater calls of a male leopard seal (L). J. Acoust. Soc Am. 136, 1495–1498. doi: 10.1121/1.4895685
Rugh D. J., Shelden K. E. W., Withrow D. E. (1997). Spotted seals, Phoca largha, in Alaska. Mar. Fish. Review. 59, 1–18.
Sauve C. C., Beauplet G., Hammill M. O., Charrier I. (2015). Acoustic analysis of airborne, underwater, and amphibious mother attraction calls by wild harbor seal pups (Phoca vitulina). J. Mammal. 96, 591–602. doi: 10.1093/jmammal/gyv064
Tripovich J. S., Rogers T. L., Arnould J. P. Y. (2005). Species-specific characteristics and individual variation of the bark call produced by male Australian fur seals, Arctocephalus pusillus doriferus. Bioacoustics 15, 79–96. doi: 10.1080/09524622.2005.9753539
Trukhin A. M., Permyakov P. A., Ryazanov S. D., Lobanov V. B., Kim H. W., Choi Y. M., et al. (2021). Migrations of young spotted seals (Phoca largha) from Peter the great bay, Sea of Japan/East Sea, and the pattern of their use of seasonal habitats. PloS One 16, e0244232. doi: 10.1371/journal.pone.0244232
Van Opzeeland I., Kindermann L., Boebel O., Van Parijs S. (2008). “Insights into the acoustic behaviour of polar pinnnipeds-current knowledge and emerging techniques of study,” in Animal Behaviour: New Research, ed. Weber E. A., Krause L. H. (Hauppage, NY: Nova Science Publishers, Inc), 133–161.
Van Parijs S. M., Kovacs K. M. (2002). In-air and underwater vocalizations of eastern Canadian harbour seals, Phoca vitulina. Can. J. Zool. 80, 1173–1179. doi: 10.1139/Z02-088
Wang R., Yu G. (1997). The open port system in northeast China. Chin. Geograph. Sci. 7, 270–277. doi: 10.1007/s11769-997-0054-5
Won C., Yoo B. H. (2004). Abundance, seasonal haul-out patterns and conservation of spotted seals Phoca largha along the coast of bak-ryoung island, south Korea. Oryx 38, 109–112. doi: 10.1017/S0030605304000171
Xu J., Li F., Suo A., Zhao J., Su X. (2019). Spatio-temporal change and carrying capacity evaluation of human coastal utilization in liaodong bay, China from 1993 to 2015. Chin. Geograph. Sci. 29, 463–473. doi: 10.1007/s11769-019-1044-0
Yang L. L., Xu X. M., Berggren P. (2022). Spotted seal Phoca largha underwater vocalisations in relation to ambient noise. Mar. Ecol. Prog. Ser. 683, 209–220. doi: 10.3354/meps13951
Yang L., Xu X., Zhang P., Han J., Li B., Berggren P. (2017). Classification of underwater vocalizations of wild spotted seals (Phoca largha) in liaodong bay, China. J. Acoust. Soc Am. 141, 2256–2262. doi: 10.1121/1.4979056
Zhang P. J., Lu J. J., Li S. H., Han J. B., Wang Q. G., Yang L. L. (2016). In-air vocal repertoires of spotted seals, Phoca largha. J. Acoust. Soc Am. 140, 1101–1107. doi: 10.1121/1.4961048
Keywords: airborne, Liaodong Bay, ontogeny, Phoca largha, vocal development
Citation: Zhang P, Yang L, Han J, Yang Y, Lu Z and Li S (2022) Age and sex differences in in-air vocalization characteristics of spotted seal pups from newborn to 1 year old in captivity. Front. Mar. Sci. 9:943030. doi: 10.3389/fmars.2022.943030
Received: 13 May 2022; Accepted: 06 December 2022;
Published: 21 December 2022.
Edited by:
Tracy A. Romano, Sea Research Foundation, Inc., United StatesReviewed by:
Michael L. Fine, Virginia Commonwealth University, United StatesYisi Zhang, Princeton University, United States
Copyright © 2022 Zhang, Yang, Han, Yang, Lu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peijun Zhang, cGp6aGFuZ0BpZHNzZS5hYy5jbg==; Zhichuang Lu, bHV6aGljaHVhbmdAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Peijun Zhang
Peijun Zhang Liangliang Yang
Liangliang Yang Jiabo Han2
Jiabo Han2 Zhichuang Lu
Zhichuang Lu Songhai Li
Songhai Li