
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
TECHNOLOGY AND CODE article
Front. Mar. Sci. , 18 August 2022
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.942094
This article is part of the Research Topic Aquaculture of Emergent Marine Invertebrates: Advances in Nutrition, Rearing Technology and End-Product Quality View all 11 articles
Interest in the rearing of jellyfish has grown exponentially over recent years due to their indisputable potential in a wide variety of uses and research. Here, we describe the design and operation of an effective modified kreisel aquarium that allows to grow of the early planktonic life stages of jellyfish in both flow-through or closed systems. Thanks to its versatile and innovative approach, the aquarium operates for species with a metagenetic life cycle, and allows the metamorphosis of ephyrae from fertilized eggs for species with a holoplanktonic life cycle, such as Pelagia noctiluca. In addition, its configuration allows the mesh screen to be changed in situ, adjusting the size of the mesh according to the growth of the jellyfish and the size of the prey offered. An accessory, named the polyp plate, suspends the polyps in a downward or sideways position, facilitating prey capture, strobilae formation and release of ephyrae. The improvements of this modified kreisel reduce the time-involvement for staff in maintenance routines, and it is an important contribution to jellyfish husbandry techniques and biomass production. These improvements especially target to the culture of the blooming and stinging P. noctiluca, which has been noted to present a host of challenges to the scientific and aquarist communities.
Jellyfish are considered an important fishery commodity for the food industry (Omori and Nakano, 2001; Dong et al., 2009) and have a high value as raw material in a wide variety of biotechnological applications (Merquiol et al., 2019; D’Ambra and Merquiol, 2022). Although wild jellyfish are exploited as a marine resource for different proposals (Omori and Nakano, 2001; Dong et al., 2009; Elliott et al., 2017), they are considered unpredictable due to the seasonality and spatiotemporal variability of their life cycles (Purcell et al., 2013; Gueroun et al., 2021; Marambio et al., 2021). From this point of view, biomass production through aquaculture activity can offer a permanent stock to supplement the fished biomass, as well as, to assures individuals not contaminated with unknown pollutants and safeguards traceability; a plus when aiming to target premium sectors associated with pharmaceutical, cosmeceutical and biomedical industries.
To raise jellyfish successfully, knowledge on the biology of target species is required including their feeding and reproductive behavior (Fu et al., 2014; Lilley et al., 2014b; Gueroun et al., 2021; Camacho-Pacheco et al., 2022). The majority of the scyphozoan species (Cnidaria: Scyphozoa) have a well-known metagenetic life cycle. Sexually mature jellyfish produce eggs or sperm which fuse to develop a ciliated planulae. Planulae settle to the marine substrate and metamorphose into the scyphistoma or polyp (benthic phase) (Helm, 2018). Through the process of strobilation, the polyps fission perpendicularly (strobilae) to release small ephyrae into the water column (planktonic phase). Ephyrae grow into metaephyrae, juvenile medusae and finally, sexually mature adults that produce eggs and sperm, completing the life cycle (Fuentes et al., 2011; Helm, 2018). Despite being the most common life cycle in scyphozoans, some species, such as the holoplanktonic Pelagia noctiluca, lack a benthic phase, and the fertilized eggs metamorphose directly into ephyrae in the water column (Sandrini and Avian, 1983; Canepa et al., 2014).
Aquaculture techniques, employed to produce jellyfish, vary according to the species and life cycle stages (Raskoff et al., 2003; Pierce, 2005; Purcell et al., 2013; Schaadt et al., 2017; Duarte et al., 2021). Polyp colonies are a continuous source of jellyfish through the strobilation process (Helm, 2018; Duarte et al., 2021), hence keeping colonies healthy is strategic for continuous production over time. Their maintenance is easier and much less time-consuming than early planktonic life stages (ephyrae and metaephyrae), which are the most complicated stages in jellyfish aquaculture and consume the majority of staff time (Duarte et al., 2021). However, although polyps are resistant to some anthropogenic stressors in marine habitats (e.g., pesticides, Olguín-Jacobson et al., 2020), to ensure successful breeding in aquaculture, it is important to maintain adequate water quality and suitable nutrition (Raskoff et al., 2003; Crow et al., 2013; Duarte et al., 2021).
Regarding life support system in jellyfish aquaculture, polyps and jellyfish can be maintained in flow-through or closed systems (Crow et al., 2013). While in a flow-through is the water is constantly replaced with new water from a source such as the ocean, in a closed system, the water needs to be recirculated (Duarte et al., 2021). In the case of a close system, the outflow drain should lead to a sump with proper mechanical and biological filtration, and UV sterilisation (Duarte et al., 2021, e.g., Figure 6). Both systems have advantages and disadvantages, but one of the drawbacks of using a closed system is the time spent on maintenance routines (Crow et al., 2013).
Depending on the life cycle stage, individuals are grown in a variety of culture vessels or aquariums in a flow-through or closed systems (Widmer et al., 2005; Purcell et al., 2013; Duarte et al., 2021, e.g., Figure 5). The best-known type of aquarium in jellyfish rearing is the kreisel tank (Greve, 1968). The kreisel is a circular aquarium with a flat back and front that contains an inlet and an outlet water chamber separated by a screen. The water inlet is generally provided by a spray bar with holes creating a circular flow that moves jellyfish away from the screen (Purcell et al., 2013, e.g., Figure 13.3). The separation of the chambers along with the water inlet allows jellyfish to swim freely without danger of being sucked down the drain (Purcell et al., 2013; Duarte et al., 2021). Despite their wide use in jellyfish aquaculture, usually, kreisel aquariums are not used as early stage grow-out tanks (Duarte et al., 2021, e.g., Figure 4). Ephyrae and metaephyrae, which are small and thin (Straehler-Pohl and Jarms, 2010; Straehler-Pohl et al., 2011), can easily be retained in the mesh screen due to the suction produced by the drain. Under this premise and to ensure their safety, they are often cultured successfully in vessels such as flasks, dishes or balls glass and plastic jars (Widmer et al., 2005; Crow et al., 2013), as well as, in larger tanks without water outlet (Duarte et al., 2021). All of these rearing systems require daily water changes to ensure its quality (Duarte et al., 2021). However, from the perspective of biomass production and scalability of jellyfish cultures, the time spent on recurrent water changes, involving the pipetting of each ephyra one by one at discrete time intervals, is unfeasible.
With the aim of reducing the time required for maintenance routines, and exploring new technologies to scale jellyfish cultures and biomass production, we present a versatile modified kreisel aquarium that allows safe cultivation early jellyfish life stages in both flow-through and closed systems. Our prototype aquarium is an important contribution to jellyfish husbandry techniques, especially for P. noctiluca, a species whose culture represents a challenge for the scientific and aquarist community due to the lack of polyp stage in its life cycle (Ramondenc et al., 2019; Ballesteros et al., 2022). Additionally, due to its versatility and accessories, this system can be used for polyps, strobilae, eggs, planulae and grow-out ephyrae and metaephyrae tanks.
The aquarium (350 mm x 350 mm x 150 mm) comprises an 8 L main PVC and methacrylate circular tank (main chamber) with a rectangular upper frame on top, through which the water inlet and jellyfish are introduced (Figures 1A–C, 2A, B). A second chamber (drainage chamber) adjoins the main chamber, separated by a long, narrow interchangeable mesh screen (Figures 1B, D, 2C). The mesh screen allows the water to enter from the main chamber to the drainage chamber (Figure 2D). An air duct (Qair = 11.5 l/h), placed in the bottom-center of the mesh screen (Figures 1A, B), introduces air into the first tank, creating a flow of bubbles which covers almost the entire mesh screen to prevent small ephyrae and metaephyrae from approaching (Figures 2E, F). The air bubbles, push the animals into the main chamber.
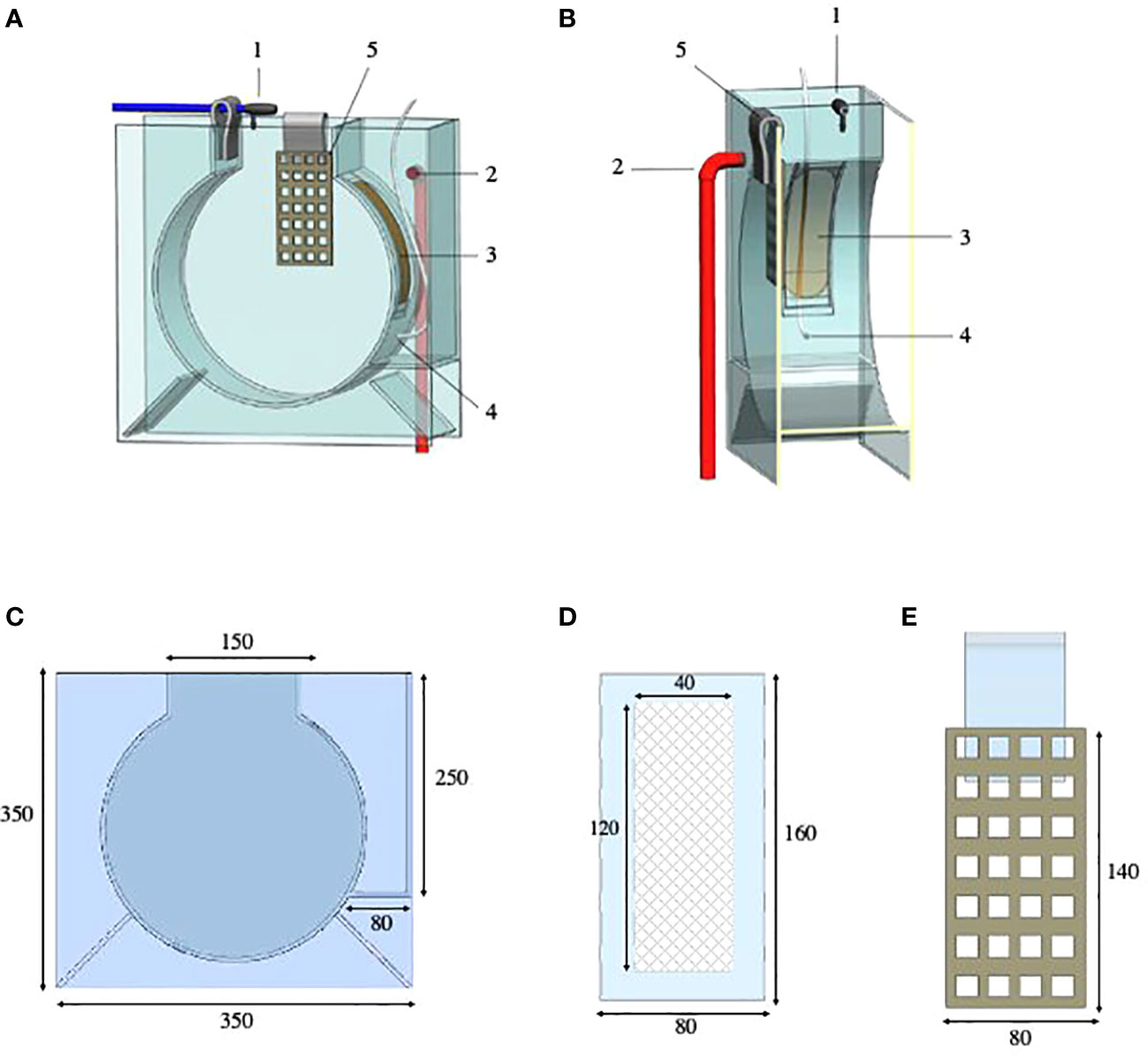
Figure 1 Schematic representation of the aquarium and accessories for the growth of the early stages of jellyfish: (A) Frontal view. (B) Transverse modified section. (C) Aquarium measures (mm). (D) Interchangeable mesh screen measures (mm). (E) Polyp plate measures (mm). Aquarium configuration: 1, water inlet in the main chamber (blue pipe, Ø ext.=8 mm); 2, water outlet in the drainage chamber (red pipe, Ø ext.=20 mm); 3, interchangeable mesh screen (150, 250, 350 and 500 µm and 1 mm); 4, air inlet in the main chamber (white tube, Ø ext.=5 mm; 5, polyp plate (square grid, 20x20 mm). Polyp plate dimensions can be varied as desired. Schematic representation by Carlos Mengod.
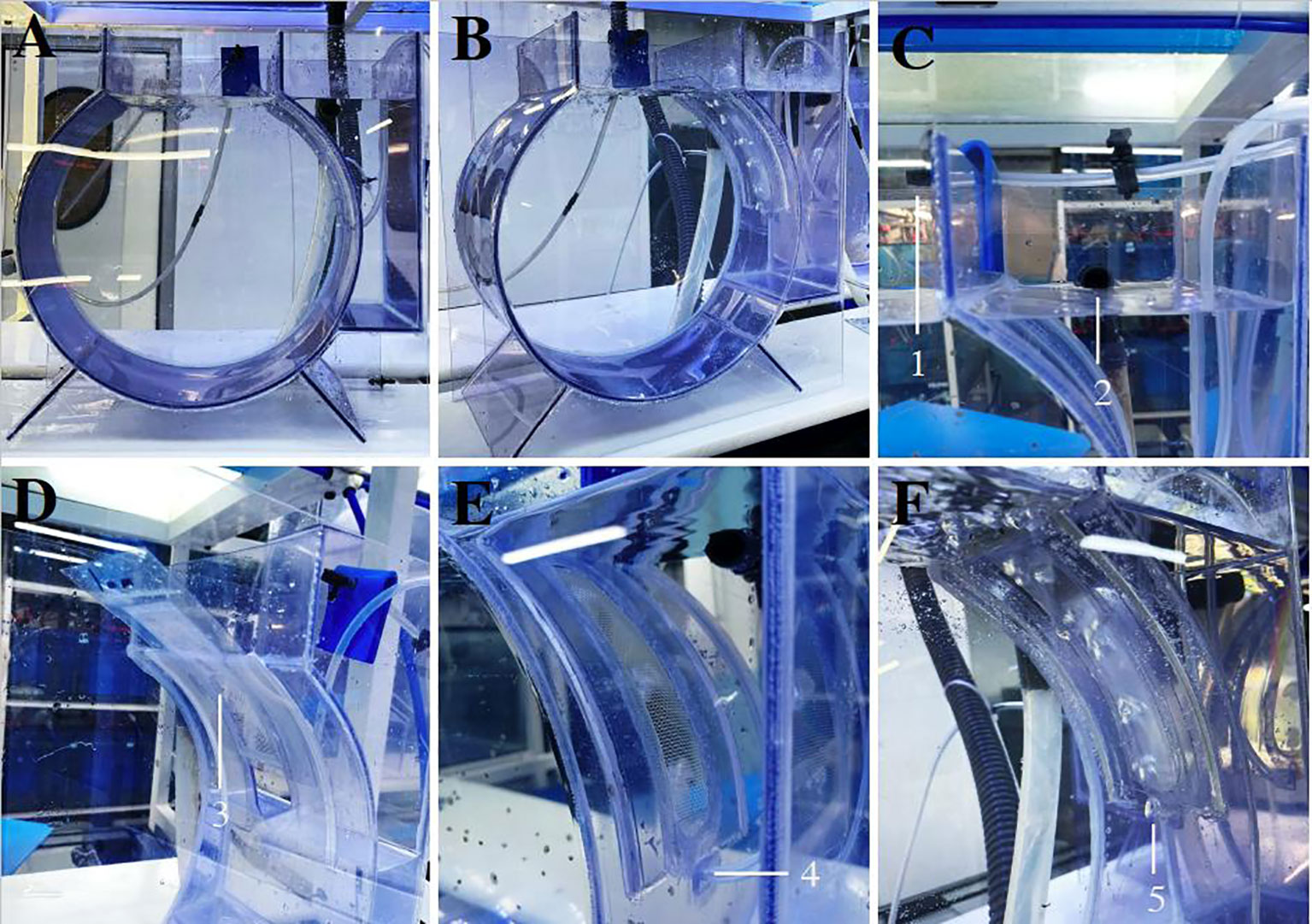
Figure 2 Aquarium for the growth out of the early jellyfish life stages: (A, B) Frontal view. (C) Drainage chamber. (D) Interchangeable mesh screen (150, 250, 350 and 500 µm and 1 mm). (E) Air inlet in the main chamber. (F) Air bubbles covering the interchangeable mesh screen. Aquarium configuration: 1, water inlet; 2, water outlet; 3, interchangeable mesh screen; 4, air inlet; 5, air bubbles.
The interchangeable mesh screen (150, 250, 350 and 500 µm and 1 mm) (Figures 1B, D, 2D) allows the changing of different sizes of mesh screen throughout the growth of the early jellyfish stages in situ without removing individuals from the tank.
An accessory, named the polyp plate (Figures 1A, E, 3A, B, E), can be easily placed to keep polyps suspended in the water column in a downward or sideways position, facilitating prey capture, strobilae formation and release of ephyrae (Figures 3C, D). The strobilation process can be induced in the tank. In jellyfish aquaculture, different methods are used to trigger the strobilation process (e.g., addition of chemicals or the up- or down-regulation of water temperature or salinity, Kuniyoshi et al., 2012; Treible and Condon, 2019). In this case, and in order to keep the desired chemical concentrations in a closed system, water entry to the system can be stopped with stopcocks.
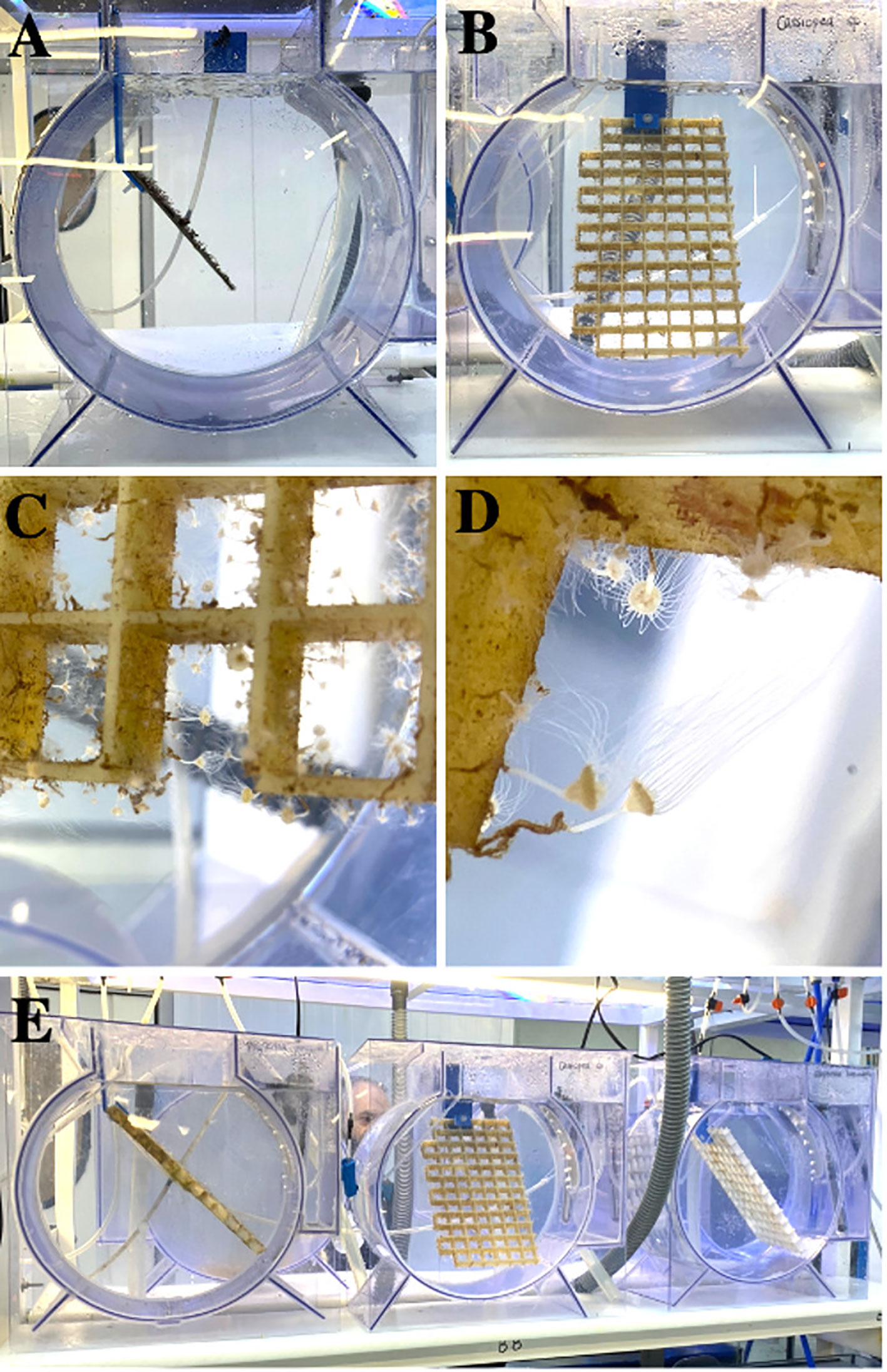
Figure 3 Polyp plates in flow-through aquarium: (A) Polyp plate of Aurelia spp. (B) Grid polyp plate of Cassiopea spp. (C) Suspended polyps in a downward or sideways position, enhancing prey capture, strobilae formation and release of ephyrae. (D) Tentacles of Cassiopea spp. polyps fully extended. (E) Polyp plates with Phyllorhiza punctata (left), Cassiopea spp. (middle) and Cotylorhiza tuberculata (right) species. Notes: The Cassiopea spp. polyp plates can be placed in rectangular tanks; The use of grid plates prevents the massive growth of algae; Multiple polyp plates can be placed in the same aquarium.
In order to increase biomass, the aquariums are placed in series in metal galvanized shelves in a temperature-controlled chamber (Figure 4). The water inlet is supplied with seawater that has been pretreated in a distribution system, obtained from a facility that provides access to high-quality seawater (e.g., Aquaria and Experimental Chambers of the Institute of Marine Science (ICM-CSIC)). The water is pretreated by mechanical (decantation and filtration with artificial polymers) and chemical (activated carbon) processes, as well as UV sterilized to remove unwanted organisms. For greater safety in case of any specific climatic event (e.g., storm), a filter system comprising filter cartridges of 20, 10 and 1 µm – the 1 µm filter cartridge can be replaced by a 5 µm cartridge – is placed before the water enters the sump (Figure 4A). For ease of emptying, there is a screw cap at the bottom (Figure 4B).
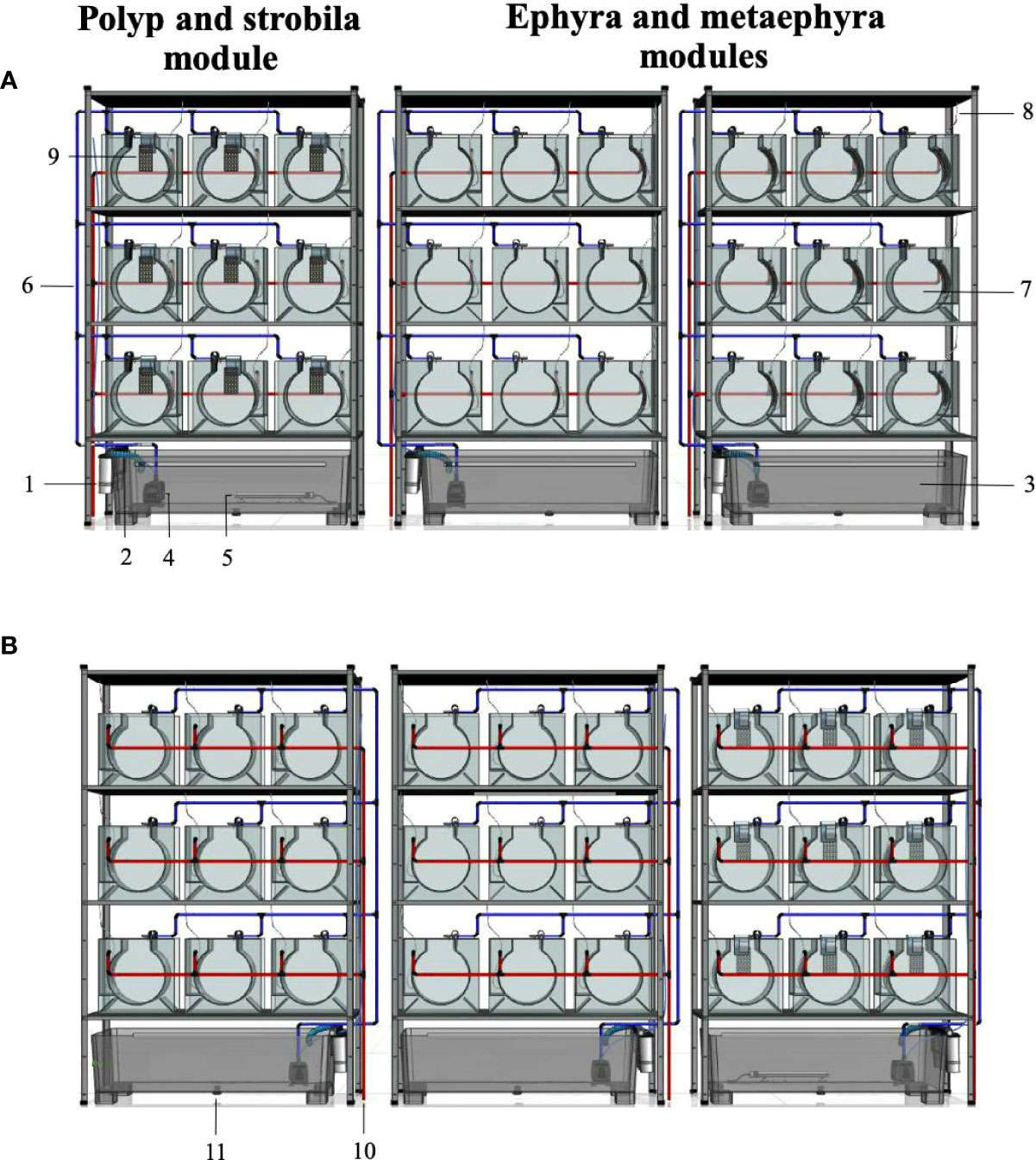
Figure 4 Schematic representation of a flow-through system of jellyfish biomass production for metagenetic and holoplanktonic species. (A) Frontal view. (B) Posterior view. System configuration: 1, filter system composed of filter cartridges; 2, water inlet towards the sump; 3, sump; 4, water pump; 5, heater; 6, individual water inlet to each kreisel (blue pipe); 7, kreisel; 8, individual air inlet to each kreisel; 9, polyp plate; 10, water drainage (red pipe); 11, screw cap for emptying the sump. Notes: Polyp plates with strobilae can be easily moved to the ephyra and metaephyra modules during the strobilation process; Fertilized eggs of Pelagia noctiluca can be metamorphosed into the ephyra and metaephyra modules.
From the sump, water is pumped through the pipes to each aquarium individually, and the inlet flow can be regulated by individual stopcocks (Figure 4A). Like the water inlet, the air inlet comes from the general system for each aquarium (Figure 4A). The air bubble flow can also be controlled with stopcocks. Lastly, the excess water stored in the drainage chamber flows into laboratory sewers through the system’s drain pipes (Figure 4B). Before being returned to the sea, the water is once again pretreated at the facility through mechanical and biological filtrations, pH control and UV sterilization.
Finally, and proving its versatility, this system has been successfully used for metagenetic species such as Aurelia sp. or Rhizostoma pulmo (Table 1), as well as for the breeding of the holoplanktonic jellyfish P. noctiluca in a flow-through system (Table 2). For P. noctiluca culture, fertilized eggs were transferred from sexually mature jellyfish to the aquariums. After three (22°C) or four days (18°C) the first ephyrae were observed in the tank, and they were grown until they reach the juvenile stage in flow-through aquariums (Figure 4). The conditions for its culture from fertilized eggs to metaephyrae in a flow-through system are given in Table 2.
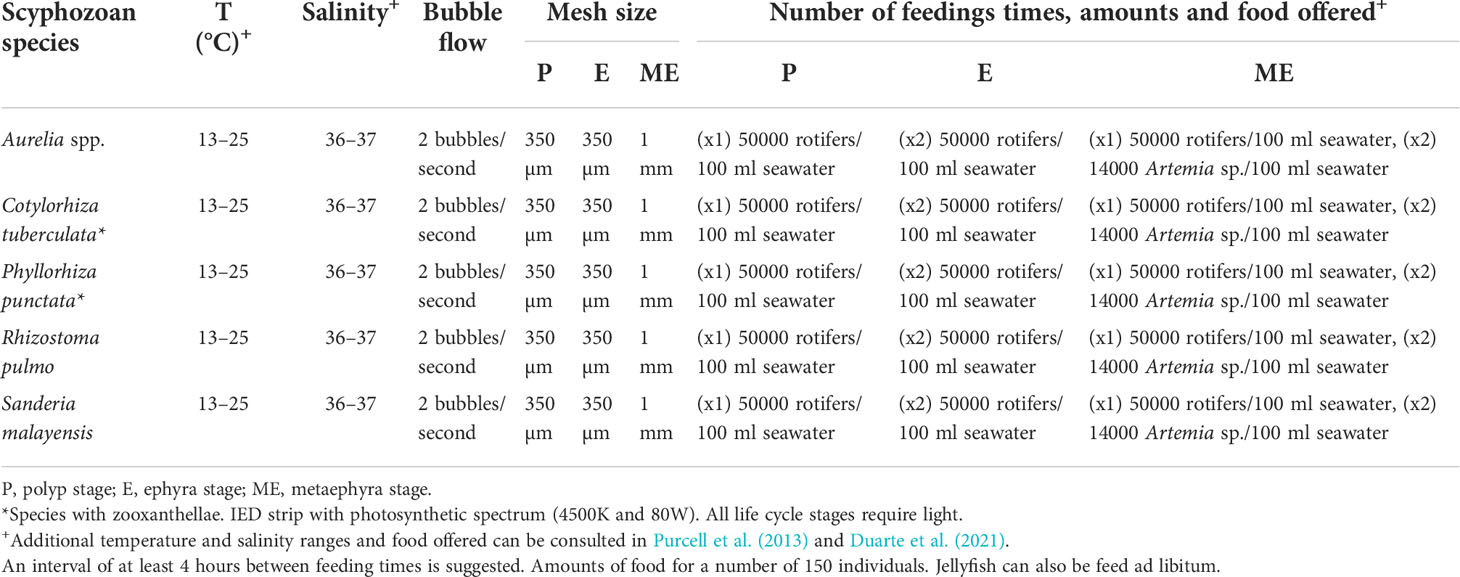
Table 1 Culture conditions for polyps, ephyrae and metaephyrae of some metagenetic species in flow-through system.
Jellyfish early life stages are commonly cultured in receptacles (e.g., balls glass and plastic jars) which involve recurring water changes as well as more staff time on maintenance routines (Duarte et al., 2021). In the recent review of jellyfish husbandry by Duarte et al. (2021), different types of ephyrae grow-out tanks have been proposed. However, of all of them, only the rectangular and V-shaped bottom aquariums allow growth in flow-through (Duarte et al., 2021, Figure 4 and Table 1). Here, we present a modified kreisel for rearing of the early planktonic stages of jellyfish in both flow-through and closed systems. The aquariums have been manufactured for an 8 L water capacity, but larger volumes can be mass-produced keeping the same configuration (Figure 1).
Collecting newly released ephyrae from polyp tanks represents a large investment of time for research staff and aquarists, who must chase ephyrae around the polyp tank to transfer them to new aquariums (Schaadt et al., 2017). To optimize jellyfish aquaculture techniques, polyp and ephyrae aquariums have been connected to each other in order to sweep ephyrae after release from the polyp towards ephyrae catch aquariums in a flow-through. An example of an ephyrae catch tank was proposed by Schaadt et al. (2017) for Aurelia spp. The system was composed of a small plastic vessel with a large parabolic arc-shaped mesh screen to one side of the tank followed by a hole allowing water drainage. Similar designs can be consulted in Raskoff et al. (2003) and Duarte et al. (2021) for any scyphozoan with a metagenetic life cycle. Here, the easily removable polyp plates allow movement between tanks (Figures 1A, 3A, B), and they can be returned to the polyp module once the strobilation process is finished (Figure 4). The suspension of the polyp plate in the kreisel allows the scyphistome to reside in a position where the tentacles are suspended downward or sideways as in the wild, improving prey capture, strobilae formation and release of ephyrae (Figures 3C, D) (Brewer, 1976). In addition, it also prevents the polyp from being covered by debris. However, for the rearing of Cassiopea spp., a benthic jellyfish, the use of the current kreisel design is not recommended, but to benefit from the improvements in polyp suspension (Figures 3C–E), the polyp plates can be placed in flow-through rectangular tanks. Those specifically interested in the culture of Cassiopea spp. should consult the system proposed by Pierce (2005).
The rearing of P. noctiluca is a challenge for the scientific and aquarist community (Ramondenc et al., 2019; Ballesteros et al., 2022). Lilley et al. (2014a) proposed a system composed of 5 to 15 L receptacles with motorized PVC paddles rotating to keep animals in suspension with recurrent water changes (Ramondenc et al., 2019, Figure 1A). According to Ramondenc et al. (2019), this system caused stress to the individuals and was not suitable for the farming of any jellyfish. Years later, Ramondenc et al. (2019) improved the rearing techniques for P. noctiluca and cultivated the individuals in a closed system kreisel aquarium, avoiding collision of the individuals with the vessel walls (Ramondenc et al., 2019, Figure 1B). The authors highlighted the difficulty of adjusting prey concentration to feed individuals ad libitum and avoiding the accumulation of debris from unassimilated food. The technical improvement of this aquarium (Figure 1) has allowed to provide large amounts of food or ad libitum thanks to the continuous renewal of new seawater (Tables 1, 2). In addition, for the first time to our knowledge, P. noctiluca to be safely maintained during their early life stages in a flow-through system (Table 2). The design of the screen mesh (Figures 1B, D), has allowed fertilized eggs metamorphose into ephyrae in the aquarium directly (Figure 4) without staff having to move the transparent ephyrae between receptacles as in Purcell et al. (2013), Lilley et al. (2014a), Ramondenc et al. (2019); Ballesteros et al. (2021) and Ballesteros et al. (2022). In disagreement with Purcell et al. (2013) and Duarte et al. (2021), we do not recommend the ephyrae grow-out of P. noctiluca in a flow-through system using conventional kreisels or rectangular tanks due to their risk of being retained on the mesh screen. Although our aquarium design has been used for different scyphomedusae (Table 1), specifically, we highlight the successful rearing of P. noctiluca from fertilized eggs to metaephyrae in flow-through, an important improvement and contribution to the rearing techniques of this scyphozoan (Table 2).
Jellyfish grow faster during the early stages of their planktonic life cycle (Widmer et al., 2005; Lilley et al., 2014a; Ramondenc et al., 2019), when the gastric system, prey capture structures and cnidome develop rapidly (Straehler-Pohl et al., 2011; Holst, 2012), increasing the possibility of eating larger amounts of food and capturing larger prey (Ballesteros et al., 2021). Like rearing vessels or aquariums, food regimens also influence the proper development of the gastric system and the growth and survival rates in jellyfish aquaculture (Widmer et al., 2005; Lilley et al., 2014a; Miranda et al., 2016; Ramondenc et al., 2019). Overall, enriched Artemia spp. nauplii and Brachiounus spp. (rotifers) are frequently used for husbandry (Raskoff et al., 2003; Purcell et al., 2013; Crow et al., 2013; Duarte et al., 2021) but some researchers have highlighted the importance of prey size for proper rearing, particularly during the polyp and early planktonic life cycle stages (Widmer et al., 2005; Purcell et al., 2013; Miranda et al., 2016; Ballesteros et al., 2022). In our system, the interchangeable mesh screen (Figures 1B, D, 2D) allows adaptation of the size of the mesh to the size of the prey in the aquarium. For example, if the prey offered is Brachionus plicatilis (≈ 100 – 200 µm) – an ideal size range for capture and ingestion by polyps (Raskoff et al., 2003) and ephyrae such as Phyllorhiza punctata (Miranda et al., 2016) and P. noctiluca (Ballesteros et al., 2022) – a mesh screen of 350 µm can be selected (Tables 1, 2). When Artemia sp. (≈ 400 µm) (Raskoff et al., 2003) is included for feeding larger individuals during the metaephyrae stage, the previous mesh screen (350 µm) can be changed for a 1 mm screen (Tables 1, 2). In this way, not only is the permanence of the feed in the tank in a flow-through system improved, but it also allows the passage and drainage of debris, avoiding the mesh screen to become clogged. The air inlet moves the individuals inside the tank without any damage (Figures 5E, F), since the air bubbles are safe for individuals with a total body diameter less than 30 mm as reported by Raskoff et al. (2003). It is important to transfer juvenile medusae to their appropriate aquariums (Purcell et al., 2013; Duarte et al., 2021) to avoid any possible damage.
Despite the advantages of the mesh screen change, screens still collect debris quickly in any system and they should be scrubbed and cleaned regularly (Raskoff et al., 2003). When screens become clogged, organisms are more likely to stick to them, and inadequate drainage can even lead to overflow involving the total loss of jellyfish. Here, the mesh screen can be replaced in situ without stressing the jellyfish on a regular basis, allowing better cleaning and disinfection of the removable mesh screen and improving water quality, a critical requirement during the husbandry of early jellyfish stages (Raskoff et al., 2003; Duarte et al., 2021).
In summary, the improvements in this modified kreisel have made it possible to optimize the time invested by staff in rearing the early life-cycle stages of jellyfish with a metagenetic life cycle, such as R. pulmo, and species with a holoplanktonic life cycle such as P. noctiluca. Its configuration and accessories offer the aquarium a versatile and innovative approach in the field of jellyfish rearing techniques, allowing the use of the same aquarium for the maintenance of fertilized eggs, planulae, polyps, strobilae, ephyrae and metaephyrae in both flow-through and closed systems to safeguard the water quality.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
AB and J-MG conceived and designed the aquarium prototype. PS manufactured the aquariums. AB wrote and edited the original manuscript. PS, EJ, and J-MG revised the manuscript and contributed to its improvement. All authors agreed to the published version of the manuscript.
The authors declare that this study received funding from ISDIN (Barcelona, Spain). The funder had the following involvement in the study: the writing of this article and the decision to submit it for publication.
The funder was not involved in the study design, collection, analysis or interpretation of data.
Many thanks to the staff of the Experimental Aquarium Zone (ZAE) of the Institute of Marine Science (ICM-CSIC), especially to Elvira Martínez. The authors also thank Carlos Mengod for the schematic representation of the aquariums in this manuscript. Authors AB, PS, and J-MG acknowledge the institutional support of the ‘Severo Ochoa Centre of Excellence’ accreditation (CEX2019-000928-S).
AB and J-MG are intellectual authors of the utility model (Industrial Property number: U202132533) solicited by the Spanish National Research Council (CSIC).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ballesteros A., Östman C., Santín A., Marambio M., Narda M., Gili J.-M. (2021). Cnidome and morphological features of Pelagia noctiluca (Cnidaria: Scyphozoa) throughout the different life cycle stages. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.714503
Ballesteros A., Páez D., Santin A., García A., Martín Y., Alonso E., et al. (2022). Successful culture of Pelagia noctiluca (Cnidaria: Scyphozoa) over time: a continuous supply of the holoplanktonic jellyfish for research and industrial applications. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.911383
Brewer R. H. (1976). Larval settling behavior in Cyanea capillata (Cnidaria: Scyphozoa). Biol. Bull. 150, 183–199. doi: 10.2307/1540467
Camacho-Pacheco A. V., Gómez-Salinas L. C., Cisneros-Mata M. Á., Rodríguez-Félix D., Díaz-Tenorio L. M., Unzueta-Bustamante M. L. (2022). Feeding behavior, shrinking, and the role of mucus in the cannonball jellyfish stomolophus sp. 2 in captivity. Diversity 14, 103. doi: 10.3390/d14020103
Canepa A., Fuentes V., Sabatés A., Piraino S., Boero F., Gili J. M. (2014). “Pelagia noctiluca in the Mediterranean sea,” in Jellyfish blooms. Eds. Pitt K. A., Lucas C. H. (Dordrecht: Springer), 237–266. doi: 10.1007/978-94-007-7015-7_11
Crow G., Howard M., Levesque V., Matsushige L., Spina S., Schaadt M., et al. (2013). “Association of zoos and aquariums aquatic invertebrate TAG.,” in Jellyfish (Cnidaria/ ctenophora) care manual. Ed. Schaadt M. (Silver Spring), 5–79. doi: 10.13140/RG.2.1.4344.3045
D’Ambra I., Merquiol L. (2022). Jellyfish from fisheries by-catches as a sustainable source of high-value compounds with biotechnological applications. Mar. Drugs 20, 266. doi: 10.3390/MD20040266
Dong J., Jiang L. X., Tan K. F., Liu H. Y., Purcell J. E., Li P. J., et al. (2009). “Stock enhancement of the edible jellyfish (Rhopilema esculentum kishinouye) in liaodong bay, China: a review,” in Jellyfish blooms: Causes, consequences, and recent advances. Eds. Pitt K. A., Purcell J. E. (Dordrecht: Springer), 113–118. doi: 10.1007/978-1-4020-9749-2_8
Duarte I. M., Marques S. C., Leandro S. M., Calado R. (2021). An overview of jellyfish aquaculture: for food, feed, pharma and fun. Rev. Aquac. 14, 265–287. doi: 10.1111/raq.12597
Elliott A., Hobson V., Tang K. W. (2017). Balancing fishery and conservation: a case study of the barrel jellyfish Rhizostoma octopus in south Wales. ICES J. Mar. Sci. 74, 234–241. doi: 10.1093/icesjms/fsw157
Fuentes V., Straehler-Pohl I., Atienza D., Franco I., Tilves U., Gentile M., et al. (2011). Life cycle of the jellyfish Rhizostoma pulmo (Scyphozoa: Rhizostomeae) and its distribution, seasonality and inter-annual variability along the Catalan coast and the mar menor (Spain, NW Mediterranean). Mar. Biol. 158, 2247–2266. doi: 10.1007/s00227-011-1730-7
Fu Z., Shibata M., Makabe R., Ikeda H., Uye S. I. (2014). Body size reduction under starvation, and the point of no return, in ephyrae of the moon jellyfish Aurelia aurita. Mar. Ecol. Prog. Ser. 510, 255–263. doi: 10.3354/meps10799
Gueroun S. K., Torres T. M., Dos Santos A., Vasco-Rodrigues N., Canning-Clode J., Andrade C. (2021). Catostylus tagi (Class: Scyphozoa, order: Discomedusae, suborder: Rhizostomida, family: Catostylidae) life cycle and first insight into its ecology. PeerJ 9, e12056. doi: 10.7717/peerj.12056
Helm R. R. (2018). Evolution and development of scyphozoan jellyfish. Biol. Rev. 93, 1228–1250. doi: 10.1111/brv.12393
Holst S. (2012). Morphology and development of benthic and pelagic life stages of north Sea jellyfish (Scyphozoa, cnidaria) with special emphasis on the identification of ephyra stages. Mar. Biol. 159, 2707–2722. doi: 10.1007/s00227-012-2028-0
Kuniyoshi H., Okumura I., Kuroda R., Tsujita N., Arakawa K., Shoji J., et al. (2012). Indomethacin induction of metamorphosis from the asexual stage to sexual stage in the moon jellyfish, Aurelia aurita. Biosci. Biotechnol. Biochem. 76, 1397–1400. doi: 10.1271/bbb.120076
Lilley M. K., Elineau A., Ferraris M., Thiéry A., Stemmann L., Gorsky G., et al. (2014b). Individual shrinking to enhance population survival: quantifying the reproductive and metabolic expenditures of a starving jellyfish, Pelagia noctiluca. J. Plankton Res. 36, 1585–1597. doi: 10.1093/plankt/fbu079
Lilley M. K. S., Ferraris M., Elineau A., Berline L., Cuvilliers P., Gilletta L., et al. (2014a). Culture and growth of the jellyfish Pelagia noctiluca in the laboratory. Mar. Ecol. Prog. Ser. 510, 265–273. doi: 10.3354/meps10854
Marambio M., Canepa A., Lòpez L., Gauci A. A., Gueroun S. K. M., Zampardi S., et al. (2021). Unfolding jellyfish bloom dynamics along the Mediterranean basin by transnational citizen science initiatives. Diversity 13, 274. doi: 10.3390/d13060274
Merquiol L., Romano G., Ianora A., D’Ambra I. (2019). Biotechnological applications of scyphomedusae. Mar. Drugs 17, 604. doi: 10.3390/md17110604
Miranda F. S., Chambel J., Almeida C., Pires D., Duarte I., Esteves L., et al. (2016). Effect of different diets on growth and survival of the white-spotted jellyfish, Phyllorhiza punctata. Front. Mar. Sci. doi: 10.3389/conf.FMARS.2016.04.00042
Olguín-Jacobson C., Pitt K. A., Carroll A. R., Melvin S. D. (2020). Polyps of the jellyfish Aurelia aurita are unaffected by chronic exposure to a combination of pesticides. Environ. Toxicol. Chem. 39, 1685–1692. doi: 10.1002/etc.4750
Omori M., Nakano E. (2001). Jellyfish fisheries in southeast Asia. Hydrobiologia 451, 19–26. doi: 10.1023/A:1011879821323
Pierce J. (2005). A system for mass culture of upside-down jellyfish Cassiopea spp as a potential food item for medusivores in captivity. Int. Zoo Yearb 39, 62–69. doi: 10.1111/j.1748-1090.2005.tb00005.x
Purcell J. E., Baxter E. J., Fuentes V. L. (2013). “Jellyfish as products and problems of aquaculture,” in Advances in aquaculture hatchery technology. Eds. Allan G., Burnell G. (Cambridge, United Kingdom: Elsevier), 404–430. doi: 10.1533/9780857097460.2.404
Ramondenc S., Ferrieux M., Collet S., Benedetti F., Guidi L., Lombard F. (2019). From egg to maturity: A closed system for complete life cycle studies of the holopelagic jellyfish Pelagia noctiluca. J. Plankton Res. 41, 207–217. doi: 10.1093/plankt/fbz013
Raskoff K. A., Sommer F. A., Hamner W. M., Cross K. M. (2003). Collection and culture techniques for gelatinous zooplankton. Biol. Bull. 204, 68–80. doi: 10.2307/1543497
Sandrini L. R., Avian M. (1983). Biological cycle of Pelagia noctiluca: morphological aspects of the development from planula to ephyra. Mar. Biol. 74, 169–174. doi: 10.1007/BF00413920
Schaadt M., Widmer C. L., Sowinski N. (2017). “Jellyfish,” in Marine ornamental species aquaculture. Eds. Calado R., Olivotto I., Oliver M., Holt J. (New Jersey: John Wiley & Sons Ltd), 457–473. doi: 10.1002/9781119169147
Straehler-Pohl I., Jarms G. (2010). Identification key for young ephyrae: a first step for early detection of jellyfish blooms. Hydrobiologia 645, 3–21. doi: 10.1007/978-90-481-9541-1_2
Straehler-Pohl I., Widmer C. L., Morandini A. C. (2011). Characterizations of juvenile stages of some semaeostome scyphozoa (Cnidaria), with recognition of a new family (Phacellophoridae). Zootaxa 2741, 1–37. doi: 10.11646/zootaxa.2741.1.1
Treible L. M., Condon R. H. (2019). Temperature-driven asexual reproduction and strobilation in three scyphozoan jellyfish polyps. J. Exp. Mar. Biol. Ecol. 520, 151204. doi: 10.1016/J.JEMBE.2019.151204
Keywords: aquaculture, ephyra, kreisel, metaephyra, Pelagia noctiluca, polyp, strobilation, tank
Citation: Ballesteros A, Siles P, Jourdan E and Gili J-M (2022) Versatile aquarium for jellyfish: A rearing system for the biomass production of early life stages in flow-through or closed systems. Front. Mar. Sci. 9:942094. doi: 10.3389/fmars.2022.942094
Received: 12 May 2022; Accepted: 02 August 2022;
Published: 18 August 2022.
Edited by:
Sílvia Lourenço, Instituto Politécnico de Leiria, PortugalReviewed by:
Tiago Filipe Baptista Da Rosa Repolho, University of Lisbon, PortugalCopyright © 2022 Ballesteros, Siles, Jourdan and Gili. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ainara Ballesteros, YmFsbGVzdGVyb3NAaWNtLmNzaWMuZXM=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.