
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 13 September 2022
Sec. Deep-Sea Environments and Ecology
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.941793
This article is part of the Research TopicManaging Deep-sea and Open Ocean Ecosystems at Ocean Basin Scale - Volume 2View all 10 articles
Humpback whales (Megaptera novaeangliae) produce song and non-song vocalisations, which allows their presence to be detected through passive acoustic monitoring. To determine the seasonal and diel acoustic presence and acoustic behaviour of humpback whales at the migratory stopover site off Bermuda, three hydrophones were deployed between March 2018 and April 2019 on Challenger Bank and the Bermuda platform. Song was the predominant vocalisation type encountered, with 65% of song recordings containing whale chorus and a clear seasonal trend of humpback whale occurrence in the spring and winter months from late December to mid-May. A strong diel pattern in singing activity was detected. Singing activity significantly increased at night relative to the daytime (p<0.01), whilst twilight periods were characterised by intermediate levels of singing. The song structure encountered in spring 2018 consisted of 18 units, 6 themes and 5 transitional phrases. The high occurrence of whale chorus and the strong seasonal and diel patterns of male humpback whale singing activity highlights the importance of Bermuda not just on their northward migration during spring, as described historically, but also on their southward migration during winter. Bermuda therefore constitutes a two-way migratory stopover site for humpback whales. The present study also provides Bermuda’s planning authorities with better constraints on the duration and intensity of anthropogenic activities in these waters.
Humpback whales (Megaptera novaeangliae) are one of the large baleen whales best known for their extremely variable vocal behaviour. “Song of the Humpback Whale”, recorded off Bermuda in the 1950s by Frank Watlington, was the first recording of humpback whale song worldwide and initiated an era of humpback whale research off Bermuda throughout the 1960s and 1970s (Payne and Payne, 1985). While humpback whales produce unstructured non-song vocalisations year-round in different behavioural contexts (Silber, 1986; Dunlop et al., 2007; Rekdahl et al., 2015), humpback whale song is the most dominant vocal display of the species. Humpback whale song is displayed exclusively by males (Herman et al., 2013), and is thought to be a multi-message reproductive display (Murray et al., 2018) involved in both inter- (Smith et al., 2008) and intra-sexual interactions (Darling and Berube, 2001; Cholewiak et al., 2018b). However, the exact function of the song remains uncertain (Herman, 2017).
Humpback whale songs differ across ocean basins, due to the geographic isolation of the three recognised humpback whale subspecies (Baker et al., 1990; Bettridge et al., 2015; Cooke, 2018), and to some extent within an ocean basin, but are the same within a breeding population (Murray et al., 2012; Garland et al., 2013; Nikšić, 2014; Darling et al., 2019). Song is defined as a repetitive, stereotyped vocal display with a hierarchical structure (Payne and McVay, 1971; Cholewiak et al., 2013). A humpback whale “song” or “song cycle” is repeated for the duration of a “song session”, i.e., the time period an individual whale sings continuously, which has been shown to last up to 22 hours (Winn and Winn, 1978). A song can be subdivided into a hierarchical order of several distinct “themes”, each of which consists of repeated “phrases”, which in turn consist of a sequence of individual “units” (Payne and McVay, 1971; Cholewiak et al., 2013). Along with songs produced by bowhead whales (Balaena mysticetus), humpback whale song is considered the most complex (Janik, 2009; Stafford et al., 2018). A population’s song undergoes constant and progressive changes through time, a phenomenon referred to as song evolution (or song revolution in the case of sudden changes) (Winn and Winn, 1978; Payne and Payne, 1985; Noad et al., 2000; Garland et al., 2011; Garland et al., 2017).
North Atlantic humpback whales undertake extensive seasonal migrations between high latitude summer feeding grounds – off northern Norway and Iceland (referred to as eastern feeding grounds), as well as western Greenland, eastern Canada and the northeastern United States (referred to as western feeding grounds) – and low latitude winter breeding grounds around the West Indies and Cape Verde (Stevick et al., 2003; Reeves et al., 2004; Wenzel et al., 2009; Ruegg et al., 2013; Bettridge et al., 2015). However, fluke identification matches now suggest that the Caribbean breeding ground might be further subdivided, as humpback whales wintering in the southeast Caribbean are behaviourally distinct from those wintering in the northwest Caribbean, in two ways (Stevick et al., 2016). First, humpback whales winter in the northwestern Caribbean between January–April with peaks between February–March, while those wintering in the southeastern Caribbean do so a bit later between March–May with peaks in April (Charif et al., 2001; Stevick et al., 2003; Gandilhon, 2012; Stevick et al., 2018; Heenehan et al., 2019). Second, re-sightings of individuals revealed a strong tendency for southeastern Caribbean humpback whales to migrate to eastern North Atlantic feeding grounds, while whales from northwestern Caribbean breeding grounds tend to migrate to western feeding grounds (MacKay, 2015; Stevick et al., 2018), causing some genetical differentiation (partly due to feeding ground destination in humpback whales showing strong maternally-directed fidelity) (Baker et al., 1990; Palsboll et al., 1995; Weinrich, 1998). However, some individuals have been matched between Cape Verde and the southeastern Caribbean, as well as between the southeastern and northwestern Caribbean, demonstrating that the population units and boundaries are not as clear as previously thought (Stevick et al., 2016; MacKay et al., 2019). While the western North Atlantic humpback whale population has been increasing in recent years after the cessation of whaling, the Cape Verde population is still of considerable concern (Wenzel et al., 2020).
Although historically believed to exclusively occur on breeding grounds (Winn and Winn, 1978), humpback whale song has been increasingly recorded on their feeding grounds during the breeding season, which suggests that some males may not migrate at all but instead remain year-round in their feeding grounds (Mattila et al., 1987; Vu et al., 2012; Baumgartner et al., 2019; Magnúsdóttir and Lim, 2019; Kowarski et al., 2021; Tyarks et al., 2021). In the North Atlantic, humpback whales start singing in early autumn (around September) and continue singing through winter, stopping in late spring (around June) (Mattila et al., 1987; Vu et al., 2012; Kowarski et al., 2019; Kowarski et al., 2021). Transitions between song and “non-song” periods at the start and end of summer are dominated by “song fragments”, i.e., only a short part of the complete song is sung (Mattila et al., 1987; Vu et al., 2012; Kowarski et al., 2019; Kowarski et al., 2021). The seasonal singing behaviour displayed by males is thought to underlie a hormonally triggered physiological mechanism (Wright and Walsh, 2010; Vu et al., 2012), as the males’ testosterone levels are the lowest during the summer months and highest during the winter months, i.e., during the breeding season (Cates et al., 2019). Thus, song fragments could be the result of spring decreases and autumnal increases in testosterone levels (Cates et al., 2019; Kowarski et al., 2019).
Notably, most of what is known about North Atlantic humpback whales and their vocalisations has come from coastal studies on feeding and breeding grounds. Thus, their migration routes and mid-ocean behaviours, including vocalisations, with the exception of a few studies tracking individual whales with satellite tags (Kennedy et al., 2014; Kennedy and Clapham, 2017), remain vastly understudied or unknown (Reeves et al., 2004; MacKay, 2015; Kowarski et al., 2018). Bermuda, being an oceanic migratory stopover site for North Atlantic humpback whales on their northward migration (Payne and McVay, 1971; Stone et al., 1987; Stevenson and Stevick, 2009), provides a unique opportunity to study vocalisations of migrating humpback whales. Individuals observed off Bermuda have been re-sighted in the northwestern Caribbean breeding grounds and to a much lesser extent in the southeastern Caribbean, as well as, in all major feeding grounds (with the exception of Norway) (Stone et al., 1987; Beaudette et al., 2009; Stevenson, 2010; MacKay, 2015; Stevenson, unpublished data), but predominantly in western North Atlantic feeding grounds (Stone et al., 1987; Beaudette et al., 2009; Kennedy et al., 2014). Thus, the waters around Bermuda most likely represent an oceanic migratory stopover site between the northwestern Caribbean breeding grounds and higher latitude western feeding grounds. While in Bermuda, humpback whales have been observed to linger for several days whilst aggregating into large groups, accompanied by male singing (Payne and McVay, 1971; Payne and Payne, 1985; Stevenson, 2011), before continuing their northward migration.
Recordings of humpback whales off Bermuda led to the first formal description and definition of humpback whale song (Payne and McVay, 1971), which is now fundamental to the field of humpback whale song research. However, there has been no acoustic recording or analysis of whale vocalisations in Bermuda since 1976 (Winn and Winn, 1978; Payne and Payne, 1985) and these initial studies did not use long-term Passive Acoustic Monitoring (PAM) deployments that permit year-round data collection (under all weather conditions and overnight) of the marine soundscape and therefore year-round acoustic detection of vocal species like humpback whales (Johnson et al., 2009; Rafter et al., 2018). Thus, in stark contrast to a good baseline knowledge of the seasonal occurrence of humpback whale vocalisations from North Atlantic feeding and breeding grounds, the acoustic presence of humpback whales at their stopover site off Bermuda has not yet been analysed.
Such knowledge on the temporal presence of whales off Bermuda is urgently needed to address potential threats posed by increasing human activities. The Government of Bermuda has enacted some protection for humpback whales under its Fisheries Act 1972 (Government of Bermuda, 1978) and its Protected Species Act 2003 (Government of Bermuda, 2003; Minister of Health Seniors and Environment, 2016) and there are voluntary whale-watching guidelines (Department of Environment and Natural Resources, 2017). Bermuda’s Exclusive Economic Zone (EEZ) is also an important migratory corridor and stopover location for various cetacean species (Klatsky et al., 2007; Hallett, 2011; Hoyt, 2011) and was designated as a Marine Mammal Sanctuary but this designation only offers data-sharing opportunities and comes with no management or protection measures (NOAAGovernment of Bermuda, 2012). With Bermuda becoming popular as a megafauna “hotspot”, as a cruise destination and sites for various international sporting events, the tourism industry including the whale-watching industry (O’Connor et al., 2009) is anticipated to further develop in line with Bermuda’s six-year National Tourism Plan 2015–2023 (Bermuda Tourism Authority, 2019). New legislation under the Superyachts and Other Vessel (Miscellaneous) Act 2019 now also allows more large yachts (>24 meters length) to secure cruising and charter permits. Thus, growth in Bermuda’s tourism industry will increase vessel traffic of all kinds (cruise liners, freight, superyachts, whale-watching boats and smaller recreational craft).
Increased vessel traffic and marine tourism can have various negative impacts on humpback whales, from behavioural disturbance, increased stress levels and physical injuries, to disturbing their crucial auditory sensory system and communication (Au and Green, 2000; Cholewiak et al., 2018a; Fiori et al., 2020; Sprogis et al., 2020; Currie et al., 2021). Humpback whales produce low to mid frequency vocalisations (ranging from 0.01-28 kHz), but like all baleen whales most energy is produced in the lower frequencies (below 2 kHz), which can propagate across an entire ocean basin (Payne and Webb, 1971; Hannay et al., 2013; Cerchio et al., 2014; Huang et al., 2016; Cholewiak et al., 2018a; Davis et al., 2020). However, vessel-generated noise, the most prevalent anthropogenic underwater noise (Cato, 2014), overlaps in frequency (Clark et al., 2009; Rolland et al., 2012) and thus interferes with the acoustic detection of mysticetes non-song and song vocalisations (“masking”) (André, 2018; Dooling and Leek, 2018) and reduces the distance over which they are able to acoustically communicate (Clark et al., 2009; Rolland et al., 2012; Cato, 2014; Cholewiak et al., 2018a; Dunlop, 2019). Thus, to mitigate impacts of increased anthropogenic noise levels on humpback whales migrating through Bermuda, knowledge of spatiotemporal patterns of humpback whale presence and vocalisations needs to be gathered and integrated by ocean planners and authorities into planning and management scenarios and decisions for sustainable developments.
The present study is the first long-term PAM study of humpback whale vocalisations in Bermuda. The aim is to investigate their seasonal and diel acoustic presence and acoustic behaviour at this migratory stopover site. To facilitate future comparisons of song structures across the North Atlantic, as well as within Bermuda (determining inter-annual song variation), the song structure encountered in spring 2018 will be described in detail at the unit, phrase and theme level.
Bermuda forms part of a small mid-ocean seamount chain of volcanic origin rising abruptly from the deep abyssal plain of the Sargasso Sea (Figure 1) (Stone et al., 1987; Vogt and Jung, 2007). Besides the topographic highs of the inhabited Bermuda platform, Bermuda’s EEZ has three large submerged seamounts, which are known for their high biodiversity: Bowditch Seamount, Challenger Bank (CB) and Plantagenet Bank (Figure 1) (Vogt and Jung, 2007; Hallett, 2011). CB and Sally Tucker (ST; located at the southwest edge of the Bermuda platform), which are 13 km apart, were chosen as recording sites for the present study (Figure 1).
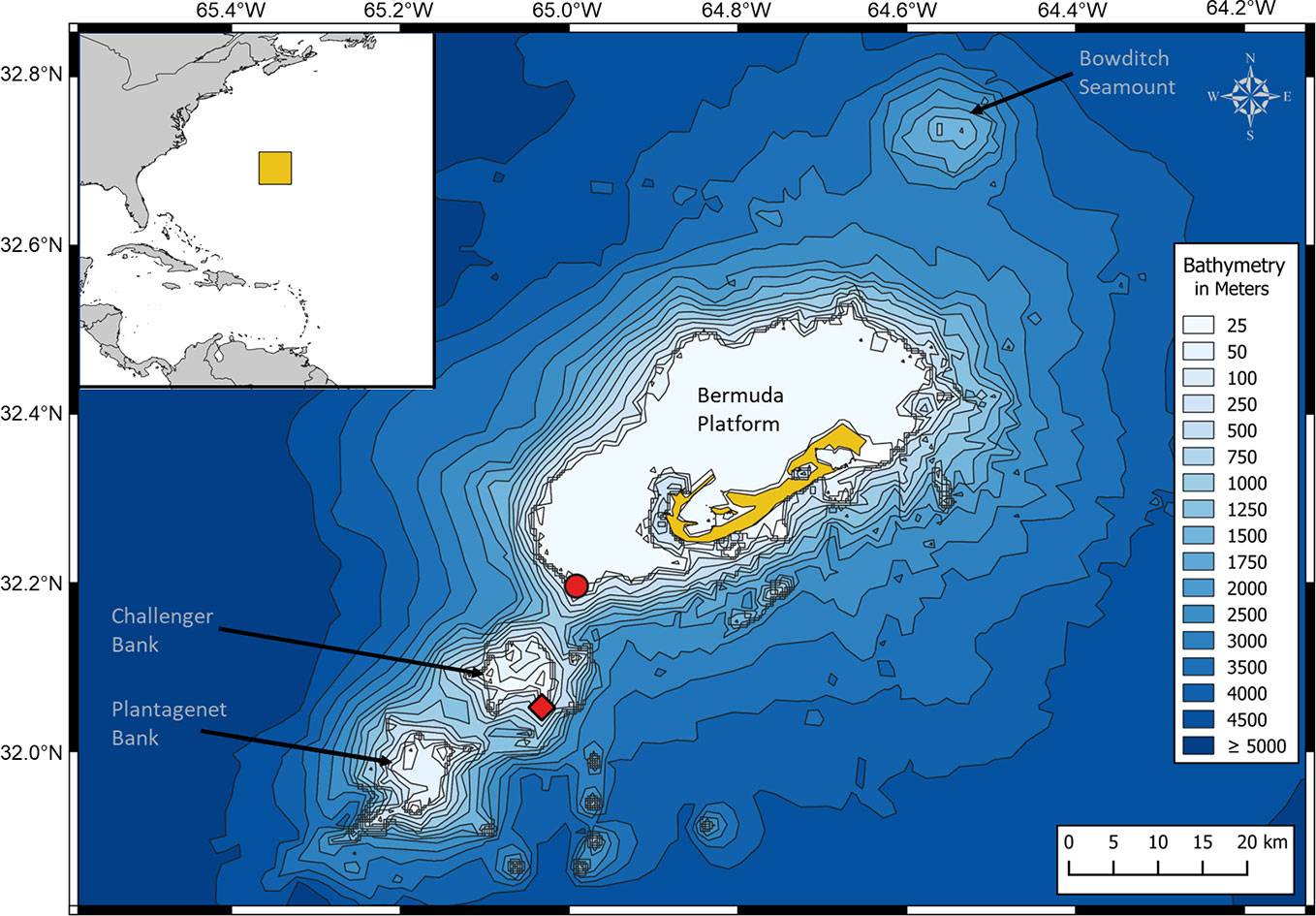
Figure 1 Map of the study area, showing Bermuda (in yellow) and the three seamounts Bowditch Seamount, Challenger Bank and Plantagenet Bank. The three hydrophones were deployed on Challenger Bank (red rhombus) and Sally Tucker (red circle), which are 13 km apart. The map was created in QGIS 3.2 software (QGIS Development Team, 2016) using the coordinate reference system WGS 84, EPSG: 4326 and a world (source: Natural Earth) and bathymetry map (source: Open Digital Elevation Model, 2019).
Two Autonomous Multichannel Acoustic Recorders (AMAR G3A; JASCO Applied Sciences) equipped with M36-V35-100 omnidirectional hydrophones (-165 ± 4 dB re 1 V/μPa sensitivity) (Supplementary Figure 1) were deployed from 31 March to 6 September 2018 on CB at a water depth of 45.7 m at 32.08746, -65.05373 and from 31 March to 10 September 2018 on ST at a water depth of 40.2 m at 32.19605, -64.99133. The AMARs were programmed to record 30 minutes of every hour. Another AMAR equipped with M36-V35-900 omnidirectional hydrophone (-165 ± 4 dB re 1 V/μPa sensitivity) was deployed on CB at a water depth of 47.7 m from 10 September 2018 to 23 April 2019 at 32.08725, -65.05386, and programmed to record 30 minutes every 75 minutes. All three hydrophones located on the seafloor recorded 29 minutes at a sampling rate of 16 kHz (24 bit resolution) and 1 minute at a sampling rate of 250 kHz (16 bit resolution). These sampling rates were chosen to detect the lower frequency vocalisations of baleen whales (Kowarski et al., 2018), as well as high-frequency clicks and whistles produced by toothed whale (Edds-Walton, 1997). For the present study, only the 29-minute recordings were analysed. Due to technical failure of the recording device, recordings from 20 September 2018 to 1 November 2018 were not usable. The AMARs were retrieved from anchors using an acoustic release (Supplementary Figure 1).
All recordings were manually scanned for humpback whale sounds using spectrograms generated with Raven Pro 1.6 sound analysis software (fast Fourier transformation [FFT] size: 2048 points, 75% overlap, Hann window, frequency resolution: 7.8 Hz, time resolution: 32 ms) (Center for Conservation Bioacoustics, 2019). As most humpback whale vocalisations are detected below 2 kHz (Silber, 1986; Cerchio et al., 2014; Huang et al., 2016), the 29-minute-long recordings were viewed zooming into the frequency band of 0–2 kHz.
Every 29-minute recording was first scanned for humpback whale song. If no song was detected then the recording was re-scanned for other humpback whale vocalisations (non-song vocalisations) to capture the acoustic presence of whales, in absence of song. Each recording was classified into one of three whale sound categories: song (song fragments were also allocated to this category), calls, and no vocalisation.
Second, all recordings allocated to the song category were re-analysed to assess the minimum number of singing whales (“singers”) per hour. Spectrograms of song-containing recordings were scanned for the first 10 minutes (Cerchio et al., 2014; Cholewiak et al., 2018b) of song detection to determine the highest number of overlapping singers in that period. Number of singers was determined by visually counting (i) overlapping units, (ii) overlapping phrases and (iii) differences in sound intensity (Cerchio et al., 2014; Magnúsdóttir and Lim, 2019) from the spectrogram (Figure 2). This is because song structures overlapping in time cannot be produced by the same whale and therefore indicate the number of simultaneously singing whales at one moment in time (Magnúsdóttir and Lim, 2019), while sounds of different intensities indicate multiple vocalising whales at different distances from the hydrophone (Cerchio et al., 2014). Up to five simultaneously singing whales could be differentiated confidently in the present dataset. Therefore, every recording was allocated the minimum number of simultaneous singers, ranging from 0 to 5 (Figure 2). In instances where five or more singers were detected, they could not be further differentiated and were allocated the category “5”.
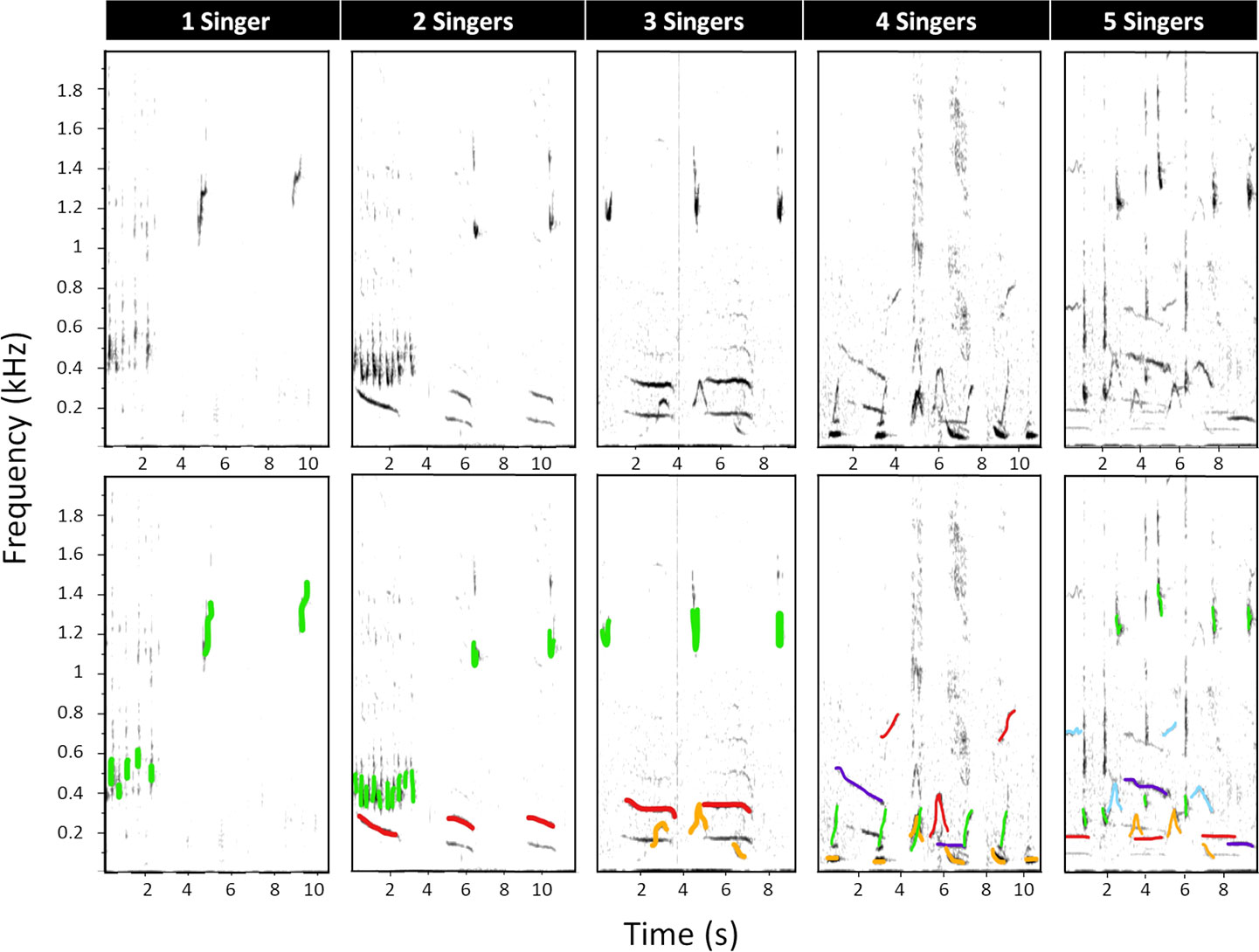
Figure 2 Spectrograms of multiple humpback whales signing, showing five different recordings that vary in their number of simultaneously singing humpback whales. In the lower panel, every singer has been allocated a colour to visualise overlapping units and/or overlapping phrases. Only the fundamental units were coloured. Differences in sound intensity can be seen in the upper panel by the darkness of a unit. Spectrogram parameters: fast Fourier transform (FFT) size = 2048 points, overlap = 75%, sample rate = 16000 Hz, frequency resolution = 7.8 Hz and time resolution = 32 ms.
Third, all 29-minute recordings where only one whale was singing one entire song cycle were graded into low, medium, and high-quality recordings (Supplementary Figure 2). Only recordings in the high-quality category were considered for the detailed 2018 Bermuda song description. To ensure consistency throughout the acoustic analysis, all recordings were analysed and categorised into vocalisation type, number of singers and quality by a single person (the first author).
Temporal patterns of humpback whale song based on the numbers of singing whales were statistically analysed in R version 3.6.2 (R Core Team, 2019), with figures created using the R-package ggplot2 (Wickham, 2016). To explore diel patterns, the entire dataset was shifted to Atlantic Standard Time (UTC -4, Bermudas standard time zone) and every day was split into four light conditions in accordance with previous studies (Cholewiak, 2008; Kowarski et al., 2018; Ryan et al., 2019): nighttime, dawn, daytime and dusk. To accommodate for the variation in day and night length over the study period, the corresponding time intervals defined by nautical dawn, sunrise, sunset and nautical dusk were determined separately for each day. The four variables were obtained from https://www.timeanddate.com/ (Time and Date, 2019a) and uniformly transformed to UTC -4. Then, the 29-minute-long audio files recorded during the around one-hour long dawn and dusk periods were allocated to the appropriate light level and the remaining recordings to the daytime or nighttime category accordingly. To further explore seasonal patterns, the entire study period was split into astronomical seasons, i.e., as defined by equinoxes and solstices (Time and Date, 2019b), giving rise to four datasets: ST-spring-2018, CB-spring-2018, CB-winter-2018/19 and CB-spring-2019.
Statistical analysis of diel patterns in singing activity focussed exclusively on days with song-containing recordings. As humpback whale songs can be detected over greater distances than the distance between CB and ST (Cholewiak et al., 2018a), we cannot exclude that individual whales were recorded simultaneously during overlapping time frames between the two sites. Given the unequal sample sizes between the light categories and seasons, the data not being normally distributed and the spring 2018 datasets potentially being dependent of each other, each dataset was analysed individually with a non-parametric Kruskal-Wallis test to determine if the mean number of singing whales significantly differed between different light conditions, to determine any diel variation. Consequently, Bonferroni post-hoc tests were conducted to identify specific pairs of conditions that differed.
Phrase and theme allocation in humpback whale song analysis is most often conducted through the subjective manual analysis of spectrograms (Cholewiak et al., 2013; Nikšić, 2014; Garland et al., 2017; Magnúsdóttir, 2017; Hauer-Jensen, 2018). Thus, it is recommended to base the song description on the review of multiple song recordings from different individuals (Cholewiak et al., 2013). Only the CB recordings from spring 2018 were used for the song description. By going through every song-containing recording (n = 777) in detail to determine the number of singers, the typical song structure became rapidly evident. Seven high-quality song recordings (Narganes Homfeldt et al., 2022), each around a week apart from the next, were chosen to describe the song in more detail. This interval was chosen to minimise the chances of describing the vocal display of the same individual (Kowarski et al., 2019; Magnúsdóttir and Lim, 2019), given that some individuals are sighted for eight consecutive days off Bermuda (Stevenson, 2011). The methodology to describe the songs generally followed approaches adopted in previous humpback whale song analyses (Nikšić, 2014; Garland et al., 2017; Magnúsdóttir, 2017; Darling et al., 2019; Kowarski et al., 2019) and is summarised below.
The seven songs were viewed as spectrograms (FFT size: 2048 points, 75% overlap, Hann window, frequency resolution: 7.8 Hz, time resolution: 32 ms) and each full song cycle was delineated at the unit and phrase level, based on aural and visual spectrographic characteristics. Distinct units and phrases were identified and allocated an alphanumeric code: units (a,b,c); phrases (1,2,3). As a theme is defined as a repeated sequence of the same or similar phrases (Payne and McVay, 1971; Cholewiak et al., 2013; Magnúsdóttir, 2017), themes were allocated the same numerical code as the phrase type it contained, e.g., phrase 1 is repeated within theme 1. The identified characteristic unit sequences were then grouped into phrases and phrase types into themes. Any natural variation in the characteristic unit sequences through addition, deletion or replacement of individual units within a phrase that would generate imperfect replicas of a phrase type (Payne and McVay, 1971; Cholewiak et al., 2013) were considered to be the same phrase type. In addition, there were “transitional phrases”, which are sometimes encountered at the transition between two subsequent themes and contain units from both themes (Payne and Payne, 1985; Garland et al., 2017). For example, a transitional phrase between themes 2 and 3 would be referred to as phrase 2/3. All seven songs were transcribed into alphanumerical sequences to facilitate the identification of the songs’ theme order.
Given the subjective nature of the song delineation process, Cholewiak et al. (2013) advised to support the alphanumerical classification with exemplar spectrograms to illustrate at least part of the song structure to the humpback whale song research field. Thus, in the present study, exemplars of the identified unit, phrase and theme order were illustrated (see Results).
The present study analysed a total of 347 days of continuous recording [excluding a 43-day period with no available recordings (Figure 3)] from 2018 and 2019 across two locations off Bermuda, scanning a total of 5417 hours of acoustic data for humpback whale vocalisations. Besides humpback whale song and non-song vocalisations, an unusually long, tonal baleen whale vocalisation, lasting for 18-seconds, was detected in the presence of multiple humpback whale singers (Supplementary Figure 3). Also, other biotic sounds emitted by dolphins (and possibly other cetacean species), fish and invertebrates, as well as anthropogenic sounds from vessels and echosounders, were regularly detected in recordings.
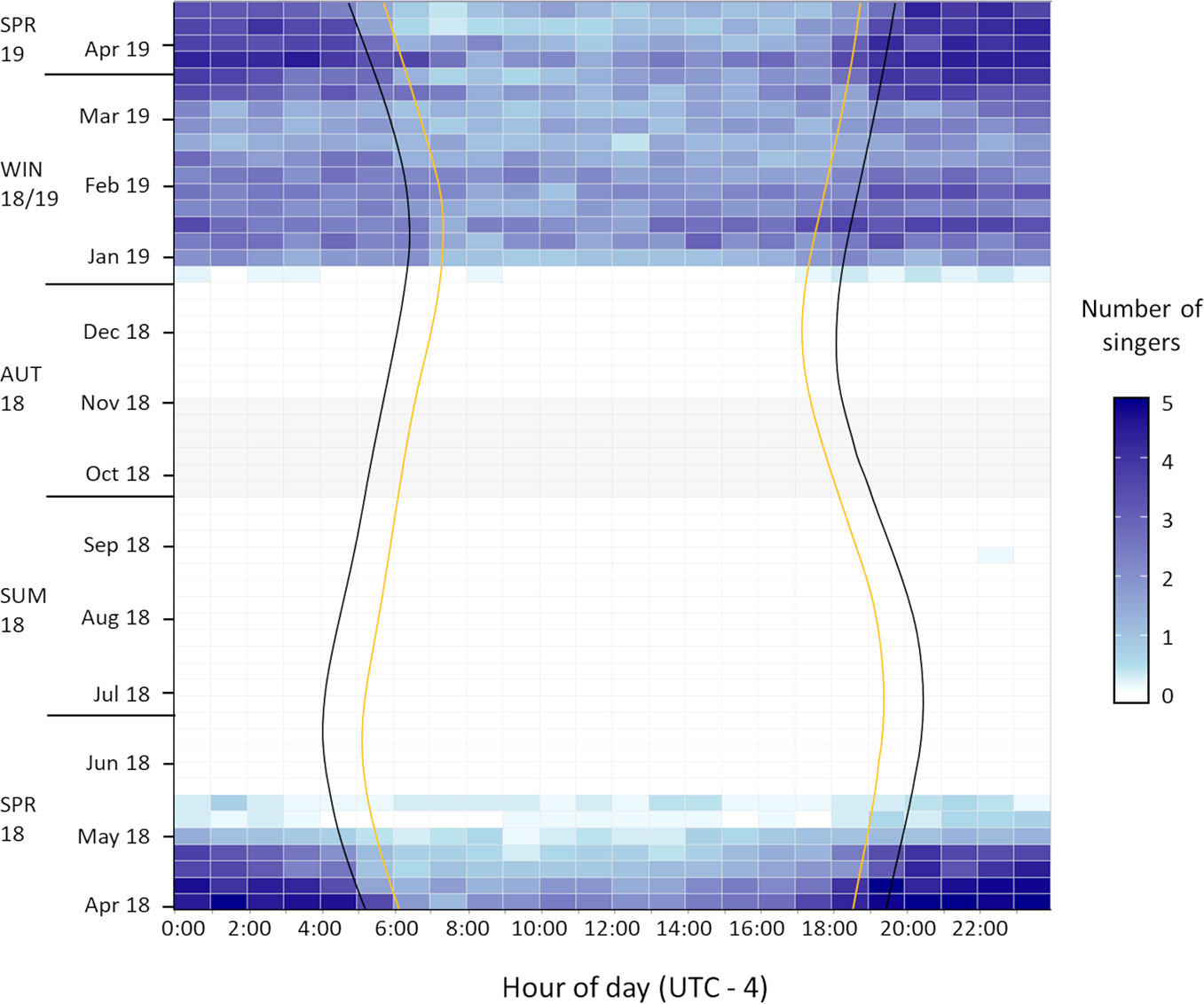
Figure 3 Seasonal occurrence and diel pattern of humpback whale song on Challenger Bank encountered across 55 weeks between 2018 and 2019. Sunset to sunrise (yellow lines) defines daytime, dusk to dawn (black lines) defines nighttime and the two remaining time intervals define the twilight periods. Grey shaded fields indicate no available recordings due to technical failure of the recording device.
Humpback whale vocalisations were heard in 32% (1733 h) of recordings. All acoustic detections occurred on 48% (166 days) of recording days, whereby social calls were only detected on days when song, the predominant vocalisation type (97%), was present too. Thus, non-song vocalisations were neglected for determining the seasonality of humpback whale vocalisations. Humpback whales were exclusively heard between 31 March (start of study) and 19 May 2018 (across both sites), as well as 26 December 2018 and 23 April 2019 (end of study) (Figure 4). There was one exception on 31 August 2018 (Figure 3) when a single song fragment was documented on CB. Overall, acoustic presences across the three deployments indicated a clear seasonal trend of humpback whale occurrence off Bermuda in the spring and winter months, ranging from late December to mid-May, hereafter referred to as “whale season” (Figures 3, 4). Notably, 65% of song recordings contained whale chorus (≥ 2 singers).
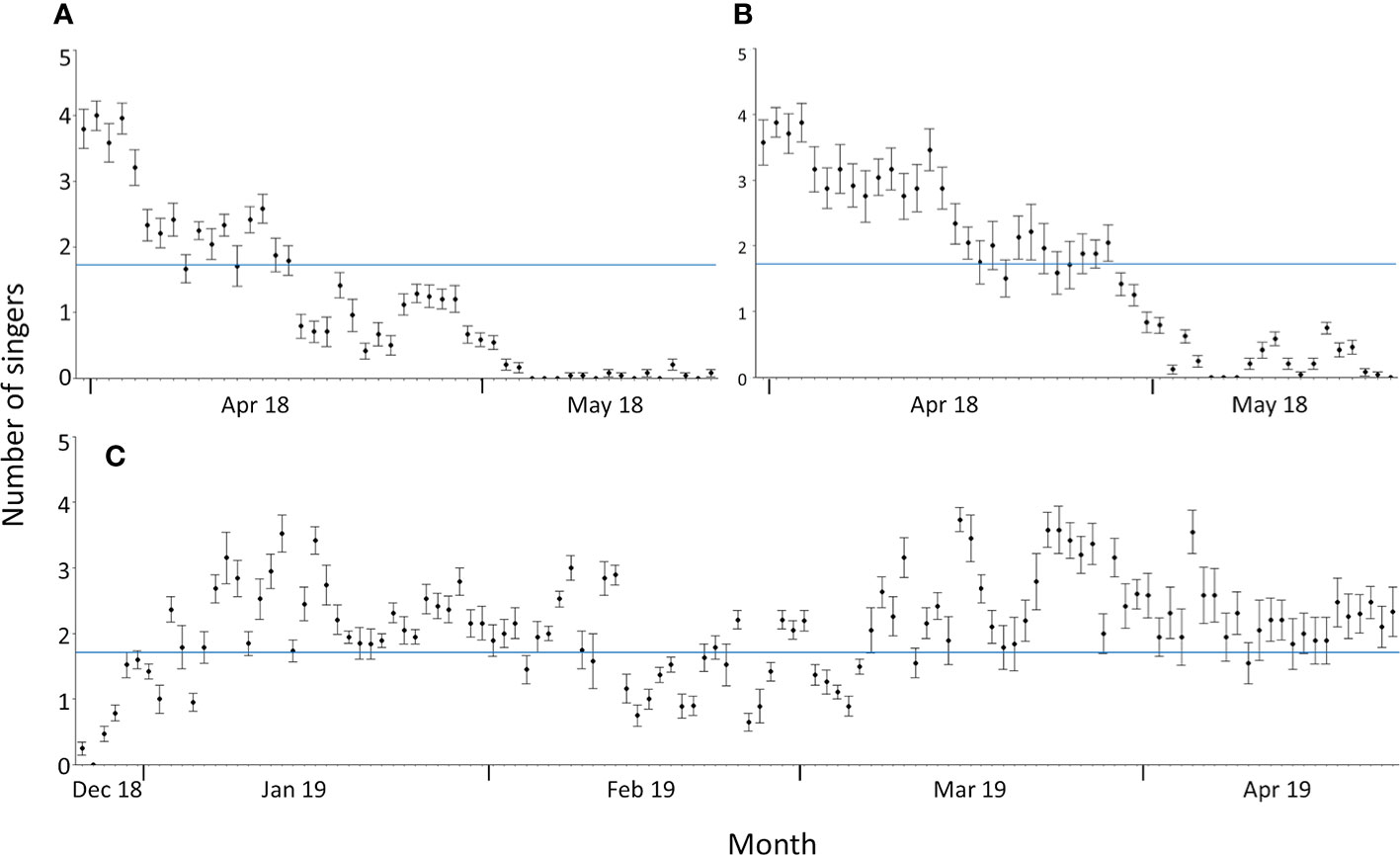
Figure 4 Daily mean number of singers during the whale season at Sally Tucker (A) and Challenger Bank between 31 March and 19 May 2018 (B) and between 26 December 2018 and 23 April 2019 (C). Error bars are ± 1 standard error. The average number of singers across the entire whale season (1.73 singers; blue line) is illustrated as a reference.
Singing activity, as quantified by the numbers of simultaneously singing whales, starts in late December and reaches the daily average across the whale season (1.7 ± 1.5 singers) quite quickly (Figure 4). The daily averages of simultaneously singing whales recorded at CB during the two spring seasons (1.921 ± 1.385 singers) and the winter season (1.926 ± 1.388 singers) were nearly identical, indicating that the intra-seasonal variation might be bigger than inter-seasonal variation. By mid-February to early March, fewer male humpback whales were recorded singing (Figures 3, 4). The highest average number of singers was detected in January, March and early April (in both 2018 and 2019 data) (Figure 4). In May, consecutive recordings were often characterised by a single singer and similar intensities, which likely represent a single male’s song session lasting several hours. Thus, although still singing for most hours of the day, the acoustic density of singing males fell below daily average in late April and ceased entirely by mid-May (Figures 3, 4). Days without song presence during the whale season only occurred at the start (27 December 2018) and end of the whale season at both CB (5-7 and 19 May 2018) and ST (5-7, 10, 13, 15, 18 May 2018) (Figure 4).
ST and CB showed broadly similar patterns in singing activity in spring 2018 (Figure 4). The concentration of singers was higher at CB, with the last humpback whale song being recorded on 18 May 2018, whilst singing activity at ST had already decreased below average by mid-April, with the last humpback whale song being recorded on 19 May 2018 (Figure 4). Therefore, humpback whale singing activity in Bermuda showed a strong seasonal pattern in the spring and winter months but with reduced activity towards the start and end of the whale season, as well as mid-season from February to early March.
Throughout the whale season moderate singing activity was detected during daylight hours and high levels of whale chorusing during the night (Figure 3). A statistically significant difference in the mean number of singing whales was detected between light conditions for all four datasets (Kruskal-Wallis; ST-spring-2018: χ2 = 13.9; df = 3; p = 0.003; CB-spring-2018: χ2 = 27.2; df = 3;
p = 5.4 x 10 -6; CB-winter-2018/19: χ2 = 26.7; df = 3; p = 6.7 x 10 -6; CB-spring-2019: χ2 = 55.0;
df = 3; p = 6.9 x 10 -12), with the number of singers being significantly lower during daytime than nighttime (ST-spring-2018: p = 0.0011; CB-spring-2018: p = 3.5 x 10 -5; CB-winter-2018/19:
p = 7.7 x 10 -7; CB-spring-2019: p = 2.1 x 10 -11) (Figure 5). At CB, the number of singers across the seasons was also significantly lower during dawn than nighttime (CB-spring-2018: p = 0.013; CB-winter-2018/19: p = 0.013; CB-spring-2019: p = 0.0053) (Figure 5). In both spring seasons at CB there was a significantly lower number of singers during daytime than dusk (CB-spring-2018:
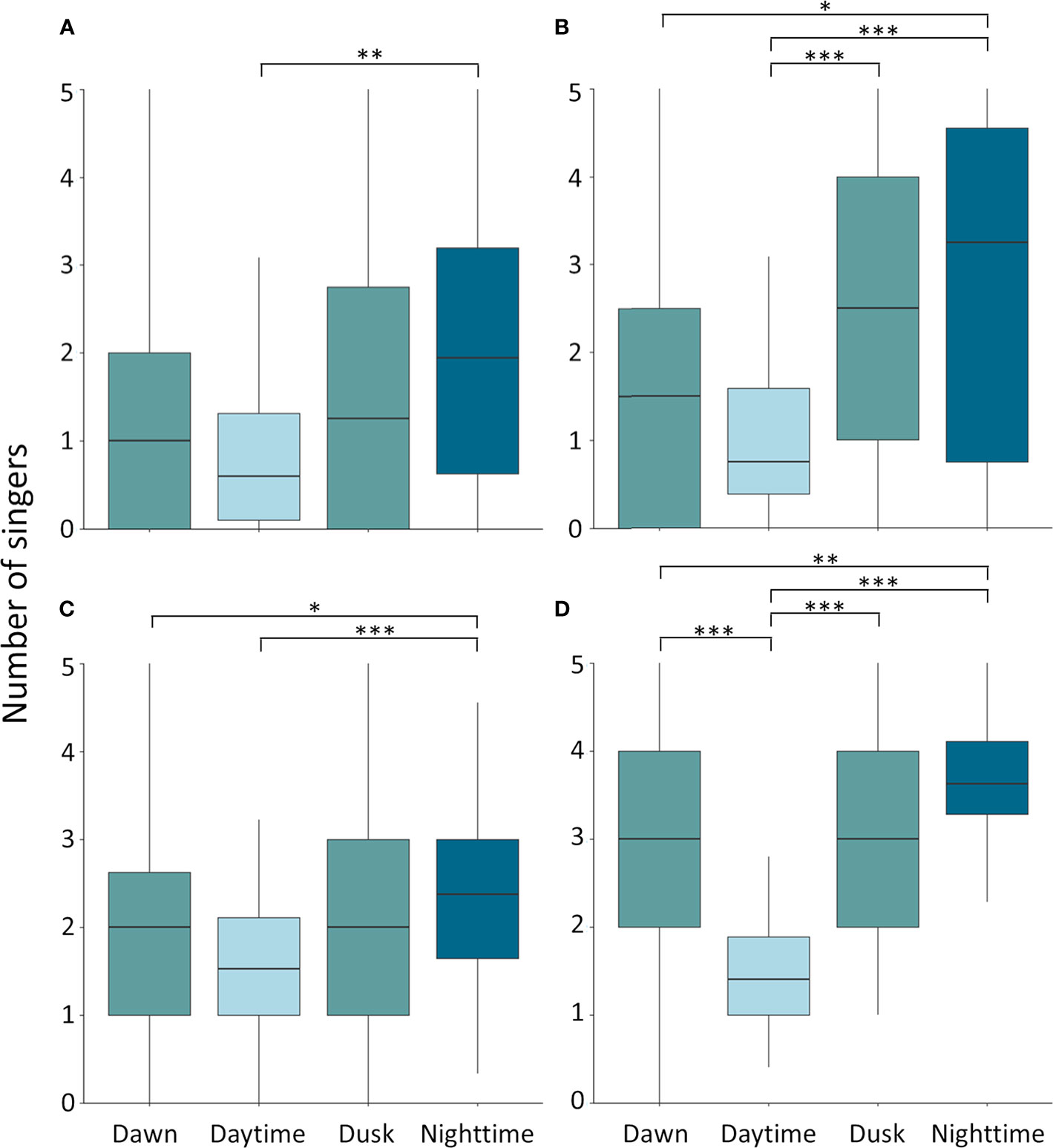
Figure 5 Diel patterns of humpback whale singing activity at Sally Tucker in spring 2018 (A) and Challenger Bank in spring 2018 (B), winter 2018/19 (C) and spring 2019 (D). The median number of singers (horizontal black line) varied significantly between daytime and nighttime in all four datasets. Boxes indicate the interquartile range and the vertical lines indicate the range of daily averages in the number of singers. Significance levels are illustrated with stars (*p < 0.05; **p < 0.01; ***p < 0.001).
p = 6.5 x 10 -4; CB-spring-2019: p = 6.5 x 10 -6) and in spring 2019 during daytime than dawn
(p = 3.3 x 10 -5) (Figure 5). Across all datasets the mean number of singers during the twilight periods did not differ significantly from each other (Figure 5). In addition, the single song fragment encountered in late August was also recorded during nighttime (Figure 3). Therefore, humpback whale singing activity in Bermuda showed a diel pattern across spring and winter months with significantly increased singing at night relative to the daytime and with twilight periods characterised by intermediate levels of singing (Figures 3, 5).
The detailed analysis of the seven humpback whale songs that were transcribed at the unit and phrase level contained 14 full song cycles (Supplementary Table 1). The coded songs revealed the stereotypical song structure of the whales migrating through Bermuda in spring 2018, consisting of: 18 distinctive unit types (Supplementary Figure 4), making up 6 phrase types and consequently 6 themes, and 5 transitional phrases (Table 1; Figure 6).
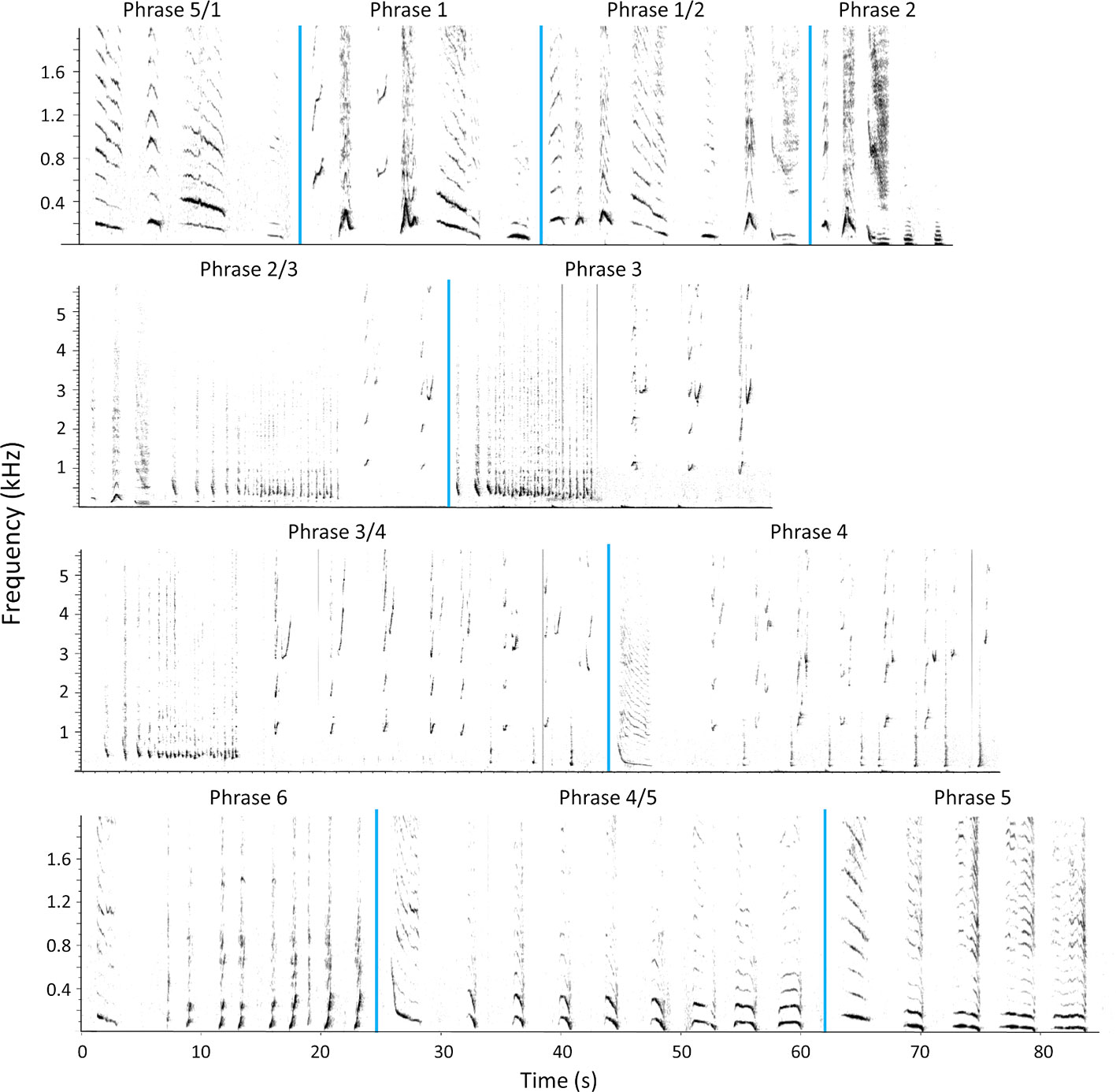
Figure 6 Humpback whale song type encountered in spring 2018 at Challenger Bank, Bermuda. A representative phrase for each theme and transitional phrase (/) is shown. The phrases are in cyclical order 5/1-1-1/2-2-2/3-3-3/4-4-6-4/5-5. Phrase 6 did not occur in every analysed song cycle. The vertical blue lines indicate divisions between phrase types. Spectrogram parameters: fast Fourier transform (FFT) size = 2048 points, overlap = 75%, sample rate = 16000 Hz, frequency resolution = 7.8 Hz and time resolution = 32 ms.
Every song cycle, even from the same individual, showed small unit variations within the same phrase type. These variations were still considered the same phrase and thus part of the same theme (Table 1). In particular, units b, c, f, p, q seemed to act as synonyms and be interchangeable to some extent within various phrase types (Table 1). Also, the amount of unit repetitions within the same phrase sequence varied (Supplementary Table 1).
All seven whales sung the theme order: 1-1/2-2-2/3-3-3/4-4-4/5-5-5/1-1 (Figure 6), which repeated itself through various song cycles (Supplementary Table 1). However, in 4 of the 14 analysed song cycles, although the transitional phrases 2/3 and 3/4 occurred, phrase 3 was not sung (Supplementary Table 1). Every song cycle contained the five transitional phrases once, while the non-transitional phrase types were repeated to a varying extent, even within an individual’s song session (Supplementary Table 1). This variation in phrase repetitions resulted in a large range of song cycle duration, ranging from 4.05 to 16.15 min for the same theme frequency (Supplementary Table 1).
Notably, phrase 6, the only phrase containing unit m (Table 1), was only sung by three of the seven whales (on 27 April, 3 and 18 May), and two of these did not sing phrase 6 in every song cycle either (Supplementary Table 1). Phrase 6 either replaced phrase 4 or occurred after phrase 4, and was always followed by the transitional phrase 4/5 (Figure 6; Supplementary Table 1). All other phrases remained stable throughout the whale season.
As all recordings obtained from the fixed AMARs contained high levels of singing activity throughout the whale season (Figures 3, 4), CB and ST seem to be an important singing habitat and stopover site for male humpback whales on their annual migrations. Seamounts with shallow summits like CB are generally known as hotspots for aggregations of migratory megafauna (Morato et al., 2008) and are considered important offshore habitats for humpback whales worldwide, on their breeding grounds but also as key sites along their trans-oceanic migration routes (Garrigue et al., 2015; MacKay, 2015; Derville et al., 2020). Garrigue et al. (2015) suggests that humpback whales visit seamounts on their migration to rest, to use them as navigational landmarks, and potentially as opportunistic feeding areas. These habitat uses could all be applicable to Bermuda (Stevenson, 2011). The Bermuda platform (ST), which is shallower and has calmer waters than CB, represents the typical protected nearshore habitat in which mother-calf pairs are frequently encountered in other regions (MacKay, 2015; Indeck et al., 2021). Although ST and CB thus might form different habitats for humpback whales, both locations showed a very similar pattern in singing activity in spring 2018 (Figure 4). However, singing activity was most prevalent at CB, which might indicate that the more sheltered waters of ST are visited less by male singers and might in fact be used more by mothers and calves (Stevenson, unpublished data).
Finally, the present study detected a long tonal vocalisation of unknown origin on 8 April 2018 within the presence of humpback whale chorus (Supplementary Figure 3). This type of vocalisation is not part of humpback whales’ described call repertoire in either the North Pacific (Fournet, 2018), Southern Hemisphere (Dunlop et al., 2007; Recalde-Salas et al., 2020; Ross-Marsh et al., 2022) or the North Atlantic (Stimpert et al., 2011). The “long cry” as defined in the repertoire of humpback whales in Western Australia is typically only 3 seconds long (Recalde-Salas et al., 2020), compared to the 18-second-long cry documented in this present study (Supplementary Figure 3). Therefore, if produced by humpback whales, this very long cry could be a rare vocalisation (from calves, females and/or males), and may serve a highly specialised, but rarely required, function. Alternatively, it could have been emitted by another baleen species, such as Bryde’s whale (Balaenoptera edeni), who are known for long moans that are in this range of duration (Rice et al., 2014) but who have not so far been recorded off Bermuda (Stevenson, unpublished data).
The present study identified a strong seasonal pattern of humpback whale singing activity, with a moderately high occurrence of chorusing whales, off Bermuda in the spring and winter months, ranging from late December to mid-May (Figure 3). This is in line with boat-based observations from Whales Bermuda (Stevenson and Stevick, 2009; Stevenson, 2011) and the historic records of humpback whales (Stone et al., 1987) and their songs being recorded off Bermuda in January, April and May (Payne and McVay, 1971; Payne and Payne, 1985). Fluctuations in numbers of singers throughout the spring and winter months (Figure 4) suggest that male humpback whales migrate through Bermuda in waves and do not stay for longer periods of time. This theory is supported by visual boat-based observations made by Whales Bermuda and studies showing North Atlantic humpback whales lingering for several days up to two weeks and aggregating around Bermuda before continuing their northward migrations in large groups (Payne and Payne, 1985; Stevenson, 2008; Stevenson and Stevick, 2009; Stevenson, 2011). Humpback whales overwintering in the northwestern Caribbean are sighted at their breeding grounds between January–April with peaks between February–March, while those wintering in the southeastern Caribbean do so a bit later between March–May with peaks in April (Charif et al., 2001; Stevick et al., 2003; Gandilhon, 2012; Stevick et al., 2018; Heenehan et al., 2019). In Bermuda, reduced singing activity was observed between mid-February to early March (Figures 3, 4), which is in line with the northwestern Caribbean peak. Detecting humpback whale song from late December onwards and to a lesser extent during the northwestern Caribbean peak breeding season (Figure 3), as well as the observed higher intra- than inter-seasonal variation in average number of singers, suggests that Bermuda, previously described as a one-way stopover on their northward migration during the spring months (Payne and McVay, 1971; Stone et al., 1987; Stevenson and Stevick, 2009) also acts as a migratory stopover site on their southward migration during the winter months.
The humpback whales recorded in the present study could have migrated to and from either Caribbean breeding ground, given the temporal overlap in both breeding grounds. However, as Bermuda’s whale season starts just before the northwestern Caribbean whale season and the northwestern peak aligns with reduced singing in Bermuda (Figure 3), it is more likely that humpback whales migrate to and from the northwestern Caribbean through Bermuda. Both, satellite-tagging (Kennedy et al., 2014) and fluke identification matches support this theory (Stone et al., 1987; Beaudette et al., 2009; MacKay, 2015). However, in 2017, humpback whales were heard singing until 13 May on the northwestern Caribbean breeding ground and until 27 May on the southeastern Caribbean breeding ground (Heenehan et al., 2019). Thus, it is possible, that humpback whales recorded towards the end of Bermuda’s whale season (Figure 3) could also be originating from the southeastern breeding ground.
The single song fragment heard on 31 August 2018 (Figure 3) matches temporarily with song fragments only starting to become more frequent from late August onwards in feeding grounds (Vu et al., 2012; Baumgartner et al., 2019). However, North Atlantic humpback whales are thought to leave their feeding grounds and start their southward migrations much later than August, usually in late autumn to early winter, or, for some whales from the eastern feeding grounds, even in late winter (Stevick et al., 2018; Heenehan et al., 2019; Kowarski et al., 2019; Magnúsdóttir and Lim, 2019). Bermudian fishermen have reported sightings of humpback whales in September (Stevenson, unpublished data). This suggests that a few individuals in some years could start their migration earlier than previously expected and the present acoustic study confirms the presence of such an individual at this time in 2018. Although no acoustic presence of humpback whales was evident in June 2018 (Figure 3), a humpback whale was observed breaching at CB two years later on 15 June 2020 (Stevenson, unpublished data). Thus, inter-annual variability of occasional single individuals migrating through Bermuda seems to occur off-season, e.g., on 31 August 2018 and 15 June 2020.
The diel singing pattern observed in our study, with peak singing activity at nighttime (Figure 3; 5), has been observed in all three humpback whale subspecies on feeding (Magnúsdóttir et al., 2014; Huang et al., 2016; Kowarski et al., 2018) and breeding (Au et al., 2000; Cholewiak, 2008; Cerchio et al., 2014; Kobayashi et al., 2021) grounds, as well as migration routes (Ryan et al., 2019; Shabangu and Kowarski, 2022), and is thus suggested to be a species-wide characteristic (Kowarski et al., 2019). Au et al. (2000) suggested that the diel pattern results from humpback whales relying on visual and acoustic cues during the day, but solely on acoustic cues during the night. For the singing whale, this means that nighttime is the most efficient time to send out the signal and may explain why the diel trend is observed across different breeding, feeding and migratory habitats and why even most non-song vocalisations increase at night (Parks et al., 2014; Huang et al., 2016; Kowarski et al., 2018). In other baleen species, diel variations in vocal activity have been linked to diel distribution in their prey: when prey aggregate, foraging becomes most efficient and whales vocalise less, unless the vocalisation is directly associated to foraging (Wiggins et al., 2005; Baumgartner and Fratantoni, 2008; Širović et al., 2013). However, this hypothesis would not explain why humpback whale singing activity also peaks at nighttime on breeding grounds where they are not exhibiting any feeding behaviour (Cholewiak, 2008). Thus, reliance on visual cues during daytime, as suggested by Au et al. (2000) is currently considered the most plausible explanation for the observed diel patterns in humpback whale singing activity.
The present study reveals the importance of Bermuda’s waters for migrating humpback whales throughout the spring and winter months. However, the high volume of vessel traffic and underwater noise that accompanies expansion of Bermuda’s tourism industry could become a key issue (Jones, 2011; Lester et al., 2016; Bermuda Tourism Authority, 2019). Bermuda’s lucrative cruise tourism season currently operates from May to October (Jones, 2011) but could be extended to last from April to December (Bermuda Tourism Authority, 2019). The presence of humpback whales, being continuously present from late December until mid-May (Figure 3), already coincides with the start of the current cruise season; should the new cruise season be rolled out, this temporal overlap will increase. Besides increased risks from ship strikes and behavioural disturbance from prolonged and more intense whale-watching, cruise and freight passages through Bermuda, growth in the tourism sector will further elevate ambient noise levels (e.g., through coastal infrastructure construction and increased vessel noise) which reduce humpback whales’ communication space (Au and Green, 2000; Jones, 2011; Micheli et al., 2012; Dunlop, 2016; Lester et al., 2016; Cholewiak et al., 2018a; Gabriele et al., 2018; Sprogis et al., 2020; Currie et al., 2021). In response to elevated noise levels male humpback whales have been shown to cease their song (Sousa-Lima and Clark, 2008; Risch et al., 2012; Cerchio et al., 2014; Tsujii et al., 2018), lengthen their song cycle (Miller et al., 2000) and amplify their vocalisation display, exhibiting the Lombard effect (Guazzo et al., 2020). Given that the present study detected humpback whales frequently chorusing, the masking of these displays by anthropogenic noise could negatively impact this behaviour in Bermuda, with currently unknown consequences on the population.
In light of this, we advocate authorities to consider precautionary and mitigation measures (Currie et al., 2021; Pires et al., 2021; Risch et al., 2021) during the whale season (December–May). The heavy cruise and shipping traffic lanes operating near CB and the Bermuda platform (ST) could be re-routed given the authorisation by the International Maritime Organization (IMO, 2014), similar to the implementation at the sister Marine Mammal Sanctuary around the Dominican Republic (Kennedy and Clapham, 2017; Heenehan et al., 2019). Dredging and construction of critical marine infrastructure to support the shipping and tourism sectors could be avoided during the night, when humpback whale vocal activity is at its highest (Figure 5), or during the whale season entirely. In addition, moving towards more sustainable noise-reduction vessel designs (Arranz et al., 2021) and implementing a 10 knot vessel speed limit across Bermuda’s EEZ for the duration of the whale season could significantly reduce noise levels and reduce masking for both mysticetes and odontocetes (IMO, 2014; Pensieri and Bozzano, 2017; Williams et al., 2019; Aschettino et al., 2020; ZoBell et al., 2021). The latter measure, would also reduce the risk of vessel collisions and cut down on the vessel’s carbon emissions (Laist et al., 2001; Lack and Corbett, 2012).
Although these precautionary measures will reduce anthropogenic noise for humpback whales when migrating through Bermuda, year-round implementation of any measure to reduce cetacean disturbance, collision risk and anthropogenic noise would benefit other marine species in Bermuda’s EEZ including the resident bottlenose dolphin (Tursiops truncatus) population (Klatsky et al., 2007), Cuvier’s beaked whales (Ziphius cavirostris) and occasional passing sperm whales (Physeter macrocephalus) (Hallett, 2011; Stevenson, unpublished data).
The present study characterised Bermuda’s humpback whale song structure, encountered in spring 2018, for the first time since 1976. As phrase 6 occurred less frequently than other phrase types and was increasingly present at the end of the 2018 spring season (Supplementary Table 1), it may represent a new phrase type that evolved in late April from phrase 4 and was slowly being introduced into the song repertoire of the predominant breeding population (northwestern Caribbean) migrating through Bermuda across the whale season. Alternatively, phrase 6 could represent the song repertoire of the southeastern breeding population migrating through Bermuda later in the spring season. Therefore, further research should compare the song type described in the present study to acoustic recordings obtained between 2017 and 2019 across the full North Atlantic humpback whale range including off Cape Verde, the southeastern and northwestern Caribbean, Bermuda, the migratory corridor off the British Isles and eastern and western feeding grounds. Analysing and identifying similarities and differences in song structure of all the above listed habitats within the same song season would help elucidate North Atlantic humpback whales’ population structure (Murray et al., 2012; Archer et al., 2020), migration paths and the role of Bermuda as a migratory stopover for both the northwestern and southeastern breeding population, which is important information required for the conservation management of this migratory species.
The present acoustic study represents the first long-term PAM study of humpback whale vocalisations off Bermuda. Our results highlight the importance of Bermuda as a key two-way migration stopover site for male North Atlantic humpback whales. They primarily display nocturnal singing activity in the spring and winter months from late December until mid-May. The strong seasonal and diel pattern of whale chorus observed in this study provides new evidence to aid Bermuda’s planning authorities with sustainable marine development around Bermuda and the wider Sargasso Sea.
The original contributions presented in the study are included in the article/Supplementary Material and an additional data publication is archived with PANGAEA at https://doi.pangaea.de/10.1594/PANGAEA.946517. Further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by School of GeoSciences, University of Edinburgh.
AS and L-AH contributed to the conception of the study. AS conducted all fieldwork. TNH and DR designed the study. TNH processed the data, performed the analysis, created the figures and took the lead in writing the manuscript. DR and L-AH supervised the project and provided critical feedback throughout the study. All authors contributed to the article and approved the submitted version.
This study has received funding from the Atlantic Conservation Partnership and the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 818123 for the iAtlantic project. This output reflects only the authors’ views and the European Union cannot be held responsible for any use that may be made of the information contained therein.
We thank JASCO Applied Sciences (Canada) Ltd. for providing the measurement equipment for the data collection and to Katie Kowarksi for discussions on data analysis and interpretation of humpback song. Data for this study were collected under a Protected Species Licence (License no: 18-12-18-73) for Scientific Research Activities issued by the Department of Environment and Natural Resources, Government of Bermuda.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.941793/full#supplementary-material
André M. (2018). Ocean noise: Making sense of sounds. Soc Sci. Inf. 57, 483–493. doi: 10.1177/0539018418793052
Archer F. I., Rankin S., Stafford K. M., Castellote M., Delarue J. (2020). Quantifying spatial and temporal variation of north pacific fin whale ( Balaenoptera physalus ) acoustic behavior. Mar. Mammal Sci. 36, 224–245. doi: 10.1111/mms.12640
Arranz P., de Soto N. A., Madsen P. T., Sprogis K. R. (2021). Whale-watch vessel noise levels with applications to whale-watching guidelines and conservation. Mar. Policy 134, 104776. doi: 10.1016/j.marpol.2021.104776
Aschettino J. M., Engelhaupt D. T., Engelhaupt A. G., DiMatteo A., Pusser T., Richlen M. F., et al. (2020). Satellite telemetry reveals spatial overlap between vessel high-traffic areas and humpback whales (Megaptera novaeangliae) near the mouth of the Chesapeake bay. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00121
Au W. W. L., Green M. (2000). Acoustic interaction of humpback whales and whale-watching boats. Mar. Environ. Res. 49, 469–481. doi: 10.1016/S0141-1136(99)00086-0
Au W. W. L., Mobley J., Burgess W. C., Lammers M. O., Nachtigall P. E. (2000). Seasonal and diurnal trends of chorusing humpback whales wintering in waters off western Maui. Mar. Mammal Sci. 16, 530–544. doi: 10.1111/j.1748-7692.2000.tb00949.x
Baker C. S., Palumbi S. R., Lambertsen R. H., Weinrich M. T., Calambokidis J., O’Brien S. J. (1990). Influence of seasonal migration on geographic distribution of mitochondrial DNA haplotypes in humpback whales. Nature 344, 238–240. doi: 10.1038/344238a0
Baumgartner M. F., Bonnell J., Van Parijs S. M., Corkeron P. J., Hotchkin C., Ball K., et al. (2019). Persistent near real-time passive acoustic monitoring for baleen whales from a moored buoy: System description and evaluation. Methods Ecol. Evol. 10, 1476–1489. doi: 10.1111/2041-210X.13244
Baumgartner M. F., Fratantoni D. M. (2008). Diel periodicity in both sei whale vocalization rates and the vertical migration of their copepod prey observed from ocean gliders. Limnol. Oceanogr. 53, 2197–2209. doi: 10.4319/lo.2008.53.5_part_2.2197
Beaudette A., Allen J., Bort J., Stevenson A., Stevick P., Stone G. (2009) Movement patterns of north Atlantic humpback whales identified at Bermuda. Available at: http://www.whalesbermuda.com/whale-diary/60-2009-diary/506-2009-10-12-presentation-to-biennial-meeting-of-the-society-for-marine-mammology (Accessed July 24, 2020).
Bermuda Tourism Authority (2019). Bermuda National tourism plan 2019-2025 (Hamilton, Bermuda: BTA). Available at: https://www.gotobermuda.com/bta.
Bettridge S., Baker C. S., Barlow J., Clapham P. J., Ford M., Gouveia D., et al. (2015). Status review of the humpback whale (Megaptera novaeangliae) under the endangered species act. (Southwest Fisheries Science Center, Miami, FL: National Oceanic and Atmospheric Administration, NOAA Fisheries)
Cates K. A., Atkinson S., Gabriele C. M., Pack A. A., Straley J. M., Yin S. (2019). Testosterone trends within and across seasons in male humpback whales (Megaptera novaeangliae)from Hawaii and Alaska. Gen. Comp. Endocrinol. 279, 164–173. doi: 10.1016/j.ygcen.2019.03.013
Cato D. H. (2014) Shipping noise impacts on marine life. in 43rd international congress and exposition on noise control engineering (Internoise 2014): Improving the world through noise control (Melbourne, VIC, Australia). Available at: http://www.acoustics.asn.au/conference_proceedings/INTERNOISE2014/papers/p888.pdf# (Accessed May 15, 2020).
Center for Conservation Bioacoustics (2019) Raven pro: Interactive sound analysis software (Version 1.6.1). Available at: http://ravensoundsoftware.com/.
Cerchio S., Strindberg S., Collins T., Bennett C., Rosenbaum H. (2014). Seismic surveys negatively affect humpback whale singing activity off northern Angola. PloS One 9:e86464. doi: 10.1371/journal.pone.0086464
Charif R. A., Clapham P. J., Clark C. W. (2001). Acoustic detections of singing humpback whales in deep waters off the British isles. Mar. Mammal Sci. 17, 751–768. doi: 10.1111/j.1748-7692.2001.tb01297.x
Cholewiak D. M. (2008). Evaluating the role of song in the humpback whale (Megaptera novaeangliae) breeding system with respect to intra-sexual interactions. (Cornell, NY: Faculty of the Graduate School of Cornell University)
Cholewiak D. M., Cerchio S., Jacobsen J. K., Urbán-R. J., Clark C. W. (2018b). Songbird dynamics under the sea: acoustic interactions between humpback whales suggest song mediates male interactions. R. Soc Open Sci. 5, 171298. doi: 10.1098/rsos.171298
Cholewiak D., Clark C. W., Ponirakis D., Frankel A., Hatch L. T., Risch D., et al. (2018a). Communicating amidst the noise: Modeling the aggregate influence of ambient and vessel noise on baleen whale communication space in a national marine sanctuary. Endanger. Species Res. 36, 59–75. doi: 10.3354/ESR00875
Cholewiak D. M., Sousa-Lima R. S., Cerchio S. (2013). Humpback whale song hierarchical structure: Historical context and discussion of current classification issues. Mar. Mammal Sci. 29, 312–332. doi: 10.1111/mms.12005
Clark C., Ellison W., Southall B., Hatch L., Van Parijs S., Frankel A., et al. (2009). Acoustic masking in marine ecosystems: intuitions, analysis, and implication. Mar. Ecol. Prog. Ser. 395, 201–222. doi: 10.3354/meps08402
Cooke J. G. (2018). Megaptera novaeangliae. IUCN Red List Threat. Species 2018, e.T13006A50362794. doi: 10.1016/B978-0-12-373553-9.00135-8
Currie J. J., McCordic J. A., Olson G. L., Machernis A. F., Stack S. H. (2021). The impact of vessels on humpback whale behavior: The benefit of added whale watching guidelines. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.601433
Darling J. D., Acebes J. M. V., Frey O., Jorge Urbán R., Yamaguchi M. (2019). Convergence and divergence of songs suggests ongoing, but annually variable, mixing of humpback whale populations throughout the north pacific. Sci. Rep. 9, 1–14. doi: 10.1038/s41598-019-42233-7
Darling J. D., Berube M. (2001). Interactions of singing humpback whales with other males. Mar. Mammal Sci. 17, 570–584. doi: 10.1111/j.1748-7692.2001.tb01005.x
Davis G. E., Baumgartner M. F., Corkeron P. J., Bell J., Berchok C., Bonnell J. M., et al. (2020). Exploring movement patterns and changing distributions of baleen whales in the western north Atlantic using a decade of passive acoustic data. Glob. Change Biol. 26, 4812–4840. doi: 10.1111/gcb.15191
Department of Environment and Natural Resources (2017) Whale watching guidelines. Available at: https://environment.bm/whale-watching-guidelines.
Derville S., Torres L. G., Zerbini A. N., Oremus M., Garrigue C. (2020). Horizontal and vertical movements of humpback whales inform the use of critical pelagic habitats in the western south pacific. Sci. Rep. 10, 1–13. doi: 10.1038/s41598-020-61771-z
Dooling R. J., Leek M. R. (2018). Communication masking by man-made noise, in Effects of anthropogenic noise on animals (New York, NY: Springer). doi: 10.1007/978-1-4939-8574-6_2
Dunlop R. A. (2016). The effect of vessel noise on humpback whale, megaptera novaeangliae, communication behaviour. Anim. Behav. 111, 13–21. doi: 10.1016/j.anbehav.2015.10.002
Dunlop R. A. (2019). The effects of vessel noise on the communication network of humpback whales. R. Soc Open Sci. 6, 190967. doi: 10.1098/rsos.190967
Dunlop R. A., Noad M. J., Cato D. H., Stokes D. (2007). The social vocalization repertoire of east Australian migrating humpback whales (Megaptera novaeangliae). J. Acoust. Soc Am. 122, 2893. doi: 10.1121/1.2783115
Edds-Walton P. L. (1997). Acoustic communication signals of mysticete whales. Bioacoustics 8, 47–60. doi: 10.1080/09524622.1997.9753353
Fiori L., Martinez E., Orams M. B., Bollard B. (2020). Using unmanned aerial vehicles (UAVs) to assess humpback whale behavioral responses to swim-with interactions in vava’u, kingdom of Tonga. J. Sustain. Tour. 28, 1743–1761. doi: 10.1080/09669582.2020.1758706
Fournet M. E. H. (2018). Humpback whale (Megaptera novaeangliae) calling behavior in southeast Alaska: A study in acoustic ecology and noise acoustic spyglass view project rapunzel project: investigating non-song vocalizations in southeast Alaska view project (Corvallis, OR: Oregon State University). doi: 10.13140/RG.2.2.35810.43200
Gabriele C. M., Ponirakis D. W., Clark C. W., Womble J. N., Vanselow P. B. S. (2018). Underwater acoustic ecology metrics in an Alaska marine protected area reveal marine mammal communication masking and management alternatives. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00270
Gandilhon N. (2012). Contribution au recensement des cétacés dans l’archipel de Guadeloupe. (Antilles-Guyane)
Garland E. C., Goldizen A. W., Rekdahl M. L., Constantine R., Garrigue C., Hauser N. D., et al. (2011). Dynamic horizontal cultural transmission of humpback whale song at the ocean basin scale. Curr. Biol. 21, 687–691. doi: 10.1016/j.cub.2011.03.019
Garland E. C., Noad M. J., Goldizen A. W., Lilley M. S., Rekdahl M. L., Garrigue C., et al. (2013). Quantifying humpback whale song sequences to understand the dynamics of song exchange at the ocean basin scale. J. Acoust. Soc Am. 133, 560–569. doi: 10.1121/1.4770232
Garland E. C., Rendell L., Lamoni L., Poole M. M., Noad M. J. (2017). Song hybridization events during revolutionary song change provide insights into cultural transmission in humpback whales. Proc. Natl. Acad. Sci. U. S. A. 114, 7822–7829. doi: 10.1073/pnas.1621072114
Garrigue C., Clapham P. J., Geyer Y., Kennedy A. S., Zerbini A. N. (2015). Satellite tracking reveals novel migratory patterns and the importance of seamounts for endangered south pacific humpback whales. R. Soc Open Sci. 2, 150489. doi: 10.1098/rsos.150489
Government of Bermuda (1978) Bermuda Fisheries (Protected species) order 1978. BR8 / 1978. Available at: http://www.bermudalaws.bm/laws/ConsolidatedLaws/Fisheries(ProtectedSpecies)Order1978.pdf.
Government of Bermuda (2003) Bermuda Protected species act 2003. Available at: http://www.bermudalaws.bm/laws/ConsolidatedLaws/ProtectedSpeciesAct2003.pdf.
Guazzo R. A., Helble T. A., Alongi G. C., Durbach I. N., Martin C. R., Martin S. W., et al. (2020). The Lombard effect in singing humpback whales: Source levels increase as ambient ocean noise levels increase. J. Acoust. Soc Am. 148, 542–555. doi: 10.1121/10.0001669
Hallett J. (2011). The importance of the Sargasso sea and the offshore waters of the bermudian exclusive economic zone to Bermuda and its people.
Hannay D. E., Delarue J., Mouy X., Martin B. S., Leary D., Oswald J. N., et al. (2013). Marine mammal acoustic detections in the northeastern chukchi Sea, September 2007-July 2011. Cont. Shelf Res. 67, 127–146. doi: 10.1016/j.csr.2013.07.009
Hauer-Jensen M. (2018). Analysis of humpback whale songs: Applying the traditional method. (Scripps College: MBARI)
Heenehan H., Stanistreet J. E., Corkeron P. J., Bouveret L., Chalifour J., Davis G. E., et al. (2019). Caribbean Sea Soundscapes: Monitoring humpback whales, biological sounds, geological events, and anthropogenic impacts of vessel noise. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00347
Herman L. M. (2017). The multiple functions of male song within the humpback whale ( Megaptera novaeangliae ) mating system: review, evaluation, and synthesis. Biol. Rev. 92, 1795–1818. doi: 10.1111/brv.12309
Herman L. M., Pack A. A., Spitz S. S., Herman E. Y. K., Rose K., Hakala S., et al. (2013). Humpback whale song: who sings? Behav. Ecol. Sociobiol. 67, 1653–1663. doi: 10.1007/s00265-013-1576-8
Hoyt E. (2011). Marine protected areas for whales, dolphins and porpoises: A world handbook for cetacean habitat conservation and planning (New York, US: Earthscan, Taylor & Francis Ltd).
Huang W., Wang D., Ratilal P. (2016). Diel and spatial dependence of humpback song and non-song vocalizations in fish spawning ground. Remote Sens 8, 712. doi: 10.3390/rs8090712
IMO (2014) Guidelines for the reduction of underwater noise from commercial shipping to address adverse impacts on marine life. Available at: http://docs.nrdc.org/water/files/wat_14050501a.pdf.
Indeck K. L., Noad M. J., Dunlop R. A. (2021). The conspecific avoidance strategies of adult female-calf humpback whales. Behav. Ecol. 32, 845–855. doi: 10.1093/beheco/arab031
Johnson M., Aguilar de Soto N., Madsen P. (2009). Studying the behaviour and sensory ecology of marine mammals using acoustic recording tags: a review. Mar. Ecol. Prog. Ser. 395, 55–73. doi: 10.3354/meps08255
Jones R. (2011). Environmental effects of the cruise tourism boom: Sediment resuspension from cruise ships and the possible effects of increased turbidity and sediment deposition on corals (Bermuda). Bull. Mar. Sci. 87, 659–679. doi: 10.5343/bms.2011.1007
Kennedy A. S., Clapham P. J. (2017). From whaling to tagging: The evolution of north Atlantic humpback whale research in the West indies. Mar. Fish. Rev. 79, 23–37. doi: 10.7755/MFR.79.2.2
Kennedy A. S., Zerbini A. N., Vásquez O. V., Gandilhon N., Clapham P. J., Adam O. (2014). Local and migratory movements of humpback whales (Megaptera novaeangliae) satellite-tracked in the north Atlantic ocean. Can. J. Zool. 92, 8–17. doi: 10.1139/cjz-2013-0161
Klatsky L. J., Wells R. S., Sweeney J. C. (2007). Offshore bottlenose dolphins ( tursiops truncatus ): Movement and dive behavior near the Bermuda pedestal. J. Mammal. 88, 59–66. doi: 10.1644/05-mamm-a-365r1.1
Kobayashi N., Okabe H., Higashi N., Miyahara H., Uchida S. (2021). Diel patterns in singing activity of humpback whales in a winter breeding area in okinawan (Ryukyuan) waters. Mar. Mammal Sci. 37, 982–992. doi: 10.1111/mms.12790
Kowarski K., Cerchio S., Whitehead H., Moors-Murphy H. (2021). Where, when, and why do western north Atlantic humpback whales begin to sing? Bioacoustics 31: 450–69. doi: 10.1080/09524622.2021.1972838
Kowarski K., Evers C., Moors-Murphy H., Martin B., Denes S. L. (2018). Singing through winter nights: Seasonal and diel occurrence of humpback whale ( megaptera novaeangliae ) calls in and around the Gully MPA,offshore eastern Canada. Mar. Mammal Sci. 34, 169–189. doi: 10.1111/mms.12447
Kowarski K., Moors-Murphy H., Maxner E., Cerchio S. (2019). Western North Atlantic humpback whale fall and spring acoustic repertoire: Insight into onset and cessation of singing behavior. J. Acoust. Soc Am. 145, 2305–2316. doi: 10.1121/1.5095404
Lack D. A., Corbett J. J. (2012). Black carbon from ships: a review of the effects of ship speed, fuel quality and exhaust gas scrubbing. Atmos. Chem. Phys. 12, 3985–4000. doi: 10.5194/acp-12-3985-2012
Laist D. W., Knowlton A. R., Mead J. G., Collet A. S., Podesta M. (2001). Collisions between ships and whales. Mar. Mammal Sci. 17, 35–75. doi: 10.1111/j.1748-7692.2001.tb00980.x
Lester S. E., White C., Mayall K., Walter R. K. (2016). Environmental and economic implications of alternative cruise ship pathways in Bermuda. Ocean Coast. Manage. 132, 70–79. doi: 10.1016/j.ocecoaman.2016.08.015
MacKay M. (2015). Occurence patterns and social behaviors of humpback whales (Megaptera novaeangliae) wintering off Puerto Rico, USA. (Corpus Christi, TX: Texas A&M University)
MacKay M. M., Bacon C. E., Bouveret L., Fossette S., Stevick P. T. (2019). Humpback whale (Megaptera novaeangliae) Intra/Inter-seasonal exchanges between Puerto Rico and the southeastern Caribbean. Anim. Behav. Cogn. 6, 98–104. doi: 10.26451/abc.06.02.02.2019
Magnúsdóttir E. E. (2017). The singing behaviour of humpback whales (Megaptera novaeangliae) in subarctic waters. (University of Iceland: Faculty of Life and Environmental Science)
Magnúsdóttir E. E., Lim R. (2019). Subarctic singers: Humpback whale (Megaptera novaeangliae) song structure and progression from an icelandic feeding ground during winter. PloS One 14, 1–26. doi: 10.1371/journal.pone.0210057
Magnúsdóttir E. E., Rasmussen M. H., Lammers M. O., Svavarsson J. (2014). Humpback whale songs during winter in subarctic waters. Polar Biol. 37, 427–433. doi: 10.1007/s00300-014-1448-3
Mattila D. K., Guinee L. N., Mayo C. A. (1987). Humpback whale songs on a north Atlantic feeding ground. J. Mammal. 68, 880–883. doi: 10.2307/1381574
Micheli F., Saenz-Arroyo A., Greenley A., Vazquez L., Espinoza Montes J. A., Rossetto M., et al. (2012). Evidence that marine reserves enhance resilience to climatic impacts. PloS One 7, e40832. doi: 10.1371/journal.pone.0040832
Miller P. J. O., Biassoni N., Samuels A., Tyack P. L. (2000). Whale songs lengthen in response to sonar. Nature 405, 903. doi: 10.1038/35016148
Minister of Health Seniors and Environment (2016) Bermuda Protected species amendment order 2016. gov. Bermuda BR4 / 2016. Available at: http://www.bermudalaws.bm/laws/AnnualLaws/2016/StatutoryInstruments/ProtectedSpeciesAmendmentOrder2016.pdf.
Morato T., Varkey D., Damaso C., Machete M., Santos M., Prieto R., et al. (2008). Evidence of a seamount effect on aggregating visitors. Mar. Ecol. Prog. Ser. 357, 23–32. doi: 10.3354/meps07269
Murray A., Cerchio S., McCauley R., Jenner C. S., Razafindrakoto Y., Coughran D., et al. (2012). Minimal similarity in songs suggests limited exchange between humpback whales (Megaptera novaeangliae) in the southern Indian ocean. Mar. Mammal Sci. 28, E41–E57. doi: 10.1111/j.1748-7692.2011.00484.x
Murray A., Dunlop R. A., Noad M. J., Goldizen A. W. (2018). Stereotypic and complex phrase types provide structural evidence for a multi-message display in humpback whales ( megaptera novaeangliae ). J. Acoust. Soc Am. 143, 980–994. doi: 10.1121/1.5023680
Narganes Homfeldt T., Risch D., Stevenson A., Henry L.-A. (2022). Spectrograms of singing humpback whales migrating through Bermuda. PANGAEA. doi: 10.1594/PANGAEA.946517
Natural Earth. Features. Available at: http://www.naturalearthdata.com/features/ (Accessed July 13, 2020).
Nikšić S. (2014). Analysis of humpback whale song from the Eastern Caribbean. (Zagreb, Croatia: University of Zagreb)
NOAA, Government of Bermuda (2012) Memorandum of understanding between the united states of America, U.S. department of commerce national oceanic and atmospheric administration, national ocean service, office of marine sanctuaries - and the government of Bermuda, ministry of the environmen. MOA-2012-0. Available at: https://nmsstellwagen.blob.core.windows.net/stellwagen-prod/media/archive/sister/pdfs/bermuda_moa12.pdf.
Noad M. J., Cato D. H., Bryden M. M., Jenner M. N., Jenner K. C. S. (2000). Cultural revolution in whale songs: Humpbacks have picked up a catchy tune sung by immigrants from a distant ocean. Nature 408, 537. doi: 10.1038/35046199
O’Connor S., Campbell R., Knowles T., Cortez H. (2009). Whale watching worldwide: tourism numbers, expenditures and expanding economic benefits, a special report from the international fund for animal welfare228. doi: 10.1115/89GT251
Open Digital Elevation Model (2019) Bathymetry. Available at: https://opendem.info/download_bathymetry.html (Accessed July 12, 2020).
Palsboll P. J., Clapham P. J., Mattila D. K., Larsen F., Sears R., Siegismund H. R., et al. (1995). Distribution of mtDNA haplotypes in north Atlantic humpback whales: The influence of behaviour on population structure. Mar. Ecol. Prog. Ser. 116, 1–10. doi: 10.3354/meps116001
Parks S. E., Cusano D. A., Stimpert A. K., Weinrich M. T., Friedlaender A. S., Wiley D. N. (2014). Evidence for acoustic communication among bottom foraging humpback whales. Sci. Rep. 4, 1–7. doi: 10.1038/srep07508
Payne R. S., McVay S. (1971). Songs of humpback whales. Sci. (80-. ). 173, 585–597. doi: 10.1126/science.173.3997.585
Payne K., Payne R. (1985). Large Scale changes over 19 years in songs of humpback whales in Bermuda. Z. Tierpsychol. 68, 89–114. doi: 10.1111/j.1439-0310.1985.tb00118.x
Payne R., Webb D. (1971). Orientation by means of long range acoustic signaling in baleen whales. Ann. N. Y. Acad. Sci. 188, 110–141. doi: 10.1111/j.1749-6632.1971.tb13093.x
Pensieri S., Bozzano R. (2017). “Active and passive acoustic methods for in-situ monitoring of the ocean status,”,” in Advances in underwater acoustics (InTechOpen). doi: 10.5772/intechopen.68998
Pires A. L. M. S., de Sá Maciel I., dos Santos Alves M. A., Tardin R. H. (2021). The effects of anthropogenic noise on cetaceans in Brazil: the need to consider recent scientific advances in environmental licensing. J. Coast. Conserv. 25, 45. doi: 10.1007/s11852-021-00832-5
QGIS Development Team (2016). “QGIS geographic information system (Version 3.2.0),” in Open source geospatial found. (Open Source Geospatial Foundation Project) Available at: http://qgis.osgeo.org.
Rafter M. A., Frasier K. E., Trickey J. S., Hildebrand J. A., Rice A. C., Thayre B. J., et al. (2018). Passive acoustic monitoring for marine mammals at Norfolk canyon April 2016 - June 2017 (San Diego, California: Marine Physical Laboratory).
R Core Team (2019) R: A language and environment for statistical computing. Available at: https://www.r-project.org/.
Recalde-Salas A., Erbe C., Salgado Kent C., Parsons M. (2020). Non-song vocalizations of humpback whales in Western Australia. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00141
Reeves R. R., Smith T. D., Josephson E. A., Clapham P. J., Woolmer G. (2004). Historical observations of humpback and blue whales in the north Atlantic ocean: Clues to migratory routes and possibly additional feeding grounds. Mar. Mammal Sci. 20, 774–786. doi: 10.1111/j.1748-7692.2004.tb01192.x
Rekdahl M. L., Dunlop R. A., Goldizen A. W., Garland E. C., Biassoni N., Miller P., et al. (2015). Non-song social call bouts of migrating humpback whales. J. Acoust. Soc Am. 137, 3042–3053. doi: 10.1121/1.4921280
Rice A. N., Palmer K. J., Tielens J. T., Muirhead C. A., Clark C. W. (2014). Potential bryde’s whale ( balaenoptera edeni ) calls recorded in the northern gulf of Mexico. J. Acoust. Soc Am. 135, 3066–3076. doi: 10.1121/1.4870057
Risch D., Calderan S., Leaper R., Weilgart L., Werner S. (2021). Current knowledge already justifies underwater noise reduction. Trends Ecol. Evol. 36, 381–382. doi: 10.1016/j.tree.2020.12.010
Risch D., Corkeron P. J., Ellison W. T., van Parijs S. M. (2012). Changes in humpback whale song occurrence in response to an acoustic source 200 km away. PloS One 7e29741. doi: 10.1371/journal.pone.0029741
Rolland R. M., Parks S. E., Hunt K. E., Castellote M., Corkeron P. J., Nowacek D. P., et al. (2012). Evidence that ship noise increases stress in right whales. Proc. R. Soc B Biol. Sci. 279, 2363–2368. doi: 10.1098/rspb.2011.2429
Ross-Marsh E. C., Elwen S. H., Fearey J., Thompson K. F., Maack T., Gridley T. (2022). Detection of humpback whale ( megaptera novaeangliae ) non-song vocalizations around the vema seamount, southeast Atlantic ocean. JASA Express Lett. 2, 041201. doi: 10.1121/10.0010072
Ruegg K., Rosenbaum H. C., Anderson E. C., Engel M., Rothschild A., Baker C. S., et al. (2013). Long-term population size of the north Atlantic humpback whale within the context of worldwide population structure. Conserv. Genet. 14, 103–114. doi: 10.1007/s10592-012-0432-0
Ryan J. P., Cline D. E., Joseph J. E., Margolina T., Santora J. A., Kudela R. M., et al. (2019). Humpback whale song occurrence reflects ecosystem variability in feeding and migratory habitat of the northeast pacific. PloS One 14, e0222456. doi: 10.1371/journal.pone.0222456
Shabangu F. W., Kowarski K. A. (2022). The beat goes on: Humpback whale song seasonality in Antarctic and south African waters. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.827324
Silber G. K. (1986). The relationship of social vocalizations to surface behavior and aggression in the Hawaiian humpback whale ( megaptera novaeangliae ). Can. J. Zool. 64, 2075–2080. doi: 10.1139/z86-316
Širović A., Williams L. N., Kerosky S. M., Wiggins S. M., Hildebrand J. A. (2013). Temporal separation of two fin whale call types across the eastern north pacific. Mar. Biol. 160, 47–57. doi: 10.1007/s00227-012-2061-z
Smith J. N., Goldizen A. W., Dunlop R. A., Noad M. J. (2008). Songs of male humpback whales, megaptera novaeangliae, are involved in intersexual interactions. Anim. Behav. 76, 467–477. doi: 10.1016/j.anbehav.2008.02.013
Sousa-Lima R. S., Clark C. W. (2008). Modeling the effect of boat traffic on the fluctuation of humpback whale singing activity in the abrolhos national marine park, Brazil. Can. Acoust. 36, 174–181.
Sprogis K. R., Videsen S., Madsen P. T. (2020). Vessel noise levels drive behavioural responses of humpback whales with implications for whale-watching. Elife 9, e56760. doi: 10.7554/eLife.56760
Stafford K. M., Lydersen C., Wiig Ø., Kovacs K. M. (2018). Extreme diversity in the songs of spitsbergen’s bowhead whales. Biol. Lett. 14, 20180056. doi: 10.1098/rsbl.2018.0056
Stevenson A. (2008)Whale behaviour in Bermuda. In: Whales Bermuda. Available at: http://www.whalesbermuda.com/all-about-humpbacks/whale-behaviour/46-general/217-whale-behaviour-in-bermuda (Accessed May 17, 2020).
Stevenson A. (2010)Fluke ID matches. In: Whales Bermuda. Available at: https://www.whalesbermuda.com/fluke-ids/57-whale-id-flukes/131-using-fluke-shots-to-identify-whales (Accessed July 18, 2020).
Stevenson A. (2011) Humpback whale research project, bermuda. Sargasso Sea alliance science report series. Available at: www.sargassoalliance.org (Accessed July 26, 2020).
Stevenson A., Stevick P. T. (2009) Habitat use of humpback whales at Bermuda. Available at: http://www.whalesbermuda.com/whale-diary/60-2009-diary/506-2009-10-12-presentation-to-biennial-meeting-of-the-society-for-marine-mammology.
Stevick P. T., Allen J., Bérubé M., Clapham P. J., Katona S. K., Larsen F., et al. (2003). Segregation of migration by feeding ground origin in north Atlantic humpback whales (Megaptera novaeangliae). J. Zool. ), 259. doi: 10.1017/S0952836902003151
Stevick P. T., Berrow S. D., Bérubé M., Bouveret L., Broms F., Jann B., et al. (2016). There and back again: Multiple and return exchange of humpback whales between breeding habitats separated by an ocean basin. J. Mar. Biol. Assoc. United Kingdom 96, 885–890. doi: 10.1017/S0025315416000321
Stevick P. T., Bouveret L., Gandilhon N., Rinaldi C., Rinaldi R., Broms F., et al. (2018). Migratory destinations and timing of humpback whales in the southeastern Caribbean differ from those off the Dominican republic. J. Cetacean Res. Manage. 18, 127–133.
Stimpert A. K., Au W. W. L., Parks S. E., Hurst T., Wiley D. N. (2011). Common humpback whale ( megaptera novaeangliae ) sound types for passive acoustic monitoring. J. Acoust. Soc Am. 129, 476–482. doi: 10.1121/1.3504708
Stone G. S., Katona S. K., Tucker E. B. (1987). History, migration and present status of humpback whales megaptera novaeangliae at Bermuda. Biol. Conserv. 42, 133–145. doi: 10.1016/0006-3207(87)90019-X
Time and Date (2019a) Hamilton, Bermuda – sunrise, sunset and daylength 2018. Available at: https://www.timeanddate.com/sun/bermuda/hamilton?month=5&year=2018 (Accessed July 20, 2020).
Time and Date (2019b) Solstices & equinoxes for Hamilton (Surrounding 10 years). Available at: https://www.timeanddate.com/calendar/seasons.html?n=38 (Accessed July 28, 2022).
Tsujii K., Akamatsu T., Okamoto R., Mori K., Mitani Y., Umeda N. (2018). Change in singing behavior of humpback whales caused by shipping noise. PloS One 13 e0204112. doi: 10.1371/journal.pone.0204112
Tyarks S. C., Aniceto A. S., Ahonen H., Pedersen G., Lindstrøm U. (2021). Humpback whale (Megaptera novaeangliae) song on a subarctic feeding ground. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.669748
Vogt P. R., Jung W.-Y. (2007). Origin of the Bermuda volcanoes and the Bermuda rise: History, observations, models, and puzzles, in Plates, plumes and planetary processes (Boulder, CO: Geological Society of America), 553–591. doi: 10.1130/2007.2430(27
Vu E., Risch D., Clark C., Gaylord S., Hatch L., Thompson M., et al. (2012). Humpback whale song occurs extensively on feeding grounds in the western north Atlantic ocean. Aquat. Biol. 14, 175–183. doi: 10.3354/ab00390
Weinrich M. (1998). Early experience in habitat choice by humpback whales (Megaptera novaeangliae). J. Mammal. 79, 163–170. doi: 10.2307/1382851
Wenzel F. W., Allen J., Berrow S., Hazevoet C. J., Jann B., Seton R. E., et al. (2009). Current knowledge on the distribution and relative abundance of humpback whales (Megaptera novaeangliae) off the cape Verde islands, Eastern north Atlantic. Aquat. Mamm. 35, 502–510. doi: 10.1578/AM.35.4.2009.502
Wenzel F. W., Broms F., López-Suárez P., Lopes K., Veiga N., Yeoman K., et al. (2020). Humpback whales (Megaptera novaeangliae) in the cape Verde islands: Migratory patterns, resightings, and abundance. Aquat. Mamm. 46, 21–31. doi: 10.1578/AM.46.1.2020.21
Wickham H. (2016) ggplot2: Elegant graphics for data analysis. Available at: https://ggplot2.tidyverse.org.
Wiggins S. M., Oleson E. M., McDonald M. A., Hildebrand J. A. (2005). Blue whale (Balaenoptera musculus) diel call patterns offshore of southern California. Aquat. Mamm. 31, 161–168. doi: 10.1578/am.31.2.2005.161
Williams R., Veirs S., Veirs V., Ashe E., Mastick N. (2019). Approaches to reduce noise from ships operating in important killer whale habitats. Mar. pollut. Bull. 139, 459–469. doi: 10.1016/j.marpolbul.2018.05.015
Winn H. E., Winn L. K. (1978). The song of the humpback whale megaptera novaeangliae in the West indies. Mar. Biol. 47, 97–114. doi: 10.1007/BF00395631
Wright A. J., Walsh L. A. (2010). Mind the gap: Why neurological plasticity may explain seasonal interruption in humpback whale song. J. Mar. Biol. Assoc. United Kingdom 90, 1489–1491. doi: 10.1017/S0025315410000913
Keywords: North Atlantic humpback whale, Bermuda, song, seasonality, diel pattern, passive acoustic monitoring, management
Citation: Narganes Homfeldt T, Risch D, Stevenson A and Henry L-A (2022) Seasonal and diel patterns in singing activity of humpback whales migrating through Bermuda. Front. Mar. Sci. 9:941793. doi: 10.3389/fmars.2022.941793
Received: 11 May 2022; Accepted: 23 August 2022;
Published: 13 September 2022.
Edited by:
Albertus J. Smit, University of the Western Cape, South AfricaReviewed by:
Fannie W. Shabangu, Department of Forestry, Fisheries and the Environment, South AfricaCopyright © 2022 Narganes Homfeldt, Risch, Stevenson and Henry. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lea-Anne Henry, TC5IZW5yeUBlZC5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.