- Key Laboratory of Inland Saline-alkaline Aquaculture, Ministry of Agriculture and Rural Affairs, East China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Shanghai, China
Background color in aquaculture has been paid more attention due to the effect on fish growth, survival, health, and reproduction. In the present study, we evaluated the background color preference of the lined seahorse (Hippocampus erectus) and its relationship with personality. Preference was assessed over 10 consecutive days through allowing the lined seahorse to freely choose six different colored compartments, i.e., white, red, green, black, yellow, and blue backgrounds. To minimize the spurious preference response, the individual preference index (PI) was applied to calculate the preference intensity of the background colors. Preference reliability was further verified by a binary choice test through the choice for the most preferred or non-preferred color. Preference consistency under stress situation was assessed by a “knock-at-the-door” test as confining the seahorse in a transparent circle after a 30-s air exposure and measuring the proportion of knock toward different colored backgrounds. The personality was conjointly analyzed by new environment test and novel object test. Overall, the lined seahorse showed a general preference for white and blue while avoidance of black and red backgrounds at either unstressed or stressed situations. The shyer the seahorse was, the more preference for white background it displayed. Thus, white and blue background colors are recommended for culturing the lined seahorse. Furthermore, white color preference is a potential indicator in personality study of the lined seahorse.
Introduction
Color patterns in the surroundings can be detected by fish via the visual pigments. Fishes use color vision to see prey against a variety of backgrounds and to detect potential mates and predators (Lythgoe and Partridge, 1989; Neumeyer, 1992; Espmark et al., 2000; Losey et al., 2003; BowmakerLoew, 2007). On the other hand, color sources, including light color and background color, prominently affect fishes’ physiological–behavioral processes, such as in relieving stress (Barcellos et al., 2009; Karakatsouli et al., 2010; Maia and Volpato, 2013), food intake (Spence and Smith, 2008; Volpato et al., 2013), growth (Ruchin, 2004; Karakatsouli et al., 2007), survival (Barcellos et al., 2009; Kang and Kim, 2013a), and reproduction (Volpato et al., 2004). Background color has been paid more attention in fish culture, as regards to the growth rate and fish welfare (Karakatsouli et al., 2007; Strand et al., 2007; Kang and Kim, 2013a; Kang and Kim, 2013b). For example, white background was recommended in flounder and the Eurasian perch culture to obtain a better growth rate (Tamazouzt et al., 2000; Takahashi et al., 2007; Sunuma et al., 2009).
The ability to sense the color enables fishes to make a choice for a preferred or avoided color. Similar to motivation for an essential resource in animals (Mason et al., 2001; Asher et al., 2009; Houpt, 2012), animals should have a motivation for choosing a favorable background color in captivity. For example, the rainbow trout (Oncorhynchus mykiss) was more motivated to access to blue background—a strongly preferred color (Maia et al., 2017). Generally, preference behaviors are consistent across time and vary among individuals, such as mating preference in the sand goby (Pomatoschistus minutus) and the treehoppers (Enchenopa binotata) (Lehtonen and Lindstrom, 2006; Fowler-Finn and Rodriguez, 2013). It is probably similar in color preference (Maia et al., 2017).
In human beings, the relationship between color preference and personality has been studied intensively (Birren, 1973; Stimpson and Stimpson, 1979; Nikolaenko and Ostrovskaia, 1989; Fetterman et al., 2015; Tao et al., 2015). Personality is defined as the inter-individual variation in behavior, characterized as stability through time and/or consistency across situations (Réale et al., 2007; Kaiser and Müller, 2021). In another word, color preference could be a personality trait as well if it was consistent across time and situations. It is interesting to know whether there is a relationship between color preference and personality in animals. Animal personality highlights the difference in physiology and behaviors, e.g., bold fishes with high adrenergic axis activity and low hypothalamo–pituitary–interrenal axis activity are more active and more likely to explore novel environments or objects versus shy fish (Sneddon, 2003; Sih et al., 2004; Carere et al., 2005; Thomson et al., 2011). Investigating whether bold–shy personality participates in fishes’ color choice would further provide insights into understanding fitness of color preference in fishes.
Seahorses are a valuable traditional Chinese medicine and fascinated aquarium fishes. All 53 recognized seahorse species were listed on Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora, due to overexploitation of the wild populations to meet growing demands in traditional Chinese medicine and ornamental markets (Vincent, 1996; Lourie et al., 1999). The commercial culture of seahorses has advanced significantly in the past 15 years to meet growing demands. More than 10 seahorse species have been reared successfully in captivity, such as Hippocampus abdominalis (Woods, 2000; Woods, 2000), H. comes (Job et al., 2006), H. erectus (Correa et al., 1989; Scarratt, 1995; Lin et al., 2008, 2009; Zhang et al., 2010), H. kuda (Job et al., 2002; Lin et al., 2006; Lin et al., 2007), H. reidi (Olivotto et al., 2008), H. subelongatus (Payne and Rippingale, 2000), H. guttulatus (Faleiro et al., 2008; Miquel et al., 2008), and H. trimaculatus (Sheng et al., 2006). The lined seahorse (H. erectus) has been considered as one of the best seahorse species for large-scale culture due to its good characteristics in growth, eurytherm, and high disease resistance (Zhang et al., 2010; Qin et al., 2016; Qu et al., 2016). Color preference of fishes in aquaculture is paid more attention (Li et al., 2016; Maia et al., 2021; Yang et al., 2021), however, only a few studies in seahorse. For example, the juveniles of the yellow seahorse (H. kuda) cultured in darker tank color had higher growth rate (Pawar et al., 2011), and mixed colors (green and orange, and green and red) resulted in higher survival than a single background color in the lined seahorse (Lin et al., 2009). Recently, our work revealed that the survival rate of the larval lined seahorse under blue and gray tanks with light intensity of 200–700 Lux was 72.0% and 49.6%, respectively (Zhang et al., 2020). Although the limited information tells us that background color should be important to culture the lined seahorse, we still do not know which background color should be chosen in practice; actually, gray, black, and white tanks provided by manufacturers are normally used in farms.
The main aim of the present study is to identify background color preference in the lined seahorse under unstressed and stressed situations and to explore the effect of personality on the color preference in the species. The present study would guide the application of background color in seahorse culture practice. Moreover, color preference could be used as a potential indicator in personality study of the lined seahorse. In the present study, we analyzed the color preference of individual adult lined seahorse by preference index (PI). PIs calculation is a history-based method to necessarily represent the consistent preference response over time and can minimize the influence of possible stress or other momentary reaction that may lead to the spurious preference response (Maia et al., 2017; Maia et al., 2021). A binary choice test and a “knock-at-the-door” test were conducted to further complement the preference reliability and consistence, respectively.
Materials and methods
Animal culture and experimental conditions
The 3-month-old lined seahorses (Hippocampus erectus Perry, 1810) (wet weight, 3.05 ± 1.07 g; height, 9.07 ± 0.94 cm) for the experiment that was conducted in December 2021 were bred at the Qionghai Research Center of the East China Sea Fisheries Research Institute, Hainan, China. The experimental seahorses were kept in a flow-through fiberglass tank (4 × 2 × 1 m) with light gray background. Seawater was sand-filtrated and ultraviolet-sterilized. Salinity, temperature, light intensity, and photoperiod were 32‰, 23°C–25°C, 800–1,000 Lux, and 13-h light:11-h dark (natural photoperiod), respectively. Transparent plastic tube was provided as holdfast. The seahorses were fed twice a day with frozen mysis (Neomysis awatschensis) purchased from a harvester/supplier located in Wudi County, Shandong Province, China. A total of 32 seahorses were tested for the background color preference. The seahorses were selected randomly from the holding tank and cultured individually in glass tanks (45 × 45 × 20 cm) a week prior to test. These flow-through glass tanks had the same culture conditions as in the holding tank, and each tank was labeled to mark the individual seahorses. All seahorses were handled in accordance with the IACUC #160413 established by the Chinese Academy of Sciences.
Background color preference test procedures
Blue, red, yellow, and green are the colors normally provided in fish color preference test (Maia et al., 2017; Maia et al., 2017; de Abreu et al., 2020), and black and white are the most common colors used in seahorse farms. Thus, six colors, i.e., blue, red, yellow, green, white, and black, were tested. The test tank was divided into six equal compartments colored with polyvinyl chloride (PVC) tape of six corresponding colors (Figure 1A). For the color preference test, seahorses were individually transferred daily with a 250-ml beaker into a transparent plastic cylinder of 18 cm in diameter, which was placed in the central of the test tank. After 5 min of acclimation to minimize the possible spurious stress reactions, the seahorse was released from the cylinder to allow the seahorse moving at will. The seahorse’s color choices among the six compartments were recorded for 1 h by a monitor (EZVIZ Company, Hanzhou, China) mounted on a custom-built wood frame. All seahorses were active to make a choice between the six colored compartments during the entire one test hour. The test was run for 10 days, including an acclimation phase, i.e., the first 3 days, and a test phase, i.e., the following 7 days. During the test phase, the total time (min) that individual seahorse visited each colored compartment was registered for calculating PI of each color. The entrance was defined as the whole body got inside a colored compartment.
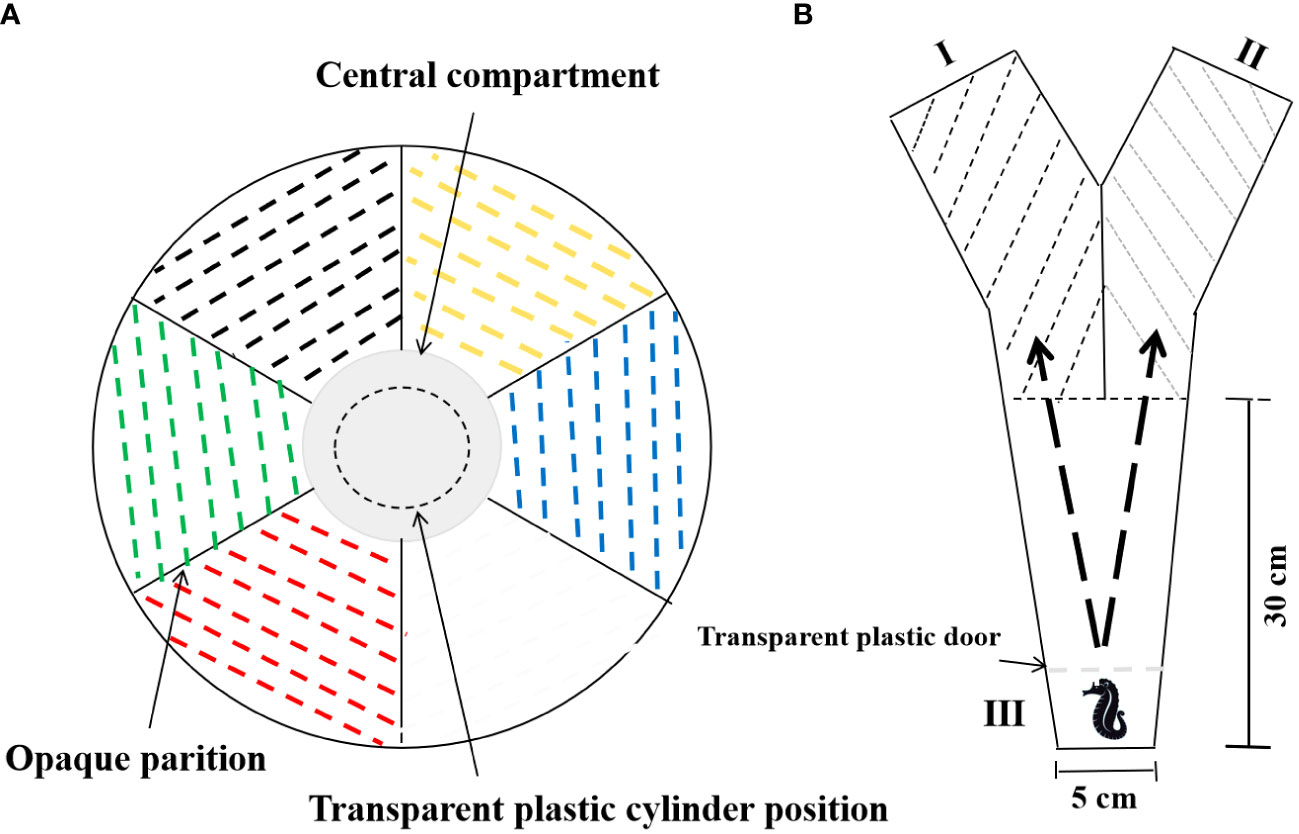
Figure 1 Top view of the aquaria for the background color preference test (A) and binary choice test (B). (A) The colored PVC adhesive tape (white, blue, yellow, black, green, and red) was used to cover the aquaria area, and identical colored foam boards (40 × 35 cm) were used to divide the tank into individual compartments. In the center, the gray color presented the central compartment for seahorse introduction and acclimation. The water column was 25 cm in depth and 100 cm in diameter. (B) The test aquarium was in “Y” shape. On the one side, the long route was in “V” shape, covered with black PVC tape (III). On the other side, two choice compartments were covered by blue or red PVC tape to create the most preferred or the most non-preferred option (I or II), respectively. The water column was 15 cm in depth.
Daily test was conducted in eight test tanks, i.e., eight seahorses were tested each batch, from 8:00 to 14:00 to ensure that the seahorse was in active status. Handling procedures were the same for the two phases. After the daily test, each seahorse was returned to the respective glass tank. All seahorses were fed at least 2 h before the test to eliminate the feeding effect on behavior. The same seahorse was always tested in the same test tank at the fixed test time (i.e., 8:00–14:00). Moreover, the test tanks were rotated daily to eliminate the possible effects of orientation memory and the light intensity. The temperature in the test tanks was the same as that in the isolation tanks.
PI calculations
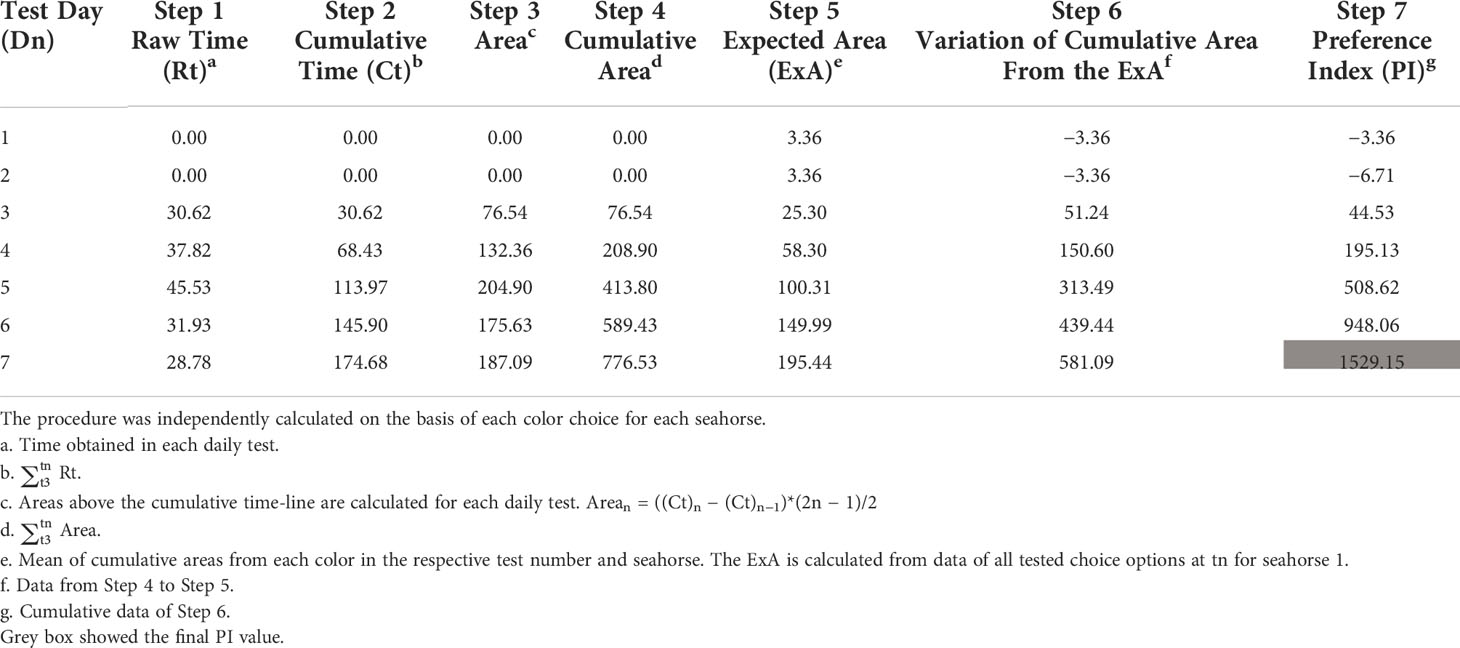
To analyze the preferred or non-preferred color choice and the intensity of the response to colored background, the PI for individual seahorses over the 7 test days was calculated according to Maia et al., 2017 with a little modification. As an example, the PI calculation of blue color preference in seahorse 1 is presented in Table 1. First, the time (Rt) that individual seahorse visited blue compartment was registered daily, from D1 to D7 (step 1). Then, the cumulative time (Ct) of seahorse 1 in blue background was calculated over the test days (step 2, e.g., when Dn = 3, Ct3 = Rt1 + Rt2 + Rt3). Considering that the most recent choices might highly impact on the preference, the area above the line built from the XY axes (X axis, test number; Y axis, step 1) was calculated to present the variation over time and more recent choices [step 3, e.g., Dn = 3, Area3 = (Ct3 – Ct2) × 5/2]. The cumulative area above the cumulative frequency line of each colored compartment was calculated (step 4, e.g., Dn = 3, Cumulative area3 = Area1 + Area2 + Area3). Expected area (step 5) was the mean of cumulative areas (step 4) from all the tested color in the respective test number of the seahorse [e.g., when Dn = 3, Expected area3 = (Cumulative area3 of blue + Cumulative area3 of red + Cumulative area3 of white + Cumulative area3 of black + Cumulative area3 of green + Cumulative area3 of yellow)/6]. Variation of cumulative area was the value that the cumulative area minus ExA (step 6 = step 4 − step 5). Finally, the PIs (step 7) were calculated from the cumulative data of step 6 (for example, when Dn = 3, PI3 = + + ). Finally, a PI of 1529.15 based on a 7-day test was calculated to present the preference for blue color in seahorse 1. The same procedure was applied to PI calculation of each seahorse for each color as well. The PI value ranges from the negative to positive, of which any positive value was considered as preferred color and negative value as non-preferred color. The value of each PI indicates the intensity of the response, which means that the larger the PI, the stronger the color preference.
Binary choice test and “knock-at-the-door” test
On the basis of the PIs, we found that the lined seahorse preferred for white and blue and avoided black and red backgrounds. To further confirm the results achieved with PI, a binary choice test and a “knock-at-the-door” test were performed.
On day 11, we applied the binary choice test to clarify the individual seahorses’ first chosen background compartment after they moved across the aversive route (Maia et al., 2017). The test aquaria were made in a “Y” shape, constituted of a long route (chamber III) with two exits at the upper end to chamber I and II, respectively (Figure 1B). The chamber III was a “V” shape and covered with black PVC tape to make it as an aversive area to make sure the seahorse would across this section to make a binary choice, which black had been manifested to be the most non-preferred color for the lined seahorse. As the lined seahorse were normally cultured in gray, white, or black tanks, to avoid a learned bias, blue and red were selected as the most preferred and the most non-preferred color, respectively. Thus, chambers I and II were covered by either blue or red PVC tape to create the most preferred or the least preferred option, respectively. A transparent plastic door was set up at the low end of chamber III to create an acclimation area. After 5 min of acclamation, the door was carefully removed, and the seahorse would cross over the black aversive route toward to chamber I or II as assumed. The behavior was recorded for 10 min, and the first chosen compartment was recorded. After the test, seahorses were returned to the respective isolation tanks.
A “knock-at-the-door” test was conducted on day 12. We supposed that the color choice of the stressed (i.e., “knock-at-the-door” test) lined seahorse would be consistent with that at unstressed (i.e., background color preference test) situation. We applied a 30-s air exposure to the lined seahorse, which has been widely used to induce an acute physiological stress response in fishes (Pickering and Pottingger, 1989; Thomson et al., 2011; Skrzynska et al., 2018), and confined the seahorse in the transparent plastic cylinder that was placed in center of the test tank (Figure 1). Behaviors in the cylinder were recorded immediately for 10 min, to allow seahorse fully expressing the response to color under stress. On the basis of preliminary observations (i.e., after a 30-s air exposure, the seahorse repetitively knocked the cylinder wall toward a specific-colored compartment with its snout in an upright posture and attempted to get access), the knock behavior is defined as the seahorse knocks the cylinder wall with its snout toward different colored compartments. Thus, we registered the frequency of the knock toward individual color compartment for further analysis. After the test, seahorses were returned as well.
Bold–shy personality screening assays
After 3 days of recovery, the bold–shy personality of all the seahorses used for background color preference test was screened by new environment and novel object test according to Freret-Meurer and Alves (2018) and Toms et al. (2011) with a little modification. All seahorses were fasting for 24 h prior to test. The assay was individually conducted in a white polyethylene tank (60 × 45 × 45 cm) with a transparent plastic tube provided as holdfast placed in the middle of the tank for the seahorse, to create a new environment different from the transparent glass tank where seahorse was cultured. Three behaviors of seahorse have been demonstrated (Freret-Meurer and Alves, 2018): a) inspecting the new habitat: seahorses exhibit the behavior of swimming throughout the aquarium; b) protection: grasping the holdfast and still; and c) rest: grasping the holdfast with active movement, such as body or head wiggling. After 5 min of acclimation in a corner of the aquarium restricted by transparent plastic cylinder, the seahorse was released gently. The following behavioral parameters were measured: 1) the total time of swimming and 2) the latency to grasp the holdfast. The behaviors were recorded for 30 min and scored later. About 1 h after the new environment test, when the seahorses were in the rest state, a green plastic plant was introduced in front of the seahorse as a novel object. Then, another 30 min was recorded: 1) the total time of the first protection state; 2) the total time of the protection state; and 3) the latency to change the holdfast. A total of 31 seahorses were individually tested due to missing one. The personality score for individual seahorse was calculated by principal components analysis (PCA).
Data analysis
The recorded behaviors were analyzed by one person to minimize the observer bias. The data of PI and knock frequency toward individual-colored backgrounds that failed to meet the normality by a Kolmogorov–Smirnov test were analyzed with a non-parametric test (i.e., Kruskal–Wallis test). If the Kruskal–Wallis test result was significant, then a post hoc test (Dunn test with Bonferroni adjustment) was applied. A Chi-square test was used to test whether the seahorse chosen the blue or red color as the expected in the binary choice test. A r × c contingency table Chi-square test was applied to test the significant difference between the colored backgrounds in the frequency of seahorses that prefer and do not prefer. If outcome variables were statistically significant, then a post hoc test using partitions of Chi-square method with Bonferroni adjustment was followed. Kendall’s tau-b correlation analysis was conducted to reveal the correlation between PI and the personality score. To screen the shy–bold continuum behavior, behavior responses from the new environment and novel object trials were standardized and collapsed into the principal component composite score by PCA. The Kaiser–Meyer–Olkin test for sample adequacy was greater than 0.5, and the Bartlett’s test of sphericity was significant (P< 0.05), indicating that PCA is suitable. The principal component scores were weighted by the contribution rate of each principal component to get the principal component composite score.
Results
The colored background preference of the lined seahorse
The final PIs of the lined seahorse that responded to the colored backgrounds over the 7 test days are presented in Figure 2. The PIs were significantly (Kruskal–Wallis test, P< 0.0001) different among individual background colors, ranking in descending order as follows: white (median PI = 900.81), blue (median PI = 385.91), green (median PI = −376.80), yellow (median PI = −518.34), black (median PI = −568.03), and red (median PI = −620.13). Post hoc pairwise comparison outcomes are presented in Figure 2. Moreover, all tested seahorses for red background had negative PI value, whereas either preference or non-preference responses for the other colors were found.
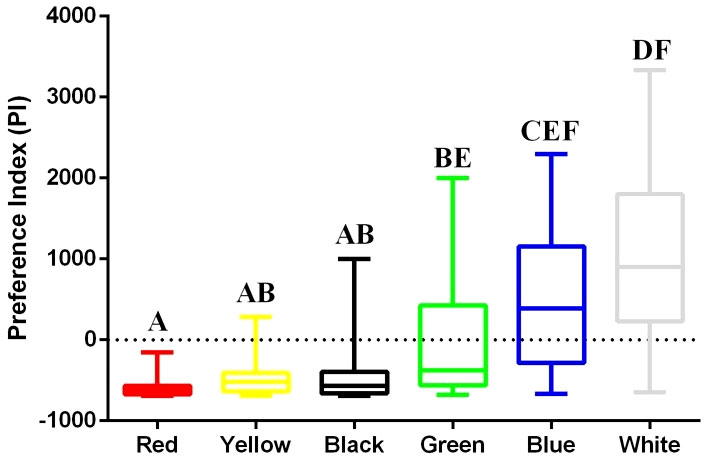
Figure 2 The final PIs of the lined seahorse indicating the intensity of preference for each color. Different capital letters indicate the significant differences of preferred options among the colors of red, yellow, black, green, blue, and white (Kruskal–Wallis test, P< 0.0001). Preference is showed as positive values and non-preference is showed as negative values. The more positive the value is, higher preference the seahorse is; N = 32.
Figure 3 shows the results of a r × c contingency table Chi-square test with the number of the seahorse showing preference (positive PIs) or non-preference (negative PIs) compared among colors (Figure 3A) and with the number of the seahorse with most preference or most non-preference compared among colors (Figure 3B), followed by a post hoc test with partitions of Chi-square method and Bonferroni adjustment. The results indicate that the two frequencies are significantly ( = 89.949, P< 0.0001, Figure 3A; = 53.826, P< 0.0001, Figure 3B) associated with colors, respectively. Post hoc tests for the two analyses indicated that more lined seahorses expressed the preference for white and blue than for red and black, whereas more seahorses disliked the red and black than white and blue (P< 0.05, Figures 3A, B).
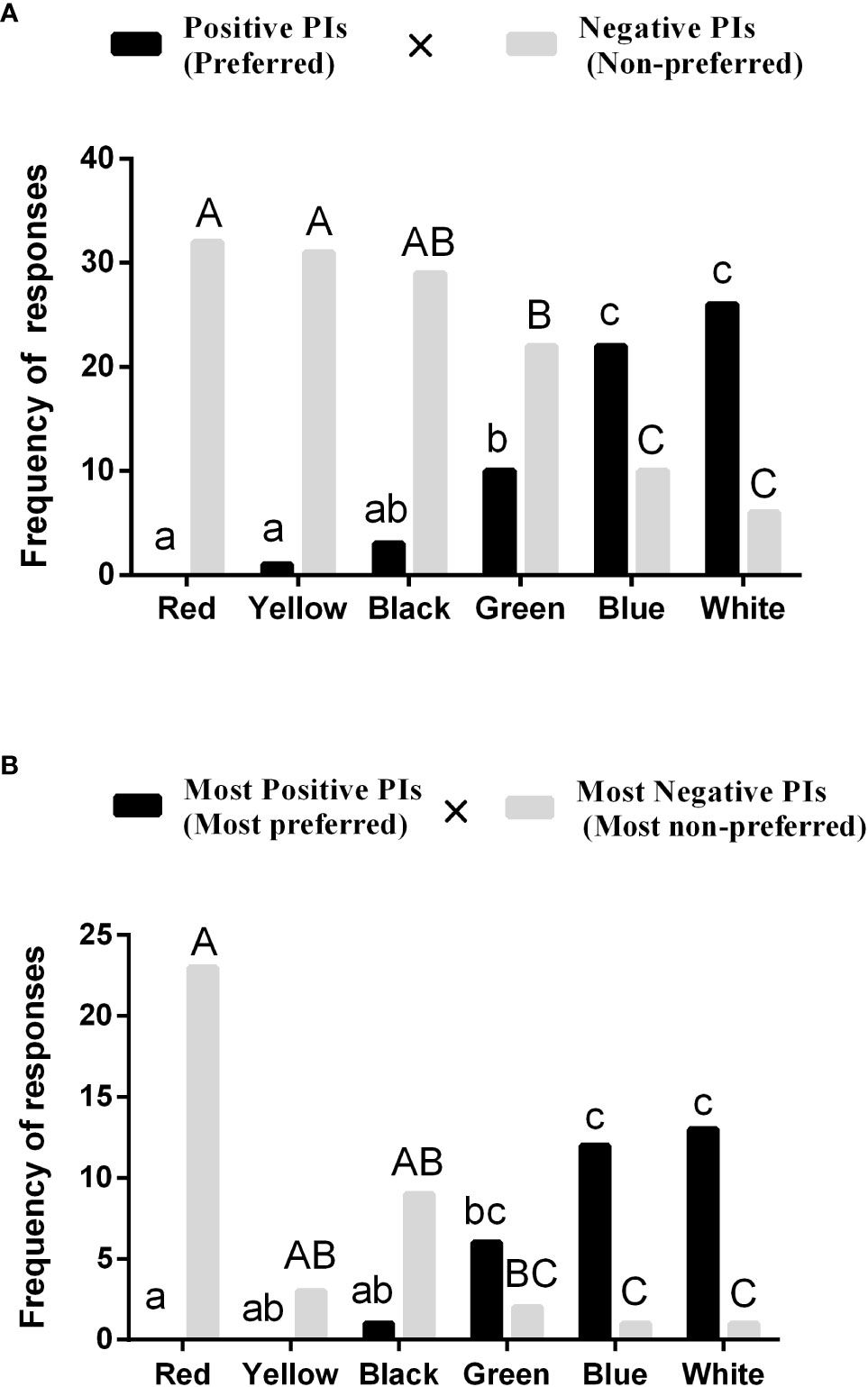
Figure 3 Frequency of preference and non-preference (A), and the most preference and most non-preference (B) responses of the seahorse to different colors. Different small or capital letters indicate significant differences among the colors in respective pairwise comparisons (N = 32).
Verification of the color preference by binary choice test and “knock-at-the-door” test
The binary choice test shows that significantly more seahorses entered the blue than red (28 versus 4, = 18, P< 0.0001) as the first chosen background compartment after across the long black route (Figure 4A). Generally, when the door opened, the seahorse would swim out of the acclimation area and crossed the black route toward either the blue or red chamber within 2 min and remained in the first chosen compartment. In the “knock-at-the-door” test (Figure 4B), the result shows that the proportion of knock the lined seahorse toward different colored compartments is significantly (Kruskal–Wallis test, P< 0.0001) associated with background color. The proportion that the seahorse knocked white background was significantly (P< 0.05) higher than the remaining background colors. The lowest frequency was found in red and black backgrounds.
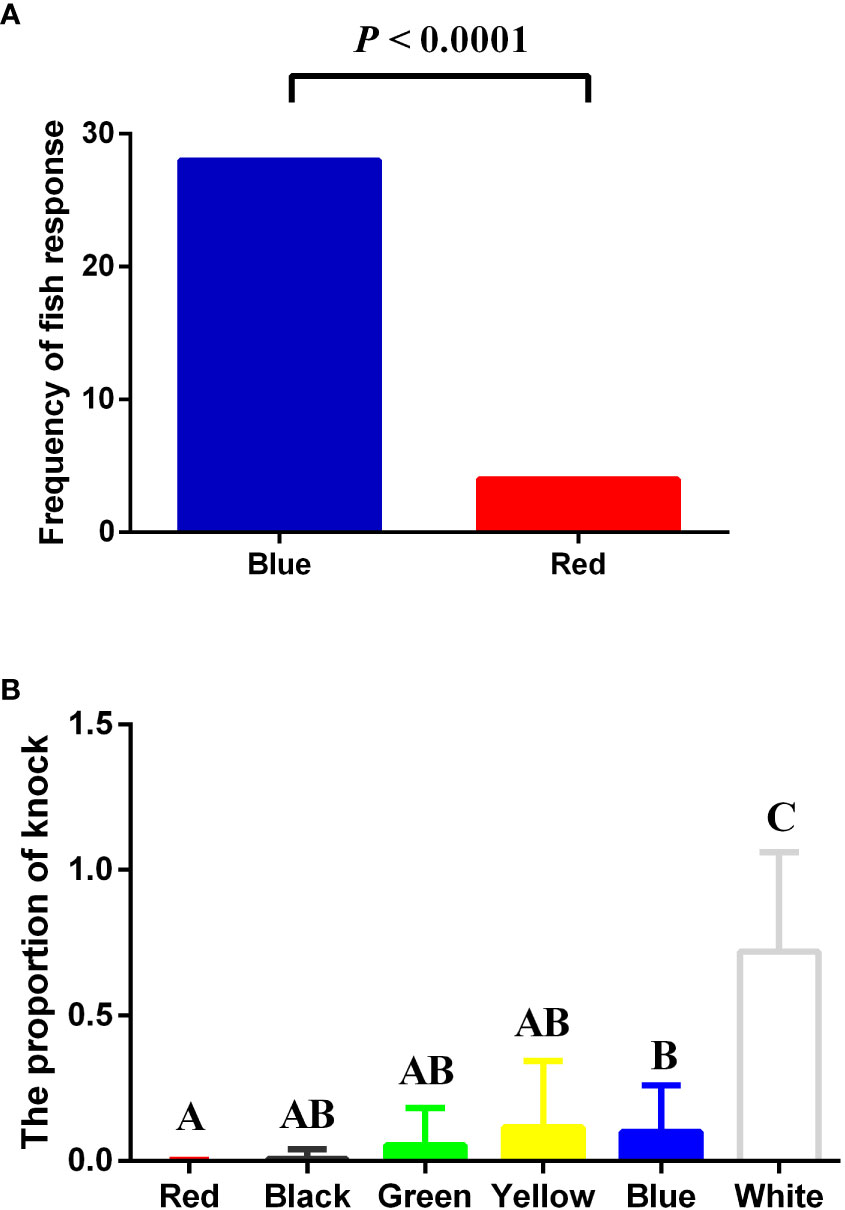
Figure 4 Binary choice test (A) and “knock-at-the-door” test (B) to verify the color preference. (A) Frequency of the seahorse entering blue chamber is significantly (Chi-square test, P< 0.0001) higher than red chamber. (B) The proportion of knock against the cylinder wall toward different colored backgrounds after a 30-s air exposure differs significantly (Kruskal–Wallis test, P< 0.0001, post hoc test with Dunn test and Bonferroni adjustment, N = 32). Different capital letters indicate significant differences among the colors.
Bold–shy personality and its relationship with color preference
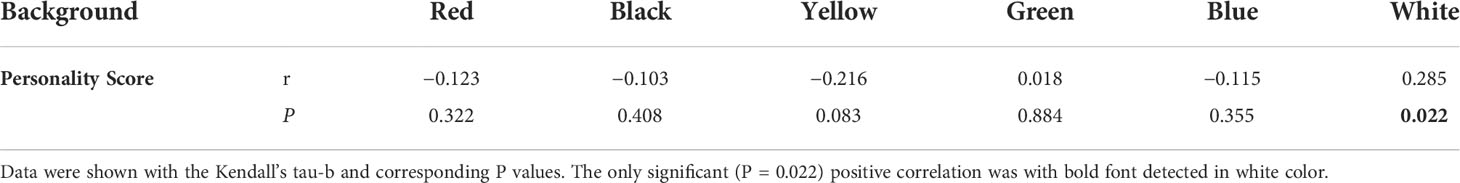
A total of five behavioral responses were recorded to structure the seahorse personality. Two components were derived (for component 1, Eigenvalue = 2.095; for component 2, Eigenvalue = 1.844), and each explains 41.909% and 36.889% variation, respectively (Table 2A). Thus, we collapsed the behaviors into the first and second component to calculate the principal component score to characterize of individual personality, as component matrix shown in Table 2B. The personality score distribution for individuals as shown in Figure 5 depicted the pronounced continuous variable with range from −1.43 to 3.06. The increasing value represented a stronger protective behavior and neophobia when faced a novel subject and inactive swimming behavior in a new environment, by which shyer seahorses were featured. Furthermore, we analyzed the correlation between personality score obtained from PCA values and PI of each color. The only significantly positive correlation was identified between personality score and the PI of white background (Kendall’s tau-b, P = 0.02, Table 3), indicating the shyer the seahorse was, the more preference white background color it displayed. The figure showing the actual data points and the correlation line tendency of PI of white background with personality score is presented in Supplementary Figure 2. No significant correlation was identified for the other background color preference with personality score (Kendall’s tau-b, P > 0.05).
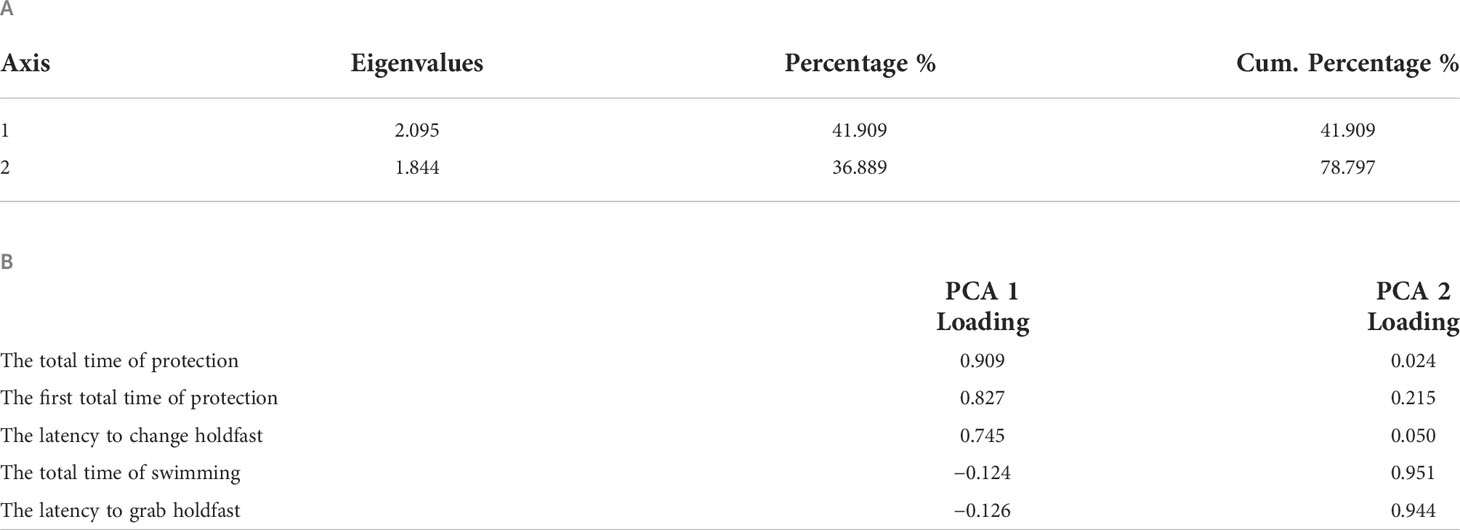
Table 2 Eigen explain (A) and component matrix (B) in terms of behavioral responses of the lined seahorse to assess the bold–shy axis for personality identification.
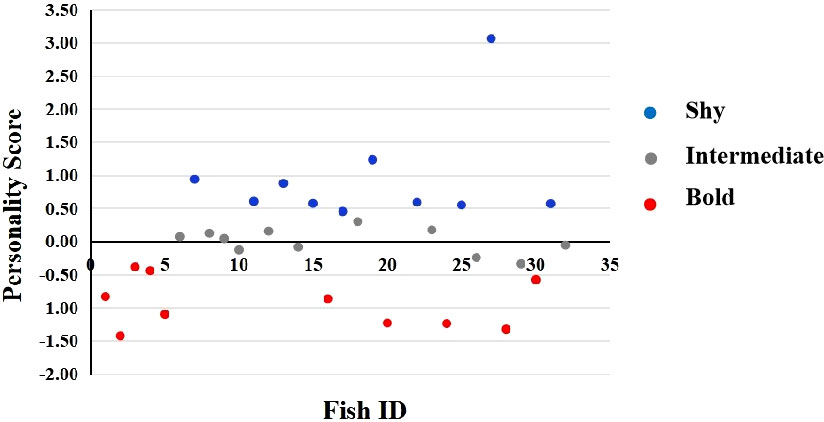
Figure 5 The bold–shy axis of the lined seahorse identified on the basis of personality score by the principal components analysis (PCA) (N = 31).
Discussion
Color resources in the environment can affect fishes’ social, cognitive, and affective responses (de Abreu et al., 2020). Many fish species exhibit specific color preference on account of the distinct evolutionary and ecological history (Egger et al., 2011; Selz et al., 2014). In the present study, we found that the lined seahorse preferred white and blue color backgrounds and avoided red and black ones in captivity, and the color preference was consistent under unstressed and stress situations. More importantly, our pioneering study explored for the first time the relationship between personality trait and color preference in fishes, as shy seahorses showed stronger protection, neophobia, and inactive swimming behavior in contrast to the bold ones. The shyer the seahorse was, the more preference for white background it displayed.
The preference for white and blue background
It has been reported that inhabiting in the adapted color enables fishes simultaneously to minimize the energy investment of color adaption to achieve growth and physical conditions and to reduce predator risk (Li et al., 2012; Rodgers et al., 2013). Many fish species have been demonstrated the preference for blue color (Li et al., 2016; Park et al., 2016; Maia et al., 2017; Roy et al., 2019), as blue color improves reproduction (Volpato et al., 2004) and growth (Ruchin, 2004) and reduces stress (Volpato and Barreto, 2001; Karakatsouli et al., 2008; Maia and Volpato, 2013). In the present study, we revealed that the lined seahorse has strong preference for blue background color as well. This may explain why the survival rate of low-quality juveniles of the lined seahorse cultured in blue tank (72.0%) was significantly higher than that in gray (49.6%) ones (Zhang et al., 2020). Seahorses prefer inhabiting shallow waters of marine and estuarine ecosystems where more blue light than green and yellow lights penetrate (Chiang et al., 2011) and grasp coral reefs, sea-grass beds, rocky, and mangrove roots to hide (Lourie et al., 1999),. Thus, it is not surprised that most fish species including the lined seahorse prefer for blue color. Meanwhile, the PIs imply that the lined seahorse mostly preferred white background. This might be related to their experience in early development that also affects the color preference (DePasquale et al., 2016). For example, Li et al. (2016) showed that juvenile turbot (Scophthalmus maximus) more frequently occupied the white background than other colored background after a 60-day adaptation to white background. In the present study, the lined seahorse was cultured originally under a light gray background, quite close to white condition. In addition, against white background would make food object more visible, probably accounting for the white background preference (Amiya et al., 2008; Sunuma et al., 2009).
The non-preference for black and red background
Red color is suggested having some disruptive effect on growth in some fish species (Ruchin, 2004; Karakatsouli et al., 2010). Maia et al. (2021) recommended red shelters should be avoided in juvenile Nile tilapia (Oreochromis niloticus) aquaculture. Unanimously, all 32 tested seahorses show a strong non-preference for black and red backgrounds. It is widely accepted that the “behavioral background matching” or “cryptic coloration” strategy is adopted in fishes to best match the habitats with coloration for concealment and avoiding predators (Kjernsmo and Merilaita, 2012; Rodgers et al., 2013; Phillips et al., 2017). Obviously, the hypothesis does not apply to the lined seahorse, because the tested seahorses were the black in body color, and 90.6% of the seahorses showed black color avoidance in the present study. Seahorses, a group of habitat mimicry or crypsis species, can rapidly translate the color that they see in the surroundings and accurately display the color through the skin (Stevens and Merilaita, 2011; Duarte et al., 2019). We inferred that the lined seahorses may adapt themselves to the color in nature or cultured environment, rather than selecting a habitat that has the similar color as their own body coloration.
Unusually, in 24 days after birth of the yellow seahorse (H. kuda), darker background (red, green, blue, and black) rearing had a higher growth than lighter background (yellow and transparent) (Pawar et al., 2011), whereas in pelagic phase longsnout seahorse (H. reidi), the tank color did not affect the growth and survival (Hora et al., 2017). Strains should be considered in the color-related behaviors (Vignet et al., 2013). Furthermore, age is demonstrated to affect the color preference in zebrafish (Danio rerio). As for larval zebrafish, the preference for light over dark background revealed the “survival” adaptive behavioral strategies (Bai et al., 2016). Nevertheless, adult zebrafish showed the dislike of bright light or white areas to avoid predators (Kysil et al., 2017). It should be mentioned here that background color preference was not biased by colored compartments position in test tank. Individual PI profile over time (Supplementary Figure 1) exhibiting consistent responses further supports the assumption. Interestingly, the PI of green background did not differ significantly from that of either blue or yellow background (Figure 2), which might be related to that green light that is the second highest amount of sunlight in shallow waters of the ocean.
Bold–shy personality involved in background color preference
The bold–shy axis in personality greatly affects the decision-making, especially in unpredictable environments, that varied greatly among individuals (Freret-Meurer and Alves, 2018). The bold individuals are more active, have courage in exploration, and are willing to take more risks and learn faster versus the shy ones (Sneddon, 2003; Martins et al., 2012). The bold longsnout seahorse readily inspected new habitats and objects, whereas the shy ones were inactivity and had neophobia response to novelties (Freret-Meurer and Alves, 2018). Similarly, the shy lined seahorse presented a stronger protection behavior and neophobia when confronted a novel object and had an inactive swimming behavior in a new environment, in contrast to the bold ones. As the color preference in the lined seahorse was consistent across situations, it is necessary to explore whether color preference could be a personality trait as well. Interestingly, the personality score has significant positive correlation with white color preference, suggesting that the white color preference could be used as an indicator to identify personality in the lined seahorse. However, it should be firmly confirmed whether the preference for blue is correlated with the personality in the future study. Nevertheless, whether background color of culture tank affects the personality, behaviors, physiology, and growth of the lined seahorse is worth further study.
Conclusion
In general, the lined seahorse displayed intense preference for white and blue background and avoidance of black and red background in either stressed or unstressed situation. Furthermore, the shy seahorses show defensive and neophobia behavior in contrast to the bold ones. A positive correlation that the shyer the individual, the more preference for white coloration suggests that color preference could be used as an indicator to identify bold–shy personality in the lined seahorse. Overall, on the basis of the findings of the present study, white or blue background color is recommended to be the optimal background colors for rearing the lined seahorse.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Chinese Academy of Sciences(IACUC #160413).
Author contributions
SL designed and conducted the experiment, analyzed the data, and prepared the draft manuscript. XL and TL provided assistance in conducting the experiment; DZ provided suggestions in experiment design and manuscript preparation. XZ helped to remold the test aquaria, cultured, and bred the seahorse for the experiment. All authors finally approved for publication.
Funding
This work was supported by the Shanghai Sailing Program (21YF1459800) and the Central Public-Interest Scientific Institution Basal Research Fund, Chinese Academy of Fishery Sciences (2020TD53, 2017HYZD0401).
Acknowledgments
We thank all laboratory members for their constructive suggestions and discussions. We are also grateful to the reviews for their valuable suggestions and modifications.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.939749/full#supplementary-material
Supplementary Figure 1 | Individual profiles of preference (positive PIs) and non-preference (negative PIs) responses of the lined seahorse to different background color conditions (X axis: Test Day; Y axis: Preference Index (PI), N = 32).
Supplementary Figure 2 | The actual data points and the correlation line tendency of PI of white background (Y axis) with personality score (X axis) (N = 31).
References
Amiya N., Amano M., Yamanome T., Yamamori K., Takahashi A. (2008). Effects of background color on GnRH and MCH levels in the barfin flounder brain. Gen. Comp. Endocrinol. 155, 88–93. doi: 10.1016/j.ygcen.2007.03.007
Asher L., Kirkden R. D., Bateson M. (2009). An empirical investigation of two assumptions of motivation testing in captive starlings (Sturnus vulgaris): do animals have an energy budget to “spend”? and does cost reduce demand? Appl. Anim. Behav. Sci. 118, 152–160. doi: 10.1016/j.applanim.2009.02.029
Bai Y., Liu H., Huang B., Wagle M., Guo S. (2016). Identification of environmental stressors and validation of light preference as a measure of anxiety in larval zebrafish. BMC Neurosci. 17 (1), 63. doi: 10.1186/s12868-016-0298-z
Barcellos J. G., Kreutz L. C., Quevedo R. M., da Rosa J. G. S., Koakoski G., Centenaro L., et al. (2009). Influence of color background and shelter availability on jundiá (Rhamdia quelen) stress response. Aquaculture 288, 51–56. doi: 10.1016/j.aquaculture.2008.11.002
Birren F. (1973). Color preference as a clue to personality. Art. Psychother. 1 (1), 13–16. doi: 10.1016/0090-9092(73)90005-7
Bowmaker J. K., Loew E. R. (2007). “Vision in fish,” in The senses: a comprehensive reference, 1st ed. Eds. Basbaum A., Kaneko A., Shepherd G., Westheimer G. (Oxford, UK: Elsevier), 53–76.
Carere C., Drent P. J., Privitera L., Koolhaas J. M., Groothuis T. G. G. (2005). Personalities in great tits, Parus major: stability and consistency. Anim. Behav. 70, 795–805. doi: 10.1016/j.anbehav.2005.01.003
Chiang J. Y., Chen Y. C., Chen Y. F. (2011). “Underwater image enhancement: using wavelength compensation and image dehazing (WCID),” in ACIVS 2011, LNCS 6915. Eds. Blanc-Talon J., Kleihorst R. P., Philips W., Popescu D. C., Scheunders P. (Dessau, Germany: Springer), 372–383.
Correa M., Chung K. S., Manrique R. (1989). Cultive experimental. del caballito de mar, Hippocapus erectus. Bol. Inst. Oceanogr. Venez 28 (1-2), 191–196.
de Abreu M. S., Giacomini A. C. V. V., Genario R., dos Santos B. E., Marcon L., Demin K. A., et al. (2020). The impact of housing environment color on zebrafish anxiety-like behavioral and physiological (cortisol) responses. Gen. Comp. Endocrinol. 294, 113499. doi: 10.1016/j.ygcen.2020.113499
DePasquale C., Neuberger T., Hirrlinger A., Braithwaite V. A. (2016). The influence of complex and threatening environments in early life on brain size and behavior. P. R. Soc B-Biol. Sci. 283, 20152564. doi: 10.1098/rspb.2015.2564
Duarte M., Gawryszewski F. M., Ramineli S., Bessa E. (2019). Disruptive coloration and habitat use by seahorses. Neotrop. Ichthyol 17 (4), e190064. doi: 10.1590/1982-0224-20190064
Egger B., Klaefiger Y., Theis A., Salzburger W. (2011). A sensory bias has triggered the evolution of egg-spots in cichlid fishes. PloS One 6, e25601. doi: 10.1371/journal.pone.0025601
Espmark Y., Amundsen T., Rosenqvist G. (2000). Animal signals: signalling and signal design in animal communication (Norway: Tapir Academic Press).
Faleiro F., Narciso L., Vicente L. (2008). Seahorse behaviour and aquaculture: How to improve Hippocampus guttulatus husbandry and reproduction? Aquaculture 282 (1-4), 33–40. doi: 10.1016/j.aquaculture.2008.05.038
Fetterman A. K., Liu T., Robinson M. D. (2015). Extending color psychology to the personality realm: interpersonal hostility varies by red preferences and perceptual biases. J. Pers. 83 (1), 106–116. doi: 10.1111/jopy.12087
Fowler-Finn K. D., Rodriguez R. L. (2013). Repeatability of mate preference functions in enchenopa treehoppers (Hemiptera: Membracidae). Anim. Behav. 85, 493–499. doi: 10.1016/j.anbehav.2012.12.015
Freret-Meurer N. V., Alves M. A. S. (2018). Personality in the longsnout seahorse, Hippocampus reidi ginsbur: Are males shyer than females? Behav. Process 157, 106–110. doi: 10.1016/j.beproc.2018.09.006.
Hora M. S. C., Joyeux J. C., Guabiroba H. C., Tsuzuki M. Y. (2017). Effect of photoperiod and tank colour on growth and survival of pelagic-phase seahorse Hippocampus reidi. Aquac. Res. 48, 1–8. doi: 10.1111/are.13252
Houpt K. A. (2012). Motivation for cribbing by horses. Anim. Welf. 21, 1–7. doi: 10.7120/096272812799129367
Job S., Buu D., Vincent A. (2006). Growth and survival of the tiger tail seahorse, Hippocampus comes. J. World Aquacult. Soc. 37, 322–327. doi: 10.1111/j.1749-7345.2006.00044.x
Job S. D., Do H. H., Meeuwigc J. J., Hall H. J. (2002). Culturing the oceanic seahorse, Hippocampus kuda. Aquaculture 214, 333–341. doi: 10.1016/S0044-8486(02)00063-7
Kaiser M. I., Müller C. (2021). What is an animal personality? Biol. Philos. 36, 1. doi: 10.1007/s10539-020-09776-w
Kang D. Y., Kim H. C. (2013a). Importance of bottom type and background color for growth and blind-side hypermelanosis of the olive flounder, Paralichthys olivaceus. aquac. Eng 57, 1–8. doi: 10.1016/j.aquaeng.2013.05.001
Kang D. Y., Kim H. C. (2013b). Influence of density and background color to stress response, appetite, growth, and blind-side hypermelanosis of flounder, Paralichthys olivaceus. fish physiol. Biochem 39, 221–232. doi: 10.1007/s10695-012-9693-2
Karakatsouli N., Papoutsoglou S. E., Manolessos G. (2007). Combined effects of rearing density and tank colour on the growth and welfare of juvenile white sea bream Diplodus sargus l. in a recirculating water system. Aquac. Res. 38, 1152–1160. doi: 10.1111/j.1365-2109.2007.01780.x
Karakatsouli N., Papoutsoglou S. E., Panopoulos G., Papoutsoglou E. S., Chadio S., Kalogiannis D. (2008). Effects of light spectrum on growth and stress response of rainbow trout Oncorhynchus mykiss reared under recirculating system conditions. Aquacult. Eng. 38, 36–42. doi: 10.1016/j.aquaeng.2007.10.006
Karakatsouli N., Papoutsoglou E. S., Sotiropoulos N., Mourtikas D., Stigen-Martinsen T., Papoutsoglou S. E. (2010). Effects of light spectrum, rearing density and light intensity on growth performance of scaled and mirror common carp Cyprinus carpio reared under recirculating system conditions. Aquacult. Eng. 42 (3), 121–127. doi: 10.1016/j.aquaeng.2010.01.001
Kjernsmo K., Merilaita S. (2012). Background choice as an anti-predator strategy: the roles of background matching and visual complexity in the habitat choice of the least killifish. Proc. Biol. Sci. 279 (1745), 4192–4198. doi: 10.1098/rspb.2012.1547
Kysil E. V., Meshalkina D. A., Frick E. E., Echevarria D. J., Rosemberg D. B., Maximino C., et al. (2017). Comparative analyses of zebrafish anxiety-like behavior using conflict-based novelty tests. Zebrafish 14 (4), 197–208. doi: 10.1089/zeb.2016.1415
Lehtonen T. K., Lindstrom K. (2006). Repeatability of mating preferences in the sand goby. Anim. Behav. 75, 55–61. doi: 10.1016/j.anbehav.2007.04.011
Li X., Chi L., Tian H., Meng L., Zheng J., Gao X., et al. (2016). Color preferences of juvenile turbot (Scophthalmus maximus). Physiol. Behav. 156, 64–70. doi: 10.1016/j.physbeh.2016.01.007
Li X., Fan L., Liu Z., Ren X., Liu Y. (2012). Preference habit of juvenile turbot for different color backgrounds based on computer vision. Trans. Chin. Soc Agric. Eng. 28, 189–193. doi: CNKI:SUN:NYGU.0.2012-10-031
Lin Q., Gao Y. L., Sheng J. Q., Chen Q. X., Zhang B., Lu J. Y. (2007). The effect of food and the sum of effective temperature on the embryonic development of the seahorse, Hippocampus kuda bleeker. Aquaculture 262, 481–492. doi: 10.1016/j.aquaculture.2006.11.011
Lin Q., Lin J., Huang L. (2009). Effects of substrate color, light intensity and temperature on survival and skin color change of juvenile seahorses, Hippocampus erectus perr. Aquaculture 298, 157–161. doi: 10.1016/j.aquaculture.2009.10.015
Lin Q., Lin J., Zhang D. (2008). Breeding and juvenile culture of the lined seahorse, Hippocampus erectus. Aquaculture 277, 287–292. doi: 10.1016/j.aquaculture.2008.02.030
Lin Q., Lu J. Y., Gao Y. L. (2006). The effect of temperature on gonad, embryonic development and survival rate of juvenile seahorses, Hippocampus kuda bleeker. Aquaculture 254, 701–713. doi: 10.1016/j.aquaculture.2005.11.005
Losey G. S., McFarland W. N., Loew E. R., Zamzow J. P., Nelson P. A., Marshall N. J. (2003). Visual biology of Hawaiian boral reef fishes. i. ocular transmission and visual pigments. Copeia 2003 (3), 433–454. doi: 10.1643/01-053
Lourie S. A., Vincent A. C., Hall H. J. (1999). Seahorse: An identification guide to the world’s species and their conservation. Proj. Seahorse London., 214.
Lythgoe J. N., Partridge J. C. (1989). Visual pigments and the acquisition of visual information. J. Exp. Biol. 146, 1–20. doi: 10.1242/jeb.146.1.1
Maia C. M., Alves N. P. C., Tatemoto P. (2021). Juvenile Nile tilapia fish avoid red shelters. J. Appl. Anim. Welf. Sci. 1, 98–106. doi: 10.1080/10888705.2020.1848567
Maia C. M., Ferguson B., Volpato G. L., Braithwaite V. A. (2017). Physical and psychological motivation tests of individual preferences in rainbow trout. J. Zool. 302 (2), 108–118. doi: 10.1111/jzo.12438
Maia C. M., Volpato G. L. (2013). Environmental light color affects the stress response of Nile tilapia. Zoology 116, 64–66. doi: 10.1016/j.zool.2012.08.001
Maia C. M., Ferguson Volpato B. G. L., Braithwaite V. A. (2017). Physical and psychological motivation tests of individual preferences in rainbow trout. Zoology 302 (2), 108–18. doi: 10.1038/srep28328
Martins C. I. M., Schaedelin F. C., Mann M., Blum C., Mandl I., Urban D., et al. (2012). Exploring novelty: a component trait of behavioural syndromes in a colonial fish. Behaviour 149, 215–231. doi: 10.1163/156853912X634430
Mason G. J., Cooper J., Clarebrough C. (2001). Frustrations of fur-farmed mink. Nature 410, 35–36. doi: 10.1038/35065157
Miquel P., Alexandro C., Patricia Q., Antonio V. (2008). Establishment and maintenance of threatened long-snouted seahorse, Hippocampus guttulatus, broodstock in captivity. Aquaculture 283 (1-4), 0–28. doi: 10.1016/j.aquaculture.2008.06.023
Neumeyer C. (1992). Tetrachromatic color-vision in goldfish-evidence from color mixture experiments. J. Comp. Physiol. A 171, 639–649. doi: 10.1007/BF00194111
Nikolaenko N. N., Ostrovskaia M. I. (1989). Color preference as an index of emotional-personality characteristics (research under conditions of transient depression of the right or left hemisphere). Fiziol. Cheloveka 15 (4), 11–15.
Olivotto I., Avella M. A., Sampaolesi G., Piccinetti C. C., Ruiz P. N., Carnevali O. (2008). Breeding and rearing the longsnout seahorse Hippocampus reidi: rearing and feeding studies. Aquaculture 283, 92–96. doi: 10.1016/j.aquaculture.2008.06.018
Park J. S., Ryu J. H., Choi T. I., Bae Y. K., Lee S., Kang H. J., et al. (2016). Innate color preference of zebrafish and its use in behavioral analyses. Mol. Cells 39 (10), 750–755. doi: 10.14348/molcells.2016.0173
Pawar H. B., Sanaye S. V., Murugan A., Sreepada R. A. (2011). Effect of background color of tanks on growth and survival of juvenile yellow seahorse, Hippocampus kuda (Bleeker 1852), in the pelagic phase. ISR. J. Aquacult-Bamid 63, 1–6. doi: 10.3750/AIP2011.41.3.14
Payne M. F., Rippingale R. J. (2000). Rearing West Australian seahorse, Hippocampus subelongatus, juveniles on copepod nauplii and enriched Artemia. Aquaculture 188, 353e361. doi: 10.1016/S0044-8486(00)00349-5
Phillips G. A. C., How M. J., Lange J. E., Marshall N. J., Cheney K. L. (2017). Disruptive colouration in reef fish: does matching the background reduce predation risk? J. Exp. Biol. 220 (11), 1962–1974. doi: 10.1242/jeb.151480
Pickering A. D., Pottinger T. G. (1989). Stress responses and disease resistance in salmonid fish-effects of chronic elevation of plasma-cortisol. Fish Physiol. Biochem. 7, 253–258. doi: 10.1007/BF00004714
Qin G., Zhang Y., Wang X., Lin Q. (2016). Effects of anesthetic disposal on the physiological and behavioral responses of the lined seahorses, Hippocampus erectus. J. World Aquacult. Soc. 47, 387–395. doi: 10.1111/jwas.12282
Qu H., Luo W., Lin Q. (2016). Development of SNP markers in lined seahorse (Hippocampus erectus) based on transcriptome sequencing. Conserv. Genet. Resour. 8, 1–4. doi: 10.1007/s12686-015-0510-y
Réale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. J. (2007). Integrating animal temperament within ecology and evolution. Biol. Rev. Camb. Philos. Soc 82 (2), 291–318. doi: 10.1111/j.1469-185X.2007.00010.x
Rodgers G. M., Gladman N. W., Corless H. F., Morrell L. J. (2013). Costs of colour change in fish: food intake and behavioural decisions. J. Exp. Biol. 216, 2760–2767. doi: 10.1242/jeb.080879
Roy T., Suriyampola P. S., Flores J., López M., Hickey C., Bhat A., et al. (2019). Color preferences affect learning in zebrafish, Danio rerio. sci. Rep 9 (1), 14531. doi: 10.1038/s41598-019-51145-5
Ruchin A. B. (2004). Influence of colored light on growth rate of juveniles of fish. Fish Physiol. Biochem. 30 (2), 175–178. doi: 10.1007/s10695-005-1263-4
Scarratt A. M. (1995). Techniques for raising lined seahorses (Hippocapus erectus). Aquarium Front. 3, 24–29.
Selz O. M., Pierotti M. E. R., Maan M. E., Schmid C., Seehausen O. (2014). Female preference for male color is necessary and sufficient for assortative mating in 2 cichlid sister species. Behav. Ecol. 25, 612–626. doi: 10.1093/beheco/aru024
Sheng J. Q., Lin Q., Chen Q. X., Gao Y. L., Shen L., Lu J. Y. (2006). Effects of food, temperature and light intensity on the feeding behavior of three-spot juveniles, Hippocampus trimaculatus leach. Aquaculture 256, 596–607. doi: 10.1016/j.aquaculture.2006.02.026
Sih A., Bell A., Johnson J. C. (2004). Behavioural syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. doi: 10.1016/j.tree.2004.04.009
Skrzynska A. K., Maiorano E., Bastaroli M., Naderi F., Míguez J. M., Martínez-Rodríguez G., et al. (2018). Impact of air exposure on vasotocinergic and isotocinergic systems in gilthead Sea bream (Sparus aurata): New insights on fish stress response. Front. Physiol. 13 (9), 96. doi: 10.3389/fphys.2018.00096
Sneddon L. U. (2003). The bold and the shy: individual differences in rainbow trout. J. Fish Biol. 62, 971–975. doi: 10.1046/j.1095-8649.2003.00084.x
Spence R., Smith C. (2008). Innate and learned colour preference in the zebrafish, Danio rerio. Ethology 114 (6), 582–588. doi: 10.1111/j.1439-0310.2008.01515.x
Stevens M., Merilaita S. (2011). Animal camouflage: mechanisms and function (Cambridge: United Kingdom at the University Press).
Stimpson D. V., Stimpson M. F. (1979). Relation of personality characteristics and color preferences. Percept. Mot. Skills 49 (1), 60–62. doi: 10.2466/pms.1979.49.1.60
Strand A., Alanärä A., Staffan F., Magnhagen C. (2007). Effects of tank colour and light intensity on feed intake, growth rate and energy expenditure of juvenile Eurasian perch, Perca fluviatilis l. Aquaculture 272, 312–318. doi: 10.1016/j.aquaculture.2007.08.052
Sunuma T., Yamanome T., Amano M., Takahashi A., Yamamori K. (2009). White background stimulates the food intake of a pleuronectiform fish the barfin flounder, Verasper moseri (Jordan and Gilbert). Aquacult. Res. 40, 748–751. doi: 10.1111/j.1365-2109.2009.02179.x
Takahashi A., Kosugi T., Kobayashi Y., Yamanome T., Schioth H. B., Kawauchi H. (2007). The melanin-concentrating hormone receptor 2 (MCH-R2) mediates the effect of MCH to control body color for background adaptation in the barfin flounder. Gen. Comp. Endocrinol. 151, 210–219. doi: 10.1016/j.ygcen.2007.01.011
Tamazouzt L., Chatain B., Fontaine P. (2000). Tank wall colour and light level affect growth and survival of Eurasian perch larvae (Perca fluviatilis l.). Aquaculture 182 (1-2), 85–90. doi: 10.1016/S0044-8486(99)00244-6
Tao B., Xu S., Pan X., Gao Q., Wang W. (2015). Personality trait correlates of color preference in schizophrenia. Transl. Neurosci. 6 (1), 174–178. doi: 10.1515/tnsci-2015-0018
Thomson J. S., Watts P. C., Pottinger T. G., Sneddon L. U. (2011). Physiological and genetic correlates of boldness: characterising the mechanisms of behavioural variation in rainbow trout, Oncorhynchus mykiss. horm. Behav 59 (1), 67–74. doi: 10.1016/j.yhbeh.2010.10.010
Vignet C., Bégout M. L., Péan S., Lyphout L., Leguay D., Cousin X. (2013). Systematic screening of behavioral responses in two zebrafish strains. Zebrafish 10 (3), 365–375. doi: 10.1089/zeb.2013.0871
Vincent A. C. J. (1996). The international trade in seahorse (Cambridge: TRAFFIC International), 164.
Volpato G. L., Barreto R. E. (2001). Environmental blue light prevents stress in the fish Nile tilapia. Braz. J. Med. Biol. Res. 34, 1041–1045. doi: 10.1590/S0100-879X2001000800011
Volpato G. L., Bovi T. S., de Freitas R. H. A., da Silva D. F., Delicio H. C., Giaquinto P. C., et al. (2013). Red light stimulates feeding motivation in fish but does not improve growth. PloS One 8, e59134. doi: 10.1371/journal.pone.0059134
Volpato G. L., Duarte C. R. A., Luchiari A. C. (2004). Environmental color affects Nile tilapia reproduction. Brazil J. Med. Biol. Res. 37 (4), 479–483. doi: 10.1590/S0100-879X2004000400004
Woods C. M. C. (2000). Improving initial survival in cultured seahorse, Hippocampus abdominalis Lesson, 1827 (Teleostei: Syngathidae). Aquaculture 190, 377–388. doi: 10.1016/S0044-8486(00)00408-7
Woods C. M.C. (2003). PGrowth and survival of juvenile seahorse Hippocampus abdominalis reared on live frozen and artificial foods. Aquaculture 220, 287–98. doi: 10.1016/S0044-8486(02)00227-2
Yang T., Kasagi S., Takahashi A., Mizusawa K. (2021). Effects of background color and feeding status on the expression of genes associated with body color regulation in the goldfish Carassius auratus. gen. Comp. Endocrinol. 312, 113860. doi: 10.1016/j.ygcen.2021.113860
Zhang D., Liu X., Lin T. T., Xin S. M. (2020). A method to improve low-quality juvenile seahorse by background color and light CN111631185A.
Keywords: the lined seahorse, background color, preference, fish personality, welfare condition
Citation: Li S, Liu X, Lin T, Zhang D and Zou X (2022) The consistent background color preference highlights the personality in the lined seahorse, Hippocampus erectus. Front. Mar. Sci. 9:939749. doi: 10.3389/fmars.2022.939749
Received: 09 May 2022; Accepted: 29 August 2022;
Published: 20 September 2022.
Edited by:
Enric Gisbert, Institute of Agrifood Research and Technology (IRTA), SpainReviewed by:
Marco Antonio Vindas, Norwegian University of Life Sciences, NorwayMartin Iversen, Nord University, Norway
Copyright © 2022 Li, Liu, Lin, Zhang and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Zhang, emRmaXQ2M0AxNjMuY29t
 Siping Li
Siping Li Xin Liu
Xin Liu Dong Zhang
Dong Zhang