- 1State Key Laboratory for Quality and Safety of Agro-Products, Ningbo University, Ningbo, China
- 2Shandong Key Laboratory of Disease Control in Mariculture, Qingdao, China
This study aims to establish a quantitative and qualitative evaluation model of sea cucumber Apostichopus japonicus research. Data from 2000 to 2021 were obtained from the Web of Science Core Collection (WoSCC) of Thomson Reuters. Bibliometrics and CiteSpace software were used to analyze authors, exporting countries, journals, influential articles, research areas, institutions, research hot spots, and trends. A total of 1,358 research papers on A. japonicus research were identified from 2000 to 2021. The number of papers published in this field is rapidly increasing, and the research phase can be divided into initial, developmental, and stabilization phases. Research on A. japonicus is mostly conducted in China, followed by Japan and the United States. Hongsheng Yang, Chenghua Li, and Shuanglin Dong are the lead authors. Research activities are focused on genetics and breeding, growth and development, immunology and disease, aestivation, regeneration, and food processing. Gut microbiota, activation, and collagen are potential research hot spots. The project highlights differences in the level of research between countries and teams, and regions with more developed industries or richer resources need further support. Governments or organizations are encouraged to 1) promote the development of the A. japonicus industry through the development or implementation of policies; 2) further participate in the research, production, and processing of A. japonicus; and 3) strengthen international exchange and cooperation to bring economic benefits to farmers in suitable breeding areas through technology sharing.
Introduction
More than 1,500 species of sea cucumbers have been recorded in the world (Liao, 1997). Sea cucumber Apostichopus japonicus (Echinodermata, Holothuroidea) was first described and reported by Selenka (1867). Considering its richness in bioactive substances such as triterpene glycosides, polysaccharides, and neuropeptides, it has been a valuable traditional food and nutraceutical, especially in East Asian countries (Bordbar et al., 2011; Ramírez-González et al., 2020). Only approximately 60 species have exploitation value, among which A. japonicus is the most economically important species (Kinch et al., 2008). In 2021, China’s total production of A. japonicus will be 350 million pounds, about 1.6 billion pieces, and the value of the whole industry chain will be about $9 billion (Sea Cucumber Industry Branch of China Fisheries Association, 2022). Wild A. japonicus is mainly found in the temperate waters of the western Pacific Ocean, including Japan, Korea, the Russian Far East, and Northeast China (Cui and Zhao, 2000). China is a major producer and consumer of A. japonicus. The economic growth and increased consumption of the Chinese society provide a good environment for the development of the A. japonicus industry (Ru et al., 2019). In addition to seabed seeding, three well-established aquaculture models, namely, shrimp pool coculture, floating raft culture, and bottom net culture, are being developed in China (Chang, 2002).
In the late 19th century, the Japanese scholar Kakichi Mitsukuri first studied the morphology and ecological habits of A. japonicus (Mitsukuri, 1897; Mitsukuri, 1905). The early research on A. japonicus was also conducted in Japan by a group of technical application talents who studied the growth and reproduction of A. japonicus. In 1938, Densaburo Inaba first tried to breed sea cucumbers in captivity (Midori and Kenichiro, 1981). In 1950, he worked with Takeshi Imai and Ryuhei Sato to develop the use of colorless flagellates as bait for sea cucumber larvae (Imai et al., 1950). Scholars Kinoshita and Tanaka reported on the short aestivation time of sea cucumbers from Hokkaido (Tanaka, 1958; Furukawa et al., 2016). A few years later, they sampled and analyzed the A. japonicus digestive tract and found that it mainly consisted of gravel, sediment, and shell fragments, providing a research basis for the culture of the species. Choe (1963) studied the genetics and regeneration of sea cucumbers. Over the next 60 years, Russia, South Korea, United States, and China will work together to improve A. japonicus breeding, disease control, and growth performance.
Zoology, agriculture, and other disciplines have begun using knowledge analysis and visualization tools, such as the CiteSpace software, to investigate and analyze water pollution (Hu et al., 2019; Liu et al., 2019), soil erosion (Liu et al., 2020; Huang et al., 2021), and marine conservation (Picone et al., 2021; Wang et al., 2021). In the case of A. japonicus, although we have a lot of basic research published in journals, access to cutting-edge data in the industry still relies on regular review articles, technical summits, and especially the support of government agencies in charge of fisheries. Scholars have not attempted to systematize and visualize the knowledge about A. japonicus developed from basic or industrial research. In the present study, we used bibliometrics and CiteSpace software to qualitatively or quantitatively analyze and visualize literature from 2000 to 2021, estimate the global scientific outputs of A. japonicus research, and analyze the hot spots and trends, which aims to provide fundamental data for further research on A. japonicus and encourage governments and organizations to promote the development of the A. japonicus industry through the development or implementation of policies.
Data and methods
Data sources
The data retrieval process was completed on 1 April 2022. This study is based on the Web of Science Core Collection (WoSCC) database, including Science Citation Index Expanded, Social Sciences Citation Index, Current Chemical Reactions, and Index Chemicus. Both A. japonicus and Stichopus japonicus were used in research papers because of the different usage habits of scholars or other factors. Therefore, the terms “Apostichopus japonicus” and “Stichopus japonicus” were used as keywords to retrieve titles, author information, abstracts, and references from 2000 to 2021, respectively. On this basis, we used the Boolean Operator “OR” to combine the two search formulas and obtained 1,358 research papers on A. japonicus. The bibliographies of 1,358 papers were downloaded and exported as “plain text files,” and the records were set to “full records and cited references.” The three downloaded text files were imported into the CiteSpace analysis software to perform taxonomy and visual analysis of the research literature.
Research method
To present the research hot spot and development trend of this subject intuitively, objectively, quantitatively, and authentically, we used bibliometrics theory and CiteSpace 5.8.R3 as research tools. As proposed by Allen Pritchard in 1969, bibliometrics is defined as “the assembling and interpretation of statistics relating to books and periodicals … to demonstrate historical movements, to determine the national or universal research use of books and journals, and to ascertain in many local situations the general use of books and journals” and is commonly used to analyze published literature and organize knowledge (Pritchard, 1969; Khalil and Crawford, 2015). CiteSpace is an excellent scientific mapping tool based on bibliometrics, and it was developed by Dr. Chaomei Chen, a tenured professor at Drexel University (Chen, 2006). It provides researchers with a powerful tool for the analysis of historical literature and creating a summary of the characteristics of the discipline through time-sharing and dynamic citation analysis visualization techniques (Chen et al., 2015). CiteSpace allows the identification of emerging trends while reducing our reliance on domain experts or prior knowledge base. This approach makes the analysis repeatable with new data and can be validated by different analysts. The evolution of the research field can be shown centrally on citation network maps. Based on the citation nodes and co-citation clusters on the map, the research frontiers and hot spots in the field can be analyzed.
Results
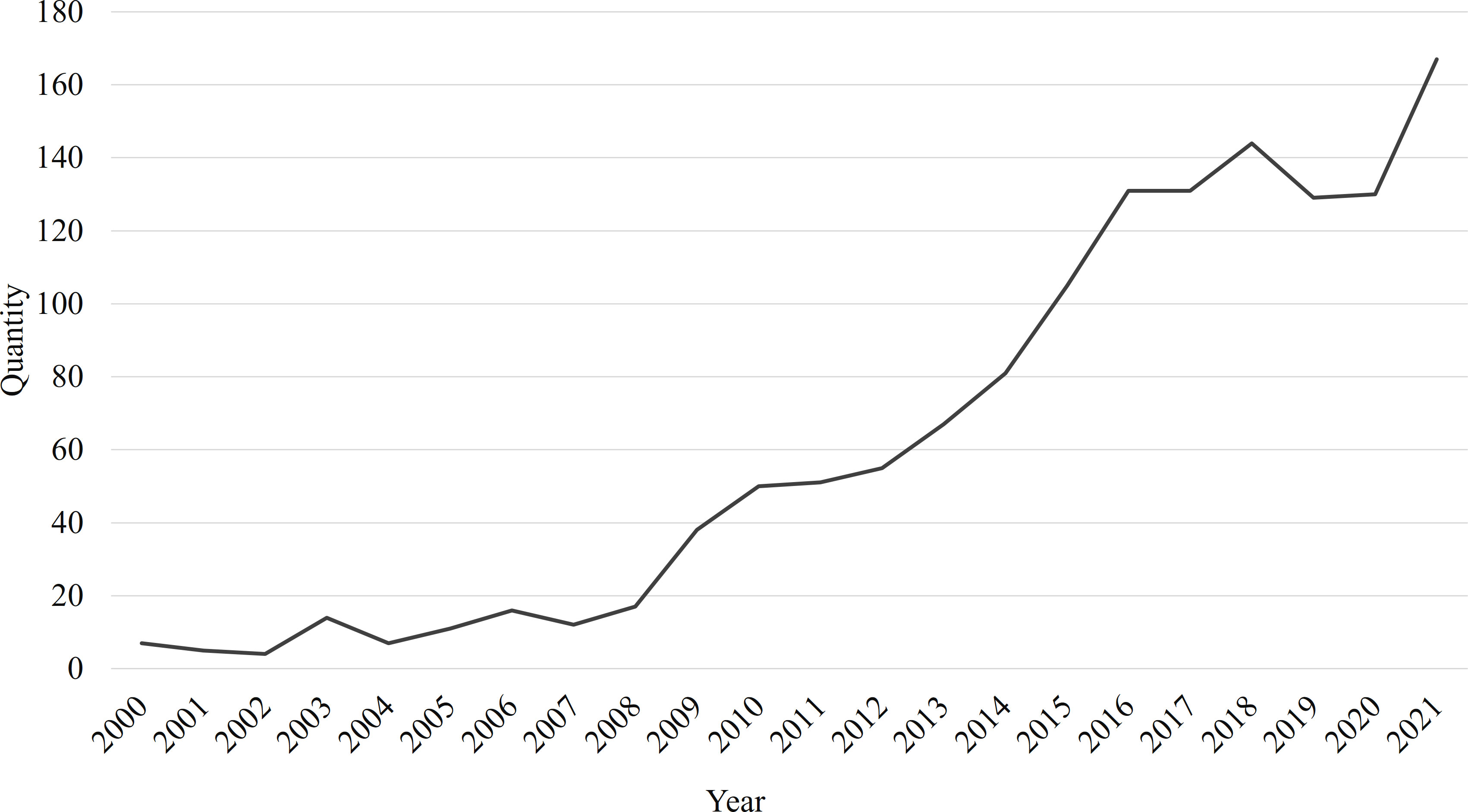
Analysis of publication quantity
There is a positive correlation between the research heat and the number of articles published. Comparing the number of publications per year is an easy way to get an idea of how hot a particular research area is. Based on the search results, annual trends in A. japonicus research publications from 2000 to 2021 are presented, as shown in Figure 1. In this period, studies on A. japonicus have increased rapidly. We divided the development of A. japonicus research into three stages. Prior to 2009, the research was at a preliminary stage, in which fewer than 20 articles were published per year. The range of studies is relatively narrow. During this period, researchers focused primarily on growth performance and immune response, accounting for 20.22% and 24.72% of 93 papers, respectively. Common keywords include temperature, salinity, oxygen consumption, arginine kinase, and microsatellite. During the development period from 2009 to 2016, A. japonicus research rapidly became a research hot spot with an average annual growth rate of 22.91%. The research direction of this period has the characteristics of diversification and refinement. It has been in a stable phase since 2017, in which the number of A. japonicus-related publications exceeded 130. The rapid rise in the number of publications signifies that A. japonicus is becoming an increasingly important vector for scientific research.
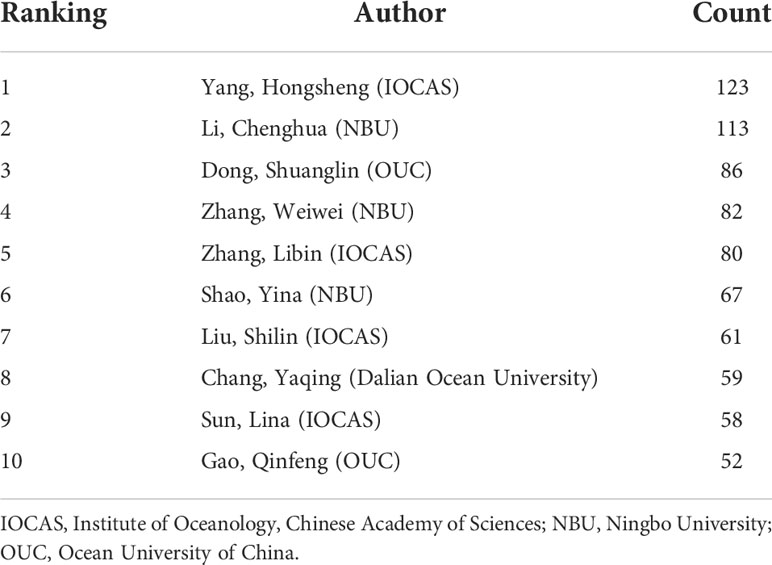
Analysis of the research teams
Research teams led by high-yielding writers are the main force behind scientific work (Wuchty et al., 2007). The core team and highly productive group of authors for the A. japonicus study were therefore identified as being necessary. Table 1 lists the top 10 authors in the field of A. japonicus. According to Price’s law of bibliometrics, which states that “On the same topic, half of all articles are written by highly effective authors” (Wei et al., 2020), we set N max as the number of publications of the most productive authors and (1,Nmax) is the total number of publications. The mathematical expression is as follows:
where m is a positive integer set by Price, and the total number of articles published by author groups with publications greater than m is approximately half of all articles (Price et al., 1984). The formula can be derived by further calculation as follows:
The positive integer m is approximately 9 when N max=NY,HS=123 . In other words, authors who have published nine or more papers in the field of A. japonicus can be considered highly productive, with a total of 134 authors, or only 4.43% of the total number of authors. Therefore, a stable core group of authors has not been formed in the field of A. japonicus research, and the concentration of authors is low. Every year, a large number of talents are absorbed into the field of A. japonicus. As shown in Table 1, the authors in the field of A. japonicus are mainly distributed in the Institute of Oceanology, Chinese Academy of Sciences (IOCAS), Ningbo University (NBU), and Ocean University of China (OUC). Team Hongsheng Yang from IOCAS, Team Chenghua Li from NBU, and Team Shuanglin Dong from OUC are top teams in the field of A. japonicus. Identifying the central research team allows us then to have a timeline of the development of each research direction through its articles.
Analysis of source countries
The country-level analysis also deserves our consideration. The count of independent studies and the frequency of inter-country cooperation can reflect the status and problems of A. japonicus research. An analysis of the main source countries for the A. japonicus study is presented in Table 2. China contributed 1,026 articles, accounting for 75.55% of the total number of articles on A. japonicus, followed by Japan with 113 published articles (8.32%). Notably, Japan is not only a pioneer in research but also the type locality of A. japonicus (Liao, 1997). Graphs indicating international comparisons of A. japonicus literature numbers and cooperative relationships were plotted (Figure 2). A total of 58 countries worldwide have been involved in A. japonicus research. However, only 115 international collaborations in the form of literature have been recorded, which is only 8.47% of the total literature. These data indicate that there is a huge scope for international collaboration in A. japonicus research.
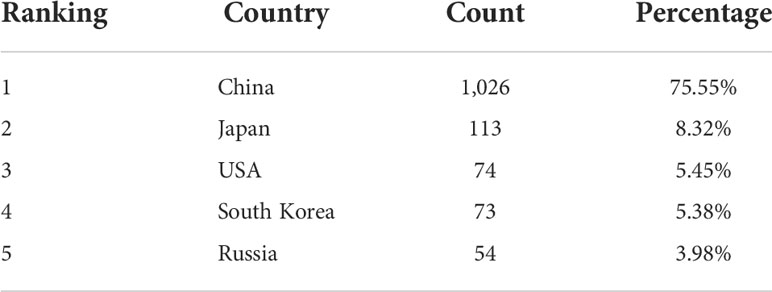
Table 2 List of five main source countries of Apostichopus japonicus research papers from 2000 to 2021.

Figure 2 The map representing the international comparison of the number of Apostichopus japonicus literature and cooperative relationships.
Analysis of participating journals
The Journal Citation Reports (JCR) Quartile and Impact Factor (IF) of journals often represent the quality of the research output (Law and Leung, 2020). The top 10 journals published in the field of A. japonicus are listed in Table 3 based on the database and comparison with the JCR 2020. More than 297 academic journals have published articles on A. japonicus research. Fish and Shellfish Immunology (Q1; IF2020, 4.581) published the most articles on the study of A. japonicus (119 articles, 8.76%), followed by Aquaculture (Q1; IF2020, 4.242; 113 articles, 8.32%) and Aquaculture Research (Q2; IF2020, 2.082; 98 articles, 7.22%). Notably, in 2019, JCR imposed a short penalty on the Q1 regional journal Fish and Shellfish Immunology for self-citing rate issues. As a result, the level of publications and research in the area of A. japonicus has declined in the short term. In comparison with other journals, articles on A. japonicus were more likely to be accepted by these active journals.
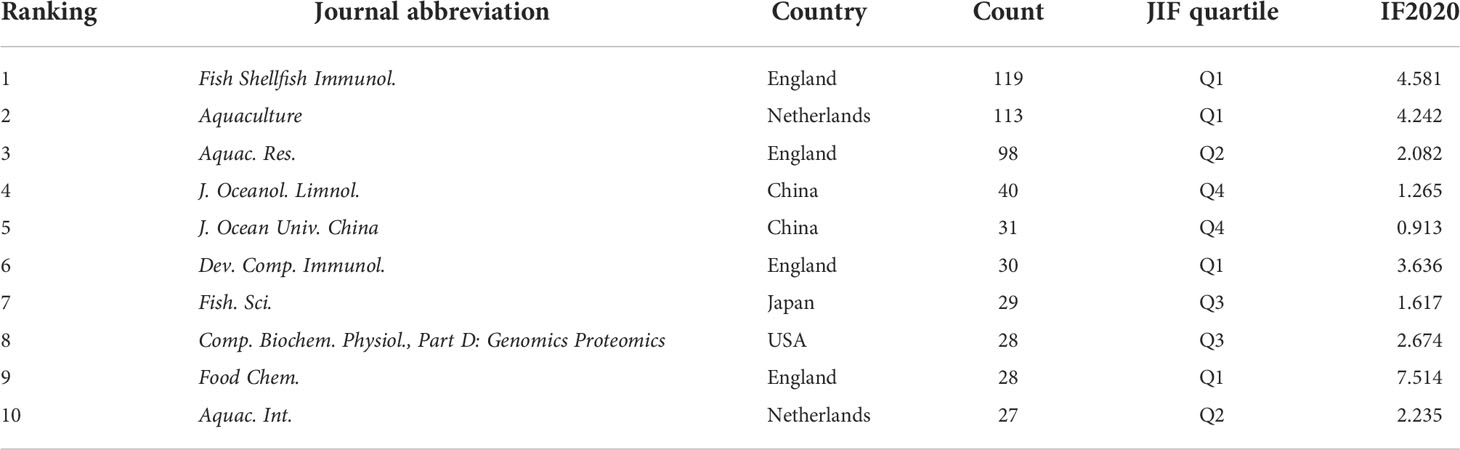
Table 3 The most active journals with publication volume on Apostichopus japonicus search from 2000 to 2021.
Analysis of the influence of the literature
Highly cited articles in the field represent the existence of more interest and affirmation by practitioners in the current state of research in a particular direction. We counted the papers published in the field of A. japonicus in the past 21 years. The 10 most influential academic papers were selected based on the number of citations as of 1 April 2022 (Table 4). The research directions of these 10 papers were briefly classified, and the general research directions were sea cucumber bioactive, fucosylated chondroitin sulfates (FCSs), diseases, growth performance, probiotics, aestivation, body wall collagen, and transcriptome sequencing.
Analysis of research directions
In the analysis of research directions, A. japonicus research is more focused on biology, marine science, and aquaculture. Based on the Web of Science subject classification method, the related literature of A. japonicus was classified (Table 5). Nearly 97% of these studies involved life sciences and biomedical research, with almost no research related to industry or economy. It will be helpful for us to follow up on the subdivision of the research direction of A. japonicus and no longer consider the research on industrial economy, history and culture, and diet.
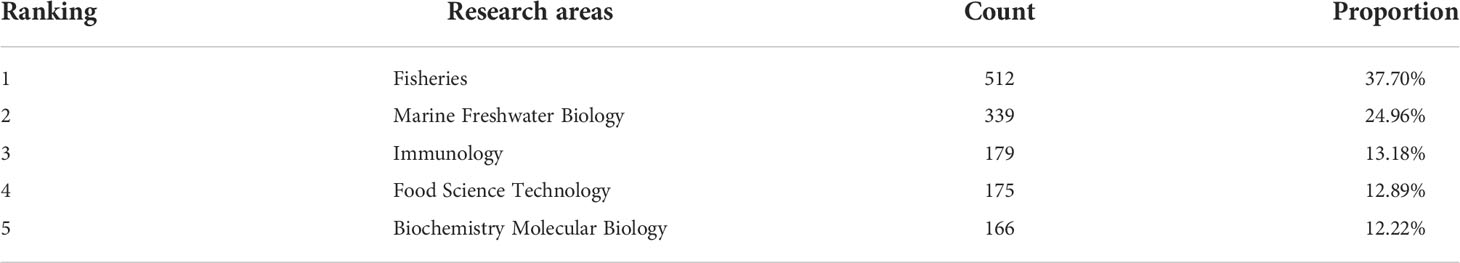
Table 5 Classification of the main research areas of Apostichopus japonicus based on the subject classification method of Web of Science (WoS) from 2000 to 2021.
Analysis of main keywords
In the keyword set of a large number of professional papers in the same discipline, hidden clues can be found about the current status of research, research hot spots, and development trends (Yin et al., 2009). Therefore, quantitative and visual analysis of keywords in A. japonicus research literature is helpful in mining knowledge in this field.
The term frequency–inverse document frequency weighted algorithm was used to calculate the importance of a keyword in a set of all keywords, highlighting key nodes and important connections, and form keyword clusters (Chen et al., 2015). The bibliography was imported into CiteSpace software, and synonym merging and keyword nonsense deletion were performed to achieve a symbiotic map of 682 keywords consisting of nodes and 1,393 links (Figure 3). One of the nodes represents a keyword. The larger the circle of nodes, the more frequently the keyword is cited, and the more frequently it can be considered a research hot spot in the A. japonicus field. As shown in Figure 3, the largest keyword node is A. japonicus, while the other nodes are more widely distributed mainly along the three branches of the distribution. In combination with the keyword frequency ranking data (Table 6), A. japonicus hot spots can be identified. The main keywords that appear in three directions are growth, protein, body wall, immune response, temperature, gene expression, and purification.
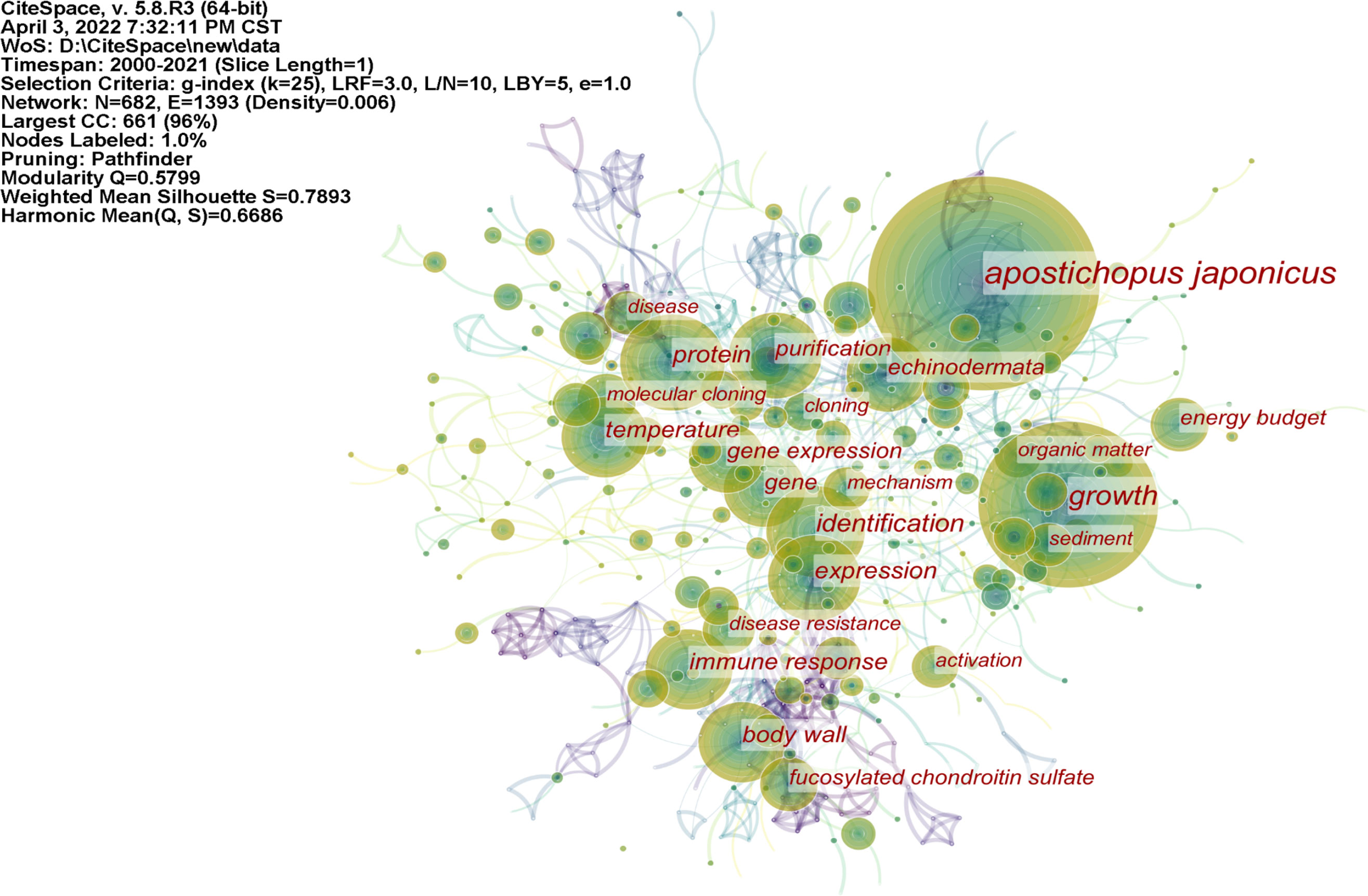
Figure 3 The map of keyword co-occurrence in articles related to the research of Apostichopus japonicus published from 2000 to 2021.
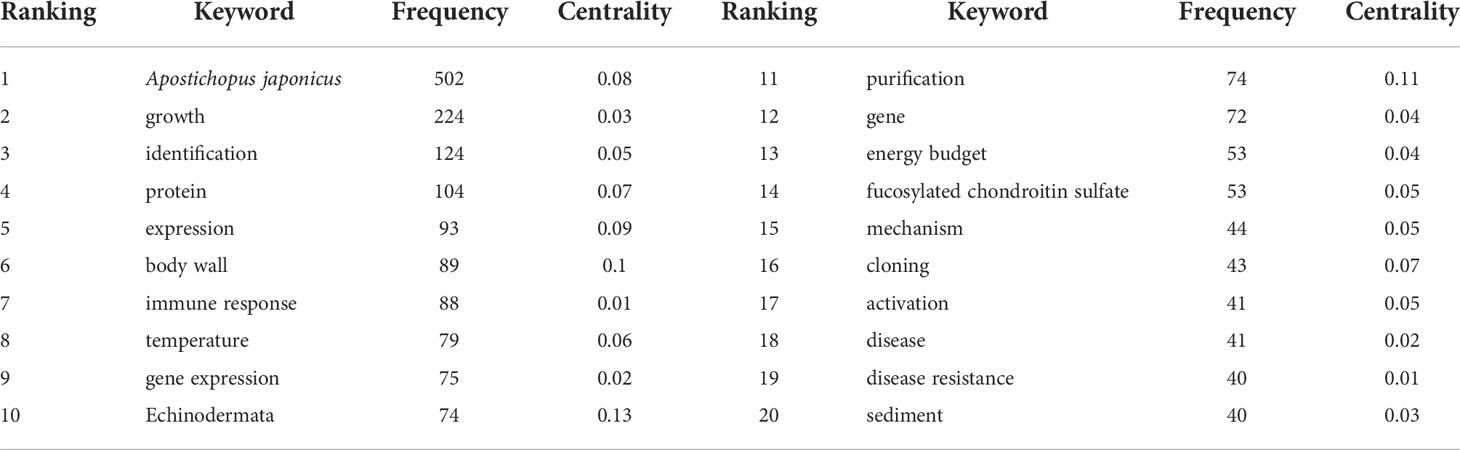
Table 6 The 20 most frequently used keywords in articles related to Apostichopus japonicus research published from 2000 to 2021.
Clustering graphs can accurately reflect the knowledge structure of different research directions (Rodriguez and Laio, 2014). We used the logarithmic likelihood ratio algorithm for keyword clustering. This algorithm can accurately reflect the contribution of low-frequency keywords to clustering and overcome the inaccuracy of the classical feature vector selection algorithm in low-frequency word estimation (Lin and Tang, 2009). By adjusting the contrast of the color blocks, a keyword network clustering map was formed (Figure 4). The hot spots in A. japonicus research over the past two decades can be briefly classified into the following categories: 1) growth, including keywords such as temperature, energy budget, and organic matter; 2) FCS, including body wall, in vitro, and glycosaminoglycan; 3) Apostichopus japonicus, including identification, protein, expression, and gene; 4) arginine kinase, including purification, mechanism, binding, phosphagen kinase, and inactivation; 5) Vibrio splendidus, including collagen, bacteria, triterpene glycoside, and resistance; 6) sea cucumber, including anticoagulant activity, NF-kappa B (NF-κB), peptide, and fucoidan. Some clusters generated by software analysis do not cover the entire content of the knowledge structure, and these clusters need to be properly integrated or renamed.
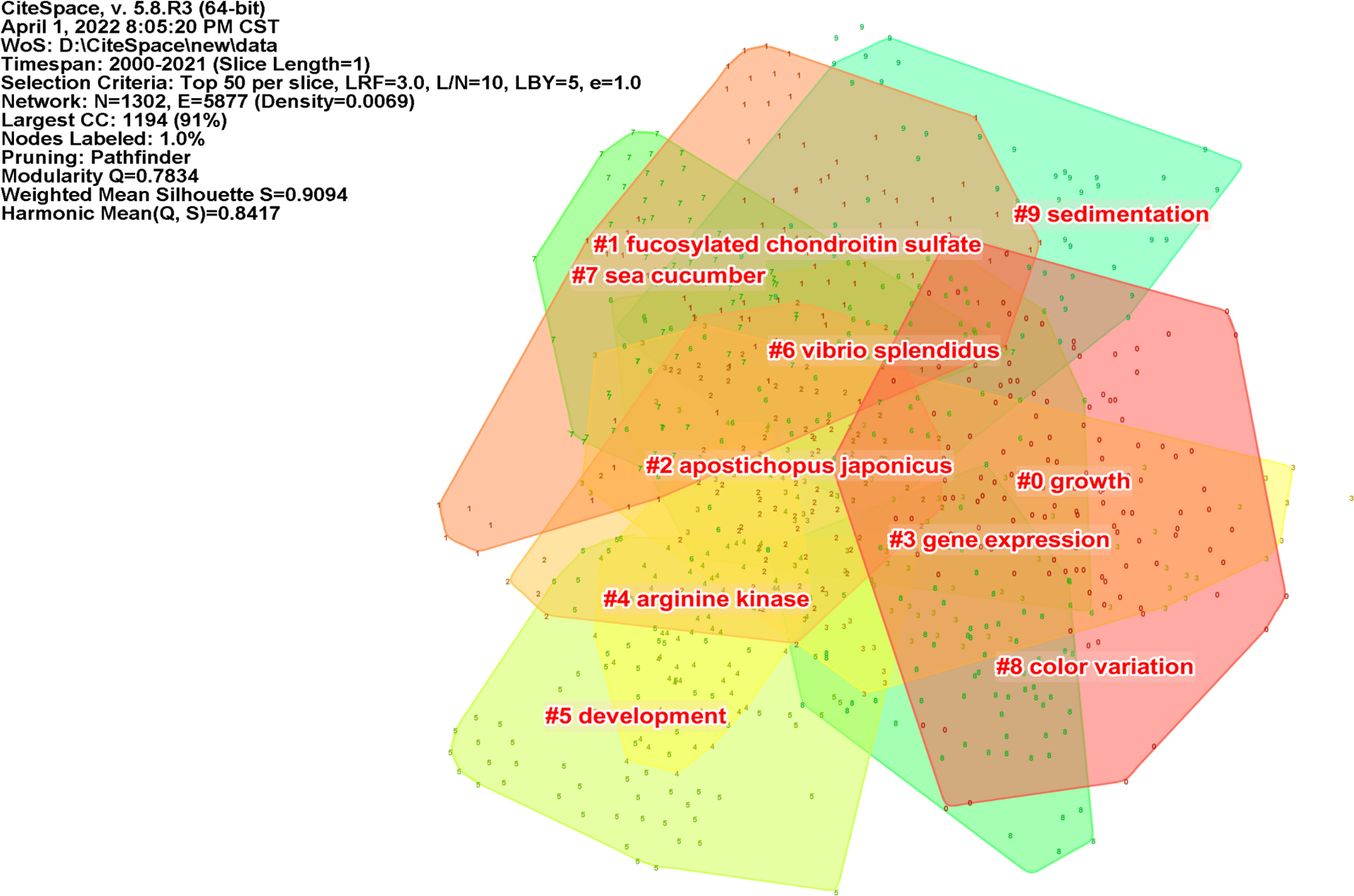
Figure 4 A network clustering map of keywords in the field of Apostichopus japonicus from 2000 to 2021.
In terms of keyword emergence time, CiteSpace uses Kleinberg’s burst word monitoring algorithm, which is based on the vending machine model, to model keyword frequency over different periods (Kleinberg, 2003). The duration of the main keyword emergence varies, as shown in Figure 5. The emergence lasted from 2000 to 2010 for brown algae, 2003 to 2011 for arginine kinase, 2010 to 2015 for consumption, 2015 to 2017 for NF-κB, and 2018 to the present for identification. These keywords have strong intensity and long duration and are all important in any research period. Recently emerging keywords included gut microbiota, activation, and collagen, which may be the main direction and technical methods of current or future research.
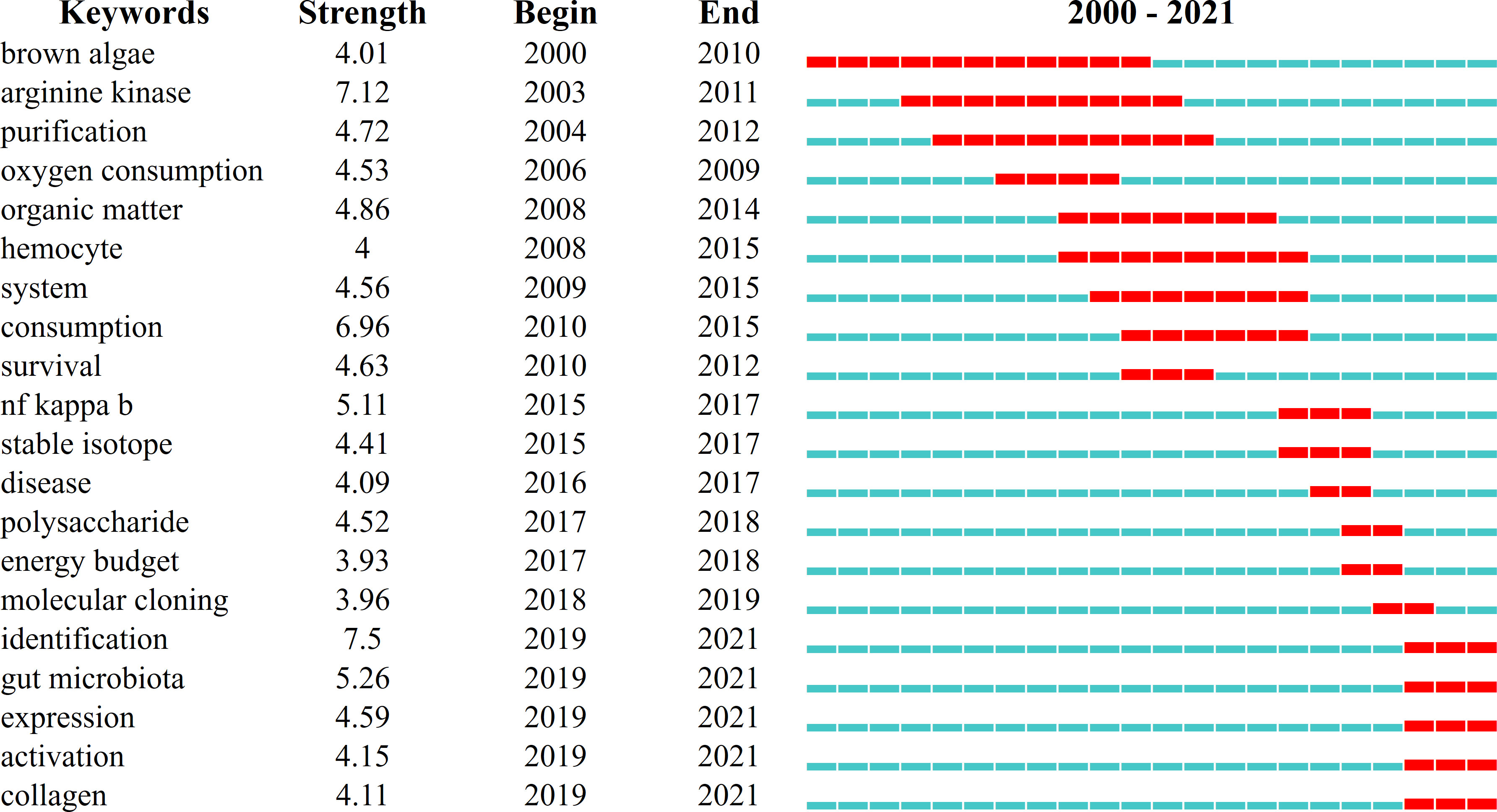
Figure 5 The emergence map of the top 20 keywords in the field of Apostichopus japonicus from 2000 to 2021.
Conclusion
As a condensation of literature knowledge, keywords deserve to be further classified and studied. The results of the software analysis can provide us with some assistance and evidence, but they do not fully represent the actual research status. Therefore, it is necessary to integrate all of the analyses and own experience to reach a final conclusion. Based on the network clustering map of keywords in Figure 4, the research direction in the A. japonicus domain was gradually clarified after integrating the citation frequency (Figure 3 and Table 6), occurrence time, and intensity (Figure 5) of keywords and the progress of large research teams. It was divided into six departments: genetics and breeding, growth and development, immunology and disease, aestivation, regeneration, and food processing.
Research on the genetics and breeding of Apostichopus japonicus
High-quality seedlings are important for the survival and development of the aquaculture industry. Research into the genetics and breeding of A. japonicus should also be the most concerning work in the A. japonicus industry. Genetics and breeding include but are not limited to genetic marker development, genetic linkage map construction, and genetic mechanism analysis of economic traits. 1) Studies on the genetic marker development of A. japonicus. The molecular genetics of A. japonicus concentrates on the development of microsatellite markers and single-nucleotide polymorphisms (SNPs). Microsatellite labeling technology is used for sifting through microsatellite locations and detecting them after PCR amplification. Kanno et al. (2005) have used magnetic bead enrichment methods by hybridization to develop 20 polymorphic microsatellite markers for sea cucumbers, which will be helpful for further research on the genetic structure and reproductive isolation of sea cucumbers. SNPs are the most common type of genetic variation among people. Each SNP represents a difference in a single DNA building block, called a nucleotide (National Library of Medicine, 2022). Based on the assembly transcriptome, Zhou et al. (2014) identified 142,511 high-quality SNPs. Attempts were made by Kang et al. (2011) to identify SNPs and to analyze differences associated with SNP genotypes between green and red color variants using heat shock protein (HSP)70 as the target gene. 2) Studies on the genetic linkage map construction of A. japonicus. Most of our earlier knowledge on echinoderm genomics came from the sea urchin Strongylocentrotus purpuratus and sea star Acanthaster planci; the need for the complete genome of A. japonicus is becoming increasingly urgent. The whole genome of A. japonicus released by team Hongsheng Yang in 2017 is a more complete one in recent years (Zhang et al., 2017). Li et al. (2018b) then updated the whole-genome landscape, perfecting the development of the whole genome of A. japonicus. 3) Studies on the genetic mechanism analysis of economic traits of A. japonicus. Growth rate, body wall thickness and flesh yield, and stress resistance are the most important economic traits of A. japonicus. Chen et al. (2020) tried to identify and screen potential molecular markers associated with the quantitative traits of “Anyuan No. 1” by miRNA-mRNA integrated analysis for future selective breeding and new seed creation in sea cucumbers. “Anyuan No. 1” is exactly the new generation breed of the Chinese, with weight, number of parapodium, and dressing-out percentage as the main target traits. The stress resistance includes indicators of a low-salt tolerance, high-temperature tolerance, and antibacterial infection. Song and Chen (2012) compared the growth traits of different pedigrees under low-salt environment by means of line selection, and finally, nine low salt-tolerant pedigrees were obtained. Zhao et al. (2014) analyzed the survival rate and HSP gene expression characteristics under high-temperature stress in the offspring of a high temperature-oriented selection population of Sargassum and preliminarily validated the heritability of heat tolerance traits in the selection population.
Research on the growth and development of Apostichopus japonicus
Researchers have focused on the growth performance of A. japonicus. The research direction of growth performance mainly includes feed formulation, temperature, salinity, and cocultivation environment. 1) Studies on the effect of feed formulation on the growth and development of A. japonicus. Qin Zhang and Yancui Zhao provided evidence that the addition of probiotic Bacillus subtilis T13 and prebiotic fructooligosaccharides to the feed has a synergistic effect on improving immunity and disease resistance of A. japonicus (Zhang et al., 2010; Zhao et al., 2012). According to the specific growth rate of sea cucumber, the formula of 75% bivalve feces and 25% powdered algae was the best diet for sea cucumber culture (Yuan et al., 2006). 2) Studies on the effect of temperature and salinity on the growth performance of A. japonicus. Dong et al. (2006) studied the effects of constant or variable temperature on the growth, proximate body composition, and oxygen consumption of juvenile sea cucumbers. Moreover, the appropriate temperature for A. japonicus growth is between 12°C and 21°C, the optimum temperature is 16°C−18°C, and the diel fluctuating temperature model promotes the growth of juvenile sea cucumbers and can be applied to the large-scale culture of A. japonicus. A temperature range of 21°C−24°C and a salinity of 30 are considered optimal for the early development of the red A. japonicus (Li and Li, 2010). 3) Studies on the effect of coculture environment on the growth performance of A. japonicus. The coculture of juvenile charm abalone Haliotis discus hannai and sea cucumber can reduce the content of inorganic nitrogen in water and promote synergistic growth (Kang et al., 2003). It can be used as an alternative culture method during the overwintering period of abalone farms.
Research on the immunology and disease of Apostichopus japonicus
The immunological research of A. japonicus mainly covers the epidemiology of pathogenic microorganisms, immunology and pathology of aquatic organisms, aquatic disease prevention, and control technology. 1) Pathogenic microorganism epidemiology mainly studies the epidemiological characteristics of diseases of farmed species, clarifies the regulatory effect of key environmental factors on pathogenic infection, reveals the molecular mechanism of epidemic outbreak and epidemic, and provides an analysis of the pathogenic principle of the main pathogens and the immune escape mechanism of the pathogens in the host. V. splendidus is the main causative agent of skin ulceration syndrome (SUS), which is the most common and serious disease in sea cucumber culture (Zhang et al., 2014). In the early stage of SUS, A. japonicus has the phenomenon of shaking its head, slow response, swelling of the mouth cannot be contracted; in the middle stage, the body of A. japonicus shrinks, the flesh spines turn white, small ulcers appear on the epidermis, and drainage of dirt; in the end stage, the epidermis ulcerates widely until death and autolysis (Li et al., 2013; Yang et al., 2016). The main causative factors identified at present of V. splendidus include adhesion molecules, iron absorption systems, and some extracellular products with toxicity (Zhang et al., 2016). Adhesion molecules in charge of contacting and binding to receptors include flagella, lipopolysaccharide, and outer membrane proteins (Dai et al., 2022). To cover the iron requirements for growth and reproduction, iron absorption systems have developed, including iron carriers and ferritin (Huang et al., 2021). Extracellular products can help defend the host immune killings, such as extracellular proteases and hemolysins (Binesse et al., 2008). 2) Immunology and pathology of aquatic organisms provide information about the changes in morphological structure, function, and metabolism of immune organs and immune cells in response to pathogenic microbial infection, revealing the occurrence and development of diseases. The immune system of A. japonicus in response to V. splendidus infection mainly consists of coelomocytes, immune factors, and gut microbiota. The defense means adopted by the coelomocytes when facing the invasion of pathogenic microorganisms are abundant, including chemotaxis (damaged cells release information to attract a large number of inflammatory cells, triggering inflammation) (Lv et al., 2022) and programmed cell death (apoptosis, autophagy, and pyroptosis may exist in A. japonicus) (Shao et al., 2016; Shao et al., 2019; Shao et al., 2020). The immune factors in A. japonicus mainly include Toll-like receptor (Sun et al., 2013), myeloid differentiation factor (Lv et al., 2019), interleukin-1 receptor-associated kinase (IRAK)-1 and IRAK-4 (Lu et al., 2015; Cui et al., 2018), NF-κB subunit (Wang et al., 2013), and signal transduction and activator of transcription-5 (Shao et al., 2015). Research on gut microbiota has been a hot topic in recent years, and we have mentioned above that the timing of their heat emergence may continue. Gut microbiota forms a biological barrier that restricts pathogen colonization in the intestine and enhances the original nonspecific immune function of A. japonicus (Li et al., 2012; Chi et al., 2014). A. japonicus gut microbiota provides a timely and accurate response to the occurrence of disease, which is directly reflected in the change of flora structure after the disease (Zhang et al., 2018; Zhang et al., 2019). 3) Aquatic disease prevention and control technology aim to solve the root cause of disease problems in aquaculture, which requires the full management of all aspects such as disease-resistant seedlings, immune enhancers, symptomatic medication, and daily monitoring of the water environment (Li, 2021). “Anyuan No. 1” and “Shenyou No. 1” are new A. japonicus breeds with better economic performance and disease resistance that have been cultivated in China in recent years, and the development of new breeds can effectively improve breeding efficiency (Chen et al., 2020; Wang et al., 2021). Immune enhancers improve nonspecific immunity and suppress invading pathogens in cultured animals to improve survival and growth rates. Numerous known substances can be used as immune enhancers for A. japonicus, mainly classified as Chinese herbal preparations (Sun et al., 2015), polysaccharides (Gu et al., 2011), and probiotics (Zhao et al., 2012). The overuse of antibiotics in animal farming is costly and undesirable and has threatened the human living environment (Harikrishnan et al., 2011). The use of antibiotic alternatives for pathogen suppression is imperative, such as the use of antivirulence biologics (Zhang et al., 2017), phages (Katharios et al., 2017), and probiotics.
Research on the aestivation of Apostichopus japonicus
Aestivation of A. japonicus has also been widely studied. Metabolic rate depression is a very common mechanism for animals to escape from environmental stress (Brooks and Storey, 1997). For A. japonicus, the temperature is the principal environmental factor that determines its growth rate (Yang et al., 2005). Moreover, aestivation is the main strategy for A. japonicus to reduce its metabolic rate to resist high temperatures (Du et al., 2013). Studies on aestivation in A. japonicus have focused on histomorphology, physiobiology, and molecular biology (Yuan et al., 2007). 1) Studies on the histomorphology of A. japonicus during aestivation. According to the changes in the gut and respiratory trees of A. japonicus, the aestivation process can be roughly divided into four stages: active period, aestivation period, deep aestivation period, and recovery period (Cheo, 1963). The most obvious physiological manifestation of A. japonicus during aestivation is the cessation of feeding and activity (Chen et al., 2016), which causes degradation of the digestive system (Gao et al., 2009) and negative body mass growth to maintain minimum metabolic requirements (Zhao et al., 2014). Wang et al. (2007) showed that the ratio of the digestive tract length to body length was only 0.8–1.1 during the aestivation from August to October, while the ratio was 2.7–5.9 for the normal growth period from January to May. Gao (2008) reported that the relative digestive mass of the intestine during aestivation was only 8.2%–35.8% of the annual maximum. Therefore, shortening the aestivation period can effectively improve the benefits of the cultivation process. 2) Studies on the physiobiology of A. japonicus during aestivation. Depending on the daily food intake of individuals, Yang et al. (2005) reported that the starting temperature at which medium and large A. japonicus (72.3–139.3 g) entered aestivation was 24.5°C–25.5°C and that of the small individual (28.9–40.7 g) was 25.5°C–30.5°C. They continued to determine the effect of water temperature on oxygen consumption rate (OCR) and ammonia-N excretion rate (AER) of aestivated A. japonicus using the Winkler and Hypobromite methods. Both OCR and AER of mature (large, 148.5 ± 15.4 g; medium, 69.3 ± 6.9 g) individuals peaked at 20.8°C, while the OCR of immature (small, 21.2 ± 4.7 g) individuals continued to increase with temperature and even beyond the aestivation temperature, while their AER peaked at 25.8°C (Yang et al., 2006). The results of previous studies have shown that both coelomocytes and immune enzymes of A. japonicus underwent significant changes during aestivation (Wang et al., 2008). Liu et al. (2016a) reported a significant effect of temperature on both immuno-enzyme activities [Superoxide dismutase, catalase, total antioxidant capacity (T-AOC)] and metabolic enzyme activities (pyruvate kinase (PK), hexokinase, malate dehydrogenase, lactate dehydrogenase (LDH)) in the non-aestivating group of A. japonicus, while the effects on T-AOC, PK, and LDH activities in the aestivating group were not significant. 3) Studies on the molecular biology of A. japonicus aestivation. At present, the molecular biology research on aestivation is still in its infancy, and the research content is mostly distributed in a dot-like distribution without systematization. Li et al.(2018b) reported that transcriptional factors Klf2 and Egr1 were identified as key regulators of aestivation, probably exerting their effects through a clock gene-controlled process. Yang (2017) investigated the changing pattern of A. japonicus 5-hydroxytryptamine (5-HT) receptor (Aj5-HTR) in the aestivation cycle and its function in metabolic regulation and, based on the results, elucidated the relationship between 5-HT and its receptor 5-HTR and the existence of metabolic regulation of aestivation in A. japonicus. Chen and Storey (2014) used high-throughput sequencing technology to detect and analyze the miRNA expression patterns in the intestine and respiratory tree before and after aestivation and screened out the differentially expressed miRNAs. They found that miR-124, miR-124-3p, miR-79, miR-9, and miR-2010 were significantly overexpressed during deep aestivation, and these miRNAs may play an important role in inhibiting the metabolic rate of A. japonicus during aestivation (Chen et al., 2013. In the follow-up study, they used tandem mass tag labeling followed by an immobilized metal-chelate affinity chromatography enrichment method to map aestivation sponsive changes in the A. japonicus intestinal phosphoproteome (Chen et al., 2016). The current research, including the above parts, has not clarified the molecular regulation mechanism of A. japonicus aestivation, which needs to be further broadened and deepened.
Research on the regeneration of Apostichopus japonicus
A growing number of scientists are attempting to demystify the organ regeneration process in invertebrates and lower vertebrates and determine whether the methods that trigger such regeneration can be applied to vertebrates (Tanaka and Reddien, 2011; Marques et al., 2019). The echinoderms to which sea cucumbers belong are grouped in the Duteromata evolutionary branch of animals and are therefore closely related to vertebrates in terms of phylogeny. Adult sea cucumbers are a useful model for organ regeneration (Garcia-Arraras et al., 2019). Current research on the regeneration of A. japonicus is mainly focused on digestive tract regeneration (Garcia-Arraras and Greenberg, 2001), and a few of them involve coelomocyte and longitudinal muscle band (LMB) regeneration. 1) Studies on digestive tract regeneration in A. japonicus. The regeneration process of the digestive tract can be divided into the rudiment formation stage, intestinal lumen formation stage, differentiation stage, and growth stage (Nie and Li, 2006). Only the calcareous ring and the stump of the pharynx-esophagus and cloaca remained in the body cavity after gutting, and two rudiments are formed by the stump of the pharynx-esophagus and cloaca in the anterior and posterior parts of the body, respectively. They grow along the free edge of the thickened mesentery toward the middle of the body, penetrate the mesenchyme, and form a fine digestive tract (Leibson, 1992). The thickened mesentery allows A. japonicus to regenerate the missing part of the intestine between the two ends much faster in order to feed earlier (Mashanov and Garcia-Arraras, 2011). Garcia-Arraras et al. (1998) described the spatial and temporal patterns of cellular events that occur during intestinal regeneration after chemically induced evisceration. It is also essential to investigate the molecular mechanism of the digestive tract regeneration in A. japonicus. The first signs of somatic epithelial cell activation on the molecular level were detected as early as 24 h after organ culling, with increased the transcription of genes involved in transcription, translation, and protein transport (Quispe-Parra et al., 2021). Data obtained by metabolomic analysis showed that the accumulation of nutrients in the residual esophageal tissue occurred after 3 days post-evisceration (dpe) (Sun et al., 2017a). The accumulation of energy reserves and their further utilization during regeneration are regulated by deacetylation (Sun et al., 2018). The expression of Hox1 and Hox3 was recorded as increased during this period, which proves that the formation site of the anterior part of the intestine is determined (Sun et al., 2017b). While Hox9/10 started to be expressed after 7 dpe, the site of formation of the posterior part of the intestine was marked (Sun et al., 2013b). Yuan et al. (2019) reported that the Wnt signaling pathway is the only pathway under positive selection in regenerating echinoderms and the only pathway enriched by differentially expressed genes during intestinal regenerating. An increase in the number of Wnt4 and Wnt6 transcripts was recorded after 7 dpe (Sun et al., 2013a). At 14–21 dpe, the main feature of this phase is the redifferentiation of enterocytes. Expression of Hox9/10 and Hox11/13 continued to increase, and marking of the anterior–posterior axis continues. The main processes occurring in A. japonicus during intestinal tube formation are extracellular matrix (ECM) remodeling, cell dedifferentiation, cell migration, cell proliferation, and apoptosis (Dolmatov, 2021). Two matrix metalloproteinase (MMP) genes, MMP-2 and MMP-16, may regulate the interaction between ECM components and growth factors by performing targeted proteolysis of ECM proteins and other biomolecules (Miao et al., 2017). The regeneration process requires a regulation between cell proliferation and cell death. Apoptotic cells greatly influence the behavior of the surrounding cells through phagocytosis and proliferation, as well as by activating the Wnt signaling (Kawamoto et al., 2016). The Wnt signaling pathway was activated during intestinal regeneration in sea cucumbers. The expression of Wnt7 and Wnt8 peaked after 1–3 dpe, while the number of Wnt6 transcripts was highest at 7 dpe and was maintained for a long time (Sun et al., 2013). 2) Studies on coelomocyte and LMB regeneration in A. japonicus. A. japonicus required depletion and regeneration of coelomocytes for effective innate immune mechanisms (Smith et al., 2010; Li et al., 2019). Available studies have shown that coelomic fluid and coelomocyte regenerate rapidly after the removal of viscera from sea cucumbers, returning to normal levels after 2 and 6 h, respectively (Li et al., 2018a; Shi et al., 2020). Dolmatov et al. (1996) demonstrated that new muscle bundles were derived from coelomic epithelial cells covering LMBs. The migration of coelomic epithelial cells to damaged LMBs and their myogenic transformation are the basic mechanisms of muscle regeneration. Ginanova (2007) reported that the coelomic epithelium of the body wall and the coelomic epithelium of the muscle of A. japonicus are involved in the regeneration of muscle tissue at the same time and have an important role in the recruitment of myogenic cell pools. The work by Qin et al. (2015) illustrates that the S7 subunit of the 19S gene or the S7-based assembled 26S proteasome may be involved in the regulation of cell migration and cell cycle during the regeneration of the A. japonicus body wall.
Research on the food processing of Apostichopus japonicus
Sea cucumber is delicious and nutritious. They are highly edible and have medicinal value. The medical value of sea cucumbers still has enormous scope for development and is not yet widespread. As an important part of the entire industrial chain of sea cucumbers, food engineering is also a hot spot in the field of A. japonicus research. Sea cucumber derivatives have many types, including polysaccharides, saponins, sea cucumber bioactive peptides, and many other biologically active compounds. At first, sea cucumber polysaccharides extracted from the body wall of sea cucumbers are unique to sea cucumber species, which are composed mainly of holothurian glycosaminoglycan and holothurian fucan (Jin et al., 2019). Current research is mainly focused on anticoagulation, antitumor, antiviral, and other directions (Pomin, 2014). 1) Study on the anticoagulation mechanism of sea cucumber polysaccharides. FCSs have stronger anticoagulant activity than sulfated fucan (Chen et al., 2011; Luo et al., 2013). The difference in the anticoagulant properties of FCSs is mainly attributed to their different sulfation patterns. 2) Study on the antitumor effect of sea cucumber polysaccharides. Low-molecular-weight FCSs could significantly inhibit the growth and metastasis of Lewis lung carcinoma in mice in a dose-dependent manner (Liu et al., 2016b). A. japonicus acid mucopolysaccharide (AJAMP) has an effective inhibitory effect on experimental hepatocellular carcinoma in rats, and the antitumor mechanism of AJAMP can protect immune organs, promote the proliferation of immune organs and tissues, and enhance cellular immunity (Song et al., 2013). 3) Study on the antiviral activity of sea cucumber polysaccharides. Through screening, the Academician of the Chinese Academy of Engineering Zhu Beiwei obtained three polysaccharides, namely, sea cucumber sulfated polysaccharide, fucoidan from brown algae, and iota-carrageenan from red algae, which have significant antiviral activities. The three antiviral polysaccharides could be employed for the treatment and prevention of coronavirus disease 2019 (COVID-19) (Song et al., 2020). Secondly, as the most important and abundant secondary metabolites in sea cucumbers, the research on saponins is also one of the hot topics (Bahrami and Franco, 2015; Bahrami and Franco, 2016). Due to their numerous and positive bioactivities, sea cucumber saponins are now high-economic value natural compounds with great market potential in the fields of medicine (Aminin et al., 2015; Dai et al., 2020), healthcare (Li et al., 2020), and cosmetics (Osbourn et al., 2011). In addition, studies on bioactive peptides and other biologically active compounds of sea cucumber have shown that they have lots of functions beneficial to the human body (Zhu et al., 2012; Pangestuti and Arifin, 2018; Zhang et al., 2018).
Discussion
This report reviews the history of A. japonicus research and the available scientific data from 2000 to 2021. Clearly, sea cucumbers should be considered a key component of aquaculture in terms of either economic or scientific value. We have divided the direction of A. japonicus research into six departments, namely, genetics and breeding, growth and development, immunology and disease, aestivation, regeneration, and food processing, which have independent and interlinked characteristics. These six research departments cover most of the problems that occurred during the production and processing of A. japonicus.
The five other research departments all serve the sea cucumber industry, and only the research on regeneration serves humans directly. Many tools and references can help improve the knowledge of regeneration phenomena in sea cucumbers and contribute to knowledge sharing. Biologically, regeneration is the process of displacing or restoring damaged or lost cells, tissues, organs, or even entire parts to respond resiliently to environmental fluctuations (Birbrair et al., 2013; National Institute of General Medical Sciences, 2021). Regeneration of tissues and organs depends on obtaining the cells required to form the new structure. These cells usually originate from two origins: 1) resident stem cells or 2) mature cells close to the injury site that lose their phenotype and dedifferentiate (Garcia-Arraras, 2017). Each metazoan species has the ability to regenerate and differs greatly (Carlson, 2007; Lindsay, 2010). In Echinodermata, tissue regeneration is well represented in Asteroidea, Holothuroidea, and Echinoidea to a higher degree (Carnevali et al., 1998). Among them, sea cucumbers show superb regenerative ability and can quickly regenerate various new functional internal organs when the environmental conditions are suitable, which is much greater than the ability of sea stars and sea urchins (Zhang et al., 2017). Therefore, they believe that studying the regeneration of sea cucumbers could inspire humans to find the key to initiating tissue regeneration in vivo. In contrast to other species, such as zebrafish (Yan and Gu, 2013; Marques et al., 2019), where various models of damage have been developed for the study, Schmidtea mediterranea (Lewallen and Burggren, 2022), where the metabolic cost of regeneration has been further investigated, and Eublepharis macularius (Austin et al., 2021), which has been found to regenerate spontaneous neurons in damaged brains, the road to regeneration research of sea cucumbers is long and difficult.
In summary, differences were observed based on the comparison of research power between regions and teams, and more extensive support is needed in regions with more developed industries or greater resource abundance. Governments or organizations are encouraged to 1) promote the development of the A. japonicus industry through the development or implementation of policies; 2) further participate in the whole process of research, production, and processing of A. japonicus; and 3) strengthen international exchange and cooperation to bring economic benefits to farmers in suitable breeding areas through technology sharing.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by JC, MG, ZL. The first draft of the manuscript was written by JC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Key Project from Science Technology Department of Zhejiang Province (2019R52016), National Natural Science Foundation of China (31902399), Natural Science Foundation of Zhejiang Province (LY22C190004), Ningbo Natural Science Foundation (2021J113), the Open Fund of Shandong Key Laboratory of Disease Control in Mariculture (KF202002), and the K.C. Wong Magna Fund in Ningbo University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aminin D. L., Menchinskaya E. S., Pisliagin E. A., Silchenko A. S., Avilov S. A., Kalinin V. I. (2015). Anticancer activity of sea cucumber triterpene glycosides. Mar. Drugs 13, 1202–1223. doi: 10.3390/md13031202
Austin L., Graham C., Vickaryous M. (2021). Spontaneous neuronal regeneration following lesioning in the brain of the leopard gecko (Eublepharis macularius). FASEB J. 35, 1. doi: 10.1096/fasebj.2021.35.S1.04331
Bahrami Y., Franco C. M. M. (2015). Structure elucidation of new acetylated saponins, lessoniosides a, b, c, d, and e, and non-acetylated saponins, lessoniosides f and G, from the viscera of the sea cucumber holothuria lessoni. Mar. Drugs 13, 597–617. doi: 10.3390/md13010597
Bahrami Y., Franco C. M. M. (2016). Acetylated triterpene glycosides and their biological activity from holothuroidea reported in the past six decades. Mar. Drugs 14, 147. doi: 10.3390/md14080147
Binesse J., Delsert C., Saulnier D., Champomier-Verges M. C., Zagorec M., Munier-Lehmann H., et al. (2008). Metalloprotease vsm is the major determinant of toxicity for extracellular products of vibrio splendidus. Appl. Environ. Microbiol. 74, 7108–7117. doi: 10.1128/aem.01261-08
Birbrair A., Zhang T., Wang Z. M., Messi M. L., Enikolopov G. N., Mintz A., et al. (2013). Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev. 22, 2298–2314. doi: 10.1089/scd.2012.0647
Bordbar S., Anwar F., Saari N. (2011). High-value components and bioactives from sea cucumbers for functional foods- a review. Mar. Drugs 9, 1761–1805. doi: 10.3390/md9101761
Brooks S. P. J., Storey K. B. (1997). Glycolytic controls in estivation and anoxia: A comparison of metabolic arrest in land and marine molluscs. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 118, 1103–1114. doi: 10.1016/s0300-9629(97)00237-5
Carlson B. M. (2007). “Chapter 1 - an introduction to regeneration,” in Principles of regenerative biology. Ed. Carlson. B. M. (Burlington: Academic Press), 1–29.
Carnevali M. D. C., Bonasoro F., Patruno M., Thorndyke M. C. (1998). Cellular and molecular mechanisms of arm regeneration in crinoid echinoderms: The potential of arm explants. Dev. Genes Evol. 208, 421–430. doi: 10.1007/s004270050199
Chang Z. Y. (2002). Study on the mode and key points of sea cucumber culture. China Fisheries 06, 55.
Chen C. M. (2006). CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc Inf. Sci. Technol. 57, 359–377. doi: 10.1002/asi.20317
Chen Y., Chen C. M., Liu Z. Y., Hu Z. G., Wang X. W. (2015). The methodological function of CiteSpace knowledge graph. Sci. Res. 33, 242–253. doi: 10.16192/j.cnki.1003-2053.2015.02.009
Chen Y., Li Y. Y., Zhan Y. Y., Hu W. B., Sun J. X., Zhang W. J., et al. (2020). Identification of molecular markers for superior quantitative traits in a novel sea cucumber strain by comparative microRNA-mRNA expression profiling. Comp. Biochem. Physiol. Part D: Genomics Proteomics 35, 100686. doi: 10.1016/j.cbd.2020.100686
Chen M. Y., Li X. K., Zhu A. J., Storey K. B., Sun L. N., Gao T. X., et al. (2016). Understanding mechanism of sea cucumber apostichopus japonicus aestivation: Insights from TMT-based proteomic study. Comp. Biochem. Physiol. Part D: Genomics Proteomics 19, 78–89. doi: 10.1016/j.cbd.2016.06.005
Chen M. Y., Storey K. B. (2014). Large-Scale identification and comparative analysis of miRNA expression profile in the respiratory tree of the sea cucumber apostichopus japonicus during aestivation. Mar. Genomics 13, 39–44. doi: 10.1016/j.margen.2014.01.002
Chen S. G., Xue C. H., Yin L. A., Tang Q. J., Yu G. L., Chai W. G. (2011). Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydr. Polym. 83, 688–696. doi: 10.1016/j.carbpol.2010.08.040
Chen M. Y., Zhang X. M., Liu J. N., Storey K. B. (2013). High-throughput sequencing reveals differential expression of miRNAs in intestine from sea cucumber during aestivation. PLoS One 8, e76120. doi: 10.1371/journal.pone.0076120
Cheo (1963). “Study of sea cucumber: morphology, ecology, and propagation of sea cucumber,” in Sea Cucumber research: reproduction of sea cucumbers (Tokyo: Kaibundou publisher), 32–40.
Chi C., Liu J. Y., Fei S. Z., Zhang C., Chang Y. Q., Liu X. L., et al. (2014). Effect of intestinal autochthonous probiotics isolated from the gut of sea cucumber (Apostichopus japonicus) on immune response and growth of a. japonicus. Fish Shellfish Immunol. 38, 367–373. doi: 10.1016/j.fsi.2014.04.001
Cui Y., Jiang L. T., Xing R. L., Wang Z. D., Wang Z. H., Shao Y. N., et al. (2018). Cloning, expression analysis and functional characterization of an interleukin-1 receptor-associated kinase 4 from apostichopus japonicus. Mol. Immunol. 101, 479–487. doi: 10.1016/j.molimm.2018.08.006
Cui G. Y., Zhao L. (2000). Identification of names and species of edible sea cucumbers. Cuisine Journal of Yangzhou University 3, 13–18.
Dai F., Guo M., Shao Y. N., Li C. H. (2022). Vibrio splendidus flagellin c binds tropomodulin to induce p38 MAPK-mediated p53-dependent coelomocyte apoptosis in Echinodermata. J. Biol. Chem. 298, 102091. doi: 10.1016/j.jbc.2022.102091
Dai Y. L., Kim E. A., Luo H. M., Jiang Y. F., Oh J. Y., Heo S. J., et al. (2020). Characterization and anti-tumor activity of saponin-rich fractions of south Korean sea cucumbers (Apostichopus japonicus). J. Food Sci. Technol.-Mysore 57, 2283–2292. doi: 10.1007/s13197-020-04266-z
Dolmatov I. Y. (2021). Molecular aspects of regeneration mechanisms in holothurians. Genes 12, 250. doi: 10.3390/genes12020250
Dolmatov I. Y., Eliseikina M. G., Bulgakov A. A., Ginanova T. T., Lamash N. E., Korchagin V. P. (1996). Muscle regeneration in the holothurian stichopus japonicus. Rouxs Arch. Dev. Biol. 205, 486–493. doi: 10.1007/bf00377230
Dong Y. W., Dong S. L., Tian X. L., Wang F., Zhang M. Z. (2006). Effects of diel temperature fluctuations on growth, oxygen consumption and proximate body composition in the sea cucumber apostichopus japonicus selenka. Aquaculture 255, 514–521. doi: 10.1016/j.aquaculture.2005.12.013
Du R. B., Zang Y. Q., Tian X. L., Dong S. L. (2013). Growth, metabolism and physiological response of the sea cucumber, apostichopus japonicus selenka during periods of inactivity. J. Ocean Univ. China 12, 146–154. doi: 10.1007/s11802-013-2076-1
Furukawa N., Furukawa Y., Yamana Y., Kashiwao S., Uekusa R., Goshima S. (2016) 66, 39–46. Growth and survival of juvenile sea cucumbers released on artificial reefs. Hokkaido University Marine Studies Department Bulletin.
Gao F. (2008). Seasonal variations of nutritional composition, food resources, and digestive physiology in sea cucumber apostichopus japonicus. Doctoral thesis. Qingdao: Institute of Oceanology, Chinese Academy of Sciences.
Gao F., Yang H. S., Xu Q., Wang F. Y., Liu G. B. (2009). Effect of water temperature on digestive enzyme activity and gut mass in sea cucumber apostichopus japonicus (Selenka), with special reference to aestivation. Chin. J. Oceanol. Limnol. 27, 714–722. doi: 10.1007/s00343-009-9202-3
Garcia-Arraras J. E. (2017). “Dedifferentiation as a cell source for organ regeneration,” in Regenerative engineering and developmental biology. Ed. Gardiner. D. (Florida: CRC Press), 373–394.
Garcia-Arraras J. E., Bello S. A., Malavez S. (2019). The mesentery as the epicenter for intestinal regeneration. Semin. Cell Dev. Biol. 92, 45–54. doi: 10.1016/j.semcdb.2018.09.001
Garcia-Arraras J. E., Estrada-Rodgers L., Santiago R., Torres I. I., Diaz-Miranda L., Torres-Avillan I. (1998). Cellular mechanisms of intestine regeneration in the sea cucumber, holothuria glaberrima selenka (Holothuroidea: Echinodermata). J. Exp. Zool. 281, 288–304. doi: 10.1002/(sici)1097-010x(19980701)281:4<288::Aid-jez5>3.0.Co;2-k
Garcia-Arraras J. E., Greenberg M. J. (2001). Visceral regeneration in holothurians. Microsc. Res. Tech. 55, 438–451. doi: 10.1002/jemt.1189
Ginanova T. T. (2007). Participation of the coelomic epithelium of the body wall in muscle regeneration in apostichopus japonicus (Holothuroidea: Aspidochirota). Russ. J. Mar. Biol. 33, 411–416. doi: 10.1134/s1063074007060089
Gu M., Ma H. M., Mai K. S., Zhang W. B., Bai N., Wang X. J. (2011). Effects of dietary beta-glucan, mannan oligosaccharide and their combinations on growth performance, immunity and resistance against vibrio splendidus of sea cucumber, apostichopus japonicus. Fish Shellfish Immunol. 31, 303–309. doi: 10.1016/j.fsi.2011.05.018
Harikrishnan R., Balasundaram C., Heo M. S. (2011). Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquaculture 317, 1–15. doi: 10.1016/j.aquaculture.2011.03.039
Huang B. W., Lv Z. M., Li Y. N., Li C. H. (2021). Identification and functional characterization of natural resistance-associated macrophage protein 2 from sea cucumber apostichopus japonicus. Dev. Comp. Immunol. 114, 12. doi: 10.1016/j.dci.2020.103835
Huang X., Wang K. R., Zou Y. W., Cao X. C. (2021). Development of global soil erosion research at the watershed scale: a bibliometric analysis of the past decade. Environ. Sci. Pollut. Res. 28, 12232–12244. doi: 10.1007/s11356-020-11888-5
Hu W., Li C. H., Ye C., Wang J., Wei W. W., Deng Y. (2019). Research progress on ecological models in the field of water eutrophication: CiteSpace analysis based on data from the ISI web of science database. Ecol. Modell. 410, 108779. doi: 10.1016/j.ecolmodel.2019.108779
Imai T., Inaba D., Sato R., Hatanaka M. (1950). Artificial breeding of sea cucumbers in colorless flagellates. Report of institute of agronomy, (Northeastern University) 2, 269–278.
Jin Q., Teng Y., Hu X. Q., Zhang C. H. (2019). Research progress on anti-tumor mechanism of sea cucumber polysaccharides. Zhejiang Med. 41, 300–303. doi: 10.12056/j.issn.1006-2785.2019.41.3.2018-2412
Kang K. H., Kwon J. Y., Kim Y. M. (2003). A beneficial coculture: charm abalone haliotis discus hannai and sea cucumber stichopus japonicus. Aquaculture 216, 87–93. doi: 10.1016/s0044-8486(02)00203-x
Kang J. H., Yu K. H., Park J. Y., An C. M., Jun J. C., Lee S. J. (2011). Allele-specific PCR genotyping of the HSP70 gene polymorphism discriminating the green and red color variants sea cucumber (Apostichopus japonicus). J. Genet. Genomics 38, 351–355. doi: 10.1016/j.jgg.2011.06.002
Kanno M., Li Q., Kijima A. (2005). Isolation and characterization of twenty microsatellite loci in Japanese sea cucumber (Stichopus japonicus). Mar. Biotechnol. 7, 179–183. doi: 10.1007/s10126-004-0006-3
Katharios P., Kalatzis P. G., Kokkari C., Sarropoulou E., Middelboe M. (2017). Isolation and characterization of a N4-like lytic bacteriophage infecting vibrio splendidus, a pathogen of fish and bivalves. PLoS One 12, e0190083. doi: 10.1371/journal.pone.0190083
Kawamoto Y., Nakajima Y., Kuranaga E. (2016). Apoptosis in cellular society: Communication between apoptotic cells and their neighbors. Int. J. Mol. Sci. 17, 2144. doi: 10.3390/ijms17122144
Khalil G. M., Crawford C. A. G. (2015). A bibliometric analysis of US-based research on the behavioral risk factor surveillance system. Am. J. Prev. Med. 48, 50–57. doi: 10.1016/j.amepre.2014.08.021
Kinch J., Purcell S., Uthicke S., Friedman K. (2008). "Population status, fisheries and trade of sea cucumbers in the Western central pacific" inToral-Granda M.V., Lovatelli A., Vasconcellos M. Eds. Sea cucumbers: a global review of fisheries and trade (Rome: Food and Agriculture Organization of the United Nations), 7–55.
Kleinberg J. (2003). Bursty and hierarchical structure in streams. Data. Min. Knowl. Disc. 7, 373–397. doi: 10.1023/a:1024940629314
Law R., Leung D. (2020). Journal impact factor: A valid symbol of journal quality? Tour. Econ. 26, 734–742. doi: 10.1177/1354816619845590
Leibson N. L. (1992). Regeneration of digestive tube in holothurians stichopus japonicus and eupentacta fraudatrix. Monogr. Dev. Biol. 23, 51–61.
Lewallen M., Burggren W. (2022). Metabolic cost of development, regeneration, and reproduction in the planarian schmidtea mediterranea. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 265, 8. doi: 10.1016/j.cbpa.2021.111127
Li C. H. (2021). Research progress on molecular regulation mechanism of skin ulcer syndrome in sea cucumber apostichopus japonicus: A review. J. Dalian Ocean Univ. 3, 355–373. doi: 10.16535/j.cnki.dlhyxb.2021-086
Liao Y. L. (1997). Fauna of China, phylum Echinodermata: Class holothuroidea (Beijing: Science Press), 147–158.
Li L., Li Q. (2010). Effects of stocking density, temperature, and salinity on larval survival and growth of the red race of the sea cucumber apostichopus japonicus (Selenka). Aquacult. Int. 18, 447–460. doi: 10.1007/s10499-009-9256-4
Lindsay S. M. (2010). Frequency of injury and the ecology of regeneration in marine benthic invertebrates. Integr. Comp. Biol. 50, 479–493. doi: 10.1093/icb/icq099
Lin S., Tang F. G. (2009). Feature selection algorithm based on log-likelihood ratio. Comput. Eng. 35, 56–58. doi: 10.3969/j.issn.1000-3428.2009.19.018
Li Q., Ren Y., Liang C. L., Qiao G., Wang Y. N., Ye S. G., et al. (2018a). Regeneration of coelomocytes after evisceration in the sea cucumber, apostichopus japonicus. Fish Shellfish Immunol. 76, 266–271. doi: 10.1016/j.fsi.2018.03.013
Li Q., Ren Y., Luan L. L., Zhang J. L., Qiao G., Wang Y. N., et al. (2019). Localization and characterization of hematopoietic tissues in adult sea cucumber, apostichopus japonicus. Fish Shellfish Immunol. 84, 1–7. doi: 10.1016/j.fsi.2018.09.058
Li Q., Sun K. T., Zhang X. Y. (2013). Research progress on “skin ulceration syndrome” of apostichopus japonicas. J. Agric. Sci. Technol. 15, 40–45. doi: 10.3969/j.issn.1008-0864.2013.06.07
Liu X. X., Liu Y., Hao J. J., Zhao X. L., Lang Y. Z., Fan F., et al. (2016b). In anti-cancer mechanism of low-molecular-weight fucosylated chondroitin sulfate (LFCS) from sea cucumber cucumaria frondosa. Molecules 21, 12. doi: 10.3390/molecules21050625
Liu Y. N., Wu K. N., Zhao R. (2020). Bibliometric analysis of research on soil health from 1999 to 2018. J. Soils Sediments 20, 1513–1525. doi: 10.1007/s11368-019-02519-9
Liu Z. Q., Yang J. Y., Zhang J. E., Xiang H. M., Wei H. (2019). A bibliometric analysis of research on acid rain. Sustainability 11, 3077. doi: 10.3390/su11113077
Liu S. L., Zhou Y., Ru X. S., Zhang M. Z., Cao X. B., Yang H. S. (2016a). Differences in immune function and metabolites between aestivating and non-aestivating apostichopus japonicus. Aquaculture 459, 36–42. doi: 10.1016/j.aquaculture.2016.03.029
Li Y. L., Wang R. J., Xun X. G., Wang J., Bao L. S., Thimmappa R., et al. (2018b). Sea Cucumber genome provides insights into saponin biosynthesis and aestivation regulation. Cell Discov 4, 17. doi: 10.1038/s41421-018-0030-5
Li H. Y., Zhang G. L., Hou H. M., Ge J. H., Sun M. Y. (2012). Study on inhibitory ability and mechanism of apostichopus japonicus-related microbes against vibrio splendidus. Food Industry 33, 117–120.
Li R., Zhang L. Y., Li Z. J., Xue C. H., Dong P., Huang Q. R., et al. (2020). Characterization and absorption kinetics of a novel multifunctional nanoliposome stabilized by sea cucumber saponins instead of cholesterol. J. Agric. Food Chem. 68, 642–651. doi: 10.1021/acs.jafc.9b06460
Luo L., Wu M. Y., Xu L., Lian W., Xiang J. Y., Lu F., et al. (2013). Comparison of physicochemical characteristics and anticoagulant activities of polysaccharides from three sea cucumbers. Mar. Drugs 11, 399–417. doi: 10.3390/md11020399
Lu M., Zhang P. J., Li C. H., Lv Z. M., Zhang W. W., Jin C. H. (2015). miRNA-133 augments coelomocyte phagocytosis in bacteria-challenged apostichopus japonicus via targeting the TLR component of IRAK-1 in vitro and in vivo. Sci. Rep. 5 1–14. doi: 10.1038/srep12608
Lv Z. M., Guo M., Zhao X. L., Shao Y. N., Zhang W. W., Li C. H. (2022). IL-17/IL-17 receptor pathway-mediated inflammatory response in apostichopus japonicus supports the conserved functions of cytokines in invertebrates. J. Immunol. 208, 464–479. doi: 10.4049/jimmunol.2100047
Lv Z. M., Li C. H., Guo M., Shao Y. N., Zhang W. W., Zhao X. L. (2019). Major yolk protein and HSC70 are essential for the activation of the TLR pathway via interacting with MyD88 in apostichopus japonicus. Arch. Biochem. Biophys. 665, 57–68. doi: 10.1016/j.abb.2019.02.019
Marques I. J., Lupi E., Mercader N. (2019). Mode systems for regeneration: zebrafish. Development 146, 13. doi: 10.1242/dev.167692
Mashanov V. S., Garcia-Arraras J. E. (2011). Gut regeneration in holothurians: A snapshot of recent developments. Biol. Bull. 221, 93–109. doi: 10.1086/BBLv221n1p93
Miao T., Wan Z. X., Sun L. N., Li X. N., Xing L. L., Bai Y. C., et al. (2017). Extracellular matrix remodeling and matrix metalloproteinases (ajMMP-2 like and ajMMP-16 like) characterization during intestine regeneration of sea cucumber apostichopus japonicus. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 212, 12–23. doi: 10.1016/j.cbpb.2017.06.011
Midori Y., Kenichiro W. (1981) 8, 51–62. About the initial breeding of sea cucumber larvae. Report of Yamaguchi Prefectural Sea Fisheries Experiment Station.
National Institute of General Medical Sciences (2021) What are regeneration and regenerative medicine. Available at: https://www.nigms.nih.gov/education/fact-sheets/Pages/regeneration (Accessed April 3, 2022).
National Library of Medicine (2022) What are single nucleotide polymorphisms (SNPs). Available at: https://medlineplus.gov/genetics/understanding/genomicresearch/snp/ (Accessed June 1, 2022).
Nie Z. L., Li X. (2006). Study on the regeneration of sea cucumber. Mar. Sci. 5, 78–82. doi: 10.3969/j.issn.1000-3096.2006.05.016
Osbourn A., Goss R. J. M., Field R. A. (2011). The saponins - polar isoprenoids with important and diverse biological activities. Nat. Prod. Rep. 28, 1261–1268. doi: 10.1039/c1np00015b
Pangestuti R., Arifin Z. (2018). Medicinal and health benefit effects of functional sea cucumbers. J. Tradit. Complement. Med. 8, 341–351. doi: 10.1016/j.jtcme.2017.06.007
Picone F., Buonocore E., Chemello R., Russo G. F., Franzese P. P. (2021). Exploring the development of scientific research on marine protected areas: From conservation to global ocean sustainability. Ecol. Inform. 61, 101200. doi: 10.1016/j.ecoinf.2020.101200
Pomin V. H. (2014). Holothurian fucosylated chondroitin sulfate. Mar. Drugs 12, 232–254. doi: 10.3390/md12010232
Qin Y. J., Zhao X. J., Li X., Wang Y. F., Liu Y. (2015). Characterization of the 26S proteasome S7 subunit gene and its expression during regeneration in apostichopus japonicus. J. World Aquacult. Soc 46, 159–170. doi: 10.1111/jwas.12176
Quispe-Parra D. J., Medina-Feliciano J. G., Cruz-Gonzalez S., Ortiz-Zuazaga H., Garcia-Arraras J. E. (2021). Transcriptomic analysis of early stages of intestinal regeneration in holothuria glaberrima. Sci. Rep. 11, 1–14. doi: 10.1038/s41598-020-79436-2
Ramírez-González J., Moity N., Andrade-Vera S., Reyes H. (2020). Overexploitation and more than a decade of failed management leads to no recovery of the galápagos sea cucumber fishery. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.554314
Rodriguez A., Laio A. (2014). Clustering by fast search and find of density peaks. Science 344, 1492–1496. doi: 10.1126/science.1242072
Ru X. S., Zhang L. B., Li X. N., Liu S. L., Yang H. S. (2019). Development strategies for the sea cucumber industry in China. J. Oceanol. Limnol. 37, 300–312. doi: 10.1007/s00343-019-7344-5
Sea Cucumber Industry Branch of China Fisheries Association (2022) Review of china’s sea cucumber industry development in 2021. Available at: http://www.haishenren.org.cn/industry_service/show-23.html (Accessed June 1, 2022).
Selenka E. (1867). Beiträge zur anatomie und systematik der holothurien (Leipzig: W. Engelmann), 291–382.
Shao Y. N., Che Z. J., Chen K. Y., Li C. H., Zhao X. D. (2020). Target of rapamycin signaling inhibits autophagy in sea cucumber apostichopus japonicus. Fish Shellfish Immunol. 102, 480–488. doi: 10.1016/j.fsi.2020.05.013
Shao Y. N., Che Z. J., Li C. H., Zhang W. W., Zhao X. L., Guo M. (2019). A novel caspase-1 mediates inflammatory responses and pyroptosis in sea cucumber apostichopus japonicus. Aquaculture 513, 11. doi: 10.1016/j.aquaculture.2019.734399
Shao Y. N., Li C. H., Zhang W. W., Duan X. M., Li Y., Han Q. X., et al. (2015). Three members in JAK/STAT signal pathway from the sea cucumber apostichopus japonicus: Molecular cloning, characterization and function analysis. Fish Shellfish Immunol. 46, 523–536. doi: 10.1016/j.fsi.2015.07.019
Shao Y. N., Li C. H., Zhang W. W., Duan X. M., Li Y., Jin C. H., et al. (2016). Molecular cloning and characterization of four caspases members in apostichopus japonicus. Fish Shellfish Immunol. 55, 203–211. doi: 10.1016/j.fsi.2016.05.039
Shi W. B., Zhang J. L., Wang Y. N., Ji J. L., Guo L. Y., Ren Y., et al. (2020). Transcriptome analysis of sea cucumber (Apostichopus japonicus) polian vesicles in response to evisceration. Fish Shellfish Immunol. 97, 108–113. doi: 10.1016/j.fsi.2019.12.016
Smith L. C., Ghosh J., Buckley K. M., Clow L. A., Dheilly N. M., Haug T., et al. (2010). “Echinoderm immunity,” in Invertebrate immunity. Ed. Soderhall. K. (Cham: Springer), 260–301.
Song J., Chen X. K. (2012). Establishment of low-salinity sea cucumber apostichopus japonicus family and comparison of their growth traits. Hebei Fisheries 11, 15–18+62. doi: 10.3969/j.issn.1004-6755.2012.11.005
Song Y., Jin S. J., Cui L. H., Ji X. J., Yang F. G. (2013). Immunomodulatory effect of stichopus japonicus acid mucopolysaccharide on experimental hepatocellular carcinoma in rats. Molecules 18, 7179–7193. doi: 10.3390/molecules18067179
Song S., Peng H. R., Wang Q. L., Liu Z. Q., Dong X. P., Wen C. R., et al. (2020). Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 11, 7415–7420. doi: 10.1039/d0fo02017f
Sun Y. X., Du X. F., Li S. Y., Wen Z. X., Li Y. J., Li X. J., et al. (2015). Dietary cordyceps militaris protects against vibrio splendidus infection in sea cucumber apostichopus japonicus. Fish Shellfish Immunol. 45, 964–971. doi: 10.1016/j.fsi.2015.05.053
Sun L. N., Lin C. G., Li X. N., Xing L., Huo D. C., Sun J. C., et al. (2018). Comparative phospho- and acetyl proteomics analysis of posttranslational modifications regulating intestine regeneration in sea cucumbers. Front. Physiol. 9. doi: 10.3389/fphys.2018.00836
Sun L. N., Sun J. C., Li X. N., Zhang L. B., Yang H. S., Wang Q. (2017a). Understanding regulation of microRNAs on intestine regeneration in the sea cucumber apostichopus japonicus using high-throughput sequencing. Comp. Biochem. Physiol. Part D: Genomics Proteomics 22, 1–9. doi: 10.1016/j.cbd.2017.01.001
Sun L. N., Xu D. X., Xu Q. Z., Sun J. C., Xing L. L., Zhang L. B., et al. (2017b). iTRAQ reveals proteomic changes during intestine regeneration in the sea cucumber apostichopus japonicus. Comp. Biochem. Physiol. Part D: Genomics Proteomics 22, 39–49. doi: 10.1016/j.cbd.2017.02.004
Sun L. N., Yang H. S., Chen M. Y., Ma D. Y., Lin C. G. (2013b). RNA-Seq reveals dynamic changes of gene expression in key stages of intestine regeneration in the sea cucumber apostichopus japonicas. PLoS One 8, 18. doi: 10.1371/journal.pone.0069441
Sun L. N., Yang H. S., Chen M. Y., Xu D. X. (2013a). Cloning and expression analysis of Wnt6 and Hox6 during intestinal regeneration in the sea cucumber apostichopus japonicus. Genet. Mol. Res. 12, 5321–5334. doi: 10.4238/2013.November.7.7
Sun H. J., Zhou Z. C., Dong Y., Yang A. F., Jiang B., Gao S., et al. (2013). Identification and expression analysis of two toll-like receptor genes from sea cucumber (Apostichopus japonicus). Fish Shellfish Immunol. 34, 147–158. doi: 10.1016/j.fsi.2012.10.014
Tanaka Y. (1958). Feeding and digestive processes of stichopus japonicus Bulletin of faculty of fisheries (Hokkaido University), 14–28.
Tanaka E. M., Reddien P. W. (2011). The cellular basis for animal regeneration. Dev. Cell 21, 172–185. doi: 10.1016/j.devcel.2011.06.016
Wang Z. P., Li B., Qin L., Wang Y. G., Liao M. J., Rong X. J., et al. (2021). Metabolic characteristics and adaptability of a new variety of sea cucumber “Shenyou No.1” under different salinities. Prog. Fishery Sci. 42, 108–115. doi: 10.19663/j.issn2095-9869.20200331002
Wang T. T., Sun Y. X., Jin L. J., Thacker P., Li S. Y., Xu Y. P. (2013). Aj-rel and aj-p105, two evolutionary conserved NF-kappa b homologues in sea cucumber (Apostichopus japonicus) and their involvement in LPS induced immunity. Fish Shellfish Immunol. 34, 17–22. doi: 10.1016/j.fsi.2012.09.006
Wang J. Q., Tang L., Xu C., cheng J. C. (2007). Histological observation of alimentary tract and annual changes of four digestive enzymes in sea cucumber (Apostichopus japonicus). Fisheries Sci. 09, 481–484. doi: 10.16378/j.cnki.1003-1111.2007.09.014
Wang F. Y., Yang H. S., Gabr H. R., Gao F. (2008). Immune condition of apostichopus japonicus during aestivation. Aquaculture 285, 238–243. doi: 10.1016/j.aquaculture.2008.08.033
Wang Z. Q., Zhou Z. Y., Xu W. C., Yang D., Xu Y., Yang L. H., et al. (2021). Research status and development trends in the field of marine environment corrosion: A new perspective. Environ. Sci. Pollut. Res 28, 54403–54408. doi: 10.1007/s11356-021-15974-0
Wei J. P., Liang G. F., Alex J., Zhang T. C., Ma C. B. (2020). Research progress of energy utilization of agricultural waste in China: Bibliometric analysis by citespace. Sustainability 12, 812. doi: 10.3390/su12030812
Wuchty S., Jones B. F., Uzzi B. (2007). The increasing dominance of teams in production of knowledge. Science 316, 1036–1039. doi: 10.1126/science.1136099
Yang Z. (2017). Characteristics and potential functions of 5-HT4R in sea cucumber (Apostichopus japonicus). Master's thesis Zhoushan:Zhejiang Ocean University. doi: 10.7666/d.Y3236272
Yan L. F., Gu A. H. (2013). Progress and application of zebrafish in regenerative medicine. Yi Chuan = Hereditas 35, 856–866. doi: 10.3724/SP.J.1005.2013.00856
Yang H. S., Yuan X. T., Zhou Y., Mao Y. Z., Zhang T., Liu Y. (2005). Effects of body size and water temperature on food consumption and growth in the sea cucumber apostichopus japonicus (Selenka) with special reference to aestivation. Aquacult. Res. 36, 1085–1092. doi: 10.1111/j.1365-2109.2005.01325.x
Yang A. F., Zhou Z. C., Pan Y. J., Jiang J. W., Dong Y., Guan X. Y., et al. (2016). RNA Sequencing analysis to capture the transcriptome landscape during skin ulceration syndrome progression in sea cucumber apostichopus japonicus. BMC Genomics 17, 1–16. doi: 10.1186/s12864-016-2810-3
Yang H. S., Zhou Y., Zhang T., Yuan X. T., Li X. X., Liu Y., et al. (2006). Metabolic characteristics of sea cucumber apostichopus japonicus (Selenka) during aestivation. J. Exp. Mar. Biol. Ecol. 330, 505–510. doi: 10.1016/j.jembe.2005.09.010
Yin X. X., Zhang G. P., Li X. F. (2009). Analysis of information science research status based on keyword statistics. J. Inf. 11, 5–8. doi: 10.3969/j.issn.1002-1965.2009.11.001
Yuan J. B., Gao Y., Sun L. N., Jin S. J., Zhang X. J., Liu C. Z., et al. (2019). Wnt signaling pathway linked to intestinal regeneration via evolutionary patterns and gene expression in the sea cucumber apostichopus japonicus. Front. Genet. 10. doi: 10.3389/fgene.2019.00112
Yuan X. T., Yang H. S., Chen M. Y., Gao F. (2007). Research advances in aestivation of sea cucumber apostichopus japonicas (Selenka): A review. Mar. Sci. 31, 88–90. doi: 10.3969/j.issn.1000-3096.2007.08.018
Yuan X. T., Yang H. S., Zhou Y., Mao Y. Z., Zhang T., Liu Y. (2006). The influence of diets containing dried bivalve feces and/or powdered algae on growth and energy distribution in sea cucumber apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea). Aquaculture 256, 457–467. doi: 10.1016/j.aquaculture.2006.01.029
Zhang W. W., Liang W. K., Li C. H. (2016). Inhibition of marine vibrio sp. by pyoverdine from pseudomonas aeruginosa PA1. J. Hazard. Mater. 302, 217–224. doi: 10.1016/j.jhazmat.2015.10.003
Zhang P., Li C. H., Li Y., Zhang P. J., Shao Y. N., Jin C. H., et al. (2014). Proteomic identification of differentially expressed proteins in sea cucumber apostichopus japonicus coelomocytes after vibrio splendidus infection. Dev. Comp. Immunol. 44, 370–377. doi: 10.1016/j.dci.2014.01.013
Zhang S. S., Liu N. N., Liang W. K., Han Q. X., Zhang W. W., Li C. H. (2017). Quorum sensing-disrupting coumarin suppressing virulence phenotypes in vibrio splendidus. Appl. Microbiol. Biotechnol. 101, 3371–3378. doi: 10.1007/s00253-016-8009-3
Zhang Z., Lv Z. M., Zhang W. W., Shao Y. N., Zhao X. L., Guo M., et al. (2019). Comparative analysis of midgut bacterial community under vibrio splendidus infection in apostichopus japonicus with hindgut as a reference. Aquaculture 513, 11. doi: 10.1016/j.aquaculture.2019.734427
Zhang Q., Ma H. M., Mai K. S., Zhang W. B., Liufu Z. G., Xu W. (2010). Interaction of dietary bacillus subtilis and fructooligosaccharide on the growth performance, non-specific immunity of sea cucumber, apostichopus japonicus. Fish Shellfish Immunol. 29, 204–211. doi: 10.1016/j.fsi.2010.03.009
Zhang X. J., Sun L. N., Yuan J. B., Sun Y. M., Gao Y., Zhang L. B., et al. (2017). The sea cucumber genome provides insights into morphological evolution and visceral regeneration. PLoS Biol. 15, e2003790. doi: 10.1371/journal.pbio.2003790
Zhang Z., Xing R. L., Lv Z. M., Shao Y. N., Zhang W. W., Zhao X. L., et al. (2018). Analysis of gut microbiota revealed lactococcus garviaeae could be an indicative of skin ulceration syndrome in farmed sea cucumber apostichopus japonicus. Fish Shellfish Immunol. 80, 148–154. doi: 10.1016/j.fsi.2018.06.001
Zhang L. Y., Zhang T. T., Ding L., Xu J., Xue C. H., Yanagita T., et al. (2018). The protective activities of dietary sea cucumber cerebrosides against atherosclerosis through regulating inflammation and cholesterol metabolism in male mice. Mol. Nutr. Food Res. 62, 1800315. doi: 10.1002/mnfr.201800315
Zhao H., Liu S. L., Yang H. S., Zhao H. L., Liu C. G. (2014). The study on thermo tolerance of juvenile offspring apostichopus japonicus (Selenka) with directive breeding. Mar. Sci. 38, 1–6. doi: 10.11759/hykx20121105001
Zhao Y., Yang H. S., Storey K. B., Chen M. Y. (2014). Differential gene expression in the respiratory tree of the sea cucumber apostichopus japonicus during aestivation. Mar. Genom. 18, 173–183. doi: 10.1016/j.margen.2014.07.001
Zhao Y. C., Zhang W. B., Xu W., Mai K. S., Zhang Y. J., Liufu Z. G. (2012). Effects of potential probiotic bacillus subtilis T13 on growth, immunity and disease resistance against vibrio splendidus infection in juvenile sea cucumber apostichopus japonicus. Fish Shellfish Immunol. 32, 750–755. doi: 10.1016/j.fsi.2012.01.027
Zhou Z. C., Dong Y., Sun H. J., Yang A. F., Chen Z., Gao S., et al. (2014). Transcriptome sequencing of sea cucumber (Apostichopus japonicus) and the identification of gene-associated markers. Mol. Ecol. Resour. 14, 127–138.doi: 10.1111/1755-0998.12147
Keywords: Apostichopus japonicus, co-citation analysis, research hot spots, frontier prediction, bibliometrics
Citation: Chen J, Lv Z and Guo M (2022) Research advancement of Apostichopus japonicus from 2000 to 2021. Front. Mar. Sci. 9:931903. doi: 10.3389/fmars.2022.931903
Received: 29 April 2022; Accepted: 02 August 2022;
Published: 24 August 2022.
Edited by:
Libin Zhang, Institute of Oceanology (CAS), ChinaReviewed by:
Lina Sun, Institute of Oceanology (CAS), ChinaZhuang Xue, Dalian Ocean University, China
Dongxue Xu, Qingdao Agricultural University, China
Copyright © 2022 Chen, Lv and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Guo, Z3VvbWluZ0BuYnUuZWR1LmNu
 Jiting Chen
Jiting Chen Zhimeng Lv1
Zhimeng Lv1 Ming Guo
Ming Guo