- Department of Fisheries, Gonbad Kavous University, Gonbad Kavous, Iran
This study provides comparative information about population dynamics for the Palaemon adspersus Rathke, 1836 and P. elegans Rathke, 1836 shrimps on the southeastern coast of the Caspian Sea. Specimens were collected on a monthly basis between April 2019 and May 2020. A total of 9,643 specimens (8,478 P. adspersus and 1,165 P. elegans) were captured and examined. The highest abundance of both shrimps was observed in autumn. The male P. elegans ranged between 16.80 and 61.99 mm in total length and the females between 16.80 and 61.99 mm. The total length for male and female P. adspersus ranged from 21.46 to 69.29 mm and 18.24 to 75.46 mm, respectively. The mean ( ± SD) total length for the males and females of P. elegans was 33.65 ± 4.12 and 40.81 ± 6.59, whereas that of P. adspersus was 48.41 ± 6.27 for males and 54.68 ± 9.90 for females. Based on the b-value of LWR, the growth type of P. adspersus is positively allometric. Likewise, the relationships of female and pooled-sexes P. elegans was positively allometric, while that of male P. elegans was negatively allometric. The estimation of the VBGF parameters resulted as Total Length∞ (TL∞)=71.93 mm, K=0.72 y-1, and t0=-0.176 for males, and TL∞=78.23 mm, K=0.64 y-1, and t0=-0.194 for the females of P. adspersus, while for P. elegans as TL∞=56.13 mm, K=0.91 y-1, and t0=-0.147 for males and TL∞=65.63 mm, K=0.87 y-1, and t0=-0.148 for females. The size-frequency analysis showed that both shrimps were made up of two age groups. The recruitment pattern of the shrimps was continuous with a unimodal pulse for both males and females and showed that P. adspersus is mainly recruited during the autumn and early winter while for P. elegans, during mid-summer and early autumn. The estimated maximum age was 3.35/year for the males and 2.66/year for the females of P. adspersus and 3.78/year for the males and 3.33/year for the females of P. elegans. Therefore, in this case, P. elegans shrimp is more sensitive to death than P. adspersus. The findings point to the underlying notable differentiation of the growth of the shrimps governing the processes of population dynamics. We assume that these differences result from the contrasting life-history strategies of these two Palaemon shrimps.
Introduction
In the Caspian Sea, two shrimp species, including Palaemon adspersus and P. elegans, have been commonly identified within the Palaemonidae family. These Palaemon shrimps are distributed predominantly in shallow waters of the southeastern areas of the sea all around the year (Ghorbani et al., 2012). Nonetheless, in the fishery point of view, they have not contributed to the local fishery in the region. Like many shrimp species, both species congregate and spawn on specific shallow bottoms in the coastal waters including the coastal lagoons and estuaries of the Caspian Sea, where they are threatened due to the depleting seawater level. Accordingly, concerns are growing regarding the sustainable management of coastal habitats and inhabitant organisms. They have thus attracted a rising interest in ecological studies in the region due to the high abundance of the shrimps that rank as one of the most abundant benthic species in the southern Caspian Sea. Given the high abundance and widespread distribution of the shrimps in the southern Caspian, they have been termed keystone benthic species for the ecological studies of the coastal areas since disturbances in the coastal areas promote changes in population parameters such as growth, abundance, and mortality. Therefore, the estimation of these parameters is essential in any population dynamics study. This is itself a useful indicator for stock assessment and the effective management of bioresources in a given area (Guerra and Sánchez, 1998; Diop et al., 2007; Kevrekidis and Thessalou-Legaki, 2011; Veloso et al., 2011; Dolbeth et al., 2012; Baker et al., 2014). Moreover, growth and mortality may show substantial variability among the species of a genus. Hence, estimating these parameters can help to establish the degree of variability and differentiation of species’ life history.
In Europe, P. adspersus and P. elegans are widely distributed (Berglund and Bengtsson, 1981; Berglund, 1982; Baden and Pihl, 1984; Campbell, 1994; d’Udekem d’Acoz, 1999; Lapinska and Szaniawska, 2006). However, there is a dearth of studies on the population dynamics of the shrimps, requiring the determination of growth and mortality rates in the field (Guerao and Ribera, 1995; Duran et al., 2006; Manent and Abella-Gutiérrez, 2006; Bilgin et al., 2009a; Bilgin et al., 2009b; Glamuzina et al., 2014). Information on the mortality and growth parameters of a population, as a basic estimation for stock assessment, is coupled with the key aspects governing the dynamics of fish stocks. To date, there is no comparative study that differentiates the growth and mortality of these shrimps. In the Iranian waters of the Caspian Sea, there is only scattered evidence on the population parameters of these shrimps. Research on the subject has been mostly restricted to limited fragmentary studies on the population dynamics (Abdolmaleki et al., 2003; Gharaei et al., 2005; Sadeghi et al., 2013; Vejan et al., 2018), and nearly nothing is known about the abundance of these species in the southern Caspian Sea. Additionally, to date, there have not been any comparative investigations that examine the population dynamics of the co-occurrence of Palaemon shrimps in the southeast Caspian Sea. The lack of awareness of the population structure and a proper evaluation of the population status of the shrimps emphasize the importance of a detailed comparative study on population dynamics and related ecological processes.
In the case of the sympatric species, those studies comparing species differences in population parameters within one study is necessary as they can estimate species-specific parameters and management strategies. Generally, understanding the biological reference points of the co-occurrence of shrimps is important to understanding population persistence.
These two shrimp species are considered as acclimatized non-indigenous crustaceans in the Caspian Sea as these species were accidentally introduced into the sea in the early 1930s (Aladin et al., 2001 and Aladin et al., 2002). Due to their relatively old history since the shrimps were being introduced into the Caspian Sea, each species has probably had its own evolutionary path of life history. The estimation of population parameters, therefore, would greatly enlighten our understanding of this shrimp evolution. Despite the fact that the two shrimps might have coextensively overlapped in geographical distributional patterns in the Caspian Sea, we hypothesized that these two shrimps differ in many aspects of their biology. These two shrimps co-occur in the southeastern Caspian around the mouth of Gorgan Bay, which is considered an estuarine habitat with the almost-virgin conditions. This provides an excellent opportunity for obtaining comparable data on their population dynamics. While some studies have been carried out as previously mentioned, the analysis reported here is the first comparative data on the growth and mortality rates of these shrimp based on which efficient sustainable management of these shrimp resources plan can be organized and implemented on a relatively non-impacted beach of the southeastern Caspian Sea. More specifically, the present study was carried out to investigate the size structure, weight–length relationship, cohorts, and recruitment patterns, VBGF parameters, natural and total mortality rates, and life span of the P. adspersus and P. elegans in Gorgan Bay—southeast Caspian Sea. Differences in these parameters have been supposed but never demonstrated in details comparatively. A detailed comparison of the life history parameters can make contributions to predict the future of different populations of these species in this ever-changing world.
Materials and methods
The study was conducted at the mouth of Gorgan Bay including the main strait and in the adjacent areas (hereafter referred to as Gorgan Bay) (Figure 1). The bay is part of a complex of the Miankaleh peninsula, known as Miankaleh Wildlife Sanctuary and Wetland, on the southeastern Iranian coast of the Caspian Sea. It encompasses an area of 100,000 ha; approximately half (45%) of that area is composed of water bodies of the wetland type. Since the bay includes different habitats, sampling from different habitats was performed to fully cover all the population in these habitats. We selected sampling sites (main strait of the bay, sea coast, approximately 1 km seaward, and areas inside the bay, approximately 5 km toward inside the bay, near the main strait of the bay) because the conditions in the study area are in constant flux between the Caspian Sea and the bay. Although the complex of the Miankaleh peninsula, including Gorgan Bay, has been designated by a protected area by the Iranian Department of Environment [as an international wetland (Ramsar site) and international UNESCO Biosphere Reserve], there are significant anthropogenic impacts on the southern coast of the bay by local people and agricultural and industrial activities. Notably, the selected sampling sites are far from urban settlements and agricultural and industrial areas, and sparsely visited by tourists, suggesting an insignificant anthropogenic impact on the shrimps.
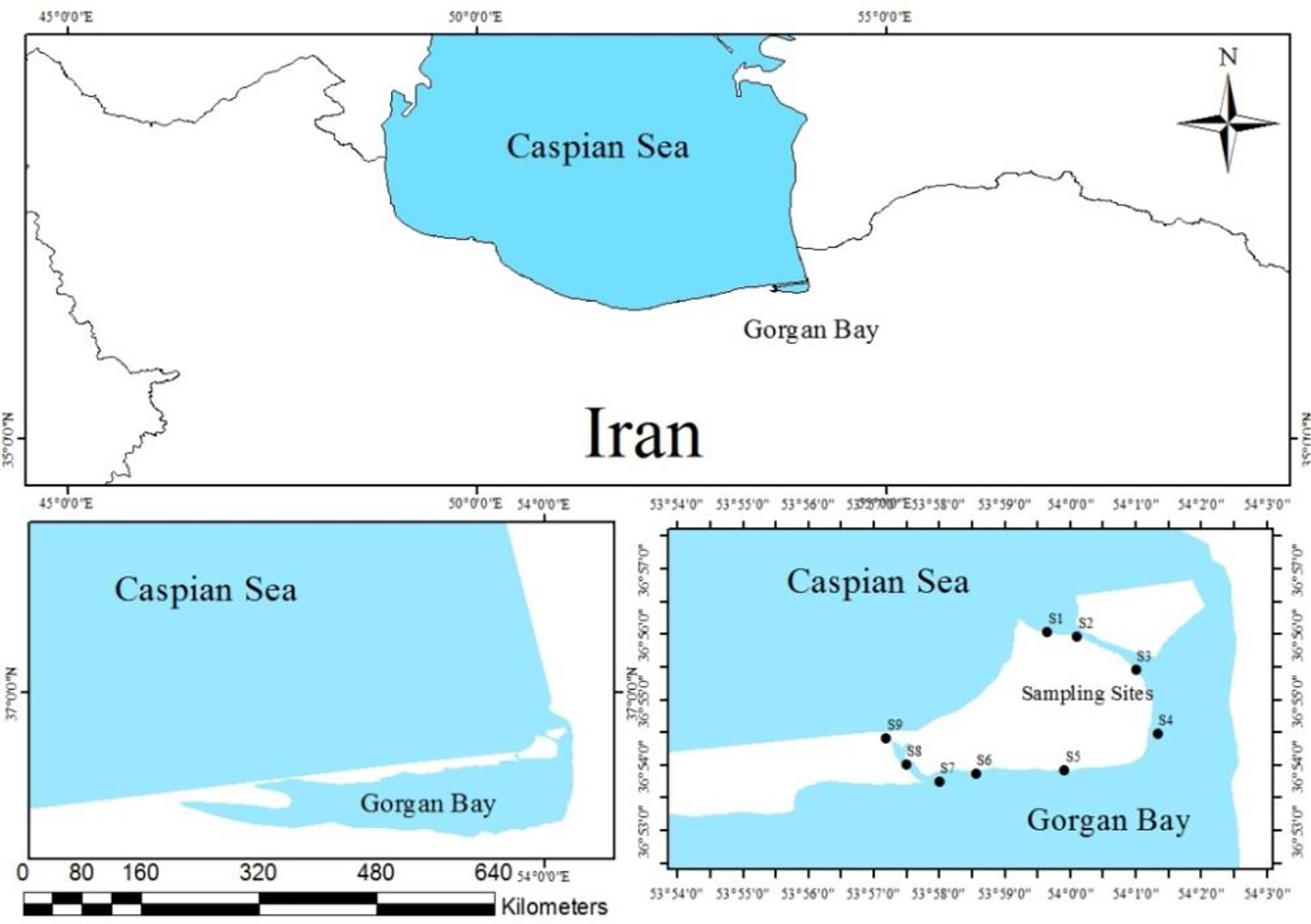
Figure 1 Sampling localities of P. elegans and P. adspersus in the southeast Caspian Sea–Iran (2019–2020).
The sampling was monthly carried out using a small beach seine net of 30 m in length with a 3-mm mesh size during the daytime from May 2018 to April 2019. Each sampling effort was four persons for 30 min of beach seining. Three transects, orthogonal to the shore, were sampled at each sampling site covering an area of approximately 500 m2 at each sampling point. All specimens collected were fixed in 4% formalin diluted with seawater in the field. A total of 8,643 specimens were collected during the 12-month period of sampling, consisting of 1,165 P. elegans and 8,478 P. adspersus. At the laboratory, each sample was first sorted into species, and then the samples were sexed, measured, and biometric- recorded.
The population structure of the shrimps was described by considering months and sexes. Size frequency distribution analysis is used to determine the distribution of the shrimps based on intervals of 3-mm-size groups. The Kolmogorov–Smirnov two-sample test (K-S test) was applied to detect any difference between the length-frequency distributions of the considered groups. In addition, the Mann–Whitney test was used to compare differences in the sizes of the shrimps.
The power function of length–weight relationships (W=aLb) was analyzed to estimate biometric relationships, where L, as the independent variable, is the total length in centimeters and W, as the dependent variable, is total weight in grams; a and b are constants (Le Cren, 1951; Lagler, 1961). The equation was performed separately for males and females, and as well as for pooled sexes. To determine the growth type (isometric or allometric), the slope (b coefficient) was statistically tested by Student’s t-test (Zar, 1984) to determine the growth type for each considered group as isometric growth when b=3 and allometric when b#3 (positively allometric growth when b>3 and negative allometric when b<3). ANCOVA was used for the comparison of the b coefficient between sexes to determine the difference in the growth pattern (Zar, 1984).
The von Bertalanffy growth curves were adjusted for the sex-segregated data of the shrimps, separately using the modal progressions of 2-mm-size classes over 12 successive months. The growth parameters were estimated by using the ELEFAN method in the FiSATII computer software package for each sex of the shrimps. It was considered as a more appropriate method for estimating the parameters because of the short life of the species. The age–length relationship was described using the VBGF equation using the ELEFAN I method in the FiSAT-II package (FAO-ICLARM Stock Assessment Tools) (Pauly, 1987; Gayanilo et al., 2005), where Lt is the total length (mm) at age t, L∞ is the theoretical maximum length or asymptotic length (mm) and K is the growth coefficient (year−1), and t0 is the theoretical age (year) when the shrimp hypothetically has zero length (mm). The growth curve of VBGF was determined by distributing the measures of total length into class intervals of 2 mm to define the mean lengths by age of males and females using the method of Bhattacharya (1967). Since ELEFAN I does not define the t0-value, it was estimated using Pauly’s equation: log(-t0) = -0.3922 – 0.2752(log(L∞)) – 1.038(log(K)) (Sparre and Venema, 1992). Instead of the comparison of L and K individually, the growth performance index (ϕ′) is preferred for the comparison of growth between populations. The results of L∞ and K values were used to estimate the index (Munro and Pauly, 1983; Pauly and Munro, 1984), as follows: ϕ′=log(K)+2*log(L∞).
The recruitment pattern was established with ELEFAN II in FISAT II (Gayanilo et al., 2005), where asymptotic length (L∞ mm) and growth coefficient (K year−1) were input data. This method reconstructs the recruitment pulses from the monthly length frequencies, to determine the number and magnitude of pulses per year as the recruitment pattern (Pauly, 1987; Moreau and Cuende, 1991).
In order to estimate the longevity (or theoretical life span) for each species (tmax), we used the following formula (Pauly, 1983): tmax=(2.996/K)+t0, where K and t0 are the growth coefficient (year-1) and the theoretical age (year) at the length zero of VBGF, respectively. The theoretical life span was calculated for males and females separately.
The method of the length-converted catch curve in FiSAT II (FAO-ICLARM-Stock Assessment Tools) was used to determine the total mortality (Z) rate (Pauly, 1983; Sparre and Venema, 1992; Gayanilo et al., 2005).
Results
Throughout the 12-month surveys (between April 2019 and May 2020), 9,633 specimens were encountered, with the majority being P. adspersus (8,468 specimens). In terms of the number percentage, both shrimps showed the highest abundance in autumn, followed by summer. Although both species were observed in all months of the year, P. adspersus had the lowest abundance in spring and P. elegans in winter (Figure 2). In comparison, P. adspersus was more seasonal than P. elegans, with their numbers differing significantly between seasons (chi-square test, P<0.05), wherein the male P. elegans ranged from 16.80 to 61.99 mm in total length and the female P. elegans between 16.80 and 61.99 mm in total length. The total length for males and females of P. adspersus ranged from 21.46 to 69.29 mm and 18.24 to 75.46 mm, respectively. Additionally, it was observed that the average size of females was larger than that of males for both species. The mean ( ± SD) total length for males and females of P. elegans was 33.65 ± 4.12 and 40.81 ± 6.59, respectively. In contrast, based on the length-frequency analysis of the P. adspersus, the mean total length ( ± SD) was 48.41 ± 6.27 for males and 54.68 ± 9.90 for females (Mann–Whitney U-test, P < 0.05). Generally, it was observed that P. adspersus attains larger sizes than P. elegans (Mann–Whitney U-test, P < 0.05). The size distributions of the shrimps showed a bimodal trend in P. elegans and unimodal in P. adspersus (Figure 3). The most abundant individuals of P. elegans had sizes between 33- and 36-mm TL, and that of P. adspersus between 51- and 54-mm TL. There was also a significant difference in the mode of length between the shrimps; the mode in the total length frequency of P. adspersus was 52.8 mm, while that for P. elegans shrimp was 33.1 mm. With regard to the comparison of the size distributions, the K–S test proved significant differences in size distribution between shrimps (P<0.05). In P. elegans length-frequency distributions, individuals were absent in the length group of 60–63 mm and larger ones, while 9.3% of the total individual abundance of P. adspersus were in these length groups.
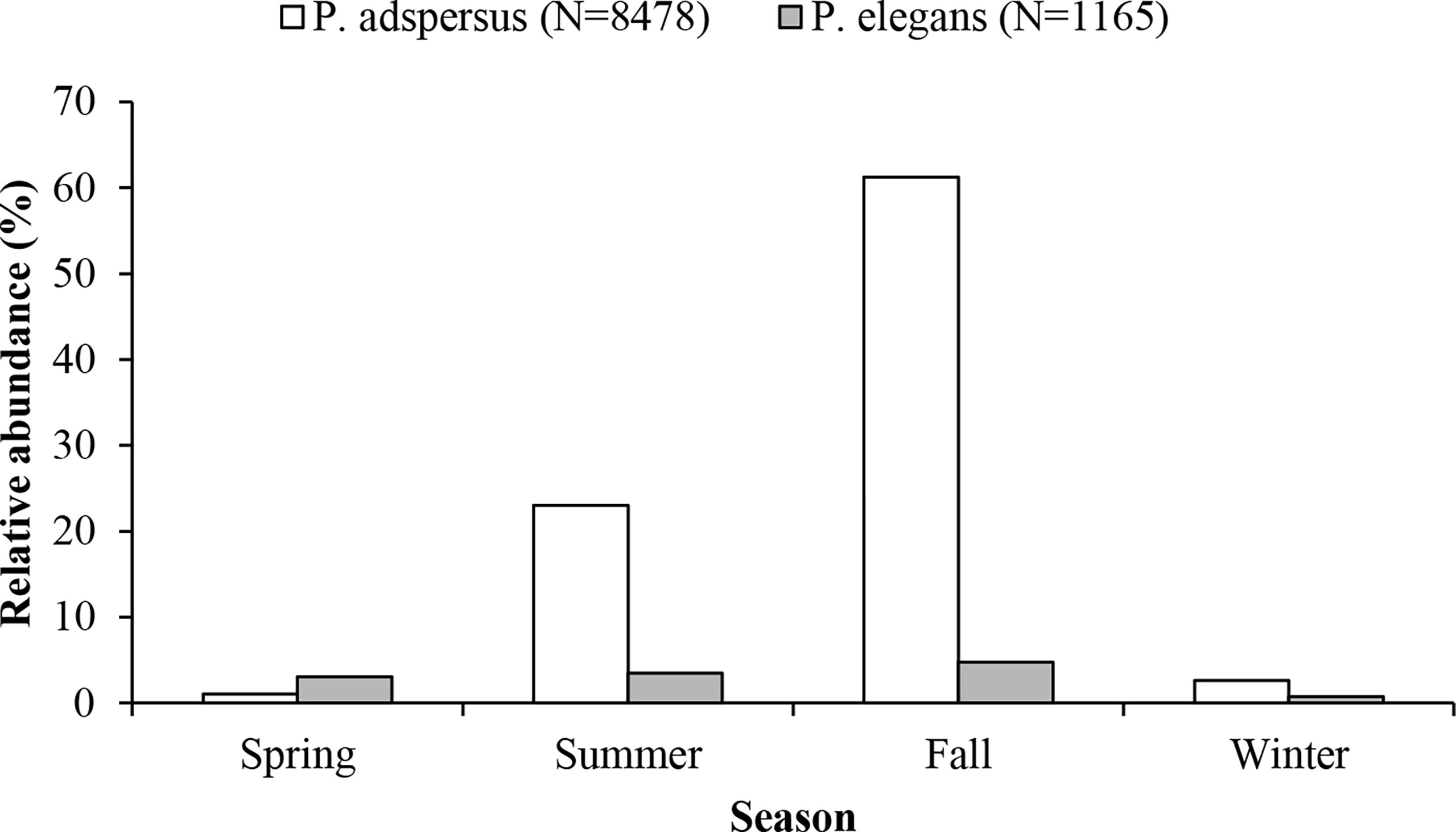
Figure 2 Comparison of relative abundance of the shrimps P. adspersus and P. elegans from the southeast Caspian Sea (2019-2020).
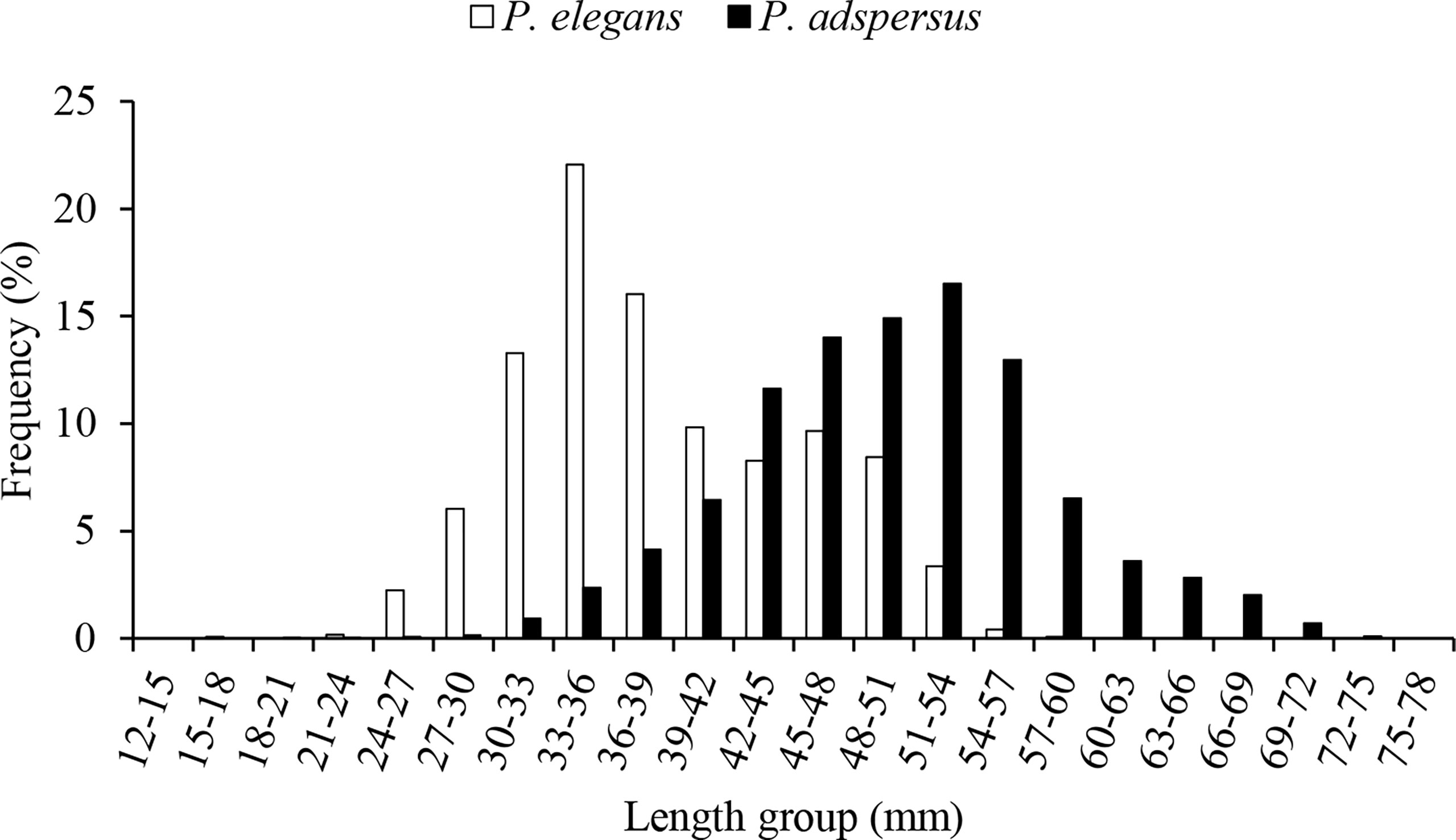
Figure 3 Length-frequency distributions of the shrimps P. adspersus and P. elegans from the southeast Caspian Sea (2019-2020).
The significant correlation between the total weight and total length was found to be a power function in both shrimps (P<0.05); the formulae are given in Figures 4 and 5. Based on the value of the correlation coefficient (r2), the relationships in general had high R2 values and a highly close correlation. Note that the R2 for the regressions of the P. adspersus was higher than of P. elegans. Based on the t-test against the value of growth coefficient (b) at 95% confidence interval, it was found that the growth pattern of P. adspersus is positively allometric (b>3, t-test, P<0.05), that is, length growth is not as fast as the weight. Likewise, from the analysis of the relationships of P. elegans, the growth type in females and pooled sexes was positively allometric (b>3, t-test, P<0.05), while in the males of P. elegans, the relationship was negatively allometric (b<3, t-test, P<0.05), meaning that the increase in length of males was accompanied by a smaller increase in weight. The relationships are statistically different between the males and females of each shrimp (ANCOVA, P<0.05). Additionally, the relationship differed significantly between the same sexes of the shrimps (ANCOVA, P<0.05). Therefore, it could be assumed that the discrepancy of b-values indicates that the growth pattern is different between these two Palaemon shrimps, a common trait of the species that usually experience changes in body form with age among crustaceans.
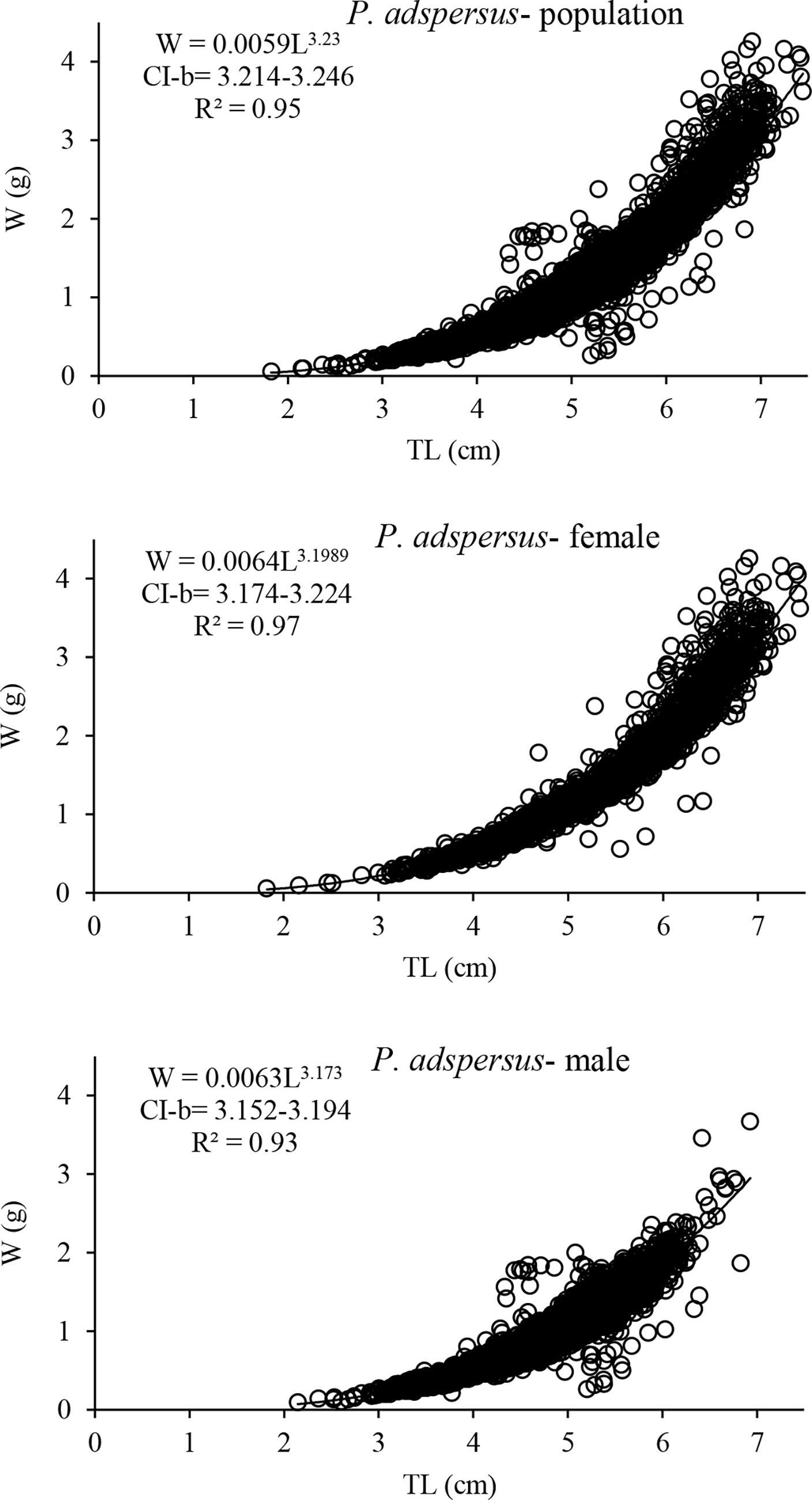
Figure 4 Length-weight relationship curves for population, females and males of P. adspersus from the southeast Caspian Sea (2019–2020).
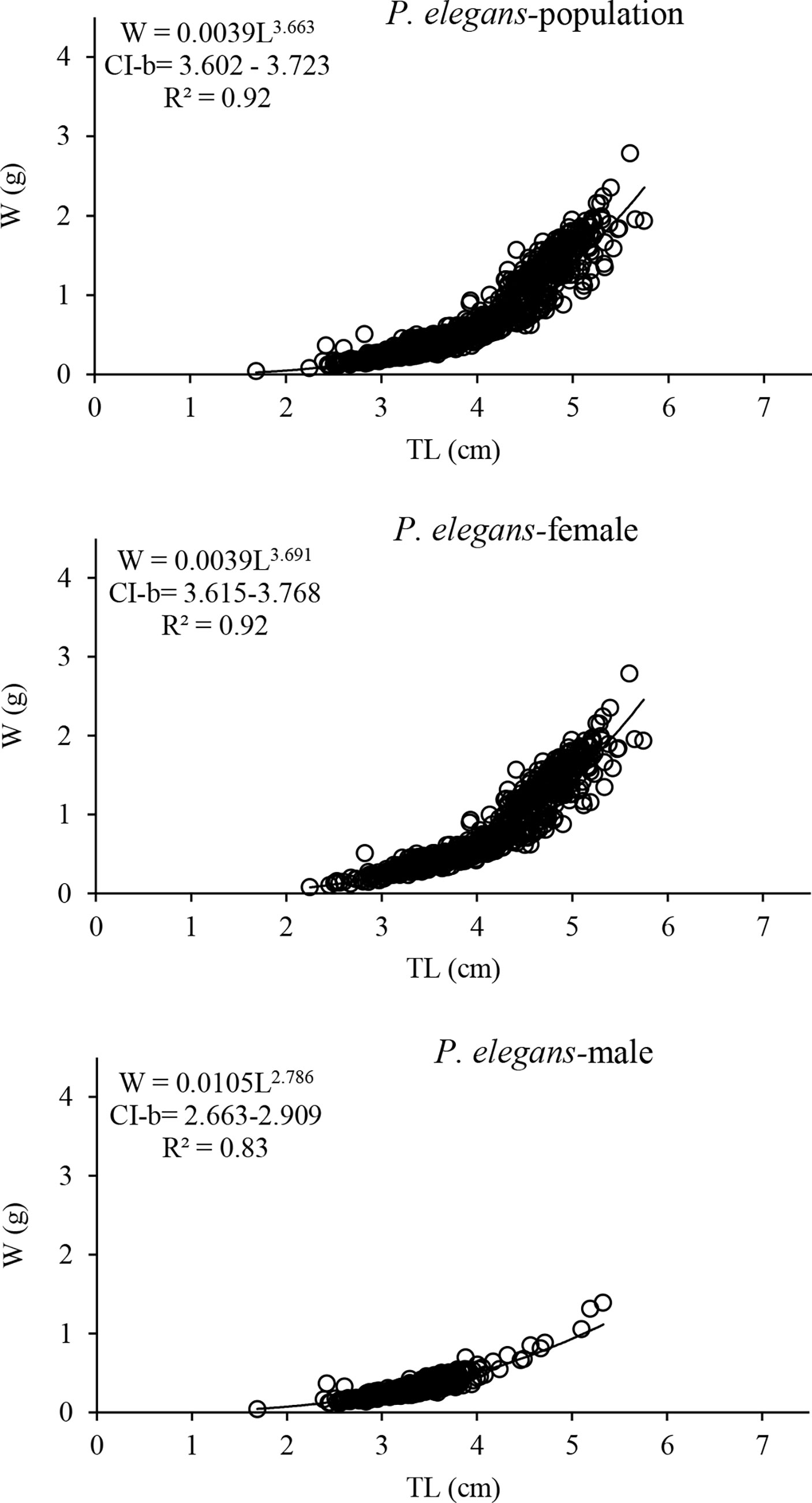
Figure 5 Length–weight relationship curves for population, females and males of P. elegans from the southeast Caspian Sea (2019–2020).
Both shrimps displayed differences in best-fitting von Bertalanffy growth curves by sex and species (Figures 6–9). The growth parameters were TL∞=71.93 mm, K=0.72 y-1, and t0=-0.176 for the males and TL∞=78.23 mm, K=0.64 y-1, and t0=-0.194 for the females of P. adspersus. The analysis of P. elegans growth parameters revealed different results. The estimated L∞, K, and t0 for males were TL∞=56.13 mm, K=0.91 y-1, and t0=-0.147 year, while the females had TL∞=65.63 mm, K=0.87 y-1, and t0=-0.148. These results revealed that P. adspersus shrimps had a lower K but a higher L∞ than the P. elegans shrimps. In the monthly adjusted VBGF parameters, the estimated growth parameters of each gender of the shrimps showed that males have slightly higher K values than females, and females have a greater L∞ than males; thus, the females of both P. adspersus and P. elegans need more time than the male individuals to get their L∞s. Moreover, the females devote more energy to reproduction than to their own growth. The growth functionality index of phi-Monro (ϕ′) was 3.57 for the males and 3.59 for the females of P. adspersus, and 3.46 for the males and 3.57 for the females of P. elegans, indicating no significant errors in the calculations.
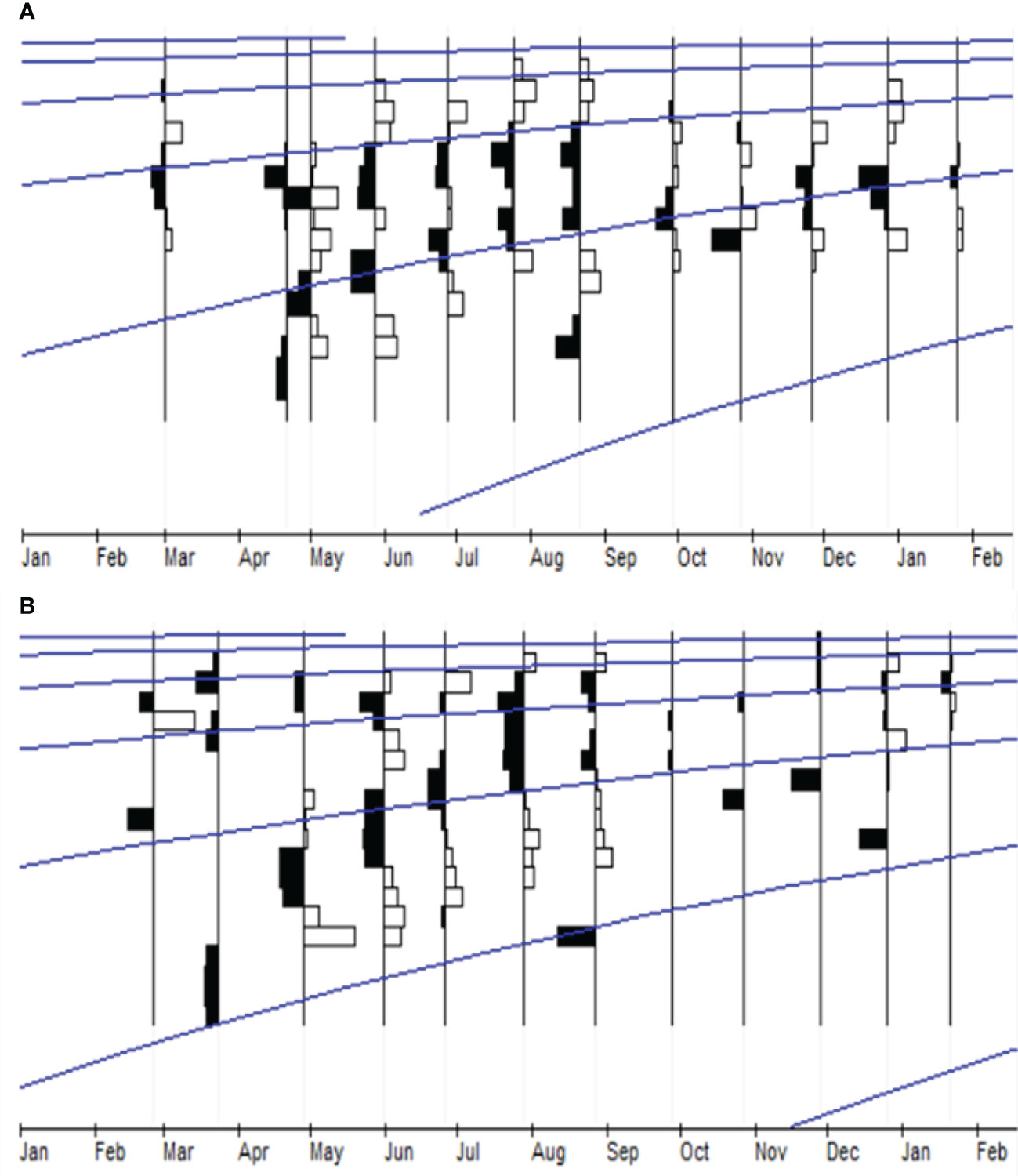
Figure 6 Length-frequency distribution data and the growth curves estimated using ELEFAN I module FISAT-II for (A) male and (B) female P. adspersus from the southeast Caspian Sea (2019–2020).

Figure 7 Length-frequency distribution data and the growth curves estimated using ELEFAN for (A) female and (B) male P. elegans from the southeast Caspian Sea (2019–2020).
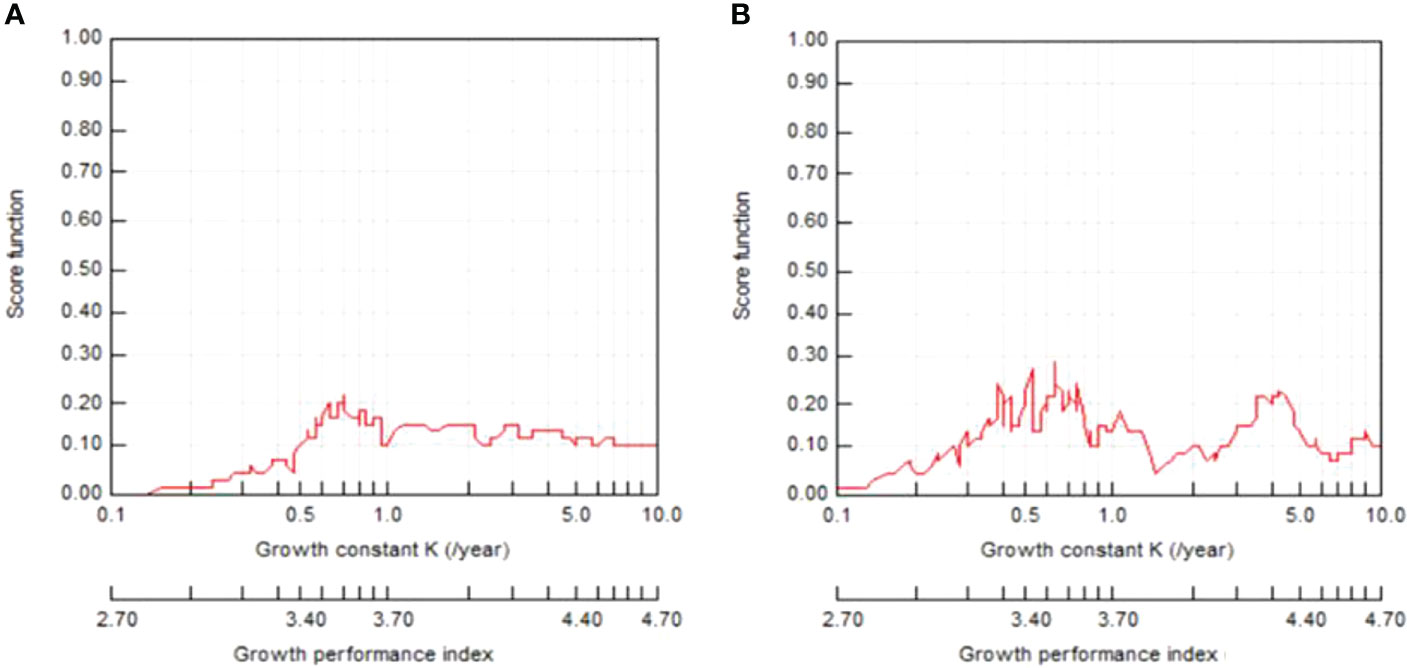
Figure 8 ELEFAN I K-scan routine FiSAT II output for (A) male and (B) female P. adspersus from the southeast Caspian Sea (2019–2020).
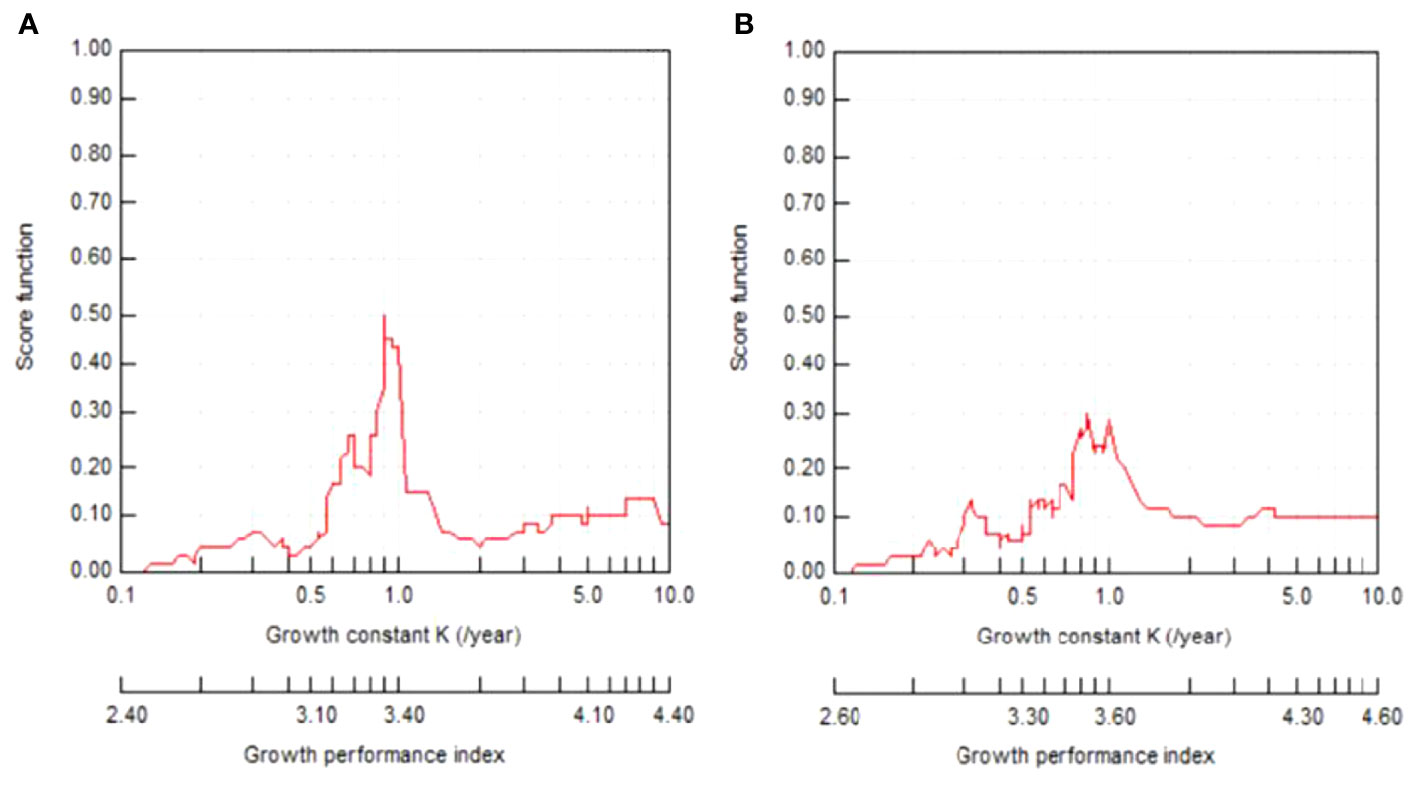
Figure 9 ELEFAN I K-scan routine FiSAT II output for (A) male and (B) female P. elegans from the southeast Caspian Sea (2019–2020).
The Bhattacharya (1967) method allowed us to determine the mean lengths by age using the cohorts in the length distribution. The size–frequency distributions showed that P. adspersus was made up of two age groups in both sexes during the study period (Figure 10), and the presence of two modes were also identified in the length–frequency distributions of P. elegans males and females (Figure 11). Thus, for the males and females of P. elegans, this method identified only two age groups. This provides a rough estimation for the shorter life cycle of both shrimps, probably as a consequence of the high mortality rate and lower maximum age of the shrimps. However, there was clear evidence for different population size structures and mean length at the age between the shrimps (Table 1).
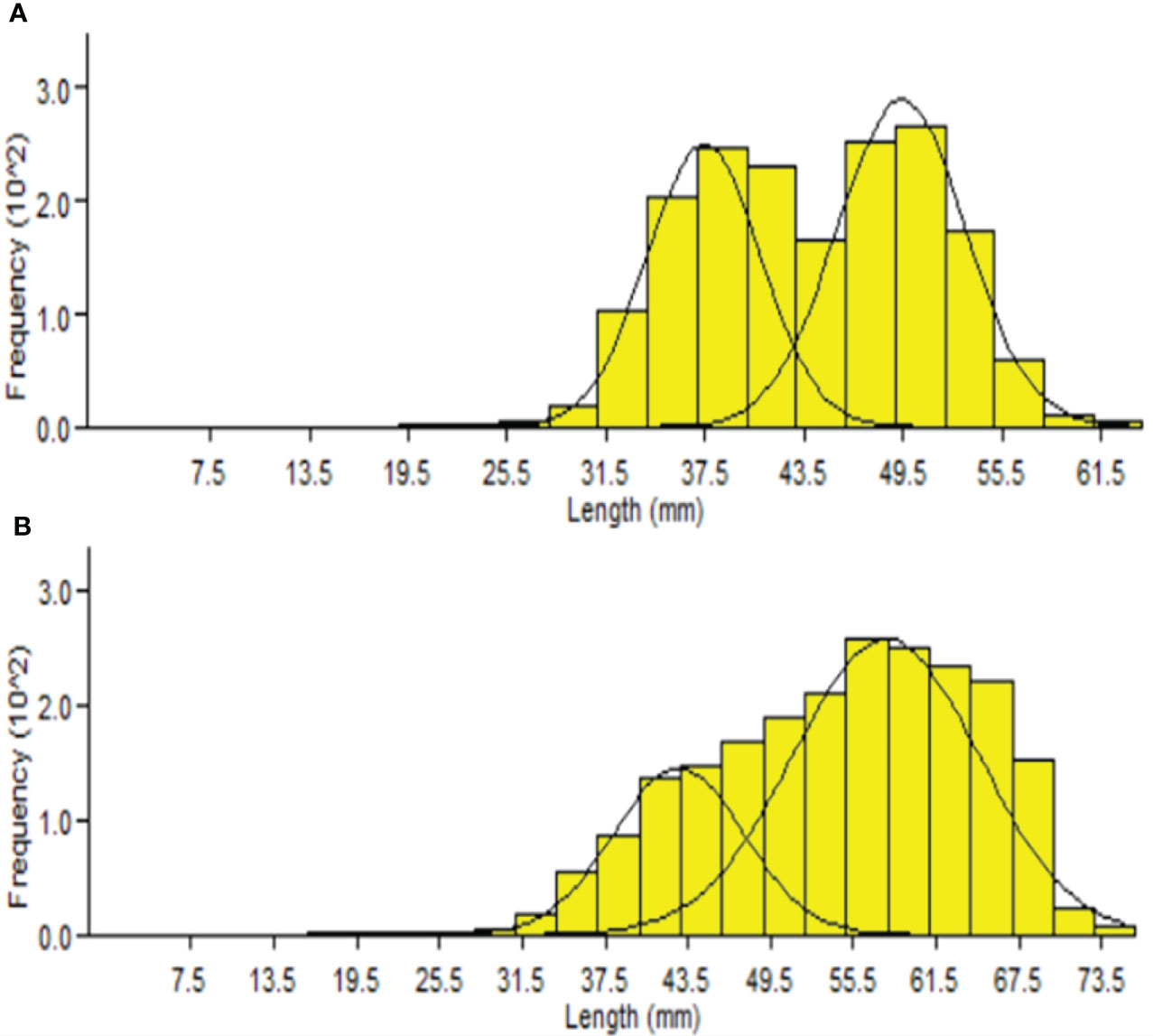
Figure 10 Cohort analysis output from FiSAT II for (A) male and (B) female P. adspersus from the southeast Caspian Sea (2019–2020).
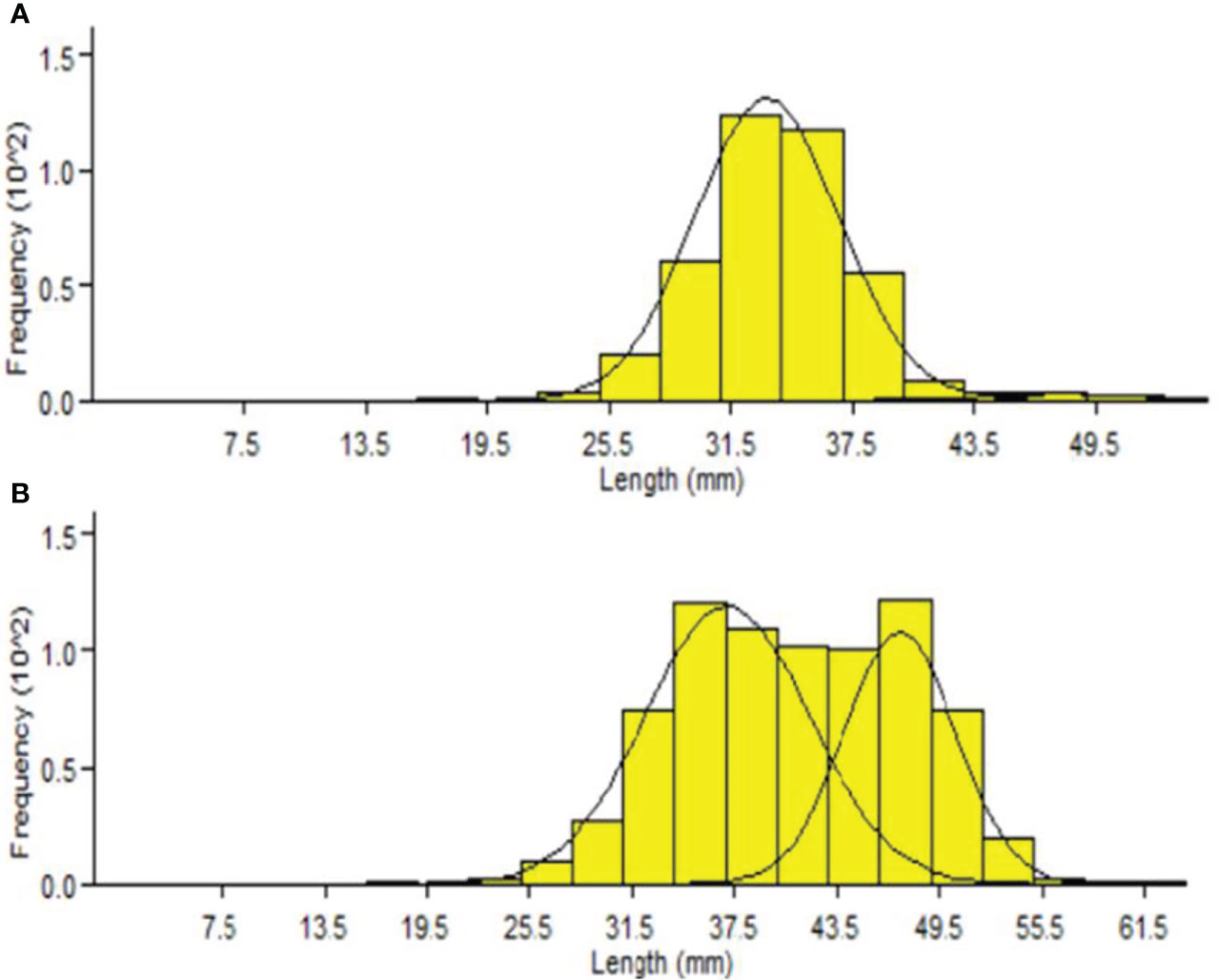
Figure 11 Cohort analysis output from FiSAT II for (A) male and (B) female P. elegans from the southeast Caspian Sea (2019–2020).
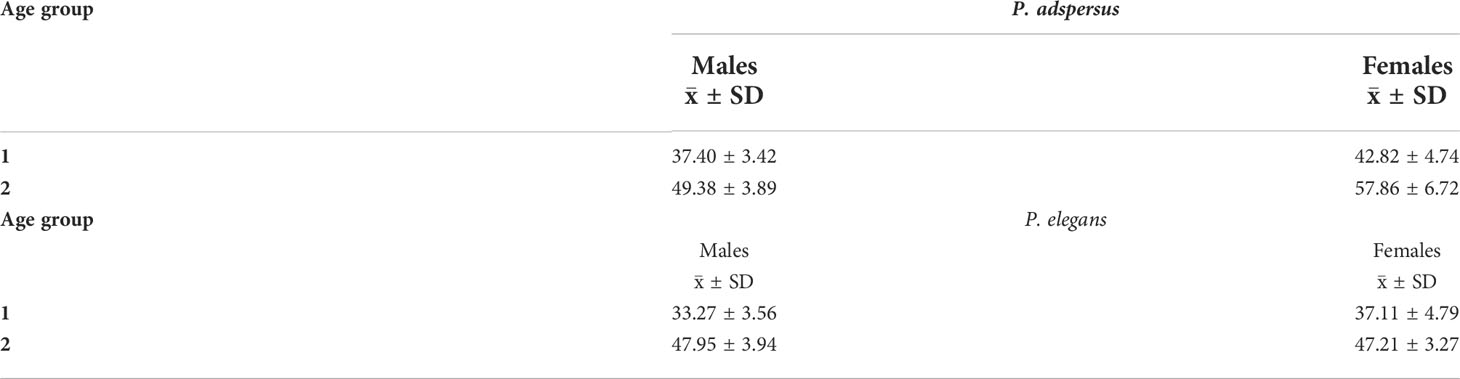
Table 1 Length at age (cohort) output from FiSAT II for (A) male and (B) female P. adspersus and P. elegans from the southeast Caspian Sea (2019–2020).
The recruitment pattern of the shrimps, obtained through FISAT II software in a 12-month period between April and March, showed that the recruitment of male P. adspersus almost peaked in November and that of female P. adspersus in December (Figure 12). The peak of recruitment of P. elegans occurred in July for males and in August for females (Figure 13). The unimodal shape of the recruitment pattern confirmed the normally distributed nature of a unique annual pulse of recruitment for both shrimps. The recruitment pattern of males and females were unequal for each shrimp. The main recruitment of P. adspersus was found in September–December for males and in October–January for females. The males of P. elegans had higher recruitments between June and August and females between July and October. However, it seems that recruitment can take place nearly during all months of the year and maybe considered seasonal-continuous for these species since recruitment appears every month with low seasonal variation of occurrence. As a general result, P. adspersus is mainly recruited during the autumn and early winter, while P. elegans during mid-summer and early autumn.
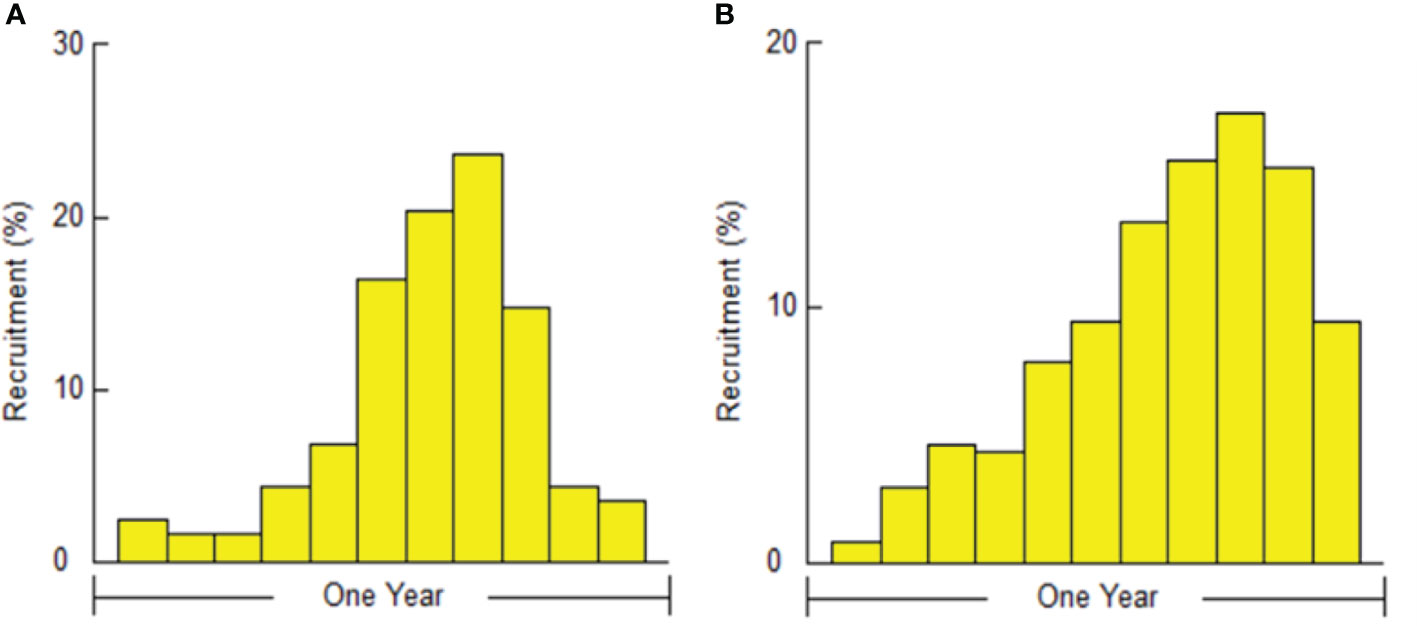
Figure 12 The perceptual recruitment pattern of (A) male and (B) female P. adspersus from the southeast Caspian Sea (2019–2020).

Figure 13 The perceptual recruitment pattern of (A) male and (B) female P. elegans from the southeast Caspian Sea (2019–2020).
The estimated theoretical longevity was 3.98 years for the males and 4.487 for the females of P. adspersus, while those of P. elegans were as 3.14 years and 3.29 years for males and females, respectively. As the findings represented, the females of both shrimps enjoyed greater longevity than the males. These findings, at least from our data, suggest that these two shrimps enjoy a longer life expectancy among shrimp species. The estimated Z, using length-converted catch curves (Figures 14, 15), were 3.35 (CI: 2.45-4.25) year-1 for the males and 2.66 (CI: 1.47-3.86) year-1 for the females of P. adspersus and 3.78 (CI: 1.68-5.88) year-1 for the males and 3.33 (CI: 2.38-4.29) year-1 for the females of P. elegans. When the Z value is transformed to percentage, a high mortality rate is obtained from 93% for P. adspersus females to 97.72% for P. elegans males, with a mean value of 94.75% for P. adspersus and 97.07% for P. elegans by the end of the first year of life. The rate of total mortality of P. elegans for both sexes was higher than that of P. adspersus. This, in turn, implies that the P. elegans shrimp is more sensitive to death than the P. adspersus. Generally, the estimated Z of males was higher than that of females regardless of the species, giving evidence for the shorter life span of the males of the shrimps.
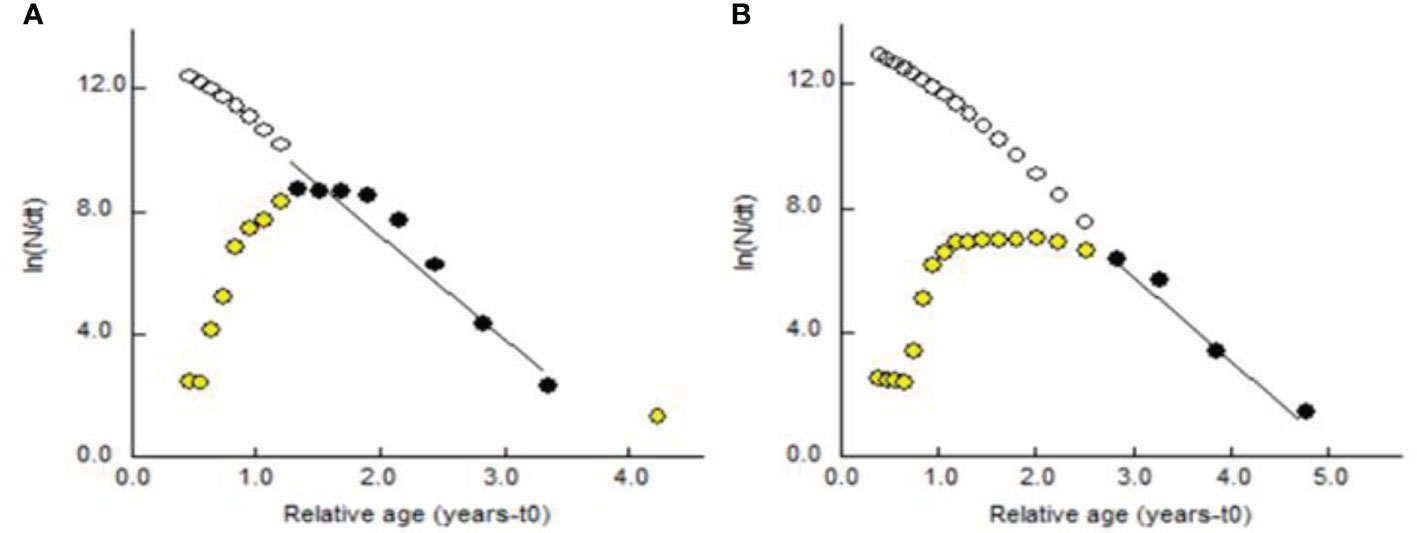
Figure 14 Length-converted catch curve of (A) male and (B) female P. adspersus from the southeast Caspian Sea (2019–2020).
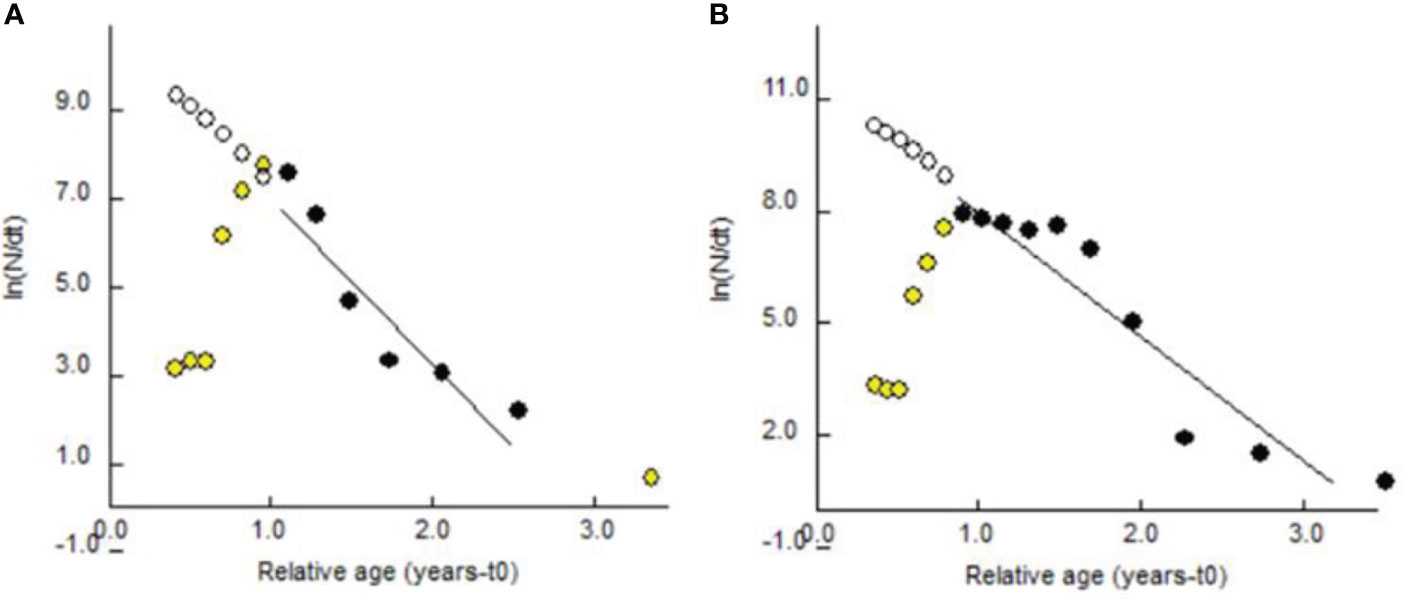
Figure 15 Length-converted catch curve of (A) male and (B) female P. elegans from the southeast Caspian Sea (2019–2020).
Discussion
In the Caspian Sea, P. adpsersus and P. elegans have been observed year-round in shallow waters (>1.5 m) in Gorgan Bay. This observation clearly reveals a continuous presence and the establishment of a self-sustaining population of the shrimps. Despite the high abundance of shrimps in the southern Caspian Sea, there is no fishery activity on the shrimps in the Iranian waters of the sea. In the existing condition, the seasonal heterogeneity of the shrimps both in distribution and abundance can be attributed to the life history strategy and population dynamics.
The shrimps are widely distributed in the coastal waters of the southern Caspian Sea. However, generally, P. adspersus–biased abundance was observed and the overall abundance of P. elegans was comparatively low in the study area. The dominance as bias toward P. adspersus seems to be a general trend between Palaemon species in the southeastern Caspian Sea, covering Anzali lagoon (southwest of the sea) eastward to Gorgan Bay (southeast of the sea) and vice versa for P. elegans (Abdolmaleki et al., 2003; Gharaei et al., 2005; Hajimoradloo et al., 2007; Sadeghi et al., 2013; Vejan et al., 2018). Because the abundance of the shrimps is different in the southern Caspian Sea regionally, we expect that the distribution pattern of the shrimps in our study area could be quite different between species. These differences can be caused by very diverse environmental factors (Berglund and Bengtsson, 1981; Lapinska and Szaniawska, 2006; Schaffmeister et al., 2006).
Additionally, there were seasonal fluctuations in the abundance of the shrimps in the study area with peak abundance in fall for both species. We could not find any comparable data on the seasonal abundance of the shrimps in the Caspian Sea. The clear differences in abundance found between the two species illustrate the important role of geographical location and season as factors regulating the abundance of the shrimps in the southern Caspian Sea. However, compared to other areas of the southern Caspian Sea, such as Anzali wetland-southwest Caspian Sea (Abdolmaleki et al., 2003; Abdolmaleki et al., 2005) and the central part of the southern coast of Caspian Sea (Gharaei et al., 2005), the coastal habitats of southeastern Caspian such as the mouth of Gorgan Bay support a much higher abundance of P. adspersus.
Variation in size among the populations of a species is pronounced on many occasions. The Palaemon shrimps from the southeast Caspian Sea are considered among the large-sized populations of the species; P. adspersus reaches up to 74-mm and P. elegans 57.47-mm TL. The shrimps reaching the largest sizes were female P. adspersus with 82-mm TL from the Black Sea (Bilgin et al., 2009b) and with 76.20 mm TL from Gorgan Bay (Vejan et al., 2018), and female P. elegans with 69 and 60 mm TL from Black Sea (Duran et al., 2006; Janas and Mańkucka, 2010). These data confirm that the populations of these shrimps present different sizes in their geographical distribution range.
Compared to previous studies, the largest individual caught was larger than other specimens observed in the southern Caspian: female P. adspersus 56.3 mm (TL) in Gomishan wetland (Hajimoradloo et al., 2007), female P. adspersus 59.3 mm (TL) in Anzali lagoon (Abdolmaleki et al., 2005), female P. elegans 71 mm (TL) in the central region of southern Caspian Sea (Gharaei et al., 2005), female P. elegans 44.7 mm (TL) in Gomishan wetland, female P. elegans 43.4 mm (TL) in Anzali wetland (Abdolmaleki et al., 2003), female P. elegans 52.2 mm (TL) in the central region of southern Caspian Sea (Gharaei et al., 2005). These results suggest that the habitat conditions in Gorgan Bay favored the growth of these shrimps, thus favoring the development of relatively large specimens of the shrimps studied herein.
Furthermore, we found that males were smaller than females, representing size-based sexual dimorphism in both P. adspersus and P. elegans. Sexual dimorphism in size observed in many species of genus Palaemon (Figueras, 1984; Berglund and Rosenqvist, 1986; Klaoudatos and Tsevis, 1987; Sanz, 1987; Omori and Chida, 1988; Guerao et al., 1994; Anger and Moreira, 1998; Guerao and Ribera, 2000). Generally, sexual dimorphism related to size is observed in the shrimp species, where female individuals are mainly bigger and heavier than male individuals, and is probably related to the reproductive process and considered an adaptation that increases energy investment in reproduction (Hartnoll, 1982; Gab-Alla et al., 1990; Yamada et al., 2007).
Based on the results, P. adspersus growth was positively allometric, where the b-value was >3. It means that based on the relationship curve between total length and the body weight, the length gain was not as fast as the increase in weight. Both male and female P. adspersus exhibit positive allometry in the relationships. On the contrary, in the P. elegans, the coefficient b of the length–weight relationship revealed a negative allometric growth for males and positive for females. It means that as the length increases, the weight is heavier in females than in males. The difference in the slopes between regression lines for males and females represents that male and female P. elegans have different growth patterns. It can also indicate a clear sexual difference in the growth type in P. elegans as interpreted different energy investment in growth between males and females.
Positive allometry has also been observed for females P. adspersus in many other studies (e.g., Conides et al., 1992; Guerao and Ribera, 1995; Hajimoradloo et al., 2007; Bilgin et al., 2009b; Vejan et al., 2018). However, in some studies with P. adspersus, negative allometry for females was found (e.g., Manent and Abella-Gutiérrez, 2006; Glamuzina et al., 2014). Also, both positive (e.g. Guerao and Ribera, 1995; Manent and Abella-Gutiérrez, 2006; Hajimoradloo et al., 2007) and negative (e.g., Conides et al., 1992; Bilgin et al., 2009b; Glamuzina et al., 2014; Vejan et al., 2018) allometric growth was observed for male P. adspersus in previous studies. In studies with P. elegans, negative allometry was found for both the males and females of most of the studied populations (e.g., Başçinar et al., 2002; Abdolmaleki et al., 2003; Duran et al., 2006; Janas and Mańkucka, 2010), and rarely positive allometry (e.g., Sanz, 1987; Bilgin et al., 2009a; Sadeghi et al., 2013).
The variations in the growth type of different populations of P. adspersus and P. elegans, such as other shrimp species, could be described mainly by variability in body measurements throughout the life process (Dall et al., 1990; Albertoni et al., 2003) and habitat condition (Schaffmeister et al., 2006) of the shrimp populations under study. Considering these factors, variability in growth type is common among different populations of a species, and it is usually recognizable between co-occurring species such as P. adspersus and P. elegans depending on the growth characteristics of the species.
P. adspersus and P. elegans exhibited a year-round recruitment due to its sinchronotopic recruitment pattern. The absence of previous studies on recruitment patterns in these palaemonids in the southern Caspian Sea makes it difficult to hypothesize on the subject and subsequent conclusion. However, the results stress different recruitment patterns between the two shrimps. This difference is probably related to variation in the reproductive activity of the shrimps. Even though both shrimps showed one peak of recruitment in one season of recruitment during a year, the differentiation of the recruitment pattern between the males and females of the shrimps is difficult to elucidate, probably as a consequence of the spatial segregation of length–frequency between males and females. This could be attributed to the unequal size distribution of males and females of the shrimps at the same spot of the study area. In general, as the recruitment analysis and these data reflect, we can make some inferences on the generation of the shrimps in the southeast Caspian Sea. The generations of the shrimps were separated, as determined by the modal distinction of the cohorts and recruitment patterns. Totally, the generation pattern of P. adspersus was different from that of P. elegans.
The estimated L∞ and K of P. adspersus and P. elegans were apparently dissimilar. Comparable interspecies dissimilarity in growth patterns has been documented for the shrimps by Abdolmaleki et al. (2003) and Vejan et al. (2018) in the southern Caspian Sea. Abdolmaleki et al. (2003) obtained L∞ of 44.5 mm and K of 2.1 year-1 for the females and L∞ of 35.3 mm and K of 2.3 year-1 for the males of P. elegans in Anzali lagoon, southwest Caspian Sea, and Vejan et al. (2018) estimated L∞ and K of 78.75 mm and 1.10 year-1 for females and L∞ and K of 66.15 mcm and 0.71 year-1 for the males of P. aspersus in Gorgan Bay, southeast Caspian Sea. The estimated asymptotic length (L∞) of P. elegans for the studied population was considerably higher than for the shrimp population from the Anzali lagoon (Abdolmaleki et al., 2003), while the results obtained for P. adspersus were approximately the same and there is an only minor difference in the asymptotic length (L∞) between the males of the two studies. The difference of approximately 5 mm between males in the two studies, however, does not seem to be significant in a species that reaches approximately 70 mm (TL).
The asymptotic length (L∞) for the southern Caspian population of P. adspersus is higher than those estimated in the Mediterranean Sea (Manent and Abella-Gutiérrez, 2006), Adriatic Sea (Glamuzina et al., 2014), and Black Sea (Bilgin et al., 2009b); however, all these populations of P. adspersus have considerably lower values of L∞: 47.8 mm for females and 34.14 mm for males in the western Mediterranean Sea (Manent and Abella-Gutiérrez, 2006), 72.45 mm for females and 58.98 mm for males in the Adriatic Sea (Glamuzina et al., 2014), and 62.99 mm for females and 49.63 for males in the Black Sea (Bilgin et al., 2009b). Thus, it is suggested that the shrimp gets larger asymptotic length in the Caspian Sea. Different L∞ of the same species from different locations can be caused by environmental conditions (Przybylski, 1996; Tsoumani et al., 2006) and/or by factors such as heredity, genetics, diseases, and parasites (Albertoni et al., 2003).
It is important to note that both L∞ and K parameters vary among the populations of these shrimp species (Conides et al., 1992; Başçinar et al., 2002). This suggests that the growth parameters of the shrimps can differ between areas and years. Generally speaking, because of the high variation in these parameters among populations and locations, the direct comparison of L∞ and K values is not biologically feasible. Therefore, using the index of growth performance (ϕ′) may be useful to compare and differentiate the confidence of the estimated growth parameters of the populations of a species (Munro and Pauly, 1983; Pauly, 1983; Pauly and Munro, 1984; Gayanilo et al., 2005). Taking into consideration the values of phi-Monro (ϕ′) obtained in this study that fall within the range of 3.09–3.83 reported for different populations of P. adspersus. Therefore, the results of the L∞ and K of this work are acceptable. According to the results, the females of both shrimps showed larger L∞ and males higher K. This fact emphasizes intrapopulation variations in the growth of Palaemon species. The results are supported by the hypothesis of sexual dimorphism in Palaemon species, explaining any difference between the sexes of a species.
Up to now, there are no published data on the longevity of P. adspersus and P. elegans in the southern Caspian Sea. However, based on the results of this study, the maximum life span differs considerably between P. adspersus and P. elegans. In the southern Caspian Sea, both the males and females of P. elegans show a longevity of more than 3 years and for male and female P. adspersus, more than 3 and 4 years, respectively.
These results also accord with our earlier observations on Palaemon species, which showed that the longevity of species may be related to species growth characteristics, leading to considerable variability in life span among the shrimp species (Conides et al., 1992; Duran et al., 2006; Bilgin et al., 2009b; Glamuzina et al., 2014). P. adspersus presented larger asymptotic length and longevity than P. elegans and, conversely, lower values of K. Therefore, it is suggested that P. adspersus has a longer life span when compared to P. elegans. This finding is in agreement with the conclusion that species with a lower K-value have longer longevity in shrimp species (Hartnoll, 1982; Pauly and Munro, 1984; Dall et al., 1990; Gab-Alla et al., 1990; Kevrekidis and Thessalou-Legaki, 2011). One of the problems with estimating longevity is that in most cases, longevity is overestimated. However, the longevity of both P. adspersus and P. elegans seems to be not overestimated and the result seems reasonable for these shrimps. Future research efforts ought to validate our preliminary longevity estimates for these Palaemon shrimps.
The estimates of the total mortality rate (Z) varied between P. adspersus and P. elegans. Abdolmaleki et al. (2003) reported the Z value of 2.38 year-1 for the females and 2.50 year-1 for the males of P. elegans in the Anzali lagoon, while Vejan et al. (2018) obtained Z of 3.74 year-1 for females and of 2.88 year-1 for males of P. adspersus in Gorgan Bay, southeast Caspian Sea. These authors partially attributed such a high estimated rate to both shrimps. In the assessment made by Glamuzina et al. (2014) of the P. adspersus shrimp population in the Adriatic Sea, the total mortality rate approached the high value of 3.72 year-1 and 3.79 year-1 for males and females, respectively. The total mortality herein reported may seem to lie in a higher range.
In terms of the comparison between the two shrimps, P. elegans had higher Z than P. adspersus. These results indicate that in the southeastern waters of the Caspian Sea, P. elegans shrimps die more than P. adspersus as a result of different causes, which takes place in both sexes of P. elegans at a relatively similar higher rate. We assumed that the total mortality rate can vary in different habitats. This could be associated with diverse factors such as food availability, competition for territories, the relative abundance of predators, and migration. From the factors mentioned, migration causes an error for the underestimation or overestimation of Z-values in most cases, which is itself correlated with variability in water temperature and the availability of food in habitats (Feng et al., 1982).
Conclusion
This study raises our awareness and understanding on the population dynamics of P. adspersus and P. elegans. Taking advantage of the unique population parameters of the shrimps in the southern Caspian Sea, the results in the present work can answer the hypotheses addressed here, contributing to the life history measures of these shrimps. A comparison between the population parameters of these two shrimp species suggests that population dynamics should differ considerably. In general, when the two species are compared, the growth parameters of P. elegans are much different from P. adspersus. Compared to P. adspersus, P. elegans showed a high K value of VBGF, small L-infinity, low longevity, and high mortality rates, presumably adaptive traits linked to its life history, which is considered to be more similar to r-selected species. The nature of differences between the shrimps can be intrinsic to the species’ life history.
The analysis here reported provides data-supporting measures to ensure shrimps’ life history differentiation. As a general conclusion, the results hint that the growth characteristics of P. adspersus differ considerably from those of the P. elegans, indicating that the shrimps have successfully evolved different growth patterns. It thus suggests an interspecific difference in life history traits between two shrimps. Given all around the year of residency in the coastal areas, the shrimps make ideal candidates as an indicator of benthic species in the dynamic nature of the southern Caspian coast ecosystem. Likewise, an analysis of the feasible migration activity that these shrimps perform is considered highly desirable since there still remains a paucity of evidence, although it seems improbable that these shrimps have a regulatory migration between the areas of the sea. Further research is required to assess the dynamic linking of the environmental condition of the region with the population parameters of these species. This would enable us to elucidate the functional role of the shrimps in the coastal habitats of the Caspian Sea, which has been previously hypothesized for many benthic organisms in the coastal estuaries.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The shrimp samples and data were collected under the approval of the Department of Environment of Golestan Province, Iran, and with the support of the Fisheries Organization of Golestan Province, Iran.
Author contributions
AV designed the study, collected field data, and carried out the analyses. RP analyzed the data and wrote the manuscript. HJ, MG, HA, and SA collected field data, gave input, and proofread the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was partially supported by Gonbad Kavous University as PhD thesis project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdolmaleki Sh., Emadi H., Ahmadi M. R., Valinasab T. (2005). Spawning season, fecundity and LM50% of the Caspian Sea prawn (Palaemon adspersus) in the coastal water of guilan province. Iran. Sci. Fish. J. 14, 59–72.
Abdolmaleki Sh., Emadi H., Nezami Sh. (2003). Populations dynamics and some biological aspects of palaemon elegans in the guilan province waters. Iran. Sci. Fish. J. 12, 109–126.
Aladin N. V., Plotnikov I. S., Filippov A. A. (2001). Opportunistic settlers in the aral Sea, firs international meeting “the invasion of the Caspian Sea by the comb jelly mnemiopsis problems, perspectives, need for action”, Baku, azarbaijan. Proceeding meeting 9, 24–26.
Aladin N. V., Plotnikov I. S., Filippov A. A. (2002). “Invaders in the Caspian sea,” in Invasive aquatic species of Europe. Eds. Leppäkoski E., Gollash S., Olenin S. (Springer, Dordrecht: Kluver Academic), 351–359.
Albertoni E. F., Palma-Silva C., Esteves F. A. (2003). Crescimento e fator de condição na fase juvenil de farfantepenaeus brasiliensis (Latreille) e f. paulensis (Pérez-farfante) (Crustacea, decapoda, penaeidae) em uma lagoa costeira tropical do Rio de Janeiro, brasil. Rev. Bras. Zool. 20, 409–418. doi: 10.1590/S0101-81752003000300008
Anger K., Moreira G. S. (1998). Morphometric and reproductive traits of tropical caridean shrimps. J. Crus. Biol. 18 (4), 823–838. doi: 10.2307/1549156
Baden S. P., Pihl L. (1984). Abundance, biomass and production of mobile epibenthic fauna in zostera marina (L.) meadows, western Sweden. Ophelia 23, 65–90. doi: 10.1080/00785236.1984.10426605
Baker R., Fujiwara M., Minello T. J. (2014). Juvenile growth and mortality effects on white shrimp litopenaeus setiferus population dynamics in the northern gulf of Mexico. Fish. Res. 155, 74–82. doi: 10.1016/j.fishres.2014.02.026
Başçinar N. S., Düzgüneş E., Başçinar N., Sağlam H. E. (2002). A preliminary study on reproductive biology of palaemon elegans rathke 1837 along the south- eastern black Sea coast. Turk. J. Fish. Aqua. Sci. 2, 09–111.
Berglund A. (1982). Coxistence, size overlap and population regulation in tidal vs. nontidal palaemon prawns. Oecol 54, 1–70. doi: 10.1007/BF00541099
Berglund A., Bengtsson J. (1981). Biotic and abiotic factors determining the distribution of two prawn species: Palaemon adspersus and p. squilla. Oecol 49, 300–304. doi: 10.1007/bf00347589
Berglund A., Rosenqvist G. (1986). Reproductive costs in the prawn palaemon adspersus: effects on growth and predator vulnerability. OIKOS 46, 349–354. doi: 10.2307/3565833
Bilgin S., Ozen O., Samsun O. (2009a). Sexual seasonal growth variation and reproduction biology of the rock pool prawn, palaemon elegans (Decapoda: Palaemonidae) in the southern black Sea. Sci. Mar. 73 (2), 239–246. doi: 10.3989/scimar.2009.73n2239
Bilgin S., Samsun O., Ozen O. (2009b). Seasonal growth and reproduction biology of the Baltic prawn, palaemon adspersus (Decapoda: Palaemonidae), in the southern black Sea. J. Mar. Biol. Assoc. UK 89 (3), 509–519. doi: 10.1017/S0025315408003056
Campbell A. (1994). Seashores and shallow seas of Britain and Europe (London: Hamlyn Publ. Group Ltd).
Conides A., Tsevis N., Klaoudatos S. (1992). Somatic measures and mortality of the prawn palaemon adspersus (Rathke 1837) in messolonghi lagoon, western Greece. Fres. Env. Bull. 1, 468–471.
Dall W., Hill B. J., Rothlisberg P. C., Sharples D. J. (1990). The biology of the penaeidae (London: Academic Press).
Diop H., Keithly W. R., Kazmierczak R. F., Shaw R. F. (2007). Predicting the abundance of white shrimp (Litopenaeus setiferus) from environmental parameters and previous life stages. Fish. Res. 86, 31–41. doi: 10.1016/j.fishres.2007.04.004
Dolbeth M., Cusson M., Sousa R., Pardal M. A. (2012). Secondary production as a tool for better understanding of aquatic ecosystems. Can. J. Fish. Aqua. Sci. 69, 1230–1253. doi: 10.1139/f2012-050
d’Udekem d’Acoz C. (1999). Inventaire et distribution des crustace ´s de´capodes de l’Atlantique nord-oriental, de la mediterrane ´e et des eaux continentales adjacentes au nord de 258N (Paris: Service Patrimoine Naturel, Muse´um National d’Histoire Naturelle).
Duran M., Suiçmez M., Kayim M., Kaynar C. (2006). Preliminary analysis of the biological characteristics of palaemon elegans (Decapoda, palaemonidae) in the coast of sinop, black Sea, n. Turkey. Pak. J. Biol. Sci. 9 (5), 848–853. doi: 10.3923/pjbs.2006.848.853
Feng Z. Q., Sun J. H., Yang G. B., An X. S. (1982). A discussion of acetes chinensis resources in the western areas of bohai Sea and their rational use. Trans. Oceanol. Limnol. 4, 62–68.
Figueras A. J. (1984). Biologia y pesca del camaron (Palaemon adspersus y palaemon serratus) en la ria de vigo (Spain: Obradoiro, Universidad de Santiago de Compostela).
Gab-Alla A. A. F. A., Hartnoll R. G., Ghobashy A. F., Mohammed S. Z. (1990). Biology of penaeid prawns in the Suez canal lakes. Mar. Biol. 107, 417–426. doi: 10.1007/BF01313423
Gayanilo F. C., Sparre P. Jr., Pauly D. (2005). FAO-ICLARM stock assessment tools II (FiSAT II) - revised version, user’s guide, no. 8 (Rome: FAO Computerized Information Series (Fisheries).
Gharaei A., Ahmadifar N., Sourinezhad I., Alavi M. S. (2005). “Investigation of some biological characteristics of two shrimps palaemon adspersus and palaemon elegans in southern Caspian Sea (Nour coast),” in Proceeding of 6th conference of marine science and technology, Tehran, Iran, publications of Atmospheric Science Centre. 1–11.
Ghorbani R., Baghfalaki M., Shalouei F. (2012). The Caspian Sea environment (Gorgan, Iran: Publications of Gorgan University of Agricultural Sciences and Natural Resources).
Glamuzina L., Conides A., Prusina I., Ćukteraš M., Klaoudatos D., Zacharaki P., et al. (2014). Population structure, growth, mortality and fecundity of palaemon adspersus (Rathke 1837; decapoda: Palaemonidae) in the parila lagoon (Croatia, SE Adriatic Sea) with notes on the population management. Turk. J. Fish. Aqua. Sci. 14, 677–687. doi: 10.4194/1303-2712-v14_3_10
Guerao G., Perez-Baquera J., Ribera C. (1994). Growth and reproductive biology of palaemon xiphias risso 1816 (Decapoda: Caridea: Palaemonidae). J. Crust. Biol. 14 (2), 280–288. doi: 10.1163/193724094X00272
Guerao G., Ribera C. (1995). Growth and reproductive ecology of palaemon adspersus (Decapoda, palaemonidae) in the western Mediterranean. Ophel 43 (3), 205–213. doi: 10.1080/00785326.1995.10429832
Guerao G., Ribera C. (2000). Population characteristics of the prawn palaemon serratus (Decapoda, palaemonidae) in a shallow Mediterranean bay. Crust 73 (4), 459–468. doi: 10.1163/156854000504543
Guerra S. A., Sánchez J. L. (1998). Fundamentos de explotación de recursos vivos marinos (Zaragoza, España: ACRIBIA).
Hajimoradloo A., Ziaei R., Chitsaz H., Ghorbani R. (2007). A survey of some morphometric and reproductive traits of palaemon adspersus rathke 1937 in gomishan lagoon (southeast of Caspian Sea). J. Agric. Sci. Nat. Res. 14 (1), 231–239.
Hartnoll R. G. (1982). “Growth,” in The biology of Crustacea. Ed. Bliss D D. (New York, NY: Academic Press), 111–185.
Janas U., Mańkucka A. (2010). Body size and reproductive traits of palaemon elegans Rathke,1837 (Crustacea, decapoda), a recent colonizer of the Baltic Sea. Oceanol. Hydrobiol Stud. XXXIV (2), 3–24. doi: 10.2478/V10009-010-0016-6
Kevrekidis K., Thessalou-Legaki M. (2011). Population dynamics of melicertus kerathurus (Decapoda: Penaeidae) in thermaikos gulf (N. Aegean Sea). Fish. Res. 107 (1–3), 46–58. doi: 10.1016/j.fishres.2010.10.006
Klaoudatos S., Tsevis N. (1987). Biological observations on palaemon adspersus (Rathke) at messolonghi lagoon. Thalassograph 10, 73–88.
Lapinska E., Szaniawska A. (2006). Environmental preferences of crangon crangon (Linnaeus 1758), palaemon adspersus, rathke 1837, and palaemon elegans rathke 1837, in the litoral zone of the gulf of gdansk. Crust 79 (6), 649–662. doi: 10.1163/156854006778026799
Le Cren E. D. (1951). The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 20, 201–219. doi: 10.2307/1540
Manent P., Abella-Gutiérrez J. (2006). Population biology of palaemon adspersus rathke 1837 (Decapoda, caridae) in fornells bay, Balearic islands, Western Mediterranean. Crust 79 (11), 1297–1308. doi: 10.1163/156854006779277286
Moreau J., Cuende F. (1991). On improving the resolution of the recruitment patterns of fishes. Fishbyte 9, 45–46.
Munro J. L., Pauly D. (1983). A simple method for comparing the growth of fishes and invertebrates. Fishbyte 1, 5–6.
Omori M., Chida Y. (1988). Life history of a caridean shrimp palaemon macrodactylus, with special reference to the difference in reproductive features among ages. Nip. Suis. Gakk 54 (3), 365–375. doi: 10.2331/suisan.54.365
Pauly D. (1983). Length-converted catch curves: a powerful tool for fisheries research in the tropics (Part II). ICLARM Fish. 2, 17–19.
Pauly D. (1987). “A review of the ELEFAN system for analysis of length-frequency data in fish and aquatic invertebrates,” in Length-based models in fisheries research, vol. Vol. 13 . Eds. Pauly D., Morgan G. R.. ICLARM Conference Proceedings. (Manila, Philippines: International Center for Living Aquatic Resources Management; Safat, Kuwait: Kuwait Institute for Scientific Research), 7–34
Pauly D., Munro J. L. (1984). Once more on the comparison of growth in fish and invertebrates. Fishbyte 1 (21).
Przybylski M. (1996). Variation in fish growth characteristics along a river course. Hydrobiol 325, 39–46. doi: 10.1007/BF00023666
Sadeghi M. S., Yelghi S., Ghorbani R., Enaiatmehr M. (2013). Survey some of biological characteristics, palaemon elegans in associated with gomishan wetland pools, Caspian Sea. New Technol. Aquacul. Devel. (J. Fish.- Azadshar Azad Uni.) 7 (2), 31–42.
Sanz A. (1987). Biologia de Palaemon elegans rathke 1837 (Natantia: Palaemonidae) en las costas del mediterráneo occidental. Inv. Pesq. 51 (Supl. 1), 177–187.
Schaffmeister B. E., Hiddink J. G., Wolff W. J. (2006). Habitat use of shrimps in the intertidal and shallow subtidal seagrass beds of the tropical banc d’Arguin, Mauritania. J. Sea Res. 55, 230–243. doi: 10.1016/j.seares.2005.10.003
Sparre P., Venema S. C. (1992). “Introduction to tropical fish stock assessment,” in Part 1. manual. FAO fisheries technical paper, no. 306. (Rome: FAO).
Tsoumani M., Liasko R., Moutsaki P., Kagalou I., Leonardos I. (2006). Length-weight relationships of an invasive cyprinid fish (Carassius gibelio) from 12 Greek lakes in relation to their trophic states. J. Appl. Ichthyol. 22, 281–284. doi: 10.1111/j.1439-0426.2006.00768.x
Vejan A., Alijanpour S., Patimar R., Jorjani E., Bahalkeh A. (2018). Some parameters of population dynamics of palaemon adspersus (Rathke 1837) in the southeast of the Caspian Sea (Gorgan bay). J. Aqua. Ecol. 8 (2), 31–40.
Veloso V. G., Neves G., Capper L. A. (2011). Sensitivity of a cirolanid isopod to human pressure. Ecol. Ind. 11, 782–788. doi: 10.1016/j.ecolind.2010.10.004
Yamada R., Kodama K., Yamakawa T., Horiguchi T., Aoki I. (2007). Growth and reproductive biology of the small penaeid shrimp trachysalambria curvirostris in Tokyo bay. Mar. Biol. 151, 961–971. doi: 10.1007/s00227-006-0536-5
Keywords: population structure, growth, recruitment, mortality, longevity, Palaemon adspersus Palaemon elegans, Caspian Sea
Citation: Vejan A, Patimar R, Jafaryan H, Gholizadeh M, Adineh H and Aghilinezhad SM (2022) Comparative population dynamics of two sympatric Palaemon shrimps (Palaemon adspersus Rathke, 1836 and Palaemon elegans Rathke, 1836) from the Southeast Caspian Sea. Front. Mar. Sci. 9:928445. doi: 10.3389/fmars.2022.928445
Received: 25 April 2022; Accepted: 01 August 2022;
Published: 19 August 2022.
Edited by:
Pedro Morais, Florida International University, United StatesReviewed by:
Enrique González-Ortegón, Spanish National Research Council (CSIC), SpainLevent Bat, Sinop University, Turkey
Copyright © 2022 Vejan, Patimar, Jafaryan, Gholizadeh, Adineh and Aghilinezhad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rahman Patimar, cnBhdGltYXJAeWFob28uY29t
 Alti Vejan
Alti Vejan Rahman Patimar
Rahman Patimar