- 1Guangdong Provincial Key Laboratory of Marine Disaster Prediction and Prevention, Shantou University, Shantou, China
- 2Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), Guangzhou, China
- 3Department of Marine Sciences, University of Connecticut, Groton, MA, United States
How coral–Symbiodiniaceae mutualistic symbiosis is established, maintained, and disrupted is arguably the most fundamental and central area of coral research. The breakdown of this symbiosis, and consequent coral bleaching, have been frequently attributed to thermal stress, although microbial attack and pollution have also been blamed. Despite the perceived intense and broad research, it is unclear whether all the potential causes have been given adequate attention and whether some important areas have been overlooked. This work aims to comprehensively review the literature on coral and Symbiodiniaceae research and provide a portrait of the current coral research landscape, hence identifying areas that require more research effort. Data of publication output were extracted from the Web of Science (WoS) from 1986 to 2022 by using the keywords “coral” and “Symbiodiniaceae.” A total of 43,089 and 3,191 papers in the coral and Symbiodiniaceae were identified, mostly published after 2002. The journal Coral Reefs was ranked first regarding the total number of publications on coral or Symbiodiniaceae. The USA, Australia, and China were the top three countries in the number of publications. The network co-occurrence analysis of all keywords in coral and Symbiodiniaceae using VOSviewer showed that biodiversity, climate change, nutrient, and survival were the central research areas in coral and Symbiodiniaceae. Among them, climate change was the most invested research field, as revealed by the high proportion of published literature, while nutrient was the most understudied area. Thematic evolution analysis revealed that nutrient enrichment combined with elevated temperature was an emerging research field about coral and Symbiodiniaceae. Besides, nitrogen is currently the most studied nutrient. The findings from this study shed light on the trends of coral and Symbiodiniaceae research in the past 36 years, current research hotspots in the field, and areas that need more research investment going forward.
Introduction
Coral reefs are one of the world’s most biodiverse and productive ecosystems (Ea and Ka-Kudla, 1997; Sully et al., 2019). Although they only cover less than 0.5% of the global oceanic area, they can provide indispensable products and services for humankind through fishery and tourism (Yap, 2013). In the coral reef ecosystem, corals are hosts to a group of exceptionally diverse dinoflagellate symbionts in the family Symbiodiniaceae (Baker, 2003). In this mutualistic relationship, coral can provide the inorganic nutrients and serve as shelter; in exchange, Symbiodiniaceae can provide its coral host with photosynthesis products (Muscatine and Porter, 1977). However, this mutualistic nutritional relationship is susceptible to environmental factors such as elevated temperature, ocean acidification, and nutrient enrichment (Davy et al., 2012; Thurber et al., 2014; Peixoto et al., 2017; Morris et al., 2019; Rädecker et al., 2021). Triggered by climate change and human activities, coral bleaching events have become widespread, and the coral reef ecosystem has been irretrievably damaged around the world in the past decades (Hughes et al., 2003; Putnam et al., 2017; Van Oppen and Lough, 2018; Sully et al., 2019). According to the IPCC Fifth Assessment Report, extensive coral bleaching is expected by 2050, and more than 95% of coral reefs will be subjected to long-term damage (Frieler et al., 2013). Therefore, how coral–Symbiodiniaceae mutualistic symbiosis is established, maintained, and disrupted is conceivably the most fundamental and central area of current coral research.
The breakdown of symbiosis between coral and Symbiodiniaceae and consequent coral bleaching have been frequently attributed to thermal stress (Baker, 2003; Yee and Barron, 2010; Lin et al., 2019). To make matters worse, one study reported that even limiting global warming to 2°C is unlikely to save most coral reefs (Frieler et al., 2013). Besides, nutrient enrichment combined with elevated temperature has been shown to cause coral bleaching historically and currently in the Red Sea (Manasrah et al., 2006; Silverman et al., 2007; Hall et al., 2018; DeCarlo et al., 2020). Furthermore, previous studies also reported that excess nutrients are detrimental to corals even at normal temperatures as they break up the symbiosis (Wiedenmann et al., 2013; Vega Thurber et al., 2014). Furthermore, microbial attack and pollution have also been blamed in recently published literature (Morris et al., 2019; Evans et al., 2020). Despite the perceived diverse and broad research, it is unclear whether all the potential causes have been given adequate attention and whether some important areas have been overlooked. Therefore, it is crucial to understand the historical development of the research field to further elucidate the transformation of the research landscape and identify future research priorities (Ubando et al., 2021). Bibliometrics, the application of mathematics and statistical methods to books and other media of communication serves as a quantitative analysis tool for written communications (Pritchard, 1969). This method is different from the traditional review of the literature and has acquired progressive importance in biomedical research (Ellegaard and Wallin, 2015; Thompson and Walker, 2015). However, the application of the method to coral and Symbiodiniaceae research is very limited, with only two examples available to us, which were limited to India coral reef research and black band disease (Mohan et al., 2011; Krishnaveni et al., 2021).
The research on coral and Symbiodiniaceae has flourished in the past decades and has accrued a considerable amount of literature parallel to the increase in coral bleaching events. This study aims to comprehensively review the research landscape of the coral and Symbiodiniaceae through a comprehensive bibliometric analysis. The first objective is to reveal the central scientific realm of current coral and Symbiodiniaceae research. The second objective is to explore potential crucial areas that are currently overlooked. The third objective is to identify a direction for future research on corals and Symbiodiniaceae based on research development trends and the research hotspots.
Methodology
Data sources
The Web of Science (WoS) database was developed by Thomson Reuters and has collected literature since 1980 (Şenel et al., 2017). Now WoS is considered a principal quality-oriented database worldwide and contains a more standardized record for retrieving the global scientific literature for multidisciplinary fields and research areas (Falagas et al., 2008; Lai et al., 2022; Zhou et al., 2022). Thus, the WoS database was used to search for the literatures on corals and Symbiodiniaceae in this study.
Search strategy
The first coral-related article recorded in the WoS database was from 1986. Therefore, we used the advanced search function to retrieve related publication on corals and Symbiodiniaceae for 36 years in the initial stage from 1986. This study defined appropriate keywords for search after reviewing highly cited literature on coral research. The search terms included the title, abstract, keywords in articles (author keywords), and keywords plus as follows: TS=((“coral”) AND (“coral” and “temperature”) AND (“Symbiodiniaceae) AND (“Symbiodiniaceae” and “temperature”) (“coral” and “Symbiodiniaceae”) AND (“coral”, “Symbiodiniaceae” and “temperature”) AND (“coral, “Symbiodiniaceae”, “temperature” and nutrient)). The keywords plus is the keyword that WoS adds relevance to the original article through clustering under a certain algorithm, which can increase the hit rate of the article relative to the author keyword alone when the reader searches for the article (Zhang et al., 2016). The name of “Symbiodiniaceae” has been changed many times, from “zooxanthella”, “zooxanthellae”, “Symbiodinium”, to “Symbiodiniaceae”. Therefore, when searching Symbiodiniaceae, the results from using these four names were synthesized. Our survey carried out on 2 January 2022 spanned 1986 to 2022. A file containing the Full Record (including author, title, source, abstract) and Cited References of each paper was downloaded and available at: https://data.mendeley.com/drafts/h6sr2ys5yf.
Summary of article information
The numbers of papers published each year, countries or regions of the authors, and the journals publishing the papers were analyzed in the WoS platform. Among them, the number of papers published each year was summarized in Microsoft Excel, and the countries or regions of the author were counted and displayed through the MapChart website. The web link of MapChart was https://mapchart.net/world.html. Only the top 10 coral or Symbiodiniaceae research countries were selected for display on the world map.
Co-occurrence of the keywords
In order to better grasp the focal areas and hotspots of coral and Symbiodiniaceae research, the bibliometric co-occurrence of keywords was conducted through VOSviewer visualization analysis (Van Eck and Waltman, 2010). The keywords used in this analysis were all keywords, including the keywords that the authors designated in the article’s keyword field and the keywords plus. Because some keywords have the same meaning in the co-occurrence map, the results from related terms such as coral-reef, coral-reefs, reef-coral, and reefs coral were merged manually. The colors of keywords indicate the cluster to which a keyword was assigned by the clustering technique developed by Van Eck (Van Eck and Waltman, 2010). Note that, in this display, and subsequently, we have ignored the search term itself (in this case, “coral” or “Symbiodiniaceae”) following previous reports, as it must always be the most frequent term and must always, therefore, dominate the map of co-occurrences (Einecker and Kirby, 2020).
Because the above-described analyses indicated that the temperature was the most extensively studied environment factor regarding corals and Symbiodiniaceae, further analyses were focused on temperature. We used a keywords scheme: TS = (“coral”, “Symbiodiniaceae”, and “temperature”) was searched again, and the relationship between temperature and other keywords and the relationship between nutrient and other keywords were highlighted in the figure. Note that the keyword “temperature” includes “temperature”, “thermal stress”, “thermal tolerance”, “heat stress”, “global warming”, “sea surface temperature”, “elevated temperature”, “temperature stress”, “temperature tolerance”, “water temperature”, “seawater temperature”, “ocean warming”, “ocean temperature”, and “heat tolerance”. Only keywords that appeared above five times were retained in the co-occurrence map.
Thematic evolution and thematic map
In order to better explore the origin, interrelationship, tendency, and value of the primary thematic areas, thematic evolution (TE) and thematic map (TM) were conducted using an R-package bibliometrix. For TE, a time span in different time slices was divided to plot and study the topic evolution using the Sankey diagram. The five time slices were set based on the marine climate change events such as El Niño in 1997, La Niña in 2007, and mass coral bleaching events in 2015, i.e., [1980–1997], [1998–2007], [2008–2014], [2015–2019], [2020–2022] for keywords plus from coral or Symbiodiniaceae research alone, and [2003–2007], [2008–2014], [2015–2017], [2019–2019], and [2020–2022] for keyword plus from the combination of coral, Symbiodiniaceae, and temperature. The parameter settings of the TM were set as follows: Field: keywords plus; Number of Words: 1000; Min Cluster Frequency: 5; Min Weight Index: 0.1; Label Size: 0.3.
For TM, each cluster/theme can be represented on a particular plot, where the X-axis is the relevance degree (Centrality) and the Y-axis is the development degree (Density) (Cobo, 2011). The Density is the strength of internal ties among all the keywords used to describe the research theme, and the Centrality is the strength of external ties to other themes by exploiting the author’s keywords field. Besides, the TM map is divided into four quadrants, namely, (1) Motor Themes, which are considered significant and well-developed in this field of research and located in the first quadrant; (2) Niche Themes, which are thought to be highly developed and isolated themes and are located in the second quadrant; (3) Emerging or Declining Themes, which are located in the third quadrant, indicating that there are no notable developments in this field of research, probably because they have just emerged or are about to disappear; and (4) Basic Themes, which are considered basic and transversal as they are located in the fourth quadrant (Cobo, 2011). The parameter settings of the TM are as follows: Field: keywords plus; Number of Words: 1000; Min Cluster Frequency: 5; Number of Labels: 3; Label Size: 0.3.
Results and discussion
Annual publication and the leading countries/regions
Totally, 43,089 and 3,191 papers related to the coral and Symbiodiniaceae were identified on WoS until 2 January 2022, respectively (Table 1). In the first 17 years (1986–2002), only 406 and 13 papers reporting coral and Symbiodiniaceae were published, respectively, and the number increased very slowly over time, only accounting for about 1% of all the studies (Figure 1). In the next 20 years (2003–2022), the number of research articles in this field increased rapidly, with the maximum number of coral research papers reaching 3,494 in 2020 alone and the maximum number of Symbiodiniaceae papers reaching 265 in 2021 alone. This may be related to the widespread coral bleaching events in 1995 and 2002 that attracted extensive attention (DeCarlo et al., 2020). Besides, 2015 was also a year of severe coral bleaching events (Johnston et al., 2020; DeCarlo et al., 2020). Because the deadline for statistics is 2 January 2022, the number of article count is small in 2022 (Figure 1).
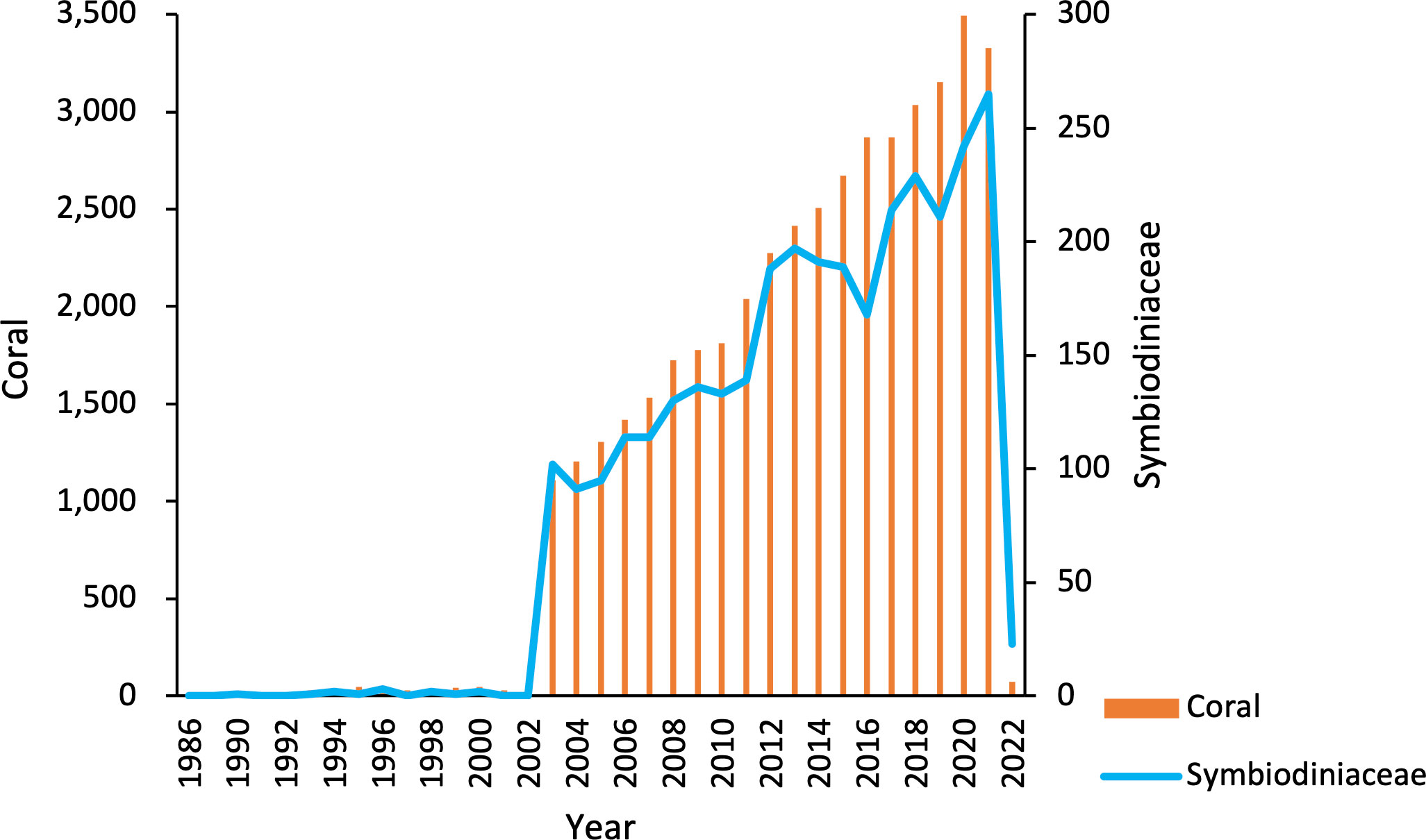
Figure 1 Annual number of published articles on coral and Symbiodiniaceae from 1986 to 2022 counted from the Web of Science (WoS).
The numbers of articles per journal for coral and Symbiodiniaceae are summarized in Tables 2, 3, respectively. The results showed that Coral Reef was the most preferred outlet not only for coral but also for Symbiodiniaceae research work. This journal published 2,102 (account for 4.88% of total publications) on corals and 293 (account for 9.18% of total publications) for Symbiodiniaceae. Marine Ecology Progress Series and PloS One were also popular journals for corals, and PloS one and the Journal of Experimental Marine Biology were another two popular journals for Symbiodiniaceae. However, most articles (77.27% for coral and 62.18% for Symbiodiniaceae) have been published in many other journals, indicating that the research works on coral and Symbiodiniaceae received the attention of many journals (Tables 2, 3).
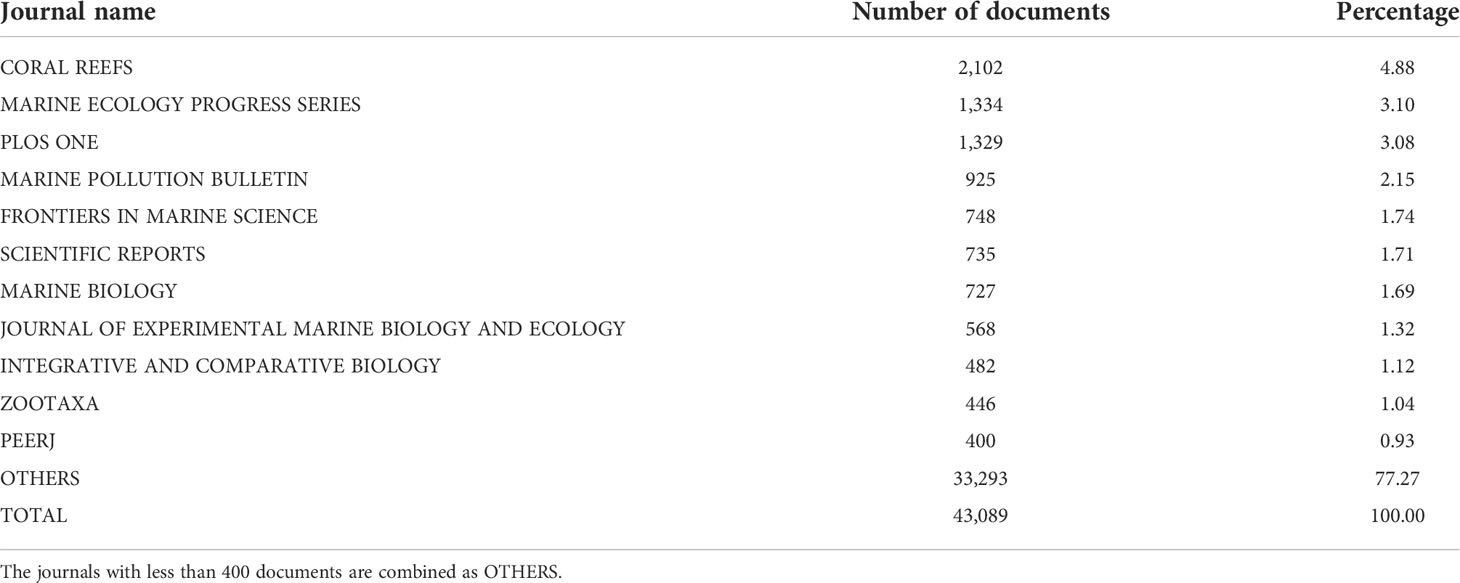
Table 2 The number of papers per journal for the keyword search on “coral” (item no. 1 in Table 1) based on the WoS (2 January 2022).
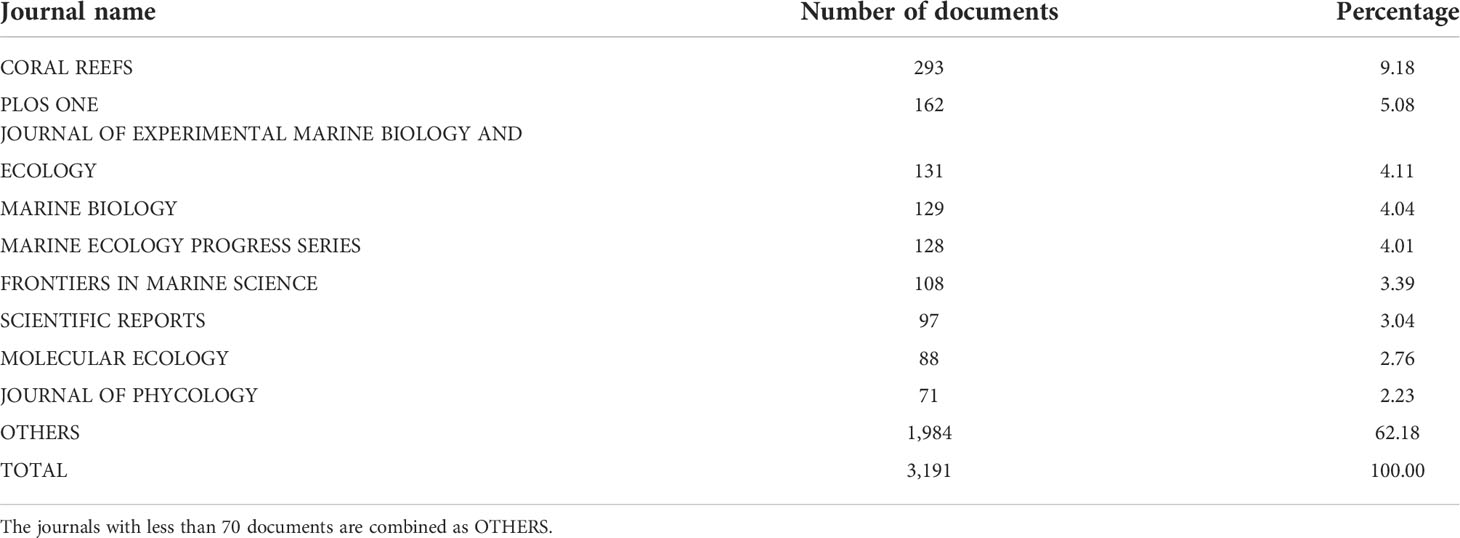
Table 3 The number of documents per journal for the keywords in Symbiodiniaceae research (item no. 3 in Table 1) based on the WoS (2 January 2022).
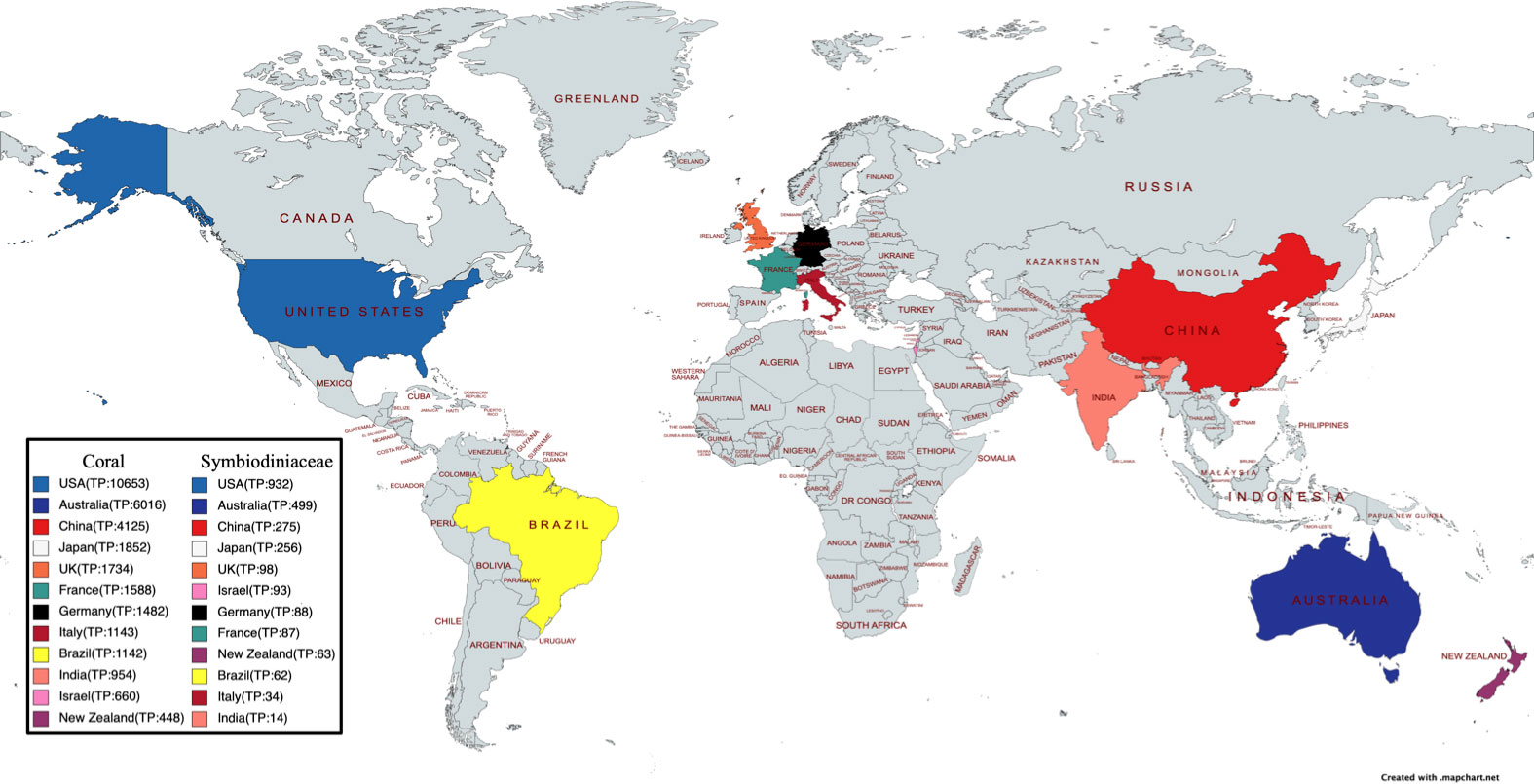
From information of the author’s affiliation, 123 countries were found to have been involved in the coral and Symbiodiniaceae research. Among them, USA (10,653 publications), Australia (6,016 publications), China (4,125 publications), Japan (1,852 publications), the UK (1,734 publications), and France (1,588 publications) were ranked the top six prolific countries in coral research. USA (932 publications), Australia (499 publications), China (275 publications), Japan (256 publications), UK (98 publications), and Israel (93 publications) were the top six productive countries in Symbiodiniaceae research. This result showed that the research on coral and Symbiodiniaceae had been mainly concentrated in economically developed countries. As such, there is a potential that the status of coral reefs in economically underdeveloped countries is less studied and understood. For example, the Philippines has a long coral reef coastline, but it is not in the top 10 countries for coral or Symbiodiniaceae research (Figure 2).
Co-occurrence of the keywords from coral or Symbiodiniaceae research
We first examined the co-occurrence of keywords related to coral research (Figure 3 and Table 4). Due to the huge number of papers published in coral, we showed the keywords through clusters and attempted to interpret the clusters by assessing the keywords most closely associated with each of the six clusters, especially the largest nodes, as size reflects the frequency of appearance (Moral-Muñoz et al., 2019). The results showed that the category (in red) including keywords of “diversity”, “pattern”, “fish”, “resilience”, “conservation”, “impacts”, and “management” was the most popular research area for corals, which can be interpreted as “biodiversity”. The second category (blue) was interpreted as “symbiosis”, which covered “Symbiodiniaceae”, “scleractinian corals”, “genome”, “mechanism”, and “coral disease”. The third category (green) can be called “climate change”, which includes “temperature”, “sea” and “sea level rise”. The fourth category (purple) can be described as “nutrient”, which contains “climate change”, “ocean acidification”, “photosynthesis”, “carbon”, “nutrient”, “phosphorus”, “nitrogen”, “copper”, and “sediment”. The fifth category (kelly) can be regarded as “survival”, which comprises “evolution”, “population”, “connectivity”, and “reproduction”. The last category (cyan), related to “growth”, “recruitment”, “mortality”, and “settlement”, was the least studied research area for corals.
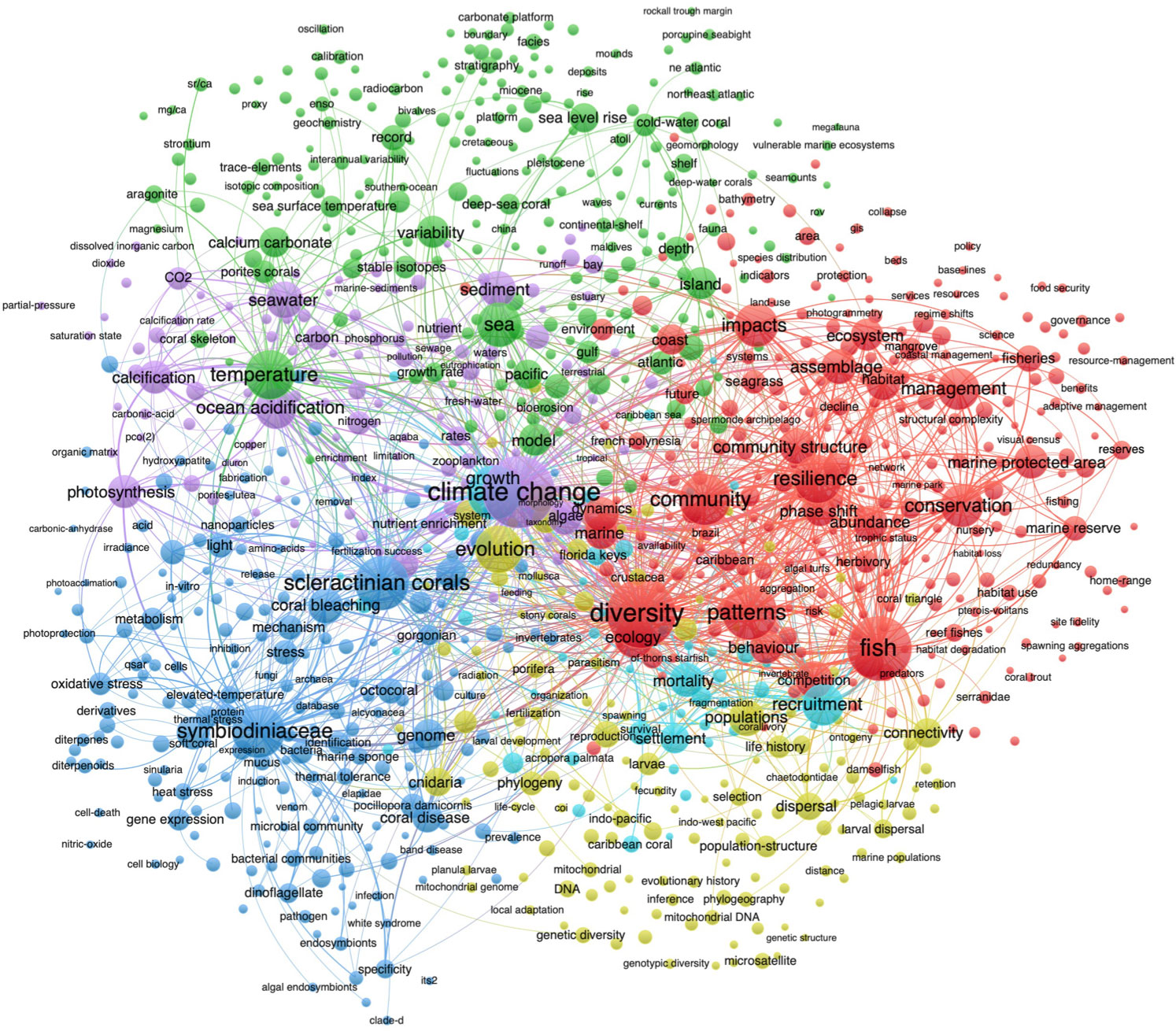
Figure 3 The bibliometric co-occurrence of the keyword clusters in coral research. Note: The color of keywords indicates the cluster to which a keyword was assigned. Only keywords that appeared over 10 times were retained. The thickness of a line indicates link strength.
For Symbiodiniaceae, the keywords used in co-occurrence analysis were from 3,191 Symbiodiniaceae articles (Figure 4; Table 5). The results showed that the first category (in Kelly), including “climate change”, “photosynthesis”, “coral bleaching”, “temperature”, “oxidative stress”, and “thermal stress”, was the most explored research area, which can be interpreted as “vulnerability”. The second popular research area (red), described as “biodiversity”, was related to “diversity”, “specificity”, “dinoflagellate”, “endosymbionts”, “genome”, and “evolution”. The third category (green) covered “coral”, “growth”, “gene expression”, “ocean acidification”, “calcification”, and “transcriptomics” and was named “mechanism”. The fourth category (blue), comprised of “coral reef”, “community”, “resilience”, “nutrient enrichment”, “nitrogen”, and “nutrient”, can be called “nutrient”. The fifth category (purple), which was related to “scleractinian coral”, “light”, “carbon”, “heterotrophy”, “fatty acid”, and “Red Sea”, was termed “CO2 fixation”. The last category (cyan), including “mortality”, “reproduction”, “microbial”, “flexibility”, “recruitment”, and “conservation”, was the least studied research area for Symbiodiniaceae and can be named “survival”.
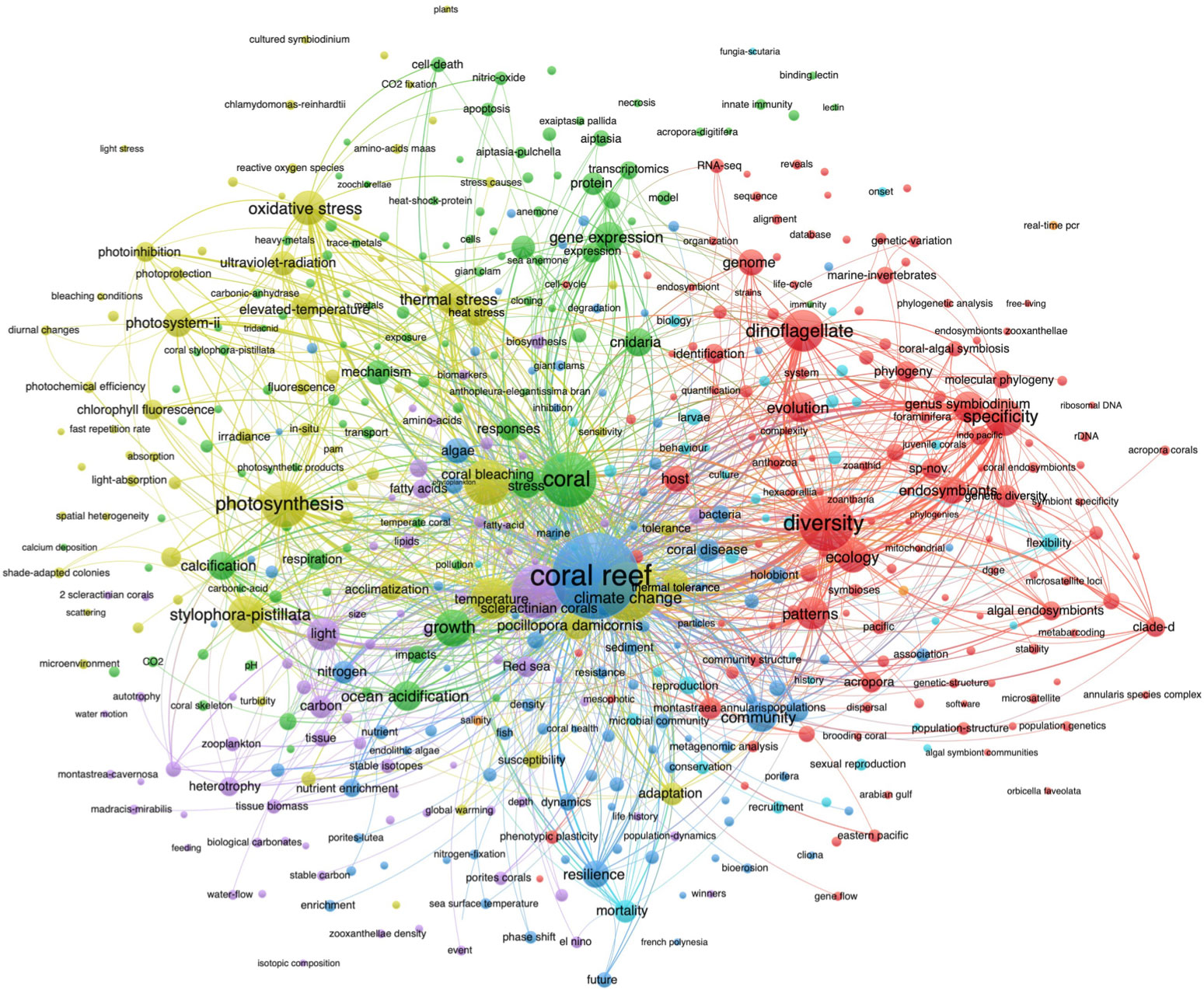
Figure 4 The bibliometric co-occurrence of the keyword clusters in Symbiodiniaceae research. Note: Only keywords that appeared over ten times were retained.
From the above result, we can see that the research fields of climate change, biodiversity, nutrient, and survival were the central research area not only for corals but also for Symbiodiniaceae (Figures 3, 4). In addition, the circle size of ocean acidification is larger than that of nutrient and most other keywords in Figure 4. However, recent indoor studies demonstrated that Symbiodiniaceae community composition was hardly affected by ocean acidification, suggesting that more field studies on Symbiodiniaceae responses to ocean acidification are required while considering the experimental period and acidification intensity (Ge et al., 2021; Zhou et al., 2021). It is worth mentioning that a field with relatively limited research is not necessarily an unpopular field but can be the latest research field. For example, the circle size of nitrogen is smaller than most other keywords in Figure 3 and Figure 4, but it is currently the prevalent environmental factor in explaining coral bleaching under elevated temperatures, especially the ammonium (Rädecker et al., 2015; Xiang et al., 2020; DeCarlo et al., 2020; Rädecker et al., 2021). Therefore, to show the development of the research associated with the latest keywords and highlight the current research hotspots, the thematic evolution over time for coral and Symbiodiniaceae was described below.
Thematic evolution of keywords over time for coral and Symbiodiniaceae
For coral, five subperiods were set to show the thematic evolution in the Sankey diagram (Figure 5). The results showed that “diversity” was the popular thematic area in coral research, which first appeared in 1998–2007 and further developed in the following three subperiods from 2008 to 2022 (Figure 5A). Besides, “climate change” was another hotspot, which appeared three times among five subperiods, and “temperature” was the only environmental factor in the Sankey diagram. Indeed, there are 7,834 reports on “coral” accompanied by “temperature”, which accounted for 18.18% of coral research (Table 1). Although extensive coral bleaching was first reported in eastern Jamaica (Goreau, 1964), the “Great Barrier Reef” was the only place in the Sankey diagram, indicating that the eastern Jamaican reef has not been a hot spot for coral research.
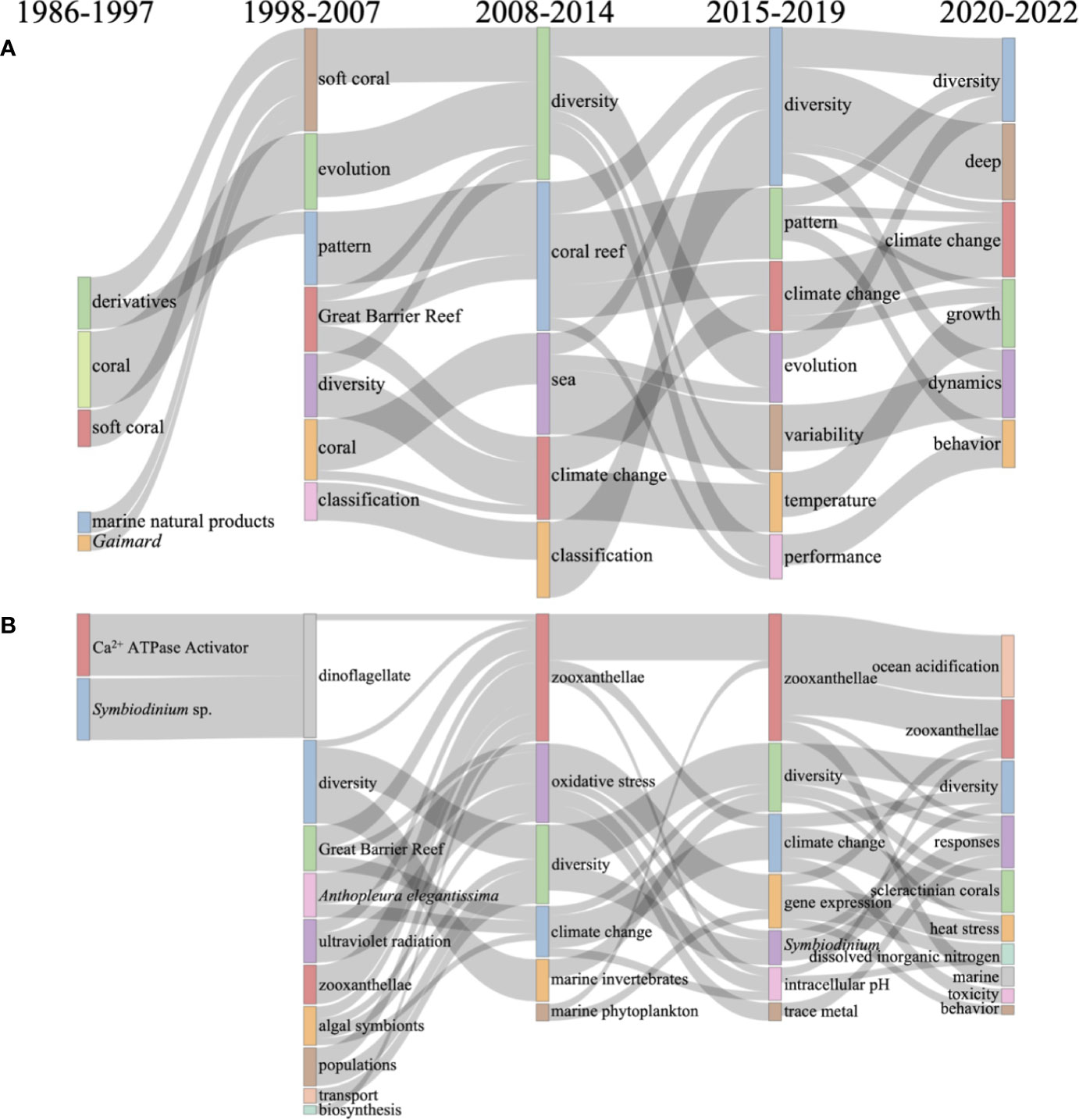
Figure 5 Thematic evolution of keywords in coral (A) and Symbiodiniaceae research (B). Each box in the map denotes a theme, and the size of the boxes is proportional to the number of documents associated with each theme. The flows connect each box showing the evolution traces of the theme, and the thicker the connecting line, the higher the linkage of the two themes.
For Symbiodiniaceae, the results showed that “Ca2+ ATPase activator” is a relatively early research field in Symbiodiniaceae as the keyword frequently appeared from 1986 to 1997, but it was not developed in the following three subperiods (Figure 5B). On the contrary, “diversity” first appeared in the subperiod of 1998 to 2007 and further developed in the following three subperiods from 2008 to 2022, showing that diversity research has always been a hot research area in Symbiodiniaceae and corals (Figure 5). Among the diversity research, metabarcoding is a crucial method for biodiversity assessment of Symbiodiniaceae communities (Smith et al., 2017). Furthermore, the ITS2 gene was the preferred primer in Symbiodiniaceae diversity research than other primers such as 23S rRNA (Thomas et al., 2014). Besides, “trace metals” was the first keyword of nutrient that appeared in the subperiod of 2015 to 2019, and another was the “dissolved inorganic nitrogen” that appeared in the subperiod of 2020 to 2022, indicating that research on nutrients in Symbiodiniaceae has attracted greater research attention in the last 7 years. Indeed, previous research found that the cnidarian host uses nitrogen limitation as a primary mechanism to control endosymbiont populations, and altered nutrient cycling (mainly nitrogen) during heat stress is a primary driver of the functional breakdown of the symbiosis (Xiang et al., 2020; Rädecker et al., 2021). However, it must be noted that the research on nutrients in coral reef ecosystems has received attention for a long time. For example, Stambler et al. reported that nutrient enrichment could slow the skeletal growth rate of Pocillopora damicornis, and Bruno et al. found that nutrient enrichment can significantly increase the severity of Gorgonia ventalina and Montastraea annularis (Stambler et al., 1991; Bruno et al., 2003). Moreover, the keyword “gene expression” appeared in the subperiod of 2015 to 2019, showing that research began to explore its internal molecular mechanisms. This is confirmed by the appearance of “RNA-seq”, “transcriptomics”, “genome”, and “mechanism” in VOSviewer (Figure 4; Table 5).
Thematic map of keywords from coral and Symbiodiniaceae research
The thematic map allows visualization of four different typologies of themes based on the two dimensions (Density and Centrality). The results showed the same distinct keyword distribution between coral and Symbiodiniaceae in the thematic map (Figure 6). For coral, the keywords “coral reef”, “climate change”, “Great Barrier Reef”, “diversity”, and “Symbiodiniaceae” were distributed in Basic Themes, the keywords “pattern”, “growth”, “communities”, “management”, and “ecology” were distributed in Motor Themes, and the keywords “evolution”, “identification”, “morphology”, “cnidaria”, “performance”, “population structure”, “natural products”, “derivatives”, “chemistry”, “toxicity” were found in Emerging or Declining Themes. Besides, no keyword was found in Niche Themes for coral (Figure 6A).
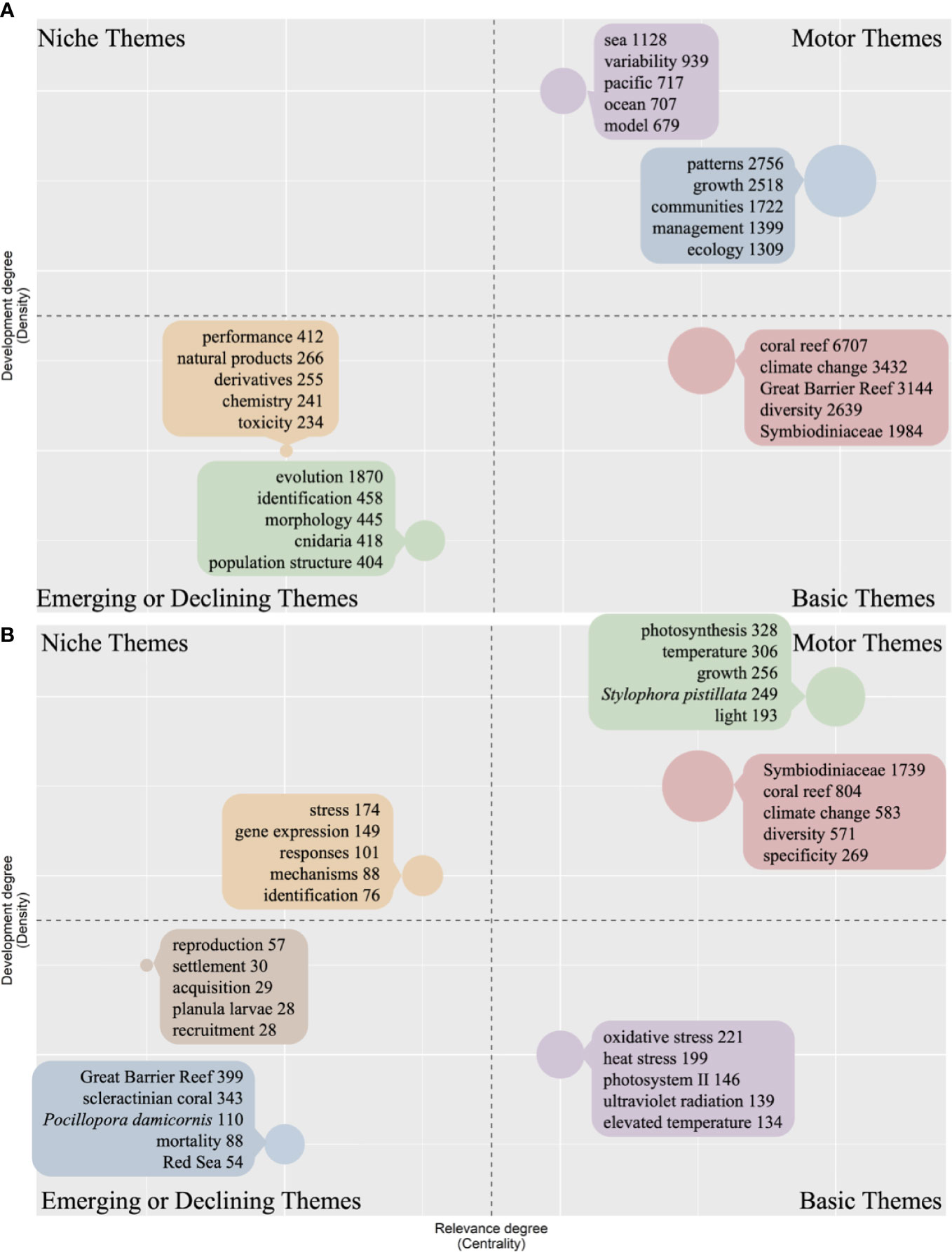
Figure 6 Thematic map of keywords in coral (A) and Symbiodiniaceae research (B). Only five keywords of each cluster were shown on the map.
For Symbiodiniaceae, the keywords “oxidative stress”, “heat stress”, “photosystem II”, “ultraviolet radiation”, and “elevated temperature” were distributed in Basic Themes, “photosynthesis”, “temperature”, “growth”, “Stylophora pistillata”, “light”, “coral reef”, “climate change”, “diversity”, and “specificity” were distributed in Motor Themes, “stress”, “gene expression”, “response”, “mechanisms”, and “identification” occurred in Niche Themes, and “reproduction”, “settlement”, “acquisition”, “planula larvae”, “recruitment”, “Great Barrier Reef”, “scleractinian coral”, “P. damicornis”, “mortality”, and “Red Sea” were found in Emerging or Declining Themes (Figure 6B). Among the above keywords, “climate change”, “diversity”, “Great Barrier Reef”, “identification”, and “growth” were the shared research area for coral and Symbiodiniaceae, indicating that they are important and need more attention (Figure 6).
Compared with corals, the keywords “gene expression” and “mechanism” appeared in Symbiodiniaceae in Niche Themes, which revealed that gene level research was increasingly becoming more popular in Symbiodiniaceae (Figure 6). So far, 12 species from five of the genera have been subjected to genome sequencing: they are Breviolum minutum (Shoguchi et al., 2013), Fugacium kawagutii (Lin et al., 2015; Liu et al., 2018; Li et al., 2020), Symbiodinium microadriacticum (Aranda et al., 2016), Cladocopium goreaui (Liu et al., 2018), Symbiodinium sp., Cladocopium sp. (Shoguchi et al., 2018), Durusdinium trenchii (Shoguchi et al., 2021), Symbiodinium tridacnidorum, Symbiodinium linucheae, Symbiodinium necroappetens, Symbiodinium natans, and Symbiodinium pilosum (González-Pech et al., 2021). This is due to advances in sequencing technology and a dramatic drop in sequencing costs. In addition, more than 15 species of corals had their genomes sequenced in recent years (Shinzato et al., 2021). The reason why “gene”, “genome”, or “mechanism” does not appear in the thematic map and thematic evolution is that the research in other fields has developed ahead.
Co-occurrence of the keywords from the combination of coral, Symbiodiniaceae, and temperature research
From the above results of co-occurrence, thematic evolution, and thematic map, we found that temperature was the most studied environmental factor in coral and Symbiodiniaceae (Figures 3–6). Therefore, coral and Symbiodiniaceae combined with temperature were used as the keywords to search on WoS, and it retrieved 1,293 articles (Table 1). Then the co-occurrence of the keywords from 1,293 articles was analyzed using VOSviewer visualization (Figure 7). The results showed that the first cluster (in green) related to “coral bleaching”, “photosynthesis”, “oxidative stress”, “photosystem II”, “photoinhibition”, “CO2 fixation”, and “ultraviolet radiation” was the most popular research area, indicating that temperature mainly influences the photosynthesis of symbionts and thus potentially affects the carbon sequestration capacity of coral reef ecosystems (Table 6). For example, Effrenium voratum and Cladocopium spp. showed a decreased maximum quantum yield of PSII (Fv/Fm) under heat stress condition (32°C) than the control condition (25°C), which can be ascribed to pigment loss, photosynthetic apparatus damage, and inhibition of the repair of photodamaged PSII under heat stress conditions (Takahashi et al., 2004; Tolleter et al., 2013; Krueger et al., 2014). To make matters worse, recent studies found that elevated temperature not only reduced symbiont primary productivity but also induced more resource sequestration in the symbionts for their own growth, thus decreasing photosynthate translation to coral host and turning the mutualistic relationship into a parasitic relationship (Baker et al., 2018; Claar and Wood, 2020). The result also indicates that temperature is likely the cause of oxidative stress (Figure 7).
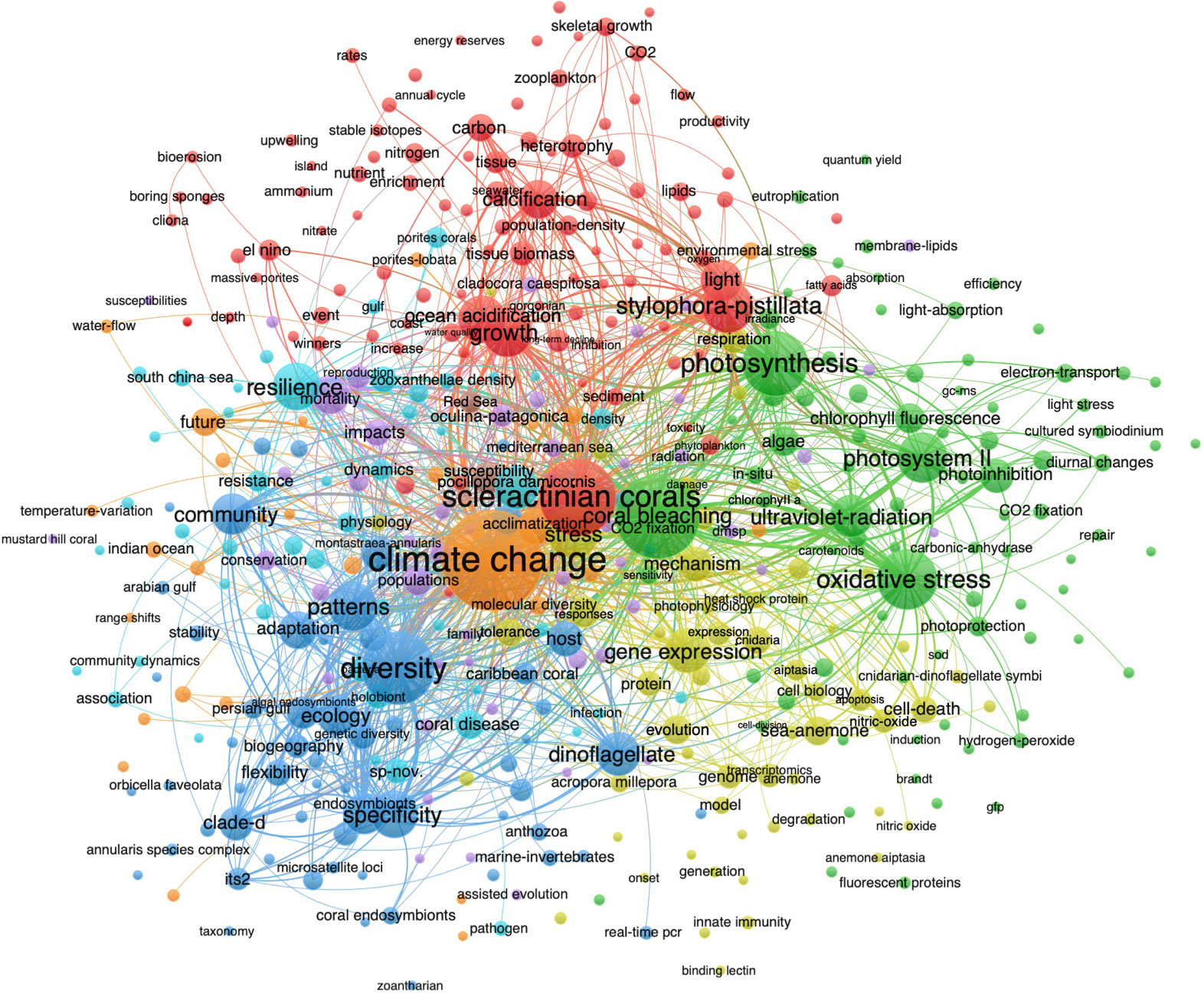
Figure 7 The bibliometric co-occurrence of the keyword clusters in coral and Symbiodiniaceae combined with temperature research. Note: Only keywords that appeared above five were retained.
The cluster (blue) of keywords “diversity”, “specificity”, “dinoflagellate”, “community”, “pattern”, and “ecology” was the second most popular research area, which can be interpreted as “biodiversity” (Table 6). The family Symbiodiniaceae is diverse, including nine genera of Symbiodinium, Breviolum, Cladocopium, Durusdinium, Effrenium, Fugacium, Gerakladium, clade H, and clade I (LaJeunesse et al., 2018). Many corals are relatively flexible in the type of algal symbiont they contain, but one type is usually dominant in any given species, such as Breviolum in the coral Madracis decactis and Durusdinium in P. damicornis (Baker et al., 2004; Varasteh et al., 2021; Zhou et al., 2022). Generally, Cladocopium and Durusdinium are dominant among Symbiodiniaceae which can establish a symbiotic relationship with corals, and most species in Durusdinium seem to be more tolerant of high temperature (Rowan, 2004; Chen et al., 2020). As such, the elevated temperature often leads to the shuffling of the symbiont community, such as from a Cladocopium-dominant community to a Durusdinium-dominant community (Baker, 2003; Baker et al., 2004; Abbott et al., 2021).
The third cluster (kelly) related to “gene expression”, “stress”, “mechanism”, “genome”, and “cell death” can be called “mechanism” (Table 6). Our understanding of the biodiversity and molecular response of coral and Symbiodiniaceae to elevated temperature is rapidly increasing with the advances in high-throughput sequencing in recent years, such as DNA-barcoding and transcriptome (Lin et al., 2019; Chen et al., 2020; Li et al., 2021). The fourth cluster (red), including “scleractinian corals”, “S. pistillata”, “growth”, “light”, “calcification”, and “ocean acidification”, was named “sufferer”. The fifth cluster (cyan) highlights the keywords of “resilience”, “P. damicornis”, “South China Sea”, and “coral disease”, indicating that the South China Sea was the main area for research on coral bleaching triggered by elevated temperature. This is consistent with the fact that China is the third most productive country in coral and Symbiodiniaceae research (Figure 2). The last cluster (orange) covers the keywords “climate change”, “acclimatization”, and “future”, which was the least studied area in the research on coral and Symbiodiniaceae combined with temperature.
Thematic evolution and thematic map of keywords from coral, Symbiodiniaceae, and temperature research
When searching for articles using the combination of keywords coral, Symbiodiniaceae, and temperature in WoS, we found that the first paper was published in 2003. Therefore, five subperiods were set to explore the thematic evolution from 2003 (Figure 8A). The results showed that the keywords “photosynthesis”, “diversity”, “climate change”, “response”, and “stress” appeared more than three times (inclusive) in the five subperiods, indicating they have been the central research areas. Besides, “evolution”, “nitrogen”, “mortality”, “population density”, and “growth” appeared twice within the five subperiods. Furthermore, the keyword “gene expression” was first found in the subperiod of 2018 to 2019, indicating that the research of temperature effects on coral and Symbiodiniaceae was rapidly advancing toward the gene level since around 2018.
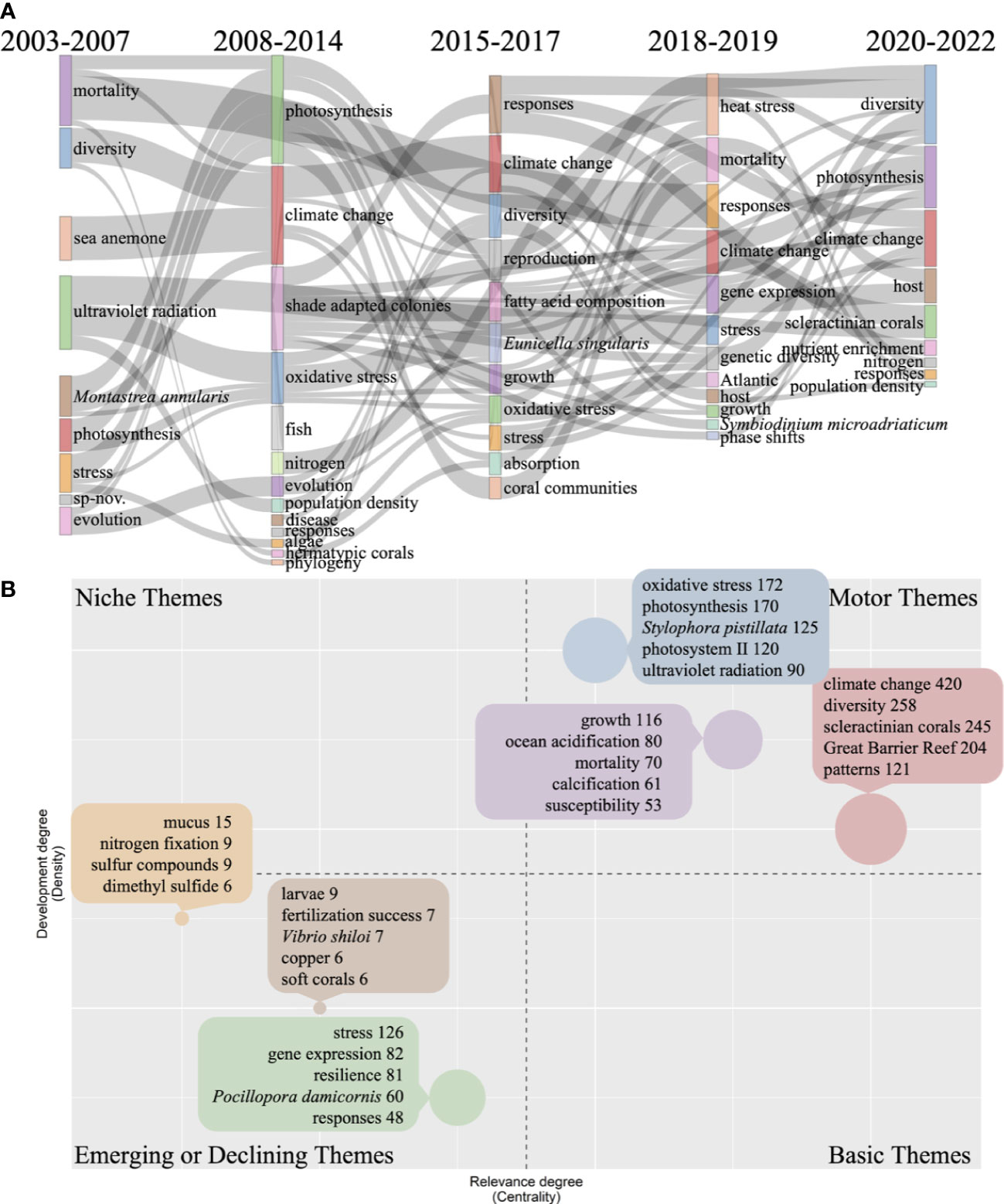
Figure 8 Thematic evolution and thematic map of the keywords from the combination of coral, Symbiodiniaceae, and temperature research. (A) thematic evolution; (B) thematic map.
Among the various nutrients, nitrogen and copper were two nutrients that appeared in this analysis (Figure 8; Figure S1). The keyword “nitrogen” appeared three times in the thematic evolution, and “nutrient enrichment” first appeared in the subperiod of 2020 to 2022, indicating that nutrients, especially nitrogen, might be the latest addition to temperature effect research on corals and Symbiodiniaceae (Figure 8A). Besides, current studies have shown that elevated nutrient loading can increase coral bleaching, and increased levels of dissolved inorganic nitrogen can aggravate the susceptibility of corals to temperature- and light-induced bleaching (Donovan et al., 2013; Wiedenmann et al., 2013; D’Angelo and Wiedenmann, 2014; Vega Thurber et al., 2014). Furthermore, recent experiments have also unveiled nutritional mechanisms that regulate bleaching and highlighted excess nutrients as a cause of converting algal symbionts into parasitism (Baker et al., 2018; Morris et al., 2019; DeCarlo et al., 2020). However, other studies also reported that the dissolved inorganic nutrients delivered by upwelling could relieve the stress caused by elevated temperature, illustrating that nutrients may have two faces in mediating coral bleaching triggered by temperature (Riegl et al., 2019). Therefore, a high concentration of nutrients can increase the temperature tolerance of corals and help corals survive under heat stress, while excess nutrients are detrimental to corals even at normal temperatures as they stimulate algal blooms causing shading over corals (Rasher et al., 2011). In addition, different forms of nitrogen (such as ammonium, nitrate, and urea) may have different impacts on the thermal tolerance of corals. For example, nitrate loading can increase coral bleaching more than urea in coral Acropora and Pocillopora, and nitrate enrichment reduces the threshold of thermal tolerance, while ammonium enrichment tends to benefit coral S. pistillata’s health (Fernandes de Barros Marangoni et al., 2020; Burkepile et al., 2020). Unlike nitrogen, many copper-related studies have focused on the toxic effects of copper enrichment combined with temperature (da Silva Fonseca et al., 2017; da Silva Fonseca et al., 2019; Banc-Prandi and Fine, 2019; Banc-Prandi et al., 2021).
In the thematic map, all keywords were distributed in Motor Theme and Emerging or Declining Themes (Figure 8B). In Motor Theme, the keywords were clustered into three categories. Among them, “climate change”, “diversity”, “scleractinian corals”, “Great Barrier Reef”, and “pattern” were clustered together (in red), “oxidative stress”, “photosynthesis”, “S. pistillata”, “photosystem”, and “ultraviolet radiation” were clustered as another category (blue), and “growth”, “ocean acidification”, “mortality”, “calcification”, and “susceptibility” formed yet another (purple). The keywords were clustered into three categories in Emerging or Declining Themes. Among them, “stress”, “gene expression”, “resilience”, “P. damicornis”, and “responses” were clustered in the green category, “mucus”, “nitrogen fixation”, “sulfur compounds”, and “dimethyl sulfide” were clustered in the orange category, and “larvae”, “fertilization success”, “Vibrio shiloi”, “copper”, and “soft corals” were clustered in the brown category. These results showed that the corals S. pistillata and P. damicornis were the preferred species for studying coral responses to elevated temperatures. Besides, V. shiloi first appeared in Emerging or Declining Themes, indicating that V. shiloi is involved in temperature-induced coral bleaching and needs further research as the keyword appeared only seven times (Figure 8B).
Conclusion and future direction
Climate change and human activities cause coral bleaching and mortality, threatening coral reefs globally. This study provides a complete description of coral and Symbiodiniaceae using the comprehensive bibliometric survey to understand the dynamics of knowledge production in the field. The results showed that elevated temperature, diversity, nutrients, survival, and mechanism are central research areas for both corals and Symbiodiniaceae. Among them, the elevated temperature was the main culprit for coral bleaching, and thus a large volume of literature reports it (accounting for 18.18% of total coral research; 42.09% of total Symbiodiniaceae research). Further analysis revealed that the elevated temperature mainly influenced the biodiversity of coral or Symbiodiniaceae and photosynthesis of symbionts, which was reflected by high-frequency keywords of diversity, photosynthesis, photosystem II, and photoinhibition.
Consequently, temperature affects the carbon sequestration capacity of coral reef ecosystems, as confirmed by keywords of CO2 fixation. Besides, coral S. pistillata and P. damicornis are the two main coral species studied in response to elevated temperature stress, and V. shiloi may be involved in coral response to elevated temperature. Furthermore, the number of sequenced Symbiodiniaceae and coral genomes has grown, suggesting that the research was intensively advancing toward the gene level, especially in Symbiodiniaceae. In addition, nitrogen and copper were involved in the research of elevated temperature, and the nutrient enrichment appeared in the subperiod of 2020 to 2022 from thematic elevation, indicating a new research direction of nitrogen nutrient and toxic copper in coral response to a high-temperature environment, which needs further attention. The present study provides the first general overview of coral reef research and sheds light on research hotspots and gaps that may inform future research direction.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
SL and TL conceived the study. JH, TL, and CZ performed the bibliometric analysis. TL, JH, HD, and XL wrote the manuscript. SL and TL reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515110001) and the Start-Up funding of Shantou University (NTF21049).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.926783/full#supplementary-material
References
Abbott E., Dixon G., Matz M. (2021). Shuffling between Cladocopium and Durusdinium extensively modifies the physiology of each symbiont without stressing the coral host. Mol. Ecol. 30 (24), 6585–6595. doi: 10.1111/mec.161190
Aranda M., Li Y., Liew Y. J., Baumgarten S., Simakov O., Wilson M. C., et al. (2016). Genomes of coral dinoflagellate symbionts highlight evolutionary adaptations conducive to a symbiotic lifestyle. Sci. Rep. 6, 39734. doi: 10.1038/srep39734
Baker A. C. (2003). Flexibility and specificity in coral-algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annu. Rev. Ecol. Evol. Syst. 34 (1), 661–689. doi: 10.1146/annurev.ecolsys.34.011802.132417
Baker D. M., Freeman C. J., Wong J. C., Fogel M. L., Knowlton N. (2018). Climate change promotes parasitism in a coral symbiosis. ISME J. 12 (3), 921–930. doi: 10.1038/s41396-018-0046-8
Baker A. C., Starger C. J., Mcclanahan T. R., Glynn P. W. (2004). Coral reefs: Corals’ adaptive response to climate change. Nature 430, 741. doi: 10.1038/430741a
Banc-Prandi G., Cerutti J. M., Fine M. (2021). Recovery assessment of the branching coral Stylophora pistillata following copper contamination and depuration. Mar. pollut. Bull. 162, 111830. doi: 10.1016/j.marpolbul.2020.111830
Banc-Prandi G., Fine M. (2019). Copper enrichment reduces thermal tolerance of the highly resistant red Sea coral Stylophora pistillata. Coral. Reefs. 38, 285–296. doi: 10.1007/s00338-019-01774-z
Bruno J. F., Petes L. E., Drew Harvell C., Hettinger A. (2003). Nutrient enrichment can increase the severity of coral diseases. Ecol. Let. 6 (12), 1056–1061. doi: 10.1046/j.1461-0248.2003.00544.x
Burkepile D., Shantz A., Adam T., Munsterman K., Speare K., Ladd M., et al. (2020). Nitrogen identity drives differential impacts of nutrients on coral bleaching and mortality. Ecosystems 23 (4), 798–811. doi: 10.1007/s10021-019-00433-2
Chen B., Yu K. F., Qin Z. J., Liang J. Y., Wang G. H., Huang X. Y., et al. (2020). Dispersal, genetic variation, and symbiont interaction network of heat-tolerant endosymbiont Durusdinium trenchii: Insights into the adaptive potential of coral to climate change. Sci. Total. Environ. 723, 138026. doi: 10.1016/j.scitotenv.2020.138026
Claar D. C., Wood C. L. (2020). Pulse heat stress and parasitism in a warming world. Trends. Ecol. Evol. 35 (8), 704–715. doi: 10.1016/j.tree.2020.04.002
Cobo M. J. (2011). An approach for detecting, quantifying, and visualizing the evolution of a research field: A practical application to the fuzzy sets theory field. JOI 5 (1), 146–166. doi: 10.1016/j.joi.2010.10.002
D’Angelo C., Wiedenmann J. (2014). Impacts of nutrient enrichment on coral reefs: new perspectives and implications for coastal management and reef survival. Curr. Opin. Environ. Sustain. 7, 82–93. doi: 10.1016/j.cosust.2013.11.029
da Silva Fonseca J., de Barros Marangoni L. F., Marques J. A., Bianchini A. (2017). Effects of increasing temperature alone and combined with copper exposure on biochemical and physiological parameters in the zooxanthellate scleractinian coral. Mussismilia. Harttii. Aquat. Toxicol. 190, 121–132. doi: 10.1016/j.aquatox.2017.07.002
da Silva Fonseca J., de Barros Marangoni L. F., Marques J. A., Bianchini A. (2019). Energy metabolism enzymes inhibition by the combined effects of increasing temperature and copper exposure in the coral Mussismilia harttii. Chemosphere 236, 124420. doi: 10.1016/j.chemosphere.2019.124420
Davy S. K., Allemand D., Weis V. M. (2012). Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol. Mol. Biol. Rev. 76, 229–261. doi: 10.1128/MMBR.05014-11
DeCarlo T. M., Gajdzik L., Ellis J., Coker D. J., Roberts M. B., Hammerman N. M., et al. (2020). Nutrient-supplying ocean currents modulate coral bleaching susceptibility. Sci. Adv. 6 (34), eabc5493. doi: 10.1126/sciadv.abc5493
Donovan M. K., Adam T. C., Shantz A. A., Speare K. E., Munsterman K. S., Rice M. M., et al (2020). Nitrogen pollution interacts with heat stress to increase coral bleaching across the seascape. Pro. Natl. Acad. Sci. 117 (10), 5351–5357. doi: 10.1073/pnas.1915395117
Ea R., Ka-Kudla M. L. (1997). The global biodiversity of coral reefs: a comparison with rainforests. Biodivers. II.: Understanding. Protecting. Our. Biol. Resour. 2, 551.
Einecker R., Kirby A. (2020). Climate change: a bibliometric study of adaptation, mitigation and resilience. Sustainability 12 (17), 6935. doi: 10.3390/su12176935
Ellegaard O., Wallin J. A. (2015). The bibliometric analysis of scholarly production: How great is the impact? Scientometrics 105 (3), 1809–1831. doi: 10.1007/s11192-015-1645-z
Evans R. D., Wilson S. K., Fisher R., Ryan N. M., Babcock R., Blakeway D., et al. (2020). Early recovery dynamics of turbid coral reefs after recurring bleaching events. J. Environ. Manage. 268, 110666. doi: 10.1016/j.jenvman.2020.110666
Falagas M., Pitsouni E., Malietzis G., Pappas G. (2008). Comparison of pubmed, scopus, web of science, and google scholar: strengths and weaknesses. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 22 (2), 338–342. doi: 10.1096/fj.07-9492LSF
Fernandes de Barros Marangoni L., Ferrier-Pagès C., Rottier C., Grover R.. (2020). Unravelling the different causes of nitrate and ammonium effects on coral bleaching. Sci. Rep. 10, 11975. doi: 10.1038/s41598-020-68916-0
Frieler K., Meinshausen M., Golly A., Mengel M., Lebek K., Donner S. D., et al. (2013). Limiting global warming to 2 degrees c is unlikely to save most coral reefs. Nat. Clim. Change. 3 (2), 165–170. doi: 10.1038/NCLIMATE1674
Ge R., Liang J., Yu K., Chen B., Yu X., Deng C., et al. (2021). Regulation of the coral-associated bacteria and symbiodiniaceae in acropora valida under ocean acidification. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.767174
González-Pech R. A., Stephens T. G., Chen Y., Mohamed A. R., Cheng Y., Shah S., et al. (2021). Comparison of 15 dinoflagellate genomes reveals extensive sequence and structural divergence in family symbiodiniaceae and genus Symbiodinium. BMC. Biol. 19 (1), 1–22. doi: 10.1186/s12915-021-00994-6
Goreau T. F. (1964). Mass expulsion of zooxanthellae from Jamaican reef communities after hurricane flora. Science 145 (3630), 383–386. doi: 10.1126/science.145.3630.383
Hall E. R., Muller E. M., Goulet T., Bellworthy J., Ritchie K. B., Fine M. (2018). Eutrophication may compromise the resilience of the red Sea coral Stylophora pistillata to global change. Mar. pollut. Bull. 131, 701–711. doi: 10.1016/j.marpolbul.2018.04.067
Hughes T. P., Baird A. H., Bellwood D. R., Card M., Connolly S. R., Folke C., et al. (2003). Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933. doi: 10.1126/science.1085046
Johnston E. C., Counsell C. W., Sale T. L., Burgess S. C., Toonen R. J. (2020). The legacy of stress: Coral bleaching impacts reproduction years later. Funct. Ecol. 34 (11), 2315–2325. doi: 10.1111/1365-2435.13653
Krishnaveni S. M. S., Sreenath K. R., Anakha M., Joshi K. K., Sobhana K. S., Dash G. (2021). Visualizing the scientific panorama of coral black band disease research: a bibliometric analysis. J. Mar. Biol. Assoc. India. 63 (2), 49–58. doi: 10.6024/jmbai.2021.63.2.2213-07
Krueger T., Becker S., Pontasch S., Dove S., Hoeghguldberg O., Leggat W., et al. (2014). Antioxidant plasticity and thermal sensitivity in four types of Symbiodinium sp. J. Phycol. 50, 1035–1047. doi: 10.1111/jpy.12232
Lai Q., Ma J., He F., Zhang A., Pei D., Wei G., et al. (2022). Research development, current hotspots, and future directions of blue carbon: A bibliometric analysis. Water 14 (8), 1193. doi: 10.3390/w14081193
LaJeunesse T. C., Parkinson J. E., Gabrielson P. W., Jeong H. J., Reimer J. D., Voolstra C. R., et al. (2018). Systematic revision of symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr. Biol. 28 (16), 2570–2580. doi: 10.1016/j.cub.2018.07.008
Li J., Long L., Zou Y., Zhang S. (2021). Microbial community and transcriptional responses to increased temperatures in coral Pocillopora damicornis holobiont. Environ. Microbiol. 23 (2), 826–843. doi: 10.1111/1462-2920.15168
Lin S., Cheng S., Song B., Zhong X., Lin X., Li W., et al. (2015). The Symbiodinium kawagutii genome illuminates dinoflagellate gene expression and coral symbiosis. Science 350, 691–694. doi: 10.1126/science.aad0408
Lin S., Yu L., Zhang H. (2019). Transcriptomic responses to thermal stress and varied phosphorus conditions in Fugacium kawagutii. Microorganisms 7 (4), 96. doi: 10.3390/microorganisms7040096
Liu H., Stephens T. G., Gonzalezpech R. A., Beltran V. H., Lapeyre B., Bongaerts P., et al. (2018). Symbiodinium genomes reveal adaptive evolution of functions related to coral-dinoflagellate symbiosis. Commun. Biol. 1, 95. doi: 10.1038/s42003-018-0098-3
Li T., Yu L., Song B., Song Y., Li L., Lin X., et al. (2020). Genome improvement and core gene set refinement of Fugacium kawagutii. Microorganisms 8 (1), 102. doi: 10.3390/microorganisms8010102
Manasrah R., Lass H. U., Fennel W. (2006). Circulation in the gulf of aqaba (Red sea) during winter–spring. J. Oceanoger. 62 (2), 219–225. doi: 10.1007/s10872-006-0046-6
Mohan V., Visakhi P., Ravi S. (2011). Coral reef research in India: A bibliometric analysis (Part-I). Indian. J. Agric. Lib. Inf. Serv. 27 (1), 48–60.
Moral-Muñoz J. A., López-Herrera A. G., Herrera-Viedma E., Cobo M. J. (2019). “Science mapping analysis software tools: A review”, in Springer handbook of science and technology indicators (Cham, Switzerland: Springer), 159–185. doi: 10.1007/978-3-030-02511-3_7
Morris L. A., Voolstra C. R., Quigley K. M., Bourne D. G., Bay L. K. (2019). Nutrient availability and metabolism affect the stability of coral–symbiodiniaceae symbioses. Trends. Microbiol. 27 (8), 678–689. doi: 10.1016/j.tim.2019.03.004
Muscatine L., Porter J. W. (1977). Reef corals: mutualistic symbioses adapted to nutrient poor environments. Biosci 27, 454–460. doi: 10.2307/1297526
Peixoto R. S., Rosado P. M., Leite D. C., Rosado A. S., Bourne D. G. (2017). Beneficial microorganisms for corals (BMC): proposed mechanisms for coral health and resilience. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.00341
Putnam H. M., Barott K. L., Ainsworth T. D., Gates R. D. (2017). The vulnerability and resilience of reef-building corals. Curr. Biol. 27, 528–540. doi: 10.1016/j.cub.2017.04.047
Rädecker N., Pogoreutz C., Gegner H. M., Cárdenas A., Roth F., Bougoure J., et al. (2021). Heat stress destabilizes symbiotic nutrient cycling in corals. Pro. Natl. Acad. Sci. 118 (5), e2022653118. doi: 10.1073/pnas.2022653118
Rädecker N., Pogoreutz C., Voolstra C. R., Wiedenmann J., Wild C.. (2015). Nitrogen cycling in corals: the key to understanding holobiont functioning? Trends. Microbiol. 23, 490–497. doi: 10.1016/j.tim.2015.03.008
Rasher D. B., Stout E. P., Engel S., Kubanek J., Hay M. E. (2011). Macroalgal terpenes function as allelopathic agents against reef corals. Pro. Natl. Acad. Sci. 108 (43), 17726–17731. doi: 10.1073/pnas.1108628108
Riegl B., Glynn P. W., Banks S., Keith I., Rivera F., Vera-Zambrano M., et al. (2019). Heat attenuation and nutrient delivery by localized upwelling avoided coral bleaching mortality in northern galapagos during 2015/2016 ENSO. Coral. Reefs. 38 (4), 773–785. doi: 10.1007/s00338-019-01787-8
Şenel E., Demir E., Alkan R. M. (2017). Bibliometric analysis on global behçet disease publications during 1980-2014: is there a silk road in the literature? J. Eur. Acad. Dermatol. Venereol. 31 (3), 518–522. doi: 10.1111/jdv.13897
Shinzato C., Khalturin K., Inoue J., Zayasu Y., Kanda M., Kawamitsu M., et al. (2021). Eighteen coral genomes reveal the evolutionary origin of acropora strategies to accommodate environmental changes. Mol. Biol. Evol. 38 (1), 16–30. doi: 10.1093/molbev/msaa216
Shoguchi E., Beedessee G., Hisata K., Tada I., Narisoko H., Satoh N., et al. (2021). A new dinoflagellate genome illuminates a conserved gene cluster involved in sunscreen biosynthesis. Genome. Biol. Evol. 13 (2), evaa235. doi: 10.1093/gbe/evaa235
Shoguchi E., Beedessee G., Tada I., Hisata K., Kawashima T., Takeuchi T., et al. (2018). Two divergent Symbiodinium genomes reveal conservation of a gene cluster for sunscreen biosynthesis and recently lost genes. BMC. Genom. 19, 458. doi: 10.1186/s12864-018-4857-9
Shoguchi E., Shinzato C., Kawashima T., Gyoja F., Mungpakdee S., Koyanagi R., et al. (2013). Draft assembly of the Symbiodinium minutum nuclear genome reveals dinoflagellate gene structure. Curr. Biol. 23, 1399–1408. doi: 10.1016/j.cub.2013.05.062
Silverman J., Lazar B., Erez J. (2007). Effect of aragonite saturation, temperature, and nutrients on the community calcification rate of a coral reef. J. Geophys. Res.: Oceans. 112, C05004. doi: 10.1029/2006JC003770
Smith K. F., Kohli G. S., Murray S. A., Rhodes L. L. (2017). Assessment of the metabarcoding approach for community analysis of benthic-epiphytic dinoflagellates using mock communities. New. Zeal. J. Mar. Fresh., 51, 1–22. doi: 10.1080/00288330.2017.1298632
Stambler N., Popper N., Dubinsky Z. V.Y., Stimson J. (1991). Effects of nutrient enrichment and water motion on the coral Pocillopora damicornis. Pacific Sci. 45, 299–307.
Sully S., Burkepile D. E., Donovan M. K., Hodgson G., van Woesik R. (2019). A global analysis of coral bleaching over the past two decades. Nat. Commun. 10, 1264. doi: 10.1038/s41467-019-09238-2
Takahashi S., Nakamura T., Sakamizu M., Woesik R. V., Yamasaki H. (2004). Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef-building corals. Plant Cell. Physiol. 45 (2), 251–255. doi: 10.1093/pcp/pch028
Thomas L., Kendrick G. A., Kennington W. J., Richards Z. T., Stat M. (2014). Exploring Symbiodinium diversity and host specificity in Acropora corals from geographical extremes of Western Australia with 454 amplicon pyrosequencing. Mol. Ecol. 23 (12), 3113–3126. doi: 10.1111/mec.12801
Thompson D. F., Walker C. K. (2015). A descriptive and historical review of bibliometrics with applications to medical sciences. Pharmacother.: J. Hum. Pharmacol. Drug Ther. 35 (6), 551–559. doi: 10.1002/phar.1586
Thurber R. L. V., Burkepile D. E., Fuchs C., Shantz A. A., McMinds R., Zaneveld J. R. (2014). Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Global. Change. Biol. 20 (2), 544–554. doi: 10.1111/gcb.12450
Tolleter D., Seneca F. O., DeNofrio J. C., Krediet C. J., Palumbi S. R., Pringle J. R., et al. (2013). Coral bleaching independent of photosynthetic activity. Cur. Biol. 23 (18), 1782–1786. doi: 10.1016/j.cub.2013.07.041
Ubando A. T., Africa A. D. M., Maniquiz-Redillas M. C., Culaba A. B., Chen W. H., Chang J. S.. (2021). Microalgal biosorption of heavy metals: a comprehensive bibliometric review. J. Hazard. Mater. 402, 123431. doi: 10.1016/j.jhazmat.2020.123431
Van Eck N., Waltman L. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84 (2), 523–538. doi: 10.1007/s11192-009-0146-3
Van Oppen M. J. H., Lough J. M. (2018). “Synthesis: coral bleaching: patterns, processes, causes and consequences”, in Coral bleaching. ecological. studies, vol. 233 . Eds. van Oppen M., Lough J. (Cham: Springer). doi: 10.1007/978-3-319-75393-5_14
Varasteh T., Salazar V., Tschoeke D. ,. D., Francini-Filho R. B., Swings J., Garcia G., et al. (2021). Breviolum and Cladocopium are dominant among symbiodiniaceae of the coral holobiont Madracis decactis. Microbiol. Ecol. doi: 10.1007/s00248-021-01868-8
Vega Thurber R. L., Burkepile D. E., Fuchs C., Shantz A. A., McMinds R., Zaneveld J. R. (2014). Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Global Change Biol. 20 (2), 544–554. doi: 10.1111/gcb.12450
Wiedenmann J., D’Angelo C., Smith E., Hunt A. N., Legiret F. E., Postle A. D., et al. (2013). Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat. Clim. Change. 3, 160–164. doi: 10.1038/NCLIMATE1661
Xiang T., Lehnert E., Jinkerson R. E., Clowez S., Kim R. G., DeNofrio J. C., et al. (2020). Symbiont population control by host-symbiont metabolic interaction in symbiodiniaceae-cnidarian associations. Nat. Commun. 11 (1), 1–9. doi: 10.1038/s41467-019-13963-z
Yee S. H., Barron M. G. (2010). Predicting coral bleaching in response to environmental stressors using 8 years of global-scale data. Environ. Monit. Assess. 161 (1), 423–438. doi: 10.1007/s10661-009-0758-3
Zhang J., Yu Q., Zheng F., Long C., Lu Z., Duan Z. (2016). Comparing keywords plus of WOS and author keywords: A case study of patient adherence research. J. Associ. Inf. Sci. Technol. 67 (4), 967–972. doi: 10.1002/asi.23437
Zhou C., Bi R., Su C., Liu W., Wang T. (2022). The emerging issue of microplastics in marine environment: A bibliometric analysis from 2004 to 2020. Mar. pollut. Bull. 179, 113712. doi: 10.1016/j.marpolbul.2022.113712
Keywords: Symbiodiniaceae, coral, bibliometric, temperature, nutrient enrichment, nitrogen
Citation: Li T, Huang J, Du H, Liu X, Zhong C and Lin S (2022) Coral bleaching from a nutrient perspective is understudied: A bibliometric survey. Front. Mar. Sci. 9:926783. doi: 10.3389/fmars.2022.926783
Received: 23 April 2022; Accepted: 05 July 2022;
Published: 02 August 2022.
Edited by:
Wei Jiang, Guangxi University, ChinaReviewed by:
Xiaopeng Yu, Guangxi University, ChinaSanqiang Gong, South China Sea Institute of Oceanology (CAS), China
Copyright © 2022 Li, Huang, Du, Liu, Zhong and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tangcheng Li, dGNobGlAc3R1LmVkdS5jbg==; Senjie Lin, c2VuamllLmxpbkB1Y29ubi5lZHU=
 Tangcheng Li
Tangcheng Li Jiahong Huang
Jiahong Huang Hong Du
Hong Du Xiaojuan Liu
Xiaojuan Liu Chongming Zhong1
Chongming Zhong1 Senjie Lin
Senjie Lin