- 1Key Laboratory of Tropical & Subtropical Fishery Resource Application & Cultivation, Ministry of Agriculture and Rural Affairs, Key Laboratory of Aquatic Animal Immune Technology of Guangdong Province, Pearl River Fisheries Research Institute, Chinese Academy of Fishery Science, Guangzhou, China
- 2Maoming Branch, Guangdong Laboratory for Lingnan Modern Agriculture, Maoming, China
- 3Institute of Semiconductors, Chinese Academy of Science, Beijing, China
The light spectrum is a vital environmental factor for the culture of fish, and the welfare of farmed fish is a crucial issue in aquaculture. In this study, Nile tilapia (Oreochromis niloticus, GIFT strain) juveniles were exposed to full-spectrum (LW), red (LR), yellow (LY), or blue (LB) light. After the 45-day experiment, growth performance, stress responses, and aggressive behaviors were evaluated, and transcriptomic analysis was carried out. The results revealed that LW and LR positively affected growth performance. At the same time, LY and LB had a negative effect. Light spectrum induced stress responses of juvenile fish exposed to LY, under which the total antioxidant capacity (T-AOC) and cortisol (COR) contents were the highest. The activities of α-amylase (AMS), protease (PES), and lipase (LPS) in the digestive tract showed a similar tendency, indicating that the light spectra altered the digestive enzyme activities and then affected growth. Behavioral analyses showed increased chase and bite activities of tilapia juveniles exposed to LW and LY. The affected functions included the nervous system, muscle morphogenesis, and immune system-related regulation. Enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways include the tryptophan metabolism signaling pathway, protein digestion and absorption signaling pathway, Jak-STAT signaling pathway, arachidonic acid metabolism signaling pathway, and alpha-linolenic acid metabolism signaling pathway. Overall, light spectra influenced the welfare of farmed tilapia juveniles in terms of growth, stress, and behavior. Our results suggested that LR should be used in juvenile tilapia culture.
1 Introduction
Aquaculture has grown faster than other modes of food production and currently represents 47% of global fish production (FAO, 2018). Nevertheless, farmed fish are subject to an unfavorable environment, such as high stocking densities and lack of control over food and habitat choice. Like other agricultural animals, such as mammals and birds, fish welfare has received considerable attention in recent decades (Ashley, 2007; Barreto et al., 2022). Fish welfare requires freedom from hunger, discomfort, pain, injury, disease, fear, and distress, as well as the freedom to express normal behavior (Ashley, 2007). Several environmental factors relevant to fish welfare have been recognized (Toni et al., 2017). With advanced breeding technology and facilities, environmental factors, including temperature, dissolved gasses, pH, salinity, nitrogen compounds, and artificial chemical pollutants are maintained within acceptable ranges for cultured fish. Another environment parameter that requires further attention for fish welfare is light.
Fishes have color vision (Cheng and Flamarique, 2004), and light, including the light spectrum, affects the growth, development, maturation, and other physiological processes of fish (Karakatsouli et al., 2010; Qiu et al., 2015; Choi et al., 2019). Providing the ideal light character for farmed aquatic animals may improve animal welfare. Orange and full-spectrum light were beneficial to turbot (Scophthalmus maximus) embryo incubation and survival of larvae (Wu et al., 2019). Giant freshwater prawn (Macrobrachium rosenbergii) larvae under green or white light expressed the fastest growth and metamorphosis (Wei et al., 2021). Green light kept olive flounder (Paralichthys olivaceus) from oxidative stress and strengthened its immunity under high density (Choi et al., 2019). Blue light could protect zebrafish (Danio rerio) against Cd toxicity (Yuan et al., 2017). At the same time, the preferred light spectrum of different fish species is varied. Scaled common carp (Cyprinus carpio) preferred red and blue light when the fish were reared at low and high stocking density, respectively, while red or blue light was unsuitable for mirror carp (C. carpio) (Karakatsouli et al., 2010). Koi carp (C. carpio) cultured under blue and green light exhibited better growth performance and improved non-specific immune ability (Bairwa et al., 2017). Nevertheless, blue light was stressful for rainbow trout (Oncorhynchus mykiss), and red was stressful for gilthead seabream (Sparus aurata) (Karakatsouli et al., 2007).
Animal behavior is flexible in response to the light environment. The red spectrum disturbed endocrine homeostasis and was associated with aggressive behaviors in spotted sea bass (Lateolabrax maculatus) culture (Hou et al., 2019). Higher light intensities and ultraviolet light altered virile (Faxonius virilis) and rusty (Faxonius rusticus) crayfish social interactions (Jackson and Moore, 2019). Collector urchins (Tripneustes gratilla) are highly sensitive to and dislike blue LED light, exhibiting the most hiding behavior and expressing a lower level of melanin (Li et al., 2021). Blue and white light could improve sea urchins’ (Strongylocentrotus intermedius) foraging and feeding abilities (Yang et al., 2020).
Improper lighting conditions may harm growth and stress responses and may negatively affect behavior. Red light disadvantaged giant freshwater prawn larval development (Wei et al., 2021). The red spectrum disturbed endocrine homeostasis associated with stress responses, growth, and behavior and so was unfavorable for spotted sea bass (Hou et al., 2019). It was found that long wavelengths hurt the growth of turbot larvae and induced stress responses (Wu et al., 2020). White and red light strongly affected the behavioral responses of European sea bass (Dicentrarchus labrax) larvae (Villamizar et al., 2011b). Sea urchins under blue light spend more time on righting response and foraging, and red light significantly inhibits growth in long-term rearing (Yang et al., 2021).
Tilapias account for 10% of current cultured fish production (FAO, 2018). The GIFT strain of Nile tilapia (Oreochromis niloticus) is well known worldwide for its high growth performance and hardiness and accounted for a significant proportion of Nile tilapia production (Khaw et al., 2012). It is necessary to evaluate the welfare of farmed tilapia with increasing public concern over the welfare of farmed fish. The importance of water quality parameters and feeding to the welfare of farmed tilapia has been recognized (Villarroel et al., 2011; Lopez-Luna et al., 2013). However, little is known about the light conditions relevant to juvenile tilapia welfare based on metabolic, behavioral, and physiological indicators (Jin et al., 2019; Toni et al., 2019). Therefore, the growth performance, stress, and behavioral responses of Nile tilapia juveniles cultured under different light spectra were assessed in the current study.
2 Materials and Methods
2.1 Juvenile Tilapia and Experiment Design
The juvenile GIFT tilapias (0.02 ± 0.00 g, 1.09 ± 0.04 cm) used in this research were 15 days post-hatching (dph) before the experiment and were randomly obtained from the same parents. The juveniles were placed into twelve glass tanks (1.0 m in length, 0.5 m in width, 0.5 m in depth, approximately 200 L of water, and 0.3 juveniles per liter). The twelve glass tanks were divided into full-spectrum, red (LR, 625–630 nm), yellow (LY, 590 nm–595 nm), and blue (LB, 450 nm–455 nm) light groups (three tanks per spectrum). Light-emitting diodes provided by Shenzhen Fluence Technology PLC (Shenzhen, China) were placed in the bottom center of the fish tank. Photosynthetic photon flux density (PPFD) of LW, LR, LY, and LB were set at 2.67 ± 0.33, 1.97 ± 0.51, 2.18 ± 0.56, and 2.21 ± 0.13 μmol/m2/s, respectively. The photoperiod was set to 12:12 h according to the natural rhythm, and the light intensity was measured with a Lighting Analyzer (PLA-20, EVERFINE Photo-e-info Co., Ltd., Hangzhou, China). The juvenile GIFT tilapias in all groups were maintained in tanks supplied with a continuous flow of fresh water. The fish tanks were wrapped with shade cloth to avoid ambient light interference. During the 45-day experiment, the juveniles were fed with artemia (Artemia salina) to apparent satiation twice a day (09:00 and 17:00), and the water temperature was maintained at 27°C ± 0.5°C. Uneaten artemia was removed 30 min after feeding by flowing water.
The tilapias used in this research were handled according to the guidelines of the Laboratory Animal Ethics Committee of the Pearl River Fisheries Research Institute, Chinese Academy of Fishery Science.
2.2 Sampling and Behavior Surveillance
After the 45-day experiment, all but ten juveniles’ body weight and length were measured. The digestive tract and liver of ten juveniles anesthetized by MS-222 were sampled and stored at −80°C. After sampling, a camera (C6TC, EZVIZ, Hangzhou, China) on top of each tank was used to capture and track the movements of the ten remaining juveniles from 10:00 to 16:00. Video recordings were subsequently replayed, and behavior was quantified. Nine videos were selected randomly from the complete video recordings (10 min per video), and the numbers of chase and bite behaviors in each video were measured. Biting was defined as mouth contact with lateral body regions, fin, tail, and eyes of other juveniles, with each time the mouth contacted another fish counting as one bite. Aggressive behavior was labeled as biting and chasings. Another thirty juveniles per tank were freeze-dried using a vacuum freeze dryer (Alpha 1-4/2-4 LD Plus, Martin Christ, Osterode am Harz, Germany) for moisture, crude protein, and lipid content quantification.
2.3 Growth Index
Condition factor (CF) and specific growth rate (SGR) were computed, as follows:
where W1 and W2 are the initial and final average body weights (g), respectively. L is the final average body length (cm). T is the number of days of experiment (days).
2.4 Biochemical Analysis
The activities of α-amylase (AMS), protease (PES), and lipase (LPS) of the digestive tract, and superoxide dismutase (SOD) of the liver, as well as total antioxidant capacity (T-AOC) and cortisol (COR) in the liver, were measured using detection kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The moisture, crude protein, and crude lipid were quantified by the drying method (Alpha 1-4/2-4 LD Plus, Martin Christ, Germany), Kjeldahl method (Kjeltec 2300, Foss, Hillerød, Denmark), and Soxhlet extraction method (Soxtec 2055, Foss, Denmark), respectively.
2.5 Transcriptomic Analysis
2.5.1 Total RNA Extraction and Sequencing
The total RNA of ten juveniles per tank was isolated using TRIzol (Invitrogen, Carlsbad, CA, USA). RNA quality was measured using Bioanalyzer 2100 (Agilent, Santa Clara, CA, USA). RNA sequence library construction and sequencing were carried out by LC-Bio (Hangzhou, China), which conducted 2 × 150 bp paired-end sequencing (PE150) on an Illumina Novaseq™ 6000.
2.5.2 The Analysis of RNA-seq Reads
After quality control using FastQC v0.10.1 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc), low-quality raw reads and adaptor sequences were removed using Cutadapt-1.9 (https://cutadapt.readthedocs.io/en/stable/) to obtain clean reads. Clean data were mapped to the O. niloticus reference genome (Accession GCA_922820385.1) using Hisat v2-2.0.4 (https://daehwankimlab.github.io/hisat2/) and assembled by StringTie v1.3.4d (http://ccb.jhu.edu/software/stringtie/). Then, the transcriptomes were merged to reconstruct a comprehensive transcriptome using Gffcompare v0.9.8 (http://ccb.jhu.edu/software/stringtie/gffcompare.shtml). The expression levels of transcripts were estimated by StringTie and ballgown (http://www.bioconductor.org/packages/release/bioc/html/ballgown.html) and calculated by FPKM. Differentially expressed genes (DEGs) were identified by edgeR (https://bioconductor.org/packages/release/bioc/html/edgeR.html) with Fold Change > 2 and p < 0.05 used as indicators for significant differences, followed by an analysis of Gene Ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment for DEGs.
2.5.3 RT-qPCR
RT-qPCR was carried out after screening the KEGG pathways to characterize the genes. A TransScript® One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China) was used for reverse transcription of total RNA. Primers were designed by Primer Premier 5.0 (http://www.premierbiosoft.com/primerdesign/index.html) (Supplementary Table 1) and synthesized by Sangon Biotech (Shanghai, China). RT-qPCR was run on the QuantStudio 3&5 Real-Time PCR System (Thermo Fisher, Waltham, MA, USA) using the 2× SG Fast qPCR Master Mix (High Rox, B639273, BBI, ABI, Foster, CA, USA) and a standard RT-qPCR amplification procedure (3 min at 95°C followed by 45 cycles including 5 s at 95°C and 30 s at 60°C).
2.6 Statistical Analysis
The data were analyzed by one-way ANOVA after the homogeneity of variance test with SPSS v19.0 and expressed as the mean ± SEM. A significance level of p < 0.05 was used.
3 Results
3.1 Effects of Different Light Spectra on the Growth and Related Enzyme Activities of GIFT Tilapia Juveniles
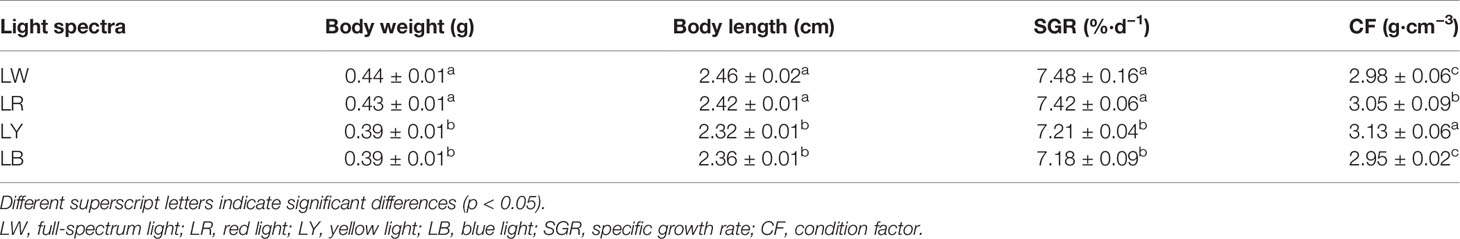
Different light spectra affected the body weight, length, and SGR of GIFT tilapia juveniles (Table 1). The body weight, length, and SGR of GIFT tilapia juveniles under LW and LR were significantly higher than those under LY and LB (p < 0.05, Table 1). No statistical differences in body weight, length, and SGR between LW and LR and LY and LB were observed (p > 0.05, Table 1). GIFT tilapia juveniles under LY exhibited a higher CF than those under LR (p < 0.05), and both groups showed significantly higher CF than the LW and LB groups (p < 0.05). CF of GIFT tilapia juveniles in the LW and LB groups showed no statistical difference (p > 0.05, Table 1).
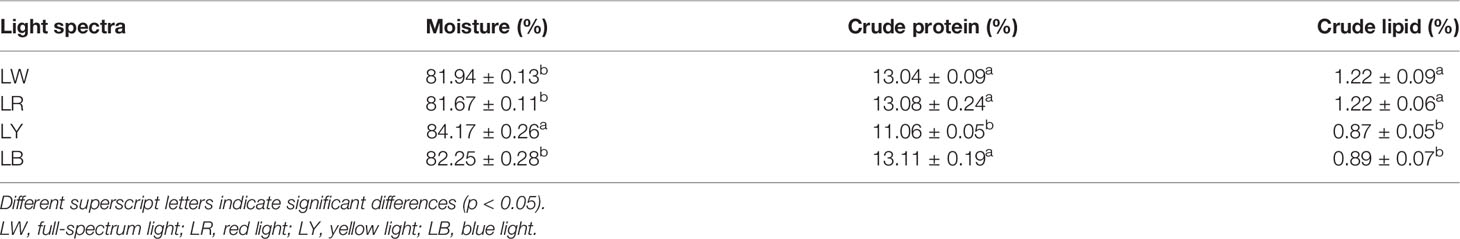
The effects of light spectra on the nutritional contents of GIFT tilapia juveniles are shown in Table 2. GIFT tilapia juveniles under LY had the highest moisture content (p < 0.05), but no statistical difference existed among those under LW, LR, and LB (p > 0.05, Table 2). There were no statistical differences in crude protein among the LW, LR, and LB groups (p > 0.05), and all showed significantly higher crude protein content than the LY group (p < 0.05, Table 2). No statistical difference existed in crude lipid between LW and LR and between LY and LB (p > 0.05, Table 2). Nevertheless, the crude lipid contents of juveniles under LW and LR were higher than those under LY and LB (p < 0.05, Table 2).
The effects of light spectra on the enzymes associated with digestion are shown in Figure 1. The AMS activities of GIFT tilapia juveniles under LR were higher than those under LW, LY, and LB (p < 0.05, Figure 1A). AMS activities of LW and LY were higher than those of LB (p < 0.05, Figure 1A), yet no statistical differences in AMS activities existed between the LW and LY groups (p > 0.05, Figure 1A). The PES activities in the LW group were higher than those in the LR, LY, and LB groups (p < 0.05), and significant differences were found between LR and LY, as well as LR and LB (p < 0.05, Figure 1B). Nevertheless, no statistical differences in PES activities existed between LY and LB (p > 0.05, Figure 1B). The LPS activities of fish under LY, which did not statistically differ from LW and LR (p > 0.05), were higher than those under LB (p < 0.05, Figure 1C). No statistical differences in LPS activities existed among the LW, LR, and LB groups (p > 0.05, Figure 1C).

Figure 1 Related digestive enzyme activities of GIFT tilapia juveniles cultured under different light spectra. (A) The α-amylase (AMS) activities of GIFT tilapia juveniles under four light spectra. (B) The protease (PES) activities of GIFT tilapia juveniles under four light spectra. (C) The lipase (LPS) activities of GIFT tilapia juveniles under four light spectra. Different letters implies statistical differences (p < 0.05).
3.2 Effects of Different Light Spectra on the Non-Specific Immune Responses and Stress of GIFT Tilapia Juveniles
The influences of light spectra on the immune and stress responses of GIFT tilapia juveniles are shown in Figure 2. The SOD activities of juveniles under LB were higher than those under LW, LR, and LY (p < 0.05), while no statistical differences existed among LW, LR, and LY (p > 0.05, Figure 2A). The T-AOC contents of fish under LY were higher than those under LW, LR, and LB (p < 0.05, Figure 2B). The T-AOC contents of fish under LW did not statistically differ from those under LR (p > 0.05) and were higher than fish under LB (p < 0.05, Figure 2B). No statistical differences in T-AOC contents were found between LR and LB (p > 0.05, Figure 2B). The COR contents of LY were higher than those under LW, LR, and LB (p < 0.05), while no statistical differences in COR contents existed among LW, LR, and LB groups (p > 0.05, Figure 2C).
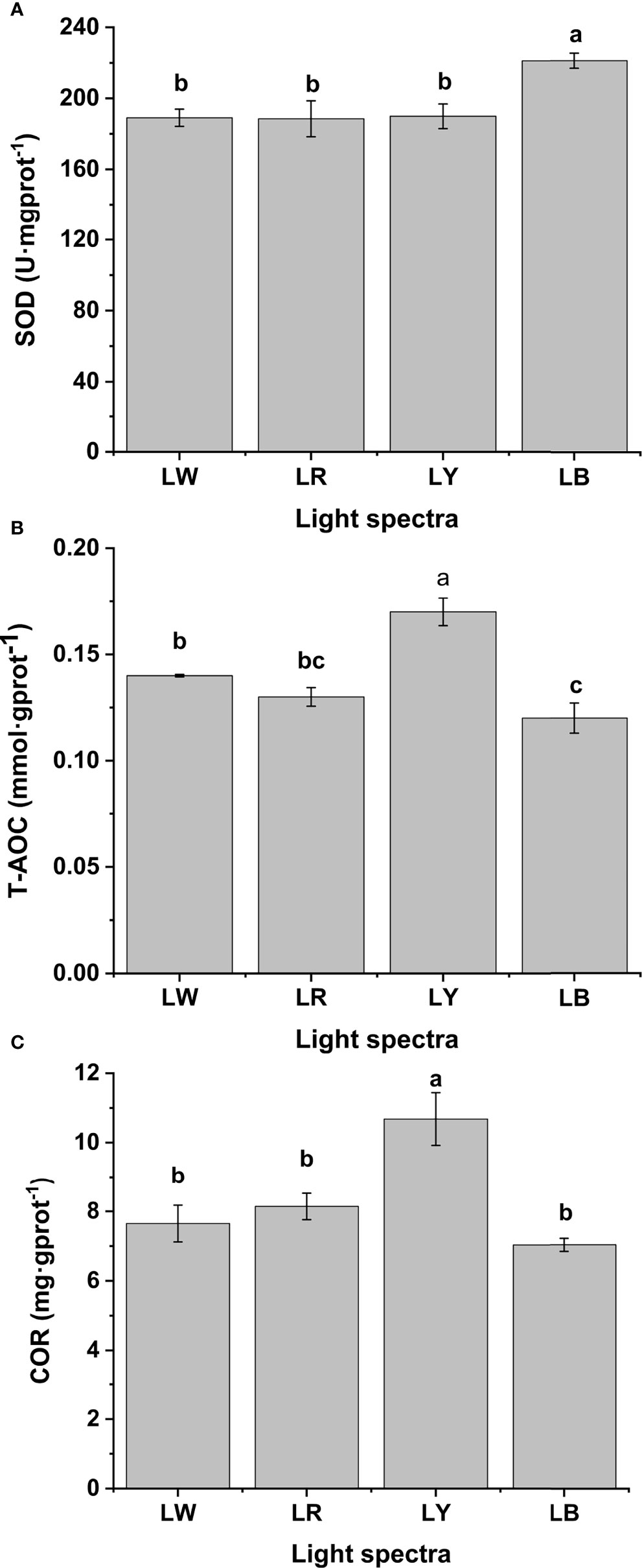
Figure 2 Related non-specific immune enzyme activities of GIFT tilapia juveniles cultured under different light spectra. (A) The superoxide dismutase (SOD) activities of GIFT tilapia juveniles under four light spectra. (B) The total antioxidant capacity (T-AOC) content of GIFT tilapia juveniles under four light spectra. (C) The cortisol (COR) content of GIFT tilapia juveniles under four light spectra. Different letters implies statistical differences (p < 0.05).
3.3 Effects of Different Light Spectra on the Behavioral Responses of GIFT Tilapia Juveniles
As shown in Figure 3, light spectra significantly influenced the frequency of aggressive behaviors of GIFT tilapia juveniles. Similar effects of different light spectra on GIFT tilapia juveniles’ chase and bite behaviors were observed. Fish under LW and LY exhibited significantly more chase and bite behaviors than those under LR and LB (p < 0.05), yet no statistical differences in the chase and bite behaviors were found between the LW and LY groups, as well as between the LR and LB groups (p > 0.05, Figures 3A, B).
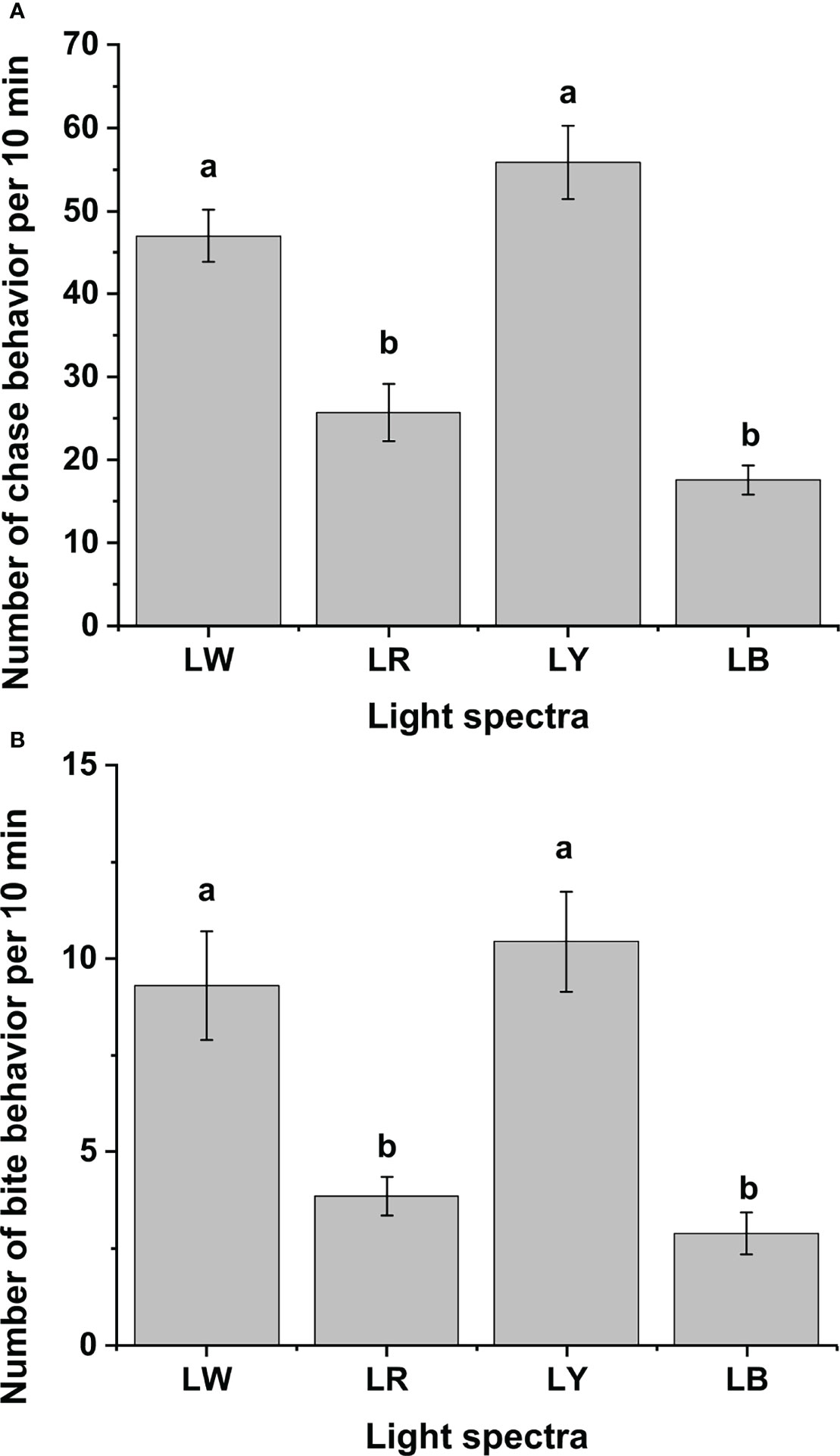
Figure 3 The aggressive behaviors of GIFT tilapia juveniles under different light spectra. (A) The chase behavior number of GIFT tilapia juveniles under different light spectra per 10 min. (B) The bite behavior number of GIFT tilapia juveniles under different light spectra per 10 min. Different letters implies statistical differences (p < 0.05).
3.4 Transcriptome Responses of GIFT Tilapia Juveniles to Different Light Spectra
RNA-seq was carried out to investigate the influences of light spectra on the expression of key compounds in GIFT tilapia juveniles. The results showed that 26 (LR vs. LW), 42 (LY vs. LW), and 24 (LB vs. LW) GO terms were upregulated and 12 (LR vs. LW), 6 (LY vs. LW), and 9 (LB vs. LW) GO terms were downregulated (Supplementary Table 2). GO pathway enrichment was re-analyzed (q-value <0.05, DEGs > 2) to explore the biological processe (BP), cellular component (CC), and molecular function (MF) pathways of DEGs, and there were 7, 21, and 7 GO terms related to BP, CC, and MF of LR vs. LW, LY vs. LW, and LB vs. LW, respectively (Figure 4).
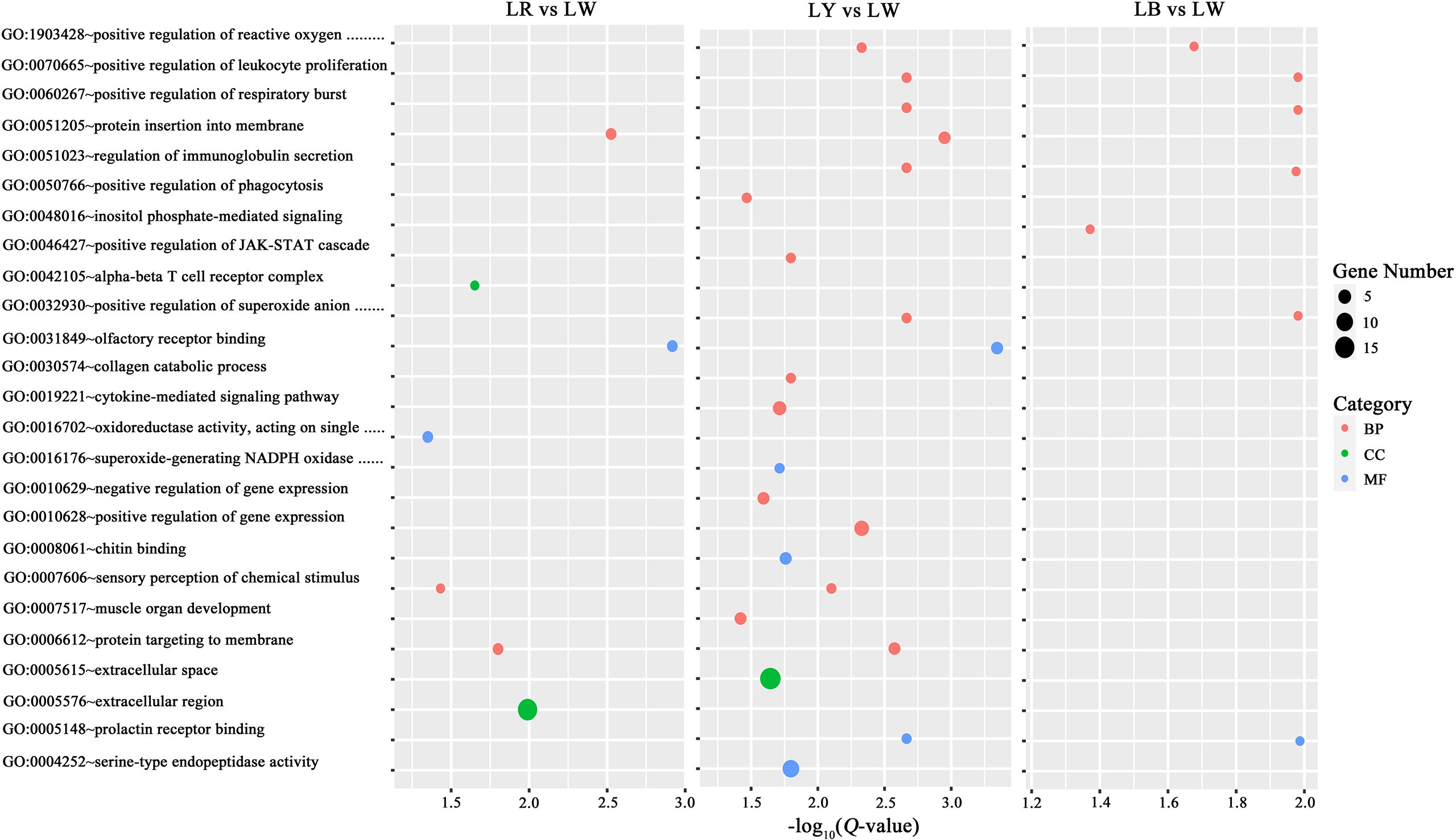
Figure 4 Enriched GO terms of biological processes (BPs; red), cellular components (CCs; green), and molecular functions (MFs; blue) between treatment groups and LW. The dots’ size represents the number of genes involved in a function. GO, Gene Ontology; LW, full-spectrum light.
The Venn diagram (Figure 5) shows that the GO BP terms of LR vs. LW are mainly related to cell growth and apoptosis, protein localization, DNA damage and repair, energy generation, neurotransmitter transport, sensory perception, immune and stress responses, and proteometabolism. GO BP terms of LY vs. LW are mainly related to immune and stress responses, proteometabolism, protein localization, DNA damage and repair, energy generation, the JAK-STAT cascade, sensory perception, neurodevelopment, osteogenesis, muscle morphogenesis, and the cytokine-mediated signaling pathway. GO BP terms of LB vs. LW are mainly related to immune and stress responses, cell growth and apoptosis, proteometabolism, DNA damage and repair, neurodevelopment, lipid metabolism, glycometabolism, the JAK-STAT cascade, and smoothened signaling pathway.
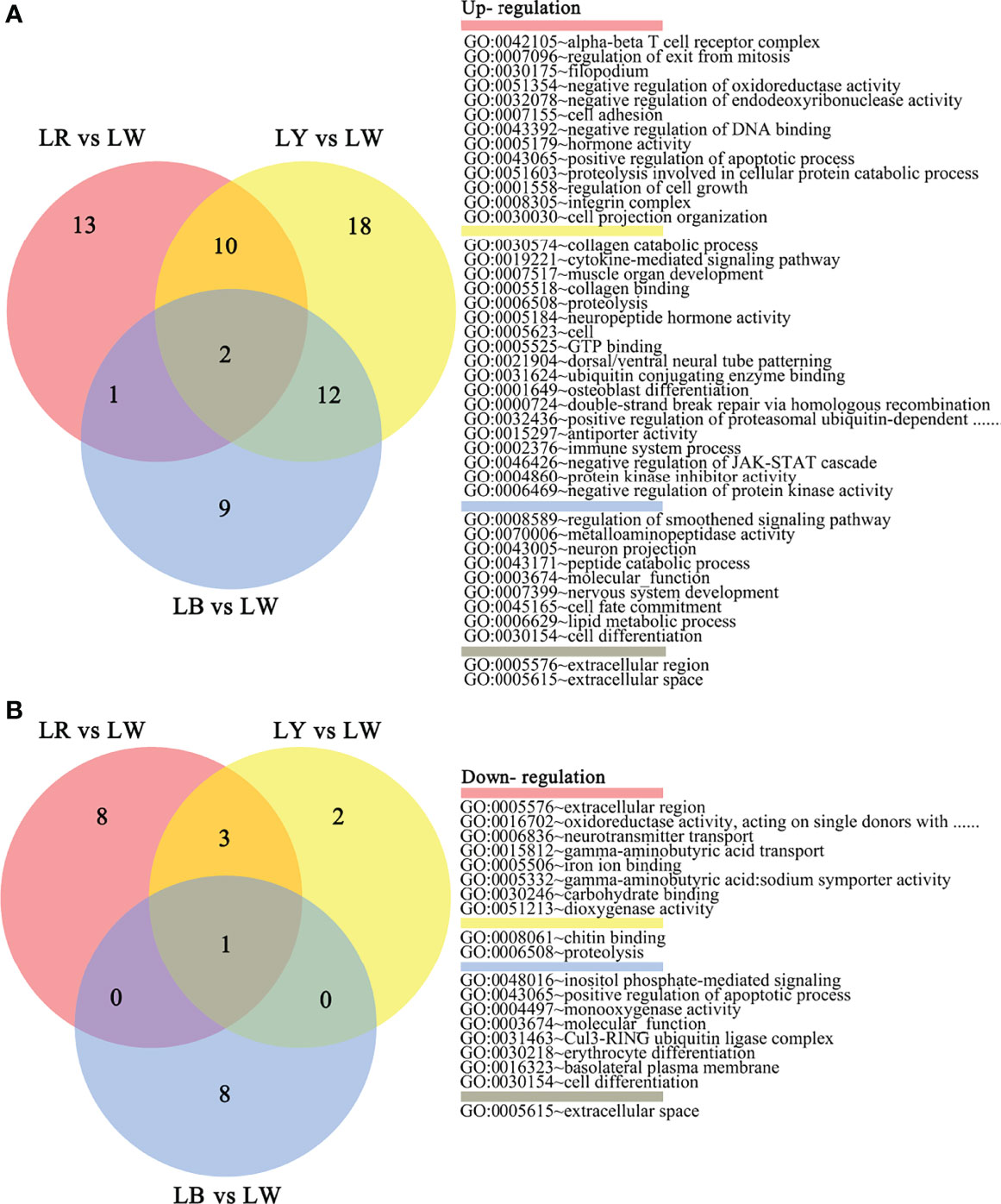
Figure 5 The number of different GO terms upregulated (A) and downregulated (B) in each treatment vs. LW of GIFT tilapia juveniles. The DEGs common to different groups were represented as overlapped regions, and those unique to each group were represented as non-overlapped regions. GO, Gene Ontology; LW, full-spectrum light; DEGs, differentially expressed genes.
KEGG results showed that the tryptophan metabolism signaling pathway, protein digestion and absorption signaling pathway, Jak-STAT signaling pathway, arachidonic acid metabolism signaling pathway, and alpha-linolenic acid metabolism signaling pathway were enriched (Supplementary Table 3). These signaling pathways play a significant role in teleost fishes’ behavior, growth, and immunology. We further verified the expression of three genes (Ccyp1a, Cyp1b1, and Cyp1c1) of the tryptophan metabolism signaling pathway, two genes (Slc7a6 and Kcnj13) of the protein digestion and absorption signaling pathway, four genes (Socs1b, Smtla, Prl, and Gh1) of the Jak-STAT signaling pathway, three genes (Cbr1, Pla2g1b, and Ggt1b) of the arachidonic acid metabolism signaling pathway, and Pla2g1b of the alpha-linolenic acid metabolism signaling pathway by RT-qPCR. A significant positive correlation between RNA-seq and RT-qPCR was shown by a principal component analysis (PCA) (r = 0.569, p < 0.0001; Figure 6), supporting the reliability of the RNA-seq data.
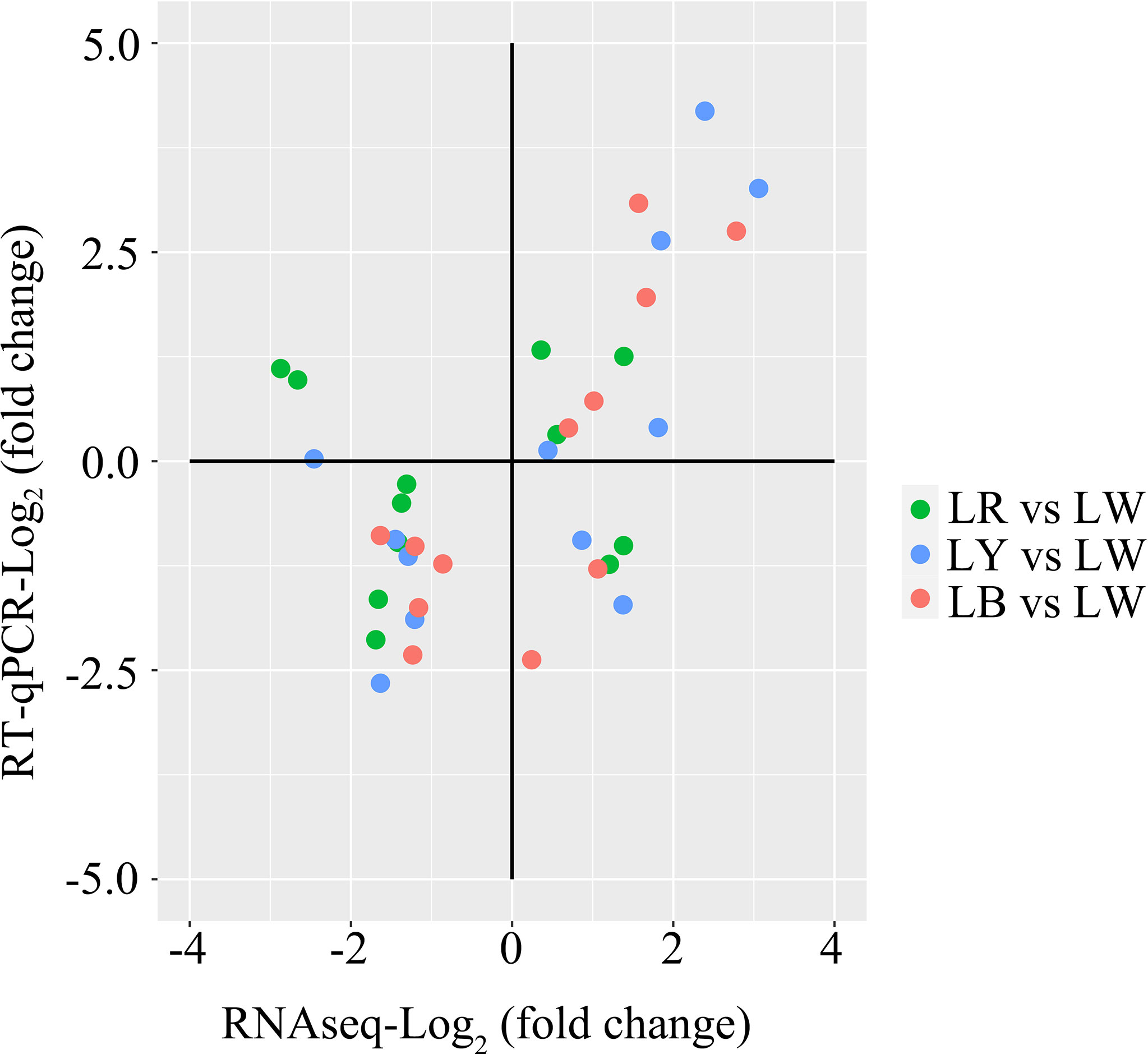
Figure 6 Correlation between RNA-seq and RT-qPCR determination of gene expression (r = 0.569, p < 0.0001). Green, comparisons between LR and LW (LR vs. LW). Blue, comparisons between LY and LW (LY vs. LW). Red, comparisons between LB and LW (LB vs. LW). LR, red light; LW, full-spectrum light; LY, yellow light; LB, blue light.
4 Discussion
Light influences fish through light intensity, spectra, and photoperiod (Karakatsouli et al., 2010; Qiu et al., 2015; Wei et al., 2021). Teleost fishes have different preferred spectral environments and exhibit different responses, including growth performance, stress responses, or behavior (Hou et al., 2019; Jin et al., 2019). The ideal light spectrum for farmed fish should be reflected in production efficiency and animal welfare. Moreover, the welfare of farmed fish should be assessed through several endpoints, such as metabolic, performance, anatomical, behavioral, and physiological indicators (Toni et al., 2019). Unfortunately, relatively few studies have shed light on farmed fishes’ welfare under different light spectra. In this study, the growth, stress, and behavioral responses of GIFT tilapia juveniles cultured in different light spectra were assessed.
More and more evidence indicates that the light spectrum influences the growth of teleost fishes (Bairwa et al., 2017; Wu et al., 2020). Body weight, body length, and SGR are growth parameters commonly used (Du and Turchini, 2021), and crude protein and lipid contents of an organism can be used to measure the compositional status of fish (Shioya et al., 2012). Our results indicated that the light spectrum affected the growth of GIFT tilapia juveniles. GIFT tilapia juveniles showed higher growth performance, measured as body weight, length, SGR, and crude protein and lipid contents under LW and LR. These findings are consistent with results on European sea bass larvae and common carp. The total length of European sea bass larvae was significantly larger when reared under white light (Villamizar et al., 2009). Common carp showed higher body weight, total length, CF, and SGR under red than under white and blue lights (Karakatsouli et al., 2010). Nevertheless, the influence of the light spectrum on fish growth varied with fish species or growth and developmental stage (Karakatsouli et al., 2007; Villamizar et al., 2009; Karakatsouli et al., 2010). Turbot larval growth performance was affected by the light spectrum, and the influence was stage-specific (Wu et al., 2020). Spotted sea bass juveniles had the highest growth performance under blue light, while red seriously hurt their growth (Hou et al., 2019). Blue and green lights accelerated the growth of koi carp (Bairwa et al., 2017). This study with the GIFT strain of Nile tilapia achieved higher body weight and SGR of fish under LW and LR in terms of higher crude protein, crude lipids, and body length. Furthermore, the lower CF of fish under LW and LR may be due to the relatively fast development of body length. Digestive enzymes play an essential role in digesting nutrients, such as starch, fat, and protein of feed (García-Meilán et al., 2016). Absorption capacities are affected by environmental factors such as photoperiod, stocking density, and biotic factors such as species and diet (Espinosa-Chaurand et al., 2017; García-Meilán et al., 2020; Hernández-López et al., 2021; Upadhyay et al., 2022). AMS, PES, and LPS activities of GIFT tilapia juveniles under LW and LR were higher, compared with LY and LB. We explain this as increased digestive enzyme activities inducing GIFT tilapia juveniles’ higher crude protein and lipid contents under LW and LR.
Reactive oxygen species (ROS) are easily induced during the oxidative decomposition of organic matter. The antioxidant defense system can scavenge ROS and protect against oxidative stress; T-AOC and SOD are important indicators for evaluating the antioxidant capacity of aquatic animals (Kong et al., 2021). Oxidative stress occurs when there is an imbalance between ROS production and the ability of the antioxidant defense system. Furthermore, COR is an important indicator of stress (Samaras et al., 2021). In this study, SOD activities of GIFT tilapia juveniles under LB, and T-AOC and COR content of fish under LY were the highest. These results indicated that tilapia juveniles reared under LY and LB suffered oxidative stress. Previous studies have reported that the light spectrum could influence the antioxidant defense system of teleost fishes. Green wavelength light could prevent oxidative stress of the olive flounder under high density (Choi et al., 2019). Nevertheless, unsuitable light spectrum conditions can cause oxidative stress. The SOD activity of zebrafish under blue light-emitting diodes was higher than that under white fluorescent bulbs (Yuan et al., 2017). A higher COR level of snubnose pompano Trachinotus blochii (Lacépède, 1801) larvae under yellow light was observed (Mapunda et al., 2021). These results support our interpretation that LY and LB induced oxidative stress in tilapia juveniles.
Individual behavior is the comprehensive reflection of the external environment and internal factors. It has considerable practical significance for understanding the behavioral ecology and improving breeding protocols for fish, so the behavior should be considered when assessing fish welfare (Barreto et al., 2022). The light spectrum is an external environmental factor affecting farmed fish behavior (Li et al., 2021). Lighting conditions strongly affected the behavioral responses of European sea bass larvae (Villamizar et al., 2011b). In this research, GIFT tilapia juveniles under LW and LY exhibited more aggressive chase and bite behaviors than fish under LR and LB, indicating that juvenile fish under LW and LY experienced poor welfare. Previous research also showed that an unsuitable light spectrum induced discomfort or abnormal animal behavior. The red spectrum, disturbing endocrine homeostasis associated with aggressive behaviors, induced stress responses, while blue light was beneficial for spotted sea bass culture (Hou et al., 2019). Exposure to ultraviolet light affected the behavior of virile (F. virilis) and rusty (F. rusticus) crayfishes, and the duration of time spent fighting and the number of fights significantly increased (Jackson and Moore, 2019). Collector urchins are highly sensitive to and dislike blue LED light, exhibiting the most hiding behavior with a lower level of melanin expression (Li et al., 2021). These results indicate that aquatic animals exhibit different preferred light spectra and that preferred light spectra for different species at different developmental stages should be determined and provided in culture systems. In the wild, Nile tilapia prefers to live in shallow waters and the upper, middle, or near surface of the water during the daytime, where the spectral composition is varied including the long wavelengths (Villamizar et al., 2011a). In the current study, GIFT tilapia juveniles under LR exhibited faster growth performance, fewer stress responses, and more aggressive behaviors as compared with those under LW, LY, and LB. Therefore, red light is the preferred light spectra for GIFT tilapia juveniles.
Results of this study suggested that the light spectrum had growth, stress, and behavioral influences on GIFT tilapia juveniles. Furthermore, the results of previous studies support our findings (Villamizar et al., 2009; Bairwa et al., 2017; Yang et al., 2021). Therefore, RNA-seq was carried out to investigate potential molecular pathways. Results of the GO BP revealed that the component of the nervous system associated with behavior (GO:0006836 neurotransmitter transport and GO:0005332~gamma-aminobutyric acid:sodium symporter activity) was significantly affected by LR, leading to decreased aggression behaviors, such as chase and bite. GIFT tilapia juveniles under LY showed that muscle morphogenesis (GO:0007517 muscle organ development and GO:0021904: dorsal/ventral neural tube patterning) and immune system (GO: 0002376 immune system process) functions associated with the growth and immune responses were significantly affected, resulting in decreased growth performance and more stress. Juvenile fish exposed to blue light showed that nervous system development and lipid metabolic processes changed significantly, resulting in decreased growth performance and aggressive behaviors.
Moreover, we analyzed the potential mechanisms of the spectrum on the growth, stress, and behavior of GIFT tilapia juveniles through the KEGG pathway. Tryptophan (Trp) is a precursor of crucial compounds related to immune and nerve development and social behavior. Disturbance of Trp metabolic pathways, as well as the content of Trp or its derivatives, can induce behavioral and neuropsychiatric disorders (Galley et al., 2021). Studies show that Cyp1a1 and Cyp1b1 of this pathway regulated Trp/5-HT metabolism and transport (Galley et al., 2021). Moreover, aberrant metabolism and transport of Trp/5-HT can affect normal neurodevelopment and behavior (Connors et al., 2014; Herculano and Maximo, 2014; Barreiro-Iglesias et al., 2015). In this study, the tryptophan metabolism signaling pathway of GIFT tilapia juveniles under different light spectra was enriched, and the behavior of fish exposed to LR and LB changed significantly as compared with fish under LW.
The JAK-STAT signal pathway is crucial to cellular proliferation, differentiation, and immunomodulation. In medaka (Oryzias melastigma), the JAK-STAT signaling pathway regulates hepcidin1 expression, which exerts an important role associated with pathogen infection or inflammation (Wrighting and Andrews, 2006; Cui et al., 2019). In our study, GIFT tilapia juveniles exposed to LY underwent stress, reflected by the increased T-AOC and COR contents. There are reports that long wavelengths of light can induce stress and immune responses in turbot larvae (Wu et al., 2020), which is consistent with our results. Moreover, the transcriptomic analysis revealed that the JAK-STAT signaling pathway associated with immunomodulator was enriched in tilapia juveniles under LY. Thus, we believe that the light spectrum regulated the JAK-STAT signaling pathway, inducing the stress responses of juvenile fish exposed to LY.
The protein digestion and absorption pathway regulate the hydrolysis and transport of proteins, peptides, and amino acids. Metabolism of protein and lipid in the diet directly influences fish growth, such as weight gain, protein content, trypsin, and chymotrypsin specific activities (Ma et al., 2019). Dietary nutrition regulated protein digestion of large yellow croaker (Larimichthys crocea) larvae through the transcription of peptide transporter 1, cholecystokinin, and trypsin, and growth performance was influenced (Cai et al., 2015). Choline deficiency impeded the absorption of intestinal amino acids in juvenile grass carp (Ctenopharyngodon idella), which might be related to the expression of the corresponding transporters, including SLC7A6 associated with protein digestion and absorption pathway (Yuan et al., 2020). Fatty acids (FAs), especially arachidonic acid and alpha-linolenic acid, are among the most important nutritional factors for successful fish production (Norambuena et al., 2013; Magalhães et al., 2021). Dietary arachidonic acid could regulate lipid metabolism and accelerate the growth of striped bass (Morone saxatilis) juveniles (Araújo et al., 2021). Dietary linoleic acid affected the growth and FA composition of rabbitfish (Siganus canaliculatus) (Xie et al., 2018), and both the growth and gonadal development of the juvenile common carp were altered (Ma et al., 2020). The main FA constituents significantly affected pike perch (Sander lucioperca) larvae tissue FA content (Lund et al., 2019). In the current research, GIFT tilapia juveniles under LY and LB exhibited poor growth performance. Considering the vital role of these signal pathways on fish growth, we believe that light spectra regulated protein digestion and absorption, arachidonic acid metabolism, and alpha-linolenic acid metabolism pathways, inducing poor growth of juvenile fish exposed to LY and LB.
5 Conclusions
This study confirmed that light spectra significantly affected the welfare of GIFT tilapia juveniles in terms of growth performance, stress responses, and behavior. LW and LR positively affected the growth performance of tilapia juveniles, while LY could induce a stress response in juvenile fish. Moreover, tilapia juveniles exposed to LW and LY exhibited more aggressive behaviors than fish under LR and LB. The transcriptomic analysis demonstrated that different light spectra could regulate signaling pathways associated with the tilapia juveniles’ growth, stress, and behavior. In terms of growth performance, stress responses, and behavior, the optimal light spectrum for the welfare of GIFT tilapia juveniles is LR.
Data Availability Statement
Illumina NovaSeq 6000 were used to generate transcriptomic data. The data are deposited in the NCBI repository, accession number PRJNA833495.
Ethics Statement
The animal study was reviewed and approved by Laboratory Animal Ethics Committee Pearl River Fisheries Research Institute, CAFS.
Author Contributions
MY: conceptualization, data curation, formal analysis, investigation, methodology, writing—original draft, and writing—review and editing. WZ: validation and formal analysis. MW: software and visualization. HW: methodology and writing—reviewing and editing. ZL: visualization. FG: data curation. XK: project administration. CS: writing—reviewing and editing. JC: resources. ML: supervision and conceptualization. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 31902428], China Agriculture Research System of MOF and MARA [grant number CARS-46], Independent Research and Development Projects of Maoming Laboratory [grant number 2021ZZ007], National Natural Science Foundation of China [grant number 31972783], and Guangdong Basic and Applied Basic Research Foundation [grant numbers 2019A1515111046, 2021A1515010852].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.924110/full#supplementary-material
References
Araújo B. C., Rodriguez M., Honji R. M., Rombenso A. N., del Rio-Zaragoza O. B., Cano A., et al. (2021). Arachidonic Acid Modulated Lipid Metabolism and Improved Productive Performance of Striped Bass (Morone Saxatilis) Juvenile Under Sub- to Optimal Temperatures. Aquaculture 530, 735939. doi: 10.1016/j.aquaculture.2020.735939
Ashley P. J. (2007). Fish Welfare: Current Issues in Aquaculture. Appl. Anim. Behav. Sci. 104, 199–235. doi: 10.1016/j.applanim.2006.09.001
Bairwa M. K., Saharan N., Rawat K. D., Tiwari V. K., Prasad K. P. (2017). Effect of Light Spectra on Growth Performance and Immune Response of Koi Carp, Cyprinus Carpio (Linnaeus 1758). Fishery Technol. 54, 100–106. doi: 10.18805/ijar.v0iof.8452
Barreiro-Iglesias A., Mysiak K. S., Scott A. L., Reimer M. M., Yang Y., Becker C. G., et al. (2015). Serotonin Promotes Development and Regeneration of Spinal Motor Neurons in Zebrafish. Cell Rep. 13, 924–932. doi: 10.1016/j.celrep.2015.09.050
Barreto M. O., Rey Planellas S., Yang Y., Phillips C., Descovich K. (2022). Emerging Indicators of Fish Welfare in Aquaculture. Rev. Aquacul. 14, 343–361. doi: 10.1111/raq.12601
Cai Z., Li W., Mai K., Xu W., Zhang Y., Ai Q. (2015). Effects of Dietary Size-Fractionated Fish Hydrolysates on Growth, Activities of Digestive Enzymes and Aminotransferases and Expression of Some Protein Metabolism Related Genes in Large Yellow Croaker (Larimichthys Crocea) Larvae. Aquaculture 440, 40–47. doi: 10.1016/j.aquaculture.2015.01.026
Cheng C. L., Flamarique I. N. (2004). New Mechanism for Modulating Colour Vision. Nature 428, 279–280. doi: 10.1038/428279a
Choi C. Y., Choi J. Y., Choi Y. J., Kim B. S., Kim J. W. (2019). Effects of Green Wavelength Light on Antioxidant and Non-Specific Immune Responses of the Olive Flounder Paralichthys Olivaceus Maintained at Different Stocking Densities. Aquacul. Eng. 84, 23–28. doi: 10.1016/j.aquaeng.2018.11.004
Connors K. A., Valenti T. W., Lawless K., Sackerman J., Onaivi E. S., Brooks B. W., et al. (2014). Similar Anxiolytic Effects of Agonists Targeting Serotonin 5-HT1A or Cannabinoid CB Receptors on Zebrafish Behavior in Novel Environments. Aquat. Toxicol. 151, 105–113. doi: 10.1016/j.aquatox.2013.12.005
Cui Q., Chen F. Y., Zhang M., Peng H., Wang K. J. (2019). Transcriptomic Analysis Revealing Hepcidin Expression in Oryzias Melastigma Regulated Through the JAK-STAT Signaling Pathway Upon Exposure to BaP. Aquat. Toxicol. 206, 134–141. doi: 10.1016/j.aquatox.2018.11.015
Du Z. Y., Turchini G. M. (2021). Are We Actually Measuring Growth?—An Appeal to Use a More Comprehensive Growth Index System for Advancing Aquaculture Research. Rev. Aquacul. 14, 525–527. doi: 10.1111/raq.12604
Espinosa-Chaurand D., Vega-Villasante F., Carrillo-Farnés O., Nolasco-Soria H. (2017). Effect of Circadian Rhythm, Photoperiod, and Molt Cycle on Digestive Enzymatic Activity of Macrobrachium Tenellum Juveniles. Aquaculture 479, 225–232. doi: 10.1016/j.aquaculture.2017.05.029
FAO (2018). The State of World Fisheries and Aquaculture 2018 - Meeting the Sustainable Development Goals (Rome).
Galley J. D., Chen H. J., Antonson A. M., Gur T. L. (2021). Prenatal Stress-Induced Disruptions in Microbial and Host Tryptophan Metabolism and Transport. Behav. Brain Res. 414, 113471. doi: 10.1016/j.bbr.2021.113471
García-Meilán I., Ordóñez-Grande B., Machahua C., Buenestado S., Fontanillas R., Gallardo M. A. (2016). Effects of Dietary Protein-to-Lipid Ratio on Digestive and Absorptive Processes in Sea Bass Fingerlings. Aquaculture 463, 163–173. doi: 10.1016/j.aquaculture.2016.05.039
García-Meilán I., Ordóñez-Grande B., Valentín J. M., Fontanillas R., Gallardo Á. (2020). High Dietary Carbohydrate Inclusion by Both Protein and Lipid Replacement in Gilthead Sea Bream. Changes in Digestive and Absorptive Processes. Aquaculture 520, 734977. doi: 10.1016/j.aquaculture.2020.734977
Herculano A. M., Maximino C. (2014). Serotonergic Modulation of Zebrafish Behavior: Towards a Paradox. Prog. Neuropsychopharmacol. Biol. Psychiatry 55, 50–66. doi: 10.1016/j.pnpbp.2014.03.008
Hernández-López I. A., Ibarra-Castro L., Álvarez-González C. A., Martínez-Brown J. M., Maytorena-Verdugo C. I., Peña-Marín E. S. (2021). Characterization of Digestive Enzymes During Early Ontogeny of White Snook (Centropomus Viridis). Aquaculture 535, 736399. doi: 10.1016/j.aquaculture.2021.736399
Hou Z. S., Wen H. S., Li J. F., He F., Li Y., Qi X., et al. (2019). Effects of Photoperiod and Light Spectrum on Growth Performance, Digestive Enzymes, Hepatic Biochemistry and Peripheral Hormones in Spotted Sea Bass (Lateolabrax Maculatus). Aquaculture 507, 419–427. doi: 10.1016/j.aquaculture.2019.04.029
Jackson K. M., Moore P. A. (2019). The Intensity and Spectrum of Artificial Light at Night Alters Crayfish Interactions. Conserv. Physiol. 8, 131–150. doi: 10.1080/10236244.2019.1663124
Jin G., Zhao J., Zhang Y. D., Liu G., Liu D. Z., Zhu S. M., et al. (2019). Light Spectrum Preference of Nile Tilapia (Oreochromis Niloticus) Under Different Hunger Levels. Int. J. Agric. Biol. Eng. 12, 51–57. doi: 10.25165/j.ijabe.20191205.4170
Karakatsouli N., Papoutsoglou S. E., Pizzonia G., Tsatsos G., Tsopelakos A., Chadio S., et al. (2007). Effects of Light Spectrum on Growth and Physiological Status of Gilthead Seabream Sparus Aurata and Rainbow Trout Oncorhynchus Mykiss Reared Under Recirculating System Conditions. Aquacul. Eng. 36, 302–309. doi: 10.1016/j.aquaeng.2007.01.005
Karakatsouli N., Papoutsoglou E. S., Sotiropoulos N., Mourtikas D., Stigen-Martinsen T., Papoutsoglou S. E. (2010). Effects of Light Spectrum, Rearing Density and Light Intensity on Growth Performance of Scaled and Mirror Common Carp Cyprinus Carpio Reared Under Recirculating System Conditions. Aquacul. Eng. 42, 121–127. doi: 10.1016/j.aquaeng.2010.01.001
Khaw H. L., Ponzoni R. W., Hamzah A., Abu-Bakar K. R., Bijma P. (2012). Genotype by Production Environment Interaction in the GIFT Strain of Nile Tilapia (Oreochromis Niloticus). Aquaculture 326-329, 53–60. doi: 10.1016/j.aquaculture.2011.11.016
Kong Y., Li M., Chu G., Liu H., Shan X., Wang G., et al. (2021). The Positive Effects of Single or Conjoint Administration of Lactic Acid Bacteria on Channa Argus: Digestive Enzyme Activity, Antioxidant Capacity, Intestinal Microbiota and Morphology. Aquaculture 531, 735852. doi: 10.1016/j.aquaculture.2020.735852
Li Y. Y., Su F. J., Hsieh Y. J., Huang T. C., Wang Y. S. (2021). Embryo Development and Behavior in Sea Urchin (Tripneustes Gratilla) Under Different Light Emitting Diodes Condition. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.684330
Lopez-Luna J., Ibanez M. A., Villarroel M. (2013). Using Multivariate Analysis of Water Quality in RAS With Nile Tilapia (Oreochromis Niloticus) to Model the Evolution of Macronutrients. Aquacul. Eng. 54, 22–28. doi: 10.1016/j.aquaeng.2012.10.005
Lund I., Rodríguez C., Izquierdo M. S., El Kertaoui N., Kestemont P., Reis D. B., et al. (2019). Influence of Salinity and Linoleic or α-Linolenic Acid Based Diets on Ontogenetic Development and Metabolism of Unsaturated Fatty Acids in Pike Perch Larvae (Sander Lucioperca). Aquaculture 500, 550–561. doi: 10.1016/j.aquaculture.2018.10.061
Magalhães R., Martins N., Fontinha F., Moutinho S., Olsen R. E., Peres H., et al. (2021). Effects of Dietary Arachidonic Acid and Docosahexanoic Acid at Different Carbohydrates Levels on Gilthead Sea Bream Growth Performance and Intermediary Metabolism. Aquaculture 545, 737233. doi: 10.1016/j.aquaculture.2021.737233
Ma R., Liu X., Meng Y., Wu J., Zhang L., Han B., et al. (2019). Protein Nutrition on Sub-Adult Triploid Rainbow Trout (1): Dietary Requirement and Effect on Anti-Oxidative Capacity, Protein Digestion and Absorption. Aquaculture 507, 428–434. doi: 10.1016/j.aquaculture.2019.03.069
Mapunda J., Mtolera M. S. P., Yahya S. A. S., Ngo V. M., Golan M. (2021). Light Colour Affect the Survival Rate, Growth Performance, Cortisol Level, Body Composition, and Digestive Enzymes Activities of Different Snubnose Pompano (Trachinotus Blochii (Lacépède 1801) Larval Stages. Aquacul. Rep. 21, 100804. doi: 10.1016/j.aqrep.2021.100804
Ma X., Wang L., Xie D., Tian X., Zhang Y., Wu L., et al. (2020). Effect of Dietary Linolenic/Linoleic Acid Ratios on Growth Performance, Ovarian Steroidogenesis, Plasma Sex Steroid Hormone, and Tissue Fatty Acid Accumulation in Juvenile Common Carp, Cyprinus Carpio. Aquacul. Rep. 18, 100452. doi: 10.1016/j.aqrep.2020.100452
Norambuena F., Morais S., Estévez A., Bell J. G., Tocher D. R., Navarro J. C., et al. (2013). Dietary Modulation of Arachidonic Acid Metabolism in Senegalese Sole (Solea Senegalensis) Broodstock Reared in Captivity. Aquaculture, 372–375, 80-88. doi: 10.1016/j.aquaculture.2012.10.035
Qiu D., Xu S., Song C., Chi L., Li X., Sun G., et al. (2015). Effects of Spectral Composition, Photoperiod and Light Intensity on the Gonadal Development of Atlantic Salmon Salmo Salar in Recirculating Aquaculture Systems (RAS). Chin. J. Oceanol. Limnol. 33, 45–56. doi: 10.1007/s00343-015-4011-3
Samaras A., Dimitroglou A., Kollias S., Skouradakis G., Papadakis I. E., Pavlidis M. (2021). Cortisol Concentration in Scales is a Valid Indicator for the Assessment of Chronic Stress in European Sea Bass, Dicentrarchus Labrax L. Aquaculture 545, 737257. doi: 10.1016/j.aquaculture.2021.737257
Shioya I., Takemura S., Ishizuka R., Yamaguchi T. (2012). Variations in the Proximate Composition of Muscle in Cultured Yellowtail Seriola Quinqueradiata at Different Anatomical Portions. Fish. Sci. 78, 725–733. doi: 10.1007/s12562-012-0477-5
Toni M., Angiulli E., Malavasi S., Alleva E., Cioni C. (2017). Variation in Environmental Parameters in Research and Aquaculture: Effects on Behaviour, Physiology and Cell Biology of Teleost Fish. J. Aquacul. Mar. Biol. 5, 137. doi: 10.15406/jamb.2017.05.00137
Toni M., Manciocco A., Angiulli E., Alleva E., Cioni C., Malavasi S. (2019). Review: Assessing Fish Welfare in Research and Aquaculture, With a Focus on European Directives. Animal 13, 161–170. doi: 10.1017/S1751731118000940
Upadhyay A., Swain H. S., Das B. K., Ramteke M. H., Kumar V., Krishna G., et al. (2022). Stocking Density Matters in Open Water Cage Culture: Influence on Growth, Digestive Enzymes, Haemato-Immuno and Stress Responses of Puntius Sarana (Ham 1822). Aquaculture 547, 737445. doi: 10.1016/j.aquaculture.2021.737445
Villamizar N., Blanco-Vives B., Migaud H., Davie A., Carboni S., Sánchez-Vázquez F. J. (2011a). Effects of Light During Early Larval Development of Some Aquacultured Teleosts: A Review. Aquaculture 315, 86–94. doi: 10.1016/j.aquaculture.2010.10.036
Villamizar N., Garcia-Alcazar A., Sanchez-Vazquez F. J. (2009). Effect of Light Spectrum and Photoperiod on the Growth, Development and Survival of European Sea Bass (Dicentrarchus Labrax) Larvae. Aquaculture 292, 80–86. doi: 10.1016/j.aquaculture.2009.03.045
Villamizar N., Garcia-Mateos G., Sanchez-Vazquez F. J. (2011b). Behavioral Responses of European Sea Bass (Dicentrarchus Labrax) Larvae and Artemia Sp Exposed to Constant Light or Darkness vs. Light/Dark Cycles of White, Red or Blue Wavelengths. Aquaculture 317, 197–202. doi: 10.1016/j.aquaculture.2011.03.036
Villarroel M., Alavrino J. M. R., Lopez-Luna J. (2011). Effect of Feeding Frequency and One Day Fasting on Tilapia (Oreochromis Niloticus) and Water Quality. Israeli. J. Aquaculture-Bamidgeh. 63, 1–6. doi: 10.46989/001c.20600
Wei J., Tian L., Wang Y., Yu L., Zhu X. (2021). Effects of Salinity, Photoperiod, and Light Spectrum on Larval Survival, Growth, and Related Enzyme Activities in the Giant Freshwater Prawn, Macrobrachium Rosenbergii. Aquaculture 530, 735794. doi: 10.1016/j.aquaculture.2020.735794
Wrighting D. M., Andrews N. C. (2006). Interleukin-6 Induces Hepcidin Expression Through STAT3. Blood 108, 3204–3209. doi: 10.1182/blood-2006-06-027631
Wu L., Han M., Song Z., Xu S., Li J., Li X., et al. (2019). Effects of Different Light Spectra on Embryo Development and the Performance of Newly Hatched Turbot (Scophthalmus Maximus) Larvae. Fish. Shellfish. Immunol. 90, 328–337. doi: 10.1016/j.fsi.2019.05.007
Wu L., Wang Y., Han M., Song Z., Song C., Xu S., et al. (2020). Growth, Stress and non-Specific Immune Responses of Turbot (Scophthalmus Maximus) Larvae Exposed to Different Light Spectra. Aquaculture 520, 734950. doi: 10.1016/j.aquaculture.2020.734950
Xie D., Liu X., Wang S., You C., Li Y. (2018). Effects of Dietary LNA/LA Ratios on Growth Performance, Fatty Acid Composition and Expression Levels of Elovl5, Δ4 Fad and Δ6/Δ5 Fad in the Marine Teleost Siganus Canaliculatus. Aquaculture 484, 309–316. doi: 10.1016/j.aquaculture.2017.08.039
Yang M. F., Chen Z. L., Hu F. Y., Sun J. N., Ding J. Y., Chang Y. Q., et al. (2020). Light Spectra Regulated Foraging and Feeding Behaviors Shed Light on Stock Enhancement of the Sea Urchin Strongylocentrotus Intermedius. Aquacul. Rep. 18, 100480. doi: 10.1016/j.aqrep.2020.100480
Yang M. F., Hu F. Y., Leng X. F., Chi X. M., Yin D. H., Ding J. Y., et al. (2021). Long-Term Effects of Light Spectra on Fitness Related Behaviors and Growth of the Sea Urchin Strongylocentrotus Intermedius. Aquaculture 537, 736518. doi: 10.1016/j.aquaculture.2021.736518
Yuan Z. H., Feng L., Jiang W. D., Wu P., Liu Y., Jiang J., et al. (2020). Choline Deficiency Decreased the Growth Performances and Damaged the Amino Acid Absorption Capacity in Juvenile Grass Carp (Ctenopharyngodon Idella). Aquaculture 518, 734829. doi: 10.1016/j.aquaculture.2019.734829
Yuan S. S., Lv Z. M., Zhu A. Y., Zheng J. L., Wu C. W. (2017). Negative Effect of Chronic Cadmium Exposure on Growth, Histology, Ultrastructure, Antioxidant and Innate Immune Responses in the Liver of Zebrafish: Preventive Role of Blue Light Emitting Diodes. Ecotoxicol. Environ. Saf. 139, 18–26. doi: 10.1016/j.ecoenv.2017.01.021
Keywords: light spectrum, GIFT tilapia juvenile, growth, stress, behavior, welfare, transcriptomics
Citation: Yi M, Zhai W, Wang M, Wang H, Liu Z, Gao F, Ke X, Song C, Cao J and Lu M (2022) The Welfare of Nile Tilapia (Oreochromis niloticus, GIFT Strain) Juveniles Cultured in Different Light Spectra. Front. Mar. Sci. 9:924110. doi: 10.3389/fmars.2022.924110
Received: 20 April 2022; Accepted: 12 May 2022;
Published: 14 June 2022.
Edited by:
Marce Herrera, IFAPA Centro Agua del Pino, SpainReviewed by:
Chao Song, Chinese Academy of Fishery Sciences, ChinaMing Jiang, Chinese Academy of Fishery Sciences, China
Eric M. Hallerman, Virginia Tech, United States
Copyright © 2022 Yi, Zhai, Wang, Wang, Liu, Gao, Ke, Song, Cao and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianmeng Cao, Y2Fvamlhbm1lbmdAYWxpeXVuLmNvbQ==; Maixin Lu, bXgtbHVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Mengmeng Yi
Mengmeng Yi Wanting Zhai
Wanting Zhai Miao Wang
Miao Wang He Wang1
He Wang1 Zhigang Liu
Zhigang Liu Jianmeng Cao
Jianmeng Cao Maixin Lu
Maixin Lu