- 1Forensic Medicine and Toxicology Department, Faculty of Veterinary Science, Zagazig University, Zagazig, Egypt
- 2Department of Zoology, Faculty of Science, Zagazig University, Zagazig, Egypt
- 3Poultry Department, Faculty of Agriculture, Zagazig University, Zagazig, Egypt
- 4Department of Veterinary Medicine, University of Bari, Valenzano, Italy
- 5School of Biosciences and Veterinary Medicine, University of Camerino, Matelica, Italy
- 6Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, Alexandria University, Alexandria, Egypt
The present study assessed the impact of Panax ginseng essential oil (GEO) supplementation on physiological parameters related to productive performance and health status in Nile tilapia reared under standard conditions and exposed to a sub-lethal atrazine (ATZ) concentration. Fish were allocated into 6 groups: the control group was reared in clean water and fed with a commercial basal diet (CNT), two groups were fed with the basal diet supplemented with two different levels of GEO (GEO1 and GEO2, respectively), one group was intoxicated with 1/5 of ATZ 96-h lethal concentration 50 (1.39 mg/L) (ATZ group), and the remaining two groups were fed with the GEO-supplemented diets and concurrently exposed to 1.39 mg ATZ/L (GEO1+ATZ and GEO2+ATZ, respectively). The experiment lasted for 60 days. GEO supplementation exerted a significantly positive influence on fish growth, feed utilization, and hepatic antioxidant defense systems at both levels of supplementation. ATZ exposure significantly reduced fish survival rates and impaired fish growth and feed utilization, with the lowest final weights, weight gain, total feed intake, and the highest feed conversion ratio being recorded in the ATZ-intoxicated group. ATZ exposure caused significant changes in intestinal digestive enzyme activity (decreased lipase activity), hematological indices (decreased hemoglobin, packed cell volume, erythrocytes, and leukocytes), blood biochemical variables (decreased total proteins, albumin, globulins, and immunoglobulin M; increased total cholesterol, triglycerides, and cortisol), and hepatic oxidative/antioxidant indices (decreased glutathione level, superoxide dismutase and catalase enzyme activity and mRNA expression levels, and increased malondialdehyde content). Moreover, in the hepatic tissue of ATZ-intoxicated Nile tilapia, histopathological alterations and upregulated mRNA expression levels of stress- and apoptosis-related genes (Hsp70, caspase 3, and p53) were observed. GEO supplementation in ATZ-treated groups significantly attenuated the aforementioned negative effects, though some parameters did not reach the CNT values. These findings provide further and partly new evidence that sub-lethal ATZ toxicity induces reduced survivability, growth retardation, impaired digestive function, anemia, immunosuppression, hepatic oxidative stress damage, and overall increased stress level in Nile tilapia, and suggest that GEO supplementation may be useful for mitigating this toxicity and provide more general support to the productive performance and health status of this fish species.
1 Introduction
In recent years, the use of essential oils (EOs) derived from medicinal herbs or plants as feed supplements in aquafeed showed a significant increase. EOs contain a high amount of functional bioactive phytochemicals, which offer some unique health benefits for fishes and shrimps farming (Chakraborty and Hancz, 2011; Chakraborty et al., 2014; Ahmadifar et al., 2021). Indeed, EOs have been shown to exert several important activities, including antimicrobial and antiparasitic (Iseppi et al., 2020; Dawood et al., 2021), immunostimulating (Dawood et al., 2020) anti-stress (Souza et al., 2019), and growth promoting (Magouz et al., 2021). Consistently, several studies provided evidence supporting the dietary use of EOs in aquaculture as possible alternative strategies for improving disease resistance, antioxidant capacity, growth performance, and the overall welfare of farmed fishes. Among EOs investigated for potential applications in fish diet were those prepared from menthol (Dawood et al., 2020; Magouz et al., 2021), Origanum vulgare (Abdel-Latif et al., 2020a; Abdel-Latif et al., 2020b; Khafaga et al., 2020), Petroselinum crispum (Farag et al., 2021; Farag et al., 2022), Thymus vulgaris (El Euony et al., 2020; Ghafarifarsani et al., 2021), Lippia alba (Sena et al., 2016; de Freitas Souza et al., 2019), Ocimum basilicum (de Souza et al., 2019), Geranium (Mohamed et al., 2020), and Aloysia triphylla (Zeppenfeld et al., 2014; de Souza et al., 2020). Recently, plant-derived essential oils and lipid-soluble bioactive compounds gained attention due to their higher bioavailability compared to water-soluble bioactive compounds. Moreover, to the best of our knowledge, no study concerning the use of P. ginseng essential oil (GEO) in aquafeed has been proposed so far.
Ginseng has long been used as a traditional remedy in several territories worldwide. Panax ginseng (known as Asian ginseng), Panax notoginseng, and Panax quinquefolius (known as North American ginseng) are the three ginseng species most commonly used for medical purposes, particularly as immunostimulating, antioxidant, cardioprotective, anti-aging, and anti-tumor agents (Yuan et al., 2010). P. ginseng is the main source of a wide range of functional bioactive compounds such as alkaloids, phenolics, vitamins, amino acids, proteins, ginsenosides, and polysaccharides (Zhao et al., 2019). The ginsenosides found in P. ginseng have powerful antioxidant, anti-inflammatory, anti-allergic, and anti-diabetic activities (Bahukhandi et al., 2021). In this sense, the use of ginseng appears promising in aquaculture. For instance, it was reported that dietary P. ginseng polysaccharides could improve the immunity of white shrimp (Liu et al., 2011). Moreover, ginseng extract was found to modulate aflatoxin-induced toxicity in Clarias lazera (Zaki et al., 2011) and positively impact on the growth and immunity of Nile tilapia (Oreochromis niloticus) (El-Sayed et al., 2014). Dietary ginseng has also been shown to enhance the reproductive efficiency of African catfish (Mehrim et al., 2022).
Atrazine (ATZ) is a highly water-soluble herbicide that is used in agriculture worldwide to control broadleaf weeds, especially for corn, sugarcane, pineapple, sorghum, and soybean crop fields (Nwani et al., 2010). Reports showed that up to 20 µg/L of ATZ are frequently detected on surface water runoff, while higher ATZ concentrations may be found in water bodies because of its repeated applications to the crop fields (Graymore et al., 2001; Selim, 2003). Indeed, it is well known that the indiscriminate, frequent and incautious use of herbicides, and/or their accidental discharge from the unhygienically treated effluents into the water flow may reach the aquatic organisms and induce harmful effects, with a long-term negative impact on the surrounding environment (Farag et al., 2021). It has been observed that the 50% lethal concentration (LC50) of ATZ varies greatly among different fish species, ranging from 3 to 100 mg/L (Solomon et al., 2008) and its exposure was able to induce various signs of toxicity in the African catfish (Michael et al., 2018), the Channa Punctatus (Nwani et al., 2010), the snow trout (Schizothorax plagiostomus) (Akhtar et al., 2021), the zebrafish (Danio rerio) (Zhu et al., 2011; Zhu et al., 2011), the common carp (Cyprinus carpio) (Neskovic et al., 1993; Paulino et al., 2012; Xing et al., 2013; Blahova et al., 2014; Wang et al., 2019), the Prochilodus lineatus (Santos and Martinez, 2012), and also in the Nile tilapia (Neamat Allah et al., 2020; Abdel-Warith et al., 2021; Ali et al., 2022).
Signs of ATZ-induced toxicity in aquatic organisms comprise growth retardation (Abdel-Warith et al., 2021; Ali et al., 2022), histopathological changes (Paulino et al., 2012; Blahova et al., 2014; Michael et al., 2018), serum biochemical alterations (Michael et al., 2018), hematological alterations (Akhtar et al., 2021; Abdel-Warith et al., 2021), disruption of the antioxidant system and oxidative stress damage (Nwani et al., 2010; Zhu et al., 2011; Zhu et al., 2011; Santos and Martinez, 2012), changes in liver function enzymes (Neskovic et al., 1993), induction of heat shock protein 70 (Hsp70), apoptotic changes in tissues and/or blood cells (Xing et al., 2013; Wang et al., 2019), and genotoxicity (Santos and Martinez, 2012; Akhtar et al., 2021).
Numerous natural feed supplements have been reported to be useful for mitigating sub-lethal ATZ toxicity in various fish species, such as vitamin E in African catfish (Kadry et al., 2012), Spirulina platensis in common carp (Khalil et al., 2017), fucoidan (Abdel-Warith et al., 2021), beta-glucans (Neamat Allah et al., 2020) and Isatis phytogenic diet in Nile tilapia (Ali et al., 2022), and herbal extracts in Labeo rohita (Pugazhendy, 2014) and Clarias gariepinus (Owolabi and Abdulkareem, 2021). However, no information is available regarding the suitability of GEO for this specific application.
Based on the above premises, this study was planned to assess the impact of a dietary supplementation with GEO on the productive performance and health/welfare status of healthy Nile tilapia, as well as its efficacy in mitigating the negative influence exerted by ATZ on such fishes.
An array of physiological parameters was selected to retrieve information regarding (a) the productivity performance of the farmed animals (assessed by measuring the growth parameters Wt60 and WG, the feed utilization parameters FI and FCR, and survival rate) and (b) particular aspects of the animal health and welfare status that are known to be important for optimal efficiency in the use of feed energy and/or affected by exposure to ATZ and other adverse environmental challenges. More specifically, such aspects included:
● the digestive functionality (assessed by measuring the activity of the digestive enzymes α-amylase, protease, and lipase);
● the health of erythrocytes, namely the blood cells involved in oxygen transport (assessed by measuring their abundance, along with their content of hemoglobin);
● the immune functionality (assessed by measuring the number of circulating leukocytes and serum levels of total globulins and immunoglobulin M);
● the liver structural integrity (assessed by a histomorphological evaluation), functionality (with particular regard to its involvement in protein synthesis, assessed by measuring the blood levels of total proteins, albumin, and globulin, in lipid metabolism, assessed by measuring total cholesterol and triglycerides, and in carbohydrate metabolism, assessed by measuring glucose levels) and oxidative status (assessed by measuring both the activity of the antioxidant enzymes superoxide dismutase and catalase and expression levels of their respective coding genes, as well as tissue contents of the non-enzymatic antioxidant GSH and of the lipid peroxidation product malondialdehyde), along with more in-depth evaluation of hepatocyte pathophysiological reactivity to stressful conditions (assessed by measuring expression levels of the stress-related gene Hsp70 and of the apoptosis-related genes caspase 3 and p53);
● the whole body’s stress level (assessed by measuring serum cortisol levels).
2 Materials and Methods
2.1 Atrazine and Panax ginseng Essential Oil
Atrazine (ATZ; 98.0% purity, molecular weight: 215.68, CAS Number: 1912-24-9) was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). The required exposure dose used in the present study was obtained by dissolving ATZ in dimethyl sulfoxide (DMSO; SERVA Electrophoresis GmbH, De69115 Heidelberg, Germany) so that the final concentration of DMSO did not exceed 0.02%. Panax ginseng essential oil (GEO) was purchased from Elhawag Company for Natural Oils, Nasr City, Cairo, Egypt. The bioactive constituents present in GEO used in the present study were identified by gas chromatography-mass spectroscopy (GC–MS) analysis (Smigielski et al., 2006) and are reported in Figure S1 and Table S1 (Supplementary Files). The main identified compounds were 9,12-octadecadienoic acid (Z,Z) (36.16%), 2,4-decadienal, (E,E)- (CAS) (13.48%), hexadecanoic acid (CAS) (11.46%) and oleic acid (6.39%) followed by isoalantolactone (3.39%), cis-asarone (3.32%), octadecanoic acid (CAS) (3.23%), furoscrobiculin B (2.97%), and eugenol (1.91%).
2.2 Ethical Statement
All experiments have been performed in accordance with the Local Experimental Animal Care Committee guidelines. Experiments have also been approved by the Institutional Ethics Committee (IEC) of the Faculty of Veterinary Medicine, Zagazig University, Egypt (ZU-IACUC/2F/189/2019).
2.3 Fish Procurement, Acclimation, and Husbandry
One hundred eighty Nile tilapia (Oreochromis niloticus) (mean weight ± SD, 40 ± 0.5 g) were enrolled in this study. Fishes were obtained from Abbassa Fish Hatchery, Sharkia, Egypt and allowed to acclimate for 14 days in fiberglass tanks. During the acclimation period, fishes were fed with a basal diet (Aller Aqua Co., Egypt). Ration ingredients and proximate chemical composition are illustrated in Table S2 (Supplementary Files). Fishes were fed thrice daily with 5% of the total fish biomass per tank. The water parameters were kept constant at 26.5–27.5°C, 6.4–7.5 mg/L of dissolved oxygen, pH 7.6–8.0, 0.02–0.04 mg/L of nitrites, 0.02–0.03 mg/L of nitrates, and 0.01–0.03 mg/L of un-ionized ammonia. The photoperiod was adjusted to be 10:14 h light and dark cycles.
2.4 Fish Grouping and Experimental Procedures
The tested GEO was added to the ingredients of the basal ration at two supplementation levels (1.0 and 2.0 mL/kg diet), thoroughly mixed to form a paste, and defined as GEO1 and GEO2 diets, respectively. The prepared paste was passed through a meat mincer to form 1.5-mm pelleted diets, allowed to dry at room temperature for 24 h and then refrigerated at 4°C until the use. After the acclimation period, 180 fishes were grouped into 6 equal groups (30 fish per group); each group was divided into 3 replicates of 10 fishes. Each replicate was allocated in a glass aquaria whose size was 0.8 m × 0.8 m × 1.0 m with a water capacity of 100 L. Fishes were reared in these aquaria for 60 days. The groups were:
● Group 1, which was used as control, reared in clean water and fed with the basal diet (CNT group).
● Groups 2 and 3, which were fed with the freshly prepared GEO1 and GEO2 diets (GEO1 and GEO2 group).
● Group 4, which was fed with the basal diet and exposed to a sub-lethal concentration of ATZ (1.39 mg/L, equal to ⅕ of the previously calculated 96-h LC50 of ATZ for Nile tilapia), based on literature findings (ATZ group) (Neamat Allah et al., 2020).
● Groups 5 and 6, which were fed with GEO1 and GEO2 diets and concomitantly exposed to 1.39 mg ATZ/L (GEO1+ATZ and GEO2+ATZ groups).
About 35% of the water in each aquarium was daily exchanged, and metabolic wastes were removed by siphoning. Due to the occurrence of ATZ degradation in the rearing water, the required exposure concentration was preserved by substituting the ATZ dose every 24 h after water siphoning. Moreover, the feed was adjusted every two weeks according to the new fishes’ weight.
2.5 Growth Parameters, Feed Utilization Parameters, and Survivability
At the end of the feeding experiment (60 days), the fishes’ productive performance was assessed by recording the fishes’ final weight (Wt60) and by estimating weight gain (WG), feed intake (FI), feed conversion ratio (FCR), and survival rate (SR, %) according to the following equations:
2.6 Sampling Procedures
For the assessment of the physiological parameters related to fish health/welfare status, different kinds of biological samples were collected from the animals at the end of the feeding experiment. The specific sampling procedures are detailed below.
2.6.1 Blood and Serum Samples
Fishes were fasted for one day after the feeding trial and before blood sampling. Fishes were sampled from the experimental aquaria and then sedated with tricaine methanesulfonate (100 μg/mL) to decrease the handling stress. Whole blood samples for the hematobiochemical analyses were collected from the caudal vessel of nine fishes per group (3 fishes/replicate) and pooled from each replicate into sterile EDTA-coated test tubes. Further nine blood samples were collected without anticoagulant, left at room temperature, and then centrifuged (2500 ×g for 15 min) to retrieve the serum. All sera were then refrigerated at - 20°C and subsequently used in the hematobiochemical analyses.
2.6.2 Intestinal and Liver Specimens for Tissue Homogenates
Fishes were aseptically necropsied after blood sampling, and intestinal and liver specimens were sampled. Such tissues were kept on ice-cold plates, cleaned with cold sterile saline, dried by filter paper, divided, and then stored frozen at - 20°C. Then 100 mg of each tissue was placed in a tube with 1 ml of a buffer (10 mM phosphate/20 mM tris-pH 7.0) and homogenated at 6000 ×g for 5 min at 4°C. After centrifugation, the supernatants were assembled and then preserved at - 80°C until use. Hepatic homogenates were used to determine oxidative stress markers, while the intestinal ones to evaluate digestive enzymes’ activity.
2.6.3 Liver Specimens for Histopathology and Gene Expression Analysis
A set of liver specimens (n = 9) were sampled, washed with cold physiological buffer saline, and then transferred in 10% buffered formalin for further histopathological studies. Another set of 100 mg of liver specimens was quickly frozen in a liquid nitrogen tank, transferred to the lab, and preserved at - 80°C for gene expression analysis.
2.7 Sample Processing and Analysis for the Assessment of Health/Welfare-Related Parameters
2.7.1 Hematological Parameters
Hematological analysis was performed on whole blood samples for determination of red blood cell (RBC) count, hemoglobin (Hb) concentration, packed cell volume (PCV, %), and white blood cell (WBC) count, using an automatic cell counter (Hospitex Hema screen 18, Italy) (Dacie and Lewis, 1991).
2.7.2 Serum Biochemical Parameters
Total protein (TP; g/dL) and albumin (ALB; g/dL) in sera samples obtained from the different experimental groups were assessed using colorimetric diagnostic kits (Biomed Diagnostic EgyChem kits, Egypt) following the manufacturer’s instructions and a method reported in the literature (Doumas et al., 1981). Globulin (GLO; g/dL) levels were calculated by the differences of TP and ALB levels. The total immunoglobulin M (IgM) level was measured in sera samples using diagnostic kits (Cusabio Biotech Co. Ltd., Wuhan, Hubei, China) (Siwicki and Anderson, 1993; Cuesta et al., 2004). Total cholesterol (mg/dL) and triglycerides (mg/dL) levels in sera samples were quantified by commercial diagnostic kits (Bio diagnostic Co., Giza, Egypt). Moreover, glucose (mg/dL) and cortisol (ng/mL) levels were assessed in sera samples by using diagnostic kits (Cayman Chemical Company, Ellsworth, Ann Arbor, USA).
2.7.3 Intestinal Digestive Enzyme Assays
The α-amylase, protease, and lipase enzymes’ activity in the intestinal homogenates from the different experimental groups were analyzed using commercial colorimetric kits (Spectrum Diagnostic Company, Egypt) following the manufacturer’s instructions.
2.7.4 Oxidative Stress Assays
The superoxide dismutase (SOD) and catalase (CAT) (U/g tissue) activity as well as the concentration of the reduced glutathione (GSH) and malondialdehyde (MDA) (nmol/g tissue) were assessed in the liver homogenates using specific Bio diagnostic kits (Giza, Egypt). Hepatic SOD, CAT, and GSH were analyzed according to the colorimetric methods reported by the literature (Beutler et al., 1963; Nishikimi et al., 1972; Aebi, 1984), while MDA concentrations were assayed using the thiobarbituric acid method reported in the literature (Mihara and Uchiyama, 1978; Ohkawa et al., 1979).
2.7.5 Histopathology
Formalin-fixed liver specimens were dehydrated in ethanol, cleared in xylene, embedded and blocked in paraffin wax, cut into numerous 5-μm sections, and then stained with hematoxylin and eosin stain according to the standard procedures (Suvarna et al., 2013). Several photomicrographs were captured using a digital camera (Leica EC3, Leica, Germany) connected to a microscope (Leica DM500).
2.7.6 Gene Expression Levels
Total RNA was extracted from the liver specimens collected from different experimental groups using TRIzol reagent (iNtRON Biotechnology, Korea) following the manufacturer’s instructions. The cDNA synthesis was performed by Quantitect® Reverse Transcription kit (Qiagen, Germany) following the manufacturer’s directions. The primer sequences of the target genes were obtained from O. niloticus specific NCBI GenBank accession number, and their efficiency was tested before RT-PCR. The primers of SOD, CAT, Hsp70, caspase 3, tumor suppressor protein p53 (p53), and the housekeeping gene beta-actin (β-actin) are illustrated in Table 1. RT-PCR analysis was performed by a Rotor-Gene Q instrument with a QuantiTect® SYBR® Green PCR kit (Qiagen, Germany). The thermocycling conditions were adjusted in the thermocycler as follows: 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s. Melting curve analysis was done to confirm the PCR specificity. The mRNA expression for each tested gene was evaluated using the comparative 2-ΔΔCt method (Livak and Schmittgen, 2001) and expressed relatively to the β-actin mRNA normalizer control.
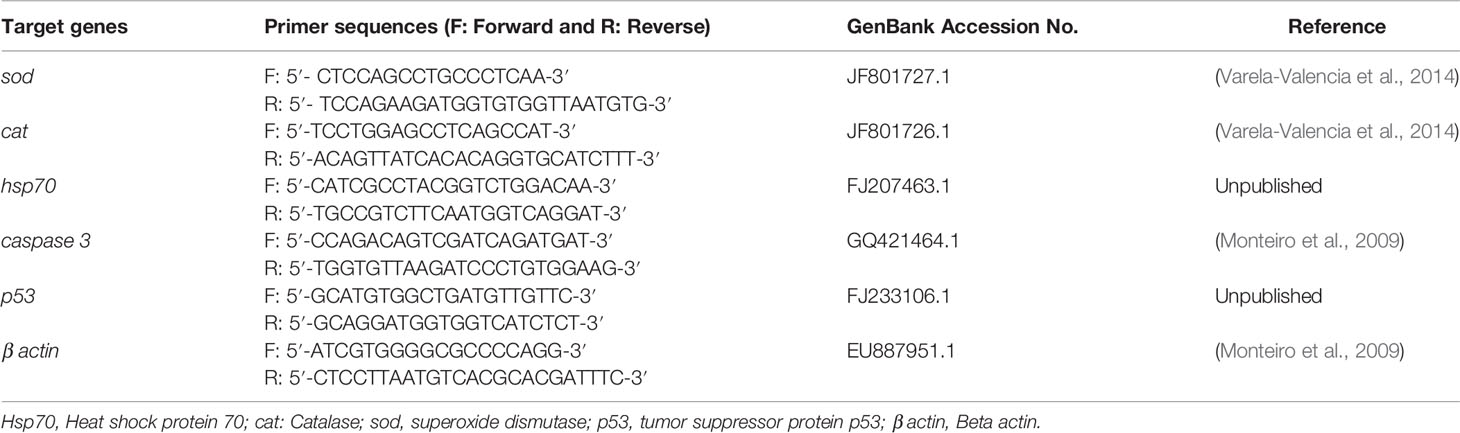
Table 1 Primers sequences of Oreochromis niloticus target genes used in the real-time quantitative PCR study.
2.8 Statistical Analysis
Data were analyzed using GraphPad Prism™ 8 (GraphPad Software, San Diego, CA, USA). All data are presented as the means ± standard error of mean (S.E.M.) and were first checked for normality using the D’Agostino-Pearson normality test. Intergroup differences in the various physiological parameters evaluated were analyzed using a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. A P < 0.05 was considered significant.
3 Results
3.1 Growth Parameters, Feed Utilization Parameters, and Survivability
As presented in Table 2, the survival rate percentages of the CNT and GEO1- and GEO2-supplemented groups were the highest among all groups and reached 99.47%, 99.51%, and 99.60%, respectively. ATZ exposure induced a significant decrease in the fish survival rates, reaching 80.51%, while GEO1- and GEO2 supplementation combined with ATZ (GEO1+ATZ and GEO2+ATZ) mitigated the negative effects of ATZ on fish survival rates in a dose-dependent manner, becoming significantly more elevated than those of the ATZ group.
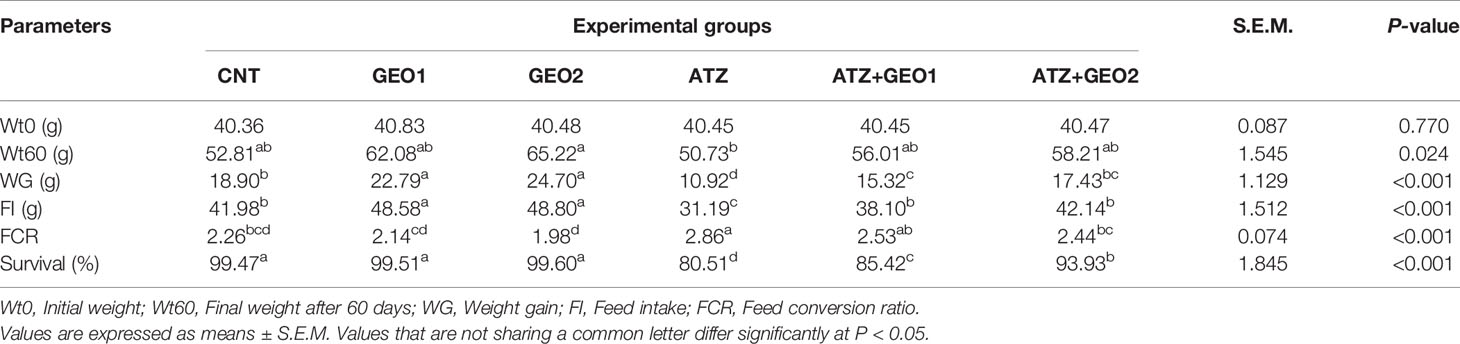
Table 2 Growth parameters, feed utilization parameters, and survival rates of Nile tilapia in response to sub-lethal atrazine (ATZ) exposure and/or dietary supplementation with Panax ginseng essential oil (GEO) for 60 days.
In GEO1- and GEO2-supplemented groups, there was a significant increase in WG and FI compared to the CNT group (P < 0.05), while Wt60 and FCR values were comparable to the CNT. No differences were observed between GEO1 and GEO2 on fishes’ growth performance. ATZ exposure for 60 days caused a depression in fishes’ growth significantly decreasing WG and FI values compared to the CNT group, while FCR was the highest value among the experimental groups. Interestingly, GEO1+ATZ and GEO2+ATZ groups elicited a significant and similar improvement in Wt60, WG, FI, and FCR values compared with the ATZ group (Table 2).
3.2 Hematological Parameters
Changes occurring in the hematological measurements of Nile tilapia in response to sub-lethal ATZ exposure and/or dietary supplementation with GEO for 60 days are summarized in Table 3. The WBC count was significantly elevated both in GEO1 and GEO2 groups compared to the CNT group. ATZ exposure elicited a significant decrease in RBC count, Hb concentration, PCV, and WBC count compared to all experimental groups. In the GEO1+ATZ and GEO2+ATZ groups, there were significant, although non-dose-dependent, improvements in the hematological variables compared to the ATZ group.
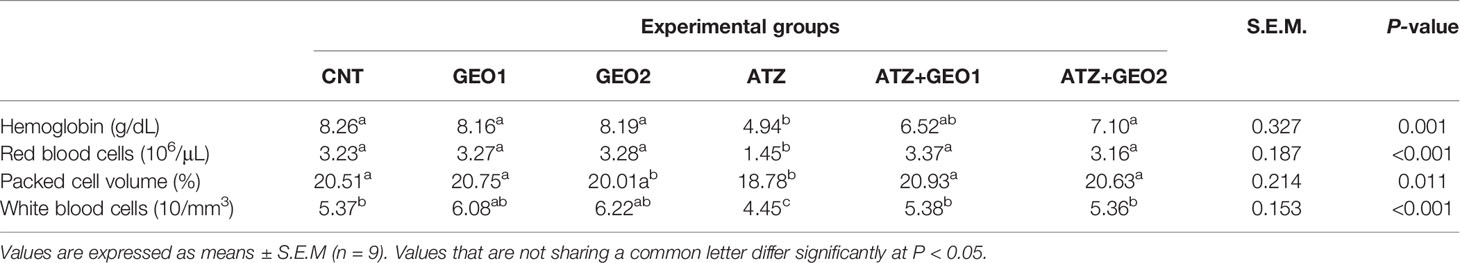
Table 3 Hematological parameters of Nile tilapia in response to sub-lethal atrazine (ATZ) exposure and/or dietary supplementation with Panax ginseng essential oil (GEO) for 60 days.
3.3 Serum Biochemical Parameters
Blood protein profiles (TP, ALB, and GLO) and total IgM levels of Nile tilapia in response to sub-lethal ATZ exposure and/or dietary supplementation with GEO for 60 days are shown in Figure 1.
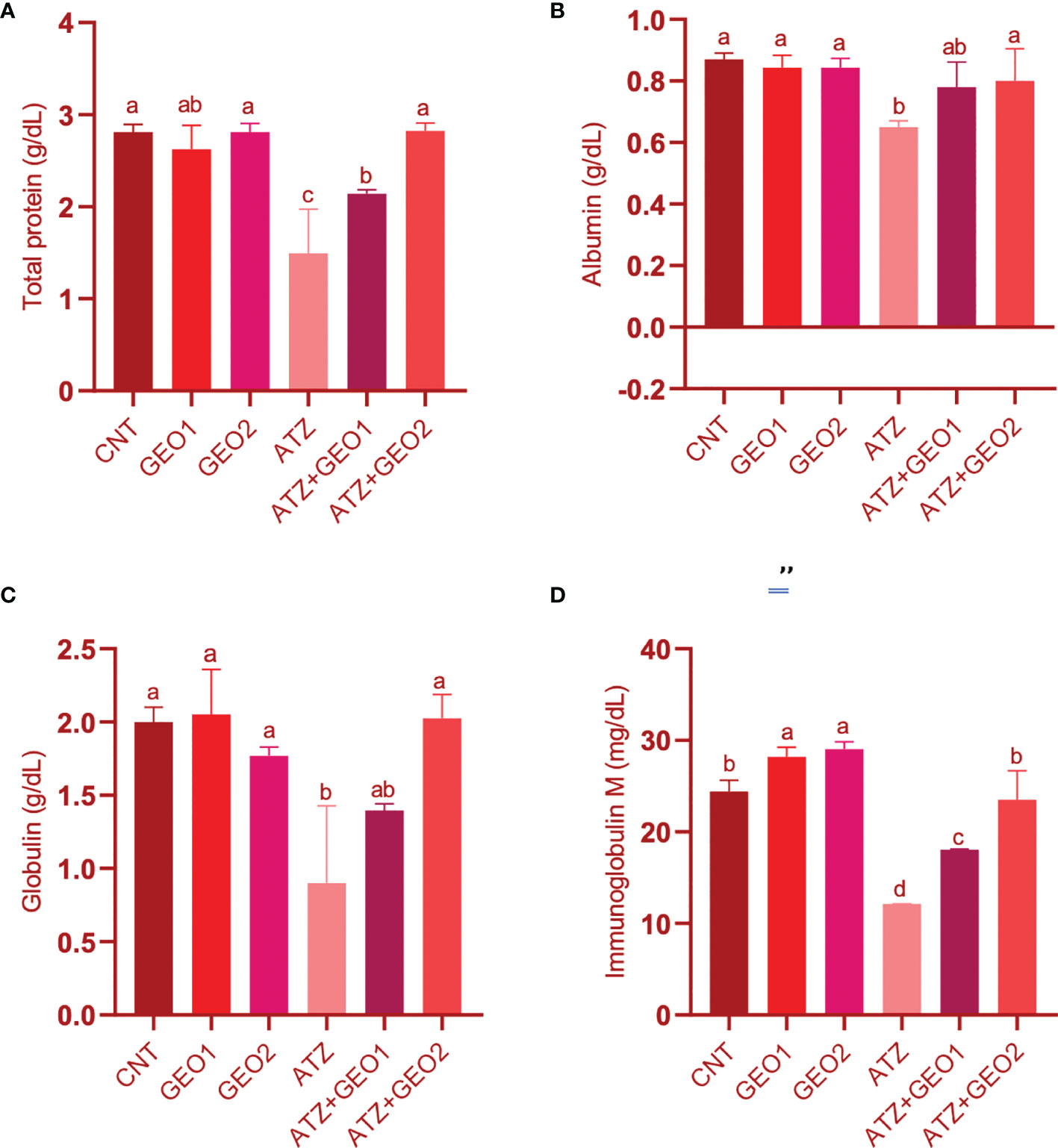
Figure 1 Blood proteins (A–C) and immunoglobulin M (D) levels of Nile tilapia in response to sub-lethal ATZ exposure and/or dietary supplementation with GEO for 60 days. Values are expressed as means ± S.E.M (n = 9). Values that are not sharing a common letter differ significantly at P < 0.05.
ATZ exposure induced significant hypoproteinemia, hypoalbuminemia, hypoglobulinemia, and decreased total IgM levels (P < 0.05) compared to the CNT, GEO1, and GEO2 groups. Dietary GEO supplementation in GEO1+ATZ and GEO2+ATZ groups significantly improved the blood protein and IgM levels compared to the ATZ group and in a dose-dependent manner for TP, GLO, and IgM, but not for ALB (P < 0.05).
As for total cholesterol, triglycerides, and cortisol, all values were significantly elevated in the ATZ group compared to CNT, GEO1, and GEO2 groups (P < 0.05; Figure 2).
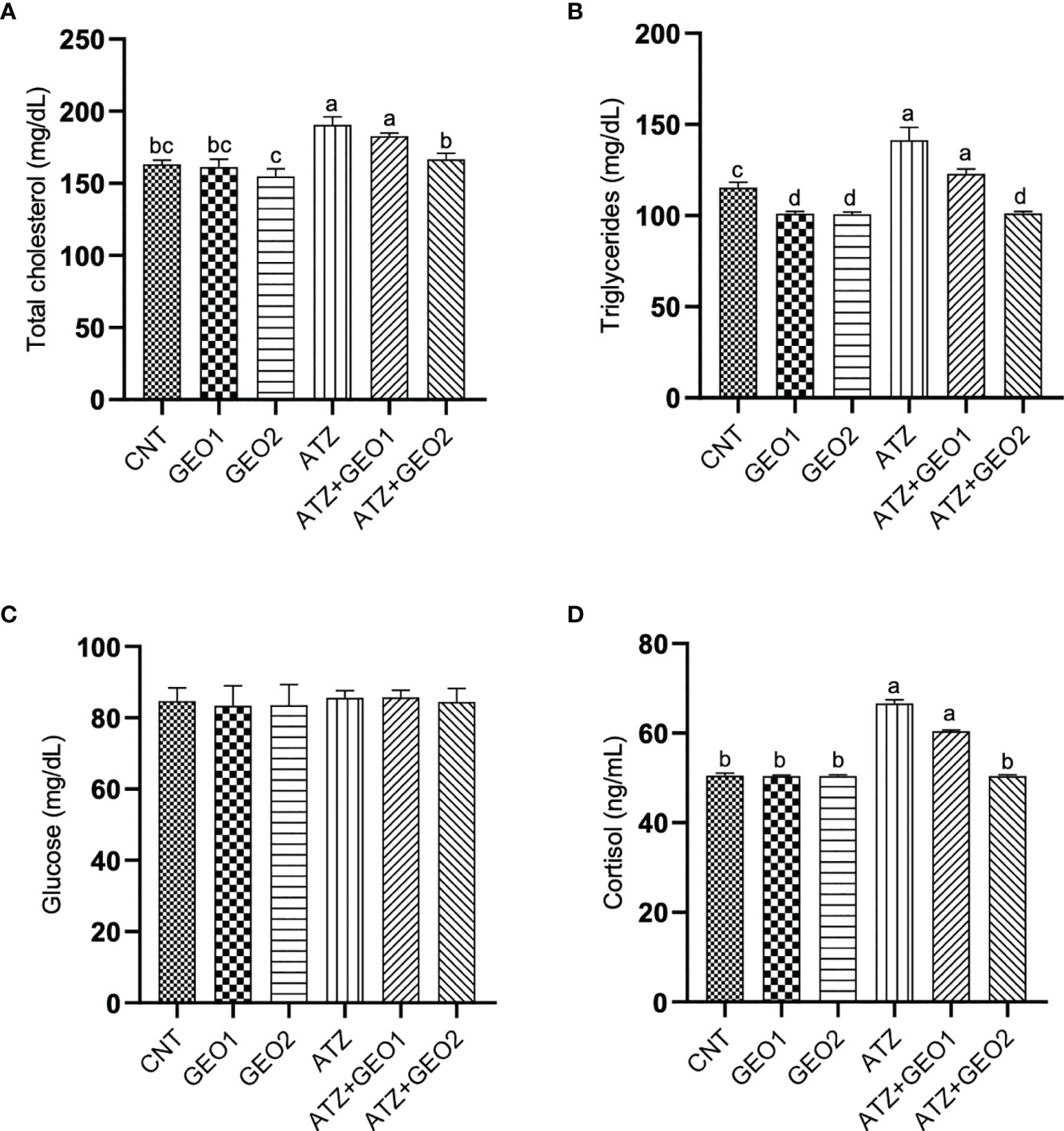
Figure 2 Serum biochemical parameters [total cholesterol (A), triglycerides (B), glucose (C) and cortisol (D)] of Nile tilapia in response to sub-lethal ATZ exposure and/or dietary supplementation with GEO for 60 days. Values are expressed as means ± S.E.M (n = 9). Values that are not sharing a common letter differ significantly at P < 0.05.
Conversely, the GEO2+ATZ group showed improved values of these parameters compared to the ATZ group. Blood glucose levels were not significantly affected among all experimental groups.
3.4 Intestinal Digestive Enzymes’ Activity
Figure 3 shows the changes that occurred in the intestinal digestive enzymes’ activity of Nile tilapia in response to sub-lethal ATZ exposure and/or dietary supplementation with GEO for 60 days.
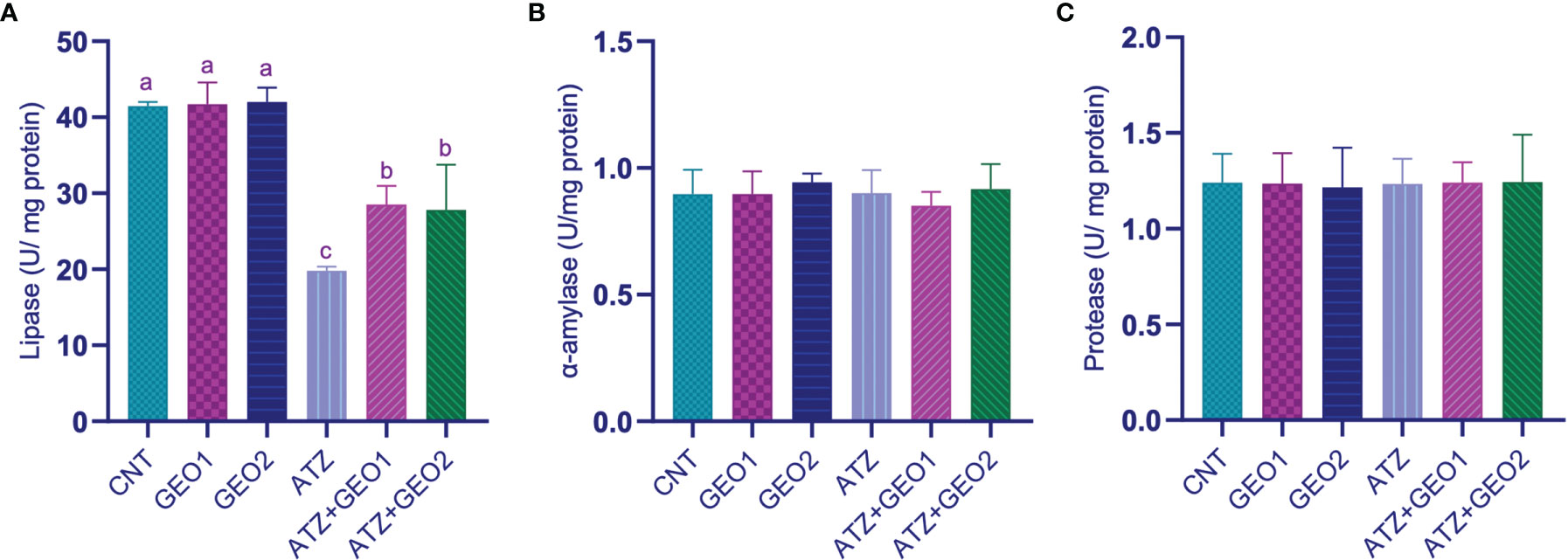
Figure 3 Intestinal digestive enzymes’ activity [Lipase (A), α-amylase (B), and protease (C)] of Nile tilapia in response to sub-lethal ATZ exposure, and/or dietary supplementation with GEO for 60 days. Values are expressed as means ± S.E.M (n = 9). Values that are not sharing a common letter differ significantly at P < 0.05.
ATZ exposure caused a significant decrease in the intestinal lipase activity compared to CNT, GEO1, and GEO2 groups. Dietary GEO supplementation in GEO1+ATZ and GEO2+ATZ groups significantly attenuated, although non-dose-dependently, the decreased lipase activity compared to the ATZ group (P < 0.05). On the other hand, intestinal α-amylase and protease activities did not significantly differ among all groups.
3.5 Oxidative Stress Biomarkers in Liver Tissue
Changes occurring in the hepatic oxidative stress markers of Nile tilapia in response to sub-lethal ATZ exposure and/or dietary supplementation with GEO for 60 days are presented in Table 4. Dietary GEO supplementation positively influenced the hepatic antioxidant defense system of the fishes. GEO1 and GEO2 groups showed the highest antioxidant markers (SOD and CAT) activity and the lowest GSH levels. Further, CNT, GEO1, and GEO2 showed the lowest hepatic levels of MDA, with no significant differences among them.
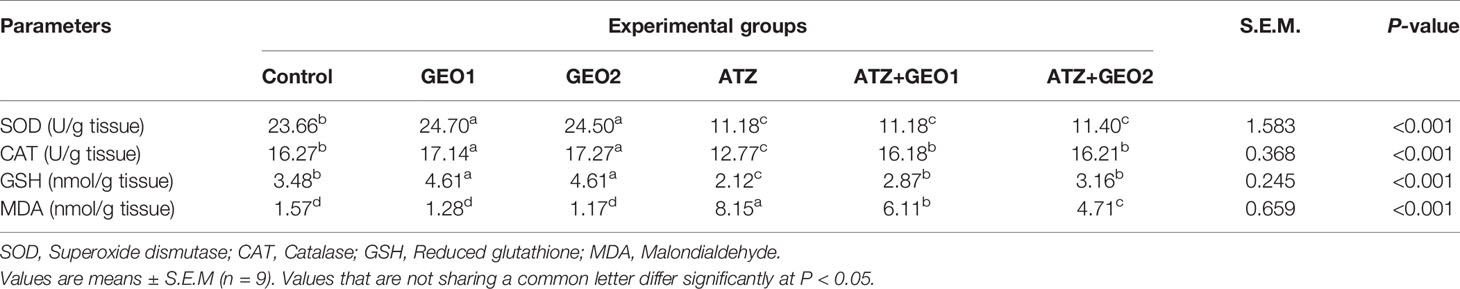
Table 4 Hepatic oxidative status biomarkers of Nile tilapia in response to sub-lethal atrazine (ATZ) exposure and/or dietary supplementation with Panax ginseng essential oil (GEO) for 60 days.
Conversely, the only ATZ exposure triggered a significant decrease in SOD and CAT activity, as well as in GSH levels (P < 0.05), while hepatic MDA levels were the highest among all experimental groups. GEO1+ATZ and GEO2+ATZ groups significantly attenuated the changes occurring in hepatic CAT activity and GSH levels and, in a dose-dependent manner, MDA levels compared to the ATZ group, although not reaching the CNT group levels (Table 4).
Moreover, SOD activity did not improve in GEO1+ATZ and GEO2+ATZ groups, remaining similar to that observed in the ATZ group.
3.6 Liver Histopathology
The hepatic tissues of Nile tilapia in the CNT group (Figure 4A) and GEO1 and GEO2 groups (Figures 4B and 4C, respectively) showed normal hepatocyte architecture with normal hepatic cords around central veins.
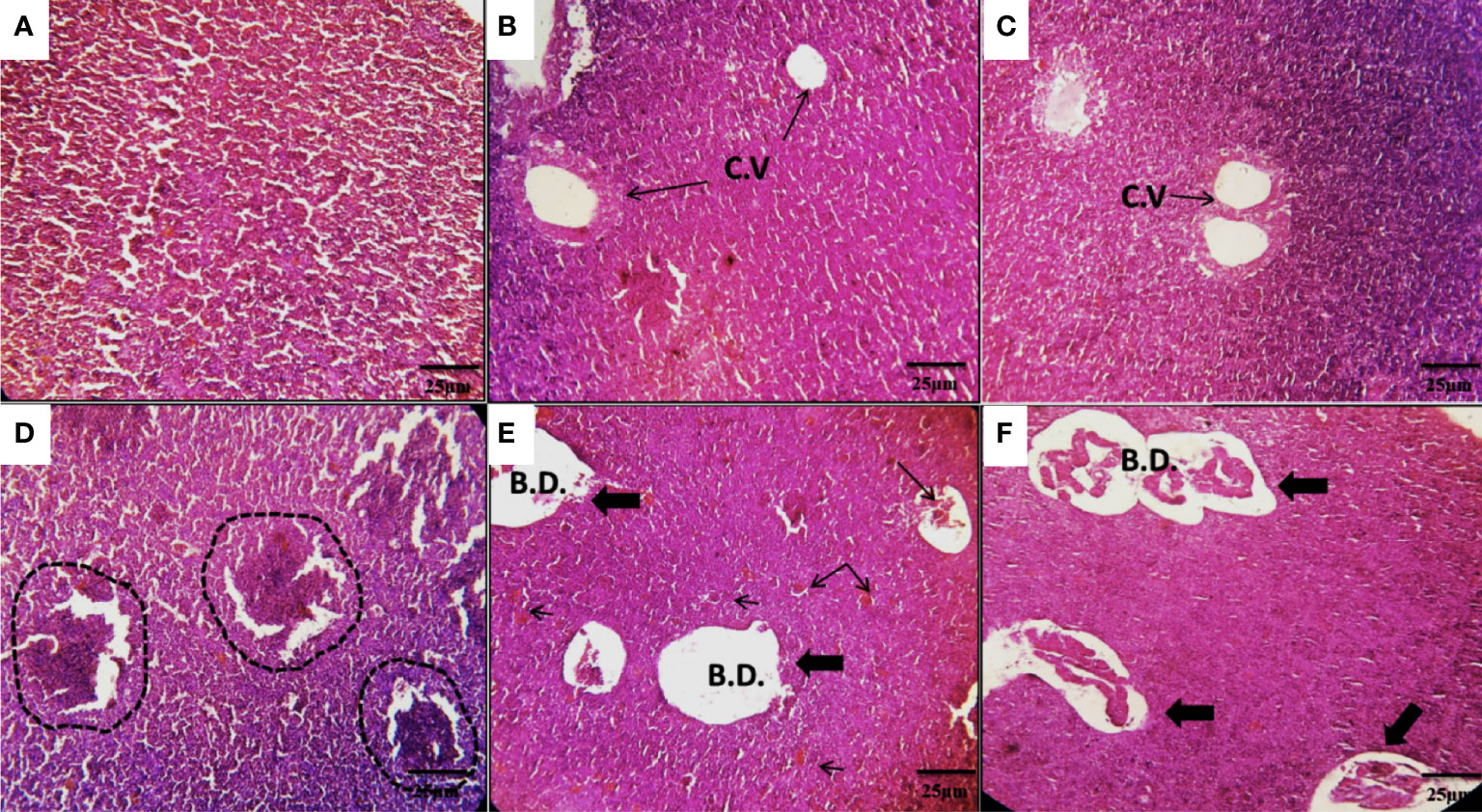
Figure 4 Photomicrographs of the hepatic tissues of Nile tilapia in the CNT group (A), and in the GEO1 and GEO2 groups for 60 days (B, C) showing normal hepatocyte architecture with normal hepatic cords around central veins. The AZT group (D) showed multi-hepatic lesions and regular condensation of hepatic tissue as a result of hypertrophy or hepatoma (dash lines). ATZ+GEO1 and ATZ+GEO2 groups (E, F) showed fewer hepatic lesions in the form of mild hepatic cholestasis with dilated bile canaliculi (thick arrows) and the trapped bile plugs diffused in the hepatic tissue (thin arrows). H&E staining, scale bar = 25 µm, C.V., central vein; B.D., bile duct.
Conversely, specimens from the ATZ group showed multi-hepatic lesions and regular condensation of hepatic tissue as a result of hypertrophy or hepatoma (Figure 4D). On the other hand, the hepatic tissues of fishes belonging to the ATZ+GEO1 and ATZ+GEO2 groups showed fewer hepatic lesions such as mild hepatic cholestasis accompanied with dilated bile canaliculi (Figures 4E, F, respectively).
3.7 Gene Expression Levels in Liver
The expression levels of stress- and apoptosis-related genes such as Hsp70, caspase 3, and p53 in the hepatic tissues of Nile tilapia was significantly upregulated in the AZT group by 4.8-, 3.85-, and 4.1-fold, respectively (Figure 5).
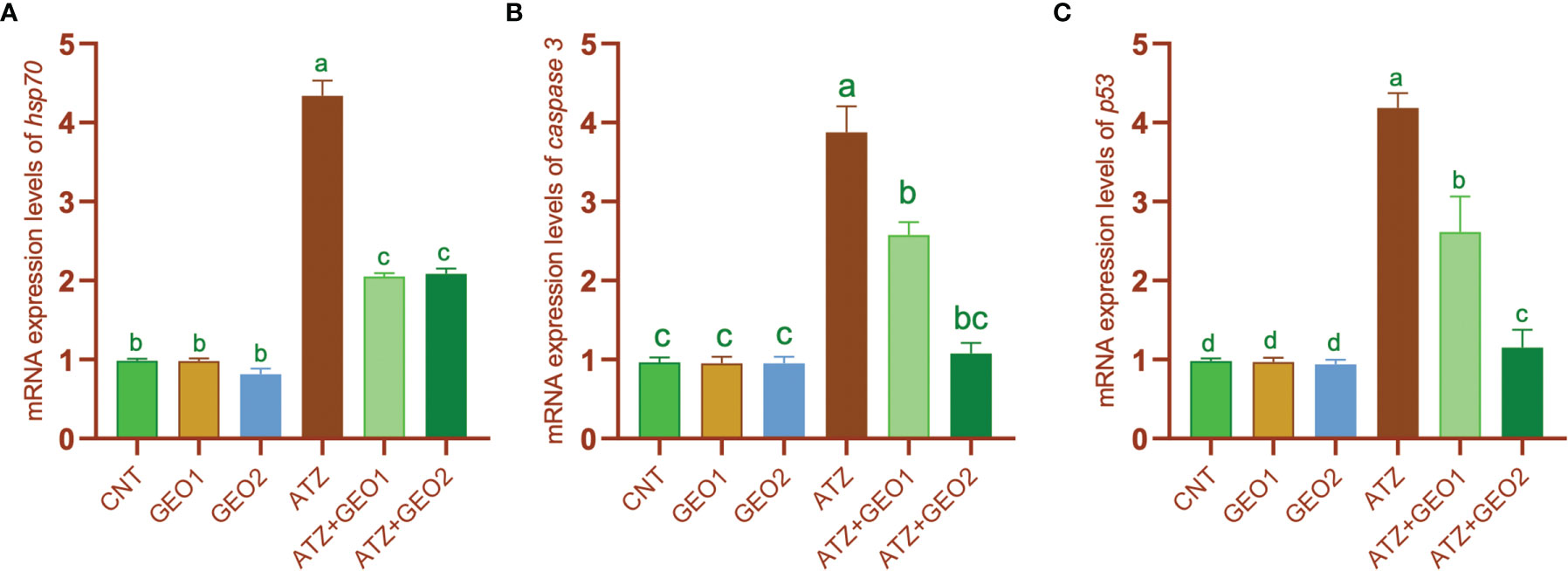
Figure 5 mRNA expression levels of heat shock protein 70 hsp70 (A), caspase 3 (B), and tumor suppressor protein p53 (C) genes relative to β-actin gene in the hepatic tissues of Nile tilapia in response to sub-lethal ATZ exposure and/or dietary supplementation with GEO for 60. Values are expressed as means ± S.E.M (n = 9). Values that are not sharing a common letter differ significantly at P < 0.05.
Such change was significantly and, for some parameters, dose-dependently mitigated by dietary GEO supplementation, with expression levels of the aforementioned genes being significantly decreased in GEO1+ATZ and GEO2+ATZ groups compared to the ATZ group, although not reaching the levels measured in CNT, GEO1, and GEO2 groups (P < 0.05). Conversely, the mRNA expression values of the antioxidant enzyme (sod and cat) genes were significantly downregulated in the ATZ group compared to the CNT, GEO1, and GEO2 groups and, additionally, GEO-2 diet significantly increased sod and cat mRNA expression levels in healthy fishes (P < 0.05; Figure 6).
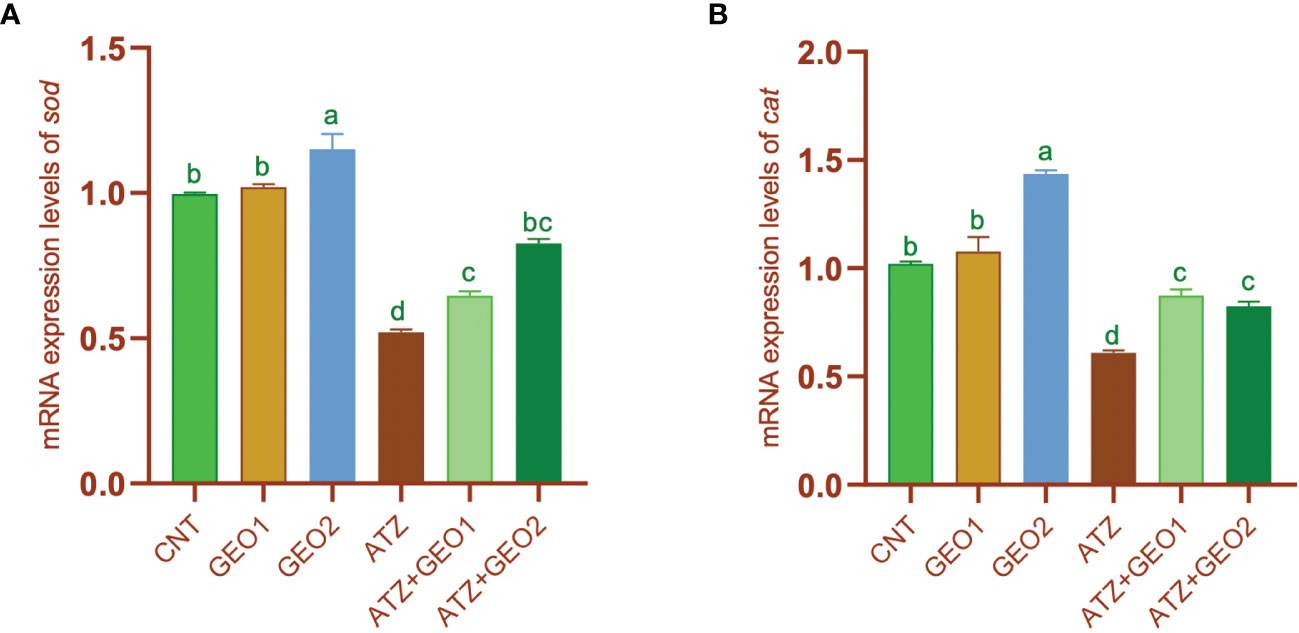
Figure 6 mRNA expression levels of sod (A) and cat (B) genes relative to β-actin gene in the hepatic tissues of Nile tilapia in response to sub-lethal ATZ exposure and/or dietary supplementation with GEO for 60 days. Values are expressed as means ± S.E.M (n = 9). Values that are not sharing a common letter differ significantly at P < 0.05.
However, the expression of the aforementioned genes significantly, but non-dose-dependently, increased in GEO1+ATZ and GEO2+ATZ groups compared to the ATZ group, although not reaching the values of CNT, GEO1, and GEO2 groups (P < 0.05).
4 Discussion
The present paper describes for the first time the effects that dietary supplementation with Panax ginseng, in the form of an essential oil, exerts on an array of physiological parameters of Nile tilapia reared under standard conditions or under sub-chronic ATZ intoxication over a 60-day feeding period.
4.1 Effects of GEO on Physiological Parameters of Healthy Nile Tilapia
In the present study, dietary supplementation with GEO for 60 days was found to exert a positive influence on the productivity performance of Nile tilapia. Indeed, all growth and feed utilization parameters were significantly improved in both GEO1 and GEO2 groups compared with the CNT group. A dose dependency was not observed, which may suggest either that the two tested supplementation levels triggered the maximal attainable response (ceiling effect), or that the difference occurring in the two supplementation levels was not great enough to elicit distinctly different effect magnitudes.
Similar to our study, previous studies showed that dietary supplementation with Panax ginseng or other ginseng species (American and Thai ginseng), in the form of herbs or extracts, is able to enhance the growth performance of Nile tilapia (Goda, 2008; Abdel-Tawwab, 2012; El-Sayed et al., 2014; Van Doan et al., 2019). Therefore, it appears that the use of Panax ginseng as an EO in fish diets can be a good option, alternative to other forms of dietary ginseng supplementation already tested, for promoting the production of Nile tilapia. Moreover, our findings revealed that in both GEO1 and GEO2 groups, most of the health and welfare parameters investigated did not differ from those of the CNT group (including the histoarchitecture of the hepatic tissue), suggesting that GEO is also safe as a feed supplement for the Nile tilapia farming.
The positive influence exerted by dietary GEO supplementation on the productive performance of Nile tilapia cannot be explained in light of the same physiological changes reported by other studies in Nile tilapia after administration of other forms of P. ginseng or other EOs. For instance, Goda found that diet supplementation with P. ginseng herb significantly improved hematological parameters of Nile tilapia such as RBC count, hematocrit, and Hb content (Goda, 2008); however, these effects were not observed in our study in response to dietary GEO supplementation. Furthermore, dietary supplementation with Ocimum basilicum EO reduced triglycerides and increased TP levels in Nile tilapia plasma samples (de Souza et al., 2019). Further, dietary Aloysia triphylla and Petroselinum crispum EOs had a positive impact on intestinal digestive enzymes such as lipase and protease in Nile tilapia (de Souza et al., 2020; Farag et al., 2022). In the present study, none of these blood characters and intestinal parameters showed significant changes in response to dietary GEO supplementation. Rather, a possible explanation for our productive performance findings may rely on an overall positive influence on the defense systems of Nile tilapia, as suggested by the significant increases that GEO diets produced in the WBC count (namely in the number of the circulating cells involved in immune response), as well as in the antioxidant equipment of the liver (SOD and CAT activities and expression levels, and GSH content).
4.2 Effects of GEO on Physiological Parameters of ATZ-Intoxicated Nile Tilapia
A further interesting finding of the present study is that dietary GEO supplementation was able to mitigate almost all of the negative effects exerted by sub-lethal ATZ toxicity in Nile tilapia over 60 days of concomitant exposure.
4.2.1 ATZ-Induced Toxicity
In this respect, it is worth mentioning that the significant ATZ-induced changes that we observed in many physiological parameters related to the productive performance and health/welfare of Nile tilapia were in agreement to those reported in the literature for this fish species (Neamat Allah et al., 2020; Abdel-Warith et al., 2021; Ali et al., 2022).
According to the literature, sub-lethal ATZ exposure significantly depressed the survival and growth rates of Nile tilapia (Abdel-Warith et al., 2021; Ali et al., 2022). This impaired productive performance in the ATZ group and is likely attributable to the negative impact exerted by ATZ on the health and welfare status of the exposed fishes in comparison with the CNT group.
Particularly, ATZ exposure impaired digestive functionality, as indicated by the significant decline in intestinal lipase enzyme activity. A similar effect has been also described for lead and bifenthrin toxicity in Nile tilapia (Alvarez-Gonzalez et al., 2020; Farag et al., 2022), although the latter pollutant also caused a significant decrease in the activity of α-amylase and protease enzymes (Farag et al., 2022), which were not affected by ATZ in our study.
ATZ exposure induced anemia and leukocytopenia (as indicated by the significant decline in RBC and WBC counts, as well as in PCV and Hb concentration). Similar alterations in hematological parameters have been reported for the ATZ-intoxicated Nile tilapia (Abdel-Warith et al., 2021) but also in several other ATZ-exposed fish species, such as common carp (Blahova et al., 2014) and Acipenser nudiventris (Naji et al., 2019).
The reduced WBC count, along with the significant decrease recorded in blood total GLO and IgM levels, suggests that sub-lethal ATZ exposure exerted immunosuppressive effects in the intoxicated Nile tilapia. Likewise, a significant decrease in α1-globulin, α2-globulin, ɤ-globulin, and IgM levels has been reported by other authors in Nile tilapia (Neamat Allah et al., 2020).
ATZ exposure induced further significant alterations in the blood protein profile of Nile tilapia, as well as in the fish blood lipid profile, as indicated by the decreased levels of TP, ALB, and GLO (hypoproteinemia), and increased levels of total cholesterol and triglycerides (hypercholesterolemia, hypertriglyceridemia). Overall, these findings suggest an impaired functionality of the fish liver, with particular regard to its ability to synthesize blood proteins and regulate lipid metabolism. Similar declines in blood TP levels were reported for ATZ-intoxicated Nile tilapia (Neamat Allah et al., 2020; Abdel-Warith et al., 2021). Moreover, significant alterations in blood protein and lipid profiles were observed to also occur in other ATZ-intoxicated fish species, such as grass carp (decreased TP, ALB, cholesterol, and triglycerides) (Soorena et al., 2011), African catfish (decreased TP, GLO and ALB levels) (Sing, 2020), and snow trout (decreased TP and cholesterol levels) (Akhtar et al., 2021).
The most likely cause for the impaired liver functionality in ATZ-intoxicated Nile tilapia was the occurrence of oxidative stress damage, as suggested by the results of the various assays performed to evaluate the liver oxidative status. Oxidative stress usually happens because of free radicals and reactive oxygen species overproduction, together with the inability of the endogenous antioxidant mechanisms such as SOD, CAT, glutathione-S-transferase (GST), and glutathione peroxidase (GPx) to counteract the negative effect of these free radicals (Lackner, 1998; Nordberg and Arner, 2001; Le Bras et al., 2005). Our study showed that ATZ exposure caused a significant decrease in hepatic SOD and CAT activity, as well as in the liver content of reduced GSH. Additionally, the hepatic MDA levels (that are index of lipid peroxidation) were the highest in the ATZ group among all experimental groups. Our findings of reduced antioxidant enzyme activity in the liver of ATZ-intoxicated Nile tilapia were consistent with results of gene expression assays. Indeed, we found the mRNA expression levels of the genes coding for these antioxidant enzymes (sod and cat) to be significantly downregulated in the hepatic tissues of the fishes exposed to sub-lethal ATZ dosage. These findings, on the whole, suggest the inability of the antioxidant system of the Nile tilapia liver to counteract the oxidative stress damage induced by the overproduction of free radicals after ATZ exposure. Previous studies evidenced a reduced activity of antioxidant enzymes SOD, CAT, and GPx and an increased MDA concentration in the liver of ATZ-intoxicated Nile tilapia (Abdel-Warith et al., 2021; Ali et al., 2022). Moreover, the negative influence of ATZ on liver oxidative status (particularly in terms of decreased activities of the antioxidant enzymes CAT, SOD, and GPx) has been documented for other fish species, such as Prochilodus lineatus (Santos and Martinez, 2012) and rohu (Labeo rohita) (Pugazhendy, 2014). Finally, it is worth noting that reduction in the expression of sod and cat genes similar to that observed in our ATZ-intoxicated Nile tilapia also occurred in C. carpio following exposure to indoxacarb (Ghelichpour et al., 2019). In contrast with all these findings, other studies and ours showed that both sub-chronic ATZ exposure in P. lineatus (Paulino et al., 2012) and chronic ATZ exposure in African catfish (Kadry et al., 2012) caused a significant increase (rather than decrease) in SOD, CAT, and GST activities, in association with increased lipid peroxidation levels. Moreover, several published studies reported overexpression (rather than downregulation) of antioxidant-related genes in fish tissues exposed to toxicants (Afifi et al., 2017; Taheri Mirghaed et al., 2020). These discrepancies may be linked to differences in toxicant types, exposure doses, exposure period, fish species, physiological and immune responses of fish, and several other factors.
Further evidence supporting the establishment of an oxidative stressful environment in the liver of ATZ-intoxicated Nile tilapia was provided in our study by the result of the assays measuring the mRNA expression levels of hsp70, caspase 3, and p53 genes in this tissue. HSPs play a chief role in the response of cells to stress (Feder and Hofmann, 1999). They can be found in fish tissues after exposure to several stressful conditions of biological and non-biological nature, such as pathogens, cold shock, heat stress, and environmental toxicants (Iwama et al., 1998). The upregulated expression of hsp70, in particular, is considered a normal pathophysiological mechanism that occurs in fish tissues to overcome the adverse environmental challenges (Basu et al., 2002; Xing et al., 2013; Iwama et al., 2015). Caspases are essential mediators involved in apoptosis (programmed cell death) (Porter and Jänicke, 1999). Particularly, caspase 3 is imposed and triggered after the occurrence of DNA damage and morphologic alterations associated with apoptosis (Porter and Jänicke, 1999). Finally, the tumor suppressor (p53) gene is considered an important biomarker for genotoxic effects and apoptotic changes after fish exposure to different aquatic toxicants and pollutants (Bhaskaran et al., 1999; Helton and Chen, 2007). Of note, it has been reported that oxidative stress triggered the activation of the p53 gene and consequently facilitated the start of apoptosis and cellular death (Mai et al., 2010). In the present study, ATZ exposure resulted in the overexpression of hsp70, caspase 3, and p53 genes in the hepatic tissues of Nile tilapia. This finding suggests that ATZ increased the stress level of the exposed fish hepatocytes and induced apoptotic changes in these cells. The capability of ATZ exposure to result in upregulated expression of hsp70, as well as of other HSPs (such as hsp60, hsp90), has also been documented in the brain and other tissues of C. carpio (Xing et al., 2013). In the latter fish species, ATZ was also shown to trigger neutrophil apoptosis by activating caspase 3 (Wang et al., 2019). Similarly to ATZ, also sub-lethal thallium (Farag et al., 2021) and bifenthrin (Farag et al., 2022) induced toxicities were found to be associated with increased expression levels of hsp70, caspase 3, and p53 genes in liver tissue of Nile tilapia.
In the present study, the damage that sub-lethal ATZ exposure produced via oxidative stress to the Nile tilapia hepatocytes was large enough to produce appreciable histopathological lesions in the examined hepatic tissues. Similar lesions were also described in other ATZ-intoxicated fish species, namely in the African catfish (Michael, 2018) and common carp (Neskovic et al., 1993).
Unsurprisingly, the multiple perturbations that sub-lethal ATZ exposure caused in our study to the health status of intoxicated fish negatively influenced the overall welfare status of the animals, leading to severely stressed fish. This was indicated by the finding that ATZ-exposed Nile tilapia had significantly elevated serum levels of the hormone cortisol, which is a well-known marker of fish exposure to stressful circumstances (Wendelaar Bonga, 1997; Barton, 2002). The occurrence of increased release of cortisol in Nile tilapia in response to sub-lethal ATZ exposure has also been reported in the literature (Abdel-Warith et al., 2021).
4.2.2 Mitigation of ATZ Toxicity by GEO
Most of the alterations that ATZ induced in the physiological parameters that were used as markers of productive performance and health/welfare status of Nile tilapia appeared significantly attenuated when the exposure of these animals to sub-lethal ATZ dosage occurred concomitantly with dietary GEO supplementation.
More specifically, dietary GEO supplementation significantly reduced the negative impact of sub-lethal ATZ toxicity on the growth, feed utilization, and survivability of exposed fishes, just as dietary menthol EO and thyme EO did on Nile tilapia and rainbow trout after the exposure to sub-lethal dosage of chlorpyrifos (Dawood et al., 2020) and aflatoxin B1 (Ghafarifarsani et al., 2021), respectively. This preserving effect of GEO on the productive performance of ATZ-intoxicated fishes was the likely consequence of all the protective effects that such a supplementation exerted on fishes’ digestive functionality, erythrocyte health, immune function, and hepatic oxidative status following the ATZ intoxication.
Indeed, dietary GEO supplementation in GEO1+ATZ and GEO2+ATZ groups significantly mitigated the negative impact of ATZ on the activity of the intestinal enzyme lipase, just as dietary Petroselinum crispum EO did in Nile tilapia exposed to bifenthrin (Farag et al., 2022).
Dietary GEO supplementation also significantly attenuated ATZ’s negative impact on Nile tilapia hematology, in this sense, producing similar beneficial effects to those recently described for dietary supplementation with fucoidan (Abdel-Warith et al., 2021).
Interestingly, supplementation of Nile tilapia diet with GEO resulted in less marked ATZ-induced disruption of most hepatic antioxidant defense systems (less marked decreases in CAT activity, sod and cat gene expression, GSH content) and consistently less marked ATZ-induced lipid peroxidation (MDA content). In all likelihood, these protective effects of GEO are attributable to the well-known antioxidant properties of the bioactive functional constituents present in the GEO (Kitts et al., 2000; Kim et al., 2002; Zhao et al., 2020; Ahmadifar et al., 2021). In producing these antioxidant effects, dietary GEO supplementation appears to behave similarly to dietary parsley EO, which attenuated bifenthrin-induced oxidative stress in Nile tilapia (Farag et al., 2022), Origanum vulgare EO, which improved CAT and SOD enzyme activities of cypermethrin-intoxicated common carp (Khafaga et al., 2020), and finally menthol EO, which negatively modulated the expression of cat and sod genes in Nile tilapia exposed to a sub-lethal chlorpyrifos dosage (Dawood et al., 2020).
As a plausible consequence of the attenuation of ATZ-induced oxidative stress in Nile tilapia liver, and also as a possible effect of the direct anti-stress and anti-apoptotic properties of GEO bioactive constituents (Rai et al., 2003; Lee et al., 2008; Souza et al., 2019), dietary GEO supplementation in GEO1+ATZ and GEO2+ATZ groups was able to significantly mitigate the toxicity associated overexpression of all stress-related (hsp70) and apoptosis-related (caspase 3 and p53) genes. In this respect, the behavior of dietary GEO seems similar to that of dietary menthol EO, which negatively modulated the expression of hsp70 genes in Nile tilapia exposed to a sub-lethal chlorpyrifos dosage (Dawood et al., 2020), to that of parsley EO, which counteracted the increase in the expression levels of hsp70, caspase3 and p53 genes in Nile tilapia exposed to a sub-lethal bifenthrin dosage (Farag et al., 2022), and finally to that of dietary Astragalus membranaceus polysaccharides, which negatively modulated the expression of hsp70 and apoptosis-related genes in Nile tilapia subjected to a sub-lethal thallium dosage (Farag et al., 2021).
Consistently with all the protective effects discussed so far, and probably also due to the direct anti-inflammatory activity of P. ginseng bioactive compounds (Paul et al., 2012; Baek et al., 2015), the present study found that dietary GEO supplementation reduced the hepatic histological lesions in ATZ-exposed Nile tilapia. In this respect, GEO seems endowed with beneficial properties similar to those reported for several other EOs in many different toxicity studies. For instance, dietary fucoidan (Abdel-Warith et al., 2021) and Isatis phytogenic-based diets were found to significantly reduce the ATZ-induced intestinal histological lesions in Nile tilapia (Ali et al., 2022). Moreover, dietary Carica papaya and Mangifera indica negatively modulated the histopathological changes in ATZ-intoxicated Clarias gariepinus (Owolabi and Abdulkareem, 2021). As far as other toxicants are considered, it should be mentioned that dietary menthol EO significantly reduced the histopathological alterations in Nile tilapia tissues exposed to a sub-lethal chlorpyrifos dosage (Dawood et al., 2020). Moreover, dietary Thymus vulgaris EO considerably mitigated the histopathological alterations in the thiamethoxam-intoxicated African catfish (El Euony et al., 2020). Finally, it was reported that supplementing diets with Origanum vulgare EO modulated the histopathological changes that occurred in common carp tissues subjected to a sub-lethal cypermethrin dosage (Khafaga et al., 2020).
Probably strictly related to the mitigating effects on ATZ-induced damage, the present study found dietary GEO supplementation in ATZ-intoxicated Nile tilapia to be associated with less marked detrimental changes in hepatic functionality (as indicated by the improved blood protein and lipid profiles) and less marked impairment of the animal welfare status (as indicated by the improved serum values of the systemic stress marker cortisol). Once again, dietary GEO seems to offer beneficial effects similar to those offered by dietary supplementation with other natural feed supplements, such as fucoidan (Abdel-Warith et al., 2021), which was reported to improve the blood levels of proteins and cortisol in ATZ-intoxicated Nile tilapia, Petroselinum crispum EO (Farag et al., 2022), which significantly improved blood proteins and serum cortisol levels in Nile tilapia intoxicated with a sub-lethal bifenthrin concentration, and Ocimum basilicum EO (de Souza et al., 2019), which was found to reduce plasma triglycerides and increased plasma TP levels in Nile tilapia.
As a final consideration, it is worth noting that concerning some of the physiological parameters examined (growth and feed utilization parameters, hematological parameters, intestinal lipase activity, blood ALB levels, liver CAT enzyme activity, GSH content, sod, cat, and hsp70 mRNA expression levels), the two different levels of dietary GEO supplementation were equally effective at protecting the fishes from the toxicity of ATZ and dose dependency was not observed. Conversely, for other physiological parameters tested (survival rate, blood levels of TP, GLO, and IgM, blood levels of total cholesterol, triglycerides and cortisol, liver MDA content, and mRNA expression levels of caspase 3 and p53 genes), a dose dependency was demonstrated, with the higher dietary GEO supplementation level (2.0 mL/kg diet) resulting to be more effective than the lower (1.0 mL/kg diet). This lack of homogeneity probably reflects the fact that the various protective activities against ATZ-induced toxicity are exerted by GEO bioactive constituents with differing potency and/or efficacy. Moreover, the sensitivity of one physiological parameter to the protective modulation exerted by GEO may vary depending on the extent to which that same parameter is altered by ATZ toxicity.
At any rate, for some ATZ-affected parameters (survival rate, intestinal lipase activity, liver MDA content, and mRNA expression levels of cat, hsp70, and p53 genes), dietary GEO supplementation, even at the higher dosage, did not completely mitigate the detrimental effects of ATZ , failing to keep the parameter values at their normal levels (at levels not different from those measured in the CNT group). In this respect, it might be expected that further increasing the GEO supplementation dose could result in even better protection of Nile tilapia against ATZ-induced toxicity.
5 Conclusions
In summary, GEO-supplemented diets significantly improved productive performance, immune function, lipid metabolism, and hepatic antioxidant status of the treated Nile tilapia. Moreover, dietary GEO considerably mitigated the negative impacts of ATZ toxicity on the productive performance and health/welfare status of the exposed Nile tilapia. Particularly, the sub-lethal ATZ exposure reduced survivability, impaired digestive functionality, and induced growth retardation, anemia, immunosuppression, and oxidative stress damage to the liver, with consequent impairment of liver functionality and appearance. Dietary GEO attenuated almost all of the ATZ-induced changes in Nile tilapia physiological parameters, indicating a significant and beneficial anti-oxidative, anti-stress, anti-apoptotic, and anti-inflammatory activity. However, GEO did not always completely mitigate ATZ toxic effects reaching the CNT values. Further investigations should be performed to better identify the bioactive constituents of GEO that are actually responsible for the observed positive effects, and to elucidate the action mechanisms by which dietary GEO modulated the productive performance and ATZ-induced toxicity in Nile tilapia.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The animal study was reviewed and approved by institutional Ethics Committee of Zagazig University, Egypt.
Author Contributions
Conceptualization: MF, AM, MH, and MMA. Methodology: MF, AM, MH, SM, MA, and MMA. Validation: MF, AM, MH, SM, MA, and MMA. Investigation: MF, AM, MH, HA-L, SM, MA, and MMA. Writing—original draft: HA-L and CZ. Data curation: HA-L, ADC. Writing—review and editing: ADC and CZ. Formal analysis: MA and ADC. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.920057/full#supplementary-material
References
Abdel-Latif H. M. R., Abdel-Tawwab M., Khafaga A. F., Dawood M. A. O. (2020a). Dietary Oregano Essential Oil Improved the Growth Performance via Enhancing the Intestinal Morphometry and Hepato-Renal Functions of Common Carp (Cyprinus Carpio L.) Fingerlings. Aquaculture 526, 735432. doi: 10.1016/j.aquaculture.2020.735432
Abdel-Latif H. M. R., Abdel-Tawwab M., Khafaga A. F., Dawood M. A. O. (2020b). Dietary Origanum Essential Oil Improved Antioxidative Status, Immune-Related Genes, and Resistance of Common Carp (Cyprinus Carpio L.) to Aeromonas Hydrophila Infection. Fish. Shellf. Immunol. 104, 1–7. doi: 10.1016/j.fsi.2020.05.056
Abdel-Tawwab M. (2012). The Use of American Ginseng (Panax Quinquefolium) in Practical Diets for Nile Tilapia (Oreochromis Niloticus): Growth Performance and Challenge With Aeromonas Hydrophila. J. Appl. Aquacult. 24 (4), 366–376. doi: 10.1080/10454438.2012.733593
Abdel-Warith A. A., Younis E. M., Al-Asgah N. A., Gewaily M. S., El-Tonoby S. M., Dawood M. A. O. (2021). Role of Fucoidan on the Growth Behavior and Blood Metabolites and Toxic Effects of Atrazine in Nile Tilapia Oreochromis Niloticus (Linnaeus, 1758). Anim. (Basel.) 11 (5), 1–14. doi: 10.3390/ani11051448
Aebi H. (1984). Catalase In Vitro. Methods Enzymol. 105, 121–126. doi: 10.1016/s0076-6879(84)05016-3
Afifi M., Alkaladi A., Abu Zinada O. A., Couderchet M. (2017). Alteration in Antioxidant Genes Expression in Some Fish Caught From Jeddah and Yanbu Coast as a Bio-Indicator of Oil Hydrocarbons Pollution. Saudi. J. Biol. Sci. 24 (7), 1580–1587. doi: 10.1016/j.sjbs.2015.06.014
Ahmadifar E., Yousefi M., Karimi M., Fadaei Raieni R., Dadar M., Yilmaz S., et al. (2021). Benefits of Dietary Polyphenols and Polyphenol-Rich Additives to Aquatic Animal Health: An Overview. Rev. Fish. Sci. Aquacult. 29 (4), 478–511. doi: 10.1080/23308249.2020.1818689
Akhtar N., Fiaz Khan M., Tabassum S., Zahran E. (2021). Adverse Effects of Atrazine on Blood Parameters, Biochemical Profile and Genotoxicity of Snow Trout (Schizothorax Plagiostomus). Saudi. J. Biol. Sci. 28 (3), 1999–2003. doi: 10.1016/j.sjbs.2021.01.001
Ali M. F., Soliman A. A., Gewaily M. S., Abdel-Kader T. Y., Amer A. A., Zaineldin A. I., et al. (2022). Isatis Phytogenic Relieved Atrazine Induced Growth Retardation, Hepato-Renal Dysfunction, and Oxidative Stress in Nile Tilapia. Saudi. J. Biol. Sci. 29 (1), 190–196. doi: 10.1016/j.sjbs.2021.08.072
Alvarez-Gonzalez C., Martinez-Sanchez L., Pena-Marin E., Guerrero Zarate R., Jesus-Ramirez F., Morales-Garcia V., et al. (2020). Effects on the Growth and Digestive Enzyme Activity in Nile Tilapia Fry (Oreochromis Niloticus) by Lead Exposure. Wat. Air. Soil Pollut. 231, 1–15. doi: 10.1007/s11270-020-04810-9
Baek K. S., Hong Y. D., Kim Y., Sung N. Y., Yang S., Lee K. M., et al. (2015). Anti-Inflammatory Activity of Ap-Sf, a Ginsenoside-Enriched Fraction, From Korean Ginseng. J. Ginsen. Res. 39 (2), 155–161. doi: 10.1016/j.jgr.2014.10.004
Bahukhandi A., Upadhyay S., Bisht K. (2021). “Chapter 3.2.2 - Panax Ginseng C.A. Meyer,” in Naturally Occurring Chemicals Against Alzheimer's Disease (Elsevier-Netherland), Eds. Belwal T., Nabavi S. M., Nabavi S. F., Dehpour A. R., Shirooie S. (Academic Press), 217–223.
Barton B. A. (2002). Stress in Fishes: A Diversity of Responses With Particular Reference to Changes in Circulating Corticosteroids. Integr. Comp. Biol. 42 (3), 517–525. doi: 10.1093/icb/42.3.517
Basu N., Todgham A. E., Ackerman P. A., Bibeau M. R., Nakano K., Schulte P. M., et al. (2002). Heat Shock Protein Genes and Their Functional Significance in Fish. Gene 295 (2), 173–183. doi: 10.1016/s0378-1119(02)00687-x
Beutler E., Duron O., Kelly B. M. (1963). Improved Method for the Determination of Blood Glutathione. J. Lab. Clin. Med. 61, 882–888.
Bhaskaran A., May D., Rand-Weaver M., Tyler C. R. (1999). Fish P53 as a Possible Biomarker for Genotoxins in the Aquatic Environment. Environ. Mol. Mutagen. 33 (3), 177–184. doi: 10.1002/(SICI)1098-2280(1999)33:3<177::AID-EM1>3.0.CO;2-X
Blahova J., Modra H., Sevcikova M., Marsalek P., Zelnickova L., Skoric M., et al. (2014). Evaluation of Biochemical, Haematological, and Histopathological Responses and Recovery Ability of Common Carp (Cyprinus Carpio L.) After Acute Exposure to Atrazine Herbicide. BioMed. Res. Int. 2014, 980948. doi: 10.1155/2014/980948
Chakraborty S. B., Hancz C. (2011). Application of Phytochemicals as Immunostimulant, Antipathogenic and Antistress Agents in Finfish Culture. Rev. Aquacult. 3 (3), 103–119. doi: 10.1111/j.1753-5131.2011.01048.x
Chakraborty S. B., Horn P., Hancz C. (2014). Application of Phytochemicals as Growth-Promoters and Endocrine Modulators in Fish Culture. Rev. Aquacult. 6 (1), 1–19. doi: 10.1111/raq.12021
Cuesta A., Meseguer J., Esteban M. A. (2004). Total Serum Immunoglobulin M Levels Are Affected by Immunomodulators in Seabream (Sparus Aurata L.). Veterin. Immunol. Immunopathol. 101 (3), 203–210. doi: 10.1016/j.vetimm.2004.04.021
Dawood M. A. O., El Basuini M. F., Zaineldin A. I., Yilmaz S., Hasan M. T., Ahmadifar E., et al. (2021). Antiparasitic and Antibacterial Functionality of Essential Oils: An Alternative Approach for Sustainable Aquaculture. Pathogens 10 (2), 1–38. doi: 10.3390/pathogens10020185
Dawood M. A. O., El-Salam Metwally A., Elkomy A. H., Gewaily M. S., Abdo S. E., Abdel-Razek M. A. S., et al. (2020). The Impact of Menthol Essential Oil Against Inflammation, Immunosuppression, and Histopathological Alterations Induced by Chlorpyrifos in Nile Tilapia. Fish. Shellf. Immunol. 102, 316–325. doi: 10.1016/j.fsi.2020.04.059
de Freitas Souza C., Dellaméa Baldissera M., Baldisserotto B., Heinzmann B. M., Martos-Sitcha J. A., Mancera J. M. (2019). Essential Oils as Stress-Reducing Agents for Fish Aquaculture: A Review. Front. Physiol. 10. doi: 10.3389/fphys.2019.00785
de Souza R. C., Baldisserotto B., Melo J. F. B., da Costa M. M., de Souza E. M., Copatti C. E. (2020). Dietary Aloysia Triphylla Essential Oil on Growth Performance and Biochemical and Haematological Variables in Nile Tilapia. Aquaculture 519, 734913. doi: 10.1016/j.aquaculture.2019.734913
de Souza E. M., de Souza R. C., Melo J. F. B., da Costa M. M., de Souza A. M., Copatti C. E. (2019). Evaluation of the Effects of Ocimum Basilicum Essential Oil in Nile Tilapia Diet: Growth, Biochemical, Intestinal Enzymes, Haematology, Lysozyme and Antimicrobial Challenges. Aquaculture 504, 7–12. doi: 10.1016/j.aquaculture.2019.01.052
Doumas B. T., Bayse D. D., Carter R. J., Peters T. Jr., Schaffer R. (1981). A Candidate Reference Method for Determination of Total Protein in Serum. I. Development and Validation. Clin. Chem. 27 (10), 1642–1650.
El Euony O. I., Elblehi S. S., Abdel-Latif H. M., Abdel-Daim M. M., El-Sayed Y. S. (2020). Modulatory Role of Dietary Thymus Vulgaris Essential Oil and Bacillus Subtilis Against Thiamethoxam-Induced Hepatorenal Damage, Oxidative Stress, and Immunotoxicity in African Catfish (Clarias Garipenus). Environ. Sci. Pollut. Res. Int. 27 (18), 23108–23128. doi: 10.1007/s11356-020-08588-5
El-Sayed S. A. A., EL-Galil S. Y. A., Rashed N. A. E. (2014). Immunomodulatory and Growth Performance Effects of Ginseng Extracts as a Natural Growth Promoter in Comparison With Oxytetracycline in the Diets of Nile Tilapia (Oreochromis Niloticus). Int. J. Livest. Res. 4, 130–142. doi: 10.5455/ijlr.20140109084346
Farag M. R., Alagawany M., Bilal R. M., Gewida A. G. A., Dhama K., Abdel-Latif H. M. R., et al. (2021). An Overview on the Potential Hazards of Pyrethroid Insecticides in Fish, With Special Emphasis on Cypermethrin Toxicity. Anim. (Basel.) 11 (7). doi: 10.3390/ani11071880
Farag M. R., Alagawany M., Khalil S. R., Abd El-Aziz R. M., Zaglool A. W., Moselhy A. A. A., et al. (2022). Effect of Parsley Essential Oil on Digestive Enzymes, Intestinal Morphometry, Blood Chemistry and Stress-Related Genes in Liver of Nile Tilapia Fish Exposed to Bifenthrin. Aquaculture 546, 737322. doi: 10.1016/j.aquaculture.2021.737322
Farag M. R., Alagawany M., Khalil S. R., Moustafa A. A., Mahmoud H. K., Abdel-Latif H. M. R. (2021). Astragalus Membranaceus Polysaccharides Modulate Growth, Hemato-Biochemical Indices, Hepatic Antioxidants, and Expression of Hsp70 and Apoptosis-Related Genes in Oreochromis Niloticus Exposed to Sub-Lethal Thallium Toxicity. Fish. Shellf. Immunol. 118, 1–17. doi: 10.1016/j.fsi.2021.09.009
Farag M. R., Alagawany M., Taha H. S. A., Ismail T. A., Khalil S. R., Abou-Zeid S. M. (2021). Immune Response and Susceptibility of Nile Tilapia Fish to Aeromonas Hydrophila Infection Following the Exposure to Bifenthrin and/or Supplementation With Petroselinum Crispum Essential Oil. Ecotoxicol. Environ. Saf. 216, 112205. doi: 10.1016/j.ecoenv.2021.112205
Feder M. E., Hofmann G. E. (1999). Heat-Shock Proteins, Molecular Chaperones, and the Stress Response: Evolutionary and Ecological Physiology. Annu. Rev. Physiol. 61, 243–282. doi: 10.1146/annurev.physiol.61.1.243
Ghafarifarsani H., Kachuei R., Imani A. (2021). Dietary Supplementation of Garden Thyme Essential Oil Ameliorated the Deteriorative Effects of Aflatoxin B1 on Growth Performance and Intestinal Inflammatory Status of Rainbow Trout (Oncorhynchus Mykiss). Aquaculture 531, 735928. doi: 10.1016/j.aquaculture.2020.735928
Ghelichpour M., Taheri Mirghaed A., Hoseinifar S. H., Khalili M., Yousefi M., Van Doan H., et al. (2019). Expression of Immune, Antioxidant and Stress Related Genes in Different Organs of Common Carp Exposed to Indoxacarb. Aquat. Toxicol. 208, 208–216. doi: 10.1016/j.aquatox.2019.01.011
Goda A. (2008). Effect of Dietary Ginseng Herb (Ginsanaæ G115) Supplementation on Growth, Feed Utilization, and Hematological Indices of Nile Tilapia, Oreochromis Niloticus (L.), Fingerlings. J. World Aquacult. Soc. 39, 205–214. doi: 10.1111/j.1749-7345.2008.00153.x
Graymore M., Stagnitti F., Allinson G. (2001). Impacts of Atrazine in Aquatic Ecosystems. Environ. Int. 26 (7), 483–495. doi: 10.1016/S0160-4120(01)00031-9
Helton E. S., Chen X. (2007). P53 Modulation of the DNA Damage Response. J. Cell Biochem. 100 (4), 883–896. doi: 10.1002/jcb.21091
Iseppi R., Di Cerbo A., Aloisi P., Manelli M., Pellesi V., Provenzano C., et al. (2020). In Vitro Activity of Essential Oils Against Planktonic and Biofilm Cells of Extended-Spectrum Beta-Lactamase (Esbl)/Carbapenamase-Producing Gram-Negative Bacteria Involved in Human Nosocomial Infections. Antibio. (Basel.) 9 (5), 1–12. doi: 10.3390/antibiotics9050272
Iwama G. K., Thomas P. T., Forsyth R. B., Vijayan M. M. (1998). Heat Shock Protein Expression in Fish. Rev. Fish. Biol. Fish. 8 (1), 35–56. doi: 10.1023/A:1008812500650
Iwama G. K., Vijayan M. M., Forsyth R. B., Ackerman P. A. (2015). Heat Shock Proteins and Physiological Stress in Fish1. Am. Zool. 39 (6), 901–909. doi: 10.1093/icb/39.6.901
Kadry S., Amer A. A., Marzouk M., Hanna M., Azmy A., Hamed H. (2012). Vitamin E as Antioxidant in Female African Catfish (Clarias Gariepinus) Exposed to Chronic Toxicity of Atrazine. Egypt. J. Aquat. Biol. Fish. 16 (2), 83–98. doi: 10.21608/ejabf.2012.2127
Khafaga A. F., Naiel M. A. E., Dawood M. A. O., Abdel-Latif H. M. R. (2020). Dietary Origanum Vulgare Essential Oil Attenuates Cypermethrin-Induced Biochemical Changes, Oxidative Stress, Histopathological Alterations, Apoptosis, and Reduces DNA Damage in Common Carp (Cyprinus Carpio). Aquat. Toxicol. 228, 105624. doi: 10.1016/j.aquatox.2020.105624
Khalil S. R., Reda R. M., Awad A. (2017). Efficacy of Spirulina Platensis Diet Supplements on Disease Resistance and Immune-Related Gene Expression in Cyprinus Carpio L. Exposed to Herbicide Atrazine. Fish. Shellf. Immunol. 67, 119–128. doi: 10.1016/j.fsi.2017.05.065
Kim Y. K., Guo Q., Packer L. (2002). Free Radical Scavenging Activity of Red Ginseng Aqueous Extracts. Toxicology 172 (2), 149–156. doi: 10.1016/s0300-483x(01)00585-6
Kitts D. D., Wijewickreme A. N., Hu C. (2000). Antioxidant Properties of a North American Ginseng Extract. Mol. Cell Biochem. 203 (1-2), 1–10. doi: 10.1023/a:1007078414639
Lackner R. (1998). ““Oxidative Stress” in Fish by Environmental Pollutants,” in Fish Ecotoxicology, vol. . p . Eds. Braunbeck T., Hinton D. E., Streit B. (Basel: Birkhäuser Basel), 203–224.
Le Bras M., Clement M. V., Pervaiz S., Brenner C. (2005). Reactive Oxygen Species and the Mitochondrial Signaling Pathway of Cell Death. Histol. Histopathol. 20 (1), 205–219. doi: 10.14670/HH-20.205
Lee L. S., Wise S. D., Chan C., Parsons T. L., Flexner C., Lietman P. S. (2008). Possible Differential Induction of Phase 2 Enzyme and Antioxidant Pathways by American Ginseng, Panax Quinquefolius. J. Clin. Pharmacol. 48 (5), 599–609. doi: 10.1177/0091270008314252
Liu X. L., Xi Q. Y., Yang L., Li H. Y., Jiang Q. Y., Shu G., et al. (2011). The Effect of Dietary Panax Ginseng Polysaccharide Extract on the Immune Responses in White Shrimp, Litopenaeus Vannamei. Fish. Shellf. Immunol. 30 (2), 495–500. doi: 10.1016/j.fsi.2010.11.018
Livak K. J., Schmittgen T. D. (2001). Analysis of Relative Gene Expression Data Using Real-Time Quantitative Pcr and the 2(-Delta Delta C(T)) Method. Methods 25 (4), 402–408. doi: 10.1006/meth.2001.1262
Magouz F. I., Mahmoud S. A., El-Morsy R. A. A., Paray B. A., Soliman A. A., Zaineldin A. I., et al. (2021). Dietary Menthol Essential Oil Enhanced the Growth Performance, Digestive Enzyme Activity, Immune-Related Genes, and Resistance Against Acute Ammonia Exposure in Nile Tilapia (Oreochromis Niloticus). Aquaculture 530, 735944. doi: 10.1016/j.aquaculture.2020.735944
Mai W.-J., Yan J.-L., Wang L., Zheng Y., Xin Y., Wang W.-N. (2010). Acute Acidic Exposure Induces P53-Mediated Oxidative Stress and DNA Damage in Tilapia (Oreochromis Niloticus) Blood Cells. Aquat. Toxicol. 100 (3), 271–281. doi: 10.1016/j.aquatox.2010.07.025
Mehrim A. I., Refaey M. M., Hassan M. A. E., Zaki M. A., Zenhom O. A. (2022). Ginseng(R) as a Reproductive Enhancer Agent for African Catfish, Clarias Gariepinus (Burchell, 1822). Fish. Physiol. Biochem. 48 (1), 15–32. doi: 10.1007/s10695-021-00969-y
Michael P. O. (2018). “Toxicity Effect of Atrazine on Histology,” in Haematology and Biochemical Indices of Clarias Gariepinus. Int. J. Fish. Aquat. Stud. 6 (3), 87–92
Michael P. O., Veronica O. B., Priscilla E. (2018). Response of Clarias Gariepinus (Juveniles) Exposed to Sublethal Concentrations of Atrazine. Aquac. Stud. 18 (1), 19–26. doi: 10.4194/2618-6381-v18_1_03
Mihara M., Uchiyama M. (1978). Determination of Malonaldehyde Precursor in Tissues by Thiobarbituric Acid Test. Anal. Biochem. 86 (1), 271–278. doi: 10.1016/0003-2697(78)90342-1
Mohamed A. A., Rahman A. N. A., Mohammed H. H., Ebraheim L. L. M., Abo-ElMaaty A. M. A., Ali S. A., et al. (2020). Neurobehavioral, Apoptotic, and DNA Damaging Effects of Sub-Chronic Profenofos Exposure on the Brain Tissue of Cyprinus Carpio L.: Antagonistic Role of Geranium Essential Oil. Aquat. Toxicol. 224, 105493. doi: 10.1016/j.aquatox.2020.105493
Monteiro S. M., dos Santos N. M., Calejo M., Fontainhas-Fernandes A., Sousa M. (2009). Copper Toxicity in Gills of the Teleost Fish, Oreochromis Niloticus: Effects in Apoptosis Induction and Cell Proliferation. Aquat. Toxicol. 94 (3), 219–228. doi: 10.1016/j.aquatox.2009.07.008
Naji M., Yousefi Jourdehi Y., Hosseinzadeh Sahafi H. (2019). Impacts of Atrazine on Some Blood and Biochemical Indices in Farmed Acipenser Nudiventris. Surv. Fish. Sci. 5 (2), 19–27. doi: 10.18331/sfs2019.5.2.3
Neamat Allah A. N. F., Abd El Hakim Y., Mahmoud E. A. (2020). Alleviating Effects of “ Glucan Inoreochromis Niloticuson Growth Performance, Immune Reactions, Antioxidant, Transcriptomics Disorders and Resistance Toaeromonas Sobriacaused by Atrazine. Aquacult. Res. 51, 1801–1812. doi: 10.1111/are.14529
Neskovic N. K., Elezovic I., Karan V., Poleksic V., Budimir M. (1993). Acute and Subacute Toxicity of Atrazine to Carp (Cyprinus Carpio L.). Ecotoxicol. Environ. Saf. 25 (2), 173–182. doi: 10.1006/eesa.1993.1016
Nishikimi M., Appaji Rao N., Yagi K. (1972). The Occurrence of Superoxide Anion in the Reaction of Reduced Phenazine Methosulfate and Molecular Oxygen. Biochem. Biophys. Res. Commun. 46 (2), 849–854. doi: 10.1016/S0006-291X(72)80218-3
Nordberg J., Arner E. S. (2001). Reactive Oxygen Species, Antioxidants, and the Mammalian Thioredoxin System. Free Radic. Biol. Med. 31 (11), 1287–1312. doi: 10.1016/s0891-5849(01)00724-9
Nwani C. D., Lakra W. S., Nagpure N. S., Kumar R., Kushwaha B., Srivastava S. K. (2010). Toxicity of the Herbicide Atrazine: Effects on Lipid Peroxidation and Activities of Antioxidant Enzymes in the Freshwater Fish Channa Punctatus (Bloch). Int. J. Environ. Res. Public Health 7 (8), 3298–3312. doi: 10.3390/ijerph7083298
Ohkawa H., Ohishi N., Yagi K. (1979). Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 95 (2), 351–358. doi: 10.1016/0003-2697(79)90738-3
Owolabi O. D., Abdulkareem S. I. (2021). Carica Papaya and Mangifera Indica Modulate Haematological, Biochemical and Histological Alterations in Atrazine-Intoxicated Fish, Clarias Gariepinus (Burchell 1822). J. Basic. Appl. Zool. 82 (1), 42. doi: 10.1186/s41936-021-00241-y
Paulino M. G., Souza N. E., Fernandes M. N. (2012). Subchronic Exposure to Atrazine Induces Biochemical and Histopathological Changes in the Gills of a Neotropical Freshwater Fish, Prochilodus Lineatus. Ecotoxicol. Environ. Saf. 80, 6–13. doi: 10.1016/j.ecoenv.2012.02.001
Paul S., Shin H. S., Kang S. C. (2012). Inhibition of Inflammations and Macrophage Activation by Ginsenoside-Re Isolated From Korean Ginseng (Panax Ginseng C.A. Meyer). Food Chem. Toxicol. 50 (5), 1354–1361. doi: 10.1016/j.fct.2012.02.035
Porter A. G., Jänicke R. U. (1999). Emerging Roles of Caspase-3 in Apoptosis. Cell Death Diff. 6 (2), 99–104. doi: 10.1038/sj.cdd.4400476
Prabakaran S., Pugazhendy K., Revathi A., Jayanthi C. (2014). Hepatoprotective Effect of Pisonia Alba and Cardiospermum Halicacabum in Atrazine Toxicity on Lpo and Some Antioxidant Activities in the Liver Tissue of Fresh Water Fish Labeo Rohita. Int. J. Pharm. Biol. Arch. 5, 1231–1237. ISSN 0976 – 3333.
Rai D., Bhatia G., Sen T., Palit G. (2003). Anti-Stress Effects of Ginkgo Biloba and Panax Ginseng: A Comparative Study. J. Pharmacol. Sci. 93 (4), 458–464. doi: 10.1254/jphs.93.458
Santos T. G., Martinez C. B. (2012). Atrazine Promotes Biochemical Changes and DNA Damage in a Neotropical Fish Species. Chemosphere 89 (9), 1118–1125. doi: 10.1016/j.chemosphere.2012.05.096
Selim H. M. (2003). Retention and Runoff Losses of Atrazine and Metribuzin in Soil. J. Environ. Qual. 32 (3), 1058–1071. doi: 10.2134/jeq2003.1058
Sena A. C., Teixeira R. R., Ferreira E. L., Heinzmann B. M., Baldisserotto B., Caron B. O., et al. (2016). Short Communication. Aquaculture 465 (C), 374–379. doi: 10.1016/j.aquaculture.2016.09.033
Sing R. (2020). Studies on Atrazine Induced Changes in Some Cat Fish: Aspects of Female African Catfish (Clarias Gariepinus). Int. J. Fauna. Biol. Stud. 7 (1), 114–117. ISSN 2347-2677
Siwicki A. K., Anderson D. P. (1993). An Easy Spectrophotometric Assay for Determining Total Protein and Immunoglobulin Levels in Fish Sera: Correlation to Fish Health. Tech. Fish. Immunol. 3, 23–30.
Smigielski K., Dolot M., Raj A. (2006). Composition of the Essential Oils of Ginseng Roots of Panax Quinquefolium L. And Panax Ginseng C.A. Meyer. J. Essential. Oil Bear. Plants 9 (3), 261–266. doi: 10.1080/0972060X.2006.10643501
Solomon K. R., Carr J. A., Du Preez L. H., Giesy J. P., Kendall R. J., Smith E. E., et al. (2008). Effects of Atrazine on Fish, Amphibians, and Aquatic Reptiles: A Critical Review. Crit. Rev. Toxicol. 38 (9), 721–772. doi: 10.1080/10408440802116496
Soorena A., Ayoub Y. J., Rezvanollah K., Ali Y. S. M. (2011). Effects of Atrazine (Herbicide) on Blood Biochemical Indices of Grass Carp (Ctenopharhyngoden Idella). J. Persian Gulf. 2 (5), 51–56
Souza C., Baldissera M. D., Baldisserotto B., Heinzmann B. M., Martos-Sitcha J. A., Mancera J. M. (2019). Essential Oils as Stress-Reducing Agents for Fish Aquaculture: A Review. Front. Physiol. 10. doi: 10.3389/fphys.2019.00785
Suvarna S. K., Layton C., Bancroft J. D. (2013). Bancroft's Theory and Practice of Histological Techniques ([Oxford]: Churchill Livingstone Elsevier).
Taheri Mirghaed A., Baes M., Hoseini S. M. (2020). Humoral Immune Responses and Gill Antioxidant-Related Gene Expression of Common Carp (Cyprinus Carpio) Exposed to Lufenuron and Flonicamide. Fish. Physiol. Biochem. 46 (2), 739–746. doi: 10.1007/s10695-019-00747-x
Van Doan H., Hoseinifar S. H., Chitmanat C., Jaturasitha S., Paolucci M., Ashouri G., et al. (2019). The Effects of Thai Ginseng, Boesenbergia Rotunda Powder on Mucosal and Serum Immunity, Disease Resistance, and Growth Performance of Nile Tilapia (Oreochromis Niloticus) Fingerlings. Aquaculture 513, 734388. doi: 10.1016/j.aquaculture.2019.734388
Varela-Valencia R., Gómez-Ortiz N., Oskam G., de Coss R., Rubio-Piña J., del Río-García M., et al. (2014). The Effect of Titanium Dioxide Nanoparticles on Antioxidant Gene Expression in Tilapia (Oreochromis Niloticus). J. Nanopart. Res. 16 (4), 2369. doi: 10.1007/s11051-014-2369-3
Wang S., Zhang Q., Zheng S., Chen M., Zhao F., Xu S. (2019). Atrazine Exposure Triggers Common Carp Neutrophil Apoptosis via the Cyp450s/Ros Pathway. Fish. Shellf. Immunol. 84, 551–557. doi: 10.1016/j.fsi.2018.10.029
Wendelaar Bonga S. E. (1997). The Stress Response in Fish. Physiol. Rev. 77 (3), 591–625. doi: 10.1152/physrev.1997.77.3.591
Xing H., Li S., Wang X., Gao X., Xu S., Wang X. (2013). Effects of Atrazine and Chlorpyrifos on the Mrna Levels of Hsp70 and Hsc70 in the Liver, Brain, Kidney and Gill of Common Carp (Cyprinus Carpio L.). Chemosphere 90 (3), 910–916. doi: 10.1016/j.chemosphere.2012.06.028
Yuan C. S., Wang C. Z., Wicks S. M., Qi L. W. (2010). Chemical and Pharmacological Studies of Saponins With a Focus on American Ginseng. J. Ginsen. Res. 34 (3), 160–167. doi: 10.5142/jgr.2010.34.3.160
Zaki M. S., Fawzi O. M., Zytuun I. M. (2011). Reduction of Alfatoxin in Clarious Lazara Catfish by Ginseng Extract and Nigella Sativa Oil. J. Am. Sci. 7 (2), 591–596. ISSN: 1545-1003
Zeppenfeld C. C., Toni C., Becker A. G., dos Santos Miron D., Parodi T. V., Heinzmann B. M., et al. (2014). Physiological and Biochemical Responses of Silver Catfish, Rhamdia Quelen, After Transport in Water With Essential Oil of Aloysia Triphylla (L'herit) Britton. Aquaculture 418-419, 101–107. doi: 10.1016/j.aquaculture.2013.10.013
Zhao B., Lv C., Lu J. (2019). Natural Occurring Polysaccharides From Panax Ginseng C. A. Meyer: A Review of Isolation, Structures, and Bioactivities. Int. J. Biol. Macromol. 133, 324–336. doi: 10.1016/j.ijbiomac.2019.03.229
Zhao B., Wang X., Liu H., Lv C., Lu J. (2020). Structural Characterization and Antioxidant Activity of Oligosaccharides From Panax Ginseng C. A. Meyer. Int. J. Biol. Macromol. 150, 737–745. doi: 10.1016/j.ijbiomac.2020.02.016
Zhu L., Dong X., Xie H., Wang J., Wang J., Su J., et al. (2011). DNA Damage and Effects on Glutathione-S-Transferase Activity Induced by Atrazine Exposure in Zebrafish (Danio Rerio). Environ. Toxicol. 26 (5), 480–488. doi: 10.1002/tox.20575
Keywords: herbal essential oils, aquafeed, animal health, animal welfare, productive performance, oxidative stress, physiological parameters, atrazine toxicity
Citation: Ahmed MM, Mohammed AT, Farag MR, Hassan MA, Mawed SA, Alagawany M, Zizzadoro C, Di Cerbo A and Abdel-Latif HMR (2022) Dietary Supplementation of Nile Tilapia (Oreochromis niloticus) With Panax ginseng Essential Oil: Positive Impact on Animal Health and Productive Performance, and Mitigating Effects on Atrazine- Induced Toxicity. Front. Mar. Sci. 9:920057. doi: 10.3389/fmars.2022.920057
Received: 14 April 2022; Accepted: 12 May 2022;
Published: 30 June 2022.
Edited by:
Rigers Bakiu, Agricultural University of Tirana, AlbaniaCopyright © 2022 Ahmed, Mohammed, Farag, Hassan, Mawed, Alagawany, Zizzadoro, Di Cerbo and Abdel-Latif. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mayada R. Farag, ZHIubWF5YWRhcmZAZ21haWwuY29t; Claudia Zizzadoro, Y2xhdWRpYS56aXp6YWRvcm9AdW5pYmEuaXQ=
 Mona M. Ahmed1
Mona M. Ahmed1 Mayada R. Farag
Mayada R. Farag Claudia Zizzadoro
Claudia Zizzadoro Alessandro Di Cerbo
Alessandro Di Cerbo