
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 17 November 2022
Sec. Marine Megafauna
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.916950
This article is part of the Research Topic Risks, Threats, and Conservation Status of Cetaceans in the Mediterranean and Black Seas View all 15 articles
The most eastern population of common dolphins (Delphinus delphis) in the Mediterranean Sea inhabits the southern coastal waters of Israel. They are mainly observed in the shallow waters off Ashdod and Ashkelon, between the 15-30 m isobaths, with no reported observations north or west of this area. These dolphins were observed and studied year-round between 2016-2021 using boat-based surveys and photo identification methods. Common dolphins were encountered and photographed 43 times during the study period, resulting in 2,851 identifications of 25 distinctive mature individuals and 12 calves. Most individuals (62%) were sighted over multiple years, with high yearly and monthly sighting rates, indicating long-term site fidelity and residency. Closed population mark-recapture models estimated a total abundance of 25 (95% CI 24 – 37) individuals in 2016 that declined to only 15 (95% CI 15 – 15) individuals in 2021. Social network analysis described these remaining individuals as one closed and well-associated social unit. Survival probabilities for this population appeared lower than those of other delphinid populations. The decrease in their abundance, coupled with their apparent isolation level, qualifies the local population for a re-assessment of their conservation status. This study first describes the Israeli local population of common dolphins, their dynamics and an assessment of their status based on the IUCN Red List framework.
Common dolphins (Delphinus delphis) were once one of the most abundant cetaceans in the Mediterranean Sea, inhabiting coastal and deep-water areas (Bearzi et al., 2003; Pace et al., 2016; Vella et al., 2021). However, culling and killing efforts since the early 19th century, followed by a reduction in prey abundance due to overfishing since the mid-20th century (Bearzi et al., 2003; FAO, 2018), has resulted in a dramatic decrease in their numbers. This trend led to their declaration as ‘Endangered’ in the Mediterranean Sea by the IUCN in 2003 (Bearzi et al., 2003), compared to their global status, classified as ‘Least Concern’ (Hammond et al., 2008). Furthermore, according to Natoli et al. (2008), genetic evidence of sub-population structure may indicate separate management units in the western and eastern Mediterranean Sea, thus differentiating between the “inner Mediterranean’’ sub-population of common dolphins and a north-eastern Atlantic population (Natoli et al., 2008; Moura et al., 2013; Bearzi et al., 2021). The “outer Mediterranean” population inhabits the north-eastern Atlantic and the Alborán Sea (Cañadas and Hammond, 2008), while the “inner Mediterranean” sub-population inhabits the Mediterranean waters east of the Almerìa – Orán thermohaline front and was reassessed as ‘Endangered’ in 2021 (Bearzi et al., 2021)
The ‘inner Mediterranean Sea’ sub-population is segregated into several small groups scattered in parts of the southern Tyrrhenian Sea, Sicily Channel, and Ionian Sea (Vella, 2004; Gannier, 2005; Arcangeli et al., 2013; Aissi and Vella, 2015; Santoro et al., 2015; Pace et al., 2016; Arcangeli et al., 2017) and more regularly in the northern and eastern Aegean Sea (Frantzis et al., 2003; Milani et al., 2021). These small groups will be referred to as local populations as they inhabit different habitats who are subjected to numerous anthropogenic effects (Bearzi and Genov, 2021). Most of them present decreasing trends in abundance (Bearzi et al., 2008; Gonzalvo and Costa, 2016; Mussi et al., 2019; Genov et al., 2020; Vella et al., 2021). In the Gulf of Corinth, for example, 22 (range 16 - 32) common dolphins are observed exclusively in mixed-species groups with the striped dolphin (Stenella coeruleoalba) (Santostasi et al., 2016). This local population was recently declared ‘Critically Endangered’ and faces a high extinction risk (≥ 50%, Santostasi et al., 2018). In the north Adriatic Sea, the numbers are even lower, as the local population underwent a dramatic decline in the 1970s, and between 2009 – 2012 only four individuals were observed (Genov et al., 2020).
The small size and separation between these local populations have led to the formation of different social structures. For example, a social study conducted in the Ionian Sea between 1996 and 1999 described a small population of 47 individuals in the area who presented a fluid social structure akin to a ‘fission-fusion’ society composed of a single social unit divided into groups with frequently changing memberships (Bruno et al., 2004). In the Tyrrhenian Sea, however, 38 individuals presented high site fidelity and stable association patterns (Pace et al., 2009), while females in the same reproductive state maintained strong and long-lasting associations for up to five years (Mussi et al., 2019).
Each local population described above might react differently to anthropogenic effects and other environmental changes in their habitats, as observed in other cetacean species worldwide (Ansmann et al., 2012; Blasi and Boitani, 2014; Genov et al., 2019). Therefore, they may be addressed to as different management units and understanding the dynamics of each one of them, such as their abundance, trends, distribution, and social structure, is necessary to inform conservation actions and apply appropriate management actions (Notarbartolo di Sciara and Birkun, 2010).
The most eastern common dolphins in the Mediterranean inhabits the southern waters of Israel, from south Tel Aviv to the southern Israeli border (Brand et al., 2019). They are mainly observed in the coastal waters off Ashdod and Ashkelon, at water depths of 15-30 m (2-4 km from shore), frequently in the same small area. Bottlenose dolphins (Tursiops truncatus) also range in this area, usually in deeper waters than the common dolphins, and the two species were never observed together. Common dolphins have been observed along the Israeli coastline since 1993, mostly during occasional sightings and second-party reports, containing a mean group size of 22.2 ± 19.1 (range 1 – 75) individuals (Kerem et al., 2012). These reports are prior to the study period, and they are the earliest known encounters with common dolphins in Israel. None of them observed the common dolphins northern or western of the study area, except for two reports north of Tel Aviv in 2009 and 2011. The southern border of their habitat is less clear as reports from north Egypt do not include this species (Farrag et al., 2019), and only rare sightings and few strandings of common dolphins have been reported from the Gaza strip in the last 20 years (Abd Rabou et al., 2021). These observations might indicate the presence of another group or groups southern to this research study area but could also be the same individuals that range the southern Israeli waters.
Several individuals have been stranded on the Israeli shore over the last two decades. Their stomach content reveals that their diet is mainly composed of the Balearic eel (Ariosoma balearicum), Klunzinger’s ponyfish (Equulites kluzingeri), and cephalopods (Brand et al., 2019), which are also common in the local fisheries catch. Dolphins are often observed feeding from the discards of the sorting process of the fishermen while pulling the net back to the boat.
The local population of common dolphins in Israel is subjected to many anthropogenic pressures as they inhabit a coastal area in proximity to a major port and a power station with various human activities such as fishing, sailing, and discharging of sewage. Additionally, their habitat is near the Israeli border with the Gaza strip, which increases the presence of navy ships in the area. Part of their ranging area has been approved as the Marine Protected Area ‘Evtach’ (Figure 1), but it is not yet declared and enforced; therefore, it is still subject to fishing pressure. Given their delicate status in the Mediterranean Sea and their regional decrease in abundance, this study aims to provide the first insight into the dynamics of the most eastern local population of common dolphins in the Mediterranean Sea, using mark-recapture models and social network analysis. In addition, this study provides a quantitative evaluation of conservation status following the IUCN Red List criteria (IUCN, 2012a; IUCN, 2012b) framework applied to the local population of common dolphins in Israel and performs a quantitative estimate of its probability of extinction.
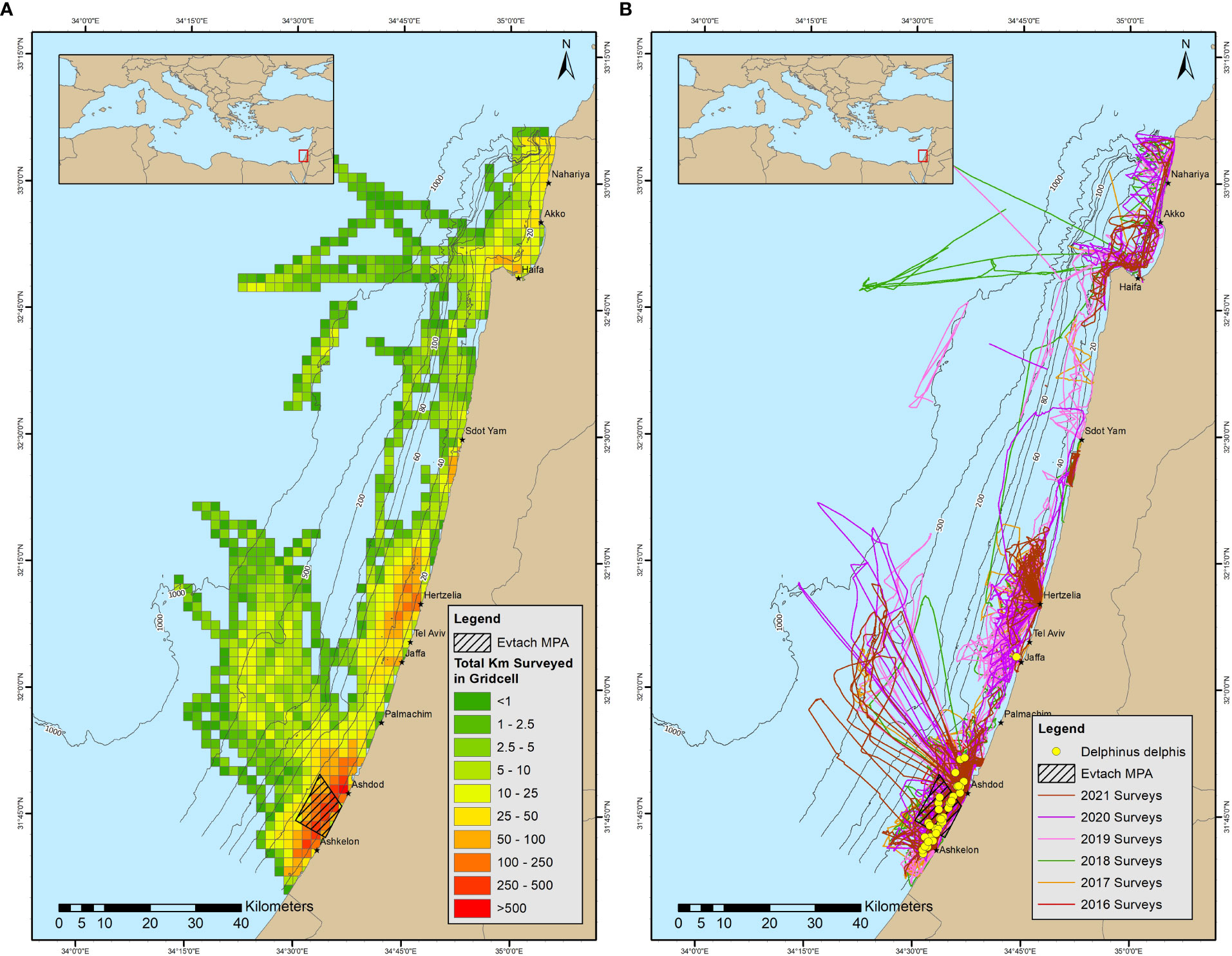
Figure 1 (A) Heatmap of survey effort. Colors represent effort in surveyed km in 2x2 grid cells. (B) Survey routs throughout the study period. Different colors represent different years. Yellow circles represent common dolphin sightings.
The study area is spread along the entire 196 km of Israel’s coastline of the Mediterranean Sea (Figure 1B and Supplementary Figures 1–6). The continental shelf extends to a depth of 200 m; gradually widens from its narrowest part in the north, approximately 10 km from shore, towards the widest part in the south, nearly 20 km from shore. Survey transects started from the marina’s exit toward the open sea, usually between 10 - 60 m in depth with some effort in deeper waters, up to 1200 meters in depth (50 km from shore).
Shipboard surveys were conducted year-round according to the protocol described by Scheinin (2010) as part of the long-term monitoring activity of Morris Kahn Marine Research Station (MKMRS) of the University of Haifa, in collaboration with the Israel Marine Mammal Research and Assistance Center (IMMRAC NGO), and Delphis NGO. The starting point of each survey was from one of eight locations along the coast grouped as South (Ashkelon and Ashdod), Center (Jaffa port, Tel Aviv, Herzliya, and Sdot–Yam), and North (Haifa, Akko, and Nahariya). The surveys were primarily opportunistic and dependent on collaborations with private yacht and boat owners, apart from a designated project funded by the Ministry of Energy between 2018 – 2020, which allowed regular surveys once a week from Ashdod, following the same protocol. On average, surveys were conducted two to five times a month throughout Israel’s continental shelf’s southern and central marine areas, while the northern area was surveyed less frequently due to fewer collaborations. The boat surveys were performed at the discretion of the research team, based on variables such as sea conditions, prior survey routes, and the locations of the last observations of dolphins. The sampling effort was distributed in an attempt to cover the entire study area equally, but due to the dependency on collaborations, that was not always the case. Areas closer to the main marinas were surveyed more frequently (Figure 1A), and during the funding time of the Ministry of Energy, there was a high concentration of effort in the south. The survey route generally followed a transverse zigzag approach between 30-60 m isobaths, parallel to the coastline, at a speed of 4-12 knots. Bottom trawlers, sailing at a speed of 3 knots, following the longshore 35-60 m isobaths, were opportunistically approached, searching for dolphins foraging nearby. Deep-water surveys were conducted aboard a commercial longline pelagic fishing boat fishing for tuna fish, traveling from Ashdod up to 50 km from shore, and deploying pelagic longlines between 800 - 1200 m isobaths.
The boat’s position was recorded along the route every 20 seconds during each survey, while environmental parameters and all wildlife encounters were logged using ‘Delphis’, a designated data collection mobile application (Marco, 2017). Once dolphins were sighted, they were approached to photograph and collect group focal follow data, logged into the ‘Delphis’ application as well. Disturbances to the dolphins were reduced by following them at minimum speed from 20 - 50 meters away and avoiding sudden directional or speed changes. Close approaches, < 20 m, were only initiated by the dolphins themselves, approaching the boat from curiosity or to bow-ride. The dolphins were kept in sight until high-quality photographs of all the individuals were achieved or until vessel constraints or sea Ficonditions forced the encounter to an end. Photographs of the left and right sided of the dorsal fin were taken using a Canon EOS 7D 18mp camera with 70-200mm f2.8 EF zoom lenses.
In addition to the data collected during this study, 11 observations of common dolphins were recorded and photographed by the marine unit of the Nature and Park Authority in Israel. These observations occurred in the same area as the study area, and the dolphins were photographed following the same protocol. To enlarge the database for this study, the locations and photographs of these 11 encounters were added to the data and were considered for the analysis, summing to a total of 43 photographed encounters between 2016 - 2021.
Three age classes were considered based on visual assessments, as suggested by Mussi et al. (2019): “calf below half of an adult length, constantly in close association with an adult, with a dorsal fin typically low and rounded, a dark, lead-grey coloration with visible fetal folds, and immature swimming style with stereotyped surfacing pattern when breathing; juvenile about two‐thirds of an adult, usually swimming in association with an adult, but sometimes independently, with a coloration slightly lighter than the adult; adult approximately 2 m long”. Sex was determined when photographs of the genital area were achievable (Smolker et al., 1992) or when an adult was consistently accompanied by a calf and assumed female (Shinohara et al., 1997). A group was defined according to Shane (1990), as a “group of dolphins observed in apparent association, moving in the same direction and often, but not always, engaged in the same activity”. Members of the group usually remained within 100 m from each other and were assumed to all have the same probability of being detected and photographed (i.e., captured). All survey methods remained consistent throughout the study period.
Common dolphins were individually identified based on long-lasting markings and coloration patterns on their dorsal fins (Würsig and Würsig, 1977). Photographs were first graded by quality (Q) (Wilson et al., 1999) from 1 to 5; where 1 is assigned to photographs with no dolphins but might contain other relative information, 2 to photographs that contain dolphins but not their dorsal fins or very bad angled dorsal fins, 3 was assigned to photographs which contained less focused and tilted angled dorsal fins but with clearly visible edge, 4 to focused and good angled dorsal fins and 5 to photographs containing well lit, straight angle and focused dorsal fins. Only photographs with a Q ≥ 3 were further processed and given an additional grade by the distinctiveness of the dorsal fin (Wilson et al., 1999). Highly distinctive dorsal fins with visible and long-lasting marks or irregularly shaped ones were graded as 1. Grade 2 was assigned to the moderately marked dorsal fin, which contains, for example, only one small notch, while smooth, mark-less fins were graded as 3. Minor scratches usually heal and therefore were only used for short-term identification between observations within the same month or two and were not considered in the distinctiveness grading. The best left and right photographs of each individual from every encounter were used to create a catalog, where all uniquely identified individuals were assigned a number. Each identification was considered final when approved by the independent evaluation of at least two researchers (Berrow et al., 2012). Matches could be determined by photographs of either side of the dorsal fin, but preferably both, when photographs of both sides were available. Calves were given identification numbers in reference to their mothers for easier follow-up. Only substantially marked individuals (distinctiveness grade 1 or 2) from good-quality photographs (Q ≥ 3) were considered in the analysis to avoid misidentification of individuals that can cause biased estimations of abundance and structure (Hammond, 2010). Five common dolphin individuals were stranded along the Israeli coastline throughout the study period: three adult males, one young male, and one young female. Their dorsal fins were checked for matches in the catalog.
The site fidelity of the local population was estimated by calculating the mean yearly and monthly sighting rates for each individual as a proportion following the equation (Parra et al., 2006):
The limited distribution of the local common dolphin population, the highly localized nature of their sightings, the high resighting rate of identified individuals, and the lack of new additions to the local population suggest the application of closed population mark-recapture models (Otis et al., 1978; Schwarz and Seber, 1999; Wilson et al., 1999). The output of such models includes estimations of capture (p) and recapture (c) probabilities, applied to estimate the abundance of the local population. To test the closure assumption, capture history from each of the studied years was tested for closure using ‘CloseTest’ software (Stanley and Richards, 2004), applying two different closure tests: the Stanley and Burnham (1999) test, which was developed under a null model allowing for time-specific variation in capture probabilities under closure, and the Otis et al. (1978) test, which was developed under a null model allowing for heterogeneity in capture probabilities under closure. A suite of closed population models was fitted to the five-year database, as listed in Table 1. These models include the following scenarios: constant capture probabilities, monthly varying capture probabilities, time changing probabilities, additive affect to capture probabilities, and behavioral response between capture and recapture. They were chosen in order to account for the dynamic environment of this local population’s habitat, being so close to shore and subject to varying human activities in the area. The R (R Core Team, 2020) package RMark (Laake, 2013) to construct models for the program MARK (Cooch and White, 2014) was used to fit the models.
Each year was divided into several sampling periods, one month each, to allow mixing within the local population while maintaining the closure assumption (Seber, 1982; Thomas et al., 1986). The models estimated capture and recapture probabilities for monthly sampling occasions between April and November (Table 2). Calves were not included as their capture probability is not independent of their mothers (Hammond, 2010). The Akaike Information Criterion corrected for small sample size (AICc) (Akaike, 1973; Burnham and Anderson, 2002) was used for model selection, considering models within ΔAICc ≤ 2 as the most supported and using model averaging to account for uncertainty in model selection when more than one model had an ΔAICc value less than 2 (Burnham and Anderson, 2002). The estimated abundance was then divided by the mark ratio, calculated as the estimated proportion of animals with long-lasting marks in the local population (Wilson et al., 1999).
Two Cormack-Jolly-Seber (CJS) models (Cormack, 1964; Jolly, 1965; Seber, 1965) were fitted to the data to estimate the survival and capture probabilities of the local population over the years. Between two following years, the time interval was set to 1 and between 2016 and 2018 the time interval was set to 2 as data was missing from 2017. The first model was applied to the entire population without age discrimination, and the second included age class (i.e., calf and adult) as a group covariate. The effect of survey effort (amount of surveyed km) on capture probabilities was tested in both models. The Akaike Information Criterion corrected for small sample size (AICc) (Akaike, 1973; Burnham and Anderson, 2002) was used for model selection as for the closed population models.
A statistical power analysis was performed on the data to determine whether the model outputs can detect a trend using linear regression (Gerrodette, 1987). The power of the test is affected by the accuracy of the estimates (CV), the sample size (n), the chance for Type 1 and Type 2 errors (α and β), and the rate of change in the local population (R). The power analysis was used to measure what is the lowest rate of change (R) that can be detected with a sufficient statistical power of 0.8 (Taylor et al., 2007b) given the duration of the study (5 yearly samples of common dolphin) and the precision of the estimates. Analyses were performed using the ‘fishmethods’ package in “R” (Nelson, 2019; R Core Team, 2020), setting the parameters following Santostasi et al. (2016): one-tailed test, linear trend, and a ≤ 0.05 probability of Type 1 error. The overall CV of the study period was calculated as the mean of the annual CVs (Santostasi et al., 2016). A trend was considered significant when the regression of abundance estimates over the study period had a slope significantly distinct from zero (Gerrodette, 1987).
The social network of the local population was examined over time to observe its dynamics and the strength of the relationships between individuals. Social structure analysis was conducted on the entire network (2016 – 2021) and the network of individuals remaining in the area in 2021 after the decline in the local population size. Indices based on associations within the group were used to measure the relationship between individuals. Association was defined according to the “Gambit of the Group” assumption (Whitehead and Parijs, 2010), where two individuals observed in the same group during an encounter are assumed to be associating. The Half Weight Index (HWI) (Cairns and Schwager, 1987) was used to calculate the strength of the relationship between individuals: , when x is the number of sampling periods in which the two individuals were associated, yij is the number of sampling periods in which both were observed yet not associated. Yi or yj, are the numbers of sampling periods where only one individual was observed (Whitehead, 2008a). The HWI accounts for bias from pairs more likely to be identified when separate or when not all associations can be identified (Whitehead, 2008b). A zero value of HWI indicates that the dyad was never observed together as part of the same group, while the value of 1 indicates that the dolphins were always together. The HWI is then used to create the association matrix (N x N matrix containing the association index of each dyad of dolphins within the local population), which is the primary data structure for further social network analysis (Farine and Whitehead, 2015). Calculations were made using the ‘asnip’ package (Farine, 2013) and ‘igraph’ (Csardi, 2020) in R (R Core Team, 2020).
Network diagrams were constructed to visualize the social network’s social connections and complete structure. Each node describes an individual, and the associations between individuals are represented by lines (edges). The width of the line is relative to the strength of a dyad’s association (Farine and Whitehead, 2015), calculated by the HWI.
According to the guidelines for the application of IUCN Red List criteria (IUCN, 2012a), five criteria can be used to classify a subpopulation or regional/local population as Vulnerable (VU), Endangered (EN), or Critically Endangered (CE) as described in IUCN, 2012b. Moreover, when dealing with regional populations, it is essential to address the degree of their isolation, as their extinction risk might resemble that of an endemic taxon (Gärdenfors et al., 2001; IUCN, 2012a; IUCN, 2012b; Santostasi et al., 2018). Given this local population’s restricted distribution and the limited data regarding the existence of neighboring populations, a precautionary approach is taken to consider this local population as isolated. Therefore, in addition to the analyses described earlier to study the local population dynamics, several other measures were calculated to assess the status of the local population of common dolphins.
The extent of occurrence (EOO) and area of occupancy (AOO) are two measures usually calculated to examine the distribution of a certain population. They are used to assess the distribution range of a specific population and, according to that range, how likely it is to be isolated and, therefore, at risk of extinction (IUCN, 2012a). The extent of occurrence is defined as “the area contained within the shortest continuous imaginary boundary which can be drawn to encompass all the known, inferred or projected sites of present occurrence of a taxon, excluding cases of vagrancy”, and it is calculated by creating a minimum convex polygon around the plotted tracked positions of common dolphin groups observed in the study area (IUCN, 2012b; Santostasi et al., 2018). The area of occupancy is described as “the area within its extent of occurrence which is occupied by a taxon, excluding cases of vagrancy” (IUCN, 2012b). This measure takes into account the fact that a taxon will not always occur throughout the entire area of its extent of occurrence, which may contain unsuitable or unoccupied habitats (IUCN, 2001). As the southern distribution range of the Israeli common dolphins’ is the Israeli border, it is not clear what is their full range of distribution. Therefore, these measures were not applied to the local population, and Criteria B was not considered in the analysis.
Criteria E requires quantitative analysis showing the probability of extinction in the wild is at least 50% within ten years or three generations (IUCN, 2012b). The probability of extinction was calculated following the methods used in Santostasi et al. (2018) by multiplying the abundance estimated in this study by a range of biological plausible growth rates to cover a variety of scenarios caused by increasing levels of growth rate stochasticity (Morris and Doak, 2002; Currey et al., 2009). This is simulated by randomly drawing a growth rate value from a normal distribution where a higher SD represents a higher year-to-year variation of the growth rate. Scenario 1 indicates a constant growth rate (SD = 0), while scenarios 2 and 3 simulate increasing levels of stochasticity, a more realistic scenario considering the dynamic environment the local population inhabit, which is likely to have fluctuations (SD = 0.01 and SD = 0.02, respectively). The probability of extinction was then estimated under three quasi-extinction thresholds, chosen to provide a range of conservative values for extinction; two, four, and six reproductive individuals and over three-time scales; 45, 75, and 100 years (Taylor et al., 2007a). Modeling was programmed in R (R Core Team, 2020), as detailed in Santostasi et al. (2018).
A total of 16,531.36 km was surveyed between 2016 –2021 (Figure 1B) during 368 boat-based surveys. Common dolphins were encountered and photographed 32 times, and together with the 11 photographed encounters from the NPA, the total number of observations is 43. No photographed encounters were obtained during 2017; therefore, this year was not considered in the analysis. Most of the observations were achieved during the warm season – between April and October, while only years 2019 and 2020 contained encounters during November and December (a total of six). In order to maintain uniformity in the surveyed months, these six encountered were not considered in the mark-recapture models (Table 2).
19,569 photographs of common dolphins were taken, containing 2,851 identifications of 42 common dolphin individuals. Of them, 25 presented substantial markings while 17 did not, including 12 calves and five adults. These five were not included in any further analysis as they could not be re-identified. Mean group size was 10.2 individuals (n = 43, range = 2 – 21, SD = 4.43). 23 individuals were sighted in multiple years, with a mean number of sightings of 11.8 (n = 37, range = 1 – 37, SD = 12.2) per individual. Of the 37 common dolphins (25 adults and 12 calves), ten were identified as females after being observed with a dependent calf, one adult was recognized as male after stranding on shore in September 2020, and one calf was recognized as male after obtaining a photograph of its genital area. All the others (n = 25) were of unknown sex. Of the five stranded dolphins, two were identifiable through photo ID and were recognized as previously observed individuals.
Of the 25 identified mature individuals, three (12%) were sighted only once during the study period, eight (32%) twice, three (12%) were identified four times, and 14 (56%) were observed seven times or more (Supplementary Figure 7). The most observed individual was ‘Lavian’ (number 37 in the catalog), with 37 observations during the five years study period. Mean yearly sighting rate was 0.54 ± 0.33 (range = 0.2 - 1) and mean monthly sighting rate was 0.36 ± 0.31 (range = 0.04 - 0.91). Three females were observed with multiple calves during the study period, with a mean inter-calf interval of 1.5 ± 0.58 years. The discovery curve of the common dolphins reached a clear plateau (Supplementary Figure 8), indicating a good representation of the local population (Wilson et al., 1999).
The goodness of fit test for the CJS model to the data did not indicate a lack of fit (χ2 = 1.404, p = 0.924, df = 5). The best fitting model to estimate the survival probabilities for the entire population had constant survival and capture probabilities. An additional model was estimated within ΔAICc ≤ 2 from the best fitting model, with constant survival probability and effort variation in capture probabilities. Model averaging was applied to these two models to obtain the model-averaged parameter estimates, as shown in Table 3.

Table 3 Averaged parameter estimates for the two models within ΔAICc ≤ 2 in the CJS analysis to estimate survival and capture probability.
The best fitting model to estimate adult and calf survival probabilities had constant survival and capture probability. Three other models had ΔAICc ≤ 2: 1) constant survival probability and effort variation in capture probability, 2) age varying survival and constant capture probability, 3) constant survival and group varying capture probability (Supplementary Table 1). Model averaging was applied to these four models to obtain the model-averaged parameter estimates, as shown in Table 4.
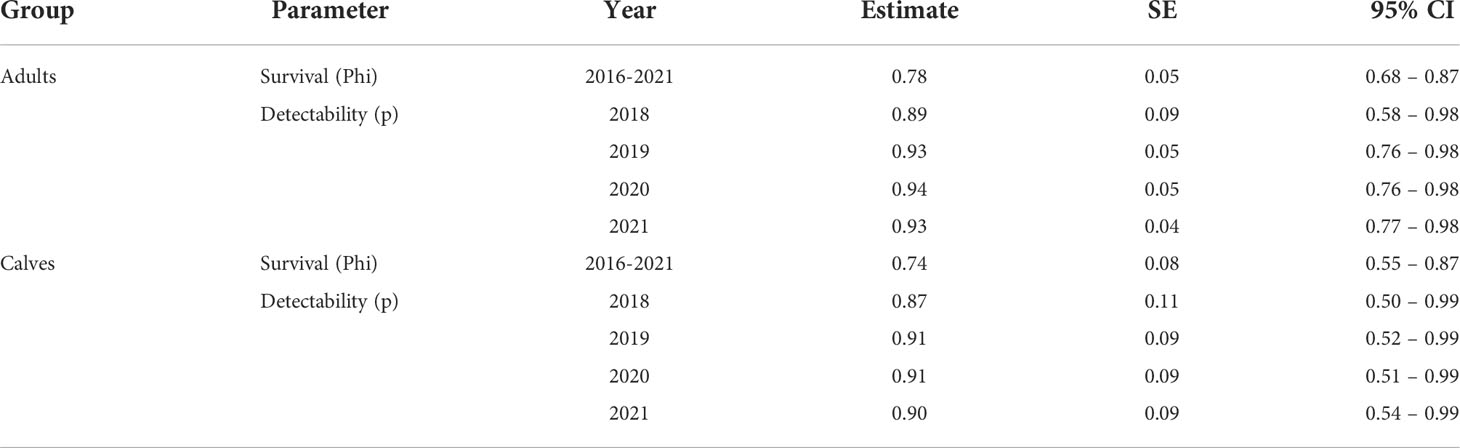
Table 4 Averaged parameter estimates for the four models within ΔAICc ≤ 2 from the best fitting model in the CJS analysis for calves’ survival.
The Otis et al. (1978) test found the local population to be closed (P > 0.05) in all of the study years, while the Stanley and Burnham (1999) test supports the population closure (P > 0.05) for the year 2016 but suggests an open population (P < 0.05) for years 2018 -2021.
Closed population models were applied to each of the study years separately. Each year yielded different models within ΔAICc ≤ 2 from the best fitting model (Supplementary Table 2) that were averaged to extract the capture and recapture probabilities as shown in Table 5 and the estimated abundance as shown in Table 6.
The mean CV of the estimates was 0.017. The minimum rate of decay of the population abundance that could be detected in five years with a 0.8 statistical power was a total decrease of 10%. The local population size in 2016 was estimated to be 25 (95% CI 24 - 37) individuals, while in 2021, there were only 15 (95% CI 15 – 15). A decrease of 40% was found in linear regression, as shown in Figure 2 (R2 = 0.76, P = 0.034, y = 3546.14 – 1.74x, n = 5), as a result of the disappearance of more than half of the local population observed at the beginning of the study period.
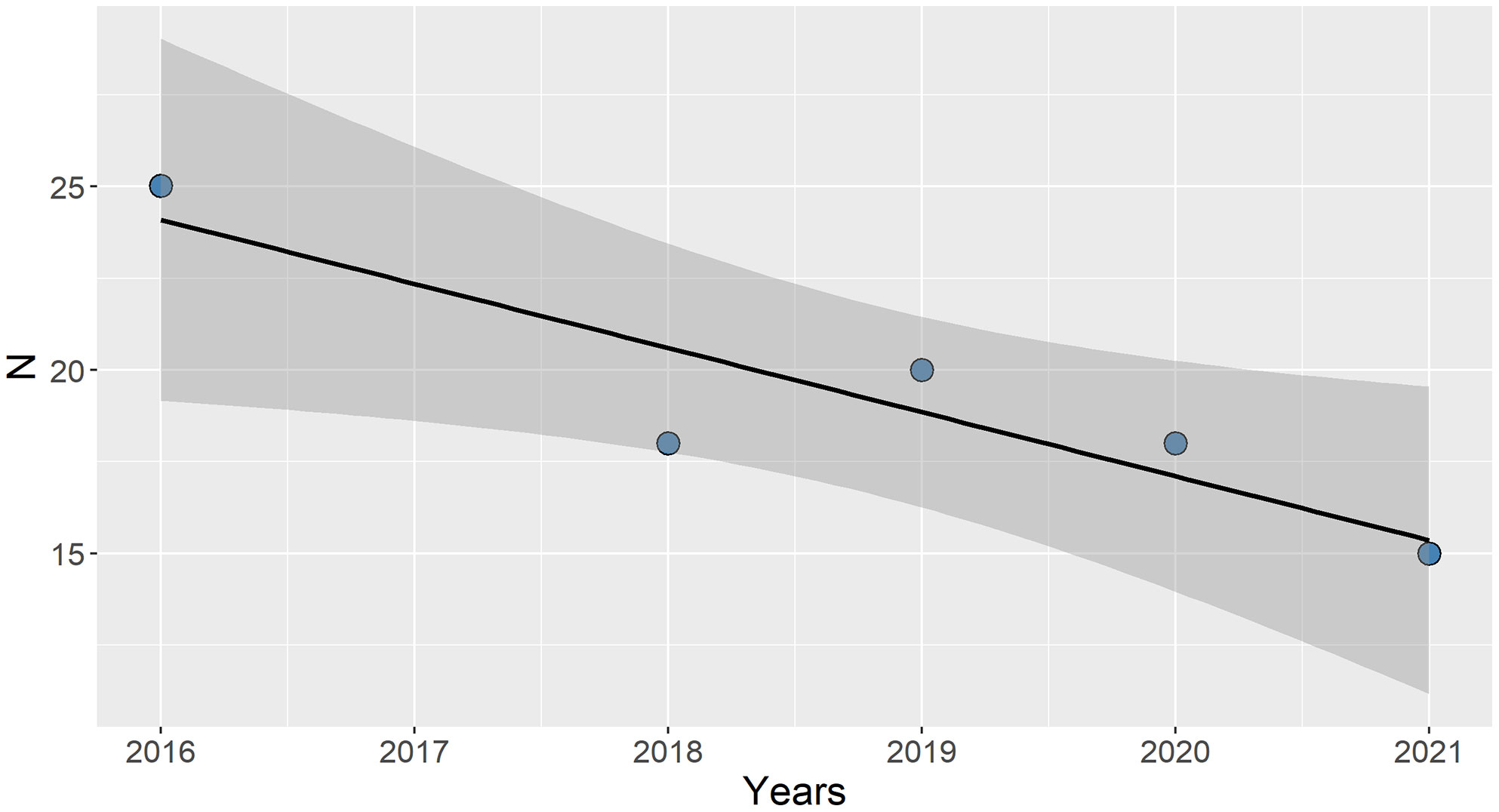
Figure 2 Abundance estimates for the common dolphins’ local population. Estimates of abundance of the common dolphins, including 95% confidence intervals, from mark-recapture models applied every year. The presented N is the total abundance after dividing the estimated abundance bythe mark ratio. The linear model is presented on the graph.
Population projection analysis placed the local population under more than 50% probability of extinction in 25 of the 27 projections (Table 7 and Figure 3). Thus, meeting criteria E for Critically endangered.
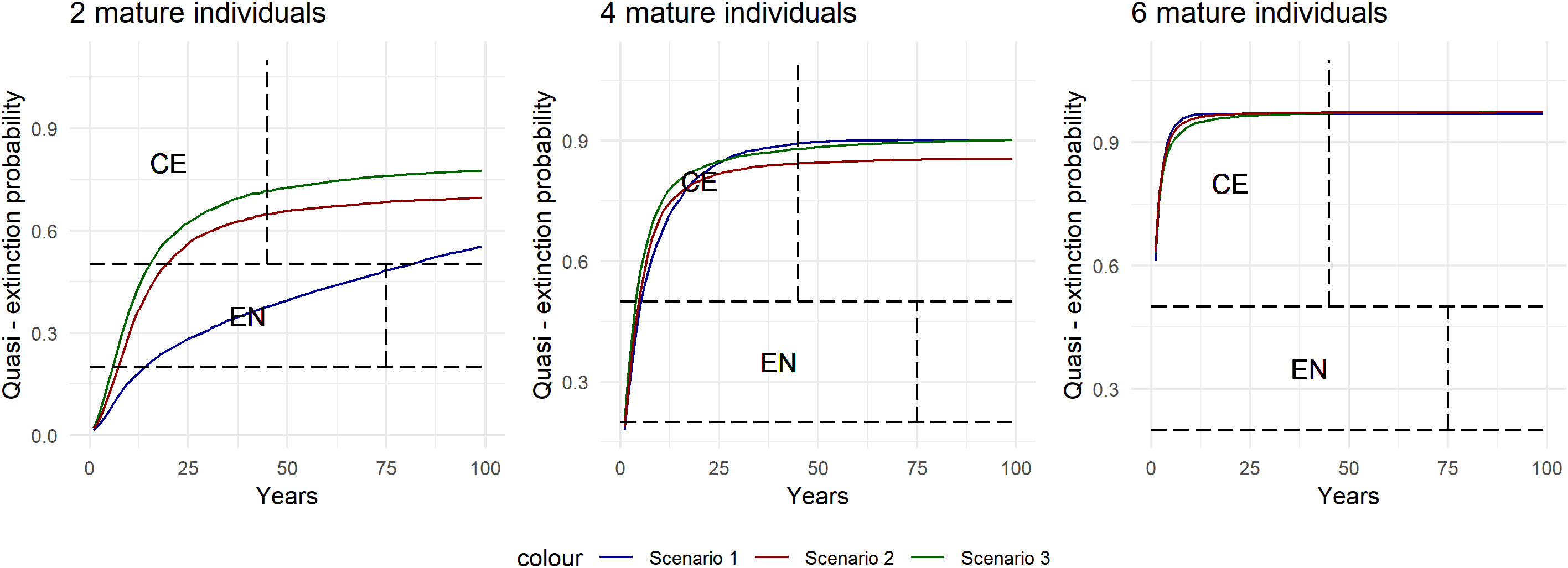
Figure 3 Quasi-extinction probability for the common dolphins. Quasi-extinction probability for common dolphins along the Israeli coast for 100 years. The y-axis is the quasi-extinction probability; the x-axis is years. The different scenarios represent increasing levels of growth rates randomness modeled from a normal distribution with increasing standard deviation (from 0 to 0.02).
From the 625 possible associations between the 25 mature individuals, 153 (25%) were zero, meaning no association was observed between the specific dyad. 188 were 0.1 (30%), indicating a low level of association, and the rest were distributed, as shown in Figure 4A. The mean association rate over the five-year study period was 0.35 ± 0.28 (range 0.05 – 1). When examining the association patterns of the local population in 2020 - 2021 (the remaining individuals after the decline in the local population size), the mean association rate rises to 0.76 ± 0.08 (range 0.5 – 0.92), and the individuals are all part of the same highly associated social unit. The remaining individuals contained six mature females, three mature individuals of unknown sex, and five calves (one of them is a male). They were all observed together more than 25 times in 72.4% of the observations between 2020-2021 (Figure 4B).
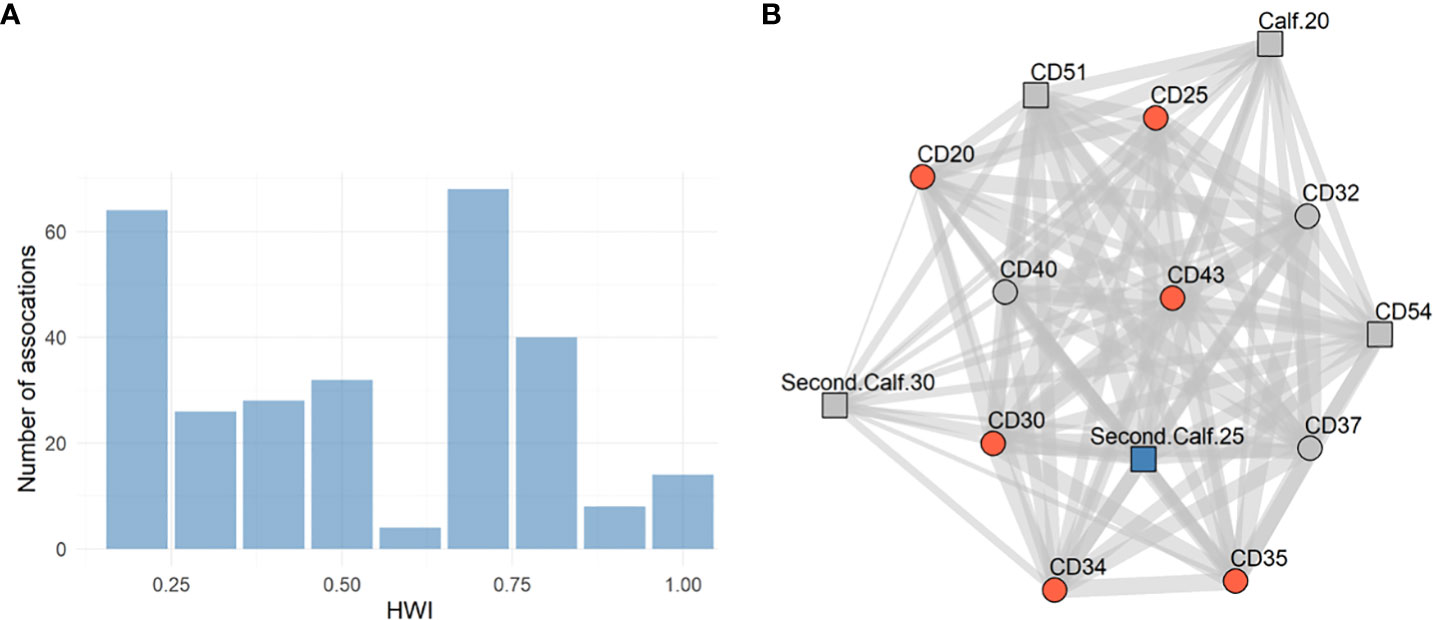
Figure 4 (A) Histogram of HWI values. HWI values higher than 0.1 for mature individuals (n = 25). (B) Social network of the common dolphins in 2021. The network structure of the local population in 2021 includes calves (n = 14). Red nodes represent females, blue represents a male, and grey represents individuals with unknown sex. Square nodes represent calves, and circles represent adults. The width of the edge represents the strength of the association between the dyad according to the HWI values.
The local population of common dolphins was observed in the southern region of Israel all year round throughout the five years of the study. The majority of individuals (62%) were sighted over multiple years with high yearly and monthly sighting rates, indicating long-term site fidelity and residency. During the last two years of the study, 2020-2021, the same group of individuals were observed in all encounters, repeatedly in the same area, in front of Ashkelon and Ashdod, and occasionally near Palmahim. This group’s small-ranging patterns and their high side fidelity indicate year-round residency in the area. The stabilization of the discovery curve (Supplementary Figure 8) and the high frequency of observations of the same individuals strongly suggest that this group is relatively closed and isolated, yet additional information is needed from south to the Israeli border to confirm this hypothesis and understand this local population’s complete distribution, and we take this opportunity to invite collaboration on this matter.
The apparent annual survival rate was 0.77 ± 0.04 (Cl 95% 0.67-0.84), which appeared constant over the years, while capture probability rose with the increasing effort. As expected, calves had a lower survival probability and were observed in other dolphin species (Currey et al., 2009; Croft et al., 2017). Their capture probability was also low due to fewer marks on their dorsal fins. These are the first survival estimates described specifically for common dolphins and their different age classes, and they appear low compared to other cetacean species worldwide and in the Mediterranean. For example, the survival rate of bottlenose dolphins in the northeastern Adriatic Sea was estimated between 0.82 and 0.93 (95% CI 0.69 – 0.98) (Fortuna, 2007). A mixed-species group of common and striped dolphins in the Gulf of Corinth presented survival probabilities of 0.94 (95% CI 0.92 – 0.96) (Santostasi et al., 2016). Even along the Israeli coastline, with the same environmental conditions, the survival of bottlenose dolphins was estimated to be higher, with a probability of 0.92 (95% CI 0.89 – 0.93) (Yaly Mevorach, unpublished data). Attempts to study the factors affecting the survival probabilities of cetaceans in the Mediterranean Sea revealed various reasons. In the Strait of Gibraltar, a local population of long-finned pilot whales appeared to be affected by epizootic episodes of morbillivirus, causing a decrease in their survival probabilities, from 0.997 ± 0.003 in the years before the epizootic episodes to 0.831 ± 0.042 after the first one and 0.649 ± 0.085 after the second one (Pons et al., 2022). Another example from the Strait of Gibraltar is the survival estimates of bottlenose dolphins, which appears to be negatively correlated with ferry traffic and are assessed between 0.918 and 0.924 (Tenan et al., 2020). The low apparent survival rate of the common dolphins in Israel could result from emigration from the study area or frequent mortality events leading to a decrease in their abundance, similar to other regions in the Mediterranean Sea.
The close tests presented controversial results as one of the tests supported the closure (Otis et al., 1978) assumption and the second one did not (Stanley and Burnham, 1999). These results should be considered carefully as close tests are usually applied to a dataset comprising many more individuals. In addition, in all the cases where the Stanley and Burnham test did not support closure, the component statistics support that there may have been population losses (not additions) which is consistent with the death or permanent emigration of individuals. If the Stanley and Burnham test is correct, the estimates for the years 2018-2021 may be an overestimation of abundance as the model includes the present individuals (birth and immigration) but does not account for the ones that are no longer in the area (deaths or emigration). However, the characteristics of the local population, such as discovery curve, high resighting rates, high site fidelity, and no new additions, fit the ones of a closed population, and therefore these models were applied.
The common dolphin local population along the Israeli coastline was found to be small (< 50), similar to other “inner” Mediterranean local populations (Bearzi et al., 2021; Vella et al., 2021). Given the relatively high yearly site fidelity of the common dolphins and the stabilization of their discovery curve, an assumption can be made that most of the individuals within this local population were identified during the study period, which suggests that the local population in Israel is a small group of year-round residents. Similar abundance patterns have been observed in the Mediterranean as described before (Santostasi et al., 2016; Bearzi et al., 2020) and could result from similar environmental conditions and anthropogenic pressures. The size of the local population during the study years (15 – 25) meet criteria D for a ‘Critically Endangered’ population as it is lower than 50 individuals (IUCN, 2012b).
An alarming decrease in the local population’s size was observed in the study area during the five-year study period, with a 40% decline in size since 2016. Historical data from occasional observations describe larger groups of common dolphins (22.2 ± 19.1, range 1 – 75) ranging over a more extensive habitat, from Ashdod to Herzliya (Kerem et al., 2012). Such large groups are no longer observed along the coast, and about half of the individuals observed in 2016 have disappeared from the area during the study period. Based on this trajectory, the complete disappearance of this species from the local waters is expected by 2029. This significant trend cannot result from decreasing effort as both effort, and capture probability increased along the study years. A similar decline was documented for the common dolphin local population in western Greece, where the local population decreased by 90% throughout 13 years of study (1995 - 2007). This decline was found to be related to prey depletion in the area, resulting from overfishing (Bearzi et al., 2008). This might also be the case for this population, but further studies regarding their prey distribution in the area is needed to test this hypothesis.
Criteria A (population size reduction over ten years or three generations) requires a decline observed, estimated, inferred, or suspected over a more extended period than the period for this study (IUCN, 2012b). The absence of previous abundance estimates for this local population and the low power of abundance estimates based on small population size (Taylor et al., 2007a; Santostasi et al., 2016) make it harder to meet this criterion adequately. Nonetheless, sharp declines in other local populations of common dolphins all over the Mediterranean Sea has led to a regional classification of this species as Endangered (Bearzi et al., 2003; Bearzi et al., 2021). These observed declines, together with the precautionary principle promoted by the IUCN Red List (Mace and Stuart, 1994), support the assumption that the decline observed in the local population in Israel (40% over five years) might be similar and even stronger than the declines observed throughout the Mediterranean Sea (50% decline over three generations; Bearzi, 2012). Criteria C (small population size and decline) requires an abundance of fewer than 250 individuals and a decline of 25% or more in three years or one generation. The observed abundance and decline meet this criterion. Therefore, the local Israeli population should be defined as ‘Critically Endangered’ under Red List Criteria C and as ‘Endangered’ under Red List criteria A.
The majority of simulated scenarios yielded a ≥ 50% probability of quasi‐extinction (Table 7) for every time interval tested (45, 75, and 100 years). Furthermore, the scenario with zero temporal growth rate stochasticity also showed a ≥ 50% probability of quasi‐extinction after five generations and 100 years, suggesting that the local population is at high extinction risk even in the most optimistic scenario. Therefore, the local population should be defined as Critically Endangered under criteria E (quantitative analysis).
In light of the reduction of the local population size between 2016 and 2020, as half of the local population disappeared from the area, it is likely that the common dolphin local population’s social structure changed during this time. Due to the low sample size in the early years of the study (2016 and 2018), it is hard to describe the social structure at the beginning of the study as the associations could not be measured with so few observations. In 2021, the local population was composed of nine mature individuals who maintain long-lasting and strong associations and are observed almost exclusively together in one social unit along with five calves (all above one year old).
This group of nine mature individuals and five calves were observed together more than 25 times during the last two years of the study in 72.4% of the observations. As these are the only individuals known to inhabit the area, they appear to maintain a strongly associated close social unit with high association indices. According to Vella et al. (2021), only 16 peer-reviewed studies have used photo identification to study common dolphins worldwide. From these, only three studied the social structure of a specific local population. Therefore, not much is known about common dolphin societies, and what is known varies greatly between populations. For example, in the eastern Ionian Sea, the common dolphin local population range over large areas of the neritic zone and present a fission-fusion social network with little evidence of long-term associations (Bruno et al., 2004). Similarly, the common dolphin local population in Hauraki Gulf, a semi-enclosed coastal body of temperate water located on the north-eastern coastline of the North Island, New Zealand, also presented a fluid social structure with a low mean association rate and very few long-lasting associations (Hupman, 2016). In contrast, the common dolphin local population’s social structure in the Tyrrhenian Sea contains a core group of 12 females that formed long-lasting associations over five years (Pace et al., 2009; Mussi et al., 2019), indicating a highly associated closed group.
The latter presents a more similar structure to the one in Israel, comprised mostly of females. However, two males were stranded ashore in 2020. Before his death, one of these males was part of the social unit and maintained strong associations with females, indicating a mixed-sex social unit. The social structure of the local population could reflect their level of isolation as observed in the bottlenose dolphin local population in Doubtful Sound (Lusseau et al., 2003), or the utilization of a specific resource as observed in the bottlenose dolphins local population in the north Adriatic Sea (Genov et al., 2019). It could also be the consequence of extensive segregation of the common dolphins in the Mediterranean, resulting in a separation into several small local populations (Bearzi and Genov, 2021).
The proximity of the Israeli common dolphin local population to the coast puts them in a constant state of vulnerability to human pressure. Several main threats have been identified in the Mediterranean Sea as the causes of the decrease in abundance of this species: historical culling and killing, bycatch in fishing gear, prey depletion, and contamination/pollution (Bearzi and Genov, 2021; Vella et al., 2021). In Israel, there is no historical data about killing of dolphins, but it can be assumed that the massive killing in the Mediterranean led to the segregation of the Mediterranean sub-population, resulting in separation to several small local populations, such as the one in Israel (Bearzi and Genov, 2021). Throughout the study years, two common dolphins have stranded ashore, showing clear signs of entanglement and drowning, thus indicating the presence of entanglement threat to this local population as well, as several fishing methods are used in their habitat, including nets, lines, and bottom trawlers.
Common dolphins are often observed feeding around trawlers while the net is being pulled back to the boat. Stomach content analysis revealed that their diet includes several species targeted or bycaught by the trawler industry (Brand et al., 2019), such as Ariosoma balearicum, which was found to be among the main prey species of common dolphins in Israel (Brand et al., 2019). This species is not abundant in the common dolphins’ diet in the Alboran Sea or the eastern Ionian Sea (Bearzi et al., 2006; Giménez et al., 2018), while the most abundant prey species in these areas are less common in the local population diet. The bottlenose dolphins in Israel also prey on Ariosoma balearicum, often from the trawler’s net as well. This behavior and prey preferences of the coastal dolphin species in Israel could indicate that the trawling industry is an important food resource in their diet (Scheinin et al., 2014; Brand et al., 2019). Further research on the abundance of common prey species of common dolphins in the area, as done in western Greece (Bearzi et al., 2008) is needed in order to understand the differences from other areas in the Mediterranean.
Contamination through the food web and pollution could also pose a threat to the local population as they inhabit an area with a busy port, power station, desalination, and sewage spill but the effects of these on the dolphins in the area are still unknown.
The Marine Protected Area, ‘Evtach’, is approved in part of the common dolphins’ habitat. Once declared, it will decrease the interaction rate between dolphins and commercial fishing boats and encourage public awareness and enforcement of conservation measures for this species. Whether it will improve the lives of the common dolphins in the area is remained to be discovered, but a change in their IUCN status will help promote the declaration of this MPA and the importance of their conservation in the area.
The local population of common dolphins in Israel presents a similar decline to other local populations in the inner Mediterranean Sea. Even with insufficient historical data, a clear trend is observed throughout the study years. These dolphins face a challenging environment and seem unable to adjust appropriately, resulting in almost half of them leaving the area or dying. A strong need for research collaboration with the neighboring countries arises to understand the full-ranging patterns and abundance of common dolphins in the Eastern Levantine Sea. The risk assessment provided in this study places the common dolphin local population in Israel as ‘Critically Endangered’ under criteria C, D, and E and as ‘Endangered’ under criteria A. Only one of these criteria needs to be met to reconsider the risk assessment of the local population (IUCN, 2012b). We highly recommend expediting this decision to promote the importance of this species conservation among the local influencing factors and the public. In addition, we encourage collaborations to construct innovative conservation actions to prevent the final disappearance of common dolphins from the most eastern part of the Mediterranean Sea.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for this animal study because the study participants adhered to the current national regulations related to observational studies of this nature in Israel. As there was no physical sampling or disturbance to the dolphins during the observational surveys, no ethical permits were required.
AS has equal contribution. DT and AS devised the project. AS, YM and OG collected the data. YM and NS performed the analysis. OG created the maps. YM wrote the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the Morris Kahn Marine Research Station, the department of Marine Biology, Leon H. Charney School of Marine Sciences, University of Haifa, as part of a long-term monitoring study of the cetacean species along the Israeli coastline and by the Ministry of Energy in Israel. Additionally, this research was partly funded by the Prince Albert II of Monaco Foundation (grant No. 2241) as part of the ACCOBAMS Survey Initiative (ASI), coordinated by the Agreement on the Conservation of Cetaceans of the Black Sea, Mediterranean Sea and Contiguous Atlantic Area (ACCOBAMS). Yaly Mevorach was supported by an M.Sc grant by the University of Haifa.
Many thanks to all our colleagues for their contribution to the data collection; Eyal Bigal, Yotam Zuriel, Meytal Markovich, Yaron Haitovich, Mia Elsar, Dror E. Vardimon and Guy Lavian. Thanks to the entire apex predator lab at MKMRS, Ort Yami Ashdod, Delphis NGO, IMMRAC NGO, Ashdod School of Marine and Sports Education, the marine unit of the Nature and Parks Authority in Israel for their support throughout this study. Special thanks to Dana Reininger and Shlomi Marco for the development of the Delphis app and to Dr Leigh Livne for editing this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.916950/full#supplementary-material
Abd Rabou A. F. N., Abd Rabou M. A., Qaraman A. F. A., Abualtayef M. T., Abd Rabou A. A., Khalaf N. A., et al. (2021). Sightings and strandings of the cetacean fauna in the Mediterranean coast of the Gaza strip, Palestine. Israa Univ. J. Appl. Sci. 5, 152–186. doi: 10.52865/NUWV7692
Aissi M., Vella A. (2015). Status and conservation of cetaceans in the Sicily Channel/Tunisian plateau. United Nations Environment Programme/Mediterranean Action Plan (UNEP/MAP), UNEP (DEPI)/MED WG.408/Inf.19 Tunisia: Regional Activity Centre for Specially Protected Areas (RAC/SPA). Available from: https://www.um.edu.mt/library/oar/bitstream/123456789/44073/1/Status_and_conservation_of_cetaceans_in_the_Sicily_channel_Tunisian_plateau.pdf. doi: 10.4324/9780429052347-62
Akaike H. (1973). Information theory and an extention of the maximum likelihood principle. Second International Symposium on Information Theory, Eds. B. Petrov N., Csaki F. (Budapest: Academiai Kiado) 267–281. doi: 10.1007/978-1-4612-0919-5_38
Ansmann I. C., Parra G. J., Chilvers B. L., Lanyon J. M. (2012). Dolphins restructure social system after reduction of commercial fisheries. Anim. Behav. 84, 575–581. doi: 10.1016/j.anbehav.2012.06.009
Arcangeli A., Campana I., Bologna M. A. (2017). Influence of seasonality on cetacean diversity, abundance, distribution and habitat use in the western Mediterranean Sea: Implications for conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 27, 995–1010. doi: 10.1002/aqc.2758
Arcangeli A., Marini L., Crosti R. (2013). Changes in cetacean presence, relative abundance and distribution over 20 years along a trans-regional fixed line transect in the central tyrrhenian Sea. Mar. Ecol. 34, 112–121. doi: 10.1111/maec.12006
Bearzi G. (2012). “Delphinus delphis,” in The IUCN red list of threatened species 2012. e.T134817215A195829089. 8235. doi: 10.2305/IUCN.UK.2012-1.RLTS.T134817215A195829089.en
Bearzi G., Agazzi S., Gonzalvo J., Costa M., Bonizzoni S., Politi E., et al. (2008). Overfishing and the disappearance of short-beaked common dolphins from western Greece. Endanger. Species Res. 5, 1–12. doi: 10.3354/esr00103
Bearzi G., Bonizzoni S., Santostasi N. L. (2020). “Delphinus delphis (Gulf of Corinth subpopulation) (errata version published in 2021),” in IUCN red list threat. species 2020 e.T156206333A194321818, 8235. doi: 10.2305/IUCN.UK.2020-2.RLTS.T156206333A194321818.en
Bearzi G., Genov T. (2021). Imperiled common dolphins of the Mediterranean Sea. in Imperiled: The Encyclopedia of Conservation. DellaSala D. A., Goldstein M. I. (Elsevier Inc.), 837–846. doi: 10.1016/b978-0-12-821139-7.00059-3.
Bearzi G., Genov T., Natoli A., Gonzalvo J., Pierce G. (2021). “Delphinus delphis (Inner Mediterranean subpopulation),” in IUCN red list threat. species 2021, 8235. e.T41762A10557372. doi: 10.2305/IUCN.UK.2021-3.RLTS.T189865869A189865884.en%0ACopyright
Bearzi G., Politi E., Agazzi S., Azzellino A. (2006). Prey depletion caused by overfishing and the decline of marine megafauna in eastern Ionian Sea coastal waters (central Mediterranean). Biol. Conserv. 127, 373–382. doi: 10.1016/j.biocon.2005.08.017
Bearzi G., Reeves R. R., Notarbartolo di Sciara G., Politi E., Canadas A., Frantis A., et al. (2003). Ecology, status and conservation of short-beaked common dolphins delphinus delphis in the Mediterranean Sea. Mamm. Rev. 33, 224–252. doi: 10.1046/j.1365-2907.2003.00032.x
Berrow S., O’Brien J., Groth L., Foley A., Voigt K. (2012). Abundance estimate of bottlenose dolphins (Tursiops truncatus) in the lower river Shannon candidate special area of conservation, Ireland. Aquat. Mamm. 38, 136–144. doi: 10.1578/AM.38.2.2012.136
Blasi M. F., Boitani L. (2014). Complex social structure of an endangered population of bottlenose dolphins (Tursiops truncatus) in the aeolian archipelago (Italy). PloS One 9, e114849. doi: 10.1371/journal.pone.0114849
Brand D., Edelist D., Goffman O., Hadar N., Scheinin A., Kerem D. (2019). Common dolphins, common in neritic waters off southern Israel, demonstrate uncommon dietary habits. Aquat. Conserv. Mar. Freshw. Ecosyst. 31, 15–21. doi: 10.1002/aqc.3165
Bruno S., Politi E., Bearzi G. (2004). “Social organization of common dolphin community in the eastern Ionian Sea: Evidence of a fluid fission-fusion society,” in European Research on cetaceans. Eds. Evans P. G. H., O’Boyle E.(Rome, Italy, European Cetacen Society), 49–51.
Burnham K. P., Anderson D. R. (2002). Model selection and inference: A practical information-theoretic approach (New York: Springer).
Cairns S. J., Schwager S. J. (1987). A comparison of association indices. Anim. Behav. 35, 1454–1469. doi: 10.1016/S0003-3472(87)80018-0
Cañadas A., Hammond P. S. (2008). Abundance and habitat preferences of the short-beaked common dolphin delphinus delphis in the southwestern Mediterranean: Implications for conservation. Endanger. Species Res. 4, 309–331. doi: 10.3354/esr00073
Cooch E. G., White G. C. (2014). Program MARK: a gentle introduction, 13th ed. (Fort Collins, Colorado: Colorado State University). Available at: http://www.phidot.org/software/mark/docs/book/.
Cormack R. M. (1964). Estimates of survival from the sighting of marked animals. Biometrika 51, 429–438. doi: 10.1093/biomet/51.3-4.429
Croft D. P., Johnstone R. A., Ellis S., Nattrass S., Franks D. W., Brent L. J. N., et al. (2017). Reproductive conflict and the evolution of menopause in killer whales. Curr. Biol. 27, 298–304. doi: 10.1016/j.cub.2016.12.015
Csardi G. (2020) Igraph: Network analysis and visualization. Available at: https://cran.r-project.org/web/packages/igraph/igraph.pdf.
Currey R. J. C., Dawson S. M., Slooten E., Schneider K., Lusseau D., Biosseau O. J., et al. (2009). Survival rates for a declining population of bottlenose dolphins in doubtful sound, new Zealand: an information theoretic approach to assessing the role of human impacts. Aquat. Conserv. Mar. Freshw. Ecosyst. 19, 658–670. doi: 10.1002/aqc
FAO (2018). The State of World Fisheries and Aquaculture 2018 - Meeting the sustainable development goals (Rome, Italy).
Farine D. R. (2013). Animal social network inference and permutations for ecologists in r using asnipe. Methods Ecol. Evol. 4, 1187–1194. doi: 10.1111/2041-210X.12121
Farine D. R., Whitehead H. (2015). Constructing, conducting and interpreting animal social network analysis. J. Anim. Ecol. 84, 1144–1163. doi: 10.1111/1365-2656.12418
Farrag M. M. S., Ahmed H. O., TouTou M. M. M., Eissawi M. M. (2019). Marine mammals on the Egyptian Mediterranean coast “Records and vulnerability.” Int. J. Ecotoxicol. Ecobiol. 4, 8. doi: 10.11648/j.ijee.20190401.12
Fortuna C. M. (2007). Ecology and conservation of bottlenose dolphins (Tursiops truncatus) in the north-eastern Adriatic Sea (University of St Andrews, Scotland). Doctoral dissertation.
Frantzis A., Alexiadou P., Paximadis G., Politi E., Gannier A., Corsini-Foka M. (2003). Current knowledge of the cetacean fauna of the Greek seas. J. Cetacean Res. Manage. 5, 219–232.
Gannier A. (2005). Summer distribution and relative abundance of delphinids in the Mediterranean Sea. Rev. d’Ecologie Terre Vie 60, 223–238.
Gärdenfors U., Hilton-Taylor C., Mace G. M., Rodriguez J. P. (2001). The application of IUCN red list criteria at regional levels. Conserv. Biol. 15, 1206–1212. doi: 10.1046/j.1523-1739.2001.00112.x
Genov T., Centrih T., Kotnjek P., Hace A. (2019). Behavioural and temporal partitioning of dolphin social groups in the northern Adriatic Sea. Mar. Biol. 166, 1–14. doi: 10.1007/s00227-018-3450-8
Genov T., Kotnjek P., Centrih T. (2020). Occurrence of common dolphins (Delphinus delphis) in the gulf of Trieste and the northern Adriatic Sea. Aquat. Conserv. Mar. Freshw. Ecosyst. 31, 69–75. doi: 10.1002/aqc.3407
Gero S., Gordon J., Whitehead H. (2013). Calves as social hubs: Dynamics of the social network within sperm whale units. Proc. R. Soc B Biol. Sci. 280. doi: 10.1098/rspb.2013.1113
Gerrodette T. (1987). A power analysis for detecting trends. Ecology 68, 1364–1372. doi: 10.2307/1939220
Giménez J., Marçalo A., García-Polo M., García-Barón I., Castillo J. J., Fernández-Maldonado C., et al. (2018). Feeding ecology of Mediterranean common dolphins: The importance of mesopelagic fish in the diet of an endangered subpopulation. Mar. Mammal Sci. 34, 136–154. doi: 10.1111/mms.12442
Gonzalvo J., Costa M. (2016). “Will there be any reward for common dolphin perseverance,” in Report of the 1st international workshop: Conservation and research networking on short-beaked common dolphin (Delphinus delphis) in the Mediterranean Sea(Ischia Island, Italy, Mediterranean Common Dolphin Working Grou), 41–42. doi: 10.13140/RG.2.1.4801.3047
Hammond P. S. (2010). “Estimating the abundance of marine mammals,” in Marine mammal ecology and conservation: a handbook of techniques, Boyd I. L., Bowen W. D., Iverson S. J. (Oxford University Press Inc.: New York), 42–67.
Hammond P. S., Bearzi G., Bjørge A., Forney K., Karczmarski L., Kasuya T., et al. (2008). “Delphinus delphis, common dolphin,” in The IUCN Red List of Threatened Species. 2008: e.T6336A12649851. doi: 10.2305/IUCN.UK.2008.RLTS.T6336A12649851.en
Hupman K. (2016). Photo-identification and its application to gregarious delphinids: Common dolphins (Delphinus sp.) in the hauraki gulf, new Zealand (Albany, New Zeal: Massey Univ). A thesis Submitt. Partial fulfilment Requir. degree Dr. Philos. Mar. Ecol. Massey Univ.
IUCN (2001). IUCN red list categories and criteria: Version 3.1., ed (Gland, Switzerland and Cambridge: World Conservation Union), 30. U. K. I. +.
IUCN (2012a). Guidelines for application of IUCN red list criteria at regional and national levels: Version 4.0 (Gland, Switzerland and Cambridge, UK: IUCN). Available at: www.iucn.org/publications.
IUCN (2012b). IUCN red list categories and criteria: Version 3.1 second edition (Gland, Switzerland and Cambridge, UK: IUCN).
Jolly G. M. (1965). Explicit estimates from capture-recapture data with both death and immigration - stochastic model. Biometrika 52, 225–247. doi: 10.1093/biomet/52.1-2.225
Kerem D., Hadar N., Goffman O., Scheinin A., Kent R., Boisseau O., et al. (2012). Update on the cetacean fauna of the Mediterranean levantine basin. Open Mar. Biol. J. 6, 6–27. doi: 10.2174/1874450801206010009
Laake J. L. (2013) RMark: An r interface for analysis of capture-recapture data with MARK. Available at: https://cran.r-project.org/web/packages/RMark/RMark.pdf.
Lusseau D., Schneider K., Boisseau O. J., Haase P., Slooten E., Steve M. D. (2003). The bottlenose dolphin community of doubtful sound features a large proportion of long-lasting associations: Can geographic isolation explain this unique trait? Behav. Ecol. Sociobiol. 54, 396–405. doi: 10.1007/s00265-003-0651-y
Marco S. (2017) Delphis; marine surveys platform. Available at: https://www.shlomimarco.com/delphis.
Milani C., Vella A., Vidoris P., Christidis A., Koutrakis E. (2021). Abundance, distribution and diet of the common dolphin, delphinus delphis, in the northern Aegean Sea (Greece). Aquat. Conserv. Mar. Freshw. Ecosyst. 31, 76–86. doi: 10.1002/aqc.3081
Morris W. F., Doak D. F. (2002). Quantitative conservation biology. Sinauer, Sunderland, Massachusetts, USA
Moura A. E., Natoli A., Rogan E., Hoelzel A. R. (2013). Atypical panmixia in a European dolphin species (Delphinus delphis): Implications for the evolution of diversity across oceanic boundaries. J. Evol. Biol. 26, 63–75. doi: 10.1111/jeb.12032
Mussi B., Vivaldi C., Zucchini A., Miragliuolo A., Pace D. S. (2019). The decline of short-beaked common dolphin (Delphinus delphis) in the waters off the island of ischia (Gulf of Naples, Italy). Aquat. Conserv. Mar. Freshw. Ecosyst. 31, 87–100. doi: 10.1002/aqc.3061
Natoli A., Cañadas A., Vaquero C., Politi E., Fernandez-Navarro P., Hoelzel A. R. (2008). Conservation genetics of the short-beaked common dolphin (Delphinus delphis) in the Mediterranean Sea and in the eastern north Atlantic ocean. Conserv. Genet. 9, 1479–1487. doi: 10.1007/s10592-007-9481-1
Nelson G. A. (2019) Fishmethods: Fishery science methods and models in r. Available at: https://cran.r-project.org/web/packages/fishmethods/fishmethods.pdf.
Notarbartolo di Sciara G., Birkun A. J. (2010). Conserving whales, dolphins and porpoises in the Mediterranean and black seas: an ACCOBAMS status report (Monaco, ACCOBAMS), 212.
Otis D. L., Burnham K. P., White G. C., Anderson D. R. (1978). Statistical inference from capture data on closed animal populations. Wildl. Monogr. 62, 3–135. doi: 10.1093/analys/31.6.177
Pace D. S., Mariani M., Miragliuolo A., Venier M., Mussi B. (2009). “Preliminary analysis of the social structure of the short-beaked common dolphin (Delphinus delphis) in the tyrrhenian Sea, Italy,” in Proceedings of the 23rd annual conference of the European cetacean society, Istanbul, Turkey. Eds. Vincent C., Pierce G. J., Öztürk A. A., Kotnjek P., Siemensma M., Tonay A. (Istanbul, Turkey: Türk Deniz Aras¸tırmaları Vakfı), 176–177.
Pace D. S., Mussi B., Vella A., Vella J., Frey S., Bearzi G., et al. (2016). Conservation and research networking on short-beaked common dolphin (Delphinus delphis) in the Mediterranean Sea (Ischia Island, Italy, Mediterranean Common Dolphin Working Group). 13–15 doi: 10.13140/RG.2.1.4801.3047
Parra G. J., Corkeron P. J., Marsh H. (2006). Population sizes, site fidelity and residence patterns of Australian snubfin and indo-pacific humpback dolphins: Implications for conservation. Biol. Conserv. 129, 167–180. doi: 10.1016/j.biocon.2005.10.031
Pons M., De Stephanis R., Verborgh P., Genovart M. (2022). Sharp decreases in survival probabilities in the long-finned pilot whales in strait of Gibraltar. Mar. Biol. 169, 1–9. doi: 10.1007/s00227-022-04030-1
R Core Team (2020) R: A language and environment for statistical computing. Available at: http://www.r-project.org/.
Santoro R., Sperone E., Tringali M., Pellegrino G., Giglio G., Tripepi S., et al. (2015). Summer distribution, relative abundance and encounter rates of cetaceans in the Mediterranean waters off southern Italy (western Ionian Sea and southern tyrrhenian Sea). Mediterr. Mar. Sci. 16, 613–620. doi: 10.12681/mms.1007
Santostasi N. L., Bonizzoni S., Bearzi G., Eddy L., Gimenez O. (2016). A robust design capture-recapture analysis of abundance, survival and temporary emigration of three odontocete species in the gulf of Corinth, Greece. PloS One 11, e0166650. doi: 10.1371/journal.pone.0166650
Santostasi N. L., Bonizzoni S., Gimenez O., Eddy L., Bearzi G. (2018). Common dolphins in the gulf of Corinth are critically endangered. Aquat. Conserv. Mar. Freshw. Ecosyst. 31, 101–109. doi: 10.1002/aqc.2963
Scheinin A. P. (2010). The population of bottlenose dolphins (Tursiops truncatus), bottom trawl catch trends and the interaction between the two along the Mediterranean continental shelf of Israel (Israel, University of Haifa).
Scheinin A. P., Kerem D., Lojen S., Liberzon J., Spanier E. (2014). Resource partitioning between common bottlenose dolphin (Tursiops truncatus) and the Israeli bottom trawl fishery? assessment by stomach contents and tissue stable isotopes analysis. J. Mar. Biol. Assoc. United Kingdom 94, 1203–1220. doi: 10.1017/S0025315414001015
Schwarz C. J., Seber G. A. F. (1999). Estimating animal abundance: Review III. Stat. Sci. 14, 427–456. doi: 10.1214/ss/1009212521
Seber G. A. F. (1965). A note on the multiple-recapture census. Biometrika 52, 249–259. doi: 10.1093/biomet/52.1-2.249
Seber G. A. F. (1982). The estimation of animal abundance and related parameters. (2nd) Ed. Arnold (London: Charles Griffin).
Shane S. H. (1990). “Behavior and ecology of the bottlenose dolphin at sanibel island, Florida,” in The bottlenose dolphin. Eds. Reeves R. R., Leatherwood S. (San Diego: Academic Press), 245–265.
Shinohara M., Domingo-Roura X., Takenaka O. (1997). Microsatellites in the bottlenose dolphin tursiops truncatus. Mol. Ecol. 6, 695–696. doi: 10.1046/j.1365-294X.1997.00231.x
Smolker R. A., Richards A. F., Connor R. C., Pepper J. W. (1992). Sex differences in patterns of association among Indian ocean bottlenose dolphins. Behaviour 123, 38–69. doi: 10.1163/156853992X00101
Stanley T. R., Richards J. D. (2004). CloseTest: a program for testing capture-recapture data for closure. US Geological Survey, Fort Collins Science Center
Taylor B. L., Chivers S. J., Larese J., Perrin W. F. (2007a). Generation length and percent mature estimates for IUCN assessments of cetaceans. (National Marine Fisheries Service, Southwest Fisheries Science Center)
Taylor B. L., Martinez M., Gerrodette T., Barlow J., Hrovat Y. N. (2007b). Lessons from monitoring trends in abundance of marine mammals. Mar. Mammal Sci. 23, 157–175. doi: 10.1111/j.1748-7692.2006.00092.x
Tenan S., Hernández N., Fearnbach H., de Stephanis R., Verborgh P., Oro D. (2020). Impact of maritime traffic and whale-watching on apparent survival of bottlenose dolphins in the strait of Gibraltar. Aquat. Conserv. Mar. Freshw. Ecosyst. 30, 949–958. doi: 10.1002/aqc.3292
Thomas J. A., Fisher S. R., Ferm L. M., Holt R. S. (1986). “Behaviour of whales in relation to management,” in Reports of the international whaling commission (Special issue). Ed. Donovan G.P. (International Whaling Commission The Red House, Cambridge, U.K.), 139–148.
Vella A. (2004). Common dolphins (Delphinus delphis) status in the central and southern Mediterranean around the Maltese Islands. in 18th Annual Conference on Common Dolphins : Current Research, Threats and Issues (Kolmarden: European Cetacen Society), 4–12.
Vella A., Murphy S., Giménez J., de Stephanis R., Mussi B., Vella J. G., et al. (2021). The conservation of the endangered Mediterranean common dolphin (Delphinus delphis): Current knowledge and research priorities. Aquat. Conserv. Mar. Freshw. Ecosyst., 31, 1–27. doi: 10.1002/aqc.3538
Whitehead H. (2008a). Analyzing animal societies: Quantitative methods for vertebrate social analysis (Chicago; University of Chicago Press). doi: 10.7208/9780226895246
Whitehead H. (2008b). Precision and power in the analysis of social structure using associations. Anim. Behav. 75, 1093–1099. doi: 10.1016/j.anbehav.2007.08.022
Whitehead H., Parijs S. V. (2010). “Studying marine mammal social systems,” in Marine mammal ecology and conservation: a handbook of techniques. Eds. Boyd I. L., Bowen W. D., Iverson S. J. (Oxford: Oxford University Press), 263–282.
Wilson B., Hammond P. S., Thompson P. M. (1999). Estimating size and assessing trends in a coastal bottlenose dolphin population. Ecol. Appl. 9, 288–300. doi: 10.1890/1051-0761(1999)009[0288:ESAATI]2.0.CO;2
Keywords: common dolphins, IUCN red list, conservation status, extinction risk, abundance, mark-recapture, social structure, delphinus delphis
Citation: Mevorach Y, Scheinin A, Galili O, Santostasi NL and Tchernov D (2022) Common dolphins (Delphinus delphis) in Israel: Unique dynamics of a critically endangered population. Front. Mar. Sci. 9:916950. doi: 10.3389/fmars.2022.916950
Received: 10 April 2022; Accepted: 17 October 2022;
Published: 17 November 2022.
Edited by:
Morgana Vighi, Consultant, Barcelona, SpainReviewed by:
Tilen Genov, Morigenos - Slovenian Marine Mammal Society, SloveniaCopyright © 2022 Mevorach, Scheinin, Galili, Santostasi and Tchernov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaly Mevorach, eWFseS5tZUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.