
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 24 May 2022
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.909237
This article is part of the Research TopicExploration and Utilization of Marine and Freshwater High-Value Biological ResourcesView all 12 articles
 Feng Li1,2,3,4
Feng Li1,2,3,4 Minggang Cai2,3,4
Minggang Cai2,3,4 Yanqi Wu1
Yanqi Wu1 Qingsheng Lian1
Qingsheng Lian1 Zuyuan Qian1
Zuyuan Qian1 Jiansen Luo1
Jiansen Luo1 Yulei Zhang1
Yulei Zhang1 Ning Zhang1
Ning Zhang1 Changling Li1*
Changling Li1* Xianghu Huang1*
Xianghu Huang1*The dietary supplementation of Haematococcus pluvialis is a natural, safe, and sustainable method for fish pigmentation. However, astaxanthin-rich H. pluvialis cysts have a poor effect in pigmenting salmonid flesh due to their rigid and thick cell wall. H. pluvialis thin-walled motile cells have recently attracted attention due to their potential advantages in maintaining compound stability, easy digestion, enhancing the bioavailability of carotenoids, and reducing production costs. This study aimed to investigate the effect of various nitrogen concentrations and light intensities on astaxanthin production in motile cells. We first investigated the effect of four different concentrations of nitrogen on astaxanthin accumulation in motile cells. According to the results, the motile cells had the highest astaxanthin concentration and content under the 0 N condition. Then, we compared the differences in astaxanthin production in motile cells under three different light intensities under 0 N conditions. The results showed that after four days of treatment, the protoplasts of the motile cells in the medium light (ML) group and the high light (HL) group had distinct granularity. The cell mortality rate in the HL group reached more than 15%, which was significantly higher than that in the low light (LL) and ML groups, indicating that high light intensity was not suitable for inducing motile cells to accumulate astaxanthin. There were no significant differences between the LL and ML groups in astaxanthin content, motile cells percentage, and cell mortality rate. Considering these indicators, we recommended inducing motile cells to produce astaxanthin under low light conditions because it is more economical in terms of electricity consumption. This study added to the knowledge that nitrogen and light affects the accumulation of astaxanthin in H. pluvialis motile cells. The results would help determine the optimal nitrogen and light conditions in astaxanthin production from motile cells.
Farmed fish is globally recognized as one of the healthiest foods (Tacon and Metian, 2013) and plays a significant role in global food security and nutrition strategies. With the continuous increase in the scale of aquaculture, people have higher and higher requirements for farmed fish. Fish flesh color plays an important role in consumer sensory evaluation and sales price (Hart and Colombo, 2022). While flesh color doesn’t directly affect flesh quality, consumers often consider it as a sign that fish is fresh and tasty or not (Yesilayer, 2020). Astaxanthin is the main carotenoid responsible for the red or pink pigmentation of most wild salmonids (Nogueira et al., 2021), and accounts for more than 85% of the total carotenoid content in salmonids fresh (Torrissen et al., 1995). Feeding salmonids’ diets containing astaxanthin has become the main means of improving their flesh and skin color (Tejera et al., 2007; Kalinowski et al., 2011; Rahman et al., 2016). At present, synthetic astaxanthin occupies more than 95% of the market share astaxanthin (Shah et al., 2016), which is widely used in aquaculture due to its lower production cost (Panis and Garreon, 2016; Li et al., 2020a). However, it has raised consumer concerns about food safety, pollution, and sustainability due to synthetic astaxanthin derived from petrochemicals (Li et al., 2011; Stachowiak and Szulc, 2021). Consumer preference is shifting towards nature astaxanthin (Young et al., 2017; Viera et al., 2018).
Microalgae as fish feed additives have advantages in terms of safe availability of ingredients, ease of cultivation operations, and sustainability of production. H. pluvialis could accumulate high levels of astaxanthin (3-5% dry weight) (Lorenz and Cysewski, 2000; Ambati et al., 2014) and is regarded as the most potent natural source of astaxanthin (Bowen et al., 2002). Several studies indicated that dietary supplementation of H. pluvialis would improve the color, immune and antioxidant capacity of fish (Moretti et al., 2006; Sheikhzadeh et al., 2012; Li et al., 2018; Yu et al., 2021). However, H. pluvialis red cysts typically have thick and less digestible cell walls (Hagen et al., 2002; Shah et al., 2016), which reduce the bioavailability of astaxanthin (Sommer et al., 1991; Choubert et al., 2006). As a result, the tissue pigmentation levels of fish fed H. pluvialis were generally lower than those in fish fed synthetic astaxanthin (Choubert et al., 2006; Li et al., 2014). Recent studies suggested that whole-celled H. pluvialis with thin cell walls achieves the same efficiency at pigmenting the flesh of rainbow trout as synthetic astaxanthin (Hart and Colombo, 2022). It was attributed to the ease of digestion of H. pluvialis thin-walled cells.
There are various types of cells in the H. pluvialis life cycle, including motile cells, nonmotile cells, and red cyst cells (Li et al., 2019a), and only motile cells have thin cell walls. More attention has been paid in the past periods to enhancing astaxanthin accumulation in the thick-walled cysts (Boussiba and Vonshak, 1991; Kobayashi et al., 1997; Li et al., 2019b; Fang et al., 2020), but less attention has been paid to motile cells. Previous studies have shown that nitrogen and light intensity play critical roles in the astaxanthin accumulation in H. pluvialis (Borowitzka et al., 1991; Fábregas et al., 1998; Orosa et al., 2005). However, little is known about the effects of nitrogen and light intensity on astaxanthin accumulation in motile cells. In this study, we first investigated the effect of four different concentrations of nitrogen on the accumulation of astaxanthin in motile cells and determined the optimal nitrogen concentration to induce motile cells to produce astaxanthin. The effect of three light intensities on the astaxanthin accumulation in motile cells were then investigated at this nitrogen concentration. The results would help determine the optimal nitrogen and light conditions in the production of astaxanthin from H. pluvialis motile cells.
H. pluvialis CCMA-451 (Genbank accession number MG847145) was acquired from the Center for Collections of Marine Algae of Xiamen University (CCMA). The motile cells were grown photoautotrophically in liquid 3N-BBM (Bold Basal Medium) at 30 μmol m-2 s-1 (side, white light) for 10 days.
The first experiment was the effect of different nitrogen concentrations on astaxanthin accumulation in motile cells. Four different nitrogen (NaNO3) concentrations were tested, including 0 N, 0.5-fold N, 1-fold N (250 mg/L), and 3N. The experiment was carried out under the condition of 30 μmol m-2 s-1 light intensity.
For the second experiment, under the conditions of nitrogen concentration screened in experiment 1, motile cells were treated under the conditions of nitrogen concentration screened in experiment 1 with three light intensities: 30, 80, and 150 μmol m-2 s-1, representing a low (LL), medium (ML), and high light (HL), respectively.
The 10-day-old motile cells were collected by centrifugation (3000 rpm, 2 min) for experiments. All experimental groups were performed in 1 L glass columns (inner diameter 5 cm) at an initial optical density of 0.5 (OD680) in 25 ± 1°C for 4 days, with three parallels per group. The cultures were mixed by continuous bubbling with 1.5% CO2 gas at 0.5 vvm.
For cell numbers determination, samples were fixed with Lugol’s iodine solution first, and then the cell number was counted by a Neubauer improved cell counting chamber under Olympus BX53 light microscope.
Dry weight (DW) is determined daily according to Li et al. (2019a). Briefly, 10 mL algal suspension were filtered by passing through a pre-weighed (m1) filter paper (47 mm, 1.2 μm, Whatman GF/C). Then dry the filter paper overnight at 90 °C to a constant weight (m2). The DW was calculated with Eq. 1.
The morphological changes of algal cells were examined daily using Olympus BX53 light microscope and the microphotographs were taken with an Olympus DP74 digital camera. Since the shape of motile cells is irregular, the cell size is characterized by the protoplast length and measured by Olympus application software with an internal reticle scale. No less than 300 cells per group were used for cell size statistics.
All data were subjected to an analysis of variance (ANOVA), and differences at p ≤ 0.05 were considered statistically significant.
The effect of different nitrogen concentrations on the dry weight is shown in Figure 1B. During the first three days of culture, the dry weight development trend of the four groups was the same, and no significant differences were observed. However, on the 4th day of treatment, the dry weight growth of the 0 N group slowed down significantly, while the other three groups continued to maintain a sustained increase. After four days of cultivation, the maximum dry weight of the 0 N, 0.5 N, 1 N and 3 N groups reached 1.02 ± 0.08 g L-1, 1.25 ± 0.02 g L-1, 1.15 ± 0.02 g L-1 and 1.19 ± 0.04 g L-1, respectively.
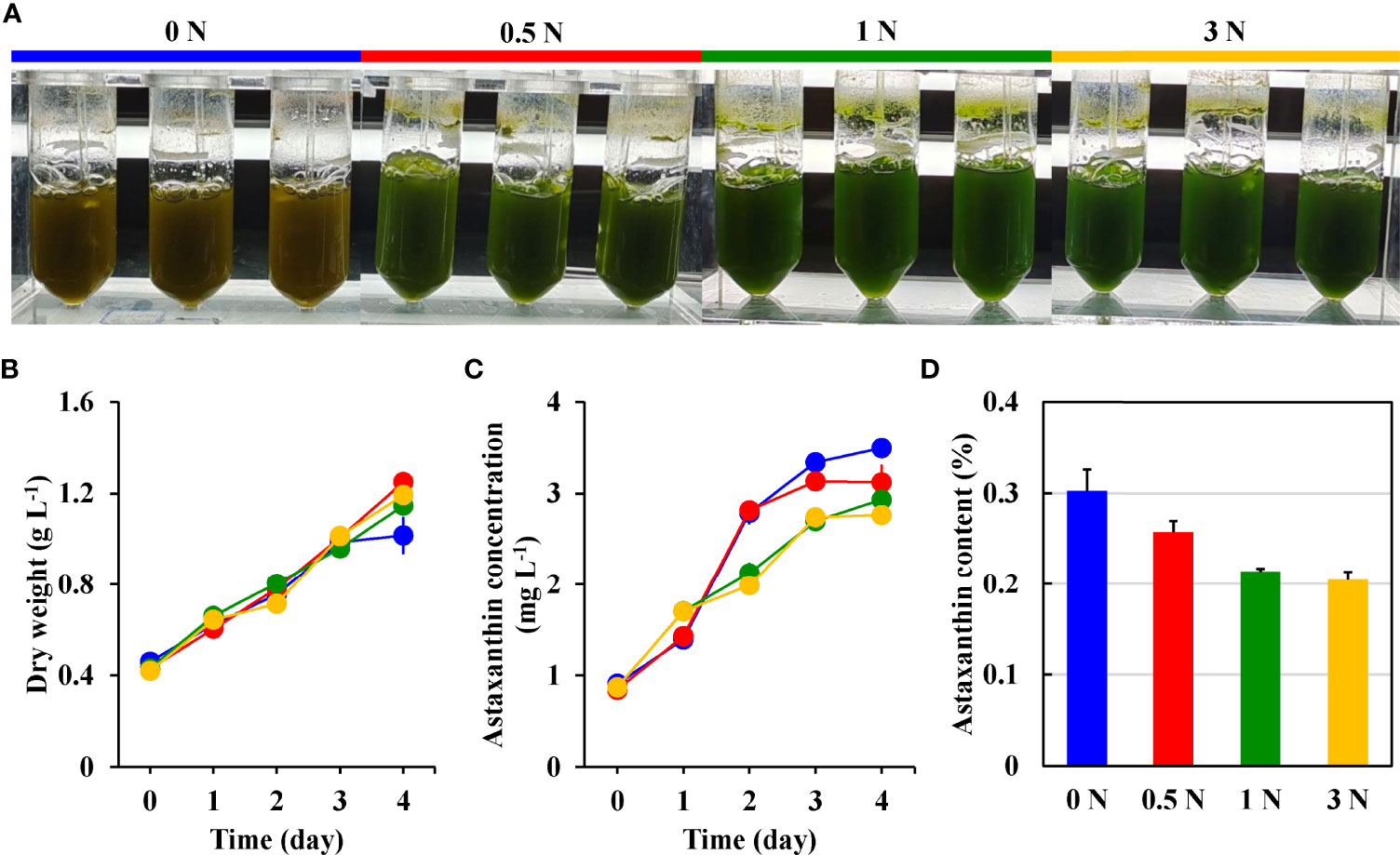
Figure 1 The color changes (A), dry weight (B), astaxanthin concentration (C), and astaxanthin content (D) of H. pluvialis motile cells in the 0 N (blue), 0.5 N (red), 1 N (green), and 3 N (yellow) group.
It can be seen from Figure 1C that the astaxanthin concentration of the 0 N and 0.5 N groups shows a similar growth trend, and the astaxanthin concentration growth trend of the 1 N and 3 N groups is similar. The 0 N and the 0.5 N groups showed rapid growth at first and then slowed down after one day of treatment, while the astaxanthin concentration of the 1 N group and the 3 N group showed a stable growth trend within four days. After four days of treatment, the astaxanthin concentration in the 0 N group has the highest value of 3.50 ± 0.10 mg L-1, followed by the 0.5 N group and the 1 N group with the value of 3.12 ± 0.19 mg L-1 and 2.93 ± 0.05 mg L-1. The 3 N group had the lowest astaxanthin concentration with a value of 2.76 ± 0.09 mg L-1.
The astaxanthin content of the four cultures at the end of cultivation is calculated, and the results is shown in Figure 1D. A trend is observed that the lower the nitrogen concentration, the higher the astaxanthin content. The 0 N group had the highest astaxanthin content at 0.31 ± 0.02%, while the 3N group had the lowest at 0.20 ± 0.01%.
As shown in Figure 2A, the motile cell morphological changes at different light intensities are observed under the light microscope. The motile cells protoplast was enclosed by a swollen and gelatinous cell wall layer. After culturing for one day under nitrogen starvation, a small amount of orange-red pigment was observed in the motile cells treated with different light intensities. With the increase of treatment time, the motile cells of the three groups gradually changed from green to red due to astaxanthin accumulation. After being exposed to HL for two days, the gelatinous extracellular matrix outside the protoplasts of some motile cells disappeared, and a few motile cells developed into cysts with thickened cell walls. After four days of treatment, the protoplasts of motile cells in the ML and HL groups had distinct granularity, but the granularity of cells in the LL group was weak.
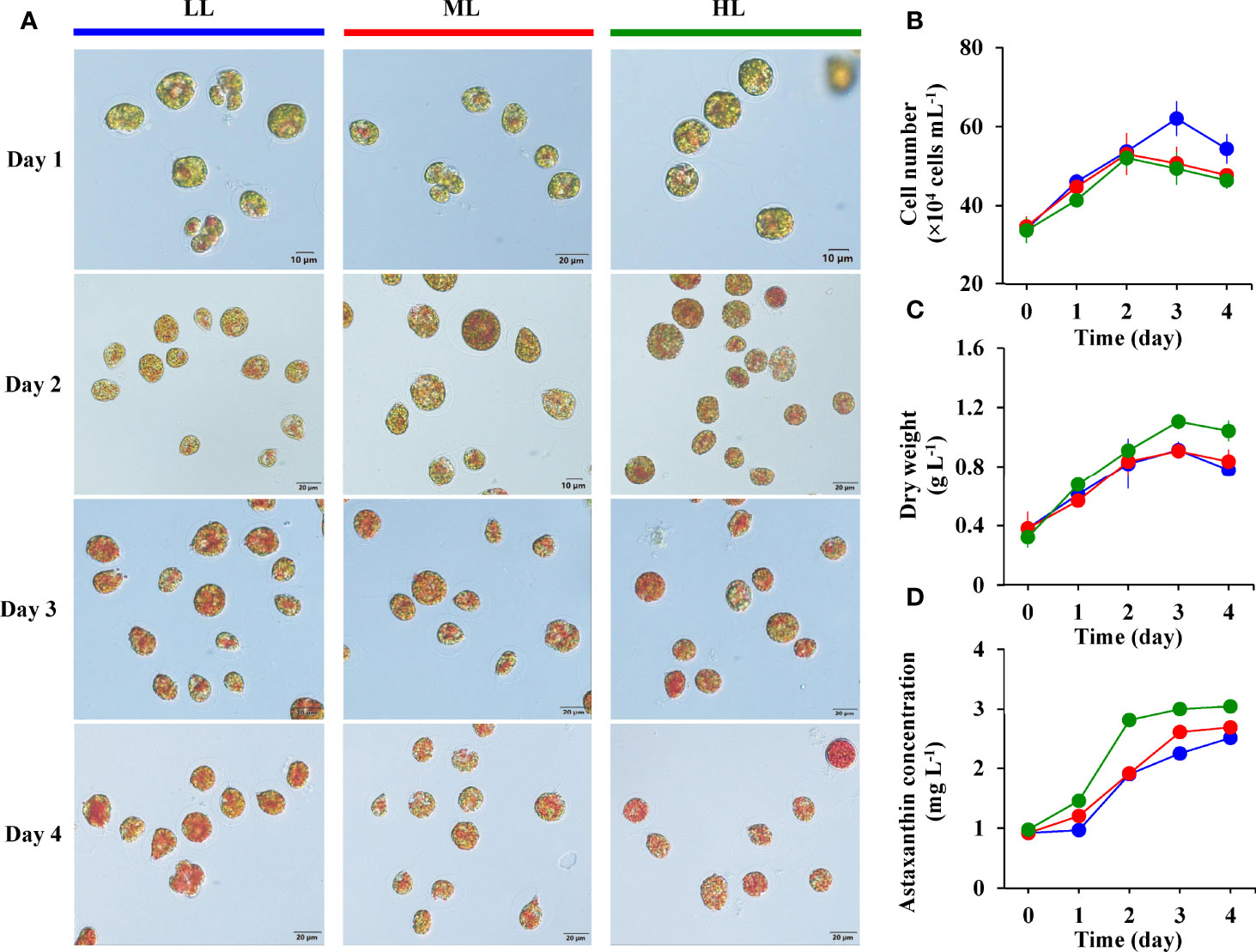
Figure 2 The cell morphological changes (A), cell number (B), dry weight (C), and astaxanthin concentration (D) of H. pluvialis in the LL (blue), ML (red), and HL (green) group.
The effect of light intensity on the cell number of motile cells under nitrogen starvation is shown in Figure 2B. During the first two days of culture under nitrogen starvation conditions, the number of cells in the three groups showed an upward trend. The cell number in the ML and HL groups reached the maximum value on the second day, at 5.3×105 cells mL-1 and 5.2×105 cells mL-1, respectively, and then rapidly decreased until the end. However, the number of cells in the LL group reached a maximum value of 6.2×105 cells mL-1 after three days of culture, significantly higher than the ML and HL group.
Figure 2C shows the changes in dry weight in different light intensity groups. Dry weights of the three groups show a similar trend, increasing with culture time, peaking on the third day, and then decreasing until the end of the 4-day cultivation. After four days of cultivation, the HL group showed the highest dry weight, followed by the ML group, and the LL group had the lowest dry weight. All the maximum dry weight of each group appeared on the third day of culture, and it was 1.11 ± 0.05 g L-1 in the HL group, 0.91 ± 0.02 g L-1 in the ML group, and 0.91 ± 0.06 g L-1 in the LL group (Table 1).

Table 1 The maximum dry weight and astaxanthin concentration, percentage of motile cells, cell mortality rate, and cell size in the LL, ML, and HL group.
The changes in astaxanthin concentration in different light intensity groups are shown in Figure 2D. The concentrations of astaxanthin in the three groups showed an upward trend with the increase of culture time. A significant difference in astaxanthin concentrations among the three groups was observed, with the highest in the HL group, followed by the ML group, and the lowest in the LL group. The maximum astaxanthin concentration values of the three groups all appeared at the end of the 4-day cultivation. As shown in Table 1, the maximum astaxanthin concentration of the HL group reached 3.04 ± 0.04 mg L-1, the ML group was 2.69 ± 0.05 mg L-1, and the LL group was 2.51 ± 0.02 mg L-1.
We calculated the astaxanthin content of the three cultures at the end of cultivation. As shown in Table 1, no difference in astaxanthin content was observed between LL and ML groups, with values of 0.32 ± 0.02% and 0.32 ± 0.04%, respectively, while the astaxanthin content in the HL group was lower than that in the other two groups, with a value of 0.29 ± 0.02%.
The motile cells percentage, cell mortality rate, and cell size at the end of the 4-day cultivation were analyzed, and the results are shown in Table 1. Among the three groups, the motile cells percentage and cell size in the ML group were 88.1 ± 1.5% and 23.67 ± 4.63 μm, which were higher than those in the LL and HL groups, respectively, while the cell mortality rate in the ML group reached 9.8 ± 1.3%, which was smaller than that in the other two groups. The HL group had the lowest motile cells percentage and highest cell mortality rate among the three groups, with the value of 82.8 ± 1.4% and 15.1 ± 0.7%, respectively. The cell size in the LL group was the lowest among the three groups, with a value of 21.72 ± 3.82 μm.
Astaxanthin accumulation in H. pluvialis is normally accompanied by the formation of encystment (Boussiba, 2000). During encystment, a voluminous multilayered cell wall, including trilaminar sheath and secondary wall, is formed with a significantly hardening and thickening (Monstsant et al., 2001; Hagen et al., 2002; Wang et al., 2003). Although the formation of thick cell walls is favorable for the large quality astaxanthin accumulation in H. pluvialis (Li et al., 2019a), it is regarded as one of the main factors in reducing the coloration efficiency and bioavailability of astaxanthin when fed to salmonids and other commercial species (Sommer et al., 1991; Young et al., 2017). Previous studies have shown that breaking H. pluvialis cysts can improve the astaxanthin deposition rate in trout flesh (Sommer et al., 1991). But, disruption of H. pluvialis cyst cell walls often require mechanical, chemical, or enzymatic means, and all of these processes are currently highly energy-intensive and expensive (Tibbetts, 2018). In addition, these processes can decrease the stability of astaxanthin and reduce the shelf life of the compound (Han et al., 2013). In contrast, thin-walled H. pluvialis can use whole cells as a source of astaxanthin, which has potential advantages in maintaining compound stability, easy digestion, enhance bioavailability of carotenoids, and reducing production costs.
Astaxanthin is accumulated in H. pluvialis under growth-limiting conditions, such as nutrient deprivation, high light, and/or high salinity (Lemoine & Schoefs, 2010; Chekanov et al., 2014; Scibilia et al., 2015; Solovchenko, 2015; Oslan et al., 2021). Nitrogen is one of the essential nutrients that affect cell growth and enzymatic activity of H. pluvialis (Zhang et al., 2018). Previous studies showed that nitrogen deficiency inhibited H. pluvialis chlorophyll biosynthesis and promoted chlorophyll b degradation, PTOX activity, and cyclic electron transport (Scibilia et al., 2015). The reduction of chlorophyll content resulted in the limitation of the cell’s ability to maintain photosynthetic function (Young and Beardall, 2003). In this case, H. pluvialis accumulated large amounts of carbon in the form of carotenoids and lipids (Sun et al., 2018). In the present study, we observed that the lower the nitrogen concentration, the higher the astaxanthin content in motile cells. The results are consistent with previous reports that nitrogen deficiency can stimulate astaxanthin synthesis (Borowitzka et al., 1991; Fábregas et al., 1998).
Light intensity is another one of the most significant factors in the induction of astaxanthin accumulation (Oslan et al., 2021). It can cause oxidative stress and promote the excess of reactive oxygen species in algal cells, thereby activating the biosynthesis of astaxanthin (Han et al., 2012). It has been reported that within the first three days of high light induction, the non-photochemical quenching (NPQ) of H. pluvialis cells decreased dramatically, while the chlorophyll a/b ratio increased transiently. In contrast, under the high light combined with nitrogen starvation, cells exhibited a sustained increase in chlorophyll a/b ratio and a rapid rise in NPQ induction. In addition, the PSII quantum yield decreased by 20% under high light conditions, while the Fv/Fm remained stable under the combined induction of high light and nitrogen deficiency (Scibilia et al., 2015). Scibilia et al. (2015) believed that nitrogen starvation enhanced the stress response of H. pluvialis, inducing cells to rapidly counteract high-irradiation-induced photooxidative stress, thereby improving the resistance of cells to photo-oxidation. However, motile cells of H. pluvialis are sensitive to light intensity, and the PSII core subunits of cells are degraded when exposed to high light conditions (Han et al., 2012). In this study, after four days of culture, the cell mortality rate in the HL group reached more than 15%, which was significantly higher than that in the LL and ML groups, indicating that high light intensity was not suitable for inducing motile cells to accumulate astaxanthin. In addition, no significant differences were observed in astaxanthin content, motile cells percentage, and cell mortality rate between the LL and ML groups. Considering low light is more economical in terms of electricity consumption, we recommend that low light as the light condition for motile cells to accumulate astaxanthin under nitrogen starvation.
We have to point out that the astaxanthin content obtained in the current study was not high. It may account for the short irradiation time. Considering that prolonged induction can lead to an increase in the number of thick-walled cysts, further work investigating the relationship between the induction period and the percentage of motile cells and astaxanthin content is needed with the aim to determine the optimal induction period. In addition, according to Butler et al. (2018), the intracellular astaxanthin content of H. pluvialis motile cells can reach up to 2.7% of dry weight. It is indicated that there is still large improvement room for astaxanthin content in motile cells. Therefore, more effort to optimize induction conditions would be needed for improving astaxanthin production by motile cells. Phosphorus deficiency (Imamoglu et al., 2009) or high salt (Tam et al., 2012) can promote astaxanthin accumulation. However, phosphorus deficiency (Boussiba and Vonshak, 1991; Li et al., 2021) and high salt (Droop, 1955; Li et al., 2021) can induce cyst formation in H. pluvialis. Further study on optimizing the astaxanthin production by motile cells should take into account this aspect.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
FL: data curation, methodology, and writing-original draft. YW: investigations. MC: writing-review and editing. QL: investigations. ZQ: investigations. JL: investigations. YZ: methodology. NZ: methodology. XH: project administration and resources. CL: project administration and resources. All authors contributed to the article and approved the submitted version.
This research was mainly supported by Program for Scientific Research Start-up Funds of Guangdong Ocean University (grant number 060302022102) and Marine Economic Development Special Fund of Fujian Province (FJHJF-L-2021-5).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ambati R. R., Phang S. M., Ravi S., Aswathanarayana R. G. (2014). Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 12 (1), 128–152. doi: 10.3390/md12010128
Borowitzka M. A., Huisman J. M., Osborn A. (1991). Culture of The Astaxanthin-Producing Green Alga Haematococcus Pluvialis 1. Effects of Nutrients on Growth and Cell Type. J. Appl. Phycol. 3 (4), 295–304. doi: 10.1007/BF02392882
Boussiba S. (2000). Carotenogenesis in The Green Alga Haematococcus Pluvialis: Cellular Physiology and Stress Response. Physiol. Plantar. 108 (2), 111–117. doi: 10.1034/j.1399-3054.2000.108002111.x
Boussiba S., Vonshak A. (1991). Astaxanthin Accumulation in The Green Alga Haematococcus Pluvialis. Plant Cell Physiol. 32 (7), 1077–1082. doi: 10.1093/oxfordjournals.pcp.a078171
Bowen J., Soutar C., Serwata R. D., Lagocki S., White D. A., Davies S. J. (2002). Utilization of (3S, 3′S) Astaxanthin Acyl Esters in Pigmentation of Rainbow Trout (Oncorhynchus Mykiss). Aquacult. Nutr. 8 (1), 59–68. doi: 10.1046/j.1365-2095.2002.00190.x
Butler T. O., McDougall G. J., Campbell R., Stanley M. S., Day J. G. (2018). Media Screening for Obtaining Haematococcus Pluvialis Red Motile Macrozooids Rich in Astaxanthin and Fatty Acids. Biology 7 (1), 2. doi: 10.3390/biology7010002
Chekanov K., Lobakova E., Selyakh I., Semenova L., Sidorov R., Solovchenko A. (2014). Accumulation of Astaxanthin by A New Haematococcus Pluvialis Strain BM1 From The White Sea Coastal Rocks (Russia). Mar. Drugs 12 (8), 4504–4520. doi: 10.3390/md12084504
Choubert G., Mendes-Pinto M. M., Morais R. (2006). Pigmenting Efficacy of Astaxanthin Fed to Rainbow Trout Oncorhynchus Mykiss: Effect of Dietary Astaxanthin and Lipid Sources. Aquaculture 257 (1-4), 429–436. doi: 10.1016/j.aquaculture.2006.02.055
Droop M. R. (1955). Some Factors Governing Encystment in Haematococcus Pluvialis. Arch. Für. Mikrobiol. 21, 267–272. doi: 10.1007/BF00412349
Fábregas J., Domínguez A., Álvarez D. G., Lamela T., Otero A. (1998). Induction of Astaxanthin Accumulation by Nitrogen and Magnesium Deficiencies in Haematococcus Pluvialis. Biotechnol. Lett. 20 (6), 623–626. doi: 10.1023/A:1005322416796
Fang L., Zhang J., Fei Z., Wang M. (2020). Astaxanthin Accumulation Difference Between Non-Motile Cells and Akinetes of Haematococcus Pluvialis Was Affected by Pyruvate Metabolism. Biores. Bioprocess. 7 (1), 1–12. doi: 10.1186/s40643-019-0293-1
Hagen C., Siegmund S., Braune W. (2002). Ultrastructural And Chemical Changes in The Cell Wall of Haematococcus Pluvialis (Volvocales, Chlorophyta) During Aplanospore Formation. Eur. J. Phycol. 37 (2), 217–226. doi: 10.1017/S0967026202003669
Han D., Li Y., Hu Q. (2013). Astaxanthin in Microalgae: Pathways, Functions and Biotechnological Implications. Algae 28 (2), 131–147. doi: 10.4490/algae.2013.28.2.131
Han D., Wang J., Sommerfeld M., Hu Q. (2012). Susceptibility And Protective Mechanisms of Motile and Non-Motile Cells of Haematococcus Pluvialis (Chlorophyceae) to Photooxidative Stress. J. Phycol. 48 (3), 693–705. doi: 10.1111/j.1529-8817.2012.01147.x
Hart B., Colombo S. M. (2022). Effects of a Novel Weakened Whole-Cell Form of Haematococcus Pluvialis on Flesh Pigmentation of Rainbow Trout (Oncorhynchus Mykiss) When Compared to Synthetic Astaxanthin. Aquacult. Res. 53 (6), 2408–2319. doi: 10.1111/are.15758
Imamoglu E., Dalay M. C., Sukan F. V. (2009). Influences of Different Stress Media and High Light Intensities on Accumulation of Astaxanthin in The Green Alga Haematococcus Pluvialis. New Biotechnol. 26 (3-4), 199–204. doi: 10.1016/j.nbt.2009.08.007
Kalinowski C. T., Robaina L. E., Izquierdo M. S. (2011). Effect of Dietary Astaxanthin on The Growth Performance, Lipid Composition and Post-Mortem Skin Colouration of Red Porgy Pagrus Pagrus. Aquacult. Int. 9 (5), 811–823. doi: 10.1007/s10499-010-9401-0
Kobayashi M., Kurimura Y., Tsuji Y. (1997). Light-Independent, Astaxanthin Production by The Green Microalga Haematococcus Pluvialis Under Salt Stress. Biotechnol. Lett. 19 (6), 507–509. doi: 10.1023/A:1018372900649
Lemoine Y., Schoefs B. (2010). Secondary Ketocarotenoid Astaxanthin Biosynthesis in Algae: A Multifunctional Response to Stress. Photosynthesis. Res. 106 (1), 155–177. doi: 10.1007/s11120-010-9583-3
Li F., Cai M., Lin M., Huang X., Wang J., Ke H., et al. (2019a). Differences Between Motile and Nonmotile Cells of Haematococcus Pluvialis in The Production of Astaxanthin at Different Light Intensities. Mar. Drugs 17 (1), 39. doi: 10.3390/md17010039
Li F., Cai M., Lin M., Huang X., Wang J., Ke H., et al. (2020b). Enhanced Biomass and Astaxanthin Production of Haematococcus Pluvialis by A Cell Transformation Strategy With Optimized Initial Biomass Density. Mar. Drugs 18 (7), 1–7. doi: 10.3390/md18070341
Li F., Cai M., Lin M., Huang X., Wang J., Zheng X., et al. (2019b). Accumulation of Astaxanthin Was Improved by The Nonmotile Cells of Haematococcus Pluvialis. BioMed. Res. Int. 2019, 1–7. doi: 10.1155/2019/8101762
Li F., Huang S., Lu X., Wang J., Lin M., An Y., et al. (2018). Effects of Dietary Supplementation With Algal Astaxanthin on Growth, Pigmentation, And Antioxidant Capacity of The Blood Parrot (Cichlasoma Citrinellum × Cichlasoma Synspilum). J. Oceanol. Limnol. 36 (5), 1851–1859. doi: 10.1007/s00343-019-7172-7
Li X., Wang X., Duan C., Yi S., Gao Z., Xiao C., et al. (2020a). Biotechnological Production of Astaxanthin From The Microalga Haematococcus Pluvialis. Biotechnol. Adv. 43, 107602. doi: 10.1016/j.biotechadv.2020.107602
Li M., Wu W., Zhou P., Xie F., Zhou Q., Mai K. (2014). Comparison Effect of Dietary Astaxanthin and Haematococcus Pluvialis On Growth Performance, Antioxidant Status and Immune Response of Large Yellow Croaker Pseudosciaena Crocea. Aquaculture 434, 227–232. doi: 10.1016/j.aquaculture.2014.08.022
Li F., Zhang N., Zhang Y., Lian Q., Qin C., Qian Z., et al. (2021). NaCl Promotes the Efficient Formation of Haematococcus Pluvialis Nonmotile Cells Under Phosphorus Deficiency. Mar. Drugs 19 (6), 337. doi: 10.3390/md19060337
Li J., Zhu D., Niu J., Shen S., Wang G. (2011). An Economic Assessment of Astaxanthin Production by Large Scale Cultivation of Haematococcus Pluvialis. Biotechnol. Adv. 29 (6), 568–574. doi: 10.1016/j.biotechadv.2011.04.001
Lorenz R. T., Cysewski G. R. (2000). Commercial Potential for Haematococcus Microalgae as A Natural Source of Astaxanthin. Trends Biotechnol. 18 (4), 160–167. doi: 10.1016/S0167-7799(00)01433-5
Montsant A., Zarka A., Boussiba S. (2001). Presence Of a Nonhydrolyzable Biopolymer in The Cell Wall of Vegetative Cells and Astaxanthin-Rich Cysts of Haematococcus Pluvialis (Chlorophyceae). Mar. Biotechnol. 3 (6), 515–521. doi: 10.1007/s1012601-0051-0
Moretti V. M., Mentasti T., Bellagamba F., Luzzana U., Caprino F., Turchini G. M., et al. (2006). Determination of Astaxanthin Stereoisomers and Colour Attributes in Flesh of Rainbow Trout (Oncorhynchus Mykiss) As A Tool to Distinguish the Dietary Pigmentation Source. Food Additive. Contaminant. 23 (11), 1056–1063. doi: 10.1080/02652030600838399
Nogueira N., Canada P., Caboz J., Andrade C., Cordeiro N. (2021). Effect of Different Levels of Synthetic Astaxanthin on Growth, Skin Color and Lipid Metabolism of Commercial Sized Red Porgy (Pagrus Pagrus). Anim. Feed. Sci. Technol. 276, 114916. doi: 10.1016/j.anifeedsci.2021.114916
Orosa M., Franqueira D., Cid A., Abalde J. (2005). Analysis and Enhancement of Astaxanthin Accumulation in Haematococcus Pluvialis. Biores. Technol. 96 (3), 373–378. doi: 10.1016/j.biortech.2004.04.006
Oslan S. N. H., Shoparwe N. F., Yusoff A. H., Rahim A. A., Chang C. S., Tan J. S., et al. (2021). A Review on Haematococcus Pluvialis Bioprocess Optimization of Green and Red Stage Culture Conditions for The Production of Natural Astaxanthin. Biomolecules 11 (2), 256. doi: 10.3390/biom11020256
Panis G., Carreon J. R. (2016). Commercial Astaxanthin Production Derived by Green Alga Haematococcus Pluvialis: A Microalgae Process Model and A Techno-Economic Assessment All Through Production Line. Algal. Res. 18, 175–190. doi: 10.1016/j.algal.2016.06.007
Rahman M. M., Khosravi S., Chang K. H., Lee S. M. (2016). Effects of Dietary Inclusion of Astaxanthin on Growth, Muscle Pigmentation and Antioxidant Capacity of Juvenile Rainbow Trout (Oncorhynchus Mykiss). Prev. Nutr. Food Sci. 21 (3), 281–288. doi: 10.3746/pnf.2016.21.3.281
Scibilia L., Girolomoni L., Berteotti S., Alboresi A., Ballottari M. (2015). Photosynthetic Response to Nitrogen Starvation and High Light in Haematococcus Pluvialis. Algal. Res. 12, 170–181. doi: 10.1016/j.algal.2015.08.024
Shah M. M. R., Liang Y., Chen J. J., Daroch M. (2016). Astaxanthin-Producing Green Microalga Haematococcus Pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00531
Sheikhzadeh N., Tayefi-Nasrabadi H., Khani Oushani A., Enferadi M. H. N. (2012). Effects of Haematococcus Pluvialis Supplementation on Antioxidant System and Metabolism in Rainbow Trout (Oncorhynchus Mykiss). Fish. Physiol. Biochem. 38 (2), 413–419. doi: 10.1007/s10695-011-9519-7
Solovchenko A. E. (2015). Recent Breakthroughs in the Biology of Astaxanthin Accumulation by Microalgal Cell. Photosynthesis. Res. 125 (3), 437–449. doi: 10.1007/s11120-015-0156-3
Sommer T. R., Potts W. T., Morrissy N. M. (1991). Utilization of Microalgal Astaxanthin by Rainbow Trout (Oncorhynchus Mykiss). Aquaculture 94 (1), 79–88. doi: 10.1016/0044-8486(91)90130-Y
Stachowiak B., Szulc P. (2021). Astaxanthin for the Food Industry. Molecules 26 (9), 26. doi: 10.3390/molecules26092666
Sun X. M., Ren L. J., Zhao Q. Y., Ji X. J., Huang H. (2018). Microalgae for the Production of Lipid and Carotenoids: A Review With Focus on Stress Regulation and Adaptation. Biotechnol. Biofuels. 11 (1), 1–16. doi: 10.1186/s13068-018-1275-9
Tacon A. G. J., Metian M. (2013). Fish Matters: Importance of Aquatic Foods in Human Nutrition and Global Food Supply. Rev. Fish. Sci. 21 (1), 22–38. doi: 10.1080/10641262.2012.753405
Tam L. T., Hoang D. D., Mai D. T. N., Thu N. T. H., Anh H. T. L., Dang D. H. (2012). Study on the Effect of Salt Concentration on Growth and Astaxanthin Accumulation of Microalgae Haematococcus Pluvialis As the Initial Basis for Two Phase Culture of Astaxanthin Production. Academia. J. Biol. 34 (2), 213–223. doi: 10.15625/0866-7160/v34n2.964
Tejera N., Cejas J. R., Rodríguez C., Bjerkeng B., Jerez S., Bolaños A., et al. (2007). Pigmentation, Carotenoids, Lipid Peroxides and Lipid Composition of Skin of Red Porgy (Pagrus Pagrus) Fed Diets Supplemented With Different Astaxanthin Sources. Aquaculture 270 (1-4), 218–230. doi: 10.1016/j.aquaculture.2007.01.019
Tibbetts S. M. (2018). “The Potential for ‘Next-Generation’, Microalgae-Based Feed Ingredients for Salmonid Aquaculture in Context of The Blue Revolution,” in Microalgal Biotechnology. Eds. Jacob-Lopes E., Zepka L. Q., Queiroz M. I., 151–175. London, United Kingdom: IntechOpen. doi: 10.5772/intechopen.73551
Torrissen O. J., Christiansen R., Struksnæs G., Estermann R. (1995). Astaxanthin Deposition in the Flesh of Atlantic Salmon, Salmo Salar L, in Relation to Dietary Astaxanthin Concentration and Feeding Period. Aquacult. Nutr. 1 (2), 77–84. doi: 10.1111/j.1365-2095.1995.tb00022.x
Viera I., Pérez-Gálvez A., Roca M. (2018). Bioaccessibility of Marine Carotenoids. Mar. Drugs 16 (10), 397. doi: 10.3390/md16100397
Wang B., Zarka A., Trebst A., Boussiba S. (2003). Astaxanthin Accumulation in Haematococcus Pluvialis (Chlorophyceae) As an Active Photoprotective Process Under High Irradiance1. J. Phycol. 39 (6), 1116–1124. doi: 10.1111/j.0022-3646.2003.03-043.x
Yeşilayer N. (2020). Comparison of Flesh Colour Assessment Methods for Wild Brown Trout (Salmo Trutta Macrostigma), Farmed Rainbow Trout (Oncorhynchus Mykiss) and Farmed Atlantic Salmon (Salmo Salar). Pakistan J. Zool. 52 (3), 1007–1014. doi: 10.17582/journal.pjz/20190520140524
Young E. B., Beardall J. (2003). Photosynthetic Function in Dunaliella Tertiolecta (Chlorophyta) During a Nitrogen Starvation and Recovery Cycle. J. Phycol. 39 (5), 897–905. doi: 10.1046/j.1529-8817.2003.03042.x
Young A. J., Pritchard J., White D., Davies S. (2017). Processing of Astaxanthin-Rich Haematococcus Cells for Dietary Inclusion and Optimal Pigmentation in Rainbow Trout, Oncorhynchus Mykiss L. Aquacult. Nutr. 23 (6), 1304–1311. doi: 10.1111/anu.12505
Yu W., Lin H., Yang Y., Zhou Q., Chen H., Huang X., et al. (2021). Effects of Supplemental Dietary Haematococcus Pluvialis on Growth Performance, Antioxidant Capacity, Immune Responses and Resistance to Vibrio Harveyi Challenge of Spotted Sea Bass Lateolabrax Maculatus. Aquacult. Nutr. 27 (2), 355–365. doi: 10.1111/anu.13189
Keywords: astaxanthin, Haematococcus pluvialis, motile cells, nitrogen starvation, light intensity
Citation: Li F, Cai M, Wu Y, Lian Q, Qian Z, Luo J, Zhang Y, Zhang N, Li C and Huang X (2022) Effects of Nitrogen and Light Intensity on the Astaxanthin Accumulation in Motile Cells of Haematococcus pluvialis. Front. Mar. Sci. 9:909237. doi: 10.3389/fmars.2022.909237
Received: 31 March 2022; Accepted: 27 April 2022;
Published: 24 May 2022.
Edited by:
Pengfei Cheng, Ningbo University, ChinaReviewed by:
Fatimah Md Yusoff, Putra Malaysia University, MalaysiaCopyright © 2022 Li, Cai, Wu, Lian, Qian, Luo, Zhang, Zhang, Li and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changling Li, bGljbEBnZG91LmVkdS5jbg==; Xianghu Huang, aHVhbmd4aEBnZG91LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.