
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 06 September 2022
Sec. Coral Reef Research
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.907362
This article is part of the Research TopicOrnamental Fishing IndustryView all 8 articles
 Kuttan Kuravamparambu Anikuttan*
Kuttan Kuravamparambu Anikuttan* Palsamy Rameshkumar
Palsamy Rameshkumar Abdul Khudus Nazar
Abdul Khudus Nazar Rengarajan Jayakumar
Rengarajan Jayakumar Govindan Tamilmani
Govindan Tamilmani Mohammed Sakthivel
Mohammed Sakthivel Murugesan Sankar
Murugesan Sankar Rajendran Bavithra
Rajendran Bavithra Belevendran Johnson
Belevendran Johnson Nataraj Krishnaveni
Nataraj Krishnaveni Augustin Angela Mercy
Augustin Angela Mercy Nallathambi Moulitharan
Nallathambi Moulitharan Gunasekharan Iyyapparaja Narasimapallavan
Gunasekharan Iyyapparaja Narasimapallavan Tinto Thomas
Tinto Thomas Galinki Hanumanta Rao
Galinki Hanumanta Rao Muthu Jayasingh
Muthu Jayasingh Imelda Joseph
Imelda Joseph Boby Ignatius
Boby Ignatius Kuttan Madhu
Kuttan Madhu Achamveetil Gopalakrishnan
Achamveetil GopalakrishnanThe global marine ornamental fish trade is dominated by wild collected fishes, and the contribution from hatchery production is less than 10% of the total. Hatchery production is considered to be the only long-term sustainable option to reduce the fishing pressure on the wild population and also to safeguard the delicate coral reef ecosystem, which houses most of the marine ornamental species. Among the hatchery-produced fishes that are being traded, clown fishes form a dominant group, and a recent addition to this list is the designer clown fishes, which are costlier than other clown fishes due to their rare and attractive color patterns. However, ambiguities about designer clown fish such as its production (wild caught, captive bred, or genetically modified) and taxonomic identity still exist among the general public and hobbyists, as scientific reports on these aspects have not yet been published, even though few aquaculture companies display the photos of designer clown fishes in their websites. The common names for designer clown fishes (such as Platinum, Picasso, and snowflake) have been given by the aquaculture companies/traders solely based on the color patterns or designs on the fish body. The paper describes in detail the production of designer clown fish through captive breeding, followed by elucidating the taxonomic identity of two easily distinguishable designer clown fishes, viz., Picasso and Platinum. Both classical taxonomic tools and molecular methods were employed to elucidate the taxonomic identity. The morpho-merisitic characteristics of Picasso and Platinum were similar to those of Amphiprion percula. The partial cytochrome c-oxidase subunit I (COI) sequences of Picasso and Platinum clown fishes were submitted to the National Center for Biotechnology Information (NCBI) GenBank with accession numbers MT947238 and MT947239, respectively, which had maximum similarity to sequences of A. percula already deposited in the NCBI GenBank. The designer clown fishes exhibited protandrous hermaphroditism with monogamous mating behavior. Their egg incubation period ranged from 7 to 8 days, and the larval rearing was carried out with live feeds such as rotifers and Artemia under the greenwater technique using microalgae, Nannochloropsis oculata. This paper can thus unravel the ambiguities related to the production of designer clown fishes as well as their taxonomy.
Aquarium keeping is one of the most favorite hobbies of the world that have witnessed remarkable changes over the years, which led to the growth of the global ornamental fish trade as a multibillion-dollar industry. The value of the global ornamental fish trade is approximately US$15–30 billion (INFOFISH, 2021), which comprises both freshwater and marine ornamental species. The bulk of the trade is contributed by the freshwater species, whereas most of the marine ornamentals command a higher unit price over their freshwater counterparts. Furthermore, wild collected fishes continue to dominate the marine ornamental fish trade where the contribution from hatchery production is minimal (less than 10% of the total). Therefore, hatchery production is considered to be the only long-term sustainable option for the trade so as to reduce the fishing pressure on the wild population and also to protect the delicate coral reef ecosystem from where most of the marine ornamentals are caught.
Among the hatchery-produced marine ornamental fishes that are being traded, clown fishes form a dominant group, which comprises varieties such as percula or orange clown (Amphiprion percula), tomato clown (Amphiprion frenatus), false percula/ocellaris clown (Amphiprion ocellaris), skunk clown (Amphiprion periderion, Amphiprion akallopisosis, and Amphiprion sandaracinos), and maroon clown (Premnas biaculeatus). A recent addition to this list is the designer clown fishes, which are more costly than other clown fishes due to their rare and attractive color patterns (Figure 1). As rightly pointed out by Ricardo Calado (2017), we have entered an era of designer clown fishes, but there exist some ambiguities or apprehensions among the hobbyists and general public as regards the production of designer clown fishes, viz., whether these are wild caught, hatchery produced, or genetically modified. There have also been reports that these are genetically modified and hence their trade should not be promoted. Nevertheless, many of the leading aquaculture companies such as ORA—Ocean Reef & Aquarium (https://www.orafarm.com), S&R—Sea and Reef Aquaculture (https://www.seaandreef.com), and Proaquatix (https://www.proaquatix.com), display various types of designer clown fish in their websites. These aquaculture companies have produced many such color variations or mutations, and presently approximately 40 different color patterns are available in the trade with different names (Klann et al., 2021).
The common names/trade names assigned are sometimes specific to the company, and many times, the exact distinguishing characteristics of each variety are confusing or highly subjective. The common names used for different types of designer clown fishes in the trade include Platinum, Picasso, snowflake, snowbrite, and gladiator. An interesting fact is that these common names have been given by the traders or breeders solely based on the color patterns or designs on the fish body without taking into account the conventional taxonomic indices like morphometric or meristic characteristics. These common names keep changing when a new variety with a different color pattern is produced through cross-breeding or selective breeding. We carried out a literature survey to check for any scientific studies or reports on the captive breeding of designer clown fishes and the taxonomic identity of designer clown fishes, which did not yield any authentic publications. It was under these circumstances that the present study was undertaken with the following two objectives:
i. to elucidate the ambiguity with regard to the taxonomic identity of designer clown fishes Picasso and Platinum, which have easily distinguishable color patterns on the body and
ii. to standardize the captive breeding and seed production of designer clown fishes and publish the same for the benefit of all stakeholders of the marine ornamental fish trade so that the misconceptions about the production of designer clown fishes can be clarified.
Sub adults of designer clown fishes (3–4-cm length) procured from commercial outlets (a total of 50 fishes) were reared in fiber-reinforced plastic (FRP) tanks (2,000-L capacity) filled with clean and filtered seawater of 32–35 ppt salinity. They were fed to satiation with formulated pellet feed (Cadalmin-Varna™) with 38% protein, 9% fat, 39% carbohydrates, 7% ash, and less than 2% fiber. Every day in the morning, settled fecal matter and feed were removed by bottom siphoning after which complete water was exchanged daily. Adequate dissolved oxygen (4–5 ppm) in the rearing water was maintained by providing vigorous aeration. The fishes were grown to adult size (6–8 cm) after which they were transferred to breeding tanks.
The broodstock of the fishes has to be maintained at optimum water quality conditions free from pathogenic organisms in order to condition them for breeding. The natural reef conditions were simulated to make the fishes feel at home, which has been found to trigger their hormonal pathways for induction of spawning and egglaying. Hence, compatible pairs were selected from the adult fishes and were then introduced into glass tanks of 150-L capacity in such a way that only one pair was maintained in one tank. All the glass tanks were connected to a recirculating aquaculture system (RAS) as shown in Figures 2, 3. Lighting was provided by installing compact fluorescent lamp (CFL) bulbs of suitable wattage above each tank, which could be controlled by a timer to maintain the optimum photoperiod. Adequate aeration was maintained in the tanks with the help of a blower of sufficient capacity and aeration lines.
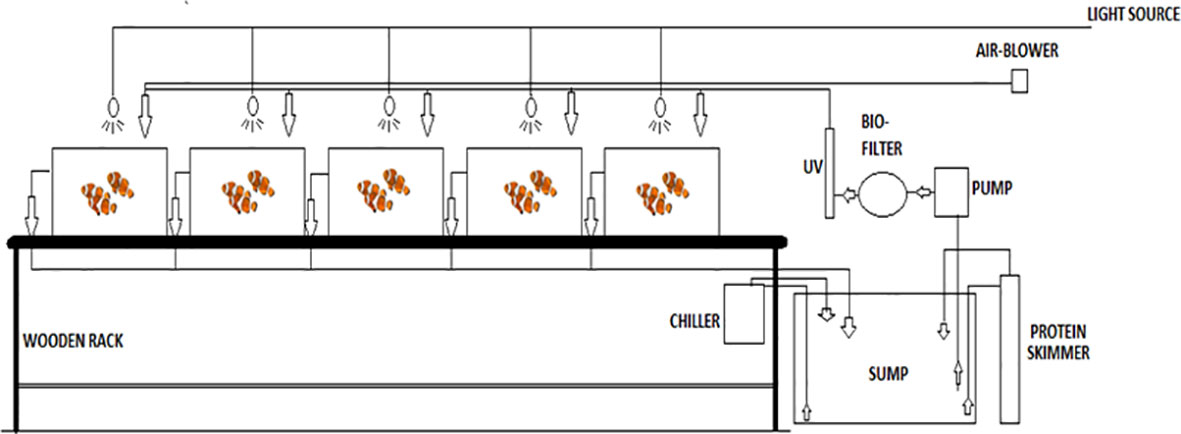
Figure 2 Schematic diagram of recirculating aquaculture system (RAS) for broodstock maintenance of designer clown fish.
As the designer clown fishes are substratum spawners, ceramic tiles or earthen pots were provided to facilitate the egg deposition. The male fertilized the eggs by its milt. The cylindrical eggs were found attached to the substratum at one end. The average number of eggs per clutch varied from 300 to 1,200 during the seed production trials. The color of freshly laid eggs was bright yellow (Figure 4), which finally became brownish black (Figure 5), and the eyes of the embryo could be clearly seen (Figure 6). The eggs hatched out after a period of 7 to 8 days, during which parental care was exhibited mostly by the male and occasionally by the female also (Figure 7). The parents were seen fanning the eggs, cleaning the area of any dirt or fungus, and removing dead eggs.
The eggs laid on the substratum were transferred carefully to hatching tanks on the day of hatching without taking the tile out of water. The hatching occurred in the evening hours after sunset. The ceramic tiles with the eggs were kept for hatching with mild aeration near the eggs.
The larvae hatched out in the evening hours after sunset and were fed on rotifers (Brachionus rotundiformis and Brachionus plicatilis) and copepod nauplii (Parvocalanus crassirostris) during the initial days followed by Artemia nauplii from 12 days post-hatch (DPH) onwards by following the green water technique wherein microalgae (Nannochloropsis occulata) were maintained at a cell density of 1 × 105 cells/ml. Weaning to a formulated feed of 0.5-mm size (Varna™, ICAR-CMFRI) started from 18 DPH onwards. The pellet size of the formulated feed was gradually increased from 0.5 to 0.75 mm and then to 1 mm, in line with the growth of the juveniles. The juveniles were further grown to a size of approximately 2- to 3-cm length so that the color patterns of each variety become expressed phenotypically. The photograph of larvae at different stages of growth is shown in Figures 8, 9. The detailed protocol for larval rearing is given in Table 1.
The designer clown fishes are given various names by the traders or breeders based on the phenotypical differences mostly differentiated by the color patterns, which become highly subjective in certain cases if there is very little difference in the color pattern between two varieties. Hence, in the present study, two varieties with highly distinctive color patterns (Picasso and Platinum) were selected so as to avoid any errors due to subjectivity. In the case of Picasso, the three white bands on the lateral side of the body do not maintain a clear banding pattern, and in most cases, the middle band expands and becomes joined with the band on the posterior side. In the case of Platinum, the body surface is completely white except for the fins, which may be either orange or black. The photograph of Picasso, Platinum, and percula clown is depicted in Figure 10. The representative samples from these two varieties were subjected to taxonomic studies. The conventional taxonomic tools, viz., morphometric and meristic characteristics, were recorded for both selected varieties of designer clown fishes, viz., Picasso and Platinum. The length measurements were carried out using a 30-cm-long plastic scale with an accuracy of 1 mm and also by using a digital Vernier caliper. The fin rays were counted under an inverted Stereo Zoom microscope (Carl Zeiss, Oberkochen, Germany).
The representative fin samples from the selected two varieties, Picasso and Platinum, were subjected to studies based on molecular tools. Total genomic DNA was isolated from the fin samples by the phenol/chloroform method. The cytochrome c-oxidase subunit I (COI) gene was amplified using universal primers FishF1-5′-TCAACCAACCACAAAGACATTGGCAC-3′ and FishR1-5′-TAGACTTCTGGG TGGCCAAAGAATCA-3′ (Ward et al., 2005) in a 50-µl reaction volume with 50–100 ng template DNA, 10 pmol of each specific primer, 0.2 mM of each dNTP, 0.75 units of Taq DNA polymerase, and 1× Taq buffer containing 1.5 mM of MgCl2. The PCR conditions were initial denaturation at 94°C for 3 min, followed by 35 cycles of 40 s at 94°C, 40 s at 54°C, 1 min at 72°C, and final extension at 72°C for 10 min. The PCR products were visualized on 1.5% agarose gel, and the amplicons were purified by a gel extraction kit (Genei, Bangalore, India) following the manufacturer’s protocol. The purified products were sequenced by Agrigenome Pvt. Ltd., Kochi, India. The COI partial gene sequences obtained were end-trimmed by b FinchTV to avoid sequencing errors. The sequence was blasted in the National Center for Biotechnology Information (NCBI) BLAST for sequence similarity and submitted to the NCBI GenBank. The phylogenetic tree was constructed by the maximum likelihood method using MEGA X.
The designer clown fishes exhibited a reproductive behavior similar to that of other members of the subfamily Amphiprioninae. Female was bigger in size than male, and monogamous pairs started laying eggs in a similar fashion to those of other clown fishes. The eggs were cylindrical in shape, and the incubation period ranged from 7 to 8 days. The average number of eggs per clutch varied from 300 to 1,200. Larvae were fed on live feeds such as rotifers and Artemia nauplii initially and later on weaned to formulated pellet feed. The survival rate from newly hatched larvae to juveniles ranged from 40% to 50%. A remarkable observation during the captive breeding was that a uniform phenotypic characteristic or color pattern was not present among the progeny produced from a pair of designer clown fish. The progeny is comprised of a mixture of various types of designer clown fishes in contrast to the case of other clown fishes such as percula or maroon clowns. For example, the progeny produced from a pair of percula clown fish would be all percula only, and that from a pair of tomato clown fish would be all tomato clown fish, whereas in the case of designer clown fish, the progeny from a pair of Picasso consisted of different types such as Picasso, Platinum, and snowflake. Furthermore, the distinguishing characteristics or color patterns in the designer clown fish become clearly visible after a period of about 2 months from hatching. The observations about the phenotype variations in the progeny produced from designer clown fish breeding have been recorded by us over a period of two years.
The taxonomic ambiguity was elucidated through both molecular methods and conventional taxonomic tools. The partial COI sequence of Picasso clown fish was submitted to the NCBI GenBank with the accession number MT947238. BLAST results indicated that the partial COI sequence of Picasso clown fish had 100% query coverage and 99.85% identity with sequences of A. percula already deposited in the NCBI GenBank. The partial COI sequence of Platinum clown fish was submitted to the NCBI GenBank with the accession number MT947239. BLAST results showed that the partial COI sequence of Platinum clown fish had 100% query coverage and 100% identity with sequences of A. percula already deposited in the NCBI GenBank. A phylogenetic tree was constructed using the maximum likelihood method in MEGA X (Figure 11). Similar tree topologies were obtained when the phylogenetic analysis was conducted using maximum parsimony, unweighted pair group method with arithmetic mean (UPGMA), and neighbor-joining methods. The tree was rooted in the COI sequence of Dascyllus aruanus.
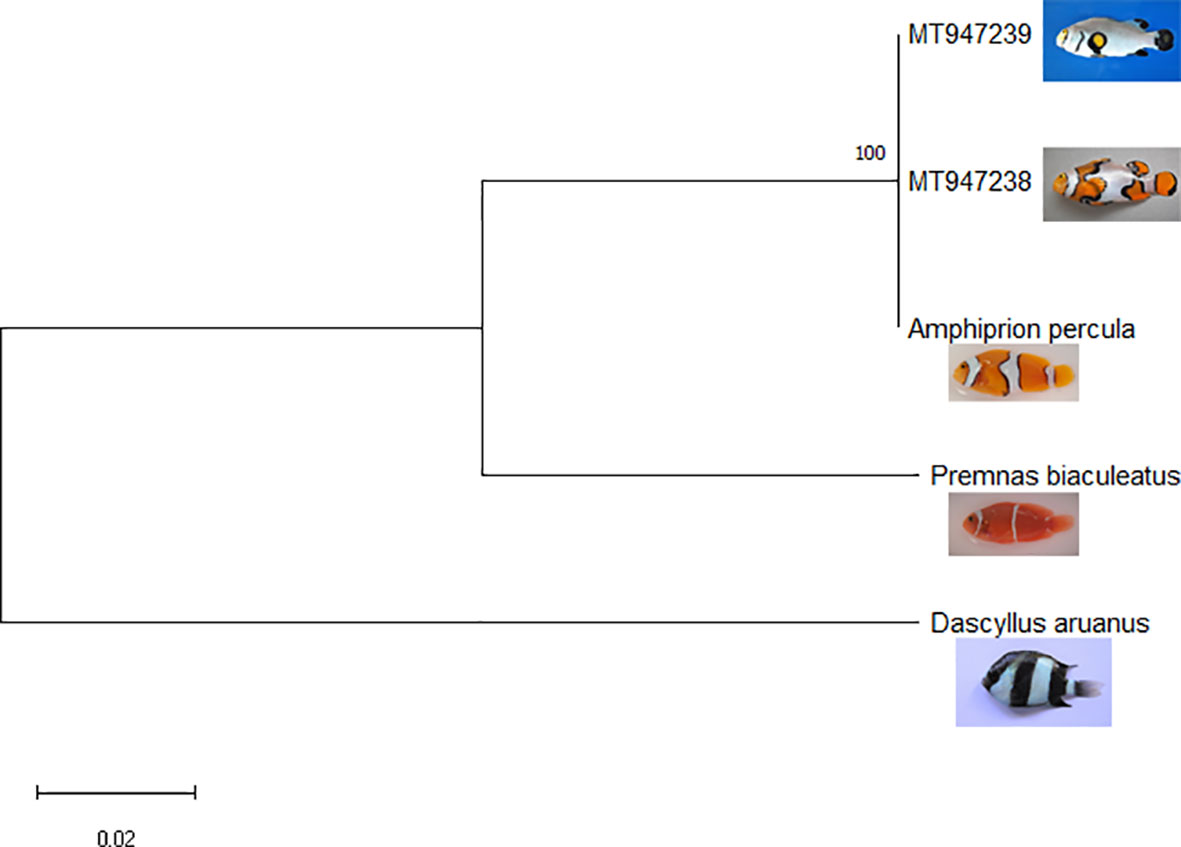
Figure 11 Maximum likelihood phylogenetic tree constructed using cytochrome c-oxidase subunit I (COI) sequences of designer clown fishes.
The molecular study revealed that both designer clown fishes had maximum similarity to A. percula. Hence, the conventional taxonomy was also carried out to reaffirm the identity of designer clowns. The morphometric and meristic characteristics of both Picasso and Platinum were recorded and then compared with those of A. percula specimens maintained in our hatchery along with the published data in FishBase (fishbase.org)1,2. The comparative analysis of morphometric and meristic characteristics is given in Table 2.
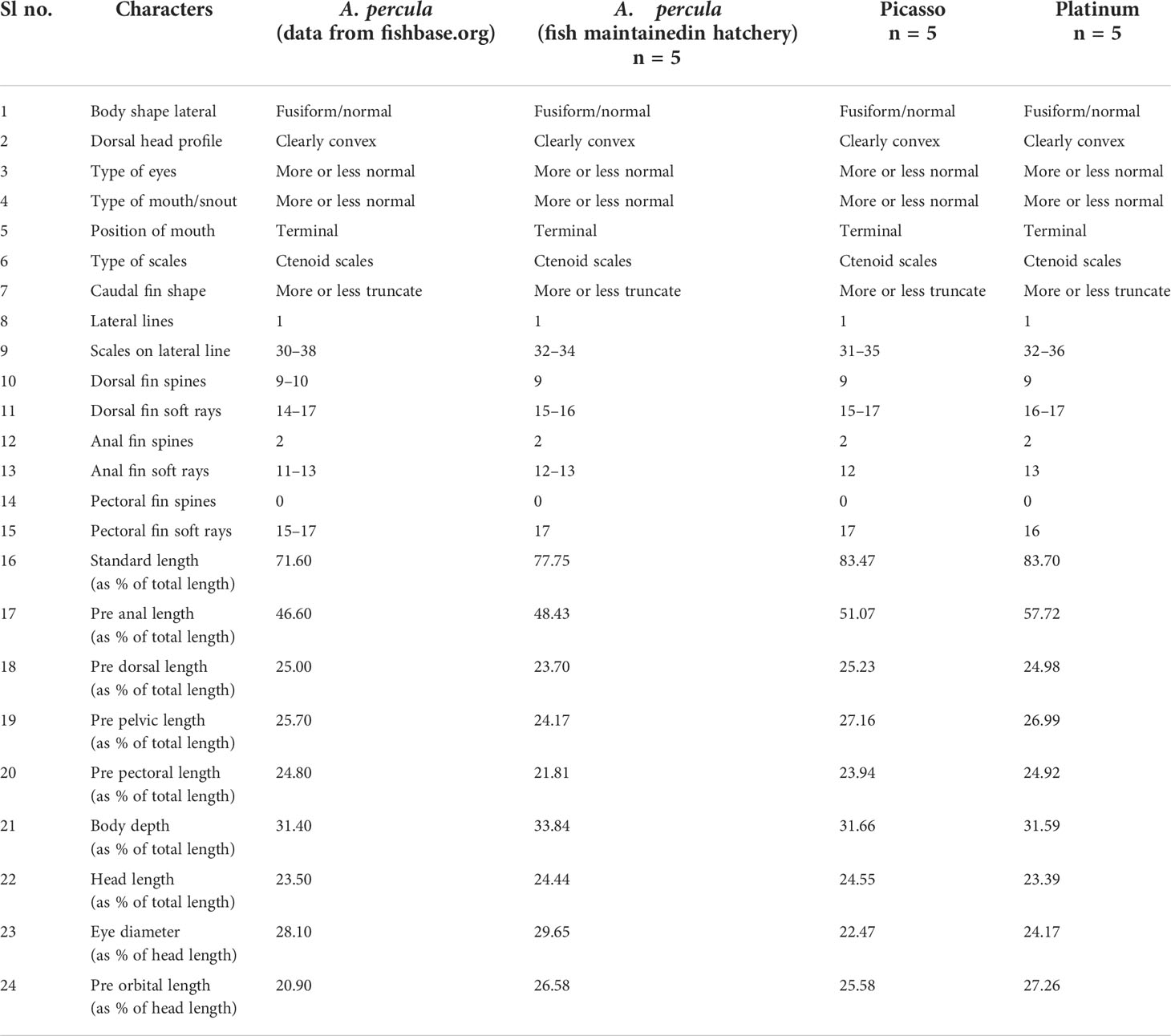
Table 2 Morphometric and meristic characteristics of designer clown fishes (Picasso and Platinum) and Amphiprion percula.
The members of the family Pomacentridae show different types of mating behaviors and reproductive strategies (Tang et al., 2021). The anemone fishes belonging to the subfamily Amphiprioninae under Pomacentridae are monogamous protandrous hermaphrodites (Fricke and Fricke, 1977; Fricke, 1979). They are oviparous in nature laying adhesive eggs on a substrate and display varying levels of parental care by either the male alone or both the parents (Breder and Rosen, 1966; Robertson, 1973; Blumer, 1979; Blumer, 1982). The reproductive strategy involves laying adhesive eggs by the female on a substratum, which will be fertilized externally by the male. The incubation period ranges from 7 to 8 days. Parental care (viz., fanning the eggs, cleaning the debris, removing dead, or weak eggs) is exhibited mostly by the male, but the female also joins the male sometimes.
In the case of designer clown fish also, the captive breeding technique followed was similar to that in other clown fishes, such as monogamous pairing and mating. The incubation period, hatching, and larval rearing methodologies were also similar to those of general clown fish breeding. However, the progeny produced from a single pair comprised of a mixture of various types of designer clown fishes. For example, from a pair of Picasso clown fish, the progeny would consist of Picasso, Platinum, snowflake, etc. We have studied the proportion of each variety in the progeny over a period of 2 years from different sets of parents, which will be published later.
Basically, among the subfamily Amphiprioninae (anemonefishes) comprising 30 species with two genera Amphiprion and Premnas (Roux et al., 2020), the interspecific variation is manifested mainly in the form of a number of white bars as well as in coloration of body and fins (Litsios and Salamin, 2014; Rolland et al., 2018; Salis et al., 2018). The designer clown fishes have not been described in earlier literature on clown fish systematics, since these were added to the trade in the recent past only. The conventional interspecific distinguishing characteristics described above cannot be applied in the case of designer clown fishes because of the presence of various types of body color patterns.
The analysis of morphometric and meristic characteristics shows that both Picasso clown fish and Platinum clown fish have similarities with A. percula in terms of the characteristics studied. Phenotypically, the designer clown fish differ from A. percula in terms of color and banding patterns. The typical banding pattern of A. percula, viz., three vertical white bands on the lateral body surface, is absent in designer clown fishes. Instead, they have rare and attractive banding patterns.
Body coloration or pigmentation is important for every animal either as a survival strategy (Klann et al., 2021) or for protection from UV radiation, reproductive success, or social interactions (Kelley et al., 2013; Dale et al., 2015; Marshall, 2000). The skin pigmentation in fish depends on the distribution of pigment cells called chromatophores, and the major teleost chromatophores are Melanophores, Xanthophores/Erythrophores, Iridophores, and Leucophores. During embryonic development, the chromatophore precursor/progenitor cells are established, which later on help to develop the color pattern in larvae or adults (Klann et al., 2021). Budi et al. (2011) have reported that in zebrafish (Danio rerio), there are two distinct precursors of chromatophores, one for melanophores and one for melano and iridophores, whereas in Medaka (Oryzias latipes), the xanthophore precursor is multipotent, capable of producing either xanthophores or closely related leucophores (Nagao et al., 2014). The studies on zebrafish and Medaka (as model fish) have helped to increase our knowledge of the color pattern formation in fishes. Among other new model fishes identified, the anemone fishes offer immense scope for research on color pattern formation (Roux et al., 2020; Salis et al., 2019). However, the anemone fishes are relatively new model organisms, and hence, scientific studies on the color variants or mutants have not yet been performed, even though many variants are available, which might have been produced from rare color variants captured from the wild and then by captive breeding (Klann et al., 2021).
Studies on the color patterns in anemone fishes are scanty. Salis et al. (2021) have demonstrated that in A. percula and A. ocellaris, the adult pigmentation pattern is controlled by thyroid hormones, and the main target of the thyroid hormones is the iridophores, responsible for white bar formation. In adult A. ocellaris, the body color pattern is formed by three chromatophore subtypes, viz., i) xanthophores interspersed with round melanophores, giving the orange skin color; ii) iridophores interspersed with stellar melanophores, giving the white color; iii) dense round melanophores produce the black color (Salis et al., 2019; Salis et al., 2019a; Salis et al., 2021). Klann et al. (2021) have demonstrated that it is possible to change the pattern of white bar formation in A. ocellaris by treating the juveniles with a chemical agent, TAE 684, which is an inhibitor of tyrosine kinase receptors alk and tlk, expressed in iridophores. It was also seen that the general development and survival were not affected by treatment with TAE 684. Thus, the possibility of altering the original body color pattern by using such chemical agents could be demonstrated. However, the mechanism behind the body color pattern of designer clown fishes is still not understood properly, and hence, further research efforts are needed to understand the various chromatophore subtypes involved and the cues for their expression in the designer clown fish.
The studies on altering the body color pattern of A. ocellaris, as well as the production of designer clown fish, show that external body color patterns cannot always be taken as a characteristic for identifying the species of a particular anemone fish. In the present study also, the scientific name of designer clown fishes Picasso and Platinum were found to be A. percula, whereas in body coloration patterns, they are entirely different from A. percula. This can be compared to the case of human beings, where Homo sapiens is the sole species name whereas external appearance varies considerably from individual to individual. This reaffirms the need for molecular tools to identify species names in the marine ornamental fish trade.
The commercial firms engaged in marine ornamental fish trade may have more designer clown fish varieties. This includes names such as gladiator, snowbrite, frostbite, snowstorm, black storm, wyoming white clown fish, premium snowflake, black snowflake, and black ice snowflake. These names are given solely based on the variations in the external color patterns, which at many times are highly subjective. It is also seen that certain websites display scientific names along with the trade names wherein either A. percula or A. ocellaris is given as the scientific name of the designer clown fish variety displayed on their website. However, the authenticity or scientific criteria for assigning such scientific names could not be verified.
It is very difficult to distinguish between A. percula and A. ocellaris (Tao et al., 2014) since both species look the same, and only with careful observation could one make out the difference. The morphological characteristics such as the number of dorsal fin spines and soft rays, anal fin spines and soft rays, pectoral fin soft rays, lateral line scales, and gill rakers are almost the same or in overlapping range for both species. For example, in the case of the number of dorsal fin spines, the range is 9–10 for A. percula and 10–11 for A. ocellaris. Hence, if there are 10 dorsal fin spines in a specimen, it becomes difficult to confirm whether it is A. percula or A. ocellaris. The one distinguishing characteristic normally used by breeders and traders is the difference in the width of the black border for the white vertical bands on the body surface. However, in the case of designer clown fish, this characteristic cannot be used since the banding pattern will be totally different. Hence, it will be difficult to assign a scientific name solely based on morphometric or meristic characters. Hence, molecular methods can also be used to elucidate such ambiguities. In our study, we found that the two designer clown fish varieties Picasso and Platinum showed maximum resemblance to A. percula in a molecular study, which was well supported by morpho-merisitic characteristics similar to A. percula. This observation is in line with the findings of He et al. (2020) describing the complete genome of Picasso clown fish, where they opined that Picasso clown fish is a variant of A. percula.
There have also been some misconceptions about designer clown fishes that they are produced through genetic engineering, and hence, its trade should not be encouraged. It has been reported that designer clown fishes occur in the wild as in the case of lightning maroon clown fish (Ricardo Calado, 2017). However, the numbers in wild are much less when compared to normal clown fishes. This may be because of predation, as the rare color patterns make them stay isolated from their normal counterparts and becomes more prone to attack by predators3. Nonetheless, with the commencement of captive breeding of designer clown fishes especially by the aquaculture companies, dependence on wild collections has decreased. However, the ambiguities about designer clown fishes regarding their mode of production (whether captive bred or genetically modified) and scientific nomenclature still exists among the general public and hobbyists, barring those who are closely associated with the trade. Hence, more studies in this line for elucidating the taxonomy as well as creating awareness about the captive breeding techniques of designer clown fish are necessary to popularize this variety of clown fish among consumers/hobbyists.
The global marine ornamental fish trade largely depends on wild-caught fishes, which in the long run is not advisable from a sustainability perspective; hence, captive breeding has been promoted to address this issue. The significance of designer clown fishes in the trade has been well established mainly due to their rare and attractive color patterns, as the ornamental fish trade is a variety-oriented one. The reproductive behavior of designer clown fish is similar to that of other anemone fishes, and the breeding technique has been developed because it is widely available in the trade. Designer clown fish is a general term that comprises different varieties known by different common names in the trade such as Picasso, Platinum, snowflake, snowbrite, and gladiator. These names are assigned solely based on differences in their body color patterns, which are highly subjective. In the present study, two clearly distinctive varieties, Picasso and Platinum, were chosen, and the ambiguity regarding their scientific name was elucidated through molecular and conventional taxonomic methods. The study revealed that both Picasso and Platinum have maximum similarity to A. percula, and hence, they can be regarded as a variant of A. percula. The findings of this study would help to understand more clearly about designer clown fishes and would also clarify the misconceptions about designer clown fish that these are genetically modified organisms, because the term designer clown fish is still not familiar to the general public, and only those who are closely associated with the trade knows about it. Thus, the false-negative propaganda about designer clown fish should be resolved, which would help the marine ornamental fish industry in the long run, as captive breeding and seed production are the ultimate solutions to ensure sustainable development of the trade.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, MT947238
https://www.ncbi.nlm.nih.gov/genbank/, MT947239.
The study involved recording of observations pertaining to reproduction and taxonomy of fishes.
KKA: Work Conceptualization, Breeding and manuscript preparation; PRK, AM, MSan, and MSak: Molecular studies; RB, NK, GNI, and NM: Conventional taxonomy studies; TT, GR, and MJ: Larval rearing and live feed culture; GT, AKA, RJ, BI, IJ, KM, and BJ: manuscript review; AG: Overall supervision. All authors contributed to the article and approved the submitted version.
We thank the Director, ICAR CMFRI, for the guidance and support as well as for the infrastructural facilities provided while conducting this study. We place on record our sincere gratitude to Dr Sandhya Sukumaran, CMFRI, Kochi, and Dr Suresh Babu PP, CMFRI, Karwar, for the critical scrutiny of the manuscript and the valuable suggestions. We also thank the following staff of CMFRI Mandapam for the help rendered during captive breeding and larval rearing of designer clown fishes: R. Suresh, K. Ganesan, J. Ramachandran, Mohamed Kaleem, Aneesh U., and M. Mahalingam.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Blumer L. S. (1982). A bibliography and categorization of bony fishes exhibiting parental care. Zoological J. Linn. Soc. 75, 1–22.
Breder C. M. Jr., Rosen D. E. (1966). Modes of reproduction in fishes (Garden City, New York: The Natural History Press).
Budi E. H., Patterson L. B., Parichy D. M. (2011). Post-embryonic nerve-associatedprecursors to adult pigment cells: genetic requirements and dynamics ofmorphogenesis and differentiation. PloS Genet. 7, e1002044.
Calado R. (2017). “The need for cultured specimens,” in Marine ornamental species aquaculture, 1st ed. Eds. Calado R., Olivotto I., Oliver M. P., Holt G.J. (Published by John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK), pp: 15–pp: 22.
Dale J., Dey C. J., Delhey K., Kempenaers B., Valcu M. (2015). The effects of life history and sexual selection on male and female plumage colouration. Nature 527, 367–370. doi: 10.1098/rstb.2005.1716
Fricke H. W. (1979). Mating system, resource defence and sex change in the anemonefish amphiprionakallopisos. Zeitschriftf¨urTierpsychologie 50, 313–326.
Fricke H. W., Fricke S. (1977). Monogamy and sex change by aggressive dominance in coral reef fish. Nature 266, 830.
He L.-b., Wu S.-q., Luo H.-y., Zheng L.-y. (2020). The complete mitochondrial genome of the Picasso clownfish: genomic comparisons and phylogenetic inference among amphiprioninae. Mitochondrial. DNA Part B 5, 2990 2991. doi: 10.1080/23802359.2020.1797554
INFOFISH (2021) in 3rd International Ornamental Fish Trade and Technical Virtual Conference. (ORNAMENTAL FISH -2021), Organised by INFOFISH and OFI. (http://ornamentalfish.infofish.org/images/pdf/3rd_ORNAMENTAL2021_brochure_fa.pdf).
Kelley J. L., Fitzpatrick J. L., Merilaita S. (2013). Spots and stripes: ecology andcolour pattern evolution in butterflyfishes. Proc. R Soc. B Biol. Sci. 280, 20122730.
Klann M, Mercader M, Carlu L, Hayashi K, Davis Reimer J, Laudet V (2021). Variation on a theme: pigmentation variants and mutants of anemone fish. EvoDevo 12, 8. doi: 10.1186/s13227-021-00178-x
Litsios G., Salamin N. (2014). Hybridisation and diversification in the adaptiveradiation of clownfishes. BMC Evol. Biol. 14, 245.
Marshall N. J. (2000). Communication and camouflage with the same “bright”colours in reef fishes. Philos. Trans. R Soc. Lond B Biol. Sci. 355, 1243–1248.
Nagao Y., Suzuki T., Shimizu A., Kimura T., Seki R., Adachi T., et al. (2014). Sox5 functions as a fate switch in medaka pigment celldevelopment. PloS Genet. 10, e1004246.
Robertson D. R. (1973). Field observations on the reproductive behaviour of a pomacentrid fish, acanthochromispolyacanthus. Zeitschriftf¨urTierpsychologie 32, 319–324.
Rolland J., Silvestro D., Litsios G., Faye L., Salamin N. (2018). Clownfishes evolutionbelow and above the species level. Proc. R Soc. B Biol. Sci, 285, 20171796. doi: 10.1098/rspb.2017.1796
Roux N., Salis P., Lee S.-H., Besseau L., Laudet V. (2020). Anemonefish, a model forEco-Evo-Devo. EvoDevo. 11, 20.
Salis P., Lorin T., Laudet V., Frederich B. (2019). Magic traits in magic fish:understanding color pattern evolution using reef fish. Trends Genet. 35, 265–278.
Salis P., Lorin T., Lewis V., Rey C., Marcionetti A., Escande M. L., et al. (2019a). Developmental andcomparative transcriptomic identification of iridophore contribution towhite barring in clownfish. Pigment. Cell Melanoma. Res. 32, 391–402.
Salis P., Roux N., Huang D., Marcionetti A., Mouginot P., Reynaud M., et al. (2021). Thyroid hormonesregulate the formation and environmental plasticity of white bars inclownfishes. PNAS. (23):e2101634118. doi: 10.1073/pnas.2101634118
Salis P., Roux N., Soulat O., Lecchini D., Laudet V., Frederich B. (2018). Ontogeneticand phylogenetic simplification during white stripe evolution in clownfishes. BMC Biol. 16, 90.
Tang K. L., Melanie L. J., Richard L. (2021). Systematics of damselfishes. Ichthyol. Herpetology109 109 (1), 258–318.
Tao Y., Li J.-L., Liu M., Hu X.-Y. (2014). Complete mitochondrial genome of the orange clownfish Amphiprionpercula(Pisces: Perciformes, Pomacentridae)Mitochondrial DNA (Informa UK Ltd). doi: 10.3109/19401736.2014.892099
Keywords: designer clown fish, marine ornamental fish, DNA sequence, taxonomy, Picasso, Platinum
Citation: Anikuttan KK, Rameshkumar P, Nazar AK, Jayakumar R, Tamilmani G, Sakthivel M, Sankar M, Bavithra R, Johnson B, Krishnaveni N, Mercy AA, Moulitharan N, Narasimapallavan GI, Thomas T, Rao GH, Jayasingh M, Joseph I, Ignatius B, Madhu K and Gopalakrishnan A (2022) Designer clown fishes: Unraveling the ambiguities. Front. Mar. Sci. 9:907362. doi: 10.3389/fmars.2022.907362
Received: 29 March 2022; Accepted: 14 July 2022;
Published: 06 September 2022.
Edited by:
Archana Sinha, Central Inland Fisheries Research Institute (ICAR), IndiaReviewed by:
Vaneet Inder Kaur, Guru Angad Dev Veterinary and Animal Sciences University, IndiaCopyright © 2022 Anikuttan, Rameshkumar, Nazar, Jayakumar, Tamilmani, Sakthivel, Sankar, Bavithra, Johnson, Krishnaveni, Mercy, Moulitharan, Narasimapallavan, Thomas, Rao, Jayasingh, Joseph, Ignatius, Madhu and Gopalakrishnan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kuttan Kuravamparambu Anikuttan, ZHJhbmlrdXR0YW5AZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.