- 1Department of Biology, University of Louisiana at Lafayette, Lafayette, LA, United States
- 2Instituto de Biociências, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil
- 3Ifremer, Centre Atlantique Ecosystèmes Microbiens et Molécules Marines pour les Biotechnologies (EM3B) Laboratory, Rue de l’Île d’Yeu, Nantes, France
- 4Centro de Ciências Biológicas, Departamento de Botânica, Universidade Federal de Santa Catarina, Florianópolis, Brazil
- 5School of Science and Technology (FCT), Research Center in Biodiversity and Genetic Resources (CIBIO), University of the Azores, Ponta Delgada, Portugal
In the past, non-geniculate coralline algae in the northwestern Gulf of Mexico have been identified based primarily on comparative morpho-anatomy. Recent studies employing DNA sequencing techniques combined with morpho-anatomical studies using SEM have revealed a wealth of previously undocumented diversity of rhodolith-forming non-geniculate coralline algae in the Corallinales, Hapalidiales and Sporolithales from mesophotic hard bank communities at 45-90 meters depth. Although many advances in the last decade have been made in clarifying species names and describing new species of corallines from offshore Louisiana and Texas, total diversity estimates are still incomplete and many species remain to be described. Collections from offshore Louisiana at Parker Bank in the newly expanded Flower Garden Banks National Marine Sanctuary yielded thin, finely branched rhodoliths. DNA sequence analyses of plastid-encoded psbA and rbcL loci, and nuclear-encoded LSU rDNA of these rhodolith-forming specimens revealed that some belong to an unnamed species of Sporolithon (Sporolithales) that we herein newly describe. Additionally, comparative DNA sequence analyses of rhodolith collections from Ewing Bank and other hard banks offshore Louisiana were conducted to assess rhodolith diversity in these mesophotic communities. The results revealed new reports of taxa for the region, including new rhodolith-forming species of Roseolithon (Hapalidiales) to be described herein as well. Our new biodiversity findings will be compared with historical studies from the NW Gulf of Mexico.
Introduction
Previously, non-geniculate coralline algae (also known as crustose coralline algae or CCA) in the northwestern Gulf of Mexico have been identified based on morpho-anatomy (Minnery et al., 1985; Rezak et al., 1985; Minnery, 1990; Fredericq et al., 2009; Fredericq et al., 2014). Recent studies employing DNA sequencing techniques combined with morpho-anatomical studies have revealed a wealth of previously undocumented diversity of non-geniculate coralline algae in the Corallinales (Richards et al., 2014; Richards et al., 2021), Hapalidiales (Krayesky-Self et al., 2016; Richards et al., 2016; Richards et al., 2020) and Sporolithales (Richards and Fredericq, 2018; Fredericq et al., 2019; Richards et al., 2019) in the region.
Recently, Richards et al. (2017) performed comparative analyses of DNA sequences including sequences of type specimens and topotype specimens of Sporolithon spp., as well as morpho-anatomical studies using images generated with SEM. This foundational study clarified species names and helped resolve taxonomic problems in the order Sporolithales (Richards et al., 2017) and provided a foundation for describing new species of Sporolithon Heydrich from mesophotic habitats in the northwestern Gulf of Mexico, Brazil, and Bermuda, including Sporolithon sinusmexicanum J.L.Richards & Fredericq (Richards and Fredericq, 2018), Sporolithon amadoi J.L.Richards & R.G.Bahia (Richards et al., 2019), Sporolithon franciscanum L.A.S.Leão & R.G.Bahia (Leão et al., 2020) and Sporolithon mesophoticum J.Richards, P.W.Gabrielson & C.W.Schneider (Richards et al., 2018b). More recent advancements in Sporolithales taxonomy include the description of a new genus and species, Roseapetra farriae W.A.Nelson, Twist & K.F.Neil, a currently monotypic genus from New Zealand that includes the taxon previously treated as Heydrichia woelkerlingii R.ATownsend, Y.M.Chamberlain & Keats (Nelson et al., 2021).
Regarding the Hapalidiales, Richards et al. (2016) reported six species of Lithothamnion Heydrich from the northwestern Gulf of Mexico and demonstrated that Lithothamnion is a polyphyletic taxon. However, at the time of the 2016 study, there was a lack of DNA sequences available from the generitype species, L. muelleri, thus determining which clade corresponded to the true Lithothamnion was not possible at that time. Recently, the lectotype specimen of L. muelleri was sequenced (Jeong et al., 2021), which helped clarify the identification of the true Lithothamnion. The study by Jeong et al. (2021) in turn formed the foundation for describing a new genus, Roseolithon L.M.Coutinho and Barros-Barreto, which accommodated taxa previously included in the genus Lithothamnion. In their study, Coutinho et al. (2021) also described seven new species of Roseolithon.
Although many advances have been made in describing and clarifying the taxonomy of non-geniculate coralline algae offshore the northwestern Gulf of Mexico, many species remain to be described and further taxonomic issues remain unresolved. Herein, we describe the newly collected Sporolithales taxon from mesophotic depth at Parker Bank offshore Louisiana, as a new species of Sporolithon from offshore the northwestern Gulf of Mexico in the newly expanded Flower Garden Banks National Marine Sanctuary. Additionally, the diversity of the genus Roseolithon from offshore Louisiana will be assessed, and we herein describe three new species of Roseolithon from the northwestern Gulf of Mexico.
Materials and Methods
Specimen Collection
Mesophotic specimens were collected aboard the R/V Pelican, the UNOLS (University National Oceanographic Laboratory System) research vessel stationed at LUMCON (Louisiana Universities Marine Consortium), using an hourglass design box dredge (Joyce and Williams, 1969) with minimum tows (usually 10 minutes or less) from offshore Louisiana in the Gulf of Mexico in the vicinity of Parker Bank (27˚ 58.189’ N; 92˚ 02.80’ W) at 92 m. depth. The rhodolith specimens were preserved in silica gel. Collection date was May 20, 2019, prior to the expansion of the Flower Garden Banks National Marine Sanctuary (the location was not part of the Marine Sanctuary at the time of collection). Additional newly collected Hapalidiales and Sporolithales specimens collected in the vicinity of Ewing Bank in May 2018-2019 were also included for comparative analyses, as well as a specimen collected from December 2010 following the Deepwater Horizon oil spill and a specimen collected from Campeche Bank in 2005. Specimens are housed at the University of Louisiana at Lafayette Herbarium (LAF). Herbarium abbreviations follow Thiers (2022, continuously updated). Supplementary Table S1 provides a list of specimens and voucher information for taxa included in the analyses.
DNA Extraction and Sequencing
DNA was extracted from the newly collected specimens using the Quick-DNA Plant/Seed Miniprep Kit (Zymo Research, Irvine, CA, USA) and also using GenCatch™ Plant Genomic DNA Purification Kit (Epoch Life Science Inc., Missouri City, TX, USA). Markers chosen for PCR and sequencing included the plastid-encoded genes psbA (encodes photosystem II reaction center protein D1 gene) and rbcL (encodes the large subunit of the enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase), and the nuclear-encoded LSU (partial 28S rDNA). PCR was performed following the protocols and primers described in Richards et al. (2014). PCR products were cleaned by the addition of 2 µl of ExoSAP-IT™ (USB, Cleveland, Ohio) per 5 µl of amplified DNA product. Reactions were incubated at 37°C for 15 min, followed by inactivation of ExoSAP-IT™ at 80°C for 15 min. Purified PCR products were subsequently cycle-sequenced using the BrightDye® Terminator Cycle Sequencing Kit (Molecular Cloning Laboratories [MCLAB], South San Francisco, CA, USA). Resulting cycle sequence reactions were purified with ETOH/EDTA precipitation and were sequenced in-house at the UL Lafayette campus on an ABI Model 3130xl Genetic Analyzer. The resulting chromatograms were assembled and edited using Sequencher 5.1 (Gene Codes Corp., Ann Arbor, MI, USA) and exported as individual “.FASTA” files. Newly generated sequences were accessed in GenBank (https://www.ncbi.nlm.nih.gov/genbank/) (Supplementary Table S1).
Phylogenetic Analysis
Single loci analyses were conducted for psbA, rbcL, and LSU. Available sequences were downloaded from GenBank (Supplementary Table S1) and aligned with newly generated sequences. Alignment was performed using MUSCLE in MEGA X (Stecher et al., 2020). Exploratory analyses were conducted in MEGA X using the Maximum Likelihood method and Tamura-Nei model (Tamura and Nei, 1993; Stecher et al., 2020). Final Maximum Likelihood analyses were conducted with CIPRES Science Gateway (Miller et al., 2010) using the RAxML-HPC2 program on XSEDE with 1,000 alternative runs on distinct starting trees and 1,000 bootstrap replicates.
Sequence Divergence Analysis
Single loci alignments of psbA and rbcL were constructed for Sporolithon spp. and Roseolithon spp. Alignments were cropped at the 5’ and 3’ ends to minimize missing data. For Sporolithon spp., a 472 bp alignment and a 366 bp alignment was constructed for psbA and rbcL, respectively. For Roseolithon spp., a 716 bp alignment was constructed for psbA, and both long and short alignments were constructed for rbcL and analyzed separately. The long rbcL alignment was 623 bp and did not include the sequence of LAF 7384; the short rbcL alignment included the sequence of LAF 7384 and was 366 bp in length. Sequence divergence values were calculated as the number of pairwise base pair differences in MEGA X (Kumar et al., 2018) and presented as a percentage (the number of base pair differences divided by the alignment length).
Microscopy
Thallus fragments were removed from the same specimens that were DNA-sequenced and fractured using a single edge razor blade. Specimens were mounted and viewed according to the protocol of Richards et al. (Richards et al., 2017; Richards et al., 2018b) using a Hitachi S-3000N Scanning Electron Microscope (SEM), and also using a Scios 2 Dual Beam Focused Ion Beam scanning electron microscope (FIB-SEM) at an accelerating voltage of 15 kV.
Results
The psbA and rbcL analyses (Figures 1, 2) showed a wealth of previously reported and newly reported diversity of non-geniculate corallines in the northwestern Gulf of Mexico, including 17 species. In the Sporolithales, two previously reported Sporolithon species were shown in the analyses, as well as a third species of Sporolithon that is described herein as a new species. In the Hapalidiales, the analyses revealed a newly reported range extension for Roseolithon purii, and showed that three taxa from the northwestern Gulf of Mexico previously reported as Lithothamnion spp. are members of Roseolithon as well, and are herein described as new species. The analyses also showed three species of Lithothamnion and Mesophyllum erubescens (Foslie) Me.Lemoine are present in the northwestern Gulf of Mexico. In the Corallinales, three species of Harveylithon A.Rösler, Perfectti, V.Peña & J.C.Braga were shown from the northwestern Gulf of Mexico, one species of Lithophyllum Philippi, and two species of “Titanoderma” Nägeli.
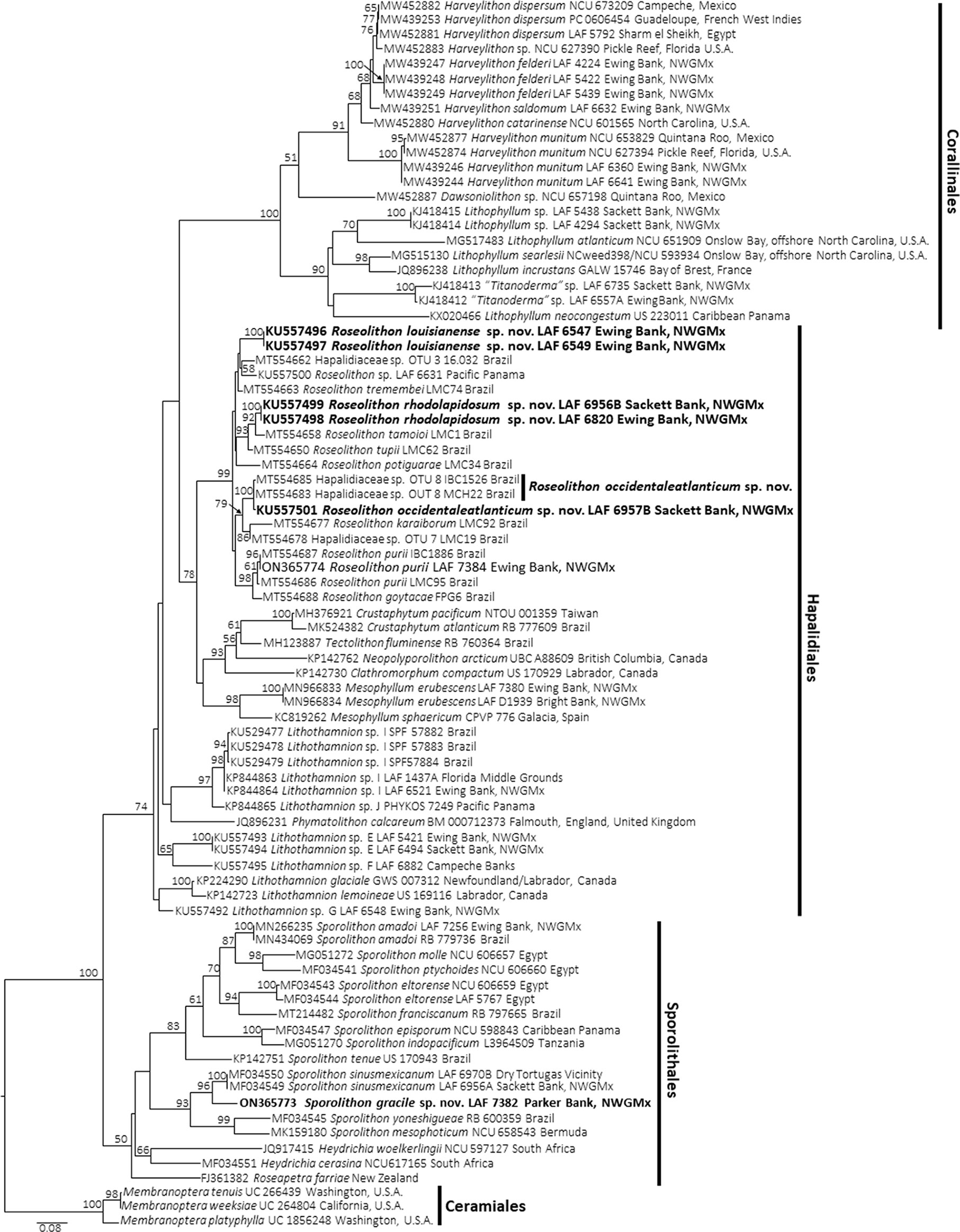
Figure 1 Maximum Likelihood analyses of psbA. Node values represent bootstrap values out of 1,000 replicates. Newly described species shown in bold. NWGMx, northwestern Gulf of Mexico.
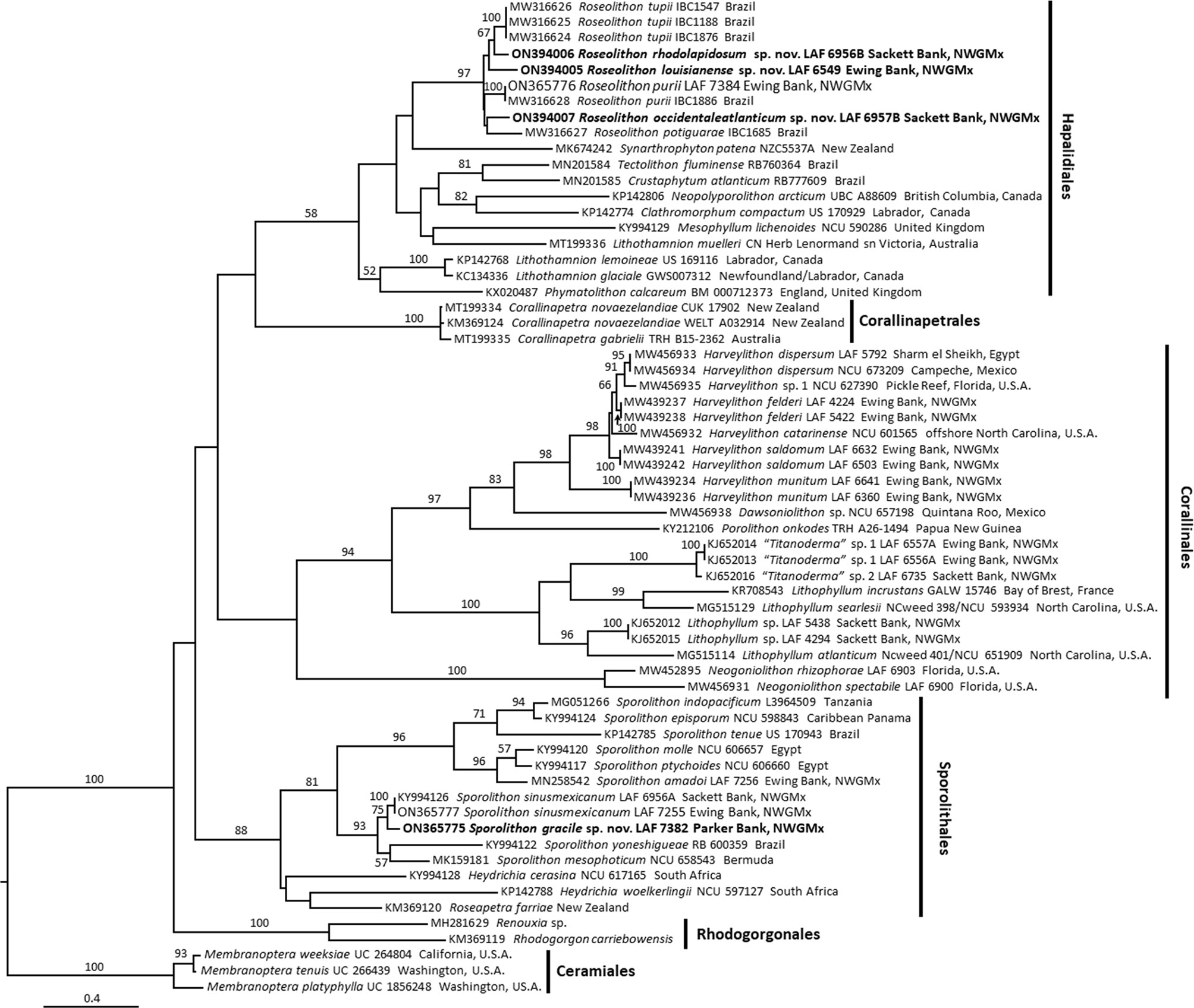
Figure 2 Maximum Likelihood analyses of rbcL. Node values represent bootstrap values out of 1,000 replicates. Newly described species shown in bold. NWGMx, northwestern Gulf of Mexico.
LSU analyses (Figure 3) showed at least three species of Sporolithon and a topology similar to the psbA and rbcL trees, with Sporolithon gracile J.Richards, Kittle & Fredericq sp. nov. (described below) sister to S. sinusmexicanum. The LSU tree revealed a putative fourth species of Sporolithon as well. This putative fourth species of Sporolithon included only one specimen, LAF 6726, that was sister to Sporolithon amadoi. The LSU tree also showed four clades of Roseolithon, that have a similar topology as shown in the psbA and rbcL trees.
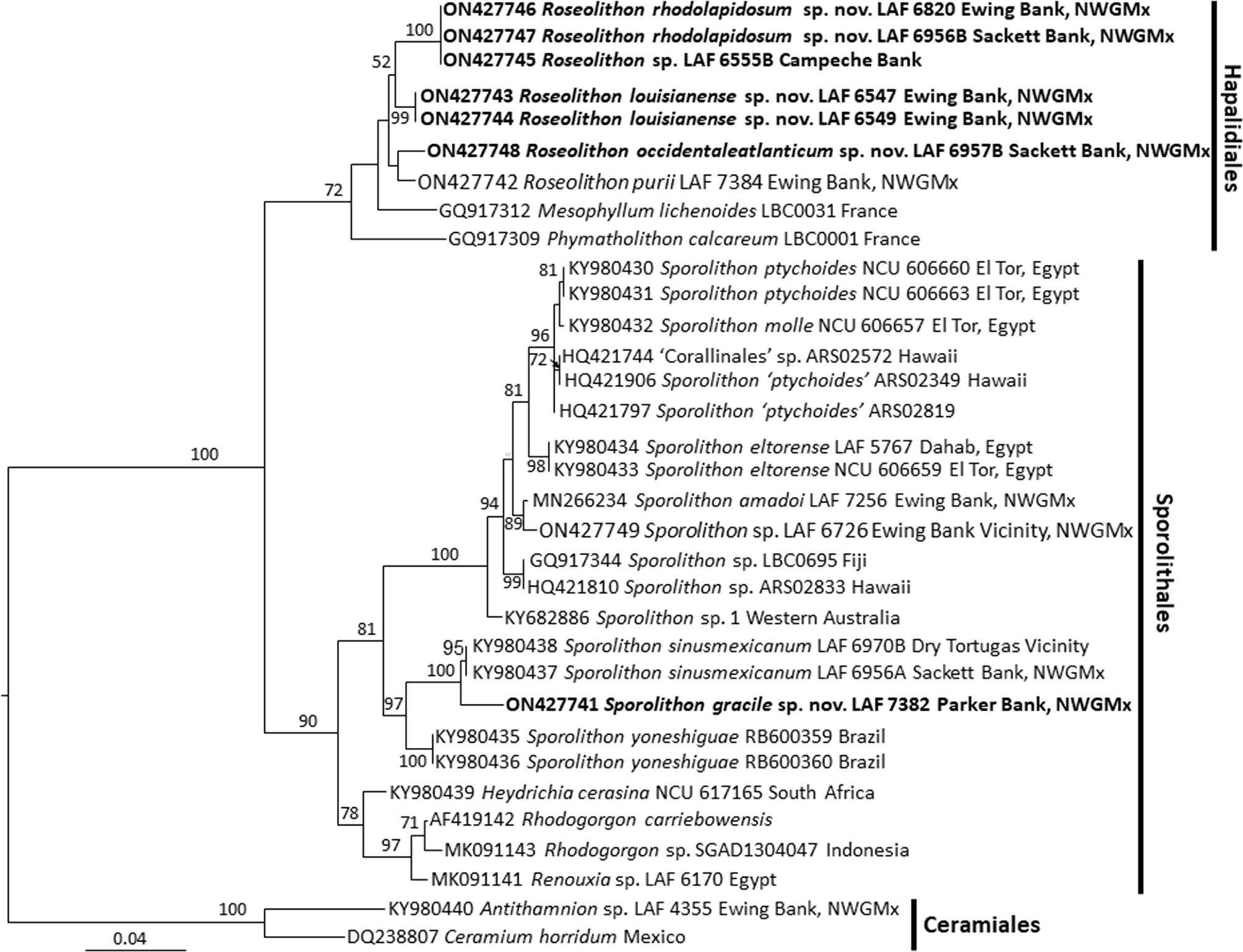
Figure 3 Maximum Likelihood analyses of LSU. Node values represent bootstrap values out of 1,000 replicates. Newly described species shown in bold. NWGMx, northwestern Gulf of Mexico.
The psbA sequence of S. gracile was 3.39% diverged from S. sinusmexicanum, and 6.99% and 7.63% diverged from S. yoneshigueae Bahia, Amado-Filho, Maneveldt & W.H.Adey and S. mesophoticum, respectively (Supplementary Table S2). The rbcL sequence of LAF 7255 was identical to the holotype sequence of S. sinusmexicanum, LAF 6956A (Supplementary Tables S3). The rbcL sequence of S. gracile was 2.46% diverged from sequences of S. sinusmexicanum and 11.46% and 8.47% diverged from sequences of S. yoneshigueae and S. mesophoticum, respectively (Supplementary Table S3). The psbA and rbcL sequences of S. gracile diverged 9.7% -12.3% from Sporolithon amadoi (Supplementary Tables S2, S3).
The psbA sequence of LAF 7384 was 0.0%-0.4% diverged from psbA sequences of Brazilian specimens of Roseolithon purii L.M.Coutinho & Barros-Barreto (Supplementary Table S4). Interspecific divergence for psbA sequences of Roseolithon spp. ranged from 1.49% - 5.46%. The rbcL sequence of LAF 7384 was identical to the sequence of Roseolithon purii IBC1886 from Brazil (Supplementary Table S5). Interspecific divergence for rbcL sequences of Roseolithon spp. ranged from 2.73% - 6.10% (Supplementary Table S6).
Sporolithon gracile J.L.Richards, Kittle & Fredericq sp. nov.
Holotype: LAF 7382 (field ID no. 5-20-19-5-1): Parker Bank, offshore Louisiana, U.S.A. (27˚ 58.180’ N; 92˚ 02.80’ W), Gulf of Mexico, western Atlantic Ocean, 20.v.2019, depth 92m, leg. S. Fredericq, R. P. Kittle III, W. E. Schmidt.,
Etymology: The specific epithet refers to the slender form of the protuberances that this rhodolith species is comprised of.
Description
DNA sequences: psbA and rbcL sequences (GB accessions ON365773, ON365775) diagnostic for this species. LSU sequence is also provided (GB accession ON427741) (Supplementary Table S1).
Habit and vegetative anatomy (Figures 4, 5): Thallus non-geniculate, forming free-living biogenic rhodoliths that consist entirely of thin, branching protuberances. The habitat of this species is mesophotic rhodolith beds at a depth of 92 m. Protuberances with radial construction. Secondary hypothallus weakly developed with monomerous construction and 1-2 layers of rectangular hypothallial cells that formed new growth layers. Perithallus with cell fusions; secondary pit connections not observed. Perithallial cells 9-28 µm long x 8-15 µm wide. Pseudodichotomous branching was observed in the perithallus that contributed to protuberance widening. Intercalary meristem was observed with meristematic cells 5-9 µm long x 9-15 µm wide. Epithallus consisted of one layer of armored epithallial cells 2.9-4 µm long x 5-7 µm wide, with thick, heavily calcified cell walls and epithallial cell roof that was observed intact in some cells and missing in others.
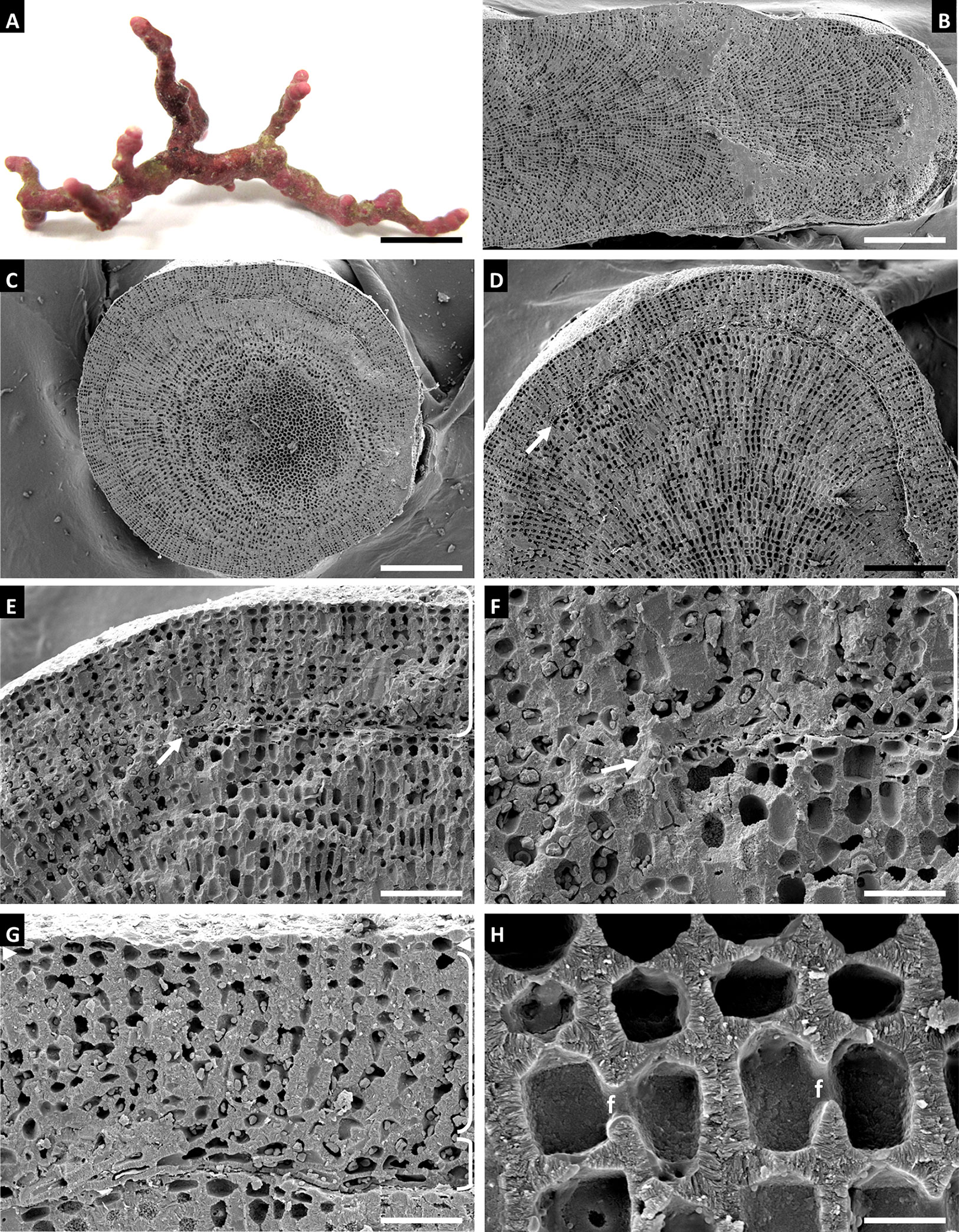
Figure 4 Sporolithon gracile Holotype, voucher no. LAF 7382 (A–H). (A) Habit of holotype. Scale bar = 0.4 cm. (B) Longitudinal section of thallus protuberance showing radial construction. Scale bar = 350 µm. (C) Cross section of thallus protuberance showing radial construction. Scale bar = 335 µm. (D) Longitudinal section showing origin of the new growth layer (arrow) over older portion of thallus. Scale bar = 225 µm. (E, F) Magnified views showing detail of new growth layer (arrow, bracket) over older portion of thallus. Scale bars = 90, 35 µm. (G) Detail of new growth layer showing secondary hypothallus with monomerous construction (lower bracket), perithallus (upper bracket), and intercalary meristem (arrowheads) over older thallus layer. Scale bar = 45 µm. (H) Perithallus with cell fusions (f). Scale bar = 11 µm.
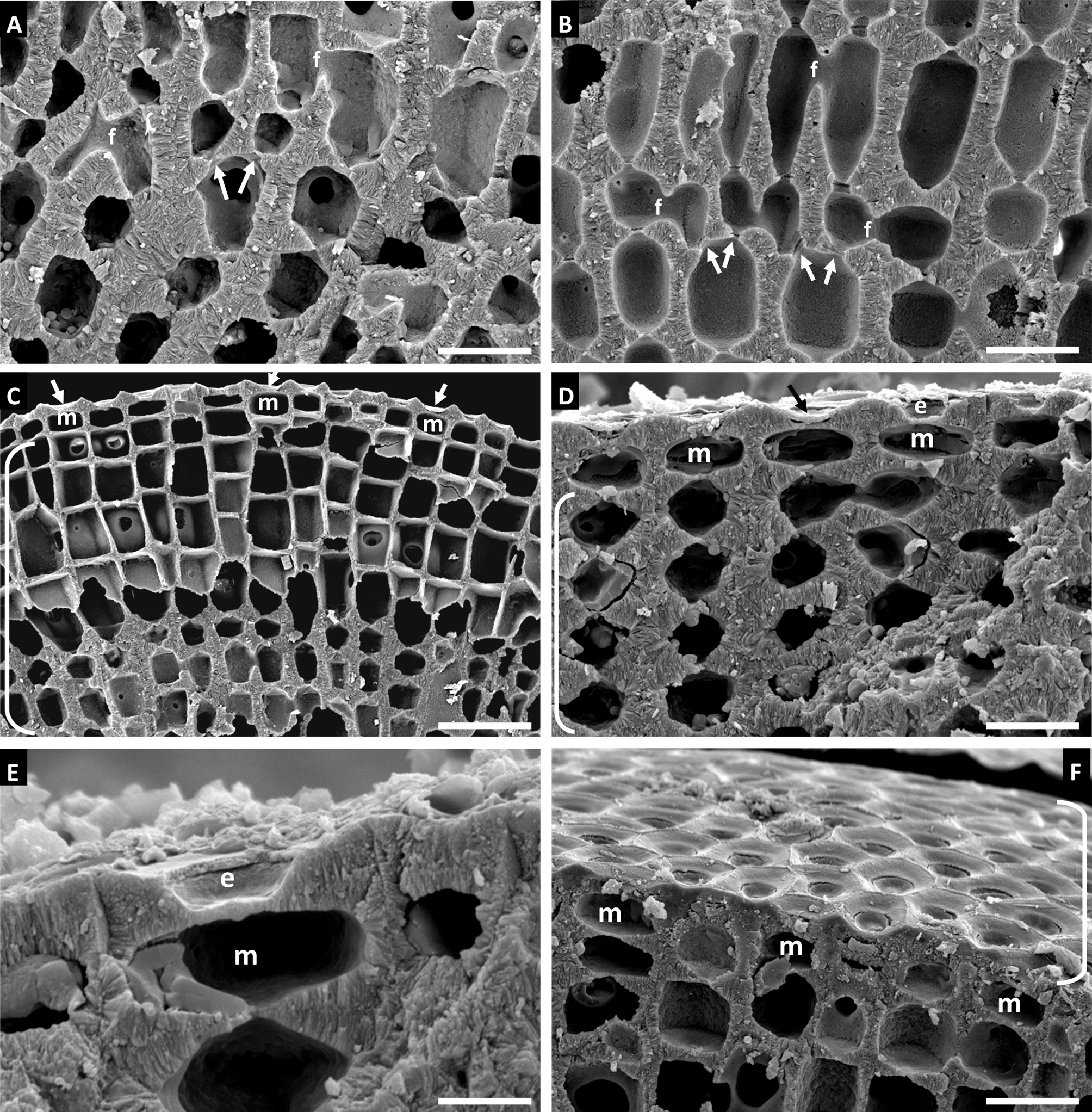
Figure 5 Sporolithon gracile Holotype, voucher no. LAF 7382 (A–F). (A, B) Perithallus showing locations of pesudodichotomous branching (arrows) and cell fusions (f). Scale bars = 18 µm. (C) Perithallus (bracket), meristematic cells (m), and epithallial cells (arrows). Scale bar = 37 µm. (D) Epithallus showing epithallial cells with roof intact (arrow) and with roof missing (e), intercalary meristematic cells (m), and perithallus (lower bracket). Scale bar = 15 µm. (E) Magnified view of epithallial cell lacking epithallial cell roof (e) and intercalary meristematic cell (m). Scale bar = 5.5 µm. (F) Surface view of epithallial cells (bracket) and partial section view showing intercalary meristematic cells (m). Scale bar = 18 µm.
Reproduction: No reproductive structures were observed.
Distribution: Known only from Parker Bank, offshore Louisiana, U.S.A.
Sporolithon sinusmexicanum J.L.Richards & Fredericq
DNA sequences: an rbcL sequence is herein provided for the newly collected specimen of this species (voucher no. LAF 7255, GB accession ON365777) (Supplementary Table S1).
Habit and vegetative anatomy (Figure 6): The specimen examined in this study was a biogenic rhodolith found growing inside of a marine sponge from mesophotic rhodolith beds at a depth of 75 m. Specimen was non-reproductive with a perithallus showing cell fusions and lacking secondary pit connections, an intercalary meristem, and an epithallus with armored epithallial cells.
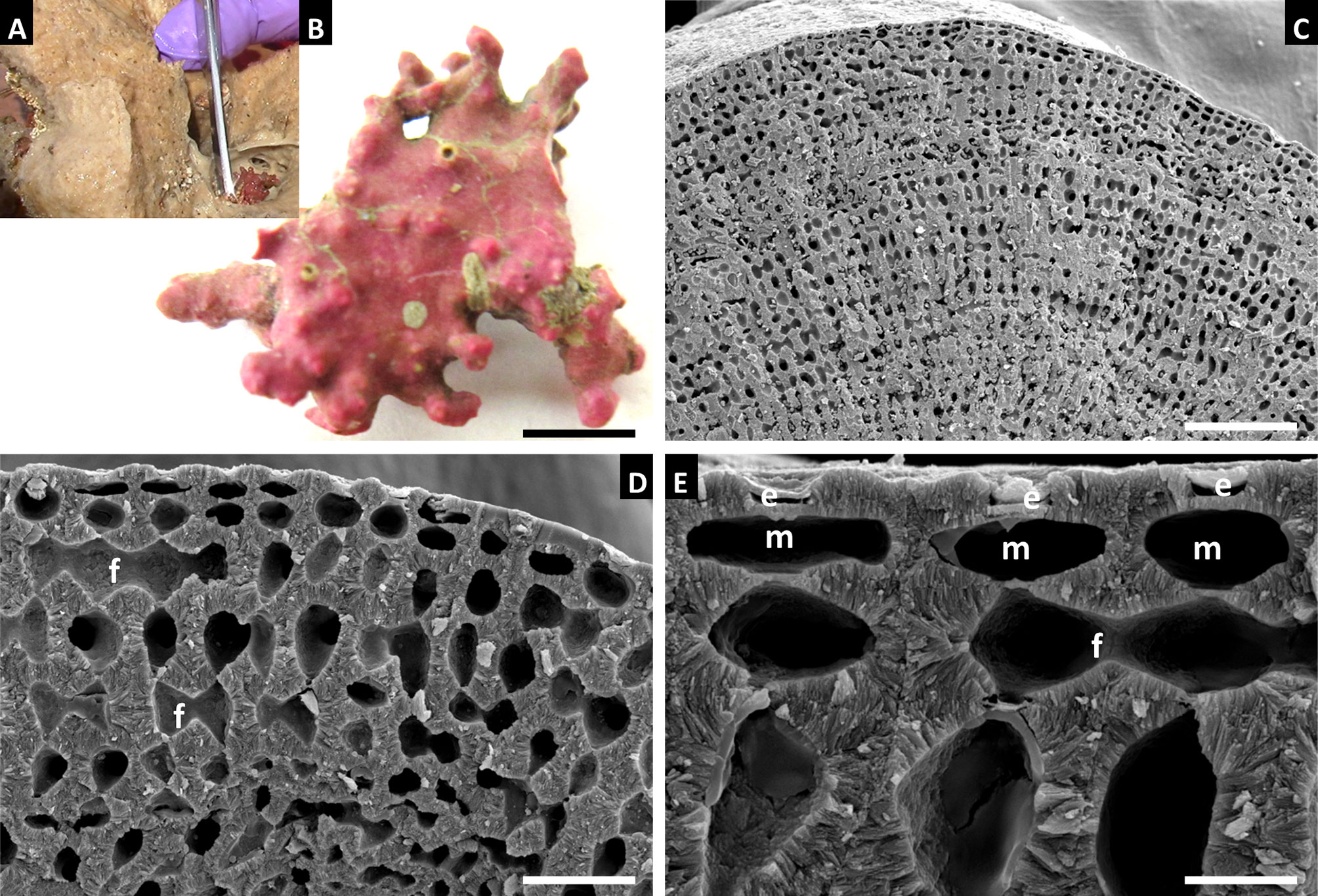
Figure 6 Sporolithon sinusmexicanum, voucher no. LAF 7255 (A-E). (A) Thallus habit in situ growing inside of a marine sponge. (B) Thallus habit. Scale bar = 0.4 cm. (C) Longitudinal section of rhodolith protuberance. Scale bar = 110 µm (D) Magnified view of longitudinal section of protuberance showing cell fusions between perithallial cells (f). Scale bar = 25 µm (E) Magnified view of epithallial cells (e), some with intact epithallial cell roofs, intercalary meristematic cells (m), and cell fusions between perithallial cells (f) Scale bar = 7 µm.
Distribution: Ewing Bank and Sackett Bank, northwestern Gulf of Mexico, and the vicinity of the Dry Tortugas, southeastern Gulf of Mexico.
Sporolithon sp.
DNA sequence: An LSU sequence (GB accession ON427749) is provided for this taxon (Supplementary Table S1).
Habit and vegetative anatomy (Figure 7): This species was found epizoic on a Naria acicularis (Gmelin, 1791) shell. Hypothallus was incompletely shown in section view. Perithallus with cells linked by both secondary pit connections and cell fusions. Epithallus consisted of one layer of armored epithallial cells.
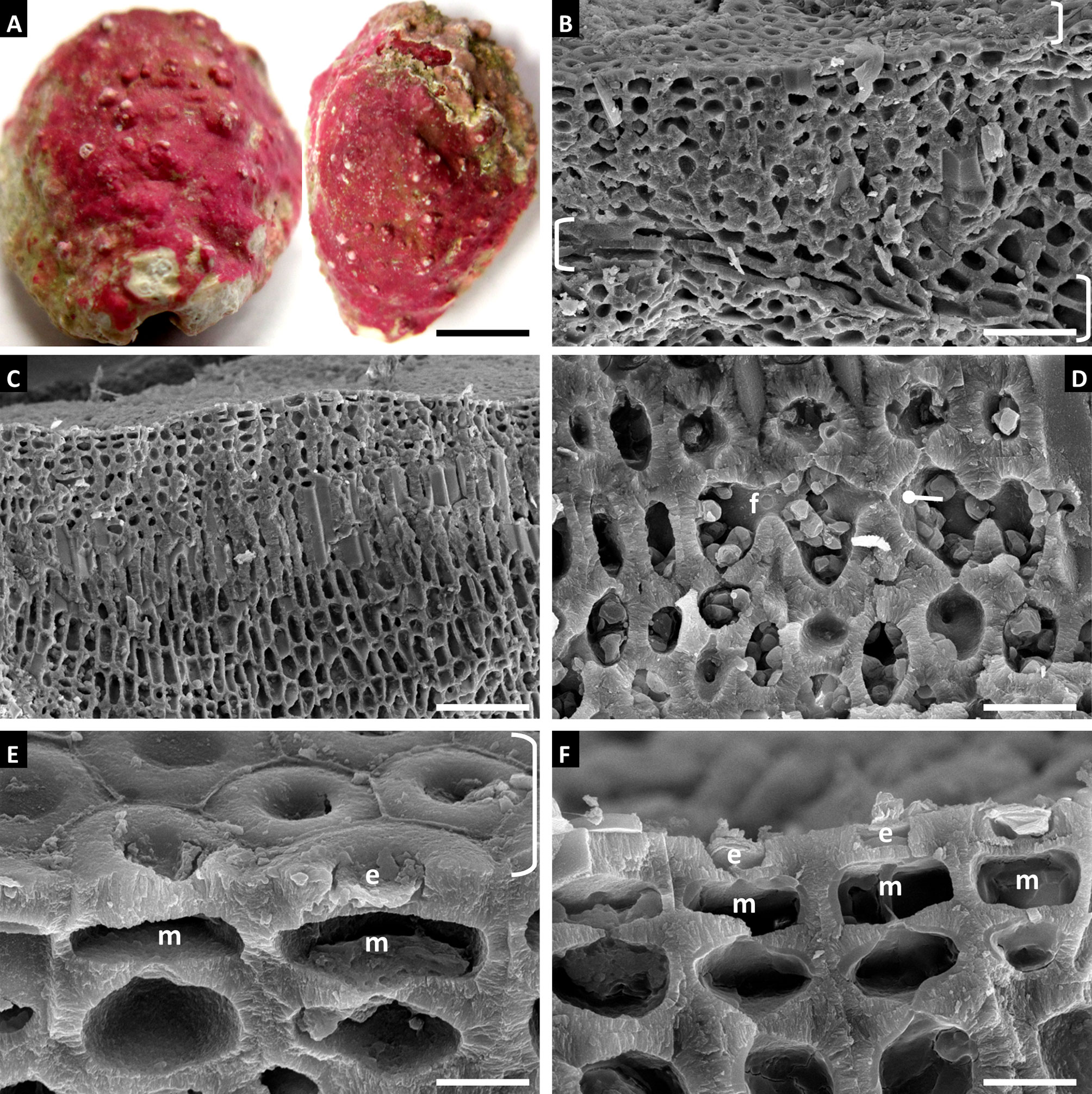
Figure 7 Sporolithon sp., voucher no. LAF 6726. (A) Thallus habit epizoic upon Naria acicularis shell. Scale bar = 0.4 cm. (B) Vertical fracture showing incomplete view of hypothallus (lower brackets), perithallus, and partial surface view of epithallus (upper bracket). Scale bar = 30 µm. (C) Vertical fracture of thallus showing perithallus. Scale bar = 55 µm. (D) Magnified view of perithallus showing cell fusion (f) and secondary pit connection (circle pointer). Scale bar = 11 µm. (E) Surface view of epithallus (bracket) and partial section view showing epithallial cells (e) and intercalary meristematic cells (m). Scale bar = 5 µm. (F) Vertical fracture showing epithallial cells (e) and intercalary meristematic cells (m). Scale bar = 6 µm.
Reproduction: No reproductive structures were observed.
Note: A single specimen of this species was identified from collections taken shortly after the Deepwater Horizon oil spill and has not been found in any collections since.
Roseolithon purii L.M.Coutinho & Barros-Barreto
DNA sequences: psbA, rbcL, and LSU sequences herein provided for the Gulf of Mexico specimen of this species (GB accessions ON365774, ON365776, ON427742) (Supplementary Table S1).
Habit and vegetative anatomy of Gulf of Mexico specimen (Figure 8): Thallus forming biogenic rhodoliths with thin warty protuberances. Protuberances have radial construction; secondary hypothallus with monomerous construction and 3-8 layers of rectangular hypothallial cells observed grown over older growth layers. Perithallus with cell fusions and lacking secondary pit connections between adjacent filaments. Intercalary meristematic cells approximately as long as wide. Epithallus with a single layer of epithallial cells; epithallus sloughing was also observed.
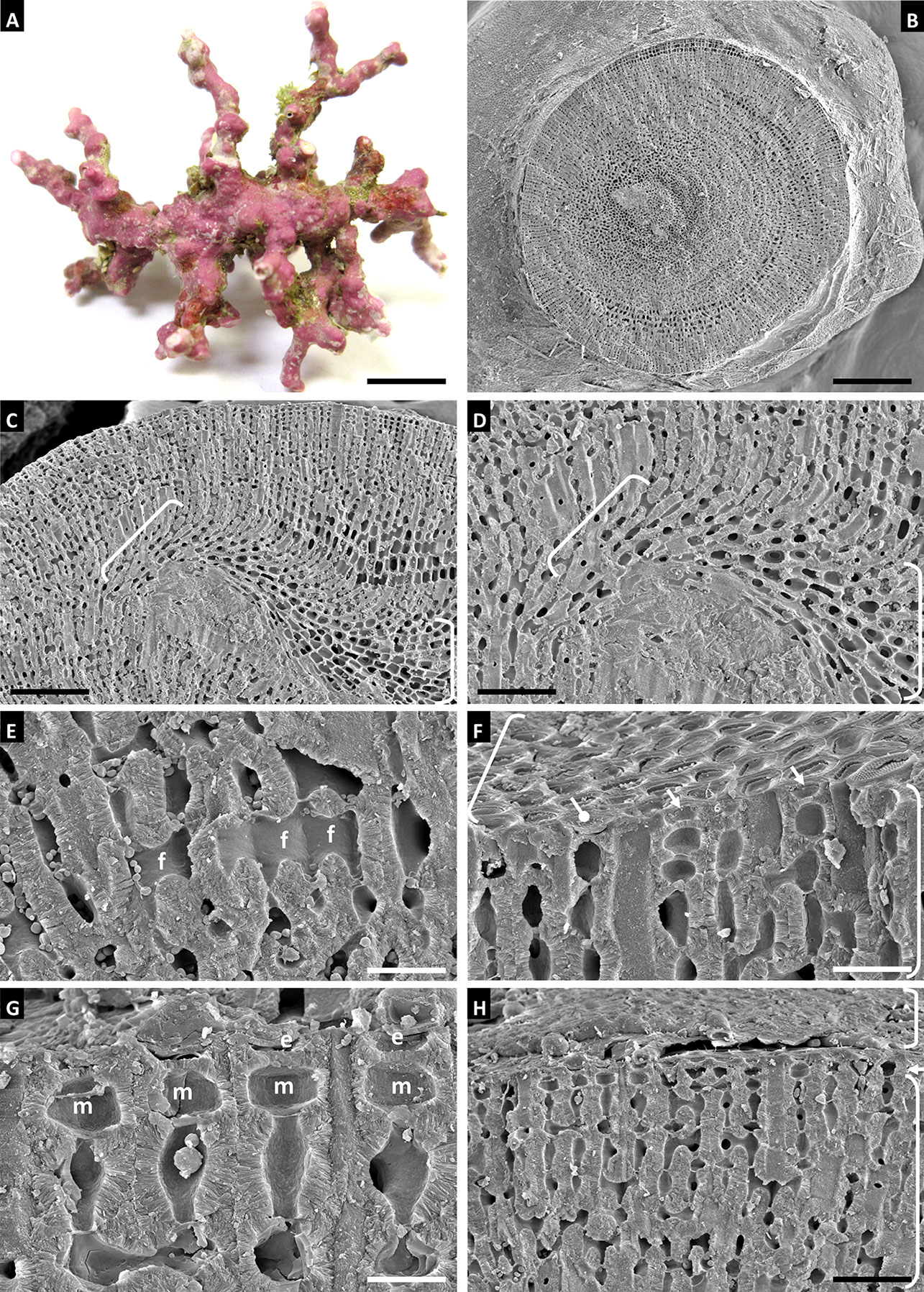
Figure 8 Roseolithon purii, voucher no. LAF 7384 (A-H). (A) Thallus habit. Scale bar = 0.4 cm. (B) Protuberance cross section showing radial construction and partial surface view of thallus at base of protuberance. Scale bar = 260 µm. (C) Protuberance section showing location of new growth layer with secondary hypothallus (brackets) over older growth layer. Scale bar = 100 µm. (D) Magnified view of secondary hypothallus (brackets). Scale bar = 53 µm. (E) Perithallus with cell fusions (f). Scale bar = 18 µm. (F) Surface view showing epithallus (upper bracket) and section view showing perithallus (lower bracket), and epithallial cells with roof intact (circle pointer) and roofs missing (arrows). Scale bar = 18 µm. (G) Intercalary meristematic cells (m) and epithallial cells in the process of sloughing (e). Scale bar = 10 µm. (H) Perithallus (lower bracket), intercalary meristem (arrow), and epithallus in the processing of sloughing off. Scale bar = 35 µm.
Distribution: This species is currently distributed in Brazil and the Gulf of Mexico.
Roseolithon louisianense J.L.Richards & Fredericq sp. nov.
Holotype: LAF 6549 (field ID no. 11-16-12): Ewing Bank, offshore Louisiana, U.S.A. (28° 5.936’N; 91° 2.112’W), Gulf of Mexico, western Atlantic Ocean, 16.xi.12, depth 55-58 meters, leg. J. Richards & S. Fredericq.
Isotype: LAF 6547 (field ID no. 11-16-12).
Etymology: The specific epithet refers to the locality where it was collected offshore Louisiana.
Description
DNA sequences: psbA, rbcL, and COI sequences diagnostic for this species (GB accessions for the holotype: psbA = KU557497, rbcL = ON394005, COI = KU514420; GB accession for the isotype: psbA = KU557496). UPA and LSU sequences are also provided (GB accessions for the holotype: UPA = KU514426, LSU = ON427744; GB accessions for the isotype: UPA = KU514425, LSU = ON427743) (Supplementary Table S1; see also Richards et al. (2016).
Habit and vegetative anatomy: Vegetative anatomy as for the genus, including non-geniculate thallus habit with monomerous thallus construction, hypothallus growing parallel to substratum, adjacent perithallial cells linked by cell fusions, secondary pit connections and trichocytes absent, and a single layer of armored (i.e. flared) epithallial cells. See Richards et al. (2016) for detailed habit and vegetative anatomy description (“as Lithothamnion sp. A”), as well as thallus habit image and SEM images.
Reproduction: This species produces multiporate tetrasporangial conceptacles with pores surrounded by 5-7 rosette cells. The rosette cells and pores have a pitted appearance that is characteristic of the genus, which appears to be from a disintegration of surface cells surrounding the pores as previously reported by the genus authorities. See Richards et al. (2016) for images of multiporate conceptacles.
Distribution: Currently known only from Ewing Bank, offshore Louisiana, U.S.A.
Roseolithon occidentaleatlanticum J.L.Richards & Fredericq sp. nov.
Holotype: LAF 6957B (field ID no. 9-7-14-1-3): Sackett Bank, offshore Louisiana, U.S.A. (28° 38.0’ N; 89° 33.028’ W), Gulf of Mexico, western Atlantic Ocean, 7.ix.2014, depth 65-68 meters, leg. J. Richards & S. Fredericq.
Etymology: The specific epithet refers to the western Atlantic Ocean.
Description:
DNA sequences: psbA and rbcL sequences diagnostic for this species (GB accessions KU557501, ON394007). UPA and LSU sequences are also provided (GB accessions KU514429, ON427748) (Supplementary Table S1; see also Richards et al., 2016).
Habit and vegetative anatomy: Vegetative anatomy as for the genus, including non-geniculate thallus habit with monomerous thallus construction, plumose hypothallus growing parallel to substratum, adjacent perithallial cells linked by cell fusions, secondary pit connections and trichocytes absent, and a single layer of armored (ie. flared) epithallial cells. See Richards et al. (2016) for detailed habit and vegetative anatomy description (“as Lithothamnion sp. B”), as well as thallus habit image and SEM images.
Reproduction: No reproductive structures were observed in the holotype of this species.
Distribution: Currently known from the Gulf of Mexico and Brazil.
Roseolithon rhodolapidosum J.L.Richards & Fredericq sp. nov.
Holotype: LAF 6820: Ewing Bank, offshore Louisiana, U.S.A. (28° 05.041’N; 91° 01.648’W), Gulf of Mexico, western Atlantic Ocean, 19.x.2013, depth 70-75 meters, leg. J. Richards & S. Fredericq.
Additional specimen examined: LAF 6956B: Sackett Bank, offshore Louisiana, U.S.A. (28° 38.0’N; 89° 33.028’W), Gulf of Mexico, western Atlantic Ocean, 7.ix.2014, depth 65-68 meters, leg. J. Richards & S. Fredericq.
Etymology: The specific epithet refers to the stony rhodolith-forming habit of this species.
Description
DNA sequences: psbA and rbcL sequences diagnostic for this species (GB accessions for the holotype: psbA = KU557498; GB accession for the additional specimen examined: psbA = KU557499, rbcL = ON394006). UPA and LSU sequences are also provided (GB accessions for the holotype: UPA = KU514427, LSU = ON427746; GB accessions for the addition specimen examined: UPA = KU514428, LSU = ON427747) (Supplementary Table S1; see also Richards et al., 2016).
Habit and vegetative anatomy: Vegetative anatomy as for the genus, including non-geniculate thallus habit forming free-living biogenic rhodoliths, adjacent perithallial cells linked by cell fusions, secondary pit connections and trichocytes absent, and a single layer of armored (i.e. flared) epithallial cells. See Richards et al. (2016) for detailed habit and vegetative anatomy description (“as Lithothamnion sp. C”).
Reproduction: This species produces abundant uniporate gametangial conceptacles that become overgrown by new layers of vegetative thallus. It was not determined if these uniporate conceptacles are male or female conceptacles. See Richards et al. (2016) for images of uniporate conceptacles.
Distribution: Currently known only from offshore Louisiana, U.S.A.
Discussion
Our phylogenetic analyses (Figures 1, 2) show at least 17 species of rhodolith-forming coralline algae are present in the northwestern Gulf of Mexico. Minnery (1990) reported 18 species of non-geniculate coralline algae from the Flower Garden Banks in the northwestern Gulf of Mexico, including 13 species currently classified in the Corallinales, four species currently classified in the Hapalidiales, and one species currently classified in the Sporolithales. However, it is difficult to make meaningful direct comparisons to the reports of Minnery (1990) because 1) the taxonomy of these corallines has undergone significant changes since the time that study was conducted, and 2) at the time of the study, Minnery identified corallines based on morpho-anatomy alone. Therefore, it is difficult to parse the names of those taxa reported by Minnery (1990) with the names verified by DNA sequencing that are reported in this current study.
Phylogenetic analyses (Figures 1–3) and sequence divergence values (Supplementary Tables S2, S3) show that Sporolithon gracile is a distinct species of biogenic rhodolith-forming coralline algae that is sister to S. sinusmexicanum and also closely related to S. mesophoticum and S. yoneshigueae. For context, the psbA and rbcL sequences of Sporolithon gracile were 3.39% and 2.46% diverged, respectively, from S. sinusmexicanum. These divergence values are similar to other closely related species of Sporolithon, for example, psbA and rbcL sequences of S. episporum and S. indopacificum are 3.1%, and 2.7% diverged, respectively (Maneveldt et al., 2017).
Sporolithon sp. LAF 6726 and Roseolithon sp. LAF 6555B are represented in this study by the LSU marker alone, thus a marker that is more diagnostic at the species level, such as psbA, rbcL, or COI, should be generated to confirm their species identity, especially for LAF 6726, which showed a short branch length from its closest sister taxon (Figure 3). LAF 6726 may represent a separate species sister to Sporolithon amadoi, although this needs to be assessed by sequencing additional markers in a future study. Although previous studies have shown that LSU is more conserved and thus has more limited utility in distinguishing species compared to other markers such as psbA, rbcL, and COI (Sherwood et al., 2010; Richards et al., 2014; Richards et al., 2017) it is still useful in assessing the diversity of rhodoliths in the northwestern Gulf of Mexico and worldwide. LSU may also amplify easier than other markers for coralline algae (Sherwood et al., 2010), and for some samples in this study it was the only marker that could be amplified as was the case with Sporolithon sp. LAF 6726 and Roseolithon sp. LAF6555B. LSU rDNA has also recently been used in environmental DNA metabarcoding studies (Bombin et al., 2021). Therefore, sequencing LSU rDNA of preserved algal specimens, as was performed in this study, is also important to link sequences generated from environmental samples to those sequences generated from algal specimens.
Regarding the Hapalidiales, four species of Roseolithon are present in the northwestern Gulf of Mexico, including Roseolithon purii, R. louisianaensis sp. nov., R. rhodolapidosum sp. nov. and R. occidentaleatlanticum sp. nov. Species delimitation analyses (ABGD and GMYC) of these newly described species was conducted previously in Richards et al. (2016) and the results indicated these taxa are unique species. These Roseolithon taxa were also delimited as separate species by Coutinho et al. (2021) using ABGD, GMYC, and bPTP delimitation methods. Additionally, Mesophyllum erubescens is also present offshore Louisiana (Richards et al., 2020; Figures 1, 2, present study), as well as three species tentatively identified as “Lithothamnion sp. E”, “Lithothamnion sp. G” and “Lithothamnion sp. I”. The taxonomy of these three taxa tentatively identified as “Lithothamnion” needs to be reassessed in future studies in light of the Lithothamnion muelleri sequence data that was provided in Jeong et al. (2021).
Although the morpho-anatomical characters for the genus Roseolithon were observed in the Roseolithon species described in this study (namely non-geniculate thallus habit with monomerous thallus construction, plumose hypothallus, adjacent perithallial cells linked by cell fusions, secondary pit connections and trichocytes absent, a single layer of armored/flared epithallial cells, and multiporate tetrasporangial conceptacles with rosette cells that have a pitted appearance), this suite of characters overlaps with other genera of Hapalidiales. Moreover, there is considerable overlap in character states between species within Roseolithon (Richards et al., 2016; Coutinho et al., 2021; present study). Thus, DNA sequencing is needed to identify species within this genus.
Roseolithon species present in both the Gulf of Mexico and Brazil highlight the presence of this genus in both the northern and southern hemisphere. This distribution is interesting considering the presence of Roseolithon in rhodolith beds associated with salt domes (diapirs) rich in petroleum deposits in offshore mesophotic habitats in the Gulf of Mexico and offshore Brazil (Amado-Filho et al., 2012; Coutinho et al., 2021). Other non-geniculate coralline algae, for example Sporolithon amadoi, show a similar distribution in both the Gulf of Mexico and Brazil (Richards et al., 2019). Additional sampling and sequencing should be done worldwide to determine the full range of the species described in this study and in Coutinho et al. (2021) and to identify and describe additional species worldwide. Currently, sample sizes are too low to confirm if some species are endemic to the Gulf of Mexico. For example, R. louisianense and R. rhodolapidosum are each only represented only by two specimens (or possibly three specimens for R. rhodolapidosum, see above note about specimen LAF 6555B). Moreover, additional sampling should be conducted in the southeastern, southwestern, and northeastern Gulf of Mexico to determine the full ranges of these species within the Gulf of Mexico.
Regarding the Corallinales, Lithophyllum sp. and “Titanoderma” spp. that are present offshore the northwestern Gulf of Mexico are different taxa than those Lithophyllum spp. found in the Western Atlantic offshore North Carolina (Richards et al., 2014; Richards et al., 2018a; Figures 1, 2, present study). The Lithophylloideae spp. present offshore the northwestern Gulf of Mexico need to be described in future studies. Likewise, the three species of Harveylithon, H. munitum (Foslie & M.Howe) A.Rösler, Perfectti, V.Peña & J.C.Braga, H. saldomum J.Richards, W.E.Schmidt & Fredericq, and H. felderii J.Richards, W.E.Schmidt & Fredericq, that are found offshore the northwestern Gulf of Mexico are different species than the Harveylithon species, H. catarinense I.O.Costa, P.A.Horta & J.M.C.Nunes, that is found offshore North Carolina (Richards et al., 2021; Figures 1, 2, present study).
Conclusion
The northwestern Gulf of Mexico is a hotspot for non-geniculate, rhodolith-forming coralline algae. In total, at least 17 species of rhodolith-forming coralline species are found offshore the northwestern Gulf of Mexico including six species of Corallinales, eight species of Hapalidiales, and three species of Sporolithales. Four species of corallines are newly described in this study and one species is newly reported for the region. Regarding the status of names, 11 species from the northwestern Gulf of Mexico have been assigned names verified by comparative DNA sequence analyses. Continuing to assess the diversity of rhodolith-forming corallines, including naming other new species of Hapalidiales and Corallinales, is of critical importance to conservation efforts in the region.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
JR, RK, WS, and SF conceived the study. JR, RK, TS, WS, DG, CG and SF collected the samples. JR, WS, and RK conducted the laboratory work. JR, RK, and TS performed the data analyses. JR and SF wrote the manuscript with contributions from RK, WS, TS, DG, and CG. All authors edited the manuscript before submission.
Funding
This study was funded by NSF grant DEB–1754504 to SF for rhodolith research in the Gulf of Mexico. NSF grants MRI-1920166 and DEB-0315995 also provided funding. CFDG acknowledges CNPq grant 306304/2019-8 and 437115/2018-6.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the crews of the R/V Pelican and R/V Manta, as well as Jason White and Eric Glidden from the University of North Carolina-Undersea Vehicle Program (WUNCW-UVP), for help with sampling protocols during rhodolith collection expeditions offshore the Gulf of Mexico. We also thank our close research collaborators Darryl L. Felder, Sherry Krayesky-Self, Paul W. Gabrielson, and Emma Hickerson (FGBNMS). Sincere thanks to Dr. Tom Pesacreta, Mike Purpera, and Lily Ann Hume from the Microscopy Center at UL Lafayette for help and advice while using the SEM and FIB-SEM, and Emilio Garcia for help with Mollusca identification.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.906679/full#supplementary-material
Supplementary Table 1 | List of taxa names, voucher numbers, localities, GenBank numbers and reference information for newly generated sequences and sequences downloaded from GenBank of taxa included in phylogenetic analyses. Newly generated sequences are shown in bold. N.A. = not available.
Supplementary Table 2 | Divergence values of psbA sequences for Sporolithon spp.
Supplementary Table 3 | Divergence values of rbcL sequences for Sporolithon spp.
Supplementary Table 4 | Divergence values of psbA sequences for Roseolithon spp.
Supplementary Table 5 | Divergence values of rbcL sequences (366 bp) for Roseolithon spp.
Supplementary Table 6 | Divergence values of rbcL sequences (623 bp) for Roseolithon spp.
References
Amado-Filho G. M., Moura R. L., Bastos A. C., Salgado L. T., Sumida P. Y., Guth A. Z., et al. (2012). Rhodolith Beds are Major CaCO3 Bio-Factories in the Tropical South West Atlantic. PloS One 7 (4), e35171. doi: 10.1371/journal.pone.0035171
Bombin S., Wysor B., Lopez-Bautista J. M. (2021). Assessment of Littoral Algal Diversity From the Northern Gulf of Mexico Using Environmental DNA Metabarcoding. J. Phycol 57, 269–278. doi: 10.1111/jpy.13087
Coutinho L. M., Gomes F. P., Sissini M. N., Vieira-Pinto T., Muller de Oliveira Henriques M. C., Oliveira M. C., et al. (2021). Cryptic Diversity in non-Geniculate Coralline Algae: A New Genus Roseolithon (Hapalidiales, Rhodophyta) and Seven New Species From the Western Atlantic. Eur. J. Phycol. 57, 227–250. doi: 10.1080/09670262.2021.1950839
Fredericq S., Arakaki N., Camacho O., Gabriel D., Krayesky D., Self-Krayesky S., et al. (2014). A Dynamic Approach to the Study of Rhodoliths: A Case Study for the Northwestern Gulf of Mexico. Cryptogamie Algologie 35, 77–98. doi: 10.7872/crya.v35.iss1.2014.77
Fredericq S., Cho T. O., Earle S. A., Gurgel C. F., Krayesky D. M., Mateo-Cid L. E., et al. (2009). “Seaweeds of the Gulf of Mexico,” in Gulf of Mexico: Its Origins, Waters, and Biota. I. Biodiversity. Eds. Felder D. L., Camp D. K. (College Station, Texas A&M Univ. Press), 187–259.
Fredericq S., Krayesky-Self S., Sauvage T., Richards J., Kittle R., Arakaki N., et al. (2019). The Critical Importance of Rhodoliths in the Life Cycle Completion of Both Macro- and Microalgae, and as Holobionts for the Establishment and Maintenance of Biodiversity. Front. Mar. Sc.– Mar. Ecosyst. Ecol. 5. doi: 10.3389/fmars.2018.00502
Jeong S. Y., Nelson W. A., Sutherland J. E., Peña V., Le Gall L., Diaz-Pulido G., et al. (2021). Corallinapetrales and Corallinapetraceae: A New Order and Family of Coralline Red Algae Including Corallinapetra Gabrielii Comb. Nov. J. Phycol 57, 849–862. doi: 10.1111/jpy.13115
Krayesky-Self S., Richards J. L., Rahmatian M., Fredericq S. (2016). Aragonite Infill in Overgrown Conceptacles of Coralline Lithothamnion Spp. (Hapalidiaceae, Hapalidiales, Rhodophyta): New Insights in Biomineralization and Phylomineralogy. J. Phycol 52, 161–173. doi: 10.1111/jpy.12392
Kumar S., Stecher G., Li M., Knyaz C., Tamura K. (2018). MEGA X: Molecular Evolutionary Genetics Analysis Across Computing Platforms. Mol. Biol. Evol. 35, 1547–1549. doi: 10.1093/molbev/msy096
Leão L. A. S., Bahia R. G., Jesionek M. B., Adey W. H., Johnson G., Salgado L. T., et al. (2020). Sporolithon Franciscanum Sp. Nov. (Sporolithales, Rhodophyta), a New Rhodolith-Forming Species From Northeast Brazil. Diversity 12, 199. doi: 10.3390/d12050199
Maneveldt G. W., Gabrielson P. W., Kangwe J. (2017). Sporolithon Indopacificum Sp. Nov. (Sporolithales, Rhodophyta) From Tropical Western Indian and Western Pacific Oceans: First Report, Confirmed by DNA Sequence Data, of a Widely Distributed Species of Sporolithon. Phytotaxa 326, 115–128. doi: 10.11646/phytotaxa.326.2.3
Miller M. A., Pfeiffer W., Schwartz T. (2010). “Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees,” in Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, 14 Nov. 2010. 1–8. doi: 10.1109/GCE.2010.5676129
Minnery G. A. (1990). Crustose Coralline Algae From the Flower Garden Banks, Northwestern Gulf of Mexico; Controls on Distribution and Growth Morphology. J. Sedim Petrol 60, 992–1007.
Minnery G. A., Rezak R., Bright T. J. (1985). ““Chapter 18: Depth Zonation and Growth Form of Crustose Coralline Algae: Flower Garden Banks, Northwestern Gulf of Mexico,”,” in Paleoalgology: Contemporary Research and Applications. Eds. Toomey D. F., Titecki M. H. (Berlin; Heidelberg: Springer-Verlag), 238–246. doi: 10.1306/D4267663-2B26-11D7-8648000102C1865D
Nelson W. A., Neill K. F., Twist B. A., Sutherland J. E. (2021). A New Genus and Species in the Sporolithales (Corallinophycidae, Rhodophyta) From Northern New Zealand: Roseapetra Farriae Sp. Nov. Phytotaxa 490, 35–46. doi: 10.11646/phytotaxa.490.1.3
Rezak R., Bright T. J., McGrail D. W. (1985). Reefs and Banks of the Northwestern Gulf of Mexico: Their Geological, Biological, and Physical Dynamics (New York, NY: Wiley), 259.
Richards J. L., Bahia R. G., Jesionek M. B., Fredericq S. (2019). Sporolithon Amadoi Sp. Nov. (Sporolithales, Rhodophyta), a New Rhodolith-Forming non-Geniculate Coralline Alga From Offshore the Northwestern Gulf of Mexico and Brazil. Phytotaxa 423, 49–67. doi: 10.11646/phytotaxa.423.2.1
Richards J. L., Fredericq S. (2018). Sporolithon Sinusmexicanum Sp. Nov. (Sporolithales, Rhodophyta): A New Rhodolith-Forming Species From Deepwater Rhodolith Bed in the Gulf of Mexico. Phytotaxa 350, 135–146. doi: 10.11646/phytotaxa.350.2.2
Richards J. L., Gabrielson P. W., Fredericq S. (2014). New Insights Into the Genus Lithophyllum (Lithophylloideae, Corallinaceae, Corallinales) From Deepwater Rhodolith Beds Offshore the NW Gulf of Mexico. Phytotaxa 190, 162–175. doi: 10.11646/phytotaxa.190.1.11
Richards J. L., Gabrielson P. W., Hughey J. R., Freshwater D. W. (2018a). A Re-Evaluation of Subtidal Lithophyllum Species (Corallinales, Rhodophyta) From North Carolina, USA, and the Proposal of L. Searlesii Sp. Nov. Phycologia 57, 318–330. doi: 10.2216/17-110.1
Richards J. L., Gabrielson P. W., Schneider C. W. (2018b). Sporolithon Mesophoticum Sp. Nov. (Sporolithales, Rhodophyta) From Plantagenet Bank Off Bermuda at a Depth of 178 M. Phytotaxa 385, 67–76. doi: 10.11646/phytotaxa.385.2.2
Richards J. L., Kittle R. P. III, Abshire J. R., Fuselier D., Schmidt W. E., Gurgel C. F., et al. (2020). Range Extension of Mesophyllum Erubescens (Foslie) Me. Lemoine (Hapalidiales, Rhodophyta): First Report From Mesophotic Rhodolith Beds in the Northwestern Gulf of Mexico Offshore Louisiana and Texas, Including the Flower Garden Banks National Marine Sanctuary. Check List 16, 513–519. doi: 10.15560/16.3.513
Richards J. L., Sauvage T., Schmidt W. E., Fredericq S., Hughey J. R., Gabrielson P. W. (2017). The Coralline Genera Sporolithon and Heydrichia (Sporolithales, Rhodophyta) Clarified by Sequencing Type Material of Their Generitypes and Other Species. J. Phycol 53, 1044–1059. doi: 10.1111/jpy.12562
Richards J. L., Schmidt W. E., Fredericq S., Sauvage T., Peña V., Le Gall L., et al. (2021). DNA Sequencing of Type Material and Newly Collected Specimens Reveals Two Heterotypic Synonyms for Harveylithon Munitum (Metagoniolithoideae, Corallinales, Rhodophyta) and Three New Species. J. Phycol 57, 1234–1253. doi: 10.1111/jpy.13161
Richards J. L., Vieira-Pinto T., Schmidt W. E., Sauvage T., Gabrielson P. W., Oliveira M. C., et al. (2016). Molecular and Morphological Diversity of Lithothamnion Spp. (Hapalidiales, Rhodophyta) From Deepwater Rhodolith Beds in the Northwestern Gulf of Mexico. Phytotaxa 278, 81–114. doi: 10.11646/phytotaxa.278.2.1
Sherwood A. R., Sauvage T., Kurihara A., Conklin K. Y., Presting G. G. (2010). A Comparative Analysis of COI, LSU and UPA Marker Data for the Hawaiian Florideophyte Rhodophyta: Implications for DNA Barcoding of Red Algae. Cryptogamie Algologie 31, 451–465.
Stecher G., Tamura K., Kumar S. (2020). Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. doi: 10.1093/molbev/msz312
Tamura K., Nei M. (1993). Estimation of the Number of Nucleotide Substitutions in the Control Region of Mitochondrial DNA in Humans and Chimpanzees. Mol. Biol. Evol. 10, 512–526. doi: 10.1093/oxfordjournals.molbev.a040023
Thiers B. (2022). Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff (New York: New York Botanical Garden’s Virtual Herbarium). Available at: http://sweetgum.nybg.org/ih.
Keywords: CCA, coralline algae, FGBNMS, Gulf of Mexico, mesophotic, rhodoliths
Citation: Richards J, Kittle RP III, Schmidt WE, Sauvage T, Gurgel CFD, Gabriel D and Fredericq S (2022) Assessment of Rhodolith Diversity in the Northwestern Gulf of Mexico Including the Description of Sporolithon gracile sp. nov. (Sporolithales, Rhodophyta), and Three New Species of Roseolithon (Hapalidiales, Rhodophyta). Front. Mar. Sci. 9:906679. doi: 10.3389/fmars.2022.906679
Received: 28 March 2022; Accepted: 16 May 2022;
Published: 04 July 2022.
Edited by:
Daniela Basso, University of Milano-Bicocca, ItalyReviewed by:
Gavin William Maneveldt, University of the Western Cape, South AfricaDonatella Serio, University of Catania, Italy
Copyright © 2022 Richards, Kittle, Schmidt, Sauvage, Gurgel, Gabriel and Fredericq. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph Richards, am9lcjIwN0BnbWFpbC5jb20=
 Joseph Richards
Joseph Richards Ronald P. Kittle III
Ronald P. Kittle III William E. Schmidt
William E. Schmidt Thomas Sauvage
Thomas Sauvage Carlos F. D. Gurgel
Carlos F. D. Gurgel Daniela Gabriel
Daniela Gabriel Suzanne Fredericq
Suzanne Fredericq