- 1Department of Biological Sciences, Graduate School of Science, The University of Tokyo, Tokyo, Japan
- 2Faculty of Science, Hokkaido University, Sapporo, Japan
- 3Zhirmunsky National Scientific Centre of Marine Biology, Far Eastern Branch, Russian Academy of Sciences, Vladivostok, Russia
- 4Sugashima Marine Biological Laboratory, Graduate School of Science, Nagoya University, Toba, Japan
- 5Centre for Marine and Coastal Studies, Universiti Sains Malaysia, Penang, Malaysia
- 6Department of Natural History Sciences, Graduate School of Science, Hokkaido University, Sapporo, Japan
- 7Misaki Marine Biological Station, School of Science, The University of Tokyo, Miura, Japan
- 8Faculty of Human Environmental Studies, Hiroshima Shudo University, Hiroshima, Japan
- 9Department of Geology and Palaeontology, National Museum of Nature and Science, Tsukuba, Japan
- 10Research Institute for Global Change (RIGC), Japan Agency for Marine-Earth Science and Technology (JAMSTEC), Yokosuka, Japan
Nemerteans, or ribbon worms, have been reported from intertidal to hadal depths, often showing bathymetrically wide distribution in genus levels. Although current nemertean systematics practices require to provide DNA sequences and infer phylogenetic relationships with suitable molecular markers, previous molecular systematics on nemerteans are mostly biased toward shallow-water species. Members in the genus Nipponnemertes occur worldwide, from tropical to polar waters and intertidal to bathyal waters. Molecular phylogenetic studies are scarce for the genus; only six shallow-water species of 18 species in the genus were subject to molecular phylogeny. Thus, Nipponnemertes is one candidate that needs to be assessed by genetic approaches. In this study, we performed molecular phylogenetic analyses using 59 specimens in 23 species based on partial sequences of two mitochondrial (16S rRNA and cytochrome c oxidase subunit I) and three nuclear gene markers (18S rRNA, 28S rRNA, and histone H3). Our extensive sampling from intertidal to bathyal waters in the Northwest Pacific significantly updated the fauna of Nipponnemertes in this region from four to 17 species. We herein establish 10 new species and provide an updated species list concisely summarizing all the congeners known from the world. Our phylogenetic tree indicated three major lineages within the genus (herein referred to as “Clade A, B, and C”), each presumably characterized by the combination of morphological characters in the head region. Members in Clade A are: Nipponnemertes pulchra (Johnston, 1837), Nipponnemertes ogumai (Yamaoka, 1947), and several unidentified congeners, characterized by having demarcated head without cephalic patches; members in Clade B are: Nipponnemertes crypta sp. nov., Nipponnemertes jambio sp. nov., Nipponnemertes neonilae sp. nov., and Nipponnemertes ojimaorum sp. nov., species having demarcated head with cephalic patches; members in Clade C are: Nipponnemertes ganahai sp. nov., Nipponnemertes kozaensis sp. nov., Nipponnemertes lactea sp. nov., Nipponnemertes notoensis sp. nov., Nipponnemertes ornata sp. nov., Nipponnemertes sugashimaensis sp. nov., and two unidentified forms collected off Jogashima (Japan) and Guam (USA), species with non-demarcated head lacking cephalic patches. Furthermore, we discuss the evolution of remarkably small body size retained among Clade C.
Introduction
Ribbon worms (phylum Nemertea) are commonly known as predators or scavengers in a wide variety of marine, freshwater, and terrestrial ecosystems (McDermott & Roe, 1985; Thiel & Kruse, 2001). The phylum currently contains around 1300 species (Chernyshev, 2021; Kajihara, 2021a), of which about 594 species in 127 genera are members in the order Monostilifera (Kajihara, 2021b). The Monostilifera can be divided into the suborders Cratenemertea and Eumonostilifera chiefly by a combination of three characters: the arrangement of the rhynchocoel musculature (a wickerwork of longitudinal and circular muscle fibres in Cratenemertea as well as in some Eumonostililfera vs separate inner longitudinal and outer circular muscle layers in Eumonostilifera), the vascular plug (single plug in Cratenemertea vs variously 0–2 plugs in Eumonostilifera), and the position of the cerebral organs (mostly extending behind brain in Cratenemertea vs at most to the brain region in Eumonostilifera) (Chernyshev, 2021; Kajihara, 2021b). While these taxonomic characters seem to have evolved homoplastically (Kajihara, 2021b), the distinction between Cratenemertea and Eumonostilifera is supported by molecular phylogenetic analyses based on multiple loci in nuclear and mitochondrial genomes (Andrade et al., 2012; Kvist et al., 2014; Kvist et al., 2015; Chernyshev & Polyakova, 2019).
Molecular systematics within Cratenemertea is still in progress. Although 28 species in three families have been recognized in this taxon, phylogenetic relationships within Cratenemertea are not well resolved due to a lack of molecular data. So far, species in two genera, Nipponnemertes Gibson & Crandall, 1989 and Uniporus Brinkmann, 1914–1915a, and unidentified specimens collected from the Far Eastern seas of Russia, have been included in molecular phylogeny (e.g., Kvist et al., 2015; Chernyshev & Polyakova, 2018; Chernyshev and Polyakova, 2019). Recent molecular phylogenetic analyses suggest that ‘Cratenemertea sp. 25DS’ (Chernyshev & Polyakova, 2019) and ‘Cratenemertidae sp. IZ-45644’ (Kvist et al., 2015) are the sister taxon to Uniporus (Chernyshev & Polyakova, 2019). ‘Cratenemertea sp. 25DS’ is likely to be a planktonic juvenile of ‘Cratenemertidae sp. IZ-45644’ (Chernyshev & Polyakova, 2019). Although ‘Cratenemertea sp. 25DS’ is morphologically similar to Achoronemertes scorebyi (Wheeler, 1934) and Korotkevitschia pelagica (Korotkevitsch, 1961) (Chernyshev & Polyakova, 2019), a comparison of both morphological and molecular data is required for accurate species identification.
Another issue that needs to be investigated with the molecular approach is the generic distinction between Cratenemertes Friedrich, 1955 and Nipponnemertes. Friedrich (1955) established Cratenemertes for a species originally described by Bürger (1890), Amphiporus amboinensis Bürger, 1890; in Friedrich (1968), the generic name was attributed to species with a small cerebral organ posteriorly fused with dorsal cerebral ganglia. Whether smaller cerebral organs in Cratenemertes—the only difference between the two genera—are a phylogenetic signal or not need to be verified with molecular data.
Species in the genus Nipponnemertes—18 are currently recognized as valid—represent the majority of Cratenemertea (Gonzalez-Cueto et al., 2017; Kajihara, 2021b). They are marine benthic, usually feeding on small crustaceans such as amphipods (Brunberg, 1964; Berg, 1972; McDermott, 1984; McDermott, 1993; McDermott, 1997) and caridean shrimps (Kajihara et al., 2015) by sucking out body fluid and internal tissue of those prey organisms. Nipponnemertes was established by Friedrich (1968) for the seven nominal species Amphiporus bergendali Gering, 1912, Amphiporus drepanophoroides Griffin, 1898, Amphiporus pacificus Coe, 1905, Amphiporus occidentalis Coe, 1905, Amphiporus punctatulus Coe, 1905, Cratenemertes danae Friedrich, 1957, and Cratenemertes madagascarensis Kirsteuer, 1965 without type designation, and thus was unavailable under Article 13.3 of the International Code of Zoological Nomenclature (International Commission on Zoological Nomenclature, 1999). To make Nipponnemertes nomenclaturally available, Gibson & Crandall (1989) designated A. drepanophoroides as the type species and provided a morphological diagnosis for the genus; the authorship of Nipponnemertes is thus ascribed to Gibson & Crandall (1989). Phylogenetic relationships among some members of Nipponnemertes were inferred by Kajihara et al. (2015), where four described—Nipponnemertes bimaculata (Coe, 1901), Nipponnemertes ogumai (Yamaoka, 1947), Nipponnemertes pulchra (Johnston, 1837), and Nipponnemertes punctatula (Coe, 1905)—and two undescribed congeners—Nipponnemertes sp. 1 (MCZ DNA105622) and Nipponnemertes sp. 2 (MCZ DNA105589) (Andrade et al., 2012)—were analysed. The resulting tree indicated that the six species were divided into two groups, which seemed to be characterized by the presence and absence of a cephalic patch (Kajihara et al., 2015); however, it has never been confirmed based on a more comprehensive dataset.
From the Northwest Pacific in eastern Asia, including the Sea of Okhotsk, the Sea of Japan, and the East and South China Seas, four species of Nipponnemertes have been recorded: Nipponnemertes arenaria (Ushakov, 1927), N. bimaculata, N. ogumai, and N. punctatula (Kajihara, 2007; Kajihara, 2017; Chernyshev, 2020). Besides, several authors reported unidentified forms of Nipponnemertes from Japanese (Yamaoka, 2005; Kajihara, 2017), Vietnamese (Chernyshev, 2016), and Far East Russian waters (Chernyshev, 2020), suggesting there is hidden nemertean diversity.
With the advent of molecular tools—DNA barcoding and molecular phylogeny—in monostiliferan systematics (e.g., Andrade et al., 2012; Kvist et al., 2014; Kvist et al., 2015; Leasi et al., 2016; Hookabe et al., 2020; Hookabe et al., 2021), we are now in a position to obtain a more accurate picture of the species diversity. Some of the diagnostic characters traditionally used in monostiliferan taxonomy are often ambiguous and thus need to be revisited with molecular phylogeny. In the present study, we describe 10 new species of the genus from Japanese and Far East Russian waters. Since previous studies on molecular phylogenetic study in Nipponnemertes were biased toward shallow-water species (e.g., Kajihara et al., 2015) despite the bathymetrically wide distribution of this taxon, we extensively sampled specimens from intertidal to bathyal waters by various methods: by hand for intertidal species, SCUBA, dredging, sledging, and a remotely operated vehicle (ROV) equipped with a slurp gun. In the description part, histological observation (including cerebral organ, which is an important character for distinguishing Nipponnemertes from Cratenemertes) was performed to characterize the internal morphology of each taxon. Using molecular data obtained from the newly discovered species and described species from Japanese and Far East Russian waters, phylogenetic analyses based on partial sequences of nuclear and mitochondrial genes are performed to assess phylogenetic relationships as well as species boundaries within the genus.
Materials and Methods
Taxon Sampling and Morphological Examination
In the present study, we collected cratenemertids from Japanese and Far East Russian waters in 2014–2021 by multiple methods (Figure 1 and Table 1). Specimens were photographed with digital still cameras NIKON D5600 (NIKON, Japan) or OM-D E-M1 Mark II (Olympus, Japan) while they were alive. Worms were anaesthetized in 7.5% MgCl2 and gently placed between a slide glass and cover slip with a drop of anaesthetic solution so that ocelli and stylet apparatus could be observed under a light microscope. Afterwards, worms were picked up from the slides and the posterior tips were preserved in 99% ethanol for DNA extraction while the rest of the body was pre-fixed in 10% formalin in seawater, then post-fixed in Bouin’s fluid for 24 hours, and transferred to 70% ethanol. For histological preparation, samples preserved in 70% ethanol were dehydrated in a series of ethanol solutions (70%, 80%, 90%, 95%, and 99% ethanol), cleared in xylene, and embedded in Surgipath Paraplast paraffin (Leica, Germany). Paraffin blocks were serially sectioned at 7 µm thickness and stained with Mallory’s trichrome method (Gibson, 1994).
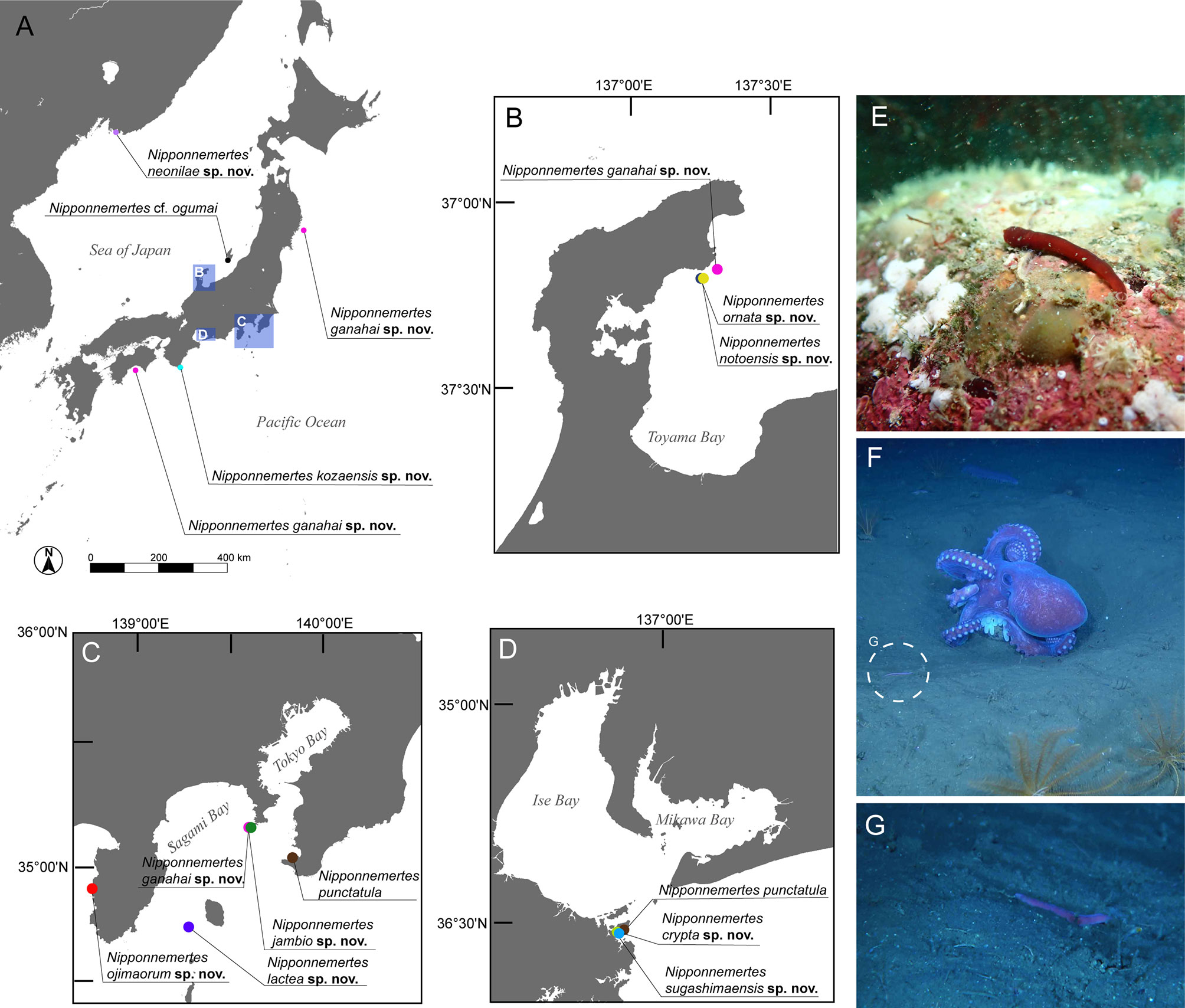
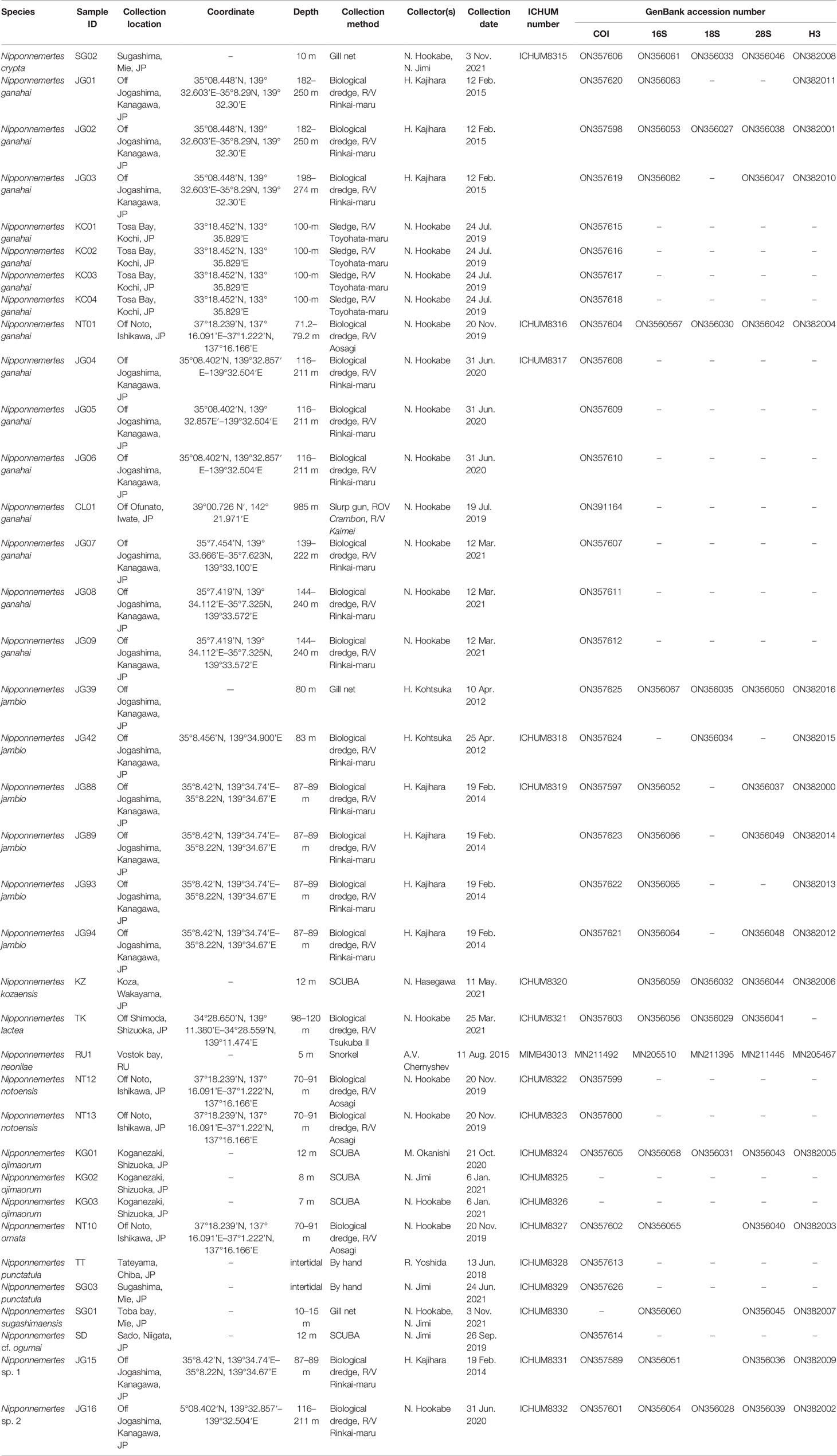
Figure 1 Sampling sites of specimens used in the present study. (A) Overview map. (B) Collection sites in Noto, Japan. (C) Collection sites in Kanto region and adjacent areas, Japan, including Tateyama, off Jogashima, and off Izu Peninsula. (D) Collection sites around Sugashima, Japan. (E) In-situ photographs of Nipponnemertes ojimaorum sp. nov., Koganezaki, Shizuoka, Japan. (F) In-situ photograph of N. ganahai sp. nov., crawling near an octopus brooding eggs (circle with broken line), off Ofunato, Iwate, Japan. (G) Close-up of N. ganahai sp. nov. in Figure 1F.
DNA Extraction, PCR Amplification, and Sequencing
Total DNA was extracted using the DNeasy Blood &Tissue Kit (Qiagen, Germany) according to the manufacturer’s protocol. Each partial sequence was amplified with primer pairs summarized in Table 2 according to the following PCR protocol: preheating at 94°C for 2 min; 35 cycles of 94°C for 40 s, 52°C for 60–75 s, and 72°C for 60 s; then a final extension at 72°C for 7 min. PCR products were purified using Exo-SAP-IT (Thermo Fisher Scientific, USA). Sequencing reaction was performed with BigDye Terminator ver. 3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, USA); for partial sequences of 18S rRNA (18S) and 28S rRNA (28S), internal primers listed in Table 2 were additionally used. The resulting dye-labelled products were purified with the EDTA/ethanol precipitation method and sent to GENEWIZ (Tokyo, Japan) for nucleotide sequencing. Sequences newly obtained in this study have been deposited in DNA Data Bank of Japan (DDBJ) under accession numbers listed in Table 1. Type and voucher specimens have been deposited in Invertebrate Collection of the Hokkaido University Museum (ICHUM), Sapporo, Japan and Zoological Museum of Far East Federal University, Vladivostok, Russia.
Sequence Alignment and Phylogenetic Analyses
Sequencing chromatographs were checked and edited with GeneStudio Pro ver. 2.2 (GeneStudio, Inc., USA). Sequences newly obtained in this study were combined with sequences available in GenBank listed in Table 3. For 16S, 18S, and 28S, data sets were aligned by MAFFT ver. 7 (Katoh & Standley, 2013) using the automatically selected strategy L-INS-i. After being aligned with ClustalW algorithm (Thompson et al., 1994) implemented in MEGA ver. 7 (Kumar et al., 2016), COI and H3 sequences were checked by translating into amino acids to see if they did not contain stop codons. Ambiguous sites were removed by Gblocks ver. 0.91b (Castresana, 2000) under a less stringent option; a 657-bp sequence for COI, a 458-bp for16S, a 1740-bp for 18S, a 1221-bp for 28S, and a 330-bp for H3 finally remained. After tree topology is checked for each marker and consistent with each other, partial sequences of COI, 16S, 18S, 28S, and H3 were concatenated using MEGA ver. 7.
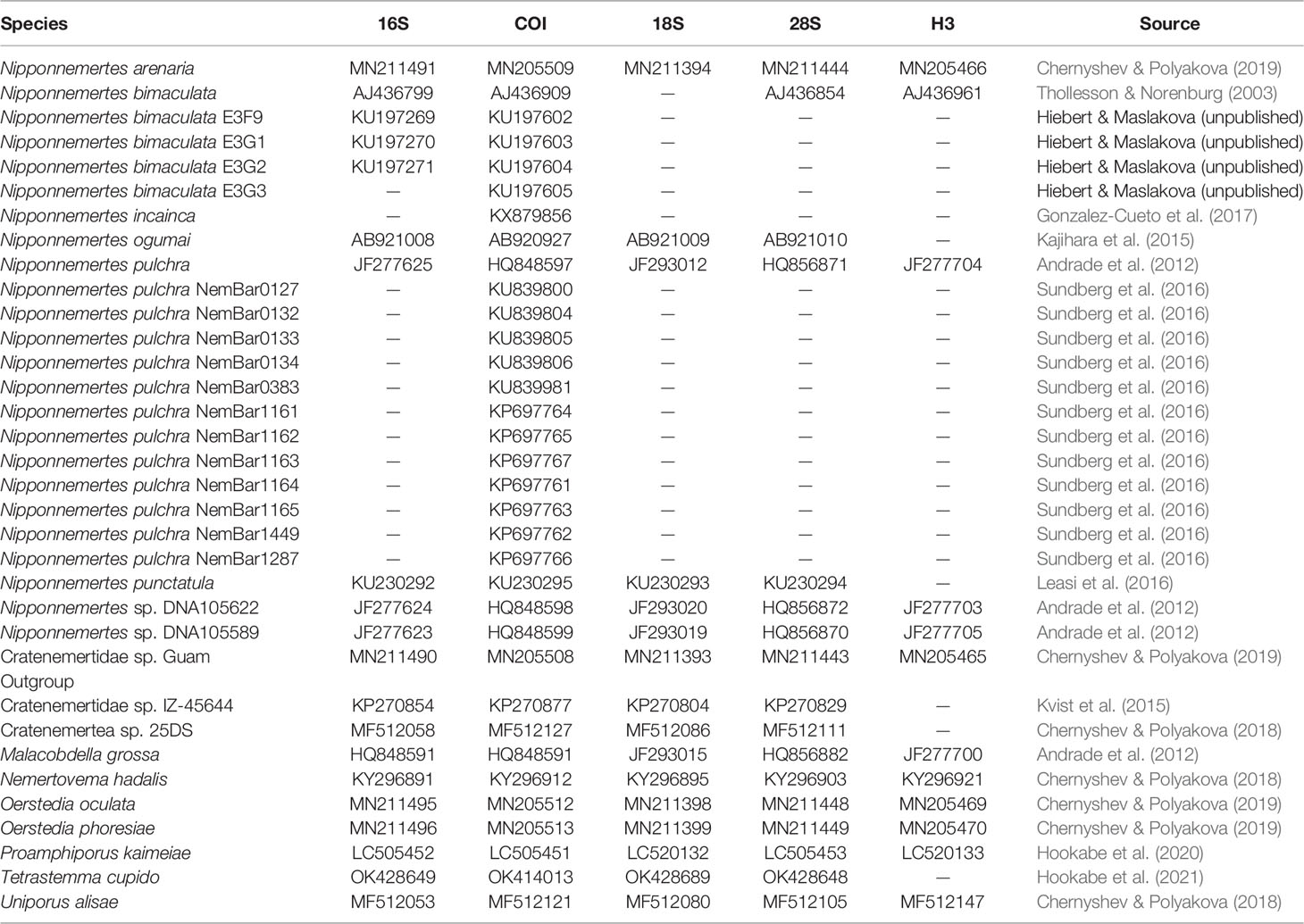
Table 3 GenBank accession numbers of eumonostiliferans except species herein described, which are used in the molecular phylogenetic analyses.
To infer phylogenetic relationships among cratenemertids examined in the present study, molecular phylogenetic trees were reconstructed based on maximum-likelihood (ML) and Bayesian inference (BI) analyses. The ML analysis was performed with RaxML ver. 8.0.0 (Stamatakis, 2014) under GTR + G + I selected as an optimum nucleotide substitution model by PartitionFinder ver. 2.1.1 (Lanfear et al., 2016) employing the greedy algorithm. Nodal support values were obtained from 1000 bootstrap pseudoreplicates. The BI tree was obtained using MrBayes ver. 3.2.3 (Ronquist et al., 2012) under GTR + G + I model selected by PartitionFinder ver. 2.1.1, launching two independent Metropolis-coupled analyses with four Markov chains for 107 generations and sampling every 100 generations from the chains. Run convergence was assessed by Tracer ver. 1.7 (Rambaut et al., 2018).
Uncorrected pairwise genetic distances were calculated based on 657-bp COI by MEGA ver. 7.
Species Delimitation Analyses
Using 658 bp of COI dataset, species delimitation analyses for Nipponnemertes species with cephalic patches were performed employing three methods: Automatic Barcode Gap Discovery (ABGD) (Puillandre et al., 2012), Bayesian implementation of the PTP (bPTP) (Zhang et al., 2013), and statistical parsimony (Templeton et al., 1992). The ABGD analyses was performed on the online server (https://bioinfo.mnhn.fr/abi/public/abgd/abgdweb.html) with default settings. The bPTP analyses were performed based on a ML tree generated by RaxML ver. 8.0.0 with the COI dataset. The statistical parsimony analysis was performed using the TCS algorithm (Clement et al., 2000) implemented in PopART (Leigh & Bryant, 2015). Haplotype networks for individual loci were generated for visualizing relationships between haplotypes by using PopART.
Results
Phylogenetic Relationships Among Nipponnemertes
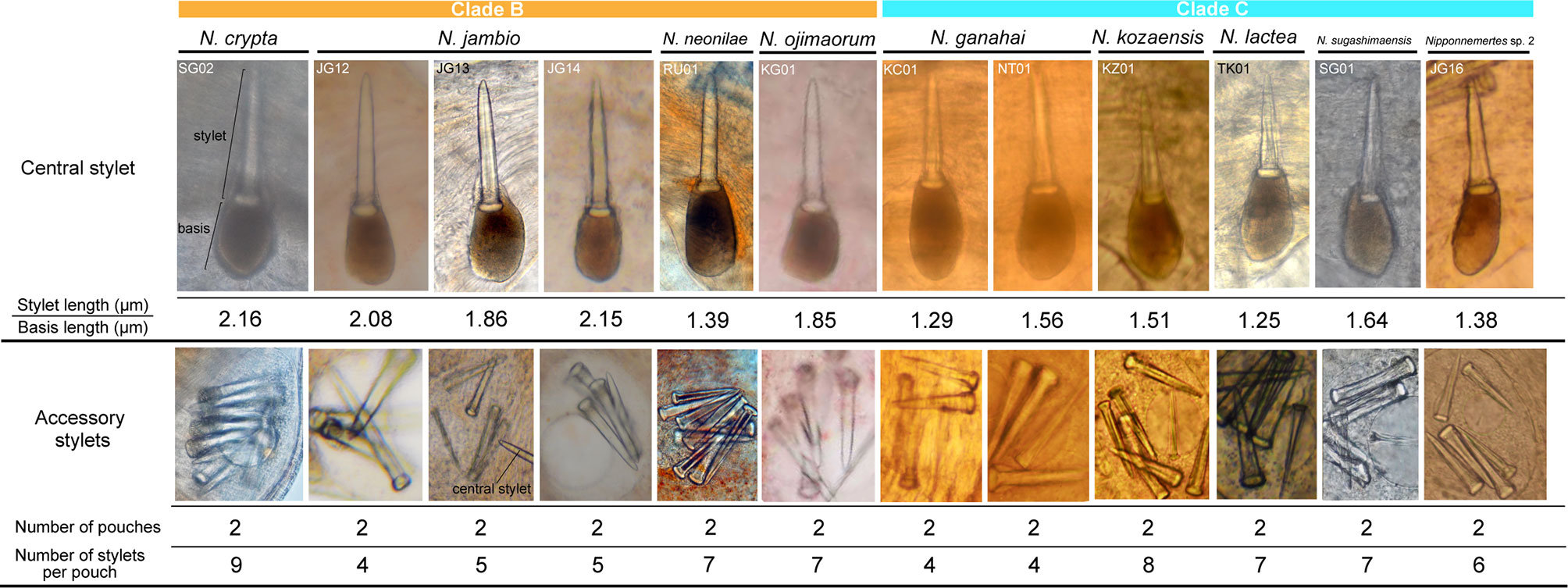
In the present study, we performed molecular phylogenetic analyses with 59 specimens of Nipponnemertes (Figures 2, 3). Of these, 10 species turned out to be new to science and described based on external (Figures 4–6) and internal morphology (Figures 7–16).
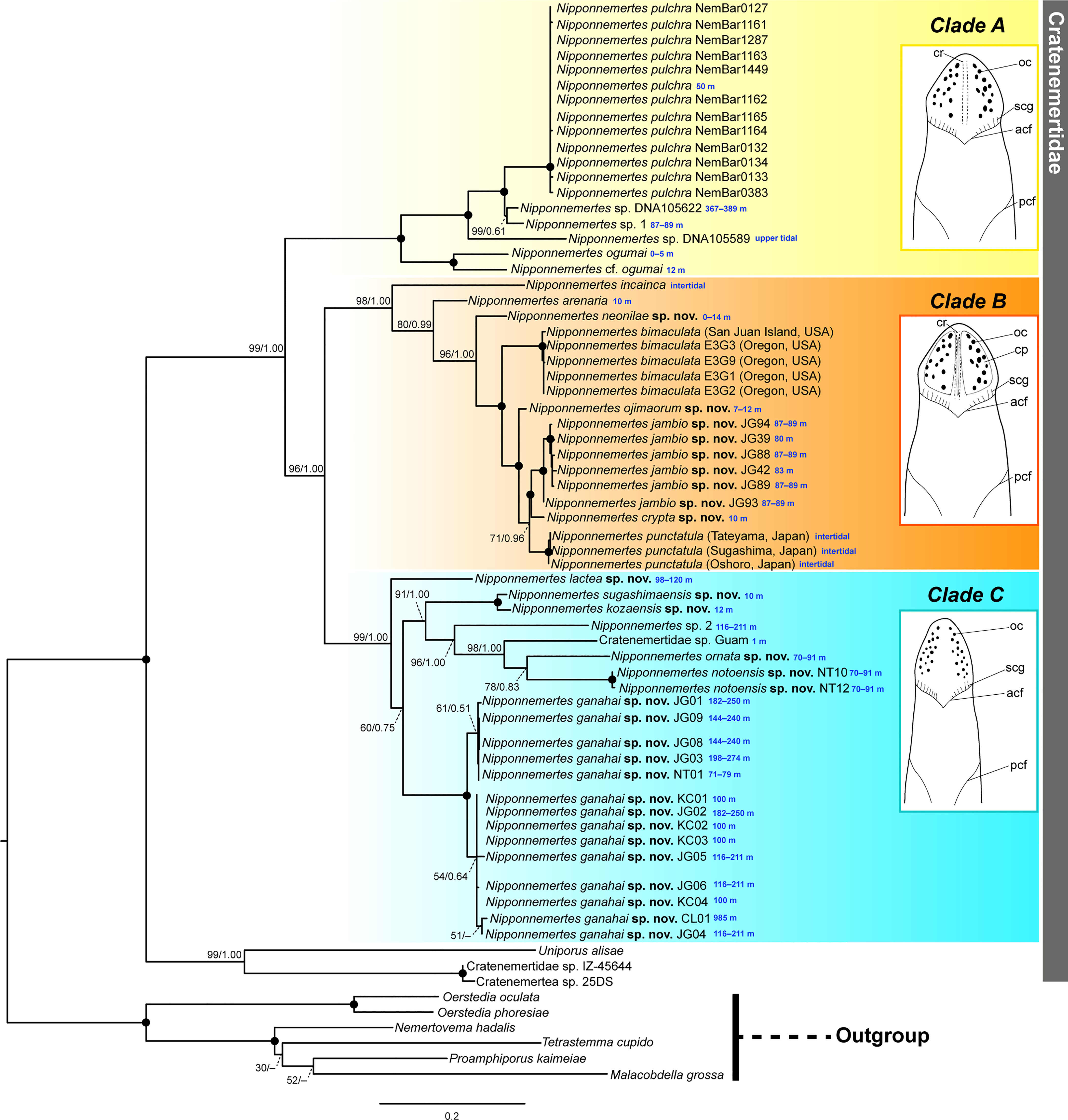
Figure 2 Molecular phylogenetic tree reconstructed based on ML analyses using concatenated sequences of 16S rRNA, COI, 18S rRNA, 28S rRNA, and histone H3 genes, with illustrations showing general head morphology of species constituting each clade. Tree topology obtained by BI was generally identical with ML. Numbers near nodes indicate bootstrap support values (BS) generated by 1000 replicates and posterior probability (PP) of a separate partitioned BI analysis (BS/PP). Solid circles indicate full support values (100/1.00). Collection depths are provided in blue letters. acf, anterior cephalic furrow; cp, cephalic patch; cr, mid-dorsal cephalic ridge; oc, ocelli; pcf, posterior cephalic furrow; scg, secondary transverse groove.
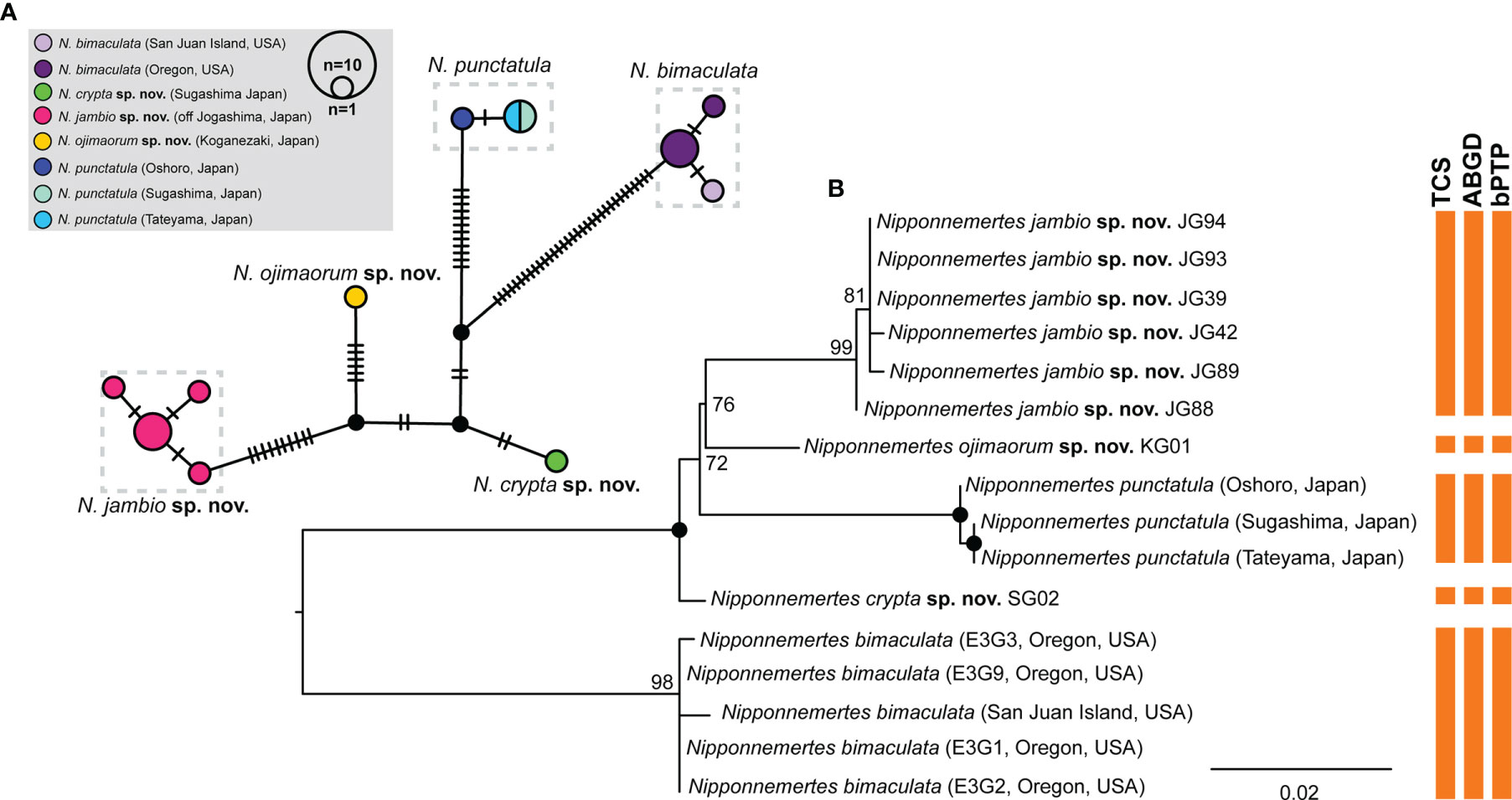
Figure 3 Results of species delimitation analyses for Nipponnemertes spp. in Clade (B). (A) A haplotype network generated by statistical parsimony analysis based on partial sequence (658 bp) of COI. Numbers of black bars correspond to the numbers of nucleotide substitutions between the haplotypes. (B) A ML tree based on 658 bp of COI. Vertical bars indicate the putative species delineated with TCS, ABGD, and bPTP methods. Solid circles indicate 100% of bootstrap values.

Figure 4 Images of Nipponnemertes spp., taken in life. (A, B), Nipponnemertes cf. ogumai, Sado (A, whole body; B, magnification of head). (C, D) Nipponnemertes sp. 1, off Jogashima (C, whole body; D, magnification of head). acf, anterior cephalic furrow; cr, mid-dorsal cephalic ridge. Scale bars: 1 cm (A, C), 5 mm (B, D).

Figure 5 Images of Nipponnemertes spp., taken in life. (A) Nipponnemertes arenaria, Peter the Great Bay. (B–D) Nipponnemertes crypta sp. nov., Sugashima (B, whole body; C, magnification of head, dorsal; D, ventral). (E–H) Nipponnemertes jambio sp. nov., off Jogashima (E), whole body; (F), magnification of head, dorsal; (G), ventral; (H), lateral). (I–K), Nipponnemertes ojimaorum sp. nov., Koganezaki [(I), whole body; (J), magnification of head, dorsal; (K), ventral]. (L–P), Nippponnemertes neonilae sp. nov., Vostok Bay [(L), whole body; (M), magnification of head, dorsal, living specimen; (N), magnification of head, dorsal, fixed specimen in formalin; (O), magnification of head, ventral, living specimen; (P), microphotograph of a live squeezed specimen, dorsal], (Q–T), Nippponnemertes punctatula [(Q), Tateyama; (R, S), Misaki; (T), Sugashima]. Each arrowhead points to posterior cephalic furrows. acf, anterior cephalic furrow; cg, cerebral ganglia; co, cerebral organ; cr, mid-dorsal cephalic ridge; ic, lateral diverticulum of intestinal caecum; pp, proboscis pore; py, pylorus. Scale bars: 1 cm (A, B, L, S), 5 mm (B, C, I, M, N, Q, T); 2.5 mm (D); 1 mm (E, R); 500 μm (F–H, J, K, O, P); 1.5 mm (I).
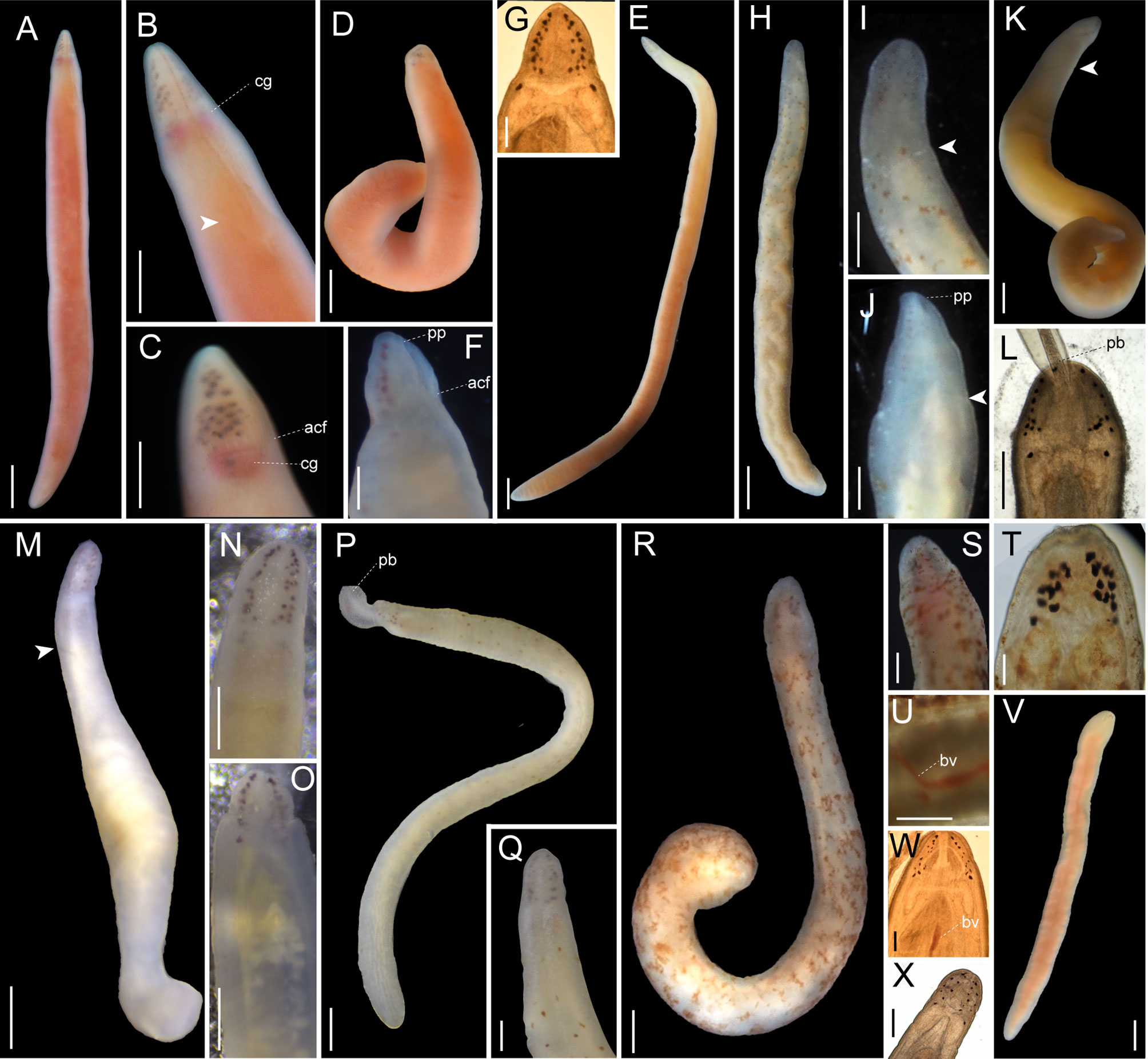
Figure 6 Images of Nipponnemertes spp., taken in life. (A–F), Nipponnemertes ganahai sp. nov., collected off Ofunato (A–C), off Jogashima (D), Tosa Bay (E–G) [(A), CL01, whole body; (B), magnification of head, dorsal; (C), lateral; (D), JG01, whole body; (E), KC01, whole body; (F), magnification of head, ventral; (G), microphotograph of a live squeezed specimen, dorsal]. (H–J), Nipponnemertes kozaensis sp. nov., Koza [(H), whole body; (I), magnification of head, ventral; (J), dorsal]. (K, L), Nipponnemertes lactea sp. nov., off Izu [(K), whole body; (L), microphotograph of a live squeezed specimen, ventral]. (M), Nipponnemertes notoensis sp. nov. [(M), whole body; (N), magnification of head, dorsal; (O), ventral]. (P, Q), Nipponnemertes ornata sp. nov., off Noto [(P), whole body; (Q), magnification of head, dorsal]. (R–U), Nipponnemertes sugashimaensis sp. nov., Sugashima [(R), whole body; (S), magnification of head, dorsal; (T), microphotograph of a live squeezed specimen, dorsal; (U), lateral blood vessel]. (V, W), Nipponnemertes sp. 2, off Jogashima [(V), whole body; (W), microphotograph of a live squeezed specimen, dorsal]. (X), Cratemertidae sp. Guam, a live squeezed specimen. Each arrowhead points to posterior cephalic furrows. acf, anterior cephalic furrow; bv, blood vessel; cg, cerebral ganglia; pb, proboscis; pp, proboscis pore. Scale bars: 2 mm (A, D, E), 1.5 mm (B); 1 mm (C, H, M, P, V); 500 μm (F, K, L, N, O, U); 300 μm (G, J, Q, S); 700 μm (I, R); 100 μm (T, W, X).
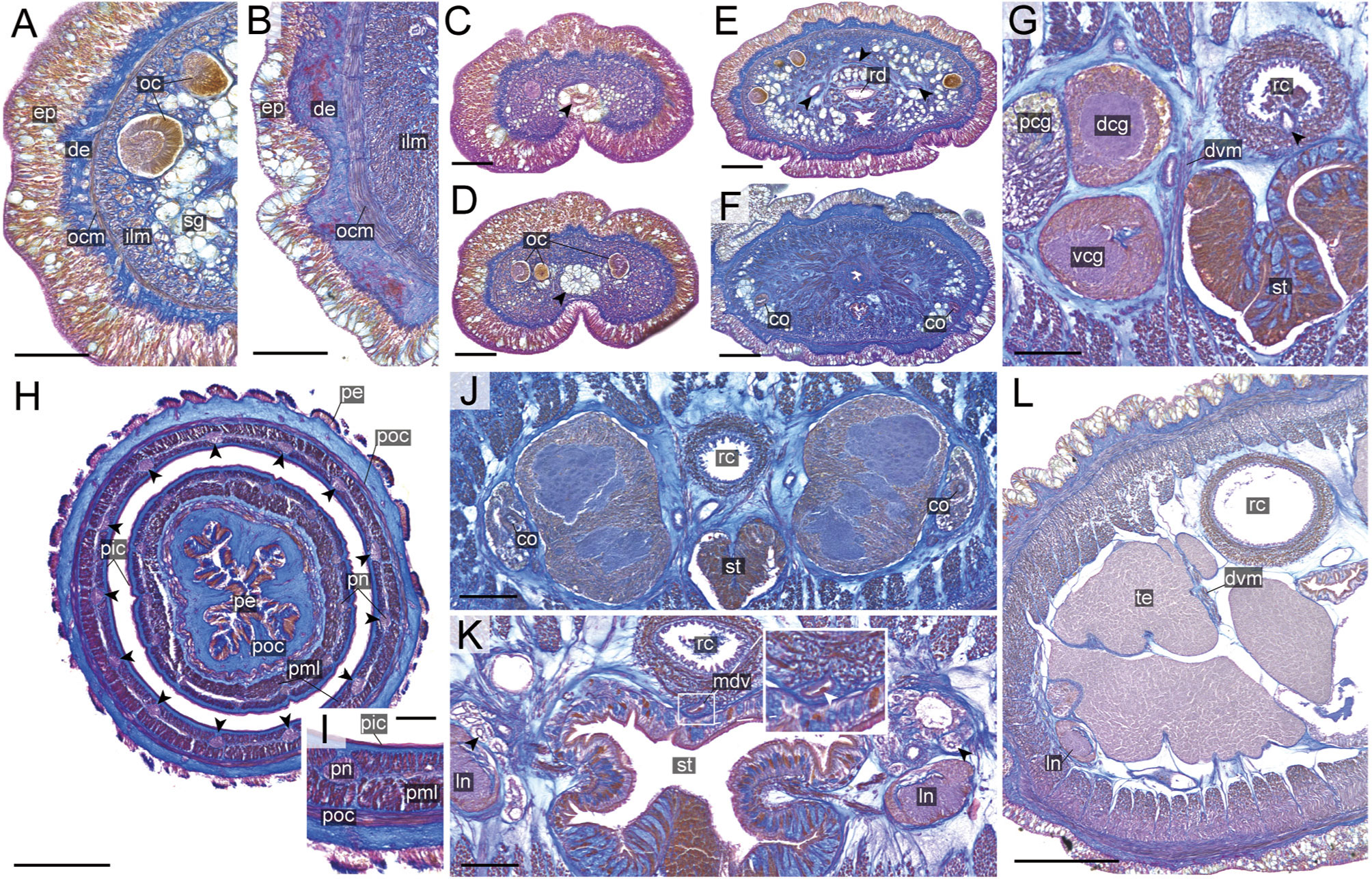
Figure 8 Nipponnemertes crypta sp. nov., transverse sections. (A) Body wall in precerebral region. (B) Body wall in foregut region. (C) Apical organ (arrowhead). (D) Cephalic glands (arrowhead). (E) Precerebral vessels (arrowheads). (F) Precerebral septum. (G) Vascular plug (arrowhead). (H) Proboscis, arrowheads pointing to proboscis nerves. (I) Magnification of proboscis musculature. (J) Posterior region of cerebral ganglia. (K) Nephridial canals (black arrowheads) and mid-dorsal vessel (white box, a white arrowhead pointing to a blood cell in mid-dorsal vessel). (L) Testes. co, cerebral organ; dcg, dorsal cerebral ganglia; de, dermis; dvm, dorso-ventral muscle in body wall; ep, epidermis; ilm, body-wall inner longitudinal muscle; mdv, mid-dorsal vessel; oc, ocelli; ocm, body-wall outer circular muscle; pcg, postcerebral glands of cerebral organ; pe, proboscis epithelium; pic, proboscis inner circular muscle; pml, proboscis middle longitudinal muscle; pn, proboscis nerve; poc, proboscis outer circular muscle; rc, rhynchocoel; rd, rhynchodaeum; st, stomach; sg, submuscular glands; te, testis; vcg, ventral cerebral ganglia. Scale bars: 50 μm (A–D, I), 100 μm (E, F, K), 200 μm (G), 150 μm (H, J), 250 μm (L).
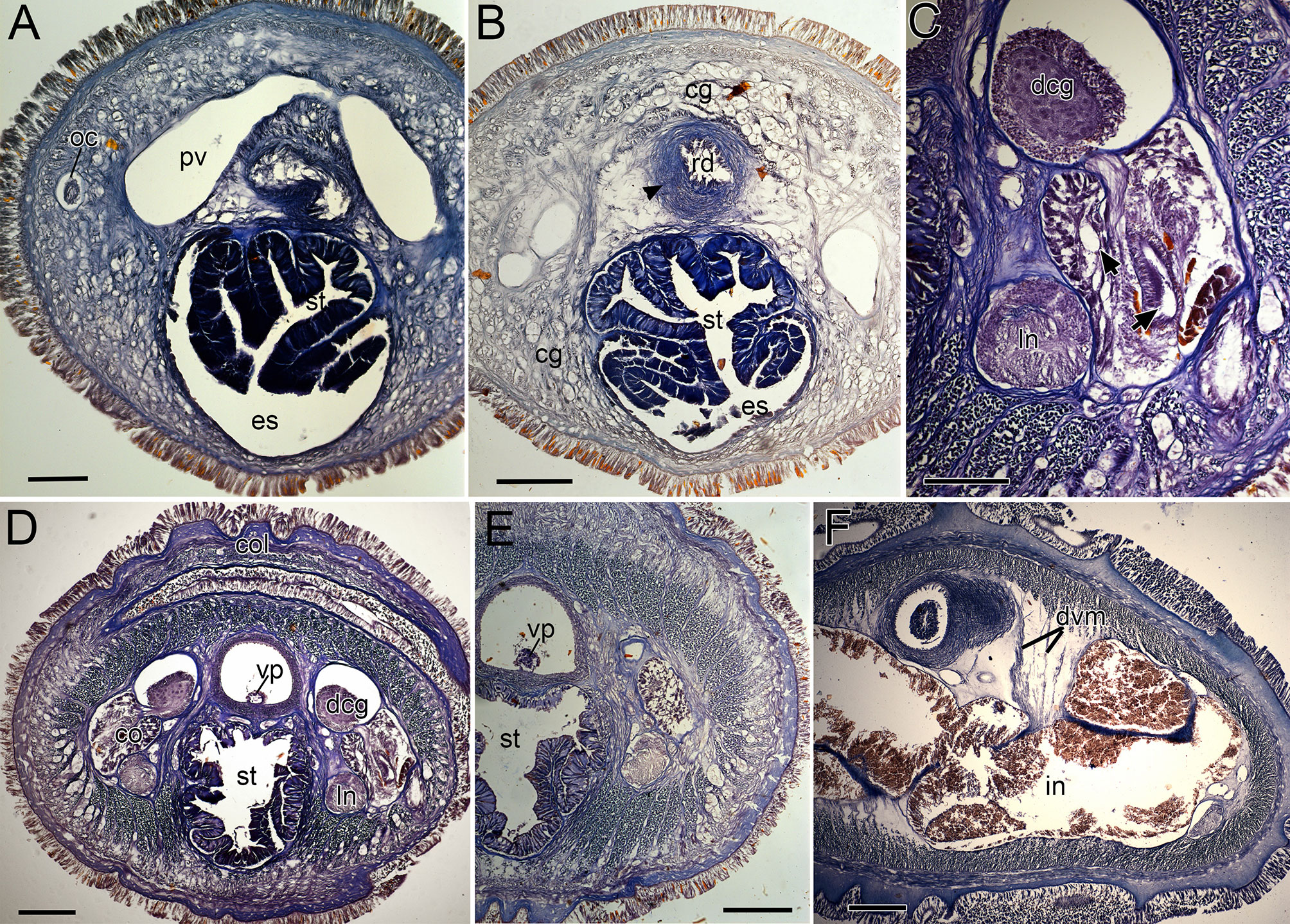
Figure 9 Nipponnemertes neonilae sp. nov., transverse sections. (A) Anterior part of cephalic blood lacuna. (B) Rhynchodeal sphincter (arrowhead). (C) Cerebral organ (arrows indicate bifurcate canal of cerebral organ). (D) Collar. (E) Stomach. (F) Intestine. cg, cephalic glands; co, cerebral organ; col, collar; dcg, dorsal cerebral ganglia; dvm, dorso-ventral muscles; es, esophagus; in, intestine; ln, lateral nerve; oc, ocelli; pv, precerebral vessel; rd, rhynchodaeum; st, stomach; vp, vascular plug. Scale bars: 100 μm (A, C), 200 μm (B, D–F).
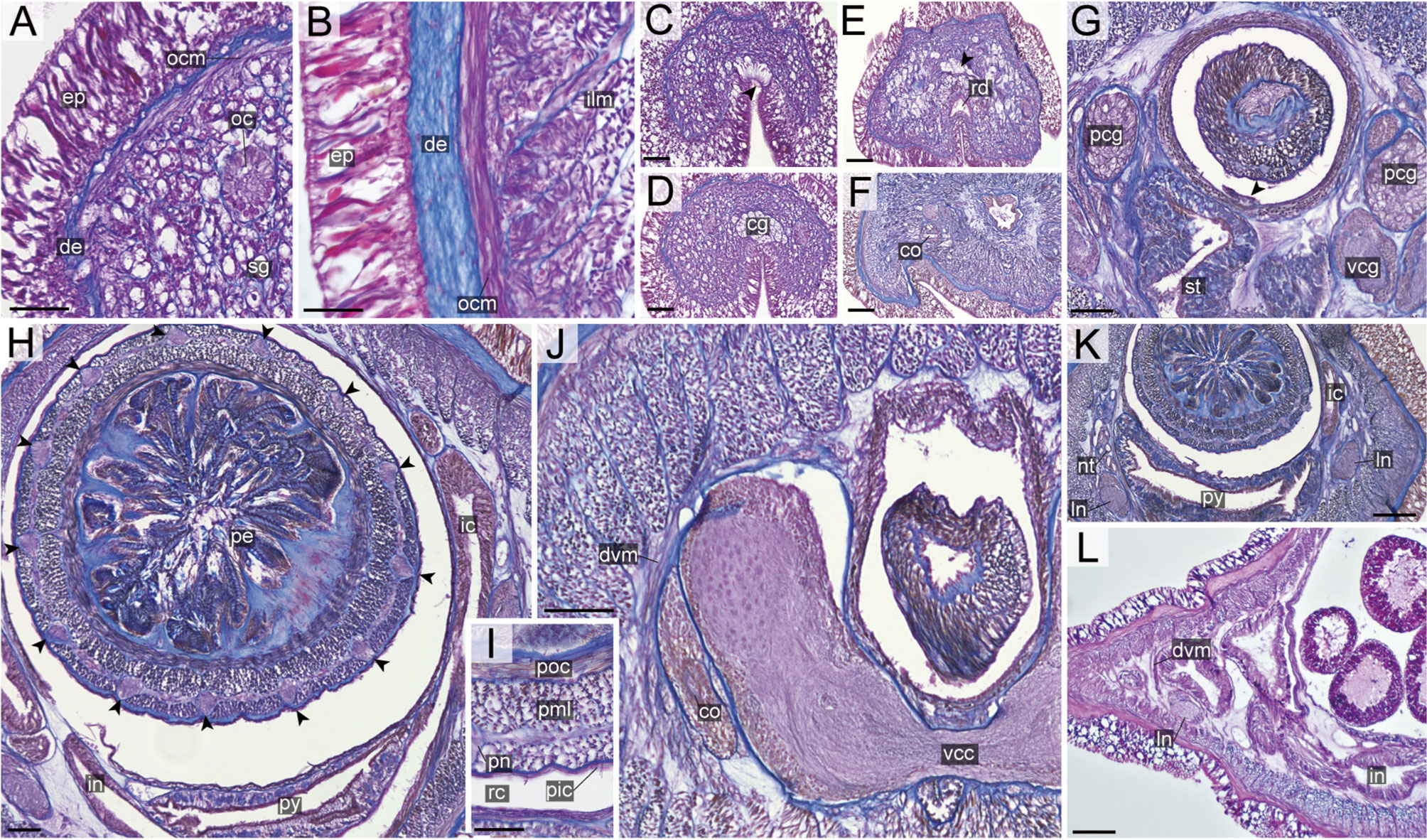
Figure 10 Nipponnemertes ojimaorum sp. nov., transverse sections. (A) Body wall in precerebral region. (B) Body wall in foregut region. (C) Apical organ (arrowhead). (D) Cerebral glands. (E) Precerebral vessel (arrowhead). (F) Cerebral organ opening. (G) Mid-dorsal vessel (arrowehead). (H) Proboscis, arrowheads pointing to proboscis nerves. (I) Magnification of proboscis musculature. (J) Ventral cerebral commissure. (K) Nephridial tubules. (L) Intestine. co, cerebral organ; dcg, dorsal cerebral ganglia; de, dermis; dvm, dorso-ventral muscle in body wall; ep, epidermis; ic, lateral diverticulum of intestinal caecum; ilm, body-wall inner longitudinal muscle; in, intestine; ln, lateral nerve; mdv, mid-dorsal vessel; nt, nephridial tubule; oc, ocelli; ocm, body-wall outer circular muscle; pcg, postcerebral glands of cerebral organ; pe, proboscis epithelium; pic, proboscis inner circular muscle; pml, proboscis middle longitudinal muscle; pn, proboscis nerve; poc, proboscis outer circular muscle; rc, rhynchocoel; rd, rhynchodaeum; sg, submuscular glands; st, stomach; vcg, ventral cerebral ganglia. Scale bars: 50 μm (A, C, D, E), 25 μm (B, F), 100 μm (G, J–L), 75 μm (H), 30 μm (I).
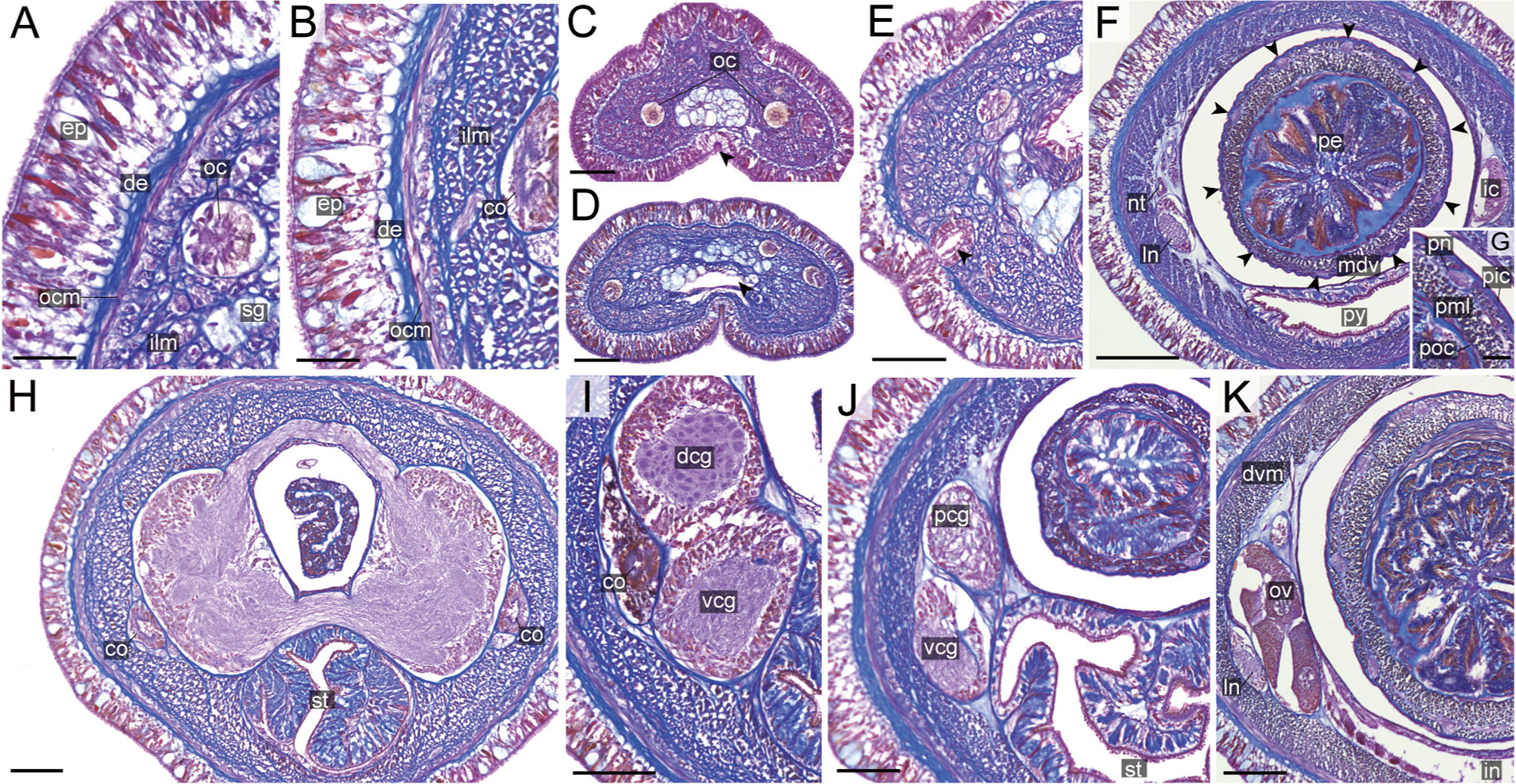
Figure 11 Nipponnemertes ganahai sp. nov., transverse sections. (A) Body wall in precerebral region. (B) Body wall in foregut region. (C) Apical organ (white arrowhead) and cephalic glands (black arrowhead). (D) Precerebral vessels (arrowhead). (E) Cerebral organ opening (arrowhead). (F) Proboscis, arrowheads pointing to proboscis nerves. (G) Magnification of proboscis musculature. (H) Dorsal and ventral cerebral commissure. (I) Cerebral organ. (J) Posterior region of cerebral ganglia. (K) Ovaries. co, cerebral organ; dcg, dorsal cerebral ganglia; de, dermis; dvm, dorso-ventral muscle in body wall; ep, epidermis; ic, lateral diverticulum of intestinal caecum; ilm, body-wall inner longitudinal muscle; in, intestine; ln, lateral nerve; mdv, mid-dorsal vessel; nt, nephridial tubule; oc, ocelli; ocm, body-wall outer circular muscle; ov, ovaries; pcg, postcerebral glands of cerebral organ; pe, proboscis epithelium; pic, proboscis inner circular muscle; pml, proboscis middle longitudinal muscle; pn, proboscis nerve; poc, proboscis outer circular muscle; py, pylorus; sg, submuscular glands; st, stomach; vcg, ventral cerebral ganglia. Scale bars: 30 μm (A, B, G), 50 μm (C–E, J, K), 150 μm (F), 75 μm (H, I).
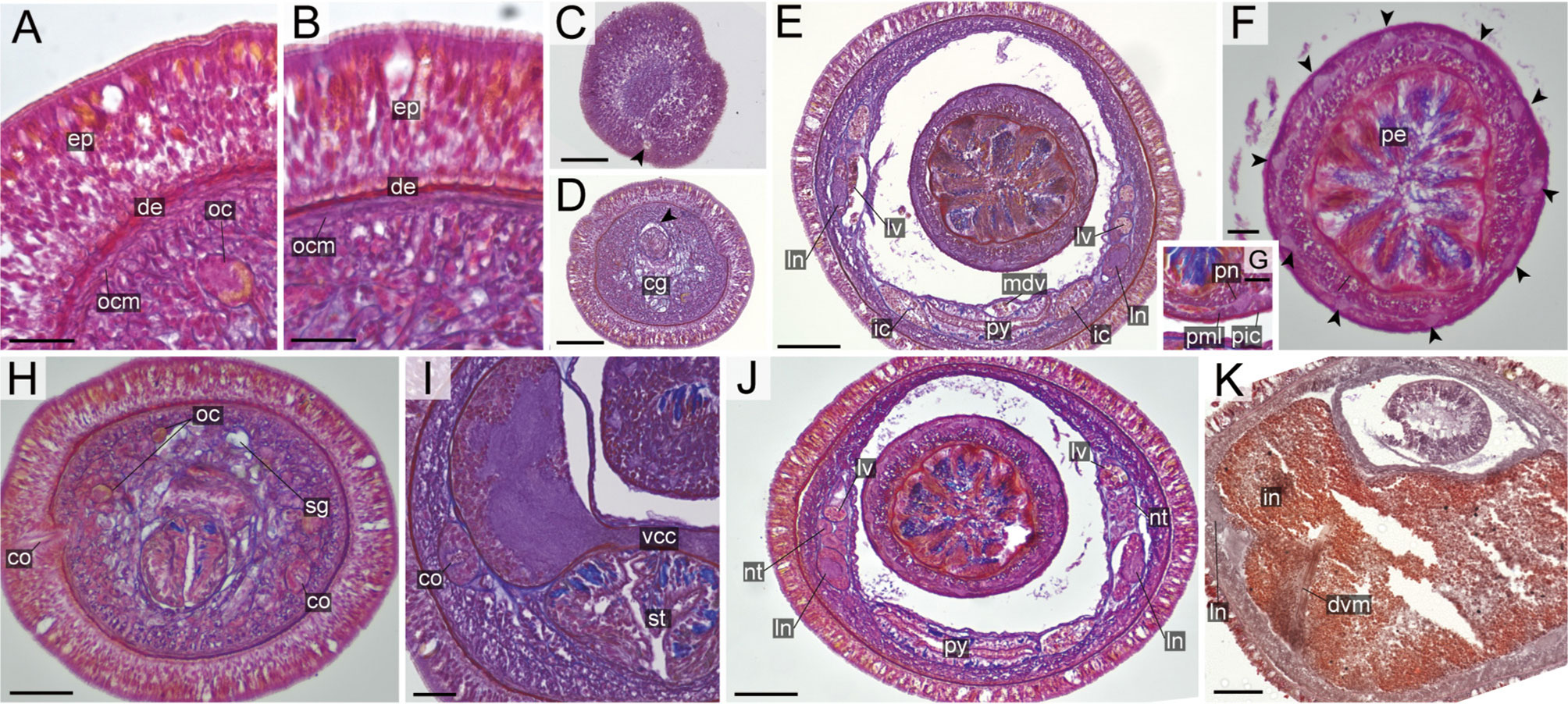
Figure 12 Nipponnemertes kozaensis sp. nov., transverse sections. (A) Body wall in precerebral region. (B) Body wall in foregut region. (C) Apical organ (arrowhead). (D) Precerebral vessel (arrowhead) running above cerebral glands. (E) Mid-dorsal vessel. (F) Proboscis, arrowheads pointing to proboscis nerves. (G) Magnification of proboscis musculature. (H) Cerebral organ opening. (I) Cerebral organ. (J) Nephridial tubules. (K) Intestine. cg, cephalic glands; co, cerebral organ; de, dermis; dvm, dorso-ventral muscle in body wall; ep, epidermis; ic, lateral diverticulum of intestinal caecum; in, intestine; ln, lateral nerve; lv, lateral vessel; mdv, mid-dorsal vessel; nt, nephridial tubule; oc, ocelli; ocm, body-wall outer circular muscle; pcg, postcerebral glands of cerebral organ; pe, proboscis epithelium; pic, proboscis inner circular muscle; pml, proboscis middle longitudinal muscle; pn, proboscis nerve; py, pylorus; st, stomach; vcc, ventral cerebral commissure. Scale bars: 15 μm (A, B), 50 μm (C, D, F), 75 μm (E, H, J, K), 25 μm (G), 30 μm (I).
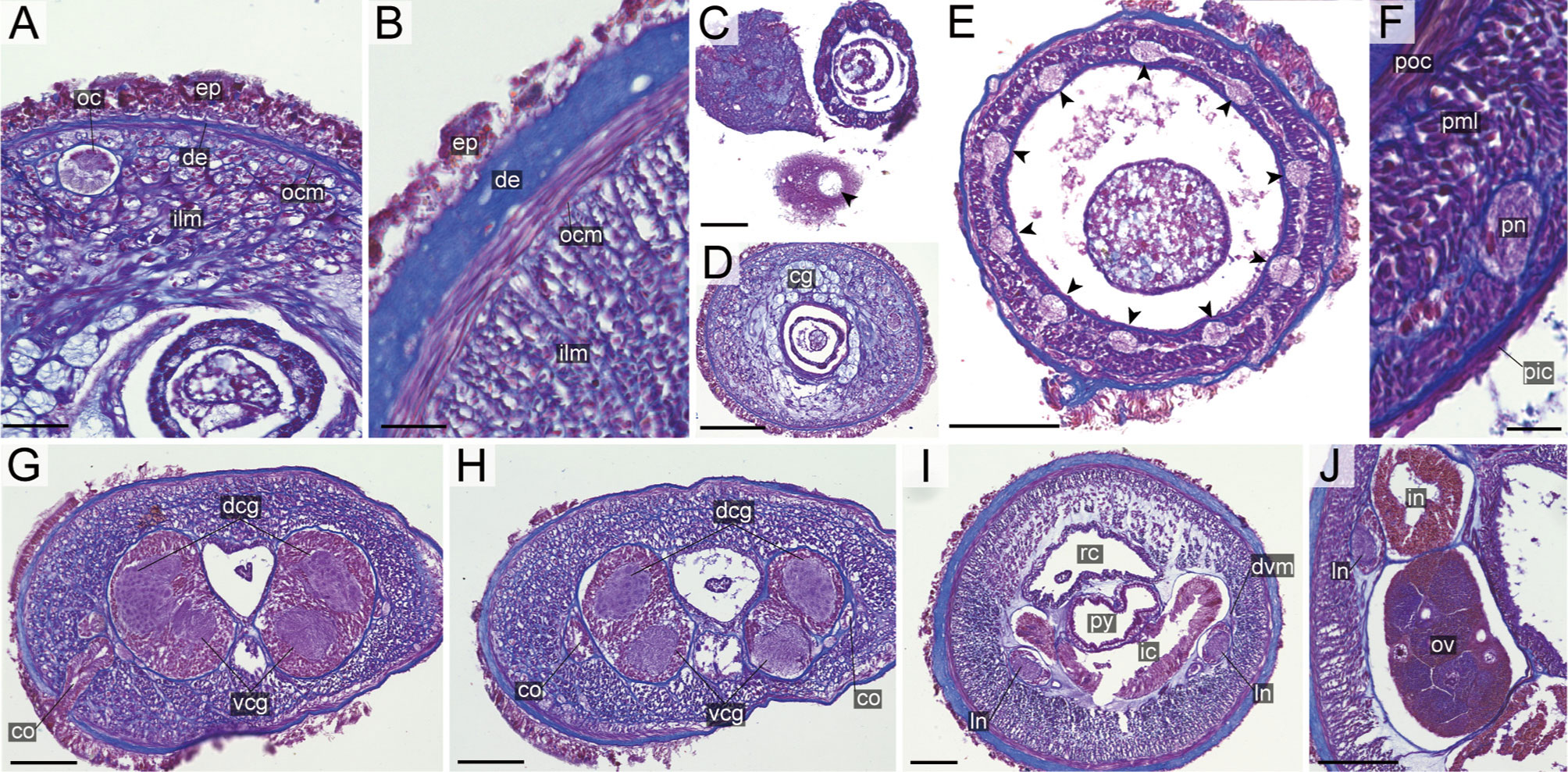
Figure 13 Nipponnemertes lactea sp. nov., transverse sections. (A) Body wall in precerebral region. (B) Body wall in foregut region. (C) Apical organ (arrowhead). (D) Cerebral glands. (E) Proboscis, arrowheads pointing to proboscis nerves. (F) Magnification of proboscis musculature. (G) Cerebral organ opening. (H) Posterior region of cerebral ganglia. (I) Pylorus. (J) Ovaries. co, cerebral organ; dcg, dorsal cerebral ganglia; de, dermis; dvm, dorso-ventral muscle in body wall; ep, epidermis; ic, lateral diverticulum of intestinal caecum; ilm, body-wall inner longitudinal muscle; in, intestine; ln, lateral nerve; oc, ocelli; ocm, body-wall outer circular muscle; ov, ovary; pe, proboscis epithelium; pic, proboscis inner circular muscle; pml, proboscis middle longitudinal muscle; pn, proboscis nerve; poc, proboscis outer circular muscle; py, pylorus; rc, rhynchocoel; vcg, ventral cerebral ganglia. Scale bars: 30 μm (A, F), 25 μm (B, C), 100 μm (D, E), 75 μm (G–I), 50 μm (J).
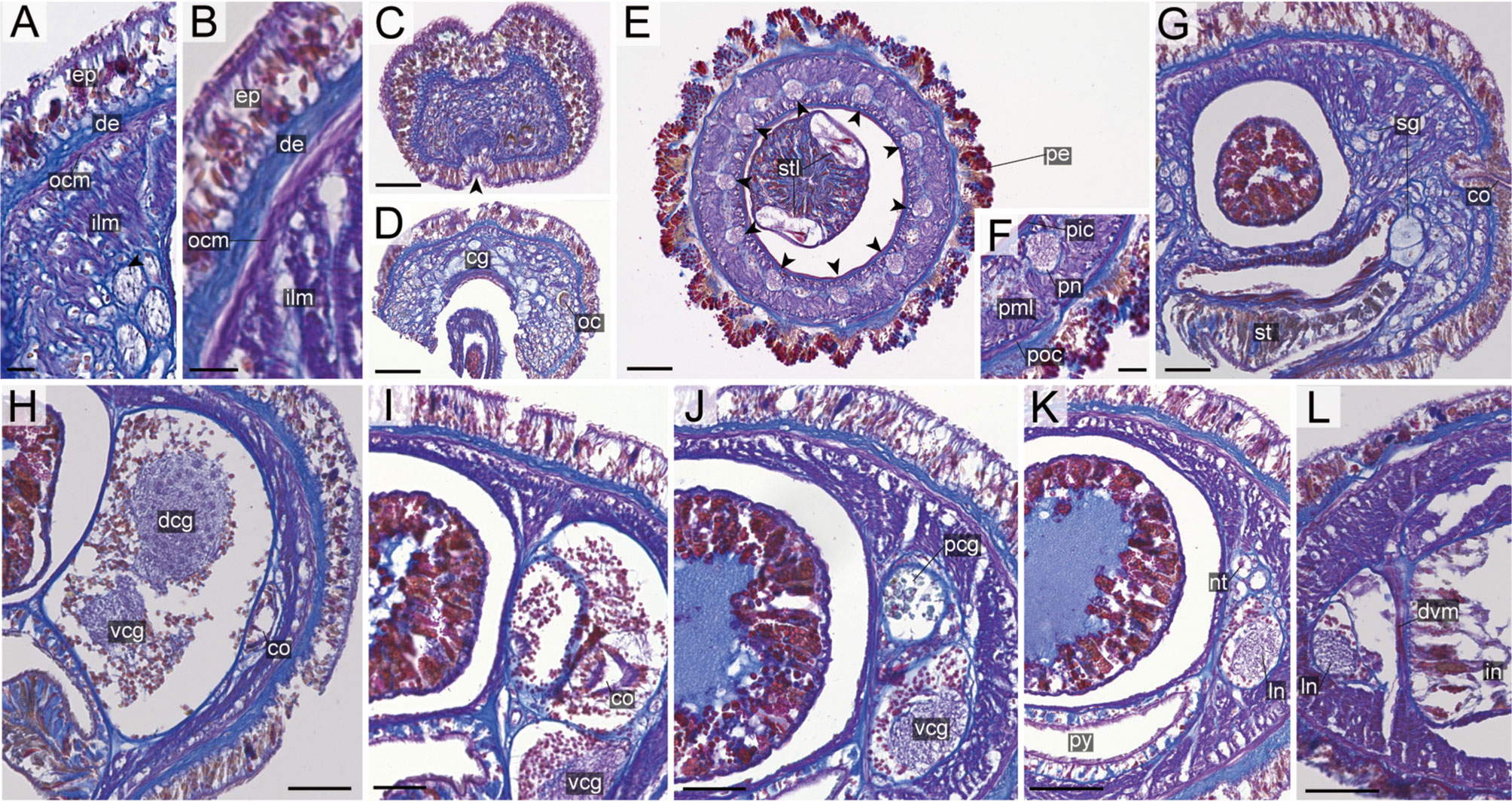
Figure 14 Nipponnemertes notoensis sp. nov., transverse sections. (A) Body wall in precerebral region. (B) Body wall in foregut region. (C) Apical organ (arrowhead). (D) Cerebral glands. (E) Proboscis, arrowheads pointing to proboscis nerves. (F) Magnification of proboscis musculature. (G) Cerebral organ opening. (H) Middle region of cerebral organ (I) posterior region of cerebral organ. (J) Postcerebral glands of cerebral organ. (K) Nephridial tubules. (L) Intestine. cg, cephalic glands; co, cerebral organ; dcg, dorsal cerebral ganglia; de, dermis; dvm, dorso-ventral muscle in body wall; ep, epidermis; ilm, body-wall inner longitudinal muscle; in, intestine; ln, lateral nerve; nt, nephridial tubule; oc, ocelli; ocm, body-wall outer circular muscle; pcg, postcerebral glands of cerebral organ; pe, proboscis epithelium; pic, proboscis inner circular muscle; pml, proboscis middle longitudinal muscle; pn, proboscis nerve; poc, proboscis outer circular muscle; py, pylorus; sg, submuscular glands; st, stomach; stl, stylet; vcg, ventral cerebral ganglia. Scale bars: 15 μm (A, B), 60 μm (C, D, L), 75 μm (E), 30 μm (F, I, J), 50 μm (G, H, K).
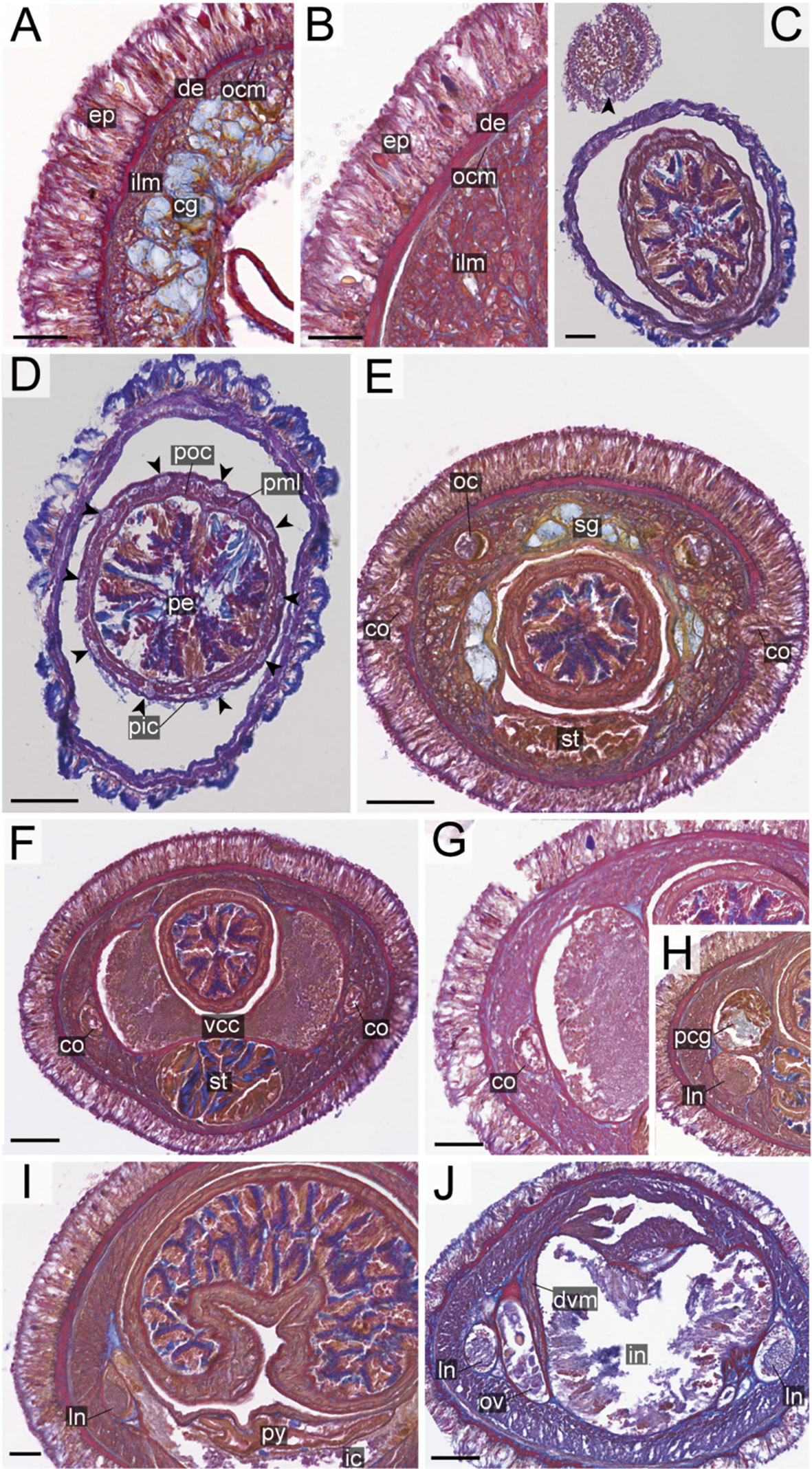
Figure 15 Nipponnemertes ornata sp. nov., transverse sections. (A) Body wall in precerebral region. (B) Body wall in foregut region. (C) Apical organ (arrowhead). (D) Proboscis, arrowheads pointing to proboscis nerves. (E) Cerebral organ opening. (F) Ventral cerebral commissure. (G) Cerebral organ. (H) Postcerebral glands. (I) Pylorus and intestinal caecum. (J) Ovaries. cg, cephalic glands; co, cerebral organ; de, dermis; dvm, dorso-ventral muscle in body wall; ep, epidermis; ic, lateral diverticulum of intestinal caecum; ilm, body-wall inner longitudinal muscle; in, intestine; ln, lateral nerve; nt, nephridial tubule; ocm, body-wall outer circular muscle; ov, ovaries; pcg, postcerebral glands of cerebral organ; pe, proboscis epithelium; pic, proboscis inner circular muscle; pml, proboscis middle longitudinal muscle; pn, proboscis nerve; poc, proboscis outer circular muscle; py, pylorus; st, stomach; sg, submuscular glands; vcc, ventral cerebral commissure. Scale bars: 20 μm (A, B, H, I), 50 μm (C, E, F, J), 75 μm (D), 30 μm (G).
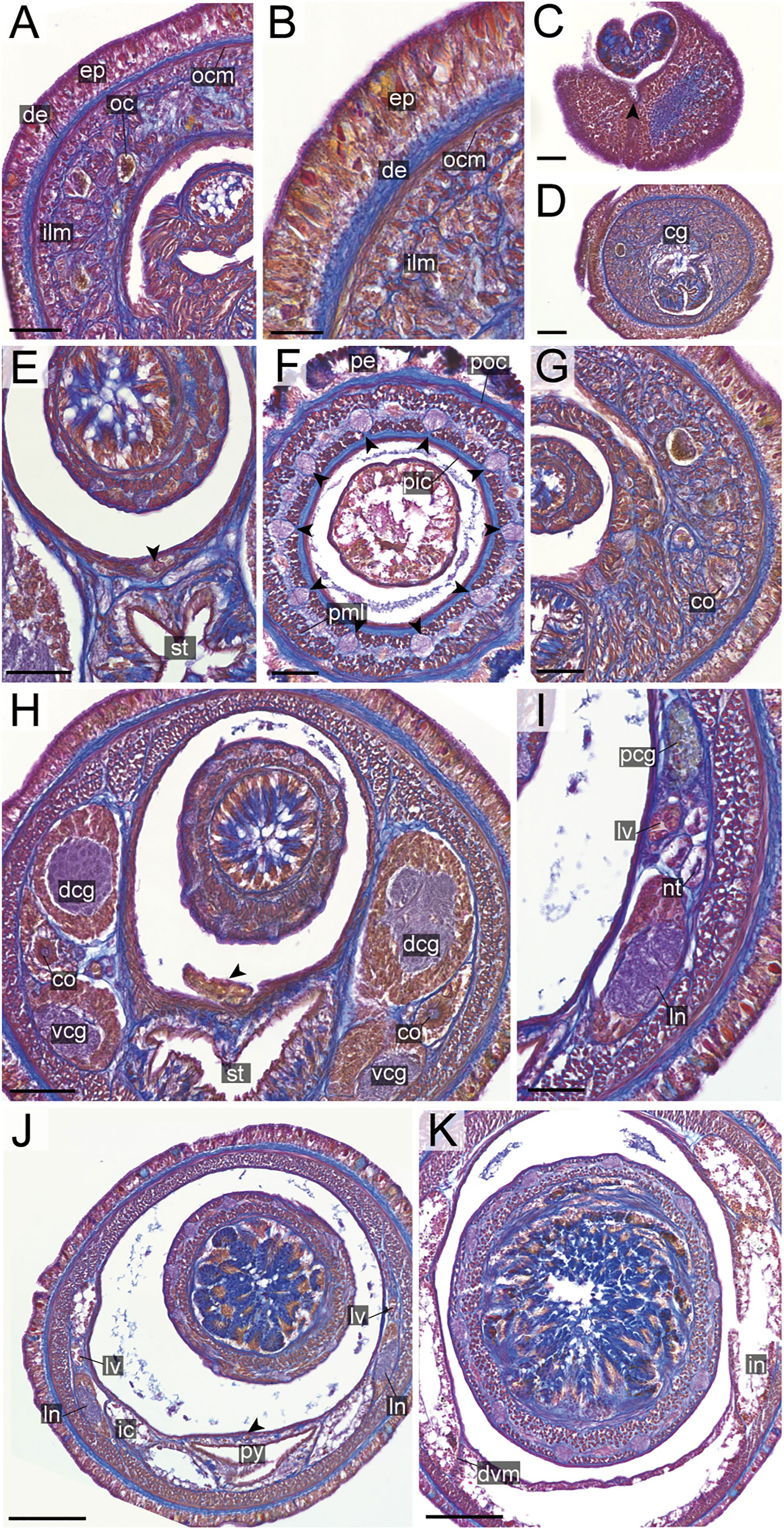
Figure 16 Nipponnemertes sugashimaensis sp. nov., transverse sections. (A) Body wall in precerebral region. (B) Body wall in foregut region. (C) Apical organ (arrowhead). (D) Cerebral glands. (E) Mid-dorsal vessel (arrowhead). (F) Proboscis, arrowheads pointing to proboscis nerves, (G) Cerebral organ opening. (H) Posterior region of cerebral ganglia, arrowhead pointing to vascular plug. (I) Nephridial tubules. (J) Pylorus, arrowhead pointing to mid-dorsal vessel. (K) Intestine. cg, cephalic glands; co, cerebral organ; dcg, dorsal cerebral ganglia; de, dermis; dvm, dorso-ventral muscle in body wall; ep, epidermis; ic, lateral diverticulum of intestinal caecum; ilm, body-wall inner longitudinal muscle; in, intestine; ln, lateral nerve; lv, lateral vessel; nt, nephridial tubule; oc, ocelli; ocm, body-wall outer circular muscle; pcg, postcerebral glands of cerebral organ; pe, proboscis epithelium; pic, proboscis inner circular muscle; pml, proboscis middle longitudinal muscle; pn, proboscis nerve; poc, proboscis outer circular muscle; py, pylorus; vcg, ventral cerebral ganglia. Scale bars: 30 μm (A), 20 μm (B), 50 μm (C–F, G–I), 150 μm (J), 100 μm (K).
In the resulting tree (Figure 2), a clade constituted by 59 specimens of Nipponnemertes was sister to another clade formed by the two bathyal species, Cratenemertidae sp. IZ-45644 and Cratenemertea sp. 25DS (possibly pelagic juvenile of Cratenemertidae sp. IZ-45644) and an abyssal species, Uniporus alisae (Chernyshev & Polyakova, 2018). The Nipponnemertes clade was divided into three major clades (Figure 2). The tree topology reconstructed by the ML analysis was almost identical with the BI tree. Below, we refer to the “N. pulchra–Nipponnemertes cf. ogumai” clade as Clade A, the “N. incainca–N. punctatula” clade as Clade B, and the “N. lactea–N. ganahai” clade as Clade C.
In Clade A, Nipponnemertes cf. ogumai from Sado (the Sea of Japan) (Figures 4A, B) was a sister taxon of N. ogumai from Araihama (Pacific Ocean); they were nested in a clade along with N. pulchra, Nipponnemertes sp. DNA105622, Nipponnemertes sp. DNA105589 (Andrade et al., 2012), and Nipponnemertes sp. 1 (Figures 4C, D). Nipponnemertes sp. 1 and Nipponnemertes sp. DNA105622 (Andrade et al., 2012) from California constituted a clade supported by 99% of bootstrap value (BS); between the two species, the genetic distances based on COI were 3.4% in uncorrected p-distance (Table S1), smaller than interspecific genetic distances previously reported in Nipponnemertes (Chernyshev & Polyakova, 2022).
Within Clade B, N. incainca was first branched from the rest of the members in this clade. Nipponnemertes neonilae sp. nov. collected from the Sea of Japan and Kuril Islands (Figures 5L–P) was sister-related with a clade constituted by “N. bimaculata–N. punctatula” clade with full support value. Nipponnemertes ojimaorum sp. nov. collected from Koganezaki was nested in the “N. bimaculata–N. punctatula” clade, together with Nipponnemertes jambio sp. nov. and Nipponnemertes crypta sp. nov.
Clade C was detected for the first time in this study; all members were herein newly included in molecular analyses. In this clade, Nipponnemertes lactea sp. nov. (Figures 6K, L) was sister to the rest of the members. Nipponenemertes ganahai sp. nov. (Figures 6A–G) formed a clade weakly supported by 60% of BS and 0.75 of PP with N. kozaensis sp. nov. (Figures 6H–J), N. notoensis sp. nov. (Figures 6M–O), N. ornata sp. nov. (Figures 6P, Q), N. sugashimaensis sp. nov. (Figures 6R–U), Nipponnemertes sp. 2 (Figures 6V, W), and Cratenemertidae sp. Guam (Figure 6X) (Chernyshev & Polyakova, 2019).
Species Delimitation for Clade B
We performed species delimitation analyses using partial sequences of COI (658 bp) to examine putative species boundaries among Clade B. Five distinct lineages (each corresponding to N. bimaculata, N. crypta, N. jambio, N. ojimaorum, and N. punctatula) were identified by all three methods (TCS, ABGD, and bPTP) (Figure 3). Among them, the maximum value of intraspecific genetic distance was 7.9% of uncorrected p-distance (N. bimaculata–N. jambio and N. bimaculata–N. punctatula); the minimum value was 1.6% between N. crypta and N. ojimaorum.
Systematics
Family Cratenemertidae Friedrich, 1968
Genus Nipponnemertes Gibson & Crandall, 1989
(= Collarenemertes Chernyshev, 1993)
Type species: Nipponnemertes drepanophoroides Griffin, 1898.
Diagnosis [given by Friedrich (1968); Gibson & Crandall (1989), and Riser (1998)]: Cratenemertids with generally more than 10 eyes on each side of head in adults. Mouth and rhynchodaeum opening via common pore. Anterior cephalic furrows with secondary longitudinal grooves; latter faintly visible or invisible in small individuals. Mid-dorsal cephalic ridge present or absent. Rhynchocoel extending to posterior end of body, consisting of interwoven longitudinal and circular muscle fibres, without lateral pouches. Mid-dorsal vessel penetrating into rhynchocoel to form a single vascular plug. Apical organ and cephalic glands present. Large cerebral organs extending behind brain.
1 Nipponnemertes cf. ogumai
Material examined: Posterior tip used for DNA extraction, collected at depths of 8 m by SCUBA diving, Shukunegi, Sado Island, Niigata Prefecture, Japan (Figure 1A), by Naoto Jimi, on 26 September 2019.
Description: Body 4.5 cm in length, 4.2–6.0 mm in width; body uniformly orange coloured (Figures 4A, B). Head conspicuously demarcated from trunk; cephalic patch absent; proboscis pore depressed; mid-dorsal cephalic ridge present but not well developed (Figure 4B). Anterior cephalic furrows completely encircling body, meeting at midline on dorsal surface (Figure 4B). Ocelli numerous, arranged in lateral margin of head (Figure 4B).
Remarks: In the present study, we refer to the Sado specimen as Nipponnemertes cf. ogumai due to morphological similarity in the uniformly orange body. A genetic distances between N. ogumai collected from intertidal zone of Araihama, Pacific coast of Japan (Kajihara et al., 2015) is 6.6% in uncorrected p-distance (Table S1), comparable with interspecific COI divergences among hoplonemerteans (Sundberg et al., 2016). Without samples for deposition in museum except the total DNA, we leave a species description to a future study when additional materials for further observation and museum deposition are available.
2 Nipponnemertes sp. 1
Material examined: ICHUM 8331, preserved in 99% ethanol; posterior tip used for DNA extraction, collected at depths of 87–89 m by dredging, off Jogashima, Kanagawa Prefecture (35°8.42′N, 139°34.74′E–335°8.22′N, 139°34.67′E), by Hiroshi Kajihara, on 19 February 2014.
Description: Body 1.2 cm in length, 0.8–1.2 mm in width; body uniformly pale-orange coloured (Figures 4C, D). Head demarcated from trunk; cephalic patch absent; mid-dorsal cephalic ridge present (Figure 4D). Anterior cephalic furrow completely encircling body, nearly M-shaped on dorsal surface (Figure 4D). As many as 19 ocelli irregularly arranged on each side (Figure 4D).
Remarks: A genetic distance between the present specimen and Nipponnemertes sp. DNA105622 collected from California at depths of 367–389 m (Andrade et al., 2012) is 3.2% in uncorrected p-distance (Table S1); these values fall within previously reported intraspecific COI divergences among hoplonemerteans (Sundberg et al., 2016). Due to the lack of museum deposition, we leave a species description to a future study.
3 Nipponnemertes crypta sp. nov. Hookabe, Kajihara & Chernyshev
[New Japanese name: kakure-omen-himomushi]
(Figures 5B–D, 7, 8)
Material examined: Holotype: ICHUM 8315, serial transverse sections of anterior tip to intestine, a posterior tip used for DNA extraction, bycaught with a gill net from 10 m depth, Sugashima, Mie Prefecture, Japan (Figures 1A, D), collected by Natsumi Hookabe and Naoto Jimi, on 3 November 2021.
Habitat and geographic distribution: The species is only known from the type locality, off Sugashima, Mie, Japan, and collected from rock samples bycaught with a gill net. After rock samples were broken into pieces using a hammer, the specimen was found in a rock crack, probably as a result of erosion by rock boring sipunculans, which sympatrically occur around the studied area in Sugashima.
Etymology: The specific name is derived from Greek, kryptós (concealed, hidden), referring to cryptic habitat of the new species, among a subtidal rock crack.
Description: External features. Body 4.2 cm in length, 5 mm in width; body dorsally pale brown and ventrally lighter coloured than dorsal surface (Figures 5B–D). Head slightly demarcated from trunk, with a pair of pale brown cephalic patches (Figures 5C, D). Narrow longitudinal midline present between cephalic patches but not reaching to anterior tip of head (Figure 5C). Anterior cephalic furrow incompletely encircling body, opening at mid-dorsal line (Figure 5C). Posterior cephalic furrow V-shaped on dorsal surface (Figure 5C). Twenty ocelli in an irregular cluster on each side of head (Figure 5C). Cerebral ganglia visible through body wall as a pair of red spots (Figures 5C, D).
Internal morphology. Epidermis 30–38 μm thick, with numerous red- and yellow-stained acidophilic cells, blue-stained basophilic cells, and ciliated cells (Figures 8A, B). Dermis up to 39 μm thick, forming cup-like structures on distal surface (Figures 8A, B). Body wall musculature with outer circular and inner longitudinal muscle layers (Figures 8A, B); distinct diagonal muscle layers not found. Precerebral septum present (Figure 8F). Oesophagus with red-stained acidophilic cells, blue-stained basophilic cells, and ciliated cells (Figures 8G, J, K). Dorso-ventral muscles present in cerebral region (Figure 8G) and between intestinal diverticula (Figure 8L). Cephalic glands (Figure 8D) leading to well-developed submuscular glands (Figures 8A, E). A single apical organ present (Figure 8C). Precerebral vessels transversely connected above rhynchodaeum at anterior tip of head, running posteriorly in both sides of rhynchodaeum (Figure 8E). Mid-dorsal vessel protruding into rhynchocoel to form a single vascular plug in posterior region of brain (Figure 8G); mid-dorsal vessel containing red- to orange-coloured blood cells (Figure 8K).
Proboscis pore opening ventrally (Figure 8D). Rhynchocoel muscular wall interwoven with circular and longitudinal musculatures (Figures 8G, J-L). Proboscis epithelium with well-developed papilla (Figures 8H, I). Proboscis musculature composed of outer circular, middle longitudinal, and inner circular muscle layers (Figures 8H, I). Proboscis nerves 13 in number (Figure 8H). Stylet basis oval, 33.6 μm in length and 22.0 μm in maximum width; central stylet smooth, 72.5 μm in length; stylet-to-basis-length (S/B) ratio 2.16 (Figure 7). Two accessory stylet pouches present, each containing 9 stylets (Figure 7).
Pigment-cup ocelli embedded in body-wall longitudinal muscle layer (Figures 8A, D, E). Cerebral organs unbranched, extending to posterior region of brain (Figures 8F,J) and posteriorly followed by developed gland cells (Figure 8G). Brain separated from body wall by outer neurilemma; inner neurilemma absent (Figures 8G, J); dorsal lobe with glomerular structures (Figure 8J). Lateral nerves without accessory nerves and neurochords (Figure 8L).
Nephridial tubules anteriorly extending to brain region, forming a bundle of tubules behind brain region (Figure 8K). Testes between intestinal diverticula (Figure 8L).
Remarks: Nipponnemertes crypta sp. nov. is distinguished from all the congeners in the body colouration and cephalic patches (Figures 5C, D). Body colouration of N. pacifica (Coe, 1905) described based on preserved specimens is most similar to, but distinguished from N. crypta sp. nov. in lacking cephalic patches. The same is true for the Antarctic species N. variabilis (Korotkevitsch, 1983); in addition, N. crypta sp. nov. differs from N. variabilis in the absence of accessory stylet in a central stylet basis (Figure 7).
4 Nipponnemertes jambio sp. nov. Hookabe, Kajihara & Chernyshev
[New Japanese name: jyambio-omen-himomushi]
(Figures 5E–H, 7)
Cf. Cosmocephala japonica: Kajihara, 2017, p. 436, Figure 16F, G.
Material examined: Holotype: ICHUM 8319, preserved in 99% ethanol, posterior tip used for DNA extraction, collected by dredging from 87–89 m depth, off Jogashima, Sagami Bay, Kanagawa Prefecture, Japan (Figures 1A, C), by Hiroshi Kajihara, on 19 February 2014. Paratype: ICHUM 8318, preserved in 99% ethanol, posterior tip used for DNA extraction, collected by dredging from 80 m depth, off Jogashima, Sagami Bay, Kanagawa Prefecture, Japan (Figures 1A, C), by Hisanori Kohtsuka, on 25 April 2012.
Habitat and geographic distribution: The species is only known from the type locality, off Jogashima, Miura (Kanagawa), Japan.
Description: External features. Body 2.0–2.2 cm in length, 1.0–1.4 mm in width; in trunk region, dorsal body surface uniformly brownish orange; ventral surface slightly lighter coloured than dorsal surface (Figures 5E–H). Head with a pair of cephalic patches and a narrow longitudinal midline between cephalic patches on dorsal surface (Figures 5E, F); mid-longitudinal line without reaching to anterior end of cephalic patches (Figures 5E, F); ventral surface pale coloured (Figure 5G). Anterior cephalic furrows incompletely encircling body, dorsally discontinuous at midline (Figure 5F), ventrally extending anteriorly and meeting at midline (Figure 5G). Posterior cephalic furrows V-shaped on dorsal side (Figure 5H). Eyes 14–17 in number, present on each side of lateral margin of head (Figures 5F, H).
Internal morphology. Stylet basis oval, 45.0–60.0 μm in length and 35.0–48.0 μm in maximum width; central stylet smooth, 95.0–120.0 μm in length; S/B ratio 1.86–2.15 (Figure 7). Two accessory stylet pouches present, each containing 4–5 stylets (Figure 7).
Remarks: In external morphology, N. jambio sp. nov. is extremely similar to N. ojimaorum sp. nov. However, it is distinguished from the latter in the following features: i) trunk region ventrally lighter coloured than dorsal surface (Figure 5G), ii) a narrow longitudinal midline (mid-dorsal cephalic ridge) between cephalic patches reaching more anteriorly than N. ojimaorum sp. nov., without fusing with cephalic patches (Figure 5F).
Intraspecific COI divergences in this species are 0.0–0.3% in uncorrected p-distance (Table S2).
5 Nipponnemertes neonilae sp. nov. Hookabe, Kajihara & Chernyshev
[Japanese name: rishiri-himomushi]
(Figures 5L–P, 7, 9)
Amphiporus bimaculatus: Iwata, 1954
Collarenemertes bimaculatus: Chernyshev, 1993
Collarenemertes cf. bimaculata: Chernyshev & Polyakova, 2019
Material examined: Holotype: MIMB 43013, preserved specimen, a proboscis used for DNA extraction, collected by Alexei V. Chernyshev from 5 m depth, among Modiolus kurilensis, Vostok Bay, Peter the Great Bay (Figure 1A), Sea of Japan, Russia, 11 August 2015. Paratypes: two specimens, MIMB 43014 and 43015, transverse and sagittal sections of anterior tips, collected by Alexei V. Chernyshev from topotype. Additional specimens: (GenBank specimen ID NipbimIt15), collected by V.G. Kuznetzov from 5–15 m depth, Iturup Island, 45°22.397′N, 148°27.296′E, Sea of Okhotsk (Figure 1A), 19 August 2019; 16 specimens from Sea of Japan and Kuril Islands (Zoological Museum of Far East Federal University and personal collection of AVC).
Habitat and geographic distribution: The species is known from the type locality (Vostok Bay) and Kuril Islands (Iturup Island). Externally identical specimens were collected in Posyet, Ussuri, and Vladimir Bays, Valentin Bight (Sea of Japan), Aniwa Bay (South Sakhalin), Shikotan Island (Kuril Island), and Rausu, Hokkaido (Sea of Okhotsk) (Hookabe & Jimi, personal observation) from the intertidal to a depth of 14 m. As well, the species was reported from Rishiri Island, Hokkkaido (Sea of Japan) (Iwata, 1954). The present species is extracted from substrate samples, in which sedentary mytilids (Modiolus kurilensis and Crenomytilus grayanus) and calcareous algae (Bossiella sp.) were included.
Etymology: The specific name is a noun in the genitive case, after Dr. Neonila Polyakova, to whom we acknowledge her significant contribution to molecular systematics of nemerteans.
Description: External features. Body 5–16 cm in length (12 cm in holotype), 5–6 mm in width (up to 12–15 mm in contracted specimens); body dorsally brownish-red or brick-red and ventrally beige or whitish (Figure 5L). Head triangular from above, with a pair of darker triangular cephalic patches and a longitudinal midline between cephalic patches on dorsal surface; mid-longitudinal line not reaching to anterior tip of head and not fused with cephalic patches (Figures 5M, N). Narrow longitudinal midline between cephalic patches not reaching to anterior tip of head (Figures 5M, N, P). When contracted, head can be retracted into body, forming a collar (Figure 9D). Anterior cephalic furrow incompletely encircling body, opening at mid-dorsal line, with secondary grooves (Figure 5O). Posterior cephalic groove V-shaped on dorsal surface (Figure 5M). Eyes 12–23 in number, present on each side of head (Figure 5P).
Internal morphology. Epidermis with numerous ciliated and orange-stained glandular cells. Dermis up to 60–80 μm thick in contracted specimens. Body-wall musculature as in other species in the genus, diagonal muscles detected by confocal laser scanning microscopy (Chernyshev, 2010: Figure 3). Pylorus sporadically with glandular cells; intestinal caecum anteriorly extending beneath pylorus (Figure 5P), with 5–6 pairs of lateral pouches. Dorso-ventral muscles present between intestinal diverticula (Figure 9F). Cephalic glands (Figure 9B) and the subsequent submuscular glands as in other species in the genus. Precerebral vessel extending above rhynchodaeum at anterior tip of head and posteriorly bifurcated (Figure 9A). Mid-dorsal vessel protruding into rhynchocoel to form a single vascular plug in posterior region of brain (Figures 9D, E); blood cells colourless.
Proboscis pore sub-terminal, opening ventrally. Rhynchocoel musculature consisting of interwoven longitudinal and circular muscle fibres (Figures 9E, F). Proboscis nerves 16–17 in number. Stylet basis oval, 118–156 (156 in holotype) μm in length and 80–97 (94 in holotype) μm in maximum width; central stylet smooth,152–222 (218 in holotype) μm in length; S/B ratio 1.14–1.67 (1.39 in holotype) (Figure 7). Two (rarely four or five) accessory stylet pouches present, each containing 3–10 (7 in holotype) stylets (Figure 7).
Pigment-cup ocelli embedded in body-wall muscle layer (Figure 9A). Cerebral organs extending to posterior region of brain, posteriorly bifurcated (Figure 9C); posterior glandular part located between lateral blood vessel and lateral nerve (Figure 9E). Brain and lateral nerves as in other species of the genus.
Nephridial tubules branching near lateral blood vessels. Gonads, gonoducts, and gonopores not found in the material examined.
Remarks: In external morphology, N. neonilae sp. nov. is very similar to N. bimaculata. However, it is distinguishable from the latter by the presence of a narrow longitudinal midline (mid-dorsal cephalic ridge) between cephalic patches (Figures 5M, N, P). In the original description of N. bimaculata, Coe (1901) wrote that the head had two oval, black or very dark brown patches in material from Victoria (British Columbia, Canada), Sitka (Alaska, USA), and Puget Sound (Washington, USA). Later, Coe (1905: pl. 2, figure 21) illustrated a specimen with two narrow triangular cephalic patches. Photographs of the similar specimens are provided in BOLD (http://v3.boldsystems.org/index.php/Taxbrowser_Taxonpage?taxid=939114). Dr. Frank Crandall (personal communication) regarded that specimens of N. bimaculata with oval and triangular cephalic patches belong to two different species. If this is the case, specimens with oval head spots should be assigned to N. bimaculata. Nipponnemertes neonilae sp. nov. is distinguished from N. bimaculata in the Northeast Pacific in having triangular patches by wider and darker cephalic patches.
From Japanese waters, Iwata (1954) reported a similar form with the present species from sublittoral among laminarian holdfasts, Rishiri Island (Hokkaido), the Sea of Japan. As noted in Crandall et al. (2002), the form differs from Coe’s (1901) description of N. bimaculata in having triangular cephalic patches, and Crandall et al. (2002) noted that the form identified by Iwata (1954) differs from Coe’s (1901) taxon in having a pair of quadrangular head markings, and rather resembles N. neonilae sp. nov. Therefore, we herein redescribe Iwata’s (1954) taxon as N. neonilae sp. nov.
6 Nipponnemertes ojimaorum sp. nov. Hookabe, Kajihara & Chernyshev
[New Japanese name: benishouga-omen-himomushi]
(Figures 5I–K, 7, 10)
Material examined: Holotype: ICHUM 8324, serial transverse sections of anterior tip to intestine, posterior tip used for DNA extraction, collected by SCUBA diving from 12 m depth, Koganezaki, Shizuoka Prefecture, Japan (Figures 1A,C), by Masanori Okanishi, on 21 October 2020. Paratypes: ICHUM 8325, fixed in Bouin’s fluid and later preserved in 70% ethanol, collected by SCUBA diving from 8 m depth, Koganezaki, Shizuoka Prefecture, Japan (Figures 1A, C), by Natsumi Hookabe, on 6 January 2021; ICHUM 8326, fixed in Bouin’s fluid and later preserved in 70% ethanol, collected by SCUBA diving from 7 m depth, Koganezaki, Shizuoka Prefecture, Japan (Figures 1A, C), by Naoto Jimi, on 6 January 2021.
Habitat and geographic distribution: The species has been currently known only from Koganezaki (Shizuoka), Japan, inhabiting subtidally under rocks on sandy substrates (Figure 1E).
Etymology: The specific name is a noun in the genitive plural, named after Tomohito Ojima and Masako Ojima, who organized a field survey on subtidal invertebrate fauna in Koganezaki; all the specimens of the new species were collected during the survey.
Description: External features. Body 2.2 cm in length, 1.2 mm in width; in trunk region, dorsal body surface uniformly bright red coloured; ventral surface the same colour as dorsal surface (Figures 5I–K). Head with a pair of cephalic patches on dorsal surface (Figure 5J); ventral surface pale coloured (Figure 5K). Narrow longitudinal midline between cephalic patches not reaching to anterior tip of head (Figure 5J). Anterior cephalic furrows incompletely encircling body, dorsally discontinuous at midline (Figure 5J), ventrally extending anteriorly and meeting at midline (Figure 5K). Posterior cephalic furrows V-shaped on dorsal side (Figure 5J). Eyes arranged on each side of lateral margin (Figure 5J); number of eyes not counted for the present species.
Internal morphology. Epidermis with numerous red-stained acidophilic cells and ciliated cells (Figures 10A, B). Dermis up to 30 μm thick, forming cup-like structures on distal surface in precerebral region (Figure 10A); in foregut region more flattened and without forming conspicuous cup-like structure (Figure 10B). Body-wall musculature (Figures 10A, B), esophagus (Figure 10G), pylorus, intestinal caecum (Figures 10H, K) as in other species in the genus. Dorso-ventral muscles present in cerebral region (Figure 10J) and in intestinal region between intestinal diverticula (Figure 10L). Cephalic glands and the subsequent submuscular glands as in other species in the genus (Figure 10A). A single apical organ present (Figure 10C). Precerebral vessels transversely connected above rhynchodaeum at anterior tip of head (Figure 10E). A single vascular plug present in posterior region of brain (Figure 10G); mid-dorsal vessel not well distinguished thorough histology in the present species.
Proboscis pore positioned sub-terminally (Figure 5K). Rhynchocoel musculature (Figure 10G), proboscis epithelium (Figure 10H), and proboscis musculature (Figures 10H, I) as in other species in the genus. Proboscis nerves 13 in number (Figure 10H). Stylet basis oval, 53.0 μm in length and 32.0 μm in maximum width; central stylet smooth, 98.0 μm in length; S/B ratio 1.85 (Figure 7). Two accessory stylet pouches present, each containing 7–8 stylets (Figure 7).
Pigment-cup ocelli embedded in body-wall muscle layer (Figure 10A). Cerebral organs unbranched (Figures 9F, J) and followed by glands (Figure 10G) as in other species in the genus. Lateral nerves lacking accessory nerves and neurochords (Figures 10K, L).
Nephridial tubules forming a bundle of tubules behind brain region (Figure 10K). Gonads, gonoducts, and gonopores not found in the specimen examined.
Remarks: Nipponnemertes ojimaorum sp. nov. is similar to N. arenaria (Figure 5A), N. bimaculata, N. crypta sp. nov. (Figures 5B–D), N. jambio sp. nov. (Figures 5E–H), N. neonilae sp. nov. (Figures 5L–P), and N. punctatula (Figures 5Q–T) because these species possess cephalic patches in common. However, N. ojimaorum sp. nov. can be differentiated from these species by the colouration of the dorsal and ventral body surfaces, which is the same in N. ojimaorum sp. nov. (Figures 5I–K), whereas the dorsal surface is darker than the ventral in other species. In terms of the colour contrast between the dorsal and ventral body surfaces, N. ojimaorum sp. nov. most closely resembles N. jambio sp. nov. because the ventral surface in the latter is only slightly lighter than the dorsal surface; in other species (N. arenaria, N. bimaculata, N. crypta sp. nov., N. neonilae sp. nov., and N. punctatula), the contrast is more prominent with a darker dorsal surface against paler ventral one. Still, N. ojimaorum sp. nov. is distinguishable from N. jambio sp. nov. by the mid-dorsal cephalic line, which is i) not reaching to the anterior end of the cephalic patches, ii) laterally fusing to the cephalic patches, and iii) posteriorly fusing to the dorsal dark colour (Figure 5J); in N. jambio sp. nov., the mid-dorsal cephalic line i) extends anteriorly almost reaching to the level of the anterior end of the cephalic patches, ii) is laterally separated from the cephalic patches, and iii) is not continuous with the dorsal dark colour (Figure 5F).
Cosmocephala japonica Stimpson, 1857—currently Amphiporus japonica (Stimpson, 1857)—is anticipated to be a species in Nipponnemertes (Kajihara et al., 2015; Kajihara, 2017); it was originally described based on specimens intertidally collected from Shimoda (Shizuoka), geographically close to Koganezaki, the type locality of N. ojimaorum. sp. nov. However, by having ventrally pale-coloured body surface, Cosmocephala japonica is distinguished from N. ojimaorum sp. nov.
7 Nipponnemertes punctatula (Coe, 1905)
[Japanese name: madara-himomushi]
Material examined: ICHUM 8328, preserved in formalin, posterior tip used for DNA extraction, collected at intertidal, Tateyama, Mie Prefecture, Japan (Figures 1A, C), by Ryuta Yoshida, on 13 June 2018. ICHUM 8329, preserved in formalin, posterior tip used for DNA extraction, collected at intertidal, Sugashima, Mie Prefecture, Japan (Figures 1A, D), by Naoto Jimi, on 24 June 2021.
Habitat and geographic distribution: The species is widely known from Japanese coastal waters from Hokkaido to Nagasaki.
Description: External features. Body 4.0–5.2 cm in length, 8–12 mm in width; in trunk region, dorsal body surface with a dorsal brown blotch pattern (Figures 5Q, R) or uniformly brownish (Figures 5S, T); ventral surface pale-coloured (Figure 5T). Head with a pair of cephalic patches and a narrow longitudinal midline between cephalic patches on dorsal surface; mid-longitudinal line without reaching to anterior end of cephalic patches (Figures 5Q–T)). Posterior cephalic furrows V-shaped on dorsal side (Figure 5Q).
Remarks: Body colouration pattern of the present species varies from previously known brown blotch pattern (Figures 5Q, R) to uniformly brown colour without blotched (Figures 5S, T). Intraspecific COI divergences in this species are 0.0–0.2% in uncorrected p-distance (Table S2), suggesting intraspecific variation of body colouration pattern in N. punctatula.
8 Nipponnemertes ganahai sp. nov. Hookabe, Kajihara & Chernyshev
[New Japanese ganaha-omen-himomushi]
(Figures 6A–G, 7, 11)
?Nipponnemertes sp. 1: Yamaoka, 2005, p. 151–152.
Material examined: Holotype: ICHUM 8317, serial transverse sections of anterior tip to intestine, a posterior tip used for DNA extraction, collected by dredging from 116–211 m depth, off Jogashima, Miura, Kanagawa Prefecture, Japan (Figures 1A, D), by Natsumi Hookabe, on 31 July 2020. Paratypes: ICHUM 8316, serial transverse sections of anterior tip to intestine, a posterior tip used for DNA extraction, collected by dredging from 71.2–79.2 m depth, off Noto, Ishikawa Prefecture, Japan (Figures 1A, B), by Natsumi Hookabe, on 20 November 2019.
Habitat and geographic distribution: The species has been collected off Jogashima (Kanagawa Prefecture), Noto (Ishikawa Prefecture), Ofunato (Iwate Prefecture), and Tosa (Kochi Prefecture), Japan, likely preferring muddy to sandy sediments (Figures 1F, G).
Etymology: The specific name is a noun in the genitive case, after Ikumasa Ganaha, to whom we acknowledge his significant contribution to molecular experiments including DNA extraction, PCR amplification, sequencing, and organizing the obtained molecular data for several specimens used in the present study.
Description: External features. Body 1.2–1.6 cm in length, 1.5–2.0 mm in width; body uniformly pale pink to yellow coloured (Figures 6A–E). Head not conspicuously demarcated from trunk; cephalic patch absent (Figures 6A–D). Anterior cephalic furrows completely encircling body, extending anteriorly and meeting at midline on ventral side (Figure 6F). Posterior cephalic furrows V-shaped on dorsal surface, completely encircling body (Figure 6B). As many as 15–21 ocelli irregularly arranged on each side, varying in sizes; 2 to 3 ocelli fused in several specimens (Figures 6C, F, G).
Internal morphology. Epidermis with numerous red-, yellow-, and blue-stained cells and ciliated cells (Figures 11A, B). Dermis up to 25 μm thick, forming cup-like structures on distal surface (Figures 11A, B). Body-wall musculature (Figures 11A, B), oesophagus (Figures 11H, I), pylorus, and intestinal caecum (Figure 11F) as in other species in the genus. Dorso-ventral muscles present between intestinal diverticula (Figure 11K). Cephalic glands (Figure 11C) leading to well-developed submuscular glands (Figures 11A, D). A single apical organ present (Figure 11C). Precerebral vessels transversely connected above rhynchodaeum at anterior tip of head (Figure 11D). Mid-dorsal vessel present between rhynchocoel and pylorus (Figure 11F); vascular plug not detected in the present species.
Proboscis pore sub-terminal, opening ventrally (Figure 6F). Rhynchocoel musculature, proboscis epithelium, and proboscis musculature (Figures 11F, G) as in other species in the genus. Proboscis nerves 10 in number (Figure 11F). Stylet basis oval, 57.0–71.0 μm in length 45.0–47.0 μm in maximum width; central stylet vertically sculptured, 89.0–92.0 μm in length; S/B ratio 1.29–1.56 (Figure 7). Two accessory stylet pouches present, each containing four stylets (Figure 7).
Pigment-cup ocelli embedded sub-dermally (Figures 11A, C, D). Cerebral organs ventrolaterally opening in precerebral region (Figure 11E) and extending to posterior region of brain (Figures 11H, I), followed by glandular cells (Figure 11J). Brain (Figures 11H–J) and lateral nerves (Figures 11F, K) as in other species of the genus.
Nephridial tubules branching near lateral nerves (Figure 11F). Ovaries between intestinal diverticula; each ovary containing 10 or more oocytes; oocytes 100–150 μm in diameter (Figure 11K).
Remarks: Nipponnemertes ganahai resembles N. africana (Wheeler, 1940), N. danae (Friedrich, 1957) (probably synonym of N. pulchra in morphological basis; Berg, 1985), N. magna (Punnett, 1903), N. pulchra (Johnston, 1837), and N. sanguinea Riser, 1998 in having pale pink to yellow body (Figures 6A–E) and intestinal caecum extending to pyloric region (Figure 11F) and anteriorly extending in posterior region of esophagus. However, it is distinguished from N. pulchra (also probably from N. danae) in lacking an accessory stylet in the basis of the central stylet armature (Figure 7). Between the remaining three species, the number of the proboscis nerves can be useful for species distinction; it is 10 in N. ganahai sp. nov., 20 in N. magna (Punnett, 1903), and 12 in N. sanguinea (Riser, 1998). Furthermore, N. sanguinea is distinguished from N. ganahai in the red-coloured fluid contained in blood vessels due to red blood corpuscles (Riser, 1998); red blood was not visible in a live squeezed specimen of N. ganahai sp. nov. through a light microscope (Figure 6G).
Intraspecific COI divergences in this species are 0.0–0.8% in uncorrected p-distance (Table S3).
9 Nipponnemertes kozaensis sp. nov. Hookabe, Kajihara & Chernyshev
[New Japanese name: koza-sujinashi-himomushi]
(Figures 6H–J, 7, 12)
?Nipponnemertes sp. 2: Yamaoka, 2005, p. 152–153.
Material examined: Holotype: ICHUM 8320, serial transverse sections of anterior tip to intestine, posterior tip used for DNA extraction, found during anaesthetization for ascidians in menthol-floated seawater; the ascidians were collected by Naohiro Hasegawa from 12 m depth by SCUBA diving, Kaminoshima, Koza, Wakayama Prefecture (Figure 1A), Japan, on 11 May 2021.
Habitat and geographic distribution: The present specimen was found among rocky substrate samples. In the substrate samples, several sessile invertebrates such as sponges, ascidians, and bryozoans were colonized. Currently known from the type locality, Koza (Wakayama Prefecture), Japan.
Etymology: The specific name is derived from the type locality, Koza, Wakayama Prefecture, Japan.
Description: External features. Body 1.5 cm in length, 1.0 mm in width; background generally pale-coloured, mottled with red-brown and white dots; red-brown dots more densely distributed in dorso-anterior region of body; white dots uniformly distributed on both dorsal and ventral surfaces (Figures 6H–J). Head devoid of red-brown pigments and slightly demarcated from body (Figure 6I). Anterior cephalic furrows not conspicuous, probably discontinuous on dorsal midline, but ventrally meeting at midline (Figures 6I, J). Posterior cephalic furrows completely encircling body and V-shaped on dorsal side (Figures 6I, J). As many as 14 eyes arranged in two irregular rows at lateral margin on each side of head (Figure 6I). Internal organs visible through body wall; proboscis pure white, extending three-fourths the body; foregut and intestine yellow coloured (Figure 6H).
Internal morphology. Epidermis with numerous red-, yellow-, blue-stained cells and ciliated cells (Figures 12A, B). Dermis thin, up to 20 μm thick, forming cup-like structures on distal surface (Figures 12A, B). Body-wall musculature (Figures 12A, B), oesophagus (Figure 12I), pylorus, and intestinal caecum (Figure 12E) as in other species in the genus. Intestinal caecum anteriorly extending beneath pylorus (Figure 12E). Dorso-ventral muscles present between intestinal diverticula (Figure 12K). Cephalic glands (Figure 12D) followed by subsequent submuscular glands (Figure 12H). A single apical organ present (Figure 12C). Precerebral vessels as in other species in the genus (Figure 12D). Mid-dorsal vessel passing above pylorus and containing red to orange blood cells (Figure 12E); vascular plug indistinguishable in the specimen examined.
Proboscis pore sub-terminal, opening ventrally (Figure 6J). Rhynchocoel musculature (Figures 12E, J), proboscis epithelium (Figures 12E, F, J), and proboscis musculature (Figures 12E–G, J) as in other species in the genus. Proboscis nerves 10 in number (Figure 12F). Stylet basis oval, 49.6 μm in length and 32.0 μm in maximum width; central stylet vertically sculptured, 75.0 μm in length; S/B ratio 1.51 (Figure 7). Two accessory stylet pouches present, each containing 8 stylets (Figure 7).
Pigment-cup- ocelli embedded sub-dermally (Figures 12A, H). Cerebral organs extending to posterior region of brain (Figure 12I). Brain (Figure 12I) and lateral nerves (Figures 12E, J, K) as in other species of the genus.
Nephridial tubules branching near lateral blood vessels, latter containing red to orange blood cells (Figure 12J). Gonads, gonoducts, and gonopores not found in the specimen examined.
Remarks: Currently five species have been known to possess pale coloured body mottled with red to brown dots in the genus: N. occidentalis (Coe, 1905), N. madagascarensis (Kirsteuer, 1965), N. kozaensis sp. nov., N. ornata sp. nov., and N. sugashimaensis sp. nov. (Figure 6). Nipponnemertes kozaensis sp. nov. is distinguished from the two previously known species, N. occidentalis and N. madagascarensis, by having submuscular glands originated from cephalic glands, although it could not be traced because of poor fixation or staining in N. occidentalis and N. madagascarensis and its validity as a diagnostic character needs to be reexamined in future study. In addition, N. kozaensis sp. nov. differs from N. madagascarensis in having 10 proboscis nerves (Figure 12F) and 8 accessory stylets in each pouch (Figure 7), whereas N. madagascarensis possesses 9 proboscis nerves and 2–3 accessory stylets per pouch (Kirsteuer, 1965). No information as to the proboscis in N. occidentalis is available, because the proboscis was lost in the type material (Coe, 1905). According to the original description, one of the notable anatomical features found in N. occidentalis is the complex alimentary canal with oesophageal and cardiac caeca (Coe, 1905, pl. XX, fig. 121). In the species herein described, including N. kozaensis sp. nov., complex alimental canals as depicted by Coe (1905) were not observed. This would also allow the distinction of N. occidentalis from N. kozaensis sp. nov.
In contrast to N. madagascarensis and N. occidentalis, no conspicuous difference is found in the internal morphology between N. kozaensis sp. nov., N. ornata sp. nov., and N. sugashimaensis sp. nov. Nipponemertes ornata sp. nov. differs from N. kozaensis sp. nov. in the lower density of brown dots on the body surface (Figures 6P, Q). Although the species distinction is molecularly supported by the resulting phylogenetic tree, where the two species were not in a sister relation (Figure 2), further observation based on additional materials is required to examine if any subtle differences in the spotting pattern might represent interspecific variation and thus could be used for species discrimination. A brown colour pattern similar to the one found in N. kozaensis is also present in N. sugashimaensis but the pattern is irregular in the latter specie (Figure 6R) rather than the uniformly distributed dots in N. kozaensis (Figure 6H).
In the resulting molecular phylogenetic tree (Figure 2), N. kozaensis formed a clade with N. sugashimaensis with high support values. Although we could not obtain COI sequences for them even with specific primers for their delimitation, the nucleotide differences between the two species were comparable with interspecific divergences observed in Nipponnemertes.
10 Nipponnemertes lactea sp. nov. Hookabe, Kajihara & Chernyshev
[New Japanese name: irojiro-sujinashi-himomushi]
(Figures 6K, L, 7, 13)
?Nipponnemertes sp. 1: Yamaoka, 2005, p. 151–152
Material examined: Holotype: ICHUM 8321, serial transverse sections of anterior tip to intestine, a posterior tip used for DNA extraction, collected at depths of 98–120 m by dredging, Takase, off Shimoda, Shizuoka Prefecture, Japan (Figures 1A, C), by Natsumi Hookabe, on 25 March 2021.
Habitat and geographic distribution: The species is only known from the type locality, off Shimoda (Shizuoka), Japan.
Etymology: The specific name is derived from the Latin lacteus, meaning “milky”, after the uniformly pale body colouration in this species.
Description: External features. Body 0.7 cm in length, 0.8 mm in width; body uniformly pale; intestinal region pale-orange due to internal organs (Figure 6K). Head demarcated from trunk and without cephalic patches. Anterior cephalic furrows completely encircling body. Posterior cephalic furrows V-shaped on dorsal surface, completely encircling body (Figure 6K). As many as 12 eyes arranged on each side of lateral margin (Figure 6L).
Internal morphology. Epidermis with numerous red-stained cells and ciliated cells (Figures 13A, B). Dermis up to 25 μm thick, forming cup-like structures on distal surface in precerebral region (Figure 13A); in foregut to intestine regions, dermis becoming thicker and more flattened without forming cup-like structure (Figure 13B). Body-wall musculature (Figures 13A, B); oesophagus, pylorus, and intestinal caecum (Figure 13I) as in other species of the genus. Dorso-ventral muscles present between intestinal diverticula (Figure 13I). Cephalic glands (Figure 13D) leading to well-developed submuscular glands. A single apical organ present (Figure 13C). Precerebral vessels, mid-dorsal vessel, and vascular plug difficult to be confirmed in type material.
Proboscis pore opening ventrally (Figure 6L). Rhynchocoel musculature (Figures 13G–I), proboscis epithelium (Figure 13E), and proboscis musculature (Figure 13F) as in other species in the genus. Proboscis nerves 10 in number (Figure 13E). Stylet basis oval, 72.0 μm in length and 40.0 μm in maximum width; central stylet smooth, 90.0 μm in length; S/B ratio 1.25 (Figure 7). Two accessory stylet pouches present, each containing 7 stylets (Figure 7).
Pigment-cup ocelli embedded sub-dermally (Figure 13A). Cerebral organs unbranched, extending to posterior region of brain (Figures 13G, H). Brain (Figures 13G, H) and lateral nerves (Figures 13I, J) as in other species in the genus.
Nephridial tubules difficult to be confirmed in type material. Ovaries between intestinal lateral diverticula; each ovary containing 20 or more oocytes; oocytes 42 μm in diameter (Figure 13J).
Remarks: Nipponnemertes lactea sp. nov. resembles N. africana (Wheeler, 1940), N. notoensis sp. nov. (present study), and N. variabilis (Korotkevitsch, 1983) in having uniformly pale coloured body, posterior cephalic furrows completely encircling body, and the circulatory system lacking red blood corpuscles. Nipponnemertes lactea sp. nov. is characterized by the ocelli arranged in a single row at lateral margin of the head (Figure 6L). As depicted in the original illustration based on a preserved specimen (Wheeler, 1940: Figures 9B, C), the ocelli in N. africana are arranged irregularly, and the similar arrangement is found in N. notoensis sp. nov. (Figure 6N). Nipponnemertes lactea sp. nov. differs from N. variabilis in the absence of accessory stylet in the central stylet basis (Figure 7).
Nipponnemertes schollaerti (Wheeler, 1934), for which no information on posterior cephalic furrows is available from Wheeler (1934)’s original description, is distinguished from N. lactea sp. nov. in the larger numbers of the ocelli on each side of head—55 in N. schollaerti (Berg, 1985) vs 12 in N. lactea sp. nov. (Figure 6L)—and more than ten times larger body size—97 mm in N. schollaerti (preserved, mature male) vs 7 mm in N. lactea (living, mature female).
11 Nipponnemertes notoensis sp. nov. Hookabe, Kajihara & Chernyshev
[New Japanese name: noto-sujinashi-himomushi]
(Figures 6M–O, 14)
Material examined: Holotype: ICHUM 8322, serial transverse sections of anterior tip to intestine, a posterior tip used for DNA extraction, collected by dredging from 70–91 m depth, off Noto, Ishikawa Prefecture, Japan (Figures 1A, B), by Natsumi Hookabe, on 20 November 2019. Paratype: ICHUM 8323, fixed in 10% formalin and later preserved in 70% ethanol, a posterior tip used for DNA extraction, collected by dredging from 70–91 m depth, off Noto, Ishikawa Prefecture, Japan (Figures 1A, B), by Natsumi Hookabe, on 20 November 2019.
Habitat and geographic distribution: The species has been only known from the type locality, off Noto (Ishikawa Prefecture), Japan.
Etymology: The specific name is derived from the type locality, Noto, Ishikawa Prefecture, Japan.
Description: External features. Body 1.2 cm in length, 1.2 mm in width; body uniformly pale to pale-yellow coloured (Figure 6M). Head not demarcated from trunk, mottled with white spots along dorsal midline (Figures 6N, O); cephalic patch absent (Figures 5N, O). Anterior cephalic furrows not well developed. Posterior cephalic furrow V-shaped on dorsal surface (Figure 6M), completely encircling body. As many as 19–20 reddish brown ocelli irregularly arranged on each side; each ocelli irregular in size and shape; 2 to 3 ocelli fusing with each other (Figure 6N).
Internal morphology. Epidermis with numerous red-, orange-, and blue-stained cells and ciliated cells (Figures 14A, B). Dermis up to 21 μm thick, forming cup-like structures on distal surface in both precerebral (Figure 14A) and foregut regions (Figure 14B). Body-wall musculature as in other species in the genus (Figures 14A, B). Oesophagus (Figure 14G) and pylorus (Figure 14K) as in other species in the genus. Dorso-ventral muscles present between intestinal diverticula (Figure 14L). Cephalic (Figure 14D) and submuscular glands present (Figure 13G). A single apical organ present (Figure 14C). Precerebral and mid-dorsal vessel not observed.
Rhynchocoel musculature (Figures 14G–K), proboscis epithelium (Figure 14E), and proboscis musculature (Figures 14E, F) as in other species in the genus. Proboscis nerves 10 in number (Figure 14E). Stylet armature not observed in the specimen examined. Two accessory stylet pouches present, each containing more than two stylets (Figure 14E).
Pigment-cup ocelli embedded in body-wall longitudinal muscle layer (Figure 14D). Cerebral organs extending to posterior region of brain (Figures 14G–I), followed by glandular cells (Figure 14J). Brain (Figures 14H, I) and lateral nerves (Figures 14K,L) as in other species in the genus.
Nephridial tubules branching near lateral blood vessel (Figure 14K). Gonads and gonoducts not found in the specimen examined.
Remarks: Among 19 species previously described and 13 species herein reported, N. sanguinea and N. schollaerti are most similar to N. notoensis sp. nov. due to the uniformly pale-coloured body (Figure 6M) and the irregular arrangement of ocelli (Figures 6N, O) (see the Remarks section of N. lactea sp. nov.). However, N. notoensis sp. nov. can be differentiated from the two species in the number of proboscis nerves: 10 in N. notoensis sp. nov. (Figure 14E) vs 11 in N. sanguinea vs 14 in N. schollaerti.
12 Nipponnemertes ornata sp. nov. Hookabe, Kajihara & Chernyshev
[New Japanese name: kazari-sujinashi-himomushi]
(Figures 6P, Q, 15)
?Nipponnemertes sp. 1: Yamaoka, 2005, p. 152–153.
Material examined: Holotype: ICHUM 8327, serial transverse sections of anterior tip to intestine, a posterior tip used for DNA extraction, collected at depths of 70–91 m by dredging, off Noto, Ishikawa Prefecture, Japan (Figures 1A, B), by Natsumi Hookabe, on 20 November 2019.
Habitat and geographic distribution: The species is only known from the type locality, off Noto (Ishikawa Prefecture), Japan.
Etymology: The specific name is a feminine form of Latin ornatus, meaning “adorned or decorated”, derived from the body coloration mottled with white and brown dots in the present species.
Description: External features. Body 1.8 cm in length, 0.9 mm in width; background uniformly pale coloured, mottled with white and brown dots; white dots conspicuous in dorsal surface of head region; brown dots more densely distributed in dorso-anterior region of body than ventral and posterior region (Figure 6P). Head not well demarcated from trunk; cephalic patch absent (Figure 6Q). Anterior cephalic furrows not well developed (Figure 6Q). Posterior cephalic groove V-shaped on dorsal surface, completely encircling body (Figure 6Q). Ocelli 16 or 17 in number, not regularly arranged in rows, varying in size (Figure 6Q).
Internal morphology. Epidermis with numerous red-and yellow-stained cells and ciliated cells (Figures 15A, B). Dermis thin, up to 20 μm thick, forming cup-like structures on distal surface in precerebral (Figure 15A) and foregut regions (Figure 15B). Body-wall musculature (Figures 15A, B) and oesophagus (Figures 15E, F) as in other species in the genus. Intestinal caecum anteriorly extending beneath pylorus (Figure 15I). Dorso-ventral muscles present between intestinal diverticula (Figure 15J). Cephalic glands (Figure 15A) and the subsequent submuscular glands (Figure 15E) as in other species in the genus. A single apical organ present (Figure 15C). Precerebral and mid-dorsal vessel not well observed in our histological sections.
Proboscis pore opening ventrally (Figure 6P). Rhynchocoel musculature (Figures 15F, I), proboscis epithelium (Figure 15D), and proboscis musculature (Figure 15D) as in other species in the genus. Proboscis nerves 10 in number (Figure 15D). Stylet not observed in the specimen examined.
Pigment-cup ocelli embedded sub-dermally (Figure 15E). Cerebral organs extending to posterior region of brain (Figures 15E–G), followed by glandular cells (Figure 15H). Brain (Figures 15F, G) and lateral nerves (Figures 15I, J) as in other species of the genus.
Nephridial tubules not observed in the present specimen. Ovaries developing between intestinal diverticula (Figure 15J).
Remarks: The present species is extremely similar to N. kozaensis sp. nov. in having white and brown dots on uniformly pale body; however, it can be differentiated from the latter in brown dots more densely scattering on body surface (Figure 6H) (see also the Remarks section of N. kozaensis sp. nov.). Internal morphology of the two species is not remarkably different, although there might be other useful characters which are not obtained for N. ornata sp. nov. in this study (e.g., number of accessory stylets in proboscis).
Our molecular phylogenetic tree shows that N. ornata is sister-related with N. notoensis (Figure 2). Nipponnemertes notoensis differs from N. ornata in lacking dotted pattern (Figure 6M). Moreover, uncorrected p-distance between the two species was 9%, higher than the threshold value (5%) used for species separation in nemertean systematics (e.g., Sundberg et al., 2016).
13 Nipponnemertes sugashimaensis sp. nov. Hookabe, Kajihara & Chernyshev
[New Japanese Sugashima-sujinashi-himomushi]
(Figures 6R–U, 7, 16)
Material examined: Holotype: ICHUM 8330, serial transverse sections of anterior tip to intestine, a posterior tip used for DNA extraction, bycaught with a gill net from 10 m depth, Sugashima, Mie Prefecture, Japan (Figures 1A,D), collected by Natsumi Hookabe and Naoto Jimi, on 3 November 2021.
Habitat and geographic distribution: The species is only known from the type locality. Worms were picked up from rocky substrate samples bycaught with a gill net.
Etymology: The specific name is derived from the type locality, Sugashima (Mie Prefecture), Japan.
Description: External features. Body 0.9 cm in length, 1.1 mm in width; background uniformly pale coloured, with irregular, leopard-like brown mottling (Figure 6R); mottling pattern ventrally faded. Head not well demarcated from trunk; cephalic patch lacking (Figure 6S). Anterior cephalic furrows not well developed (Figure 6S). Posterior cephalic furrows not found in the specimen examined. 12 or 13 ocelli irregularly arranged (Figure 6T). Proboscis white in colour when everted.
Internal morphology. Epidermis with numerous red- and yellow-stained cells and ciliated cells (Figures 16A, B). Dermis up to 24 μm thick, forming cup-like structures on distal surface in precerebral (Figure 16A) and foregut regions (Figure 16B). Body-wall musculature (Figures 16A, B), oesophagus (Figures 16E, H), pylorus, and intestinal caecum (Figure 16J) as in other species in the genus. Dorso-ventral muscles present between intestinal diverticula (Figure 16K). Cephalic glands (Figure 16D) and the subsequent submuscular glands as in other species in the genus. A single apical organ present (Figure 16C). Mid-dorsal vessel (Figures 16E, J) protruding into rhynchocoel to form a single vascular plug in posterior region of brain (Figure 16H); mid-dorsal vessel (Figures 16E, J) as well as lateral vessels (Figures 16I, J) containing red to orange coloured blood cells.
Rhynchocoel musculature (Figures 16E, H, J, K), proboscis epithelium (Figure 16F), and proboscis musculature (Figure 16F) as in other species in the genus. Proboscis nerves 10 in number (Figure 16F). Stylet basis oval, 75.0 μm in length and 48.0 μm in maximum width; central stylet vertically sculptured, 115.0 μm in length; S/B ratio 1.38 (Figure 7). Two accessory stylet pouches present, each containing 7 stylets (Figure 7).
Pigment-cup ocelli embedded sub-dermally (Figure 16I). Cerebral organs extending to posterior region of brain (Figures 16G–I), followed by glandular cells (Figure 16I). Brain (Figure 16H) and lateral nerves (Figures 16I, J) as in other species of the genus.
Nephridial tubules branching near lateral blood vessels (Figure 16K). Gonads, gonoducts, and gonopores not found in the specimen examined.
Remarks: Apart from N. sugashimaensis sp. nov., irregular brown mottling on uniformly pale body is known in N. madagascarensis (see the Remarks section of N. ornata sp. nov.). However, N. sugashimaensis sp. nov. is discriminated from N. madagascarensis by having submuscular glands (Figure 16A) and the larger number of accessory stylets per pouch: 7 in N. sugashimaensis sp. nov. (Figure 6T) and 2–3 in N. madagascarensis (Kirsteuer, 1965).
14 Nipponnemertes sp. 2
(Figures 6V, W, 7)
Material examined: Holotype: ICHUM 8332, preserved in 70% ethanol, a posterior tip used for DNA extraction, collected at depths of 116–211 m by dredging, off Jogashima, Miura, Kanagawa Prefecture, Japan (Figures 1A, C), by Natsumi Hookabe, on 30 July 2021.
Habitat and geographic distribution: The species is only known from Jogashima, Kanagawa Prefecture, Japan.
Description: External features. Body 0.9 cm in length, 0.8 mm in width; body uniformly pale; intestinal region visible through body wall as pinkish (Figure 6V). Head not demarcated from trunk, without cephalic patches (Figure 6V). Anterior cephalic furrows not well developed. Posterior cephalic furrow not visible. As many as 13 ocelli present (Figure 6W). Precerebral and lateral vessels filled with red corpuscles (Figure 6W).
Proboscis pore sub-terminal. Stylet basis oval, 76.0 μm in length and 42.0 μm in maximum width; central stylet smooth, 96.0 μm in length; S/B ratio 1.38 (Figure 7). Two accessory stylet pouches present, each containing six stylets (Figure 7).
Remarks: The species is characterized by pinkish intestine visible through body wall (Figure 6V). Due to the absence of morphological voucher specimen, we leave the species as an unidentified form in the present study.
Discussion
Molecular Systematics of Nipponnemertes
In this study, species diversity of Nipponnemertes in Japanese and the Far Eastern Russian waters was significantly updated from 4 to 14 species, in which newly discovered 9 species as well as herein redescribed N. bimaculata sensu Iwata (1954) (= N. neonilae sp. nov.) are included (Table 4). Our molecular phylogenetic analyses recovered three major lineages in Nipponnemertes (Figure 2). In a previous study based on six congeners (Kajihara et al., 2015), two well-supported clades each corresponding to Clade A and Clade B (Figure 2) have been detected. With increased taxon sampling especially in subtidal to deep-sea species, Clade C was newly confirmed in the present study.
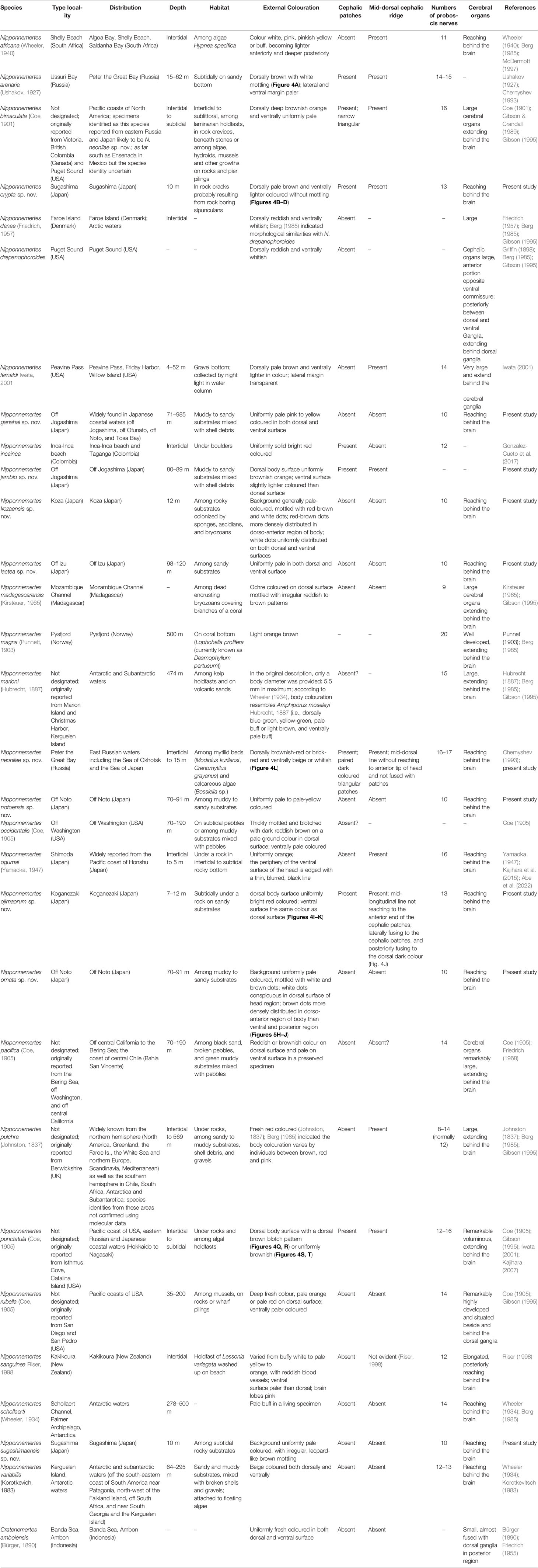
Table 4 An updated species list in Nipponnemertes and Cratenemertes amboiensis with selected morphological characters and ecological information.
Apart from relatively small body size in Clade C (see the discussion below), each clade is not characterized by any apparent synapomorphy, but is mutually distinguishable by a combination of three external characters—demarcation between the head and body, mid-dorsal ridge on head, and cephalic patches (Figure 2). The former two characters are likely plesiomorphic and retained in Clade A (Figure 4) and Clade B (Figure 5) while the last one is an apomorphy for Clade B (Figure 4). In members in Clade C, the head is not demarcated from the body and does not have a mid-dorsal ridge and cephalic patches (Figure 6). Although it remains unclear in Clade A due to a lack of data, stylet-to-basis-length ratio is also different between Clade B (1.85–2.16) and Clade C (1.25–1.64) (Figure 7). Neither clade-specific characters nor combinations of characters are found in internal morphology in the species examined in the present study.
As mentioned above, species in each clade are differentiated by external morphological characters of the head: species possessing demarcated head without cephalic patches (Clade A), demarcated head with cephalic patches (Clade B), and non-demarcated head lacking cephalic patches (Clade C) (Figure 2). Although the three lineages are presumably characterized by the combination of morphological characters of the head and could be regarded as separate genera, we place all the species in Nipponnemertes since molecular data for the type species, N. drepanophoroides is currently unavailable. In possessing heads without cephalic patches, N. drepanophoroides is probably included in Clade A rather than Clade C; however, no information is available as to the head–trunk demarcation and the mid-dorsal ridge in the original description by Griffin (1898) and redescription by Coe (1905). Molecular confirmation using topotype specimens is required for future taxonomic reappraisal.
In the present study, greater external morphological disparity between specimens (body coloration and spotting pattern) generally turned out to be a manifestation of species difference with greater phylogenetic/genetic distances (Figure 2). However, this was not the case in the N. kozaensis–N. ornata species pair. Despite having extremely similar body coloration (pale body with reddish to brownish ornamentation; Figures 5H–J, P, Q), the two did not form a single clade (Figure 2). On the contrary, the converse was not true for the “N. ojimaorum–N. punctatula” subclade in Clade B, consisting of N. crypta, N. jambio, N. ojimaorum, and N. punctatula. Their interspecific genetic distances based on COI were 1.6–4.4% in uncorrected p-distance and (Table S2), lower than interspecific COI divergences previously reported in Nipponnemertes (3.8–4.3% between N. pulchra and N. cf. rubella) (Chernyshev & Polyakova, 2022) and other nemerteans (Sundberg et al., 2016). The most conspicuous case was observed between N. crypta and N. ojimaorum showing 1.6% in p-distance, albeit apparently different body coloration (Figures 4C, D, I–K) (similar phenomena have been found in other invertebrates such as bobtail squids (Fernández-Álvarez et al., 2021) and mites (García-Jiménez et al., 2017). Moreover, the two species were not sister taxa in our tree based on the concatenated dataset (Figure 2). One thing we can deduce from the result is that the use of COI-based genetic distances might underestimate the true intraspecific divergences due to mutational saturation in COI region among the “N. ojimaorum–N. punctatula” subclade.
Our species delimitation analyses for Clade B show the consistent result, where five lineages are identified, preferring to separate N. crypta and N. ojimaorum as different species in all three methods (TCS, ABGD, and bPTP) (Figure 3). The threshold used to separate nemertean species based on COI is mostly around 5% (e.g., Chen et al., 2010; Kang et al., 2015; Krämer et al., 2017; Chernyshev et al., 2018; Hookabe et al., 2020). Although the values we obtained were lower than 5%, we describe N. crypta and N. ojimaorum as separate species based on the differences in body colouration and internal morphology, as well as the result of species delimitation analyses (Figure 3).
Reduction in Body Size and Blood-Circulatory System in Clade C
The newly confirmed Clade C is formed by relatively smaller species (7–20 mm in the maximum body lengths) than the other two clades (12–110 mm in Clade A and 11.7–160 mm in Clade B) although we could not obtain mature adults for all species in Clade C. The smallest species in Clade C is N. lactea with developed gonads. Members in Clade C were collected from subtidal (8 m~) to deep sea bottoms (~985 m). In the deep-sea environment, body-size trends across environmental gradients (hydrostatic pressure, light, water temperature, and amount of food resources) have been demonstrated in invertebrates and demersal fish (Collins et al., 2005). It is difficult to determine which variable(s) most critically influences body size; still, food availability is known as one the of major factors in these animals (Collins et al., 2005). The body size reduction in Clade C in contrast with Clade A and B might reflect an adaptation to food-limitation in deep-water bottoms (herein referring to water depths deeper than 70 m) as inferred in non-scavenging fish (Collins et al., 2005) or predatory invertebrates (Van der Grient & Rogers, 2015). If species in Clade C are suctorial predators on small crustaceans as reported in Nipponnemertes cf. africana (McDermott, 1997), N. pulchra (Brunberg, 1964; Berg, 1972; McDermott, 1984, 1993), and N. ogumai (Kajihara et al., 2015), smaller body sizes might allow them to require less food demand for survival and reproduction. The phylogenetic positions among Clade C also indicate that N. kozaensis collected at a depth of 12 m, N. sugashimaensis collected at a depth of 10 m, and Cratenemertidae sp. Guam collected at a depth of 1 m (Chernyshev & Polyakova, 2019) might have secondarily colonized from deep (>70 m) to shallow waters (<12 m). Since ecological factors other than amount of potential food resources can be also related to the body size evolution, the speculation above should be accompanied with further examination of ecological data on species collected from various water depths.
The vascular plug, by which nutrients and other substances are supposedly delivered from the blood vascular system to the rhynchocoel (Crandall, 1993), is considered to be important to maintain the function of proboscis apparatus principally used to capture prey items. In small-bodied hoplonemerteans, in contrast, this structure seems to have been often lost, suggesting that the proboscis could function without a vascular plug by diffusion through the rhynchocoel wall (Kajihara, 2021b). In Clade C, a vascular plug is not observed except for N. sugashimaensis. This might be actually resulted from a small body size; however, the other possibility is that a vascular plug is easily affected by body contraction during fixation and/or difficult to find through histological sectioning in small-sized species. Additional morphological examination with broader taxon sampling as well as alternative methods for observation of the fine muscular structure (e.g., phalloidin labelling) is expected in future studies.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
NHo conceived the present study, executed molecular experiments, and analyzed the molecular data. NHo, HKa, and AC performed morphological observation and analyzed morphological data. All authors took part in the collection of animal samples and field data used in this study. The manuscript was drafted by NHo and critically reviewed by the other authors. All authors approved the final manuscript for submission and publication.
Funding
Part of this study was supported by the Japan Society for the Promotion of Science (21J14807 for NHo; 25650133, 26282067, 26304011, 20H03304, 20K06780 for HKa).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer AV declared a past collaboration with one of the authors HKa to the handling Editor
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
NHo and HKa thank Mamoru Sekifuji, Michiyo Kawabata (Misaki Marine Biological Station, the University of Tokyo) for help in collecting samples off Jogashima and Hiroaki Nakano (Shimoda Marine Research Center, University of Tsukuba) for organizing JAMBIO Coastal Organism Joint Surveys. NHo also thank Shouzo Ogiso (Noto Marine Laboratory, Kanazawa University) and the all the other participants in the 22nd JAMBIO Coastal Organism Joint Surveys in Noto. NHo, NJ, ST, and YF are thankful to Kensuke Yanagi (Natural History Museum and Institute, Chiba) and all the other participants in the Tohoku Ecosystem-Associated Marine Sciences (TEAMS) research project for generous help in sample collection; the captain and the crew of R/V Kaimei during KM19-05C cruise. NHo, NJ, and KT thank Hironori Komatsu (National Museum of Nature and Science, Tokyo (NSMT)), Akito Ogawa (JAMSTEC), Itaru Kobayashi, Gregorius Altius Pratama, Takumi Matsuo (the University of Tokyo) for help in sample collection in Shimoda. NHo thanks Ryuta Yoshida (Marine and Coastal Research Center, Ochanomizu University) for sincerely providing specimens used in this study with NHo. NHo, NJ, and MO are grateful to Masanori Kawai, Tomohito Ojima, Masako Ojima, Masanori Taru (Tokyo Bay Ecosystem Research Center, Toho University), and Hiroyuki Yokooka (Institute of Environmental Ecology, IDEA Consultants, Inc.) for sincere help in collecting specimens in Koganezaki. NHo, NJ, and NHa thank Takeo Sugimoto (South Wakayama Fishery Cooperative), Naofumi Ueda, Takashi Okada, Ayumi Mizutani (DIVE KOOZA) and Cati (Association for the Protection of the Sea and Marine Life, Umimorinchu) during sampling in Wakayama. Special thanks to Masashi Fukuoka, Maki Shirae-Kurabayashi, and Gohta Goshima (Sugashima Marine Biological Laboratory, Nagoya University) for kindly helping in sample collection as well as providing facilities during NHo’s sampling trip. NHo thanks Hiroshi Namikawa (NSMT), Hiroshi Saito (NSMT), Keiichi Kakui, Yuki Oya (Hokkaido University), Itaru Kobayashi (the University of Tokyo), Tomomi Saito, and Kouki Tanaka (Kochi University) for their generous help during sampling in Tosa Bay. This study was supported by the research project Tohoku Ecosystem-Associated Marine Sciences from the Ministry of Education, Culture, Sports, Science, and Technology and National Museum of Nature and Science integrated research on “Geological, biological, and anthropological histories in relation to the Kuroshio Current”.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.906383/full#supplementary-material
References
Abe H., Kan K., Matsumasa M., Suzuki T., Kajihara H. (2022). Northernmost Record of Nipponnemertes ogumai (Nemertea: Monostilifera) in Otomo-Ura (Iwate Prefecture, Japan: Michi. Ben), 6.
Andrade S. C. S., Strand M., Schwartz M., Chen H., Kajihara H., Sun S., et al. (2012). Disentangling Ribbon Worm Relationships: Multi-Locus Analysis Supports Traditional Classification on the Phylum Nemertea. Cladistics 28, 141–159. doi: 10.1111/j.1096-0031.2011.00376.x
Berg G. (1972). Studies on Nipponnemertes Friedrich 1968 (Nemertini, Hoplonemertini) I. Redescription of Nipponnemertes pulcher (Johnston 1837) With Special Reference to Intraspecific Variation of the Taxonomical Characters. Zool. Scr. 1, 211–225. doi: 10.1111/j.1463-6409.1972.tb00580.x
Berg G. (1985). Studies on Nipponnemertes Friedrich (Nemertini, Hoplonemertini). II. Taxonomy of Nipponnemertes pulcher (Johnston) and Some Other Species. Zool. Scr. 14, 239–246. doi: 10.1111/j.1463-6409.1985.tb00194.x
Brinkmann A. (1914–1915). Uniporus, Ein Neues Genus Der Familie Drepanophoridae Verrill. Ber. Mus. Aar. 6, 1–29.
Brunberg L. (1964). On the Nemertean Fauna of Danish Waters. Ophelia 1, 77–111. doi: 10.1080/00785326.1964.10416273
Bürger O. (1890). Untersuchungen über die Anatomie und Histologie der Nemertinen nebst Beiträgen zur Systematik. Z. Wiss. Zool. 50, 1–277.
Castresana J. (2000). Selection of Conserved Blocks From Multiple Alignments for Their Use in Phylogenetic Analysis. Mol. Biol. Evol. 17, 540–552. doi: 10.1093/oxfordjournals.molbev.a026334
Chen H., Strand M., Norenburg J. L., Sun S., Kajihara H., Chernyshev A. V., et al. (2010). Statistical Parsimony Networks and Species Assemblages in Cephalotrichid Nemerteans (Nemertea). PloS One 5, e12885. doi: 10.1371/journal.pone.0012885
Chernyshev A. V. (1993). Novuie Svedeniya O Sistematike Nemertin Semeistva Cratenemertidae (Enopla, Monostilifera). Vest. Zool. 1, 72–75.
Chernyshev A. V. (2010). Confocal laser scanning microscopy analysis of the phalloidin-labelled musculature in nemerteans. J. Nat. Hist. 44, 2287–2302.
Chernyshev A. V. (2016). “Nemerteans of the Coastal Waters of Vietnam”, in Biodiversity of the Western Part of the South China Sea. Eds. Adrianov A. V., Lutaenko K. A. (Vladivostok, RU: Dalnauka), 279–314.
Chernyshev A. V. (2020). Nemerteans From the Far Eastern Seas of Russia. Russ. J. Mar. Biol. 46, 141–153. doi: 10.1134/S1063074020030049
Chernyshev A. V. (2021). An Updated Classification of the Phylum Nemertea. Invertebrate. Zool. 18, 188–196. doi: 10.15298/invertzool.18.3.01
Chernyshev A. V., Polyakova N. E. (2018). Nemerteans of the Vema-TRANSIT Expedition: First Data on Diversity With Description of Two New Genera and Species. Deep-Sea. Res. II.: Top. Stud. Oceanogr. 148, 64–73. doi: 10.1016/j.dsr2.2017.06.004
Chernyshev A. V., Polyakova N. E. (2019). Nemerteans From the Deep-Sea Expedition KuramBio II With Descriptions of Three New Hoplonemerteans From the Kuril-Kamchatka Trench. Prog. Oceanogr. 178, 102–148. doi: 10.1016/j.pocean.2019.102148
Chernyshev A. V., Polyakova N. E. (2022). Nemerteans Collected in the Bering Sea During the Research Cruises Aboard the R/V Akademik MA Lavrentyev in 2016, 2018, and 2021 With an Analysis of Deep-Sea Heteronemertean and Hoplonemertean Species. Deep. Sea. Res. Part II. Top. Stud. Oceanogr., 199, 105081. doi: 10.1016/j.dsr2.2022.105081
Chernyshev A. V., Polyakova N. E., Turanov S. V., Kajihara H. (2018). Taxonomy and Phylogeny of Lineus torquatus and Allies (Nemertea, Lineidae) With Descriptions of a New Genus and a New Cryptic Species. Syst. Biodiv. 16, 55–68. doi: 10.1080/14772000.2017.1317672
Clement M., Posada D., Crandall K. A. (2000). TCS: A Computer Program to Estimate Gene Genealogies. Mol. Ecol. 9, 1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x
Coe W. R. (1901). Papers From the Harriman Alaska Expedition. XX. The Nemerteans. Proc. Wash. Acad. Sci. 3, 1–110.
Coe W. R. (1905). Nemerteans of the West and Northwest Coasts of America. Bull. Mus. Comp. Zool. 47, 1–318.
Colgan D. J., McLauchlan A., Wilson G. D. F., Livingston S. P., Edgecombe G. D., Macaranas J., et al. (1998). Histone H3 and U2 snRNA DNA Sequences and Arthropod Molecular Evolution. Aust. J. Zool. 46, 419–437. doi: 10.1071/ZO98048
Collins M. A., Bailey D. M., Ruxton G. D., Priede I. G. (2005). Trends in Body Size Across an Environmental Gradient: A Differential Response in Scavenging and non-Scavenging Demersal Deep-Sea Fish. Proc. R. Soc B.: Biol. Sci. 272, 2051–2057.
Crandall F. B. (1993). Major Characters and Enoplan Systematics. Hydrobiologia 266, 115–140. doi: 10.1007/BF00013363
Crandall F. B., Crandall J. L., Chernyshev A. V., Maslakova S. A., Schwartz & H. Kajihara M. L. (2002). Checklist of the nemertean fauna of Japan and northeastern Asia. Washington D.C:Smithsonian Institution, 1–44. Available from http://pages.uoregon.edu/svetlana/Crandall2002.pdf
Fernández-Álvarez F.Á., Sánchez P., Villanueva R. (2021). Morphological and Molecular Assessments of Bobtail Squids (Cephalopoda: Sepiolidae) Reveal a Hidden History of Biodiversity. Front. Mar. Sci. 7, 632261. doi: 10.3389/fmars.2020.632261
Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R. (1994). DNA Primers for Amplification of Mitochondrial Cytochrome C Oxidase Subunit I From Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 3, 294–299.
Friedrich H. (1955). Beiträge Zu Einer Synopsis Der Gattungen Der Nemertini Monostilifera Nebst Bestimmungsschlüssel. Z. Wiss. Zool. 158, 133–192.
Friedrich H. (1957). Beiträge Zur Kenntnis Der Arktischen Hoplonemertinen. Vidensk. Meddel. Natuirist. Foren. Kjobenhavn. 119, 129–154.
Friedrich H. (1968). Sagaminemertes, Eine Bemerkenswerte Neue Gattung Der Hoplonemertinen Und Ihre Systematische Stellung. Zool. Anz. 180, 33–36.
García-Jiménez R., Horreo J. L., Valdecasas A. G. (2017). Minimal Barcode Distance Between Two Water Mite Species From Madeira Island: A Cautionary Tale. Exp. Appl. Acarol. 72, 133–143. doi: 10.1007/s10493-017-0147-5
Gibson R. (1995). Nemertean Genera and Species of the World: An Annotated Checklist of Original Names and Description Citations, Synonyms, Current Taxonomic Status, Habitats and Recorded Zoogeographic Distribution. J. Nat. Hist. 29, 271–562. doi: 10.1080/00222939500770161
Gibson R., Crandall F. B. (1989). The Genus Amphiporus Ehrenberg (Nemertea, Enopla, Monostiliferoidea). Zool. Scr. 18, 453–470. doi: 10.1111/j.1463-6409.1989.tb00140.x
Giribet G., Carranza S., Baguñà J., Riutort M., Ribera C. (1996). First Molecular Evidence for the Existence of a Tardigrada + Arthropoda Clade. Mol. Biol. Evol. 13, 76–84. doi: 10.1093/oxfordjournals.molbev.a025573
Gonzalez-Cueto J., Castro L. R., Quiroga S. (2017). Nipponnemertes incainca sp. n. Adoption of the New Taxonomic Proposal for Nemerteans (Nemertea, Cratenemertidae). ZooKeys 693, 1–15. doi: 10.3897/zookeys.693.12015
Griffin B. B. (1898). Description of Some Marine Nemerteans of Puget Sound and Alaska. Ann. N. Y. Acad. Sci. 11, 193–217. doi: 10.1111/j.1749-6632.1898.tb54969.x
Hillis D. M., Dixon M. T. (1991). Ribosomal DNA: Molecular Evolution and Phylogenetic Inference. Q. Rev. Biol. 66, 411–453. doi: 10.1086/417338
Hookabe N., Kohtsuka H., Kajihara H. (2021). A Histology-Free Description of Tetrastemma cupido sp. nov. (Nemertea: Eumonostilfera) From Sagami Bay, Japan. Mar. Biol. Res. 17, 467–474. doi: 10.1080/17451000.2021.1979236
Hookabe N., Tsuchida S., Fujiwara Y., Kajihara H. (2020). A New Species of Bathyal Nemertean, Proamphiporus kaimeiae sp. nov., Off Tohoku, Japan, and Molecular Systematics of the Genus (Nemertea: Monostilifera). Spec. Div. 25, 183–188. doi: 10.12782/specdiv.25.183
Hubrecht A. A. W. (1887). Report on the Nemertea Collected by H.M.S. Challenger During the Years 1873–76. Rep. Sci. Res. Chall. 19, 1–150.
International Commission on Zoological Nomenclature (1999). International Code of Zoological Nomenclature. 4th Edition (London: International Trust for Zoological Nomenclature).
Iwata F. (1954). The Fauna of Akkeshi Bay. XX. Nemertini in Hokkaido (Revised Report). J. Fac. Sci. Hokkaido. Univ. 6 Zool. 12, 1–39.
Iwata F. (2001). Nipponnemertes fernaldi, a New Species of Swimming Monostiliferous Hoplonemertean From the San Juan Archipelago, Washington, USA. Proc. Biol. Soc Wash. 114, 833–857.
Johnston G. (1837). Miscellanea Zoologica. II. A Description of Some Planarian Worms. Mag. Zool. Bot. 1, 529–538.
Kajihara H. (2007). A Taxonomic Catalogue of Japanese Nemerteans (Phylum Nemertea). Zool. Sci. 24, 287–326. doi: 10.2108/zsj.24.287
Kajihara H. (2017). “Species Diversity of Japanese Ribbon Worms (Nemertea)” in Species Diversity of Animals in Japan. Eds. Motokawa M., Kajihara H. (Tokyo: Springer Japan), 419–444.
Kajihara H. (2021a). “Phylum Nemertea" in Invertebrate Zoology. A Tree of Life Approach. Eds. Schierwater B., DeSalle R. (Boca Raton: CRC Press), 357–368.
Kajihara H. (2021b). Higher Classification of the Monostilifera (Nemertea: Hoplonemertea). Zootaxa 4920, 151–199. doi: 10.11646/zootaxa.4920.2.1
Kajihara H., Nishi E., Kawabata M., Kohtsuka H., Uyeno D. (2015). Records of the Poorly Known Ribbon Worm Nipponnemertes ogumai (Nemertea: Monostilifera) and its Phylogenetic Position. Mar. Biodiv. 45, 175–182. doi: 10.1007/s12526-014-0252-1
Kang X. X., Fernández-Álvarez F.Á., Alfaya J. E., Machordom A., Strand M., Sundberg P., et al. (2015). Species Diversity of Ramphogordius sanguineus/Lineus ruber-Like Nemerteans (Nemertea: Heteronemertea) and Geographic Distribution of. R. sanguineus Zool. Sci. 32, 579–589. doi: 10.2108/zs150064
Katoh K., Standley D. M. (2013). MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Kirsteuer E. (1965). Über Das Vorkommen Von Nemertinen in Einem Tropischen Korallenriff. 4. Hoplonemertini Monostilifera. Zool. Jahrb. Abt. Syst. Geog. Biol. Tiere. 92, 289–326.
Korotkevitsch V. S. (1961). A New Nemertean Species and its Position in the System. Zool. Zhurnal. 40, 1416–1420.
Korotkevitsch V. S. (1983). O Stiletakh Nemertin I Novom Vide Cratenemertes (Hoplonemertini, Amphiporidae) Iz Antarktiki. Antark. Dok. Kom. 22, 137–143.
Krämer D., Schmidt C., Podsiadlowski L., Beckers P., Horn L., Döhren J.v. (2017). Unravelling the Lineus ruber/viridis Species Complex (Nemertea, Heteronemertea). Zool. Scr. 46, 111–126. doi: 10.1111/zsc.12185
Kumar S., Stecher G., Tamura K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Kvist S., Chernyshev A. V., Giribet G. (2015). Phylogeny of Nemertea With Special Interest in the Placement of Diversity From Far East Russia and Northeast Asia. Hydrobiologia 760, 105–119. doi: 10.1007/s10750-015-2310-5
Kvist S., Laumer C. E., Junoy J., Giribet G. (2014). New Insights Into the Phylogeny, Systematics and DNA Barcoding of Nemertea. Invert. Syst. 28, 287–308. doi: 10.1071/IS13061
Lanfear R., Frandsen P. B., Wright A. M., Senfeld T., Calcott B. (2016). PartitionFinder 2: New Methods for Selecting Partitioned Models of Evolution for Molecular and Morphological Phylogenetic Analyses. Mol. Biol. Evol. 34, 772–773. doi: 10.1093/molbev/msw260
Leasi F., Andrade S. D. S., Norenburg J. (2016). At Least Some Meiofaunal Species are Not Everywhere. Indication of Geographic, Ecological and Geological Barriers Affecting the Dispersion of Species of Ototyphlonemertes (Nemertea, Hoplonemertea). Mol. Ecol. 25, 1381–1397. doi: 10.1111/mec.13568
Leigh J. W., Bryant D. (2015). Popart: Full-Feature Software for Haplotype Network Construction. Methods Ecol. Evol. 6, 1110–1116. doi: 10.1111/2041-210X.12410
Littlewood D. T. J. (1994). Molecular Phylogenetics of Cupped Oysters Based on Partial 28S rRNA Gene Sequences. Mol. Phylogenet. Evol. 3, 221–229. doi: 10.1006/mpev.1994.1024
McDermott J. J. (1984). The Feeding Biology of Nipponnemertes pulcher (Johnston) (Hoplonemertea), With Some Ecological Implications. Ophelia 23, 1–21. doi: 10.1080/00785236.1984.10426601
McDermott J. J. (1993). Nemertea Inhabiting the Haploops (Amphipoda) Community of the Northern Øresund With Special Reference to the Biology of Nipponnemertes pulcher (Hoplonemertea). Hydrobiologia 266, 15–28. doi: 10.1007/BF00013356
McDermott J. J. (1997). Observations on Feeding in a South African Suctorial Hoplonemertean, Nipponnemertes sp. (Family Cratenemertidae). Hydrobiologia 365, 251–256. doi: 10.1023/A:1003114117397
McDermott J. J., Roe P. (1985). Food, Feeding Behavior and Feeding Ecology of Nemerteans. Am. Zool. 25, 113–125. doi: 10.1093/icb/25.1.113
Palumbi S. R., Martin A., Romano S., McMillan W. O., Stice L., Grabowski G. (1991). The Simple Fool’s Guide to PCR, V. 2.0 (Honolulu: Department Zoology, Kewalo Marine Laboratory, University of Hawaii).
Puillandre N., Lambert A., Brouillet S., Achaz G. (2012). ABGD, Automatic Barcode Gap Discovery for Primary Species Delimitation. Mol. Ecol. 21, 1864–1877. doi: 10.1111/j.1365-294X.2011.05239.x
Rambaut A., Drummond A. J., Xie D., Baele G., Suchard M. A. (2018). Posterior Summarisation in Bayesian Phylogenetics Using Tracer Ver. 1.7. Syst. Biol. 67, 901–904. doi: 10.1093/sysbio/syy032
Riser N. W. (1998). New Zealand Nemertines From Kelp Holdfasts: Hoplonemertinea 1. Nipponnemertes sanguinea sp. N. Z. J. Zool. 25, 287–294. doi: 10.1080/03014223.1998.9518157
Ronquist F., Teslenko M., van der Mark P., Ayres D. L., Darling A., Höhna S., et al. (2012). MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Schwendinger P. J., Giribet G. (2005). The Systematics of the Southeast Asian Genus Fangensis Rambla (Opiliones: Cyphophthalmi: Stylocellidae). Invert. Syst. 19, 297–323. doi: 10.1071/IS05023
Stamatakis A. (2014). RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Stimpson W. (1857). Prodromus Descriptionis Animalium Evertebratorum, Quae in Expeditione ad Oceanum Pacificum Septentrionalem,a Republica Federata missa, Cadwaladaro Ringgold et Johanne Rodgers Ducibus, observavit et descripsit. ParsII. Turbellarieorum Nemertineorum.. Proceedings of the Academy of Natural Sciences of Philadelphia 9, 159–165.
Sundberg P., Kvist S., Strand M. (2016). Evaluating the Utility of Single-Locus DNA Barcoding for the Identification of Ribbon Worms (Phylum Nemertea). PloS One 1, e0155541. doi: 10.1371/journal.pone.0155541
Templeton A. R., Crandall K. A., Sing C. F. (1992). A Cladistic Analysis of Phenotypic Associations With Haplotypes Inferred From Restriction Endonuclease Mapping and DNA Sequence Data. III. Cladogram Estimation. Genetics 132, 619–633.
Thiel M., Kruse I. (2001). Status of the Nemertea as Predators in Marine Ecosystems. Hydrobiologia 456, 21–32. doi: 10.1023/A:1013005814145
Thollesson M., Norenburg J. L. (2003). Ribbon Worm Relationships: A Phylogeny of the Phylum Nemertea. Proc. R. Soc. B: Biol. Sci. 270, 407–415. doi: 10.1098/rspb.2002.2254
Thompson J. D., Higgins D. G., Gibson T. J. (1994). CLUSTAL W: Improving the Sensitivity of Progressive Multiple Sequence Alignment Through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Res. 22, 4673–4680. doi: 10.1093/nar/22.22.4673
Van der Grient J. M., Rogers A. D. (2015). Body Size Versus Depth: Regional and Taxonomical Variation in Deep-Sea Meio-And Macrofaunal Organisms. Adv. Mar. Biol. 71, 71–108. doi: 10.1016/bs.amb.2015.07.002
Wheeler J. F. G. (1934). Nemerteans From the South Atlantic and Southern Oceans. ‘Discovery’. Rep 9, 215–294.
Wheeler J. F. G. (1940). Some Nemerteans from South Africa and a Note on Lineus corrugatus McIntosh. Zool. J. Linn. Soc. 41, 20–49.
Whiting M. F., Carpenter J. M., Wheeler Q. D., Wheeler W. C. (1997). The Strepsiptera Problem: Phylogeny of the Holometabolous Insect Orders Inferred From 18S and 28S Ribosomal DNA Sequences and Morphology. Syst. Biol. 46, 1–68. doi: 10.1093/sysbio/46.1.1
Yamaoka T. (1947). “Amphiporus ogumai”, in Revised Edition, Illustrated Encyclopedia of the Fauna of Japan (Exclusive of Insects). Ed. Okuda S. (Tokyo: Hokuryukan). 1468, fig. 4138 (1–3).
Yamaoka T. (2005). On Fourteen Monostiliferous Hoplonemerteans From the Izu Peninsula, Middle Japan. Publ. Seto. Mar. Biol. Lab. 40, 141–158. doi: 10.5134/176324
Keywords: Hoplonemertea, ROV, Nemertea, systematics, ribbon worm, SCUBA
Citation: Hookabe N, Kajihara H, Chernyshev AV, Jimi N, Hasegawa N, Kohtsuka H, Okanishi M, Tani K, Fujiwara Y, Tsuchida S and Ueshima R (2022) Molecular Phylogeny of the Genus Nipponnemertes (Nemertea: Monostilifera: Cratenemertidae) and Descriptions of 10 New Species, With Notes on Small Body Size in a Newly Discovered Clade. Front. Mar. Sci. 9:906383. doi: 10.3389/fmars.2022.906383
Received: 28 March 2022; Accepted: 16 May 2022;
Published: 26 July 2022.
Edited by:
Jin Sun, Ocean University of China, ChinaReviewed by:
Jose Alfaya, CONICET Instituto de Biología de Organismos Marinos (IBIOMAR), ArgentinaAida Verdes, Department of Biodiversity and Evolutionary Biology, National Museum of Natural Sciences, Spanish National Research Council (CSIC), Spain
Fernando Ángel Fernández-Álvarez, Institute of Marine Sciences, Spanish National Research Council (CSIC), Spain
Copyright © 2022 Hookabe, Kajihara, Chernyshev, Jimi, Hasegawa, Kohtsuka, Okanishi, Tani, Fujiwara, Tsuchida and Ueshima. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natsumi Hookabe, sofeechan312@gmail.com
 Natsumi Hookabe
Natsumi Hookabe Hiroshi Kajihara
Hiroshi Kajihara Alexei V. Chernyshev
Alexei V. Chernyshev Naoto Jimi
Naoto Jimi Naohiro Hasegawa6
Naohiro Hasegawa6 Masanori Okanishi
Masanori Okanishi Kenichiro Tani
Kenichiro Tani Yoshihiro Fujiwara
Yoshihiro Fujiwara Shinji Tsuchida
Shinji Tsuchida