
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 24 May 2022
Sec. Aquatic Physiology
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.902633
This article is part of the Research Topic Nutritional Physiology of Aquacultured Species View all 12 articles
A 63-day feeding trial with 640 juvenile L. maculatus was conducted to assess the effects of dietary CT on serum metabolites, antioxidant and immune response, liver histomorphology and glycometabolism enzyme activities of fish. Four diets were formulated to contain 0 (T1), 1 (T2), 2 (T3), and 2 g/kg of CT plus 4 g/kg of polyethylene glycol (PEG) (T4). PEG specifically binds with CT to neutralize CT activity. Fish were randomly distributed into 16 tanks (4 tanks per group and 40 fish per tank) and were fed to apparent satiation twice daily. Results indicated that fish fed T2 and T3 had lower (P<0.05) concentrations of serum albumin, total cholesterol and triacylglycerol, but higher (P<0.05) alanine aminotransferase activity than T1 and T4. T3 had lower (P<0.05) serum glucose but higher (P<0.05) insulin concentrations than other groups. Fish fed T2 and T3 had higher (P<0.05) liver superoxide dismutase, catalase, lysozyme and immune globulin M than T1 and T4. Compared with T1 and T4, hepatocytes in T2 and T3 were shown to have obvious vacuolar degeneration along with different degree of inflammatory cell infiltration. Fish fed T2 and T3 had higher (P<0.05) values of eosinophilic infiltrate and necrosis and greater (P<0.05) pyruvate kinase, but lower (P<0.05) glucokinase activities than those of fish fed T1 and T4. All parameters were similar (P>0.05) between T1 and T4. In summary, dietary CT up to 2 g/kg reduced serum lipid and glucose concentrations, enhanced liver antioxidant and immune response, improved glucose utilization but of L. maculatus. CT induced liver injury of L. maculatus which provided a caution for its application in aquaculture.
Chinese seabass (Lateolabtax maculatus), an important commercial fishery species in China, whose production was nearly 200,000 tons and the annual output value exceed 3 billion yuan in 2020 according to the data from China Fishery Statistics Yearbook (Fishery Administration of Ministry of Agriculture and Rural Affairs, 2021). In recent years, the ever-increasing breeding density of L. maculatus driven by commercial interests compels fish under stress or in sub-health state for a long time, which threats to fish health and food safety. On the other hand, since antibiotics were completely banned, challenges including metabolic disorders, disease and oxidative stress faced by intensive breeding have increased severely. Therefore, there is an urgent need to find new alternatives to antibiotics to maintain healthy and sustainable development of aquaculture.
Condensed tannins (CT) is a kind of polyphenol compound which widely exists in the plant kingdom (Peng et al., 2016). CT was regarded as a promising alternative to antibiotics owing to its strong antioxidant, anti-inflammatory and antibacterial activities and etc. (Huang et al., 2018). However, limited information is available about the evaluation of effects of CT on physiology and health of fish. It has been reported that the biological activity of CT is closely associated with its dietary concentrations (Waghorn, 2008). Our recent studies showed that dietary CT less than 1 g/kg did not affect growth performance but improved stress resistance of L. maculatus (Peng et al., 2020a; Peng et al., 2022a; Peng et al., 2022b). In contrast, other studies documented that dietary CT exceeded 1 g/kg induced intestinal injury of shrimp (Litopenaeus vannamei) (Peng et al., 2021a) and impaired immune function of grass carp (Ctenopharyngodon idella) (Li et al., 2020). Furthermore, dietary CT up to 1 g/kg was observed to reduce the serum lipid and glucose levels of L. maculatus (Peng et al., 2020a), whereas this has not been assessed for high concentration of CT.
Although CT enhanced antioxidant and immune capacity and improved physical health of L. maculatus, dietary CT exceeded 1 g/kg may have adverse effects on fish, including growth inhibition and cellular damage according to the observations by previous studies. To verify this hypothesis, this study is conducted to assess the effects of dietary CT on serum metabolites, antioxidant and immune response, liver histomorphology and glycometabolism enzyme activities of L. maculatus to provide a rational for the application of CT in fish diets.
Condensed tannins (purity ≥99.5%) was extracted from grape seed and purified according to the method described by Peng et al. (2022a). Polyethylene glycol (PEG, MW 3350, Sigma) specifically binds with CT to neutralize CT activity (Peng et al., 2016). Therefore, comparison between diets with and without PEG supplementation would define the effects of CT. In total of four isoproteic and isolipidic diets (Table 1) were formulated to contain 0 (T1), 1 (T2), 2 (T3), and 2 g/kg of CT plus 4 g/kg of PEG (T4). PEG was added to diet by spraying 30 g/mL of PEG solution onto CT to achieve a CT-to-PEG ratio of 1:2 (Makkar et al., 1995). All ingredients were smashed to pass through a 320 μm sieve, mixed thoroughly and then extruded into 2 mm pellets, dried at 55 °C and stored at -20°C until use.
A 63-day feeding trial was carried out using 640 disease-free L. maculatus provided by a commercial fish farm in Zhangzhou, Fujian, China. Fish with a mean initial body weight of 2.75 (± 0.03) g were randomly distributed into 16 tanks (four tanks per diet and 40 fish per tank) and were fed to apparent satiation twice daily (08:00 and 20:00). Water temperature was 26.5 ± 1.0°C, dissolved oxygen > 5.0 mg/L, pH was 7.7 ± 0.3, salinity was 1‰, ammonia nitrogen and nitrite < 0.01 mg/L, and the photoperiod regime was 12 h light and 12 h dark. The protocol (no. SC20210402) of this study was approved by the Animal Care and Use Committee of Guangdong Academy of Agricultural Sciences (Guangzhou, China).
Before sampling, fish were fasted for 24 h and anesthetized with 40 mg/L of tricaine methanesulfonate (Sigma, USA). Blood were collected from the caudal veins of twelve fish per tank, kept at 25°C for 30 min, and centrifuged at 8000 × g for 10 min. The resultant serum was determined for the serum metabolite concentrations. Livers of six fish per tank were randomly collected, of which three livers were analyzed for antioxidant and immune parameters, and another three livers were used for histological examination following the procedures described by Peng et al. (2021a). Livers of three fish per tank were randomly collected for measuring glycometabolism enzyme activities.
The crude protein, crude lipid and ash contents of the experimental diets were measured using AOAC method (AOAC, 1999). Serum metabolites, including albumin (ALB), globulin (GLOB), total cholesterol (TCHO), triacylglycerol (TG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), high density lipoprotein cholesterol (HDLC), low density lipoprotein cholesterol (LDLC), glucose (GLU), insulin (INS) and glucagon (GLG), and liver antioxidant and immune parameters, including total antioxidant capacity (TAOC), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), malondialdehyde (MDA), lysozyme (LZM), immune globulin M (IgM) and complement 3 (C3), and liver glycometabolism enzyme activities, including phosphofructokinase (PFK), pyruvate kinase (PK) and glucokinase (GK) were determined using commercial kits provided by Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to corresponding manufacture’s instructions.
For liver histological analysis, score were attributed to histological changes according to the degree of intensity: 0 (absence of change), 1 (mild change, corresponding to <25% of the organ area), 2 (moderate change, 25% to 50% of the organ area) and 3 (severe alteration, >50% of the organ area) following the method described by Schwaiger et al. (1997) modified by Brum et al. (2018). The following hepatic changes were evaluated: nucleus hypertrophy, hepatocyte hypertrophy, eosinophilic infiltrate, lymphocytic infiltrate, necrosis, karyolysis, karyorrhexis and pyknosis.
All data were subjected to a one-way analysis of variance using SPSS 17.0 analysis software for Windows followed by the Duncan’s multiple-range test with tank as statistical unit and treatment as fixed effect. Differences were regarded as significance when P<0.05 and the results are presented as means ± standard error.
Fish fed T2 and T3 had lower (P < 0.05) ALB, TCHO and TG, but higher (P < 0.05) ALT than those of fish fed T1 and T4 (Table 2). T3 had lower (P < 0.05) ALB, and similar (P > 0.05) TCHO and TG, and higher (P < 0.05) ALT than T2. T4 had similar (P > 0.05) ALB, TCHO, TG and ALT than T1. T3 had lower (P < 0.05) GLU but higher (P < 0.05) INS than other groups. GLU and INS were similar (P > 0.05) among T1, T2 and T4. All fish had similar (P > 0.05) GLOB, AST, HDLC, LDLC and GLG among groups.
Fish fed T2 and T3 had higher (P < 0.05) SOD, CAT, LZM and IgM than those of fish fed T1 and T4 (Table 3). T3 had higher (P < 0.05) SOD and CAT, but similar (P > 0.05) LZM and IgM than T2. T4 had similar (P > 0.05) SOD, CAT, LZM and IgM than T1. All fish had similar (P > 0.05) TAOC, GPx, MDA and C3 among groups.
As shown in Figure 1, hepatocytes in T1 and T4 were loose, polyhedral in shape with vacuolated cytoplasm, and no obvious inflammatory cell infiltration was detected. However, hepatocytes in T2 and T3 were shown to have obvious vacuolar degeneration along with different degree of inflammatory cell infiltration.
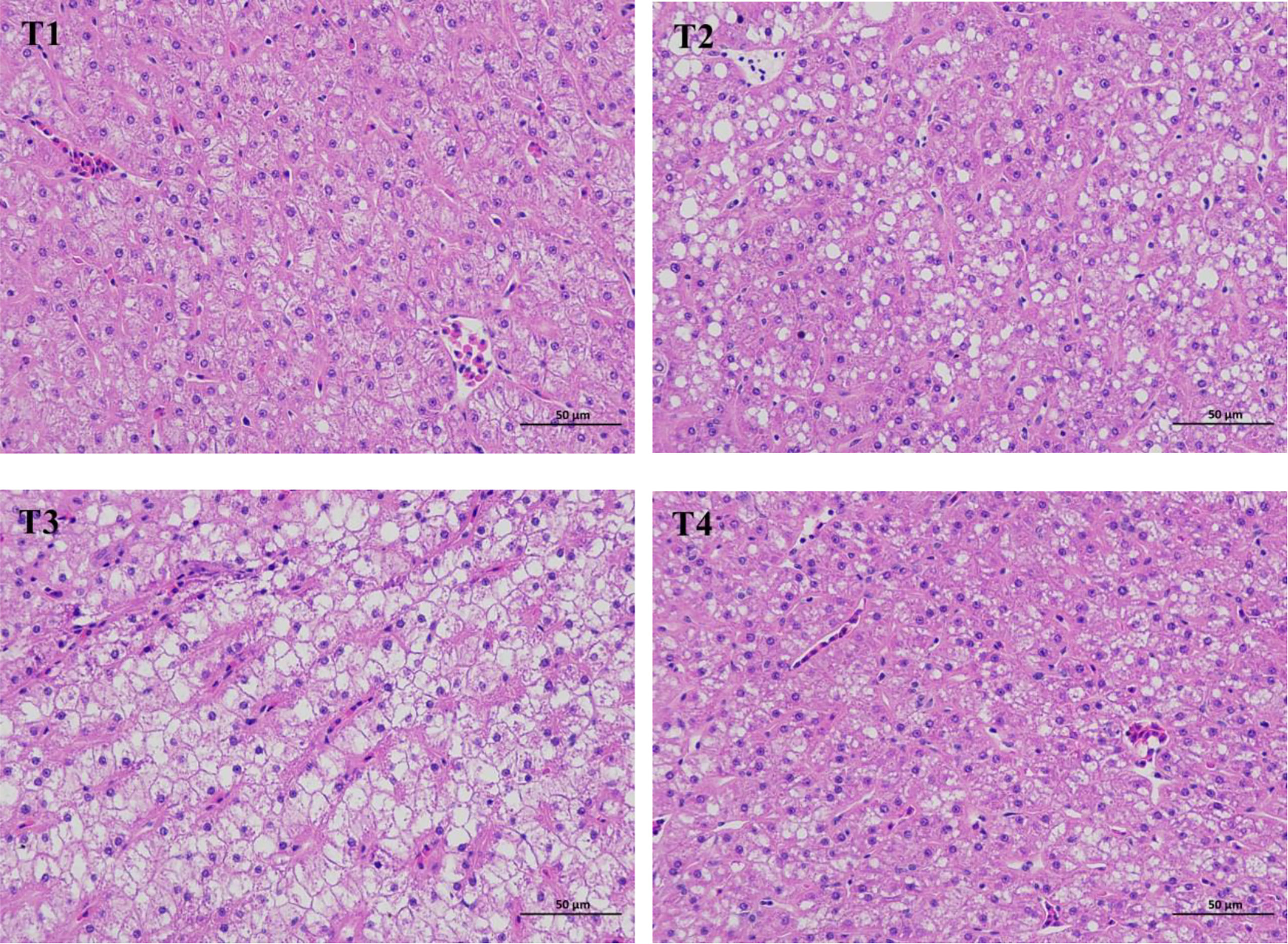
Figure 1 Liver histomorphology (haematoxylin-eosin stain, × 400) of L. maculatus fed experimental diets. T1, basal diet; T2, basal diet supplemented with 1 g/kg of condensed tannins; T3, basal diet supplemented with 2 g/kg of condensed tannins; T4, basal diet supplemented with 2 g/kg of condensed tannins and 4 g/kg of polyethylene glycol.
Fish fed T2 and T3 had higher (P < 0.05) values of eosinophilic infiltrate and necrosis than those of fish fed T1 and T4 (Table 4). T4 had similar (P > 0.05) values of eosinophilic infiltrate and necrosis than T1. All fish had similar (P > 0.05) values of nucleus hypertrophy, lymphocytic infiltrate, karyolysis, karyorrhexis and pyknosis among treatments.
All fish had similar (P < 0.05) PFK activity among groups (Figure 2). The PK activity was ranked as T1<T2<T3 (P < 0.05), and was similar (P > 0.05) between T1 and T4. Fish fed T2 and T3 had lower (P < 0.05) GK activity than T1 and T4. T4 had similar (P > 0.05) GK activity than T1.
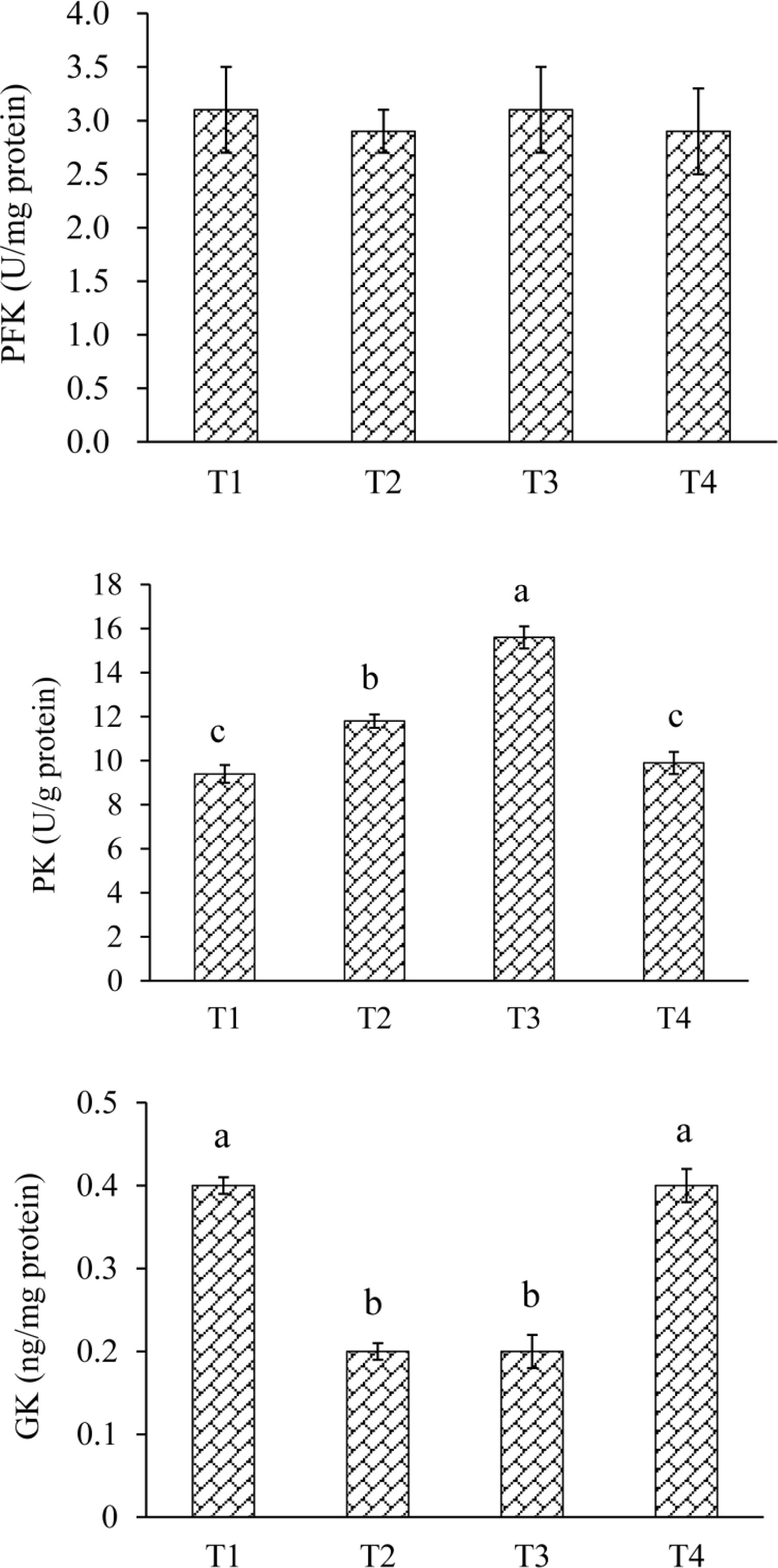
Figure 2 Liver glycometabolism enzyme activities of L. maculatus fed experimental diets. PFK, phosphofructokinase; PK, pyruvate kinase; GK, glucokinase. T1, basal diet; T2, basal diet supplemented with 1 g/kg of condensed tannins; T3, basal diet supplemented with 2 g/kg of condensed tannins; T4, basal diet supplemented with 2 g/kg of condensed tannins and 4 g/kg of polyethylene glycol. (a-c) Different lowercase letters above the bars denote significant differences among diets (P < 0.05).
In this study, PEG specifically binds with CT to neutralize CT activity, therefore comparison between diets with and without PEG supplementation would define the effects of CT. This is a typical method used in previous studies (Huang et al., 2015; Peng et al., 2016; Peng et al., 2018; Peng et al., 2021b). Serum metabolites are one of the best non-invasive means for evaluating health condition, as they are stable end-products of biochemical processes in animals. The serum metabolite profiles in this study suggest that dietary CT partly affected physiological metabolism processes of L. maculatus as reflected by the corresponding variation parameters. ALB is synthesized mainly in liver which is the predominant protein filtered by the glomerulus and the major serum protein targeted to oxidant stress in fish (Liu et al., 2018). Generally, injured liver cause decreased concentration of serum ALB (Zhang et al., 2018). Conversely, increased serum ALT activity is commonly related to liver damage or hepatocyte necrosis (Wang et al., 2014). Therefore, the decreased serum ALB concentrations along with increased ALT activities in L. maculatus fed CT-treated diets indicated CT induced liver injury of fish. This finding suggested that inclusion of CT in fish diet should be cautious, and the liver damage induced by CT should be considered in their application. It is recommended that the supplemental level of CT in L. maculatus diets should be less than 1 g/kg. Blood TCHO and TG indexes are critical factors affecting lipid accumulation which has been commonly proved in fish studies (Torstensen et al., 2011; Jiang et al., 2015; Peng et al., 2020a). In this study, the decreased serum concentrations of TCHO and TG in fish fed CT-treated diets indicated that CT decreased blood lipid content. Similarly, the lipid-lowing effect of CT has been previously observed in Nile tilapia (Oreochromis niloticus) (Aiura and Carvalho, 2007), Chinese seabass (L. maculatus) (Peng et al., 2020a) and grass carp (Ctenopharyngodon idellus) (Yao et al., 2022). INS plays a key role in maintaining glucose homeostasis and a negative correlation between INS and GLU was generally observed in fish (Brauge et al., 1994; Liang et al., 2017; Peng et al., 2020b). Declined GLU concentration along with increased INS level in L. maculatus fed diet containing 2 g/kg of CT indicated that CT may decrease blood glucose level by stimulating insulin secretion. Peng et al. (2020b) documented that CT improved glucose utilization of L. maculatus by the promotion of glycolysis and inhibition of gluconeogenesis. This may account for the hypoglycemic activity of CT as observed in this study. Furthermore, dietary CT was reported to decrease serum GLU concentration of sheep, owing to CT induced pancreatic β-cell regeneration (Peng et al., 2016).
Condensed tannins has been shown to possess strong antioxidant activity both in vitro and in vivo studies (Beninger and Hosfield, 2003; Peng et al., 2020a; Peng et al., 2021a; Peng et al., 2022a). In this study, dietary CT increased liver SOD and CAT activities. Antioxidant enzymes such as SOD and CAT play a key role in alleviating oxidative stress by scavenging reactive oxygen species (Peng et al., 2022a). SOD catalyzes the dismutation of superoxide anion free radical and plays an important role in defensing oxidant stress (Paulton et al., 2012). CAT protects cells from oxidative damage via catalyzing hydrogen peroxide decomposition (Peng et al., 2016). Our previous studies also showed that dietary CT improved antioxidant enzymes activities of L. maculatus by activating the Keap1-Nrf2/ARE signaling pathway (Peng et al., 2020a; Peng et al., 2020c). LZM is a key defense protein in innate immune system which plays a critical role in defending pathological change (Brott and Clarke, 2019). IgM is regarded as the first antibody responded to an antigen (Semple et al., 2018). In this study, the increased liver LZM and IgM in fish fed CT-treated diets suggested that CT enhanced immune response of L. maculatus. This is similar with our previous observation by Peng et al. (2020a). Generally, CT was regarded as an immunopotentiator (Huang et al., 2018) which enhanced immune function by inhibiting the activation of signaling pathways, such as mitogen activated protein kinases (MAPKs) and nuclear factor kappa B (NF-κB) (Peng et al., 2020d). It has been reported that the biological activity of CT is closely associated with their dietary concentrations (Waghorn, 2008). Previously, Peng et al. (2020a) documented that dietary CT up to 1 g/kg enhanced antioxidant and immune capacity of L. maculatus. This study indicated that CT up to 2 g/kg in L. maculatus diet still has significantly antioxidant and immune activities.
As is known to us, histological change is an important aspect in the understanding of pathological alteration related to nutritional intervention in fish (Shi et al., 2017). Although CT was documented to alleviate the stress-induced damage to the liver of fish (Peng et al., 2022b), our recent study reported that no obvious degeneration and inflammatory cell infiltration were observed in the liver of L. maculatus fed diets containing CT less than 1 g/kg (Peng et al., 2020a). However, this study indicated that dietary CT exceed 1 g/kg injured the liver of L. maculatus accompanied by obvious vacuolar degeneration and inflammatory cell infiltration along with increased values of eosinophilic infiltrate and necrosis, suggesting that effects of CT on liver histomorphology depend on the concentrations of CT in diets. In this study, the histomorphology observation is also consistent with the results of serum metabolites, as reflected by the decreased serum ALB concentrations and the increased ALT activities of fish fed CT-treated diets. This is the first study indicating that dietary CT induced liver damage of fish which provided a caution for the application of CT in aquaculture.
As the main control center for glycogenesis, glycogenolysis, glycolysis and gluconeogenesis, liver plays a critical role in the regulation of glucose metabolism. Among the glycometabolism enzymes, the PK and GK are critical indicators to diagnose the glucose metabolism in the liver of fish (Peng et al., 2020b). It has been reported that PK is a major enzyme that participates in the last step of glycolysis (Lu et al., 2018), whereas GK promotes glycogen synthesis in the liver (Li et al., 2015). In this study, the increased PK and decreased GK activities in the liver of L. maculatus fed CT-treated diets suggested that CT promotes glucose utilization of fish. This is similar with the observation by Peng et al. (2020b) that CT improved glucose utilization of L. maculatus due to its positive effects on the activity and gene expression of glycometabolism enzymes. As is well known to us, most carnivorous fish have a low glucose tolerance and poor utilization of carbohydrate (Tan et al., 2009). Our previous study reported that high dietary carbohydrate induced hyperglycemia and abnormal of liver glucose metabolism, and therefore depressed the growth of L. maculatus (Peng et al., 2020b). Results of this study provide a new insight for improving the glucose utilization of fish by application of CT in fish diet. Despite the reason why CT improved glucose utilization of fish is not fully understood, CT and its precursors were reported to accelerate the absorption rate of glucose and promote the synthesis of glycogen by up-regulating gene expression of GLUT-2 (a primary glucose transporter) (Cordero-Herrera et al., 2014) and inhibiting hepatic GK activity (Huang et al., 2013). Howsoever, the findings of this study provided a new explanation from a different perspective to reveal the underlying mechanism regarding CT improve the glucose utilization of fish. Further study is still needed to elucidate the mechanism by which CT improve the glycometabolism of L. maculatus.
In conclusion, dietary CT up to 2 g/kg reduced serum lipid and glucose concentrations, enhanced liver antioxidant and immune response, and improved glucose utilization of L. maculatus. However, dietary CT at 1 and 2 g/kg induced liver injury of L. maculatus which provided a caution for their application in aquaculture. It is recommended that the supplemental level of CT in L. maculatus diets should be less than 1 g/kg. Further study is still needed to elucidate the mechanism by which CT improve the glycometabolism of fish.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by Animal Care and Use Committee of Guangdong Academy of Agricultural Sciences.
KP and WH conceived and designed the experiments. KP, BC, HZ, and YW performed the experiments. KP analyzed the data and wrote the manuscript. All authors approved the submitted version.
This study was funded by National Natural Science Foundation of China (31902388), Natural Science Foundation of Guangdong Province of China (2021A1515010850, 2022A1515010545), Science and Technology Planning Project of Guangzhou (202002030378) and Special Fund for Scientific Innovation Strategy-Construction of High-Level Academy of Agriculture Science (R2018QD-075, R2021PY-QY001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We acknowledge those who donated the experimental animals and those who helped support this study at the Institute of Animal Science, Guangdong Academy of Agricultural Sciences.
Aiura F. S., Carvalho M. R. B. (2007). Body Lipid Deposition in Nile Tilapia Fed on Rations Containing Tannin. Pesqui. Agropecu. Bras. 42, 51–56. doi: 10.1590/S0100-204X2007000100007
AOAC (1999). Official Methods of Analysis of the Association of Official Agricultural Chemists. 16th ed (Gaithersburg: AOAC International, 5th rev).
Beninger C. W., Hosfield G. L. (2003). Antioxidant Activity of Extracts, Condensed Tannin Fractions, and Pure Flavonoids From Phaseolus Vulgaris L. Seed Coat Color Genotypes. J. Agr. Food Chem. 51 (27), 7879–7883. doi: 10.1021/jf0304324
Brauge C., Medale F., Corraze G. (1994). Effect of Dietary Carbohydrate Levels on Growth, Body Composition and Glycaemia in Rainbow Trout, Oncorhynchus Mykiss, Reared in Seawater. Aquaculture 123, 109–120. doi: 10.1016/0044-8486(94)90123-6
Brott A. S., Clarke A. J. (2019). Peptidoglycan O-Acetylation as a Virulence Factor: Its Effect on Lysozyme in the Innate Immune System. Antibiotics 8, 94. doi: 10.3390/antibiotics8030094
Brum A., Cardoso L., Chagas E. C., Chaves F. C. M., Mourino J. L. P., Martins M. L. (2018). Histological Changes in Nile Tilapia Fed Essential Oils of Clove Basil and Ginger After Challenge With Streptococcus Agalactiae. Aquaculture 490, 98–107. doi: 10.1016/j.aquaculture.2018.02.040
Cordero-Herrera I., Martin M., Goya L., Ramos S. (2014). Cocoa Flavonoids Attenuate High Glucose Induced Insulin Signalling Blockade and Modulate Glucose Uptake and Production in Human HepG2 Cells. Food Chem. Toxicol. 64, 10–19. doi: 10.1016/j.fct.2013.11.014
Fishery Administration of Ministry of Agriculture and Rural Affairs (2021). China Fishery Statistical Yearbook (Beijing, China: China Agriculture Press).
Huang P. L., Chi C. W., Liu T. Y. (2013). Areca Nut Procyanidins Ameliorate Streptozocin-Induced Hyperglycemia by Regulating Gluconeogenesis. Food Chem. Toxicol. 55, 137–143. doi: 10.1016/j.fct.2012.12.057
Huang Q., Jin L., Xu Z., Barbieri L. R., Acharya S., Hu T., et al. (2015). Effects of Purple Prairie Clover (Dalea Purpurea Vent.) on Feed Intake, Nutrient Digestibility and Faecal Shedding of Escherichia Coli O157:H7 in Lambs. Anim. Feed Sci. Technol. 207, 51–61. doi: 10.1016/j.anifeedsci.2015.06.009
Huang Q., Liu X., Zhao G., Hu T., Wang Y. (2018). Potential and Challenges of Tannins as an Alternative to in-Feed Antibiotics for Farm Animal Production. Anim. Nutr. 4, 137–150. doi: 10.1016/j.aninu.2017.09.004
Jiang S., Wu X., Li W., Wu M., Luo Y., Lu S., et al. (2015). Effects of Dietary Protein and Lipid Levels on Growth, Feed Utilization, Body and Plasma Biochemical Compositions of Hybrid Grouper (Epinephelus Lanceolatus ♂ × Epinephelus Fuscoguttatus ♀) Juveniles. Aquaculture 446, 148–155. doi: 10.1016/j.aquaculture.2015.04.034
Liang H., Habte-Tsion H. M., Ge X., Ren M., Pan W. (2017). Dietary Arginine Affects the Insulin Signaling Pathway, Glucose Metabolism and Lipogenesis in Juvenile Blunt Snout Bream Megalobrama Amblycephala. Sci. Rep. 7, 7864. doi: 10.1038/s41598-017-06104-3
Li M., Feng L., Jiang W. D., Wu P., Liu Y., Jiang J., et al. (2020). Condensed Tannins Decreased the Growth Performance and Impaired Intestinal Immune Function in on-Growing Grass Carp (Ctenopharyngodon Idella). Br. J. Nutr. 123 (7), 737–755. doi: 10.1017/S0007114519003295
Liu Y., Yang P., Hu H., Li Y., Dai J., Zhang Y., et al. (2018). The Tolerance and Safety Assessment of Taurine as Additive in a Marine Carnivorous Fish, Scophthalmus Maximus L. Aquac. Nutr. 24, 461–471. doi: 10.1111/anu.12579
Li J. N., Xu Q. Y., Wang C. A., Wang L. S., Zhao Z. G., Luo L. (2015). Effects of Dietary Glucose and Starch Levels on the Growth, Haematological Indices and Hepatic Hexokinase and Glucokinase mRNA Expression of Juvenile Mirror Carp (Cyprinus Carpio). Aquac. Nutr. 22, 550–558. doi: 10.1111/anu.12278
Lu S., Wu X., Gao Y., Gatlin D. M., Wu M., Yao W., et al. (2018). Effects of Dietary Carbohydrate Sources on Growth, Digestive Enzyme Activity, Gene Expression of Hepatic Gluts and Key Enzymes Involved in Glycolysis-Gluconeogenesis of Giant Grouper Epinephelus Lanceolatus Larvae. Aquaculture 484, 343–350. doi: 10.1016/j.aquaculture.2017.07.033
Makkar H. P., Blummel M., Becker K. (1995). Formation of Complexes Between Polyvinyl Pyrrolidones or Polyethylene Glycols and Tannins, and Their Implication in Gas Production and True Digestibility in In Vitro Techniques. Br. J. Nutr. 73, 897–913. doi: 10.1079/BJN19950095
Paulton M. P., Thomas P. C., Vijayan K. K. (2012). Identification of Antioxidant Enzyme Genes of the Indian Edible Oyster, Crassostrea Madrasensis (Preston) Through Polymerase Chain Reaction. Indian J. Fish. 59 (4), 179–181. doi: 10.1016/j.aquaculture.2012.08.041
Peng K., Chen B., Zhao H., Zheng C., Wang Y., Luo C., et al. (2022b). Condensed Tannins Alleviate Aflatoxin B1-Induced Injury in Chinese Sea Bass (Lateolabrax Maculatus). Aquaculture 552, 738029. doi: 10.1016/j.aquaculture.2022.738029
Peng K., Huang W., Zhao H., Sun Y., Chen B. (2021a). Dietary Condensed Tannins Improved Growth Performance and Antioxidant Function But Impaired Intestinal Morphology of Litopenaeus Vannamei. Aquac. Rep. 21, 100853. doi: 10.1016/j.aqrep.2021.100853
Peng K., Jin L., Niu Y., Huang Q., McAllister T. A., Yang H. E., et al. (2018). Condensed Tannins Affect Bacterial and Fungal Microbiomes and Mycotoxin Production During Ensiling and Upon Aerobic Exposure. Appl. Environ. Microbiol. 84, e02274–e02217. doi: 10.1128/AEM.02274-17
Peng K., Lv X., Zhao H., Chen B., Chen X., Huang W. (2022a). Antioxidant and Intestinal Recovery Function of Condensed Tannins in Lateolabrax Maculatus Responded to In Vivo and In Vitro Oxidative Stress. Aquaculture 547, 737399. doi: 10.1016/j.aquaculture.2021.737399
Peng K., Shirley D. C., Xu Z., Huang Q., McAllister T. A., Chaves A. V., et al. (2016). Effect of Purple Prairie Clover (Dalea Purpurea Vent.) Hay and Its Condensed Tannins on Growth Performance, Wool Growth, Nutrient Digestibility, Blood Metabolites and Ruminal Fermentation in Lambs Fed Total Mixed Rations. Anim. Feed Sci. Technol. 222, 100–110. doi: 10.1016/j.anifeedsci.2016.10.012
Peng K., Wang G., Wang Y., Chen B., Sun Y., Mo W., et al. (2020c). Condensed Tannins Enhanced Antioxidant Capacity and Hypoxic Stress Survivability But Not Growth Performance and Fatty Acid Profile of Juvenile Japanese Seabass (Lateolabrax Japonicus). Anim. Feed Sci. Technol. 269, 114671. doi: 10.1016/j.anifeedsci.2020.114671
Peng K., Wang Y., Wang G., Huang Y. (2020d). Biological Function of Condensed Tannins and Their Application in Animal Production. Chin. J. Anim. Nutr. 32 (8), 3451–3460. doi: 10.3969/j.issn.1006-267x.2020.08.001
Peng K., Wang G., Zhao H., Wang Y., Mo W., Wu H., et al. (2020b). Effect of High Level of Carbohydrate and Supplementation of Condensed Tannins on Growth Performance, Serum Metabolites, Antioxidant and Immune Response, and Hepatic Glycometabolism Gene Expression of Lateolabrax Japonicus. Aquac. Rep. 18, 100515. doi: 10.1016/j.aqrep.2020.100515
Peng K., Xu Z., Nair J., Jin L., McAllister T. A., Acharya S., et al. (2021b). Conserving Purple Prairie Clover (Dalea Purpurea Vent.) as Hay and Silage Had Little Effect on the Efficacy of Condensed Tannins in Modulating Ruminal Fermentation In Vitro. J. Sci. Food Agric. 101, 1247–1254. doi: 10.1002/jsfa.10913
Peng K., Zhou Y., Wang Y., Wang G., Huang Y., Cao J. (2020a). Inclusion of Condensed Tannins in Lateolabrax Japonicus Diets: Effects on Growth, Nutrient Digestibility, Antioxidant and Immune Capacity and Copper Sulphate Stress Resistance. Aquac. Res. 18, 100525. doi: 10.1016/j.aqrep.2020.100525
Schwaiger J., Wanke R., Adam S., Pawert M., Honnen W., Triebskorn R. (1997). The Use of Histopathological Indicators to Evaluate Contaminant-Related Stress in Fish. J. Aquat. Ecosyst. Stress Recov. 6, 75–86. doi: 10.1023/A:1008212000208
Semple S. L., Kellendonk C. J., Al-Hussinee L., MacInnes J. I., Lumsden J. S., Dixon B. (2018). Serum IgM, MH Class Iiβ Genotype and Respiratory Burst Activity do Not Differ Between Rainbow Trout Families Displaying Resistance or Susceptibility to the Coldwater Pathogen, Flavobacterium Psychrophilum. Aquaculture 483, 131–140. doi: 10.1016/j.aquaculture.2017.10.020
Shi X., Luo Z., Chen F., Wei C. C., Wu K., Zhu X. M., et al. (2017). Effect of Fish Meal Replacement by Chlorella Meal With Dietary Cellulase Addition on Growth Performance, Digestive Enzymatic Activities, Histology and Myogenic Genes’expression for Crucian Carp Carassius Auratus. Aquac. Res. 48, 3244–3256. doi: 10.1111/are.13154
Tan Q., Wang F., Xie S., Zhu X., Lei W., Shen J. (2009). Effect of High Dietary Starch Levels on the Growth Performance, Blood Chemistry and Body Composition of Gibel Carp (Carassius Auratus Var. Gibelio). Aquac. Res. 40, 1011–1018. doi: 10.1111/j.1365-2109.2009.02184.x
Torstensen B. E., Espe M., Stubhaug I., Lie O. (2011). Dietary Plant Proteins and Vegetable Oil Blends Increase Adiposity and Plasma Lipids in Atlantic Salmon (Salmo Salar L.). Brit. J. Nutr. 106, 633–647. doi: 10.1017/S0007114511000729
Waghorn G. (2008). Beneficial and Detrimental Effects of Dietary Condensed Tannins for Sustainable Sheep and Goat Production-Progress and Challenges. Anim. Feed Sci. Technol. 147, 116–139. doi: 10.1016/j.anifeedsci.2007.09.013
Wang L. N., Liu W. B., Lu K. L., Xu W. N., Cai D. S., Zhang C. N., et al. (2014). Effects of Dietary Carbohydrate/Lipid Ratios on Non-Specific Immune Responses, Oxidative Status and Liver Histology of Juvenile Yellow Catfish Pelteobagrus Fulvidraco. Aquaculture 426-427 (1), 41–48. doi: 10.1016/j.aquaculture.2014.01.022
Yao J., Liu N., Li N., Li X., Hua X. (2022). Different Metabolomic Responses of Grass Carp (Ctenopharyngodon Idellus) to Dietary Tannin and Rapeseed Meal. Aquac. Fish. 7, 40–51. doi: 10.1016/j.aaf.2020.06.002
Keywords: condensed tannins, feed additive, glycolipid metabolism, physical health, fish
Citation: Peng K, Chen B, Zhao H, Wang Y and Huang W (2022) Condensed Tannins Improve Glycolipid Metabolism but Induce Liver Injury of Chinese Seabass (Lateolabrax maculatus). Front. Mar. Sci. 9:902633. doi: 10.3389/fmars.2022.902633
Received: 23 March 2022; Accepted: 25 April 2022;
Published: 24 May 2022.
Edited by:
Helena Peres, University of Porto, PortugalReviewed by:
Josef Velíšek, University of South Bohemia, CzechiaCopyright © 2022 Peng, Chen, Zhao, Wang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen Huang, aHVhbmd3ZW41NDlAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.