- 1Marine Biology Branch, Zoology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt
- 2Department of Biology, College of Science, Taif University, Taif, Saudi Arabia
- 3Zoology Department, Faculty of Science, Zagazig University, Zagazig, Egypt
- 4PhD Program in Evolutionary Biology and Ecology, Department of Biology, University of Rome “Tor Vergata”, Rome, Italy
Medicinal plants are a potential safe source of bioactive compounds. Fish diet supplemented with the medicinal plant bottle gourd (Lagenaria siceraria) seed powder was evaluated in this study for the potential effect on growth performance, antioxidative status, immunological response, and resistance to infectious pathogens in the Nile tilapia (Oreochromis niloticus). Nile tilapia fingerlings with mean weight ( ± SD)= (25.64 ± 0.17 g), were fed four L. siceraria seed supplemented diets (LSSD) at 0.0, 1, 2, and 3% for 60 days. Specimens were then challenged with the bacterium Aeromonas hydrophila for 10 days. Also, three different concentrations (2.5, 5, and 10 ml/L) of Lagenaria siceraria ethanolic extract (LSEE) were tested for their antibacterial and antiparasitic efficacy on four selected bacterial and one parasitic species. All parameters’ values generally improved with elevating the content of the L. siceraria seed powder in the diet. Dietary administration of LSSD-3% enabled significant (P < 0.05) higher growth performance, and feed utilization efficiency. It reduced the mortality induced by A. hydrophila infection, increased crude protein content in the fish body and exhibited the highest in vitro antibacterial and antiparasitic efficiency. RBCs, WBCs, Hb, PCV, MCV, MCH, and total serum protein values in pre- and post-challenge groups were significantly higher (P < 0.05) in the LSSD-3% group. While MCHC, ALT, AST and glucose levels were significantly lower (P < 0.05) than those of the other groups. Lysozyme and antioxidant enzyme activities in pre- and post-challenge groups were also higher (P < 0.05) in the LSSD-3% group compared to the other groups. LSEE provided good efficacy against Gram-negative bacterial strains, mild efficacy against Gram-positive bacterial strains, and an antagonistic effect on the parasite Cichlidogyrus Tilapiae. The 10 ml/L concentration was the most effective against the pathogens followed by the 5 ml/L concentration and then 2.5 ml/L. Our findings suggest the feasibility of supplementation of Nile tilapia (O. niloticus) diet with L. siceraria seed powder by 3% to improve the growth performance, immunity, and vital parameters.
Introduction
The challenges of food security considering the global population increase require concerted efforts between researchers and animal protein producers in the fish sector to implement scientific solutions in line with the global trend to reduce pressure on wild stocks and increase production through aquaculture. This leads to new concerns related to the production of fish that are safe for human consumption in so-called “green fish” or fish raised in an antibiotic-free environment. Antibiotics are among the most widely used chemicals in the aquaculture sector, especially in intensive systems to control outbreaks of fungal, bacterial, and parasitic pathogens (Sapkota et al., 2008). The use of several compounds classified as antibiotics has been reported in the majority of aquaculture producing countries (Lulijwa et al., 2019). Despite the remarkable positive effect of these antibiotics in resisting infection, they have various concerns related to human health such as the development of antibiotic resistance to bacterial pathogens and adverse drug reactions (ADR), as well as possible chronic toxicity in the case of bioaccumulation of residues in the consumer’s body (Lulijwa et al., 2019). Therefore, there was a need for natural immunostimulants as an alternative to antibiotics for infectious diseases in cultivated fish (Meena et al., 2013; Song et al., 2014; Soares et al., 2020). Medicinal plants are one of those sources that have shown effectiveness in improving the immune status, stress resistance, growth performance, and nutrient utilization in cultivated fish as well as deterring infection from pathogens during early life stages (Newaj-Fyzul and Austin, 2015; Hoseinifar et al., 2017; Mansouri Taee et al., 2017; Safari et al., 2017; Nawaz et al., 2018; Hoseinifar et al., 2019; Kesbiç et al., 2020; Soares et al., 2020; Fazio et al., 2022). The bottle gourd Lagenaria siceraria (Molina) is a big pubescent, annual, prostrate, or ascending plant native to Africa and Asia. The plant is a member of the Cucurbitaceae family that is widely dispersed throughout the world’s warmer climates (Yetişir et al., 2008). This plant has traditionally been used to treat asthma, fever, hypertension, jaundice, ulcer, trypanosomiasis, myiasis, and some ectoparasite infestations in tropical regions (Habibur Rahaman, 2003; Tadeg et al., 2005; Hussain et al., 2008; Yirga et al., 2012). Various studies have revealed good therapeutic potential of the fruits and seeds of L. siceraria as an effective antibiotic, antioxidant, antihyperglycemic, anticancer, analgesic, antidiabetic, antihepatotoxic, anti-inflammatory, anthelmintic, antimicrobial, cardioprotective, and diuretic (Prajapati et al., 2010; Saha et al., 2011; Gill et al., 2012; Ahmed et al., 2014; Dar et al., 2014; Ahmed et al., 2017; Ferdaus et al., 2020). Furthermore, the biochemical composition of different parts of the plant presents a good nutritional profile due to the presence of important nutrients and bioactive compounds such as triterpenoids, saponins, polyphenols, flavonoids (Chen et al., 2008), pectin, β-carotene, amino acids, vitamin B, vitamin C (Ogunbusola et al., 2010; Roopan et al., 2016), and cucurbitacin I (Attar and Ghane, 2018).
In aquaculture, there is a scarcity of studies dealing with dietary supplementation with cucurbits in aquatic feeds, hence the novelty of this study, especially as it investigates the effect of this supplement on improving growth performance and enhancing immune resistance toward bacterial and parasitic pathogens in the Nile tilapia (Oreochromis niloticus) as a species of high economic importance. The Nile tilapia is the third most produced species globally, representing more than 8% of the total species produced globally by aquaculture (FAO, 2020). Many communities, especially Africans, depend on this species specifically for their daily sustenance as a cheap source of animal protein, encouraging farmers to cultivate it to meet the great demand.
Aeromonas hydrophila is a very common bacterium pathogen that is involved in a disease condition inducing hemorrhage and septicemia and may lead to significant economic losses in cultivated fish (Bailone et al., 2010). Thus, the present study aims to investigate whether dietary supplementation with L. siceraria seed powder has a significant effect on growth performance and immune response to bacterial and parasitic pathogens in juveniles of the Nile tilapia O. niloticus.
Materials and Methods
Preparation of the Experimental Diet
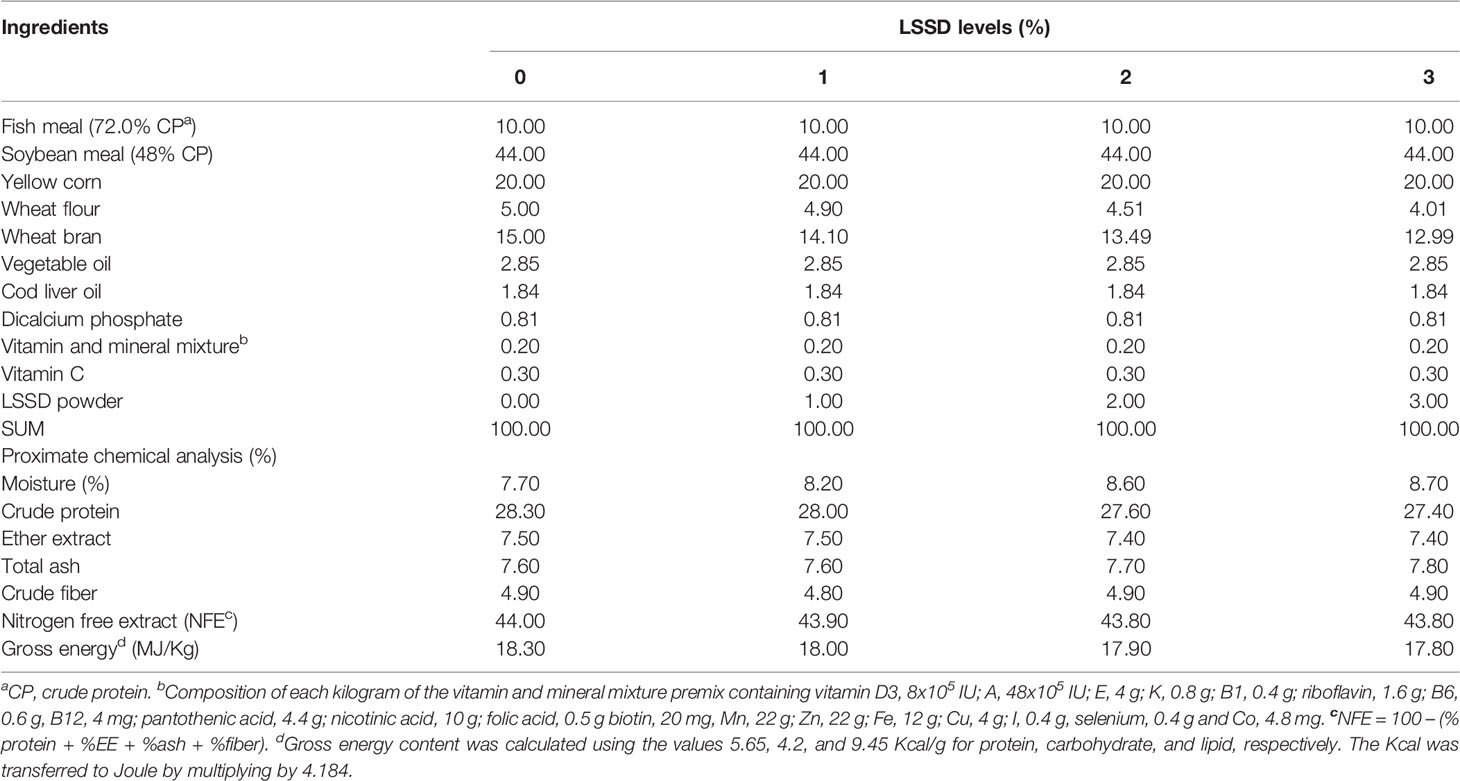
Different concentration levels (0, 1, 2, and 3%) of Lagenaria siceraria seed powder (LSSD) were selected according to Fay et al. (2014). All ingredients of the 4 experimental diets shown in Table 1 were mingled with oil, water was added until a solid paste was obtained, and then each formulated diet was extruded using a mincer. The obtained diet pellets were air-dried and stored at 4°C in plastic bags until use.
Feeding Trial
There were 350 juvenile specimens of Oreochromis niloticus with mean weight (± SD)= 25.64 ± 0.17 g) obtained from a private fish farm, Abbassa, Sharkia Governorate, Egypt. They were transferred in polyethylene bags filled with dechlorinated water and supplied with aeration to an experimental unit of a private fish farm at Abbassa, Sharkia Governorate, Egypt. Once they arrived at the experimental unit, they were allocated into (1 × 1 x 1) m3 fiberglass tanks during 14 days for acclimatization. All specimens appeared healthy, and no injuries or lesions were recorded on the visual examination (Schmitt et al., 2004).
After acclimatization, a total of 320 specimens were randomly separated into 4 groups corresponding to the 4 formulated diets. Each group consisted of 80 specimens and was evenly quadrupled (20 specimens/replicate). Specimens were then placed in (1.50 x 1.50 x 1.10) m3 experimental concrete ponds and fed with the formulated diet twice daily, at 9:00 and 14:00 for 60 days during June and July 2021. The ponds were provided with dechlorinated water (10% daily exchange) and aeration and were siphoned daily to remove solid waste. The feeding trial was conducted under natural photoperiod (12L:12D) and water temperature of 26 ± 2°C. Water quality parameters such as pH, DO, and total ammonia values were stable throughout the trial and were found to be within the normal ranges reported by Boyd and Tucker (2012).
Growth Performance and Feed Efficiency Indices
At the end of the feeding trial, the fish were collected, counted, and the final wet weight of all specimens was recorded. Weight Gain (WG), Specific Growth Rate (SGR), and Feed Conversion Ratio were estimated according to the formulas:
WG (g) = Wf − Wi
SGR (%.day-1) = 100 * (ln Wf − ln Wi)/number of days
FCR = FI/WG
where Wi is the fish initial wet weight (g), Wf is the fish final wet weight (g), and FI is the feed intake (g).
Chemical Analysis
The chemical composition of the formulated diet and fish (8 specimens/replicate) was measured according to standard methods of Thiex et al. (2012) to estimate the dry matter, crude protein, total lipids, crude fiber, and ash content as illustrated in Table 1. The nitrogen-free extract (NFE) was calculated using the following formula: NFE (g.kg-1) = 1000 – (crude protein + crude lipids + ash + crude fiber). In addition, the gross diet energy was estimated based on the values of protein, lipid, and carbohydrates as 5.65, 9.45, and 4.2 Kcal/g, respectively. All analyses were performed in triplicate.
The Pathogenic Bacterium Challenge Test
A bacterial strain (Aeromonas hydrophila) was used in the challenge test and was obtained from the Department of Microbiology, Faculty of Science, Al-Azhar University, Egypt. It was cultured using Tryptic Soy Broth (Himedia, Mumbai, India) and then incubated for 24 h at 25°C. The culture broth solution was centrifuged at 3000 rpm for 10 min. The supernatant was discarded, and the obtained pellets were washed twice with phosphate-buffered saline as described by Naiel et al. (2020). The optical density (OD) was then measured using the prepared solution at 456 nm, which parallels 1 × 107 cells.ml-1 (Bailone et al., 2010).
Twenty-four hours after the end of the feeding trial, the pathogenic bacterium challenge test was performed on 40 specimens of each diet group (10 specimens/replicate). The specimens were injected intraperitoneally with 0.1 ml of bacterial suspension (Zahran et al., 2018a; Zahran et al., 2018b). During the 10-day trial period, the fish were fed once daily on the control diet (LSSD-0). Dead specimens were removed continuously and recorded daily.
The mortality data were used to calculate the relative live percentage (RLP) according to the Amend (1981) equation:
RLP = 1 - [(recorded mortality percentage in treated groups (%))/(recorded mortality percentage in non-treated groups (%))] × 100.
Blood Samples Collection
After 60 days of the feeding trial (Phase I), fish were starved for 24 h before being removed from the holding ponds and immersed in an anaesthetic solution (Shah and Altindag, 2004). Using a plastic syringe, 2 ml of blood samples was collected from the caudal peduncle of the fish specimens in each group replicate (n=3). A small portion was mixed with EDTA dipotassium salt as an anticoagulant and placed in Eppendorf tubes, while the other portion was allowed to coagulate at room temperature for serum in a plain centrifuge tube. Serum was extracted from a blood sample and centrifuged at 4°C for 10 min at 3000 rpm before being stored in Eppendorf tubes at -20°C until analysis. Red blood cells (RBCs) and white blood cells (WBCs) were counted under the recommendations of Blaxhall and Daisley (1973). The mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentrations (MCHC) were estimated as described by Dacie and Lewis (1991). While samples for enzymes such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), glucose, and total protein were preserved in bottles without anticoagulants, they were also estimated using the standard Dacie and Lewis (1975) formula. The turbidimetric assay (Ellis, 1990) based on the lysis of Micrococcus lysodeikticus (Sigma Chemical Co.) was used to measure serum lysozyme activity, with some modifications as described elsewhere (Zahran et al., 2018a; Zahran et al., 2018b). All these procedures have been repeated after completing the bacterial challenge test (Phase II).
Determination of Lipid Peroxidation
Lipid peroxidation serum was measured by estimation of the hepatic malondialdehyde (MDA), following procedures of the Diamond Diagnostic Co. of Modern Chemicals Laboratory, Egypt. In a test tube, 1 ml of thiobarbituric acid reagent was mixed with 0.2 ml of each sample and standard. The mixture was thoroughly mixed and heated for 30 min at 95°C in a boiling water bath to form thiobarbituric acid reacting substances measured at 534 nm. MDA was measured as nmol/g tissue.
Hepatic Oxidative Stress Biomarkers
The activity of superoxide dismutase (SOD) was measured using commercial kit procedures (Bio-diagnostic, Egypt). According to Aebi (1984), catalase (CAT) activity was measured spectrophotometrically (spectrophotometer, 510 nm) by measuring the decrease in H2O2 concentration at 240 nm. The activity of glutathione peroxidase (GPx) was measured using commercial kits (Biodiganostic, Egypt) according to the manufacturer’s instructions. In addition, the activity of glutathione peroxidase (GPx) was determined using commercial kits (Biodiganostic, Egypt), under the manufacturer’s instructions with reading absorbance at 340 nm. The activity of all enzymes was expressed as U/g tissue.
Preparation of L. siceraria Ethanolic Extract
The fruits of the bottle gourd, Lagenaria siceraria, were collected from a private vegetable and fruit farm in Kafr El-Sheikh Governorate, Egypt, and were identified by the research staff of the botany department, Faculty of Science, Al-Azhar University, Egypt. The fruits were cut lengthwise to reveal the seeds. The seeds were collected, washed, and then shade-dried for 3 days and crushed with the peel. The pulverized powder of the seeds was defatted by maceration using petroleum ether for 48 h. The solution was then subjected to Soxhlet extraction with ethanol (95%) for 72 h. The extract was concentrated in vacuo to obtain the L. siceraria ethanolic extract (LSEE). LSEE was kept in the refrigerator in a closed glass flask. The dose-calculated amount of extract was dissolved in distilled water for desired experimental use (Rajput et al., 2014).
GC-MS of L. siceraria Seed Extract
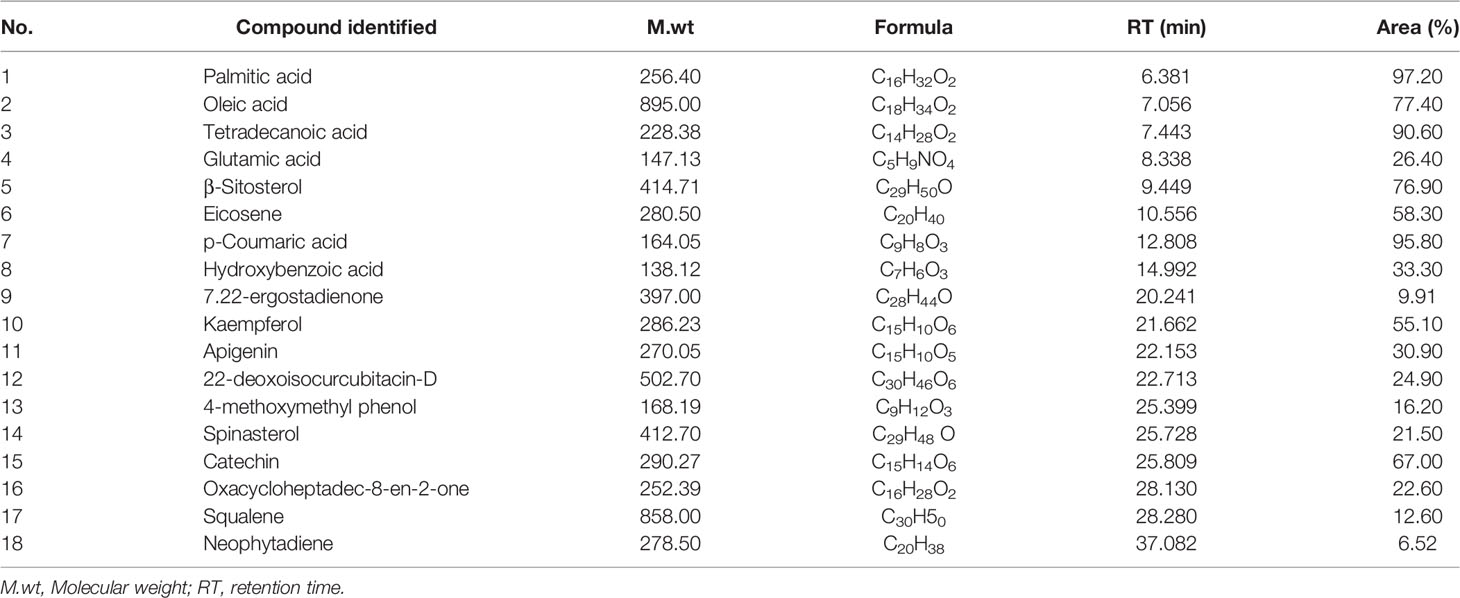
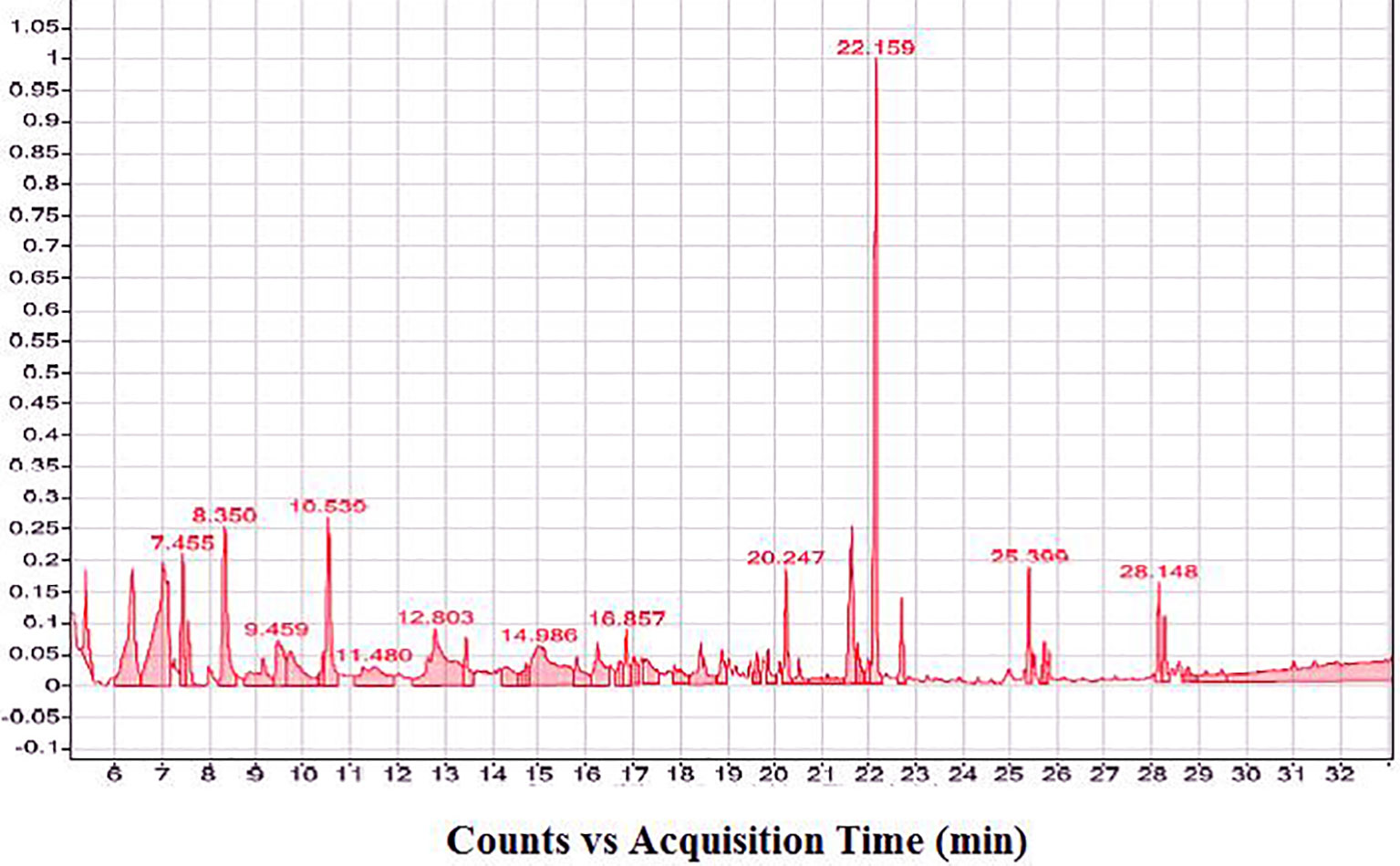
The composition of the prepared concentrated seed extract was analyzed and identified by gas chromatography-mass spectrometer method (GC-MS) (Table 2 and Figure 1). The analysis was done with standard specification by using GC-MS Agilent 7890A Series GC system interfaced to 5975C inert MSD with a Triple-Axis detector with a 7697A autosampler (Agilent Technologies, US), and an HP-5MS 5% phenyl methyl silox-bonded phase column (30 m in length × 250 μm in diameter × 0.25 μm in thickness of film) (Agilent Technologies, US). The total GC run time was 62 min and the carrier gas was helium at a flow rate of 1.22 ml/min at a constant pressure of 22.231 psi. The initial oven temperature was held at 90°C for 1 min and then raised to 205°C at a rate of 8 ml/min for 1 min, followed by raising to 240°C at a rate of 5 ml/min for 1 min and finally held at 300°C at a rate of 8 ml/min for 30 min. An injection volume of 1 μl was used. Compound identification was performed by comparison with chromatographic retention characteristics, a mass spectral library of the GC-MS data system (Sigma-Aldrich), and quantified using total ion peak area and calibration curves of the external standards (Agricultural Research Center, Dokki, Giza).
In Vitro Antibacterial Activity of LSEE
The 3 LSEE levels (2.5, 5, and 10 ml/L) were tested for their antibacterial ability on 4 selected bacterial species. First, the weight of each selected level was extracted using 100 ml of ethanol (80%) by distillation and stored in a dark Durham’s bottle. The bottles were then incubated at <24°C for 24 h in a 130-rpm shaking water bath (Hashemi Karouei et al., 2012). After obtaining the solution, it was separated by centrifugation at 5000 rpm for 15 min and then filtered by Whatman filter paper (150 mm, Ashless). The solvent was evaporated to one-fifth of the original volume and the extract was stored in closed bottles at 4°C. The four bacterial strains that were tested are Aeromonas hydrophilia, Aeromonas sobria, Streptococcus agalactiae, and Streptococcus iniae, and they were obtained from the Department of Microbiology, Faculty of Science, Al-Azhar University, Egypt.
In Vitro Anti-Parasitic Activity of LSEE
Infected Nile tilapia (O. niloticus) with parasite Cichlidogyrus tilapiae was obtained from Abbassa, Sharkia Governorate, Egypt, in July 2021. The target parasite was identified according to Radwan (2022). The fish were sacrificed by a blow to the head, then the gill arches were extracted and placed in Petri dishes containing distilled water. Parts of gill filaments holding parasites were cut and placed in 12-well plates, each containing 10 ml of distilled water and 10 parasites. The three LSEE concentrations (2.5, 5, and 10 ml/L for 120 min, 4 replicates each), were applied against C. tilapiae when the time was defined as zero. Each treatment had control wells containing distilled water without the addition of the LSEE. The parasites were observed every 10 min using a dissecting microscope and mortality was recorded. Parasites were considered dead if they did not respond to touch and did not show any reaction when being transferred to clean wells containing distilled water. According to Zhang et al. (2014), treatment can be considered effective if 100% parasite mortality is achieved within 24 h. Finally, the anti-parasitic efficacy of each treatment and control group was calculated using the Wang et al. (2009) formula:
AE = [B – T] x 100%/B
where AE is the anti-parasitic efficacy, B is the mean number of survival in control, and T is the mean number of survival in the treatment group.
Data Analysis
The obtained data were examined for normality and homogeneity distribution using Levene’s test. All the data expressed in percentage was arcsine transformed before performing the statistical analysis. One-way ANOVA was applied to test the differences between LSSD levels in 95% confidence value (P < 0.05) using the SPSS software (v.22.0). Tukey’s HSD was used when significant differences (P < 0.05) between data were identified. Data were expressed as mean ± SE, except for the antibacterial and antifungal data that were expressed as mean ± SD.
Results
Growth and Survival Performance
The growth performance of O. niloticus presented in Table 3 and Figure 2 reveals increases in all parameters with a significant difference (P < 0.05) between all supplemented groups, where the values improved with the increasing proportion of the LSSD in the diet. The LSSD-3% diet showed the best growth performance, followed by LSSD-2%, then LSSD-1%, with a significant difference (P < 0.05) from the control group. Consistently, the survivability represented by SR and RPS increased in the supplemented groups with the increasing proportion of LSSD in the diet, after a challenge with A. hydrophila. The RPS of LSSD-1, LSSD-2, and LSSD-3% was 75%, 90%, and 95%, respectively, compared to the 70% LSSD-0 control group.
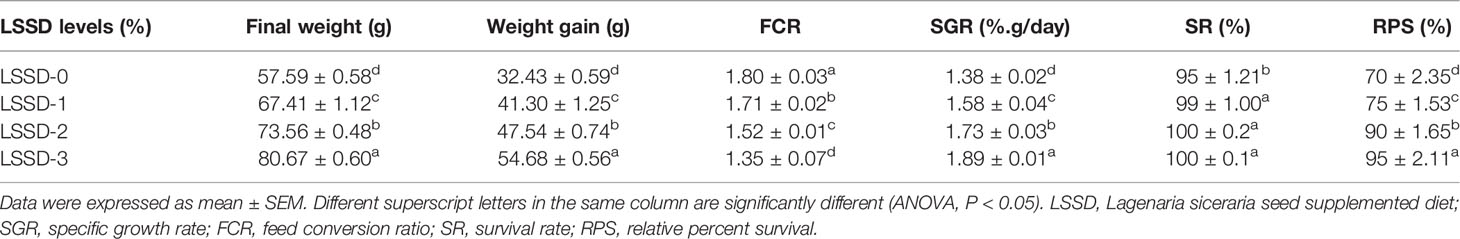
Table 3 Growth performance of O. niloticus fed 0, 1, 2, and 3% diet of L. siceraria seeds supplemented diets (LSSD) for 60 days, and relative survival after bacterial challenge with A. hydrophila for 10 days.

Figure 2 Growth performance of O. niloticus fed 0, 1, 2, and 3% diet of L. siceraria seeds supplemented diets (LSSD) for 60 days. Different superscript letters indicate significantly different (ANOVA, P < 0.05).
Biochemical Composition of Fish Body
The results of the body biochemical analysis showed that the crude protein content in the fish was significantly higher (P < 0.05) in the supplemented groups with higher LSSD than in those fed with a lower level of LSSD and the control group. In contrast, the ash content was significantly lower (P < 0.05) in the supplemented groups with higher LSSD than in those fed with a lower level of LSSD and the control group. While no significant difference (P < 0.05) was observed in the crude fat content among all groups as observed in Table 4.
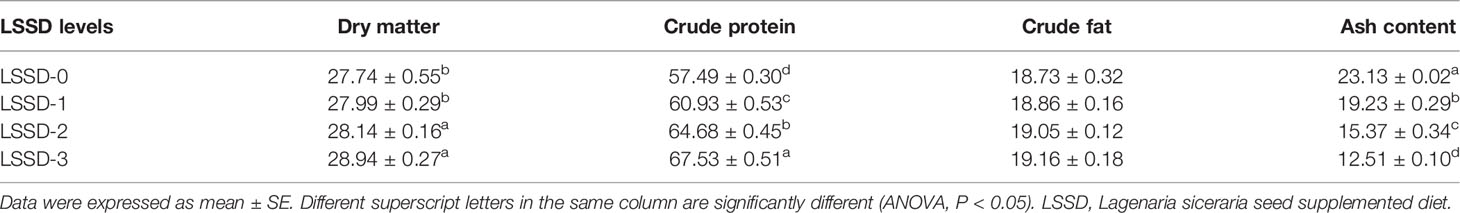
Table 4 Biochemical composition of O. niloticus (%, dry weight basis) fed 0, 1, 2, and 3% diet of L. siceraria seeds supplemented diets (LSSD).
Blood Parameters
Table 5 reveals that WBCs were significantly higher (P < 0.05) in the LSSD supplemented diet groups in both phases I and II in agreement with the elevated LSSD content compared to the control diet group without LSSD supplementation. Likewise, the values of other indices including RBCs, Hb, PCV, MCV, and MCH were significantly higher (P < 0.05) in the diet groups with LSSD supplementation at both phases compared to the control diet. On the other hand, MCHC decrease significantly (P < 0.05) in the LSSD supplemented diet groups compared to the control group in phases I and II.
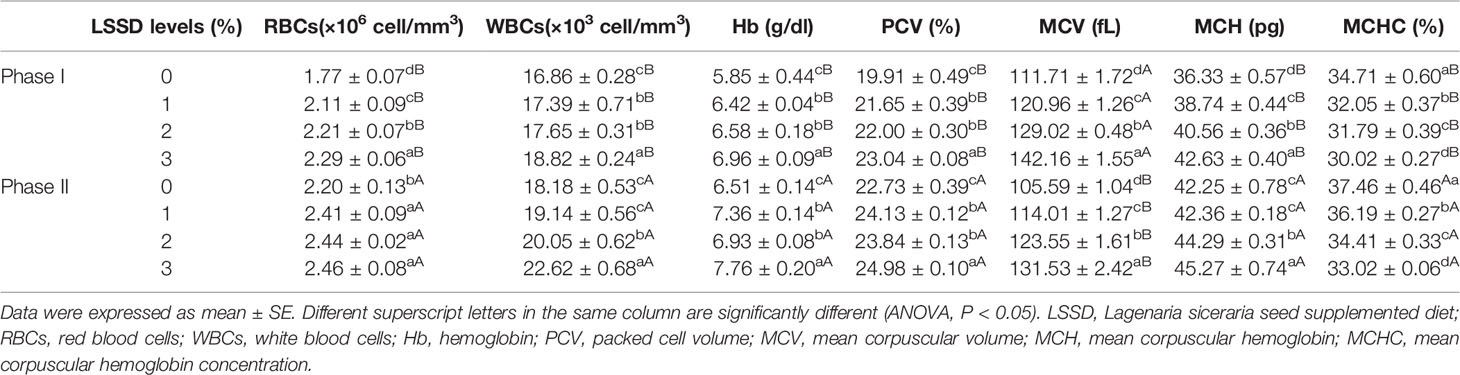
Table 5 Hematological indices of O. niloticus fed 0, 1, 2, and 3% diet of L. siceraria seeds supplemented diets (LSSD) for 60 days (Phase I) and after exposure to A. hydrophila (Phase II).
The values of other blood parameters such as ALT, AST, and glucose were generally lower in the diet groups with the highest LSSD content than in the lower and control groups in the first and second phases, dissimilar, the total serum protein value increased in the diet groups with the highest LSSD content than in the lower and control groups (Table 6).
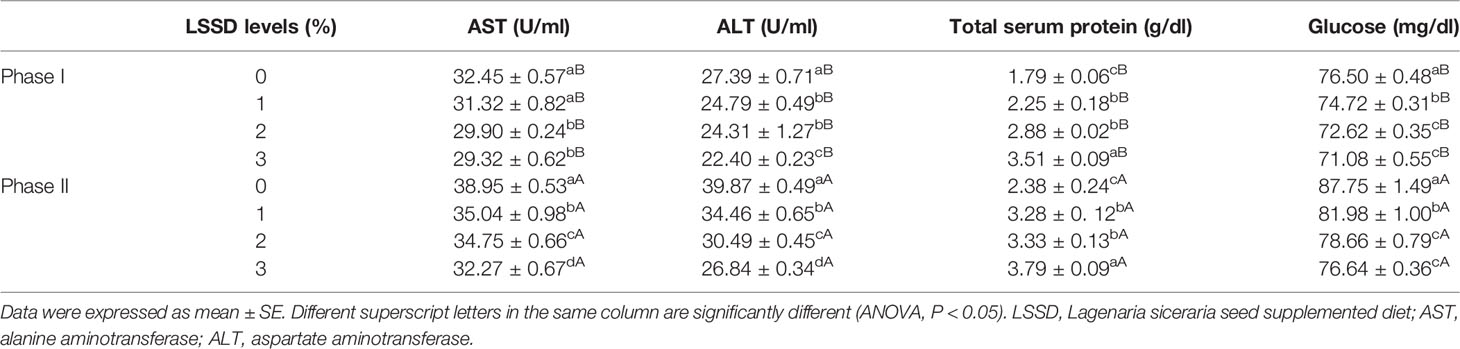
Table 6 Biochemical indices of O. niloticus fed 0, 1, 2, and 3% diet of L. siceraria seeds supplemented diets (LSSD) for 60 days (Phase I) and after exposure to A. hydrophila (Phase II).
Stress Biomarkers and Lipid Peroxidation
The lysozyme activity was found to differ significantly (P < 0.05) between the supplemented diet groups in phases I and II with the best activity observed in the LSSD-3% group and the lowest in the LSSD-0 group in both phases. In parallel, the enzymes SOD, GPx, and CAT had a similar trend with the highest activity observed in the LSSD-3% group and the lowest in the LSSD-0 group in both phases. The CAT activity in phase II was significantly higher (P < 0.05) in the higher supplemented LSSD groups (LSSD-2 and LSSD-3%) than in the lower groups (LSSD-0% and LSSD-1%) (Table 7). Clearly, the increase in the LSSD content was accompanied by a decrease in the production of the MDA, as the lowest MDA was found in LSSD-2 and LSSD-3 supplemented diet groups in both phases.
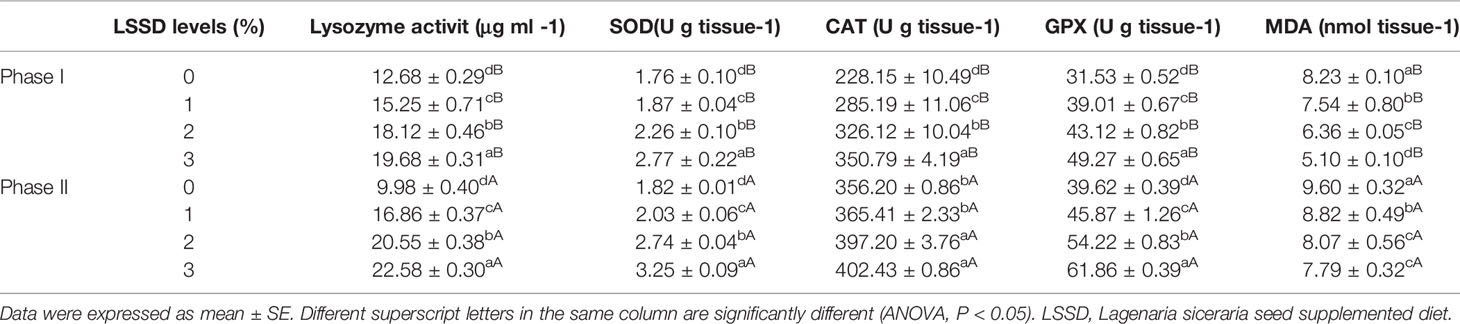
Table 7 Variations in lysozyme activity, superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX) activities and malondialdehyde (MDA) value and of Nile tilapia, O. niloticus, fed 0, 1, 2, and 3% diet of L. siceraria seeds supplemented diets (LSSD) for 60 days (Phase I) and after exposure to A. hydrophila (Phase II).
In Vitro Antibacterial Activity of LSEE
The result of the antibacterial examination is represented in Figure 3. It showed good efficacy of the LSEE against gram-negative bacterial strains (Aeromonas hydrophilia and A. sobria), and mild efficacy against gram-positive bacterial strains (Streptococcus agalactiae and Staphylococcus iniae). The higher concentration of the LSEE exhibited higher antibacterial activity in both gram-negative and gram-positive bacteria than the lower concentration groups of LSEE.
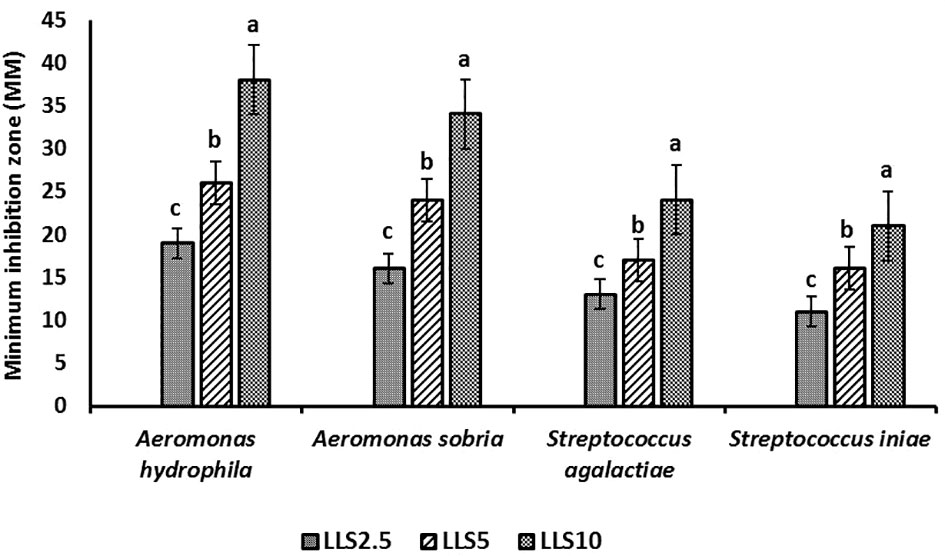
Figure 3 Antibacterial activity (safe zone, mm) of L. siceraria ethanolic extract (LSEE) against 4 selected bacterial strains. Data were presented as mean ± SD. Different superscript letters indicate significantly different (ANOVA, P < 0.05).
In Vitro Anti-Parasitic Activity of LSEE
An antagonistic effect was observed on C. tilapiae parasite following the addition of the LSEE. The parasites were initially noticed moving turbulently, after which time they began to contract and twitch violently. This was followed by a detachment of the parasite from the host. Then the parasites continued to contract, but at a slower rate until their final death. Parasites in the control group were not affected to the same degree as those in the LSEE treated groups, as the number of dead parasites was increasing with increasing LSEE concentration over time. In the control group, the number of dead parasites increased from about 12% after 30 min to 50% after 60 min, while at the higher concentration of the LSEE 10 ml/L, about 41% of the parasites were reported to die after only 10 min while the complete parasite death occurred after 40 min (Table 8).
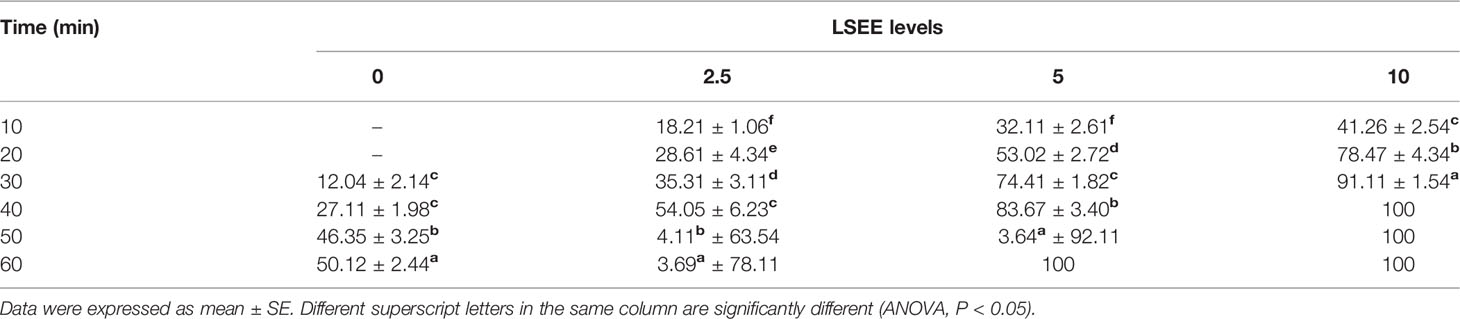
Table 8 Anti-parasitic efficacy of different concentrations of L. siceraria ethanolic extract on time after treatment (min) to death of C. tilapiae (%).
Discussion
The current study contributes to enhancing research studies on the potential use of medicinal plants in aquaculture for growth promotion, immunity stimulation, and disease resistance. The results of this study demonstrate that O. niloticus diets supplemented with L. siceraria seed powder can improve feed utilization efficiency, fish growth, and fish body biochemical composition. This advantage of supplemented feed can be attributed to the distinct nutritional profile of the bottle gourd seed powder being rich in amino acids, dietary fibers,carbohydrates along with several bioactive compounds such as saponins and triterpenoid glycosides that have been reported to actively promote growth and feed utilization in Nile tilapia (Goda, 2008). The presence of flavonoids, di- and triterpenes found in the seed extract suggests antimicrobial capability that may explain the better feed and growth efficiency due to potential inhibition of pathogens and thus the increased beneficial microbial activity leading to improved feed digestibility and nutrient absorption as well as better resistance of fish specimens to the pathogenic A. hydrophila bacteria. A study conducted on possible alternatives to fish meal has also reported a better growth performance of O. niloticus fingerlings when fed on a diet supplemented with squash meal (Ajuru and Nmom, 2017). Ahmed et al. (2011) stated that L. siceraria is a rich source of prebiotics that are believed to enhance probiotic populations that affect intestinal enteric metabolism and physiology, leading to the synthesis and release of various beneficial biomolecular substances.
Dietary supplementation with L. siceraria seeds may also contribute to improving hematological parameters that mainly reflect fish health status and their stress responses (Osman et al., 2018; Fazio, 2019). WBCs, RBCs, Hb, and PCV values in this study increased significantly in all groups of LSSD diets compared to the control group, suggesting an immunostimulant and protective ability of LSSD against stressors. This may be due to the content of bioactive compounds including saponins, glycosides, flavonoids, terpenoids, tannins, and phenols that are reported to boost hematological parameters in fish as immunostimulants (Talpur and Ikhwanuddin, 2012; Antache et al., 2014; Roohi et al., 2017; Abd El-Gawad et al., 2020). Fish diet supplemented with ginseng herb was found to improve hematological indices, increasing Hb and WBCs in the Nile tilapia O. niloticus and the effect was also attributed to similar bioactive compounds to that reported in the current study (Goda, 2008). Recent studies have shown that a variety of medicinal plants are rich in secondary metabolites that may induce immune-modulating activities during stress events (Hoseinifar et al., 2021). Similarly, elevated serum total protein levels can be linked to the innate immune response in fish that are fed on diets typically enhanced with immunostimulants (Wiegertjes et al., 1996; Choudhury et al., 2005; Rodneva and Kovyrshina, 2011). Thus, the significant elevated total serum protein in fish groups fed on LSSD supplemented diets pre- and post-challenge with A. hydrophila in this study suggests that LSSD-based diets increase innate immunity in Nile tilapia. Other herbal plants such as the ginseng herb were found to elevate total plasma protein and globulin in fish when used as a supplementation in the feed diet (Goda, 2008). When mixes of sterols and flavonoids were isolated from L. siceraria fruit and tested on experimental rats, they presented increased WBCs and hemagglutination antibody titers and inhibited delayed-type hypersensitivity response which indicate an immunomodulatory activity and phagocytosis action of this plant (Deshpande et al., 2008; Gangwal et al., 2008; Gangwal et al., 2009). Regarding alanine aminotransferase (ALT) and aspartate aminotransferase (AST), they are liver enzymes that have the function of transferring the amino group from alpha-amino acids to alpha-keto acids and are often released in large quantities into the blood during liver cell damage (Ye et al., 2011). Clearly, LSSD has an influence in reducing the release of these two enzymes in the blood, likely due to the presence of phenolic and flavonoid groups, which act as hepatoprotective agents (Deshpande et al., 2008; Owais, 2018). Lin et al. (2012) had similar observations on the values of ALT and AST of the pompano Trachinotus ovatus fish when fed on a fermented soybean diet without presenting a possible mechanism or explanation for their findings. On the other hand, the lower glucose level associated with LSSD supplemented diets in pre- and post-challenge conditions compared to the control group, appears to be due to the hypoglycemic effect of L. siceraria seeds caused by the presence of bioactive components, particularly phenols, flavonoids, and saponins. Flavonoid and phenolic compounds are well known for their ability to reduce blood glucose levels, which may be attributed to their potent antidiabetic activity (Parwata et al., 2018). Saponins are also phytochemicals that exhibit a wide range of biological activities, including lowering blood glucose by inhibiting the enzymes that break down disaccharides into monosaccharides (Oishi et al., 2007). Many authors have reported that several different parts of the bottle gourd have efficacy against hyperglycemia and dyslipidemia (Deshpande et al., 2008; Kumar et al., 2012; Bhattacharya and Das, 2012; Sharmin et al., 2012; Charu et al., 2013; Rajasree et al., 2016; Juee and Naqishbandi, 2020). Thus, the current findings present a good scientific base to use bottle gourd in the diet of diabetic patients.
Lysozyme is a cationic enzyme that has phagocytic and antimicrobial activity through the splitting of peptidoglycan in bacterial cell walls which allows bacterial cell lysis (Alexander and Ingram, 1992; Magnadottir, 2010). Thus, serum lysozyme is used as an indicator of the innate immune response in fish (Magnadottir, 2010; Uribe et al., 2011), and therefore, the increased level of serum lysozyme activity as observed in the current study is an indicator of beneficial use of LSSD in Nile tilapia diet to promote the innate immunity against potential bacterial infections and pathogens. These findings support other studies investigating chemical extracts of plant origin to raise immunity in cultivated fish where several bioactive molecules and phytochemicals including alkaloids, flavonoids, pigments, phenolics, terpenoids, and steroids promote several biological activities such as immunostimulant, anti-stress, phagocytic, and complement system activation in cultivated fish (Citarasu, 2010; Chakraborty and Hancz, 2011; Chakraborty et al., 2014). The benefits of herbal supplements extend to providing appropriate oxidative conditions for animals and preventing oxidative stress by scavenging free radicals and/or stimulating antioxidant enzymes (Sönmez et al., 2015; Bilen et al., 2020; Elbesthi et al., 2020; Yousfi et al., 2020). Among those enzymes, SOD, CAT, and GPx represent the first line of defense against oxidative stress (Farombi et al., 2007), and their values are used as biomarkers that reflect animals’ physical health (Ding et al., 2015). As for MDA, it is formed as an end product of lipid peroxidation and is used as an indicator of the toxic process caused by reactive oxygen species (ROS) (Lushchak, 2011). In the present study, LSSD significantly reduced hepatic MDA and raised enzymatic antioxidant levels pre- and post-challenge compared to the control group. These results can also be explained considering the bioactive compounds present in L. siceraria seeds, phenols, flavonoids, tannins, and terpenoids that play a significant role in the antioxidant and lipid peroxidation potential (Attar and Ghane, 2019). These phytochemicals are excellent reducing agents, hydrogen donors, singlet oxygen quenchers, and metal chelators (Sharma et al., 2013; Sulaiman et al., 2013; Nagarani et al., 2014; Attar and Ghane, 2017a; Attar and Ghane 2017b; Ghane et al., 2018; Patel et al., 2018). They can react with the stable free radicals by donating electrons or hydrogen atoms and converting them to non-radical form molecules (Benzie and Strain, 1996; Gill et al., 2012; Antia et al., 2015).
Regarding the results of in vitro antimicrobial trials, the ethanolic extract of L. sicereria showed an anti-parasitic and anti-bacterial efficacy against the tested strains. Apparently, the higher concentration of LSEE enabled better antimicrobial efficacy, thus suggesting an optimum concentration of 0.5% for the ideal anti-parasitic and antibacterial activity. Indeed, the general antimicrobial activity of LSEE in this study was attributed to constituents, phenols, sterols, terpenoids, flavonoids, and saponins, which exist in the seed extract. These results were generally consistent with several studies revealing a potent efficacy of L. sicereria seed extract against gram-positive and gram-negative bacteria including Staphylococcus aureus, Pseudomonas sp., Escherichia coli, and Bacillus subtilis in addition to some fungal strains such as Candida sp. and Aspergillus niger (Goji et al., 2006; Essien et al., 2015). Moreover, Smita et al. (2009) and Ramalingam and Patel (2010) both reported the anthelmintic activity of L. sicereria seed and leaf extract against the earthworm Pheretima posthuma as well as antibacterial and antifungal activity. Joseph et al. (2001) stated that the plant’s medicinal properties and biological activities are usually due to its phytochemical profile and bioactive molecules. This may explain the antimicrobial effects exhibited in this study for the constituents, saponins, alkaloids, or terpenoids in the potent diethyl ether extract of L. siceraria.
Conclusion
Based on these findings, Nile tilapia diets supplemented with different levels of Lagenaria siceraria seed powder can promote growth and enhance feed utilization and resistance against stressors. High levels of LSSD may be ascribed to activate antioxidant and immune status and exhibited potential anti-bacterial and anti-parasitic properties against common infected pathogens. LSSD up to 1% in this study presented the best performance in the Nile tilapia Oreochromis niloticus in all parameters. Our findings support the use of medicinal plants as dietary supplementation in fish feeds as an eco-friendly approach for sustainable aquaculture.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
MR: Writing – original draft, formal analysis, methodology, investigation, visualization. MA: Formal analysis, statistical analysis, methodology, editing. AM: Formal analysis, methodology, conceptualization, editing. JA: Formal analysis, methodology, visualization, editing. SM: Chemical analysis, GC-MS data interpretation, editing. MM: Writing – original draft, methodology, visualization, writing – review and editing. All authors read and approved the final manuscript.
Funding
This research was funded by The Taif University researchers, supporting project number (TURSP-2020/299), Taif University, Taif, Saudi Arabia.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research, Taif University for the financial support. The authors would also like to thank the research staff of the departments of Zoology, Al-Azhar University for the scientific guidance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.901439/full#supplementary-material
References
Abd El-Gawad E. A., El Asely A. M., Soror E. I., Abbass A. A., Austin B. (2020). Effect of Dietary Moringa Oleifera Leaf on the Immune Response and Control of Aeromonas Hydrophila Infection in Nile Tilapia (Oreochromis Niloticus) Fry. Aquac. Int. 28 (1), 389–402. doi: 10.1007/s10499-019-00469-0
Ahmad I., Irshad Md., Rizvi M. (2011). Nutritional and Medicinal Potential of Lagenaria Siceraria. Int. J. Vegetable Sci. 17, 157– 170. doi: 10.1080/19315260.2010.526173
Ahmed D., Dar P., Chaudhery R., Masih R. (2017). Chemical Constituents of Lagenaria Siceraria Meso Carp and its Xanthine Oxidase and Alpha-Amylase Inhibitory Activities. Int. J. Fruit Sci. 17 (3), 310–322. doi: 10.1080/15538362.2017.1315628
Ahmed D., Fatima M., Saeed S. (2014). Phenolic and Flavonoid Contents and Anti-Oxidative Potential of Epicarp and Mesocarp of Lagenaria Siceraria Fruit: A Comparative Study. Asian Pac J. Trop. Med. 7, S249–S255. doi: 10.1016/S1995-7645(14)60241-8
Ajuru M., Nmon F. (2017). A Review on the Economic Uses of Species of Cucurbitaceae and Their Sustainability in Nigeria. Am. J. Plant Biol. 2 (1), 17–24. doi: 10.11648/j.ajpb.20170201.14
Alexander J., Ingram G. (1992). Noncellular Nonspecific Defence Mechanisms of Fish. Annu. Rev. Fish Dis. 2, 249–279. doi: 10.1016/0959-8030(92)90066-7
Amend D. F. (1981). Potency Testing of Fish Vaccines. Fish Biologics: Serodiagnostics Vaccines 49, 447–454.
Antia B. S., Essien E. E., Udoh B. (2015). Antioxidant Capacity of Phenolic Fromseed Extracts of Lagenaria Siceraria (Short-Hybrid Bottle Gourd). Eur. J. Med. Plants. 9, 1–9. doi: 10.9734/EJMP/2015/18242
Antache A., Cristea V., Grecu I., Dediu L., Cretu M., Bocioc E., et al. (2014). Effects of Dietary Supplementation at Nile Tilapia With Thymus Vulgaris, Trigonela Foenum Graecum and Azadirachta Indica on Welfare Status. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Anim. Sci. Biotechnologies 71 (2), 115–122.
Attar U. A., Ghane S. G. (2017a). Phytochemicals, Antioxidant Activity and Phenolic Profiling of Diplocyclos Palmatus (L.) C. Jeffery. Int. J. Pharm. Pharmaceut. Sci. 9, 101–106. doi: 10.22159/ijpps.2017v9i4.16891
Attar U. A., Ghane S. G. (2017b). Proximate Composition, Antioxidant Activities and Phenolic Composition of Cucumissativus Forma Hardwickii (Royle) WJ De Wilde & Duyfjes. Int. J. Phytomed. 9, 101–112. doi: 10.5138/09750185.1922
Attar U. A., Ghane S. G. (2018). Optimized Extraction of Anti-Cancer Compound – Cucurbitacin I and LC-MS Identification of Major Metabolites From Wild Bottle Gourd (Lagenaria Siceraria (Molina) Standl.). SA J. Bot. 119, 181–187. doi: 10.1016/j.sajb.2018.09.006
Attar U. A., Ghane S. G. (2019). In Vitro Antioxidant, Antidiabetic, Antiacetylcholine Esterase, Anticancer Activities and RP-HPLC Analysis of Phenolics From the Wild Bottle Gourd (Lagenaria Siceraria (Molina) Standl.). S. Afr. J. Bot. 125, 360–370. doi: 10.1016/j.sajb.2019.08.004
Bailone R., Martins M., Mourino J., Vieira F., Pedrotti F., Nunes G., et al. (2010). Hematology and Agglutination Titer After Polyvalent Immunization and Subsequent Challeng With Aeromonas Hydrophila in Nile Tilapia (Oreochromis Niloticus). Arch. Med. Vet. 42, 221–227. doi: 10.4067/S0301-732X2010000300015
Benzie I. F.F., Strain J. J. (1996). The Ferric Reducing Ability of Plasma (FRAP) as a Measure of Antioxidant Power” the FRAP Assay. Anal. Biochem. 239, 70–76.
Bhattacharya S., Das B. (2012). Anti-Diabetic Activity of Lagenaria Siceraria Pulp and Seed Extract in Normal and Alloxan-Induced Diabetic Rats. Int. J. Pharm. Sci. Res. 3, 3362–3369. doi: 10.13040/IJPSR.0975-8232.3(9).3362-69
Bilen S., Altief T., Özdemir K. Y., Salem M., Terzi E., Güney K. (2020). Effect of Lemon Balm (Melissa Officinalis) Extract on Growth Performance, Digestive and Antioxidant Enzyme Activities, and Immune Responses in Rainbow Trout (Oncorhynchus Mykiss). Fish Physiol. Biochem. 46 (1), 471–481. doi: 10.1007/s10695-019-00737-z
Blaxhall P. C., Daisley K. W. (1973). Routine Haematological Methods for Use Fish With Blood. J. Fish Biol. 5, 771–781. doi: 10.1111/j.1095-8649.1973.tb04510.x
Boyd C. E., Tucker C. S. (2012). Pond Aquaculture Water Quality Management (Springer Science & Business Media).
Chakraborty S. B., Hancz C. (2011). Application of Phytochemicals as Immunostimulant, Antipathogenic and Anti-Stress Agents in Finfish Culture. Rev. Aquacul. 3, 103–119. doi: 10.1111/j.1753-5131.2011.01048.x
Chakraborty S. B., Horn P., Hancz C. (2014). Application of Phytochemicals a Growth Promoters and Endocrine Modulators in Fish Culture. Rev. Aquacult. 6, 1–19. doi: 10.1111/raq.12021
Charu K., Sonali S., Supriya A., Prasad G. B.K.S. (2013). Alleviation of Diabetes Induced Dyslipidemia by Lagenariasiceraria Fruit Extract in Human Type 2 Diabetes. J. Herb. Med. 3, 1–8. doi: 10.1016/j.hermed.2012.11.002
Chen L., Jin H., Ding L., Zhang H., Li J., Qu C., et al. (2008). Dynamic Microwave-Assisted Extraction of Flavonoids From Herba Epimedii. Sep. Purif. Technol. 59, 50–57. doi: 10.1016/j.seppur.2007.05.025
Choudhury D., Pal A. K., Sahu N. P., Kumar S., Das S. S., Mukherjee S. C. (2005). Dietary Yeast RNA Supplementation Reduces Mortality by Aeromonas Hydrophila in Rohu (Labeo Rohita L.) Juveniles. Fish shellfish Immunol. 19 (3), 281–291. doi: 10.1016/j.fsi.2005.01.004
Citarasu T. (2010). Herbal Biomedicines: A New Opportunity for Aquaculture Industry. Aquacult. Int. 18, 403–414. doi: 10.1007/s10499-009-9253-7
Dacie J., Lewis S. (1991). “Reference Ranges and Normal Values,” in Practical Haematology (New York: Churchill Livingstone), 9–17, pp.
Dar P., Ahmed D., Waqas U., Saeed R., Chaudhery R. (2014). Comparative Analysis of Antimicrobial Potential of Peel and Mesocarp of Lagenaria Siceraria Fruit Extracts in Various Solvents Against Clinically Important Pathogens. Pharmacology 3, 100–105. doi: 10.1080/15538362.2017.1315628
Deshpande J. R., Choudhari A. A., Mishra M. R., Meghre V. S., Wadodkar S. G., Dorle A. K. (2008). Beneficial Effects of Lagenaria Siceraria (Mol.) Standley Fruit Epicarp in Animal Models. Indian J. Exp. Biol. 46 (4), 234–242.
Ding Z., Zhang Y., Ye J., Du Z., Kong Y. (2015). An Evaluation of Replacing Fish Meal With Fermented Soybean Meal in the Diet of Macrobrachium Nipponense: Growth, Nonspecific Immunity, and Resistance to Aeromonas Hydrophila. Fish shellfish Immunol. 44 (1), 295–301. doi: 10.1016/j.fsi.2015.02.024
Elbesthi R., Özdemir K. Y., Taştan Y., Bilen S., Sönmez A. Y. (2020). Effects of Ribwort Plantain (Plantago Lanceolata) Extract on Blood Parameters, Immune Response, Antioxidant Enzyme Activities, and Growth Performance in Rainbow Trout (Oncorhynchus Mykiss). Fish Physiol. Biochem. 46 (4), 1295–1307. doi: 10.1007/s10695-020-00790-z
Ellis A. E. (1990). “Lysozyme Assays,” in Techniques in Fish Immunology USA. Eds. Stolen J. S., Fletcher T. C., Anderson D. P., Roberson B. S., Muiswinkel W. B. (Fair Haven, USA: SOS publications), 101–103, p.
Essien E., Antia B., Udoh B. I. (2015). Phytochemical Screening and Antimicrobial Activity of Lagenaria Siceraria Seeds Extracts. Int. J. Pharmacogn. Phytochem. Res. 7, 554–558.
FAO (2020). “The State of World Fisheries and Aquaculture 2020,” in Sustainability in Action (Rome, Italy: The Food and Agriculture Organization). doi: 10.4060/ca9229en
Farombi E. O., Adelowo O. A., Ajimoko Y. R. (2007). Biomarkers of Oxidative Stress and Heavy Metal Levels as Indicators of Environmental Pollution in African Cat Fish (Clarias Gariepinus) From Nigeria Ogun River. Int. J. Environ. Res. Public Health 4 (2), 158–165. doi: 10.3390/ijerph2007040011
Fay C. J. T., Noelle M. M. F., Ragaza J. A. (2014). Effects of Replacing Fishmeal With Squash Seed Meal (Cucurbita Maxima) on Performance of Juvenile Nile Tilapia (Oreochromis Niloticus). J. AACL 7 (2), 68–75.
Fazio F. (2019). Fish Hematology Analysis as an Important Tool of Aqua-Culture: A Review. Aquaculture 500, 237– 242. doi: 10.1016/j.aquaculture.2018.10.030
Fazio F., Habib S. S., Naz S., Filiciotto F., Cicero N., Rehman H., et al. (2022). Effect of Fortified Feed With Olive Leaves Extract on the Haematological and Biochemical Parameters of Oreochromis Niloticus (Nile Tilapia). Natural Product Res. 36, 1575–1580, 6.
Ferdaus M. J., Ferdous Z., Sara R. J., Mahin M. G., Faruque M. O. (2020). Total Antioxidants Activity and Proximate Analysis of Selected Fruits and Vegetables in Jashore Region, Bangladesh. Curr. Res. Nutr. Food Sci. 8, 785–797. doi: 10.12944/CRNFSJ.8.3.11
Gangwal A., Parmar S. K., Gupta G. L., Rana A. C., Sheth N. R. (2008). Immunomodulatory Effects of Lagenaria Siceraria Fruits in Rats. Pharmacognosy Mag. 4, S234–S238.
Gangwal A., Parmar S., Sheth N. (2009). Isolation and Immunomodulatory Activity of Phytoconstituents of Lagenaria Siceraria. InPharm Communique 2, 46–50.
Ghane S. G., Attar U. A., Yadav P. B., Lekhak M. M. (2018). Antioxidant, Anti-Diabetic, Acetylcholinesterase Inhibitory Potential and Estimation of Alkaloids (Lycorine and Galanthamine) From Crinum Species: An Important Source of Anticancer and Anti- Alzheimer Drug. Indus. Crops Prod. 125, 168–177. doi: 10.1016/j.indcrop.2018.08.087
Gill N. S., Singh S., Arora R., Bali M. (2012). Evaluation of Ethanolic Seed Extract of Lagenaria Siceraria for Their Therapeutic Potential. J. Med. Sci. 12, 78–84.
Goda A. M. A. S. (2008). Effect of Dietary Ginseng Herb (Ginsana G115) Supplementation on Growth, Feed Utilization, and Hematological Indices of Nile Tilapia, Oreochromis Niloticus (L.), Fingerlings. J. World Aquacult. Soc. 39 (2), 205–214. doi: 10.1111/j.1749-7345.2008.00153.x
Goji M., Gebre-Mariam T., Asres K., Lemma H., Gemeda N., Yirsaw K. (2006). Screening of Antimicrobial Activities of Some Plants Used Traditionally in Ethiopia for the Treatment of Skin Disorders. J. Ethiopian Pharmaceut. 24, 130–135. doi: 10.4314/epj.v24i2.35108
Habibur Rahaman A. S. (2003). Bottle Gourd (Lagenaria Siceraria)—a Vegetable for Good Health. Nat. Prod. Rad. 2, 249–256.
Hashemi Karouei S. M., Sadeghpour Haji M., Azizi I. G. (2012). Isolation of Saprolegnia and the Influence of Root Ethanolic Extract of Ruta graveolens on Saprolegnia. Spp Growth. Int. J. Biosci. Biochem. Bioinform. 2 (1), 64–67. doi:–10.7763/IJBBB.2012.V2.72
Hoseinifar S. H., Dadar M., Ringo E. (2017). Modulation of Nutrient Digestibility and Digestive Enzyme Activities in Aquatic Animals: The Functional Feed Additives Scenario. Aquac. Res. 48, 3987–4000. doi: 10.1111/are.13368
Hoseinifar S. H., Dadar M., Van Doan D. H., Harikrishnan R. (2019). Feed Additives Impacts on Shellfish Microbiota, Health, and Development. In: Derome N. (Ed.). Microbial Communities in Aquaculture Ecosystems. (Berlin: Springer), pp. 143–163. doi: 10.1007/978-3-030-16190-3_7
Hoseinifar S. H., Yousefi S., Van Doan H., Ashouri G., Gioacchini G., Maradonna F., et al. (2021). Oxidative Stress and Antioxidant Defense in Fish: The Implications of Probiotic, Prebiotic, and Synbiotics. Rev. Fish. Sci. Aquac. 29, 198–217. doi:–10.1080/23308249.2020.1795616
Hussain A., Khan M. N., Iqbal Z., Sajid M. S. (2008). An Account of the Botanical Anthelmintics Used in Traditional Veterinary Practices in Sahiwal District of Punjab, Pakistan. J. Ethnopharmacol. 119, 185–190.
Joseph B., Otta S. K., Karunasagar I., Karunasagar I. (2001). Biofilm Formation by Salmonella Spp. on Food Contact Surfaces and Their Sensitivity to Sanitizers. Int. J. Food Microbiol. 64, 367–372. doi: 1016/s0168-1605(00)00466-9
Juee L. Y., Naqishbandi A. M. (2020). Calabash (Lagenaria Siceraria) Potency to Ameliorate Hyperglycemia and Oxidative Stress in Diabetes. J. Funct. Foods 66, 103821–103829. doi: 10.1016/j.jff.2020.103821
Kesbiç O. S., Parrino V., Acar U, Yilmaz S., Lo Paro G., Fazio F.. (2020). Effects of Monterey Cypress (Cupressus Macrocarpa Hartw) Leaf Essential Oil as a Dietary Supplement on Growth Performance and Haematological and Biochemical Parameters of Common Carp (Cyprinus Carpio L.). Ann. Anim. Sci. 20, 1411–1426.
Kumar A., Partap S., Sharma N. K., Jha K. K. (2012). Phytochemical, Ethnobotanical and Pharmacological Profile of Lagenaria Siceraria: - a Review. J. Pharmacog. Phytochem. 1, 24–31.
Lin H., Chen X., Chen S., Zhuojia L., Huang Z., Niu J., et al. (2012). Replacement of Fish Meal With Fermented Soybean Meal in Practical Diets for Pompano Trachinotus Ovatus. Aquacult. Res. 44 (1), 151–156. doi: 10.1111/j.1365-2109.2011.03000x
Lulijwa R., Rupia E. J., Alfaro A. C. (2019). Antibiotic Use in Aquaculture, Policies and Regulation, Health and Environmental Risks: A Review of the Top 15 Major Producers. Rev. Aquac. 12, 640–663.
Lushchak V. I. (2011). Environmentally Induced Oxidative Stress in Aquatic Animals. Aquat. Toxicol. 101 (1), 13–30. doi: 10.1016/j.aquatox.2010.10.006
Magnadottir B. (2010). Immunological Control of Fish Diseases. Mar. Biotechnol. 12 (4), 361–379. doi: 10.1007/s10126-010-9279-x
Mansouri Taee H., Hajimoradloo A., Hoseinifar S. H., Ahmadvand H. (2017). Dietary Myrtle (Myrtus Communis L.) Improved non-Specific Immune Parameters and Bactericidal Activity of Skin Mucus in Rainbow Trout (Oncorhynchus Mykiss) Fingerlings. Fish shellfish Immunol. 64, 320–324. doi: 10.1016/j.fsi.2017.03.034
Meena D. K., Das P., Kumar S., Mandal S. C., Prusty A. K., Singh S. K., et al. (2013). Beta-Glucan: An Ideal Immunostimulant in Aquaculture (a Review). Fish Physiol. Biochem. 39 (3), 431–457. doi: 10.1007/s10695-012-9710-5
Nagarani G., Abirami A., Siddhuraju P. (2014). A Comparative Study on Antioxidant Potentials, Inhibitory Activities Against Key Enzymes Related to Metabolic Syndromeand Anti-Inflammatory Activity of Leaf Extract From Different Momordica Species. Food Sci. Hum. Wellness 3, 36–46. doi: 10.1016/j.fshw.2014.02.003
Naiel M. A. E., Ismael N. E., Negm S. S., Ayyat M. S., Al-Sagheer A. A. (2020). Rosemary Leaf Powder–Supplemented Diet Enhances Performance, Antioxidant Properties, Immune Status, and Resistance Against Bacterial Diseases in Nile Tilapia (Oreochromis Niloticus). Aquaculture 526, 735370. doi: 10.1016/j.aqrep.2021.100707
Nawaz A., Bakhsh javaid A., Irshad S., Hoseinifar S. H., Xiong H. (2018). The Functionality of Prebiotics as Immunostimulant: Evidences From Trials on Terrestrial and Aquatic Animals. Fish Shellfish Immunol. 76, 272–278. doi: 10.1016/j.fsi.2018.03.004
Newaj-Fyzul A., Austin B. (2015). Probiotics, Immunostimulants, Plant Products and Oral Vaccines, and Their Role as Feed Supplements in the Control of Bacterial Fish Diseases. J. fish Dis. 38 (11), 937–955. doi: 10.1111/jfd.12313
Ogunbusola E. M., Fagbemi T. M., Osundahunsi O. F. (2010). Amino Acidcomposition of Lagenaria Sicerariaseed Flour and Protein Fractions. J. Food Sci. Tech 6, 656–661.
Oishi Y., Sakamoto T., Udagawa H., Taniguchi H., Kobayashi-Hattori K., Ozawa Y. (2007). Inhibition of Increases in Blood Glucose and Serum Neutral Fat by Momordica Charantia Saponin Fraction. Biosci. Biotechnol. Biochem. 71 (3), 735–740.
Osman A. G., AbouelFadl K. Y., Abd El Baset M., Mahmoud U. M., Kloas W., Moustafa M. A. (2018). Blood Biomarkers in Nile Tilapia Oreochromis Niloticus Niloticus and African Catfish Clarias Gariepinus to Evaluate Water Quality of the River Nile. Fish. Sci. 12 (1), 1–15.
Owais F. M. (2018). Hepatoprotective Effect of Lagenaria Siceraria (Linn) in Carbamazepine Induced Hepatotoxicity in Rabbits. Isra Med. J. 10 (6), 345–348.
Parwata A., Laksmiwati L., Sudiarta S., Dina M. N., Yasa S. (2018). The Contents of Phenol and Flavonoid Compounds in Water Extract of Gyrinops Versteegii Leaves Have Potentially as Natural Antioxidants and Hypoglicemic in Hyperglycemic Wistar Rats. BioMed. Pharmacol. J. 11 (3), 1543–1552. doi: 10.13005/bpj/1521
Patel S. B., Attar U. A., Ghane S. G. (2018). Antioxidant Potential of Wild Lagenaria Siceraria (Molina) Standl. Thai J. Pharm. Sci. 42, 90–96. doi: 10.13140/RG.2.2.22813.36326
Prajapati R. P., Kalariya M., Parmar S. K., Sheth N. R. (2010). Phytochemical and Pharmacological Review of Lagenaria Sicereria. J. Ayurveda Integr. Med. 1 (4), 266–272. doi: 10.4103/0975-9476.74431
Radwan M. (2022). Vital Economic Threat of Predatory Birds and Parasites to Cultivated Fishes in Egypt. Aquaculture 548, 737666. doi: 10.1016/j.aquaculture.2021.737666
Rajasree R. S., Sibi P. I., Francis F., William H. (2016). Phytochemicals of Cucurbitaceae Family – A review. Int. J. Pharmacog. Phytochem. Res. 8, 113–123.
Rajput M. S., Balekar N., Jain D. K. (2014). Inhibition of ADP-Induced Platelet Aggregation and Involvement of non-Cellular Blood Chemical Mediators are Responsible for the Antithrombotic Potential of the Fruits of Lagenaria Siceraria. Chin. J. Natural Medicines 12 (8), 599–606. doi: 10.1016/S1875-5364(14)60091-1
Ramalingam B., Patel C. N. (2010). Studies on Anthelmintic and Antimicrobial Activity of the Leaf Extracts of Lagenaria Siceraria. J. Glob. Pharm. Technol. 2, 66–70.
Roohi Z., Imanpoor M. R., Jafari V., Taghizadeh V. (2017). The Use of Fenugreek Seed Meal in Fish Diets: Growth Performance, Haematological and Biochemical Parameters, Survival and Stress Resistance of Common Carp (Cyprinus Carpio L.). Aquac. Res. 48 (3), 1209–1215. doi: 10.1111/are.12962
Roopan S. M., Devi Rajeswari V., Kalpana V. N., Elango G. (2016). Biotechnology and Pharmacological Evaluation of Indian Vegetable Crop Lagenaria Siceraria: An Overview. Appl. Microbiol. Biotechnol. 100 (3), 1153–1162. doi: 10.1007/s00253-015-7190-0
Rudneva I. I., Kovyrshina T. B. (2011). Comparative Study of Electrophoretic Characteristics of Serum Albumin of Round Goby Neogobius Melanostomus From Black Sea and Azov Sea. Int. J. Sci. Nat. 1, 131–136.
Safari R., Hoseinifar S. H., Van Doan H., Dadar M. (2017). The Effects of Dietary Myrtle (Myrtus Communis) on Skin Mucus Immune Parameters and mRNA Levels of Growth, Antioxidant and Immune Related Genes in Zebrafish (Danio Rerio). Fish shellfish Immunol. 66, 264–269. doi: 10.1016/j.fsi.2017.05.007
Saha P., Mazumder U. K., Haldar P. K., Sen S. K., Naskar S. (2011). Anti Hyperglycemic Activity of Lagenaria Siceraria Aerial Parts on Streptozotocin Induced Diabetes in Rats. Diabetol. Croat. 40 (2), 49–60.
Sapkota A., Sapkota A. R., Kucharski M., Burke J., McKenzie S., Walker P., et al. (2008). Aquaculture Practices and Potential Human Health Risks: Current Knowledge and Future Priorities. Environ. Int. 34, 1215–1226.
Schmitt C. J., Dethloff G. M., Hinck J. E., Bartish T. M., Blazer V. S., Coyle J. J., et al. (2004). “Biomonitoring of Environmental Status and Trends (BEST) Program: Environmental Contaminants and Their Effects on Fish in the Rio Grande Basin,” in U.S. Geological Survey, Columbia Environmental Research Center (Columbia, Missouri: Scientific Investigations Report), 2004—5108117p. doi: 10.3133/sir20045108
Shah S. L., Altindag A. (2004). Hematological Parameters of Tench (Tinca Tinca L.) After Acute and Chronic Exposure to Lethal and Sublethal Mercury Treatments. Bull. Environ. Contam Toxicol. 73 (5), 911–918. doi: 10.1007/s00128-004-0513-y
Sharma N., Yadav P., Singh H., Shrivastava A. (2013). In Vitro Antioxidant Activity of Lagenaria Siceraria Leaves. Malay. J. Pharm. Sci. 11, 1–11.
Sharmin R., Khan M. R.I., Akhtar M. A., Alim A., Islam M. A., Anisuzzaman A. S.M., et al (2012). Hypoglycemic and Hypolipidemic Effects of Cucumber, White Pumpkin and Ridge Gourd in Alloxan Induced Diabetic Rats. J. Sci. Res. 5, 161–170. doi: 10.3329/jsr.v5i1.10252
Smita T., Rashmi T., Farooque P. M., Daud P. S. (2009). In-Vitro Anthelmintic Activity of Seed Extract of Lagenaria Siceraria (Molina.) Standley Fruit. J. Pharm. Res. 2 (7), 1194–1195.
Soares M. P., Cardoso I. L., Ishikawa M. M., De Oliveira A. D. S. S., Sartoratto A., Jonsson C. M., et al. (2020). Effects of Artemisia Annua Alcohol Extract on Physiological and Innate Immunity of Nile Tilapia (Oreochromis Niloticus) to Improve Health Status. Fish Shellfish Immunol. 105, 369–377. doi: 10.1016/j.fsi.2020.07.035
Song X., Zhao J., Bo J., Liu Z., Wu K., Gong C. (2014). Aeromonas Hydrophila Induces Intestinal Inflammation in Grass Carp (Ctenopharyngodon Idella): An Experimental Model. Aquaculture 434, 171–178. doi: 10.1016/j.aquaculture.2014.08.015
Sönmez A. Y., Bilen S., Alak G., Hisar O., Yanık T., Biswas G. (2015). Growth Performance and Antioxidant Enzyme Activities in Rainbow Trout (Oncorhynchus Mykiss) Juveniles Fed Diets Supplemented With Sage, Mint and Thyme Oils. Fish Physiol. Biochem. 41 (1), 165–175. doi: 10.1007/s10695-014-0014-9
Sulaiman S. F., Ooi K. L., Supriatno A. (2013). Antioxidant and α-Glucosidase Inhibitory Activities of Cucurbit Fruit Vegetables and Identification of Active and Major Constituents From Phenolic-Rich Extracts ofLagenaria Siceraria and Sechium Edule. J. Agric. Food Chem. 61 (42), 10080–10090. doi: 10.1021/jf4031037
Tadeg H., Mohammed E., Asres K., Gebre-Mariam T. (2005). Antimicrobial Activities of Some Selected Traditional Ethiopian Medicinal Plants Used in the Treatment of Skin Disorders. J. Ethnopharmacology 100, 166–175. doi: 10.1016/j.jep.2005.02.031
Talpur A. D., Ikhwanuddin M. (2012). Dietary Effects of Garlic (Allium Sativum) on Haemato-Immunological Parameters, Survival, Growth, and Disease Resistance Against Vibrio Harveyi Infection in Asian Sea Bass, Lates Calcarifer (Bloch). Aquaculture 364–365, 6–12. doi: 10.1016/j.aquaculture.2012.07.035
Thiex N., Novotny L., Crawford A. (2012). Determination of Ash in Animal Feed: AOAC Official Method 942.05 Revisited. J. AOAC Int. 95 (5), 1392–1397. doi: 10.5740/jaoacint.12-129
Uribe C., Folch H., Enríquez R., Moran G. (2011). Innate and Adaptive Immunity in Teleost Fish: A Review. Vet. Med. 56 (10), 486–503. doi: 10.17221/3294-VETMED
Wang G. X., Han J., Cheng C., Feng T. T., Fu-yuan L., Zhu B. (2009). Bioassayguided Isolation and Identification of Active Compounds From Fructus Cnidii Against Dactylogyrus Intermedius (Monogenea) in Goldfish (Carassius Auratus). ParasitolRes 106, 247–255. doi: 10.1007/s00436-009-1659-7
Wiegertjes G. F., Stet R. M., Parmentier H. K., van Muiswinkel W. B. (1996). Immunogenetics of Disease Resistance in Fish: A Comparative Approach. Dev. Comp. Immunol. 20 (6), 365–381. doi: 10.1016/s0145-305x(96)00032-8
Ye J., Liu X., Wang Z., Wang K. (2011). Effect of Partial Fish Meal Replacement by Soybean Meal on the Growth Performance and Biochemical Indices of Juvenile Japanese Flounder Paralichthys Olivaceus. Aquac. Int. 19 (1), 143–153. doi: 10.1007/s10499-010-9348-1
Yetişir H., Şakar M., Serçe S. (2008). Collection and Morphological Characterization of Lagenaria Siceraria Germplasm From the Mediterranean Region of Turkey. Genet. Resour. Crop Evol. 55, 1257‒1266. doi: 10.1007/s10722-008-9325-y
Yirga G., Teferi M., Brhane G., Amare S. (2012). Plants Used in Ethnoveterinary Practices in Medebay-Zana District, Northern Ethiopia. J. Med. Plants Res. 6, 433–438. doi: 10.5897/JMPR11.1133
Yousefi M., Vatnikov Y. A., Kulikov E. V., Plushikov V. G., Drukovsky S. G., Hoseinifar S. H., et al. (2020). The Protective Effects of Dietary Garlic on Common Carp (Cyprinus Carpio) Exposed to Ambient Ammonia Toxicity. Aquaculture 526, 735400. doi: 10.1016/j.aquaculture.2020.735400
Zahran E., El-Gawad E. A. A., Risha E. (2018a). Dietary Withania Sominefera Root Confers Protective and Immunotherapeutic Effects Against Aeromonas Hydrophila Infection in Nile Tilapia (Oreochromis Niloticus). Fish Shellfish Immunol. 80, 641–650. doi: 10.1016/j.fsi.2018.06.009
Zahran E., Risha E., Awadin W., Palić D. (2018b). Acute Exposure to Chlorpyrifos Induces Reversible Changes in Health Parameters of Nile Tilapia (Oreochromis Niloticus). Aquat. Toxicol. 197, 47–59. doi: 10.1016/j.aquatox.2018.02.001
Keywords: dietary supplements, cucurbits, hematological and biochemical parameters, growth promoters, immunomodulatory diet, Nile tilapia feed
Citation: Radwan M, Abbas MMM, Mohammadein A, Al Malki JS, Elraey SMA and Magdy M (2022) Growth Performance, Immune Response, Antioxidative Status, and Antiparasitic and Antibacterial Capacity of the Nile Tilapia (Oreochromis niloticus) After Dietary Supplementation With Bottle Gourd (Lagenaria siceraria, Molina) Seed Powder. Front. Mar. Sci. 9:901439. doi: 10.3389/fmars.2022.901439
Received: 21 March 2022; Accepted: 04 May 2022;
Published: 02 June 2022.
Edited by:
Khor Waiho, University of Malaysia Terengganu, MalaysiaReviewed by:
Victor Tosin Okomoda, Federal University of Agriculture Makurdi (FUAM), NigeriaEhab El-Haroun, Cairo University, Egypt
Francesco Fazio, University of Messina, Italy
Copyright © 2022 Radwan, Abbas, Mohammadein, Al Malki, Elraey and Magdy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahmoud Radwan, TWFobW91ZEtocml5LjIwMUBhemhhci5lZHUuZWc=
 Mahmoud Radwan
Mahmoud Radwan Mahmoud Mahrous M. Abbas
Mahmoud Mahrous M. Abbas Amaal Mohammadein2
Amaal Mohammadein2 Mohammad Magdy
Mohammad Magdy