
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Mar. Sci. , 08 September 2022
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.899789
 Hanafiah Fazhan1,2,3
Hanafiah Fazhan1,2,3 Mohamad N. Azra4
Mohamad N. Azra4 Siti Aisah Halim1
Siti Aisah Halim1 Muhamad Naimullah5
Muhamad Naimullah5 Muyassar H. Abualreesh6
Muyassar H. Abualreesh6 Alexander Chong Shu-Chien3,7
Alexander Chong Shu-Chien3,7 Youji Wang8
Youji Wang8 Yushinta Fujaya9
Yushinta Fujaya9 Mohammad Syahnon1,10
Mohammad Syahnon1,10 Hongyu Ma2,11
Hongyu Ma2,11 Khor Waiho1,2,3*
Khor Waiho1,2,3* Mhd Ikhwanuddin1,2*
Mhd Ikhwanuddin1,2*The aim of the present study was to determine the movement patterns of mud crabs, genus Scylla, within the mangrove area of the Setiu Wetland in Terengganu, Malaysia. Mark-release-recapture technique were conducted during 24 sampling trips. A fluorescent visible implant elastomer (VIE) tag was inserted within the crab’s shell and they were released at the same capture stations over an interval of two weeks before the next sampling. A total of 288 crabs of various sizes, sex and species were identified, measured, tagged and recorded. The numbers of male crabs were higher than females. Three species were present, dominated by S. olivacea at 54.2% and S.tranquebarica at 26.4%, with S. paramamosain in third place at 19.4%. Among the 288 crabs that were tagged and released, 26.38% were recaptured. The tagged crabs did not move very far from the release station based on the recapture data. The greatest mean chance of being recaptured within 24 h was 44.5%, and there were no tagged crabs recaptured after 48 h even on sampling trips at several points from the release site. Knowledge of movement patterns is essential to understand migration and population dynamics and the relationship between reproduction and local distribution.
Mud crabs of the genus Scylla are ecologically and economically important crustacean species commonly found in the Indo-West Pacific region (Azra & Ikhwanuddin, 2016; Waiho et al., 2018; Fazhan et al., 2022). Although in general, they are known to inhabit intertidal mangrove zones and subtidal estuaries with fluctuating salinity (Keenan et al., 1998; Fazhan et al., 2021a), previous studies showed that the mud crab species within the genus Scylla tend to inhabit a fairly limited area (Keenan, 1999). Scylla olivacea are typically found in burrows within dense coastal mangrove forests (Kathiresan & Bingham, 2001; Walton et al., 2006; Waiho et al., 2021). As for Scylla paramamosain species, they live in the sub-tidal zones while their early instars and juveniles inhabit the fringes of the mangrove ecosystem (Le Vay, 2001). S. olivacea were commonly found together with Scylla tranquebarica within the mangroves, but there appeared to be some niche separation between the two species, with S. tranquebarica prefer the outer zone of mangrove forests whereas S. olivacea inhabit the inner mangrove forest with higher fluctuation in water level and salinity (Ikhwanuddin et al., 2011; Fazhan et al., 2021b).
Information on the movement patterns of crustaceans could reveal their migratory patterns, dispersal and home ranges, and their habitat preference, all of which have strong ecological and management implications (Follesa et al., 2009; Froehlich et al., 2014). Movement patterns are influenced by various factors, including habitat quality, sex and age (Quinn & Brodeur, 1991; Froehlich et al., 2014). Most tagging procedures aim to collect behavioural and demographic data as well as to track movements (Macaulay et al., 2021). Tagging of crustaceans can be done either using the anchor T-bar (Follesa et al., 2009) or the visible implant elastomer (VIE) (Liu et al., 2011; Ikhwanuddin et al., 2012b), acoustic transmitter (Froehlich et al., 2014; Wada et al., 2016) and ultrasonic transmitting tag (Carr et al., 2004; Ikhwanuddin et al., 2012a). The mark-release-recapture technique is then being applied after tag insertion to estimate biological information such as survival, movement, growth, age-at-maturity, migration and distribution of the targeted species (Carr et al., 2004; Gunnarson et al., 2005; Le Vay et al., 2007; Ikhwanuddin et al., 2012a).
There has been no previous movement pattern study of mud crabs, especially of genus Scylla, along the coast of peninsular Malaysia. Correlations between size-at-maturity and preferred habitat have been identified among associated species, but there is still a lack of data on the differences in habitat requirement and population dynamics of specific species of portunid crabs especially genus Scylla. The distribution seems to be influenced by salinity, temperature, substrate, sex and age-at-maturity (Walton et al., 2006; Azra et al., 2018).
In the present study, the movements of Scylla mud crabs were tracked within the mangrove ecosystem of Setiu Wetland, Terengganu, Malaysia, with regard to species, size, weight and sex ratio. The information gathered in this study can be used by managers of fisheries, crustacean aquaculturists and conservationists. The data will ensure a better understanding of the ecology of Scylla species which can be used to predict their feeding behaviour and habitat preferences for development of aquaculture technology and fisheries conservation.
This study was conducted from November 2015 to April 2016 at Setiu Wetlands, Terengganu, Malaysia, representing the coastal waters of the South China Sea (Figure 1A). Twelve sampling sites were selected, with a distance of 1 to 2 km between sites. 24 sampling trips, with 2 trips per site. Crabs were captured by means of baited collapsible crab traps made of 25 mm mesh nylon net stretched over a rectangular steel frame (L50 × W30 × H16 cm). The traps, which have two 15 cm entrance funnels, are the same type as commonly used by the local fishermen in Setiu. Chopped fish were placed in the traps as bait. The locations of all mud crabs sampled were marked with GPS coordinates and their species was identified (Keenan et al., 1998). The weight and carapace width of the crabs were measured, and their sex determined according to Ikhwanuddin et al. (2011). Crabs were then tagged with VIE tags and released at the capture sites. Damaged crabs were released without tagging. VIE tags were chosen over ultrasonic transmitting tags as previous study showed the retrieval of poor signals if the tags were buried in mud bottom or opposite and away from the hydrophone (Ikhwanuddin et al., 2012a).
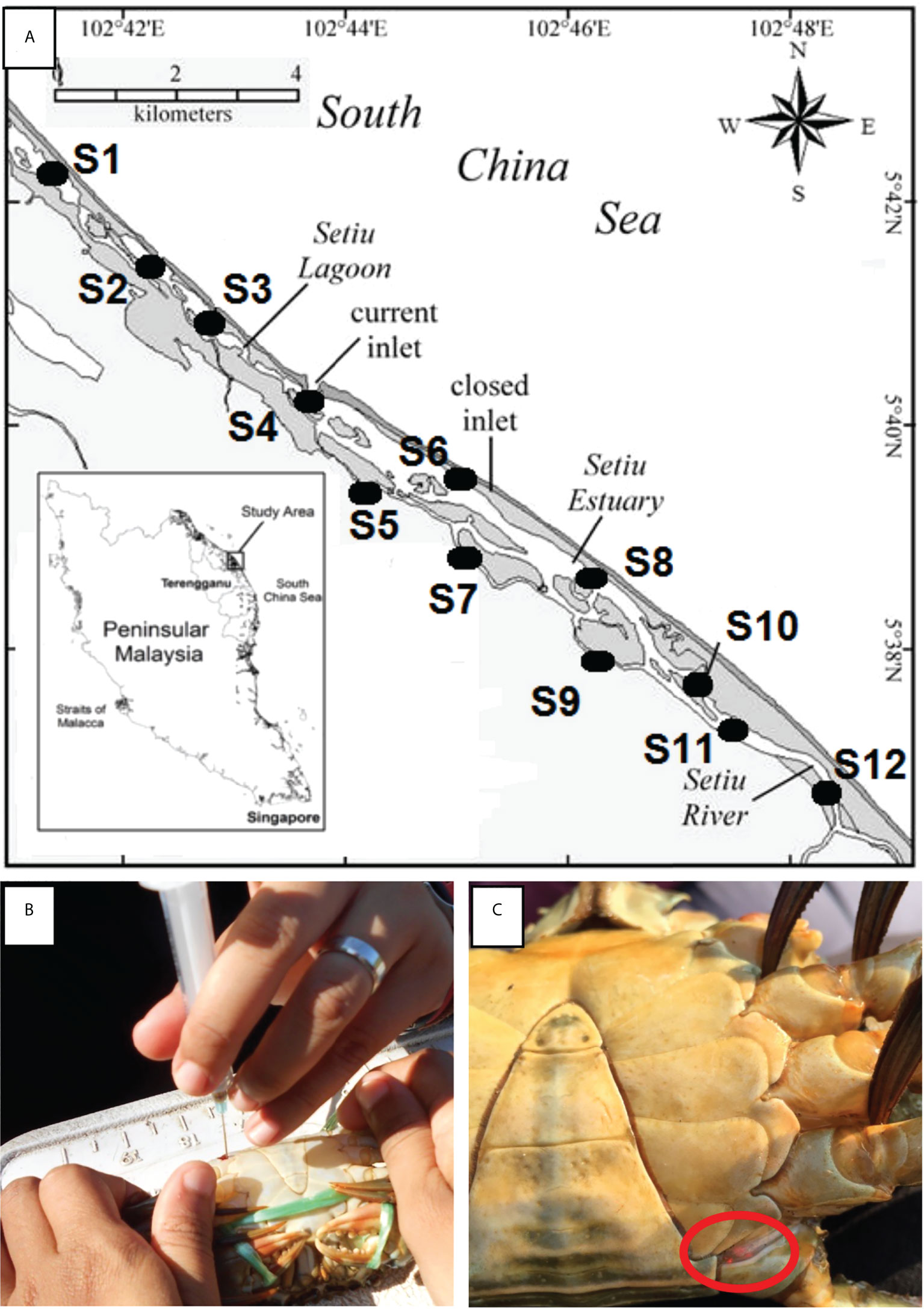
Figure 1 (A) Location of sampling stations in Setiu Wetland, Terengganu, bordering the South China Sea. The stations were divided into (1) mangrove forests – Station 5, 7, 9, 10; (2) Estuaries – Station 3, 4, 6, 8; and (3) river channels – Station 1, 2, 11, 12. Mangrove forests are characterized by the presence of mangrove trees, estuaries are wetlands surrounding by bodies of brackish water, and river channels are characterised by the presence of river banks and beds, with lower salinity compared to estuaries; (B) Tagging of crab sample at the joint of appendage using orange VIE tag; (C) The presence of orange VIE tag on recaptured samples.
Visible implant elastomer (VIE) tags and tagging equipment were supplied by Northwest Marine Technology, USA. The VIE tag is a two-part silicone-based material that is mixed just before use. The colour component and curing agent are mixed in a 10:1 ratio and taken up into a 1 ml syringe. From the time of mixing, the user will have from 45 min (in warm environments) to 2 h (in cold environments) of working time during which the VIE can be injected. The nontoxic tagging mixture is implanted beneath translucent tissue and remains externally visible. Captured crabs were injected with different coloured VIEs at different joints of the walking or swimming legs (e.g. at the fused basis-ischium of either left or right of any walking or swimming legs) to identify the specific release site and sampling station (Figures 1B, C). This differential marking technique indicated the sampling trip on which the crab was captured.
Thirty traps were placed at each of twelve sampling stations, which were located along the channel and also inside the mangrove forest at the site. The traps were placed during the lowest water level of low tide in sheltered areas and the distance between each station was measured. Each crab trap was checked at 6, 12 and 24 h to prevent cannibalism if there was more than one crabs were in the same crab trap for an extended period. The captured crabs were weighed, measured, tagged, recorded and released at the capture site. Crab traps were deployed up to a maximum of 24 h after tagged crabs were released. The first sampling trip was conducted 24-h after the release of tagged crabs. The same procedure was repeated during the next sampling. The interval between the two sampling trips was two months. The tags on the legs were used to determine the release sites of the crabs and the GPS coordinates allowed calculation of the distance moved in meters over a certain time. The distance between stations was known and the numbers of mud crab species moving from one sampling station to another were counted. Data on the size, weight, sex and species of the recaptured crabs were also recorded. These data will be used to determine the preferred habitat of each Scylla mud crab species.
Data are given as means ± SD. One-way Analysis of Variance (ANOVA) was used to test for differences between CPUE (catch per unit effort), carapace width (CW), and body weight (BW). Poisson regression analysis was used to determine if the number of crabs was influenced by the environment types and species. Data were checked for normality and homogeneity of variance using Shapiro-wilk test and Leven’s test, respectively before proceeding with parametric test. To analyse the numbers of each Scylla species and the ratio of male to female crabs recaptured at each sampling station, chi-square goodness-of-fit test was used to determine if the male:female (M:F) sex ratio significantly differ from the 1:1 expected ratio.
The summary of the results from sampling trips in the study area is shown in Supplementary Table 1. A total of 360 crab traps were used during the study resulting in the capture of 288 crabs of which 212 were captured only once and 76 were recaptured. S. olivacea was the dominant species of mud crab at 54.2% of the total (34.0% male, 20.1% female), while S. tranquebarica was also relatively common at 26.4% (14.6% male, 11.8% female). S. paramamosain was less common at 19.4% of the total (10.4% male and 9.0% female) (Figure 2). Scylla olivacea showed significantly higher number in river channels and mangrove forests () whereas S. tranquebarica and S. paramamosain were equally found in mangrove forests and estuaries (). The environment type had no significant influence on the total number of crabs captured ().
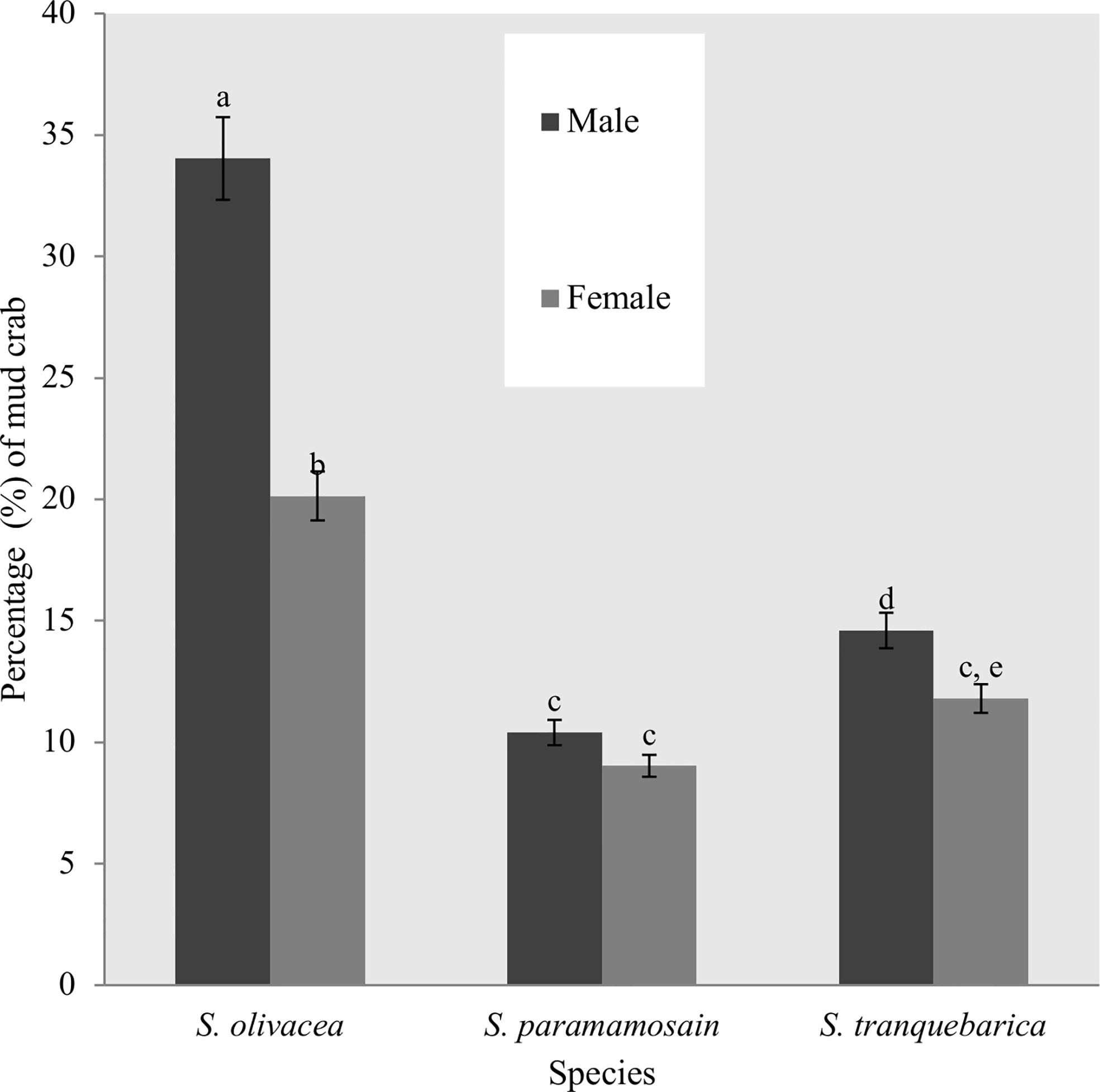
Figure 2 Percentage of mud crab species captured during the sampling trips of the study period at Setiu Wetlands. *Different alphabets between each bars showed the significant difference (p < 0.05) between species.
The mean CPUE was 0.80 crabs/trap (range, 0.60-1.2 crabs/trap; n = 360). The fourth sampling station had the highest CPUE at 1.23 crabs/trap. During the initial crab collection stage at each station, sampling station 6 showed the lowest CPUE (0.37 crabs/trap), although the total number of marked crabs captured between types of location (e.g. mangrove forests, estuaries, and river channels) was not significant (F2,9= 0.360,P= 0.707 ). Out of twenty-four sampling trips, tagged crabs were only retrieved on trips numbered thirteen to twenty-four. Most crabs were recaptured in sampling station 4 (n = 12) and sampling station 12 (n = 10), but the number of crabs recaptured was similar across mangrove forests, estuaries, and river channels (F2,9= 0.703,P= 0.520 ) (Supplementary Table 1).
The sex ratio of different Scylla species differed from the expected 1:1 (M:F) ratio in river channels and mangrove forests, but not estuaries (Supplementary Table 2). The highest M:F ratio (2.43:1) was S. tranquebarica at river channels (). Similar sex ratio pattern, where males were of higher number than females, was observed in S. olivacea at river channels (M:F ratio = 1.71:1; ) and mangrove forests (M:F ratio = 1.77:1; ).
The average CW and BW of crabs from different sampling stations are shown in Supplementary Table 3. Significant differences in CW and BW were observed among different environment categories (CW: F2,285= 7.075 , P = 0.001; BW: F2,285= 4.734,P= 0.009 ). Subsequent posthoc Tukey test revealed similar patterns in both CW and BW of captured crabs, where crabs captured in the estuaries were larger and heavier than that of river channels and mangrove forests, with no significant difference in the CW and BW of crabs found in river channels and mangrove forests (CW: P = 0.619; BW: P = 0.861).
Among the 288 tagged crabs captured, 76 were recaptured (26.4%) (Table 1). The largest numbers of crabs recaptured were S. olivacea at 20 males (20.4% out of total tagged and released male S. olivacea) and 20 females (34.5% out of total tagged and released female S. olivacea). The lowest recapture rate was males of S. paramamosain, i.e., only four out of 30 tagged crabs were recaptured. However, the females of S. paramamosain had the highest (53.85%) recapture rate. For S. tranquebarica, the recapture rate for males and females were almost similar, at approximately 23%. Scylla olivacea had the tendency of being recaptured twice (8 males and 8 females), whereas that of S. paramamosain and S. tranquebarica were only recaptured once, except for four S. tranquebarica males that were recaptured twice. The recapture rate over the selected time intervals is shown in Table 2. The crabs were all recaptured within the 6, 12 and 24 h ‘soaking’ crab trap period. None of the crabs were recaptured on a different sampling trip from the release site, which indicates that they did not move very far from the release point where the chance of being recaptured within 24 h was the greatest at 44.5%. The mean recapture rate was 19.0% at 6 h and 32.8% at 12 h. This shows that the crabs covered a relatively small area over a 24-hour period.
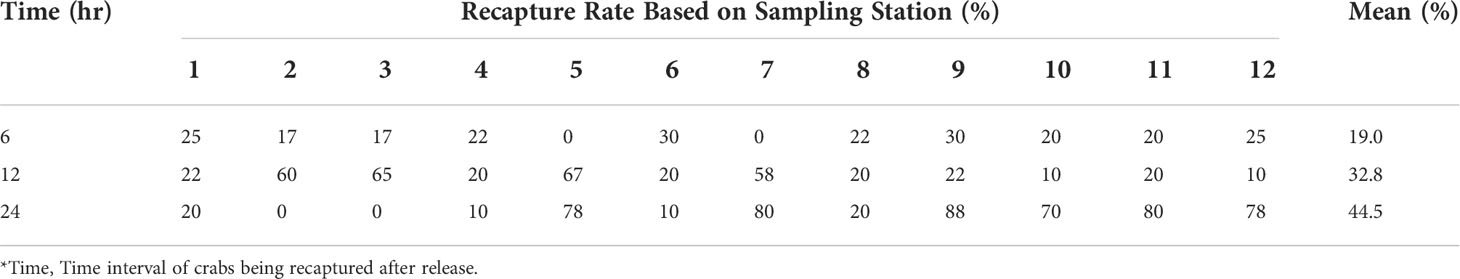
Table 2 Recapture rate (%) after 6, 12 and 24 hours of soaking crab trap period at each sampling station at Setiu Wetlands.
Scylla olivacea was the most abundant species captured in the Setiu Wetlands, followed by S. tranquebarica and S. paramamosain. The results obtained confirmed the observation by Keenan et al. (1998) and Fazhan et al. (2017) that the three species of Scylla, S. olivacea, S. tranquebarica and S. paramamosain, were commonly found from the South China Sea to specific locations around the Indo-Pacific, specifically in Malaysia (Fazhan et al., 2020). The finding of S. olivacea as the most abundant species among the captured crabs suggests that this species may be a permanent resident in the mangroves due to its feeding habits. According to Walton et al. (2006), mature S. olivacea crabs are much more likely than other species to enter the mangroves to feed during high tide and to become permanent residents. The relatively low number of S. tranquebarica captured in this study is probably due to the fact that this species requires a higher salinity and that unlike S. olivacea, the population distribution of S. tranquebarica and S. paramamosain showed seasonal fluctuation (Fazhan et al., 2017).
The sixth sampling trip showed the lowest CPUE, 0.37 crabs/trap. According to local fisherman, the crabs will move deeper into the mangroves as the tides become higher. The habitat of S. olivacea has been described as limited to the coastal mangroves and other coastline areas with reduced salinity especially during the rainy season suggesting that they tend to avoid high salinity. S. olivacea in Australia, have been reported to be more tolerant of low salinity (Keenan et al., 1998). S. paramamosain also appears to be tolerant of extended periods of low salinity with recruitment peaking at a salinity of 5-7 ppt (Le Vay, 2001; Walton et al., 2006). While physical parameters such as salinity and seasonality can influence the CPUE of crabs, there are other unforeseen factors as well. During low tide along the mangrove fringes, monitor lizards were seen to disturb the bait in the trap which could reduce the crab catch. The sampling sites are also commercial fishing grounds for local fisherman which could influence CPUE.
In general, the sex ratio data from the study showed that males were more likely to be trapped than females in the study area. The higher male to female ratio was also reported in Scylla species within similar equatorial region, including Malaysia (Fazhan et al., 2017), Thailand (Moser et al., 2005), India (Devi, 1985), and South Africa (Robertson, 1996). Hill (1978) pointed out that movement in crabs, including Scylla, could be divided into three categories. The first category was restricted movement centring on a more permanent home site. The second category was free-ranging movement during which crabs may forage over extensive distances and not return to a fixed place each day. The third category was migration associated with reproduction. The tagging experiments carried out in Moreton Bay, Queensland, Australia on Scylla spp. categorised movements into two types: a free-ranging type and an offshore migration by females (Hyland et al., 1984). Therefore, one possible postulate for the higher male to female ratio is that mature Scylla females tend to migrate offshore for spawning purposes (Hill, 1994; Koolkalya et al., 2006), thereby reducing the number of females in the intertidal zones. Future study on the relationship of sex ratio with spawning season and fluctuating seasonal parameters in tropical wetlands would shed light on the distribution and recruitment of Scylla along the equatorial region.
The study conducted by Ikhwanuddin et al. (2011) using telemetry showed that S. tranquebarica migrate offshore during the spawning season while S. olivacea do not. Female S. olivacea do not migrate too far offshore but remain in the muddy areas for spawning, as befits a mud crab, and only migrate offshore during monsoon season when salinity decreases. According to Shelley and Lovatelli (2011), a muddy bottom enhances maturation and spawning of mud crabs. S. olivacea females also prefer to make burrows in the mud into which they can retreat safely. Mud crabs may be found foraging for food on the surface of the mud among mangrove roots, but when resting they usually occupy burrows within the mud. In the studies by Ikhwanuddin et al. (2010; 2011; 2012a) it was stated that S. tranquebarica showed free-ranging activity while the movements of S. olivacea were more limited. The results of our investigation revealed a pattern in which S. olivacea was mainly found in the river channels and mangrove forests, whereas S. paramamosain and S. tranquebarica were concentrated at mangrove forests and estuaries. It is suggested that both S. tranquebarica and S. paramamosain crabs exhibit free-ranging activity compared to S. olivacea which is primarily an intertidal species.
The movements of the crabs in this study were determined by tag returns from each station. The results with the three different species showed that more males than females were recaptured. A study from Sematan, Sarawak reported that S. olivacea females complete their spawning cycle within the intertidal area where they are commonly captured (Ikhwanuddin et al., 2012a). The reproductive seasonality of S. paramamosain with peak female maturity in September was reported in Southern Vietnam but does not correspond with the timing of this study (Le Vay, 2001). S. olivacea crabs prefer to inhabit the coastal areas and have a greater tendency to be recaptured. The lower numbers of S. paramamosain and S. tranquebarica recaptured are due in part to their characteristically lower tendency to inhabit or remain in a limited area for a period of time. In Thailand, for example, there appears to be some niche separation where S. olivacea were reported to live within the mangrove root system while S. paramamosain lived sub-tidally (Overton and Macintosh, 2002).
Scylla olivacea is more likely to stay within a relatively limited area with 20.4% (male) and 34.5% (female) of the crabs being recaptured near the release station. S. olivacea shows little movement within a limited home range. The maximum period during which S. olivacea were recaptured was at the time interval of 24 h, suggesting that the crabs, mostly males, were moving around due to foraging. The mud crabs were observed migrating into the intertidal zone at high tide in order to feed (Le Vay, 2001). Our recapture rate data shows that the highest mean chance of being recaptured after 24 h was 44.5%. Hill et al. (1982) demonstrated that mud crabs moved freely within their environment, but their activities were largely restricted to the mangroves, the estuarine water channel and the shallow tidal flats. S olivacea showed a movement pattern that was mainly confined to the intertidal area at high tide and to burrows in the mud among the mangrove roots during low tide. Movement activities from the study area were not necessarily limited because S. paramamosain were not being recaptured twice, indicating that they were most likely to move around within a short time. Le Vay et al. (2007) reported that recaptured juvenile S. paramamosain had maximum recorded movements of 5.8 to 12.3 km after 20 weeks. Due to the limited area of the study site, only 26.4% of tagged crabs were recaptured within a short period of time.
The results from this study allowed us to identify the dominant species, the size distribution and sex ratio among the three common species of Scylla mud crabs. S. olivacea was the dominant species in the Setiu Wetland comprising 54.2% of the 288 crabs captured and tagged. Next most abundant was S. tranquebarica at 26.4%, followed by S. paramamosain at 19.4%. Most of the recaptured crabs, especially S. olivacea, were from the same release station. The numbers of male crabs were higher than females. The results show that the collapsible crab traps used were quite efficient for trapping small crabs within the mangroves. This study also demonstrated that S. olivacea was mainly an intertidal species, preferring to inhabit the mangroves with limited movement around a home range and returning to the same area after foraging. S. paramamosain has a free-range movement pattern, mainly sub-tidal, with a low tendency to stay in the same area for extended periods of time. For future studies, it is suggested that more physical, biological, chemical and ecological parameters such as temperature, salinity, monsoon season, predation, reproduction, habitat vegetation and food availability be investigated for a better understanding of the factors that influence the movement patterns of Scylla mud crabs.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
HF: Investigation, Formal analysis, Writing – Original Draft, Writing – Review and Editing; MNA: Methodology, Investigation, Formal analysis; SH: Investigation, Formal analysis; MN: Formal analysis, Writing – Review and Editing; MA: Formal analysis, Writing – Review and Editing; AS-C: Formal analysis, Writing – Review and Editing; YW: Formal analysis, Writing – Review and Editing; YF: Visualisation, Writing – Review and Editing; MS: Visualisation, Writing – Review and Editing; HM: Validation, Writing – Review and Editing; KW: Conceptualization, Methodology, Investigation, Formal analysis, Writing – Original Draft, Writing – Review and Editing; MI: Conceptualization, Methodology, Writing – Original Draft, Writing – Review and Editing. All authors contributed to the article and approved the submitted version.
This study was funded by the Malaysian Ministry of Higher Education under Higher Institution Centre of Excellence (HICoE) Program accredited to Institute of Tropical Aquaculture and Fisheries, Universiti Malaysia Terengganu (Vot. No.: 63933 & 56046). The International Partnership Research Grant by Universiti Malaysia Terengganu and Hasanuddin University (Vot. No.: 55300) was also acknowledged.
Thanks to Prof. Gary Bentley for providing English revision on an earlier draft of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.899789/full#supplementary-material
Azra M. N., Chen J. C., Ikhwanuddin M., Abol-Munafi A. B. (2018). Thermal tolerance and locomotor activity of blue swimmer crab Portunus pelagicus instar reared at different temperatures. J. Therm Biol. 74, 234–240. doi: 10.1016/j.jtherbio.2018.04.002
Azra M. N., Ikhwanuddin M. (2016). A review of maturation diets for mud crab genus Scylla broodstock: Present research, problems and future perspective. Saudi J. Biol. Sci. 23, 257–267. doi: 10.1016/j.sjbs.2015.03.011
Carr S. D., Tankersley R. A., Hench J. L., Forward R. B. Jr., Luettich R. A. Jr. (2004). Movement patterns and trajectories of ovigerous blue crabs Callinectes sapidus during the spawning migration. Estuar Coast. Shelf Sci. 60, 567–579. doi: 10.1016/j.ecss.2004.02.012
Fazhan H., Waiho K., Al-Hafiz I., Kasan N. A., Ishak S. D., Afiqah-Aleng N., et al. (2021a). Composition, size distribution, length-weight relationship of sympatric mud crab species (Scylla) and the case of presumed hybrids. Estuarine Coast. Shelf Sci. 250, 107154. doi: 10.1016/j.ecss.2020.107154
Fazhan H., Waiho K., Darin Azri M. F., Al-Hafiz I., Wan Norfaizza W. I., Megat F. H., et al. (2017). Sympatric occurrence and population dynamics of Scylla spp. in equatorial climate: Effects of rainfall, temperature and lunar phase. Estuarine, Coast. Shelf Sci. 198, 299–310. doi: 10.1016/j.ecss.2017.09.022
Fazhan H., Waiho K., Fujaya Y., Rukminasari N., Ma H., Ikhwanuddin M. (2021n). Sexual dimorphism in mud crabs: a tale of three sympatric Scylla species. PeerJ 9, e10936. doi: 10.7717/peerj.10936
Fazhan H., Waiho K., Ikhwanuddin M., Shu-Chien A. C., Fujaya Y., Wang Y., et al. (2022). Limb loss and feeding ability in the juvenile mud crab Scylla olivacea: Implications of limb autotomy for aquaculture practice. Appl. Anim. Behav. Sci. 247, 105553. doi: 10.1016/j.applanim.2022.105553
Fazhan H., Waiho W., Quinitio E., Baylon J. C., Fujaya Y., Rukminasari N., et al. (2020). Morphological descriptions and morphometric discriminant function analysis reveal an additional four groups of Scylla spp. PeerJ 8, e8066. doi: 10.7717/peerj.8066
Follesa M. C., Cuccu D., Cannas R., Sabatini A., Deiana A. M., Cau A. (2009). Movement patterns of the spiny lobster Palinurus elephas (Fabriciu) from a central western Mediterranean protected area. Sci Mar 73, 499–506. doi: 10.3989/scimar.2009.73n3499
Froehlich H. E., Essington T. E., Beaudreau A. H., Levin P. S. (2014). Movement patterns and distributional shifts of dungeness crab (Metacarcinus magister) and English sole (Parophyrys vetulus) during seasonal hypoxia. Estuar Coasts 37, 449–460. doi: 10.1007/s12237-013-9676-2
Gunnarson T. G., Jennifer A. G., Peter. M. P., Philip W. A., Ruth E. C., Gelinaud G., et al. (2005). Estimating population size in black-tailed godwits limosa limosa islandica by color-marking. Bird Study 52, 153–158. doi: 10.1080/00063650509461385
Hill B. J. (1978). Activity, track and speed of movement of the crab Scylla serrata in an estuary. Mar. Biol. 47, 135–141. doi: 10.1007/BF00395634
Hill B. J. (1994). Offshore spawning by the portunid crab Scylla serrata (Crustacea: Decapoda). Mar. Biol. 120, 379–384. doi: 10.1007/BF00680211
Hill B. J., Williams M. J., Dutton P. (1982). Distribution of juvenile, sub-adult and adult Scylla serrata (Crustacea: Portunidae) on tidal flats in Australia. Mar. Biol. 69, 117–120. doi: 10.1007/BF00396967
Hyland S., Hill B. J., Lee C. P. (1984). Movement within and between different habitats by the portunid crab Scylla serrata. Mar. Biol. 80, 57–61. doi: 10.1007/BF00393128
Ikhwanuddin M., Azmie G., Juariah H. M., Abol-Munafi A. B., Zakaria M. Z., Ambak M. A. (2012a). Tracking the movement of mud crabs, genus Scylla from mangrove area using telemetry system. Borneo Sci. 30, 40–57.
Ikhwanuddin M., Azmie G., Juariah H. M., Zakaria M. Z., Ambak M. A. (2011). Biological information and population features of mud crab, genus Scylla from mangrove areas of Sarawak, Malaysia. Fish Res. 108, 299–306.
Ikhwanuddin M., Bachok Z., Hilmi M. G., Azmie G., Zakaria M. Z. (2010). Species diversity, carapace width-body weight relationship, size distribution and sex ratio of mud crab, genus Scylla from setiu wetlands of terengganu coastal waters, Malaysia. J. Sustain Sci. Manage. 5, 97–109.
Ikhwanuddin M., Nurfaseha A. H., Abol-Munafi A. B., Shabdin M. L. (2012b). Movement patterns of blue swimming crab, Portunus pelagicus in the Sarawak coastal water, south China Sea. J. Sustain Sci. Manage. 7, 8–15.
Kathiresan K., Bingham B. I. (2001). Biology of mangrove and mangrove ecosystems. Adv. Mar. Biol. 40, 81–251.
Keenan C. P. (1999). “Aquaculture of the mud crab, genus Scylla – past, present and future,” in Mud crab aquaculture and biology, vol. 78 . Eds. Keenan C. P., Blackshaw A. (Canberra:Australian Center for Information Agricultural Research Proceedings), 9–13.
Keenan C. P., Davie P. J. F., Mann D. L. (1998). A revision of the genus Scylla de haa (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bull. Zool 46, 217–245.
Koolkalya S., Thapanand T., Tunkijjanujij S., Havanont V., Jutagate T. (2006). Aspects in spawning biology and migration of the mud crab Scylla olivacea in the Andaman Sea, Thailand. Fish Manage. Ecol. 12, 391–397. doi: 10.1111/j.1365-2400.2006.00518.x
Le Vay L. (2001). Ecology and management of mud crab, Scylla spp. Asian Fish Sci. 14, 101–111. doi: 10.33997/j.afs.2001.14.2.001
Le Vay L., Ut V. N., Walton M. (2007). Population ecology of the mud crab Scylla paramamosain (Estampador) in an estuarine mangrove system; a mark-recapture study. Mar. Biol. 151, 1127–1135. doi: 10.1007/s00227-006-0553-4
Liu Z. M., Wang G. Z., Ye H. H., Li S. J., Tao Y., Lin Q. W., et al. (2011). Tag performance and physiological responses of juvenile mud crabs Scylla paramamosain tagged with visible implant elastomer. Fish Res. 110, 183–188. doi: 10.1016/j.fishres.2011.04.005
Macaulay G., Warren-Myers F., Barret L. T., Oppedal F., Føre M., Dempster T. (2021). Tag use to monitor fish behaviour in aquaculture: a review of benefits, problems and solutions. Rev. Aquac 13, 1565–1582. doi: 10.1111/raq.12534
Moser S., Macintosh D., Laoprasert S., Tongdee N. (2005). Population ecology of the mud crab Scylla olivacea: a study in the ranong mangrove ecosystem, Thailand, with emphasis on juvenile recruitment and mortality. Fish Res. 71, 27–41. doi: 10.1016/j.fishres.2004.07.008
Overton J. L., Macintosh. D. J. (2002). Estimated size at sexual maturity for female mud crabs (Genus Scylla) From two sympatric species within the ban don bay, Thailand. J. Crust. Biol. 22, 790–797. doi: 10.1163/20021975-99990293
Quinn T. P., Brodeur R. D. (1991). Intra-specific variations in the movement patterns of marine animals. Am. Zool 31, 231–241. doi: 10.1093/icb/31.1.231
Robertson W. D. (1996). Abundance, population structure and size at maturity of Scylla serrata (korskål) (Decapoda: Portunidae) in Eastern cape estuaries, south Africa. South Afr. J. Zool 31, 177–185.
Wada T., Mitsushio T., Inoue S., Koike H., Kawabe R. (2016). Movement patterns and residency of the critically endangered horseshoe crab Tachypleus tridentatus in a semi-enclosed bay determined using acoustic telemetry. PloS One 11, e0147429. doi: 10.1371/journal.pone.0147429
Waiho K., Fazhan H., Quinitio E. T., Baylon J. C., Fujaya Y., Azmie G., et al. (2018). Larval rearing of mud crab (Scylla): What lies ahead. Aquaculture 493, 37–50. doi: 10.1016/j.aquaculture.2018.04.047
Waiho K., Ikhwanuddin M., Abualreesh M. H., Shu-Chien A. C., Ishak S. D., Jalilah M., et al. (2021). Intra- and interspecific variation in sexual dimorphism patterns of mud crab genus Scylla along the equatorial region. Front. Mar. Sci. 8, 690836. doi: 10.3389/fmars.2021.690836
Keywords: mangrove, movement pattern, recapture, Scylla, tagging
Citation: Fazhan H, Azra MN, Halim SA, Naimullah M, Abualreesh MH, Shu-Chien AC, Wang Y, Fujaya Y, Syahnon M, Ma H, Waiho K and Ikhwanuddin M (2022) Species composition, abundance, size distribution, sex ratios, and movement of Scylla mud crabs within the mangrove ecosystem at Setiu Wetland, Terengganu, Malaysia. Front. Mar. Sci. 9:899789. doi: 10.3389/fmars.2022.899789
Received: 19 March 2022; Accepted: 24 August 2022;
Published: 08 September 2022.
Edited by:
Michael Phillips, WorldFish, MalaysiaReviewed by:
Carlos Rosas, National Autonomous University of Mexico, MexicoCopyright © 2022 Fazhan, Azra, Halim, Naimullah, Abualreesh, Shu-Chien, Wang, Fujaya, Syahnon, Ma, Waiho and Ikhwanuddin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khor Waiho, d2FpaG9AdW10LmVkdS5teQ==; Mhd Ikhwanuddin, aWtod2FudWRkaW5AdW10LmVkdS5teQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.