- 1South African Institute for Aquatic Biodiversity, Makhanda, South Africa
- 2Department of Ichthyology and Fisheries Science, Rhodes University, Makhanda, South Africa
The Blue Economy is a global initiative aimed at using marine resources to create economic viability and environmental sustainability. While successes have been reported, for example, in Europe and China, examples of African successes are notably missing. Abject poverty, unemployment and food insecurity are everyday concerns on the African continent; however, its large latitudinal coverage gives rise to extremely biodiverse marine fauna, which could promote socio-economic development of coastal communities through initiatives such as sustainably-managed fisheries. In order to improve sustainability via improved management, information on a species and its habitat is needed, particularly how it moves and in which areas it occurs. Acoustic telemetry is a powerful tool used to determine the movements of aquatic animals, the success of which has led to the development of several large-scale networks throughout the globe, including South Africa’s Acoustic Tracking Array Platform. This network, formally in place for the last decade, has now matured, and data are revealing insights into residency, habitat connectivity and transboundary movements of a multitude of animals, with some species having been continually detected for the past 10 years. These data are also actively being incorporated into marine spatial planning efforts, with the aim of protecting threatened and endemic species. Due to knowledge generation, successful benefit-sharing arrangements, and dedication to engage with the public and other stakeholder groups, the ATAP represents a highly successful example of ocean stewardship in Africa.
1 Introduction
The United Nations Sustainable Development Goals (SDGs) are a list of 17 goals, whose collective implementation aim to eradicate poverty, while balancing economic and social development, as well as environmental protection and sustainable resource use (Lee et al., 2020). The concept of the ‘Blue Economy’ (or ‘Oceans Economy’), whose origin is linked with the SDGs (particularly SDG 14: Life below water) has gained significant traction over the past decade, and aims to improve human well-being and social equity through the sustainable use of aquatic resources for economic growth while maintaining ecosystem health (Smith-Godfrey, 2016; Wenhai et al., 2019). Successful applications of this concept have included the assessment of harmful algal blooms around aquaculture hubs in European Atlantic shelf seas, conducting ship-based oil spill risk assessments in Atlantic Basin coasts, and the ecological restoration of wetlands in China (Wenhai et al., 2019).
Despite these success stories, African examples are notably missing. Africa is surrounded by three oceans, and represents the largest latitudinal coverage of any continent resulting in a richly biodiverse marine fauna (Decker et al., 2003) with large fisheries resources (Belhabib et al., 2016; Muringai et al., 2021). However, many African countries are amongst the most impoverished, with abject poverty, high unemployment rates, and high levels of food insecurity being an every-day concern (Belhabib et al., 2015; Battersby and Watson, 2018). Consequently, many marine species are heavily targeted by African fisheries, spanning the recreational, commercial, and small-scale/informal sector. Together, these sectors contribute approximately US$ 24 billion annually, constituting 1.26% of the region’s Gross Domestic Product (Chan et al., 2019). Unfortunately, stocks are largely being harvested unsustainably, resulting in declining resources, which further negatively impacts food security (Belhabib et al., 2015; Temple et al., 2018; Muringai et al., 2021). For example, all West African fishing grounds are considered fully or over-exploited, with an estimated 6.7 million people relying on fishing activities for both food and livelihoods (Belhabib et al., 2015). Sustainably managed fisheries are essential for the development of Africa through alleviating poverty and promoting socio-economic growth (Chan et al., 2019). However, promoting growth and development is often at heads with conservation efforts, particularly when it comes to the protection needs of threatened and vulnerable species (Voyer et al., 2018). Many Blue Economy initiatives across the African continent also place significant emphasis on economic gains, with little to no regard to both environmental sustainability and conservation, and social inclusion (Okafor-Yarwood et al., 2020).
On the journey towards the sustainable development and use of aquatic resources, Africa’s Blue Economy Strategy was developed in 2018, with the aim of implementing this plan between 2021 and 2025 (Failler et al., 2020). This strategy built on global policies and initiatives to which African countries were already committed, including the 2014 Africa’s Integrated Maritime Strategy (2050 AIMS) and the SDGs, with emphasis being placed on SDG 14: Life below water (Failler et al., 2020). Five overarching themes were identified as being critical to the growth of the Blue Economy in Africa, all of which are similar to the implementation of the Blue Economy across the globe. These themes encompass renewable innovative marine energy industries; maritime transport, safety and enforcement; improved policy and governance; sustainable fishing, aquaculture and conservation; and marine and coastal tourism (Failler et al., 2020). Linked to the sustainable use and development goals of the African Blue Economy Strategy is the concept of ‘ocean stewardship’. The term stewardship broadly encompasses the principles and actions that aim to improve sustainability and resilience of social-ecological systems, that are grounded on values of voluntary altruism and long-term benefits (Barendse et al., 2016). Peçanha Enqvist et al. (2018) highlights that there are multiple meanings and frameworks of stewardship as it is used across disciplines, but argues that it can be considered as a conceptual ‘boundary object’ encompassing three components: care, knowledge and agency.
The knowledge dimension of stewardship specifically refers to the basic information and understanding about a species, habitat, or other resource that is being stewarded (Berkes et al., 2008; Peçanha Enqvist et al., 2018). While such knowledge can come from a variety of different knowledge systems, the conventional scientific method is highly relevant (Hansen, 2014; Peçanha Enqvist et al., 2018). In the context of stewardship as it applies to the ocean, knowledge generation around both marine species and habitats is vital, as these are both essential components that need to be managed sustainably under the umbrella of the African Blue Economy Strategy. Spatial management tools, such as Marine Protected Areas (MPAs), can be considered as good management practices in an African context. They provide ecological benefits by protecting both species and habitat (Roberts et al., 2005; Lester et al., 2009; Bennett and Dearden, 2014; Kirkman et al., 2021), social benefits such as rebuilding depleted fish stocks and supporting livelihoods, and providing recreation/tourism opportunities (Roberts et al., 2005; Lopes et al., 2015; Kirkman et al., 2021) all of which encompass the social-ecological aspects of ocean stewardship. However, in order for these benefits to be realised, spatial management tools need to be implemented effectively. For example, if MPAs are to be effective in protecting vulnerable species, information on animal movement is essential (Kerwath et al., 2007; Afonso et al., 2009; Mann et al., 2016). More specifically, knowing where and when an animal moves provides critical insights into ecologically and/or biologically significant areas (Kock et al., 2018).
Acoustic telemetry is a method used to study aquatic animal spatial ecology that has gained significant popularity over recent decades, and is being used to investigate and answer diverse questions for a multitude of animal groups including teleosts, elasmobranchs, marine mammals, reptiles and invertebrates (Hussey et al., 2015). This method involves attaching an acoustic transmitter to an aquatic animal which transmits uniquely coded acoustic signals that are detected, recorded and stored by acoustic receivers. Acoustic telemetry has allowed for the successful elucidation of various aspects of animal movement including residency and site affinity, home range size, seasonal migratory behaviours, and habitat connectivity (Heupel et al., 2005; Hussey et al., 2015; Cowley et al., 2017). More specifically, the success and popularity of passive acoustic telemetry, where acoustic receivers are stationed at fixed locations, has led to the development of several large-scale collaborative networks of acoustic receivers distributed across the globe. These include Australia’s Integrated Marine Observing System (IMOS) Animal Tracking Facility (Steckenreuter et al., 2017; Hoenner et al., 2018; Huveneers et al., 2021), the European Tracking Network (Abecasis et al., 2018; Reubens et al., 2019), the US’s FACT (Young et al., 2020) and Atlantic Cooperative Tracking networks (Block et al., 2016), and South Africa’s Acoustic Tracking Array Platform ATAP (Cowley et al., 2017). These large-scale networks are collecting movement data on a multitude of aquatic species, and are now in a position to incorporate these data into improved fisheries management (Nguyen et al., 2018; Lowerre-Barbieri et al., 2019).
The ATAP is the only network of its kind on the African continent. It represents a significant example of the successful implementation of ocean stewardship principles that can directly contribute towards sustainable resource management. Cowley et al. (2017) reflected on the first five years of the ATAP (2011 – 2016). This article aims to provide a decadal overview of the ATAP highlighting it as a successful ocean stewardship example for Africa, and in particular (i) detailing how the data collected are generating important ecological and biological knowledge, (ii) highlighting how this can be utilised in management and conservation tools through the provision of two case studies, (iii) discussing how the data-sharing arrangements benefit multiple stakeholders, and (iv) briefly touching on how stakeholder engagement and community outreach are also important aspects being practised by the ATAP.
2 ATAP as a Knowledge Generation Platform
2.1 Background and Spatial Coverage of the ATAP
The ATAP was first formalised in 2011 with a signed partnership agreement with the Canadian-based global Ocean Tracking Network (OTN; http://oceantrackingnetwork.org/) project and is managed by the South African Institute for Aquatic Biodiversity (SAIAB), a National Facility of South Africa’s National Research Foundation (NRF). The ATAP is a collaborative network of acoustic receivers (and temperature loggers) that provide the backbone of acoustic telemetry hardware to facilitate the large-scale, long-term monitoring of acoustically-tagged marine animals along the South African coastline. Furthermore, the ATAP maintains all metadata associated with receivers and transmitters, as well as a national database of detection data, which are shared with the relevant researchers (transmitter owners).
Since inception, the ATAP has had a mean of 179 (± 43) active acoustic receiver stations per year, and currently spans approximately 2200 km of the South African coastline (for further details on equipment and deployment, see Cowley et al., 2017). The focal monitoring sites are three large coastal embayments (False Bay, Mossel Bay and Algoa Bay; Figure 1), with receivers being deployed in one or more lines representing listening curtains. Additional receivers are deployed in the nearshore environment along the majority of the South African coastline, including (from west to east) Walker Bay, Gansbaai, Plettenberg Bay, Port Alfred, Port St Johns, Protea Banks, Jesser Point and Ponta do Ouro at the South Africa-Mozambique border (Figure 1). A number of selected estuaries also have at least one receiver deployed in them. Together these receivers allow for the assessment of residency, site fidelity, localised movement patterns, habitat connectivity, large-scale coastal movements, migration biology, and estuarine-marine connectivity of marine animals (Cowley et al., 2017). Additionally, where receivers lack water temperature sensors (VR2W as opposed to VR2AR; Innovasea, Halifax, Canada), temperature loggers are attached to selected receivers, which allows for the assessment of the influence of temperature on animal movement, a crucial aspect given the ectothermic nature of the majority of acoustically-tagged species.
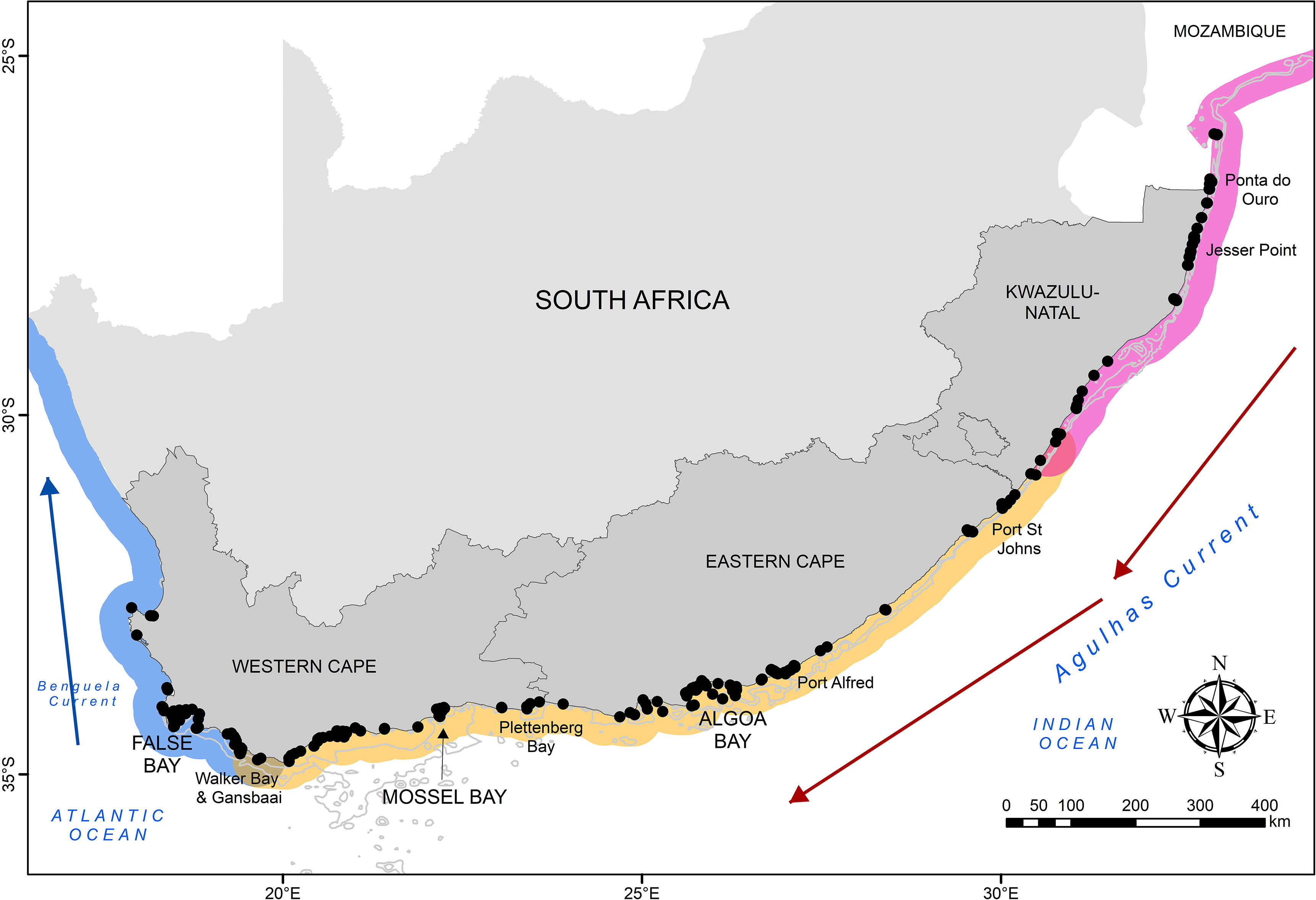
Figure 1 The locations of receivers (black dots) forming the greater Acoustic Tracking Array Platform currently deployed along the South African coastline. Influential ocean boundary currents are indicated, as are important sites mentioned in the text. The cool-temperate, warm-temperate and subtropical biogeographic regions are indicated by blue, yellow, and pink areas, respectively.
2.2 Importance of Location
The ATAP sits at an important geographical location on the African continent. Located at the southern tip of Africa, the coastal waters of South Africa are strongly influenced by two contrasting boundary currents – the cool Benguela associated with coastal upwelling and significant productivity (Harrison, 2003), and the warm Agulhas associated with warm tropical waters flowing down from the equator (Roberts, 2010). Together, these currents give rise to three distinct biogeographic regions: cool-temperate, warm-temperate and subtropical (Turpie et al., 2000) (Figure 1), making the region a global biodiversity hotspot with a rich diversity of species and a high degree of endemism (averaging 28% across all marine taxa [Griffiths and Robinson, 2016)].
Many fish species occurring in the estuarine, coastal and offshore southern African waters are targeted by both commercial and recreational fisheries; for example, Cape hakes Merluccius capensis and M. paradoxus, geelbek Atractoscion aequidens, yellowtail Seriola lalandi, soupfin Galeorhinus galeus and smoothhound Mustelus mustelus sharks (commercially exploited) [DEFF (Department of Environment, Forestry and Fisheries), 2020)], as well as dusky kob Argyrosomus japonicus and spotted grunter Pomadasys commersonnii (recreationally exploited) (Cowley et al., 2013; Childs et al., 2015; Dames et al., 2017). Both of these fishery sectors are highly important industries, providing economic and food security to millions of South Africans, but have had, and continue to have, a considerable impact on many marine species (Pradervand and Baird, 2002; Lamberth and Turpie, 2003; Cowley et al., 2013; da Silva et al., 2015; Lallemand et al., 2016). Additionally, the small-scale/informal sector places further stress on selected species, albeit in significantly smaller quantities relative to the former sectors (Baust et al., 2015). Subsequently, the stocks of many fish species have declined with others completely collapsing, including dusky kob, seventy-four seabream Polysteganus undulosus and red steenbras Petrus rupestris. This exemplifies the need for sustainable resource management through the African Blue Economy and ocean stewardship principles in South Africa.
South Africa has a rich history of marine spatial planning in an attempt to manage its marine resources. The first MPA was declared in 1964 at Tsitsikamma (Tsitsikamma National Park) on the south coast (Attwood et al., 1997) and by 2011, the year in which ATAP was formalised, 25 MPAs had been declared in South African waters. However, these MPAs only provided protection to <0.5% of South Africa’s Exclusive Economic Zone (EEZ), and lacked ecosystem representivity, especially of offshore habitats (Kirkman et al., 2021). Subsequently, systematic conservation planning was undertaken to identify priority areas to address the gaps in protection which fell under the government’s Operation Phakisa, a strategic initiative that would support sustainable development of economic opportunities in South Africa’s ocean space (Sink, 2016). Through this process, 20 new MPAs (mainly offshore) were declared and some existing coastal MPAs were expanded in 2019 (Findlay, 2020). While this significantly increased the MPA spatial coverage to 5.4% of the EEZ, and increased ecosystem representivity (87% of ecosystem types are now protected), only benthic habitat types were considered during this planning, and no data on the habitat use or movement patterns of marine animals were considered. Subsequently, a review of South African MPAs found that only about half of the assessed marine fish groups have been seen to occur within these MPAs (Kirkman et al., 2021).
2.3 What Has Been Monitored to Date?
TThere has been a total steady growth in both the numbers of individuals and species tagged with acoustic transmitters by researchers within the ATAP network since its inception (Figure 2). To date, there has been a total of 1579 individuals tagged spanning 48 species. The taxonomic group with both the most numbers of individuals and species tagged is chondrichthyans (924 individuals, 32 species), followed by teleosts (640 individuals, 12 species), turtles (11 individuals, 3 species), and birds (4 individuals, 1 species) (Figure 3 and Table 1). A mean of 33 (± 38) individuals per species has been tagged (range 1 - 158) and 24.6% of tagged individuals were endemic to the southern African region (Table 1). Given the three focal monitoring bays, 29% of individuals have been tagged within these bays (6%, 7% and 16% in False Bay, Mossel Bay, and Algoa Bay, respectively; these include tagging within estuaries in these bays). Despite this, tagging effort has been well spread out along the South African coastline for teleosts and chondrichthyans, stretching from the west coast eastwards to Mozambique (Figure 4).
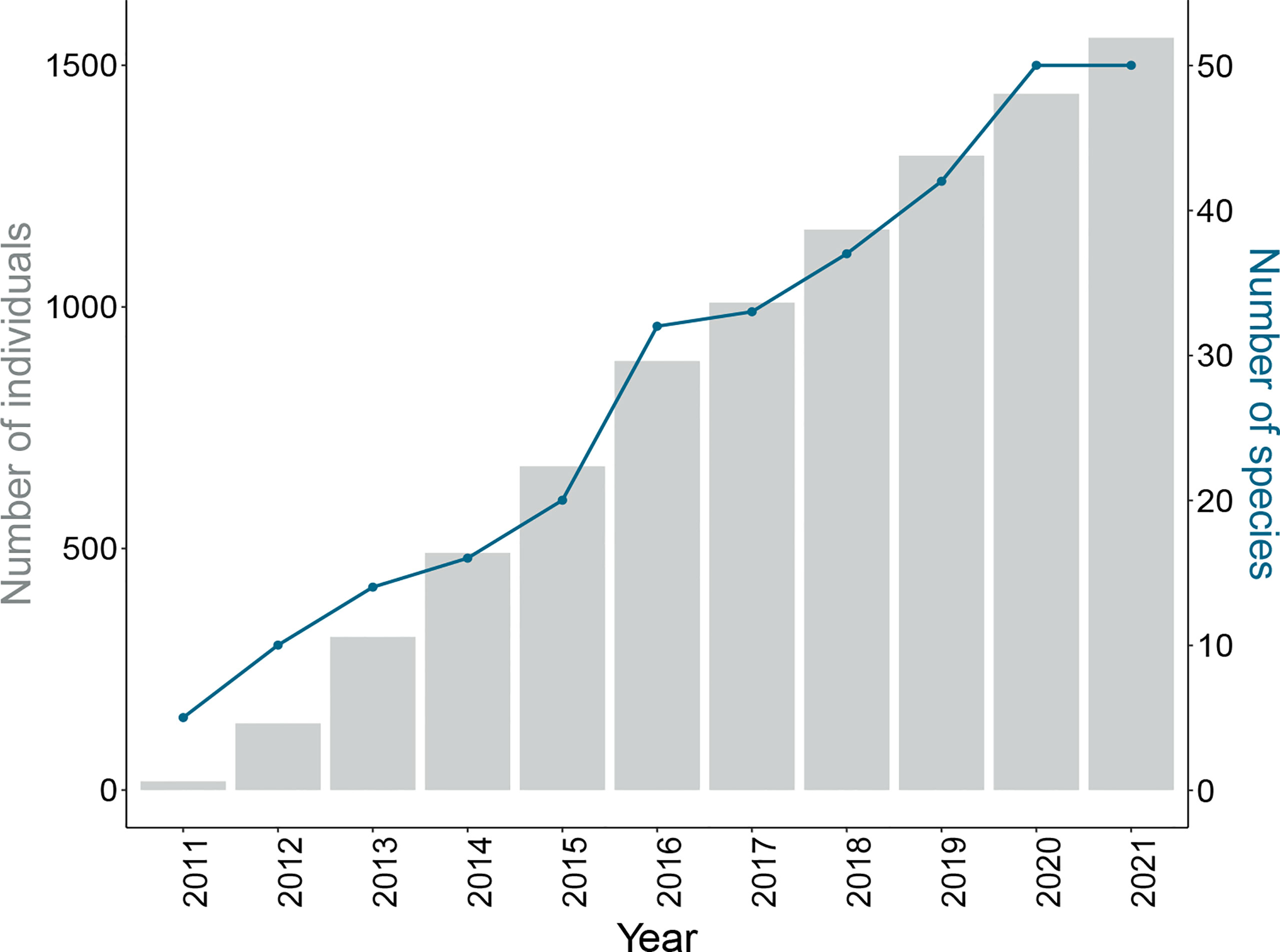
Figure 2 Cumulative number of individuals (grey bars) and species (blue line) tagged with acoustic transmitters per year since the inception of ATAP.
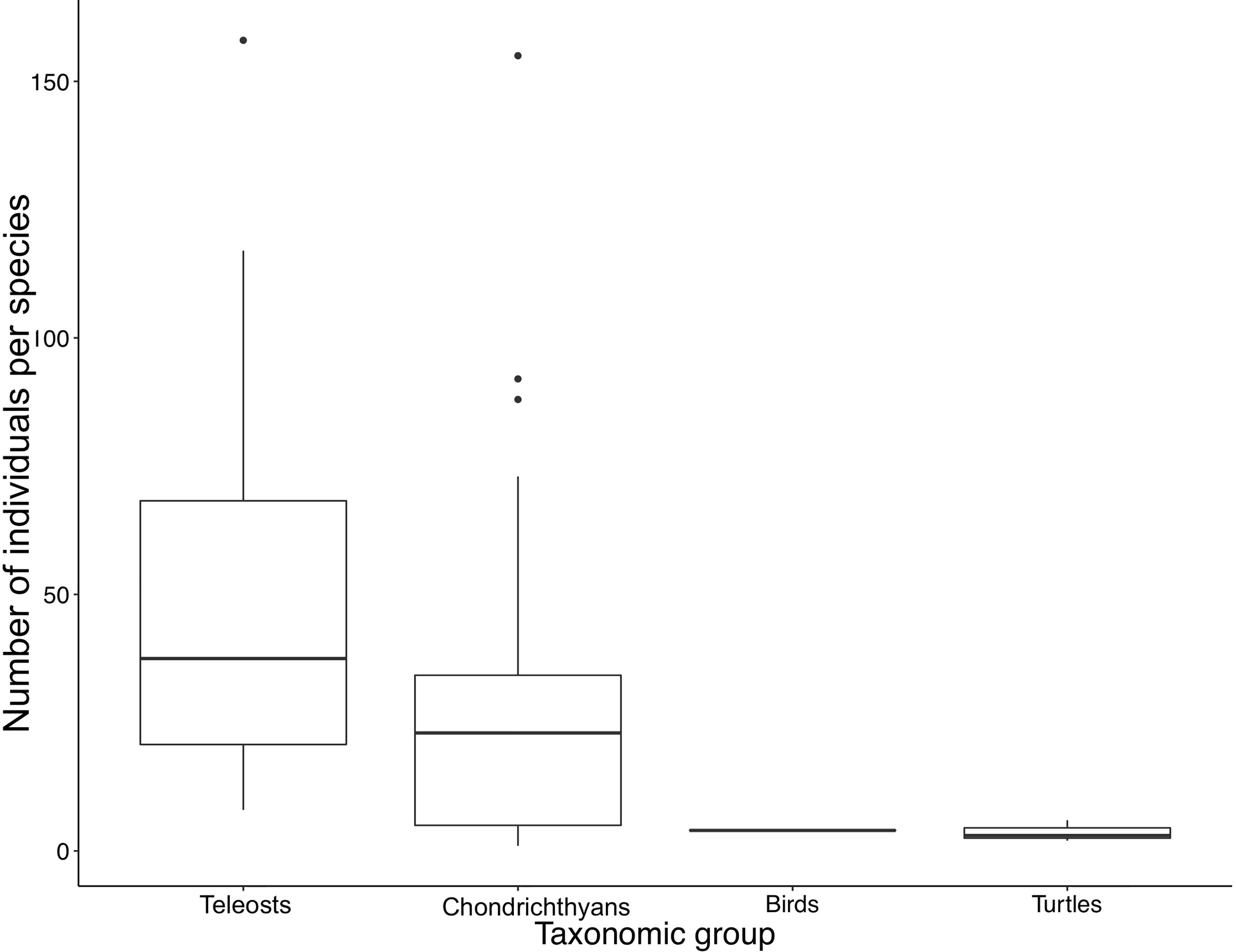
Figure 3 Boxplot (median, first and third quartiles, 1.5*IQR, outliers) of the number of individuals tagged per species within each taxonomic grouping.
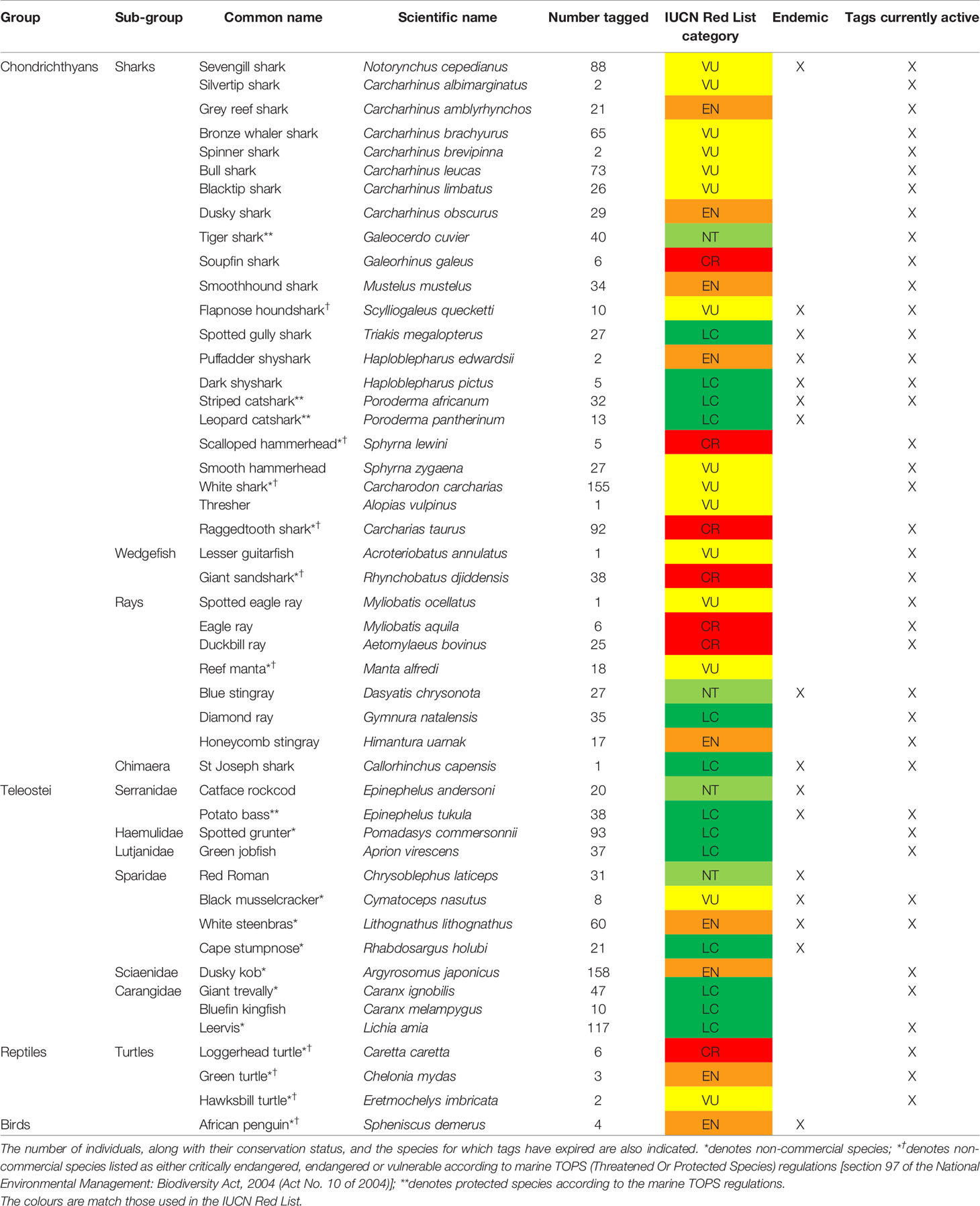
Table 1 List of species which have been tagged by researchers in South Africa and have been, or are currently, monitored by the nationwide Acoustic Tracking Array Platform.

Figure 4 The distribution of tagging effort for both chondrichthyans (blue circles) and teleosts (yellow triangles) has been nationwide along the entire South African coastline.
Prominent species tagged within the teleost group include dusky kob, leervis Lichia amia, spotted grunter and white steenbras Lithognathus lithognathus, while prominent species in the chondrichthyan group were all larger shark species (white shark Carcharodon carcharias, raggedtooth shark Carcharias taurus, sevengill shark Notorynchus cepedianus) (Table 1). This is reflective of the research interests of platform users, which include extensive work on estuary-associated teleosts in order to gain a better understanding of estuary use, estuary-marine connectivity and ontogenetic habitat shifts by these species (Næsje et al., 2007; Childs et al., 2008a; Childs et al., 2008b; Bennett et al., 2011; Bennett et al., 2012; Bennett et al., 2015; Childs et al., 2015; Dames et al., 2017; Murray et al., 2018), and learning more about the spatial ecology of large predatory sharks, especially given their relative proximity to water users (Kock et al., 2013; Daly et al., 2014; Engelbrecht et al., 2017; Kock et al., 2018). In more recent years, other more diverse species have been tagged. These include smaller endemic catsharks, with researchers aiming to answer questions related to spatio-temporal movements and the relative importance of MPAs to these species, as well as endemic and threatened rays (blue stingray Dasyatis chrysonota, common eagle ray Myliobatis aquila, duckbill ray Aetomylaeus bovinus, diamond ray Gymnura natalensis) and wedgefishes (white spotted wedgefish Rhynchobatus djiddensis, lesser guitarfish Acroteriobatus annulatus), with researchers aiming to assess both the local coastal movements and longshore transboundary movements between South Africa and southern Mozambique (the latter particularly for the wedgefish).
In order for these tagging efforts to be useful in the elucidation of movement behaviour, sufficient data need to be collected; in this case, a sufficient number of date/time- and location-stamped detections recorded by the acoustic receivers. The ATAP has gone through various iterations of the geographic placement of acoustic receivers, with some locations being discontinued indefinitely due to low numbers of detections on those receivers (particularly those deployed in deeper water - see Cowley et al., 2017; currently 76% of receivers are deployed in waters with a depth of 30 m or less). Additionally, receiver locations are often altered to suit the research objectives of active projects at the time. Consequently, and at the time of writing, the receivers of the ATAP have recorded more than 20 million detections between 2011 and 2021, with 41.7% of those being detected on receivers deployed in the nearshore (marine environment), and 58.3% in estuaries and harbours (Figure 5). Of the 1579 animals tagged over the past decade, at least 73% have been detected, with taxonomic groups being detected to varying degrees. Approximately 78.7% of all tagged chondrichthyans have been detected, 63.7% of teleosts and 55.6% of turtles. This relatively high rate of detection has resulted in extensive datasets, with some species accumulating almost consistent, daily detections over a 10-year period; for example, the large predatory bull, raggedtooth and white sharks, and the piscivores dusky kob and leervis. This illustrates the success of the configurations of the ATAP receivers, with the concentrations of receivers in select focal areas, linked by more sparsely distributed receivers in between, being able to detect and monitor the movements of the majority of tagged individuals across a wide taxonomic and movement behaviour spectrum.
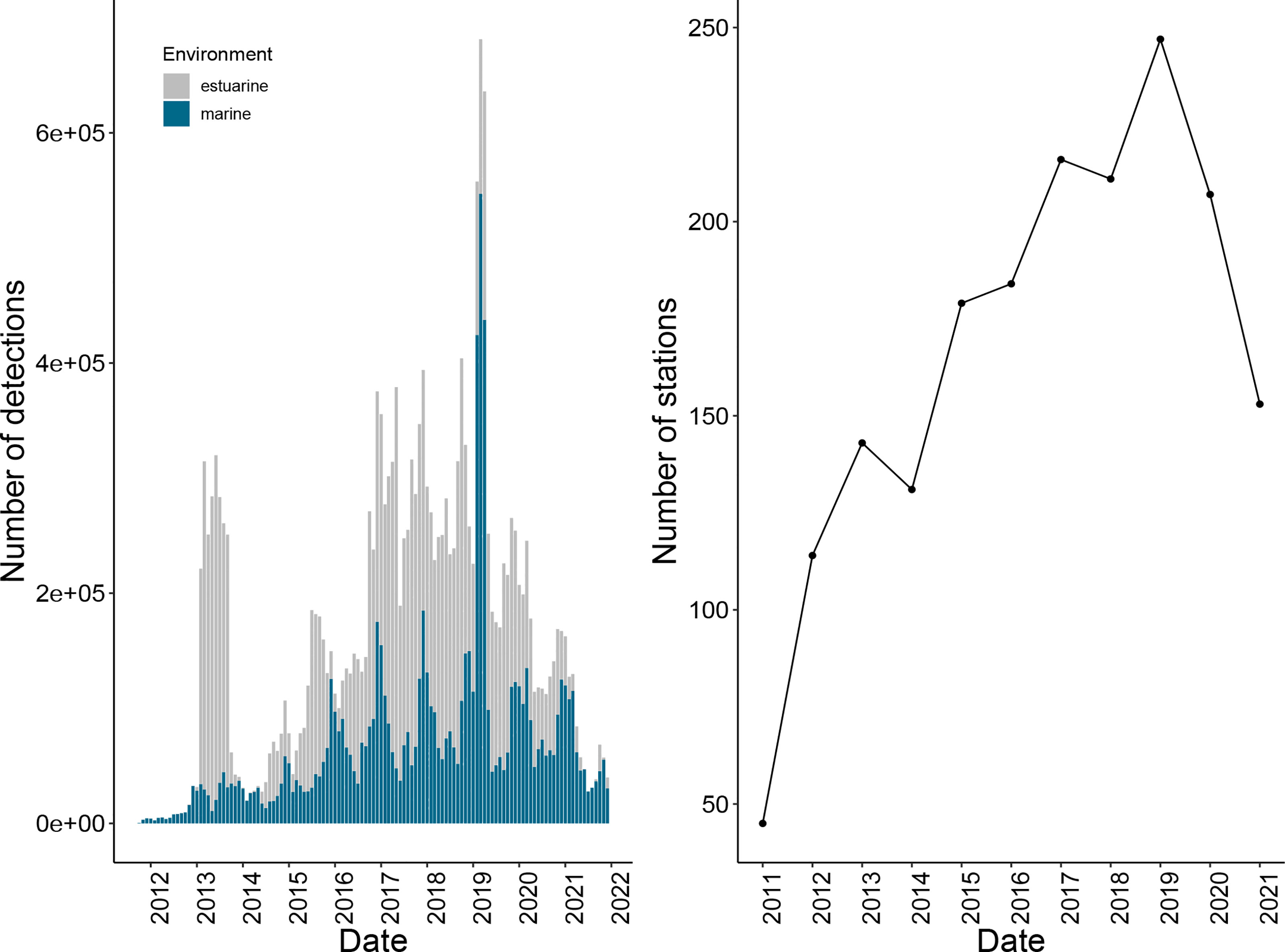
Figure 5 Number of detections recorded monthly on ATAP receivers between October 2011 and December 2021 (left). Blue bars denote detections recorded on receivers deployed in the nearshore environment (marine), and grey bars denote detections recorded on estuarine receivers. The number of receivers deployed and active in the ATAP by year from 2011 - 2021 (right).
3 Conservation Potential of ATAP-Generated Knowledge
One of the key management and conservation goals of the ATAP put forward by Cowley et al. (2017) was the provision of data to assist in the planning of conservation/management zones, particularly MPAs. Other global studies have highlighted how acoustic telemetry data can be used to assist in the planning of MPAs and to provide valuable insights on relative MPA use by both teleosts (Alós et al., 2011; La Mesa et al., 2012; Abecasis et al., 2014; Aspillaga et al., 2016; Novak et al., 2020) and chondrichthyans (Lea et al., 2016; Jacoby et al., 2020; Elston et al., 2021; van Zinnicq Bergmann et al., 2022). Despite significant investment in telemetry science, the documentation and assessment of the impact of telemetry research is still lacking (McGowan et al., 2016). However, in South Africa, the intention of using movement data collected by the ATAP to assist with marine spatial planning efforts, and to evaluate the effectiveness of the country’s existing MPAs to protect vulnerable and endemic species, is being realised.
Through the years the ATAP has supported, and continues to support, a number of acoustic telemetry projects of conservation concern, both in terms of the species being monitored and the placement of effort within several southern African MPAs. Firstly, almost two-thirds of all individuals tagged and monitored by the ATAP over the past decade were of conservation concern as defined by the International Union for Conservation of Nature (IUCN) Red List categories, one of the most objective and authoritative systems available to assess the extinction risk of a species (Mace et al., 2008). However, it is recognised that these category designations may not necessarily consider the use-related data associated with each species. Indeed, Marsh et al. (2021) found that 98.5% of all chondrichthyans, and 82.1% of all teleosts, irrespective of conservation status, are used as a biological resource, creating a conflict between resource use for economic gain and food security, and conservation measures. Specifically, 62.4% (n = 985) of tagged individuals detected within the ATAP were threatened with extinction, being classed as Critically Endangered (n = 178), Endangered (n = 328), or Vulnerable (n = 479), while 37.6% (n = 594) of tagged individuals were classed as Near Threatened (n = 118), or Least Concern (n = 476). Furthermore, approximately one-third of all individuals have been tagged within South African MPAs and, at time of writing, approximately 40% (n = 103) of the 251 active receivers were situated within MPAs (Figure 6). These partner projects within MPAs aim to (i) evaluate the efficacy of the De Hoop Nature Reserve in protecting endemic and threatened shark species, including endemic catsharks (Albano et al., 2021); (ii) investigate the movements of giant kingfish Caranx ignobilis in the Mtentu Estuary within the Pondoland MPA and their connectivity with surrounding areas (Dixon, 2022), including their known spawning aggregation site in southern Mozambique (Daly et al., 2019); (iii) investigate the connectivity between the Pondoland and St Lucia MPAs using catface rockcod Epinephelus andersoni as the focal species; (iv) investigate the movements of green jobfish Aprion virescens and potato bass Epinephelus tukula in the iSimangaliso Wetland Park (an MPA); (v) assess the influence of fishing pressure on the behaviour and activity of an endemic sparid red Roman Chrysoblephus laticeps tagged in the Tsitsikamma National Park MPA; and (vi) understand how the resident sparid white steenbras responds to oceanographic features within the Greater Addo Elephant National Park MPA (Figure 6). Together, these projects highlight the conservation potential that the ATAP has to inform whether vulnerable and endemic species are being effectively protected by the current MPA zonation in South Africa. Furthermore, telemetry data collected by the ATAP is also currently being used to advise spatial management plans for both chondrichthyans and teleosts. These are presented as two case studies below.
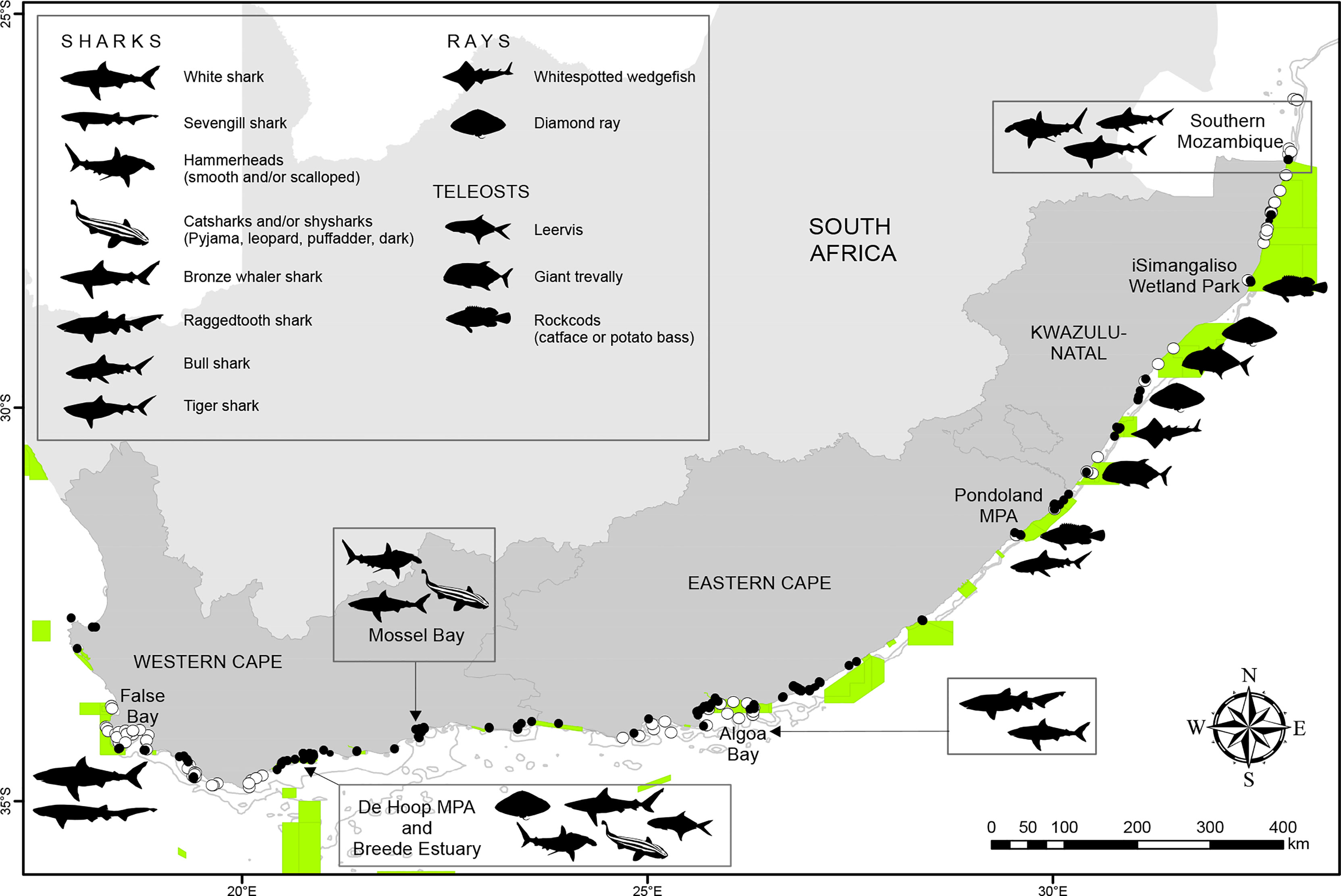
Figure 6 The current MPA network in South Africa (green areas) covers approximately 5.4% of the country Exclusive Economic Zone. The majority of smaller research projects in the country are taking place in inshore MPAs, with focal study species (presented as silhouettes) being placed near the relevant MPAs. Note that not all focal species per region are shown. ATAP receivers are indicated by black dots and ATAP partner receivers are indicated by white dots. ATAP receivers are indicated by black dots and ATAP partner receivers are indicated by white dots.
3.1 Chondrichthyan Case Study
There has been a large effort, particularly in recent years, to tag chondrichthyans in South Africa as the region is a global hotspot for threatened and endemic chondrichthyan species, with 193 out of the ≈1188 known species worldwide occurring here (Ebert et al., 2021). However, 29% of these species occurring in South Africa are considered at risk of extinction (Ebert and van Hees, 2015), and South Africa has been identified as a priority region for conservation efforts (Davidson and Dulvy, 2017). In South Africa, coastal chondrichthyans are subjected to overfishing and habitat degradation, with species such as the common smoothhound shark and soupfin shark targeted by commercial shark fisheries (da Silva et al., 2015).
While there remains uncertainty in the contribution that spatial protection can have in shark conservation, especially for larger and more mobile species who frequently move outside MPA boundaries (Dulvy et al., 2014; Davidson and Dulvy, 2017; MacKeracher et al., 2019), there have been some studies showing the positive effect that localised MPAs can have on shark abundance (Goetze and Fullwood, 2013; Speed et al., 2016; Bond et al., 2017; MacNeil et al., 2020). However, on a global scale, existing MPAs do not overlap in space with threatened endemic chondrichthyans (Davidson and Dulvy, 2017). For conservation efforts to be successful, MPAs must encompass suitable habitat for these threatened species, which requires species-specific knowledge on movement and habitat use (Birkmanis et al., 2020). The planning of the current South African MPA network did not take into account any movement or habitat use data on focal marine animals, including chondrichthyans. To combat this, the WILDOCEANS Programme produced a systematic conservation plan (SCP) for sharks and rays in South Africa, which aims to improve the protection of the IUCN Red List threatened (Vulnerable, Endangered, and Critically Endangered) sharks and rays found within South Africa’s oceans, prioritising threatened endemics. The main outcome of the SCP was to utilise distribution data to develop a GIS-based SCP that identified candidate sites for the area-based management of threatened and endemic chondrichthyans (Beaulieu et al., 2022). The ATAP provided data in the form of GPS coordinates per station on which a species was detected. This provided an overall detection distribution per species. Data from 23 species, including 18 sharks and 5 stingrays, belonging to 11 researchers from nine different organisations, were ultimately included in the species distribution modelling phase of the SCP. This conservation plan will be presented to the South African government (Department of Forestry, Fisheries and the Environment), who will consider the data when developing a new National Plan of Action for Elasmobranchs. The MPAs are all currently promulgated but the intent of the plan is to highlight important areas for multiple species, which when identified, could be put forward or motivated for as needing increased protection.
3.2 Teleost Case Study
Despite immense MPA efforts including expansion of existing borders, the level of protection afforded to some species can remain low, largely because environments or habitats most important to those species have not been incorporated. This is particularly applicable to estuary-associated species which utilise estuaries predominantly as juveniles, but also to a large extent as adults. A spatial management tool which would provide significant protection to estuary-associated species is the designation of strategically-placed Estuarine Protected Areas (EPAs) which could either be extensions of existing MPAs [thus creating larger Marine and Estuarine Protected Areas (MEPAs)], or stand-alone EPAs in biologically or ecologically significant estuaries. EPAs have been described as the missing armour for the conservation of estuarine-associated species (Whitfield et al., 2020), and could be the key to increased catches of important vulnerable fishery species (including dusky kob, spotted grunter and white steenbras) for multiple fishing sectors along the majority of the South African coastline. Without this last line of conservation defence, catches will continue to decline (of already collapsed stocks) which will be detrimental to the people whose livelihoods and food security relies on these resources and have significant economic impacts on the recreational fishing sector (Whitfield et al., 2020).
Dusky kob, a large estuary-dependent piscivorous sciaenid, is arguably the most iconic recreational fishery species in South Africa, with the landing of larger individuals being particularly coveted. The South African population was declared collapsed more than two decades ago, with adult spawning biomass estimated to be between 1 and 4.5% of pristine levels (Griffiths, 1997), showing no signs of recovery since then (Kerwath, 2020). Despite the poor state of the population, one last ecological stronghold of large adults remains - the Breede Estuary and its adjacent coastal habitats in the Western Cape. Large adults are critical for maintaining populations and are crucial for the recovery of stocks. This requires improved conservation and management strategies, specifically aimed at rebuilding the stock. In order to protect these large adults, a night-time fishing ban on the Breede Estuary was gazetted in 2013, and a slot limit for this species has been approved (not yet gazetted) which prohibits the retention of all dusky kob <50 cm total length (TL) and >110 cm TL. Despite these slightly improved management regulations, the population continues to decline, with up to 150 - 300 large adult dusky kob being removed from the water every year. As such, it is crucial to gain a better understanding of the movements of these large fish and the variables (both environmental and cyclical) driving them. This would provide invaluable information for the development of appropriate species-specific conservation measures. Since 2016, 81 dusky kob (comprising juveniles and adults) have been tagged in the Breede Estuary and the adjacent De Hoop MPA. The results of this ongoing study have revealed many new insights into the movement behaviour of this species, including (i) broad-scale longshore movements have been far more limited than previously thought, with these fish tagged in the Western Cape never being recorded further than 150 km from their tagging sites; (ii) the majority of dusky kob tagged in the De Hoop MPA have mostly been detected within the MPA boundaries, showing how important existing spatial management plans can be to threatened species; and (iii) those adults tagged in the Breede Estuary have displayed extremely high levels of philopatry, consistently returning to this estuary every year. The predictability and restrictive nature of their movements, along with the high fishing pressure experienced in the estuary, has significant implications for this species, highlighting the necessity for more stringent management if this stock is to be sustained. While the estuary management plan for the Breede Estuary will be updated in 2025, these data will undoubtedly be extremely valuable in aiding the development of more appropriate estuary-specific management regulations for this severely threatened species.
4 ATAP and Benefit-Sharing Arrangements
There is great potential for acoustic telemetry to track animals over vast distances, and answer complex questions about animal movement behaviour across large spatial scales. However, this can only be effectively achieved with sufficient established infrastructure and the cooperation of scientists willing to share resources and data (Young et al., 2020; Reubens et al., 2021). Such efforts have been realised in large-scale collaborative telemetry networks around the world, where the necessary infrastructure includes both compatible hardware (i.e the acoustic telemetry receivers allowing transmitters to operate without hindrance within the network) (Harcourt et al., 2019) and centralised databases, as telemetry data are moving into the realm of ‘big data’ and there needs to be sufficient capacity to store, manage, access and share the large amounts of data generated (Nguyen et al., 2017). Often these collaborative networks are made up of nation-wide receivers provided through the network facilitator, as well as smaller local arrays of receivers that are provided by interested research partners (for e.g. non-profit organisations NPOs, university researchers and research institutes). The benefits of these collaborative networks are numerous, and at minimum include the opportunity for researchers to expand the spatial extent over which they can detect their tagged animals, as well as increasing opportunities for collaboration and access to additional tools and community knowledge (Young et al., 2020).
There have been published examples detailing how these collaborative receiver networks have provided distinct benefits to partners. For example, in the Australian IMOS network of receivers, bronze whaler Carcharhinus brachyurus and dusky C. obscurus sharks were detected significantly more often when including data recorded by nation-wide (IMOS) receivers compared to local array (non-IMOS) receivers, which provided important insights into inter-state movements that would have otherwise been under-represented or undetected (Huveneers et al., 2021). Huisman et al. (2016) observed European silver eel Anguilla anguilla tagged in catchments from three different European countries moving to the Dutch-Belgian coastal zone, which would have remained undetected if receivers were not present in the coastal zone of each country and data had not been shared. Through the collaborative efforts of several acoustic telemetry networks deployed in the US (including iTAG, FACT and ACT networks), stretching from North Carolina to Florida (approximately 2000 km), Griffin et al. (2018) were able to characterise the spatial ecology of Atlantic tarpon Megalops atlanticus, including migratory connectivity. Using the same US networks, DeGroot et al. (2021) was able to characterise intra-specific variability in migration patterns of white-spotted eagle rays Aetobatus narinari, which has important implications for population structuring and management efforts.
When considering South Africa’s current ATAP network, it comprises receivers belonging to multiple institutions, NPOs and individual researchers in addition to the receivers provided through the platform itself (Table 2). Prior to the formalisation of the ATAP in 2011, three smaller local acoustic receiver arrays were already operational; namely in False Bay, Gansbaai and Algoa Bay. In partnering with Canada’s OTN, which saw a large investment in telemetry infrastructure in the country, as well a substantial capital equipment grant from South Africa’s National Research Foundation, a nationwide backbone of receivers could then be deployed by the platform. Since then, several other smaller local receiver arrays were established by partners to fill in the gaps between ATAP receivers (Figure 6). Over the past decade, at least 216 partner receivers have been deployed along the South African coastline and of those, 61.1% (n = 132) remain active at the time of writing.
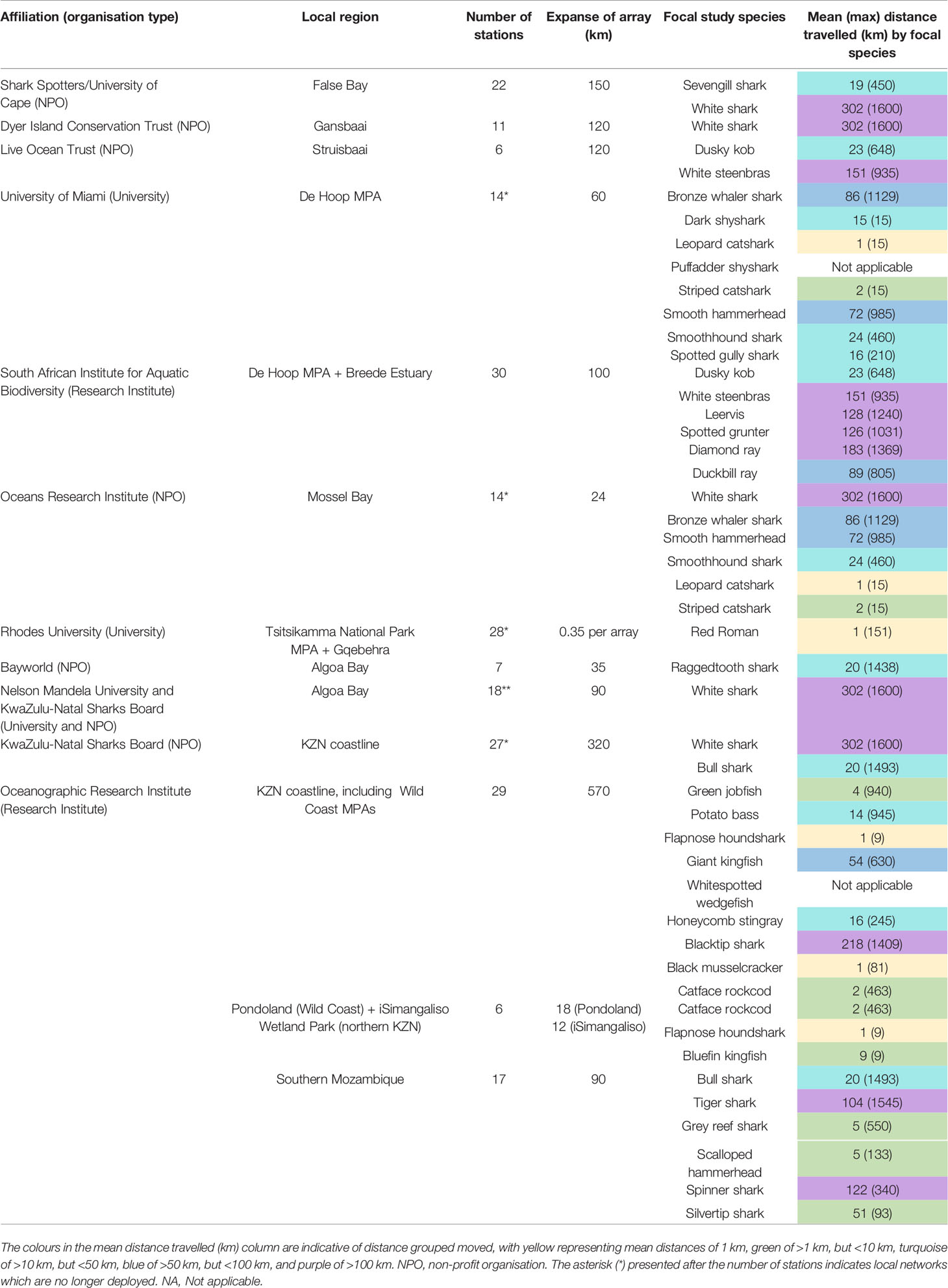
Table 2 Organisational names (and type) of ATAP collaborators who run their own smaller local networks along the South African coastline, details of the networks, and the focal study species for which the network was initially established.
The benefits of this equipment and data sharing are two-fold: they increase the spatial coverage of the overall ATAP network providing a benefit to the platform and in return, the local receiver owners/researchers have access to the telemetry data collected on all of the receivers within the network (for their tagged animals) as opposed to only data collected by their respective receivers. Empirically, and as seen in other similar networks, this collaboration and data sharing within the ATAP network has resulted in significant increases in the data richness collected at larger spatial scales. Inherently, the vast majority of local partner receiver arrays are small-scale in nature, with only half of localised arrays having receivers covering >100 km of coastline (maximum straight-line distance), while only 20% of partner arrays had receivers covering >250 km of the coastline (Table 2). These spatial scales over which local receiver arrays were deployed were compared to the mean and maximum distances that all tagged species travelled in coastal waters during the 10 years of ATAP’s detection collection (this excluded estuarine detections). This revealed that a significant number of animals often travelled further distances from their tagging locations. Specifically, 10.8% (n = 781 345) of all detections, from 34 of the 48 tagged species, occurred further than 100 km from an individual’s tagging location and 5.7% (n = 412 647) of all detections, spanning 29 of the 48 tagged species, occurred further than 250 km from an individual’s tagging location. Additionally, for 76% of local receiver arrays, the focal species that receiver owners concentrated tagging effort on displayed greater average distances travelled (from their tagging locations) compared to the spatial extent of their local array (Table 2). Finally, while only one localised array extended further than 500 km (Table 2), 18 species travelled further than 500 km (Figure 7). These insights highlight the extra data that the ATAP, a large collaborative network of receivers, has collected when compared to small localised arrays of receivers. It is important to understand the large-scale, longshore movements that animals make to gain a better understanding of migratory behaviours, habitat connectivity, and to what extent spatial protection measures may be suitable for them.
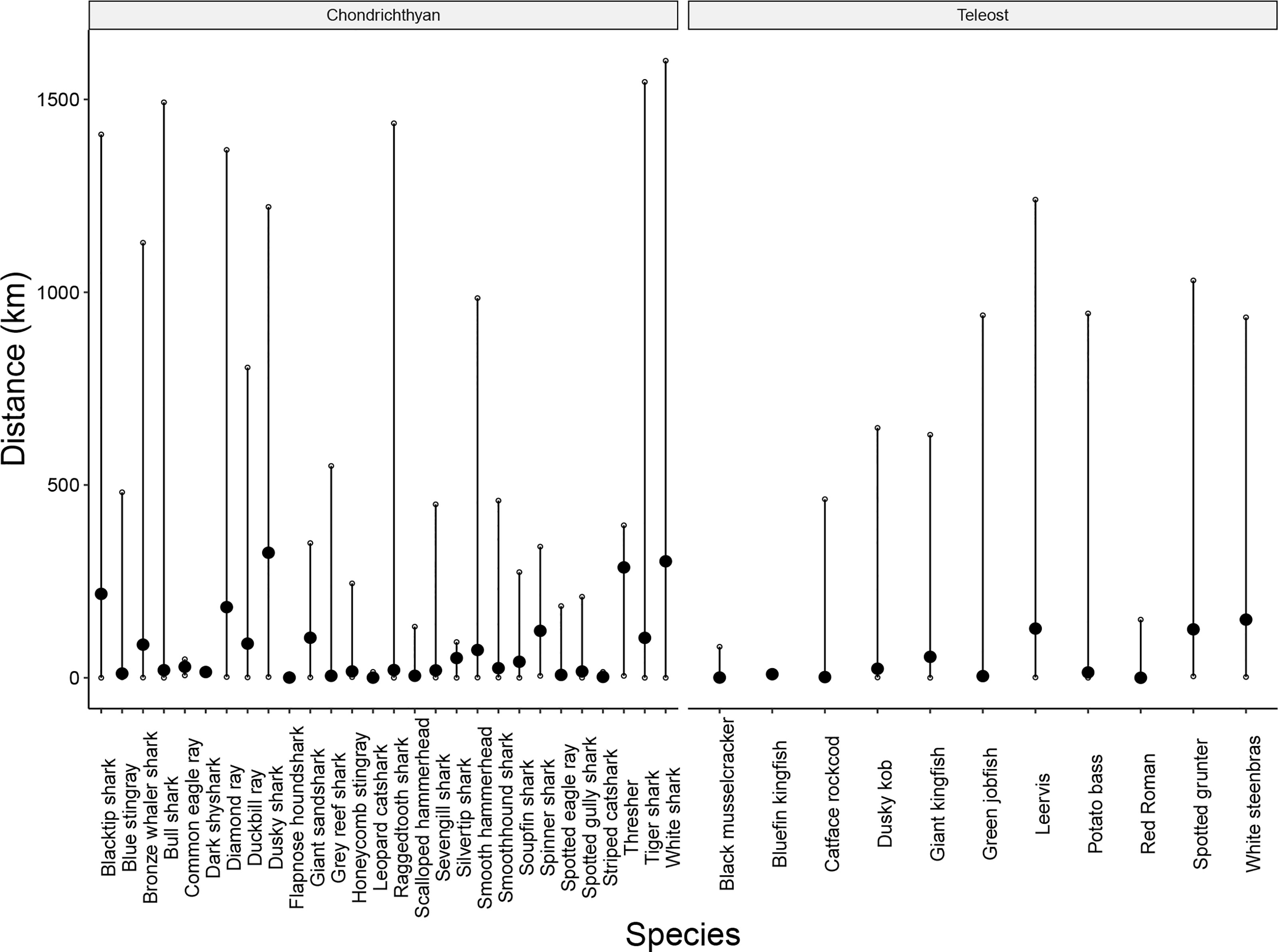
Figure 7 Average distance moved from tagging location (black circle, with confidence intervals indicating minimum and maximum distances) per species monitored by the ATAP between 2011 and 2021. Only chondrichthyans and teleosts were considered for this analysis.
5 Stakeholder Engagement and Development
Acoustic telemetry results can potentially impact society, particularly in relation to changes in existing management strategies e.g. declaration of MPAs or EPAs in popular fishing areas, or changes to existing traditional management measures, such as a reduction in both size and bag limits (Nguyen et al., 2019). Additionally, a significant portion of receivers in the greater network, along with the transmitters with which animals have been tagged, have been funded by government capital equipment grants, which is ultimately public money. As such, open and transparent communication with the public is crucial not only for buy-in on new or amended management interventions, but also for the public to build an active interest in the resources on which people rely for livelihoods and/or enjoyment and sport. Indeed, Cooke et al. (2016) highlighted several case studies where conservation of targeted species was a success due to active engagement by the angling community, including catch-and-release angling for Atlantic bluefin tuna Thunnus thynnus as an alternative revenue stream for displaced commercial fishers (Stokesbury et al., 2011), and increased revenue of sharks through tourism initiatives as opposed to consumptive use, including non-harvest angling (Gallagher et al., 2012).
The ATAP has always made an attempt to actively engage with the public using numerous avenues, which include participation in science festivals (e.g. SciFest - Africa’s largest school science festival), public presentations at angling clubs, and informal lectures to schools or universities, and with the scientific community via local and international conference presentations and platform user workshops. Given the enormous developments in online social media platforms over the past decade (Kumar and Nanda, 2019), and the subsequent exponential uptake of these platforms by the public, and the increasingly important role these platforms play in research and education (Zimba and Gasparyan, 2021), the next logical step was to start engaging in the virtual realm. As such, the ATAP now manages accounts on three social media platforms including Facebook (ATAP - Tracking fish movements), Twitter (@ATAP_ZA) and Instagram (@atap_za), and through regular efforts in content creation, there has been a steady increase in the number of followers per platform, ranging from >500 followers on Instagram, to almost 2,000 followers on Facebook.
Capacity building and skills development are important for enriching knowledge, expertise, and skill (Findlay and Bohler-Muller, 2018). However, many developing countries, including South Africa, face challenges associated with basic scientific capacity (Hassan, 2007; Miloslavich et al., 2019). Over the past five years, the ATAP has aimed to develop human capacity by running Summer Schools – a 4-day fieldtrip targeting undergraduate students hoping to pursue postgraduate degrees in the aquatic sciences. The emphasis of these Summer Schools is on training students from historically disadvantaged institutions – a user group severely under-represented in the biological science field, and involving female researchers, addressing the United Nations’ Sustainable Development Goals 4: Quality education and 5: Gender equality. Although the COVID-19 global pandemic has hampered the running of this school in recent times, the first three schools were successful, with uptake of postgraduate projects across all years.
6 Future Directions
The current ATAP network has an unmistakeable lack of listening power along the west coast of the country (Figure 1), with the closest station to the South African-Namibian border in the Berg Estuary. While good working terms exist between South African and Mozambican researchers, the lack of reliable deployment collaborators, as well as a general lack of research capacity, has seen no receivers being deployed further north, or into southern Namibia. However, the ATAP and the Namibian Rays and Sharks (NaRaS) Project have started a tentative collaboration, where the ATAP (at this stage) is acting in an advisory position, with both parties already committing to data sharing. NaRaS will, at a later stage, deploy their own transmitters on species which occur in both countries, including white sharks, sevengill sharks, spotted gully sharks, bronze whaler sharks and blue stingrays. The transmitters of species tagged in False Bay whose populations occupy both South African and Namibian waters are beginning to, or have expired (e.g. white sharks and sevengill sharks). However, other important mesopredators, including bronze whaler sharks, which have been acoustically tagged, are genetically indistinct between the two countries (Benavides et al., 2011), and have been recorded making transboundary movements, will still be monitored for approximately four years. This collaboration will see not only an increase in listening power along the west coast, but will also increase our understanding of the transboundary connectivity of numerous chondrichthyan and teleost species.
Climate change is anticipated to create a more volatile ocean, with, amongst other things, increases in marine heat waves (Frölicher and Laufkötter, 2018), a relative increase in ocean acidification (Kapsenberg and Cyronak, 2019), and an increase in ocean temperature, which alone is recognised as one of the biggest threats to marine ecosystems (Freer et al., 2018). Therefore, understanding how certain environmental variables may change is crucial to understanding how aquatic animals may, in turn, respond. Water temperature is arguably the most important abiotic variable influencing the movements of aquatic ecotherms such as fish (Little et al., 2020). In species that remain resident, sudden and extreme fluctuations in temperature can have devastating effects, resulting in fish kills (Durham et al., 2006; Stauffer et al., 2012). In mobile species, there is more room for adaption, where an animal can use movement as a coping mechanism to extreme fluctuations (Heupel and Simpfendorfer, 2008). Due to the close relationship between water temperature and fish movement, the importance of recording this abiotic variable is becoming increasingly evident. The ATAP has had temperature loggers (U22-001, Onset®, Cape Cod, Massachusetts, USA) in place at selected sites since its formalisation in 2011. As such, this network of temperature data, most of which has been recorded at a depth of 30 m, is becoming an invaluable source of information, which can be linked to a suite of studies, including movement assessments in relation to temperature, and ocean modelling. Moving forwards, the ATAP aims to maintain this nationwide temperature network, and is also hoping to expand recording power to a depth of 100 m (with temperature loggers being moored using acoustic receivers, model VR2AR, Innovasea), which will not only increase our understanding of the temperature of these more offshore regions, but will also provide new insights into the relative importance of offshore regions to inshore coastal species.
7 Conclusion
Despite some of Africa’s current failures to sustainably develop and use its rich resources, it is a place of hope and potential. The core values of ocean stewardship, knowledge generation around resources and the altruistic care of those resources for long-term benefit, are vital to see the potential of Africa’s Blue Economy realised. The ATAP represents a highly successful example of ocean stewardship in Africa. First and foremost, it is a platform for knowledge generation. The receivers of the ATAP situated in focal bays (eg, False Bay, Mossel Bay and Algoa Bay) allow researchers to gain insights into fine-scale habitat use and residency behaviours of tagged animals, while the linking receivers along the coastline allow for the determination of habitat connectivity, longshore movements, and migration patterns. Not only is this ecological knowledge valuable in and of itself, but it has direct and applied management and conservation benefits and, as outlined in the case studies, the information collected through the ATAP is currently being used in management planning of marine resources in South Africa. Furthermore, the ATAP has successful benefit-sharing arrangements in place with multiple NPOs, research institutions and universities in South Africa who provide additional acoustic receivers at certain locations along the coastline. The ATAP benefits from further receivers being in the water, while the partners benefit from having data collected on a much larger scale. These arrangements are built on principles of voluntary data-sharing and long-term benefits, both of which are core-values of ocean stewardship (Barendse et al., 2016; Peçanha Enqvist et al., 2018). Finally, the ATAP is dedicated to education, outreach and awareness through various platforms, to ensure the platform is as transparent and impactful as possible. Initiatives built on similar principles will help Africa to rise to its challenges, to understand its rich resources, and the best ways in which to sustainably develop and use those resources for important long-term socio-economic benefits.
Data Availability Statement
The datasets created and/or analyzed during the current study are unavailable due to various data-sharing agreements in place.
Author Contributions
TM, CE, and PC conceived the study. TM and CE analysed the data and wrote the manuscript. MP and JF contributed significantly to the fieldwork and data management, and all authors edited and approved the manuscript.
Funding
Capital equipment funding was provided by the Ocean Tracking Network and National Research Foundation and the Department of Science and Innovation-Shallow Marine and Coastal Research Infrastructure (DSI-SMCRI) programme, with running expenses funded by the African Coelacanth Ecosystem Programme and the Save Our Seas Foundation (Keystone Grant 227). Funding for CE and JF was provided by the NRF’s Postdoctoral Professional Development Programme, and funding for research in the Breede Estuary as part of JF’s postdoctoral study was provided by the SANOCEAN Programme (project number: 287015).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The Ocean Tracking Network (Canada) and South Africa’s National Research Foundation are thanked for the provision of capital equipment. The Save Our Seas Foundation and the African Coelacanth Ecosystem Programme (ACEP) of the National Research Foundation South African Institute for Aquatic Biodiversity (NRF-SAIAB) are thanked for funding to service and maintain the equipment. Logistical support in the form of manpower, access to boats, and provision of mooring anchors is provided by the South African Environmental Observation Network (SAEON) Elwandle Node, ACEP and the Acoustic Tracking Array Platform (ATAP) stakeholders at each of the deployment sites, which include Shark Spotters, South African National Parks, South African Shark Conservancy, Dyer Island Conservation Trust, Marine Dynamics, Live Ocean Trust, CapeNature, Oceans Research Institute, SAEON, Rhodes University, Eastern Cape Parks and Tourism Agency, and Oceanographic Research Institute. ATAP data provided for the chondrichthyan case study falls within the greater ‘Shark and Ray Project’ funded by Shark Conservation Fund and implemented by the WILDTRUST, through its WILDOCEANS programme, and the NRF-SAIAB. Data collected for the teleost case is part of the greater SANOCEAN project ‘Benchmarking knowledge-based adaptive management of estuarine fisheries in South Africa for a sustainable development’, project number: 287015.
References
Abecasis D., Afonso P., Erzini K. (2014). Combining Multispecies Home Range and Distribution Models Aids Assessment of MPA Effectiveness. Mar. Ecol. Prog. Ser. 513, 155–169. doi: 10.3354/meps10987
Abecasis D., Steckenreuter A., Reubens J., Aarestrup K., Alós J., Badalamenti F., et al. (2018). A Review of Acoustic Telemetry in Europe and the Need for a Regional Aquatic Telemetry Network. Anim. Biotelem 6, 1–7. doi: 10.1186/s40317-018-0156-0
Afonso P., Fontes J., Holland K. N., Santos R. S. (2009). Multi-Scale Patterns of Habitat Use in a Highly Mobile Reef Fish, the White Trevally Pseudocaranx Dentex, and Their Implications for Marine Reserve Design. Mar. Ecol. Prog. Ser. 381, 273–286. doi: 10.3354/meps07946
Albano P. S., Fallows C., Fallows M., Schuitema O., Bernard A. T., Sedgwick O., et al. (2021). Successful Parks for Sharks: No-Take Marine Reserve Provides Conservation Benefits to Endemic and Threatened Sharks Off South Africa. Biol. Conserv. 261, 109302. doi: 10.1016/j.biocon.2021.109302
Alós J., March D., Palmer M., Grau A., Morales-Nin B. (2011). Spatial and Temporal Patterns in Serranus Cabrilla Habitat Use in the NW Mediterranean Revealed by Acoustic Telemetry. Marine Ecol. Prog. Ser. 427, 173–186. doi: 10.3354/meps09042
Aspillaga E., Bartumeus F., Linares C., Starr R. M., López-Sanz À., Díaz D., et al. (2016). Ordinary and Extraordinary Movement Behaviour of Small Resident Fish Within a Mediterranean Marine Protected Area. PloS One 11, 1–19. doi: 10.1371/journal.pone.0159813
Attwood C. G., Mann B. Q., Beaumont J., Harris J. M. (1997). Review of the State of Marine Protectes Areas in South Africa. South Afr. J. Mar. Sci. 14, 17131. doi: 10.2989/025776197784160910
Barendse J., Roux D., Currie B., Wilson N., Fabricius C. (2016). A Broader View of Stewardship to Achieve Conservation and Sustainability Goals in South Africa. South Afr. J. Sci. 112, 1–15. doi: 10.17159/sajs.2016/20150359
Battersby J., Watson V. (2018). Addressing Food Security in African Cities. Nat. Sustainability 1, 153–155. doi: 10.1038/s41893-018-0051-y
Baust S., Teh L., Harper S., Zeller D. (2015). South Africa's Marine Fisheries Catches (1950-2010). pp. 129-150. In: Le Manach F., Pauly D. (eds.) Fisheries catch reconstructions in the Western Indian Ocean, 1950-2010. Fisheries Centre Research Reports 23(2). Fisheries Centre, University of British Column.
Beaulieu N. F., Obers J., Goodall V., Lombard P. A., Harris J. (2022). A Systematic Conservation Plan for Sharks and Rays in South Africa. Tech. Rep. WILDOCEANS. 76
Belhabib D., Lam V. W., Cheung W. W. (2016). Overview of West African Fisheries Under Climate Change: Impacts, Vulnerabilities and Adaptive Responses of the Artisanal and Industrial Sectors. Mar. Policy 71, 15–28. doi: 10.1016/j.marpol.2016.05.009
Belhabib D., Sumaila U. R., Pauly D. (2015). Feeding the Poor: Contribution of West African Fisheries to Employment and Food Security. Ocean Coastal Manage. 111, 72–81. doi: 10.1016/j.ocecoaman.2015.04.010
Benavides M. T., Feldheim K. A., Duffy C. A., Wintner S., Braccini J. M., Boomer J., et al. (2011). Phylogeography of the Copper Shark (Carcharhinus Brachyurus) in the Southern Hemisphere: Implications for the Conservation of a Coastal Apex Predator. Mar. Freshw. Res. 62, 861–869.
Bennett R. H., Childs A. R., Cowley P. D., Næsje T. F., Thorstad E. B., Økland F. (2011). First Assessment of Estuarine Space Use and Home Range of Juvenile White Steenbras, Lithognathus Lithognathus. Afr. Zool. 46, 32–38. doi: 10.3377/004.046.0115
Bennett R. H., Cowley P. D., Childs A. R., Næsje T. F. (2015). Movements and Residency of Juvenile White Steenbras Lithognathus Lithognathus in a Range of Contrasting Estuaries. Estuarine Coastal Shelf Sci. 152, 100–108. doi: 10.1016/j.ecss.2014.11.015
Bennett R. H., Cowley P. D., Childs A. R., Whitfield A. K. (2012). Area-Use Patterns and Diel Movements of White Steenbras Lithognathus Lithognathus in a Temporarily Open/Closed South African Estuary, Inferred From Acoustic Telemetry and Long-Term Seine-Netting Data. Afr. J. Mar. Sci. 34, 81–91. doi: 10.2989/1814232X.2012.673287
Bennett N. J., Dearden P. (2014). From Measuring Outcomes to Providing Inputs: Governance, Management, and Local Development for More Effective Marine Protected Areas. Mar. Policy 50, 96–110. doi: 10.1016/j.marpol.2014.05.005
Berkes F., Colding J., Folke C. (2008). Navigating Social-Ecological Systems: Building Resilience for Complexity and Change (Cambridge: Cambridge University Press).
Birkmanis C. A., Partridge J. C., Simmons L. W., Heupel M. R., Sequeira A. M. (2020). Shark Conservation Hindered by Lack of Habitat Protection. Global Ecol. Conserv. 21, e00862. doi: 10.1016/j.gecco.2019.e00862
Block B. A., Holbrook C. M., Simmons S. E., Holland K. N., Ault J. S., Costa D. P., et al. (2016). Toward a National Animal Telemetry Network for Aquatic Observations in the United States. Anim. Biotelem 4, 4–11. doi: 10.1186/s40317-015-0092-1
Bond M. E., Valentin-Albanese J., Babcock E. A., Abercrombie D., Lamb N. F., Miranda A., et al. (2017). Abundance and Size Structure of a Reef Shark Population Within a Marine Reserve has Remained Stable for More Than a Decade. Mar. Ecol. Prog. Ser. 576, 1–10. doi: 10.3354/meps12241
Chan C. Y., Tran N., Pethiyagoda S., Crissman C. C., Sulser T. B., Phillips M. J. (2019). Prospects and Challenges of Fish for Food Security in Africa. Global Food Secur. 20, 17–25. doi: 10.1016/j.gfs.2018.12.002
Childs A. R., Cowley P. D., Næsje T. F., Bennett R. H. (2015). Habitat Connectivity and Intra-Population Structure of an Estuary-Dependent Fishery Species. Marine Ecol. Prog. Ser. 537, 233–245. doi: 10.3354/meps11456
Childs A. R., Cowley P. D., Næsje T. F., Booth A. J., Potts W. M., Thorstad E. B., et al. (2008a). Do Environmental Factors Influence the Movement of Estuarine Fish? A Case Study Using Acoustic Telemetry. Estuarine Coastal Shelf Sci. 78, 227–236. doi: 10.1016/j.ecss.2007.12.003
Childs A. R., Cowley P. D., Næsje T. F., Booth A. J., Potts W. M., Thorstad E. B., et al. (2008b). Estuarine Use by Spotted Grunter Pomadasys Commersonnii in a South African Estuary, as Determined by Acoustic Telemetry. Afr. J. Mar. Sci. 30, 123–132. doi: 10.2989/AJMS.2008.30.1.12.462
Cooke S. J., Hogan Z. S., Butcher P. A., Stokesbury M. J., Raghavan R., Gallaghe A. J., et al. (2016). Angling for Endangered Fish: Conservation Problem or Conservation Action? Fish. Fisheries 17, 249–265. doi: 10.1111/faf.12076
Cowley P. D., Bennett R. H., Childs A. R., Murray T. S. (2017). Reflection on the First Five Years of South Africa’s Acoustic Tracking Array Platform (ATAP): Status, Challenges and Opportunities. Afr. J. Marine Sci. 39, 363–372. doi: 10.2989/1814232X.2017.1399927
Cowley P. D., Childs A. R., Bennett H. R. (2013). The Trouble With Estuarine Fisheries in Temperate South Africa, Illustrated by a Case Study on the Sundays Estuary. Afr. J. Mar. Sci. 35, 117–128. doi: 10.2989/1814232X.2013.789079
Daly R., Filmalter J. D., Daly C. A., Bennett R. H., Pereira M. A., Mann B. Q., et al. (2019). Acoustic Telemetry Reveals Multi-Seasonal Spatiotemporal Dynamics of a Giant Trevally Caranx Ignobilis Aggregation. Mar. Ecol. Prog. Ser. 621, 185–197. doi: 10.3354/meps12975
Daly R., Smale M. J., Cowley P. D., Froneman P. W. (2014). Residency Patterns and Migration Dynamics of Adult Bull Sharks (Carcharhinus Leucas) on the East Coast of Southern Africa. PloS One 9, (10), e109357. doi: 10.1371/journal.pone.0109357
Dames M. H., Cowley P. D., Childs A. R., Bennett R. H., Thorstad E. B., Næsje T. F. (2017). Estuarine and Coastal Connectivity of an Estuarine-Dependent Fishery Species, Pomadasys Commersonnii (Haemulidae). Afr. J. Mar. Sci. 39, 111–120. doi: 10.2989/1814232X.2017.1305991
da Silva C., Booth A. J., Dudley S. F. J., Kerwath S. E., Lamberth S. J., Leslie R. W., et al. (2015). The Current Status and Management of South Africa’s Chondrichthyan Fisheries. Afr. J. Mar. Sci. 2338, 232–248. doi: 10.2989/1814232X.2015.1044471
Davidson L. N., Dulvy N. K. (2017). Global Marine Protected Areas to Prevent Extinctions. Nat. Ecol. Evol. 1, 1–6. doi: 10.1038/s41559-016-0040
Decker C., Griffiths C., Prochazka K., Ras C., Whitfield A. (2003). “Marine Biodiversity in Sub-Saharan Africa: The Known and the Unknown Edited by,” in Mar. Biodiversity in Sub-Saharan Africa: The Known and the Unknown (Cape Town: Proceedings of the Marine Biodiversity in Sub-Saharan Africa), 284–312.
DEFF (Department of Environment, Forestry and Fisheries) (2020). Status of the South African Marine Fishery Resources and Status of the South African marine fishery resources 2020. Tech. Rep. Department Environment Forestry Fisheries. Cape Town: DEFF. 132 https://www.dffe.gov.za/sites/default/files/docs/publications/statusofsouthafrican_marinefisheryresources2020.pdf.
DeGroot B. C., Bassos-Hull K., Wilkinson K. A., Lowerre-Barbieri S., Poulakis G. R., Ajemian M. J. (2021). Variable Migration Patterns of Whitespotted Eagle Rays Aetobatus Narinari Along Florida’s Coastlines. Marine Biol. 168:21. doi: 10.1007/s00227-021-03821-2
Dixon (2022). Movement Patterns of the Iconic Giant Kingfish Caranx Ignobilis From Southern Africa. Msc Rhodes Univ. 171
Dulvy N. K., Fowler S. L., Musick J. A., Cavanagh R. D., Kyne P. M., Harrison L. R., et al. (2014). Extinction Risk and Conservation of the World’s Sharks and Rays. eLife 3, 1–35. doi: 10.7554/eLife.00590
Durham B. W., Wilde G. R., Pope K. L. (2006). Temperature-Caused Fish Kill in a Flowing Great Plains River. Southwestern Nat. 51, 397–401.
Ebert D., van Hees K. (2015). Beyond Jaws: Rediscovering the ‘Lost Sharks’ of Southern Africa. Afr. J. Mar. Sci. 37, 141–156. doi: 10.2989/1814232X.2015.1048730
Ebert D. A., Wintner S. P., Kyne P. M. (2021). An Annotated Checklist of the Chondrichthyans of South Africa. Zootaxa 4947, 1–127. doi: 10.11646/zootaxa.4947.1.1
Elston C., Cowley P. D., Brandis R. G. V., Lea J. (2021). Residency and Habitat Use Patterns by Sympatric Stingrays at a Remote Atoll in the Western Indian Ocean. Mar. Ecol. Prog. Ser. 662, 97–114. doi: 10.3354/meps13632
Engelbrecht T., Kock A., Waries S., O’Riain M. J. (2017). Shark Spotters: Successfully Reducing Spatial Overlap Between White Sharks (Carcharodon Carcharias) and Recreational Water Users in False Bay, South Africa. PloS One 12, 1–15. doi: 10.1371/journal.pone.0185335
Failler P., Karani P., Gilau A. M., Hamukuaya H., Diop S. (2020). Africa Blue Economy Strategy – Implementation Plan 2021-2025 (Kenya: The African Union Inter-African Bureau for Animal Resources).
Findlay K. (2020). Challenges Facing Marine Protected Areas in Southern African Countries in Light of Expanding Ocean Economies Across the Sub-Region. in. ‘Mar. Protected Areas: Science, Policy and Management. Editors: Humphreys John, Robert W.E., Clark. doi: 10.1016/C2017-0-02525-9 ISBN: 978-0-08-102698-4 Elsevier
Findlay K., Bohler-Muller N. (2018). South Africa's ocean economy and Operation Phakisa: lessons learned. In: : Attri V.N., Bohler-Muller N. (eds.), The Blue Economy Handbook of the Indian Ocean Region vol. 231 Humphreys J, Clark RWEElsevier.
Freer J. J., Partridge J. C., Tarling G. A., Collins M. A., Genner M. J. (2018). Predicting Ecological Responses in a Changing Ocean: The Effects of Future Climate Uncertainty. Mar. Biol. 165, 1–18. doi: 10.1007/s00227-017-3239-1
Frölicher T. L., Laufkötter C. (2018). Emerging Risks From Marine Heat Waves. Nat. Commun. 9, 2015–2018. doi: 10.1038/s41467-018-03163-6
Gallagher A. J., Kyne P. M., Hammerschlag N. (2012). Ecological Risk Assessment and its Application to Elasmobranch Conservation and Management. J. Fish. Biol. 80, 1727–1748. doi: 10.1111/j.1095-8649.2012.03235.x
Goetze J. S., Fullwood L. A. (2013). Fiji’s Largest Marine Reserve Benefits Reef Sharks. Coral Reefs 32, 121–125. doi: 10.1007/s00338-012-0970-4
Griffin L. P., Brownscombe J. W., Adams A. J., Boucek R. E., Finn J. T., Heithaus M. R., et al. (2018). Keeping Up With the Silver King: Using Cooperative Acoustic Telemetry Networks to Quantify the Movements of Atlantic Tarpon (Megalops Atlanticus) in the Coastal Waters of the Southeastern United States. Fish. Res. 205, 65–76. doi: 10.1016/j.fishres.2018.04.008
Griffiths M. H. (1997). Management of South African Dusky Kob Argyrosomus Japonicus (Sciaenidae) Based on Per-Recruit Models. South Afr. J. Mar. Sci. 18, 213–228.
Griffiths C. L., Robinson T. B. (2016). Use and Usefulness of Measures of Marine Endemicity in South Africa. South Afr. J. Sci. Vol. 112, 1–7. doi: 10.17159/sajs.2016/20150249
Hansen W. D. (2014). Generalizable Principles for Ecosystem Stewardship-Based Management of Social-Ecological Systems: Lessons Learned From Alaska. Ecol. Soc. 19(4), 13. doi: 10.5751/ES-06907-190413
Harcourt R., Sequeira A. M., Zhang X., Roquet F., Komatsu K., Heupel M., et al. (2019). Animal-Borne Telemetry: An Integral Component of the Ocean Observing Toolkit. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00326
Harrison T. D. (2003). Biogeography and Community Structure of Fishes in South African Estuaries. Ph.D. Thesis (Makhanda, South Africa: Rhodes University).
Hassan M. H. (2007). Building Capacity in the Life Sciences in the Developing World. Cell 131, 433–436. doi: 10.1016/j.cell.2007.10.020
Heupel M. R., Simpfendorfer C. A. (2008). Movement and Distribution of Young Bull Sharks Carcharhinus Leucas in a Variable Estuarine Environment. Aquat. Biol. 1, 277–289. doi: 10.3354/ab00030
Heupel M., Simpfendorfer C., Lowe C. (2005). Passive Acoustic Telemetry Technology: Current Applications and Future Directions. Results VR2 Workshop Held 98.
Hoenner X., Huveneers C., Steckenreuter A., Simpfendorfer C., Tattersall K., Jaine F., et al. (2018). Data Descriptor: Australia’s Continental-Scale Acoustic Tracking Database and its Automated Quality Control Process. Sci. Data 5, 1–10. doi: 10.1038/sdata.2017.206
Huisman J., Verhelst P., Deneudt K., Goethals P., Moens T., Nagelkerke L. A., et al. (2016). Heading South or North: Novel Insights on European Silver Eel Anguilla Anguilla Migration in the North Sea. Mar. Ecol. Prog. Ser. 554, 257–262. doi: 10.3354/meps11797
Hussey N. E., Kessel S. T., Aarestrup K., Cooke S. J., Cowley P. D., Fisk A. T., et al. (2015). Aquatic Animal Telemetry: A Panoramic Window Into the Underwater World. Science 348, 1255642. doi: 10.1126/science.1255642
Huveneers C., Niella Y., Drew M., McAuley R., Butcher P., Peddemors V., et al. (2021). Continental-Scale Network Reveals Cross-Jurisdictional Movements of Sympatric Sharks With Implications for Assessment and Management. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.697175
Jacoby D. M., Ferretti F., Freeman R., Carlisle A. B., Chapple T. K., Curnick D. J., et al. (2020). Shark Movement Strategies Influence Poaching Risk and can Guide Enforcement Decisions in a Large, Remote Marine Protected Area. J. Appl. Ecol., 57(9): 1782–1792. doi: 10.1111/1365-2664.13654
Kapsenberg L., Cyronak T. (2019). Ocean Acidification Refugia in Variable Environments. Global Change Biol. 25, 3201–3214. doi: 10.1111/gcb.14730
Kerwath S. E., Winker H., Parker D., da Silva C., Attwood C.G. (2020). An Introduction to the Rich Methodology to Assess Data-Poor South African Linefishes,” in 5th Southern African Marine Linefish Symposium (Makhanda: Rhodes University). eds. Murray T.S., Duncan M.I., Winkler A.C., Childs A-R., Mann B.Q., Potts W.M. 113 pp. doi: 10.5281/zenodo.3959750
Kerwath S. E., Götz A., Attwood C. G., Sauer W. H., Wilke C. G. (2007). Area Utilisation and Activity Patterns of Roman Chrysoblephus Laticeps (Sparidae) in a Small Marine Protected Area. Afr. J. Mar. Sci. 29, 259–270. doi: 10.2989/AJMS.2007.29.2.10.193
Kirkman S. P., Mann B. Q., Sink K. J., Adams R., Livingstone T. C., Mann-Lang J. B., et al. (2021). Evaluating the Evidence for Ecological Effectiveness of South Africa’s Marine Protected Areas. Afr. J. Mar. Sci. 43, 389–412. doi: 10.2989/1814232X.2021.1962975
Kock A., O’Riain M. J., Mauff K., Meÿer M., Kotze D., Griffiths C. (2013). Residency, Habitat Use and Sexual Segregation of White Sharks, Carcharodon Carcharias in False Bay, South Africa. PloS One 8, e55048. doi: 10.1371/journal.pone.0055048
Kock A. A., Photopoulou T., Durbach I., Mauff K., Meÿer M., Kotze D., et al. (2018). Summer at the Beach: Spatio-Temporal Patterns of White Shark Occurrence Along the Inshore Areas of False Bay, South Africa. Movement Ecol. 6, 1–13. doi: 10.1186/s40462-018-0125-5
Kumar V., Nanda P. (2019). Social Media as a Tool in Higher Education Handbook of research on Diverse teaching strategies for the technology-rich classroom. 239–253. Information Science Reference doi: 10.4018/978-1-7998-0238-9.ch016
Lallemand P., Bergh M., Hansen M., Purves M. (2016). Estimating the Economic Benefits of MSC Certification for the South African Hake Trawl Fishery. Fish. Res. 182, 98–115. doi: 10.1016/j.fishres.2016.02.003
Lamberth S. J., Turpie J. K. (2003). The Role of Estuaries in South African Fisheries: Economic Importance and Management Implications. Afr. J. Mar. Sci., 25 131–157. doi: 10.2989/18142320309504005
La Mesa G., Consalvo I., Annunziatellis A., Canese S. (2012). Movement Patterns of the Parrotfish Sparisoma Cretense in a Mediterranean Marine Protected Area. Mar. Environ. Res. 82, 59–68. doi: 10.1016/j.marenvres.2012.09.006
Lea J. S. E., Humphries N. E., von Brandis R. G., Clarke C. R., Sims D. W. (2016). Acoustic Telemetry and Network Analysis Reveal the Space Use of Multiple Reef Predators and Enhance Marine Protected Area Design. Proc. R. Soc. B.: Biol. Sci. 283, 20160717. doi: 10.1098/rspb.2016.0717
Lee K. H., Noh J., Khim J. S. (2020). ). The Blue Economy and the United Nations’ Sustainable Development Goals: Challenges and Opportunities. Environ. Int. 137, 105528. doi: 10.1016/j.envint.2020.105528
Lester S. E., Halpern B. S., Grorud-Colvert K., Lubchenco J., Ruttenberg B. I., Gaines S. D., et al. (2009). Biological Effects Within No-Take Marine Reserves: A Global Synthesis. Mar. Eco. 384, 33–46. doi: 10.3354/meps08029
Little A. G., Loughland I., Seebacher F. (2020). What do Warming Waters Mean for Fish Physiology and Fisheries? J. Fish. Biol. 97, 328–340. doi: 10.1111/jfb.14402
Lopes P. F., Pacheco S., Clauzet M., Silvano R. A., Begossi A. (2015). Fisheries, Tourism, and Marine Protected Areas: Conflicting or Synergistic Interactions? Ecosyst Serv. 16, 333–340. doi: 10.1016/j.ecoser.2014.12.003
Lowerre-Barbieri S. K., Kays R., Thorson J. T., Wikelski M. (2019). The Ocean’s Movescape: Fisheries Management in the Bio-Logging Decad-2028). ICES J. Mar. Sci. 76, 477–488. doi: 10.1093/icesjms/fsy211
Mace G. M., Collar N. J., Gaston K. J., Hilton-Taylor C., Akçakaya H. R., Leader-Williams N., et al. (2008). Quantification of Extinction Risk: IUCN’s System for Classifying Threatened Species. Conserv. Biol. 22, 1424–1442. doi: 10.1111/j.1523-1739.2008.01044.x
MacKeracher T., Diedrich A., Simpfendorfer C. A. (2019). Sharks, Rays and Marine Protected Areas: A Critical Evaluation of Current Perspectives. Fish. Fisheries 20, 255–267. doi: 10.1111/faf.12337
MacNeil M. A., Chapman D. D., Heupel M., Simpfendorfer C. A., Heithaus M., Meekan M., et al. (2020). Global Status and Conservation Potential of Reef Sharks. Nature 583, 801–806. doi: 10.1038/s41586-020-2519-y
Mann B. Q., Cowley P. D., Kyle R. (2016). Estimating the Optimum Size for Inshore No-Take Areas Based on Movement Patterns of Surf-Zone Fishes and Recommendations for Rezoning of a World Heritage Site in South Africa. Ocean Coastal Manage. 125, 8–19. doi: 10.1016/j.ocecoaman.2016.03.006
Marsh S. M., Hoffmann M., Burgess N. D., Brooks T. M., Challender D. W., Cremona P. J., et al. (2021). Prevalence of Sustainable and Unsustainable Use of Wild Species Inferred From the IUCN Red List of Threatened Species. Conserv. Biol., 36(2) e13844. doi: 10.1111/cobi.13844
McGowan J., Beger M., Lewison R. L., Harcourt R., Campbell H., Priest M., et al. (2016). Integrating Research Using Animal-Borne Telemetry With the Needs of Conservation Management. J. Appl. Ecol. 54, 423–429. doi: 10.1111/1365-2664.12755
Miloslavich P., Seeyave S., Muller-Karger F., Bax N., Ali E., Delgado C., et al. (2019). Challenges for Global Ocean Observation: The Need for Increased Human Capacity. J. Operation. Oceanogr. 12, S137–S156. doi: 10.1080/1755876X.2018.1526463
Muringai R. T., Mafongoya P. L., Lottering R. (2021). Climate Change and Variability Impacts on Sub-Saharan African Fisheries: A Review. Rev. Fisheries Sci. Aquacult. 29, 706–720. doi: 10.1080/23308249.2020.1867057
Murray T. S., Cowley P. D., Bennett R. H., Childs A.-R. (2018). Fish on the Move: Connectivity of an Estuary-Dependent Fishery Species Evaluated Using a Large-Scale Acoustic Telemetry Array. Can. J. Fisheries Aquat. Sci. 75, 2038–2052.
Næsje T. F., Childs A. R., Cowley P. D., Potts W. M., Thorstad E. B., Økland F. (2007). Movements of Undersized Spotted Grunter (Pomadasys Commersonnii) in the Great Fish Estuary, South Africa: Implications for Fisheries Management. Hydrobiologia 582, 25–34. doi: 10.1007/s10750-006-0563-8
Nguyen V. M., Brooks J. L., Young N., Lennox R. J., Haddaway N., Whoriskey F. G., et al. (2017). To Share or Not to Share in the Emerging Era of Big Data: Perspectives From Fish Telemetry Researchers on Data Sharing. Can. J. Fisheries Aquat. Sci. 74, 1260–1274. doi: 10.1139/cjfas-2016-0261
Nguyen V. M., Young N., Brownscombe J. W., Cooke S. J. (2019). Collaboration and Engagement Produce More Actionable Science: Quantitatively Analyzing Uptake of Fish Tracking Studies. Ecol. Appl. 29, 1316–1330. doi: 10.1002/eap.1943
Nguyen V. M., Young N., Cooke S. J. (2018). Applying a Knowledge–Action Framework for Navigating Barriers to Incorporating Telemetry Science Into Fisheries Management and Conservation: A Qualitative Study. Can. J. Fisheries Aquat. Sci. 75, 1733–1743. doi: 10.1139/cjfas-2017-0303
Novak A. J., Becker S. L., Finn J. T., Danylchuk A. J., Pollock C. G., Hillis-Starr Z., et al. (2020). Inferring Residency and Movement Patterns of Horse-Eye Jack Caranx Latus in Relation to a Caribbean Marine Protected Area Acoustic Telemetry Array. Anim. Biotelem 8, 1–13. doi: 10.1186/s40317-020-00199-8
Okafor-Yarwood I., Kadagi N. I., Miranda N. A., Uku J., Elegbede I. O., Adewumi I. J. (2020). The Blue Economy-Cultural Livelihood-Ecosystem Conservation Triangle: The African Experience. Front. Marine Sci. 7. doi: 10.3389/fmars.2020.00586
Peçanha Enqvist J., West S., Masterson V. A., Haider L. J., Svedin U., Tengö M. (2018). Stewardship as a Boundary Object for Sustainability Research: Linking Care, Knowledge and Agency. Landscape Urban Plann. 179, 17–37. doi: 10.1016/j.landurbplan.2018.07.005
Pradervand P., Baird D. (2002). Assessment of the Recreational Linefishery in Selected Eastern Cape Estuaries: Trends in Catches and Effort. South Afr. J. Mar. Sci. 24, 87–101.
Reubens J., Aarestrup K., Meyer C., Moore A., Økland F., Afonso P. (2021). Compatibility Acoustic Telemetry. Anim. Biotelem. 9, 4–9. doi: 10.1186/s40317-021-00253-z
Reubens J., Verhelst P., van der Knaap I., Wydooghe B., Milotic T., Deneudt K., et al. (2019). The Need for Aquatic Tracking Networks: The Permanent Belgian Acoustic Receiver Network. Anim. Biotelem 7, 1–6. doi: 10.1186/s40317-019-0164-8
Roberts M. J. (2010). Coastal Currents and Temperatures Along the Eastern Region of Algoa Bay, South Africa, With Implications for Transport and Shelf-Bay Water Exchange. Afr. J. Mar. Sci. 32, 145–161. doi: 10.2989/1814232X.2010.481153
Roberts C. M., Hawkins J. P., Gell F. R. (2005). The Role of Marine Reserves in Achieving Sustainable Fisheries. Philos. Trans. R. Soc. B.: Biol. Sci. 360, 123–132. doi: 10.1098/rstb.2004.1578
Sink K. (2016). The Marine Protected Areas Debate: Implications for the Proposed Phakisa Marine Protected Areas Network. South Afr. J. Sci. 112, 1–4. doi: 10.17159/sajs.2016/a0179
Smith-Godfrey S. (2016). Defining the Blue Economy. Maritime Affairs 12, 58–64. doi: 10.1080/09733159.2016.1175131
Speed C. W., Meekan M. G., Field I. C., McMahon C. R., Harcourt R. G., Stevens J. D., et al. (2016). Reef Shark Movements Relative to a Coastal Marine Protected Area. Reg. Stud. Mar. Sci. 3, 58–66. doi: 10.1016/j.rsma.2015.05.002
Stauffer B. A., Gellene A. G., Schnetzer A., Seubert E. L., Oberg C., Sukhatme G. S., et al. (2012). An Oceanographic, Meteorological, and Biological “Perfect Storm” Yields a Massive Fish Kill. Mar. Ecol. Prog. Ser. 468, 231–243. doi: 10.3354/meps09927
Steckenreuter A., Hoenner X., Huveneers C., Simpfendorfer C., Buscot M. J., Tattersall K., et al. (2017). Optimising the Design of Large-Scale Acoustic Telemetry Curtains. Mar. Freshw. Res. 68, 1403–1413. doi: 10.1071/MF16126
Stokesbury M. J., Neilson J. D., Susko E., Cooke S. J. (2011). Estimating Mortality of Atlantic Bluefin Tuna (Thunnus Thynnus) in an Experimental Recreational Catch-and-Release Fishery. Biol. Conserv. 144, 2684–2691. doi: 10.1016/j.biocon.2011.07.029
Temple A. J., Kiszka J. J., Stead S. M., Wambiji N., Brito A., Poonian C. N. S., et al. (2018). Marine Megafauna Interactions With Small-Scale Fisheries in the Southwestern Indian Ocean: A Review of Status and Challenges for Research and Management. Rev. Fish. Biol. Fisheries 28, 89–115. doi: 10.1007/s11160-017-9494-x
Turpie J. K., Beckley L. E., Katua S. M. (2000). Biogeography and the Selection of Priority Areas for Conservation of South African Coastal Fishes. Biol. Conserv. 92, 59–72. doi: 10.1016/S0006-3207(99)00063-4
van Zinnicq Bergmann M. P., Guttridge T. L., Smukall M. J., Adams V. M., Bond M. E., Burke P. J., et al. (2022). Using Movement Models and Systematic Conservation Planning to Inform Marine Protected Area Design for a Multi-Species Predator Community. Biol. Conserv. 266, 109469. doi: 10.1016/j.biocon.2022.109469
Voyer M., Quirk G., McIlgorm A., Azmi K. (2018). Shades of Blue: What do Competing Interpretations of the Blue Economy Mean for Oceans Governance? J. Environ. Policy Plann. 20, 595–616. doi: 10.1080/1523908X.2018.1473153
Wenhai L., Cusack C., Baker M., Tao W., Mingbao C., Paige K., et al. (2019). Successful Blue Economy Examples With an Emphasis on International Perspectives. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00261
Whitfield A. K., Attwood C. G., Cowley P. D., Lamberth S. J., Mann B. Q. (2020). No-Take Estuarine-Protected Areas: The Missing Armour for the Conservation of Fishes. Koedoe 62, 1–7. doi: 10.4102/koedoe.v62i1.1648
Young J. M., Bowers M. E., Reyier E. A., Morley D., Ault E. R., Pye J. D., et al. (2020). The FACT Network: Philosophy, Evolution, and Management of a Collaborative Coastal Tracking Network. Mar. Coastal Fisheries 12, 258–271. doi: 10.1002/mcf2.10100
Keywords: acoustic telemetry, conservation, movement behaviour, sustainable development, resource management, ocean optimism
Citation: Murray TS, Elston C, Parkinson MC, Filmalter JD and Cowley PD (2022) A Decade of South Africa’s Acoustic Tracking Array Platform: An Example of a Successful Ocean Stewardship Programme. Front. Mar. Sci. 9:886554. doi: 10.3389/fmars.2022.886554
Received: 28 February 2022; Accepted: 20 April 2022;
Published: 23 May 2022.
Edited by:
Jaco Barendse, Nelson Mandela University, South AfricaReviewed by:
David Abecasis, University of Algarve, PortugalJoy Young, Florida Fish and Wildlife Research Institute, United States
Copyright © 2022 Murray, Elston, Parkinson, Filmalter and Cowley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Taryn S. Murray, dHMubXVycmF5QHNhaWFiLm5yZi5hYy56YQ==
 Taryn S. Murray
Taryn S. Murray Chantel Elston
Chantel Elston Matthew C. Parkinson1,2
Matthew C. Parkinson1,2 John D. Filmalter
John D. Filmalter