- 1College of Ocean and Meteorology, Guangdong Ocean University, Zhanjiang, China
- 2Marine Environmental Monitoring Centre of Beihai, State Oceanic Administration, Beihai, China
- 3Key Laboratory of Climate, Resources and Environment in Continental Shelf Sea and Deep Sea of Department of Education of Guangdong Province, Guangdong Ocean University, Zhanjiang, China
- 4Laboratory for Coastal Ocean Variation and Disaster Prediction, College of Ocean and Meteorology, Guangdong Ocean University, Zhanjiang, China
- 5Key Laboratory of Marine Environmental Survey Technology and Application, Ministry of Natural Resources, Guangzhou, China
The eutrophication degree in the coastal bay has been increasing significantly for the past years, due to the increasing nutrient discharge. However, the factors controlling sources and nitrogen (N) cycling in the different types of bays are still poorly understood. In this comparative study, nitrate dual isotopes and ammonium nitrogen isotope , were used to determine the origin of nitrate in Qinzhou Bay (with riverine input) and Tieshangang Bay (without riverine input) in Beibu Gulf and to study biogeochemical processes associated with nitrogen cycling. The nutrient concentrations generally showed a decreased trend from the upper bay to the outer bay. The isotopic values in Tieshangang Bay were generally higher than that in the Qinzhou Bay, suggesting that there are differences in nitrate sources and transformation processes between the two bays. The dominant sources from manure and sewage (41%) and soil N (30%) from runoff input were responsible for the high nitrate observed in the upper Qinzhou Bay. Though manure and sewage (59%) were also dominant nitrate sources in the upper Tieshangang Bay, a decrease in source from soil N (20%) occurred due to less runoff input. Nutrients were retained in the upper Tieshangang Bay due to weak hydrodynamic conditions, which caused higher concentrations in the upper bay. Significant nitrate loss occurred in the outer Qinzhou Bay, which was related to the intense hydrodynamic condition. Moreover, phytoplankton assimilation mainly utilized due to sufficient in the outer Qinzhou Bay. In contrast, nitrate loss was also found in the outer Tieshangang Bay, which is mainly related to the phytoplankton assimilation due to the weak hydrodynamic condition. In addition, the greater enrichment of than during both bays suggests that atmospheric deposition also contributes to the nitrate pool in the water and the impact of atmospheric deposition on the whole Beibu Gulf is relatively consistent. By this comparative study, we found that different nitrate biogeochemical processes occurred in these two types of bays, which were mainly determined by the difference of human activities and hydrodynamic conditions.
Introduction
As a transition zone between ocean and land, coastal oceans receive anthropogenic nutrient inputs, which have significantly increased over the past decades (Gruber and Galloway, 2008; Sharples et al., 2017; Yan et al., 2017; Lao et al., 2019a; Chen et al., 2020). This has resulted in serious nutrient load in the coastal environment and has caused a host of environmental problems such as eutrophication, harmful algal blooms, and seasonal hypoxia in the coastal areas, which greatly impacts the original functions of coastal ecosystems and biogeochemical cycles (Carstensen and Conley, 2019; Gobler, 2020; Yu et al., 2020; Anderson et al., 2021; Lao et al., 2021a). In addition, nitrogen (N) is also a key element that regulates marine primary productivity and its availability influences the storage of anthropogenic carbon dioxide in coastal areas and climate change (Moore et al., 2013; Tremblay et al., 2015). Thus, tracing nitrogen sources and its cycles are very important to design effective management practices to protect coastal ecosystems.
Among many methods for studying N dynamics, stable nitrogen isotope (δ15N) of various N pools combined with oxygen isotope of nitrate , is considered a powerful tool to trace sources and biogeochemical processes of N. Generally, various sources of N can be distinguished by their different ranges of nitrogen and oxygen isotopic values (Kendall, 1998; Xue et al., 2009). For example, nitrate sources originating from sewage and manure are more enriched in δ15N (4-25‰) than fertilizer and atmospheric deposition due to the volatilization of 15N-depleted ammonia from human and animal waste (Kendall, 1998; Xue et al., 2009), while values from atmospheric deposition are generally high (>50‰) compared to those from other sources (<25‰) (Ye et al., 2016). In addition, the isotopic ratios can also reflect biological processes. For example, assimilation and denitrification cause a synchronous increase of δ15N and δ18O in the residual water as the lighter isotopes (14N and 16O) are preferentially utilized by microorganisms (Granger et al., 2004; Sigman et al., 2005). Therefore, a better understanding of sources and biogeochemical processes of N in the coastal ecosystem could be achieved by the distribution and variation of isotopic signatures of various N pools.
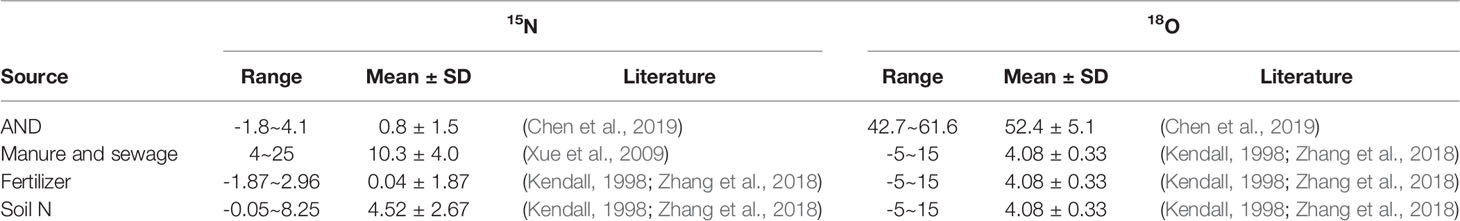
The Beibu Gulf located in the northwestern South China Sea (SCS), is a newly developing port, mariculture, and industrial area in south China. Its rich biology and high productivity make the gulf a key fishing ground and source of fisheries products in China (Liu et al., 2020; Lao et al., 2021b; Xu et al., 2021). However, the increasing population and intensification of industrial and agriculture activities around the coastal gulf for the past years have greatly increased nutrient loading to the gulf (Lao et al., 2020; Lao et al., 2021a; Lao et al., 2021c; Xu et al., 2021). This has resulted in an increase in the degree of eutrophication in the coastal gulf for the past years, particularly in the coastal bay of the Beibu Gulf (Lao et al., 2021a; Lao et al., 2021c). There are several rivers that flow into the Beibu Gulf (Figure 1), and the nutrient input into the gulf shows a different seasonal change between the rivers (Lao et al., 2020). However, due to the difference in river distribution (most of which are concentrated in the middle of the Beibu Gulf) and different depths, there are obvious differences in hydrodynamic conditions between the coastal bays of the Beibu Gulf. For example, there are many rivers around the Qinzhou Bay in the gulf (Figure 1), which carry a large amount of nutrients such as nitrogen and phosphorus into the bay every year, and thus causing high nutrient load and increasing the eutrophication in the bay (Lao et al., 2020; Lao et al., 2021a; Lao et al., 2021c). Different from Qinzhou Bay, Tieshangang Bay is a natural deep-water port in Guangxi Province and an important port area in the Beibu Gulf. There are no large rivers flowing to the Tieshanggang Bay, thus hydrodynamic conditions are weak and the water exchange is mainly affected by the tide (Jiang et al., 2017). In addition, due to the unique ecological resources and environment of freshwater and saltwater in Qinzhou Bay, the bay is an important aquaculture area in Guangxi Province and the largest natural oyster spawning and breeding area in China (Liu et al., 2020; Xu et al., 2020; Lao et al., 2021c). However, since there is less influence from freshwater input and weak hydrodynamic conditions in the Tieshangang Bay, the aquaculture scale is much smaller than that of Qinzhou Bay. But as an industrial area and port, the ecological environment of the Tieshangang Bay is significantly affected by human activities (Gan et al., 2013; Lao et al., 2019b; Lao et al., 2021d). Thus, with such great differences in hydrological conditions, their nutrient sources are different between the two bays in the Beibu Gulf. However, the factors controlling sources and nitrogen cycling in the different types of bays are still poorly understood.
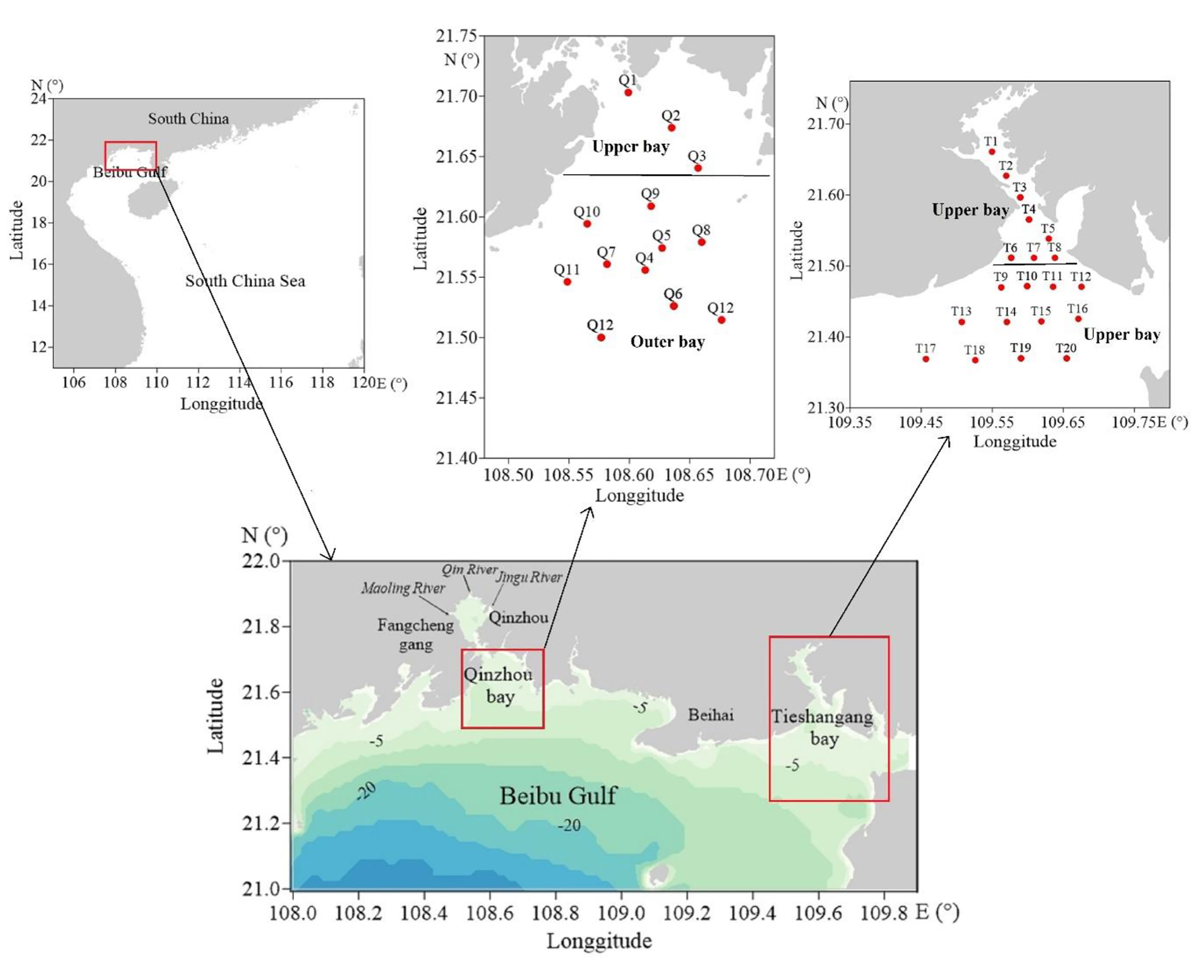
Figure 1 Map of Beibu Gulf and sampling stations. The red boxes are the sampling areas, including Qinzhou Bay and Tieshangang Bay in the Beibu Gulf.
In this comparative study, nitrate dual isotopes and ammonium nitrogen isotope , were used to determine the origin of nitrate in two types of bays in Beibu Gulf (Qinzhou Bay and Tieshanggang Bay) and to study biogeochemical processes associated with nitrogen cycling.
Materials And Methods
Study Area and Sampling
The Beibu Gulf is a semi-enclosed gulf located in south China and the northwestern part of the SCS. The climate of Beibu Gulf is affected by the East Asian monsoon, whereas the northeast monsoon prevails in winter and the southwest monsoon prevails in summer. The annual rainfall in the gulf is 1,775mm and most occurs in the rainy season (April to October, accounting for nearly 90% of the annual total) (http://data.cma.cn). Qinzhou Bay and Tieshangang Bay are two semi-enclosed bays in the center and northeastern coastal Guangxi Province (Figure 1). They are all bays that are significantly affected by industrial and aquaculture activities but they are still quite different. Geographically, Qinzhou Bay is a shallow semi-enclosed bay located in the northernmost part of the Beibu Gulf, with a water depth of 2-18 m. In addition, there are many rivers around Qinzhou Bay, including Maoling River, Qin River, Jingu River (Figure 1). The annual freshwater discharge from Qin River and Maoling River (the two largest rivers around the bay) is 599.33×108 m3 and 885.98×108 m3, respectively (Lao et al., 2020). By contrast, there are no large river flowing into the Tieshanggang Bay and the hydrodynamic conditions are weak.
In this study, the cruises were carried out in Tieshangang Bay and Qinzhou Bay from March 31 to April 1, 2021 and April 8, 2021, respectively. A total of 13 and 20 stations were conducted in Qinzhou Bay and Tieshangang Bay, respectively (Figure 1). A total of 13 and 20 seawater samples were collected from the surface water (0.5 m) in Qinzhou Bay and Tieshangang Bay, respectively, using a rosette sampler fitted with 10 L Niskin bottles. The temperature, salinity, pH, and DO were measured in the field. For nutrients and isotopic samples, seawater was filtered using precombustion (450°C, 4h) glass fiber filters (47 mm diameter, Whatman GF/F). Then, the filtrate was transferred into a precleaned (acid-washed) polyethylene bottle and stored at -20°C until analysis. For Chl a, about 1000-2000 mL of seawater samples were filtered using the GF/F and stored at -20°C until further analysis.
Chemical Analysis
The Chl a samples of GF/F filter were extracted using 90% acetone and determined by a spectrophotometer (Lorenzen, 1967). DO samples were measured using the Winkler titration method. The nutrient samples were measured using a San++ continuous flow analyzer (Skalar, Netherlands), with detection limits for and were 0.02 µmol L-1, 0.1 µmol L-1, 0.1 µmol L-1 and 0.1 µmol L-1, respectively. The analysis of and samples were using a cadmium-azide method modified from Mcilvin et al. (2005). The sulfamic acid was added to each sample to remove preexisting , followed the procedure from Granger and Sigman (2009). Spongy cadmium (Cd) was added to reduce to , and further reduce to N2O, sodium azide buffered with acetic acid to pH 4-5 was added. The samples were first quantitatively oxidized to by adding BrO- at pH 12, and then excess BrO- was removed by NaAsO2 and the yield was verified via colorimetric nitrite determination. Then, was further reduced to N2O by adding sodium azide buffered with acetic acid (Zhang et al., 2007). N2O was separated and analyzed for and by a Precon-GasBench II-253 plus (253 Plus, Thermo Scientific, United States). To ensure the quality of monitoring data, a standard sample (IAEA-N-1) was inserted into each batch of samples (10 samples) to monitor the stability of the instrument. In addition, the values were calibrated by the international standards of USGS 25, USGS26, and IAEA-N1. The and values were calibrated by the IAEA-N3 international standard. The reproducibility of duplicate analysis of , , and were <0.3‰ (mean ± 0.1‰), <0.3‰ (mean ± 0.1‰), and <0.6‰ (mean ± 0.3‰), respectively.
Mixing Model
To evaluate the behavior of nitrogen along the salinity gradient, nitrogen concentration and the isotopic values from simple physical mixing between two endmembers (coastal diluted water and outer seawater) can be calculated by a salinity-based conservative mixing model (Fry, 2002), as follows:
where q terms denote the fractional contributions of the two endmembers (denoted by the subscripts qr and qm). The S, N, δ terms denote the parameters being mixed: salinity, or concentration and or value. Smix, Nmix and δmix terms denote the theoretical value of a mixture from the two endmembers. Based on equation (1)-(4):
According to the above equations, the salinity-based or mixing shows linear conservative mixing (equation 6), whereas the salinity-based isotopic mixing shows curvilinear, which reflects the or -based weighting of endmember isotopic contributions. The or concentration and the isotopic value distributions, are expected to fall on the physical mixing line between the two endmembers. However, deviations from the mixing line indicate the presence of N transformation processes or other sources or both (Wankel et al., 2007). For example, the processes of nitrification and mineralization cause nitrate distribution above the mixing line, whereas and cause distributions below the mixing line (Yang et al., 2018). In contrast, the processes of assimilation and denitrification cause nitrate distribution below the mixing line whereas and distributions above the mixing lines (Yang et al., 2018).
SIAR Mixing Model
The contribution of potential nitrate sources to nitrate in water can be quantified by using a Bayesian mixing model created by and run in the stable isotope analysis in the R (SIAR) package This model has been widely used to estimate the proportional contribution of different nitrate sources (Korth et al., 2014; Davis et al., 2015; Zhang et al., 2018; Lao et al., 2019a; Torres-Martínez et al., 2020; Lao et al., 2021b). The framework is as follows (Moore and Semmens, 2008; Xue et al., 2009):
where Xij and Sjk represent nitrate dual isotope values in a mixed sample and the values of nitrate dual isotopes from nitrate sources, respectively; μjk and ωjkrepresent mean value and standard deviation from the normally distributed of Sjk; Pk is the proportional contribution of source k; cjk is the fractionation factor for nitrate dual isotopes on source k; and λjk and τjkare the mean value and standard deviation from the normally distributed of cjk; ϵjk is the residual error of the additional unquantified variation between individual samples, and 0 and σj are the normally distributed of ϵjk. The detail information of the model can be found in Moore and Semmens (2008); Xue et al. (2009) and Zhang et al. (2018).
Results
Distribution Characteristics of Physicochemical Parameters
The distribution characteristics of temperature, salinity, DO, and Chl a in Qinzhou Bay and Tieshangang Bay are presented in Figure 2. The temperature in Qinzhou Bay and Tieshangang Bay ranged from 24.4°C to 26.0°C and from 24.0°C to 27.3°C, with an average of 24.9°C and 25.3°C, respectively. The salinity in Qinzhou Bay and Tieshangang Bay increases seaward, i.e., from the inner bay to the outer bay, ranging overall from 29.89 to 31.54 and 30.59 to 31.82, with an average of 30.79 and 31.54 during the sampling period, respectively. The lowest salinity was observed in the inner Qinzhou Bay (station Q1), which could be influenced by the freshwater input from the rivers around the bay. The DO levels in Qinzhou Bay (ranging from 6.44 to 7.01 mg L-1, an average of 6.68 mg L-1) were slightly higher than those in Tieshangang Bay (ranging from 5.53 to 7.72 mg L-1, an average of 6.42 mg L-1). A relatively low DO level (< 6.5 mg L-1) was also found in the upper Qinzhou Bay (Figure 2E). Similarly, a significantly low DO level (< 6.0 mg L-1) was observed in the upper Tieshanggang Bay, whereas a higher DO level was in the outer bay (Figure 2F). The Chl a levels in Qinzhou Bay and Tieshangang Bay ranged from 1.70 to 4.50 µg L-1 and 0.90 to 2.80 µg L-1, with an average of 2.45 µg L-1 and 1.89 µg L-1, respectively. Except for a lower Chl a level was found in the station Q12 in the most seaward in Qinzhou Bay, the higher Chl a level (>2.0µg L-1) was found in other stations. In contrast, a lower Chl a level was found in the inner Tieshangang Bay whereas a higher Chl a level in the outer bay (Figure 2H). Generally, the Chl a level in Qinzhou Bay was higher than that in the Tieshangang Bay. The pH ranged from 7.86 to 8.07 and 7.76 to 8.21 in Qinzhou Bay and Tieshangang Bay, respectively, and generally showed an increasing trend from the upper bay to the outer bay in both bays (Figures 3A, B). The total suspended matter (TSM) ranged from 13.7 to 30.8 mg L-1 and 16.6 to 37.6 mg L-1 in Qinzhou Bay and Tieshangang Bay, respectively. Corresponding to the lower temperature in the outer Qinzhou Bay, a higher TSM level also occurred in the outer bay (Figure 3C), suggesting that the vertical mixing of water may be strong in the outer bay. The TSM level in Tieshanggang Bay generally showed a decreasing trend from the upper bay to the outer bay (Figure 3D).
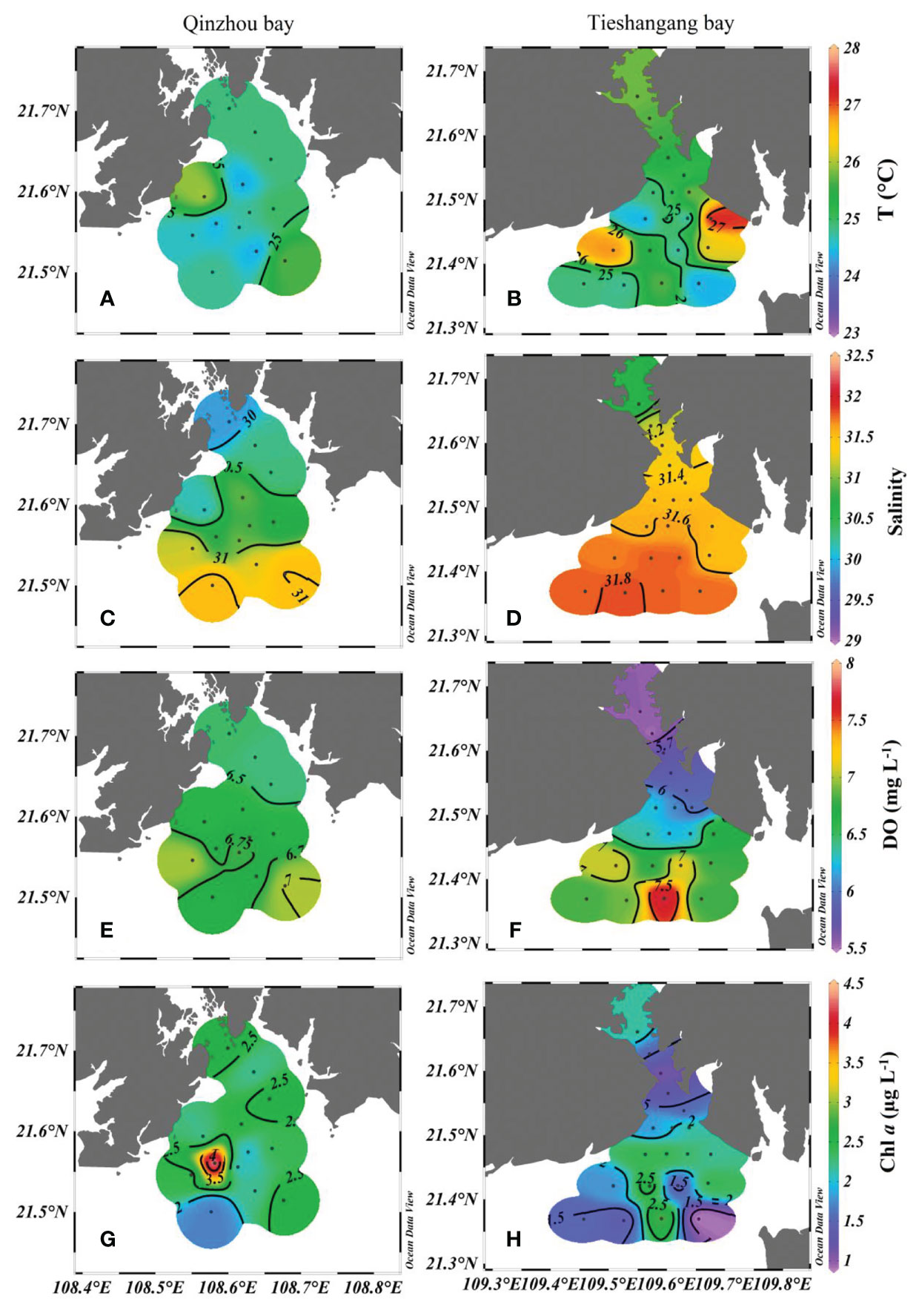
Figure 2 Spatial distributions of temperature (A, B), salinity (C, D), DO (E, F) and Chl a level (G, H) in Qinzhuo Bay and Tieshangang Bay during the sampling period.
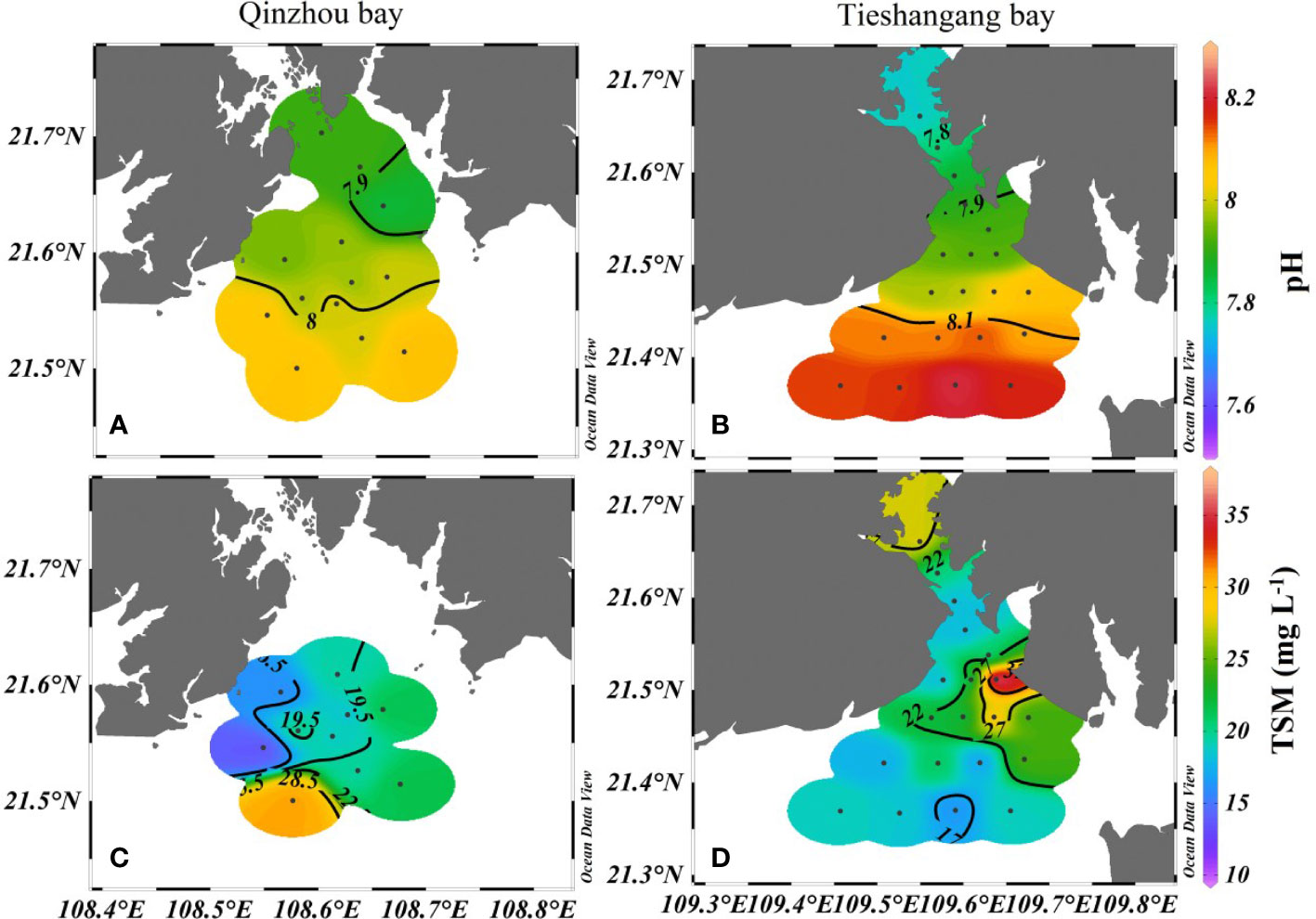
Figure 3 Spatial distributions of pH (A, B) and TSM (C, D) in Qinzhuo Bay and Tieshangang Bay during the sampling period.
Nutrient Concentrations
The distribution characteristics of and concentrations were similar in Qinzhou Bay and Tieshangang Bay, which exhibited the highest concentration in the most upper station (Q1 and T1), and increasing trend from the upper bay to the outer bay (Figure 4). The concentrations of and were ranged 0.15 to 1.06 µmol L-1, 0.37 to 0.82 µmol L-1, 1.24 to 19.21 µmol L-1 and 0.65 to 1.16 µmol L-1, with an average of 0.48 µmol L-1, 0.53 µmol L-1, 6.23 µmol L-1 and 0.93 µmol L-1 in Qinzhou bay, respectively; concentrations of and ranged from 0.02 to 0.92 µmol L-1, 0.05 to 1.28 µmol L-1, 0.23 to 10.07 µmol L-1 and 0.60 to 2.31 µmol L-1, with an average of 0.25 µmol L-1, 0.46 µmol L-1, 3.17 µmol L-1 and 1.21 µmol L-1 in Tieshangang Bay, respectively. Generally, the concentrations of nutrients decreased from the upper bay to outer bay in both Qinzhou Bay and Tieshangang Bay (except for in Qinzhou Bay) (Figure 4). This suggests that it is greatly influenced by terrigenous input in both two bays. However, the hydrodynamic condition may be another important factor affecting the spatial distribution of nutrients. For example, in Qinzhou Bay, with strong hydrodynamic conditions, the difference between nutrient concentration in the upper bay and outer bay is significantly higher than that in Tieshangang Bay. This is mainly due to the strong hydrodynamic conditions, which are conducive to the diffusion of nutrients and easily diluted by the low nutrient concentration in the outer bay seawater. Notably, the concentration in the upper Qinzhou Bay (average of 17.05 µmol L-1) was significantly higher than that in the upper Tieshangang Bay (average of 5.96 µmol L-1). In contrast, the concentration in the upper Tieshangang Bay (average of 1.74 µmol L-1) was higher than that in the upper Qinzhou Bay (average of 1.05 µmol L-1) (Figure 4). Among the components of dissolved inorganic nitrogen (DIN, including and ), the proportion of was the highest in both Qinzhou Bay (80%) and Tieshangang Bay (65%), followed by (13% in Qinzhou Bay and 25% in Tieshangang Bay), and the lowest by (7% in Qinzhou Bay and 9% in Tieshangang Bay). The ratio of N/P ranged from 6.9 to 36.1 (average of 16.23) in Qinzhou Bay and from 8.4 to 120.4 (average of 29.7) in Tieshangang Bay. The ratios in Qinzhou Bay and Tieshangang Bay were all in the range of previous studies in the Beibu Gulf (Lai et al., 2014; Lao et al., 2021b). Comparing with the rivers around the bay, the N/P ratio in the Qinzhou Bay was higher than that in Maoling River (11) but significantly lower than in the Qin River (145) during a similar period (Lao et al., 2020). This suggested that the river input may change the nutrient structure in the bay. The N/P ratio in Qinzhou Bay was similar to the Redfield ratio (i.e., the ratio of the nutrients utilized by marine phytoplankton) of 16 (Justić et al., 1995). However, the N/P ratio in Tieshangang Bay was higher than the Redfield ratio, suggesting that phosphorus (P) could be limited to producing phytoplankton in the bay; and this is consistent with the results of the previous studies on the coast of Beihai city (Lao et al., 2021b) and the western coastal Beibu Gulf (Lao et al., 2021a).
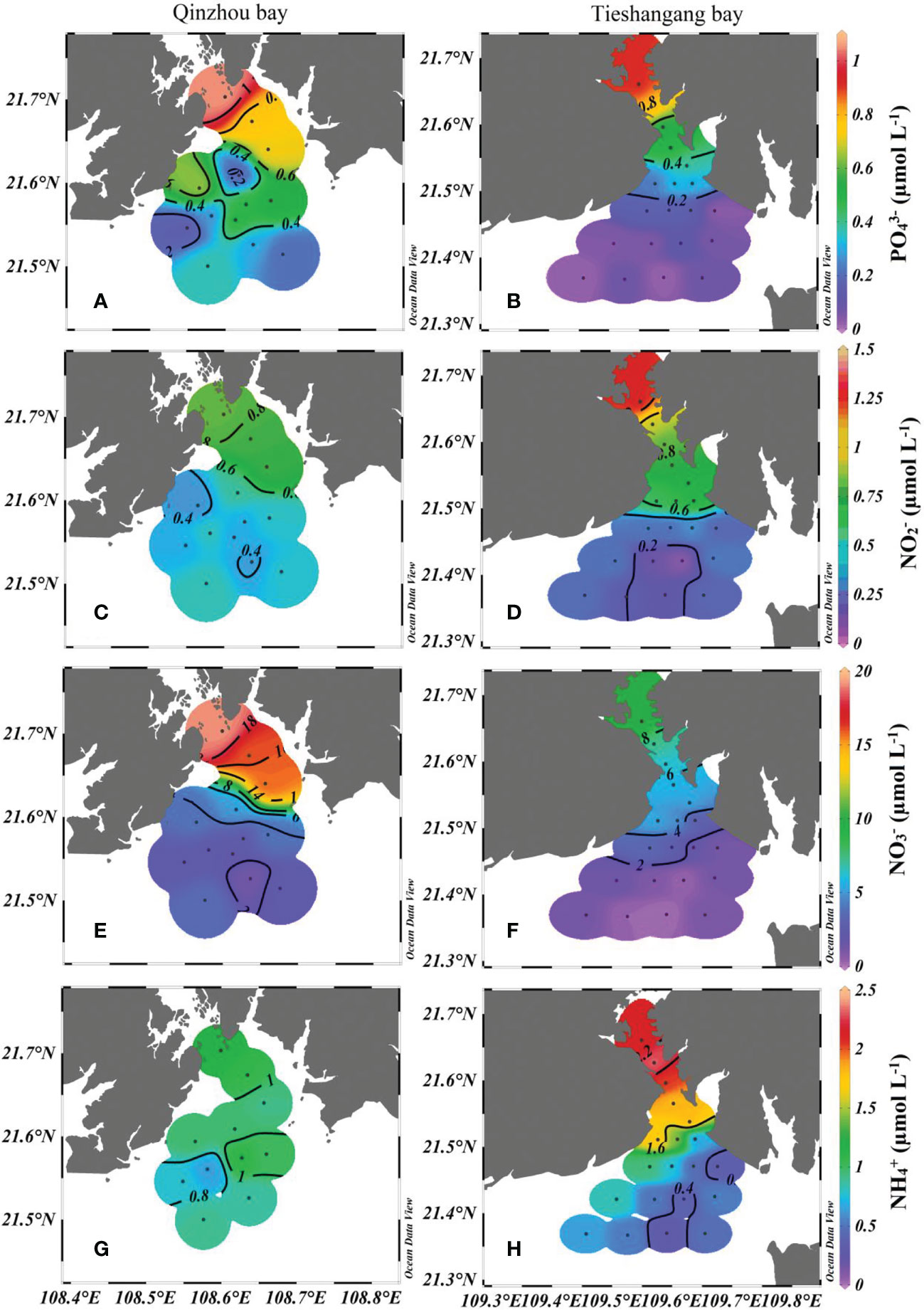
Figure 4 Spatial distributions of nutrients (A, B) (C, D), (E, F) and (G, H)] in Qinzhuo Bay and Tieshangang Bay during the sampling period.
Isotopic Compositions
The distribution characteristics of , and in Qinzhou Bay and Tieshangang Bay during the sampling period were presented in Figure 5. The , and values in Qinzhou Bay ranged from 11.4 to 14.2‰, 1.3 to 13.5‰ and -35.6 to 16.7‰, with an average of 13.0‰, 8.2‰ and -4.8‰, respectively; the values in Tieshangang Bay ranged from 9.8 to 18.3‰, 2.7 to 18.0‰ ang -18.6 to 18.9‰, with an average of 15.2‰, 11.7‰ and 4.98‰, respectively. The lower , and values were found in the upper Qinzhou Bay and Tieshangang Bay and exhibited an increasing trend from the upper bay to the outer bay (Figures 5A–D). In contrast, the highest value was found in the upper Tieshangang Bay and the value exhibited a decreasing trend from the upper to the outer bay (Figure 5F). While in the Qinzhou Bay, fewer spatial variations of value were found in Qinzhou Bay (Figure 5E). Generally, isotopic values in the Tieshangang Bay were higher than those in the Qinzhou Bay, suggesting that there may be differences in nutrient sources and transformation processes between the two bays.
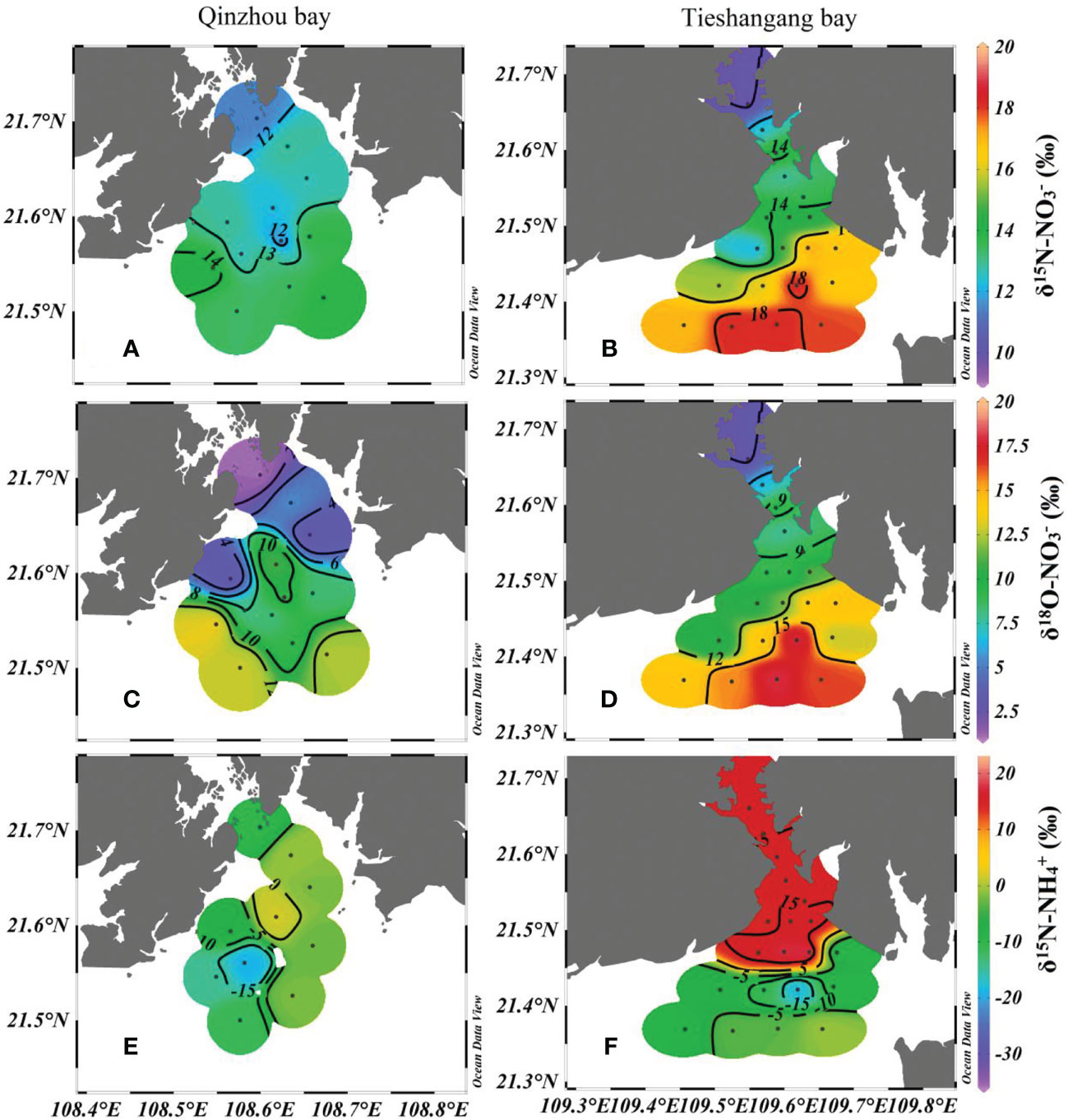
Figure 5 Spatial distributions of (A, B), (C, D) and (E, F) in Qinzhuo Bay and Tieshangang Bay during the sampling period.
Discussion
To further analyze the nitrogen sources and their biological transformations in the Qinzhou Bay and Tieshangang Bay, we classified the sampling sites into two analysis regions based on the characteristics of physicochemical parameters, nutrients, and isotopic compositions: the upper bay, including stations Q1-Q3 in Qinzhou Bay and stations T1-T8 in Tieshangang Bay, was characterized by the effect of terrestrial freshwater discharge having lower salinity and higher nutrient concentration; and the outer bay, including other stations except for the upper bay in Qinzhou Bay and Tieshangang Bay, was characterized by higher salinity and lower nutrient concentrations (Figure 1). The physico-chemical parameters of the two end-members of Tieshangang Bay and Qinzhou Bay are presented in Table 1.

Table 1 Endmember parameters of Qinzhou Bay and Tieshangang Bay, including salinity, nutrient concentrations and nitrate dual isotopes, used in the two-endmember mixing model in equation (5–7) .
Nitrate Sources and Their Biogeochemical Processes in Qinzhou Bay
In the Qinzhou Bay, nutrient concentrations in the upper bay were significantly higher than that in the outer bay (Figure 4), which has also been observed previously, particularly in rainy seasons, as reported by Lao et al. (2021a) and Lao et al. (2021c). In addition, the nutrients showed significantly negative correlation with salinity in both Qinzhou Bay and Tieshangang Bay (Figure 6), suggesting that the lower salinity water with higher nutrient concentrations in the upper bay is well mixed with the higher salinity water with lower nutrient concentrations in the outer bay. In the Beibu Gulf, the heavy rainfall mainly occurred from April to October, and the runoff in the rivers around the gulf increased significantly from April (Lao et al., 2020). More importantly, although the runoff was higher in the rainy seasons, the nutrient concentrations in some rivers around the Beibu Gulf and the coastal areas during the rainy season were still higher than that in the dry seasons, indicating that the significant influence by the heavy land-sources discharge from anthropogenic activities (Lao et al., 2020; Lao et al., 2021a). Thus, the high nutrient concentration in the upper bay may be largely influenced by the land-source inputs. The increasing land-source input has resulted in the significant increase of eutrophication in Qinzhou Bay over the past 40 years (Lao et al., 2021c). In addition, the submarine groundwater discharge (SGD) was also considered as another nutrient source in the Maowei Sea, which is a semi-enclosed bay located close to the upper Qinzhou Bay, and the SGD-derived nutrients were even more important than the amounts in the local river input (Chen et al., 2018). Thus, the higher nutrient concentration in the upper bay may be also influenced by the SGD. In the upper bay, the N/P (ranged from 19.9 to 24.0, an average of 22.4) was higher than the throughout Qinzhou Bay (average of 16.2), as well as the Redfield ratio (16.0), suggesting that higher DIN concentrations input in the upper bay compared to the P. However, the high concentrations [with a minimum of >0.70 µmol L-1] suggested that neither N nor P acted as a limiting nutrient in the upper bay, which were suitable for phytoplankton blooms. Notably, the N/P ratio also decreased significantly and closer to the Redfield ratio for the past years, mainly due to the increasing P input (Lao et al., 2021c). This has caused the harmful algal blooms to increase in frequency over the past ten years, particularly in the spring (Xu et al., 2019; Kang et al., 2020; Guan et al., 2022). This may be the reason that the higher Chl a level was observed in Qinzhou Bay during the sampling period (Figure 2G).
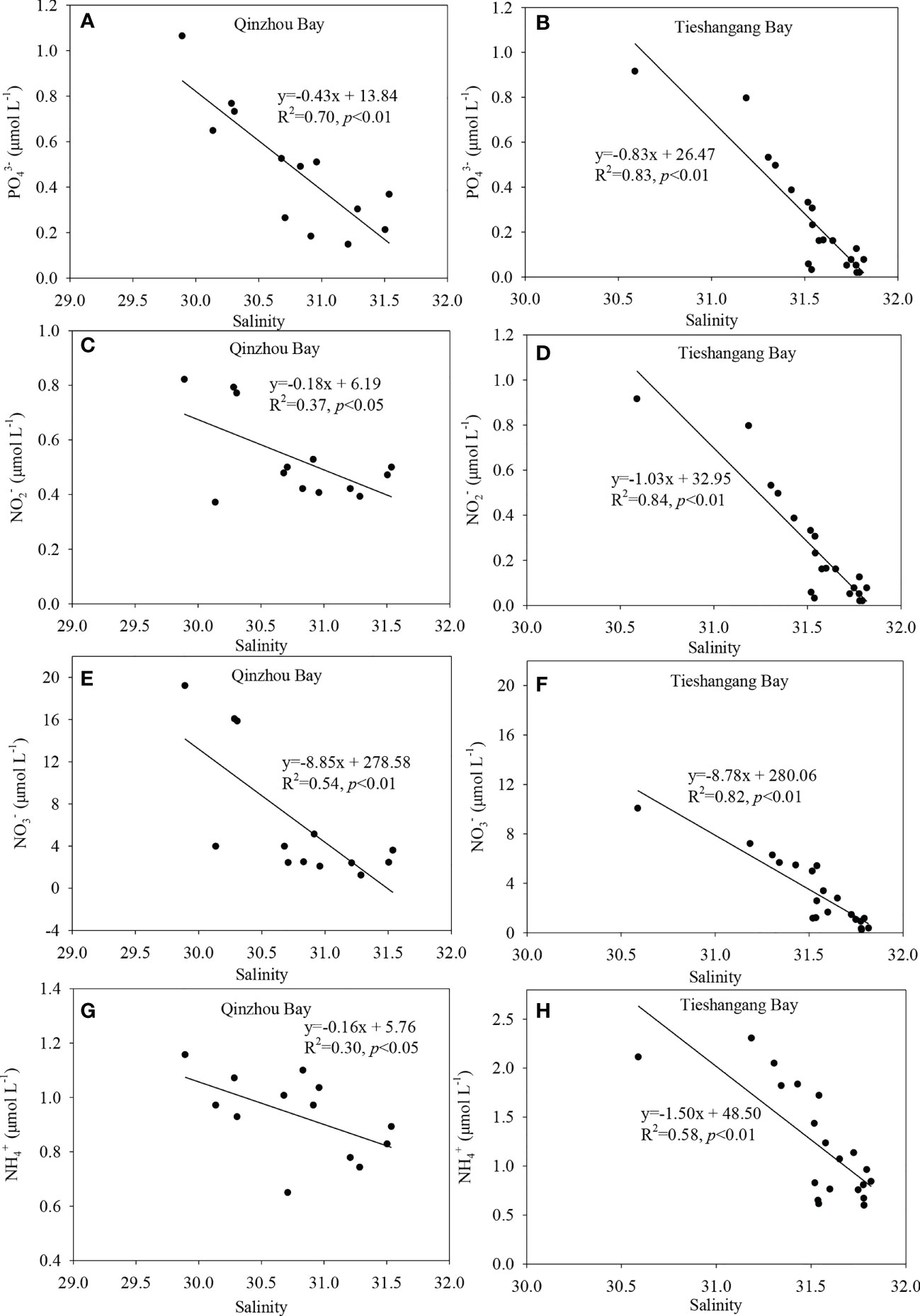
Figure 6 Linear relationship between nutrient (A, B), (C, D), (E, F), and (G, H) ] and salinity in the Qinzhou Bay and Tieshangang Bay.
Lower and values but relatively high values were found in the upper bay (Figures 5A, C, E), suggesting that nitrification may occur in the area. The light N in the presence of excess is preferentially used by the microorganisms, which could result in heavier 15N in the residual pool, whereas adding, depleted 14N to the residual pool (Sigman et al., 2005; Chen et al., 2009; Ye et al., 2016). Isotope fractionation during the biological processes of nitrification can be approximated by the open-system Rayleigh fractionation (Altabet, 2006; Ye et al., 2016), the equation is as follows:
where δm and δi represent the measured and initial values for , respectively; ϵ represents the fractionation factor; fNH4 represent the fraction of measured relative to the initial . However, there is no relationship between and fNH4 (p>0.05). Moreover, the overall estimated ϵ (6.7‰) in the upper bay was significantly deviated from the reported values for N fractionation factors in nitrification (-14 to -38‰) (Casciotti et al., 2003; Ye et al., 2016). This suggested that microbial nitrification was not the dominant biological process in the upper bay and the isotopic signal in this area may reflect the mixing of sources. According to a classical nitrate dual isotopic approach (Xue et al., 2009; Zhang et al., 2018), the (from 11.4 to 12.9‰) and (from 1.3 to 5.3‰) values in the upper bay would suggest that sewage and manure might be the dominant nitrate sources to this region (Figure 7).
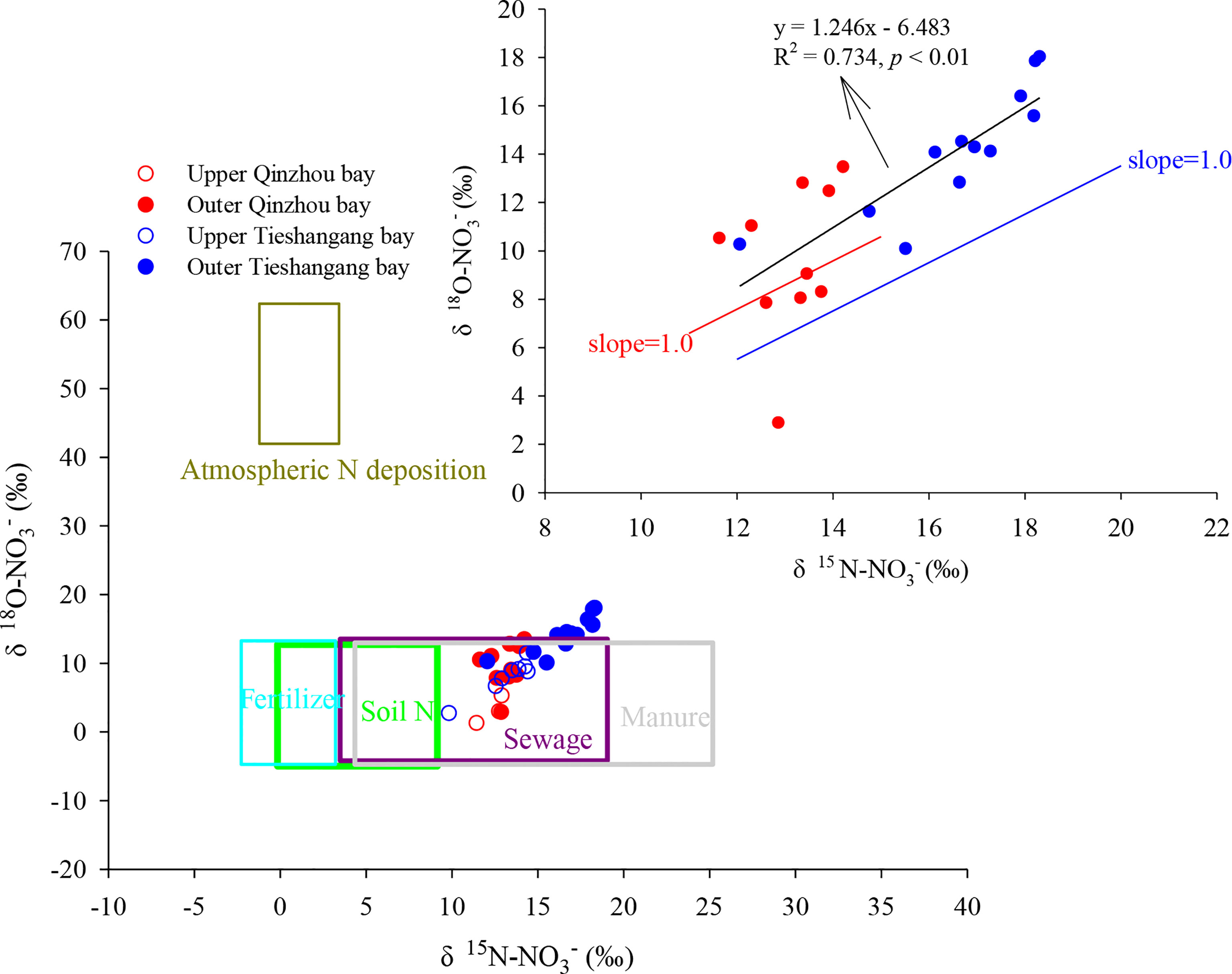
Figure 7 Values of and in Qinzhou Bay (red dots and circles) and Tieshangang Bay (blue dots and circles) and in various source reservoirs (boxes). In the larger graph, the isotopic composition of five potential nitrate sources are adapted from Chen et al. (2019), (Kendall 1998), Xue et al. (2009) and Zhang et al. (2018). The inset in the upper right corner is the plots of and in outer Qinzhou Bay (red dots) and outer Tieshangang Bay (blue dots). Assimilative uptake would cause nitrate dual isotopes to increase along a slope of 1.
In the outer bay, there was a clear and values increase. In addition, significantly loss and relatively high offset and values were found in the outer Qinzhou Bay (Figure 8), suggesting that substantial loss or consumption occurred in the outer bay. There could be several reasons for the increase of isotopic values, including phytoplankton assimilation, denitrification, and intense physical sediment-water interaction. However, denitrification could be firstly ruled out as the cause of the increase of isotopic anomalies due to high DO levels (>6.50 mg L-1) in the outer bay (Figure 2E). Hence, phytoplankton assimilation may be responsible for the loss and relatively positive isotope values in the outer bay. Phytoplankton assimilation can cause isotopic enrichment of , and , during which fractionation factors vary among different species (Granger et al., 2004; Wankel et al., 2009; Yan et al., 2017). Indeed, a spring phytoplankton bloom was indicated by a higher Chl a level (average of 2.5 µg L-1) in the outer bay and the highest Chl a level (4.5 µg L-1) was observed in station Q7 (Figure 2G). However, there is no relationship between and values in the outer bay and the increase of isotopic values were noticeably deviated from the assimilation line (1:1) (Figure 7). The uptake of by phytoplankton causes isotope enrichment in the residual pool due to preferential consumption of light nitrate by phytoplankton, with coupled and fractionation effects (18ϵ:15ϵ) of ~1 (Sigman et al., 1999; Granger et al., 2004). Moreover, based on the open-system Rayleigh fractionation (equation 10), the fractionation factor associated with the assimilation process was calculated. No relationship between and fNO3 (p>0.05) was found in the outer bay and the overall estimated ϵ (-0.7‰) in the outer bay was significantly deviated from the range of reported values (-3 to -9‰) for the process of assimilation (York et al., 2007; Ye et al., 2016). Additionally, the concentration of does not decrease too much in the outer bay (average of 0.90 µmol L-1, which is close to the upper bay [average of 1.05µmol L-1)]. Thus, when is sufficient, phytoplankton preferentially assimilates (Glibert et al., 2016). However, the values did not increase significantly in the outer bay (Figure 5E), which may be related to the continuous outward transportation of in the upper bay under the strong hydrodynamic force, resulting in the isotopic signal of sources covering the signal of isotopic fractionation caused by assimilation. Thus, phytoplankton assimilation is unlikely to be the dominant cause for the increase of nitrate dual isotopes in the outer bay. The intense physical sediment-water interaction may be responsible for the nitrate loss in the outer bay. Previous studies suggest that active consumption of nitrate due to denitrification in the sediments, causing an efflux of nitrate from the water column to the sediments, which resulted in the enrichment of and concurrent nitrate loss in the water (Zhang et al., 2013; Ye et al., 2016). Because advective flux returning nitrate to the upper overlying water environment caused by wind-induced mixing and tidal pumping, and values in the overlying water are substantially high, reflecting more closely nitrate isotopic fractionation by denitrification (Lehmann et al., 2007; Wankel et al., 2009). This suggestion was agreeable with the previous work that suggested the physical perturbation in the surface sediments could be very significant in spring period, when wind-induced mixing and tidal pumping in the northern Beibu Gulf was strong (Lao et al., 2021b). Indeed, the depth of the Qinzhou Bay is shallow (most stations are less than 6m, and the deeper depth in station Q12 is only 10.1m), and wind-induced mixing could aggravate the exchange of bottom and surface water. In addition, the air temperature has increased from the spring (March) in Beibu Gulf, but lower surface temperature (average of 24.7°C) in the Qinzhou Bay was found when compared to that in Tieshangang Bay (average of 25.4°C) during the same sampling period (Figures 2A, B), suggesting that the lower surface temperature in Qinzhou Bay originated from the bottom water upwelling. Moreover, an increasing TSP level (average of 20.0 mg L-1) was also found in the outer bay (Figure 3C). Such a dynamic environment would result in the bidirectional exchange of substances, including nitrate, between the overlying water and the sediment pore water. This process would finally lead to the increase of nitrate isotopic values but the decrease of nitrate concentration in the bay due to denitrification-induced isotopic enrichment in sediments. However, due to the lack of other water layers in the bay, i.e., the bottom layer, this hypothesis still needs further evaluation in the field investigation.
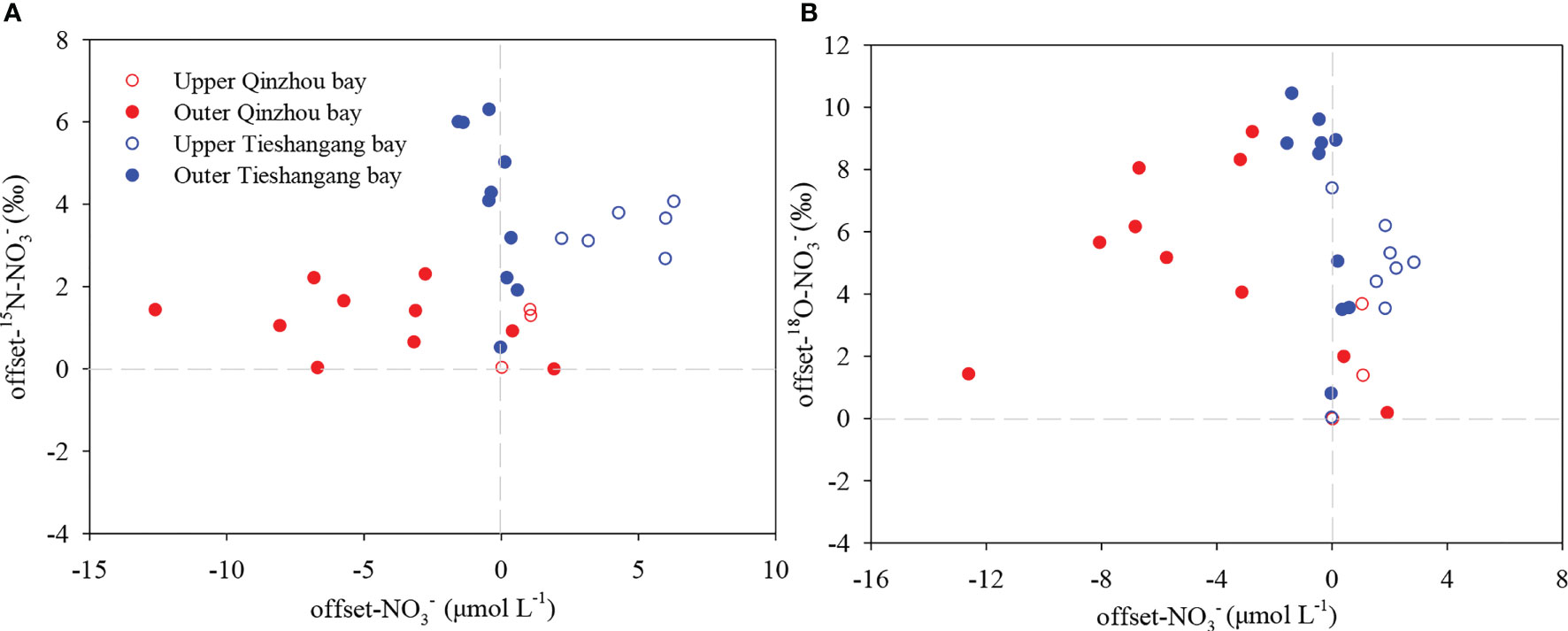
Figure 8 Relationships between offset (A) and (B) and offset in the Qinzhou Bay and Tieshangang Bay. The offset values are the difference between measured and expected values, which area calculated using the end-member mixing model.
Nitrate Sources and Its Biogeochemical Processes in Tieshangang Bay
In the Tieshangang Bay, significantly high nutrient concentrations were observed in the upper bay and showed a decrease trend from the upper bay to the outer bay (Figure 4). Different from Qinzhou Bay, there is no large rivers around the bay. Thus, the nutrient sources in the bay are mainly influenced by local human activities. In the upper bay, significantly positive offset values were found, with the values ranging from 0.01 to 2.85 µmol L-1 (average of 1.77 µmol L-1) (Figure 8), which could be new nitrate added into the waters in the upper bay. The new nitrate could be sourced from direct nitrate input, and/or decomposition and/or nitrification of in the upper bay. Indeed, significantly low DO level (Figure 2F) and lower pH (Figure 3B) and nitrate dual isotope values (Figures 4B, D) were found in the upper bay. This suggested that oxygen consumption processes could have occurred in the upper bay, which is probably decomposition. Moreover, a lower Chl a level was found in the upper bay (Figure 2H), indicating that oxygen consumption by decomposition is more important than photosynthesis. A large oxygen consumption and a large amount of CO2 release caused by the decomposition process could be responsible for the low DO level and pH in the upper bay. Similar results were also reported in the other coastal bays in Beibu Gulf (Lao et al., 2021a; Lao et al., 2021c). Correspondingly, a higher concentration occurred in the upper bay (Figure 4H). The rainy seasons in the coastal Beibu Gulf would carry a relatively huge transport of sewage from the coastal cities and soil organic nitrogen (SON) from the soil to the coastal bay (Lao et al., 2020), where SON could have been easily remineralized due to the priming effect to (Bianchi et al., 2015), and then nitrified to nitrate in the water (Guo et al., 2015; Ye et al., 2015). However, if the mineralization/nitrification processes are the dominant in the upper bay, the values should be close to 0‰, because the process generally adds isotopically light isotopes to the nitrate pool (Burns and Kendall, 2002; Ye et al., 2015). In contrast, although relatively low values occurred in the upper bay compared to the outer bay, the most values were still higher than 12‰ (average of 13.2‰) (Figure 5B), which is much higher than 0‰. This suggests that the mineralization/nitrification processes are unlikely to be the dominant cause for the increase of and in the outer bay. Notably, significantly high values (average of 15.1‰) were also found in the upper bay (Figure 5F), which are possibly associated with municipal sewage effluents from the coastal area. Generally, nitrate originated from sewage and manure is usually characterized by high isotopic values of nitrate (4 to 25‰) due to the volatilization of 15N-depleted ammonia formed from human and animal waste, which results in an enrichment of 15N in residual nitrate pool (Xue et al., 2009). Thus, the nitrate sources from sewage and manure may be responsible for the higher isotopic signal in the upper bay (Figure 7).
In the outer bay, the nutrient concentrations decrease but nitrate dual isotopic values increase. In addition, a relatively negative offset (average of -0.29 µmol L-1) but positive offset (average of 4.0‰) and value (average of -6.8‰) was found in the outer bay, suggesting that nitrate loss or consumption occurred in the outer bay. However, the denitrification could be ruled out due to the high DO level (>7.0 mg L-1) in the outer bay (Figure 2F), which did not favor the occurrence of denitrification. Moreover, the influence of physical sediment-water interaction should be less in the outer bay. Different from the Qinzhou Bay, the depth of outer Tieshangang Bay is deeper (most station >10m, and the deepest station in T19 reach 20m). With the air temperature increasing in spring, the surface water temperature increases in the outer bay (average of 25.4°C), opposite to the outer Qinzhou Bay (Figures 2A, B). This suggests that with the increase of surface water temperature, there is no bottom water upwelling in Tieshangang Bay as in Qinzhou Bay. Thus, the phytoplankton assimilation may be related to the nitrate loss but increasing isotopic nitrate values in the outer bay. Indeed, a higher Chl a level was found in the outer bay (average 2.41 µg L-1) (Figure 2H). In addition, although the slope (1.25) has slightly deviated from the assimilation line (1:1), a significantly positive relationship between and values were found in the outer bay (Figure 7). Moreover, the fractionation factor associated with assimilation was calculated and the 15N fractionation with isotope effects of -3.3‰ in the outer bay was also within the range of reported values (-3 to -9‰) for phytoplankton assimilation (York et al., 2007). This suggests that uptake by phytoplankton was the dominant process for decrease and its isotopic increase in the outer bay. However, the value did not increase in the outer bay. This may be because the assimilation by phytoplankton mainly utilizes due to significantly low concentration in the outer bay (Figure 4H). is assimilated by phytoplankton when is insufficient in the water environment (Glibert et al., 2016). However, extremely higher values were also observed in the outer bay (>10‰, average of 14.1‰) (Figure 5E). Neither phytoplankton assimilation nor mixing with other water masses from outer Beibu Gulf or the northern South China Sea is enough to lead to values higher than 10 ‰ (Ye et al., 2015; Ye et al., 2016; Lao et al., 2021b). Synthetic fertilizer and atmospheric N precipitation have much higher values (Chen et al., 2009; Chen et al., 2019), and those sources may be related to the high values in the outer bay. However, the source from synthetic fertilizer can be readily ruled out since it only accounts for <2% of the synthetic N fertilizer applied in China (Chen et al., 2009). Thus, the high values are partly influenced by the atmospheric deposition, which could be responsible for the nitrate dual isotopes slightly deviation of assimilation line (1:1).
To quantify the contribution of atmospheric N deposition to the pool in the outer bay, a simple isotope mass balance model was utilized [equations (11) and (12)]. This model is based on nitrate isotope anomalies, such as the deviation from the conservative mixing, which has also been used to calculate the relative contribution of atmospheric deposition in the coastal area (Ye et al., 2016; Lao et al., 2019a; Lao et al., 2021b). In this model, we assumed that nitrate isotope anomalies are mainly influenced by assimilation and atmospheric deposition in the outer Tieshangang Bay.
where △δ15N and △δ18O are the measured and anomalies relative to the mixing line. The process of assimilation cause and values increase of 1:1 (15ϵ =18ϵ) (Granger et al., 2004). Thus,
where we define Natmosphere/(Natmosphere + Nmixing) as fatmosphere, and obtained
where fatmosphere is the proportional contribution of atmospheric deposition. The and values of atmospheric deposition were obtained from Zhanjiang, where located at the eastern coast of the Tieshangang Bay (distance less than 60 km). The result shows that the proportional contribution of atmospheric deposition to the pool in the outer bay ranged from 0 to 17%, with an average of 7%. This is similar to the previous study in the northeastern Beibu Gulf (more seaward of Tieshangang Bay), which suggests that the contribution of from atmospheric deposition in the offshore area of Beibu Gulf was 6% (Lao et al., 2021b).
Quantification of Nitrate Sources in Qinzhou Bay and Tieshangang Bay
Since the biological processes were not the dominant factor that affected the nitrate in the upper bay of both Qinzhou Bay and Tieshangang Bay, the isotopic characteristics may provide a signal on the various sources that contributed to the mixture. According to a classical nitrate dual isotopic approach in Figure 7, the nitrate dual isotopic values in the two bays were significantly deviated from the potential nitrate source from fertilizer, since the and values were generally higher than that from the fertilizer. Thus, the nitrate source from fertilizer could be less in the Beibu Gulf. Although the manure and sewage might be the dominant nitrate sources in both two upper bays, the isotopic fingerprints in the upper bays were close to the source from soil N, and the extremely high values that were influenced by atmospheric deposition as discussed above, were considered. Thus, four potential nitrate sources, including manure and sewage, soil N, fertilizer, and atmospheric deposition, were applied to quantify the proportional contribution of the nitrate sources by a Bayesian mixing model. The and values of these four potential sources are presented in Table 2. The result is presented in Figure 9. The manure and sewage are the dominant nitrate sources in the coastal bays and the contribution in Tieshangang Bay (a total of 59%) was higher than that in Qinzhou Bay (41%), while the nitrate source from soil N in Qinzhou Bay (30%) was higher than that in the Tieshangang Bay (20%). Although both bays are affected by industrial and aquaculture activities, Tieshangang Bay does not have the rivers around the bay input as Qinzhou Bay. Thus, less soil N was carried into the Tieshangang Bay, and the dominant sources from sewage and manure could also bring the into the bay. In addition, the would retain in the bay due to weak hydrodynamic conditions (Jiang et al., 2017), resulting in a high concentration in the upper Tieshangang Bay. In contrast, river input could bring more soil nitrogen from the coastal cities into Qinzhou Bay. This may be responsible for the high concentration observed in the upper Qinzhou Bay. In the river basin, the nitrification coupled denitrification process in the soil can convert ammonium into nitrate and the river can bring it into the coastal environment (Chen et al., 2009). In addition, although the dominant sources from sewage and manure could bring the into the bay, the strong hydrodynamic conditions accelerate the exchange of seawater between the upper bay and the outer bay, resulting in the dilution of concentration. Thus, the concentration of in Qinzhou Bay is not as high as that in Tieshangang Bay due to accumulation. The contribution of atmospheric deposition was similar between the two upper bays (Figure 9), and also similar to the outer Tieshanggang Bay (8%) and the offshore area of the northeastern Beibu Gulf (6%) reported by previous studies (Lao et al., 2021b). This suggests that the impact of atmospheric deposition on the Beibu Gulf is relatively consistent.
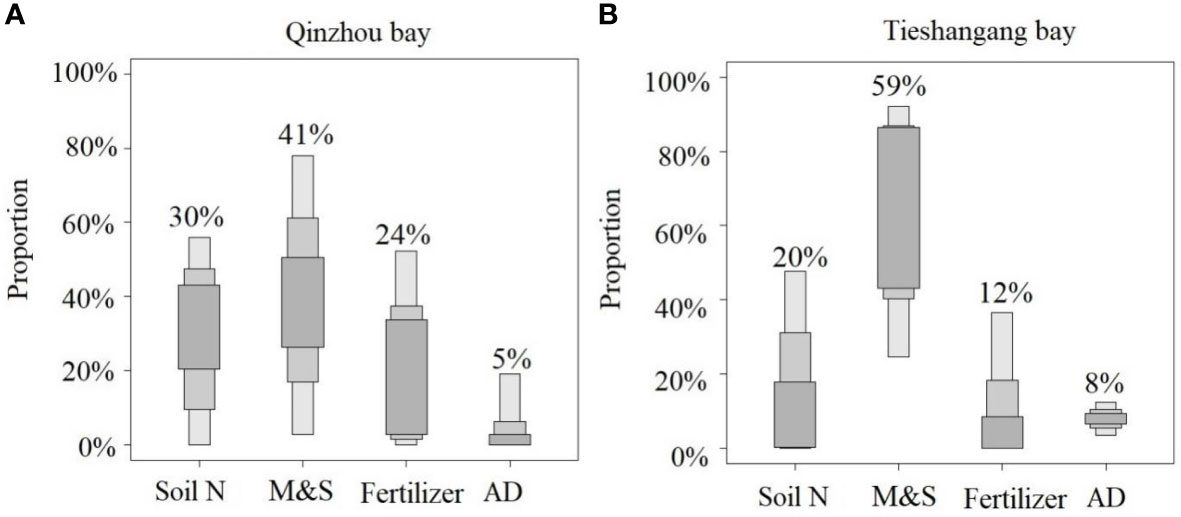
Figure 9 The proportional contribution of potential sources of nitrate in the water of upper bay of Qinzhou Bay (A) and Tieshangang Bay (B), including soil N, manure and sewage (M&S), fertilizer, and atmospheric deposition (AD).
According to the results of quantitative calculation, the sources of nitrate in the two bays are obviously different. In the Qinzhou Bay with riverine input, the contribution of the soil N and fertilizer is higher than that without riverine input in the Tieshangang Bay, while the source from manure and sewage in the Tieshangang Bay is higher than that in the Qinzhou Bay. This suggests that for the bay with riverine input, rivers can import a large number of land-based pollutants into the coastal waters, while the bay without riverine input reflects the discharge of industrial or urban manure and sewage, which needs the attention of environmental managers.
Conclusion
Nitrate dual isotopes and ammonium nitrogen isotope , were used to determine the origin of nitrate in the coastal bays of Beibu Gulf (Qinzhou Bay and Tieshanggang Bay) and to study biogeochemical processes associated with nitrogen cycling. The summary of the nitrate sources and its biogeochemical processes is presented in Figure 10. The nutrient concentrations generally showed a decreased trend from the upper bay to the outer bay, while the and values increased seaward in both bays. The values decreased from the upper Tieshangang Bay to the outer bay. Although the isotopic distribution trends of the two bays are similar, the isotopic values in Tieshangang Bay were higher than that in the Qinzhou Bay, suggesting that there may be differences in nutrient sources and transformation processes between the two bays. Manure and sewage were the dominant nitrate sources in the both upper bays, but the contribution in Tieshangang Bay (59%) was higher than that in the Qinzhou Bay (41%). While the nitrate source from soil N in upper Qinzhou Bay (30%) was higher than that in the upper Tieshangang Bay (20%), which may relate to the runoff input from the coastal cities around the Qinzhou Bay. Moreover, nutrients were retained in the upper Tieshangang Bay due to weak hydrodynamic conditions, which caused higher concentrations in the upper bay. Significant nitrate loss occurred in the outer Qinzhou Bay, which is related to the intense physical sediment-water interaction. Moreover, phytoplankton assimilation mainly utilized due to sufficient in the outer Qinzhou Bay. Nitrate loss was also found in the outer Tieshangang Bay which mainly related to the phytoplankton assimilation. In addition, the greater enrichment of than in both bays suggests that atmospheric deposition also contributes to the nitrate pool in the water and the impact of atmospheric deposition on the Beibu Gulf is relatively consistent.
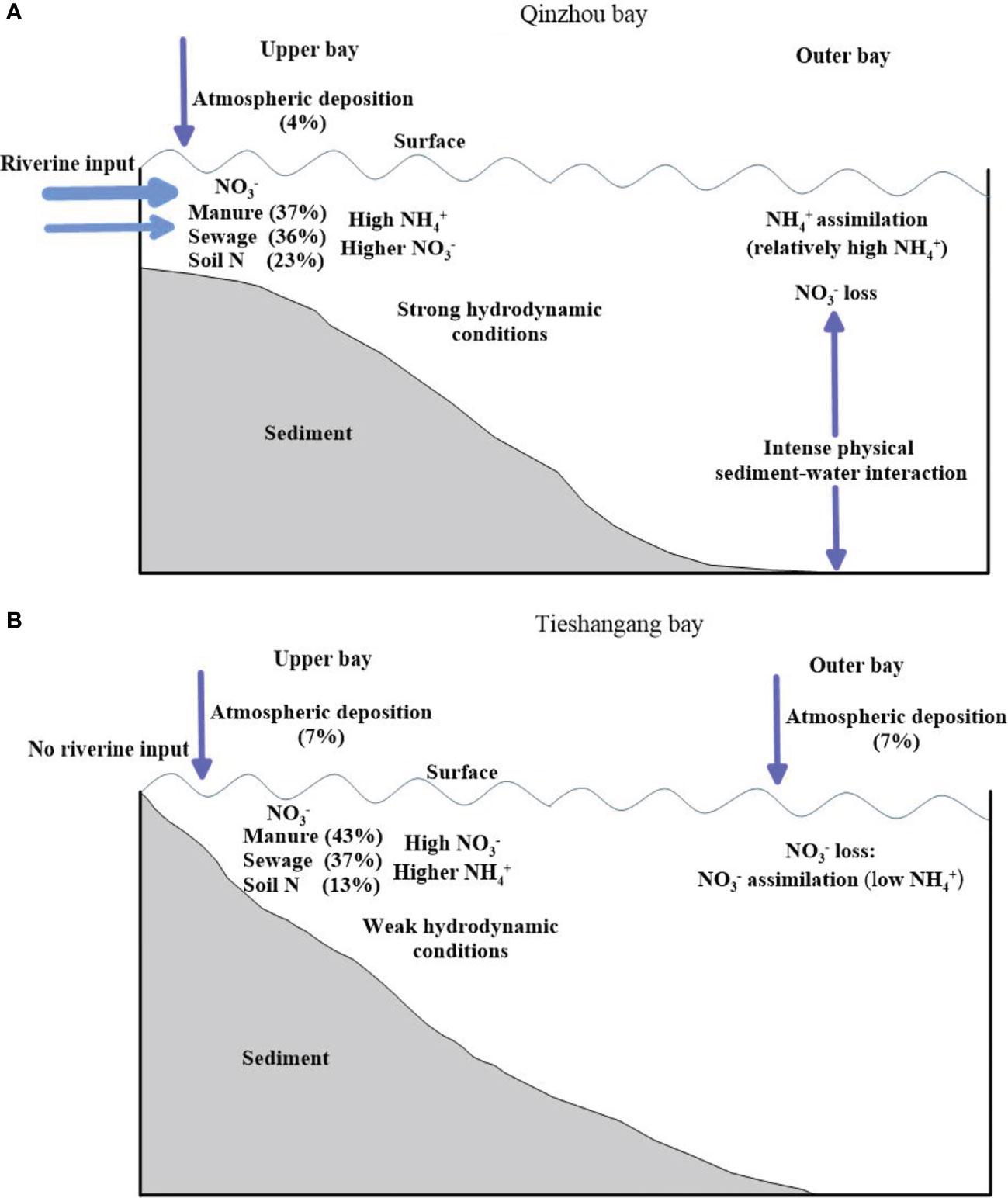
Figure 10 Conceptual diagram of sources and cycling of nitrogen between a bay with riverine input (A) and a bay without riverine input (B) in the Beibu Gulf.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
FC was responsible for the conceptualization. CC and QL prepared and wrote the original draft. QL, FC, and CC wrote, reviewed, and edited the manuscript. YS and GJ were responsible for the data curation. QZ, XLu, and XZ were responsible for the experimental operation. QS, XLe, and GL were responsible for field sampling. FC and QL funded the acquisition. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (U1901213), Guangxi Natural Science Foundation of China (2020GXNSFBA297065), Guangdong Natural Science Foundation of China (2019B1515120066, 2016A030312004), and Scientific Research Start-Up Foundation of Shantou University (NTF20006).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Altabet M. A. (2006). “Isotopic Tracers of the Marine Nitrogen Cycle: Present and Past,” in Marine Organic Matter: Biomarkers, Isotopes and DNA (Berlin, Heidelberg: Springer), 251–293.
Anderson D. M., Fensin E., Gobler C. J., Hoeglund A. E., Hubbard K. A., Kulis D. M., et al. (2021). Marine Harmful Algal Blooms (HABs) in the United States: History, Current Status and Future Trends. Harmf. Algae. 102, 101975. doi: 10.1016/j.hal.2021.101975
Bianchi T. S., Thornton D. C. O., Yvon-Lewis S. A., King G. M., Eglinton T. I., Shields M. R., et al. (2015). Positive Priming of Terrestrially Derived Dissolved Organic Matter in a Freshwater Microcosm System. Geophys. Res. Lett. 42 (13), 5460–5467. doi: 10.1002/2015GL064765
Burns D. A., Kendall C. (2002). Analysis of δ15n and δ18o to Differentiate NO3– Sources in Runoff at Two Watersheds in the Catskill Mountains of New York. Water Resour. Res. 38 (5), 9–1. doi: 10.1029/2001WR000292
Carstensen J., Conley D. J. (2019). Baltic Sea Hypoxia Takes Many Shapes and Sizes. Limnol. Oceanog. Bull. 28 (4), 125–129. doi: 10.1002/lob.10350
Casciotti K. L., Sigman D. M., Ward B. B. (2003). Linking Diversity and Stable Isotope Fractionation in Ammonia-Oxidizing Bacteria. Geomicrobio. J. 20 (4), 335–353. doi: 10.1080/01490450303895
Chen F., Jia G., Chen J. (2009). Nitrate Sources and Watershed Denitrification Inferred From Nitrate Dual Isotopes in the Beijiang River, South China. Biogeochemistry 94 (2), 163–174. doi: 10.1007/s10533-009-9316-x
Chen F., Lao Q., Jia G., Chen C., Zhu Q., Zhou X. (2019). Seasonal Variations of Nitrate Dual Isotopes in Wet Deposition in a Tropical City in China. Atmos. Environ. 196, 1–9. doi: 10.1016/j.atmosenv.2018.09.061
Chen X., Lao Y., Wang J., Du J., Liang M., Yang B. (2018). Submarine Groundwater-Borne Nutrients in a Tropical Bay (Maowei Sea, China) and Their Impacts on the Oyster Aquaculture. Geochem. Geophys. Geosyst. 19 (3), 932–951. doi: 10.1002/2017GC007330
Chen F., Lao Q., Zhang S., Bian P., Jin G., Zhu Q., et al. (2020). Nitrate Sources and Biogeochemical Processes Identified Using Nitrogen and Oxygen Isotopes on the Eastern Coast of Hainan Island. Continent. Shelf. Res. 207, 104209. doi: 10.1016/j.csr.2020.104209
Davis P., Syme J., Heikoop J., Fessenden-Rahn J., Perkins G., Newman B., et al. (2015). Quantifying Uncertainty in Stable Isotope Mixing Models. J. Geophys. Res.: Biogeosci. 120 (5), 903–923. doi: 10.1002/2014JG002839
Fry B. (2002). Conservative Mixing of Stable Isotopes Across Estuarine Salinity Gradients: A Conceptual Framework for Monitoring Watershed Influences on Downstream Fisheries Production. Estuaries 25, 264–271. doi: 10.1007/BF02691313
Gan H., Lin J., Liang K., Xia Z. (2013). Selected Trace Metals (As, Cd and Hg) Distribution and Contamination in the Coastal Wetland Sediment of the Northern Beibu Gulf, South China Sea. Mar. Poll. Bull. 66 (1-2), 252–258. doi: 10.1016/j.marpolbul.2012.09.020
Glibert P. M., Wilkerson F. P., Dugdale R. C., Raven J. A., Dupont C. L., Leavitt P. R., et al. (2016). Pluses and Minuses of Ammonium and Nitrate Uptake and Assimilation by Phytoplankton and Implications for Productivity and Community Composition, With Emphasis on Nitrogen-Enriched Conditions. Limnol. Oceanog. 61 (1), 165–197. doi: 10.1002/lno.10203
Gobler C. J. (2020). Climate Change and Harmful Algal Blooms: Insights and Perspective. Harmf. Algae. 91, 101731. doi: 10.1016/j.hal.2019.101731
Granger J., Sigman D. M. (2009). Removal of Nitrite With Sulfamic Acid for Nitrate N and O Isotope Analysis With the Denitrifier Method. Rapid Commun. Mass. Spectromet. 23 (23), 3753–3762. doi: 10.1002/rcm.4307
Granger J., Sigman D. M., Needoba J. A., Harrison P. J. (2004). Coupled Nitrogen and Oxygen Isotope Fractionation of Nitrate During Assimilation by Cultures of Marine Phytoplankton. Limnol. Oceanog. 49 (5), 1763–1773. doi: 10.4319/lo.2004.49.5.1763
Gruber N., Galloway J. N. (2008). An Earth-System Perspective of the Global Nitrogen Cycle. Nature 451, 293–296. doi: 10.1038/nature06592
Guan W., Bao M., Lou X., Zhou Z., Yin K. (2022). Monitoring, Modeling and Projection of Harmful Algal Blooms in China. Harmf. Algae. 111, 102164. doi: 10.1016/j.hal.2021.102164
Guo W., Ye F., Xu S., Jia G. (2015). Seasonal Variation in Sources and Processing of Particulate Organic Carbon in the Pearl River Estuary, South China. Estua. Coast. Shelf. Sci. 167, 540–548. doi: 10.1016/j.ecss.2015.11.004
Jiang C., Liu Y., Long Y., Wu C. (2017). Estimation of Residence Time and Transport Trajectory in Tieshangang Bay, China. Water 9 (5), 321. doi: 10.3390/w9050321
Justić D., Rabalais N. N., Turner R. E., Dortch Q. (1995). Changes in Nutrient Structure of River-Dominated Coastal Waters: Stoichiometric Nutrient Balance and its Consequences. Estua. Coast. Shelf. Sci. 40 (3), 339–356. doi: 10.1016/S0272-7714(05)80014-9
Kang Z., Yang B., Lai J., Ning Y., Zhong Q., Lu D., et al. (2020). Phaeocystis Globosa Bloom Monitoring: Based on P. Globosa induced seawater viscosity modification adjacent to a nuclear power plant in Qinzhou Bay, China. J. Ocean. Univ. China 19 (5), 1207–1220. doi: 10.1007/s11802-020-4481-6
Kendall C. (1998). “Tracing Nitrogen Sources and Cycling in Catchments,” in Isotope Tracers in Catchment Hydrology. Eds. Kendall C., McDonnel J. J. (New York: Elsevier), 519–576.
Korth F., Deutsch B., Frey C., Moros C., Voss M. (2014). Nitrate Source Identification in the Baltic Sea Using its Isotopic Ratios in Combination With a Bayesian Isotope Mixing Model. Biogeosciences 11 (17), 4913–4924. doi: 10.5194/bg-11-4913-2014
Lai J., Jiang F., Ke K., Xu M., Lei F., Chen B. (2014). Nutrients Distribution and Trophic Status Assessment in the Northern Beibu Gulf, China. Chin. J. Oceanol. Limnol. 32 (5), 1128–1144. doi: 10.1007/s00343-014-3199-y
Lao Q., Chen F., Liu G., Chen C., Jin G., Zhu Q., et al. (2019a). Isotopic Evidence for the Shift of Nitrate Sources and Active Biological Transformation on the Western Coast of Guangdong Province, South China. Mar. Poll. Bull. 142, 603–612. doi: 10.1016/j.marpolbul.2019.04.026
Lao Q., Liu G., Gao J., Shen Y., Guo Z., Qing S., et al. (2021c). Study on the Characteristics and Eutrophication of Nutrients in the Mariculture Farms of Qinzhou Bay, South China. Mar. Environ. Sci. 40 (3), 407–416. doi: 10.13634/j.cnki.mes.2021.03.011
Lao Q., Liu G., Gao J., Shen Y., Su Q., Chen C., et al. (2021b). Seasonal Sources and Cycling of Nitrogen Revealed by Stable Isotopes in the Northeastern Beibu Gulf, China. J. Mar. Sci. Eng. 9 (10), 1123. doi: 10.3390/jmse9101123
Lao Q., Liu G., Shen Y., Su Q., Gao J., Chen F. (2020). Distribution Characteristics and Fluxes of Nutrients in the Rivers of the Beibu Gulf. Haiyang Xuebao 42 (12), 93–100. doi: 10.3969/j.issn.0253−4193.2020.12.010
Lao Q., Liu G., Shen Y., Su Q., Lei X. (2021a). Biogeochemical Processes and Eutrophication Status of Nutrients in the Northern Beibu Gulf, South China. J. Earth Syst. Sci. 130 (4), 1–14. doi: 10.1007/s12040-021-01706-y
Lao Q., Liu G., Zhou X., Chen F., Zhang S. (2021d). Sources of Polychlorinated Biphenyls (PCBs) and Dichlorodiphenyltrichloroethanes (DDTs) Found in Surface Sediment From Coastal Areas of Beibu Gulf: A Reflection on Shipping Activities and Coastal Industries. Mar. Poll. Bull. 167, 112318. doi: 10.1016/j.marpolbul.2021.112318
Lao Q., Su Q., Liu G., Shen Y., Chen F., Lei X., et al. (2019b). Spatial Distribution of and Historical Changes in Heavy Metals in the Surface Seawater and Sediments of the Beibu Gulf, China. Mar. Poll. Bull. 146, 427–434. doi: 10.1016/j.marpolbul.2019.06.080
Lehmann M. F., Sigman D. M., McCorkle D. C., Granger J., Hoffmann S., Cane G., et al. (2007). The Distribution of Nitrate 15N/14N in Marine Sediments and the Impact of Benthic Nitrogen Loss on the Isotopic Composition of Oceanic Nitrate. Geochim. Cosmochim. Acta 71 (22), 5384–5404. doi: 10.1016/j.gca.2007.07.025
Liu G., Lao Q., Su Q., Shen Y., Chen F., Qing S., et al. (2020). Spatial and Seasonal Characteristics of Dissolved Heavy Metals in the Aquaculture Areas of Beibu Gulf, South China. Hum. Ecol. Risk Assess. 26 (7), 1957–1969. doi: 10.1080/10807039.2019.1629273
Lorenzen C. J. (1967). Determination of Chlorophyll and Pheopigments: Spectrophotometric Equations. Limnol. Oceanog. 12, 343–346. doi: 10.4319/lo.1967.12.2.0343
McIlvin M. R., Altabet M. A. (2005). Chemical Conversion of Nitrate and Nitrite to Nitrous Oxide for Nitrogen and Oxygen Isotopic Analysis in Freshwater and Seawater. Analytic. Chem. 77 (17), 5589–5595. doi: 10.1021/ac050528s
Moore J. M., Mills M. M., Arrigo K. R., Berman-Frank I., Bopp L., Boyd P. W., et al. (2013). Processes and Patterns of Oceanic Nutrient Limitation. Nat. Geosci. 6 (9), 701–710. doi: 10.1038/ngeo1765
Moore J. M., Semmens B. X. (2008). Incoporating Uncertainty and Prior Information Into Stable Isotope Mixing Models. Ecol. Lett. 11, 470–480. doi: 10.1111/j.1461-0248.2008.01163.x
Sharples J., Middelburg J. J., Fennel K., Jickells T. D. (2017). What Proportion of Riverine Nutrients Reaches the Open Ocean? Global Biogeochem. Cycle. 31, 39–58. doi: 10.1002/2016GB005483
Sigman D. M., Altabet M. A., McCorkle D. C., Francois R., Fischer G. (1999). The δ15n of Nitrate in the Southern Ocean: Consumption of Nitrate in Surface Waters. Global Biogeochem. Cycle. 13 (4), 1149–1166. doi: 10.1029/1999GB900038
Sigman D. M., Granger J., DiFiore P. J., Lehmann M. M., Ho R., Cane G., et al. (2005). Coupled Nitrogen and Oxygen Isotope Measurements of Nitrate Along the Eastern North Pacific Margin. Global Biogeochem. Cycle. 19 (4), GB4022. doi: 10.1029/2005GB002458
Torres-Martínez J. A., Mora A., Knappett P. S., Ornelas-Soto N., Mahlknecht J. (2020). Tracking Nitrate and Sulfate Sources in Groundwater of an Urbanized Valley Using a Multi-Tracer Approach Combined With a Bayesian Isotope Mixing Model. Water Res. 182, 115962. doi: 10.1016/j.watres.2020.115962
Tremblay J.É., Anderson L. G., Matrai P., Coupel P., Bélanger S., Michel C., et al. (2015). Global and Regional Drivers of Nutrient Supply, Primary Production and CO2 Drawdown in the Changing Arctic Ocean. Prog. Oceanog. 139, 171–196. doi: 10.1016/j.pocean.2015.08.009
Wankel S. D., Kendall C., Paytan A. (2009). Using Nitrate Dual Isotopic Composition (δ15n and δ18o) as a Tool for Exploring Sources and Cycling of Nitrate in an Estuarine System: Elkhorn Slough, California. J. Geophys. Res. 114, G01011. doi: 10.1029/2008JG000729
Wankel S. D., Kendall C., Pennington J. T., Chavez F. P., Paytan A. (2007). Nitrification in the Euphotic Zone as Evidenced by Nitrate Dual Isotopic Composition: Observations From Monterey Bay, California. Global Biogeochem. Cycle. 21, 1–13. doi: 10.1029/2006GB002723
Xu C., Dan S. F., Yang B., Lu D., Kang Z., Huang H., et al. (2021). Biogeochemistry of Dissolved and Particulate Phosphorus Speciation in the Maowei Sea, Northern Beibu Gulf. J. Hydrolog. 593, 125822. doi: 10.1016/j.jhydrol.2020.125822
Xue D., Botte J., De Baets B., Accoe F., Nestler A., Taylor P., et al. (2009). Present Limitations and Future Prospects of Stable Isotope Methods for Nitrate Source Identification in Surface-and Groundwater. Water Res. 43 (5), 1159–1170. doi: 10.1016/j.watres.2008.12.048
Xu C., Yang B., Dan S. F., Zhang D., Liao R., Lu D., et al. (2020). Spatiotemporal Variations of Biogenic Elements and Sources of Sedimentary Organic Matter in the Largest Oyster Mariculture Bay (Maowei Sea), Southwest China. Sci. Tot. Environ. 730, 139056. doi: 10.1016/j.scitotenv.2020.139056
Xu Y., Zhang T., Zhou J. (2019). Historical Occurrence of Algal Blooms in the Northern Beibu Gulf of China and Implications for Future Trends. Front. Microbiol. 10, 451. doi: 10.3389/fmicb.2019.00451
Yan X., Xu M. N., Wan X. S., Yang J.-Y. T., Trull T. W., Dai M., et al. (2017). Dual Isotope Measurements Reveal Zoning of Nitrate Processing in the Summer Changjiang (Yangtze) River Plume. Geophys. Res. Lett. 4 (12), 289–12,297. doi: 10.1002/2017GL075951
Yang Z., Chen J., Li H., Jin H., Gao S., Ji Z., et al. (2018). Sources of Nitrate in Xiangshan Bay (China), As Identified Using Nitrogen and Oxygen Isotopes. Estuar. Coast. Shelf Sci. 207, 109–118. doi: 10.1016/j.ecss.2018.02.019
Ye F., Jia G., Xie L., Wei G., Xu J. (2016). Isotope Constraints on Seasonal Dynamics of Dissolved and Particulate N in the Pearl River Estuary, South China. J. Geophys. Res.-Oceans 121 (12), 8689–8705. doi: 10.1002/2016JC012066
Ye F., Ni Z., Xie L., Wei G., Jia G. (2015). Isotopic Evidence for the Turnover of Biological Reactive Nitrogen in the Pearl River Estuary, South China. J. Geophys. Res.-Biogeosciences 120, 661–672. doi: 10.1002/2014JG002842
York J. K., Tomasky G., Valiela I., Repeta D. J. (2007). Stable Isotopic Detection of Ammonium and Nitrate Assimilation by Phytoplankton in the Waquoit Bay Estuarine System. Limnol. Oceanog. 52, 144–155. doi: 10.4319/lo.2007.52.1.0144
Yu X., Shen J., Du J. (2020). A Machine-Learning-Based Model for Water Quality in Coastal Waters, Taking Dissolved Oxygen and Hypoxia in Chesapeake Bay as an Example. Water Resour. Res. 56 (9), e2020WR027227. doi: 10.1029/2020WR027227
Zhang L., Altabet M. A., Wu T., Hadas O. (2007). Sensitive Measurement of NH4+ 15n/14n (δ15nh4+) at Natural Abundance Levels in Fresh and Saltwaters. Analytic. Chem. 79 (14), 5297–5303. doi: 10.1021/ac070106d
Zhang L., Wang L., Yin K., Lu Y., Zhang D., Yang Y., et al. (2013). Pore Water Nutrient Characteristics and the Fluxes Across the Sediment in the Pearl River Estuary and Adjacent Waters, China. Estuar. Coast. Shelf Sci. 133, 182–192. doi: 10.1016/j.ecss.2013.08.028
Keywords: nitrogen, stable nitrogen isotopes, biogeochemical processes, nitrogen cycling, Beibu Gulf
Citation: Chen C, Lao Q, Shen Y, Jin G, Chen F, Su Q, Lei X, Zhou X, Lu X, Zhu Q and Liu G (2022) Comparative Study of Nitrogen Cycling Between a Bay With Riverine Input and a Bay Without Riverine Input, Inferred From Stable Isotopes. Front. Mar. Sci. 9:885037. doi: 10.3389/fmars.2022.885037
Received: 27 February 2022; Accepted: 31 March 2022;
Published: 10 May 2022.
Edited by:
Meilin Wu, South China Sea Institute of Oceanology (CAS), ChinaCopyright © 2022 Chen, Lao, Shen, Jin, Chen, Su, Lei, Zhou, Lu, Zhu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fajin Chen, ZmpjaGVuQGdkb3UuZWR1LmNu
 Chunqing Chen
Chunqing Chen Qibin Lao
Qibin Lao Youli Shen2
Youli Shen2 Guangzhe Jin
Guangzhe Jin Fajin Chen
Fajin Chen