- 1Key Laboratory of Tropical Marine Bio-resources and Ecology, Guangdong Provincial Key Laboratory of Applied Marine Biology, Innovation Academy of South China Sea Ecology and Environmental Engineering, South China Sea Institute of Oceanology, Chinese Academy of Science, Guangzhou, China
- 2University of Chinese Academy of Sciences, Beijing, China
- 3Southern Marine Science and Engineering Guangdong Laboratory, Guangzhou, China
- 4Hainan Key Laboratory of Tropical Marine Biotechnology, Hainan Sanya Marine Ecosystem National Observation and Research Station, Sanya Institute of Oceanology Chinese Academy of Sciences, Sanya, China
P-element-induced wimpy testis (Piwi) is a key gene involved in germ cell development in a diverse range of organisms. However, in giant clams, the function of Piwi remains unclear. In the present study, we isolated the full-length cDNA of Piwi ortholog (Tc-Piwi1) and analyzed its expression patterns in the gonads of adult and juvenile Tridacna crocea. The results of qPCR showed that the transcript of Tc-Piwi1 was mainly expressed in gonad tissue. In addition, the relative expression level of Tc-Piwi1 increased with the proliferation of male and female germ cells during the adult gonad development stage, suggesting that Tc-Piwi1 might be involved in gametogenesis. In situ hybridization revealed that Tc-Piwi1 RNA was located in female and male germ cells and strongly expressed in male germ cells in the early stage. Furthermore, immunohistochemical experiments further confirmed that Tc-Piwi1 was mainly located in primordial germ cells (PGCs), germ stem cells (GSCs), and female and male germ cells of early development, so it could be used as a marker gene of T. crocea germ cells. Whole-mount in situ hybridization suggested that Tc-Piwi1 was of maternal origin and located in two clusters of cells in the trochophore-larvae stage, implying that these cells might be putative PGCs during the embryo development. Finally, Tc-Piwi1 was used as a molecular marker to elucidate the gonadal formation, sex differentiation, and gonadal maturation process of juvenile T. crocea for the first time in the Tridacna family. Collectively, all these results revealed that Tc-Piwi1 was involved in germline formation and sex differentiation in T. crocea.
Introduction
Giant clams are tropical marine shellfish with an important ecological value, which live in and around coral reefs in the tropical waters of the Indo-Pacific. They are effective ecosystem engineers and play multiple roles in coral reefs, including acting as important food suppliers for predators and scavengers, providing shelter for coral reef fish, and enhancing the stability of reef frameworks due to their dense population (Neo et al., 2015). In addition, the giant clam aquaculture industry has been supplying the food and marine aquarium trade market for about 40 years, which has gradually declined as a result of overexploitation, changes in the marine environment, and habitat degradation (Pandolfi et al., 2003; Zhao et al., 2020; He et al., 2021). Giant clams are simultaneous hermaphroditic marine bivalves. They initially reach sexual maturity as males and then develop ovaries and function simultaneously with the testes (Soo and Todd, 2014).
Germline is a cell lineage that segregates in the early stage of embryogenesis (Milani et al., 2017). The first germ cell is the primordial germ cells (PGCs), which are critical for animal reproductive development. In most organisms, PGCs are specified by maternally inherited cytoplasmic determinants (germplasm) during embryogenesis and then migrated to future gonad tissues (Sellars et al., 2007). Finally, PGCs reached an ultimate location and are encased in a somatic microenvironment called a niche to become the germ stem cells (GSCs) (Milani et al., 2017). Germ stem cells are specialized stem cells that can form gametes in the process of sexual reproduction. When an organism reaches sexual maturity, germ stem cells undergo self-renewal and differentiate into female and male germ cells and meiosis to produce functional gametes.
Argonaute/Piwi (Ago/Piwi, also known as PAZ Piwi domain proteins) family of protein has been discovered because of its function in stem cell self-renewal and germline development. Piwi proteins are predominantly present in germline and associated with a novel class of small RNAs known as Piwi-interacting, which serve diverse functions in germline development and gametogenesis (Cox et al., 1998). Substantial studies have shown that Piwi protein is necessary for germline specification and development (Cox et al., 1998; Carmell et al., 2007; Thomson and Lin, 2009). For instance, a murine homolog of Piwi (miwi), which encoded a cytoplasmic protein especially located in spermatocytes and spermatids, has been proven to be necessary for the process of spermatogenesis (Deng and Lin, 2002). In Piwi knockout mice, the genetic defects directly led to male infertility (Gou et al., 2017). In Danio rerio, zili and ziwi (homolog of Piwi in zebrafish) mutant fish have defects in oogenesis, as both mutations lead to female sterility (Cox et al., 1998). Piwi proteins can maintain germ stem cells by interacting with Polycomb group complexes PRC1 and PRC2 in niche and germline cells, thereby regulating ovarian germline stem cells and oogenesis in Drosophila melanogaster (Peng et al., 2016). Thus, the Piwi gene is an ideal target for regulatory studies to understand sexual differentiation and control sexual reproduction. In addition, Piwi is a component of germplasm that regulates the specification of PGCs during embryo development (Megosh et al., 2006). Immunofluorescence assays showed that Piwi protein colocalizes with germplasm markers such as Vasa, a highly conserved DEAD-box RNA helicase needed for germ cell formation (Lasko and Ashburner, 1988; Lasko and Ashburner, 1990). Thus, the Piwi gene could also be used to track the migration of PGCs and a germ-specific marker. For example, the expression patterns of Vasa and Piwi both showed that PGCs occurred in late embryogenesis (Schwager et al., 2015). In the centipede Strigamia maritima, Vasa and Piwi were used as marker genes, showing that the centipede PGCs are specified by zygotic mechanism (Green and Akam, 2014). In Crassostrea gigas, the Piwi-like gene was mainly expressed in the gonad, especially in the ovary. In addition, Piwi-like gene mRNA localized at a two-cluster cell which might be the putative PGCs by the gastrula stage, implying its potential to be used as a germ cell marker (Xu et al., 2020). In mollusk Chlamys farreri, Piwi1 is essential for gametogenesis because the knockdown of this gene causes apoptosis in spermatocytes and oocytes of early development (Ma et al., 2017).
In bivalve mollusks, especially giant clams, data on the origin and development of germline are very scarce. In aquaculture, the knowledge of these processes will facilitate better control of the reproduction of giant clams, which still depends largely on empirical techniques. Besides, it is difficult to accurately distinguish germ cells from surrounding somatic cells, especially in the early development stages. Thus, here, we report the isolation of a Piwi homolog from T. crocea and its expression pattern during gonadal development and embryogenesis. This paper aims to study the expression characteristics of Tc-Piwi1 and the origin and migration of PGCs and attempt to use Tc-Piwi1 as a molecular marker to track the gonadogenesis, sex differentiation, and sex maturation of junior T. crocea. These data can be applied to the boring giant clam reproduction and to help understand better the developmental processes of the hermaphrodite giant clam’s germline development.
Materials and Methods
Animals and Samples Collection
All the experimental giant clams were reared at the tropical marine biological research station in Sanya, Hainan province, China. Three individuals at the hermaphrodite stage were sacrificed, and eight tissues, including the heart, gills, hemocytes, gonads, pedis, siphonal mantle, digestive gland, and adductor muscle, were collected for tissue distribution analysis. The gonad tissue sampling method is as follows: for each individual, a part of the gonad tissue was collected and immediately immersed in TRIzol Reagent (Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA, United States), and the rest of the gonad tissues were fixed with 4% paraformaldehyde overnight for later histology examination. The gonad tissues of giant clams at six different gonad developmental stages (the undifferentiated stage, male-dominated bisexual-phase gonad, the oocyte growing stage in ovotestis, the oocyte mature stage I in ovotestis, the oocyte mature stage II in ovotestis, and the oocyte regression stage in ovary) described by Zhou (unpublished) were collected and immersed in TRIzol Reagent.
Animals that reach sex maturity were allowed to spawn and fertilized artificially. After fertilization, samples at various embryo and larvae stages (including fertilized eggs, 2-cell, 4-cell, 8-cell, blastula, gastrula embryos, trochophore, D-shaped larvae, veliger larvae, metamorphosed larvae) were collected for expression pattern analysis. Besides, for juvenile T. crocea, the whole gonad was sampled at regular intervals for hematoxylin and eosin staining (H&E staining) and immunohistochemistry experiments to track the development of germline cells. The whole tissue was fixed in Bouin’s solution for 12 h and then transferred to 70% alcohol according the protocol of H&E staining.
RNA Extraction and cDNA Synthesis
The total RNAs were isolated by TRIzol according to the manufacturer’s instructions. Briefly, each sample was immersed in 1 ml of TRIzol reagent and then added 200 μl of chloroform. The sample was vortexed vigorously for 10 s and placed at room temperature for 3 min. The sample was then centrifuged at the speed of 12,000×g at 4°C for 15 min, carefully absorbed 400–500 μl supernatant, and mixed with the same volume of isopropyl alcohol to precipitate RNA at −20°C. The samples were centrifuged at 12,000×g at 4°C for 10 min, the supernatant threw away, and washed the RNA pellet twice with 75% ethanol. Finally, RNA pellet was air dried and dissolved in DEPC-treated water. The RNA quality and quantity were assessed with 1.0% agarose gel electrophoresis and Nanodrop 2000 (Thermo, USA). The first-strand cDNA synthesis was carried out by PrimeScript™ RT reagent Kit with gDNA Eraser (Takara, Dalian, China). Fresh RNA samples extracted from gonads were pooled (4 µg in total), and the RACE-PCRs’ templates were synthesized using SMARTer RACE 5’/3’Kit (Takara, Dalian, China).
Cloning of Tc-Piwi1 and Sequence Analysis
The full-length Tc-Piwi1 cDNA sequence was acquired by 3′RACE and 5′RACE PCR using the above RACE-PCRs template. The 3′- and 5′-UTR were obtained by nested PCR, and the primers used are listed in Table 1 (designed by Primer 6.0 software based on the Piwi sequence of T. crocea transcriptome). PCR conditions were as follows: initial denaturation at 95°C for 3 min, 35 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 1 min, and a final extension at 72°C for 10 min. The initial product was diluted 50 times and used as the template for the second round of PCR reaction. The second PCR procedure consisted of denaturation at 94°C for 5 min, followed by 35 amplifications at 94°C for 15 s, 55°C–50°C for 30 s, and 72°C for 1 min, and a final extension at 72°C for 10 min. All these procedures were set according to the operation manual. The amplified DNA fragment was gel-purified and cloned into the PMD-19T vector (Takara, Dalian, China) for sequencing.
The amino acid sequence of Tc-Piwi1 protein was predicted by DNA star software. Conserve domain search was conducted by SMART; multiple sequence aliment was performed using the online tool Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/). The sequence-specific identities among aligned sequences were assessed by BLASTP at NCBI, and the phylogenetic tree of Piwi proteins was constructed using the MEGA 7.0 software with 1,000 bootstrap trials with the neighbor-joining (NJ) algorithm. The protein mass of Tc-Piwi1 and theoretical isoelectric point (PI) was obtained using the Compute PI/Mw tool (https://web.expasy.org/compute_pi/).
Transcriptional Analysis by Quantitative RT-PCR
Sequence-specific primers were designed, β-actin of T. crocea was used as a reference gene, and 60S ribosomal protein L5 (RPL5) was used as the reference gene only when calculating the relative expression of Tc-Piwi1 at different embryo development stages. The amplification was carried out in a total volume of 20 μl using the LightCycler 480II system (Roche, USA) with SYBR Green Master Mix (Takara, Dalian, China), following the manufacturer’s instructions. The qRT-PCR procedure consisted of denaturation at 94°C for 1 min, 40 cycles of amplification at 95°C for 15 s, 55°C for 30 s, and 72°C for 20 s, and 1 min signal collection at 85°C for 15 s in each cycle. At the end of each reaction, dissociation curve analysis was conducted to verify amplification specificity. The relative expression level of Tc-Piwi1 was calculated using the 2−ΔΔt method (Livak and Schmittgen, 2001). All data were presented as mean ± SD from three samples with three parallel repetitions.
In Situ Hybridization
According to the cDNA sequence of the Tc-Piwi1 gene, the 541-bp cDNA fragment of Tc-Piwi1 was amplified with sense and antisense primers. After the gene-specific fragment was acquired, the probe of Tc-Piwi1 was labeled with digoxigenin (DIG; Roche Applied Science, Germany). Gonadal tissues were fixed overnight with 4% RNase-free paraformaldehyde fixative at 4°C. The next day, the tissues were washed three times with PBS and dehydrated with 30% sucrose solution. Finally, dehydrated tissues were embedded with OCT and preserved at −80°C. This experiment was performed on the tissue sections as described by Liang et al. (2018). The whole-mount in situ hybridization (WISH) experiment was conducted according to the protocol described by Fabioux with some modifications using the same probe as above (Fabioux et al., 2004a; Fabioux et al., 2004b). Nikon e80i microscope was used for observation and digital image shooting.
Immunohistochemistry
The antigens of Tc-piwi1 (a.a. 384–724) were generated by the Escherichia coli recombinant protein expression system. The polyclonal antibody against Tc-Piwi1 was purified from the serum of a rabbit with antigen injection of Freund’s adjuvant four times at 12-day intervals (Genecreate Biological Engineering Company, Wuhan, China). Gonadal tissues were fixed in Bouin’s fixative for 24 h, dehydrated, and embedded in paraffin. Sections were cut at 5 mm thickness and incubated overnight at 37°C. Immunohistochemistry methods were described previously (Kobayashi et al., 2009). Briefly, sections were deparaffinized in xylene and hydrated in descending ethanol concentration, then antigen retrieval in 0.01 M citrate buffer (pH 6.0) was performed at 95°C for 10 min. When the temperature dropped to 37°C, sections were treated with 3% H2O2 in 80% methanol for 15 min and washed with PBS for 15 min. After that, samples were blocked in bovine serum albumin (3%) for 1 h and incubated with preimmune serum (for the negative control) and anti-Piwi1 primary antibody (1:250 dilution) at room temperature for 1 h. Specific goat-anti-rabbit IgG (1:1,000 dilution) was used as a secondary antibody and incubated for 1 h at room temperature. After washing with PBST, the sections were color developed with 3,3’-diaminobenzidine (DAB) and counterstained with hematoxylin. Tissue sections were then observed, and images were captured using a Nikon E80i microscope.
Statistical Analysis
All data were analyzed by SPSS18.0 and expressed as mean ± SD based on three biological replicates. The significance was analyzed by one-way ANOVA, and the detected difference was considered significant at p < 0.05.
Results
Characteristics of Tc-Piwi1
The full-length cDNA of Tc-Piwi1 was 3,492 bp, consisting of a 2,637-bp ORF, a 90-bp 5′-UTR, and a 765-bp 3′-UTR (GenBank accession OM372430). The ORF of Tc-Piwi1 encoded a deduced protein of 878 amino acids with a calculated molecular mass of 99.51 kDa and the theoretical isoelectric point (PI) of 9.36. The conserved domain analysis completed by SMART showed that Tc-Piwi1 protein contained two conserved domains: PAZ (a.a 294–430) and Piwi (a.a 571–864) (Supplementary Figure S1). Multiple sequence alignment showed that the Tc-Piwi1 protein shared high sequence identity with mollusks’ Piwi homologs, such as 65.7% identical to C. gigas, 67.1% to Mytilus galloprovincialis, and 60.8% to C. farreri. Besides, the sequence identity of Tc-Piwi1 shared 50.3%, 49.3%, and 49.1% similarity with Homo sapiens, Mus musculus, and Gallus gallus, respectively. The sequence similarity of Piwi between T. crocea and zebrafish was 50% (Figure 1). The phylogenetic tree showed that Tc-Piwi1 was first clustered with mollusks’ Piwi1 homologs, then clustered with a branch of Piwi homologs from H. sapiens, M. musculus, G. gallus, and D. rerio (Figure 2).
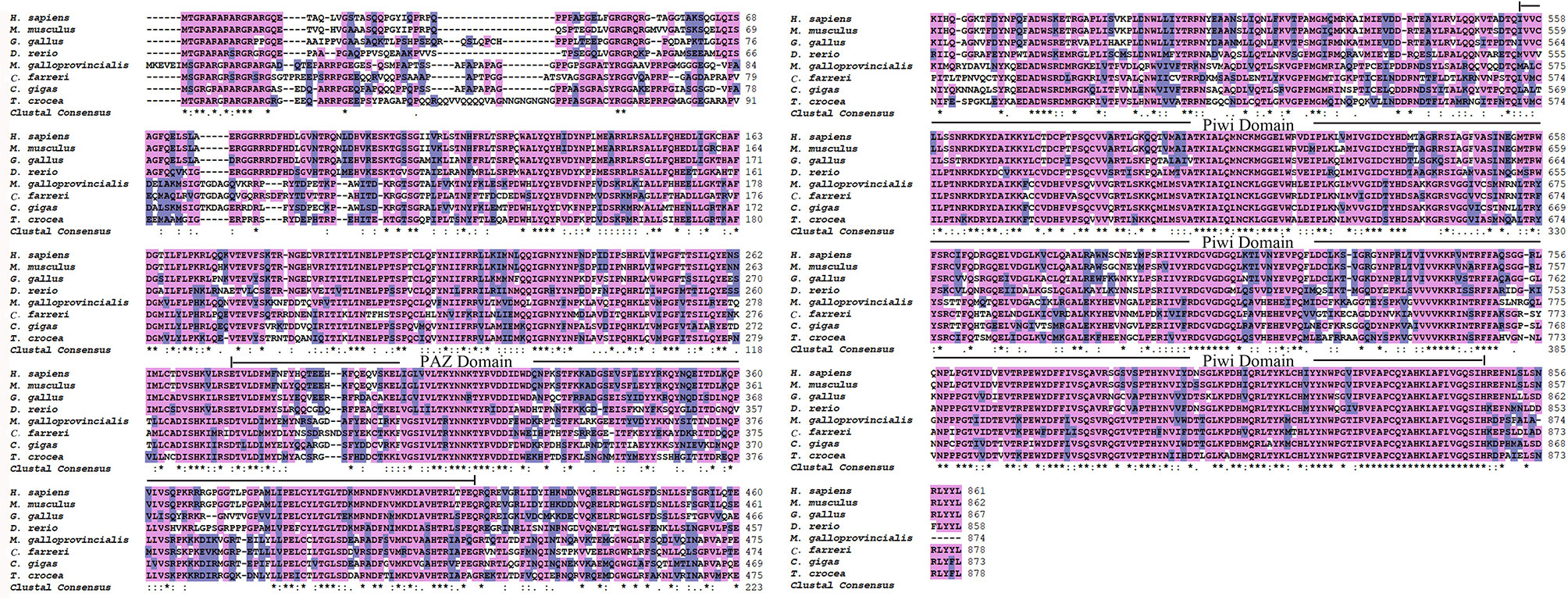
Figure 1 Multiple sequence alignment of Piwi1 proteins. The PAZ and Piwi domain are marked with a continuous line. The identical and similar sequences are highlighted in purple and gray, respectively. The accession numbers for the analyzed sequences are as follows: H. sapiens (NP 004755.2), M. musculus (NP067286.1), G. gallus (NP001092322.1), D. rerio (AAL57170.1), M. galloprovincialis (AMN88361.1), C. farreri (ALK82294.1), and C. gigas (XP 034338428.1) ‘*’ indicates positions which have a single, fully conserved residue; ‘:’ represent a similar amino acid residue position.
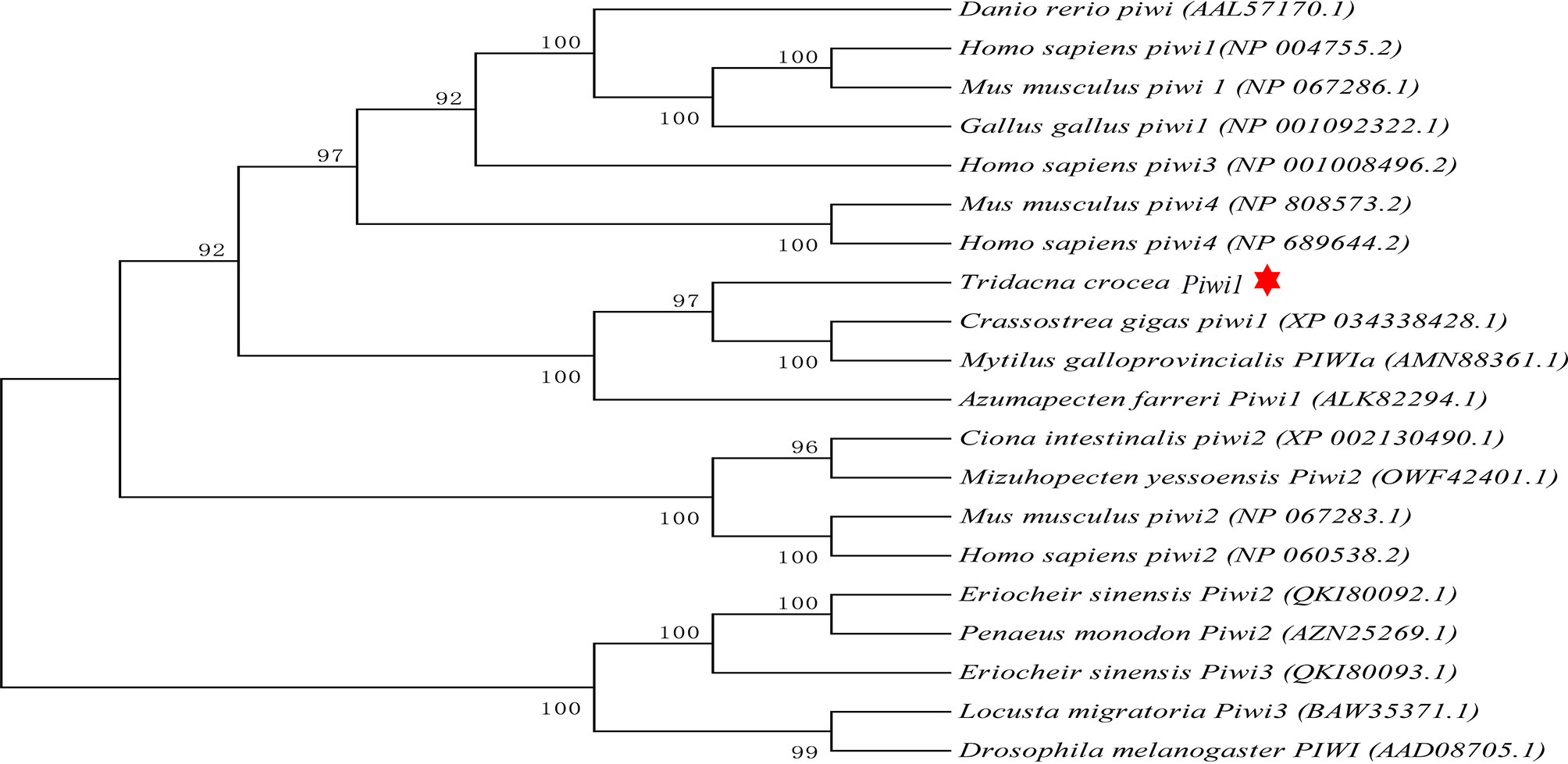
Figure 2 Phylogenetic analysis of Piwi orthologs using the neighbor-joining method with 1,000 bootstrap replications. Tc-Piwi1 protein was marked by a red star.
Expression Profiles of Tc-Piwi1 in Different Tissues and Adult Six Gonadal Developmental Stages
The spatial expression pattern of the Tc-Piwi1 gene in eight tissues of an adult hermaphroditic individual was analyzed by qRT-PCR. High levels of transcripts were observed in hermaphroditic gonadal tissues (ovotestis). In addition, the expression of the Tc-Piwi1 gene in gills, hemocytes, and digestive glands was very low, while it was almost not expressed in other tissues (Figure 3A). In the development stage of adult gonads, the result of qRT-PCR showed that the expression of Tc-Piwi1 increased significantly in stages I and II (p < 0.05) and gradually declined with the maturation of female germ cells. Finally, the expression of Tc-Piwi1 reached a low level in stages IV and V, which is comparable with that in stage 0 (p > 0.05) (Figure 3B).
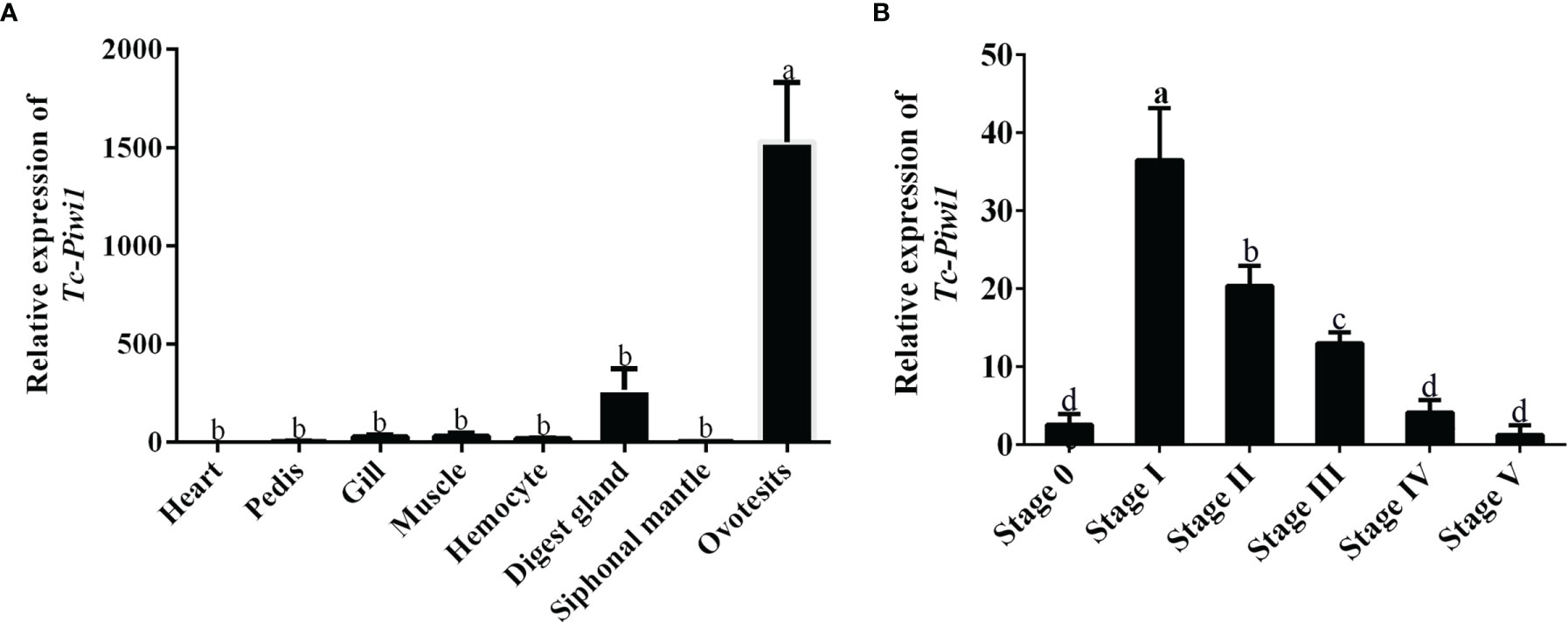
Figure 3 Expression profiles of Tc-Piwi1 in (A) different tissues and (B) adult six gonad developmental stages by qRT-PCR. Stage 0 to stage V represent the undifferentiated stage, the male-dominated bisexual-phase gonad, the oocyte growing stage in ovotestis, the oocyte mature stage I in ovotestis, the oocyte mature stage II in ovotestis, and the oocyte regression stage in the ovary, respectively. β-Actin was used as a reference gene. Data are represented as the mean ± SD (N = 3), bars not sharing with the same letter are significantly differed at p < 0.05.
Location of Tc-Piwi1 in Adult Gonad Tissues of T. crocea
The location of Tc-Piwi1 mRNA in the gonad of adult T. crocea was confirmed by the result of in situ hybridizations (ISH) in the male and female gonads. Tc-Piwi1 mRNA was found in both female and male germ cells and showed a stronger signal in male germ cells at the early development stage. No signal was observed in somatic cells of female and male gonad tissues. A relatively weak signal of Tc-Piwi1 was observed in mature oocyte cytoplasm, a strong signal was observed in spermatogonia and spermatocytes, but no signal was detected in mature spermatids (Figure 4).
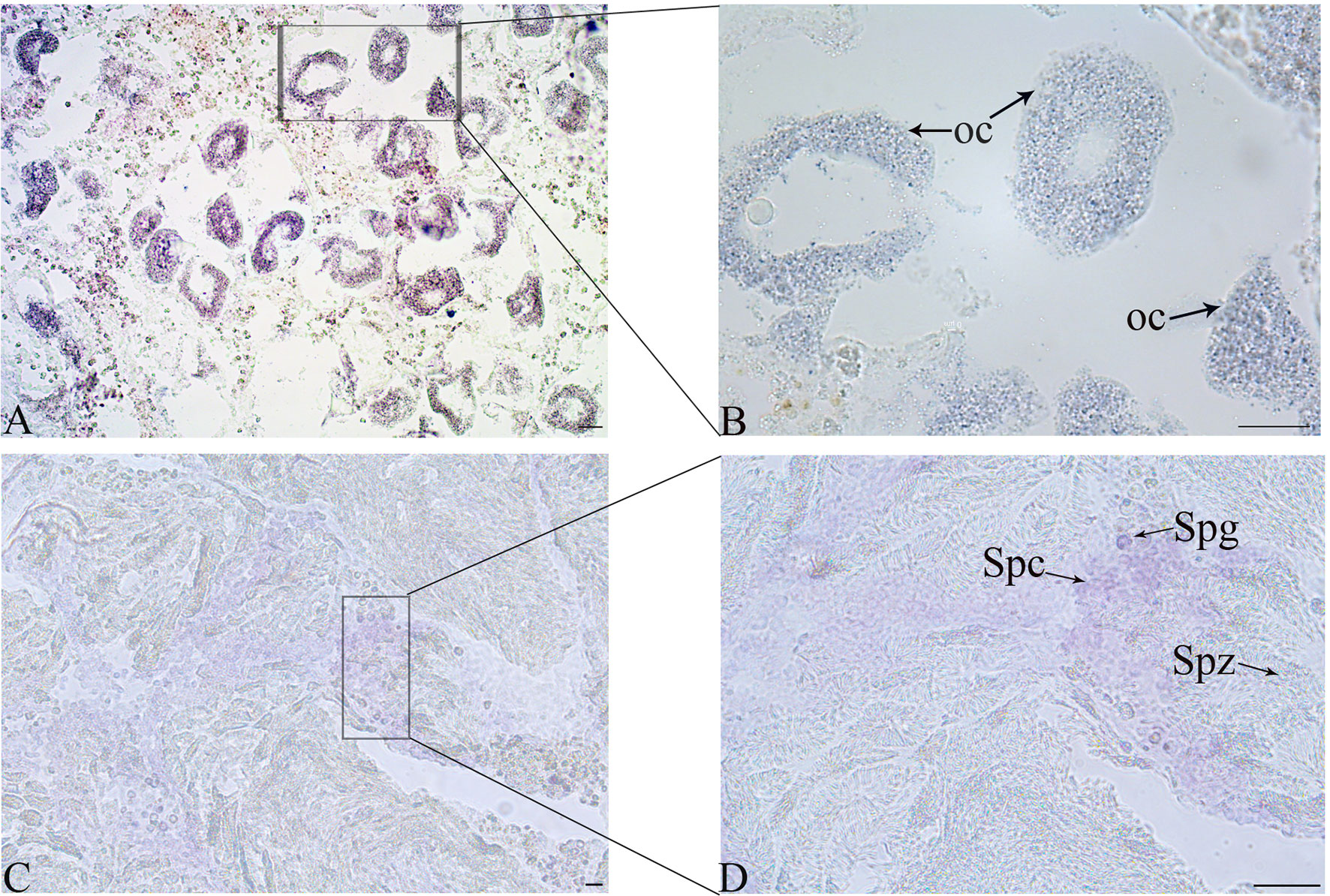
Figure 4 Cellular location of Tc-Piwi1 in female and male gonad tissues demonstrated by in situ hybridization (ISH). (A, B) The results of ISH with Tc-Piwi1 mRNA antisense probe in the ovary (Scale bar = 40 µm). (C, D) represent the results of ISH in testis by Tc-Piwi1 mRNA antisense probe (Scale bar = 25 μm). The positive signals were stained with dark-blue or purple. The cells in histological sections include spermatozoa (Spz), spermatocyte (Spc), spermatogonia (Spg), and oocyte (Oc).
Temporal Expression Pattern of Tc-Piwi1 During Embryogenesis
The result of qRT-PCR showed that Tc-Piwi1 mRNA was maternally deposited in oocytes which showed higher expression in the early embryo from fertilized eggs to blastula stages. While the expression level became lower from gastrulation to the juvenile (Figure 5A). The results of WISH showed that positive hybridization signals of Tc-Piwi1 mRNA appeared in the fertilized eggs. As the fertilized eggs developed into 2-cells, 4-cells, and 8-cells, the transcripts of Tc-Piwi1 were located uniformly at the cleavage cells. At the trochophore-larvae stage, the uniform distribution disappeared. Tc-Piwi1 mRNA gradually centralized and progressively become restricted to two small descendent micromeres in mesoderm at the trochophore-larvae stage; we speculated that these preliminary cells in these two clusters are PGCs or the founders of PGCs in T. crocea. At the D-larvae stage and middle veliger larvae stage, only a smaller spot can be detected around the digestive tissues (Figure 5B).
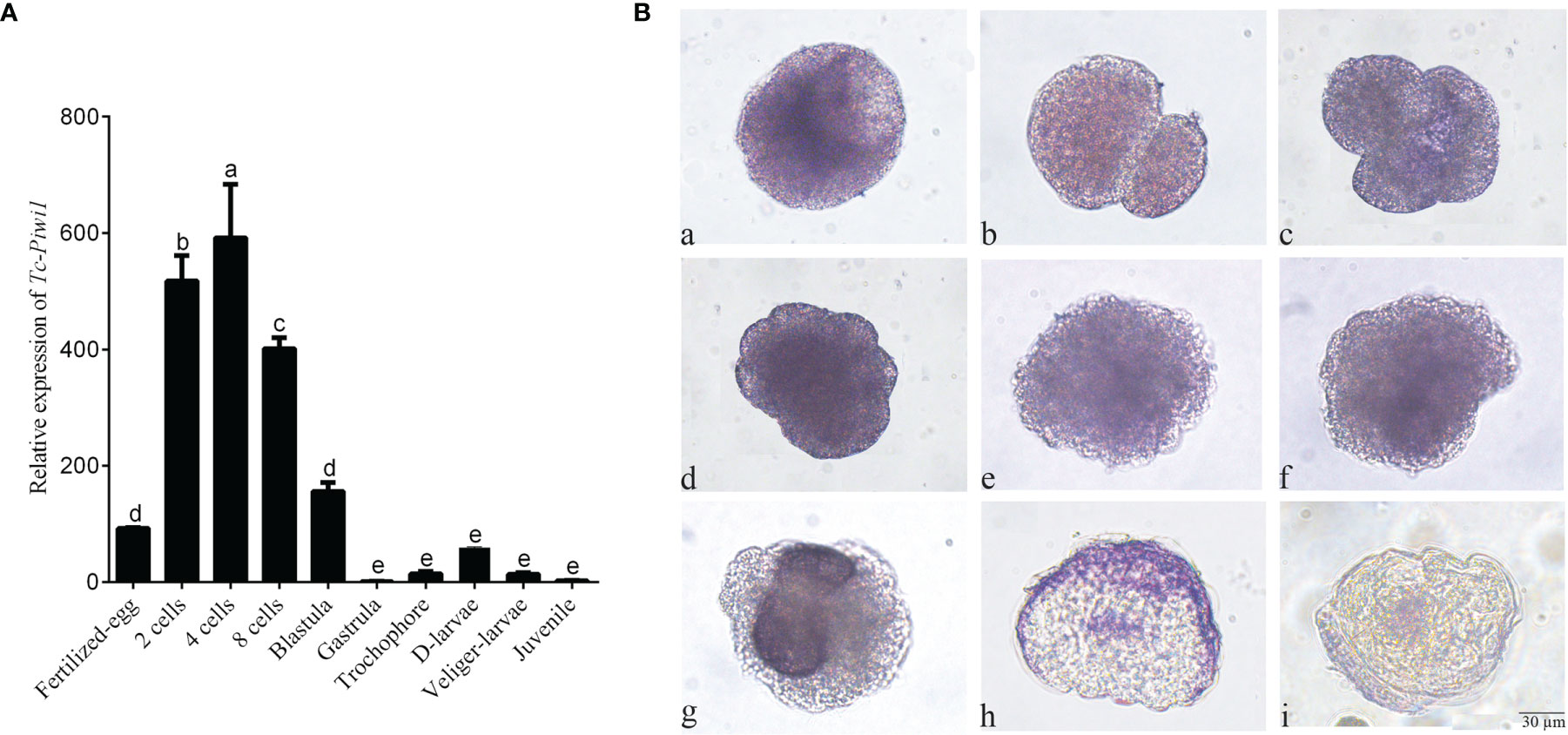
Figure 5 Expression of Tc-Piwi1 gene in fertilized oocytes and various embryonic stages of T. crocea. (A) Quantitative real-time PCR results for Tc-Piwi1, and RPL5 was used as a reference gene. Data are represented as the mean ± SD (N = 3), bars not sharing with the same letter are significantly differed at p < 0.05. (B) Location of Tc-Piwi1 in the fertilized egg and various developmental stages of embryos by WISH. (a) Fertilized eggs; (b) 2 cells; (c) 4 cells; (d) 8 cells; (e) Blastula; (f) Gastrula; (g) trochophore larvae; (h) D-shaped larvae; (i) Veliger larvae. Scale bar = 30 µm.
Development and Differentiation of Germ Cell in Juvenile T. crocea
The identification of cell types was performed according to the description of Awaji and Hamano (2004). Gonads of most juveniles under 4-month old remained undifferentiated. However, the gonads of 5-month-old individuals (shell length 4.00 ± 1.00 mm) contained several PGCs, which are larger in size and have an intense positive signal of Tc-Piwi1, which occurred in the gonadal cavity and could be distinguished from the somatic cells of gonads (Figure 6A). At 6-month of age (shell length 15.00 ± 1.00 mm), the number of PGCs increased and enclosed by a lay of somatic cells; thus, these PGCs were also germ stem cells (GSCs) (Figure 6B). The number of GSCs increased with the development of giant clams to 8-month old. Meanwhile, with the proliferation of GSCs and somatic cells, the volume of the gonadal cavity increased (Figure 6C). As gonadal development progresses, a small number of spermatogonia were observed on the gonad of several T. crocea individuals of 10-month old, and a strong signal of Tc-Piwi1 was observed in spermatogonia (Figure 6D). At the age of 12 months, a large number of individuals reached the testicular maturation stage, with a large amount of sperm and a small number of spermatogonia and spermatocytes. Tc-Piwi1 was strongly expressed in spermatogonia, and a relatively weak signal was detected in spermatocytes (Figure 6E). Testicular maturation lasts for several months and may experience the sperm release phase. In the gonads of 17-month-old individuals, several oogonia were found in ovotestis, and a strong signal of Tc-Piwi1 was observed in oogonia (Figure 6F). However, when the season shifted to winter, most oogonia stopped further development and entered the resting stage. The resting stage is characterized by connective tissue fulling in the gonad, and a small number of gonad cavities fulling with undifferentiated germ cells were visible, which may differentiate into male and female germ cells (Figure 6G). Until the age of 25 months (the second reproduction cycle), substantial individuals developed into the mature stage of ovotestis, which was characterized by a large number of mature oocytes and sperm, and the signals of Tc-Piwi1 protein in mature oocyte was weaker than that in oogonia (Figure 6H). The hermaphroditic stage lasted about 5 months.
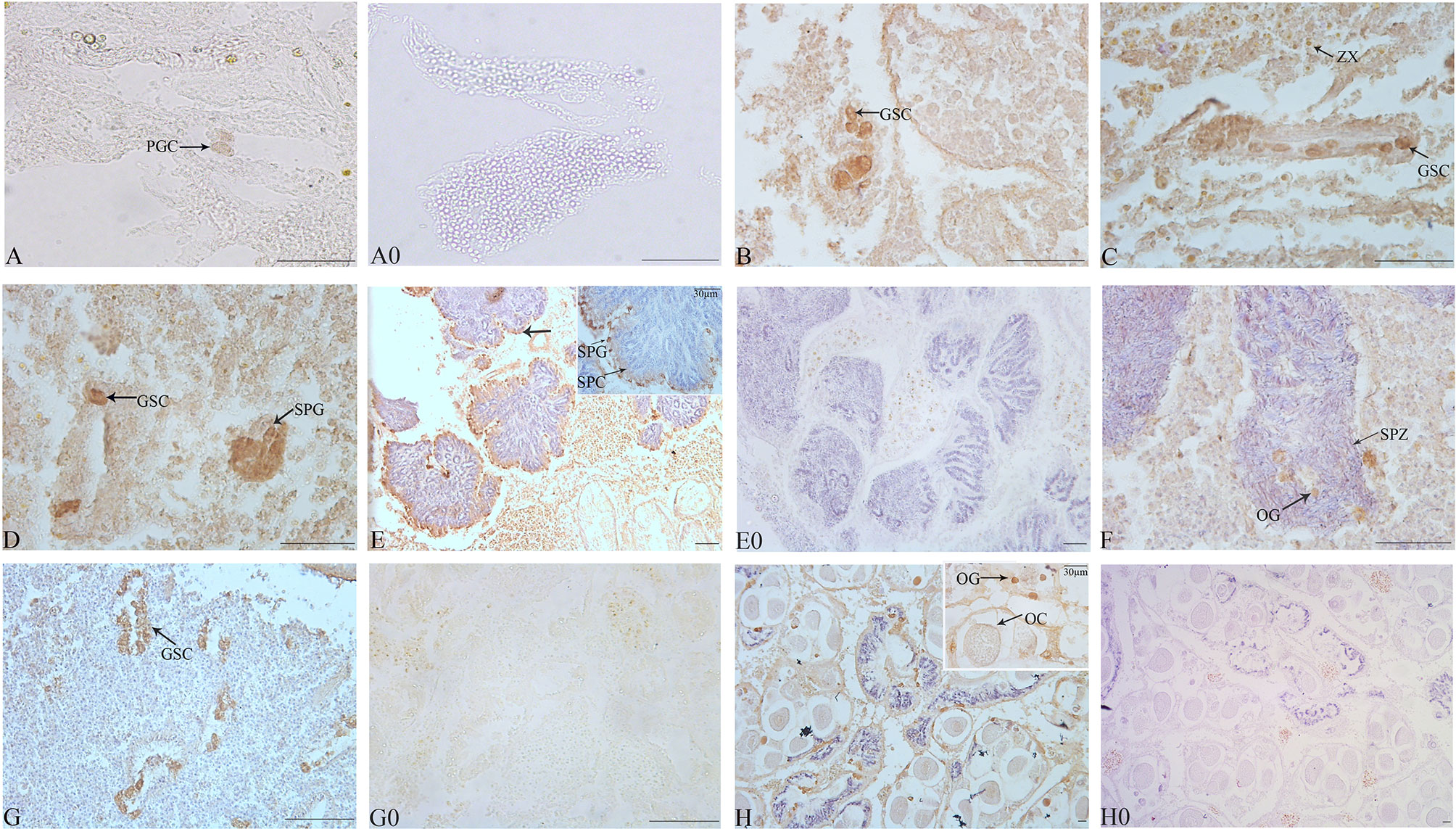
Figure 6 Observation of the gonad at the early development of juvenile T. crocea by immunohistochemistry. (A–H) The immunohistochemistry of gonads of T. crocea at 5, 6, 8, 10, 12, 17, 21, and 25 months, respectively. Germ cells specifically stained by anti-Tc-Piwi1 antibody are in dark brown. The sections incubated without the primary antibodies did not show any immunostainings (A0, E0, G0, and H0). The cells in histological sections include primordial germ cells (PGC), germ stem cells (GSC), spermatozoa (SPZ), spermatocyte (SPC), spermatogonia (SPG), oocyte (OC), oogonia (OG), and symbiotic dinoflagellates (zooxanthellae (ZX)). Scale bar = 20 µm.
Discussion
Understanding the gonadal development of giant clams is conducive to the better development of the artificial breeding of Tridacna. PGCs generation and gametogenesis are two key components of animal reproduction. Previous studies have reported that the Piwi gene plays an important role in the reproduction system (Houwing et al., 2007; Thomson and Lin, 2009). At present, a Piwi1 gene of T. crocea was cloned; Tc-Piwi1 protein contains conserved PAZ and Piwi domains, which has a high sequence identity with the Piwi homolog of vertebrates, which might imply that Tc-Piwi1 protein also has the same conserved role in invertebrate giant clams.
The result of tissue distribution analysis of Tc-Piwi1 showed that the gene was mainly expressed in gonads, which was consistent with the prediction that this gene had important functions in the reproductive system. In addition, the expression of Tc-Piwi1 is relatively low in other tissues, such as gills, hemocytes, digestive glands, and muscles, which means that it may have other functions in these organs, such as the involvement in the immune response. Gills, hemocytes, and digestive glands are regarded as immune organs involved in the innate immune response and phagocytosis of microbes in mollusks. In mosquitoes, Piwi exhibited an antiviral response mediated with Piwi RNAs (piRNAs) (Hess et al., 2011; Leger et al., 2013). In Lymnaea stagnalis and C. gigas, there is high expression of Piwi in muscle and reproduction tract due to the active Piwi-piRNA-dependent transposon silencing events in these tissues (Jehn et al., 2018). It has been reported that the Piwi gene plays an important function in gametogenesis, germ cell maintenance, and germline fate specification (Thomson and Lin, 2009). At present, the high expression of Tc-Piwi1 in stages I and II of adult gonadal development indicated that Tc-Piwi1 is also involved in the process of gametogenesis, which has been confirmed in C. farreri (Ma et al., 2017). It needs to be further verified by functional experiments in T. crocea.
Although Piwi was mainly expressed in gonad tissues, the cellular localization of Piwi in gonads among different species is different. Ziwi gene of zebrafish, the homolog of Piwi, is expressed at various stages of oocytes, showing a higher level of expression in oogonia and early-stage oocytes and a lower expression level in later-stage oocytes (Houwing et al., 2007). In T. crocea, the Tc-Piwi1 signal in the mature oocyte is relatively weak. During spermatogenesis, Tc-Piwi1 mRNA was abundant in spermatogonia and then decreased in spermatocytes and almost not expressed in mature sperm. This expression pattern was similar to that in fish and most mollusks (Houwing et al., 2008; Ma et al., 2017). While, a murine Piwi gene showed high expression in spermatids (Deng and Lin, 2002). Combined with the result of ISH in the present study, Tc-Piwi1 was mainly located in male germ cells in early development, and its expression was low in mature germ cells, which was different from mammals.
In this study, the results of qPCR showed that Tc-Piwi1 mRNA was of maternal origin, which was consistent with the expression pattern of the Piwi gene in Pacific oysters (Rui et al., 2020), Drosophila (Mani et al., 2014), and zebrafish (Tan et al., 2002). In mollusks, maternal-to-embryonic transition (MET) occurs from fertilized eggs to gastrula, showing that the expression levels of Piwi, Nanos, and Vasa decreased gradually (Fabioux et al., 2004b; Xu et al., 2018; Xu et al., 2020). However, at the egg cleavage phase, the expression of Tc-Piwi1 mRNA increased sharply from the 2-cell stage to the 8-cell stage, which is similar to the Piwi expression in bovine early embryo (Russell et al., 2016). In the MET period of an early bovine embryo, maternal transcripts and protein were gradually replaced, and reprogramming events occurred promptly after fertilization and persisted through the MET phase but are accompanied by an increase in retrotransposon expression (Russell et al., 2016). Thus, we speculated that the increased expression level of Tc-Piwi1 (from 2-cell stage to 8-cell stage) was mainly to protect the genome from the increase of retrotransposon activity. While the increased expression of Tc-Piwi1 in the cleavage stage is part of the regulatory pathway that constrains the expression of retrotransposon, the exact reason needs to be confirmed by further research. The results of WISH also showed that Tc-Piwi1 mRNA had maternal supply and was uniformly distributed in early cleavage. In early trochophore larvae, Tc-Piwi1 mRNA was detected on the right and left of the midline of the larvae, which was similar to the location of oyster vasa-like gene (Fabioux et al., 2004b) and Pacific oyster Nanos gene (Xu et al., 2020), indicating that these two cluster cells were the PGCs or the founders of PGCs. Finally, Tc-Piwi1 mRNA remained at a low expression level in the area of the future gonadal tissues near the digestive gland. There are two basic models of the original of PGCs: one is maternally provided germplasms determine germ cell fate (preformation), and another is cell types inducing PGCs fate (epigenesis) (Herpin et al., 2007; Feng et al., 2011). This conserved expression pattern of Tc-Piwi1 might suggest that both preformation and epigenesis of germ cell specification mechanisms might coexist in giant clams during embryonic development (Kranz et al., 2010; Xu et al., 2020).
Through the immunohistochemical method, it was confirmed for the first time that Tc-Piwi1 was specifically expressed in germ cells and could be used as a marker gene of germ cells. This result was consistent with the Piwi gene in Pacific oysters (Xu et al., 2020). In addition, the process of gonad formation, sex differentiation, and gonad maturation of juvenile T. crocea were clarified for the first time in the family Tridacna. The first step of gonadogenesis is the appearance of the gonad cavity, which was constituted of squamous endothelial cells (Awaji and Hamano, 2004). This step was not observed in the present research because the gonad tissues of a juvenile under 5-month old were too small to obtain the complete structure of the gonadal cavity. The PGCs were observed histologically in the gonad of 5-month T. crocea individuals for the first time, which occurred 2 months later than that in juvenile abalone (Awaji and Hamano, 2004). In Viviparus viviparus, PGCs are originated from pericardial tissues (Griffond, 1977). In the present study, we inferred that PGCs were located in the digestive gland according to the result of WISH. The emergence of spermatogonia, which are larger cells with a central coarse nucleus according to Franco’s description, confirms the beginning of male sex differentiation (Franco et al., 2008). Spermatogonia of juvenile T. crocea were first observed at about 10 months of age and mature sperm were observed at about 12 months of age, implying that T. crocea was male in the first year, which is consistent with that of Pinctada maxima (Adzigbli et al., 2019). The sex differentiation of females was confirmed by the appearance of oogonia in the gonad. The oogonia of T. crocea were first observed at about 17 months of age, and the mature oocytes were observed at about 25 months of age (in the second reproduction season), implying that T. crocea juveniles reached hermaphrodite at about 25 months of age, much earlier than T. gigas, which take about 10 years to achieve functional reproduction (Gomez et al., 2000). Thus, T. crocea, the giant clam species with the fastest sexual maturity, is a good material to study the mechanism of gonadal development of the family Tridacna.
Conclusion
In the present study, we obtained a Piwi1 gene and explored its potential role in the gonadal development of T. crocea. The expression profile showed that Tc-Piwi1 had a high expression level in gonad tissues, mainly located in PGCs, GSCs, female and male germ cells of early development, suggesting that Tc-Piwi1 could be used as a germ cell marker. In addition, the relative expression level increased with the proliferation of male and female germ cells during adult gonadal development, especially in stages I and II, revealing that Tc-Piwi1 might involve in gametogenesis. Furthermore, WISH examination showed that Tc-Piwi1 was of maternal origin, and the localization of Tc-Piwi1 mRNA in mesodermal cells might be the putative PGCs in T. crocea during the embryo development stage. Finally, using Tc-Piwi1 as a molecular marker, the process of sex differentiation and gonad maturation of T. crocea juvenile was also clarified for the first time in the family Tridacna. Collectively, all these results indicated that Tc-Piwi1 is involved in germline formation and differentiation in T. crocea.
Data Availability Statement
‘The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Author Contributions
JL and ZY: conceived the study. YYZ: performed experiments and drafted the manuscript; YL, QL, GS, and YQ carried out the laboratory work and participated in the data analysis. HM and YHZ collected the giant clams. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the National Key Research and Development Program of China (2020YFD0901102; 2018YFD0901400); the Chinese Ministry of Science and Technology through the National Science Foundation of China (31872566; 31702340; 32002387 ;42076121, M-0163); Key Special Project for Introduced Talents Team of Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou) (GML2019ZD0402); the Network Service Local Plan STS of the Chinese Academy of Sciences (KFJ-STS-QYZD-158); the Innovation Academy of South China Sea Ecology and Environmental Engineering, Chinese Academy of Sciences (ISEE2018PY01; ISEE2018ZD02); the Open Foundation of the State Key Laboratory of Loess and Quaternary Geology (SKLLQG1813; SKLLQG1918); Guangdong Basic and Applied Basic Research Foundation (2021A1515011181); National Marine Genetic Resource Center; China Agriculture Research System of MOF and MARA (CARS-49); Guangxi innovation-driven development program (No. AA19254032); and the Science and Technology Planning Project of Guangdong Province, China (2020B1212060058).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.883661/full#supplementary-material
Supplementary Figure 1 | Full-length of the Tc-Piwi1 cDNA and its conserved domains of predicted amino acid in T. crocea.(A) The start codon and termination codon are in red, the sequence of predicted amino acid sequence is in blue, the PAZ and Piwi domain are shadowed and marked by black box, respectively. (B) Two conserved domains of Tc-Piwi1 in T. crocea.
References
Adzigbli L., Wang Z., Lai Z., Li J., Deng Y. (2019). Sex Determination in Pearl Oyster: A Mini Review. Aquac. Rep. 15, 100214. doi: 10.1016/j.aqrep.2019.100214
Awaji M., Hamano K. (2004). Gonad Formation, Sex Differentiation and Gonad Maturation Processes in Artificially Produced Juveniles of the Abalone, Haliotis Discus Hannai. Aquaculture 239, 397–411. doi: 10.1016/j.aquaculture.2004.03.010
Carmell M. A., Girard A., Van de Kant H. J. G., Bourc'his D., Bestor T. H., de Rooij D. G., et al. (2007). MIWI2 is Essential for Spermatogenesis and Repression of Transposons in the Mouse Male Germline. Dev. Cell. 12, 503–514. doi: 10.1016/j.devcel.2007.03.001
Cox D. N., Chao A., Baker J., Chang L., Qiao D., Lin H. F. (1998). A Novel Class of Evolutionarily Conserved Genes Defined by Piwi are Essential for Stem Cell Self-Renewal. Genes Dev. 12, 3715–3727. doi: 10.1101/gad.12.23.3715
Deng W., Lin H. F. (2002). Miwi, a Murine Homolog of Piwi, Encodes a Cytoplasmic Protein Essential for Spermatogenesis. Genes Dev. 2, 819–830. doi: 10.1016/s1534-5807(02)00165-x
Fabioux C., Huvet A., Lelong C., Robert R., Pouvreau S., Daniel J. Y., et al. (2004b). Oyster Vasa-Like Gene as a Marker of the Germline Cell Development in Crassostrea Gigas. Biochem. Bioph. Res. Co. 320, 592–598. doi: 10.1016/j.bbrc.2004.06.009
Fabioux C., Pouvreau S., Le Roux F., Huvet A. (2004a). The Oyster Vasa-Like Gene: A Specific Marker of the Germline in Crassostrea Gigas. Biochem. Biophys. Res. Commun. 315, 897–904. doi: 10.1016/j.bbrc.2004.01.145
Feng Z.-F., Zhang Z.-F., Shao M.-Y., Zhu W. (2011). Developmental Expression Pattern of the Fc-Vasa-Like Gene, Gonadogenesis and Development of Germ Cell in Chinese Shrimp, Fenneropenaeus Chinensis. Aquaculture 314, 202–209. doi: 10.1016/j.aquaculture.2011.02.017
Franco A., Heude Berthelin C., Goux D., Sourdaine P., Mathieu M. (2008). Fine Structure of the Early Stages of Spermatogenesis in the Pacific Oyster, Crassostrea Gigas (Mollusca, Bivalvia). Tissue Cell. 40, 251–260. doi: 10.1016/j.tice.2007.12.006
Gomez E., Mingoa-Licuanan S., Roa-Quaoit H. (2000). “The Culture of the True Giant Clam Tridacna Gigas for Conservation in the Philippines,” in Conference proceedings: special session on mollusk research in Asia (Los Baños, Philippines: University of the Philippines), 159–163.
Gou L.-T., Kang J.-Y., Dai P., Wang X., Li F., Zhao S., et al. (2017). Ubiquitination-Deficient Mutations in Human Piwi Cause Male Infertility by Impairing Histone-To-Protamine Exchange During Spermiogenesis. Cell 169, 1090–1104. doi: 10.1016/j.cell.2017.04.034
Green J. E., Akam M. (2014). Germ Cells of the Centipede Strigamia Maritima are Specified Early in Embryonic Development. Dev. Biol. 392, 419–430. doi: 10.1016/j.ydbio.2014.06.003
Griffond B. (1977). Individualisation Et Organogenèse De La Gonade Embryonnaire Deviviparus Viviparus L.(Mollusque Gastéropode Prosobranche À Sexes Séparés). Wilhelm. Roux's. Arch. Dev. Biol. 183, 131–147. doi: 10.1007/BF00848783
He G., Liu X., Xu Y., Liang J., Deng Y., Zhang Y., et al. (2021). Repeated Exposure to Simulated Marine Heatwaves Enhances the Thermal Tolerance in Pearl Oysters. Aquat. Toxicol. 239, 105959. doi: 10.1016/j.aquatox.2021.105959
Herpin A., Rohr S., Riedel D., Kluever N., Raz E., Schartl M.J.B.D.B. (2007). Specification of Primordial Germ Cells in Medaka (Oryzias Latipes). BMC Dev. Biol. 7, 1–10. doi: 10.1186/1471-213X-7-3
Hess A. M., Prasad A. N., Ptitsyn A., Ebel G. D., Olson K. E., Barbacioru C., et al. (2011). Small RNA Profiling of Dengue Virus-Mosquito Interactions Implicates the PIWI RNA Pathway in Anti-Viral Defense. BMC Microbiol. 11(1), 1–12. doi: 10.1186/1471-2180-11-45
Houwing S., Berezikov E., Ketting R. F. (2008). Zili is Required for Germ Cell Differentiation and Meiosis in Zebrafish. EMBO J. 27, 2702–2711. doi: 10.1038/emboj.2008.204
Houwing S., Kamminga L. M., Berezikov E., Cronembold D., Girard A., van den Elst H., et al. (2007). A Role for Piwi and piRNAs in Germ Cell Maintenance and Transposon Silencing in Zebrafish. Cell 129, 69–82. doi: 10.1016/j.cell.2007.03.026
Jehn J., Gebert D., Pipilescu F., Stern S., Kiefer J. S. T., Hewel C., et al. (2018). PIWI Genes and piRNAs are Ubiquitously Expressed in Mollusks and Show Patterns of Lineage-Specific Adaptation. Commun. Biol. 1, 1–11. doi: 10.1038/s42003-018-0141-4
Kobayashi T., Chang X. T., Nakamura M., Kajiura H., Nagahama Y. (2009). Fish 3β-Hydroxysteroid Dehydrogenase/Δ5-Δ4isomerase: Antibody Production and Their Use for the Immunohistochemical Detection of Fish Steroidogenic Tissues. Zool. Sci. 13, 909–914. doi: 10.2108/zsj.13.909
Kranz A. M., Tollenaere A., Norris B. J., Degnani B. M., Degnani S. M. (2010). Identifying the Germline in an Equally Cleaving Mollusc: Vasa and Nanos Expression During Embryonic and Larval Development of the Vetigastropod Haliotis Asinina. J. Exp. Zool. Part B. 314B, 267–279. doi: 10.1002/jez.b.21336
Lasko P. F., Ashburner M. (1988). The Product of the Drosophila Gene Vasa is Very Similar to Eukaryotic Initiation Factor-4A. Nature 335, 611–617. doi: 10.1038/335611a0
Lasko P. F., Ashburner M. (1990). Posterior Localization of Vasa Protein Correlates With, But is Not Sufficient for, Pole Cell Development. Genes Dev. 4, 905–921. doi: 10.1101/gad.4.6.905
Leger P., Lara E., Jagla B., Sismeiro O., Mansuroglu Z., Coppee J. Y., et al. (2013). Dicer-2-and Piwi-Mediated RNA Interference in Rift Valley Fever Virus-Infected Mosquito Cells. J. Virol. 87, 1631–1648. doi: 10.1128/jvi.02795-12
Liang T., Jia Y., Zhang R., Du Q., Chang Z. (2018). Identification, Molecular Characterization and Analysis of the Expression Pattern of SoxF Subgroup Genes the Yellow River Carp, Cyprinuscarpio. J. Genet. 97(1), 157–172. doi: 10.1007/s12041-018-0898-8
Livak K. J., Schmittgen T. D. (2001). Analysis of Relative Gene Expression Data Using Real-time Quantitative PCR and the 2−ΔΔCT Method. Methods 25(4), 402–408. doi: 10.1006/meth.2001.1262
Ma X., Ji A., Zhang Z., Yang D., Liang S., Wang Y., et al. (2017). Piwi1 is Essential for Gametogenesis in Mollusk Chlamys Farreri. PeerJ 5, e3412. doi: 10.7717/peerj.3412
Mani S. R., Megosh H., Lin H. (2014). PIWI Proteins are Essential for Early Drosophila Embryogenesis. Dev. Biol. 385, 340–349. doi: 10.1016/j.ydbio.2013.10.017
Megosh H. B., Cox D. N., Campbell C., Lin H. (2006). The Role of PIWI and the miRNA Machinery in Drosophila Germline Determination. Curr. Biol. 16, 1884–1894. doi: 10.1016/j.cub.2006.08.051
Milani L., Pecci A., Ghiselli F., Passamonti M., Bettini S., Franceschini V., et al. (2017). VASA Expression Suggests Shared Germ Line Dynamics in Bivalve Molluscs. Histochem. Cell Biol. 148, 157–171. doi: 10.1007/s00418-017-1560-x
Neo M. L., Eckman W., Vicentuan K., Teo S. L. M., Todd P. A. (2015). The Ecological Significance of Giant Clams in Coral Reef Ecosystems. Biol. Conserv. 181, 111–123. doi: 10.1016/j.biocon.2014.11.004
Pandolfi J. M., Bradbury R. H., Sala E., Hughes T. P., Bjorndal K. A., Cooke R. G., et al. (2003). Global Trajectories of the Long-Term Decline of Coral Reef Ecosystems. Science 301, 955–958. doi: 10.1126/science.1085706
Peng J. C., Valouev A., Liu N., Lin H. (2016). Piwi Maintains Germline Stem Cells and Oogenesis in Drosophila Through Negative Regulation of Polycomb Group Proteins. Nat. Genet. 48, 283–291. doi: 10.1038/ng.3486
Russell S. J., Stalker L., Gilchrist G., Backx A., Molledo G., Foster R. A., et al. (2016). Identification of PIWIL1 Isoforms and Their Expression in Bovine Testes, Oocytes, and Early Embryos. Biol. Reprod. 94, 75. doi: 10.1095/biolreprod.115.136721
Schwager E. E., Meng Y., Extavour C. G. (2015). Vasa and Piwi are Required for Mitotic Integrity in Early Embryogenesis in the Spider Parasteatoda Tepidariorum. Dev. Biol. 402, 276–290. doi: 10.1016/j.ydbio.2014.08.032
Sellars M. J., Lyons R. E., Grewe P. M., Vuocolo T., Leeton L., Coman G. J., et al. (2007). A PL10 Vasa-Like Gene in the Kuruma Shrimp, Marsupenaeus Japonicus, Expressed During Development and in Adult Gonad. Mar. Biotechnol. (NY). 9, 377–387. doi: 10.1007/s10126-007-6118-9
Soo P., Todd P. A. (2014). The Behaviour of Giant Clams (Bivalvia: Cardiidae: Tridacninae). Mar. Bio. 161, 2699–2717. doi: 10.1007/s00227-014-2545-0
Tan C.-H., Lee T.-C., Weeraratne S. D., Korzh V., Lim T.-M., Gong Z. (2002). Ziwi, the Zebrafish Homologue of the Drosophila Piwi: Co-Localization With Vasa at the Embryonic Genital Ridge and Gonad-Specific Expression in the Adults. Mech. Dev. 119, S221–S224. doi: 10.1016/s0925-4773(03)00120-5
Thomson T., Lin H. (2009). The Biogenesis and Function of PIWI Proteins and piRNAs: Progress and Prospect. Annu. Rev. Cell Dev. Biol. 25, 355–376. doi: 10.1146/annurev.cellbio.24.110707.175327
Xu R., Li Q., Yu H., Kong L. (2018). Oocyte Maturation and Origin of the Germline as Revealed by the Expression of Nanos-Like in the Pacific Oyster Crassostrea Gigas. Gene 663, 41–50. doi: 10.1016/j.gene.2018.04.021
Xu R, Li Q, Yu H (2020). Expression Pattern of Piwi-Like Gene Implies the Potential Role in Germline Development in the Pacific Oyster Crossosrea Gigas. Aquac. Rep. 18, 100486. doi: 10.1016/j.aqrep.2020.100486
Keywords: Piwi1, germ cell, molecular maker, giant clams, Tridacna crocea
Citation: Zhou Y, Li Y, Liao Q, Shi G, Qin Y, Zhang Y, Ma H, Li J and Yu Z (2022) Developmental Expression Pattern of the Piwi1 Gene, Timing of Sex Differentiation and Maturation in Artificially Produced Juvenile Boring Giant Clam, Tridacna crocea. Front. Mar. Sci. 9:883661. doi: 10.3389/fmars.2022.883661
Received: 25 February 2022; Accepted: 30 March 2022;
Published: 06 May 2022.
Edited by:
Xiaotong Wang, Ludong University, ChinaReviewed by:
Xiaoting Huang, Ocean University of China, ChinaMi Zhao, Temple University, United States
Copyright © 2022 Zhou, Li, Liao, Shi, Qin, Zhang, Ma, Li and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Li, anVuLmxpQHNjc2lvLmFjLmNu; Ziniu Yu, Y2FybHp5dUBzY3Npby5hYy5jbg==
 Yinyin Zhou
Yinyin Zhou Yunqing Li
Yunqing Li Qingliang Liao
Qingliang Liao Gongpengyang Shi
Gongpengyang Shi Yanpin Qin
Yanpin Qin Yuehuan Zhang
Yuehuan Zhang Haitao Ma
Haitao Ma Jun Li
Jun Li Ziniu Yu
Ziniu Yu