
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Mar. Sci., 31 March 2022
Sec. Marine Biology
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.880337
 Yijing Han1†
Yijing Han1† Chaoyi Xie1†
Chaoyi Xie1† Nini Fan2
Nini Fan2 Hongce Song1
Hongce Song1 Xiaomei Wang2
Xiaomei Wang2 Yanxin Zheng2
Yanxin Zheng2 Meiwei Zhang1
Meiwei Zhang1 Yaqiong Liu1
Yaqiong Liu1 Baoyu Huang1
Baoyu Huang1 Lei Wei1*
Lei Wei1* Xiaotong Wang1*
Xiaotong Wang1*The Pacific oyster, Crassostrea gigas, is widely distributed and is substantially important to shellfish cultivation. Oysters with melanin-colored shells and soft parts are increasingly popular and are of high economic value. However, the distribution and synthesis of melanin in C. gigas remains unclear and there have been only few relevant studies on it. In this study, transmission electron microscopy (TEM) was used to observe melanin in the epidermis of dark mantle tissue. Melanocytes containing melanosomes were observed below the epidermis, suggesting the possibility of a gradual transfer of melanin from the mantle tissue to the epithelium. The frozen section technique and three melanin staining methods were used to confirm the presence of melanin. The results suggest that enzyme identification (DOPA) is a suitable method of melanin staining in the mantle tissues of C. gigas. This study preliminarily identified the existence of melanin in the mantle tissues of C. gigas and is a foundation for the study of melanin synthesis. Furthermore, it provides new insights into the mechanism of shell color formation.
The diversity of mollusk shell colors has attracted increased attention in recent decades (Comfort, 1951; Pietak, 1998; Kocot et al., 2016; Huang et al., 2021). Colorful shells have an esthetic value that has attracted the attention and appreciation of many scientists (Canales-Gómez et al., 2010; Cai et al., 2011; Zhu et al., 2021). Shell colors, such as black, white, gold, and orange, have become one of the selection factors for consumers, driving researchers and farmers to study, select, and breed oysters with different shell colors (Nell, 2001; Brake et al., 2004; Zhu et al., 2018). It is generally believed that beautiful and diverse shell colors are determined by pigments, such as melanin, carotenoids, and tetrapyrrole (Williams, 2017). Furthermore, shell color may be influenced by genetic control, diet, and the environment (Liu et al., 2009; Zhu et al., 2018). However, the mechanism of pigment deposition in mollusk shells is not fully understood (Williams, 2017).
Crassostrea gigas is a globally distributed mollusk that has been well-studied in the fields of ecology, genetics, and biology. It is also a potential model organism for marine mollusks (Zhang et al., 2012; Yu et al., 2016). There are many studies on shell color in C. gigas (Ge et al., 2015; Xu et al., 2017; Han and Li, 2021). The mantle, located near the shell surface, is a key organ for shell formation (Ivanina et al., 2017). The mollusk mantle can secrete organic matrix components responsible for controlling shell formation (McDougall and Degnan, 2018). Proteomic data also show that a variety of proteins related to shell formation are expressed in the mantle (Zhang and Zhang, 2006; Marin et al., 2007; Kocot et al., 2016). A study revealed the positive correlation between pigmentation at the edge of the mantle and shell color in C. gigas (Brake et al., 2004). After several generations of genetic breeding, there is a high probability of pigmentation on the edge of the mantle of C. gigas that is consistent with the occurrence of the black shell (Kang et al., 2013). Therefore, the mantle may be an important factor affecting the shell color. Although, the black mantle of C. gigas is more popular than it once was (Nell, 2001; Kang et al., 2013), the precise mechanism of melanin formation, and evidence of the relationship between melanin and shell color are unknown. Studies show that tyrosinase is important in the synthesis of oyster melanin, and the promotion of shell growth and pigmentation (Yu et al., 2014; Feng et al., 2019; Zhu et al., 2021). However, the location of melanin and the biological process of melanin formation in the mantle tissue of oysters are still unclear.
Several methods are currently used to identify melanin and melanocytes, including the reduction method (Masson-Fontana silver staining), enzyme identification (DOPA reaction), and ferrous ion uptake (ferrous sulfate staining) (Barbosa et al., 1984; Wu et al., 2010; Pang et al., 2016). In Saccostrea glomerata hemocytes, melanin was detected using the Fontana-Masson staining method (Butt and Raftos, 2008). Melanin distribution in Balanus eburneus epidermal cells was measured using the ferrous sulfate staining method (Barden and Koulish, 1983). However, few studies have focused on melanin distribution in the mantle of C. gigas. Therefore, this experiment was conducted to detect melanin in the mantle of C. gigas, as a foundational study on melanin formation and shell coloration in mollusks.
Healthy C. gigas individuals (shell length: 8-10 cm) were selected from Changdao Farm, Yantai, Shandong province, China. Before the experiment, the animals were domesticated for two weeks in aerated seawater (pH 8.1, salinity, 29‰ and temperature 18°C). The mantle tissues were obtained using a scalpel to quickly open the oyster. During the collection of transmission electron microscopy (TEM) samples, the mantle tissues were cut into cuboids (1 × 1 × 3 mm3 in volume) under an anatomical microscope and immediately placed in 2.5% glutaraldehyde with phosphate buffer saline (PBS) for fixation. During the collection of frozen section samples, the mantle tissues were cut into tissue blocks with a volume of less than 6 mm3, placed in liquid nitrogen for rapid freezing and stored in a refrigerator at -80°C.
The mantle samples were fixed with glutaraldehyde for 4 h and postfixed with 1% osmium tetroxide for 1 h. Tissue blocks were dehydrated with 50%, 70%, 90%, and 100% acetone for 15 min each. The dehydrated tissue blocks were embedded in Epon 812 resin and cured at 37°C, 45°C, and 60°C for 24 h each. Using semi-thin sections, toluidine blue staining was used to determine the areas required for the research. Samples were cut into ultra-thin sections of 70 nm with ultratome (Reichert-Jung ULTRACUT E, Austria) and stained with uranium acetate and lead citrate double staining. The samples were observed using TEM (JEOL, JEM1200, Japan).
The frozen section experiment was performed as described by Pandiar et al. (2021), with minor modifications. After the frozen slicer (Leica, CM1950, USA) was pre-cooled to -20°C, a layer of OCT embedding agent (Solarbio, China) was added to the chucks and completely frozen. Subsequently, the frozen tissues were placed on the chucks and additional OCT embedding agent was added until they were submerged. The tissue-filled chucks were placed on a freezing stand for freezing and then placed in a frozen slicer for sectioning at a thickness of 10 μm.
Dopa staining method was performed according to procedures reported previously with minor modifications (Yamashita et al., 2005). A mixture of 0.1 mol/L disodium hydrogen phosphate solution and 0.1 mol/L potassium dihydrogen phosphate solution in a ratio of 9:1 (pH = 7.4) was used to prepare 0.0056 mol/L 3,4-dihydroxy-L-phenylalanine (Macklin Reagent Company, China) solution, also called DOPA staining solution. Sample sections (10 μm thick) were immersed in DOPA staining solution and incubated at 37.5°C. After 30 min, the pre-cooled DOPA staining solution was replaced and microscopic examination was performed every 30 min. After approximately 4 h, the staining solution turned brown and the sample sections were immediately washed with ultrapure water to stop the reaction. Neutral red staining solution was counterstained for 1 min under a biological microscope (Nikon Corporation, Tokyo, Japan). Brown or black particles indicated positive results.
Masson-Fontana melanin staining was performed using a Masson-Fontana staining kit (Solarbio, G2032, China) according to the manufacturer’s instructions. The sample sections (10 μm thick) were transferred to Fontana ammoniac silver solution and dyed in the dark at 56°C for 30–40 minutes. During dyeing process, the microscope was continuously used for observation to avoid excessive dyeing. After washing with ultrapure water, the samples were treated with Hypo solution for 3 min and washed with running water for 5 min. The sections were counterstained with neutral red staining solution for 1 min and observed under microscope (Nikon Corporation, CI-L, Japan). Brown or black particles in the tissue samples indicated positive results.
Ferrous sulfate melanin staining was performed using a ferrous sulfate staining kit (Solarbio, G2031, China) according to the manufacturer’s instructions. Specifically, the sample sections were placed in ferrous solution for 1.5 hours and washed with ultrapure water five times. The samples were placed in acid potassium ferricyanide solution for 30 min and then counterstained with nuclear fast red solution for 2 min. After washing, the tissue samples viewed under microscope (Nikon Corporation, CI-L, Japan) showed melanin in green and nuclei in red.
As shown in Figure 1A, epithelial cells are closely aligned to form the epidermis of the mantle. A nucleus containing dense chromatin was also observed. In addition, many electron-dense components of different sizes were found in the upper, middle, and lower epidermis. These were speculated to be pigment particles in the epidermis. In addition to the epidermis, the mantle tissue also contained muscles and fibers below the basement membrane (Figure 1B). Cells located below the basement membrane were loosely distributed, but intact melanocytes and pigment granules were observed (Figure 1B). Among them, melanosomes with different electron-density components and irregularly shaped membranes were distributed in the cytoplasm of melanocytes.
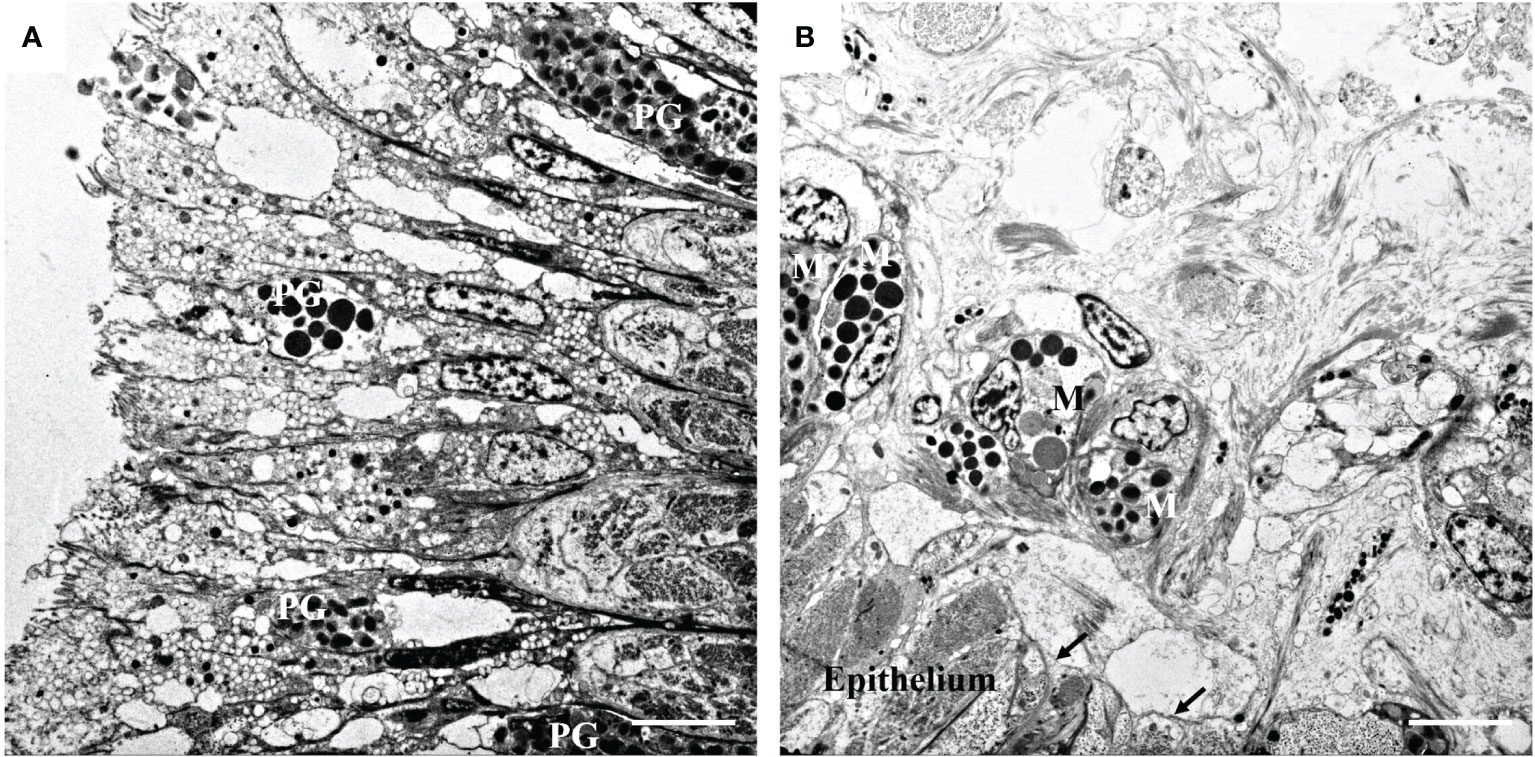
Figure 1 Transmission electron microphotographs of the mantle. (A) Pigmented granules (PG) in mantle epithelium. (B) General view showing the melanocytes (M) and the melanosomes in the melanocytes beneath the basement membrane (arrow). Bar, 5 μm.
The locations of the frozen sections selected on the mantle are shown in Figure 2A. Based on the ability of melanin to reduce the silver ions in silver ammonia solution to metallic silver, the presence of melanin was indirectly identified through an argyrophilic reaction. As shown in Figure 2B, the black area of the mantle surface contained a substantial number of black particles and melanin was located there. The white area of the mantle surface contained few black particles and less melanin.
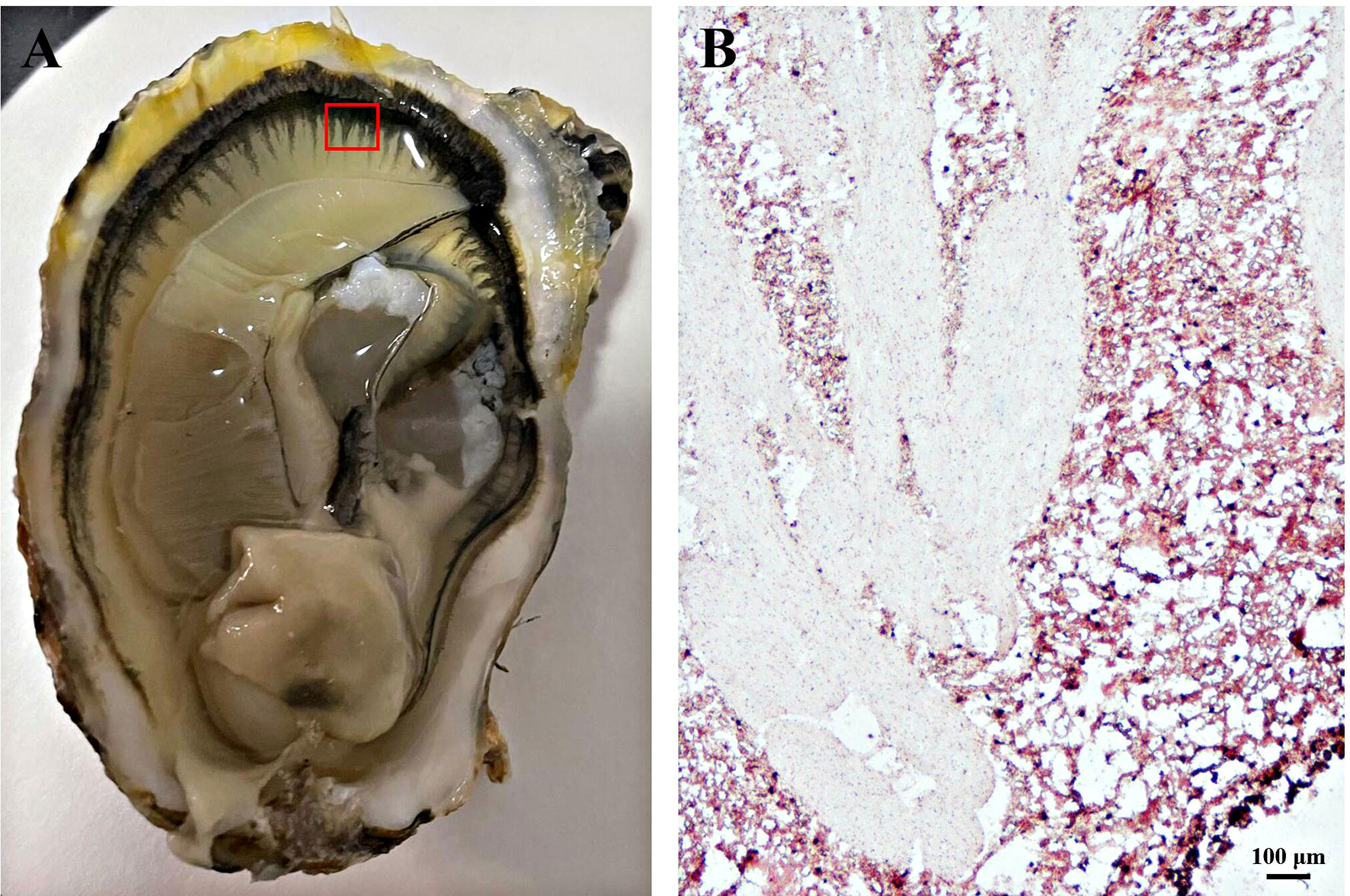
Figure 2 Masson-Fontana staining of melanin in frozen-sectioned mantle tissue. (A) The area of mantle tissue used for frozen section analysis. (B) Masson-Fontana staining of melanin in mantle tissue and counterstaining with neutral red stain. Brown or black particles indicate positive results. Bar, 100 μm.
The DOPA staining method is a better method for staining melanin because it cannot stain silver particles, chromaffin particles, or lipofuscin. As shown in Figures 3A, B, DOPA-positive staining positions were observed in many parts of the mantle tissue. Consistent with the results in Section 3.2, more melanin was detected in the black part of the mantle surface, whereas less melanin existed in the white part (Figures 3A, B). Additionally, there was a large number of black particles in the mantle epidermis, indicating that melanin may be more distributed in the black part of the epidermis. The nuclei, fibers, and cuticle of the epidermis became red after neutral red counterstaining (Figure 3B).
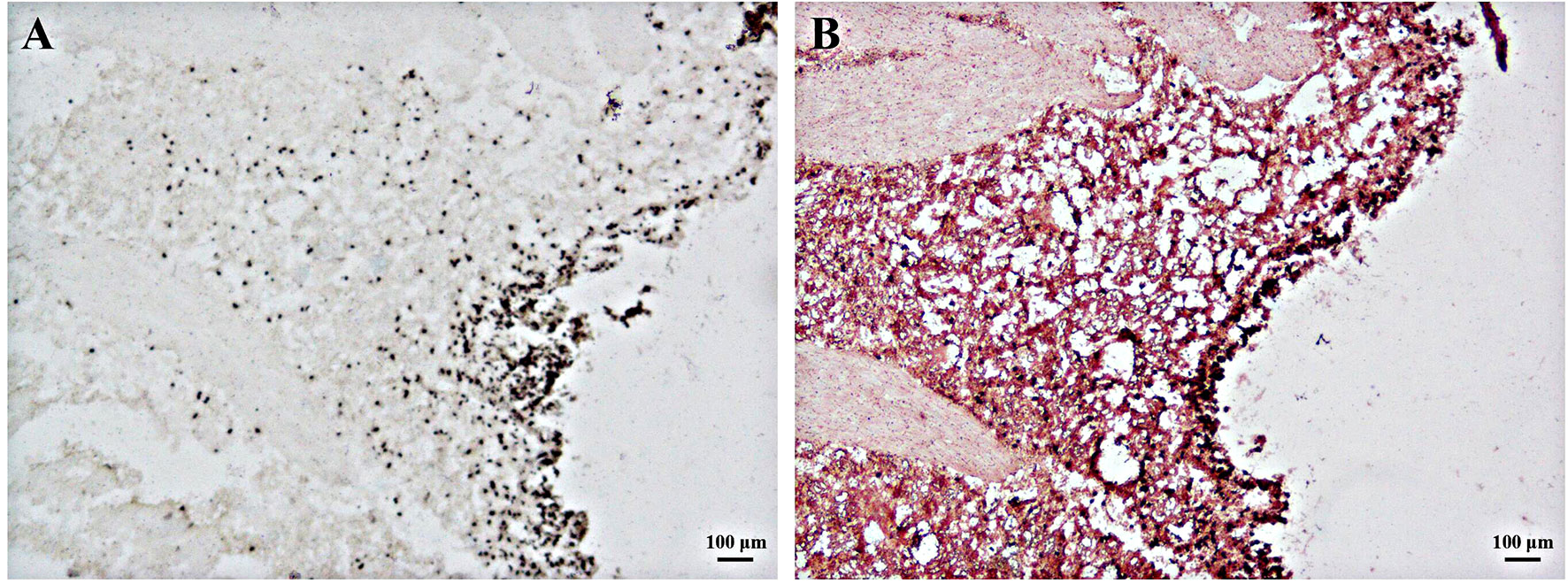
Figure 3 DOPA staining of melanin in frozen-sectioned mantle tissue. (A) DOPA staining of melanin in mantle tissue with (B) and without (A) neutral red counterstaining. Brown or black particles indicate positive results. Bar, 100 μm.
As shown in Figure 4, the ferrous sulfate staining method was used to detect the distribution of melanin in mantle tissues. Part of the mantle region showed positive light green staining (Figure 4A). After counterstaining with nuclear fast red dye, the green positive part was less obvious (Figure 4B). This may be because the green color shown by the ferrous sulfate method was not evident in the tissue sections with a background color.
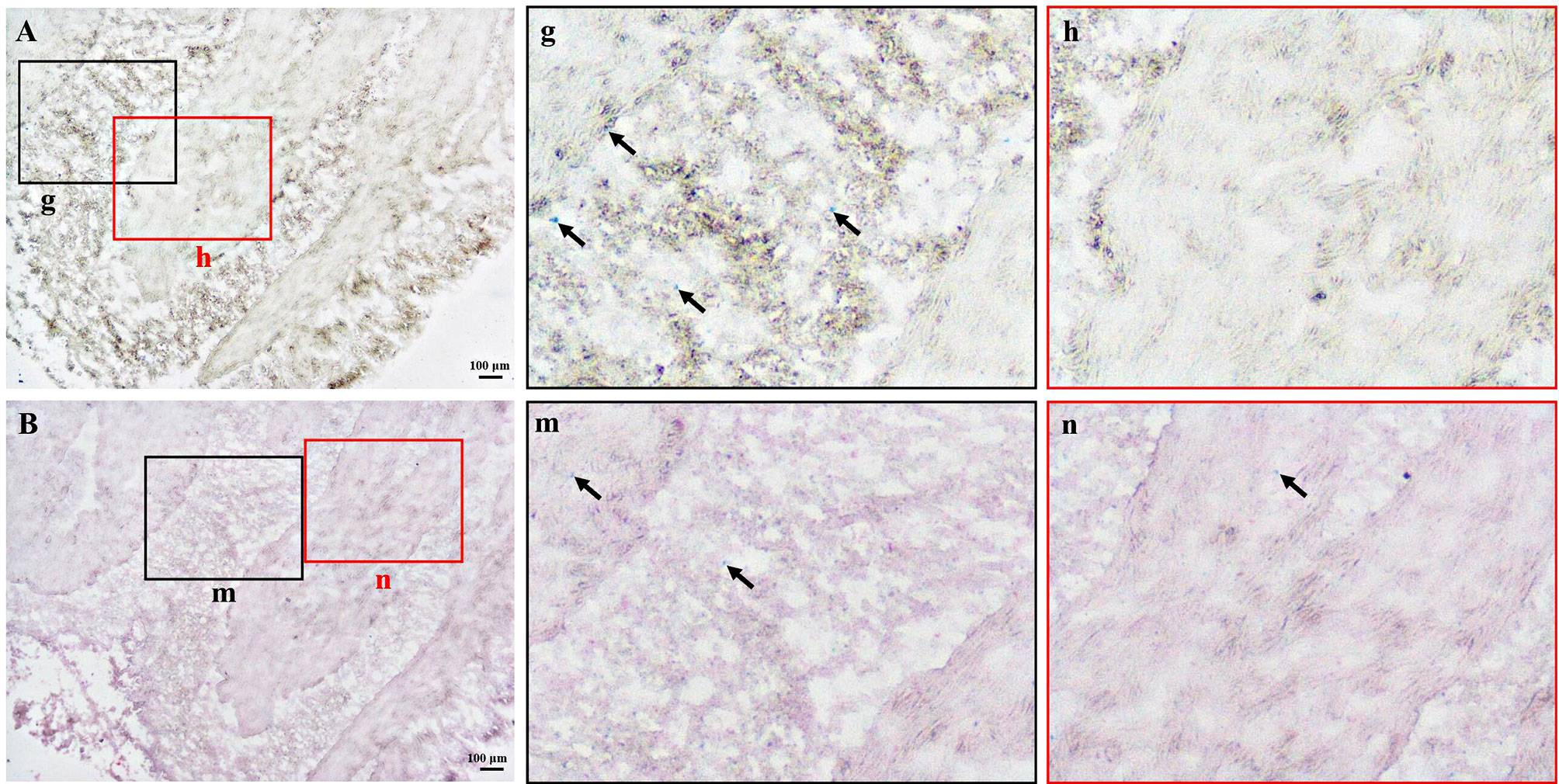
Figure 4 Ferrous sulfate staining of melanin in frozen-sectioned mantle tissue. (A) Ferrous sulfate staining of melanin in mantle tissue with (B) and without (A) nuclear fast red counterstaining. The enlarged area in the black and red boxes corresponding to Figure A or B (g, h, m, n). The green areas (arrow) represent positive results. Bar, 100 μm.
In this study, pigment particles were observed in the epidermis of the mantle tissue of C. gigas. It is generally believed that the amount of melanin in the epidermis determines the depth of skin color. This indicates that the amount of melanin in the mantle of C. gigas is closely related to the number of pigment particles in the mantle tissue. Similar results were found in the foot epithelium of abalone. The existence of melanocytes with high electron density particles in the deeper side of the foot epithelium was identified using ultrastructural analysis, but not in the light-colored sole foot epithelium (Bravo Portela et al., 2012). In normal human skin, a large amount of melanin exists in the basal layer of the epidermis and after UV stimulation melanin is transferred to the epidermal cells through a long process to prevent UV radiation (Nielsen et al., 2006). However, in this study, TEM results showed that the basal layer was not observed in the mantle tissues. Notably, pigment particles were found in the mantle epidermis and melanocytes were found below the basement membrane of the mantle tissues. It has been speculated that melanocytes secrete melanin to the epidermis by transferring melanin.
Melanin is produced by melanocytes in melanosomes through complex cellular processes (D’Alba and Shawkey, 2019). Melanosomes gradually mature after stages I to IV, and the melanin in the melanosomes is then transferred to keratinocytes resulting in skin pigmentation (Ebanks et al., 2011; Benito-Martínez et al., 2020). The results of TEM showed that the granular material in melanocytes, below the basement membrane, had an irregular shape and contained membranous or granular matter presumed to be melanosomes. Studies of the cuttlefish, Sepia officinalis, show that early melanosomes are mostly irregular in shape, contain vesicular or granular material inside, and may be derived from the Golgi complex or endoplasmic reticulum (Schraermeyer, 1994). In abalone, Haliotis tuberculata, the Golgi complex was found in melanocytes and the Golgi was shown to promote the formation of early melanosomes (Bravo Portela et al., 2012). This suggests that the Golgi complex can be further studied to explore the source of melanosomes in the mantle of C. gigas.
Three frozen section staining techniques were used in this study to identify the presence of melanin in the mantle of C. gigas. Among them, DOPA staining is a better detection method that can clearly observe the presence of melanin particles and exclude the interference of argyrophilic and chromaffin granules, as well as lipofuscin. Melanin formation is the final product of a complex transformation process involving tyrosine, which can affect melanin production (Slominski et al., 2004; Ando et al., 2007). DOPA staining, which detects the location of melanocytes based on tyrosinase content, has been used in a variety of mammals, such as cattle, mice, and guinea pigs (Amakiri, 1979; Yamashita et al., 2005). This method is mainly used to stain melanocytes that secrete melanin particles, whereas melanocytes that are unable to secrete melanin are not stained (Na et al., 2006). Therefore, it is one of the methods used to stain active melanocytes. Masson-Fontana staining was initially used to detect the presence of enterochromaffin cells (Pearse et al., 1974). This method is known as the “gold standard” for melanin particle identification in paraffin and frozen sections, owing to its ability to reduce the silver ions in silver ammonia solution to black material for experimental observation (Joly-Tonetti et al., 2016). However, Masson-Fontana staining cannot distinguish argyrophilic cells that also have the ability to reduce silver nitrate, which is a shortcoming of this staining method (Shataer et al., 2020). The ferrous sulfate method is also a conventional method for melanin detection, but it cannot distinguish melanin from other argyrophilic and chromophilic particles (Valembois et al., 1994; Wu et al., 2010; Du et al., 2017). In addition to these methods, immunohistochemistry can be used to detect melanin-specific antigens more sensitively, but this method has high requirements for antigen preservation and is affected by various experimental conditions (Joly-Tonetti et al., 2016). Moreover, it is difficult to obtain specific antibodies against marine shellfish and it is difficult to use immunohistochemical methods to identify melanin.
In this study, TEM was used to measure the ultrastructure of mantle tissue with a black surface and pigmented particles were observed in the mantle tissues of C. gigas. Three melanin staining methods were used to identify the presence of melanin in the mantle tissue. From the perspective of observation and the specificity of staining, DOPA staining is a more suitable staining method for mantle sections of C. gigas. In bivalve shellfish, the formation of shell color is very complex. An increasing number of studies have shown a correlation between the mantle and shell color (Brake et al., 2004; Kang et al., 2013; Li et al., 2014; Zheng et al., 2021). This preliminary study proved the existence of melanin in the marginal tissue of the mantle, in hopes of providing ideas for related studies on shell color. In addition, most studies on melanin formation in bivalves have focused on tyrosinase (Nagai et al., 2007; Zhu et al., 2021) and relatively few studies have explored pigmentation from the perspective of cellular processes. In this study, melanosomes were observed in the mantle tissue of C. gigas as a foundation for the study of melanin synthesis.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
LW and XTW designed the experiments. YH, CX, NF, and HS carried out this experiment. XMW and YZ contributed to the sample collection. MZ, YL, and BH contributed to data analysis. YH and LW wrote this paper. All authors reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This research was supported by the National Natural Science Foundation of China (No. 41876193, 41906088, 42076088 and 31802328), the Agricultural Variety Improvement Project of Shandong Province (No. 2019LZGC020), the National Key R&D Program of China (No. 2018YFD0901400), the Shandong Provincial Natural Science Foundation, China (No. ZR2019MC002), the Special Funds for Taishan Scholars Project of Shandong Province, China (No. tsqn201812094), the Modern Agricultural Industry Technology System of Shandong Province, China (SDAIT-14-03), and the Plan of Excellent Youth Innovation Team of Colleges and Universities in Shandong Province, China (2019KJF004).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Amakiri S. F. (1979). Melanin and DOPA-Positive Cells in the Skin of Tropical Cattle. Cells Tissues Organs 103 (4), 434–444. doi: 10.1159/000145045
Ando H., Kondoh H., Ichihashi M., Hearing V. J. (2007). Approaches to Identify Inhibitors of Melanin Biosynthesis via the Quality Control of Tyrosinase. J. Invest. Dermatol. 127 (4), 751–761. doi: 10.1038/sj.jid.5700683
Barbosa A. J., Castro L. P., Margarida A., Nogueira M. F. (1984). A Simple and Economical Modification of the Masson-Fontana Method for Staining Melanin Granules and Enterochromaffin Cells. Stain Technol. 59 (4), 193–196. doi: 10.3109/10520298409113855
Barden H., Koulish S. (1983). The Dark Brown Integumentary Pigment of a Barnacle (Balanus Eburneus). Histochemistry 78 (1), 41–52. doi: 10.1007/BF00491110
Benito-Martínez S., Zhu Y., Jani R. A., Harper D. C., Marks M. S., Delevoye C. (2020). Research Techniques Made Simple: Cell Biology Methods for the Analysis of Pigmentation. J. Invest. Dermatol. 140 (2), 257–268. doi: 10.1016/j.jid.2019.12.002
Brake J., Evans F., Langdon C. (2004). Evidence for Genetic Control of Pigmentation of Shell and Mantle Edge in Selected Families of Pacific Oysters, Crassostrea Gigas. Aquaculture 229 (1-4), 89–98. doi: 10.1016/S0044-8486(03)00325-9
Bravo Portela I., Martinez-Zorzano V. S., Molist-Perez I., Molist Garcia P. (2012). Ultrastructure and Glycoconjugate Pattern of the Foot Epithelium of the Abalone Haliotis Tuberculata (Linnaeus 1758)(Gastropoda, Haliotidae). Sci. World J. 2012, 960159. doi: 10.1100/2012/960159
Butt D., Raftos D. (2008). Phenoloxidase-Associated Cellular Defence in the Sydney Rock Oyster, Saccostrea Glomerata, Provides Resistance Against QX Disease Infections. Dev. Comp. Immunol. 32 (3), 299–306. doi: 10.1016/j.dci.2007.06.006
Cai Z., Wu J., Chen L., Guo W., Li J., Wang J., et al. (2011). Purification and Characterisation of Aquamarine Blue Pigment From the Shells of Abalone (Haliotis Discus Hannai Ino). Food Chem. 128 (1), 129–133. doi: 10.1016/j.foodchem.2011.03.006
Canales-Gómez E., Correa G., Viana M. T. (2010). Effect of Commercial Carotene Pigments (Astaxanthin, Cantaxanthin and β-Carotene) in Juvenile Abalone Haliotis Rufescens Diets on the Color of the Shell or Nacre. Veterinaria México 41 (3), 191–200.
Comfort A. (1951). The Pigmentation of Molluscan Shells. Biol. Rev. 26 (3), 285–301. doi: 10.1111/j.1469-185X.1951.tb01358.x
D’Alba L., Shawkey M. D. (2019). Melanosomes: Biogenesis, Properties, and Evolution of an Ancient Organelle. Physiol. Rev. 99 (1), 1–19. doi: 10.1152/physrev.00059.2017
Du Z., Huang K., Zhao J., Song X., Xing X., Wu Q., et al. (2017). Comparative Transcriptome Analysis of Raccoon Dog Skin to Determine Melanin Content in Hair and Melanin Distribution in Skin. Sci. Rep. 7 (1), 1–11. doi: 10.1038/srep40903
Ebanks J. P., Koshoffer A., Wickett R. R., Schwemberger S., Babcock G., Hakozaki T., et al. (2011). Epidermal Keratinocytes From Light vs. Dark Skin Exhibit Differential Degradation of Melanosomes. J. Invest. Dermatol. 131 (6), 1226–1233. doi: 10.1038/jid.2011.22
Feng D., Li Q., Yu H. (2019). RNA Interference by Ingested Dsrna-Expressing Bacteria to Study Shell Biosynthesis and Pigmentation in Crassostrea Gigas. Marine Biotechnol. 21 (4), 526–536. doi: 10.1007/s10126-019-09900-2
Ge J., Li Q., Yu H., Kong L. (2015). Mendelian Inheritance of Golden Shell Color in the Pacific Oyster Crassostrea Gigas. Aquaculture 441, 21–24. doi: 10.1016/j.aquaculture.2015.01.031
Han Z., Li Q. (2021). Relationship Between Shell Color and Growth and Survival Traits in the Pacific Oyster Crassostrea Gigas. J. Ocean Univ. China 20 (4), 985–991. doi: 10.1007/s11802-021-4676-5
Huang S., Jiang H., Zhang L., Gu Q., Wang W., Wen Y., et al. (2021). Integrated Proteomic and Transcriptomic Analysis Reveals That Polymorphic Shell Colors Vary With Melanin Synthesis in Bellamya Purificata Snail. J. Proteomics 230, 103950. doi: 10.1016/j.jprot.2020.103950
Ivanina A. V., Falfushynska H. I., Beniash E., Piontkivska H. (2017). Biomineralization-Related Specialization of Hemocytes and Mantle Tissues of the Pacific Oyster Crassostrea Gigas. J. Exp. Biol. 220 (18), 3209–3221. doi: 10.1242/jeb.160861
Joly-Tonetti N., Wibawa J. I. D., Bell M., Tobin D. (2016). Melanin Fate in the Human Epidermis: A Reassessment of How Best to Detect and Analyse Histologically. Exp. Dermatol. 25 (7), 501–504. doi: 10.1111/exd.13016
Kang J. H., Kang H. S., Lee J. M., An C. M., Lee Y. M., Kim J. J. (2013). Characterizations of Shell and Mantle Edge Pigmentation of a Pacific Oyster, Crassostrea Gigas, in Korean Peninsula. Asian-Australasian J. Anim. Sci. 26 (12), 1659. doi: 10.5713/ajas.2013.13562
Kocot K. M., Aguilera F., McDougall C., Jackson D. J., Degnan B. M. (2016). Sea Shell Diversity and Rapidly Evolving Secretomes: Insights Into the Evolution of Biomineralization. Front. Zool. 13 (1), 1–10. doi: 10.1186/s12983-016-0155-z
Li X., Bai Z., Luo H., Wang G., Li J. (2014). Comparative Analysis of Total Carotenoid Content in Tissues of Purple and White Inner-Shell Color Pearl Mussel, Hyriopsis Cumingii. Aquaculture Int. 22 (5), 1577–1585. doi: 10.1016/j.gene.2014.01.046
Liu X., Wu F., Zhao H., Zhang G., Guo X. (2009). A Novel Shell Color Variant of the Pacific Abalone Haliotis Discus Hannai Ino Subject to Genetic Control and Dietary Influence. J. Shellfish Res. 28 (2), 419–424. doi: 10.2983/035.028.0226
Marin F., Luquet G., Marie B., Medakovic D. (2007). Molluscan Shell Proteins: Primary Structure, Origin, and Evolution. Curr. Topics Dev. Biol. 80, 209–276. doi: 10.1016/S0070-2153(07)80006-8
McDougall C., Degnan B. M. (2018). The Evolution of Mollusc Shells. Wiley Interdiscip. Rev.: Dev. Biol. 7 (3), e313. doi: 10.1002/wdev.313
Nagai K., Yano M., Morimoto K., Miyamoto H. (2007). Tyrosinase Localization in Mollusc Shells. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 146 (2), 207–214. doi: 10.1016/j.cbpb.2006.10.105
Na G. Y., Paek S. H., Park B. C., Kim D. W., Lee W. J., Lee S. J., et al. (2006). Isolation and Characterization of Outer Root Sheath Melanocytes of Human Hair Follicles. Br. J. Dermatol. 155 (5), 902–909. doi: 10.1111/j.1365-2133.2006.07502.x
Nielsen K. P., Zhao L., Stamnes J. J., Stamnes K., Moan J. (2006). The Importance of the Depth Distribution of Melanin in Skin for DNA Protection and Other Photobiological Processes. J. Photochem. Photobiol. B: Biol. 82 (3), 194–198. doi: 10.1016/j.jphotobiol.2005.11.008
Pandiar D., Ramani P., Poothakulath K. R., Behera A., Monica K. (2021). A Two Steps Embedding Technique for Frozen Sections. Oral. Oncol. 123, 105631–105631. doi: 10.1016/j.oraloncology.2021.105631
Pang Y., Geng J., Qin Y., Wang H., Fan R., Zhang Y., et al. (2016). Endothelin-1 Increases Melanin Synthesis in an Established Sheep Skin Melanocyte Culture. In Vitro Cell. Dev. Biology-Animal 52 (7), 749–756. doi: 10.1007/s11626-016-0042-0
Pearse A. G. E., Polak J. M., Bloom S. R., Adams C., Dryburgh J. R., Brown J. C. (1974). Enterochromaffin Cells of the Mammalian Small Intestine as the Source of Motilin. Virchows Archiv. B 16 (1), 111. doi: 10.1007/BF02894069
Pietak L. M. (1998). Body Symbolism and Cultural Aesthetics: The Use of Shell Beads and Ornaments by Delaware and Munsee Groups. North Am. Archaeologist 19 (2), 135–161. doi: 10.2190/0BV6-Q0N1-37VU-PEQ7
Schraermeyer U. (1994). Fine Structure of Melanogenesis in the Ink Sac of Sepia Offidnalis. Pigment Cell Res. 7 (1), 52–60. doi: 10.1111/j.1600-0749.1994.tb00018.x
Shataer M., Shataer S., Libin L., Tian L., Shengbin B. (2020). Histological Comparison of Two Special Methods of Staining Melanin in Human Skin. Int. J. Morphol. 38 (6), 1535–1538. doi: 10.4067/S0717-95022020000601535
Slominski A., Tobin D. J., Shibahara S., Wortsman J. (2004). Melanin Pigmentation in Mammalian Skin and its Hormonal Regulation. Physiol. Rev. 84 (4), 1155–1228. doi: 10.1152/physrev.00044.2003
Valembois P., Seymour J., Lassègues M. (1994). Evidence of Lipofuscin and Melanin in the Brown Body of the Earthworm Eisenia Fetida Andrei. Cell Tissue Res. 277 (1), 183–188. doi: 10.1007/BF00303095
Wu X., Xu A., Song X., Zheng J. H., Wang P., Shen H. (2010). Clinical, Pathologic, and Ultrastructural Studies of Progressive Macular Hypomelanosis. Int. J. Dermatol. 49 (10), 1127–1132. doi: 10.1111/j.1365-4632.2010.04492.x
Xu L., Li Q., Yu H., Kong L. (2017). Estimates of Heritability for Growth and Shell Color Traits and Their Genetic Correlations in the Black Shell Strain of Pacific Oyster Crassostrea Gigas. Marine Biotechnol. 19 (5), 421–429. doi: 10.1007/s10126-017-9772-6
Yamashita T., Kuwahara T., Gonzalez S., Takahashi M. (2005). Non-Invasive Visualization of Melanin and Melanocytes by Reflectance-Mode Confocal Microscopy. J. Invest. Dermatol. 124 (1), 235–240. doi: 10.1111/j.0022-202X.2004.23562.x
Yu X., Yu H., Kong L., Guo F., Zhu G., Li Q. (2014). Molecular Cloning and Differential Expression in Tissues of a Tyrosinase Gene in the Pacific Oyster Crassostrea Gigas. Mol. Biol. Rep. 41 (8), 5403–5411. doi: 10.1007/s11033-014-3412-2
Yu H., Zhao X., Li Q. (2016). Genome-Wide Identification and Characterization of Long Intergenic Noncoding Rnas and Their Potential Association With Larval Development in the Pacific Oyster. Sci. Rep. 6 (1), 1–8. doi: 10.1038/srep20796
Zhang G., Fang X., Guo X., Li L., Luo R., Xu F., et al. (2012). The Oyster Genome Reveals Stress Adaptation and Complexity of Shell Formation. Nature 490 (7418), 49–54. doi: 10.1038/nature11413
Zhang C., Zhang R. (2006). Matrix Proteins in the Outer Shells of Molluscs. Marine Biotechnol. 8 (6), 572–586. doi: 10.1007/s10126-005-6029-6
Zheng J., Nie H., Yan X. (2021). Analysis of Differential Gene Expression by SRAP-Cdna in Mantle Tissue of Meretrix Petechialis With Different External Shell Color. Anim. Biotechnol. 32 (1), 31–37. doi: 10.1080/10495398.2019.1642907
Zhu Y., Li Q., Yu H., Kong L. (2018). Biochemical Composition and Nutritional Value of Different Shell Color Strains of Pacific Oyster Crassostrea Gigas. J. Ocean Univ. China 17 (4), 897–904. doi: 10.1007/s11802-018-3550-6
Zhu Y., Li Q., Yu H., Liu S., Kong L., et al. (2021). Shell Biosynthesis and Pigmentation as Revealed by the Expression of Tyrosinase and Tyrosinase-Like Protein Genes in Pacific Oyster (Crassostrea Gigas) With Different Shell Colors. Marine Biotechnol. 23 (5), 777–789. doi: 10.1007/s10126-021-10063-2
Keywords: Crassostrea gigas, mantle, melanin, melanocyte, frozen section
Citation: Han Y, Xie C, Fan N, Song H, Wang X, Zheng Y, Zhang M, Liu Y, Huang B, Wei L and Wang X (2022) Identification of Melanin in the Mantle of the Pacific Oyster Crassostrea gigas. Front. Mar. Sci. 9:880337. doi: 10.3389/fmars.2022.880337
Received: 21 February 2022; Accepted: 07 March 2022;
Published: 31 March 2022.
Edited by:
Menghong Hu, Shanghai Ocean University, ChinaCopyright © 2022 Han, Xie, Fan, Song, Wang, Zheng, Zhang, Liu, Huang, Wei and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Wei, bGVpODE5QDEyNi5jb20=; Xiaotong Wang, d2FuZ3hpYW90b25nOTk5QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.