- 1Chulabhorn International College of Medicine, Thammasat University, Rangsit Campus, Pathumthani, Thailand
- 2Division of Health and Applied Sciences, Faculty of Science, Prince of Songkla University, Hat Yai, Songkhla, Thailand
- 3Coastal Aquaculture Research and Development Regional Center 2 (Samut Sakhon), Department of Fisheries, Ministry of Agriculture and Cooperatives, Samut Sakhon, Thailand
- 4Department of Anatomy, Faculty of Science, Mahidol University, Bangkok, Thailand
In the present study, double strand RNA technology (dsRNA) was used to inhibit transcripts of vitellogenesis-inhibiting hormone (VIH) that mainly synthesized and secreted from the central nervous system in Scylla olivacea females. The results presented in this study clearly demonstrate the potential dsRNA-VIH was highly effective to inhibit VIH in the eyestalks of females injected with dsRNA-VIH on the 3rd, 7th and 14th day, respectively. The dsRNA-VIH injections were performed at 14-day intervals, a single dsRNA dose of 0.6 µg/gram body weight was enough to suppress VIH expression until 14th day after injection. The dsRNA-VIH injection significantly increased gonad-somatic index (GSI) and hemolymph vitellin level at day 14 and 28 when compared with control groups. The histological observation found that the number of oocyte step 4 in dsRNA-VIH group was significantly higher than that of the control group. Also, dsRNA-VIH has stimulatory function on other reproduction-related genes such as the Scyol-PGES and Scyol-ESULT that both genes gradually increased their expressions in brain and ventral nerve cord. In conclusion, the silence of VIH gene could reduce the production of VIH from eyestalk and brain that affected other downstream genes related to ovarian maturation in the mud crab.
Introduction
The vitellogenesis-inhibiting hormone (VIH), also known as gonad-inhibiting hormone (GIH), is produced and secreted by neuroendocrine cells of the X-organ-sinus gland complex (XO-SG) in the eyestalk of various crustaceans (Treerattrakool et al., 2011; Feijo et al., 2016). This peptide hormone is a member of crustacean hyperglycemic hormone (CHH) family which their candidates include the CHH, GIH/VIH, molt-inhibiting hormone (MIH), and mandibular organ-inhibiting hormone (MOIH) (Kegel et al., 1989; Hsu et al., 2006; Webster et al., 2012). Commonly, the CHH family peptides, as well as the VIH, contain six cysteine residues forming three intramolecular disulfide bonds in their molecules (Lacombe et al., 1999; Webster et al., 2012). Functional studies of this peptide hormone family revealed diverse physiological involvements in crustaceans include metabolism, osmoregulation, molting, and reproduction (Chen et al., 2014). Considering the VIH function, it was mostly recognized as a potent negative regulator of crustacean reproduction (Chen et al., 2014). It has been reported that VIH diminished the synthesis of vitellogenin (VTG) from the ovary (Tsukimura, 2015). The characterization of VIH have been reported in several crustacean species such as Penaeus monodon, Rimicaris kairei, Litopenaeus vannamei, Scylla paramamosain and Scylla olivacea (Treerattrakool et al., 2008; Qian et al., 2009; Chen et al., 2014; Liu et al., 2018; Kornthong et al., 2019). The white leg shrimp, L. vannamei, presented high rate of ovarian maturation (Kang et al., 2014) and increased hepatopancreatic VTG expression (Chen et al., 2014) after the eyestalk ablation. Moreover, dsRNA-mediated GIH silencing have been reported to increase ovarian maturation and eventual spawning in both domesticated and wild female broodstock, particularly with a comparable effect to eyestalk ablation in wild shrimp in P. monodon (Treerattrakool et al., 2011).
Ovarian maturation period of mud crabs (Scylla spp.) is a time-consuming process which takes approximately 55-60 days depending on the environmental conditions, i.e., hormonal control, water salinity, natural diet, biochemical compositions, and enzyme activities (Nagaraju, 2011; Amin-Safwan et al., 2016; Azra and Ikhwanuddin, 2016; Hidir et al., 2018; Muhd-Farouk et al., 2019; Hidir et al., 2021). The vitellin (Vn), a set of multiple cleaved products of VTG, is a key molecule of crustacean yolk proteins that plays a significant role in the embryonic development (Volz et al., 2002; Quackenbush, 2015). In the Scylla serrata, vitellogenesis takes place in both hepatopancreas and ovary while the cleaved Vn subunits being processed in hepatopancreas, and hemolymph was sequestered by the growing oocytes (Rani and Subramoniam, 1997; Subramoniam, 2011). Therefore, various researchers have suggested that Vn is the major egg yolk protein and a high density lipoglycoprotein frequently associated with carotenoid pigments and the common form of yolk stored in oocytes and a nutrient source for developing embryos. In many species, VTG, the precursor molecule to Vn, is transported through the hemolymph to developing oocytes in the marine shrimp Penaeus semisulcatus (Avarre et al., 2003). Previous studies have found that high hemolymph VTG levels result to late ovarian development as histologically demonstrated by the vitellogenic oocytes become larger and they are filled with yolk globules in Pandalus hypsinotus and Marsupenaeus japonicus (Okumura et al., 2004; Okumura, 2007).
Normal processes of crab reproduction consume long period to complete its cycle. The eyestalk ablation, therefore, was introduced to remove the reproductive inhibiting hormones in crustaceans, including CHH peptide family-the VIH and MIH (Okumura and Aida, 2001; Nagaraju, 2011; Kang et al., 2014). By microarray analysis, ablation of eyestalk positively increased the vitellogenesis, ovarian weight as well as altered gene expressions that participate in reproduction, immune response and calcium signaling transduction in the Penaeus monodon (Uawisetwathana et al., 2011). Therefore, this procedure has been used for ripening ovarian development and maturation of many crustaceans i.e., the P. monodon, Procambarus clarkia and M. japonicus (Tan-Fermin, 1991; Okumura et al., 2007; Guan et al., 2017). Recent study also showed that eyestalk ablation in the freshwater crab, Barytelphusa lugubris, was able to induce breeding by rapid ripening the ovary and shortening the molting cycle (Rana, 2018). Besides, an alternative technique to eyestalk ablation by neuropeptide and hormone administration such as the serotonin (5-HT) and dopamine antagonist was also noted (Alfaro-Montoya et al., 2004).
The dsRNA technique has been used to silence the hormonal target transcripts by RNA interference (Alfaro-Montoya et al., 2004; Treerattrakool et al., 2011; Kang et al., 2021). This method post-transcriptionally silenced the target gene using specific dsRNA (Meister and Tuschl, 2004; Pak and Fire, 2007; Kang et al., 2019; Kang et al., 2021). The administration of dsRNA to silence P. monodon GIH gene was reported giving the known function of GIH in inhibiting VTG gene expression (Treerattrakool et al., 2008). In addition, the injections of dsRNA of CHH1 and MIH1 reduced the hemolymph glucose level and days of molting period in the P. monodon. The dsRNA of GIH gene application also promoted the VTG gene expression in the same species (Sukumaran et al., 2017). Although eyestalk ablation in crab is quite limited since it provides physiological abnormalities and low survival rate (Sroyraya et al., 2010). Using dsRNA to silence the VIH gene is therefore an alternative method to stimulate vitellogenesis and ovarian maturation in the female S. olivacea.
Therefore, in this study we successfully used specific dsRNA to knockdown the VIH transcript of S. olivacea and demonstrated its effects in temporary silencing VIH gene. In addition, the dsRNA-VIH could significantly increase Vn level in hemolymph and enhance ovarian maturation as well as promote some reproduction-related gene expression in the central nervous system of the female mud crab.
Material and Methods
Animals
The female mud crabs, S. olivacea, were cultured in Coastal Aquaculture Research and Development Regional Center 2, Samut Sakhon, Thailand. Identification of maturity stage of each crab was followed the previous study (Overton and Macintosh, 2002). All procedures on experimental animals were approved by Animal Care and Use Committee of Thammasat University, National Research Council of Thailand (NRCT), Protocol Number 020/2561 in which all efforts were made to minimize the suffering of animals. Crabs were maintained in the concrete tanks filled with approximately 1000 L natural seawater (30 ppt) at 26-28°C, under a 12 h light and 12 h dark photoperiod. Crabs were fed twice daily (at 8:00 AM and 4:00 PM) with commercial fresh food throughout this study. The intermolt female mud crabs (n = 60) with average weight of 45-55 g were used in the dsRNA optimization assay. The mature female mud crabs at the same molting stage with average weight of 135-145 g, together with gonadsomatic index (GSI) less than 0.2, and the distance between the two tips of the 9th spine of anterolateral carapace approximately 85-92 mm (Ghazali et al., 2017; Amin-Safwan et al., 2018), were used to study the prolonged inhibitory effect of VIH knockdown and effect of dsRNA on ovarian maturation.
Construction of Recombinant Plasmids
To engineer the recombinant plasmid for dsRNAs expression, the DNA fragments corresponding to VIH sequence (380 bp) with loop sequence (580 bp) were amplified from pUC57 containing synthetic VIH gene (GenBank accession no. MH882453). The enhanced green fluorescent protein (EGFP) fragments with loop (570 bp) and without loop sequences (380 bp) were amplified by PCR using pUC-EGFP as the template. The specific primers used in this study were shown in Table 1. The PCR condition was set as follows: 95°C for 5 min, 35 cycles of 95°C for 30 s, 55°C for 30 s and 72°C for 1 min, followed by final extension at 72°C for 5 min. The PCR fragments (fragment with and without loop sequences) of VIH or EGFP were cloned into pET17b at XbaI and XhoI sites. The DNA fragments with and without loop sequence of each gene were linked together by using HindIII site to generate opposite direction. The ligated product was introduced into Escherichia coli DH5α for propagating the plasmids. The recombinant plasmids were digested with XbaI and XhoI and then confirmed the sequences by DNA sequencing. For template of dsRNAs production in vitro, both stem loop fragments were excised from pET17b and cloned into pDrive cloning vector at the XbaI and XhoI sites.
Production of DsRNA-VIH and DsRNA-EGFP
For in vitro dsRNA production, XhoI-linearized recombinant plasmids were used as the templates to produce the dsRNAs using SP6 RiboMAX™ Express Large-Scale RNA Production System (Promega, USA) kit according to the manufacturer’s protocol. The remaining template was removed by RQ1 RNase-free DNase (Promega, USA) treatment at 37°C for 15 min and dsRNA was extracted by TriPure Isolation reagent (Roche, Germany) as described by manufacturer’s protocol. dsRNA pellet was solubilized by 150 mM NaCl and characterized by RNase III and RNase A digestion. dsRNA was verified the integrity by agarose gel electrophoresis and estimated the concentration by Nanodrop.
Dose Optimization of Double-Stranded RNAs (DsRNAs) and Prolonged Inhibitory Effect of DsRNAs
After acclimatization for 14 days, thirty-five crabs were divided equally into 7 groups (n = 5 each); (1) Vehicle control group, in which the animals were administered with 100 μl of 0.9% normal saline (NSS); (2-4) dsRNA-VIH groups in which the animals were administered with 100 μl of dsRNA-VIH at 0.2, 0.4, 0.6 μg/g BW dissolved in 0.9% normal saline; (5-7) dsRNA-EGFP groups in which the animals were administered with 100 μl of dsRNA-EGFP at 0.2, 0.4, 0.6 μg/g BW dissolved in 0.9% normal saline. Administration was performed intramuscularly at the base of the fifth walking leg. Animals were sacrificed at 24 h post injection, then the eyestalk and brain from individuals were collected for determining the VIH and EGFP genes expression. Prolonged inhibition effect of the dsRNAs was verified by the VIH and EGFP genes expression in both organs after 3rd day, 7th day and 14th day post injection.
VIH Gene Expression Analysis by Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from eyestalk and brain using TriPure Isolation reagent (Roche, Germany), following the manufacturer’s protocol, and kept at -80°C until use. The purity and quantity of RNA were measured by using a nanodrop spectrophotometer. Total RNA (2 μg) were synthesized the first-strand cDNA synthesis using QuantiNova Reverse Transcription Kit (Qiagen, Germany), following the manufacturer’s protocol. To determine the inhibition effect of dsRNA-VIH and dsRNA-EGFP, the VIH gene expression was performed using VIHq-F and VIHq-R primers (Table 1). Thermocycling condition was set as follows: One cycle at 95°C for 5 minutes followed by 30 seconds at 94°C, 45 seconds at 55°C, and 30 seconds at 72°C for 35 cycles and a final extension of 10 minutes at 72°C. The PCR product was analyzed by electrophoresis with 1.5% agarose gels. The amplification of β-actin was used as internal control.
Effect of DsRNA-VIH on Ovarian Maturation Determining by Gonad Somatic Index (GSI) and Histological Analysis
Ninety crabs were divided equally into 3 groups (n = 30 each); (1) Vehicle control group in which the animals were administered with 100 μl of 0.9% normal saline (NSS); (2) dsRNA-VIH groups in which the animals were administered with 100 μl of dsRNA-VIH at 0.6 μg/g BW dissolved in 0.9% normal saline; (3) dsRNA-EGFP groups in which the animals were administered with 100 μl of dsRNA-EGFP at 0.6 μg/g BW dissolved in 0.9% normal saline. The injections were performed at day 0 and 14 of the experimental period, and ten crabs from each group were randomly collected and sacrificed at day 0, 14 and 28. Gonadal development was examined, based on external morphology (Stewart et al., 2007; Islam et al., 2010; Ghazali et al., 2017; Hidir et al., 2018) and routine histology (Saetan et al., 2017). To validate the histological differences between groups, the numbers of Oc1-Oc4 taken from ovary samples of day 0, 14 and 28 were randomly selected for four fields of non-consecutive sections (at a magnification of x200) taken from each crab (n=3). The sections were viewed and photographed under a Leica compound microscope equipped with a digital camera (Leica, Germany). Data on gonadosomatic index (GSI) was collected following the previous described protocol (Tinikul et al., 2016; Saetan et al., 2017). The eyestalk, brain, and ventral nerve cord (VNC) were collected for subsequent molecular analysis and the hemolymph was collected for Vn level measurement.
Standard Preparation of S. olivacea Vn
Different stages of S. olivacea ovaries (5 crabs each stage) were homogenized in 0.5 M Tris-HCl buffer containing 0.5 M NaCl, pH 7.5, at 4°C. Each homogenate was then centrifuged at 10,000 x g, for 30 min at 4°C, and the supernatant was retained for further purification. Western blot was used to test the specificity of anti-Vn which was raised against the M. rosenbergii Vn (anti-MrVn) in our laboratory (Soonklang et al., 2012). The 30 μg of ovarian total protein from stage 1 to stage 4 ovaries of ovary were loaded and run through the 12% tris-glycine gels for SDS polyacrylamide gel electrophoresis. Separated proteins were transferred to 0.45 μm nitrocellulose membrane and the membranes were blocked in 5% skim milk diluted in 0.1 M TBS containing 0.1% tween 20 (TBST) for 1 h and then were incubated in anti-MrVn (1:1000) diluted in blocking solution at 4°C for an overnight. After washing, the membranes were probed with the horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:5000) (Abcam, USA) at room temperature for 1 h. The immunoreactive protein bands were visualized by adding the ECL chemiluminescence substrate (Thermo Fisher Scientific Inc., Pittsburgh, PA) and all bands were finally visualized by Amersham Imager (GE Healthcare, Sweden).
To separate the Vn and use as standard in ELISA, the early- and late-stage 4 ovarian extracts were pooled and concentrated by ultrafiltration through the molecular weight-cut off concentrator (Ultra-30kDa, Amicon), followed by a final buffer exchange with 50-mM Tris-HCl buffer, pH 7.5, at 4 °C. The concentrated extract was then fractionated by fast protein liquid chromatography (FPLC) (GE Healthcare, Sweden) on an ion exchange column (HiScreen™ Q HP columns, 100mm x 7.7 mm i.d., GE Healthcare, Sweden) equilibrated with 50-mM Tris-HCl buffer, pH 7.5, as described by (Chen and Kuo, 1998). The percentage of solution A (Tris-HCl) was gradually decreased from 100 to 0, while the percentage of solution B, elution buffer (Tris-HCl + 1N NaCl) was gradually increased from 0 to 100 at a flow rate of 1 mL/min, for 35 min with absorbencies monitored at 280 nm and 474 nm (Chen and Kuo, 1998). Presence of the S. olivacea Vn in each fraction was tested by dot blot. One hundred nanogram of each fraction collected from 22nd to 28th min was dotted onto a 0.45 µm PVDF membrane (Merck, Darmstadt, Germany). The membranes were blocked for non-specificity by incubating with 5% skim milk in TBST for 1 h at room temperature. The membranes were then incubated with anti-MrVn, at a 1:1000 dilution, for overnight at 4°C. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Abcam, USA) was applied, at a 1:5,000 dilution, for 1 h at room temperature. An enhanced chemiluminescence kit (Thermo Fisher Scientific Inc., Pittsburgh, PA) was used to develop the membranes and the result was detected and photographed by Amersham Imager (GE Healthcare, Sweden).
Effect of DsRNA-VIH on Ovarian Maturation Determining by Vn Level in Hemolymph
The hemolymph Vn level of female S. olivacea was determined by an indirect ELISA (Tinikul et al., 2016; Saetan et al., 2017) with some modifications. Hemolymph was collected from the base of the fifth walking leg and transferred into a vial containing anticoagulant solution [0.45 M NaCl, 0.1 M glucose, 30 mM tri-sodium citrate, 26 mM citric acid and 10 mM EDTA (pH4.6)] at equal volume as hemolymph. The mixture was separated by centrifugation at 9000 x g, 4°C, 10 min and the supernatants were frozen at -20°C. One hundred microliters of all supernatant, and our purified Vn with known concentration, were diluted in coating buffer (15 mM Na2CO3 and 35 mM NaHCO3, pH 9.6) and coated on 96 well ELISA plate for overnight at 4°C. Each well was subsequently washed three times with 0.01 M PBS containing 0.05% tween 20 (PBST) and blocked non-specific binding with 0.25% bovine serum albumin (BSA) in PBST, at 37°C for 30 minutes. The primary antibody (anti-MrVn) diluted at 1:2000 in blocking buffer was applied and incubated at 37°C for 2 h. The goat anti-rabbit IgG-HRP (Abcam, USA) diluted at 1:10000 in blocking buffer was added to each well and incubated at 37°C for 1 h. The plate was then washed in triplicate and the color was developed by adding TMB substrate for 15 min. The reaction was stopped by adding 1 N HCl. Then, the absorbance was measured by microplate spectrophotometer (Thermo Fisher, USA) at a wavelength of 450 nm. Calculation of the Vn level of each sample was based on the standard curve of purified Vn. Determination of hemolymph Vn level was performed in triplicate.
Relative Abundance of Reproductive-Related Genes Expression Following DsRNA-VIH Administration
To study the effect of dsRNA-VIH on gene expression, the S. olivacea reproduction genes were selected from previous reports (Kornthong et al., 2014; Duangprom et al., 2018). The brain and ventral nerve cord (VNC) were collected from day 0, 7 and 14 of the experimental periods. The cDNAs were then prepared as described in the previous section of this manuscript. Real-time PCR for amplification of reproduction related genes, including S. olivacea prostaglandin E synthase (Scyol-PGES) and S. olivacea estrogen sulfotransferase (Scyol-ESULT) was performed in duplicate for each sample (n = 10 per group). Amplifications were conducted on a CFX-96 (Bio-Rad, USA) using iTaq Universal SYBR Green Supermix (Bio-Rad, USA). Scyol-PGES and Scyol-ESULT primers were shown in Table 1. Relative expression of Scyol-PGES and Scyol-ESULT against β-actin expression and a variance between individual treatments were performed, calculated, and statistically analyzed followed the methods described in (Kornthong et al., 2013; Kornthong et al., 2014; Duangprom et al., 2017; Duangprom et al., 2018).
Statistical Analysis
Values of the GSI, hemolymph Vn and relative gene expression levels of Scyol-PGES and Scyol-ESULT genes to β-actin, in different sample groups were tested by one-way analysis of variance (ANOVA) followed by Tukey test. All tests were performed using GraphPad Prism 9.0 software for Windows (GraphPad Software, USA). Mean values were identified as significantly different if the p-value was less than 0.05.
Results
Determination of DsRNA-VIH and DsRNA-EGFP Production
The coding sequence of S. olivacea VIH (MH882453; nucleotide 167 to 537) were used as the template for dsRNA-VIH production. To determine the quality of dsRNA, a large fragment of the dsRNA-VIH (380 bp) genes and dsRNA-EGFP (380 bp) were amplified using RT-PCR. After purification, the non-digested, RNase A-digested and RNase III-digested dsRNAs were visualized by agarose gel electrophoresis (Figure 1). The non-digested dsRNA-VIH and dsRNA-EGFP were shown approximately 1200 bp in lane 1 and 4 (Figure 1), while those of RNase A-digested dsRNA-VIH and dsRNA-EGFP were shown approximately 600 bp in lane 2 and 5 (Figure 1). Lastly, there was no band detected for both RNase III-digested dsRNA-VIH and dsRNA-EGFP (Figure 1, lane 3 and 6).
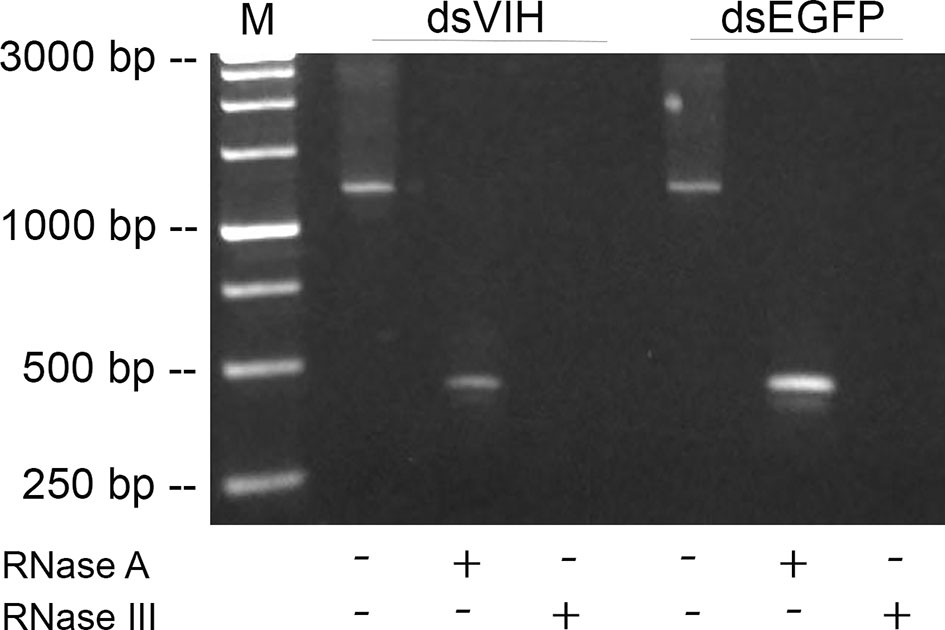
Figure 1 Visualization of the dsRNA production. Lane M was the 3kb DNA ladder marker. Lanes 1 and 4 were shown approximately 1200 bp of non-digested dsRNA-VIH and dsRNA-EGFP. Lanes 2 and 5 were shown approximately 600 bp of RNase A-digested dsRNA-VIH and dsRNA-EGFP. Lanes 3 and 6 were the RNase III-digested dsRNA-VIH and dsRNA-EGFP revealed no product detected.
Dose-Dependent Study of the DsRNA Induced VIH Knockdown and Prolonged Inhibitory Effect of VIH Knockdown
To determine the potent dose of dsRNA-VIH in sequestering the S. olivacea VIH expression, we administrated different doses of 0.2, 0.4, and 0.6 μg/g BW for the dsRNA-VIH and those of 0.2, 0.4 and 0.6 μg/g BW for dsRNA-EGFP and vehicle control (NSS) for injecting the female mud crabs. At 24 h post-injection, the VIH expressions of eyestalk and brain were inhibited by the 0.6 μg/g BW of dsRNA-VIH (Figures 2A, B). On the contrary, 0.2 and 0.4 μg/g BW of dsRNA-VIH failed to inhibit the VIH expressions in both organs (Figures 2A, B). According to these, the 0.6 μg/g BW of dsRNA-VIH was retained for further experiments. The selected dose of 0.6 μg/g BW dsRNA-VIH successfully displayed the prolonged inhibitory effect of VIH knockdown in the eyestalk for 3-, 7- and 14-days post-injection (Figure 3). However, at day 14 one of three crab was able to express VIH again while the remaining two ones were not (Figure 3). The irrelevant dsRNA-EGFP and vehicle control (NSS) had no effect on VIH inhibition (Figures 2A, B, and 3).
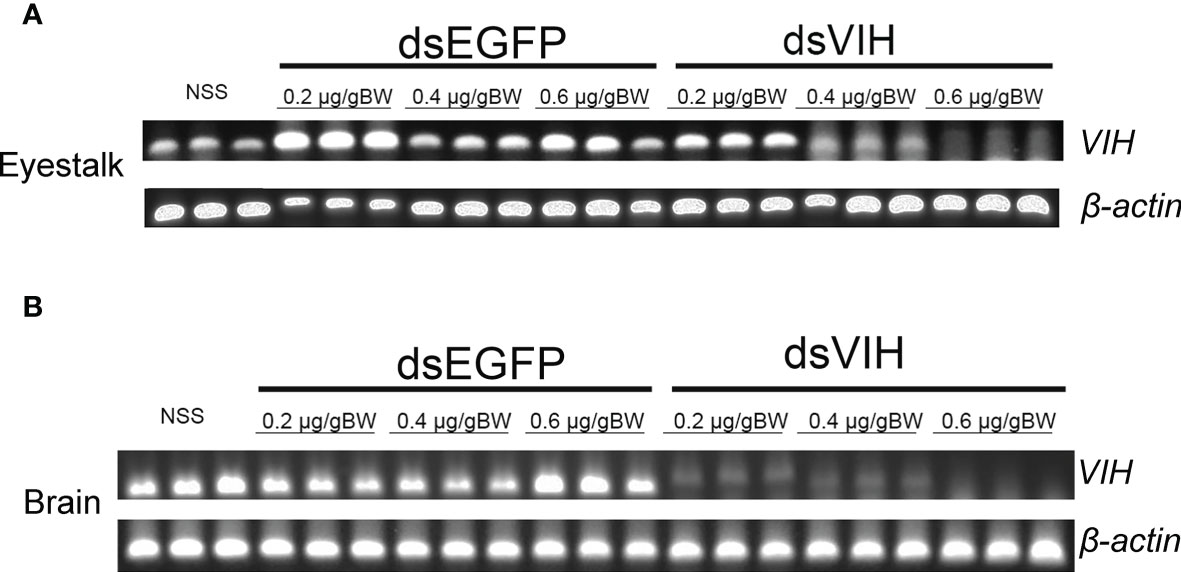
Figure 2 Dose-dependent effects of dsRNA-VIH, dsRNA-EGFP, and vehicle control (NSS) in sequestering the VIH gene expression in (A) eyestalk and (B) brain. In both organs, the most potent dose of dsRNA-VIH to inhibit the VIH gene expression was 0.6 µg/g BW. No inhibitory effect on VIH gene expression was observed by the injection of either the dsRNA-EGFP and vehicle control (NSS).
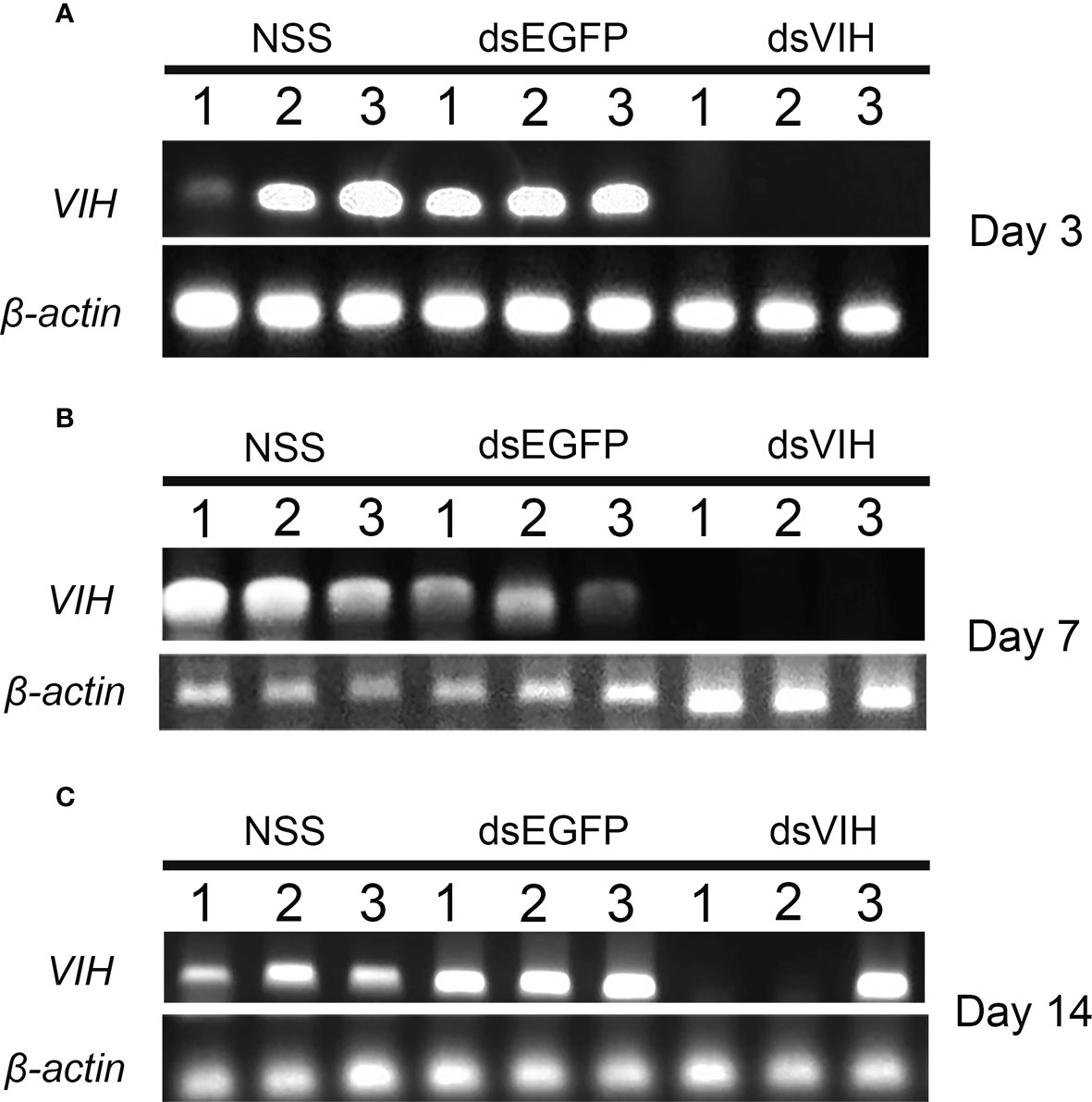
Figure 3 Prolonged effect of the 0.6 μg/g BW of dsRNA-VIH on inhibiting the eyestalk VIH gene expression at day 3 (A), day 7 (B) and day 14 (C) post-injection. No inhibitory effect on VIH gene expression was observed by the injection of either the dsRNA-EGFP and vehicle control (NSS).
Validation of the Anti-MrVn and Standard Vn Preparation
To determine the antibody reactivity between anti-MrVn and S. olivacea Vn, the western blot analysis was performed. The anti-MrVn was able to depict 2 molecular subunits of Vn comprising the 85 kDa and 105 kDa respectively. In stage 1 of ovary, the Vn subunits were not appeared. In stage 2-3 of ovary, the two subunits of Vn were slightly observed while they were mostly intensely expressed in the stage 4 of ovary (Figure 4A). Fractionation of the S. olivacea Vn by ion exchange FPLC and dot blot with MrVn antibody revealed the immunoreactivity in the fractions of 24-28 min (Figure 4B) Therefore, these fractions were combined, measured the concentration and used as standard Vn in ELISA.
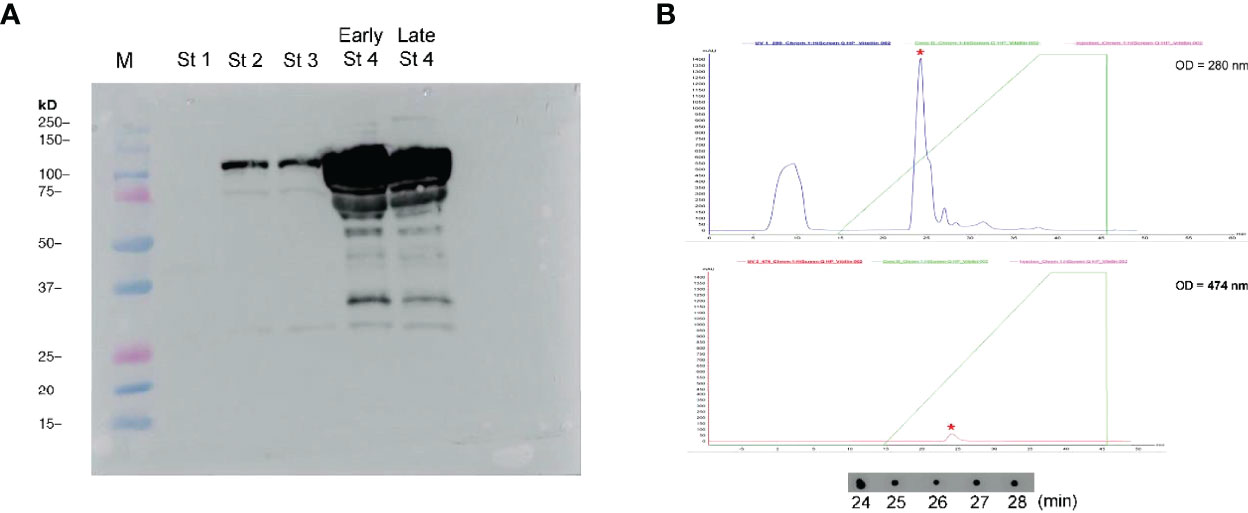
Figure 4 (A) Validation of anti-MrVn in probing the Vn subunits of the S. olivacea, lane M referred to molecular ladder, St1-Late St4 referred to the ovary samples taken from stage 1 to late stage 4, respectively. (B) Chromatogram of ovarian extract of S. olivacea as fractionated by ion exchange FPLC on a HiScreen™ Q HP columns and monitored at wavelengths of 280 nm and 474 nm, respectively. Asterisk showed the immunoreactive peak whose fractions (24-28 min) were confirmed by dot blot with anti-MrVn.
Effect of DsRNA-VIH on Ovarian Development of the S. olivacea
At day 14 and day 28 post injection, the ovarian development was determined by GSI value and hemolymph Vn level. The dsRNA-VIH treated crabs significantly produced higher GSI compared with the control group (Figure 5A). Moreover, the hemolymph Vn level of the dsRNA-VIH treated crabs at day was significantly greater than that of the control crabs at the same day of comparison (Figure 5B).
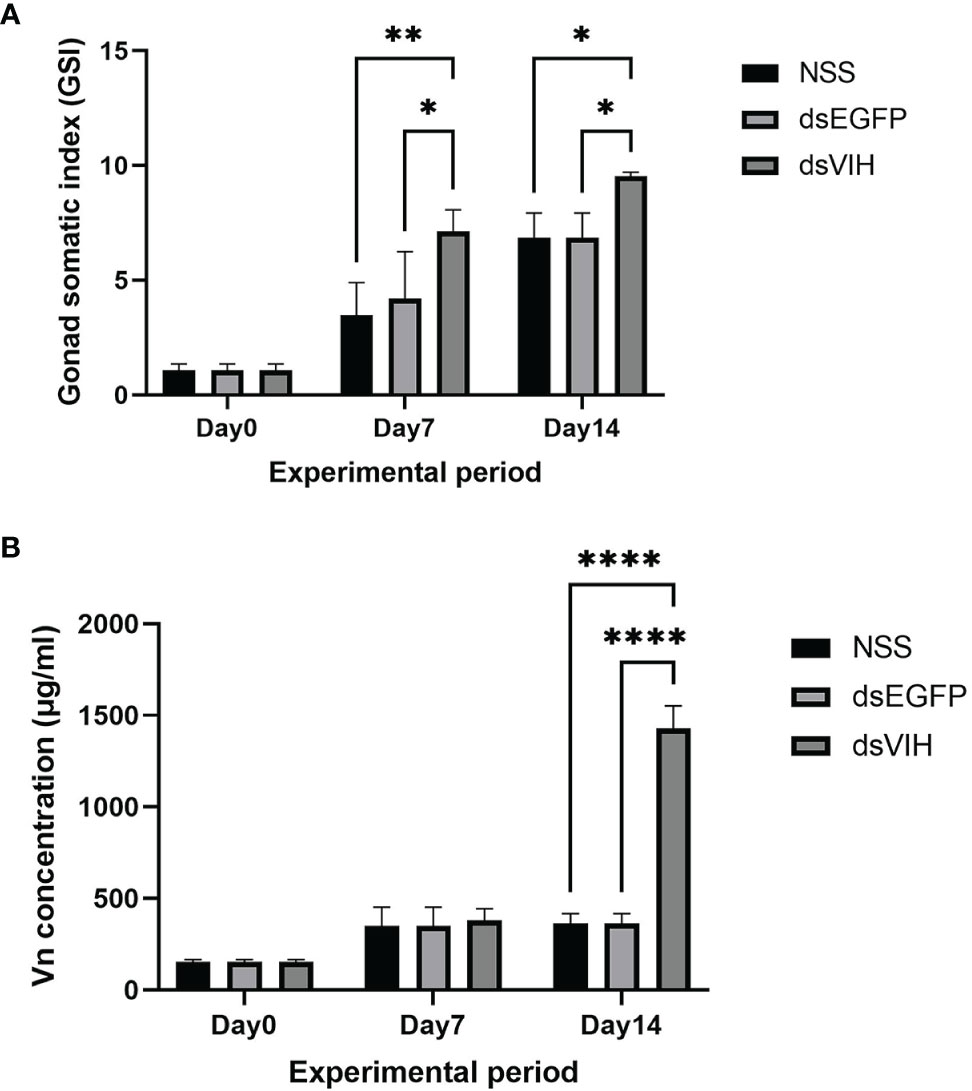
Figure 5 The effects of dsRNA-VIH on (A) GSI and (B) hemolymph Vn level of the dsRNA-VIH treated crabs and control crabs at day 14 and day 28 post-injection. Bars represented the standard error of the mean (SEM). *p < 0.05 **p < 0.005, and ****p < 0.0001.
Moreover, the histology of ovaries was observed at day 0, day 14 and day 28 of the experimental period. The histology of S. olivacea ovary, including oogonia and four steps of developing oocyte (Oc1-Oc4) was analyzed according to the previous reports (Stewart et al., 2007; Saetan et al., 2017). At day 0, ovaries in dsRNA-VIH and 0.9% normal saline (NSS) injected groups, showed three steps of oocyte (Oc1-Oc3) and oogonia (Og). The number of each stage of oocyte was similar between these two groups. (Figures 6A, B). At day 14, the NSS injected group showed Oc1-Oc3 in the ovaries, while the dsRNA-VIH injected group showed Oc4 that are fully mature oocyte (Figures 6C, D). At day 28, the NSS injected group showed Oc4 and some of Oc1-Oc2 in the ovaries, while the dsRNA-VIH injected group showed only Oc4 in the ovaries (Figures 6E, F). The number of Oc4 observed in the ovaries of dsRNA-VIH injected groups were significantly higher than those observed in the ovaries of females injected with NSS on the day 14, and 28 (Figures 6G–I).
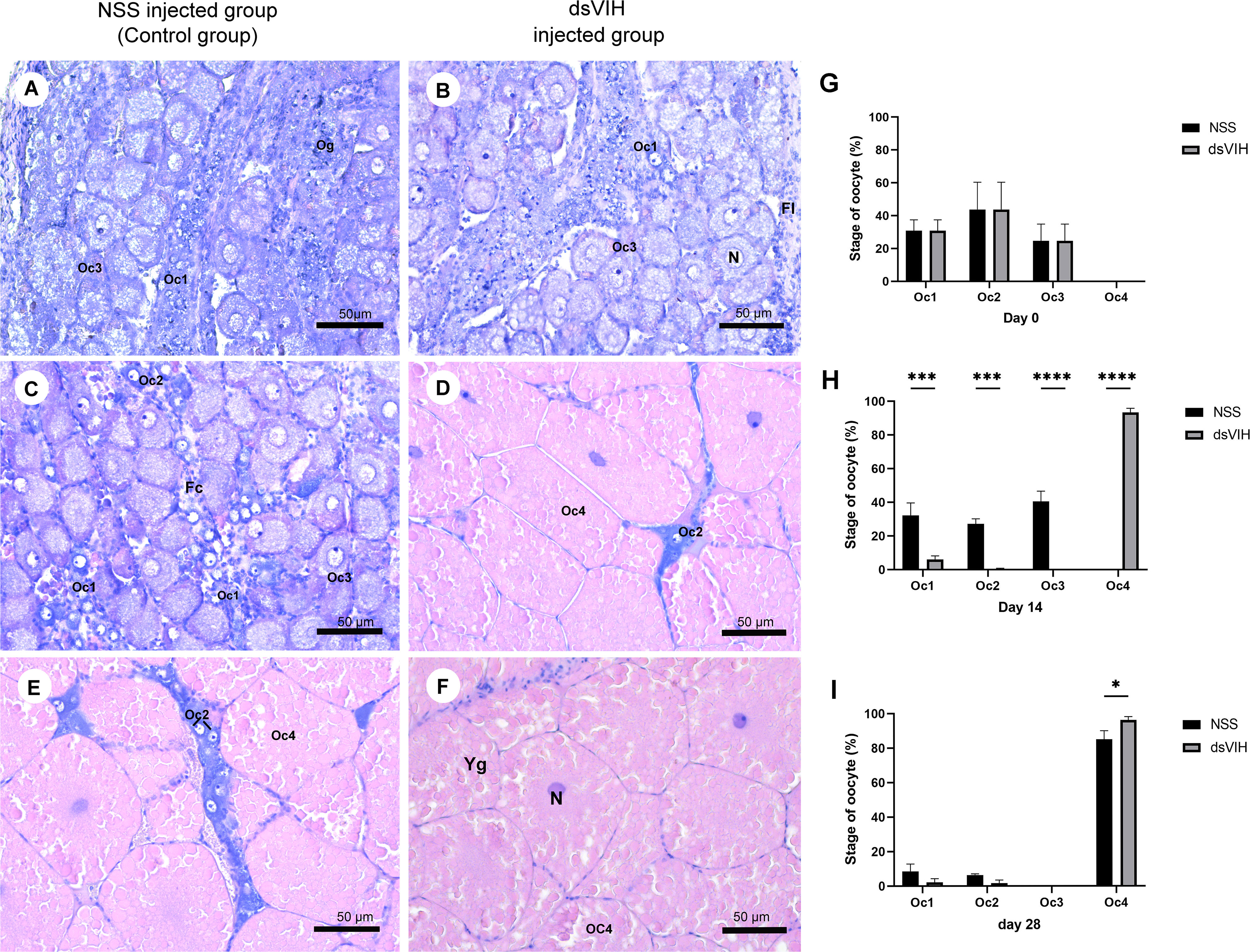
Figure 6 Histological analysis of the ovaries of dsRNA-VIH and NSS treated crabs. A representative sample from each group is shown. (A, B) At day 0, ovaries in both group showed oogonia (Og) and three steps of oocyte (Oc1-Oc3) (C, D) At day 14, NSS injected group and dsRNA-VIH injected group showed Oc1 that clearly displayed euchromatic nucleus, Oc2 that was oval and was surrounded by follicular cells, Oc3 that was oval and it contained mostly euchromatin and prominent nucleolus with small lipid droplets in cytoplasm, and Oc4 that was fully mature oocyte with large spherical ovoid shape and heavy lipid droplets in cytoplasm. (E, F) At day 28, the dsRNA-VIH injected group showed more Oc4 than that of the NSS injected group while the latter also showed some pre-mature stage (Oc1-Oc2). (G–I) Quantification of the oocyte stage on day 0, 14 and 28. Asterisks indicate significant differences compared to the NSS injected group and dsRNA-VIH injected group with *p < 0.05, ***p < 0.001, ****p < 0.0001. Oocyte developmental features are indicated as Yg, yolk granule oocyte; Oc, oocyte, Og, oogania; Fc, foccicle cell; N, nucleus.
Effect of DsRNA-VIH on the Upregulation of Reproductive-Related Genes
To study the effect of dsRNA-VIH, relative abundances of the S. olivacea prostaglandin E synthase (Scyol-PGES) and S. olivacea estrogen sulfotransferase (Scyol-ESULT) transcripts in brain and ventral nerve cord (VNC) were validated. The relative abundances of these two genes were normalized using abundance of β-actin transcript for both treatment and control groups. After dsRNA-VIH administration at day 7, relative abundance of the Scyol-PGES in the brain and VNC was greater when compared with those expressed in control crabs (Figures 7A, B). The relative abundance of Scyol-ESULT of dsRNA-VIH group in the brain and VNC were also significantly greater than those of control group at day 7 and day 14 post-injection (Figures 7C, D).
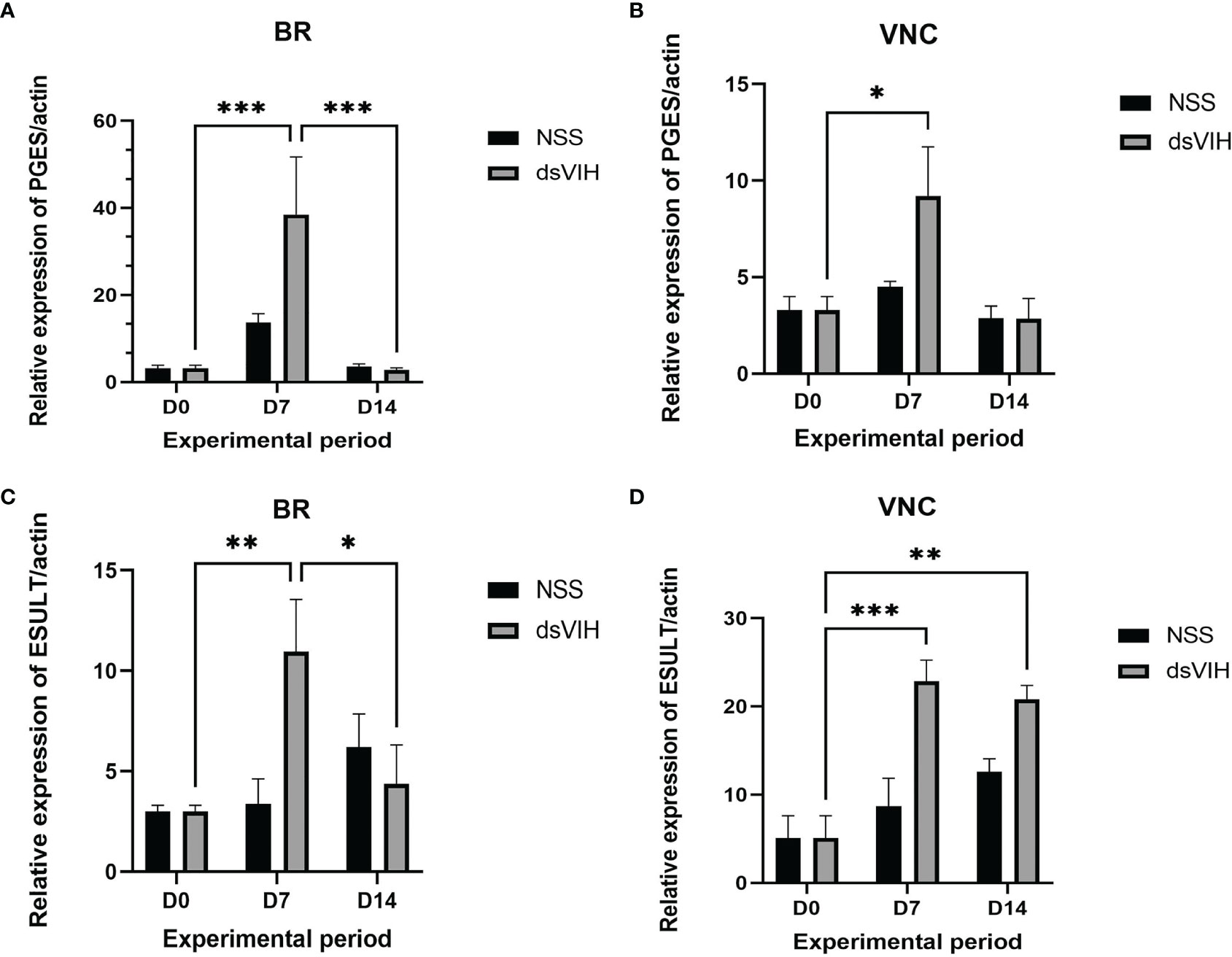
Figure 7 Effect of dsRNA-VIH on the Scyol-PGES and Scyol-ESULT transcript abundances using quantitative RT-PCR. (A, B) The histograms showed the level of Scyol-PGES which significantly expressed higher in brain and VNC after dsRNA-VIH administration for 7 days. (C, D) The histograms showed the level of Scyol-ESULT which significantly expressed higher in brain and VNC after dsRNA-VIH administration for 7 and 14 days. BR, brain; VNC, ventral nerve cord. Bars represent the standard error of the mean (SEM). *p < 0.05, **p < 0.005 and ***p < 0.0005.
Discussion
In this study, we demonstrated the use of dsRNA-VIH to promote ovarian development in the Scylla olivacea. As the reproduction of crustaceans was naturally promoted by groups of neurotransmitter (NT)/neurohormone (NH) i.e., the serotonin (Tinikul et al., 2016), the GnRH-like peptide (Guan et al., 2014; Bao et al., 2015), the RPCH (Zeng et al., 2016; Jayasankar et al., 2020) while it was together suppressed by some particular NT/NH, i.e., the dopamine (Alfaro-Montoya et al., 2004; Tinikul et al., 2016), oxytocin/vasopressin-like peptide (Saetan et al., 2018; Lin et al., 2020; Saetan et al., 2021) and the VIH (Kang et al., 2021). In the mud crab, the VIH gene was characterized and demonstrated its expression in the eyestalk, brain, and ventral nerve cord (VNC) (Kornthong et al., 2019) while this gene expressed specifically in the eyestalk of the S. paramamosain (Liu et al., 2018). The expression of VIH gene detected in eyestalk, brain, VNC as well as in other peripheral organs was also mentioned in the L. vannamei (Chen et al., 2014). Since there is no direct evidence of VIH in inhibiting vitellogenesis in this species, however, the VIH inhibited hepatopancreas vitellogenesis was reported in the L. vannamei (Chen et al., 2014). Referring the common practice for enhancing crustacean reproduction, as reported in the L. vannamei (Chen et al., 2014; Kang et al., 2014; Feijo et al., 2016), Penaeus monodon (Treerattrakool et al., 2008) and Barytelphusa lugubris (Rana, 2018), is the unilateral eyestalk ablation which resembled the removal of eyestalk GIH/VIH synthesis and release, this practice may cause significant hormonal imbalance and severe injury to the animal that affect the quantity and quality of larvae (Okumura, 2007; Uawisetwathana et al., 2011). Hence, based on the S. olivacea VIH sequence (GenBank accession no. MH882453.1), we successfully produced the dsRNA-VIH with expectation to silence the endogenous eyestalk and brain VIH expression that in turn helped promoting mud crab reproduction without torturing the animals and hence, can sustainably employ them in aquaculture.
As the VIH in S. olivacea was demonstrated to express in the eyestalk, brain and VNC, and the treatment of dopamine was found to promote the eyestalk VIH gene expression in this species (Kornthong et al., 2019). In this study, injection of dsRNA-VIH potentially inhibited the eyestalk and brain VIH transcripts for 24 h, with prolonged effect for 3 to 14 days for eyestalk VIH suppression. In P. monodon, the dsRNA-GIH, once being applied into the animal, could suppress the GIH gene expression for 24 h to 60 h (Sukumaran et al., 2017). The use of RNAi against the GIH in the same species displayed longer suppressing effect up to 30 days post-injection (Treerattrakool et al., 2011). Moreover, in L. vannamei injection of the various dsRNA-VIHs to the animals could suppress multiple VIH candidates for 10 to 30 days (Kang et al., 2019; Kang et al., 2021). Since the suppressing effect of dsRNA-VIH on its target gene was varied among species, however, it could say that the dsRNA-VIH produced in this study was efficient to silence the VIH transcript in mud crab.
The effect of dsRNA-VIH on the VTG expression was assumed by the stage of ovarian maturation and level of hemolymph Vn. The VTG, a key protein of yolk protein, has been used as indicator for determining ovarian maturation in many crustaceans (Soonklang et al., 2012; Guan et al., 2014), in this study, we fractionated the Vn from crab ovary and used it as standard protein in our Vn indirect-ELISA (Tinikul et al., 2016; Saetan et al., 2017). However, the anti-MrVn which was produced against the Vn of Macrobrachium rosenbergii was used (Soonklang et al., 2012). We, therefore, validated the antibody specificity by probing it against the S. olivacea ovarian proteins. The anti-MrVn was able to depict 2 Vn subunits which sizes were about 85 and 111 kDa and this result corresponded with previous report of 2 Vn subunits in the S. olivacea (Chen et al., 2007). Moreover, the well-organized standard curve of Vn in ELISA (data not shown) also conformed the ability of anti-MrVn to effectively probe the mud crab Vn in our study.
A single dose of 0.6 µg/g BW of dsRNA-VIH was enough to suppress VIH gene expression, without the repeat injection of dsRNA-VIH until day 14 after injection. Expectedly, the dsRNA-VIH injected crabs significantly had higher GSI value at day 28 with ovarian histology showing more mature oocytes. As similar with Vn level, the dsRNA-VIH injected crabs significantly had higher Vn level in hemolymph at day 28 compared to the control group. This was in concerted with other studies that the dsRNA-VIH injection can trigger vitellogenesis and ovarian maturation for example, in the P. monodon (Treerattrakool et al., 2008); L. vannamei (Feijo et al., 2016) and M. rosenbergii (Cohen et al., 2021). In contrast, the dsRNA-VIH injected in L. vannamei showed no significant elevation of either hemolymph Vg or ovarian Vg transcripts (Kang et al., 2019; Kang et al., 2021), while the authors suggested that the synthesized VIH was probably kept in the eyestalk and released during the time the VIH gene was silenced (Kang et al., 2021). In addition, we purposed that the dsRNA-VIH itself might not fully exert the stimulatory effects on S. olivacea reproduction in this time since other unidentified factors might probably affect. Removal of VIH by dsRNA-VIH might change their expression thresholds that in turn possibly decelerated some reproductive parameters in the S. olivacea. However, combination of dsRNA-VIH with other known stimulating agents, i.e., serotonin (Tinikul et al., 2016); or spiperone (Alfaro-Montoya et al., 2004) might be more effective in promoting female mud crab reproduction.
The S. olivacea prostaglandin E synthase (Scyol-PGES) and S. olivacea estrogen sulfotransferase (Scyol-ESULT) were identified in our previous studies (Kornthong et al., 2014; Duangprom et al., 2018). The PGES functioned in biogenesis of PGE2 which was reported in participating ovarian maturation in many crustaceans (Sarojini et al., 1988; Reddy et al., 2004; Sumpownon et al., 2015). The Scyol-PGES was highly expressed in stage 4 ovary and abundantly found in the small- and medium-sized neurons of the brain (Duangprom et al., 2018). In this study, the absence of VIH by our dsRNA-VIH administration enhanced the expression of Scyol-PGES in brain and VNC at day 7 post-injection. These findings provide the first insight into a relationship between the VIH and Scyol-PGES in a crustacean related with ovarian maturation. As well, the dsRNA-VIH could enhance expression of the Scyol-ESULT which presumably functioned in solubility of estradiol in hemolymph (Cole et al., 2010). The ESULT plays an essential role by adding a sulfate group to estradiol which play role in gonadal maturation in the S. serrata and P. monodon (Quinitio et al., 1994; Warrier et al., 2001). Therefore, this gene has also been a potential candidate for reproduction in mud crab and the Scyol-ESULT expressed in the brain and VNC was negatively regulated by the VIH. Since the direct connection between VIH and these two genes was not reported yet in any crustaceans, however, it can be assumed that the lack of VIH by the dsRNA-VIH turned other stimulating molecules, i.e., serotonin, to positively regulated the expression of these two genes, and probably of others. For example, the serotonin injection was able to increase the Scyol-ESULT expression in both brain and ovary of the mud crab (Kornthong et al., 2014). Since the serotonin injection had no effect on the VIH expression in the same species (Kornthong et al., 2019), we could therefore not set any direct link between these two molecules in the mud crab.
In conclusion, the present study was successful in synthesizing the dsRNA-VIH for applying in the S. olivacea. The dsRNA-VIH was proved to last inhibit the VIH synthesis in eyestalk and brain of the mud crabs. Injection of the dsRNA-VIH at the dose of 0.6 µg/gram body weight to the crabs could promote many reproductive parameters as well as the expression of Scyol-PGES and Scyol-ESULT which reflected the effectiveness of dsRNA-VIH. However, to get better reproductive stimulation, the combination of the dsRNA-VIH with other stimulating neuropeptides/neurohormones was suggested.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
SD, TP, SS, JS, MT and NK designed experiment, analyzed data and wrote manuscript. SD, SS, TP, PSu, and MT performed experiments. SD, JS, PSo, and NK conceptual designed the experiment, provided experimental tools and made manuscript revisions. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Thammasat University Research Unit in Biotechnology and its application of aquatic animals. The authors also gratefully acknowledge the financial support provided by Thammasat University under the TU Research scholar (Grant No. TUGT5/2562) and Thai Government Research Fund (Integrated Research and Innovation Fund) through Thammasat University, Contract No. 38/2562.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are especially grateful to Thammasat University Research Unit in Biotechnology and its application of aquatic animals, Thammasat University under the TU Research scholar and Thai Government Research Fund (Integrated Research and Innovation Fund) through Thammasat University. The authors also gratefully thank Center of Scientific Equipment for Advanced Research, Office of Advanced Science and Technology, Thammasat University and Coastal Aquaculture Research and Development Regional Center 2, Samut Sakhon, Thailand for support the research instrument and crab culture area in this work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.880235/full#supplementary-material
References
Alfaro-Montoya J., Zúñiga Calero G., Komen H. (2004). Induction of Ovarian Maturation and Spawning by Combined Treatment of Serotonin and a Dopamine Antagonist, Spiperone in Litopenaeus Stylirostris and Litopenaeus Vannamei. Aquaculture 236, 511–522. doi: 10.1016/j.aquaculture.2003.09.020
Amin-Safwan A., Harman M. F., Mardhiyyah M., Nadirah M., Ikhwanuddin M. (2018). Does Water Salinity Affect the Level of 17β-Estradiol and Ovarian Physiology of Orange Mud Crab, Scylla Olivacea (Herbst 1796) in Captivity? J. King Saud Univ. Sci. 31, 827–835. doi: 10.1016/j.jksus.2018.08.006
Amin-Safwan A., Muhd-Farouk H., Nadirah M., Ikhwanuddin M. (2016). Effect of Water Salinity on the External Morphology of Ovarian Maturation Stages of Orange Mud Crab, Scylla Olivacea (Herbst 1796) in Captivity. Pak. J. Biol. Sci. 19, 219–226. doi: 10.3923/pjbs.2016.219.226
Avarre J.-C., Michelis R., Tietz A., Lubzens E. (2003). Relationship Between Vitellogenin and Vitellin in a Marine Shrimp (Penaeus Semisulcatus) and Molecular Characterization of Vitellogenin Complementary Dnas1. Biol. Reprod. 69, 355–364. doi: 10.1095/biolreprod.102.011627
Azra M. N., Ikhwanuddin M. (2016). A Review of Maturation Diets for Mud Crab Genus Scylla Broodstock: Present Research, Problems and Future Perspective. Saudi J. Biol. Sci. 23, 257–267. doi: 10.1016/j.sjbs.2015.03.011
Bao C., Yang Y., Huang H., Ye H. (2015). Neuropeptides in the Cerebral Ganglia of the Mud Crab, Scylla Paramamosain: Transcriptomic Analysis and Expression Profiles During Vitellogenesis. Sci. Rep. 5, 17055. doi: 10.1038/srep17055
Chen H.-Y., Ho S.-H., Chen T.-I., Soong K., Chen I. M., Cheng J.-H. (2007). Identification of a Female-Specific Hemocyanin in the Mud Crab, Scylla Olivacea (Crustacea: Portunidae). Zool. Stud. 46, 194–202.
Chen Y.-N., Kuo C. (1998). Purification and Characterization of Vitellin From the Freshwater Giant Prawn, Macrobrachium Rosenbergii. Zool. Stud. 37, 126–136.
Chen T., Zhang L. P., Wong N. K., Zhong M., Ren C. H., Hu C. Q. (2014). Pacific White Shrimp (Litopenaeus Vannamei) Vitellogenesis-Inhibiting Hormone (VIH) is Predominantly Expressed in the Brain and Negatively Regulates Hepatopancreatic Vitellogenin (VTG) Gene Expression. Biol. Reprod. 90, 47. doi: 10.1095/biolreprod.113.115030
Cohen S., Ilouz O., Manor R., Sagi A., Khalaila I. (2021). Transcriptional Silencing of Vitellogenesis-Inhibiting and Molt-Inhibiting Hormones in the Giant Freshwater Prawn, Macrobrachium Rosenbergii, and Evaluation of the Associated Effects on Ovarian Development. Aquaculture 538, 736540. doi: 10.1016/j.aquaculture.2021.736540
Cole G. B., Keum G., Liu J., Small G. W., Satyamurthy N., Kepe V., et al. (2010). Specific Estrogen Sulfotransferase (SULT1E1) Substrates and Molecular Imaging Probe Candidates. Proc. Natl. Acad. Sci. U.S.A. 107, 6222–6227. doi: 10.1073/pnas.0914904107
Duangprom S., Ampansri W., Suwansa-Ard S., Chotwiwatthanakun C., Sobhon P., Kornthong N. (2018). Identification and Expression of Prostaglandin E Synthase (PGES) Gene in the Central Nervous System and Ovary During Ovarian Maturation of the Female Mud Crab, Scylla Olivacea. Anim. Reprod. Sci. 198, 220–232. doi: 10.1016/j.anireprosci.2018.09.022
Duangprom S., Kornthong N., Suwansa-ard S., Srikawnawan W., Chotwiwatthanakun C., Sobhon P. (2017). Distribution of Crustacean Hyperglycemic Hormones (CHH) in the Mud Crab (Scylla Olivacea) and Their Differential Expression Following Serotonin Stimulation. Aquaculture 468, 481–488. doi: 10.1016/j.aquaculture.2016.11.008
Feijo R. G., Braga A. L., Lanes C. F., Figueiredo M. A., Romano L. A., Klosterhoff M. C., et al. (2016). Silencing of Gonad-Inhibiting Hormone Transcripts in Litopenaeus Vannamei Females by Use of the RNA Interference Technology. Mar. Biotechnol. (NY) 18, 117–123. doi: 10.1007/s10126-015-9676-2
Ghazali A., Mat Noordin N., Abol-Munafi A. B., Azra M. N., Ikhwanuddin M. (2017). Ovarian Maturation Stages of Wild and Captive Mud Crab, Scylla Olivacea Fed With Two Diets. Sains Malays. 46, 2273–2280. doi: 10.17576/jsm-2017-4612-03
Guan Z.-B., Shui Y., Liao X.-R., Xu Z.-H., Zhou X. (2014). Primary Structure of a Novel Gonadotropin-Releasing Hormone (GnRH) in the Ovary of Red Swamp Crayfish Procambarus Clarkii. Aquaculture 418-419, 67–71. doi: 10.1016/j.aquaculture.2013.10.010
Guan Z.-B., Yi H.-X., Zhao H., Shui Y., Cai Y.-J., Liao X.-R. (2017). Cell Division Cycle 2 Participates in Eyestalk Ablation-Induced Ovarian Maturation of Procambarus Clarkii. Aquaculture 468, 115–119. doi: 10.1016/j.aquaculture.2016.10.009
Hidir A., Aaqillah-Amr M. A., Azra M. N., Shahreza M. S., Abualreesh M. H., Peng T. H., et al. (2021). Sexual Dimorphism of Mud Crab, Genus Scylla Between Sexes Based on Morphological and Physiological Characteristics. Aquac. Res. 52, 5943–5961. doi: 10.1111/are.15497
Hidir A., Aaqillah-Amr M. A., Noordiyana M. N., Ikhwanuddin M. (2018). Diet and Internal Physiological Changes of Female Orange Mud Crabs, Scylla Olivacea (Herbst 1796) in Different Ovarian Maturation Stages. Anim. Reprod. Sci. 195, 216–229. doi: 10.1016/j.anireprosci.2018.05.026
Hsu Y. W., Messinger D. I., Chung J. S., Webster S. G., de la Iglesia H. O., Christie A. E. (2006). Members of the Crustacean Hyperglycemic Hormone (CHH) Peptide Family are Differentially Distributed Both Between and Within the Neuroendocrine Organs of Cancer Crabs: Implications for Differential Release and Pleiotropic Function. J. Exp. Biol. 209, 3241–3256. doi: 10.1242/jeb.02372
Islam M. S., Kodama K., Kurokora H. (2010). Ovarian Development of the Mud Crab Scylla Paramamosain in a Tropical Mangrove Swamps, Thailand. J. Sci. Res. 2, 380–389. doi: 10.3329/jsr.v2i2.3543
Jayasankar V., Tomy S., Wilder M. N. (2020). Insights on Molecular Mechanisms of Ovarian Development in Decapod Crustacea: Focus on Vitellogenesis-Stimulating Factors and Pathways. Front. Endocrinol. 11. doi: 10.3389/fendo.2020.577925
Kang B. J., Bae S.-H., Suzuki T., Niitsu S., Wilder M. N. (2019). Transcriptional Silencing of Vitellogenesis-Inhibiting Hormone (VIH) Subtype-I in the Whiteleg Shrimp, Litopenaeus Vannamei. Aquaculture 506, 119–126. doi: 10.1016/j.aquaculture.2019.03.028
Kang B. J., Okutsu T., Tsutsui N., Shinji J., Bae S. H., Wilder M. N. (2014). Dynamics of Vitellogenin and Vitellogenesis-Inhibiting Hormone Levels in Adult and Subadult Whiteleg Shrimp, Litopenaeus Vannamei: Relation to Molting and Eyestalk Ablation. Biol. Reprod. 90, 12. doi: 10.1095/biolreprod.113.112243
Kang B. J., Sultana Z., Wilder M. N. (2021). Assessment of the Effects of Double-Stranded Rnas Corresponding to Multiple Vitellogenesis-Inhibiting Hormone Subtype I Peptides in Subadult Female Whiteleg Shrimp, Litopenaeus Vannamei. Front. Endocrinol. 12. doi: 10.3389/fendo.2021.594001
Kegel G., Reichwein B., Weese S., Gaus G., Peter-Katalinic J., Keller R. (1989). Amino Acid Sequence of the Crustacean Hyperglycemic Hormone (CHH) From the Shore Crab, Carcinus Maenas. FEBS Lett. 255, 10–14. doi: 10.1016/0014-5793(89)81051-8
Kornthong N., Chotwiwatthanakun C., Chansela P., Tinikul Y., Cummins S. F., Hanna P. J., et al. (2013). Characterization of Red Pigment Concentrating Hormone (RPCH) in the Female Mud Crab (Scylla Olivacea) and the Effect of 5-HT on its Expression. Gen. Comp. Endocrinol. 185, 28–36. doi: 10.1016/j.ygcen.2013.01.011
Kornthong N., Cummins S. F., Chotwiwatthanakun C., Khornchatri K., Engsusophon A., Hanna P. J., et al. (2014). Identification of Genes Associated With Reproduction in the Mud Crab (Scylla Olivacea) and Their Differential Expression Following Serotonin Stimulation. PloS One 9, e115867. doi: 10.1371/journal.pone.0115867
Kornthong N., Duangprom S., Suwansa-Ard S., Saetan J., Phanaksri T., Songkoomkrong S., et al. (2019). Molecular Characterization of a Vitellogenesis-Inhibiting Hormone (VIH) in the Mud Crab (Scylla Olivacea) and Temporal Changes in Abundances of VIH mRNA Transcripts During Ovarian Maturation and Following Neurotransmitter Administration. Anim. Reprod. Sci. 208, 106122. doi: 10.1016/j.anireprosci.2019.106122
Lacombe C., Greve P., Martin G. (1999). Overview on the Sub-Grouping of the Crustacean Hyperglycemic Hormone Family. Neuropeptides 33, 71–80. doi: 10.1054/npep.1999.0016
Lin D., Wei Y., Ye H. (2020). Role of Oxytocin/Vasopressin-Like Peptide and Its Receptor in Vitellogenesis of Mud Crab. Int. J. Mol. Sci. 21, 2297. doi: 10.3390/ijms21072297
Liu C., Jia X., Zou Z., Wang X., Wang Y., Zhang Z. (2018). VIH From the Mud Crab is Specifically Expressed in the Eyestalk and Potentially Regulated by Transactivator of Sox9/Oct4/Oct1. Gen. Comp. Endocrinol. 255, 1–11. doi: 10.1016/j.ygcen.2017.09.018
Meister G., Tuschl T. (2004). Mechanisms of Gene Silencing by Double-Stranded RNA. Nature 431, 343–349. doi: 10.1038/nature02873
Muhd-Farouk H., Nurul H. A., Abol-Munafi A. B., Mardhiyyah M. P., Hasyima-Ismail N., Manan H., et al. (2019). Development of Ovarian Maturations in Orange Mud Crab, Scylla Olivacea (Herbst 1796) Through Induction of Eyestalk Ablation and Methyl Farnesoate. Arab. J. Basic Appl. Sci. 26, 171–181. doi: 10.1080/25765299.2019.1588197
Nagaraju G. P. C. (2011). Reproductive Regulators in Decapod Crustaceans: An Overview. J. Exp. Biol. 214, 3–16. doi: 10.1242/jeb.047183
Okumura T. (2007). Effects of Bilateral and Unilateral Eyestalk Ablation on Vitellogenin Synthesis in Immature Female Kuruma Prawns, Marsupenaeus Japonicus. Zoolog. Sci. 24, 233–240. doi: 10.2108/zsj.24.233
Okumura T., Aida K. (2001). Effects of Bilateral Eyestalk Ablation on Molting and Ovarian Development in the Giant Freshwater Prawn, Macrobrachium Rosenbergii. Fish. Sci. 67, 1125–1135. doi: 10.1046/j.1444-2906.2001.00370.x
Okumura T., Yamano K., Sakiyama K. (2007). Vitellogenin Gene Expression and Hemolymph Vitellogenin During Vitellogenesis, Final Maturation, and Oviposition in Female Kuruma Prawn, Marsupenaeus Japonicus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 147, 1028–1037. doi: 10.1016/j.cbpa.2007.03.011
Okumura T., Yoshida K., Nikaido H. (2004). Ovarian Development and Hemolymph Vitellogenin Levels in Laboratory-Maintained Protandric Shrimp, Pandalus Hypsinotus: Measurement by a Newly Developed Time-Resolved Fluoroimmunoassay (TR-FIA). Zoolog. Sci. 21, 1037–1047. doi: 10.2108/zsj.21.1037
Overton J. L., Macintosh D. J. (2002). Estimated Size at Sexual Maturity for Female Mud Crabs (Genus Scylla) From Two Sympatric Species Within Ban Don Bay, Thailand. J. Crust. Biol. 22, 790–797. doi: 10.1163/20021975-99990293
Pak J., Fire A. (2007). Distinct Populations of Primary and Secondary Effectors During RNAi in C. Elegans. Science 315, 241–244. doi: 10.1126/science.1132839
Qian Y.-Q., Dai L., Yang J.-S., Yang F., Chen D.-F., Fujiwara Y., et al. (2009). CHH Family Peptides From an ‘Eyeless’ Deep-Sea Hydrothermal Vent Shrimp, Rimicaris Kairei: Characterization and Sequence Analysis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 154, 37–47. doi: 10.1016/j.cbpb.2009.04.013
Quackenbush L. S. (2015). Yolk Synthesis in the Marine Shrimp, Penaeus Vannamei. Am. Zool. 41, 458–464. doi: 10.1093/icb/41.3.458
Quinitio E. T., Hara A., Yamauchi K., Nakao S. (1994). Changes in the Steroid Hormone and Vitellogenin Levels During the Gametogenic Cycle of the Giant Tiger Shrimp, Penaeus Monodon. Comp. Biochem. Physiol. Part C: Pharmacol. Toxicol. Endocrinol. 109, 21–26. doi: 10.1016/0742-8413(94)00044-B
Rana S. K. (2018). Eye Stalk Ablation of Freshwater Crab, Barytelphusa Lugubris: An Alternative Approach of Hormonal Induced Breeding. Int. J. Pure Appl. Zool. 6, 30–34.
Rani K., Subramoniam T. (1997). Vitellogenesis in the Mud Crab Scylla Serrata—an in Vivo Isotope Study. J. Crust. Biol. 17, 659–665. doi: 10.1163/193724097x00080
Reddy P. S., Reddy P. R., Nagaraju G. P. (2004). The Synthesis and Effects of Prostaglandins on the Ovary of the Crab Oziotelphusa Senex Senex. Gen. Comp. Endocrinol. 135, 35–41. doi: 10.1016/j.ygcen.2003.08.002
Saetan J., Kornthong N., Duangprom S., Phanthong P., Kruangkum T., Sobhon P. (2021). The Oxytocin/Vasopressin-Like Peptide Receptor mRNA in the Central Nervous System and Ovary of the Blue Swimming Crab, Portunus Pelagicus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 258, 110983. doi: 10.1016/j.cbpa.2021.110983
Saetan J., Kruangkum T., Phanthong P., Tipbunjong C., Udomuksorn W., Sobhon P., et al. (2018). Molecular Cloning and Distribution of Oxytocin/Vasopressin-Like mRNA in the Blue Swimming Crab, Portunus Pelagicus, and its Inhibitory Effect on Ovarian Steroid Release. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 218, 46–55. doi: 10.1016/j.cbpa.2018.01.012
Saetan J., Senarai T., Thongbuakaew T., Kruangkum T., Chansela P., Khornchatri K., et al. (2017). The Presence of Abalone Egg-Laying Hormone-Like Peptide in the Central Nervous System and Ovary of the Blue Swimming Crab, Portunus Pelagicus, and its Effect on Ovarian Maturation. Aquaculture 479, 412–422. doi: 10.1016/j.aquaculture.2017.06.007
Sarojini R., Jayalakshmi K., Rao S. S., Nagabhushanam R. (1988). Stimulation of Oogenesis in the Freshwater Prawn, Macrobrachium Lamerri by Prostagalndin E2 and Follicle Stimulating Hormone. Indian J. Fish 25, 283–287.
Soonklang N., Wanichanon C., Stewart M. J., Stewart P., Meeratana P., Hanna P. J., et al. (2012). Ultrastructure of Differentiating Oocytes and Vitellogenesis in the Giant Freshwater Prawn, Macrobrachium Rosenbergii (De Man). Microsc. Res. Tech. 75, 1402–1415. doi: 10.1002/jemt.22081
Sroyraya M., Chotwiwatthanakun C., Stewart M. J., Soonklang N., Kornthong N., Phoungpetchara I., et al. (2010). Bilateral Eyestalk Ablation of the Blue Swimmer Crab, Portunus Pelagicus, Produces Hypertrophy of the Androgenic Gland and an Increase of Cells Producing Insulin-Like Androgenic Gland Hormone. Tissue Cell. 42, 293–300. doi: 10.1016/j.tice.2010.07.003
Stewart M., Soonklang N., Stewart P., Hanna P., Wanichanon C., Parratt A., et al. (2007). Histological Studies of the Ovaries of Two Tropical Portunid Crabs, Portunus Pelagicus (L.) and Scylla Serrata (F.). Inver. Reprod. Dev. 50, 85–97. doi: 10.1080/07924259.2007.9652231
Subramoniam T. (2011). Mechanisms and Control of Vitellogenesis in Crustaceans. Fish. Sci. 77, 1–21. doi: 10.1007/s12562-010-0301-z
Sukumaran V., Reshmi C., Jose S., Peter R., Kk V., Philip R., et al. (2017). Crustacean Hyperglycemic Hormone Family Gene Silencing in Penaeus Monodon Mediated Through dsRNA Synthesized In Vitro From Genomic and Cdna. Indian J. Biotechnol. 16, 37–43.
Sumpownon C., Engsusophon A., Siangcham T., Sugiyama E., Soonklang N., Meeratana P., et al. (2015). Variation of Prostaglandin E2 Concentrations in Ovaries and its Effects on Ovarian Maturation and Oocyte Proliferation in the Giant Fresh Water Prawn, Macrobrachium Rosenbergii. Gen. Comp. Endocrinol. 223, 129–138. doi: 10.1016/j.ygcen.2015.04.019
Tan-Fermin J. D. (1991). Effects of Unilateral Eyestalk Ablation on Ovarian Histology and Oocyte Size Frequency of Wild and Pond-Reared Penaeus Monodon (Fabricius) Broodstock. Aquaculture 93, 77–86. doi: 10.1016/0044-8486(91)90206-M
Tinikul Y., Poljaroen J., Tinikul R., Sobhon P. (2016). Changes in the Levels, Expression, and Possible Roles of Serotonin and Dopamine During Embryonic Development in the Giant Freshwater Prawn, Macrobrachium Rosenbergii. Gen. Comp. Endocrinol. 225, 71–80. doi: 10.1016/j.ygcen.2015.09.018
Treerattrakool S., Panyim S., Chan S. M., Withyachumnarnkul B., Udomkit A. (2008). Molecular Characterization of Gonad-Inhibiting Hormone of Penaeus Monodon and Elucidation of its Inhibitory Role in Vitellogenin Expression by RNA Interference. FEBS J. 275, 970–980. doi: 10.1111/j.1742-4658.2008.06266.x
Treerattrakool S., Panyim S., Udomkit A. (2011). Induction of Ovarian Maturation and Spawning in Penaeus Monodon Broodstock by Double-Stranded RNA. Mar. Biotechnol. (NY) 13, 163–169. doi: 10.1007/s10126-010-9276-0
Tsukimura B. (2015). Crustacean Vitellogenesis: its Role in Oocyte Development1. Am. Zool. 41, 465–476. doi: 10.1093/icb/41.3.465
Uawisetwathana U., Leelatanawit R., Klanchui A., Prommoon J., Klinbunga S., Karoonuthaisiri N. (2011). Insights Into Eyestalk Ablation Mechanism to Induce Ovarian Maturation in the Black Tiger Shrimp. PloS One 6, e24427. doi: 10.1371/journal.pone.0024427
Volz D. C., Kawaguchi T., Chandler G. T. (2002). Purification and Characterization of the Common Yolk Protein, Vitellin, From the Estuarine Amphipod Leptocheirus Plumulosus. Prep. Biochem. Biotechnol. 32, 103–116. doi: 10.1081/PB-120004123
Warrier S. R., Tirumalai R., Subramoniam T. (2001). Occurrence of Vertebrate Steroids, Estradiol 17beta and Progesterone in the Reproducing Females of the Mud Crab Scylla Serrata. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 130, 283–294. doi: 10.1016/s1095-6433(01)00385-3
Webster S. G., Keller R., Dircksen H. (2012). The CHH-superfamily of Multifunctional Peptide Hormones Controlling Crustacean Metabolism, Osmoregulation, Moulting, and Reproduction. Gen. Comp. Endocrinol. 175, 217–233. doi: 10.1016/j.ygcen.2011.11.035
Keywords: vitellogenesis-inhibiting hormone (VIH), double strand RNA, Scylla olivacea, ovarian maturation, reproductive-related genes
Citation: Duangprom S, Saetan J, Phanaksri T, Songkoomkrong S, Surinlert P, Tamtin M, Sobhon P and Kornthong N (2022) Acceleration of Ovarian Maturation in the Female Mud Crab With RNA Interference of the Vitellogenesis-Inhibiting Hormone (VIH). Front. Mar. Sci. 9:880235. doi: 10.3389/fmars.2022.880235
Received: 21 February 2022; Accepted: 19 April 2022;
Published: 02 June 2022.
Edited by:
Haihui Ye, Jimei University, ChinaCopyright © 2022 Duangprom, Saetan, Phanaksri, Songkoomkrong, Surinlert, Tamtin, Sobhon and Kornthong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Napamanee Kornthong, bmFwYW1hbmVlbmF0dEBnbWFpbC5jb20=
 Supawadee Duangprom
Supawadee Duangprom Jirawat Saetan2
Jirawat Saetan2 Napamanee Kornthong
Napamanee Kornthong