
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Mar. Sci. , 19 May 2022
Sec. Global Change and the Future Ocean
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.870228
This article is part of the Research Topic Blue Carbon: Beyond the Inventory View all 17 articles
 Shunyang Chen1,2†
Shunyang Chen1,2† Shiquan Chen3†
Shiquan Chen3† Bin Chen1,2,4
Bin Chen1,2,4 Zhongjie Wu3
Zhongjie Wu3 Wenshuo An1,2
Wenshuo An1,2 Lizhen Luo3
Lizhen Luo3 Jing Wang5
Jing Wang5 Limei Xie6
Limei Xie6 Jing Zhang5
Jing Zhang5 Guangcheng Chen1,2,4*
Guangcheng Chen1,2,4*The adverse impact of macroalgal blooms associated with nutrient enrichment on seagrass habitats and carbon storage potential is an ecological concern. In the present study, the soil carbon stock and sources were compared between a seagrass meadow where a serious macroalgal bloom occurred (site M) and an adjacent site without apparent macroalgae (site R) in a nutrient-enriched lagoon in South Hainan Island, China, to test whether macroalgal blooms associated with nutrient enrichment would impact the soil carbon in seagrass meadows. The soil organic carbon (OC) and total nitrogen contents in the top 30 cm at site M were significantly lower than those at site R. The soil OC stocks (top 30 cm) were 3.4 and 5.4 Mg C ha-1 at site M and site R, respectively, and no difference was observed between sampling stations with different distances offshore at either site. Soil δ13C was more enriched and closer to the δ13C of seagrass tissues at site R than at site M. Bayesian stable isotope mixing model analyses suggested that seagrass-derived material contributed ~50% to soil OC at site R, while at site M, the contribution was reduced to ~25%. The results suggested that macroalgal blooms associated with nutrient enrichment could drive the loss of seagrass-derived OC and the OC stock in the soil, which is worthy of full attention for blue carbon conservation.
Seagrass meadows are important blue carbon ecosystems that occur in all coastal areas of the world, except along Antarctic shores (Hemminga and Duarte, 2000). The organic carbon (OC) stored in the top metre of soils was estimated to range from 9.8 to 19.8 Pg C in global seagrass meadows, comparable to the organic carbon stored in the world’s mangrove forests and tidal salt marshes (Fourqurean et al., 2012). However, due to various anthropogenic impacts, seagrass meadows are disappearing or degraded (Dunic et al., 2021). A global assessment by Waycott et al. (2009) suggested that seagrasses disappeared at a rate of 110 km2 yr-1 between 1980 and 2006, and the rates of decline have accelerated since 1990 relative to those before 1940. The decrease in seagrass extent has resulted in a loss of OC stored in seagrass soils, in an annual loss of between 63 and 297 Tg C, since the beginning of the twentieth century (Fourqurean et al., 2012).
Declines of seagrass populations have been observed in many estuarine embayments, which are often associated with anthropogenic nutrient loading (Waycott et al., 2009). Nutrient enrichment by nitrogen fertilization has been found to increase seagrass biomass and litter production (Armitage and Fourqurean, 2016; Howard et al., 2016; Qin et al., 2021), which provide more seagrass-derived detritus to be incorporated into the soil. However, increased availability of nutrients in the embayments may lead to blooms of macroalgae, phytoplankton and epiphytes, which reduces the light available for photosynthesis and decreases seagrass productivity and populations, leading to habitat loss and fragmentation (Burkholder et al., 2007; Han and Liu, 2014; Santos et al., 2020). Moreover, nutrient enrichment and macroalgal blooms may impact the quantity and quality of OC storage in seagrass soils by a ‘priming effect’, which trigger an extra decomposition of OC after providing exogenous inorganic nitrogen and labile OC to the soils (Kuzyakov et al., 2000). The priming effect of exogenous OC input on the soil mineralization of OC have been found through experimental addition of algal OC to seagrass soil (Trevathan-Tackett et al., 2018; Liu et al., 2019), or through comparing the OC contents and compositions among sites subjected to different nutrient loadings (Liu et al., 2016; Jiang et al., 2018). These results suggest that macroalgal blooms in nutrient-enriched embayments may lead to the loss of soil OC in seagrass meadows, while few studies have examined this effect.
Seagrass meadows are commonly found in China either in the tropical/subtropical regions in the South China Sea or in the temperate northern provinces. Hainan Island within the tropical Indo-Pacific bioregion has been suggested to have the greatest extent and number of species of seagrass in China (Zheng et al., 2013), where seagrass mostly occurs in lagoons and on coral platforms (Wang et al., 2012). Due to threats from sea reclamation, marine aquaculture and harvest activities, a massive loss in the seagrass extent has taken place since the 2000s (Chen et al., 2015). In some embayment areas, the nutrient input from adjacent mariculture has resulted in nutrient enrichment, macroalgal blooms, and the degradation of seagrass meadows (Wang et al., 2012). In this study, we investigated the soil OC stocks and sources in seagrass meadows in a nutrient-enriched lagoon, namely, Xincun Bay in southeastern Hainan Island, to test whether macroalgal blooms would result in a loss of soil organic carbon. We hypothesized a lower soil organic carbon stock associated with the macroalgal bloom in the nutrient-enriched area, and the macroalgal bloom also resulted in a change in the carbon composition of the seagrass soil.
The Xincun Bay Lagoon is located southeast of Hainan Island, between 18°22′ –18°47′ N and 109°45′ –110°08′ E (Figure 1A). The region has a tropical monsoon climate, and the monthly mean temperature ranged from 20°C to 29°C from 2009 to 2018, with the highest temperature recorded in June (Weather China, 2022). The monthly mean precipitation ranges from 6-445 mm, and the annual precipitation is 2011 mm. Tides in the Xincun Bay area are mixed semidiurnal, with an annual tidal range of 1.34 m.
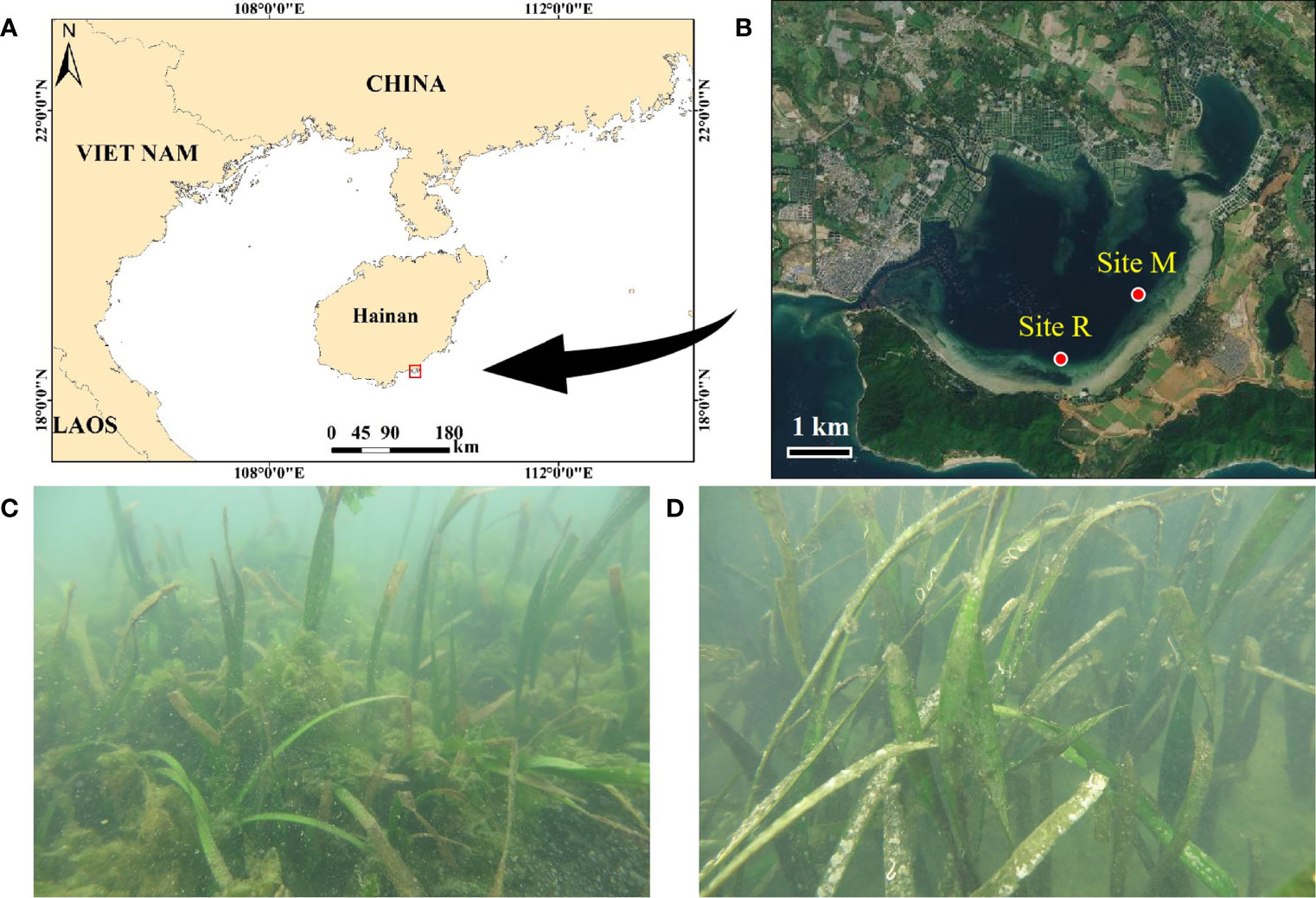
Figure 1 Locations of Xincun Bay Lagoon (A) and the two seagrass sites (B), and respective typical scenes from the two seagrass sites, site M (C) and site R (D). Site M represents the nutrient enrichment site with macroalgal blooms, and site R represents the reference site without apparent macroalgal blooms. The panels A and B were created with image obtained from ArcGIS version 10.3 and National Platform for Common Geospatial Information Services (https://map.tianditu.gov.cn), respectively.
In the Xincun Bay Lagoon, seagrass meadows occur mainly along its southern coast on the sandy substrate. The seagrass is dominated by Enhalus acoroides in terms of biomass, while Thalassia hemprichii is also commonly found. However, nutrient enrichment of the lagoon area has occurred in recent decades due to various anthropogenic activities, including offshore restaurants and residences, marine aquaculture and shipping. The inorganic nitrogen concentration in the seawater around the seagrass sites increased by ~1.5 times between year 2005 and 2013 (Jiang et al., 2018). A recent study reported an inorganic nitrogen concentration of the seawater with a range of 0.10-0.38 mg N L-1 in 2017 in the lagoon area (Fang et al., 2021). The nutrient enrichment has resulted in adverse impacts on seagrass ecosystems, e.g., habitat fragmentation and macroalgal blooms. A macroalgal biomass up to 19 g m-2 was reported at a seagrass site close to fish cage culture area in the Xincun Bay, ~5 time as that at a seagrass site with greater distance (~800m versus 3 km) off the culture area (Liu et al., 2016).
In this study, sampling was carried out at two E. acoroides-dominated seagrass sites (site M and site R) on the southern coast of the lagoon (Figure 1B). Site M had a longer semi-exchange time of seawater and stronger cumulative impacts of anthropogenic nutrient input than site R (Fang et al., 2020), where intensive overgrowth of macroalgae was observed (Figures 1C, D). The macroalgae was observed at this location in 2008, indicating that the algal bloom had been lasted for a decade before our sampling. Site R represented a reference site without apparent macroalgal blooms. The two seagrass sites had similar canopy coverage, density and biomass of E. acoroides (Table S1), while site M, as observed, presented more fragmentation of the seagrass canopy. At each of the two sites, two sampling stations (LW and SW) with different distances offshore were established. LW was designated the landward station of the E. acoroides zone, while SW (~150 m away from LW) signified the seaward zone. The substrate was sandy at these two sites, consisting of >90% sand (Table S1).
Soil cores were collected in January 2018 using PVC tubes (inner diameter 70 mm) with metal cutters on their bottom edges. The tubes were manually inserted into the soil at the E. acoroides-covered areas by gently turning the tube until a depth of 30 cm was reached at each sampling plot. At each sampling station, whole seagrass samples of E. acoroides (including leaves, roots and rhizomes) were collected in three replicates and transported to the laboratory. Moreover, macroalgal samples of the dominant species, Ulva lactuca were collected at site M, and seawater was sampled in triplicates using plastic bottles in the central lagoon area during the high tide period to collect the suspended particulate organic matter (SPOM).
The soils were extruded in the laboratory by inserting a plunger at the bottom of the cores and carefully drawing the PVC liner down over the plunger. The soil cores were divided into subsections at 10 cm intervals. Each subsection was weighed and then sliced into two halves, with one half oven-dried at 60°C to determine the water content of fresh samples. The other half was then air-dried after removing visible animals, plant residues and stones (> 2 mm). The whole-plant tissues of seagrass were cleaned of epiphytes and sand and dried at 60°C for 24 h. The seawater samples, after passing through a 75 μm mesh, were filtered through a precombusted 0.7 μm GF/F filter to collect the SPOM. Epiphytes on seagrass leaves were scraped and rinsed using Milli-Q water and then filtered through a filter.
The OC, total nitrogen (TN) and δ13C in the soil and plant samples were measured using a Thermo Flash EA 1112 HT-Delta V Advantages system. Air-dried subsamples of soils and seagrass tissues were placed into silver cups, acidified with diluted HCl (5%) and then oven-dried at 40°C to remove the carbonates. Filtered samples of SPOM and epiphytes for isotope analysis were acidified by fumigation overnight over 1 mol L−1 HCl to remove inorganic carbonates. The stable carbon isotopic composition is reported in the δ notation as the ratio of the heavy to the light stable isotope in the sample relative to that of a standard. The reproducibility of OC and stable isotopic analysis were 1.2% and 0.2‰, respectively.
The normality and homogeneity of variables were checked using the Shapiro–Wilk test and Levene’s test, respectively, and if necessary, data were transformed with the Blom method to follow normality and homogeneity. A parametric three-way analysis of variance (ANOVA) was conducted to test for any effects of the sampling site, sampling station and soil depth and their interactive effects. Differences in the soil OC stocks and variables of the seagrass samples between the two sites and sampling stations were tested using two-way ANOVA. One-way ANOVA was used to compare the differences in variables among the carbon sources. The potential contributions of the primary sources (seagrass, SPOM, macroalgae and epiphyte at Site M; seagrass, SPOM and epiphyte at Site R) to the soil carbon composition were estimated using a Bayesian stable isotope mixing model, SIMMR.
The soil bulk density of the top 30 cm was comparable between the two seagrass sites and between the two sampling stations according to the three-way ANOVA test (Figure 2A and Table S2), while a difference was found between the surface (0-10 cm) and the 20-30 m layer (Table S3). The OC content in the top 30 cm of the soil showed significant differences with the seagrass site and soil depth. The degraded site had OC values ranging between 0.47 mg g-1 and 1.06 mg g-1, which were lower than those measured at the site R (0.80-1.86 mg g-1). No significant difference in soil OC content was found with distance offshore (Figure 2B), and its value was significantly lower in the bottom layer than in the two upper layers. Similar spatial variation patterns of the soil OC density (0.78-2.61 g cm-3) to those of the soil OC content were observed in this study (Figure 2C), and higher values were measured at the site R and in the top 20 cm soil layers.
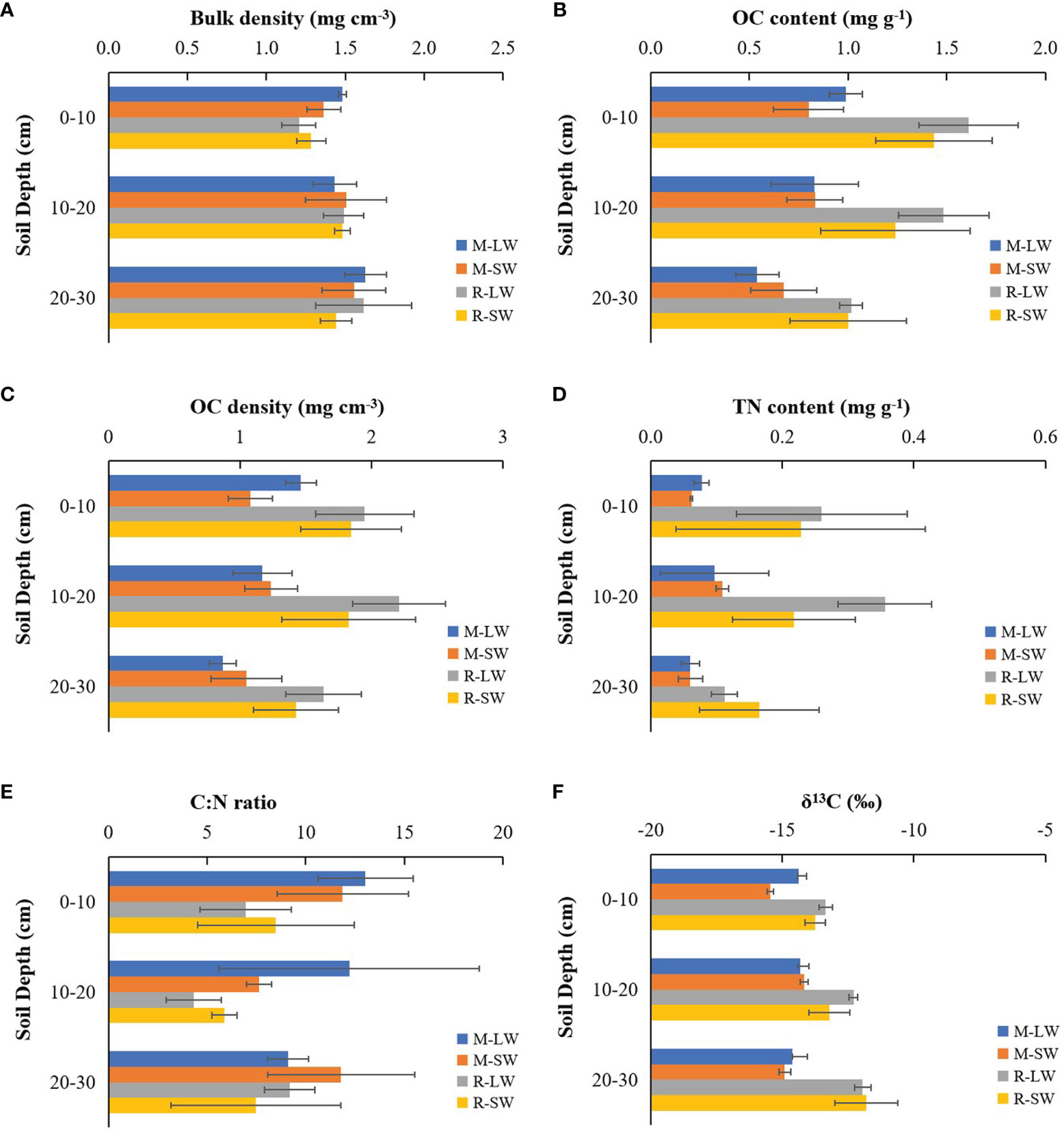
Figure 2 Soil parameters of the two sampling stations at the two seagrass sites (M and R) in the Xincun Bay lagoon: (A) bulk density, (B) OC content, (C) OC density, (D) TN content, (E) C:N ratio and (F) δ13C. LW, landward station; SW, seaward station.
The mean soil TN content was < 0.11 mg g-1 at site M (Figure 2D), while at site R, the value was higher and reached 0.36 mg g-1. In contrast, site R presented a significantly lower C:N ratio (in weight) than the values at site M (Figure 2E). There was no significant change in soil TN and the C:N ratio with either soil depth or sampling station.
The soil OC stock was higher at site R than at degraded site M (F=22.661, p<0.01) and was similar between the two sampling stations (F=0.974, p>0.05). The OC stocks were 3.5 ± 0.4 Mg C ha-1 (M-LW), 3.4 ± 0.4 Mg C ha-1 (M-SW), 5.8 ± 0.8 Mg C ha-1 (R-LW) and 5.1 ± 1.1 Mg C ha-1 (R-SW) in the top 30 cm soil at the four seagrass stations.
The δ13C and C:N ratios of the SPOM were -21.89 ± 0.32‰ and 2.44 ± 0.14, respectively, in the Xincun Bay lagoon. The seagrass tissues had comparable δ13C, OC, TN contents and C:N ratios between the two seagrass sites and between the two sampling stations (Table 1 and Table S4). Seagrass tissues had the most enriched 13C, and their δ13C values were -8‰. The epiphytes had lower mean δ13C and C:N ratios than the macroalgal and seagrass tissues; the macroalgal and seagrass tissues had comparable C:N ratios.
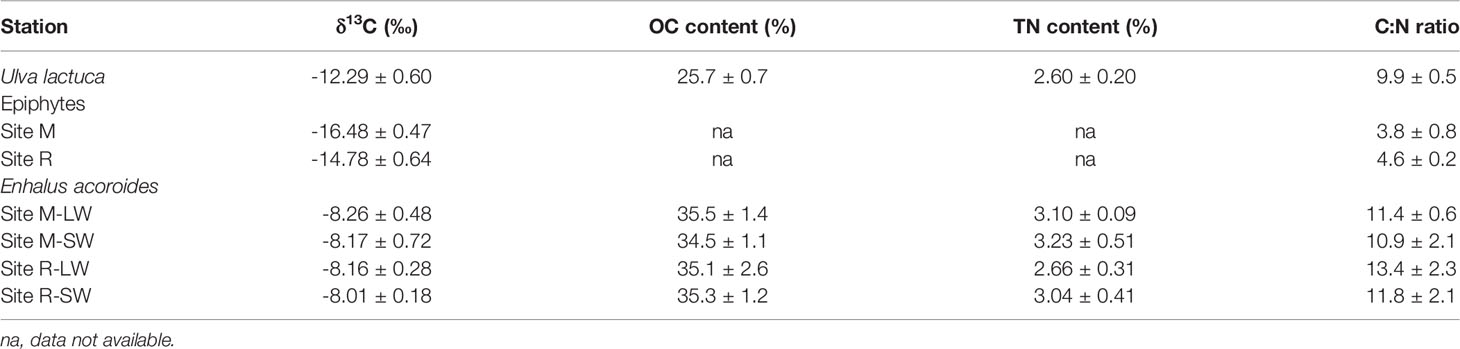
Table 1 δ13C, organic carbon (OC) content, total nitrogen (TN) content and C:N ratio (in weight) of macroalgae (Ulva lactuca), epiphytes and seagrass (Enhalus acoroides) samples in the Xincun Bay Lagoon.
There were significant main effects of the seagrass site, sampling stations and soil depth on the soil δ13C, and a significant interaction of the seagrass site with sampling depth was found (Figure 2F and Table S3). The soil δ13C were more enriched at site R and were closer to the δ13C of the seagrass tissues at each soil layer. No significant difference in soil δ13C with soil depth was found at site M, while the value became less negative when soil depth increased at site R. The soil δ13C followed an increasing trend with soil OC (r=0.553, p<0.01). The SIMMR mixing model estimations showed a dissimilarity in the composition of carbon sources as assigned to the top 30 cm of the soil (Table 2). At site R, seagrass-derived material likely contributed more than the epiphytes and SPOM, and its respective proportional contribution was similar between the two stations. At site M, the seagrass reduced its contribution to the soil OC relative to its performance at site R, and macroalgae had a greater contribution than seagrass at both sampling stations.
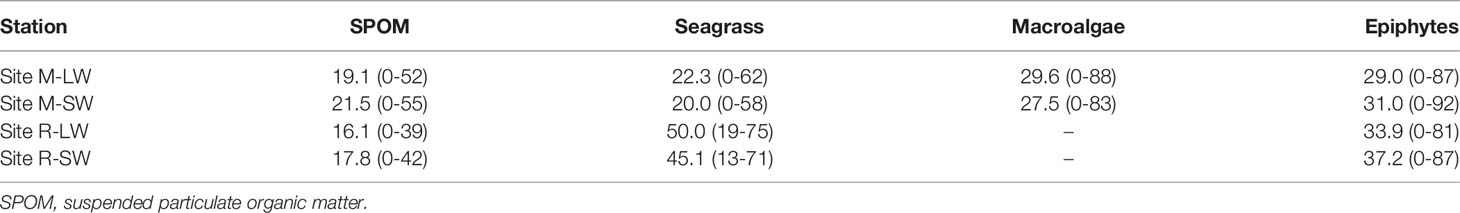
Table 2 Means and ranges of proportional contributions of the potential sources to the soil organic carbon at the two seagrass sites in the Xincun Bay Lagoon.
The present study compared the ecosystem OC stocks and soil carbon sources between the two seagrass sites in the nutrient-enriched lagoon, and the results showed that the OC content in the top 30 cm soil at the degraded seagrass site where macroalgae overgrew (site M) was 37% lower than that at the site R less impacted by macroalgae, with a low soil OC stock and more negative δ13C value. The results suggest that macroalgal blooms due to nutrient enrichment would impact the soil OC stock and the carbon composition of seagrass soil. We also found differences in the soil TN content and C:N ratio between the two seagrass sites.
The OC stocks in the Xincun Bay were 3.4 and 5.4 Mg C ha-1 in the top 30 cm of the soil at site M and site R, respectively, which were lower than the soil OC stocks at the same depth reported in other seagrass meadows in the tropical and subtropical areas (Lavery et al., 2013; Miyajima et al., 2015; Serrano et al., 2021; Table S5), especially those in North Sulawesi in Indonesia (32-68 Mg C ha−1, Chen et al., 2017) and the Colombian Caribbean (50-85 Mg C ha−1, Serrano et al., 2021). The low soil OC stock in the Xincun Bay was attributed to the low OC content in the sandy seagrass soil. The soil OC contents (0.47-1.86 mg g-1) at our sampling sites are comparable to those reported by Jiang et al. (2018) at other seagrass sites (0.5-2.3 mg g-1) in Xincun Bay and are much lower than the values reported at other seagrass sites (Table S5) and the global average OC content, 25 mg g−1, in seagrass soils (Fourqurean et al., 2012). Previous studies have suggested a low OC content strongly related to a high proportion of coarse grain size of seagrass soils (Dahl et al., 2016), and that the OC in coarse-grained soils is more refractory than in fine-grained soils (Howard et al., 2021).
The present study measured a lower soil OC content and stock at the site M where macroalgal bloom had occurred for no less than a decade than at the site R, and we consider that the macroalgal bloom due to nutrient enrichment may result in the loss of OC in seagrass soils. The macroalgal bloom reduces advective water exchange in seagrass meadows, and the rapid decomposition of macroalgal materials produces more labile carbon as a potential source of soil OC. Experimental additions of algal tissues to seagrass soils have shown promoted invertase and polyphenol oxidase activities in the soils (Liu et al., 2019) and stimulated metabolism and loss of recalcitrant components (lignin and lipids) in the seagrass litter (Liu et al., 2020) or in the soils (Trevathan-Tackett et al., 2018). During our sampling, we observed a deposited liquid layer with a dark brown colour over the soil surface from macroalgal decomposition at site M. The priming effect of the labile OC released from the rapid decomposition of macroalgae would stimulate the mineralization and loss of intrinsic OC in the seagrass soil at site M in this study. The soils in fertilized nutrient-enriched seagrass meadows were also found to present stimulated exocellular enzyme activities associated with carbon cycling (López et al., 1998; Liu et al., 2017), which are suggested to enhance the decomposition and consequent loss of soil OC in coastal wetlands (Luo et al., 2017). The soil OC contents at our sites before the macroalgal bloom were not available; however, we suspect that the soil OC content before macroalgal bloom at site M was no less than that at site R, because site M had had a longer semi-exchange time of seawater and reduced wave energy, which favours the burial of more organic carbon in the soil (Mazarrasa et al., 2021). The lower soil OC content and stock at the site M therefore indicate a loss of OC relative to the site R.
Decrease in OC content with depth is a common feature of soil profile of OC in seagrass meadows (Fourqurean et al., 2012), as was found at the site R in this study. The upper layer at the site M as the interface of macroalgae and soil, receives high macroalgal OC loading. A more pronounced loss of soil OC therefore may be expected at the upper layer relative to the deeper layers, leading to different soil profile of OC at the site M from that at site R. This is contrarily to our result at the site M showing a lower OC content in the 20-30cm layer than the upper layers. We consider that the loss of soil OC in the deeper layer could also be apparent because the macroalgal OC could reach the deeper layer of the sandy soil through penetration and bioturbation, and trigger the mineralization of OC at a higher rate than that in the upper layers (Trevathan-Tackett et al., 2018). Soil profile of δ13C at the two sites suggested a more pronounced impact of macroalgal bloom on the OC composition in the deeper layer. Moreover, the low OC content in the deeper layer may also be attributed to continuous burial of depleted OC by macroalgal bloom along with the sedimentation. However, the lack of dating of soil in this study would affect the interpretation of the observed differences in soil OC with seagrass site and soil depth; further studies are therefore deserved to investigate the priming effects of macroalgal bloom on the soil profile of OC content and composition, with the duration of macroalgal bloom and dating of soil considered.
Seagrasses contribute to the OC sequestration in their soils in the form of their above-/belowground litter, and ~16% of their net primary production was estimated to be buried in the soil pool of the global seagrass meadows (Duarte and Krause-Jensen, 2017). The seagrass canopy also enhances the deposition of imported organic matter by tides by trapping seston particles by seagrass leaves and reducing the particle-carrying capacity of the water (Chen et al., 2017). Studies have demonstrated that the soil OC sequestration and stock in seagrass meadows are highly related to the vegetation population (Serrano et al., 2019; Bedulli et al., 2020), while the loss of the seagrass canopy would result in the erosion of soil carbon stocks (Marbà et al., 2015). In this study, there was no significant difference in the seagrass population of the sampled patches between the two sites (Table S1); however, the fragmentation of the seagrass habitat, a finding also reported by Santos et al. (2020), would also contribute to the loss of OC in the seagrass soils at site M because continuous meadows have a stronger carbon-holding capacity than patchy ones (Ricart et al., 2015; Ricart et al., 2017).
The soil δ13C values measured at the two seagrass sites reflect a different carbon composition between these two sites. Organic carbon in seagrass soil is potentially derived from seagrass and allochthonous sources, including phytoplankton and epiphytes (Kennedy et al., 2004). Previous studies recognized seagrass tissue as the major contributor to the soil carbon pool under the seagrass canopy, with a global mean of ~50% of the carbon in the surface soil derived from seagrass sources (Kennedy et al., 2010). The soil δ13C in this study fell within a narrow range between -11.8‰ and -13.8‰ at the site R, closer to the seagrass signature than the SPOM, indicating a potentially major contribution of seagrass material to the soil OC composition. The soil δ13C was more negative at the degraded site M due to the incorporation of more 13C-depleted sources (e.g., macroalgae) and mineralization of seagrass detritus in the soil, as reflected by the increase in soil OC with less negative δ13C. In our study, soil cores were collected in the E. acoroides-covered area, and E. acoroides and U. lactuca were sampled as the single sources of seagrass and macroalgal, representatively. During our sampling, T. hemprichii and another macroalgal Enteromorpha sp. were also found, and they had a similar δ13C to those of E. acoroides and U. lactuca, respectively (Tables 1, S6). Other studies also reported a similar δ13C of whole plant samples between T. hemprichii and E. acoroides, and a similar δ13C of Hypnea boergeseni, another species ever observed in the seagrass sites, to that of Ulva species in the Xincun Bay (Liu et al., 2016; Jiang et al., 2018). Regarding the dominance of the E. acoroides and U. lactuca and the similarity in δ13C between seagrass/macroalgae species, we consider that the carbon sources extrapolated using E. acoroides and U. lactuca signatures could be representative.
In the present study, we found a more rapid decline in the soil TN content than the OC content, leading to a higher soil C:N ratio at the degraded site, suggesting that nutrient enrichment and macroalgae would impact N metabolism in seagrass soil. We suspect that the soil microbes supplied with labile macroalgal or epiphytic sources with lower C:N ratios than the soil values would consume nitrogen in the seagrass soils. Further studies are needed to investigate the soil nitrogen metabolism in seagrass meadows under nutrient enrichment and macroalgal impacts.
In this study, we measured a lower soil OC content and stock at the nutrient-enriched seagrass site where macroalgae bloom than at the site without apparent macroalgae located in the same lagoon and suggest that the degradation in the seagrass habitat and the macroalgal bloom due to nutrient enrichment drove the loss of seagrass-derived OC in the soil pool. The seagrass soils at Xincun Bay are relatively OC-poor and have low OC stocks, and we consider that the loss of the limited OC sequestered in the soil due to the priming effect of macroalgal bloom is worthy of full attention. Our results also suggest that nutrient enrichment and macroalgae would impact nitrogen metabolism, which deserves future detailed studies.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
GC contributed to the study conception. SYC, SQC, JW, JZ, LL, and WA performed the sample collection and data acquisition. LX, ZW, and BC performed the data analysis. All authors contributed to the article and approved the submitted version.
The work described in this paper was funded by the Provincial Natural Science Foundation of Fujian (2020J06030) and Hainan Provincial Basic and Applied Basic Research Fund for High-Level Talents in Natural Science (421RC662). The Scientific Research Foundation of the Third Institute of Oceanography, MNR (2020017) and the National Natural Science Foundation of China (42166006) also contribute to this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are grateful to Dr. Xijie Yin, Mr. Jiahui Chen, Mr. Pengfei Lin, and Mr. Yanmin Fu for their assistance with field sampling and laboratory analysis. We also appreciate the comments of two reviewers and those of Dr. Guanglong Qiu, which helped improve the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.870228/full#supplementary-material
Armitage A. R., Fourqurean J. W. (2016). Carbon Storage in Seagrass Soils: Long-Term Nutrient History Exceeds the Effects of Near-Term Nutrient Enrichment. Biogeosciences 13 (1), 313–321. doi: 10.5194/bg-13-313-2016
Bedulli C., Lavery P. S., Harvey M., Duarte C. M., Serrano O. (2020). Contribution of Seagrass Blue Carbon Toward Carbon Neutral Policies in a Touristic and Environmentally-Friendly Island. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00001
Burkholder J. M., Tomasko D. A., Touchette B. W. (2007). Seagrasses and Eutrophication. J. Exp. Mar. Biol. Ecol. 350, 46–72. doi: 10.1016/j.jembe.2007.06.024
Chen G., Azkab M. H., Chmura G. L., Chen S., Sastrosuwondo P., Ma Z., et al. (2017). Mangroves as a Major Source of Soil Carbon Storage in Adjacent Seagrass Meadows. Sci. Rep. 7, 42406. doi: 10.1038/srep42406
Chen S., Wang D., Wu Z., Zhang G., Li Y., Tu Z., et al. (2015). Discussion of the Change Trend of the Seagrass Beds in the East Coast of Hainan Island in Nearly a Decade. Mar. Environ. Sci. 34 (1), 48–53. doi: 10.3969/j.issn.0253-4193.2015.06.01
Dahl M., Deyanova D., Gütschow S., Asplund M. E., Lyimo L. D., Karamfilov V., et al. (2016). Sediment Properties as Important Predictors of Carbon Storage in Zostera Marina Meadows: A Comparison of Four European Areas. PloS One 11 (12), e0167493. doi: 10.1371/journal
Duarte C. M., Krause-Jensen D. (2017). Export From Seagrass Meadows Contributes to Marine Carbon Sequestration. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00013
Dunic J. C., Brown C. J., Connolly R. M., Turschwell M. P., Côté I. M. (2021). Long-Term Declines and Recovery of Meadow Area Across the World’s Seagrass Bioregions. Global Change Biol. 27, 4096–4109. doi: 10.1111/gcb.15684
Fang X., Li X., Xiang Y., Hao C., Zhao Y., Zhang Y. (2020). Cumulative Impact of Anthropogenic Nutrient Inputs on Lagoon Ecosystems - a Case Study of Xincun Lagoon, Hainan, China. Reg. Stud. Mar. Sci. 35, 101213. doi: 10.1016/j.rsma.2020.101213
Fang X., Li X., Zhang Y., Zhao Y., Qian J., Hao C., et al (2021). Random Forest-Based Understanding and Predicting of the Impacts of Anthropogenic Nutrient Inputs on the Water Quality of a Tropical Lagoon. Environ. Res. Lett. 16, 055003. doi: 10.1088/1748-9326/abf395
Fourqurean J. W., Duarte C. M., Kennedy H., Marbà N., Holmer M., Mateo M. A., et al. (2012). Seagrass Ecosystems as a Globally Significant Carbon Stock. Nat. Geosci. 5, 505–509. doi: 10.1038/ngeo1477
Han Q., Liu D. (2014). Macroalgae Blooms and Their Effects on Seagrass Ecosystems. J. Ocean Univ. China (Oceanic Coast. Sea Res.) 13 (5), 791–798. doi: 10.1007/s11802-014-2471-2
Howard J. L., Lopes C. C., Wilson S. S., McGee-Absten V., Carrión C. I., Fourqurean J. W. (2021). Decomposition Rates of Surficial and Buried Organic Matter and the Lability of Soil Carbon Stocks Across a Large Tropical Seagrass Landscape. Estuaries Coasts 44, 846–866. doi: 10.1007/s12237-020-00817-x
Howard J. L., Perez A., Lopes C. C., Fourqurean J. W. (2016). Fertilization Changes Seagrass Community Structure But Not Blue Carbon Storage: Results From a 30-Year Field Experiment. Estuaries Coasts 39, 1422–1434. doi: 10.1007/s12237-016-0085-1
Jiang Z., Liu S., Zhang J., Wu Y., Zhao C., Lian Z., et al. (2018). Eutrophication Indirectly Reduced Carbon Sequestration in a Tropical Seagrass Bed. Plant Soil 426, 135–152. doi: 10.1007/s11104-018-3604-y
Kennedy H., Beggins J., Duarte C. M., Fourqurean J. W., Holmer M., Marbà N., et al. (2010). Seagrass Sediments as a Global Carbon Sink: Isotopic Constraints. Global Biogeochem. Cycles 24, GB4026. doi: 10.1029/2010GB003848
Kennedy H., Gaciab E., Kennedy D. P., Papadimitriou S., Duarte C. M. (2004). Organic Carbon Sources to SE Asian Coastal Sediments. Estuar. Coast. Shelf Sci. 60, 59–68. doi: 10.1016/j.ecss.2003.11.019
Kuzyakov Y., Friedel J. K., Stahr K. (2000). Review of Mechanisms and Quantification of Priming Effects. Soil Biol. Biochem. 32, 1485–1498. doi: 10.1016/S0038-0717(00)00084-5
Lavery P., Mateo M-Á., Serrano O., Rozaimi M. (2013). Variability in the Carbon Storage of Seagrass Habitats and its Implications for Global Estimates of Blue Carbon Ecosystem Service. PloS One 8 (9), e73748. doi: 10.1371/journal.pone.0073748
Liu S., Jiang Z., Wu Y., Deng Y., Chen Q., Zhao C., et al. (2019). Macroalgae Bloom Decay Decreases the Sediment Organic Carbon Sequestration Potential in Tropical Seagrass Meadows of the South China Sea. Mar. Pollut. Bull. 138, 598–603. doi: 10.1016/j.marpolbul.2018.12.009
Liu S., Jiang Z., Wu Y., Zhang J., Arbi I., Ye F., et al. (2017). Effects of Nutrient Load on Microbial Activities Within a Seagrass-Dominated Ecosystem: Implications of Changes in Seagrass Blue Carbon. Mar. Pollut. Bull. 117, 214–221. doi: 10.1016/j.marpolbul.2017.01.056
Liu S., Jiang Z., Zhang J., Wu Y., Lian Z., Huang P. (2016). Effect of Nutrient Enrichment on the Source and Composition of Sediment Organic Carbon in Tropical Seagrass Beds in the South China Sea. Mar. Pollut. Bull. 110, 274–280. doi: 10.1016/j.marpolbul.2016.06.054
Liu S., Trevathan-Tackett S. M., Lewis C., Huang X., Macreadie P. I. (2020). Macroalgal Blooms Trigger the Breakdown of Seagrass Blue Carbon. Environ. Sci. Technol. 54 (22), 14750–14760. doi: 10.1021/acs.est.0c03720
López N. I., Duarte C. M., Vallespinós F., Romero J., Alcoverro T. (1998). The Effect of Nutrient Additions on Bacterial Activity in Seagrass (Posidonia Oceanica) Sediments. J. Exp. Mar. Biol. Ecol. 224, 155–166. doi: 10.1016/S0022-0981(97)00189-5
Luo L., Han M., Wu R., GU J. (2017). Impact of Nitrogen Pollution/Deposition on Extracellular Enzyme Activity, Microbial Abundance and Carbon Storage in Coastal Mangrove Sediment. Chemosphere 177, 275–283. doi: 10.1016/j.chemosphere.2017.03.027
Marbà N., Arias-Ortiz A., Masqué P., Kendrick G. A., Mazarrasa I., Bastyan G. R., et al. (2015). Impact of Seagrass Loss and Subsequent Revegetation on Carbon Sequestration and Stocks. J. Ecol. 103 (2), 296–302. doi: 10.1111/1365-2745.12370
Mazarrasa I., Lavery P., Duarte C. M., Lafratta A., Lovelock C. E., Macreadie P. I., et al. (2021). Factors Determining Seagrass Blue Carbon Across Bioregions and Geomorphologies. Global Biogeochem. Cycles 35, e2021GB006935. doi: 10.1029/2021GB006935
Miyajima T., Hori M., Hamaguchi M., Shimabukuro H., Adachi H., Yamano H., et al. (2015). Geographic Variability in Organic Carbon Stock and Accumulation Rate in Sediments of East and Southeast Asian Seagrass Meadows. Global Biogeochem. Cycles 29, 397–415. doi: 10.1002/2014GB004979
Qin L., Suonan Z., Kim S. H., Lee K. (2021). Coastal Sediment Nutrient Enrichment Alters Seagrass Blue Carbon Sink Capacity. Environ. Sci. Technol. 55, 15466–15475. doi: 10.1021/acs.est.1c03782
Ricart A. M., Pérez M., Romero J. (2017). Landscape Configuration Modulates Carbon Storage in Seagrass Sediments. Estuar. Coast. Shelf Sci. 185, 69–76. doi: 10.1016/j.ecss.2016.12.011
Ricart A. M., York P. H., Rasheed M. A., Pérez M., Romero J., Bryant C. V., et al. (2015). Variability of Sedimentary Organic Carbon in Patchy Seagrass Landscapes. Mar. Pollut. Bull. 100, 476–482. doi: 10.1016/j.marpolbul.2015.09.032
Santos R. O., Varona G., Avila C. L., Lirman D., Collado-Vides L. (2020). Implications of Macroalgae Blooms to the Spatial Structure of Seagrass Seascapes: The Case of theAnadyomene Spp. (Chlorophyta) Bloom in Biscayne Bay, Florida. Mar. Pollut. Bull. 150, 110742. doi: 10.1016/j.marpolbul.2019.110742
Serrano O., Gómez-López D. T., Sánchez-Valencia L., Acosta-Chaparro A., Navas−Camacho R., González−Corredor J., et al. (2021). Seagrass Blue Carbon Stocks and Sequestration Rates in the Colombian Caribbean. Sci. Rep. 11 (1), 11067. doi: 10.1038/s41598-021-90544-5
Serrano O., Lovelock C. E., Atwood T. B., Macreadie P. I., Canto R., Phinn S., et al. (2019). Australian Vegetated Coastal Ecosystems as Global Hotspots for Climate Change Mitigation. Nat. Commun. 10, 4313. doi: 10.1038/s41467-019-12176-8
Trevathan-Tackett S. M., Thomson A. C. G., Ralph P. J., Macreadie P. I. (2018). Fresh Carbon Inputs to Seagrass Sediments Induce Variable Microbial Priming Responses. Sci. Total Environ. 621, 663–669. doi: 10.1016/j.scitotenv.2017.11.193
Wang D., Wu Z., Chen C., Lan J., Chen X., Wu R., et al. (2012). Distribution of Sea-Grass Resources and Existing Threat in Hainan Island. Mar. Environ. Sci. 31 (1), 34–38. doi: 10.3969/j.issn.1007-6336.2012.01.008
Waycott M., Duarte C. M., Carruthers T. J. B., Orth R. J., Dennison W. C., Olyaynik S., et al. (2009). Accelerating Loss of Seagrasses Across the Globe Threatens Coastal Ecosystems. Proc. Natl. Acad. Sci. U.S.A. 106, 12377–12381. doi: 10.1073/pnas.0905620106
Weather China. (2022) History.Shtml. Available at: http://www.weather.com.cn/forecast/history.sht-ml (Accessed January 4, 2022).
Keywords: soil carbon stock, soil δ13C, total nitrogen, carbon sources, Enhalus acoroides, priming effect
Citation: Chen S, Chen S, Chen B, Wu Z, An W, Luo L, Wang J, Xie L, Zhang J and Chen G (2022) Implication of Macroalgal Bloom to Soil Organic Carbon Stock in Seagrass Meadows - A Case Study in South Hainan, China. Front. Mar. Sci. 9:870228. doi: 10.3389/fmars.2022.870228
Received: 06 February 2022; Accepted: 19 April 2022;
Published: 19 May 2022.
Edited by:
Hilary Anne Kennedy, Bangor University, United KingdomReviewed by:
Ursula Felicitas Marianne Witte, University of Aberdeen, United KingdomCopyright © 2022 Chen, Chen, Chen, Wu, An, Luo, Wang, Xie, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangcheng Chen, Z2MuY2hlbkB0aW8ub3JnLmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.