
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 09 May 2022
Sec. Marine Ecosystem Ecology
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.869138
 Raouia Ghanem1
Raouia Ghanem1 Wafa Rjiba Bahri1
Wafa Rjiba Bahri1 Amani Chaffai1
Amani Chaffai1 Jeanne Zaouali2
Jeanne Zaouali2 Bilel Hassen3
Bilel Hassen3 Sahar Karray3
Sahar Karray3 Monia El Bour3
Monia El Bour3 Jamila Ben Souissi1,2*
Jamila Ben Souissi1,2* Ernesto Azzurro4,5
Ernesto Azzurro4,5On 14th October 2021, a single specimen of Sargocentron caudimaculatum was captured in the locality of Cape Bon, Eastern Tunisia, by a local fisher using gillnets at 60 m depth, on rocky bottom. This observation represents the first record of this species in the Mediterranean Sea. General information on the squirrelfishes and the importance of molecular tools for the proper identification of morphologically challenging non-indigenous organisms were raised and discussed.
The squirrelfishes and soldierfishes (Holocentridae) are reef associated species, widely distributed from tropical to warm temperate waters of Indian, Pacific and Atlantic Oceans. Based on swimbladder characteristics, they are separated in two valid subfamilies: Holocentrinae and Myripristinae (Nelson, 1955). The Holocentrinae subfamily includes only three genera: Holocentrus Scopoli, 1777, Neoniphon Castelnau, 1875 and Sargocentron Fowler, 1904. The genus Sargocentron includes 33 species (Froese and Pauly, 2022). Eight species, out of the 85 valid species listed in the Holocentridae family (Eschmeyer and Fong, 2017), naturally occur in the Red Sea (Golani and Bogorodsky, 2010), whilst no native species occur in the Mediterranean Sea. The first documented occurrence of squirrelfishes in the Mediterranean Sea was made by Haas and Steinitz (1947) from Palestine, who reported Holocentrus ruber (Forsskål, 1775), which was later transferred to the genus Sargocentron. The Redcoat Sargocentron rubrum (Forsskål, 1775) is one of the first Red Sea species that entered the Mediterranean Sea via the Suez Canal (Golani and Ben-Tuvia, 1985) and it is recognized today as one of the most successful invaders of the Basin (Azzurro et al., 2014; Golani et al., 2021). In recent times, the species expanded geographically (Golani et al., 2021) reaching the coasts of Tunisia in 2013 (Ounifi-Ben Amor et al., 2016; Bradai et al., 2019).
Until recent times, S. rubrum was considered to be the only representative of the squirrelfish family in the Mediterranean. However, Deef (2021a) reported the occurrence of two squirrelfishes, Sargocentron spinosissimum (Temminck & Schlegel, 1843) and S. tiereoides (Bleeker, 1853) from the Egyptian Mediterranean coast.
The silverspot squirrelfish Sargocentron caudimaculatum (Rüppell, 1838), is a reef associated species, native to the Indian and Pacific Oceans from East Africa to Japan and northern Australia and as far east as the Marshall Islands. It is usually found at depths between 2 and 40 m (Randall and Heemstra, 1984) and to the best of our knowledge it has been never reported from Mediterranean waters.
On 14th October 2021, a single specimen of Sargocentron caudimaculatum was captured in the locality of Cape Bon, Eastern Tunisia, (37°02’57’’N and 10°54’18’’E) by a local fisher using gillnets at 60 m depth, on rocky bottom. Immediately after capture, specimen was provided by fishermen to researchers. The same individual was later photographed (Figure 1), measured (to the nearest millimeter) weighted (to the nearest gram) and frozen for subsequent study. Meristic and morphometric analyses were performed in the laboratory on the defrosted individual following the taxonomic keys provided by Randall and Heemstra (1984); Randall (1998) and Randall and Greenfield (1999). Finally, the collected specimen was stored in ethanol 80% and deposited to the Ichthyological collection of INAT, under the accession number: INAT-HOL-SA- cau01.

Figure 1 The silverspot squirrelfish Sargocentron caudimaculatum caught out of the Cape Bon locality.
DNA was isolated from fin samples using the Pure Link™ Genomic DNA Mini Kit (Thermo Fisher Scientific) following the manufacturer’s protocol, under sterile conditions. The concentration of the isolated DNA was measured in Nano Drop™ spectrophotometer to evaluate its quality and quantity. A 658 bp long fragment from the 5′ region of the COI gene was PCR-amplified using the primer pair as recommended by Ward et al. (2005):
FishF2 5 ′TCGACTAATCATAAAGATATCGGCAC3′ and FishR2 5 ′ACTTCAGGGTGACCGAAGAATCAGAA3.
The PCR amplification of each sample was conducted in a 25 µl volume, including 13.25 µl ultrapure water, 2.5 µl of 10x PCR buffer, 2 µl MgCl2 (25 mM), 1 µl each primer (10 mM), 1 µl (10 mM) of total dNTPs Mix, 0.3 µl of 5 U/µl Taq DNA polymerase (HOT FIREPol® DNA Polymerase), and 4 µl DNA template (ca. 10–100 ng).
The PCR amplification was carried out in a Bio-Rad iCycler Thermocycler where the thermocycling profile was customized as follows: an initial denaturation at 94°C for 3 min followed by 35 cycles of denaturation at 94°C for 50 s, primer annealing at 50°C for 2 min, extension for 72°C for 90 s, and a final extension at 72°C for 6 min. Amplification success was checked. Sequencing in one direction (forward) was performed and newly obtained sequence was uploaded in the BLASTn suite to verify whether they meet the threshold value of ≥97% for both the percent identity and query coverage. Two phylogenetic trees were constructed using neighbor-joining (NJ) (Figure 2A) and fast minimal evolution methods (Figure 2B). The sequences of the high-fidelity amplicon were submitted to the Gen Bank, assisted by the Barcode Submission Tool with detailed source information and feature annotation.

Figure 2 Phylogenetic tree created with (A), the neighbor joining method (NJ), (B), The fast minimum evolution method by using Blast tree viewer to show the relationships between aligned cytochrome c oxidase subunit I (COI) gene partial sequence of S. caudimaculatum with comparison to Genbank records.
The specimen under consideration (Figure 1) measured 214 mm total length (TL), weighed 187 g and it is described as follows: Head slightly convex and very large eyes; a long preopercular spine; head and body red; edges of scales silver; spinous portion of dorsal fin whitish dappled with light red except for spines and adjacent edges and triangular outer part of each membrane which are bright red. Dorsal fin with XI spines and 14 soft rays, deeply notched between spinous and soft portions; last dorsal-fin spine shortest, anal fin with IV spines and 10 soft rays; pelvic fins with I spine and 7 soft rays; oblique rows of scales on cheek 5; anterior end of nasal bone with 2 short diverging spines; preopercular spine long, subequal to orbit diameter, uppermost of 2 large spines posteriorly on opercle the longest; caudal fin forked. Meristic and morphometric characters are reported in Table 1.
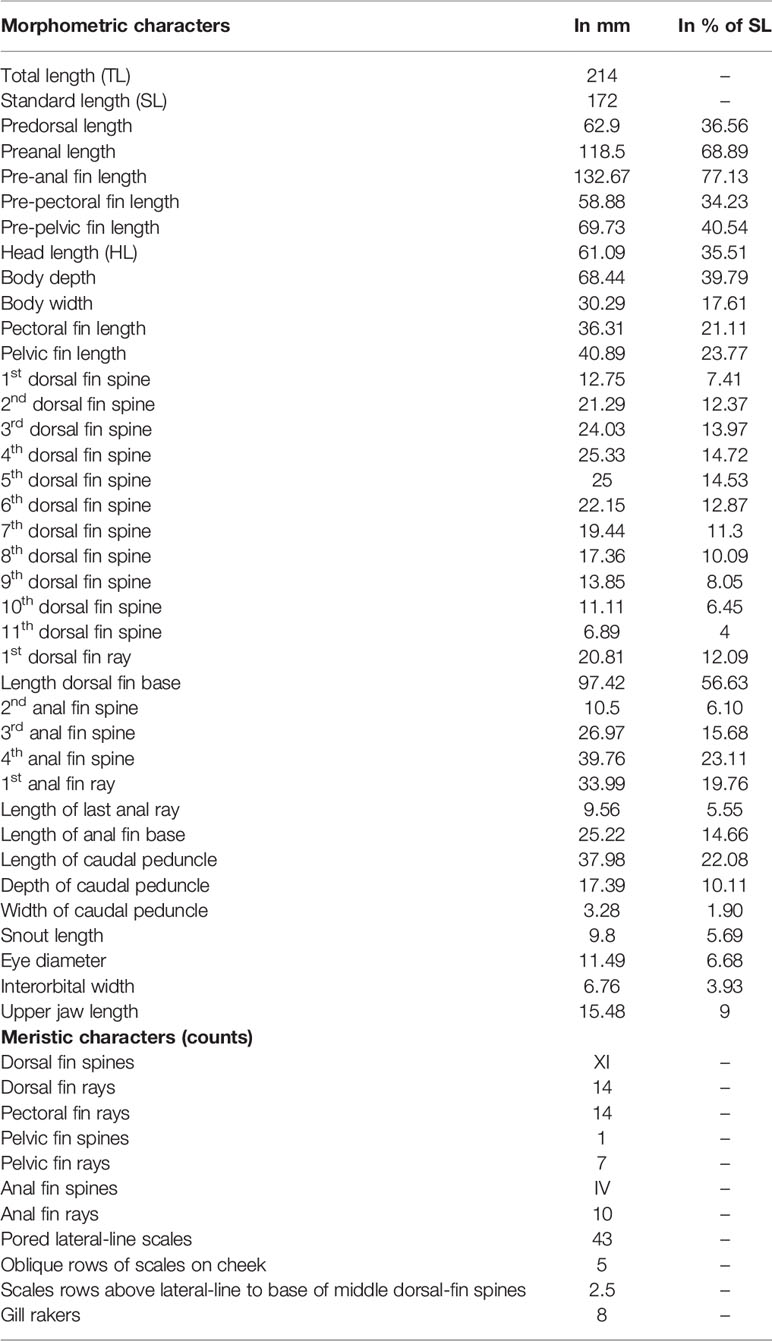
Table 1 Morphometric and meristic characters of Sargocentron caudimaculatum caught out of the Cape Bon locality.
Genetic sequences: The alignment of 100 sequences of cytochrome c oxidase subunit I (COI) gene and the resulting phylogenetic tree supported by a total of 644 nucleotide informative positions, revealed that the sequences of the Cape Bon specimen were identical to the sequences of Sargocentron caudimaculatum present in GenBank (collected in the in the Northeastern of the Red Sea (Gulf of Aqaba; MF124029) (Figure 2), with a high bootstrap support (97%).
The new sequences of S. caudimaculatum were deposited in GenBank and the accession number obtained (COI gene: OM265164).
Morphological traits, color and both morphometric and meristic characters are in accordance with previous descriptions of Sargocentron caudimaculatum (Randall and Heemstra, 1984; Randall, 1998; Randall and Greenfield, 1999). Further molecular identification using DNA barcoding confirmed the taxonomic identity of the species. Considering that S. caudimaculatum naturally occurs in the Red Sea (Golani and Bogorodsky, 2010), the species could be likely added to the list of Lessepsian immigrants (Golani et al., 2021), even if the long distance between the Suez Canal exit and the record location (> 2000 Km) could also suggest a possible introduction through ship transport. In addition, the specimen was captured near Sidi Daoud harbor which could be a further evidence to support a ship born introduction. This latter is indeed an increasingly effective vector for the introduction of exotic species in the Mediterranean Sea (Zenetos and Galanidi, 2020).
The high number of nominal species, their partial descriptions, loss of holotypes, and synonymies (Schmitz and Wainwright, 2011), as well as the similarity in color pattern and external morphology of squirrelfishes makes the identification of these ones particularly challenging (Deidun et al., 2016; Deef, 2021b). This taxonomic uncertainty also applies to S. rubrum, indeed recent barcoding analyses carried out on Lebanese specimens (Bariche et al., 2015), highlighted different sequences from any currently genetically barcoded Sargocentron species (see Keskin and Atar, 2013 for the Mediterranean Sea) and a close match with Sargocentrum seychellense (Smith & Smith, 1963). These results led to hypothesize the occurrence of a species complex for S. rubrum and to raise the possibility that unnoticed species of Sargocentron had entered the Mediterranean from the Red Sea (Bariche et al., 2015; Deidun et al., 2016; Vella et al., 2016). Hence we stress the importance of molecular tools for the proper identification of morphologically challenging non indigenous organisms, in addition to the precious collaboration with local communities, especially fishers, which always amplify our potential to detect these new arrivals.
The datasets presented in this study can be found in online repositories. The names of the repository and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, OM265164.
Ethical review and approval was not required for the animal study because It’s a new record of non-indigenous fish species (not protected).
RG performed the data analyses and wrote the first draft of manuscript. All the authors contributed to manuscript revision, read and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to acknowledge the cross-border project Italy-Tunisia BLEU-ADAPT_IS_3.1_0.21 co-financed by the European Union grant (CUP: B74I19001090002) for partially supporting this study. They also express gratitude to fishermen (Mr Ben Dafer) who provided the specimen.
Azzurro E., Victor M. T., Lombarte A., Maynou M., Simberloff D., Rodríguez-Pérez A., et al. (2014). External Morphology Explains the Success of Biological Invasions. Ecol. Lett. 17, 1455–1463. doi: 10.1111/ele.12351
Bariche M., Torres M., Smith C., Sayar N., Azzurro E., Baker R., et al. (2015). Red Sea Fishes in the Mediterranean Sea: A Preliminary Investigation of a Biological Invasion Using DNA Barcoding. J. Biogeog 42 (12), 2363–2373. doi: 10.1111/jbi.12595
Bradai M. N., Enajjar S., Saidi B. (2019). New Occurence and New Records of Fish Species of Tunisian Coasts. Bull. Inst. Natn Scien Tech Mer Salammbô 46, 9–14.
Deef L. E. M. (2021a). First Record of Two Squirrelfishes, Sargocentron spinosissimum and Sargocentron tiereoides (Actinopterygii, Beryciformes, Holocentridae) From the Egyptian Mediterranean Coast. Acta Ichthyol Piscat 51 (1), 107–112. doi: 10.3897/aiep.51.63216
Deef L. E. M. (2021b). Evaluation of Genetic Relationship of Some Squirrelfishes Through DNA Barcode. Braz. Arch. Biol. Technol. 64, 1–9. doi: 10.1590/1678-4324-2021210076
Deidun A., Attard S., Camilleri M., Vella Gaffiero J., Hampson D., Said A., et al. (2016). The First Record of the Sargocentron Genus From the Maltese Islands (Central Mediterranean)-Who Will Unravel the Current Conundrum? BioInvasions Rec 5 (2), 123–126. doi: 10.3391/bir.2016.5.2.10
Eschmeyer W. N., Fong J. (2017) Species by Family/Subfamily in the Catalog of Fishes. Electronic Version, Updated 04 January 2022. Available at: http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp.
Froese R., Pauly D. (2022). Fish Base (World Wide Web electronic publication). Available at: http://www.fishbase.org.
Golani D., Azzurro E., Dulčić J., Massutí E., Orsi-Relini L. (2021). Atlas of Exotic Fishes in the Mediterranean Sea. 2nd edition. Ed. Briand F. (Paris, Monaco: CIESM Publishers), 365 pages.
Golani D., Ben-Tuvia A. (1985). The Biology of the Indo-Pacific Squirrelfish, Sargocentron rubrum (Forsskål 1775), a Suez Canal Migrant to the Eastern Mediterranean. J. Fish Biol. 27 (3), 249–258. doi: 10.1111/j.1095-8649.1985.tb04025.x
Golani D., Bogorodsky S. V. (2010). The Fishes of the Red Sea—Reappraisal and Updated Checklist. Zootaxa 2463 (1), 1–135. doi: 10.11646/zootaxa.2463.1.1
Haas G., Steinitz H. (1947). Erythrean Fishes on the Mediterranean Coast of Palestine. Nature 160 (4953), 28–28.
Keskin E., Atar H. H. (2013). DNA Barcoding Commercially Important Fish Species of Turkey. Mol. Ecol. Resour. 13 (5), 788–797. doi: 10.1111/1755-0998.12120
Nelson E. M. (1955). The Morphology of the Swim Bladder and Auditory Bulla in the Holocentridae. Fieldiana Zool. 37, 121–130. doi: 10.5962/bhl.title.2938
Ounifi-Ben Amor K., Rifi M, Ghanem R., Draeif I., Zaouali J., Ben Souissi J. (2016). Update of Alien Fauna and New Records From Tunisian Marine Waters. Mediterr. Mar. Sci. 17 (1), 124–143. doi: 10.12681/mms.1371
Randall J. E. (1998). Revision of the Indo-Pacific Squirrelfishes (Beryciformes: Holocentridae: Holocentrinae) of the Genus Sargocentron, With Descriptions of Four New Species. Indo Pac Fishes 27), 105 pp.
Randall J. E., Greenfield D. W. (1999). Holocentridae: Squirrelfishes (Soldierfishes). FAO Species Identification Guide For Fishery Purposes: The Living Marine Resources of the Western Central Pacific: Bony Fishes, Part, 2. (Rome: FAO) 2225–2256.
Randall J. E., Heemstra P. C. (1984). A Review of the Squirrelfishes of the Subfamily Holocentrinae From the Western Indian Ocean and Red Sea (Ichthyological Bulletin of the J.L.B. Smith Institute of Ichthyology. No. 49), 29 pages, 2 plates.
Schmitz L., Wainwright P. C. (2011). Nocturnality Constrains Morphological and Functional Diversity in the Eyes of Reef Fishes. BMC Evol. Biol. 11 (338), 2–13. doi: 10.1186/1471-2148-11-338
Vella A., Vella N., Darmanin S. A. (2016). The First Record of the Longjaw Squirrelfish, Holocentrus Adscensionis (Osbeck 1765) (Holocentriformes: Holocentridae), in the Mediterranean Sea. Natural Eng. Sci. 1 (3), 78–85. doi: 10.28978/nesciences.286371
Ward R. D., Zemlak T. S., Innes B. H., Last P. R., Hebert P. D. (2005). DNA Barcoding Australia's Fish Species. Philosoph Trans. R. Soc B Biol Sci. 360 (1462), 1847–1857.
Keywords: Sargocentron caudimaculatum, Tunisian coasts, climate change, non-indigenous species, citizen, DNA barcoding
Citation: Ghanem R, Rjiba Bahri W, Chaffai A, Zaouali J, Hassen B, Karray S, El Bour M, Ben Souissi J and Azzurro E (2022) First Record of the Silverspot Squirrelfish Sargocentron caudimaculatum (Rüppell, 1838) in Mediterranean Waters. Front. Mar. Sci. 9:869138. doi: 10.3389/fmars.2022.869138
Received: 03 February 2022; Accepted: 08 April 2022;
Published: 09 May 2022.
Edited by:
Filipe Martinho, University of Coimbra, PortugalReviewed by:
Francesco Tiralongo, University of Catania, ItalyCopyright © 2022 Ghanem, Rjiba Bahri, Chaffai, Zaouali, Hassen, Karray, El Bour, Ben Souissi and Azzurro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jamila Ben Souissi, amJlbnNvdWlzc2lAeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.