
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 25 May 2022
Sec. Marine Megafauna
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.868464
This article is part of the Research Topic Risks, Threats, and Conservation Status of Cetaceans in the Mediterranean and Black Seas View all 15 articles
Depredation by cetaceans on fisheries is a major issue globally, both in terms of conservation and fisheries economics. The present study conducted in Cyprus, Eastern Mediterranean Sea, aimed to understand the extent, level, and type of cetacean depredation on the albacore tuna pelagic longline fishery, and in particular to quantify and evaluate the economic consequences of depredation and identify potential dolphin-longline conflict areas and mitigation practices for management. The data were obtained from fisher’s logbooks, interviews and onboard observations between June and August 2018. A novel and simple approach was applied to estimate the depredation rate and economic loss by using simple calculations including the number and weight of depredated fish, landings and fishing effort. The results revealed that there is an estimated economic loss per fishing trip of 313.07± 486.19 EUR and an estimated annual economic loss for the entire fleet of 259,272 EUR from depredation caused by cetaceans. The study also estimated that 16,639 albacore tunas were depredated in 2018 and the depredation rate ranged between 0% to 100% with a mean depredation rate of 17% per fishing trip. Depredation by the common bottlenose dolphin and striped dolphin was reported in more than 50% of their fishing trips. Other species that were found to be involved in depredation were the neon flying squid, the shortfin mako shark and the Risso’s dolphin. This is the first official record worldwide of depredation from the common bottlenose dolphin, the striped dolphin and the neon flying squid on the pelagic longline albacore tuna fishery. A total bycatch of 62 individuals of common bottlenose dolphins and one individual of stripped dolphin were reported in interviews as a result of depredation on bait and catch. The study also identified depredation hotspots and possible depredation mitigation measures. Such information could support the development of management action plans and measures to minimise interactions between cetaceans and pelagic longlines.
Fisheries interactions with cetaceans have been well documented in almost all existing fishing gears (Northridge and Hofman, 1999; Dalla Rosa and Secchi, 2007; Forney et al., 2011; Guinet et al., 2015) with different targeted species (Hamer et al., 2012) and at different geographical areas (Lauriano, 2004; Díaz López, 2006; Brotons et al., 2008; Maccarrone et al., 2014; Gonzalvo et al., 2015). These interactions are associated with negative economic and conservation consequences (Hall and Donovan, 2002; Lauriano, 2006; Zollet and Read, 2006; Brotons et al., 2008), which may lead to controversial practises like culling of cetaceans to avoid depredation (Bearzi et al., 2004). The interactions between cetaceans and fisheries, can be biological or operational. Biological (indirect) interaction refers to the competition for the same biological resource at the population level (Northridge and Hofman, 1999), whereas operational (direct) interaction refers to the physical interaction of cetaceans with fishing gear and catch by removing the bait and/or catch, usually with negative consequences like bycatch (Harwood, 1992). Depredation is a form of operational interaction, which refers to the damage or removal of captured fish or bait from fishing gear by marine predators (Gilman et al., 2006). These interactions often result in significant damage to the fishing gear and catch and to the bycatch of cetaceans with consequences that may lead to dolphins’ injury, death from drowning, and sometimes to the direct killing by angry fishers as a retaliatory measure (Zollett and Read, 2006).
Cetaceans are well known for their advanced learning abilities and the fast knowledge transfer within populations, enabling them to quickly discover new foraging grounds and opportunities (Whitehead et al., 2004). An example is their ability to develop familiarity with the sound produced by fishing vessels, including the sound produced by the engine, fishing gear and electric equipment facilitating the cetaceans to follow vessels or identify fishing grounds to take advantage of the catch (Gilman et al., 2006; Hernandez-Milian et al., 2008). Chilvers and Corkeron (2001) studied bottlenose dolphin (Tursiops aduncus) populations in Australia and found that some communities could become fully dependent on fisheries as an easily accessible feeding source. Many studies dealing with depredation and cetacean-fisheries interactions showed that this is a common strategy among cetaceans and that depredation on fishing gear is a practice that is taught within populations (Pennino et al., 2013). Consequently, the practise of depredation seems to be increasing compared to previous decades and is, therefore, more frequently reported in the literature (Hamer et al., 2012).
Interactions with cetaceans and longlines have been reported since 1952 when the global pelagic tuna longline fishing began in the Indian, Atlantic and Pacific Oceans (Sivasubramaniam, 1964). Among other fishing methods, longline is the most impacted from depredation worldwide (Northridge and Hofman, 1999; Gilman et al., 2006; Garrison, 2007; Hamer et al., 2012) with more than 31 odontocete species, six mysticete species, 15 pinniped species and two sirenian species been reported to interact with longline fisheries (Werner et al., 2015). From the fishermen perspective, depredation on longlines is known to cause significant damage on fishing gear and catch and is also related with increased fishing effort to avoid competition with cetaceans and reach quota levels and annual profits (Peterson et al., 2014; Tixier et al., 2015; Werner et al., 2015). Depredation on longlines provides an easy access to prey resource that could modify the energy balance of local ecosystem dynamics due to the changes in the natural predator-prey interactions (Northridge and Hofman, 1999; Morissette et al., 2012). Depredation could also lead to overexploitation and biased stock assessments, if losses due to depredation are not accounted for in fish stock assessments (Roche and Guinet, 2007). As most Mediterranean (Vasilakopoulos et al., 2014; Froese et al., 2018) and global (Zeller and Pauly, 2005; FAO, 2020) fish and invertebrate stocks are declining, prey availability for megafauna is also declining (Bearzi et al., 2006). This could explain, to some extent, the increased frequency of conflicts between fisheries and dolphins (Bearzi, 2002).
To the best of our knowledge, information on the interactions between cetaceans and the pelagic longline fishery has never been published in the Mediterranean Sea. In Cypriot waters, depredation by dolphins has already been reported in the literature for set-nets (Snape et al., 2018); however, in the pelagic longline fishery, though known for many years, it has not been described or quantified/estimated prior to this study. Previous personal author’s communication with the pelagic longline fishers revealed that cetaceans are the main species impacting their fishing operations and secondarily impacted by other taxa like elasmobranchs and cephalopods. Hence, the main objective of this study was to describe the interactions and impact primarily caused by cetaceans and secondarily by other megafauna on the pelagic longline fishery targeting albacore tuna (Thunnus alalunga) in Cyprus. This study, specifically aimed to 1) identify megafauna species that interact with the pelagic longline fishery, 2) evaluate and quantify the interactions between cetaceans and the albacore tuna longline fishery and 3) identify potential dolphin-longline conflict areas and mitigation practices employed by longline fishers in response to cetacean depredation.
The study took place within the Exclusive Economic Zone of the Republic of Cyprus, in the marine areas off Larnaca Bay and Paphos – Limassol (southeastern and western coasts of Cyprus, respectively to a maximum distance of 40 nautical miles from the nearest shore. The total fishing effort of the entire fleet targeting albacore tuna in 2018 was 600 days. The albacore tuna fishing period lasts approximately three months, from late May to August. The pelagic longline fleet consisted of 30 vessels of lengths between 12 and 18 meters capable of using various gears (polyvalent vessels) permitted to adjust fishing activities according to the season and the presence and movement of various species of fish. The main fishing gear used is the drifting longline and secondary gears are trammel nets, gillnets, bottom longlines, and traps. The longline fishery is active within small distances from the shore, targeting demersal and mesopelagic species using bottom longline and nets, and away from the shore and outside the territorial waters, using drifting longlines, mainly targeting swordfish and albacore tuna, and other pelagic species (Department of Fisheries and Marine Research, 2019). The swordfish fishing period is between September to December whereas demersal species are targeted between December to May. Sardine is always used as a bait for fishing albacore tuna, and mackerel and squid is always used for swordfish fishing.
The sampling scheme for data collection was fishery-dependent and the data were derived using three methodologies: self-reporting in logbooks, onboard observations, and interviews. Fishers who may participated in one or more of the three different methodologies provided different information according to aims of each method and therefore there was no effect on the data gathered.
Four professional longline fishers (out of 30 licensed vessels) were provided with logbooks for data collection during 71 fishing trips (days) that took place between June to August 2018. A fishing trip could last between one to three days maximum and each different day in the sea was counted as one fishing trip. Every day is a new setting and hauling for the pelagic longline and only one longline was set per day. For every fishing trip/day, fishers reported the fishing gear characteristics including the number of hooks set on the pelagic longline, length on longline, type of bait, soak time, the depth of fishing, bottom depth, the coordinates of the position of longline and if they used or not acoustic deterrent devices. In all cases, they were targeting albacore tuna. Information was also recorded about catch and depredation, including the caught species, the number of individuals caught, the total weight of landed fish, the number of individuals depredated, and the species involved in depredation events. Depredation was only recorded when the predators were visible and identified otherwise the data were excluded from the analysis. The length of each longline per trip was approximately 50 km with 4,000 hooks set and a soak time ranging between six to 12 hours. No acoustic deterrent devices were used during the fishing trips recorded in logbooks.
Fishers who participated in the study were all trained in self-reporting methods and on the identification of cetaceans and elasmobranchs species that are encountered and often incidentally caught on pelagic longlines through the ‘Cyprus Bycatch Project’ (Papageorgiou et al., 2020). This was done to ensure the validity of the data recorded on logbooks. In addition to the logbooks, fishers were provided with an identification guide for vulnerable species, including cetaceans, elasmobranchs, marine turtles and seabirds. Only fishers that committed to completing and returning the logbooks and showed great interest in the study during the training sessions were selected to participate in the study. Fishers were regularly visited at ports and monitored, and the logbooks were checked to ensure that were filled correctly. Fishers were also asked to provide photographic evidence, when possible, that was recorded from their cell phones as a confirmation material of depredation.
A small number of onboard observations (N = 9) were conducted as part of the ‘Cyprus Bycatch Project’ survey (Papageorgiou et al., 2020). This was used as an opportunity to train fishers on self-reporting and dolphin identification and also check the validity of the data recorded by fishers on logbooks. During this time, the observer confirmed that depredation on albacore tuna was caused by the common bottlenose dolphin and the striped dolphin as well as other species and photographic and video evidence was collected.
Interviews is a very useful qualitative data collection tool for collecting narrative data that allow investigating people’s knowledge, experiences, perspectives, and views in great depth (Kvale and Brinkmann, 2009). The value of this method is to help build the complete picture about a subject, to report detailed views of the people in the sample and to enable participants to tell their own story and express their feelings, thoughts and perspectives (Berg, 2004).
Structured interviews were conducted to 20 pelagic longline fishers (who are also the owners of vessels) in September 2020. The sample size (20 vessels) represented 63% of the entire fleet. Interviews seek to gain a better understanding of fishers’ knowledge relating to depredation and dolphin behaviour. In the beginning, fishers were introduced to the study and were ensured that all data would be anonymous. This was an important step to gain trust among fishers and to confirm that this study had no associations with regulatory and fishing authorities. Also, the fact that many fishers already knew the interviewer from previous collaborations (e.g., Giannakis et al., 2020; Papageorgiou et al., 2020) played a significant role in the clarity and consistency of their responses. Based on this pre-existing relationship and trust with the fishers, the interviewer was recommended to other fellow fishers. The snowball sampling method was used to interview the participants of the study (Goodman, 1961). This technique is used to interview people in the sample that are referred from the person being interviewed based on their knowledge and experience of the topic, in this case, dolphin depredation. This method has been previously used in numerous fisheries science studies (e.g., Braga and Schiavetti, 2013; Dmitrieva et al., 2013; Peterson and Carothers, 2013; Zappes et al., 2016). A pilot survey among four fishers was conducted to test the questionnaire prior to the study. The structure of the interview was composed of three main parts: 1) fisher’s personal information; 2) information on depredation, dolphin population size, fish stocks and identification of dolphin depredation hotspots, and 3) information on depredation mitigation measures. An open-ended interview (unstructured) was followed after the end of the structured interview where fishers’ empirical knowledge on longline-dolphins interactions was obtained as well.The interviewer (the leading author, native speaker of Greek) followed standardized interview methodologies to maximise clarity and consistency in the way the questions were asked and recorded. The interviewer appeared neutral during interviews to avoid influencing fishers’ responses and to assure fishers that there was no risk in participating in the study and therefore minimise concerns on reporting bycatch. The interviewer recorded any strange responses during interviews and assessed the reliability of the information provided for each fisher. The interviewer is an experienced onboard observer in small-scale and pelagic longline fisheries and has extensive knowledge of the fishing sector in Cyprus.
The analysis of the data was conducted in four phases. First, the rates and estimates for depredation and economic loss were calculated. Second, correlations and comparisons between parameters and variables were conducted. The third step aimed to qualitatively and quantitatively understand depredation, bycatch and mitigation practices based on fishers’ knowledge using descriptive statistics. Fourth, a non-parametric spatial analysis method was used to identify possible fisheries-dolphin conflict areas.
All the statistical analyses were performed using the R software (R Core Team, 2020). Statistical significance set at a<0.01 was used for all statistical tests to account for the increased probability of type I error multiple testing. The standard deviation (SD) for all averages is given unless specified otherwise.
Only fish that were landed and sold to retailers were included in the Landings Per Unit of Effort (LPUE), and only the fish that were depredated and discarded were included in the Depredated Per Unit of Effort (DPUE).
The Landings Per Unit of Effort (LPUE) was calculated as:
The Depredated Per Unit of Effort (DPUE) was calculated as:
Then, Catch Per Unit of Effort (CPUE) was calculated as:
whereas other discards refer to any other species that were caught and discarded. No other discards were recorded in the current study except the depredated tunas.
To estimate the economic loss and depredation rate, the calculations from (FAO 2019a; 2019b) manuals that were developed to estimated bycatch and discard rates and were modified and used accordingly for the purpose of this study. To the best of our knowledge, this is the first time such an approach was used to estimate the depredation rate and economic loss.
The percentage depredation rate (D%) was calculated for each fishing trip and it was defined as the percentage of the total depredated fish per fishing trip. The following equation was used:
The depredation rate (R) estimates the rate of depredation on the targeted species based on the number of sampled fishing trips. Knowing the number of depredated fish and the number of sampled fishing trips (71), it was possible to calculate the depredation rate for each depredated species using the following equation:
Then, the estimated annual depredation rate (EDR) of each species was calculated as follows:
The second step of the calculations concerns the estimation of the economic loss (EL) per fishing trip, which is essential to evaluate the impact of depredation on the catch. For the calculations of the economic loss, only the damaged catch was considered and not the damage on bait or fishing gear. The economic loss was calculated for each fishing trip by knowing the total average weight of individuals depredated, which was obtained by multiplying the average weight of tunas landed per fishing trip with the total number of individuals depredated of the specific fishing trip. The average weight of tunas landed was estimated by calculating the average weight of all individuals which landed from each specific fishing trip recorded in logbooks. Following up, the total average weight of individuals depredated was multiplied by the price per kilo of each species as of 2018. The average price sold from the fisher to the retailer was 2.30 EUR per kilo. In 2018, the price was stable with only 0.10EUR variation within the three months. The equation to estimate the economic loss per fishing trip is as follows:
Then, the economic loss rate (ER) was calculated as follows:
Therefore, the annual economic loss rate (EER) was calculated as:
A Shapiro-Wilk’s normality test, frequency density and q-q plots were used to test for normality prior to any other statistical analysis tests. The Mann-Witney test was used to compare the LPUE at the presence and absence of dolphins and also to compare the fishing area with D%, DPUE and LPUE. To compare means of LPUE, DPUE, CPUE, D% and EL with the month of fishing, the non-parametric Kruskal-Wallis test was conducted.
Descriptive statistics were conducted to show the results of the interviews including information on fish stocks, interactions, depredation, bycatch and mitigation practices. A Spearman rank correlation test was conducted to explore the relationship between the total bycatch of dolphins and years in the profession as well as between the fishing effort (days at sea) and the total number of days with depredation in 2019.
Based on fishers’ knowledge, a heat map was created to show dolphin depredation hotspots based on the number of times specific areas have been reported with dolphin depredation. For the heat-map generation, the Kernel Density Estimation (KDE) has been used, as it is one of the most classic spatial statistical algorithms to capture spatial point patterns that obeys Tobler’s First Law of Geography by introducing kernel function and attenuation effect (Yuan et al., 2019). The algorithm behind the tool fits smoothly curved surface over each point. The surface value is highest at the point location and diminishes as the distance from the point increases. It becomes zero at the search radius (bandwidth) distance from the point. Bandwidth selection is a critical step while applying KDE-based heat maps (Lampe and Hauser, 2011; Li et al., 2014). The bandwidths can either be invariant (fixed KDE) or spatially variant (adaptive KDE) across sample points. In order to be able to tell where any clusters in our data exist, several kernel bandwidths have been examined and analyzed, choosing as more suitable for our case and for visualization purposes, the search radius of 10km (based on the linear unit of the projection of the output spatial reference). The output cell size has been set to 20x20m and the output density value (is dolphins count divided by area) on the map has been set to HIGH and LOW. The analysis was performed using ArcGIS™ (Esri Inc, 2013).
Four different species were identified depredating on the albacore tuna from the board observations by an onboard observer (leading author). These were: the common bottlenose dolphin (Tursiops truncatus), the striped dolphin (Stenella coeruleoalba), the neon flying squid (Ommastrephes bartrami) and the shortfin mako shark (Isurus oxyrinchus). There are distinctive differences of depredation between the different species that can be identified from the photos taken onboard (Figure 1). Fishers were able to identify if depredation was caused by dolphins or the other predators by the damage caused on the catch, even without the visual observation of predators. Confirmation of the species producing the damage to the catch comes from direct observation or bycatch of the depredator (Figure 2).
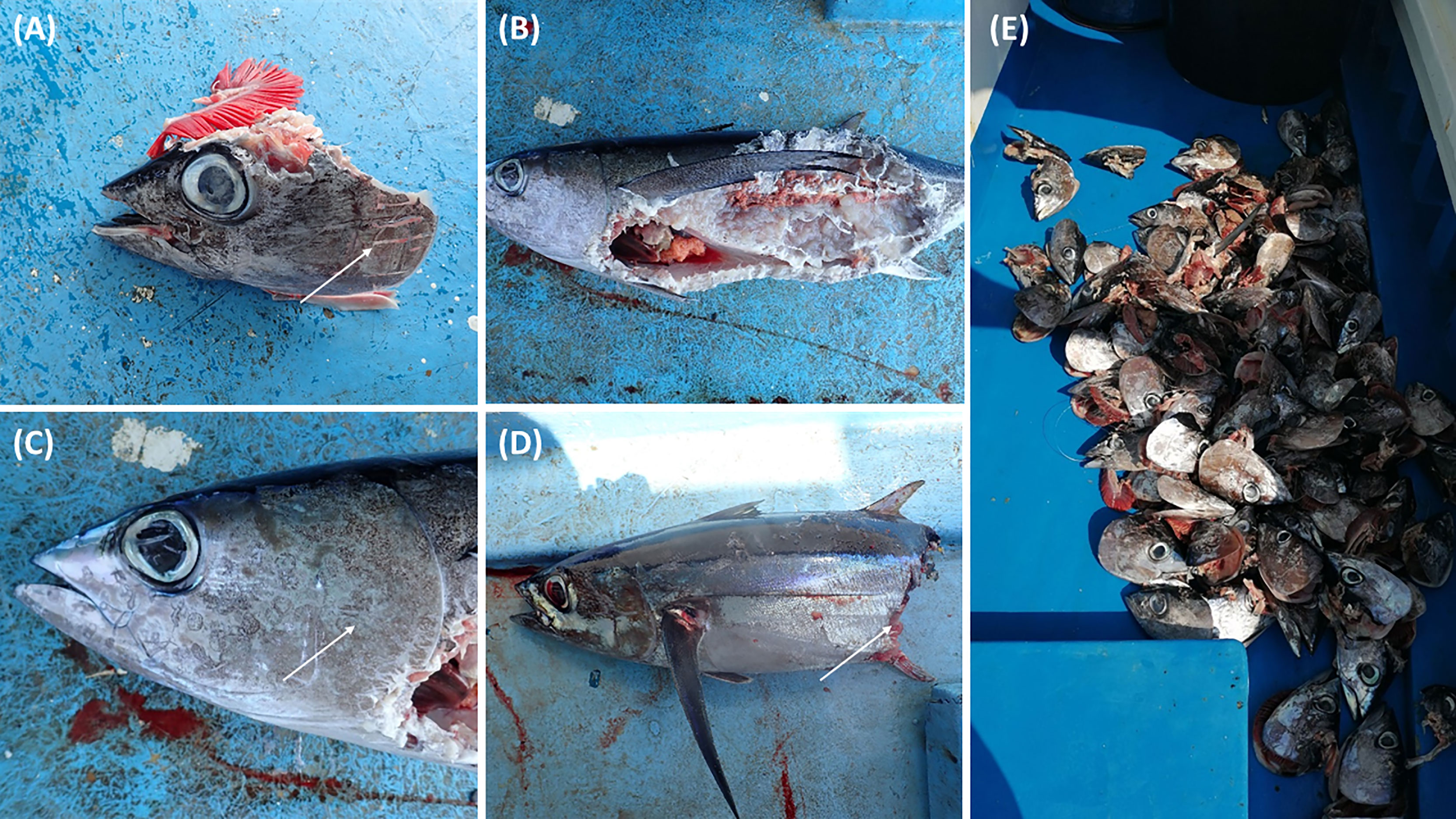
Figure 1 Examples of depredated albacore tuna (Thunnus alalunga) by common bottlenose dolphin (Tursiops truncatus) (A; arrow shows marks odontocete tooth lacerations on head), as opposed to neon flying squid (Ommastrephes bartrami) (B, C; arrow shows marks of the squid suckers) and shortfin mako shark (Isurus oxyrinchus) (D; arrow indicates the sharp cut) during pelagic longline fishing in July 2018. Depredated tunas (>200 heads) by the common bottlenose dolphin on a single fishing trip in July 2018 (E).

Figure 2 A neon flying squid that was incidentally caught on the pelagic longline fishery in May 2019 when attempting to depredate the albacore tuna.
Depredation by cetaceans is most often caused by the common bottlenose dolphin and less often by the striped dolphin. However, there are cases where the two species are found in the same pod and simultaneously depredate on catch or bait. This observation has been confirmed by the onboard observer and by the fishers when asked to explain which cetacean species are involved in depredation. For this reason, depredation recorded in the logbooks that was caused by cetaceans was not differentiated between the two species to avoid misidentification, misinformation and bias on results. Hereafter, cetacean depredation refers to the depredation that was caused by the common bottlenose dolphin or the striped dolphin or both.
The total fishing effort in June, July and August was 15, 39 and 44 days, respectively. The total number of days with depredation was 10 in June and July, and 5 in August. According to the fishing effort of each month, dolphin depredation was higher in June followed by July and August (Figure 3). The mean LPUE was compared in the presence and absence of depredation, and the results were 89.81 ± 92.65 and 117.71 ± 121.82, respectively. Results from the Mann-Whitney test revealed no significant difference (W = 1002.5, p-value = 0.47) between the mean LPUE at presence and absence of dolphins. Days with depredation were classified as days with the presence of dolphins whereas days with no depredation were classified as days with the absence of dolphins. The total DPUE, LPUE and CPUE were 3334.85, 10838.2 and 14173, respectively. This indicates that 23.53% of the total catch (CPUE) was depredated (DPUE).
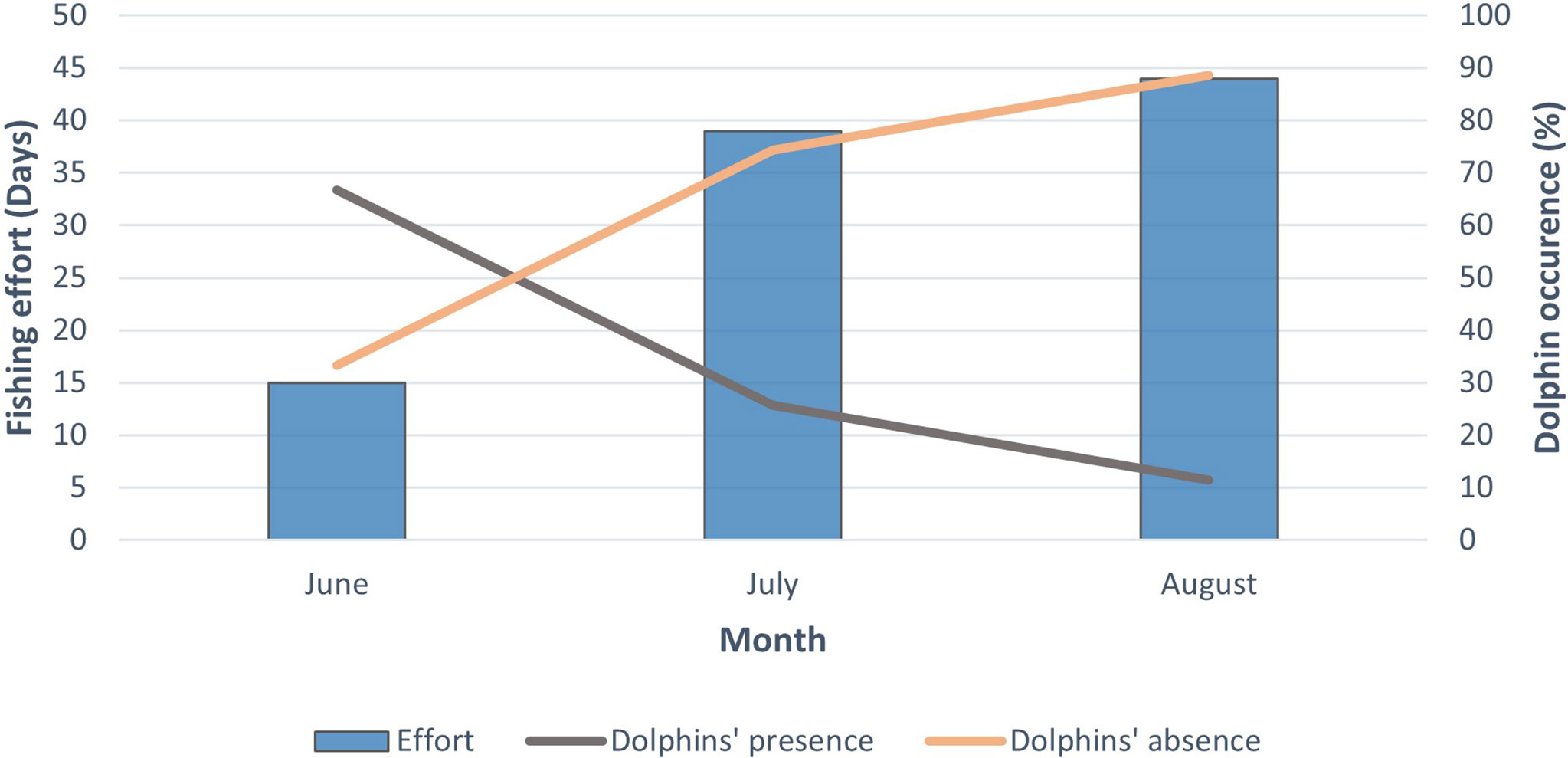
Figure 3 Relationship between fishing effort (days) and days with and without dolphin depredation (%) for each month.
Cetacean depredation on albacore tuna occurred between June – August 2018 with a higher number of depredated fish during June and July 2018, where the catchability and presence of albacore tuna are higher. Depredated albacore tunas ranged from 0 to 120 individuals with a mean of 28.04 ± 33.7 per fishing trip (N=71). The D% varied from 0% to 100% with a mean of 16.9 ± 23.01%. The estimated R was 27.8 and the estimated EDR was 16,639, meaning that each year this amount of albacore tunas is estimated to be depredated in the entire fleet. The EL ranged from 0 to 1,800.00 EUR with a total EL of 30,680.60 EUR and a mean of 313.07 ± 486.19 EUR per fishing trip. The estimated ER was 423.12 EUR and the estimated EER was 259,272.68 EUR for the entire fleet in 2018. The Kruskal-Wallis test revealed no significant differences between the month of fishing with D% (χ2 = 50.66, df = 47, df = 47, p-value = 0.33) and EL (χ2 = 50.66, df = 47, p-value = 0.33) (Figure 4).
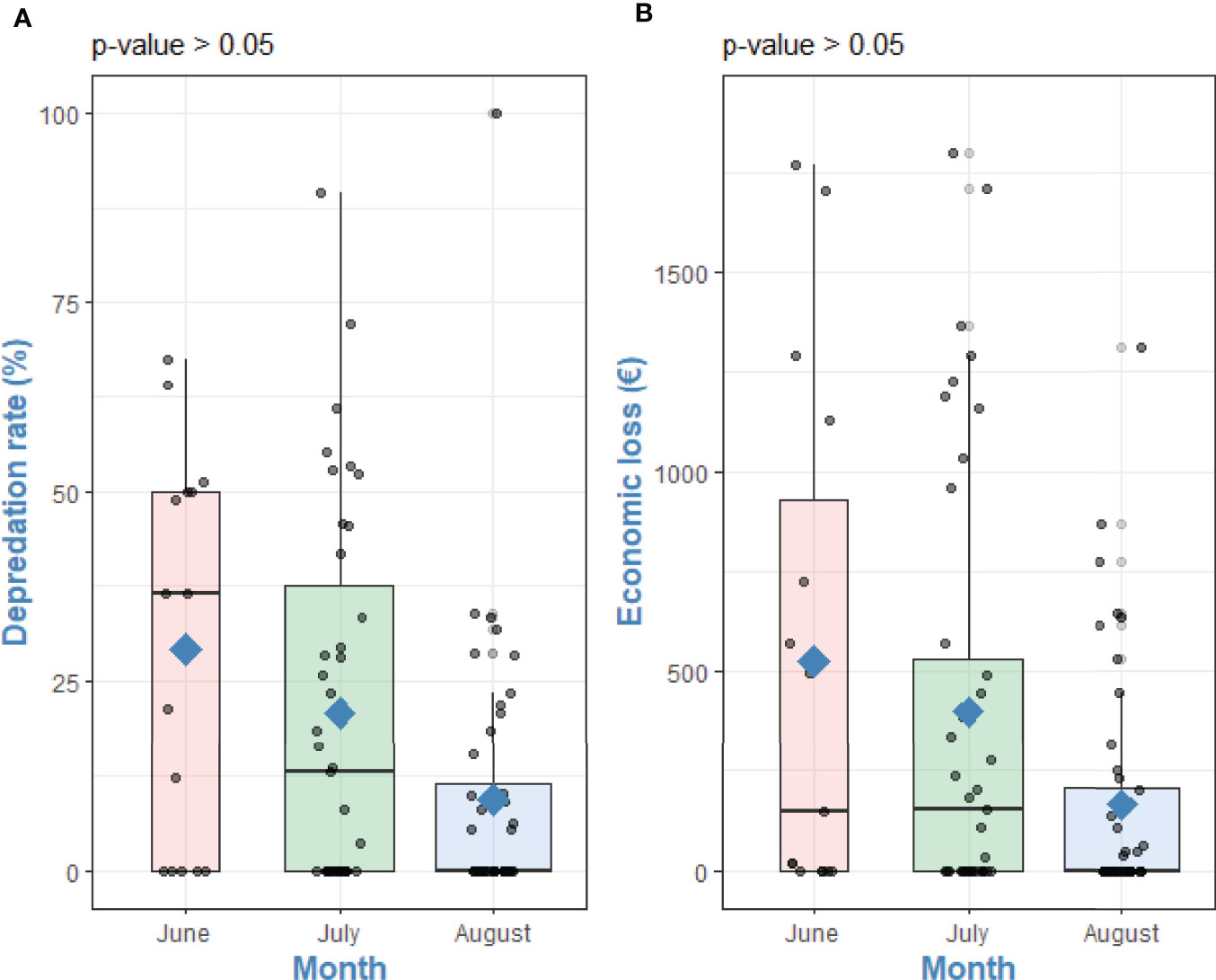
Figure 4 Differences between (A) Depredation rate (%) and (B) Economic loss (€) and with month of fishing. Kruskal-Wallis tests p-values. Points are raw survey data. Rhombuses represent the mean of each group.
The mean D% in June, July and August was 29.2 ± 25.6, 20.8 ± 24.7 and 9.3 ± 17.8, respectively. The mean EL in June, July and August was 524.6 ± 653.5, 398.7 ± 542.3 and 165.0 ± 299.3, respectively. The mean D% of the three different months of the albacore tuna fishing period in relation to the mean ER is shown in Figure 5.
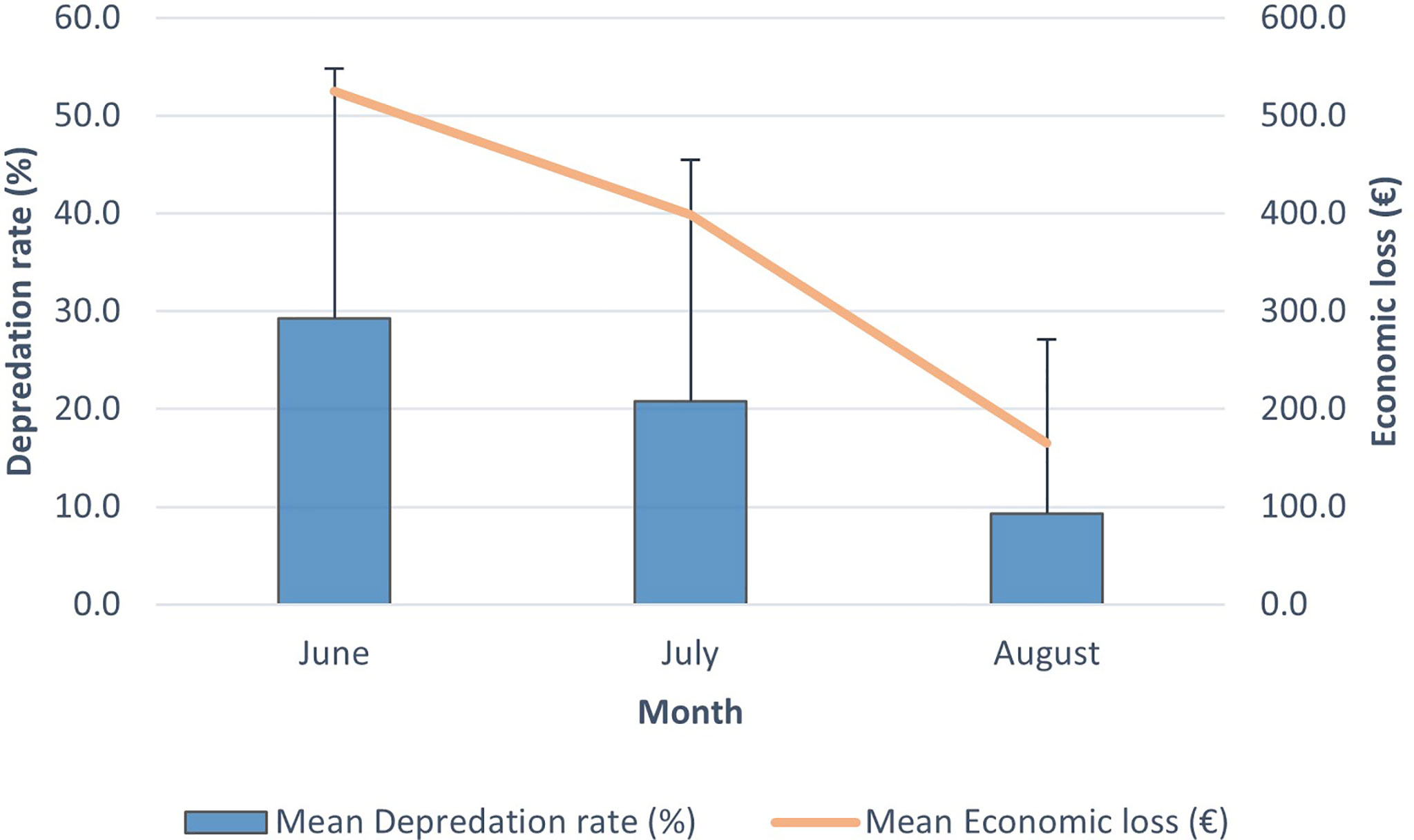
Figure 5 Mean depredation rate (%) and mean economic loss (€) for each of the three fishing months in 2018. Error bars indicate standard deviation.
No significant trend in DPUE, LPUE and CPUE was detected between the months of fishing, range and mean values are shown in Table 1. The Kruskal-Wallis test showed no significant differences between month of fishing with DPUE (χ2 = 50.66, df = 47, p-value = 0.33), LPUE (χ2 = 78.39, df = 70, p-value = 0.23) and CPUE (χ2 = 86.56, df =80, p-value = 0.29) (Figure 6).

Table 1 Range and monthly means (± SD) of Landings Per Unit of Effort (LPUE), Depredation Per Unit of Effort (DPUE), and Catch Per Unit of Effort (CPUE) during the 3-month survey in 2018.
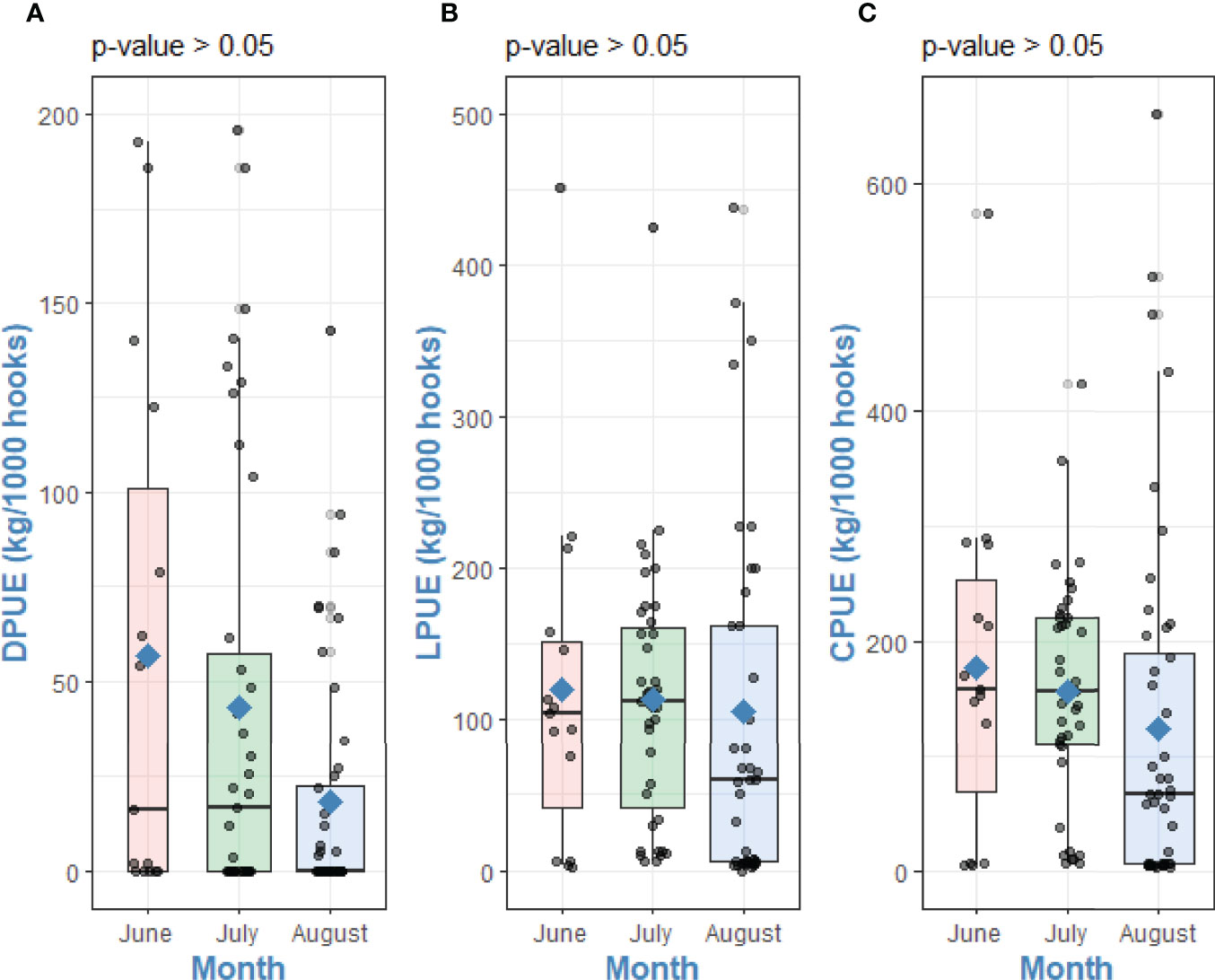
Figure 6 Differences between (A) DPUE, (B) LPUE and (C) CPUE with month of fishing. Kruskal-Wallis tests p-values. Points are raw survey data. Rhombuses represent the mean of each group.
Fishing grounds were situated in two broad geographical areas and grouped as Larnaca and Paphos-Limassol. In total, 36 and 35 fishing trips were conducted at Larnaca and Paphos-Limassol, respectively. The days with depredation and the D% were slightly higher in Larnaca than in Paphos-Limassol area. Days with depredation were 24 (67% out of total fishing trips) and 22 (63%) at Larnaca and Paphos-Limassol, respectively. At Larnaca and Paphos-Limassol the mean D% was 26.9 ± 24.8 and 15.2 ± 22.4, the mean DPUE was 62.3 ± 64.8 and 30.8 ± 43.5, and the mean LPUE was 153 ± 122.1 and 148.6 ± 101.6, respectively. The results from the Mann-Whitney test revealed a significant difference between D% and fishing area (W = 794, p = 0.05) but no significant difference between fishing area and DPUE (W = 785, p-value = 0.07) and LPUE (W = 622, p-value = 0.93). The fishing trips with information regarding depredation events and D% are shown in Figure 7; DPUE and LPUE per fishing trip are shown in Figure 8.
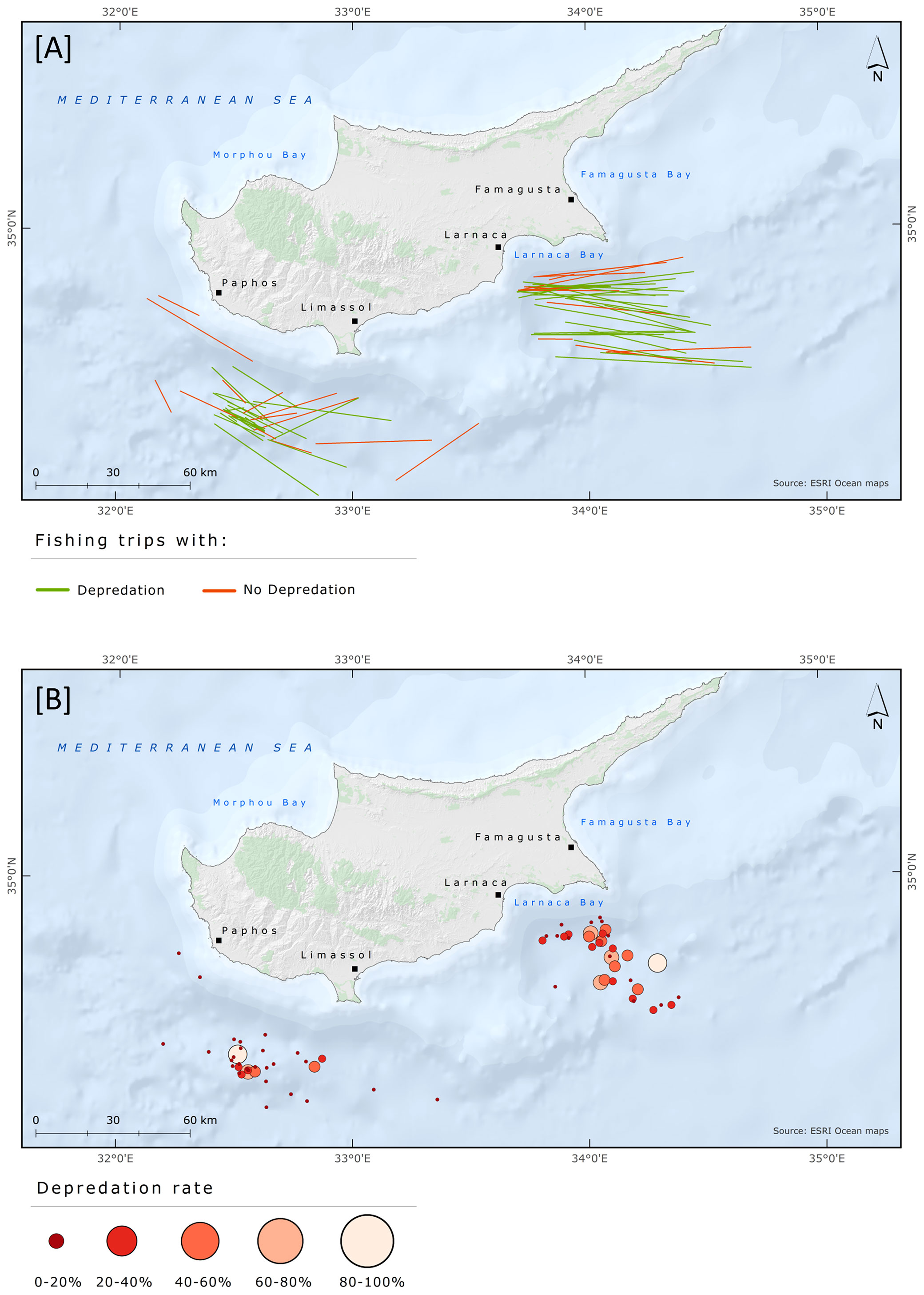
Figure 7 Spatial distribution of pelagic longline fishing trips targeting albacore tuna between June and August 2018 with information regarding (A) depredation events and (B) Depredation rate (%).
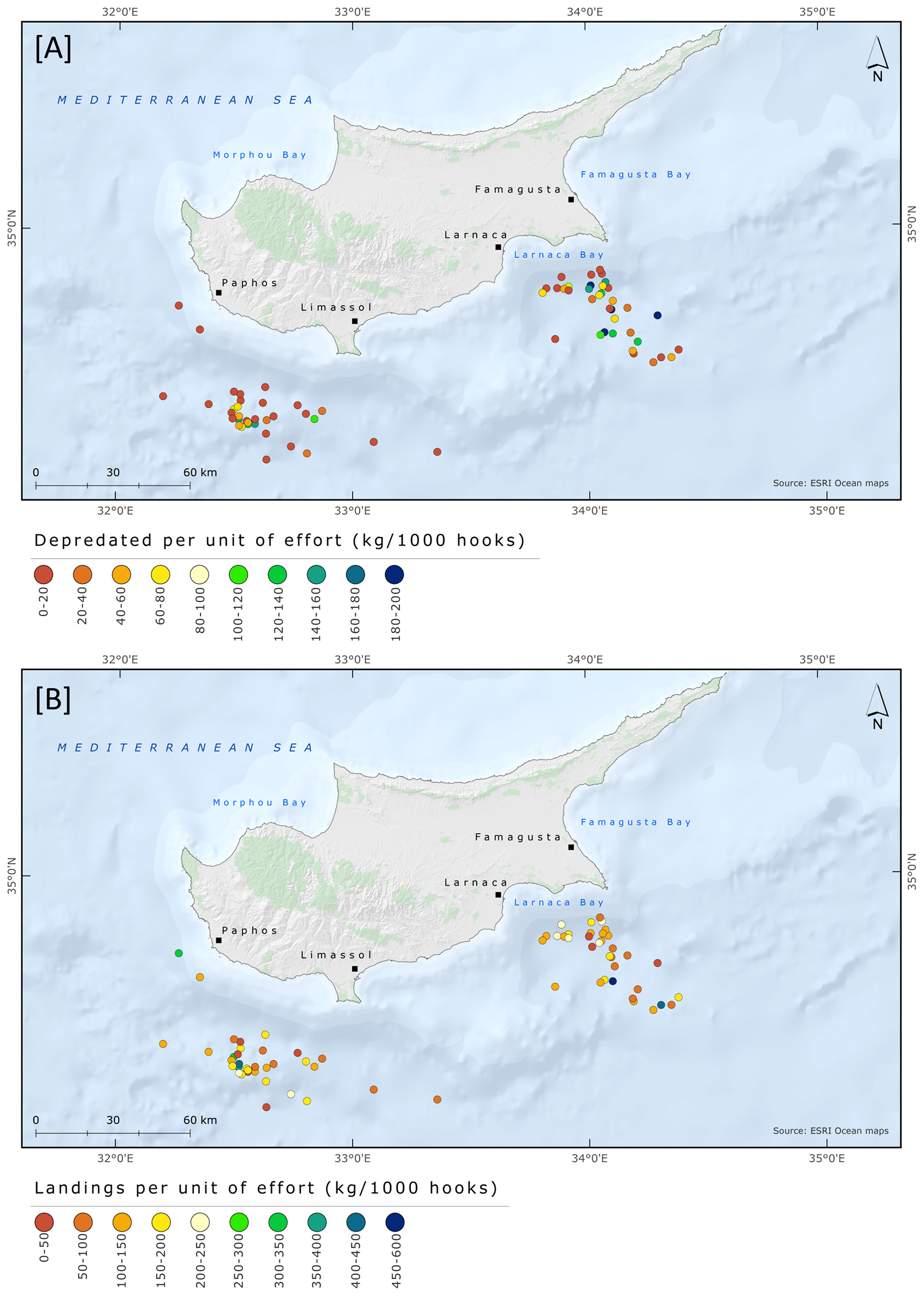
Figure 8 Spatial distribution of pelagic longline fishing trips targeting albacore tuna between June and August 2018 with information regarding (A) Discards Per Unit of Effort and (B) Landings Per Unit of Effort.
The mean age of pelagic longline fishers interviewed was 51 ± 8.2 years old with 19 ± 12.6 years in the profession. Without any exception, all the fishers interviewed reported that they had experienced depredation of their catch at some point in their career. The most common species reported in the interviews to depredate the catch was the common bottlenose dolphin (100%) followed by the striped dolphin (85%), the neon flying squid (80%), the shortfin mako shark (75%) and the Risso’s dolphin (35%) (Figure 9).
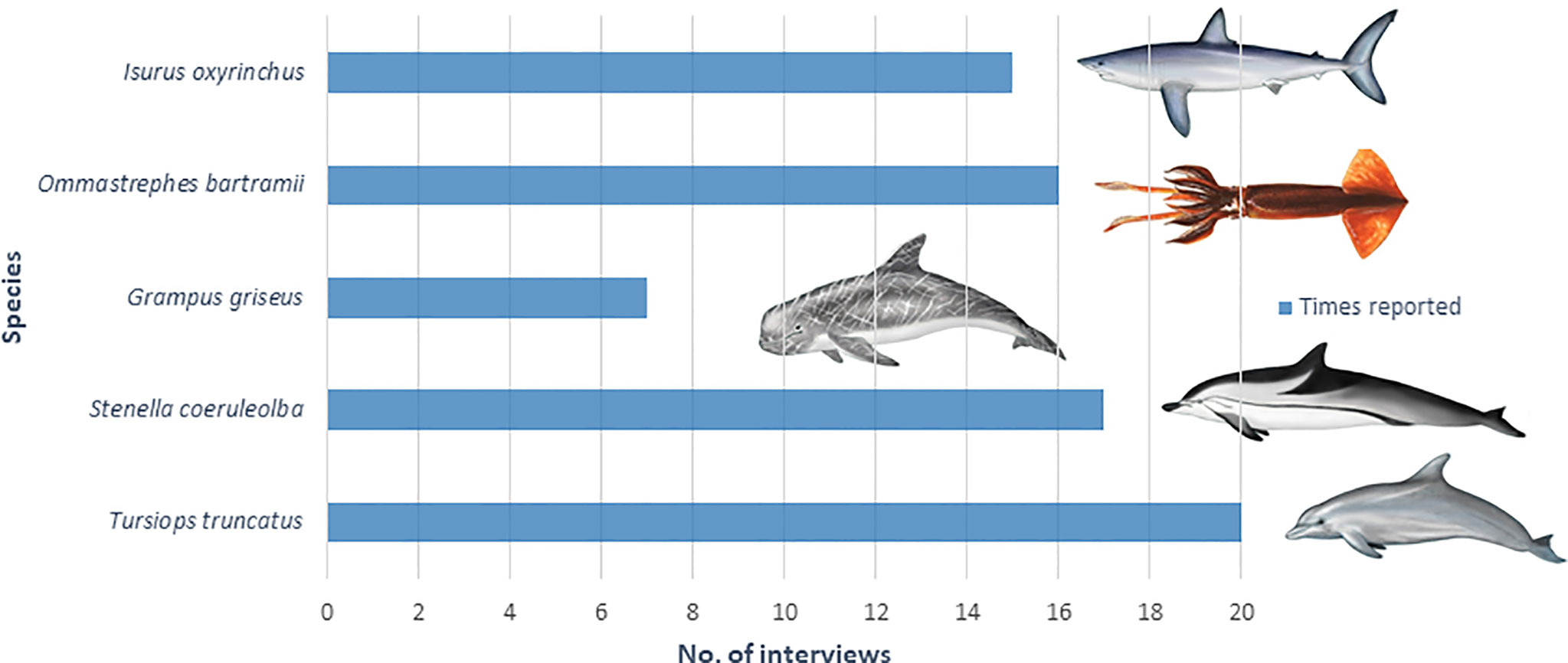
Figure 9 Number of times in which each species was reported to depredate the catch during the interviews (N = 20).
All of the respondents (100%) reported that the most common species depredating the catch is T. truncatus and the species mostly depredated is T. alalunga. All respondents reported that the interactions between dolphins and fisheries have increased in the past 10 years. These interactions mostly occur between May and August during the tuna fishing period (100% of responses). Ninety per cent of the respondents reported that depredation events with dolphins happen very often during their fishing trips targeting albacore tuna whilst only ten per cent reported that depredation happens often. Sixty per cent of the respondents reported that over the past 10 years the tuna stocks have decreased while 40% said that they remained the same. Respondents reported that over the past ten years the swordfish stocks have decreased (70% of respondents), remained the same (20% of respondents) and ‘didn’t know’ (10% of respondents). All respondents reported that all dolphins cause significant damage to the gear when depredation events occur and that depredation causes serious economic losses on their business.
Results from the Spearman rank correlation coefficients test revealed a quasi-significant positive relationship (rho = 0.53, p-value = 0.017) between dolphin bycatch and years in professional fishing. Out of the total 20 interviews conducted, 15 fishers (75%) reported bycatch of the common bottlenose dolphin, five (25%) reported no dolphin bycatch and one (5%) reported bycatch of the striped dolphin. The mean fishing effort of responders in 2019 was 74 ± 34.4 days and the mean number of days that had experienced depredation in 2019 was 37 ± 16, essentially 50% of their fishing trips. Results from the Spearman rank correlation coefficients test revealed that there is a significant positive relationship (rho = 0.69, p-value < 0.001) between the fishing effort (days at sea) and the total number of days with depredation in 2019. Fishers reported a total of 62 individuals of T. truncatus and one individual of S. coerueoalba bycaught throughout their fishing careers. Five fishers reported no bycatch of dolphins and one fisher reported the bycatch of about 20 individuals of T. truncatus throughout his career. A juvenile common bottlenose dolphin was incidentally caught on the pelagic longline during the albacore tuna fishing period in June 2019. The incident was video recorded and can be found in the Supplementary Video (S1). The video clearly shows other two dolphins (perhaps family members) that did not leave the area until the juvenile dolphin was released. The fisher reported that the incident happened during the attempt of the juvenile dolphin to depredate the catch and he also reported high dolphin depredation on that day. Recently, a juvenile S. coerueoalba was bycaught on pelagic longlines targeting swordfish in September 2021 during the attempt to depredate the bait and drowned (Figure 10).

Figure 10 Bycatch of a juvenile Stenella coerueoalba on pelagic longlines targeting swordfish in September 2021. Photograph taken onboard by the vessel’s captain.
Based on the information provided by fishers about areas where dolphins (T. truncatus and S. coeruleoabla) were most commonly encountered, a heat map was created to show the distribution of dolphin depredation hotspots based on the number of records in each area (Figure 11). The areas of Larnaca (Southeast) and Protaras (East) were found to have the highest probability of dolphin occurrence followed by the areas in the Northwest (Chrysochou Bay). There were also records of dolphin depredation in the areas of Akamas, Pegeia, Paphos, Pissouri, north of Morphou Bay and east of Apostolos Andreas.
All fishers interviewed (N=20) were aware of dolphin anti-depredation devices (pingers) and all of them were willing to explore and test possible mitigation measures. Fishers were then asked to report if they take any depredation mitigation measures. Fifteen fishers (75%) reported that they were not taking any measures to avoid dolphin depredation and only five (25%) reported they were using dolphin anti-depredation devices (Table 2). Another depredation measure reported which is a common practice among longline fishers was the avoidance of areas with high cetacean abundance and fishers moving to other fishing grounds.
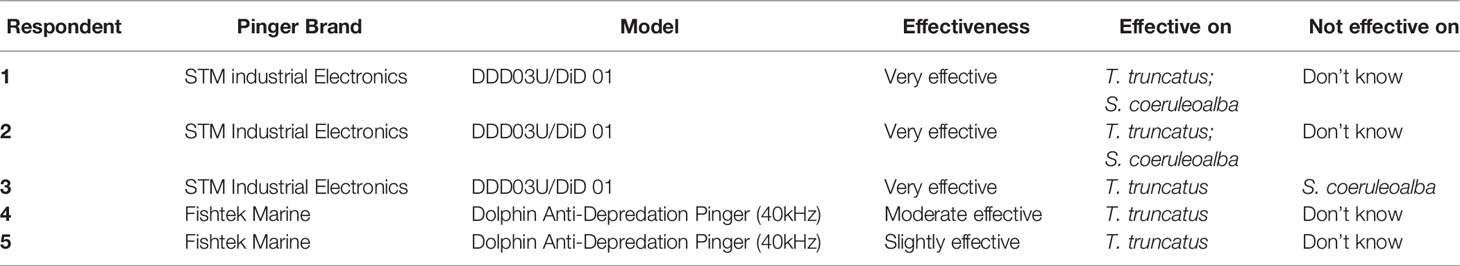
Table 2 Depredation mitigation measure (dolphin anti-depredation pinger) used by longline fishers and their effectiveness on mitigating dolphin interactions.
There is a common belief among fishers that since the beginning of the swordfish longline fishing in Cyprus in 1973, depredation levels have gradually increased over the years. They accept as true that the practice of depredation is taught and passed on to the new generations of dolphins. Fishers reported that in just two years after the beginning of the albacore tuna fishing in Cyprus in 2004, dolphins identified their fishing grounds and learned to depredate the catch. Six fishers reported that only in the last four years (since 2018) dolphins have learned to depredate the bait, whereas in the previous years’ depredation was only happening on the catch.
All fishers who participated in the interviews and have reported cetacean bycatch have also reported that in all cases the individuals bycaught were juveniles and, in most cases, they were drowned, indicating a high chance of mortality when bycaught. Some fishers have also reported that the pod did not leave the area until the bycaught dolphin was disentangled and released back to the sea. During these events, fishers have noticed sounds described as mourning coming from dolphins from the pod. One fisher has reported a rare event where other dolphins from the pod were observed carrying the dead young dolphin on their backs outside the water, presumably to allow it to breathe. In most cases of a dead dolphin, fishers reported a “vengeful” behaviour by the pod, where dolphins frantically removed caught fish from longlines and caused substantial damage to the gear.
Another common observation by fishers was that when the pod had consumed enough tunas caught on the longline, the pod displayed a playful behaviour, often swimming and “porpoising” around the boat and in some cases playing with the depredated tunas by throwing them outside the water and up in the air. In other reports, dolphin pods follow the boat during setting to depredate on the bait (sardine) and during hauling to depredate on the catch. Four fishers alleged that depredation events are more frequent during the full moon.
Two fishers have reported rare depredation events by the species G. griseus, mentioning that the species is very shy, not “showing off” and that its behaviour is not aggressive like T. truncatus and S. coeruleoalba. Fishers also mentioned that the latter has a more aggressive behaviour than the T. truncatus and even if depredation events are less frequent with this species, the damage on the catch and gear is higher when it happens.
By examining the results of the self-reporting, the onboard observations and the interviews, the study confirmed for the first time the depredation by the common bottlenose dolphin, the striped dolphin and the neon flying squid on the albacore tuna pelagic longline fishery in Cyprus. To the best of our knowledge, this is also the first time that the two cetacean species and the cephalopod species have been reported to depredate on pelagic longlines worldwide. The study shows that depredation has been responsible for causing serious socioeconomic and ecological problems, often leading to catch and gear loss, cetacean bycatch and in some extreme cases, death. Most worrying is the rise on the frequency of depredation events and the increased negative impacts on both, fishers, and dolphins. The very few available estimates on depredation rates and economic loss found in the literature, followed different approaches, thus precluding any possible direct comparison with the current study.
The findings of this study have shown that the species that are most frequently involve in depredation and cause the highest impact on fishing operations are the common bottlenose dolphin and the striped dolphin. The common bottlenose dolphin is the most frequently reported species to interact with small-scale fishing activities in the Mediterranean Sea (Lauriano, 2004; Díaz López, 2006; Brotons et al., 2008; Rocklin et al., 2009; Blasi et al., 2015; Pennino et al., 2015; Snape et al., 2018; Pardalou and Tsikliras, 2020), probably due to its opportunistic feeding habits and wide spatial distribution (Barros and Odell, 1990). This species has a flexible and cosmopolitan diet (Shane et al., 1986; Barros and Odell, 1990; Connor et al., 2000) and its distribution is often related to prey distribution, which in many cases overlaps with the distribution of species targeted by a fishery (Barros et al., 2000), and thus, inevitably leading to conflicts between dolphins and fisheries. There are several reports worldwide of the common bottlenose dolphin interacting with fishing gear involving hook and line (Werner et al., 2015). Interactions between the striped dolphin Stenella coeruleoalba and fisheries have been also documented in the Mediterranean with small-scale and purse seine fisheries (Magnaghi and Podestà, 1987; Di Natale and Notarbartolo di Sciara, 1994; Bearzi, 2002; Northridge et al., 2013; Crosti et al., 2017; Bruno et al., 2021).
The results revealed that there is an estimated mean economic loss per day of about 313 EUR and an estimated annual economic loss for the entire fleet of about 260,000 EUR from depredation. In other regions, the economic loss by cetacean depredation on set demersal longlines targeting halibut (Reinhardtius hippoglossoides), arrowtooth flounder (Atheresthes stomias) and Patagonian toothfish (Dissostichus eleginoides) has been estimated to range between 1,034 USD to 8,449 USD per day (Roche and Guinet, 2007; Tixier et al., 2010; Hamer et al., 2012 and references therein), which shows substantially higher economic loss per day from the findings of the current study. Regarding the percentage of depredation rate (D%), we found that it ranges from 0 to 100 (mean D% = 17%), which is similar to the depredation rate (17.7%) found by Tixier et al. (2010) regarding the longline depredation on Patagonian toothfish, while other studies which included depredation on species of tuna, provided a broad range of depredation rates (0.5-100%) (Secchi and Vaske, 1998; Purves et al., 2004; Williams et al., 2009; Hamer et al., 2012) It has to be addressed that, the actual cost and rate of depredation from the current study, may be underestimated, as fish completely removed from the hooks were not possible to quantify. The rate of depredation may be affected either by the number of fish available or by the size of the group or from both factors. Both, D% and LPUE decrease from June to August, whilst the fishing effort increases. This can be explained by the albacore tuna migration at the end of the fishing season in August which corresponds to less catch for fishers and LPUE, and subsequently less available depredation resources for dolphins and D%.
Currently, pelagic longline fishers in Cyprus are being compensated annually by the government 300 EUR per fisher, for the damage caused on their fishing gear due to dolphin depredation (Ministry of Agriculture, Rural Development and the Environment, Department of Fisheries and Marine Research, 2020). This is considered by the fishers to be very low compared to the actual damage they are experiencing. Our findings support their arguments since we estimated the mean economic loss, during the fishing seasons, to be 313EUR daily per fisher. Personal author’s communication with fishers, unveiled that many fishers expressed aggression towards dolphins, often considering harming them, especially during fishing trips on days with high depredation. However, this was not always the case as a minority of fishers consider themselves as “outsiders”, meaning that they did not naturally belong in the dolphin’s environment and that they should respect and protect them.
The photographic evidence collected from onboard observations confirmed the depredation by the common bottlenose dolphin and striped dolphin, the shortfin mako shark and the neon flying squid on albacore tuna. Depredation by the cetacean species was distinguishable from a shark- and squid-damaged fish. Odontocetes removed the entire torso and left only the head of the fish up to the gills and in some cases up to the jaw. In other cases, dolphins tore the body of the fish, leaving bites with ragged borders and with the head of the fish left on the hook. Also, in all cases, marks from their pencil-like teeth were visible on the wounded flesh (Figure 1A). Apart from that, depredation from dolphins is in almost all cases verified by the presence of dolphins close to the vessel.
The depredation caused by squid was distinguished from dolphins and sharks, by the small beak bites throughout the torso of the caught fish. In most cases, squids attack the belly and middle body of the caught fish, possibly aiming for the highly nutritious eggs (Figure 1B). Another distinguishable feature was the visible marks from the squid suckers on the body and head of the caught fish (Figure 1C). The squid was also reported by fishers to depredate on the bait and often get caught on the hook. Apart from the photographic evidence showing an individual neon flying squid that was bycaught during depredation (Figure 2), the fishers who participated in the interviews were asked to confirm the species by showing them pictures of the Mediterranean squids and they all identified it as O. bartrami. Fishers also reported catches of the neon flying squid of individuals weighing between 15 - 20 kg. Taking this information into consideration as well as the specimen photographed in Figure 2, no other known squid species in the Mediterranean can reach this body weight.
Shark depredation was distinguishable by the clean cuts, and bite-shaped portionsof flesh from the torso of the caught fish, leaving the surrounding body of the fish undamaged (Figure 1D). The species of the shark was identified by the observer since the depredation event happened exactly at the time of towing the longline. The shark arrived at the surface to depredate on the caught fish while towing the longline and dove back in the water. Similar results on the identification of odontocetes and shark depredation on the depredated catch with almost identical marks have been reported in the literature from other parts of the world (Dalla Rosa and Secchi, 2007; Gilman et al., 2007; Hamer et al., 2012).
Pelagic shark and squid species have been also reported in the literature to depredate on the catch on different fisheries and fishing gears. Shark depredation on the longline fishery targeting mainly different tuna species, swordfish and toothfish, has been previously documented (Gilman et al., 2008; Mandelman et al., 2008; Hamer et al., 2012; Rabearisoa et al., 2018; Tixier et al., 2021) however, such information was none existed in the Mediterranean Sea until very recently. Malara et al. (2021) reported for the first time shark depredation in the Mediterranean Sea on the swordfish harpoon fishery. The shark species reported in the study that potentially depredated on swordfish were the blue shark (Prionace glauca), the shortfin mako (I. oxyrinchus) and great white shark (Carcharodon carcharias) (Malara et al., 2021). The blue shark, the shortfin mako and the bigeye thresher (Alopias superciliosus) are the most commonly bycaught shark species in the Cypriot pelagic longline fishery (Department of Fisheries and Marine Research 2016 - 2021). The very limited published information on squid depredation mentioned the depredation by the colossal squid (Mesonychoteuthis hamiltoni) on longlines targeting the Antarctic toothfish in the Southern Ocean (Remeslo et al., 2015) and by the purpleback flying squid (Sthenoteuthis oualaniensis) on gillnets targeting tuna in the Northern Arabian Sea (Moazzam, 2019). However, squid depredation has never been reported before in the Mediterranean fisheries.
The results from the interviews showed that the species causing depredation on the albacore tuna were the T. truncatus, followed by S. coeruleoalba, O. bartrami, I. oxyrinchus and G. griseus. The results from the interviews confirmed the findings from the logbooks and onboard observations. The interviews also revealed another species that is involved in depredation for which little is known about as it appears scarcer in the Levantine basin, the Risso’s dolphin (Boisseau et al., 2017). Even the though the depredation by this species is considered less frequent and rare as described by some fishers, there are a few known areas that this species is known to inhabit of which the fishers of the area know and try avoiding fishing there. The depredation by the neon flying squid is becoming a great concern for longline fishers as this species depredates on bait and on the albacore tuna as well as on swordfish (Xiphias gladius). However, the species is edible, not prohibited to catch and sell, and according to fishers, taste-wise it is highly valued. This could be an opportunity for fishers to target the species in the future and support their income, in case of available market niche.
The results also showed the high probability of dolphin bycatch during depredation on both bait and catch. Fishers reported that in most cases the bycaught dolphins were juveniles that often drowned. The entanglement and hooking of cetaceans on longlines have been previously reported as an occasional event often leading to serious injuries and mortality (e.g. Forney and Kobayashi, 2007; Garrison, 2007). Even if the fishers cut the branchline to release the bycaught dolphin, it is very likely that the hook and the line will be ingested, injure vital organs and hence, the animal will die as a result (Wells et al., 2008). Considering that fishers have reported a decrease in tuna and swordfish stocks and significant damage on gear, catch and bait from depredation, it is very likely that they resort to extreme measures, using harmful methods to prevent depredation and sustain their operational costs. Practices to prevent depredation, such as direct shooting or using explosives have been reported previously in the literature (e.g. Northridge and Hofman, 1999; Gilman et al., 2006; Zollett and Read, 2006). This is a major concern for cetacean conservation because mortality and injury resulting from those actions may have significant consequences on a population level, especially on small, isolated populations associated, with islands such as Cyprus. However, there are no official records of cetacean harassment in Cyprus.
Although both dolphin species are responsible for depredation, it is unclear from the data which species has the greater impact. Fishers participated in the study reported that depredation by the common bottlenose dolphin is more frequent than the striped dolphin, however, they mentioned that in many cases have observed both species in the same group. Therefore, it is particularly important to understand the precise depredation rate by each species as well as their population dynamics. This will require intense sampling of direct observations by trained and experienced observers.
The study also aimed to understand fishing practices and strategies that are employed by fishers which seek to minimize interactions, depredation and bycatch of the odontocetes with the pelagic longline fishery. It was possible to identify several applicable mitigation measures that if applied alone or in combination could potentially decrease longline-dolphin interactions. However, cetaceans are well known for their adaptive capabilities and a single, universal solution is unlikely to exist.
The ‘move-on’ rule is another practical method to avoid longline-dolphin interactions that is applied among fishers and require collaborative behaviour on their part. When a triggered event occurs between dolphins and fisheries, all fishers of the area are informed and are expected to avoid this specific fishing ground for a specific period (Gilman et al., 2006; Dunn et al., 2014). This method requires good communication and coordination by the fishing industry and fishery associations (Gilman et al., 2006). The method is already practised by fishers in Cyprus as they retain good collaboration among them but with low effectiveness on avoiding dolphin interactions. This is mainly because the tuna fishing grounds are restricted to specific areas and the options for the tuna longline fishery are limited. Additionally, when a pod of dolphins identifies a vessel or a fishing activity it can follow the vessel for miles away.
Moreover, the cause of acoustic discomfort to dolphins with the use of acoustic deterrent devices is a promising mitigation technique, although scientific evidence of their effectiveness is contradicting (Northridge et al., 2003; Brotons et al., 2008; Gazo et al., 2008; Buscaino et al., 2009) and habituation to the acoustic harassment device may occur (Tixier et al., 2015). The current study identified deterrent devices that are currently used by longline fishers in Cyprus and their effectiveness in mitigating depredation (Table 2). Although no scientific experiment was conducted in the current study to prove their effectiveness, the feedback from fishers is that only one type of high-intensity acoustic deterrent devices (DDDs/DiD from STM industrial Electronics) seem to be effective in mitigating interaction with the common bottlenose dolphin and striped dolphin, even after a long period of usage (more than two years). Other studies have tested the DDDs devices and showed positive results on cetacean bycatch and depredation (Buscaino et al., 2009; Northridge et al., 2011). Based on fishers’ feedback, dolphin acoustic deterrent devices are considered for the moment as the only practical solution to mitigate interactions and depredation by dolphins. It is important to mention that in 2020 the government of the Republic of Cyprus announced a call for grant proposals for the purchase of repellent equipment (specifically dolphin anti-depredation pingers) for the protection of marine protected species. The percentage of co-funding to the beneficiaries (fishers) ranged from 30% to 80%, depending on the case and the remaining percentage was private participation (Thalassa 2014-2020, 2020). However, the potential ecological adverse effects of using high-intensity acoustic deterrent devices have not been assessed in the current study and the current knowledge on their short- and long-term effects is limited. It is likely that using such devices could alter the cetacean foraging strategies and distribution and in some cases damage hearing (Gilman et al., 2006).
Another alternative option, less invasive than deterrent devices, is the predictive forecasting; a method that identifies areas with fishing-dolphin conflicts to avoid these areas in the future through habitat modelling (Peterson and Carothers, 2013; Passadore et al., 2015). However, this method requires a high effort of data collection and analysis over a long period and the quality of such models is variable. In this study, a map (Figure 11) was developed to show areas of highly probable longline-dolphin interactions and the probability of dolphins’ occurrence in these areas based on fishers’ experience. This approach for data collection and analysis was low-cost and could be easily applied to other regions and fishing gears; these results could be used to inform fishers about conflict-prone regions and could also be applied for management measures, such as temporally spatial closures. It is also important to mention that areas identified as longline-dolphin conflict areas are also important fishing grounds with high catchability rates making any management recommendations of spatial closures difficult. It is strongly suggested that any decisions taken by policy-makers should ideally result in following consultations with stakeholders, in this case, the fishers.
The current study showed that feeding on the albacore tuna caught on pelagic longlines represents an alternative foraging strategy for dolphins. The albacore tuna is not included in the diet neither of the common bottlenose dolphin (Blanco et al., 2001; Nowacek, 2002; Bearzi et al., 2005; Díaz López, 2006; Bearzi et al., 2009; Pardalou and Tsikliras, 2020) nor of the striped dolphin (Würtz and Marrale, 1993; Blanco et al., 1995; Spitz et al., 2006; Perrin et al., 2008; Matsuda et al., 2020), however, both species are known to exhibit high dietary plasticity. Other studies from the Mediterranean Sea and around the world have shown similar foraging strategies between cetaceans and static nets (Lauriano, 2004; Díaz López, 2006; Brotons et al., 2008; Bearzi et al., 2011; Maccarrone et al., 2014; Snape et al., 2018), trawling (Zollett and Read, 2006) and longlines (Dalla Rosa and Secchi, 2007; Ramos-Cartelle and Mejuto, 2008; Rabearisoa et al., 2015). Such findings support the optimal foraging theory where an animal makes foraging decisions according to prey type and availability and based on its individual fitness benefits with the aim to increase the net energy intake per unit of time spent foraging (MacArthur and Pianka, 1996). Considering that the literature on the diet of the common bottlenose dolphin and the striped dolphin the albacore tuna is not included; depredation as a foraging strategy intends to increase foraging efficiency while decreasing foraging energy costs.
All fishers interviewed in the study strongly believed that dolphins were able to identify their fishing ground by hearing the engine sound and even follow their vessels from hours to days. Fishers who had previously used low-intensity acoustic deterrent devices (from Fishtek Marine) reported that they were effective only at the beginning while after some time of usage they became ineffective which even acted as a signal for food for dolphins that were previously exposed and had learned the sound. Similar findings were reported by Pardalou and Tsikliras (2018). The behaviour of dolphins to relate acoustic signals from the vessel engines and acoustic deterrent devices to the presence of prey is clear evidence of the ‘dinner-bell’ effect (e.g., Visser, 2000; Cox et al., 2004; Carretta and Barlow, 2011; Wargo Rub and Sandford, 2020).
The gradual increase of depredation incidents could be the result of foraging decisions and due to cetaceans advanced learning abilities and knowledge transfer within populations (Whitehead et al., 2004; Pennino et al., 2013). However, depredation incidents may have also become more frequent due to the increased fishing effort as a result of the increasing demand for seafood worldwide (FAO, 2020). The increased incidents of cetacean depredation have also motivated researchers worldwide to study cetacean depredation and since the 2000s, the number of studies published on the topic has significantly increased (Hamer et al., 2012).
The main limitation of the study is the data collection methodology. Certainly, logbooks cannot replace the quality and accuracy of the data that can be collected from onboard observations by experienced researchers. To achieve optimal coverage of onboard observations and collect sufficient data that are representative of the entire fleet, it is often very expensive, especially when fishers require compensation for their services. On the other hand, self-reporting (logbooks) and interviews are relatively inexpensive, but the data gathered can be inaccurate and biased. However, this approach is widely used and has proven to be an important tool of research if its adequately implemented and with clear protocols for the surveys (e.g. Azzurro et al., 2011; Azzurro et al., 2019; Lopes et al., 2019). To limit the factors that could lead to inaccurate and biased data, the participants in our study were carefully selected and under certain criteria (see Methods section). In addition, the fishers who participated in the self-reporting were previously actively participating and collaborating through the actions of the Cyprus Bycatch Project (2018 – 2022) and other projects (in previous years) and this was also an important criterion during the selection process. The existing trust and respect that was built between the authors of this study and the selected fishers also played a significant role in the quality of the data recorded in logbooks. We rule out any bias in the data arising from the professional relationship with the interviewees (i.e., reporting what is expected to conform to the norm); the onboard surveys confirmed the veracity of the data. This was also another reason why only four out of the 30 licensed vessels/fishers were selected for the self-reporting and anonymity was assured. Definitely, the more the participants the better the extrapolations and representation of the fleet. Nevertheless, the selected participants are among the most active pelagic longline fishers in Cyprus.
Even though the identification of the different depredators from the fish carcasses is in most cases obvious as shown in Figure 1, there might be cases which are not. For this reason, a possible misidentification might occur when fishers reported dolphin depredation in their logbooks. To limit this bias, fishers were asked to send photographic evidence of the depredated tunas when possible.
Another limiting factor is that the studies undertaken to assess the economic costs from cetacean depredation on pelagic longlines are very limited and for the Mediterranean do not exist yet. In addition to that, the lack of a standardized methodology that assesses depredation rate and costs is preventing any direct comparison with other studies.
The combination of the different data collection methodologies makes the study particularly dynamic and have strengthen the efforts made to understand the issue of depredation. In data deficient research topics and difficult-to-reach populations, it is important and efficient to combine a variety of data collection methods to address an issue. The results from the logbooks and interviews as well as personal communication from the leading author with fishers showed that depredation by the common bottlenose dolphin and the striped dolphin on pelagic longlines during the albacore tuna longline fishery is a frequent event in Cyprus. The findings of this study show for the first time the detrimental effects of species depredation on longline fisheries in Cyprus and vice versa, mainly from the cetaceans but also from other taxa including a shark and a cephalopod species. Considering the increasing competition between cetaceans and fisheries, mitigation of this problem should become a high priority by researchers and authorities whilst more effort should be placed by government bodies to support fishers and their fishing operations to mitigate interactions with dolphins, minimize economic loss and avoidance of dolphin bycatch on longlines. Alternative fishing methods and acoustic deterrent devices could support this action. The results could be used as a reference for future work on the taxa causing depredation on the catch to help the correct calculation of the damage caused from different depredating taxa and apply the suitable mitigation measures.
Beyond the economic consequences from depredation and bycatch, the evaluation methodology for depredation and the results from the current study can have important implications on the conservation and management of the albacore tuna stocks as losses due to depredation are not counted for in quota allocation processes and fish stock assessments. The results and the method developed here could also support the development of a standardised data collection methodology and methods for depredation assessment and quantification. Additionally, the data presented in this study could support national management action plans and set the foundations for future research on depredation and bycatch mitigation practices as well as to develop fishery-specific assessments on species interactions.
The empirical knowledge from longline fishers to reduce cetacean-longline interactions as well as to address other future industry problems should always be taken into consideration. Fishers have considerable ecological and empirical knowledge (i.e., traditional knowledge) that could significantly contribute to any scientific experiment and the development of potential management actions. We are certain that fishers should be active participants in fisheries research and collaboratively participate in the development of any management activities and best fishing practices to ensure the long-term effectiveness of these actions. However, this approach should never, in any case, replace scientific research by experienced researchers in the field but rather be used as a tool that supplements fisheries research and to build trust, cooperation and a sense of responsibility among fishery stakeholders.
Depredation from cetaceans is an evolving practice that seems to change from time to time. Research that aims to understand and quantify depredation must be continued. Future research that will aim to understand and quantify bait depredation and its associated costs as well as other indirect costs including gear loss, fuel, salaries, etc. is especially important. Most importantly, future research should aim to mitigate this global issue by testing new mitigation measures and technologies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MP conceptualized, administrated, and designed the study, developed methodology, cured data, performed the statistical analysis and visualized results, and wrote the original draft. CJ and AP supervised and reviewed the manuscript and approved the submitted version. AG performed the maps. LH acquired project funding, reviewed the manuscript and approved the submitted version. All authors contributed to the article and approved the submitted version.
This work was financially supported by MAVA Foundation under the framework of the Cyprus Bycatch Project (Project No. 2794).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank all fishers who participated in the study and shared their knowledge and experience.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.868464/full#supplementary-material
Azzurro E., Moschella P., Maynou F. (2011). Tracking Signals of Change in Mediterranean Fish Diversity Based on Local Ecological Knowledge. PloS One 6 (9), e24885. doi: 10.1371/journal.pone.0024885
Azzurro E., Sbragaglia V., Cerri J., Bariche M., Bolognini L., Ben Souissi J., et al. (2019). Climate Change, Biological Invasions, and the Shifting Distribution of Mediterranean Fishes: A Large-Scale Survey Based on Local Ecological Knowledge. Global Change Biol. 25, 2779– 2792. doi: 10.1111/gcb.14670
Barros N. B., Odell D. K. (1990). “Food Habits of Bottlenose Dolphins in the Southeastern United States.” in The Bottlenose Dolphin. Eds. Leatherwood S., Reeves R. R. (San Diego, CA: Academic Press), 309–328, 653pp.
Barros N. B., Parsons E. C. M., Jefferson T. A. (2000). Prey of Offshore Bottlenose Dolphins From the South China Sea. Aquat. Mamm. 26 (1), 2–6.
Bearzi G. (2002). “Interactions Between Cetacean and Fisheries in the Mediterranean Sea.” in Cetaceans of the Mediterranean and Black Seas: State of Knowledge and Conservation Strategies. Ed. Notarbartolo Di Sciara G. (Monaco: A report to the ACCOBAMS Secretariat), 20 pages.
Bearzi G., Bonizzoni S., Gonzalvo J. (2011). Dolphins and Coastal Fisheries Within a Marine Protected Area: Mismatch Between Dolphin Occurrence and Reported Depredation. Aquat. Conserv.: Mar. Freshw. Ecosyst. 21 (3), 261–267. doi: 10.1002/aqc.1179
Bearzi G., Fortuna C., Reeves R. (2009). Ecology and Conservation of Common Bottlenose Dolphins Tursiops Truncatus in the Mediterranean Sea. Mamm. Rev. 39 (2), 92. doi: 10.1111/j.1365-2907.2008.00133.x
Bearzi G., Holcer D., Notarbartolo di Sciara G. (2004). The Role of Historical Dolphin Takes and Habitat Degradation in Shaping the Present Status of Northern Adriatic Cetaceans. Aquat. Conserv.: Mar. Freshw. Ecosyst. 14 (4), 363. doi: 10.1002/aqc.626
Bearzi G., Politi E., Agazzi S., Azzellino A. (2006). Prey Depletion Caused by Overfishing and the Decline of Marine Megafauna in Eastern Ionian Sea Coastal Waters (Central Mediterranean). Biol. Conserv. 127 (4), 373–382. doi: 10.1016/j.biocon.2005.08.017
Bearzi G., Politi E., Agazzi S., Bruno S., Costa M., Bonizzoni S. (2005). Occurrence and Present Status of Coastal Dolphins (Delphinus Delphis and Tursiops Truncatus) in the Eastern Ionian Sea. Aquat. Conserv.: Mar. Freshw. Ecosyst. 15 (3), 243–257. doi: 10.1002/aqc.667
Berg B. L. (2004). “Methods for the Social Sciences,” in Qualitative Research Methods for the Social Sciences (Boston: Pearson Education).
Blanco C., Aznar J., Raga J. A. (1995). Cephalopods in the Diet of the Striped Dolphin Stenella Coeruleoalba From the Western Mediterranean During an Epizootic in 1990. J. Zool. 237 (1), 151–158. doi: 10.1111/j.1469-7998.1995.tb02753.x
Blanco C., Salomón O., Raga J. A. (2001). Diet of the Bottlenose Dolphin (Tursiops Truncatus) in the Western Mediterranean Sea. Mar. Biol. Assoc. Unit. Kingd. 81 (6), 1053. doi: 10.1017/S0025315401005057
Blasi M. F., Giuliani A., Boitani L. (2015). Influence of Trammel Nets on the Behaviour and Spatial Distribution of Bottlenose Dolphins (Tursiops Truncatus) in the Aeolian Archipelago, Southern Italy. Aquat. Mamm. 41 (3), 295–310. doi: 10.1578/AM.41.3.2015.295
Boisseau O., Frantzis A., Petrou A., Van Geel N., McLanaghan R., Alexiadou P., et al. (2017). “Visual and Passive Acoustic Survey Report,” in Report Submitted to the Department of Fisheries and Marine Research by the AP Marine Environmental Consultancy Consortium, 49. Nicosia: AP Marine Environmental Consultancy.
Braga H. D. O., Schiavetti A. (2013). Attitudes and Local Ecological Knowledge of Experts Fishermen in Relation to Conservation and Bycatch of Sea Turtles (Reptilia: Testudines), Southern Bahia, Brazil. J. Ethnobiol. Ethnomed. 9 (1), 1–13. doi: 10.1186/1746-4269-9-15
Brotons J. M., Grau A. M., Rendell L. (2008). Estimating the Impact of Interactions Between Bottlenose Dolphins and Artisanal Fisheries Around the Balearic Islands. Mar. Mamm. Sci. 24 (1), 112–127. doi: 10.1111/j.1748-7692.2007.00164.x
Bruno C. A., Caserta V., Salzeri P., Ferraro G. B., Pecoraro F., Lucchetti A., et al. (2021). Acoustic Deterrent Devices as Mitigation Tool to Prevent Dolphin-Fishery Interactions in the Aeolian Archipelago (Southern Tyrrhenian Sea, Italy). Mediter. Mar. Sci. 22 (2), 408–421. doi: 10.12681/mms.23129
Buscaino G., Buffa G., Sara G., Bellante A., Tonello A. J., Hardt F. A. S., et al. (2009). Pinger Affects Fish Catch Efficiency and Damage to Bottom Gill Nets Related to Bottlenose Dolphins. Fish. Sci. 75 (3), 537–544. doi: 10.1007/s12562-009-0059-3
Carretta J. V., Barlow J. (2011). Long-Term Effectiveness, Failure Rates, and “Dinner Bell” Properties of Acoustic Pingers in a Gillnet Fishery. Mar. Technol. Soc. J. 45 (5), 7–19. doi: 10.4031/MTSJ.45.5.3
Chilvers L. B., Corkeron P. J. (2001). Trawling and Bottlenose Dolphins' Social Structure. Proc. R. Soc. Lond. Ser. B.: Biol. Sci. 268 (1479), 1901–1905. doi: 10.1098/rspb.2001.1732
Connor R. C., Wells R. S., Mann J., Read A. J. (2000). “The Bottlenose Dolphin: Social Relationships in a Fission-Fusion Society.” in Cetacean Societies: Field Studies of Dolphins and Whales. Eds. Mann J., Connor R. C., Tyack P. L., Whitehead H. (Chicago, IL: Chicago University Press), 91–126.
Cox T. M., Read A. J., Swanner D., Urian K., Waples D. (2004). Behavioral Responses of Bottlenose Dolphins, Tursiops Truncatus, to Gillnets and Acoustic Alarms. Biol. Conserv. 115 (2), 203–212. doi: 10.1016/S0006-3207(03)00108-3
Crosti R., Arcangeli A., Romeo T., Andaloro F. (2017). Assessing the Relationship Between Cetacean Strandings (Tursiops Truncatus and Stenella Coeruleoalba) and Fishery Pressure Indicators in Sicily (Mediterranean Sea) Within the Framework of the EU Habitats Directive. Eur. J. Wildl. Res. 63 (3), 1–13. doi: 10.1007/s10344-017-1111-8
Dalla Rosa L., Secchi E. R. (2007). Killer Whale (Orcinus Orca) Interactions With the Tuna and Swordfish Longline Fishery Off Southern and South-Eastern Brazil: A Comparison With Shark Interactions. J. Mar. Biol. Assoc. Unit. Kingd. 87 (1), 135–140. doi: 10.1017/S0025315407054306
Department of Fisheries and Marine Research (2016 - 2021). Cyprus Annual Report for Data Collection in the Fisheries and Aquaculture Sectors (Nicosia: Ministry of Agriculture, Rural Development and Natural Resources). Available at: https://datacollection.jrc.ec.europa.eu/ars.
Department of Fisheries and Marine Research (2019). Annual Report 2018 (Nicosia: Department of Fisheries and Marine Research).
Díaz López B. (2006). Interactions Between Mediterranean Bottlenose Dolphins (Tursiops Truncatus) and Gillnets Off Sardinia, Italy. ICES J. Mar. Sci. 63 (5), 946–951. doi: 10.1016/j.icesjms.2005.06.012
Di Natale A., Notarbartolo di Sciara G. (1994). A Review of the Passive Fishing Nets and Trap Fisheries in the Mediterranean Sea and of Cetacean Bycatch. Rep. Int. Whal. Comm. Special Issue 15), 189–202.
Dmitrieva L., Kondakov A. A., Oleynikov E., Kydyrmanov A., Karamendin K., Kasimbekov Y., et al. (2013). Assessment of Caspian Seal by-Catch in an Illegal Fishery Using an Interview-Based Approach. PloS One 8 (6), e67074. doi: 10.1371/journal.pone.0067074
Dunn D. C., Boustany A. M., Roberts J. J., Brazer E., Sanderson M., Gardner B., et al. (2014). Empirical Move-on Rules to Inform Fishing Strategies: A N Ew E Ngland Case Study. Fish. Fish. 15 (3), 359–375. doi: 10.1111/faf.12019
Esri Inc (2013). ArcGIS Desktop (Version 10.1) (Esri Inc). Available at: https://www.esri.com/en-us/arcgis/products/arcgis-desktop/overview.
FAO (2019a). “Monitoring Discards in Mediterranean and Black Sea Fisheries: Methodology for Data Collection,” in FAO Fisheries and Aquaculture Technical Paper No. 639 (Rome: FAO).
FAO (2019b). “Monitoring the Incidental Catch of Vulnerable Species in Mediterranean and Black Sea Fisheries: Methodology for Data Collection,” in FAO Fisheries and Aquaculture Technical Paper No. 640 (Rome: FAO).
FAO (2020). The State of World Fisheries and Aquaculture 2020. Sustainability in Action (Rome: FAO). doi: 10.4060/ca9229en
Forney K. A., Kobayashi D. R. (2007) Updated Estimates of Mortality and Injury of Cetaceans in the Hawaii-Based Longline Fishery, 1994-2005, Report, November 2007; United States (University of North Texas Libraries: Libraries Government Documents Department). Available at: https://digital.library.unt.edu/ark:/67531/metadc1050058https://digital.library.unt.edu (Accessed July 5, 2021)).
Forney K. A., Kobayashi D. R., Johnston D. W., Marchetti J. A., Marsik M. G. (2011). What’s the Catch? Patterns of Cetacean Bycatch and Depredation in Hawaii-Based Pelagic Longline Fisheries. Mar. Ecol. 32 (3), 380–391. doi: 10.1111/j.1439-0485.2011.00454.x
Froese R., Winker H., Coro G., Demirel N., Tsikliras A. C., Dimarchopoulou D., et al. (2018). Status and Rebuilding of European Fisheries. Mar. Policy 93, 159–170. doi: 10.1016/j.marpol.2018.04.018
Garrison L. P. (2007). Interactions Between Marine Mammals and Pelagic Longline Fishing Gear in the US Atlantic Ocean Between 1992 and 2004. Fish. Bull. 105 (3), 408–417. doi: hdl.handle.net/1834/25527
Gazo M., Gonzalvo J., Aguilar A. (2008). Pingers as Deterrents of Bottlenose Dolphins Interacting With Trammel Nets. Fish. Res. 92 (1), 70–75. doi: 10.1016/j.fishres.2007.12.016
Giannakis E., Hadjioannou L., Jimenez C., Papageorgiou M., Karonias A., Petrou A. (2020). Economic Consequences of Coronavirus Disease (COVID-19) on Fisheries in the Eastern Mediterranean (Cyprus). Sustainability 12 (22), p.9406. doi: 10.3390/su12229406
Gilman E., Brothers N., McPherson G., Dalzell P. (2006). A Review of Cetacean Interactions With Longline Gear. J. Cetac. Res. Manage. 8 (2), 215.
Gilman E., Clarke S., Brothers N., Alfaro-Shigueto J., Mandelman J., Mangel J., et al. (2007). “Shark Depredation and Unwanted Bycatch in Pelagic Longline Fisheries: Industry Practices and Attitudes, and Shark Avoidance Strategies,” Honolulu: Western Pacific Regional Fishery Management Council.
Gilman E., Clarke S., Brothers N., Alfaro-Shigueto J., Mandelman J., Mangel J., et al. (2008). Shark Interactions in Pelagic Longline Fisheries. Mar. Policy 32 (1), 1–18. doi: 10.1016/j.marpol.2007.05.001
Gonzalvo J., Giovos I., Moutopoulos D. K. (2015). Fishermen's Perception on the Sustainability of Small-Scale Fisheries and Dolphin–Fisheries Interactions in Two Increasingly Fragile Coastal Ecosystems in Western Greece. Aquat. Conserv.: Mar. Freshw. Ecosyst. 25 (1), 91–106. doi: 10.1002/aqc.2444
Goodman L. A. (1961). Snowball Sampling. Ann. Math. Stat, 32 (1), 148–170. doi: 10.1214/aoms/1177705148
Guinet C., Tixier P., Gasco N., Duhamel G. (2015). Long-Term Studies of Crozet Island Killer Whales are Fundamental to Understanding the Economic and Demographic Consequences of Their Depredation Behaviour on the Patagonian Toothfish Fishery. ICES J. Mar. Sci. 72 (5), 1587–1597. doi: 10.1093/icesjms/fsu221
Hall M. A., Donovan G. P. (2002). Environmentalists, Fishermen, Cetaceans and Fish: Is There a Balance and Can Science Help to Find It? Mar. Mamm., 491–521 (Boston, MA: Springer). doi: 10.1007/978-1-4615-0529-7_14
Hamer D. J., Childerhouse S. J., Gales N. J. (2012). Odontocete Bycatch and Depredation in Longline Fisheries: A Review of Available Literature and of Potential Solutions. Mar. Mamm. Sci. 28 (4), E345–E374. doi: 10.1111/j.1748-7692.2011.00544.x
Harwood J. (1992). Assessing the Competitive Effects of Marine Mammal Predation on Commercial Fisheries. South Afr. J. Mar. Sci. 12 (1), 689–693. doi: 10.2989/02577619209504733
Hernandez-Milian G., Goetz S., Varela-Dopico C., Rodriguez-Gutierrez J., Romón-Olea J., Fuertes-Gamundi J. R., et al. (2008). “Results of a Short Study of Interactions of Cetaceans and Longline Fisheries in Atlantic Waters: Environmental Correlates of Catches and Depredation Events,” in Essential Fish Habitat Mapping in the Mediterranean (Dordrecht: Springer), pp. 251–268. doi: 10.1007/978-1-4020-9141-4_19
Kvale S., Brinkmann S. (2009). Interviews: Learning the Craft of Qualitative Research Interviewing (Thousand Oaks, CA: Sage Publications).
Lampe O. D., Hauser H. (2011). “Interactive Visualization of Streaming Data With Kernel Density Estimation,” in 2011 IEEE Pacific Visualization Symposium (IEEE: Hong Kong), (pp. 171–178). doi: 10.1109/PACIFICVIS.2011.5742387
Lauriano G. (2004). Interactions Between Bottlenose Dolphins (Tursiops Truncatus) and the Artisanal Fishery in Asinara Island National Park (Sardinia): Assessment of Catch Damage and Economic Loss. J. Cetac. Res. Manage. 6 (2), 165–173.
Li C., Baciu G., Han Y. (2014). “Interactive Visualization of High Density Streaming Points With Heat-Map,” in 2014 International Conference on Smart Computing (IEEE: Hong Kong), (pp. 145–149). doi: 10.1109/SMARTCOMP.2014.7043852
Lopes P. F., Verba J. T., Begossi A., Pennino M. G. (2019). Predicting Species Distribution From Fishers’ Local Ecological Knowledge: A New Alternative for Data-Poor Management. Can. J. Fish. Aquat. Sci. 76 (8), 1423–1431. doi: 10.1139/cjfas-2018-0148
MacArthur R. H., Pianka E. R. (1966). On Optimal Use of a Patchy Environment. Am. Nat. 100 (916), 603–609. doi: 10.1086/282454
Maccarrone V., Buffa G., Di Stefano V., Filiciotto F., Mazzola S., Buscaino G. (2014). Economic Assessment of Dolphin Depredation Damages and Pinger Use in Artisanal Fisheries in the Archipelago of Egadi Islands (Sicily). Turk. J. Fish. Aquat. Sci. 14 (1), 173–181. doi: 10.4194/1303-2712-v14_1_19
Magnaghi L., Podestà M. (1987). “An Accidental Catch of 8 Striped Dolphins, Stenella Coeruleoalba (Meyen 1833), in the Ligurian Sea (Cetacea Delphinidae),” in Atti Società Italiana Di Scienze Naturali, Museo Civico Di Soria Naturale, Milano, vol. 128, 235–239.
Malara D., Battaglia P., Consoli P., Arcadi E., Longo F., Stipa M. G., et al. (2021). When Opportunistic Predators Interact With Swordfish Harpoon Fishing Activities: Shark Depredation Over Catches in the Strait of Messina (Central Mediterranean Sea). Eur. Zool. J. 88 (1), 226–236. doi: 10.1080/24750263.2021.1879284
Mandelman J. W., Cooper P. W., Werner T. B., Lagueux K. M. (2008). Shark Bycatch and Depredation in the US Atlantic Pelagic Longline Fishery. Rev. Fish. Biol. Fish. 18 (4), 427–442. doi: 10.1007/s11160-008-9084-z
Matsuda A., Yamada T. K., Tajima Y., Kunisue T., Amano M., Matsuishi T. F. (2020). Diet of Mass-Stranded Striped Dolphins (Stenella Coeruleoalba) in Southern Japan (East China Sea). Mamm. Stud. 46 (1), 1–8. doi: 10.3106/ms2020-0005
Ministry of Agriculture, Rural Development and the Environment, Department of Fisheries and Marine Research (2020). “ΠPOTEPAIOTHTA THΣ ENΩΣHΣ 1,” in Πρoώθηςη Tης ΠϵριBαλλoντικά Bιώςιμης, Aπoδoτικής Ως Πρoς Toυς Πόρoυς, Kαινoτόμoυ, Aνταγωνιςτικής Kαι Bαςιζόμϵνης Στη Γνώςη Aλιϵίας. Mέτρo 1.19: Πρoςταςία Kαι Aπoκατάςταςη Tης Θαλάςςιας Bιoπoικιλότητας - Συςτήματα Για Tην Aπoζημίωςη Tων Zημιών Πoυ Πρoκαλoύνται Στα Aλιϵύματα Aπό Πρoςτατϵυόμϵνα Θηλαςτικά Kαι Πτηνά – APΘPO 40.1H «Σχέδιo Για Tην Aπoζημίωςη Tων Zημιών Πoυ Πρoκαλoύνται Στα Aλιϵύματα Aπό Πρoςτατϵυόμϵνα Θηλαςτικά» (Nicosia: Department of Fisheries and Marine Research).
Moazzam M. (2019). “Depredation of Purpleback Flying Squid (Sthenoteuthis Oualaniensis) on Tuna Caught by Gillnet Fisheries in the Northern Arabian Sea: A Major Cause of Concern for Fishermen,” in IOTC Working Party on Ecosystem and Bycatch, vol. 3-7. (France September: WPEB15 La Reunion), 11p. 2019. IOTC-2019-WPEB15-13.
Morissette L., Christensen V., Pauly D. (2012). Marine Mammal Impacts in Exploited Ecosystems: Would Large Scale Culling Benefit Fisheries? PloS One 7 (9), e43966. doi: 10.1371/journal.pone.0043966
Northridge S., Hofman R. J. (1999). “Marine Mammal-Fishery Interactions,” in Conservation and Management of Marine Mammals. Eds. Twiss J. R., Reeves R. R. (Washington, DC: Smithsonian Institution Press), 99–119.
Northridge S., Kingston A., Mackay A., Lonergan M. (2011). “Bycatch of Vulnerable Species: Understanding the Process and Mitigating the Impacts,” in Final Report to Defra Marine and Fisheries Science Unit, Project No MF1003 (Defra, London: University of St Andrews), 99pp.
Northridge S., Vernicos D., Raitsos-Exarchopolous D. (2003). Net Depredation by Bottlenose Dolphins in the Aegean: First Attempts to Quantify and to Minimise the Problem. IWC Sc/55/Sm25 (Cambridge: International Whaling Commission).
Northridge S., Waples D., Read A. J. (2013). To Ping or Not to Ping: The Use of Active Acoustic Devices in Mitigating Interactions Between Small Cetaceans and Gillnet Fisheries. Endanger. Spe. Res. 19 (3), pp.201–pp.221. doi: 10.3354/esr00464
Nowacek D. (2002). “Sequential Foraging Behaviour of Bottlenose Dolphins, Tursiops Truncatus,”, vol. 139. (Sarasota Bay: FL. Behaviour) 139 (9), pp.1125–1145. doi: 10.1163/15685390260437290
Papageorgiou M., Papadopoulou A., Hadjioannou L. (2020). “Cyprus Bycatch Project “Understanding Multi-Taxa ‘Bycatch’ of Vulnerable Species and Testing Mitigation a Collaborative Approach in Cyprus”,” in Technical Report: Results of Phase 1 (2018-20019) of the Bycatch Monitoring Programme in Cyprus (Nicosia: BirdLife Cyprus and Enalia Physis Environmental Research Centre), Pp32.
Pardalou A., Tsikliras A. C. (2018). Anecdotal Information on Dolphin-Fisheries Interactions Based on Empirical Knowledge of Fishers in the Northeastern Mediterranean Sea. Ethic. Sci. Environ. Polit. 18, pp.1–pp.8. doi: 10.3354/esep00179
Pardalou A., Tsikliras A. C. (2020). Factors Influencing Dolphin Depredation in Coastal Fisheries of the Northern Aegean Sea: Implications on Defining Mitigation Measures. Mar. Mamm. Sci. 36 (4), 1126–1149. doi: 10.1111/mms.12702
Passadore C., Domingo A., Secchi E. R. (2015). Analysis of Marine Mammal Bycatch in the Uruguayan Pelagic Longline Fishery Operating in the Southwestern Atlantic Ocean. ICES J. Mar. Sci. 72 (5), 1637–1652. doi: 10.1093/icesjms/fsu250
Pennino M. G., Mendoza M., Pira A., Floris A., Rotta A. (2013). Assessing Foraging Tradition in Wild Bottlenose Dolphins (Tursiops Truncatus). Aquat. Mamm. 39 (3), 282. doi: 10.1578/AM.39.3.2013.282
Pennino M. G., Rotta A., Pierce G. J., Bellido J. M. (2015). Interaction Between Bottlenose Dolphin (Tursiops Truncatus) and Trammel Nets in the Archipelago De La Maddalena, Italy. Hydrobiologia 747 (1), 69–82. doi: 10.1007/s10750-014-2127-7
Perrin W. F., Robertson K. M., Walker W. A. (2008). “Diet of the Striped Dolphin, Stenella Coeruleoalba,” in The Eastern Tropical Pacific Ocean (Agencies and Staff of the U.S. Department of Commerce). Available at: http://digitalcommons.unl.edu/usdeptcommercepub/23.
Peterson M. J., Carothers C. (2013). Whale Interactions With Alaskan Sablefish and Pacific Halibut Fisheries: Surveying Fishermen Perception, Changing Fishing Practices and Mitigation. Mar. Policy 42, 315–324. doi: 10.1016/j.marpol.2013.04.001
Peterson M.J., Mueter F., Criddle K., Haynie A.C. (2014). Killer Whale Depredation and Associated Costs to Alaskan Sablefish, Pacific Halibut and Greenland Turbot Longliners. PLoS One 9(2), p.e88906. doi: org/10.1371/journal.pone.0088906
Purves M. G., Agnew D. J., Balguerias E., Moreno C. A., Watkins B. (2004). Killer Whale (Orcinus Orca) and Sperm Whale (Physeter Macrocephalus) Interactions With Longline Vessels in the Patagonian Toothfish Fishery at South Georgia, South Atlantic. CCAMLR. Sci. 11, 111–26.
Rabearisoa N., Bach P., Marsac F. (2015). Assessing Interactions Between Dolphins and Small Pelagic Fish on Branchline to Design a Depredation Mitigation Device in Pelagic Longline Fisheries. ICES J. Mar. Sci. 72 (5), 1682–1690. doi: 10.1093/icesjms/fsu252
Rabearisoa N., Sabarros P. S., Romanov E. V., Lucas V., Bach P. (2018). Toothed Whale and Shark Depredation Indicators: A Case Study From the Reunion Island and Seychelles Pelagic Longline Fisheries. PloS One 13 (8), 1–26 doi: 10.1371/journal.pone.0202037
Ramos-Cartelle A., Mejuto J. (2008). “Interaction of the False Killer Whale (Pseudorca Crassidens) and Depredation on the Swordfish Catches of the Spanish Surface Longline Fleet in the Atlantic, Indian and Pacific Oceans,” in Report, International Commission for the Conservation of Atlantic Tunas (ICCAT), Collective Volume of Scientific Papers (SCRS/2007/025), vol. 62. , 1721–1783.
R Core Team (2020). “R: A Language and Environment for Statistical Computing,” (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
Remeslo A. V., Yakushev M. R., Laptikhovsky V. (2015). Alien vs. Predator: Interactions Between the Colossal Squid (Mesonychoteuthis Hamiltoni) and the Antarctic Toothfish (Dissostichus Mawsoni). J. Natural Histor. 49 (41-42), pp.2483–2491. doi: 10.1080/00222933.2015.1040477
Roche C., Guinet C. (2007). Marine Mammals and Demersal Longline Fishery Interactions in Crozet and Kerguelen Exclusive Economic Zones: An Assessment of Depredation Levels. CCAMLR. Sci. 14, pp.67–pp.82.
Rocklin D., Santoni M. C., Culioli J. M., Tomasini J. A., Pelletier D., Mouillot D. (2009). Changes in the Catch Composition of Artisanal Fisheries Attributable to Dolphin Depredation in a Mediterranean Marine Reserve. ICES J. Mar. Sci. 66 (4), 699–707. doi: 10.1093/icesjms/fsp036
Rub A. M. W., Sandford B. P. (2020). Evidence of a ‘Dinner Bell’effect From Acoustic Transmitters in Adult Chinook Salmon. Mar. Ecol. Prog. Ser. 641, pp.1–pp11. doi: 10.3354/meps13323
Secchi E. R., Vaske T. J. (1998). Killer Whale (Orcinus Orca) Sightings and Depredation on Tuna and Swordfish Longline Catches in Southern Brazil. Aquat. Mamm. 24, 117–122.
Shane S. H., Wells R. S., Würsig B. (1986). Ecology, Behavior and Social Organization of the Bottlenose Dolphin: A Review. Mar. Mamm. Sci. 2 (1), 34–63. doi: 10.1111/j.1748-7692.1986.tb00026.x
Sivasubramaniam K. (1964). Predation of Tuna Longline Catches in the Indian Ocean, by Killer-Whales and Sharks. Bull. Fish. Res. Stat. Ceyl. 17 (2), 221–236. doi: hdl.handle.net/1834/32494
Snape R. T. E., Broderick A. C., Çiçek B. A., Fuller W. J., Tregenza N., Witt M. J., et al. (2018). Conflict Between Dolphins and a Data-Scarce Fishery of the European Union. Hum. Ecol. 46 (3), 423–433. doi: 10.1007/s10745-018-9989-7
Spitz J., Richard E., Meynier L., Pusineri C., Ridoux V. (2006). Dietary Plasticity of the Oceanic Striped Dolphin, Stenella Coeruleoalba, in the Neritic Waters of the Bay of Biscay. J. Sea. Res. 55 (4), 309–320. doi: 10.1016/j.seares.2006.02.001
Thalassa 2014-2020 (2020) Σχέδιo Xoρηγιών Για Aγoρά Aπωθητικoύ Eξoπλιςμoύ Για Πρoςταςία Tων Θαλάςςιων Πρoςτατϵυόμϵνων Eιδών - 1H Πρόςκληςη ΥπoBoλής Πρoτάςϵων - 05/05/2020. Available at: http://www.moa.gov.cy/moa/opf/opf2014.nsf/All/98ECCEEADB3C0335C225855F00513070?OpenDocument.
Tixier P., Gasco N., Duhamel G., Guinet C. (2015). Habituation to an Acoustic Harassment Device (AHD) by Killer Whales Depredating Demersal Longlines. ICES J. Mar. Sci. 72 (5), 1673–1681. doi: 10.1093/icesjms/fsu166
Tixier P., Gasco N., Duhamel G., Viviant M., Authier M., Guinet C. (2010). Interactions of Patagonian Toothfish Fisheries With Killer and Sperm Whales in the Crozet Islands Exclusive Economic Zone: An Assessment of Depredation Levels and Insights on Possible Mitigation Strategies. CCAMLR. Sci. 17, pp.179–pp.195.
Tixier P., Lea M. A., Hindell M. A., Welsford D., Mazé C., Gourguet S., et al. (2021). When Large Marine Predators Feed on Fisheries Catches: Global Patterns of the Depredation Conflict and Directions for Coexistence. Fish. Fish. 22 (1), pp.31–pp.53. doi: 10.1111/faf.12504
Vasilakopoulos P., Maravelias C. D., Tserpes G. (2014). The Alarming Decline of Mediterranean Fish Stocks. Curr. Biol. 24 (14), 1643–1648. doi: 10.1016/j.cub.2014.05.070
Visser I. N. (2000). Killer Whale (Orcinus Orca) Interactions With Longline Fisheries in New Zealand Waters. Aquat. Mamm. 26 (3), 241–252.
Wells R. S., Allen J. B., Hofmann S., Bassos-Hull K., Fauquier D. A., Barros N. B., et al. (2008). Consequences of Injuries on Survival and Reproduction of Common Bottlenose Dolphins (Tursiops Truncatus) Along the West Coast of Florida. Mar. Mamm. Sci. 24 (4), 774–794. doi: 10.1111/j.1748-7692.2008.00212.x
Werner T. B., Northridge S., Press K. M., Young N. (2015). Mitigating Bycatch and Depredation of Marine Mammals in Longline Fisheries. ICES J. Mar. Sci. 72 (5), 1576–1586. doi: 10.1093/icesjms/fsv092
Whitehead H., Rendell L., Osborne R. W., Würsig B. (2004). Culture and Conservation of Non-Humans With Reference to Whales and Dolphins: Review and New Directions. Biol. Conserv. 120 (3), 427–437. doi: 10.1016/j.biocon.2004.03.017
Williams A. J., Petersen S. L., Goren M., Watkins B. P. (2009). Sightings of Killer Whales Orcinus Orca From Longline Vessels in South African Waters, and Consideration of the Regional Conservation Status. Afr. J. Mar. Sci. 31 (1), 81–86. doi: 10.2989/AJMS.2009.31.1.7.778
Würtz M., Marrale D. (1993). Food of Striped Dolphin, Stenella Coeruleoalba, in the Ligurian Sea. J. Mar. Biol. Assoc. Unit. Kingd. 73 (3), 571–578. doi: 10.1017/S0025315400033117
Yuan K., Cheng X., Gui Z., Li F., Wu H. (2019). A Quad-Tree-Based Fast and Adaptive Kernel Density Estimation Algorithm for Heat-Map Generation. Int. J. Geograph. Inf. Sci. 33 (12), 2455–2476. doi: 10.1080/13658816.2018.1555831
Zappes C. A., Simões-Lopes P. C., Andriolo A., Di Beneditto A. P. M. (2016). Traditional Knowledge Identifies Causes of Bycatch on Bottlenose Dolphins (Tursiops Truncatus Montagu 1821): An Ethnobiological Approach. Ocean. Coast. Manage. 120, 160–169. doi: 10.1016/j.ocecoaman.2015.12.006
Zeller D., Pauly D. (2005). Good News, Bad News: Global Fisheries Discards are Declining, But So are Total Catches. Fish. Fish. 6 (2), 156–159. doi: 10.1111/j.1467-2979.2005.00177.x
Keywords: dolphin-fisheries interactions, depredation, economic loss, bycatch, mitigation measures, common bottlenose dolphin, striped dolphin, eastern Mediterranean Sea
Citation: Papageorgiou M, Hadjioannou L, Jimenez C, Georgiou A and Petrou A (2022) Understanding the Interactions Between Cetaceans and Other Megafauna With the Albacore Tuna Fishery: A Case Study From the Cyprus’ Pelagic Longline Fishery. Front. Mar. Sci. 9:868464. doi: 10.3389/fmars.2022.868464
Received: 02 February 2022; Accepted: 27 April 2022;
Published: 25 May 2022.
Edited by:
Roberto Carlucci, University of Bari Aldo Moro, ItalyReviewed by:
Dimitrios K. Moutopoulos, University of Patras, GreeceCopyright © 2022 Papageorgiou, Hadjioannou, Jimenez, Georgiou and Petrou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marios Papageorgiou, bS5wYXBhZ2Vvcmdpb3VAZW5hbGlhcGh5c2lzLm9yZy5jeQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.