- 1Department of Marine Organism Taxonomy and Phylogeny, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China
- 2Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao, China
- 3College of Biological Sciences, University of Chinese Academy of Sciences, Beijing, China
- 4Shandong Province Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China
The Formosa Ridge methane seep is one of three known active methane seepage areas discovered on the northern slope of the South China Sea. Cyphocaris is a genus of pelagic amphipods. Two new species of Cyphocaris collected from water columns above the Formosa Ridge methane seep are reported in the present study. Cyphocaris lubrica sp. nov. collected at a depth of 1,118 m is characterized by pereonite 1 dorsal projection not extending beyond head, and the pereopod 5 having a spur beyond pereonite 7. Cyphocaris formosa sp. nov. collected at a depth of 500–800 m is characterized by well-developed pereonite 1 dorsal projection extending beyond head and pereopod 5 without spur. The present work describes these two new species and compares them with closely related species, and provides a modified key of world Cyphocarididae species.
Introduction
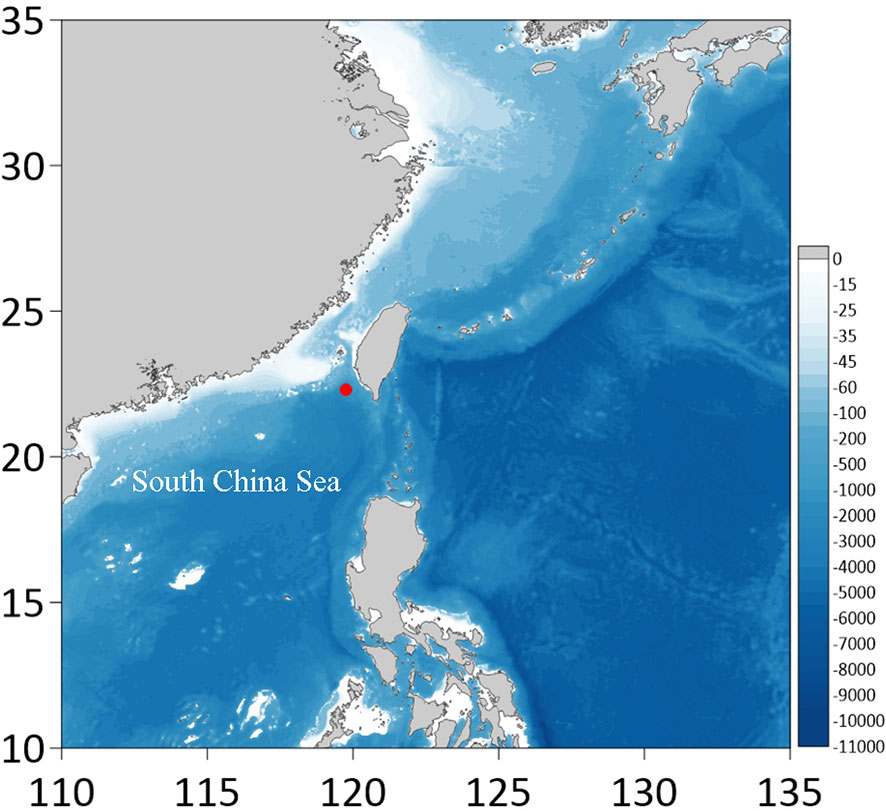
The Formosa Ridge is an active methane seep on the northern South China Sea continental slope (Feng and Chen, 2015; Feng et al., 2018; Kuo et al., 2019), located in the passive China continental margin about 100 km southwest of Taiwan (Lin et al., 2007a; Lin et al., 2007b; Machiyama et al., 2007). The community of the Formosa Ridge methane seep is dominated by two epibenthic species: the bathymodioline mussel Gigantidas platifrons (Hashimoto and Okutani, 1994) and the galatheid squat lobster Shinkaia crosnieri (Baba and Williams, 1998; Zhao et al., 2020). Although a number of studies have been conducted to understand the biodiversity of the epifauna of this methane seep (Li, 2017; Zhao et al., 2020), and the connectivity of such epifauna with those of the open western Pacific (Xu et al., 2022), nothing is known about the zooplankton in the water columns above the seep.
Amphipoda is one of the largest orders of the Crustacea and the most widespread crustacean taxa, occurring in almost all aquatic environments, some subterranean habitats and some terrestrial habitats (Barnard and Karaman, 1991; Lowry and Myers, 2017). Cyphocaridids are pelagic amphipods found throughout the oceans of the world from 90 m to thousands of meters deep (Hughes and Lowry, 2015). The Cyphocarididae is characterized by having reduced coxae 1–3, a well-developed lacinia mobilis, outer plate of maxilla 1 with an unmodified 6/5 setal-tooth arrangement, and a deeply cleft telson (Lowry and Stoddart, 1997). Cyphocarididae contains two genera Cyphocaris (Boeck, 1871) and Procyphocaris J.L. (Barnard, 1961). Procyphocaris is a monotypic genus that can be morphologically distinguished from Cyphocaris by the basis of pereopod 7 having smooth, rather than serrate posterior margin, and by having only coxae 1–2 reduced and covered by coxa 3 (Hughes and Lowry, 2015).
The genus Cyphocaris was reviewed by Hughes and Lowry (2015), and updated by Andrade et al. (2021). The genus, currently containing 18 species, includes members that are widely reported from the Pacific, the Atlantic, the Indian Ocean, the Arctic and the Antarctic Oceans. Six Cyphocaris species (C. anonyx, C. bouvieri, C. challengeri, C. faurei, C. Polaris, and C. richardi) have been reported from more than one ocean (Hughes and Lowry, 2015). For instance, C. anonyx (Boeck, 1871) has been reported from the Indian Ocean (Barnard, 1937; Birstein & Vinogradov, 1964; Vinogradov, 2004), the South and North Pacific (e.g. Schellenberg, 1929; Pirlot, 1933; Shoemaker, 1945; Birstein and Vinogradov, 1963; Barnard, 1967; Sanger, 1974; Vinogradov, 1990), the South and North Atlantic (e.g. Schellenberg, 1955; Thurston, 1976; Gislason and Astthorsson, 1992), the Great Australian Bight (Birstein and Vinogradov, 1962), and the Subantarctic (Kane, 1962). The rest of the Cyphocaris species have been reported from just one ocean, namely, two species (C. boecki and C. pedroi) from the South Atlantic (Sorrentino et al., 2016; Andrade et al., 2021); two species (C. geyserensis and C. cornuta) form the Indian Ocean (Ledoyer, 1978; Ledoyer, 1986; Vinogradov, 2004); two species (C. johnsoni and C. tunicola) from the North Atlantic (Shoemaker, 1934; Lowry and Stoddart, 1997); two species(C. latirama and C. ohtsukai) from the North Pacific (Hendrycks and Conlan, 2003; Tomikawa, 2009); and four species (C. ananke, C. bellona, C. nesoi, and C. tartaros) from the South Pacific (Lowry and Stoddart, 1994; Hughes and Lowry, 2015).
During research cruises on board the Chinese research vessel KEXUE to survey the biodiversity of the Formosa Ridge methane seep (Figure 1) in the South China Sea in 2020 and 2021, two individual of Cyphocarididae were collected at different depths by using MultiNet. The two specimens are identified to Cyphocaris on the basis of pereopod 7 having serrate posterior margin, and reduced coxae 1–3 partly covered by coxa 4. These two individuals exhibit some characteristics distinct from other described Cyphocaris species, so they are identified as new species, described morphologically and compared with other similar congeneric species. The provided key is modified from Hughes and Lowry (2015) and Sorrentino et al. (2016).
Material and Methods
Zooplankton samples were taken on board the research vessel KEXUE by MultiNet (Multi Plankton Sampler XL MPS XL, 200-mesh sieve, sampling for about 3 h), during expeditions to the Formosa Ridge methane seep by the Institute of Oceanology, Chinese Academy of Sciences (IOCAS) in May 2020 and June 2021, respectively. Type material of C. lubrica sp. nov. was collected at 1,118 m depth [temperature (4.1°), salinity (34.63‰) and oxygen (4.9 mg/l)], and was sorted on board and fixed in 96% ethanol, then transferred to 75% ethanol in the laboratory; type material of C. formosa sp. nov. was collected at 500–800 m depth [temperature (6–9°), salinity (34.56–34.63‰) and oxygen (5.25–5.15 mg/l)] and was sorted on board and fixed in 10% formalin, then transferred to 75% ethanol in the laboratory. Both specimens were deposited in the Marine Biological Museum (MBM), Chinese Academy of Sciences (CAS), Qingdao, China.
The individuals were examined and dissected with a ZEISS Discovery V20dissecting microscope. Scanning electron microscope (SEM) observation of the distal part of the outer plate and palp of maxilla 1 of C. lubrica sp. nov., was conducted using Hitachi S-3400N SEM at an accelerating voltage of 5 kV. Line drawings were first created from images from the dissecting microscope, and then completed using the software Adobe Photoshop CS6 with a graphics tablet (Wacom PTH-851; Coleman, 2003). Length measurements were made by using a LINKS caliper along the outline of the animal, beginning from the rostrum to the posterior margin of telson.
The following abbreviations were used in Figures 2–6: A, antenna; G, gnathopod; H, head; L, left; LL, lower lip; Md, mandible; Mx1, maxilla 1; Mx2; maxilla 2; Mxp, maxilliped; P, pereopod; R, right; T, telson; U, uropod; UL, upper lip.
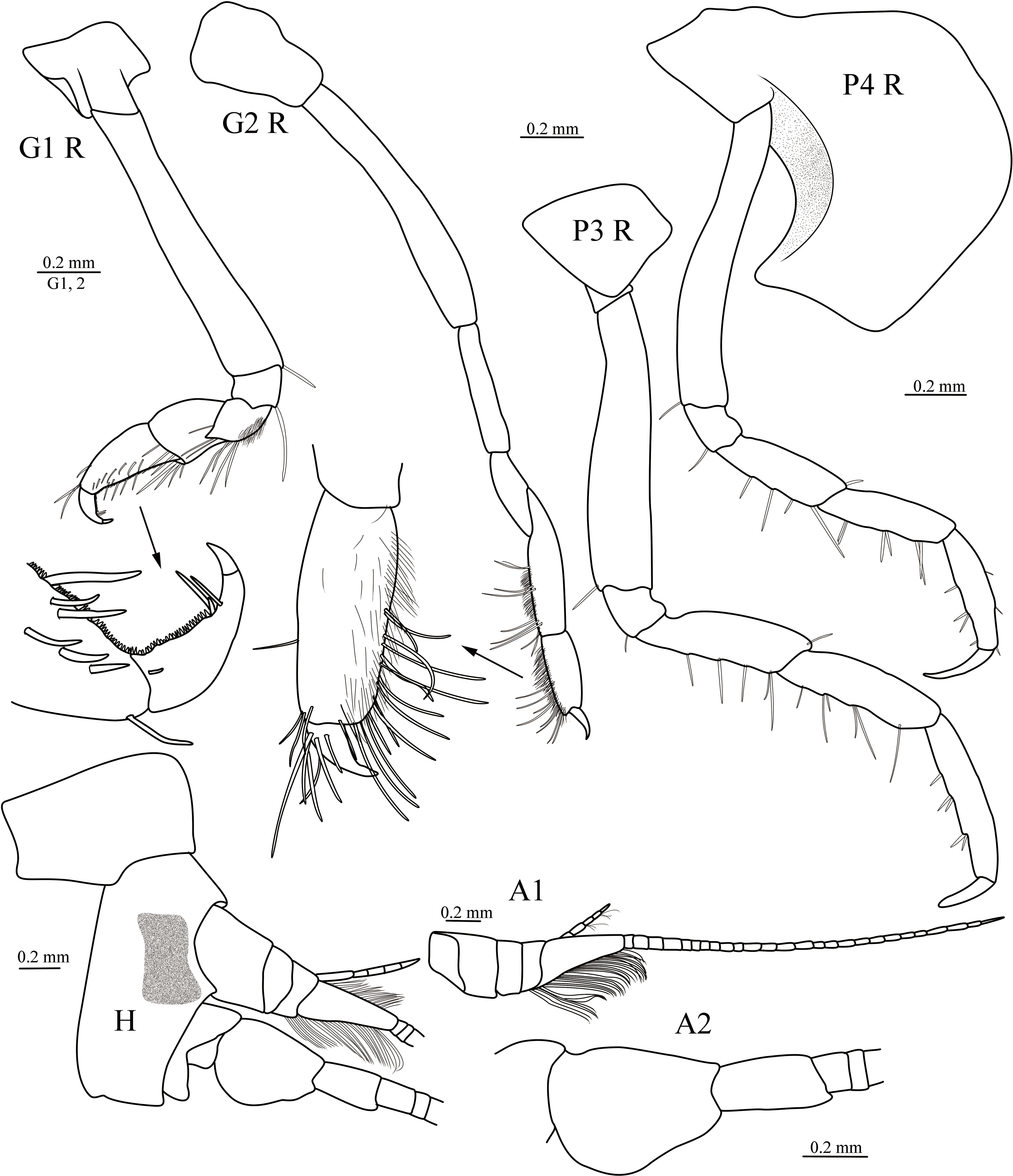
Figure 2 Cyphocaris lubrica sp. nov., MBM 286819, male holotype (9.1 mm), showing head, antenna 1 and 2, gnathopod 1 and 2 and pereopod 3 and 4. For abbreviations, see text.
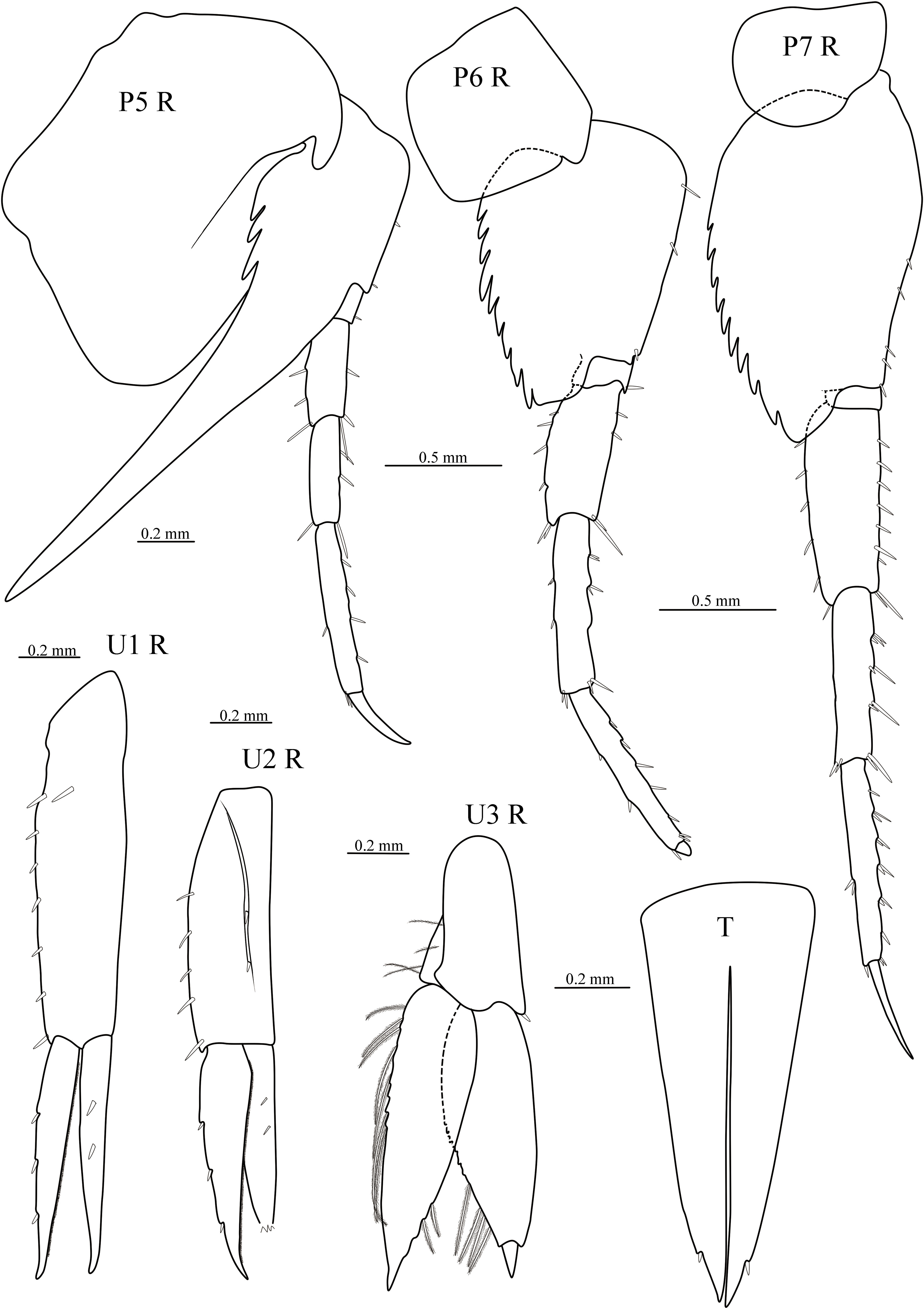
Figure 3 Cyphocaris lubrica sp. nov., MBM 286819, male holotype (9.1 mm), showing pereopod 5–7, uropod 1–3 and telson. For abbreviations, see text.
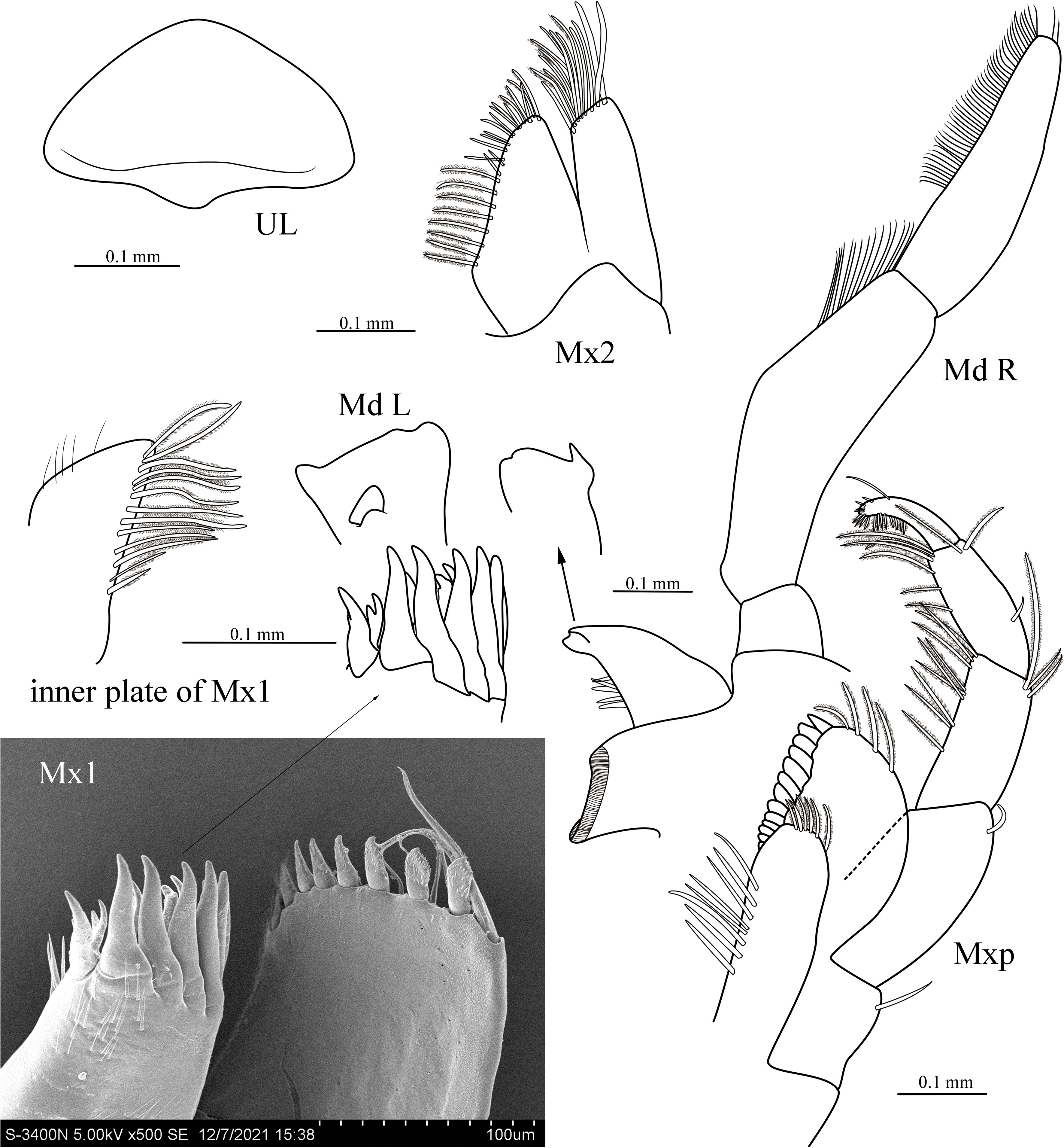
Figure 4 Cyphocaris lubrica sp. nov., MBM 286819, male holotype (9.1 mm), showing mouthparts, SEM picture showing outer plate of distal part of outer plate and palp of maxilla 1. For abbreviations, see text.
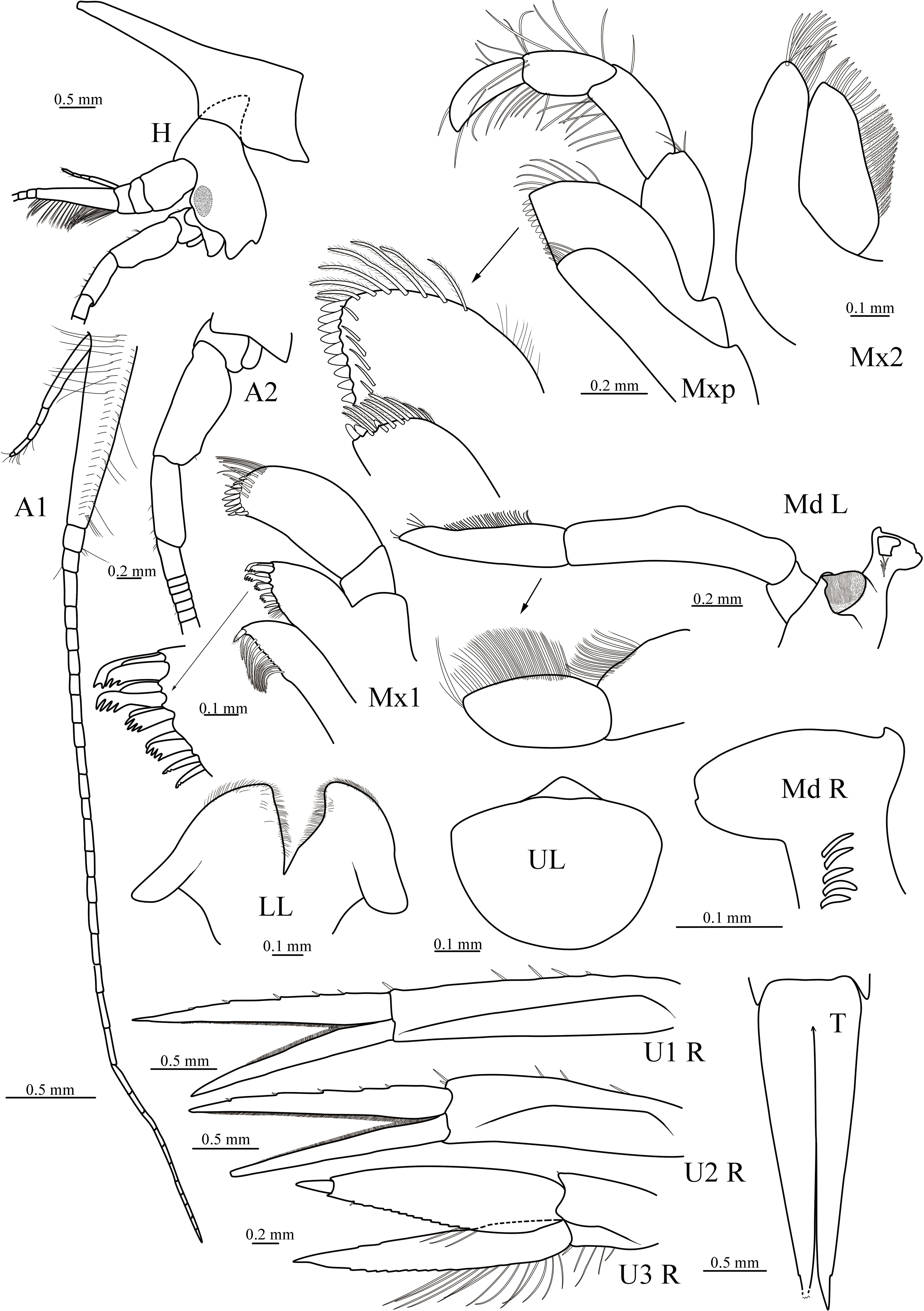
Figure 5 Cyphocaris formosa sp. nov., MBM 286820, male holotype (19 mm), showing head, antenna 1 and 2, mouthparts, uropod 1–3 and telson. For abbreviations, see text.
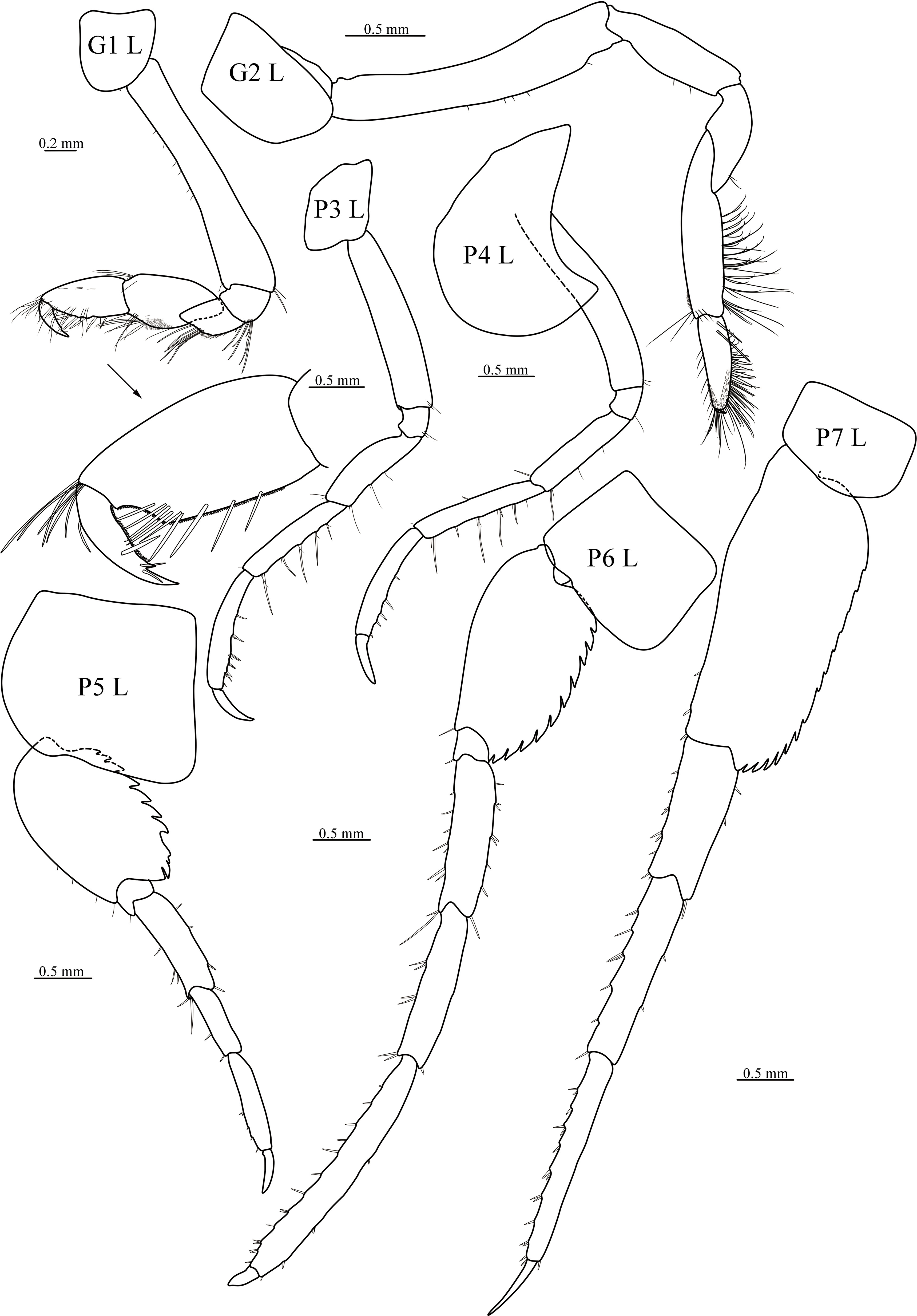
Figure 6 Cyphocaris formosa sp. nov., MBM 286820, male holotype (19 mm), showing gnathopod 1 and 2, pereopod 3–7. For abbreviations, see text.
Results
We described two new species of Cyphocaris, and each new species was described based on one individual. The classification system follows that of Lowry and Myers (2017).
Order Amphipoda (Latreille, 1816).
Suborder Amphilochidea (Boeck, 1871).
Superfamily Lysianassoidea (Dana, 1849).
Family Cyphocarididae (Lowry and Stoddart, 1997).
Genus Cyphocaris (Boeck, 1871).
Diagnosis: Head deeper than long. Flagella of antennae long; first peduncular article of antenna 1 with callynophore, accessory flagellum present. Mouthparts forming quadrate bundle; upper lip and epistome separate; mandible incisor ordinary, molar triturative, palp well setose, attached opposite molar; inner plate of maxilla 1 strongly setose; palp 2-articulate, large; inner and outer plates of maxilliped well-developed, plap strongly exceeding outer plate, dactylus well-developed. Coxae 1–3 small, strongly shortened and partly covered by coxa 4; coxa 4 large and visible, strongly lobate and excavate; coxa 5 usually large. Gnathopod 1 simple, carpus and propodus subequal in length, dactylus large. Propodus of gnathopod 2 slightly to greatly shorter than carpus, often both very elongate and linear, propodus minutely subchelate. Uropod 3 ordinary to elongate, peduncle elongate, aequiramous, outer ramus uni- or 2-articulate. Telson elongate, deeply cleft (Barnard and Karaman, 1991).
Cyphocaris lubrica sp. nov. (Figures 1, 2, 6, 7)
lsid:zoobank.org:act:B7BECCC6-0681-4E32-A36F-75E00CB68311
Type Material: Holotype: MBM 286819, ♂ (9.1 mm), dissected, the Formosa Ridge methane seep, coll. No. TXNLQ044, 22°07′N 119°17′E, depth 1118 m, 19 June 2021, MultiNet, collected by Min Hui and Ziming Yuan.

Figure 7 Cyphocaris lubrica sp. nov., MBM 286819, male holotype (9.1 mm), photographed immediately after sorting from the zooplankton samples by Ziming Yuan.
Description
Holotype, male, 9.1 mm. Body smooth, without setae. Pereonite 1 conspicuously produced anterodorsally, length subequal to basal width. Head narrow than long, eyes present, dumbbell-shaped; rostrum absent; lateral cephalic lobe acute, anteroventral corner produced rounded, ventral margin convex. Antenna 1 much shorter than antenna 2; peduncular article 1 longer than articles 2 and 3 combined; accessory flagellum short, 5-articulate, have tufts of setae, almost reaching to end of primary flagellum first article; primary flagellum article 1 very long, ventrally with well-developed callynophore in two fields, calceoli absent. Antenna 2 slightly shorter than body, peduncular article 4 strongly broadened, nearly as long as body, flagellum calceoli absent.
Epistome and upper lip separate, unequally produced. Mandible incisor smooth, toothed laterally; right lacinia mobilis absent, left one smooth, blade-like; right accessory setal with 5 setae; molar well-developed, triturative; palp 3-articulate, article 1 shortest, article 2 longer than article 3, posterior margin nearly straight, anterior margin with medial bulge, with dense row of submarginal setae distally, article 3 anterior margin with dense row of setae. Maxilla 1 outer plate with 11 setal teeth; inner plate tapering distally, inner margin with 13 plumose setae; palp with 8 robust dentate setae and 5 slender plumose setae. Maxilla 2 inner plate broad, triangular, with marginal plumose setae and slender setae; outer plate with apical setae. Maxilliped inner plate rectangular, with 3 nodular apical robust setae, distal margin lined with 6 plumose setae; outer plate subovate, apicolateral margin with long setae, medial margin with 13 nodular robust setae; palp 4-articulate, article 2 subequal in length to article 1, mesial margin with row of plumose setae, distal of lateral margin with three plumose setae; dactylus slightly shorter than article 3, mesial margin with short dense plumose setae.
Gills present on Gnathopod 2 to pereopod 7, gill on pereopod 7 not reduced.
Gnathopod 1 weakly subchelate; coxa small; basis long, slightly widened distally; merus posteriorly produced, acute, posterior surface with short setae; carpus length 0.6 × propodus; propodus with palm finely serrate; dactylus posterior margin serrated, with one and a pair distal robust setae, nail present. Gnathopod 2 minutely subchelate; coxa small, deeper than long; basis elongate, margins naked; ischium elongate, margins naked; merus length 0.7 × ischium subtriangular; carpus length 1.1 × merus, anterior margin setose; propodus length 0.8 × carpus, subrectangular, posterior and distal margins with slender setae, palm reduced, palmer corner not defines by stout seta; dactylus small, curved, reaching to palmar corner. Pereopod 3 slender; coxa subquadrate; basis elongate, posterodistal corner with one long seta; ischium short; merus and carpus subequal in length, posterior margins with long setae; propodus subequal in length to carpus, posterior margin with one and two pairs of robust setae; dactylus slightly curved, apical rounded. Pereopod 4 slender, coxa large, expanded and round anteriorly, posteroventral corner forming subacute projection, partially overlapping coxae 2–3; basis elongate, posterodistal corner with one long seta; ischium to propodus similar to that of pereopod 3, but propodus subequal in length to carpus; dactylus slender, longer than 1/2 length of propodus. Pereopod 5–7 increasing in length. Pereopod 5 coxa large, subrectangular, anterodistal corner forming an elongate subacute projection, posteroventral corner expanded and rounded; basis anterior margin broadly rounded, with two small setae, posterior margin with large subacute spur, straight, beyond pereonite 7, ventral margin smooth, dorsal margin proximal with three teeth; ischium short, anterior margin with one seta; merus and carpus subequal in length, anterior and posterior margins with robust setae; propodus elongate; dactylus slightly curved, nearly 1/2 length of propodus. Pereopod 6 coxa small, subquadrate; basis suboval, anterior margin with several small setae, posterior margin expanded, serrated, posteroventral lobe truncatus; merus slightly expanded posteriorly; carpus longer than merus, posterior and anterior margins with small setae; propodus slender; dactylus small, only 1/10 length of propodus, rounded. Pereopod 7 coxa small, ventral margin convex; basis suboval, anterior margin with several small robust setae, posterior margin serrated, posteroventral lobe subacute; merus and carpus subequal in length, merus slightly expanded posteriorly, anterior and posterior margins with robust setae; propodus slender, anterior margin with several pairs of robust setae; dactylus slender, nearly straight, longer than 1/2 length of propodus.
Uropod 1 elongate, peduncle much longer than rami, with 8 marginal robust setae; rami subequal in length margins with robust setae. Uropod 2 peduncle slightly longer than outer ramus, with 7 marginal robust setae; inner ramus broken, margins with robust setae. Uropod 3 peduncle short, marginal with 4 short plumose setae, ventrodistally with one robust seta; outer ramus slightly longer than inner ramus, outer margin with several long plumose setae, inner margin with two short plumose setae; inner ramus 2-articulate, inner margin with four short plumose setae. Telson elongate, deeply cleft to 87%, lobes tapering distally, each with one subapical robust seta.
Etymology
From the Latin lubric (=smooth), referring to the spur of pereopod 5 having smooth ventral margin and dorsal margin only having three teeth at base.
Distribution
Northwest Pacific, South China Sea, the Formosa Ridge methane seep at a depth of 1118 m.
Remarks
Cyphocaris lubrica sp. nov. is most similar to C. bouvieri, but can be easily distinguished from the latter by pereonite 1 having well-developed dorsal projection, which is absent in C. bouvieri (Gurjanova, 1962, Figure 7; Hughes and Lowry, 2015, Figure 9); the spur of pereopod 5 beyond pereonite 7, and dorsal margin of it having only three large teeth, while the dorsal margin of spur having more than 7 teeth in C. bouvieri (Gurjanova, 1962, Figure 7; Tencati and Geiger, 1968, Figure 4; Hughes and Lowry, 2015, Figure 12); eyes absent in C. bouvieri (Gurjanova, 1962, Figure 7; Hughes and Lowry, 2015, Figure 9).
C. lubrica sp. nov. is similar to five species: C. ananke (Hughes and Lowry, 2015), C. anonyx (Boeck, 1871), C. bouvieri (Chevreux, 1916), C. challengeri (Stebbing, 1888), C. faurei (Barnard, 1916) and C. pedroi (Sorrentino et al., 2016) for the spur of pereopod 5 not beyond pleonite 1. The new species differs from C. challengeri, C. faurei and C. pedroi by the produced pereonite 1 (Stebbing, 1888; Hughes and Lowry, 2015; Sorrentino et al., 2016); differs from C. ananke and C. anonyx the posterior margin of basis of pereopod 5 not completely serrate, and the spur of pereopod reaching to middle length of pleonite (Hughes and Lowry, 2015).
Cyphocaris formosa sp. nov. (Figures 3, 4, 8)

Figure 8 Cyphocaris formosa sp. nov., MBM 286820, male holotype (19 mm), photographed immediately after sorting from the zooplankton samples by Ziming Yuan.
lsid:zoobank.org:act:337E3D54-C517-446A-9811-0FD4E05C9F0C
Type material: Holotype: MBM 286820, ♂ (19 mm), dissected, the Formosa Ridge methane seep, coll. No. CS051, 22°07′N 119°17′E, depth 500–800 m, 17 May 2020, MultiNet, collected by Min Hui and Ziming Yuan.
Description
Holotype, male, 19 mm. Body smooth, without setae. Pereonite 1 conspicuously produced anterodorsally. Head narrow, much deeper than long, eyes present, subovate; rostrum absent; lateral cephalic lobe rounded, anteroventral corner produced acute, ventral margin convex. Antenna 1 much shorter than antenna 2; peduncular article 1 longer than articles 2 and 3 combined; accessory flagellum short, 5-articulate, having tufts of setae, not reaching end of primary flagellum first article; primary flagellum article 1 very long, ventrally with well-developed callynophore in two fields, calceoli absent. Antenna 2 length slightly longer than body, peduncular article 4 not strongly broadened; flagellum calceoli absent.
Epistome and upper lip separate, unequally produced. Mandible incisor smooth, toothed laterally; right lacinia mobilis absent, left one smooth, boot-shaped; accessory setal row with 4 (left) and 5 (right) setae; molar well-developed, triturative; palp 3-articulate, article 1 much shorter than 2 and 3, article 2 posterior margin nearly straight, anterior margin with medial bulge, with dense row of submarginal setae distally, article 3 shorter than 2, anterior margin with dense row of setae. Maxilla 1 inner plate triangular, tapering distally, inner margin lined with 1 robust plumose seta and 11 slender plumose setae; outer plate with 11 setal teeth; palp 2-articulate, with 9 robust setae and with 9 slender setae in two rows. Maxilla 2 inner plate triangular, lined with 9 slender setae and 21 slender plumose setae; outer plate with 13 apical setae. Maxilliped inner rectangular, with 3 nodular apical robust setae, distal margin lined with 9 plumose setae; outer plate subovate, apicolateral margin with long plumose setae, medial margin with 9 nodular robust setae and row of submarginal slender setae; palp 4-articulate, article 1 longest, article 2 longer than article 3, inner margin with row of plumose setae, article 2 margins with dense plumose, dactylus subequal to article 3, margins have plumose.
Gnathopod 1 subchelate; coxa small, ventrally rounded; basis long, slightly widened distally; merus posteriorly produced, acute, posterior surface with short setae; carpus length 1.2 × propodus; propodus with palm finely serrate, defined by weak corner; dactylus curved, posterior margin finely serrate, with 3 subapical slender setae, with nail and subterminal spine. Gnathopod 2 minutely subchelate; coxa tapering ventrally; basis with triangular lobe anterodistally; ischium elongate; merus length 0.8 × ischium, subtriangular; carpus twice longer than propodus, posterior margin lined with long setae, surface without short setae; propodus with palm smooth; dactylus small. Pereopod 3 slender; coxa subquadrate; basis straight; merus to propodus gradually increasing in length; dactylus shorter than 1/2 length of propodus. Pereopod 4 coxa anterior margin rounded, posteroventral lobe produced acute. Pereopod 5 shorter than pereopods 6 and 7; coxa subquadrate; basis posterior margin not produced, serrate, posterodistal lobe not much beyond distal margin of ischium; merus distinctly longer than carpus; propodus subequal in length to merus; dactylus length 0.45 × propodus. Pereopod 6 distinctly shorter than pereopod 7; coxa subquadrate; basis posterior margin serrate, posterodistal lobe not beyond distal margin of ischium; merus and carpus subequal in length, and much shorter than propodus; dactylus recurved. Pereopod 7 coxa small, subquadrate; basis subrectangular, posterior margin nearly straight, serrate, posterodistal lobe beyond distal margin of ischium, corner serrate; merus shorter than carpus; propodus longer than carpus; dactylus slender, straight.
Uropod 1 elongate, peduncle longer than inner ramus, with 5 marginal robust setae; outer ramus shorter than inner one. Uropod 2 reaching end of uropod 3; peduncle shorter than inner ramus; outer ramus shorter than inner one. Uropod 3 slightly beyond posterior margin of telson; peduncle much shorter than rami; rami subequal in length; outer ramus 2-articulate. Telson elongate, deeply cleft to 87%, lobes tapering distally, distal of right lobe broken.
Etymology
The species is named after the type locality “the Formosa Ridge methane seep”.
Distribution
Northwest Pacific, South China Sea, the Formosa Ridge methane seep at a depth of 500–800 m.
Remarks
Cyphocaris formosa sp. nov. is most similar to C. nesoi (Hughes and Lowry, 2015) and C. richardi (Chevreux, 1905) for having the peduncular article 4 of antenna 2 not strongly broadened; the pereonite 1 having slender dorsal projection extending well beyond bead and the basis of pereopod 5 without spur. The new species differs from C. nesoi by the dactylus of gnathopod 1 having subterminal spine; the accessory flagellum 5-articulate; only outer ramus of uropod 3 2-articulate, but both rami having two articles in C. nesoi (Hughes and Lowry, 2015, Figure 19); lateral margin of telson without setae. With the limited original illustration of C. richardi, C. formosa sp. nov. differs from it by the posterior margin of basis of pereopod 5 not strongly serrate, and not beyond distal margin of ischium, while the posterodistal corner of basis far beyond distal margin of ischium (Chevreux, 1905, Figure 2); the shape of dorsal projection of pereonite 1 columned, not triangular (Chevreux, 1905, Figure 1; Birstein and Vinogradov, 1955, Figure 2).
Key to World Cyphocarididae Species
1. Pereopod 7 basis posterior margin smooth; coxae 1–2 reduced, covered by coxa 3 Procyphocaris indurata (Barnard, 1925).
–Pereopod 7 basis posterior margin serrate; coxae 1–3 all small, partly covered by coxa 4 2 (Cyphocaris).
2. Pereopod 5 basis posterior margin with spur 3
–Pereopod 5 basis posterior margin without spur 16
3. Pereopod 5 basis posterior margin spur extremely elongate, reaching beyond pleonite 1 4
–Pereopod 5 basis posterior margin spur not reaching beyond pleonite 1 10
4. Pereopod 5 basis posterior margin spur ventral margin with serrations 5
–Pereopod 5 basis posterior margin spur ventral margin smooth, without serration 8
5. Pereopod 5 basis posterior margin spur ventral margin with single serration 6
–Pereopod 5 basis posterior margin spur ventral margin with multiple serrations 7
6. Pereonite 1 dorsal projection short, not extending beyond head C. geyserensis (Ledoyer, 1986).
–Pereonite 1 dorsal projection long, extending well beyond head C. tartaros (Hughes and Lowry, 2015).
7. Gnathopod 2 coxa sub-triangular; epimeron 2 posteroventral corner quadrate; telson almost reaching posterior end of uropod 3 C. ohtsukai (Tomikawa, 2009).
–Gnathopod 2 coxa sub-quadrate; epimeron 2 posteroventral corner rounded; telson not reaching mid-length of inner ramus of uropod 3 C. cornuta (Ledoyer, 1978).
8. Head eye not greatly enlarged, length twice as long as wide; telson reaching posterior end of uropod 3. C. bellona (Lowry and Stoddart, 1994).
–Head eye greatly enlarged, length at least 3 times longer than wide; telson exceeding posterior end of uropod 3 9
9. Pereonite 1 forming long, narrow, slightly up-turned process in male, not developed in female. Telson 1.5 × as long as uropod 3 C. johnsoni (Shoemaker, 1934).
–Pereonite 1 forming long, narrow, slightly down-turned process in both sexes. Telson 1.8 × as long as uropod 3 C. tunicola (Lowry and Stoddart, 1997).
10. Pereopod 5 basis posterior margin spur margins serrate 11
–Pereopod 5 basis posterior margin spur margins smooth 12
11. Pereopod 4 coxa anterior margin is subacute; telson short not reaching the end of uropod 3 C. anonyx (Boeck, 1871).
–Pereopod 4 coxa anterior margin is broadly rounded; telson long reaching end of uropod 3 C. ananke (Hughes and Lowry, 2015).
12. Pereopod 5 basis proximal posterior margin smooth 13
–Pereopod 5 basis proximal posterior margin serrate 14
13. Gnathopod 1 merus with subtrapezoid serrated structure on the posterodistal corner; pereopod 5 coxa well-developed lobe on anterodistal margin, broadly rounded and apically reverted backwards C. pedroi (Sorrentino et al., 2016).
–Gnathopod 1 merus anterodistal margin tapered; pereopod 5 coxa, anterodistal margin expanded posteriorly, rounded C. faurei (Barnard, 1916).
14. Pereopod 5 basis posterior margin spur exceeding length of merus 15
–Pereopod 5 basis posterior margin spur not exceeding merus C. challengeri (Stebbing, 1888).
15. Eyes absent; pereonite 1 dorsal projection absent; spur of pereopod 5 not beyond pereonite 7 C. bouvieri (Chevreux, 1916).
–Eyes present; pereonite 1 dorsal projection present; spur of pereopod 5 beyond pereonite 7 C. lubrica sp. nov.
16. Pereonite 1 dorsal projection extending well beyond head 17
–Pereonite 1 dorsal projection absent or extending slightly beyond head 19
17. Pereopod 5 basis posterior margin weakly serrate (or strongly in male), posterodistal corner rounded 18
–Pereopod 5 basis posterior margin strongly serrate, posterodistal corner subacute C. richardi (Chevreux, 1905).
18. Accessory flagellum 7-articulate; dactylus of gnathopod 1 without subterminal spine; inner ramus of uropod 3 2-articulate C. nesoi (Hughes and Lowry, 2015).
–Accessory flagellum 5-articulate; dactylus of gnathopod 1 with subterminal spine; inner ramus of uropod 3 uniarticulate C. formosa sp. nov.
19. Head lateral cephalic lobe concave C. latirama (Hendrycks and Conlan, 2003).
–Head lateral cephalic lobe subacute C. polaris (Gurjanova, 1951).
Discussion
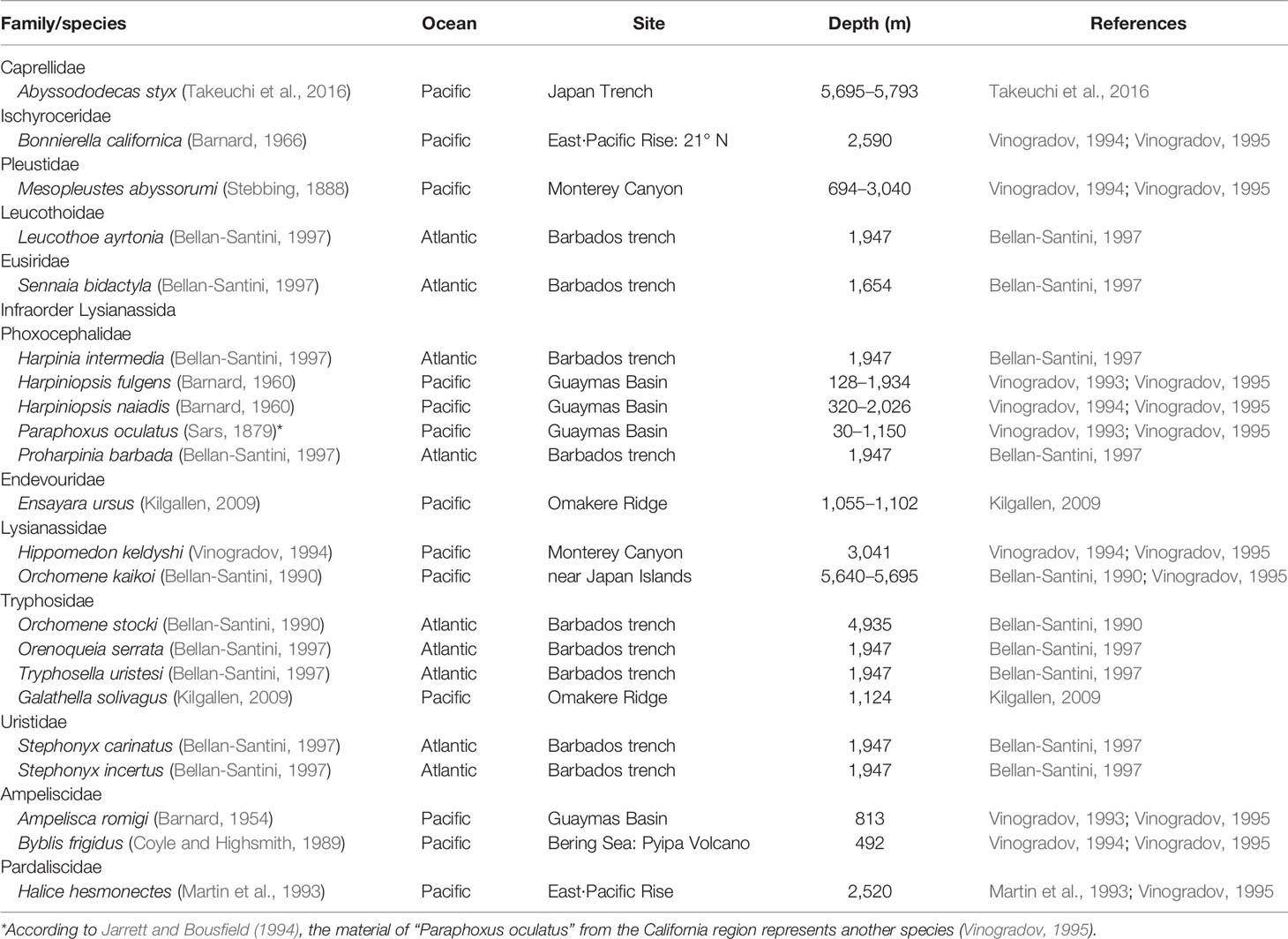
The amphipod fauna described from methane seeps comprises, up to now, 22 species spread over 12 families (Table 1). Moreover, 23 species were reported from oil and methane seep at a large freshwater lake, Lake Baikal (Mekhanikova and Sitnikova, 2014). The present two new species were collected by MultiNet, which means they are pelagic amphipods. Among all species reported from methane seeps (Table 1), Harpiniopsis fulgens (Barnard, 1960), Harpiniopsis naiadis (Barnard, 1960), Paraphoxus oculatus (Sars, 1879) and Ampelisca romigi (Barnard, 1954) are also pelagic amphipods and found in trawls (Vinogradov, 1995).
Excepting for the present two new species, only two Cyphocaris species have been reported from NW Pacific: C. polaris and C. ohtsukai. C. lubrica sp. nov. can be distinguished from C. polaris by the presence of spur on pereopod 5, and from C. ohtsukai by dorsal projection of pereonite 1 not exceeding beyond head. C. formosa sp. nov. can be distinguished from C. polaris by the presence of acute dorsal projection of pereonite 1 exceedingly well beyond head, and from C. ohtsukai by the absence of spur of pereopod 5.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: ZooBank [urn:lsid:zoobank.org:pub:F5856D5A-4A03-4E80-9A16-08B74516D507].
Author Contributions
Data curation: YW. Funding acquisition: ZS. Illustrations: YW. Writing original draft: YW. Writing review & editing: YW, ZS, XR and XR. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the National Science Foundation for Distinguished Young Scholars (42025603), Key Research Program of Frontier Sciences, CAS (QYZDBSSW-DQC036), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA22050302; XDB42000000), the National Key R&D Program of China (2018YFC0310802), and the Special Research Assistant Project of Chinese Academy of Sciences (E2KY031).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to express our sincere thanks to the crews of KEXUE for their assistance during the survey. We are very grateful to Dr. Min Hui and Ziming Yuan for collecting the specimens. Many thanks to Dr. Charles Oliver Coleman from the Museum für Naturkunde—Leibniz Institute for Research on Evolution and Biodiversity, Berlin, Germany, for providing some references.
References
Andrade L. F., Alves-Júnior F. A., Bertrand A., Senna A. R. (2021). A New Species of Cyphocaris Boec(Amphipoda: Lysianassoidea: Cyphocarididae): Found Off the Rocas Atoll, Northeastern Brazil. Taxonomy 1 (4), 360–373. doi: 10.3390/taxonomy1040027
Baba K., Williams A. B. (1998). New Galatheoidea (Crustacea, Decapoda, Anomura) From Hydrothermal Systems in the West Pacific Ocean Bismarck Archipelago and Okinawa Trough. Zoosystema 20, 143–156.
Barnard J. L. (1961). Gammaridean Amphipoda from Depths of 400-6000 Meters. Galathea Report 5, 23–128.
Barnard K. H. (1916). Contributions to the Crustacean Fauna of South Africa n° 5. The Amphipoda. Ann. S. Afr. Mus. 15 (3), 105–302.
Barnard K. H. (1925). Contributions to the Crustacean Fauna of South Africa n° 8. Further Additions to the List of Amphipoda. Ann. S. Afr. Mus. 20 (5), 319–380.
Barnard J. L. (1954). Amphipoda of the Family Ampeliscidae Collected in the Eastern Pacific Ocean by the Velero III an Velero IV. Allan Hancock Pacific Expeditions 18 (1), 1–137.
Barnard J. L. (1960). The Amphipod Family Phoxocephalidae in the Eastern Pacific Ocean With Analyses of Other Species and Notes for a Revision of the Family. Allan Hancock Pacific Expeditions 18 (3), 175–368.
Barnard J. L. (1966). Submarine canyons of southern California. Part V. Systematics: Amphipoda. Allan Hancock Pacific Expeditions 27 (5), 1–166.
Barnard J. L. (1967). Bathyal and Abyssal Gammaridean Amphipoda of Cedros Trench, Baja California. US Natl. Mus. Bull. 260, 1–205. doi: 10.5479/si.03629236.260.1
Barnard J. L., Karaman G. S. (1991). The Families and Genera of Marine Gammaridean Amphipoda (Except Marine Gammaroids). Rec. Aust. Mus. 13 (2), 1–866.
Boeck A. (1871). Crustacea Amphipoda Borealia et Arctica. Forhandlinger fra Videnskabernes Selskab Christiania 1871, 83–280. doi: 10.5962/bhl.title.2056
Bellan-Santini D. (1990). Nouvelles Espèces D’Orchomene s.L. (Crustacea - Amphipoda) Des Fonds Abyssaux. Affinités Avec Les Autres Orchomene Profonds. Beaufortia 41 (3), 15–23.
Bellan-Santini D. (1997). Amphipods of the Cold Seep Community on the South Barbados Accretionary Prism. Crustaceana 70 (1), 1–30. doi: 10.1163/156854097X00311
Birstein J. A., Vinogradov M. E. (1955). [Pelagic Gammarideans (Amphipoda-Gammaridea) From the Kurile-Kamchatka Trench]. Akademya Nauk SSSR Trudy Inst. Okean 12, 210–287.
Birstein J. A., Vinogradov M. E. (1962). [Pelagic Gammaridea (Amphipoda, Gammaridea) Collected by the Soviet Antarctic Expedition on the M/V “Ob”, South of 40°s]. Akademya Nauk SSSR Issled. Fauny Morei 1, 33–56.
Birstein J. A., Vinogradov M. E. (1963). [The Deep Sea Pelagic Amphipods of the Philippine Trench]. Akademya Nauk SSSR Trudy Inst. Okean 71, 81–93.
Birstein J. A., Vinogradov M. E. (1964). [Pelagic Gammarid Amphipods of the Northern Part of the Indian Ocean]. Akademya Nauk SSSR Trudy Inst. Okean 65, 152–195.
Coyle K. O., Highsmith R. C. (1989). Arctic Ampeliscid Amphipods: Three New Species. J. Crustacean Biol. 9 (1), 157–175. doi: 10.1163/193724089X00296
Chevreux E. (1905). Description D’un Amphipode (Cyphocaris Richardi Nov. Sp.) Provenant Des Pêches Au Filet À Grande Ouverture De La Dernière Campagne Du Yacht Princesse-Alic). Bull. Mus. Océanogr. Monaco 24, 1–5.
Chevreux E. (1916). Sur les amphipodes du genre Cyphocaris Boeck recueillis par la “Princesse Alice” au moyen du filet Richard à grande ouverture. Bull. Mus. Océanogr. Monaco 319, 1–6.
Coleman C. O. (2003). “ Digital Inking”: How to Make Perfect Line Drawings on Computers. Org. Divers. Evol. 3 (4), 303. doi: 10.1078/1439-6092-00081
Feng D., Chen D. (2015). Authigenic Carbonates From an Active Cold Seep of the Northern South China Sea: New Insights Into Fluid Sources and Past Seepage Activity. Deep-Sea Res. Part II, 122, 74–83. doi: 10.1016/j.dsr2.2015.02.003
Feng D., Qiu J. W., Hu Y., Peckmann J., Guan H., Tong H., et al. (2018). Cold Seep Systems in the South China Sea: An Overview. J. Asian Earth Sci., 168, 3–16. doi: 10.1016/j.jseaes.2018.09.021
Gislason A., Astthorsson O. S. (1992). Zooplankton Collected by Sediment Trap Moored in Deep Water South of Iceland. Sarsia 77, 219–224. doi: 10.1080/00364827.1992.10413507
Gurjanova E. F. (1951). Bokoplavy morei SSSR i sopredelnykh vod (Amphipoda,Gammaridea). [Amphipods of the seas of USSR and adjacent waters (Amphipoda, Gammaridea)]. Opredeliteli po faune SSSR, Akademiya Nauk SSSR 41, 1–1029.
Gurjanova E. F. (1962). [Amphipods of the Northern Part of the Pacific Ocean (Amphipoda-Gammaridea). Part 1]. Akademya Nauk SSSR Opredeliteli po Faune SSSR 74, 1–440.
Hashimoto J., Okutani T. (1994). Four New Mytilid Mussels Associated With Deepsea Chemosynthetic Communities Around Japan. Venus 53, 61–83.
Hendrycks E. A., Conlan K. E. (2003). New and Unusual Abyssal Gammaridean Amphipoda From the North-East Pacific. J. Nat. Hist. 37, 2303–2368. doi: 10.1080/00222930210138926
Hughes L. E., Lowry J. K. (2015). A Review of the World Cyphocarididae With Description of Three New Species (Crustacea, Amphipoda, Lysianassoidea). Zootaxa 4058 (1), 1–40. doi: 10.11646/zootaxa.4058.1.1
Jarrett N. E., Bousfield E. L. (1994). The Amphipod Superfamily Phoxocephalidea on the Pacific Coast of North America. Family Phoxocephalidae. Part H. Subfamilies Pontharpiniidae, Parbarpiniinae, Brolginae, Phoxocephalinae and Harpiniinae. Systematics and Distributional Ecology. Amphipacifica 1, 71–150.
Kilgallen N. M. (2009). New Species of Lysianassoid Amphipoda (Crustacea) Associated With Seamounts, Marine Canyons and Cold Seeps of New Zealand. Zootaxa 2298, 1–30. doi: 10.11646/zootaxa.2298.1.1
Kuo M. Y., Kang D. R., Chang C. H., Chao C. H., Wang C. C., Chen H. H., et al. (2019). New Records of Three Deep-Sea Bathymodiolus Mussels (Bivalvia: Mytilida: Mytilidae) From Hydrothermal Vent and Cold Seeps in Taiwan. J. Mar. Sci. Technol. 27 (4), 6. doi: 10.6119/JMST.201908_27(4).0006
Latreille P. A. (1816). Amphipoda. Nouveau Dictionaire d’Histoire naturelle, appliquée aux Arts, à l’Agriculture, à l’Économie rurale et domestique, à la Médecine, etc. Par une société de Naturalistes et d’Agriculteurs (2nd ed.) Tome 1. Deterville, Paris, 467–469. doi: 10.5962/bhl.title.20211
Ledoyer M. (1978). Contribution À L’étude Des Amphipodes Gammariens Profonds De Madagascar (Crustacea). Téthys 8, 365–382.
Ledoyer M. (1986). Crustacés Amphipodes Gammariens. Familles Des Haustoriidae À Vitjazianidae. Faune Madag. 59, 599–1112.
Li X. (2017). Taxonomic Research on Deep-Sea Macrofauna in the South China Sea Using the Chinese Deep-Sea Submersible Jiaolong. Integr. Zool. 12, 270–282. doi: 10.1111/1749-4877.12254
Lin S., Lim Y. C., Liu C. S., Yang T. F., Chen Y. G., Machiyama H., et al. (2007a). Formosa Ridge, a Cold Seep With Densely Populated Chemosynthetic Community in the Passive Margin, Southwest of Taiwan. Geochim. Cosmochim. Acta 71 (Supplement 1), A582.
Lin S., Machiyama H., Chen Y. G., Soh W., Yang T. F., Wang Y., et al. (2007b). Near Sea Floor Gas Hydrate Formation and Influence on Pore Water Chemistry and Authigenic Carbonate at the Formosa Ridge, South China Sea. EOS Trans. AGU 88 (52), OS21A–OS205.
Lowry J., Myers A. (2017). A Phylogeny and Classification of the Amphipoda With the Establishment of the New Order Ingolfiellida (Crustacea: Peracarida). Zootaxa 4265 (1), 1–89. doi: 10.11646/zootaxa.4265.1.1
Lowry J. K., Stoddart H. E. (1994). Crustacea Amphipoda: Lysianassoids From the Tropical Western South Pacific Ocean. Mém. Mus. Nutt. Hist. Nut 161, 127–223.
Lowry J. K., Stoddart H. E. (1997). Amphipoda Crustacea IV. Families Aristiidae, Cyphocarididae, Endevouridae, Lysianassidae, Scopelocheiridae, Uristidae. Mem. Hourglass Cruises 10 (1), 1–148.
Machiyama H., Lin S., Fujikura K., Huang C. Y., Ku C. Y., Lin L. H., et al. (2007). Discovery of “Hydrothermal” Chemosynthetic Community in a Cold Seep Environment, Formosa Ridge: Seafloor Observation Results From First ROV Cruise, Off Southwestern Taiwan. EOS Trans. AGU 88 (52), OS23A–O1041.
Martin J. W., France S. C., Van Dover C. L. (1993). Halice Hesmonectes, a New Species of Pardaliscid Amphipod (Crustacea, Peracarida) From Hydrothermal Vents in the Eastern Pacific. Can. J. Zool. 71, 1724–1732. doi: 10.1139/z93-244
Mekhanikova I. V., Sitnikova T. Y. (2014). Amphipods (Amphipoda, Gammaridea) at the Gorevoy Utes Oil and Methane Seep, Lake Baikal. Crustaceana 87 (13), 1500–1520. doi: 10.1163/15685403-00003367
Pirlot J. M. (1933). Les Amphipodes De L’expédition Du Siboga. Deuxième Partie: Les Amphipodes Gammarides, II: - Les Amphipodes De La Mer Profonde. 1. (Lysianassidae, Stegocephalidae, Stenothoidae, Pleustidae, Lepechinellidae). Siboga-Expeditie Monogr. 33c, 114–167.
Sanger G. A. (1974). Pelagic Amphipod Crustaceans From the Southeastern Bering Sea, June 1971. NOAA Tech. Rep. NMFS SSRF-580, iii and 1–8.
Sars G. O. (1879). Crustacea et Pycnogonida nova in itinere 2do et 3tio expeditionis Norvegicae anno 1877 & 78 collecta (prodromus descriptionis). Arch. Math. Naturvidensk 4, 427–476.
Shoemaker C. R. (1934). Reports on the Collections Obtained by the First Johnson-Smithsonian Deep-Sea Expedition to the Puerto Rican Deep. Three New Amphipods. Smithson. Misc. Collect. 91, 1–6.
Shoemaker C. R. (1945). The Amphipoda of the Bermuda Oceanographic Expedition–1931. Zoologica 30, 185–266.
Sorrentino R., Alves J., Johnsson R., Senna A. R. (2016). A New Species of Cyphocarididae (Crustacea, Amphipoda, Lysianassoidea) From Off the Northeastern Brazilian Coast. Zootaxa 4161, 3, 345–356. doi: 10.11646/zootaxa.4161.3.3
Stebbing T. R. R. (1888). Report on the Amphipoda Collected by H.M.S. Challenger During the Years 1873-1876. Rep. Sci. Voyage H.M.S. Challenger Zool. 29, 1–1737.
Takeuchi I., Lindsay D., Tomikawa K. (2016). A New Genus and Species of Phtisicidae (Crustacea: Amphipoda) From Abyssal Depths in the Japan Trench, With Special Reference to Similarities With Southern Ocean Genera. J. Crust. Biol. 36 (4), 495–506. doi: 10.1163/1937240x-00002457
Tencati J. R., Geiger S. R. (1968). Pelagic Amphipods of the Slope Waters of Northeast Greenland. J. Fish. Res. Bd. Canada 25, 1637–1650. doi: 10.1139/f68-147
Thurston M. H. (1976). The Vertical Distribution and Diurnal Migration of the Crustacea Amphipoda Collected During the SOND Cruise I. J. Mar. Biol. Ass. U.K. 56, 143–159. doi: 10.1017/S002531540001897X
Tomikawa K. (2009). A New Species of the Genus Cyphocaris (Crustacea: Amphipoda: Cyphocarididae) From Japan. Bull. Natl. Mus. Nat. Sci. Ser. A Suppl. 3, 37–46.
Vinogradov G. M. (1990). [Pelagic Amphipods (Amphipoda, Crustacea) From the South-Eastern Pacific]. Akademya Nauk SSSR Trudy Inst. Okean 124, 27–104.
Vinogradov G. M. (1993). Amfipody (Crustacea) s “Černych Kuril’ščikov” Iz Rajonov Gidrotermal’noj Aktivnosti Vostočnoj Časti Tichogo Okeana [Amphipods (Crustacea) From the “Black Smokers” From Hydrothermal Vents Areas of the Eastern Pacific]. Zool. Zh 72 (2), 40–53.
Vinogradov G. M. (1994). Amphipods From the Seeps Fields and Nearby Benthic Communities From Hydrothermal-Active Areas of the Northern and Eastern Pacific Ocean. Trudy Inst. Okean 131, 100–125.
Vinogradov G. M. (1995). Colonization of Pelagic and Hydrothermal Vent Habitats BY Gammaridean Amphipods: An Attempt of Reconstruction. Pol. Arch. Hydrobiol. 42 (4), 417–430.
Vinogradov G. M. (2004). Near-Bottom and Pelagic Gammaridean Amphipods in the Western Indian Ocean. Ann. S. Afr. Mus. 112, 39–88.
Xu T., Wang Y., Sun J., Chen C., Watanabe H. K., Chen J., et al. (2022). Hidden Historical Habitat-Linked Population Divergence and Contemporary Gene Flow of a Deep-Sea Patellogastropod Limpet. Mol. Biol. Evol. 38 (12), 5640–5654. doi: 10.1093/molbev/msab278
Keywords: Amphipoda, Cyphocaris, taxonomy, deep sea, new species
Citation: Wang Y, Sha Z and Ren X (2022) Two New Species of Cyphocaris (Amphipoda, Amphilochidea, Cyphocarididae) From Water Columns Above a Methane Seep in the South China Sea. Front. Mar. Sci. 9:849449. doi: 10.3389/fmars.2022.849449
Received: 06 January 2022; Accepted: 08 March 2022;
Published: 07 April 2022.
Edited by:
Jian-Wen Qiu, Hong Kong Baptist University, Hong Kong SAR, ChinaReviewed by:
Wonchoel Lee, Hanyang University, South KoreaJohanna N. J. Weston, Newcastle University, United Kingdom
Yu Zhao, Xiamen University, China
Copyright © 2022 Wang, Sha and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongli Sha, c2hhemxAcWRpby5hYy5jbg==
 Yanrong Wang1,2,3
Yanrong Wang1,2,3 Zhongli Sha
Zhongli Sha