
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 05 July 2022
Sec. Marine Megafauna
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.844893
This article is part of the Research Topic Ecological and Behavioral Traits of Apex Predators in Oceanic Insular Ecosystems: Advances and Challenges in Research and Conservation View all 14 articles
The increased risk of local extinction becomes critical for sharks depending on the narrow and isolated coastal habitats of oceanic islands. This includes large pelagic oceanic sharks that use such habitats as nurseries, as previously hypothesized for the smooth hammerhead Sphyrna zygaena, the least known of cosmopolitan large hammerhead sharks. We used a combination of acoustic and satellite telemetry in a juvenile population of Faial and Pico islands, Azores, mid-north Atlantic, to confirm if this isolated archipelago holds nurseries, and to answer questions related to their function and spatial–temporal stability. Our long-term acoustic tracking data showed a cluster of individual core home ranges in specific areas of north shore Faial, and surface positions from five Argos-linked tagged individuals also showed a clustering overlap in those areas for up to 1 year. These patterns seem to reveal a true habitat preference within the Faial-Pico island (sub) population of juvenile smooth hammerhead shark, and thus constitute strong evidence for this area to be considered a nursery. Some individuals remained in this nursery for up to 4 years, especially during summers. Sharks also showed a strong diel behavior, typically using the inshore nurseries during the day and moving further offshore during the night, during which they increased activity and dove deeper, most possibly to feed. We speculate that a combination of increased feeding opportunities, expanded trophic niche, and reduced predatory pressure may be a key evolutionary driver for the existence, prolonged use, and even preference of coastal nurseries at oceanic islands by juvenile smooth hammerhead shark. Given that these nurseries may constitute essential fish habitat for this species, they should be explicitly included in spatial management measures at the local and regional scales, as they may also play a role of greater importance to the north Atlantic population of this oceanic species.
Sharks are key top predators in the marine food web. They are globally threatened by fisheries given the high numbers taken driving over one-third of all elasmobranchs towards extinction (Dulvy et al., 2021) and because their K-selected life-history traits (slow growth, late maturity, and low fecundity) bring very slow recovery rates in heavily impacted populations (Stevens et al., 2000). Due to this vulnerability and their importance in balancing marine ecosystems, there is an emerging consensus on the urgent need to improve shark management strategies.
International agencies [e.g., Food and Agriculture Organization (FAO) and European Commission (EC)] and regional fisheries management organizations [e.g., International Commission for the Conservation of Atlantic Tunas (ICCAT)] now propose the use of Ecosystem-Based Management (EBM) for the sustainable management of sharks and to reduce conflicts between sharks and humans. However, this approach relies on the science-based knowledge of their spatial distribution and its relationships with ecosystem components. At present, such efforts are critically impaired by the lack of data on shark spatial ecology, especially with regard to their essential fish habitats (EFHs) such as nursery grounds and mating aggregations (Heupel et al., 2007; Kinney and Simpfendorfer, 2009; Meyer et al., 2009). Because sharks play a key role as moderators of trophic food webs and ecosystem functioning, protection of their EFHs should bring major benefits to many other species that make use of these habitats (Heupel et al., 2007; Kinney and Simpfendorfer, 2009; Meyer et al., 2009). As a result, those agencies emphasize that threats to shark populations must be assessed and their EFH must be identified and protected.
Coastal sharks face increased risks of local extinction due to fishing as well as through habitat degradation or severe climate change, as they frequently show high site fidelity or philopatry (they return to natal grounds to lay eggs or to pup) (Hueter et al., 2005; Field et al., 2009; Meyer et al., 2009). These threats may become critical for sharks depending on the narrow, isolated coastal habitats of oceanic islands, such as the Macaronesian archipelagos in the north Atlantic, including some oceanic migratory species. For example, adult pregnant female smooth hammerhead shark (Sphyrna zygaena Linnaeus, 1758) are thought to migrate and pup in coastal nurseries in the Azores, where juveniles grow until they are large enough to become oceanic (Afonso et al., 2014; Das and Afonso, 2017). This species is cosmopolitan, migratory, and Red-listed as Vulnerable to extinction by the International Union for Conservation of Nature (IUCN). It is frequently by-caught in the industrial longline and purse seine oceanic fisheries in sub-tropical regions (Rigby et al., 2019). This indicates that the island EFHs may be important to support populations that move throughout the Atlantic basin.
Yet, there is a great deal of uncertainty regarding the spatial and temporal location of the putative EFHs of smooth hammerhead shark (or most other pelagic sharks), or the present and future conditions of these areas under continued human disturbance. Commitments to conservation policies [e.g., the EU Marine Strategy Framework Directive (MSFD), the Oslo-Paris Convention (OSPAR), and the Convention on Biological Diversity (CBD)], coastal development, conflicting marine uses, and the emergence of local ecotourism industry all pose new challenges to the management of these island shark populations. Understanding the role of EFH for island shark ecology and conservation thus emerges as an urgent and difficult mission, given the ubiquitous lack of baseline ecological information and the challenge of studying their populations in the remote habitats across their distribution range, including oceanic seamounts and the open ocean.
The two central research questions addressed in this study were as follows: (i) Do juvenile smooth hammerhead sharks segregate from adults in space and time, i.e., are there juvenile EFH nursery areas non-overlapping with the usual adult grounds? (ii) Are these nurseries discrete in space and temporally stable at the individual and (sub)population levels? We used a combination of acoustic and satellite telemetry to address these questions. As this was the first multi-electronic tagging study on juvenile smooth hammerhead shark known at the time it started, testing and refining tagging and detection methodologies was also an objective in view of future, larger-scale studies.
This study was conducted between 2010 and 2020 in the Azores, the most remote oceanic archipelago in the north Atlantic. Located right on the Mid-Atlantic Ridge (MAR), it comprises nine islands roughly spanning 600 km and hundreds of seamounts surrounded by depths regularly exceeding 1,500 m within the region’s (sub) Exclusive Economic Zone (EEZ) of 1 million km2. We focused on the putative nurseries along the north coast of Faial Island, but the adopted multi-scale approach spans the neighboring island of Pico (the two islands being separated only by a shallow 9-km-wide channel), the chain of seamounts close to the two islands, and, eventually, the whole archipelago. The islands’ coastal habitats are greatly influenced by the region’s ecotone position and dominant oceanographic regime whereby the southern branch of the warm Gulf Current, which passes south of the islands, and its eddies and filaments promote a dynamic sub-tropical influence on its warm-temperate general character (Santos et al., 1995; Afonso et al., 2020). The shelves of the islands are very narrow, typically dropping from the shore to the break (at ca. 200 m) in less than 3 km on average. Around Faial and Pico, this shelf is less than 1 km wide on average, with the north shore of Faial being the widest (Figure 1B). The substrate is a mix of sandy bottom and rocky basaltic reef resulting from the volcanic eruptions and the dismantling of the steep shores, and the tidal regime largely determines the local scale circulation pattern. The west/north shores are typically subjected to higher swells along the year and especially during autumn–winter.
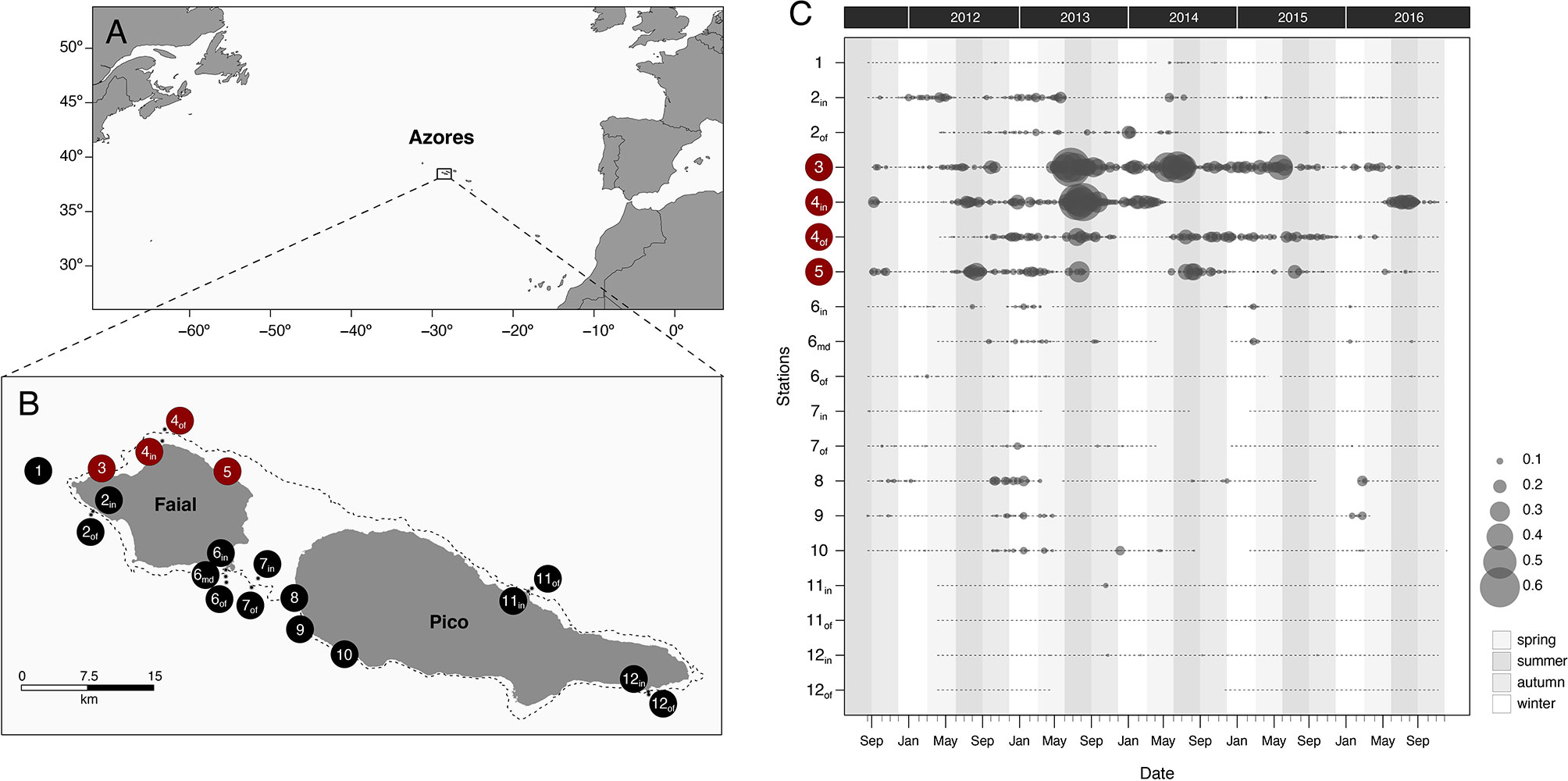
Figure 1 (A) The Azores in the north Atlantic. (B) Locations of the acoustic receivers around Faial and Pico islands with the nursery sites highlighted in red, the dashed line marking the edge of the island shelf. (C) Inter-annual pattern of weekly presence of acoustically tagged juvenile smooth hammerhead shark (Sphyrna zygaena) at receivers around Faial and Pico; gray circles represent the total detections averaged per shark at each station, vertical shaded bars represent seasons, and interruptions in the horizontal dotted lines mark the inactivity periods of a receiver.
The sharks were caught during the summer aggregation period (July–September) along the north shore of Faial. These aggregations are well known by local fishers and by the authors to occur during summer, and we therefore used the surface aggregations (anywhere from 2 to over 20 sharks swimming at the surface with i.e., <50 m of each other) as a proxy for the presence of the putative nurseries. We used a modified bottom-fixed mini-longline (ca. 20 circle hooks size 9/0 baited with sardine or squid) set in midwater. Prior to setting the line, we searched the coastline for the presence of aggregations of hammerheads at the surface and to select the fishing site. We always found these aggregations in the north shore of Faial either at the Salao, Cedros Point, or Praia do Norte areas and over bottoms of ca. 40 m depth at the transition from predominantly rocky to sandy substrate. The gear was set at sunset and left to soak for 1 h maximum before hauling.
Upon retrieval of the gear, sharks were restrained alongside the boat, kept in tonic immobility, and, if considered in good general condition, tagged at the surface with one or more of the three various types of electronic tags: single-position-only-transmitting satellite tag (Wildlife Computers SPOT5), archival pop-up satellite tag (Wildlife Computers MiniPAT), and passive acoustic tag (Innovasea/Vemco V16-4H or V13AP-1H) (Table 1). Each tag was used for a specific purpose reflecting the spatial and temporal scales offered/limited by each technology: Argos-linked SPOT tags, which make use of the sharks’ occasional surfacing behavior, allowed the study of horizontal shark movements for up to 1 year at the local to broad spatial scales depending on the error in the obtained ARGOS positions (from a few hundred meters to tens of kilometers); PAT tags allowed the study of the shark’s vertical behavior and its surrounding environment (temperature) for up to 1 year, its geolocation position estimates only allowing to detect eventual large-scale movements (i.e., oceanic migrations) of the sharks given their large error in latitudinal estimates (up to hundreds of kilometers); acoustic tags were used to monitor the long-term (up to 4 years) horizontal movements and habitat use at the local scale (i.e., nurseries) as determined by the positions (see below) and detection range of the fixed underwater acoustic receivers. A small subset of shorter-term acoustic tags is equipped with accelerometer and pressure (depth) sensors to complement the information regarding these two variables in the shark movements. Argos-linked SPOT tags were fin-mounted on the first dorsal fin through four nylon threaded rods across the fin fixed with stainless steel washers and nuts while miniPAT tags were fixed through inserting the stainless steel anchor into the musculature and through the pterygiophores (see details in Vandeperre et al., 2014). Acoustic tags were surgically inserted into the body cavity through a 3-cm ventral incision closed with catgut absorbable suture (see details in Afonso et al., 2016).
A total of 18 juvenile smooth hammerhead sharks ranging in size from 90 to 149 cm total length were electronically tagged in various tag combinations: 15 sharks were tagged with acoustic tags between 2010 and 2013, 3 of which were double-tagged with a SPOT tag and another 5 were double-tagged with a miniPAT tag; an additional 3 sharks were single-tagged in 2019 with a SPOT tag. Thus, we double-tagged 8 sharks, single-tagged 7 sharks with an acoustic tag only, and single-tagged 3 sharks with a satellite tag only, resulting in 15 acoustic tag datasets, 5 miniPAT datasets, and 6 SPOT datasets (Table 1). These 3 sharks were brought onboard the tagging vessel and maintained in a large tank (2,000 L) with hyper-saturated (ca. 120% O2) running seawater during the procedure. All animals were released at the site of capture after ensuring they had recovered swimming behavior in the upright position.
To quantify the long-term movements of the 15 acoustically tagged sharks, we deployed an array of underwater acoustic receivers (hereafter referred to as the array) fixed above the seafloor. The array was primarily designed to study the habitat use of the juvenile sharks in the putative nurseries in the north shore of Faial (the three fishing areas), as well as the movements across the contiguous Faial-Pico island shelf, but also to detect potential migrations to or between this coastal habitat and the neighboring seamount habitats. Therefore, the array included (i) 20 receivers at specific locations (“stations”) along the Faial-Pico shelf (Figure 1), mostly deployed in a “listening gate” fashion with 2 to 3 receivers from inshore (in) to middle (mid) to offshore (of) at each location to minimize the chance of a tagged shark swimming across it without being detected; (ii) 22 receivers on the summits and flanks of the nearby (ca. 18–80 km) Princess Alice seamount complex, including the Condor, S. Mateus, Açores, and Princess Alice banks; and (iii) 10 receivers at more distant and isolated seamounts (Gigante bank, 130 km; Formigas bank, 370 km) and islands (Santa Maria, 400 km) (Figure S1). The array was kept active for the whole study period (2010–2017), but a few stations were temporarily disabled due to malfunction or decommissioned after 2015 (in the more distant seamounts and islands).
We used Innovasea/Vemco VR2/VR2W acoustic receivers. Receivers at shallow stations (<40 m bottom depth) were rigged on a 3-m rope mooring suspended with small buoys and retrieved by SCUBA diving. Receivers at deeper stations (100–500 m) were rigged similarly except for the use of an acoustic release (AR50/60 SubSeaSonics, San Diego USA or ORE EdgeTech, USA) for retrieval from the surface. Stations were serviced every 6 to 12 months to download stored information. Range tests revealed a 50% detection probability of acoustic transmissions to be logged by receivers at approximately 450 m and 800 m radius for V13 and V16 transmitters of similar power output and in comparable environments to those in this study, respectively (Afonso et al., 2011; Afonso et al., 2012).
Data processing and analysis were conducted in R (R Core Team, 2014). Detections were first screened for false detections by excluding any detection that would occur isolated within the whole acoustic array in over 24 h. Data patterns were initially examined visually by plotting detections independently per fish and receiver across the study duration. This allowed verifying site fidelity of individuals to the putative nursery sites (or to any other site) by assessing whether they would return annually to these sites after long periods of absence (Chapman et al., 2015). To evaluate overall residency in the islands shelf, a residency index (IR) was then calculated for each shark, dividing the number of days with actual detections (DD) by the number of days between release and the last detection (i.e., detection span; TP). IR ranges between 0 for non-resident fish and 1 for full-time residents and was calculated for the whole acoustic array.
To estimate habitat use and movement metrics, detections were pooled into 60-min bins and used to calculate the center of activity positions (COAs) following Simpfendorfer et al. (2002). Movement trajectories of each shark were then projected based on the shortest path between each two consecutive positions using a least-cost distance approach (Dijkstra’s algorithm, “gdistance” package; van Etten, 2017) to avoid intersection with land surfaces. Therefore, these tracks correspond to an approximation of the minimum possible distance travelled. The rate of movement (ROM) of each shark was estimated by interpolating travelled distances over each hour (i.e., splitting the distance between each two successive detections in different stations by the number of time bins in between). Additionally, a linearity index (LI) was calculated for each individual dividing the distance between the first and last registered positions by the total distance travelled. This parameter ranges between 0 and 1, with higher values indicating strong directional movement and lower values indicating site attachment and high reuse of the same activity space (Villegas-Ríos et al., 2013).
To assess habitat use, COAs were used to estimate bivariate kernel utilization distributions (KUDs; Worton, 1989) for each shark using the “adehabitatHR” package (Calenge, 2006). These KUDs were then translated into core activity and home-range areas by calculating the area lying within a 50% and 95% occurrence probability threshold, respectively (Afonso et al., 2008). All distributions were calculated using a 1,000 fixed kernel bandwidth (h), selected after taking into consideration both the species ecology and the array characteristics, and corrected by excluding areas overlapping with land surfaces.
The potential occurrence of aggregative behavior (“aggregations”) was investigated by calculating a spatiotemporal overlap index between each possible pair of tagged individuals (see Gandra et al., 2020 for details). This parameter is analogous to the “simple-ratio” association index (Cairns and Schwager, 1987; Ginsberg and Young, 1992), ranging from 0% (no overlap) to 100% (complete overlap). This metric was also used to test size-mediated differences in joint space usage, that is, the effect of both individual size and size similarity in the extent of overlaps registered. With this aim, we fitted both linear and quadratic regressions and computed Pearson’s correlation coefficients between the modeled equations and the observed data.
Temporal patterns in movements and habitat use were investigated by calculating the aforementioned metrics separately for each diel period and season. Diel periods were assigned based on sunrise and sunset times (UTC −1), while boreal seasonal phases were defined on a monthly basis, with spring ranging from March to May, summer from June to August, autumn from September to November, and winter from December to February. Differences were first visually investigated through boxplots and contour plots, and then statistically tested for diel (day vs. night) and seasonal differences through pairwise Wilcoxon signed-rank tests using Bonferroni correction to adjust p-values and correct for Type I errors, given that most data were non-normally distributed. Finer-scale rhythmic patterns were also assessed using Fast Fourier Transform (FFT) on the hourly number of detections of each tagged shark across the entire array. This analysis decomposes data series into the frequency domain enabling the identification of spectral peaks that may reveal, for example, tidal (6–12 h) or diel (24 h) cyclic patterns in habitat use (Afonso et al., 2009).
Geographical positions of the SPOT tag transmissions and the popup locations of the miniPAT tags were obtained through the CLS-Argos satellite system.
Most probable geolocations from the miniPAT tags were reconstructed from archived light intensity curves transmitted by the tags after popup or retrieved after physical recovery of the tag. Light geolocations were estimated using a discretized hidden Markov model (HMM) provided by the tag manufacturer (WC-GPE3 software). The WC-GPE3 algorithm uses observations of light level, sea surface temperature, maximum depths, and any known locations from different sources, and incorporates a movement model based on a speed parameter chosen by the user (Pedersen et al., 2011). The output was provided on a 0.25° by 0.25° grid with an associated probability that the animal was in each grid cell at each time step.
The SPOT tags geolocation estimates and associated errors were inspected for any arbitrary position fixes. The LC-Z class locations were discarded and the unlikely swimming speeds (>2 m s−1) between two consecutive locations were filtered using the R package argosfilter (Freitas, 2012). The Argos tracks, together with the locations of acoustic detections for double-tagged sharks (#1 and #3), were subsequently corrected in a state-space model framework using a continuous-time correlated random walk taking location errors into account (Johnson et al., 2008). KUDs using the SPOT tags were then calculated as above.
Thirteen out of 15 acoustic transmitters implanted in juvenile smooth hammerhead sharks were detected in the array of acoustic receivers for periods ranging from 49 to 1,555 days (4.26 years), totalling 173,000 detections across the 20 coastal stations located on the Faial-Pico shelf (Table 1, Figure 1C). There were no detections on any of the remaining receivers of the Azorean acoustic telemetry network, such as the nearby seamounts. Two very contrasting patterns of individual acoustic detection emerged: either sharks were detected at a large number of stations (stations 10 to 19) over medium to long periods (94 to 1,555 days), or they were undetected or detected only at one single station (Table 1; Figure S2).
Three out of five miniPAT and eight out of nine SPOT tags reported data (Table 1). Three double-tagged (i.e., satellite and acoustic) individuals most probably died upon release: sharks #2 and #6 never reported from either tag, and shark #9 stayed in a vertical stationary position at 50 m depth (i.e., on the bottom) for 48 h upon release, after which the PAT tag (i.e., the shark’s carcass) was apparently ingested by a deepwater shark for 4 months while doing reversed diel vertical migrations between 140 and 650 m, until it finally popped offshore. This shark’s acoustic tag was occasionally detected at Salao (station 7) for about 1 year, but its pattern is very unexpected for a shark and also indicates that the acoustic tag was lying in the bottom at a detectable distance from the receiver (Figure S2). Shark #1 stopped transmitting its SPOT tag after 72 days of regular movements around Faial’s north and west shores (Figure 3), a period during which there were yet no acoustic stations deployed in Faial’s north shore. Although it was consistently detected at the Cedros deep station (station 6) over 2 years later, these are also in great contrast with the detections of most other sharks and point out the possibility that this animal was potentially predated by large top predators that frequently visit the islands’ shelf such as bluntnose sixgill shark (Hexanchus griseus) or shortfin mako shark (Isurus oxyrhinchus). Shark #8 was only first detected nearly 3 months upon release at Salao (1 detection) and Cedros Point, and it is unclear whether this was just a different behavioral pattern, or if it was predated or even dead upon release. Shark #15 was also never detected, but this tag was only set for 75 days battery life, thus making it impossible to properly evaluate the animal’s fate. Finally, all three single-SPOT-tagged sharks in 2019 survived and successfully transmitted positions.
Taken together, these results indicate that at least 13 out of 18 (72%) tagged juvenile smooth hammerhead sharks survived and behaved consistently for long periods. Therefore, for the purpose of this paper, and specifically for the spatial analysis based on acoustic telemetry, we used only data for which we could be reasonably certain about the natural behavior of the animal in the longer term, i.e., the nine individuals (hereafter referred to as the “multiple detection group”; Table 1).
The nine sharks in the multiple detection group exhibited a markedly vagile coastal behavior. They moved widely over the contiguous island shelf of Faial-Pico (Figure 2), being detected at anywhere from 10 to 20 stations over a median 929-day period (for the eight long-lasting transmitters) and eventually roaming around the shelf (Figure S2). Shark #14 also moved around the north and southwest shores of Faial Island during its short-term (75 days) acoustic tag lifetime. This behavior is reflected in their relatively low residency (RI median = 0.49, 0.34 to 0.68), the long total distance covered (average 3,338 km), and the large home range (KUD 77.6 km2) and even core activity (11.0 km2) area that these individuals used during the whole study period (Table 2). The three satellite SPOT tags deployed in 2019 showed similar movement patterns. All five transmitting SPOT-tagged individuals revealed a constant moving pattern over the island shelf for months even when away from the acoustic receiver detection range: sharks #1, 16, 17, and 18 roamed Faial’s north shore back and forth while #3 was detected all around the two neighbor islands before going silent (Figure 3). In spite of this mobility, all acoustically tagged sharks showed greater site fidelity and resulting home ranges centered at the sites located in the north shore of Faial, especially at stations 3 and 4 (Figures 1 and 2).
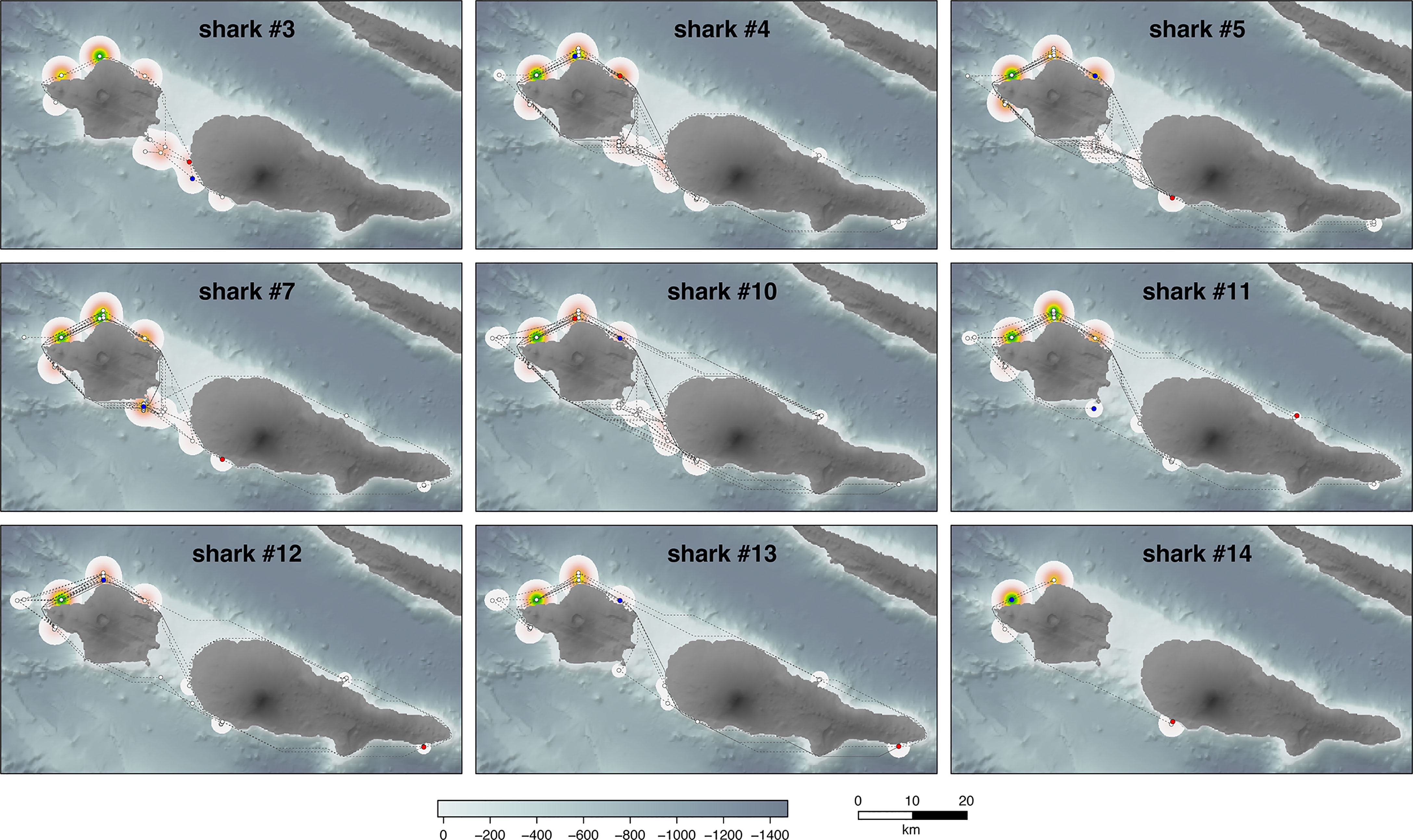
Figure 2 Individual trajectory paths (dotted lines) and home ranges (kernel utilization distributions) of nine acoustically tagged juvenile smooth hammerhead shark (Sphyrna zygaena). Trajectories were calculated based on the shortest path between each two consecutive COAs (centre of activity positions) taking into account land surfaces; white dots mark the COA, and blue and red dots signal the first and last positions, respectively, for each individual.

Table 2 Habitat use and movement statistics estimated for nine acoustically tagged smooth hammerhead shark Sphyrna zygaena (ID), including total distance travelled (km), mean (ROM) and maximum (Max ROM) rates of movement (m h−1), linearity index (LI), core activity (KUD 50%, km2), and home range (KUD 95%, km2) areas.

Figure 3 The most probable positions estimated for five SPOT satellite-tagged smooth hammerhead sharks (Sphyrna zygaena) around Faial and Pico islands; acoustic detections of double tagged individuals (#1 and #3) were used in improving the ARGOS estimates; shaded areas denote the kernel utilization distribution (KUD) home ranges.
Tagged individuals were co-detected at a given station (i.e., within the receiver listening range) and time (i.e., within 1-h bin) in numerous occasions (1,398 co-occurrences). These co-occurrences were predominantly at nursery stations, and consisted largely of two co-occurring tagged sharks (78.8%) and decreasing co-occurrence of three (14.8%), four (4.9%), and five (1.4%) tagged sharks. Six tagged sharks eventually co-occurred in two occasions. Certain individual pairs had a much higher probability of co-occurrence than others (e.g., #5 and #14, #4 and #12; Figure 4). This overlap network was related to individual length: the probability of co-occurrence between given pairs was higher at mid-range sizes (ca. 110–130 cm FL, Figure 4A) than in the lower and upper size limits. There was also a size-assortative effect in these co-occurrences; i.e., the probability of co-occurrence was higher the smaller was the size difference between two given individuals (Figure 4B).
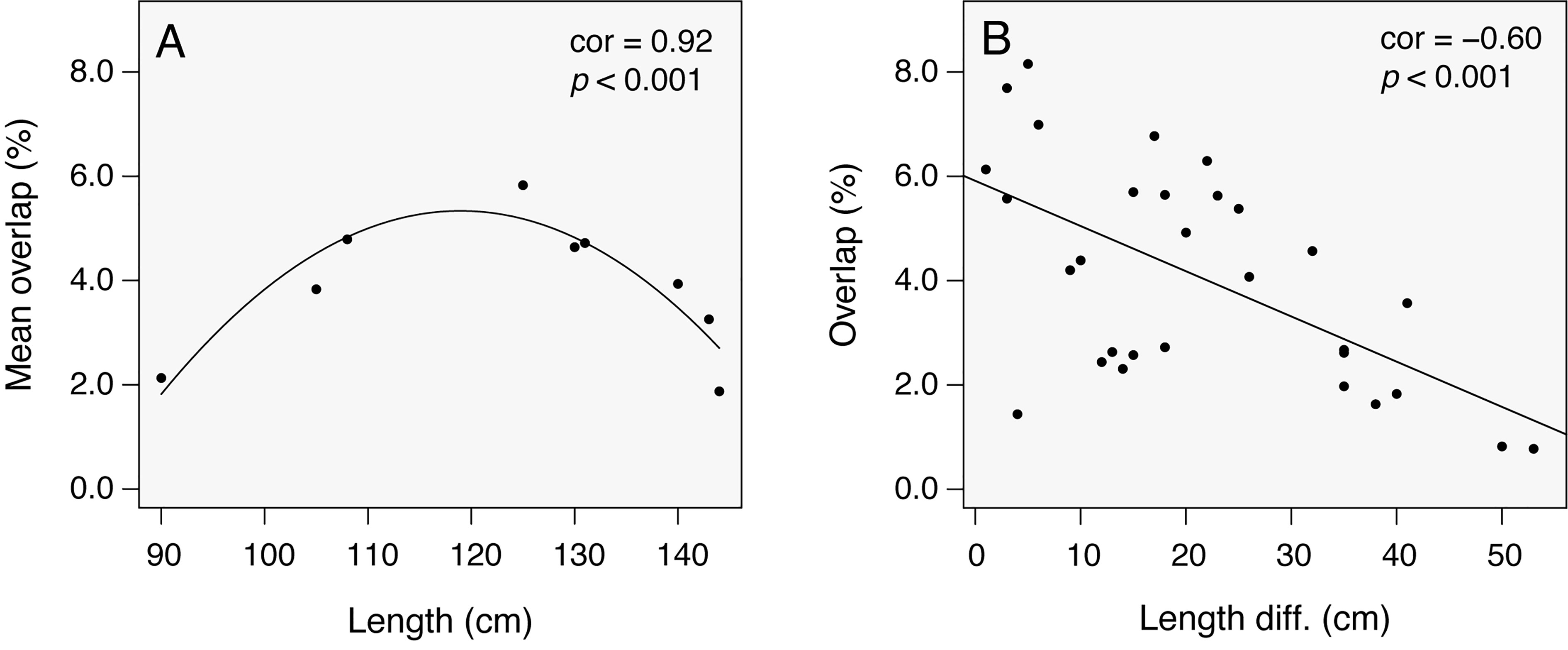
Figure 4 The influence of individual size in the probability of co-occurrence (overlap) of nine acoustically tagged smooth hammerhead shark (Sphyrna zygaena): (A) body length vs. overlap (nonlinear regression using a quadratic equation); (B) body length vs. overlap scores (linear regression).
Seasonal patterns were clearly discernible in the acoustic detection data of juvenile smooth hammerhead sharks. There was an increase in both the number of detections and number of co-occurring sharks during the warm season months (Figures 5A, B, Table S1), which resulted in a statistically significant difference between summer and the remaining seasons (Figures 5A, B, Table S2). There was also a seasonal trend in habitat use, with a peak in HR and COA areas in winter followed by a decrease through spring and summer and then an increase again in autumn (Figure S3). This pattern resulted in a significant contraction of HR and COAs during the spring and summer periods, largely due to a significant increase in detections at the receivers along the north shore of Faial during summer (Figures 6A, D, E; Table S3, as well as co-detections (Figure 6D). This pattern was consistent during consecutive years for most individuals, albeit small differences within seasons as to the specific sites along the north shore (sharks #4, #5, #10, #11, #12, #13, Figure S2). ROM was also significantly higher during these months (Figure 5C).
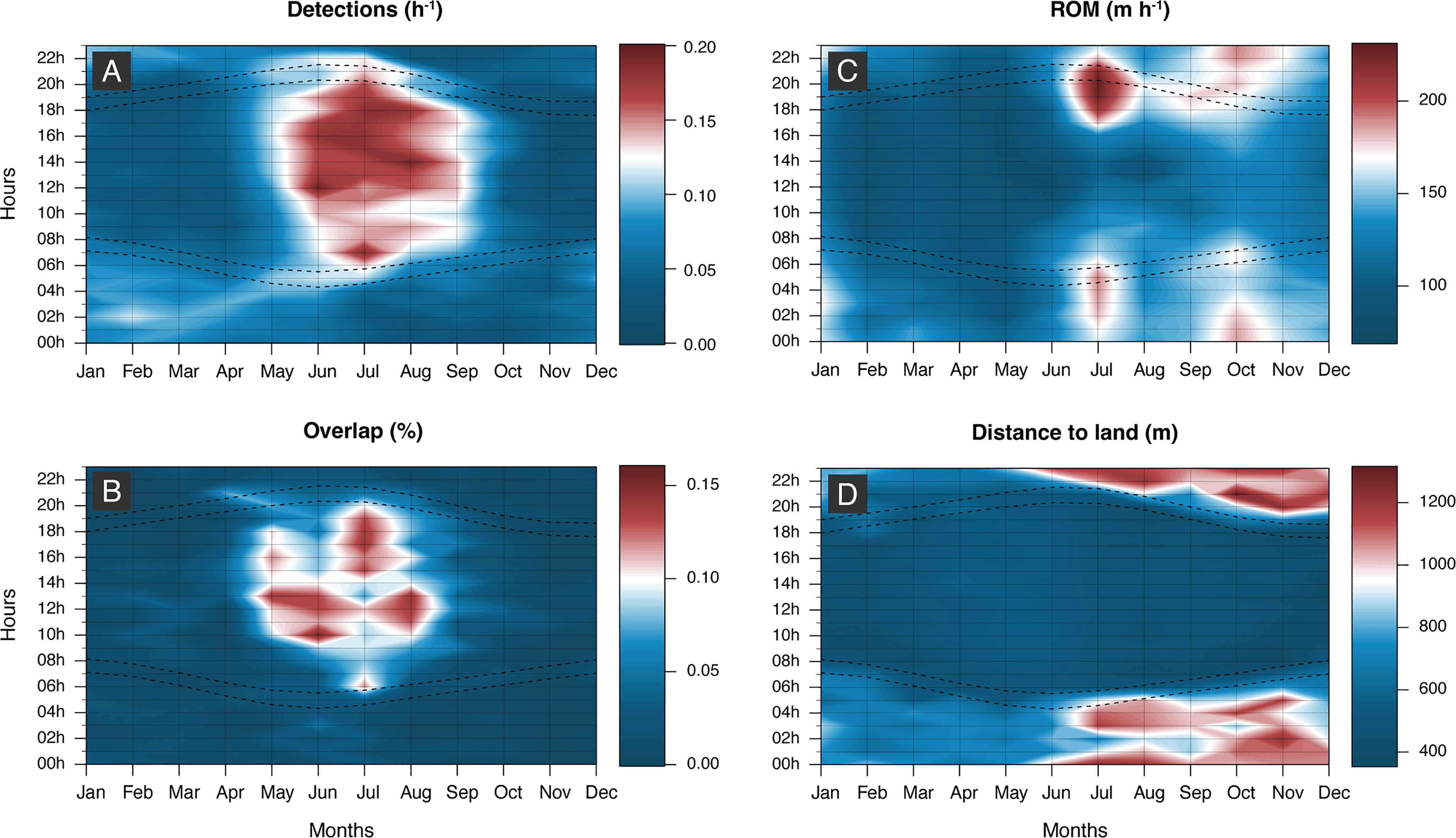
Figure 5 Contour plots representing the overall (A) acoustic detection frequencies, (B) overlap, (C) rate of movement (ROM), and (D) distance to nearest coast averaged per hour and month of nine acoustically tagged smooth hammerhead shark (Sphyrna zygaena). Dashed lines mark the times of dawn and dusk estimated for the study site illustrating the annual variation in daylight period.
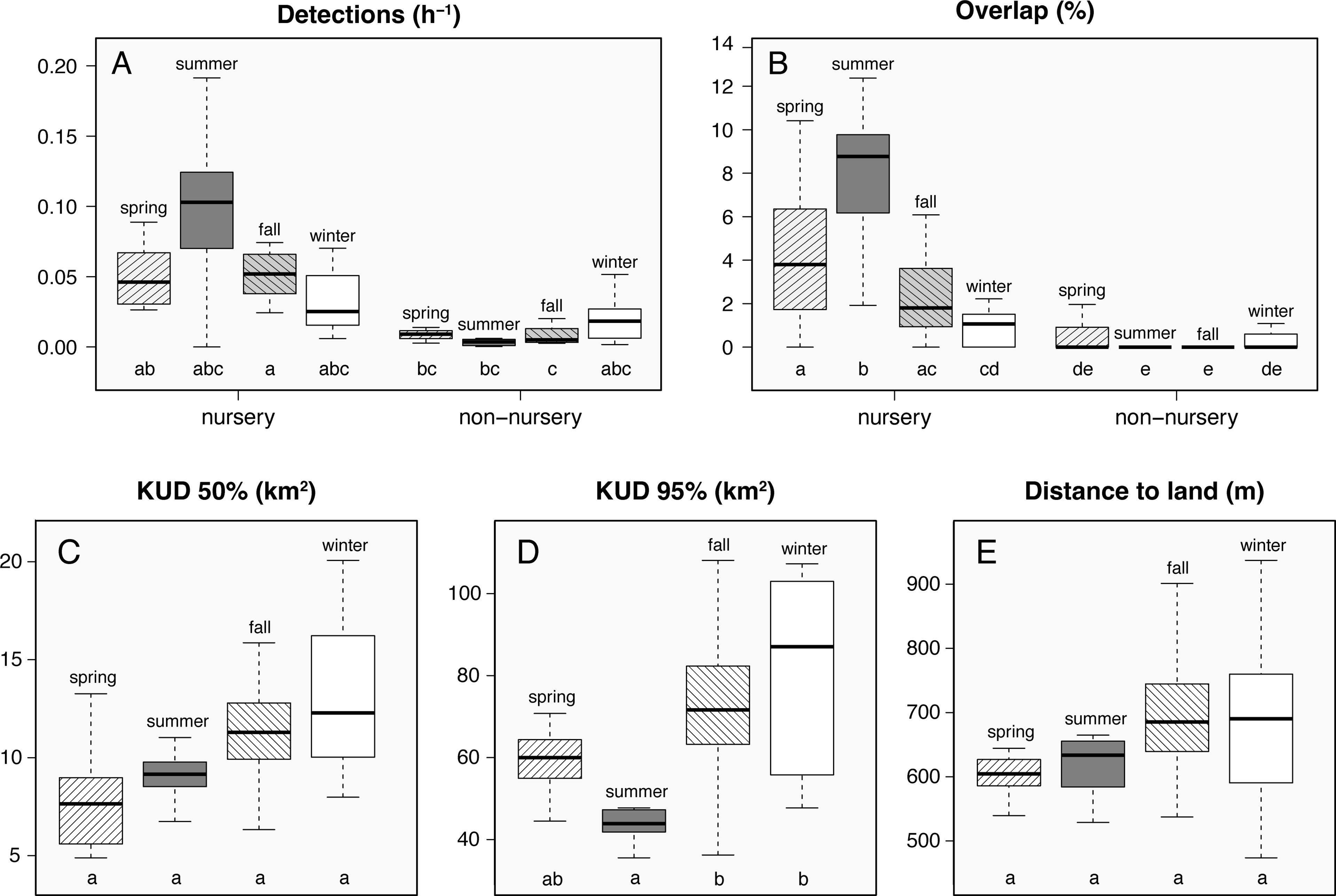
Figure 6 Detection frequency of smooth hammerhead shark (Sphyrna zygaena) (detections h-1; A), overlap (%; B), distance to nearest land surface (m; C), core activity areas (km2; D), and home range areas (km2; E) estimated per season. Boxes’ upper and lower limits represent the 75th and 25th quartiles, horizontal lines represent medians, and whiskers represent values within 1.5 interquartile ranges; outliers were removed in order to simplify visual interpretation; lowercase letters below the boxes represent significance groupings after a pairwise comparison (using Bonferroni correction) where groups sharing the same letter are not significantly different at p < 0.05.
A diel, fine-scale temporal rhythm was also quite evident in both the acoustic and satellite data. In general, sharks were much more often detected during daytime when at the three inshore stations of north shore Faial vs. at nighttime in all other stations (Figures 7, 8A, B). The most visited deeper offshore stations had almost no daytime detections (stations 3 and 6; Figure 1). As a result, sharks significantly moved offshore (Figures 5D, 8C) and increased their ROM at night (Figures 5C, 8D). The summer increase in detections was also essentially the result of daytime presence close to receivers (Figure 5A), as was the concomitant increase in daytime vs. nighttime co-occurrences (Figure 5B). This behavior resulted in a clear 24-h periodicity in detection patterns, as all individuals showed a clear peak at ca. 24 h based on the FFT analysis (Figure S4).

Figure 7 Proportion of detection frequencies per diel phase (white for daytime, black for nighttime) and receiver for nine acoustically tagged smooth hammerhead sharks (Sphyrna zygaena). Node size is proportional to the average of relative detection frequencies; the dashed line marks the edge of the island shelf.
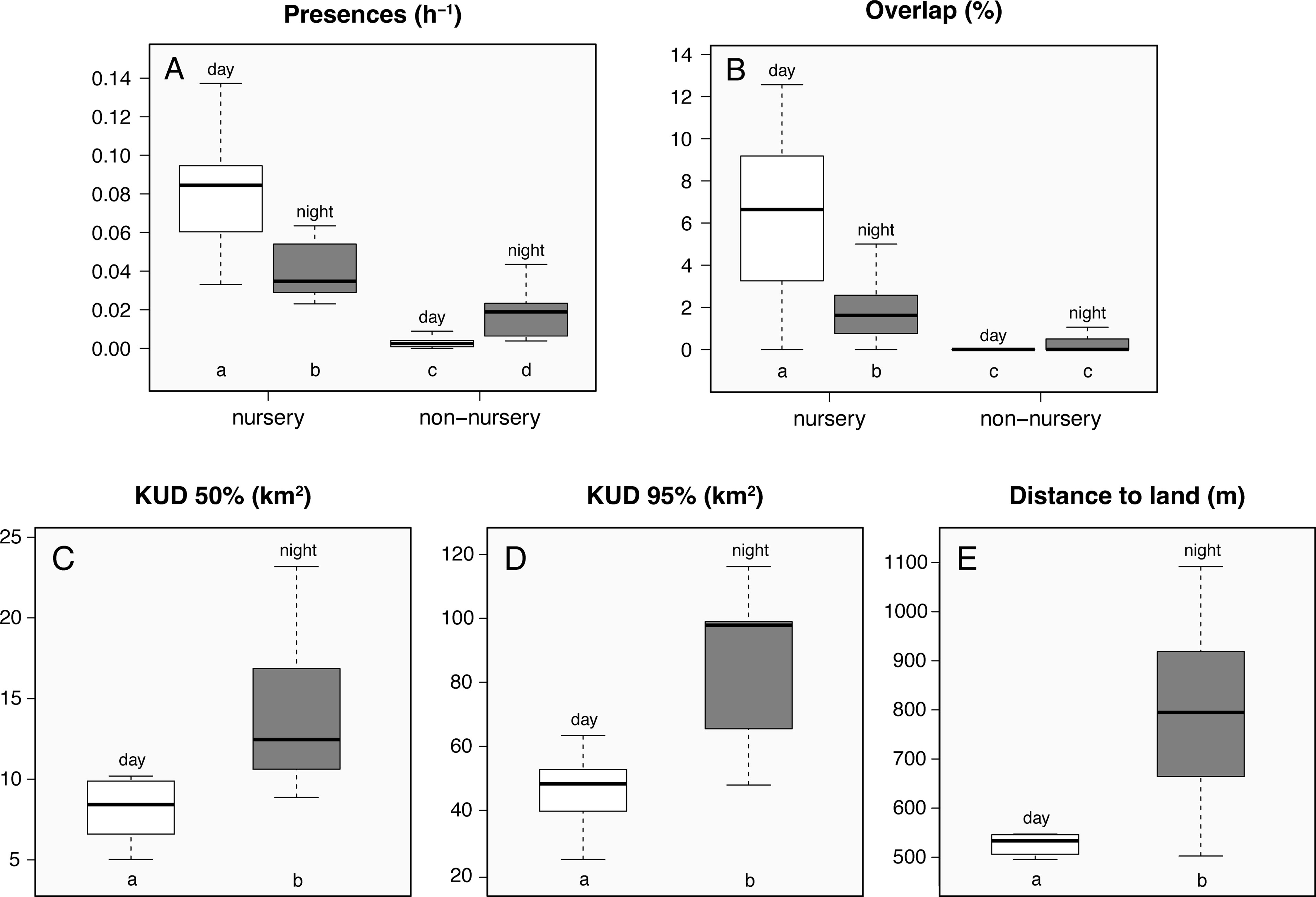
Figure 8 Acoustic detection frequency of smooth hammerhead shark (Sphyrna zygaena) (detections h-1; A), overlap (%; B), distance to nearest land surface (m; C), core activity (km2; D), and home range areas (km2; E) estimated for each diel phase. Boxes’ upper and lower limits represent the 75th and 25th quartiles, horizontal lines represent medians, and whiskers represent values within 1.5 interquartile ranges; outliers were removed in order to simplify visual interpretation; lowercase letters below the boxes represent significance groupings after a pairwise comparison (using Bonferroni correction) where groups sharing the same letter are not significantly different at p < 0.05.
The sharks’ vertical behavior closely matched the diel rhythm described above. The two sharks tagged with PAT tags that provided useful data (#4 and #5) showed a reversed diel vertical migration pattern (RDVM), staying within the shallow (<20 m) mixed layer during daytime but frequently descending down to 60 m and beyond at nighttime (Figure 9). This pattern was remarkable in matching the times of dawn and dusk for the initiation of each diel phase behavior. Nocturnal descents showed a “yo-yo” dive pattern whereby individuals intersperse dives to deeper and cooler (22–17°C, depending on the season) water with frequent, rapid ascents to superficial warmer waters (25–17°C). Occasionally, the two sharks dove below the thermocline (180 m and 130 m, respectively) down to 15°C ambient temperature in the warm season. Both sharks stayed in shallower waters (<20 m) during the first week after tagging.
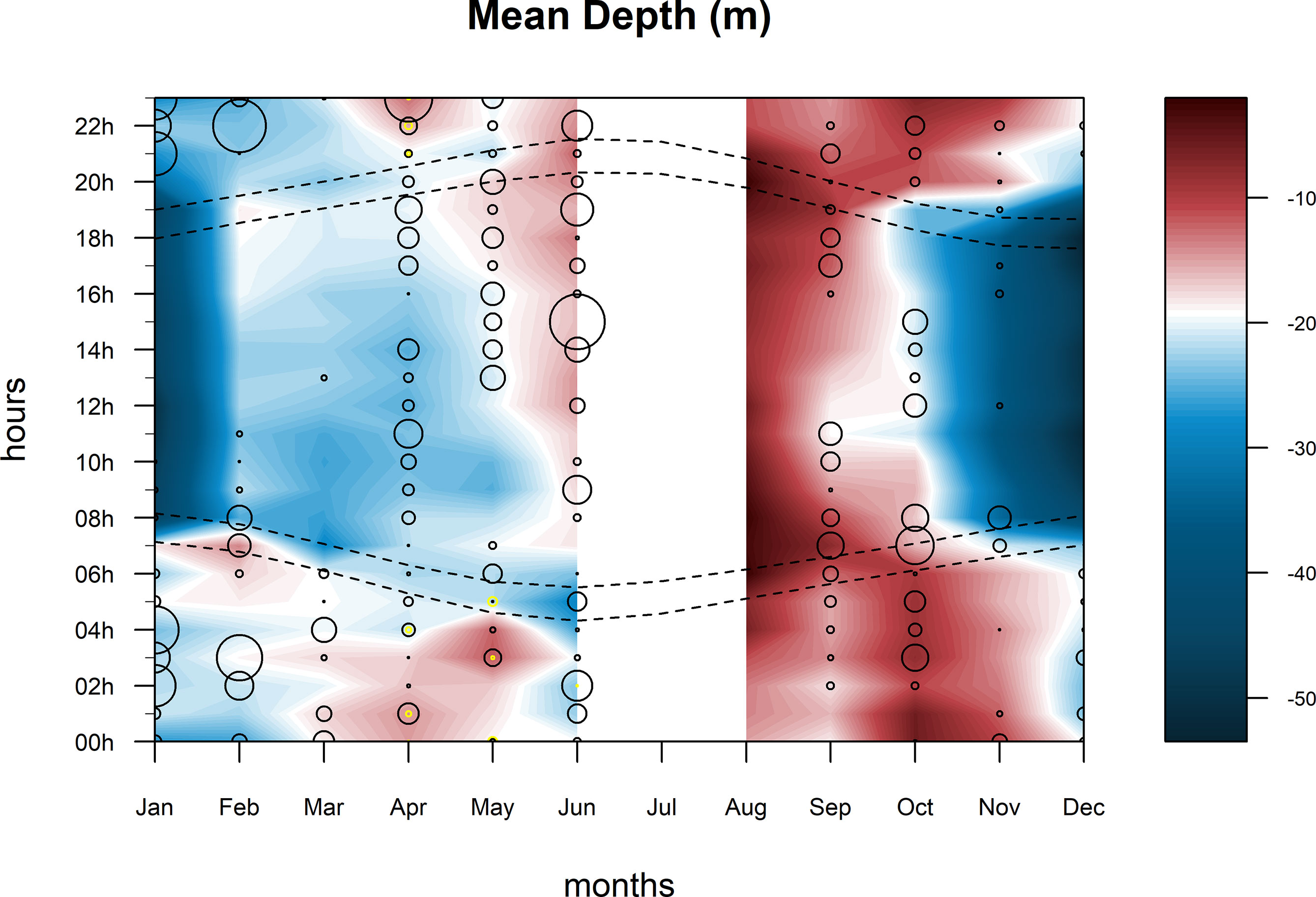
Figure 9 Abacus plot of the average depth across a 300-day deployment period for #4 smooth hammerhead shark (Sphyrna zygaena) double-tagged (acoustic and PAT) in the Azores. Dashed lines indicate the times of dawn and dusk across the year; circles represent the detections of this individual in acoustic receivers with the size being proportional to the number of detections; yellow dots signal receivers moored at deep (200 m) acoustic stations on the shelf edge, and hollow circles signal those at shallow (ca. 30 m) stations.
This is the fourth study worldwide to evaluate the movements and habitat use of the globally distributed and vulnerable smooth hammerhead shark using electronic tagging, but the second only to address its juvenile phase, and the first ever to offer any long-term movement data for this species—up to 4 years of individual tracking. It is also one of a few studies on nurseries of a pelagic/semi-pelagic shark species in a warm temperate region, and the most complete and multi-scale movement study of any hammerhead shark species to date, adding significant fine-scale data to the two previous satellite tagging studies of smooth hammerhead shark via eight successful archival/position tag deployments. Taken collectively, these results substantially increase our limited knowledge of the movement ecology of this species at relevant scales, especially with regard to the critical conservation aspect of their nurseries.
The smooth hammerhead shark is particularly vulnerable to hooking mortality in pelagic longline fisheries, with up to 71% of caught individuals being already dead upon gear retrieval (Coelho et al., 2012). Scalloped (S. lewini) and greater (S. mokarran) hammerhead sharks have also shown high sensitivity to capture and handling, substantially increasing their potential mortality upon release (Gallagher et al., 2014; Gulak et al., 2015). Although there are no direct studies on post-release mortality of smooth hammerhead shark, our results seem to indicate that this species is also vulnerable to increased potential mortality.
In total, we cannot exclude potential mortality upon release in five out of 18 (28%) tagged sharks. There is strong evidence from the patterns in our acoustic detection and satellite transmission data (see the Survival and Data Throughput section for details) that this was indeed the case in three out of eight double-tagged individuals versus two out of seven single-tagged individuals of undetermined fate (i.e., they might not have died) during the 2010–2013 tagging experiences. This difference indicates heavier physiological stress induced by the extended handling time (20 vs. 10 min max) and the more invasive manipulation of the double tagging, especially during the SPOT tag fixation. Our results match those of Francis (2016) in New Zealand and Santos and Coelho (2018) in the northeast tropical Atlantic, with three out of four and three out of eight SPOT-tagged smooth hammerhead sharks that did not transmit or did so for less than a week, respectively. There was no indication of size-related potential mortality in our study, as all surviving individuals provided very long acoustic tracking data and abundant high-quality satellite fixes, with no apparent behavioral change indicating long-term degradation of their condition.
In addition, all three single SPOT-tagged individuals in 2019 survived, even after being brought onboard and kept in a large oxygen-enriched saltwater tank. In spite of our initial tagging procedure with the sharks partially submersed in saltwater reducing their physiological stress in comparison to a classic handling procedure on deck, the combination of an oxygen-rich environment on the tank and reduced handling time (6 min maximum) proved efficient in reducing potential post release mortality and the animals were very active upon release. We therefore advise the use of this refined methodology when studying particularly sensitive individuals such as juvenile smooth hammerhead shark. These results highlight both the usefulness of multi-electronic tagging to study their multi-scale spatial ecology and the need to use refined tagging methodologies to reduce the handling time and physiological stress of this rather sensitive shark.
The 18 sharks tagged in this study ranged in size from 90 to 147 cm total length (avg. 128 cm). During the 2010–2013 fishing sets, we caught only one slightly larger (Tl = 162 cm) and one smaller young-of-the-year (62 cm Tl) individual (total n = 28, avg. 123.4 cm). According to previous age-and-growth studies for the northeast Atlantic population (Rosa et al., 2017) and elsewhere (e.g., López-Martínez et al., 2020), all tagged individuals were immature juveniles between their second and fourth years of age. We argue that these juveniles typify the smooth hammerhead shark population in the Azores, and that the island shelves host nursery (growth) and, most probably, also pupping (parturition) juvenile habitats for this species within their broader life history context in the north Atlantic.
Our acoustic tracking data showed an agglomeration of detections and resulting cluster of individual core home ranges in the sites along the north shore of Faial. The five SPOT tags also showed that their estimated surface positions clustered in this coastal area even if the sharks occasionally moved offshore and out of the receivers’ detection ranges. Importantly, they also showed that juvenile sharks stayed within or just next to the continuous Faial-Pico island shelf, a behavioral pattern broadly concurred by the miniPAT geolocation position estimates, which included this shelf in all the position error radius estimates, and even by their vertical behavior, as those sharks never dove into mesopelagic or deep-sea depths. Thus, these juveniles seem to adopt an essentially coastal lifestyle and reside (Chapman et al., 2015) in the shelf during their first years of life, during which they use the north shore of Faial as their preferred habitat.
According to Heupel et al. (2007), three criteria should be met to define a shark nursery: “(1) sharks are more commonly encountered in the area than other areas; (2) sharks have a tendency to remain or return for extended periods; and (3) the area or habitat is repeatedly used across years”. Traditional surveys (e.g., fishing or underwater censuses) in combination with acoustic tracking inside and outside assumed nursery habitats can be used to address the three criteria (Heupel et al., 2019). Although we did not perform long-term traditional surveys, the acoustic telemetry clearly showed higher presence and residency inside the putative nursery areas along the north shore of Faial Island than in the remaining stations year-round (Figures 8A, B) and seasonally (Figures 6A, B). Also, overlapped detections of 2 to 6 out of 9 successfully tagged individuals at these stations (Figure 4) confirm that juveniles are more commonly encountered and have a tendency to aggregate in these areas (Criterion 1). The persistence of use (Criterion 2) and residency (sensu Chapman et al., 2015) (Criterion 3) of juveniles in the nursery habitat along 4 years (Table 1) were confirmed by the long-term acoustic telemetry.
Although the listening range of the acoustic receiver array used in this study was far from fully covering the Faial-Pico shelf, it was designed (1) to broadly distribute receivers along the two contiguous islands, and (2) to reduce the chances of a shark moving along the shelf without being detected by using an “acoustic gate” approach with receivers closely placed perpendicular from the shoreline to the shelf break (Figure 1B). As in virtually all other tracking studies, we are not sure if sharks tagged elsewhere (e.g., in east Pico or along the south shores) would reveal a different use of the nursery areas. However, these sites were initially selected for tagging because they are well known by local fishers to hold larger quantities of juvenile hammerhead sharks, and subsequently proven to be so after our catch trials, which also included areas in south shore Faial. In addition, the temporal patterns in detections showed that the increased use of Faial north shore areas varies along the year and that sharks eventually move all around the two contiguous islands with no apparent habitat barriers to this behavior. Thus, the high use of Faial’s north shore seems to be, to a large extent, a true habitat preference within the local shark (sub) population.
The few studies that have addressed the movements of juvenile smooth hammerhead shark concluded that there are higher residency areas in coastal habitats, both for neonates and for larger, older juveniles, and that was considered to provide direct evidence for pupping and nursery areas, respectively (Diemer et al., 2011; Francis, 2016). Apparently, our results also indicate that the juvenile residency of smooth hammerhead shark may be higher than in other continental shelf areas, where there seem to be stronger seasonal migrations linked to seasonal changes in sea surface temperature and primary productivity (Diemer et al., 2011; Francis, 2016; Logan et al., 2020). This is not surprising given the fragmented nature of the coastal nursery habitat in oceanic islands such as the Azores, which apparently constrains the juvenile sharks to stay within coastal (shelf) habitat by preventing them from crossing the open ocean even between relatively close islands with a distance of only tens of kilometers from each other. However, those studies also found high potential residency within a given season, most notably during local summer. Thus, both our results and those of other studies show a clear seasonal trend in the strength of the spatial behavior during the nursery habitat use phase.
Finally, although we did not directly assess pregnancy or studied the pups in this study, we have only observed sharks the size of those tagged in this study at the surface-oriented aggregations in multiple years of observations, and never larger adult sharks (>200 cm). These adults are only occasionally seen in nearshore habitats by fishers, divers, and researchers (personal observation), always isolated, and in the summer. One adult pregnant female was reportedly caught inshore in Faial by a fixed gillnet in August 1997 (personal observation). Also, we caught some individuals in their first and second years of age, and filmed pups in Faial shores using underwater baited cameras as part of another study (unpublished results). These findings broadly support the hypothesis that pregnant females come in the Azorean summer to give birth (Afonso et al., 2014), as in other hammerhead shark populations (e.g., Guttridge et al., 2017; Félix-López et al., 2019), and that these pups reside and grow in the island where the pupping ground is located, spending most of its time in the nursery areas for up to 4 years. More research on the adult female and pup behavior is needed to confirm this hypothesis.
Juvenile smooth hammerhead shark seems to inhabit the shelves and upper slope of the islands in the Azores until they reach a certain age (this study), upon which they supposedly leave the coastal habitat and switch to an oceanic lifestyle (Afonso et al., 2014). Yet, their spatial behavior and nursery habitat use during the juvenile (post-pup) coastal phase is both typified by certain traits and variable across individuals and size.
The most striking movement characteristic of all tracked sharks was the clear and ubiquitous long-term preference for the nurseries located in the monitored coastal habitats located along the north shore of Faial. However, there were individual differences in the use of the remaining coastal areas around Faial and Pico, from individuals residing in the nursery sites to others that were occasionally detected all around the two islands. As there was no clear relationship between these patterns and either individual size or sex, they seem to reflect a true individual variability in space use and range of movements. This finding adds to the large body of evidence that individual variability is a widespread characteristic in the movement ecology of reef fishes (e.g., Afonso et al., 2008; Afonso et al., 2009; Villegas-Ríos et al., 2013; Afonso et al., 2016) and sharks (e.g., Matich and Heithaus, 2015; Munroe et al., 2016), including this species (Francis, 2016; Santos and Coelho, 2018; Logan et al., 2020).
We also saw a general diel trend in their use of the coastal nursery habitat; that is, they used the inshore sites of the nursery areas typically during the day and moved further offshore around the island shelf break during the night. This diel pendular movement was accompanied by an increase in activity, as indicated by the horizontal movements and by the frequent and deeper diving behavior at nighttime revealed in the vertical miniPAT data. Pre-adult smooth hammerhead shark also displays this behavior when in the open ocean, but not the adults (Santos and Coelho, 2018). Noticeably, we did not see this pattern when sharks were close to stations located away from the nursery, as the presence in the other coastal areas was always more frequent at nighttime than at daytime regardless of their shallower or deeper location. However, these detections were typically isolated and in areas where the island shelf is much narrower than in the north shore Faial. Diel movement patterns recorded from tracking studies have generally found that sharks increase their activity and home range at night, which has been attributed to increased foraging activity (Speed et al., 2010), but the 24-h periodicity of the use of nurseries, at least in the shorter term, is a relatively undescribed behavior in sharks.
Although based on few observations, the report of a narrow and shallow vertical habitat envelope used by smooth hammerhead shark, restricted to the first 50–60 m of water except for sporadic deeper dives, is remarkably consistent across regions (Azores, tropical eastern Atlantic, and New Zealand), habitats (oceanic island shelf, continental shelf, and open ocean), and even life stages (juvenile, pre-adult, and adult) (this study, Francis, 2016; Santos and Coelho, 2018). In the juvenile coastal phase, this behavior is most probably associated to a bentho-pelagic feeding behavior. Immature smooth hammerheads feed primarily on cephalopods and teleost fishes within the pelagic and bento-pelagic zone of shallow coastal habitats in the South-African continental shelf (Smale, 1991; Dicken et al., 2018). There is no information published on the diet of juvenile smooth hammerhead shark in the Azores or in any other oceanic island, but a recent study revealed that the Azorean juvenile population shows stable isotope values highly consistent with a coastal-associated diet (Priester, 2020). Thus, their diel forays may well reflect a nighttime foraging behavior. Future studies should investigate the dependency of the juveniles on coastal prey and their nighttime, more offshore predatory behavior, as both aspects have an implication for their conservation.
Our results provide compelling evidence that juvenile smooth hammerhead sharks are full-time residents in the coastal shelf habitat around Azorean islands. They also show that individuals aggregate seasonally in discrete areas during their first years of life, and that they consistently show seasonal high residency and annual site fidelity to these areas, configuring what is usually termed as nursery areas (Heupel et al., 2007). These findings pose questions about the function of these nurseries, their connectivity with other coastal and oceanic habitats in the north Atlantic, and the implications for the conservation and management of the north Atlantic population of smooth hammerhead shark.
As in many shark studies (e.g., Heupel et al., 2007; Heupel et al., 2019), it was postulated that nurseries of smooth hammerhead shark are located in areas of abundant food, either permanently (Smale, 1991) or seasonally (Logan et al., 2020), and reduce chances of cannibalism from the adults (Smale, 1991). One should therefore ask whether these assumptions are still valid and remain the main evolutionary drivers for the existence of coastal nurseries in highly constrained and fragmented coastal habitats around oceanic islands, such as the Azores.
The combination of an increased productivity due to local to regional oceanographic phenomena with the very existence of a shallow seabed habitat around oceanic islands and nearby shallow seamounts is thought to create favorable conditions for higher local food resources when compared with the oligotrophic open ocean, including in the Azores region (Santos et al., 1995; Morato et al., 2010; Caldeira and Reis, 2017). For a coastal species of bentho-pelagic feeding habits such as juvenile smooth hammerhead shark, this could include small coastal pelagic fishes, squid, and even other smaller elasmobranchs such as stingrays and skates, all of which occur in abundance and support local fisheries (Torres et al., 2022). and constitute their main staple elsewhere (Smale, 1991; Bornatowski et al., 2007). The little information available about the trophic ecology of the species in the Azores seems to support this theory (Priester, 2020). Juveniles may also benefit from increased opportunities to feed on the vast mesopelagic resources that occur in the region and frequently invade the coastal pelagic habitat during their nighttime diel vertical migrations (DVMs). This combination would offer an expanded trophic niche during their juvenile phase as well as a more heterogeneous diet as juveniles grow and approach their adult oceanic lifestyle, after which they will essentially depend on mesopelagic resources, especially squid (Galván-Magaña et al., 2013; Estupiñán-Montaño et al., 2019).
The risk of cannibalistic predation should also be much lower in these nursery areas given that the occurrence of adults is rare and probably limited to pregnant females (Afonso et al., 2014). This advantage may, however, be offset by the potential increased predation from other large predators, namely, sharks. However, the only other coastal shark species occurring abundantly in the region and broadly sharing the same habitat is the tope shark Galeorhinus galeus (Das and Afonso, 2017). In contrast to other apex predator reef sharks typical of tropical regions but absent in the Azores, such as carcharhinids and tiger sharks, tope shark predates small fishes and squid rather than other elasmobranchs (Morato et al., 2003). Additionally, the one deepwater shark potentially predating these juveniles, the locally abundant bluntnose sixgill shark, occurs in shallower habitats but typically at depths greater than 100 m (unpublished data). Thus, it is very likely that juvenile hammerhead sharks also benefit from reduced predation at these coastal oceanic nurseries. Interestingly, individuals tended to co-occur (aggregate) at the nurseries with other similarly sized individuals and less so among the larger juveniles, which may indicate a release of predatory pressure by local predators as they grow and, possibly, a dilution effect against predation for smaller individuals. It would be useful to validate this assumption by investigating if pups aggregate in higher numbers than larger juveniles.
In conclusion, the combination of increased feeding opportunities and expanded trophic niche with reduced predatory pressure may be a key evolutionary driver for the existence, prolonged use, and even preference of coastal nurseries at oceanic islands by juvenile smooth hammerhead shark. Aggregations at coastal nurseries could additionally increase survival of juveniles through socialization benefits, such as cooperative learning in hunting and predator avoidance, as previously hypothesized for juvenile lemon shark (Guttridge et al., 2009; Jacoby et al., 2012; Heupel et al., 2019).
At the local scale, it is also worth considering why the areas in north shore Faial are selected as nurseries. This area is the widest, with more sandy habitat around the two islands combined, with the exception of the channel between them. This may implicate that those habitats offer more feeding opportunities upon sandy associated prey and/or less predation opportunities from vertically migrating deepwater sharks or other ambushing reef predators such as dusky grouper (Mycteroperca marginatus), although we have no means of validating these assumptions. It is thus vital to assess if other nurseries exist across the islands in the archipelago and if they are located in the same type of coastal habitat. This would allow translating the knowledge gathered in this study to support the species conservation via protection of its coastal EFH, the nurseries. It follows that the threats to such EFH should also be objectively assessed. Although there is no indication of appreciable directed catch or by-catch of this species in the Azores (Fauconnet et al., 2019), it is possible that the bycatch of juveniles and even pupping females from the hook-and-line and especially gillnet coastal fisheries impacts the population, especially if they are carried out in the nurseries. A potential precautionary measure could be the inclusion of these nurseries in areas closed to fishing.
Finally, these putative EFHs may also play a role of greater importance to the north Atlantic smooth hammerhead population(s) the juveniles (and pupping females) belong to. For example, it is very possible that the oceanic adults and pre-adults in the tropical north-east Atlantic were born at nurseries located in the Azores and other oceanic islands (Afonso et al., 2020), and that females return to these nurseries to pup later in life via philopatric behavior, as seems to be the case in the Northern Mexican Pacific (Félix-López et al., 2019). In this case, oceanic island nurseries/EFH should explicitly be put in context with other (adult) EFH, including the pupping migrating corridors, in current international fisheries management approaches as implemented by the international bodies (e.g., ICAAT, CBD, and OSPAR).
The raw data supporting the conclusions of this article may be made available by the authors upon request.
This study was performed according to national Portuguese laws for the use of vertebrates in research, and the animal handling and tagging protocols approved by the Azorean Directorate of Sea Affairs of the Azores Autonomous region (permits DRAM/SRRN ref. 24/2010), which oversees and issues permits for scientific activities. All procedures followed the guidelines for the use of fishes in research of the American Fisheries Society. No animals were sacrificed, and procedures for reduction, replacement and refinement were thoroughly adopted.
PA and JF designed the study. PA, GG, FV, BM, and JF conducted the study. PA drafted the manuscript, and all other authors improved the draft and critically reviewed the manuscript. MG performed the acoustic telemetry analyses. BM and FV performed the satellite telemetry analyses. PA secured funding for fieldwork. All authors contributed to the article and approved the submitted version.
We acknowledge funds provided by the Portuguese Science & Technology Foundation (FCT) through individual contracts/grants to PA (IF/01640/2015), MG (UI/BD/151309/2021), JF (M3.1.a/F/062/201), and FV (CEECIND/03469/2017), through the research project IslandShark (PTDC/BIA-BMA/32204/2017), and through the Strategic Program to Okeanos R&D Centre; by the Oceanário de Lisboa and the Shark Conservation Fund through the IslandShark project; and by the EC through the Mission Atlantic research project H2020-LC-BG-08-2018. This is a contribution to the European Tracking Network (ETN) and the Ocean Tracking Network (OTN).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank D. Das, R. Priester, S. Solleliet-Ferreira, and the crews of R/Vs Archipelago and Caroline for their support in field data collection.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.844893/full#supplementary-material
Afonso P., Abecasis D., Santos R. S., Fontes J. (2016). Contrasting Movements and Residency of Two Serranids in a Small Macaronesian MPA. Fisheries Res. 177. doi: 10.1016/j.fishres.2015.12.014
Afonso P., Fontes J., Giacomello E., Magalhães M. C., Martins H. R., Morato T., et al. (2020). The Azores: A Mid-Atlantic Hotspot for Marine Megafauna Research and Conservation. Front. Mar Sci. 6. doi: 10.3389/fmars.2019.00826
Afonso P., Fontes J., Holland K. N., Santos R. S. (2008). Social Status Determines Behaviour and Habitat Usage in a Temperate Parrotfish: Implications for Marine Reserve Design. Marine Ecol. Prog. Ser. 359. doi: 10.3354/meps07272
Afonso P., Fontes J., Holland K. N., Santos R. S. (2009). Multi-Scale Patterns of Habitat Use in a Highly Mobile Reef Fish, the White Trevally Pseudocaranx Dentex, and Their Implications for Marine Reserve Design. Marine Ecol. Prog. Ser. 381. doi: 10.3354/meps07946
Afonso P., Fontes J., Santos R. S. (2011). Small Marine Reserves can Offer Long Term Protection to an Endangered Fish. Biol. Conserv. 144. doi: 10.1016/j.biocon.2011.07.028
Afonso P., Fontes J., Vandeperre F., Porteiro F. (2014). Conservation of Pelagic Elasmobranchs in the Azores. Arquipélago. Life Marine Sci., 25–30.
Afonso P., Graça G., Berke G., Fontes J. (2012). First Observations on Seamount Habitat Use of Blackspot Seabream (Pagellus Bogaraveo) Using Acoustic Telemetry. J. Exp. Marine Biol. Ecol. 436–437. doi: 10.1016/j.jembe.2012.08.003
Bornatowski H., Costa L., Robert M., Pina J. (2007). Feeding Habits of Young Smooth Hammerhead Sharks, Sphyrna Zygaena (Carcharhiniformes: Sphyrnidae), in the Southern Coast of Brazil. Biota Neotropica 7. doi: 10.1590/S1676-06032007000100025
Cairns S. J., Schwager S. J. (1987). A Comparison of Association Indices. Anim. Behav. 35. doi: 10.1016/S0003-3472(87)80018-0
Caldeira R. M. A., Reis J. C. (2017). The Azores Confluence Zone. Front. Mar Sci. 4. doi: 10.3389/fmars.2017.00037
Calenge C. (2006). The Package “Adehabitat” for the R Software: A Tool for the Analysis of Space and Habitat Use by Animals. Ecol. Modelling 197. doi: 10.1016/j.ecolmodel.2006.03.017
Chapman D. D., Feldheim K. A., Papastamatiou Y. P., Hueter R. E. (2015). There and Back Again: A Review of Residency and Return Migrations in Sharks, With Implications for Population Structure and Management. Annu. Rev. Marine Sci. 7. doi: 10.1146/annurev-marine-010814-015730
Coelho R., Fernandez-Carvalho J., Lino P. G., Santos M. N. (2012). An Overview of the Hooking Mortality of Elasmobranchs Caught in a Swordfish Pelagic Longline Fishery in the Atlantic Ocean. Aquat. Living Resour. 25. doi: 10.1051/alr/2012030
Das D., Afonso P. (2017). Review of the Diversity, Ecology, and Conservation of Elasmobranchs in the Azores Region, Mid-North Atlantic. Front. Mar Sci. 4. doi: 10.3389/fmars.2017.00354
Dicken M. L., Winker H., Smale M. J., Cliff G. (2018). Sharks Caught in the KwaZulu-Natal Bather Protection Programme, South Africa. 14. The Smooth Hammerhead Shark Sphyrna Zygaena (Linnaeus). Afr. J. Marine Sci. 40. doi: 10.2989/1814232X.2018.1470031
Diemer K. M., Mann B. Q., Hussey N. E. (2011). Distribution and Movement of Scalloped Hammerhead Sphryna Lewini and Smooth Hammerhead Sphyrna Zygaena Sharks Along the East Coast of Southern Africa. Afr. J. Marine Sci. 33. doi: 10.2989/1814232X.2011.600291
Dulvy N. K., Pacoureau N., Rigby C. L., Pollom R. A., Jabado R. W., Ebert D. A., et al. (2021). Overfishing Drives Over One-Third of All Sharks and Rays Toward a Global Extinction Crisis. Curr. Biol. doi: 10.1016/j.cub.2021.08.062
Estupiñán-Montaño C., Cedeño-Figueroa L., Estupiñán-Ortiz J. F., Galván-Magaña F., Sandoval-Londoño A., Castañeda-Suarez D., et al. (2019). Feeding Habits and Trophic Level of the Smooth Hammerhead Shark, Sphyrna Zygaena (Carcharhiniformes: Sphyrnidae), Off Ecuador. J. Marine Biol. Assoc. United Kingdom 99. doi: 10.1017/S0025315418000474
Fauconnet L., Pham C. K., Canha A., Afonso P., Diogo H., Machete M., et al. (2019). An Overview of Fisheries Discards in the Azores. Fisheries Res. 209. doi: 10.1016/j.fishres.2018.10.001
Félix-López D. G., Bolaño-Martinez N., Díaz-Jaimes P., Oñate-González E. C., Ramírez-Pérez J. S., García-Rodríguez E., et al. (2019). Possible Female Philopatry of the Smooth Hammerhead Shark Sphyrna Zygaena Revealed by Genetic Structure Patterns. J. Fish Biol. 94, 671–679. doi: 10.1111/jfb.13949
Field I. C., Meekan M. G., Buckworth R. C., Bradshaw C. J. A. (2009). Susceptibility of Sharks, Rays and Chimaeras to Global Extinction. Adv. Marine Biol. 56. doi: 10.1016/S0065-2881(09)56004-X
Francis M. P. (2016). Distribution, Habitat and Movement of Juvenile Smooth Hammerhead Sharks (Sphyrna Zygaena) in Northern New Zealand. New Z. J. Marine Freshwater Res. 50. doi: 10.1080/00288330.2016.1171244
Gallagher A. J., Serafy J. E., Cooke S. J., Hammerschlag N. (2014). Physiological Stress Response, Reflex Impairment, and Survival of Five Sympatric Shark Species Following Experimental Capture and Release. Marine Ecol. Prog. Ser. 496. doi: 10.3354/meps10490
Galván-Magaña F., Polo-Silva C., Berenice Hernández-Aguilar S., Sandoval-Londoño A., Ruth Ochoa-Díaz M., Aguilar-Castro N., et al. (2013). Shark Predation on Cephalopods in the Mexican and Ecuadorian Pacific Ocean. Deep-Sea Res. Part II: Topical Stud. Oceanography 95. doi: 10.1016/j.dsr2.2013.04.002
Gandra M., Afonso P., Fontes J. (2020). Intra- And Interspecific Associations in Two Predatory Reef Fishes at a Shallow Seamount. Marine Ecol. Prog. Ser. 654. doi: 10.3354/meps13501
Ginsberg J. R., Young T. P. (1992). Measuring Association Between Individuals or Groups in Behavioural Studies. Anim. Behav. 44. doi: 10.1016/0003-3472(92)90042-8
Gulak S. J. B., de Ron Santiago A. J., Carlson J. K. (2015). Hooking Mortality of Scalloped Hammerhead Sphyrna Lewini and Great Hammerhead Sphyrna Mokarran Sharks Caught on Bottom Longlines. Afr. J. Marine Sci. 37. doi: 10.2989/1814232X.2015.1026842
Guttridge T. L., Gruber S. H, Gledhill K. S, Croft D. P, Sims , D. W, Krause J. (2009). Social Preferences of Juvenile Lemon Sharks, Negaprion brevirostris.. Anim. Behav 78(2), 543–548. doi: 10.1016/j.anbehav.2009.06.009
Guttridge T. L., van Zinnicq Bergmann M. P. M., Bolte C., Howey L. A., Finger J. S., Kessel S. T., et al. (2017). Philopatry and Regional Connectivity of the Great Hammerhead Shark, Sphyrna Mokarran in the U.S. And Bahamas. Front. Mar Sci. 4. doi: 10.3389/fmars.2017.00003
Heupel M. R., Carlson J. K., Simpfendorfer C. A. (2007). Shark Nursery Areas: Concepts, Definition, Characterization and Assumptions. Marine Ecol. Prog. Ser. 337, 287–297. doi: 10.3354/meps337287
Heupel M. R., Kanno S., Martins A. P. B., Simpfendorfer C. A. (2019). Advances in Understanding the Roles and Benefits of Nursery Areas for Elasmobranch Populations. Marine Freshwater Res. 70. doi: 10.1071/MF18081
Hueter R. E., Heupel M. R., Heist E. J., Keeney D. B. (2005). Evidence of Philopatry in Sharks and Implications for the Management of Shark Fisheries. J. Northwest Atlantic Fishery Sci. 35, 239–247. doi: 10.2960/j.v35.m493
Jacoby D. M. P., Croft D. P., Sims D. W. (2012). Social Behaviour in Sharks and Rays: Analysis, Patterns and Implications for Conservation. Fish Fisheries 13, 399–417. doi: 10.1111/j.1467-2979.2011.00436.x
Johnson D. S., London J. M., Lea M.-A., Durban J. W. (2008) CONTINUOUS-TIME CORRELATED RANDOM WALK MODEL FOR ANIMAL TELEMETRY DATA. Available at: https://www.argos-system.org/manual/i.
Kinney M. J., Simpfendorfer C. A. (2009). Reassessing the Value of Nursery Areas to Shark Conservation and Management. Conserv. Lett. 2, 53–60. doi: 10.1111/j.1755-263x.2008.00046.x
Logan R. K., Vaudo J. J., Sousa L. L., Sampson M., Wetherbee B. M., Shivji M. S. (2020). Seasonal Movements and Habitat Use of Juvenile Smooth Hammerhead Sharks in the Western North Atlantic Ocean and Significance for Management. Front. Mar Sci. 7. doi: 10.3389/fmars.2020.566364
López-Martínez J., Cabanilla-Carpio C., Ruiz Choez W., Arzola-Sotelo E. A. (2020). Interannual Variability of Distribution, Abundance and Population Dynamics of the Smooth Hammerhead Sphyrna Zygaena (Linnaeus, 1758) in the Central-Southeast Pacific Ocean. J. Fish Biol. 97. doi: 10.1111/jfb.14360
Matich P., Heithaus M. R. (2015). Individual Variation in Ontogenetic Niche Shifts in Habitat Use and Movement Patterns of a Large Estuarine Predator (Carcharhinus Leucas). Oecologia 178. doi: 10.1007/s00442-015-3253-2
Meyer C. G., Clark T. B., Papastamatiou Y. P., Whitney N. M., Holland K. N. (2009). Long-Term Movement Patterns of Tiger Sharks Galeocerdo Cuvier in Hawaii. Marine Ecol. Prog. Ser. 381. doi: 10.3354/meps07951
Morato T., Hoyle S. D., Allain V., Nicol S. J. (2010). Seamounts are Hotspots of Pelagic Biodiversity in the Open Ocean. Proc. Natl. Acad. Sci. U.S.A. 107. doi: 10.1073/pnas.0910290107
Morato T., Solà E., Grós M. P., Menezes G. (2003). Diets of Thornback Ray (Raja Clavata) and Tope Shark (Galeorhinus Galeus) in the Bottom Longline Fishery of the Azores, Northeastern Atlantic. Fishery Bull. 101.
Munroe S. E. M., Simpfendorfer C. A., Heupel M. R. (2016). Variation in Blacktip Shark Movement Patterns in a Tropical Coastal Bay. Environ. Biol. Fishes 99. doi: 10.1007/s10641-016-0480-2
Pedersen M. W., Patterson T. A., Thygesen U. H., Madsen H. (2011). Estimating Animal Behavior and Residency From Movement Data. Oikos 120, 1281–1290. doi: 10.1111/j.1600-0706.2011.19044.x
Priester C. R. (2020). Trophic Ecology and Coastal Habitat Use of Two Sympatric Shark Species in the Azores Using CNS Stable Isotope Analysis.
R Core Team (2014). R: A Language and Environment for Statistical Computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: http://www.R-project.org/.
Rigby C. L., Barreto R., Carlson J., Fernando D., Fordham S., Herman K., et al. (2019). Sphyrna Zygaena. IUCN Red List Threatened Species. doi: 10.2305/IUCN.UK.2019-3Affilia
Rosa D., Coelho R., Fernandez-Carvalho J., Santos M. N. (2017). Age and Growth of the Smooth Hammerhead, Sphyrna Zygaena, in the Atlantic Ocean: Comparison With Other Hammerhead Species. Marine Biol. Res. 13. doi: 10.1080/17451000.2016.1267366
Santos C. C., Coelho R. (2018). Migrations and Habitat Use of the Smooth Hammerhead Shark (Sphyrna Zygaena) in the Atlantic Ocean. PloS One 13. doi: 10.1371/journal.pone.0198664
Santos R. S., Hawkins S., Monteiro L. R., Alves M., Isidro E. J. (1995). Marine Research, Resources and Conservation in the Azores. Aquat. Conservation: Marine Freshwater Ecosystems 5. doi: 10.1002/aqc.3270050406
Simpfendorfer C. A., Heupel M. R., Hueter R. E. (2002). Estimation of Short-Term Centers of Activity From an Array of Omnidirectional Hydrophones and its Use in Studying Animal Movements. Can. J. Fisheries Aquat. Sci. 59. doi: 10.1139/f01-191
Smale M. J. (1991). Occurrence and Feeding of Three Shark Species, Carcharhinus Brachyurus, C. Obscurus and Sphyrna Zygaena, on the Eastern Cape Coast of South Africa. South Afr. J. Marine Sci. 11. doi: 10.2989/025776191784287808
Speed C. W., Field I. C., Meekan M. G., Bradshaw C. J. A. (2010). Complexities of Coastal Shark Movements and Their Implications for Management. Marine Ecol. Prog. Ser. 408. doi: 10.3354/meps08581
Stevens J. D., Bonfil R., Dulvy N. K., Walker P. A. (2000). The Effects of Fishing on Sharks, Rays, and Chimaeras (Chondrichthyans), and the Implications for Marine Ecosystems. ICES J. Marine Sci. doi: 10.1006/jmsc.2000.0724
Torres P., Milla i Figueras D., Diogo H., Afonso P. (2022). Risk Assessment of Coastal Fisheries in the Azores (North-Eastern Atlantic). Fisheries Res. 246. doi: 10.1016/j.fishres.2021.106156
Vandeperre F., Aires-da-Silva A., Fontes J., Santos M., Serrão Santos R., Afonso P. (2014). Movements of Blue Sharks (Prionace Glauca) Across Their Life History. PloS One 9. doi: 10.1371/journal.pone.0103538
van Etten J. (2017). R Package Gdistance: Distances and Routes on Geographical Grids. J. Stat. Software 76. doi: 10.18637/jss.v076.i13
Villegas-Ríos D., Alós J., March D., Palmer M., Mucientes G., Saborido-Rey F. (2013). Home Range and Diel Behavior of the Ballan Wrasse, Labrus Bergylta, Determined by Acoustic Telemetry. J. Sea Res. 80. doi: 10.1016/j.seares.2013.02.009
Keywords: Sphyrna zygaena, essential fish habitat, shark nursery, acoustic telemetry, satellite telemetry
Citation: Afonso P, Gandra M, Graça G, Macena B, Vandeperre F and Fontes J (2022) The Multi-Annual Residency of Juvenile Smooth Hammerhead Shark in an Oceanic Island Nursery. Front. Mar. Sci. 9:844893. doi: 10.3389/fmars.2022.844893
Received: 28 December 2021; Accepted: 16 May 2022;
Published: 05 July 2022.
Edited by:
Nuno Queiroz, Centro de Investigacao em Biodiversidade e Recursos Geneticos (CIBIO-InBIO), PortugalReviewed by:
Píndaro Díaz-Jaimes, National Autonomous University of Mexico, MexicoCopyright © 2022 Afonso, Gandra, Graça, Macena, Vandeperre and Fontes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pedro Afonso, cGFmb25zb3BpbUBnbWFpbC5jb20=; Jorge Fontes, am9yZ2UubXIuZm9udGVzQHVhYy5wdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.