- 1Pós-graduação em Biologia Animal, Instituto de Biologia, Universidade Estadual de Campinas, Campinas, Brazil
- 2Instituto Do Mar, Universidade Federal de São Paulo, Santos, Brazil
- 3Departamento de Biologia Animal, Instituto de Biologia, Universidade Estadual de Campinas, Campinas, Brazil
Nematoda is a very species-rich phylum that has successfully adapted to almost all types of ecosystems. Despite their abundance and ecological importance, the taxonomic knowledge of nematodes is still limited and the identification of species is not trivial. In Cyatholaimidae, a relatively common and abundant family of free-living nematodes, the identification of organisms is challenging due to the overlap of some generic diagnoses and the absence of updated systematic reviews. Here we systematically reviewed the knowledge about the family diversity, providing a list of valid species, the diagnostic characters to genus level, and the geographical and habitat distribution of species. The review systematized a total of 619 records. The occurrences were classified into biogeographic ecoregions and habitats. Cyatholaimidae includes 211 valid species, classified in 20 genera. The genera can be differentiated based on six diagnostics characters, namely: pattern of cuticle ornamentation; number of longitudinal rows of pore-complex in cuticle; structures of the buccal cavity; presence/absence of pharyngeal bulb; pre-cloacal supplements aspect; and the shape of gubernaculum. Cyatholaimidae includes mainly marine species, mostly occurring in the Coastal Zone. Four and three species were registered in freshwater and terrestrial habitats, respectively, all classified in the genus Paracyatholaimus. About 38% of the valid species occur in more than one type of habitat, under very different environmental conditions, suggesting a broad niche. The occurrence of congeneric species in different habitats types indicates that, throughout the evolutionary history of the family, multiple ecological shift events have occurred. The family occurs worldwide in 74 ecoregions, and the majority of the records and species are in the North Sea and Western Mediterranean. Most species are endemic to one ecoregion, and examples of broadly distributed ones may be a result of misidentifications or cases of long-distance dispersal, especially for those associated with biological substrates.
Introduction
Nematodes are the most diverse and abundant representative of meiofauna in many marine environments (Giere, 2009); yet, it is estimated that more than half of existent species are yet to be discovered (Appeltans et al., 2012). The taxonomic impediment is the result of a combination of different aspects, such as the small size of the individuals, the apparent few morphological change over the evolutionary time, and the limited number of specialists (Giere, 2009; Fonseca et al., 2017). The presence of substantial cryptic diversity in marine nematodes (Derycke et al., 2005; Bhadury et al., 2008; De Oliveira et al., 2012) and the absence of data from many geographical regions (Venekey et al., 2010; Garraffoni et al., 2021), are also an obstacle for the taxonomy of the group. Such a lack of taxonomic knowledge hampers the assessment of species inventories which are the basis of ecological, monitoring, and impact assessment studies (Hortal et al., 2015). In these surveys, the marine nematodes are commonly identified only to genera or morphospecies level (e.g., Leduc et al., 2015; Corte et al., 2017; Netto and Fonseca, 2017; Spedicato et al., 2020), and even the identification to genus level may be challenging. This is particularly evident for the Cyatholaimidae Filipjev, 1918, where descriptions of some genera overlap with each other and there is no information about the taxonomic importance of the diagnostic characters (Miljutina and Miljutin, 2015; Leduc and Zhao, 2016). Given that Cyatholaimidae is a relatively diverse group with more than 200 valid species, and is among the most abundant free-living marine nematodes (e.g., Coull et al., 1982; Zeppilli and Danovaro, 2009; Santos and Venekey, 2017; Zhao et al., 2020), the misidentification of specimens from this family may cause a substantial effect on the study's conclusions.
Recent and complete taxonomic review represent the first step in achieving a rigorous species delimitation (Dayrat, 2005). A review including character diagnosis and information of species geographical and habitat distribution can help taxa identification and support future taxonomic, systematic, and biogeography studies (e.g., Fonseca and Decraemer, 2008; Venekey et al., 2014, 2019). Normally, in this type of survey, the data is presented following the political geographical units, a delimitation without biological meaning that may bias the conclusions (Whittaker et al., 2005). The application of biogeographic classifications is thus fundamental to guide conservation planning and to provide a framework to analyze the patterns of biodiversity (Olson et al., 2001; Spalding et al., 2007).
Despite its broad distribution and diversity, there is no updated taxonomic review and a list of the valid species of Cyatholaimidae. The most recent list was published in the past century (Gerlach and Riemann, 1973). Since then, many new species have been described (e.g., Vincx et al., 1982; Tchesunov, 2008; Huang and Xu, 2013) and many others were synonymized (e.g., Platt and Warwick, 1988; Cidreira et al., 2019) or considered inquerenda (e.g., Miljutina and Miljutin, 2015). The information available about the family is sometimes incongruent and incomplete, which represents an obstacle to species identification and descriptions and hinders the phylogenetic analysis of Cyatholaimidae.
In 1918, Filipjev established the subfamily Cyatholaimi within the Chromadoridae family. The systematic relationships of marine nematodes were reanalyzed by De Coninck and Schuurmans-Stekhoven (1933) and Cyatholaimidae was considered a family within Chromadoroidea order (currently Chromadorida Chitwood, 1933). The synapomorphy of Cyatholaimidae was defined as the position of the outer labial and cephalic setae in a single crown by Lorenzen (1981, 1994), however, this is not a unique characteristic within the order, and the diagnosis of the family is made with the combination of that character with the presence of 12 distinctly cuticularized rugae in the cheilostoma, multi-spiral amphids, cuticle covered with punctations, and anterior and posterior gonads always on opposite sides of the intestine (Lorenzen, 1981, 1994). Molecular phylogenies show that the group is monophyletic (Meldal et al., 2007; Holterman et al., 2008; Van Megen et al., 2009); nevertheless, the four subfamilies that are included in the family (Cyatholaiminae, Paracanthonchinae, Pomponematinae, and Xenocyatholaiminae) are not supported by morphological synapomorphies and are probably paraphyletic (Lorenzen, 1981; Leduc and Zhao, 2016).
In the present study, we review and organize the knowledge about the family, with a list of valid species, comments about the species distribution, and for each genus, we provide the diagnostic characters as well as taxonomical issues. A critical analysis of the geographical and habitat distribution patterns of these species and some future perspectives about the study of these taxa are elaborated.
Materials and Methods
The nematode checklist of Gerlach and Riemann (1973) was used as the starting point and all taxonomical studies published thereafter were considered in the present review. The diagnosis characters and comments about taxonomical issues are given for each genus. Since the relationship within the family is unknown and the subfamilies are probably non-monophyletic, we listed the genera in alphabetical order. A valid species list is provided including the species synonymized in the present study.
To investigate the distribution of the species we included data from ecological and taxonomic publications with identification to species level. Studies recording species of the family were searched in Nemys database (Bezerra et al., 2021) and Google Scholar. The search was made with each species name, full and abbreviated, within quotation marks (i.e., “Cyatholaimus gracilis” and “C. gracilis”). We also looked for records of synonymous species and did an active search for older publications. Given the lack of diagnostic characters of females and juveniles, we did not consider articles that recorded only them.
The habitat of occurrence of each species record was defined among eight categories: Soil (terrestrial environment), Hypersaline waters (hypersaline lakes), Freshwater (rivers and freshwater lakes), Brackish waters (estuaries, mangroves, brackish lagoons, and marshes), Coastal Zone (intertidal and subtidal regions of beaches, rocky shores and artificial coastal habitats, like pillars bridges), Continental Shelf (offshore until 200 m depth), Continental Slope (201 to 4,000 m depth), and Abyssal Zone (more than 4,000 m depth). The geographic distribution of each species recorded in Brackish waters, Coastal Zone, and Continental Shelf habitats was classified following the biogeographic regionalization for coastal and shelf areas proposed by Spalding et al. (2007). This system is composed of 12 Realms (largest spatial units), 62 Provinces (nested within the realms), and 232 Ecoregions (smallest-scale units with relatively homogeneous species composition, distinct from adjacent areas). The deep-sea environment (Continental Slope and Abyssal) was not considered in this classification, and we indicated the ocean and the hemisphere of occurrence for the species found there.
Results
Since Gerlach and Riemann (1973), three new genera and more than 60 Cyatholaimidae species have been described (see section Taxonomic Review: Genus Diagnoses, Distribution, and Relationships and the list of valid species in Supplementary Material 1). On average, almost two species per year have been described. The peak of taxonomic studies of the family happened between the decades of 1950 and 1970 when more than 80 species were described (Figure 1). The main contributors to this “Golden-Age” for cyatholaimids were Sebastian Gerlach and Wolfgang Wieser. Gerlach discovered a total of 19 Cyatholaimidae species considered valid today in materials from Italy (Gerlach, 1953a), Chile (Gerlach, 1953b), Madagascar (Gerlach, 1953c), France (Gerlach, 1954), El Salvador (Gerlach, 1955), Germany (Gerlach, 1956), Brazil (Gerlach, 1957a,b), Egypt (Gerlach, 1964a), Maldives (Gerlach, 1964b), and Saudi Arabia (Gerlach, 1967). Wieser also worked with material from different regions all over the world and described 28 species alone (1954a-Chile, 1954b-Italy, 1955-Japan, 1959-USA) and with Hopper (1967-USA).
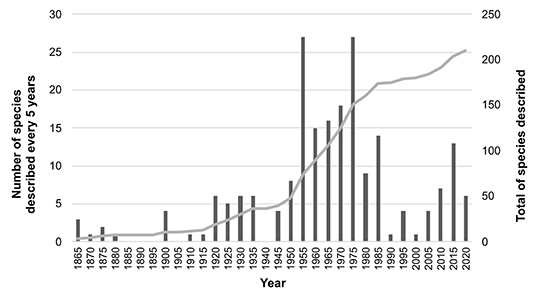
Figure 1. Number of species descriptions of Cyatholaimidae along the years. The bars indicated the number of descriptions every 5 years and the line shows the accumulated data.
Taxonomic Review: Diagnosis and Relationships of the Family Cyatholaimidae
Class Chromadorea Inglis, 1983
Order Chromadorida Chitwood, 1933
Family Cyatholaimidae Filipjev, 1918
Diagnosis [from Leduc and Zhao (2016)]: Cuticle with transverse rows of punctations. Lateral punctations may be larger, irregular, or arranged in longitudinal rows. Longitudinal rows of circular or elliptical cuticular structures, called lateral pore-like structures, often present along mediolateral lines; up to 12 longitudinal rows of pore complexes may also be present. Inner labial sensilla often setiform; six outer labial setae and four cephalic setae in a single crown; outer labial setae longer than the cephalic setae. Multispiral amphideal fovea. Cheilostoma with 12 distinctly cuticularised rugae. Pharyngostoma with a large dorsal tooth, and usually with two smaller ventrosublateral teeth, which may be single or double. Pharynx usually without a posterior bulb. Female didelphic-amphidelphic with reflexed anterior and posterior gonads always on opposite sides of the intestine. Male usually with two testes, rarely with one. Pre-cloacal supplements may be present or absent.
Phylogenetic relationships: Cyatholaimidae seems to be monophyletic according to different molecular phylogenies, however, all these studies used an average of five sequences from species of the family (Meldal et al., 2007; Holterman et al., 2008; Van Megen et al., 2009; Avó et al., 2017). The non-monophyly of the subfamilies (Cyatholaiminae Filipjev, 1918, Paracanthonchinae De Coninck, 1965, Pomponematinae Gerlach and Riemann, 1973, and Xenocyatholaiminae Gerlach and Riemann, 1973) was suggested due to the absence of synapomorphic characters (Gerlach and Riemann, 1973; Lorenzen, 1981, 1994), and it was corroborated by the molecular phylogenetic tree available for the family (Leduc and Zhao, 2016). This phylogeny was based on only a few rDNA sequences of small subunit (SSU) and D2-D3 of the large subunit (LSU) from a few species (14 and six species, respectively).
Remarks: Drawings of head and tails of typical species of Cyatholaimidae genera are available in the Handbook of Zoology (Tchesunov, 2014—Figure 7.96; 7.97—p. 386 and 388). In this chapter, the genera Minolaimus Vitiello, 1970, Parapomponema Ott, 1972, and Propomponema Ott, 1972 were listed within Cyatholaimidae; however, the first had previously been transferred to Comesomatidae family (Hope and Zhang, 1995), and the other two were later synonymized with Pomponema (Cidreira et al., 2019). Schemes of the principal structures that are necessary for the identification may be more informative than an illustration of the type species, given the great morphological variety of most genera. Six diagnostics characters are generally sufficient to differentiate the genera and are presented in the polytomous identification key (Table 1). The table is organized in alphabetical order. The different ornamentation patterns of the cuticle and the shape of pre-cloacal supplements are schematized in Figures 2, 3, respectively. The shape of the gubernaculum (copulatory structure) is commonly considered in genus delimitation; however, this character hampers the identification of females and juveniles, and it can be very variable even within the genera (Figure 4). The pore complex, a structure commonly present in the cuticle of Cyatholaimidae species, is frequently poorly described and currently, it is only considered to separate Longicyatholaimus and Marylynnia genus. Nevertheless, it was suggested that this character may be phylogenetic informative (Leduc and Zhao, 2016), thus we included it in the Table 1.
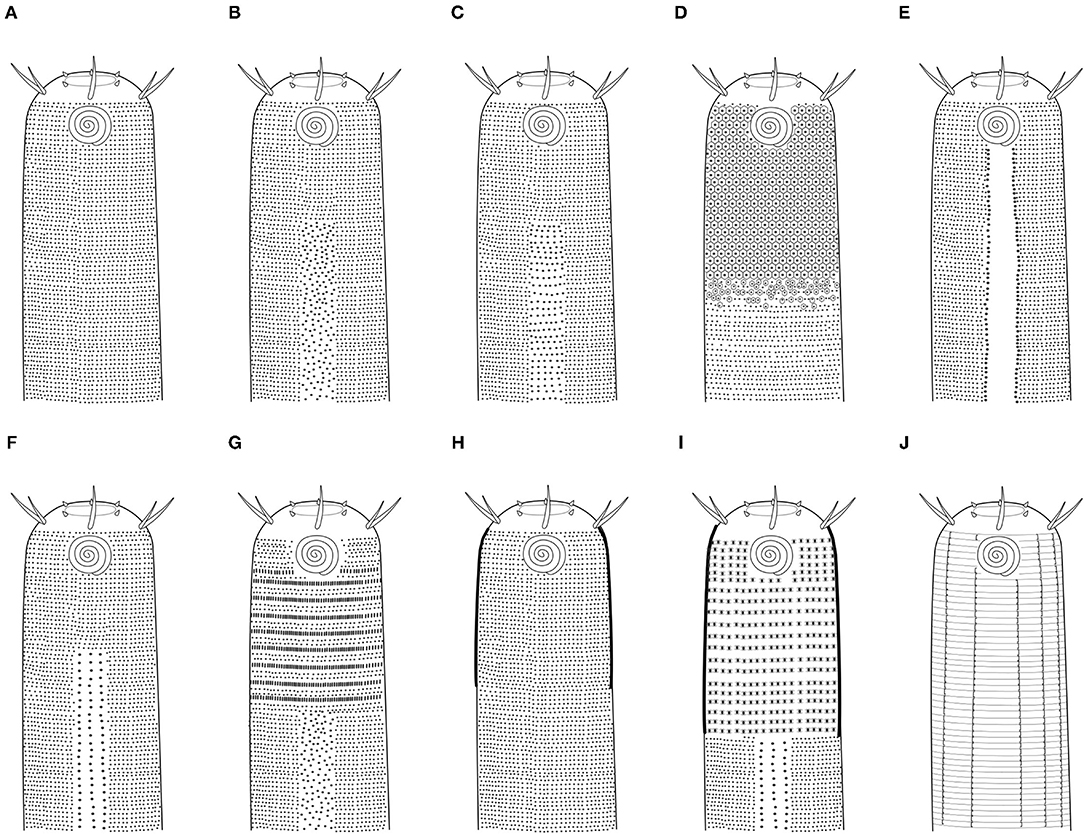
Figure 2. Cuticle pattern of Cyatholaimidae species. (A) Homogeneous, without lateral differentiation; (B) Homogeneous, with lateral differentiation of larger and more sparsely dots irregularly arranged; (C) Homogeneous, with lateral differentiation of larger and more sparsely dots regularly arranged; (D) Heterogeneous, with enlarged hexagonal punctations in the anterior end; (E) Homogeneous, with lateral differentiation of longitudinal rows of enlarged punctations with broad lateral fields between them; (F) Homogeneous, with lateral differentiation of longitudinal rows of punctations; (G) Heterogeneous, with alternating pattern of one or two rows of simple dots and one row of longitudinal bars consisting of two fused dots in anterior end; (H) Heterogeneous, with cuticle in the head thicker; (I) Heterogeneous, with cuticle in the head thicker, and with lateral differentiation of longitudinal rows of enlarged dots; (J) Homogeneous, with rings smooth, not dotted and adorned with 10 marked longitudinal lines.
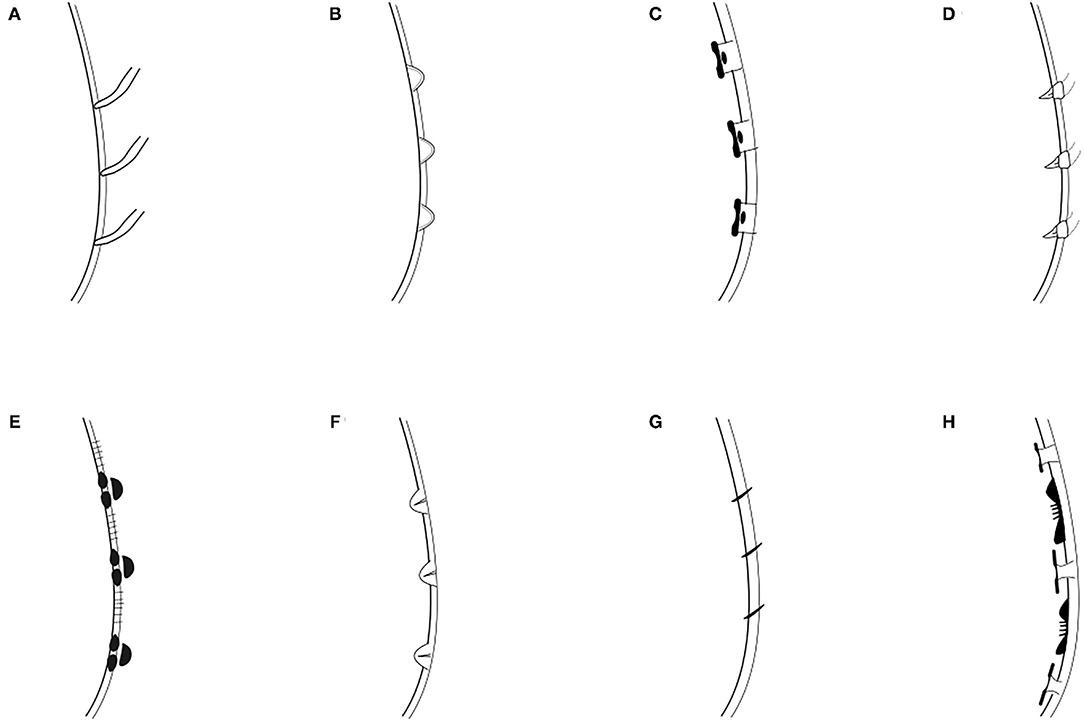
Figure 3. Pre-cloacal supplements aspects of Cyatholaimidae species. (A) Tubular; (B) cup-shaped; (C) complex, consisting of a stout cylindrical body having a flat cover with a central opening; (D) papilla outer piece and inner tubular piece; (E) plate-shaped outer piece and inner cup-shaped piece; (F) pappiloid; (G) setae-like; (H) complex, with cuticle lamellated between supplements.
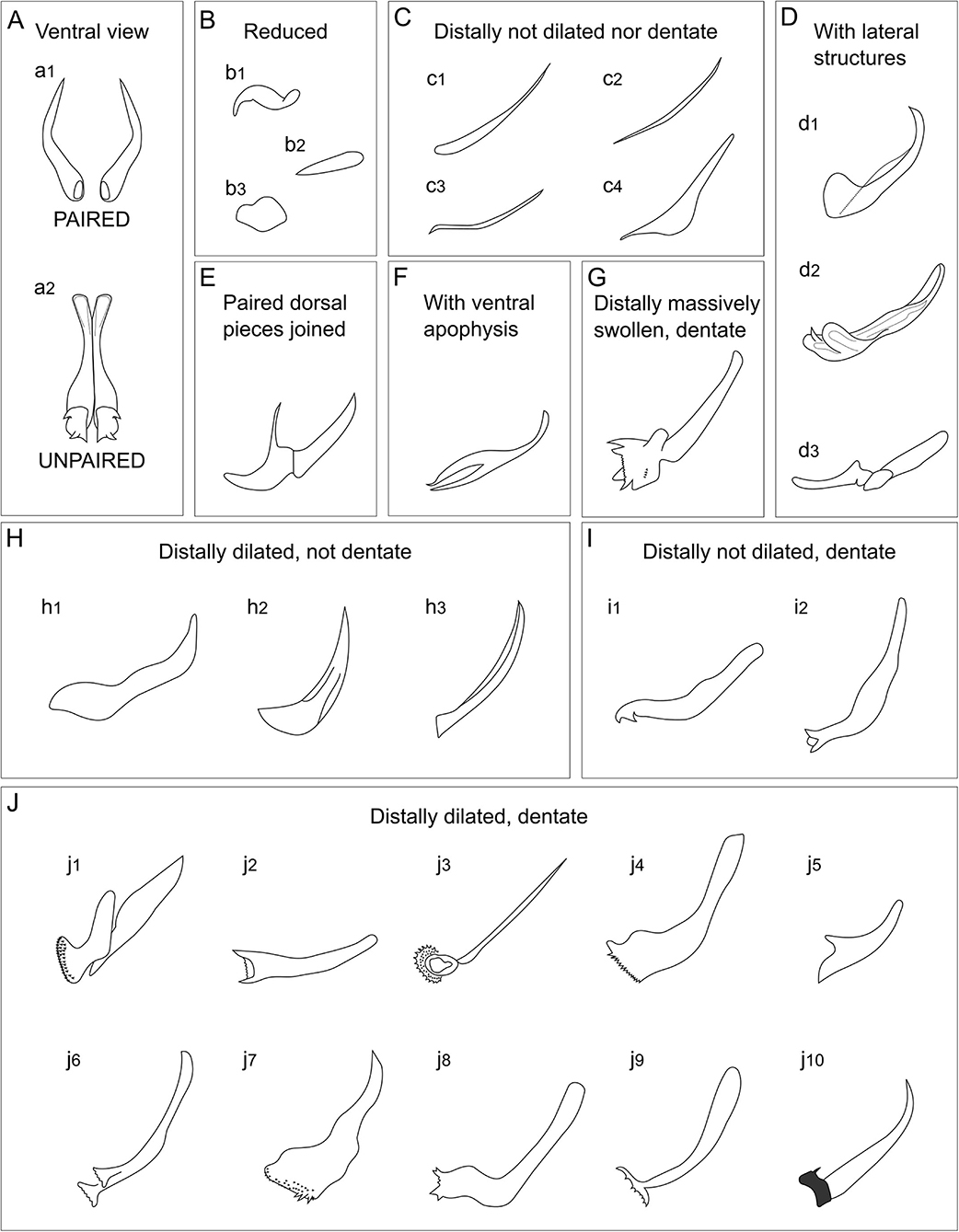
Figure 4. Examples of the different gubernaculum types of Cyatholaimidae species. (A) Ventral view showing a gubernaculum (a1) paired and an (a2) unpaired. (B–J) Lateral view. (B) Reduced, (b1) Paracyatholaimoides asymmetricus, (b2) Craspodema octogoniata, (b3) Xenocyatholaimus delamarei; (C) Distally not dilated nor dentate, (c1) Biarmifer laminatus, (c2) Paracyatholaimoides mutispiralis, (c3) Paracyatholaimus diva, (c4) Paralongicyatholaimus minutus; (D) With lateral structures, (d1) Nannolaimoides decoratus, (d2) Xyzzors fitzgeraldae, (d3) Pomponema reductum; (E) Paired dorsal pieces joined, Craspodema reflectans; (F) With ventral apophysis, Isacanthonchus obesus; (G) Distally massively swollen, dentate, Praeacanthonchus punctatus; (H) Distally dilated, not dentate, (h1) Paracanthonchus platypus, (h2) Paramarylynnia subventrosetata, (h3) Paracyatholaimus intermedius; (I) Distally not dilated, dentate, (i1) Paracanthonchus cochlearis, (i2) Marylynnia annae; (J) Distally dilated, dentate, (j1) Biarmifer madrynensis, (j2) Cyathoshiva amaleshi, (j3) Longicyatholaimus longicaudatus, (j4) Marylynnia preclara, (j5) Metacyatholaimus cylindribucca, (j6) Metacyatholaimus delicatus, (j7) Paracanthonchus macrodon, (j8) Paracanthonchus caecus, (j9) Paracyatholaimus pesavis, (j10) Phyllolaimus tridentatus.
Taxonomic Review: Genus Diagnoses, Distribution, and Relationships
Genus Acanthonchus Cobb, 1920
Diagnosis: Cuticle with lateral differentiation absent or present of larger and more wide-spaced dots (Figures 2A–C); pre-cloacal supplements tubular (Figure 3A), the anterior-most much larger than the others; and gubernaculum paired (Figure 4A) usually dilated and dentated distally (similar to Figures 4J4,7) (Wieser, 1955). The species are differentiated mostly by the presence or absence of a dorsal tooth, the size of amphids in relation to the corresponding diameter of the body, the number of pre-cloacal supplements, and the number of post cloacal setae (Wieser, 1955).
Remarks: The Acanthonchus species were found in eight different realms, mostly in the Temperate Northern Pacific (six species). Except for A. arcuatus, A. singaporensis, and A. tridentatus that presents a distribution restricted to only one ecoregion, the other species have a broader distribution and occur in two to six ecoregions sometimes very far apart. A. gracilis, for example, was recorded in the North Sea (Ditlevsen, 1918; Gerlach, 1958), Baltic Sea (Allgén, 1929, 1953), Yellow Sea (Steiner, 1921), and Panama Bight (Allgén, 1947), and A. viviparus was registered in six ecoregions (Table 2). Most species were found on the Coastal Zone and/or in the Continental Shelf associated with macroalgae. Wieser (1955) classified the subgenera Acanthonchus and Seuratiella that are distinguished only by the presence or absence of a dorsal tooth and ocelli. These are previously separated genera that need to be carefully studied and they might prove to be synonymous (Wieser, 1955).
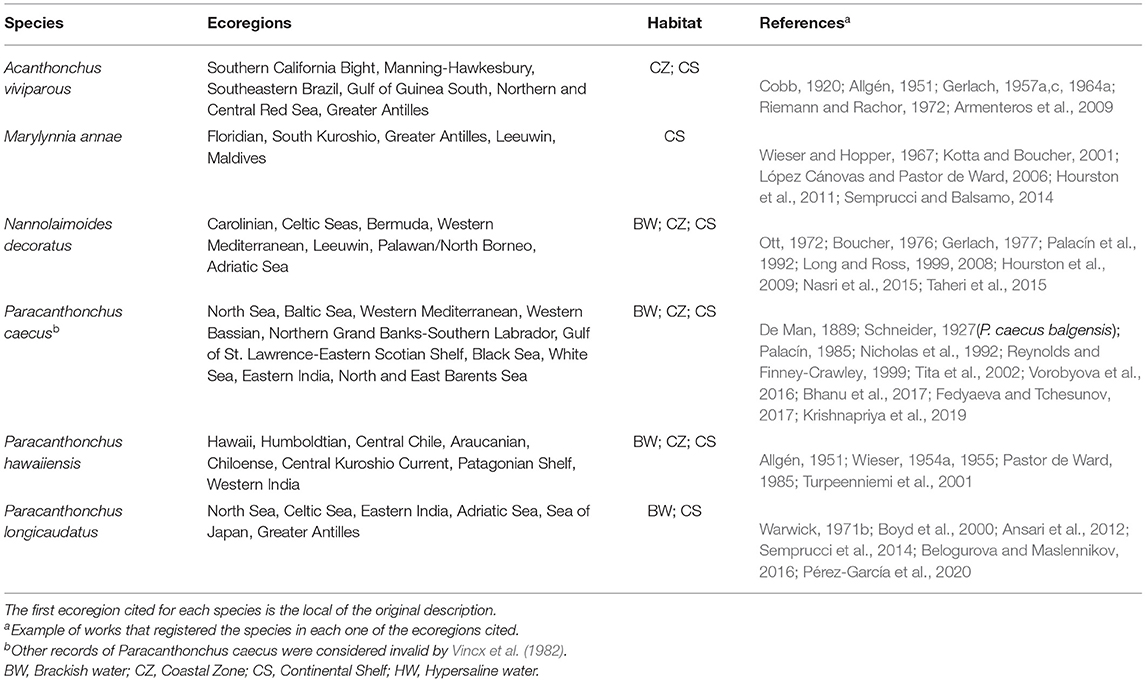
Table 2. Cyatholaimidae species most broadly distributed, recorded in more than five ecoregions nested in at least four different realms.
Genus Biarmifer Wieser, 1954
Diagnosis
Cuticle configuration heterogeneous, with enlarged hexagonal punctations in transverse rows from the anterior end to the nerve ring and transverse rows of simple punctations on the rest of the body (Figure 2D), unique in the family; pre-cloacal supplements cup-shaped (Figure 3B); and gubernaculum aspect variable, distally dilated or not, dentate or not (Figures 4C1,J1; Pastor de Ward, 2001). The species can be differentiated for each other mostly by the length of the head sensilla, the shape of copulatory organs, and the format of the tail.
Remarks
Until now, there were only three Biarmifer species described, recorded in the Coastal Zone or Continental Shelf habitats on Temperate South American realm. Here, we transfer three Marylynnia species to this genus (see section Genus Marylynnia Hopper, 1972; Hopper, 1977). B. hopperi (Sharma and Vincx, 1982) comb. n. were found on a coastal habitat of the Temperate Northern Pacific. B. dayi (Inglis, 1963) comb. n. and B. punctata (Jensen, 1985) comb. n. were described from the Continental Shelf of the Temperate Southern Africa and Temperate Northern Atlantic, respectively. The species of the genus have been found in sublittoral sand, and B. laminatus was also found associated with algae.
Genus Craspodema Gerlach, 1956
Diagnosis
Presence of lateral differentiation in the cuticle very prominent as longitudinal rows of enlarged punctations with broad lateral fields between them (Figure 2E); pre-cloacal supplements complex, consisting of a stout cylindrical body having a flat cover with a central opening (Figure 3C); and gubernaculum reduced (Figure 4B2) or consisting of paired dorsal pieces joined (Figure 4E; Semprucci and Burattini, 2015). The species can be differentiated by amphideal fovea and buccal cavity structure (Semprucci and Burattini, 2015).
Remarks
There are two species described for the genus, both recently reanalyzed by Semprucci and Burattini (2015). C. octogoniata, first described for the Coastal Zone of the Western Mediterranean, was posteriorly found on the Continental Shelf (Boucher, 1976) and the Brackish water habitats of the ecoregion (Nasri et al., 2015). C. reflectans was described for Continental Shelf from the Maldives and was also found on Western Mediterranean, on the Continental Shelf (Beyrem and Aissa, 1998), and Coastal Zone (Boufahja et al., 2015).
Genus Cyatholaimus Bastian, 1865
Diagnosis
Cuticle homogeneous without lateral differentiation (Figure 2A); pre-cloacal supplements absent; and gubernaculum unpaired (Figure 4B), distally swollen, squarish, with large pointed teeth (similar to Figure 4G; Tchesunov, 2014). The differences between the species are mostly based on the body size, length of head sensilla and reproductive male structures.
Remarks
The majority of species classified in the type genus of the family were described in the past century and many were transferred to other genera or considered taxon inquerendum (Gerlach and Riemann, 1973; Bezerra et al., 2021). Here we add three more species as inquerendae for having been described based only on females and/or juveniles: C. canariensis Steiner, 1921, C. crassus Kreis, 1963, and C. unalaskensis Allgén, 1957. Two-thirds of the valid species were first described in the Temperate Northern Atlantic realm. The majority seems to have a more restricted distribution and only C. gracilis was recorded in several locations, including the Temperate South America and in the Southern Ocean (Allgén, 1959). Nevertheless, the records of this species outside the Temperate Northern Atlantic were considered unreliable based on the descriptions provided (Inglis, 1961). After that, C. gracilis was recorded from the Red Sea (Western Indo-Pacific realm; Riemann and Rachor, 1972). Most valid species were recorded in Coastal habitats, only one is also found on Continental Shelf (C. microsetosus) and a few were found in the Brackish water. The unique species of the family that is probably a parasite of an Isopoda is classified in this genus (C. cirolanae). The diagnostics characteristics of this genus are not always sufficient for classification, since it can also occur in other genera, such as Paracanthonchus, and the arrangement of the gubernaculum is difficult to observe, visible only in ventral view (Wieser, 1954b). Therefore, the group urgently needs a systematic revaluation.
Genus Cyathoshiva Datta, Miljutin, Chakraborty and Mohapatra, 2016
Diagnosis
Cuticle transversely punctated with lateral differentiation in form of slightly enlarged dots hardly visible along the body, except in tail (Figure 2C); buccal cavity with well-developed dorsal tooth and two subventral teeth; supplements having basal tubular part inserted mostly within the body and distal part in shape of indistinct papilla or short setae (Figure 3D); and proximally unpaired and distally paired gubernaculum, dilated and dentate (Figure 4J2; Datta et al., 2016).
Remarks
A monospecific genus recorded exclusively on the Coastal Zone of Northern Bay of Bengal.
Genus Dispira Cobb, 1933
A monospecific genus found only once on a beach in the ecoregion Virginian. Here we considered Dispira as incertae sedis based on the organization of the head sensilla in three circles with six setae each, which goes against the synapomorphy of the family.
Genus Isacanthonchus Gagarin and Nguyen Vu Thanh, 2008
Diagnosis
Lateral differentiation of cuticle formed by two longitudinal rows of dots (Figure 2F); pre-cloacal supplement tubular (Figure 3A) arranged in two rows; and gubernaculum paired (Figure 4A) with ventral apophysis (Figure 4F; Gagarin and Nguyen Vu Thanh, 2008).
Remarks
The single species described in this genus was recorded on a mangrove in the Gulf of Tonkin, in Vietnam.
Genus Longicyatholaimus Micoletzky, 1924
Diagnosis
Presence of four complete longitudinal rows of pore complex in the cuticle; none or only one lateral pore-like structure on the tail; lateral differentiation of cuticle of larger and more wide-spaced dots (Figure 2C) sometimes in form of longitudinal rows of punctations (Figure 2F); pre-cloacal supplement usually present, cup-shaped and non-sclerotized (Figure 3B) (conical setae protruding from the body of the cup-shaped supplements in L. maldivarum Gerlach, 1964); and gubernaculum distally dilated with numerous denticles (Figure 4J3; Hopper, 1972; Semprucci et al., 2017). The species can be differentiated mostly by the type of lateral differentiation of the cuticle, the length of the head sensilla, and the shape of gubernaculum and spicules.
Remarks
The records of Longicyatholaimus species came from Brackish water, Coastal Zone, Continental Shelf, and Continental Slope habitats of six different realms from all oceans. The only species broader distributed is L. longicaudatus found in six ecoregions (see Table 2). The classification in this genus of species with lateral fields arranged in form of longitudinal rows is debatable and their taxonomy position needs careful attention [see Hopper (1972) and Semprucci et al. (2017)].
Genus Marylynnia Hopper, 1972; Hopper, 1977
Diagnosis
Presence of eight, 10, or 12 longitudinal rows of pore complex on the cuticle; lateral differentiation on the cuticle with larger and more widely spaced punctations (Figure 2C); lateral pore-like structures present on the tail; pre-cloacal supplements cup-shaped (Figure 3B); and gubernaculum distally dentate, dilated or not (Figures 4I2,J4). Marylynnia species can be differentiated from each other by the length of the head sensilla, the shape of the gubernaculum, and the format of the tail (Hopper, 1972).
Remarks
There are records of Marylynnia species in many regions all over the world, occurring on Brackish water, Continental Slope, and most on Coastal Zone and Continental Shelf habitats. Most species seem to have a restricted geographical distribution, and 10 were recorded in more than one ecoregion. M. annae have a broader distribution and was found in five ecoregions nested in four different realms (Table 2). The genus was established by Hopper (1972) for Longicyatholaimus species having eight, ten, and 12 longitudinal rows of pore complex on the cuticle. We here transfer the species M. denticulata Kim Tchesunov and Lee, 2015 for the genus Paracanthonchus, based on the presence of tubular pre-cloacal supplements. M. dayi (Inglis, 1963) Hopper, 1977, M. hopperi Sharma and Vincx (1982), and M. punctata Jensen, 1985 are transferred to Biarmifer genus by having the typical cuticle configuration of it, with enlarged hexagonal punctations in transverse rows in the anterior end. Also, M. dayi and M. punctata have only four longitudinal rows of pore complex.
Genus Metacyatholaimus Schuurmans Stekhoven, 1942
Diagnosis
Lateral differentiation of three to five longitudinal rows of punctations on the cuticle (Figure 2F; except in M. effilatus, which possesses 4–12 longitudinal rows of punctations); buccal cavity with small to medium-sized dorsal tooth and smaller subventral teeth usually present; pharynx with posterior bulb; pre-cloacal supplements absent; and gubernaculum distally dilated or not, with or without denticles or horns (Figures 4J5,6; Leduc and Zhao, 2016). The species are distinguished among them mostly by the number of longitudinal rows of dots in the lateral differentiation of cuticle, position and the number of turns of amphideal fovea, and in the shape of spicules and gubernaculum (Vidakovic et al., 2003; Leduc and Zhao, 2016).
Remarks
Despite the majority of species registers being from the Continental Shelf, there are representatives of Metacyatholaimus in all marine habitats categorized here, including M. chabaudi and M. delicatus from the deep-sea. Only M. cylindribucca was recorded in the Brackish water (Jouili et al., 2018), however, it had been first described for Continental Shelf habitat. Most species were found in the Mediterranean Sea, but there are also records in other regions of the world, like M. delicatus from Temperate Australasia realm and M. spatiosus from Temperate South America. The position of M. brevicollis, and M. papillatus in this genus is doubtful due to the presence of pre-cloacal supplements, however, we opt to maintain them within the genus for now. Both possess complex supplements with several elements separated by a lamellated cuticle, a structure similar to supplements from individuals of the Pomponema genus.
Genus Nannolaimoides Ott, 1972
Diagnosis
Cuticle homogeneous or heterogeneous with lateral differentiation irregular (Figures 2B,G); buccal armature weakly developed with no denticles; pre-cloacal supplements consisting of several elements (Figure 3E); and lateral flanges in the distal end of gubernaculum (Figure 4D1; Ott, 1972). The species can be differentiated by the length of head sensilla, tail length, and cuticle ornamentation homogeneous or heterogeneous throughout the body (Ott, 1972; Platt and Warwick, 1988).
Remarks
Despite there being only three species described of this genus, it presents a broad habitat distribution and was recorded on Brackish water, Coastal Zone, and Continental Shelf habitats. The type species is N. armatus described for a Continental Shelf habitat in Maldives ecoregions. N. decoratus and N. effilatus were first described in the Temperate Northern Atlantic realm and posteriorly registered in many other regions around the world. The first one, for example, was recorded in seven ecoregions included in four different realms (see Table 2).
Genus Nyctonema Bussau, 1993
This monospecific genus was considered unaccepted since it was described only in gray literature. Recently, the nomenclatural status of the nematode nomina included in this thesis was evaluated and considered valid (Holovachov, 2020). However, the six outer labial setae and four cephalic setae are organized in two separated crowns in Nyctonema, and here we considered this genus as incertae sedis.
Genus Paracanthonchus Micoletzky, 1924
Diagnosis
All characteristics used on identification can be found in other genera of the family, and it is diagnosed only by the unique combination of the characters. Cuticle homogeneous with or without lateral differentiation of slightly larger and/or more sparsely, irregularly arranged dots (Figures 2A–C); buccal cavity in the vast majority of species with large pointed dorsal tooth and smaller ventro-sublateral teeth; tubular supplements (Figure 3A); and proximally paired gubernaculum (Figure 4A), frequently distally dilated, very variable in shape (Figures 4H1,I1,J7,8; Miljutina and Miljutin, 2015).
Remarks
Species of the Paracanthonchus genus were described from all oceans and latitudes, most of them recorded in the Temperate Northern Atlantic realm (20 species). More than 90% of species are described in the coastal habitats and a few were recorded from the Continental Shelf and Abyssal region. The species are found not only in soft sediments but also on algae as well as on other hard substrates. This species-rich genus was recently revised by Miljutina and Miljutin (2015) where it was listed 72 species, of which 20 were designated as species inquirenda. P. medius as omitted of this revision without explanation, and we here considered the species as valid. Since then, another four species have been described. Here we transfer two species to this genus (see sections Genus Marylynnia Hopper, 1972; Hopper, 1977 and Genus Paracyatholaimus Micoletzky, 1922): Paracanthonchus denticulata (Kim, Tchesunov and Lee, 2015) comb. n. and Paracanthonchus duplicatus (Gerlach, 1964) comb. n. Besides being commonly found in meiofauna surveys, the taxonomy of Paracanthonchus species is challenging mainly due to the absence of apomorphic morphological characters and the great morphological variation between species that in many cases lead to overlap between the descriptions and the diagnosis of other genera. The molecular phylogeny study of Cyatholaimidae species suggests that Paracanthonchus is not a monophyletic group (Leduc and Zhao, 2016).
Genus Paracyatholaimoides Gerlach, 1953
Diagnosis
Cuticle in the head region thickened (Figure 2H); tight spiral turn of the amphid; conspicuous dimorphism sexual in the size of amphideal fovea; pre-cloacal supplements papilloid (Figure 3F); and gubernaculum distally not dilated nor dentate (Figures 4B1,C2; Gerlach, 1953a; Riemann, 1966). The species can be distinguished from each other by the form of buccal sensilla and the shape of the gubernaculum.
Remarks
There are three species of this genus described for the Temperate Northern Atlantic and only one, P. serpens, that was first recorded in the Temperate South America realm on a Coastal habitat. The Paracyatholaimoides species seems to have restricted distribution, except by P. multispiralis, that was registered in the Coastal Zone and Continental Shelf habitats in five ecoregions nested in two different realms, Temperate Northern Atlantic (Gerlach, 1953a,d,e,f; Somerfield et al., 2007; Ellis et al., 2010) and Tropical Atlantic (Pérez-García et al., 2020). The systematic value of the characteristics on which the genus erection was based is controversial and a taxonomy review is needed (Riemann, 1966).
Genus Paracyatholaimus Micoletzky, 1922
Diagnosis
Cuticle without lateral differentiation (Figure 2A); pre-cloacal supplements papilloid or as setae-like organs half inserted into the body (Figures 3F,G); and gubernaculum hardly dilated at the distal end, without pronounced teeth (Figures 4C3,H3), but sometimes plate-shaped with denticles (Figure 4J9; Platt and Warwick, 1988; Tchesunov, 2008). The species of Paracyatholaimus can be distinguished from each other mainly by the length of the head sensilla, the number of turns in the amphideal fovea, supplementary organs, and copulatory apparatus shape (Tchesunov, 2008).
Remarks
Species classified in the Paracyatholaimus genus were recorded in all oceans and latitudes and were found in all types of habitats. P. intermedius has a remarkable distribution: it is a mainly brackish species (e.g., de Man, 1880; Warwick, 1971a), however, it was also recorded from marine (e.g., Kreis, 1963; Gerlach, 1965), freshwater (e.g., Riemann, 1966; Gagarin and Nguyen Vu Thanh, 2012; Gusakov and Gagarin, 2012), and terrestrial environment (Loof, 1961; Nzeako et al., 2019). Three species were found exclusively in the Freshwater habitats: P. lewisi Coomans, Vincx and Decraemer, 1985, P. paramonovi, and P. truncatus. Here we considered P. lewisi as species inquirenda because the original description is based on only females and juvenile specimens. There are also two species recorded in the abyssal zone, P. comatus from the Southeast Pacific Ocean and P. diva found in the Southeast Atlantic Ocean. Andrássy (1973) transferred the species Xyzzors inglisi Wieser and Hopper, 1967 to this genus based on the pre-cloacal supplement and gubernaculum shape. This new combination was omitted by Tchesunov (2008) and Huang and Xu (2013) without explanations, however, we agree with Andrássy (1973). When describing P. duplicatus, Gerlach (1964b) raised the possibility that the species may be classified in Paracanthonchus genus due to the tubular aspect of pre-cloacal supplements. Here we decide to make that transfer based on the shape of supplements and the strong expansion of the gubernaculum in the distal part, typical of Paracanthonchus.
Genus Paralongicyatholaimus Schuurmans Stekhoven, 1950
Diagnosis
Cuticle without lateral differentiation (Figure 2A); buccal cavity without tooth; pharynx with posterior bulb; pre-cloacal supplements absent; and the distal end of gubernaculum not dilated nor dentated (Figure 4C4; Warwick, 1971b). The species are differentiated mostly by the form of labial sensilla, the shape of gubernaculum, and the size of amphids in relation to the corresponding diameter of the body.
Remarks
Most Paralongicyatholaimus species were described for the Continental Shelf of Temperate Northern Atlantic realm, except for P. complicatus from the Coastal Zone in Temperate South America. P. minutus and P. macramphis are the broader distributed, recorded in five (Warwick, 1971b; Boyd et al., 2000; Chinnadurai and Fernando, 2007; Sajan and Damodaran, 2007; Ansari et al., 2012; Lampadariou and Eleftheriou, 2018) and three ecoregions (Lorenzen, 1972; Long and Ross, 1999; Pérez-García et al., 2020), respectively.
Genus Paramarylynnia Huang and Zhang, 2007
Diagnosis
Cuticle without lateral differentiation (Figure 2A); buccal cavity with a dorsal tooth and paired subventral teeth; and distal region of gubernaculum dilated no dentate (Figure 4H2; Huang and Zhang, 2007). The pre-cloacal supplements were observed only in P. stenocervica and the authors indicated the aspect tubular of the supplements, however, this is not clear on drawings and images of the description and the character needs to be interpreted with caution. The species are differentiated mostly by the tail shape and by cuticle ornamentation homogeneous or heterogeneous throughout the body (Huang and Sun, 2011).
Remarks
All three species of the genus were described and only recorded on the Continental Shelf of the Yellow Sea.
Genus Phyllolaimus Murphy, 1963
Diagnosis
Lips with distinctive foliaceous development, unique in the family; cuticle without lateral differentiation (Figure 2A); pre-cloacal supplements papilloid (Figure 3F); and gubernaculum distally dilated, dentate, and heavily sclerotized (Figure 4J10). They can be distinguished from each other by the number of amphid turns and the number of subventral teeth on the buccal cavity (Murphy, 1963).
Remarks
Both species of this genus were recorded on the intertidal region of beaches one in the Temperate Northern Atlantic and the other in the Temperate Northern Pacific realm. The two species were described based on a few specimens and the structure of the lips, on which the genus erection was based, may be artifacts of the fixation (Wieser, 1959), so the validity of the genus remains to be confirmed.
Genus Pomponema Cobb, 1917
Diagnosis
Cuticle heterogeneous, commonly thicker in head with the punctations appearing Y-shaped in lateral view and/or with more widely spaced dots, with or without lateral differentiation of longitudinal rows of enlarged dots occasionally with slit-like markings (Figures 2C,H,I); buccal cavity armed with a pointed dorsal tooth, subventral teeth, and with or without additional minute denticles; pre-cloacal supplements complex consisting of several elements and cuticle between supplements lamellated (Figure 3H); and gubernaculum distally with various structures, sometimes with L-shaped lateral plates or lateral flanges bearing blunt teeth (Figure 4D3; Wieser and Hopper, 1967; Cidreira et al., 2019). The valid species can be differentiated mostly by the lateral differentiation of the cuticle, the presence or absence of denticles in the buccal cavity, the length of head sensilla, the number of amphideal turns, the gubernaculum shape, and the number of pre-cloacal supplements.
Remarks
The Pomponema species were found in almost all oceans, but more than half of them were first described in the Temperate Northern Atlantic realm. They were recorded in all marine habitats here categorized, mostly in Coastal Zone (19 species) and Continental Shelf (18 species) habitats. P. tautraense seems to be the species more broadly geographically distributed of the genus and was recorded in eight ecoregions nested in two realms (see Supplementary Material 2). Here we considered P. multisupplementa Huang and Zhang, 2014 as incertae sedis for having tubular supplements, amphideal fovea circular, and a pharyngeal bulb.
Genus Praeacanthonchus Micoletzky, 1924
Diagnosis
Cuticle without lateral differentiation (Figure 2A); buccal cavity without subventral teeth; pre-cloacal supplements tubular (Figure 3A); and gubernaculum proximally unpaired with massive squarish distal ends with teeth (Figure 4G; Platt and Warwick, 1988). The main characteristics used to differentiate the species are the shape of spicules and gubernaculum.
Remarks
All Praeacanthonchus species are found on coastal habitats, however, a few species are also recorded on Brackish water and/or on Continental Shelf locations. They can be associated with algae substrate or live within the sediment. Most were first described from the province of Northern European Seas, and P. cygnis was described from the Southwest Australian Shelf. P. kreisi and P. punctatus were recorded in Temperate Northern Atlantic and Temperate South America, two realms that are very far apart. The first one was also recorded in the Southern Ocean (Allgén, 1959).
Genus Xenocyatholaimus Gerlach, 1953
Diagnosis
Cuticle made up of rings smooth, not dotted, and adorned with ten marked longitudinal lines (Figure 2J), configuration unique in the family; pre-cloacal supplements absent; and gubernaculum reduced (Figure 4B3).
Remarks
The single species of this genus was recorded on the Coastal Zone of Southeast Madagascar.
Genus Xyzzors Inglis, 1963
Diagnosis
Presence of irregular lateral differentiation in the cuticle (Figure 2B); cuticular rings on the buccal cavity; spicules with double proximal ends; pre-cloacal supplements cup-shaped (Figure 3B); and gubernaculum with a lateral process (Figure 4D2; Inglis, 1963). The species are mostly differentiated by the shape of the copulatory apparatus and the length of the head sensilla.
Remarks
There are two valid Xyzzors species from the Atlantic Ocean. X. fitzgeraldae was recorded only in a Continental Shelf region in ecoregion Namaqua (Temperate Southern Africa realm). X. iubatus was found on coastal habitats in the ecoregion Carolinian (Temperate Northern Atlantic realm).
Habitat Distribution
A total of 288 studies were considered in the present review, including the publications with the original taxonomic descriptions (see Supplementary Material 3). The data of geographical and habitat distribution retrieved from a total of 619 records are available in Supplementary Material 2. About 48% of valid species (101 out of 211) were recorded only from their original description. Cyatholaimidae species are mainly marine, but they are also found in freshwater and terrestrial environments, and one species, Cyatholaimus cirolanae, is possibly a parasite of Isopoda. The majority of species are recorded exclusively in one habitat (~62%), and 81 species are present in more than one (Figure 5). The habitat distribution of species from most genera is broad, being that only Metacyatholaimus, Pomponema, and Paracyatholaimus occur in all marine habitats, and this last is the single one recorded in a terrestrial and freshwater environment (Figure 6).
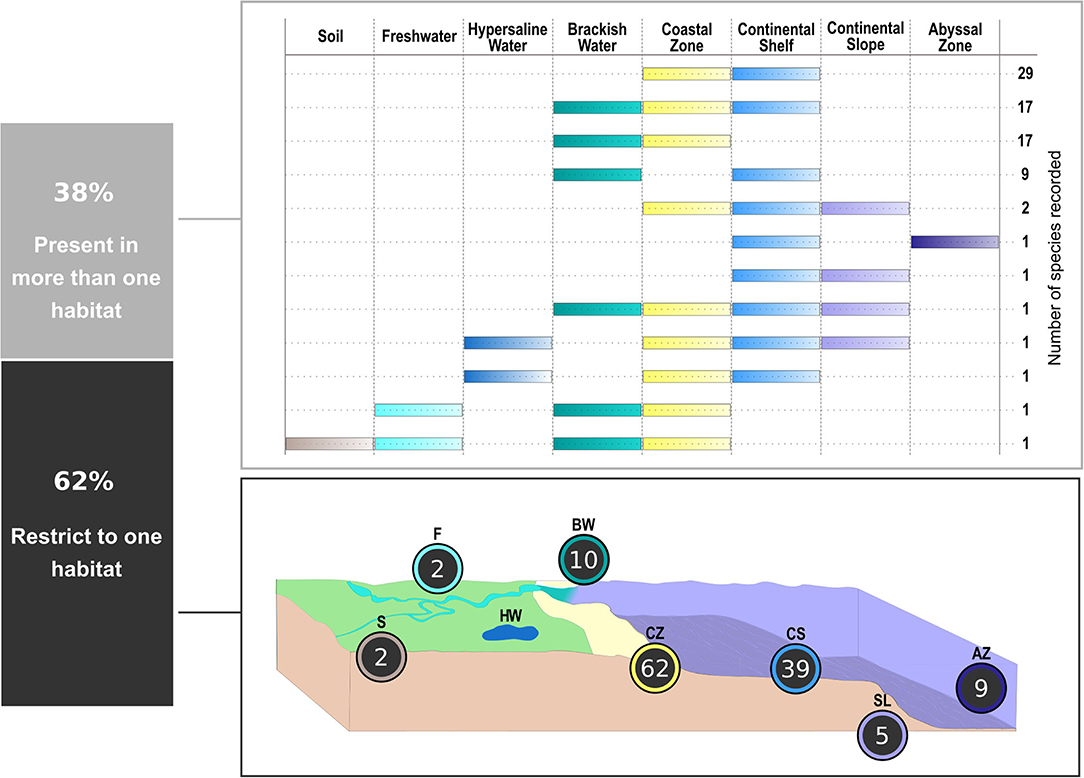
Figure 5. Habitat distribution of the Cyatholaimidae species. The panel above shows the number of species occurring in more than one habitat. Below are the numbers of species exclusive of each habitat. S, Soil; F, Freshwater; HW, Hypersaline Water; BW, Brackish Water; CZ, Coastal Zone; CS, Continental Shelf; SL, Continental Slope; AZ, Abyssal Zone.
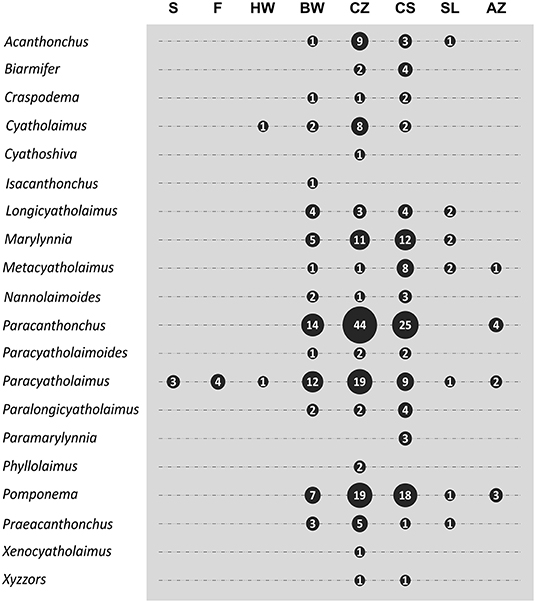
Figure 6. The number of species registered in each habitat by genus. S, Soil; F, Freshwater; HW, Hypersaline Water; BW, Brackish Water; CZ, Coastal Zone; CS, Continental Shelf; SL, Continental Slope; AZ, Abyssal Zone.
From the three species recorded in Soil, two are found in sand dunes and are exclusive of terrestrial habitats (Paracyatholaimus botosaneanui and Paracyatholaimus papillatus). The third one is P. intermedius, which is considered a brackish species, but it was identified in clayey soil in Holland (Loof, 1961) and soil covered with grass from a hill in Uganda (Nzeako et al., 2019). This species was also found in brackish and freshwater sediments of the Elbe River, Germany (Riemann, 1966) and sediments from rivers and freshwater lakes from Spain (Ocaña et al., 1990), Russia (Gusakov and Gagarin, 2012), and Vietnam (Gagarin and Nguyen Vu Thanh, 2012). P. ternus is also a brackish species that was found in a Freshwater habitat (a river from Colombia – Riemann, 1970). Including the two species exclusive of this type of habitat (P. paramonovi and P. truncatus), there are a total of four species registered in Freshwater. In the Hypersaline habitat, only two species were recorded: P. pesavis, and C. gracilis. The first was described based on specimens from the Coastal Zone of Floridian ecoregion and then it was found in the Continental Shelf (Tietjen, 1971), Continental Slope (Tietjen, 1976), and in the Salton Sea, a highly saline body of water in the U.S. state of California (Warwick et al., 2002). The second one is mainly a coastal species (e.g., De Man, 1889; Filipjev, 1918; Allgén, 1935), but it has been also recorded in the Continental Shelf (e.g., Southern, 1914; Allgén, 1959) and in the hypersaline lagoon of Bay Sivash (Shadrin et al., 2019).
The Coastal Zone is the habitat where most of the species occur, followed by the Continental Shelf (Figure 5). The Continental Slope and Abyssal Zone are the marine habitats with fewer records, with only 10 species in each one. Of all 56 species recorded from the Brackish waters, only 10 are exclusive to this habitat, the others also occur in the Coastal Zone and/or in the Continental Shelf. Among the exclusive species, only two were recorded more than once. The estuarine species Longicyatholaimus falcatus was also found in a brackish lagoon (Boufahja and Semprucci, 2015) and Pomponema reductum, as found in an estuary at the Celtic Seas and in an estuary from the North Sea.
Geographic Distribution
There are records of Cyatholaimidae species in all the 12 Realms of coastal and shelf areas. The Temperate Northern Atlantic (TNA) has the largest number of records with 99 species from 16 genera (only the monospecific genera and Paramarylynnia were not recorded in TNA). At the Province level, there are records of the family in about 60% of the units (37 out of 62). The species were reported from 74 ecoregions, covering ~32% of the 232 marine ecoregions in the world (Figure 7). The North Sea and Western Mediterranean, both nested on the realm TNA, are the richest ecoregions (43 and 39 species, respectively).

Figure 7. World Map showing the marine ecoregions of Spalding et al. (2007) colored accordingly the number of Cyatholaimidae species recorded in each one.
Most of the marine species are restricted/endemic to an ecoregion (~55%) and a few have a relatively wide distribution, for example, Paracanthonchus caecus occur in ten ecoregions (Table 2). Other species are also widely distributed, despite occurring in two or three ecoregions. Pomponema segregatum, for example, described in the Puget Trough/Georgia Basin ecoregion (realm Temperate Northern Pacific), also occur in ecoregions Carolinian (realm Temperate Northern Atlantic; Tietjen, 1976) and Western India (realm Western Indo-Pacific; Sajan and Damodaran, 2007). Among all the non-monospecific genera, only Paramarylynnia have species exclusively in one realm, the Temperate Northern Pacific. All other genera have representatives in at least two realms (see Supplementary Material 2). Considering the species from deep-sea (continental slope and abyssal zone), there are records in the Atlantic Ocean (13 species), Pacific Ocean (four species), Arctic Ocean (two species), and Southern Ocean (one species).
The species recorded exclusively in Soil habitats were found in Cuba (Paracyatholaimus botosaneanui) and Italy (Paracyatholaimus papillatus). There are two species restricted to the Freshwater: Paracyatholaimus truncatus from the USA (Cobb, 1914) and Colombia (Riemann, 1970); and Paracyatholaimus paramonovi from Russia (Gagarin, 2004).
Discussion
Taxonomic Issues
Like for other marine nematodes, the delimitation among Cyatholaimidae genera is not a trivial task and most are not defined by autoapomorphic characters (Lorenzen, 1994). The weight given to each character is variable. The most important characters seem to be the cuticle pattern, which is sufficient to identify the genera Biarmifer, Craspodema, and Xenocyatholaimus, and the male copulatory structures for the remaining genera. However, such importance is far from conclusive and other neglected traits may be more relevant. The descriptions of the pore patterns on the cuticle of cyatholaimids for example, are frequently inadequate and limited by light microscopy, and the use of advanced microscopies technics will certainly improve taxa diagnosis (Semprucci and Burattini, 2015; Leduc and Zhao, 2016).
The limitations of morphological data to taxon identification reveal the urgent need for high-quality reference sequence databases from free-living nematodes species, ideally combining morphological data and different molecular approaches (Avó et al., 2017; Macheriotou et al., 2019; Pereira et al., 2020). Datasets of ribosomal 18S and/or mitochondrial CO1 sequences from marine nematodes associated with voucher specimens identified morphologically may serve as reference to species identification (Macheriotou et al., 2019; Pantó et al., 2021). Although the initiatives for the acquisition of DNA sequence are increasing, the data is still reduced and limited to a few locations and habitats (Macheriotou et al., 2019). In SILVA, a digital repository for quality check and aligned ribosomal RNA sequences, from the 20 known genera and 211 described species of Cyatholaimidae, there are only 51 sequences from nine genera, of which 22 are identified to species level. The identified sequences are from eight species, namely: Longicyatholaimus egregius, Longicyatholaimus subtenuis, Metacyatholaimus delicatus, Paracanthonchus caecus, Paracanthonchus mamubiae, Paracanthonchus miltommatus, Paracyatholaimus intermedius, and Praeacanthonchus punctatus. There are three sequences identified as Paracyatholaimus oistospiculoides (Allgén, 1935) Wieser, 1954, a species considered inquerenda by Tchesunov (2008). Despite being abundant in many surveys, only a few Cyatholaimidae genera/species is found in high frequencies, and most of the taxa are considered rare (e.g., Maria et al., 2013; Santos et al., 2018), what makes it even more difficult to obtain the sequences. While the successful DNA barcoding of nematodes is not feasible, the use of robust and updated identification keys enables the standardization and comparisons across studies.
Habitat Transitions
The occurrence of congeneric species in different habitats, including soil, freshwater, and marine, indicates that multiple ecological shift events have occurred along with the evolutionary history of the family Cyatholaimidae. Interestingly, these changes seem to be linked with subtle morphological modifications. In Paracyatholaimus, for example, which contains species in soil, freshwater, brackish water, hypersaline and marine habitats, the differentiation from one another is based on slight morphological details (Tchesunov, 2008). P. paucipapillatus and P. botosaneanui, for example, are differentiated only by the length of the body and the number of pre-cloacal supplements (Andrássy, 1973) and they occur in marine and terrestrial habitats, respectively. Paracanthonchus bulbicola and Paracanthonchus longicaudatus are also morphologically very similar, but the former occurs in the abyssal, while the latter is coastal. Habitats shifts between marine, freshwater, and terrestrial habitats at a low taxonomic level were also observed in genera from other families, such as Theristus Bastian, 1865 (Xyalidae), Phillometra Costa, 1845 (Philometridae), and Procamallanus Baylis, 1923 (Camallanidae; Holterman et al., 2019).
The phylum Nematoda arose from the marine environment and later colonized the land (Rota-Stabelli et al., 2013). Nevertheless, the changes in habitat occurrence and ecological preferences happened a few times along the evolutionary history of nematodes and the frequency and directions of these habitat transitions vary within the group (Holterman et al., 2019). The ecological transitions seem to be more common among free-living nematodes with highly water-permeable cuticles and a relatively simple secretory-excretory (S-E) system (Holterman et al., 2019). Although the water permeability of the cuticle of Cyatholaimidae species is not yet studied, the S-E system consists of a single ventral gland cell, usually with a non-cuticularized terminal duct, or it can be absent in some species. It was suggested that the pore complex structure, commonly present in the cuticle of cyatholaimids, resembles a structure of trichuroid nematodes that may function in water/ion regulation (Wright, 1963), but their true role remains to be proven (Wright and Hope, 1968).
The morphological variation can also be small within species that occupy very distinct habitats. The specimens of Paracyatholaimus quadriseta from an estuarine habitat, differ from individuals from the continental shelf only by a higher relation between body length and tail length (c rate), which may represent a local variation (Pastor de Ward, 1985). However, the acquisition of high-quality morphological data of organisms from populations of various habitats may reveal previously overlooked characters (Fonseca et al., 2017). The use of advanced techniques, like confocal laser scanning microscopy, enabled the detailed observation of the morphology and the re-description of species, such as Craspodema reflectans (Semprucci and Burattini, 2015). For instance, the variations of pore complex and pore-like structures, may be meaningful for genera, species, or even populations differentiation (Leduc and Zhao, 2016).
The case of P. intermedius, which was found in soil, freshwater, brackish water, and marine habitat, is worth noting. The majority of studies that recorded this species did not make a description and a comparison between the organisms found. Among those who did, a few morphological variations were observed. The most striking difference among some specimens from brackish water and terrestrial habitats, for example, was the number of pre-cloacal supplements, varying between four to nine (De Man, 1880; Gerlach, 1953g; Paetzold, 1958; Loof, 1961). Tchesunov (2015) shows that the variability in number and shape of this feature can be notable even within individuals of Paracanthonchus olgae from the same site. Therefore, he stated that this character may not be useful to species identification, and the same can be held for Paracyatholaimus species. Population genetics studies are the first step required to investigate the relationship between organisms occurring in different locations. Understanding the evolutionary history of a taxon like P. intermedius may lead to a comprehension of mechanisms related to plasticity and adaptability to different environmental conditions.
Geographic Distribution
The higher number of species registers in the northern hemisphere is common for inconspicuous marine species and it is related to the sampling intensity in this area, due to the long tradition of marine nematode taxonomic studies and a greater number of specialists in institutions from Europe and North America (Campos and Garraffoni, 2019; Garraffoni et al., 2021). Thus, the knowledge about the biogeography of these organisms is biased by the absence of sampling in many regions all over the world (Venekey et al., 2010). Despite this bias, the number of records from Cyatholaimidae in South America is similar to that from North America (9 vs. 10 genera and 30 vs. 47 species).
The high endemism observed for most cyatholaimid species suggests a low dispersal capability, however, there are a few examples of a relatively broad distribution (see Table 2). Examples of widely distributed meiofauna species may represent one of two distinct scenarios: false records or a high dispersal potential (Cerca et al., 2018). The apparent morphological stasis in the meiofauna group and the tradition of using taxonomic keys and systematic reviews from European species, when the information for the study area is absent, lead to a greater number of species records that were proved to be incorrect (Cerca et al., 2018). Nevertheless, species associated with biological substrates, for example, may present a high dispersal potential and be transported across the oceans by rafting (Thiel and Gutow, 2005). Until now, the only study dealing with morphological and molecular data of different populations of a Cyatholaimidae species suggested that Paracanthonchus gynodiporata have been able to disperse over large distances (Oliveira et al., 2017). They found that the populations of P. gynodiporata are genetically similar, but with considerable differences between them in the morphometrics characters. P. gynodiporata is associated with seaweeds and was recorded more than 1,000 km apart (Oliveira et al., 2017). Besides being an important mechanism of dispersion, the structural complexity of macroalgae provides numerous microhabitats, refuges for predation (Coull and Wells, 1983), protection from tidal and waves strength (Gibbons, 1988), and food resources, especially for epi-growth feeders (Da Rocha et al., 2006), such as cyatholaimids.
Conclusion
Most Cyatholaimidae genera lack information about synapomorphic characters and are possibly non-monophyletic. Also, the definition of genus and species, or even of the subfamily, are based on the combination of multiple non-phylogenetic informative characters, which makes essential the systematic and taxonomic review. This issue difficult the identification of taxa and hamper the advance of studies in other disciplines that depend on taxonomic knowledge. All these caveats can be bypassed by embracing phylogenetics studies of the group, especially those integrating molecular and morphological data. Knowing the relationship within the family is fundamental to understanding the habitat transitions that occur along with the evolutionary history and may help the comprehension of the mechanisms that underlie the ecological flexibility of Nematoda (Holterman et al., 2019).
The present work represents a foundation to uncover and understand the diversity and biogeographic patterns of the Cyatholaimidae family. For the first time, the compilation of distributional data of marine nematodes was based on the ecoregion system of classification. Cyatholaimidae is a family that includes organisms found worldwide, but with most taxa endemic to one ecoregion. The few examples of broadly distributed species might be misidentifications, however, long-distance dispersal may be possible in species associated with biological substrates, such as algae and turtles. The occurrence in more than one type of habitat, under very different environmental conditions, indicates that many cyatholaimid species have broad ecological niches.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
BC, GF, and AA conceived the idea. BC conducted the literature review and data syntheses and wrote the first draft of the manuscript. GF and AA contributed to writing and editing. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by the São Paulo Research Foundation – FAPESP (grant number 2017/21986-2 and 2018/10313-0). Financial support was provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq (301551/2019-7) to AA.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are very thankful to Virág Venekey for providing references to the study. We are also grateful to the reviewers for their constructive criticisms of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.836670/full#supplementary-material
References
Allgén, C. A. (1929). Neue freilebende marine Nematoden von der Westküste Schwedens. Zoologische Jahrbucher. 57, 431–496.
Allgén, C. A. (1935). Zur Kenntnis norwegischer Nematoden V. Weitere neue oder wenig bekannte freilebende Nematoden aus der Strandzone bei Tarva. K. Norske Vidensk. Selsk. Forh. 8, 47–50.
Allgén, C. A. (1947). Papers from Dr. Th. Mortensen's Pacific Expedition 1914–16. LXXV. West American Marine Nematode. Vidensk. Medd. fra Dansk Naturh. Foren. Bd. 110, 65–219.
Allgén, C. A. (1951). Papers from Dr. Th. Mortensen's Pacific Expedition 1914-16. LXXVI. Pacific Freeliving Marine Nematodes. Vidensk. Medd. fra Dansk Naturh. Foren. 113, 263–411.
Allgén, C. A. (1953). Revision der freilebenden marinen Nematoden aus der Umgebung der Staatlichen Zoologischen Station Kristineberg an der Westküste Schwedens. Zoologische Jahrbucher. 81, 548–603.
Allgén, C. A. (1959). Freeliving Marine Nematodes. Further Zoological Results of the Swedish Antarctic Expedition, 1901–1903 Under the Direction of Dr. Otto Nordenskjold, V(2). Stockholm: P.A. Norstedt & Söner.
Andrássy, I. (1973). Nematoden aus Strand - und Höhlenbiotopen von Kuba. Acta Zoologica Academiae Scientiarum Hungaricae. 19, 233–270.
Ansari, K. G. M. T., Manokaran, S., Raja, S., Khan, S. A., and Lyla, S. (2012). Checklist of nematodes (Nematoda: Adenophorea) from southeast Continental Shelf of India. Check List 8, 414–420. doi: 10.15560/8.3.414
Appeltans, W., Ahyong, S. T., Anderson, G., Angel, M. V., Artois, T., Bailly, N., et al. (2012). The magnitude of global marine species diversity. Curr. Biol. 22, 2189–2202. doi: 10.1016/j.cub.2012.09.036
Armenteros, M., Ruiz-Abierno, A., Fernández-Garcés, R., Pérez-García, J. A., Díaz-Asencio, L., Vincx, M., et al. (2009). Biodiversity patterns of free-living marine nematodes in a tropical bay: Cienfuegos, Caribbean Sea. Estuar. Coast. Shelf Sci. 85, 179–189. doi: 10.1016/j.ecss.2009.08.002
Avó, A. P., Daniell, T. J., Neilson, R., Oliveira, S., Branco, J., and Adão, H. (2017). DNA barcoding and morphological identification of benthic nematodes assemblages of estuarine intertidal sediments: advances in molecular tools for biodiversity assessment. Front. Mar. Sci. 4:66. doi: 10.3389/fmars.2017.00066
Belogurova, L. S., and Maslennikov, S. I. (2016). Meiobenthos under mariculture conditions of the brown seaweed Saccharina japonica in Rifovaya Bay, Peter the Great Gulf, Sea of Japan. Oceanology 56, 546–551. doi: 10.1134/S0001437016030012
Beyrem, H., and Aissa, P. (1998). Evolution spatio - temporelle de la composition des peuplements de nematodes libres en reponse a la pollution petroliere sevissant dans la Baie de Bizerte. Bulletin de l'Institut National des Sciences et Technologies de la Mer 25, 81–100.
Bezerra, T. N., Eisendle, U., Hodda, M., Holovachov, O., Leduc, D., Mokievsky, V., et al. (2021). Nemys: World Database of Nematodes. Available online at: http://nemys.ugent.be (accessed November 20, 2021).
Bhadury, P., Austen, M. C., Bilton, D. T., Lambshead, P. J. D., Rogers, A. D., and Smerdon, G. R. (2008). Evaluation of combined morphological and molecular techniques for marine nematode (Terschellingia spp.) identification. Mar. Biol. 154, 509–518. doi: 10.1007/s00227-008-0945-8
Bhanu, C., Rao, M. S., Annapurna, C., and Ambedkar, A. (2017). Functional diversity of nematode communities in the Nizampatnam Bay, Bay of Bengal. Indian J. Geo Mar. Sci. 46, 322–337. Available online at: http://nopr.niscair.res.in/handle/123456789/40776
Boucher, G. (1976). Nématodes des sables fins infralittoraux de la Pierre Noire (Manche occidentale) II. Chromadorida. Bull. Mus. nat. Hist. Nat. Zoologie 352, 25–61.
Boufahja, F., Ismaïly, S., and Beyrem, H. (2015). Experimental evidence of the effects of an antimitotic agent, Colchicine, on a nematode community through a microcosm approach. Cah. Biol. Mar. 56, 39–48. doi: 10.21411/CBM.A.3955B0A
Boufahja, F., and Semprucci, F. (2015). Stress-induced selection of a single species from an entire meiobenthic nematode assemblage: is this possible using iron enrichment and does pre-exposure affect the ease of the process? Environ. Sci. Pollut. Res. 22, 1979–1998. doi: 10.1007/s11356-014-3479-2
Boyd, S. E., Rees, H. L., and Richardson, C. A. (2000). Nematodes as sensitive indicators of change at dredged material disposal sites. Estuar. Coast. Shelf Sci. 51, 805–819. doi: 10.1006/ecss.2000.0722
Campos, A., and Garraffoni, A. R. S. (2019). A synopsis of knowledge, zoogeography and an online interactive map of Brazilian marine gastrotrichs. PeerJ 7:e7898. doi: 10.7717/peerj.7898
Cerca, J., Purschke, G., and Struck, T. H. (2018). Marine connectivity dynamics: clarifying cosmopolitan distributions of marine interstitial invertebrates and the meiofauna paradox. Mar. Biol. 165:123. doi: 10.1007/s00227-018-3383-2
Chinnadurai, G., and Fernando, O. J. (2007). Meiofauna of mangroves of the southeast coast of India with special reference to the free-living marine nematode assemblage. Estuar. Coast. Shelf Sci. 72, 329–336. doi: 10.1016/j.ecss.2006.11.004
Cidreira, G., Pinheiro-Junior, E. P., Venekey, V., and de S Alves, O. F.. (2019). A new species of Pomponema Cobb, 1917 (Nematoda: Cyatholaimidae) from Northeast of Brazil, with reference to the taxonomic status of the genera Parapomponema Ott, 1972 and Propomponema Ott, 1972. Zootaxa 4691, 063–077. doi: 10.11646/zootaxa.4691.1.5
Cobb, N. A. (1914). The North American free-living fresh-water nematodes. Trans. Am. Microscopical. Soc. 33, 69–119. doi: 10.2307/3221617
Cobb, N. A. (1920). One hundred new nemas (type species of 100 new genera). Contributi. Sci. Nematol. 9, 217–343.
Corte, G. N., Checon, H. H., Fonseca, G., Vieira, D. C., Gallucci, F., Di Domenico, M., et al. (2017). Cross-taxon congruence in benthic communities: searching for surrogates in marine sediments. Ecol. Indic. 78, 173–182. doi: 10.1016/j.ecolind.2017.03.031
Coull, B. C., and Wells, J. B. J. (1983). Refuges from fish predation: experiments with phytal meiofauna from the New Zealand rocky intertidal. Ecology 64, 1599–1609. doi: 10.2307/1937513
Coull, B. C., Zo, Z., Tietjen, J. H., and Williams, B. S. (1982). Meiofauna of the Southeastern United States continental shelf. Bull. Mar. Sci. 32, 139–150.
Da Rocha, C. M. C., Venekey, V., Bezerra, T. N. C., and Souza, J. R. B. (2006). Phytal marine nematode assemblages and their relation with the macrophytes structural complexity in a Brazilian tropical rocky beach. Hydrobiologia 553, 219–230. doi: 10.1007/s10750-005-0923-9
Datta, T. K., Miljutin, D. M., Chakraborty, S. K., and Mohapatra, A. (2016). Cyathoshiva amaleshi gen. n.sp.n. (Nematoda: Cyatholaimidae) from the coast of India. Zootaxa 4126, 577–586. doi: 10.11646/zootaxa.4126.4.8
Dayrat, B. (2005). Towards integrative taxonomy. Biol. J. Linn. Soc. 85, 407–415. doi: 10.1111/j.1095-8312.2005.00503.x
De Coninck, L. A., and Schuurmans-Stekhoven, J. H. (1933). The freeliving marine nemas of the Belgian Coast. II With general remarks on the structure and the system of nemas. Mém. Mus. r. Hist. nat. Belg. 58, 3–163.
De Man, J. G. (1880). Die einheimischen, frei in der reinen Erde und im süßen Wasser lebende Nematoden monographisch bearbeitet. Vorläufiger Bericht und descriptiv-systematischer Theil. Tijdschr. Ned. Dierkd. Ver. 5, 1–104.
De Man, J. G. (1889). Troisième note sur les nématodes libres de la mer du Nord et de la Manche. Mém. Soc. zool. Fr. 2, 182–216.
De Oliveira, D. A. S., Decraemer, W., Holovachov, O., Burr, J., Tandingan de Ley, I., de Ley, P., et al. (2012). An integrative approach to characterize cryptic species in the Thoracostoma trachygaster Hope, 1967 complex (Nematoda: Leptosomatidae). Zool. J. Linn. Soc. 164, 18–35. doi: 10.1111/j.1096-3642.2011.00758.x
Derycke, S., Remerie, T., Vierstraete, A., Backeljau, T., Vanfleteren, J. R., Vincx, M., et al. (2005). Mitochondrial DNA variation and cryptic speciation within the free-living marine nematode Pellioditis marina. Mar. Ecol. Prog. Ser. 300, 91–103. doi: 10.3354/meps300091
Ditlevsen, H. (1918). Marine freeliving nematodes from Danish waters. Vidensk. Meddr. dansk naturh. Foren. 70, 147–214.
Ellis, J. R., Maxwell, T., Schratzberger, M., and Rogers, S. I. (2010). The benthos and fish of offshore sandbank habitats in the southern North Sea. J. Mar. Biol. Assoc. U. K. 91, 1319–1335. doi: 10.1017/S0025315410001062
Fedyaeva, M. A., and Tchesunov, A. V. (2017). Fine structure of midgut cells of some White Sea free-living nematodes. Invertebrate Zool. 14, 8–13. doi: 10.15298/invertzool.14.1.02
Filipjev, I. N. (1918). Free-living marine nematodes of the Sevastopol area. Trans. Zool. Lab. Sevastopol. Biol. Stat. Rus. Acad. Sci. 2, 1–255.
Fonseca, G., and Decraemer, W. (2008). State of the art of the free-living marine Monhysteridae (Nematoda). J. Mar. Biol. Assoc. U. K. 88, 1371–1390. doi: 10.1017/S0025315408001719
Fonseca, G., Fontaneto, D., and Di Domenico, M. (2017). Addressing biodiversity shortfalls in meiofauna. J. Exp. Mar. Biol. Ecol. 502, 26–38. doi: 10.1016/j.jembe.2017.05.007
Gagarin, V. G. (2004). “Some data on free-living nematodes from Kunashir (Kuril Islands, Russia) reservoirs”, in Parasitic Nematodes of Plants and Insects, eds M. D. Sonin (Moscow: Nauka), 17–31.
Gagarin, V. G., and Nguyen Vu Thanh (2008). A new genus and three new species of free-living nematodes from mangroves of the Red River Estuary, Vietnam. Tp chí Sinh hc. 30, 3–11.
Gagarin, V. G., and Nguyen Vu Thanh (2012). Free-living nematodes of the Tra Ly River in the Red River Mouth, Vietnam. Inland Water Biol. 5, 11–18. doi: 10.1134/S1995082912010038
Garraffoni, A., Sørensen, M. V., Worsaae, K., Di Domenico, M., Sales, L. P., Santos, J., et al. (2021). Geographical sampling bias on the assessment of endemism areas for marine meiobenthic fauna. Cladistics 37, 571–585. doi: 10.1111/cla.12453
Gerlach, S. A. (1953a). Die Nematodenbesiedlung des Sandstrandes und des Küstengrundwassers an der italienischen Küste. I. - Systematischer Teil. Archo. Zool. Ital. 37, 517–640.
Gerlach, S. A. (1953b). Freilebende marine Nematoden aus dem Küstengrundwasser und aus dem Brackwasser der Chilenischen Küste. Acta Univ. Lund. 49, 1–37.
Gerlach, S. A. (1953c). Recherches sur la faune des eaux interstitielles de Madagascar. III. Sur quelques Nématodes libres des eaux souterraines littorales de Madagascar. Mém. Inst. scient. Madagascar. 8, 73–86.
Gerlach, S. A. (1953d). Nouveaux nématodes libres des eaux souterraines littorales Françaises. Vie et Milieu Life Environ. 4, 95–110.
Gerlach, S. A. (1953e). Les nématodes marins libres des eaux souterraines littorales d'Esposende (Portugal). Vie et Milieu. 4, 83–94.
Gerlach, S. A. (1953f). Contributions a l'étude de la faune souterraine littorale nematodes marins libres des eaux souterraines littorales de Tunisie et d'Algerie. Vie et Milieu 219–237.
Gerlach, S. A. (1953g). Die Nematodenfauna der Uferzone und des Küstengrundwassers am finnischen Meerbusen. Acta Zool. Fenn. 73, 1–32.
Gerlach, S. A. (1954). Nouveaux Nématodes libres des eaux souterraines littorales françaises. Vie Milieu. 4, 95–110.
Gerlach, S. A. (1955). Zur Kenntnis der freilebenden marinen Nematoden von San Salvador. Z. wiss. Zool. Band 158, 249–303.
Gerlach, S. A. (1956). Diagnosen neuer Nematoden aus der Kieler Bucht. Kieler Meeresforsch. 12, 85–109.
Gerlach, S. A. (1957a). Die Nematodenfauna des Sandstrandes an der Küste von Mittelbrasilien (Brasilianische Meerse-Nematoden IV). Mitt. zool. Mus. Berl. 33, 411–459. doi: 10.1002/mmnz.19570330206
Gerlach, S. A. (1957b). Marine Nematoden aus dem Mangrove-Gebiet von Cananéia (Brasilianische Meeres-Nematoden III). Abh. Math.-naturw. Kl. Akad. Wiss. Mainz. 5, 129–176.
Gerlach, S. A. (1957c). Marine Nematoden von der Kongo-Mündung. Bull. Inst. r. Sci. nat. Belg. 33, 1–16.
Gerlach, S. A. (1958). Die Nematodenfauna der sublitoralen Region in der Kieler Bucht. Kieler Meeresforsch. 14, 64–90.
Gerlach, S. A. (1964b). Neue Cyatholaimidae (Nematoda Chromadorida) von den Malediven. Veröff. Inst. Meeresforsch. Bremerh. 9, 70–78.
Gerlach, S. A. (1965). Über die Fauna in der Gezeitenzone von Spitzbergen. Botanica Gothoburgensia. 3, 81–92.
Gerlach, S. A. (1967). Freilebende Meeres-Nematoden von den Sarso-Inseln (Rotes Meer). Meteor-Forschungsergebnisse (D). 2, 19–43.
Gerlach, S. A. (1977). Attraction to decaying organisms as a possible cause for patchy distribution of nematodes in a Bermuda beach. Ophelia 16, 151–165. doi: 10.1080/00785326.1977.10425467
Gerlach, S. A., and Riemann, F. (1973). The Bremerhaven Checklist of Aquatic Nematodes: a catalog of Nematoda Adenophorea excluding the Dorylaimida. Bremerhaven: Veroffentlichungen des Instituts fur Meeresforschung in Bremerhaven, Supplement 4.
Gibbons, M. J. (1988). The impact of wave exposure on the meiofauna of Gelidium pristoides (Turner) Kuetzing (Gelidiales: Rhodophyta). Estuar. Coast. Shelf Sci. 27, 581–593. doi: 10.1016/0272-7714(88)90070-4
Giere, O. (2009). The Microscopic Motile Fauna of Aquatic Sediments. Meiobenthology. Berlin: Springer.
Gusakov, V. A., and Gagarin, V. G. (2012). Meiobenthos composition and structure in highly mineralized tributaries of Lake El'ton. Arid Ecosyst. 2, 232–238. doi: 10.1134/S2079096112030067
Holovachov, O. (2020). The nomenclatural status of new nematode nomina proposed in 1993 in the doctoral thesis of Christian Bussau, entitled Taxonomische und ökologische Untersuchungen an Nematoden des Peru-Beckens (Nematoda). Bionomina 19, 86–99. doi: 10.11646/bionomina.19.1.5
Holterman, M., Holovachov, O., van den Elsen, S., van Megen, H., Bongers, T., Bakker, J., et al. (2008). Small subunit ribosomal DNA-based phylogeny of basal Chromadoria (Nematoda) suggests that transitions from marine to terrestrial habitats (and vice-versa) require relatively simple adaptations. Mol. Phylogenet. Evol. 48, 758–763. doi: 10.1016/j.ympev.2008.04.033
Holterman, M., Schratzberger, M., and Helder, J. (2019). Nematodes as evolutionary commuters between marine, freshwater and terrestrial habitats. Biol. J. Linn. Soc. 128, 756–767. doi: 10.1093/biolinnean/blz107
Hope, W. D., and Zhang, Z. (1995). New nematodes from the Yellow Sea, Hopperia hexadentata nsp. and Cervonema deltensis nsp. (Chromadorida: Comesomatidae), with observations on morphology and systematics. Invertebr. Biol. 114, 119–138. doi: 10.2307/3226884
Hopper, B. E. (1972). Free-living marine nematodes from Biscayne Bay, Florida IV. Cyatholaimidae: On the occurrence of Marilynia n. gen. and Longicyatholaimus Micoletzky, 1924 in Biscayne Bay, with a description of L. longicaudatus De Man, (1876) from the type locality. Zool. Anz. 189, 64–88.
Hortal, J., de Bello, F., Diniz-Filho, J. A. F., Lewinsohn, T. M., Lobo, J. M., and Ladle, R. J. (2015). Seven shortfalls that beset large-scale knowledge of biodiversity. Annu. Rev. Ecol. Evol. Syst. 46, 523–549. doi: 10.1146/annurev-ecolsys-112414-054400
Hourston, M., Potter, I. C., Warwick, R. M., and Valesini, F. J. (2011). The characteristics of the nematode faunas in subtidal sediments of a large microtidal estuary and nearshore coastal waters differ markedly. Estuar. Coast. Shelf Sci. 94, 68–76. doi: 10.1016/j.ecss.2011.05.022
Hourston, M., Potter, I. C., Warwick, R. M., Valesini, F. J., and Clarke, K. R. (2009). Spatial and seasonal variations in the ecological characteristics of the free-living nematode assemblages in a large microtidal estuary. Estuar. Coast. Shelf Sci. 82, 309–322. doi: 10.1016/j.ecss.2009.01.018
Huang, Y., and Sun, J. (2011). Two new free-living marine nematode species of the genus Paramarylynnia (Chromadorida: Cyatholaimidae) from the Yellow Sea, China. J. Mar. Biol. Ass. U. K. 91, 395–401. doi: 10.1017/S0025315410001177
Huang, Y., and Xu, K. (2013). Two new free-living nematode species (Nematoda: Cyatholaimidae) from intertidal sediments of the Yellow Sea, China. Cah. Biol. Mar. 54, 1–10. doi: 10.21411/CBM.A.103E1CF8
Huang, Y., and Zhang, Z. (2007). A new genus and new species of free-living marine nematodes from the Yellow Sea, China. J. Mar. Biol. Ass. U. K. 87, 717–722. doi: 10.1017/S002531540705432X
Inglis, W. G. (1961). Three species of Cyatholaimus Bastian, 1865 (Nematoda: free-living: marine). Bull. Soc. Zool. Fr. 86, 73–86.
Inglis, W. G. (1963). New marine nematodes from off the coast of South Africa. Bull. Br. Mus. nat. Hist. 10, 529–552. doi: 10.5962/bhl.part.20531
Jouili, S., Semprucci, F., Nasri, A., Saidi, I., Mahmoudi, E., and Essid, N. (2018). Inventory of the free–living marine nematode species from el Bibane Lagoon (Tunisia). Arx. misc. zool. 16, 1–19. doi: 10.32800/amz.2018.16.0001
Kotta, J., and Boucher, G. (2001). Interregional variation of free-living nematode assemblages in tropical coral sands. Cah. Biol. Mar. 42, 315–326. doi: 10.21411/CBM.A.95E4A366
Krishnapriya, P. P., Minu, M., Regina Hershey, N., Bijoy Nandan, S., Hari Krishnan, K., and Krishnan, K. P. (2019). Inter fjord variations as a key contributor in the meiobenthic faunal distribution in the Arctic Kongsfjord, Svalbard. Regional Stud. Mar. Sci. 32:100817. doi: 10.1016/j.rsma.2019.100817
Lampadariou, N., and Eleftheriou, A. (2018). Seasonal dynamics of meiofauna from the oligotrophic continental shelf of Crete (Aegean Sea, eastern Mediterranean). J. Exp. Mar. Biol. Ecol. 502, 91–104. doi: 10.1016/j.jembe.2017.12.014
Leduc, D., Nodder, S. D., Berkenbusch, K., and Rowden, A. A. (2015). Effect of core surface area and sediment depth on estimates of deep-sea nematode genus richness and community structure. Mar. Biodiv. 45, 349–356. doi: 10.1007/s12526-014-0271-y
Leduc, D., and Zhao, Z. Q. (2016). Phylogenetic relationships within the Cyatholaimidae (Nematoda: Chromadorida), the taxonomic significance of cuticle pore and pore-like structures, and a description of two new species. Mar. Biodiv. 48, 217–230. doi: 10.1007/s12526-016-0605-z
Long, S. M., and Ross, O. B. H. (1999). Vertical distribution of nematodes (Nematoda) and harpacticoid copepods (Copepoda: Harpacticoida) in muddy and sandy bottom of intertidal zone at Lok Kawi, Sabah, Malaysi. Raffles Bull. Zool. 47, 349–363.
Long, S. M., and Ross, O. B. H. (2008). Horizontal distribution of intertidal nematode from Sabah, Malaysia. J. Trop. Biol. Conserv. 4, 39–53. Available online at: https://jurcon.ums.edu.my/ojums/index.php/jtbc/article/view/97
López Cánovas, C. I., and Pastor de Ward, C. (2006). Lista de los nemátodos (Adenophorea: Chromadoria, Enoplia) de los pastos marinos del Archipiélago de Sabana-Camagüey, Cuba. COCUYO 16, 6–10. Available online at: http://repositorio.geotech.cu/jspui/handle/1234/703
Lorenzen, S. (1972). Die Nematodenfauna im Verklappungsgebiet für Industrieabwässer nordwestlich von Helgoland: III. Cyatholaimidae, mit einer Revision von Pomponema Cobb, 1917. Veröff. Inst. Meeresforsch. Bremerh. 13, 285–306.
Lorenzen, S. (1981). Entwurf eines phylogenetischen systems der freilebenden nematoden. Veröffentlichungen de Instituts für Meeresforschung in Bremerhaven 7:472S.
Macheriotou, L., Guilini, K., Bezerra, T. N., Tytgat, B., Tu Nguyen, D., Phuong Nguyen, T. X., et al. (2019). Metabarcoding free-living marine nematodes using curated 18S and CO1 reference sequence databases for species-level taxonomic assignments. Ecol. Evol. 9, 1211–1226. doi: 10.1002/ece3.4814
Maria, T. F., Paiva, P., Vanreusel, A., and Esteves, A. M. (2013). The relationship between sandy beach nematodes and environmental characteristics in two Brazilian sandy beaches (Guanabara Bay, Rio de Janeiro). An. Acad. Bras. Ciênc. 85, 257–270. doi: 10.1590/S0001-37652013005000019
Meldal, B. H. M., Debenham, N. J., De Ley, P., De Ley, I. T., Vanfleteren, J. R., Vierstraete, A. R., et al. (2007). An improved molecular phylogeny of the Nematoda with special emphasis on marine taxa. Mol. Phylogenet. Evol. 42, 622–636. doi: 10.1016/j.ympev.2006.08.025
Miljutina, M. A., and Miljutin, D. M. (2015). A revision of the genus Paracanthonchus (Cyatholaimidae, Nematoda) with a tabular key to species and a description of P. mamubiae sp. n. from the deep North-Western Pacific. Deep-Sea Res II 111, 104–118. doi: 10.1016/j.dsr2.2014.08.002
Murphy, D. G. (1963). A new genus and two new species of nematodes from Newport, Oregon. Proc. Helm. Soc. Wash. 30, 73–78.
Nasri, A., Boufahja, F., Hedfi, A., Mahmoudi, E., Aïssa, P., and Essid, N. (2015). Impact of penicillin G on the trophic diversity (Moens & Vincx, 1997) of marine nematode community: results from microcosm experiments. Cah. Biol. Mar. 56, 65–72. doi: 10.21411/CBM.A.7AB8BEF0
Netto, S. A., and Fonseca, G. (2017). Regime shifts in coastal lagoons: evidence from freeliving marine nematodes. PLoS ONE 12:e0172366. doi: 10.1371/journal.pone.0172366
Nicholas, W. L., Bird, A. F., Beech, T. A., and Stewart, A. C. (1992). The nematode fauna of the Murray River estuary, South Australia; the effects of the barrages across its mouth. Hydrobiologia 234, 87–101. doi: 10.1007/BF00010864
Nzeako, S. O., Talwana, H., Teye, E., Sekanjako, I., Nabweteme, J., and Businge, M. A. (2019). Characterization of the Soil Nematode Fauna of Makerere Hill, Kampala, Uganda. J. Entomol. Nematol. 11, 70–84. doi: 10.5897/JEN2019.0239
Ocaña, A., Picazo, J., and Jimenez-Millan, F. (1990). First record of nematode species in continental water from Spain: taxonomic and ecological considerations. Nematol. Medito. 18, 179–188.
Oliveira, D. A. S., de Decraemer, W., Moens, T., Dos Santos, G. A. P., and Derycke, S. (2017). Low genetic but high morphological variation over more than 1,000 km coastline refutes omnipresence of cryptic diversity in marine nematodes. BMC Evolution. Biol. 17:71. doi: 10.1186/s12862-017-0908-0
Olson, D. M., Dinerstein, E., Wikramanayake, E. D., Burgess, N. D., Powell, G. V. N., Underwood, E. C., et al. (2001). Terrestrial ecoregions of the world: a new map of life on earth. BioScience 51, 933–938. doi: 10.1641/0006-3568(2001)0510933:TEOTWA2.0.CO;2
Ott, J. A. (1972). Twelve new species of nematodes from an intertidal sandflat in North Carolina. Int. Rev. Ges. Hydrobiol. Hydrogr. 57, 463–496. doi: 10.1002/iroh.19720570307
Paetzold, D. (1958). Beitrage zur Nematodenfauna mitteldeutscher Salzstellen im Raum von Halle. Wiss.Z. Martin-Luther-Univ.Halle-Wittenb. 8, 17–48.
Palacín, C. (1985). Nematodos marinos de las algas fotófilas del litoral de Menorca II. Chromadorida y Monhysterida. Misc. Zool. 9, 31–48.
Palacín, C., Gill, J. M., and Martin, D. (1992). Evidence for coincidence of meiofauna spatial heterogeneity with eutrophication processes in a shallow-water Mediterranean bay. Estuar. Coast. Shelf Sci. 35, 1–16. doi: 10.1016/S0272-7714(05)80053-8
Pantó, G., Pasotti, F., Macheriotou, L., and Vanreusel, A. (2021). Combining traditional taxonomy and metabarcoding: assemblage structure of nematodes in the shelf sediments of the Eastern Antarctic Peninsula. Front. Mar. Sci. 8:629706. doi: 10.3389/fmars.2021.629706
Pastor de Ward, C. (2001). A new nematode from West Patagonian coasts, Biarmifer madrynensis spn., with a redefinition of the genus Biarmifer Wieser 1954 (Nematoda: Cyatholaimidae). Bull. Inst. r. sci. nat. Belg. 71, 139–149. Available online at: http://biblio.naturalsciences.be/rbins-publications/bulletin-of-the-royal-belgian-institute-of-natural-sciences-biologie/71-2001
Pastor de Ward, C. T. (1985). Free-living marine nematodes of the Deseado river estuary (Chromadoroidea: Chromadoridae, Ethmolaimidae, Cyatholaimidae and Choniolaimidae) Santa Cruz, Argentina. 5. Centro Nacional Patagónico Publcaciones Especiales. 6, 1–83.
Pereira, T. J., Santiago, A., de Schuelke, T., Hardy, S. M., and Bik, H. M. (2020). The impact of intragenomic rRNA variation on metabarcoding-derived diversity estimates: a case study from marine nematodes. Environ. DNA 2, 519–534. doi: 10.1002/edn3.77
Pérez-García, J. A., Ruiz-Abierno, A., and Armenteros, M. (2020). A checklist of aquatic nematodes from Cuban Archipelago. Zootaxa 4731, 301–320. doi: 10.11646/zootaxa.4731.3.1
Platt, H. M., and Warwick, R. M. (1988). “Free-living marine nematodes part II, british chromadorids,” in Synopses of the British Fauna (New Series), eds E. J. Brill and W. Backhuys (London: The Linnean Society & The Estuarine and Brackish-Water Sciences Association).
Reynolds, P., and Finney-Crawley, J. R. (1999). A survey of nematode fauna associated with some marine algae in Newfoundland. Russian J. Nematol. 7, 19–28.
Riemann, F. (1966). Die interstitielle fauna im Elbe-Aestuar. Verbreitung und Systematik. Arch. Hydrobiol. Suppl. 1/2, 1–279.
Riemann, F. (1970). Frelebende Nematoden aus dem Grenzbereich Meer-Süss-Wasser in Kolumbien, Südamerika. Veröffentlichungen des Instituts für Meeresforschung in Bremerhaven 12, 365–412.
Riemann, F., and Rachor, R. (1972). Freilebende Nematoden aus dem Suezkanal. Israel J. Zool. 21, 167–187.
Rota-Stabelli, O., Daley, A. C., and Pisani, D. (2013). Molecular timetrees reveal a Cambrian colonization of land and a new scenario for ecdysozoan evolution. Curr. Biol. 23, 392–398. doi: 10.1016/j.cub.2013.01.026
Sajan, S., and Damodaran, R. (2007). Faunal composition of meiobenthos from the shelf regions off the west coast of India. J. Mar. Biol. Ass. India 49, 19–26. Available online at: http://mbai.org.in/php/journaldload.php?id=19&bkid=19
Santos, G. A. P., dos Corrêa, G. V. V., Valdesa, Y., Apolônio Silva de Oliveira, D., Fonsêca-Genevois, V. G., Silva, A. C., et al. (2018). Eretmochelys imbricata shells present a dynamic substrate for a facilitative epibiont relationship between macrofauna richness and nematode diversity, structure and function. J. Exp. Mar. Biol. Ecol. 502, 153–163. doi: 10.1016/j.jembe.2017.08.009
Santos, T. M. T., and Venekey, V. (2017). Meiofauna and free-living nematodes in volcanic sands of a remote South Atlantic, oceanic island (Trindade, Brazil). J. Mar. Biol. Assoc. U. K. 98, 1919–1934. doi: 10.1017/S0025315417001710
Schneider, G. (1927). Dritter Beitrag zur Kenntnis der Brackwassernematoden Finnlands. Acta Soc. Fauna Flora fenn. 56, 1–24.
Semprucci, F., and Balsamo, M. (2014). New records and distribution of marine free-living nematodes in the Maldivian Archipelago. Proc. Biol. Soc. Wash. 127, 35–46. doi: 10.2988/0006-324X-127.1.35
Semprucci, F., Balsamo, M., and Frontalini, F. (2014). The nematode assemblage of a coastal lagoon (Lake Varano, southern Italy): ecology and biodiversity patterns. Sci. Mar. 78, 579–588. doi: 10.3989/scimar.04018.02A
Semprucci, F., and Burattini, S. (2015). Re-description of Craspodema reflectans (Nematoda, Cyatholaimidae) using confocal laser scanning microscopy. Zootaxa 3972, 407–418. doi: 10.11646/zootaxa.3972.3.6
Semprucci, F., Burattini, S., Falcieri, E., and Balsamo, M. (2017). A re-description of Longicyatholaimus maldivarum Gerlach, 1964 (Nematoda, Cyatholamidae) with an emended identification key of the genus. Zootaxa 4323, 096–108. doi: 10.11646/zootaxa.4323.1.7
Shadrin, N., Kolesnikova, E., Revkova, T., Latushkin, A., Chepyzhenko, A., Drapun, I., et al. (2019). Do separated taxa react differently to a long-term salinity increase? The meiobenthos changes in Bay Sivash, largest hypersaline lagoon worldwide. Knowl. Manag. Aquat. Ecosyst. 420:36. doi: 10.1051/kmae/2019028
Somerfield, P. J., Dashfield, S. L., and Warwick, R. M. (2007). Three-dimensional spatial structure: nematodes in a sandy tidal flat. Mar. Ecol. Prog. Ser. 336, 177–186. doi: 10.3354/meps336177
Southern, R. (1914). Nemathelmia, Kinorhyncha, and Chaetognatha. Clare Island Survey 54. Proc. Royal Irish Acad. 31, 1–80.
Spalding, M. D., Fox, H. E., Allen, G. R., Davidson, N., Ferdana, Z. A., Finlayson, M., et al. (2007). Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. BioScience 57, 573–583. doi: 10.1641/B570707
Spedicato, A., Sánchez, N., Pastor, L., Menot, L., and Zeppilli, D. (2020). Meiofauna community in soft sediments at TAG and Snake Pit hydrothermal vent fields. Front. Mar. Sci. 4:200. doi: 10.3389/fmars.2020.00200
Taheri, M., Grego, M., Riedel, B., Vincx, M., and Vanaverbeke, J. (2015). Patterns in nematode community during and after experimentally induced anoxia in the northern Adriatic Sea. Mar. Environ. Res. 110, 110–23. doi: 10.1016/j.marenvres.2015.08.004
Tchesunov, A. V. (2008). Three new species of free-living nematodes from the South-East Atlantic Abyss (DIVA I Expedition). Zootaxa 1866, 151–174. doi: 10.11646/zootaxa.1866.1.7
Tchesunov, A. V. (2014). “Order Chromadorida Chitwood, 1933,” in Handbook of Zoology: Gastrotricha, Cycloneuralia, Gnathifera, ed A. Schmidt-Rhaesa (Germany: De Gruyter), 373–398. doi: 10.1515/9783110274257.373
Tchesunov, A. V. (2015). Free-living nematode species (Nematoda) dwelling in hydrothermal sites of the North Mid-Atlantic Ridge. Helgol. Mar. Res. 69, 343–384. doi: 10.1007/s10152-015-0443-6
Thiel, M., and Gutow, L. (2005). The ecology of rafting in the marine environment. II. The rafting organisms and community. Oceanogr Mar Biol. 43, 279–418. doi: 10.1201/9781420037449.ch7
Tietjen, J. H. (1971). Ecology and distribution of deep-sea meiobenthos off North Carolina. Deep-Sea Res. 18, 941–957. doi: 10.1016/0011-7471(71)90001-5
Tietjen, J. H. (1976). Distribution and species diversity of deep-sea nematodes off North Carolina. Deep-Sea Res. 23, 755–768. doi: 10.1016/S0011-7471(76)80018-6
Tita, G., Desrosiers, G., Vincx, M., and Clement, M. (2002). Intertidal meiofauna of the St Lawrence estuary (Quebec, Canada): diversity, biomass and feeding structure of nematode assemblages. J. Mar. Biol. Ass. U. K. 82, 779–791. doi: 10.1017/S0025315402006148
Turpeenniemi, N. K., Nasira, K., and Maqbool, M. A. A. (2001). A new genus, five new and five known species of free-living marine nematodes (Nematoda: Monhysterida; Chromadorida) from Arabian Sea of Pakistan. Pakistan J. Nematol. 19, 1–31.
Van Megen, H., van den Elsen, S., Holterman, M., Karssen, G., Mooyman, P., Bongers, T., et al. (2009). A phylogenetic tree of nematodes based on about 1200 full-length small subunit ribosomal DNA sequences. Nematology 11, 927–995. doi: 10.1163/156854109X456862
Venekey, V., Fonseca-Genevois, V. G., and Santos, P. J. P. (2010). Biodiversity of free-living marine nematodes on the coast of Brazil: a review. Zootaxa 2568, 39–66. doi: 10.11646/zootaxa.2568.1.2
Venekey, V., Gheller, P. F., Kandratavicius, N., Cunha, B. P., Vilas-Boas, A. C., Fonseca, G., et al. (2019). The state of the art of Chromadoridae (Nematoda, Chromadorida): a historical review, diagnoses and comments about valid and dubious genera and a list of valid species. Zootaxa 4578, 001–067. doi: 10.11646/zootaxa.4578.1.1
Venekey, V., Gheller, P. F., Maria, T. F., Brustolin, M. C., Kandratavicius, N., Vieira, D. C., et al. (2014). The state of the art of Xyalidae (Nematoda, Monhysterida) with reference to the Brazilian records. Mar. Biodiv. 44, 367–390. doi: 10.1007/s12526-014-0226-3
Vidakovic, J., Travizi, A., and Boucher, G. (2003). Two new species of the genus Metacyatholaimus (Nematoda, Cyatholaimidae) from the Adriatic Sea with a key to the species. Cah. Biol. Mar. 44, 111–120. doi: 10.21411/CBM.A.21870002
Vincx, M., Sharma, J., and Smol, N. (1982). On the identity and nomenclature of Paracanthonchus caecus (Bastian, 1865), with a redefinition of the genus Paracanthonchus Micoletzky (Nematoda, Cyatholaimidae). Zool. Scr. 11, 243–263. doi: 10.1111/j.1463-6409.1982.tb00537.x
Vorobyova, L., Kulakova, I., Bondarenko, O., Portyanko, V., and Uzun, E. (2016). Meiofauna of the periphytal of the Odessa coast, Ukraine. J. Black Sea 22, 60–73. Available online at: https://blackmeditjournal.org/volumes-archive/vol22-2016/vol-22-2016-no-1/539-2/
Warwick, R. M. (1971a). Nematode associations in the Exe estuary. J. Mar. Biol. Ass. U. K. 51, 439–454. doi: 10.1017/S0025315400031908
Warwick, R. M. (1971b). The Cyatholaimidae (Nematoda, Chromadoroidea) off the coast of Northumberland. Cah. Biol. Mar. 12, 95–110.
Warwick, R. M., Dexter, D. M., and Kuperman, B. (2002). Freeliving nematodes from the Salton Sea. Hydrobiologia 473, 121–128. doi: 10.1023/A:1016533801827
Whittaker, R. J., Araújo, M. B., Jepson, P., Ladle, R. J., Watson, J. E. M., and Willis, K. J. (2005). Conservation biogeography: assessment and prospect. Diversity Distrib. 11, 3–23. doi: 10.1111/j.1366-9516.2005.00143.x
Wieser, W. (1954b). Beiträge zur Kenntnis der Nematoden submariner Höhlen. Ergebnisse der österreichischen Tyrrhenia-Expedition 1952, Teil II. Ost. Zool. Z. 5, 172–230.
Wieser, W. (1955). A collection of marine nematodes from Japan. Publ. Seto Mar. Biol. Lab. 4, 159–181. doi: 10.5134/174529
Wieser, W. (1959). Free-living Nematodes and Other Small Invertebrates of Puget Sound Beaches. Seattle, DC: University of Washington Press, 1–179.
Wieser, W., and Hopper, B. (1967). Marine nematodes of the east coast of North America. I. Florida Bull. Mus. comp. Zool. Harv. 135, 239–344.
Wright, K. A. (1963). Cytology of the bacillary bands of the nematode Capillaria hepatica (Bancroft, 1893). J. Morphol. 112, 233–259. doi: 10.1002/jmor.1051120304
Wright, K. A., and Hope, W. D. (1968). Elaborations of the cuticle of Acanthonchus duplicatus Wieser, (Nematoda: Cyatholaimidae) as revealed by light and electron microscopy. Can. J. Zool. 46, 1005–1011. doi: 10.1139/z68-140
Zeppilli, D., and Danovaro, R. (2009). Meiofaunal diversity and assemblage structure in a shallow-water hydrothermal vent in the Pacific Ocean. Aquat. Biol. 5, 75–84. doi: 10.3354/ab00140
Keywords: meiofauna, nematode, taxonomy, identification key, habitat transition, species distribution
Citation: Cunha BP, Fonseca G and Amaral ACZ (2022) Diversity and Distribution of Cyatholaimidae (Chromadorida: Nematoda): A Taxonomic and Systematic Review of the World Records. Front. Mar. Sci. 9:836670. doi: 10.3389/fmars.2022.836670
Received: 15 December 2021; Accepted: 24 February 2022;
Published: 18 March 2022.
Edited by:
Jeroen Ingels, Florida State University, United StatesReviewed by:
Maickel Armenteros, National Autonomous University of Mexico, MexicoAlexei Tchesunov, Lomonosov Moscow State University, Russia
Punyasloke Bhadury, Indian Institute of Science Education and Research Kolkata, India
Copyright © 2022 Cunha, Fonseca and Amaral. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beatriz P. Cunha, YmVhdHJpei5wZWN1bmhhQGdtYWlsLmNvbQ==
 Beatriz P. Cunha
Beatriz P. Cunha Gustavo Fonseca
Gustavo Fonseca A. Cecilia Z. Amaral
A. Cecilia Z. Amaral