
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Mar. Sci. , 03 May 2022
Sec. Deep-Sea Environments and Ecology
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.834839
 Abigail Powell1*
Abigail Powell1* M. Elizabeth Clarke2
M. Elizabeth Clarke2 Melissa A. Haltuch2
Melissa A. Haltuch2 Erica Fruh3
Erica Fruh3 Jeffrey Anderson4
Jeffrey Anderson4 Curt E. Whitmire3
Curt E. Whitmire3 Melanie M. Johnson5
Melanie M. Johnson5Understanding the timing and reproductive behavior of commercial fish species is a key part of well-informed stock assessments and fishery management, but this information is often limited, particularly for species that spawn in deep water. Petrale sole (Eopsetta jordani) is one of the most commercially important flatfish species in the US and is known to spawn off the West Coast during winter months. A number of spawning grounds have been identified using catch data and tagging studies, but to our knowledge there have been no direct visual observations of aggregating petrale sole. In 2018, we observed unusually high densities of petrale sole in autonomous underwater vehicle (AUV) imagery collected at a feature inshore of Santa Lucia Bank off the California coast. In addition to aggregations of fish, we observed fish in positions and configurations that we believe to be related to spawning behavior including physical contact between individuals and evidence of consistent size differentiation within clusters. We present images of the aggregating petrale sole and information on the physical and environmental conditions at this site. We also compare these observations to the results of AUV surveys carried out in 2005 at the same site. Analysis of commercial catch data from this area supports the hypothesis that this is a seasonal petrale sole aggregation as fishing at this location occurs mainly during winter months with catches dominated by petrale sole. In addition to the potential identification of a spawning aggregation, these observations show how advanced technologies can provide insight into the reproductive behavior of a commercially important species in-situ.
Many fish migrate seasonally between regions to carry out feeding and spawning activities (Banks, 1969; Bailey, 1982; Cooper and McDermott, 2011; Greenley et al., 2016). Migratory behavior allows fish to take advantage of the fact that optimal conditions for activities such as spawning and feeding (e.g., increased food availability or lower predation) vary through time and space. A number of northeast Pacific flatfish species exhibit seasonal migrations: Pacific halibut (Hippoglossus stenolepis) exhibit long northward migrations from summer feeding grounds in Canada to winter spawning grounds in the Gulf of Alaska (Loher and Seitz, 2006); English sole (Parophrys vetulus) tagged off British Columbia make latitudinal migrations to spawning sites; and arrowtooth flounder (Atheresthes stomias) in the Gulf of Alaska move to deeper water to spawn (Forrester, 1969; Blood et al., 2007). While migrations can be advantageous to species, fish that are targeted as aggregate spawners are particularly vulnerable to overfishing so understanding the timing and location of spawning is important for the management of these species (Sadovy de Mitcheson, 2016).
Petrale sole (Eopsetta jordani) is one of the most valuable flatfish species caught off the US West Coast (Ketchen and Forrester, 1966; Mcknight, 2013). This species occurs from Alaska to California and is associated with mud and sandy substrates down to 550 m (Kamikawa, 2017). Female petrale spawn once a year between late fall and spring (Alverson and Chatwin, 1957; Harry, 1959) and commercial catch data and tagging studies suggest that this species spawns at specific aggregation sites. In general, adult petrale sole exhibit northward inshore movements in the spring and summer to feeding areas, then migrate southward and offshore to deeper spawning aggregation sites during the fall and winter (Alverson and Chatwin, 1957; Best, 1963; Pedersen, 1975). This seasonal and spatial variation is reflected in the US West Coast commercial fishery data, which show that most catches from March to October are from 70-220 m while winter catches are from 330-372 m (Wetzel, 2019). Catches from spawning aggregations are characterized by the presence of mature female fish and the absence of immature, small adults (Alverson and Chatwin, 1957; Best, 1963; Hannah et al., 2002). The first petrale sole spawning aggregation, the Esteban Deep, was discovered in 1953 off the west coast of Vancouver Island, Canada. Early tagging and sampling studies at the Esteban Deep indicated that some petrale sole individuals migrated considerable distances (> 350 miles) to reach the spawning ground (Alverson and Chatwin, 1957). The subsequent discovery of additional deep spawning aggregations led to the growth of a winter fishery that targets these aggregations (see Haltuch et al., 2013 for a review of the fishery). Four petrale sole spawning areas have been delineated in California in the Cape Mendocino, Point Delgado, Point Montara and Point Sal areas (Leet et al., 2001). However, information on the precise location and timing of aggregations and the spawning behavior of petrale sole remains limited. Increased understanding of petrale sole seasonal migrations and the impacts of fishing on spawning aggregations has been identified as a key component of improving stock assessment models for this commercially important species (Haltuch et al., 2013).
During a coast wide research cruise in October 2018, we collected images of high densities of petrale sole in groups at a rocky feature inshore of Santa Lucia Bank off the California coast using an autonomous underwater vehicle (AUV). The primary aim of the cruise was to collect baseline information on fish, sponges and corals at various sites from Washington to California. The observations of petrale sole were an unexpected discovery. The aim of this report is to describe these novel observations and assess whether this could be a spawning aggregation site. In addition to the number and size of petrale sole observed in imagery and the environmental and physical characteristics of the site, we also recorded any signs of reproductive behavior. The specific reproductive behaviors of petrale sole are undescribed so we looked for evidence of known spawning behavior of other flatfish species. In addition, we examined imagery collected in 2005 at the same rocky feature at the same time of year to compare petrale sole densities. We hypothesized that aggregating petrale sole would also be evident in these images. Finally, in the absence of imagery collected from different seasons we analyzed commercial catch data to look for seasonal patterns in petrale sole abundance at this site. We hypothesized that if this was a transient spawning aggregation petrale sole catches would vary throughout the year and be higher during the winter months.
Surveys were carried out in October 2018 during a US West Coast research cruise on the NOAA ship Bell M. Shimada. The images of aggregating petrale were captured at a rocky feature inshore of Santa Lucia Bank off the coast of California (Figure 1). This region is located in the southern California Current System (CCS) in an area characterized by complex topography and a diverse community of marine mammals and birds (Croll et al., 1998; Yen et al., 2006). Photographic fish surveys were carried out by the bottom-tracking SeaBED AUV Popoki deployed from the research vessel. The SeaBED AUV is a two-hulled vehicle designed for the collection of high-resolution imagery close to the sea floor (Singh et al., 2004). In total, 24 AUV dives were carried out off the US West Coast in 2018. High-resolution photographic images (2448 X 2050 pixels) were collected with three Prosilica machine vision cameras. Two of the cameras were downward-facing and mounted in a stereo configuration. These images were used to measure the size of fish in photographs using custom image annotation software, OneTwoRedBlue. Images from the downward-looking port side camera were used for fish counts and to calculate the area surveyed. The third camera was pointed forward angled down from horizontal and was used to collect imagery to assist with species identification. The AUV was pre-programmed before each dive to survey a track taking photographs every 8 s at an altitude of 2.5 m over the seafloor. The mean area in each photograph was 3.2 m2 (SD 0.18). One image was excluded from the analysis because it overlapped with the previous image. At the end of each dive, the images were downloaded, color corrected and rectified. Back on land, they were analyzed by experts in OneTwoRedBlue who recorded all fish, sponges and corals to the lowest possible taxonomic level. All petrale sole that could be identified were counted even if part of an individual was out of the frame. The total lengths of fish (tip of the snout to tip of the caudal fin) were also recorded for all fish that were entirely visible in the images. When there was more than one petrale sole in an image we recorded the number of individuals in a cluster, any contact between individuals, their position relative to each other, and the lengths of individuals within the group. In addition to cameras, the AUV was equipped with a number of sensors that recorded environmental data including a model 49 FastCat CTD (Seabird Electronics, Inc.) that measured salinity, temperature and pressure, an oxygen sensor (Aanderaa oxygen optode 4831F) and a Miniature Autonomous Plume Recorder (MAPR) for identifying methane seeps (Walker et al., 2007).
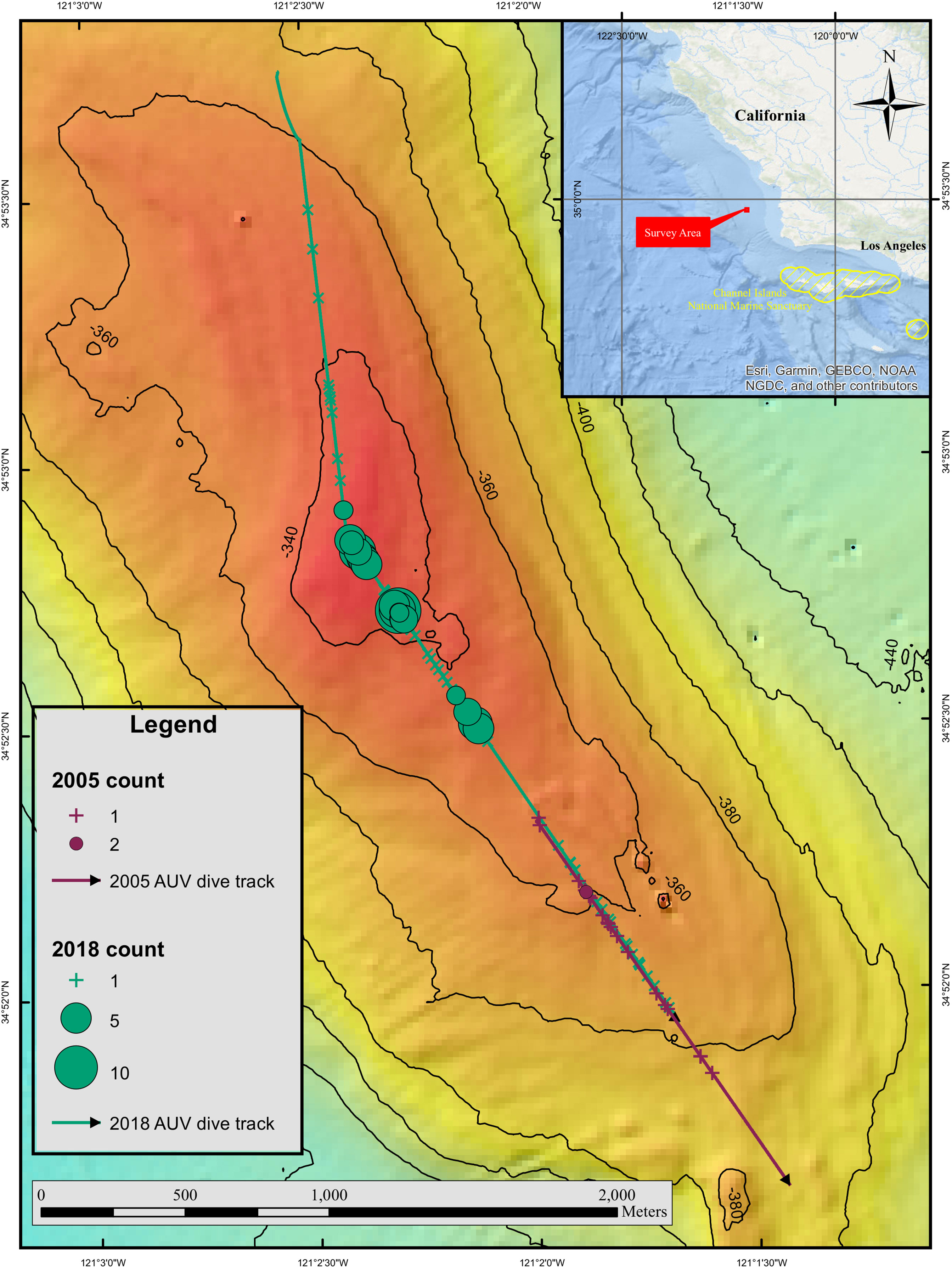
Figure 1 Inset map shows the location of the area where high densities of petrale sole were observed. Close up map shows the feature where the petrale sole were located and distribution of fish along the AUV dive track. The 2005 dive track is represented by a purple line and the 2018 dive track is shown in green. Crosses represent locations where only a single petrale sole was present in an image, whereas circles highlight images with more than one individual. The size of the circles is related to the number of fish observed in an image and smaller circles represent fewer fish than larger circles.
Data from October 2005 were also collected using a SeaBED bottom-tracking AUV during a cruise aboard R/V Thomas G. Thompson. The structure of the AUV was the same as the one used in 2018 but there were some differences in the sensors that were equipped. In 2005 there was a single downward looking 1 megapixel camera and the AUV did not have an oxygen sensor or MAPR mounted (see Tolimieri et al., 2008 for more details). In 2005, there was extensive overlap in the AUV images so every other image was excluded to avoid double counting fish and overestimating the survey area. The area surveyed was calculated by summing the areas of the images that were analyzed. Petrale sole in the images were counted and each image was scrutinized for the presence of groups of fish and potential indicators of reproductive behavior. In 2005, the AUV was only configured with one camera so no stereo length measurements were available for comparison. Summary statistics were used to examine petrale sole densities and environmental conditions along the AUV dive tracks. The phase in the lunar cycle when dives took place was compared for the 2005 and 2018 surveys. Fish and environmental data were analyzed in R version 3.4.1 (R Core Team, 2017).
We examined commercial bottom trawl catch data collected by the state of California and submitted to the Pacific Fisheries Information Network (PacFIN). Catch data from 2002 to 2019 for the area around the aggregation site were analyzed to determine whether commercial trawlers were targeting petrale sole at the same location the AUV sampled in 2005 and 2018. Data from any tows that intersected with the rocky feature were included in the analysis. Although trawling activity at this site undoubtedly predates 2002, this is the earliest time for which the coordinates of trawl set and up points are readily available. Prior to 2002, only set points or the management block for which trawling occurred was recorded, and this lack of spatially-explicit catch data precluded the finer scale examination of spawning site fidelity. We also investigated the seasonality of fishing patterns and timing of AUV observations of aggregating petrale sole. Trawl patterns were analyzed with ArcGIS Pro v.2.7 (ESRI) and R v.4.0.2.
On October 28, 2018 we observed what we think are the first visual observations of a petrale sole spawning aggregation. The rocky feature where we observed high densities of petrale sole was characterized by alternating patches of mud and low relief hard substratum. We did not observe high densities of sponges, hard corals or other sessile benthic invertebrates at this site. Total area photographed by the AUV and included in the analysis was 5873 m2. The dive track was 3720 m long with a depth range from 330-372 m and the average temperature along the dive was 7.45°C.
In total, 150 petrale sole were observed in 2018. Of these, 62 were solitary (only one observed in an image) and 88 were observed in clusters of two or more (~20% of the images where petrale sole were observed). The maximum number of petrale sole observed in an image was 11 individuals. Mean petrale density along the dive track was 0.25 (SD 1.66) per 10 m2 with a maximum density of 30.14 per 10 m2 in the image with the most individuals. The aggregating petrale sole were concentrated on the shallowest part of the bank (Figure 1). The grouped individuals were observed lying near or on top of each other (Figure 2). We were able to measure the lengths of 92 fish and the mean length of the petrale sole was 42.21 (SD 3.84) cm with a maximum length of 57.03 cm and minimum of 35.94 cm. The size distribution of petrale sole in our imagery suggests that the majority of individuals observed were large, mature individuals (Figure 3) (Best, 1961; Hannah et al., 2002). Petrale sole exhibit sexually dimorphic growth with females growing larger relative to males (Wetzel, 2019). We hypothesize that the large outliers we observed are large females. We observed that the individuals at the base of each cluster appeared larger than the others. In most cases, we were not able to confirm this with measurements as the head or tail of the bottom fish were obscured. However, for three images we were able to measure all the fish in a group and found that the fish at the base of each cluster was approximately 5-10 cm longer than the next longest in the group. Petrale sole were observed at all depths sampled and temperatures ranging from 7.42 – 7.53°C. Supplementary Material 1 summarizes the range and mean conditions associated with images with and without petrale sole. In 2018, images with groups of petrale sole were observed at slightly shallower depths where temperatures were slightly higher than areas without petrale sole.
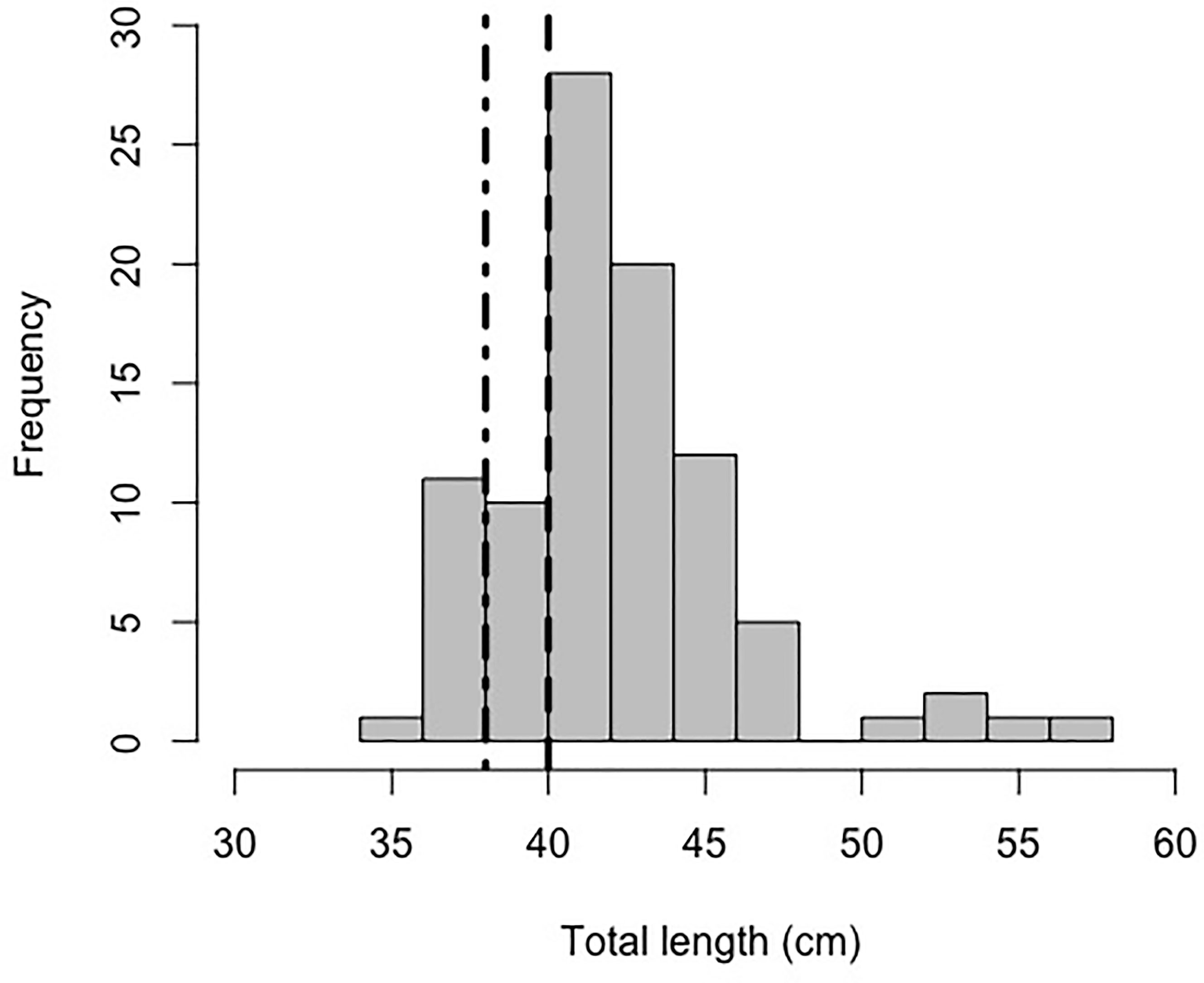
Figure 3 Histogram of petrale sole total length (cm) measured in 2018 at eastern Santa Lucia Bank. The thick dot dashed line at 38 cm is the length at which all male petrale sole are likely mature (Harry, 1959) and the dotted line at 40 cm is the length at which all females are likely mature (Hannah et al., 2002).
The 2005 dive was 1566 m long and partially overlapped the 2018 dive but started deeper (344 m) on the slope and extended further down the bank to the southeast (398 m, Figure 1). Mean water temperature along the dive track was 6.8°C. No clusters of petrale sole were observed in 2005. The total number of petrale sole observed was 21 and the maximum number in a single image was two individuals. The total area of the non-overlapping AUV images included in the analysis was 2742 m2. Mean petrale density was 0.076 (SD 0.5) per 10 m2 and the maximum density per image was 6.05 per 10 m2. Petrale sole were observed at depths of 344 – 369 m and at temperatures from 6.76 – 7.07°C (see Supplementary Material 1 for more details). Petrale sole mean densities in the same area surveyed during 2005 and 2018 dives were similar (0.12 (SD 0.63) and 0.13 (SD 0.62) per 10 m2 respectively). The surveys were carried out just before a new moon (waning crescent) in 2005 and just after a full moon (waning gibbous) in 2018.
Analysis of commercial trawl fishing data from 2002-2019 showed that effort (i.e., number of hauls) at this site was higher during the autumn and winter months (October to January) than the rest of the year (Figure 4). Petrale sole also dominated catches by weight during those winter months whereas summer hauls had a larger proportion of bank rockfish (Sebastes rufus).
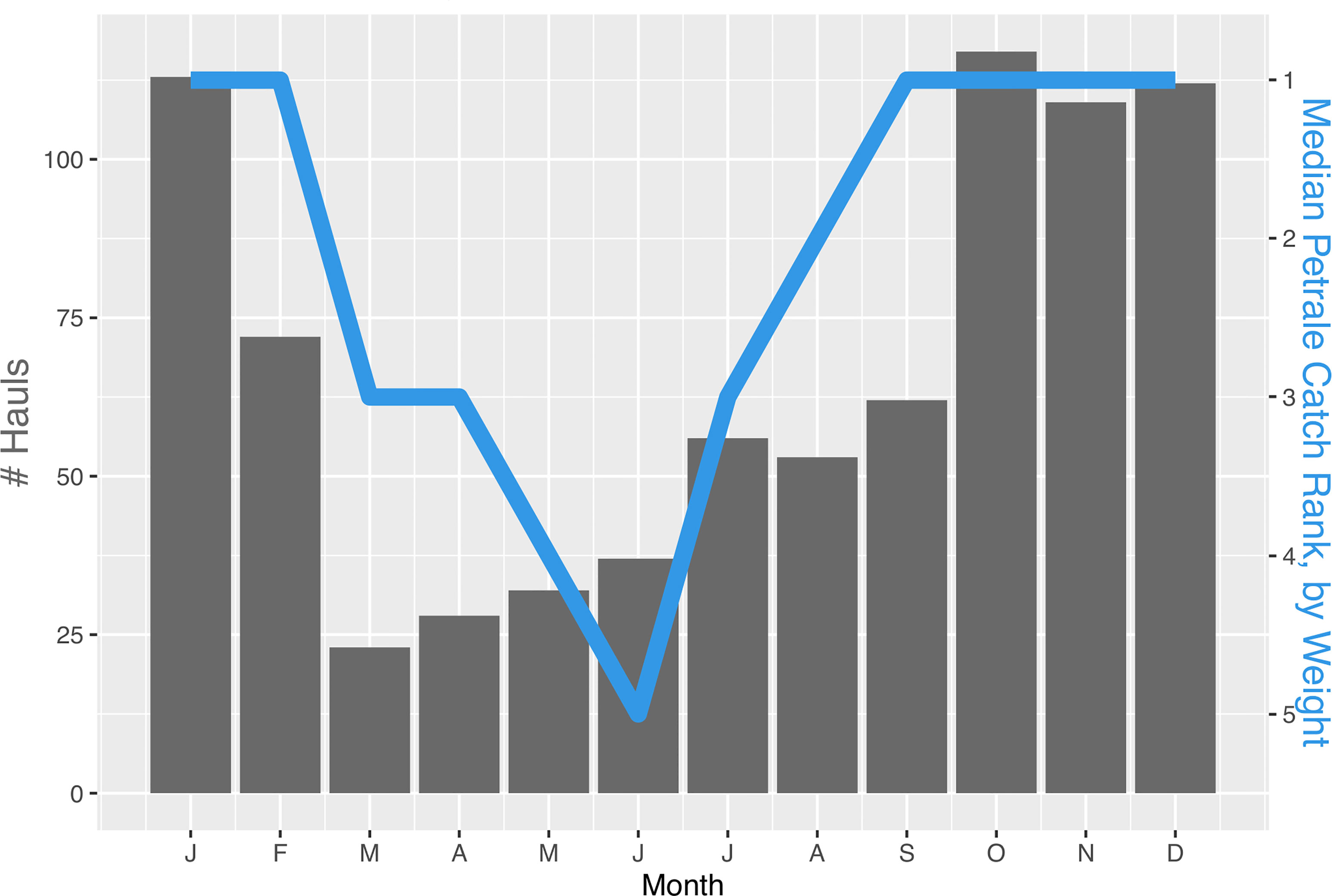
Figure 4 Histogram of the total number of limited-entry, bottom trawl hauls at eastern Santa Lucia Bank from 2002 to 2019 broken down by month. The blue line represents the median rank of petrale sole by weight in hauls for each month.
The main aim of this report is to document observations of unusually high petrale sole densities and determine whether this is a potential spawning site. In October of 2018, which is early in the fall to spring spawning season (Alverson and Chatwin, 1957; Harry, 1959), we observed maximum densities of 30 petrale sole per 10 m2 at a rocky feature near Santa Lucia Bank. Limited data are available but these densities are significantly higher than those observed in October 2011 at two banks (The Footprint and Piggy Bank) in the Southern California Bight that were surveyed with the same AUV (Clarke et al., 2020). Petrale sole density at the Footprint was 0.0018 per 10 m2 (depth range 80-500 m) and none were observed at Piggy Bank (275-900 m). While data from more sites with similar geomorphic characteristics are needed, these results suggest that the densities we observed represent a localized concentration of petrale sole as would be expected at a spawning aggregation.
Another key aspect of determining whether this is a transient spawning aggregation site is whether the high petrale sole densities observed are seasonal. Domeier and Colin (1997) suggest that a 3-fold increase in the density during reproductive periods compared to non-reproductive periods constitutes a spawning aggregation. A limitation of this research report is that we only have petrale sole densities from two surveys carried out during the same season. However, examination of the bottom trawl catch data from this location indicates that petrale abundance varies seasonally. During fall and winter, catches contained a higher proportion of petrale sole than in summer months. Fishing effort at this location is also concentrated during fall and winter months suggesting that fishers may be targeting these areas seasonally for petrale sole. These observations and the fact that fall and winter is the time when petrale sole are known to spawn (Harry, 1959; Hannah et al., 2002) suggest that petrale sole may be migrating to this site to reproduce. Petrale sole collections throughout the year to examine maturity and reproductive condition would be extremely useful to confirm the presence of a fall/winter spawning population (Domeier and Colin, 1997). Additional visual surveys at different times of the year would be useful to determine the duration of the aggregation, and surveys at other depths and habitats would increase our understanding of petrale sole distributions.
Incorporating knowledge of the mating systems of commercially important fish species has been identified as an essential component of population assessments (Rowe and Hutchings, 2003) but studying the spawning behavior of flatfishes and particularly those that occur beyond SCUBA depths is challenging. Observations of spawning flatfish in captivity and in situ with SCUBA surveys have shown that spawning behavior varies between species but there are some common characteristics. In general, spawning occurs when a male and female swim together off the bottom and release gametes into the water column during a spawning rise or rush. In all species observed this is preceded by some form of interaction between the sexes (Konstantinou and Shen, 1995; Manabe and Shinomiya, 2001; Carvalho et al., 2003; Pankhurst and Fitzgibbon, 2005; Carazo et al., 2016). Domeier and Colin (1997) state that direct observations of spawning are the best way to confirm that a group of conspecific fish are aggregated to spawn. Unfortunately, we did not observe petrale sole spawning rises but we hypothesize that clustering and petrale sole lying on top of each other is a behavior related to spawning. This type of behavior has been observed in captive Senegalese sole (Solea senegalensis) where males rested their heads on top of females prior to spawning (Carazo et al., 2016). In flatfish species the olfactory organs are located on the dorsal and ventral surfaces, so close contact between individuals may provide the opportunity for the exchange of chemical cues (Cox, 2008; Carazo et al., 2016). Konstantinou and Shen (1995) observed that male Caribbean eyed flounder (Bothus ocellatus) approached females after a spawning rise and appeared to use either visual or chemical cues to determine whether a spawning rise had been successful. In our imagery, the size difference between the bottom fish and the others could be related to the sex of the fish present. We hypothesize that the larger fish we observed were females with smaller males resting above as petrale sole are sexually dimorphic with females reaching larger sizes than males (Wetzel, 2019). A study of the spawning behavior of captive Dover sole (Solea solea) found that the female was larger than the male in all the pairs observed (Baynes et al., 1994).
To our knowledge there are no published reports of the physical characteristics of any petrale sole spawning sites. In the present study the aggregating petrale sole were most common at the shallowest part of the bank. One possible reason for this could be the presence of strong currents at the top of the bank that could influence larval dispersal. In coral reef fishes it is common for spawning aggregations to occur at specific sites and particularly near reef drop-offs and convex features (Domeier and Colin, 1997; Kobara and Heyman, 2010). Current speeds as measured at a promontory spawning site in Belize were shown to be twice those at non-promontory sites (Heyman and Kjerfve, 2008). Increases in petrale sole recruitment patterns have been related to winter conditions that favor the transport of eggs and larvae from offshore to nearshore areas are associated with increased recruitment (Castillo and Li, 1994; Castillo, 1995). A recent investigation attributed a large proportion of the variation in recruitment strength to temperature experienced by females prior to spawning, the mixed-layer depth during the egg stage, and cross-shelf transport during the larval and early benthic juvenile stages (Haltuch et al., 2020). More research is needed to determine whether local factors such as the physical characteristics of spawning sites play a role in reproductive success.
The lack of clusters of petrale sole in the 2005 imagery was unexpected and may have been due to differences in dive locations. The 2005 AUV dive only partially overlapped the 2018 dive track and did not include the area at the top of the bank where we observed the clusters of petrale sole. The differences could also have been due to changes in habitat between 2005 and 2018. We did not observe large differences along the dive tracks or between years but a more detailed analysis would be useful to explore if there are associations between petrale densities and habitat variables. It is also possible that petrale sole did aggregate at this site in 2005 but at a different time than our AUV dive. Spawning may be constrained to a particular set of environmental conditions that were not met when the dive took place in 2005, when there were differences in the lunar phase and temperature recorded. Finally, it is also possible that a reduction in fishing pressure and a large recruitment event since 2005 has resulted in an increase in the number of petrale sole at this site in 2018.
This study shows the value of AUVs for studying flatfish distributions and their behavior in deep-sea environments. It contributes to filling fundamental gaps in our knowledge of the mating behavior, timing and location of petrale sole spawning aggregations. This information begins to provide valuable insights for fisheries management regarding spawning environments and behavior that can play a role in preventing spawning stock depletion as spawning aggregations are highly vulnerable to fishing and once eliminated spawning aggregations may not reform (Aguilar-Perera, 2006). Recommendations for the future include implementing reproductive state studies in combination with imagery to confirm that this is a spawning population and the duration of the aggregation. Tagging and image-based surveys at this site and other spawning grounds would also increase our understanding of petrale sole migration patterns. There is some evidence of natal homing of petrale to spawning grounds in the California Current, but data to investigate migration of petrale sole to specific spawning grounds are required.
The raw data supporting the conclusions of this article will be made available by the authors, except for the fishing logbook and fish ticket data, which are considered confidential under U.S. law (MSA 2006) Reference: Magnuson-Stevens Fishery Conservation and Management Act of 1976. 2006. Magnuson-Stevens Conservation and Management Reauthorization Act of 2006. U.S. Code, volume 16 section 1881 a(b).
Ethical review and approval was not required for the animal study because this was an observational study where fish were observed in situ.
AP: wrote the manuscript, participated in fieldwork, analyzed biological and environmental data, and Figures 2, 3. MC: chief scientist on the research cruise, edited manuscript, and contributed to project design. MH: subject matter expert and contributed to writing of the manuscript. EF: participated in fieldwork, image analysis, and edited manuscript. JA: participated in fieldwork, Figure 1, contributed to data analysis, and edited manuscript. CW: participated in fieldwork, Figure 4, contributed to data analysis, and edited manuscript. MJ: image analysis and edited manuscript. All authors contributed to the article and approved the submitted version.
This work was funded by the National Ocean Partnership Program and Deep Sea Coral Research and Technology Program and the Department of Interior’s Bureau of Ocean Energy Management.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
With many thanks to the officers and crew of the NOAA ship Bell M. Shimada for their skill and enthusiasm. Thanks also to the scientists and collaborators from NOAA, the Bureau of Ocean Energy Management and the United States Geological Survey who made the 2018 EXPRESS cruise a success. We also wish to thank Nick Tolimieri and Jason Cope whose comments improved the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.834839/full#supplementary-material
Aguilar-Perera A. (2006). Disappearance of a Nassau Grouper Spawning Aggregation Off the Southern Mexican Caribbean Coast. Mar. Ecol. Prog. Ser. 327, 289–296. doi: 10.3354/meps327289
Alverson D. L., Chatwin B. M. (1957). Results From Tagging Experiments on a Spawning Stock of Petrale Sole, Eopsetta jordani (Lockington) J. Fish Res. Bd Canada 14 (6), 953–974. doi: 10.1139/f57-042
Bailey (1982). “The Life History and Fishery of Pacific Whiting,” in Merluccius Productus. Processed Report, Northwest and Alaska Fish. Cent., Natl. Mar. Fish. Serv (2725 Montlake Blvd. E., Seattle, WA 98112: NOAA).
Banks (1969). A Review of the Literature on the Upstream Migration of Adult Salmonids. J. Fish Biol. 1, 85–136. doi: 10.1111/j.1095-8649.1969.tb03847.x
Baynes S. M., Howell B. R., Beard T. W., Hallam J. D. (1994). A Description of Spawning Behaviour of Captive Dover Sole, Solea Solea (L.) Neth. J. Sea Res. 32 (3/4), 271–275. doi: 10.1016/0077-7579(94)90004-3
Best E. A. (1961). Savings Gear Studies on Pacific Coast Flatfish. Pac. Mar. Fish Commun. Bull. 5, 25–47.
Best E. (1963). Movements of Petrale Sole, Eopsetta Jordani, Tagged Off of California. Pac. Mar. Fish. Comm. Bull. 6, 24–38.
Blood D. M., Matarese A. C., Busby M. S. (2007). Spawning, Egg Development and Early Life History Dynamics of Arrowtooth Flounder (Atheresthes Stomias) in the Gulf of Alaska Vol. 7 (NOAA Professional Paper NMFS) Seattle, Washington, USA, 28.
Carazo I., Chereguini O., Martin I., Huntingford F., Duncan N. (2016). Reproductive Ethogram and Mate Selection in Captive Wild Senegalese Sole (Solea Senegalensis) Span. J. Agric. Res. 14 (4), c 0401, 13. doi 10.5424/sjar/2016144-9108
Carvalho N., Afonso P., Serrao Santos R. (2003). The Haremic Mating System and Mate Choice in the Wide-Eyed Flounder Bothus Podas. Environ. Biol. Fish 66, 249–258. doi: 10.1023/A:1023957825568
Castillo G. (1995). Latitudinal Patterns in Reproductive Life History Traits of Northeast Pacific Flatfish, in Proceedings of the International Symposium on North Pacific Flatfish, Lowell Wakefield Fisheries Symposium, University of Alaska Sea Grant Program, Report No. 95-04.
Castillo G., Li H. W. (1994). Environmentally Induced Recruitment Variation in Petrale Sole, Eopsetta Jordani. Fish. Bull. 92, 481–493.
Clarke M. E., Fruh E. L., Powell A., Anderson J., Taylor J. C., Whitmire C. E. (2020). Autonomous Underwater Vehicle (AUV) Survey at the Footprint and Piggy Bank in the Southern California Bight 2011. U.S. Department of Commerce Vol. 161 (NOAA Technical Memorandum NMFS-NWFSC) Seattle, Washington, USA.
Cooper D., McDermott S. (2011). Seasonal, Small-Scale Distribution of Atka Mackerel in the Aleutian Islands, Alaska, With Respect to Reproduction. Mar. Coast Fish 3 (1), 10–20. doi: 10.1080/19425120.2011.558439
Cox J. P. L. (2008). Hydrodynamic Aspects of Fish Olfaction. J. R. Soc Interface 5, 575–593. doi: 10.1098/rsif.2007.1281
Croll D. S., Tershy B. R., Hewitt R. P., Demer D. A., Fiedler P. C., Smith S. E., et al. (1998). An Integreated Approach to the Foraging Ecology of Marine Birds and Mammals. Deep-Sea Res. II 45, 1353–1371. doi: 10.1016/S0967-0645(98)00031-9
Domeier M. L., Colin P. L. (1997). Tropical Reef Fish Spawning Aggregations: Defined and Reviewed. Bull. Mar. Sci. 60 (3), 698–726.
Forrester C. R. (1969). Results of English Sole Tagging in British Columbia Waters. Pac. Mar. Fish Commun. Bull. 7, 1–10.
Greenley A., Green K., Starr R. M. (2016). Seasonal and Ontogenetic Movements of Lingcod (Ophiodon Elongatus) in Central California, With Implications for Management Vol. 57 (CalCOFI Rep.) Sacramento, California, USA.
Haltuch M. A., Ono K., Valero J. (2013). Status of the U.S. Petrale Sole Resource in 2012. Pacific Fishery Management Council, 7700 Ambassador Place NE, Suite 101, Portland, OR 97220, USA.
Haltuch M. A., Tolimieri N., Lee Q., Jacox M. G. (2020). Oceanographic Drivers of Petrale Sole Recruitment in the California Current Ecosystem. Fish Oceanogr. 29, 122–136. doi: 10.1111/fog.12459
Hannah R. W., Parker S. J., Fruh E. L. (2002). Length and Age at Maturity of Female Petrale Sole (Eopsetta Jordani) Determined From Samples Collected Prior to Spawning Aggregation. Fish Bull. 100, 711–719.
Harry G. Y. (1959). Time of Spawning, Length at Maturity of the English, Petrale, and Dover Soles (Parophrys Vetulus, Eopsetta Jordani, and Microstomus Pacificus, Respectively). Fish Commun. Oregon. Res. Briefs 7 (1), 4–13.
Heyman W. D., Kjerfve B. (2008). Characterization of Transient Multi-Species Reef Fish Spawning Aggregations at Gladden Spit, Belize. Bull. Mar. Sci. 83 (3), 531–551.
Kamikawa D. J. (2017). Survey Fishes: An Illustrated List of the Fishes Captured During the Northwest Fisheries Science Center’s Fishery Resource Analysis and Monitoring Division’s West Coast Surveys Vol. 138 (NOAA Technical Memorandum NMFS-NWFSC) Sacramento, California, USA.
Ketchen K. S., Forrester C. R. (1966). Population Dynamics of the Petrale Sole, Eopsetta Jordani, in Waters Off Western Canada. Rish Res. Board Can. Bull. 153, 195.
Kobara S., Heyman W. D. (2010). Sea Bottom Geomorphology of Multi-Species Spawning Aggregation Sites in Belize. Mar. Ecol. Prog. Ser. 405, 243–254. doi: 10.3354/meps08512
Konstantinou H., Shen D. C. (1995). The Social and Reproductive Behavior of the Eyed Flounder, Bothus Ocellatus, With Notes on the Spawning of Bothus Lunatus and Bothus Ellipticus. Environ. Biol. Fish 44, 311–324. doi: 10.1007/BF00008245
Leet W. S., Dewees C. M., Klingbeil R., Larson E. J. (Eds.) (2001). California’s Living Marine Resources: A Status Report (California Department of Fish and Game) Sacramento, California, USA.
Loher T., Seitz A. (2006). Seasonal Migration and Environmental Conditions of Pacific Halibut Hippoglossus Stenolepis, Elucidated From Pop-Up Archival Transmitting (PAT) Tags. Mar. Ecol. Prog. Ser. 317, 259–271. doi: 10.3354/meps317259
Manabe H., Shinomiya A. (2001). Two Spawning Season and Mating System of the Bastard Halibut, Tarphops Oligolepis. Ichthyol. Res 48, 421–424. doi: 10.1007/s10228-001-8167-9
Mcknight C. (2013). “19 Petrale Sole, Eopsetta Jordani,” in Status of the Fisheries Report - An Update Through 2011. Ed. Larinto T. (California Department of Fish and Wildlife) Sacramento, California, USA.
Pankhurst N. W., Fitzgibbon Q. P. (2005). Characteristics of Spawning Behaviour in Cultured Greenback Flounder Rhombosolea Tapirine. Aquaculture 253, 279–289. doi: 10.1016/j.aquaculture.2005.01.014
Pedersen M. G. (1975). Movements and Growth of Petrale Sole (Eopsetta Jordani) Tagged Off Washington and Southwest Vancouver Island. J. Fish Res. Board Can. 32, 2169–2177. doi: 10.1139/f75-255
R Core Team (2017). R: A Language and Environment for Statistical Computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org.
Rowe S., Hutchings J. A. (2003). Mating Systems and the Conservation of Commercially Exploited Marine Fish. Trends Ecol. Evol. 18 (11), 567–572. doi: 10.1016/j.tree.2003.09.004
Sadovy de Mitcheson Y. (2016). Mainstreaming Fish Spawning Aggregations Into Fishery Management Calls for a Precautionary Approach. Bioscience 66. 4, 295–306. doi: 10.1093/biosci/biw013
Singh H., Can A., Eustice R., Lerner S., McPhee N., Pizarro O., et al. (2004). Seabed AUV Offers New Platform for High-Resolution Imaging. EOS 85 (31), 289–295. doi: 10.1029/2004EO310002
Tolimieri N., Clarke M. E., Singh H., Goldfinger C. (2008). “Evaluating the SeaBED AUV for Monitoring Groundfish in Untrawlable Habitat,” in Marine Habitat Mapping Technology for Alaska. Eds. Reynolds J. R., Green H. G. (Alaska Sea Grant College Program, University of Alaska Fairbanks) Fairbanks, Alaska, USA. doi: 10.4027/mhmta.2008.09
Walker S. L., Baker E. T., Resing J. A., Nakamura K., McLain P. D. (2007). A New Tool for Detecting Hydrothermal Plumes: An ORP Sensor for the PMEL MAPR. Eos Trans. AGU88 (52). Fall Meet Suppl. Abstract V21D-0753.
Wetzel C. R. (2019). Status of Petrale Sole (Eopsetta Jordani) along the U.S. West Coast in 2019 Pacific Fishery Management Council 7700 Ambassador Place NE, Suite 101, Portland, OR 97220, USA.
Keywords: Petrale sole, Eopsetta jordani, autonomous underwater vehicle, spawning aggregation, spawning behavior, Pacific flatfish, flatfish behavior
Citation: Powell A, Clarke ME, Haltuch MA, Fruh E, Anderson J, Whitmire CE and Johnson MM (2022) First Autonomous Underwater Vehicle Observations of a Potential Petrale Sole (Eopsetta jordani) Spawning Aggregation Off the US West Coast. Front. Mar. Sci. 9:834839. doi: 10.3389/fmars.2022.834839
Received: 13 December 2021; Accepted: 21 March 2022;
Published: 03 May 2022.
Edited by:
Ana Colaço, Marine Research Institute (IMAR), PortugalReviewed by:
Jorge Paramo, University of Magdalena, ColombiaCopyright © 2022 Powell, Clarke, Haltuch, Fruh, Anderson, Whitmire and Johnson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abigail Powell, YWJpZ2FpbC5wb3dlbGxAbm9hYS5nb3Y=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.