- 1Laboratory of Aquatic Nutrition and Feed, College of Fisheries, Guangdong Ocean University, Zhanjiang, China
- 2Aquatic Animals Precision Nutrition and High Efficiency Feed Engineering Research Center of Guangdong Province, Zhanjiang, China
- 3Guangdong XIPU Biotechnology Co., Ltd., Guangzhou, China
The effect of hydrolyzed fish protein powder (HFP) on the growth, intestinal development, gene mRNA expression, and enzyme activity in the intestine and liver of juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ and Epinephelus lanceolatus ♂) was assessed after an 8-week feeding trial. Seven isonitrogenous (50%) and isolipidic (9%) diets were fed to hybrid grouper with 0% (CT), 1% (H1), 1.5% (H2), 2% (H3), 2.5% (H4), 3% (H5), and 4% (H6) HFP. No significant difference (p > 0.05) in weight gain rate (WGR), specific growth rate (SGR), feed conversion ratio (FCR), and survival rate (SR) was observed in all the groups. The crude protein content in the H6 group was significantly higher than in the other groups (p < 0.05). Intestinal lipase and trypsin activity were significantly higher in H3 and H5 groups (p < 0.05). In the serum, superoxide dismutase (SOD) activity was significantly higher in H5 and H6 groups, while malondialdehyde (MDA) activity was lower (p < 0.05) compared to other treatments. Insulin-like growth factor (IGF-I) and target of rapamycin (TOR) mRNA expression levels in the intestine and muscle were significantly higher in the H2 group and H1 group (p < 0.05), respectively. The most abundant intestinal bacteria found at the genus level are Acinetobacter, Vibrio, and Flavobacteriaceae. The villus was significantly longer in hybrid grouper fed with different levels of HFP compared to the control, and fish in the H2 group had thicker intestinal muscle compared to the other groups (p < 0.05). In conclusion, the addition of HFP to the low fishmeal (FM) diets of juvenile grouper improved the intestinal development and increased the levels of intestinal digestive enzymes.
Introduction
Aquaculture is anticipated to fulfill the global request for aquatic animals due to the reduction in capture fisheries since the 1990s (FAO, 2020). In aquaculture practice, feed accounts for over 50% of production cost, which is mainly composed of protein (Tacon and Metian, 2008). About 22 million tonnes of the world’s fish production in 2018 was used for non-food purposes, of which 18 million tonnes were used to make fish oil and fishmeal (FM) (FAO, 2020). Traditionally, the most preferred dietary source of protein, which is the most expensive and important nutrient influencing fish growth and feed cost, especially in carnivorous fish, is obtained from FM due to its high digestibility, well-balanced amino acid, and rich source of essential n-3 fatty acids (Tacon and Metian, 2008; Olsen and Hasan, 2012). Environmental and ecological distress on the use of marine pelagic fish, limited supply, and increasing demand for FM has resulted in the intensive study of identifying viable alternative protein sources in aquafeed (FAO, 2020).
Processing fish produces a substantial amount of waste, which includes skin/scales, bones, swim bladders, roes, intestines, blood, and liver, representing about 57% of total weight. Large portions of these by-products, which contain a large amount of bioactive-rich materials, are wasted, discarded, or underutilized (Meeker, 2009; Kumar et al., 2018). Karayannakidis and Zotos (2016) stated that recycling these by-products into profitable goods for agriculture can be a waste management scheme. Quality protein in animal by-products can be hydrolyzed to obtain small molecular peptides which can act as a flavoring and good source of amino acids (Choi et al., 2012; Kumar et al., 2012).
Groupers are among the most common fish and extremely merchandized seafood in the Asia-Pacific region (FAO, 2020). Due to its rapid growth rate, hardiness to environmental conditions, high disease resistance, and high nutritional value, it is an ideal species for intensive aquaculture (Ch’ng and Senoo, 2008; Jiang et al., 2015; Arrokhman et al., 2017; Bunlipatanon and U-taynapun, 2017). With regard to marine fish culture output in China, groupers are ranked third (Yang et al., 2021). Hybrid grouper with a higher disease resistance, faster growth rate, and better feed conversion ratio (FCR) was produced at the University of Malaysia Sabah from brown-marbled grouper (Epinephelus fuscoguttatus ♀) and giant grouper (Epinephelus lanceolatus ♂) (Rahimnejad et al., 2015; Firdaus et al., 2016). The hybrid produced grows to 1 kg in a period of 6–7 months, while the parents require 8 months to 1 year to attain a similar weight (Arrokhman et al., 2017).
Nutrient utilization and growth performance are the conditions mostly used to assess substitute protein sources in aquafeeds, whereas intestinal health and immunity are often overlooked (Ye et al., 2019). Using hydrolyzed fish protein powder (HFP) to replace FM has been studied by Nguyen et al. (2012); Egerton et al. (2020), and Rimoldi et al. (2020) in gilthead sea bream (Sparus aurata), Pacific white shrimp (Litopenaeus vannamei), and Atlantic salmon (Salmo salar), respectively, but they focused mainly on growth and intestinal microbiota. This study aims to evaluate the effect of HFP on the growth, survival, whole-body composition, serum and liver physiological and biochemical indexes, intestinal morphology, digestive enzymes, gene mRNA expression, and intestinal microbiota in juvenile hybrid grouper.
Materials and Methods
Experimental Diets
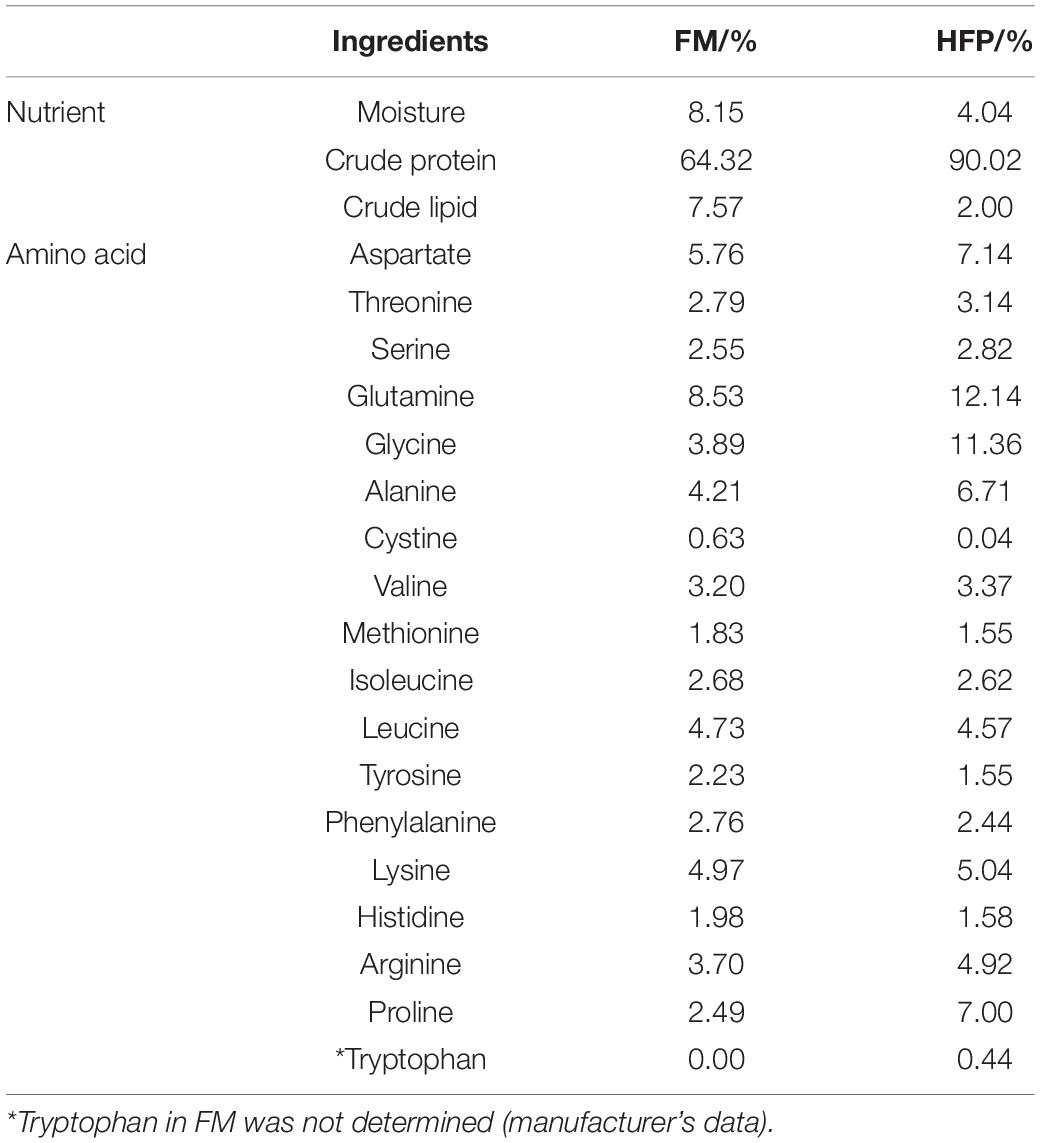
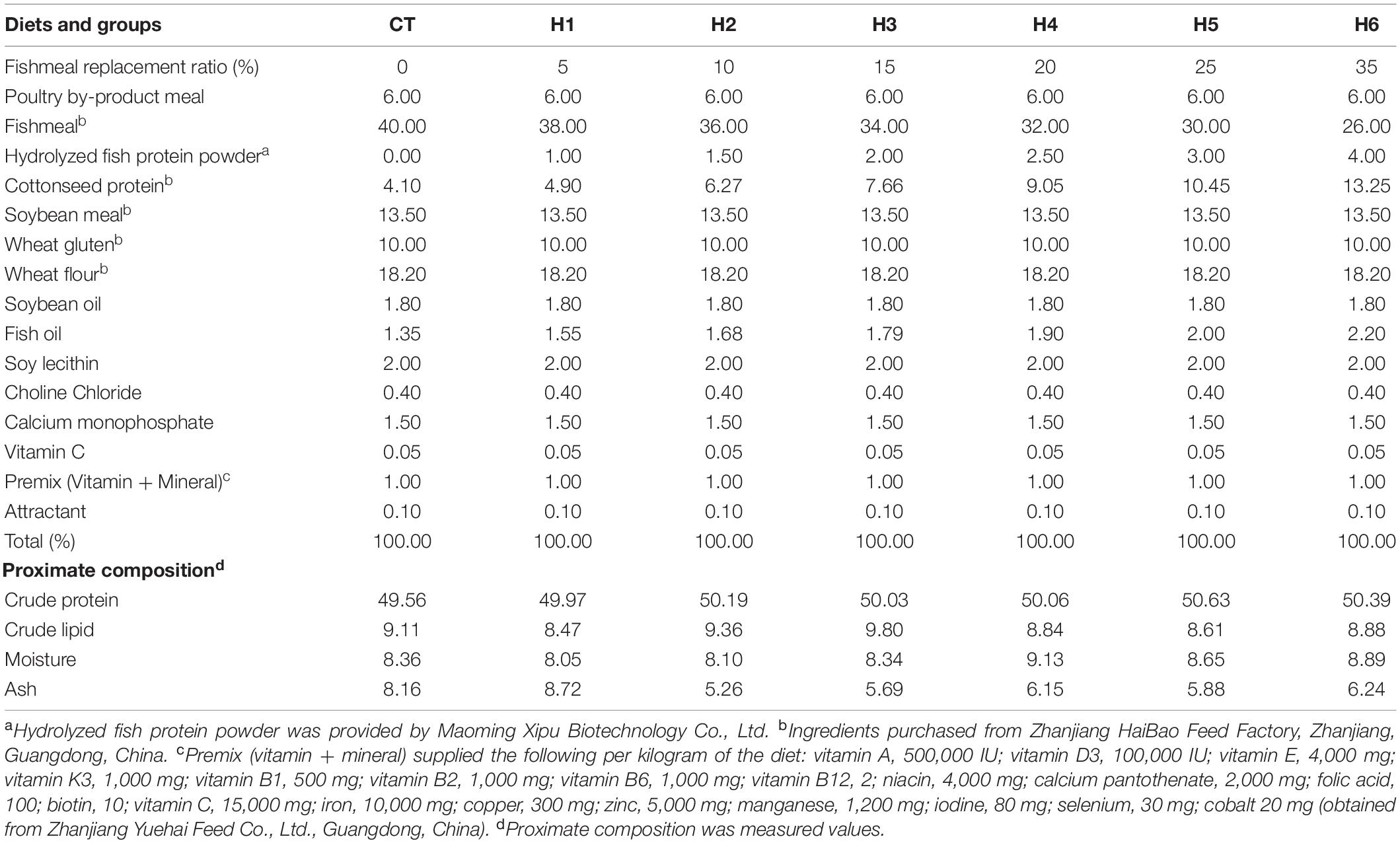
The HFP with 90% crude protein and 2% crude lipid (obtained from Maoming Xipu Biotechnology Co., Ltd.) was used as a substitute for FM in this experiment (Table 1). Seven fish diets containing crude protein (50%) and crude lipid (9%) were formulated, as shown in Table 2. The protein level of the formulated feed was balanced using cottonseed protein, which is in abundance and a good ingredient for fish feed, as observed by Yin et al. (2018). The group CT was fed with a diet that had no HFP replacing FM. Due to the high small peptides level, the experimental groups, namely, H1, H2, H3, H4, H5, and H6 were fed with diets containing 1, 1.5, 2, 2.5, 3, and 4% of HFP replacing the corresponding amount of FM, respectively. The raw materials were crushed after visual inspection and sieved using the 60 mm mesh screen. The raw ingredients were then weighed and mixed thoroughly using the V-mixer-type machine (JS-14S, Zhejiang Zhengtai Electrical Appliance Co., Ltd., China). Using a Hobart-type mixer (Food Mixer B60, Guangdong Henglian Food Machinery Co., Ltd., China), the ingredients were mixed with water and choline chloride to form a moist dough. The feed was pelletized into 2 and 2.5 mm granules, air-dried for 48 h, and the dried feed was stored in sealed plastic bags at −20°C until the experiment started.
Experimental Fish and Feeding Trial
Healthy pearl gentian groupers purchased from Hongyun seedling farm were used for the study. They were kept in aerated cement tanks for an acclimatization period of 3 weeks and hand-fed commercial diets (Dongwan No. 5 feed, China). This study was conducted using indoor fiberglass tanks (0.3 m3) at the Marine Biological Research Base of Guangdong Ocean University with a natural photoperiod (12 h light/12 h dark) regime. A total of 630 hybrid groupers with no signs of disease were starved for 24 h, batch-weighed to determine an initial average weight (31.56 ± 0.04 g), and randomly distributed at a stocking density of 30 fish per tank. Each treatment was assigned to three replicate tanks. The experimental feed was fed manually twice a day (08:00 and 16:00) until a visually apparent satisfied state was reached, thus as much as they consume during feeding for 56 days. The amount of feed consumed for the period was recorded to check feed intake. Using single-air stones, aeration was provided, and the water temperature was 27.2 ± 1.32°C. Culture water was maintained by changing about 70% in the tanks daily. Daily, mortalities were checked, weighed, and recorded.
Sample Collection
Before the feeding trial, about 20 fish were randomly sampled and stored at −20°C for the initial chemical proximate composition analysis. To obtain the optimum levels of body metabolism, fish were starved for 24 h before the cessation of the experiment. The final number of fish and body weight were checked and recorded. Survival rate (SR), weight gain rate (WGR), FCR, and specific growth rate (SGR) were calculated based on the recordings. The weight and length of five fish per replicate were checked after which their viscera were harvested and weighed to establish their viscerosomatic index (VSI) and condition factor (CF).
Nutrient Composition
Nutrient composition (e.g., moisture, crude protein, crude lipid, and ash) in feed and fish was determined using the standard methodology of the Association of Official Analytical Chemists (Baur and Ensminger, 1977).
Intestinal Enzyme Evaluation
Intestinal samples of 3 fish per replicate were weighed and homogenized in 0.9% aseptic saline as described by the specific operation method provided by the commercial kits obtained from the Nanjing Jiancheng Institute of Biological Engineering, China. Using the specific operation method and calculation method provided by the kit, amylase and lipase in the intestine were determined, and absorbance was measured using the microplate reader. Chymotrypsin, trypsin, and total protease activity in the intestine were checked by Shanghai Enzyme-Linked Biotechnology Co., Ltd., China.
Determination of Enzyme Activities
Blood was pooled from 3 fish per replicate using a 1-ml sterile syringe. Blood obtained was transferred into 1.5 ml Eppendorf tubes and stored at 4°C overnight. The blood samples were centrifuged at 4°C at 4,000 rpm for 15 min. The supernatant (serum) was collected and stored at −80°C to check the levels of total protein (TP), albumin (ALB), superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione peroxidase (GHS-PX) using the test kits (Nanjing Jiancheng Institute of Biological Engineering, China). The liver was harvested from the fish and stored at −80°C to check SOD and MDA activities using the test kits (Nanjing Jiancheng Institute of Biological Engineering, China). The absorbance was read with a microplate reader (Thermo Fisher Scientific, United States). The specific operation methods and calculation formulas were used referring to the test kit instructions. Using the isotope method, growth hormone (GH) and insulin (INS) in the serum were checked by Beijing North Biotechnology Research Co., Ltd.
RNA Extraction and Real-Time Quantitative Reverse Transcriptase PCR
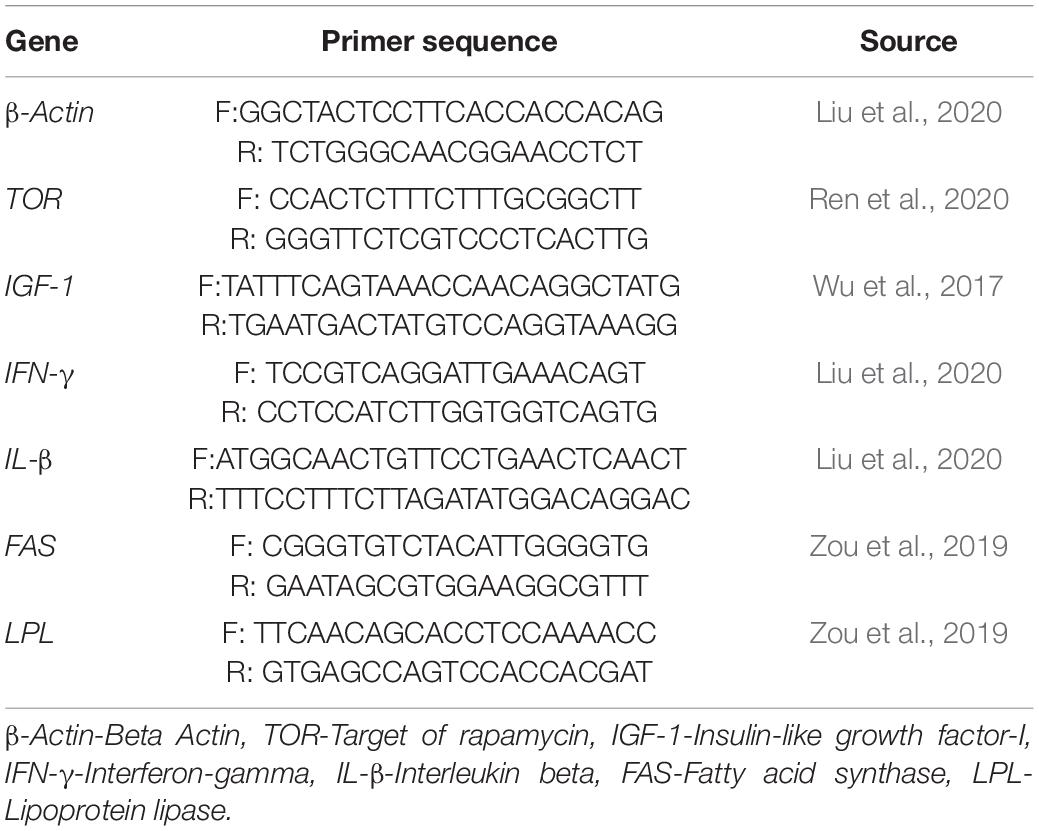
The samples of the muscle, liver, and intestine were taken from 3 fish per replicate, and total RNA was extracted using the specific operation method of TRIzol (Invitrogen, United States) reagent. Using 1% agarose gel electrophoresis, the integrity of the extracted RNA was verified, and a spectrophotometer [NanoDrop-2000 spectrophotometer (Thermo Fisher Scientific, United States)] was used to measure the concentration and purity of RNA. Reverse transcription was performed using the PrimeScript™ kit (TaKaRa, China) and its method. The β-actin gene was used as the housekeeping gene, and real-time fluorescent quantitative PCR assays were conducted to detect gene expression levels for genes shown in Table 3 using a quantitative thermal cycler (Bio-Rad CFX96; Bio-Rad Labs, Hercules, CA, United States). Relative expression levels of genes were calculated by 2–△△CT.
Intestinal Microbiota Analysis
The intestinal samples were sent to Beijing Biomarker Technologies Co., Ltd., for DNA extraction and PCR amplification using Illumina MiSep sequencing analysis. Using NucleoSpin Soil kit, the total genome DNA in the stool of the intestinal sample was extracted. Universal primers (338F: 5′-ACTCCTACGGGAGGCAGCA-3′ and 806R: 5′-GGACTACHVGGGTW TCTAAT-3′) were used to amplify the V3-V4 region of the 16S rRNA gene for Illumina deep sequencing. The microbiota alpha diversity, community composition, and community abundance at phylum and genus levels were performed by using the mothur (Schloss et al., 2009), a free online platform.
Intestinal Morphology
A grouper per replicate was randomly selected, and its mid intestine was harvested and stored in formaldehyde solution for hematoxylin and eosin (H&E) staining using the sectioning method. Results obtained were used for histological examination of the villus length (VL), villus width (VW), and muscle thickness (MT) using ImageJ software.
Statistical Analysis
All original data were subjected to statistical verification using one-way analysis of variance (ANOVA) after consistency and normality of data variance was checked. All statistical analyses were performed using the SPSS 22.0 for Windows and general differences were found to be significant at p < 0.05. Tukey’s honest significant difference (HSD) test was used to compare the mean values between individual treatments. Data are represented as mean values of each group of hybrid grouper (three replicates) ± standard error (SE).
Results
Growth Performance, Survival, and Body Composition
The hybrid grouper did not have a significant difference in WGR, SGR, FCR, SR, and VSI (p > 0.05) in all groups. CF of hybrid grouper in the CT group was not significantly different with H1, H2, H3, and H5 groups (p > 0.05) as summarized in Table 3.
In Table 4, it was observed that body moisture, crude lipid, and PPV did not show significant differences (p > 0.05) in all groups. The crude protein content in the H6 group was significantly higher than CT and H1 groups (p < 0.05). Ash content in H1, H2, H4, and H5 groups was significantly higher than the CT group but significantly lower than in the H3 group (p < 0.05).
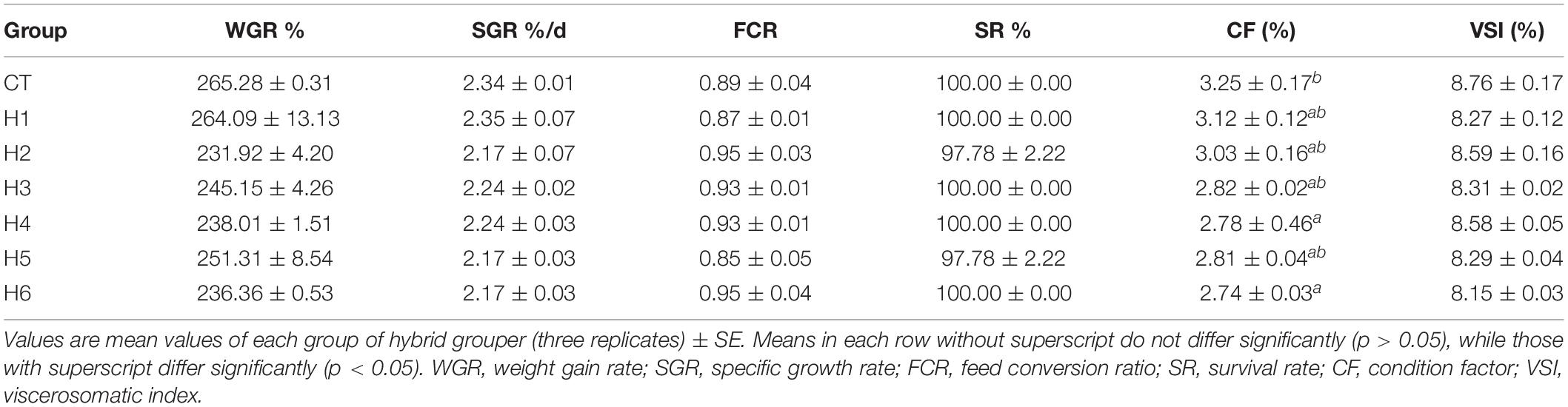
Table 4. Effect of hydrolyzed fish protein powder on the growth and survival of juvenile pearl gentian hybrid grouper.
Intestinal Digestive Enzyme Activity
No significant difference in intestinal amylase activity was observed among all the groups (p > 0.05), while lipase activity was significantly higher in the H3 group compared to H5 and H6 groups (p < 0.05). Intestinal trypsin activity was significantly lower in the H1 group (p < 0.05). Intestinal chymotrypsin activity was significantly lower in CT, H1, and H2 groups (p < 0.05). The highest intestinal protease activity was observed in the H4 group which did not differ significantly from H2, H3, and H5 groups (Figure 1).
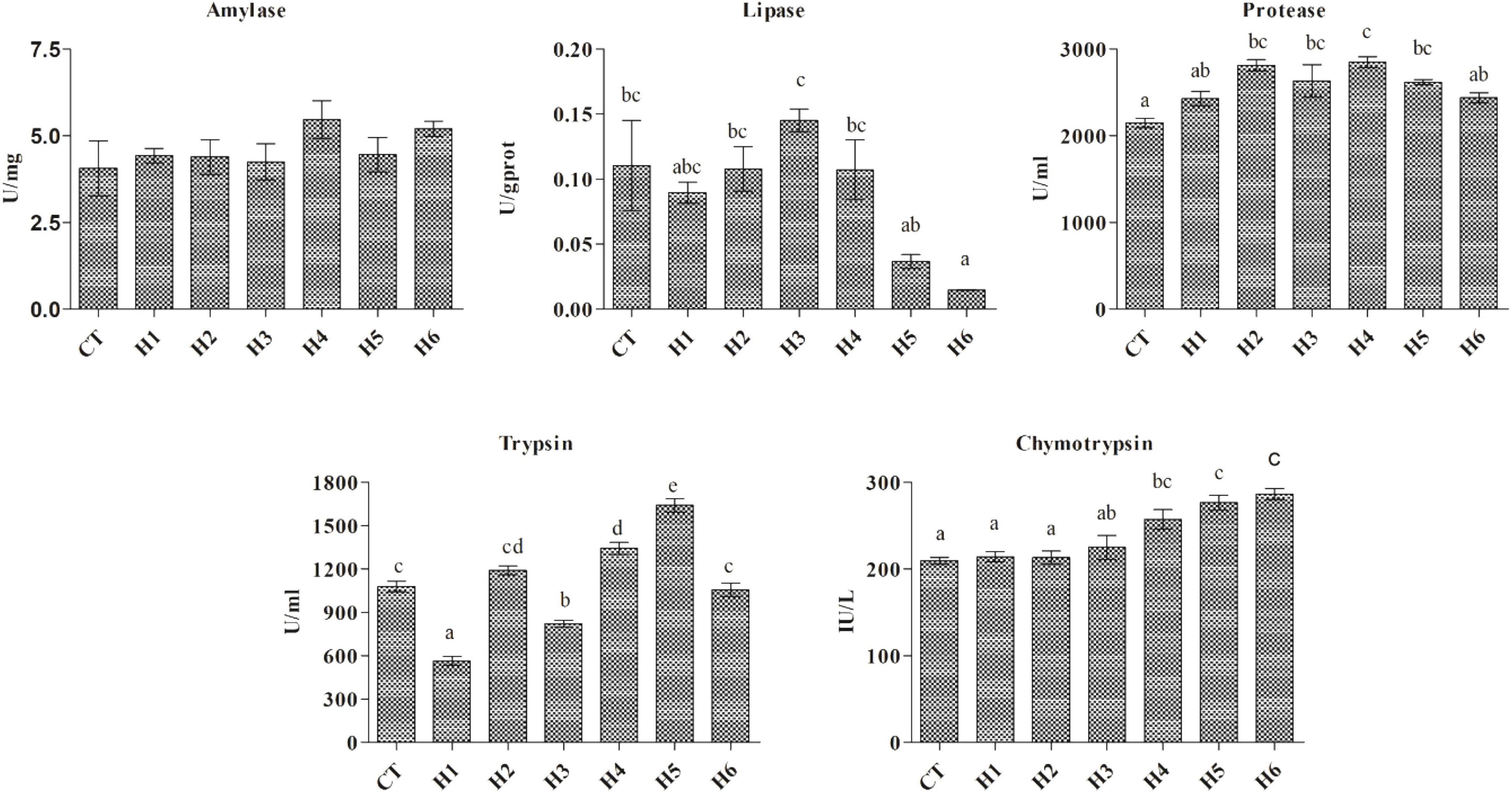
Figure 1. Effect of HFP on digestive enzymes activity in the intestine of juvenile pearl gentian hybrid grouper. Data are presented as Means ± SE. Means without superscript do not differ significantly (P < 0.05), whiles those with superscript differ significantly (P < 0.05).
Enzyme Activities in the Serum and Liver
In Table 5, the serum TP level in the H3 group was not significantly different from that of the H2, H4, H5, and H6 groups (p > 0.05) but significantly higher than CT and H1 groups (p < 0.05). There were no significant differences in the level of ALB and GSH-PX activities in the serum in all the groups (p > 0.05). Serum SOD was significantly higher in H5 and H6 groups in comparison with groups H2 and H3 (p < 0.05) but similar to the remaining groups (p > 0.05). Serum MDA in the H6 group was significantly lower but highest in H3 and CT groups (p < 0.05). No significant difference in MDA and SOD activity in the liver was observed for all groups (p > 0.05).
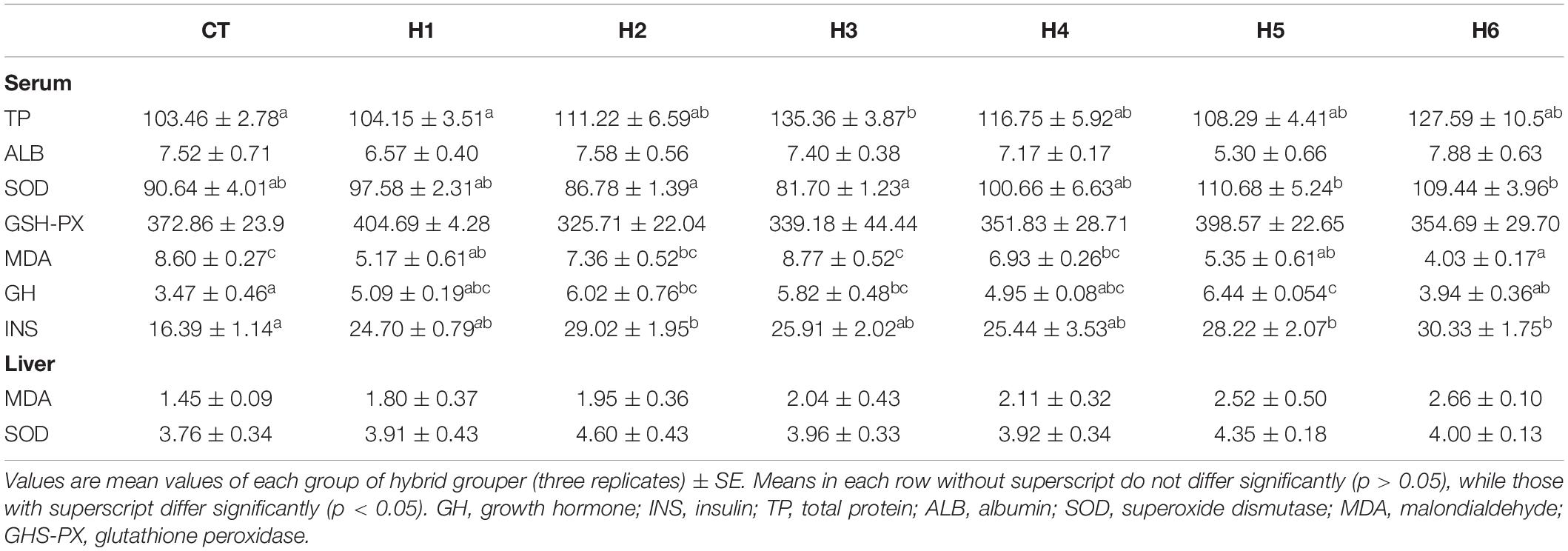
Table 5. Effect of hydrolyzed fish protein powder on the enzyme activities in the serum and liver of juvenile pearl gentian hybrid grouper.
The highest level of GH was observed in the H5 group compared to CT and H6 groups (p < 0.05) but did not differ significantly from H1, H2, and H3 groups (p > 0.05). For serum INS levels, the CT group was significantly lower than H2, H5, and H6 groups (p < 0.05) but did not have any significant difference with H1, H3, and H4 groups (p > 0.05).
mRNA Gene Expression in the Intestine, Muscle, and Liver
In the fish intestine, insulin-like growth factor (IGF-I) expression in H1, H2, H3, H4, and H6 groups was significantly higher than that in the CT and H5 groups (p < 0.05). The target of rapamycin (TOR) expression level in the intestine was significantly higher in the H2 group (p < 0.05), which was not significantly different from the H3 group (p > 0.05).
The IGF-I expression level in the muscle was significantly lower in the H4 group than the H1 group (p < 0.05) but was not significantly different from CT, H2, H3, H5, and H6 groups (p > 0.05). TOR expression level in the muscle was significantly higher in the H1 group (p < 0.05) but was not significantly different when compared to CT, H2, and H6 groups (p > 0.05).
The IGF-I expression level in the liver was significantly higher in the CT group compared to H2, H3, and H4 groups (p < 0.05), but no significant difference in liver TOR expression was observed (p > 0.05), as shown in Table 6. As shown in Figure 2, fatty acid synthase (FAS) expression levels in the liver were significantly higher in H3 and H4 groups than those in other groups (p < 0.05). The higher lipoprotein lipase (LPL) levels were observed in CT and H1 groups compared to the remaining groups (p < 0.05). In the liver, the interleukin beta (IL-β) expression level in the H6 group was significantly higher than that in CT group (p < 0.05). No significant difference in interferon-gamma (IFN-γ) expression levels was observed in all the groups (p > 0.05).
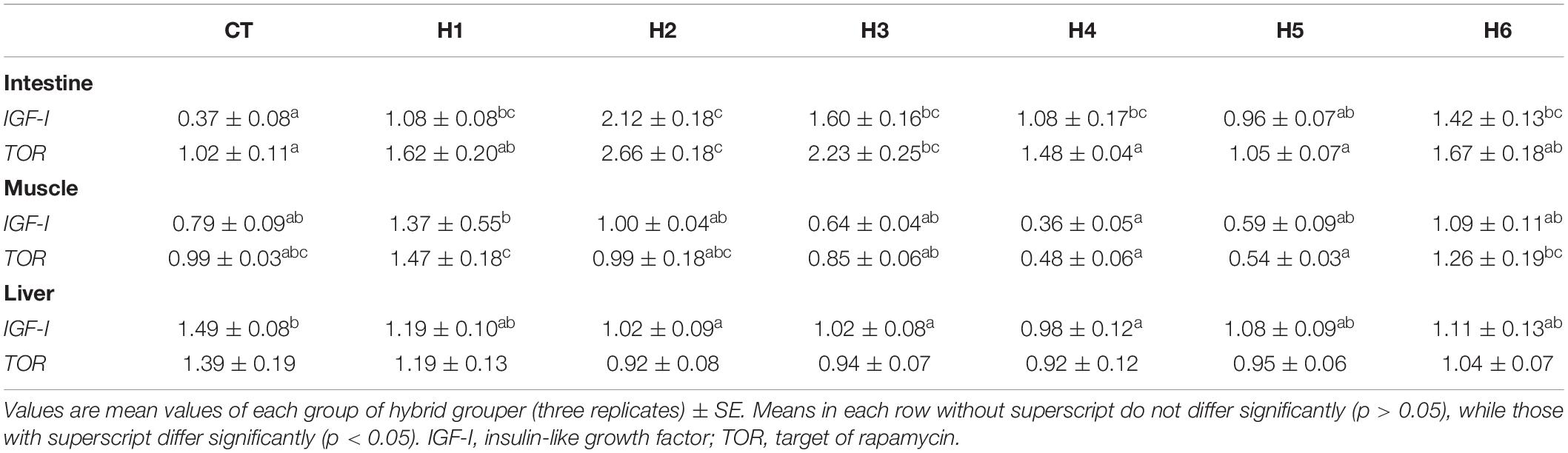
Table 6. Effect of hydrolyzed fish protein powder on mRNA IGF-I and TOR expression in the intestine, muscle, and liver of juvenile pearl gentian hybrid grouper.
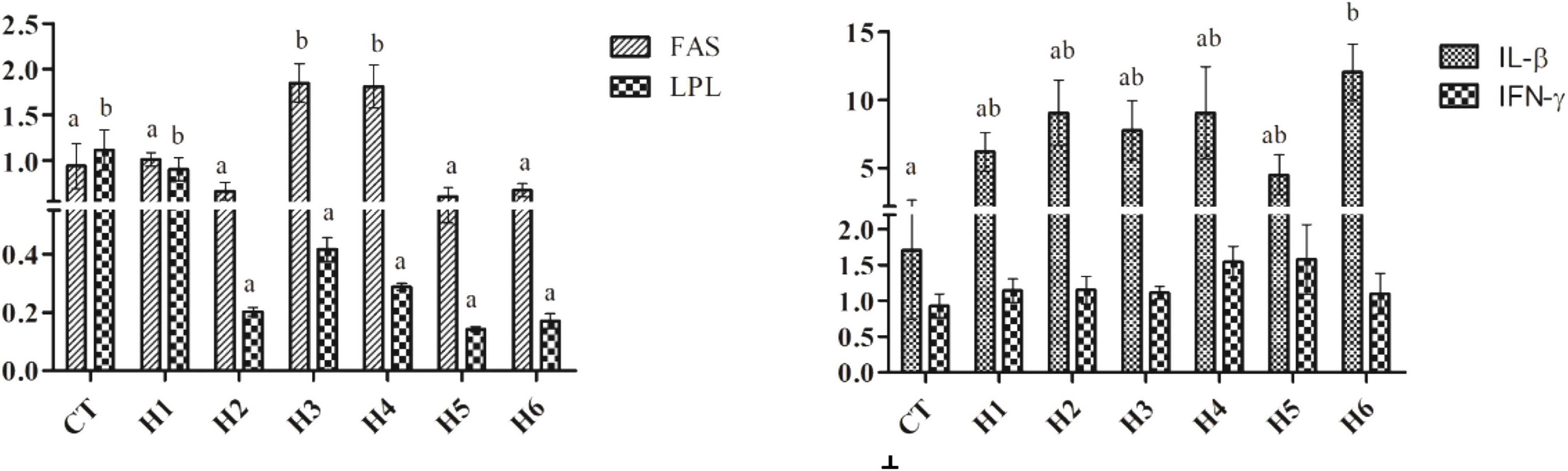
Figure 2. Effect of HFP on the expression of FAS, LPL, IL-β, and IFN-γ in the hepatopancreas of juvenile pearl gentian hybrid grouper. FAS, fatty acid synthase; LPL, lipoprotein lipase; IL-β, interleukin beta; IFN-γ, interferon-gamma.
Intestinal Microbiota
In the gut of juvenile hybrid grouper, the most abundant bacteria found at the genus level are Acinetobacter, Vibrio, and Flavobacteriaceae (Figure 3A). Proteobacteria, Bacteroidetes, and Actinobacteria were observed to be the most abundant bacteria at the phylum level, as shown in Figure 3B. As shown in Figure 4, a total of 653 operational taxonomic units (OTUs) were shared among all the treatments, and only H5 group had 1 unique OTU. Vibrio, Flavobacteriaceae, and Acinetobacter were significantly higher in H3, H4, and H2 groups, respectively, according to the heatmap (Figure 5). From the beta diversity distance matrix presented in Figure 6, the unweighted pair group method with arithmetic mean (UPGMA) tree showed a distinct dissociation from the control group. This indicates that HFP modified the overall structure of intestinal microbiota in hybrid grouper.
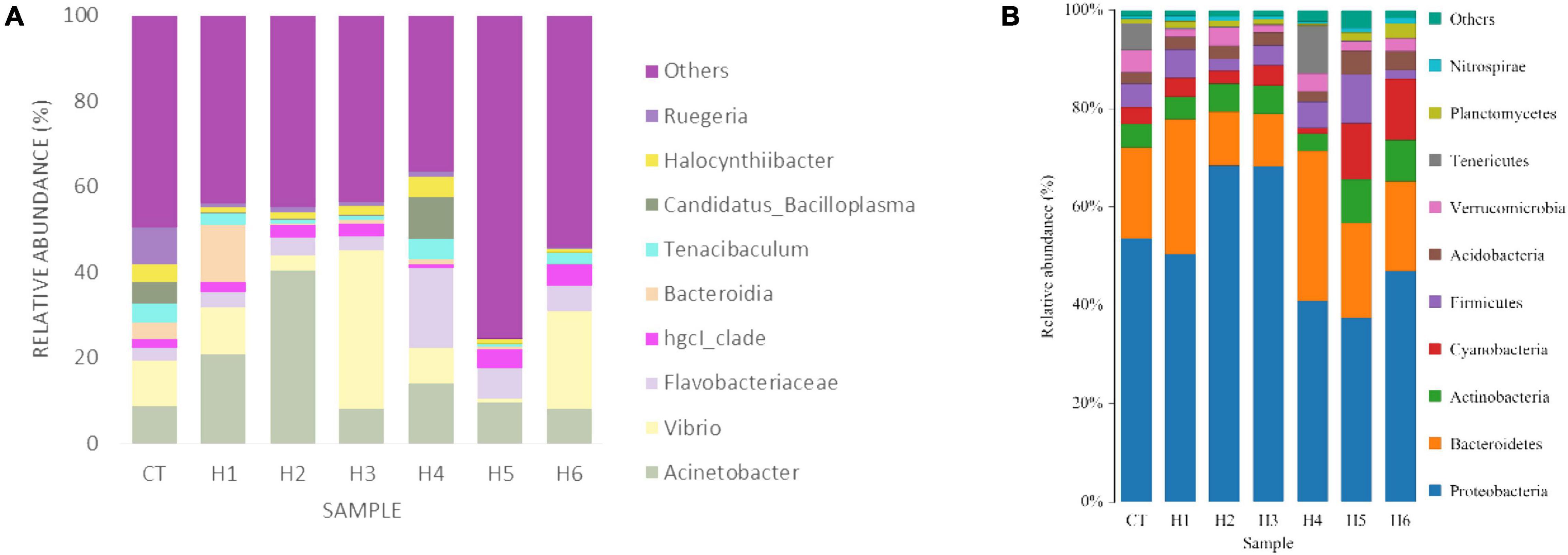
Figure 3. Effect of HFP on genus (A) and phylum (B) diversity in the intestine of juvenile pearl gentian hybrid grouper.
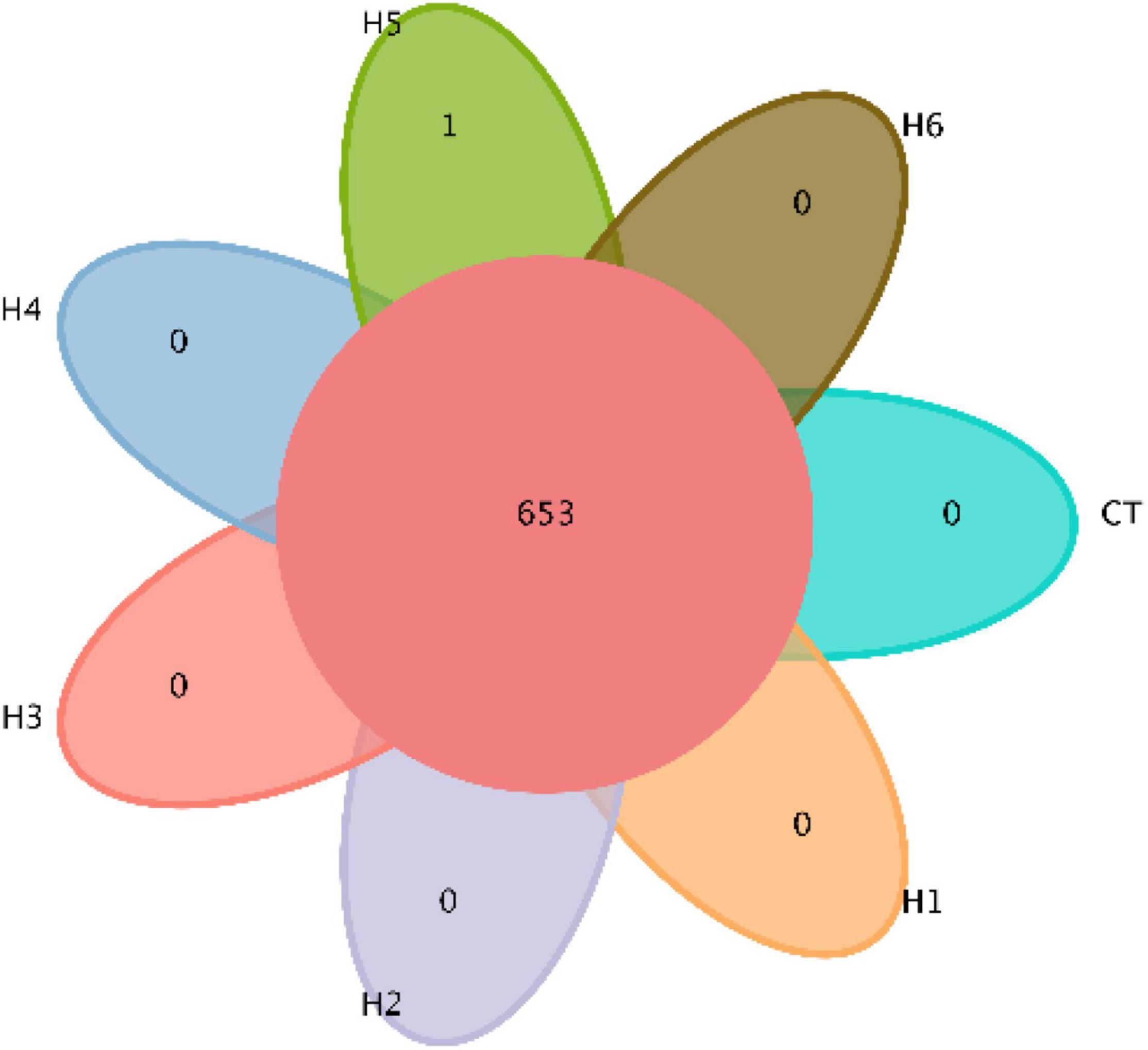
Figure 4. Venn diagram demonstrating the distribution of operational taxonomic units (OTUs) shared by juvenile pearl gentian hybrid grouper fed with different levels of HFP.
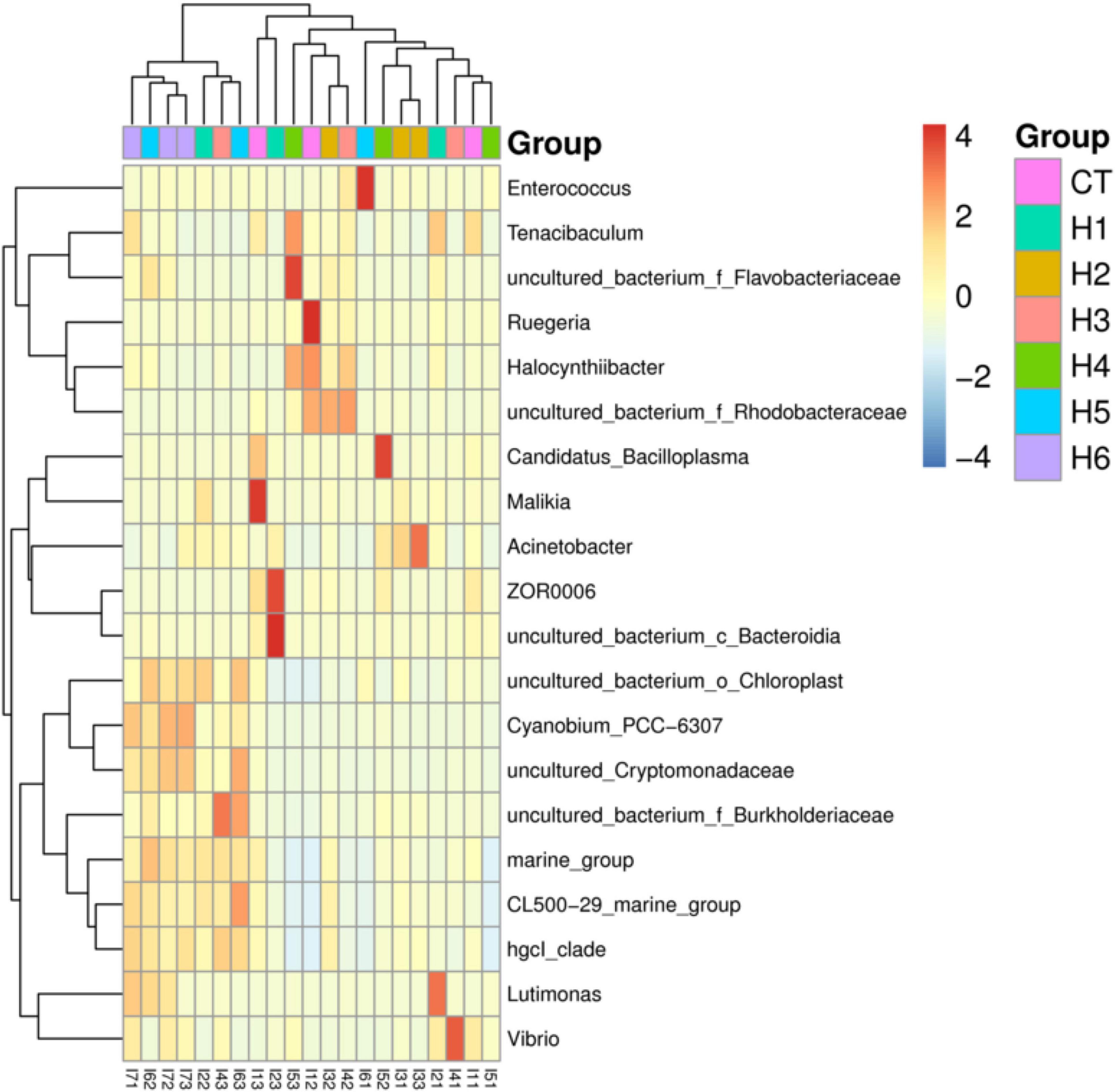
Figure 5. Heatmap of the abundance of intestinal bacteria at the genus level of juvenile pearl gentian hybrid grouper fed with different levels of HFP. Color intensity indicates the relative enrichment of OTUs.
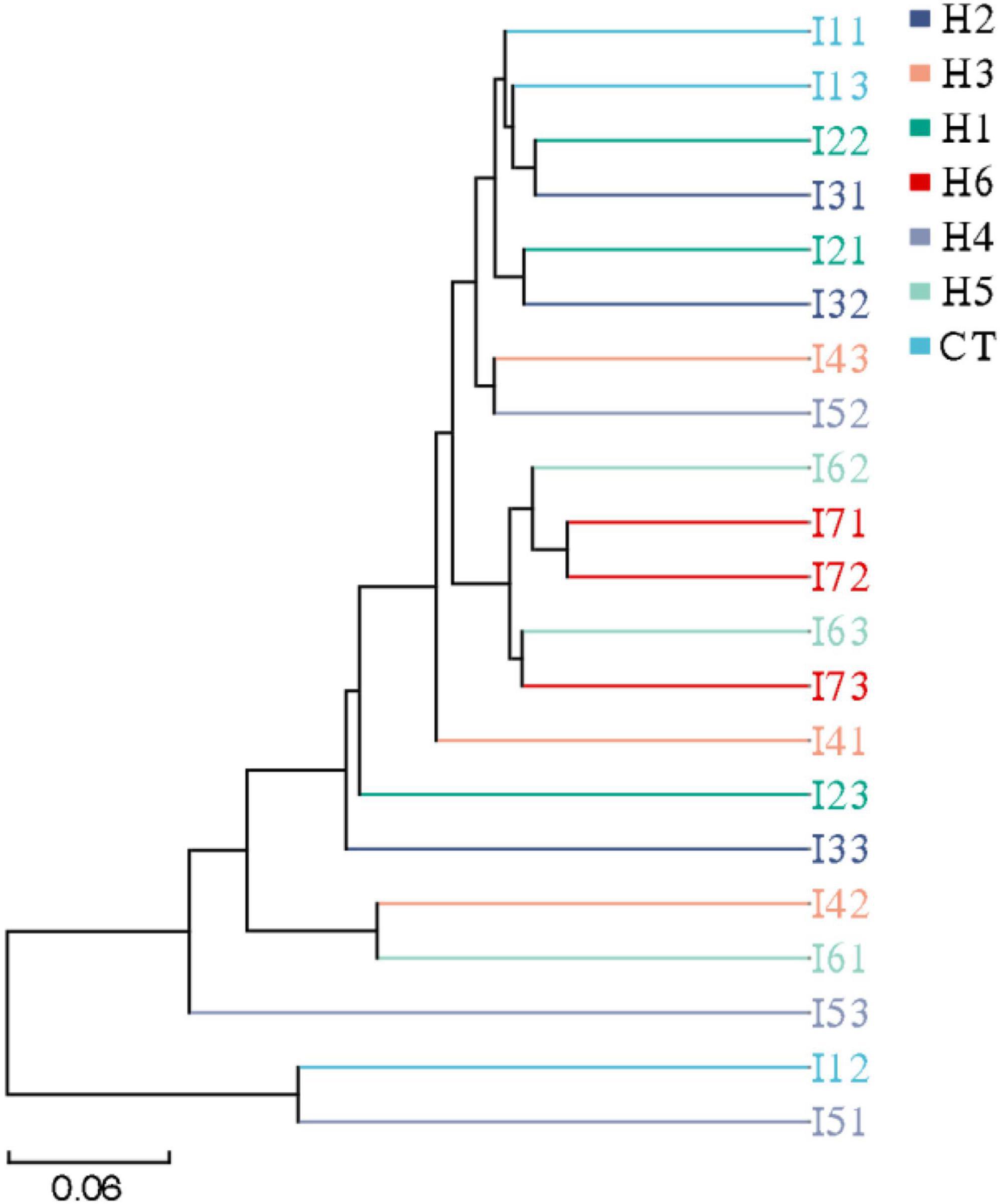
Figure 6. Unweighted pair group method with arithmetic mean-clustering tree of juvenile pearl gentian hybrid grouper fed with different levels of HFP.
Intestinal Morphology
The VL was significantly longer in the H2 group compared with the CT group (p < 0.05). Compared to the other groups, the villus was significantly wider in H5 and H6 groups (p < 0.05), and the least VW was recorded in the H4 group. The intestinal muscle layer in CT and H1 groups was significantly thicker than that of the H2 group but significantly thinner than that of the three other groups (p < 0.05), as summarized in Table 7 and Figure 7.
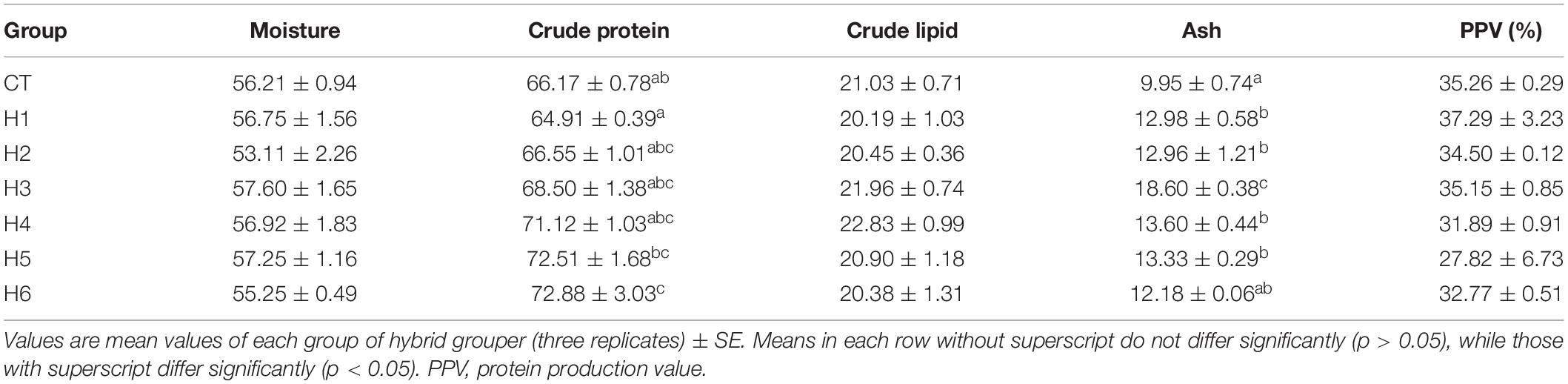
Table 7. Effect of hydrolyzed fish protein powder on the body composition of juvenile pearl gentian hybrid grouper.
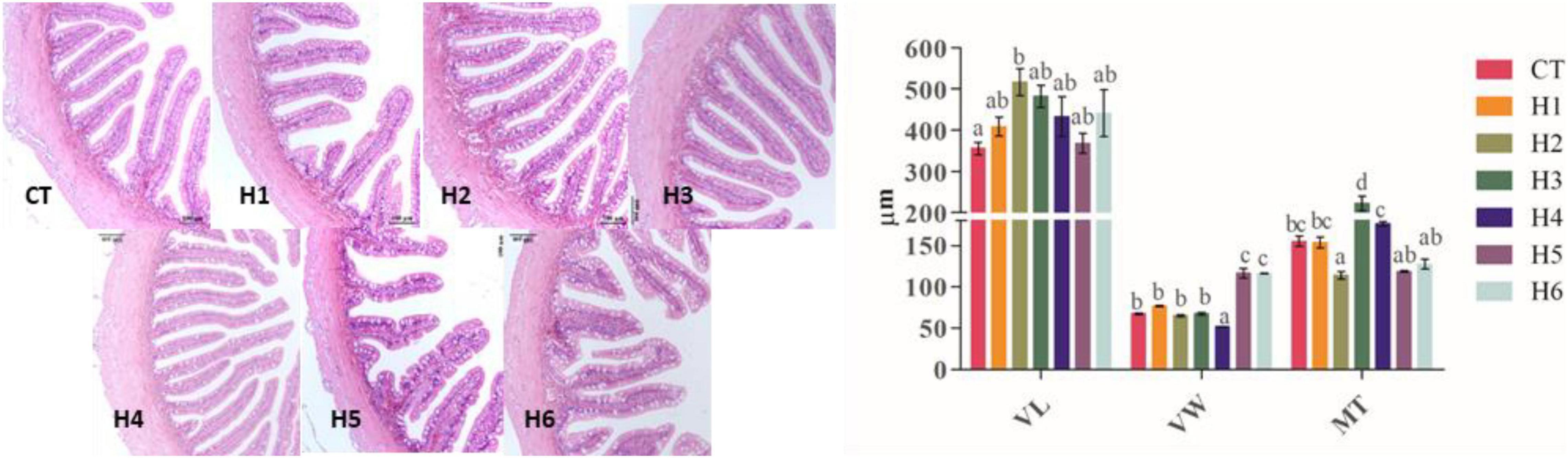
Figure 7. Effect of HFP on the intestinal morphology of juvenile pearl gentian hybrid grouper (H&E, 20×). VL, villus length; VW, villus width; MT, muscle thickness.
Discussion
The HFP is a promising ingredient used in the diet of aquatic animals due to its potential function to improve growth as well as immune status (Siddik et al., 2019b). HFP used to partially replace FM in the experimental diets in this study contains about 78.5% of small peptides less than 1,000 Da (Figure 8). No significant difference and high SR were observed in this study, which showed that all nutritional requirements were met in all the diets and experimental conditions were suitable for hybrid grouper in this study (Wei et al., 2016). The growth performance of hybrid grouper fed with different levels of HFP was slightly reduced, but there was no significant difference to the control group. This reduction in growth performance was also observed by Oliva-Teles et al. (1999) and Khieokhajonkhet and Surapon (2020) when HFP was added to the diet of Nile tilapia (Oreochromis niloticus), and juvenile turbot (Scophthalmus maximus), respectively. However, results observed in this study were not in accordance with the study conducted in juvenile barramundi (Lates calcarifer) fed with different levels of HFP which contained peptides with a molecular size of <3,000 Da (Siddik et al., 2019b). It might be a result of an increase in the catabolism of small peptides and amino acids via the gut wall due to the limited availability of peptide transporters (Bakke-McKellep et al., 2000). This can be related to reduce growth rate performance in this study. It is also possible that the macronutrients’ requirements by hybrid grouper were met by FM, hence, masking the profitable effect of HFP (Wei et al., 2016).
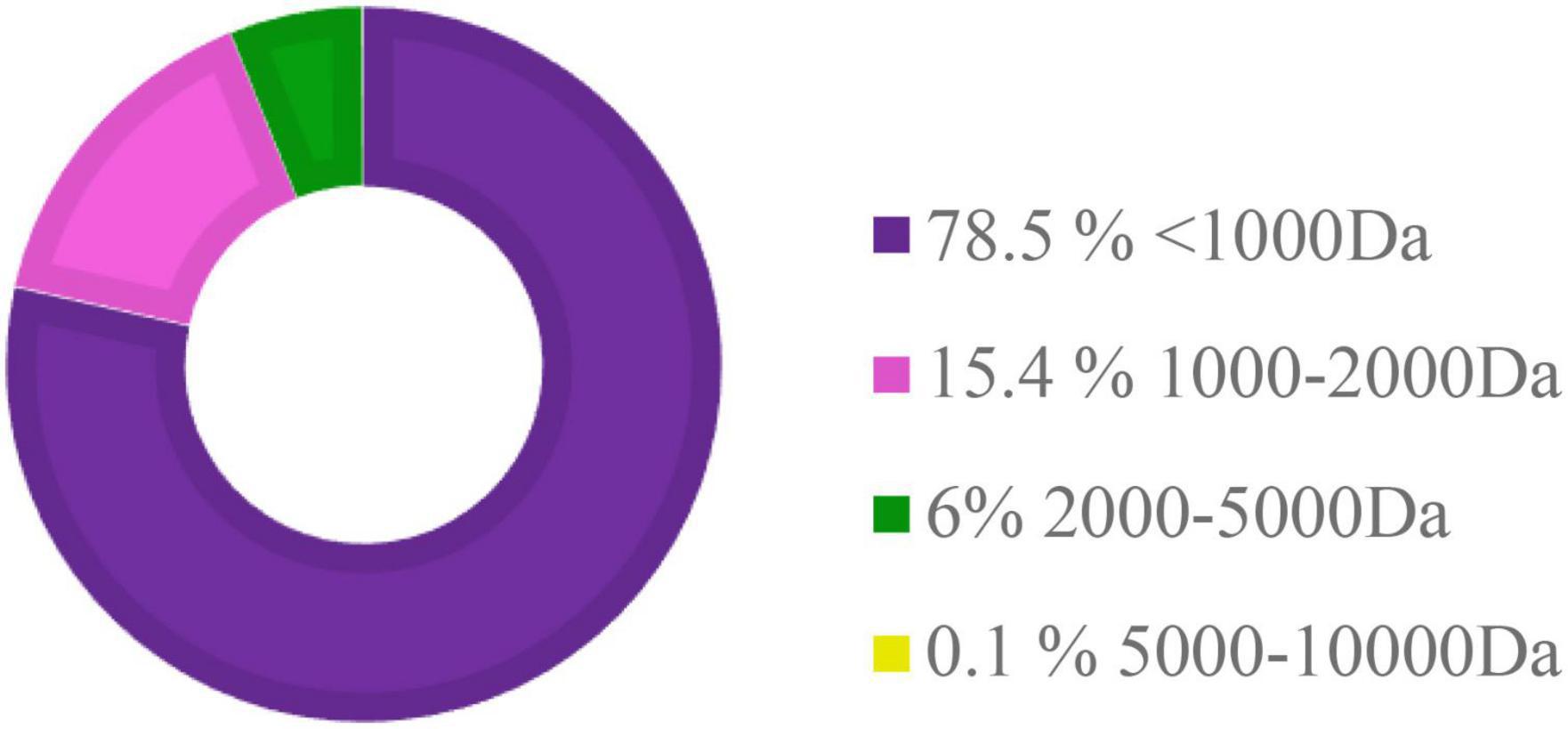
Figure 8. The molecular weight distribution of peptides in hydrolyzed fish protein powder (HFP). Da, Dalton.
At various inclusion levels of HFP, whole-body ash and crude protein contents were altered similar to the results observed by Zheng et al. (2012) in Japanese flounder (Paralichthys olivaceus) when fed with feed containing HFP with about 66.4% small peptides with a size between 100 and 1,000 Da. Sources of hydrolysates and small peptide-sized molecules may have influenced these results. Fish fed with different levels of HFP obtained significantly higher crude protein levels compared to those fed the control diet. In aquatic animal nutrition, levels of TP have been regarded as an indicator of the health and physiological condition of an aquatic organism (Harikrishnan et al., 2003).
The IGF-I and TOR levels have been evaluated to correlate with growth in different aquatic animals, such as European sea bass (Dicentrarchus labrax) (Carnevali et al., 2006), tilapia (O. niloticus) (Yan et al., 2013), juvenile turbot (S. maximus) (Wang et al., 2016), and pacific white shrimp (Liu et al., 2018). In this study, it was observed that hybrid grouper fed with different levels of HFP obtained a significantly higher total crude protein level compared to the control. A higher level of IGF-I and TOR mRNA gene expression in the intestine of hybrid grouper was also observed in fish fed with different levels of HFP compared to the control. Wei et al. (2020) also observed a higher IGF-I mRNA gene expression when FM was replaced with HFP in the diet of turbot. This could be due to better digestion and absorption of hydrolysate protein and signifies an improvement in general fish health (Khosravi et al., 2015).
The addition of suitable amounts of ingredients with small-sized peptides in feed increased digestive enzyme activity in the intestine of hybrid grouper (Yang et al., 2021), turbot (Jia et al., 2019), and sea bass (Zambonino Infante et al., 1997). Small-size peptide in HFP may play essential roles in regulating enzyme activity by promoting the secretion of digestive enzymes in aquatic animals (Madeira and Paula-Barbosa, 1999). In this study, intestinal trypsin and chymotrypsin were significantly high in the H5 group. These results show that 3% HFP in the diet of hybrid grouper could efficiently increase trypsin and chymotrypsin activity; hence, the presence of rich small-sized peptides could be linked to high levels of digestive enzymes.
The immunity and health of fish are greatly linked to the antioxidant defense system (Ahmadifar et al., 2019). Small-sized molecular peptides can improve and stimulate the capacity and activity of antioxidants in fish (Moure et al., 2006; Wu et al., 2018), and resistance in oxide damage is performed by antioxidant capacity (Lopes et al., 2001). The highest SOD activity in the serum was observed in the H6 group which contained 4% HFP. This shows that a 35% replacement ratio of dietary FM with HFP can increase the level of SOD in the serum (Moure et al., 2006).
Cytokines, which are small glycoprotein messengers, help in intercellular communication to support adaptive and innate immune responses against parasites, bacteria, and viruses (Bruce and Brown, 2017). Kotzamanis et al. (2007) stated that bioactive peptides with antibacterial and immunostimulating properties are produced during the procedure of hydrolysis. HFP used in this study is assumed to contain these peptides. IL-β is a pro-inflammatory cytokine in fish, which enhances lysozyme synthesis and defense mechanism with regard to bacterial colonization (Kim and Austin, 2006; Giri et al., 2015). IL-β was significantly higher in groups fed with different levels of HFP compared to the control. This was in agreement with Siddik et al. (2019a) who observed an upregulation of IL-β when HFP was included in the diet of juvenile barramundi. Results observed in this study could be due to the sufficient content of bioactive peptides in HFP used (Tang et al., 2008; Bui et al., 2014).
Fish gut comprises different groups of microbial communities, including virus, protists, fungi, and bacteria. The bacterial community, which is the dominant group found in the intestine, affects the immune system, metabolism, and health (Wardwell et al., 2011; Tran et al., 2018). The intestinal bacteria community can be influenced by feed composition (Merrifield et al., 2009). The results of this study were not entirely different from Kuebutornye et al. (2020) and Amoah et al. (2021) in tilapia (O. niloticus) and northern whiting fish (Sillago sihama), respectively. They noted that prevalent bacteria phyla found in the gut of fishes include Actinobacteria, Bacteroidetes, and Proteobacteria. At this level, Proteobacteria were the most abundant bacteria which increased and later decreased with increasing HFP inclusion in this study.
In this study, VL was significantly affected by HFP with the highest value observed when 1.5% HFP was used to replace FM. Improvement in the intestinal morphology, thus VL, is a positive indication of the fish’s ability to digest feed and absorb nutrients in the digestive canal (Dimitroglou et al., 2009; Tan et al., 2018). Villi growth and intestinal digestive enzymes are effectively stimulated by small peptides (Zhang et al., 2017; Jia et al., 2019). The intestinal surface available for nutrient absorption can be expanded by VL and VW, while the efficiency of nutrient absorption is determined by MT in the intestine (Geda et al., 2012; Lauriano et al., 2016).
Conclusion
The addition of HFP to a low FM diet of hybrid grouper will increase the activity of intestinal trypsin and chymotrypsin and the deposition of crude protein and ash in hybrid groupers. An improvement in antioxidant capacity and the development of the intestine in hybrid grouper fed with different levels of HFP was observed. A 5% replacement ratio using 1% HFP is suggested in the diet of hybrid grouper due to a higher WGR compared to the other groups containing HFP.
Data Availability Statement
The original contributions presented in the study are included in the article.
Ethics Statement
The animal study was reviewed and approved by Ethics Review Board of Guangdong Ocean University.
Author Contributions
SC aided in the experimental design, fund acquisition, supervision of the project, and review and editing of the manuscript. VH conducted the study, analyzed the data, and drafted the original manuscript. BT and TL aided in funding acquisition. HL, QY, XD, SZ, JW, and ZC aided in the experimental design. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by the National Key R&D Program of China (2019YFD0900200), the Special Project on Key Fields of Guangdong Universities (Rural Revitalization) (2020ZDZX1034), and the Science-Technology Plan of Zhanjiang (2020A02001).
Conflict of Interest
JW, TL, and ZC were employed by Guangdong XIPU Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmadifar, E., Dawood, M. A. O., Moghadam, M. S., Sheikhzadeh, N., Hoseinifar, S. H., and Musthafa, M. S. (2019). Modulation of immune parameters and antioxidant defense in zebrafish (Danio rerio) using dietary apple cider vinegar. Aquaculture 513:734412. doi: 10.1016/j.aquaculture.2019.734412
Amoah, K., Dong, X., Tan, B., Zhang, S., Chi, S., Yang, Q., et al. (2021). Effects of three probiotic strains (Bacillus coagulans, B. licheniformis and Paenibacillus polymyxa) on growth, immune response, gut morphology and microbiota, and resistance against Vibrio harveyi of northern whitings, sillago sihama forsskál (1775). Animal Feed Sci. Technol. 277:114958. doi: 10.1016/j.anifeedsci.2021.114958
Arrokhman, S., Wijayanti, N., and Soegianto, A. (2017). Survival and osmoregulation of juvenile of hybrid grouper (Epinephelus fuscoguttatus× Epinephelus lanceolatus) during acclimation in calcium-supplemented freshwater. Aquacu. Int. 25, 693–704. doi: 10.1007/s10499-016-0069-y
Bakke-McKellep, A. M., Nordrum, S., Krogdahl, Å, and Buddington, R. K. (2000). Absorption of glucose, amino acids, and dipeptides by the intestines of Atlantic salmon (Salmo salar L.). Fish Physiol. Biochem. 22, 33–44. doi: 10.1023/A:1007872929847
Baur, F. J., and Ensminger, L. G. (1977). The association of official analytical chemists (AOAC). J. Am. Oil Chem. Soc. 54, 171–172. doi: 10.1007/BF02670789
Bruce, T. J., and Brown, M. L. (2017). A review of immune system components, cytokines, and immunostimulants in cultured finfish species. Open J. Animal Sci. 7:267. doi: 10.4236/ojas.2017.73021
Bui, H. T. D., Khosravi, S., Fournier, V., Herault, M., and Lee, K.-J. (2014). Growth performance, feed utilization, innate immunity, digestibility and disease resistance of juvenile red seabream (Pagrus major) fed diets supplemented with protein hydrolysates. Aquaculture 418, 11–16. doi: 10.1016/j.aquaculture.2013.09.046
Bunlipatanon, P., and U-taynapun, K. (2017). Growth performance and disease resistance against V ibrio vulnificus infection of novel hybrid grouper (E pinephelus lanceolatus× E pinephelus fuscoguttatus). Aquaculture Research 48, 1711–1723. doi: 10.1111/are.13008
Carnevali, O., de Vivo, L., Sulpizio, R., Gioacchini, G., Olivotto, I., Silvi, S., et al. (2006). Growth improvement by probiotic in European sea bass juveniles (Dicentrarchus labrax, L.), with particular attention to IGF-1, myostatin and cortisol gene expression. Aquaculture 258, 430–438.
Ch’ng, C. L., and Senoo, S. (2008). Egg and larval development of a new hybrid grouper, tiger grouper Epinephelus fuscoguttatus× giant grouper E. lanceolatus. Aquacul. Sci. 56, 505–512.
Choi, J., Sabikhi, L., Hassan, A., and Anand, S. (2012). Bioactive peptides in dairy products. Int. J. Dairy Technol. 65, 1–12. doi: 10.1111/j.1471-0307.2011.00725.x
Dimitroglou, A., Merrifield, D. L., Moate, R., Davies, S. J., Spring, P., Sweetman, J., et al. (2009). Dietary mannan oligosaccharide supplementation modulates intestinal microbial ecology and improves gut morphology of rainbow trout, Oncorhynchus mykiss (walbaum). J. Animal Sci. 87, 3226–3234. doi: 10.2527/jas.2008-1428
Egerton, S., Wan, A., Murphy, K., Collins, F., Ahern, G., Sugrue, I., et al. (2020). Replacing fishmeal with plant protein in Atlantic salmon (Salmo salar) diets by supplementation with fish protein hydrolysate. Sci. Rep. 10, 1–16. doi: 10.1038/s41598-020-60325-7
Firdaus, R. F., Lim, L.-S., Kawamura, G., and Shapawi, R. (2016). Assessment on the acceptability of hybrid grouper, Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂ to soybean meal-based diets. Aquacul. Aquar. Conserv. Legis. -Int. J. Bioflux Soc. (AACL Bioflux) 9, 284–290.
Geda, F., Rekecki, A., Decostere, A., Bossier, P., Wuyts, B., Kalmar, I. D., et al. (2012). Changes in intestinal morphology and amino acid catabolism in common carp at mildly elevated temperature as affected by dietary mannanoligosaccharides. Animal Feed Sci. Technol. 178, 95–102. doi: 10.1016/j.anifeedsci.2012.09.008
Giri, S. S., Sen, S. S., Chi, C., Kim, H. J., Yun, S., Park, S. C., et al. (2015). Effect of cellular products of potential probiotic bacteria on the immune response of Labeo rohita and susceptibility to Aeromonas hydrophila infection. Fish Shellfish Immunol. 46, 716–722. doi: 10.1016/j.fsi.2015.08.012
Harikrishnan, R., Rani, M. N., and Balasundaram, C. (2003). Hematological and biochemical parameters in common carp, Cyprinus carpio, following herbal treatment for Aeromonas hydrophila infection. Aquaculture 221, 41–50. doi: 10.1016/S0044-8486(03)00023-1
Jia, G., Guo, R., Zhang, Y., Li, X., Xia, H., Gong, C., et al. (2019). Effects of partial replacement of fish meal by peptides hydrolyzed from poultry by-products on growth performance, digestive indices and specific immune indices of turbot. Chin. J. Animal Nutr. 31, 2201–2211.
Jiang, S., Wu, X., Li, W., Wu, M., Luo, Y., Lu, S., et al. (2015). Effects of dietary protein and lipid levels on growth, feed utilization, body and plasma biochemical compositions of hybrid grouper (Epinephelus lanceolatus ♀× Epinephelus fuscoguttatus ♂) juveniles. Aquaculture 446, 148–155. doi: 10.1016/j.aquaculture.2015.04.034
Karayannakidis, P. D., and Zotos, A. (2016). Fish processing by-products as a potential source of gelatin: a review. J. Aquat. Food Prod. Technol. 25, 65–92. doi: 10.1080/10498850.2013.827767
Khieokhajonkhet, A., and Surapon, K. (2020). Effects of fish protein hydrolysate on the growth performance, feed and protein utilization of Nile tilapia (Oreochromis niloticus). Int. J. Agricul. Technol. 16, 641–654.
Khosravi, S., Bui, H. T. D., Rahimnejad, S., Herault, M., Fournier, V., Jeong, J. B., et al. (2015). Effect of dietary hydrolysate supplementation on growth performance, non-specific immune response and disease resistance of olive flounder (Paralichthys olivaceus) challenged with edwardsiella tarda. Aquacul. Nutr. 21, 321–331. doi: 10.1111/anu.12157
Kim, D.-H., and Austin, B. (2006). Cytokine expression in leucocytes and gut cells of rainbow trout, Oncorhynchus mykiss walbaum, induced by probiotics. Vet. Immunol. Immunopathol. 114, 297–304. doi: 10.1016/j.vetimm.2006.08.015
Kotzamanis, Y. P., Gisbert, E., Gatesoupe, F. J., Zambonino Infante, J., and Cahu, C. (2007). Effects of different dietary levels of fish protein hydrolysates on growth, digestive enzymes, gut microbiota, and resistance to Vibrio anguillarum in European sea bass (Dicentrarchus labrax) larvae. Comparat. Biochem. Physiol. Mol. Integrat. Physiol. 147, 205–214. doi: 10.1016/j.cbpa.2006.12.037
Kuebutornye, F. K. A., Wang, Z., Lu, Y., Abarike, E. D., Sakyi, M. E., Li, Y., et al. (2020). Effects of three host-associated Bacillus species on mucosal immunity and gut health of Nile tilapia, Oreochromis niloticus and its resistance against Aeromonas hydrophila infection. Fish Shellfish Immunol. 97:46. doi: 10.1016/j.fsi.2019.12.046
Kumar, N. S. S., Nazeer, R. A., and Ganesh, R. J. (2012). Functional properties of protein hydrolysates from different body parts of horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber). Med. J. Nutr. Metab. 5, 105–110. doi: 10.1007/s12349-011-0078-3
Kumar, V., Muzaddadi, A. U., Mann, S., Balakrishnan, R., Bembem, K., and Kalnar, Y. (2018). Utilization of Fish Processing Waste: A Waste to Wealth Approach. Ludhiana: ICAR-CIPHET.
Lauriano, E. R., Pergolizzi, S., Capillo, G., Kuciel, M., Alesci, A., and Faggio, C. (2016). Immunohistochemical characterization of toll-like receptor 2 in gut epithelial cells and macrophages of goldfish Carassius auratus fed with a high-cholesterol diet. Fish Shellfish Immunol. 59, 250–255. doi: 10.1016/j.fsi.2016.11.003
Liu, X., Shi, H., He, Q., Lin, F., Wang, Q., Xiao, S., et al. (2020). Effect of starvation and refeeding on growth, gut microbiota and non-specific immunity in hybrid grouper (Epinephelus fuscoguttatus ♀×E. lanceolatus ♂). Fish Shellfish Immunol. 97, 182–193. doi: 10.1016/j.fsi.2019.11.055
Liu, X., Wang, M., Sun, G., Wang, B., Jiang, K., Shao, J., et al. (2018). Mechanistic target of rapamycin inhibition with rapamycin induces autophagy and correlative regulation in white shrimp (Litopenaeus vannamei). Aquac. Nutr. 24, 1509–1520.
Lopes, P. A., Pinheiro, T., Santos, M. C., da Luz Mathias, M., Collares-Pereira, M. J., and Viegas-Crespo, A. M. (2001). Response of antioxidant enzymes in freshwater fish populations (Leuciscus alburnoides complex) to inorganic pollutants exposure. Sci. Total Environ. 280, 153–163. doi: 10.1016/S0048-9697(01)00822-1
Madeira, M. D., and Paula-Barbosa, M. M. (1999). Effects of alcohol on the synthesis and expression of hypothalamic peptides. Brain Res. Bull. 48, 3–22. doi: 10.1016/S0361-9230(98)00131-2
Meeker, D. L. (2009). North American Rendering: processing high quality protein and fats for feed. Rev. Brasileira De Zoot. 38, 432–440. doi: 10.1590/S1516-35982009001300043
Merrifield, D. L., Burnard, D., Bradley, G., Davies, S. J., and Baker, R. T. M. (2009). Microbial community diversity associated with the intestinal mucosa of farmed rainbow trout (Oncoryhnchus mykiss walbaum). Aquacul. Res. 40, 1064–1072. doi: 10.1111/j.1365-2109.2009.02200.x
Moure, A., Domínguez, H., and Parajó, J. C. (2006). Antioxidant properties of ultrafiltration-recovered soy protein fractions from industrial effluents and their hydrolysates. Proc. Biochem. 41, 447–456. doi: 10.1016/j.procbio.2005.07.014
Nguyen, H. T. M., Pérez-Gálvez, R., and Bergé, J. P. (2012). Effect of diets containing tuna head hydrolysates on the survival and growth of shrimp Penaeus vannamei. Aquaculture 324–325, 127–134. doi: 10.1016/j.aquaculture.2011.11.014
Oliva-Teles, A., Cerqueira, A. L., and Gonçalves, P. (1999). The utilization of diets containing high levels of fish protein hydrolysate by turbot (Scophthalmus maximus) juveniles. Aquaculture 195:201. doi: 10.1016/S0044-8486(99)00162-3
Olsen, R. L., and Hasan, M. R. (2012). A limited supply of fishmeal: impact on future increases in global aquaculture production. Trends Food Sci. Technol. 27, 120–128. doi: 10.1016/j.tifs.2012.06.003
Rahimnejad, S., Bang, I. C., Park, J.-Y., Sade, A., Choi, J., and Lee, S.-M. (2015). Effects of dietary protein and lipid levels on growth performance, feed utilization and body composition of juvenile hybrid grouper, Epinephelus fuscoguttatus× E. lanceolatus. Aquaculture 446, 283–289. doi: 10.1016/j.aquaculture.2015.05.019
Ren, Z., Wang, S., Cai, Y., Wu, Y., Tian, L., Wang, S., et al. (2020). Effects of dietary mannan oligosaccharide supplementation on growth performance, antioxidant capacity, non-specific immunity and immune-related gene expression of juvenile hybrid grouper (Epinephelus lanceolatus♀× Epinephelus fuscoguttatus♂). Aquaculture 523:735195. doi: 10.1016/j.aquaculture.2020.735195
Rimoldi, S., Gini, E., Koch, J. F. A., Iannini, F., Brambilla, F., and Terova, G. (2020). Effects of hydrolyzed fish protein and autolyzed yeast as substitutes of fishmeal in the gilthead sea bream (Sparus aurata) diet, on fish intestinal microbiome. BMC Vet. Res. 16:1–13. doi: 10.1186/s12917-020-02335-1
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. doi: 10.1128/AEM.01541-09
Siddik, M. A. B., Howieson, J., and Fotedar, R. (2019b). Beneficial effects of tuna hydrolysate in poultry by-product meal diets on growth, immune response, intestinal health and disease resistance to Vibrio harveyi in juvenile barramundi, Lates calcarifer. Fish Shellfish Immunol. 89, 61–70. doi: 10.1016/j.fsi.2019.03.042
Siddik, M. A. B., Chaklader, M. R., Foysal, M. J., Howieson, J., Fotedar, R., and Gupta, S. K. (2019a). Influence of fish protein hydrolysate produced from industrial residues on antioxidant activity, cytokine expression and gut microbial communities in juvenile barramundi Lates calcarifer. Fish Shellfish Immunol. 97, 465–473. doi: 10.1016/j.fsi.2019.12.057
Tacon, A. G. J., and Metian, M. (2008). Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: trends and future prospects. Aquaculture 285, 146–158. doi: 10.1016/j.aquaculture.2008.08.015
Tan, X., Sun, Z., Zhou, C., Huang, Z., Tan, L., Xun, P., et al. (2018). Effects of dietary dandelion extract on intestinal morphology, antioxidant status, immune function and physical barrier function of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol. 73, 197–206. doi: 10.1016/j.fsi.2017.12.020
Tang, H. G., Wu, T. X., Zhao, Z. Y., and Pan, X. D. (2008). Effects of fish protein hydrolysate on growth performance and humoral immune response in large yellow croaker (Pseudosciaena crocea R.). J. Zhejiang Univ. Sci. B 9, 684–690. doi: 10.1631/jzus.B0820088
Tran, T. N., Xiong, F., Hao, Y.-T., Zhang, J., Wu, S.-G., and Wang, G.-T. (2018). Starvation influences the microbiota assembly and expression of immunity-related genes in the intestine of grass carp (Ctenopharyngodon idellus). Aquaculture 489, 121–129. doi: 10.1016/j.aquaculture.2018.02.016
Wang, Q., He, G., Mai, K., Xu, W., and Zhou, H. (2016). Fishmeal replacement by mixed plant proteins and maggot meal on growth performance, target of rapamycin signalling and metabolism in juvenile turbot (Scophthalmus maximus L.). Aquac. Nutr. 22, 752–758.
Wardwell, L. H., Huttenhower, C., and Garrett, W. S. (2011). Current concepts of the intestinal microbiota and the pathogenesis of infection. Curr. Infect. Dis. Rep. 13, 28–34. doi: 10.1007/s11908-010-0147-7
Wei, Y., Liang, M., Mu, Y., Zheng, K., and Xu, H. (2016). The effect of ultrafiltered fish protein hydrolysate level on growth performance, protein digestibility and m RNA expression of P ep T 1 in juvenile turbot (S cophthalmus maximus L.). Aquacul. Nutr. 22, 1006–1017. doi: 10.1111/anu.12319
Wei, Y., Liang, M., and Xu, H. (2020). Fish protein hydrolysate affected amino acid absorption and related gene expressions of IGF-1/AKT pathways in turbot (Scophthalmus maximus). Aquacul. Nutr. 26, 145–155. doi: 10.1111/anu.12976
Wu, D., Zhou, L., Gao, M., Wang, M., Wang, B., He, J., et al. (2018). Effects of stickwater hydrolysates on growth performance for yellow catfish (Pelteobagrus fulvidraco). Aquaculture 488, 161–173. doi: 10.1016/j.aquaculture.2018.01.031
Wu, M., Lu, S., Wu, X., Jiang, S., Luo, Y., Yao, W., et al. (2017). Effects of dietary amino acid patterns on growth, feed utilization and hepatic IGF-I, TOR gene expression levels of hybrid grouper (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂) juveniles. Aquaculture 468, 508–514. doi: 10.1016/j.aquaculture.2016.11.019
Yan, B., Zhu, C.-D., Guo, J.-T., Zhao, L.-H., and Zhao, J.-L. (2013). miR-206 regulates the growth of the teleost tilapia (Oreochromis niloticus) through the modulation of IGF-1 gene expression. J. Exp. Biol. 216, 1265–1269.
Yang, X., Wang, G., Zhao, X., Dong, X., Chi, S., and Tan, B. (2021). Addition of hydrolysed porcine mucosa to low-fishmeal feed improves intestinal morphology and the expressions of intestinal amino acids and small peptide transporters in hybrid groupers (Epinephelus fuscoguttatus♀× E. lanceolatus♂). Aquaculture 535:736389. doi: 10.1016/j.aquaculture.2021.736389
Ye, H., Zhou, Y., Su, N., Wang, A., Tan, X., Sun, Z., et al. (2019). Effects of replacing fish meal with rendered animal protein blend on growth performance, hepatic steatosis and immune status in hybrid grouper (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂). Aquaculture 511:734203. doi: 10.1016/j.aquaculture.2019.734203
Yin, B., Liu, H., Tan, B., Dong, X., Chi, S., Yang, Q., et al. (2018). Cottonseed protein concentrate (CPC) suppresses immune function in different intestinal segments of hybrid grouper♀ Epinephelus fuscoguttatus×♂ Epinephelus lanceolatu via TLR-2/MyD88 signaling pathways. Fish Shellfish Immunol. 81, 318–328. doi: 10.1016/j.fsi.2018.07.038
Zambonino Infante, J. L., Cahu, C. L., and Peres, A. (1997). Partial substitution of di-and tripeptides for native proteins in sea bass diet improves Dicentrarchus labrax larval development. J. Nutr. 127, 608–614. doi: 10.1093/jn/127.4.608
Zhang, D., Zhang, Y., Liu, B., Jiang, Y., Zhou, Q., Wang, J., et al. (2017). Effect of replacing fish meal with fermented mushroom bran hydrolysate on the growth, digestive enzyme activity, and antioxidant capacity of allogynogenetic crucian carp (Carassius auratus gibelio). Turkish J. Fish. Aquatic Sci. 17, 1039–1048.
Zheng, K., Liang, M., Yao, H., Wang, J., and Chang, Q. (2012). Effect of dietary fish protein hydrolysate on growth, feed utilization and IGF-I levels of Japanese flounder (Paralichthys olivaceus). Aquacul. Nutr. 18, 297–303. doi: 10.1111/j.1365-2095.2011.00896.x
Zou, C., Xu, M., Chen, L., Liu, Q., Zhou, Y., Sun, Z., et al. (2019). Xiaochaihu decoction reduces hepatic steatosis and improves D-GalN/LPS-induced liver injury in hybrid grouper (Epinephelus lanceolatus ♀× Epinephelus fuscoguttatus ♂). Fish Shellfish Immunol. 91, 293–305. doi: 10.1016/j.fsi.2019.05.025
Keywords: hydrolyzed fish protein, pearl gentian hybrid grouper, small peptide, intestinal development, fishmeal
Citation: Hlordzi V, Wang J, Li T, Cui Z, Tan B, Liu H, Yang Q, Dong X, Zhang S and Chi S (2022) Effects of Lower Fishmeal With Hydrolyzed Fish Protein Powder on the Growth Performance and Intestinal Development of Juvenile Pearl Gentian Grouper (Epinephelus fuscoguttatus ♀ and Epinephelus lanceolatus ♂). Front. Mar. Sci. 9:830398. doi: 10.3389/fmars.2022.830398
Received: 07 December 2021; Accepted: 27 January 2022;
Published: 14 March 2022.
Edited by:
Jin Niu, Sun Yat-sen University, ChinaCopyright © 2022 Hlordzi, Wang, Li, Cui, Tan, Liu, Yang, Dong, Zhang and Chi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuyan Chi, Y2hpc2h1eWFuNzdAMTYzLmNvbQ==
†These authors share first authorship
 Vivian Hlordzi
Vivian Hlordzi Junqing Wang3†
Junqing Wang3† Beiping Tan
Beiping Tan Qihui Yang
Qihui Yang Xiaohui Dong
Xiaohui Dong Shuang Zhang
Shuang Zhang Shuyan Chi
Shuyan Chi