- 1Key Laboratory of Exploration and Utilization of Aquatic Genetic Resources Conferred by Ministry of Education, Shanghai Ocean University, Shanghai, China
- 2International Research Center for Marine Biosciences Conferred by Ministry of Science and Technology, Shanghai Ocean University, Shanghai, China
Saccharina japonica, an importantly industrial species in brown seaweeds, has a typical alternate life history of heteromorphic generations and UV sex determination system. But up to now, the sex chromosomes, U and V, in this kelp remain to be evidenced cytologically. In the present study, the female-linked (SJ-f_000170 and MSj68-58-2) and male-linked (SJ-13_001840 and MSj68-16-2) molecular markers developed on the basis of Ectocarpus sex-determining regions (SDRs) were selected after PCR amplification and Southern blotting analysis. Mono-color fluorescence in situ hybridization (FISH) to the kelp metaphase chromosomes with each of these confirmed sex-linked markers as a probe indicated that the hybridization signals were confined to either female or male gametophytes exclusively. The shape and size of hybridized chromosomes and the relative location of these sex-linked markers on them suggested that the marked chromosomes in a sex were the same, which was further confirmed by dual-color FISH observations. SJ-f_000170 and SJ-13_001840, corresponding to each sex, were therefore used to map the sporophyte metaphase chromosomes by dual-color FISH technique, and they were hybridized on different chromosomes as expected. The sporophyte metaphase chromosomes as hybridized by SJ-f_000170 and SJ-13_001840 resembled those in either female or male gametophytes, suggesting that they were the putative U and V, respectively, chromosomes in S. japonica. To further demonstrate the reliability of this inference, genomic information of the screened bacterial artificial chromosome (BAC) clones carrying two female-linked markers, for example, from the constructed BAC libraries of the kelp female gametophytes was provided. The insert sequencing of two selected BAC clones revealed that repetitive elements were rich whereas gene density was poor, which was characterized as non-recombining SDR sequences in brown seaweed Ectocarpus sp. and liverwort Marchantia polymorpha. The present study laid a solid foundation for unveiling the mystery of S. japonica sex chromosomes.
Introduction
Saccharina japonica (Areschoug) C. E. Lane, C. Mayes, Druehl et G. W. Saunders (syn. Laminaria japonica Aresch.) possesses a heteromorphic haploid-diploid life cycle involving macroscopic phylloidal sporophytes (2N) and microscopic filamentous gametophytes (N) (Wu and Suo, 1962). When the sporophytes developed to maturity, zoospores were released from the unilocular sporangia of matured sporophytes and adhered to a substratum. Under appropriate environmental conditions for germination, the attached embryospores developed into female and male gametophytes at a sex ratio of 1 to 1 (Schreiber, 1930). Large eggs and small spermatozoids were then produced from the female and male gametangia, respectively, implying the sexual dimorphism in S. japonica (commonly referred to as kelp). This kelp is hence an oogamous species with dioicous gametophytes as proposed by Luthringer et al. (2014); Coelho et al. (2019), and Coelho and Umen (2021). In addition, several experiments on parthenogenesis (Tai and Fang, 1977; Fang et al., 1978; Motomura, 1991; Dai et al., 1993; Lewis et al., 1993) pointed out that parthenogenetic sporophytes which derived from female gametophytes via apomixis or apogamy could grow to maturity. All the produced zoospores from the parthenogenetic sporophytes were able to germinate and develop into female gametocytes exclusively. From these findings, Fang et al. (1978) inferred that an XY-like sex-determination system existed in S. japonica, and that the diploid sporophyte was XY type whereas the haploid female and male gametophytes were X- and Y-type, respectively. Now, this type of chromosomal sex determination in the haploid phase of life cycle has been termed UV sex-determination system by Bachtrog et al. (2011) to discriminate from diploid XY or ZW systems of animals and higher plants.
In cytological observations of the gametophytes of S. latissima and other species of Laminaria by an acetocarmine squash method, Evans (1963) found that the female gametophytes had a very large chromosome which was absent in male gametophytes. Since the large chromosome paired with a small one at meiosis of sporophytes in S. latissima, it was proposed to be a U (formerly X) chromosome by Evans (1965). This finding was supported by a cytological observation on Laminaria yendoana chromosomes (Yasui, 1992). Nonetheless, such a conspicuously large sex chromosome was not observed in S. japonica, while both research groups of Tai and Fang (1977) and Yabu and Yasui (1991) cytologically examined the kelp chromosomes. Such an incongruous view of whether there were sex chromosomes in S. japonica could be resolved by cytogenetic evidence. The physical features, however, of the kelp chromosomes in number, size, and morphology as reviewed by Lewis (1996) limited further cytogenetic studies. Fortunately, this situation now has improved by applying the DNA-binding fluorochrome 4′, 6-diamidino-2-phenylindole (DAPI) and the technique fluorescence in situ hybridization (FISH) to the observations on the kelp chromosomes (Liu et al., 2012). Using this established protocol, telomere and rDNAs were successfully localized on the kelp chromosomes (Liu et al., 2017; Yang et al., 2017; Liu Y. et al., 2021). Meanwhile, some female or male gametophyte-specific rather than genetically linked markers were developed and localized on S. japonica chromosomes using FISH technique, too (Liu et al., 2009, 2012; Gu et al., 2014). Accordingly, the available sex-linked molecular markers developed from S. japonica would facilitate the application of FISH to discerning U and V chromosomes in this kelp.
By use of several strategies for identification of the sex-determining regions (SDRs) in Ectocarpus siliculosus, Ahmed et al. (2014) estimated that the male and female SDRs of E. siliculosus were 920 and 929 kbp in size, respectively. While performing a homology search on S. japonica genome database (Ye et al., 2015) using the SDR-linked genes of E. siliculosus, Lipinska et al. (2017) found that four genes were linked to S. japonica U chromosome and four to V one. Zhang et al. (2018) developed four new male gametophyte-specific markers and one female gametophyte-specific marker in S. japonica based on the SDR genes of E. siliculosus reported by Ahmed et al. (2014). In this case, these female-linked genes and molecular markers are expected to be co-located on the same U chromosome, whereas the male-linked ones are to be on the same V chromosome of S. japonica.
In order to visualize these abovementioned sex-linked genes or markers to discern the putative U and V chromosomes of S. japonica using FISH technique, at first, we verified these sex-linked genes or markers from the genomic DNA of this kelp by polymerase chain reaction (PCR) amplification and Southern blotting analysis. Based upon mono-color FISH profiles with each of these confirmed sex-linked markers as a probe, two of them associated to each sex were then used as probes to detect if they were co-localized on the same chromosome of male or female gametophytes in S. japonica using dual-color FISH technique. In the same manner, at last, two (one each sex) of them were labeled as probes to illustrate their co-localization on the sporophyte metaphase chromosomes of this kelp. In addition, bacterial artificial chromosome (BAC) clones carrying the U chromosome-linked markers were screened, sequenced, and characterized to exhibit their genomic features. The present study suggested that the putative U and V chromosomes in S. japonica could be distinguished using FISH with sex-linked molecular markers as probes.
Materials and Methods
Algal Material and Culture
Saccharina japonica was selected as the algal material in the present study. The female and male gametophyte clones germinated from zoospores were isolated under a microscope as reported by Zhou and Wu (1998). They were kept under the vegetative growth conditions of 50 μmol photons⋅m–2⋅s–1 and 17 ± 1°C with a photoperiod 12 h/12 h (light/dark). PES medium (Starr and Zeikus, 1993) was used for this culture and replaced once every 2 weeks.
To compare the transcript levels of annotated genes from the sequenced BAC clones between male and female gametophytes, quantitative real-time PCR (qRT-PCR) was used. The gametophytes were cut into fragments and cultured in PES medium as previously described by Bi et al. (2021), but under the illumination of 80 μmol photons⋅m–2⋅s–1 with a 10 h/14 h (light/dark) regimen at 10 ± 1°C. Eight days later, the male and female gametophytes were centrifuged as previously described (Zhou and Wu, 1998) for RNA isolation.
Juvenile sporophytes (approximately 2 cm in length), developed from the isolated male and female gametophytes of this kelp as described by Zhou and Wu (1998), were presented kindly by Oriental Ocean Sci-tec Co., Ltd., Yantai, Shandong province, China.
Selection of Sex-Linked Molecular Markers
Comparing Ectocarpus siliculosus, E. fasciculatus, and Scytosiphon lomentaria in Ectocarpales, with other three brown seaweeds S. japonica, Macrocystis pyrifera, and Undaria pinnatifida in Laminariales, Lipinska et al. (2017) found that 20 of the 34 Ectocarpus sp. SDR genes had orthologs whereas only 9 of the 20 orthologous genes were sex-linked. Among the nine sex-linked genes in E. siliculosus, one gametologue pair, Ec-13_001840 and Ec-sdr_f_000170, was highly conserved in all the examined seaweeds, implying that these two genes were derived from the same ancestor and located within the non-recombining region of opposite sex chromosomes as defined by Coelho et al. (2018). These two gametologues were referred to as SJ-13_001840 and SJ-f_000170, respectively, in S. japonica by Lipinska et al. (2017) and therefore chosen for the present study.
Meanwhile, MSj68-16-2 was also chosen for the present study since it was proposed to be a male-linked marker which was developed by Zhang et al. (2018) on the basis of S. japonica transcriptomic and genomic sequences. It shared 100% similarity with partial coding sequence (170 bp in length) of the kelp HMG-box-containing gene (SjHMG) (Zhang et al., 2019), suggesting that this marker was a part of DNA sequence of this gene. Moreover, Zhang et al. (2019, 2021) provided experimental evidence that the HMG domain-containing protein, a transcription factor (Ec-13_001750) in Ectocarpus as annotated by Ahmed et al. (2014), was expressed exclusively in the kelp male gametophytes and was thus proposed to be a candidate male-determining gene. In addition, MSj68-58-2, a developed female-specific marker in S. japonica by Zhang et al. (2018), was selected in the present study as a candidate for sex-linked marker. The main reason for this was that MSj68-58-2 was developed based on the sequence of U. pinnatifida and M. pyrifera female-specific marker, M_68_58_2, which was originally derived from the Ectocarpus SDR gene as described by Lipinska et al. (2015).
DNA Isolation
About 0.1 g of S. japonica gametophytes were collected by centrifugation. After rinsing thrice with sterilized seawater, the harvested gametophytes were ground into powder in liquid nitrogen. Genomic DNA was extracted from them by use of a modified cetyltrimethyl ammonium bromide (CTAB) method as described previously (Hu and Zhou, 2001). The extracted genomic DNA was dissolved in 100 μL 1 × Tris-EDTA (TE) buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) and stored at −20°C.
Extraction of genomic DNA from S. japonica sporophytes was pre-treated with a mixed enzymatic solution as described by Liu et al. (2012), and the subsequent DNA was extracted in the same way as that from the gametophytes.
Quality and quantity of the extracted nucleic acids were determined by the ratios of measured optical density (OD) at wavelengths of 260 to 280 nm (i.e., OD260/OD280) and of OD260 to OD230 using a NanoDrop 2000C spectrophotometer (Thermo Fisher Scientific, Wilmington, United States). Integrity of the extracted nucleic acids was evaluated by 1% agarose gel electrophoresis.
Polymerase Chain Reaction Amplification of Sex-Linked Molecular Markers
Two pairs of primers, f170-F and f170-R, and m1840-F and m1840-R (Supplementary Table 1), were designed according to the SDR gene sequences of SJ-f_000170 and SJ-13_001840, which were developed from the female and male gametophytes, respectively, of S. japonica by Lipinska et al. (2017). The other two pairs, f58-F1 and f58-R1, and m16-F and m16-R (Supplementary Table 1), were used for amplification of the SDR markers MSj68-58-2 (GenBank accession No: MF850255) and MSj68-16-2 (GenBank accession No: MF135546) in the kelp female and male gametophytes, respectively, as developed by Zhang et al. (2018). The amplification was conducted in a total volume of 25 μL reaction system containing 9.5 μL distilled deionized (dd) H2O, 12.5 μL 2 × Taq PCR MasterMix (100 μM each), 2 μL genomic DNA (200 ng⋅μL–1), and 1 μL forward and reverse primers (0.2 μM each). PCR was performed in a gradient Mastercycler (Eppendorf, Hamburg, Germany) and programmed as described by Zhang et al. (2018), but the annealing temperature was listed in Supplementary Table 1.
The amplified products were purified and ligated into a pMD19-T Simple vector (TaKaRa, Kyoto, Japan) and transformed into the competent cells of Escherichia coli DH5α cells (Tiangen Biotech, Beijing, China). The recombinants were screened via blue-white selection on ampicillin-containing Luria-Bertani (LB) plates. White or positive colonies were verified by PCR amplification with the general primers RV-M and M13-20 (TaKaRa). The positive clones were then selected and sent to Genewiz Biological Technology Co., Ltd. (Suzhou, China) for sequencing using an automated DNA sequencer ABI Prism 3730 (Foster City, United States).
Southern Blotting Analysis
Aliquots of the isolated genomic DNA (approximately 20 μg per sample) from the S. japonica gametophytes were digested to completion at 37°C for 6 h, independently, with endonucleases either SpeI that could not digest the male-linked markers or XbaI that could not digest the female-linked markers. The digested DNA samples were fractionated on a 1.0% agarose gel, blotted onto a positively charged nitrocellulose filter membrane (Pall, Exton, United States), and subsequently hybridized with the target markers as probes. The probes which were inserted into the vector pMD19-T were labeled with biotin-dUTP using a North2South Biotin Random Prime Labeling Kit (Thermo Fisher Scientific, Rockford, United States). Hybridized bands were detected using a North2South Chemiluminescent Hybridization and Detection Kit (Thermo Fisher Scientific) following the manufacturer’s guidelines, and signals were visualized by automatic exposure with Amersham™ Imager 600 (GE Healthcare, Uppsala, Sweden).
Chromosome Preparation
Chromosome preparation of the S. japonica gametophytes and sporophytes was conducted referring to Liu et al. (2012). In brief, appropriate amount of S. japonica gametophytes or sporophytes was added into sterilized sea water containing 0.02% colchicine to arrest metaphase nuclei at 4°C for 8 h and then washed with sterilized sea water twice. The samples were fixed in a freshly prepared Carnoy’s fixative fluid (glacial acetic acid/ethanol, 3/1, v/v) at 4°C for more than 24 h (Schweizer, 1976). The fixed samples were washed with deionized water for 2 or 3 times, taken into pieces with a dissecting needle, and added into the mixed enzyme solution (cellulase/pectinase/macerozyme R-10/freshly extracted abalone alginate lyase, 2/1/1/1.5, v/v/v/v) to digest enzymatically at 37°C for 14 − 20 h. The chromosome preparation was obtained by spreading the digested samples on poly-L-lysine coated slides. The slides were screened under an upright phase contrast microscope, and the well-spread slides were selected and stored at − 20°C for FISH experiments.
Fluorescence in situ Hybridization Analysis
Prior to FISH experiments, the constructed plasmids carrying the four sex-linked molecular markers were extracted from the transgenic Escherichia coli following the protocol of Plasmid Extraction Kit (Tiangen). The extracted plasmids, at a concentration of not less than 200 ng⋅μL–1, were used as templates to prepare for FISH probes by Nick translation (Rigby et al., 1977) using an ADVANCE™ Nick Translation Kit (Sigma-Aldrich, St. Louis, United States) under the guidance of the manufacturer’s instructions. The probes of the sex-linked genes SJ-f_000170 and SJ-13_001840 were labeled with Texas Red-5-dCTP (Invitrogen, Carlsbad, United States), whereas the sex-linked markers MSj68-58-2 and MSj68-16-2 were done with Alex Flour Green-5-dUTP (PerkinElmer, Boston, United States) for the hybridization to gametophyte metaphase nuclei. If for the hybridization to sporophyte metaphase nuclei to discern U and V chromosomes simultaneously with two different colors, the probe of the sex-linked gene SJ-f_000170 was labeled with Alex Flour Green-5-dUTP (PerkinElmer) instead with Texas Red-5-dCTP (Invitrogen) used in observations on gametophyte nuclei.
The FISH experiment was conducted according to the previously described method (Liu et al., 2012). Briefly, the slides containing chromosomes were placed in an ultraviolet cross-linker BLX-E254 (Vilbert Lourmat, France), and they were cross-linked twice by ultraviolet at 254 nm for 15 s each time. The slide was then placed on ice, and hybridization solution containing 2 × saline sodium citrate (SSC; 0.3 M NaCl, 0.03 M sodium citrate, pH 7.0), 1 × TE buffer, and 10 ng of the labeled probes, was dropped onto it. After that, it was covered with a slip of plastic sheet, and placed in a water bath at 100°C for 5 min. The slides were then transferred to a DNA hybridization chamber for incubation overnight at 55°C. Post-hybridization washes of the slides were carried out with 2 × SSC hybridization buffer in a shaker for 2 min to remove non-specifically unbound hybridized probes. Before microscopic observation, the oven-dried slides were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI; 2 μg⋅mL–1) containing anti-quencher (Vector Laboratories, Burlingame, United States) and left still in the dark for 10 min.
To detect the hybridization sites on the S. japonica sporophyte chromosomes, the well-spread mitotic chromosomes were pre-treated with an appropriate amount of freshly extracted abalone alginate lyase at 37°C for 1 h. Subsequently, they were subjected to FISH detection as described above.
Fluorescence Microscopy and Image Acquisition
Images were taken with a Leica DM4000B epifluorescent microscope (Wetzlar, Germany) equipped with different filter set and a digital camera DFC550 (Heerbrugg, Switzerland). Three kinds of fluorescent signals, Alex Flour (green), DAPI (blue), and Texas Red (red), were detected at the emission wavelength of 488, 495, and 612 nm, respectively. All images were processed using ImageJ1 software for color contrast and brightness uniformity. The karyotypic patterns or ideograms of the gametophytes and sporophytes were edited using Adobe Photoshop 7.02 based on the chromosome size.
Polymerase Chain Reaction-Based Screening for Bacterial Artificial Chromosome Libraries
To provide genomic information of the U-linked markers, the strategy of “three-dimensional” screen from the constructed female gametophyte BAC libraries (Liu P. F. et al., 2021) was performed following the established protocol by Yasukochi (2002).
Every ten of 384-well microtiter plates were mixed in equal amounts (2 μL) to generate a primary DNA pool or superpool (Supplementary Figure 1). After culture with addition of 50 μL LB medium containing chloramphenicol (12.5 g⋅L–1) at 37°C for 8 h, the bacterial cells in the superpool were used to isolate the plasmid DNA using a QIAwell 96 Ultra Plasmid Kit (Qiagen, Düsseldorf, Germany) according to the supplier’s manual. Using the isolated DNA as a template, PCR was performed to screen the library with the two pairs of primers f170-F and f170-R, and f58-F2 and f58-R2 (Supplementary Table 1). The amplification reaction system and program were the same as described above, with exception of annealing temperature at 62°C (Supplementary Table 1).
If the target band was electrophoretically detected from a given primary pool, the secondary pools were constructed according to each row, column, or plate constituting this selected superpool for the “three-dimensional” screen (Supplementary Figure 1). After culture and DNA isolation, PCR was performed as mentioned above to screen positive BAC clones.
The screened BAC positive clones harboring the target markers were then sent to Bacgene Biotechnology Co., Ltd. (Wuhan, China) for sequencing analysis using PacBio Sequel System (Pacific Biosciences, Menlo Park, United States).
Sequence Analysis of Inserts in Bacterial Artificial Chromosome Clones
In order to analyze the insert sequence characteristics of BAC clones, VecScreen3 were used to remove the vector sequences. Repetitive sequences in the inserts of screened BAC clones were analyzed using RepeatMasker program.4 Physical maps of the insert sequences of BAC clones were drawn using IBS1.0 (Liu et al., 2015) and MapChart2.32 (Voorrips, 2002). Both AUGUSTUS5 (Stanke and Waack, 2003) and software TopHat (v.2.0.8)6 and Cufflinks (v.2.1.1)7 were used to de novo annotate gene information. Blastn8 was used to estimate the sequence similarity.
Quantitative Real-Time PCR
Total RNA was isolated from the freshly collected gametophytes of S. japonica using TRIzol reagent (Invitrogen). First-strand cDNA was synthesized using the Reverse Transcribed Kit II (TaKaRa). Quantitative RT-PCR amplification was conducted using a SYBR® RT-PCR Kit (TaKaRa) in a Bio-Rad iQ™ 5 multicolor real-time PCR detection system (Bio-Rad) following the previous protocols (Ye et al., 2014). The 18S rRNA gene (GenBank Accession No. EU293553) of S. japonica was used as a house-keeping one, and all primers used for qRT-PCR analysis were listed in Supplementary Table 1. The relative abundance of gene transcripts from triplicate reactions performed for each sample was expressed as the mean ± standard deviation (SD) using the 2–ΔΔCt method (Livak and Schmittgen, 2001). The significance was estimated by the two-tailed Student’s t-test using SPSS 22.0 software.9
Results
Polymerase Chain Reaction Amplification of the Selected Sex-Linked Markers and Their Southern Blotting Analyses
Based on the sequences of four selected sex-linked markers SJ-f_000170, MSj68-58-2, SJ-13_001840, and MSj68-16-2, four pairs of primers (f170-F and f170-R, f58-F1 and f58-R1, m1840-F and m1840-R, and m16-F and m16-R; Supplementary Table 1) were designed. With these primers, their corresponding amplified products were obtained by PCR amplification (Supplementary Figure 2). Sequencing analyses of these products showed that the sequences and sizes (Supplementary Table 1) were the same as expected. It was noteworthy that the markers SJ-f_000170 and MSj68-58-2 were amplified only from the female gametophytes of S. japonica (Lanes 2 and 4 in Supplementary Figure 2), whereas SJ-13_001840 and MSj68-16-2 were done only from the male gametophytes (Lanes 7 and 9 in Supplementary Figure 2). These results pointed out that they were male- and female-specific markers in S. japonica as documented by Lipinska et al. (2015) and Zhang et al. (2018). When the genomic DNA extracted from sporophyte was taken as a template, the electrophoresis profiles of amplicons demonstrated that all these markers could be detected (Lanes 12-15 in Supplementary Figure 2), suggesting that they all could be inheritable in the sporophyte and its corresponding gametophytes of S. japonica.
To further validate the natural existence in the kelp genome, the four sex-linked markers were labeled as probes independently into digested genomic DNA (upper panels in Figure 1) extracted from the either female or male gametophytes of S. japonica. Southern blotting profiles (lower panels in Figure 1) showed that only one signal was present in the female gametophytes after hybridization with the labeled SJ-f_000170 or MSj68-58-2 probes (Lanes 3 or 5 in Figure 1, respectively). With these two labeled probes for Southern blotting, on the contrary, there was no any signal in the male gametophytes (Lanes 2 and 4 in Figure 1), further suggesting that SJ-f_000170 and MSj68-58-2 were female-specific in S. japonica. Similarly, SJ-13_001840 and MSj68-16-2 were unique to the kelp male gametophytes (Lanes 8 and 9 in Figure 1, respectively) as shown by Southern blotting profiles. It was of interest to find that each of the four markers had only one hybridization signal, suggesting that they were located on the chromosomes only at one locus in their corresponding gametophytes.
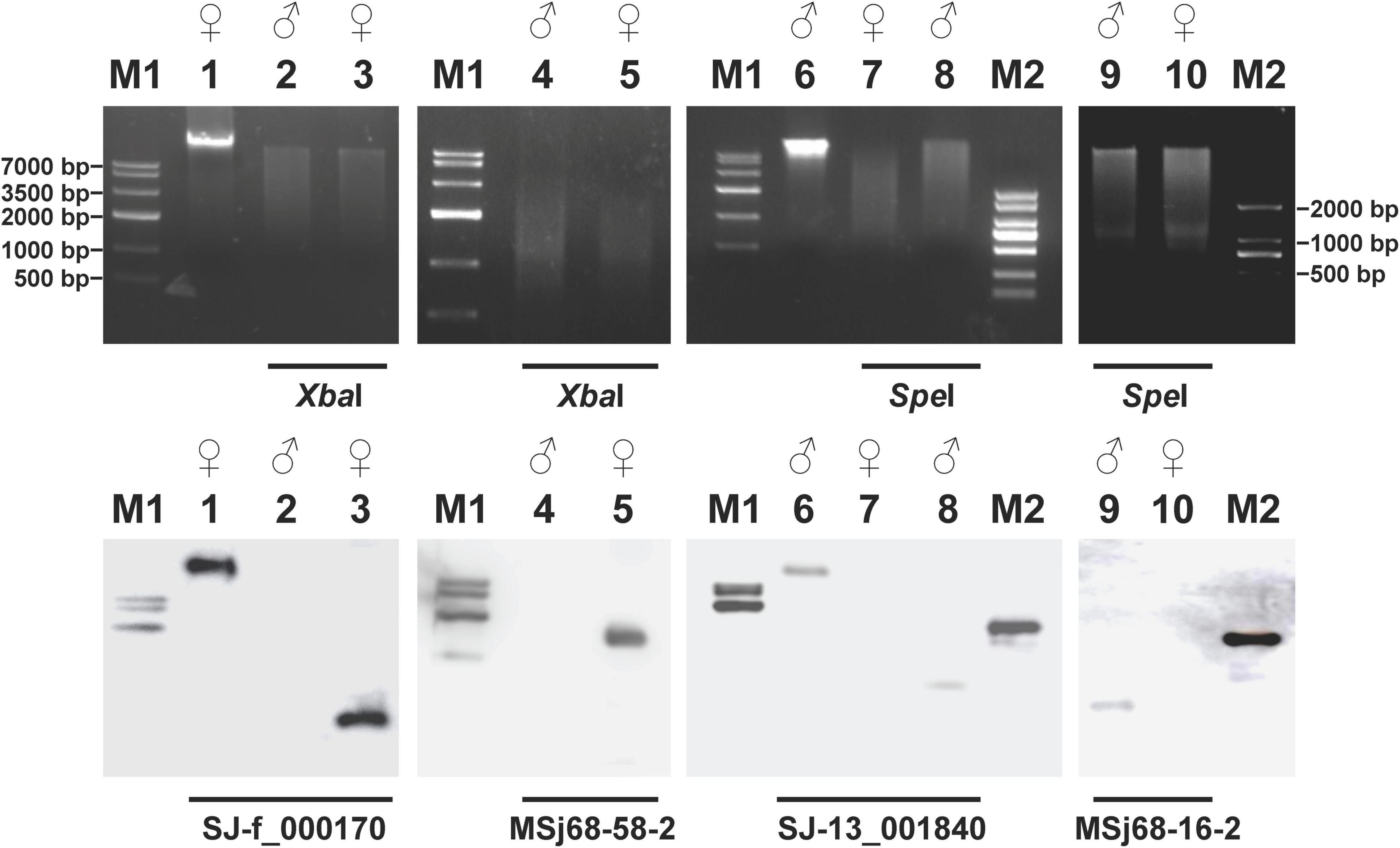
Figure 1. Electrophoresis profiles (upper panels) of the digested genomic DNA from Saccharina japonica gametophytes and their corresponding Southern blotting patterns (lower panels) hybridized with the sex-linked markers as probes. The restriction endonucleases XbaI and SpeI used to digest the genomic DNA from the gametophytes of S. japonica were shown under the upper panels. The labeled sex-linked markers used as probes for Southern blotting analysis were denoted under the lower panels. The un-digested genomic DNA from the female (Lane 1) and male (Lane 6) gametophytes of S. japonica were regarded as positive controls during the Southern blotting analysis. Lanes M1 and M2 represented the D2000 Marker and Marker IV DNA ladder molecular standard (TaKaRa, Kyoto, Japan), respectively. All the DNA markers were labeled with radionuclides.
Fluorescence in situ Hybridization Mapping of the Sex-Linked Markers to Saccharina japonica Chromosomes
Prior to FISH experiments, the quality of synthesized probes was assessed by electrophoresis. Electrophoretic results (Supplementary Figure 3) showed that all the labeled probes appeared smear with a peak intensity between 100 and 500 bp, thus being suitable for subsequent experiments. To determine the chromosomal location of each sex-linked marker, a total of at least 20 metaphase nuclei of the female or male samples was examined using mono-color FISH technique. Of the observed mitotic metaphase nuclei, 10 of the 20 female nuclei hybridized with SJ-f_000170 as a probe, 16 of the 25 female nuclei with MSj68-58-2, 16 of the 24 male nuclei with MSj68-16-2, and 12 of the 23 male nuclei with SJ-13_001840, had only one fluorescent signal as shown in Supplementary Figure 4. These FISH images illustrated that SJ-f_000170 and MSj68-58-2 were unique to female chromosomes whereas SJ-13_001840 and MSj68-16-2 to male chromosomes. When the probes SJ-f_000170 and MSj68-58-2 were used to hybridize the female gametophyte metaphase nuclei, it was interesting to find that each signal was situated at the interstitial region of the short arm of hybridized chromosomes (Supplementary Figure 4). Meanwhile, the probes SJ-13_001840 and MSj68-16-2 were hybridized to the male gametophyte chromosomes near the centromeres (Supplementary Figure 4). In the latter case, both the arms of hybridized chromosomes were nearly equal to each other in length. On the average, the length of chromosome hybridized by the probe SJ-f_000170 (2.068 ± 0.180, n = 10) was nearly equal to that by MSj68-58-2 (2.106 ± 0.272, n = 16) in the female gametophytes (Table 1). Similarly, in the male gametophytes of S. japonica, the size of chromosome probed by SJ-13_001840 (1.662 ± 0.305, n = 12) was not significantly different from that by MSj68-16-2 (1.584 ± 0.162, n = 16) (Table 1). Although the mono-color FISH images showed the similar appearance and signal locations of the hybridized chromosomes within the female or male gametophytes (Supplementary Figure 4 and Table 1), it was not understood that whether the chromosome hybridized by the labeled SJ-f_000170 as a probe might be the same as that by MSj68-58-2, and whether the labeled SJ-13_001840 might be hybridized to the same chromosome as the marker MSj68-16-2.
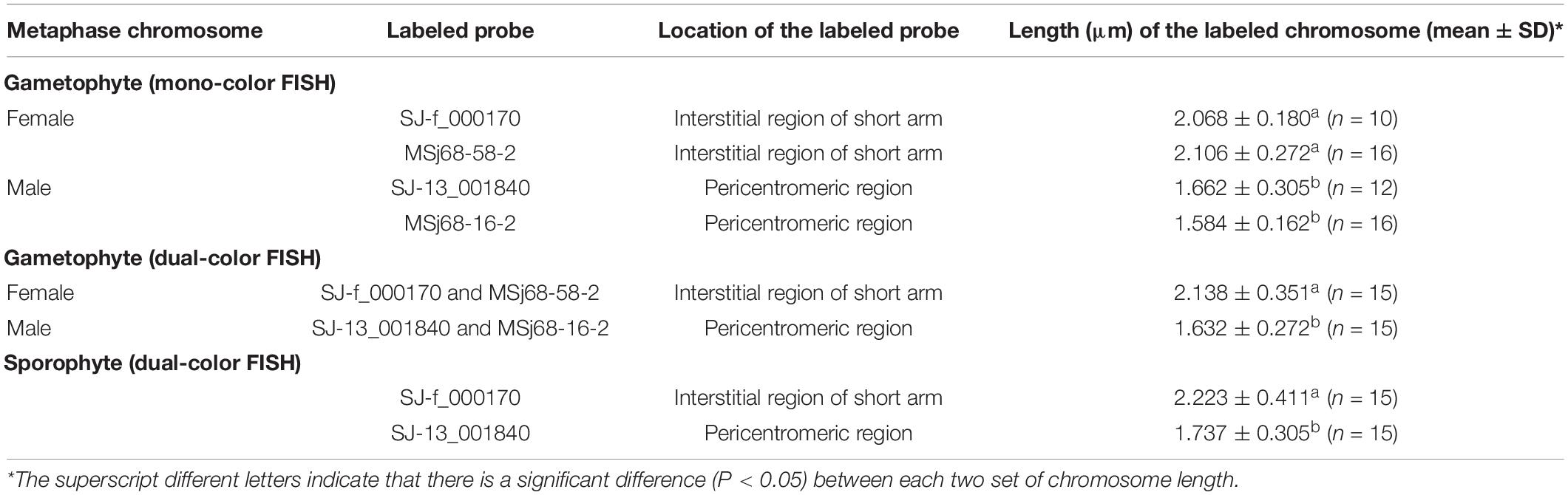
Table 1. Localization of the labeled probes on chromosomes and length of the hybridized chromosomes prepared from the female and male gametophytes and the sporophytes of Saccharina japonica.
To provide experimental evidence, dual-color FISH technique was employed. With the probes labeled by different colors, the hybridized metaphase chromosome image (upper panels in Figure 2) showed that SJ-f_000170 and MSj68-58-2 were co-localized at the same chromosome of the kelp female gametophytes. They occurred in the interstitial region of the short arm of the hybridized chromosomes (Figure 2), consistent with the mono-color FISH results (Supplementary Figure 4). In the same manner, the male-specific markers SJ-13_001840 and MSj68-16-2 were observed to be present near the centromeres of the hybridized chromosome of the kelp male gametophytes (upper panels in Figure 3). The arranged chromosomes by their decreasing length indicated that the markers SJ-f_000170 and MSj68-58-2 were situated on Chromosome 2 of the kelp female gametophytes (lower panel in Figure 2) while the markers SJ-13_001840 and MSj68-16-2 resided on Chromosome 8 of the male gametophytes (lower panel in Figure 3). As aforementioned, these selected markers were developed on the basis of the sequences of several sex-linked markers from the SDRs of Ectocarpus (Lipinska et al., 2015; Zhang et al., 2018), and MSj68-16-2 was the partial DNA sequence of the gene SjHMG that was reported to be expressed only in the male gametophytes of S. japonica (Zhang et al., 2019, 2021). Taken together with the dual-color FISH images (Figures 2, 3), SJ-f_000170 and MSj68-58-2 were proposed to be located at the female SDR while SJ-13_001840 and MSj68-16-2 at the male SDR of S. japonica. As a result, one of SJ-f_000170 and MSj68-58-2 or of SJ-13_001840 and MSj68-16-2 could be chosen as reliable markers to discern the putative sex chromosomes in the sporophyte metaphase nuclei of S. japonica.
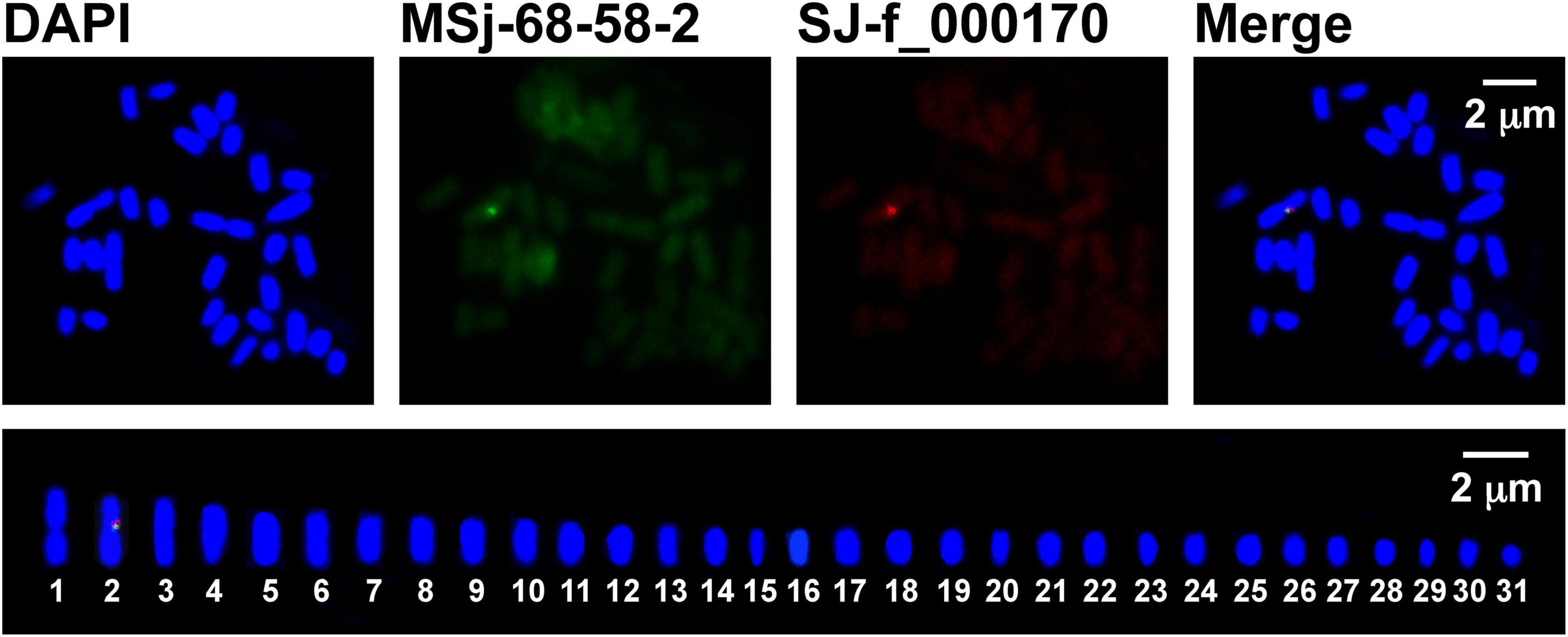
Figure 2. Dual-color FISH mapping (upper panels) and idiogram (lower panel) of the sex-linked markers SJ-13_000170 (red) and MSj68-58-2 (green) on Saccharina japonica female gametophyte metaphase chromosomes counterstained with DAPI (blue). In the lower panel, the female metaphase chromosomes were ordered only in their decreasing size using Adobe Photoshop.
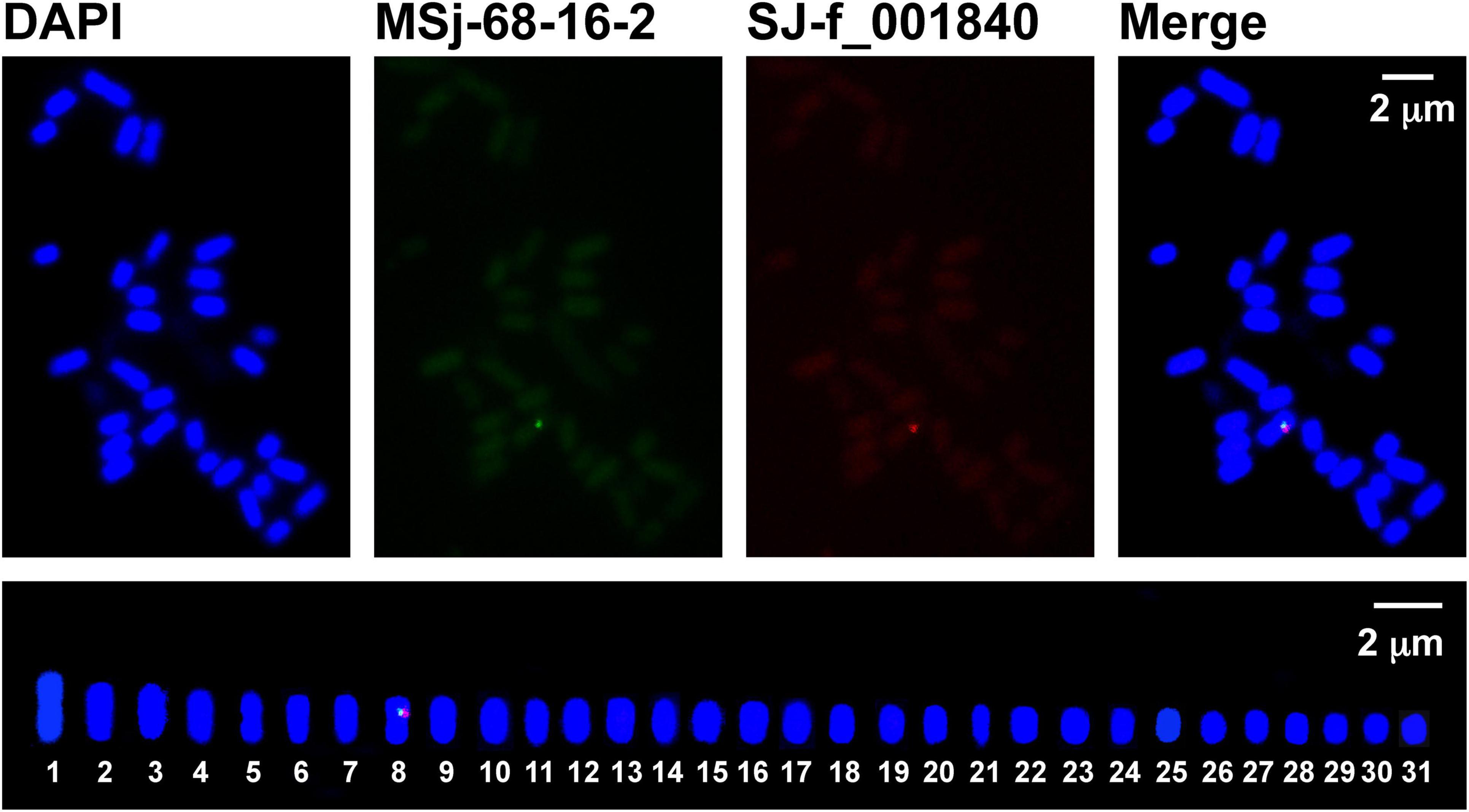
Figure 3. Dual-color FISH mapping (upper panels) and idiogram (lower panel) of the sex-linked markers SJ-13_001840 (red) and MSj68-16-2 (green) on Saccharina japonica male gametophyte metaphase chromosomes counterstained with DAPI (blue). In the lower panel, the male metaphase chromosomes were arranged only in their decreasing size using Adobe Photoshop.
To aim at simultaneously discerning the putative sex chromosomes, SJ-f_000170 and SJ-13_001840 were taken as probes into S. japonica sporophyte chromosomes using dual-color FISH technique. The FISH images (Figure 4 and Supplementary Figure 5) illustrated that the two markers SJ-f_000170 and SJ-13_001840 were located on different chromosomes in the sporophyte metaphase nuclei as expected. Upon karyotype analysis only according to the decreasing length of chromosomes, it was found that SJ-f_000170 and SJ-13_001840 were situated on the paired Chromosomes 2 and 8 (data not shown), the same order as those in the gametophyte nuclei (Figures 2, 3, respectively). It should be pointed out that, however, when homologous chromosomes paired in the prophase stage of meiotic nuclei, SJ-f_000170 and SJ-13_001840 should reside on the independently paired chromosomes as shown in the lower panel of Figure 4 if the hybridized chromosomes were the putative sex ones U and V in S. japonica. In addition, the locations of these markers on the sporophyte metaphase chromosomes hybridized by SJ-f_000170 and SJ-13_001840 were observed in the interstitial region of short arm and pericentromeric region (Figure 4 and Table 1), respectively, which was similar to the observations in the gametophyte nuclei (Figures 2, 3). Length of the hybridized chromosome by the labeled probe SJ-f_000170 (2.223 ± 0.411 μm, n = 15) was different from that by SJ-13_001840 (1.737 ± 0.305 μm, n = 15), also corresponding well to the obtained data from the gametophytes (Table 1). These pieces of information demonstrated that the putative sex chromosome for the determination of female was larger than that for male in S. japonica, thus consistent interestingly with the observations by Evans (1963, 1965) and Yabu and Yasui (1991) in several species of the Laminariales, even if this putative large U chromosome was not too conspicuous.
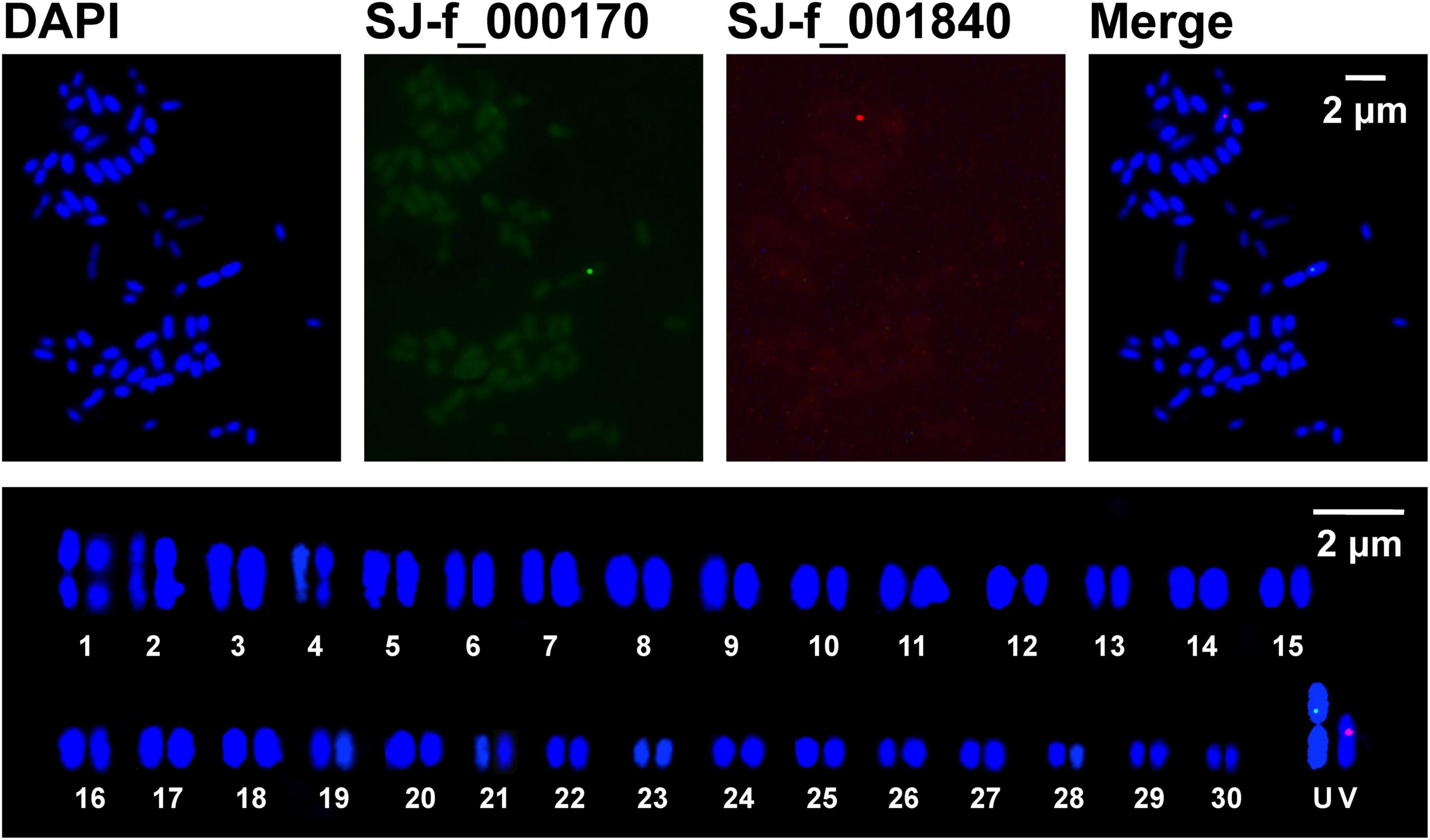
Figure 4. Physical mapping (upper panels) and idiogram (lower panel) of two sex-linked markers SJ-13_001840 (red) and SJ-f_000170 (green) on Saccharina japonica sporophyte metaphase chromosomes counterstained with DAPI (blue). In the lower panel, the sporophyte metaphase chromosomes were ordered and paired in their decreasing size using Adobe Photoshop, but the putative U and V chromosomes were grouped independently.
Genomic Features of the Selected Bacterial Artificial Chromosome Clones Containing the Kelp U-Linked Markers
As suggested by Ming and Moore (2007) and confirmed experimentally by Lipinska et al. (2017), genomic sequence comparisons of sex chromosomes could uncover their common features. Accordingly, several pieces of genomic information on the aforementioned four sex-linked markers and their surroundings in S. japonica were searched and compiled in Supplementary Table 2. Blast searching the assembled genomes of S. japonica (Ye et al., 2015; Shao et al., 2019; Fan et al., 2020) with each of the U-linked markers as a query indicated that SJ-f_000170 was not able to be searched from the kelp gametophyte genome (Ye et al., 2015; Fan et al., 2020), but it matched with Contig4871 (6,269 bp) from the sporophyte one (Shao et al., 2019) with 100% sequence identity (639 bp) (Supplementary Table 2). In the same way, MSj68-58-2 was found to match with Contig3481 (188,527 bp) from the sporophyte one (Shao et al., 2019) with 100% sequence identity (1,105 bp), whereas it matched with Scaffold220 (444,739 bp) from the kelp gametophyte genome (Ye et al., 2015) only with 52% (573/1,105 bp) sequence identity (Supplementary Table 2). Furthermore, while aligning one searched Contig3481 with the other Scaffold220 (Supplementary Figure 6A), it was found there was no identity between them. The uncertainty of the screened two assembled sequences Contig3481 and Scaffold220 prevented them from applying to the genomic feature elucidation. The present study therefore provided some pieces of information inferred from a BAC library of S. japonica. Since this BAC library has been constructed only from the kelp female gametophytes (Liu P. F. et al., 2021), the genomic feature related to U chromosome was elucidated.
With the designed primers based on the sequences of SJ-f_000170 and MSj68-58-2, two BAC clones, BAC669-A11 and BAC652-P6 encompassing the two markers, respectively, were screened using PCR technique (Supplementary Figure 7). Using PacBio RS II sequencing platform, total 1,600,988,320 and 1,500,167,532 bases (Supplementary Table 3) corresponding to the BAC clones BAC669-A11 and BAC652-P6 were obtained. Through raw reads assembling, full-length sequences of these two inserts were sequenced, and they were 110,085 and 104,111 bp in length (Supplementary Table 3). While querying these two sequences to the primarily assembled genome of S. japonica (Ye et al., 2015), it was found that 55.69% of BAC669-A11 (from 10,880 to 72,183 bp) could be assigned to several scaffolds of this kelp genome whereas only 25.81% BAC652-P6 (from 88 to 28,496 bp) could be done (Supplementary Table 4 and Supplementary Figures 8A,C). Excitedly, the coverage was evidently increased while searching the target sequences in another currently released genome of S. japonica (Shao et al., 2019). The coverage was up to 82.59 and 92.56% for BAC669-A11 (from 1 to 85,983 bp) and BAC652-P6 (from 1 to 96,362 bp), respectively, even if each BAC clone was still assigned to a number of different contigs (Supplementary Table 4 and Supplementary Figures 8B,D). The difference of the blast search results between the two assembled kelp genomes possibly resulted from the number and length of gaps as shown in Supplementary Figure 8. While searching the assembled 31 pseudo-chromosomes of the S. japonica gametophyte genome (Fan et al., 2020), the coverage was up to 94.13 and 98.76% for BAC669-A11 (from 1,601 to 105,224 bp) and BAC652-P6 (from 1 to 102,820 bp), respectively, even if each BAC clone was still assigned to at least 9 and 12 pseudo-chromosomes (Supplementary Table 4). From these blast results, it was inferred that our sequencing data from the selected BAC clones might be authentic, though there was no well match in the 580.5 million base pairs (Mbps) of genome sequence of S. japonica (Liu et al., 2019) due to the only available raw data deposited under the Bioproject accession of PRJNA280905. The insert sequences of the two BAC clones have been deposited in GenBank under the accession Nos. MZ285758 and MZ285759.
Physical mapping (Figure 5) of the inserts of the BAC clones indicated that the U-linked marker SJ-f_000170 was located upstream of BAC669-A11, while the U-linked marker MSj68-58-2 was at the beginning of BAC652-P6. A great number of repetitive sequences (Supplementary Table 5), such as short tandem repeats (STRs) and retrotransposons including long terminal repeat (LTR)/Gypsy, LTR/Copia, long interspersed nuclear element (LINE)/L1, and LINE/RTE-BovB, scattered in the inserts of these two BAC clones. The STRs mainly contained mono-(e.g., A, G, C, and T), di-(e.g., GT, AT, and AC), tri-(e.g., CAG, GCA, TGC, ACA, CTG, and GCT), and tetra-(e.g., ATGT, GTAC, TGTT, and GGTG) nucleotide repeats (Supplementary Table 5). The repetitive elements including LTR, LINE, and STR, accounted for 45% of the full-length sequences of inserts (Table 2), which was higher than the average one that was 39 or 43% of S. japonica genome as estimated by Ye et al. (2015) and Liu et al. (2019), respectively, suggesting they were non-randomly distributed in the kelp genome. In addition, four genes were de novo annotated in these two sequenced inserts of BAC clones, and they were designated as Genes 1, 2, and 3 in BAC669-A11, while only one gene (Gene 4) was annotated in BAC652-P6 (Figure 5). The gene density (0.78%, Table 2 and Supplementary Table 6) corresponding to 18.69 genes per Mbp in these two BAC clones, therefore, was significantly poorer than the average one that was 3.66% (i.e., 34.07 genes per Mbp) or 6.99% (i.e., 61.54 genes per Mbp) (Table 2) as surveyed by Ye et al. (2015) and Liu et al. (2019), respectively, in their reported kelp genomes.
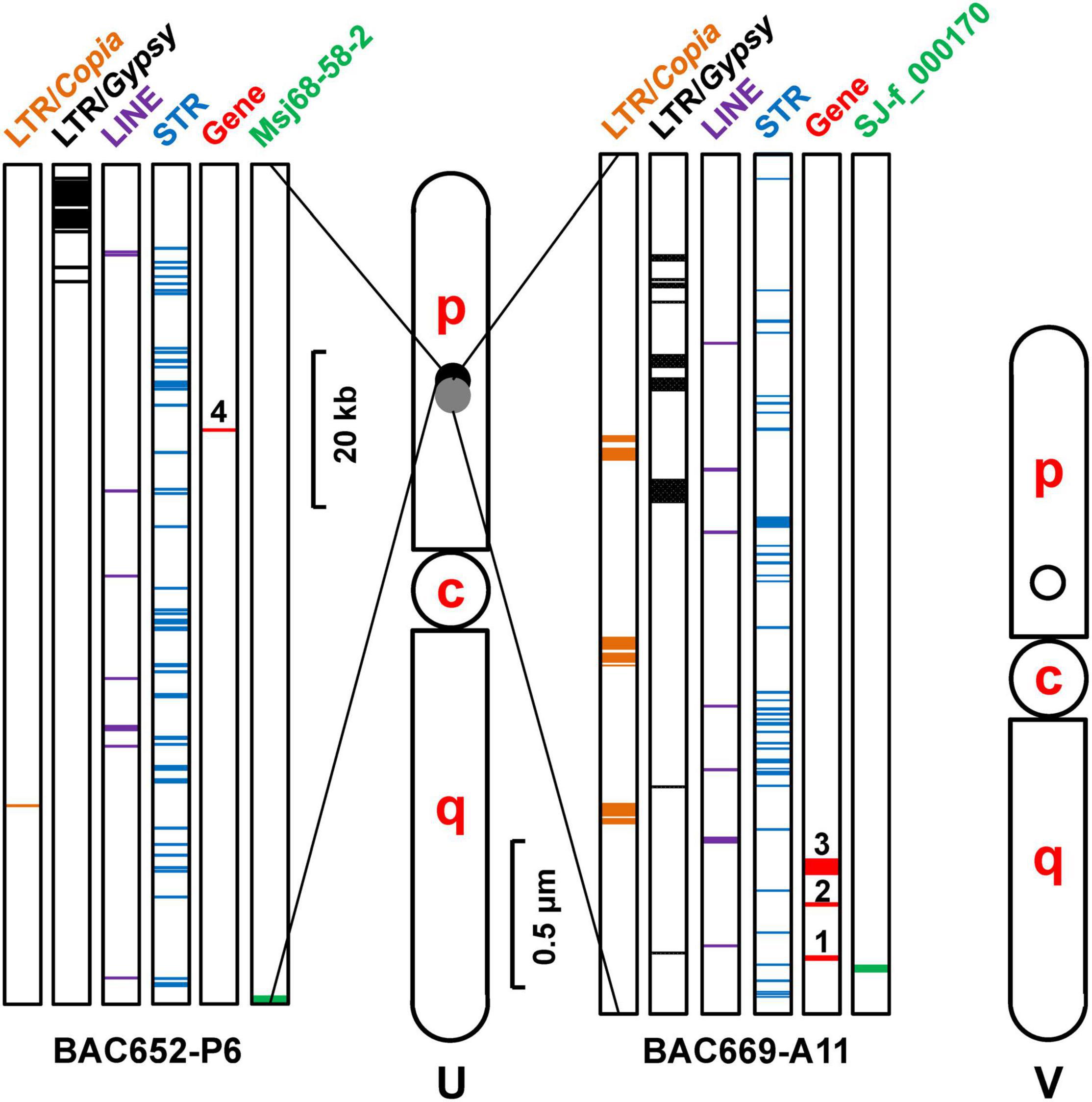
Figure 5. Schematic diagram of the putative U and V chromosomes in Saccharina japonica indicating their relative length and the loci of sex-linked molecular markers. Light gray and black spots represent the U-linked markers, while black circle denotes the V-linked marker. The distribution of genes and various types of repetitive DNA sequences in the sequenced BAC clones carrying the U-linked markers was illustrated on both sides of the putative U chromosome. c, centromere; p, short arm; q, long arm.
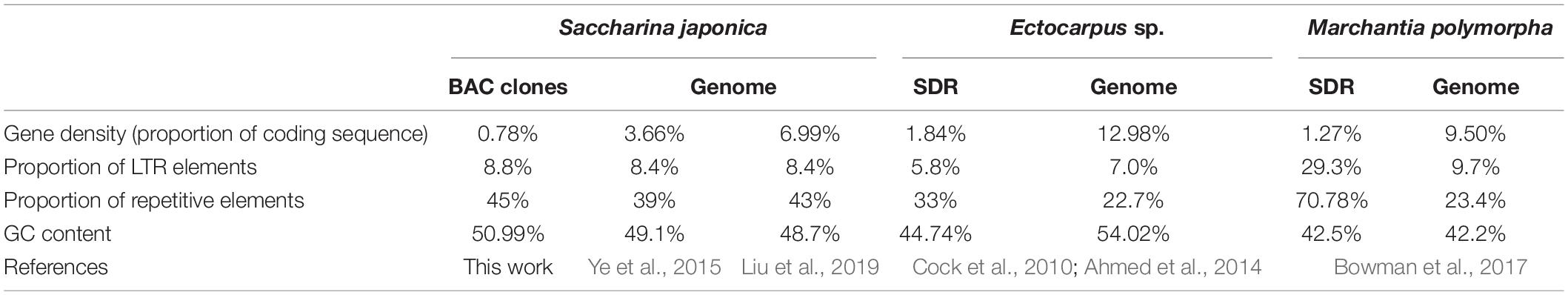
Table 2. Information comparison between SDR of U chromosome and its corresponding genome from various eukaryotes.
In short, both relatively high proportion of repetitive elements and poor gene density in the sequenced inserts of the BAC clones shared the genomic features about the SDR of Ectocarpus sp. or Marchantia polymorpha U chromosome (Table 2). Taken together with the similar location of the two markers SJ-f_000170 and MSj68-58-2 on the same chromosome in the kelp female gametophyte nuclei (Figure 2), the region where the two markers located was proposed to be the non-recombining SDR of the S. japonica U chromosome.
Annotated U-Linked Genes and Their Transcription in the Male and Female Gametophytes
As described above, four genes were de novo annotated using AUGUSTUS program from these two sequenced BAC clones. Among them, Genes 1 and 2 were also annotated by Ye et al. (2015) and Shao et al. (2019) in the released S. japonica genomes. Using the genomic DNA extracted from S. japonica female gametophyte as a template (Supplementary Figure 9), these four genes could be amplified successfully. Sequencing analyses of the amplified products demonstrated that Gene 1 had one intron whereas all the other three genes had no intron (Supplementary Table 7). The results suggested that all the four annotated genes would be active in the kelp female gametophyte genome, but their functions remained to be characterized. Surprisingly, Genes 1, 2, and 3 were also amplified (Supplementary Figure 9 and Supplementary Table 7) from the male gametophyte of S. japonica. Comparative analysis of these amplificons showed that these annotated three genes have conserved intron and exon structures but varied sequences between the kelp male and female gametophytes (Supplementary Table 7).
Gene 1, locating just downstream (from 5,400 to 6,034 bp) of the sex-linked marker SJ-f_000170 in BAC669-A11 (Figure 5), was found to completely overlap the DNA sequence of SJ-f_000080 in S. japonica. SJ-f_000080, an ortholog of Ec-sdr-f_000080 in Ectocarpus sp., has been already described as one of the sex-linked ancestral genes in S. japonica by Lipinska et al. (2017). Genes 2 and 3 also annotated from BAC669-A11 were 258 and 2,163 bp in length, respectively, while Gene 4 from the other BAC clone, BAC652-P6, was 252 bp long. Function of these gene products was unknown. However, it was of interest that part (from 77 to 134 bp) of Gene 4 shared highly similarity with the partial sequence (from 401 to 458 bp) of a sex discriminating marker m68_58_3f (GenBank accession No. KP994179) which was developed from Laminaria digitata by Lipinska et al. (2015).
To understand transcription of these annotated four genes in the male and female gametophytes of S. japonica, qRT-PCR was employed in the present study. The conserved coding sequences of these genes were used to design the qRT-PCR primers. Genes 1 and 3 were expressed fivefold more abundant at the transcription level in the female gametophytes than them in the male ones (t2 = 17.183, P = 2.02 × 10–5 < 0.01 for Gene 1; t2 = 3.562, P = 0.009 < 0.01 for Gene 3) whereas Gene 2 was transcribed in the similar abundance or constitutively (t2 = − 2.58, P = 0.975 > 0.05) between the male and female gametophytes (Figure 6). Interestingly, Gene 4, annotated from the insert sequence of BAC652-P6, was found to be expressed exclusively in the female gametophytes (Supplementary Figure 10). It was thus referred to a sex-specific gene as defined by Ellegren and Parsch (2007), while Genes 1 and 3 were the sex-biased genes. Combining the transcription of Gene 4 with its un-amplified product with the extracted genomic DNA as a template from the kelp male gametophytes suggested that Gene 4 was a potential candidate for the female sex-determining factor. In contrast to this, the other three genes, being transcribed differentially (for Genes 1 and 3) or similarly (for Gene 2) in abundance between the male and female gametophytes (Figure 6), were proposed to be gametologue pairs in S. japonica according to the termed definition by Coelho et al. (2018) and Umen and Coelho (2019). These annotated genes were thereby characterized as SDR genes in transcription as suggested by Lipinska et al. (2015).
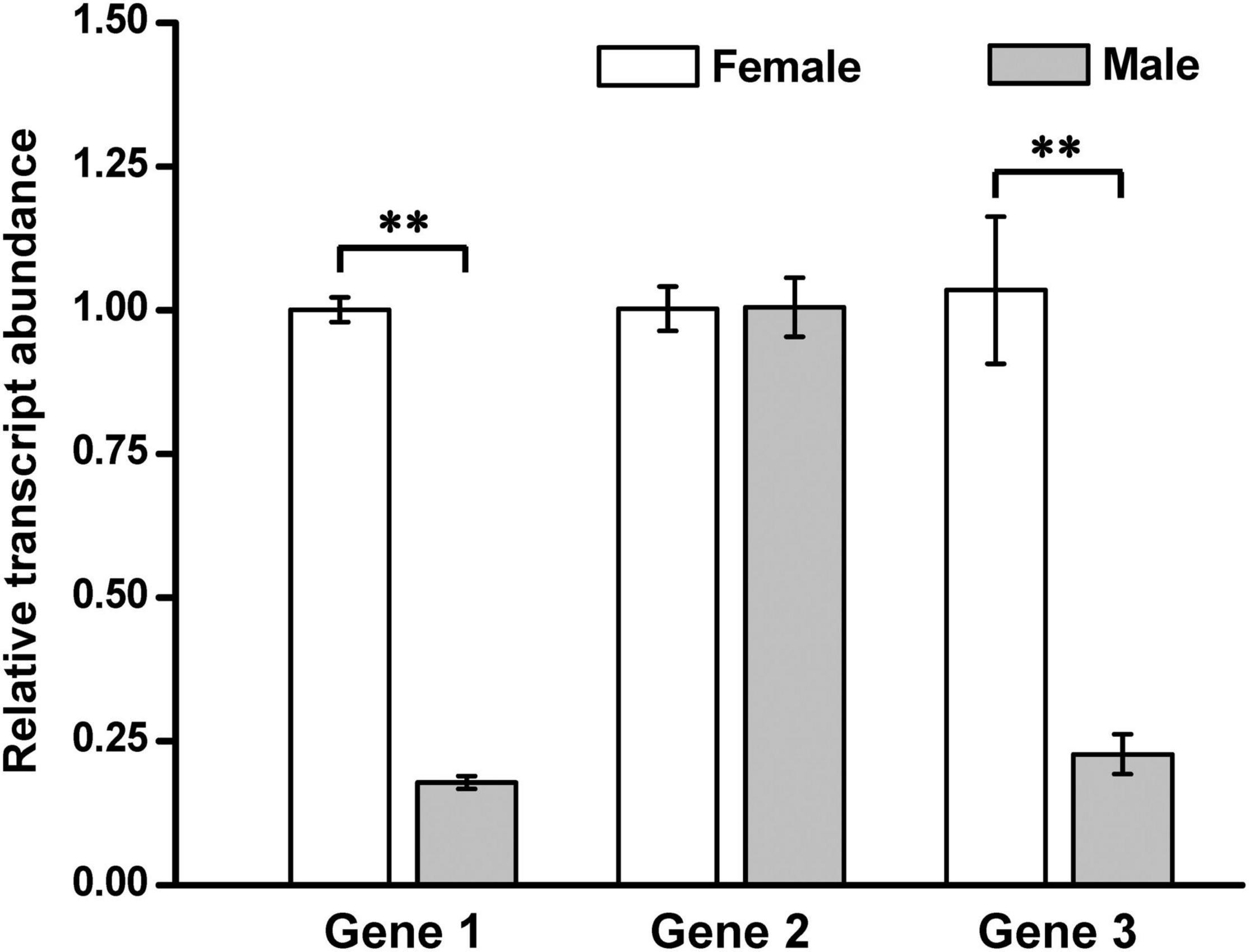
Figure 6. Expression comparison of the three annotated genes from the sequenced BAC clones between the kelp male and female gametophytes. Each value is the mean ± standard deviation (SD) of three independent biological replicates, and the significance level was set at P < 0.01 (**).
Discussion
For more than half a century since the finding of a large chromosome in the laminarian nucleus (Evans, 1963), the existence of sex chromosomes in S. japonica has been an open problem to be solved (Tai and Fang, 1977; Yabu and Yasui, 1991). But now, the developed sex-linked markers has provided us with an opportunity to understand whether S. japonica has sex chromosomes by use of FISH technique. Two sex-linked markers SJ-f_000170 and SJ-13_001840 have been proved present in the kelp genome by Southern blotting analysis (Figure 1) and then co-localized on different chromosomes in the sporophyte metaphase nuclei of S. japonica by dual-color FISH technique (Figure 4 and Supplementary Figure 5). The two different chromosomes hybridized by the two sex-linked markers are suggested to be the putative sex chromosomes U and V in this kelp for the following three reasons.
First, the two sex-linked markers SJ-f_000170 and SJ-13_001840 employed in the present study were developed by Lipinska et al. (2017) on the basis of one gametologue pair, Ec-sdr_f_000170 and Ec-13_001840 in the Ectocarpus SDRs, respectively. The latter two were found to be highly conserved in six brown seaweeds including S. japonica by Lipinska et al. (2017) via genomic comparison. In the present study, the hybridization signals on the chromosomes by the labeled sex-linked markers SJ-f_000170 and SJ-13_001840 (Figure 4 and Supplementary Figure 5) illustrated that such a chromosomal SDR could be visualized first by cytogenetic technique, thus suggesting that it would be present evidentially in S. japonica. Dual-color FISH mapping of the other two markers, MSj68-58-2 and MSj68-16-2, showed that they were co-localized at the similar loci with SJ-f_000170 (Figure 2) and SJ-13_001840 (Figure 3), respectively. Moreover, the marker MSj68-58-2 was originally derived from the Ectocarpus SDR genes as described by Lipinska et al. (2015). It was hence inferred that all the four sex-linked markers used for FISH mapping were located at the chromosomal SDRs of S. japonica. Since SDRs carried a set of genes that determined the sex of individuals (Coelho et al., 2018), the chromosomes where these sex-linked genes resided were referred to U and V sex chromosomes of S. japonica.
Second, SDR is a non-recombining region of sex chromosomes (Hobza et al., 2015; Umen and Coelho, 2019) though the size of this region differs between species. The absence of recombination leads to gene degeneration and repetitive DNA sequence accumulation on plant (Kejnovsky et al., 2009) and brown seaweed (Ahmed et al., 2014) SDRs. The present study exemplifies only the U chromosome SDR for lack of information about large fragment of V chromosome so far in S. japonica, even if 59 V-linked scaffolds have been identified from the S. japonica female gametophyte reference genome sequence (Ye et al., 2015) by Lipinska et al. (2017) via data mining. The repetitive elements distributed in the inserts of two sequenced BAC clones (Figure 5) account for 45%. This percentage is intermediate between Ectocarpus SDR (33%) and liverwort SDR (70.78%), but higher than the average one of the S. japonica genome (Table 2). These data at least suggests that the inserts of the two BAC clones from S. japonica female gametophytes share a common genomic feature with other SDRs in repetitive sequences. In addition, Fraser and Heitman (2005) and Hobza et al. (2015) proposed that rapid accumulation of repeat sequences especially of the transposable elements as reviewed by Gschwend et al. (2012) and Charlesworth (2013) contributed well to SDR expansion. As a result of accumulation of more repeat sequences (Table 2 and Figure 5), the SDR of S. japonica U chromosome is expected to be expanded in size as compared to the U SDR of Ectocarpus (see below for more details).
Third, it is generally accepted that sex chromosomes evolve from autosomes through the acquisition of genes involved in sex determination (Kejnovsky et al., 2009; Coelho et al., 2018). In the process of evolution, the gene density in SDRs tends to decline accompanied by repetitive elements acquisition with concomitant gene loss. This tendency has been found in several eukaryotes such as Volvox carteri (Ferris et al., 2010), Ectocarpus sp. (Ahmed et al., 2014), and Marchantia polymorpha (Bowman et al., 2017) with UV or UV-like (mating-type loci same as SDRs) sex determination system. Compared to the genomic data of S. japonica as documented by Ye et al. (2015) and Liu et al. (2019), the gene density of the two sequenced inserts of BAC clones in the present study is nearly one fifth or even down to approximately one ninth, respectively, of that in genome (Table 2). The low gene density from these sequenced BAC clones is comparable to that in either Ectocarpus or M. polymorpha U chromosome SDR (Table 2), thus exhibiting the common feature of the SDRs of U chromosomes in this regard.
In brief, both the employed sex-linked markers in the cytogenetic mapping and genomic features of the insert sequences of two BAC clones showed that they have the SDR characteristics, thus implying that the chromosomes where they colonized might be sex chromosomes of S. japonica.
Now let’s go back to the question of estimating the size of S. japonica U chromosome SDR. As mentioned above, either U-linked genes or markers SJ-f_000170, MSj68-58-2, SJ-f_000010, and SJ-f_000080 are, respectively, corresponding to the Ec-sdr_f_000170, Esi_0068_0058, Ec-sdr_f_00010, and Ec-sdr_f_00080 in Ectocarpus SDR (Lipinska et al., 2017) by synteny analysis (Supplementary Figure 11). Of them, SJ-f_000170, SJ-f_000080 (or Gene 1), and MSj68-58-2 are found to be present in the sequenced inserts of the screened two BAC clones BAC669-A11 and BAC652-P6 (Figure 5 and Supplementary Figure 8). Although SJ-f_000010 is not screened from these two BACs, it is co-located with MSj68-58-2 in Contig3481 screened from the assembled kelp sporophyte genome (Shao et al., 2019) as shown in Supplementary Figure 11. The sequence of Contig3481 indicates that SJ-f_000010 is downstream of MSj68-58-2 approximately 0.028 Mbp. Therefore, the size between SJ-f_000170 and SJ-f_000010 is estimated to be 0.238 (= 0.102 + 0.108 + 0.028) Mbp (Supplementary Figure 11), regardless of the physical gap present between the two BAC clones. This size is thus larger than that of the syntenic one which has been estimated by Ahmed et al. (2014) to be around 0.176 Mbp apart from one homologous marker Ec-sdr_f_000170 to the other Ec-sdr_f_000010 in the female SDR of Ectocarpus. In addition, Pedersen and Linde-Laursen (1995) suggested that a minimum of 5 − 10 Mbp distance would be necessary to resolve FISH signals of two DNA probes on metaphase chromosomes in a FISH mapping profile. From both the similar site but in-completely overlapping location of SJ-f_000170 and MSj68-58-2 on the hybridized metaphase chromosome (Figure 2) and the real presence of physical gap between these two sequenced BAC clones, SDR of S. japonica U chromosome is inferred to be unambiguously larger than the Ectocarpus one. This is also reflected by the aforementioned prediction based upon the higher proportion of repetitive elements in the inserts of the two selected BAC clones (Table 2). The complete SDR sequence and its size of the kelp U chromosome will be unveiled, once it is assembled by screening and sequencing of more BAC clones.
With respect to V chromosome, the unavailable BAC library constructed from the male gametophytes of S. japonica limits the exploration in the present study. Nevertheless, while searching the assembled genomes of S. japonica (Ye et al., 2015; Shao et al., 2019; Fan et al., 2020) with each of the V-linked markers as a query, SJ-13_001840 was found to match with both Contig4772 (10,276 bp) from the sporophyte one (Shao et al., 2019) and Scaffold3074 (24,639 bp) from the kelp gametophyte genome (Ye et al., 2015; Supplementary Figure 6B). In the same way, Scaffold1744 and Contig2278 have been searched online with the V-linked marker MSj68-16-2 as a query. Upon aligning, several identical regions are found to be present between Contig4772 and Scaffold3074 or between Scaffold1744 and Contig2278. These regions look matched better than the alignment with the searched sequences (Supplementary Figure 6A) using each of the U-linked markers as a query, even if there remained a lot of gaps (Supplementary Figures 6B,C). In addition, the V-linked marker MSj68-16-2 is a part of DNA sequence of this gene SjHMG which has been expressed exclusively in the kelp male gametophytes (Ahmed et al., 2014; Lipinska et al., 2017; Zhang et al., 2019, 2021), and the cumulative size of 59 V-linked identified scaffolds is up to approximately 4.91 Mbp (Lipinska et al., 2017). These data are expected to help uncover the genomic features of V chromosome SDR, once the BAC library is constructed from the male gametophytes of S. japonica. It is pointed out here, however, that the reference genomic draft of S. japonica has been sequenced and assembled from the female gametophytes rather than male ones as stated by Ye et al. (2015). Since the gene SjHMG, which has been exclusively expressed in male gametophytes as experimentally proved by Zhang et al. (2019, 2021), could be screened from the genomic data by Lipinska et al. (2017), the female gametophyte materials used for the obtaining of the kelp genomic data are at least supposed to be contaminated by male gametophyte.
In a word, the present study offers first insights into cytogenetic localization of sex-linked molecular markers, thus enabling us to distinguish the putative sex chromosomes U and V from the autosomes in S. japonica.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, MZ285758; https://www.ncbi.nlm.nih.gov/genbank/, MZ285759.
Author Contributions
YD carried out most experiments and written the original draft. P-FL searched sex-linked markers in S. japonica and screened the target BAC clones. ZL and QZ repeated the FISH experiment. Y-HB analyzed all these data with YD and confirmed the results in addition to the primary writing of this manuscript. Z-GZ made a significant contribution to the conceptual design of this work and the writing and polishing of this manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant nos. 32172963 and 41376136 to Z-GZ), the National Key Research and Development Program of China (grant no. 2018YFD0901500 to Y-HB), and the World Class Discipline Project of Aquaculture (to Z-GZ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are very grateful to Xiao-Jie Li from Oriental Ocean Sci-tec Co., Ltd., Yantai, Shandong province, China, for her generous help in the supply of kelp juvenile sporophytes.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.821603/full#supplementary-material
Footnotes
- ^ https://imagej.en.softonic.com/
- ^ https://www.adobe.com/products/photoshop.html
- ^ https://www.ncbi.nlm.nih.gov/tools/vecscreen/
- ^ http://repeatmasker.org/
- ^ http://augustus.gobics.de
- ^ http://ccb.jhu.edu/software/tophat/index.shtml
- ^ http://cole-trapnell-lab.github.io/cufflinks/
- ^ https://blast.ncbi.nlm.nih.gov/Blast.cgi
- ^ https://www.ibm.com/products/spss-statistics
References
Ahmed, S., Cock, J. M., Pessia, E., Luthringer, R., Cormier, A., Robuchon, M., et al. (2014). A haploid system of sex determination in the brown alga Ectocarpus sp. Curr. Biol. 24, 1945–1957. doi: 10.1016/j.cub.2014.07.042
Bachtrog, D., Kirkpatrick, M., Mank, J. E., McDaniel, S. F., Pires, J. C., Rice, W. R., et al. (2011). Are all sex chromosomes created equal? Trends Genet. 27, 350–357. doi: 10.1016/j.tig.2011.05.005
Bi, Y.-H., Qiao, Y.-M., Wang, Z., and Zhou, Z.-G. (2021). Identification and characterization of a periplasmic α-carbonic anhydrase (CA) in the gametophytes of Saccharina japonica (Phaeophyceae). J. Phycol. 57, 295–310. doi: 10.1111/jpy.13091
Bowman, J., Kohchi, T., Yamato, K., Jenkins, J., Shu, S., Ishizaki, K., et al. (2017). Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 171, 287–304. doi: 10.1016/j.cell.2017.09.030
Charlesworth, D. (2013). Plant sex chromosome evolution. J. Exp. Bot. 64, 405–420. doi: 10.1093/jxb/ers322
Cock, J. M., Sterck, L., Rouzé, P., Scornet, D., Allen, A. E., Amoutzias, G., et al. (2010). The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 465, 617–621. doi: 10.1038/nature09016
Coelho, S. M., Gueno, J., Lipinska, A. P., Cock, J. M., and Umen, J. G. (2018). UV chromosomes and haploid sexual systems. Trends Plant Sci. 23, 794–807. doi: 10.1016/j.tplants.2018.06.005
Coelho, S. M., Mignerot, L., and Cock, J. M. (2019). Origin and evolution of sex-determination systems in the brown algae. New Phytol. 222, 1751–1756. doi: 10.1111/nph.15694
Coelho, S. M., and Umen, J. (2021). Switching it up: algal insights into sexual transitions. Plant Reprod. 34, 287–296. doi: 10.1007/s00497-021-00417-0
Dai, J., Cui, J., Ou, Y., and Fang, Z. (1993). Genetical study on the parthenogenesis in Laminaria japonica. Acta Oceanol. Sin. 12, 295–298.
Ellegren, H., and Parsch, J. (2007). The evolution of sex-biased genes and sex-biased gene expression. Nat. Rev. Genet. 8, 689–698. doi: 10.1038/nrg2167
Evans, L. V. (1963). A large chromosome in the laminarian nucleus. Nature 198:215. doi: 10.1038/198215a0
Evans, L. V. (1965). Cytological studies in the Laminariales. Ann. Bot. 29, 541–562. doi: 10.1093/oxfordjournals.aob.a083971
Fan, X., Han, W., Teng, L., Jiang, P., Zhang, X., Xu, D., et al. (2020). Single-base methylome profiling of the giant kelp Saccharina japonica reveals significant differences in DNA methylation to microalgae and plants. New. Phytol. 225, 234–249. doi: 10.1111/nph.16125
Fang, T. C., Tai, C. H., Ou, Y. L., Tcuei, C. C., and Chen, T. C. (1978). Some genetic observations on the monoploid breeding of Laminaria japonica. Sci. Sin. 21, 401–408. doi: 10.1360/ya1978-21-3-401
Ferris, P., Olson, B. J., De Hoff, P. L., Douglass, S., Casero, D., Prochnik, S., et al. (2010). Evolution of an expanded sex-determining locus in Volvox. Science 328, 351–354. doi: 10.1126/science.1186222
Fraser, J. A., and Heitman, J. (2005). Chromosomal sex-determining regions in animals, plants and fungi. Curr. Opin. Genet. Dev. 15, 645–651. doi: 10.1016/j.gde.2005.09.002
Gschwend, A. R., Weingartner, L. A., Moore, R. C., and Ming, R. (2012). The sex-specific region of sex chromosomes in animals and plants. Chromosome Res. 20, 57–69. doi: 10.1007/s10577-011-9255-y
Gu, J.-G., Sun, Y.-P., Liu, Y., Bi, Y.-H., and Zhou, Z.-G. (2014). Sex identification and genetic variation of Saccharina (Phaeophyta) gametophytes as revealed by inter-simple sequence repeat (ISSR) markers. J. Appl. Phycol. 26, 635–646. doi: 10.1007/s10811-013-0089-1
Hobza, R., Kubat, Z., Cegan, R., Jesionek, W., Vyskot, B., and Kejnovsky, E. (2015). Impact of repetitive DNA on sex chromosome evolution in plants. Chromosome Res. 23, 561–570. doi: 10.1007/s10577-015-9496-2
Hu, Y.-J., and Zhou, Z.-G. (2001). Extraction of RAPD-friendly DNA from Laminaria japonica (Phaeophyta) after enzymatic dissociation of the frozen sporophyte tissues. J. Appl. Phycol. 13, 415–422. doi: 10.1023/A:1011920213639
Kejnovsky, E., Hobza, R., Cermak, T., Kubat, Z., and Vyskot, B. (2009). The role of repetitive DNA in structure and evolution of sex chromosomes in plants. Heredity 102, 533–541. doi: 10.1038/hdy.2009.17
Lewis, R. J. (1996). Chromosomes of the brown algae. Phycologia 35, 19–40. doi: 10.2216/i0031-8884-35-1-19.1
Lewis, R. J., Jiang, B. Y., Neushul, M., and Fei, X. G. (1993). Haploid parthenogenetic sporophytes of Laminaria japonica (Phaeophyceae). J. Phycol. 29, 363–369. doi: 10.1111/j.0022-3646.1993.00363.x
Lipinska, A. P., Ahmed, S., Peters, A. F., Faugeron, S., Cock, J. M., and Coelho, S. M. (2015). Development of PCR-based markers to determine the sex of kelps. PLoS One 10:e0140535. doi: 10.1371/journal.pone.0140535
Lipinska, A. P., Toda, N. R., Heesch, S., Peters, A. F., Cock, J. M., and Coelho, S. M. (2017). Multiple gene movements into and out of haploid sex chromosomes. Genome Biol. 18:104. doi: 10.1186/s13059-017-1201-7
Liu, L., Yang, Q.-F., Dong, W.-S., Bi, Y.-H., and Zhou, Z.-G. (2017). Characterization and physical mapping of nuclear ribosomal RNA (rRNA) genes in the haploid gametophytes of Saccharina japonica (Phaeophyta). J. Appl. Phycol. 29, 2695–2706. doi: 10.1007/s10811-017-1206-3
Liu, P. F., Gu, J.-G., Bi, Y.-H., and Zhou, Z.-G. (2021). Construction of a bacterial artificial chromosome (BAC) library of the female gametophytes of Saccharina japonica and map-based cloning and sequencing of genes neighboring with a female-specific marker FRML-1488. J. Fish. China 45, 672–681. doi: 10.11964/jfc.20200112143
Liu, Y., Liu, P.-F., Bi, Y.-H., and Zhou, Z.-G. (2021). Chromosomal mapping of 5S and 18S-5.8S-25S rRNA genes in Saccharina japonica (Phaeophyceae) as visualized by dual-color fluorescence in situ hybridization. J. Oceanol. Limnol. 39, 714–720. doi: 10.1007/s00343-020-9276-5
Liu, T., Wang, X., Wang, G., Jia, S., Liu, G., Shan, G., et al. (2019). Evolution of complex thallus alga: genome sequencing of Saccharina japonica. Front. Genet. 10:378. doi: 10.3389/fgene.2019.00378
Liu, W., Xie, Y., Ma, J., Luo, X., Nie, P., Zuo, Z., et al. (2015). IBS: an illustrator for the presentation and visualization of biological sequences. Bioinformatics 31, 3359–3361. doi: 10.1093/bioinformatics/btv362
Liu, Y., Bi, Y.-H., Gu, J.-G., Li, L.-H., and Zhou, Z.-G. (2012). Localization of a female-specific marker on the chromosomes of the brown seaweed Saccharina japonica using fluorescence in situ hybridization. PLoS One 7:e48784. doi: 10.1371/journal.pone.0048784
Liu, Y.-S., Li, L.-H., Wu, W.-K., and Zhou, Z.-G. (2009). A SCAR molecular marker specifically related to the female gametophytes of Saccharina (Laminaria) japonica (Phaeophyta). J. Phycol. 45, 894–897. doi: 10.1111/j.1529-8817.2009.00719.x
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Luthringer, R., Lipinska, A. P., Roze, D., Cormier, A., Macaisne, N., Peters, A. F., et al. (2014). The pseudoautosomal regions of the U/V sex chromosomes of the brown alga Ectocarpus exhibit unusual features. Mol. Biol. Evol. 32, 2973–2985. doi: 10.1093/molbev/msv173
Ming, R., and Moore, P. H. (2007). Genomics of sex chromosomes. Curr. Opin. Plant Biol. 10, 123–130. doi: 10.1016/j.pbi.2007.01.013
Motomura, T. (1991). Immunofluorescence microscopy of fertilization and parthenogenesis in Laminaria angustata (Phaeophyta). J. Phycol. 27, 248–257. doi: 10.1111/j.0022-3646.1991.00248.x
Pedersen, C., and Linde-Laursen, I. (1995). The relationship between physical and genetic distances at the Hor1 and Hor2 loci of barley estimated by two-colour fluorescent in situ hybridization. Thero. Appl. Genet. 91, 941–946. doi: 10.1007/BF00223904
Rigby, P. W., Dieckmann, M., Rhodes, C., and Berg, P. (1977). Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J. Mol. Biol. 113, 237–251. doi: 10.1016/0022-2836(77)90052-3
Schreiber, E. (1930). Untersuchungen über parthenogenesis, geschlechtsbestimmung und bastardierungsvermögen bei laminarien. Planta 12, 331–353. doi: 10.1007/BF01948810
Schweizer, D. (1976). Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma 58, 307–324. doi: 10.1007/BF00292840
Shao, Z., Wang, W., Zhang, P., Yao, J., Wang, F., and Duan, D. (2019). Genome-wide identification of genes involved in carbon fixation in Saccharina japonica and responses of putative C4-related genes to bicarbonate concentration and light intensity. Plant Physiol. Biochem. 137, 75–83. doi: 10.1016/j.plaphy.2019.01.032
Stanke, M., and Waack, S. (2003). Gene prediction with a hidden Markov model and a new intron submodel. Bioinformatics 19, ii215–25. doi: 10.1093/bioinformatics/btg1080
Starr, R. C., and Zeikus, J. A. (1993). UTEX—The culture collection of algae at the University of Texas at Austin 1993 list of cultures. J. Phycol. 29, 1–106. doi: 10.1111/j.0022-3646.1993.00001.x
Tai, S. H., and Fang, T. C. (1977). The chromosomes of Laminaria japonica Aresch. Acta Genet. Sin. 4, 325–328.
Umen, J., and Coelho, S. (2019). Algal sex determination and the evolution of anisogamy. Annu. Rev. Microbiol. 73, 267–291. doi: 10.1146/annurev-micro-020518-120011
Voorrips, R. E. (2002). MapChart: software for the graphical presentation of linkage maps and QTLs. J. Heredity 93, 77–78. doi: 10.1093/jhered/93.1.77
Wu, C. Y., and Suo, R. Y. (1962). “Morphology, reproduction and life history of Laminaria,” in Manual of Haidai (Laminaria japonica Aresch.) Cultivation, eds C. K. Tseng and C. Y. Wu (Beijing: Science Press), 14–33.
Yabu, H., and Yasui, H. (1991). Chromosome number in four species of Laminaria (Phaeophyta). Jpn. J. Phycol. 39, 185–187.
Yang, Q.-F., Liu, L., Bi, Y.-H., and Zhou, Z.-G. (2017). Telomeric localization of the Arabidopsis-type heptamer repeat, (TTTAGGG)n, at the chromosome ends in Saccharina japonica (Phaeophyta). J. Phycol. 53, 235–240. doi: 10.1111/jpy.12497
Yasui, H. (1992). Chromosome numbers and a sex chromosome of Laminaria yendoana Miyabe (Phaeophyta). Nippon Suisan Gakkaishi 58:1385. doi: 10.2331/suisan.58.1385
Yasukochi, Y. (2002). PCR-based screening for bacterial artificial chromosome libraries. Methods Mol. Biol. 192, 401–410. doi: 10.1385/1-59259-177-9:401
Ye, N., Zhang, X., Miao, M., Fan, X., Zheng, Y., Xu, D., et al. (2015). Saccharina genomes provide novel insight into kelp biology. Nat. Commun. 6:6986. doi: 10.1038/ncomms7986
Ye, R.-X., Yu, Z., Shi, W.-W., Gao, H.-J., Bi, Y.-H., and Zhou, Z.-G. (2014). Characterization of α-type carbonic anhydrase (CA) gene and subcellula localization of α-CA in the gametophytes of Saccharina japonica. J. Appl. Phycol. 26, 881–890. doi: 10.1007/s10811-013-0221-2
Zhang, J., Li, Y., Luo, S., Cao, M., Zhang, L., and Li, X. (2021). Differential gene expression patterns during gametophyte development provide insights into sex differentiation in the dioicous kelp Saccharina japonica. BMC Plant Biol. 21:335. doi: 10.1186/s12870-021-03117-z
Zhang, L., Cui, C., Li, Y., Wu, H., and Li, X. (2018). A genome screen for the development of sex-specific DNA markers in Saccharina japonica. J. Appl. Phycol. 30, 1239–1246. doi: 10.1007/s10811-017-1295-z
Zhang, L., Li, J., Wu, H., and Li, Y. (2019). Isolation and expression analysis of a candidate gametophyte sex determination gene (SjHMG) of kelp (Saccharina japonica). J. Phycol. 55, 343–351. doi: 10.1111/jpy.12821
Keywords: bacterial artificial chromosome (BAC), fluorescence in situ hybridization (FISH), gametophyte, kelp, Saccharina japonica, sex-determining region (SDR), sex-linked markers, sporophyte
Citation: Du Y, Liu P-F, Li Z, Zheng Q, Bi Y-H and Zhou Z-G (2022) Discerning the Putative U and V Chromosomes of Saccharina japonica (Phaeophyta) by Cytogenetic Mapping of Sex-Linked Molecular Markers. Front. Mar. Sci. 9:821603. doi: 10.3389/fmars.2022.821603
Received: 24 November 2021; Accepted: 31 January 2022;
Published: 18 February 2022.
Edited by:
Andrew Stanley Mount, Clemson University, United StatesReviewed by:
Daniel Garcia-Souto, University of Vigo, SpainGuanpin Yang, Ocean University of China, China
Copyright © 2022 Du, Liu, Li, Zheng, Bi and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Gang Zhou, emd6aG91QHNob3UuZWR1LmNu
†These authors have contributed equally to this work
 Yu Du
Yu Du Peng-Fei Liu
Peng-Fei Liu Zhi Li
Zhi Li Qian Zheng
Qian Zheng Yan-Hui Bi
Yan-Hui Bi Zhi-Gang Zhou
Zhi-Gang Zhou