
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 15 February 2022
Sec. Marine Megafauna
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.809902
This article is part of the Research Topic Ecological and Behavioral Traits of Apex Predators in Oceanic Insular Ecosystems: Advances and Challenges in Research and Conservation View all 14 articles
 Anja Badenas1†
Anja Badenas1† Ana Dinis1,2
Ana Dinis1,2 Rita Ferreira1,2,3
Rita Ferreira1,2,3 Annalisa Sambolino1,3
Annalisa Sambolino1,3 Eliette Hamard2,4
Eliette Hamard2,4 Leonardo G. Berninsone5
Leonardo G. Berninsone5 Marc Fernandez1,6
Marc Fernandez1,6 Filipe Alves1,2*†
Filipe Alves1,2*†
Knowledge of the role of individual associations has provided an insightful understanding of the structures of animal societies, especially in highly social mammals such as primates. Yet, this is unbalanced towards marine mammals, particularly to beaked whales, due to their elusive nature. In addition, information on the fundamental drivers of the social structure of these deep-diving animals is still scarce. Here, the hypothesis of female defence polygyny was tested in Blainville’s beaked whales (Mesoplodon densirostris) and discussed within the context of marine and terrestrial organisms displaying similar patterns, by (i) estimating residency times to obtain information on the movements into and out of the area, (ii) analysing social networks to assess individual association metrics, (iii) measuring the strength of the associations to assess the existence of preferred or avoided relationships among individuals, and (iv) modelling different social structures to address temporal patterns in social relationships. Using a 9-year photographic dataset derived from the pelagic habitat, individual associations were inferred based on likelihood techniques. This approach allowed to infer on the species’ social structure in relation to age class, sex, residency status, and spatio-temporal patterns, which can be a good practice to be applied for other taxa. Heterogeneity in capture probability and residency times was observed between age-sex classes, with adult females exhibiting long-term site fidelity. This suggests different habitat roles and spatial structuring within this social organisation. Strong and long dyadic associations occurred between adult females and immatures, contrarily to between males, and the best-fitting models of the temporal patterns suggested long-lasting and temporary associations. The present findings unravel a complex social structure stratified by age-sex class and influenced by female philopatry and defence polygyny, like an unimale group mating system, which varies from other beaked whales but is similar to some birds, pinnipeds, or non-human primates.
Movement patterns and habitat composition are known to influence complex animal social structures (He et al., 2019). Animal movements are generally driven by resource availability, mating accessibility, predator avoidance and habitat complexity (e.g., Bailey and Thompson, 2006; Duijns et al., 2019), which in turn modulate social behaviour, group composition and population dynamics at a broader scale (Morales et al., 2010). The resulting social structure plays a pivotal role in the population’s ecology since it influences the spread of diseases and genetic flow (Sutherland, 1998). Therefore, social structure is considered a determining factor in conservation management plans (Wilson, 1975).
To study a population’s social structure, data on interactions between known individuals through time are needed (Hinde, 1976; Whitehead, 2008), which can sometimes be challenging to witness. Once associations are established, various tools exist to describe and model social structure (reviewed in Farine and Whitehead, 2015), which has provided a profound understanding of animal associations and their socio-ecological processes in highly social terrestrial mammals (e.g., Schreier and Swedell, 2009; Foerster et al., 2015; Berger et al., 2021). However, little attention had been given to the societies of species whose meaningful relationships are difficult to assess, such as cetaceans (whales and dolphins) (Lusseau et al., 2006; Rendell et al., 2019). Cetaceans associate with conspecifics (and occasionally interspecifically) when survival, access to resources and reproductive success are increased by group formation (Gowans et al., 2007). Nonetheless, the longevity and strength of those associations, and hence social structures, are modulated by the individuals’ residency patterns and habitat selection, which differ among and within species. As a result, cetacean’s social structures range from highly fluid, so-called fission-fusion societies, where individuals associate in groups that change in composition and size daily (e.g., bottlenose dolphin Tursiops truncatus, Connor et al., 2000), to permanent associated matrilineal groups (e.g., killer whales Orcinus orca, Baird and Whitehead, 2000).
Nevertheless, most information on cetacean social systems is from common and accessible species (i.e., abundant, inhabiting coastal waters, or easily approachable), thus exists a gap in the knowledge of the societal patterns of lesser studied Families such as beaked whales (Li and Rosso, 2021; Weiss et al., 2021). This is mainly because beaked whales inhabit pelagic and difficult-to-access environments and because of their short surfacing periods and cryptic behaviour (Tyack et al., 2006). Although research on beaked whales has increased in the last two decades (reviewed in Hooker et al., 2019) due to their high vulnerability to anthropogenic noise and specifically to navy sonar (Tyack et al., 2011), studies on these animals’ social structures cover only four species (out of at least 24 extant species, Carroll et al., 2021) and are still on their early stages (Baird, 2019; Weiss et al., 2021). In Blainville’s beaked whales (Mesoplodon densirostris), resident populations of this medium-sized (4–5 m long) deep-diver have only been described in a few remote archipelagos (Hooker et al., 2019) and only one peer-reviewed study, wherever known, has provided insights on its social structure (McSweeney et al., 2007). That study suggested ephemeral relationships and female defence polygyny (characterised when males control females directly by defending them against conspecifics males; Shuster and Wade, 2003), yet it was based on descriptive analyses.
Here, photographic data of Blainville’s beaked whales from an oceanic pelagic habitat was used to analyse individual associations and consequently to serve as a model system to infer the social structure in relation to age class, sex, residency status, and spatio-temporal patterns. To better understand the species’ socio-behavioural traits and test the hypothesis of female defence polygyny, likelihood techniques were used to (i) estimate residency times, thus providing information on the movements into and out of the area, (ii) analyse the social network to assess individual association metrics, (iii) measure the strength of the associations to assess the existence of preferred or avoided relationships among individuals, and (iv) model different social structures to address temporal patterns in social relationships. Such an integrative approach is expected to contribute to a more comprehensive view of the species’ social structure and enlighten the mysterious life habits of elusive and lesser-known animals. Finally, it is expected to advance the theoretical understanding of a specific social strategy related to philopatry, mating, and parental care in free-ranging animals, given that cetaceans provide a powerful outgroup for inferring the evolution of the social structure of other highly related mammals (Pearson, 2011).
Photographic data of Blainville’s beaked whales were collected in the southern waters of Madeira Island (Portugal, 32°N 017°W), in a core area covering about 1,000 km2 up to 15 km off the coast (see Fernandez et al., 2021). The study area is surrounded by warm-temperate Atlantic waters and is characterised by a narrow continental shelf and steep submarine canyons (Geldmacher et al., 2000; Martins et al., 2007). These characteristics offer a privileged access to the pelagic environment where the target species commonly occurs throughout the year (Alves et al., 2018; Fernandez et al., 2021) and where some individuals are likely island-associated (Dinis et al., 2017). The data were collected year-round through whale-watching platforms (see Acknowledgments; procedures detailed in Alves et al., 2018) and in research trips, between 2011 and 2019 (Table 1 and Supplementary Table 1). On each occasion with Blainville’s beaked whales, animals were approached and photographed using digital reflex cameras with zoom lenses, independently of age class, sex, and distinctiveness.
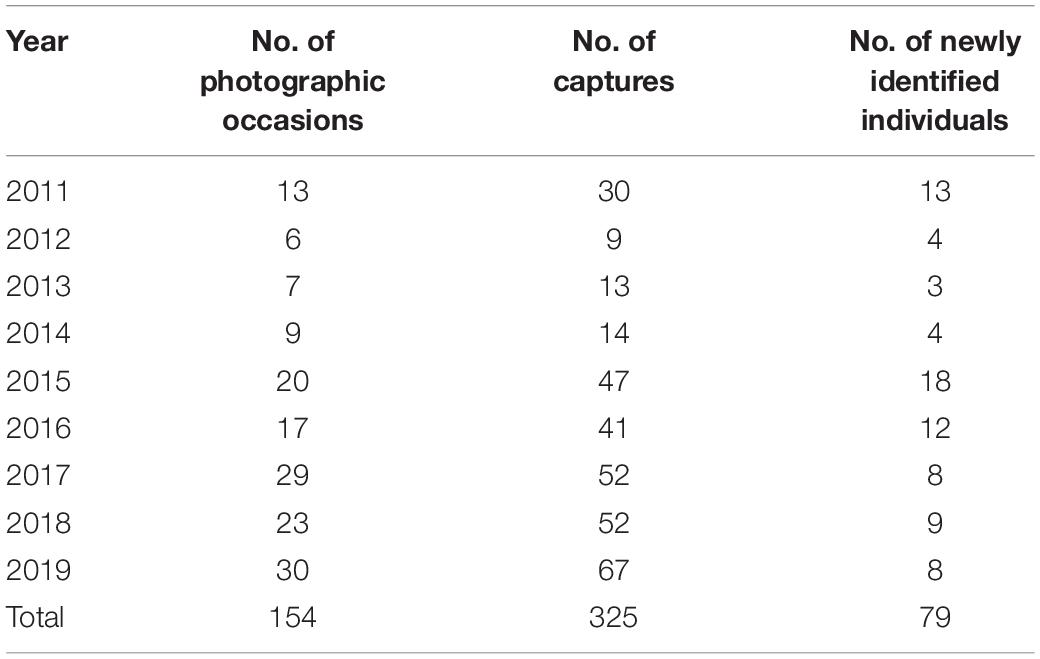
Table 1. Summary of the photographic data used in the analysis, i.e., truncated to distinctive animals and good quality pictures.
A previous photographic-identification (hereafter “photo-id”) catalogue and dataset of all animals’ capture histories from 2011 to 2016 (Dinis et al., 2017) was updated with the newly collected data following standardised procedures (Würsig and Jefferson, 1990). Individuals were identified using unique scarring patterns on the body as well as nicks on dorsal fins (McSweeney et al., 2007; Dinis et al., 2017). The distinctiveness of each whale was rated from 1 (poorly distinctive) to 4 (very distinctive) following McSweeney et al. (2007), and the photographs were assigned a quality grade ranging from 1 to 4 (low- to high-quality) based on Alves et al. (2013). Only good quality pictures (grade 3 or 4) and individuals with distinctiveness 2–4 (slightly to very distinctive) were used in the analyses to enhance the reliability of the data. A capture was defined as an individual identification from a photographic occasion (Würsig and Jefferson, 1990).
Three catalogues were compiled: one for females, one for males, and one for immature whales of unknown sex. Sex and age class (immature, subadult male, adult male, or adult female) was determined based on body size and colour, body scarring, association with calves, and the presence or absence of erupted teeth in the lower jaws (Claridge, 2006; McSweeney et al., 2007; Dinis et al., 2017). Photographic comparisons were performed visually (Robbins et al., 2011; Alves et al., 2019), and at least two co-authors confirmed individual matches, sex, and age class.
Additionally, a residency pattern was attributed to each individual. Three residency patterns were established based on the capture histories. Individuals that exhibited multi-year and year-round site fidelity (captured in at least 4 years and three seasons, i.e., January–March, April–June, July–September, October-December) were termed residents; individuals captured only once or a few times within a week and never captured again were termed transients; and individuals that fell between these two categories were considered emigrants/immigrants or regular visitors and were consequently termed visitors (adapted from Alves et al., 2013). Two exceptions were made for whales Md99 and Md119 that were only seen during 3 (consecutive) years but during the four seasons and always with resident whales, and therefore were termed residents.
A discovery curve was plotted using the capture histories, and the recapture rates (RRs) per age class were calculated to assess site fidelity. The discovery curve was created by plotting the cumulative number of identified individuals against the cumulative number of captures. The RRs were calculated by dividing the number of individuals with ≥2 captures by the total number of individuals. Recaptures within the same day were excluded.
The amount of time animals spent within the study area was examined using Lagged Identification Rates (LIR). The LIR is the probability that an individual observed in the area at a given time will still be present τ time-lags later (Whitehead, 2001). Given the difference between the capture histories of the age classes and the fact that immatures were almost always seen with an adult female, two different LIR were performed: one for all males and one for females and immatures. All individuals captured between 2015 and 2019 (years with the higher number of captures, Table 1), independently of the number of captures, were included in the analysis. Four models were fitted to each dataset using maximum likelihood, binomial loss, and bootstrapped standard error (SE), following Whitehead (2009). The model with the lowest Quasi Akaike Information Criteria (QAIC) was selected as the best-fitting model (Whitehead, 2008, 2009). The sampling period was defined as day, and associations were defined as individuals grouped within an occasion. LIR was conducted using SOCPROG version 2.9 (Whitehead, 2009).
A social network diagram was created using NetDraw 2.158 (Borgatti, 2002) to illustrate the species social structure (Kappeler, 2019). All occasions between 2011 and 2019 were considered, excluding only those resulting in single captures as they did not provide any linking information (and such cases likely represented incomplete sampling effort in photographing the entire group instead of solitary animals, given these were never recorded during the research trips). Residency pattern and age-sex class were included as individual attributes. Nodes correspond to individuals, while lines between nodes represent the strength of association among dyads, with thicker lines indicating stronger associations.
Two network metrics (strength and clustering coefficient) were obtained using SOCPROG’s network statistics (Whitehead, 2009). The strength is the sum of the weights of all links of a given node, and can be used as a measure of individual social centrality (Barrat et al., 2004). The clustering coefficient measures whether the associates of an individual are themselves associated (Barrat et al., 2004; Lusseau, 2007). Significant differences in the network measures among attributes of age/sex and residency patterns were tested with a double permutation test (Farine and Carter, 2022) using the aspine R package (Farine, 2013).
To reduce the chance of including spurious associations, only whales with ≥3 captures from occasions with medium- (where at least half of the group was photographed) and high-coverage (where essentially all individuals were photographed) between 2011 and 2019 were included in this analysis. Because of the opportunistic nature of data, the photographic coverage of individuals during sightings was unknown. To overcome this issue, 50 high-quality photographs, including at least three whales in the frame, were selected to calculate the percentage of marked individuals in a group and infer photographic coverage. The rate of distinctive individuals was obtained by dividing the number of marked individuals by the total individuals captured in the frame for each photograph and calculating an overall mean for all pictures. This resulted in 71% (±19%) of the individuals in a group being marked. Based on this and on the Blainville’s beaked whales mean group size of 3.7 (SD = 1.7) individuals in the study area (Alves et al., 2018; which is similar to other regions, see Ritter and Brederlau, 1999; Claridge, 2006; McSweeney et al., 2007), sightings with ≥2 captures were considered to be of medium- and high-coverage, and were therefore used in the subsequent analyses.
The half-weight association index (HWI) (Cairns and Schwager, 1987) was used to represent the strength of the association between beaked whale dyads (following Whitehead, 2008; Hoppitt and Farine, 2018), where “0” indicated that individuals were never captured together and “1” that individuals were always together. The mean and the maximum of associations were also calculated.
Permutation tests were used to assess whether preferred or avoided relationships among individuals and among age classes existed (Bejder et al., 1998; Whitehead, 1999). The null hypothesis was that individuals were associated with the same probability with other individuals. Observed coefficients of variation (CV) of the pairwise association indices significantly higher than those from permuted datasets were taken as evidence that individuals had preferred companions (Whitehead, 1999; Whitehead et al., 2005). The number of permutations generated was increased until the p-values stabilised (Bejder et al., 1998; Whitehead, 1999), at 1,000 trial flips per permutation. Based on the LIR results, three different sampling periods were used to assess associations between individuals and among age classes (adapted from Gero et al., 2015): (1) “hour,” hourly sampling period to test for short-term associations, (2) “month,” a monthly sampling period to test for medium-term associations, and (3) “year,” yearly sampling period to test for long-term associations. This procedure removes any existing autocorrelation between groups that have been sighted together for short periods (hours or days) (personal communication, H. Whitehead, 2020). The association was defined as individuals grouped within an occasion for all three sampling periods, and associations were permuted between sampling periods.
Standardised lagged association rates (SLAR) were used to address temporal patterns in social relationships (Whitehead, 1995). SLAR assessed the probability that two associated individuals at a given time would still be associated at a certain time-lag in the future. To aid in the interpretation of SLAR, the null association rate was also considered (Whitehead, 2009). The moving average was chosen to adjust best between precision and smoothing, and SE was estimated using the temporal jackknife method on each sampling period (Whitehead, 2009). Four exponential models that represented simulated social structures were fitted to the SLAR: the first model had no decay and suggests permanent associations; the second model had a decay down to zero and suggests that associations decrease until complete disassociation; the third model had a decay that levelled off and suggests long-lasting and temporary associations; and the fourth model had two decays and suggests two levels of disassociation, one at shorter and one at longer time lags (Whitehead, 2008). The best fitting model was chosen through the lowest QAIC (Whitehead, 2008, 2009). Since the patterning of all associations is important, data from 2011 to 2019 including all individuals, independently of the number of times captured, during medium- and high-coverage events were used for two SLARs: one for all individuals and other for adult female associations. The sampling period was defined as day, and associations were defined as individuals grouped within an occasion. Preferred association and temporal pattern analyses were performed using SOCPROG 2.9 (Whitehead, 2009).
A total of 325 captures based on good quality pictures were obtained from 154 photographic occasions, allowing the identification of 79 distinctive animals (Table 1). There was a mean of 2.1 captures (SD = 1.1, range 1–5) per occasion, and of the total catalogued whales, 29 were adult females, 19 were adult males, 12 were subadult males, and 19 were immature (Table 2).
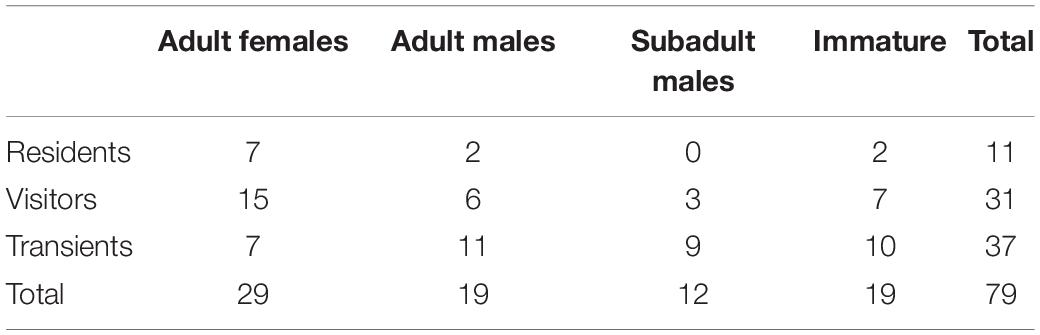
Table 2. Frequency table of age-sex class per residency patterns of Blainville’s beaked whales in Madeira.
Sixty-six percent of the animals (n = 52) were captured on multiple occasions (mean = 4 captures, range 2–31). Fifty-seven percent of the animals (n = 45) were captured intra-annually and the remaining were captured inter-annually [of which 53% (n = 18) were adult females, 21% (n = 7) adult males, 9% (n = 3) subadult males, and 18% (n = 6) immatures]. Inter-annual recaptures ranged from 2 to 8 years, but only adult females were recaptured in >5 years (Supplementary Table 1 and Supplementary Figure 1).
Residents comprised 14% of the animals (n = 11), visitors 39% (n = 31), and transients 47% (n = 37). Adult females were the dominant age-sex class of residents (64%, 7/11) and visitors (48%, 15/31), whereas adult and subadult males and immatures were mainly transients (Table 2).
The overall RR was 0.66. Adult females presented the highest value (RR = 0.86), followed by immature individuals (RR = 0.63) and adult males (RR = 0.58), while subadult males had the lowest (RR = 0.42). The discovery curve showed a slight decrease between 200 and 300 cumulative captures, and thereafter the curve begins to stabilise (Figure 1).
The best-fitting LIR model for the adult females and immatures, and for the adult and subadult males, was the “Emigration + Reimmigration + Mortality” (Table 3). The model indicates that 11 adult females and immatures (SE = 2) spent an average of 214 days (SE = 99) in the area before leaving to return after 158 days (SE = 448) with a mortality rate of < 0.001 (Figure 2A). It also estimated that 1 male (SE = 0.94) spends an average of 1 day (SE = 21) before leaving to return to the study area 14 days later (SE = 44) with a mortality rate of <0.001 (Figure 2B).
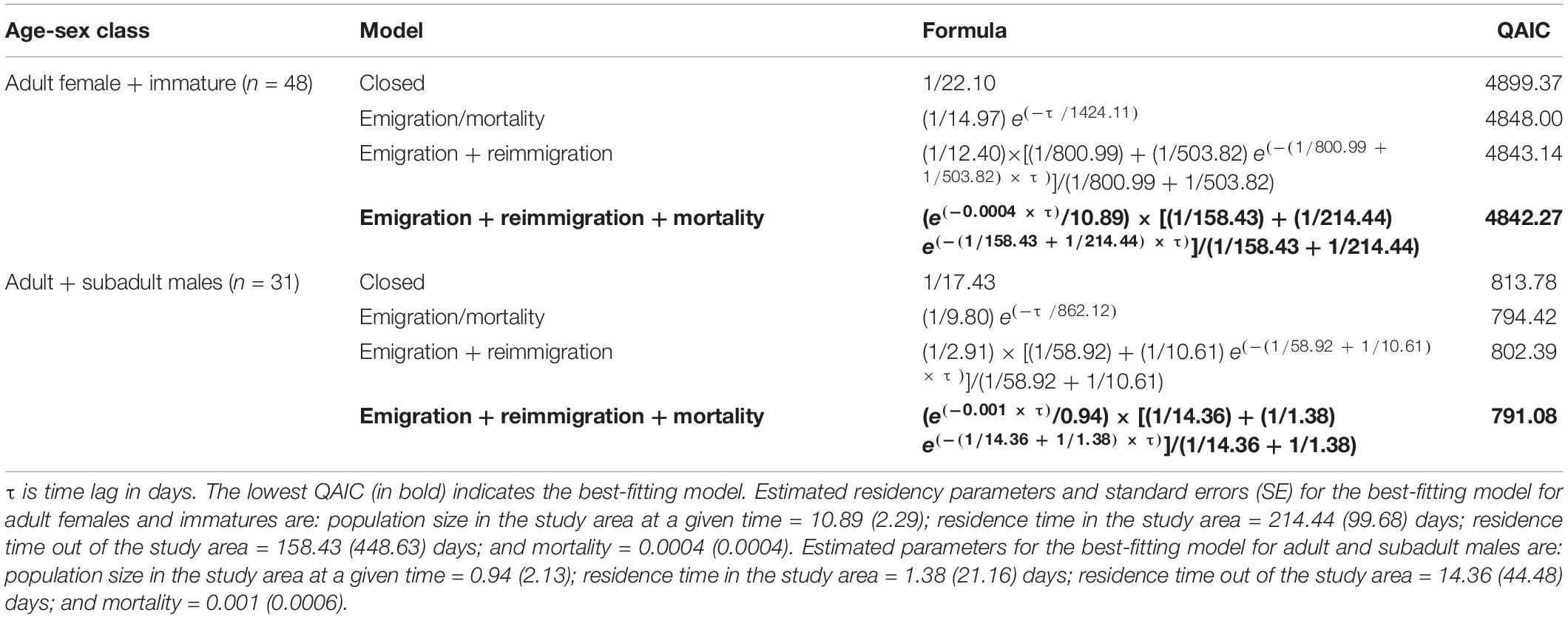
Table 3. Models fitted to Lagged Identification Rates (LIR) for adult females and immatures, and for adult and subadult males of Blainville’s beaked whales captured between 2015 and 2019.
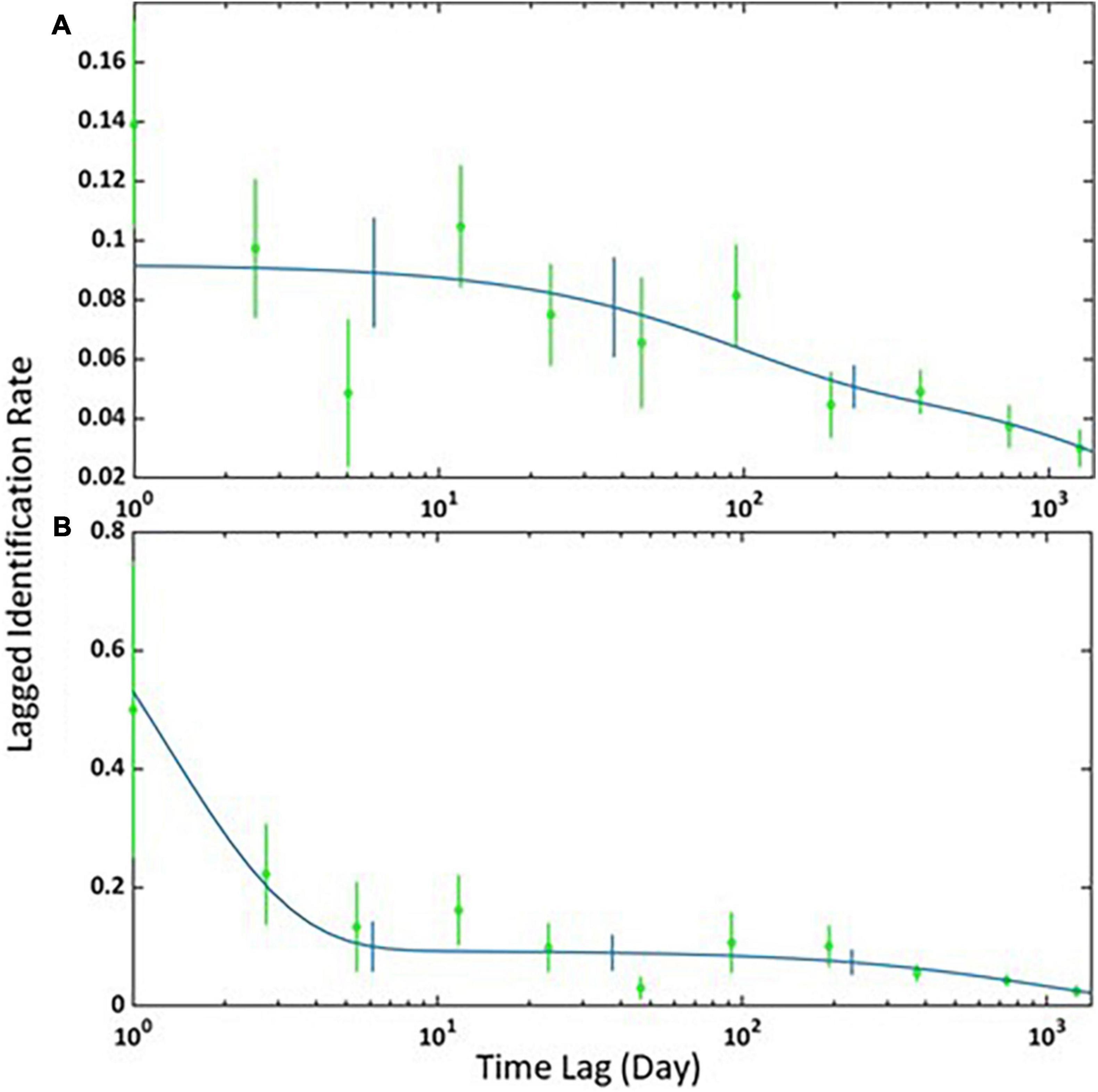
Figure 2. Lagged Identification Rates (LIR) for (A) adult females and immatures and (B) adult and subadult males in Madeira between 2015 and 2019. The graph shows the probability that an individual Blainville’s beaked whale captured at an initial time “0” will be captured again at x time later in the study area. Blue lines represent the best-fitting model according to Table 3, green circles represent the data, and vertical bars indicate SE calculated using a bootstrap method on each sampling period.
The social network analyses comprised 70 individuals from 97 photographic occasions with a total of 267 captures, of which 28 were adult females, 18 adult males, 9 subadult males, and 15 immature individuals. The network diagram shows that most individuals (92%; n = 64) are linked by association in the main social core, while the remaining six individuals (8%) form satellite dyads (Figure 3). The main cluster includes all the resident individuals, 29 visitors (93%) and 24 transients (86%). In contrast, the remaining three satellite clusters only include transients and visitors (two visitor-transient dyads and one transient-transient dyad).
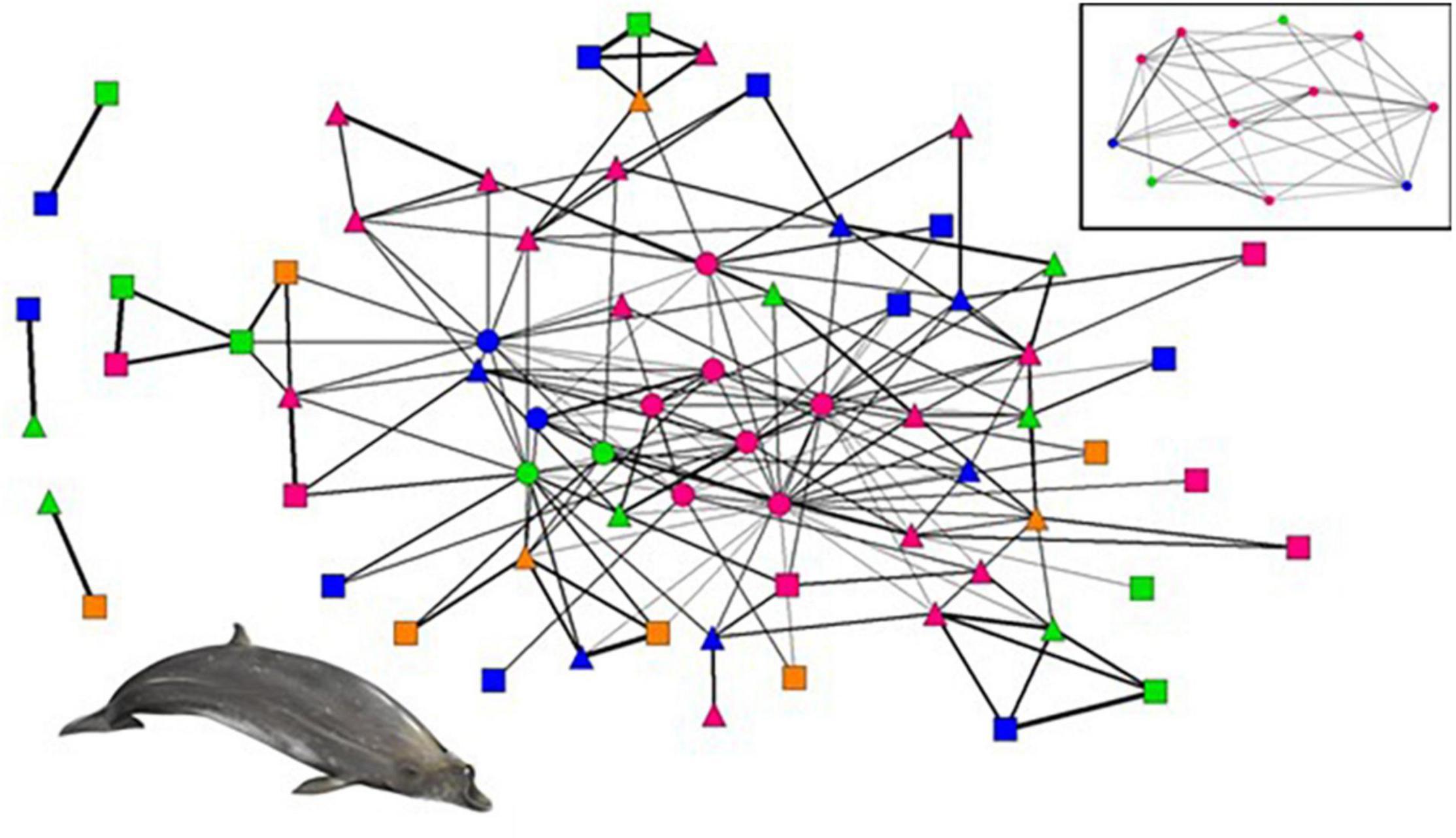
Figure 3. Network diagram of Blainville’s beaked whales. Nodes represent individuals and are coloured and shaped for age-sex class and residency attributes: pink- adult female, blue- adult male, orange- subadult male, green- immature; circle- resident, triangle- visitor, and square- transient. The thickness of the lines between nodes represents the strength of association among dyads, where thicker lines correspond to stronger associations. The embedded network at the top right is the network of the resident whales only. Illustration© ARDITI/E. Berninsone.
Network measures varied greatly between residency patterns but were similar between age classes and sexes (Figure 4 and Supplementary Table 2). The double permutation test showed no significant differences in the strength and clustering coefficient when testing the influence of age-sex classes and residency patterns.
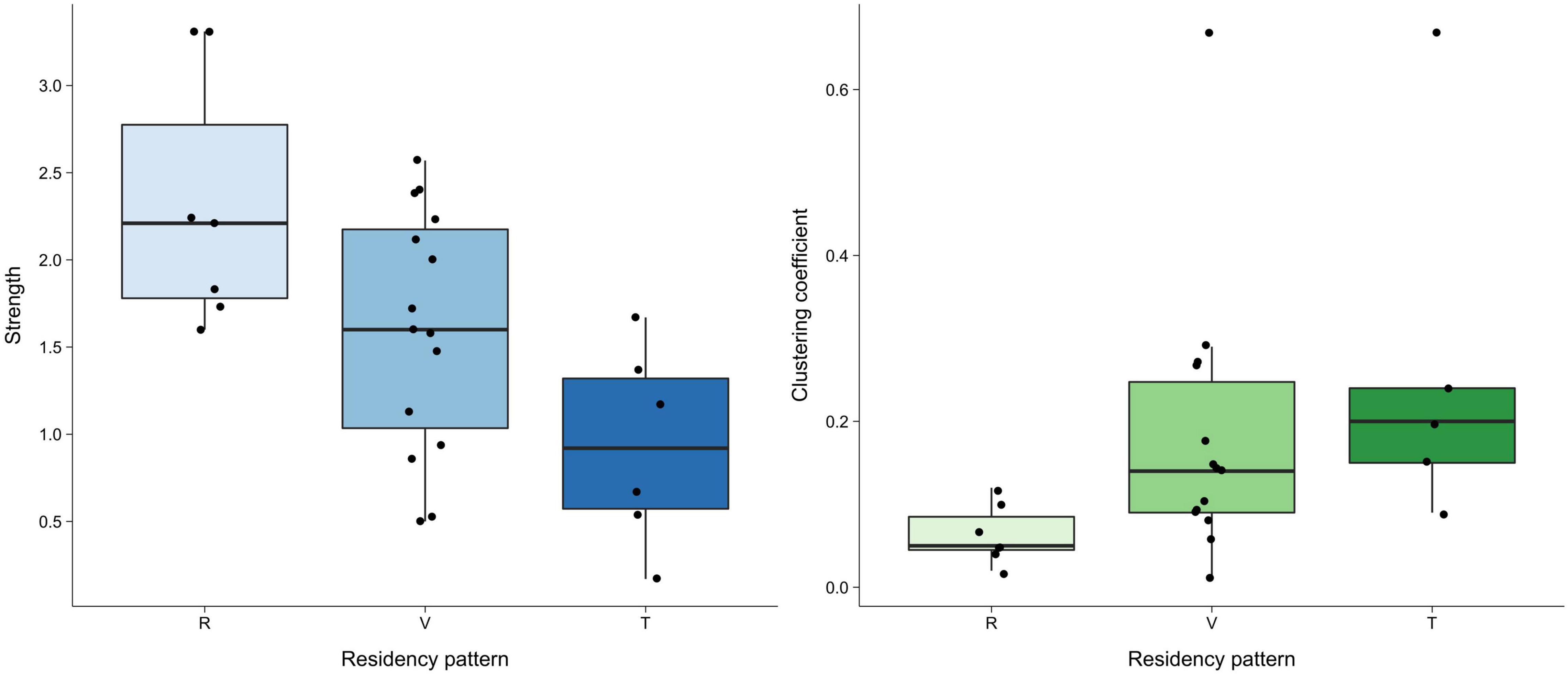
Figure 4. Boxplot for strength and clustering coefficient for each residency pattern (R–resident, V–visitor, T–transient) in adult females (the most representative group). The horizontal line in the box represents the median, the bottom and top of the boxes represent the first and third quartiles and whiskers show values within 1.5 times of the interquartile range from the boxes. Raw data are plotted as single points. A double permutation test showed no significant differences on the influence of residency patterns in the social network structure.
The mean association indices were low (HWI = 0.04 ± 0.02), but the maximums were high (Supplementary Figure 2). The highest maximum association indices were found between immature whales and adult females (0.51 ± 0.13), followed by adult males with adult females (0.34 ± 0.12), and within adult females (0.25 ± 0.13). The lowest mean and maximum association indices were found for all combinations of subadult males and adult males’ dyads (HWI = 0 for subadult-subadult and male-male dyads, HWI = 0.01 ± 0.01 for subadult male—adult male dyads). The sum of association indices indicated that the associates per individual ranged from 1.3 to 4 whales (mean = 2.48 ± 0.59, Supplementary Figure 3).
Permutation tests within and between adult males, subadult males, and immature whales, could not be permuted, for which the null hypothesis that individuals associate randomly could not be rejected. However, preferred associations within adult females and between adult females and the other age-sex classes varied for short-, medium-, and long-term sampling periods (Table 4). The observed CV was significantly higher than the random CV for the dataset, including all the individuals for short-, medium-, and long-term periods. Adult females had short- and medium-term preferred associations within their category and between all age-sex classes (p < 0.05) except for subadult males with whom they associated randomly. The significant monthly associations between adult females and adult males revealed that Md119 (adult resident male) had strong associations with Md55 and Md99 (adult resident females, HWI = 0.45 and 0.48 respectively, p < 0.05). Long-term preferred associations occurred within adult females, and between adult females and immatures (p < 0.05), and long-term random associations occurred between adult females and all males independently of age class.
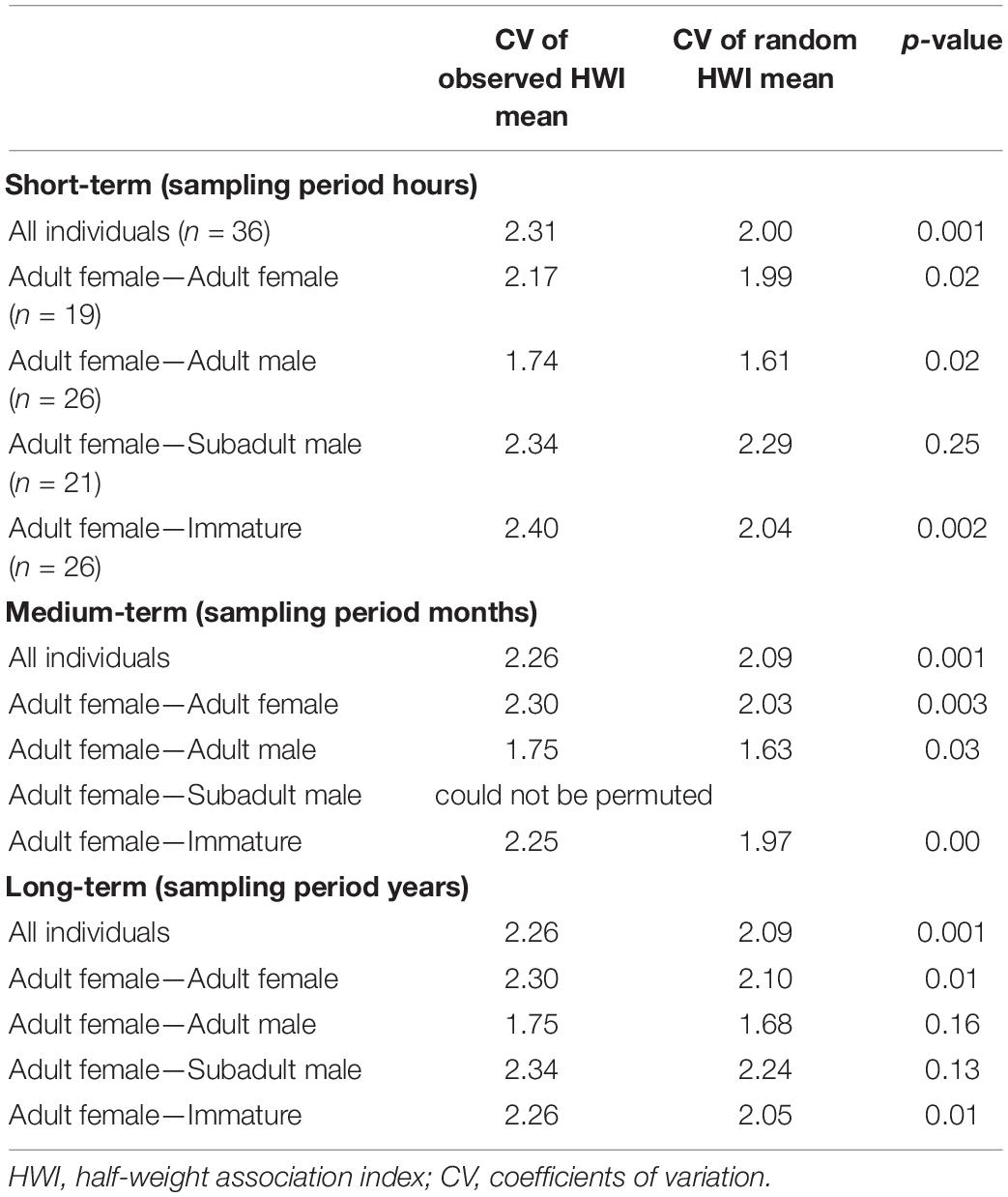
Table 4. Permutation tests for preferred associations within and between age classes and sexes for individuals captured on ≥ 3 occasions for short-, medium-, and long-term between 2011 and 2019.
The third model was the best-fitting model for all individuals and for adult females (Table 5). For all individuals, SLAR was highest for short time lags and started decreasing fast after 100 days, reaching the null association rate in about 1 year (Figure 5A). Adult females showed a similar pattern, except that SLAR constantly declined from the short time lags and that the null association rate was reached later (Figure 5B).
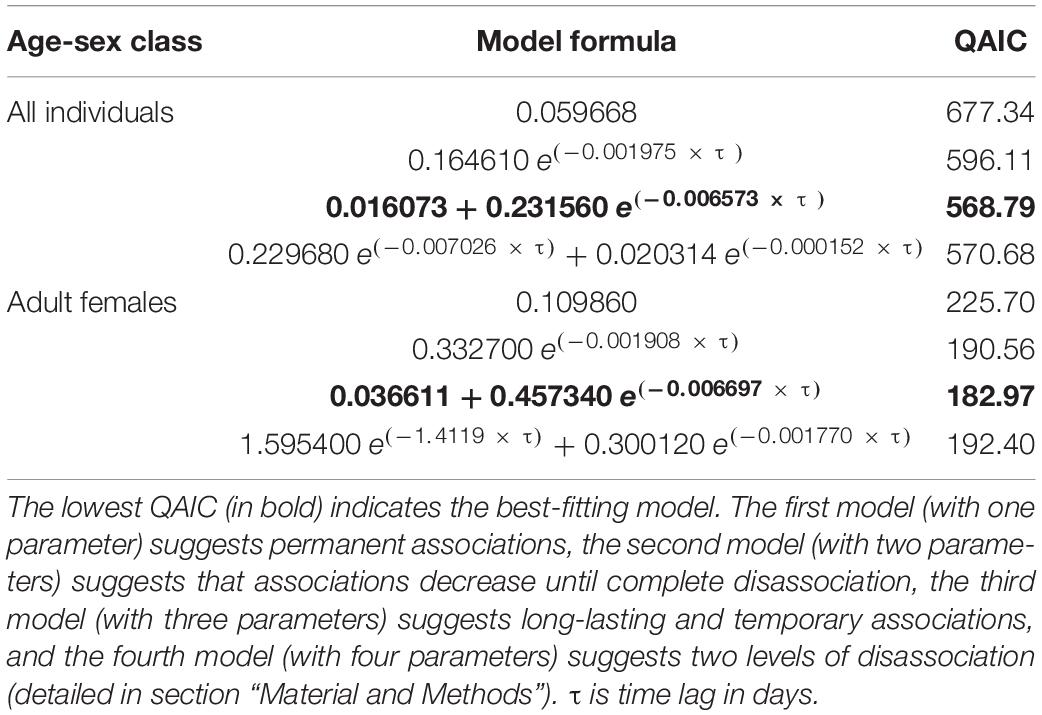
Table 5. Models fitted to the standardised lagged association rate (SLAR) for all individuals and for adult females.
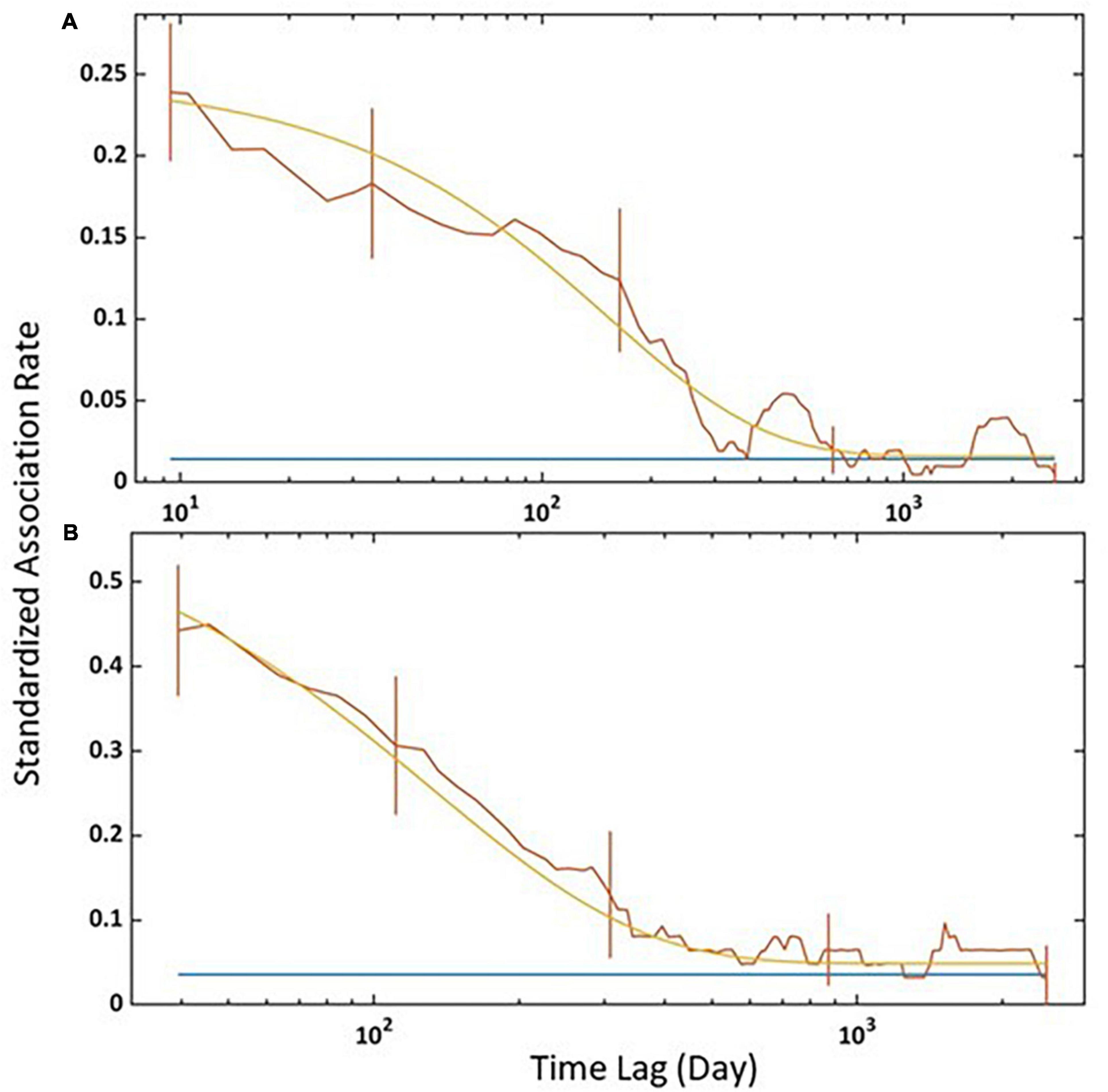
Figure 5. Standardised lagged association rate (SLAR) for (A) all individuals and (B) adult females captured between 2011 and 2019. Vertical bars indicate SE calculated using the temporal jackknife method. The brown line is the observed data, the yellow line is the best-fitting model according to Table 5, and the blue line is the standardised null association rate (i.e., if individuals associated randomly).
This study provides an analysis of the social structure of Blainville’s beaked whales in insular oceanic ecosystems, which are characterised by specific topographic and oceanographic variables that are known to influence predators’ habits, such as cetaceans (Abecassis et al., 2015; Fernandez et al., 2021). The analysis of a longitudinal dataset on individual associations in relationship to age class, sex, residency status, and spatio-temporal patterns showed a social structure modulated by adult females. This agrees with analyses of mammalians’ social complexity where a female-based sociality prevails (Lukas and Clutton-Brock, 2018), in which cetaceans, and especially toothed whales, are no exception (Rendell et al., 2019). The present findings support the hypothesis of female defence polygyny suggested by McSweeney et al. (2007) for a population in Hawaii’, as well as reported in the Bahamas and Canary Islands (Claridge, 2006; Suárez, 2018). In addition, the present findings support information on female philopatry based on higher residency levels by adult females and provide a discussion on this social strategy.
The findings obtained in this study are inferred from a good agreement of the combination of movement and social analyses, from which five broad main results have emerged. First, there was heterogeneity in capture probability, given that 60% of the animals were captured on multiple occasions and most of the inter-annual recaptures (and the longer ones; >5 years) were from adult females. Island-associated animals (i.e., residents and visitors) were also mainly adult females, which presented the highest RR (0.86). The asymptotic discovery curve, observed from approximately > 300 cumulative captures, reveals that the studied island-associated population seems relatively small (likely < 50 animals). This also shows that recruits are less common throughout the years and some adult females exhibit high site fidelity, supporting Dinis et al. (2017).
Second, the best-fitting models from the LIR analysis included emigration and reimmigration with (residual rates of) mortality, which suggests temporary migration into and out of the area and transiency, with mortality within expected values given the duration of the study and the long-living nature of this mammal species. Additionally, it shows heterogeneity in residency times between age-sex classes. A higher number of adult females and immatures spent more time in the area than adult and subadult males, but also left for more extended periods. This reinforces the more constant presence of adult females and immatures in Madeira. The differentiated habitat use by Blainville’s beaked whales of different age classes, sexes and residency patterns has also been described in the Hawaii’, Bahamas and Canaries from photo-id analysis (Claridge, 2006; McSweeney et al., 2007; Suárez, 2018). Moreover, the heterogeneity in residency times between age-sex classes suggests different habitat roles, supporting spatial structuring within the species social organisation, with an indication of female philopatry.
Third, the social network analyses shows that the main cluster includes all the resident individuals and 93% of the visitors, supporting an island-associated population in the study area. This agrees with the previous points and with Dinis et al. (2017). Central individuals can be information carriers in dolphin societies (Lusseau and Newman, 2004), yet there was no significant influence of the age-sex classes and residency patterns in the network measures. This could be related to the sampling area that likely represents a portion of the home range of these animals, especially of the transients, making it difficult to confirm the role that different residency patterns might play in the social network analyses of Blainville’s beaked whales.
Fourth, the tests for preferred associations show strong dyadic associations between adult females and immatures, contrarily to between males, as expected for a long-lived mammal species (Lukas and Clutton-Brock, 2018). This is based on the highest maximum association indices between adult females and immatures and the lowest mean and maximum association indices among all combinations of subadult males and adult males’ dyads. The sum of association indices indicated that the typical number of associates per individual ranged from 1.3 to 4, indicating small group sizes similar to other areas (reviewed in Baird, 2019). Adult females exhibited short- and medium-term preferred associations between all age-sex classes, except for subadult males with whom they associated randomly. The significant monthly associations between adult females and adult males were likely influenced by pairs of dyads composed by a particular adult resident male (Md119) with two adult resident females (Md55 and Md99; based on their capture histories, Supplementary Table 1). In the long-term, preferred associations occurred only within adult females, and between adult females and immatures, supporting the hypothesis of female defence polygyny in this species.
Fifth, the best-fitting models from the analysis of the temporal pattern included a decay that levelled off and suggested long-lasting and temporary associations. The fast decrease of SLAR after 100 days for all individuals, indicated that many associations between individuals did not last longer than 3 months. The null association rate was reached in about 1 year, meaning that individuals associated randomly more often than expected for about a year. The peaks formed at 400 and ≈1,500 days are, most probably, a sampling by-product. For adult females, the null association rate was reached later (approximately 3.5 years). Therefore, adult females associated more often than expected if they associated randomly for periods of at least 3.5 years, although the SLAR stays above the null association rate for more extended periods. Such period could be related to time invested in nursing and/or alloparental care, which is a socioecological strategy commonly displayed by mammals (Greenwood, 1980; Berger et al., 2021), and particularly by deep-diving cetaceans like pilot (Globicephala spp.) or sperm whales (Physeter macrocephalus) (Whitehead, 1996; Augusto et al., 2017). While the obtained SLAR slope for adult females declines faster, their association rates are higher than those from all individuals, indicating that other age-sex classes, such as adult males, subadult males, and immatures, have more ephemeral associations between individuals and hence decreased the values of the association rates. The jackknife precision estimates indicate that associations between adult females last longer than all age classes combined.
As an overview, there were intra- and inter-annual preferred associations between females, but preferred associations with males occurred only intra-annually. In addition, adult females and immatures stayed extended periods in the area when compared to adult and subadult males. It is suggested that Blainville’s beaked whales exhibit a general pattern of one adult male leading a small group of females during a short- to mid-period of time (hours to months) and that females are the ones “controlling” the area (i.e., higher site fidelity) and displaying longer-term associations; thus having a social structure driven by female philopatry and defence polygyny. This agrees with the unimale group mating system described in Clutton-Brock (1989) where the cost-effectiveness of territoriality declines and males are more likely to defend groups of females or to search receptive females, as observed in several terrestrial mammals (Clutton-Brock, 1989) and similar to sperm whales (Rendell et al., 2019). It can therefore be inferred that Blainville’s beaked whales have a social structure stratified by age-sex class, and that they can combine a mix of the sociality found in smaller delphinids where ephemeral relationships usually take place (Gowans et al., 2007) and in mid- to large-sized toothed whales where “matrifocal” or matrilineal systems occur (Rendell et al., 2019). Different or mixed social structures are not uncommon among mammals, such as, for example, the stratified community and the multi-male mating system of Risso’s dolphins (Grampus griseus) (Hartman et al., 2008, 2015), which differs from the fission-fusion and matrilineal society models.
The findings presented here shed light on a single species of the second-most speciose family (Ziphiidae) of cetaceans. Studies on the social structure of beaked whales exist only for four species (17% of all known beaked whale’s species; Carroll et al., 2021), derived from restricted areas (reviewed in Weiss et al., 2021). While for Cuvier’s beaked whales (Ziphius cavirostris), sperm competition seems to play a role in the mating system (Baird, 2019), in northern bottlenose whales (Hyperoodon ampullatus), there are strong associations between males (Gowans et al., 2001), whereas, in Baird’s beaked whales (Berardius bairdii), there are stable associations among more scarred (old and/or male) individuals (Fedutin et al., 2015). Thus, beaked whales’ social structures should not be generalised, given that association patterns, mating structures and societies vary between species (Hooker et al., 2019).
This study also increases our knowledge of the social strategy related to female philopatry and female defence polygyny. Here, both are present, but one does not necessarily imply the other. One and/or the other has been described for birds, pinnipeds, deers, or non-human primates (Greenwood, 1980; Le Boeuf, 1991; Koening et al., 2013; Bose et al., 2017), thus suggesting interspecific flexibility of mating systems and social structure, which could arise from several factors such as more cooperative male resource defence (Koening et al., 2013). The formation of stable associations can be correlated with biological and ecological factors (Morales et al., 2010), with long-term bonds being recorded within age-sex classes and with female-biased kinship organisation being found among larger species (Weiss et al., 2021). Such stable relationships provide certain advantages over sporadic bonds, like allomaternal care in deep-diving species (Rendell et al., 2019) or increased male mating success and herding of females during breeding seasons (Clutton-Brock, 1989; Connor et al., 1992), which could partially explain the described social system of the Blainville’s beaked whales.
Potential biases in the present study, especially those related to heterogeneity in capturability and residency, could be related to using data from platforms of opportunity. Nevertheless, likelihood techniques were used to estimate parameters of movement models (Whitehead, 2001), which allowed dealing with the effort associated with collecting the individual identifications that had been neither randomly nor systematically distributed in space-time. In addition, the fact that the data were collected year-round over a relatively long period, and that it was restricted to good quality pictures and distinctive individuals, helped minimising biases. Moreover, the HWI was selected to represent the strength of the behavioural relationships between dyads since it is potentially less biased and recommended to be used when not all individuals have been identified in a sampling period (Whitehead, 2008), as in this case. Although other indices such as the Simple Ratio (Ginsberg and Young, 1992) could also be a good candidate (following Hoppitt and Farine, 2018), the inferences drawn from that index (not shown) were similar to the HWI and therefore the latter was preferred based on Whitehead (2008), even being aware that it does not fully correct biases. Another issue is that, when there is a difference in QAIC by less than two, the second best-fitting model should not be disregarded since it still offers substantial support (Burnham and Anderson, 2002). In this study, there was one case in the LIR and another in the SLAR analyses. In the former case, the only difference with the best model was that the second-best did not include mortality. Given that it makes sense to consider mortality in a real-life scenario, the model with the lowest QAIC was in accordance with being the best one. In the latter case, the third model was the best-fitting and the fourth model was the second best. Again, the best-fitting suggests being the most logical because permanent association (or preferred companions) occurred to some extent (i.e., long-term associations among adult females) in the targeted population. Finally, analyses on the social structure of animals that spend most of its time submerged, such as beaked whales (Tyack et al., 2006), should be inferred with caution. However, we believe that the findings obtained from surface data should reflect, in a general way, the species social system.
To further assess the socioecology of deep-diving species within an evolutionary approach, future studies should combine photo-id analyses with genomics and/or biotelemetry (e.g., Aguilar de Soto et al., 2012; Abecassis et al., 2015; Visser et al., 2021). Although challenging, targeting several individuals of the same group would help clarify these elusive species’ matrilineal kinship. Comprehensive studies, such as the present one, allow incrementing our knowledge on the social behaviour of beaked whales and identifying resident populations. This information is necessary to better understand, manage and protect such cryptic species from increasing anthropogenic disturbances for which they are vulnerable.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the animal study because we used photographic-identification data that are based on photographs of wild animals (i.e., a non-invasive method).
FA and AD conceived the study and validated the dataset (matches). AB, AD, RF, AS, LB, and FA compiled the photographic data. AB, AD, RF, and EH prepared the dataset. AB and MF performed the analyses. FA contributed to its interpretation. AB and FA led the writing of the manuscript. All authors contributed with data, made significant contributions to the drafts, and approved it for publication.
The Oceanic Observatory of Madeira and the Portuguese Foundation for Science and Technology (FCT) throughout the strategic projects M1420-01-0142-FEDER-000001 and UIDB/04292/2020, respectively. AB was supported by a MARE grant throughout the project UIDB/04292/2020, AD by ARDITI throughout the project M1420-09-5369-FSE-000002, RF by the FCT grant SFRH/BD/147225/2019, AS by the FCT grant SFRH/BD/141609/2018, MF by the MAC2/1.1a/385 in the framework of INTERTAGUA (MAC INTERREG 2014-2020), and FA by the FCT project UIDP/04292/2020 granted to MARE.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all researchers, volunteers, and whale-watching operators who contributed with photographic data, especially to Ventura | nature emotions, H2O-Madeira, VMT Madeira, and Lobosonda. We thank Hal Whitehead for valuable inputs in the social analysis. We also thank Ashlie McIvor and both reviewers for valuable comments.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.809902/full#supplementary-material
Abecassis, M., Polovina, J., Baird, R. W., Copeland, A., Drazen, J. C., Domokos, R., et al. (2015). Characterising a foraging hotspot for short-finned pilot whales and Blainville’s beaked whales located off the west side of Hawai‘i Island by using tagging and oceanographic data. PLoS One 10:e0142628. doi: 10.1371/journal.pone.0142628
Aguilar de Soto, N., Madsen, P. T., Tyack, P., Arranz, P., Marrero, J., et al. (2012). No shallow talk: Cryptic strategy in the vocal communication of Blainville’s beaked whales. Mar. Mamm. Sci. 28, E75–E92. doi: 10.1111/j.1748-7692.2011.00495.x
Alves, F., Alessandrini, A., Servidio, A., Mendonça, A. S., Hartman, K. L., Prieto, R., et al. (2019). Complex biogeographical patterns support an ecological connectivity network of a large marine predator in the north-east Atlantic. Divers. Distrib. 25, 269–284. doi: 10.1111/ddi.12848
Alves, F., Ferreira, R., Fernandes, M., Halicka, Z., Dias, L., and Dinis, A. (2018). Analysis of occurrence patterns and biological factors of cetaceans based on long-term and fine-scale data from platforms of opportunity: Madeira Island as a case study. Mar. Ecol. 39:e12499. doi: 10.1111/maec.12499
Alves, F., Quérouil, S., Dinis, A., Nicolau, C., Ribeiro, C., Freitas, L., et al. (2013). Population structure of short-finned pilot whales in the oceanic archipelago of Madeira based on photo-identification and genetic analyses: Implications for conservation. Aquat. Conserv.: Mar. Freshw. Ecosyst. 23, 758–776. doi: 10.1002/aqc.2332
Augusto, J. F., Frasier, T. R., and Whitehead, H. (2017). Characterizing alloparental care in the pilot whale (Globicephala melas) population that summers off Cape Breton, Nova Scotia, Canada. Mar. Mamm. Sci. 33, 440–456. doi: 10.1111/mms.12377
Bailey, H., and Thompson, P. (2006). Quantitative analysis of bottlenose dolphin movement patterns and their relationship with foraging: Movement patterns and foraging. J. Anim. Ecol. 75, 456–465. doi: 10.1111/j.1365-2656.2006.01066.x
Baird, R. W. (2019). “Behavior and ecology of not-so-social odontocetes: Cuvier’s and Blainville’s beaked whales,” in Ethology and Behavioral Ecology of Odontocetes, ed. B. Würsig (Cham: Springer International Publishing), 305–329. doi: 10.1007/978-3-030-16663-2_14
Baird, R. W., and Whitehead, H. (2000). Social organisation of mammal-eating killer whales: group stability and dispersal patterns. Can. J. Zool. 78, 2096–2105. doi: 10.1139/z00-155
Barrat, A., Barthelemy, M., Pastor-Satorras, R., and Vespignani, A. (2004). The architecture of complex weighted networks. Proc. Natl. Acad. Sci. 101, 3747–3752. doi: 10.1073/pnas.0400087101
Bejder, L., Fletcher, D., and Bräger, S. (1998). A method for testing association patterns of social animals. Anim. Behav. 56, 719–725. doi: 10.1006/anbe.1998.0802
Berger, V., Reichert, S., Lahdenperä, M., Jackson, J., Htut, W., and Lummaa, V. (2021). The elephant in the family: costs and benefits of elder siblings on younger offspring life-history trajectory in a matrilineal mammal. J. Anim. Ecol. 2021:13573. doi: 10.1111/1365-2656.13573
Bose, S., Forrester, T. D., Brazeal, J. L., Sacks, B. N., Casady, D. S., and Wittmer, H. U. (2017). Implications of fidelity and philopatry for the population structure of female black-tailed deer. Behav. Ecol. 28, 983–990. doi: 10.1093/beheco/arx047
Burnham, K. P., and Anderson, D. R. (2002). Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York, NY: Springe-Verlag.
Cairns, S. J., and Schwager, S. J. (1987). A comparison of association indices. Anim. Behav. 35, 1454–1469. doi: 10.1016/S0003-3472(87)80018-0
Carroll, E. L., McGowen, M. R., McCarthy, M. L., Marx, F. G., Aguilar, N., Dalebout, M. L., et al. (2021). Speciation in the deep: genomics and morphology reveal a new species of beaked whale Mesoplodon eueu. Proc. R. Soc. B 288:20211213. doi: 10.1098/rspb.2021.1213
Claridge, D. E. (2006). Fine-scale distribution and habitat selection of beaked whales. M.Sc. thesis. Aberdeen: University of Aberdeen.
Clutton-Brock, T. H. (1989). Mammalian mating systems. Proc. Royal Soc. Lond. B 236, 339–372. doi: 10.1098/rspb.1989.0027)
Connor, R. C., Smolker, R. A., and Richards, A. F. (1992). Two levels of alliance formation among male bottlenose dolphins (Tursiops sp.). Proc. Natl. Acad. Sci. 89, 987–990. doi: 10.1073/pnas.89.3.987
Connor, R. C., Wells, R. S., Mann, J., and Read, A. J. (2000). “The bottlenose dolphin: social relationships in a fission-fusion society,” in Cetacean Societies, eds J. Mann, R. C. Connor, P. L. Tyack, and H. Whitehead (London: University of Chicago Press).
Dinis, A., Marques, R., Dias, L., Sousa, D., Gomes, C., Abreu, N., et al. (2017). Site Fidelity of Blainville’s Beaked Whale (Mesoplodon densirostris) off Madeira Island (Northeast Atlantic). Aquat. Mamm. 43, 387–390. doi: 10.1578/AM.43.4.2017.387
Duijns, S., Anderson, A. M., Aubry, Y., Dey, A., Flemming, S. A., Francis, C. M., et al. (2019). Long-distance migratory shorebirds travel faster towards their breeding grounds, but fly faster post-breeding. Sci. Rep. 9:9420. doi: 10.1038/s41598-019-45862-0
Farine, D. R. (2013). Animal social network inference and permutations for ecologists in R using asnipe. Methods Ecol. Evol. 4, 1187–1194. doi: 10.1111/2041-210X.12121
Farine, D. R., and Carter, G. G. (2022). Permutation tests for hypothesis testing with animal social network data: Problems and potential solutions. Methods Ecol. Evol. 13, 144–156. doi: 10.1111/2041-210X.13741
Farine, D. R., and Whitehead, H. (2015). Constructing, conducting and interpreting animal social network analysis. J. Anim. Ecol. 84, 1144–1163. doi: 10.1111/1365-2656.12418
Fedutin, I. D., Filatova, O. A., Mamaev, E. G., Burdin, A. M., and Hoyt, E. (2015). Occurrence and social structure of Baird’s beaked whales, Berardius bairdii, in the Commander Islands, Russia. Mar. Mamm. Sci. 31, 853–865. doi: 10.1111/mms.12204
Fernandez, M., Alves, F., Ferreira, R., Fischer, J.-C., Thake, P., Nunes, N., et al. (2021). Modeling fine-scale cetaceans’ distributions in oceanic islands: Madeira Archipelago as a case study. Front. Mar. Sci. 8:688248. doi: 10.3389/fmars.2021.688248
Foerster, S., McLellan, K., Schroepfer-Walker, K., Murray, C. M., Krupenye, C., Gilby, I. C., et al. (2015). Social bonds in the dispersing sex: partner preferences among adult female chimpanzees. Anim. Behav. 105, 139–152. doi: 10.1016/j.anbehav.2015.04.012
Geldmacher, J., van den Bogaard, P., Hoernle, K., and Schmincke, H.-U. (2000). The 40Ar/39Ar age dating of the Madeira Archipelago and hotspot track (eastern North Atlantic). Geochem. Geophys. Geosystems 1:1008. doi: 10.1029/1999GC000018
Gero, S., Gordon, J., and Whitehead, H. (2015). Individualised social preferences and long-term social fidelity between social units of sperm whales. Anim. Behav. 102, 15–23. doi: 10.1016/j.anbehav.2015.01.008
Ginsberg, J. R., and Young, T. P. (1992). Measuring association between individuals or groups in behavioural studies. Anim. Behav. 44, 377–379. doi: 10.1016/0003-3472(92)90042-8
Gowans, S., Whitehead, H., and Hooker, S. K. (2001). Social organisation in northern bottlenose whales (Hyperoodon ampullatus): not driven by deep-water foraging. Anim. Behav. 62, 369–377. doi: 10.1006/anbe.2001.1756
Gowans, S., Würsig, B., and Karczmarski, L. (2007). The Social Structure and Strategies of Delphinids: Predictions Based on an Ecological Framework. Adv. Mar. Biol. 53, 195–294. doi: 10.1016/S0065-2881(07)53003-8
Greenwood, P. J. (1980). Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140–1162. doi: 10.1016/S0003-3472(80)80103-5
Hartman, K. L., Fernandez, M., Wittich, A., and Azevedo, J. M. N. (2015). Sex differences in residency patterns of Risso’s dolphins (Grampus griseus) in the Azores: causes and management implications. Mar. Mamm. Sci. 31, 1153–1167. doi: 10.1111/mms.12209
Hartman, K. L., Visser, F., and Hendriks, A. J. E. (2008). Social structure of Risso’s dolphins (Grampus griseus) at the Azores: A stratified community based on highly associated social units. Can. J. Zool. 86, 294–306. doi: 10.1139/Z07-138
He, P., Maldonado-Chaparro, A. A., and Farine, D. R. (2019). The role of habitat configuration in shaping social structure: a gap in studies of animal social complexity. Behav. Ecol. Sociobiol. 73:9. doi: 10.1007/s00265-018-2602-7
Hinde, R. A. (1976). Interactions, Relationships and Social Structure. Man 11, 1–17. doi: 10.2307/2800384
Hooker, S. K., de Soto, N. A., Baird, R. W., Carroll, E. L., Claridge, D., Feyrer, L., et al. (2019). Future directions in research on beaked whales. Front. Mar. Sci. 5:514. doi: 10.3389/fmars.2018.00514
Hoppitt, W. J. E., and Farine, D. R. (2018). Association indices for quantifying social relationships: how to deal with missing observations of individuals or groups. Anim. Behav. 136, 227–238. doi: 10.1016/j.anbehav.2017.08.029
Kappeler, P. M. (2019). A framework for studying social complexity. Behav. Ecol. Sociobiol. 73:13. doi: 10.1007/s00265-018-2601-8
Koening, A., Scarry, C. J., Wheeler, B. C., and Borries, C. (2013). Variation in grouping patterns, mating systems and social structure: what socio-ecological models attempt to explain. Philos. Trans. R. Soc. B 368:20120348. doi: 10.1098/rstb.2012.0348
Le Boeuf, B. J. (1991). “Pinniped mating systems on land, ice and in the water: Emphasis on the Phocidae,” in The Behaviour of Pinnipeds, ed. D. Renouf (Dordrecht: Springer), doi: 10.1007/978-94-011-3100-1_2
Li, S., and Rosso, M. (2021). Lack of knowledge threatens beaked whales. Science 371:791. doi: 10.1126/science.abg8922
Lukas, D., and Clutton-Brock, T. (2018). Social complexity and kinship in animal societies. Ecol. Lett. 21, 1129–1134. doi: 10.1111/ele.13079
Lusseau, D. (2007). Why are male social relationships complex in the doubtful sound bottlenose dolphin population? PLoS One 2:e348. doi: 10.1371/journal.pone.0000348
Lusseau, D., and Newman, M. E. J. (2004). Identifying the role that animals play in their social networks. Proc. R. Soc. Lond. B 271, S477–S481. doi: 10.1098/rsbl.2004.0225
Lusseau, D., Wilson, B., Hammond, P. S., Grellier, K., Durban, J. W., Parsons, K. M., et al. (2006). Quantifying the influence of sociality on population structure in bottlenose dolphins. J. Anim. Ecol. 75, 14–24. doi: 10.1111/j.1365-2656.2005.01013.x
Martins, A. M., Amorim, A. S. B., Figueiredo, M. P., Souza, R. J., Mendonça, A. P., Bashmachnikov, I. L., et al. (2007). Sea surface temperature (AVHRR, MODIS) and ocean colour (MODIS) seasonal and interannual variability in the Macaronesian islands of Azores, Madeira, and Canaries. Florence: SPIE Remote Sensing. doi: 10.1117/12.738373
McSweeney, D. J., Baird, R. W., and Mahaffy, S. D. (2007). Site Fidelity, Associations, and Movements of Cuvier’s (Ziphius cavirostris) and Blainville’s (Mesoplodon densirostris) Beaked Whales Off the Island of Hawai’i. Mar. Mamm. Sci. 23, 666–687. doi: 10.1111/j.1748-7692.2007.00135.x
Morales, J. M., Moorcroft, P. R., Matthiopoulos, J., Frair, J. L., Kie, J. G., Powell, R. A., et al. (2010). Building the bridge between animal movement and population dynamics. Philos. Trans. R. Soc. B 365, 2289–2301. doi: 10.1098/rstb.2010.0082
Pearson, H. C. (2011). Sociability of female bottlenose dolphins (Tursiops spp.) and chimpanzees (Pan troglodytes): Understanding evolutionary pathways toward social convergence. Evol. Anthropol. 20, 85–95. doi: 10.1002/evan.20296
Rendell, L., Cantor, M., Gero, S., Whitehead, H., and Mann, J. (2019). Causes and consequences of female centrality in cetacean societies. Philos. Trans. R. Soc. B 374:20180066. doi: 10.1098/rstb.2018.0066
Ritter, F., and Brederlau, B. (1999). Behavioural observations of dense beaked whales (Mesoplodon densirostris) off La Gomera, Canary Islands (1995-1997). Aquat. Mamm. 25, 55–61.
Robbins, J., Rosa, L. D., Allen, J. M., Mattila, D. K., Secchi, E. R., Friedlaender, A. S., et al. (2011). Return movement of a humpback whale between the Antarctic Peninsula and American Samoa: A seasonal migration record. Endanger. Species Res. 13, 117–121. doi: 10.3354/esr00328
Schreier, A. L., and Swedell, L. (2009). The fourth level of social structure in a multi-level society: ecological and social functions of clans in hamadryas baboons. Am. J. Primatol. 71, 948–955. doi: 10.1002/ajp.20736
Shuster, S. M., and Wade, M. J. (2003). Mating systems and strategies. Princeton: Princeton University Press.
Suárez, C.R. (2018). Abundance estimate, survival and site fidelity patterns of Blainville’s (Mesoplodon densirostris) and Cuvier’s (Ziphius cavirostris) beaked whales off El Hierro (Canary Islands). M.Sc. thesis. Scotland: University of Saint Andrews.
Sutherland, W. J. (1998). The importance of behavioural studies in conservation biology. Anim. Behav. 56, 801–809. doi: 10.1006/anbe.1998.0896
Tyack, P. L., Johnson, M., Aguilar Soto, N., Sturlese, A., and Madsen, P. T. (2006). Extreme diving of beaked whales. J. Exp. Biol. 209, 4238–4253. doi: 10.1242/jeb.02505
Tyack, P. L., Zimmer, W. M. X., Moretti, D., Southall, B. L., Claridge, D. E., Durban, J. W., et al. (2011). Beaked whales respond to simulated and actual navy sonar. PLoS One 6:e17009. doi: 10.1371/journal.pone.0017009
Visser, F., Merten, V. J., Bayer, T., Oudejans, M. G., de Jonge, D. S. W., Puebla, O., et al. (2021). Deep-sea predator niche segregation revealed by combined cetacean biologging and eDNA analysis of cephalopod prey. Sci. Adv. 7:eabf5908. doi: 10.1126/sciadv.abf5908
Weiss, M. N., Ellis, S., and Croft, D. P. (2021). Diversity and consequences of social network structure in toothed whales. Front. Mar. Sci. 8:688842. doi: 10.3389/fmars.2021.688842
Whitehead, H. (1995). Investigating structure and temporal scale in social organisations using identified individuals. Behav. Ecol. 6, 199–208. doi: 10.1093/beheco/6.2.199
Whitehead, H. (1996). Babysitting, dive synchrony, and indications of alloparental care in sperm whales. Behav. Ecol. Sociobiol. 38, 237–244. doi: 10.1007/s002650050238
Whitehead, H. (1999). Testing association patterns of social animals. Anim. Behav. 57, F26–F29. doi: 10.1006/anbe.1999.1099
Whitehead, H. (2001). Analysis of animal movement using opportunistic individual identifications: Application to Sperm Whales. Ecology 82, 1417–1432. doi: 10.2307/2679999
Whitehead, H. (2008). Analysing animal societies: quantitative methods for vertebrate social analysis. Chicago: University of Chicago Press.
Whitehead, H. (2009). SOCPROG programs: analysing animal social structures. Behav. Ecol. Sociobiol. 63, 765–778. doi: 10.1007/s00265-008-0697-y
Whitehead, H., Bejder, L., and Ottensmeyer, A. C. (2005). Testing association patterns: issues arising and extensions. Anim. behav. 69:e1. doi: 10.1016/j.anbehav.2004.11.004
Keywords: age/sex-specific, beaked whales, individual temporal associations, island systems, megafauna, movement and residency patterns, pelagic habitat, social network
Citation: Badenas A, Dinis A, Ferreira R, Sambolino A, Hamard E, Berninsone LG, Fernandez M and Alves F (2022) Behavioural Ecology Traits of Elusive Deep-Diver Whales Unravel a Complex Social Structure Influenced by Female Philopatry and Defence Polygyny. Front. Mar. Sci. 9:809902. doi: 10.3389/fmars.2022.809902
Received: 05 November 2021; Accepted: 25 January 2022;
Published: 15 February 2022.
Edited by:
Lyne Morissette, M – Expertise Marine, CanadaReviewed by:
Mauricio Cantor, Max Planck Institute of Animal Behaviour, GermanyCopyright © 2022 Badenas, Dinis, Ferreira, Sambolino, Hamard, Berninsone, Fernandez and Alves. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Filipe Alves, ZmlsaXBlLmFsdmVzQG1hcmUtY2VudHJlLnB0
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.