- 1Instituto Federal de Educação Ciência e Tecnologia do Pará, Campus Abaetetuba, Abaetetuba, Brazil
- 2Instituto de Estudos Costeiros, Universidade Federal do Pará, Bragança, Brazil
- 3Laboratório de Genética Aplicada, Instituto de Estudos Costeiros, Universidade Federal do Pará, Bragança, Brazil
- 4Núcleo de Ecologia Aquática e Pesca da Amazônia, Universidade Federal do Pará, Belém, Brazil
- 5Laboratório de Biologia Pesqueira e Manejo dos Recursos Aquáticos, Grupo de Pesquisa em Ecologia, Manejo e Pesca na Amazônia, Universidade Federal do Pará, Belém, Brazil
The Southern red snapper, Lutjanus purpureus, is a valuable economic resource, especially in international market. However, no recent data are available on the reproductive biology of this species in Brazilian waters, in particular, the stock found on the Amazon reef. The present study evaluated the population structure and reproductive biology of L. purpureus, and the volume of the catch of juveniles taken by the local commercial fisheries. A total of 923 specimens were analyzed, of which, 577 were female and 346 male (sex ratio: 0.6:1; p < 0.05). The analysis of the gonadosomatic index (GSI) and the distribution of maturation stages indicated that the males and females are able to reproduce throughout the year, however the reproductive peak occurs simultaneously with the increase in the water discharge of the Amazon River. The spawning females were registered in the study area, indicating that L. purpureus does not perform long distance reproductive migrations, different from the previously proposed. The Fork length at first sexual maturation (FL50) was estimated to be 35.2 cm in the males and 32.1 cm in the females. The high frequency of juveniles (< FL50) captured by the commercial snapper fleet using longlines (33.67% of the total catch) and traps (42.1%) indicates the poor selectivity of the fishing gear used by the snapper fleet, which may contribute to the accelerated depletion of the L. purpureus stock. Although the results of this study show a catch percentage of juveniles >50%, it is still a significant percentage of juveniles that are being caught due to little or no guidance and enforcement of fishing gear to ensure compliance with current legislation. Considering the data presented in this study and the current situation of declining fish stocks of L. purpureus, an assessment is recommended for the inclusion of the species in the IUCN Red List of Threatened Species, which is currently a species categorized as “vulnerable” in the Brazilian Red List.
Introduction
The Southern red snapper – Lutjanus purpureus (Poey, 1866) – is an economically important fish species, found in the Caribbean Sea, south of Cuba, and off northern South America, from Colombia to northern and northeastern Brazil (Allen, 1985; Anderson, 2002). The species has already had its taxonomic validity questioned, being considered synonymous with Lutjanus campechanus (Gomes et al., 2008) but it is currently known that the two red snappers are separate taxonomic units, with gene introgression of L. campechanus due to recent speciation (Del Pedraza-Marrón et al., 2019; Da Silva et al., 2020).
In Brazil, L. purpureus fisheries began in the 1960s over oceanic banks and on the continental platform of the northeastern coast (Fonteles-Filho, 1972; Ivo and Hanson, 1982). Due to the depletion of these stocks in the 1980s, the fisheries are now focused off the northern coast of the country (Ivo and Hanson, 1982; Fonteles-Filho, 2007). The available data on the L. purpureus stock of the Amazon coast indicate overfishing due to intense fishery pressure and the large volume of juvenile specimens landed (Furtado-Júnior et al., 2006; Souza et al., 2008).
As an economically valuable fishery resource, L. purpureus reproduction was studied by Manickchand-Heileman and Phillip (1996) in Trinidad and Tobago. In Brazil, most of the data on their reproductive patterns date from the 1960s and 1980s, and refer specifically to the northeastern coast (Almeida, 1964; Alves, 1971; Fonteles-Filho, 1972; Gesteira and Ivo, 1973; Ivo and Souza, 1988). Only one study is available for the stock of the Amazon coast (Souza et al., 2003).
The Amazon reef plays a fundamental role in the reproductive ecology of the species. Maps of benthic habitats and plume dynamics are predictors of the biodiversity and distribution of the L. purpureus fishing fleet (Francini-Filho et al., 2018; Araújo et al., 2021).
Although L. purpureus is an economically valuable fishery resource, most of the data on its reproductive patterns in Brazilian waters date back to the 1960-1980s, and refer specifically to the northeastern coast (Almeida, 1964; Alves, 1971; Fonteles-Filho, 1972; Gesteira and Ivo, 1973; Ivo and Sousa, 1988). Only one study is available for the stock of the Amazon coast (Souza et al., 2003).
Reliable data on the reproductive biology of a species are essential for any ecosystem approach to fishery management (Morgan, 2008), and the lack of recent studies of the commercial L. purpureus fisheries in Brazil raises concerns on the sustainability of the stocks of this species. Catches that exceed the maximum sustainable yield may result in decreased abundance and body length, and early sexual maturation, as well as provoking changes in the genetic structure of the stocks and other modifications that reduce the sustainability of fisheries (Morgan et al., 2011).
The aim of this work was to determine the sexual maturity, sex ratio, gonadosomatic index, and the proportion of juveniles in commercial catches of southern red snapper, Lutjanus purpureus, from fishery dependent samples of the northern Brazilian coast. These results provides an important insights for the development of effective fishery management strategies to ensure the sustainable management of Brazilian Southern red snapper stocks.
Materials and Methods
Study Area
The fishing grounds of the lutjanid fleet of the Brazilian North coast extend from Cape Orange, in Amapá state (51° W) to São Marcos Bay, in Maranhão (46° W). The coastal area is divided into two sedimentary watersheds, that of the Amazon delta and the Pará-Maranhão watershed (Mohriak, 2003).
The Pará-Maranhão continental shelf is dominated by sedimentary deposits of sand with some gravel (Gualberto and El-Robrini, 2015). By contrast, the substrates of the continental shelf off the Amazon mouth are dominated by sand, silt and clay derived primarily from the sediment load of the Amazon River (Silva et al., 2009). The enormous discharge of water and sediments of this river forms an extensive plume that stretches out into the tropical North Atlantic, and varies in size according to the seasonal fluctuations in rainfall patterns in the Amazon region (Silva et al., 2009). This plume extends out into the Atlantic, forming a barrier to the vertical mixing of the oceanic waters (Coles et al., 2013).
From November to April, the Northwest flow is associated to the combination of strong trade winds and stronger North Brazilian Current (NBC) transport, whereas from May to October the plume retroflex eastward due to less trade winds’ stress and reduced NBC transport (Geyer et al., 1996). A digital terrain model allowed us to discriminate eight megahabitats in the the Amazon Continental Margin: Regular Mud, Irregular Sand, and Carbonate Continental Shelf, Shelf–Slope Transition, Featured Slope, Shallow Gentle Slope (<2,000 m), and Steep Slope, Deep Gentle Slope (>2,000 m). The distribution of these megahabitats is related to distinct geological and oceanographic processes that operate over different time scales (Lavagnino et al., 2020).
The Amazon River plume is superficial (maximum depth is 25m) and driven by seasonal winds and by the NBC, which flows northwestward into the Caribbean and retroflects eastward during September and October (Nittrouer and DeMaster, 1986).
The extensive area of reef system off the Amazon River mouth was first hypothesized the 40 years ago by Collette and Ruetzler (1977), however, only now have more specific studies been carried out, proving the existence the great area reef mesophotic (~56,000 km) in the Brazilian Amazon coast denominate Great Amazon Reef System (GARS) complex hard bottoms created by living organisms. (Moura et al., 2016; Francini-Filho et al., 2018). The GARS is currently considered as one of the most important mesophotic reef ecosystems of the South Atlantic (Soares et al., 2019) is composed of a greater complexity and diversity of habitats than previously recognized, where occurs reef platforms, reef walls, rhodolith beds, and sponge bottoms (Francini-Filho et al., 2018; Araújo et al., 2021).
The GARS extends much deeper than previously predicted, with a clear slope from its deepest portion (~220 m depth), where laterite outcrops alternate with areas mainly sponges, octocorals, and black corals), to its shallowest portion (~70 m), which is almost completely covered by sand. At depths of 80-100 m, marine snow may also temporarily cover rhodolite beds and algal structures The rhodolite beds and biogenic limestone platforms are the dominant features at depths between 70 and 180 m (Francini-Filho et al., 2018).
The criticism on the existence of a living reef in the Amazon River mouth was raised (see Mahiques et al., 2019), but the recent studies demonstrate that the reef is alive and growing, with living organisms inhabiting the GARS in its totality (Mahiques et al., 2019; Lavagnino, et al., 2020) but, possibly with very small growth index (Lavagnino, et al., 2020). Additionally, more recently Omachi et al., (2019) have demonstrated that there is enough light for photosynthetic organisms to thrive in the area of the GARS.
A number of commercial fisheries operate in this area, targeting a variety of reef species, including L. purpureus (Moura et al., 2016, Freire et al., 2021).
Data Collection
The L. purpureus specimens were obtained on board commercial fishing vessels operating within the study area during two periods, the first between May 2009 and October 2011, and the second between May 2016 and February 2018 (Figure 1). The specimens were collected during the fishing season, between May 1st and December 14th. During the close season, the L. purpureus samples were obtained from the bycatch of commercial vessels licensed to fish for a diversity of other fish species. In both situations, samples were collected using gears typical of commercial snapper fisheries, which were described by Costa et al. (2017) and Freire (2019).
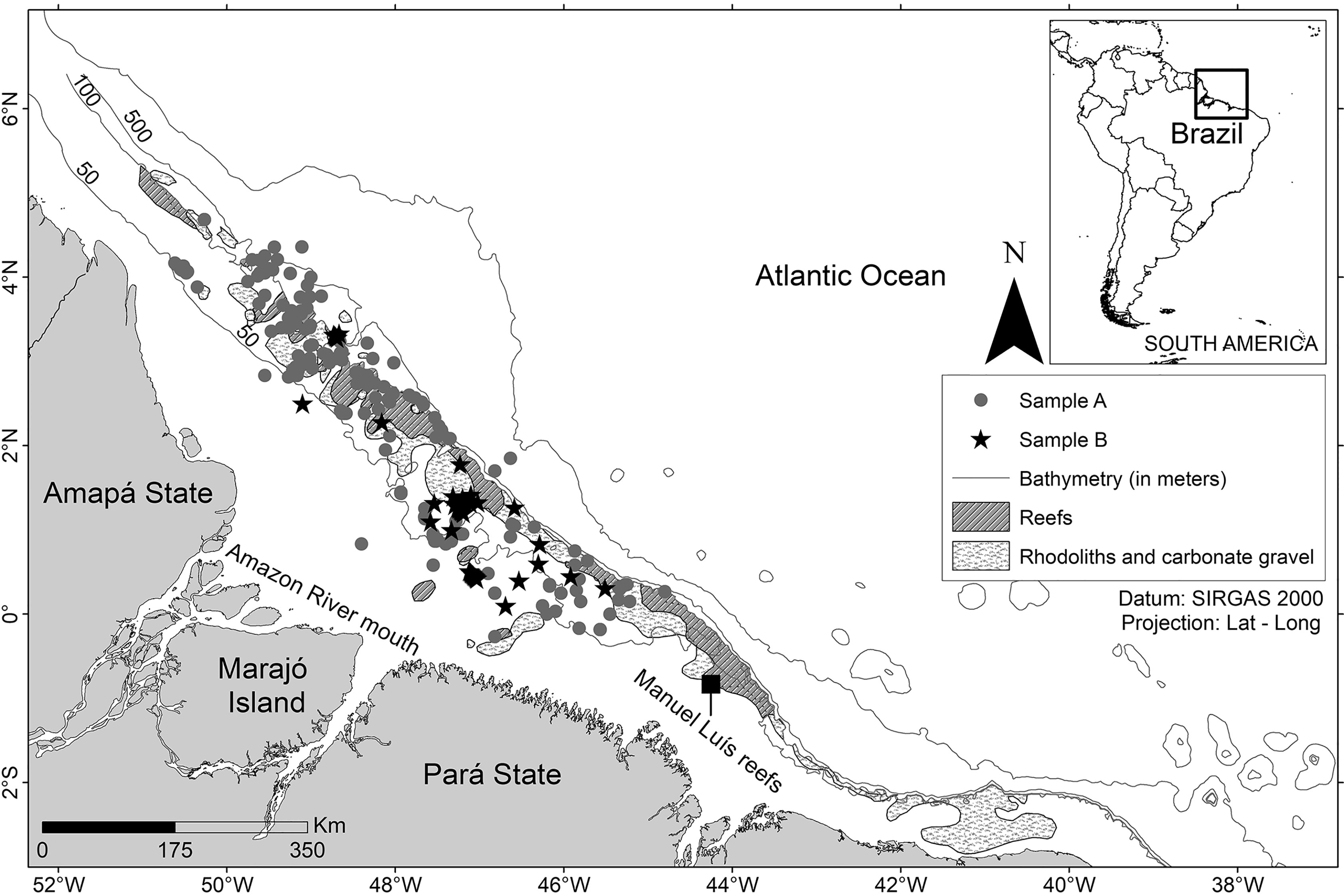
Figure 1 Study area off the Amazon coast (Brazil). Environmental data (reef, rhodoliths, and bathymetry) obtained from Moura et al. (2016).
Captured specimens collected by fishermen partners of the project, in which 30 specimens/month were requested and stored in identified bags and stored on ice in the hold of the vessel. The date and geographic coordinates of each catch were also recorded. Considering the dependence of the project on donations of specimens, in some months there were problems in the delivery of specimens, in quantities higher or lower than requested.
Reproductive Traits
In the laboratory, the specimens were identified using taxonomic keys (Allen, 1985). Each specimen was also measured (Fork length, FL, in cm: the distance between the tip of the snout and the posterior extremity of the tail fin bifurcation) and weighed (Total Weight, TW, in kg: weight of the wet specimen).
The specimens were classified according to their sex (male or female) and gonadal maturity, which was assigned to one of four classes, based on the classification of Alves (1971): IMM (immature); DEV (developing); SPW (spawning capable), and REG (regressing or regenerating) for females and IMM, SPW and REG, for males. In males, the DEV class was agrouped into IMN.
The spawning period was identified by the relative frequency of the different stages of gonadal maturation and the Gonadosomatic Index (GSI), based on Vazzoler (1996): GSI = (GW/TW)*100, where GW = gonadal weight, and TW = total weight of the individual.
The mean fork length at first sexual maturity (FL50, at which 50% of the fish have reached sexual maturity) was calculated using a logistic curve adjusted by the least squares method, based on King (2013): Pi = [1/(1 + e-r (FLi - FL50))]100, where Pi = the proportion of spawning capable individuals in class i; FL50 = Fork length with 50% of adults, and r = slope of the logistic curve.
Length Frequency of Commercial Fisheries
To analyze variation in body size, a sample of approximately 250 specimens was collected randomly each week from the catches landed by the commercial snapper fleet at Bragança (Pará, Brazil) in the 2016, 2017 and 2019 fishing seasons. The mean fork length (cm) of these specimens was determined. The type of fishing gear used by the vessels was also recorded in order to evaluate the possible impact of the type of technique used on the capture of the juvenile stock. Two types of gear are used by the Amazon snapper fleet: (i) the manzuá trap, a cylindrical, unfixed trap made of plastic-coated iron, and (ii) vertical longlines, with 20–25 secondary lines equipped with hooks of varying sizes, operated manually or with the assistance of a manual winch, known locally as a “Bicicleta” or “bicycle” (Bentes et al., 2012; Costa et al., 2017).
Data Analysis
A one-way Analysis of Variance (ANOVA) was used to evaluate the variation in the mean fork length (FL) and total weight (TW) between sexes and among months (α = 0.05). Prior to this analysis, the data were evaluated for the assumptions of normality (Kolmogorov-Smirnov test) and homoscedasticity (Levene’s test). Tukey’s post-hoc test was used to determine significant differences between the sexes and pairs of months (Zar, 2009). The sex ratio was calculated per month and body length class (1 cm intervals). Deviations of the sex ratio from expected (1:1) were evaluated using Chi square (α = 0.05).
As the assumptions necessary for a parametric analysis were not satisfied here, the nonparametric Kruskal-Wallis test was used to analyze the variation in the GSI of the sexually mature specimen (DEV, SPW and REG) among months (α = 0.05). The mean monthly discharge (m³/s) of the Amazon River (the hydrological data -10 last years- were obtained using the HIDROWEB platform, of the Water National Agency (ANA) of the Brazilian Government) was plotted against the mean monthly GSI to evaluate the possible influence of the discharge on sexual maturation. The analysis of the relationship between the average monthly discharge (m³/s) of the Amazon River and the average monthly GSI was performed using Spearman correlation (α = 0.05).
A one-way ANOVA (α = 0.05) was used to evaluate the differences in the mean fork length of the L. purpureus catches obtained using the two different types of fishing gear (trap or vertical longline). The data were log [y=log(x)] transformed to satisfy the assumptions necessary for parametric analyses. The relative frequency of juveniles (<FL50) harvested using each type of fishing gear was also determined. The maps were plotted in QGIS version 3.8.1 (https://www.qgis.org).
Results
A total of 923 specimens of L.purpureus were analyzed, including 577 (62.5%) females and 346 (37.5%) males (Table 1). The sex ratio deviated significantly from 1:1 (X² = 6.26, df = 1, p < 0.05). A predominance of females was also found in most of the fork length classes, with a significant deviation being recorded in 12 of the 18 classes analyzed (Figure 2).
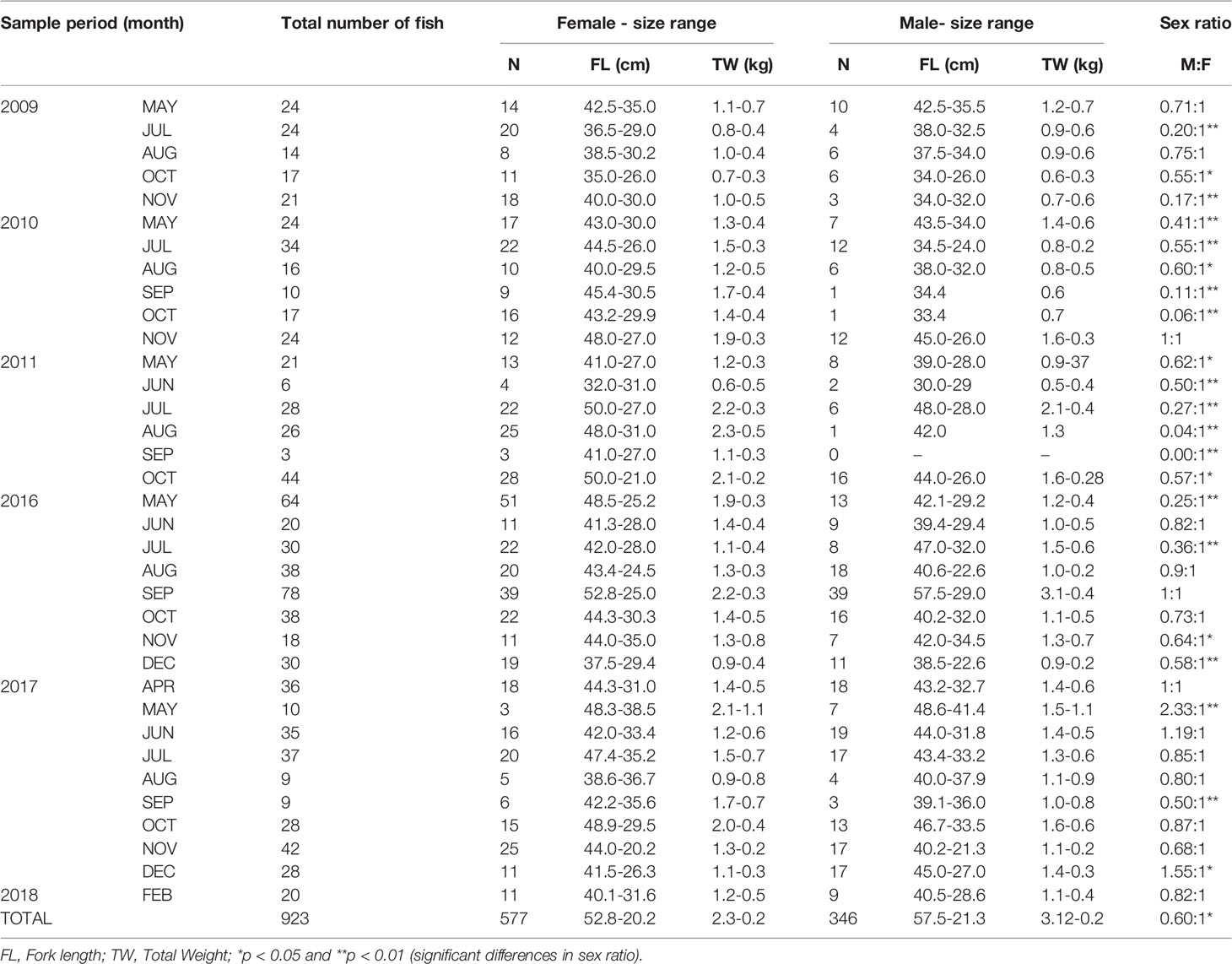
Table 1 Descriptive parameters and sex ratios of the Lutjanus purpureus specimens collected of the Amazon coast (Brazil).
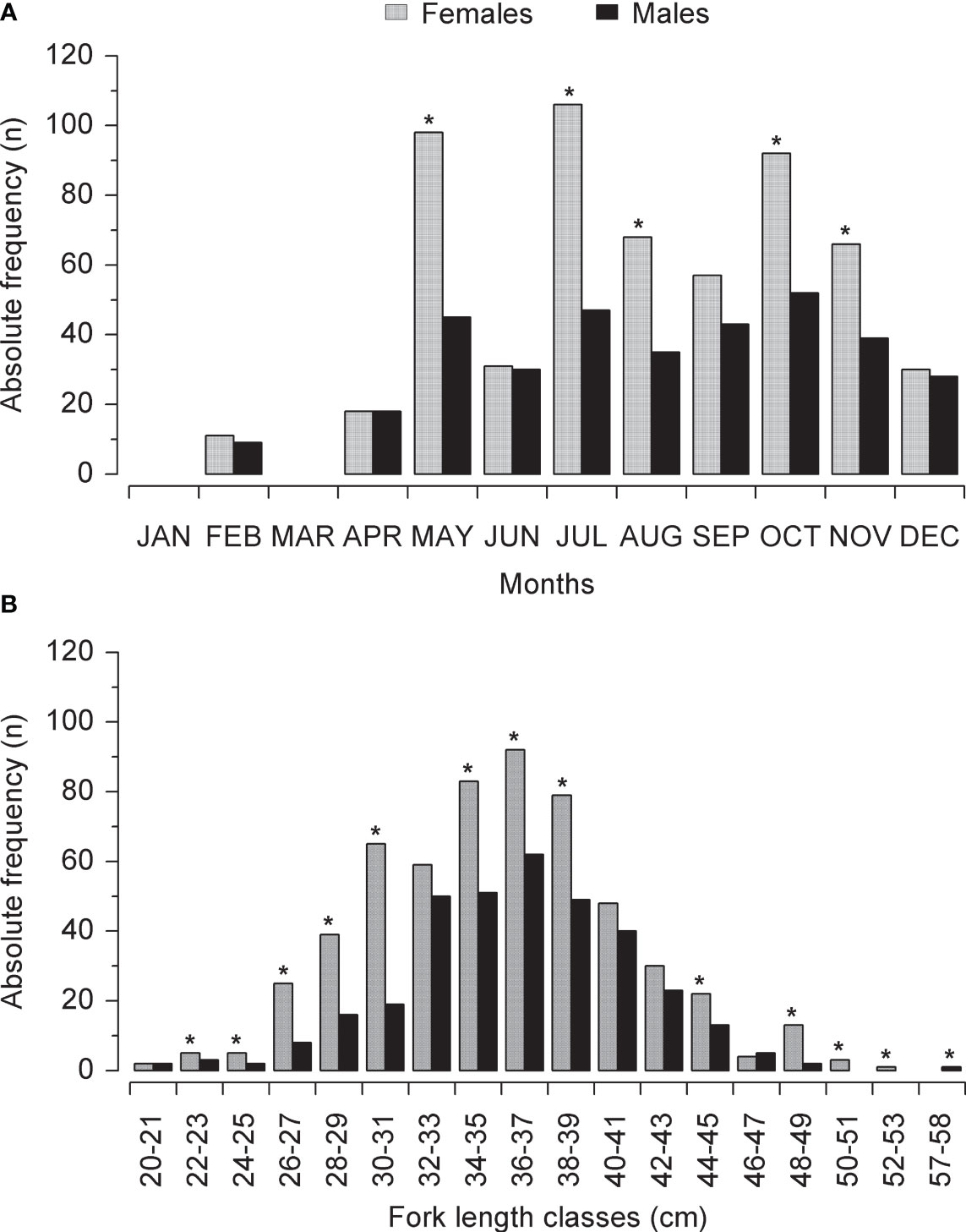
Figure 2 (A) Frequency of male and female Lutjanus purpureus captured per month of the study period (see Table 1) off the Amazon coast (Brazil). (B) Frequency of male and female Lutjanus purpureus captured per fork length class. The asterisks indicate a significant (p < 0.05) deviation in the sex ratio per month or size class.
During this study, the smaller ones had sexually mature male and female FL of 31.0 cm and 26.5 cm, respectively. Overall, 37.8% of the L. purpureus specimens were classified as juveniles (immature). While the number of specimens in the different gonadal stages was relatively constant over the study period, the number of mature (SPW) and regenerating individuals peaked during the first months of the year, indicating an intense period of reproductive activity during this part of the year (Figure 3).
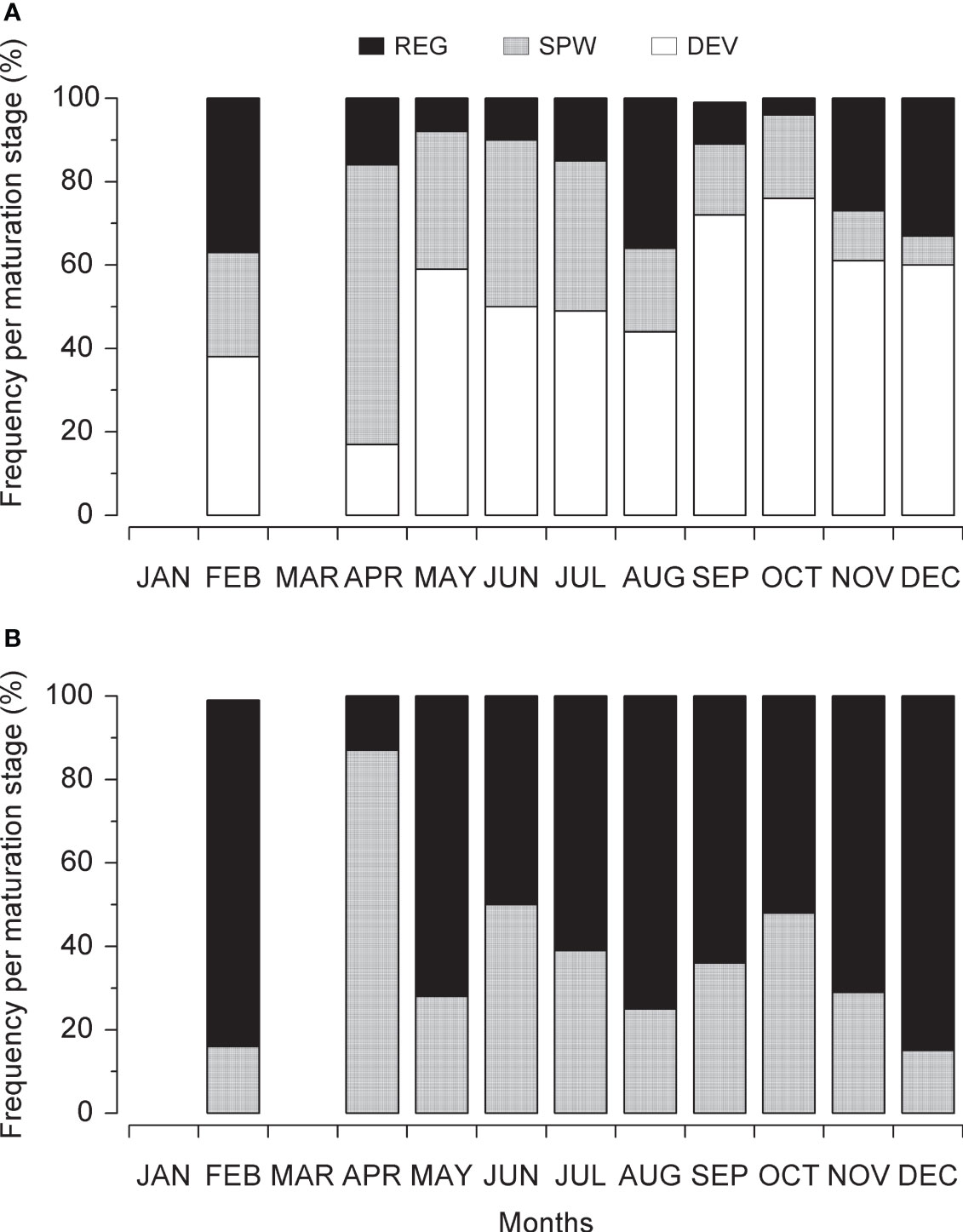
Figure 3 Monthly variation in the Lutjanus purpureus maturation stages over the whole study period (see Table 1) off the Amazon coast (Brazil). (A) Female. (B) Male. DEV, developing; SPW, spawning capable; REG, regressing or regenerating.
The highest individual GSI values recorded during the annual cycle (Figure 4) were obtained in May (4.37, male) and October (5.23, female). However, the highest mean female GSI was recorded in April, which was significantly different from all other months except June and July (F = 4.16; p < 0.001, df = 9). The mean GSI in February was low due to the number of REG individuals, which indicates a high rate of spawning during the previous month (January). The peak of reproductive activity recorded in L. purpureus during the early months of the year coincides with that in the discharge of the Amazon River, which is driven by the rainy season (Figure 4). The Spearman's correlation analysis revealed a significant positive correlation between the discharge of the Amazon River and the GSI of females (S = 50; p = 0.031; r = 0.697) and grouped sexes (S = 48; p = 0.027; r = 0.709), not being significant for male (S = 64; p = 0.066; r = 0.612).
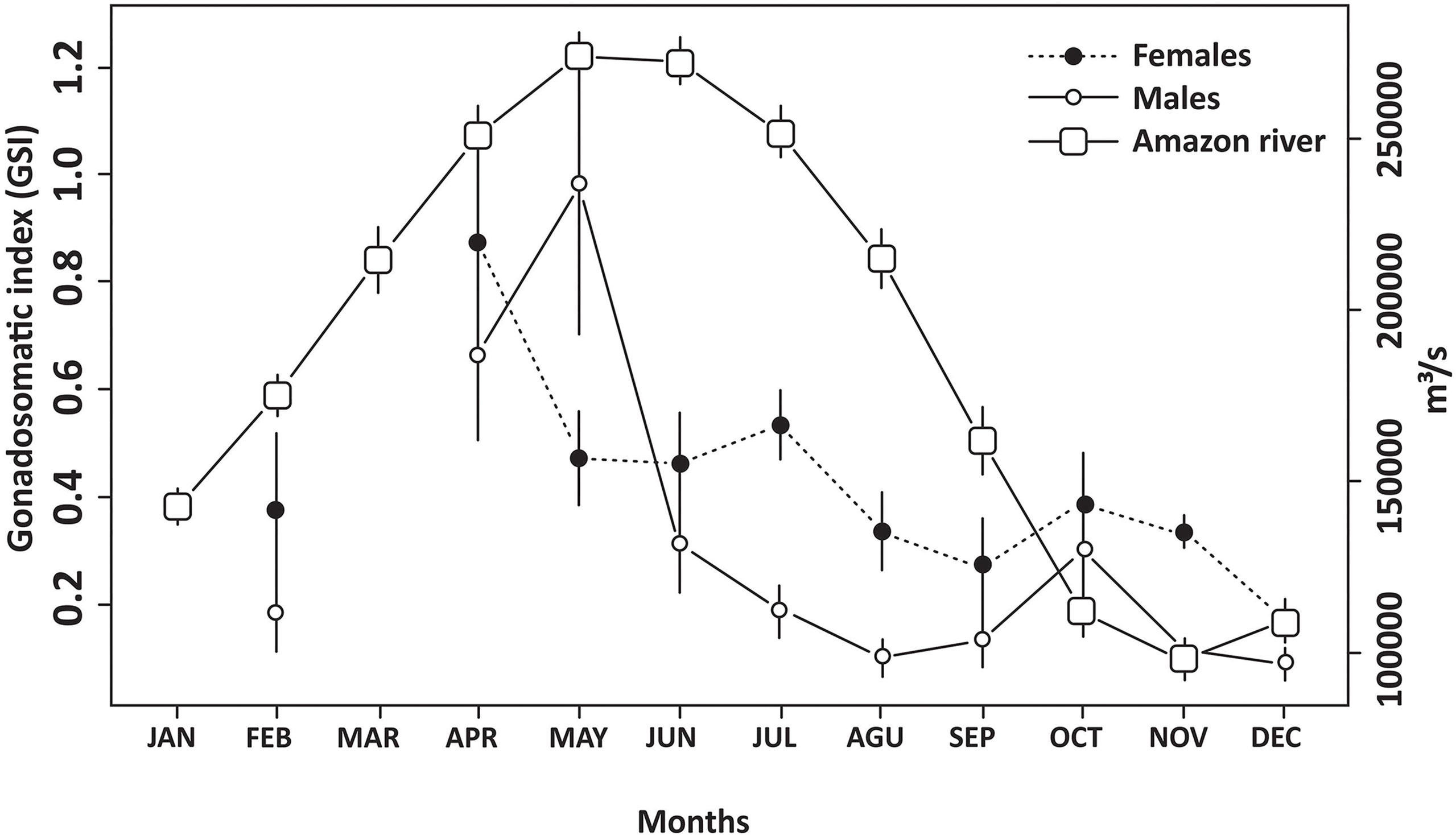
Figure 4 Mean (and standard deviation) monthly discharge (m³/s) of the Amazon River and the mean monthly gonadosomatic indices (GSI) recorded for the Lutjanus purpureus specimens collected off the Amazon coast (Brazil).
Spawning capable females (SPW) were recorded throughout the study region on the Amazon continental shelf, which indicates the presence of spawning aggregations, whose exact location has yet to be mapped (Figure 5).
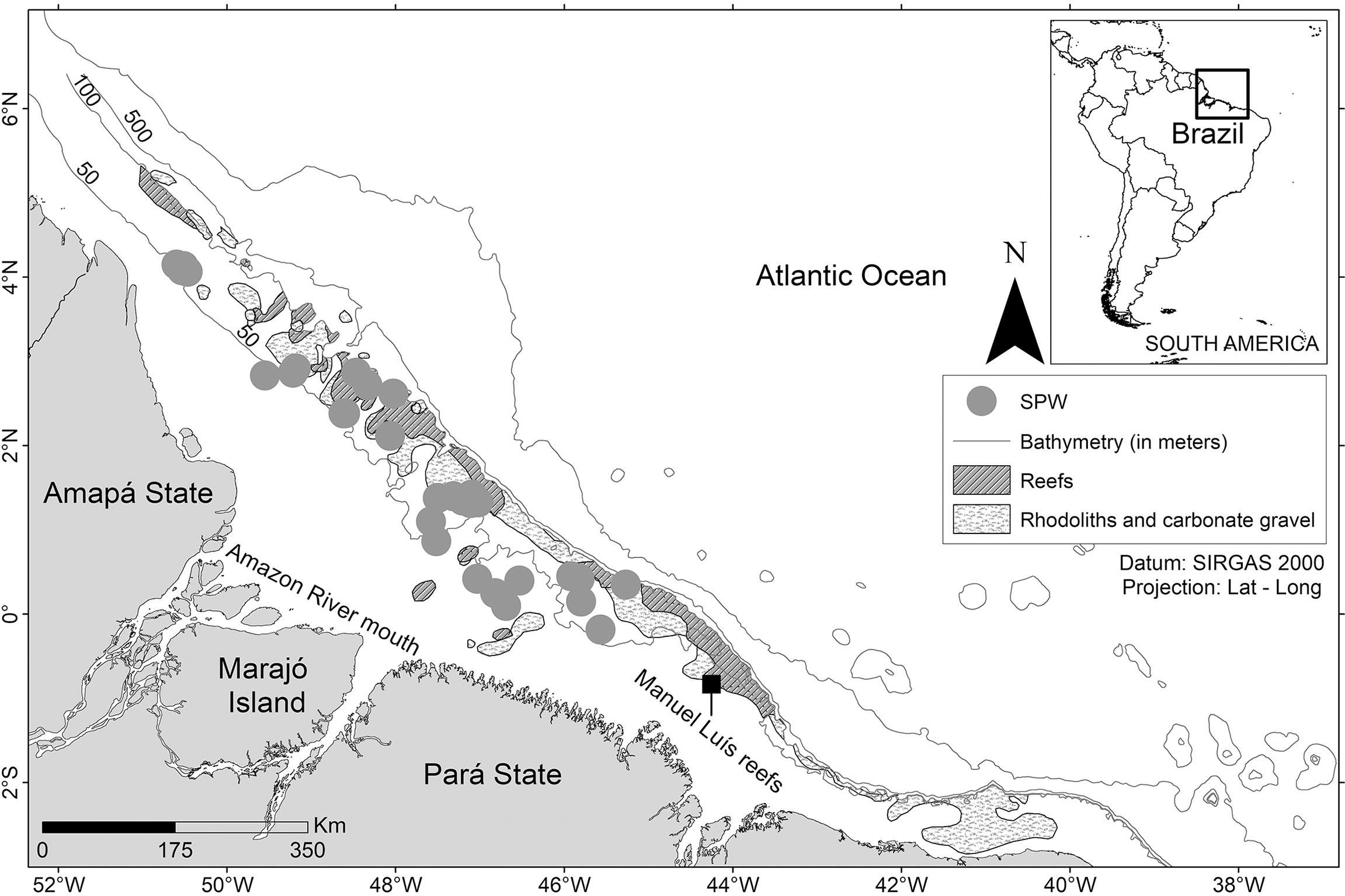
Figure 5 Distribution of females spawning capable (pooled samples) off the Amazon coast (Brazil). Environmental data (reef, rhodoliths and bathymetry) obtained from Moura et al. (2016).
The mean fork length at first gonadal maturation (FL50) was estimated at between 34.2 cm and 36.2 cm in the male L. purpureus and between 30.8 cm and 33.8 cm in the females (Table 2). The overall analysis indicated that the L50 of the females was 3.3 cm smaller than that of the males (Figure 6). The adjusted L50 was 39.3 cm (CI ±0.61) in the males and 35.7 cm (CI ±1.03) in the females.
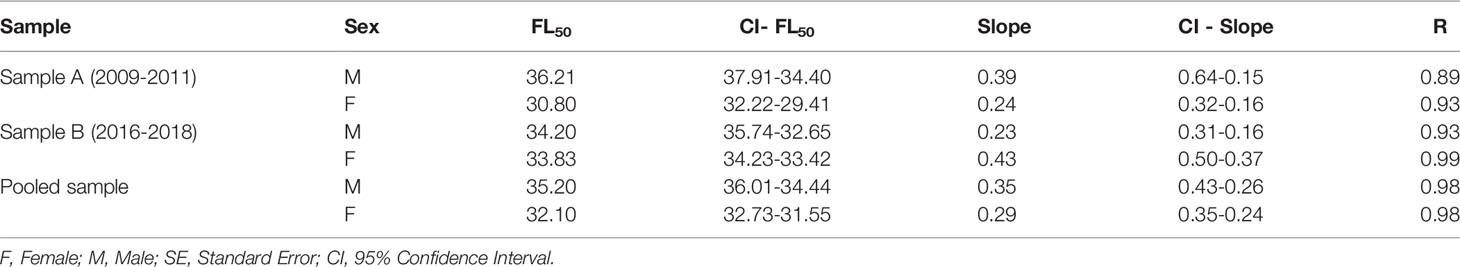
Table 2 Parameters of the nonlinear (least squares) estimates of the fork length at first gonadal maturity (FL50) of the female and male Lutjanus purpureus specimens collected off the Amazon coast (Brazil).
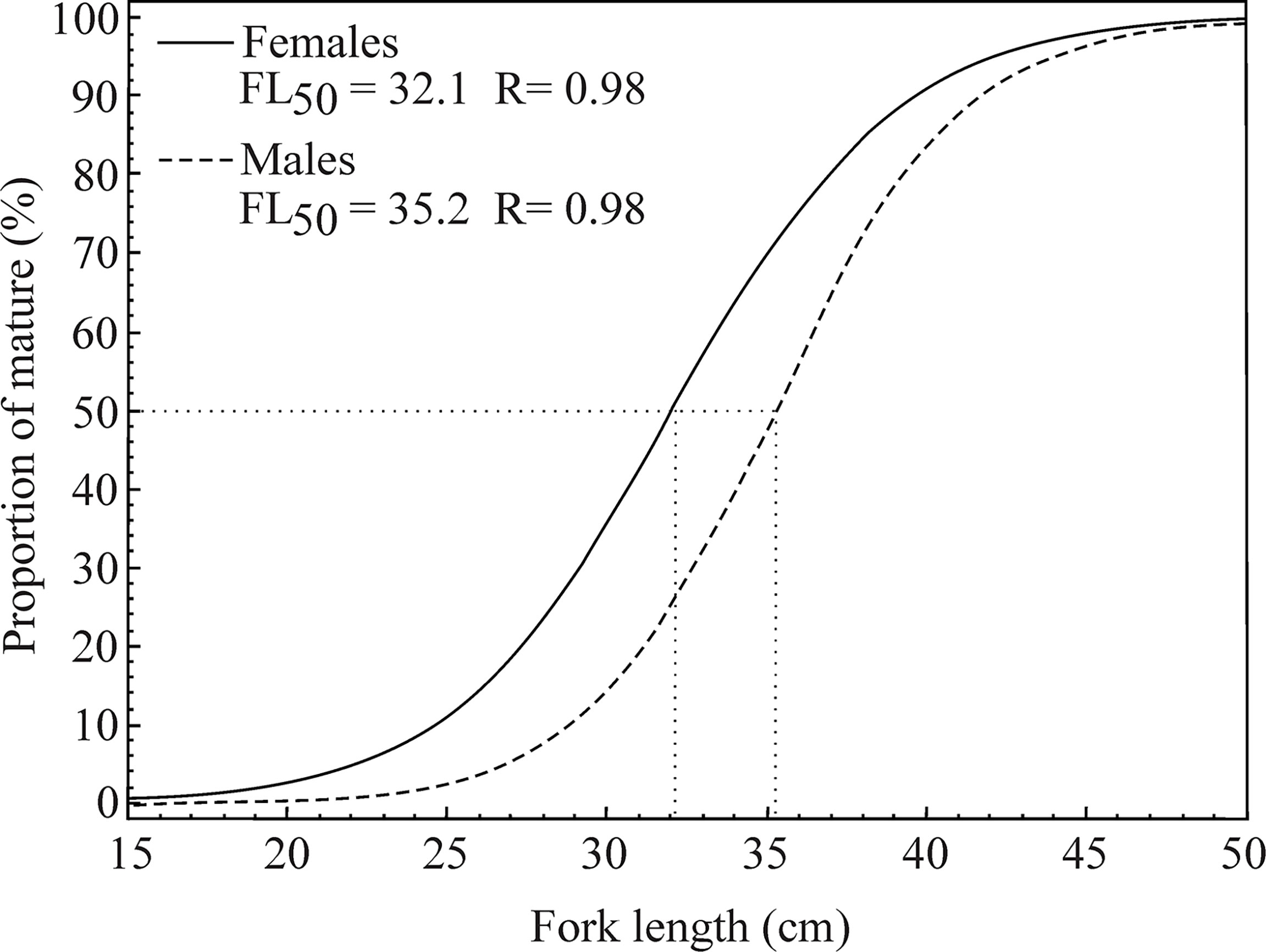
Figure 6 Fork length at first sexual maturity (FL50, cm) of the female and male Lutjanus purpureus specimens collected off the Amazon coast (Brazil).
The total of 25,905 FL specimens were measured, of which 8,687 were caught with a trap and 17,218 with a longline. This difference between the number of specimens measured of each gear type is related to the restricted access of many vessels. The overall mean FL of the L. purpureus specimens caught by the commercial snapper fleet in 2016 and 2017 (Sample B) was 35.8 cm (SD: ±7.3). The snapper caught in the traps were significantly smaller (F=544; df=1; p < 0.05), on average, i.e., 33.2 cm (SD: ±5.6), in comparison with those caught using longlines, with a mean FL of 36.6 cm (SD: ±7.6). Based on the female FL50 of 32.1 cm (all samples pooled), 42.1% of the L. purpureus caught using traps were juveniles (smaller than FL50), whereas 33.7% of the fish caught using longlines were juvenile (Figure 7).
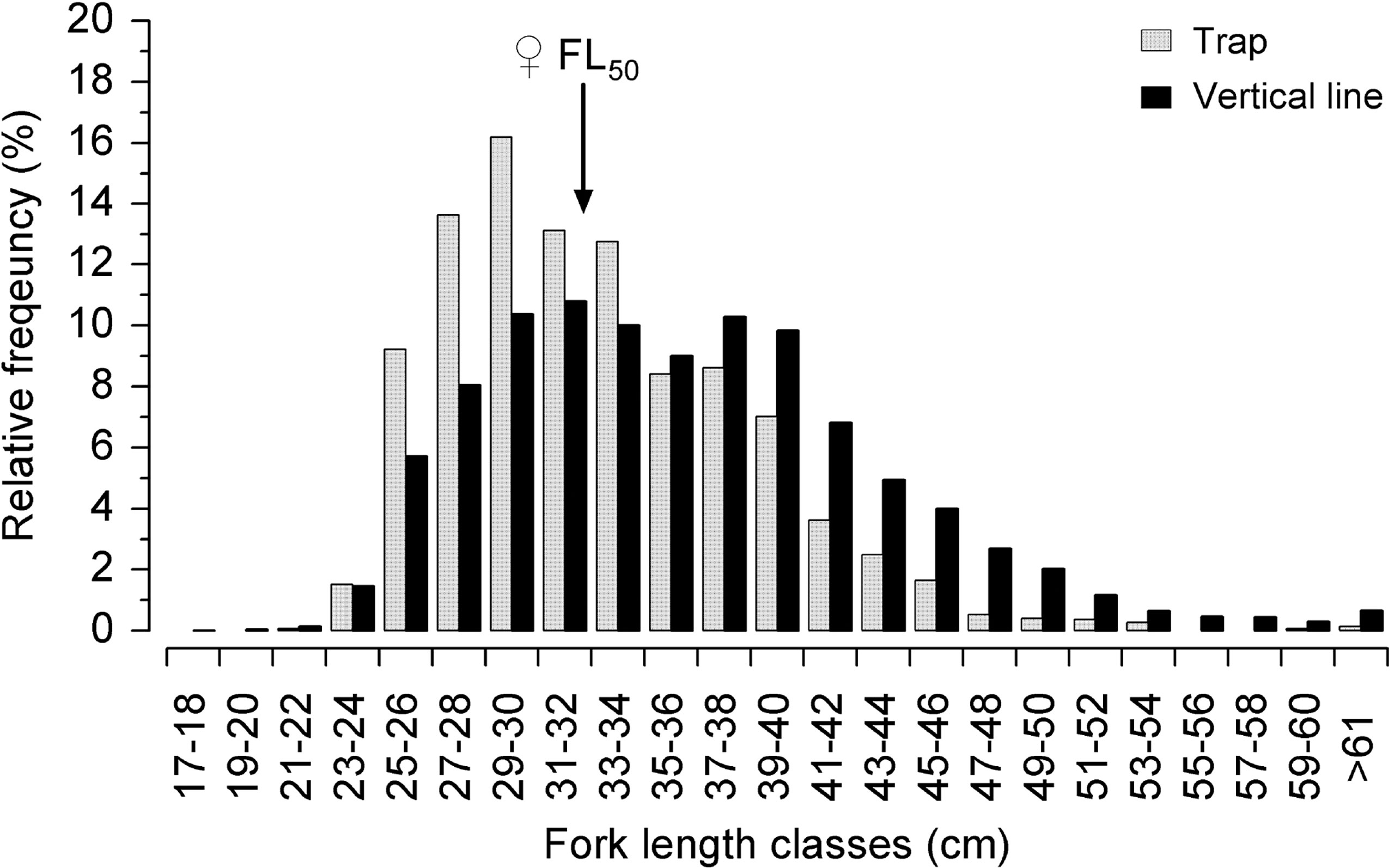
Figure 7 Relative frequency of the fork length classes of the Lutjanus purpureus specimens collected in 2016 and 2017, caught using different fishing techniques off the Amazon coast (Brazil). Theindicates the ♀FL50 of 32.1cm (see Figure 6).
Discussion
The understanding of the reproductive parameters of fish populations is fundamental to the development of effective management strategies for the maintenance of stocks (Grandcourt et al., 2009; Jakobsen et al., 2016). In this study, new information on the reproduction of the species reflects the severity of the commercial catches of L. purpureus in the Amazon continental shelf, highlighting the maintenance of the species and of fishing activity itself.
Some studies indicate that members of the family Lutjanidae that inhabit the coastal waters of the Atlantic Ocean have balanced sex ratios (Collins et al., 2001; Brulé et al., 2010; Porch et al., 2015). Significant deviations have been recorded in a number of taxa, however, with male-biased sex ratios being found in Lutjanus alexandrei (Fernandes et al., 2012) and Lutjanus guttatus (Arellano-Martínez et al., 2001), and female-biased ratios in Lutjanus peru (Santamaria and Villalejo, 2003), Lutjanus erythropterus, and Lutjanus malabaricus (Fry et al., 2009), thus, it is observed that there is no standard for the taxon.
In Amazon coast, the sex ratio of L. purpureus was significantly female-biased, as also recorded by Souza et al., 2003 in this region. Sex ratios vary considerably in other L. purpureus populations found around the Brazilian coast (Table 3), indicating that this parameter may be influenced by a range of factors, including the type of marine environment, latitude, fishing pressure, and even the sampling method (Rijnsdorp et al., 2010).
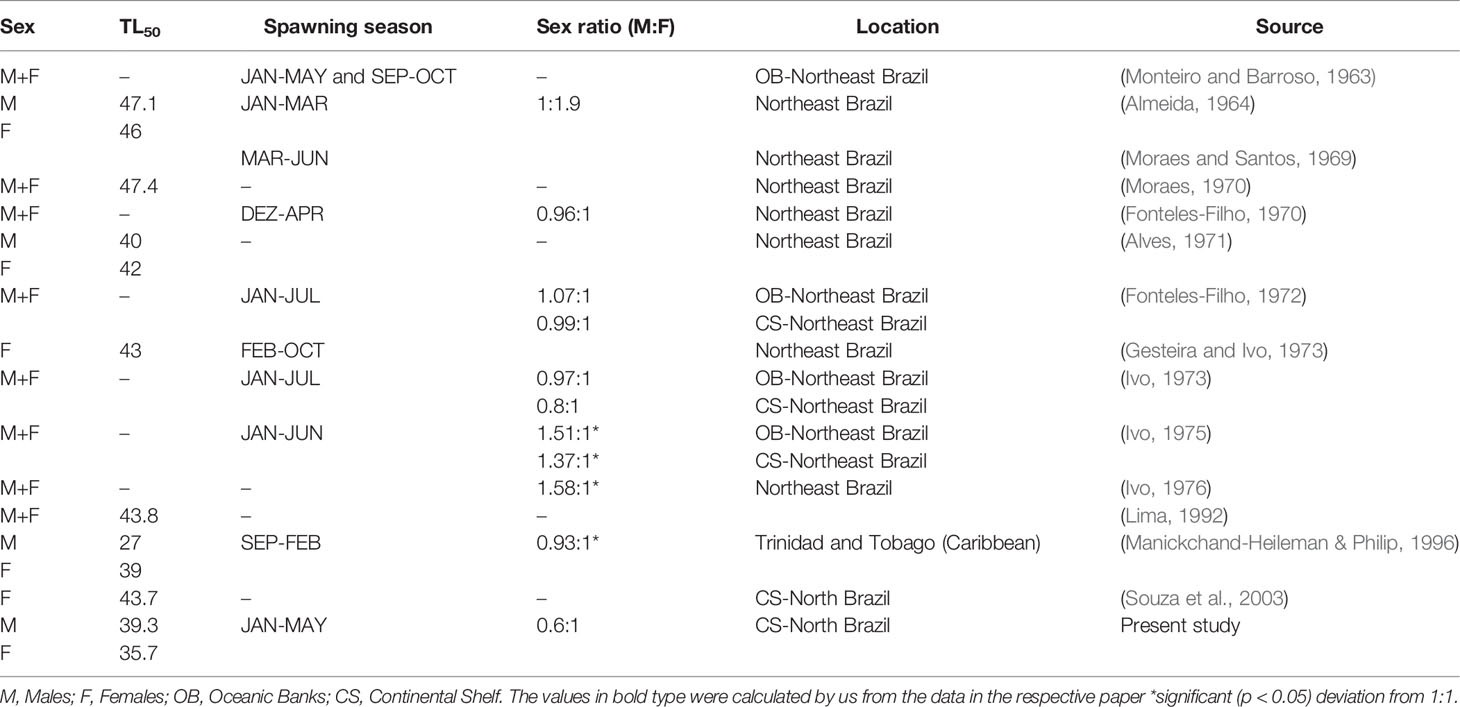
Table 3 Variation in the reproductive parameters of Lutjanus purpureus populations from other regions of South America.
While L. purpureus is potentially reproductive throughout the year, a peak in spawning during the first half of the year is typical of the populations found off the South American coast (Table 3). Some studies have reported a second, less intense spawning peak during the second half of the year, although no evidence of such a peak was found in the present study (Table 3).
Extended spawning periods are typical of marine fish populations at latitudes close to the Equator (Johannes, 1978), and the year-round presence of mature lutjanid females is common in tropical coastal regions (Grimes, 1987; Claro et al., 2003; Fry et al., 2009; Lowerre-Barbieri et al., 2015; Kulaw et al., 2017), although a peak (collective spawning) may typically occur when water temperatures increase or during the summer rains (Grimes, 1987). The results of the present study are consistent with this pattern, given the observed peak in spawning during the peak discharge of the Amazon River, between January and May.
Considering that L. purpureus and L. campechanus are highly similar, both morphologically and genetically, and probably diverged as distinct species very recently (Anderson, 1967; Gomes et al., 2008; Del Pedraza-Marrón et al., 2019), the peak spawning of L. purpureus recorded off the Amazon coast during the rainy season is possibly related to the greater availability of resources due to the increased volume of the Amazon River discharge.
The ACM represents one of the world’s most complex and dynamic continental margins, due to its long-term interaction with the Amazon River (Lavagnino et al., 2020). The freshwater of the Amazon River, apparently, functions as an important biogeographic barrier separating the coastal fish faunas of Brazil and the Caribbean (Floeter and Gasparini, 2000). In addition, the presence of the Brazilian North Coastal Current retroflexion that occurs in this environment may support self-recruitment of L. purpureus, as has been observed for lobster in this environment (Cruz et al., 2021).
In addition to an extended reproductive period, several lutjanid species have a multiple spawning pattern. These species include L. purpureus (González and Lugo, 1997), L. campechanus (Brulé et al., 2010; Kulaw et al., 2017), Lutjanus synagris (Freitas et al., 2014), Lutjanus peru (Santamaria and Villalejo, 2003), Lutjanus guttatus (Arellano-Martínez et al., 2001), and Lutjanus alexandrei (Fernandes et al., 2012), all of which present asynchronous oocyte development in the mature (SPW) females, which indicates the production of several lots of eggs during the spawning period. Porch et al. (2015) estimated spawning frequencies in L. campechanus ranging from 29 (age = 2 years) to 71 (age >10 years) spawns per year. Lutjanids spawn typically in reproductive aggregations, which can be mapped by in loco observation (by divers or subaquatic camera systems) or the analysis of the variation in catches, with areas with greater than average CPUEs (Catch Per Unit Effort) indicating reproductive aggregations, as observed in Lutjanus jocu and L. synagris (França and Olavo, 2015) and Lutjanus gibbus (Nanninga and Spaet, 2017).
Based on the relative abundance of the different stages of sexual maturation, Ivo and Hanson (1982) suggested two hypotheses of reproductive migration for L. purpureus. One hypothesis is that there is just one stock of mature (SPW) adults, which migrates from the Amazon continental shelf (feeding grounds) to oceanic banks (areas of reproductive aggregations) off the northeastern coast of Brazil (between 32° W and 39° W) twice a year, in the first and third quarters. This process entails a migration of approximately 1,300 km. In the second hypothesis, the migratory cycle is the same, but the migration is undertaken by two stocks, rather than a single one. In this case, each stock would migrate once a year in either the first or the third quarter. In both cases, the eggs and larvae would be transported back to the Amazon coast by the North Brazil current and other currents that run parallel to the coast. These hypotheses were based on the data available from previous studies, which indicated a high frequency of spawning capable females (65–85% of the sample) at oceanic banks, which decreased progressively toward the Amazon continental shelf. In the present study, however, as well as Souza et al. (2003), a considerable percentage of mature (SPW) females was recorded off the Amazon coast, which indicates the existence of reproductive aggregations within this area.
The evidence on the movement patterns of L. campechanus in the Gulf of Mexico, based on recapture and telemetry data, indicates that these snapper school on natural and artificial reefs, showing high site fidelity and short migrations to breeding grounds (Szedlmayer and Shipp, 1994; Schroepfer and Szedlmayer, 2006; Strelcheck et al., 2007; Campbell et al., 2011; Topping and Szedlmayer, 2011a, 2011b; Piraino et al., 2014). Therefore, this pattern of behaviour might be is expected in L. purpureus due to the phylogenetic proximity with L. campechanus, but further studies should be conducted to better understand this hypothesis.
Several studies of reproductive aggregations indicate that snappers prefer environments adjacent to coral reefs in the outer continental shelf or shelf edge (Claro et al., 2003; Heyman et al., 2005; Heyman and Kjerfve, 2008; Coleman et al., 2011; Farmer et al., 2017). Johnson et al. (2009) observed that, after L. campechanus spawns, its eggs and larvae are transported by currents, moving up to 480 km from the spawning grounds in approximately four weeks. These currents favor the transportation of the initial, planktonic stages and may account for the lack of genetic divergence in the population (Gold et al., 1997; Hollenbeck et al., 2015). This dispersion of the initial ontogenetic stages also appears to occur in L. purpureus, given that a multi-locus genetic analysis revealed an overall genetic homogeneity within the distribution of the specie off the Brazilian coast (Da Silva et al., 2016). Considering that the northern shelf of Brazil represents one of the most complex and dynamic continental margins in the world, due to its long-term interaction with the Amazon River (Lavagnino et al., 2020). The freshwater of the Amazon River apparently acts as an important biogeographic barrier separating the coastal fish faunas of Brazil and the Caribbean (Floeter and Gasparini, 2000). In addition, the presence of the Brazilian North Coastal Current retroflexion that occurs in this environment may support self-recruitment of L. purpureus, as has been observed for lobsters from this environment (Cruz et al., 2021). Even so, further, more detailed studies are needed to better evaluate the larval dispersion of L. purpureus in this region.
After the planktonic stage, the larvae metamorphose at 26–30 days and are recruited in environments over sandy, muddy or shell substrates (Szedlmayer and Howe, 1997; Szedlmayer and Conti, 1999; Rooker et al., 2004; Dance et al., 2018). The juveniles then migrate to deeper waters, where they develop and grow, inhabiting vertical substrates, including rock formations and coral reefs (Gallaway et al., 2009; Dance et al., 2018).
Although the ontogenetic migrations of lutjanids and their association with reefs are well documented (Szedlmayer and Howe, 1997; Aschenbrenner et al., 2016), the existence of a major reef (~56,000 km2, at depths of 70–220 m) on the Amazon continental shelf was confirmed recently (Moura et al., 2016; Francini-filho et al., 2018). In this area there are many commercially important species, such as crustaceans (e.g. Palinuridae) and fish of various taxonomic groups, mainly Epinephelidae and Lutjanidae (Moura et al., 2016), in which L. purpureus stands out due to high productivity and is mainly marketed to the USA (Freire, 2019).
Reproductive aggregations of commercially important reef fishes have lost in many areas of tropical region due to intense pressure from fisheries (Sadovy, 1996; Mitcheson et al., 2008; Mitcheson and Colin, 2012). The mapping and management of the areas in which reproductive aggregations occur are essential for the maintenance of stocks. Without protection, the exploitation of these areas can lead to the rapid exhaustion of fishery stocks (Sadovy and Cheung, 2003; Sala et al., 2003). This emphasizes the need to identify the areas in which reproductive aggregations of L. purpureus form off the Amazon coast, in order to develop adequate fishery management strategies. Furthermore, the progressive increase in pressure from various sectors, such as oil, gas and mining, to implement blocks in this environment has been a matter of great concern, with more than a hundred oil and gas exploration blocks having been auctioned by Brazil since 2012 (Francini-Filho et al., 2018, Lavagnino et al 2020, Araújo et al., 2021) and it is therefore that new studies be conducted immediately.
In the present study, the length at first gonadal maturity (L50) of L. purpureus was larger in the males than in the females, contradicting the pattern observed in previous studies of this species, in which the females generally have a larger L50 (Table 3). The differences in the L50 values between northern and northeastern Brazil may be associated with the differences in sampling, given that the species is traditionally captured in the vicinity of islands and oceanic banks, where reproductive aggregations are found. These areas tend to be inhabited by individuals of greater mean body length. In the present study on the Amazon coast, the estimated length at first maturity was 18.2% (7.95 cm) smaller than that estimated by Souza et al., 2003. This may reflect a process of adaptation to the intense fishing pressure on the local L. purpureus stock, which typically results in a reduction in this parameter (Trippel, 1995).
In Brazil, commercial fishing of L. purpureus began in the 1960s with a fishery fleet operating off the northeastern coast, initially over oceanic banks, in areas occupied by reproductive aggregations, shifting subsequently to the continental shelf, near the 50 m isobath (Fonteles-Filho, 1972; Ivo and Hanson, 1982). As stocks declined in response to intense fishing pressure, the snapper fleet migrated to the Amazon shelf, where it was consolidated during the 1990s (Ivo and Rocha, 1988; Paiva, 1997; Fonteles-Filho, 2007). It is believed that the depletion of stocks resulted in particular from intense fishing over the oceanic banks where reproductive aggregations are located, resulting in an increase in the catches of juveniles, and the reduction of the L50 from 46 cm to 42 cm (4 cm) in 10 years (Almeida, 1964; Gesteira and Ivo, 1973; Ximenes and Fonteles-Filho, 1988).
An increase in the numbers of individuals that have reached a body size and sexual maturity (L50) precociously is an effective mechanism for the maintenance of the genetic variability of subsequent generations, although it also results in reduced fertility and a decline in new recruitment (Trippel, 1995; Vazzoler, 1996). Estimates of L50 are essential fishery management tools, because they can be used to define the minimum size at which fish should be harvested, to avoid the significant capture of juveniles. While Brazilian legislation regulates snapper fisheries through controls on the specifications of the fishing gear (SEAP, 2018) to avoid the selection of immature specimens (> L50), such as size of hook (longline) and mesh opening (trap), the harvesting of large numbers of juveniles by commercial fisheries is still common, as observed in this study.
The fishing pressure on the immature stock or recently matured individuals may threaten the sustainability of fisheries (Claro et al., 2003, 2009; Freitas et al., 2011), which led Souza et al. (2008) to propose a prohibition on the use of traps by commercial fisheries. Although the traps present a high frequency of young people (42.1%), the longline also increases preoccupation, as it captures 33.7% of juveniles. In both cases, the high occurrence of juveniles is probably related to the lack of inspection of the gear used in the fisheries, as Freire (2019) noted that the fishing gear used by the commercial fleet off the Amazon coast does not comply with regulations. However, due to this problem and the large number of fishermen specialized in these types of capture, it is necessary for the government to implement guidance and inspection measures for fishing gear to ensure compliance with the legislation in force (see SEAP, 2018).
Additionally, considering the data presented in this study and the current situation of declining fish stocks of L. purpureus, an assessment is recommended for the inclusion of the species in the IUCN Red List of Threatened Species, which is currently a species categorized as "vulnerable" in the Brazilian Red List (see Ferreira et al., 2018).
Conclusions
A new insight for reproduction of L. purpureus was proposed, in which we declined the previously accepted hypothesis for the species, since there is clear evidence that L. purpureus reproduces on the Amazon coast of Brazil. We also estimated size at first maturity (L50 = 32.1 cm in females), which indicated a decline in mean size, which is probably being influenced by intense fishing pressure.
Although the results of this study show a catch percentage of juveniles >50%, it is still a significant percentage of juveniles that are being caught due to little or no guidance and enforcement of fishing gear to ensure compliance with current legislation.
In addition, detailed research on stock movements and identification of areas of spawning aggregation are essential for the development of fisheries management measures, as well as indicating areas of protection due to increasing pressure from other sectors such as the oil and gas.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The data were from monitored fishing landings, so the animal study was reviewed and approved by Comissão de Ética no Uso de Animais.
Author Contributions
JLF: Conceptualization; data curation; formal analysis; investigation; methodology; resources; visualization; roles/writing - original draft and writing - review e editing. GCS: Data curation; investigation; supervision; visualization and roles/writing - original draft. IL: Data curation; investigation; visualization; roles/writing - original draft and writing - review e editing. BB: Conceptualization; formal analysis; funding acquisition; methodology; resources; supervision; validation and roles/writing - original draft. VJI: Conceptualization; formal analysis; resources; validation; visualization. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), by granting a master's scholarship to JLF. The Article Processing Charges (APCs) were granted by the Programa de Apoio à Publicação Qualificada (PAPQ)/Universidade Federal do Pará (UFPA).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to the owners and crews of the fishing vessels, who donated samples for analysis in this study.
References
Allen G. R. (1985). Snappers of the World: An Annotated and Illustrated Catalogue of the Lutjanid Species Known to Date. Rome: FAO Species Catalogue vol 6.
Almeida N. U. M. (1964). Estudos Preliminares Sobre a Primeira Maturação Sexual, Época De Desova E ‘Sex-Ratio’ Do Pargo (Lutjanus aya) Na Costa Nordeste Do Brasil. Trop. Oceanogr. 5, 147–58. doi: 10.5914/tropocean.v5i1.2496
Almeida N. U. M. (1971). Sobre a Maturação Sexual Do Pargo, Lutjanus purpureus (Poey, 1866), Do Nordeste Brasileiro. Arq. Cienc 11, 153–58.
Alves M. I. M (1971). Sobre a Maturação Sexual Do Pargo, Lutjanus purpureus (Poey, 1866), Do Nordeste Brasileiro. Arq. Cienc 11, 153–58.
Araújo L. S , Magdalena R., Louzada T. S., Salomon P. S., Moraes C., Ferreira B. P., et al (2021). Growing Industrialization and Poor Conservation Planning Challenge Natural Resources’ Management in the Amazon Shelf off Brazil. Mar. Pol. 128, 104465. doi: 10.1016/j.marpol.2021.104465
Arellano-Martínez M., Rojas-Herrera A., García-Domínguez F., Ceballos-Vázquez B. P., Villalejo-Fuerte M. (2001). Ciclo Reproductivo Del Pargo Lunarejo Lutjanus guttatus (Steindachner, 1869) en Las Costas De Guerrero, México. Rev. Biol. Mar. Oceanog 36, 1–8. doi: 10.4067/S0718-19572001000100001
Aschenbrenner A., Hackradt C. W., Ferreira B. P. (2016). Spatial Variation in Density and Size Structure Indicate Habitat Selection Throughout Life Stages of Two Southwestern Atlantic Snappers. Mar. Environ. Res. 113, 49–55. doi: 10.1016/j.marenvres.2015.10.013
Anderson W. D. (1967). Field Guide to the Snappers (Lutjanidae) of the Western Atlantic. (United States Department of the Interior:Fish and Wildlife Service, Bureau of Commercial Fisheries).
Anderson W. D. (2002). Suborder Percoidei: Lutjanidae" in The Living Marine Resources of the Western Central Atlantic - Volume 3: Bony Fishes Part 2 (Opistognathidae to Molidae), Sea Turtles and Marine Mammals. Eds. K. E. Carpenter. (Rome: FAO Species Identification Guide for Fishery Purposes and American Society of Ichthyologists and Herpetologists, Special Publication, No. 5), 1479–1504
Bentes B., Isaac V. J., Espírito-Santo R. V., Frédou T., Almeida M. C., Mourão K. R. M., et al (2012). Multidisciplinary Approach to Identification of Fishery Production Systems on the Northern Coast of Brazil. Biota Neotrop 12, 81–92. doi: 10.1590/S1676-06032012000100006
Brulé T., Colás-Marrufo T., Pérez-Díaz E., Sámano-Zapata J. (2010). Red Snapper Reproductive Biology in the Southern Gulf of Mexico. Trans. Am. Fish. Soc. 139, 957–68. doi: 10.1577/T09-125.1
Campbell M. D., Rose K. A., Cowan J. (2011). Individual-Based Modeling of an Artificial Reef Fish Community: Effects of Habitat Quantity and Degree of Refuge. Ecol. Modell. 222, 3895–3909. doi: 10.1016/j.ecolmodel.2011.10.009
Claro R., Lindeman K.C. (2003). Spawning Aggregation Sites of Snapper and Grouper Species (Lutjanidae and Serranidae) on the Insular Shelf of Cuba. Gulf Caribb. Res 14, 91–106. doi: 10.18785/gcr.1402.07
Claro R., Mitcheson Y.S., de Lindeman K.C., García-Cagide A.R (2009). Historical Analysis of Cuban Commercial Fishing Effort and the Effects of Management Interventions on Important Reef Fishes From 1960-2005. Fish. Res 99, 7–16. Cepnor. doi: 10.1016/j.fishres.2009.04.004
Coleman F. C., Scanlon K. M., Koenig C. C. (2011). Groupers on the Edge?: Shelf Edge Spawning Habitat in and Around Marine Reserves of the Northeastern Gulf of Mexico. Prof. Geogr. 63, 456–74. doi: 10.1080/00330124.2011.585076
Coles V. J., Brooks M. T., Hopkins J., Stukel M. R., Yager P. L., Hood R.R. (2013). The Pathways and Properties of the Amazon River Plume in the Tropical North Atlantic Ocean. J. Geophys. Res. Oceans 118, 6894–6913. doi: 10.1002/2013JC008981
Collette B.B., Rutzler K. (1977). “Reef Fishes Over Sponge Bottoms of the Mouth of Amazon River, ” in Proceedings of the Third International Coral Reef SymposiumBol. Téc. Cient. 305–310. Miami, FL.
Collins L. A., Fitzhugh G. R., Mourand L., Lombardi L. A., Walling W. T., Fable W. A., et al (2001). Preliminary Results From a Continuing Study of Spawning and Fecundity of the Red Snapper (Lutjanidae: Lutjanus campechanus) From the Gulf of Mexico, 1998 - 1990. Gulf and Caribbean Fisheries Institute Proceedings.
Costa G.F., Holanda F.C.A.F., Furtado-Júnior I., Silva J.A. (2017). A Tecnologia de pesca Industrial do pargo, Lutjanus purpureus (Poey, 1876) da frota Bragantina-Pará- Brasil Bol. Téc. Cient. Cepnor 17, 21–27. doi: 10.32519/tjfas.v17i1.2142.
Cruz R., Torres M. T., Santana J. V. M., Cintra I .H .A. (2021). Lobster Distribution and Biodiversity on the Continental Shelf of Brazil: A Review. Diversity 13, 507. doi: 10.3390/d13110507
Da Silva R., Sampaio I., Schneider H., Gomes G. (2016). Lack of Spatial Subdivision for the Snapper Lutjanus purpureus (Lutjanidae: Perciformes) From Southwest Atlantic Based on Multi-Locus Analyses. PLoS One 11, e0161617. doi: 10.3390/d13110507
Da Silva R., Pedraza-Marrón C.D.R., Sampaio I., Betancur-R R., Gomes G., Schneider H. (2020). New Insights About Species Delimitation in Red Snappers (Lutjanus purpureus and L. campechanus) Using Multilocus Data. Mol. Phylogenet. Evol. 147, 106780. doi: 10.1016/j.ympev.2020.106780
Del Pedraza-Marrón C.R., Silva R., Deeds J., Van Belleghem S.M., Mastretta-Yanes A., Domínguez-Domínguez O., et al (2019). Genomics Overrules Mitochondrial DNA, Siding With Morphology on a Controversial Case of Species Delimitation Proc. R. Soc. B 286 20182924. doi: 10.1098/rspb.2018.2924.
Farmer N. A., Heyman W. D., Karnauskas M., Kobara S., Smart T. I., Ballenger J. C., et al (2017). Timing and Locations of Reef Fish Spawning Off the Southeastern United States PLoS One 12, e0172968. doi: 10.1371/journal.pone.0172968.
Fernandes C. A. F., de Oliveira P. G. V., Travassos P. E. P, Hazin F. H. V. (2012). Reproduction of the Brazilian Snapper, Lutjanus alexandrei Moura & Lindeman, 2007 (Perciformes: Lutjanidae), Off the Northern Coast of Pernambuco, Brazil Neotrop. Ichthyol. 10, 587–92. doi: 10.1590/S1679-62252012005000022.
Ferreira B.F., Di Dario F., Frédou F.L., Dias-Neto J., Santos R.A., Freitas M., et al (2018). “Lutjanus purpureus”. In ICMBIO (Instituto Chico Mendes de Conservação da Biodiversidade) (Org.), Livro Vermelho da Fauna Brasileira Ameaçada de Extinção: Peixes. Brasília: ICMBio Vol. VI, 844–850.
Floeter S. R., Gasparini. J. L. (2000). The Southwestern Atlantic Reef Fish Fauna: Composition & Zoogeographic Patterns. J. Fish Biol. 56, 1099–1114. doi: 10.1006/jfbi.2000.1231
Fonteles-Filho A. A. (1970). Estudo sobre a Biologia da pesca do Pargo Lutjanus purpureus (Poey, 1866) no Nordeste Brasileiro: dados de 1969. Arq. Cienc. Mar. 10, 73–78.
Fonteles-Filho A. A. (1972). Estudo Sobre a Biologia Da Pesca Do Pargo Lutjanus purpureus (Poey 1866), No Nordeste Brasileiro: Dados De 1970 E 1971. Arq. Cienc. Mar. 12, 21–26.
Fonteles-Filho A. A. (2007). Síntese Sobre a Distribuição, Abundância, Potencial Pesqueiro, E Biologia Do Pargo, Lutjanus purpureus, (Poey 1866) Da Zona Econômica Exclusiva Do Nordeste Do Brasil.
França A. R., Olavo G. (2015). Indirect Signals of Spawning Aggregations of Three Commercial Reef Fish Species on the Continental Shelf of Bahia, East Coast of Brazil. Braz. J. Oceanogr. 63, 289–301. doi: 10.1590/S1679-87592015087506303
Freire J. L. (2019). Pesca, reprodução e crescimento de Lutjanus purpureus (Lutjanidae-Perciformes) no recife Amazônico. [thesis/doctor thesis]. Belém (PA)]: Universidade Federal do Par
Freire K. M. F., Almeida Z. D. S. D., Amador J. R. E. T., Aragão J. A., Araújo A. R. D. R., Ávila-da-Silva A. O., et al (2021). Reconstruction of Marine Commercial Landings for the Brazilian Industrial and Artisanal Fisheries From 1950 to 2015. Front. Mar. Sci. 8, 659110. doi: 10.3389/fmars.2021.659110
Freitas M. O., Moura R. L. D. E., Bastos R., Minte-vera C. V. (2011). Spawning Patterns of Commercially Important Reef Fish (Lutjanidae and Serranidae) in the Tropical Western South Atlantic. Sci. Mar. 75, 135–146. doi: 10.3989/scimar.2011.75n1135
Freitas M. O., Rocha G. R. A., Chaves P. D. T. D. C., De Moura R. L. (2014). Reproductive Biology of the Lane Snapper, Lutjanus synagris, and Recommendations for Its Management on the Abrolhos Shelf. Brazil. J. Mar. Biol. Assoc. U. K. 94, 1711–20. doi: 10.1017/S0025315414001088
Fry G., Milton D.A., Van Der Velde T., Stobutzki I., Andamari R., Badrudin , et al (2009). Reproductive Dynamics and Nursery Habitat Preferences of Two Commercially Important Indo-Pacific Red Snappers Lutjanus erythropterus and L. malabaricus. Fisheries Sci. 75, 145–58. doi: 10.1007/s12562-008-0034-4
Furtado-Júnior I., Tavares M. C. S., Brito C. S. F. (2006). Estatísticas Das Produções De Pescado Estuarino E Marítimo Do Estado Do Pará E Políticas Pesqueiras Pará. Bol. Mus. Pará Emílio Goeldi Cienc. Hum. 1, 95–111.
Francini-Filho R.B., Asp N.E., Siegle E., Hocevar J., Lowyck K., D'Avila N., et al (2018). Perspectives on the Great Amazon Reef: Extension, Biodiversity and Threats. Front. Mar. Sci. 5, 142. doi:
Gallaway B. J., Szedlmayer S. T., Gazey W. J. (2009). A Life History Review for Red Snapper in the Gulf of Mexico With an Evaluation of the Importance of Offshore Petroleum Platforms and Other Artificial Reefs. Rev. Fish. Sci. 17, 48–67. doi: 10.1080/10641260802160717
Gesteira T. C. V., Ivo C. T. C. (1973). Estudo Da Reprodução E Fecundidade Do Pargo, Lutjanus purpureus Poey, Do Norte E Nordeste Do Brasil. Arq. Cienc. Mar 13, 109–112.
Geyer W., Beardsley R. C., Lentz S.J., Candela J., Limeburner R., Johns W. E., et al (1996). Physical Oceanography of the Amazon Shelf. Cont. Shelf Res. 16, 575–616. doi: 10.1016/0278-4343(95)00051-8
Gold J. R., Sun F., Richardson L. R. (1997). Population Structure of Red Snapper from the Gulf of Mexico as Inferred from Analysis of Mitochondrial DNA. Trans. Am. Fish. Soc. 126, 386–96. doi: 10.1577/1548-8659(1997)126<0386:PSORSF>2.3.CO;2
Gomes G., Schneider H., Vallinoto M., Santos S., Orti G. (2008). Can Lutjanus purpureus (South Red Snapper) Be “ Legally ” Considered a Red Snapper (Lutjanus campechanus). Genet. Mol. Biol. 31, 372–76.
González L. W., Lugo T. (1997). Ovogenesis De Lutjanus purpureus Poey, 1867 (Pisces: Lutjanidae) De La Region Oriental De Venezuela. Bol. Invest. Mar. Cost. 26, 53–60.
Grandcourt E. M., Francis F., Hartmann S. (2009). Reproductive Biology and Implications for Management of the Orange-Spotted Grouper Epinephelus coioides in the Southern Arabian Gulf. J. Fish Biol. 74, 820–41. doi: 10.1111/j.1095-8649.2008.02163.x
Grimes E. M., Francis F., Hartmann S. (1987). ’Reproductive Biology of the Lutjanidae: A Review’, in Tropical Snappers and Groupers: Biology and Fisheries Management ed. Polovina J. J., Ralston S.. (Boulder: Westview Press), 271–94.
Gualberto L. P. S., El-Robrini M. (2015). Faciologia Da Cobertura Sedimentar Superficial Da Plataforma Continental Do Maranhão. Estud. Geol. 15, 234–43.
Heyman W. D., Kjerfve B., Graham R. T., Rhodes K. L., Garbutt L. (2005). Spawning Aggregations of Lutjanus Cyanopterus (Cuvier) on the Belize Barrier Reef Over a 6 Year Period. J. Fish Biol. 67, 83–101. doi: 10.1111/j.0022-1112.2005.00714.x
Heyman W. D., Kjerfve B. (2008). Characterization of Transient Multi-Species Reef Fish Spawning Aggregations at Gladden Spit, Belize. Bull. Mar. Sci. 83, 531–51.
Hollenbeck C. M., Portnoy D. S., Saillant E., Gold J. R. (2015). Population Structure of Red Snapper (Lutjanus campechanus) in U.S. Waters of the Western Atlantic Ocean and the Northeastern Gulf of Mexico. Fish. Res. 172, 17–25. doi: 10.1016/j.fishres.2015.06.020
Ivo C.T.C., Sousa M.J.B. (1988). Sinopse de informações sobre o pargo, Lutjanus purpureus Poey (Pisces: Lutjanidae) no Norte e Nordeste do Brasil. Arq. Cienc. Mar. 27, 57–67.
Ivo C.T.C. (1973). Estudo Da Biologia E Pesca Do Pargo, Lutjanus purpureus Poey, No Nordeste Brasileiro: Dados De 1973. Arq. Cienc. Mar. 13, 113–16.
Ivo C.T.C. (1975). Estudo Da Biologia E Pesca Do Pargo, Lutjanus purpureus Poey, No Nordeste Brasileiro: Dados De 1974. Arq. Cienc. Mar. 15, 119–23.
Ivo C.T.C. (1976). Estudo Da Biologia E Pesca Do Pargo, Lutjanus purpureus Poey, No Nordeste Brasileiro: Dados De 1975. Arq. Cienc. Mar. 16, 87–91.
Ivo C.T.C., Hanson A. J. (1982). Aspectos da biologia e dinâmica populacional do pargo Lutjanus purpureus Poey, no Norte e Nordeste do Brasil. Arq. Cienc. Mar. 22, 1–41.
Ivo C.T.C., Rocha C. A. S. (1988). Estudo da seletividade do Anzol na captura do pargo Lutjanus purpureus Poey (Pisces: Lutjanidae), no Norte e Nordeste do Brasil. Arq. Cienc. Mar. 27, 79–56.
Jakobsen T., Fogarty M. J., Megrey B. A., Moksness E. (2016). Fish Reproductive Biology: Implications for Assessment and Management. West Sussex: John Wiley & Sons
Johannes R. E. (1978). Reproductive Strategies of Coastal Marine Fishes in the Tropics. Environ. Biol. Fishes 3, 65–84. doi: 10.1007/BF00006309
Johnson D. R., Perry H. M., Lyczkowski-Shultz J., Hanisko D. (2009). Red Snapper Larval Transport in the Northern Gulf of Mexico. Trans. Am. Fish. Soc. 138, 458–70. doi: 10.1577/T08-008
King M. (2013). Fisheries Biology, Assessment and Management. 2nd edn. Oxford: Blackwell Publishing.
Kulaw D. H., Cowan J. H., Jackson M. W. (2017). Temporal and Spatial Comparisons of the Reproductive Biology of Northern Gulf of Mexico (USA) Red Snapper (Lutjanus campechanus) Collected a Decade Apart. PLoS One 12, 1–39. doi: 10.1371/journal.pone.0172360
Lavagnino A.C., Bastos A.C., Amado-Filho G.M., De Moraes F.C., Araujo de Moura L.S. R.L. (2020). Geomorphometric Seabed Classification and Potential Megahabitat Distribution in the Amazon Continental Margin. Front. Mar. Sci. 7, 190. doi: 10.3389/fmars.2020.00190
Lima , A. C. N. D. (1992). Aspectos do Estudo da Biologia Pesqueira do pargo, Lutjanus purpureus Poey, no Norte e Nordeste do Brasil. [undergraduate thesis]. [Fortaçeza]: Universidade Federal do Ceará.
Lowerre-Barbieri S., Crabtree L., Switzer T., Burnsed S. W., Guenther C. (2015). Assessing Reproductive Resilience: An Example With South Atlantic Red Snapper Lutjanus campechanus. Mar. Ecol. Prog. Ser. 526, 125–41. doi: 10.3354/meps11212
Mahiques M. M., Siegle E., Francini-Filho R. B., Thompson F. L., Rezende C. E., Gomes J. D., et al (2019). Insights on the Evolution of the Living Great Amazon Reef System, Equatorial West Atlantic. Sci. Reports 9, 13699. doi: 10.1038/s41598-019-50245-6
Manickchand-Heileman S. C., Philip S. A. T. (1996). ’Reproduction, Age and Growth of the Caribbean Red Snapper (Lutjanus purpureus) in the Waters Off Trinidad and Tobago’ in Biology, Fisheries and Culture of Tropical Groupers and Snappers ed. Arreguln-Sanchez F., Munro J. L., Balgos M. C., Pauly D.. (ICLARM Conference Proceedings), 137–49.
Mitcheson Y. S., Cornish A., Domeier M., Colin P. L., Russell M., Lindeman K. C., et al (2008). A Global Baseline for Spawning Aggregations of Reef Fishes. Conserv. Biol. 22, 1233–44. doi: 10.1111/j.1523-1739.2008.01020.x
Mitcheson Y. S., Colin P. L. (2012). Reef Fish Spawning Aggregations: Biology, Research and Management. New York: Springer Science & Business Media.
Morgan M. J. (2008). Integrating Reproductive Biology Into Scientific Advice for Fisheries Management. J. Northwest Atl. Fishn. Sci. 41, 37–51. doi: 10.2960/J.v41.m615
Morgan M. J., Perez-Rodriguez A., Saborido-Rey F., Marshall C. T. (2011). Does Increased Information About Reproductive Potential Result in Better Prediction of Recruitment?. Can. J. Fish. Aquat. Sci. 68, 1361–68. doi: 10.1139/f2011-049
Mohriak W. U. (2003). ’Bacias Sedimentares Da Margem Continetal Brasileira’ in Geologia, Tectônica e Recursos Minerais do Brasil: textos, mapas & SIG ed. Bizzi L. A., Schobbenhaus C., Vidotti R. M., Gonçalves J. H. (Brasília), 87–94.
Monteiro N. U., Barroso L. (1963). Estudo Sobre O Ciclo Sexual E O Regime Alimentar Do Pargo. Bol. Inst. Pesca 3, 13–20.
Moraes N. U. A., Santos E. P. (1969). Sobre a Curva De MaturaÇão Do Pargo, Lutjanus purpureus - Poey. Bol. Inst. Pesca 9, 52–7.
Moraes N. U. A. (1970). Sobre a Curva De MaturaÇão Do Pargo, Lutjanus purpureus - Poey. Bol. Inst. Pesca 10, 7–20.
Moraes N. U. A. (1970). Sobre a Curva De MaturaÇão Do Pargo, Lutjanus purpureus - Poey. Bol. Inst. Pesca 10, 7–20.
Moura R. L., Amado-Filho G. M., Moraes F. C., Brasileiro P. S., Salomon P. S., Mahiques M. M., et al (2016). An Extensive Reef System at the Amazon River Mouth. Sci. Adv. 2, e1501252. doi: 10.1126/sciadv.1501252
Nanninga G. B., Spaet J. L. Y. (2017). Spawning Aggregations of the Humpback Red Snapper, Lutjanus gibbus, in the Tuamotus, French Polynesia. Mar. Biodivers 47, 375–76. doi: 10.1007/s12526-016-0497-y
Nittrouer C.A., DeMaster D.J. (1986). Sedimentary processes on the Amazon Continental Shelf: Past, Present and Future Research. Cont. Shelf. Res. 6, 5–30. doi: 10.1016/0278-4343(86)90051-8
Omachi C.Y., Asp N.E., Siegle E., Couceiro M.A.A., Francini-filho R.B., Thompson F.L., et al (2019). An Extensive Reef System at the Amazon River Mouth. Sci. Adv. 2, e1501252. doi: 10.1126/sciadv.1501252
Piraino M. N., Szedlmayer S. T., Piraino M. N., Szedlmayer S. T. (2018). Feeding Ecology of Fishes Associated With Artificial Reefs in the Northwest Gulf of Mexico. PLoS One 13, 1–25. doi: 10.1371/journal.pone.0203873
Porch C. E., Fitzhugh G. R., Lang E. T., Lyon H. M., Linton B. C. (2015). Estimating the Dependence of Spawning Frequency on Size and Age in Gulf of Mexico Red Snapper. Mar. Coast Fish 7, 233–45. doi: 10.1080/19425120.2015.104056
Rijnsdorp A. D., Damme C. J. G. V., Witthames P. R. (2010). Implications of Fisheries Induced Changes Stock Structure and Reproductive Potencial for Stock Recovery of a Sex Dimorphic Species, North Sea Plaice. ICES J. Mar. Sci. 67, 1931–38. doi: 10.1093/icesjms/fsq049
Rooker J. R., Landry A. M., Geary B. W., Harper J. A. (2004). Assessment of a Shell Bank and Associated Substrates as Nursery Habitat of Post Settlement Red Snapper. Estuar. Coast. Shelf Sci. 59, 653–61. doi: 10.1016/j.ecss.2003.11.009
Sadovy Y. (1996). ’Reproduction of Reef Fishery Species’, in . Reef Fisheries ed. Polunin N. V. C., Roberts C. M. (Dordrecht: Springer), 15–59.
Sadovy Y., Cheung W. L. (2003). Near Extinction of a Highly Fecund Fish: The One That Nearly Got Away. Fish Fish 4, 86–89. doi: 10.1046/j.1467-2979.2003.00104.x
Sala E., Aburto-Oropeza O., Paredes G., Thompson G. (2003). Spawning Aggregations and Reproductive Behavior of Reef Fishes in the Gulf of California. Bull. Mar. Sci. 72, 103–21.
Santamaria A., Villalejo M. (2003). Desarrollo Gonadal Y Ciclo Reproductivo De Lutjanus peru (Pisces?: Lutjanidae) en Guerrero, México. Rev. Biol. Trop. 51, 1–12.
SEAP. Secretaria Especial da Aquicultura e da Pesca (2018). Portaria Interministerial N°42, de 27 de Julho de 2018. Define regras para o uso sustentável e a recuperação dos estoques da espécie Lutjanus purpureus (pargo). Presidência da República, Casa Civil
Schroepfer R. L., Szedlmayer S. T. (2006). Estimates of Residence and Site Fidelity for Red Snapper Lutjanus campechanus on Artificial Reefs in the Northeastern Gulf of Mexico. Bull. Mar. Sci. 78, 93–101.
Strelcheck A. J., Cowan J. H., Patterson W. F. (2007). ’Site fidelity, movement, and growth of red snapper: Lutjanus campechanus: Implications for artificial reef management’, in Population Ecology and Fisheries of U.S. Gulf of Mexico Red Snapper ed. Patterson W. F., Cowan J. H., Fitzhugh G. R., Nieland D. J. (Bethesda, Maryland: American Fisheries Society Symposium 60), 135–48.
Silva A. C., Santos M. L. S., Araujo M. C., Bourlés B. (2009). Observações Hidrológicas E Resultados De Modelagem No Espalhamento Sazonal E Espacial Da Pluma De Áagua Amazônica. Acta Amazon. 39, 361–69.
Soares M.D.O., Tavares T.C.L., Carneiro P.B.D.M. (2019). Mesophotic Ecosystems: Distribution, Impacts and Conservation in the South Atlantic. Divers. Distrib. 25, 255–268. doi: 10.1111/ddi.12846
Souza R. F. C., Ivo C. T. C., Souza R. A. L. (2003). Aspectos Da Reprodução Do Pargo, Lutjanus purpureus (Poey, 1875), Na Costa Norte Do Brasil. Bol. Tec. Cient. 3, 107–21.
Souza R. F. C., Pantaleão G. S. L., Fonseca A. F., Ivo C. T. C. (2008). Mesophotic Ecosystems: Distribution, Impacts and Conservation in the South Atlantic. Divers. Distrib. 25, 255–268. doi: 10.1111/ddi.12846
Szedlmayer T.S., Shipps R.L. (1994). Movement and Growth of Red Snapper, Lutjanus campechanus, from an Artificial Reef Area in the Northeastern Gulf of Mexico. Bull. Mar. Sci. 55, 887–896. doi: 10.1111/ddi.12846
Szedlmayer S. T., Conti J. (1999). Nursery Habitats, Growth Rates, and Seasonality of Age-0 Red Snapper, Lutjanus campechanus, in the Northeast Gulf of Mexico. Fish. B-NOAA. 97, 626–35.
Szedlmayer S. T., Howe J. C., Howe J.C. (1997). Substrate Preference in Age-0 Red Snapper, Lutjanus campechanus. Environ. Biol. Fishes. 50, 203–207. doi: 10.1023/A:1007371514250
Trippel E. A. (1995). Age at Maturity as a Stress Indicator in Fisheries. Bioscience. 45, 759–71. doi: 10.2307/1312628
Topping D. T., Szedlmayer S. T. (2011a). Site fidelity, Residence Time and Movements of Red Snapper Lutjanus campechanus Estimated with Long-Term Acoustic Monitoring. Mar. Ecol. Prog. Ser. 437, 183–200. doi: 10.3354/meps09293
Topping D. T., Szedlmayer S. T (2011b). Home Range and Movement Patterns of Red Snapper (Lutjanus campechanus) on Artificial Reefs. Fish. Res. 112, 77–84. doi: 10.1016/j.fishres.2011.08.013
Vazzoler A. E. A. M. (1996). Biologia da reprodução de peixes Teleósteos: teoria e prática. Maringá: EDUEM.
Ximenes M. O. C., Fonteles-Filho A. A. (1988). Estudo de idade e crescimento do pargo, Lutjanus purpureus Poey (Pisces: Lutjanidae), no Norte e Nordeste do Brasil. Arq. Cienc. Mar. 27, 69–81.
Keywords: spawning, juvenile catch, FL50, fishing gear, Amazon Reef, fishing resource
Citation: Freire JL, Sarmento GC, Lutz Í, Bentes B and Isaac VJ (2022) New Insight Into the Reproductive Biology and Catch of Juveniles of the Lutjanus purpureus in a Portion of the Great Amazon Reef System Off the Northern Brazilian Coast. Front. Mar. Sci. 9:804648. doi: 10.3389/fmars.2022.804648
Received: 29 October 2021; Accepted: 30 May 2022;
Published: 18 July 2022.
Edited by:
Çetin Keskin, Istanbul University, TurkeyReviewed by:
Alfonso Aguilar-Perera, Universidad Autónoma de Yucatán, MexicoOumar Sadio, Institut de recherche pour le développement, Senegal
Copyright © 2022 Freire, Sarmento, Lutz, Bentes and Isaac. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ítalo Lutz, aXRhbG9mcmVpdGFzOTFAaG90bWFpbC5jb20=
 Julliany L. Freire
Julliany L. Freire Gabriela C. Sarmento
Gabriela C. Sarmento Ítalo Lutz
Ítalo Lutz Bianca Bentes
Bianca Bentes Victoria J. Isaac5
Victoria J. Isaac5