
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Mar. Sci., 03 March 2022
Sec. Marine Conservation and Sustainability
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.804218
The genetically and geographically isolated Cook Inlet beluga whale (CIB) was listed as endangered under the Endangered Species Act in 2008 and a federal recovery plan was adopted in 2016. Despite these measures, the population has failed to make demonstrable progress toward recovery. Data and knowledge gaps exist, as well as high uncertainty in the recovery plan, regarding the impact and severity of identified threats on CIB health and recovery, particularly for threats driven by anthropogenic factors, and cumulative effects. These data deficiencies may hinder threat prioritization and conservation and management actions. Odontocete populations in similarly ecologically precarious situations may serve as research surrogates to help fill information gaps and guide future CIB research and conservation. Through a systematic review of CIB and selected surrogate species [St. Lawrence beluga (SLB), Hector’s dolphins (HD), and southern resident killer whales (SRKW)], we identify gaps associated with threats described and ranked in the CIB recovery plan. All threats identified by the National Marine Fisheries Services as “high”-concern to CIB recovery, except noise, are lower in publication volume compared to publications related to high concern threats in SLB and SRKW. “Medium” or “low” threats to CIB, such as prey reduction and contaminants, respectively, are identified as higher priority threats in surrogate populations. These topics have been more heavily researched for surrogates and suggests that synthesis of this work may help reduce uncertainty, to aid in informing management actions for CIB. Specifically, publishing volume suggests SLB and SRKW are valuable surrogates for understanding the impacts of noise, prey, and contaminants. Publishing volume is necessary to choose a surrogate, but is not sufficient. Surrogates were chosen based on physiological similarities to CIB as well as their comparable management situations. Therefore, these lower-ranked threats should be ranked more highly and researched specifically in regard to CIB. We use this review to offer management recommendations based on current CIB and surrogate literature regarding listed threats in the CIB recovery plan. Our analyses suggest that CIB may benefit from a revision to and elevation of some low and medium-concern threats such as contaminants, habitat degradation, and prey reduction.
The Cook Inlet beluga (CIB) (Delphinapterus leucas) is an endangered beluga stock that is genetically and geographically isolated in the estuary of southcentral Alaska for which it is named (Figure 1). CIB are both ecosystem and cultural sentinels. As apex predators of the Cook Inlet estuary, CIB can serve as indicators of ecosystem health. For millennia, the Tubughna, the Beach People of the Native Village of Tyonek, have been connected to this iconic species. Despite the cessation of subsistence hunting, which the National Marine Fisheries Service (NMFS) identified as the main historical threat (NMFS, 2016), the population continued to decline and was subsequently listed as endangered under the Endangered Species Act (ESA). Uncertainty regarding key threats is well documented by the recovery plan. Many unknowns remain regarding CIB conservation and recovery, including those pertaining to major threats and factors inhibiting population growth (NMFS, 2016). As a result, CIB conservation and management are hindered by an incomplete understanding of the direct and indirect impacts of these threats and their potential interactions, making it difficult to assess the immediacy of threats and to prioritize management actions. Several reviews of CIB conservation status have been completed in the past three decades (NMFS, 1992; Moore and DeMaster, 2000; Hobbs et al., 2006, 2008; Hobbs and Shelden, 2008; Shelden et al., 2017). While these reviews document a substantial increase in knowledge of CIB biology and ecology since their original listing, research efforts and actions to date have not specifically identified the major contributor(s) to the continued decline of the CIB. Relatively more progress has been accomplished for other endangered cetacean populations such as southern resident killer whales (SRKW) (Orcinus orca) in the northeastern Pacific Ocean (NMFS, 2008) and St. Lawrence beluga (SLB) in eastern Canada (COSEWIC, 2014). The difficult in gathering more in-depth knowledge on CIB is likely due to several factors such as the difficulty in studying this species in a challenging environment, potentially less funding to support research compared to other endangered populations, and the likely complexity of contributors to the decline. In this study, we explore ways to address knowledge gaps for CIB and to reduce uncertainty in management and conservation actions using a research synthesis of surrogate populations.
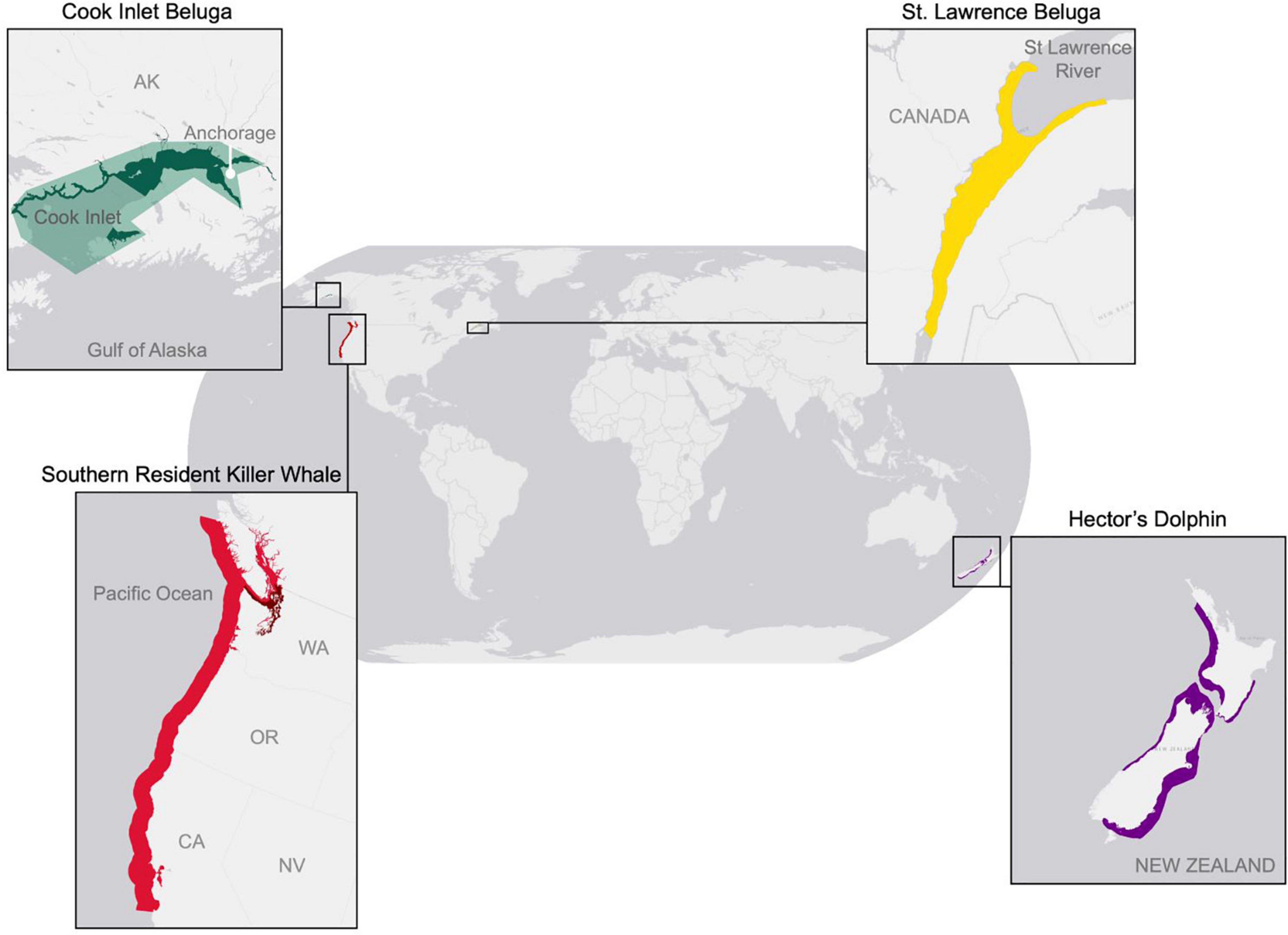
Figure 1. Map of geographic locations of CIB (green) and the three surrogate odontocete populations: St Lawrence beluga (yellow), Hector’s dolphins (purple), and southern resident killer whales (red).
Surrogate populations are populations with similar biology and ecology to the population of interest; for the CIB, these will be other populations of belugas and small odontocetes that live in habitats similar to the CIB. Surrogate populations provide examples of the response of similar populations to changes in the environment and as such are considered an effective means of conservation planning and have been used in various ways such as promoting public concern for an endangered species, serving as indicators of ecosystem health, or addressing biodiversity issues when data for a species of conservation concern is deficient (Favreau et al., 2006; Pullin and Stewart, 2006; Meurant et al., 2018; Zhang et al., 2020). Surrogates can provide insight for potential conservation and management actions through a similarly endangered population. For instance, the federally threatened bull trout (Salvelinus confluentus), found in southern British Columbia, Canada, and the northern region of western North America, was used as a surrogate species for the westslope cutthroat trout (Oncorhynchus clarki lewisi), a species of concern that is in decline, but not ESA-listed, in the bull trout’s Habitat Conservation Plan (Hitt and Frissell, 2004). Surrogate odontocete species such as SRKW have been used for comparative purposes in studies of CIB (e.g., Norman et al., 2020). Systematic reviews, have been used for conservation purposes by summarizing evidence about the effectiveness of conservation interventions, evaluating the contribution to management decisions, addressing interventions for conservation relevant to policy decisions and those for practical on-the-ground management (Cook et al., 2014; Braulik et al., 2015; Richardson et al., 2020). It has been acknowledged that an evaluation of threats that constrain productivity in other odontocete populations may provide insights into the stressors and underlying processes impeding CIB recovery (NMFS, 2016). The present review combines species comparisons and systematic literature review into a single effort. Due to the relatively small body of literature on CIB and to compare similar threats, the examination of historical data from other surrogate odontocete populations may provide complementary scientific narrative on recovery successes and challenges.
At the time the Marine Mammal Protection Act was enacted in 1972 and through the late 1970s, the CIB abundance was approximately 1,300 individuals (NMFS, 2016). Anchorage, the largest and most populous city in Alaska, located at the northern end of Cook Inlet (Figure 1), saw increases in human population growth and land development in the 1990s, creating easier access to Cook Inlet for hunters and boaters (NMFS, 2016; Figure 2). The CIB population declined steeply during this time period, late 70’s to late 90’s. Hunting was presumed to be the single major threat to the population and scientists and managers anticipated a reversal in population decline with greater regulation of hunting beginning in 1999 (Rugh et al., 2010). Realizing the diminished population status of CIB, Alaska Natives drastically reduced their hunting practices voluntarily between 2000 and 2004 through co-management agreements. The last CIB confirmed take by subsistence hunting was in 2005, with no co-management agreements being requested for CIB harvest since the end of the 2006 season (Huntington, 2000; NMFS, 2016; Jacobson et al., 2020). A “take” is defined by the U. S. Marine Mammal Protection Act as any action that seeks “to harass, hunt, capture, or kill or attempt to harass, hunt, capture, or kill any marine mammal” [16 U.S.C. §1,362 (13)]. Due to unknown factors, a brief period of mild population growth was observed beginning around 2004, and continued until 2010, based on annual best estimates of abundance (Table 3 in Shelden and Wade, 2019). Despite this small, temporary, increase in growth, the CIB population was ESA-listed in 2008. After listing, the CIB population declined at approximately 2.3 percent annually between 2010 and 2018 and is projected to continue its downward trajectory (Shelden and Wade, 2019). The most recent abundance estimate was 279 individuals in 2018, a decrease from 381 estimated individuals in 2016 (Boyd et al., 2019; Shelden and Wade, 2019). Subsistence hunting continues to be prohibited as the population has remained, on average, below 350 whales. Although this practice is no longer occurring with this population, subsistence hunting is still listed as a threat of low concern in the recovery plan. With subsistence hunting no longer featured as a major contributor to present-day population threats, research and recovery efforts focus on other potential stressors as identified in the recovery plan (NMFS, 2016).
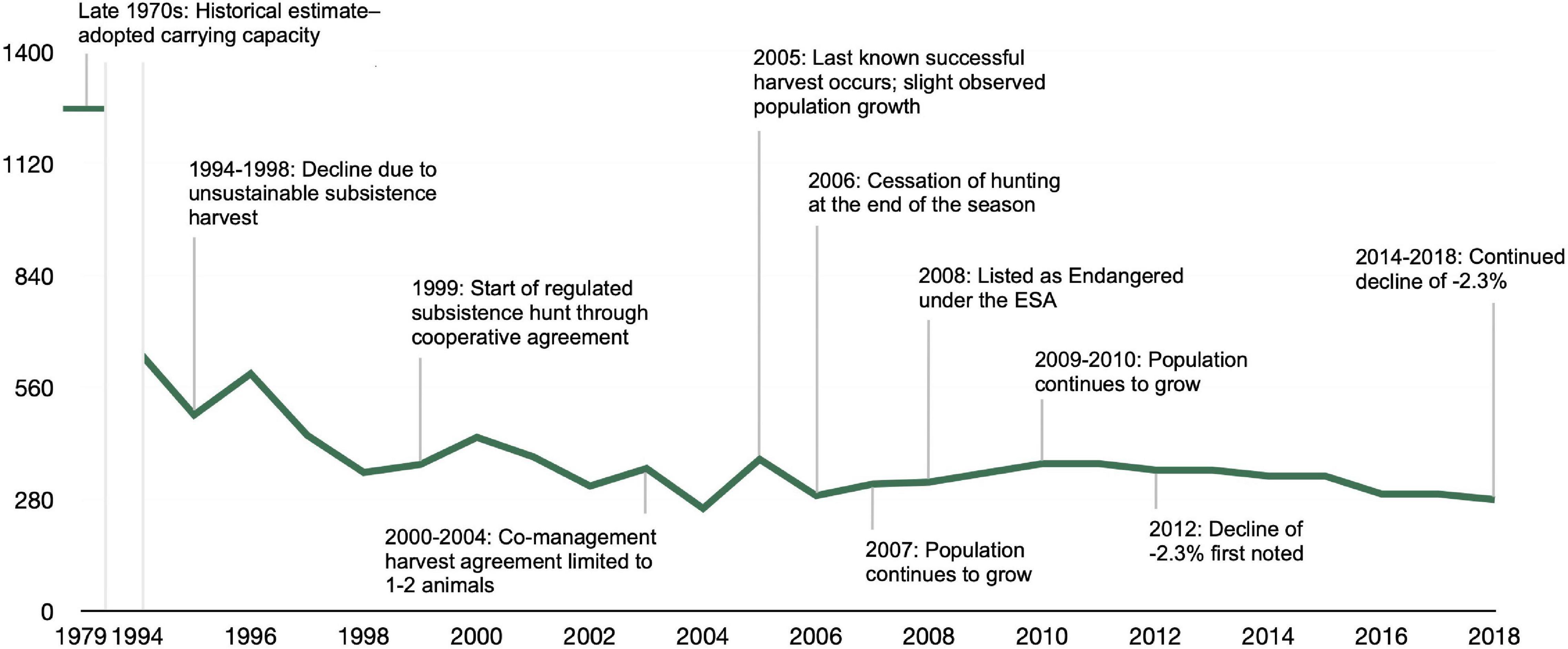
Figure 2. Timeline of Cook Inlet beluga abundance estimates and population trend from 1994 to 2018. Abundance estimates for 1994–2003 were obtained from the Cook Inlet beluga recovery plan (NMFS, 2016; Table 3) which cited Hobbs et al. (2015a,Figure 5) and Shelden et al. (2015, Figure 16) as sources for this time period. The estimates for 2004–2018 were obtained from Shelden and Wade (2019) (Table 3).
As mandated by the ESA, a recovery planning process for CIB was finalized at the end of 2016 (NMFS, 2016). Within the recovery plan, the following key points are described: (1) a narrative of site-specific management actions essential to conservation of CIB, and long-term survival of CIB; (2) measurable, objective criteria, that when met, would authorize CIB delisting (NMFS, 2016). In addition, the recovery plan compiled a list of threats considered to be potential obstacles to CIB recovery and ranked these by level of concern by NMFS (Table 1—adapted from Table 6 in NMFS, 2016). The recovery plan states threat assessments and rankings were made based on the information and data gaps outlined in the Background section of The Plan (NMFS, 2016).
The threats currently listed in the recovery plan that were evaluated in this review include those of “high-concern” such as catastrophic events (i.e., natural disasters, toxic spills, disease outbreaks) (Lacy et al., 2017), cumulative effects (i.e., contaminants, prey reduction, stress) (Hoguet et al., 2013), and noise (i.e., shipping, natural resource extraction) (Kendall et al., 2013; Castellote et al., 2016). Other CIB threats were identified as “medium- or low-level” of concern, which may reflect lack of available research data on their impacts on CIB. For example, prey reduction (listed as medium concern) is a great threat to SRKW and may also be an important threat for the beluga; Chinook salmon (Oncorhynchus tshawytscha) are a critical food source for SRKW and may also be a significant part of the beluga diet (Ford et al., 2010). Dwindling Chinook return runs were noted in the CIB recovery plan. Additionally, while pollution is listed as a “low” concern, it could interact with other threats, negatively impacting CIB and its food sources as it does in SRKW, as well as contribute to cumulative effects (Cullon et al., 2009).
Generally, threat identification and ranking are intended to prioritize management actions. However, this may prove difficult if literature on the present status of these threats, particularly for high-concern threats, is lacking or unavailable. Likewise, if there is little knowledge about specific threats (e.g., pollution) to the species in question, that threat may be improperly ranked. The current CIB threat rankings were based on the data available at the time the original recovery plan was published, though the recovery plan recognizes the need for more research to update rankings with the passage of time. Recommended Recovery Plan Action #57 describes completing a meta-analysis of previously documented cumulative effects for other populations and species, based on known threats for CIB, and prioritizing risk to CIB based on how these threats have been shown to negatively affect other beluga populations and other odontocetes (NMFS, 2016). Greater inventory and synthesis of the current literature could help assess the efficacy of this ranking system and the resulting recovery actions assigned based on threats.
Here we present a systematic review of peer-reviewed and gray literature of the endangered CIB population, and three surrogate odontocete populations. We quantify and synthesize the available research on CIB and the identified threats to their recovery. We also quantify and synthesize the available research on the surrogate populations and compare the information available on the surrogates to the CIB. The aims of the review were to examine trends within the information on each population and across populations, to identify areas in need of greater research for the CIB, determine the relationship of the available information to the threat rankings and consider, whether or not, those gaps might be filled by research on surrogate populations. Ultimately, the outcome of this work can be used to direct mitigation measures and serve to inform effective conservation and management actions for CIB recovery based on appropriate surrogate species.
We selected three odontocete populations to serve as surrogates for CIB. Surrogate species were chosen based on fulfilling select criteria. First, the candidate population’s International Union for the Conservation of Nature (IUCN) status had to be “Endangered” or “Threatened” (Lowry et al., 2019). The population also had to be subject to similar types of threats or pressures as CIB (e.g., small population size, proximity to anthropogenic activities, similar ecology and biology, alterations in prey abundance or distribution, etc.; NMFS, 2008, 2016; Department of Fisheries and Oceans [DFO] Canada, 2012; MPI, 2019). Last, the surrogate population candidate needed a robust body of corresponding published and gray literature that demonstrated efforts to address threats to that population. In our systematic review of the scientific and policy literature on the threats and recovery actions, we focused on SRKW (coastal waters of British Columbia, Canada; Washington State and Oregon, United States), St. Lawrence estuary beluga whale (SLB) (eastern Canada), and Hector’s dolphin (HD) (coastal waters of New Zealand).
In carrying out this systematic review of peer-reviewed and gray literature, we followed guidelines from Pullin and Stewart (2006) and Siddaway et al. (2019). Peer-reviewed (primary) literature consisted mostly of academic journal articles and scientific books. Gray literature sources encompassed any search result that was not under the auspices of a commercial publisher and may have undergone varying degrees of peer-review (e.g., technical reports, theses, conference symposia, and workshop proceedings). We excluded websites and webpage content and defined “record” as a search result that represented a hit of any form, whether an academic peer-reviewed article or gray literature item.
We selected primary online search engines based on their precision, reproducibility, high recall, and efficiency (Gusenbauer and Haddaway, 2020). Therefore, we searched peer-reviewed and gray literature using the online publication databases Web of Science and MEDLINE as the primary engines. We used Google Scholar as a supplementary search source, especially for gray literature, and gained additional access to publications not readily available on-line through various other collections (see Supplementary Material).
We also accessed publications not readily available on-line through regional institutional library collections, including the NMFS Regional Science Centers (Alaska and West Coast Region); Alaska Department of Fish and Game (ADFG); Washington Department of Fish and Wildlife (WDFW); Ontario and Quebec (Canada) Provincial Wildlife Fisheries websites; Fisheries and Oceans Canada website; Department of Conservation (Te Papa Atawhai) New Zealand, and the Ministry for Primary Industries (MPI) (New Zealand). Additional sources for gray literature searches included the IUCN website, and one online database, Wildlife and Ecology Studies Worldwide [EBSCO]). We explored bibliographies of peer-reviewed publications and potentially relevant studies not found through other sources and extracted them for inclusion.
We initially selected search terms based on the threats listed in the CIB recovery plan (NMFS, 2016; Table 1) and extracted similar search terms from corresponding recovery plans of the surrogate populations, and terms related to the biology and management of populations. The terms were generally grouped by subject to cover anthropogenic and natural threats to their populations, or management-related actions (Table 2). For example, the category “Climate” included “Anthropogenic”-related search terms, due to the strong influence of humans on climate change, but exclusive of “Noise” which was evaluated as a separate category. Although climate change is considered a potential threat to CIB recovery, it is not specifically addressed as a separate threat in the recovery plan, but rather was discussed in the general context of how it may affect the other listed threats, and thus is not specifically addressed by itself in this review. Under the “Management” category, search terms related to conservation were included, as well as critical habitat since the latter is a function of conservation and management of an endangered population (Small et al., 2017). “Status/Range” search terms related to updated status reviews of the population, or discussion of population ranges. The “Population status/trajectory” category served to capture records on population surveys and abundance estimates. The search period spanned from 1975 through 2020, the former being the earliest a record was found for CIB ahead of the formal search. Although records from 2021 (n = 12) were included in the search record total, they were not included in analyses and graphs, unless indicated, because the year 2021 was not yet completed during the review.
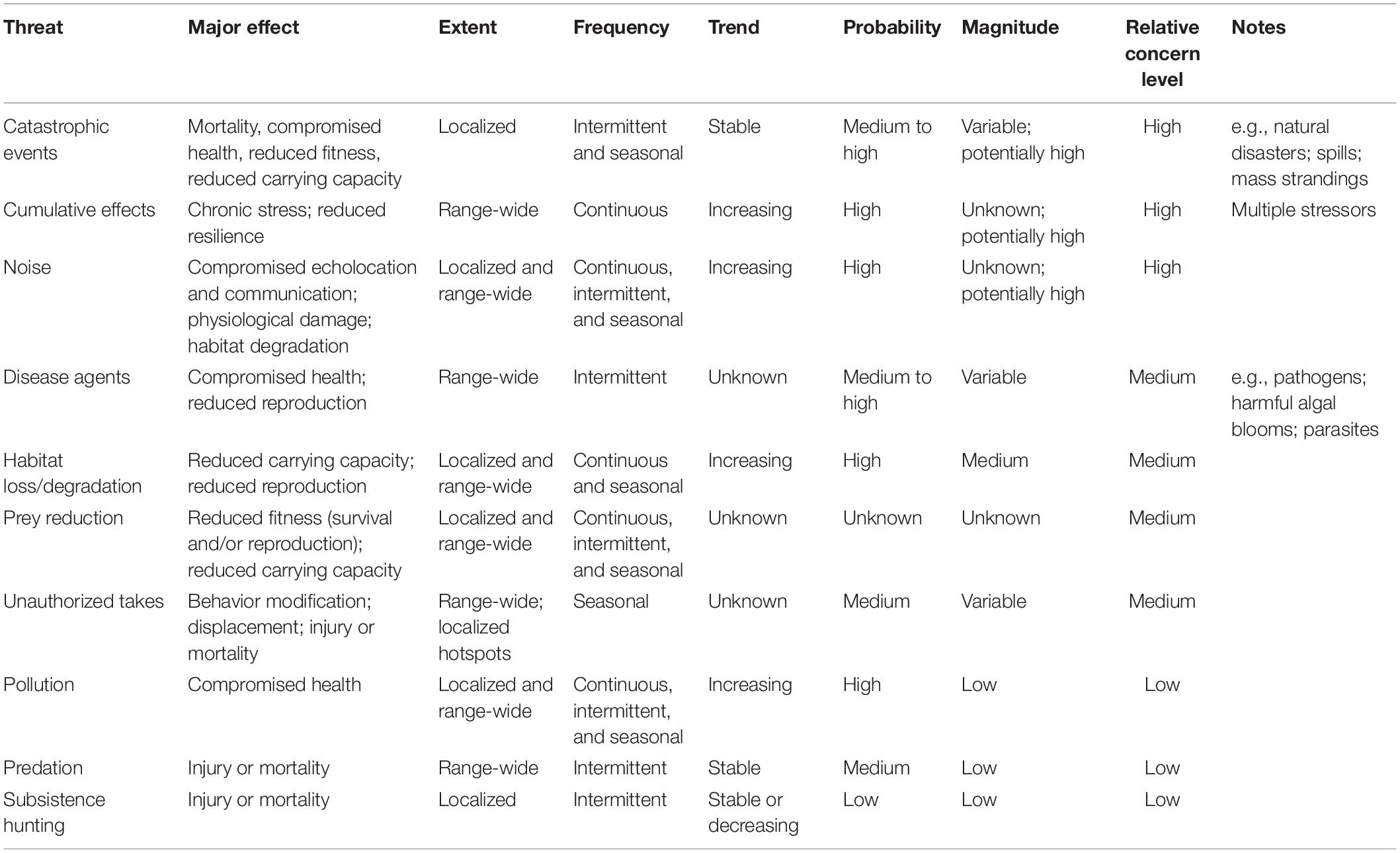
Table 1. Potential threats to Cook Inlet belugas identified in their recovery plan (adapted from Table 6, NMFS, 2016).
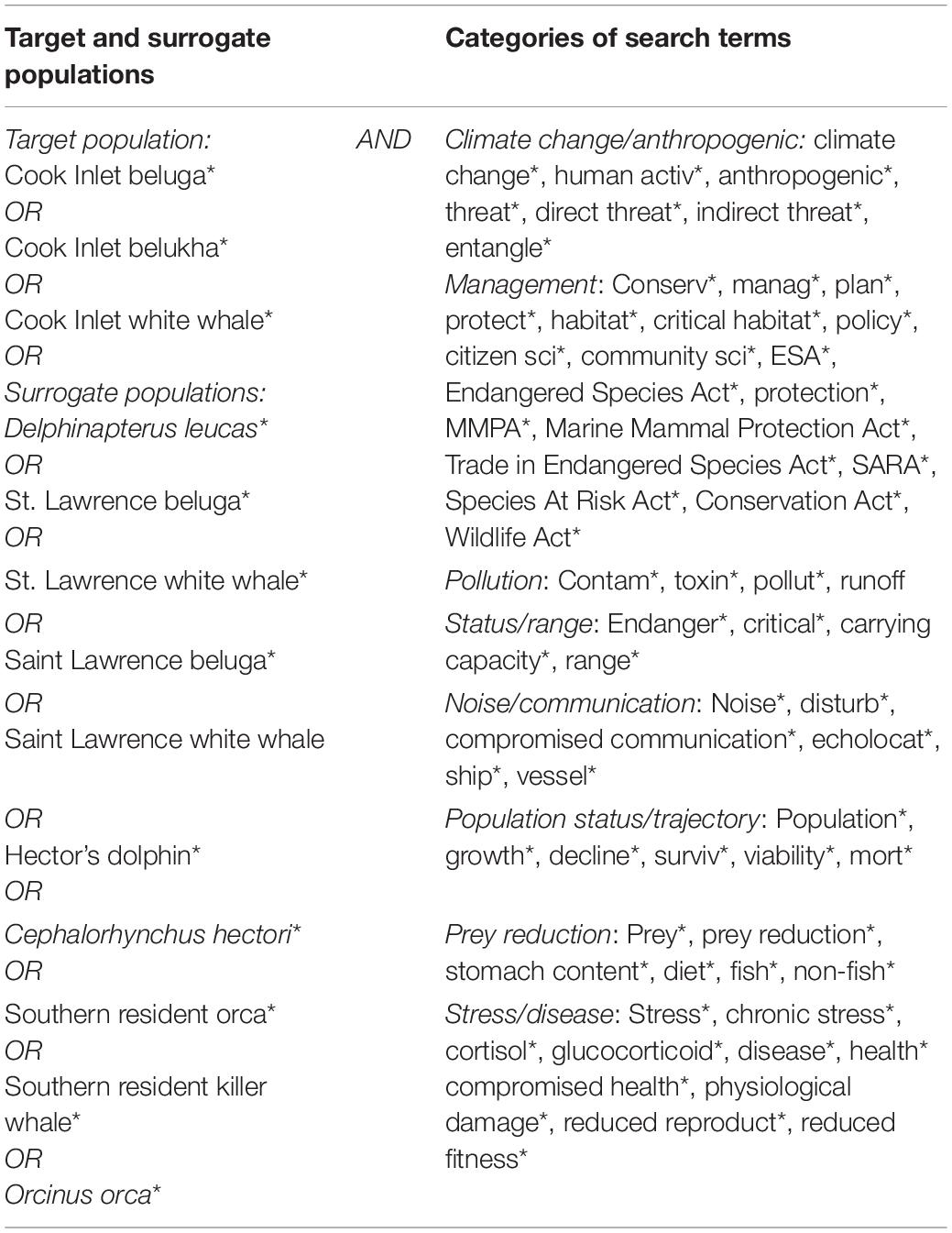
Table 2. Search terms used for a systematic review of threats to Cook Inlet belugas, and surrogate species (St. Lawrence beluga, Hector’s dolphins, and southern resident killer whales).
To capture all records that included the populations of interest, we used various keyword combinations (Table 2). The keywords within each category (e.g., “Species,” “Threat,” “Conservation/Management Action,” and “Population Status/Trajectory”) were combined with the Boolean operator “OR,” followed by the operator “AND.” Asterix (*) symbols were used at the end of a term to represent any group of characters after the search term such as the partial word “fisher*,” which could indicate fishery or fisheries or a fisherman. We excluded records on odontocetes in zoological facilities due to potential differences in exposure to environmental health risks that may differ from free-ranging populations. The specific keywords representing threats were not initially included in the peer-reviewed literature search in order to search as broadly as possible, and to capture literature whose content was related to threats for the species of focus yet may not have explicitly used those terms in the title or abstract. When a large (i.e., > 800) number of records were identified using any combination of search terms, we focused the search on sources most relevant to the research questions. A “report” was any document not published by a commercial publishing house.
We applied the PRISMA approach (Moher et al., 2009) to the process of screening and selecting literature (Figure 3). Potentially relevant papers that remained after application of the inclusion criteria were read in full and extracted from eligible records. These remaining records were categorized by source (peer-reviewed journal/book or gray literature) and focal population(s). Only records related to the populations of interest, or which mentioned a threat that implied an impact to one of the target populations, were included in this analysis. When available, information on focal population, threat category, study method, and conservation implications, were extracted from the records and presented in a spreadsheet listing all records retained from the search and used in the analyses. Records ultimately selected for the review were maintained and curated in Zotero, an open-source reference management program.1
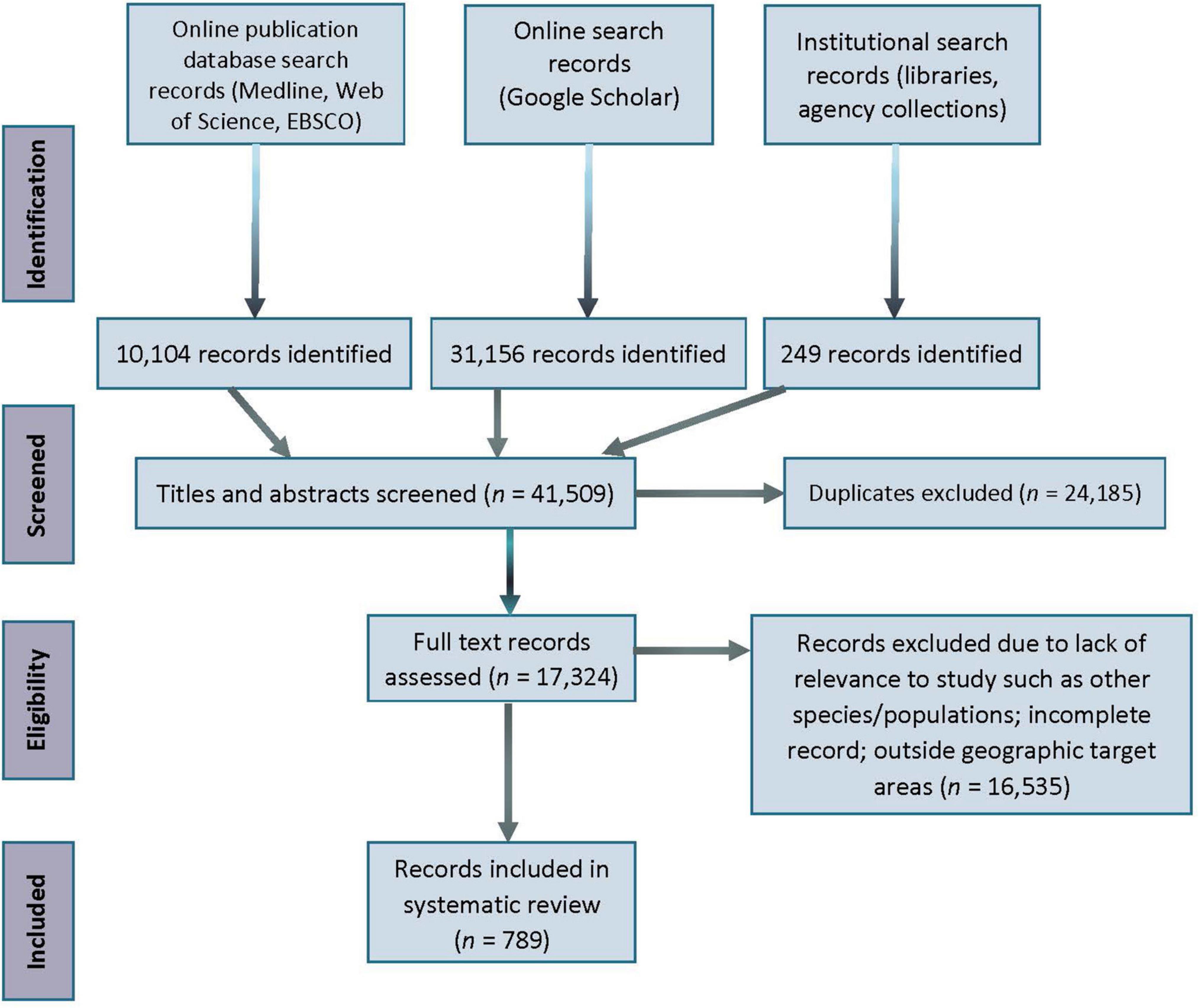
Figure 3. PRISMA literature record search flow diagram. At each stage of the review process, the number of records that were located, discarded, and retained are indicated at each stage (1975–2020). Relevant publications from January to May 2021 are included in the count.
We used five bivariate negative binomial regression models to assess for any significant trends in the total count of overall records over time, as well as total number of records over time for each of the four populations separately, and within and across population search term categories. For assessing trends across search term categories, models were selected using the Akaike criterion. Model assumptions of normality and homoscedasticity of variance were assessed with diagnostic plots. To assess gaps in the literature across the four populations, we used Pearson’s chi-square goodness of fit tests (and Fisher’s exact tests if cell number was ≤ 5) to determine if the distribution of records differed significantly from an expected distribution at a significance level of 0.05. For each population of interest, the expected distribution was the proportion of records out of the total number of records that belonged to each population. Each record was assigned to one of the focal populations (Table 2). Each of these categories was treated as mutually exclusive. Search results were classified by the categories of search terms which were used in the initial record search and that were relatively common to recovery plans for the target and surrogate populations (NMFS, 2008; Department of Fisheries and Oceans [DFO] Canada, 2012, 2020; Table 3). Search categories were not always mutually exclusive, so a record could fall into more than one search term category if the study included multiple search terms. In these cases, the record was categorized according to the predominant search term(s) discussed in the record.
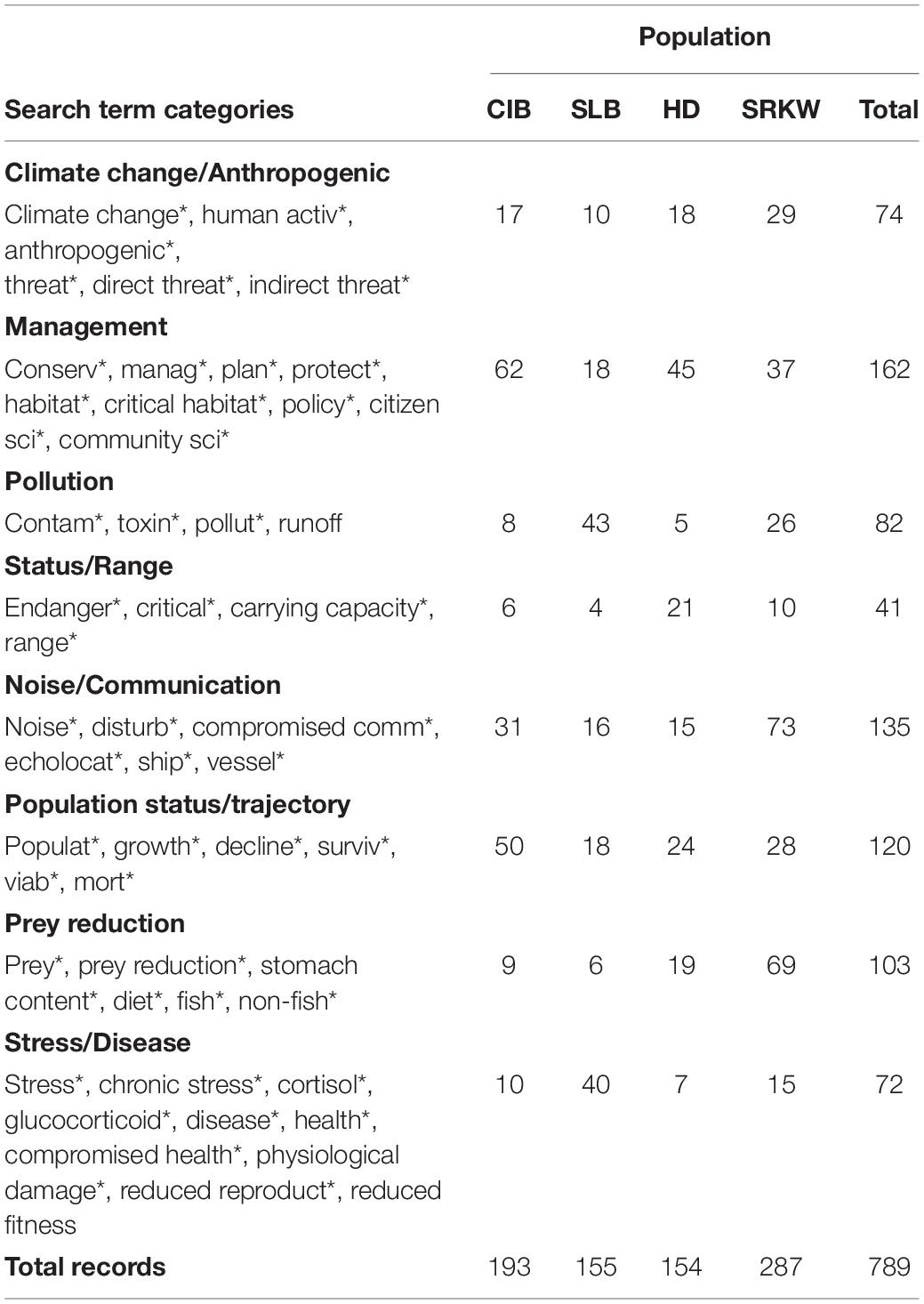
Table 3. Number of records (1975-May 2021) by search term category and population [Cook Inlet beluga (CIB), St. Lawrence beluga (SLB), southern resident killer whale (SRKW), and Hector’s dolphin (HD)].
We retained a total of 789 records in the analysis after applying criteria and removing duplicates (see Figure 3 and Supplementary Table 1). From the search period (1975–2020), the peak year was 2019 and contained 63 records across CIB and the surrogate populations. The earliest record, from 1975, was on SLB (Sergeant and Brodie, 1975). For the other populations, CIB, HD, and SRKW, the earliest records were dated 1979, 1986, and 1976, respectively (Supplementary Table 2). The following models fit the data best in evaluating over time: negbinom (#records ∼ year) for all populations combined and negbinom (#records/population ∼ year) for each population. The number of all records per year that met the criteria for inclusion increased 7.6% on average from 1975 to 2020 (95% CI = 7.604, 7.607; P = < 0.0001), with the most marked increases occurring around 2000 and 2010 (Figure 4). For each of the four populations, there was a significant increasing trend over the study period in number of records (all P = < 0.0001; individual βs not shown). In 2000, a collection of journal articles was published in a special volume of the journal Marine Fisheries Review (Supplementary Figure 1). The second peak of CIB records was noted starting in 2010, which was 2 years after the population was federally listed as endangered under the ESA (Figure 4 and Supplementary Figure 1). The increase in CIB records continued until 2016, then rose again in 2020. Of the 193 CIB records, 37 (17.6%) occurred after the recovery plan came out in 2016. Records for SRKW increased exponentially starting around 2006–2008, soon after SRKW were listed in 2005 as endangered under the ESA and have continued to trend upward ever since. Journal articles represented more than half of all records (56.8%), with gray literature being the second largest source (35.6%) (Table 4). Additionally, 7.3% of records were those originating from student-led studies, ranging from undergraduate theses to Ph.D. dissertations (CIB = 6, SLB = 4, HD = 18, SRKW = 30) (Table 4). The HD records demonstrated the prolific use of thesis-based graduate student studies to explore the severe threat of fishery interactions, as well as other research and policy topics (Supplementary Table 1).
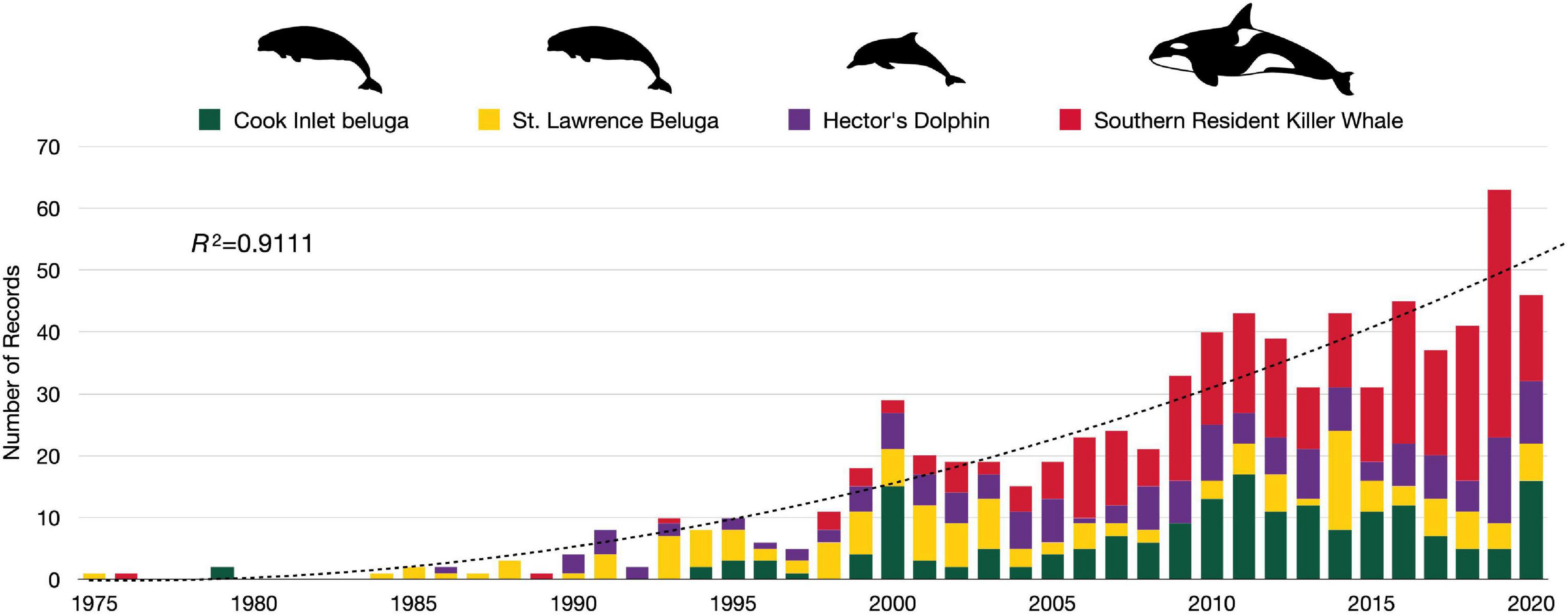
Figure 4. Total number of records for Cook Inlet belugas, St. Lawrence belugas, Hector’s dolphins, and southern resident killer whales, collectively by year (1975–May 2021) (the trendline represents the overall negbinom (#records ∼ year) model.
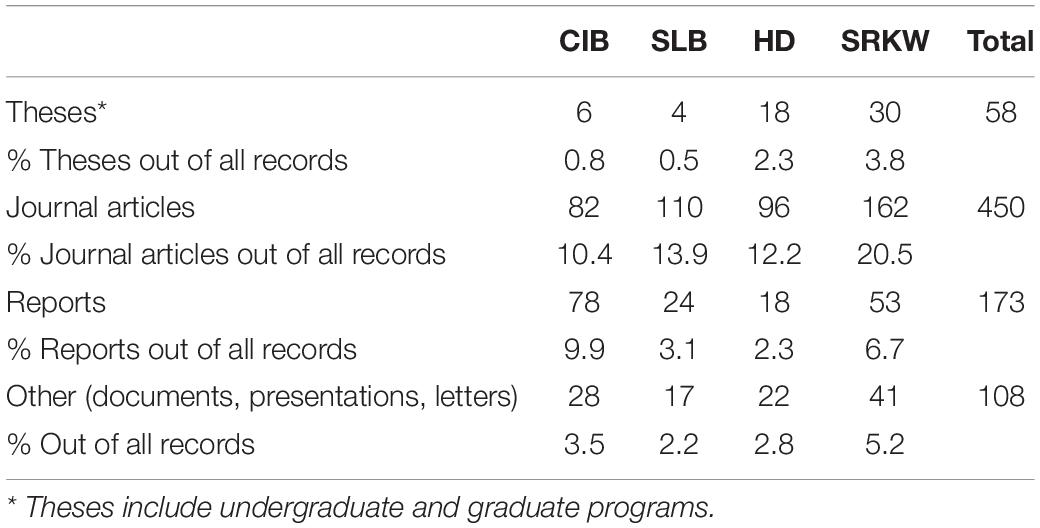
Table 4. Most common types of records (1975-May 2021) obtained for four target odontocete populations [Cook Inlet beluga (CIB), St. Lawrence beluga (SLB), Hector’s dolphins (HD), and southern resident killer whale (SRKW)].
We noted a few trends over the last 10 years in numbers of records. After CIB were listed under the ESA in 2008, the number of records related to management increased from two records in 2009 to eight in 2011 and then maintained at least four per year through 2013. Population status records were heavily represented in the search results and reported surveys for abundance estimates. Noise was the only one of the three CIB “high”-concern threats that was represented in the results of the review, and appeared consistently (i.e., 2–3 records/year) in the years after ESA listing. However, this systematic review reveals that opportunities exist to fill knowledge gaps concerning threats to CIB recovery as there is sufficient corresponding literature on similar threats for other odontocete populations, notably SLB and SRKW. For example, pollution has been a long-standing concern for SLB survival and is heavily represented by consistent records over the past 20 years. The three primary threats for SRKW, decreased prey, excessive noise, and contaminants are all heavily represented consistently in the years since their ESA listing, 2005.
We compared record quantities to the concern level of the threat according to the recovery plan and search term categories to assess the focus of research and management efforts. The CIB records had a strong bias toward two of the categories that were defined at the start of the search: “Management,” that included topics such as conservation actions, and habitat and critical habitat designation, and “Population status/trajectory” (Figure 5). This reflects the multiple habitat distribution studies in the former category, of which 50% were contract reports, and in the latter category, aerial survey reports which constituted 40% of the 50 records in that category.
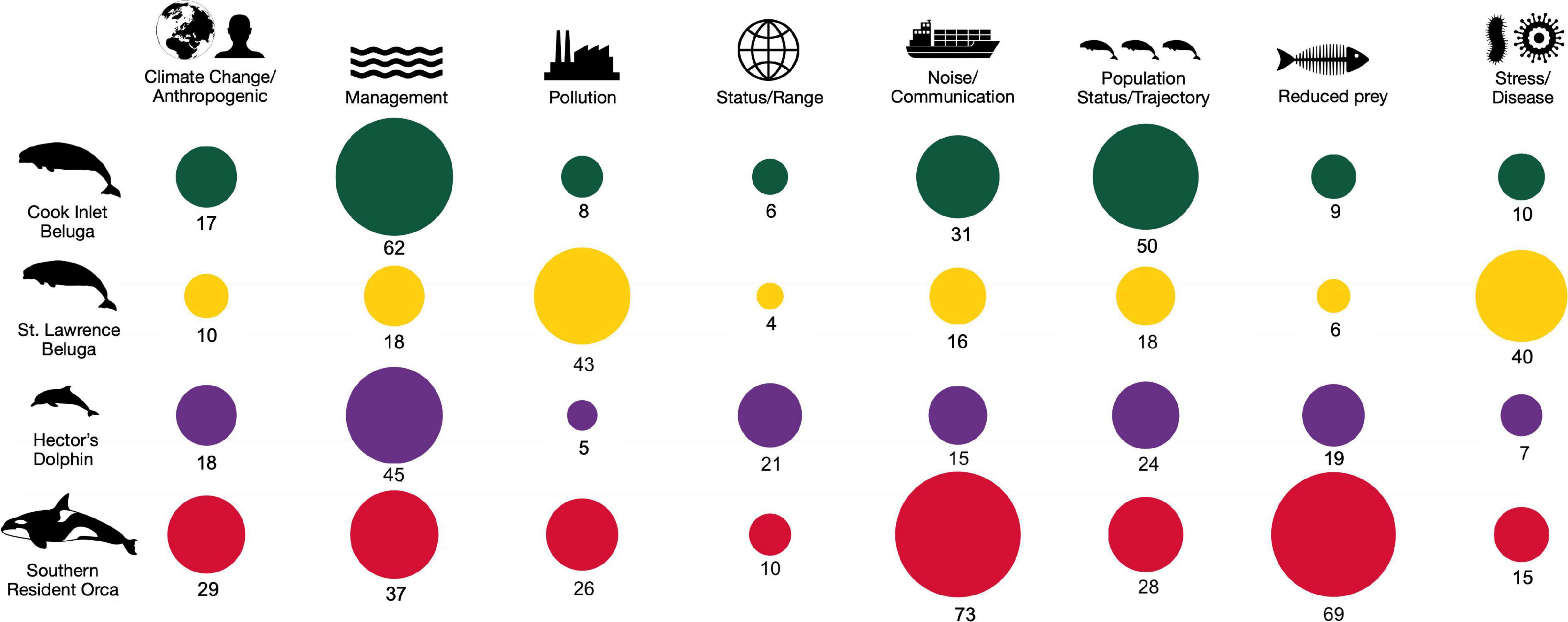
Figure 5. Proportional distribution of records by threat/search term group for Cook Inlet belugas, St. Lawrence belugas, Hector’s dolphins, and southern resident killer whales, by search term category (x-axis) for each population (1975-May 2021). The numbers of articles are shown as raw numbers.
Relating directly to the threat rankings (Table 1), we found no records to directly address cumulative effects, a threat ranked as “high” concern for CIB. Catastrophic events such as oil spills, mass strandings, and natural disasters are listed as a “high” concern in the recovery plan; however, fewer than five records specifically addressed this threat. The third “high” ranked threat, noise, was more robustly represented in the record search by 31 records, representing 16% of the CIB records, and with the majority (77%) appearing after 2010 (Table 3). Of the 31 CIB noise records, 14 (45.2%) were peer-reviewed journal articles describing the negative behavioral effects of noise on the animals during activities such as pile-driving or the excessive noise levels detected during such activities.
When evaluating differences in number of records within search term categories and populations, the number of records differed significantly (χ2 = 280.5, df = 21, P < 0.001 and χ2 = 39.9, df = 3, P < 0.0001 by population and search term category, respectively). Following model selection, the final binomial model for search term categories was: negbinom (#records ∼ searchterm + population). This analysis showed significant differences in record numbers were observed for the search categories “Management,” which included conservation, critical habitat, and legislation (β = −0.326; 95% CI = −0.542, −0.110; P = 0.003), “Population status/trajectory” (β = −0.400; 95% CI = −0.634, −0.166; P = 0.001), “Prey reduction” (β = 0.303; 95% CI = 0.097, 0.509; P = 0.004), and marginally for “Stress/disease” (β = −0.259; 95% CI = −0.514, −0.005; P = 0.046). Within the “Management” category the greatest number of records were related to CIB (39.9%).
Within the search years (1975–2020), the publication volume for CIB remained relatively small (7.9% of publication volume of all four odontocete populations combined in the peak year 2019) compared with the surrogate populations (Figure 4). The records were most heavily represented by SRKW, by total number overall, and by number of journal articles. Approximately 16.3% of reviewed records for CIB related directly to threats of high concern outlined in the CIB recovery plan, and were exclusively those related to noise. Noise-related records in CIB began to appear yearly in steady numbers of 2–3 per year starting in 2009, the year after ESA-listing. Records specifically addressing stress and disease of CIB also started to increase in 2009 with at least 10 produced through 2020. Records related to the other two threats of “high” concern, cumulative effects and catastrophic events, were not readily represented as distinct, stand-alone records.
Records for SLB focused on several of the specific recovery objectives identified by Fisheries and Oceans Canada that targeted the most pressing threats to the population. As outlined in the most recent SLB recovery strategy plan, those threats included contaminants, anthropogenic disturbances such as noise, and threats to SLB habitat throughout their range. A large proportion of the SLB records addressed pollution and disease (total n = 84; 54.6% of SLB records) as these are threats of high concern; however, other priority threats such as noise (n = 16) and inadequate prey (n = 6) were less represented in the search results. Greater than half (59.4%) of the 286 SRKW records focused on the highest priority threats facing that population: contaminants, prey reduction, and noise. Though the number of studies in SRKW conducted on contaminants, compared to their other two “high” concern threats, is fewer, findings about the impacts of contaminants indicate that it is an important area for continued future study. Records for the surrogate populations, however, did not always align with that species’ highest priority threats. For example, the HD Threat Management Plan highlighted fishery bycatch and disease as major threats. However, the HD records primarily targeted studies focusing on management and threats from climate/anthropogenic factors such as bycatch, and less for disease, even though it is a threat of high concern.
Considerable knowledge gaps are apparent in the CIB literature and could result in impediments to recovering the population. Gaps in the CIB literature were greatest for catastrophic events and cumulative effects, which are recognized as high-concern threats by the agency (i.e., NMFS) recommended recovery actions. Additional gaps were present in lower ranked threats such as pollution and prey reduction. However, our analysis reveals that opportunities exist to fill these gaps, as there is sufficient literature on these particular threats for other odontocete populations, notably SLB and SRKW. In addition, the results suggest more research on the impacts of catastrophic events such as oil spills or mass strandings, and cumulative effects to CIB recovery, is needed to better inform management actions (e.g., Vos and Shelden, 2005; Hobbs et al., 2015b). For CIB, SLB, and SRKW, listing decisions have had a clear impact on both the volume of publications and the themes being researched in the years following listing (Supplementary Figure 1). For HD, this pattern is more evident in the table of records by threat category and year (Supplementary Table 2), compared to the graph (Supplementary Figure 1), and is more subtle compared to the other populations. Last, consideration for re-ranking the levels of concern for some of the lesser ranked CIB threats (i.e., pollution, prey reduction) can better reflect what have been shown to be key threats in SLB and SRKW.
The higher prevalence of records on noise may be because this is easier to measure and track on a regular basis and obtain sufficient data to analyze, compared to threats such as catastrophic events that happen infrequently, resulting in few if any data points available for analysis. The threat of noise pollution was the only threat of “high” concern for CIB with a relatively large body of literature supporting it. Even for this and other “high” concern threats, there are still unknowns such as the magnitude of the threat (Table 1). Some of the issues that may be relevant to CIB conservation and management such as pollution and prey reduction (lower concern threats) are data deficient as evident by fewer numbers of records compared to surrogates where these two threats are of “high” concern and represented by a higher number of records compared to CIB. CIB literature in combination with appropriate surrogate literature can provide information about identified CIB threats to recovery that may aid in conservation management actions and the development of more robust threat mitigation practices.
While data gaps exist, literature from surrogate populations create opportunities to obtain realistic and comparable data on threats to CIB that are lesser known. Given that CIB are difficult to study, this is an additional justification to place emphasis on what is known about surrogates, such as prey reduction and the potential impact on overall stress and health of individual population members (Ward et al., 2013; Fearnbach et al., 2018). More specifically, this review indicates that, SLB serves well as a surrogate for pollution and disease threats, while SRKW literature provides information on impacts of pollution and prey reduction. Hector’s dolphins were not as strong a surrogate population as SLB and SRKW because most of the threats to Hector’s dolphins, with the exception of fisheries bycatch (Slooten, 2013), did not overlap as well with those of CIB. For example, the MPI’s Management Trust Plan for Hector’s dolphins cited toxoplasmosis as a significant human-caused threat to some subpopulations of HD, an assertion that was based on very limited data and uncertainty in the estimated number of deaths due to this pathogen (Roe et al., 2013).
The current body of CIB literature is relatively successful at addressing the potential threat of noise on population recovery with a number of studies characterizing sources and potential harmful effects (e.g., Small et al., 2017; Castellote et al., 2018). Literature on noise appeared consistently in the years post ESA listing. Considerable research characterizing noise sources and sound levels in Cook Inlet has focused on commercial shipping and construction noise (e.g., pile driving) as prominent sources (Kendall and Cornick, 2016; Small et al., 2017; Castellote et al., 2018). Changes in CIB behaviors were observed during these studies such as an increase in traveling through the study areas, increased diving with decreased observed feeding, changes in group composition, and increased spatial displacement. There is concern belugas are lacking safe passage from biologically important areas during key seasons. There are seasons (summer) and areas with greater noise (e.g., Cairn Point) (Castellote et al., 2018). As CIB are highly gregarious animals that rely on calls for many social and biological behaviors and communication for finding ice holes, masking of important vocalizations could have negative implications for communications, foraging, and predator avoidance (Erbe et al., 2016; Small et al., 2017). However, the acoustic footprint of other sources, such as the oil and gas industry, has yet to be fully described in Cook Inlet (Castellote et al., 2018).
In the surrogate species, noise is characterized as a threat of high concern for SLB and SRKW, and is closely monitored in HD. Exposure of SLB to marine traffic and noise has been chronic and increasing from shipping traffic and other marine activities such as dredging, with much concern about some of these activities overlapping habitat key to SLB reproduction and social activities (Department of Fisheries and Oceans [DFO] Canada, 2014, 2016; Lesage et al., 2014). The SRKW are of high public concern because anthropogenic sound sources subject the whales to auditory masking that can interfere with foraging strategies or cause annoyance and disorientation (Holt et al., 2008, 2011). This is of particular concern within Haro Strait, the western entrance to Puget Sound from the Pacific Ocean, a key transit and foraging area, where they are subject nearly continuous ship noise both directly and via complicated reverberations (Jones and Wolfson, 2005). With regard to Hector’s dolphins, activities such as pile-driving, especially in partially enclosed bodies of water such as harbors, produced noise that may cause behavioral changes and temporary hearing threshold shifts in this species (Pine et al., 2016; Leunissen and Dawson, 2018; Leunissen et al., 2019). Records addressing noise disturbance from vessels such as whale watch boats were represented in the records for HD and could have applicability for CIB. For example, these studies concluded that noise from anthropogenic activities likely had negative impacts on HD behavior by displacing them from their preferred habitat hot spots or through masking (Pine et al., 2016; Leunissen et al., 2019).
There is sufficient information (n = 31 records) for NMFS to be greatly concerned about acoustic masking of essential beluga communication over a wide temporal and spatial scale within their critical habitat. Castellote et al. (2018) recommends NMFS consider the cumulative temporal and spatial overlap of anthropogenic activities and an additive increase in noise disturbance as a factor in the permitting process. A revision of the spatial extent of the current critical habitat exclusion zone (around the Port of Anchorage) may be warranted, with the attendant implications for anthropogenic noise management at this site (Castellote et al., 2018).
In the recovery plan, reduction in prey is considered a threat of “medium” concern for CIB; however, the trend, probability, and magnitude of this threat are considered as “unknown.” There are discrepancies between the level of concern of a threat and other characteristics of the threat such as extent, frequency, and trend for CIB (Table 1). Therefore, it is unclear how the level of “medium” concern was assigned for this threat. Recent research indicates that the availability of key salmon prey could be a particularly important recovery factor, a strong indication that CIB are dependent on access to relatively dense concentrations of high value prey species, particularly in the spring and throughout the summer months, similarly to SRKW (Chasco et al., 2017; Norman et al., 2020). This finding plus the fact that sufficient prey is more susceptible to managerial actions with a timely measurable impact suggest that adequate prey availability should be ranked as “high” concern rather than “medium.” This is especially important since reduced quality nutrition may contribute to physiological stress as observed in at least one of the surrogate species, SRKW (Ayres et al., 2012). The ranking of prey reduction as a “high” threat in the SRKW Recovery Plan may have prompted research efforts to assess prey quality, abundance, and distribution within their summer range, the Pacific Northwest, and the relationship between fecundity and mortality of SRKW to prey levels (e.g., Ford et al., 2010; O’Neill et al., 2014; Ward et al., 2016). The number of SRKW records (both peer-reviewed and gray literature) related to prey reduction, doubled from 2018 to 2019 (e.g., Joy et al., 2019; Tennessen et al., 2019), suggesting an increased focus on investigating the role of prey reduction on stress and reproduction in that population, studies that could potentially be applied to CIB.
The CIB recovery plan lists pollution as a threat of “low” relative concern (Table 1). A number of sources of chemical and biological pollution have been identified in and around Cook Inlet, but a comprehensive water quality survey of Cook Inlet is not available (NMFS, 2016). Only a few studies have been completed that document the presence of contaminants in CIB at levels higher than other Alaskan beluga stocks for some contaminant congeners and temporal increases over time for others (Becker et al., 2000; Krahn et al., 2009; Reiner et al., 2011; Hoguet et al., 2013). Pollution is documented as an important factor in two of the three surrogate populations, SLB and SRKW (Lachmuth et al., 2011; Mongillo et al., 2012; Lundin et al., 2015, 2016). There is a large number of records for both SLB and SRKW populations, n = 44 and n = 25, respectively, that address consequences of pollution such as disease and reproductive failure (e.g., Wasser et al., 2017; Bernier-Graveline et al., 2021). Declines in reproductive success are likewise a concern in CIB (Booth et al., 2020); however, only eight publications address pollution for CIB. Pollution is widely considered a danger to marine mammals, but the extent of which has yet to be thoroughly documented in CIB. Pollution was listed as a “low” priority threat in the recovery plan, but few studies have investigated its impacts on CIB since 2017 to corroborate this threat is indeed of low concern to recovery. One recent study appears to confirm that environmental pollution is a threat to recovery, demonstrating that CIB and SLB are prone to bioaccumulation of significantly higher levels of polycyclic aromatic hydrocarbons through their prey than Arctic and aquarium belugas (Poirier et al., 2019). Furthermore, several other studies have suggested a strong link between body pollution burdens and alterations of hormones such as thyroid, and increased incidence of cancer (Béland et al., 1993; De Guise et al., 1995; Poirier et al., 2019; Simond et al., 2019, 2020).
The CIB recovery plan includes oil and hazardous substance spills and natural gas blowouts among the potential sources of concern, but few records discuss these threats (e.g., Norman et al., 2015). While the recognition that a large magnitude pollution event could have population-level impacts for belugas is appropriate, the available pollution information indicates that chronic pollution is a separate threat with a different profile of risks and mitigation strategies (NMFS, 2016). Larger and less frequent events seem to better fit the notion of being potentially catastrophic depending on location and season in which a spill was to occur. For example, one catastrophic event of sufficient quantity of noxious material could injure or even kill a significant number of animals (Reed et al., 2003; Wade et al., 2012; Hobbs et al., 2015b). In contrast, smaller spills are relatively more frequent in Cook Inlet, based on reported figures for the years 2004–2016 (NMFS, 2016). The probability of additive pollution from relatively small spills is likely, particularly if the chemicals settle into the sediment (NMFS, 2016). Effects of exposure to toxic chemicals from spills would be similar to that obtained from exposure to other sources such as contaminated sediment or other environmental sources of deposition. Studies in SLB and SRKW suggest potential detrimental effects that could include decline of fecundity and adult mortality from cancers due to persistent organic pollutants (Martineau et al., 1994; Lair et al., 2015; Pearce, 2018; Manteufel, 2019).
Scientific literature has an important role to play in species conservation and management. Beyond informing species listings, recovery plans, and other documents ensuring better policy implementation for biodiversity conservation, research provides sound, evidence-based data to inform management decisions. As an example, the sufficient and consistent literature on the impacts of noise on the endangered SRKW serves as a model for other populations exposed to excessive noise and demonstrates the role of noise in cumulative effects on populations (Murray et al., 2021). Aggregating and assessing cumulative “takes” resulting from noise is consistent with the “high” relative concern given the “cumulative impact of multiple stressors” threat to recovery and is an important and viable action to take to address this threat. Records specific to simultaneous assessment of multiple stressors are not available for any of the populations in this review; however, theoretical efforts have been made to explore the consequences of multiple disturbances in marine mammal populations that would promote the design of future marine mammal monitoring programs to inform population-level analysis (Tollit et al., 2016; Booth et al., 2020). A need exists for research on threats to CIB that are not necessarily covered by the surrogates such as climate change, interactions between threats, and more complex threats such as cumulative effects. Climate change in particular is a growing threat for changes in disease virulence or emergence that could extirpate a vulnerable population such as CIB (Lafferty, 2009; McCallum, 2012). Of the 789 records, 17 either made a reference to the term “climate change” or directly addressed it (4 in CIB, 6 in SLB, 5 in SRKW, and 2 related to general marine mammals). Even though the CIB recovery plan does not discuss climate change as a separate threat, it is considered in the context of its potential impact on the population in the presence of other identified threats, even those of lower concern. However, empirical research on the combined effects of climate change and other threats is generally still lacking. For example, theoretical ecosystem models have been developed to demonstrate higher bioaccumulation of contaminants in a marine food web in the northeastern Pacific Ocean under climate change; these pollutant accumulations have amplified in marine top predators such as resident killer whales under high carbon emissions (Alava et al., 2018), and potentially could in Cook Inlet belugas. Additionally, small populations such as CIB that have limited ranges may experience increased vulnerability to climate change through habitat perturbations or ecological shifts that are larger in scale (Silber et al., 2017). Furthermore, recent work has reviewed the decline in the size of individual salmon, an important prey for CIB, across Alaska over the last 60 years, which has primarily resulted from shifting age structure, and are secondly associated with climate change and competition with other predators at sea. A mismatch between prey abundance and the corresponding threatened or endangered population has a potential to decrease the latter’s viability and increase its extinction risks (Durant et al., 2007; Bell et al., 2017; Oke et al., 2020), while also negatively impacting survival rates and reproductive success (Lesage, 2021). Furthermore, a systematic review of cumulative effects assessments revealed that, in general, climate change intensified the effects of anthropogenic stressors at the species level, while at trophic or ecosystem level the effect of climate change with anthropogenic stressors depended on the trophic group or the environmental conditions in question (Gissi et al., 2021). These highlights confirm the need for further research focused on climate change’s effects on belugas (both direct and cumulative with other threats) (Tollit et al., 2016).
Noting the impact noise has on CIB, a remedy order was recently finalized stating that noise generated from tugs in tow cause take by harassment of CIB and that a previous NMFS’ finding of “no significant impact” was “arbitrary and capricious,” therefore, not justified in court (U.S. District Court, 2021). The current literature warrants stronger mitigation actions such as previously suggested in Castellote et al. (2018). An adaptive approach to managing noise could be applied when considering incidental take authorizations, this would involve trying alternative ways to mitigate noise, as suggested by research studies (e.g., Castellote et al., 2018), at a level predicted to mitigate noise impacts in a test area. A recent paper, calling for NMFS to implement Recovery Plan Action # 62 in the CIB recovery plan to review how harassment takes are allocated, suggests takes be apportioned more comprehensively instead of by individual project, to reduce cumulative effects of harassment by multiple takes (Migura and Bollini, 2021). As one or more of the suggested mitigation measures is implemented, the noise levels and behaviors of the CIB would be monitored to learn about the value of these management actions. The results obtained could then be used to update knowledge on the success of the mitigation measures and adjust future management actions as needed (Agardy et al., 2019).
In all four populations, the observation that management-focused records appeared in greater numbers in the late 2000s and 2010s represents a positive evolution toward the use of biological research to inform management decisions. For example, researchers who initially documented the biology of these populations (e.g., Sergeant and Hoek, 1988), went on to focus more specifically on the application of biological findings to management decisions as the population declined, prompting the need for conservation actions (e.g., McGuire et al., 2020). Contributions by graduate student research efforts can provide additional avenues to help fill knowledge gaps similar to what is happening with the HD and SRKW surrogate populations (e.g., Rayment, 2008; Bassett, 2010; Strange, 2016; Cross, 2019; Fraser, 2020). Furthermore, the surrogate population literature demonstrated the use of various conservation tools to help with management. For instance, a large number of HD records demonstrated management efforts at delineating key hotspots for preferred foraging sites at a fine scale for this species (Brough et al., 2020) and supporting the use of tools such as conservation genetics to delineate and monitor stocks (Hamner, 2014), which have been applied in CIB management (O’Corry-Crowe et al., 2018, 2020).
Management action and planning could benefit from greater clarity on threat rankings and priorities and the linkage to scientific literature. While some threats ranked as “high” or “medium” concern (Table 1) have been accorded responsive actions and attention in the recovery plan, those ranked of “low” concern are often not as well-investigated. As such, they will remain poorly understood unless they are more thoroughly studied (e.g., pollution; Hoguet et al., 2013). For CIB, there are discrepancies between the level of concern of a threat and other characteristics of the threat such as extent, frequency, and trend (Table 1). For example, prey reduction is considered a threat of “medium” concern for CIB; however, the trend, probability, and magnitude of this threat are listed as “unknown.” CIB, like SRKW, may be dependent on access to relatively dense concentrations of high value prey species, particularly in the spring and throughout the summer months for successful reproduction and health (Ward et al., 2009; Ford et al., 2010). Continued identification of unknowns can help direct research needs for more effective management and decision making.
The CIB will require an adaptive management approach (deFur and Kaszuba, 2002): a method that emphasizes learning and continued incorporation of new information into the management process. This involves exploring alternative ways to meet management objectives; predicting the outcomes of the alternatives based on the current state of knowledge; implementing one or more of these alternatives; then monitoring to learn about the impacts of those management actions (Gerber et al., 2007). Results are used to update scientific knowledge and adjust subsequent management actions. Adaptive management focuses on learning and adapting, through partnerships of managers, scientists, and stakeholders who learn together how to create and maintain sustainable resource systems that can be adapted to populations living in urban ecosystems such as Cook Inlet (Tyre and Michaels, 2011; Kelly et al., 2017; Kehoe et al., 2020). As an example, a community driven or bottom-up adaptive management process was adopted to implement and evaluate whale watching guidelines for SRKW, that were enforced through a partnership between the whale watch industry and local non-governmental organizations concerned about harm to SRKW from disturbances caused by whale watch vessels. This concern spurred the development of transboundary whale watch guidelines for Canada and the United States (Giles, 2014).
The use of adaptive management strategies, would allow NMFS the flexibility to consider new information from various sources to determine annually if mitigation or monitoring measures should be modified, based on new research findings and the response of the CIB population to previous management actions. Performance indicators for effectiveness of recovery measures have been used for SLB and could be applied to CIB (Department of Fisheries and Oceans [DFO] Canada, 2020). For example, monitoring of noise levels in CIB habitat, and contaminant levels in CIB, can be instated and monitored to assess trends in the threats and how they are affecting CIB recovery over time in order to inform adaptive management. Acoustic monitoring of SRKW within the inland waters of British Columbia, Canada and Washington State, United States provides data on sound levels and sources that could interfere with their communication, cause behavioral avoidance, and possibly hearing loss (Jones and Wolfson, 2005; Williams et al., 2014, 2019; Cominelli et al., 2019).
Ecological mechanisms, such as Allee effects, lack of resilience to exploitation by humans, or disruption of social behaviors can explain the lack of recovery in small populations especially when social systems provide plausible mechanisms for such populations dynamics (Courchomp et al., 1999; Ward et al., 2011; Wade et al., 2012). For example, in northern resident killer whales, certain individuals seem to be more crucial than others in maintaining the social network throughout the population. Williams and Lusseau (2006) found that anthropogenic removals targeting certain age or sex classes might have different population-level effects than random removal. Several potential biases were revealed during this systematic review. The record search resulted in a very limited contribution of Traditional Ecological Knowledge (TEK) to the CIB body of records, and essentially none in any of the surrogate populations (Huntington, 2000; Breton-Honeyman et al., 2016). Additionally, no searches for oral literature (e.g., recordings, transcripts of interviews) of TEK were included thus some TEK that could have contributed to the library of CIB records could have been missed. These findings highlight the need for management agencies to authentically seek feedback from indigenous stakeholders for future species management plans (Huntington, 2000; Breton-Honeyman et al., 2016).
The search results contained a large number of gray literature records. Although some gray literature is well-cited (e.g., NMFS, 2008, 2016), much of it may receive very little attention despite its valuable contribution (e.g., Goetz et al., 2012). This highlights the importance of including gray literature such as documents and reports when conducting a systematic review. The review may also risk bias by the selection of the surrogate species. We selected surrogates based on certain minimum criteria, which included a requirement for a significant body of literature. There may have been other applicable populations from which equivalent data on specific threats, could have been extracted, However, other endangered beluga populations (e.g., Ungava Bay, Canada) lacked significant bodies of literature thus the available information was insufficient to serve as a surrogate (Reeves and Mitchell, 1987; Gosselin et al., 2017). Lastly, placement of the records into a particular search term category may have been biased, but attempts were made to minimize this by identifying the dominant threat theme of the record and ensuring it matched one of the search terms of that category.
This review revealed that due to relatively low numbers of records for CIB, odontocete populations with larger bodies of relevant literature (e.g., SLB and SRKW) could serve as appropriate surrogates in informing the direction of future CIB studies and management strategies and that re-ranking the levels of concern for some of the CIB threats (i.e., pollution, prey reduction) may be warranted. Although differences in the respective management situations of each of the four populations can limit the applicability of our findings arising from studies of surrogates, these surrogate populations may be appropriate to address uncertainty with documented threats in the CIB recovery plan that are listed as high/medium/low concern, such as noise and cumulative effects/prey reduction/pollution, respectively. Our review demonstrates the need for NMFS to proactively seek to synthesize information about other appropriate odontocetes and threats to help fill data gaps for CIB (Action #57 in the recovery plan). Most importantly, these gaps should also be filled specifically by new studies on CIB for these threats. By highlighting comparable threats in surrogate species, we hope to stimulate dialogue and promote the creation of resources to better address uncertainty with threat management concerning the CIB population. Suggested immediate management actions have been put forth for SLB which strive to improve recovery and help mitigate the effects of other threats to population recovery. As the CIB populations continues to show no progress toward recovery, bold, well-informed management strategies are needed to recover the endangered CIB.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
SN conducted the systematic review. LD provided intellectual input and critical review into the manuscript. TN provided intellectual input and critical review of the manuscript, as well as designed and constructed the figures. KN conceived the idea of conducting a systematic review of Cook Inlet beluga literature with comparison to surrogate species. All authors contributed to manuscript revisions and read and approved the final submission.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Roderick C. Hobbs (NOAA, Marine Mammal Laboratory, retired) and Jacob W. Malcom (former Director of the Center for Conservation Innovation, Defenders of Wildlife) for critically reviewing the manuscript. We would also like to thank Nicole Whittington-Evans for providing logistical support for this project.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.804218/full#supplementary-material
DFO, Department of Fisheries and Oceans Canada; CIB, Cook Inlet belugas; SLB, St. Lawrence belugas; SRKW, Southern resident killer whales; HD, Hector’s dolphins; NMFS, National Marine Fisheries Service; TEK, Traditional Ecological Knowledge.
Agardy, T., Cody, M., Hastings, S., Hoyt, E., Nelson, A., Tetley, M., et al. (2019). Looking beyond the horizon: An early warning system to keep marine mammal information relevant for conservation. Aquat. Conserv. 29, 71–83. doi: 10.1002/aqc.3072
Alava, J. J., Cisneros-Montemayor, A. M., Sumaila, U. R., and Cheun, W. W. L. (2018). Projected amplification of food web bioaccumulation of MeHg and PCBs under climate change in the Northeastern Pacific. Sci. Rep. 8:13460. doi: 10.1038/s41598-018-31824-5
Ayres, K. L., Booth, R. K., Hempelmann, J. A., Koski, K. L., Emmons, C. K., Baird, R. W., et al. (2012). Distinguishing the impacts of inadequate prey and vessel traffic on an endangered killer whale (Orcinus orca) population. PLoS One 7:e36842. doi: 10.1371/journal.pone.0036842
Bassett, C. (2010). Underwater ambient noise at a proposed tidal energy site in Puget Sound. [Master’s thesis]. Seattle, WA: University of Washington.
Becker, P. R., Krahn, M. M., Mackey, E. A., Demiralp, R., Schantz, M. M., Epstein, M. S., et al. (2000). Concentrations of polychlorinated biphenyls (PCB’s), chlorinated pesticides, and heavy metals and other elements in tissues of belugas, Delphinapterus leucas, from Cook Inlet, Alaska. Mar. Fish. Rev. 62, 81–98.
Béland, P., De Guise, S., Girard, C., Lagacé, A., Martineau, D., Michaud, R., et al. (1993). Toxic compounds and health and reproductive effects in St. Lawrence beluga whales. J. Great Lakes Res. 19, 766–775. doi: 10.1016/S0380-1330(93)71264-2
Bell, D. A., Kovach, R. P., Vulstek, S. C., Joyce, J. E., and Tallmon, D. A. (2017). Climate-induced trends in predator-prey synchrony differ across life-history stages of an anadromous salmonid. Can. J. Fish. Aquat. Sci. 74, 1431–1438. doi: 10.1139/cjfas-2016-0309
Bernier-Graveline, A., Lesage, V., Cabrol, J., Lair, S., Michaud, R., Rosabal, M., et al. (2021). Lipid metabolites as indicators of body condition in highly contaminant-exposed belugas from the endangered St. Lawrence Estuary population (Canada). Environ. Res. 192:110272. doi: 10.1016/j.envres.2020.110272
Booth, C. G., Sinclair, R. R., and Harwood, J. (2020). Methods for monitoring for the population consequences of disturbance in marine mammals: a review. Fron. Mar. Sci. 7:115. doi: 10.3389/fmars.2020.00115
Boyd, C., Hobbs, R. C., Punt, A. E., Shelden, K. E. W., Sims, C. L., and Wade, P. (2019). Bayesian estimation of group sizes for a coastal cetacean using aerial survey data. Mar. Mamm. Sci. 35, 1322–1346. doi: 10.1111/mms.12592
Braulik, G. T., Noureen, U., Arshad, M., and Reeves, R. R. (2015). Review of status, threats, and conservation management options for the endangered Indus River blind dolphin. Biol. Conserv. 192, 30–41. doi: 10.1016/j.biocon.2015.09.008
Breton-Honeyman, K., Furgal, C. M., and Hammill, M. O. (2016). Systematic review and critique of the contributions of Traditional Ecological Knowledge of beluga whales in the marine mammal literature. Arctic 69, 37–46. doi: 10.14430/arctic4543
Brough, T., Rayment, W., Slooten, E., and Dawson, S. (2020). Spatiotemporal distribution of foraging in a marine predator: behavioural drivers of hotspot formation. Mar. Ecol. Prog. Ser. 635, 187–202. doi: 10.3354/meps13198
Castellote, M., Thayre, B., Mahoney, M., Mondragon, J., Lammers, M. O., and Small, R. J. (2018). Anthropogenic noise and the endangered Cook Inlet beluga whale, Delphinapterus leucas: acoustic considerations for management. Mar. Fish. Rev. 80, 63–88. doi: 10.7755/MFR.80.3.3
Castellote, M., Thayre, B., Mahoney, M., Mondragon, J., Schmale, C., and Small, R. J. (2016). Anthropogenic noise in Cook Inlet beluga habitat: sources, acoustic characteristics, andfrequency of occurrence. Alaska Department of Fish and Game, Final Wildlife Research Report, ADF&G/DWC/WRR-2016-4, Juneau. Available online at https://www.adfg.alaska.gov/static/home/library/pdfs/wildlife/research_pdfs/wrr_2016_4_anthropogenic_noise_cook_inlet_beluga_habitat.pdf (accessed August 31, 2021)
Chasco, B. E., Kaplan, I. C., Thomas, A. C., Acevedo-Gutiérrez, A., Noren, D. P., Ford, M. J., et al. (2017). Competing tradeoffs between increasing marine mammal predation and fisheries harvest of Chinook salmon. Sci. Rep. 7:15439. doi: 10.1038/s41598-017-14984-8
Cominelli, S., Leahy, M., Devillers, R., and Hall, G. B. (2019). Geovisualization tools to inform the management of vessel noise in support of species’ conservation. Ocean Coast. Manage 169, 113–128. doi: 10.1016/j.ocecoaman.2018.11.009
Cook, C. N., Possingham, H. P., and Fuller, R. A. (2014). Contribution of systematic reviews to management decisions. Conserv. Biol. 27, 902–915. doi: 10.1111/cobi.12114
COSEWIC (2014). COSEWIC assessment and status report on the Beluga Whale Delphinapterus leucas, St. Lawrence Estuary population, in Canada. Ottawa: Committee on the Status of Endangered Wildlife in Canada.
Courchomp, F., Clutton-Brock, T., and Grenfell, B. (1999). Inverse density dependence and the Allee effect. Trends Ecol. Evol. 14, 405–410. doi: 10.1016/S0169-5347(99)01683-3
Cross, C. L. (2019). Spatial ecology of delphinids in Queen Charlotte Sound, New Zealand : implications for conservation management. [Dissertation]. Palmerston North, NZ: Massey University.
Cullon, D. L., Yunker, M. B., Alleyne, C., Dangerfield, N. J., O’Neill, S., Whiticar, M. J., et al. (2009). Persistent organic pollutants in chinook salmon (Oncorhynchus tshawytscha): implications for resident killer whales of British Columbia and adjacent waters. Environ. Toxicol. Chem. 28, 148–161. doi: 10.1897/08-125.1
De Guise, S., Martineau, D., BeIand, P., and Fournier, M. (1995). Possible mechanisms of action of environmental contaminants on St. Lawrence beluga whales (Delphinapterus leucas). Environ. Health Persp. 103(Suppl. 4), 73–77. doi: 10.1289/ehp.95103s473
deFur, P. L., and Kaszuba, M. (2002). Implementing the precautionary principle. Sci. Total Environ. 288, 155–165. doi: 10.1016/s0048-9697(01)01107-x
Department of Fisheries and Oceans [DFO] Canada (2012). Recovery Strategy for the Beluga Whale (Delphinapterus leucas) St. Lawrence Estuary Population in Canada. Species at Risk Act Recovery Strategy Series. Ottawa, ON: Fisheries and Oceans Canada, 88+X.
Department of Fisheries and Oceans [DFO] Canada (2014). Impacts of Rerouting Marine Traffic in the St. Lawrence Estuary on Beluga (Delphinapterus leucas): Science in Support of Risk Management. Canadian Science Advisory Secretariat Science Advisory Report 2014/004, Quebec Region. Ottawa, ON: Fisheries and Oceans Canada, 9.
Department of Fisheries and Oceans [DFO] Canada (2016). Effects of Dredging Activities on St. Lawrence Beluga and Their Habitat. Canadian Science Advisory Secretariat Science Response 2016/033, Quebec Region. Ottawa, ON: Fisheries and Oceans Canada, 17.
Department of Fisheries and Oceans [DFO] Canada (2020). Review of the Effectiveness of Recovery Measures for St. Lawrence Estuary Beluga. Ottawa, ON: Fisheries and Oceans Canada, 64.
Durant, J. M., Hjermann, D. O., Ottersen, G., and Stenseth, N. D. (2007). Climate and the match or mismatch between predator requirements and resource availability. Clim. Res. 33, 271–283. doi: 10.3354/cr033271
Erbe, C., Reichmuth, C., Cunningham, K., Lucke, K., and Dooling, R. (2016). Communication masking in marine mammals: a review and research strategy. Mar. Pollut. Bull. 103, 15–38. doi: 10.1016/j.marpolbul.2015.12.007
Favreau, J. M., Drew, C. A., Hess, G. R., Rubino, M. J., Koch, F. H., and Eschelbach, K. A. (2006). Recommendations for assessing the effectiveness of surrogate species approaches. Biodivers. Conserv. 15, 3949–3969. doi: 10.1007/s10531-005-2631-1
Fearnbach, H., Durban, J. W., Ellifrit, D. K., and Balcomb, K. C. (2018). Using aerial photogrammetry to detect changes in body condition of endangered southern resident killer whales. Endang. Species R. 35, 175–180. doi: 10.3354/esr00883
Ford, J. K. B., Ellis, G. M., Olesiuk, P. F., and Balcomb, K. C. (2010). Linking killer whale survival and prey abundance: food limitation in the oceans’ apex predator? Biol. Lett. 6, 139–142. doi: 10.1098/rsbl.2009.0468
Fraser, M. (2020). Whale and small vessel interactions: exploring regulatory compliance and management implications in the Salish Sea. [Master’s Thesis]. Victoria, BC: University of Victoria.
Gerber, L. R., Wielgus, J., and Sala, R. (2007). A decision framework for the adaptive management of an exploited species with implications for marine reserves. Conserv. Biol. 21, 1594–1602. doi: 10.1111/j.1523-1739.2007.00824.x
Giles, D. A. (2014). Southern Resident Killer Whales (Orcinus orca): The evolution of adaptive management practices for vessel-based killer whale watching in the Salish Sea, A novel non-invasive method to study southern resident killer whales (Orcinus orca) and vessel compliance with regulations, and The effect of vessels on group cohesion and behavior of southern resident killer whales (Orcinus orca). [Dissertation]. Davis, CA: University of California, Davis.
Gissi, E., Manea, E., Mazaris, A. D., Fraschetti, S., Almpanidou, V., Bevilacqua, S., et al. (2021). A review of the combined effects of climate change and other local human stressors on the marine environment. Sci. Total Environ. 755:142564. doi: 10.1016/j.scitotenv.2020.142564
Goetz, K. T., Robinson, P. W., Hobbs, R. C., Laidre, K. L., Huckstadt, L. A., and Shelden, K. E. W. (2012). Movement and dive behavior of belugsa whales in Cook Inlet, Alaska. AFSC Processed Rep. 2012-03, 40 p. Alaska Fish. Sci. Cent., NOAA, Natl. Mar. Fish. Serv., 7600 Sand Point Way NE, Seattle WA 98115. Available at https://www.fisheries.noaa.gov/resource/document/movement-and-dive-behavior-beluga-whales-cook-inlet-alaska (accessed February 17, 2021)
Gosselin, J.-F., Hammill, M. O., and Mosnier, A. (2017). Indices of abundance for beluga (Delphinapterus leucas) in James Bay and eastern Hudson Bay in summer 2015. DFO Can. Sci. Advis. Sec. Res. Doc. 2017/067. iv + 25 p. Available online at https://waves-vagues.dfo-mpo.gc.ca/Library/40653018.pdf (accessed September 3, 2021).
Gusenbauer, M., and Haddaway, N. R. (2020). Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of Google Scholar, PubMed, and 26 other resources. Res. Synth. Methods 11, 181–217. doi: 10.1002/jrsm.1378
Hamner, R. M. (2014). All in a DNA’s work: conservation genetics and monitoring of the New Zealand endemic Maui’s and Hector’s dolphins.[Dissertation]. Corvallis, OR: Oregon State University.
Hitt, N. P., and Frissell, C. A. (2004). A case study of surrogate species in aquatic conservation planning. Aquatic Conserv: Mar. Freshw. Ecosyst. 14, 625–633. doi: 10.1002/aqc.638
Hobbs, R. C., and Shelden, K. E. W. (2008). Supplemental status review and extinction assessment of Cook Inlet belugas (Delphinapterus leucas). AFSC Processed Rep. 2008-08, 76 p. Alaska Fish. Sci. Cent., NOAA, Natl. Mar. Fish. Serv., 7600 Sand Point Way NE, Seattle WA 98115. Available at https://repository.library.noaa.gov/view/noaa/9027 (accessed March 29, 2021).
Hobbs, R. C., Shelden, K. E. W., Rugh, D. J., and Norman, S. A. (2008). 2008 status review and extinction risk assessment of Cook Inlet belugas (Delphinapterus leucas). AFSC Processed Rep. 2008-02, 116 p. Alaska Fish. Sci. Cent., NOAA, Natl. Mar. Fish. Serv., 7600 Sand Point Way NE, Seattle WA 98115. Available at https://archive.fisheries.noaa.gov/afsc/Publications/ProcRpt/PR%202008-02.pdf (accessed April 21, 2021).
Hobbs, R. C., Shelden, K. E. W., Sims, C. L., Waite, J. M., and Rugh, D. J. (2015a). Estimated abundance and trend in aerial counts of beluga whales, Delphinapterus leucas, in Cook Inlet, Alaska, 1994–2012. Mar. Fish. Rev. 77, 11–31. doi: 10.7755/MFR.77.1.2
Hobbs, R. C., Wade, P. R., and Shelden, K. E. W. (2015b). Viability of a small, geographically isolated population of beluga whale, Delphinapterus leucas: effects of hunting, predation, and mortality events in Cook Inlet, Alaska. Mar. Fish. Rev. 77, 59–88. doi: 10.7755/MFR.77.2.4
Hobbs, R. C., Shelden, K. E. W., Vos, D. J., Goetz, K. T., and Rugh, D. J. (2006). Status review and extinction assessment of Cook Inlet belugas (Delphinapterus leucas). AFSC Processed Rep. 2006-16, 74 p. Available at http://www.afsc.noaa.gov/Publications/ProcRpt/PR%202006-16.pdf (accessed March 26, 2021)
Hoguet, J., Keller, J. M., Reiner, J. L., Kucklick, J. R., Bryan, C. E., Moors, A. J., et al. (2013). Spatial and temporal trends of persistent organic pollutants and mercury in beluga whales (Delphinapterus leucas) from Alaska. Sci. Total Environ. 449, 285–294. doi: 10.1016/j.scitotenv.2013.01.072
Holt, M. M., Noren, D. P., and Emmons, K. C. (2011). Effects of noise levels and call types on the source levels of killer whale calls. J. Acoust. Soc. Am. 130:3100. doi: 10.1121/1.3641446
Holt, M. M., Viers, V., and Viers, S. (2008). Noise effects on the call amplitude of southern resident killer whales (Orcinus orca). Bioacoustics 17, 164–166. doi: 10.1080/09524622.2008.9753802
Huntington, H. P. (2000). Traditional knowledge of the ecology of Belugas, Delphinapterus leucas, in Cook Inlet, Alaska. Mar. Fish. Rev. 62, 134–140.
Jacobson, E. K., Boyd, C., McGuire, T. L., Shelden, K. E. W., Boor, G. K. H., and Punt, A. E. (2020). Assessing cetacean populations using integrated population models: an example with Cook Inlet beluga whales. Ecol. Appl. 30:e02114. doi: 10.1002/eap.2114
Jones, C., and Wolfson, M. (2005). The acoustic environment of the southern resident killer whales in Haro Strait: propagation modeling and analysis of field measurements. J. Acoust. Soc. Am. 117:2526. doi: 10.1121/1.4809428
Joy, R., Tollit, D., Wood, J., MacGillivray, A., Li, Z., Trounce, K., et al. (2019). Potential benefits of vessel slowdowns on endangered southern resident killer whales. Front. Mar. Sci. 6:344. doi: 10.3389/fmars.2019.00344
Kehoe, L. J., Lund, J., Chalifour, L., Asadian, Y., Balke, E., Boyd, S., et al. (2020). Conservation in heavily urbanized biodiverse regions requires urgent management action and attention to governance. Conserv. Sci. Pract. 3:e310. doi: 10.1111/csp2.310
Kelly, R. P., Levin, P. S., and Lee, K. N. (2017). Science, policy, and data-driven decisions in a data vacuum. Ecol. Law Quart. 44, 7–40. doi: 10.15779/Z380G3GX60
Kendall, L. S., and Cornick, L. A. (2016). Behavior and distribution of Cook Inlet beluga whales, Delphinapterus leucas, before and during pile driving activity. Mar. Fish. Rev. 77, 106–114. doi: 10.7755/MFR.77.2.6
Kendall, L. S., Široviæ, A., and Roth, E. H. (2013). Effects of construction noise on the Cook Inlet beluga whale (Delphinapterus leucas) vocal behavior. Can. Acoust. 41, 3–13.
Krahn, M. M., Hanson, M. B., Schorr, G. S., Emmons, C. K., Burrow, D. G., Bolton, J. L., et al. (2009). Effects of age, sex and reproductive status on persistent organic pollutant concentrations in ‘Southern Resident’ killer whales. Mar. Pollut. Bull. 58, 1522–1599. doi: 10.1016/j.marpolbul.2009.05.014
Lachmuth, C. L., Barrett-Lennard, L. G., Steyn, D. Q., and Milsom, W. K. (2011). Estimation of southern resident killer whale exposure to exhaust emissions from whale-watching vessels and potential adverse health effects and toxicity thresholds. Mar. Pollut. Bull. 62, 792–805. doi: 10.1016/j.marpolbul.2011.01.002
Lacy, R. C., Williams, R., Ashe, E., Balcomb, K. C. III, Brent, L. J. N., Clark, C. W., et al. (2017). Evaluating anthropogenic threats to endangered killer whales to inform effective recovery plans. Sci. Rep. 7:14119. doi: 10.1038/s41598-017-14471-0
Lafferty, K. D. (2009). The ecology of climate change and infectious diseases. Ecology 90, 888–900. doi: 10.1890/08-0079.1
Lair, S., Measures, L., and Martineau, D. (2015). Pathologic findings and trends in mortality in the beluga (Delphinapterus leucas) population of the St Lawrence Estuary, Quebec, Canada, from 1983 to 2012. Vet. Pathol. 53, 22–36. doi: 10.1177/0300985815604726
Lesage, V. (2021). The challenges of a small population exposed to multiple anthropogenic stressors and a changing climate: the St. Lawrence Estuary beluga. Polar Res. 40:5523. doi: 10.33265/polar.v40.5523
Lesage, V., McQuinn, I. H., Carrier, D., Gosselin, J.-F., and Mosnier, A. (2014). Exposure of the beluga (Delphinapterus leucas) to marine traffic under various scenarios of transit route diversion in the St. Lawrence Estuary. DFO Can. Sci. Advis. Sec. Res. Doc. 2013:28.
Leunissen, E. M., and Dawson, S. M. (2018). Underwater noise levels of pile-driving in a New Zealand harbour, and the potential impacts on endangered Hector’s dolphins. Mar. Pollut. Bull. 135, 195–204. doi: 10.1016/j.marpolbul.2018.07.024
Leunissen, E. M., Rayment, W. J., and Dawson, S. M. (2019). Impact of pile-driving on Hector’s dolphin in Lyttelton Harbour. New Zealand. Mar. Pollut. Bull. 142, 31–42. doi: 10.1016/j.marpolbul.2019.03.017
Lowry, L., Hobbs, R. C., and O’Corry-Crowe, G. (2019). Delphinapterus leucas (Cook Inlet subpopulation). The IUCN Red List of Threatened Species 2018:e.T61442A50384653. doi: 10.2305/IUCN.UK.2019-1.RLTS.T61442A50384653.en Available online at https://www.iucnredlist.org/species/61442/50384653(accessed 2 March 2021)
Lundin, J. I., Dills, R., Ylitalo, G. M., Hanson, M. B., Emmons, C. K., Schorr, G. S., et al. (2015). Persistent organic pollutant determination in killer whale scat samples: optimization and validation of the method, and application to real samples. Arch. Environ. Contam. Toxicol. 70, 9–19. doi: 10.1007/s00244-015-0218-8
Lundin, J. I., Ylitalo, G. M., Booth, R. K., Anulacion, B., Hempelmann, J. A., Parsons, K. M., et al. (2016). Modulation in persistent organic pollutant concentration and profile by prey availability and reproductive status in southern resident killer whale scat samples. Environ. Sci. Technol. 50, 6506–6516. doi: 10.1021/acs.est.6b00825
Manteufel, N. L. (2019). Effects of increased PCB & PBDE levels on survival and reproduction of Southern Resident Killer Whales. [Master’s Thesis]. Olympia, WA: The Evergreen State College.
Martineau, D., De Guise, S., Fournier, M., Shugart, L., Girard, C., Lagacé, A., et al. (1994). Pathology and toxicology of beluga whales from the St. Lawrence Estuary, Quebec, Canada.Past, present and future. Sci. Total Environ. 154, 201–215. doi: 10.1016/0048-9697(94)90088-4
McCallum, H. (2012). Disease and the dynamics of extinction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 2828–2839. doi: 10.1098/rstb.2012.0224
McGuire, T. L., Boor, G. K. H., McClung, J. R., Stephens, A. D., Garner, C., Shelden, K. E. W., et al. (2020). Distribution and habitat use by endangered Cook Inlet beluga whales: Patterns observed during a photo-identification study, 2005–2017. Aquat. Conserv. 30, 2402–2427. doi: 10.1002/aqc.3378
Meurant, M., Gonzalez, A., Doxa, A., and Albert, C. H. (2018). Selecting surrogate species for connectivity conservation. Biol. Conserv. 227, 326–334. doi: 10.1016/j.biocon.2018.09.028
Migura, M., and Bollini, C. (2021). To take or not take? Examination of the status quo process for issuing take authorizations of endangered Cook Inlet beluga whales and implications for their recovery. Cons. Sci. Pract. 2021:e590. doi: 10.1111/csp2.590
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097
Mongillo, T. M., Holmes, E. E., Noren, D. P., VanBlaricom, G. R., Punt, A. E., O’Neill, S. M., et al. (2012). Predicted polybrominated diphenyl ether (PBDE) and polychlorinated biphenyl (PCB) accumulation in southern resident killer whales. Mar. Ecol. Prog. Ser. 453, 263–277. doi: 10.3354/meps09658
Moore, S. E., and DeMaster, D. P. (2000). Cook Inlet belugas, Delphinapterus leucas: Status and overview. Mar. Fish. Rev. 62, 1–5.
MPI (2019). Protecting Hector’s and Mâui Dolphins - Consultation on proposals for an updated Threat Management Plan. Using aerial photogrammetry to detect changes in body condition of endangered southern resident. New Zealand: Ministry for Primary Industries.
Murray, C. C., Hannah, L. C., Doniol-Valcroze, T., Wright, B. M., Stredulinsky, E. H., Nelson, J. C., et al. (2021). A cumulative effects model for population trajectories of resident killer whales in the Northeast Pacific. Biol. Conserv. 257:109124. doi: 10.1016/j.biocon.2021.109124
NMFS (1992). Status report on Cook Inlet belugas (Delphinapterus leucas). Anchorage, AK: National Marine Fisheries Service.
NMFS (2008). Recovery Plan for Southern Resident Killer Whales (Orcinus orca). Seattle, WA: National Marine Fisheries Service.
NMFS (2016). Recovery Plan for the Cook Inlet Beluga Whale (Delphinapterus leucas). Juneau, AK: National Marine Fisheries Service.
Norman, S. A., Hobbs, R. C., Beckett, L., Trumble, S., and Smith, W. (2020). Relationship between per capita births of Cook Inlet belugas and summer salmon runs: age-structured population modeling. Ecosphere 11:e02955. doi: 10.1002/ecs2.2955
Norman, S. A., Hobbs, R. C., Goertz, C. E. C., Burek-Huntington, K. A., Shelden, K. E. W., et al. (2015). Potential natural and anthropogenic impediments to the conservation and recovery of Cook Inlet Beluga Whales, Delphinapterus leucas. Mar. Fish. Rev. 77, 89–105. doi: 10.7755/MFR.77.2.5
O’Corry-Crowe, G., Suydam, R., Quakenbush, L., Potgieter, B., Harwood, L., Litovka, D., et al. (2018). Migratory culture, population structure and stock identity in North Pacific beluga whales (Delphinapterus leucas). PLoS One 13:e0194201. doi: 10.1371/journal.pone.0194201
O’Corry-Crowe, G., Suydam, R., Quakenbush, L., Smith, T. G., Lydersen, C., Kovacs, K. M., et al. (2020). Group structure and kinship in beluga whale societies. Sci. Rep. 10:11462. doi: 10.1038/s41598-020-67314-w
Oke, K. B., Cunningham, C. J., Westley, P. A. H., Baskett, M. L., Carlson, S. M., Clark, J., et al. (2020). Recent declines in salmon body size impact ecosystems and fisheries. Nat. Comm. 11:4155. doi: 10.1038/s41467-020-17726-z
O’Neill, S. M., Ylitalo, G. M., and West, J. E. (2014). Energy content of Pacific salmon as prey ofnorthern and southern resident killer whales. Endang. Species R. 25, 265–281. doi: 10.3354/esr00631
Pearce, R. (2018). Bioaccumulation of PCBs in Southern Resident Killer Whales in the Salish Sea.[Master’s Thesis]. Vancouver, BC: Simon Fraser University.
Pine, M. K., Jeffs, A. G., Wang, D., and Radford, C. A. (2016). The potential for vessel noise to mask biologically important sounds within ecologically significant embayments. Ocean Coast. Manage. 127, 63–73. doi: 10.1016/j.ocecoaman.2016.04.007
Poirier, M. C., Lair, S., Michaud, R., Hernández-Ramon, E. E., Divi, K. V., Dwyer, J. E., et al. (2019). Intestinal polycyclic aromatic hydrocarbon-DNA adducts in a population of beluga whales with high levels of gastrointestinal cancers: PAH-DNA adducts in intestine of whales with cancer. Environ. Mol. Mutagen. 60, 29–41. doi: 10.1002/em.22251
Pullin, A. S., and Stewart, G. B. (2006). Guidelines for systematic review in conservation and environmental management. Conserv. Biol. 20, 1647–1656. doi: 10.1111/j.1523-1739.2006.00485.x
Rayment, W. (2008). Distribution and ranging of Hector’s dolphins: implications for protected area design. [Dissertation]. Dunedin, NZ: University of Otago.
Reed, D. H., O’Grady, J. J., Ballou, J. D., and Frankham, R. (2003). The frequency and severity of catastrophic die-offs in vertebrates. Anim. Conserv. 6, 109–114. doi: 10.1017/S1367943003003147
Reeves, R. R., and Mitchell, E. (1987). Catch history, former abundance and distribution of white whales in Hudson Strait and Ungava Bay. Can. Field-Nat. 114, 1–65.
Reiner, J. L., O’Connell, S. G., Moors, A. J., Kucklick, J. R., Becker, P. R., and Keller, J. M. (2011). Spatial and temporal trends of perfluorinated compounds in beluga whales (Delphinapterus leucas) from Alaska. Environ. Sci. Technol. 45, 8129–8136. doi: 10.1021/es103560q
Richardson, S., Mill, A. C., Davis, D., Jam, D., and Ward, A. I. (2020). A systematic review of adaptive wildlife management for the control of invasive, non-native mammals, and other human–wildlife conflicts. Mamm. Rev. 50, 147–156. doi: 10.1111/mam.12182
Roe, W., Howe, L., Baker, E. J., Burrows, L., and Hunter, S. A. (2013). An atypical genotype of Toxoplasma gondii as a cause of mortality in Hector’s dolphins (Cephalorhynchus hectori). Vet. Parasitol. 192, 67–74. doi: 10.1016/j.vetpar.2012.11.001
Rugh, D. J., Shelden, K. E. W., and Hobbs, R. C. (2010). Range contraction in a beluga whale population. Endang. Species R. 12, 68–75. doi: 10.3354/esr00293
Sergeant, D. E., and Brodie, P. F. (1975). Identity, abundance, and present status of populations of white whales, Delphinapterus leucas, in North America. J. Fish. Res. Board Can. 32, 1047–1054. doi: 10.1139/f75-123
Sergeant, D. E., and Hoek, W. (1988). An update of the status of white whales Delphinapterus leucas in the Saint Lawrence estuary, Canada. Biol. Conserv. 45, 287–302. doi: 10.1016/0006-3207(88)90060-2
Shelden, K. E. W., and Wade, P. R. (eds) (2019). Aerial surveys, distribution, abundance, and trend of belugas (Delphinapterus leucas) in Cook Inlet, Alaska, June 2018. AFSC Processed Rep. 2019-09, 93 p. Alaska Fish. Sci. Cent., NOAA, Natl. Mar. Fish. Serv., 7600 Sand Point Way NE, Seattle WA 98115. Available online at https://apps-afsc.fisheries.noaa.gov/documents/PR2019-09.pdf. (accessed April 20, 2021).
Shelden, K. E. W., Migura, M. A., Hobbs, R. C., and Balough, G. (2017). Status Review: Beluga Whale Cook Inlet DPS (Delphinapterus leucas) 5-Year Review: Summary and Evaluation. Alaska Fish. Sci. Cent., NOAA, Natl. Mar. Fish. Serv., 7600 Sand Point Way NE, Seattle WA 98115. Available online at https://repository.library.noaa.gov/view/noaa/17047 (accessed April 11, 2021).
Shelden, K. E. W., Sims, C. L., Vate Brattström, L., Goetz, K. T., and Hobbs, R. C. (2015). Aerial Surveys of Beluga Whales (Delphinapterus leucas) in Cook Inlet, Alaska, June 2014. AFSC Processed Rep. 2015-03. Seattle, WA: National Marine Fisheries Service.
Siddaway, A. P., Wood, A. M., and Hedges, L. V. (2019). How to do a systematic review: a best practice guide for conducting and reporting narrative reviews, meta-analyses, and meta-syntheses. Annu. Rev. Psychol. 70, 747–770. doi: 10.1146/annurev-psych-010418-102803
Silber, G. K., Lettrich, M. D., Thomas, P. O., Baker, J. D., Baumgartner, M., Baker, E. M., et al. (2017). Projecting marine mammal distribution in a changing climate. Fron. Mar. Sci. 4:413. doi: 10.3389/fmars.2017.00413
Simond, A. E., Houde, M., Lesage, V., Michaud, R., and Verreault, J. (2020). Metabolomic profiles of the endangered St. Lawrence Estuary beluga population and associations with organohalogen contaminants. Sci. Total Environ. 717:137204. doi: 10.1016/j.scitotenv.2020.137204
Simond, A. E., Houde, M., Lesage, V., Michaud, R., Zbinden, D., and Verreault, J. (2019). Associations between organohalogen exposure and thyroid- and steroid-related gene responses in St. Lawrence Estuary belugas and minke whales. Mar. Pollut. Bull. 145, 174–184. doi: 10.1016/j.marpolbul.2019.05.029
Slooten, E. (2013). Effectiveness of area-based management in reducing bycatch of the New Zealand dolphin. Endang. Species R. 20, 121–130. doi: 10.3354/esr00483
Small, R. J., Brost, B., Hooten, M., Castellote, M., and Mondragon, J. (2017). Potential for spatial displacement of Cook Inlet beluga whales by anthropogenic noise in critical habitat. Endang. Species R. 32, 43–57. doi: 10.3354/esr00786
Strange, E. L. (2016). Assessing Southern Resident killer whale (Orcinus orca) prey abundance: the effects of Chinook salmon (Oncorhynchus tshawystcha) ocean fishery reductions and related hatchery production.[Master’s Thesis]. Sacramento, CA: California State University - Sacramento.
Tennessen, J. B., Holt, M. M., Hanson, M. B., Emmons, C. K., Giles, D. A., and Hogan, J. T. (2019). Kinematic signatures of prey capture from archival tags reveal sex differences in killer whale foraging activity. J. Exp. Biol. 222:jeb191874. doi: 10.1242/jeb.191874
Tollit, D., Harwood, J., Booth, C. G., Thomas, L., New, L., and Wood, J. D. (2016). Cook INLET BELUga Whale PCoD Expert Elicitation Workshop Report, September 29, 2016. Prepared by SMRU Consulting North America, SMRUC-NA-NOAA0915. Available online at: https://smruconsulting.com/wp-content/uploads/2016/10/Cook-Inlet-Beluga-PCoD-Expert-Elicitation-Workshop-Report_2016-SMRU-Consulting-29Sept2016-final-pdf.pdf (accessed 30 November 2021).
Tyre, A. J., and Michaels, S. (2011). Confronting socially generated uncertainty in adaptive management. J. Environ. Manage. 92, 1365–1370. doi: 10.1016/j.jenvman.2010.10.014
U.S. District Court (2021). U.S. District Court for the District of Alaska. Cook Inletkeeper and the Center for Biological Diversity V. Gina Raimondo, Secretary of Commerce et al., and Hilcorp Alaska, LLC. Case No. 3:19-cv-00238-SLG, Document 85. 18., Available online at https://www.govinfo.gov/content/pkg/USCOURTS-akd-3_19-cv-00238/pdf/USCOURTS-akd-3_19-cv-00238-1.pdf (accessed June 16, 2021).
Vos, D. J., and Shelden, K. E. W. (2005). Unusual mortality in the depleted Cook Inlet beluga (Delphinapterus leucas) population. Northwest. Nat. 86, 59–65.
Wade, P. R., Reeves, R. R., and Mesnick, S. L. (2012). J. Mar. Sci. 2012:567276. doi: 10.1155/2012/567276
Ward, E. J., Dahlheim, M. E., Waite, J. M., Emmons, C. K., Marshall, K. N., Chasco, B. E., et al. (2016). Long-distance migration of prey synchronizes demographic rates of top predators across broad spatial scales. Ecosphere 7:e01276. doi: 10.1002/ecs2.1276
Ward, E. J., Ford, M. J., Kope, R. G., Ford, J. K. B., Velez-Espino, L. A., Parken, C. K., et al. (2013). Estimating the impacts of Chinook salmon abundance and prey removal by ocean fishing on Southern Resident Killer Whale population dynamics. U.S. Dept. Commer., NOAA Tech. Memo. NMFS-NWFSC-123. Available online at https://repository.library.noaa.gov/view/noaa/4527 (accessed August 29, 2021).
Ward, E. J., Holmes, E. E., and Balcomb, K. C. (2009). Quantifying the effects of prey abundance on killer whale reproduction. J. Appl. Ecol. 46, 632–640. doi: 10.1111/j.1365-2664.2009.01647.x
Ward, E. J., Semmens, B. X., Holmes, E. E., and Balcomb, K. C. III (2011). Effects of multiple levels of social organization on survival and abundance. Conserv. Biol. 25, 350–355.
Wasser, S. K., Lundin, J. I., Ayres, K., Seely, E., Giles, D. A., Balcomb, K., et al. (2017). Population growth is limited by nutritional impacts on pregnancy success in endangered Southern Resident killer whales (Orcinus orca). PLoS One 12:e0179824. doi: 10.1371/journal.pone.0179824
Williams, R., Erbe, C., Ashe, E., Beerman, A., and Smith, J. (2014). Severity of killer whale behavioral responses to ship noise: a dose–response study. Mar. Pollut. Bull. 79, 254–260. doi: 10.1016/j.marpolbul.2013.12.004
Williams, R., and Lusseau, D. (2006). A killer whale social network is vulnerable to targeted Removals. Biol. Lett. 2, 497–500. doi: 10.1098/rsbl.2006.0510
Williams, R., Veirs, S., Veirs, V., Ashe, E., and Mastick, N. (2019). Approaches to reduce noise from ships operating in important killer whale habitats. Mar. Pollut. Bull. 139, 459–469. doi: 10.1016/j.marpolbul.2018.05.015
Keywords: Cook Inlet beluga, endangered species, recovery, St. Lawrence beluga, Hector’s dolphin, southern resident killer whale, systematic literature review, threat
Citation: Norman SA, Dreiss LM, Niederman TE and Nalven KB (2022) A Systematic Review Demonstrates How Surrogate Populations Help Inform Conservation and Management of an Endangered Species—The Case of Cook Inlet, Alaska Belugas. Front. Mar. Sci. 9:804218. doi: 10.3389/fmars.2022.804218
Received: 29 October 2021; Accepted: 07 February 2022;
Published: 03 March 2022.
Edited by:
Vitor H. Paiva, University of Coimbra, PortugalReviewed by:
Elisabetta Manea, Institute of Marine Science (CNR), ItalyCopyright © 2022 Norman, Dreiss, Niederman and Nalven. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephanie A. Norman, c3RlcGhhbmllQG1hcmluZS1tZWQuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.