
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 17 February 2022
Sec. Coastal Ocean Processes
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.784238
This article is part of the Research Topic Eutrophication, Algal Bloom, Hypoxia and Ocean Acidification in Large River Estuaries View all 11 articles
Benthic diatoms are the main primary producers and are sensitive to environmental changes in the estuarine ecosystem. Therefore, it is critical to evaluate the impact of environmental stress on the benthic diatom community in the estuarine ecosystem. In this study, the sediment samples from the five sampling sites were collected from the Yellow River Delta in the four seasons, and the abundance of benthic diatoms were determined using the high-throughput sequencing of 18S rRNA genes. The results showed that the motile guild taxa, such as Navicula, Nitzschia, and Amphora, was dominated in the benthic diatom the community throughout the sampling period. The structure of the benthic diatom community was significantly different among seasons (ANOSIM P < 0.01), especially between summer and winter. Redundancy analysis showed that water temperature and the concentrations of silicate, nitrate, ammonium, and pH value are the main driving factors shaping the seasonal assembly of the benthic diatom community. The results will improve knowledge about the benthic diatom community in the estuarine ecosystem and provide a theoretical foundation for estuary environmental management.
The estuary is the key transition zone between rivers and seas which is subject to strong continental-oceanic interactions (Sun et al., 2020; Deng et al., 2021). Estuarine zones are a complex and important ecosystem with a high biodiversity value, providing diversified ecological services, such as ecological habitat for many organisms, and maintaining ecological security in the coastal areas (Barbier et al., 2011; Frankenbach et al., 2020; Chi et al., 2021). Meanwhile, the estuary is a sensitive and vulnerable ecosystem and is affected easily by anthropogenic activity (e.g., ocean acidification, climate change, eutrophication) and natural stresses (e.g., temperature, salinity, nutrient loadings) which have been reported to influence the community structure and diversity of the coastal ecosystem (Hollister et al., 2010; Osland et al., 2014, 2016; Gabler et al., 2017; Kafouris et al., 2019). Accordingly, the analysis of the community structure and environmental drivers could not only improve the understanding of the estuary ecosystem but also provide for theoretical support about the ecological protection and restoration of the coastal ecosystem.
The diatom is photosynthetic, unicellular, eukaryotic microalgae that distributes in almost all aquatic environments (Maher et al., 2018; Merz et al., 2021). The benthic diatom is an important component of the estuarine ecosystem which plays indispensable roles in driving the biological pump, shaping the carbon cycle of the coastal environments, and providing energy to herbivores (Amin et al., 2012; Marques da Silva et al., 2017; Wang et al., 2019; Virta et al., 2020). The diatom responds quickly to environmental changes as a result of its short life cycle and has been widely regarded as the good bio-indicator tool (Potapova and Charles, 2007; Clark et al., 2020).
The Yellow River Delta is the youngest estuarine wetland in the warm temperate zone of China (Yang et al., 2016; Ma et al., 2019). Sediments have been depositing and oscillating because of the combined action of marine dynamics and the Yellow River runoff in the Yellow River Delta. Further, the intensive anthropogenic activities (e.g., aquaculture development, oil field mining) in this region have brought damage to the local organisms and destroyed their habitat (Xu et al., 2017; Zhao et al., 2017). Previous studies focused on the response of bacteria (He et al., 2020; Zou et al., 2020; Chi et al., 2021; Lee et al., 2021), archaea (Li et al., 2018) communities to environmental factors in the Yellow River Delta. In spite of the importance of benthic diatoms in the functioning of coastal ecosystems, little is known on environmental factors driving their structure and composition in the Yellow River Delta. Therefore, in this study, the concentrations of 11 environmental variables, and the seasonal benthic diatom abundance data were obtained using the high-throughput sequencing of 18S rRNA genes from five sampling sites in the Yellow River Delta. This study aimed to (1) investigate the structure of the seasonal benthic diatom community in the Yellow River Delta and (2) identify the main environmental variables driving the structure of the seasonal benthic diatom community. Our results provide insights for understanding the seasonal succession of benthic diatom in the Yellow River Delta.
The Yellow River Delta (N37°40′ –38°10′, E118°41′ –119°16′) is located in Dongying City, Shandong Province, China. The sample collections were taken from five sites along a 2.5 km transect in the Yellow River Delta (Figure 1). The sample collections were taken on 25 August 2019 (summer), 19 November 2019 (fall), 11 January 2020 (winter), and 10 April 2021 (spring, site B was not collected). At each site, surface sediment samples (upper 2 mm) were collected using the disposable sterilization plastic syringes with a diameter of 3.5 cm and were placed in sterile polyethylene bags. The collected samples were then put in a portable cooler, and immediately transported to the laboratory. Sediment samples were stored at –80°C until analysis. Pore water was filtered in a 0.22 μm filter membrane and then kept at –20°C until analysis.

Figure 1. The geographical location of the sampling sites. A total of five sites were set up around the Yellow River Delta. The Yellow River flows in Shandong Province, China, and the red circle represents the study area (version ArcGIS 10.2).
A total of 11 environmental variables were obtained for each sampling site. Water temperature was acquired from the Dongying municipal bureau of marine development and fisheries. Salinity, and pH were in situ measured. The concentrations of nitrate, nitrite, ammonium, orthophosphate, silicate were measured with the standard colorimetric methods using a continuous flow analyzer (Auto-Analyzer 3, Seal Analytical Ltd., United Kingdom). The water content of sediment was measured as a percentage of weight loss by drying the sediment at 60°C for 48 h. The sediment grain size was performed using a Laser Diffraction Particle Size Analyzer (Cilas 940L). Chlorophyll a (Chl-a) was extracted from freeze-dried sediment samples in 15 ml of 90% acetone for 24 h. Acetone extracts were quantified using a Trilogy Fluorometer (Turner Designs, United States) to give Chl-a concentration in microgram per gram.
DNA from sediment samples was extracted using the E.Z.N.A.® Soil DNA Kit (D4015, Omega, Inc., United States) according to the manufacturer’s instructions. V8-V9 variable region of the 18S rRNA genes was amplified using the primer sets of V8f (5′-ATAACAGGTCTGTGATGCCCT-3′) (Bradley et al., 2016) and 1510r (5′-CCTTCYGCAGGTTCACCTAC-3′) (Amaral-Zettler et al., 2009). Nuclear-free water was used for blank. PCR amplification was performed in a total volume of 25 μL reaction mixture containing 25 ng of template DNA, 12.5 μL PCR Premix, 2.5 μL of each primer, and PCR-grade water to adjust the volume. PCR amplification was performed as follows: initial denaturation at 98°C for 30 s; 32 cycles of denaturation at 98°C for 10 s, annealing at 54°Cfor 30 s, and extension at 72°C for 45 s, and then final extension at 72°C for 10 min. The PCR products were purified by AMPure XT beads (Beckman Coulter Genomics, Danvers, MA, United States) and quantified by Qubit (Invitrogen, United States), and then sequencing was conducted using on an Illumina NovaSeq 6000 platform at LC-Bio Technologies (Hangzhou, P. R. China) Co., Ltd.
Paired-end reads were assigned to samples based on their unique barcode and truncated by cutting off the barcode and primer sequence. Paired-end reads were merged using the FLASH program. Chimeric sequences were filtered using Vsearch software (v2.3.4) (Rognes et al., 2016). After dereplication using DADA2 (Divisive Amplicon Denoising Algorithm) (Callahan et al., 2016), the sequences of 18S rRNA genes were assigned to the same amplicon sequence variants (ASVs). To identify taxonomically ASVs, the representative ASV was annotated by database SILVA (release 132). All sequence analyses were conducted in QIIME2 (Bolyen et al., 2019) and its plugins (Caporaso et al., 2010).
All statistical analyses and graphics were conducted with R 3.4.2. Alpha diversity index (Chao1 richness, Shannon diversity index) was calculated with the vegan package. Seasonal difference in Alpha diversity index was compared by the stats package using Kruskal-Wallis test. Principal co-ordinates analysis (PCoA) was performed to visualize the dissimilarity of the benthic diatom community based on the Bray-Curtis distance matrix among seasons. Analysis of similarity (ANOSIM) was conducted to test whether the benthic diatom community dissimilarities are significant among different seasons and sampling sites, with the Bray-Curtis distance. Linear discriminant analysis (LDA) effect size (LEfSe) (Segata et al., 2011) was conducted to identify high-dimensional biomarkers and explain benthic diatom taxa differences in the four seasons. The LEfSe biomarker detection was performed using the logarithmic LDA threshold >4 and the statistical parameters of P < 0.05. Spearman correlation coefficients method were used to identify the correlation between the seasonal difference of the benthic diatom genera (obtained by the LEfSe) as well as Alpha diversity index with the environmental factors. Redundancy discriminant analysis (RDA) was performed to determine the environmental variables associated with changes in the benthic diatom community structure using the vegan package.
The seasonal variations of environmental factors are shown in Table 1. The pore water salinity ranged from 16.4 to 22.8 in the four seasons and had the highest value in spring. The sediment was alkaline throughout the sampling period, and pH value was the highest in spring. The nitrate and nitrite concentrations were the highest in spring and the lowest in winter. Sediment grain size remained similar among seasons. Silicate and Chl-a concentrations were the highest in summer.
A total of 3,078,045 V8-V9 18S rRNA reads were obtained in our study. All sequencing reads were classified into 1,040 ASVs. Rarefaction curves ranged from 0.9854 to 0.9987 can represent the benthic diatom community reasonably. Across all samples, the classification of the benthic diatom ASVs results showed 4 classes, 18 orders, 31 families, 143 diatoms genera.
The richness and diversity of the benthic diatom community were compared among seasons (Figure 2). The Chao1 richness was higher significantly in spring (735 ± 130) and winter (536 ± 52) than in summer (239 ± 112), and fall (166 ± 27), indicating that fewer diatom species were observed in summer and fall than in spring and winter. Similarly, the Shannon diversity index was higher significantly in spring (4.86 ± 0.34) and winter (4.83 ± 0.25) than in summer (4.14 ± 0.47), and fall (3.80 ± 0.21), suggesting that a lower diatom diversity was observed in summer and fall.
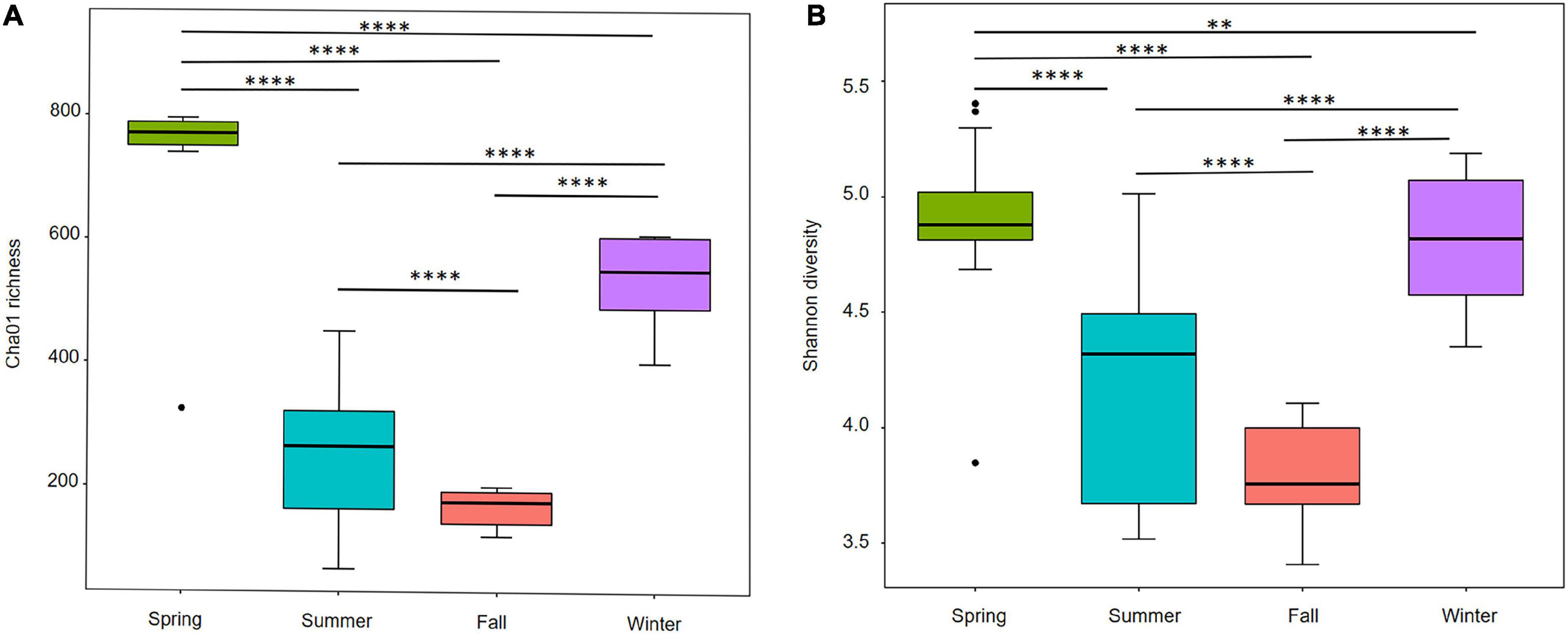
Figure 2. The Alpha diversity index of the Yellow River Delta benthic diatoms communities at different sampling seasons. (A) Chao1 richness. (B) Shannon diversity. ** indicates that p < 0.01, **** indicates that p < 0.0001.
PCoA analysis was employed to study the dissimilarities of the benthic diatom community in the Yellow River Delta among seasons (Figure 3). Samples from summer and winter separated from each other, which indicated that the structure of the benthic diatom community had changed greatly among seasons. Then, the ANOSIM results demonstrated that the structure of the benthic diatom community was significantly different among seasons (R = 0.54, P = 0.001) (Supplementary Figure 1A). However, there are no significant spatial variations in the benthic diatom community structure among different sites (R = –0.21, P = 0.999) (Supplementary Figure 1B).
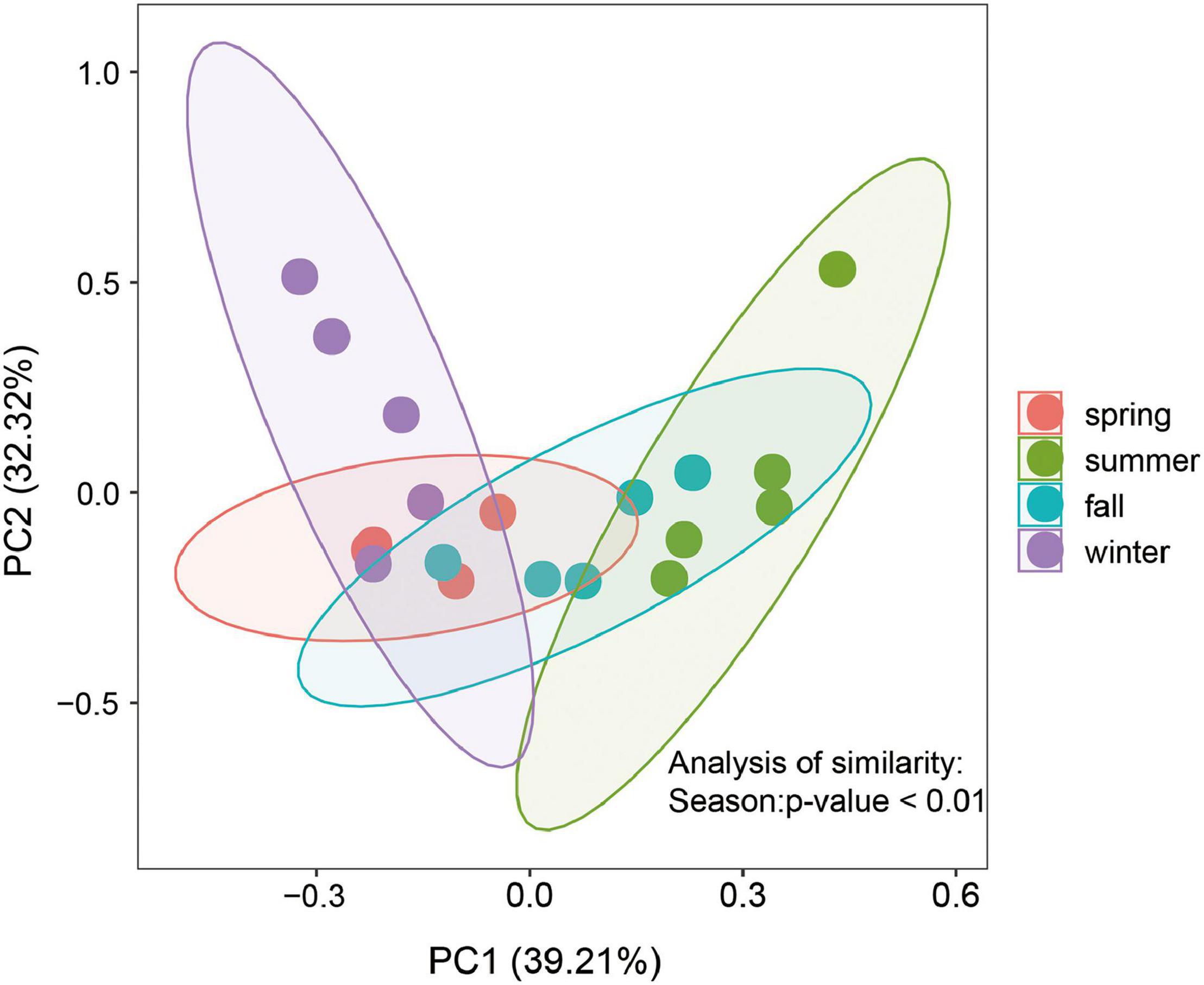
Figure 3. Principal co-ordinates analysis diagram of the Bray-Curtis distance between benthic the diatom community for all samples.
The composition of the benthic diatom community at the top 10 genera level in different sampling sites is shown in Figure 4. The top 10 genera accounted for 75.35∼84.90% throughout the sampling period. Benthic diatoms were classified into guilds (Passy, 2007; Rimet and Bouchez, 2012), and motile guild taxa (e.g., Navicula, Nitzschia, and Amphora) dominated all communities among seasons. Then the linear discriminant analysis effect size (LEfSe) was conducted to identify the significant difference of the benthic diatom taxa among seasons (Figure 5 and Supplementary Table 1). For example, the Navicula and Seminavis abundances are higher significantly in spring than in other seasons; six genera, namely, the Cylindrotheca, Nitzschia, Pseudogomphonema, Gyrosigm, Pleurosiga, and Cyclotella abundances were higher in summer; the Entomoneis, Halamphora, Craticula, and Surirella abundances are higher significantly in winter.
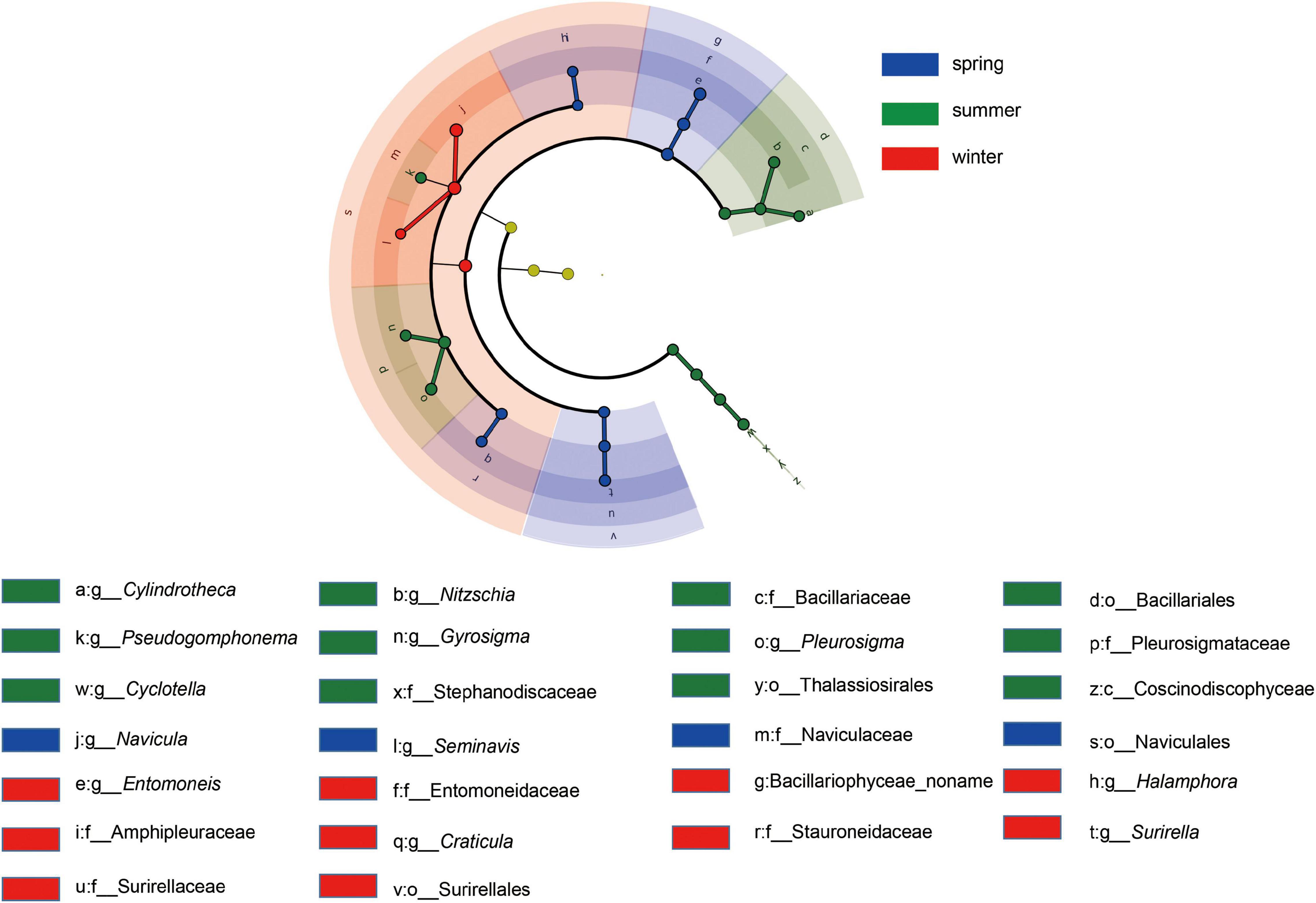
Figure 5. LEfSe cladogram of benthic diatom community for different seasons. Benthic diatom taxa in all samples, assigned to the kingdom (innermost), phylum, class, order, family, and genera (outermost), are used to determine taxa most likely to explain differences for different seasons. Differentially abundant taxa are colored according to the slope regions in which they are most abundant; i.e., blue, green and red circles stand for biomarkers in spring, summer, and winter.
The relationship between the benthic diatom diversity and environmental factors was evaluated in the Yellow River Delta among seasons (Figure 6). The Shannon diversity and Chao1 richness of the benthic diatom community were negatively correlated with the water temperature, the orthophosphate, silicate concentrations. The water temperature and silicate concentration were the main environmental factors affecting the dominant benthic diatom taxa (Figure 6). Water temperature and silicate concentration were positively correlated with Cyclotella, Cylindrotheca, Gyrosigma, Pleurosigma abundances, while were negatively correlated with Entomoneis, Halamphora, Surirella abundances. The Navicula and Pleurosigma were most closely correlated with the environmental factors. The Navicula abundance was positively correlated with salinity, nitrate concentration, pH value, but was negatively correlated with the water content of sediments and ammonium concentration. The Pleurosigma abundance was positively correlated with the orthophosphate, silicate concentrations, water temperature, and the water content of sediments. Additionally, the Pleurosigma abundance had a stronger positive correlation with silicate concentration (Figure 7).
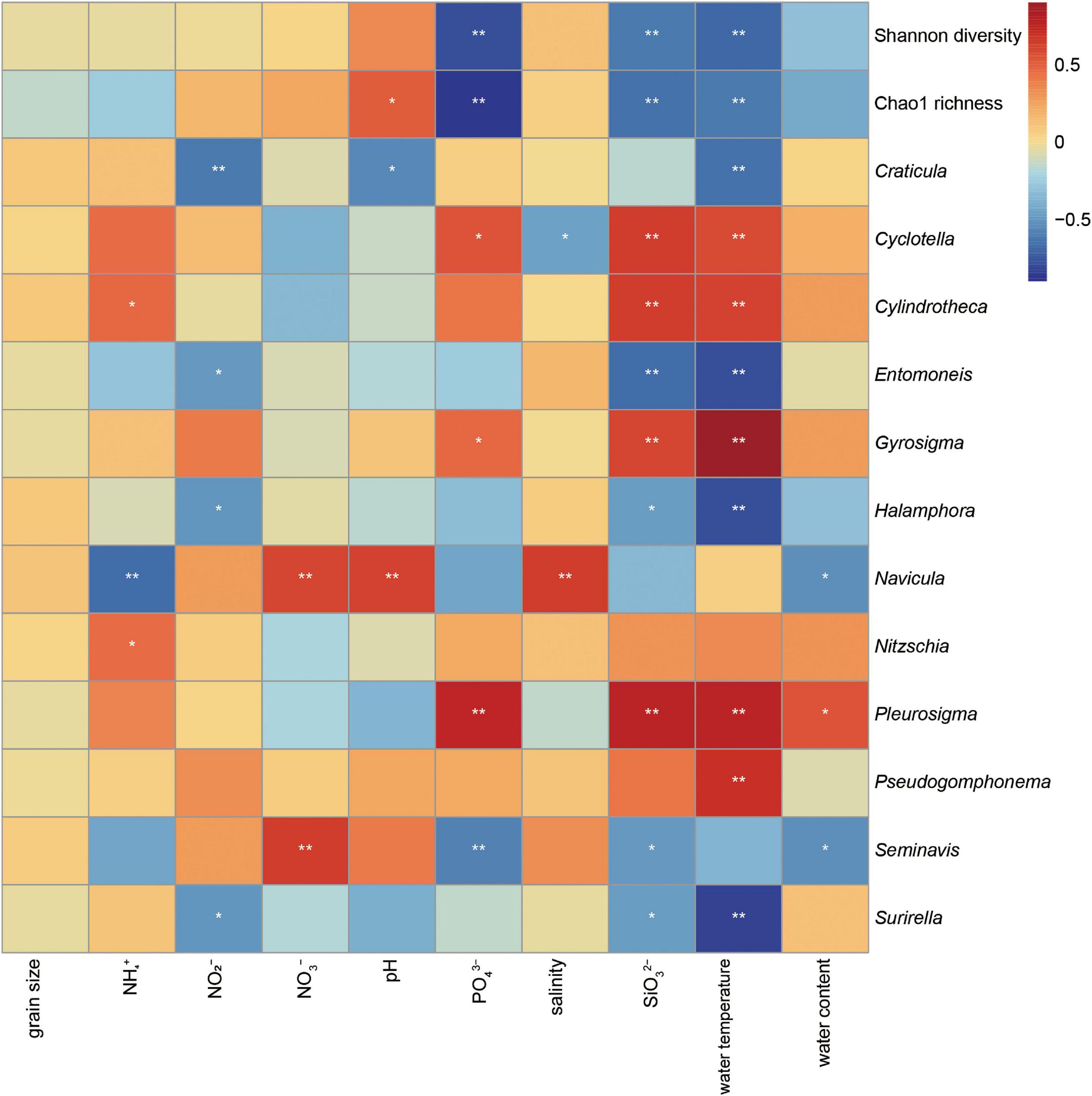
Figure 6. Heatmap of the correlation between the dominant benthic diatom taxa as well as diversity and environmental factors in the Yellow River Delta. Spearman correlation coefficient was displayed by the color of each cell in the heatmap. A significant correlation was confirmed if the p-value was less than 0.05 (*) or 0.01 (**).
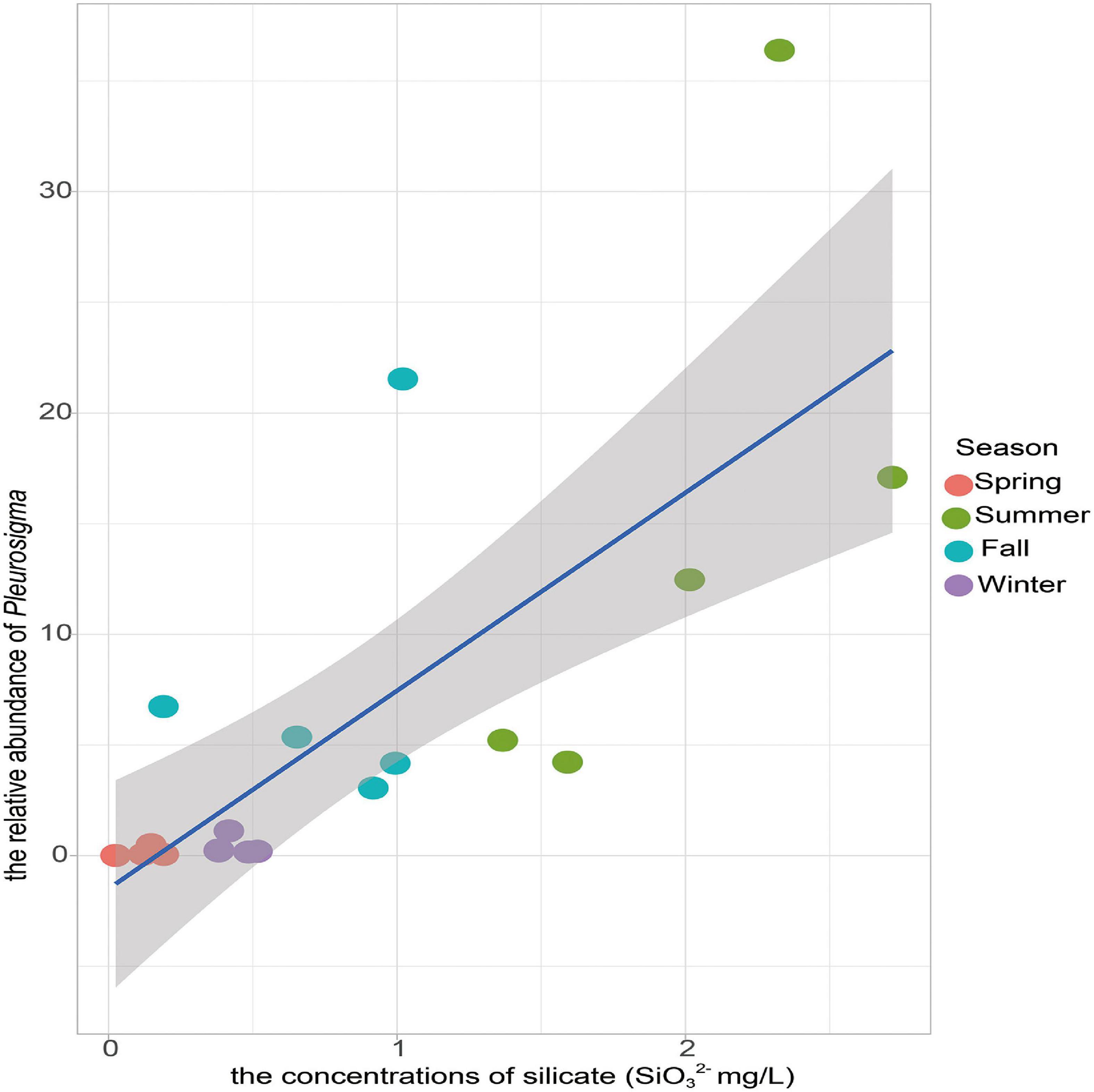
Figure 7. The relationship between the relative abundance of the Pleurosigma and silicate concentration. Values of Pearson correlation coefficient (r) and probability (P) are provided. Blue lines indicate ordinary least squares linear regression across all samples.
Redundancy analysis was used to analyze the change of the benthic diatom community structure associated with the environmental variables (Figure 8), which the first two axes explained up to 36.22% of RDA 1 and 19.70% of RDA 2 of the total variation in the benthic diatom community, indicating that environmental factors drove the difference of the benthic diatom community. Water temperature (R2 = 0.78, p = 0.001) apparently had the most significant effect on the benthic diatom community, and the most variances can be explained by the silicate concentration (R2 = 0.61, p = 0.001), pH value (R2 = 0.50, p = 0.005), the nitrate concentration (R2 = 0.44, p = 0.009), and the ammonium concentration (R2 = 0.43, p = 0.012).
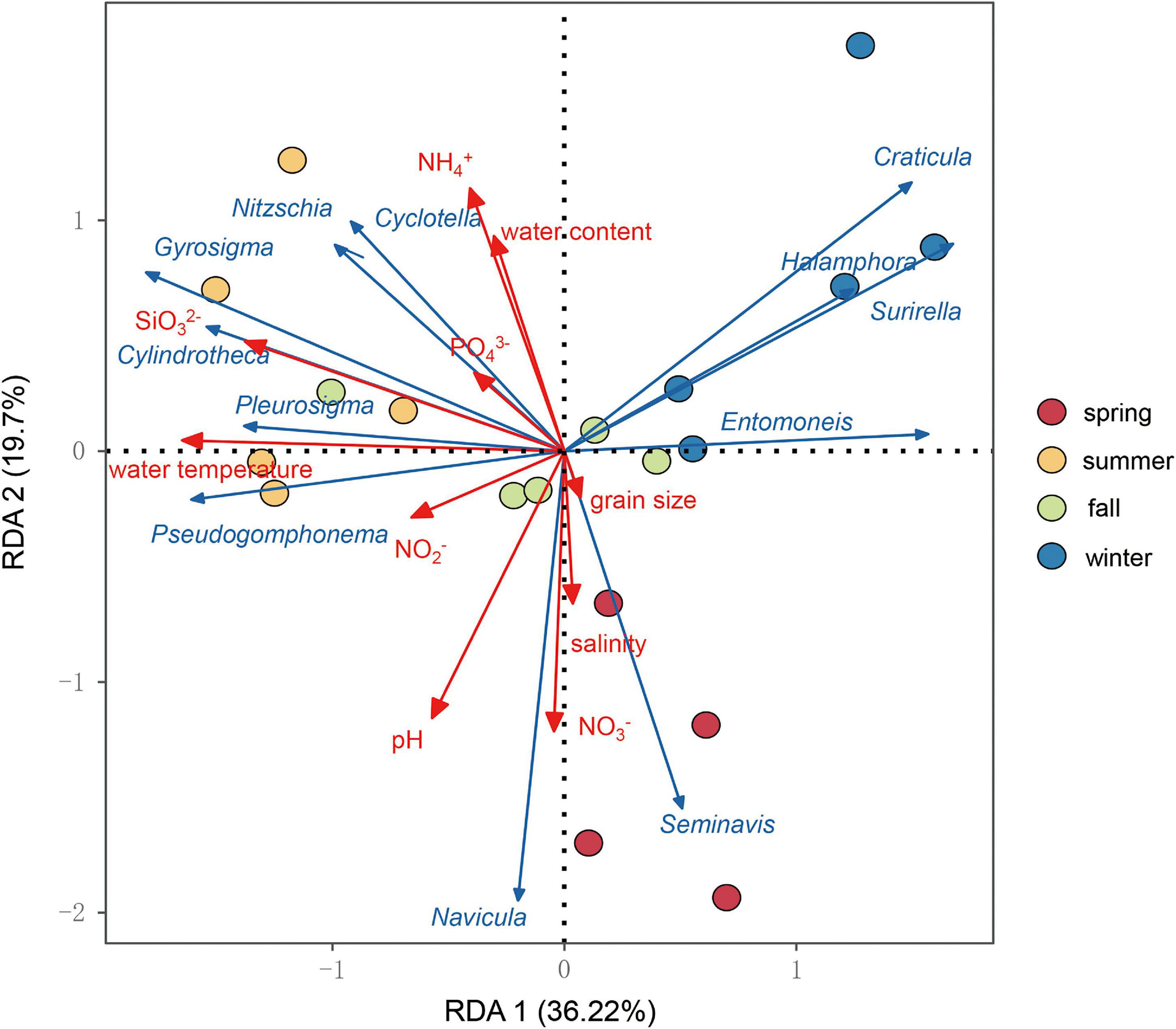
Figure 8. Results of redundancy analysis graph for the environmental variables associated with changes in the benthic diatom community structure in the Yellow River Delta.
Our observations obtained by a high-coverage taxonomic survey have provided the first description of the seasonal assembly of the benthic diatom community in the Yellow River Delta, then the response of the benthic diatom community to environmental factors was analyzed. Our results provide insights for further understanding the seasonal succession of biota in the Yellow River Delta.
The local benthic diatom taxa are the result of natural adaptation and are associated closely with the change of the local environment. First, the motile-guild taxa (e.g., Navicula, Nitzschia, and Amphora) were dominant in the Yellow River Delta (Figure 4), and the result is similar to other studies of the benthic diatom compositions in the estuary (Admiraal et al., 1984; Oh and Koh, 1995; Cibic et al., 2012; Yamamoto et al., 2017; An et al., 2020). Some researchers reported that the motile guild taxa can respond quickly to environmental change and choose suitable habitat (Berthon et al., 2011). Different from the Oregon and Washington estuaries, United States and the Westerschelde estuary, Netherlands, the Pleurosigma, Entomoneis are dominant genera in the Yellow River Delta (Forster et al., 2006; Sawai et al., 2016).
Second, the structure of the seasonal benthic diatom community was significantly different, especially in summer and winter (Figure 3). Previous studies showed that the nutrient supply carried by rivers was important for affecting diatom growth and distribution (Passy and Larson, 2011). Redundancy analysis revealed that water temperature and silicate concentration were the main factors that affected the structure of the benthic diatom community (Figure 8), and the main source of silicon in the delta was the weathering and transport of the Yellow River (Gong et al., 2015). Therefore, the seasonal fluctuations in nutrients input to the Yellow River Delta maybe play an important role in influencing the seasonal patterns of the benthic diatom assemblage.
Finally, the heterogeneity of the local environment, which was caused by the freshwater and seawater erosion in the different seasons, makes common species different remarkably in the local sites. The Cyclotella abundance was significantly higher in summer than in other seasons, but the abundance of Cyclotella at site E was 22.75% and at site A 0.39% in summer (Figure 4). The variations of the distribution of the most common taxa have been reported previously (Zong and Horton, 1999; Roe et al., 2009; Watcham et al., 2013). It was observed that the seasonal environment heterogeneity had a stronger effect on the benthic diatom assemblages in the Yellow River Delta, especially in summer.
The salinity is one of the most likely drivers of the benthic diatom community (Oppenheim, 1991; Underwood et al., 1998; Ulanova et al., 2009; Yamamoto et al., 2017), but it did not affect significantly the benthic diatom community in our study (Figure 8). For one thing, the salinity remained similar throughout the sampling seasons due to the mixing of seawater intrusion and the Yellow River fresh runoff in the Yellow River Delta. For another, we used the genera level (DNA barcoding can give more accurate information at the genera level) to analyze the relationship between the benthic diatom community and environment variables. The high taxa may cover up the sensitive species, in other words, the same diatom genera may have ecological niche differentiation for better environmental adaptation.
The molecular method used in this paper can establish a better correspondence with the morphological (unpublished) identification at the genus level. Nistal-García et al. (2021) found that molecular and morphological methods provided similar information when it comes to the underlying processes determining variation in diatom assemblages. This is also one of the reasons why the analysis in this paper was based on genus level. Further, molecular markers are good methods to identify several semi-cryptic or cryptic species which make it difficult to understand how diatom assemblages vary in response to environmental variables (Créach et al., 2006). DNA metabarcoding using high-throughput sequencing platforms, have been developed drastically in recent years, overcoming the biases related to morphological identification (Zimmermann et al., 2015; Apothéloz-Perret-Gentil et al., 2021). However, the completeness of the reference database is a key factor that strongly limits the taxonomy assignment of OTUs (Malviya et al., 2016; Weigand et al., 2019; Nistal-García et al., 2021). At present, various DNA barcoding studies have been successfully conducted, based on different maker genes, including rbcL (An et al., 2020), COI (Evans et al., 2007), ITS (Moniz and Kaczmarska, 2010), and 18S rDNA (Visco et al., 2015). Therefore, there is no unified standard primer and analysis process (such as sequencing depth, a threshold for assign taxonomy), so it is difficult to compare different research results. In the future, unified standard primer and analysis process will need. Meanwhile, we suggest that combining molecular and morphological approaches provide complementary information on each other, especially when completeness of the reference databases improves and bioinformatics biases are overcome.
In summary, this study used 18S rRNA gene-based high-throughput sequencing technology to analyze the structure of the benthic diatom community in the Yellow River Delta for the first time. The results show that the diversity of the benthic diatom community is higher significantly in spring and winter than in summer and fall. The dominant benthic diatom groups at the genera level are Navicula, Nitzschia, and Amphora. The structure of the benthic diatom community was significantly different among seasons. Redundancy analysis showed that water temperature and the concentrations of silicate, nitrate, ammonium, and pH value are the main factors driving the seasonal assembly of the benthic diatom community. Overall, this study reveals the composition of the benthic diatom community and its relationship with environmental factors, providing a certain theoretical sight for understanding microbial ecology and environmental protection in the Yellow River Delta.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, PRJNA764319.
KP, JH, and YnL contributed to the conception and design of the study. XL wrote the manuscript and was responsible for nutrient measurement. BZ, YhL, and KP gave suggestive comments on this draft, revised the manuscript. All authors read and approved the final manuscript to be published.
This study was supported by the National Key Research and Development Program of China (2018YFD0900703) and the Marine S&T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao) (2018SDKJ0406-3).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.784238/full#supplementary-material
Admiraal, W., Peletier, H., and Brouwer, T. (1984). The seasonal succession patterns of diatom species on an intertidal mudflat: an experimental analysis. Oikos 42, 30–40. doi: 10.2307/3544606
Amaral-Zettler, L. A., McCliment, E. A., Ducklow, H. W., and Huse, S. M. (2009). A method for studying protistan diversity using massively parallel sequencing of V9 hypervariable regions of small-subunit ribosomal RNA genes. PLoS One 4:e6372. doi: 10.1371/journal.pone.0006372
Amin, S. A., Parker, M. S., and Armbrust, E. V. (2012). Interactions between Diatoms and Bacteria. Microbiol. Mol. Biol. Rev. 76, 667–684. doi: 10.1128/MMBR.00007-12
An, S. M., Choi, D. H., and Noh, J. H. (2020). High-throughput sequencing analysis reveals dynamic seasonal succession of diatom assemblages in a temperate tidal flat. Estuar. Coast. Shelf Sci. 237:106686. doi: 10.1016/j.ecss.2020.106686
Apothéloz-Perret-Gentil, L., Bouchez, A., Cordier, T., Cordonier, A., Guéguen, J., Rjmet, F., et al. (2021). Monitoring the ecological status of rivers with diatom eDNA metabarcoding: a comparison of taxonomic markers and analytical approaches for the inference of a molecular diatom index. Mol. Ecol. 30, 2959–2968. doi: 10.1111/mec.15646
Barbier, E. B., Hacker, S. D., Kennedy, C., Koch, E. W., Stier, A. C., and Silliman, B. R. (2011). The value of estuarine and coastal ecosystem services. Ecol. Monogr. 81, 169–193. doi: 10.1890/10-1510.1
Berthon, V., Bouchez, A., and Rimet, F. (2011). Using diatom life-forms and ecological guilds to assess organic pollution and trophic level in rivers: a case study of rivers in south-eastern France. Hydrobiologia 673, 259–271. doi: 10.1007/s10750-011-0786-1
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al-Ghalith, G. A., et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. doi: 10.1038/s41587-019-0209-9
Bradley, I. M., Pinto, A. J., and Guest, J. S. (2016). Design and evaluation of Illumina MiSeq-compatible, 18S rRNA gene-specific primers for improved characterization of mixed phototrophic communities. Appl. Environ. Microb. 82, 5878–5891. doi: 10.1128/AEM.01630-16
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J. A., and Holmes, S. P. (2016). DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. doi: 10.1038/nmeth.3869
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Chi, Z., Zhu, Y., Li, H., Wu, H., and Yan, B. (2021). Unraveling bacterial community structure and function and their links with natural salinity gradient in the Yellow River Delta. Sci. Total Environ. 773:145673. doi: 10.1016/j.scitotenv.2021.145673
Cibic, T., Comici, C., Bussani, A., and Del Negro, P. (2012). Benthic diatom response to changing environmental conditions. Estuar. Coast. Shelf Sci. 115, 158–169. doi: 10.1016/j.ecss.2012.03.033
Clark, D. E., Pilditch, C. A., Pearman, J. K., Ellis, J. I., and Zaiko, A. (2020). Environmental DNA metabarcoding reveals estuarine benthic community response to nutrient enrichment – Evidence from an in-situ experiment. Environ. Pollut. 267:115472. doi: 10.1016/j.envpol.2020.115472
Créach, V., Ernst, A., Sabbe, K., Vanelslander, B., Vyverman, W., and Stal, L. J. (2006). Using quantitative PCR to determine the distribution of a semicryptic benthic diatom, Navicula phyllepta (Bacillariophyceae). J. Phycol. 42, 1142–1154. doi: 10.1111/j.1529-8817.2006.00268.x
Deng, H., Li, R., Yan, B., Li, B., Chen, Q., Hu, H., et al. (2021). PAEs and PBDEs in plastic fragments and wetland sediments in Yangtze estuary. J. Hazard. Mater. 409:124937. doi: 10.1016/j.jhazmat.2020.124937
Evans, K. M., Wortley, A. H., and Mann, D. G. (2007). An assessment of potential diatom “barcode” genes (cox1, rbcL, 18S and ITS rDNA) and their effectiveness in determining relationships in Sellaphora (Bacillariophyta). Protist 158, 349–364. doi: 10.1016/j.protis.2007.04.001
Forster, R. M., Creach, V., Sabbe, K., Vyverman, W., and Stal, L. J. (2006). Biodiversity-ecosystem function relationship in microphytobenthic diatoms of the Westerschelde estuary. Mar. Ecol. Prog. Ser. 311, 191–201. doi: 10.3354/meps311191
Frankenbach, S., Ezequiel, J., Plecha, S., Goessling, J. W., Vaz, L., Kühl, M., et al. (2020). Synoptic spatio-temporal variability of the photosynthetic productivity of microphytobenthos and phytoplankton in a tidal estuary. Front. Mar. Sci. 7:170. doi: 10.3389/fmars.2020.00170
Gabler, C. A., Osland, M. J., Grace, J. B., Stagg, C. L., Day, R. H., Hartley, S. B., et al. (2017). Macroclimatic change expected to transform coastal wetland ecosystems this century. Nat. Clim. Change 7, 142–147. doi: 10.1038/nclimate3203
Gong, Y., Yu, Z., Yao, Q., Chen, H., Mi, T., and Tan, J. (2015). Seasonal variation and sources of dissolved nutrients in the Yellow River, China. Int. J. Environ. Res. Public Health 12, 9603–9622. doi: 10.3390/ijerph120809603
He, H., Miao, Y., Gan, Y., Wei, S., Tan, S., Rask, K. A., et al. (2020). Soil bacterial community response to long-term land use conversion in Yellow River Delta. Appl. Soil Ecol. 156:103709. doi: 10.1016/j.apsoil.2020.103709
Hollister, E. B., Engledow, A. S., Hammett, A. J. M., Provin, T. L., Wilkinson, H. H., and Gentry, T. J. (2010). Shifts in microbial community structure along an ecological gradient of hypersaline soils and sediments. ISME J. 4, 829–838. doi: 10.1038/ismej.2010.3
Kafouris, S., Smeti, E., Spatharis, S., Tsirtsis, G., Economou-Amilli, A., and Danielidis, D. B. (2019). Nitrogen as the main driver of benthic diatom composition and diversity in oligotrophic coastal systems. Sci. Total Environ. 694:133773. doi: 10.1016/j.scitotenv.2019.133773
Lee, H., Heo, Y. M., Kwon, S. L., Yoo, Y., Kim, D., Lee, J., et al. (2021). Environmental drivers affecting the bacterial community of intertidal sediments in the Yellow Sea. Sci. Total Environ. 755:142726. doi: 10.1016/j.scitotenv.2020.142726
Li, M., Wei, G., Shi, W., Sun, Z., Li, H., Wang, X., et al. (2018). Distinct distribution patterns of ammonia-oxidizing archaea and bacteria in sediment and water column of the Yellow River estuary. Sci. Rep. 8:1584. doi: 10.1038/s41598-018-20044-6
Ma, T., Li, X., Bai, J., Ding, S., Zhou, F., and Cui, B. (2019). Four decades’ dynamics of coastal blue carbon storage driven by land use/land cover transformation under natural and anthropogenic processes in the Yellow River Delta, China. Sci. Total Environ. 655, 741–750. doi: 10.1016/j.scitotenv.2018.11.287
Maher, S., Kumeria, T., Aw, M. S., and Losic, D. (2018). Diatom silica for biomedical applications: recent progress and advances. Adv. Healthc. Mater. 7:1800552. doi: 10.1002/adhm.201800552
Malviya, S., Scalco, E., Audic, S., Vincent, F., and Veluchamy, A. (2016). Insights into global diatom distribution and diversity in the world’s ocean. Proc. Natl. Acad. Sci. U. S. A. 113, E1516–E1525. doi: 10.1073/pnas.1509523113
Marques da Silva, J., Cruz, S., and Cartaxana, P. (2017). Inorganic carbon availability in benthic diatom communities: photosynthesis and migration. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372:20160398. doi: 10.1098/rstb.2016.0398
Merz, E., Dick, G. J., Beer, D., Grim, S., Hübener, T., Littmann, S., et al. (2021). Nitrate respiration and diel migration patterns of diatoms are linked in sediments underneath a microbial mat. Environ. Microbiol. 23, 1422–1435. doi: 10.1111/1462-2920.15345
Moniz, M. B., and Kaczmarska, I. (2010). Barcoding of diatoms: nuclear encoded ITS revisited. Protist 161, 7–34. doi: 10.1016/j.protis.2009.07.001
Nistal-García, A., García-García, P., García-Girón, J., Borrego-Ramos, M., Blanco, S., and Bécares, E. (2021). DNA metabarcoding and morphological methods show complementary patterns in the metacommunity organization of lentic epiphytic diatoms. Sci. Total Environ. 786:147410. doi: 10.1016/j.scitotenv.2021.147410
Oh, S. H., and Koh, C. H. (1995). Distribution of diatoms in the surficial sediments of the Mangyung-Dongjin tidal flat, west coast of Korea (Eastern Yellow Sea). Mar. Biol. 122, 487–496. doi: 10.1007/BF00350883
Oppenheim, D. R. (1991). Seasonal changes in epipelic diatoms along an intertidal shore, Berrow flats, Somerset. J. Mar. Biol. Assoc. UK 71, 579–596. doi: 10.1017/S0025315400053169
Osland, M. J., Enwright, N., and Stagg, C. L. (2014). Freshwater availability and coastal wetland foundation species: ecological transitions along a rainfall gradient. Ecology 95, 2789–2802. doi: 10.1890/13-1269.1
Osland, M. J., Enwright, N. M., Day, R. H., Gabler, C. A., Stagg, C. L., and Grace, J. B. (2016). Beyond just sea-level rise: considering macroclimatic drivers within coastal wetland vulnerability assessments to climate change. Glob. Change Biol. 22, 1–11. doi: 10.1111/gcb.13084
Passy, S. I. (2007). Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters. Aquat. Bot. 86, 171–178. doi: 10.1016/j.aquabot.2006.09.018
Passy, S. I., and Larson, C. A. (2011). Succession in stream biofilms is an environmentally driven gradient of stress tolerance. Microb. Ecol. 62, 414–424. doi: 10.1007/s00248-011-9879-7
Potapova, M., and Charles, D. F. (2007). Diatom metrics for monitoring eutrophication in rivers of the United States. Ecol. Indic. 7, 48–70. doi: 10.1016/j.ecolind.2005.10.001
Rimet, F., and Bouchez, A. (2012). Life-forms, cell-sizes and ecological guilds of diatoms in European rivers. Knowl. Manage. Aquat. Ecosyst. 406, 1–14. doi: 10.1051/kmae/2012018
Roe, H. M., Doherty, C. T., Patterson, R. T., and Swindles, G. T. (2009). Contemporary distributions of saltmarsh diatoms in the Seymour–Belize Inlet Complex, British Columbia, Canada: implications for studies of sea-level change. Mar. Micropaleontol. 70, 134–150. doi: 10.1016/j.marmicro.2008.12.001
Rognes, T., Flouri, T., Nichols, B., Quince, C., and Mahé, F. (2016). VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584. doi: 10.7717/peerj.2584
Sawai, Y., Horton, B. P., Kemp, A. C., Hawkes, A. D., Nagumo, T., and Nelson, A. R. (2016). Relationships between diatoms and tidal environments in Oregon and Washington, USA. Diatom Res. 31, 17–38. doi: 10.1080/0269249X.2015.1126359
Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W. S., et al. (2011). Metagenomic biomarker discovery and explanation. Genome Biol. 12:R60. doi: 10.1186/gb-2011-12-6-r60
Sun, Y., Li, H., Yang, Q., Liu, Y., Fan, J., and Guo, H. (2020). Disentangling effects of river inflow and marine diffusion in shaping the planktonic communities in a heavily polluted estuary. Environ. Pollut. 267:115414. doi: 10.1016/j.envpol.2020.115414
Ulanova, A., Busse, S., and Snoeijs, P. (2009). Coastal diatom-environment relationships in the brackish Baltic sea. J. Phycol. 45, 54–68. doi: 10.1111/j.1529-8817.2008.00628.x
Underwood, G., Phillips, J., and Saunders, K. (1998). Distribution of estuarine benthic diatom species along salinity and nutrient gradients. Eur. J. Phycol. 33, 173–183. doi: 10.1080/09670269810001736673
Virta, L., Soininen, J., and Norkko, A. (2020). Stable seasonal and annual alpha diversity of benthic diatom communities despite changing community composition. Front. Mar. Sci. 7:88. doi: 10.3389/fmars.2020.00088
Visco, J. A., Apothéloz-Perret-Gentil, L., Cordonier, A., Esling, P., Pillet, L., and Pawlowski, J. (2015). Environmental monitoring: inferring the diatom index from next-generation sequencing data. Environ. Sci. Technol. 49, 7597–7605. doi: 10.1021/es506158m
Wang, J., Liu, Q., Zhao, X., Borthwick, A. G. L., Liu, Y., Chen, Q., et al. (2019). Molecular biogeography of planktonic and benthic diatoms in the Yangtze River. Microbiome 7:153. doi: 10.1186/s40168-019-0771-x
Watcham, E. P., Shennan, I., and Barlow, N. L. M. (2013). Scale considerations in using diatoms as indicators of sea-level change: lessons from Alaska. J. Quat. Sci. 28, 165–179. doi: 10.1002/jqs.2592
Weigand, H., Beermann, A. J., Èiampor, F., Costa, F. O., Csabai, Z., Duarte, S., et al. (2019). DNA barcode reference libraries for the monitoring of aquatic biota in Europe: gap-analysis and recommendations for future work. Sci. Total Environ. 678, 499–524. doi: 10.1016/j.scitotenv.2019.04.247
Xu, Y., Cai, Y., Sun, T., Yin, X., and Tan, Q. (2017). Development of an integrated indicator system to assess the impacts of reclamation engineering on a river estuary. Mar. Pollut. Bull. 119, 50–59. doi: 10.1016/j.marpolbul.2017.03.015
Yamamoto, M., Chiba, T., and Tuji, A. (2017). Salinity responses of benthic diatoms inhabiting tidal flats. Diatom Res. 32, 243–250. doi: 10.1080/0269249X.2017.1366951
Yang, W., Sun, T., and Yang, Z. (2016). Effect of activities associated with coastal reclamation on the macrobenthos community in coastal wetlands of the Yellow River Delta, China: a literature review and systematic assessment. Ocean Coast Manage 129, 1–9. doi: 10.1016/j.ocecoaman.2016.04.018
Zhao, Z., Wang, J., Han, Y., Chen, J., Liu, G., Lu, Y., et al. (2017). Nutrients, heavy metals and microbial communities co-driven distribution of antibiotic resistance genes in adjacent environment of mariculture. Environ. Pollut. 220, 909–918. doi: 10.1016/j.envpol.2016.10.075
Zimmermann, J., Glöckner, G., Jahn, R., Enke, N., and Gemeinholzer, B. (2015). Metabarcoding vs. morphological identification to assess diatom diversity in environmental studies. Mol. Ecol. Resour. 15, 526–542. doi: 10.1111/1755-0998.12336
Zong, Y., and Horton, B. P. (1999). Diatom-based tidal-level transfer functions as an aid in reconstructing Quaternary history of sea-level movements in the UK. J. Quat. Sci. 14, 153–167. doi: 10.1002/(SICI)1099-1417(199903)14:2<153::AID-JQS425<3.0.CO;2-6
Keywords: the Yellow River Delta, high-throughput sequencing, benthic diatom community structure, environment factors, redundancy analysis
Citation: Liu X, Han J, Li Y, Zhu B, Li Y and Pan K (2022) The Structure of the Seasonal Benthic Diatom Community and Its Relationship With Environmental Factors in the Yellow River Delta. Front. Mar. Sci. 9:784238. doi: 10.3389/fmars.2022.784238
Received: 27 September 2021; Accepted: 26 January 2022;
Published: 17 February 2022.
Edited by:
Daji Huang, Fourth Institute of Oceanography, Ministry of Natural Resources, ChinaReviewed by:
Zhaoqing Yang, Pacific Northwest National Laboratory (DOE), United StatesCopyright © 2022 Liu, Han, Li, Zhu, Li and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Li, c3hzZGx3bEBvdWMuZWR1LmNu; Kehou Pan, cWRraHBhbkAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.