
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 11 April 2022
Sec. Aquatic Microbiology
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.780055
This article is part of the Research TopicAssembly and Functions of Gut Microbiota in Aquatic AnimalsView all 28 articles
 Xiaohui Sun1*
Xiaohui Sun1* Jia Liu1
Jia Liu1 Shijing Deng1
Shijing Deng1 Renhe Li1
Renhe Li1 Wenhua Lv1
Wenhua Lv1 Shufeng Zhou1
Shufeng Zhou1 Xu Tang2*
Xu Tang2* Yun-zhang Sun3*
Yun-zhang Sun3* Mingyue Ke4
Mingyue Ke4 Kunming Wang4
Kunming Wang4
Aquatic pathogens such as Vibrio parahaemolyticus cause a bacterial infection that reduces the economic benefits of aquaculture and affects the food quality and safety of human beings. Quorum quenching (QQ) is considered a novel strategy of microbial antagonism that inhibits pathogens and reduces the abuse of antibiotics. This study investigates a QQ bacterial strain, Bacillus velezensis DH82 from the deep sea Yap trench, in vitro to examine the effects of DH82 and its functional products against V. parahaemolyticus, focusing on the Quorum sensing (QS) regulation and the inhibition of pathogenicity and bacterial growth. The study also conducted in vivo investigation in the aquaculture of Litopenaeus vannamei challenged with V. parahaemolyticus by immersion and injection challenge. The results of the QS regulator transcription level demonstrated the multiple QQ enzymes in DH82 regulated the pathogenicity but could not fully control the biofilm formation; the effective antibacterial activity of extracellular peptides on microbial antagonism verified the inhibition on bacterial growth of V. parahaemolyticus. The in vivo experiment in aquaria demonstrated the effective enrichment of DH82 and inhibition of Vibrio in both the aquatic system and the shrimp intestine. The dietary DH82 relieved the negative effect of Vibrio on the activity of enzyme acid phosphatase (ACP), alkaline phosphatase (AKP), superoxide dismutase (SOD) under challenge of Vibrio pathogens, and was not harmful to host according to lysozyme (LZM) activity. DH82 also ameliorated the damage to the intestine and muscles induced by V. parahaemolyticus infection according to tissue imaging. Though DH82 did present some dose-dependent adverse effects to the host, the findings revealed the effective QQ and antibacterial activity of DH82 on emerging biocontrol against V. parahaemolyticus, therefore indicating the potential application of DH82 as a biological control reagent in the sustainable and green production of aquaculture.
As one of the most important economic activities for food production, marine aquaculture provides high-quality protein resources and therefore is required with high yield and food safety. Though high stocking density aquaculture could maximize the commercial profits on aquatic animal production, the farming conditions would inevitably lead to the frequent occurrence of aquatic diseases (Long et al., 2019), among which, Vibrio parahaemolyticus is a common pathogenic bacterium causing diseases to fish, shrimp, and other aquatic animals (Fuente et al., 2015; Jin et al., 2017). Besides, V. parahaemolyticus is also the primary food-borne pathogen causing infection in humans (Baker-Austin et al., 2018). V. parahaemolyticus could invade aquatic animals via a wound and form a biofilm in the host (Sun et al., 2007), causing a bacterial infection through the released virulence factors under the regulation of quorum sensing (QS) (Chang and Lee, 2018), or intermediate bacterial resistance against antibiotics through the regulation of outer membrane protein and efflux pump protein (Zhao et al., 2018).
There is a lack of effective solutions for dealing with the problems of bacterial infection caused by pathogenic Vibrio. Farmers in less developed regions use an abundance of antibiotics when facing outbreaks of Vibrio to reduce their losses. Due to the abuse of antibiotics, there is now widespread drug resistance in V. parahaemolyticus from aquatic products and aquatic environment (Elmahdi et al., 2016), which not only increases the difficulty of prevention and treatment of V. parahaemolyticus, but also makes it more difficult for scientific researchers to develop novel antibiotics. With the probiotics and immune stimulators being applied as feed additives in aquaculture (Ganguly and Mukhopadhayay, 2010), microbial antagonism based on QS inhibition is regarded as an effective alternative to replacing antibiotics (Defoirdt, 2013) on the biological control of aquatic pathogens.
Bacillus is one of the main antagonistic bacteria widely used for biological control in agriculture (Jiang et al., 2018) and aquaculture (Wang C. et al., 2019). Under the regulation of QS (Bareia et al., 2018), Bacillus can produce lipopeptides, polyketones, and other metabolites with antibacterial, antiviral, or antitumor activities (Wu et al., 2019). In addition, the acyl-homoserine lactonase AiiA (Dong et al., 2000) widely exists in Bacillus, is one of the famous quorum quenching (QQ) enzymes that could recognize and degrade AHLs signal molecules produced by Gram-negative bacteria, and achieve QS inhibition by intervening in the biofilm formation and toxin release of AHLs/Lux mediated pathogens. Nevertheless, the application of QQ enzymes is limited by the enzyme activity and stability under complex environmental conditions of aquaculture and feed production, therefore the in vivo study of QQ is more focused on the utilization of Bacillus strain. Though Bacillus had been verified to modulate gut microbiota and control pathogens as probiotics in shrimp aquaculture (Olmos Soto, 2021), the effects of probiotics with QQ capacity on outbreaks of Vibrio pathogens and the host response still need to be verified by in vivo experiments, especially under the synergistic effect of the antibacterial metabolites.
In previous work, targeting the prevention and disease control of aquatic pathogens, a marine bacterium, Bacillus velezensis DH82 strain, was isolated from underlying seawater of the Western Pacific Yap trench at a depth of 6,000 m (Wang Q. H. et al., 2019) and identified as a QQ bacteria with multiple functional products for microbial antagonism, including the verified extracellular secreted anti-bacterial peptides (Wang et al., 2020) and intracellular products QQ enzymes of AiiA (Liu et al., 2020) and YtnP (Sun et al., 2021). Aiming to qualify the efficiency of DH82 on Vibrio control for sustainable aquaculture, especially under the circumstance of Vibrio outbreaks, DH82 is in vitro investigated against V. parahaemolyticus to assess the antibacterial and QQ ability of its functional products and is also used as biocontrol reagents to culture white shrimp Litopenaeus vannamei, to investigate the probiotic properties and in vivo effects on the host against the infection of V. parahaemolyticus by bath and injection challenge under high stocking density.
Bacillus velezensis DH82 strain (GenBank accession no. MK203035) was isolated from the seawater of the Western Pacific Yap trench at the depth of 6,000 m and was kindly provided by the Third Institute of Oceanography (Xiamen, China). The engineered Escherichia coli BL21 (DE3) strains respectively carry the expression clone of AiiA and YtnP from DH82, E. coli:pET28a/AiiA (Liu et al., 2020) and E. coli:pET28a/YtnP (Sun et al., 2021), which were constructed in this lab in previous work. V. parahaemolyticus 17SZ strain was isolated from the infected shrimp sample in the Vibrio breakout case at Xiamen in 2017 and was provided by the Center for Disease Control of Siming District, Xiamen, China. The AHLs, C6-HSL (Cat. 56395), 3-O-C6-HSL (Cat. K3255), 3-O-C10-HSL (Cat. O9014), and 3-O-C12-HSL (Cat. O9139), were purchased from Sigma-Aldrich.
The QQ enzyme expressing strain was cultured in Luria–Bertani (LB) media containing 50 mg/L kanamycin with shaking at 180 rpm at 37°C, 0.2 mM IPTG was inoculated after 2-h incubation of bacterial culture to induce the protein expression. The overnight cultured bacteria were washed and concentrated 5:1 with PBS (pH 7.0), performed ultrasonic breaking (operate for 3 s at 300 W then break for 6 s, and repeat for 60 times) on ice and centrifuged at 4°C at 8,000 g for 15 min to harvest the crude extract in the supernatant. The supernatant containing crude enzyme was loaded to the high affinity NI-NTA chromatography, washed using a lysis buffer (300 mM NaCl and 50 mM NaH2PO4, pH 7.4), then washed with an imidazole elution buffer (300 mM NaCl, 200 mM imidazole, and 50 mM NaH2PO4, pH 7.4) to purify the His-tagged enzyme. The purified enzyme was sterilized by filtration using 0.22 μm syringe filter (Millipore, Cat. SLGP033R) and analyzed by SDS-PAGE. The concentration of protein was qualified using the Bradford assay.
The overnight culture of DH82 was inoculated 1% (v/v) in 1 L fresh LB broth (10 g/L Tryptone, 5 g/L Yeast extract, and 10 g/L NaCl, pH7.0) and cultured with shaking at 180 rpm at 37°C for 24 h. The bacterial culture was centrifuged at 10,000 rpm at 4°C for 10 min to harvest supernatant, then filter sterilized with 0.22 μm membrane. The filtered supernatant was added slowly with (NH4)2SO4 to 70% of saturability, standing overnight at 4°C, and was centrifuged at 10,000 rpm at 4°C for 20 min to collect the salting-out proteins. The salting-out proteins were re-dissolved in 10 mL PBS (pH 7.0) to harvest crude proteins. The crude proteins were then desalted using Solarbio MD44 dialysis membrane (Cat. YA1078) by soaking in distilled water for 18 h and stored for further study.
DH82 were additionally prepared in a 200 L fermentation tank (15 g/L glucose, 20 g/L bean pulp, 10 g/L peptone, 5 g/L corn flour, 0.5 g/L MgSO4, 1 g/L K2HPO4, 2 g/L NaCl, 3 g/L CaCO3, pH 7.0), attached with corn starch and dried for granulation to produce bacterial powder, at a final concentration of 1011 CFU/g, for further in vivo study.
A 1.5 mL overnight culture of 17SZ in LB broth was mixed with 150 mL fresh LB medium (1.5% agar) maintained at 50°C. The agar-culture solution was immediately poured as 20 mL portions in petri dishes. Each 2 mL DH82 overnight culture in LB broth was centrifuged at 6,000 g for 5 min, then filtered using 0.22 μm membrane to collect the supernatant. The pellets were resuspended in PBS and then treated with 1 μg/mL lysozyme at 37°C for 30 min, then centrifuged at 8,000 g for 5 min to harvest the bacterial lysate from the supernatant. 50 μL of above prepared supernatant, cell pellets, and cell lysate were respectively spotted in wells punched in the solidified agar, compared with 50 μL of salting-out antibacterial protein as a positive control. The plates were incubated at 30°C for 24 h for examination of the bacterial inhibition zone.
The overnight culture of 17SZ was diluted to OD600 value at 0.1, then 1:100 (v/v) inoculated in 40 mL fresh LB broth for incubation with shaking at 30°C for 12 h, with additional QQ enzyme at a final concentration of 50 μg/mL for treatment, and the same volume of sterile water as the negative control. The bacterial density at OD600 was measured to obtain the growth curve.
Each 1 mL of 17SZ culture was sampled at 1, 2, 4, 6, and 12 h during growth, centrifuged at 6,000 g for 5 min, and resuspended with the same volume of sterile water to harvest the bacterial pellets. RNA samples were respectively extracted from the pellets using Trizol RNA extraction kit (Takara, Dalian) and then used as a template for reverse transcription by PrimeScriptRT reagent Kit with gDNA Eraser (Takara) to construct cDNA for the further qPCR program. The primers listed in Table 1 were respectively used to amplify the sequence of 16S rRNA, aphA, opaR, tlh by qPCR using Bio-Rad CFX Connect. The transcription levels of key regulators on QS in V. parahaemolyticus were analyzed, compared with that of 16S rRNA, to determine the activity of QQ enzymes on bacterial inhibition against V. parahaemolyticus.
One microliter of 17SZ overnight culture was inoculated in 200 μL of Biofilm medium [BM, filter sterilized tap water supplemented with 5 mM sodium citrate, 0.5% casamino acids (Difco) and 0.5% v/v standard brain heart infusion broth (Difco)] in 96 well microplates, with additional 50 μg/mL QQ enzyme for treatment in triplicate, compared with sterilized water as a negative control. Bacterial cultures were grown statically at 30°C for 3 days when a luxuriant biofilm was apparent. After this time the wells were subsequently washed with phosphate buffered saline (PBS). Two hundred microliters of 0.1% crystal violet solution was then added to each well and left for 15 min at room temperature, after which the crystal violet was removed prior to three washes with PBS. Two hundred microliters of ethanol was then added to each well to dissolve any crystal violet bound to the well and any remaining biofilm. After 15 min at room temperature, the absorbance of the wells was measured at 580 nm using Tecan Infinite M200 Pro.
Each 100 μL DH82 overnight culture in LB broth was centrifuged at 6,000 g for 5 min to collect the supernatant and cell pellets. The pellets were resuspended in PBS and then treated with 1 μg/mL lysozyme at 37°C for 30 min, then centrifuged at 8,000 g for 5 min to harvest the bacterial lysate from the supernatant. Each 100 μL of DH82 supernatant and lysate, 0.1, 1, and 10 μL DH82 overnight culture, 0.1, 1 and 10 μL crude QQ enzyme as well, were respectively mixed with 1 μL of 17SZ overnight culture in 200 BM medium in 96 well microplates, to assess the effect of different fractions of DH82 on the inhibition of biofilm formation and planktonic bacterial amount of 17SZ, compared with PBS as a negative control.
The above mixtures were incubated in BM at 30°C for 3 days to form biofilms. The biofilms were analyzed by crystal violet assay, and the supernatants of the biofilm cultures were spread on LB agar plates and incubated at 37°C for 24 h to calculate the planktonic bacterial amount by plate counting method.
The trial experiments of shrimp farming were performed in aquaria located at Zhaoan, Zhangzhou, Fujian Province, China (longitude 117.17501, latitude 23.71148). White shrimp (L. vannamei), with a length of 10.0 ± 1.0 cm and the weight of 13.3 ± 0.8 g, were grown in an aquarium (length × width × depth = 96 mm × 74 mm × 66 mm) containing 300 L fresh seawater and continuous aeration under high stocking density. The cultures in each aquarium were absorbed by siphon to remove excreta of shrimps and pumped in 30 L fresh seawater to refill the aquarium at noon every day. The aquaria were placed indoors with good air circulation.
The shrimps were fed using non-antibiotic commercial feed thrice daily (6 a.m., 12 a.m., and 6 p.m.) for 1 week to acclimatize to the diet before use. The experiments were approved by the institutional research ethics committee of Huaqiao University and carried out in accordance with the PRC National Standard GB T 35823-2018.
150 shrimps per aquaria were randomly allocated for five treatments in duplicate for 28 days, respectively treated with 100 mL of fresh LB broth (blank) as control (group A), same volume of 17SZ (group B), DH82 (group C), continuous daily dietary of DH82 with the same volume (group D), and the challenge with the same volume of 17SZ on day 1 along with daily dietary of DH82 (group E). The bacterial culture of 17SZ (OD600nm 0.6, 108 CFU/mL) was directly poured into the aquaria to simulate the emerging out-breaking of V. parahaemolyticus, whilst the 0.3 g pre-made DH82 powder was activated in 300 mL of 100 g/L brown sugar water and mixed with feeding stuff to feed the shrimps, to achieve the bacterial inoculation with a final concentration of 105 CFU/mL (Ottaviani et al., 2020) to both 17SZ and DH82 after seawater exchanging.
The water samples in each group were collected and spread on an LB agar medium to calculate the planktonic bacterial number, the Sodium Chloride Polymyxin Base (SCPB) medium containing 250 U/mL Polymyxin B was used to calculate the bacterial number of Vibrio sp.
Total genomic DNA of microbial community were extracted from the intestine samples of the shrimps in blank groups (int_12), treated with DH82 in the first week (int_13), infected with 17SZ (int_20), and those treated with continuous dietary DH82 after 17SZ infection (int_15), using an MP FastDNA Spin Kit for Soil (MP Biomedicals, Santa Ana, CA, United States) according to the manufacturer’s instructions. The DNA extract was checked on 1% agarose gel, and DNA concentration and purity were determined with NanoDrop 2000 UV-vis spectrophotometer (Thermo Scientific, Shanghai) and used as template DNA to amplify the hypervariable region V3-V4 of the bacterial 16s rRNA with the primer pairs 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) by PCR using Phusion ® High-Fidelity PCR Master Mix kit (NEB). The PCR amplification of 16s rRNA was performed as follows: initial denaturation at 95°C for 3 min, followed by 30 cycles of denaturing at 98°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 15 s, and single extension at 72°C for 5 min, and end at 4°C. The PCR products were extracted from 2% agarose gel and purified using a DNA Gel Extraction Kit (Omega Bio-tek, China) according to the manufacturer’s instructions and quantified using Quantus™ Fluorometer (Promega, Beijing).
The purified amplicons were pooled in equimolar and paired-end sequenced on Illumina MiSeq PE300 platform/NovaSeqPE250 platform (Illumina, San Diego, CA, United States) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).
The raw 16S rRNA gene sequencing reads were demultiplexed, quality-filtered by fastp version 0.20.0 (Chen et al., 2018), and merged by FLASH version 1.2.7 (Magoč and Salzberg, 2011) with the following criteria: (i) the 300 bp reads were truncated at any site receiving an average quality score of <20 over a 50 bp sliding window, and the truncated reads shorter than 50 bp were discarded, reads containing ambiguous characters were also discarded; (ii) only overlapping sequences longer than 10 bp were assembled according to their overlapped sequence. The maximum mismatch ratio of the overlap region is 0.2. Reads that could not be assembled were discarded; (iii) Samples were distinguished according to the barcode and primers, and the sequence direction was adjusted with exact barcode matching or a maximum two nucleotide mismatch in primer matching.
Operational taxonomic units (OTUs) with 97% similarity cutoff were clustered using the UPARSE version7.1 (Edgar, 2013), and the chimeric sequences were identified and removed. The taxonomy of each OTU representative sequence was analyzed by RDP Classifier version 2.2 (Wang et al., 2007) according to the SILVA ribosomal RNA database using a confidence threshold of 0.7. Alpha diversity index including observed OTUs, richness estimators, such as Ace, Chao, Shannon, and Simpson index, were calculated based on the frequency of OTUs and genera in the assigned sequence collections after rare sequences were removed. The OTUs of int_15 and int_20 were analyzed by Fisher’s exact test to compare the difference of the abundance on genus level.
50 shrimps were randomly allocated for 10 treatments for 7 days (five replicates per treatment), respectively injected on day 1 by syringe with 1 mL of 17SZ and DH82 mixture (1:1) at high dose (OD600 nm 0.6, 108 CFU/mL), medium dose (OD600 nm 0.06, 107 CFU/mL) and low dose (OD600 nm 0.006, 106 CFU/mL), compared with those individually injected with the same volume of PBS (pH 7.0), 17SZ and DH82 at the same dose (1:1 mixed with PBS at pH 7.0) as control. The injections were performed on the muscle of the shrimp between the 5th and 6th uromere. The shrimps under injection challenge in each group were partitioned by nets in the aquarium and continuously fed with non-antibiotic feed as normal. The survival number and elapsed days were recorded and analyzed using GraphPad Prism 6 software.
Approximate 1 mL of hemolymph were sampled from the ventral sinus of each shrimp on day 28 after the bath challenge, incubated at 4°C overnight to separate the serum. The serums were centrifuged at 1,000 g for 15 min at 4°C to harvest the supernatants. The enzyme activities of alkaline phosphatase (AKP), acid phosphatase (ACP), superoxide dismutase (SOD), catalase (CAT), and lysozyme (LZM) in the supernatants were respectively assessed using commercial detection kits (Nanjing Jiancheng Bioengineering Institute) and measured using MD microplate reader (SpectraMax M5).
The infected tissues of white shrimps (intestines from bath challenge and muscles from injection challenge) were immersed in Bouin’s Fixative for 4 h and transferred to 70% ethanol. The individual lobes of infected tissue biopsy material were placed in processing cassettes, dehydrated through a serial alcohol gradient, and embedded in paraffin wax blocks. The tissue sections were dewaxed twice in xylene for 20 min, rehydrated through decreasing concentrations of ethanol from 100% to 75% for 5 min in each step, and washed in sterile water. The sections were then stained with hematoxylin for 5 min, rinse with sterile water, followed with increasing concentration of ethanol from 85, 95% for 5 min in each step, and stained with eosin for 5 min. After staining, the sections were dehydrated through 100% ethanol thrice and xylene twice for 5 min in each step and sealed with neutral gum. The tissue sections were imaged using a brightfield microscope (Nikon Eclipse E100) and an imaging system (Nikon DS-U3).
The experimental data were analyzed by the software GraphPad Prism 6. P value from T-test were used to determine the difference between each experimental group.
The recombinant QQ enzymes were investigated on the in vitro effect of QQ against V. parahaemolyticus, as shown in Figure 1, the treatment of AiiA and YtnP both present a tendency of depression on the gene expression level of key regulatory proteins AphA and OpaR during bacterial growth. AiiA significant functioned at 2 h (P-value was 0.00027) and 12 h (P-value was 0.029) on aphA and significantly depressed on opaR at 4 h (P-value was 0.018); by contrast, YtnP was observed with higher activity on the down-regulation, significantly depressed aphA at early stage of 1 h (P-value was 5.6E-05), 2 h (P-value was 3.9E-07) and 4 h (P-value was 0.049), and down-regulated opaR at 4 h (P-value was 0.013), which indicated the potential cooperation of AiiA and YtnP, and possibly involving other unidentified QQ enzymes in DH82, on the inhibition against pathogens at different growth stages.
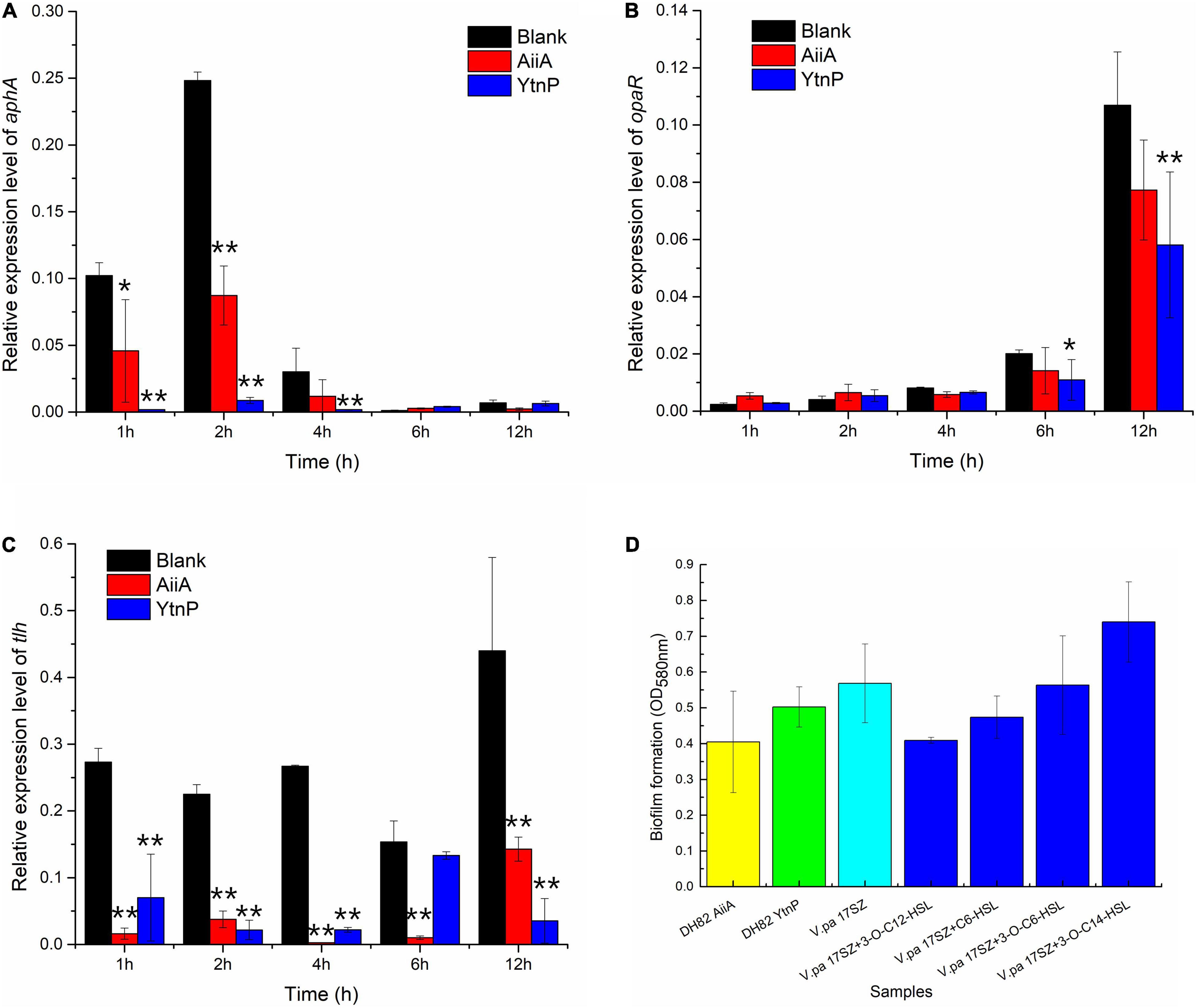
Figure 1. Effect of quorum quenching (QQ) enzymes on the regulation of QS against Vibrio parahaemolyticus. Fifty microgram/milliliter QQ enzyme was used to treat the 17SZ culture [OD600 0.1, 1:100 (v/v) inoculation] in LB at 30°C. RNA from bacterial culture sampled at 1, 2, 4, 6, and 12 h was used as temperate for RT-qPCR, using 16S rRNA as internal reference. Panel (A) is the effect on the expression of aphA; Panel (B) is the effect on the expression of opaR; Panel (C) is the effect on the expression of tlh; Panel (D) is the effect of QQ enzyme on biofilm formation of 17SZ. The error bars are presented as the standard deviation. Statistical analysis results are presented by a significant difference indicated by ** where p < 0.01 and * where p < 0.05.
As shown in Figure 1C, the presence of QQ enzymes both remarkably down-regulated the expression level of virulence factors, thermolabile hemolysin (TLH), that the treatment of AiiA present significant difference during the whole test period of 12 h (P-value at 1, 2, 4, and 6 h were all lower than 0.001, and was 0.022 at 12 h), and YtnP functioned with an extremely significant difference at 1, 2, 4, and 12 h (P-value all lower than 0.001).
The effects of QQ enzymes on biofilm formation of V. parahaemolyticus were later investigated (Panel D). The results demonstrated a slight decrease but no significant difference under the treatment of respectively QQ enzyme of AiiA and YtnP (P-value was 0.19 and 0.41). Besides, the addition of different types of exogenous AHLs presented no difference on biofilm formation of 17SZ (P-value all above 0.05), which indicated that AHL mediated QS might not be the only pathway on the combined intermediation of 17SZ on biofilm formation.
Considering the unsatisfactory inhibition on biofilm formation by QQ enzymes, the DH82 pellets and the functional products in different fractions, intracellular QQ enzymes in the lysate fraction, extracellular antibacterial peptides in the supernatant of bacterial culture, and the salting-out crude antimicrobial proteins, were investigated for their effects on the growth of V. parahaemolyticus. As shown in Table 2, the results of antagonistic activity demonstrated positive antibacterial ability by bacterial supernatant (with inhibitory zone at a diameter of 19.4 ± 0.2 mm) and cell pellets of DH82 (with inhibitory zone at a diameter of 16.5 ± 0.3 mm), and negative inhibition by the cell lysate; whilst the crude proteins extracted from the supernatant (approximately 100 times concentrated) presented a larger inhibitory zone at a diameter of 24.3 ± 0.2 mm. The results indicated that the antibacterial property of DH82 was contributed by the secreted extracellular peptides, but not the intracellular products containing QQ enzymes in cell lysate.
As the status transition between biofilm and planktonic cells of V. parahaemolyticus, an in vitro assessment was designed to investigate the synergistic effects of functional products from DH82 on the Vibrio control. The cell pellets, cell lysate, supernatant of overnight culture, and an enzyme mixture of AiiA and YtnP were used to assess the function of different fractions from DH82 on microbial antagonism against V. parahaemolyticus. As shown in Figure 2, the DH82 lysate significantly decreased the biofilm formation of 17SZ (P-value was 0.0045) and showed no difference to the planktonic bacteria; whilst the supernatant of DH82 containing secreted anti-microbial peptides also limited the biofilm formation (P-value was 0.0026) and reduced approximately half of the planktonic 17SZ. The whole pellets of DH82 demonstrated a dose dependent magnitude reduction on planktonic 17SZ and slight down-regulation of the biofilm formation, including the few biofilms formed by DH82, compared with the control groups of 17SZ and different dose injections of DH82. The treatment of mixed enzymes of AiiA and YtnP also presented a similar tendency on biofilm control to that of bacterial pellets and lysate, but no significant reduction on planktonic 17SZ.
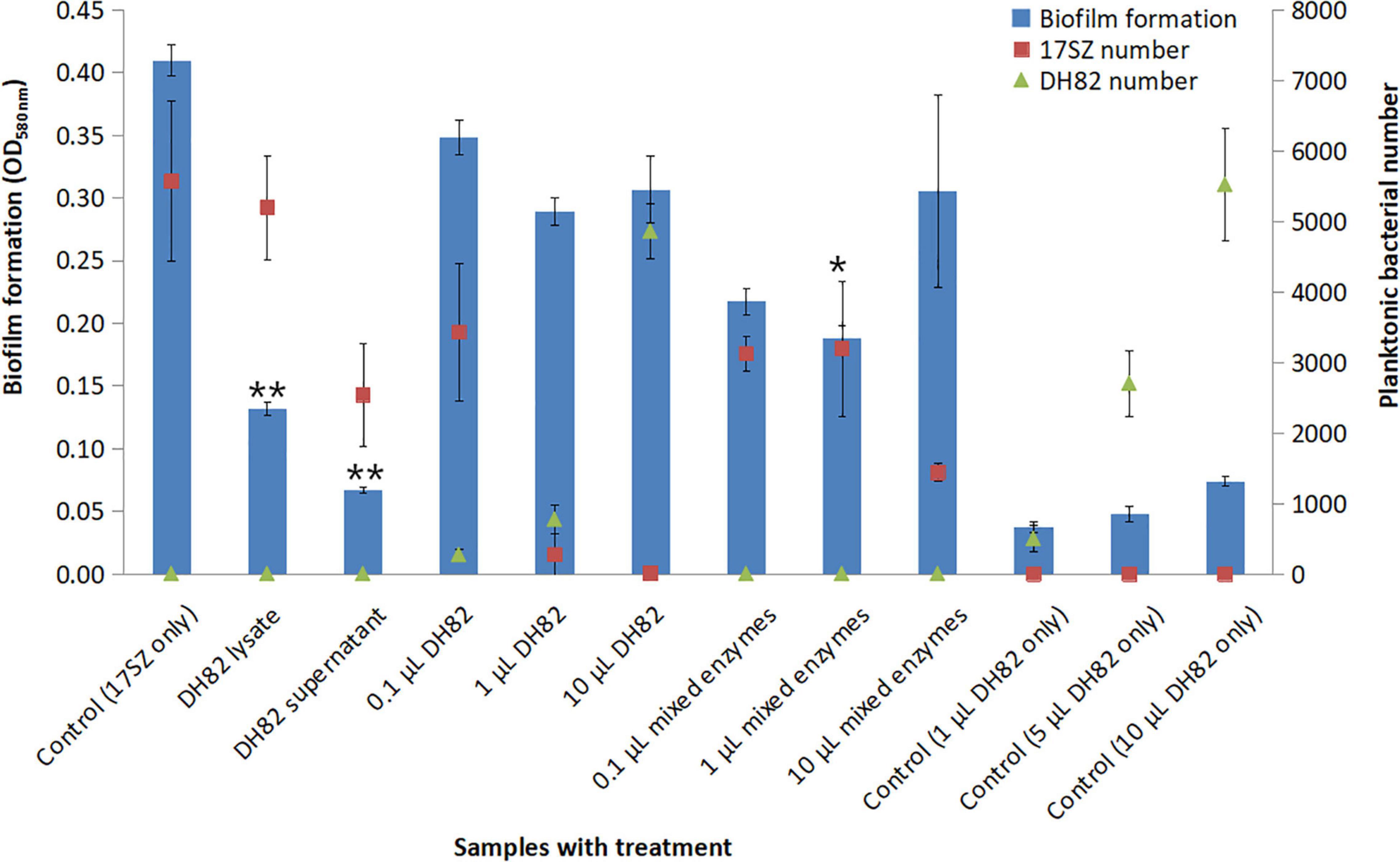
Figure 2. Effect of fractions of DH82 on the biofilm and planktonic bacterial amount of Vibrio parahaemolyticus. Each 100 μL of DH82 supernatant and lysate, 0.1, 1, and 10 μL DH82 overnight culture (107 CFU/mL), 0.1, 1, and 10 μL QQ enzyme (AiiA and YtnP mixture) were respectively mixed with 1 μL of 17SZ overnight culture (OD600 nm 0.1, 108 CFU/mL) in 200 BM medium, compared with 17SZ with PBS as a negative control, the mixtures were incubated in 30°C for 3 days. The biofilms were analyzed by crystal violet assay, and the supernatants of biofilm cultures were spread on Luria–Bertani (LB) agar plates to calculate the planktonic bacterial number. The biofilms are presented by blue bars, the number of planktonic bacteria of 17SZ is presented ■ in red, the DH82 by ▲ in green. The error bars are presented as the standard deviation. The error bars are presented as the standard deviation. Statistical analysis results are presented by the significant difference indicated by * where p < 0.05 and ** where p < 0.01.
Compared with the in vitro experiment, the in vivo experiment of bacterial challenge in shrimp trial experiment is designed to assess the synergistic effects of DH82 strain with the multiple functional products on Vibrio control in aquaria with pump-in seawater, which contained the inoculated Bacillus and Vibrio and other microorganisms from marine environments. We monitored the total amount of planktonic microorganisms in the culture system, as shown in Figure 3, compared with the blank control, the total number of Bacillus and Vibrio in the treatment group with DH82 and 17SZ alone in the system showed quantitative advantages respectively 1 day after the bacterial solution was added for bath challenge. In the treatment group with a mixture of DH82 and 17SZ, although Vibrio was still countable, its number was significantly lower than that in the single challenge group with 17SZ alone, which indicated the bacterial inhibition of DH82 against Vibrio.
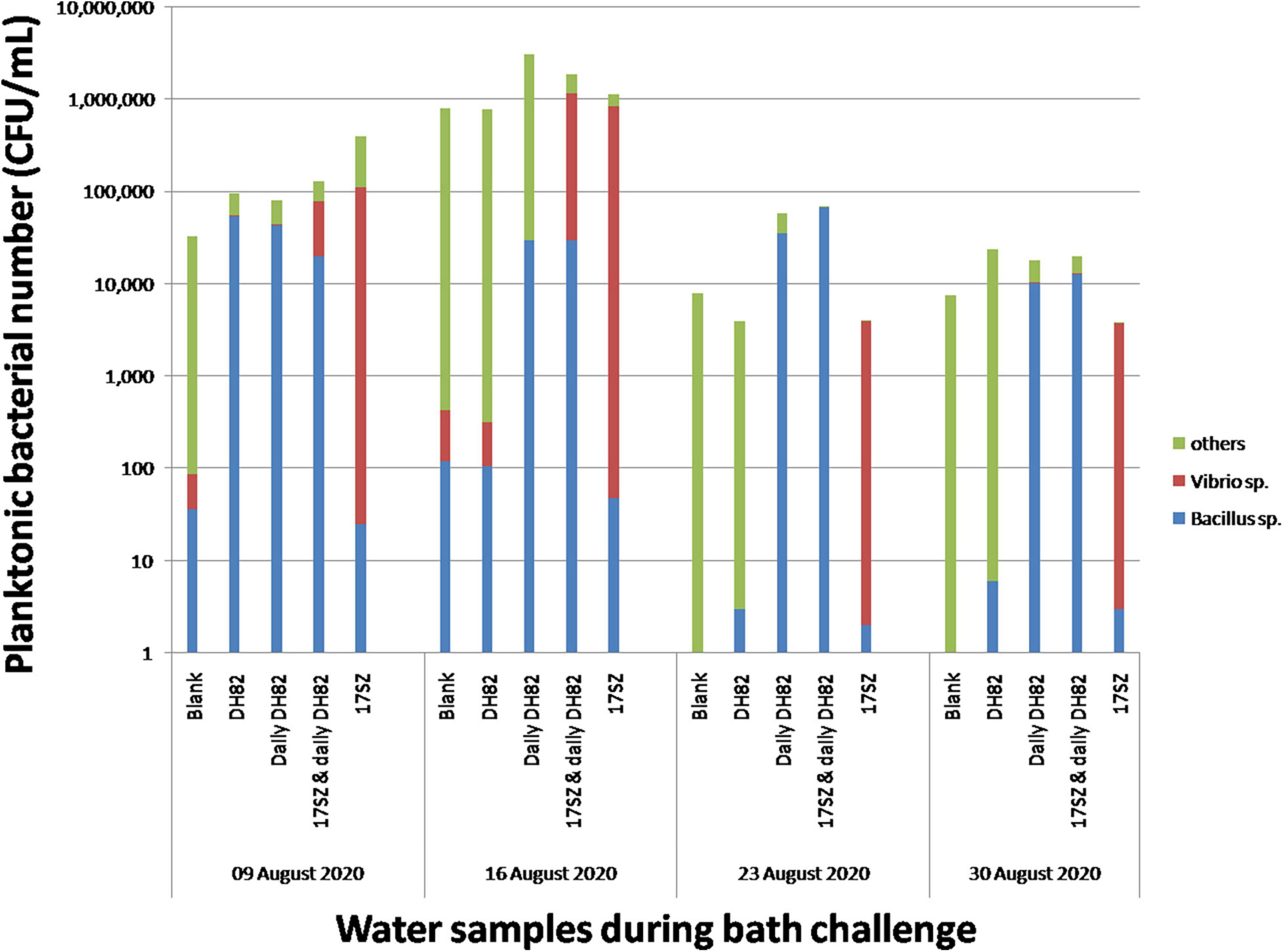
Figure 3. Effects of DH82 on Vibrio control under bath challenge. Each 100 mL bacterial culture (OD600nm 0.1) was individually poured into aquaria for bacterial bath challenge to white shrimps with the final concentration of 105 CFU/mL. The water samples in each group were collected before water exchange and spread on Luria–Bertani (LB) agar plates to calculate the bacterial number of Bacillus sp. and total amount, and on SCPB agar plates to calculate the number of Vibrio sp. The number of Bacillus sp. is presented by column in blue, Vibrio sp. in red and other bacteria in green. The vertical axis in the stacked column graph is marked as a logarithmic scale.
In the subsequent weekly sampling, the total amount of planktonic bacteria in the aquaria fluctuated greatly affected by the environment. The addition of DH82 in the first week did not show the quantitative advantage of Bacillus in the following 3 weeks, whilst Bacillus was continuously detected and dominant in the treatment group of continuous addition of DH82, and Vibrio was inhibited at a low level. In addition, it could be seen that a high number of Vibrios were detected in the treatment group of 17SZ alone during the whole bacterial bath challenge period. Since there was no artificial addition of Vibrios after the first day, considering the good biofilm-forming capacity of 17SZ observed in the in vitro experiment, and compared with the Vibrio number in other treatment groups, it could be inferred that the planktonic Vibrios might transform from the biofilm statues and cause continuous influence to the shrimps.
High throughput sequencing on 16s rRNA was also used to analyze the bacterial community of gut microbiota on genus level, between the 17SZ infected shrimps (int_20) and those treated with continuous dietary DH82 after Vibrio infection (int_15), compared with the blank groups (int_12) and those treated with DH82 in the first week (int_13) as control.
The richness of microbial community from each sample was analyzed by the Alpha index, as shown in Table 3. The results of OTU coverage in each group were all above 99.84%, which indicated the good recovery and reliable reflection of the tested samples. The results of a comprehensive assessment on the four indexes of Ace, Chao, Shannon, and Simpson demonstrated adverse impact on the richness of bacterial community caused by Vibrio infection, and recovery under the treatment of continuous DH82.
The microbial community of each group is shown in Figure 4. The DH82 treated shrimps were observed with significantly more abundant Bacillus sp. existed (respectively took 7.266% out of 7.457% in the gut of DH82 feed shrimps, and only 0.03143% out of 0.03404% in 17SZ infected ones), and significant lower richness and abundance of Vibrio sp. in the intestine (respectively 0.3842% in DH82 treated groups and 5.57% in 17SZ infected groups), where both P-values were lower than 1E-15. Among these, the Bacillus community mostly contributed B. velezensis on a species level, whose corresponding OTU sequence was 100% matched to that of 16s rDNA of B. velezensis, according to the analysis of further alignment. The results verified the successful colonization of DH82 and the effective inhibition against pathogenic Vibrio in shrimp guts.
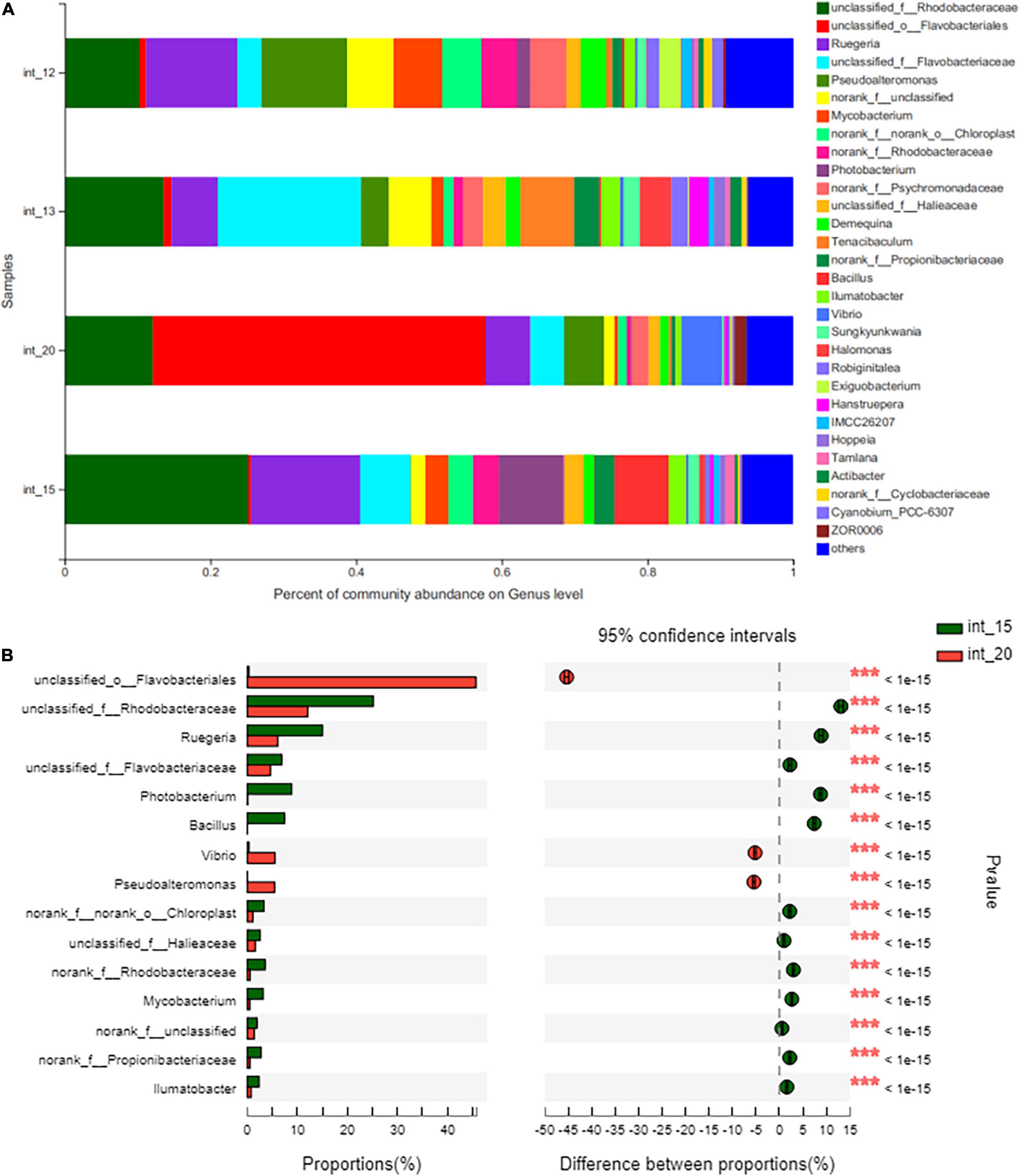
Figure 4. Intestinal bacterial community of shrimps under bath challenge. The total genomic DNA from the intestine sample of the shrimps under the treatment of blank (int_12), DH82 in the first week (int_13), 17SZ then continuous with DH82 (int_15), and the 17SZ only (int_20) were respectively performed high throughput sequencing on the 16s rRNA. The taxonomy of each OTU representative sequence was analyzed by RDP Classifier version 2.2 and classified on genus level. The OTUs were analyzed by Fisher’s exact test, to compare the difference of abundance between treatments. Panel (A) is the microbial structure presented by relative community abundance, the bar plot of Bacillus is arrowed in red and that of Vibrio in blue; Panel (B) is the difference of abundance with DH82 treatment after 17SZ infection, the top 15 genera with the highest abundance were listed, including the Bacillus sp. and Vibrio sp. Statistical analysis results are presented by a significant difference indicated by *** where p ≤ 0.001.
The enzyme activities of ACP, AKP, SOD, and LZM in shrimp serum were assessed to demonstrate the non-specific immune response of shrimp to the challenge by bacterial bath, as shown in Figure 5.
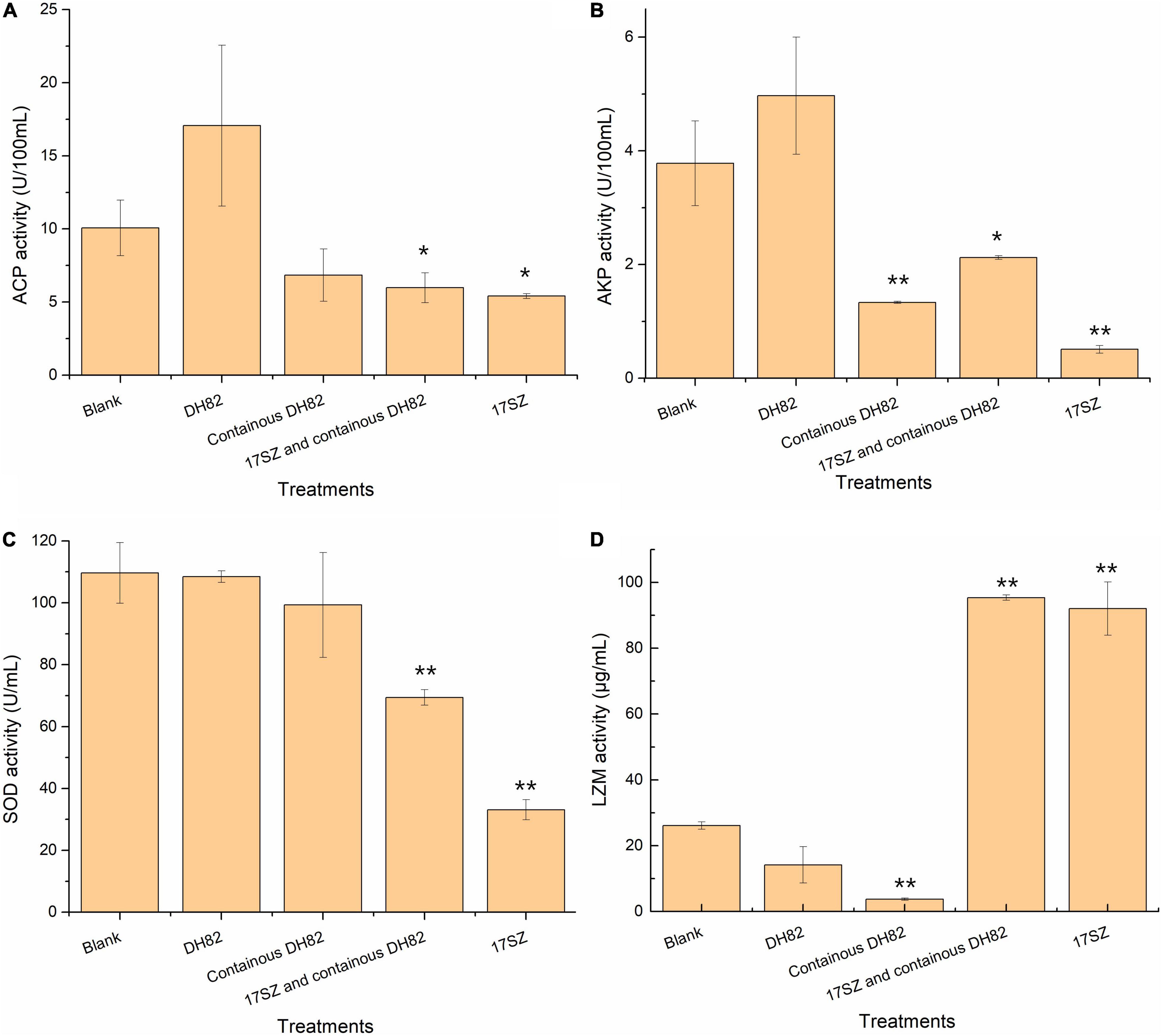
Figure 5. Effect of bacterial treatments to acid phosphatase (ACP), alkaline phosphatase (AKP), superoxide dismutase (SOD), and lysozyme (LZM) activities in the serum of shrimps. Each 100 mL bacterial culture (OD600nm 0.1) was individually poured into aquaria for bacterial bath challenge to shrimps with final concentration of 105 CFU/mL. The enzyme activities of serum under the treatment of blank (negative control), DH82 in the first week, continuous DH82 in each week, 17SZ then continuous with DH82, and the 17SZ only as infection control are presented, panel (A) is ACP, panel (B) is AKP, panel (C) is SOD, and panel (D) is LZM. The error bars are presented as the standard deviation. Statistical analysis results are presented by significant difference that indicated by ** where p < 0.01 and * where p < 0.05.
The results demonstrated that ACP activity (Panel A) significantly decreased under the challenge of V. parahaemolyticus (P-value was 0.013), even with the control of DH82 (P-value was 0.030), which indicated the irreversible adverse effects of the pathogen to shrimp on the growth factor of ACP. Moreover, the additional DH82 in the first week improved the ACP activities, however, there was no significant difference to the blank group, and somehow the continuous treatment of DH82 also presented adverse effects to ACP activity.
A similar tendency was observed in the data of AKP activities (Panel B), that the initial delivery of DH82 showed no difference on the effect of the host. However, the long-term giving of DH82 led to a significant reduction in AKP activity, the continuous dietary DH82 significantly relieved the adverse effects caused by V. parahaemolyticus (P-value was 3.2E-06) and showed no difference to that of the blank (P-value was 0.018).
The result of SOD activity (Panel C) also presented a consistent tendency under the challenge of V. parahaemolyticus to that of phosphatases, where both the initial treatment and continuous treatment of DH82 showed no difference in SOD activity to that of the control. In addition, even though the infection of 17SZ generated a decrease in SOD activities, the emerging treatment of DH82 significantly relieved the influence (P-value was 0.00011), which indicated that the treatment of DH82 at the presence of V. parahaemolyticus did contribute to the non-response immune response scavenging for free radicals.
The data of LZM activity (Panel D) presented an interesting result. The treatment of DH82 gradually reduced the congenital immune response of shrimp on the expression of lysozyme, and the continuous addition of DH82 was observed to have a significant difference on LZM activity (P-value was 0.0016), whilst the challenge of V. parahaemolyticus remarkably promoted the LZM activity of host (P-value was 0.0076) despite the addition of DH82 (P-value was 0.00020 compared with the blank). Considering the functional products of QQ enzymes and antibacterial peptides in DH82, the results verified the strategy of “inhibit but not kill” by DH82 against V. parahaemolyticus and also indicated the non-harmful property of DH82 to the shrimps.
The intestines of shrimps in each group were sampled to analyze the effect of bacteria on the intestinal structure of shrimps by bath challenge. The intestines in the blank (negative control), DH82 treated, and mixture treated groups all had thick intestinal walls and were full of feedstuffs when observed by the naked eye, compared with those of the 17SZ challenged group, which were observed to have an empty intestine, thin intestinal well, and clear inflammation.
The sections of the intestines were microscopically examined, as shown in Figure 6. The intestinal epithelial cells of healthy shrimp in the control group (Panel a) were closely arranged with a thick edge of intestinal epithelial cells without any swelling. The intestines of shrimps with DH82 challenge (Panel b) were observed to have a slightly thinner edge with damage to intestinal epithelial cells. However, in the group infected with V. parahaemolyticus (Panel c), the intestinal epithelial tissue of shrimp were nearly exfoliated and shed in the intestinal cavity, with a swollen intestinal wall. For the group treated with DH82 together with 17SZ (Panel d), the symptoms of intestinal damage were milder and they continued to have complete intestinal epithelial cells, though there were few epithelial cells separated from lamina propria and swelling on the intestinal wall was observed. The symptoms of the four groups of shrimps under bath challenge demonstrated the inhibition of DH82 on anti-inflammation and damage-reduction against V. parahaemolyticus.
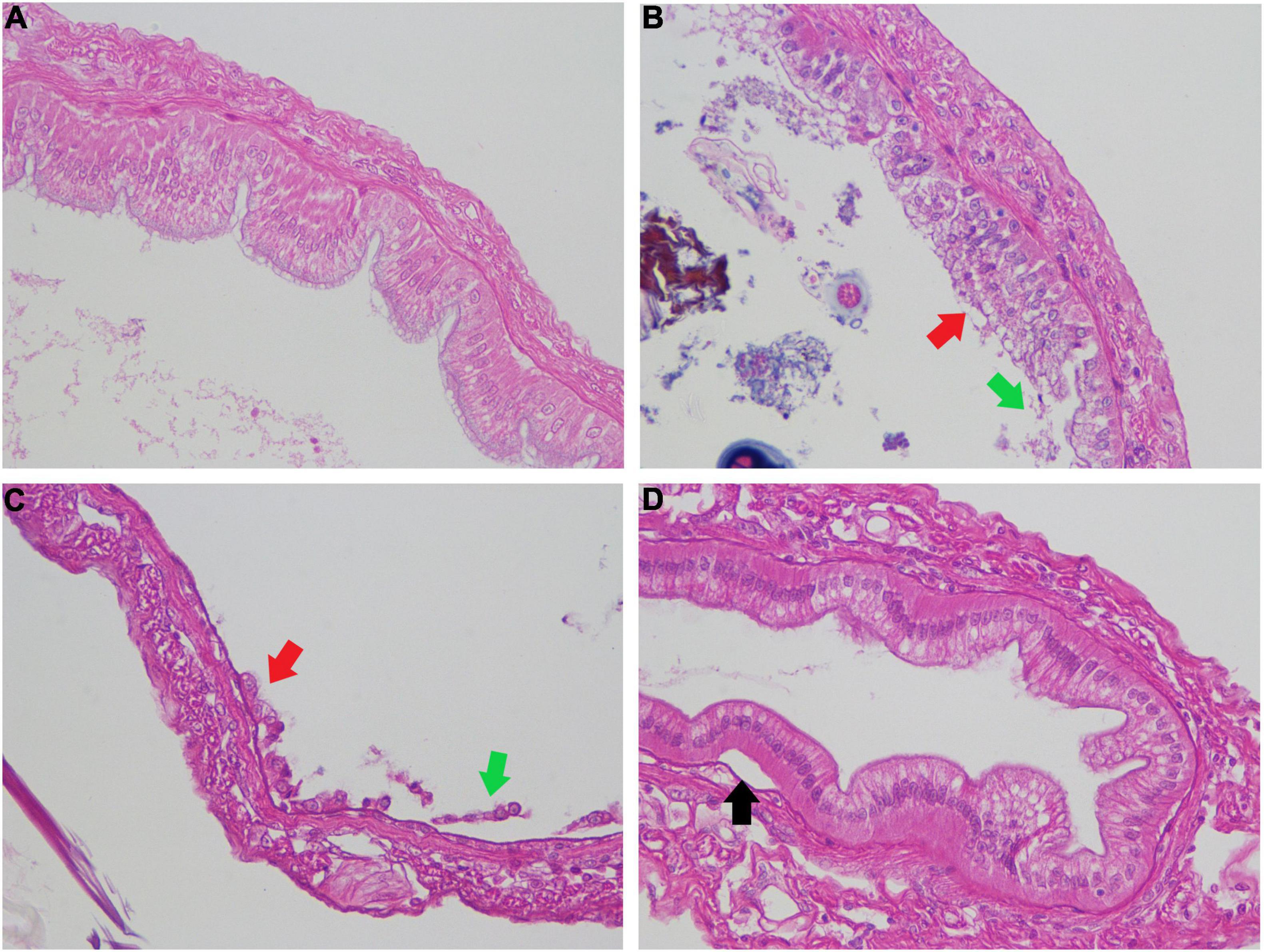
Figure 6. Pathological analysis of intestinal tissue of shrimps challenged with Vibrio parahaemolyticus. Each 100 mL bacterial culture (OD600nm 0.1) was individually poured into aquaria for bacterial bath challenge to shrimps with the final concentration of 105 CFU/mL. The intestine samples were performed H&E staining and imaged by microscopy. Panel (A) is the intestine of blank, panel (B) is intestine challenged with DH82, panel (C) is intestine challenged with 17SZ, panel (D) is intestine challenged with 17SZ and treated with continuous DH82. The arrows point out where the intestinal epithelial cells detach from the basement membrane (black arrows), the occurrence of basophilic hypertrophied nuclei with a reduced volume of eosinophilic cytoplasm (red arrows), and the disintegrated intestinal epithelial cells (green arrows).
The survival rate of shrimps after injection challenge, which is shown in Figure 7, demonstrated a dose-dependent decreasing tendency under the bacterial treatments, and higher percent survival. Compared with the 100% survival of blank in the control group, the shrimps treated by DH82 at a dose of 106 CFU/mL also fully survived after 7 days, and with 80 and 40% survival respectively, when the dose of DH82 was increased to 107 and 108 CFU/mL. The challenge of 17SZ sharply reduced the number of surviving shrimp, especially at the injection dose higher than 107 CFU/mL, whilst the coexisting DH82 raised the survival rate at a dose of 106 CFU/mL and 107 CFU/mL and prolonged the survival time of shrimps at a dose of 108 CFU/mL.
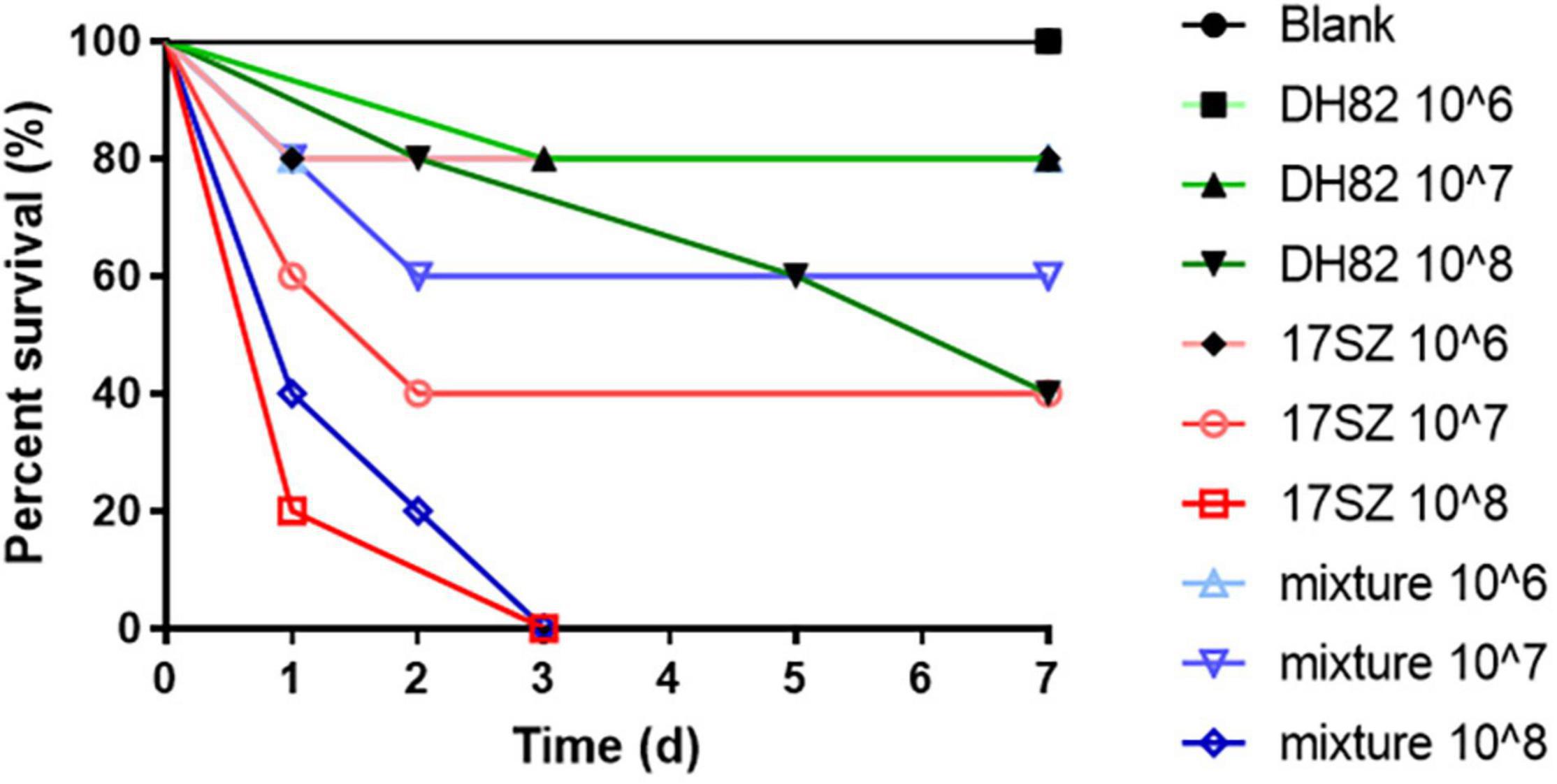
Figure 7. Survival rate of shrimps after injection challenge. Fifty shrimps were randomly allocated to 10 groups, respectively injected at the muscle between 5th and 6th uromere with 1 mL of 17SZ and DH82, and the same volume of the mixture (1:1) of 17SZ and DH82 at different doses, compared with the blank of injection with PBS as control. The survival rates of each treatment are presented: the blank is presented by ● in black line; DH82 at a dose of 106 CFU/mL is presented by ■ in light green line, 107 CFU/mL by ▲ in green line, DH82 at 108 CFU/mL by ▼ in dark green line; 17SZ at 106 CFU/mL by ♦ in pink line, 107 CFU/mL by ○ in light red line, 108 CFU/mL by □ in red line; mixture at 106 CFU/mL by △ in light blue line, 107 CFU/mL by ▽ in light purple line, 108 CFU/mL by ◊ in blue line.
As for the bacterial infection to the muscle, the shrimps in the blank and DH82 treated groups were all observed with no whitish symptoms, and recovered with a black scar at the injection spot; whilst the 17SZ infected shrimps were observed with obvious symptoms of muscle gonorrhea. The muscle sections from survived shrimps were further performed H&E staining and examined by brightfield microscope. As shown in Figure 8, the muscles of shrimp infected by 17SZ (Panels c and d) were observed with serious disorder and large gaps between the muscle fibers, whilst the muscles in the group that were individually injected with DH82 (Panels a and b) were more compact and thicker for the fiber bundle. Besides, both groups were observed to have dose dependent histopathological changes on gaps between the muscle fibers, where a higher dose of bacteria challenge refers to larger gaps and more serious whitish symptoms. For the group with a mixed injection of 17SZ and DH82 (Panels e and f), the muscle fibers of challenged shrimp were tightly arranged with no histopathological changes observed, which indicated the inhibition of DH82 on the pathogenicity of 17SZ.
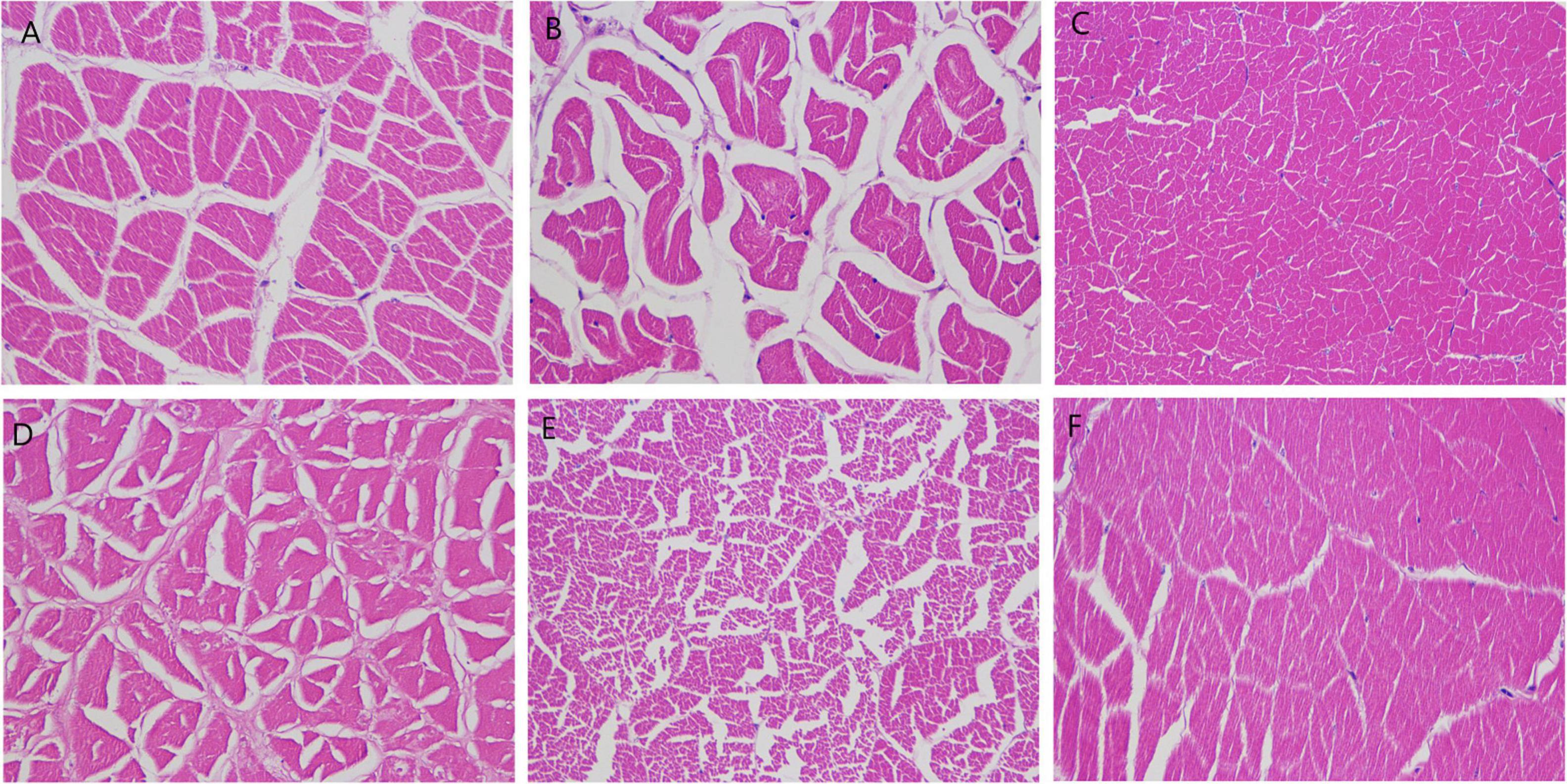
Figure 8. Pathological analysis of muscle tissue of shrimps challenged with Vibrio parahaemolyticus. The shrimps were injected at the muscle between the 5th and 6th uromere with 1 mL of 17SZ and DH82 and the same volume of the mixture (1:1) of 17SZ and DH82. The muscle sections were performed using H&E staining and imaged by brightfield microscope with magnification of 400 times. Panel (A) is a medium dose of DH82 (107 CFU/mL), panel (B) is a low dose of DH82 (106 CFU/mL); Panel (C) is a medium dose of 17SZ (107 CFU/mL), panel (D) is a low dose of 17SZ (106 CFU/mL); Panel (E) is the medium dose of the mixture of DH82 and 17SZ (1:1, 107 CFU/mL), panel (F) is a low dose of the mixture of DH82 and 17SZ (1:1, 107 CFU/mL).
Using microbial antagonism to inhibit pathogen infection, by controlling the biofilm formation and virulence factor expression through QS regulation (Kalia et al., 2019), had become a novel strategy of biocontrol to replace antibiotics for the prevention and control of aquatic pathogenic bacteria such as V. parahaemolyticus (Defoirdt, 2013). It is currently known that the pathogenicity of Vibrio sp. is regulated by three parallel QS pathways, LuxM/LuxN related AHLs, CqsA/CqsS related CAI-1, and LuxS/LuxP related AI-2, all lead to the regulation of core regulatory protein LuxO (Herzog et al., 2019), then to the synergistic action of key regulatory proteins AphA (Lu et al., 2018) and OpaR (Zhang et al., 2016), which both regulate downstream exopolysaccharide synthesis genes to affect biofilm formation and virulence relative genes to release virulence factors including hemolysin.
In this article, the in vitro assessment of QQ enzymes demonstrated that AHL-lactonases significantly down-regulated the expression of AphA, which is mainly regulated at an early stage of low cell density, and OpaR, which plays the leading role at a later stage of high cell density, and the virulence factor tlh (Figures 1A–C), which verified the primary function of AHL mediated QS pathway on the pathogenicity of V. parahaemolyticus, and was consistent to other reports (Bzdrenga et al., 2017; Torres et al., 2018). The performance of biofilm formation under the treatment of inhibition (by AHLs degradation) or induction (by additional exogenous AHLs), both demonstrated the direct relationship between biofilm forming and the AHL mediated QS pathway (Vinoj et al., 2014; Paluch et al., 2020), which could be intervened by free AHL-lactonases (Figure 1D) and DH82 cell pellets (Figure 2), and the phenomenon of non-significant difference also indicated the possibility that other two QS pathways (Henke and Bassler, 2004) might involve the regulation of biofilm formation of V. parahaemolyticus. Besides, the extracellular products, including anti-microbial peptides in the supernatant of the bacterial culture, also contributed to inhibition against V. parahaemolyticus either on the agar plate (Table 2) or in broth media under both planktonic and biofilm-status (Figure 2), which indicated the mechanism of DH82 on microbial antagonism, by using the above described dual functional products in DH82, QQ enzymes, and antibacterial peptides, to control the biomass accumulation and pathogenicity of Vibrio pathogens (Bai et al., 2008; Cai et al., 2019) with a synergistic effect.
Pathogenic Vibrio could exist as planktonic and biofilm status in an aquatic water system, and invade shrimps via gill to cause bacterial gill-rot disease (Shi et al., 2017) through contaminated feed and cause intestine infection (Zhou et al., 2016), or via wounds, it could cause whitish muscle disease (Sun et al., 2007). The environmental conditions such as high stocking density would motivate the agonistic behavior of shrimps (Yuchao et al., 2016), thus increasing the probability of death from wound infection, intestinal inflammation, and cross predation.
The in vivo study of bacterial challenge on shrimp simulated the two pathways of bacterial infection, respectively through the digestive tract and surface wounds. The findings indicate that the shrimps showed intestinal inflammation, cross contamination caused by predation, or decreased exercise ability caused by white muscle turbidity, and the vicious circle, which led to the rapid increase of mortality in the Vibrio infection groups and therefore make the survival rate uncountable in the experiment of bath challenge.
Since the trial experiment was completed in an open system, the newly pumped water would bring environmental microorganisms to the aquaria, and the bacteria attached sediment rolling up by typhoon attack would aggravate this problem. The results of bacterial counting from the water samples (Figure 3) did point out the phenomenon that the continuous addition of DH82 formed a dominant flora in the environment and effectively inhibited the Vibrios in aquaria (not just 17SZ but also other Vibrios from pump-in seawater), these findings were consistent with the data on the bacterial community of gut microbiota analyzed by high throughput sequencing (Figure 4).
Although Typhoon Mekkhala attacked the farming base in the first week of the experiment period after bacteria inoculation, the biochemical indexes of white shrimps under treatments all demonstrated the antibacterial and probiotic properties of DH82, in which the initial dietary DH82 promoted the growth of shrimps by increasing the ACP and AKP activities (Figures 5A,B), both of which were the growth factors of phosphoric acid metabolism, and further leads to weakening of shell-changing, which were regulated by ACP and AKP. However, the continuous delivery of DH82 reduced the ACP and AKP activities instead, the reason was unknown, yet based on the current experiment, the daily inoculation of DH82 with a dose of 105 CFU/mL consumed a lot of oxygen, which might cause additional stress to the shrimps and affect growth. Besides, DH82 enhanced the stress resistance ability of the host by increasing the SOD activity (Figure 5C), the additional DH82 also assists the host with antibacterial ability by increasing the LZM activity when challenged with pathogens and reducing the LZM activity when there was an absence of V. parahaemolyticus, which also verified the antibacterial activity of DH82 (Figure 5D).
Considering that the bacterial challenge might be synergistically affected by other potentially opportunistic pathogens, the subsequent injection challenge was designed to further confirm the direct effect of Vibrio via shrimp wound infection and the pathogenic inhibition of DH82 against 17SZ.
The physical signs of shrimps under bath challenge and injection challenge both supported evidence of the potential probiotic properties of DH82. Additional DH82 presented obvious rehabilitation on intestinal tissue against V. parahaemolyticus, compared with the damage caused by the pathogens in control groups (Figure 6), which indicated the consistent probiotic effects of DH82 on the digestion ability of the host (Vinoj et al., 2014); as for the whitish muscle disease caused by bacterial infection, the presence of DH82 remarkably raised the survival rate of shrimps (Figure 7), and reduced the damage of athletic ability from V. parahaemolyticus (Figure 8), which indicated the effective protection of DH82 as probiotics during shrimp growth, especially to prevent bacterial infection through wounds from fights or the shell-changing of shrimp during growth (Zhou et al., 2012).
Traditional studies on probiotic applications have investigated the immune enhancement of aquatic animals when probiotics are used as feed additives. This article was more focused on emergency treatment against Vibrio outbreak. The trial experiment of dietary DH82 on white shrimp farming verified the effective in vivo bacterial inhibition against V. parahaemolyticus, and also revealed the increasing survival rate of shrimp and the rehabilitation on infected intestine and muscle with additional DH82 when challenged with V. parahaemolyticus. The in vitro and in vivo investigation in this study both verified the QQ capacity and probiotic properties of DH82 and the functional products working along with both QQ and antibiotic against V. parahaemolyticus, and indicated potential application as biocontrol reagents. For example, they could be used for emergency treatment when the breaking out of pathogenic Vibrio, in sustainable aquaculture and green production (Interaminense et al., 2019; Lukwambe et al., 2019).
The in vitro and in vivo assessments undertaken in this study revealed that the DH82 strain could significantly reduce the biomass accumulation of V. parahaemolyticus 17SZ on an agar plate and in broth media, including the planktonic bacteria number and biofilm formation. It could inhibit the QS regulation of 17SZ to reduce the pathogenicity by down-regulating the expression level of primary regulator AhpA, OpaR, and the virulence factor of tlh. The delivery of dietary DH82 could enrich their abundance, richness, and quantity in both the aquatic system and the shrimp intestine, and also inhibit the biomass of Vibrio, thus reducing the damage to the non-specific immune system of ACP, AKP, and SOD activities, and assisting host to undertake antibacterial activity. This can reduce the damage to the intestine, infected muscles, and survival rate caused by infection of V. parahaemolyticus. The findings indicated the potential application of DH82 as a biocontrol reagent to prevent and undertake biological control of V. parahaemolyticus. This is a potentially effective strategy for enabling immune regulation in aquatic white shrimp, especially when there is an outbreak of pathogens, which might contribute to the sustainable and green production of aquaculture.
The data would be available on request.
The animal study was reviewed and approved by the institutional research Ethics Committee of Huaqiao University.
XS contributed to the conception of the study and drafted the manuscript. JL performed the experiments of quorum sensing inhibition. JS, RL, and WL performed the experiment of bacterial challenge and enzyme activities assessment. Y-ZS contributed to design the experiment of bacterial challenge. XT and SZ helped analysis with constructive discussions. MK and KW provided the bacterial strain. XS, MK, KW, and Y-ZS contibuted to the review and correction in the revision. XS offered the funding acquisition. All authors reviewed the results and approved the final version of the manuscript.
This project was sponsored by the Fujian Provincial Department of Science and Technology (2021N0012), the Fund of the Technology Innovation Center for Exploitation of Marine Biological Resources, MNR (TICMBR202103), the Start-up research funding from Huaqiao University (605-50Y19014), and the cooperative research project between Huaqiao University and Fujian Huisheng Biotech Ltd. (605-54319210). We would also like to thank funding support from the National Innovation and Entrepreneurship Training Program for College Students.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank CDC Siming District, Xiamen, China for providing the bacterial isolates of Vibrio parahaemolyticus 17SZ for this study.
Bai, F., Han, Y., Chen, J., and Zhang, X. H. (2008). Disruption of quorum sensing in Vibrio harveyi by the AiiA protein of Bacillus thuringiensis. Aquaculture 274, 36–40. doi: 10.1016/j.aquaculture.2007.11.024
Baker-Austin, C., Oliver, J. D., Alam, M., Ali, A., Waldor, M. K., Qadri, F., et al. (2018). Vibrio spp. infections. Nat. Rev. Dis. Prim. 5:8.
Bareia, T., Pollak, S., and Eldar, A. (2018). Self-sensing in Bacillus subtilis quorum-sensing systems. Nat. Microbiol. 3, 83–89. doi: 10.1038/s41564-017-0044-z
Bzdrenga, J., Daudé, D., Rémy, B., Jacquet, P., Plener, L., and Elias, M. (2017). Biotechnological applications of quorum quenching enzymes. Chem. Biol. Interact. 267, 104–115. doi: 10.1016/j.cbi.2016.05.028
Cai, Y., Yuan, W., Wang, S., Guo, W., Li, A., Wu, Y., et al. (2019). In vitro screening of putative probiotics and their dual beneficial effects: to white shrimp (Litopenaeus vannamei) postlarvae and to the rearing water. Aquaculture 498, 61–71. doi: 10.1016/j.aquaculture.2018.08.024
Chang, S. C., and Lee, C. Y. (2018). OpaR and RpoS are positive regulators of a virulence factor PrtA in Vibrio parahaemolyticus. Microbiology 164, 221–231. doi: 10.1099/mic.0.000591
Chen, S., Zhou, Y., Chen, Y., and Gu, J. (2018). Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890. doi: 10.1093/bioinformatics/bty560
Defoirdt, T. (2013). Antivirulence therapy for animal production: filling an arsenal with novel weapons for sustainable disease control. PLoS Pathog. 9:e1003603. doi: 10.1371/journal.ppat.1003603
Dong, Y., Xu, J., Li, X., and Zhang, L. (2000). AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. PNAS 97, 3526–3531. doi: 10.1073/pnas.97.7.3526
Edgar, R. C. (2013). UPARSE:Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998. doi: 10.1038/nmeth.2604
Elmahdi, S., Dasilva, L. V., and Parveen, S. (2016). Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: a review. Food Microbiol. 57, 128–134. doi: 10.1016/j.fm.2016.02.008
Fuente, M., De Miranda, C. D., Jopia, P., and González-rocha, G. (2015). Growth inhibition of bacterial fish pathogens and quorum-sensing blocking by bacteria recovered from chilean salmonid farms. J. Aquat. Anim. Health 27, 112–122. doi: 10.1080/08997659.2014.1001534
Ganguly, S., and Mukhopadhayay, S. K. (2010). Immunostimulant, probiotic and prebiotic- their applications and effectiveness in aquaculture: a Review. Isr. J. Aquac. 66, 130–138.
Henke, J. M., and Bassler, B. L. (2004). Three parallel quorum-sensing systems regulate gene expression in Vibrio harveyi. J. Bacteriol. 186, 6902–6914. doi: 10.1128/JB.186.20.6902-6914.2004
Herzog, R., Peschek, N., Fröhlich, K. S., Schumacher, K., and Papenfort, K. (2019). Three autoinducer molecules act in concert to control virulence gene expression in Vibrio cholerae. Nucleic Acids Res. 47, 3171–3183. doi: 10.1093/nar/gky1320
Interaminense, J. A., Vogeley, J. L., Gouveia, C. K., Portela, R. S., Oliveira, J. P., and Silva, S. M. B. C. (2019). Effects of dietary Bacillus subtilis and Shewanella algae in expression profile of immune-related genes from hemolymph of Litopenaeus vannamei challenged with Vibrio parahaemolyticus. Fish Shellfish Immunol. 86, 253–259. doi: 10.1016/j.fsi.2018.11.051
Jiang, C., Liao, M., Wang, H., Zheng, M., and Xu, J. (2018). Bacillus velezensis, a potential and efficient biocontrol agent in control of pepper gray mold caused by Botrytis cinerea. Biol. Control 126, 147–157. doi: 10.1016/j.biocontrol.2018.07.017
Jin, P., Li, S., Sun, L., Lv, C., and Ma, F. (2017). Transcriptome-wide analysis of microRNAs in Branchiostoma belcheri upon Vibrio parahemolyticus infection. Dev. Comp. Immunol. 74, 243–252. doi: 10.1016/j.dci.2017.05.002
Kalia, V. C., Patel, S. K. S., Kang, Y. C., and Lee, J. (2019). Quorum sensing inhibitors as antipathogens: biotechnological applications. Biotechnol. Adv. 37, 68–90. doi: 10.1016/j.biotechadv.2018.11.006
Liu, J., Sun, X., Ma, Y., Zhang, J., and Xu, C. (2020). Quorum quenching mediated bacteria interruption as a probable strategy for drinking water treatment against bacterial pollution. Int. J. Environ. Res. Public Health 17:9539. doi: 10.3390/ijerph17249539
Long, L., Zhang, H., Ni, Q., Liu, H., Wu, F., and Wang, X. (2019). Effects of stocking density on growth, stress, and immune responses of juvenile Chinese sturgeon (Acipenser sinensis) in a recirculating aquaculture system. Comp. Biochem. Physiol. Part C 219, 25–34. doi: 10.1016/j.cbpc.2019.02.002
Lu, R., Osei-Adjei, G., Huang, X., and Zhang, Y. (2018). Role and regulation of the orphan AphA protein of quorum sensing in pathogenic Vibrios. Future Microbiol. 13, 383–391. doi: 10.2217/fmb-2017-0165
Lukwambe, B., Nicholaus, R., Zhang, D., Yang, W., Zhu, J., and Zheng, Z. (2019). Successional changes of microalgae community in response to commercial probiotics in the intensive shrimp (Litopenaeus vannamei Boone) culture systems. Aquaculture 511:734257. doi: 10.1016/j.aquaculture.2019.734257
Magoč, T., and Salzberg, S. L. F. L. A. S. H. (2011). Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963. doi: 10.1093/bioinformatics/btr507
Niu, B., Hong, B., Zhang, Z., Mu, L., Malakar, P. K., and Liu, H. (2018). A novel qPCR method for simultaneous detection and quantification of viable pathogenic and non-pathogenic Vibrio parahaemolyticus (tlh+, tdh+, and ureR+). Front. Microbiol. 9:1747. doi: 10.3389/fmicb.2018.01747
Olmos Soto, J. (2021). Feed intake improvement, gut microbiota modulation and pathogens control by using Bacillus species in shrimp aquaculture. World J. Microbiol. Biotechnol. 37:28. doi: 10.1007/s11274-020-02987-z
Ottaviani, D., Pieralisi, S., Chierichetti, S., Rocchegiani, E., Hattab, J., and Mosca, F. (2020). Vibrio parahaemolyticus control in mussels by a Halobacteriovorax isolated from the Adriatic sea, Italy. Food Microbiol. 92:103600. doi: 10.1016/j.fm.2020.103600
Paluch, E., Rewak-Soroczyńska, J., Jędrusik, I., Mazurkiewicz, E., and Jermakow, K. (2020). Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 104, 1871–1881. doi: 10.1007/s00253-020-10349-w
Shi, G., Zhao, C., Fu, M., and Qiu, L. (2017). The immune response of the C-Jun in the black tiger shrimp (Penaeus monodon) after bacterial infection. Fish Shellfish Immunol. 61, 181–186. doi: 10.1016/j.fsi.2016.12.025
Sun, B., Zhang, X. H., Tang, X., Wang, S., Zhong, Y., Chen, J., et al. (2007). A single residue change in Vibrio harveyi Hemolysin results in the loss of phospholipase and hemolytic activities and pathogenicity for turbot (Scophthalmus maximus). J. Bacteriol. 189, 2575–2579. doi: 10.1128/JB.01650-06
Sun, X., Hill, P., Liu, J., Qian, J., Ma, Y., and Zhou, S. (2021). Marine-source quorum quenching enzyme YtnP to improve hygiene quality in dental units. Mar. Drugs 19:225. doi: 10.3390/md19040225
Torres, M., Reina, J. C., Fuentes-Monteverde, J. C., Fernández, G., Rodríguez, J., and Jiménez, C. (2018). AHL-lactonase expression in three marine emerging pathogenic Vibrio spp. reduces virulence and mortality in brine shrimp (Artemia salina) and Manila clam (Venerupis philippinarum). PLoS One 13:e0195176. doi: 10.1371/journal.pone.0195176
Vinoj, G., Vaseeharan, B., Thomas, S., Spiers, A. J., and Shanthi, S. (2014). Quorum-Quenching activity of the AHL-Lactonase from Bacillus licheniformis DAHB1 inhibits Vibrio biofilm formation In Vitro and reduces shrimp intestinal colonisation and mortality. Mar. Biotechnol. 16, 707–715. doi: 10.1007/s10126-014-9585-9
Wang, C., Liu, Y., Sun, G., Li, X., and Liu, Z. (2019). Growth, immune response, antioxidant capability, and disease resistance of juvenile Atlantic salmon (Salmo salar L.) fed Bacillus velezensis V4 and Rhodotorula mucilaginosa compound. Aquaculture 500, 65–74. doi: 10.1016/j.aquaculture.2018.09.052
Wang, Q. H., Sun, X. H., Tang, X., Wan, J. L., and Xu, C. A. (2019). Screening and identification of Bacillus velezensis strain DH82 and the characterization of the crude antimicrobial protein. Mar. Sci. Bull. 38, 63–69.
Wang, Q., Garrity, G. M., Tiedje, J. M., and Cole, J. R. (2007). Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267. doi: 10.1128/AEM.00062-07
Wang, Q. H., Sun, X. H., Tang, X., Wan, J. L., and Xu, C. A. (2020). Purification of antimicrobial substance produced by deep sea Bacillus velezensis strain DH82 and its inhibition spectrum. J. Appl. Oceanogr. 39, 20–26.
Wu, Q., Zhi, Y., and Xu, Y. (2019). Systematically engineering the biosynthesis of a green biosurfactant surfactin by Bacillus subtilis 168. Metab. Eng. 52, 87–97. doi: 10.1016/j.ymben.2018.11.004
Yuchao, Z., Hao, Q., Yuquan, L., and Qingyin, W. (2016). Effects of stocking density and food types on growth and agonistic behavior in Pacific white leg shrimp Litopenaeus vannamei. Chin. J. Fish. 29, 44–48.
Zhang, Y., Zhang, L., Hou, S., Huang, X., Sun, F., and Gao, H. (2016). The master quorum-sensing regulator OpaR is activated indirectly by H-NS in Vibrio parahaemolyticus. Curr. Microbiol. 73, 71–76. doi: 10.1007/s00284-016-1018-8
Zhao, Y., Li, H., Zhang, Z., Liu, H., and Pan, Y. (2018). Progress in studying antimicrobial resistance of foodborne pathogenic bacteria. Chin. J. Bioprocess Eng. 16, 1–10.
Zhou, J., Fang, W., Yang, X., Zhou, S., Hu, L., and Li, X. (2012). A nonluminescent and highly virulent Vibrio harveyi strain is associated with ‘bacterial white tail disease’ of Litopenaeus vannamei shrimp. PLoS One 7:e29961. doi: 10.1371/journal.pone.0029961
Keywords: Bacillus velezensis, microbial antagonism, biological control, sustainable aquaculture, Litopenaeus vannamei, Vibrio pathogens, quorum quenching (QQ)
Citation: Sun X, Liu J, Deng S, Li R, Lv W, Zhou S, Tang X, Sun Y-z, Ke M and Wang K (2022) Quorum Quenching Bacteria Bacillus velezensis DH82 on Biological Control of Vibrio parahaemolyticus for Sustainable Aquaculture of Litopenaeus vannamei. Front. Mar. Sci. 9:780055. doi: 10.3389/fmars.2022.780055
Received: 20 September 2021; Accepted: 21 February 2022;
Published: 11 April 2022.
Edited by:
Jinbo Xiong, Ningbo University, ChinaReviewed by:
Graciela Dias, Federal University of Rio de Janeiro, BrazilCopyright © 2022 Sun, Liu, Deng, Li, Lv, Zhou, Tang, Sun, Ke and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohui Sun, c3VueGlhb2h1aUBocXUuZWR1LmNu; Xu Tang, dGFuZ3h1QHRpby5vcmcuY24=; Yun-zhang Sun, am11c3VueXVuemhhbmdAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.