- 1College of Science and Engineering, James Cook University, Townsville, QLD, Australia
- 2Center for Sustainable Tropical Fisheries and Aquaculture, College of Science and Engineering, James Cook University, Townsville, QLD, Australia
- 3Australian Research Council Center of Excellence for Coral Reef Studies, James Cook University, Townsville, QLD, Australia
Marine mammal interactions with fisheries, such as bycatch and depredation, are a common occurrence across commercial and small-scale fisheries. We conducted a systematic review to assess the management responses to marine mammal interactions with fisheries. We analyzed literature between 1995 and 2021 to measure research trends in studies on direct and indirect interactions for: (i) high and low to middle-income countries, (ii) fishery operations (commercial and small-scale), and (iii) taxonomic groups. Management responses were categorized using the framework described previously in peer-reviewed studies. Marine mammal bycatch remains a major conservation concern, followed by marine mammal depredation of fishing gear. A high proportion of studies concentrated on commercial fisheries in high-income countries, with an increase in small-scale fisheries in low to middle-income countries between 1999 and 2020. The insufficient understanding of the social dimensions of interactions and the inevitable uncertainties concerning animal and human behaviors are major challenges to effective management. Despite the key role of human behavior and socioeconomics, we found only eight articles that incorporate human dimensions in the management context. Integrating social dimensions of marine mammal interactions with fisheries could help in setting pragmatic conservation priorities based on enhanced understanding of critical knowledge gaps. An area-specific adaptive management framework could be an effective tool in reducing the risk to marine mammals from fisheries by coupling technical solutions with socio-economic and political interventions. We conclude that despite the vast body of literature on this subject, a “silver bullet” management solution to marine mammal interactions with fisheries does not yet exist.
Introduction
As a result of resource overlap, interactions between marine mammals and fisheries are a common occurrence (Trites et al., 1997; Liu et al., 2019). A wide spectrum of these interactions has been documented, from the mutually beneficial to the detrimental. Marine mammals have taken advantage of human activities, particularly, fishing, for the nutritional gain (Bearzi, 2002; Allen et al., 2014). In several parts of the world, “co-operative” or “associative” fishing between marine mammal species and fishing communities has led to mutually beneficial interactions, in that both the fishermen and the animals find easy target and forage fish, respectively (Northridge and Hofman, 1999; Neil, 2002; Peterson et al., 2008; Kumar et al., 2012; D’Lima et al., 2014). On the other end of the spectrum, interactions such as depredation and bycatch negatively affect and alter marine social-ecological systems (Tixier et al., 2021; Box 1). Depredation affects both marine mammal populations and fishery socioeconomics due to gear damage and catch loss, by creating artificial resource provisioning due to the introduction of novel prey resources, leading to higher chances of incidental entanglements (Werner et al., 2015). Bycatch is a major threat to the conservation and recovery of marine mammal population(s) worldwide (Avila et al., 2018) leading to the declines of a wide range of species such as dugongs (Dugong dugon) (Marsh et al., 2011), monk seals (Monachus monachus) (Woodley and Lavigne, 1991; Guüçluüsoy et al., 2004), and Hector’s dolphins (Cephalorhyncus hectori) (Slooten and Dawson, 2010), amongst others. The recent “human caused” extinction of the Baiji (Lipotes vexillifer) (Turvey et al., 2007), and the expected extinction of the vaquita (Phocaena sinus) (Rojas-Bracho et al., 2006; Jaramillo-Legorreta et al., 2007; Morzaria-Luna et al., 2012) have further added to the growing concerns of the impact of fisheries on marine mammals.
Box 1. Marine mammal interactions with fisheries: Categories and definitions of different types of interactions with commercial and small-scale fisheries.
Based on their effects on marine mammal species and fishery socioeconomics, the interactions between fishing operations and marine mammals have been categorized as “direct” or “indirect” (adapted from Beverton, 1985; Hall, 1996; DeMaster et al., 2001; Read, 2008).
Direct interactions occur when marine mammals come into direct or close contact with fishing gear (Read, 2008). These interactions include bycatch, accidental entanglements in fishing gear and depredation (Silva et al., 2002; Read et al., 2006; Gerrodette, 2009).
Bycatch is defined as the unintended capture of marine biota in fishing gear during an operation targeting a different species (Gray and Kennelly, 2018). Bycatch in fishing gear is a persistent threat for many marine mammal species (Read, 2008).
Depredation is also a direct interaction where marine mammals remove or damage fish (Tixier et al., 2019), leading to catch loss and gear damage for fishers. Depredation is recorded for several coastal and offshore odontocete species, over a range of fishery operations (Bearzi et al., 2019), for example, gillnet depredation by bottlenose dolphins (Tursiops truncatus) (Ayadi et al., 2013; Rechimont et al., 2018) and that of longline gear by sperm whales (Physeter macrocephalus) and killer whales (Orcinus orca) (Werner et al., 2015; Towers et al., 2019).
Indirect interactions arise due to fishery-induced ecological changes and resource competitions (i.e., habitat and prey overlap between fisheries and marine mammals) (Plagányi and Butterworth, 2009). Over the past five decades, marine fishery operations have advanced technologically, and fishery production has increased as a result. These changes have resulted either in gross overfishing or local reductions in the biomass of the target species that marine mammals depend on for prey (DeMaster et al., 2001), therefore influencing species compositions of marine communities, particularly top predators like marine mammals. For example, reduced prey availability due to overfishing has led to local declines in striped dolphin (Stenella coeruleoalba) population(s) in the Mediterranean Sea (Aguilar, 2000) and harbor seal (Phoca vitulina) population(s) in the Aleutian Islands (Plagányi and Butterworth, 2009).
The large-scale capture of pantropical spotted (Stenella attenuata), spinner (S. longirostris) and common (Delphinus delphis) dolphins (Gosliner, 1999; Wade et al., 2007) in the Easter Tropical Pacific (ETP) tuna fishery was instrumental in the recognition of bycatch as a global threat to marine mammal populations and the subsequent development and implementation of the United States Marine Mammal Protection Act (MMPA) in 1972 (Gerrodette, 2009). Since then, large-scale commercial operations using gear such as gillnets, purse-seines, hook and line, and trawlers have been extensively reviewed to develop management strategies to reduce their impacts on marine mammals (Gilman et al., 2007; Tixier et al., 2021). Several aspects of these interactions, particularly, marine mammal entanglement in fisheries have been widely studied (Read et al., 2006; Moore et al., 2010; Reeves et al., 2013), especially: (i) well-documented bycatch in gillnets, trawls, longlines, purse-seines, pots and traps, etc.; (ii) poorly documented bycatch in artisanal or small-scale fisheries in developing countries, and (iii) the transition of incidental marine mammal catch in fishing gear from being discarded to gaining market value (Read, 2008). This increased understanding has provided the impetus for the development and implementation of marine mammal population recovery and maintenance measures (Perrin et al., 1994).
Despite this documentation, the management and mitigation of these interactions is a pressing concern (Anderson et al., 2020; Hines et al., 2020). The k-selected life-history traits of marine mammals: long-life spans coupled with relatively late sexual maturity and low reproductive rates, severely limit conservation efforts (Brown et al., 2014; Mannocci et al., 2014).
In their review of a decade of bycatch management in commercial fisheries, Dawson et al. (2013) categorized management measures into three main technical approaches to increase the understanding of their efficacy in attaining management goals: (i) strategies that change human behavior, either mandated or voluntary: e.g., spatial or place-based management, monetary incentives, etc.; (ii) strategies that change the nature of interactions: e.g., technological interventions like bycatch reduction devices in trawl fisheries, and gear modifications such as changes in hook design in longline fisheries; and (iii) strategies that change animal behavior: e.g., acoustic deterrent devices, acoustic harassment devices. These management strategies were developed and evaluated in the context of commercial fishery operations. The high economic and social costs of management, such as, heavy reliance on expensive technology, marginalization of fishing communities, inadequate governance and enforcement, limit their wider implementation in small-scale fisheries (Brotons et al., 2008; Mangel et al., 2013; Brownell et al., 2019) but these aspects were not explicitly considered in the Dawson et al. (2013) typology.
An alternative multidisciplinary framework for bycatch mitigation has recently been proposed by Squires et al. (2021). This “Bycatch mitigation hierarchy framework” offers a systematic order of actions to manage and mitigate bycatch that considers the human elements of bycatch reduction. This framework comprises four basic approaches: (1) private solutions, including voluntary, moral suasion, and intrinsic motivation; (2) direct or “command-and-control” regulation starting from the fishery management authority down to the vessel; (3) incentive- or market-based approach to alter producer and consumer behavior and decision-making; and (4) a hybrid of direct and incentive-based regulation through liability laws.
Managing marine mammal interactions with commercial and small-scale fisheries requires deep understanding of their social, economic, and cultural linkages to fishers’ livelihoods (Carvalho et al., 2011). However, the current definitions of fishery operations are relatively simplistic in the context of management (Box 2). For small-scale fisheries in particular, these definitions are nuanced, and vary spatially (Teh and Pauly, 2018). Therefore, finding an appropriate balance between the ecological and human dimensions of marine mammal interactions with fisheries is a major challenge (D’Lima et al., 2014).
Box 2. Definitions of fishery operations based on scale, and social and economic factors.
Fisheries are classified as “small” or “commercial” (also industrial) based on their overall size, presumed technological differences, capital investment and market, areas of operations, and production output (Béné, 2006; Pauly, 2009).
Commercial fisheries include industrial or large-scale operations with substantial technological and capital investments. These operations generally supply fish to international as well as domestic markets and use specific types of fishing gear, for example, trawlers, longlines, gillnets (drift, bottom-set), and purse seines [Food and Agriculture Organization, 2001–2016, 2001-2019]. As a result of their high profit margins and international supply chains, commercial fishery statistics are available in national and international reports, particularly the data on catch characteristics, fishing activities and demography (Christensen and Pauly, 2004; Pauly, 2006).
Small-scale fisheries employ about 90% of the human population in low-income countries and provide food security to over 45% of the human population worldwide (Chuenpagdee et al., 2006; Batista et al., 2014; Zeller et al., 2015; Fisher et al., 2018). Based on their market volumes, small-scale fisheries are classified as commercial or non-commercial (also subsistence) small-scale fisheries (Gillett et al., 2001). Commercial small-scale fisheries are those that cater to local or regional markets, whereas non-commercial or subsistence fisheries provide food security locally and to marginalized socio-economic classes (Pauly, 1997; Zeller et al., 2015). Small-scale fisheries are highly diverse, in that they target multiple species using a variety of gears. This diversity severely limits a comprehensive accounting of these operations in national policies (Gillett and Lightfoot, 2001).
Despite the acknowledgment of these challenges, the long-term efficacy of management measures implemented so far, is seldom considered by stakeholders in the overall conservation management process. This ambiguity leads to a general lack of focus and consensus on the critical role of place-based management measures to mitigate these interactions. The overall aim of this review, therefore, is to understand the scope of research on marine mammal interactions with fisheries and discuss the implications of the management measures aimed at reducing the impacts of these interactions, particularly in the context of small-scale fisheries.
Materials and Methods
We used two bibliographic databases, SCOPUS, and Web of Science to identify a range of peer-reviewed articles, published and unpublished reports, and conference papers related to primary research on marine mammal interactions with fisheries. We did not use Google Scholar as it is not recommended for systematic or scoping reviews due to its unreliability in identifying articles specific to the keywords (Haddaway et al., 2015).
After initial deletions based on duplicates and relevance to the search terms, the list was reduced to 784 articles. Of these, 489 articles were excluded by screening the titles and abstracts for relevance to key words (Supplementary Material). Further stringent inclusion and exclusion criteria were applied to the remaining articles, based on the overall aims of the review, i.e., to understand the overall research trends in marine mammal interactions with fisheries. Review articles, fisheries management per se, marine mammal behavior and ecology studies, that were not directly related to interactions, were excluded from the review.
Relevant additional articles and gray literature (e.g., reports, conference, and meeting proceedings, etc.) were mined through the available reference lists of articles and were analyzed based on the above criteria. Limited or poor bibliographic information, however, is a major challenge in the accessibility of gray literature. Several reports and proceedings were inaccessible using search engines like Google Scholar, Elsevier, Scopus, etc., or through university libraries.
Thematic analyses were conducted on the final list of manuscripts (271 articles: published articles 263, gray literature: 8 documents) to understand the general trends in research, based on the year of publication, region where research was conducted, type of fishery operation (commercial or small-scale fishery, Box 1), and taxonomic group (cetacean, pinniped, sirenian, etc.). The literature was then segregated into two main emergent themes related to the interactions between marine mammals and fisheries (Box 2): (A) Direct interactions: mainly, (i) bycatch, and (ii) depredation, and (B) Indirect interactions: (i) trophic interactions, to compare trends over time across fishery operations and, to understand which of these interactions are the most reported in the literature.
Further, management strategies and the challenges faced to mitigate marine mammal interactions with fisheries were collated and discussed in accordance with the technical categories described by Dawson et al. (2013) (see Introduction) because we considered these categories appropriate for the current literature.
There are two limitations to our study: (1) the literature reviewed is a non-random sample from the available literature driven by the key-words we used in our search; (2) our analyses are based on the results described in this literature and are not a statistical comparison between experimental or control studies.
Results
We first document the global trends in research on marine mammal interactions across commercial and small-scale fisheries, and various fishing gears. We then present the thematic analyses of research on direct and indirect interactions, followed by a discussion of the observed management responses and challenges across fishery operations and interactions.
Global Research Trends Across Commercial and Small-Scale Fisheries
There was a high degree of consistency (90%) between the bibliographic databases, SCOPUS and Web of Science. In the final analyses (271 articles), research was mainly focused on marine mammal interactions with commercial fisheries particularly in high income countries (108 articles). This trend is more pronounced in the early 1990’s, in high-income countries such as United States, New Zealand, Great Britain, Ireland, Spain, France, Greece, and Australia. Studies on commercial fisheries in low-middle income countries were not so common (15 articles). The proportion of studies on small-scale fisheries in low to middle-income countries has increased since the mid-2000’s (124 articles: high-income countries: 25, low to middle-income countries: 99) (Figures 1, 2). Cetaceans remain the prime focus of research followed by pinnipeds and sirenians (263 articles where taxa were recorded: 189 cetaceans, 66 pinnipeds, 8 sirenians, 8 marine megafauna, 14 marine mammals in general) (Figure 3).
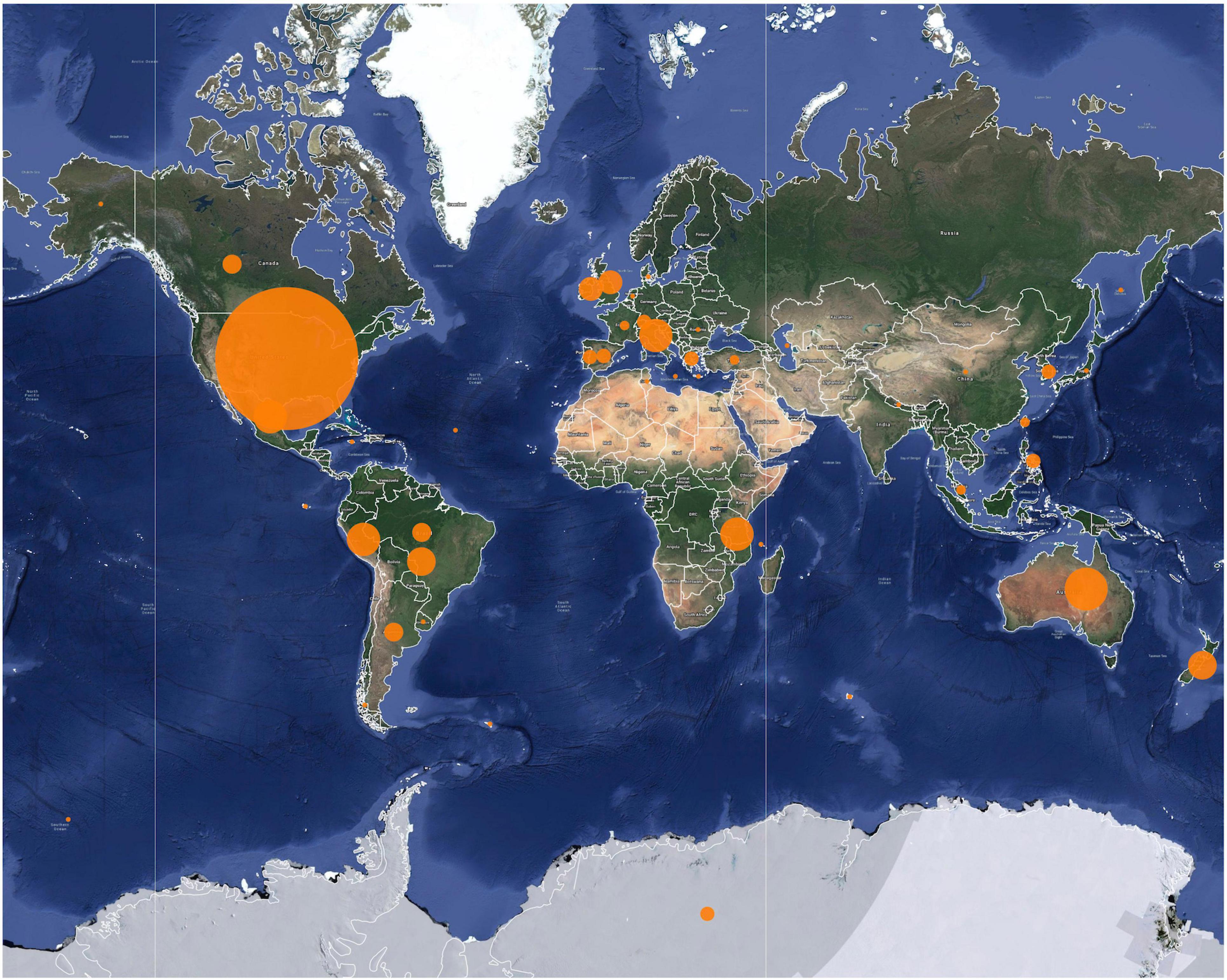
Figure 1. Geographic distribution of the publications reviewed in this study. The size of the orange circles across the map depicts the number of publications in the given field site where studies were conducted, and/or from regions from where data for those studies originated.
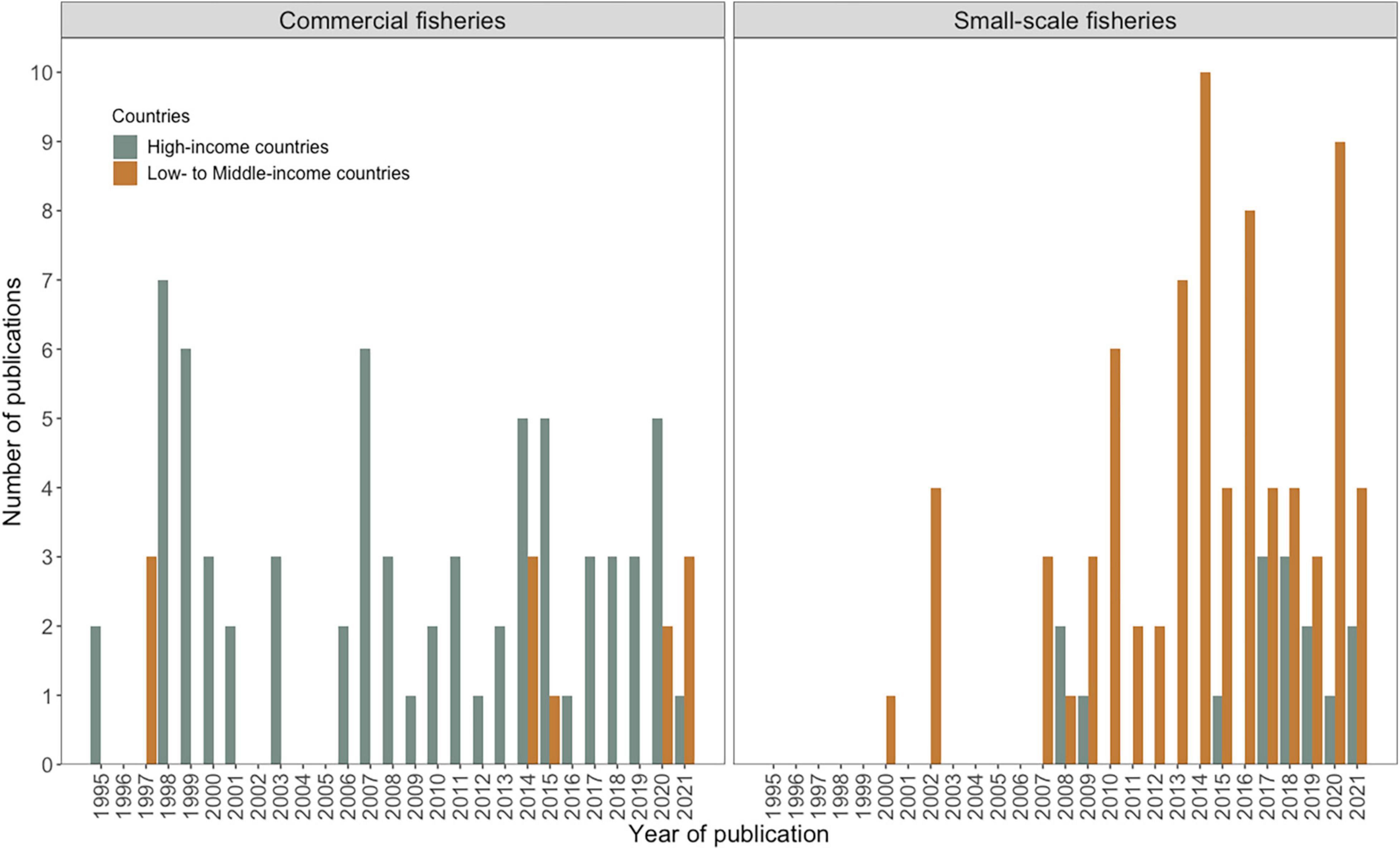
Figure 2. Trends in number of published studies over time (1995–2020) across commercial and small-scale fishery operations in high-income and low to middle income countries. These data are segregated by commercial and small-scale fishery operations, gray signifying high income countries and orange, low to middle income countries.
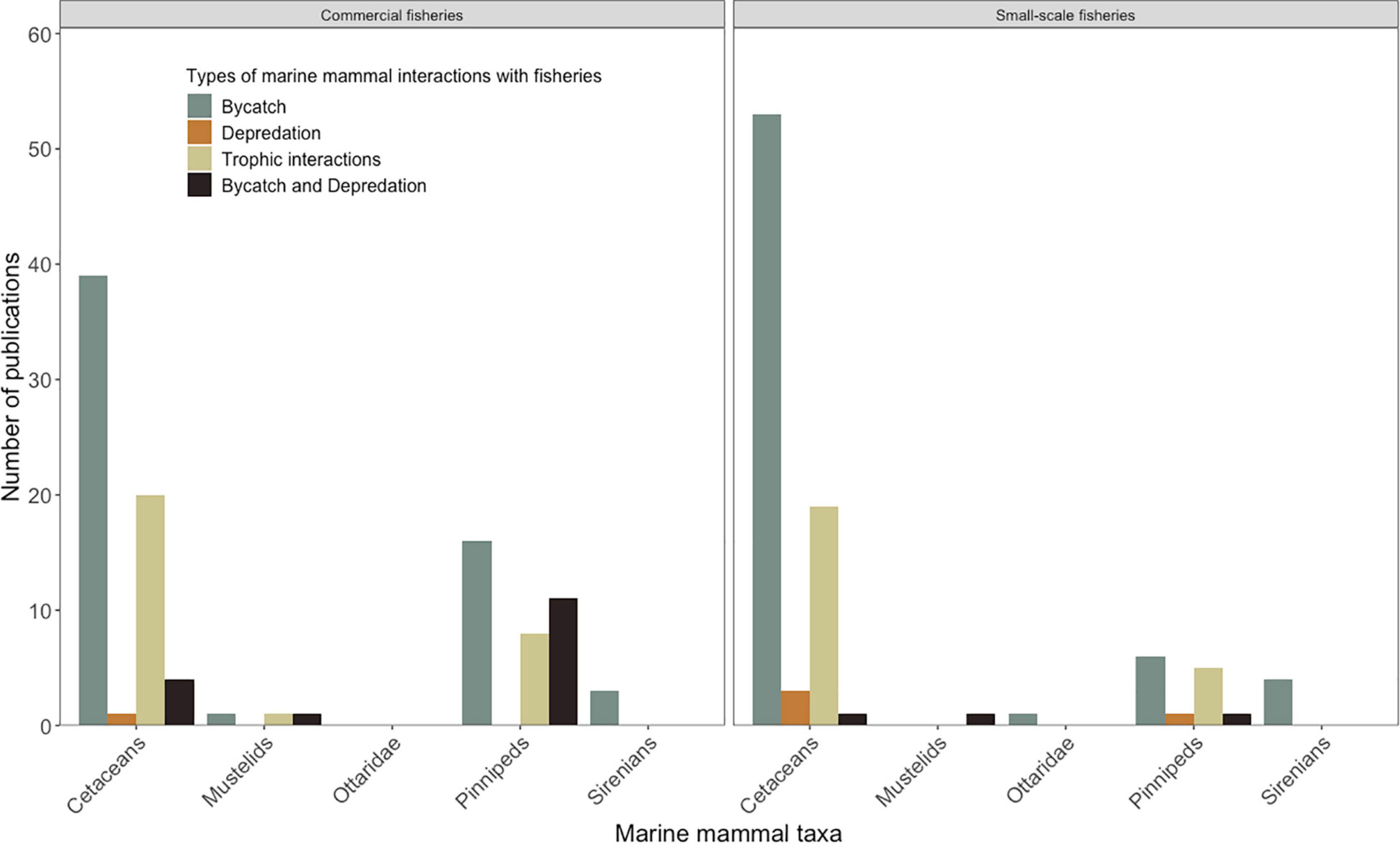
Figure 3. Trends in number of published studies of marine mammal interactions with fisheries across marine mammal taxa (cetaceans, mustelids, pinnipeds and sirenians) across commercial and small-scale fisheries, and, bycatch, depredation, and trophic interactions).
Research Trends Across Fishing Gears
Marine mammal interactions with fisheries also vary across gear types in both commercial and small-scale fisheries. For both commercial and small-scale fisheries, marine mammal interactions were the reported most commonly in gillnets (27 and 79 published studies respectively). These variations are further described for each interaction type below in section “Thematic Analyses of Marine Mammal Interactions With Fisheries.”
Thematic Analyses of Marine Mammal Interactions With Fisheries
These analyses reflect the number of studies and trends in these studies across both commercial and small-scale fisheries. Bycatch remains the main area of research, with 187 studies, followed by depredation with 56 studies. Trophic interactions between marine mammal and fisheries have attracted research attention since the late 2000’s, particularly, studies on prey consumption by marine mammals and their dietary overlaps with fisheries, as an aid to understand the potential risks from fisheries to mammal population(s) and vice versa (22 studies) (Figure 4). These themes are described in detail below.
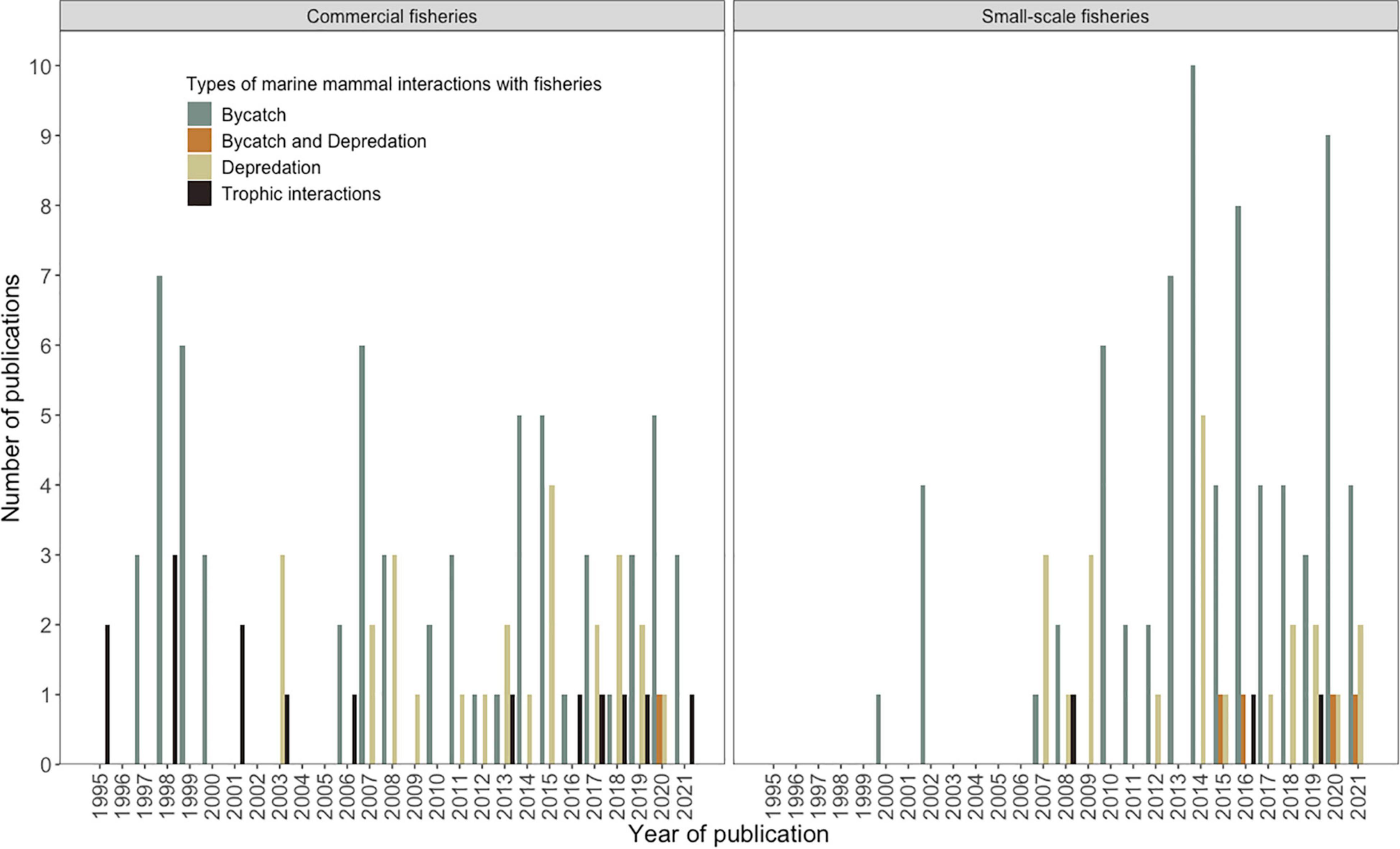
Figure 4. Trends over time (1995–2020) in number of published studies on bycatch, depredation, and trophic interactions, across commercial and small-scale fisheries.
Direct Interactions: Bycatch
Between 1997 and 2021, 164 publications documented the scale of marine mammal bycatch, including fishery and gear-specific bycatch estimates (Julian and Beeson, 1998; Allen, 2000; D’Agrosa et al., 2000; Ortega-Argueta et al., 2005). Marine mammal bycatch is spatially variable, with larger bycatch estimates coinciding with areas of higher marine mammals or fish (prey species) abundance (Lewison et al., 2004, 2014; de Godoy et al., 2020; Baird et al., 2021), reflecting the increasing resource overlap between marine mammals and fishery operations. In many regions, spatial, temporal, and oceanographic factors affect bycatch. For example, management area and season were not associated with bycatch in Australian trawl fisheries (Hamer et al., 2012; Allen et al., 2014), whereas, in the gillnet fisheries of Peru, bycatch was higher in certain geographic locations, but was not associated with seasons (Majluf et al., 2002; Mangel et al., 2010; Ayala et al., 2019). Bycatch estimates also vary across different gear types. Bycatch estimates for gillnets are the highest in both commercial and small-scale fisheries (29 and 63 published studies, respectively), compared with other gear types such as longlines, trawlers, and purse seines (Figure 5).
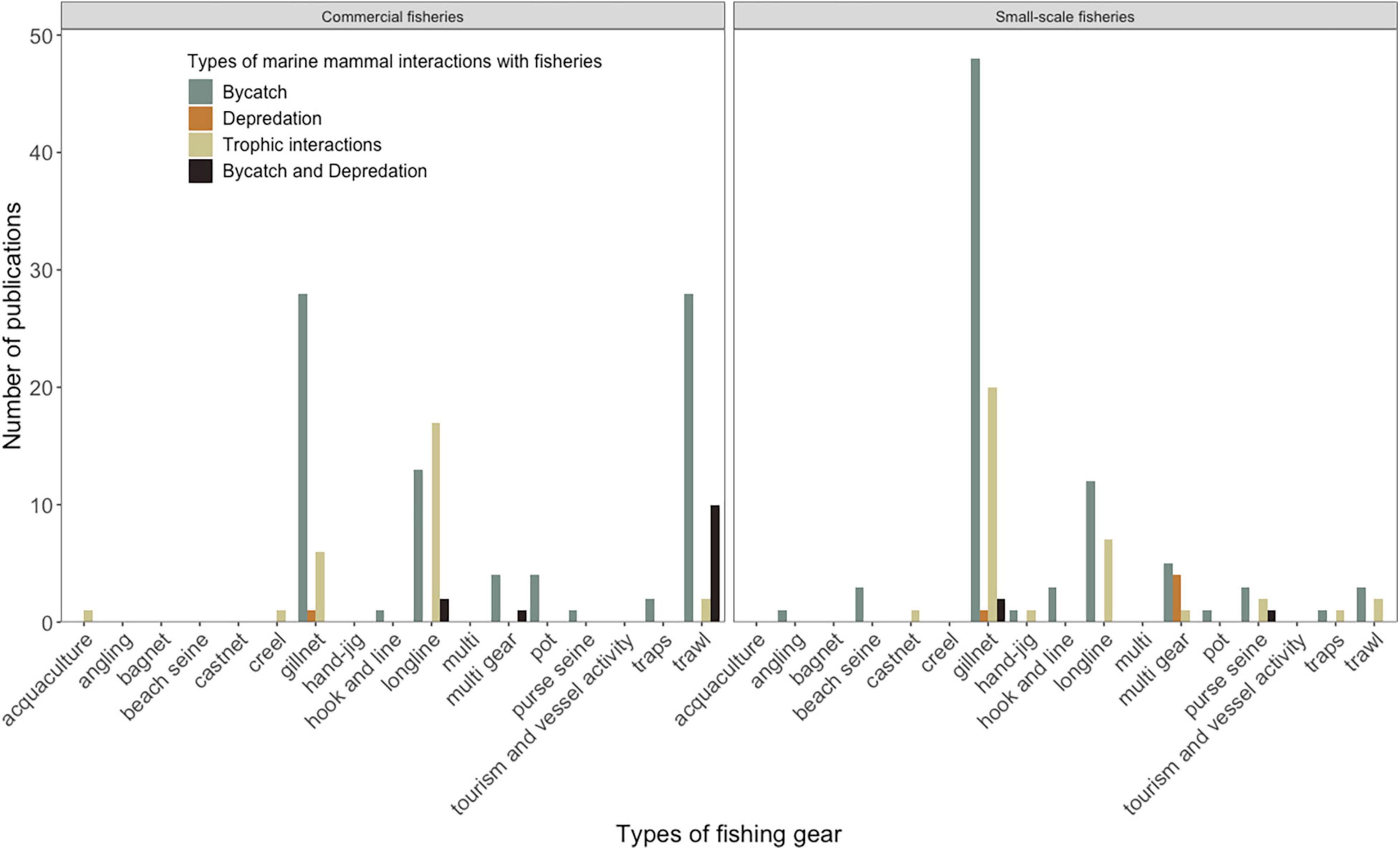
Figure 5. Trends in the number of published articles on marine mammal interactions with fisheries (Bycatch, Depredation and Trophic interactions), across commercial and small-scale fishing gears.
Direct Interactions: Depredation
Fifty-six studies examined depredation, in both commercial (28 studies) and small-scale fisheries (28 studies). The impacts of depredation vary across fisheries and marine mammal populations. For some gear, depredation commonly leads to both gear damage and catch loss, in other gears, economic losses to fisheries are uncommon. For example, for commercial longline fisheries, significant reduction in catch rates occurred due to sperm whale and killer whale depredation (Tixier et al., 2017). In areas where overfishing has caused stock declines, these losses may compound social-economic costs, leading to the implementation of retaliatory measures like intentional shooting or hunting marine mammals, allegedly to protect livelihoods (Gilman et al., 2007; Lauriano et al., 2009).
For commercial fisheries, longlines have the highest proportion of reported marine mammal depredation with gear damage and catch loss (19 studies) (Hernandez-Milian et al., 2008; Huang, 2011; Towers et al., 2019). Depredation of other commercial gear such as purse seines, gillnets, and trawlers, is relatively less common (Goldsworthy et al., 2001; Hall et al., 2013). Gillnets are a still a cause for concern in small-scale fisheries (20 studies), with a higher proportion of reported marine mammal depredation than any other gear (Bordino et al., 2002; Rechimont et al., 2018). In small-scale fisheries, gear damage and catch loss were reported for most gear types, irrespective of the level of reported depredation (Bearzi et al., 2011; Monaco et al., 2019; Figure 4).
Cetacean depredation of commercial fishing gear has been reported from species such as bottlenose dolphins (Tursiops spp.) (Paudel et al., 2016; Bayless et al., 2017; Wild et al., 2017; Rechimont et al., 2018; Revuelta et al., 2018), killer whales (Orcinus orca) and sperm whales (Physeter macrocephalus) (Peterson et al., 2013, 2014; O’Connell et al., 2015; Thode et al., 2015; Bayless et al., 2017; Hanselman et al., 2018; Richard et al., 2018; Tixier et al., 2019; Towers et al., 2019), short-finned pilot whales (Globicephala macrorhyncus) and false killer whales (Pseudorca crassidens) (Hernandez-Milian et al., 2008; Rabearisoa et al., 2015; Bayless et al., 2017). Pinniped depredation has been documented for gray (Halichoerus grypus) (Moore, 2003), fur (Arctocephalus gazelle, A. australis) (Croll and Tershy, 1998; Goldsworthy et al., 2001; Bombau and Szteren, 2017), harbor (Phoca vitulina) (Moore, 2003; Rafferty et al., 2012) and elephant seals (Mirounga spp.) (Green et al., 1998; van den Hoff et al., 2017), and South American sea lions (Otaria flavescens) (Sepulveda et al., 2007; de la Torriente et al., 2010; de Maria et al., 2014).
Species such as bottlenose dolphins, Boto (Inia geoffrensis), Risso’s dolphin (Grampus griseus), striped (Stenella coeruleoalba), spinner (Stenella longirostris) and common dolphins (Delphinus spp.) have been reported depredating small-scale gear (Lauriano et al., 2009; Cruz et al., 2014; Mintzer et al., 2015). Pinniped depredation of small-scale fisheries is less frequently reported than for commercial fisheries, possibly reflecting the variability in the distributions of small-scale fisheries and pinnipeds (Panagopoulou et al., 2017; Sepulveda et al., 2018). Small-scale fisheries occur mainly in the tropics whereas pinnipeds mostly occur at higher latitudes (Chuenpagdee et al., 2006).
Indirect Interactions: Trophic Interactions Between Marine Mammals and Fisheries
Twenty-two studies discussed predator overlap with fishery operations and prey species to understand the prey requirements of top predators and the potential threats posed to both fisheries and animals (Kellert et al., 1995; Croll and Tershy, 1998). The overlap between the diet of the alleged marine mammal predator and the target species of commercial fisheries, such as longlines, gillnets and trawlers was described in 14 articles. Two articles quantified this overlap, highlighting marine mammal predation pressures on fishery resources and competition with fisheries (Li et al., 2010; Reinaldo et al., 2016). Diet overlap for both pinnipeds and cetaceans with small-scale fisheries were documented but not quantified.
The pressures exerted by fisheries on marine mammal energy intake and predation are orders of magnitude higher than the effect of marine mammal predation on the fish catch of commercial and small-scale fisheries (Croll and Tershy, 1998; Weinstein et al., 2017). An indirect effect of this trophic competition also leads to the shift of marine mammal diet from higher to lower trophic level species. Little is known about how this shift manifests across marine mammal populations. Nonetheless, it is clear that overfishing, rather than marine mammal predation has resulted in fishery stock declines that in turn affect marine mammal population(s) (Goldsworthy et al., 2001; Etnier and Fowler, 2010).
Management Response to Marine Mammal Interactions With Fisheries
Of the reviewed literature, 129 studies described and discussed management responses to various types of interactions observed (commercial fisheries: 63 studies, small-scale fisheries: 56 studies, both commercial and small-scale fisheries: 10 studies) (Table 1). The management responses and recommendations observed are categorized below using the framework described by Dawson et al. (2013). This framework mainly demonstrates the variations in management responses in commercial fisheries. We adapted this framework in this literature review to highlight the general challenges and opportunities in management response, in both commercial and small-scale fisheries.
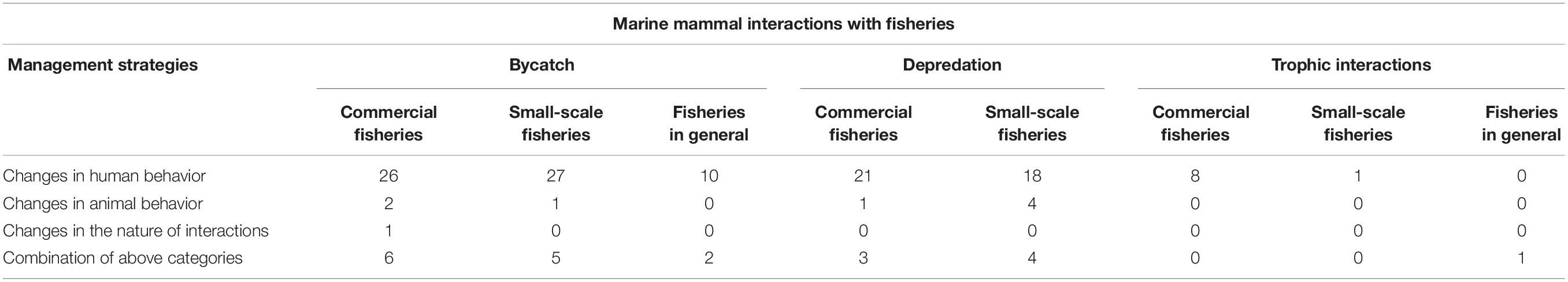
Table 1. Number of publications describing management strategies across marine mammal interactions with fisheries for both direct (bycatch, depredation) and indirect (trophic) interactions and fishery operations (commercial and small-scale fisheries); as per the management categories described by Dawson et al. (2013).
Management Response Categories
Measures That Aim to Change Human Behavior
This strategy includes spatial or place-based conservation measures and, modifications in fishing practices, or a combination of both, usually to mitigate marine mammal bycatch and incidental injuries or entanglements.
Place-based conservation measures, such as, Marine Protected Areas, have been implemented since the 1970’s (Hoyt, 2011). Permanent or temporary fishing closures (di Sciara et al., 2016) are a common conservation approach for habitats and species impacted by fishing activity. In response to the spatial and temporal variations in marine mammal bycatch and depredation events, selective fishing practices, particularly, in recognized sensitive habitats, and/or seasonal variations in fishing practices have also been implemented (Lauriano et al., 2009; Hanselman et al., 2018). The efficacy of these attempted solutions has rarely been rigorously tested.
Monetary compensation (Güçlüsoy, 2008) and subsidies on more selective gear and mitigation devices (Monaco et al., 2019) have also been implemented to offset the cost of gear damage and catch loss to fishers caused by depredation or incidental entanglements. Levies and subsidies on commercial fisheries, based on allotted quotas, have been introduced to encourage adequate reporting, and reduction of marine mammal bycatch (Bisack and Das, 2015). Developing alternative sources of income to deter the use of fishing gear(s) most prone to interactions, have also been suggested (Majluf et al., 2002). Alternative livelihoods such as eco-tourism have shown partial success in mitigating these interactions through poverty alleviation in certain regions of the world (Ermolin and Svolkinas, 2018; Berninsone et al., 2020).
Technological Interventions to Change the Nature of Interactions
Technological interventions, mainly for commercial fisheries, include bycatch reduction devices and modifications to gear that reduce bycatch rates, facilitating the safe escape of animals caught in fishing gear, or to reduce the instances of depredation (Allen et al., 2014). Gear modifications such as bottom-set gillnets illuminated with LED lights (Bielli et al., 2020), and depredation mitigation devices (Rabearisoa et al., 2015) can reduce cetacean, sea turtle, and sea-bird bycatch. Modifications to longlines have been extensively trialed. Hook modifications (McLellan et al., 2015; Hamilton and Baker, 2019), gangions (O’Connell et al., 2015) and decoys (Wild et al., 2017), have significantly reduced the rate of killer whale and sperm whale depredation in longline gear.
Several other technological modifications to fishing gear, particularly gillnets, have been developed, and reviewed (Harwood and Hembree, 1987; Trippel et al., 2003; Uhlmann and Broadhurst, 2015; Hamilton and Baker, 2019). However, these modifications are generalist in their approach and often of limited value to small-scale fisheries because of the spatial, temporal, and cultural variations in fishery operations (Teh et al., 2015; Davies et al., 2018).
Measures That Aim to Change Animal Behavior
This approach includes technological interventions that help to deter marine mammals from fishing gear. Active sound emitting devices, mainly acoustic deterrent devices, are used for bycatch mitigation, and acoustic harassment devices have been tested to reduce the instances of depredation (Reeves et al., 2001) on both commercial and small-scale fisheries.
Acoustic deterrent devices or pingers, actively emit mid to high frequency signals (2.5 to 10 kHz) at a low intensity (< 150 dB, 1 μPa at 1 m) that “deter” marine mammals from approaching fishing gear. Pingers have been shown to reduce the bycatch of bottlenose dolphins (Cox et al., 2004), harbor porpoises (Phocaena phocaeana) and Franciscana (Pontoporia blainvillei) (Dawson et al., 2013; Mangel et al., 2013; Chladek et al., 2020) in gillnet fisheries. Pingers have also proven successful in reducing pinniped interactions with aquaculture operations and have reduced the bycatch of some (but not all) species of cetaceans in gillnets (Clay et al., 2019).
Acoustic harassment devices are relatively high output sound emitters (>185 dB) primarily used to deter pinnipeds from mariculture or aquaculture operations by causing discomfort to the animals (Quick et al., 2004). Concerns about the effect of depredation on catch rates have led to their widespread use in commercial fisheries and aquaculture (Dawson et al., 2013).
Challenges, Uncertainties, and Opportunities for Management Actions
Based on the intensity of interactions, or their effects on marine mammal populations or fishery operations, management approaches aim to achieve set goals or conservation priorities. The success or failure of these goals can be recognized by certain indicators, for example, increased recruitment in and/or mortality reduction of the target population(s), and improved usage of the area by stakeholders, etc. (Hoyt, 2011; di Sciara et al., 2016). All efficacy metrics are dependent on prior information about the system under consideration, such as: (i) baseline population or abundance estimates, (ii) mortality records of the target species, (iii) stakeholder usage of the area, and (iv) the scale of the fishery operation. However, there are inevitable uncertainties regarding data on marine mammal population(s), fishery socioeconomics, and both human and animal behavioral ecology that limit the assessment of management efficacy in several ways (Table 2).
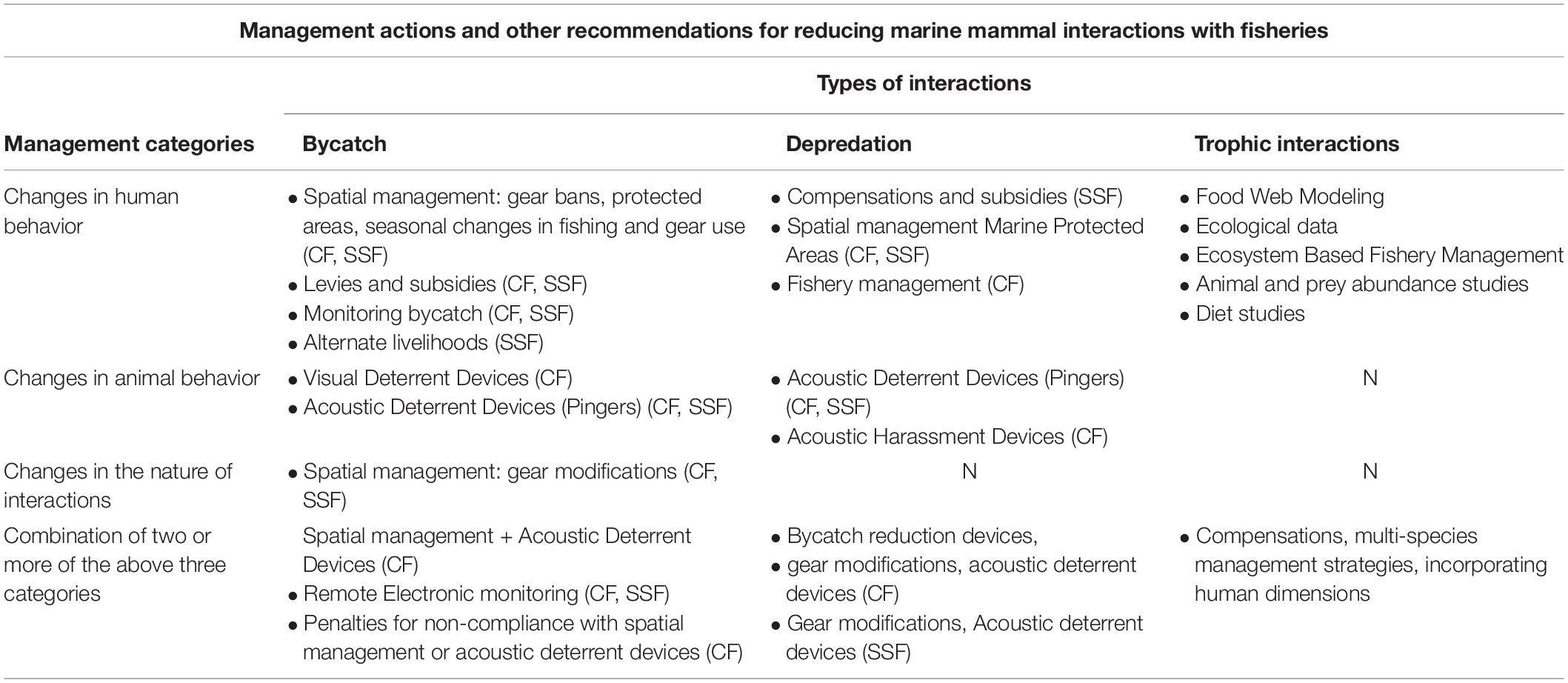
Table 2. Management actions and recommendations for direct (bycatch, depredation) and indirect (trophic) interactions, with commercial and small-scale fisheries. [Commercial fisheries (CF), Small-scale fisheries (SSF); No management action (N), as per the management categories described by Dawson et al. (2013)].
First, evaluating the success of place-based conservation measures and technological interventions is challenging because of the uncertainties associated with prior information on marine mammal abundance and identification of strategic habitats. In many instances, information on marine mammal abundance is inadequate, and data on most species are restricted to stranding records or from fisheries landing centers (IUCN, 2019). For example, for coastal species like the Indian Ocean humpback dolphin (Sousa plumbea), which is widely distributed around the peninsular Indian coastline, population estimates are fragmented, and most of the information on bycatch comes from incidental strandings and is thus mainly anecdotal (Sutaria et al., 2015; Braulik et al., 2017).
Second, robust and long-term population estimates require significant financial investment over extended periods. Such information is difficult to obtain, especially in developing countries (Moore et al., 2010; Lewison et al., 2014). In these scenarios without prior information, designing place-based measures poses a challenge to setting realistic goals and evaluating their success in the long run (di Sciara et al., 2016).
Third, bycatch projections for commercial fishery operations are based on existing bycatch estimates which are derived from fishery-observer surveys, and compiling and collating logbook data (Julian and Beeson, 1998; Morizur et al., 1999; Norman, 2000; Majluf et al., 2002; Underwood et al., 2008; Kindt-Larsen et al., 2016). While observer surveys have been an invaluable source of data (Edwards and Perrin, 1993; Morgan et al., 2002), accurately projecting these estimates over entire fishing fleets is not a straightforward process since observer coverage is often inadequate and the presence of an observer can influence fishing practices (Curtis and Carretta, 2020).
Small-scale fisheries present additional challenges with respect to bycatch monitoring, because of their spatial and operational variability, and unregulated, unstructured working environment (Hines et al., 2020). Methods such as rapid survey interviews, spatial risk assessments and monitoring catch at landing centers have provided baseline bycatch estimates for small-scale fisheries in developing countries (Pilcher et al., 2017; Temple et al., 2019; Hines et al., 2020; Verutes et al., 2020). However, fragmented monitoring efforts in small scale fisheries and the resultant inconsistencies in or the lack of bycatch data hamper the successful implementation of mitigation measures in small-scale fisheries (Gilman et al., 2010; Teh et al., 2015).
Data on marine mammal space use and behavioral observations, in the context of fishery operations, are being used for spatial risk assessments, understanding the drivers of bycatch, and assessing the effectiveness of implemented management measures (Grech et al., 2008; Marsh et al., 2011; Cerutti-Pereyra et al., 2020). Studies on acoustic activity and behavior of marine mammals have also assisted in understanding the precursors and the intensities of these interactions (Iriarte and Marmontel, 2013; Lewison et al., 2014; Kindt-Larsen et al., 2016; Lopes et al., 2016; Clay et al., 2019). Over the past decade, researchers have stressed the importance of ecological studies to understand the underlying causes of bycatch (Northridge et al., 2017).
Marine debris and the transition of bycatch from discard to commodity are two emerging concerns documented in the available literature. Since the mid to late-2010, data from stranding records and carcass examinations has been used to augment bycatch estimates to evaluate the risks due to all anthropogenic activities, mainly fisheries, and to understand the fate of bycaught animals. Analyses of injuries on carcasses have revealed significant number of entanglements of marine mammals in marine debris from small-scale fisheries, indicating interactions with fishing gear or ghost nets (Kaiser et al., 1996; Franco-Trecu et al., 2017).
The transition of bycatch from discard to commodity is also a growing concern in the light of fishery resource depletions (Ermolin and Svolkinas, 2018). Incidental catches create opportunities for food procurement, as declining fish catches widen the gap between supply and demand for food and income, particularly in developing countries (Robards and Reeves, 2011; Leeney et al., 2015; de Boer et al., 2016). Recent studies have highlighted the urgency of the issue. Marine mammals have been targeted for bait (Campbell et al., 2020; Briceno et al., 2021) or to attract fish to modified fish aggregating devices (Castro et al., 2020).
This process increases the likelihood of a fishery transitioning from bycatch to unregulated and directed harvest of marine mammals (DeMaster et al., 2001), exacerbating the threats to marine mammal populations. These factors should also be included in existing management strategies.
Human behavior is another major challenge to the uptake and application of management. Some measures are unlikely to be adopted, unless mandated and enforced. Even with mandates and regulations in place, the compliance levels depend upon the local social and economic conditions (Whitty, 2018). For example, fishing restrictions, quotas, or blanket bans on fisheries, can adversely affect community livelihoods and introduce human-human conflict within social-ecological systems, making management of interactions even more complicated (Dickman, 2010). Furthermore, in areas where marine mammals are protected by law, fishers are often unwilling to report interactions altogether for fear of persecution (Torres et al., 2018). For interactions such as marine mammal depredation of fisheries, the observed disparity between reported and actual depredation levels, particularly for small-scale fisheries, is high (Bearzi et al., 2011). Therefore, although the frequency of depredation may be lower than reported, the perceived economic damages due to depredation are likely to be higher than they actually are (Sepulveda et al., 2018). In certain cases, there may not be a link between depredation events and the involvement of marine mammals (Bearzi et al., 2008). Several other factors may cause gear damage and catch loss, for example, fish or other invertebrate species, or marine debris (Lauriano et al., 2009), which need to be considered in management strategies.
Incentive-based management measures, such as alternative livelihoods have been advocated as a practical and successful measure to manage interactions. However, such approaches may have limited success in poverty alleviation or be unsuitable for certain areas, particularly remote areas in low income countries, and for small-scale fisheries (Marsh et al., 2011; Squires et al., 2021). If implemented at all, such activities need to be carried out in accordance with proper guidelines (Armitage et al., 2009) and regulations, if unintended consequences to local communities, are to be avoided.
The wider implementation and effective enforcement of conservation measures can be severely limited by social-economic factors (Mendoza-Portillo et al., 2020). For example, small-scale fishers in the developing world are unlikely to have the financial resources to implement technological interventions. Even with subsidies to purchase these devices and adequate participant training and awareness, their voluntary long-term usage and maintenance and ultimately, their efficacy is highly unlikely (Brotons et al., 2008). For example, the vaquita population is dangerously low, despite the considerable investments in the gillnet ban by the Mexican government. This population decline is largely fueled by the high demand for and high market value of the totoaba (Totoaba macdonaldi) coupled with corruption (Brownell et al., 2019).
We were concerned to find that, despite the key role of human behavior and socioeconomics in the management context, only eight of the articles we considered propose the incorporation of human dimensions, mainly, socioeconomics of fisheries, cultural significance of livelihoods, and stakeholder belief systems (Szteren and Lezama, 2006; Bearzi et al., 2011; Morteo et al., 2012; Iriarte and Marmontel, 2013; Cook et al., 2015; Leeney et al., 2015; Snape et al., 2018; Mustika et al., 2021).
In addition to human behavior, the behavioral ecology and life-history traits of marine mammals are also major challenges to evaluating the long-term success of management measures. Marine mammal behavior plays an important role in the design and testing of technological interventions. For example, acoustic deterrent devices, or pingers have been shown to be unsuccessful in the long term in deterring marine mammals that exhibit behavioral plasticity. Especially for species with high site fidelity, pingers usually cause “habituation” or act as a “dinner bell,” facilitating higher interaction rates (Brownell et al., 2019). For instance, pingers successfully reduced Franciscana bycatch in bottom-set gillnet fisheries, and common dolphins and beaked whale bycatch in drift gillnet operations. However, Cox et al. (2004) concluded that after the initial success in bottlenose dolphin bycatch reduction, these devices were unlikely to further reduce their bycatch rates in gillnets.
Integrating data on marine mammal life-history traits is also vital to setting pragmatic goals for the management of interactions, particularly for bycatch mitigation. Fisheries bycatch models generally assume relatively a large population sizes of the by-caught species and seldom consider the impacts of the Allee effect (Wade and Slooten, 2020), i.e., reduced population growth rate at small population sizes. The Allee effect can have a significant influence on small, restricted populations, for example, coastal small cetaceans (Allee et al., 1949; Berec et al., 2007). Furthermore, the k-selected life-history traits of marine mammals, i.e., low reproductive rate and relatively slow rate of population growth, further limit population recovery (Mannocci et al., 2014). In such scenarios, even if fisheries-induced risks remain constant, bycatch could be a rare event with very deleterious population impacts, especially on small declining populations (di Sciara et al., 2016).
To effectively manage the resource overlap between marine mammals and fisheries, management guidelines should include social and economic dimensions of fisheries and marine mammal ecology. These measures could help in setting pragmatic and achievable conservation priorities based on enhanced understanding of critical knowledge gaps. The heterogeneity in the cultural, social and political nature of small-scale fisheries warrants a holistic understanding of these knowledge systems to implement temporal and spatial management measures. Bycatch is a major conservation concern in marine ecosystems, that requires a multidisciplinary approach to mitigation (Lent and Squires, 2017; Squires et al., 2021).
In addition to the occurrence, abundance and behavior of marine mammals, information on the effects of artificial resource provisioning on predators can shed light on how trophic interactions affect other functional groups in the ecosystem, including prey species (mainly fish stocks), and therefore fisheries (Tixier et al., 2019). However, there is also a need to develop social and economic perspectives of these interactions for the application of any mitigation measures to be contextually successful, both temporally and spatially. An area-specific adaptive management framework could therefore be an effective tool in reducing the risk to marine mammals from fisheries by coupling technical solutions with socio-economic and political interventions.
Conclusion
Marine mammal interactions with fisheries occur frequently. Marine mammal bycatch in fisheries is widely studied in literature and remains a major conservation concern due to its detrimental effects on marine mammal populations. However, despite its occurrence in many fishery operations, the effects of depredation on marine mammal ecology and fishery operations have rarely been quantified (Mooney et al., 2009). Depredation poses short-term benefits for marine mammals, creating new foraging opportunities directly facilitated by fishing operations (Tixier et al., 2015, 2020; Esteban et al., 2016). Such interactions could also result in increased chances of entanglement, injuries, and bycatch (Lewison et al., 2014; Guinet et al., 2015; Werner et al., 2015) due to the proximity of marine mammals to active fishing gear.
The biggest management challenge is our inadequate understanding of the social dimensions of these interactions and the inevitable uncertainties concerning both animal and human behaviors (Panagopoulou et al., 2017; Whitty, 2018; Mendoza-Portillo et al., 2020). Human behavior and fishery socioeconomics, in particular, play a key role in effective management of interactions and very few studies address these limitations (Northridge et al., 2017; Lewison et al., 2018).
An adaptive management response, i.e., (i) identifying management challenges and (ii) addressing the related data gaps in both the ecological and human dimensions should be used to set context-specific research and management priorities. Such a multidisciplinary approach could help in underlining the best possible measures for mitigating bycatch based on the local social-ecological conditions (Lent and Squires, 2017). For example, for a given region, with prior information on the fishery operations, and available data on marine mammal interactions with fisheries, the challenges could be evaluated against any existing management actions. This exercise could be then used to identify area-specific research gaps and to set pragmatic targets for research, management and social well-being in that area.
Clear conservation policies coupled with political will and capacity are important for the meaningful implementation of mitigation measures (Kuiper et al., 2018). With the limitations of technological solutions, coupled with socio-economic and political challenges, an adaptive management framework can be an effective tool in reducing the risk to marine mammals from fisheries. Nonetheless, we conclude that despite the vast body of literature on this subject, a “silver bullet” management solution to marine mammal interactions with fisheries does not yet exist (Bearzi et al., 2011; Snape et al., 2018).
Author Contributions
KJ, HM, and DS contributed to the conception of the study. KJ conducted the review, analyzed the data, and wrote the first draft of the manuscript. All authors contributed to the editorial and review process and approved the final version of the manuscript.
Funding
This study was funded by the Marine Conservation Action Fund (New England Aquarium) and the Society for Marine Mammalogy Small Grants for Research.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the reviewers and the editor for their comments which significantly improved this article.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.758013/full#supplementary-material
References
Aguilar, A. (2000). Population biology, conservation threats and status of Mediterranean striped dolphins (Stenella coeruleoalba). J. Cetacean Res. Manag. 2, 17–26.
Allee, W. C., Park, O., Emerson, A. E., Park, T., and Schmidt, K. P. (1949). Principles of Animal Ecology. Philadelphia: W. B. Saundere Co. Ltd.
Allen, L. K. (2000). Protected species and New England fisheries: an overview of the problem and conservation strategies. Northeast. Nat. 7, 411–418.
Allen, S. J., Tyne, J. A., Kobryn, H. T., Bejder, L., Pollock, K. H., and Loneragan, N. R. (2014). Patterns of dolphin bycatch in a North-Western Australian trawl fishery. PLoS One 9:e93178. doi: 10.1371/journal.pone.0093178
Anderson, R. C., Herrera, M., Ilangakoon, A. D., Koya, K. M., Moazzam, M., Mustika, P. L., et al. (2020). Cetacean bycatch in Indian Ocean tuna gillnet fisheries. Endanger. Species Res. 41, 39–53. doi: 10.3354/esr01008
Armitage, D. R., Plummer, R., Berkes, F., Arthur, R. I., Charles, A. T., Davidson-Hunt, I. J., et al. (2009). Adaptive co-management for social–ecological complexity. Front. Ecol. Environ. 7, 95–102. doi: 10.1890/070089
Avila, I. C., Kaschner, K., and Dormann, C. F. (2018). Current global risks to marine mammals: taking stock of the threats. Biol. Conserv. 221, 44–58.
Ayadi, A., Ghorbel, M., and Bradai, M. N. (2013). Do pingers reduce interactions between bottlenose dolphins and trammel nets around the Kerkennah Islands (Central Mediterranean Sea)? Cahiers Biol. Mar. 54, 375–383.
Ayala, L., Ortiz, M., and Gelcich, S. (2019). Exploring the role of fisher’s knowledge in assessing marine megafauna bycatch: insights from the Peruvian longline artisanal fishery. Anim. Conserv. 22, 251–261. doi: 10.1111/acv.12460
Baird, R. W., Anderson, D. B., Kratofil, M. A., and Webster, D. L. (2021). Bringing the right fishermen to the table: indices of overlap between endangered false killer whales and nearshore fisheries in Hawai’i. Biol. Conserv. 255:108975.
Batista, V. S., Fabré, N. N., Malhado, A. C., and Ladle, R. J. (2014). Tropical artisanal coastal fisheries: challenges and future directions. Rev. Fish. Sci. Aquac. 22, 1–15.
Bayless, A. R., Oleson, E. M., Baumann-Pickering, S., Simonis, A. E., Marchetti, J., Martin, S., et al. (2017). Acoustically monitoring the Hawai‘i longline fishery for interactions with false killer whales. Fish. Res. 190, 122–131. doi: 10.1016/j.fishres.2017.02.006
Bearzi, G. (2002). Interactions Between Cetacean and Fisheries in the Mediterranean Sea. Cetaceans of the Mediterranean and Black Seas: State of Knowledge and Conservation Strategies. A Report to the ACCOBAMS Secretariat. Monaco: ACCOBAMS.
Bearzi, G., Agazzi, S., Gonzalvo, J., Costa, M., Bonizzoni, S., Politi, E., et al. (2008). Overfishing and the disappearance of short-beaked common dolphins from western Greece. Endanger. Species Res. 5, 1–12.
Bearzi, G., Bonizzoni, S., and Gonzalvo, J. (2011). Dolphins and coastal fisheries within a marine protected area: mismatch between dolphin occurrence and reported depredation. Aquat. Conserv. Mar. Freshw. Ecosyst. 21, 261–267. doi: 10.1002/aqc.1179
Bearzi, G., Piwetz, S., and Reeves, R. R. (2019). “Odontocete adaptations to human impact and vice versa,” in Ethology and Behavioral Ecology of Odontocetes, ed. B. Würsig (Cham: Springer), 211–235.
Béné, C. (2006). Small-Scale Fisheries: Assessing Their Contribution to Rural Livelihoods in Developing Countries. FAO Fisheries Circular No. 1008, Rome: FAO, 46.
Berec, L., Angulo, E., and Courchamp, F. (2007). Multiple Allee effects and population management. Trends Ecol. Evol. 22, 185–191.
Berninsone, L. G., Bordino, P., Gnecco, M., Foutel, M., Mackay, A. I., and Werner, T. B. (2020). Switching Gillnets to Longlines: an Alternative to Mitigate the Bycatch of Franciscana Dolphins (Pontoporia blainvillei) in Argentina. Front. Mar. Sci. 7:699. doi: 10.3389/fmars.2020.00699
Beverton, R. J. H. (1985). “Analysis of marine mammal-fisheries interactions,” in Marine Mammals and Fisheries, eds J. R. Beddington, R. J. H. Beverton, and D. M. Lavigne (London: George Allen & Unwin), 3–33.
Bielli, A., Alfaro-Shigueto, J., Doherty, P. D., Godley, B. J., Ortiz, C., Pasara, A., et al. (2020). An illuminating idea to reduce bycatch in the Peruvian small-scale gillnet fishery. Biol. Conserv. 241:108277.
Bisack, K. D., and Das, C. (2015). Understanding non-compliance with protected species regulations in the northeast USA gillnet fishery. Front. Mar. Sci. 2:91. doi: 10.3389/fmars.2015.00091
Bombau, A., and Szteren, D. (2017). Seasonal variability of South American fur seals (Arctocephalus australis) and sea lions (Otaria flavescens) in two haulouts and interactions with small-scale fisheries off the coast of Montevideo, Uruguay. Aquat. Mamm. 43, 479–491. doi: 10.1578/am.43.5.2017.479
Bordino, P., Kraus, S., Albareda, D., Fazio, A., Palmerio, A., Mendez, M., et al. (2002). Reducing incidental mortality of franciscana dolphin Pontoporia blainvillei with acoustic warning devices attached to fishing nets. Mar. Mamm. Sci. 18, 833–842. doi: 10.1111/j.1748-7692.2002.tb01076.x
Braulik, G. T., Findlay, K., Cerchio, S., Baldwin, R., and Perrin, W. (2017). Sousa plumbea. The IUCN Red List of Threatened Species (2017: e. T82031633A82031644).
Briceno, Y., Sanchez, L., Trujillo, F., von Fersen, L., and Ramirez, S. (2021). Aquatic Wildmeat Consumption of Guiana Dolphins (Sotalia guianensis) in Lake Maracaibo System, Venezuela. Front. Mar. Sci. 8:625801. doi: 10.3389/fmars.2021.625801
Brotons, J. M., Grau, A. M., and Rendell, L. (2008). Estimating the impact of interactions between bottlenose dolphins and artisanal fisheries around the Balearic Islands. Mar. Mamm. Sci. 24, 112–127. doi: 10.1111/j.1748-7692.2007.00164.x
Brown, S., Reid, D., and Rogan, E. (2014). Characteristics of fishing operations, environment and life-history contributing to small cetacean bycatch in the northeast Atlantic. PLoS One 9:e104468. doi: 10.1371/journal.pone.0104468
Brownell, R. L. Jr., Reeves, R. R., Read, A. J., Smith, B. D., Thomas, P. O., Ralls, K., et al. (2019). Bycatch in gillnet fisheries threatens critically endangered small cetaceans and other aquatic megafauna. Endanger. Species Res. 40, 285–296.
Campbell, E., Mangel, J. C., Alfaro-Shigueto, J., Mena, J. L., Thurstan, R. H., and Godley, B. J. (2020). Coexisting in the Peruvian Amazon: interactions between fisheries and river dolphins. J. Nat. Conserv. 56:125859. doi: 10.1016/j.jnc.2020.125859
Carvalho, N., Edwards-Jones, G., and Isidro, E. (2011). Defining scale in fisheries: small versus large-scale fishing operations in the Azores. Fish. Res. 109, 360–369. doi: 10.1016/j.fishres.2011.03.006
Castro, C., Van Waerebeek, K., Cardenas, D., and Alava, J. J. (2020). Marine mammals used as bait for improvised fish aggregating devices in marine waters of Ecuador, eastern tropical Pacific. Endanger. Species Res. 41, 289–302. doi: 10.3354/esr01015
Cerutti-Pereyra, F., Moity, N., Dureuil, M., Ramirez-Gonzalez, J., Reyes, H., Budd, K., et al. (2020). Artisanal longline fishing the Galapagos Islands -effects on vulnerable megafauna in a UNESCO World Heritage site. Ocean Coast. Manag. 183:104995. doi: 10.1016/j.ocecoaman.2019.104995
Chladek, J., Culik, B., Kindt-Larsen, L., Albertsen, C. M., and von Dorrien, C. (2020). Synthetic harbour porpoise (Phocoena phocoena) communication signals emitted by acoustic alerting device (Porpoise ALert, PAL) significantly reduce their bycatch in western Baltic gillnet fisheries. Fish. Res. 232:105732. doi: 10.1016/j.fishres.2020.105732
Christensen, V., and Pauly, D. (2004). Placing fisheries in their ecosystem context, an introduction. Ecol. Model. 172, 103–107.
Chuenpagdee, R., Liguori, L., Palomares, M. L. D., and Pauly, D. (2006). Bottom-up, global estimates of small-scale marine fisheries catches. Fish. Cent. Res. Rep. 14:105.
Clay, T. A., Alfaro-Shigueto, J., Godley, B. J., Tregenza, N., and Mangel, J. C. (2019). Pingers reduce the activity of Burmeister’s porpoise around small-scale gillnet vessels. Mar. Ecol. Prog. Ser. 626, 197–208. doi: 10.3354/meps13063
Cook, T. C., James, K., and Bearzi, M. (2015). Angler perceptions of California sea lion (Zalophus californianus) depredation and marine policy in Southern California. Mar. Policy 51, 573–583. doi: 10.1016/j.marpol.2014.09.020
Cox, T. M., Read, A. J., Swanner, D., Urian, K., and Waples, D. (2004). Behavioral responses of bottlenose dolphins, Tursiops truncatus, to gillnets and acoustic alarms. Biol. Conserv. 115, 203–212. doi: 10.1016/S0006-3207(03)00108-3
Croll, D. A., and Tershy, B. R. (1998). Penguins, fur seals, and fishing: prey requirements and potential competition in the South Shetland Islands, Antarctica. Polar Biol. 19, 365–374. doi: 10.1007/s003000050261
Cruz, M. J., Jordao, V. L., Pereira, J. G., Santos, R. S., and Silva, M. A. (2014). Risso’s dolphin depredation in the Azorean hand-jig squid fishery: assessing the impacts and evaluating effectiveness of acoustic deterrents. ICES J. Mar. Sci. 71, 2608–2620. doi: 10.1093/icesjms/fsu073
Curtis, K. A., and Carretta, J. V. (2020). ObsCovgTools: assessing observer coverage needed to document and estimate rare event bycatch. Fish. Res. 225:105493. doi: 10.1016/j.fishres.2020.105493
D’Agrosa, C., Lennert-Cody, C. E., and Vidal, O. (2000). Vaquita bycatch in Mexico’s artisanal gillnet fisheries: driving a small population to extinction. Conserv. Biol. 14, 1110–1119. doi: 10.1046/j.1523-1739.2000.98191.x
Davies, T. E., Epstein, G., Aguilera, S. E., Brooks, C. M., Cox, M., and Evans, L. S. (2018). Assessing trade-offs in large marine protected areas. PLoS One 13:e0195760. doi: 10.1371/journal.pone.0195760
Dawson, S. M., Northridge, S., Waples, D., and Read, A. J. (2013). To ping or not to ping: the use of acoustic devices in mitigating interactions between small cetaceans and gillnet fisheries. Endanger. Species Res. 19, 201–221.
de Boer, M. N., Saulino, J. T., Van Waerebeek, K., and Aarts, G. (2016). Under Pressure: cetaceans and Fisheries Co-occurrence off the Coasts of Ghana and Cote d’Ivoire (Gulf of Guinea). Front. Mar. Sci. 3:178. doi: 10.3389/fmars.2016.00178
de Godoy, D. F., Mendonca, J. T., and Andriolo, A. (2020). Occurrence of Guiana dolphin (Sotalia guianensis) in southeast of Brazil: driven by prey distribution or human fishing activity?. Aquat. Conserv. 30, 1910–1921. doi: 10.1002/aqc.3367
de la Torriente, A., Quinones, R. A., Miranda-Urbina, D. A., and Echevarria, F. (2010). South American sea lion and spiny dogfish predation on artisanal catches of southern hake in fjords of Chilean Patagonia. ICES J. Mar. Sci. 67, 294–303. doi: 10.1093/icesjms/fsp235
de Maria, M., Barboza, F. R., and Szteren, D. (2014). Predation of South American sea lions (Otaria flavescens) on artisanal fisheries in the Rio de la Plata estuary. Fish. Res. 149, 69–73. doi: 10.1016/j.fishres.2013.09.006
DeMaster, D. P., Fowler, C. W., Perry, S. L., and Richlen, M. F. (2001). Predation and competition: the impact of fisheries on marine-mammal populations over the next one hundred years. J. Mammal. 82, 641–651. doi: 10.1644/1545-15422001082
di Sciara, G. N., Hoyt, E., Reeves, R., Ardron, J., Marsh, H., Vongraven, D., et al. (2016). Place-based approaches to marine mammal conservation. Aquat. Conserv. 26, 85–100.
Dickman, A. J. (2010). Complexities of conflict: the importance of considering social factors for effectively resolving human–wildlife conflict. Anim. Conserv. 13, 458–466.
D’Lima, C., Marsh, H., Hamann, M., Sinha, A., and Arthur, R. (2014). Positive interactions between Irrawaddy dolphins and artisanal fishers in the Chilika Lagoon of Eastern India are driven by ecology, socioeconomics, and culture. Ambio 43, 614–624. doi: 10.1007/s13280-013-0440-4
Edwards, E. F., and Perrin, C. (1993). Effects of dolphin group type, percent coverage, and fleet size on estimates of annual dolphin mortality derived from 1987 U.S. tuna-vessel observer data. Fish. Bull. 91, 628–640.
Ermolin, I., and Svolkinas, L. (2018). Assessment of the sturgeon catches and seal bycatches in an IUU fishery in the Caspian Sea. Mar. Policy 87, 284–290. doi: 10.1016/j.marpol.2017.09.022
Esteban, R., Verborgh, P., Gauffier, P., Giménez, J., Guinet, C., and de Stephanis, R. (2016). Dynamics of killer whale, bluefin tuna and human fisheries in the Strait of Gibraltar. Biol. Conserv. 194, 31–38.
Etnier, M. A., and Fowler, C. W. (2010). Size Selectivity in Marine Mammal Diets as a Guide to Evolutionarily Enlightened Fisheries Management. N. Am. J. Fish. Manag. 30, 588–603. doi: 10.1577/m09-086.1
Fisher, E., Bavinck, M., and Amsalu, A. (2018). Transforming asymmetrical conflicts over natural resources in the Global South. Ecol. Soc. 23:28.
Food and Agriculture Organization [FAO] (2001–2016). Fishing Gear Types. Beach seines. Technology Fact Sheets. Rome: FAO Fisheries and Aquaculture Department.
Food and Agriculture Organization [FAO] (2001-2019). Fishing Gear Types. Technology Fact Sheets. Rome: FAO Fisheries and Aquaculture Department.
Franco-Trecu, V., Drago, M., Katz, H., Machín, E., and Marín, Y. (2017). With the noose around the neck: Marine debris entangling otariid species. Environ. Pollut. 220, 985–989. doi: 10.1016/j.envpol.2016.11.057
Gerrodette, T. (2009). “The tuna-dolphin issue,” in Encyclopedia of marine mammals, eds W. F. Perrin, B. Würsig, and J. G. M. Thewissen (San Diego: Academic Press), 1192–1195. doi: 10.1016/b978-0-12-373553-9.00272-8
Gillett, R., and Lightfoot, C. (2001). The contribution of fisheries to the economies of Pacific Island countries. Pacific studies series, Asian Development Bank. Manila: Forum Fisheries Agency and World Bank.
Gillett, R., McCoy, M., Rodwell, L., and Tamate, J. (2001). Tuna: A Key Economic Resource in the Pacific. Manila: ADB.
Gilman, E., Brothers, N., McPherson, G., and Dalzell, P. (2007). A review of cetacean interactions with longline gear. J. Cetacean Res. Manag. 8:215.
Gilman, E., Gearhart, J., Price, B., Eckert, S., Milliken, H., and Wang, J. (2010). Mitigating sea turtle by-catch in coastal passive net fisheries. Fish Fish. 11, 57–88. doi: 10.1111/j.1467-2979.2009.00342.x
Goldsworthy, S. D., He, X., Tuck, G. N., Lewis, M., and Williams, R. (2001). Trophic interactions between the Patagonian toothfish, its fishery, and seals and seabirds around Macquarie Island. Mar. Ecol. Prog. Ser. 218, 283–302. doi: 10.3354/meps218283
Gosliner, M. L. (1999). “The tuna-dolphin controversy,” in Conservation and management of marine mammals, eds J. R. Twiss and R. R. Reeves (Washington: Smithsonian Institution Press), 133–134.
Gray, C. A., and Kennelly, S. J. (2018). Bycatches of endangered, threatened and protected species in marine fisheries. Rev. Fish Biol. Fish. 28, 521–541. doi: 10.1007/s11160-018-9520-7
Grech, A., Marsh, H., and Coles, R. (2008). A spatial assessment of the risk to a mobile marine mammal from bycatch. Aquat. Conserv. 18, 1127–1139. doi: 10.1002/aqc.943
Green, K., Slip, D. J., and Moore, G. J. (1998). The take of fish species by seabirds and marine mammals in the Australian Fisheries Zone around Heard Island: the potential for competition with a commercial fishery. Polar Biol. 20, 273–280. doi: 10.1007/s003000050303
Güçlüsoy, H. (2008). Damage by monk seals to gear of the artisanal fishery in the Foça Monk Seal Pilot Conservation Area, Turkey. Fish. Res. 90, 70–77. doi: 10.1016/j.fishres.2007.09.012
Guinet, C., Tixier, P., Gasco, N., and Duhamel, G. (2015). Long-term studies of Crozet Island killer whales are fundamental to understanding the economic and demographic consequences of their depredation behaviour on the Patagonian toothfish fishery. ICES J. Mar. Sci. 72, 1587–1597. doi: 10.1093/icesjms/fsu221
Guüçluüsoy, H., Kira, C. O., Veryeri, N. O., and Sava, Y. (2004). Status of theMediterranean Monk Seal Monachus monachus (Hermann, 1779) in the coastalwaters of Turkey– Ege University. J. Fish. Aquat. Sci. 21, 201–210.
Haddaway, N. R., Woodcock, P., Macura, B., and Collins, A. (2015). Making literature reviews more reliable through application of lessons from systematic reviews. Conserv. Biol. 29, 1596–1605. doi: 10.1111/cobi.12541
Hall, A. G., McNeill, J. B., Conn, P. B., Davenport, E., and Hohn, A. A. (2013). Seasonal co-occurrence of sea turtles, bottlenose dolphins, and commercial gill nets in southern Pamlico and northern Core Sounds, and adjacent coastal waters of North Carolina, USA. Endanger. Species Res. 22, 235–249. doi: 10.3354/esr00539
Hamer, D. J., Childerhouse, S. J., and Gales, N. J. (2012). Odontocete bycatch and depredation in longline fisheries: a review of available literature and of potential solutions. Mar. Mamm. Sci. 28, E345–E374.
Hamilton, S., and Baker, G. B. (2019). Technical mitigation to reduce marine mammal bycatch and entanglement in commercial fishing gear: lessons learnt and future directions. Rev. Fish Biol. Fish. 29, 223–247. doi: 10.1007/s11160-019-09550-6
Hanselman, D. H., Pyper, B. J., and Peterson, M. J. (2018). Sperm whale depredation on longline surveys and implications for the assessment of Alaska sablefish. Fish. Res. 200, 75–83. doi: 10.1016/j.fishres.2017.12.017
Harwood, M. B., and Hembree, D. (1987). Incidental catch of small cetaceans in the offshore gillnet fishery in northern Australian waters: 1981-1985. Rep. Int. Whaling Comm. 37:7.
Hernandez-Milian, G., Goetz, S., Varela-Dopico, C., Rodriguez-Gutierrez, J., Romon-Olea, J., and Fuertes-Gamundi, J. R. (2008). Results of a short study of interactions of cetaceans and longline fisheries in Atlantic waters: environmental correlates of catches and depredation events. Hydrobiologia 612, 251–268. doi: 10.1007/s10750-008-9501-2
Hines, E., Ponnampalam, L. S., Junchompoo, C., Peter, C., Vu, L., Huynh, T., et al. (2020). Getting to the bottom of bycatch: a GIS-based toolbox to assess the risk of marine mammal bycatch. Endanger. Species Res. 42, 37–57. doi: 10.3354/esr01037
Hoyt, E. (2011). Marine Protected Areas for Whales, Dolphins and Porpoises: A World Handbook for Cetacean Habitat Conservation and planning. London: Routledge.
Huang, H. W. (2011). Bycatch of high sea longline fisheries and measures taken by Taiwan: actions and challenges. Mar. Policy 35, 712–720. doi: 10.1016/j.marpol.2011.02.012
Iriarte, V., and Marmontel, M. (2013). River Dolphin (Inia geoffrensis, Sotalia fluviatilis) Mortality Events Attributed to Artisanal Fisheries in the Western Brazilian Amazon. Aquat. Mamm. 39, 116–124. doi: 10.1578/am.39.2.2013.116
Jaramillo-Legorreta, J., Rojas-Bracho, L., Brownell, R. L. Jr., Read, A. J., Reeves, R. R., Ralls, K., et al. (2007). Saving the Vaquita: immediate Action, Not More Data. Conserv. Biol. 21, 1653–1655. doi: 10.1111/j.1523-1739.2007.00825.x
Julian, F., and Beeson, M. (1998). Estimates of marine mammal, turtle, and seabird mortality for two California gillnet fisheries: 1990-1995. Fish. Bull. 96, 271–284.
Kaiser, M. J., Bullimore, B., Newman, P., Lock, K., and Gilbert, S. (1996). Catches in ’ghost fishing” set nets. Mar. Ecol. Prog. Ser. 145, 11–16. doi: 10.3354/meps145011
Kellert, S. R., Gibbs, J. P., and Wohlgenant, T. J. (1995). Canadian perceptions of commercial fisheries management and marine mammal conservation in the northwest atlantic-ocean. Anthrozoos 8, 20–30. doi: 10.2752/089279395787156518
Kindt-Larsen, L., Berg, C. W., Tougaard, J., Sørensen, T. K., Geitner, K., and Northridge, S. (2016). Identification of high-risk areas for harbour porpoise Phocoena phocoena bycatch using remote electronic monitoring and satellite telemetry data. Mar. Ecol. Prog. Ser. 555, 261–271. doi: 10.3354/meps11806
Kuiper, T., Dickman, A. J., Hinks, A. E., Sillero-Zubiri, C., Macdonald, E. A., and Macdonald, D. W. (2018). Combining biological and socio-political criteria to set spatial conservation priorities for the endangered African wild dog. Anim. Conserv. 21, 376–386. doi: 10.1111/acv.12405
Kumar, A. B., Smrithy, R., and Sathasivam, K. (2012). Dolphin-assisted cast net fishery in the Ashtamudi Estuary, south-west coast of India. Indian J. Fish. 59, 143–148.
Lauriano, G., Caramanna, L., Scarno, M., and Andaloro, F. (2009). An overview of dolphin depredation in Italian artisanal fisheries. J. Mar. Biol. Assoc. U. K. 89, 921–929. doi: 10.1017/s0025315409000393
Leeney, R. H., Dia, I. M., and Dia, M. (2015). Food, Pharmacy, Friend? Bycatch, Direct Take and Consumption of Dolphins in West Africa. Hum. Ecol. 43, 105–118. doi: 10.1007/s10745-015-9727-3
Lent, R., and Squires, D. (2017). Reducing marine mammal bycatch in global fisheries: an economics approach. Deep Sea Res. II Top. Stud. Oceanogr. 140, 268–277. doi: 10.1111/cobi.13418
Lewison, R. L., Crowder, L. B., Read, A. J., and Freeman, S. A. (2004). Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol. Evol. 19, 598–604. doi: 10.1016/j.tree.2004.09.004
Lewison, R. L., Crowder, L. B., Wallace, B. P., Moore, J. E., Cox, T., and Zydelis, R. (2014). Global patterns of marine mammal, seabird, and sea turtle bycatch reveal taxa-specific and cumulative megafauna hotspots. Proc. Natl. Acad. Sci. U. S. A. 111, 5271–5276. doi: 10.1073/pnas.1318960111
Lewison, R. L., Johnson, A. F., and Verutes, G. M. (2018). Embracing complexity and complexity-awareness in marine megafauna conservation and research. Front. Mar. Sci. 5:207. doi: 10.3389/fmars.2018.00207
Li, L. B., Ainsworth, C., and Pitcher, T. (2010). Presence of harbour seals (Phoca vitulina) may increase exploitable fish biomass in the Strait of Georgia. Prog. Oceanogr. 87, 235–241. doi: 10.1016/j.pocean.2010.09.006.54
Liu, M. M., Lin, M. L., Turvey, S. T., and Li, S. H. (2019). Fishers’ experiences and perceptions of marine mammals in the South China Sea: insights for improving community-based conservation. Aquat. Conserv. 29, 809–819. doi: 10.1002/aqc.3073
Lopes, K., Passos, L., Rodrigues, J. G., Koenen, F., Stiebens, V., Székely, T., et al. (2016). Sea turtle, shark, and dolphin bycatch rates by artisanal and semi-industrial fishers in Maio Island, Cape Verde. Chelonian Conserv. Biol. 15, 279–288.
Majluf, P., Babcock, E. A., Riveros, J. C., Schreiber, M. A., and Alderete, W. (2002). Catch and bycatch of sea birds and marine mammals in the small-scale fishery of Punta San Juan, Peru. Conserv. Biol. 16, 1333–1343. doi: 10.1046/j.1523-1739.2002.00564.x
Mangel, J. C., Alfaro-Shigueto, J., Van Waerebeek, K., Cáceres, C., Bearhop, S., Witt, M. J., et al. (2010). Small cetacean captures in Peruvian artisanal fisheries: high despite protective legislation. Biol. Conserv. 143, 136–143. doi: 10.1016/j.biocon.2009.09.017
Mangel, J. C., Alfaro-Shigueto, J., Witt, M. J., Hodgson, D. J., and Godley, B. J. (2013). Using pingers to reduce bycatch of small cetaceans in Peru’s small-scale driftnet fishery. Oryx 47, 595–606. doi: 10.1017/s0030605312000658
Mannocci, L., Catalogna, M., Dorémus, G., Laran, S., Lehodey, P., and Massart, W. (2014). Predicting cetacean and seabird habitats across a productivity gradient in the South Pacific gyre. Prog. Oceanogr. 120, 383–398. doi: 10.1016/j.pocean.2013.11.005
Marsh, H., O’Shea, T. J., Reynolds, J. E., and Reynolds, J. E. III (2011). Ecology and Conservation of the Sirenia: Dugongs and Manatees, Vol. 18. Cambridge: Cambridge University Press.
McLellan, W. A., Arthur, L. H., Mallette, S. D., Thornton, S. W., McAlarney, R. J., Read, A. J., et al. (2015). Longline hook testing in the mouths of pelagic odontocetes. ICES J. Mar. Sci. 72, 1706–1713. doi: 10.1093/icesjms/fsu181
Mendoza-Portillo, F. J., Ramírez-Rodríguez, M., and Vargas-López, V. (2020). Interactions of small-scale fisheries in Mexico’s northwest Pacific. Lat. Am. J. Aquat. Res. 48, 94–105. doi: 10.3856/vol48-issue1-fulltext-2176
Mintzer, V. J., Schmink, M., Lorenzen, K., Frazer, T. K., Martin, A. R., and da Silva, V. M. F. (2015). Attitudes and behaviors toward Amazon River dolphins (Inia geoffrensis) in a sustainable use protected area. Biodivers. Conserv. 24, 247–269. doi: 10.1007/s10531-014-0805-4
Monaco, C., Cavalle, M., and Peri, I. (2019). Preliminary study on interaction between dolphins and small-scale fisheries in Sicily: learning mitigation strategies from agriculture. Qual. Access Success 20, 400–407.
Mooney, T. A., Pacini, A. F., and Nachtigall, P. E. (2009). False killer whale (Pseudorca crassidens) echolocation and acoustic disruption: implications for longline bycatch and depredation. Can. J. Zool. 87, 726–733. doi: 10.1139/Z09-061
Moore, J. E., Cox, T. M., Lewison, R. L., Read, A. J., Bjorkland, R., McDonald, S. L., et al. (2010). An interview-based approach to assess marine mammal and sea turtle captures in artisanal fisheries. Biol. Conserv. 143, 795–805. doi: 10.1016/j.biocon.2009.12.023
Moore, P. G. (2003). Seals and fisheries in the Clyde Sea area (Scotland): traditional knowledge informs science. Fish. Res. 63, 51–61. doi: 10.1016/s0165-7836(03)00003-1
Morgan, M. J., Brodie, W. B., and Kulka, D. W. (2002). Was overexploitation the cause of the decline of the American plaice stock off Labrador and northeast Newfoundland?. Fish. Res. 57, 39–49. doi: 10.1016/S0165-7836(01)00331-9
Morizur, Y., Berrow, S. D., Tregenza, N. J. C., Couperus, A. S., and Pouvreau, S. (1999). Incidental catches of marine-mammals in pelagic trawl fisheries of the northeast Atlantic. Fish. Res. 41, 297–307. doi: 10.1016/s0165-7836(99)00013-2
Morteo, E., Rocha-Olivares, A., Arceo-Briseño, P., and Abarca-Arenas, L. G. (2012). Spatial analyses of bottlenose dolphin–fisheries interactions reveal human avoidance off a productive lagoon in the western Gulf of Mexico. J. Mar. Biol. Assoc. U. K. 92, 1893–1900.
Morzaria-Luna, H. N., Ainsworth, C. H., Kaplan, I. C., Levin, P. S., and Fulton, E. A. (2012). Exploring Trade-Offs between Fisheries and Conservation of the Vaquita Porpoise (Phocoena sinus) Using an Atlantis Ecosystem Model. PLoS One 7:e42917. doi: 10.1371/journal.pone.0042917
Mustika, P. L. K., Wonneberger, E., Erzini, K., and Pasisingi, N. (2021). Marine megafauna bycatch in artisanal fisheries in Gorontalo, northern Sulawesi (Indonesia): an assessment based on fisher interviews. Ocean Coast. Manag. 208:105606. doi: 10.1016/j.ocecoaman.2021.105606
Neil, D. T. (2002). Cooperative fishing interactions between Aboriginal Australians and dolphins in eastern Australia. Anthrozoös 15, 3–18. doi: 10.2752/089279302786992694
Norman, F. I. (2000). Preliminary investigation of the bycatch of marine birds and mammals in inshore commercial fisheries, Victoria, Australia. Biol. Conserv. 92, 217–226. doi: 10.1016/s0006-3207(99)00055-5
Northridge, S., Coram, A., Kingston, A., and Crawford, R. (2017). Disentangling the causes of protected-species bycatch in gillnet fisheries. Conserv. Biol. 31, 686–695. doi: 10.1111/cobi.12741
Northridge, S. P., and Hofman, R. J. (1999). “Marine mammal interactions with fisheries,” in Conservation and Management of Marine Mammals, eds J. R. Twiss, and R. R. Reeves (Washington: Smithsonian Institution), 99–119. doi: 10.1007/s13280-018-1131-y
O’Connell, V., Straley, J., Liddle, J., Wild, L., Behnken, L., Falvey, D., et al. (2015). Testing a passive deterrent on longlines to reduce sperm whale depredation in the Gulf of Alaska. ICES J. Mar. Sci. 72, 1667–1672. doi: 10.1093/icesjms/fsv014
Ortega-Argueta, A., Pérez-Sánchez, C. E., Gordillo-Morales, G., Gordillo, O. G., Pérez, D. G., and Alafita, H. (2005). Cetacean strandings on the southwestern coast of the Gulf of Mexico. Gulf Mex. Sci. 23, 179–185.
Panagopoulou, A., Meletis, Z. A., Margaritoulis, D., and Spotila, J. R. (2017). Caught in the Same Net? Small-Scale Fishermen’s Perceptions of Fisheries Interactions with Sea Turtles and Other Protected Species. Front. Mar. Sci. 4:180. doi: 10.3389/fmars.2017.00180
Paudel, S., Levesque, J. C., Saavedra, C., Pita, C., and Pal, P. (2016). Characterization of the artisanal fishing communities in Nepal and potential implications for the conservation and management of Ganges River Dolphin (Platanista gangetica gangetica). PeerJ 4:e1563. doi: 10.7717/peerj.1563
Pauly, D. (1997). Small-scale fisheries in the tropics: marginality, marginalization, and some implications for fisheries management. Glob. Trends Fish. Manag. 20, 40–49.
Pauly, D. (2006). Major trends in small-scale marine fisheries, with emphasis on developing. countries, and some implications for the social sciences. MAST 4, 7–22.
Pauly, D. (2009). Beyond duplicity and ignorance in global fisheries. Sci. Mar. 73, 215–224. doi: 10.3989/scimar.2009.73n2215
Perrin, W. F., Donovan, G. P., and Barlow, J. (1994). Gillnets and Cetaceans. Cambridge: International Whaling Commission.
Peterson, D., Hanazaki, N., and Simoes-Lopes, P. C. (2008). Natural resource appropriation in cooperative artisanal fishing between fishermen and dolphins (Tursiops truncatus) in Laguna, Brazil. Ocean Coast. Manag. 51, 469–475. doi: 10.1016/j.ocecoaman.2008.04.003
Peterson, M. J., Mueter, F., Criddle, K., and Haynie, A. C. (2014). Killer Whale Depredation and Associated Costs to Alaskan Sablefish, Pacific Halibut and Greenland Turbot Longliners. PLoS One 9:e88906. doi: 10.1371/journal.pone.0088906
Peterson, M. J., Mueter, F., Hanselman, D., Lunsford, C., Matkin, C., and Fearnbach, H. (2013). Killer whale (Orcinus orca) depredation effects on catch rates of six groundfish species: implications for commercial longline fisheries in Alaska. ICES J. Mar. Sci. 70, 1220–1232. doi: 10.1093/icesjms/fst045
Pilcher, N. J., Adulyanukosol, K., Das, H., Davis, P., Hines, E., and Kwan, D. (2017). A low-cost solution for documenting distribution and abundance of endangered marine fauna and impacts from fisheries. PLoS One 12:e0190021. doi: 10.1371/journal.pone.0190021
Plagányi, ÉE., and Butterworth, D. S. (2009). “Competition with fisheries,” in Encyclopedia of Marine Mammals, eds W. F. Perrin, B. Würsig, and J. G. M. Thewissen (Cambridge: Academic Press), 269–275. doi: 10.1016/b978-0-12-373553-9.00065-1
Quick, N. J., Middlemas, S. J., and Armstrong, J. D. (2004). A survey of antipredator controls at marine salmon farms in Scotland. Aquaculture 230, 169–180. doi: 10.1016/s0044-8486(03)00428-9
Rabearisoa, N., Bach, P., and Marsac, F. (2015). Assessing interactions between dolphins and small pelagic fish on branchline to design a depredation mitigation device in pelagic longline fisheries. ICES J. Mar. Sci. 72, 1682–1690. doi: 10.1093/icesjms/fsu252
Rafferty, A. R., Brazer, E. O., and Reina, R. D. (2012). Depredation by harbor seal and spiny dogfish in a Georges Bank gillnet fishery. Fish. Manag. Ecol. 19, 264–272. doi: 10.1111/j.1365-2400.2011.00837.x
Read, A. J. (2008). The looming crisis: interactions between marine mammals and fisheries. J. of Mammal. 89, 541–548. doi: 10.1644/07-mamm-s-315r1.1
Read, A. J., Drinker, P., and Northridge, S. (2006). Bycatch of marine mammals in US and global fisheries. Conserv. Biol. 20, 163–169. doi: 10.1111/j.1523-1739.2006.00338.x
Rechimont, M. E., Lara-Domínguez, A. L., Morteo, E., Martínez-Serrano, I., and Equihua, M. (2018). Depredation by coastal bottlenose dolphins (Tursiops truncatus) in the southwestern Gulf of Mexico in relation to fishing techniques. Aquat. Mamm. 44, 469–481. doi: 10.1578/AM.44.5.2018.469
Reeves, R. R., McClellan, K., and Werner, T. B. (2013). Marine mammal bycatch in gillnet and other entangling 2234 net fisheries, 1990 to 2011. Endanger. Species Res. 20, 71–97. doi: 10.3354/esr00481
Reeves, R. R., Read, A. J., and di Sciara, G. N. (eds) (2001). Report of the Workshop on Interactions Between Dolphins and Fisheries in the Mediterranean, Evaluation of Mitigation Alternatives. Roma: ICRAM.
Reinaldo, M. O., Milessi, A. C., Romero, M. A., Crespo, E., Wolff, M., and Gonzalez, R. A. (2016). Assessing the effects of demersal fishing and conservation strategies of marine mammals over a Patagonian food web. Ecol. Model. 331, 31–43. doi: 10.1016/j.ecolmodel.2015.10.025
Revuelta, O., Domenech, F., Fraija-Fernandez, N., Gozalbes, P., Novillo, O., Penades-Suay, J., et al. (2018). Interaction between bottlenose dolphins (Tursiops truncatus) and artisanal fisheries in the Valencia region (Spanish Mediterranean Sea). Ocean Coast. Manag. 165, 117–125. 001 doi: 10.1016/j.ocecoaman.2018.08
Richard, G., Guinet, C., Bonnel, J., Gasco, N., and Tixier, P. (2018). Do commercial fisheries display optimal foraging? The case of longline fishers in competition with odontocetes. Can. J. Fish. Aquat. Sci. 75, 964–976. doi: 10.1139/cjfas-2016-0498
Robards, M. D., and Reeves, R. R. (2011). The global extent and character of marine mammal consumption by humans: 1970–2009. Biol. Conserv. 144, 2770–2786. doi: 10.1016/j.biocon.2011.07.034
Rojas-Bracho, L., Reeves, R. R., and Jaramillo-Legorreta, A. (2006). Conservation of the vaquita Phocoena sinus. Mamm. Rev. 36, 179–216. doi: 10.1111/j.1365-2907.2006.00088.x
Sepulveda, M., Martinez, T., Oliva, D., Couve, P., Pavez, G., and Navarro, C. (2018). Factors affecting the operational interaction between the South American sea lions and the artisan gillnet fishery in Chile. Fish. Res. 201, 147–152. doi: 10.1016/j.fishres.2018.01.014
Sepulveda, M., Perez, M. J., Sielfeld, W., Oliva, D., Duran, L. R., and Rodriguez, L. (2007). Operational interaction between South American sea lions Otaria flavescens and artisanal (small-scale) fishing in Chile: results from interview surveys and on-board observations. Fish. Res. 83, 332–340. doi: 10.1016/j.fishres.2006.10.009
Silva, M. A., Feio, R., Prieto, R., Gonçalves, J. M., and Santos, R. S. (2002). Interactions between cetaceans and the tuna fishery in the Azores. Mar. Mamm. Sci. 18, 893–901. doi: 10.1111/j.1748-7692.2002.tb01080.x
Slooten, E., and Dawson, S. M. (2010). Assessing the effectiveness of conservation management decisions: likely effects of new protection measures for Hector’s dolphin (Cephalorhynchus hectori). Aquat. Conserv. 20, 334–347. doi: 10.1002/aqc.1084
Snape, R. T. E., Broderick, A. C., Cicek, B. A., Fuller, W. J., Tregenza, N., Witt, M. J., et al. (2018). Conflict between Dolphins and a Data-Scarce Fishery of the European Union. Hum. Ecol. 46, 423–433. doi: 10.1007/s10745-018-9989-7
Squires, D., Ballance, L. T., Dagorn, L., Dutton, P. H., and Lent, R. (2021). Mitigating Bycatch: novel Insights to Multidisciplinary Approaches. Front. Mar. Sci. 8:613285. doi: 10.3389/fmars.2021.613285
Sutaria, D., Panicker, D., Jog, K., Sule, M., Muralidharan, R., and Bopardikar, I. (2015). Humpback dolphins (Genus Sousa) in India: an overview of status and conservation issues. Adv. Mar. Biol. 72, 229–256. doi: 10.1016/bs.amb.2015.08.006
Szteren, D., and Lezama, C. (2006). “Southern sea lions and artisanal fisheries in Piriápolis, Uruguay: interactions in 1997, 2001, and 2002,” in Sea Lions of the World. Alaska Sea Grant College Program, eds A. W. Trities, S. K. Atkinson, D. P. De Master, and L. W. Frits (Fairbanks: University of Alaska), 591–604.
Teh, L. C., and Pauly, D. (2018). Who brings in the fish? The relative contribution of small-scale and industrial fisheries to food security in Southeast Asia. Front. Mar. Sci. 5:44. doi: 10.3389/fmars.2018.00044
Teh, L. S., Teh, L. C., Hines, E., Junchompoo, C., and Lewison, R. L. (2015). Contextualising the coupled socio-ecological conditions of marine megafauna bycatch. Ocean Coast. Manag. 116, 449–465. doi: 10.1016/j.ocecoaman.2015.08.019
Temple, A. J., Wambiji, N., Poonian, C. N. S., Jiddawi, N., Stead, S. M., Kiszka, J. J., et al. (2019). Marine megafauna catch in southwestern Indian Ocean small-scale fisheries from landings data. Biol. Conserv. 230, 113–121. doi: 10.1016/j.biocon.2018.12.024
Thode, A., Mathias, D., Straley, J., O’Connell, V., Behnken, L., Falvey, D., et al. (2015). Cues, creaks, and decoys: using passive acoustic monitoring as a tool for studying sperm whale depredation. ICES J. Mar. Sci. 72, 1621–1636. doi: 10.1093/icesjms/fsv024
Tixier, P., Authier, M., Gasco, N., and Guinet, C. (2015). Influence of artificial food provisioning from fisheries on killer whale reproductive output. Anim. Conserv. 18, 207–218. doi: 10.1111/acv.12161
Tixier, P., Barbraud, C., Pardo, D., Gasco, N., Duhamel, G., and Guinet, C. (2017). Demographic consequences of fisheries interaction within a killer whale (Orcinus orca) population. Mar. Biol. 164:170.
Tixier, P., Burch, P., Massiot-Granier, F., Ziegler, P., Welsford, D., Lea, M. A., et al. (2020). Assessing the impact of toothed whale depredation on socio-ecosystems and fishery management in wide-ranging subantarctic fisheries. Rev. Fish Biol. Fish. 30, 203–217. doi: 10.1007/s11160-020-09597-w
Tixier, P., Burch, P., Richard, G., Olsson, K., Welsford, D., Lea, M. A., et al. (2019). Commercial fishing patterns influence odontocete whale-longline interactions in the Southern Ocean. Sci. Rep. 9:1904. doi: 10.1038/s41598-018-36389-x
Tixier, P., Lea, M. A., Hindell, M. A., Welsford, D., Mazé, C., Gourguet, S., et al. (2021). When large marine predators feed on fisheries catches: global patterns of the depredation conflict and directions for coexistence. Fish Fish. 22, 31–53. doi: 10.1111/faf.12504
Torres, D. F., Oliveira, E. S., and Alves, R. R. N. (2018). “Understanding human–wildlife conflicts and their implications,” in Ethnozoology, eds R. R. N. Alves and U. P. de Albuquerque (Cambridge: Academic Press), 421–445. doi: 10.1016/b978-0-12-809913-1.00022-3
Towers, J. R., Tixier, P., Ross, K. A., Bennett, J., Arnould, J. P. Y., Pitman, R. L., et al. (2019). Movements and dive behaviour of a toothfish-depredating killer and sperm whale. ICES J. Mar. Sci. 76, 298–311. doi: 10.1093/icesjms/fsy118
Trippel, E. A., Holy, N. L., Palka, D. L., Shepherd, T. D., Melvin, G. D., and Terhune, J. M. (2003). Nylon barium sulphate gillnet reduces porpoise and seabird mortality. Mar. Mamm. Sci. 19, 240–243. doi: 10.1111/j.1748-7692.2003.tb01106.x
Trites, A. W., Christensen, V., and Pauly, D. (1997). Competition between fisheries and marine mammals for prey and primary production in the Pacific Ocean. J. Northwest Atl. Fish. Sci. 22, 173–187. doi: 10.1007/s00442-017-3883-7
Turvey, S. T., Pitman, R. L., Taylor, B. L., Barlow, J., Akamatsu, T., and Barrett, L. A. (2007). First human-caused extinction of a cetacean species?. Biol. Lett. 3, 537–540. doi: 10.1098/rsbl.2007.0292
Uhlmann, S. S., and Broadhurst, M. K. (2015). Mitigating unaccounted fishing mortality from gillnets and traps. Fish Fish. 16, 183–229. doi: 10.1111/faf.12049
Underwood, J. G., Hernandez Camacho, C. J., Aurioles-Gamboa, D., and Gerber, L. R. (2008). Estimating sustainable bycatch rates for California sea lion populations in the Gulf of California. Conserv. Biol. 22, 701–710. doi: 10.1111/j.1523-1739.2008.00919.x
van den Hoff, J., Kilpatrick, R., and Welsford, D. (2017). Southern elephant seals (Mirounga leonina Linn.) depredate toothfish longlines in the midnight zone. PLoS One 12:e0172396. doi: 10.1371/journal.pone.0172396
Verutes, G. M., Johnson, A. F., Caillat, M., Ponnampalam, L. S., Peter, C., Vu, L., et al. (2020). Using GIS and stakeholder involvement to innovate marine mammal bycatch risk assessment in data-limited fisheries. PLoS One 15:e0237835. doi: 10.1371/journal.pone.0237835
Wade, P. R., and Slooten, E. (2020). Extinction risk from human impacts on small populations of marine mammals. bioRxiv [Preprint]. doi: 10.1101/2020.03.28.013698
Wade, P. R., Watters, G. M., Gerrodette, T., and Reilly, S. B. (2007). Depletion of spotted and spinner dolphins in the eastern tropical Pacific: modelling hypotheses for their lack of recovery. Mar. Ecol. Prog. Ser. 343, 1–14. doi: 10.3354/meps07069
Weinstein, B. G., Double, M., Gales, N., Johnston, D. W., and Friedlaender, A. S. (2017). Identifying overlap between humpback whale foraging grounds and the Antarctic krill fishery. Biol. Conserv. 210, 184–191. doi: 10.1016/j.biocon.2017.04.014
Werner, T. B., Northridge, S., Press, K. M., and Young, N. (2015). Mitigating bycatch and depredation of marine mammals in longline fisheries. ICES J. Mar. Sci. 72, 1576–1586.
Whitty, T. S. (2018). Conservationscapes: an interdisciplinary framework to link species-focused conservation to human systems. Front. Ecol. Environ. 16, 44–52. doi: 10.1002/fee.1750
Wild, L., Thode, A., Straley, J., Rhoads, S., Falvey, D., and Liddle, J. (2017). Field trials of an acoustic decoy to attract sperm whales away from commercial longline fishing vessels in western Gulf of Alaska. Fish. Res. 196, 141–150.
Woodley, T. H., and Lavigne, D. M. (1991). Incidental Capture of Pinnipeds in Commercial Fishing gear. International Marine Mammal Association Technical Report. Canada: IMMA.
Keywords: bycatch, depredation, trophic interactions, competition, direct interactions, commercial fisheries, small-scale fisheries, marine mammals
Citation: Jog K, Sutaria D, Diedrich A, Grech A and Marsh H (2022) Marine Mammal Interactions With Fisheries: Review of Research and Management Trends Across Commercial and Small-Scale Fisheries. Front. Mar. Sci. 9:758013. doi: 10.3389/fmars.2022.758013
Received: 20 August 2021; Accepted: 19 January 2022;
Published: 10 March 2022.
Edited by:
Dale Edward Squires, National Oceanic and Atmospheric Administration (NOAA), United StatesReviewed by:
Lemnuel De Vera Aragones, University of the Philippines Diliman, PhilippinesEllen Hines, San Francisco State University, United States
Copyright © 2022 Jog, Sutaria, Diedrich, Grech and Marsh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ketki Jog, a2V0a2kuam9nQG15LmpjdS5lZHUuYXU=
 Ketki Jog
Ketki Jog Dipani Sutaria
Dipani Sutaria Amy Diedrich
Amy Diedrich Alana Grech
Alana Grech Helene Marsh
Helene Marsh