
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 14 February 2022
Sec. Marine Evolutionary Biology, Biogeography and Species Diversity
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.657124
This article is part of the Research TopicBenthic Biodiversity of the Indian OceanView all 14 articles
The radula is the main feeding organ and also very significant to the majority of the mollusks (especially gastropod) taxonomy. With shell morphology, radular morphology is the key characteristic for the identification of gastropod species. The shape and structure of the radular teeth are unique from family to species level. In this study, five basic types of radula (i.e., docoglossan, rhipidoglossan, taenioglossan, stenoglossan, and toxoglossan), which were observed from a total of 23 different species belonging to 12 families, were examined. Collection of the voucher intertidal gastropod specimen for the study had initiated during May–October 2019 in the rocky intertidal area near Veraval of the south Saurashtra coastline. Direct handpicking methods were used for the collection of the specimen for experiments.
The radula, a unique taxonomical characteristic and feeding organ of mollusks, is found in every class except the class Bivalvia (Arularasan et al., 2011). This ribbon-like structure is found and used to slide on odontophore (another supportive part to radula) in the mouth and supports a variety of different feeding mechanisms. This odontophore is embedded with rows of many tiny denticles or teeth. From the front side, new fragments of denticles will be produced constantly when old ones are worn out by abrasion. The radula has attached at both ends, and it grows continuously during the life of the gastropod. With the help of this cumulative mechanism, food particles or pieces of food and debris go into the esophagus of the animal. The outline and the radular arrangement are the significant tools for species identification (Mutlu, 2004). Researchers currently understood that modifications of the radular arrangements have long been recognized as a key feature for the diversity of molluscans which is related to the important process of gaining energy from the surrounding environment (Cruz et al., 1998; Guralnick and Smith, 1999). In the case of molluscan taxonomy, marine intertidal gastropods, in particular, were identified on the basis of diverse morphological characteristics such as shape, size, color, and band pattern, which were not sufficient for an individual to be identified up to species level. Shell pattern and its coloration pattern vary due to several reasons such as coastal environment (wave action, light, humidity, salinity, and temperature), geographical distribution, and age-related variations, which may inhibit proper identification up to species level (Vakani et al., 2020). Other than the shell pattern, characteristics are needed for species identification. The radula can be a useful potential source of such characteristics. After a long research, we understood that radula is important not only for nutrition but also for classification and phylogenetic studies, which is responsible for the differences in intraspecific classification better than the morphology of foot and shell (Mutlu, 2004; Andrade and Solferini, 2006; Mutaf and Aksit, 2009). Generally, it indicates similarities up to family level and compatibility differences up to species level. The shapes and structures of molluscan radular teeth are often unique to a species or a genus, and these structures have traditionally been one of the most widely used sources of data for studies on molluscan systematics. Some features of the radula have also been used to study higher-level molluscan taxonomic relationships (Roberts, 2000; Kruta et al., 2013, 2014).
Gastropoda is represented by species with tremendous adaptative success, specifically regarding the strategies of food search and food capture (Taylor et al., 1980; Hughes, 1986), which can be well understood by the example of the toxoglossan radular structure. This radula is totally different from the radula of the other gastropod. The teeth are usually arrow-shaped and not fixed to a base plate. Central (rachidian) teeth are fully destroyed from the base plate. These teeth are long and linked to the toxin channel. Each tooth can be individually moved to the snout similar to a spear that is ready to be thrown to hunt (Kantor, 1990).
As molluscan is the second most diverse group in the animal kingdom, they are existing in all kinds of habitats available worldwide (Mutlu, 2004). Habitat-wise, every molluscan family has different kinds of feeding organ radula (Padilla, 1989). Few examples are discussed in this study for a better understanding of the radular pattern of the group: the very first example is the single-shelled mollusks true limpets possess radula covered with denticles, which enables the animal to scrape tiny vegetation from the rocky surface (Guralnick and Smith, 1999). Whelks have a stalk radula that can be extended outside to the shell and be helped to bore toxicant digestive juice into the shells of other gastropods or other animals and suck out the flesh that was digested and became jelly or semifluid of the prey. Similarly, the cone radula is modified into a poisoned harpoon-like structure with which they throw like a spear to paralyze their prey. In still more active carnivores, the heavy shell is reduced in size and may even be lost as has occurred in the sea slugs that have an upper surface covered with tentacles. One species of sea slugs actively hunts jellyfish and ingests the stinging cells of these animals, which it then concentrates in the tentacles and uses them for protection.
In this study, we have tried to find different types of radulae and their basic structure and relationship between radular size and shell size for a particular family of the selected abundant gastropods of the intertidal area of the south Saurashtra coast of India. The radula of different gastropods was analyzed by compound stereomicroscope.
For this study, the sample was collected from Veraval, south Saurashtra coast of Gujarat, India. Veraval is one of the largest fish landing sites in India.
The molluscan species selected for radular analysis were selected based on their occurrence during all seasons of the study period. For a long-term study on the same aspect, we also ensured that all these species were moreover reported as Inter-coast species (non-migrant). We have also used few previous research reports such as Vaghela (2010) and Vakani (2013) as references for selecting the species. Few species that were also used to compare species-level differentiation were from the previous collections of the Museum of the Department of Biosciences.
Samples are collected from the intertidal zone of the Veraval coastal area during the low tide. Before the sample was collected for the laboratory experiment, it was identified by using different field manuals and available literature such as Apte (1998). The sample was preserved in 10% formalin or 70% alcohol for the laboratory experiment and identification.
For radular analysis, an individual sample of gastropod mollusk was separated from the shell, and a small cut was made on the dorsal surface of the head until radula was not exposed. Then, dissection was performed until the end of the snout, and the tongue-like odontophore was detached from the muscle fibers attached to it. The separated radula was washed or cleaned in dilute sodium hypochlorite, and a soft brush was used to remove the adhering tissue (Vakani et al., 2020). In this study, we have observed the pattern of teeth and the variation of shape, size, and formation of radular teeth in different species. The full amylaceous radular strip was detached from the buccal mass of each species, and the radular structure was examined under a dissecting microscope (Vakani and Kundu, 2021). Scanning electron microscopy (SEM) was also performed on the radular teeth of the observed species.
The teeth of radular (except in toxoglossan) are always found to attach with the odontophore in transverse rows consisting of two or more teeth that are mirror images about the midpoint, and for that, all gastropods have been found with bilateral symmetry in radular ribbon. Each transverse row is identical to the adjacent rows, producing columns of identical teeth (Smith, 1988; Shaw et al., 2008). The teeth are mostly odd in number when rachidian or central or middle teeth are present. In case of no rachidian (or central or middle) teeth, radula has an even number of teeth. The common formula of radula is M + L + R + L + M. Each row in the radula has one central or rachidian tooth (R): on each side, a few lateral teeth (L), and then beyond that, a few marginal teeth (M). Different species could have different lateral teeth, and then, numerous marginal teeth may be uncountable. All studied animals are described in Table 1. In this study, we have posted only half a row (only left side) of the counting of teeth of ribbon (R + L + M) of radular pattern to avoid repetition and to save space (Table 1).
Cytochrome oxidase I (COI) gene sequence of gastropods was retrieved from the NCBI database. The phylogenetic tree for gastropods was constructed by unweighted pair group method with arithmetic mean (UPGMA) using Jones-Taylor-Thornton (JTT) estimate models of amino acid replacement with 1,000 bootstrap replicates in Molecular Evolutionary Genetics Analysis (MEGA11) software.
The communication was deal with the study of the radular morphology of the selected dominating intertidal gastropod mollusks and found the radular pattern that was made by the combination of different kinds of denticles (teeth). This report creates baseline data about the radular patterns and radular types of dominating intertidal gastropod mollusks of the Saurashtra region. In total, 23 different species belonging to 12 different gastropod families were analyzed for the undersetting of the radula and its pattern into the different families as well as species. The results showed that all selected gastropods were followed by all five basic different types of radular patterns, namely, docoglossan, rhipidoglossan, taenioglossan, stenoglossan, and toxoglossan. A graphical representation of species and family number against radula types showed that among the 23 species, 8 from 3 families represent rhipidoglossan radula while among the 12 families, 4 families represent taenioglossan radula. Both docoglossan and toxoglossan radulae were represented by the very least species as well as family (Figure 1).
Different families of the gastropod showed obvious different types of radula among five known types. Individuals from the same family were observed with more or less differentiation in the same types of radula (Table 1). To be more precise, it can be said that, interfamily variation was observed but no intrafamily variation was observed in the case of radular type. With the same types of radulae, different species showed variation in radular pattern for the same family (Figures 2–5).
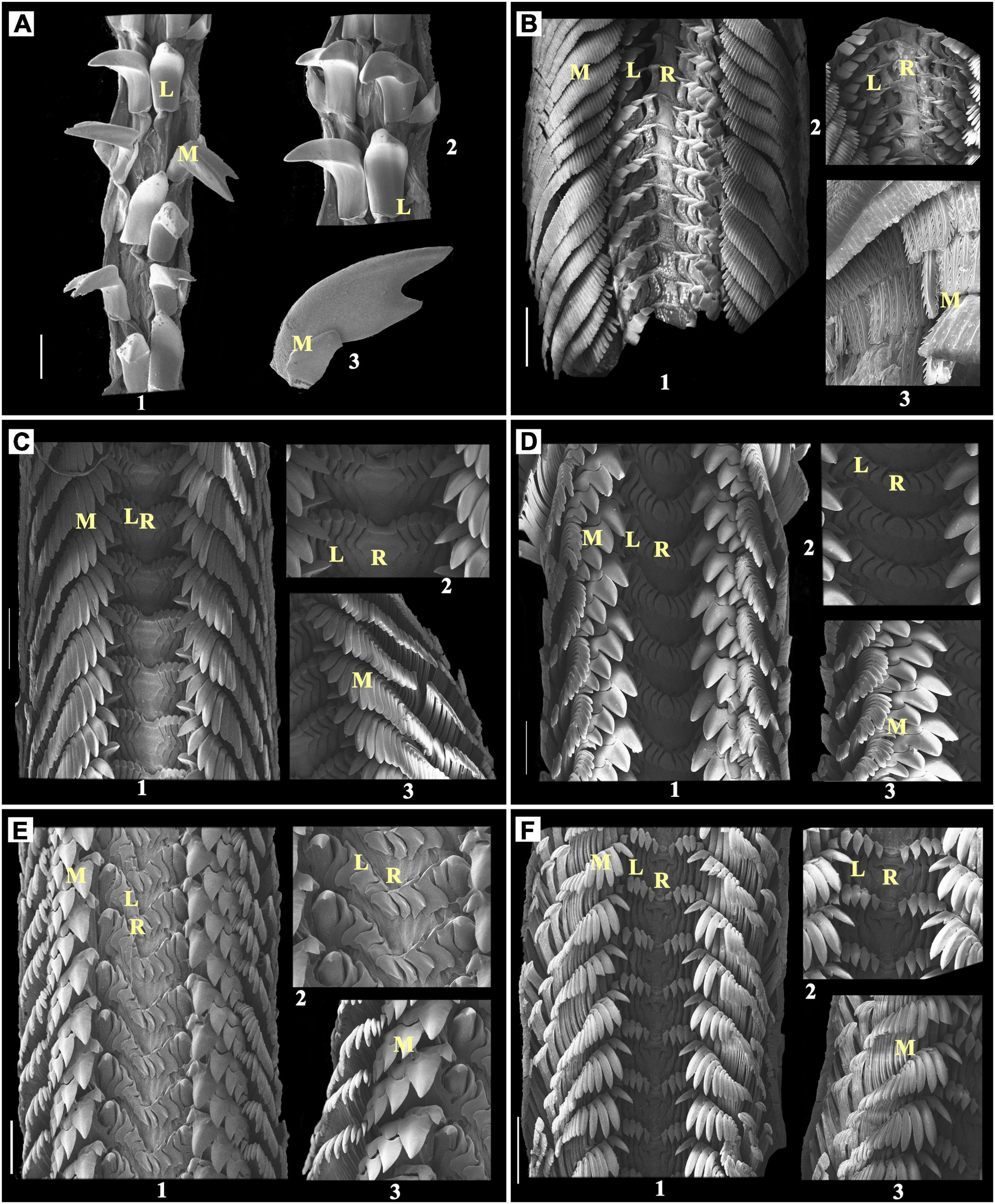
Figure 2. (A) Cellana karachiensis, Scale bar:100 μm; (B) Monodonta australis, Scale bar: 200 μm; (C) Lunella coronata, Scale bar: 300 μm; (D) Turbo bruneus, Scale bar: 200 μm; (E) Turbo intercostalis, Scale bar: 300 μm; (F) Astralium semicostatum, Scale bar: 300 μm; 1: radula; 2 and 3: enlarged part of radula; R, rachidian teeth; L, lateral teeth; M, marginal teeth.
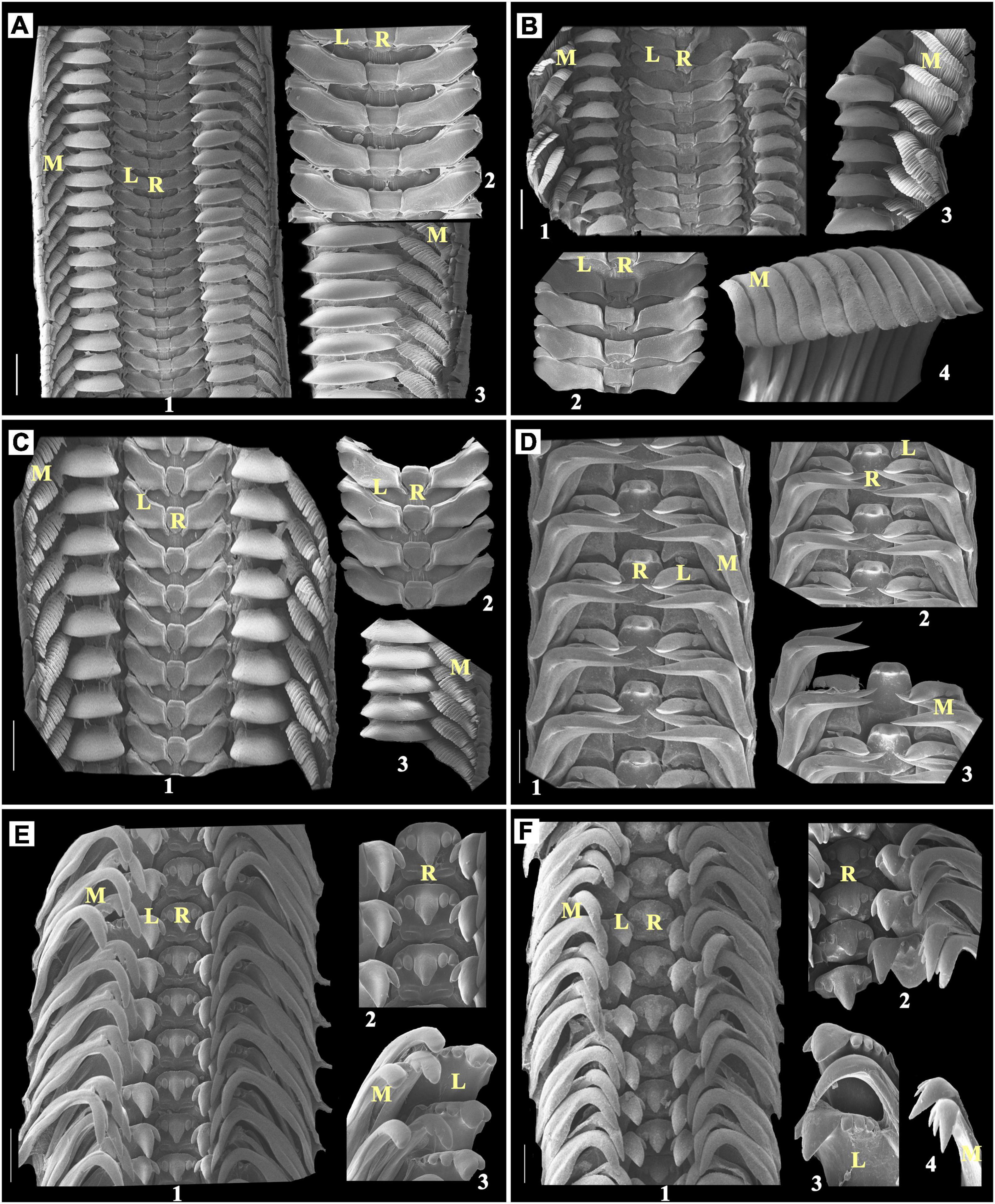
Figure 3. (A) Nerita albicilla, Scale bar: 300 μm; (B) Nerita costata, Scale bar: 300 μm; (C) Nerita undata, Scale bar: 200 μm; (D) Naria sp., Scale bar: 200 μm; (E) Cerithium coralium, Scale bar: 100 μm; (F) Rhinoclavis sinensis, Scale bar: 100 μm; 1: radula; 2 and 3: enlarged part of radula; R, rachidian teeth; L, lateral teeth; M, marginal teeth.
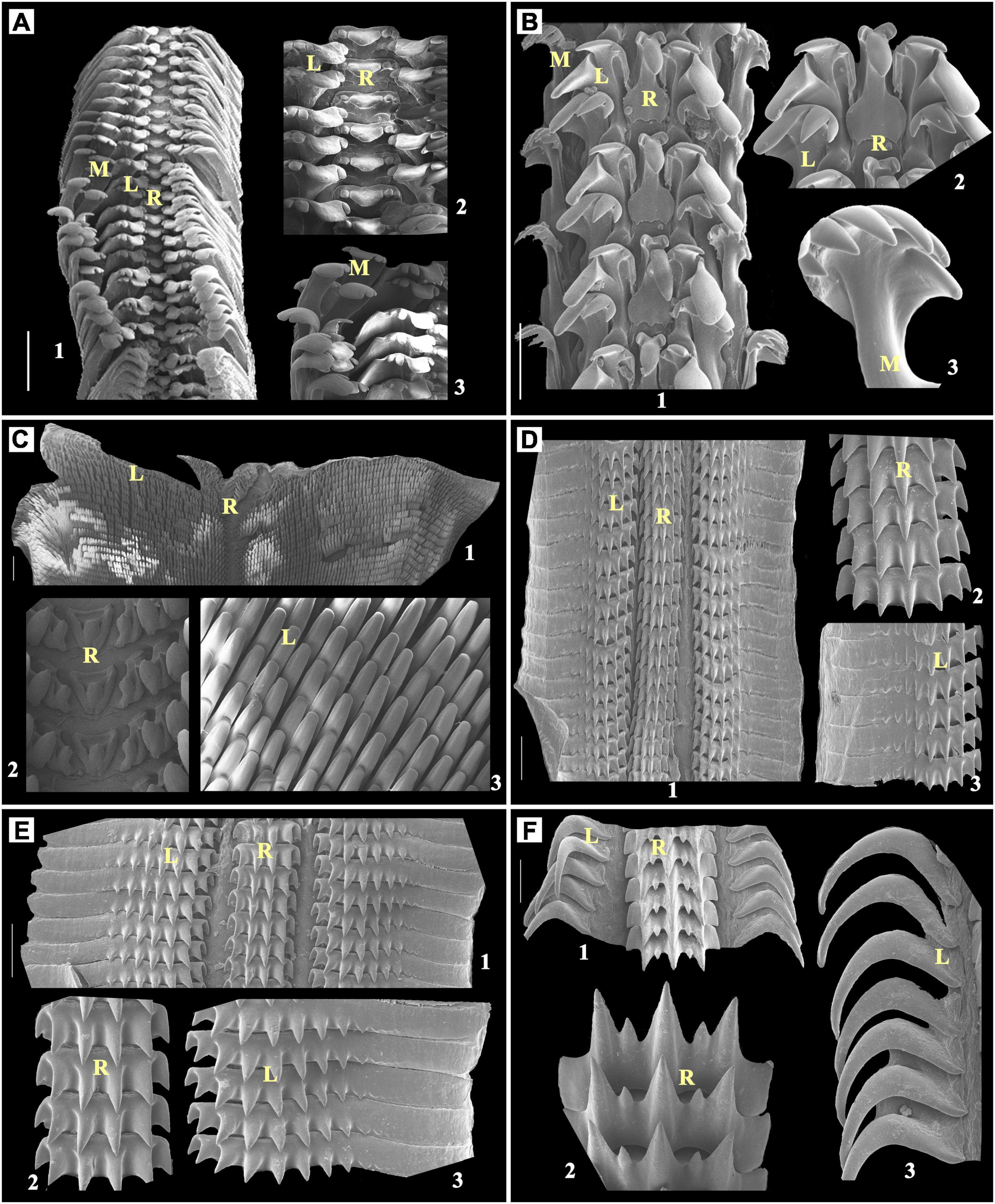
Figure 4. (A) Clypeomorus bifasciata, Scale bar: 200 μm; (B) Echinolittorina pascua, Scale bar: 50 μm; (C) Peronia verruculata, Scale bar: 500 μm; (D) Scabricola guttata, Scale bar: 50 μm; (E) Strigatella scutulata, Scale bar: 100 μm; (F) Murex sp., Scale bar: 50 μm; 1: radula; 2 and 3: enlarged part of radula; R, rachidian teeth; L, lateral teeth; M, marginal teeth.
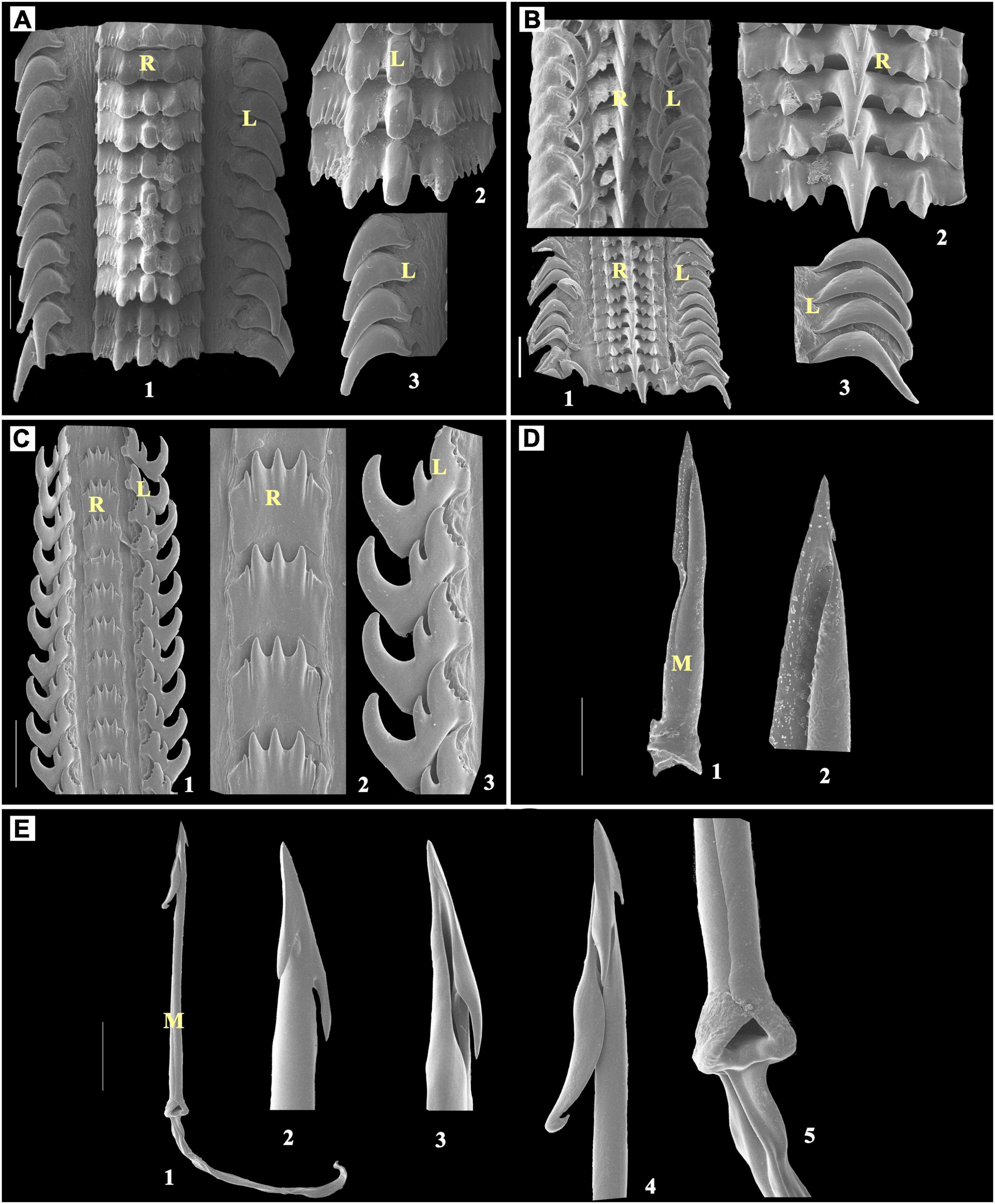
Figure 5. (A) Thais sp., Scale bar: 100 μm; (B) Tylothais savignyi, Scale bar: 50 μm; (C) Pollia undosa, Scale bar: 100 μm; (D) Conus figulinus, Scale bar: 100 μm; (E) Conus achatinus, Scale bar: 100 μm; 1: radula; 2–5: enlarged part of radula; R, rachidian teeth; L, lateral teeth; M, marginal teeth.
The docoglossan radula observed in family Nacellidae with only one representative species has a dwarf rachidian tooth with one lateral and one marginal tooth on each side of the radular ribbon.
Almost concealed or dwarf and narrow unicuspid rachidian tooth, one unicuspid pointed lateral tooth, and one bicuspid marginal tooth were observed on each side of the radular ribbon of Cellana karachiensis (Figure 2A).
The rhipidoglossan radula was represented by three different families of the gastropod, namely, Trochidae with one species, Turbinidae with four species, and Neritidae with three species. In this study, the types of radula were found similar in these three families with quite spectacular modifications of the denticles. All individuals of these three families have a single central/rachidian tooth. Among these three families, two families such as Trochidae and Turbinidae were observed with 5 or [4 + D] lateral teeth (Figure 2B–F). Marginal teeth were observed with quite different shapes and very numerous to count. In Nerita, a narrow and blunt unicuspid central/rachidian tooth was present with plate-like lateral teeth and many narrow pointed marginal teeth (Figures 3A–C).
Monodonta australis has a broad unicuspid central/rachidian tooth that has multiple serrations at the broad anterior part. The rachidian tooth is followed by a unicuspid bent finger such as five lateral teeth having multiple serrations at the sideward part of each cusp. These lateral teeth have a broader basal part and are sharp at the squared anterior edge. There are numerous narrow bases and multilayered feathery marginal teeth on both sides of lateral teeth (Figure 2B).
Lunella coronata has a broad-based unicuspid rachidian tooth, narrow unicuspid five adjacent lateral teeth and marginals looks narrow, triangular blunt at a tip on each side of the radular ribbon. The size of the marginal teeth decreases in descending array as to outer marginal teeth (Figure 2C).
Turbo bruneus has a flat, ellipsoid shape, unicuspid rachidian teeth. The size of inner marginal teeth is in the descending array while outer marginals are similar in size. Inner marginal teeth are tricuspid with a central larger cusp enclosed by two similar-shaped cusps. The multilayered feathery outer marginal teeth are multicuspid (Figure 2D).
Turbo intercostalis has a single, vase-shaped rachidian teeth followed by four rhomboid lateral teeth on each side and an immediate one pointed dominant lateral tooth at each side. Among the marginal teeth, four inner marginals are tricuspid with middle larger pointed triangular cusp surrounded by two blunt triangular cusps. These are followed by feathery multicuspid outer marginal teeth on each side (Figure 2E).
Astralium semicostatum has a single saucer-shaped rachidian tooth followed by five falcate-shaped lateral teeth. This is also followed by the bifurcated leaflet-shaped, multiple feathery marginal teeth (Figure 2F).
Nerita albicilla has one central mortar or hyoid bone-shaped rachidian teeth, which is followed by the footstep of shoe-shaped lateral teeth. The single lateral teeth are followed by an inverted saucer-shaped second lateral plate and many feather-like marginal teeth (Figure 3A). The central/rachidian tooth of Nerita costata are slightly concave shaped at both the sides, i.e., up and down (Figure 3B). The rachidian teeth of Nerita undata are urn-shaped. The shapes of lateral teeth, plates, and marginal teeth of N. costata and N. undata are similar to those of the N. albicilla with bit modifications, which can be observed in Figures 3A–C.
The taenioglossan radulae were observed in a diverse range of families. Nearly 4 representative families, namely, Cypraeidae with 1 species, Cerithiidae with 3 species, Littoridae with 1 species, and Onchidiidea with 1 species from 12 families, were identified. They all have a single functional and primarily analogous (interfamily level) central tooth. Most of the individuals have one lateral tooth while very few consist of more than one lateral tooth. In the case of marginal teeth, most have been observed with a gradient change in the pattern of tooth, and the first lateral tooth is, in most cases, dominated or bigger than others (Table 1).
In Cypraeidae, radula, the rachidian tooth observed with a moderately broad base which is curved and less pointed from the front, also has one lateral and two marginal teeth (Table 1). In Naria sp., a single rachidian tooth looks similar to Serpentes hood shape followed by one lateral tooth, where marginal teeth are observed bifurcated anteriorly into two sharp pointed structure (Figure 3D) (Naria sp.).
Cerithiidae has a broad-based rake-like multicuspid rachidian tooth with one lateral and two marginal teeth in each row of the radular ribbon.
Cerithium coralium has a single but pentafurcated rachidian tooth, in which the middle is a larger round pointed tip with two comparatively smaller similar-shaped teeth found sideways to the middle one. The single lateral teeth with rake like (mostly 5) in which the second one was found to be dominated and larger than the other. In this study, it was followed by two boomerang-shaped marginals that are also unequally bifurcated from the top (Figure 3E).
Rhinoclavis sinensis has a radula that looks exactly similar to C. coralium. When observed carefully, the radular arrangement was found to be highly overlapped, and the structure appears tightly packed as compared to C. coralium (Figure 3F).
Clypeomorus bifasciata has a pentafurcated rachidian tooth, which has a central serpent hood-shaped furcation with a broad-based curved blunt cusp with two smaller than central cusps at each side. This structure is followed by a single pentacuspid lateral tooth on each side having a larger broader blunt end. From the rachidian tooth, the inner second cusp was found broadly larger than the remaining cusps with a broader rounded tip. Then, two marginal multicuspid teeth were observed at each side, followed by these lateral teeth (Figure 4A).
Echinolittorina pascua has a maple leaf-shaped rachidian tooth with a very broader end, two lateral teeth with four cusps, i.e., outer cusps of rectangular chisel-shaped, the second one from outside cusp larger and pointed, and last one with a dwarf growth, bent, fork-shaped marginal tooth with many pointed cusps and narrow neck on each side (Figure 4B).
Each radular row of Peronia verruculata contains a tricuspid rachidian tooth. The middle cusp of the rachidian tooth is rounded and blunt at the tip. Many unicuspidal lateral teeth at each side of the rachidian tooth look similar to chisel shape, except few inner- and outermost lateral teeth that look similar to blunt triangular. Radula of P. verruculata is broader than other selected species. This kind of radular arrangement is commonly found in Siphonariids (Figure 4C).
It was observed in three different families, namely, Mitridae with two species, Muricidae with three species, and Pisaniidae with one species. In stenoglossan radula, a central tooth was found extremely variable than the other observed radular types (Figures 4D–5C) and looked similar to the bunch of small furcated pieces of a central tooth (5–8 furcation), but those together originated from a same or single broad base. A vast range of variation is observed in the pattern of lateral teeth in different families that have this type of radula. No marginal teeth were observed here.
In the representative of Mitridae family, such as Scabricola guttata has a triseriated radula, each radular row contains broad-based multicuspid rachidian tooth which has seven very short and looks similar to robust cusps. Lateral multicuspid about ten short and robust cusps situated proximally with their distal halves has smooth and wavy plate-like formation on each side of the rachidian tooth (Figure 4D).
Strigatella scutulata have been observed with similar radular structure as S. guttata. In this study, the difference was found with the rachidian tooth that has six rather than seven, which is also the same as S. guttata, very short and robust cusps (Figure 4E).
Muricidae family has three representatives. In this study, the rachidian tooth was observed with uneven multicusps with boomerang- or sickle-like distal lateral teeth.
Murex sp. contains broad-based multicuspid (seven) rachidian teeth, including a central, a consecutive cusp that is sharp and pointed, and the outermost two cuspids that look undeveloped. There are single long boomerang-shaped lateral teeth pointed at the basal end on each side of the rachidian teeth (Figure 4F).
Thais sp. was observed with a multicuspid broad-based rachidian tooth that had central bezel-shaped; this was followed by maple leaf-like trifurcated cusps at each side. These cusps are followed by multiple narrow similar-sized denticles that are single sickle-shaped lateral teeth with a blunt-ended base on each side of the rachidian teeth (Figure 5A).
Tylothais savignyi also has a multicuspid broad-based rachidian tooth similar to Thais sp. In this study, the arrangement was found to be quite loosely attached as compared to Thais sp., in which central longer sharp and pointed cusp, and small and narrower cusp on each side of the central longer cusp are followed by broad-tipped cusp and two rounded blunts and look similar to undeveloped denticles at each side. There are single falcated lateral teeth with a pointed tip with a broader base on each side of the rachidian teeth (Figure 5B).
There was only one representative of the family Pisaniidae observed during the field survey, i.e., Pollia undosa.
The rachidian tooth of P. undosa was observed with a broad base and multicuspid. It has a central triangular pointed cusp and one instant outward pointed similar-sized cusp on each side of the central cusp, which are followed by one smaller denticle that has a blunt tip. There is one lateral tooth on each side of the rachidian teeth, which has a tricuspid shape that looks similar to three attached bezels. From that, the innermost cusp is larger than the other two cusps and also has small denticles (approximately 3–4). The middle one cusp is short and sharp with a pointed tip (Figure 5C).
The toxoglossan radula has neither a central tooth nor a lateral tooth, and the structure has only marginal harpoon-like teeth observed on each side.
Conus figulinus has only single lateral harpoon-shaped tooth with a single barb at the apex and a blade on its opposite side. The longer serration opposite to the blade terminates to the waist region. The knob at the terminal end has a single prominent spur at the side opposite to the barb (Figure 5D).
In this study, in Conus achatinus, the harpoon has three barbs at the tooth apex; among them, two are short and a third is long with a recurved tip. The long shaft lacks serration and cusp. The shaft ends in an enlarged terminal knob without a spur (Figure 5E).
In this research perspective, all 23 species belonging to 12 families were observed for their shell length measurement and radular length (Figure 6). Among these families, the Nacellidae family persists in the docoglossan type of radula which can be represented in C. karachiensis in which radula is almost about five times longer than the shell length. In genus Turbo of the family Turbinidae, the radular length is found to be half of the shell length. Individuals of the family Neritidae reported almost the same ratio of shell length to the radular length. In the Cerithiidae family, shell length was noted with approximately five times larger than the radular length. Shell length of E. pascua representative of family Littorinidae was found to be very less as compared to radula, which is a very distinctive feature. In P. verruculata, the radular ribbon was very small and broad in contrary to body size as it was a shell-less organism. Individuals of the Mitridae family were observed with a small radular ribbon than the shell length, most probably either half or less than half of the shell length. Most of the individuals of the family Muricidae were observed with three times smaller radula than the shell length. In the Conidae family, the harpoon-like structure was observed differently in every species. For an instance, in Conus achatinus and in Conus figulinus, shell size was found to be very high as compared to the harpoon length (Figure 7).
The ratio of shell length and radular length is low in the animals possessing rhipidoglossan and docoglossan types of radula as compared to taenioglossan types of radula. The gastropods that contain taenioglossan types of radula were observed with a comparatively high ratio of up to 6 excluding E. pascua and Naria sp. which showed very low ratios of about 0.3 and 1.6, respectively. Individuals of stenoglossan type bearing radula have a moderate ratio. A harpoon-like radula observed in Conus sp. was identified as toxoglossan radula in which ratio is dependent on the shell size of the individuals (Figure 7).
Cellana karachiensis is the most primitive species and is shown in Figure 8, which shows a distinct clade in the phylogenetic tree. This species represents the docoglossan type of radula. In our study, rhipidoglossan types of radula are the most representative radula in the group of herbivores. The families such as Turbinidae and Neritidae show a clear difference in the phylogenetic tree, and the cladistic representation also supports our description on the radular arrangements of those families (Figure 8). In other cases, representative families in the phylogenic tree support our observed results (Figure 8). Out of 22 species, 10 are herbivores (Figure 8, green color square), 9 are carnivores (Figure 8, red color square), and 3 are detritus feeders (Figure 8, gray color square). Naria sp. is a carnivore, but its radular formula is similar to cerithiids.
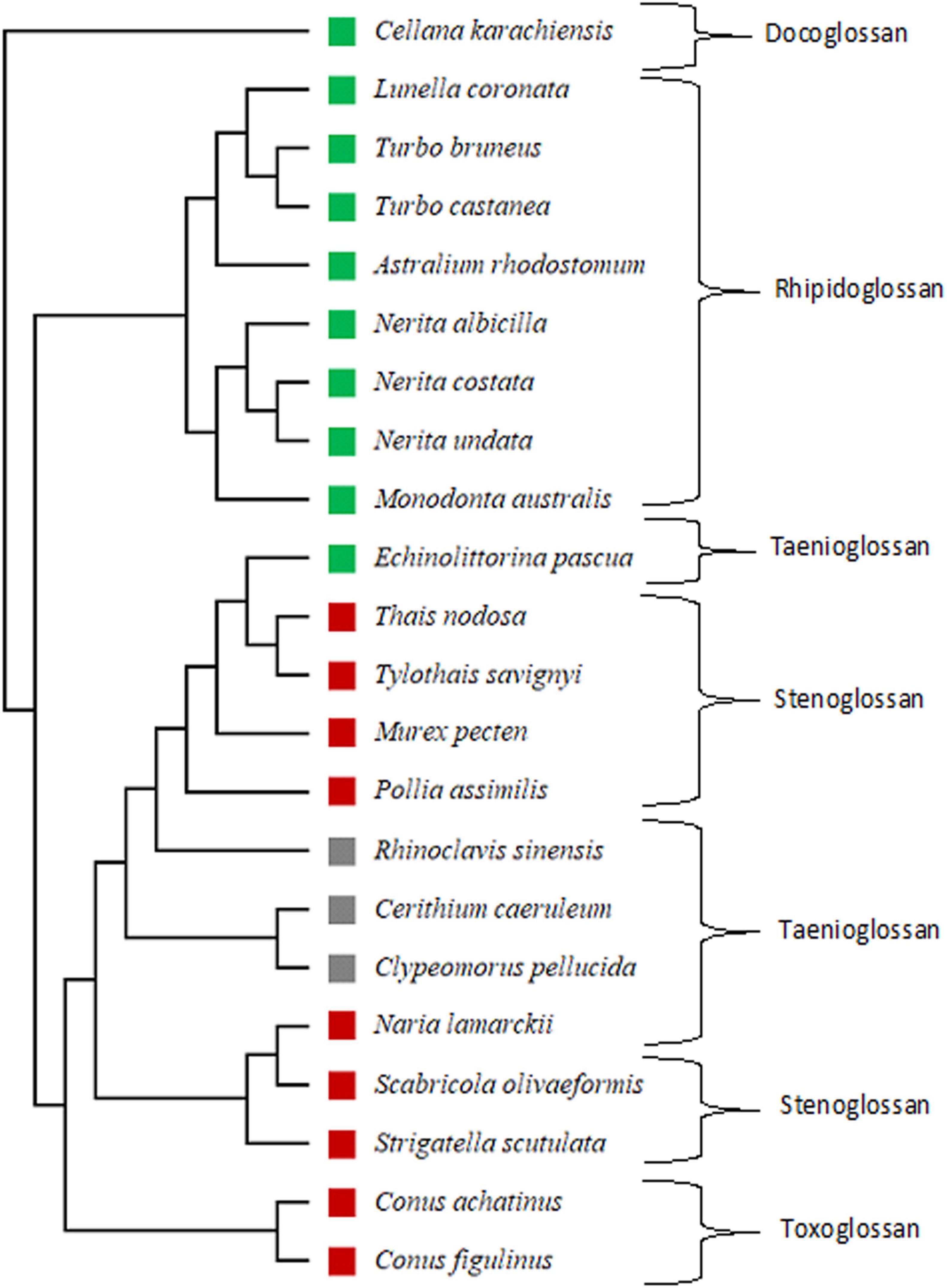
Figure 8. Phylogenetic tree based on cytochrome oxidase I (COI) gene for gastropod analyzed using UPGMA (without out-group) with Jones-Taylor-Thornton (JTT) estimate models of amino acid replacement with 1,000 bootstrap replicates in Molecular Evolutionary Genetics Analysis (MEGA11) software. Green color square: herbivore; gray color square: detritus feeder; red color square: carnivore.
The results of the phylogenetic tree with the JTT estimation model revealed that Patellogastropoda may be a primitive group and more ancient lineage among the studied gastropods. The docoglossan type of radula is a primitive and simple herbivore radula compared to the rhipidoglossan and taenioglossan. While the radula types of the carnivore indicated a gradual evolution through taenioglossan to stenoglossan and toxoglossan. Thus, the toxoglossan radula is a modern radula among all radular types. During this gradual evolution, the changes in radula from herbivores to carnivores was observed in teeth morphology and revealed from simple arrangements to complex ones. This study also revealed the evolution of radular arrangements which shows the conversation of chitinous ribbon in limpets to harpoon shape in Conus. Thus, we can conclude that the development of the radular morphology is passing through the evolutionary processes and is strongly dependent on the feeding guilds of the organisms.
In this study, the radular morphology of 23 species from 12 different families were analyzed. The collected samples from the south Saurashtra coastline were fixed and analyzed. This feeding organ is very significant in molluscan, especially in the taxonomy of the gastropod. As they are limited to a species or genus, it is useful to identify the feeding habitat of the animal (Vakani et al., 2020). Radula reveals the variances in intraspecific level better than the morphology of shell, foot, and other significant characteristics (Mutlu, 2004; Mutaf and Aksit, 2009; Vakani et al., 2020). Only through radular morphology, we could point out similarities in the family level as well as in the species level.
The docoglossan is known as the very earliest radula, which is commonly found in Patellogastropoda. The habitation of this group has been found in the initial rocky intertidal area, which itself is harsh to survive due to less algal growth and tearing wave actions of the intertidal zone. Toxoglossan radula that has the harpoon shape of denticles has been found in carnivorous species such as Conus. Thus, harpoons can be useful to sting prey and paralyze it (Kantor, 1990). The structure of radula is dependent on their size, form, the material of teeth, and the reactions between teeth and nutrition material on which they feed. This condition creates few specifications in radula which can lead to species-specific radular ribbon (Eisapour et al., 2015). A number of each type of radula, specific arrangement and structure of teeth, different functions of teeth, different diets, and different nutritional methods indicate different ecological conditions of an animal. The habitat of various species or families directly concerns with the diet, which can lead to understand the interactions and competitions between the species or family.
The docoglossan and rhipidoglossan types of radula were found in herbivore organisms. According to this study, carnivorous animals have smaller radula than herbivorous ones. In this study, C. karachiensis inhabits in spray and upper littoral zone and feeds on microalgal film from rock that represents the docoglossan type of radula in which the radular size was found to be very large as compared to shell length. The families of Turbinidae (Foster et al., 1999), Trochidae (Wakefield and Murray, 1998), and Neritidae (Aliakrinskaia, 2003) have rhipidoglossan type of radula. The enlargement of the radula in gastropods with rhipidoglossan radula type made a possible use of a larger area of substratum during the food scraping (Fretter and Graham, 1994), and they have broad radula with many narrow marginal teeth. Animals from the family Cerithiidae are mostly detritivores which consist of long marginal teeth, well adapted for capturing particles from the substratum (Morton, 1968). Cerithiids were observed with very small radula as compared to the shell size, and hence, the length of marginal teeth seems to be efficient for capturing food. E. Pascua and P. verruculata have taenioglossan type of radula and both of them are herbivores. E. Pascua lives in the upper portions of the upper littoral zone, feeds on microfilm of algae from the rock, and has a very long radular ribbon that helps to scrape microalgae and nutrients from the rock surface. P. verruculata has a very shorter radular length as compared to body length. A very broad radular ribbon was observed as compared to other gastropods with many lateral teeth that made possible use of the larger area of substratum during food scraping. Muricidae and Conidae families in the Neogastropoda group are carnivorous, but the mechanism of capturing prey is different (Watanabe and Young, 2006). Muricids have access to the soft parts of the prey which is typically obtained by boring a hole through the shell by means of a softening secretion and the scraping action of the radula. The Conidae family has a special type of toxoglossan radula, and compared to other gastropods, they use a completely different way of capturing prey. They have a movable harpoon-like structure in the radular pouch and a separate poisonous gland that is used for paralyzing their prey.
According to the study by Guralnick and De Maintenon (1997), the radula type or diet affects not only the morphological characteristics of each individual from each species but also the prey-capturing strategies and the food processing mechanism (how the whole radula teeth are used and time taken for being reconstructed), the environmental pressures (phenotypic plasticity and food competition), and the species ontogeny.
Different kinds of radula also vary in structure and arrangement of denticles of radular teeth. The carnivore animals (toxoglossan radula) required less teeth, and herbivores (rhipidoglossan radula) need more teeth than carnivores; this feature can be used to study the evolution of gastropods. A total of 23 studied species belong to 12 different families of gastropods. These types of enlisting data can be used for further experimental purposes in future. Basic knowledge about the dentition details will be useful for true inter-anticipation of taxonomy and ecology of gastropods.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
RK was a guiding faculty for this work. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are thankful to the UGC, Government of India, for support through its CAS Program. RK received the Mid-Career Award grant, and BV received the Meritorious Research Fellowship. Various related Government Agencies such as the ScHeme Of Developing High-quality research (SHODH) fellowship (NG) and Non-Governmental organizations are also acknowledged for extending their support and active help during the study. The authors are greatly thankful to the Saurashtra University, Department of Biosciences, for providing the necessary facility and giving various permissions to conduct our research. They are also thankful to Soniya Jethva, Drushita Aghera, Bhavendrakumar Chaudhry, Hitisha Baroliya, and Vishal Handa for helping them in various stages of manuscript preparations. In addition, Authors are also thankful to Rohit Bhalara, Food Testing Laboratory, Junagadh Agriculture University, for the generous support in capturing SEM images and for providing SEM facility. They also thank their reviewers for their constructive suggestions to make this manuscript more precise.
Aliakrinskaia, O. (2003). Certain Biological Traits and Morphobiochemical Adaptations to Nutrition in Strombus decorus raybaudii Nicolay and Manoja, 1983. Izv. Akad. Nauk. Ser. Biol. 30, 214–224. doi: 10.1023/A:1023297424087
Andrade, S. C. S., and Solferini, V. N. (2006). The influence of size on the radula of Littoraria angulifera (Gastropoda : Littorinidae). Malacologia 49, 1–5.
Arularasan, S., Kesavan, K., and Lyla, P. S. (2011). Scanning electron microscope (SEM) studies of Radula of the Dog Conch Strombus canarium (Gastropoda: Prosobranchia: Strombidae). Eur. J. Exp. Biol. 1, 122–127.
Cruz, R., Lins, U., and Farina, M. (1998). Minerals of the radular apparatus of Falcidens sp. (Caudofoveata) and the evolutionary implications for the phylum Mollusca. Biol. Bull. 194, 224–230. doi: 10.2307/1543051
Eisapour, M., Seyfabadi, S. J., and Daghooghi, B. (2015). Comparative radular morphology in some intertidal gastropods along Hormozgan province, Iran. J. Aquac. Res. Dev. 6:4.
Foster, G. G., Hodgson, A. N., and Balarin, M. (1999). Effect of diet on growth rate and reproductive fitness of Turbo sarmaticus (Mollusca: Vetigastropoda: Turbinidae). Mar. Biol. 134, 307–315. doi: 10.1007/s002270050548
Guralnick, R., and De Maintenon, M. J. (1997). Formation and homology of radular teeth: a case study using columbellid gastropods (Neogastropoda: Columbellidae). J. Molluscan Stud. 63, 65–77.
Guralnick, R., and Smith, K. (1999). Historical and biomechanical analysis of integration and dissociation in molluscan feeding, with special emphasis on the true limpets (Patellogastropoda: Gastropoda). J. Morphol. 241, 175–195. doi: 10.1002/(SICI)1097-4687(199908)241:2<175::AID-JMOR7>3.0.CO;2-0
Kantor, Y. I. (1990). Anatomical basis for the origin and evolution of the toxoglossan mode of feeding. Malacologia 32, 3–18.
Kruta, I., Landman, N., Rouget, I., Cecca, F., and Tafforeau, P. (2013). The radula of the Late Cretaceous scaphitid ammonite Rhaeboceras halli (Meek and Hayden, 1856). Palaeontology 56, 9–14. doi: 10.1111/j.1475-4983.2012.01188.x
Kruta, I., Landman, N. H., Mapes, R., and Pradel, A. (2014). New insights into the buccal apparatus of the Goniatitina: palaeobiological and phylogenetic implications. Lethaia 47, 38–48. doi: 10.1111/let.12036
Mutaf, B. F., and Aksit, D. (2009). Further SEM assessment of radular characters of the limpets Patella caerulea Linneaus 1758 and P. rustica Linneaus 1758 (Mollusca: Gastropoda) from Antalya Bay, Turkey. Turk. J. Zool. 33, 359–365.
Mutlu, E. (2004). Sexual dimorphisms in radula of Conomurex persicus (Gastropoda: Strombidae) in the Mediterranean Sea. Mar. Biol. 145, 693–698.
Padilla, D. K. (1989). Algal structure defenses: form and calcification in resistance to tropical limpets. Ecology 70, 835–842. doi: 10.2307/1941352
Roberts, A. (2000). A Comparison of the Feeding Behaviour and the Functional Morphology of Radula Structure in Nudibranchs. Durham: Van Mildert College, University of Durham. [PhD Thesis].
Shaw, J. A., Macey, D. J., and Brooker, L. R. (2008). Radula synthesis by three species of iron mineralizing molluscs: production rate and elemental demand. J. Mar. Biol. Assoc. U.K. 88, 597–601. doi: 10.1017/s0025315408000969
Smith, D. A. (1988). Radular kinetics during grazing in Helisoma trivolvis (Gastropoda: Pulmonata). J. Exp. Biol. 136, 89–102. doi: 10.1242/jeb.136.1.89
Taylor, J. D., Morris, N. J., and Taylor, C. N. (1980). Food specialization and the evolution of predatory prosobranch gastropods. Palaeontology 23, 375–409.
Vaghela, A. (2010). “Spatial and Temporal Variations in Population Dynamics of Few Key Rocky Intertidal Macrofauna at Anthropogenically Influenced Intertidal Shoreline. Rajkot: Saurashtra University. [PhD Thesis].
Vakani, B. (2013). Studies on the Structure of a Rocky and Muddy Inter-Tidal Assemblage. M. Phil. thesis. Rajkot: Saurashtra University.
Vakani, B., and Kundu, R. (2021). Diversity and Distribution of Siphonariid Limpets Along the Coastline of Mainland India. J. Bombay Nat. Hist. Soc. 118, 131–140. doi: 10.17087/jbnhs/2021/v118/143634
Vakani, B., Nakano, T., and Kundu, R. (2020). Diversity and taxonomy of the intertidal patellogastropod limpets of the mainland Indian coastline. Zootaxa 4728, 211–226. doi: 10.11646/zootaxa.4728.2.3
Wakefield, R. L., and Murray, S. N. (1998). Factors influencing food choice by the seaweed-eating marine snail Norrisianorrisi (Trochidae). Mar. Biol. 130, 631–642. doi: 10.1007/s002270050285
Keywords: intertidal, Gastropoda, radular morphology, shell morphology, Veraval coast
Citation: Gajera N, Vakani B and Kundu R (2022) Radular Morphology and Relationship Between Shell Size and Radula Size of Few Dominating Intertidal Gastropod Mollusks of Veraval Coast, Gujarat. Front. Mar. Sci. 9:657124. doi: 10.3389/fmars.2022.657124
Received: 22 January 2021; Accepted: 13 January 2022;
Published: 14 February 2022.
Edited by:
Mandar Nanajkar, National Institute of Oceanography, Council of Scientific and Industrial Research (CSIR), IndiaReviewed by:
Prasad Tudu, Zoological Survey of India, IndiaCopyright © 2022 Gajera, Vakani and Kundu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bhavik Vakani, YmhhdmlrLnZha2FuaUBnbWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.