- 1Laboratory of Marine Biodiversity, Third Institute of Oceanography, Ministry of Natural Resources, Xiamen, China
- 2Fisheries College, Jimei University, Xiamen, China
- 3Environment and Emergency Department, Fujian Fuqing Nuclear Power Co., Ltd., Fuqing, China
Biofouling is one of the main factors affecting the efficiency and safety of cooling water systems in coastal nuclear power plants. Understanding the population dynamics, succession rules and cumulative effects of major fouling organisms is the basis for targeted prevention and control. A 1-year simulated concrete panel test was conducted from December 2020 to November 2021 in Xinghua Bay, China. A total of 78 species of fouling organisms were recorded by combining the monthly, seasonal, semiannual, annual and monthly cumulative panels, and the community composition was dominated by nearshore warm-water species, making for a typical subtropical inner bay-type community. The fouling organisms had a peak attachment period from June to October. Significantly more attachment was observed during summer (from June to August) than during the other three seasons. The attachment amount in the second half-year (from June to November) was much higher than that in the first half-year (from December to May). The attachment thickness, density, and biomass of the bottom summer panels reached 20 cm, 105,150 ind./m2, and 19,274.50 g/m2, respectively, while those of the bottom annual panels were 40 cm, 27,300 ind./m2, and 17,762.50 g/m2, respectively. The dominant fouling organisms with calcified shells mainly included Amphibalanus reticulatus and Pernaviridis. These species had high attachment amounts,could accumulate attachments for a long time, and even might cause secondary blockage, making them the most detrimental to the safety of a cooling system. Moreover,the seasonal upward growth of hydroids and bryozoans can also significantly reduce the efficiency of cooling water intake. We suggest that targeted prevention and control should be carried out according to the larval attachment period of different dominant groups of fouling organisms during June-October, which can greatly improve the prevention and control efficiency. Strengthening the research on the biological cycle phenomenon of the main species and their main environmental impact factors, and establishing a scientific and effective early-warning model are the governance direction of formulating and implementing scientific pollution prevention and control in the future.
1. Introduction
Biofouling is a key factor affecting the safety of the water intake of the cooling water systems of coastal nuclear power plants. In recent decades, biofouling has affected the safety of the cooling water systems of coastal nuclear power plants in various countries around the world. For example, in 1981, the broken shells of Crassostrea virginica completely damaged the residual heat removal exchanger of the Brunswick Nuclear Power Plant in the United States. The attachment of fouling organisms hindered the normal cooling water intake of the Madras Atomic Power Station in India (Rajagopal et al., 1991a; Rajagopal et al., 1991b). The attachment of numerous Dreissena polymorpha lowered the safety of the cooling water systems of the Kalinin Nuclear Power Plant in Russia and the Pickering Nuclear Generating Station in Canada (Florin et al., 2013; Dixon, 2015). In 2009 and 2016, broken shells impaired the cooling water systems of the Daya Bay Nuclear Power Plant and the Fuqing Nuclear Power Plant in China (Information from internal communication).
Biofouling can reduces the efficiency or causes the failure of water intake facilities (such as barrier nets, tunnels, and gratings). Hard calcified shells in the water flow damage small pipelines, heat exchangers, and valves, thereby affecting the safety of equipment and causing serious economic losses. The reported annual global expenditure on biofouling treatment and prevention in marine projects has been estimated to be 10 billion British pounds (Satpathy, 1990). Biofouling restricts the operational safety of coastal nuclear power plants. Therefore, targeted biofouling prevention is an urgent task for coastal nuclear power plants to reduce the impact of fouling organisms on the water intake of cooling water systems.
Past studies have mostly investigated the impact of biofouling on ships (Callow, 1990), buoys (Yan et al., 2009; Zhang et al., 2015), oil and gas drilling platforms (Sammarco et al., 2004; Yan et al., 2006), farming cages (Greene and Grizzle, 2007), simulated concrete panel test (Wu et al., 2019; Liu et al., 2020; Peng et al., 2020), and other facilities (Qvarfordt et al., 2006; Lin et al., 2017), mainly from the perspectives of the species composition, attachment mechanisms, control technology, ecological effects of biofouling, and alien species (Yan and Yan, 2003; Maruzzo et al., 2011; Pradhan et al., 2011; Zhang et al., 2011; Tasso et al., 2012; Cao et al., 2013). Research on the biofouling of cooling systems of coastal power plants and related prevention and control measures began in the 1970s (Graham et al., 1975). In the early 1990s, Rajagopal et al., (1991a; b) investigated the attachment of fouling organisms to the cooling water pipes of the Madras Atomic Power Station in India through a remotely operated vehicle. Since 2000, the number of relevant studies has gradually increased (Satpathy et al., 2010; Nápoles-Rivera et al., 2012; George et al., 2016; Barath Kumar et al., 2017; Xu et al., 2021). For instance, Florin et al. traced the mussels in the Bothnian Sea that appeared in the vicinity of a nuclear power plant in Sweden (Florin et al., 2013). In recent years, several monitoring and early-warning systems and decision support systems for marine organisms in the cooling water systems of nuclear power plants have been developed and put into practice. (Tang et al., 2017; Zhang et al., 2017; Lu et al., 2018). Wood and Marsh (1999) conducted a comprehensive study on the control of bryozoans. A targeted prevention and control experiment was carried out on the attachment of Dreissena polymorpha to the Pickering Nuclear Generating Station. The focus of research on the prevention and control of blockages in the cooling water systems of nuclear power plants has shifted from passive defense measures to active prevention measures and mechanisms.
The impact of biofouling on cooling water systems has gradually attracted the attention of nuclear power plants and safety management departments. However, the relevant research work that has been carried out at present is not targeted enough, and the understanding of the temporal and spatial variation law of the species, quantity, accumulation and succession of marine fouling organisms is insufficient. The investigation and research work is often disconnected from the application management work, so that the corresponding prevention and removal measures are inappropriate. The effective prevention and control of biofouling rely on a full understanding of the attachment patterns and basic biological information of fouling organism communities. Therefore, in this study, we conducted a 1-year simulated concrete panel test in Xinghua Bay to systematically understand the community composition of fouling organisms and the larval attachment period, seasonal succession, cumulative attachment, and spatial distribution of the key fouling organism populations in this sea area, evaluated the current status and impact of biofouling, and developed economical and efficient treatment programs.
2. Materials and methods
2.1. Study area
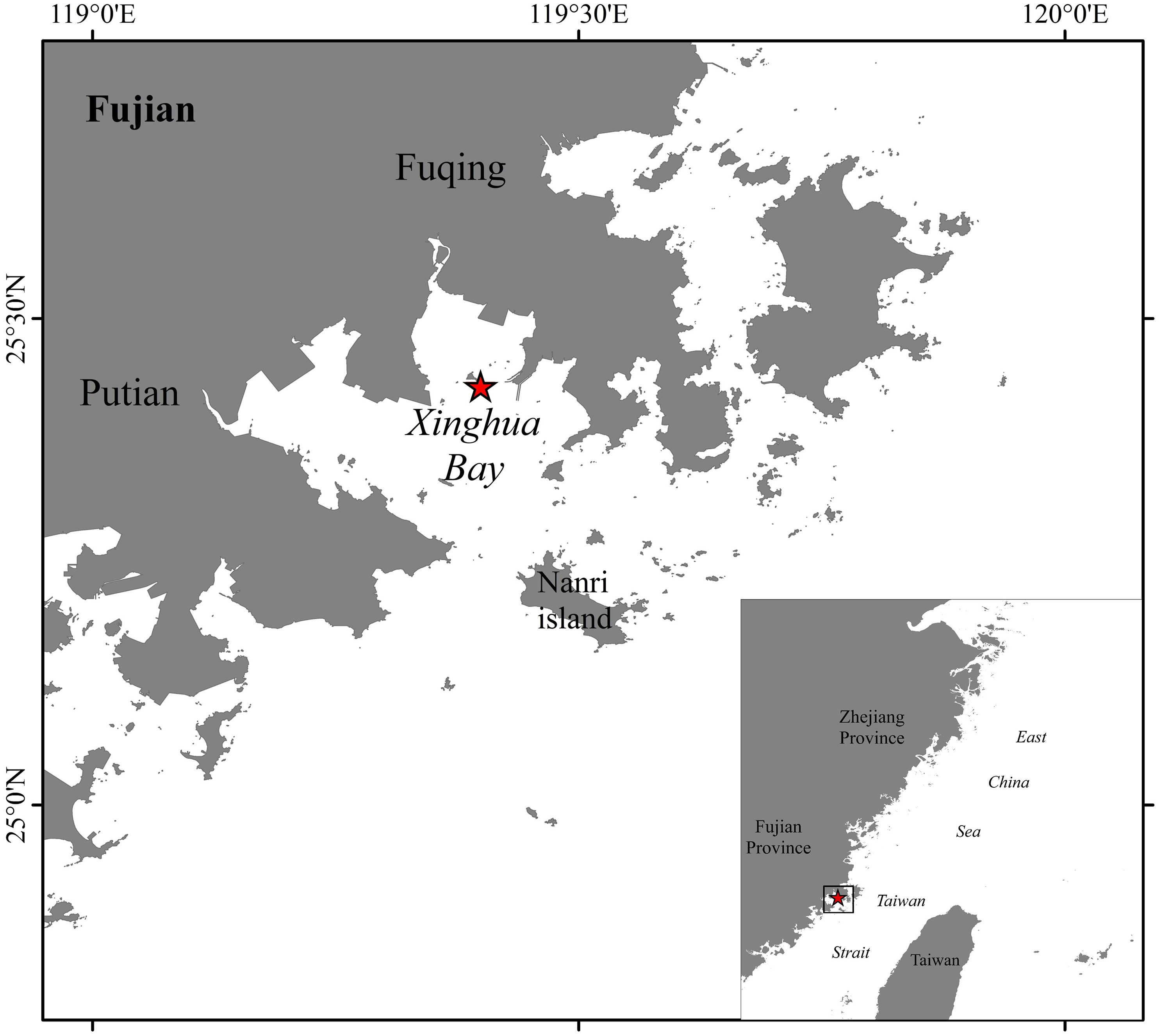
Xinghua Bay is located northwest of the Taiwan Strait. It is a semiclosed coastal bay. The mouth of the bay faces southeast, and the water flow from the bay passes the Nanri Islands and goes into the Taiwan Strait through the Xinghua waterway and the Nanri waterway. The bay has strong semidiurnal tides. This area is dominated by a subtropical marine monsoon climate, with an average annual water temperature of 19.7°C and a multiyear average seawater salinity of 31.0. The experimental site is located in the northeast of Xinghua Bay, near the Xiaomai Island, about 2.8km away from the water intake of Fuqing Nuclear Power Plant. The sea area is relatively open and the water flow is smooth (Figure 1).
2.2. Sampling strategy and laboratory methods
An experimental floating raft (Figure 2) was set up in Xinghua Bay at a water depth of 9 m. Each panel consisted of a 15-cm-long × 15-cm-wide × 2-cm-thick cement concrete test panel (Figure 2) with a surface roughness close to that of the tunnel wall to faithfully simulate the attachment of fouling organisms in actual water intake facilities. A screw hole with a diameter of approximately 0.8-1 cm was drilled at the center of each of the four corners (2.5 cm × 2.5 cm) of each cement slab, and appropriate stainless steel screws were used for fixation. The effective sampling area of each test panel was 200 cm2. The immersion cycles of the monthly test panels (MTPs), seasonal test panels (STPs), semiannual test panels (SATPs), and annual test panels (ATPs) were monthly, quarterly, semiannually, and annually, respectively. A total of 12 groups of monthly accumulation test panels (MATPs) were immersed in the water at the start, and one group of MATPs was removed every month until all groups of MATPs had been removed after 12 months (Figure 3). Each group of test panels was placed perpendicular to the sea surface in two layers (a top layer and a bottom layer). The top test panels were placed 20-30 cm below the water surface, and the bottom test panels were approximately 5 m below the water surface. The test panels and samples were preserved in a 5% formalin solution. All individuals were identified to the species level or the lowest taxonomic level possible. The biomass was recorded as the wet weight.
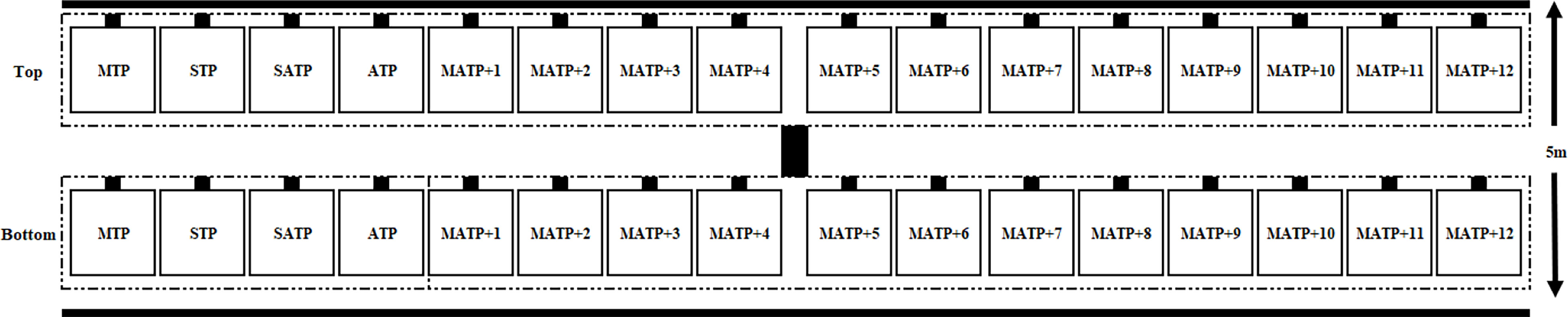
Figure 3 Deployment schematic diagram of the test panels installation. MTP, monthly test panel; STP, seasonal test panel; SATP, semi-annual test panel; ATP, annual test panel; MATP, monthly accumulation test panel.
A UTBI-001 water temperature data logger was used to simultaneously measure the water temperature of the top and bottom layers once every 2 hours. The measure of transparency was calculated using the secchi disc. The salinity, pH, and dissolved oxygen (DO) of the water body were measured with a YSI ProDSS multiparameter water quality meter.
The dominant species of the fouling organisms were analyzed using the index of relative importance (IRI) as follows:
where W is the percentage of the biomass of a particular species out of the total biomass, N is the percentage of the density of a particular species out of the total density, and F is the frequency of a particular species.
Analyses of the community structure and Bray–Curtis similarity were performed using Primer 7.0. One-way analysis of variance was conducted with SPSS 19.0 to examine the differences in parameters (such as species number, density, and biomass) of the marine fouling community. Diagrams were produced using ArcMap 10.7.
3. Results
3.1. Water temperatures
No significant difference was observed between the water temperatures of the top and bottom layers where the test panels were placed throughout the year. The average water temperature in July–September was higher than 30°C, and the average water temperature in January was the lowest out of the entire year (Figure 4).
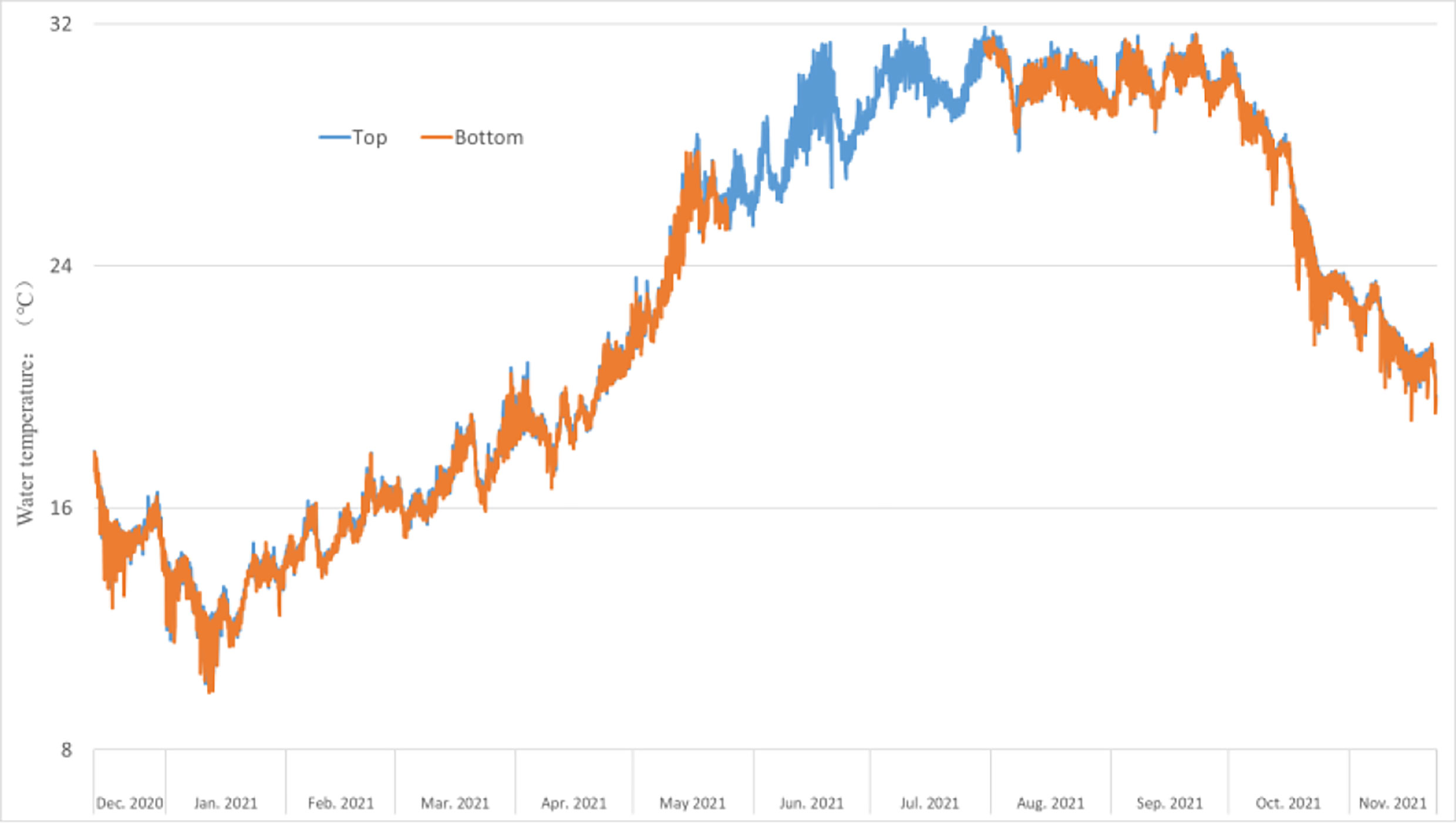
Figure 4 Trend chart of monthly average water temperature change from December 2020 to November 2021.
3.2. Species composition
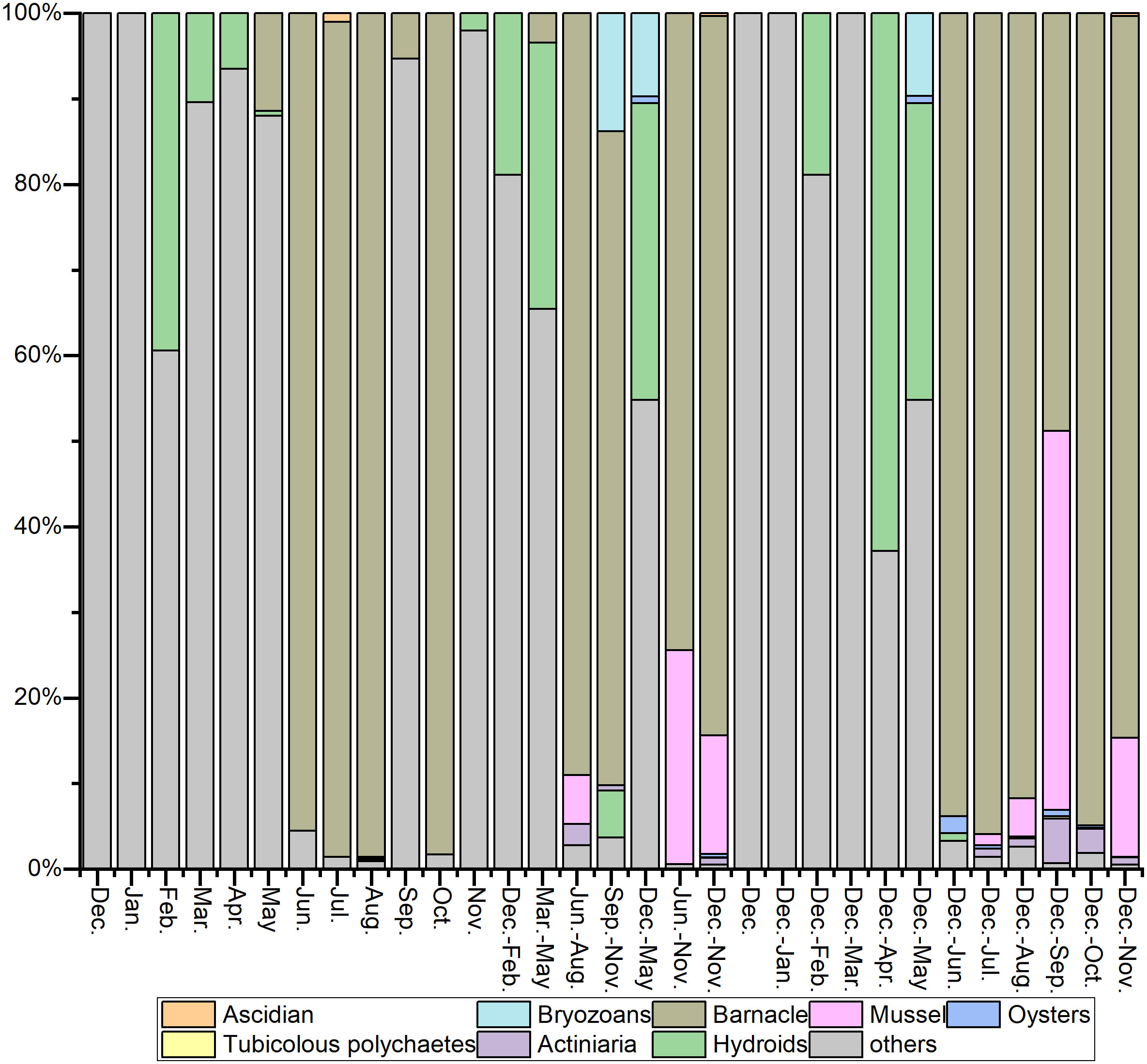
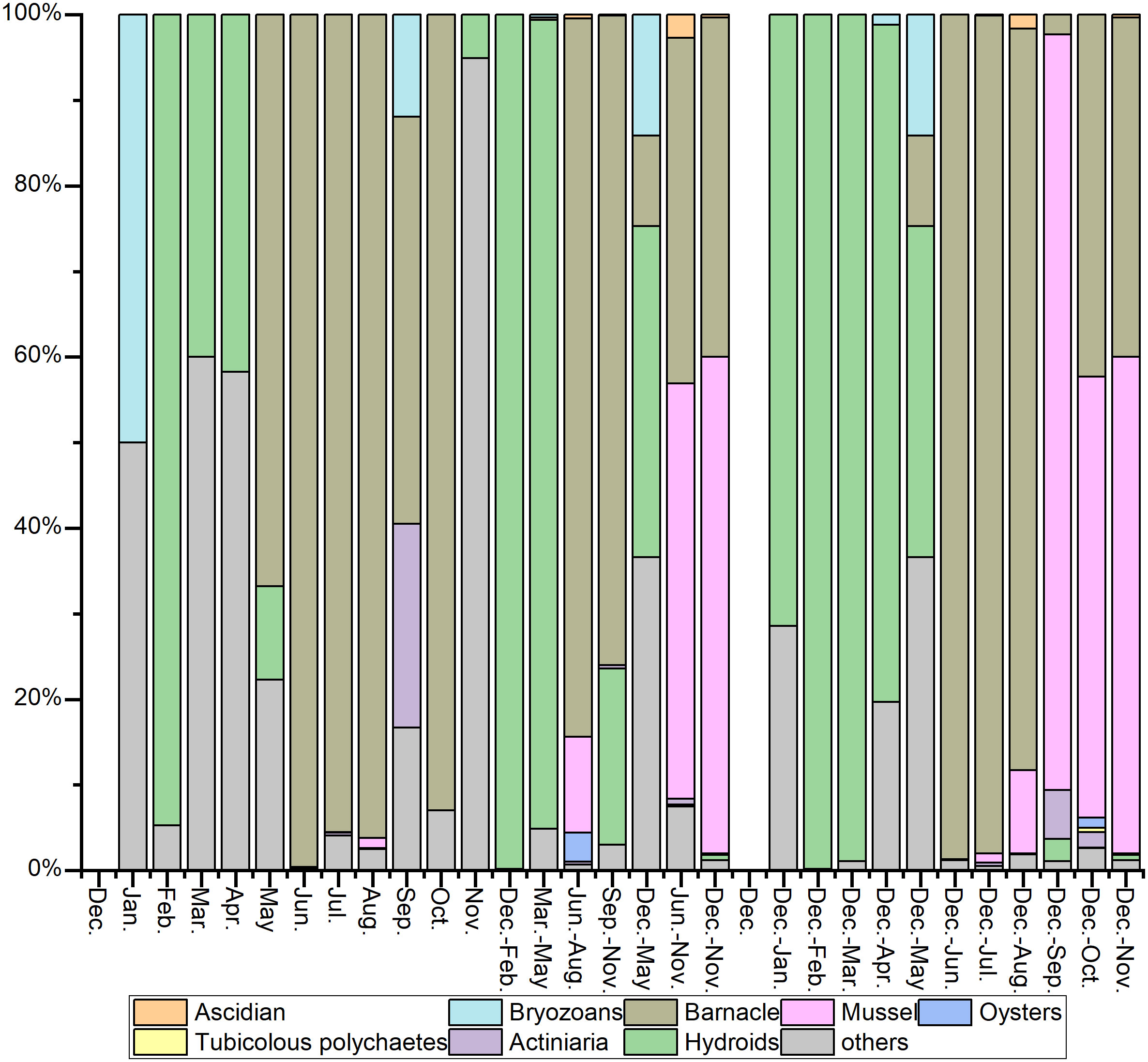
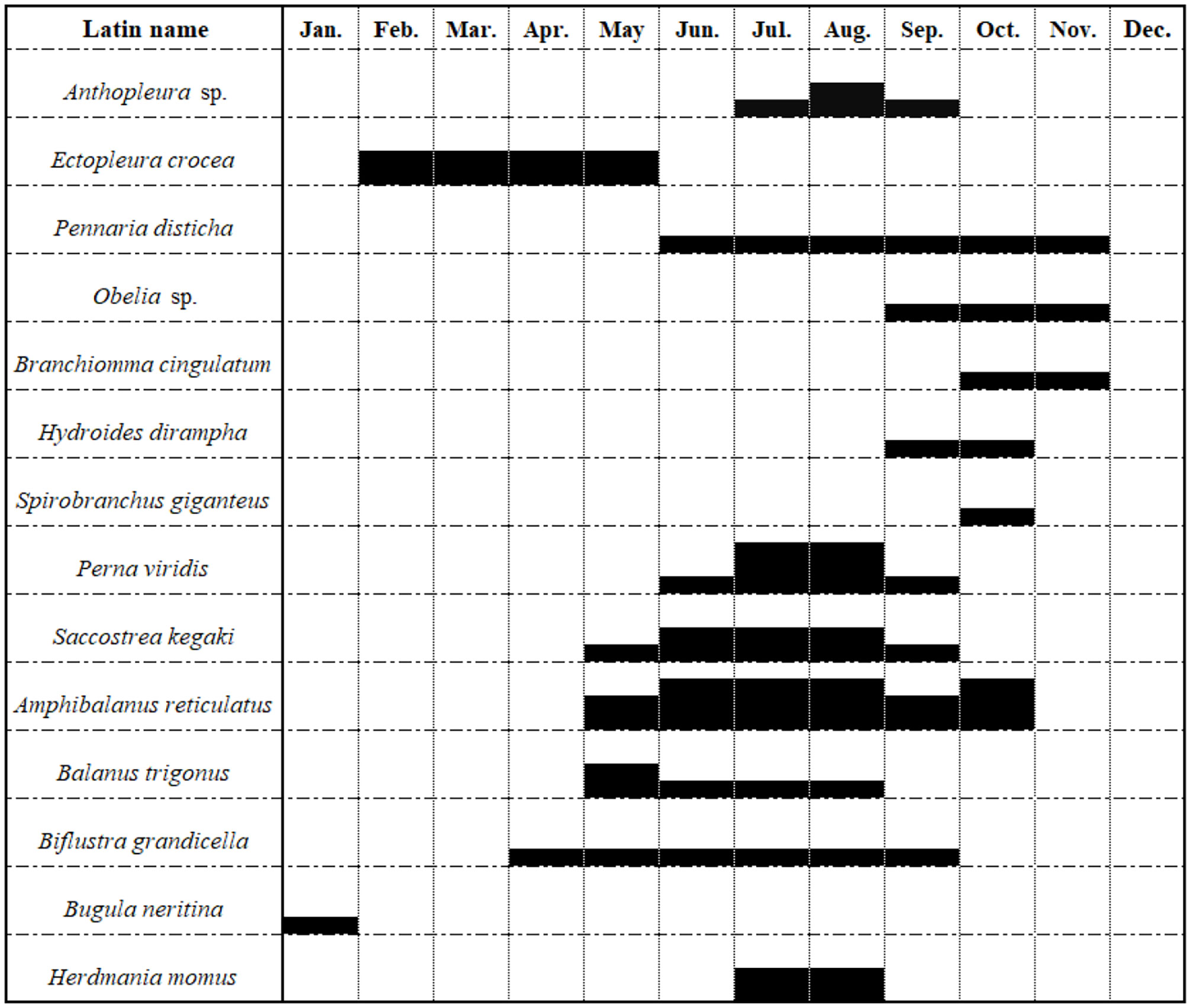
A total of 78 species of fouling organisms were recognized in this sea area based on the MTPs, STPs, SATPs, ATPs, and MATPs. According to the occurrence frequency and attachment amount, Amphibalanus reticulatus was the absolute dominant species in the fouling organism community in this sea area. Other dominant species on the STPs, SATPs, ATPs, and MATPs included Perna viridis, Podocerus brasiliensis and Caprella equilibra. Anthopleura sp., Ectopleura crocea, Pennaria disticha, Platynereis bicanaliculata and Herdmania momus were also common species in the fouling organism community in this sea area. The adherent algae on the top test panels were the main difference from the bottom test panels. The other species were not significantly different (Figures 5, 6).
3.3. Attachment amount and its spatio-temporal variation
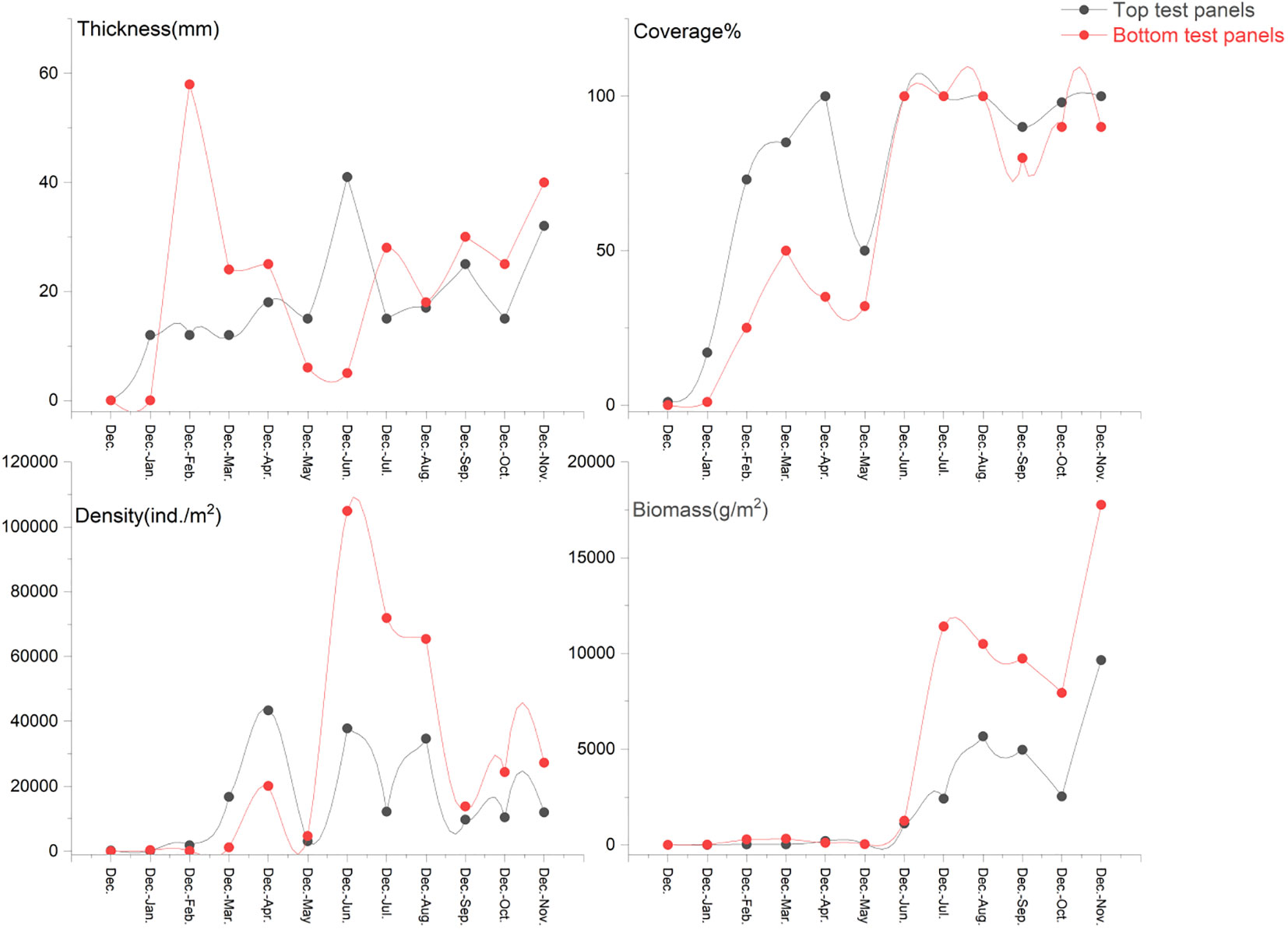
June–October was the peak period of attachment. The attachment density on the bottom panels was the highest in June, reaching 103,650 ind./m2, and the attachment biomass of the top panels was the highest in July, reaching 7085.5 g/m2. November to March was the off-season for attachment (Figures 7, 8). Significantly more attachment was observed in summer than in the other three seasons, and the same trend was observed for the second half of the year compared to the first half (Figure 9). The attachment thickness, density, and biomass of the summer (June-August) bottom STP reached 20 cm, 105,150 ind./m2, and 19,274.50g/m2, respectively. The attachment thickness, density, and biomass of the annual (December to November of next year) bottom ATP reached 40 cm, 27,300 ind./m2, and 17,762.50 g/m2, respectively. The attachment amount on the bottom test panels was slightly higher than that on the top test panels.
3.4. Seasonal succession of communities
The attachment period of the fouling organism community in this sea area can be roughly divided into four stages (Figures 10, 11).
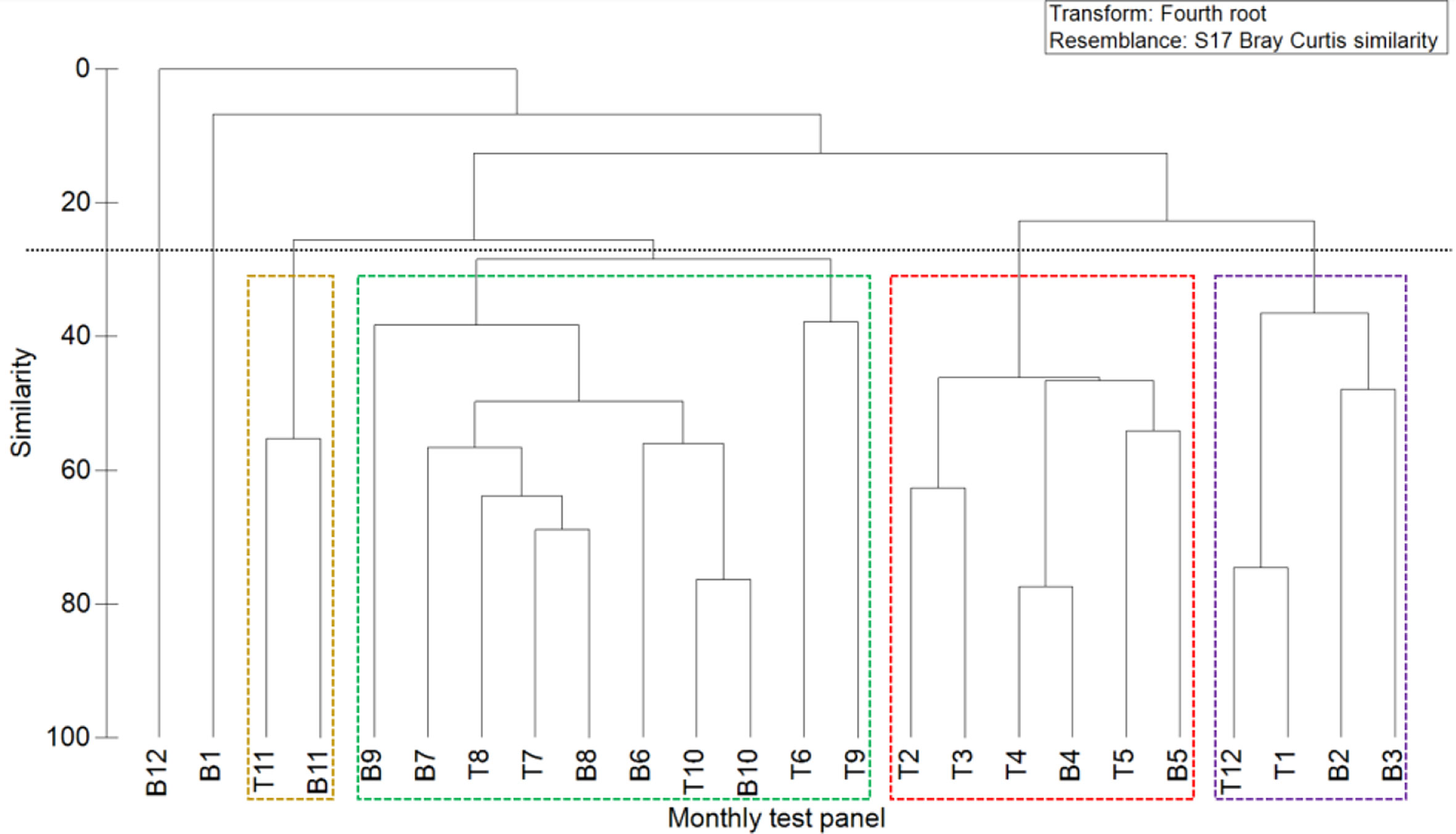
Figure 10 Clustering of the Bray–Curtis similarity of the marine fouling communities on monthly test panels.
Stage I: From December to February of the following year, the attachment density and biomass were low, and only a small number of hydroids and amphipods were attached. The representative species were E. crocea and C. equilibra.
Stage II: From March to May, the community composition was similar to that in Stage I, although the attachment amount was much higher than that in Stage I. The main populations included algae, hydroids and amphipods. The representative species were Ulva lactuca, Stenothoe gallensis, C. equilibra, Caprella scaura, and E. crocea.
Stage III: From June to October, when attachment plateaued, the attachment density and biomass were high, and the dominant fouling organisms, such as barnacles, mussels, oysters, actiniaria, tubicolous polychaetes, and bryozoans, were all observed. In particular, barnacles were absolutely dominant, and their representative species included A. reticulatus, P. viridis, Saccostrea kegaki, P. brasiliensis, and Elasmopus pectenicrus.
Stage IV: In November, attachment started gradually declining, and only a small number of hydroids and amphipods were observed. The representative species were P. brasiliensis and P. disticha.
4. Discussion
4.1. Species composition and its harm
The dominant sessile species with calcified shells are the most detrimental to the safety of cooling water systems, and the most representative sessile species is A. reticulatus, which was the dominant species by attachment amount in the fouling organism community in Xinghua Bay. During the peak period, A. reticulatus occupied almost the entire surface of the attachment substrate, and the empty shells of A. reticulatus and spaces between A. reticulatus individuals could also provide habitats, foraging space, and shelter for other migratory species.
The second most dominant species were P. viridis and S. kegaki, which had relatively large amounts of attachment. These animals can accumulate for a long time and squeeze out other small and medium-sized individuals or even occupy the entire surface of an attachment substrate. Other common fouling species with calcified shells included tubicolous polychaetes (such as Sabellidae and Hydroides).
In addition, although algae (such as Ulva), hydroids (such as E. crocea, P. disticha, Obelia), Actiniaria (such as Anthopleura), Bryozoans (such as Biflustra grandicella, Bugula neritina), and ascidians (such as H. momus) do not firmly attach to the facilities via calcified shells, they grow upward in clusters and thus reduce the water intake efficiency (Huang and Cai, 1984; Huang, 2008; Yan et al., 2021).
4.2. Community succession
The biofouling in this sea area underwent approximately four stages. The attachment density and biomass of the fouling organisms were both extremely low from December to February of the following year (E. crocea was the dominant species in Stage I), slightly increased from March to May (Ulva and E. crocea were the dominant species in Stage II), and reached their highest values in June–October when the fouling organisms fully entered the blooming period (especially in July, when the attachment density and biomass of the top MTPs reached 65,500 ind./m2 and 7085.5 g/m2, respectively, and the attachment density and biomass of the bottom MTPs reached 59450 ind./m2 and 6327.5 g/m2, respectively). Almost all dominant fouling organism populations were observed, and the representative dominant species included A. reticulatus, P. viridis, S. kegaki, Anthopleura sp., Branchiomma cingulatum, B. grandicella, and H. momus. Stage IV (after November) was the off-season for attachment, and only a small number of P. brasiliensis and P. disticha were observed.
Compared with the results of the previous study conducted in the southeastern waters of the Xinghua Bay (Wu et al., 2019), there is little difference in the community composition, and the main dominant species are still represented by A. reticulatus, P. viridis, E. crocea, B. grandicella, etc.. However, the attachment amount of fouling organisms in this survey is significantly higher than the survey results of 2017 to 2018. Taking the bottom summer panels (June-August) as an example, the density and biomass of 2020 to 2021 can reach 105150 ind/m2 and 19260.0 g/m2 respectively, which are significantly higher than the 24375 ind/m2 and 8742.1 g/m2 investigated during 2017 to 2018. This may be due to the fact that the survey site is located in the central waters, far from the shore, and the environmental conditions are more beneficial for the attachment and growth of fouling organisms.
Barnacles are often the large pioneer adherent species in fouling communities, playing an important role in the succession and development of the communities from scratch and from simple to complex (Yan et al., 2012). In general, barnacle larvae can experience two peaks of attachment. The first-generation larvae reproduced by the previous generation begin to attach in the second half of May, and their number first peaks in June or July and drops greatly in August and September (Huang and Cai, 1984; Lin et al., 2017). In the initial stage, A. reticulatus and Balanus trigonus were comparable in number, but starting in June, A. reticulatus almost completely overwhelmed B. trigonus.
With the extension of the immersion cycle of the test panels, P. viridis gradually replaced the dominant position of barnacles and even occupied most of the surface of the attachment substrate, squeezing out the living space of other dominant species, such as A. reticulatus and S. kegaki. At this point, the fouling organism community reached a relatively stable state (Huang and Cai, 1984). In addition, the attachment substrate provided more diverse and complex habitat spaces, including habitat spaces for organisms with low requirements for attachment space, such as sponges, actiniaria, ascidian, migratory polychaetes, amphipods, and crabs.
4.3. Influencing factors and prevention suggestions
Compared with other facilities, cooling water ystem facilities can significantly strengthen and accelerate the biofouling process. First, the concrete wall of the cooling water system can be an excellent habitat for the attachment and growth of fouling organisms. Second, the continuous flow of water rich in oxygen and food allows for a constant supply of new larvae. Third, the reduction in sediment deposition increases the survival rate of fouling organisms. Fourth, there is a lack of biological competition and predator threat (Huang and Cai, 1984; Huang, 2008; Satpathy et al., 2010; Lin et al., 2012; Lin et al., 2014). Temperature is the most important environmental factor in the geographical distribution of fouling organisms (Yan et al., 2020). The temperature adaptability of organisms is the essential determinant of the spatial differences of fouling organism communities across different climatic zones and is the dominant factor in the seasonal succession of biological communities on a small scale. The seasonal succession pattern in this sea area showed that 24-30°C is a relatively suitable temperature range for fouling organism larvae and that 15°C is the tolerance limit of most larvae (Yan et al., 2020). Light affects the vertical distribution of fouling organisms (Cai and Huang, 1988). Due to the high turbidity and no much light reaches deep-sea areas, almost no algae were observed below a water depth of 5 m in the study waters.
Scientifically effective control plans for fouling organisms need to be based on the environmental conditions and a comprehensive understanding of the composition of the fouling organisms in the sea area and their attachment patterns. We should determine these through targeted preliminary investigations and full research studies, especially on the breeding period, population dynamics, succession pattern, and cumulative effect of the main prevention and control strategies. Targeted and differentiated prevention and control strategies should be implemented at the initial stage of larval attachment, and the frequency and intensity of prevention and control should be increased during the peak attachment season. Only in this way can a cost-effective treatment effect be achieved. The biological control of fouling should be avoided in the adult phase to reduce the use of biological control agents and avoid secondary blockage caused by a large number of broken shells.
The focus months of the prevention and control of fouling organisms in this sea area are June to October, when both the frequency and intensity of prevention and control need to be strengthened. To ensure the continuity of prevention and control, targeted prevention and control measures can be started in late May. The focus species of prevention and control are A. reticulatus and P. viridis. The larvae of the two species have roughly the same peak season and the largest proportions of the attachment amount. In addition, their bodies can create a broader three-dimensional space for the attachment of other fouling species. A. reticulatus is superior to P. viridis in its short-term attachment strength, but the long-term threat of P. viridis is greater. The attachment intensity is low from November to early the following May, so a less strict prevention and control measure can be adopted for this period.
After decades of development of antifouling technologies for coastal power plants, a variety of prevention and control measures have been devised, including physical isolation, the high flow rate method, heat treatment, mechanical removal, chlorine production from seawater by electrolysis, and so on. The methods can be used alone or in combination. In addition, we must also better understand the life history and physiological characteristics of fouling organisms, explore the influencing factors of fouling organism attachment (temperature, water depth, flow rate, light, water quality, and attachment substrate), and establish scientific and effective early-warning models for fouling organisms. To provide scientific basis for inhibiting the attachment of fouling organisms, the formation of mucous membrane and disinfection and sterilization.
5. Conclusions
Fully understanding the composition of fouling organisms and their attachment patterns (especially the breeding periods, population dynamics, succession patterns, and cumulative effects of the main control species) is a prerequisite for the scientific prevention and control of biofouling. The research shows that the biofouling in Xinghua Bay is approximately classified into four stages, of which the attachment peak of fouling organisms is from June to October, with a large attachment density and biomass. The dominant species include A. reticulatus, P. viridis, S. kegaki, Anthopleura, Branchiomma cingulatum, B. grandicella and H. momus, etc. Of them A. reticulatus is the pioneer of fouling community, but with the extension of the immersion cycle of the test panels, P. viridis gradually replaced the dominant position and even occupied most of the surface of the attachment substrate. To implement targeted prevention and control strategies in the prosperous period of larvae of fouling organisms, and to increase the frequency and intensity of prevention and control during this period, is the key to achieve economic and efficient treatment effect. Better understanding the life history and ecological characteristics of the main control species of fouling organisms, clarifying the effects of the influencing factors on fouling organisms (such as temperature, water depth, flow rate, light, water quality, and attachment substrate), and establishing scientific and effective early-warning models are future directions for the development and implementation of scientific prevention and control measures for fouling organisms.
Contribution to the field
Fully understanding the composition of fouling organisms and their attachment patterns (especially the breeding periods, population dynamics, succession patterns, and cumulative effects of the main control species) is a prerequisite for the scientific prevention and control of biofouling. The main dominant species of fouling organisms have relatively stable attachment cycles. Adopting targeted and differentiated prevention and control strategies at the initial stage of larval attachment and increasing the frequency and intensity of prevention and control during the peak attachment period are the keys to economic and efficient control. The biological control of fouling should be avoided in the adult phase to reduce the use of biological control agents and avoid secondary blockage caused by a large number of broken shells. Better understanding the life history and ecological characteristics of the main control species of fouling organisms, clarifying the effects of the influencing factors on fouling organisms, and establishing scientific and effective early-warning models are future directions for the development and implementation of scientific prevention and control measures for fouling organisms.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
HL: Investigation and sampling, data analysis and processing, paper writing. YH: Sample identification. YL: Investigation assistance. SZ: Sample identification, data processing. SY: Data processing. KL: Data processing, drafting. JM: Investigation and sampling, sample identification. JL: Investigation and sampling, sample identification. XH: Sample identification. SF: Sample identification. WX: Investigation and sampling. ZL: Proofread and guide. All authors contributed to the article and approved the submitted version.
Acknowledgments
This work was supported by the National Science Foundation of China under contract (No. 42076237), the Scientific Research Foundation of the Third Institute of Oceanography, MNR, (No. 2018023). We thank Qiang Wang, Xiao Fan and Jie Wang from Fujian Fuqing Nuclear Power Co., Ltd. for the assistance of this study.
Conflict of interest
YL is employed by Fujian Fuqing Nuclear Power Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.1107087/full#supplementary-material
References
Barath Kumar S., Mohanty A. K., Das N. P. I., Satpathy K. K., Sarkar S. K. (2017). Impingement of marine organisms in a tropical atomic power plant cooling water system. Mar. pollut. Bull. 124 (1), 555–562. doi: 10.1016/j.marpolbul.2017.07.067
Cai R. X., Huang Z. G. (1988). Studies on the orientation of cirripedes II orientation on hosts and natural habitats. Oceanol. Limnol. Sin. 19 (4), 321–328. doi: 10.1300/J496v17n03_02
Callow M. (1990). Ship fouling: problems and solutions. Chem. Ind. Lond 54, 123–127. doi: 10.1016/0033-0655(90)85007-K
Cao W., Yan T., Li Z., Li J., Cheng Z. (2013). Fouling acorn barnacles in China–a review. Chin. J. Oceanol. Limnol. 31 (4), 699–711. doi: 10.1007/s00343-013-2275-z
Dixon D. (2015). Best management practices manual for preventing cooling water intake blockages (Palo Alto, CA: EPRI). 3002006735.
Florin A.-B., Mo K., Svensson F., Schagerstrm E., Kautsky L., Bergstrm L. (2013). First records of conrad’s false mussel, mytilopsis leucophaeata (Conrad 1831) in the southern bothnian Sea, Sweden, near a nuclear power plant. BioInvasions Records 2 (4), 1–8. doi: 10.3391/bir.2013.2.4.07
George R. P., Kamachi Mudali U., Raj B. (2016). Characterizing biofilms for biofouling and microbial corrosion control in cooling water systems. Anti-Corrosion Methods Materials 63 (6), 477–489. doi: 10.1108/acmm-07-2014-1401
Graham J. W., Moncreiff R. W., Benson P. H., Stock J. N. (1975). Heat treatment for the control of marine fouling at coastal electric generating stations. OCEAN 75 Conference. IEEE, 926–930. doi: 10.1109/oceans.1975.1154027
Greene J. K., Grizzle R. E. (2007). Successional development of fouling communities on open ocean aquaculture fish cages in the western gulf of Maine, USA. Aquaculture 262 (2-4), 289–301. doi: 10.1016/j.aquaculture.2006.11.003
Huang Z. G., Cai R. X. (1984). Marine fouling and its prevention (I) (Beijing, China: China Ocean Press).
Lin H. S., Wang J. J., Liu W., Liu K., Zhang S. Y., He X. B., et al. (2017). Fouling community characteristics in subtropical coastal waters of the southwestern East China Sea. Acta Oceanol. Sin. 36 (10), 70–78. doi: 10.1007/s13131-017-1007-1
Lin H. S., Wang J. J., Zheng C. X., Li R. G., Zheng F. W., Lin J. H., et al. (2012). Ecological research of marine fouling in Dongshan Bay, China. Acta Oceanol. Sin. 34 (6), 160–169. doi: 0253-4193(2012)06-0160-10
Lin H. S., Wang J. J., Zheng C. X., Lin J. H., Huang Y. Q., He X. B., et al. (2014). Marine fouling in quanzhou bay, China. Acta Oceanol. Sin. 36 (4), 100–109. doi: 10.3969/j.issn.0253-4193.2014.04.007
Liu K., Lin H. S., Li Z., He X. B., Huang Y. Q., Lin J. H., et al. (2020). Community structure of macro-fouling organisms in the northeastern waters of the pingtan island, East China Sea. Haiyang Xuebao 42 (6), 70–82. doi: 10.3969/j.issn.0253–4193.2020.06.009
Lu H., Meng Y., Duan Y. (2018). Research on monitoring and early-warning system of marine organisms for the intake of nuclear power plants. Anim. Husbandry Feed Sci. 10 (4), 236–240.
Maruzzo D., Conlan S., Aldred N., Clare A. S., Høeg J. T. (2011). Video observation of surface exploration in cyprids of balanus amphitrite: the movements of antennular sensory setae. Biofouling 27 (2), 225–239. doi: 10.1080/08927014.2011.555534
Nápoles-Rivera F., Bin-Mahfouz A., Jiménez-Gutiérrez A., El-Halwagi M. M., Ponce-Ortega J. M. (2012). An MINLP model for biofouling control in seawater-cooled facilities. Comput. Chem. Eng. 37, 163–171. doi: 10.1016/j.compchemeng.2011.09.008
Peng Y., Li Z., Lin H. S., Liu K., Huang Y. Q., Lin J. H., et al. (2020). Community structure and its spatio-temporal patterns of fouling organism in nearshore waters of putian, China. J. Appl. Oceanogr. 39 (1), 35–41. doi: 10.3969/J.ISSN.2095-4972.2020.01.005
Pradhan N. N., Gohad N. V., Orihuela B., Burg T. C., Birchfield S. T., Rittschof D., et al. (2011). Development of an automated algorithm for tracking and quantifying barnacle cyprid settlement behavior. J. Exp. Mar. Biol. Ecol. 410, 21–28. doi: 10.1016/j.jembe.2011.10.001
Qvarfordt S., Kautsky H., Malm T. (2006). Development of fouling communities on vertical structures in the Baltic Sea. Estuar. Coast. Shelf Sci. 67 (4), 618–628. doi: 10.1016/j.ecss.2006.01.004
Rajagopal S., Sasikumar N., Azariah J., Nair K. V. K. (1991a). Some observations on biofouling in the cooling water conduits of a coastal power plant. Biofouling 3 (4), 311–324. doi: 10.1080/08927019109378185
Rajagopal S., Venugopalan V. P., Nair K. V. K., Azariah J. (1991b). Biofouling and its control in a tropical coastal power station: A case study. Biofouling 3 (4), 325–338. doi: 10.1080/08927019109378186
Sammarco P. W., Atchison A. D., Boland G. S. (2004). Expansion of coral communities within the northern gulf of Mexico via offshore oil and gas platforms. Mar. Ecol. Prog. Ser. 280, 129–143. doi: 10.3354/meps280129
Satpathy K. K. (1990). Biofouling control measures in power plant cooling systems - a brief overview. In Nair K. V. K., Venugopalan V. P. (eds) Marine Biofouling and Power Plants, Bhabha Atomic Research Centre, Bombay (Kalpakkam, India). 153–156.
Satpathy K. K., Mohanty A. K., Sahu G., Biswas S., Selvanayagam M. (2010). Biofouling and its control in seawater cooled power plant cooling water system - a review. Nuclear power, Pavel Tsvetkov (Ed.), InTech, 191–242.Available at: http://www.intechopen.com/books/nuclear-power/biofouling-and-its-control-in-seawater-cooled-power-plantcooling-water-system-a-review-
Tang Z., Cheng F., Jin X., Sun L., Bao R., Liu Y. (2017). An automatic marine-organism monitoring system for the intake water of the nuclear power plant. Ann. Nucl. Energy 109, 208–211. doi: 10.1016/j.anucene.2017.05.040
Tasso M., Conlan S. L., Clare A. S., Werner C. (2012). Active enzyme nanocoatings affect settlement of balanus amphitrite barnacle cyprids. Advanced Funct. Materials 22 (1), 39–47. doi: 10.1002/adfm.201101173
Wood T. S., Marsh T. G. (1999). Biofouling of wastewater treatment plants by the freshwater bryozoans, Plumatellavaihiriae (Hastings, 1929). Water Res. 33 (3), 609–614. doi: 10.1016/S0043-1354(98)00274-7
Wu J. W., Li Z., Lin H. S., Liu K., Huang Y. Q., Lin J. H., et al. (2019). Community structure and its spatio-temporal patterns of marine fouling organisms in xinghua bay, China. J. Appl. Oceanogr. 38 (4), 578–584. doi: 10.3969/J.ISSN.2095-4972.2019.04.013
Xu Z. M., Jiang X., Liu Z. D., Hu Y. H., Li Y. F. (2021). Experimental investigation of microbial fouling and heat mass transfer characteristics on Ni-p modified surface of heat exchanger. J. Thermal Sci. 30, 271–278. doi: 10.1007/s11630-020-1304-4
Yan T., Li Z. F., Hu Y. F., Li X. X., Cao W. H., Luo W. J., et al. (2012). A review on the balanomorph barnacles in the coastal waters of China. Acta Ecol. Sin. 32 (16), 5230–5241. doi: 10.5846/stxb201202130185
Yan T., Lin M., Cao W., Han S., Song X. (2021). Fouling characteristics of cnidarians (Hydrozoa and anthozoa) along the coast of China. J. Oceanol. Limnol. 39, 2220–2236. doi: 10.1007/s00343-021-0242-7
Yan J., Lin M. Q., Cao W. H., Lin Y. G., Zou L. (2020). Influence of temperature on larval development and settlement of the acorn barnacle Amphibalanus reticulatus. J. Trop. Oceanogr. 39 (5), 55–61. doi: 10.11978/2019124
Yan T., Yan W. X. (2003). Fouling of offshore structures in China-a review. Biofouling 19 (sup1), 133–138. doi: 10.1080/0892701021000057927
Yan T., Yan W., Dong Y., Wang H., Yan Y., Liang G. (2006). Marine fouling of offshore installations in the northern beibu gulf of China. Int. Biodeterioration Biodegradation 58 (2), 99–105. doi: 10.1016/j.ibiod.2006.07.007
Yan T., Yan W. X., Dong Y., Wang H. J., Yan Y., Liang G. H. (2009). Marine fouling on floating installations west of dongsha islands, the northern south China Sea. Int. Biodeterioration Biodegradation 63 (8), 1079–1087. doi: 10.1016/j.ibiod.2009.09.003
Zhang H., Cao W., Wu Z., Song X., Wang J., Yan T. (2015). Biofouling on deep-sea submersible buoy systems off xisha and dongsha islands in the northern south China Sea. Int. Biodeterioration Biodegradation 104, 92–96. doi: 10.1016/j.ibiod.2015.05.003
Zhang Y. F., Wang G. C., Xu Y., Sougrat R., Qian P. Y. (2011). The effect of butenolide on behavioural and morphological changes of marine fouling species balanus amphitrite and bugula neritina. Biofouling 27 (5), 467–475. doi: 10.1080/08927014.2011.583985
Keywords: biofouling, succession, prevention and control, Xinghua Bay, cooling water system, nuclear power plant
Citation: Lin H, Huang Y, Lin Y, Zhang S, Yu S, Liu K, Mou J, Lin J, He X, Fu S, Xie W and Li Z (2023) Biofouling characteristics in Xinghua Bay of Fujian, China. Front. Mar. Sci. 9:1107087. doi: 10.3389/fmars.2022.1107087
Received: 24 November 2022; Accepted: 13 December 2022;
Published: 06 January 2023.
Edited by:
Huang Honghui, South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences (CAFS), ChinaReviewed by:
Li-Zhe Cai, Xiamen University, ChinaTao Yan, South China Sea Institute of Oceanology, Chinese Academy of Sciences (CAS), China
Copyright © 2023 Lin, Huang, Lin, Zhang, Yu, Liu, Mou, Lin, He, Fu, Xie and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heshan Lin, bGluaGVzaGFuQHRpby5vcmcuY24=; Zhongbao Li, MjAwMDYxMDAwMDEzQGptdS5lZHUuY24=
†These authors have contributed equally to this work
 Heshan Lin
Heshan Lin Yaqin Huang1,2†
Yaqin Huang1,2† Jianfeng Mou
Jianfeng Mou