
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 18 January 2023
Sec. Aquatic Physiology
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.1095246
Among all the mucosal barriers, the skin and its surrounding mucus are possibly the main defensive tool against changes in the environment that can be harmful for fish. Due to the extraction of this mucus being less invasive, the study of its production and functions has attracted great interest in recent years. However, there are still many gaps concerning the sampling process as well as the possible alterations in skin integrity and mucus composition. In the current study, the effects of skin mucus extraction were determined by comparing the effects of a single extraction (single extraction group, SEG) with those of three successive extractions separated by 3 days (repetitive extractions group, REG). Intact skin histology without mucus extraction (ØEG) and both plasma and skin mucus biomarkers and antibacterial capacities were also assessed. Regarding the skin histology and skin barrier properties, both the SEG and REG did not show differences in the intact skin. Interestingly, repetitive mucus extractions seemed to activate skin mucus turnover, significantly increasing the number of small-sized mucous cells (cell area< 100 µm2) and reducing the number of large-sized mucous cells (cell area > 150 µm2). Repetitive extractions significantly decreased the amounts of soluble protein and increased cortisol secretion. These metabolites remained unaltered in the plasma, indicating different responses in the plasma and mucus. Despite changes in the mucus biomarkers, antibacterial capacity against pathogenic bacteria (Pseudomonas anguilliseptica and Vibrio anguillarum) was maintained in both the plasma and mucus irrespective of the number of mucus extractions. Overall, the mucus sampling protocol had little effect on skin integrity and mucus antibacterial properties, only modifying the amounts of soluble protein exuded and stimulating mucous cell replacement. This protocol is a feasible and minimally invasive way of studying and monitoring fish health and welfare and can be used as an alternative or a complement to plasma analysis. This methodology can be transferred to farm culture conditions and be very useful for studying threatened species in order to preserve fish welfare.
Fish skin has evolved to perform several different functions. The skin mucosa (skin layers plus the exuded mucus) acts as a dynamic and semipermeable barrier that is involved in several functions in fish, such as osmoregulation, respiration, nutrition, and locomotion (Shephard, 1994; Esteban, 2012; Sanahuja and Ibarz, 2015). Several sensory receptors are also present on the skin surface together with chromatophores, often located under iridocytes or leucophores, which one of their function is effectively camouflaging the animal by reproducing the colors of the surroundings (Weitzman and Parenti, 2021). As a layer of active living cells, fish skin also has a set of cells specialized in the secretion of a mucus substance that covers and protects the entire surface of the fish. The main mucus-producing cells are the goblet cells that are almost universally present in the skin of fishes, proving its important secretory function. However, there are some exceptions such as the ancient lampreys and some teleost fish (Elliott, 2011). In these species, mucus is secreted by epithelial cells, which have been suggested to be the precursors of the common goblet cells. This indicates that the skin mucus acquires its composition from the skin epidermal cell complex.
Skin mucus is a complex matrix with several functions. It is mainly composed of water and mucins, which are specialized glycoprotein molecules that provide rheological, viscoelastic, and adhesive characteristics to the surface of the fish body (Fernández-Alacid et al., 2018). Proteomic studies in several fish species have revealed that skin mucus is a complex matrix with numerous proteins (Cordero et al., 2015; Sanahuja and Ibarz, 2015; Patel and Brinchmann, 2017; Fæste et al., 2020), some of which have been identified as biomarkers of stress (Pérez-Sánchez et al., 2017; Sanahuja et al., 2019a; Fernández-Montero et al., 2021). Mucus also contains several proteins with defensive enzymatic activity, such as lysozyme and several isoforms of esterases, proteases, and antiproteases, among others (Firth et al., 2000; Sanahuja et al., 2019b; Sanahuja et al., 2020; Sridhar et al., 2021). This immune capacity has been investigated in several studies, which have demonstrated the antimicrobial and antiparasitic capacities of mucus (Conforto et al., 2021; Fernández-Montero et al., 2021; Firth et al., 2000; Sanahuja et al., 2019b).
In addition to the defensive role in fish health, skin mucus possesses other interesting qualities that can be determined to evaluate fish welfare. Some studies have shown parallel reactions in the skin and its mucus in response to dietary modifications (Reyes-López et al., 2021) and in response to physical damage (Saleh et al., 2018; Sveen et al., 2019). Therefore, the sum of the properties offered by both matrices, the skin and its mucus, is crucial for the survival of these aquatic animals and for them to cope with changes in the environment. Natural changes in the water, such as in the salinity, temperature, and pH (Balebona et al., 1995; Ordóñez-Grande et al., 2020; Ordóñez-Grande et al., 2021; Wang et al., 2021), as well as the presence of contaminants (Dallarés et al., 2020; Omidi et al., 2020; Mosley et al., 2018) elicit physiological changes in the composition of skin mucus. Due to its dynamism and plasticity as well as its modulation by physiological factors, this matrix has an interesting and potentially useful role in aquaculture as an accessible indicator to evaluate the effects of new feeds and dietary additives (Firmino et al., 2021; Gisbert et al., 2021) or culture conditions in farmed fish (Vakili et al., 2021). It could also be used as an ecological indicator reflecting the impact of changes in natural environmental conditions on wild fish (Fernández-Alacid et al., 2018).
Skin mucus differs from other viscous/liquid matrices (such as blood, feces, seminal fluid, and urine) in its external location, thus being more accessible and avoiding excessive manipulation of the fish in order to obtain it. Its collection can be, at least, less invasive than that of the other above-mentioned matrices. As skin mucus is a non-conventional matrix, there is no consensus for its collection, storage, or analysis. Thus, existing methodologies in the literature use different materials and techniques. Mucus sampling procedures may alter the external barrier by affecting the upper layers of the skin, which are involved in mucus production and secretion (Elliott, 2011; Ivanova et al., 2018; Tartor et al., 2020), thus changing its efficiency as well as impacting the validity of subsequent samplings. The potential defensive ability of the skin barrier, which is composed of skin and mucus layers, can be inferred from the mucus-producing capacity and by measuring the morphological traits of mucus-related cells (Pittman et al., 2013; Dang et al., 2020). Variations in these traits indicate the health of the barrier and its potential to cope with environmental challenges. The degradation of this barrier offers access to potential opportunistic pathogens or deleterious chemicals present in the surrounding water that can affect fish health and lead to a decline in fish welfare.
In view of the recent studies proposing the evaluation of skin mucus biomarkers as a minimally-invasive method and regarding the controversy on the possible deleterious impact of its collection on skin integrity and functional capacity, we aimed to evaluate the feasibility of skin mucus utilization as a non-invasive tool by: (1) determining the systemic response against the repetitive extraction of skin mucus by analyzing several mucus and plasma biomarkers, and (2) determining the skin barrier status and the effectiveness of skin mucus against possible infections. This knowledge will be valuable in determining the utility of mucus biomarkers and developing best practices for its collection and usage to monitor fish statuses in both “indoor” and field studies.
Juvenile gilthead sea bream (277.3 ± 11.7 g) from local providers were acclimated indoors at the Center of Marine Sciences (CCMAR) in the Ramalhete marine station (Faro, Portugal). The fish were reared for one month in open-flow 1000-L fiberglass tanks supplied with running seawater pumped from a marine environment, under natural temperature (17.9 ± 0.2°C) and salinity (34.5 ± 0.1‰) conditions. The fish were exposed to a simulated natural photoperiod (October-November) and fed twice a day (2.5% w/w) with a commercial diet (Sparos, Portugal). Oxygen, pH, and ammonia were monitored daily to ensure the best culture conditions. Two weeks before the start of the experiment, 30 fish were randomly distributed between three groups in six 500-L fiberglass tanks (5 fish per tank, density 2.8 kg m-3 per tank; two tanks per group) under the same conditions as described above.
The three separate experimental groups were designed as follows: (1) a control group (Ø Extraction Group, ØEG) was kept untouched until the end of the experiment for histological purposes; (2) a Single Extraction Group (SEG), in which both skin mucus and blood were extracted at the end of the experiment; (3) and a Repetitive Extractions Group (REG), in which skin mucus was collected three times total, with a 3-day interval between the samplings, and blood was extracted at the end of the experiment.
For the first two samplings of the repetitive extraction group, the fish were lightly anesthetized (buffered 150 mg/L of MS-222, Sigma-Aldrich, Spain) to facilitate handling, avoid fish injuries and mucus loss through rubbing against the tank and other fish, and reduce stress due to manipulation. Skin mucus was immediately collected following the method described by Fernández-Alacid et al. (2018). To minimize the stress and harm to the animals, mucus was collected quickly (time per fish< 1 min) using sterile glass slides, moving from behind the operculum in a front to caudal direction on the dorsal region over the lateral line. A sterile slide was gently wiped along both sides of the animal, and the epidermal mucus was carefully pushed and collected in a sterile tube (2 mL), taking care to avoid wounds and/or any urinogenital and intestinal excretions. At the end of each extraction of the Repetitive Extraction Group (REG), fish were recovered and returned to the same tank. For the final single and repetitive samplings and after the skin mucus collection as described above, the fish were deeply anesthetized with a lethal dose of MS-222. Fish weight, standard length, and the skin mucus extraction area were measured. Blood was obtained from the caudal vein with a 2-mL heparinized syringe fitted with a 21G needle and the fish were sacrificed by cervical dislocation.
The collected mucus samples were homogenized using a sterile Teflon pestle to desegregate the mucus matrix before centrifugation at 14,000 g. The resulting mucus supernatants were collected, aliquoted, and stored at −80°C. Blood was centrifugated (13,000 g for 30 min at 4°C) to obtain plasma samples, which were stored at −80°C until use. For histological purposes, the skin was rinsed in seawater and 1 cm2 of the skin from the dorsal anterior region of the body was dissected (N = 5 fish per tank) and fixed in Bouin’s solution (Sigma-Aldrich, Madrid, Spain) for 24 h at room temperature. Overall, there were 10 histology samples per treatment: without mucus sampling, ØEG (Day 0); after one mucus sampling, SEG (Day 2); or after three mucus samplings, REG (Day 10, after three mucus extractions, with a 3-day interval between each sampling).
To determine the exudation values through single and multiple extractions, total mucus exudation was calculated by measuring: the amount of mucus collected (mg), the amount of skin mucus produced per area of extraction (mg of skin mucus·cm-2), and the quantity of skin mucus produced per fish weight (mg of skin mucus·100 g-1 of fish), following the method described in Ordóñez-Grande et al. (2020).
Both glucose and lactate were measured using commercial kits (LO-POD Glucose and LO-POD Lactate, respectively; SPINREACT®, Barcelona, Spain), which were adapted to microplates as described in Fernández-Alacid et al. (2018). Briefly, following the protocols of the manufacturers, skin mucus extract and plasma optical density (OD), in triplicate, were analyzed at λ = 505 nm with a microplate reader (Infinite 200 PRO spectrophotometer, Tecan, Spain). Values are expressed as μg of metabolite·mL−1 of skin mucus and mg of metabolite·dL−1 of plasma.
Cortisol levels were measured using an ELISA kit (IBL International, Germany), as described in Fernández-Alacid et al. (2018). Following the manufacturer’s instructions, the OD was determined (after adding 50 μL of the mucus extract, plasma, or standard solutions to the reaction solutions), in triplicate, at λ = 450 nm in a microplate reader (Infinite 200 PRO spectrophotometer, Tecan, Spain). The cortisol values are expressed as ng cortisol·mL-1 of sample.
The soluble protein concentration of homogenized mucus and plasma was determined using the Bradford assay (Bradford, 1976), with bovine serum albumin (BSA; Sigma) as the standard. Mucus samples, plasma or standard solutions (from 0 to 1.41 mg·mL-1), in triplicate, were mixed with 250 μL of the Bradford reagent and incubated for 5 min at room temperature. The OD was determined at λ = 596 nm in a microplate reader (Infinite 200 PRO spectrophotometer, Tecan, Spain). The protein values are expressed as mg protein·mL−1 of sample.
The study of mucus and plasma antibacterial activity in gilthead sea bream was performed as described in Fernandez-Alacid et al. (2021), using two different pathogenic bacteria for marine fish species: Vibrio anguillarum (CECT522T) and Pseudomonas anguilliseptica (CECT899T). V. anguillarum and P. anguilliseptica were grown in Marine Broth culture media (MB, Difco Laboratories, Detroit, MI, USA). The effect of skin mucus on bacterial growth was determined by monitoring the absorbance of the bacterial cultures grown in flat-bottomed 96-well plates. Each well was filled with 100 μL of the bacterial suspension (OD = 0.2) in the culture media plus 100 μL of skin mucus (4 μg·μL−1 of mucus protein) to obtain a final volume of 200 μL. Additionally, a bacterial growth control was prepared by adding 100 μL of the bacterial suspension (OD = 0.2) to 100 μL of the culture media. The absorbance was measured at λ= 400 nm every 30 min for 14 h at 25°C in flat-bottomed 96-well plates. All assays were performed in triplicate (methodological replicates). Data are presented as growth curves (increased absorbance at λ = 400 nm per unit of time) and as a percentage of inhibition with respect to bacterial growth for every two hours of co-culture with skin mucus.
After 24 h of fixation with Bouin’s solution at room temperature, the samples were dehydrated in a graded series of ethanol and stored at 4°C. The samples were cleaned with xylene, embedded in paraffin, and cut into serial sections (3-µm thick). After dewaxing and rehydration, the sections were mounted on glass slides. The slides were stained using a periodic acid-Schiff (PAS) and Alcian blue (AB) staining protocol. The morphometric slides were digitalized (Hamamatsu NanoZoomer 2.0-HT, Hamamatsu Photonics K.K., Hamamatsu City, Japan). Digital images (600 dpi) were processed and analyzed using an image analysis software (ImageJ 1.52, National Institutes of Health, USA). Measurements of epidermis thickness as well as mucous cell measurements were based on the analysis of randomly chosen fields from each skin sample.
The skin barrier status was assessed as described in Dang et al. (2020) using 3 different indices:
(a) Mean mucous cell (MC) area (µm2)
(b)
(c)
Results are presented as mean values ± standard error of the mean (SEM). The data were checked for normality and homoscedasticity prior to analysis. The Shapiro-Wilk test was first used to ensure the normal distribution of the data, while the uniformity of the variances was determined by Levene’s test. Comparison between the skin mucus and plasma parameters as well as of the antibacterial activity between the SEG and REG were evaluated by Student’s t-test. Histological parameters were analyzed by one-way ANOVA followed by the post-hoc Bonferroni test (if variances among the groups were equal) or Dunnett’s test (for unbalanced variances) to assess the effect of the extractions (ØEG, SEG, and REG). Statistical analysis was performed using SPSS Statistics for Windows, version 22.0 (IBM Corp.; Armonk, NY, USA).
The comparisons of the mucus exudation parameters between a single mucus sampling and repetitive mucus sampling, including the amount of collected mucus as well as the mucus biomarkers, are summarized in Table 1. No differences were observed in the amount of mucus collected, which was slightly, but not significantly, higher (by around 20%) in the fish subjected to repetitive extractions. Consequently, the relationship of the amount of mucus collected with the extraction area or fish weight was also not affected by the number of extractions.
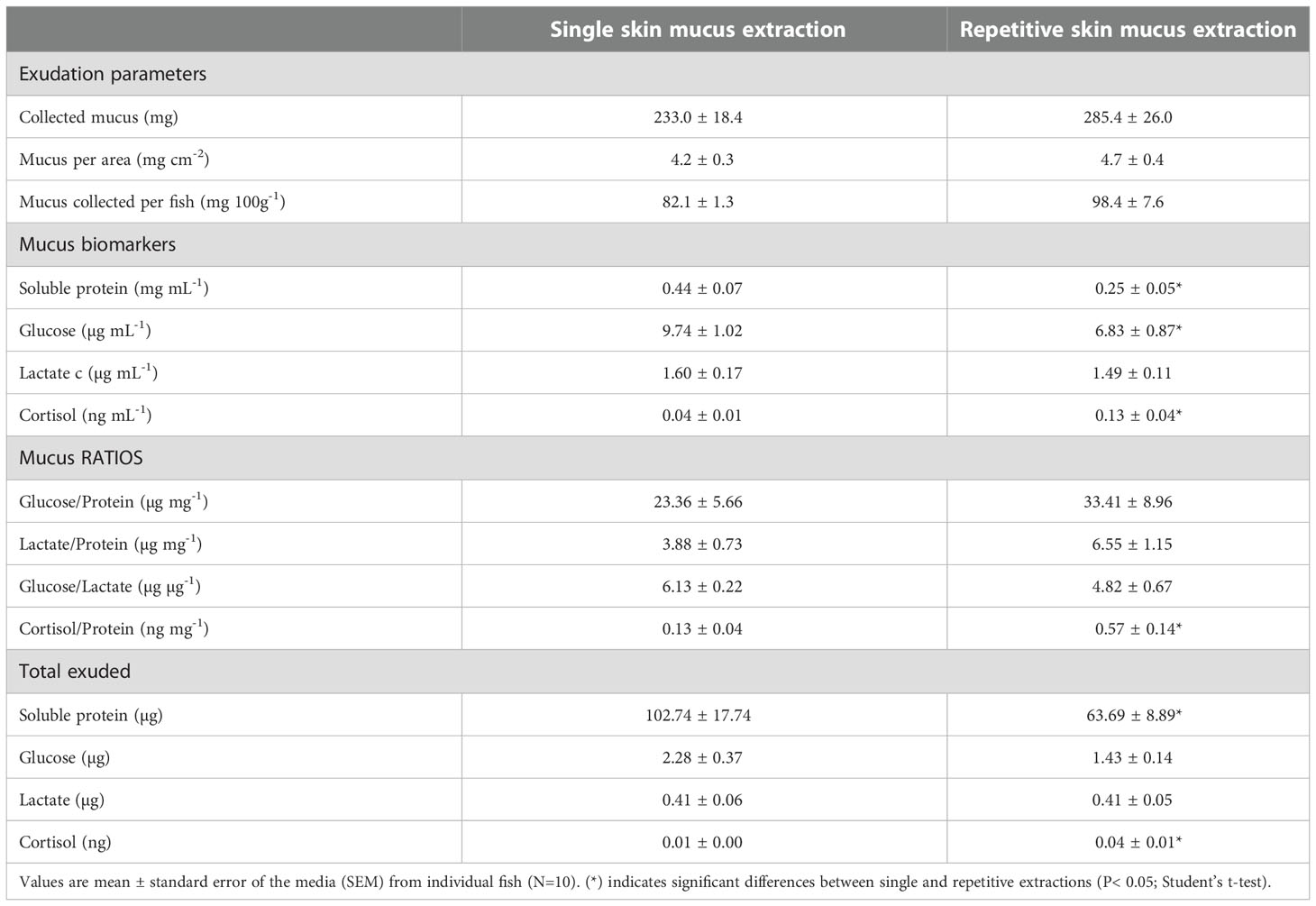
Table 1 Exudation parameters and Skin Mucus-Associated Biomarkers (SMABs) of gilthead sea bream skin mucus subjected to single or repetitive extractions.
Regarding the main mucus biomarkers, the abundance of the soluble protein content was lower (P< 0.05) after repetitive extractions. In parallel, the mucus glucose level was significantly reduced by about 30% (P< 0.05), whereas the mucus lactate level was not modified. Moreover, the mucus cortisol levels increased 3-fold in response to the repetitive extractions (P< 0.05). Despite a slight increase in the total amount of mucus exuded under repetitive extractions, the total levels of the mucus biomarkers showed the same composition as in the relative amount (per mL of mucus). However, when the mucus ratios were calculated (glucose/protein; lactate/protein; glucose/lactate; and cortisol/protein), no differences were observed between the single extraction and the repetitive extractions, except for the cortisol/protein ratio.
The impact of the repetitive extractions on the antibacterial activity of skin mucus against the pathogenic bacteria V. anguillarum and P. anguilliseptica in co-culture is shown in Figure 1, which displays the respective growth curves (Figures 1A, B) and the calculated inhibitory effect (Figures 1C, D). Regarding the antibacterial capacity of the mucus against V. anguillarum, both types of mucus samples (from the SEG and REG) delayed bacterial growth throughout the experimental period when compared to mucus-free bacterial growth (Figure 1A), inducing a growth inhibition of about 20-30% (Figure 1C). A slight decrease in the inhibitory power was detected for the samples obtained from repetitive extractions at the end of the co-culture period (10 h -14 h, P< 0.05). Skin mucus from both the SEG and REG showed a great capacity of inhibiting P. anguilliseptica (Figure 1B), reaching inhibitory values of over 60% at 8-10 h of co-culture, with no differences between the SEG and REG (Figure 1D).
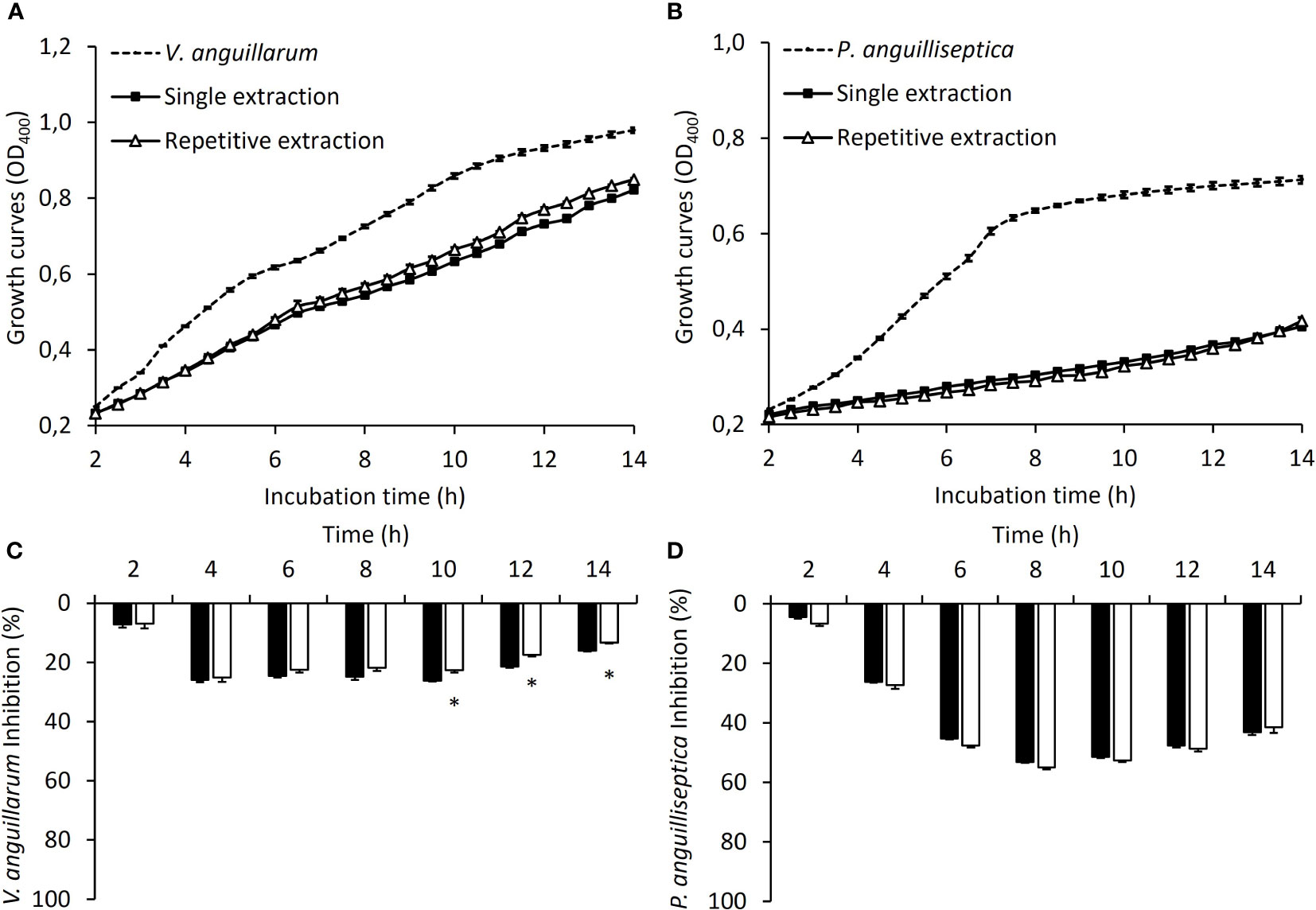
Figure 1 The antibacterial properties of juvenile gilthead sea bream skin mucus. Antibacterial activity against V. anguillarum (A) and P. anguilliseptica (B) of skin mucus obtained from a single extraction (SEG; black squares) or after repetitive extractions (REG; white triangles). Dashed lines correspond to the growth of both pathogenic bacteria used as a control. Inhibition rates against V. anguillarum (C) and P. anguilliseptica (D) for the skin mucus obtained from a single extraction (SEG; black bars) or after repetitive extractions (REG; white bars). (*) indicates significant differences between the single extraction and repetitive extractions (P< 0.05; Student’s t-test; N = 3).
Plasma soluble biomarkers (Table 2), in contrast to the mucus biomarkers, did not show differences between the SEG and REG. Their levels were 23-25 mg mL-1 for soluble protein, 60-70 mg dL-1 for glucose, 24-25 mg dL-1 for lactate, and 3 ng mL-1 for cortisol, indicating a lack of effect of the repetitive extractions with respect to the single extraction.
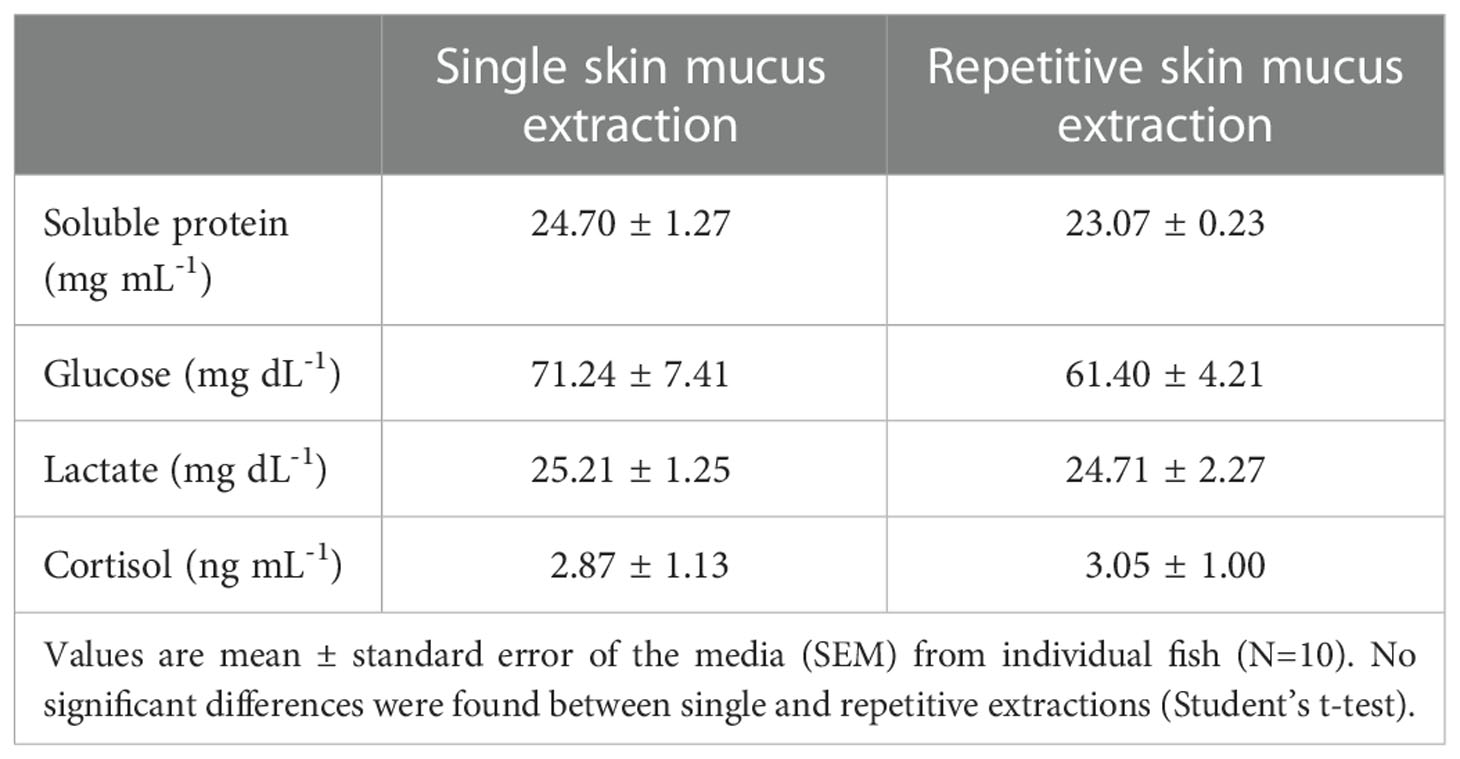
Table 2 Plasma biomarkers of gilthead sea bream subjected to single or repetitive skin mucus extractions.
Figure 2 shows plasma antibacterial activity against the two pathogenic bacterial strains in co-culture. Regarding the plasma antibacterial capacity against V. anguillarum, both conditions (single extraction and repetitive extractions) showed little capacity to inhibit bacterial growth during the first hours of co-culture, with the inhibitory growth capacity becoming evident only at the end of co-culture (10-12 h of co-culture), with no differences between the SEG and REG. By contrast, plasma from both conditions promoted an OD increase in P. anguilliseptica co-culture from 6 h onwards.
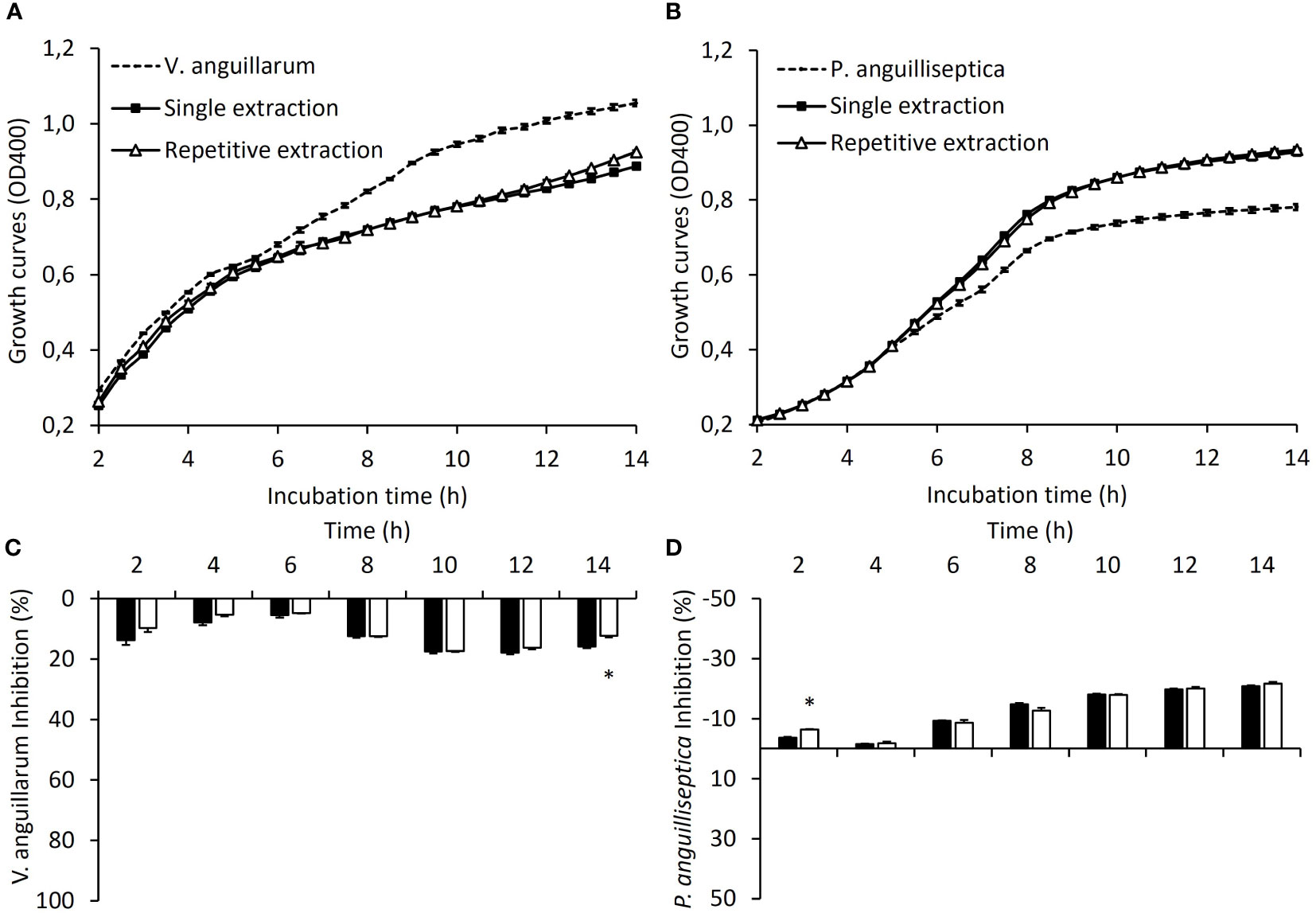
Figure 2 The antibacterial properties of juvenile gilthead sea bream plasma. Antibacterial activity against V. anguillarum (A) and P. anguilliseptica (B) of plasma obtained after a single skin mucus extraction (SEG; black squares) or after repetitive skin mucus extractions (REG; white triangles). Dashed lines correspond to the growth of both pathogenic bacteria used as a control. Inhibition rates against V. anguillarum (C) and P. anguilliseptica (D) for plasma obtained after a single skin mucus extraction (black bars) or after repetitive skin mucus extractions (white bars). (*) indicates significant differences between the single extraction and repetitive extractions (P< 0.05; Student’s t-test; N = 3).
To study the effects of the mechanical impact of a single mucus extraction or repetitive mucus extractions on the integrity of the skin layers, a control group of fishes was used as the reference condition (Figure 3). Skin integrity was evaluated by measuring the histological properties of the epidermal layer (thickness, mucous cell density, and relative mucus exudation) together with the indices of the skin mucus barrier properties (data in Table 3). Interestingly, the mucus extraction protocol for single or repetitive extractions did not alter the epidermal thickness or the indices of the barrier properties. Mucous cell density tended to increase with the number of mucus extractions, but this was not statistically significant. However, a deeper analysis of the mucous cell shape showed that the percentage of larger mucous cells (> 150 µm2) had decreased by half (P< 0.05) for both mucus extraction conditions, whereas the number of smaller mucous cells (< 100 µm2) increased with the number of mucus extractions (Figure 4).
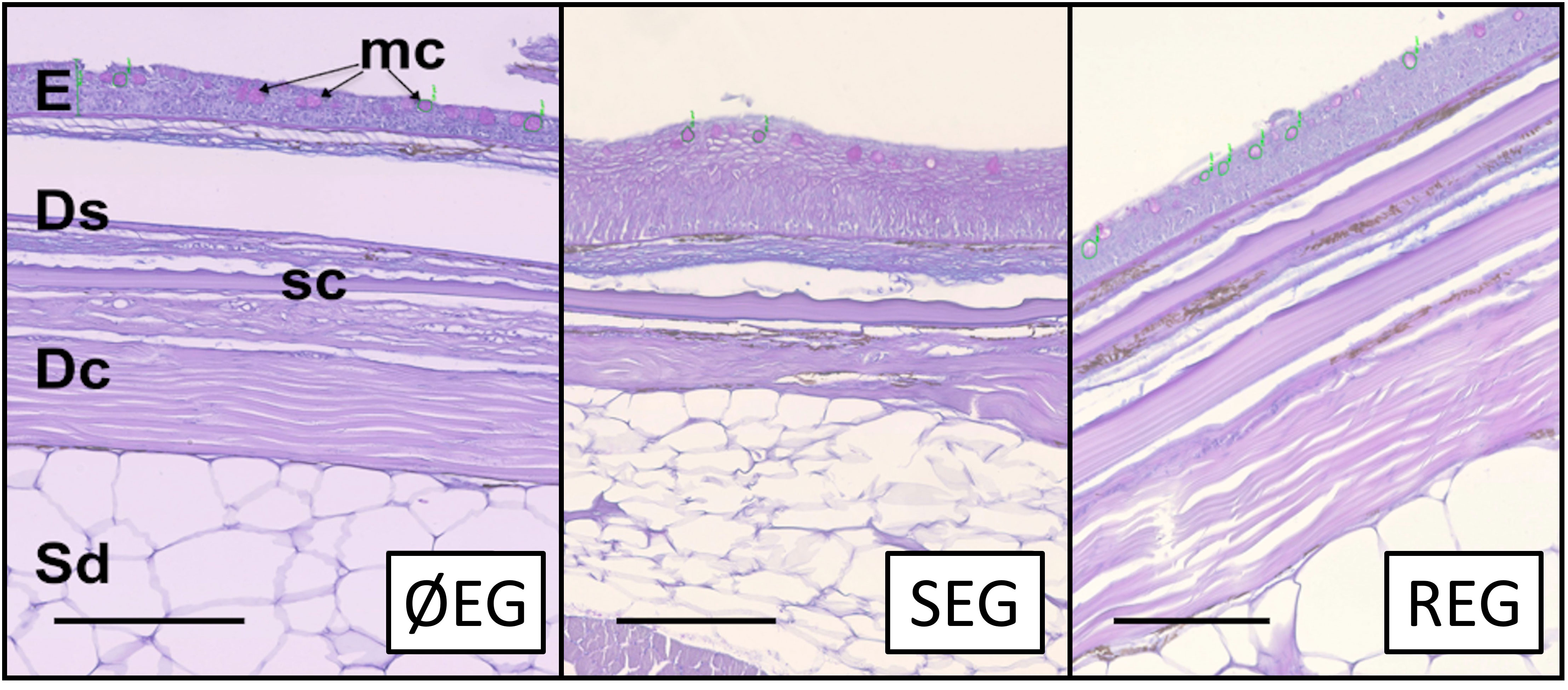
Figure 3 Histological sections for non-sampled (ØEG), single-sampling (SEG), and repeated-sampling (REG) treatments (N = 5). The epidermis (E) presented magenta-stained mucous cells (mc) showing neutral mucin. The stratum spongiosum (Ds) and stratum compactum (Dc) of the dermis as well as the scales (sc) and subdermal space (Sd) presented no abnormalities. Stain: PAS and Alcian blue. Scale bar: 100 µm.
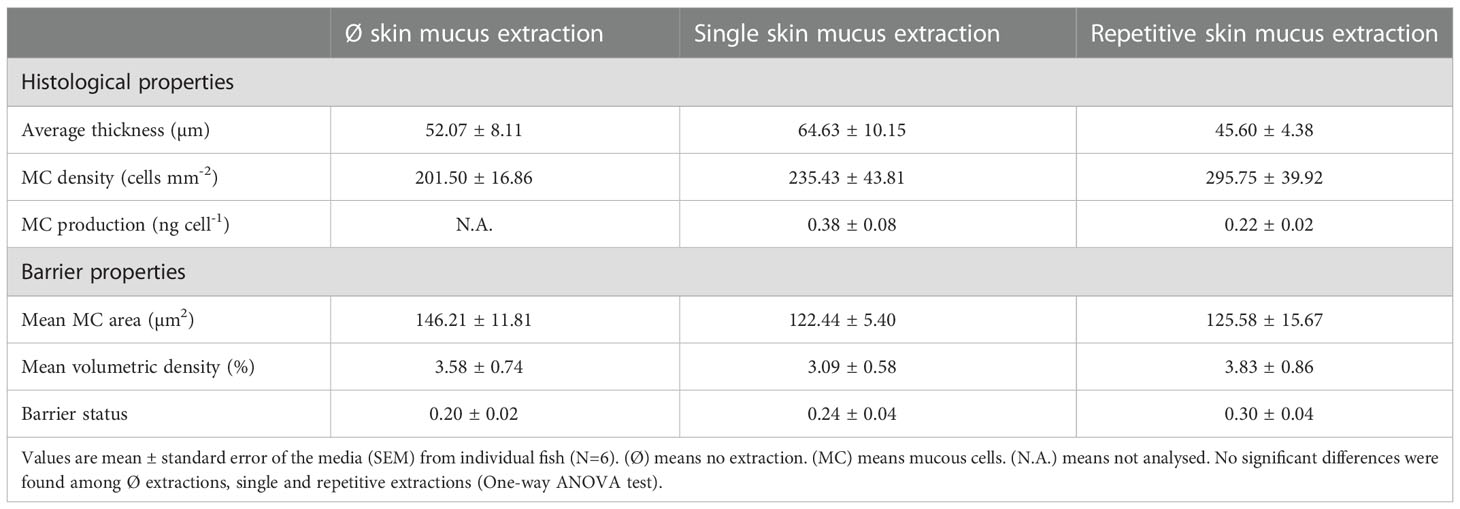
Table 3 Histological and barrier properties of gilthead sea bream skin subjected to zero, single or repetitive skin mucus extractions.
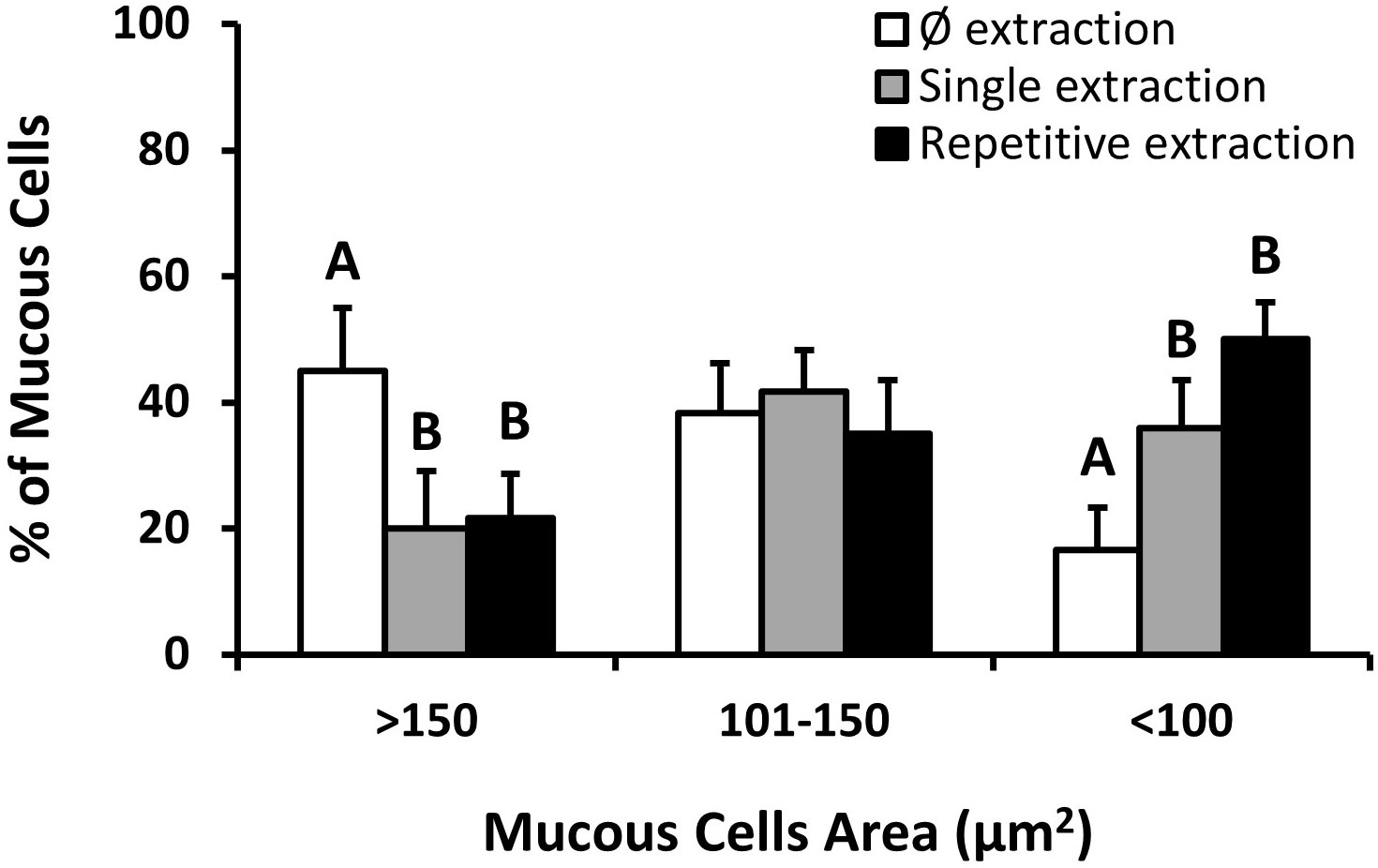
Figure 4 Mucous cell grouping by cell area. Data correspond to the mean percentage ± standard error of the mean (SEM) of the mucous cells of fish skin with: Ø skin mucus extractions (white bars), a single skin mucus extraction (gray bars), and repetitive skin mucus extractions (black bars). Letters indicate significant differences between no extractions (ØEG), a single extraction (SEG), and repetitive extractions (REG) (one-way ANOVA; N = 5).
Skin mucus is considered one of the most novel and promising tools for studying fish health and welfare, mainly due to its barrier function and adaptative responses to environmental and physiological changes as well as the minimally invasive procedure to collect it (Esteban, 2012; Sanahuja and Ibarz, 2015; Dash et al., 2018; Fernández-Alacid et al., 2018; Tiralongo et al., 2020; Franco-Martinez et al., 2022). Mucus samples are obtained through a non-invasive or minimally invasive collection process that is a particular advantage in evaluating the fish condition and physiological status in conservation studies, such as those dealing with protected species or those concerning animal care and welfare in production systems (Ekman et al., 2015; Fernández-Alacid et al., 2019a; Sanahuja et al., 2019b; Ivanova et al., 2021). Although mucus sampling is widely classified as or suggested to be minimally invasive, there is a lack of studies on the skin status and putative lesions occurring from mucus sampling as well as of assessments of mucus properties upon repetitive sampling. This information is necessary to consider whether skin mucus extraction is non-invasive and whether it is an accurate, sensitive, and reproducible tool to study fish.
Several studies have evaluated comparative methodologies of extraction, indicating different skin mucus composition depending on whether the mucus was scraped off, wiped, or absorbed (Stabell and Selset, 1980; Ivanova et al., 2018; Tartor et al., 2020). Moreover, post-extraction treatments crucially define the quality and composition of skin mucus, which must be considered prior to analytical procedures. Distinct metabolic profiles have been observed among the different procedures due to the limiting factors inherent to each extraction method. For example, in the wiped and absorbed methods, some of the components might remain partially absorbed on the wipes, while in the scraping method, components will inevitably include epidermal cells or even scales. To our knowledge and based on the results of previous studies, the quality of the resulting sample and potential skin injuries are mainly associated with the skill and practice employed during the extraction and the subsequent preparation of the sample rather than the tool or method used. Nevertheless, this must be contrasted with histological studies. Therefore, our main goal was to study the effects of skin mucus removal on mucosal integrity, using our established technique, by analyzing the effects on cell structure and the classic properties of mucus. Thus, in the current work, skin mucus was obtained by carefully scraping/wiping the skin surface with an easily sterilizable glass slide, following the method reported in Fernández-Alacid et al. (2018).
Skin mucus is mainly produced by cells in the epidermis (Elliott, 2011; Esteban and Cerezuela, 2015). Due to its viscous properties, the mucus remains adherent to the epithelial surface, protecting the entire body of the fish against changes in the environment that can be harmful for fish (Cone, 2009). In nature, the skin and its adhering mucus layer can be disturbed, for example, by contact with the benthic surface or with other fish or due to abrupt or prolonged swimming, resulting in a potential risk to fish health. As skin mucus is constantly renewed and replaced if removed (Ingram, 1980; Benhamed et al., 2014; Ibarz et al., 2019), it provides constant protection to the animal and also allows the study of the mucus to be feasible if its extraction is carried out under controlled conditions. However, the scenario changes if the epidermal surface is compromised. Healing depends on several factors (Sveen et al., 2020; Yun et al., 2021), and its response to possible superficial injuries is relatively quick (Raj et al., 2011). As mentioned before, the scraping/wiping method could impact the upper layers. However, at the histological level, our results showed no obvious changes in the epidermis. The skin sections analyzed, where mucus had been removed, maintained their structure and integrity after one or several extractions. Thus, the scraping method with the proper use of a hard glass slide enables an injury-free collection, similar to that demonstrated in previous works with other methods using soft materials as collection tools, although they performed only one extraction (Raj et al., 2011; Tartor et al., 2020). Our data further show that despite small modifications, there were no significant differences in skin thickness or mucous cell density between untouched skin sections and those used for single or repetitive scrapings.
As skin mucus is constantly secreted, the skin mucosa is forced to expend energy to maintain its homogeneity and mucus characteristics (Peatman et al., 2015; Ibarz et al., 2019). It is well known that epidermal mucous cells are differentiated and recruited from the basal layers of the epidermis, maturing while migrating to the upper layers and later releasing their contents to the surface (Chambraud et al., 1989; Kim and Ho, 2010; Elliott, 2011). We observed that after the mechanical extraction of skin mucus (sampling), this pathway was stimulated by a change in the proportions of small and large mucous cells (which could be indicative of an increased turnover), with the global mean area and volumetric density showing only a slight non-significant upward trend in parallel to the number of extractions (from one to three extractions). Studies in this field show an interesting relationship between mucous cell density and its area, which is also described as the “barrier status”, indicating the reactive capacity of the epithelium (Dang et al., 2020). The barrier status can be altered by several factors such as nutrition and contaminants (Dang et al., 2019; Sørensen et al., 2021), denoting the feasibility of its use. Using this novel matrix, single or repetitive mucus extractions did not significantly alter the histological properties or the barrier capacity in our study, indicating the non-aggressive nature of the applied technique. These results also reinforce the fact that practice and skills are crucial for extraction and will determine the quality and quantity of the obtained skin mucus.
Classic skin mucus-associated biomarkers (SMABs), such as soluble protein, glucose, lactate, and cortisol, are used to evaluate, in a minimally invasive way, the effects of several biotic and abiotic factors on fish welfare (Fernández-Alacid et al., 2018; Carbajal et al., 2019; Dallarés et al., 2020; Fernández-Montero et al., 2020). Alterations in their relative amounts have been linked to physiological modifications. For example, elevated mucus glucose and mucus cortisol levels have been observed after acute stress, while low mucus glucose levels have been reported after a period of fasting and increased soluble protein levels have been detected during infections (Fernández-Alacid et al., 2018). It is well known that one of the first responses of fish to being captured is a rapid mucus exudation (Shephard, 1994; Reverter et al., 2018). Due to the repetitive extractions, we observed an increase, although not significant, in the volume of exuded mucus, which showed a lower concentration of soluble protein, but no changes in the other biomarkers such as lactate and glucose. In previous works, we considered this reduction as non-favorable for the fish condition (Fernández-Alacid et al., 2018; Fernández-Alacid et al. 2019b) due to the putative loss of defensive properties. However, when the same biomarkers were analyzed in the plasma, no differences were observed between the single extraction and repetitive extractions, which indicated that a systemic response to cope with the repetitive mucus extractions was efficient in maintaining plasma homeostasis. These results could also reinforce the idea that mucus can provide information on some alterations that are not provided by plasma biomarkers. However, it may also be that such responses have different time windows that must be considered when using these matrices as proxies for physiology. In fact, recent studies in sea bass have demonstrated that mucus biomarkers are more sensitive than plasma ones to acute stress (Ordóñez-Grande et al., 2020) compared to chronic stress (Ordóñez-Grande et al., 2021).
As one of its main functions is defense, when the skin surface suffers any alteration, damage or infection, the exuded mucus responds accordingly (Cordero et al., 2017; Saleh et al., 2018; Sveen et al., 2018). Mucus presents different physical properties and consists of enzymatic and molecular components that, all together, create a powerful shield against possible changes in the environment that can be harmful for fish. Mucus glycoproteins, or mucins, produce a gel structure that generates an inhospitable environment for parasites and microorganisms (McAuley et al., 2007; Sveen et al., 2017). Furthermore, the distinct molecules with important enzymatic activities, such as lysozyme and proteases, continuously protect against external agents (Sanahuja et al., 2019b; Wang et al., 2019; Sanahuja et al., 2020), with their levels varying under challenging conditions (Sanahuja et al., 2019a; Espinosa-Ruíz and Esteban, 2021). To evaluate the defensive capacities of skin mucus in fish exposed to repetitive extractions, we developed the bacteria-mucus co-culture assays to ascertain the antibacterial capacity of the mucus (Sanahuja et al., 2019b). We analyzed the response against two of the most recurrent marine bacteria: Pseudomonas anguilliseptica and Vibrio anguillarum. Our results showed that the protective defense capacity of the mucus remained unaltered, as the reduced growth pattern of these bacteria and, thus, the antibacterial effects were similar in the cultures exposed to mucus collected in a single extraction and to that obtained after repetitive extractions. Mucus obtained from both conditions (single extraction and repetitive extractions) showed an antibacterial capacity similar to that reported in other studies with gilthead sea bream (Firmino et al., 2021; Gisbert et al., 2021). This suggests that a recovery period of three days is enough for the fish to regain the protective mucus layer, validating and confirming the type of extraction method proposed. Whether this period can be even shorter remains to be tested and may very much depend on the number of repeated extractions as well as on the initial condition of the fish.
Skin mucus is being increasingly studied mainly due to its potential in indicating the health and welfare of fish and the minimally invasive methods of collecting it. In this study, we demonstrate the non-aggressive and minimally harmful nature of the applied technique. Single extraction or repetitive extractions of the exuded mucus did not affect the skin barrier of the handled and sampled animals when compared to the pristine skin of untouched fish. Repetitive skin mucus extractions produced a response in the epidermal layers, increasing de novo cell formation. However, the main defensive function of this mucus seemed to be unaffected, as shown by its effects on two of the major aquaculture pathogenic bacteria, P. anguilliseptica and V. anguillarum. Although, in biological terms, any change in a natural behavior is sufficient to indicate invasiveness, this study demonstrates the minimally invasive nature of a protocol for repetitive skin mucus extractions as well as the feasibility of using skin mucus to determine fish health and welfare.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The animal study was reviewed and approved by Experiments were conducted following the guidelines established by the EU Directive 2010/63/EU and the Portuguese Decree Law n° 113/2013 on “The protection of animals used for scientific purposes”. Experimental design was previously approved by the CCMAR ethical committee for Managing Animal Welfare (ORBEA) and by the Portuguese Veterinary Authority (DGAV) under permit 013158. Fish manipulation was performed by accredited scientists in laboratory animal science by the Portuguese DGAV, following FELASA category C recommendations.
The conceptualization of the experiment was performed by IS, PG, and AI. The methodology carried out was originally proposed by IS, PG, LF-A, and AI. The trial was performed by PG and IS, while the sampling was conducted by IS, PG, and LF-A. The procedure related to skin mucus, including processing and data analysis, was undertaken by IS, LF-A, and AI. The co-culture challenges, including processing and data analysis, were conducted by LF-A. The histological experiments, including processing and data analysis, were performed by AG. The conceptualization and design of the figures and tables were overseen by IS and AI. All the authors contributed to the data analysis. IS, PG, and AI wrote the original draft. Funding acquisition was placed under the charge of AI. All authors contributed to the article and approved the submitted version.
This work was financially supported by the project PID2019-106878RB-I00 (Ministerio de Ciencia e Innovación, Spain), and through ASSEMBLE Plus Transnational Access programme PID13105 (Agreement No. 730984). IS was granted a Spanish postdoctoral fellowship (FJC2020-043933-I). This study received Portuguese national funds from FCT-Foundation for Science and Technology through project UIDB/04326/2020, UIDP/04326/2020 and LA/P/0101/2020, and from the operational programmes CRESC Algarve 2020 and COMPETE 2020 through project EMBRC.PT ALG-01-0145-EDER-022121.
The authors thank João Reis and the technical crew of the CCMAR-UALG Ramalhete Marine station for their support. They are also grateful to Elsa Couto for her laboratory assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Balebona M. C., Morifiigo M. A., Faris A., Krovacek K., Mhsson I., Bordas M. A., et al. (1995). Influence of salinity and pH on the adhesion of pathogenic vibrio strains to Sparus aurata skin mucus. Aquaculture 132, 113–120. doi: 10.1016/0044-8486(94)00376-Y
Benhamed S., Guardiola F. A., Mars M., Esteban M. A. (2014). Pathogen bacteria adhesion to skin mucus of fishes. Vet. Microbiol. 171, 1–12. doi: 10.1016/j.vetmic.2014.03.008
Bradford M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Carbajal A., Reyes-López F. E., Tallo-Parra O., Lopez-Bejar M., Tort L. (2019). Comparative assessment of cortisol in plasma, skin mucus and scales as a measure of the hypothalamic-pituitary-interrenal axis activity in fish. Aquaculture 506, 410–416. doi: 10.1016/j.aquaculture.2019.04.005
Chambraud L., Bernadac A., Gorvel J.-P., Maroux S. (1989). Renewal of goblet cell mucus granules during the cell migration along the crypt-villus axis in rabbit jejunum: an immunolabeling study. Biol. Cell 65, 151–162. doi: 10.1111/j.1768-322x.1989.tb00784.x
Cone R. A. (2009). Barrier properties of mucus. Adv. Drug Deliv. Rev. 61, 75–85. doi: 10.1016/j.addr.2008.09.008
Conforto E., Vílchez-Gómez L., Parrinello D., Parisi M. G., Esteban M. Á., Cammarata M., et al. (2021). Role of mucosal immune response and histopathological study in European eel (Anguilla anguilla l.) intraperitoneal challenged by Vibrio anguillarum or Tenacibaculum soleae. Fish. Shellfish. Immunol. 114, 330–339. doi: 10.1016/j.fsi.2021.05.011
Cordero H., Brinchmann M. F., Cuesta A., Esteban M. A. (2017). Chronic wounds alter the proteome profile in skin mucus of farmed gilthead seabream. BMC Genomics 18. doi: 10.1186/s12864-017-4349-3
Cordero H., Brinchmann M. F., Cuesta A., Meseguer J., Esteban M. A. (2015). Skin mucus proteome map of European sea bass (Dicentrarchus labrax). Proteomics 15, 4007–4020. doi: 10.1002/pmic.201500120
Dallarés S., Dourado P., Sanahuja I., Solovyev M., Gisbert E., Montemurro N., et al. (2020). Multibiomarker approach to fipronil exposure in the fish Dicentrarchus labrax under two temperature regimes. Aquat. Toxicol. 219. doi: 10.1016/j.aquatox.2019.105378
Dang M., Pittman K., Bach L., Sonne C., Hansson S. V., Søndergaard J., et al. (2019). Mucous cell responses to contaminants and parasites in shorthorn sculpins (Myoxocephalus scorpius) from a former lead zinc mine in West Greenland. Sci. Total. Environ. 678, 207–216. doi: 10.1016/j.scitotenv.2019.04.412
Dang M., Pittman K., Sonne C., Hansson S., Bach L., Søndergaard J., et al. (2020). Histological mucous cell quantification and mucosal mapping reveal different aspects of mucous cell responses in gills and skin of shorthorn sculpins (Myoxocephalus scorpius). Fish. Shellfish. Immunol. 100, 334–344. doi: 10.1016/j.fsi.2020.03.020
Dash S., Das S. K., Samal J., Thatoi H. N. (2018). Epidermal mucus, a major determinant in fish health: a review. Iran J. Vet. Res. 19, 72–81.
Ekman D. R., Skelton D. M., Davis J. M., Villeneuve D. L., Cavallin J. E., Schroeder A., et al. (2015). Metabolite profiling of fish skin mucus: A novel approach for minimally-invasive environmental exposure monitoring and surveillance. Environ. Sci. Technol. 49, 3091–3100. doi: 10.1021/es505054f
Elliott D. G. (2011). “The skin | functional morphology of the integumentary system in fishes,” in Encyclopedia of fish physiology (Elsevier Inc.), 476–488. doi: 10.1016/B978-0-12-374553-8.00108-8
Espinosa-Ruíz C., Esteban M. Á. (2021). Wound-induced changes in antioxidant enzyme activities in skin mucus and in gene expression in the skin of gilthead seabream (Sparus aurata l.). Fishes 6. doi: 10.3390/fishes6020015
Esteban M. A. (2012). An overview of the immunological defenses in fish skin. ISRN. Immunol. 2012, 1–29. doi: 10.5402/2012/853470
Esteban M. A., Cerezuela R. (2015). Fish Mucosal Immunity: Skin. In: Beck and Peatman. In: Beck and Peatman Eds. Mucosal Health in Aquaculture. (Cambridge: Academic Press) 67–92.
Fæste C. K., Tartor H., Moen A., Kristoffersen A. B., Dhanasiri A. K. S., Anonsen J. H., et al. (2020). Proteomic profiling of salmon skin mucus for the comparison of sampling methods. J. Chromatogr. B.: Anal. Technol. Biomed. Life Sci. 1138. doi: 10.1016/j.jchromb.2019.121965
Fernández-Alacid L., Firmino J. P., Sanahuja I., Madrid C., Polo J., de Borba M. R., et al. (2021). Impact of dietary porcine blood by-products in meagre (Argyrosomus regius) physiology, evaluated by welfare biomarkers and the antibacterial properties of the skin mucus. Fish. Shellfish. Immunol. 118, 241–250. doi: 10.1016/j.fsi.2021.09.011
Fernández-Alacid L., Sanahuja I., Ordóñez-Grande B., Sánchez-Nuño S., Herrera M., Ibarz A. (2019a). Skin mucus metabolites and cortisol in meagre fed acute stress-attenuating diets: Correlations between plasma and mucus. Aquaculture 499, 185–194. doi: 10.1016/j.aquaculture.2018.09.039
Fernández-Alacid L., Sanahuja I., Ordóñez-Grande B., Sánchez-Nuño S., Herrera M., Ibarz A. (2019b). Comparison between properties of dorsal and ventral skin mucus in Senegalese sole: Response to an acute stress. Aquaculture 513. doi: 10.1016/j.aquaculture.2019.734410
Fernández-Alacid L., Sanahuja I., Ordóñez-Grande B., Sánchez-Nuño S., Viscor G., Gisbert E., et al. (2018). Skin mucus metabolites in response to physiological challenges: A valuable non-invasive method to study teleost marine species. Sci. Total. Environ. 644, 1323–1335. doi: 10.1016/j.scitotenv.2018.07.083
Fernández-Montero Á., Torrecillas S., Montero D., Acosta F., Prieto-Álamo M. J., Abril N., et al. (2021). Proteomic profile and protease activity in the skin mucus of greater amberjack (Seriola dumerili) infected with the ectoparasite Neobenedenia girellae — an immunological approach. Fish. Shellfish. Immunol. 110, 100–115. doi: 10.1016/j.fsi.2021.01.001
Fernández-Montero A., Torrecillas S., Tort L., Ginés R., Acosta F., Izquierdo M. S., et al. (2020). Stress response and skin mucus production of greater amberjack (Seriola dumerili) under different rearing conditions. Aquaculture 520. doi: 10.1016/j.aquaculture.2020.735005
Firmino J. P., Fernández-Alacid L., Vallejos-Vidal E., Salomón R., Sanahuja I., Tort L., et al. (2021). Carvacrol, thymol, and garlic essential oil promote skin innate immunity in gilthead seabream (Sparus aurata) through the multifactorial modulation of the secretory pathway and enhancement of mucus protective capacity. Front. Immunol. 12. doi: 10.3389/fimmu.2021.633621
Firth K. J., Johnson S. C., Ross N. W. (2000). Characterization of proteases in the skin mucus of Atlantic salmon (Salmo salar) infected with the salmon louse (Lepeophtheirus salmonis) and in whole-body louse homogenate. J. Parasitol. 86.
Franco-Martinez L., Brandts I., Reyes-López F., Tort L., Tvarijonaviciute A., Teles M. (2022). Skin mucus as a relevant low-invasive biological matrix for the measurement of an acute stress response in rainbow trout (Oncorhynchus mykiss). Water 14, 1754. doi: 10.3390/w14111754
Gisbert E., Ibarz A., Firmino J. P., Fernández-Alacid L., Salomón R., Vallejos-Vidal E., et al. (2021). Porcine protein hydrolysates (Pepteiva®) promote growth and enhance systemic immunity in gilthead sea bream (Sparus aurata). Animals 11. doi: 10.3390/ani11072122
Ibarz A., Ordónez-Grande B., Sanahuja I., Sánchez-Nunõ S., Fernández-Borras J., Blasco J., et al. (2019). Using stable isotope analysis to study skin mucus exudation and renewal in fish. J. Exp. Biol. 222. doi: 10.1242/jeb.195925
Ingram G. A. (1980). Substances involved in the natural resistance of fish to infection-a review. J. Fish. Biol. 16. doi: 10.1111/j.1095-8649.1980.tb03685.x
Ivanova L., Rangel-Huerta O. D., Tartor H., Gjessing M. C., Dahle M. K., Uhlig S. (2021). Fish skin and gill mucus: A source of metabolites for non-invasive health monitoring and research. Metabolites 12, 28. doi: 10.3390/metabo12010028
Ivanova L., Tartor H., Grove S., Kristoffersen A. B., Uhlig S. (2018). Workflow for the targeted and untargeted detection of small metabolites in fish skin mucus. Fishes 3. doi: 10.3390/fishes3020021
Kim Y. S., Ho S. B. (2010). Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 12, 319–330. doi: 10.1007/s11894-010-0131-2
McAuley J. L., Linden S. K., Chin W. P., King R. M., Pennington H. L., Gendler S. J., et al. (2007). MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J. Clin. Invest. 117, 2313–2324. doi: 10.1172/JCI26705
Mosley J. D., Ekman D. R., Cavallin J. E., Villeneuve D. L., Ankley G. T., Collette T. W. (2018). High-resolution mass spectrometry of skin mucus for monitoring physiological impacts and contaminant biotransformation products in fathead minnows exposed to wastewater effluent. Environ. Toxicol. Chem. 37, 788–796. doi: 10.1002/etc.4003
Omidi F., Jafaryan H., Patimar R., Harsij M., Paknejad H. (2020). Biochemical biomarkers of skin mucus in Neogobius melanostomus for assessing lead pollution in the gulf of gorgan (Iran). Toxicol. Rep. 7, 109–117. doi: 10.1016/j.toxrep.2019.12.003
Ordóñez-Grande B., Guerreiro P. M., Sanahuja I., Fernández-Alacid L., Ibarz A. (2020). Evaluation of an acute osmotic stress in European sea bass via skin mucus biomarkers. Animals 10, 1–19. doi: 10.3390/ani10091546
Ordóñez-Grande B., Guerreiro P. M., Sanahuja I., Fernández-Alacid L., Ibarz A. (2021). Environmental salinity modifies mucus exudation and energy use in European sea bass juveniles. Animals 11. doi: 10.3390/ani11061580
Patel D. M., Brinchmann M. F. (2017). Skin mucus proteins of lumpsucker (Cyclopterus lumpus). Biochem. Biophys. Rep. 9, 217–225. doi: 10.1016/j.bbrep.2016.12.016
Peatman E., Lange M., Zhao H., Beck B. H. (2015). Physiology and immunology of mucosal barriers in catfish (Ictalurus spp.). Tissue Barriers. 3, 1–14. doi: 10.1080/21688370.2015.1068907
Pérez-Sánchez J., Terova G., Simó-Mirabet P., Rimoldi S., Folkedal O., Calduch-Giner J. A., et al. (2017). Skin mucus of gilthead sea bream (Sparus aurata l.). protein mapping and regulation in chronically stressed fish. Front. Physiol. 8. doi: 10.3389/fphys.2017.00034
Pittman K., Pittman A., Karlson S., Cieplinska T., Sourd P., Redmond K., et al. (2013). Body site matters: An evaluation and application of a novel histological methodology on the quantification of mucous cells in the skin of Atlantic salmon, Salmo salar l. J. Fish. Dis. 36, 115–127. doi: 10.1111/jfd.12002
Raj V. S., Fournier G., Rakus K., Ronsmans M., Ouyang P., Michel B., et al. (2011). Skin mucus of Cyprinus carpio inhibits cyprinid herpesvirus 3 binding to epidermal cells. Vet. Res. 42. doi: 10.1186/1297-9716-42-92
Reverter M., Tapissier-Bontemps N., Lecchini D., Banaigs B., Sasal P. (2018). Biological and ecological roles of external fish mucus: A review. Fishes 3. doi: 10.3390/fishes3040041
Reyes-López F. E., Ibarz A., Ordóñez-Grande B., Vallejos-Vidal E., Andree K. B., Balasch J. C., et al. (2021). Skin multi-Omics-Based interactome analysis: Integrating the tissue and mucus exuded layer for a comprehensive understanding of the teleost mucosa functionality as model of study. Front. Immunol. 11. doi: 10.3389/fimmu.2020.613824
Sørensen S. L., Park Y., Gong Y., Vasanth G. K., Dahle D., Korsnes K., et al. (2021). Nutrient digestibility, growth, mucosal barrier status, and activity of leucocytes from head kidney of Atlantic salmon fed marine- or plant-derived protein and lipid sources. Front. Immunol. 11. doi: 10.3389/fimmu.2020.623726
Saleh M., Kumar G., Abdel-Baki A. A., Dkhil M. A., El-Matbouli M., Al-Quraishy S. (2018). Quantitative shotgun proteomics distinguishes wound-healing biomarker signatures in common carp skin mucus in response to Ichthyophthirius multifiliis. Vet. Res. 49. doi: 10.1186/s13567-018-0535-9
Sanahuja I., Dallarés S., Ibarz A., Solé M. (2020). Multi-organ characterisation of b-esterases in the European sea bass (Dicentrarchus labrax): Effects of the insecticide fipronil at two temperatures. Aquat. Toxicol. 228. doi: 10.1016/j.aquatox.2020.105617
Sanahuja I., Fernández-Alacid L., Ordóñez-Grande B., Sánchez-Nuño S., Ramos A., Araujo R. M., et al. (2019b). Comparison of several non-specific skin mucus immune defences in three piscine species of aquaculture interest. Fish. Shellfish. Immunol. 89, 428–436. doi: 10.1016/j.fsi.2019.04.008
Sanahuja I., Fernández-Alacid L., Sánchez-Nuño S., Ordóñez-Grande B., Ibarz A. (2019a). Chronic cold stress alters the skin mucus interactome in a temperate fish model. Front. Physiol. doi: 10.3389/fphys.2018.01916
Sanahuja I., Ibarz A. (2015). Skin mucus proteome of gilthead sea bream: A non-invasive method to screen for welfare indicators. Fish. Shellfish. Immunol. 46, 426–435. doi: 10.1016/j.fsi.2015.05.056
Sridhar A., Sekar R. K., Manikandan D. B., Arumugam M., Veeran S., Ramasamy T. (2021). Activity profile of innate immune-related enzymes and bactericidal of freshwater fish epidermal mucus extract at different pH. Environ. Sci. pollut. Res. 28, 33914–33926. doi: 10.1007/s11356-020-11173-5
Stabell O. B., Selset R. (1980). Comparison of mucus collecting methods in fish olfaction. Acta Physiol. Scand. 108. doi: 10.1111/j.1748-1716.1980.tb06504.x
Sveen L. R., Grammes F. T., Ytteborg E., Takle H., Jørgensen S. M. (2017). Genome-wide analysis of Atlantic salmon (Salmo salar) mucin genes and their role as biomarkers. PloS One 12. doi: 10.1371/journal.pone.0189103
Sveen L., Karlsen C., Ytteborg E. (2020). Mechanical induced wounds in fish – a review on models and healing mechanisms. Rev. Aquacult. 12, 2446–2465. doi: 10.1111/raq.12443
Sveen L. R., Timmerhaus G., Krasnov A., Takle H., Handeland S., Ytteborg E. (2019). Wound healing in post-smolt Atlantic salmon (Salmo salar l.). Sci. Rep. 9. doi: 10.1038/s41598-019-39080-x
Sveen L. R., Timmerhaus G., Krasnov A., Takle H., Stefansson S. O., Handeland S. O., et al. (2018). High fish density delays wound healing in Atlantic salmon (Salmo salar). Sci. Rep. 8. doi: 10.1038/s41598-018-35002-5
Tartor H., Monjane A. L., Grove S. (2020). Quantification of defensive proteins in skin mucus of Atlantic salmon using minimally invasive sampling and high-sensitivity Elisa. Animals 10, 1–17. doi: 10.3390/ani10081374
Tiralongo F., Messina G., Lombardo B. M., Longhitano L., Li Volti G., Tibullo D. (2020). Skin mucus of marine fish as a source for the development of antimicrobial agents. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.541853
Vakili F., Roosta Z., Hoseinifar S. H., Akbarzadeh A. (2021). Effects of thermal stress and hypoxia on skin mucus immune and stress responses in blue gourami (Trichogaster trichopterus) cultured in intensive recirculation aquaculture system and semi-intensive systems. Aquacult. Res. 52, 6581–6590. doi: 10.1111/are.15529
Wang B., Ma G., Liu Y., Wang Y., Du X., Shi Q., et al. (2021). Effects of different temperatures on the antibacterial, immune and growth performance of crucian carp epidermal mucus. Fishes 6. doi: 10.3390/fishes6040066
Wang H., Tang W., Zhang R., Ding S. (2019). Analysis of enzyme activity, antibacterial activity, antiparasitic activity and physico-chemical stability of skin mucus derived from Amphiprion clarkii. Fish. Shellfish. Immunol. 86, 653–661. doi: 10.1016/j.fsi.2018.11.066
Keywords: antibacterial activity, skin mucus-associated biomarkers (SMABs), mucous cells, mucus barrier, marine fish
Citation: Sanahuja I, Guerreiro PM, Girons A, Fernandez-Alacid L and Ibarz A (2023) Evaluating the repetitive mucus extraction effects on mucus biomarkers, mucous cells, and the skin-barrier status in a marine fish model. Front. Mar. Sci. 9:1095246. doi: 10.3389/fmars.2022.1095246
Received: 10 November 2022; Accepted: 28 December 2022;
Published: 18 January 2023.
Edited by:
Ming Li, Ningbo University, ChinaReviewed by:
Lluis Tort, Universitat Autònoma de Barcelona, SpainCopyright © 2023 Sanahuja, Guerreiro, Girons, Fernandez-Alacid and Ibarz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ignasi Sanahuja, aWduYXNpLnNhbmFodWphQGlydGEuY2F0; Laura Fernandez-Alacid, ZmVybmFuZGV6X2FsYWNpZEB1Yi5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.