
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
DATA REPORT article
Front. Mar. Sci. , 23 December 2022
Sec. Global Change and the Future Ocean
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.1094814
This article is part of the Research Topic The Adaptation and Response of Aquatic Animals in the Context of Global Climate Change View all 18 articles
 Da Huo1,2,3,4,5
Da Huo1,2,3,4,5 Fang Su1,2,3,4,5,6
Fang Su1,2,3,4,5,6 Libin Zhang1,2,3,4,5,6
Libin Zhang1,2,3,4,5,6 Hongsheng Yang1,2,3,4,5,6,7
Hongsheng Yang1,2,3,4,5,6,7 Lina Sun1,2,3,4,5,6*
Lina Sun1,2,3,4,5,6*Aquatic species naturally live in water environment, therefore stress brought on by alterations in the environmental conditions directly affects them (Huo et al., 2021). The growth, survival and distribution of marine organisms are largely influenced by environmental factors like water temperature and dissolved oxygen (DO) (Coutant, 1985; Gobler et al., 2014). By the end of the century, it is expected that global temperatures will rise by at least 2°C, but ocean DO concentrations will drop by 4-7% (Matear and Hirst, 2003; Hoegh-Guldberg et al., 2007). Since oxygen becomes less soluble as temperature rises, heat stress and hypoxic stress frequently coexist (Huo et al., 2020).
The sea cucumber Apostichopus japonicus is an echinoderm with considerable commercial and ecological significance (Huo et al., 2020). As an aquatic poikilothermal animal, the physiological activities (i.e., digestive function, immunity, and antioxidant defense) of sea cucumber are directly influenced by water temperature and dissolved oxygen. The suitable temperature range for A. japonicus growth is between 15°C and 18°C (Dong et al., 2006), and when the temperature exceeds 26°C and persists for more than 10 days, massive mortality would occur in farmed sea cucumbers. Moreover, hypoxia is typically seen as occurring when dissolved oxygen levels drop below 2 mg·L-1 (Wu, 2002). An earlier study illustrated that A. japonicus could survive at hypoxic condition (2 mg·L-1) in a short time, but its physical status and movement would be affected (Huo et al., 2018). The A. japonicus mainly relies on non-specific immunity, and the humoral immune response is one of its main defense reactions (Shao et al., 2018). The coelomic fluid of A. japonicus is similar to lymphatic fluid, and the cells inside work together with various humoral immune factors to form an immune response. Therefore, it is necessary to investigate the variations of enzyme activity in the coelomic fluid of A. japonicus to reveal how that species reacts to environmental challenges.
The activity of digestive enzymes is one of the most commonly used indicators to evaluate the digestive capacity, nutritional biochemistry and physiological status of the organism (Zhang et al., 2014). A. japonicus would reduce feeding and the digestive tract would be degraded when water temperature increases and dissolved oxygen decreases (Xu et al., 2015; Huo et al., 2018), and the digestive functions were potentially negatively affected (Huo et al., 2018). Therefore, the digestive function of A. japonicus may be altered under environmental stress, and to investigate this change, we could check digestive enzyme activities.
Environmental stresses could lead to an increase in reactive oxygen species (ROS) in the organism (Das and Roychoudhury, 2014). To avoid the damage caused by ROS, organisms have evolved various types of antioxidant systems, including non-enzymatic antioxidants represented by vitamin C and vitamin E and enzymatic antioxidants represented by superoxide dismutase (SOD) and catalase (CAT) (Tan et al., 2020). The antioxidant enzyme family members are widely distributed in the organism and regulate ROS levels thus acting as antioxidants and play crucial roles in response to stress. It is necessary to identify the changes of antioxidant enzymes in A. japonicus under adverse environment. In this study, 16 enzymes related to immune defense, digestive function, and antioxidant level were measured to reveal the physiological response characteristics in A. japonicus exposed to environmental stress. Our findings would provide insight into the response and adaptation of sea cucumber under the context of global climate change.
Experimental A. japonicus were collected from the coast of Weihai, China, with a wet weight of 90-110 g. One-week acclimatation in a tank containing aeration sand-filtered seawater at a temperature of 16 ± 0.5°C before the formal experiment. The normal control (NC) group was maintained at a temperature of 16°C with sufficient aeration; the high temperature (HT) group (heat stress group) was maintained at a temperature of 26°C with sufficient aeration; the low dissolved oxygen (LO) group (hypoxic stress group) was maintained at a temperature of 16°C and dissolved oxygen concentration of 2 mg·L-1; the high temperature and low dissolved oxygen (HL) group (heat combined with hypoxic stress group) maintains at a temperature of 26°C and a DO concentration of 2 mg·L-1. The equipment used for temperature and DO change, and the changing rate were same with the previous study (Huo et al., 2020). Five replicates were set in each group and cultured in separate tanks during the experiment. After 48h exposure, the coelomic fluid of each A. japonicus was collected by sterile syringe and rapidly frozen in liquid nitrogen, and then transferred to a refrigerator at -80°C for storage.
A total of 16 enzyme activities involving immunity, digestion and antioxidant ability were measured in this study, including acid phosphatase (ACP), alkaline phosphatase (AKP) and lysozyme (LZM), lipase (LPS), α-amylase (AMS), pepsin (PEP), trypsin (TRY), SOD, glutathione peroxidase (GSH-PX), CAT, succinate dehydrogenase (SDH), lactate dehydrogenase (LDH), total antioxidant capacity (T-AOC), malondialdehyde (MDA), peroxidase (POD), and phenol oxidase (PPO). All enzyme activities were determined within one month of sampling the coelomic fluid samples, and the commercial kits used in this study were purchased from Nanjing Jiancheng Biological Research Institute (Nan Jing, China) and tested according to the instructions. Specifically, the kit number for the enzymes assay were listed in Table S1. The obtained data were statistically analyzed by SPSS19 software (IBM Corp., Armonk, NY, USA). The significance of the differences between the treated and comparison groups for each enzyme was analyzed by t-test and the statistical significance threshold was set at P < 0.05. Bar graphs were plotted using Prism7 software (GraphPad Software Inc., USA).
As the immune response in A. japonicus is a typical non-specific immune response, enzymes like ACP, AKP, and LZM may be able to aid in the complete destruction of foreign compounds after they have passed through the organism’s first line of defense (Wang et al., 2015). In this study, the activity of the three enzymes related to immune defense was measured (Figure 1). Compared with the normal environmental condition, the activity of ACP was significantly higher in A. japonicus under hypoxic stress (P < 0.05), and the changes of AKP activity were not significant under the three environmental stresses; the activity of LZM was significantly higher in A. japonicus under heat combined with hypoxic stress (P < 0.05). This could be that more adenosine triphosphate (ATP) was needed to maintain normal metabolic level when A. japonicus exposed to environment stress, and the inorganic phosphate required for ATP synthesis can be produced by the hydrolysis of phosphate by ACP and AKP (Zheng et al., 2014). The results suggested that supply of potential metabolic high energy demand was enhanced, thus providing more energy to adapt to the adversity. Lysozyme is an important innate immune factor widely present in the endothelial cells and body fluids of echinoderms that kills germs and shields against bacterial infection (Canicatti and Roch, 1989). The increased LZM activity suggested that phagocytic activity of phagocytes may be elevated. A high temperature also caused an increase in serum lysozyme levels in Atlantic halibut Hippoglossus hippoglossus L. (Langston et al., 2002). According to the findings, the immune defense mechanisms were induced in A. japonicus in response to environmental stress, the organism’s defense against foreign substances was enhanced.
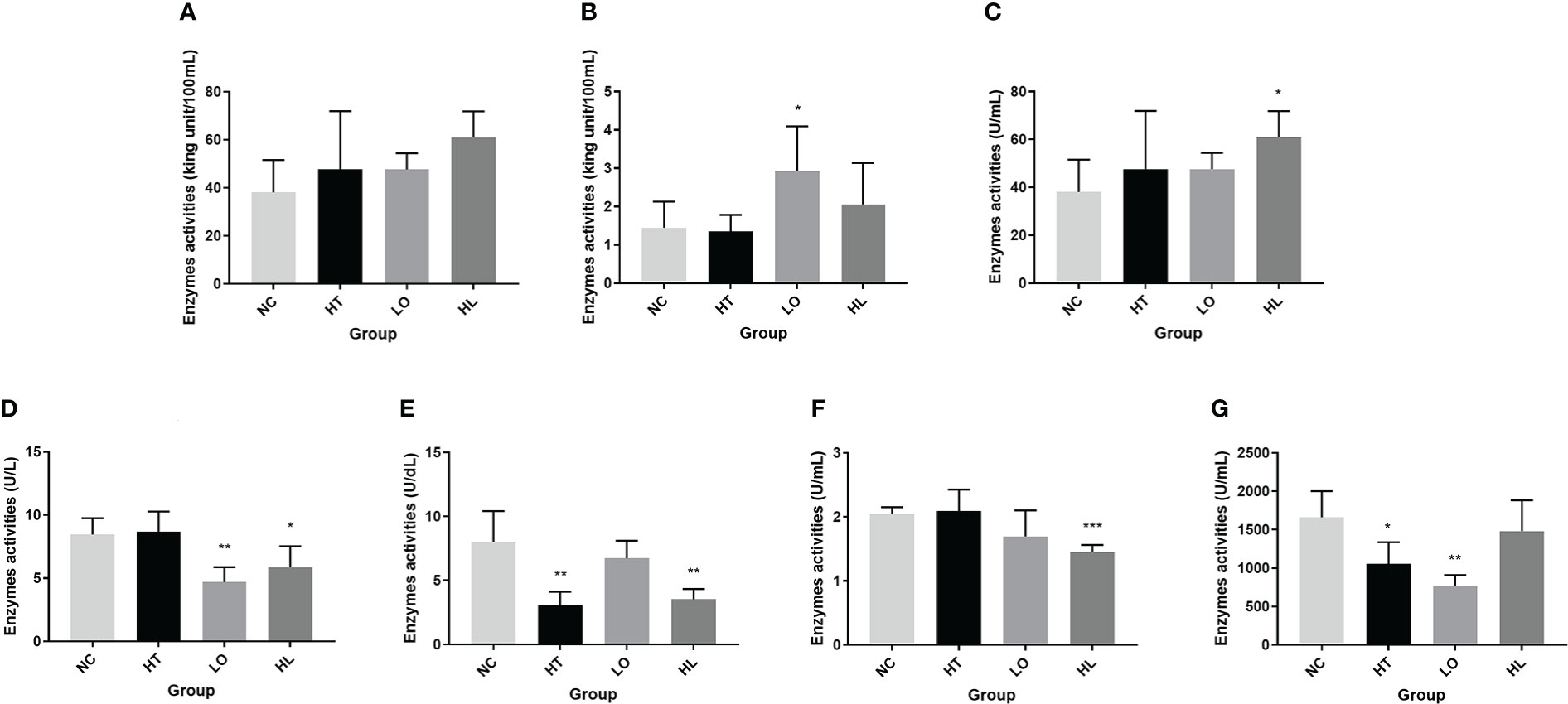
Figure 1 Activities of immune and digestive enzymes in A. japonicus under environmental stresses (A). alkaline phosphatase (AKP); (B) acid phosphatase (ACP); (C) lysozyme (LZM); (D) lipase (LPS); (E) α-amylase (AMS); (F) pepsin (PEP); and (G) trypsin (TRY); *: P < 0.05; **: P < 0.01; ***: P < 0.001).
In this study, four enzymes related to digestive function were selected for activity measurement, including LPS, AMS, PEP and TRY. The results showed that the LPS activity was significantly reduced under hypoxia (P < 0.01) and heat combined with hypoxic stress (P < 0.05); AMS activity was highly significantly reduced under heat and heat combined with hypoxic stress (P < 0.01); PEP activity was extremely significantly reduced under heat combined with hypoxic stress (P < 0.001); and TRY activity was significantly reduced under heat stress (P < 0.05) and hypoxic stress (P < 0.01) (Figure 1). In the previous of yellowtail kingfish Seriola lalandi, TRY, LPS and AMS enzyme activities were altered by temperature but did not seem to be impacted by dissolved oxygen concentration (Bowyer et al., 2014); PEP and AMS activities also significantly changed by temperature in the leopard coral grouper Plectropomus leopardus (Sun et al., 2015). Under environmental stress, A. japonicus undergoes degeneration of the intestine and respiratory tree (Xu et al., 2015; Huo et al., 2018), and may even occur evisceration. Substantial changes in these digestive organs are also responsible for the decrease in digestive enzyme secretion and activity. The decrease in digestion-related enzyme activities in A. japonicus under environmental stress indicated that there was a negative impact of environmental stress on the digestive function of A. japonicus.
Environmental stress may lead to an increase in ROS, which could oxidize cellular components and damage cell membrane. The imbalance between the production and clearance of ROS will cause oxidative stress (Halliwell and Gutteridge, 2001; Kong et al., 2012). To assess the oxidative stress response of A. japonicus under environmental stress, nine enzyme activities were measured in this study (Figure 2), including SOD, GSH-PX, CAT, SDH, LDH, MDA, POD, PPO, and T-AOC. The T-AOC was significantly reduced under heat and heat combined with hypoxic stress (P < 0.05); SOD was significantly reduced, and MDA were extremely significantly increased under hypoxia and heat combined with hypoxic stress (P < 0.001). These suggested that oxidative stress brought on by environmental stress results in lipid peroxidation and caused oxidative damage to organisms. SDH activity was significantly reduced heat combined with hypoxic stress (P < 0.05), suggesting that aerobic oxidation capacity was suppressed, and the tricarboxylic acid cycle was impacted. POD and PPO activity were significantly increased under heat stress; the activity of CAT was significantly reduced under all three types of environmental stresses. PPO could oxidize phenolic substrates to unstable quinones (Cerenius et al., 2008); POD has the property of catalyzing the oxidation reaction between hydrogen peroxide (H2O2) and hydrogen donors (Şişecioğlu et al., 2010), and CAT could catalyze the intracellular hydrogen peroxide decomposition (Wang et al., 2016). Therefore, by altering their activity to reduce the cell damage caused by excessive free radicals, A. japonicus could adapt to the adversity. The activity of GSH-PX and LDH did not significantly change; It is possible that these two enzymes did not play such crucial role in the oxidative stress defense in A. japonicus. It is also possible that these two indicators are not sensitive to environmental stress in A. japonicus coelomic fluid. The oxidation status influenced by environmental changes has been reported in aquatic animals, including the shrimp Litopenaeus vannamei (Liu et al., 2015), the crab Paralomis granulosa (Romero et al., 2011), the fish Carassius auratus (Sun et al., 2012), Micropterus salmoides (Sun et al., 2020) and the scallop Chlamys farreri (Chen et al., 2007). For example, the results of decreased SOD and CAT were in accordance of the crucian carp Carassius auratus in hypoxic condition (Sun et al., 2012). Increased T-AOC and GSH-Px activities were also found in heat-stressed largemouth bass Micropterus salmoides (Sun et al., 2020). It was suggested that various environmental stresses resulted in varing degrees of oxidative stress response, and these enzymes with altered activity may be vital in protecting A. japonicus from oxidative damage.
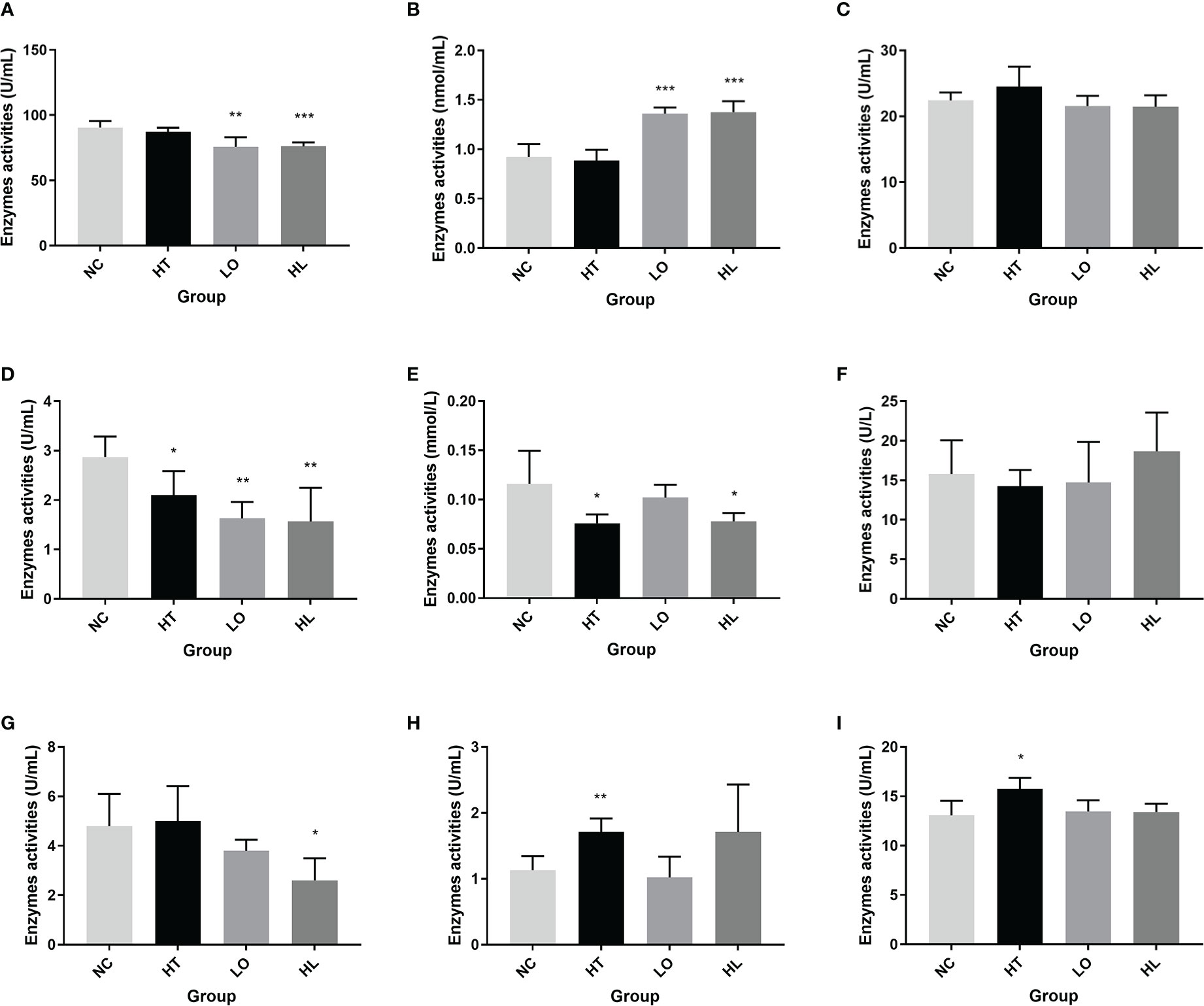
Figure 2 Activities of antioxidative enzymes in A. japonicus under environmental stresses (A). superoxide dismutase (SOD); (B) malondialdehyde (MDA); (C) glutathione peroxidase (GSH-PX); (D) catalase (CAT); (E) total antioxidant capacity (T-AOC); (F) lactate dehydrogenase (LDH); (G) succinate dehydrogenase (SDH); (H) peroxidase (POD); and (I) phenol oxidase (PPO); *: P < 0.05; **: P < 0.01; ***: P < 0.001).
To conclude, the coelomic fluid is an important component in sea cucumbers to defense against undesirable environments. When exposed to the environmental stress, digestive function was suppressed, immune system was induced, and the antioxidant enzymes changed in varying degrees in A. japonicus. The integrated regulation of immunity, digestion, oxidative stress, and other associated enzymes is a series of adaptive mechanisms made by the A. japonicus to adapt to the extreme environment. Our results provide a better understanding of how the A. japonicus survives in adversity in the context of global change.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Since culture commercial sea cucumber were used, ethical review and approval not required.
DH: Conceptualization, Investigation, Data curation, Methodology, Validation, Project administration, Funding acquisition, Writing - original draft, Writing - review & editing. FS: Validation, Writing - review & editing. LZ: Supervision, Writing - review & editing. HY: Supervision, Funding acquisition, Writing - review & editing. LS: Formal analysis, Funding acquisition, Writing - review & editing. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (grant numbers 42106106, 42030408), Postdoctoral Innovative Talents Support Program of Shandong Province (grant number SDBX2020006), the Major Scientific and Technological Innovation Projects in Shandong Province (grant number 2019JZZY010812), Shandong Provincial Natural Science Foundation (grant number ZR2021QD013), Special Research Assistant Project of Chinese Academy of Sciences, Qingdao Postdoctoral Applied Research Project (grant number 2020170), Youth Innovation Promotion Association CAS (grant number 2019209).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.1094814/full#supplementary-material
Bowyer J. N., Booth M. A., Qin J. G., D’Antignana T., Thomson M. J., Stone D. A. (2014). Temperature and dissolved oxygen influence growth and digestive enzyme activities of yellowtail kingfish Seriola lalandi (Valenciennes 1833). Aquaculture Res. 45 (12), 2010–2020. doi: 10.1111/are.12146
Canicatti C., Roch P. (1989). Studies on Holothuria polii (Echinodermata) antibacterial proteins. i. evidence for and activity of a coelomocyte lysozyme. Cell. And Mol. Life Sci. 45, 756–759. doi: 10.1007/BF01974579
Cerenius L., Lee B. L., Söderhäll K. (2008). The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 29, 263–271. doi: 10.1016/j.it.2008.02.009
Chen M., Yang H., Delaporte M., Zhao S. (2007). Immune condition of Chlamys farreri in response to acute temperature challenge. Aquaculture 271 (1-4), 479–487. doi: 10.1016/j.aquaculture.2007.04.051
Coutant C. C. (1985). Striped bass, temperature, and dissolved oxygen: a speculative hypothesis for environmental risk. Trans. Am. Fisheries Soc. 114 (1), 31–61. doi: 10.1577/1548-8659(1985)114<31:SBTADO>2.0.CO;2
Das K., Roychoudhury A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2, 53. doi: 10.3389/fenvs.2014.00053
Dong Y., Dong S., Tian X., Wang F., Zhang M. (2006). Effects of diel temperature fluctuations on growth, oxygen consumption and proximate body composition in the sea cucumber apostichopus japonicus selenka. Aquaculture 255 (1-4), 514–521. doi: 10.1016/j.aquaculture.2005.12.013
Gobler C. J., DePasquale E. L., Griffith A. W., Baumann H. (2014). Hypoxia and acidification have additive and synergistic negative effects on the growth, survival, and metamorphosis of early life stage bivalves. PloS One 9 (1), e83648. doi: 10.1371/journal.pone.0083648
Halliwell B., Gutteridge J. M. C. (2001). Free radicals in biology and medicine (New York: Oxford University Press Inc).
Hoegh-Guldberg O., Mumby P. J., Hooten A. J., Steneck R. S., Greenfield P., Gomez E., et al. (2007). Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. doi: 10.1126/science.1152509
Huo D., Sun L., Ru X., Zhang L., Lin C., Liu S., et al. (2018). Impact of hypoxia stress on the physiological responses of sea cucumber Apostichopus japonicus: respiration, digestion, immunity and oxidative damage. PeerJ 6, e4651. doi: 10.7717/peerj.4651
Huo D., Sun L., Storey K. B., Zhang L., Liu S., Sun J., et al. (2020). The regulation mechanism of lncRNAs and mRNAs in sea cucumbers under global climate changes: Defense against thermal and hypoxic stresses. Sci. Total Environ. 709, 136045. doi: 10.1016/j.scitotenv.2019.136045
Huo D., Sun L., Sun J., Lin C., Liu S., Zhang L., et al. (2021). Emerging roles of circRNAs in regulating thermal and hypoxic stresses in Apostichopus japonicus (Echinodermata: Holothuroidea). Ecotoxicol. Environ. Saf. 228, 112994. doi: 10.1016/j.ecoenv.2021.112994
Kong X., Wang S., Jiang H., Nie G., Li X. (2012). Responses of acid/alkaline phosphatase, lysozyme, and catalase activities and lipid peroxidation to mercury exposure during the embryonic development of goldfish Carassius auratus. Aquat. Toxicol. 120-121, 119–125. doi: 10.1016/j.aquatox.2012.05.005
Langston A. L., Hoare R., Stefansson M., Fitzgerald R., Wergeland H., Mulcahy M. (2002). The effect of temperature on non-specific defence parameters of three strains of juvenile Atlantic halibut (Hippoglossus hippoglossus l.). Fish Shellfish Immunol. 12 (1), 61–76. doi: 10.1006/fsim.2001.0354
Liu H. L., Yang S. P., Wang C. G., Chan S. M., Wang W. X., Feng Z. H., et al. (2015). Effect of air exposure and resubmersion on the behavior and oxidative stress of pacific white shrimp Litopenaeus vannamei. North Am. J. Aquaculture 77 (1), 43–49. doi: 10.1080/15222055.2014.955157
Matear R. J., Hirst A. C. (2003). Long-term changes in dissolved oxygen concentrations in the ocean caused by protracted global warming. Global Biogeochemical Cycles 17, 1125. doi: 10.1029/2002GB001997
Romero M. C., Tapella F., Sotelano M. P., Ansaldo M., Lovrich G. A. (2011). Oxidative stress in the subantarctic false king crab paralomis granulosa during air exposure and subsequent re-submersion. Aquaculture 319 (1-2), 205–210. doi: 10.1016/j.aquaculture.2011.06.041
Şişecioğlu M., Gülçin L., Çankaya M., Atasever A., Kaya H. B., Ozdemir H. (2010). Purification and characterization of peroxidase from Turkish black radish (Raphanus sativus l.). J. Medicinal Plants Res. 4, 1187–1196. doi: 10.5897/JMPR10.071
Shao Y., Che Z., Xing R., Wang Z., Zhang W., Zhao X., et al. (2018). Divergent immune roles of two fucolectin isoforms in Apostichopus japonicus. Dev. Comp. Immunol. 89, 1–6. doi: 10.1016/j.dci.2018.07.028
Sun H., Li J., Tang L., Yang Z. (2012). Responses of crucian carp Carassius auratus to long-term exposure to nitrite and low dissolved oxygen levels. Biochem. Systematics Ecol. 44, 224–232. doi: 10.1016/j.bse.2012.06.011
Sun Z., Xia S., Feng S., Zhang Z., Rahman M. M., Rajkumar M., et al. (2015). Effects of water temperature on survival, growth, digestive enzyme activities, and body composition of the leopard coral grouper plectropomus leopardus. Fisheries Sci. 81 (1), 107–112. doi: 10.1007/s12562-014-0832-9
Sun J. L., Zhao L. L., Liao L., Tang X. H., Cui C., Liu Q., et al. (2020). Interactive effect of thermal and hypoxia on largemouth bass (Micropterus salmoides) gill and liver: Aggravation of oxidative stress, inhibition of immunity and promotion of cell apoptosis. Fish Shellfish Immunol. 98, 923–936. doi: 10.1016/j.fsi.2019.11.056
Tan K., Zhang B., Zhang H., Ma H., Li S., Zheng H. (2020). Enzymes and non-enzymatic antioxidants responses to sequential cold stress in polymorphic noble scallop Chlamys nobilis with different total carotenoids content. Fish Shellfish Immunol. 97, 617–623. doi: 10.1016/j.fsi.2019.12.063
Wang C., Wang X., Wang P., Chen B., Hou J., Qian J., et al. (2016). Effects of iron on growth, antioxidant enzyme activity, bound extracellular polymeric substances and microcystin production of microcystis aeruginosa FACHB-905. Ecotoxicol. Environ. Saf. 132, 231–239. doi: 10.1016/j.ecoenv.2016.06.010
Wang J. H., Zhao L. Q., Liu J. F., Wang H., Xiao S. (2015). Effect of potential probiotic rhodotorula benthica D30 on the growth performance, digestive enzyme activity and immunity in juvenile sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 43, 330–336. doi: 10.1016/j.fsi.2014.12.028
Wu R. S. (2002). Hypoxia: from molecular responses to ecosystem responses. Mar. pollut. Bull. 45 (1-12), 35–45. doi: 10.1016/S0025-326X(02)00061-9
Xu D., Sun L., Liu S., Zhang L., Yang H. (2015). Histological, ultrastructural and heat shock protein 70 (HSP70) responses to heat stress in the sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 45, 321–326. doi: 10.1016/j.fsi.2015.04.015
Zhang H. C., Zhu J. Y., Chen Z. Q., Lin M. (2014). Variation of the activity of digestive enzymes involved in Apostichopus japonicus with different sizes. J. Quanzhou Normal Univ 32 (2), 27–30. doi: 10.16125/j.cnki.1009-8224.2014.02.019
Keywords: echinoderm, heat stress, hypoxia, environmental stress, immune response, oxidative stress
Citation: Huo D, Su F, Zhang L, Yang H and Sun L (2022) Temperature and dissolved oxygen influence the immunity, digestion, and antioxidant level in sea cucumber Apostichopus japonicus. Front. Mar. Sci. 9:1094814. doi: 10.3389/fmars.2022.1094814
Received: 10 November 2022; Accepted: 07 December 2022;
Published: 23 December 2022.
Edited by:
Jonathan Y.S. Leung, University of Adelaide, AustraliaCopyright © 2022 Huo, Su, Zhang, Yang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lina Sun, c3VubGluYUBxZGlvLmFjLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.