
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 12 January 2023
Sec. Marine Pollution
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.1093468
This article is part of the Research Topic Nuclear Power Cooling-Water System Disaster-Causing Organisms: Outbreak and Aggregation Mechanisms, Early-warning Monitoring, Prevention and Control View all 15 articles
In this study, we analyzed the spatial and seasonal distributions of ten species of benthic macrofauna and 12 water environmental parameters at thirty-six sampling stations in the subtidal zone near the Daya Bay Nuclear Power Plant. The results showed that there were four types of distribution characteristics for 10 species of macrobenthic animals and 12 water environmental factors near the Daya Bay nuclear power plant: (1) three species of benthic macrofauna, namely Apionsoma (Apionsoma) trichocephalus, Amphioplus (Lymanella) laevis, and P. bidentata, and six water environmental parameters, including water depth, salinity, dissolved oxygen, suspended solids, chromium, and lead increased from inside the bay to outside the bay. (2) Three species of benthic macrofauna, P. cristata, T. lata, and T. scabra, and four water environmental parameters, including oils, arsenic, total phosphorus, and silicate, decreased from inside to outside the bay. (3) Two species of benthic macrofauna, A. dibranchis, and P. undulatus and one water environmental parameter, pH, were higher in the central bay than inside and outside the bay. (4) One species of benthic macrofauna, Sigambra hanaokai, and one water environmental parameter, total nitrogen, were lower in the central bay than inside and outside the bay. Correlation and BIO-ENV analyses confirmed that water depth was the main environmental factor affecting the ten species of benthic macrofauna. Understanding the distributions of the dominant benthic macrofauna could help protect nuclear cold source systems from benthic macrofaunal blockage and explore marine ecosystem connectivity.
Daya Bay is located in the eastern Guangdong Province between Red (Honghai) Bay and Mirs Bay, with a total area of 650 km2. The Daya Bay coast contour twists and turns, with smaller bays set into the larger bay. The main bays inside Daya Bay include Chimney (Yancong) Bay, Xunliao Port, Fanhe Port, Aotou Port, and Xiaogui Bay. Daya Bay Nuclear Power Base encompasses two nuclear power stations: Daya Bay and Ling’ao.
Construction of the Daya Bay Nuclear Power Plant began in 1987 and was put into commercial operation in 1994. Subsequently, the Lingao Nuclear Power Plant was built near the Daya Bay Nuclear Power Plant, and the two nuclear power plants jointly formed a nuclear power base.
Benthic macrofauna and water environmental factors in the subtidal zone of Daya Bay were surveyed before the construction of the Daya Bay Nuclear Power Plant and have been continuously monitored since 1986. Over the past 35 years, the most dominant species have remained the same. These include T. scabra, P. undulatus, L. brevirostris, and Amphioplus (Lymanella) laevis (Jiang et al., 1990a; Jiang et al., 1990b; Du et al., 2008a; Du et al., 2008b; Du et al., 2009; Du et al., 2011; Yuan et al., 2017; Rao et al., 2020a). Terebellides stroemii, Paucibranchia belli, and Glycera alba were the dominant species in macrozoobenthic communities in the cage culture area (Huang et al., 2005), and Paraprionospio cristata was the dominant macrozoobenthic community species in the mariculture area (Rao et al., 2021). Timoclea scabra, P. undulatus, L. brevirostris, and A. (Lymanella) laevis were the dominant species of macrozoobenthic communities in the sea area around the Daya Bay Nuclear Power Station (Zhang et al., 2007) and from benthic trawling in Daya Bay (Zhang et al., 2017).
Most previous studies have divided benthic macrofauna into several communities according to the dominant species in different regions. The benthic macrofauna from 50 sampling stations in Daya Bay in 1988 and 1989 were divided into six communities (Jiang et al., 1990b). According to the survey data from four voyages in 2004, three communities of benthic macrofauna were identified (Du et al., 2009).
Some studies have focused on the effects of warm drainage in nuclear power plants on benthic macrofauna (Zhang et al., 2007), and the effects of sewage discharge and mariculture on benthic macrofauna (Huang et al., 2005; Rao et al., 2021), while others have focused on the ecological environmental changes in Daya Bay (Wang et al., 2004; Wang et al., 2008).
Although many benthic macrofaunal communities have been monitored before and after the construction of the Daya Bay nuclear power plant, little research has been conducted on the spatial and seasonal distribution of common benthic macrofauna and their relationships with the water environment. The main goals of this study were to (1) analyze the spatial and temporal distribution characteristics of common benthic macrofauna in the subtidal zone near the Daya Bay Nuclear Power Plant, (2) analyze the spatial and temporal distribution characteristics of some water environmental factors in the subtidal zone near the Daya Bay Nuclear Power Plant, (3) analyze the relationships between common benthic macrofauna and water environmental factors in the subtidal zone near the Daya Bay Nuclear Power Plant. To achieve these goals, we simultaneously sampled benthic macrofauna and measured environmental parameters in the subtidal zone near the Daya Bay Nuclear Power Plant.
Sixty-five grid-based sampling stations were established and divided into three sectors (inner, middle, and outer) according to their relative geographic locations and environmental conditions (Rao et al., 2020a; Rao et al., 2021). Sampling surveys were conducted in the autumn (November 2017), winter (January 2018), spring (April 2018), and summer (July 2018). However, in this study, we only selected data from 36 sampling stations, namely, 12 sampling stations in the inner bay, 12 sampling stations in the middle of the bay, and 12 sampling stations outside the bay (Figure 1).
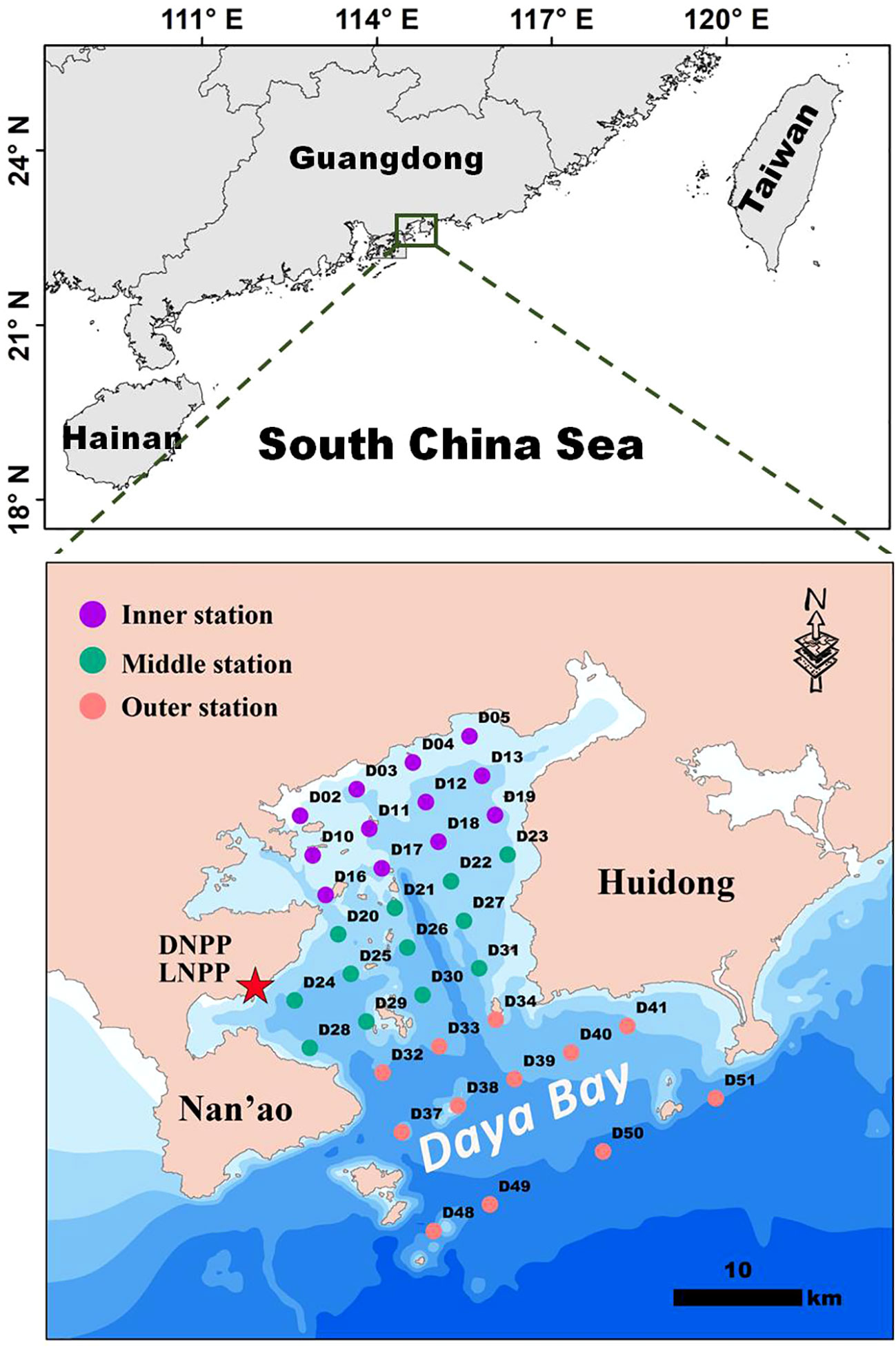
Figure 1 Schematic diagram of benthic macrofaunal sampling stations in the Daya Bay Subtidal zone. Stations are divided into three sectors: inner (purple circle, n = 12), middle (green circle, n = 12) and outer (orange circle, n = 12). DNPP, Daya Bay Nuclear Power Plant; LNPP, Ling’ao Nuclear Power Plant.
Benthic macrofauna were collected using a 0.05 m2 van Veen grab at each station. The sediments were washed through a 0.5 mm mesh sieve, and the residues were transferred to sample containers with 5% formalin buffer in situ for further identification. In the laboratory, benthic macrofauna were identified to the lowest possible taxon and enumerated under a dissecting microscope. They were then weighed using an electronic balance (0.1 mg, FA1204) after blotting surface water off with clean absorbing paper (Rao et al., 2020a; Rao et al., 2021).
There are many benthic animals in the Daya Bay subtidal zone (Jiang et al., 1990a; Jiang et al. 1990b; Du et al., 2008a; Du et al., 2008b; Du et al., 2009; Du et al., 2011; Rao et al., 2020a; Rao et al., 2021). In this study, ten species of benthic animals were considered based on the following three points: (1) according to the relative importance index (IRI) value (Zhang et al., 2017), based on four surveys in 2017 and 2018 that found Aglaophamus dibranchis, P. undulatus, L. brevirostris, A. laevis and P. cristata to be the top five species for average IRI values, and Sigambra hanaokai and T. lata to be the first seven and the first 20, respectively; (2) potential disaster-causing animals in the nuclear power coldsource system, including A. dibranchis, P. cristata, P. undulatus, L. brevirostris, Apionsoma (Apionsoma) trichocephalus, and P. bidentata (Cai et al., 2022b); (3) historically recorded dominant species, including T. scabra, P. undulatus, L. brevirostris, and A. laevis (Jiang et al., 1990a; Jiang et al., 1990b; Du et al., 2008a; Du et al., 2008b; Du et al., 2009; Du et al., 2011; Yuan et al., 2017; Rao et al., 2020a; Rao et al., 2021).
Water depth was determined using portable bathymetry (SPEEDTECH SM-5A, USA). Salinity and dissolved oxygen (DO) were measured using a portable water quality analyzer (WTW Multi 3430, Germany). Oils were extracted with n-hexane and measured using UV spectrophotometry procedures (Ehrhardt and Burns, 1993). The metal concentrations of Cr (chromium), As (arsenic), and Pb (lead) were determined using inductively coupled plasma mass spectrometry (Agilent 7700x, Agilent Technologies, USA). Quality assurance was performed using the procedure described by Wang et al. (2019). The samples for analyzing total nitrogen (TN) and total phosphorus (TP) were measured using the method of Varol and Şen (2012). Total nitrogen was measured by converting all nitrogen forms to nitrate via alkaline persulfate oxidation and subsequent analysis of nitrate was performed using spectrophotometric procedures. The total phosphorus was determined spectrophotometrically using the ascorbic acid method after persulfate digestion. The silicate content was measured using standard silicon molybdenum blue spectrophotometric procedures. Samples were filtered with 0.45 μm polycarbonate filters and analyzed later using the method of Dai et al. (2008). The samples for analyzing suspended solids (SS) were collected using 0.45 μm polycarbonate filters and were subsequently measured gravimetrically (Wang et al., 2015).
Variance analysis (ANOVA) and correlation analyses were performed using SPSS v25 software. ANOVA was used to determine whether there were significant differences in the ten species of benthic macrofauna and the 12 water environmental parameters in different seasons and regions. Correlation analysis was used to determine whether the densities and biomasses of the common benthic macrofauna were significantly correlated with the 12 water environment parameters. BIO-ENV analyses were performed using PRIMER v7 (Anderson et al., 2008). Similarities in benthic macrofauna between each pair of sites were determined using the Bray-Curtis similarity measure based on the fourth root transformed abundance data. Nonmetric multidimensional scaling (NMDS) ordination based on Bray-Curtis similarity was performed to explore the seasonal and site variation of the macrofaunal community. BIO-ENV analyses were used to examine the major environmental factors affecting the ten species of benthic macrofauna.
From the spatial distributions of ten species of benthic macrofauna in the subtidal zone near the Daya Bay Nuclear Power Plant, the densities of A. trichocephalus, A. laevis, and P. bidentata increased from inside the bay to outside the bay. The densities of P. cristata, T. lata, and T. scabra decreased from inside to outside the bay. The densities of A. dibranchis, and P. undulatus were higher in the middle bay than in the inner bay and outside the bay. The densities of S. hanaokai and L. brevirostris were lower in the middle bay than in the inner bay and outside the bay (Figure 2). In addition, the density distribution of L. brevirostris was inconsistent with the biomass distribution, while the density and biomass distributions of the other nine macrobenthic species were consistent (Figure 3).
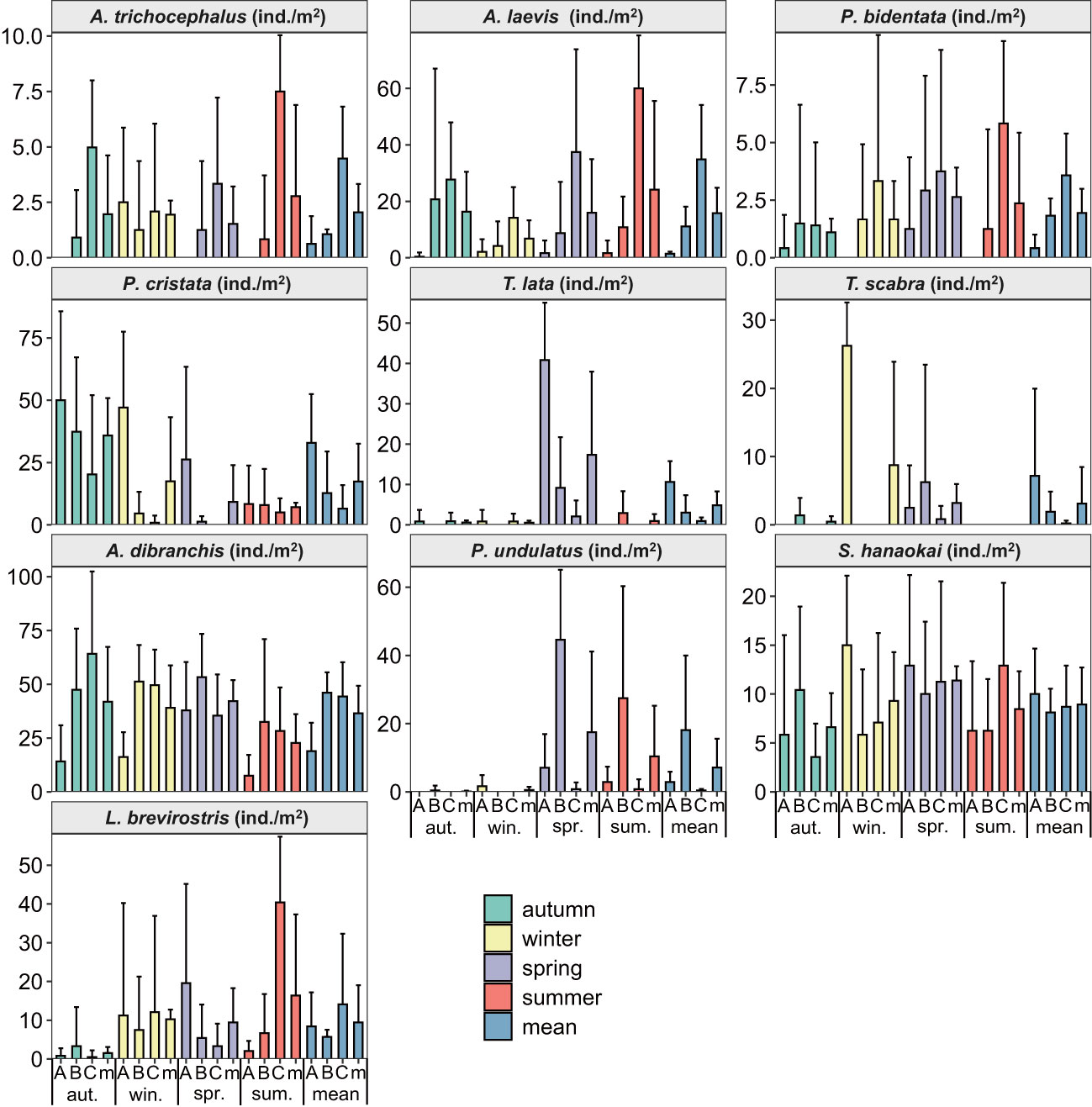
Figure 2 The density spatial and temporal distributions of ten species benthic macrofauna in subtidal zone near the Daya Bay Nuclear Power Plant. (A: represent the inner bay; B: represent the middle bay; C: represent the outside bay; m: represent the mean value).
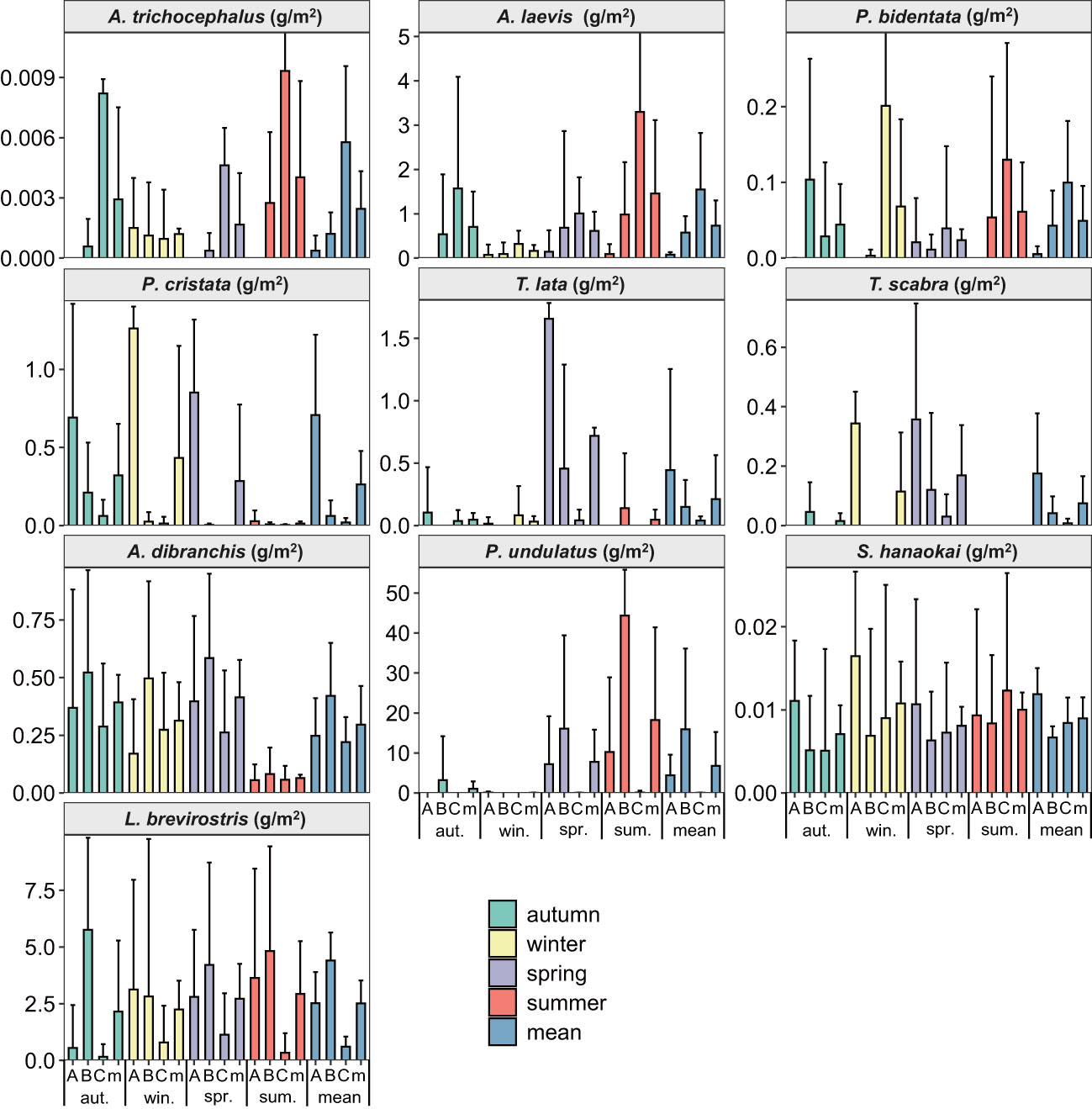
Figure 3 The biomass spatial and temporal distributions of ten species benthic macrofauna in subtidal zone near the Daya Bay Nuclear Power Plant. (A: represent the inner bay; B: represent the middle bay; C: represent the outside bay; m: represent the mean value).
Two-way ANOVA results indicated that the densities of A. trichocephalus, A. laevis, P. bidentata, P. cristata, A. dibranchis, and P. undulatus showed significant regional variation (Table 1). The biomasses of A. trichocephalus, A. laevis, P. cristata, P. undulatus, and L. brevirostris showed significant regional variation (Table 2). The densities of A. laevis, P. cristata, A. dibranchis, P. undulatus, and T. lata showed significant seasonal variation (Table 1). The biomass of A. laevis, A. dibranchis, P. undulatus, and T. lata showed significant seasonal variation (Table 2).
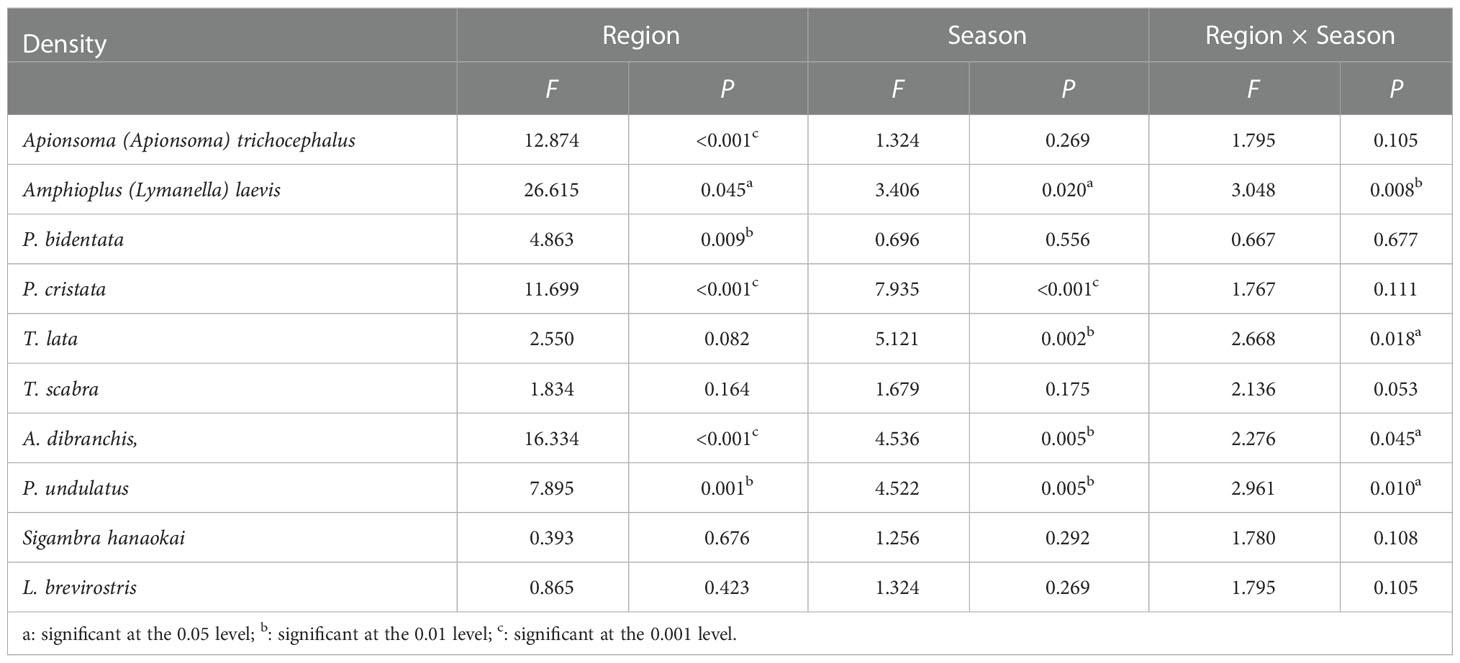
Table 1 The F and P values between seasons and regions for density of ten benthic macrofauna species in subtidal zone near the Daya Bay Nuclear Power Plant.
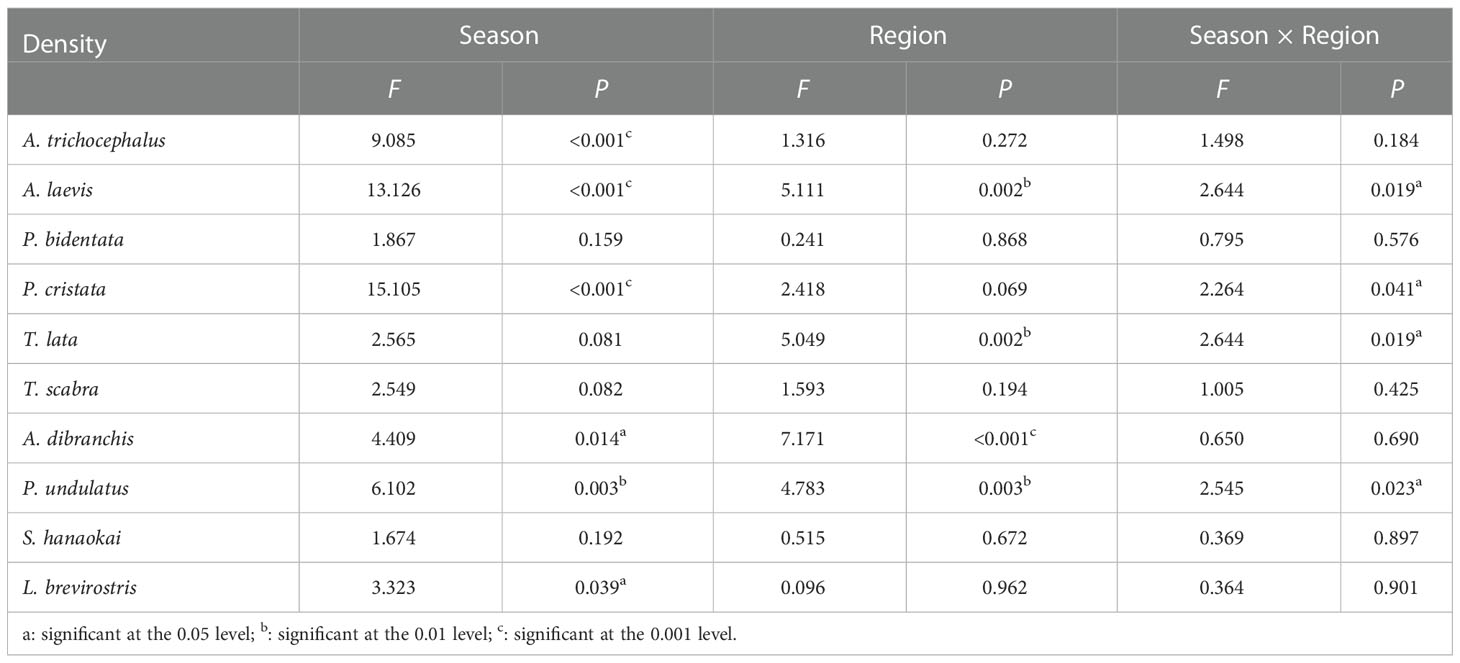
Table 2 The F and P values between seasons and regions for biomass of ten benthic macrofauna species in subtidal zone near the Daya Bay Nuclear Power Plant.
From the spatial distributions of 12 water environmental parameters in the subtidal zone near the Daya Bay Nuclear Power Plant, water depth, salinity, DO, suspended matter, Cr, and Pb increased from inside the bay to outside the bay. Oils, TP, and silicate decreased from the inside to the outside of the bay. The pH was higher in the central bay than in the inner bay and outside the bay. The TN was lower in the central bay than in the inner bay and outside the bay (Figure 4).
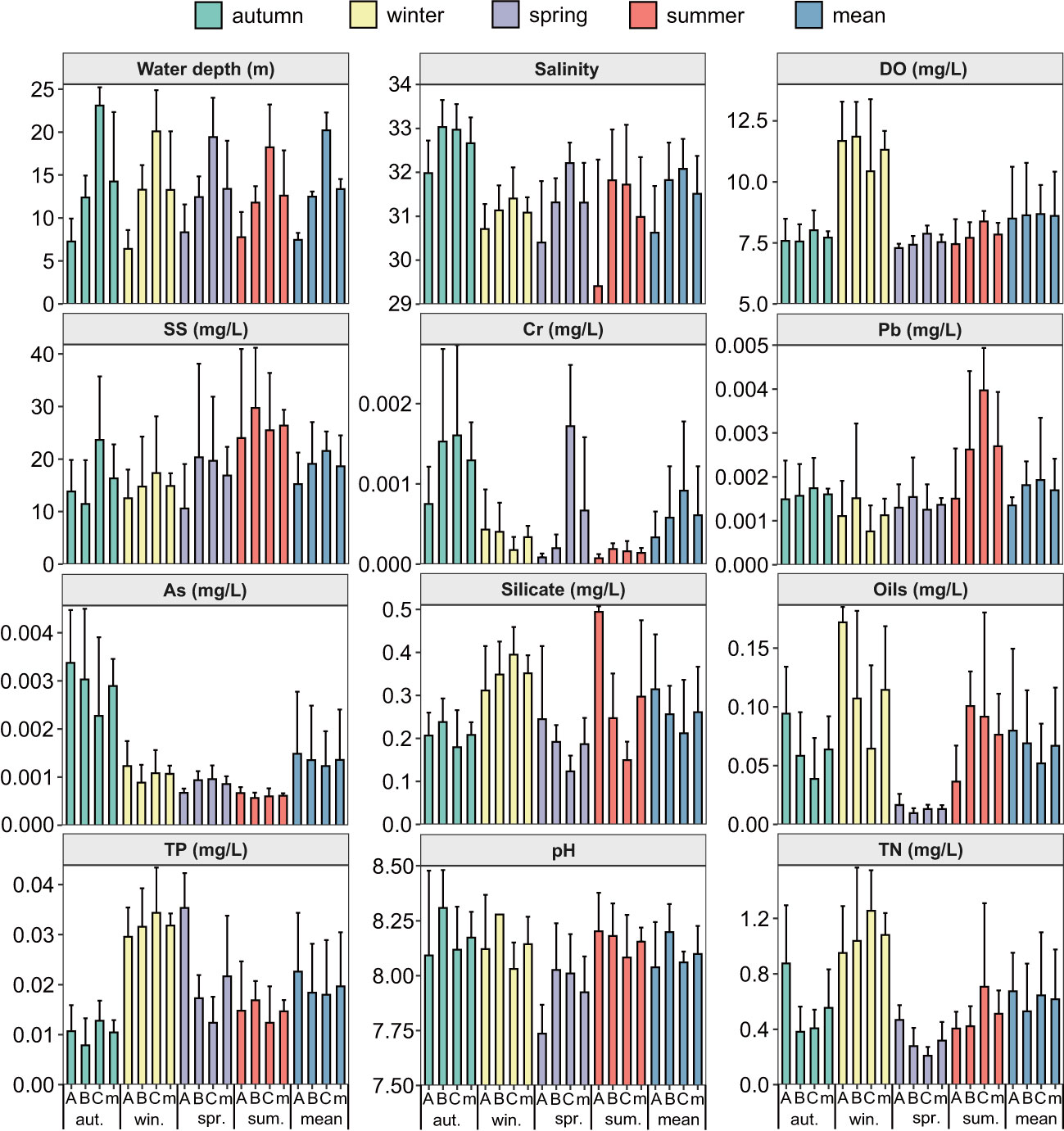
Figure 4 The spatial and temporal distributions of twelve environment factors in subtidal zone near the Daya Bay Nuclear Power Plant. (A: represent the inner bay; B: represent the middle bay; C: represent the outside bay; m: represent the mean value).
A two-way ANOVA indicated that water depth, salinity, Cr, silicate, and pH showed significant regional variation. Salinity, DO, suspended matter, Cr, Pb, silicate, oils, TP, pH, and TN showed significant seasonal variation (Table 3).
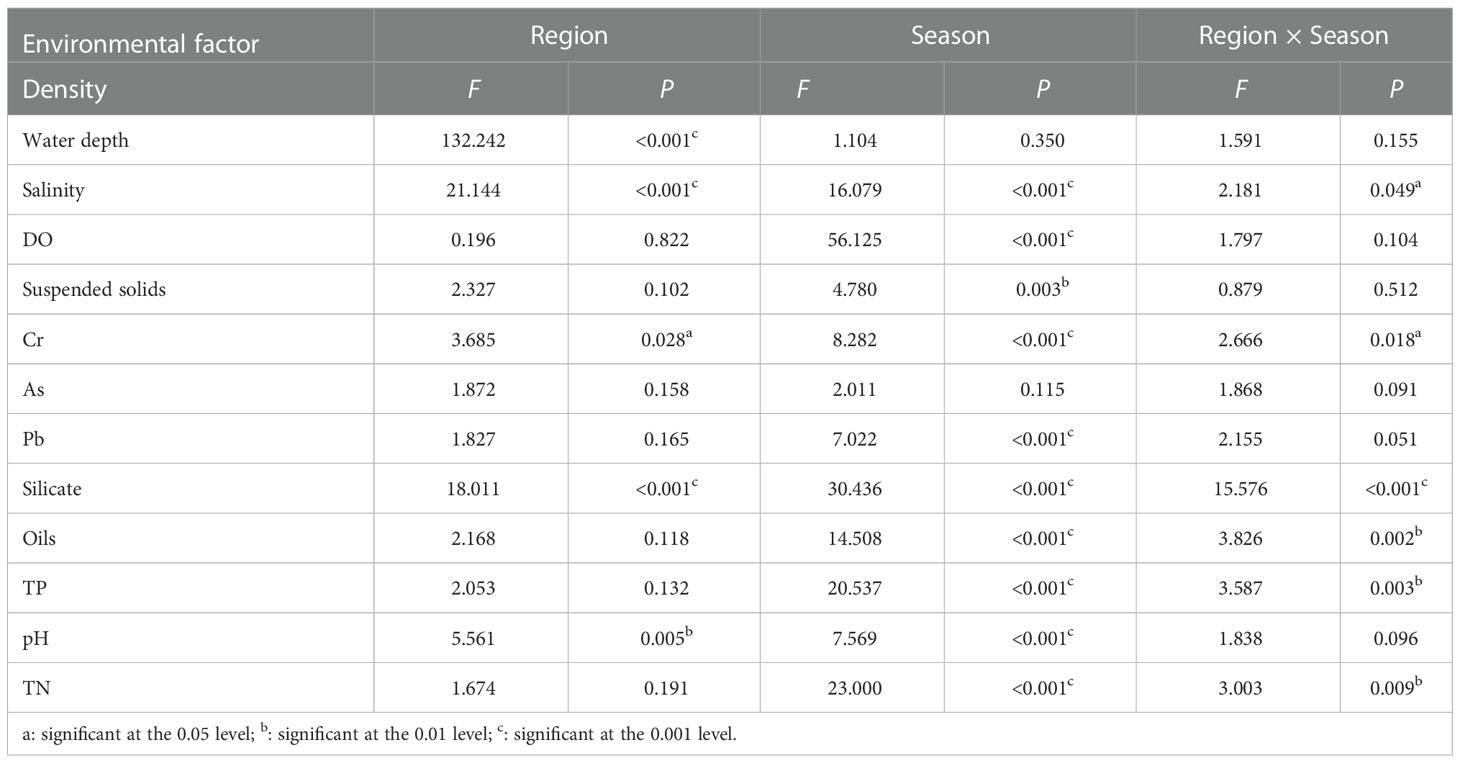
Table 3 The F and P values between seasons and regions for twelve environment factors in subtidal zone near the Daya Bay Nuclear Power Plant.
In terms of water environmental parameters, water depth was significantly correlated with the densities of six species of benthic macrofauna. There was a significant positive correlation between the densities of A. trichocephalus, A. laevis, P. bidentata, and A. dibranchis and water depth. There was a significant negative correlation between the densities of P. cristata and T. lata and water depth. Regarding benthic macrofaunal density, A. laevis and T. lata were significantly correlated with the five water environmental parameters. There was a significant positive correlation between the density of A. laevis and water depth, salinity, and Pb. There was a significant negative correlation between the densities of A. laevis, silicate, and TP. There was a significant positive correlation between the densities of T. lata, silicate, and TP. There was a significant negative correlation between the density of T. lata and water depth, salinity, and pH (Table 4).
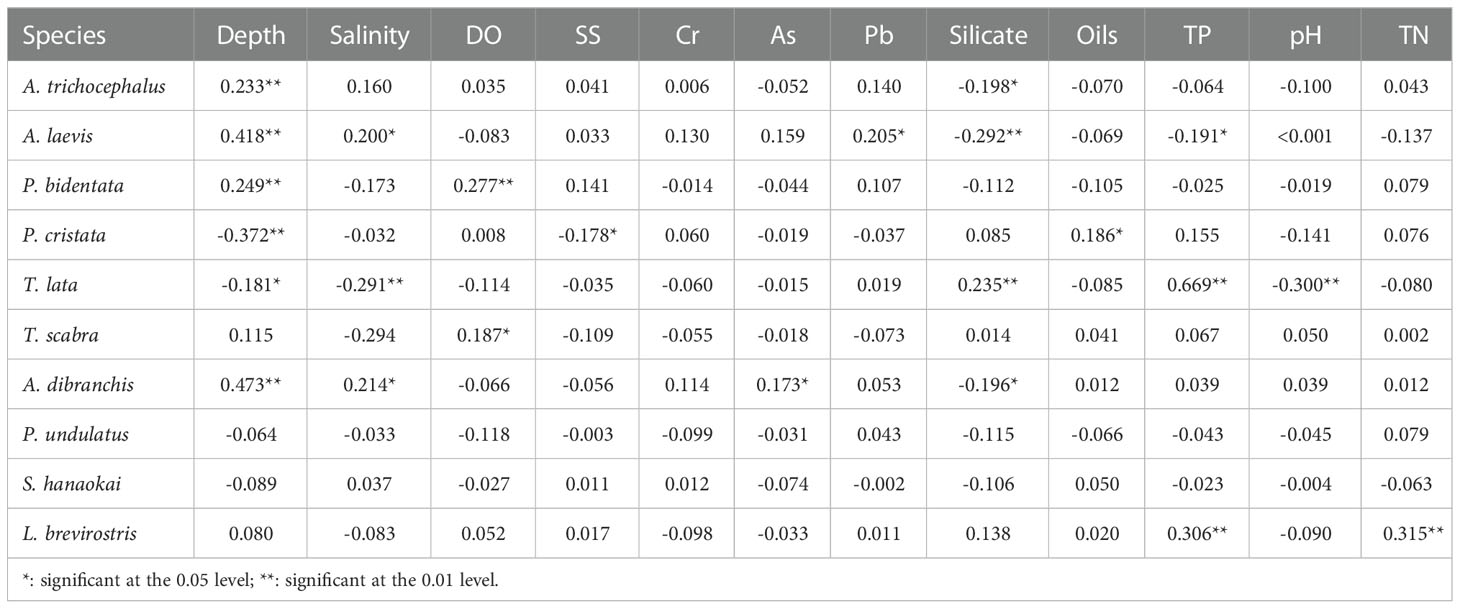
Table 4 Correlation analysis between densities of ten species benthic macrofauna and water environmental factors in subtidal zone near the Daya Bay Nuclear Power Plant.
In terms of environmental parameters, water depth was significantly correlated with the biomass of six species of benthic macrofauna. There was a significant positive correlation between the biomass of A. trichocephalus, A. laevis, and P. bidentata and water depth. There was a significant negative correlation between the biomass of P. cristata, T. lata, S. hanaokai and water depth. Among the benthic macrofaunal biomass, A. laevis and T. lata were significantly correlated with the five water environment parameters. There was a significant positive correlation between the biomass of A. laevis and water depth, salinity, As, and Pb. A significant negative correlation was observed between the biomass of A. laevis and silicate. There was a significant positive correlation between the biomass of T. lata and silicate and TP. There was a significant negative correlation between the biomass of T. lata and water depth, salinity, and pH (Table 5).
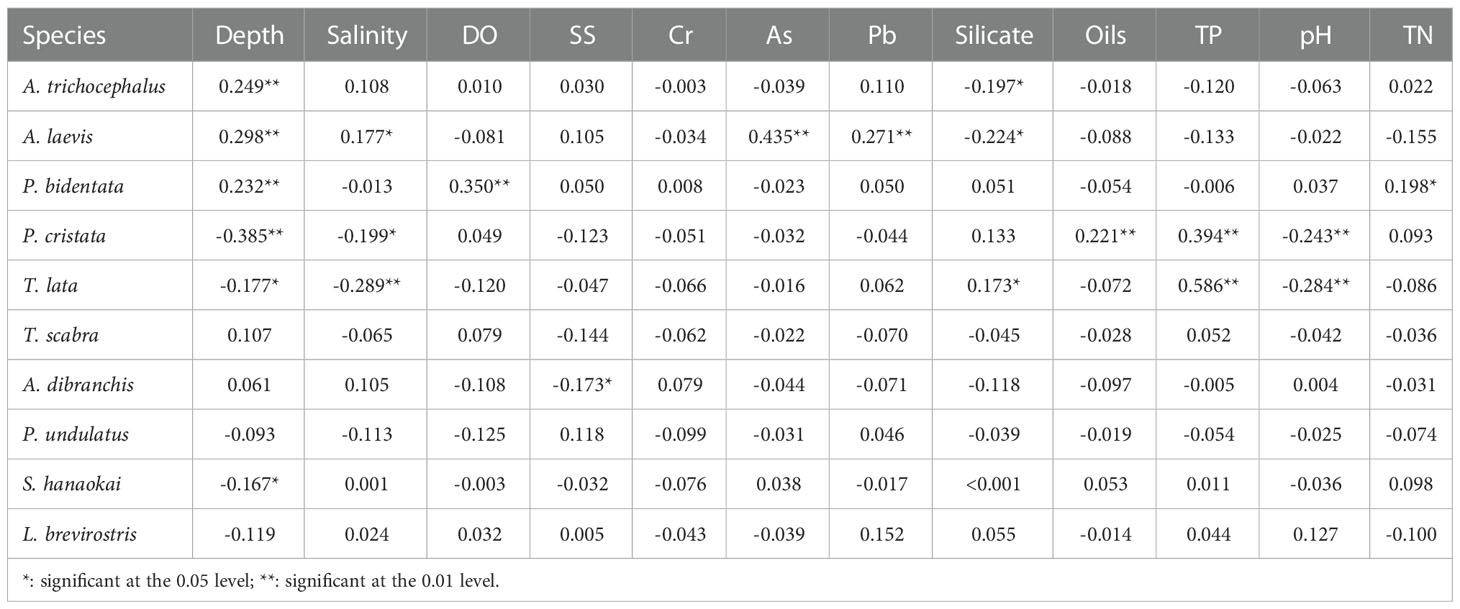
Table 5 Correlation analysis between biomass of ten species benthic macrofauna and water environmental factors in subtidal zone near the Daya Bay Nuclear Power Plant.
The BIO-ENV analysis showed that water depth was the first factor affecting 10 macrobenthic species, with a correlation coefficient of 0.191. The second factor affecting 10 macrobenthic species was salinity, with a correlation coefficient of water depth + salinity of 0.215.
This study involved three polychaetes: P. cristata, A. dibranchis, and S. hanaokai. Three species of polychaetes were the dominant species of macrozoobenthic communities in Daya Bay (Jiang et al., 1990a; Jiang et al., 1990b; Du et al., 2008a; Du et al., 2008b; Du et al., 2009; Du et al., 2011; Yuan et al., 2017; Cai et al., 2022b), and they were also the dominant species along the coast of China (Cai and Zheng, 1994; Huang et al., 2012; Chen et al., 2020; Rao et al., 2020b). Sigambra hanaokai is the dominant species in the macrobenthic community in the southern Yellow Sea (Xu et al., 2021).
P. cristata (It was previously identified as Paraprionospio pinnata in China and Korean, therefore, the P. pinnata reported in Chinese waters should be P. cristata) collected in Jiaozhou Bay and the Yellow Sea may be P. inaequibranchia and P. coora (Zhou et al., 2008). P. cristata has been widely reported in Korean waters (Yokoyama and Choi, 2010). P. cristata is considered an opportunistic species (Ji et al., 2022). In coastal waters along the Arabian Sea, Cossura coasta, the dominant species during the pre-monsoon period, was replaced by the surface deposit feeder P. pinnata during the monsoon and post-monsoon periods (Rehitha et al., 2019). Paraprionospio pinnata is only located in low-oxygen habitats in the Humboldt upwelling ecosystem (Fajardo et al., 2018) and it is the dominant species of the macrobenthic community in winter in the cage culture area of Daya Bay (Huang et al., 2005). The results of this study showed that the density of P. cristata was significantly negatively correlated with water depth and suspended solids, and significantly positively correlated with oil, whereas the biomass of P. cristata was significantly negatively correlated with water depth, salinity, and pH, and significantly positively correlated with oils and TP.
The relationship between S. hanaokai and the environmental factors in Chinese sea waters has been rarely reported, only a correlation analysis showed that there were significant negative correlations between the density of S. hanaokai and the content of organic carbon and nitrogen in the intertidal zone of Xiamen Crocodile Island (Cai et al., 2022c).
In winter and spring, the grappler method risk index (GMRI) of A. dibranchis is more than 50%, the risk of blocking the nuclear cold source system is at a medium-risk level. In summer and autumn, the GMRI of A. dibranchis is less than 50%, the risk of blocking the nuclear cold source system is at a medium-risk level. The risks of blocking the nuclear cold source system P. cristata and S. hanaokai are both at a low-risk level in Daya Bay in four seasons (Cai et al., 2022b).
Apionsoma trichocephalus was not the dominant species in previous surveys in Daya Bay (Jiang et al., 1990a; Jiang et al., 1990b; Du et al., 2008a; Du et al., 2008b; Du et al., 2009; Yuan et al., 2017), but it poses a potential risk of blocking nuclear cooling source systems (Cai et al., 2022b). Although the average density of A. trichocephala was low in all four seasons in Daya Bay (below 4 ind./m2), it was the dominant species in the subtidal zone of the East China Sea during all four seasons (Shou et al., 2018) and in the subtidal zone of the Chinese islands (Huang et al., 2012). However, there are few reports on the relationship between A. trichocephala and environmental factors. Our study found that A. trichocephala showed a significant positive correlation with water depth and a significant negative correlation with silicate.
L. brevirostris is one of the dominant species in macrozoobenthic communities in Daya Bay (Jiang et al., 1990a; Jiang et al., 1990b; Du et al., 2011; Yuan et al., 2017; Cai et al., 2022b). The results of this study showed that the density of L. brevirostris was significantly positively correlated with TN and TP. The density of L. brevirostris was high in sea areas with high organic matter content in Daya Bay (Jiang et al., 1990b).
Although the individual weight of L. brevirostris is large, its density is low and the distribution range is small; therefore, the risk of blocking the nuclear power cold source system is also low. Except for the GMRI in summer, which is over 50%, the risk of blocking is less than 50% in the other three seasons in Daya Bay (Cai et al., 2022b).
P. undulatus and T. scabra are the dominant species in macrozoobenthic communities in Daya Bay (Jiang et al., 1990a; Jiang et al., 1990b; Du et al., 2009; Du et al., 2011; Yuan et al., 2017; Cai et al., 2022a). The undulated surf clam, Paphia undulata (the new revised name in WoRMS is P. undulatus), is commercially cultured on the southern coast of China (Zhang et al., 2022a). Therefore, there have been many studies on the relationship between P. undulatus and environmental factors. P. undulatus showed a significant positive correlation with the DO, clay, and chlorophyll-a content in the Beibu Gulf (Ye et al., 2010). Mud substrates with ≥ 40% water content in the temperature range of 20–30°C and salinity range of 20–40 psu were appropriate for P. undulatus burrowing and may be appropriate for its culture (Zhang et al., 2022a). P. undulatus is not only a cultured species but also a common dominant species in the macrozoobenthic community along the southeast coast of China (Ye et al., 2010; Du et al., 2011; Shu et al., 2015; Yuan et al., 2017; Rao et al., 2021; Cai et al., 2022a).
T. scabra is not an economically important species, but it is also a common dominant species in the macrozoobenthic community along the coast of Guangdong (Du et al., 2011; Li et al., 2016). T. lata is neither an economically important nor a dominant species on the southeast coast of China (Jiang et al., 1990a; Jiang et al., 1990b; Zhang et al., 2007; Du et al., 2008a; Cai et al., 2022a). Why do heavy metals have less impact on the Daya Bay bivalves? That is, there is no significant correlation between bivalves and heavy metals. This is because the sediment quality radically improved after the late-2000s, and heavy metals in nearshore sediments of Daya Bay were all closely related to the import of anthropogenic and/or terrestrial material, whereas those offshore were likely to be related to the joint influence of anthropogenic activities and natural processes (Du et al., 2008; Qu et al., 2018).
The GMRI of P. undulatus in all four seasons was less than 50%; therefore, the risk of blocking the nuclear power cold source system was low in Daya Bay (Cai et al., 2022b).
Amphioplus (Lymanella) laevis has been a dominant species in Daya Bay for over 30 years (Jiang et al., 1990a; Jiang et al., 1990b; Zhang et al., 2007; Du et al., 2008a; Du et al., 2008b; Du et al., 2009; Huang et al., 2012; Zhang et al., 2017; Cai et al., 2022b). It is also a common dominant species on the coast and in the Gulf of China (Cai and Zheng, 1994; Chen et al., 2020). The genus Amphioplus is also dominant in some open-sea areas. Amphioplus sinicus was not only the dominant species of macrozoobenthic communities in Hailing Bay, western Guangdong, but also outside the bay, in addition to being the annual dominant species (Li et al., 2018). A. laevis is the dominant species in subtidal amphioxus habitats in Xiamen Bay (Chen et al., 2020; Rao et al., 2020b). BIO-ENV analysis revealed significant seasonal variations in environmental factors affecting community structure (including A. laevis) in semi-enclosed waters in Bohai Bay, China (Shi et al., 2022). This study found that the density of A. laevis showed a significantly positive correlation with water depth, salinity, and Pb and a significantly negative correlation with silicate and TP.
P. bidentata has not been the dominant species in previous surveys in Daya Bay (Jiang et al., 1990a; Jiang et al., 1990b; Du et al., 2008a; Du et al., 2008b; Du et al., 2009; Zhang et al., 2017; Yuan et al., 2017; Rao et al., 2020a), but it was a common species in the sea area near the nuclear power plant (Cheng et al., 2018; Li et al., 2020; Zhang et al., 2022b), It was a dominant species on the northern coast of Zhejiang Province (Yan et al., 2020) and in the surrounding waters of Qinshan Island (Mao et al., 2022). This study found that the density and biomass of P. bidentata were significantly positively correlated with water depth and DO.
The GMRI of P. bidentata in all four seasons was less than 50%, so the risk of blocking the nuclear power cold source system was low in Daya Bay (Cai et al., 2022b).
The main sources of pollution near the Daya Bay nuclear power plant were the warm drainage from the power plant and the shallow aquaculture area of Dapeng Ao, but the warm drainage from the power plant had less effect on benthic macrofauna in nearby waters (Zhang et al., 2007). From 1982 to 2004, the ecological environment of Daya Bay changed from 237 species in 1987 to 194 species in 1997 (Wang et al., 2004), and the mean biomass and species of benthic animals near power plants ranged from 317.9 g/m2 in 1991 to 45.24 g/m2 in 2004, and from 250 species in 1991 to 177 species in 2004 (Wang et al., 2008). The correlation analysis between the macrobenthos community and environmental factors indicated that secondary productivity was significantly affected by the content of inorganic nitrogen, phosphorus, dissolved oxygen of seawater, and organic carbon content of sediment (Liu et al., 2018). Changes in the ecological environment in Daya Bay are related to sewage discharge and mariculture. Small body size, short longevity, and high tolerance species are more abundant in industrial sewage discharge areas and mariculture areas (Rao et al., 2021). We believe that to accurately identify the main environmental factors affected, long-term monitoring and sufficient comparable data are needed.
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
LC conceived of the study and obtained funding. LC, YR, XYZ, DY, DW, XPZ, and XY were responsible for field and laboratory work. LC, YR, and DY conducted the identification of benthic macrofauna. The manuscript was reviewed by LC. YR and XYZ helped analyzing the data and plotting the figures. All authors contributed to the article and approved the submitted version.
This work was supported by the National Key Research and Development Program of China [2018YFC1407501] and the Coordinate Oil Subsidy Project of Huizhou [F2017-01-4].
We sincerely grateful to the Huizhou Ocean Technology Center for providing us with environmental data. We would like to thank Director Haoliang Liang, Junxing Wang, Binglin Chen, Sujing Fu and Bingwen Chen for their support in sample collection. All members involved in this project are to be acknowledged here.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Anderson M. J., Gorley R. N., Clarke K. R. (2008). PERMANOVA + for PRIMER: Guide to software and statistical methods (Plymouth, UK: PRIMER-E).
Cai L., Rao Y., Zhao X., Yang D., Lin J., Fu S., et al. (2022b). Two risk indices for benthic macrofauna entrapment evaluation on the water intake systems in coastal nuclear power plants. J. Appl. Oceanography 41, 655–662. doi: 10.11759/hykx20211003001
Cai L., Yang D., Zhao X., Lin J., Chen X., Zhou X., et al. (2022a). Progress and prospects of mollusk community and population ecology in daya bay. Mar. Sci. 46, 124–134. doi: 10.3969/J.ISSN.2095-4972.2022.04011
Cai L., Zhao X., Peng W., Lin J., Yang D., Rao Y., et al. (2022c). Comparison of the macrozoobenthic community and sedimentary environment with and without horseshoe crab presence in the crocodile island intertidal zone, xiamen, China. J. Oceanology Univ. China 21, 573–582. doi: 10.1007/s11802-022-5206-9
Cai L., Zheng T. (1994). Macrobenthic communities and evaluation of their environmental affection in subtidal zone, dongshan islands. J. Xiamen Univ. (Natural Science) 33, 37–42.
Chen B., Cai L., Rao Y., Li W., Chen X., Fu S. (2020). Effects of sediment fining on benthic macrofaunal community in subtidal amphioxus habitats in xiamen. Oceanologia Limnologia Sin. 51, 494–505. doi: 10.11693/hyhz20191200245
Cheng H., Wang J., Tang Y., Zheng B., Lu C. (2018). Research on community characteristics of macrozoobenthos and environmental quality of offshore north fujian in spring. J. Shanghai Ocean Univ. 27, 238–249. doi: 10.12024/jsou.20170902125
Dai M., Zhai W., Cai W., Callahan J., Huang B., Shang S., et al. (2008). Effects of an estuarine plume-associated bloom on the carbonate system in the lower reaches of the pearl river estuary and the coastal zone of the northern south China Sea. Cont. Shelf Res. 28, 1416–1423. doi: 10.1016/j.csr.2007.04.018
Du J., Mu H., Song H., Yan S., Gu Y., Zhang J. (2008). 100 years of sediment history of heavy metals in daya bay, China. Water Air Soil pollut. 190, 343–351. doi: 10.1007/s11270-007-9593-8
Du F., Wang X., Jia X., Yang S., Ma S., Chen H., et al. (2011). Species composition and characteristics of macrobenthic fauna in daya bay, south China Sea. J. Fishery Sci. China 18, 877–892. doi: 10.3724/SP.J.1118.2011.00877
Du F., Wang X., Li C., Zhang H., Jia X. (2008a). Study species diversity of macrobenthos in daya bay, south China Sea. South China Fishery Sci. 4, 33–41.
Du F., Wang X., Li C., Zhang H., Jia X. (2009). Macrobenthic community structure in daya bay, south China Sea. Acta Ecologica Sin. 29, 1091–1098.
Du F., Zhang H., Li C., Wang X., Jia X. (2008b). Species composition and diversity of macrobenthic fauna in daya bay. J. Fishery Sci. China 15, 252–259.
Ehrhardt M. G., Burns ,. K. A. (1993). Hydrocarbons and related photo-oxidation products in Saudi Arabian gulf coastal waters and hydrocarbons in underlying sediments and bioindicator bivalves. Mar. pollut. Bull. 27, 187–197. doi: 10.1016/0025-326X(93)90024-E
Fajardo M., Andrade D., Bonicelli J., Bon M., Gomez G., Riascos J., et al. (2018). Macrobenthic communities in a shallow normoxia to hypoxia gradient in the Humboldt upwelling ecosystem. PloS One 13, e0200349. doi: 10.1371/journal.pone.0200349
Huang H., Chen B., Chen G., Ma Z., Yu W. (2012). Spatial and temporal distribution characteristics of macrobenthos at subtidal zone in islands, China. China Environ. Sci. 32, 1888–1894.
Huang H., Lin Q., Lin Y., Jia X., Li C., Wang W. (2005). Spatial-temporal variation of large macrobenthic animals in cage culture sea area in daya bay. China Environ. Sci. 25, 412–416.
Jiang J., Cai E., Wu Q., Xu H., Li R., Zheng F., et al. (1990a). Species composition and quantitative distribution on benthic animals in daya bay. Collections papers Mar. Ecol. Daya Bay (II), 237–247.
Jiang J., Li R., Zheng F., Lu L., Wu Q., Xu H., et al. (1990b). Analysis of benthic community structure in daya bay. Collections papers Mar. Ecol. Daya Bay (II), 282–289.
Ji Y., Wang J., Zhang N., Sun B., Su K., Wang Z. (2022). Community structure and biodiversity of macrobenthos in the coastal waters of rizhao. J. Shanghai Ocean Univ. 31, 119–130. doi: 10.12024/jsou.20210103269
Li Y., Du F., Gu Y., Ning J., Wang L. (2016). Relationship between macrobenthic fauna community and environmental factors in southeast leizhou peninsula of the south China Sea. South China Fisheries Sci. 12, 33–41. doi: 10.3969/j.issn.2095-0780.2016.06.005
Li Y., Du F., Wang L., Ning J., Li C. (2018). Ecology of microbenthic fauna community in aqualture zones of hailing bay and adjacent waters along the western guangdong coast, China. Oceanologia Limnologia Sin. 49, 1294–1307. doi: 10.11693/hyhz20180300058
Li Y., Shi Z., Fan L., Huang W., Zheng B., Xia Y. (2020). Changes of population on microbenthic and its pollution index evaluation in the adjacent sea area of XX nuclear power plant. Ocean Dev. Manage. 2, 66–73. doi: 10.20016/j.cnki.hykfygl.2020.02.012
Liu K., Du F., Li Y., Wang X., Chen H., Zhang J., et al. (2018). Variation characteristics of microbenthic secondary productivity in daya bay of south China Sea for nearly 30 years. South China Fisheries Sci. 14 (2), 1–9. doi: 10.3969/j.issn.2095-0780.2018.02.001
Mao C., Wei A., Zhang Y., Yuan G., Jiao X., Cui C., et al. (2022). Health status evaluation of the macrobenthos community in surrounding waters of qinshan island. Environ. Monit. Forewarning 14, 72–78. doi: 10.3969/j.issn.1674-6732.2022.01.013
Qu B., Song J., Yuan H., Li X., Li N., Duan L. (2018). Intensive anthropogenic activities had affected daya bay in south China Sea since the 1980s: Evidence from heavy metal contaminations. Mar. pollut. Bull. 135, 318–331. doi: 10.1016/j.marpolbul.2018.07.011
Rao Y., Cai L., Chen B., Chen X., Zheng L., Lin S. (2020a). How do spatial and environmental factors shape the structure of a coastal macrobenthic community and meroplanktonic larvae cohort? evidence from daya bay. Mar. pollut. Bull. 157, 111242. doi: 10.1016/j.marpolbul.2020.111242
Rao Y., Cai L., Chen X., Zhou X., Fu S., Huang H. (2021). Responses of functional traits of macrobenthic communities to human activities in daya bay (a subtropical semi-enclosed bay), China. Front. Environ. Sci. 9, 766580. doi: 10.3389/fenvs.2021.766580
Rao Y., Cai L., Li W., Chen X., Yang D. (2020b). Spatiotemporal variations of benthic macrofaunal community in the xiamen amphioxus nature reserve, eastern south China Sea. Acta Oceanologica Sin. 39, 10–18. doi: 10.1007/s13131-020-1587-z
Rehitha T., Madhu N., Vineetha G., Vipindas P., Resmi P., Revichandran C. (2019). Spatio-temporal variability in macrobenthic communities and trophic structure of a tropical estuary and its adjacent coastal waters. Environ. Monit. Assess. 191, 341. doi: 10.1007/s10661-019-7460-x
Shi Y., Zhang G., Zhang G., Wen Y., Guo Y., Peng L., et al. (2022). Species and functional diversity of marine macrobenthic community and benthic habitat quality assessment in semi-enclosed waters upon recovering from eutrophication, bohai bay, China. Mar. pollut. Bull. 181, 113918. doi: 10.1016/j.marpolbul.2022.113918
Shou L., Liao Y., Tang Y., Chen J., Jiang Z., Gao A., et al. (2018). Seasonal distribution of macrobenthos and its relationship with environmental factors in yellow Sea and East China Sea. J. Oceanology Limnology 36, 772–782. doi: 10.1007/s00343-018-6271-1
Shu L., Chen P., Li X., Yu J., Feng X. (2015). Species composition and seasonal variation of microbenthic fauna in zhelin bay and adjacent waters. J. Appl. Oceanography 34, 124–132. doi: 10.3969/J.ISSN.2095-4972.2015.01.016
Varol M., Şen B. (2012). Assessment of nutrient and heavy metal contamination in surface water and sediments of the upper Tigris river, Turkey. Catena 92, 1–10. doi: 10.1016/j.catena.2011.11.011
Wang Q., Mei D., Chen J., Lin Y., Lu H., Yan C. (2019). Sequestration of heavy metal by glomalin-related soil protein: implication for water quality improvement in mangrove wetlands. Water Res. 148, 142–152. doi: 10.1016/j.watres.2018.10.043
Wang Y., Lou Z., Sun C., Sun S. (2008). Ecological environment changes in Daya Bay, China, from 1982 to 2004. Mar. Pollut. Bull. 56, 1871–1879. doi: 10.1016/j.marpolbul.2008.07.017
Wang Y., Wang Z., Huang L. (2004). Environment changes and trends in daya bay in recent 20 years. J. Trop. Oceanography 23 (5), 85–95.
Wang D., Yang X., Zhai W., Li Y., Hong H. (2015). Spatial distribution of dissolved cadmium in the jiulong river–estuary system: Relevance of anthropogenic perturbation. Cont. Shelf Res. 111, 279–285. doi: 10.1016/j.csr.2015.10.008
Xu J., Xin L., Liu X. (2021). Patterns of species and functional diversity of macrofaunal assemblages and the bioassessment of benthic ecological quality status in the southern yellow Sea. Mar. pollut. Bull. 171, 112784. doi: 10.1016/j.marpolbul.2021.112784
Yan R., Feng M., Wang X., Han Q. (2020). Temporal and spatial distribution of dominant macrozoobenthos species in the sea area off northern zhejiang province. Oceanologia Limnologia Sin. 51, 1162–1174. doi: 10.11693/hyhz20191200253
Ye J., Cai L., Huang R., Zhou X., Fu S., Lin H., et al. (2010). Species composition of trawling mollusk in beibu gulf and its environmental effect. Mar. Sci. Bull. 29, 617–622.
Yokoyama H., Choi J. (2010). New records of three Paraprionospio species (Polychaeta: Spionidae) from Korean waters. Ocean Sci. J. 45, 55–61. doi: 10.1007/s12601-010-0005-4
Yuan T., Li H., Li L., Wang H., Yang C. (2017). Community structure on macrobenthos in summer in daya bay. J. Trop. Oceanography 36, 41–47. doi: 10.11978/2016040
Zhang J., Gao Y., Shi X., Lü X. (2017). Species composition and diversity of marine organisms from benthic trawling in daya bay of the northern south China Sea. Biodiversity Sci. 25, 1019–1030. doi: 10.17520/biods.2017103
Zhang W., Sun W., Xu X., Wang H., Zheng B., Lu C., et al. (2022b). Ecological environment and the potential hazard-causing organisms in the sea area near the nuclear power plant. Mar. Sci. 46, 32–43. doi: 10.11759/hykx20211101001
Zhang J., Xiao Y., Wang H., Lin D., Dong Y. (2007). Analysis of community structure on macrobenthos in sea area around daya bay nuclear power station. Mar. Environ. Sci. 26, 561–564.
Zhang P., Yongo E., Feng H., Pan S., Sun A., Zhou L., et al. (2022a). Effects of substrate, temperature, salinity, size and transportation on burrowing capacity of juvenile undulated surf clam Paphia undulata. Aquaculture Res. 53, 2796–2805. doi: 10.1111/are.15794
Keywords: benthic macrofauna, environmental factor, nuclear power plant, subtidal zone, Daya Bay
Citation: Cai L, Rao Y, Zhao X, Yang D, Zhou X, Wang D and Yue X (2023) Spatial and seasonal distributions of ten species of benthic macrofauna and twelve water environmental factors in a subtidal zone near the Daya Bay nuclear power plant. Front. Mar. Sci. 9:1093468. doi: 10.3389/fmars.2022.1093468
Received: 09 November 2022; Accepted: 23 December 2022;
Published: 12 January 2023.
Edited by:
Kaizhi Li, South China Sea Institute of Oceanology (CAS), ChinaReviewed by:
Xingyu Song, South China Sea Institute of Oceanology (CAS), ChinaCopyright © 2023 Cai, Rao, Zhao, Yang, Zhou, Wang and Yue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lizhe Cai, Y2FpbGl6aGVAeG11LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.