
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 09 January 2023
Sec. Aquatic Physiology
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.1089421
 Jian-Feng Qiu1,2†
Jian-Feng Qiu1,2† Cheng Luo1,2†
Cheng Luo1,2† Li-Hua Ren3
Li-Hua Ren3 Wei Li3
Wei Li3 Tai-Ming Dai1,2
Tai-Ming Dai1,2 Guang Wang1,2
Guang Wang1,2 Xiao-Ning Sun1,2
Xiao-Ning Sun1,2 Kou-Chang Chong Moua1,2
Kou-Chang Chong Moua1,2 Yang-Hu Sima1,2
Yang-Hu Sima1,2 Shi-Qing Xu1,2*
Shi-Qing Xu1,2*Chinese mitten crabs (Eriocheir sinensis) are traditionally fed iced trash fish, but the industry is facing problems such as low breeding safety. Black soldier fly (Hermetia illucens) is an alternative protein source in animal diets, including diets for aquatic animals, due to its high nutritional value. However, studies on the effects of black soldier fly on the flavor characteristics of aquatic animals are still limited. In the present study, we investigated the effects of the complete replacement of iced trash fish with black soldier fly larvae during the fattening period of Chinese mitten crab on the flavor molecule contents and evaluation indices. The levels of free amino acids and nucleotides were determined in three edible parts (muscle, hepatopancreas, and gonads) of crab. Taste activity value analysis showed that glutamic acid, glycine, alanine, and arginine were the main amino acids contributing to the umami taste and sweetness, while histidine, lysine, valine, and methionine were the main amino acids contributing to the bitterness. Equivalent umami concentration (EUC) analysis showed that female gonads had the strongest umami taste, followed by the hepatopancreas and muscle. Sweetness value (SWT) analysis showed that the sweetness of muscle was the highest. Feeding black soldier fly larvae affected the flavor characteristics of crabs with tissue and sex differences. The EUC of the female gonads and SWT of the muscle were significantly increased. Meanwhile, the EUC of the hepatopancreas and SWT of the gonads were slightly decreased in male crabs. Our results indicate that the complete replacement of iced trash fish with black soldier fly larvae during the fattening period significantly enhances the flavor characteristics of crabs based on the contents of flavoring amino acids and nucleotides. It is important for sustainable aquaculture to replace animal protein with alternative protein sources such as black soldier fly larvae.
Chinese mitten crab (Eriocheir sinensis) is an aquaculture product that is highly efficiently produced in bulk. The crab farming area has reached 47000 hectares in China, with an annual yield of up to 80000 tons (CFSY, 2021). The Yangtze River system is the main production area of Chinese mitten crab, with the aquaculture output exceeding 90% of the national output. Jiangsu Province has a crab breeding area of 24000 hectares, and the production is more than 50% of the national production (CFSY, 2021). Farming practice, including feed composition, affects the taste, nutrition, and health of crabs (Wu et al., 2020). Iced trash fish is the traditional food of Chinese mitten crab, which plays an important role in the growth, nutritional quality, and taste characteristics of crabs. However, the use of iced trash fish as feed is associated with safety problems such as feed unstable sources and uncontrollable quality (Bunlipatanon et al., 2014). It has been reported that trash fish may carry bacterial and viral pathogens, causing the death of farmed aquatic species. In addition, uneaten trash fish in water may deteriorate the water quality, leading to environmental problems such as eutrophication (Kim et al., 2007; Xu et al., 2007). Based on the requirements of stable feed quality and safety monitoring in food production processes, it is necessary to explore the expansion or even replacement of trash fish and other animal protein feed sources. Some important achievements are mainly based on the application of formulated feed (Feng et al., 2021). However, there is a lack of research on the possibility to feed cultured crabs directly with iced (fresh) insects instead of trash fish or other animal bait.
Black soldier fly (Hermetia illucens; BSF) is an ideal alternative protein source for animal feed due to its high nutritional value. BSF has a short life cycle, high yield, and sufficient supply. BSF larvae (BSFL) contain 55% crude protein (dry weight), 35% fat (dry weight), and a balanced amino acid structure, which can meet the nutritional requirements of animals (Magalhães et al., 2017). They do not concentrate pesticides or mycotoxins, and the risk of transmitting zoonotic diseases is low (Čičková et al., 2015; Wang and Shelomi, 2017; Chia et al., 2019; Proc et al., 2020). All of these advantages make BSF a practical, sustainable source of animal feed. BSFL has been used to feed economic animals such as poultry and predatory fish as a partial alternative to corn- or soy-based feeds (Kroeckel et al., 2012). In an experiment of broiler quails (Coturnix japonica), the addition of BSFL to the diet improved the amino acid content of quail meat and the nutritional value without affecting the production performance and yield (Cullere et al., 2016; 2018). Partial or total replacement of soybean cake with BSFL has similar effects and has no effect on the health or performance of broiler chickens and laying hens (Maurer et al., 2016; Schiavone et al., 2017). Partial or complete replacement of the fish diet by BSF pupa has no significant effect on the growth and vision of rainbow trout (Oncorhynchus mykiss) and has no adverse effect on trout fillet (Sealey et al., 2011; Renna et al., 2017). Similarly, feeding BSFL or prepupae has no adverse effect on the growth and health of zebrafish (Danio rerio) (Lanes et al., 2021). There are few reports on the study of BSF as crustacean feed. The replacement of 50% fish meal in the diet by BSF for the breeding of Scylla paramamosain shows that it is beneficial to the survival rate, growth rate, and aquaculture water environment. Moreover, when the BSF completely replaces the fish meal, it will not affect the growth of crabs, and the ammonia nitrogen content in the water (Huang et al., 2021). Feeding BSFL can meet the nutritional requirements of the sub-adult growth of Portunus trituberculatus, and will not have negative effects on its growth (Zhou et al., 2021). The results showed that the BSFL, as a food, could not affect the growth and development of crabs. However, to the best of the authors’ knowledge, no studies have evaluated the BSFL to replace iced trash fish for the purpose of improving crab flavor characteristics.
The Chinese mitten crab has a strong umami taste and high sweetness, which is a unique flavor pursued by consumers (Tao et al., 2018; Wang et al., 2020; Yang et al., 2021). The umami taste and sweetness of edible tissues are largely due to delicious amino acids and nucleotides, such as glutamic acid (umami), glycine (sweet), arginine (bitter/sweet), and adenosine monophosphate (AMP). Many types of free amino acids have synergistic effects with nucleotides to produce a strong umami taste (Kong et al., 2012). Studies have shown that fish-source protein intake affects the amino acid composition of crabs (Zeng et al., 2021). In the present study, industrially cultured BSFL was used to replace iced trash fish and other animal bait in the diet of Chinese mitten crab during the fattening period, and the effects on the flavor molecule contents and evaluation indices were investigated. It is important for aquaculture sustainability to replace animal protein with alternative protein sources such as BSFL.
Chinese mitten crabs were bred in Diaoyu Town, Xinghua, Jiangsu Province. The pond sizes were 2.0–3.0 hectares, and the stocking density was 13000–15000 crabs per hectare. The crabs were raised with traditional diets before the fattening period. During the fattening period (beginning on 25 August), when the crabs had just finished their last molt, the initial mass of the crabs was 125 ± 10 g for females and 150 ± 10 g for males. The iced trash fish has completely replaced with BSF last instar larvae (prepupa) in the BSF group, but not in the control group (CK). Plant-based feed such as corn and sweet potato and formulated feed were not changed.
On 25 October, three female or male Chinese mitten crabs (female: 150 ± 19 g, male: 200 ± 22 g) were collected from a single pond as one sample. A total of nine female or male Chinese mitten crabs were collected from three adjacent ponds as three samples. The water quality and feeding management of the three ponds were consistent. The water grass coverage rate in the pond was 50%-60%, and the water transparency was 30-50 cm. Crabs were reared at 26 ± 2 °C, pH 7.5-8.5, dissolved oxygen ≥ 5mg/L, ammonia nitrogen content ≤ 0.2mg/L. The crab samples were quickly transported to the laboratory at a normal temperature in a water-free environment, and the abdominal muscle (body meat), hepatopancreas, and gonad tissues of mitten crabs were isolated. Edible tissues of three crabs of the same sex were mixed as one sample, and three samples were collected for both sexes. The samples were frozen in liquid nitrogen and stored at −80°C before amino acid and nucleotide analysis.
Free amino acids were extracted from edible tissues according to the method of Konosu et al. (1974). Tissue samples (1.00 g) were mashed with liquid nitrogen and then 2.00 mL of 3% trichloroacetic acid (v/v) was added, followed by centrifugation at 12000 g for 15 min at 4°C. The supernatant was filtered with a 0.22-µm filter membrane. The free amino acids were then separated by an Agilent 1100 HPLC (Thermo Fisher Scientific, MA, USA) (Chen and Zhang, 2007). The column was an ODS HYPERSIL (250 mm × 4.6 mm, 5 μm), at a temperature of 25°C. Mobile phase A was prepared by mixing 1000 mL of 0.65% (w/v) sodium acetate aqueous solution (pH 7.2) with 200 μL triethylamine and 5 mL tetrahydrofuran. Mobile phase B was prepared by mixing 800 mL acetonitrile and 800 mL methanol with 400 mL of 1.625% (w/v) sodium acetate aqueous solution (pH 7.2). Settings were as follows: elution time, 0-36 min; flow rate, 1.0–1.5 mL/min; detection wavelengths, 338 nm and 262 nm (hydroxyproline). The quantities of different amino acids were determined by comparing retention times and peak areas with those of amino acid standards.
The nucleotides were extracted from edible tissues according to the method of Kong et al. (2012), with some modifications. Briefly, 2.00 g tissue was mashed with liquid nitrogen and then 25.0 mL of 0.1% (v/v) trichloroacetic acid solution was added, followed by mixing for 30 min and centrifugation at 12000 g for 5 min. The supernatant was taken and sterile water was added to 50 mL. The sample solution was filtered with a 0.45-μm filter membrane. An LC20AD HPLC system (Shimadzu, Japan) equipped with a C18 column (4.6 mm × 250 mm, 5 μm) (Thermo Fisher Scientific, MA, USA) was used for HPLC analysis at a temperature of 40°C. Mobile phase A was prepared by mixing 960 mL of 0.01 M potassium dihydrogen phosphate with 40 mL of 0.1 M dipotassium hydrogen phosphate. Mobile phase B was pure methanol. The flow rate was 0.6 mL/min and the detection wavelength was 254 nm.
Taste activity value: Taste activity value (TAV) analysis was conducted according to the method of Scharbert and Hofmann (2005). The TAV was calculated as the ratio of the concentration of amino acids or nucleotides measured in edible tissues to a threshold value. Compounds with TAV greater than 1 were considered to contribute to food taste.
Sweetness: The sweetness value (SWT) of 10% sucrose aqueous solution at 20°C was taken as 1.0, and the relative SWT (sweetness coefficient) of other substances was obtained by comparison. The content of free amino acids with TAV greater than 1.0 contributing to the sweetness was used to calculate the relative SWT based on sucrose as follows: SWT = ΣAsSt. As represents the content of sweet free amino acids in tissues (g/100 g) and St is the sweetness coefficient. The sweetness coefficients of alanine, glycine, and arginine were set as 1.2, 0.8, and 1.7, respectively (Kong et al., 2017).
Equivalent umami concentration: The intensity of the umami taste due to the synergistic effects of amino acids and nucleotides was compared with that produced by monosodium glutamate (MSG; g MSG/100 g) (Yamaguchi et al., 1971). The equivalent umami concentration (EUC) was calculated as follows:
ai is the concentration of umami amino acid (Asp/Glu, g/100 g), bi is the ratio of the umami taste of an amino acid to that of MSG (Glu, 1; Asp, 0.077), aj is the concentration of umami 5’-nucleotide (inosine monophosphate [IMP], guanosine monophosphate [GMP], or AMP, g/100 g), bj is the ratio of the umami taste of a 5′-nucleotide to that of IMP (IMP, 1; GMP, 2.3; AMP, 0.18), and 1218 is a synergistic constant based on the concentration of g/100 g used.
GraphPad Prism version 8 (GraphPad, San Diego, CA, USA) was used for statistical computations and graph construction. Data are presented as the mean ± SD. Significance analysis was performed using t-tests and P< 0.05 was considered as significant.
Iced trash fish in the feed of mitten crabs was completely replaced with BSFL during the fattening period. Two months later, the free amino acid composition in the edible tissues of crabs was investigated. The results showed that 21 amino acids, including eight essential amino acids (EAAs), were detected in the muscle, hepatopancreas, and gonads of mitten crabs (Table 1). The amino acid content in the edible tissues of mitten crabs showed significant tissue and sex differences after BSFL feeding. In male mitten crabs, the total free amino acid (TAA) of muscle was highest, followed by the hepatopancreas and gonads. BSFL feeding significantly increased the TAA levels in male gonads (Figure 1A). In female mitten crabs, the TAA levels were highest in muscle and hepatopancreas, and the lowest in gonads. BSFL feeding decreased the TAA levels in the female hepatopancreas (Figure 1B). The EAA levels were the highest in the hepatopancreas of both sexes. BSFL feeding greatly affected the EAA levels of edible tissues of male mitten crabs, and the EAA levels in the hepatopancreas and gonads were significantly increased (Table 1). Further analysis of delicious amino acids (DAAs) in edible tissues showed that the muscle had the highest DAA levels in both sexes. BSFL feeding increased DAA levels in the muscle and gonads of male mitten crabs, but significantly decreased DAA levels in the hepatopancreas of female mitten crabs (Figures 1C, D). These results indicate that the complete replacement of iced trash fish by BSFL not only affected the TAA and EAA contents of edible tissues but also changed the flavor characteristics.

Table 1 Effects of BSFL feeding on amino acid concentrations in edible tissues of mitten crabs (mg/g wet weight).
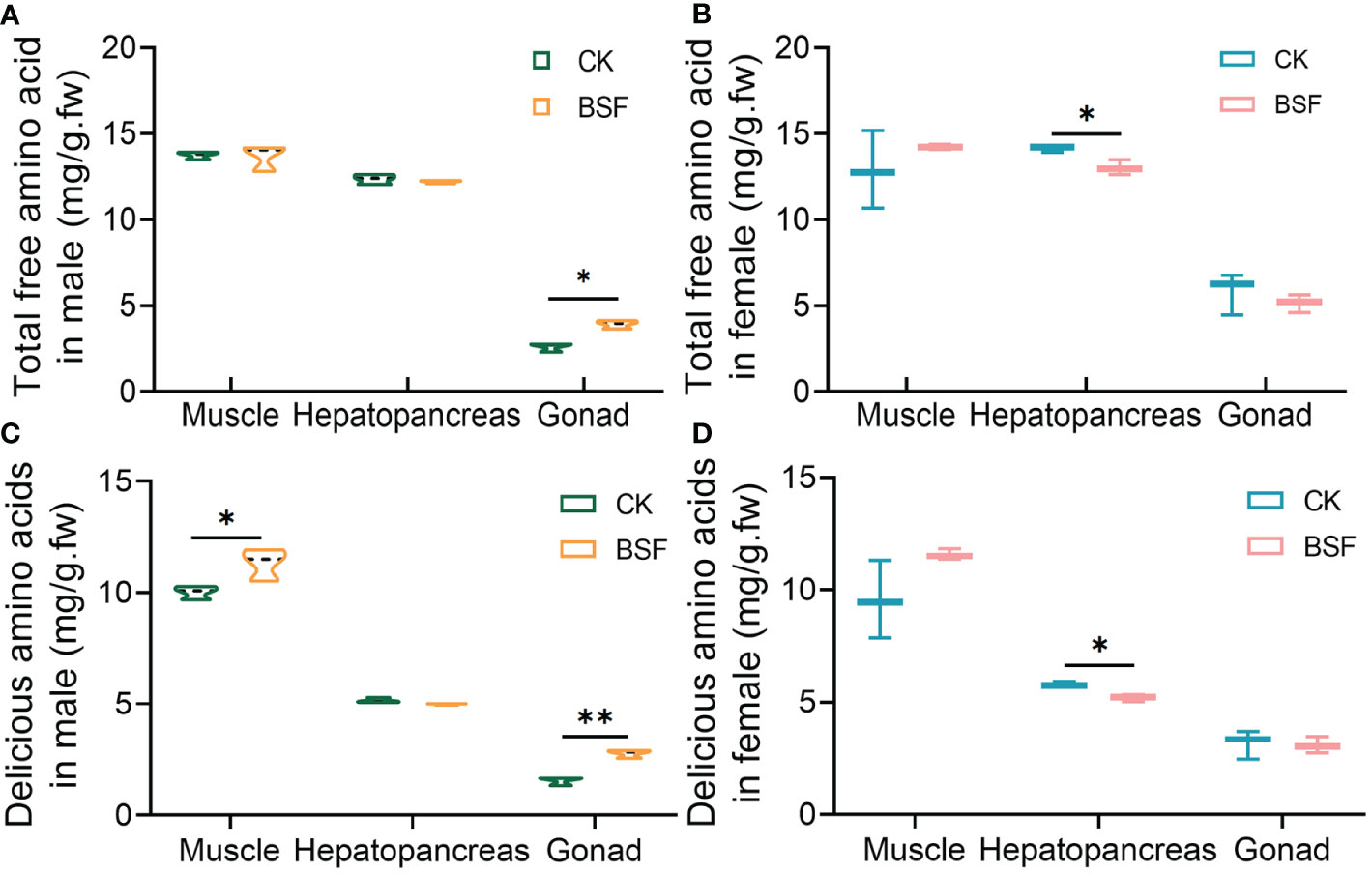
Figure 1 Effects of BSFL feeding on total free amino acid and delicious amino acid levels in edible tissues of mitten crabs. Total amino acid contents (A, B) and delicious amino acid contents (C, D) in edible tissues of male and female crabs. CK, control group; BSF, experimental group. *P ≤ 0.05; **P ≤ 0.01. n = 3.
According to the taste of amino acids, the contents of DAAs and bitter amino acids in edible tissues of mitten crabs after BSFL feeding were analyzed. The results showed that the contents of sweet amino acids Gly, Arg, and Ala in the muscle tissue of mitten crabs were higher, and the contents of Gly and Arg were significantly increased in the BSF group (Figures 2A, B). Conversely, the contents of most DAAs in the hepatopancreas were slightly decreased in the BSF group, and only the content of Arg was increased (Figures 2C, D). The contents of umami amino acids (Asp and Glu) and sweet amino acids (Ala) in male gonads of the BSF group were significantly increased (Figure 2E). The DAA levels of the female gonads were not affected (Figure 2F).

Figure 2 Effects of BSFL feeding on delicious amino acid levels in edible tissues of mitten crabs. Levels of amino acid with a sweet/umami taste in male and female muscles (A, B), hepatopancreas (C, D), and gonads (E, F). CK, control group; BSF, experimental group. H, hepatopancreas; Asp, aspartic acid; Glu, glutamic acid; Ser, serine; Gly, glycine; Thr, threonine; Arg, arginine; Ala, alanine. *P ≤ 0.05; **P ≤ 0.01. n = 3.
The changes in bitter amino acid content in edible tissues of mitten crabs after BSFL feeding were further analyzed. As shown in Figure 3, BSFL feeding during the fattening period mainly affected bitter amino acid levels in male crabs, but had little effect in female crabs. In the BSF group, the levels of seven bitter amino acids, such as Val, Met, Leu, and Lys, were significantly decreased in the male muscles (Figure 3A). Unexpectedly, the levels of most bitter amino acids in the male hepatopancreas and gonads, such as Leu and Lys, were significantly increased in the BSF group (Figures 3C, E). The hepatopancreas of mitten crabs contains the highest content of bitter amino acids (Figure 3).
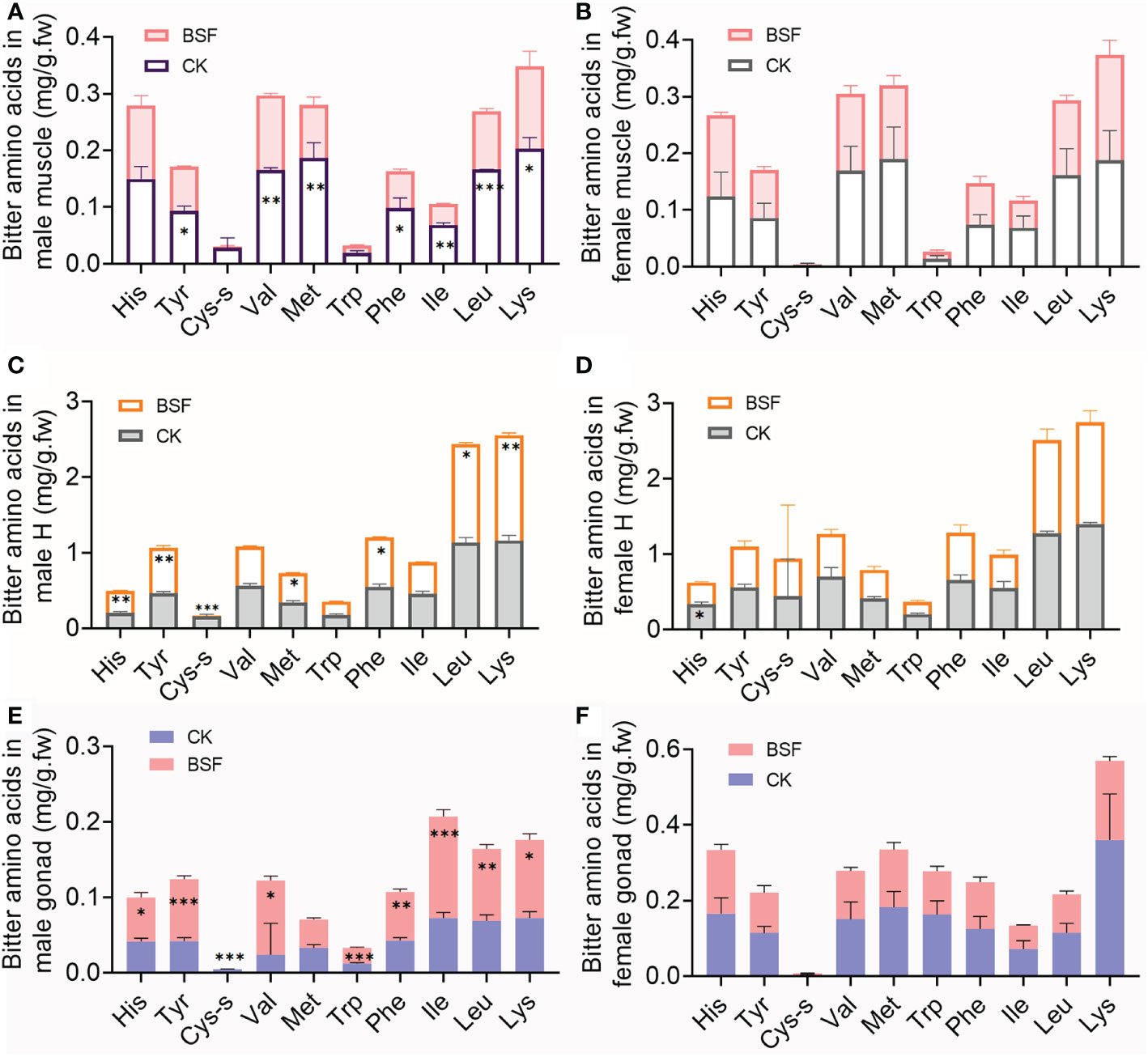
Figure 3 Effects of BSFL feeding on bitter amino acid levels in edible tissues of mitten crabs. Bitter amino acid levels in male and female muscles (A, B), hepatopancreas (C, D), and gonads (E, F). CK, control group; BSF, experimental group. H, hepatopancreas; His, histidine; Tyr, tyrosine; Cys-s, cysteine-s; Val, valine; Met, methionine; Trp, tryptophan; Phe, phenylalanine; Ile, isoleucine; Leu, leucine; Lys, lysine. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. n = 3.
These results indicate that BSFL feeding significantly increased the levels of sweet amino acids and decreased the levels of bitter amino acids in muscles of mitten crabs, increased the umami amino acids in male gonads, and did not significantly change the DAA levels, but increased the levels of bitter amino acids in the hepatopancreas.
Next, the levels of umami nucleotides, including AMP, IMP, and GMP in edible tissues of mitten crabs were analyzed (Figure 4). In muscle and gonads, only AMP and IMP were detected. AMP and GMP were detected in the hepatopancreas. The levels of other nucleotides were so low that they were undetectable. The results showed that IMP levels were significantly increased in the muscle tissue of mitten crabs in the BSF group (Figures 4A, B). There was no difference in the nucleotide content of the hepatopancreas (Figures 4C, D). It is worth noting that although there was no significant change in AMP levels in the gonads, IMP levels in the gonads of female crabs were significantly increased (Figures 4E, F).
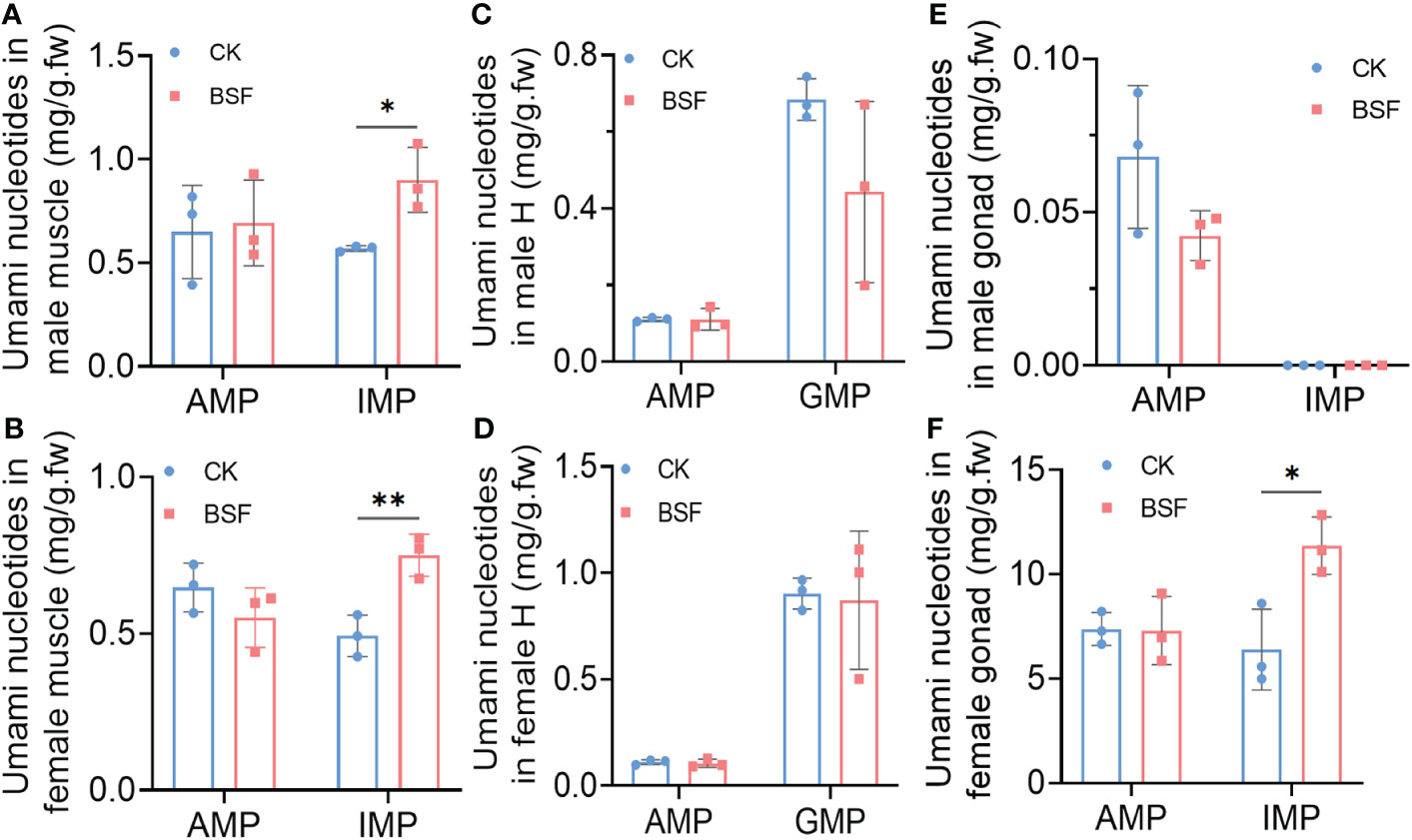
Figure 4 Effects of BSFL feeding on umami nucleotide levels in edible tissues of mitten crabs. Umami nucleotide levels in male and female muscles (A, B)), hepatopancreas (C, D), and gonads (E, F). CK, control group; BSF, experimental group. H, hepatopancreas; AMP, adenosine monophosphate; IMP, inosine monophosphate; GMP, guanosine monophosphate. *P ≤ 0.05; **P ≤ 0.01. n = 3.
To comprehensively evaluate the taste characteristics of edible tissues of Chinese mitten crab fed BSFL, the TAVs of amino acids and nucleotides were analyzed. TAV > 1 was considered to indicate a contribution to the flavor characteristics of tissues. The results showed that Glu was the main amino acid contributing to the umami taste of muscle, hepatopancreas, and gonadal tissues. Ala and Arg play important roles in the sweetness of muscle and the hepatopancreas, Gly only increases muscle sweetness, and Arg mainly contributes to female gonad sweetness. His, Lys, Val, and Met contribute to the bitterness of the hepatopancreas (Table 2). Compared with CK, the TAV of Ala in male gonads in the BSF group was greater than 1.
Nucleotide TAV analysis showed that the TAVs of IMP and AMP were the highest in the female gonads, i.e., more than 10 times higher than those in muscle. However, the TAV of GMP was greater than 1.0 only in the hepatopancreas. The TAVs of IMP in muscle and female gonads were significantly increased in the BSF group (Figure 5A). Next, the intensity of the umami taste produced by the synergistic effect of amino acids and nucleotides was analyzed. According to the EUC, the female gonads had the strongest umami taste, followed by the hepatopancreas, and the male gonads had the weakest umami taste. BSFL feeding during the fattening period has a great influence on the umami taste of edible tissues of mitten crabs, and the EUC of the female gonads was significantly increased in the BSF group (Figure 5B). Unexpectedly, the EUC of the male hepatopancreas in the BSF group was significantly reduced, and the EUCs of muscle and male gonads were not significantly affected. These results indicate that BSFL feeding could significantly improve the umami taste of female gonads.

Figure 5 Effects of BSFL feeding on umami evaluation indices in edible tissues of mitten crabs. (A) TAVs of nucleotides. White boxes indicate that TAV< 1.0. (B) EUC heatmap showing the effects of various umami amino acids and nucleotides on umami flavor. CK, control group; BSF, experimental group; ♂, female crabs; ♀, male crabs. n = 3.
Furthermore, SWT values were calculated to quantify the sweetness produced by various sweet amino acids in different tissues. As shown in Figure 6, SWT was the highest in muscle and the lowest in male gonads in both the CK and BSF groups. After BSFL feeding, SWT in the muscle of crabs of both sexes was significantly increased, while SWT in other tissues did not change significantly (Figure 6). These results indicate that BSFL feeding could significantly increase the sweetness of muscle.

Figure 6 Effects of BSFL feeding on sweetness evaluation indices in edible tissues of mitten crabs. SWT heatmap indicating the effects of various sweet amino acid contents on sweetness. CK, control group; BSF, experimental group; ♂, female crabs; ♀, male crabs. n = 3.
Chinese mitten crabs are favored by consumers in East and Southeast Asia, including China, South Korea, and Japan, due to their unique delicious flavor (Wang et al., 2016; Wang et al., 2020). At present, with the increasing scale of mitten crab breeding, the demand for trash fish for use as traditional bait is increasing, causing problems such as fishery depletion, low breeding safety, and water pollution, which limits the further development of crab breeding (Kim et al., 2007; Xu et al., 2007; Bunlipatanon et al., 2014). BSF has been widely tested and applied as bait in animal husbandry and aquatic animal cultures (Kroeckel et al., 2012). However, studies on the effects of BSF on the flavor characteristics of aquatic animals, including mitten crabs, are limited. In this study, BSFL from industrial culture was used to replace the iced trash fish in Chinese mitten crab feed during the fattening period. The results showed that BSFL feeding significantly enhanced the flavor characteristics of crabs based on the contents of flavoring amino acids and nucleotides.
The nutrients of the BSFL are not inferior to those of fish meals. On the contrary, they contain some special ingredients, such as microelements and chitin (Lu et al., 2022). Chitin is thought to improve the immune function of animals and is more conducive to animal growth (Swiatkiewicz et al., 2015). In addition, BSFL contains rich amino acids and is considered to be a more sustainable protein source than traditional soybean meal or fish meal (Crosbie et al., 2020). The most abundant essential amino acids in BSFL are leucine, lysine, and valine. The content of these three amino acids is higher than that of soybean meal, and even the content of valine is higher than that of fish meal (Müller et al., 2017; Lu et al., 2022). Surprisingly, the content of these three amino acids did not increase when the mitten crabs were fed BSFL (Table 1). The contents of DAA in BSFL were not different from those of fish meal (Muller et al., 2017). However, after feeding BSFL, the DDA content of male mitten crabs was significantly increased in muscles and gonads (Figure 1), and the major contributions were Arg, Asp, Glu, and Ala (Figure 2). The content of DDA varies significantly with different tissues and genders, which may be because the amino acid absorption of mitten crabs is gender- and tissue-specific in order to meet the amino acid requirements of various tissues. The nucleotide content in BSFL was not determined. We speculate that similar to amino acids, mitten crabs also have a selective absorption of nucleotides. Our study shows that feeding BSFL does not lead to a significant increase in amino acid and nucleotide content of mitten crabs instead of the iced trash fish, but rather the tissue-specific absorption of required amino acids or nucleotides. Therefore, feeding the BSFL did not affect the growth and development of mitten crabs, but affected the taste characteristics of the edible tissues.
Farming practice, including animal protein sources in feed, affects the composition of amino acids and nucleotides of aquatic animals (Cheng et al., 2021; Zeng et al., 2021). Recent studies have shown that the replacement of traditional feed with formulated feed alters amino acid metabolism and the relative abundance of gut microbiota in mitten crabs (Feng et al., 2021). Compared with the traditional diet, the mixed diet could improve the amino acid balance in the edible tissues of juvenile crabs (Han et al., 2021). The palatability of the Litopenaeus vannamei diet was significantly improved by adding the BSFL protein hydrolysate, which may be due to the high content of free amino acids and water solubility of BSFL protein hydrolysate (Terrey et al., 2021). In the present study, Glu was the only umami amino acid with TAV greater than 1.0 in edible tissues of mitten crabs after feeding the BSFL, and there was no difference with the CK group (Table 2). After feeding the BSFL, the content of umami nucleotides in the gonads of female mitten crabs was significantly increased. (Figure 4), and the TAVs of AMP, IMP, and GMP were greater than 1.0 in at least one edible tissue (Figure 5). We speculated that single amino acid or nucleotide contributed little to the formation of umami flavor in the edible tissues of Chinese mitten crab. EUC analysis showed that the umami taste of edible mitten crab tissues was strongest in the female gonads and weakest in the male gonads. This is consistent with previously reported tasting analysis results (Xie et al., 2021). Our results indicate that the synergistic effect of amino acids and nucleotides was the main contributor to the umami flavor of mitten crab. BSFL feeding during the fattening period significantly increased the EUC of female gonads of Chinese mitten crab.
The effect of feeding BSFL on the sweetness or bitter taste of animal tissue has not been reported. The sweet amino acids Arg, Ala, Gly, Pro, and Thr have effects on the sweetness characteristics of edible tissues of Chinese mitten crab (Kong et al., 2012; Zhuang et al., 2016). SWT was calculated to quantify the effects of sweet amino acids on sweetness. The results showed that the sweetness of the edible tissues was the highest in muscle and the lowest in male gonads. After BSFL feeding, SWT in muscle increased significantly, while SWT in male gonads decreased slightly (Figure 6). The hepatopancreas of mitten crabs is the most bitter, and the bitterness of muscles and gonads is almost impossible to taste (Zhang et al., 2022). After feeding the BSFL, the TAVs of bitter amino acids in the muscle and gonads of the mitten crab were less than 1.0, while the TAVs of bitter amino acids His, Lys, Val, and Met in the hepatopancreas were significantly higher than 1.0, which had no difference with CK group (Figure 3). These results indicated that BSFL feeding did not affect the levels of bitter amino acids in the hepatopancreas.
Taken together, the replacement of iced trash fish by BSFL significantly enhanced the flavor characteristics of Chinese mitten crab based on the contents of flavoring amino acids and nucleotides. The umami taste of female gonads and the sweetness of male and female muscles were significantly improved.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
J-FQ: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Writing original draft, Writing review and editing, Funding acquisition. CL: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Writing original draft. L-HR: Conceptualization, Methodology. WL: Conceptualization, Investigation. T-MD: Investigation, Funding acquisition. GW: Investigation, Data curation. X-NS: Investigation, Data curation. K-CC: Investigation, Data curation. Y-HS: Data curation, Writing review and editing. S-QX: Conceptualization, Writing original draft, Writing review and editing, Funding acquisition. All authors contributed to the article and approved the submitted version.
This work was supported by Science and Technology Program of Suzhou, China (Grant No. SNG2021016 to S.-Q.X.), Science and Technology Program of Kunshan Yangcheng Lake Crab Industrial Research Institute (Grant No. 202104 to S.-Q.X.). China Postdoctoral Science Foundation (Grant No. 2021M692343 to J.-F.Q.), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD to S.-Q.X.), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (Grant No.KYCX22_3209 to T.-M.D.). We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bunlipatanon P., Songseechan N., Kongkeo H., Abery N. W., De Silva S. S. (2014). Comparative efficacy of trash fish versus compounded commercial feeds in cage aquaculture of Asian seabass (Lates calcarifer) (Bloch) and tiger grouper (Epinephelus fuscoguttatus) (Forsskål). Aquac. Res. 45 (3), 373–388. doi: 10.1111/j.1365-2109.2012.03234.x
Cheng X., Li M., Leng X., Wen H., Wu F., Yu L., et al. (2021). Creatine improves the flesh quality of pacific white shrimp (Litopenaeus vannamei) reared in freshwater. Food Chem. 354, 129498. doi: 10.1016/j.foodchem.2021.129498
Chen D. W., Zhang M. (2007). Non-volatile taste active compounds in the meat of Chinese mitten crab (Eriocheir sinensis). Food Chem. 104 (3), 1200–1205. doi: 10.1016/j.foodchem.2007.01.042
Chia S. Y., Tanga C. M., van Loon J. J. A., Dicke M. (2019). Insects for sustainable animal feed: Inclusive business models involving smallholder farmers. Curr. Opin. Environ. Sustain. 41, 23–30. doi: 10.1016/j.cosust.2019.09.003
Čičková H., Newton G. L., Lacy R. C., Kozánek M. (2015). The use of fly larvae for organic waste treatment. Waste Manage. 35, 68–80. doi: 10.1016/j.wasman.2014.09.026
Crosbie M., Zhu C., Shoveller A. K., Huber L. A. (2020). Standardized ileal digestible amino acids and net energy contents in full fat and defatted black soldier fly larvae meals (Hermetia illucens) fed to growing pigs. Transl. Anim. Sci. 4 (3), txaa104. doi: 10.1093/tas/txaa104
Cullere M., Tasoniero G., Giaccone V., Miotti-Scapin R., Claeys E., De Smet S., et al (2016). Black soldier fly as dietary protein source for broiler quails: apparent digestibility, excreta microbial load, feed choice, performance, carcass and meat traits. Animal. 10 (12), 1923–1930. doi: 10.1017/S1751731116001270
Cullere M., Tasoniero G., Giaccone V., Acuti G., Marangon A., Dalle Zotte A. (2018). Black soldier fly as dietary protein source for broiler quails: Meat proximate composition, fatty acid and amino acid profile, oxidative status and sensory traits. Animal. 12 (3), 640–647. doi: 10.1017/S1751731117001860
Feng W., Feng W., Ge J., Li J., Su S., Jia R., et al. (2021). Alterations of amino acid metabolism and intestinal microbiota in Chinese mitten crab (Eriocheir sinensis) fed on formulated diet and iced trash fish. Comp. Biochem. Physiol. Part. D Genomics Proteomics. 40, 100924. doi: 10.1016/j.cbd.2021.100924
Han W., Sun Y., Liu J., Zhang Y., Lu Z., Cheng Y. (2021). Effect of different feeding modes on the growth, biochemical composition, and living environment of the juvenile Chinese mitten crab eriocheir sinensis. Aquaculture. 541, 736687. doi: 10.1016/j.aquaculture.2021.736687
Huang W. Q., Wang Y. G., Zhang Y., Wu X. Z., Li B. F., Shi L., et al. (2021). Effect of substitution of fish meal with black soldier fly larvae meal on survival,growth,aquaculture environment of Scylla paramamosain. Feed Res. 44 (16), 48–51. doi: 10.13557/j.cnki.issn1002-2813.2021.16.011
Kim J. H., Gomez D. K., Choresca C. H., Park S. C. (2007). Detection of major bacterial and viral pathogens in trash fish used to feed cultured founder in Korea. Aquaculture 272, 105–110. doi: 10.1016/j.aquaculture.2007.09.008
Kong L., Cai C., Ye Y., Chen D., Wu P., Li E., et al. (2012). Comparison of non-volatile compounds and sensory characteristics of Chinese mitten crabs (Eriocheir sinensis) reared in lakes and ponds: Potential environmental factors. Aquaculture 364-365, 96–102. doi: 10.1016/j.aquaculture.2012.08.008
Kong Y., Zhang L. L., Sun Y., Zhang Y. Y., Sun B. G., Chen H. T. (2017). Determination of the free amino acid, organic acid, and nucleotide in commercial vinegars. J. Food Sci. 82 (5), 1116–1123. doi: 10.1111/1750-3841.13696
Konosu S., Watanabe K., Shimizu T. (1974). Distribution of nitrogenous constituents in the muscle extracts of eight species of fish. Nippon Suisan Gakkaishi 40, 909–914. doi: 10.2331/suisan.40.909
Kroeckel S., Harjes A. G. E., Roth I., Katz H., Wuertz S., Susenbeth, et al. (2012). When a turbot catches a fly: Evaluation of a pre-pupae meal of the black soldier fly (Hermetia illucens) as fish meal substitute-growth performance and chitin degradation in juvenile turbot (Psetta maxima). A. Aquaculture. 364, 345–352. doi: 10.1016/j.aquaculture.2012.08.041
Lanes C. F. C., Pedron F. A., Bergamin G. T., Bitencourt A. L., Dorneles B. E. R., Villanova J. C. V., et al. (2021). Black soldier fly (Hermetia illucens) larvae and prepupae defatted meals in diets for zebrafish (Danio rerio). Anim. (Basel). 11 (3), 720. doi: 10.3390/ani11030720
Lu S., Taethaisong N., Meethip W., Surakhunthod J., Sinpru B., Sroichak T., et al. (2022). Nutritional composition of black soldier fly larvae (Hermetia illucens l.) and its potential uses as alternative protein sources in animal diets: A review. Insects. 13 (9), 831. doi: 10.3390/insects13090831
Magalhães R., Sánchez-López A., Leal R. S., Martínez-Llorens S., Oliva-Teles A., Peres H. (2017). Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture. 476, 79–85. doi: 10.1016/j.aquaculture.2017.04.021
Maurer V., Holinger M., Amsler Z., Früh B., Wohlfahrt J., Stamer A., et al. (2016). Replacement of soybean cake by hermetia illucens meal in diets for layers. J. Insects Food Feed. 2 (2), 83–90. doi: 10.3920/JIFF2015.0071
Müller A., Wolf D., Gutzeit H. O. (2017). The black soldier fly, hermetia illucens - a promising source for sustainable production of proteins, lipids and bioactive substances. Z. Naturforsch. C. J. Biosci. 72 (9-10), 351–363. doi: 10.1515/znc-2017-0030
Proc K., Bulak P., Wiącek D., Bieganowski A. (2020). Hermetia illucens exhibits bioaccumulative potential for 15 different elements - implications for feed and food production. Sci. Total Environ. 723, 138125. doi: 10.1016/j.scitotenv.2020.138125
Renna M., Schiavone A., Gai F., Dabbou S., Lussiana C., Malfatto V., et al. (2017). Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens l.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss walbaum) diets. J. Anim. Sci. Biotechnol. 8, 57. doi: 10.1186/s40104-017-0191-3
Scharbert S., Hofmann T. (2005). Molecular definition of black tea taste by means of quantitative studies, taste reconstitution, and omission experiments. J. Agric. Food Chem. 53 (13), 5377–5384. doi: 10.1021/jf050294d
Schiavone A., De Marco M., Martínez S., Dabbou S., Renna M., Madrid J., et al. (2017). Nutritional value of a partially defatted and a highly defatted black soldier fly larvae (Hermetia illucens l.) meal for broiler chickens: Apparent nutrient digestibility, apparent metabolizable energy and apparent ileal amino acid digestibility. J. Anim. Sci. Biotechnol. 8, 51. doi: 10.1186/s40104-017-0181-5
Sealey W. M., Gaylord T. G., Barrows F. T., Tomberlin J. K., McGuire M. A., Ross C., et al. (2011). Sensory analysis of rainbow trout, oncorhynchus mykiss, fed enriched black soldier fly prepupae, hermetia illucens. J. World Aquac. Soc 42 (1), 34–45. doi: 10.1111/j.1749-7345.2010.00441.x
Swiatkiewicz S., Swiatkiewicz M., Arczewska-Wlosek A., Jozefiak D. (2015). Chitosan and its oligosaccharide derivatives (chito-oligosaccharides) as feed supplements in poultry and swine nutrition. J. Anim. Physiol. Anim. Nutr. (Berl). 99 (1), 1–12. doi: 10.1111/jpn.12222
Tao H., Du B. B., Wang H., Dong H. L., Yu D. S., Ren L. H., et al. (2018). Intestinal microbiome affects the distinctive flavor of Chinese mitten crabs in commercial farms. Aquaculture. 483, 38–45. doi: 10.1016/j.aquaculture.2017.09.031
Terrey D., James J., Tankovski I., Dalim M., van Spankeren M., Chakraborty A., et al. (2021). Palatability enhancement potential of hermetia illucens larvae protein hydrolysate in litopenaeus vannamei diets. Molecules. 26 (6), 1582. doi: 10.3390/molecules26061582
Wang S., He Y., Wang Y., Tao N., Wu X., Wang X., et al. (2016). Comparison of flavour qualities of three sourced eriocheir sinensis. Food Chem. 200, 24–31. doi: 10.1016/j.foodchem.2015.12.093
Wang Y. S., Shelomi M. (2017). Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods. 6 (10), 91. doi: 10.3390/foods6100091
Wang M., Tang Y., Yu J., Su S., Li J., Yu F., et al. (2020). Molecular insights into the sex-differential regulation of signal transduction in the cerebral ganglion and metabolism in the hepatopancreas of eriocheir sinensis during reproduction. Genomics. 112 (1), 71–81. doi: 10.1016/j.ygeno.2019.10.014
Wu H. R., Ge M. T., Chen H. F., Jiang S. T., Lin L., Lu J. F. (2020). Comparison between the nutritional qualities of wild-caught and rice-field male Chinese mitten crabs (Eriocheir sinensis). LWT-Food Sci. Technol. 117, 108663. doi: 10.1016/j.lwt.2019.108663
Xie H., Yin M. Y., Zhang Y. F., Wang X. C. (2021). The effect of different culturing region on the sensory and taste quality of Chinese mitten crab (Eriocheir sinensis). Food Fermentation Industries. 47, 114–120. doi: 10.13995/j.cnki.11-1802/ts.025575
Xu Z., Lin X., Qin L., Yang Y., Wang Y. (2007). Nitrogen, phosphorus, and energy waste outputs of four marine cage-cultured fish fed with trash fish. Aquaculture 263, 130–141. doi: 10.1016/j.aquaculture.2006.10.020
Yamaguchi S., Yoshikawa T., Ikeda S., Ninomiya T. (1971). Measurement of the relative taste intensity of some l-α-amino acids and 5'-nucleotides. J. Food Sci. 36 (6), 846–849. doi: 10.1111/j.1365-2621.1971.tb15541.x
Yang F., Guo H., Gao P., Yu D., Xu Y., Jiang Q., et al. (2021). Comparison of methodological proposal in sensory evaluation for Chinese mitten crab (Eriocheir sinensis) by data mining and sensory panel. Food Chem. 356, 129698. doi: 10.1016/j.foodchem.2021.129698
Zeng Q., Xu Y., Jeppesen E., Gu X., Mao Z., Chen H. (2021). Farming practices affect the amino acid profiles of the aquaculture Chinese mitten crab. Peer J. 9, e11605. doi: 10.7717/peerj.11605
Zhang L., Tao N. P., Wu X., Wang X. (2022). Metabolomics of the hepatopancreas in Chinese mitten crabs (Eriocheir sinensis). Food Res. Int. 152, 110914. doi: 10.1016/j.foodres.2021.110914
Zhou J., Huang Y. D., Zhou X., Yang J. Y., Qiao X. T., Cheng Z. Y. (2021). Effects of two kinds of diets and feeding frequency on growth, digestion and immune response of portunus trituberculatus. J. Tianjin Agric. University. 28 (04), 41–46. doi: 10.19640/j.cnki.jtau.2021.04.010
Keywords: Chinese mitten crab, animal diet, black soldier fly, umami, sweet
Citation: Qiu J-F, Luo C, Ren L-H, Li W, Dai T-M, Wang G, Sun X-N, Moua K-CC, Sima Y-H and Xu S-Q (2023) Black soldier fly larvae replace traditional iced trash fish diet to enhance the delicious flavor of Chinese mitten crab (Eriocheir sinensis). Front. Mar. Sci. 9:1089421. doi: 10.3389/fmars.2022.1089421
Received: 04 November 2022; Accepted: 20 December 2022;
Published: 09 January 2023.
Edited by:
Samad Rahimnejad, University of South Bohemia in České Budějovice, CzechiaReviewed by:
Xiaohui Dong, Guangdong Ocean University, ChinaCopyright © 2023 Qiu, Luo, Ren, Li, Dai, Wang, Sun, Moua, Sima and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi-Qing Xu, c3pzcXh1QHN1ZGEuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.