
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mar. Sci., 04 January 2023
Sec. Marine Biotechnology and Bioproducts
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.1075498
This article is part of the Research TopicGenomic Cell Preservation in Aquatic Animals: With Emphasis on CryopreservationView all 4 articles
 Michele Di Iorio1
Michele Di Iorio1 Giusy Rusco1
Giusy Rusco1 Stefano Esposito2
Stefano Esposito2 Mariasilvia D’Andrea1
Mariasilvia D’Andrea1 Alessandra Roncarati3
Alessandra Roncarati3 Nicolaia Iaffaldano1*
Nicolaia Iaffaldano1*The Mediterranean brown trout is one of the most endangered freshwater species. A complicated network of climate and human influences has severely harmed its biodiversity. The introduction of alien trout is one of the most serious threats to native populations’ intraspecific diversity. In Molise region (south-Italy) an important conservation program (LIFE Nat.Sal.Mo project) has recently been proposed to preserve the genetic integrity of native Mediterranean trout. This project, alongside safeguarding and re-establishing the habitats’ usefulness aims to restore the genetic integrity of the autochthonous population. This is one of the major goals, and it is accomplished by employing frozen wild breeder semen in conjunction with proper fertilization techniques to carry out artificial reproduction to enhance genetic diversity in the progeny and maintain fitness within self-sustaining populations. In this regard, the implementation of the first European semen cryobank has played a strategic role for conserving extant genomic diversity of native population. The goal of this review is to outline the procedures developed and guidelines established for the creation of a Mediterranean trout sperm cryobank. Here, we specifically provide an overview of some of the main challenges associated with the implementation of semen cryobank, the results achieved, the prospects for restoring genetic integrity in native populations, and lastly, future views for hatchery management to preserve the wild biodiversity of native salmonid species. During the project timeframe 1,683 semen doses, from 150 native breeders were stored inside the cryobank. Our results clearly showed the efficiency of the freezing procedure used, both in vitro and in vivo. In fact, we recorded satisfactory values of post-thaw sperm motility and viability that ranged from 40% to 80%, and excellent fertilization rate in vivo, which ranged from 64% to 81%.
The Mediterranean brown trout is a significant freshwater fish with economic value for fishery management and conservation biology. It is a widely distributed species found in Eurasia and North Africa, showing a significant morphological, ecological, and genetic variation among its populations (Larios-López et al., 2015). In the Italian distribution range, the Mediterranean trout is referred to as Salmo cettii syn. Salmo macrostigma (Querci et al., 2013; Lorenzoni et al., 2019; Splendiani et al., 2019; Carosi et al., 2020). However, according to recent genetic findings the Italian peninsular Mediterranean brown trout belongs to a different taxon known as S. ghigii, therefore limiting the name S. cettii to Sicilian trout (Lorenzoni et al., 2019; D’Agaro et al., 2022; Polgar et al., 2022). Although, we agree with this recent evidence, we still use the name S. cettii, because Mediterranean brown trout populations are still protected by the Habitat Directive and subsequent conservation status updates under this taxon.
Over the past decades, it has become one of the mostly threatened freshwater fish on the verge of extinction (Marzano et al., 2003; Lorenzoni et al., 2019; Rossi et al., 2019). In Italy, the populations of native Mediterranean brown trout are in constant and rapid decline and the loss of biodiversity appears to be troubling and unavoidable if appropriate recovery and safeguard action are not implemented.
The decrease of these native populations is the result of a series of anthropogenic activities, such as the river pollution, habitat deterioration, water withdrawals, largely unregulated fishing activities and the introduction of alien species (Iaffaldano et al., 2016a; Iaffaldano et al., 2016b; Lorenzoni et al., 2019; Carosi et al., 2020). In Italian freshwaters, including Biferno and Volturno rivers (Molise region), the introgressive hybridization with the alien trout species is the most important threat to the native species survival (Lorenzoni et al., 2019; Carosi et al., 2020). In such contest Mediterranean brown trout is currently classified in the Italian IUCN Red List as ‘critically endangered’ and its population is declining (www.iucn.it, accessed on: 28 July 2022). This situation has prompted the development of many conservation projects aiming at restoring the genetic integrity of Mediterranean trout populations in Italy (Sabatini et al., 2018; Lorenzoni et al., 2019; Splendiani et al., 2019; Rossi et al., 2022), including Molise region (Rusco et al., 2019; Rusco et al., 2020), where, in 2018 the “LIFE” Nat.Sal.Mo project was born.
Our project beyond protecting and re-establishing the functionality of habitats aims to ensure the native population genetic integrity. This is one of the main goals, and it is attained by using frozen wild breeder semen associated with appropriate fertilization schemes to carry out artificial reproduction to maximize genetic diversity in the progeny and maintain fitness within self-sustaining populations. In this regard, the implementation of the first European semen cryobank has played a strategic role in conserving extant genomic diversity of native population.
Cryopreserving gametes is an effective method for preserving the genetic profile of native populations with a particular genotype in threatened aquatic species. (Robles et al., 2003). Studies on fish germplasm cryobanking have been carried out on different cell types, mainly on sperm (Martínez-Páramo et al., 2017; Diwan et al., 2020), oocytes and embryos (Zhang et al., 2003; Diwan et al., 2020) and less on spermatogonia and primordial germ cells (Labbè et al., 2013; Comizzoli and Holt, 2014; Robles et al., 2017).
Studies done so far on the freezing of fish oocytes have shown that early-stage ovarian follicles, primarily stage I and stage II follicles, can yield some promising outcomes. (Martínez-Páramo et al., 2017). However, the efforts to cryopreserve fish embryos have frequently failed, therefore, cryopreservation of fish embryo remains elusive (Diwan et al., 2020). Accordingly, the sperm cells are the main type of cell used for cryobanking purposes in aquatic species (Martínez-Páramo et al., 2017). In fact, thanks to their small size and relatively high resistance to chilling, sperm cryopreservation is a more feasible method compared to the ones performed on other cell types.
The purpose of the present report is to describe the activities developed and the rules adopted for the realization of a sperm cryobank of autochthonous Mediterranean trout and its practical use in artificial fertilization schemes. In particular, here we give an overview of 1) the efforts incurred to obtain an effective semen freezing protocol, which has been essential for the realization of a semen cryobank, 2) the results achieved over four years using the frozen semen, 3) the opportunities provided in the restoration of genetic integrity in native populations and finally 4) the future perspectives supporting the hatcheries’ management in order to maintain the wild biodiversity of native salmonids species.
The main challenge for the implementation of a sperm cryobank is the development of a successful semen freezing protocol. The cryopreservation procedure results as highly stressful for sperm cells (Cabrita et al., 2010), in fact, the subcellular compartments of fish spermatozoa, including nucleus (head), cytosol, plasma membrane, midpiece and flagellum (Cabrita et al., 2010), are susceptible to cryodamage with consequent compromises of cell function and structure, DNA alteration (Gwo and Arnold, 1992; Zilli et al., 2003), loss of motility (Linhart et al., 2000), plasma membrane disruption (Lahnsteiner et al., 1996) and impaired fertilization ability (Nynca et al., 2014; (Bozkurt and Yavaş, 2021).
Under this regard, it should be remembered that the salmonids semen is among the most complicated to cryopreserve because of its distinctive traits (reduced adenosine triphosphate production, short duration of motility, high sensitivity to osmotic stress and large number of sperm cells needed to fertilize a single egg), which render them more susceptible to the freezing procedure (Martínez-Páramo et al., 2009; Bozkurt et al., 2019; Bozkurt et al., 2021). As a result, cell survival and its functionality entirely depend on the adopted cryopreservation protocol (Bozkurt and Yavaş, 2021).
Therefore, in order to identify a reference cryopreservation procedure, during the timeframe of the project, our research group has focused its efforts on the key factors involved in sperm cryosurvival, by designing several experiments on basic extenders, dilution rates, cryoprotectants (CPAs) and their concentration, freezing and thawing rates (Iaffaldano et al., 2016a; Di Iorio et al., 2019; Rusco et al., 2019; Rusco et al., 2020; Giametta et al., 2021).
These investigations helped us to identify an effective freezing protocol for in vitro processing of trout semen, to be adopted for the realization of a cryobank. Specifically, this protocol involved the use of a glucose–methanol extender and the dilution of semen to reach a standardized concentration before freezing (i. e. 3 × 109/mL) (Rusco et al., 2020). This freezing procedure was found to have a positive impact on post-thawing sperm total motility, with values exceeding 70%. These findings were noticeably superior to previous findings, which ranged between 33% and 52% (Iaffaldano et al., 2016a; Di Iorio et al., 2019; Rusco et al., 2019).
As reported in the project proposal, the native breeders (males and females) were caught during upstream spawning migration in specific capture stations, with fixed traps located on Biferno river (loc. Bojano; latitude 41°28’52.4”N, longitude 14°28’51.4”E) and a Volturno river tributary (loc. Rio Caprionero; latitude 41°31’18.2”N, longitude 14°08’27.1”E) in easily reachable sites to facilitate both installation and daily maintenance. The cages were installed at the beginning of each breeding season in December, and they were monitored from January to March every day, twice a day (morning and evening). The traps were used also as an unusual eradication system because due to the ineffectiveness of classic electrofishing eradication in the main waterways of the project area, non-native breeders were removed from the controlled spawning sites.
Moreover, in support of the fixed traps, electrofishing was also used to capture native specimens in other spawning spots.
The location of fixed traps and other sampling sites in Volturno and Biferno rivers are shown in Figure 1. We identified 3 sites in Biferno (1 fixed trap + 2 electrofishing sites) and 3 in Volturno (1 fixed trap + 2 electrofishing sites).
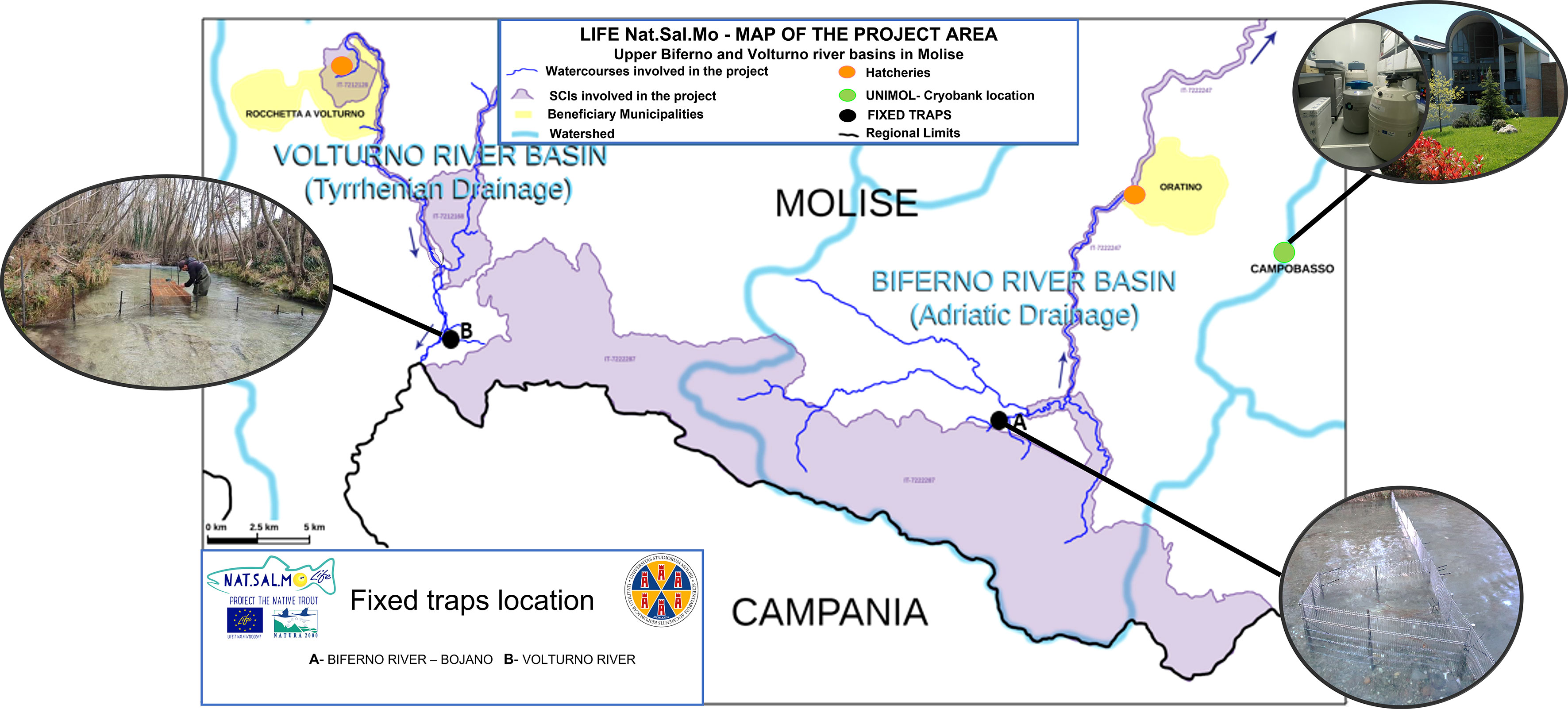
Figure 1 Location of the frozen gene bank (University of Molise), hatcheries (Rocchetta a Volturno and Oratino) and fixed traps (Biferno and Volturno rivers).
In order to select only native specimens, all sampled individuals were marked by passive transponders (PIT-TAG) and/or T-anchor tags, for subsequent recaptures and a portion of adipose fin tissue was taken from live animals that had previously been given a clove powder anesthetic. Fin tissue fragments preserved in ethanol allowed the isolation of individual genomic DNA, which was genotyped in accordance with Palombo et al. (2021). More in detail, since the beginning of the project, N=672 individuals were genotyped through the 57 K rainbow trout Axiom SNP array. The genetic ancestry was estimated using ADMIXTURE software v.1.3.0 with K = 2 population cluster, roughly corresponding to “native” and “alien” trout population inhabiting Molise rivers (Palombo et al., 2021; Salvatore et al., 2022). SNP’s analysis was combined withPCR-RFLP analysis of mitochondrial (16s rDNA; McMeel et al., 2001) and nuclear (LDH-C1*; Chiesa et al., 2016) markers, which provide an extensive genetic characterization of Mediterranean brown trout population in many studies (Penserini et al., 2006; Splendiani et al., 2006; Rossi et al., 2019; Lorenzoni et al., 2019; Salvatore et al., 2022), For gametes collection we prioritized tagged individuals genotyped by the combination of SNP array and PCR-RFLP analysis.
Semen was collected from each native male using the abdominal massage technique; drying urogenital papilla before stripping, special care was needed to prevent the contamination of semen with blood cells, urine, and river water. Semen samples were directly collected in 10 mL graduated tubes and stored in a cooler containing crushed ice, subsequently the samples were transferred to the laboratory within a variable interval time (from 30 minutes to 4 hours) depending on the river and the sampling site, in our previous research we found that the Mediterranean brown trout semen can be frozen even up to 6 h post-collection without losing its freezability and fertilizing ability (Rusco et al., 2021).
Eggs were gathered by stripping the females in a dry metal bowl, prior to collection, it was determined that each female had mature eggs by hand inspection of the abdomen. After collecting, the eggs were visually inspected to ensure that the ones used in the fertilization practice were well-rounded and transparent. The mature eggs were yellow to orange with a diameter ranging from 3 to 4 mm.
In the laboratory, firstly the sperm motility for each semen sample was checked by using a Computer-Assisted Sperm Analysis (CASA) system connected to a phase contrast microscope (Nikon model Ci-L) employing the Sperm Class Analyzer (SCA) software (VET Edition, Barcelona, Spain). Before analysis, spermatozoa were activated in a specific solution containing 1 mM CaCl2, 20 mM Tris, 30 mM glycine and 125 mM NaCl, at pH 9.0 and supplemented with 0.5% bovine albumin, using a dilution rate 1:300 or 1:30 for fresh or frozen semen, respectively (Judycka et al., 2018). An aliquot (0.7 µL) of this solution was immediately placed into a well (diameter 5 mm) of a 12-well multitest glass slide (TEKDON Inc., Florida, USA) and a coverslip was applied. The sperm motility parameters considered were: motile spermatozoa (MOT, (%)), curvilinear velocity (VCL, (μm/s)), straight-line velocity (VSL, (μm/s)), average path velocity (VAP, (μm/s)).
Only sperm samples with a total motility rate greater than 70% and containing at least 6 × 109 spermatozoa/mL were cryopreserved and stored in the cryobank (see Figure 2). Sperm volume from each donor was quantified using a calibrated micropipette. To determine the sperm concentration the photometric method was used, semen was diluted in 0.9% of NaCl at a 1:200 (v:v) ratio, and the optical density was measured at 530 nm using a portable photometer DR 1900 (HACH company, Loveland, CO, USA). The sperm concentration value was obtained using a standard curve that had been developed previously, by relating the optical density with the sperm concentration expressed as × 109 spermatozoa/mL, following the procedure described by Nynca and Ciereszko (2009).
Sperm viability in fresh and frozen semen was evaluated with flow cytometry (Muse© Cell Analyser; Luminex corporation, 12212 Technology Blvd Suite 130, Austin, TX 78727, United States) taking into consideration the manufacturer’s protocol. Firstly, semen was diluted in PBS to reach a concentration ranging from 1 × 105 to 1 × 107 spermatozoa/mL. Subsequently, 20 µL was added to 780 µL (dilution factor 1:40) of Muse Count & Viability Kit© in an Eppendorf tube and incubated for 5 min in the dark (at room temperature). Lastly, the sperm suspension was examined, and the results were displayed in two dot plots: (1) nucleated cells and (2) viability percentage.
The semen from native individual males deemed suitable for the cryopreservation process (Figure 2), was diluted with a specific freezing medium to reach a final extender concentration of 0.15 M glucose and 7.5% methanol. Diluted semen was stuffed into 0.25 mL plastic straws through the aid of a manual micro-aspirator (IMV-Technologies) obtaining a final sperm concentration of 3.0 × 109 sperm/mL, corresponding to 750 × 106 sperm/straw. The straws were then set on a 3 cm-high frame for the equilibration phase (15 min on ice). Following this phase, the straws were cryopreserved by exposure to liquid nitrogen vapor at 3 cm above its level for 5 min. They were then plunged into liquid nitrogen; the straws of each donor were rapidly collected and transferred into a goblet marked with donor ID, which were finally stored in the cryobank. Frozen semen was thawed at 40°C for 5 seconds, for in vitro analysis or for its use in artificial fertilization activities.
In order to ensure the correct management of semen cryobank an appropriate system of semen doses traceability was necessary. Thus, each straw was identified by means of a code bearing the donor information (serial number).
Simultaneously, to identify each straw a database including the following information was created:
- donor identification which coincides with the identification code of the genetic analysis;
- number of straws for each semen donor;
- straw colour;
- list of alpha-numeric code;
- date of semen collection and freezing;
- fresh semen quality of each donor (including volume, concentration, motility and viability);
- frozen/thawed semen quality (motility, viability);
- n° of straws used for in vitro analysis and in vivo fertilization;
- position in the cryogenic tank;
- sampling site (river and position of fixed trap);
- any other useful information.
This database is constantly updated to guarantee proper and successful cryobank maintenance. The liquid nitrogen tanks (dewars) were stored in an air-conditioned room with a controlled temperature of 5°C. In order to ensure an appropriate level of liquid nitrogen, the storage dewars were refilled with liquid nitrogen, at regular intervals of one week.
The main usefulness of our cryobank is represented by its practical application in artificial reproduction practices to maximize the genetic variability. Indeed, using frozen semen of native males in appropriate fertilization schemes allows an increase of the genetic variability in the offspring.
The activities were accomplished in the field (e.g., riverbank), briefly semen samples were thawed using a portable water bath, the post-thaw motility and viability of each donor were previously checked in our laboratory and that showed at least 50% of motile and viable spermatozoa were considered suitable.
The eggs from each native female were splitted into equal aliquots (3-5) containing around 1000 eggs and each of them were fertilized with different male according to the cross-fertilization matrix scheme (Figure 3). The sperm was introduced into eggs and gently mixed for 10 s, subsequently roughly 20 mL of river water was added. After 2 min, the eggs were rinsed with river water and moved to the hatcheries, the eggs from Volturno and Biferno river were incubated at two hatcheries close to the Rocchetta a Volturno and the Oratino municipalities, respectively.
The eggs from each specific cross-fertilization were placed in a frame with running water at about 9-10°C, until the eye stage (after ~25–30 days from fertilization) (Rusco et al., 2020; Rusco et al., 2021). Dead and unfertilized eggs were monitored and removed twice a week. The fertilization rate was calculated with the formula below:
In order to avoid the domestication of wild stock, the restocking of the suitable areas was carried out using eyed eggs with the “artificial nesting” method. The technique assured a more successful approach by avoiding the exposure of larvae and early life stages to an artificial environment.
Briefly, this technique consists in recreating an artificial nest in the river gravel, with the aid of a tube, to a depth of about 20-30 cm, subsequently the eyed eggs were deposited inside it and were covered with gravel and other natural material.
Independent t-test was used to investigate the effect of river populations (Biferno and Volturno) in regard of semen volume and sperm concentration.
Other sperm traits (total motility, VCL, VSL, VAP and viability) were measured across fresh or frozen semen and were compared by analysis of variance (ANOVA) followed by Scheffe’s comparison test. Lastly, a generalized linear model (GLM) was used to establish the fixed effects of treatment (implied as fresh or frozen semen) and river. The level of significance was set at p < 0.05 and all statistical analysis were carried out using the commercial software SPSS (SPSS 15.0 for Windows, 2006; SPSS, Chicago, IL, USA).
The consistency of cryobank is reported in Table 1, in total about 1,700 semen doses were obtained during three spawning seasons. Specifically, almost 1,000 straws from 85 native males from Volturno river and 700 doses from 65 native donors from Biferno river were stored in the cryobank.
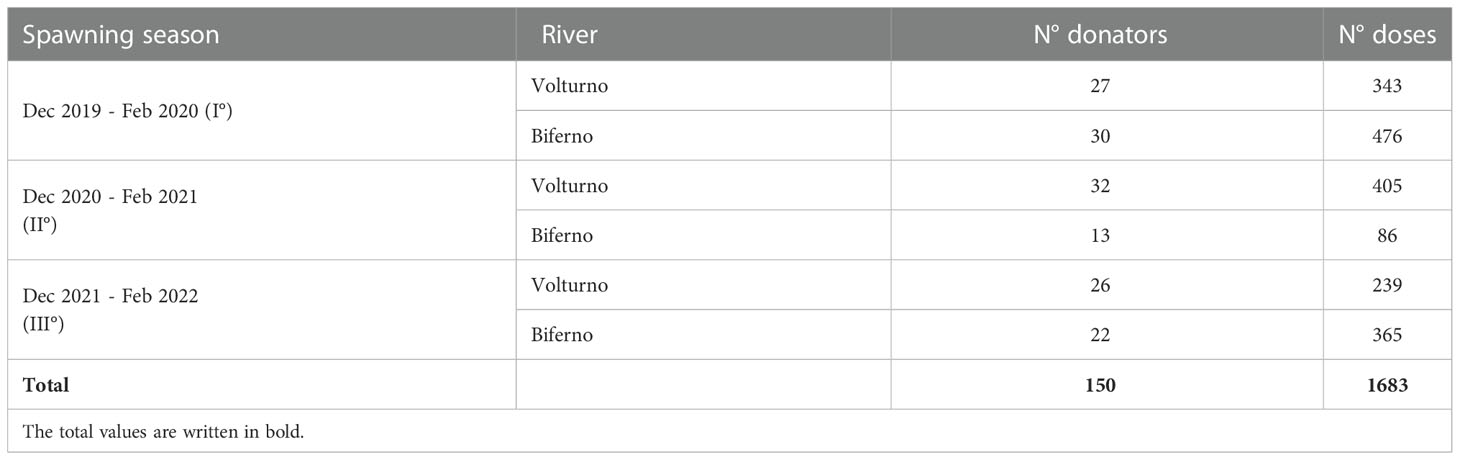
Table 1 Semen doses stored within the semen cryobank of Mediterranean brown trout during the project timeframe.
The results of artificial fertilization achieved with frozen semen are summarized in Table 2, the total number of fertilized and eyed eggs were quite similar in both watercourses in each spawning season. Fertilization rate ranged from 64 to 81% in the Volturno river and from 66 to 80% in the Biferno river.
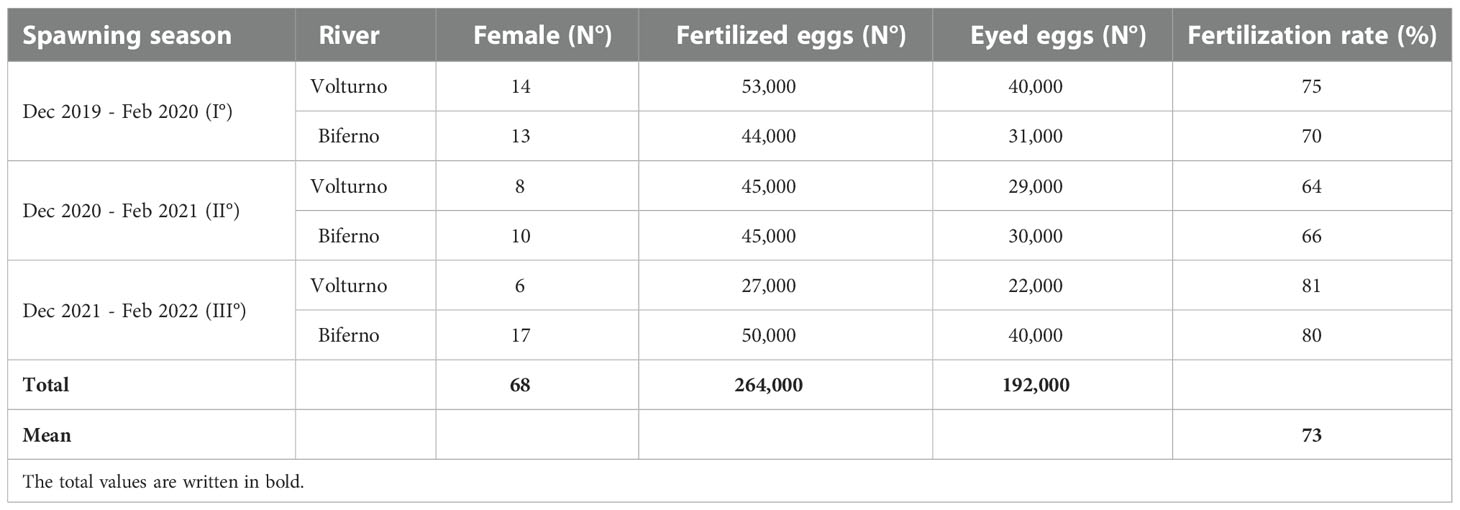
Table 2 Number of fertilized and eyed eggs and fertilization ability recorded with frozen semen, along the spawning seasons.
The semen quality parameters recorded in fresh semen of native males captured in the two rivers are shown in Table 3, mean ejaculate volumes and concentrations were similar across the two populations, while sperm motility and viability values were slightly higher in the males of the Volturno, however, no significant differences for each sperm trait were found.
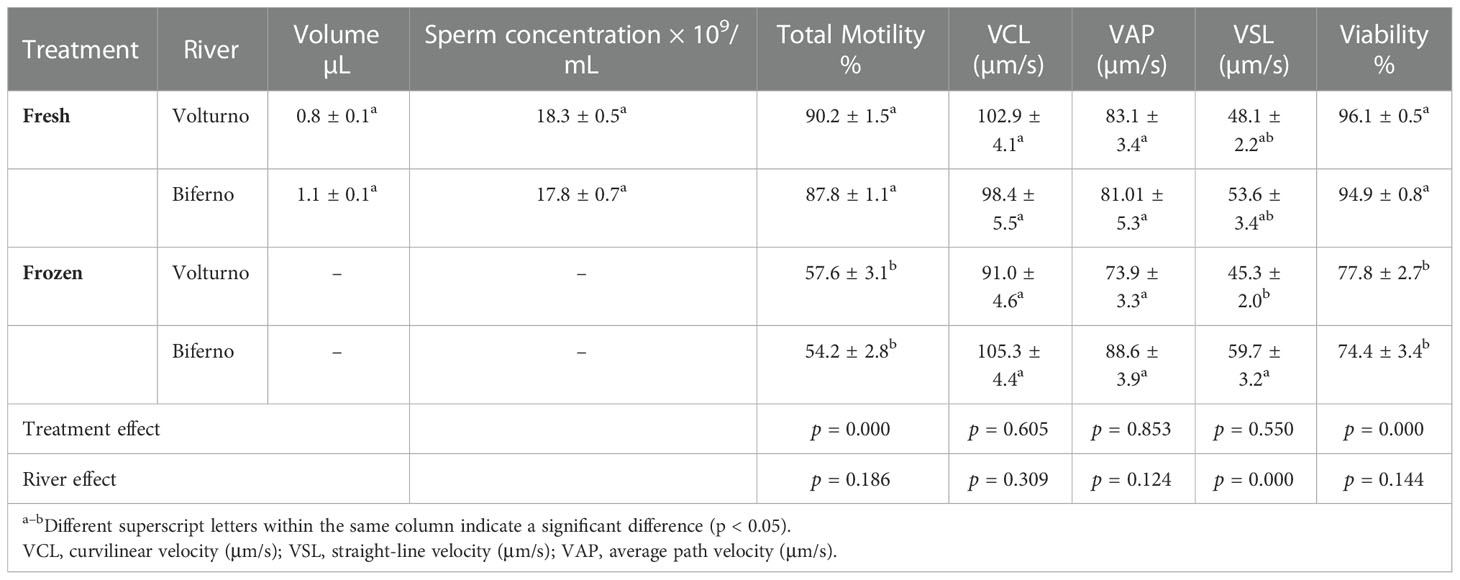
Table 3 Quality of fresh and frozen semen (means ± SEM) in brown trout population inhabiting Biferno and Volturno river (Molise region, Italy).
In line with our previous research, the cryopreservation of sperm resulted in a significant decrease in post-thaw sperm motility and viability (Rusco et al., 2019; Rusco et al., 2020), however, no significant differences were found for kinetic parameters considered, except for VSL, as a significantly higher value of this parameter was obtained in frozen semen of Biferno river compared with frozen semen of Volturno.
The GLM analysis indicated a significant effect for semen treatment (fresh or frozen semen) on total sperm motility and viability, and significant effect for river on VSL.
The average values in terms of sperm motility and viability obtained turned out to be satisfactory as reported in table 3. We achieved a recovery rate (value in frozen semen/value in fresh semen × 100) of about 65% for motility and 80% for spermatozoa viability in both rivers.
During the last decades the cryopreservation of fish spermatozoa has evolved due to the growing number of potential applications, including its application in aquaculture, enhancing hatchery broodstock management, conserving the genetically selected strains generated from genetic improvement programs (Asturiano et al., 2017; Judycka et al., 2019).
Recently, the use of cryopreserved semen is finding wide use for the safeguard of biodiversity in endangered species for preserving the genetic material of native population with a specific genotype and through the creation of semen cryobanks (Martínez-Páramo et al., 2017; Judycka et al., 2019; Bøe et al., 2021). Currently, in fish, the semen cryopreservation represents the only acceptable and valid technology to establish ex situ conservation programmes because the freezing procedures of oocytes and embryos results as still unsatisfactory.
The semen cryobank realised within our project represents an important tool to safeguard the genetic integrity and biodiversity of Mediterranean brown trout that populate the rivers in Molise. Its main purpose was to preserve the natural genetic variation as much as possible by creating a repository of genetic material from representative populations of Mediterranean brown trout inhabiting Biferno and Volturno rivers (Molise - Italy). During the project timeframe approximately 1,700 semen doses, from 150 native breeders were stored inside the cryobank representing a very important “tank” of genetic variability, as it includes a large number of native donors captured in the Molise rivers during the spawning seasons: 2019- 20; 2020-2021 and 2021-2022. The semen cryobank within the project assured the maximum genetic variability during the artificial supportive breeding.
The results obtained during the project were very satisfactory because 264,000 eggs from 68 native females were fertilized with 298 semen doses from 150 native males for a total of 298 unique male × female crosses to increase the genetical variability (see fertilization schemes). The fertilized eggs incubated at two hatcheries, one for each basin produced with fertility rates averaging at 75% therefore resulting in about 200,000 eyed eggs that were planted using nesting techniques in suitable sites for the restocking program of native trout. The nesting techniques assure a more successful approach because decreasing the non-adaptive selective pressure as much as possible by planting eyed eggs avoids the exposure of larvae and early life stages to an artificial environment.
Accordingly, to our project, the main goals of conservation programs are the maintenance of low inbreeding degree and high levels of genetic variation (Machado-Schiaffino et al., 2007); under this view, genetic variability is among the most important requisite for endangered populations to deal with any upcoming environmental changes by ensuring a long-term reply to natural and artificial selection, as well as for economic and cultural interests (Fernández et al., 2005; Frankham, 2008). It is to be noted that the introgression/inbreeding grade should be kept as low as possible to prevent harmful effects on fitness-related features, which might jeopardize the survival of the populations (Fernández et al., 2005).
Currently, about 30% of the semen doses stored in the cryobank were used for fertilization purposes and in vitro analysis. The remaining part of the cryobank’s consistency represents a valuable repository of genetic material for future use, to preserve this native species in conservation and management programs even after the end of the project.
Sperm cryopreservation includes many advantages for biodiversity conservation, this technique could also reduce inbreeding and reduce domestication selection. This is just the case with the Nat.Sal.Mo project, the semen stripping from native male to be frozen for the fertilization with cryopreserved semen were performed on the river. This allowed us to avoid transferring wild fish into an artificial environment, that frequently causes significant loss by stress or domestication and in the meantime the frozen semen associated to the fertilization schemes assured the genetic variability of the native population.
In light of these good practices developed in the project, the advantages of using frozen sperm in a gene bank namely the transport of genetic material over time as well as space (Bøe et al., 2021) must be highlighted. Cryopreserved semen can be stored almost indefinitely without undergoing substantial changes on cell motility and viability (Bøe et al., 2021). This means that frozen sperm can be used over different generations and places, in this regard we successfully used semen frozen during the first breeding season (2019-2020) to fertilize eggs obtained in subsequent spawning seasons (2020-2021 and 2021-2022), allowing us to keep the evolutionary potential of the natural population (Charlesworth, 2009; Witzenberger and Hochkirch, 2011; Bøe et al., 2021).
Cryopreservation approach can help manage species having trouble reproducting, such as those where the production of both sexes’ gametes is not synchronized (Anguilla anguilla; Asturiano et al., 2004); in species characterized by low sperm production (i.e., Solea senegalensis; Cabrita et al., 2006);when capture of females and males at the same time is not possible (i.e., European sturgeon (Acipenser sturio; Williot et al., 2011); or to allow time for the genetic analysis of a specific individual to establish its use in a conservation program (Horvath et al., 2012).
Hence, variations in male or female maturity timing results in uneven sex-ratios during spawning season, or, in the worst-case circumstance an increased male mortality could occur. Because unequal sex ratios result in imbalanced reproductive contribution among individuals, using cryopreserved sperm can reduce the risk of unwanted selection as a result of certain males contributing more than others (Bøe et al., 2021). Within the project we just guaranteed that the eggs from each native female were fertilized by frozen semen of different native males (at least 3) using fertilization matrix schemes, ensuring a wider genetic variability of the offspring.
Sperm cryobanking has significant advantages compared to breeding in captivity considering labour, costs, and security; since a large number (hundreds/thousands) of samples from different generations can be preserved in relatively small spaces, without the threat of damage from disease or genetic drift over time (Martínez-Páramo et al., 2009). Since, the genes within individual frozen spermatozoa remain largely unchanged, genetic variation is not lost from the sample and no directional changes in allele frequencies can occur (O’Reilly and Doyle, 2007). Furthermore, according to Wedekind et al. (2007), depending on the fertilization procedures, sperm competition caused by hatchery practices may also result in artificial selection and in increasing the loss of genetic diversity. Therefore, it’s crucial to reduce the genetic risks associated with in vitro fertilization.
However, the loss of maternal genetic information and the risk of epigenetic hereditary changes due to cryopreservation are still to be looked into. At the moment, cryopreservation of semen remains the most feasible compared to oocytes, embryos and primordial cells. In addition, advanced cryopreservation techniques must not only maintain high sperm motility and viability after freezing-thawing process, but also guard against potentially harmful changes of the genome and epigenome (Zhang et al., 2022). In fact, there is an increasing concern about the epigenetic implications of cryopreservation process on the sperm DNA methylation and offspring performances (Pérez-Cerezales et al., 2010; Labbé et al., 2017). During the freezing process, the cryoprotectant could induce a possible harmful epigenetic modification (Zhang et al., 2022). Specifically, cryoprotectant-induced abnormal methylation changes in cryopreserved semen, involving both hypomethylation and hypermethylation mechanisms (Kawai et al., 2010; Zhang et al., 2022). However, encouraging results were obtained through the use of methanol as a cryoprotectant in the semen freezing protocols on DNA methylation in the European eel (Anguilla anguilla) (Herranz-Jusdado et al., 2019), in zebrafish (Depincé et al., 2020), and in rainbow trout (El Kamouh et al., 2022).
Hence, these results are inspiring because methanol as a cryoprotectant would avoid possible harmful epigenetic modification, further studies are intended to further deepen information on this.
An obvious but crucial aspect for the successful implementation of sperm cryobanks, for hatchery practices or for conservation program purposes, requires that the biological material stored is of an acceptable quality in terms of motility/viability and even of a suitable quantity (Judycka et al., 2019). In order to produce the number of eggs needed for population restoration or the creation of new broodstock generations, an acceptable quantity of viable and motile sperm cells must be available (Bøe et al., 2021). Several factors, such as collection methods, handling techniques, and transport circumstances before preservation, might have an impact on the post-quality of cryopreserved sperm (Kommisrud et al., 2020; Rusco et al., 2021), as well as the freezing procedure used (Cabrita et al., 2010; Nynca et al., 2014; Nynca et al., 2017; Judycka et al., 2018; Bozkurt et al., 2019; Bozkurt et al., 2021). Cryopreservation may generate damages in spermatozoa, which impair different cell compartments, such as mitochondria, plasma membrane, and chromatin structure resulting in a negative impact on fertilization success (Cabrita et al., 2010; Figueroa et al., 2016; Mayer, 2019).
Our results clearly showed the effectiveness of the freezing protocol adopted, both in vitro but chiefly in vivo. In fact, we recorded satisfactory values of post-thaw sperm quality that ranged from 40% to 80% for total motility and from 50% to 85% for viability. Remarkably, the validity of freezing procedure can be demonstrated by the fertilization rate achieved in vivo, which, according to the breeding season, ranged from 64% to 81%.
Furthermore, the semen cryobank realized within our project opens new opportunities such as supporting the hatchery management practices. The implementation of a native broodstock park (live gene bank) is expected shortly in order to restock the waterways of our Molise region (south of Italy) with only autochthonous materials. However, the impoverishment of genetic variability and the loss of “rusticity” are among the main negative effects caused by the broodstock breeding in captivity that are transmitted and amplified to future generations. Breeding in captivity could lead to a dilution of “wild” genetic characters, in the course of generations dangerously exposing the populations at risk of extinction. According to different authors (O’Reilly and Doyle, 2007; Fraser, 2008), cryopreservation methodologies have the potential to minimize the losses of fitness and genetic diversity in long-term live-gene banking applications.
The eggs of future generations could be fertilized using the doses of cryopreserved sperm produced by males in the founder generations (Sonesson et al., 2002; Fraser, 2008). The frozen semen can maintain the genes within sperm fully unchanged for extended periods of time, however the sperm in frozen semen state has several benefits for biodiversity conservation. Primarily, because founder female alleles would be present in the sperm of the first male generation, it could aid in the preservation of a significant portion of the genetic variation in the original generation (up to 50%) (Sonesson et al., 2002; Fraser, 2008). Secondly, the method could keep inbreeding to a minimum and reduce domestication selection in captivity because half of the gametes used to create subsequent generations would come from individuals who were originally from the wild (O’Reilly and Doyle, 2007; Fraser, 2008).
Summarized, in order to develop an effective conservation program in salmonids the frozen semen in gene bank in combination with the live gene bank 1) would allow the fertilization of the eggs for the restoration of the lost or near extinct populations, 2) would increase the capacity of a live gene bank by replacing older males with frozen sperm doses; 3) would maintain the high ratio males to females that must be in favour of the male, reducing the number of males in captivity breeding.
The establishment of the first sperm cryobank for populations of native Mediterranean trout is a priceless tool for the protection and conservation of this species’ biodiversity. The effectiveness and usefulness of our semen cryobank is demonstrated by the excellent results obtained, either in terms of fertilization rates achieved with frozen semen that confirm the efficient sperm protocol used and in the terms of genetic conservation, as suggested by our preliminary data about the monitoring activities (unpublished data).
The remaining genetic material of the semen cryobank could be useful even after the end of the project for Molise River basins as well as for other Italian basins where the native populations of S. cettii are threatened and near to extinction risk. Aside from being a helpful tool in ensuring the project’s long-term viability, our cryobank represents a powerful multiplier effect that will affect other neighbouring areas on a national scale as well as other European river basins. Our approach could overcome the limits of the artificial reproduction and supportive breeding programs, potentially affected by domestication and undesired artificial selection. Our results suggest that semen cryobanks could represent an effective tool to support the hatchery management of all endangered salmonids species, in order to maintain the wild diversity of native populations.
MDI and NI, conceptualization, investigation, visualization, and writing. MDI, GR, SE and MD’A, data curation, formal analysis. NI, SE, GR, and AR, review, and editing. NI, funding acquisition. All authors contributed to the article and approved the submitted version.
This study was funded by the LIFE Nat.Sal.Mo. project (LIFE17 NAT/IT/000547).

The authors thank Amber Burchell and Antonietta Di Giovanni for the English revision of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Asturiano J. F., Cabrita E., Horváth A. (2017). Progress, challenges and perspectives on fish gamete cryopreservation: A mini-review. Gen. Comp. Endocrinol. 245, 69–76. doi: 10.1016/j.ygcen.2016.06.019
Asturiano J. F., Pérez L., Garzón D. L., Marco-Jiménez F., Peñaranda D. S., Vicente J. S., et al. (2004). Physio-chemical characteristics of seminal plasma and development of media and methods for cryopreservation of European eel sperm. Fish Physiol. Biochem. 30, 283–293. doi: 10.1007/s10695-005-1553-x
Bøe K., Bjøru B., Tangvold Bårdsen M., Nordtug Wist A., Wolla S., Sivertsen A. (2021). Opportunities and challenges related to sperm cryopreservation in Atlantic salmon gene banks. Conserv. Sci. Pract. 3 (12), e552. doi: 10.1111/csp2.552
Bozkurt Y., Yavaş İ. (2021). Effect of supplementations of docosahexaenoic acid (DHA) into a tris-glucose based extender on the post-thaw sperm quality, fertility and hatching rates in brown trout (Salmo trutta macrostigma) following cryopreservation. Int. Aquatic. Res. 13 (2), 147–154. doi: 10.22034/IAR.2021.1926852.1151
Bozkurt Y., Yavaş İ., Bucak M. N., Kıran T. R., Gül A. (2021). Cryoprotective effect of vitamin e supplementation of different extenders on quality and fertilizing ability of frozen-thawed brown trout sperm. Biopreserv. Biobank. 19 (3), 171–177. doi: 10.1089/bio.2020.0062
Bozkurt Y., Yavaş İ., Gül A., Bucak M. N., Yeni D., Avdatek F. (2019). Effect of extender supplemented with boron on post-thaw motility, viability, DNA damage and fertilization ability of cryopreserved brown trout (Salmo trutta macrostigma) spermatozoa. CryoLetters 40 (5), 275–283. Available at: http://www.cryoletters.org/Abstracts/vol_40_5_2019.htm#275.
Cabrita E., Sarasquete C., Martínez-Páramo S., Robles V., Beirao J., Pérez-Cerezales S., et al. (2010). Cryopreservation of fish sperm: Applications and perspectives. J. Appl. Ichthyol. 26, 623–635. doi: 10.1111/j.1439-0426.2010.01556.x
Cabrita E., Soares F., Dinis M. T. (2006). Characterization of Senegalese sole, Solea senegalensis, male broodstock in terms of sperm production and quality. Aquaculture 261, 967–975. doi: 10.1016/j.aquaculture.2006.08.020
Carosi A., Bonomo G., Lorenzoni M. (20202020). Effectiveness of alien brown trout salmo trutta l. removal activities for the native trout conservation in Mediterranean streams. J. Appl. Ichthyol. 36, 461–471. doi: 10.1111/jai.14063
Charlesworth B. (2009). Effective population size and patterns of molecular evolution and variation. Nat. Rev. Genet. 10, 195–205. doi: 10.1038/nrg2526
Chiesa S., Filonzi L., Ferrari C., Vaghi M., Bilò F., Piccinini A., et al. (2016). Combinations of distinct molecular markers allow to genetically characterize marble trout (Salmo marmoratus) breeders and stocks suitable for reintroduction plans. Fish. Res. 176, 55–64. doi: 10.1016/j.fishres.2015.12.009
Nynca J., Ciereszko A. (2009). Measurement of concentration and viability of brook trout (Salvelinus fontinalis) spermatozoa using computer-aided fluorescent microscopy. Aquaculture 292, 256–258. doi: 10.1016/j.aquaculture.2009.04.020
Comizzoli P., Holt W. V. (2014). Recent advances and prospects in germplasm preservation of rare and endangered species. Adv. Exp. Med. Biol. 753, 331–356. doi: 10.1007/978-1-4939-0820-2_14
D’Agaro E., Gibertoni P., Marroni F., Messina M., Tibaldi E., Esposito S. (2022). Genetic and phenotypic characteristics of the Salmo trutta complex in Italy. Appl. Sci. 12 (7), 3219. doi: 10.1111/jai.14063
Depincé A., Gabory A., Dziewulska K., Le Bail P. Y., Jammes H., Labbé C. (2020). DNA Methylation stability in fish spermatozoa upon external constraint: impact of fish hormonal stimulation and sperm cryopreservation. Mol. Reprod. Dev. 87 (1), 124–134. doi: 10.1002/mrd.23297
Di Iorio M., Esposito S., Rusco G., Roncarati A., Miranda M., Gibertoni P. P., et al. (2019). Semen cryopreservation for the Mediterranean brown trout of the biferno river (Molise-italy): Comparative study on the effects of basic extenders and cryoprotectants. Sci. Rep. 9, 9703. doi: 10.1038/s41598-019-45006-4
Diwan A. D., Harke S. N., Gopalkrishna, Panche A. N. (2020). Cryobanking of fish and shellfish egg, embryos and larvae: an overview. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00251
El Kamouh M., Brionne A., Laurent A., Labbè C. (2022). Book of abstract of 8th International Workshop on the Biology of Fish Gametes. (Gdansk (Poland): Institute of Animal Reproduction and Food Research, Polish Academy of Sciences
Fernández J., Villanueva B., Pong-Wong R., Toro M. A. (2005). Efficiency of the use of pedigree and molecular marker information in conservation programs. Genetics 170 (3), 1313–1321. doi: 10.1534/genetics.104.037325
Figueroa E., Valdebenito I., Merino O., Ubilla A., Risopatrón J., Farias J. G. (2016). Cryopreservation of Atlantic salmon Salmo salar sperm: Effects on sperm physiology. J. Fish Biol. 89, 1537–1550. doi: 10.1111/jfb.13052
Frankham R. (2008). Genetic adaptation to captivity in species conservation programs. Mol. Ecol. 17, 325–333. doi: 10.1111/j.1365-294X.2007.03399.x
Fraser D. J. (2008). How well can captive breeding programs conserve biodiversity? a review of salmonids. Evol. Appl. 1 (4), 535–586. doi: 10.1111/j.1752-4571.2008.00036.x
Giametta F., Perone C., Di Iorio M., Rusco G., Catalano P., Iaffaldano N. (2021). A new freezing box for the managing of semen cryopreservation process. Chem. Engineer. Trans. 87, 265–270. doi: 10.3303/CET2187045
Gwo J.-C., Arnold C. R. (1992). Cryopreservation of Atlantic croaker spermatozoa: evaluation of morphological changes. J. Exp. Zool. 264, 444–453. doi: 10.1002/jez.1402640410
Herranz-Jusdado J. G., Gallego V., Morini M., Rozenfeld C., Pérez L., Kása E., et al. (2019). Comparison of European eel sperm cryopreservation protocols with standardization as a target. Aquaculture 498, 539–544. doi: 10.1016/j.aquaculture.2018.09.006
Horváth Á., Jesenšek D., Csorbai B., Bokor Z., Raboczki É., Kaczkó D., et al. (2012). Application of sperm cryopreservation to hatchery practice and species conservation: a case of the Adriatic grayling (Thymallus thymallus). Aquaculture 358–359, 213–215. doi: 10.1016/j.aquaculture.2012.07.012
Iaffaldano N., Di Iorio M., Manchisi A., Esposito S., Gibertoni P. P. (2016a). Effective freezing rate for semen cryopreservation in endangered Mediterranean brown trout (Salmo trutta macrostigma) inhabiting the biferno river (South Italy). Zygote 24, 668–675. doi: 10.1017/S0967199415000647
Iaffaldano N., Di Iorio M., Manchisi A., Gibertoni P. P., Esposito S. (2016b). Semen quality of threatened native population of Mediterranean brown trout (Salmo cettii, rafinesque 1810) in the biferno river (Molise region -south Italy). Turk. J. Fish. Aquat. Sci. 16, 259–266. doi: 10.4194/1303-2712-v16_2_05
Judycka S., Nynca J., Ciereszko A. (2019). Opportunities and challenges related to the implementation of sperm cryopreservation into breeding of salmonid fishes. Theriogenology 132, 12–21. doi: 10.1016/j.theriogenology.2019.03.022
Judycka S., Nynca J., Liszewska E., Dobosz S., Grudniewska J., Ciereszko A. (2018). Optimal sperm concentration in straws and final glucose concentration in extender are crucial for improving the cryopreservation protocol of salmonid spermatozoa. Aquaculture 486, 90–97. doi: 10.1016/j.aquaculture.2017.12.019
Kawai K., Li Y. S., Song M. F., Kasai H. (2010). DNA Methylation by dimethylsulfoxide and methionine sulfoxide triggered by hydroxyl radical and implications for epigenetic modifications. Bioorg. Med. Chem. Lett. 20 (1), 260–265. doi: 10.1016/j.bmcl.2009.10.124
Kommisrud E., Myromslien F. D., Stenseth E. B., Zeremichael T. T., Hofman N., Grevle I., et al. (2020). Viability, motility, ATP content and fertilizing potential of sperm from Atlantic salmon (Salmo salar l.) in milt stored before cryopreservation. Theriogenology 151, 58–65. doi: 10.1016/j.theriogenology.2020.04.008
Labbé C., Robles V., Herraez M. P. (2013). “Cryopreservation of gametes for aquaculture and alternative cell sources for genome preservation,” in Advances in aquaculture hatchery technology. Eds. Allan G., Burnell G. (Elsevier: Woodhead Publishing), 76–116.
Labbé C., Robles V., Herraez M. P. (2017). Epigenetics in fish gametes and early embryo. Aquaculture Elsevier 472, 93–106. doi: 10.1016/j.aquaculture.2016.07.026
Lahnsteiner F., Berger F., Weismann T., Patzner R. (1996). The influence of various cryoprotectants on semen quality of the rainbow trout (Oncorhynchus mykiss) before and after cryopreservation. J. Appl. Ichthyol. 12, 99–106. doi: 10.1111/j.1439-0426.1996.tb00070.x
Larios-López J. E., Figueroa J. M. T. D., Galiana-García M., Gortázar J., Alonso C. (2015). Extended spawning in brown trout (Salmo trutta) populations from the southern Iberian peninsula: the role of climate variability. J. Limnol. 74 (2), 394–402. doi: 10.4081/jlimnol.2015.1089
Linhart O., Rodina M., Cosson J. (2000). Cryopreservation of sperm in common carp cyprinus carpio: sperm motility and hatching success of embryos. Cryobiology 41, 241–250. doi: 10.1006/cryo.2000.2284
Lorenzoni M., Carosi A., Giovannotti M., Porta G. L., Splendiani A., Barucchi V. C. (2019). Ecology and conservation of the Mediterranean trout in the central Apennines (Italy). J. Limnol. 78 (1), 1–13. doi: 10.4081/jlimnol.2018.1806
Machado-Schiaffino G., Dopico E., Garcia-Vazquez E. (2007). Genetic variation losses in Atlantic salmon stocks created for supportive breeding. Aquaculture 264, 59–65. doi: 10.1016/j.aquaculture.2006.12.026
Martínez-Páramo S., Horváth Á., Labbé C., Zhang T., Robles V., Herráez P., et al. (2017). Cryobanking of aquatic species. Aquaculture 472, 156–177. doi: 10.1016/j.aquaculture.2016.05.042
Martínez-Páramo S., Pérez-Cerezales S., Gómez-Romano F., Blanco G., Sánchez J. A., Herráez M. P. (2009). Cryobanking as tool for conservation of biodiversity: effect of brown trout sperm cryopreservation on the male genetic potential. Theriogenology 71, 594–604. doi: 10.1016/j.theriogenology.2008.09.034
Marzano F. N., Corradi N., Papa R., Tagliavini J., Gandolfi G. (2003). Molecular evidence for introgression and loss of genetic variability in Salmo (trutta) macrostigma as a result of massive restocking of apennine populations (Northern and central Italy). Environ. Biol. Fishes 68, 349–356. doi: 10.1023/B:EBFI.0000005762.81631.fa
Mayer I. (2019). The role of reproductive sciences in the preservation and breeding of commercial and threatened teleost fishes. Adv. Exp. Med. Biol. 1200, 187–224. doi: 10.1007/978-3-030-23633-5_7
McMeel O. M., Hoey E. M., Ferguson A. (2001). Partial nucleotide sequences, and routine typing by polymerase chain reaction–restriction fragment length polymorphism, of the brown trout (Salmo trutta) lactate dehydrogenase, LDH-C1*90 and *100 alleles. Mol. Ecol. 10, 29–34. doi: 10.1046/j.1365-294X.2001.01166.x
Nynca J., Dietrich G. J., Dobosz S., Grudniweska J., Ciereszko A. (2014). Effect of cryopreservation on sperm motility parameters and fertilizing ability of brown trout semen. Aquaculture 433, 62–65. doi: 10.1016/j.aquaculture.2014.05.037
Nynca J., Judycka S., Liszewska E., Dobosz S., Ciereszko A. (2017). Standardization of spermatozoa concentration for cryopreservation of rainbow trout semen using a glucose-methanol extender. Aquaculture 477, 23–27. doi: 10.1016/j.aquaculture.2017.04.036
O’Reilly P., Doyle R. W. (2007). “Live gene banking of endangered populations of Atlantic salmon,” in The Atlantic salmon: Genetics, conservation and management. Eds. Verspoor E., Stradmeyer L., Nielsen J. L. (The Netherlands: Springer), 346–380. doi: 10.1002/9780470995846.ch14
Palombo V., De Zio E., Salvatore G., Esposito S., Iaffaldano N., D’Andrea M. (2021). Genotyping of two Mediterranean trout populations in central-southern Italy for conservation purposes using a rainbow-trout-derived SNP array. Animals 11, 1803. doi: 10.3390/ani11061803
Pensierini M., Nonnis Marzano F., Gandolfi G., Maldini M., Marconato E., Gibertoni P. (2006). Genotypes and phenotypes of Mediterranean brown trout: molecular investigation combined to morphological characterization for identification of autochthonous specimens. Quaterni ETP-J. Freshw. Biol. 34, 69–75. Available at: https://www.igiardinidellacqua.com/downloads/penserini%20et%20al%202006.pdf.
Pérez-Cerezales S., Martínez-Páramo S., Beirão J., Herráez M. P. (2010). Fertilization capacity with rainbow trout DNA damaged sperm and embryo developmental success. Reproduction 139, 989–997. doi: 10.1530/REP-10-0037
Polgar G., Iaia M., Righi T., Volta P. (2022). The Italian alpine and subalpine trouts: taxonomy, evolution, and conservation. Biology 11 (4), 576. doi: 10.1111/jai.14063
Querci G., Pecchioli E., Leonzio C., Frati F., Nardi F. (2013). Molecular characterization and hybridization in Salmo (trutta) macrostigma morphotypes from central Italy. Hydrobiologia 702, 191–200. doi: 10.1007/s10750-012-1320-9
Robles V., Cabrita E., Cuñado S., Herráez M. P. (2003). Sperm cryopreservation of sex-reversed rainbow trout (Oncorhynchus mykiss): parameters that affect its ability for freezing. Aquaculture 224, 203–212. doi: 10.1016/S0044-8486(03)00221-7
Robles V., Riesco M. F., Psenicka M., Saito T., Valcarce D. G., Cabrita E., et al. (2017). Biology of teleost primordial germ cells (PGCs) and spermatogonia: biotechnological applications. Aquaculture 472, 4–20. doi: 10.1016/j.aquaculture.2016.03.004
Rossi A. R., Petrosino G., Milana V., Martinoli M., Rakaj A., Tancioni L. (2019). Genetic identification of native populations of Mediterranean brown trout Salmo trutta l. complex (Osteichthyes: Salmonidae) in central Italy. Eur. Zool. J. 86 (1), 424–431. doi: 10.1080/24750263.2019.1686077
Rossi A. R., Talarico L., Petrosino G., Crescenzo S., Tancioni L. (2022). Conservation genetics of Mediterranean brown trout in central Italy (Latium): A multi-marker approach. Water 2022 (14), 937. doi: 10.3390/w14060937
Rusco G., Di Iorio M., Gibertoni P. P., Esposito S., Penserini M., Roncarati A., et al. (2019). Optimization of sperm cryopreservation protocol for Mediterranean brown trout: a comparative study of non-permeating cryoprotectants and thawing rates in vitro and in vivo. Animals 9, 304. doi: 10.3390/ani9060304
Rusco G., Di Iorio M., Iampietro R., Esposito S., Gibertoni P. P., Penserini M., et al. (2020). A simple and efficient semen cryopreservation method to increase the genetic variability of endangered Mediterranean brown trout inhabiting molise rivers. Animals 10, 403. doi: 10.3390/ani10030403
Rusco G., Di Iorio M., Iampietro R., Roncarati A., Esposito S., Iaffaldano N. (2021). Cryobank of Mediterranean brown trout semen: evaluation of the use of frozen semen up to six hours post-collection. Fishes 6, 26. doi: 10.3390/fishes6030026
Sabatini A., Podda C., Frau G., Cani M. V., Musu A., Serra M., et al. (2018). Restoration of native Mediterranean brown trout Salmo cettii rafinesque 1810 (Actinopterygii: Salmonidae) populations using an electric barrier as a mitigation tool. Eur. Zool. J. 85, 137–149. doi: 10.1080/24750263.2018.1453554
Salvatore G., Palombo V., Esposito S., Iaffaldano N., D’Andrea M. (2022). Identification of ancestry informative markers in Mediterranean trout populations of molise (Italy): a multi-methodological approach with machine learning. Genes 13, 1351. doi: 10.3390/genes13081351
Sonesson A. K., Goddard M. E., Meuwissen T. H. E. (2002). The use of frozen semen to minimize inbreeding in small populations. Genet. Res. 80, 27–30. doi: 10.1017/s0016672302005712
Splendiani A., Giovannotti M., Cerioni P. N., Caniglia M. L., Caputo V. (2006). Phylogeographic inferences on the native brown trout mtDNA variation in central Italy. Ital. J. Zool. 73, 179–189. doi: 10.1080/11250000600679751
Splendiani A., Palmas F., Sabatini A., Barucchi V. C. (2019). The name of the trout: Considerations on the taxonomic status of the Salmo trutta l. 1758Complex (Osteichthyes: Salmonidae) in Italy. Eur. Zool. J. 86 (1), 432–442. doi: 10.1080/24750263.2019.1686544
Wedekind C., Rudolfsen G., Jacob A., Urbach D., Muller R. (2007). The genetic consequences of hatchery-induced sperm competition in a salmonid. Biol. Conserv. 137, 180–188. doi: 10.1016/j.biocon.2007.01.025
Williot P., Rochard E., Desse-Berset N., Kirschbaum F., Gessner J. (2011). Biology and conservation of the European sturgeon acipenser sturio l. 1758: the reunion of the European and Atlantic sturgeons (Berlin Heidelberg: Springer-Verlag).
Witzenberger K. A., Hochkirch A. (2011). Ex situ conservation genetics: A review of molecular studies on the genetic consequences of captive breeding programmes for endangered animal species. Biodivers. Conserv. 20, 1843–1861. doi: 10.1007/s10531-011-0074-4
Zhang S., Cheng Y., Vĕchtová P., Boryshpolets S., Shazada N. E., Hadi Alavi S. M., et al. (2022). Potential implications of sperm DNA methylation functional properties in aquaculture management. Rev. Aquac. 2022, 1–21. doi: 10.1111/raq.12735
Zhang T., Liu X. H., Rawson D. M. (2003). Effects of methanol and developmental arrest on chilling injury in zebrafish (Danio rerio) embryos. Theriogenology 59, 1545–1556. doi: 10.1016/s0093-691x(02)01199-8
Keywords: Mediterranean brown trout, semen cryobank, safeguarding biodiversity, hatcheries’ management, genetic conservation
Citation: Di Iorio M, Rusco G, Esposito S, D’Andrea M, Roncarati A and Iaffaldano N (2023) The role of semen cryobanks for protecting endangered native salmonids: Advantages and perspectives as outlined by the LIFE Nat.Sal.Mo. project on Mediterranean brown trout (Molise region – Italy). Front. Mar. Sci. 9:1075498. doi: 10.3389/fmars.2022.1075498
Received: 20 October 2022; Accepted: 14 December 2022;
Published: 04 January 2023.
Edited by:
Yusuf Bozkurt, Iskenderun Technical University, TurkeyReviewed by:
P. Routray, Central Institute of Freshwater Aquaculture (ICAR), IndiaCopyright © 2023 Di Iorio, Rusco, Esposito, D’Andrea, Roncarati and Iaffaldano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicolaia Iaffaldano, bmljb2xhaWFAdW5pbW9sLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.