
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mar. Sci. , 13 December 2022
Sec. Marine Biotechnology and Bioproducts
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.1074135
This article is part of the Research Topic Bioactive Terpenoids from Marine Organisms and Their Pharmacological Activities View all 5 articles
The fungi Eutypella could metabolize a myriad of natural products with unique structures and diverse bioactivities, which were deemed as key sources for lead compounds in drug discovery. Since the first research on the genus Eutypella in 2009, a myriad of secondary metabolites including terpenoids, alkaloids, and polyketides have been discovered in this genus, and most of them exhibited significant pharmacological activities. However, there are no systematic reviews that reported about the structures and bioactivities of Eutypella up to now. In this review, a total of 153 secondary metabolites and 42 references have been systematically summarized, and we found that the terpenoids (68.09%) and alkaloids (19.15%) were the new main components of this fungi, and the primary antiproliferative activity (64.89%) was mainly derived from the terpenoids and alkaloids. Thus, this review about the chemical diversity and biological activities of the metabolites from the fungus Eutypella provided a new perspective for the development of new drugs for pharmacologists.
Eutypella are cosmopolite and diverse fungi, which are included in phylum Diatrypaceae, subclass Xylariomycetidae, and genus Eutypella (Acero et al., 2017). They are commonly isolated from various terrestrial and marine environments (Da Silva et al., 2008; Moyo et al., 2016; Zhang, 2019) and play an important ecological role as a decomposer (Lee et al., 2011; Deng et al., 2019; Brglez et al., 2020). Meanwhile, the fungi Eutypella are reported to be valuable biological resources due to their ability to produce bioactive secondary metabolites such as terpenoids, alkaloids, and polyketides (Sun et al., 2011; Liu et al., 2014b; Niu et al., 2017a; Zhang et al., 2020; Zhang et al., 2021b). Accumulated reports suggest that the secondary metabolites represent a wide range of biological activities including antiproliferative, anti-inflammatory, antibacterial, and antiviral activities (Palem et al., 2015; Kuriakose et al., 2016; Liao et al., 2017; Niu et al., 2018; Mao et al., 2021). For example, libertellenone H (LH) (Zhang et al., 2021c) was isolated from the arctic fungus Eutypella sp. D-1 as antiproliferative against various cancer cells. Diaporthein B (Isaka et al., 2011), from the endophyte Eutypella sp. BCC 13199, displayed significant inhibitory activities against NCI-H187 human small-cell lung cancer cells with an IC50 value of 0.15 μM. Therefore, the fungi Eutypella can produce secondary metabolites with novel structures and have extensive pharmacological properties, which is of great research significance for drug discovery.
According to the previous literature reported, domestic and foreign researchers have studied 10 strains of this genus [Eutypella sp. BCC 13199 (Isaka et al., 2011), E. scoparia PSU-D44 (Pongcharoen et al., 2006), E. scoparia FS46 (Liu et al., 2017b), E. scoparia FS26 (Sun et al., 2012a), E. scoparia PSU-H267 (Kongprapan et al., 2015), E. sp. D-1 (Lu et al., 2014), E. scoparia ICB-OBX (Ciavatta et al., 2008), E. scoparia 1-15 (Qi et al., 2015), E. scoparia SCBG-8 (Zhang et al., 2020), and E. sp. MCCC 3A00281 (Niu et al., 2017a)] for their secondary metabolites. However, there is no systematic review on the structural diversity and biological activities for the fungi Eutypella. Therefore, in this review, we focused on the structural diversities and relevant biological activities of 153 metabolites from the fungi Eutypella, which provide new insights for chemists and pharmacologists in drug discovery.
Eutypellol A (1), the first norsesquiterpenoid of sesquicarene family, and a rare 7-methyl oxidized 2-carene derivative eutypellol B (2) (Liu et al., 2017b) were isolated from the marine-derived fungi Eutypella scoparia FS46, which was found to show potent anti-Staphylococcus aureus and cytotoxic activities. Ent-4(15)-eudesmen-11-ol-1-one (3) and ent-4(15)-eudesmen-1R, 11-diol (4) (Isaka et al., 2009) were enantiomers of known compounds, respectively, which were isolated from Eutypella sp. BCC 13199. Furthermore, 3 exhibited weak cytotoxicity towards NCI-H187, MCF-7, KB, and noncancerous Vero cells at concentrations ranging from 11 to 32 μM.
3,7,10-Trihydroxy-6,11-cyclofarnes-1-ene (5) and 8-(hydroxymethyl)-1-(2-hydroxy-1-methylethyl)-4-methylspiro [4.5]-dec-8-en-7-ol (6) (Sun et al., 2012a), which were identified as monocyclofarnesane-type and acorane-type sesquiterpene, respectively, were constituents of Eutypella scoparia FS26. In the bioassays, both of them showed weak inhibitory activities against the MCF-7 cell line at the concentration of 100 μM by the MTT method.
Scopararane C (7), a new β-eudesmol sesquiterpene, was produced by a fungal strain, Eutypella scoparia 1-15, which was isolated from mangrove rhizosphere soil of Jimei, Fujian Province, China (Qi et al., 2015). Eutypella sp. D-1 yielded a minor fermentation product, eut-guaiane sesquiterpene (8) (Zhou et al., 2017). When 50 μg of sesquiterpene (8) acted on the antibacterial paper (Φ = 6 mm), the results showed that 8 had strong antibacterial activities against Bacillus subtilis, S. aureus, and Escherichia coli such as the positive control ampicillin. Moreover, 8 also exhibited in vitro weak cytotoxicity towards the SGC7901 cell line.
To investigate the bioactive metabolites, isolation of four new eudesmanolides of a subgroup of 12,8-eudesmanolides (9–12)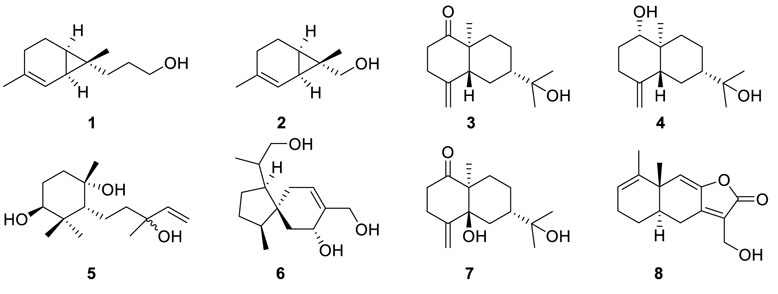
from soil-related mangrove fungus Eutypella sp. 1-15 had been performed (Wang et al., 2017). Notably, compound 9 displayed cytotoxic activities towards liver cancer cells HepG2 and human sleeve lymphoma cancer cells JEKO-1 with IC50 values of 28.5 and 8.4 μM, respectively. Moreover, compound 9 also showed moderate antibacterial activity against Bacillus pumilus CMCC63202 and B. subtilis CMCC63501 with IC50 values of 23.8 and 18.1 μM, respectively.
Cultivation of fungus Eutypella sp. MCCC 3A00281 using two chemical epigenetic manipulation reagents suberohydroxamic acid (SBHA) and histone deacetylase inhibitor (HDI) led to the isolation of a variety of novel eremophilane-type sesquiterpenoids eutyperemophilanes A-Z (13−38) (Niu et al., 2018). Among them, eutyperemophilane B (14), eutyperemophilane I (21), and eutyperemophilane J (22) showed weak cytotoxic activity with CC50 > 100 μM on RAW264.7 cells. In particular, eutyperemophilane I (21) and eutyperemophilane J (22) were further evaluated as anti-inflammatory agents with IC50 values of 8.6 and 13 μM, respectively.
As part of an ongoing investigation into fungus Eutypella sp. MCCC 3A00281, a suberohydroxamic acid (SBHA) and a DNA methyltransferase inhibitor (5-azacytidine) were co-treated with the fungus, which led to the isolation of 17 sesquiterpenes eutypeterpenes A−Q (39−55) (Niu et al., 2021). They are classified into bergamotane-type (39−44),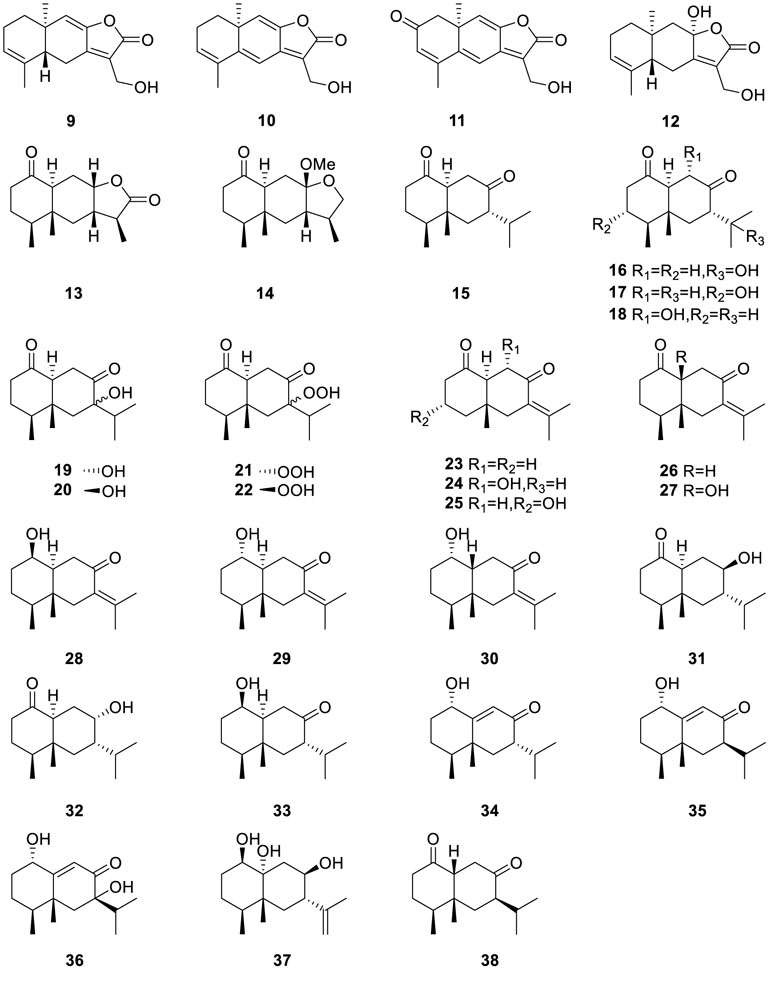
bisabolane-type (45−50), cadinene-type (51), carabrane-type (52), and an undescribed subtype of sesquiterpenes with a cyclopentane ring (53−55). Except for eutypeterpene K (49) and eutypeterpene L (50), all these metabolites inhibited the production of NO in lipopolysaccharide (LPS)-induced RAW 264.7 macrophages. Among them, eutypeterpene N (52) was the most effective sesquiterpene to inhibit the NO production, with an IC50 of 8.6 μM, which has the potential to treat inflammatory diseases.
Chromatographic separation of the bioactive extract (anti-MRSA) of the endophytic fungus Eutypella scoparia SCBG-8 resulted in the isolation of a group of new sesquiterpenes, named eutyscoparins A−H (56−63) (Zhang et al., 2021a). Eutyscoparin G (63) exhibited antibacterial activity against MRSA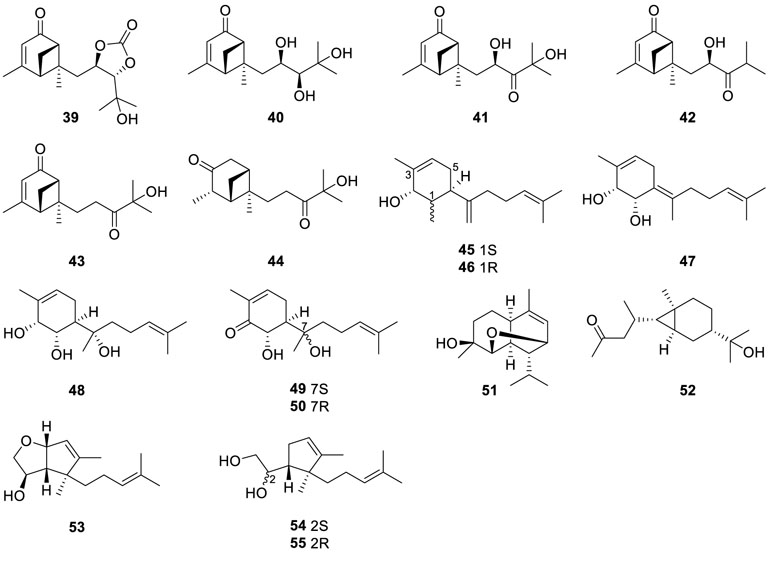
(methicillin-resistant S. aureus) and S. aureus with MIC values of 6.3 μg/ml.
Scopararanes A and B (64 and 65), as well as two known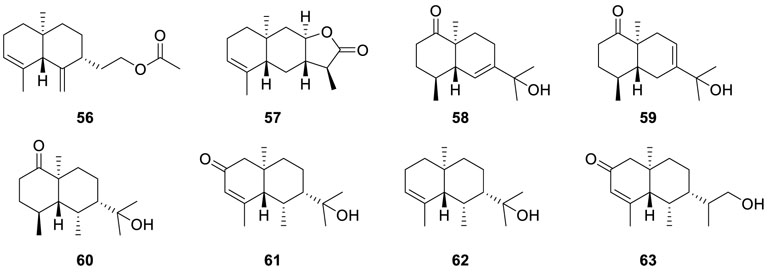
diterpenes diaportheins A and B (66 and 67) (Pongcharoen et al., 2006), were isolated from the Eutypella sp. PSU-D44. Of note, all of them showed weak antibacterial activities against Microsporum gypseum SH-MU-4 and S. aureus ATCC 25923. Investigation on the endophyte Eutypella scoparia BCC 13199, which was fermented in a bioreactor containing malt extract broth after shake incubation in PDB, resulted in the isolation of two novel γ-lactones diterpenes eutypellones A and B (68 and 69) and three known diterpenes scopararane A (64), diaporthein B (67), and libertellenone C (70) (Isaka et al., 2011). Meanwhile, antiproliferative activity demonstrated that scopararane A (64) and diaporthein B (67) displayed significant inhibitory activities against human small-cell lung cancer cells NCI-H187 with IC50 values of 0.024 and 0.15 μM, respectively.
A strain of Eutypella scoparia FS26 yielded a series of oxygenated pimarane diterpenes scopararanes C–G (71−75)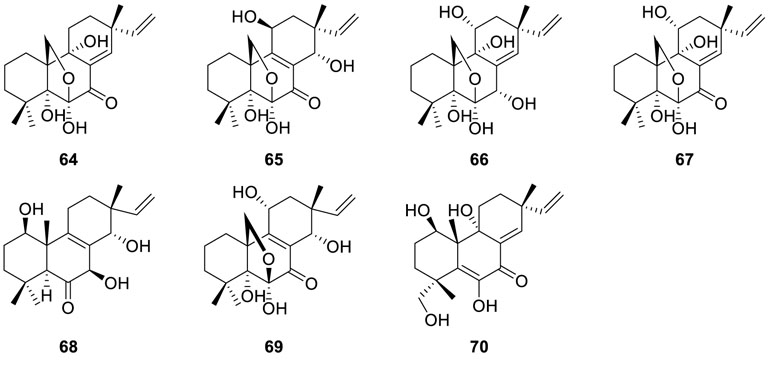
(Sun et al., 2012b). Among them, scopararanes C (71) and D (72) exhibited moderate cytotoxic activities with IC50 values of 35.9 μM and 25.6 μM in the tumor cell line MCF-7. Furthermore, the MTT method was subjected to two known compounds diaporthein B (67) and libertellenone A (76), and both of them exhibited strong cytotoxicity against NCI-H460, SF-268, and MCT-7 ranging from 4.4 to 9.9 µM and from 12.0 to 40.2 µM, respectively. Bioassay-guided separation of the EtOAc extract of the fungus Eutypella sp. D-1 resulted in the isolation of two novel diterpenes libertellenones G (77) and H (78), along with two known ones libertellenone C (70) and libertellenone A (76) (Lu et al., 2014). Meanwhile, libertellenone G (77) displayed moderate antibacterial activities against B. subtilis, S. aureus, and E. coli. In addition, LH (78) showed significant cytotoxic activities with IC50 values in the range of 3.31 to 44.1 µM in an array of cancer cell lines (U251 cells, MCF-7 cells, SG7901 cells, H460 cells, Huh-7 cells, Hela cells, and SW-1990 cells).
Investigation on the E. sp. D-1 obtained from soil of the high-latitude Arctic led to the isolation of three special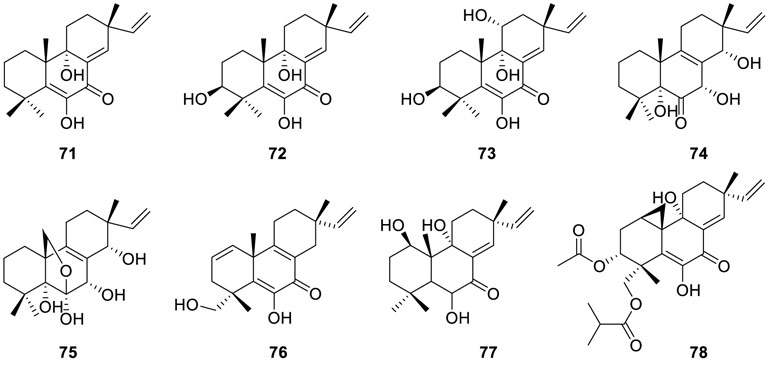
pimarane-type diterpenes eutypenoids A–C (79–81) (Zhang et al., 2016). Among them, 79 and 81 are ring A rearranged pimarane diterpenes. The NMR data suggested that 79 is a diterpenoid with a new carbon skeleton, which possessed a tetracyclic system consisting of two hexatomic rings, the furan ring and the oxepane skeleton of ring A. Eutypenoid C (81) is a disubstituted tetracyclic pimarane diterpenoid with an additional pyranoid ring compared to the typical pimarane diterpenoid. Moreover, eutypenoid B (80) is a nitrogenous diterpene, which showed strong antiproliferative activity of splenocyte under concanavalin A induction within the concentration from 0.16 to 40 μM.
Scopararanes H and I (82 and 83) (Liu et al., 2017a), identified as new pimarane diterpenes, were isolated from fungus Eutypella sp. FS46. Furthermore, scopararane I (82) exhibited inhibitory activities against NCI-H460 (human non-small cell lung cancer cell line), MCF-7 (human breast adenocarcinoma cell line), and SF-268 (human glioma cell line) with IC50 values of 13.5, 25.3, and 83.9 μM, respectively.
Large-scale fermentation of the fungus Eutypella sp. D-1 resulted in the characterization of seven new pimarane-type diterpenes, libertellenones O−S (84–88), eutypellenone A (89), and eutypellenone B (90) (Yu et al., 2018b). Most importantly, all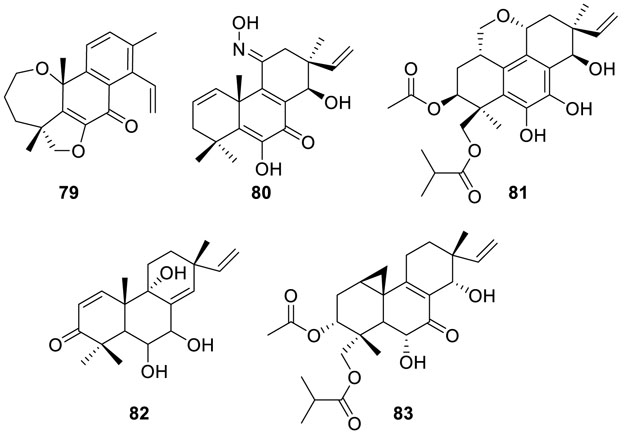
of them showed different levels of cytotoxic activities towards SW1990, MCF-7, PANC-1, HCT-116, and HeLa cells, with IC50 values in the range of 0.8 to 29.4 μM. Furthermore, eutypellenones A (89) and B (90) could inhibit the activity of NF-κB in a dose-dependent manner and exerted significant inhibitory effects to reduce NO production induced by LPS with IC50 < 10 μM. A further study on Eutypella sp. D-1 resulted in the discovery of three pimarane-type diterpenes eutypellenoids A–C (91–93) (Yu et al., 2018a), which possessed an infrequent tetrahydrofuran-fused pimarane diterpene skeleton. Significantly, antifungal tests demonstrated that eutypellenoid B (92) showed strong inhibitory activities on Candida albicans, Candida parapsilosis, Candida glabrata, and Candida tropicalis with MIC values of 8.0, 8.0, 16.0, and 32.0 μg/ml, respectively, and also displayed antibacterial activity against E. coli and S. aureus with an MIC value of 8 μg/ml for both. Meanwhile, eutypellenoid B (92), which was assessed for its cytotoxic activity, could reduce the proliferation of the HCT-116 cell line with an IC50 value of 3.7 μM.
In-depth investigation on the CH2Cl2 extract of Eutypella sp. D-1 led to the identification of two new pimarane-type diterpenes, libertellenone M (94) and libertellenone N (95), and five known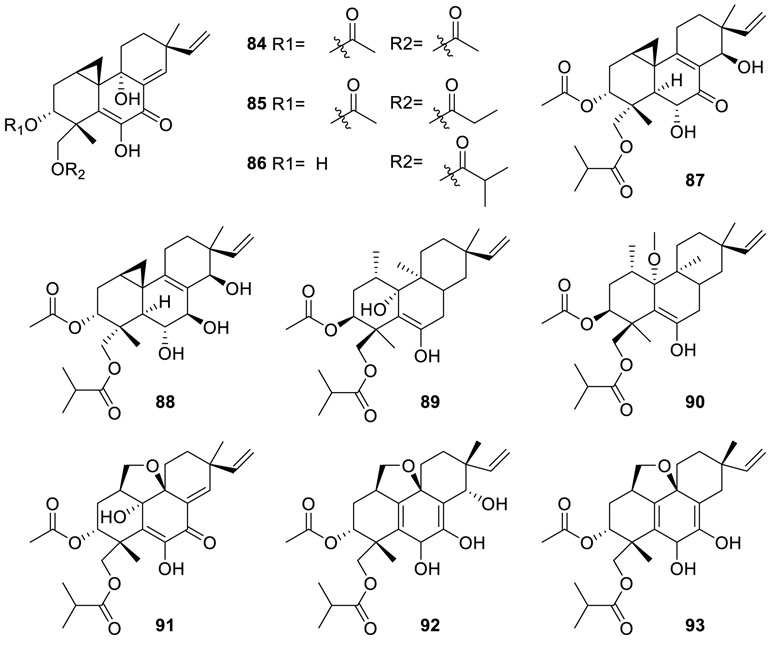
compounds, libertellenones A–C (76, 96, 70), eutypellone A (97), and kaempulchraol W (98) (Wang et al., 2018). Among them, libertellenone M (94) exhibited antibacterial activity against V. vulnificus, S. aureus, and E. coli, while libertellenone A (76) showed extra antibacterial activity against B. subtilis. Moreover, cytotoxicity assay indicated that libertellenone N (95) showed excellent antiproliferative activity with an IC50 value of 7.67 μM in the K562 cell line. Apart from the reported MCF-7 (Lu et al., 2014), libertellenone A (76) also exhibited moderate cytotoxicity against four human cancer cells SW1990, HCT-116, K562, and HeLa with IC50 values of 17.31, 15.39, 14.81, and 14.31 μM, respectively.
Four unusual meroterpenoids eutypellacytosporins A–D (99–102) (Zhang et al., 2019), structurally related to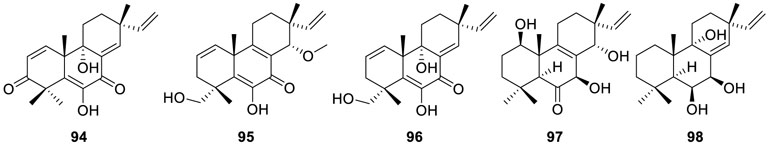
cytosporins and decipienolides, were acquired from an Arctic stain of Eutypella sp. D-1. In particular, the research proposed 99–102 may be constructed by two moieties cytosporin D and decipienolide A or decipienolide B through a 12,32-ester linkage. Bioactivity evaluation suggested that all the compounds possessed cytotoxic activity against PANC-1, Huh7, DU145, and SW1990 cell lines with IC50 values in the range of 4.9 to 17.1 μM.
Marine-derived fungus Eutypella scoparia SCBG-8 extract was subjected to a chemical research, yielding a unique meroterpenoid eutyscoparol J (103) (Zhang et al., 2021b). Interestingly, 103 was endowed with an unusual 6/6/6/6/6/6/6 polycyclic ring system based on the dimeric isoprenylated epoxyquinol skeleton. Meanwhile, eutyscoparol J (103) exhibited a moderate antiproliferative effect.
Antimicrobial activity-guided fractionation of fungus Eutypella scoparia PSU-D44 produced two new alkaloids scoparasin A (104) and scoparasin B (105) (Pongcharoen et al., 2006). Interestingly, scoparasin B (105) displayed sensible inhibitory activity against M. gypseum SH-MU-4 with an MIC value of 30.3 μM.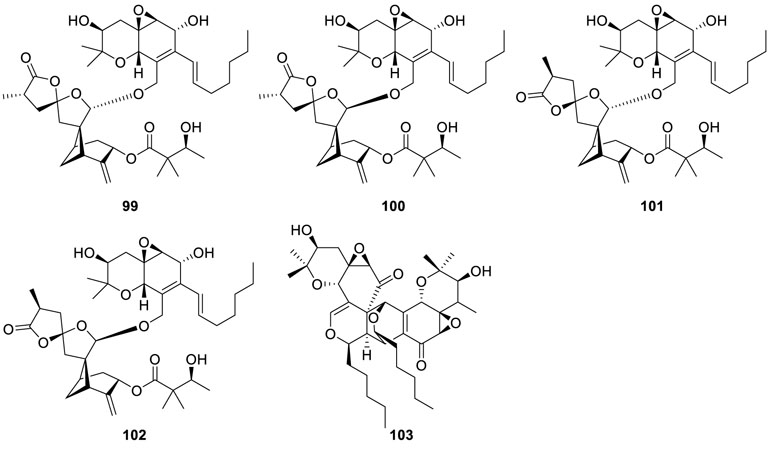
Chromatographic separation of a crude extract obtained from the fungus Eutypella sp. D-1 yielded three new alkaloids Z24−Z26 (106–108) and a known one alkaloid scoparasin B (105) (Liu et al., 2014a). Furthermore, cytochalasin Z24 (106) exhibited strong cytotoxicity against the MCF-7 human breast cancer cell line with an IC50 value of 9.33 μM. Moreover, scoparasin B (105) was selective for the H460 lung cell line with an IC50 of 3.9 μM.
A new alkaloid, scoparasin C (109), and four known alkaloid derivatives, [12]-Cytochalasin (110), phenochalasin B (111), isomer of 5,6-dehydro-7-hydroxy-29-methoxycytochalasin E (112), and scoparasin A (104) (Kongprapan et al., 2015), were isolated from Eutypella sp. PSU−H267. Of note, phenochalasin B (111), the methyl ether derivative of 109, was remarkable against KB cell lines with an IC50 value of 2.46 μM. Meanwhile, scoparasin C (109), phenochalasin B (111), and isomer of 5,6-dehydro-7-hydroxy-29-methoxycytochalasin E (112) displayed substantial cytotoxic activity toward Vero cell lines with IC50 values of 1.19, 0.04, and 1.01 μM, respectively. [12]-Cytochalasin (110) (Sun et al., 2013) exhibited mild cytotoxic activities on MCF-7 and SF-268 with IC50 values of 47.2 and 35.4 mM, respectively.
Extraction of a stain of fungus Eutypella scoparia 1-15 yielded two new alkaloids, including an open-chain alkaloid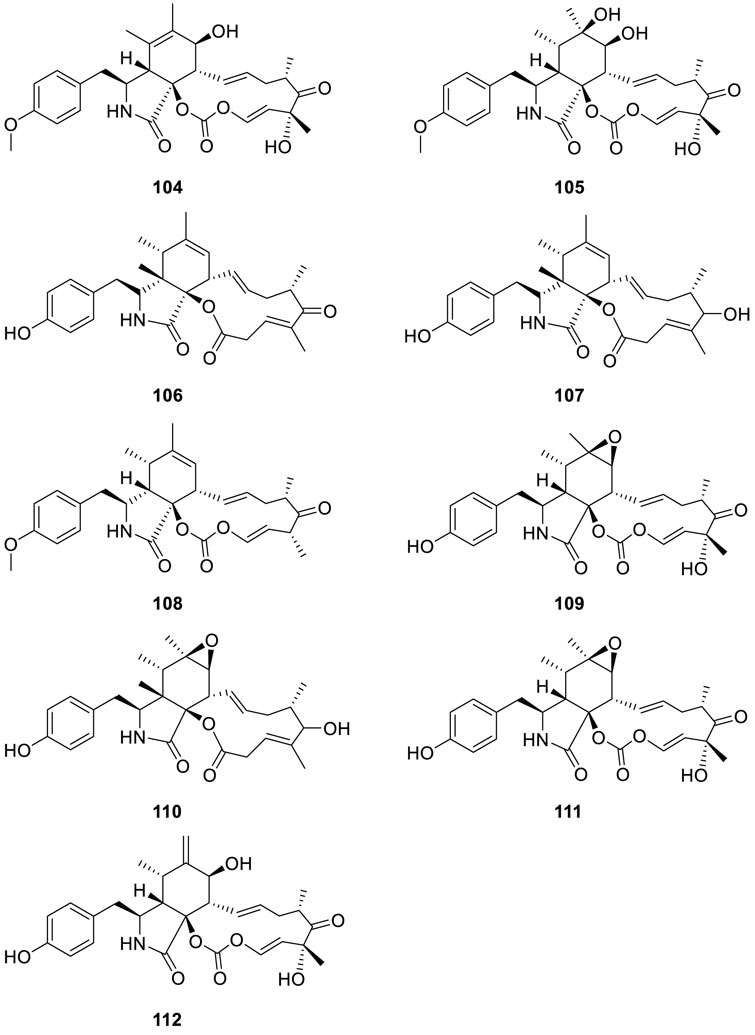
scoparasin C (113) and a pyrichalasan scoparasin D (114) (Qi et al., 2015). Scoparasin D (114) had potent inhibitory activities against A375, A549, MCF-7, and HepG2 cancer cell lines with very low IC50 values of 1.08, 2.25, 3.40, and 3.51 μM, respectively.
In the ongoing search for bioactive thiodiketopiperazine-type alkaloids, a class of new congeners named eutypellazines A−M (115−127) were disclosed from fungus Eutypella sp. MCCC 3A00281 based on bioassay and the NMR/MS spectroscopic data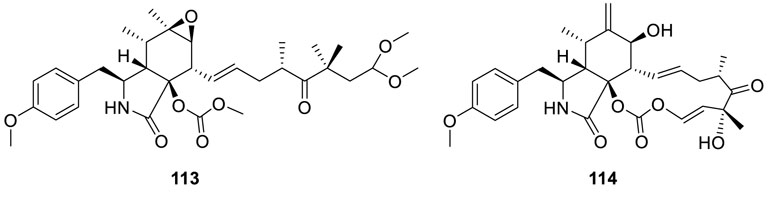
(Niu et al., 2017a). Significantly, eutypellazine E (115) exhibited the strongest anti-HIV activity with an IC50 value of 3.2 μM, while other alkaloids caused HIV-1 inhibition with IC50 values from 5.0 to 20 μM.
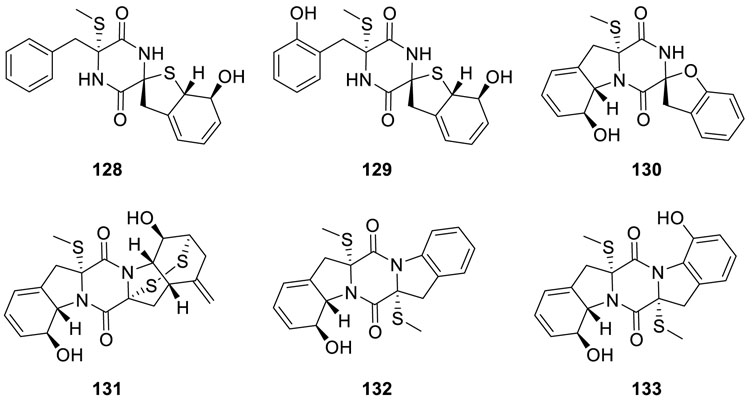
Eutypellazines N−S (128−133) (Niu et al., 2017b), six thiodiketopiperazine-type alkaloids, were isolated from fungus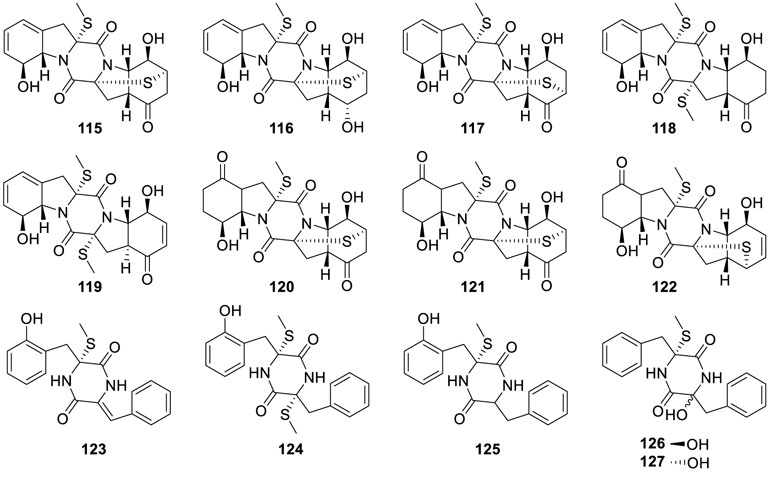
Eutypella sp. MCCC 3A00281. Among them, eutypellazines N−P (128−130) contained a special ring structure, while eutypellazines N–O (128–129) possessing a spirocyclic tetrahydrobenzothiophene motif were a new case of fungus. Furthermore, the inhibitory effects of eutypellazines P−S (130−133) against vancomycin-resistant enterococci indicated them as potential drug-resistant pathogens with MIC values of 32, 16, and 32 μM, respectively.
The marine-derived fungus Eutypella scoparia FS26, from the South China Sea, yielded two polyketides 7,8-dihydroxy-3 5,7-trimethyl-8,8a-dihydro-1H-isochromen-6 (7H)-one (134) and 6-(hydroxymethyl)-2,2-dimethyl-3,4-dihydro-2H-chromene-3,4-diol (135) (Sun et al., 2013). Three benzo[c]oxhepteng polyketides were isolated from Eutypella sp. D-1 and identified as cladoacetal C (136), benzophomopsin A (137), and peastalospirane B (138) (Yu et al., 2020). Among them, cladoacetal C (136) was a new compound and peastalospirane B (138) exerted moderate cytotoxicity towards PANC-1 and SW1990 cells with IC50 values of 13.4 and 10.3 μM, respectively. Subsequent fractionation of the leaves of Leptospermum brachuandrum endophytic fungus Eutypella scoparia SCBG-8 led to the identification of six new polyketides eutyscoparols A–F (139–144), together with a new natural product eutyscoparol G (145) (Zhang et al., 2020).
In addition to the three types of metabolites summarized above, many scholars discovered some other types of compounds from the genus Eutypella, including three benzopyran derivatives (146–148) (Ciavatta et al., 2008; Liao et al., 2017), four lactones (149–152) (Isaka et al., 2009; Zhang et al., 2021b), and a steroid (153) (Zhang et al., 2021a).
Research on a strain of Eutypella sp. ICB-OBX resulted in the discovery of novel benzopyran derivatives cytosporin D (146) and cytosporin E (147) (Ciavatta et al., 2008) from the marine pulmonate mollusc Onchidium sp. Most significantly, cytosporin E (147) was identified as containing an unusual cyclic carbonate functionality by an analysis of 2D-NMR techniques and modified Mosher’s method. Investigation on the fungus Eutypella sp. originated from gorgonian corals; one novel enzopyran derivative cytosporin L (148) and two known derivatives cytosporin D (103) and cytosporin E (147) (Liao et al., 2017) were separated and purified. Furthermore, all of them were evaluated for their antiviral activities; the research showed that cytosporin D (103) and cytosporin L (148) were able to obviously inhibit the respiratory virus (RSV) with IC50 values of 30.2 and 72.0 μM, respectively.
Two lactones, eutypellins A and B (149 and 150) (Isaka et al., 2009), were separated from the endophyte Eutypella sp. BCC 13199, of which eutypellin A (149) was able to inhibit proliferation of human small-cell lung cancer cells NCI-H187 with an IC50 value of 12 μM. Eutypella scoparia SCBG-8 produced two lactones eutyscoparols H–I (151–152) (Zhang et al., 2021b). In addition, both showed weak activities against HeLa, MDA-MB-231, and MCF-7 tumor cells.
Based on the antibacterial activity, 11 compounds were isolated from Eutypella sp. SCBG-8. Among them, eutyscoparene A (153) (Zhang et al., 2021a) was identified as a new steroid.
This review provides a comprehensive overview of the diverse chemical structures and bioactive properties of new metabolites that have been isolated from the fungi Eutypella in the last 20 years. Up to 141 new compounds have been discovered and 12 known compounds were first isolated from the 10 Eutypella fungal strains. Among 141 new compounds, terpenoids accounted for 68.09%, which were further divided into monoterpenes (2, 1.42%), sesquiterpenes (61, 43.26%), diterpenes (28, 19.86%), and meroterpenoids (5, 3.55%), and the rest were composed of alkaloids (19.15%), polyketides (7.09%), and others (5.67%) (Figure 1). According to the data above, terpenoids comprised the highest proportion of all metabolites. Simultaneously, the antiproliferative (64.89%), anti-inflammatory (19.15%), and antibacterial activities (13.83%) were the top three ranked in biological activity, followed by antiviral (2.13%) activity (Figure 2). In particular, the antiproliferative activity might be the main pharmacological value for the genus Eutypella, and we also found that the alkaloids and diterpenes contributed to the antiproliferative activity (37.7% and 31.1%, respectively). Secondly, the sesquiterpenes played a key role in the anti-inflammatory activity. Thus, due to the chemical diversity and medicinal values of the metabolites from the fungi Eutypella, it is worth studying the fungi further to find the promising lead compounds for the development of future drug discovery.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. YZ was responsible for designing, searching and writing literature, and JH was responsible for statistics and writing literature.
The project was funded by the State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources (Guangxi Normal University) CMEMR2021-B09.
The authors thank the System Resource of Tianhe-2 of National Supercomputer Center in Guangzhou for its generous support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.1074135/full#supplementary-material
Acero F. J., González V., Sánchez-Ballesteros J., Rubio V., Checa J., Bills G. F., et al. (2017). Molecular phylogenetic studies on the diatrypaceae based on rDNA-ITS sequences. Mycologia 96 (2), 249–259. doi: 10.1080/15572536.2005.11832975
Brglez A., Piškur B., Ogris N. (2020). In vitro interactions between Eutypella parasitica and some frequently isolated fungi from the wood of the dead branches of young sycamore maple (Acer pseudoplatanus). Forests 11 (10), 1072. doi: 10.3390/f11101072
Ciavatta M. L., Lopez-Gresa M. P., Gavagnin M., Nicoletti R., Manzo E., Mollo E., et al. (2008). Cytosporin-related compounds from the marine-derived fungus Eutypella scoparia. Tetrahedron 64 (22), 5365–5369. doi: 10.1016/j.tet.2008.03.016
Da Silva M., Passarini M. R., Bonugli R. C., Sette L. D. (2008). Cnidarian-derived filamentous fungi from Brazil: Isolation, characterisation and RBBR decolourisation screening. Environ. Technol. 29 (12), 1331–1339. doi: 10.1080/09593330802379466
Deng Y., Wang C., Liu Y., Chen P., Lin X., Zhang Y. (2019). The first demonstration of a novel isolated fungus Eutypella sp. BJ associated with the biodegradation of polyvinyl alcohol. RSC. Adv. 9 (47), 27398–27405. doi: 10.1039/c9ra04410h
Isaka M., Palasarn S., Lapanun S., Chanthaket R., Boonyuen N., Lumyong S. (2009). Gamma-lactones and ent-eudesmane sesquiterpenes from the endophytic fungus Eutypella sp. BCC 13199. J. Nat. Prod. 72 (9), 1720–1722. doi: 10.1021/np900316x
Isaka M., Palasarn S., Prathumpai W., Laksanacharoen P. (2011). Pimarane diterpenes from the endophytic fungus Eutypella sp. BCC 13199. Chem. Pharm. Bull. (Tokyo). 59 (9), 1157–1159. doi: 10.1248/cpb.59.1157
Kongprapan T., Rukachaisirikul V., Saithong S., Phongpaichit S., Poonsuwan W., Sakayaroj J. (2015). Cytotoxic cytochalasins from the endophytic fungus Eutypella scoparia PSU-H267. Phytochem. Lett. 13, 171–176. doi: 10.1016/j.phytol.2015.06.010
Kuriakose G. C., Palem P. P., Jayabaskaran C. (2016). Fungal vincristine from Eutypella spp - CrP14 isolated from Catharanthus roseus induces apoptosis in human squamous carcinoma cell line -A431. BMC Complement. Altern. Med. 16 (1), 302. doi: 10.1186/s12906-016-1299-2
Lee J. B., Park S. Y., Shin K. S., Jeon C. P., Kim J. E., Kwon G. S. (2011). Biodegradation of organochlorine insecticide endosulfan by the fungus Eutypella sp. KEF-1. Korean. J. Environ. Agric. 30 (2), 202–208. doi: 10.5338/kjea.2011.30.2.202
Liao H. X., Sun D. W., Zheng C. J., Wang C. Y. (2017). A new hexahydrobenzopyran derivative from the gorgonian-derived fungus Eutypella sp. Nat. Prod. Res. 31 (14), 1640–1646. doi: 10.1080/14786419.2017.1285301
Liu J. T., Hu B., Gao Y., Zhang J. P., Jiao B. H., Lu X. L., et al. (2014a). Bioactive tyrosine-derived cytochalasins from fungus eutypella sp. d-1. Chem. Biodivers. 11 (5), 800–806. doi: 10.1002/cbdv.201300218
Liu J. T., Lu X. L., Zheng H., Liu X. Y., Jiao B. H. (2014b). Pimarane diterpenes from Arctic fungus Eutypella sp. d-1. Nat. Prod. Res. Dev. 26 (01), 56–59. doi: 10.16333/j.1001-6880.2014.01.030
Liu H., Zhang L., Chen Y., Li S., Tan G., Sun Z., et al. (2017a). Cytotoxic pimarane-type diterpenes from the marine sediment-derived fungus eutypella sp. FS46. Nat. Prod. Res. 31 (4), 404–410. doi: 10.1080/14786419.2016.1169418
Liu H. X., Zhang L., Chen Y. C., Sun Z. H., Pan Q. L., Li H. H., et al. (2017b). Monoterpenes and sesquiterpenes from the marine sediment-derived fungus Eutypella scoparia FS46. J. Asian Nat. Prod. Res. 19 (2), 145–151. doi: 10.1080/10286020.2016.1189906
Lu X. L., Liu J. T., Liu X. Y., Gao Y., Zhang J., Jiao B. H., et al. (2014). Pimarane diterpenes from the Arctic fungus eutypella sp. d-1. J. Antibiot. (Tokyo). 67 (2), 171–174. doi: 10.1038/ja.2013.104
Mao Z., Zhang W., Wu C., Feng H., Peng Y., Shahid H., et al. (2021). Diversity and antibacterial activity of fungal endophytes from Eucalyptus exserta. BMC Microbiol. 21 (1), 155. doi: 10.1186/s12866-021-02229-8
Moyo P., Mostert L., Bester M., Halleen F. (2016). Trunk disease fungi associated with diospyros kaki in south Africa. Plant Dis. 100 (12), 2383–2393. doi: 10.1094/pdis-02-16-0245-re
Niu S., Liu D., Shao Z., Liu J., Fan A., Lin W. (2021). Chemical epigenetic manipulation triggers the production of sesquiterpenes from the deep-sea derived Eutypella fungus. Phytochemistry 192, 112978. doi: 10.1016/j.phytochem.2021.112978
Niu S., Liu D., Shao Z., Proksch P., Lin W. (2017a). Eutypellazines a–m, thiodiketopiperazine-type alkaloids from deep sea derived fungus Eutypella sp. MCCC 3A00281. RSC. Adv. 7 (53), 33580–33590. doi: 10.1039/c7ra05774a
Niu S., Liu D., Shao Z., Proksch P., Lin W. (2017b). Eutypellazines n–s, new thiodiketopiperazines from a deep sea sediment derived fungus Eutypella sp. with anti-VRE activities. Tetrahedron. Lett. 58 (38), 3695–3699. doi: 10.1016/j.tetlet.2017.08.015
Niu S., Liu D., Shao Z., Proksch P., Lin W. (2018). Eremophilane-type sesquiterpenoids in a deep-sea fungus Eutypella sp. activated by chemical epigenetic manipulation. Tetrahedron 74 (51), 7310–7325. doi: 10.1016/j.tet.2018.10.056
Palem P. P., Kuriakose G. C., Jayabaskaran C. (2015). An endophytic fungus, Talaromyces radicus, isolated from Catharanthus roseus, produces vincristine and vinblastine, which induce apoptotic cell death. PloS One 10 (12), e0144476. doi: 10.1371/journal.pone.0144476
Pongcharoen W., Rukachaisirikul V., Phongpaichit S., Rungjindamai N., Sakayaroj J. (2006). Pimarane diterpene and cytochalasin derivatives from the endophytic fungus Eutypella scoparia PSU-D44. J. Nat. Prod. 69 (5), 856–858. doi: 10.1021/np0600649
Qi S., Wang Y., Zheng Z., Xu Q., Deng X. (2015). Cytochalasans and sesquiterpenes from Eutypella scoparia 1-15. Nat. Prod. Commun. 10 (12), 2027–2030. doi: 10.1177/1934578X1501001203
Sun L., Li D. L., Chen Y. C., Tao M. H., Dan F. J., Zhang W. M. (2011). Purification, identification and antitumor activities of secondary metabolites from marine fungus Eutypella scoparia FS26. Chin. Tradit. Herbal. Drugs 42 (03), 432–436.
Sun L., Li D., Tao M., Chen Y., Dan F., Zhang W. (2012b). Scopararanes c-G: New oxygenated pimarane diterpenes from the marine sediment-derived fungus Eutypella scoparia FS26. Mar. Drugs 10 (3), 539–550. doi: 10.3390/md10030539
Sun L., Li D., Tao M., Chen Y., Zhang Q., Dan F., et al. (2013). Two new polyketides from a marine sediment-derived fungus Eutypella scoparia FS26. Nat. Prod. Res. 27 (14), 1298–1304. doi: 10.1080/14786419.2012.733393
Sun L., Li D. L., Tao M. H., Dan F. J., Zhang W. M. (2012a). Two new sesquiterpenes from the marine fungus Eutypella scoparia FS26 from the south China Sea. Helv. Chim. Acta 95 (1), 157–162. doi: 10.1002/hlca.201100275
Wang X., Sun K., Wang B. (2018). Bioactive pimarane diterpenes from the Arctic fungus Eutypella sp. d-1. Chem. Biodivers. 15 (2), e1700501. doi: 10.1002/cbdv.201700501
Wang Y., Wang Y., Wu A. A., Zhang L., Hu Z., Huang H., et al. (2017). New 12,8-eudesmanolides from eutypella sp. 1-15. J. Antibiot. (Tokyo). 70 (10), 1029–1032. doi: 10.1038/ja.2017.89
Yu H. B., Wang X. L., Hu B., Zhang J. P., Lu X. L., Liu X. Y., et al. (2020). Studies on bioactive components of the Arctic fungus eutypella sp. d-1. Chin. J. Mar. Drug 39 (02), 38–41. doi: 10.13400/j.cnki.cjmd.2020.02.005
Yu H. B., Wang X. L., Xu W. H., Zhang Y. X., Qian Y. S., Zhang J. P., et al. (2018a). Eutypellenoids A-C, new pimarane diterpenes from the Arctic fungus eutypella sp. d-1. Mar. Drugs 16 (8), 284. doi: 10.3390/md16080284
Yu H. B., Wang X. L., Zhang Y. X., Xu W. H., Zhang J. P., Zhou X. Y., et al. (2018b). Libertellenones O-s and eutypellenones a and b, pimarane diterpene derivatives from the Arctic fungus eutypella sp. d-1. J. Nat. Prod. 81 (7), 1553–1560. doi: 10.1021/acs.jnatprod.8b00039
Zhang Y. X. (2019). Reasesrches on the secondary metabolites of fungus eutypella sp. d-1 from high latitudes area of the Arctic (Shanghai, China: Master, Naval Medical University).
Zhang L. Q., Chen X. C., Chen Z. Q., Wang G. M., Zhu S. G., Yang Y. F., et al. (2016). Eutypenoids a-c: Novel pimarane diterpenoids from the Arctic fungus eutypella sp. d-1. Mar. Drugs 14 (3), 14. doi: 10.3390/md14030044
Zhang W., Lu X., Huo L., Zhang S., Chen Y., Zou Z., et al. (2021a). Sesquiterpenes and steroids from an endophytic Eutypella scoparia. J. Nat. Prod. 84 (6), 1715–1724. doi: 10.1021/acs.jnatprod.0c01167
Zhang W., Lu X., Wang H., Chen Y., Zhang J., Zou Z., et al. (2021b). Antibacterial secondary metabolites from the endophytic fungus Eutypella scoparia SCBG-8. Tetrahedron. Lett. 79, 153314. doi: 10.1016/j.tetlet.2021.153314
Zhang W., Wang M., Zhang S., Xu K., Tan G., Qiu S., et al. (2020). Eutyscoparols a-G, polyketide derivatives from endophytic fungus Eutypella scoparia SCBG-8. Fitoterapia 146, 104681. doi: 10.1016/j.fitote.2020.104681
Zhang Y. X., Yu H. B., Xu W. H., Hu B., Guild A., Zhang J. P., et al. (2019). Eutypellacytosporins a-d, meroterpenoids from the Arctic fungus eutypella sp. d-1. J. Nat. Prod. 82 (11), 3089–3095. doi: 10.1021/acs.jnatprod.9b00700
Zhang W., Zhu Y., Yu H., Liu X., Jiao B., Lu X. (2021c). Libertellenone h, a natural pimarane diterpenoid, inhibits thioredoxin system and induces ROS-mediated apoptosis in human pancreatic cancer cells. Molecules 26 (2), 315. doi: 10.3390/molecules26020315
Keywords: fungi, Eutypella sp., terpenoids, bioactive metabolites, antiproliferative (cytotoxic) activity
Citation: Zhou Y, He J and Cui H (2022) Terpenoids and other secondary metabolites produced by the Eutypella fungi and their bioactivities. Front. Mar. Sci. 9:1074135. doi: 10.3389/fmars.2022.1074135
Received: 19 October 2022; Accepted: 24 November 2022;
Published: 13 December 2022.
Edited by:
Ling Liu, Institute of Microbiology (CAS), ChinaCopyright © 2022 Zhou, He and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Cui, Y3VpaHVpQGd6dWNtLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.