- 1Department of Biology, National Changhua University of Education, Changhua, Taiwan
- 2Graduate Program of Biotechnology, National Changhua University of Education, Changhua, Taiwan
- 3Land Crabs Ecology Research Laboratory, Jubei, Taiwan
Developmental profile of the female reproductive apparatus (the externa) of Polyascus planus parasitizing the thukuhar shore-crab Metopograpsus thukuhar is described. The development is divided into 4 stages, which are named based on the distinct color of the externa at each stage. The effects of parasitism on the gonad and hepatopancreas of the host were then examined. The ovary was heavily infiltrated by the rootlet of the parasite, with the oocytes being arrested at the pre-vitellogenic state, whereas the testis, not being infiltrated, still contained abundant amounts of spermatozoa. The hepatopancreas of the hosts of both sexes was heavily infiltrated by the rootlets. Regarding the synthesis of vitellogenin (Vg), the hemolymph precursor of the major yolk protein (vitellin, Vn), expression of host Vg gene was detected in the ovary and hepatopancreas of both non-parasitized and parasitized females, and, importantly, its expression found to be induced in the hepatopancreas of parasitized males. Furthermore, results of quantitative gene expression assays showed that expression levels of the host Vg in the ovary and hepatopancreas did not change significantly among the various parasitization status (i.e., host bearing externa of different developmental stages), nor did those of the parasite Vg in the externa among the developmental stage of the externa. The Vg expression levels in the hepatopancreas of parasitized males and in the externa were however negatively correlated. Finally, analysis of tryptic peptide fragments derived from hemolymph proteins of high molecular weight in the parasitized males confirmed the induction of synthesis of the host Vg, whereas analysis of externa proteins of similar molecular weight revealed that Vg of both host and parasite origins contributes to the formation of parasite Vn. The combined results indicate that the parasitized hosts, regardless of sex, synthesize Vg for the ovarian maturation of the parasite, to which Vg of parasite origin also contributes. Discussion is also made with regard to the strategy the parasite adopts toward hosts of different sexes. The profile of the development of externa established would serve as a temporal framework against which studies of host/parasite interaction can be conducted in a developmental context.
Introduction
Parasitism occurred independently multiple times in various major taxa during the course of evolution, hence is a biological phenomenon existing in a wide variety of organisms (Poulin, 2011; Poulin and Randhawa, 2015). Phenotypes of the host are often altered by the parasite − on behavioral, physiological, or morphological level − in order for the parasite to successfully complete its life cycle. Many of the host/parasite system provide intriguing models for the study of mechanism of host control, offering insight into adaptation to parasitism (Thomas et al., 2005; Biron and Loxdale, 2013; Poulin and Randhawa, 2015).
Rhizocephala, an infraclass of Cirripedia, comprises a taxon of highly evolved and specialized barnacles that mainly parasitize decapod hosts. While its planktonic larvae are similar to those of other cirripedes, the rhizocephalan′s life cycle also includes both endo- and ecto-parasitic stages (Høeg, 1995; Høeg et al., 2020). The female cypris larva, which in many rhizocephalans metamorphoses from naupliar larva, settles on a potential host. For akentrogonid rhizocephalans, the parasitic tissue is introduced into the host through penetration of the cyprid antennule, whereas for kentrogonid rhizocephalans, the settled cyprids metamorphose into a kentrogon, which injects the parasite into the host via a hollow cuticular structure (the stylet) (see Høeg, 1995; Walker, 2001; Høeg et al., 2020).
In several species examined, early stages of the inoculated parasite that have been observed have already differentiated into an epithelium-enclosed tumor containing a mass of cells (the nucleus) (see Høeg, 1995; Høeg and Lützen, 1995). During the internal phase of growth, the epithelium develops into a ramifying internal root system, the interna, which serves to absorb nutrients from the host and is found infiltrating various host tissues (Glenner and Høeg, 1995; Glenner et al., 2000; Glenner, 2001; Bortolini and Alvarez, 2008; Miroliubov et al., 2020; Rowley et al., 2020). The nucleus develops into the visceral mass and the mantle cavity of the externa, the reproductive organ of the parasite. Eventually, the externa, still being connected with the interna via a stalk, emerges typically on the ventral surface of the abdomen of the host, beginning the ecto-parasitic stage of the life cycle (Høeg, 1995; Høeg and Lützen, 1995).
Anatomical and functional observations of the externa have been described and summarized (see Høeg, 1995; Høeg and Lützen, 1995). The visceral mass contains the ovary and the colleteric gland, through the lumens of which mature oocytes are oviposited into the mantle cavity, a chamber where the developing embryos are brooded (Lange, 2002). In most rhizocephalans, externae receive implantation of male larvae shortly after the externa emerges. The implantation is performed by a male larval instar (the trichogon) metamorphosed from the cyprids promptly after they settle around the mantle opening of the emerged externa, through which trichogons enter the externa, move through the mantle cavity, and finally reside inside the lumen of the male receptacle (Høeg, 1987). These males are parasitic males existing as sperm-producing seminiferous tissue in the receptacle and receiving nourishment from the female (Høeg, 1995; Høeg and Lützen, 1995). More recent reports on the anatomy of the externa are available (Korn et al., 2004; Alvarez et al., 2010; Nour Eldeen et al., 2019; Golubinsykaya et al., 2021) including some that emphasize on the developmental profile of the externa.
For oviparous animals, vitellogenesis, the process of egg yolk formation, is critical for ovarian maturation. It is known that in crustaceans vitellogenesis is tightly controlled by regulatory factors (see Meusy, 1980; Jayasankar et al., 2020). Vitellogenin (Vg), a female-specific hemolymph protein, is the precursor to the major yolk protein vitellin (Vn). In crustaceans, Vg is synthesized by the hepatopancreas, ovary, or both, released into hemolymph, and sequestered by developing oocytes, where it is processed to Vn (see Meusy, 1980; Jayasankar et al., 2020).
We are intrigued by the complicated and diverse life cycles of rhizocephalans and interested in elucidating the mechanism of control of host by these parasitic barnacles. As oviparous animals, female rhizocephalans undergo the process of vitellogenesis in the ovarian tissues, when the externa is developing. However, it has not yet been studied for any rhizocephalan in terms of interaction between the host and parasite during vitellogenesis of the parasite. Interesting issues include at least the following. From which source (host, parasite itself, or both) a rhizocephalan acquires Vg for its ovarian maturation? What are the effects of plundering Vg of host origin by the parasite, if occurs, on the gonadal development of the host?
Sacculina plana is an abundant species in Taiwan waters that infects several brachyurans (Huang and Lutzen, 1998; Liu and Lützen, 2000). It has been reassigned to the genus Polyascus (Glenner et al., 2003) and its species name recently been altered – P. planus – to comply with the masculine gender of the genus name (Høeg et al., 2020; WoRMS, 2022). Development and sex ratio of its larvae (Tu et al., 2009) and effects of the parasitism on the metabolic profile of its hosts (Hsiao et al., 2016) have been reported. In the present study, we first established a developmental profile of the externa of the P. planus parasitizing on one of its brachyuran hosts, the thukuhar shore-crab Metopograpsus thukuhar. The effects of parasitism by the P. planus on the host tissues were also examined. Further, we asked, for the maturation of the externa, whether Vg comes from the host, the parasite itself, or both, and whether the degree of contribution of host or parasite Vg varies with the developmental stage of the externa. Finally, if Vg of the host origin is required for the maturation of the externa, will the male host be manipulated (feminized) to synthesize it?
Materials and methods
Animals, determination of parasitization status and of duration time of developmental stages
Collection and rearing of the host animal (Metopograpsus thukuhar Owen, 1839) had been described previously (Hsiao et al., 2016). Adult animals with a carapace width larger than 2 cm were used for the experiments described in the present study. Animals that are assigned as ‘non-parasitized’ are those not bearing any externa and tested negative by a polymerase chain reaction (PCR) amplifying the parasite 18S rRNA gene in the host thoracic ganglia; those tested positive by the 18S rRNA gene-PCR, but not bearing any externa, are assigned as ‘internally parasitized’ (Hsiao et al., 2016). Animals that bear externae on the abdomen are assigned as ‘externally parasitized’. To minimize possible interactions among the externae on the same hosts, data described in this study were those derived from the hosts bearing one single externa only.
To monitor changes in the color of the externa and to determine the mean duration time of each developmental stage (see Figure 1), experimental animals were individually reared and checked at least twice a day for external changes of the externa and (for internally parasitized hosts) emergence of the externa. The duration time (in day) each developmental stage of the externa lasts is, for the Creamy stage, defined as from the day of emergence (day 1) to the day before reaching the Orange stage, and for the other stages (i.e., Orange, Yellow, and Brown stages), the day reaching the stage to the day before entering the next stage. As externae emerged from internally parasitized hosts reared individually can only develop to the Orange stage but fail to develop beyond that stage, the mean duration time for the Orange, Yellow, and Brown stages were determined using host animals already bearing externa when collected from the field.
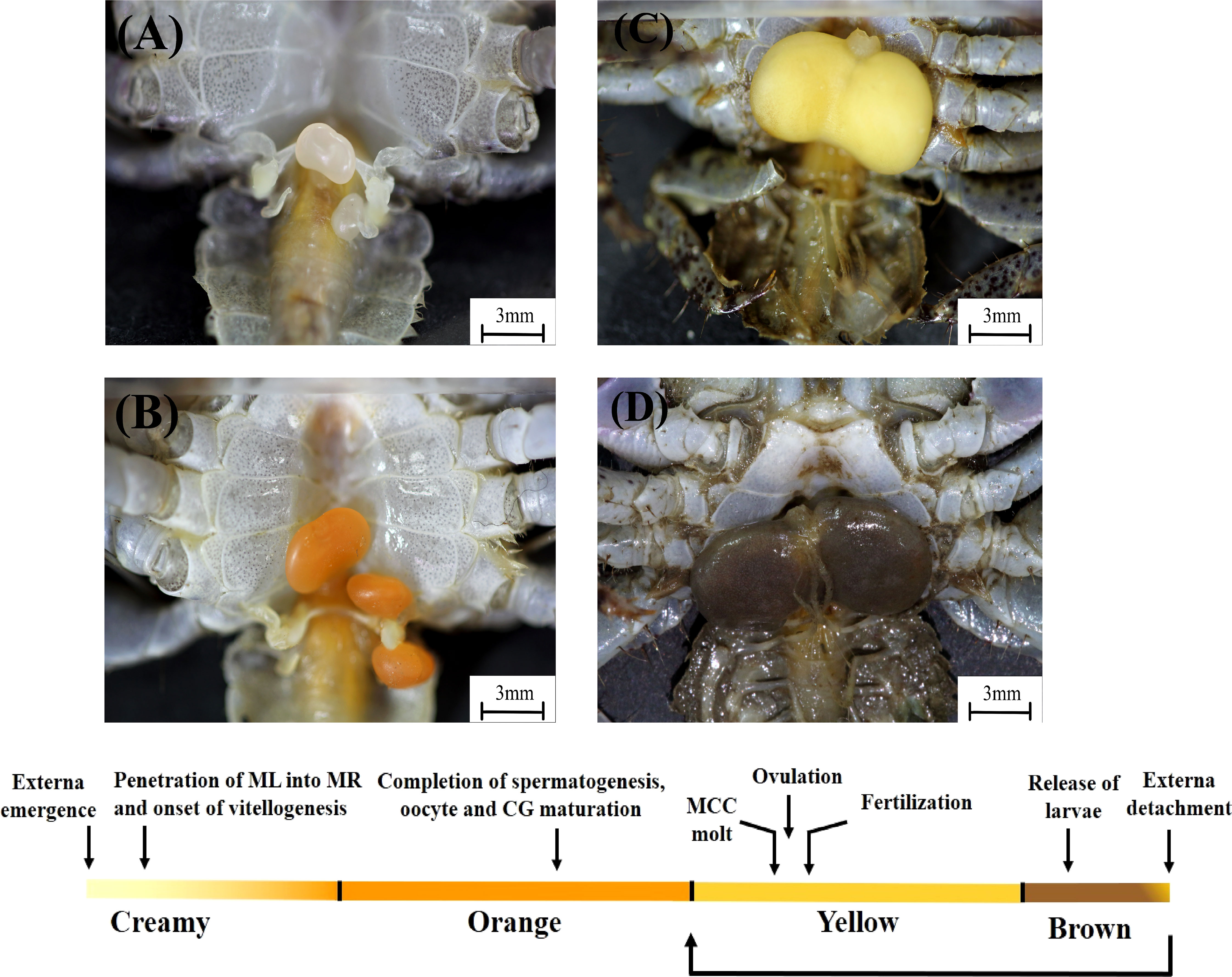
Figure 1 Development of the externa of Polyascus planus parasitizing the thukuhar shore-crab (Metopograpsus thukuhar). Upper: Development of the externa, which emerges on the ventral side of the host abdomen, is accompanied by distinct changes in the coloration of the externa, from creamy (A), orange (B), yellow (C), to brown (D). Lower: Development of the externa is divided into 4 stages, the Creamy, Orange, Yellow, and Brown, based on the color of the tissue, allowing determination of its developmental status based on an external trait. Developmental milestones (arrows) are given, with a caveat that they are intended to show the temporal order of the developmental events, but not meant to show that the events occur precisely at the time point indicated. Note that, after the release of larvae, brown externae could return to stage Yellow or become detached from the host. See text and Figures 2–5 for detailed descriptions of the development. CG, colleteric gland; ML, male larva; MCC, mantle cavity cuticle; MR, male receptacle.
Tissues sampling and processing
Animals were anesthetized with cold seawater before experimental procedures and sampling. Hemolymph was withdrawn from the anesthetized animals using syringes coupled to a 29-gauge needle, diluted 1:1 (V:V) with an anti-coagulant (0.01 M EDTA in Pantin’s crab saline, pH 7.6, Pantin, 1934), centrifuged at 16,000g for 20 min at 4°C. The resulting supernatant was collected, mixed 1:1 (V:V) with an extraction buffer (0.02M Tris-HCl, pH 7.6) containing a cocktail of serine and cysteine protease inhibitors (cOmplete™, Roche), and stored at -80°C until used for protein gel electrophoresis. Other tissues, including the gonad, thoracic ganglia, hepatopancreas, and if present, the externae, were promptly dissected out under stereoscopes (E24, Leica) using fine iris scissors and forceps (Fine Science Tools Inc.).
Depending on the purposes of the experiment, tissues were fixed in 10% neutral formalin at room temperature (for histological processing) or preserved in RNAlater (Invitrogen) at -80°C (for quantification of gene expression) until subsequent processing, or immediately homogenized in the extraction buffer containing proteinase inhibitors, and centrifuged at 16,000g for 20 min at 4°C, followed by collection of the supernatants and preservation at -80°C until used for protein gel electrophoresis.
In addition, to calculate the relative tissue weight (the tissue somatic index), host animals and freshly dissected gonad and hepatopancreas were weighed (AB104-S, Mettler Toledor). The tissue weight is expressed as a percentage of body weight, i.e., the tissue somatic index = (tissue weight in g/body weight in g) X 100.
Histology and histological observations
Animals used for histological study were field-collected animals reared and checked as described in 2.1. Externa and host tissues were dissected from animals with desired stages of externa development (see below). Tissue processing, sectioning, and staining were performed at the Rapid Science Co. Ltd (Taiwan). Briefly, tissues were fixed in 10% neutral formalin for 48 hours, dehydrated through an alcohol series (70, 80, 95, 100%), cleared through a xylene series (25, 50, 75, 100%) using an automated vacuum processor (ASP300 S, Leica), embedded in paraffin (HistoCore Arcadia, Leica), sectioned longitudinally at 3μm (Biocut, Leica), de-paraffinated, rehydrated, stained with hematoxylin/eosin Y, and coverslipped using a workstation including Autostainer XL ST5010, Transfer Station TS5015, and Coverslipper CV5030 (Leica).
Histological observations and measurements were done and imagines taken under light microscopes (DM 500, Leica) with a software platform Application Suite X (Leica). Histological observations described in the present study were based on the examination of serial tissue sections derived from at least 5 animals/developmental stage (see Figure 1), chosen from various portions of the stage. For the measurement of the diameter of parasite’s oocytes in the externae at the beginning of each developmental stage, the diameter of 100 most developmentally advanced oocytes in randomly selected visual fields/animal was measured and the mean oocyte diameter calculated.
Detection and quantification of gene expression of vitellogenin in host and parasite tissues
A reverse transcription-PCR assay was used to examine vitellogenin (Vg) gene expression in the hepatopancreas, ovary, or both in the parasitized and non-parasitized crabs. Total RNA was extracted (Direct-zol RNA Miniprep Kits, Zymo Research) from the tissues and reverse transcribed (HiScript I™ First Strand cDNA Synthesis Kit, Bionovas) according to the manufacturers′ protocols. A primer pair (Met-vg-qF1and Met-vg-qR1) was designed to amplify a 136–bp DNA fragment of the host Vg (accession number OP556484); another pair of primers (Met-beta-actin-F and Met-beta-actin-R) was designed to amplify a 193–bp DNA fragment of the host actin, which serves as the internal control (accession number OP556483) (Supplementary Table 1). PCR reactions contained 0.5 μl of the reverse transcription (RT) reaction, 0.2 mM each of dNTPs, 0.2μM each of primers, 1.25 U of GoTaq® G2 Flexi DNA Polymerase (Promega), and 1X reaction buffer (10 mM Tris–HCl, pH 9.0, 50 mM KCl, 0.1% Triton X-100, 2 mM MgCl2). The final volume was adjusted to 25μl with sterile distilled water. PCRs were performed using a DNA Thermal Cycler (MJ Research) under the following parameters: an initial denaturation (5 min, 94°C), 30 cycles of denaturation (30 s, 94°C), annealing (30 s, 54°C), and extension (30 s, 72°C), followed by a final extension (3 min, 72°C). After PCR, an aliquot of the reaction was separated on a 2.0% agarose gel and visualized with GelStar® (Cambrex). Reagents and procedures used for purification and auto-sequencing of the PCR products, as well as software resources for sequence analyses, had been described by Chen et al. (2007).
Vitellogenin gene expression in host tissues (the hepatopancreas and ovary) and in the externa was quantified using reverse transcription real-time PCR assays. RNA extraction and treatment, and reverse transcription reaction were performed on individual tissues as described above. For real-time PCR, cDNA templates were amplified in a 20-μl reaction using a commercially available kit (QuantiNova® SYBR® Green PCR Kit, QIAGEN) according to the manufacture’s protocol on a Rotor-Gene Q thermal cycler (QIAGEN). Reactions were performed under the following conditions: an initial denaturation (10 min, 94°C), 50 cycles of denaturation (10 s, 94°C), annealing (7 s, 59°C), and extension (16 s, 72°C); the rate of temperature change was 20°C/s. The primers pairs for the host Vg and actin are the same as given above. The primers pair for the parasite Vg is Sac-vg-F1/Sac-vg-R1, which was designed to amplify a 176–bp DNA fragment of the parasite Vg (accession number OP556485), whereas the primer pair for the parasite actin is EBA68842F3/EBA68842R4 designed to amplify a 238–bp DNA fragment of the parasite actin (accession number OP556486) (Supplementary Table 1).
PCR efficiency and specificity were validated as previously described (Chen et al., 2007). Briefly, sample titration curve (log dilution factor vs. threshold cycle number, Ct) for each amplicon was plotted and its slope used for calculating amplification efficiency according to Ginzinger (2002). All primers used amplified at least 90%. To ensure amplification specificity, melting curve analysis was performed from 58 to 95°C after completion of each PCR. The comparative threshold cycle method, also known as 2-ΔΔCt method (Livak and Schmittgen, 2001; Schmittgen and Livak, 2008), was used to determine transcript levels of the target genes (Vg). Briefly, transcript levels of target genes (Vg) were normalized to the corresponding endogenous reference genes (actin) according to the following equation: XN (normalized expression levels of target genes) = 2-ΔCt; ΔCt = (Ct,t - Ct,r), where Ct,t and Ct,r represent Ct of target and reference genes, respectively. Values of XN were used for statistical analysis. Further, the normalized levels of target genes in each stage were expressed relative to those in a calibrator, an arbitrarily designated stage, using the equation: XN,C (normalized and calibrated expression levels) = 2-ΔΔCt; ΔΔCt = ΔCt,a – ΔCt,b where ΔCt,a and ΔCt,b represents XN of individual stage and calibrator stage, respectively.
Protein gel electrophoresis and mass spectrometric analysis of yolk proteins
Proteins in the hemolymph and ovary of host crabs and in the externa of the parasite were separated using polyacrylamide gel electrophoresis (PAGE) performed under non-dissociating conditions (Hames, 1990). Aliquots of the samples were removed and assayed for protein content using a Bio-Rad Protein Assay kit (#500-0006) and bovine serum albumin (BSA) as standard. For electrophoresis, aliquots of the samples were mixed with the sample buffer (0.125 M Tris, 20% glycerol, 0.002% bromophenol blue) and 200 μg total proteins/sample were loaded onto the wells of the gels. For the stacking gel, the total monomer concentration (%T) was 4.0%, and the ratio of crosslinker to monomer (%C) was 2.6%. Corresponding values for the separating gel were 7.0% and 2.6%. Electrophoresis was conducted on 8 x 7-cm slab gels at constant voltage (100 V, 0.025 M Tris-0.192 M glycine, pH 8.3, Mighty Small Vertical Slab Unit, Hoefer). Proteins were stained using Coomassie Brilliant Blue R-250 (Bio-Rad) (Hames, 1990).
Protein bands predicted to be the hemolymph Vg (VgH) of the host, and the yolk protein (vitellin, Vn) of the host (VnH) and the parasite (VnP) (see Supplementary Data Sheet 1) were excised from stained gels and subjected to mass spectrometric analysis at the Mithra Biotechnology Inc. Briefly, the excised gels were reduced, alkylated, and trypsin-digested as previously described (Chang et al., 2010). The digested samples were subjected to mass spectrometric analysis using a liquid chromatography-mass spectrometry system that includes a Q Exactive mass spectrometer coupled to an Ultimate 3000 RSLCnano system (Thermo Scientific) with a C18 column (Acclaim PepMap RSLC, 75 μm x 150 mm, 2 μm, 100 Å). Full MS scan was performed with the range of m/z 300-2,000, and the selected ions from MS scan were subjected to fragmentation to obtain MS/MS spectra for peptide sequencing. The resulting set of the sequences of the tryptic peptide fragments from each protein samples were blasted against the deduced Vg amino acid sequence of the Chinese mitten crab Eriocheir sinensis (accession number: MH621336), that of the parasite (P. planus) Vg (accession number: OP556485), or both, using the function of LOCAL BLAST provided by the BioEdit (v7.0.3.5) with the expectation value set at 1. The matching fragments (the hits) were then mapped onto the vitellogenin, which the fragments were blasted against.
Statistical analysis
Data are expressed as mean values ± S.D. Differences in the measured parameters among hosts of various parasitization status or among externae of various developmental stages were analyzed using one-way ANOVA (with post hoc Tukey’s pairwise comparison) (SigmaStat v. 3.5). Correlation analysis of Vg gene expression levels in the externa and host tissues was performed using the Pearson’s Product-Moment Correlation (SigmaStat v. 3.5).
Results
Development of the externa
Development of the externa, the female reproductive organ of parasitic barnacles, is described for Polyascus planus that parasitizes its brachyuran host, the thukuhar shore-crab Metopograpsus thukuhar. It is divided into four stages, according to the developmental characteristics (see descriptions below) and named based on the distinct coloration of the externa, i.e., the Creamy, Orange, Yellow, and Brown stages (Figure 1), with the mean duration time in each stage being 8.6 ± 3.9 (n = 5), 10.5 ± 4.2 (n = 6), 10.8 ± 1.3 (n = 8), 3.9 ± 0.4 (n = 8) days, respectively. The mean diameter of the developmentally most advanced germ cells in the parasite ovaries at the beginning of each stage of the externa development is, from Creamy to Brown stage, 22.6 ± 3.4, 20.0 ± 3.6, 121.4 ± 20.0, 153.1 ± 22.1 μm (n= 3 each stage), with the diameter significantly enlarges for 6 times during the transition from the Orange to Yellow stage (p < 0.001).
Histological sections of a virgin externa are shown in Figure 2. Its mantle opening, situated anteriorly of the tissue, is still obstructed by cuticular plugs. The nascent male receptacle, opposite to the mantle opening, is composed exclusively of female cells, without sign of penetration of male larvae. A pair of receptacle ducts provide passages between the male receptacle and male cavity (Figure 2A). Inside the visceral mass, the ovary is composed of ovarian lobules and inter-lobular tissues, both of which radiate from the region where the colleteric gland is located (Figures 2A, B). Enclosed within the lobules are oogonia with a basophilic nucleus and large white cytoplasm (Figure 2C). The inter-lobular tissues are mainly packed with muscle cells that are characterized by having an elongated nucleus and eosinophilic cytoplasm; follicular cells, with a round nucleus and scanty cytoplasm, are also present in inter-lobular tissues (Figure 2C). Narrow lumen of the atrium of the colleteric glands is lined by a single layer of epithelial cells (Figure 2B).
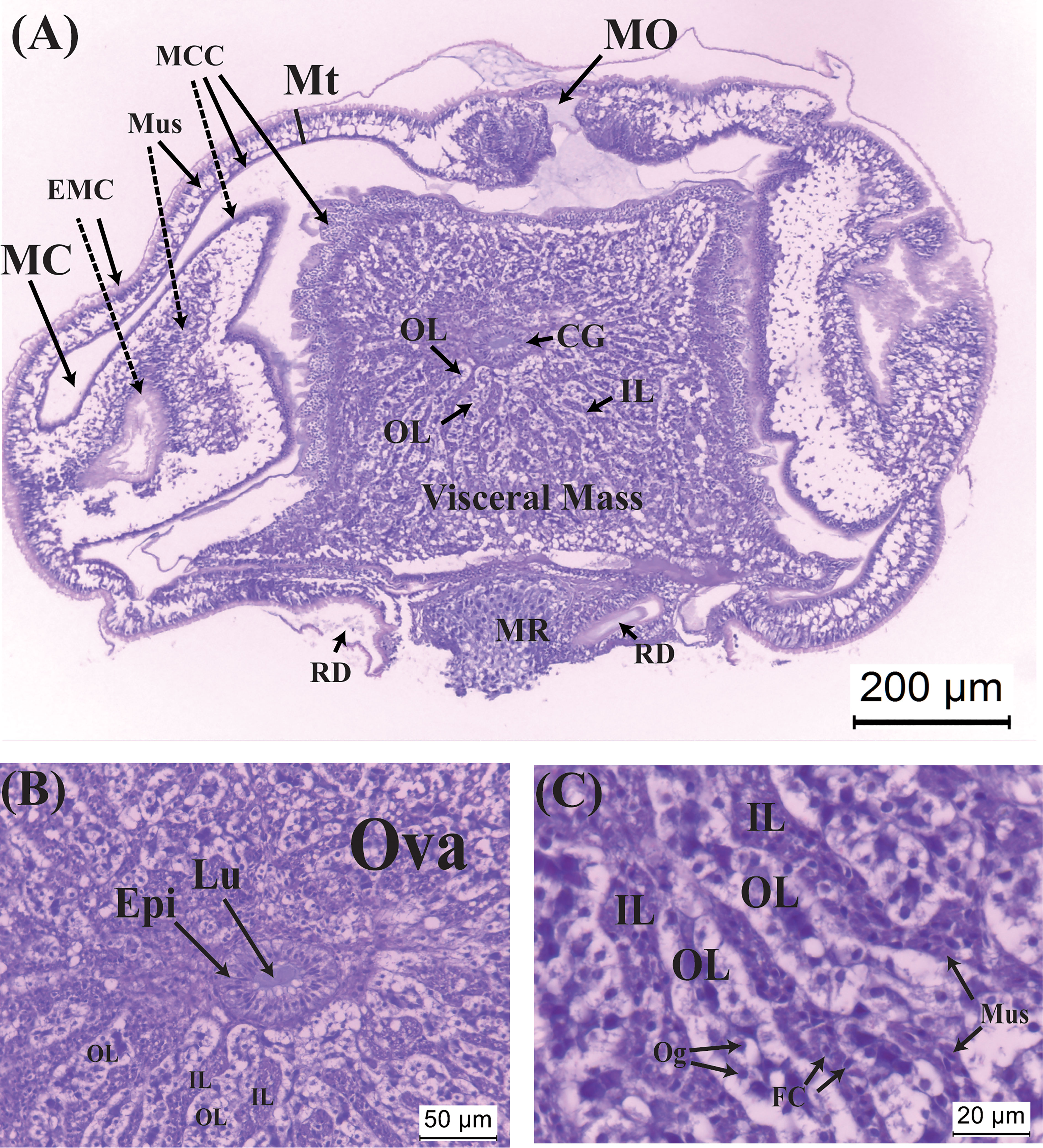
Figure 2 Histological sections of the virgin externa of Polyascus planus parasitizing the thukuhar shore-crab (Metopograpsus thukuhar). (A) A virgin externa, harvested within 12 hours of emergence, with its (B) nascent colleteric glands, and (C) ovary, which consists of ovarian lobes and inter-lobular tissues. Note that in (A) the lateral sides of the mantle invaginate toward the mantle cavity, presumably because the mantle cuticle is still soft. Dashed lines point to the invaginated areas of the mantle. CG, colleteric gland; EMC, external mantle cuticle; Epi, epithelial cells; FC, follicular cell; IL: interlobular tissue; Lu, lumen of the colleteric gland; MC, mantle cavity; MCC, mantle cavity cuticle; MO, mantle opening; Mt, mantle; Mus, muscle cell; MR, male receptacle; Og, oogonium; OL, ovarian lobe; Ova, ovary; RD, receptacle duct.
Very rapidly, within less than 2 days of emergence, as externa continues to develop, its color turns creamy. In this stage, vitellogenic oocytes, the cytoplasm of which begins accumulating eosinophilic yolk bodies, are visible inside the ovarian lobes; the muscle cells become elongated and their cytoplasm now intensely eosinophilic (Figure 3A). Penetration of male larvae into the male receptacles is already visible (Figures 3B, C). Colleteric glands begin to develop, with a central atrium and branching tubules lined with secretory epithelia (Figure 3D).
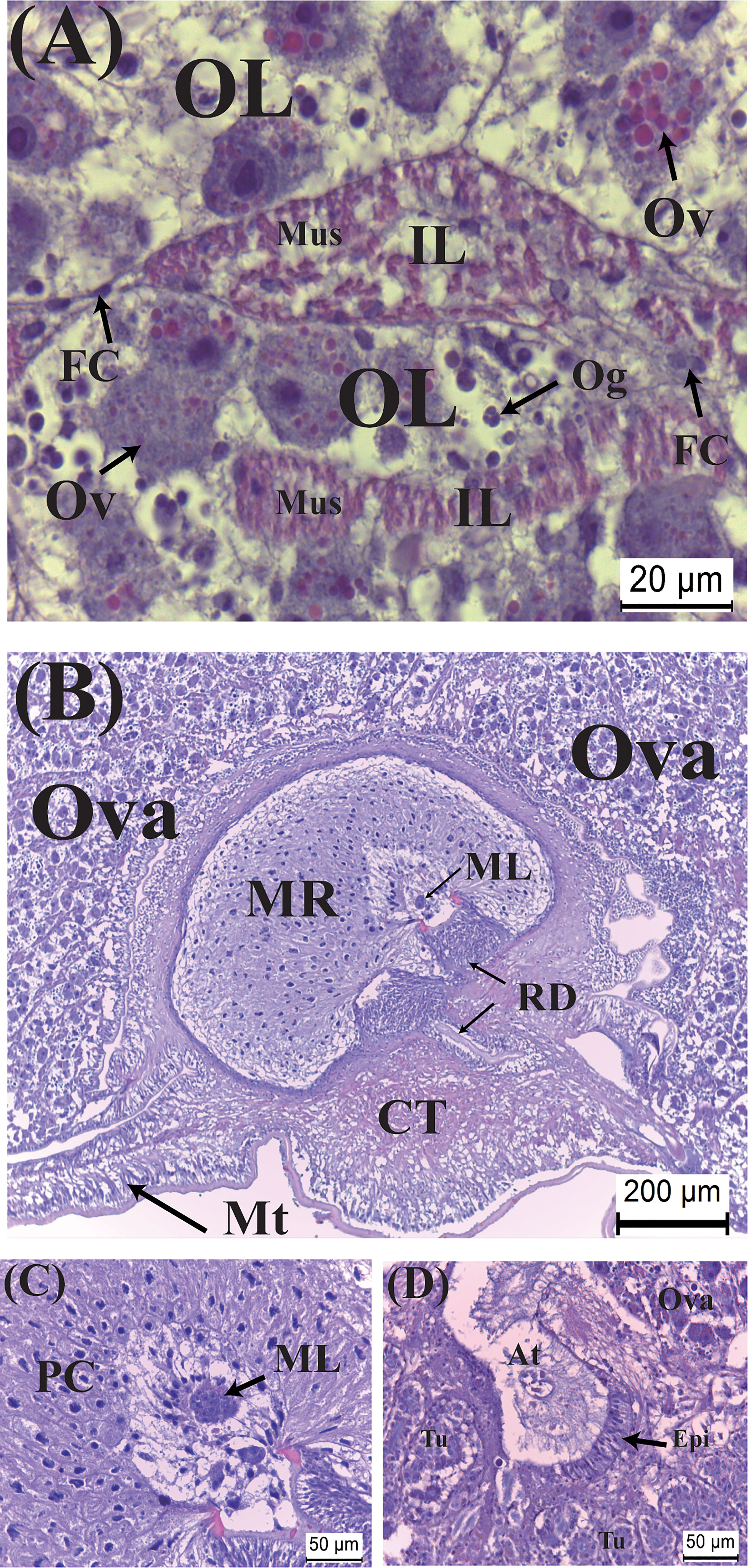
Figure 3 Developmental characteristics of the externa of Polyascus planus parasitizing the thukuhar shore-crab (Metopograpsus thukuhar) in the Creamy stage. In this stage, (A) vitellogenesis commences, with yolk granules being formed within the cytoplasm of vitellogenic oocytes, (B, C) male larvae penetrate the male receptacle, and (D) the colleteric glands begin to develop. At, atrium; CT, connective tissue; ML, male larva; Ov, vitellogenic oocyte; PC, polygonal cells; Tu, tubule. Other abbreviations are as indicated in Figure 2.
The Orange stage is characterized by marked histological changes that ultimately leads to full maturation of the externa toward the end of the stage. Initially, while vitellogenic oocytes continue to develop with bright orange yolk granules accumulating in intensely hematoxylin-stained cytoplasm, the most significant developmental event is an expansion of the interlobular tissues, with the muscle cells became more elongated and larger (Figure 4A). By the time the development passes halfway the stage, there is an abrupt and significant increase in the size of the externa, when the oocytes become fully mature with the interlobular tissues wrapping around individual oocytes (Figure 4B). Spermatogenesis is actively taking place with dividing spermatocytes lining the lumen of the male receptacle (Figure 4C). The atrium of the colleteric glands now has a large lumen lined with secretion (Figure 4D).
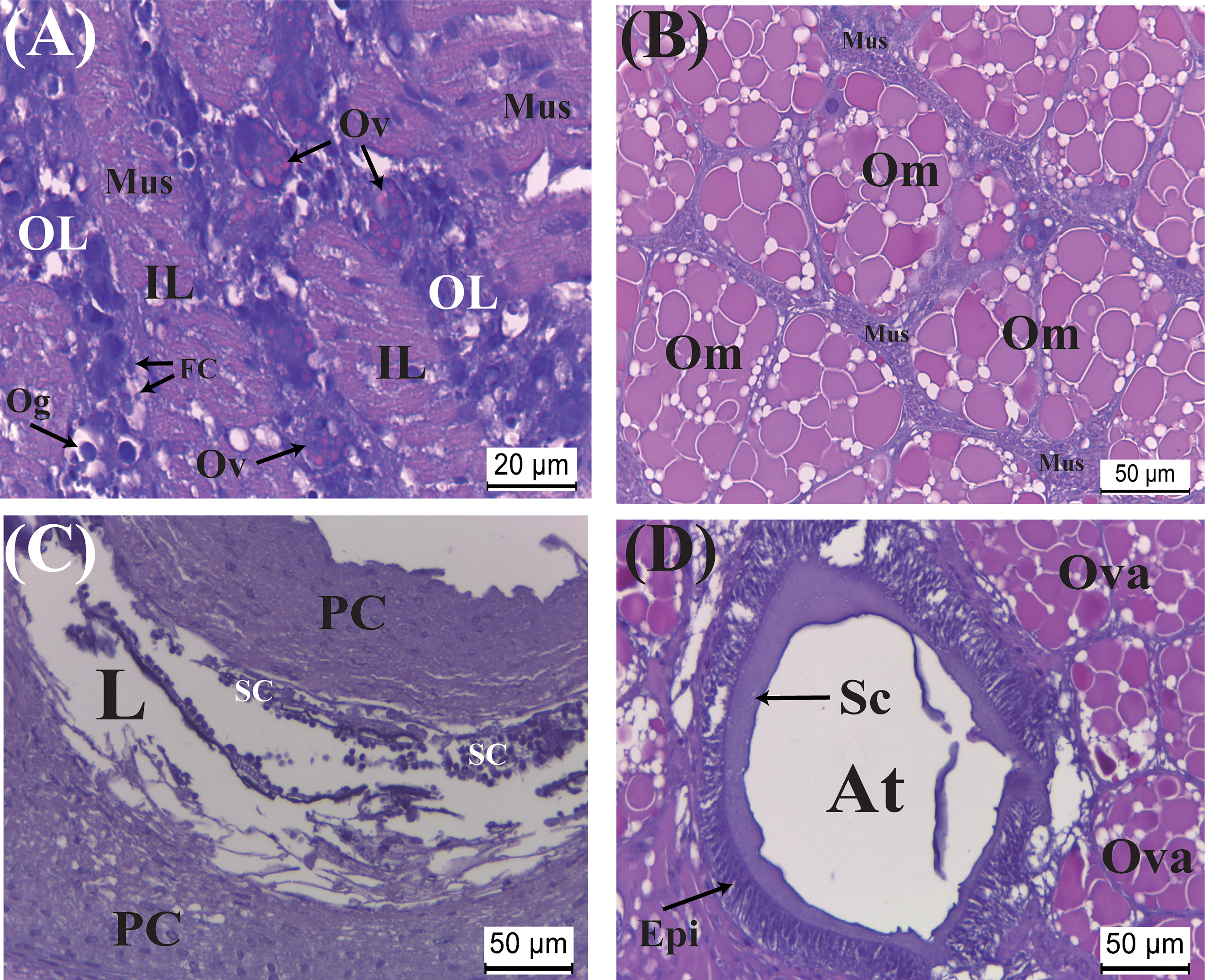
Figure 4 Developmental characteristics of the externa of Polyascus planus parasitizing the thukuhar shore-crab (Metopograpsus thukuhar) in the Orange stage. In this stage, (A) while the vitellogenic oocytes continue developing, an early event is an expansion of interlobular tissue with the muscles cell become larger and elongated. As the development continues toward the end of the stage, (B) ovarian maturation, (C) spermatogenesis inside the male receptacle, and (D) maturation of the colleteric gland are nearly completed. Om, mature oocyte; Sc, secretion; SC, spermatocyte. Other abbreviations are as indicated in Figures 2, 3.
The transition to Yellow stage from the late Orange stage is rather rapid, with the color of the externa turning into distinctly bright yellow usually within a day. Fully mature oocytes (Figure 5A) become slightly larger than those in the late Orange stage (cf. Figure 4B). Spermatozoa are now present in the wide lumen of the male receptacle (Figure 5B). Colleteric glands are also fully active with a thick basophilic epithelium and eosinophilic secretion lining the lumens of the atrium and the branching tubules (Figure 5C). Subsequently, oocytes entering the atrium of the colleteric gland could be seen (Figure 5C), followed by the presence of ova in the mantle cavity enclosed by ovisacs and fertilization taking place as balls of sperms are attached to the ovisacs (Figures 5D, E).
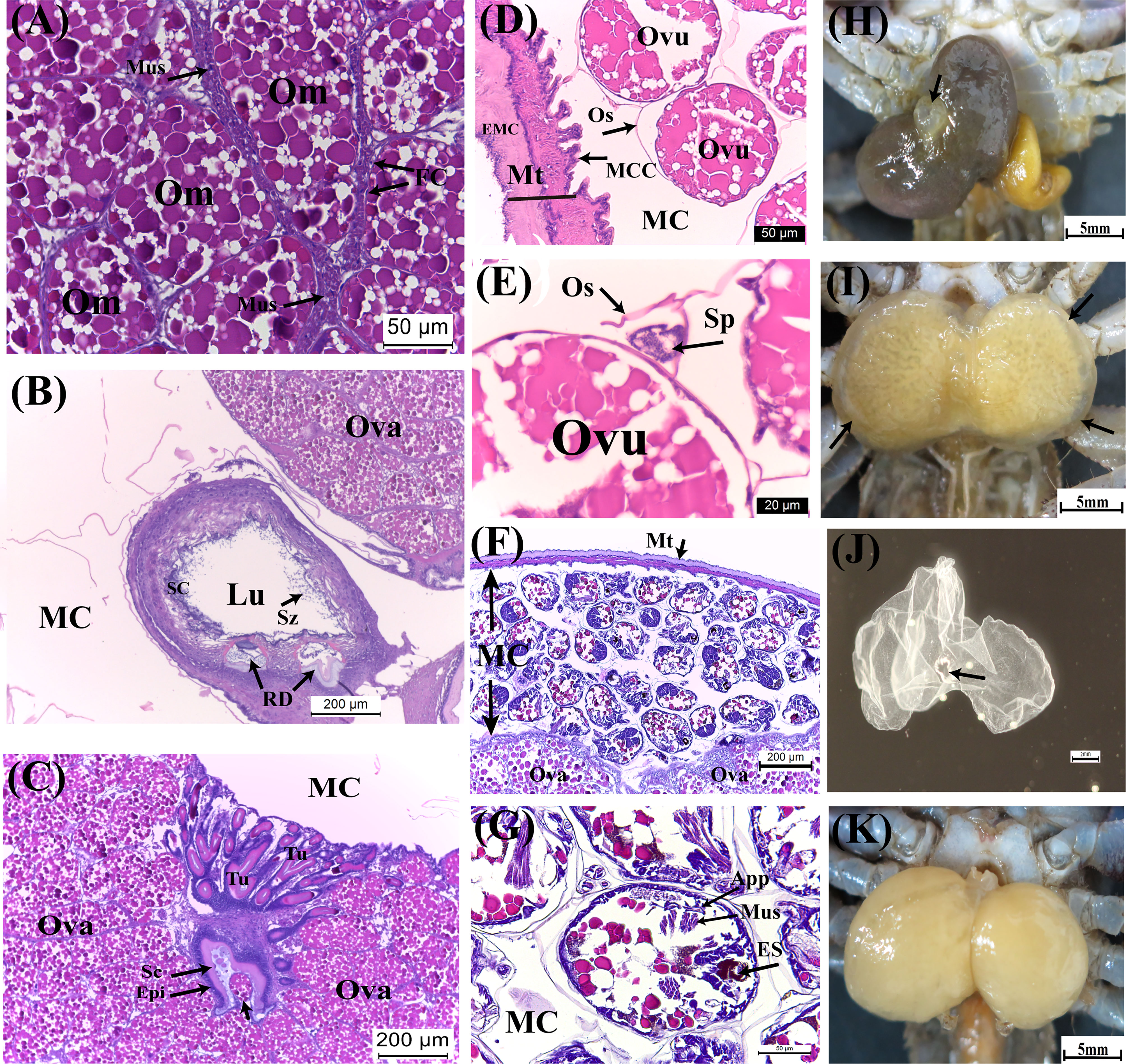
Figure 5 Developmental characteristics of the externa of Polyascus planus parasitizing the thukuhar shore-crab (Metopograpsus thukuhar) in the Yellow and Brown stages. Externa reaching Yellow stage is fully mature with (A) mature oocytes encircled by the muscle cells, (B) mature spermatozoa present in the male receptacle, and (C) the tubular lumen of the colleteric gland lined with secretion. (D, E) Following ovulation, ova enclosed by ovisac, to which ball of sperms attaches, appear within the mantle cavity. (F, G) Embryos inside the mantle cavity of the externa in Brown stage are actively undergoing organogenesis. (H) A raised mantle opening (black arrow) is indicative of imminent release of hatched nauplii. (I) After the peak of larval release, the externa returns to the Yellow stage with an opaque rim (black arrows), profiling the now vacated mantle cavity. After the mantle cavity cuticle molts, (J) the exuviae is extruded via the mantle opening, (K) followed by return of the externa to Yellow stage ready for another round of reproduction. App, appendage; ES, eye spot; Ovu, ovum; Sp, sperm ball; Sz, spermatozoa. Other abbreviations are as indicated in Figures 2–4.
As embryos within the mantle cavity develop, color of the externa gradually turns brown. When the externa become uniformly dark brown, the mantle cavity is filled with developing embryos, with the eyespots, muscle tissues, and appendages now clearly differentiated (Figures 5F, G). Release of naupillar larvae begins, through a raised mantle opening (Figure 5H), usually within 1-2 days after externa becomes dark brown. Typically, the release lasts 3 to 5 days, with a single daily surge, during which more than 80% of the larvae of a brood was released.
Color of the externa gradually loses its brown hue with the release of larvae and becomes bright yellow again immediately after the daily surge of larva release (Figure 5I). The mantle cavity cuticle that lines the entire cavity molts shortly after completion of larval release, and the exuviae is extruded (Figure 5J) via the mantle opening. Shortly after the cuticle extrusion, a new round of oviposition begins, filling the mantle cavity in the following 1 – 2 days (Figure 5K), which is followed by fertilization, embryo development, and color changes (turning dark brown) as mentioned above. For each externa, multiple (usually 3-4) broods of larvae could be produced, and therefore, the externa cycles accordingly between the Yellow and Brown stages, before it becomes spent and eventually detached from the host (see Figure 1). After the spent externa is detached, molt cycle of the host is apparently re-initiated so that the crabs would usually molt 1 month or so after the detachment and emergence of the new externa occurred concurrently with the ecdysis of the host.
Effects of parasitism on the gonads and hepatopancreas of the host
Effects of parasitism of P. planus on the development of the gonad and hepatopancreas of Metopograpsus thukuhar were examined. The gonadosomatic index indicates that the relative ovarian weight, but not the relative testicular weight, is significantly different among hosts of various parasitization status (i.e., non-parasitized hosts and hosts with externa of various developmental stages) (Ovary: F(4, 10) = 12.856, p < 0.001; Testis: F(4, 10) = 0.608, p = 0.666). The relative ovarian weight of parasitized hosts is significantly lower than that of non-parasitized animals (Table 1). The hepatopancreas-somatic index is not significantly different among hosts of various parasitization status, irrespective of the sex of the host (Female: F(4, 10) = 1.894, p = 0.188; Male: F(4, 10) = 1.772, p = 0.205) (Table 1).
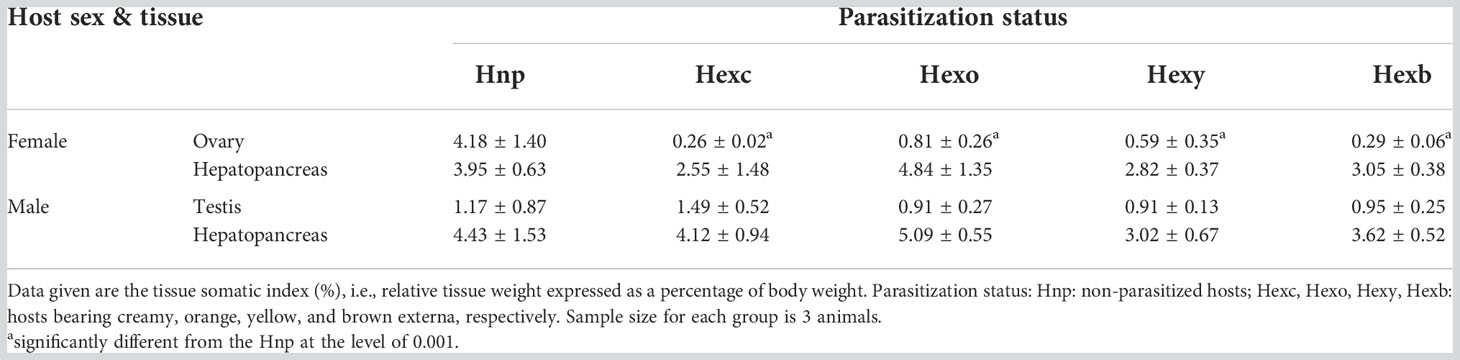
Table 1 Effect of parasitism of Polyascus planus on the tissue weight of the host thukuhar shore-crab Metopograpsus thukuhar.
Histological examination reveals that the ovary is heavily infiltrated by rootlets. Additionally, while the color of the ovary of mature parasitized hosts (i.e., black) appears similar to that of non-parasitized counterparts, histological examination indicates that most oocytes are arrested at the pre-vitellogenic stage. Degenerated oocytes are commonly observed (Figure 6A). In contrast, examination of the mature ovaries of non-parasitized crabs shows that the ovaries contain large and fully developed oocytes (Figure 6B).
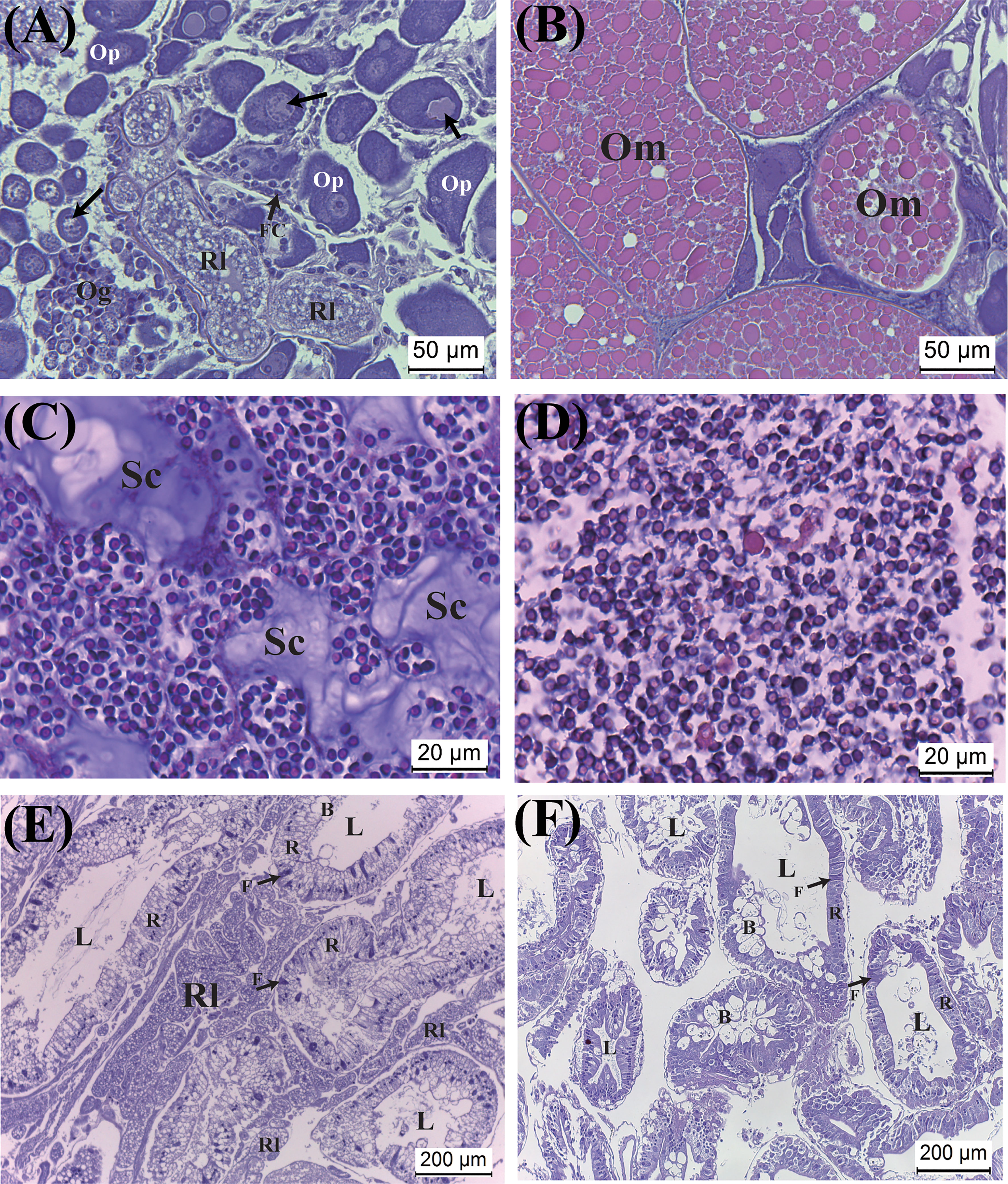
Figure 6 Effect of parasitism of Polyascus planus on the tissues of the host thukuhar shore-crab (Metopograpsus thukuhar). Tissues examined include the ovary (A, B), testis (C, D), and hepatopancreas (E, F). Note that, for the parasitized hosts, the ovary and hepatopancreas (A, E), but not the testis (C), are heavily infiltrated by rootlets. The sections (A, C, E) shown for the parasitized hosts were from hosts bearing orange externa. Arrows in (A) point to degenerating oocyte. B, F, R: the B-, F-, R-cell of the epithelia. L, lumen; Om, mature oocyte; Og, oogonium; Op, pre-vitellogenic oocyte; Rl, rootlet; Sc, secretion.
Testicular sections on the other hand shows that spermatozoa in parasitized hosts are as abundant as in non-parasitized crabs. However, there are significant amounts of secretion in the testes of parasitized hosts, which are not observed in the testes of non-parasitized hosts (Figures 6C, D). Rootlets have never been observed infiltrating the testicular tissues of the parasitized hosts.
The hepatopancreas of both sexes of hosts is heavily infiltrated by rootlets, which appear in the inter-tubular tissues. Nevertheless, tissue integrity of the infiltrated hepatopancreas appears intact, as with the hepatopancreas of the non-parasitized animals, with typical cell types of the tubular epithelia of the hepatopancreas (Figures 6E, F).
Expression of host and parasite vitellogenin genes
Expression of Vg gene was examined in the ovary, hepatopancreas, or both in non-parasitized and parasitized crabs using a primer pair specific for host Vg. A DNA band of expected size (136 bp) was amplified in the hepatopancreas and ovary of non-parasitized females, as well as those of parasitized females (Figure 7). Similarly, the 136-bp band was amplified in the hepatopancreas of parasitized males, but not in that of non-parasitized males (Figure 7).
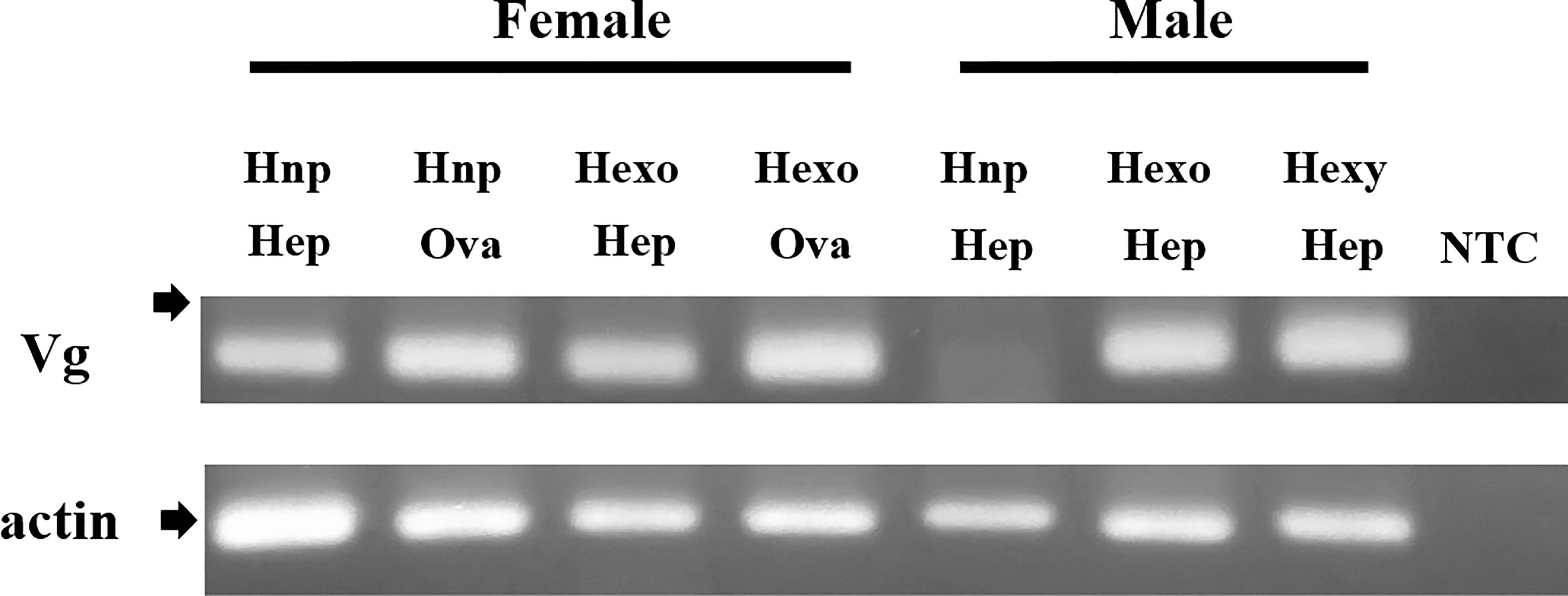
Figure 7 Induction of expression of vitellogenin gene in the male thukuhar shore-crab (Metopograpsus thukuhar) parasitized by Polyascus planus. Total RNA prepared from the tissues as indicated (Hep: hepatopancreas; Ova: Ovary) was reverse transcribed and the cDNA amplified with primers as specified in the Materials and methods. The expected size of the amplicons is 136 bp for Vg and 193 bp for actin. Arrows point to the location of a 200-bp marker. Sequence of the amplicons has been verified by DNA sequencing. Hnp: non-parasitized hosts; Hexo, Hexy: hosts bearing orange and yellow externa, respectively. NTC; no template control that did not contain any template DNA. Note especially that the Vg amplicon is amplified in the hepatopancreas of the parasitized male, but not in that of non-parasitized male.
Vitellogenin gene expression in the hepatopancreas of female and male hosts with externa of different developmental stages was quantified. The expression levels are not significantly different among parasitization status in either male (F(2, 8) = 3.913, p = 0.065) or female (F(3, 12) = 1.112, p = 0.383) hosts (Figures 8A, B). Nor are the expression levels of Vg gene in the ovary of female hosts significantly different among parasitization status (F(2, 6) = 1.210, p = 0.362) (Figure 8C).
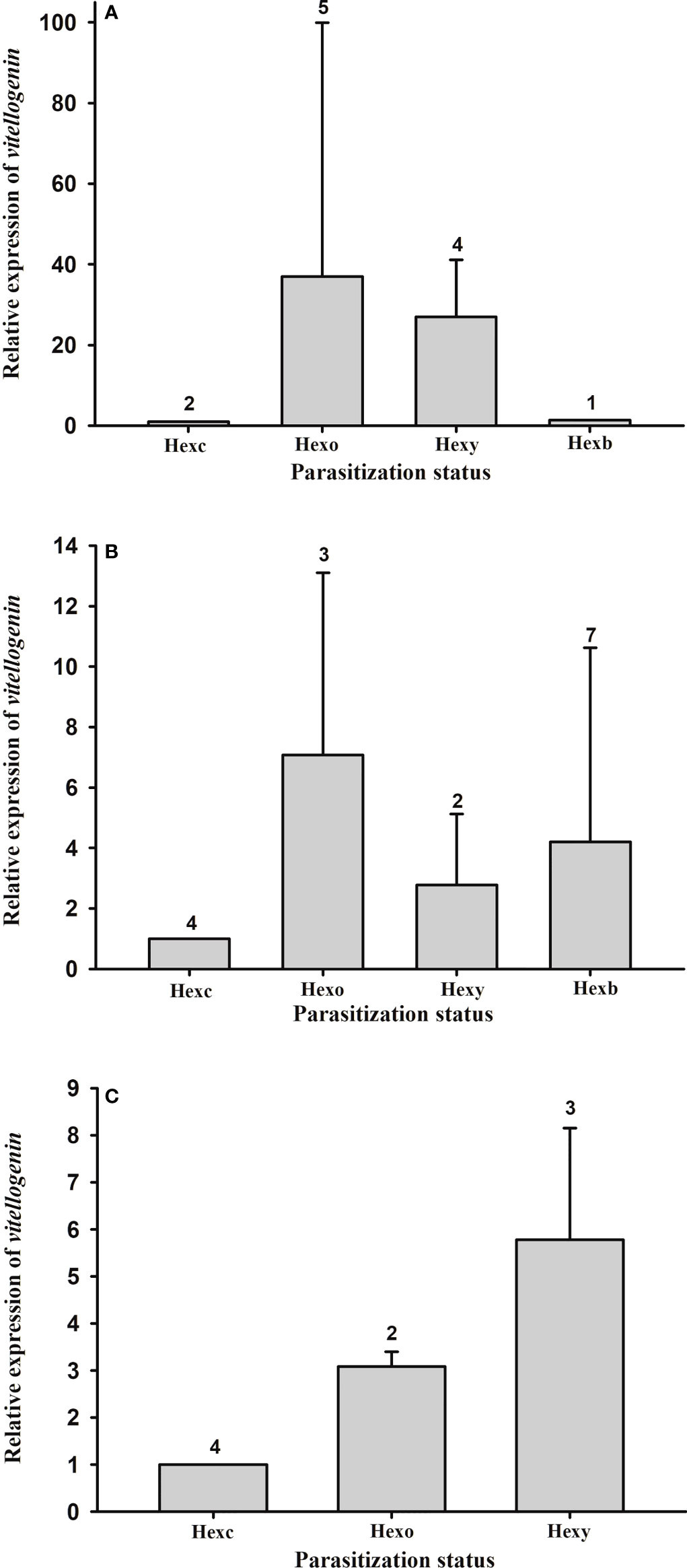
Figure 8 Expression profile of vitellogenin gene in the thukuhar shore-crab (Metopograpsus thukuhar) parasitized by Polyascus planus. Total RNA prepared from the tissues was reverse transcribed and the cDNA amplified with primers as specified in the Materials and methods. Transcript levels in the tissue (A): male hepatopancreas; (B) female hepatopancreas; (C); ovary taken from animals of various parasitization status were quantified by the real-time PCR, normalized to the reference gene (actin), and expressed relative to the levels of Hexc, the calibrator stage (Hexc = 1). Parasitization status: Hnp, non-parasitized hosts; Hexc, Hexo, Hexy, Hexb, hosts bearing creamy, orange, yellow, and brown externa, respectively. Number above the columns indicates the sample size.
Expression of the parasite Vg gene in the externa of P. planus was also quantified. Data show that there is no statistical difference in the parasite Vg expression among the various developmental stages of the externa on male (F(2, 8) = 1.744, p = 0.235) or female (F(3, 12) = 0.489, p = 0.697) hosts (Figure 9).
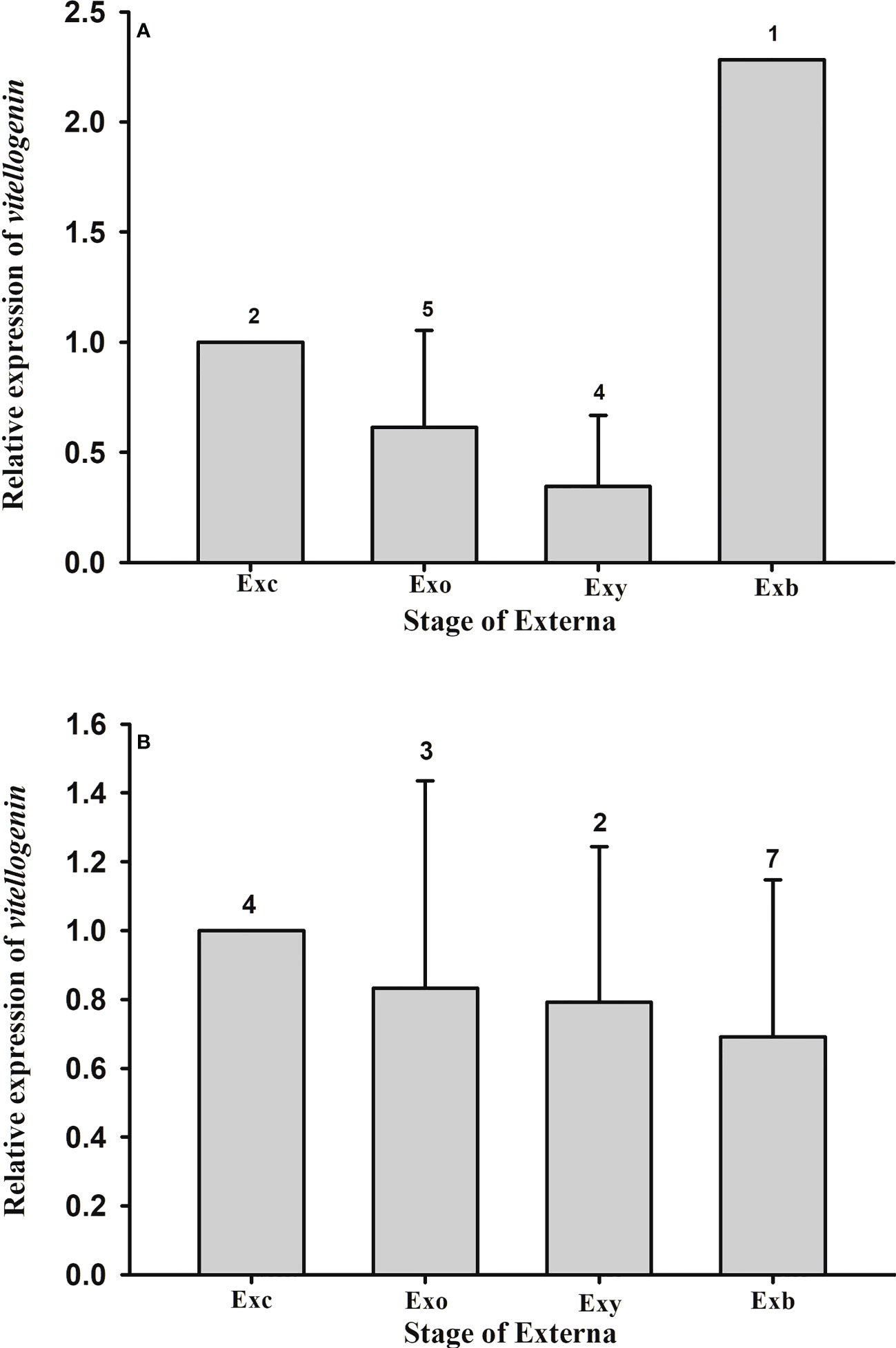
Figure 9 Expression profile of vitellogenin gene in the externa of Polyascus planus parasitizing the thukuhar shore-crab (Metopograpsus thukuhar). Total RNA prepared from the externae on the (A) male or (B) female hosts was reverse transcribed and the cDNA amplified with primers as specified in the Materials and methods. Transcript levels in the externae of various developmental stages were quantified by the real-time PCR. Transcript levels are normalized to the reference gene (actin) and expressed relative to the levels of Exc, the calibrator stage (Exc = 1). Developmental stage of the externa: Exc, Exo, Exy, and Exb indicate externa of the Creamy, Orange, Yellow, and Brown stages, respectively. Number above the columns indicates the sample size.
The expression levels of Vg in the hepatopancreas of male hosts are negatively correlated with those in the externa (Figure 10A, correlation coefficient r = -0.595; p = 0.024), whereas the levels in the hepatopancreas and ovary of female hosts are not significantly correlated with those in the externa (Figures 10B, C; B: r = -0.012; p = 0.964; C: r = -0.107, p = 0.785).
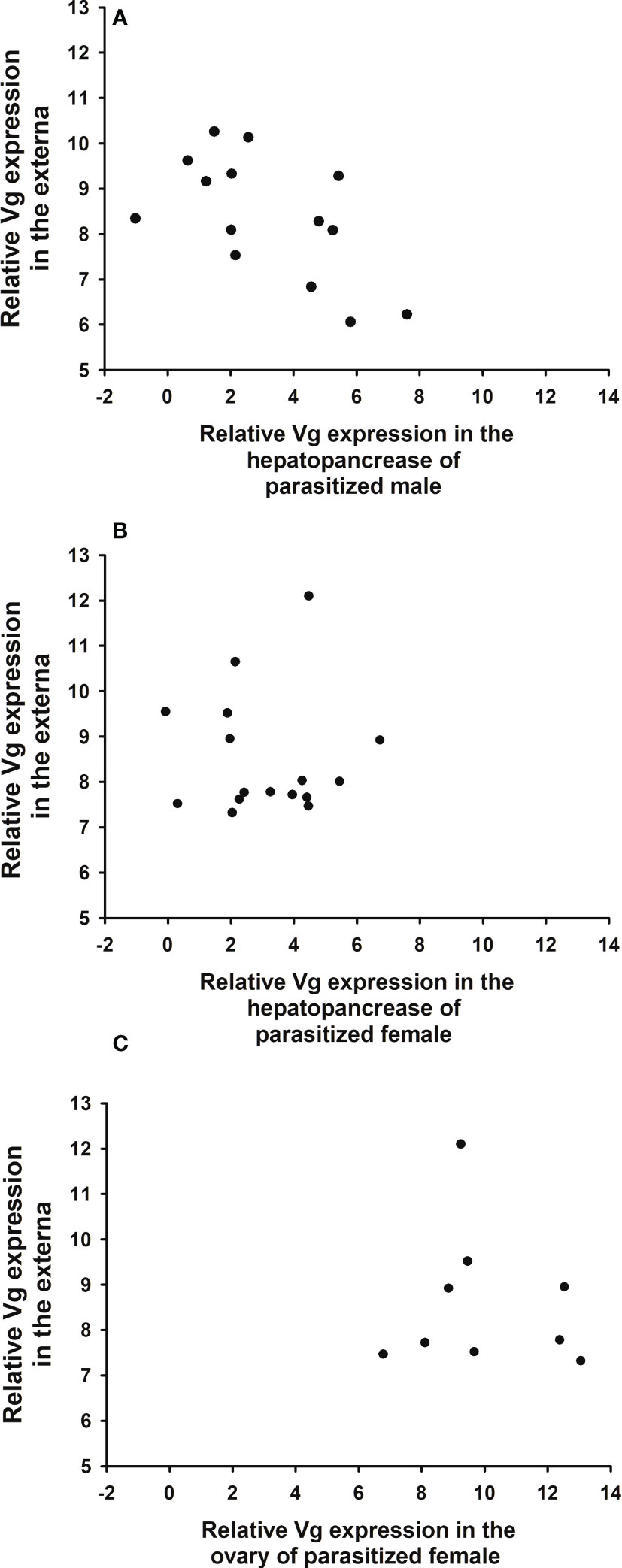
Figure 10 Relationship between expression levels of parasite (Polyascus planus) and host (Metopograpsus thukuhar) vitellogenin. Relative expression levels of parasite Vg are plotted against those of host Vg in the hepatopancreas of parasitized (A) male, (B) female, and (C) the ovary of parasitized female. Sample size for (A–C) is 14, 16, and 9, respectively. Correlation analysis shows that only the expression levels in male hepatopancreas is significantly (negatively) correlated with those in the externa (r = -0.595; p = 0.024).
Peptide fragment analysis of vitellogenin and vitellin
Hemolymph Vg of the host (VgH), and the major yolk proteins of the host (VnH) and the parasite (VnP) isolated from tissues using non-dissociating electrophoresis (Supplementary Data Sheet 1) were subjected to tryptic digestion and the tryptic fragments to mass spectrometric analysis for de novo sequencing. When the sequences of the tryptic fragments experimentally derived from these proteins are blasted against the deduced Vg sequence of the Chinese mitten crab Eriocheir sinensis (a brachyuran species that is phylogenetically close to the host species and its whose full-length Vg sequence is available), the number of hits (fragments that match in sequence to the E. sinensis) is 6, 11, and 11 for VgH, VnH, and VnP, respectively. Schematic representation of the matching fragments mapped onto the E. sinensis Vg is shown in Figure 11A. Tryptic fragments derived from VnP are also blasted against the deduced Vg sequence of the parasite, resulting in a total of 4 matching fragments (Figure 11B). No matching fragments are found when the fragments derived from VgH and VnH are blasted against the parasite Vg.
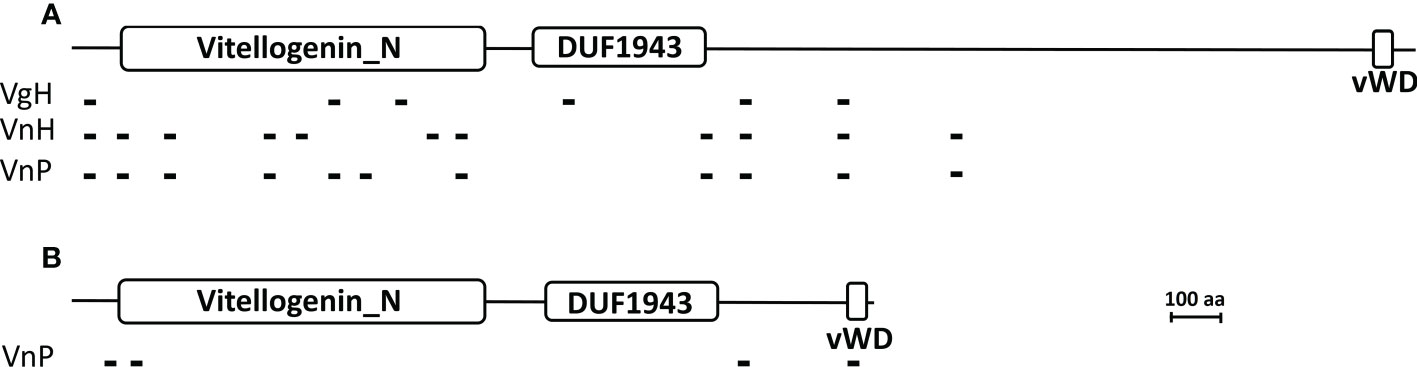
Figure 11 Mapping of the tryptic peptide fragments of vitellogenin and vitellin onto brachyuran and parasite vitellogenin. (A) Matching tryptic peptide fragments derived from the hemolymph Vg of parasitized male hosts (VgH), the ovarian Vn of non-parasitized female hosts (VnH), and the externa Vn of the parasite P. planus (VnP) are mapped onto the Chinese mitten crab Eriocheir sinensis Vg. (B) Matching tryptic peptide fragments derived from the externa Vn (VnP) are mapped onto the parasite Vg. Large white rectangles indicate the signature domains of the vitellogenin family, the N-terminal Vitellogenin-N domain (Vitellogenin_N), the domain of unknown function 1943 (DUF1943), and the C-terminal Von Willebrand D domain (vWD) (see Zhang et al., 2011). Small black blocks indicate the tryptic fragments, the sequence of which are given in Supplementary Table 2. Accession number for the Chinese mitten crab Eriocheir sinensis Vg and the parasite P. planus Vg is MH621336 and OP556485, respectively. Scale bar = 100 amino acids in length for both vitellogenins.
Sequences of the matching fragments are presented in Supplementary Table 2, which shows that VnP and VgH share 4 identical tryptic fragments and that VnP and VnH 9 identical fragments (also see Figure 11A). The 4 fragments mapped onto the deduced sequence of the parasite Vg (Figure 11B) are unique to VnP, not found in VgH or VnH (see Supplementary Table 2).
Discussion
In this study, a temporal profile of the development of the externa of the parasitic barnacle Polyascus planus parasitizing its brachyuran host, Metopograpsus thukuhar is established, highlighting developmental events during the course of externa maturation. We further characterized the sources (host and parasite) of the yolk protein precursor vitellogenin deposited in the externa, providing evidence for the first time in any rhizocephalans that both sources contribute to the process of vitellogenesis in the parasite. Importantly, parasitized male hosts are found expressing Vg, an observation suggesting that the parasitization-induced Vg expression is a molecular manifestation of parasitic feminization that has never been reported before in Rhizocephala. Effect of parasitism on the host was found sex-specific with regard to the development of the host gonad.
One of the major findings we report in this study is that both the host and parasite are involved in expressing Vg during the course of externa maturation. Further, it is suggested that the synthesized Vg, irrespective of host or parasite origin, is to be utilized solely for the maturation of the externa based on the following observations. First, the vitellin deposited in the externa contained peptide fragments of both host and parasite Vg. Second, despite both the hepatopancreas and ovary expressed Vg in the parasitized females, the oocytes were developmentally arrested at the pre-vitellogenic stage, presumably due to an inhibition of Vg uptake. Finally, it is obvious that for the parasitized male hosts there is no ovarian tissue for the sequestration of the synthesized Vg. As such, vitellogenesis in the externa of P. planus is supplied with Vg from dual sources. An obvious advantage of having dual sources of Vg is that the likelihood of ensuring that the externa can reach maturity would be higher, not relying solely on the host but with a second source from the parasite itself. The uncertainty the parasite faces perhaps is even higher when the host the P. planus parasitizes is a male, which needs to be induced (feminized) by the parasite to acquire the capability for Vg synthesis (see more below).
While the parasitized host and parasite both expressed Vg during the development of externa, there is no significant change of Vg expression over the course of the maturation process. Rather, only in parasitized male hosts, expression levels of the host and parasite Vg are inversely correlated with each other. Such an inverse correlation suggests that Vg expression levels in male hosts are negatively coupled to those in the parasite. As Vg expression in the parasitized male hosts apparently has to be induced by the parasite, it is feasible that a controlling mechanism for Vg synthesis imposed by the parasite exists that could negatively couple the expression levels of Vg in the host and parasite. In the same vein, the absence of a correlation in Vg expression levels for the pair of female host/parasite would probably indicate that expression levels of Vg in female hosts, which have innate capability of Vg synthesis, is not induced and regulated by the parasite, hence the Vg expression levels in the female host and parasite could not be coupled. For the female host, rather, it is, as suggested above, the Vg uptake by the host that is negatively regulated by the parasite. Taken together, the controlling strategy with respect to host Vg that P. planus adopts appears to be dependent on the sex of the host – induction and regulation of Vg expression when parasitizing male host but competition for Vg uptake when parasitizing female host.
The effect of parasitism by P. planus on the gonad of M. thukuhar is different between the two sexes of the host. The effects on the relative tissue weight and production of mature gametes are severe for the ovary, but moderate at most for the testis. Sex-dependent effects of rhizocephalan parasitism have been noted by previous studies (see Høeg, 1995). Interestingly, the effect is related to the tissue infiltration by the rootlet, with the ovary being heavily infiltrated, but the testis not at all. Hence, it is likely that the effects on host tissues, at least the gonad, are mainly exerted by presumptive factors secreted by the infiltrating rootlets. A recent study provides ultrastructural observations that modified structures of the rootlets of two rhizocephalan species (Peltogaster paguri and Peltogasterella gracilis) invading the nervous system of the host are the potential sites where the host and parasite interact through chemical signals (Miroliubov et al., 2020). In this regard, it is also worth noting that while the oocyte development in the female host of M. thukuhar is retarded, expression of Vg remains active, indicating that the action of the presumptive parasite-derived factors is selective and mechanistic (e.g., inhibition of Vg uptake by host oocytes), rather than non-selective and destructive in nature. The structural integrity of the examined tissues (the ovary and hepatopancreas) is preserved even though they are heavily infiltrated by the rootlet.
Rhizocephalan parasitism often results in various degrees of feminization in male hosts, the so-called parasitic feminization, which is manifested at morphological, behavioral, and physiological levels (see Høeg, 1995). Sex differentiation and development of male crustaceans is controlled by the insulin-like androgenic gland hormone (IAG), a masculinizing hormone secreted by the androgenic gland (AG) (see Charniaux-Cotton and Payen, 1985; Ventura et al., 2011). Interestingly, it has long been known that the AG of the crabs parasitized by sacculinid rhizocephalans are degenerated (Veillet, 1945; Veillet and Graf, 1959; Rubiliani et al., 1980; Rubiliani-Durozoi et al., 1980), suggesting that the parasitic feminization is a consequence of suppressing the hormonal output of the AG. Vitellogenin is initially characterized as a female-specific hemolymph protein related to vitellogenesis (see Meusy, 1980) and its expression is later found induced by silencing of the IAG expression (Levy et al., 2020). Hence, we propose that the induction of Vg expression, as shown here with the male M. thukuhar parasitized by P. planus, is a manifestation of the parasitic feminization.
It may appear paradoxical that the testis of the parasitized males is relatively unaffected in terms of spermatogenesis in the parasitized male M. thukuhar. Although they have been commonly described as “parasitic castrators”, rhizocephalans impose effect of various degrees on the host gonads (see Høeg, 1995; Waiho et al., 2021). For example, the effect of parasitism on the gonadal development of the host ranges from severe (Fazhan et al., 2020) to moderate (Rowley et al., 2020) in both sexes, to host sex-dependent with severe effect on the ovary but little on the testis (Mouritsen et al., 2018). The dependency of the effect of parasitism on host sex observed in the present report is similar to what observed in some rhizocephalan-infested pagurids (Nielsen, 1970) and shore crab C. maenas (Mouritsen et al., 2018). The infested male M. thukuhar are clearly feminized as manifested by the feminized anatomy (Hsiao et al., 2016), as well as Vg synthesis (the present study), and their AG is most likely regressed. It has also been reported that regression of AG and inhibition of spermatogenesis are not closely correlated (Rubiliani et al., 1980; Rubiliani-Durozoi et al., 1980), and that the presence of feminized appendages are not necessarily associated with histological changes in the testis in parasitized hosts (Nielsen, 1970). These observations argue that the function of the AG is not related to the functional development of the male gonad, specifically spermatogenesis. The question whether the AG and the hormone it produces and releases (the IAG) are required for the process of spermatogenesis in crustaceans remain unsettled (Okumura and Hara, 2004; Ventura et al., 2009). Other endocrine regulators, especially those synthesized in the central nervous system, are known to be involved in regulating crustacean gonadal development (Nagaraju, 2011; Chen et al., 2020). As such, study on the effects of rhizocephalan parasitism on the reproductive development of male hosts will not only provide knowledge regarding the mechanism of control of the host by the parasite in particular, but will also shed light on the hormonal regulation of sex and gonadal development of crustaceans in general.
In summary, the data presented in this report show that the parasitism of P. planus influences the thukuhar shore-crab Metopograpsus thukuhar differently depending on the sex of the host. The male host is feminized so that its hepatopancreas is induced for Vg expression, the levels of which appear coupled with the parasite’s. Relative testicular weight and spermatogenesis are not significantly affected by the parasitism. On the other hand, despite Vg synthesis in the female host remains active, relative ovarian weight and vitellogenesis are severely affected by the parasitism. Presently, we do not know if the sex of the host that P. planus parasitizes affects the development and maturation of the externa in a quantitative manner, and hence ultimately the reproductive output of the parasite, which obviously is an interesting question worth pursuing.
Data availability statement
The data presented in the study are deposited in the GenBank repository, accession number OP556483, OP556484, OP556485, OP556486.
Ethics statement
The animal study was reviewed and approved by Experimental protocols involving animals employed by the present study were approved by the Review Committee of National Changhua University of Education (Permit number: NCUE-1072900150), in full accordance with the recommendations (Guidelines for Management and Use of Experimental Animals) set by the Council of Agriculture, Taiwan.
Author contributions
Conceptualization & experimental design, C-YL, and H-YC. investigation, C-HL, H-YC, and C-CT and S-LY. data analysis: C-HL, H-YC. writing, C-YL. resource, C-HL. funding acquisition, C-YL and H-YC. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the Ministry of Science and Technology (MOST), Taiwan (110-2311-B-018-001 and 111-2311-B-018-001 to C-YL; 110-2811-B-018-002 and 111-2811-B-018-001 to H-YC).
Acknowledgments
The authors thank the financial support from the Ministry of Science and Technology.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.1073459/full#supplementary-material
References
Alvarez F., Bortolini J. L., Høeg J. T. (2010). Anatomy of virgin and mature externae of Loxothylacus texanus, parasitic on the dark blue crab Callinectes rathbunae (Crustacea: Cirripedia: Rhizocephala: Sacculinidae). J. Morphol. 271 (2), 190–199. doi: 10.1002/jmor.10790
Biron D. G., Loxdale H. D. (2013). Host-parasite molecular cross-talk during the manipulative process of a host by its parasite. J. Exp. Biol. 216 (Pt 1), 148–160. doi: 10.1242/jeb.073825
Bortolini J. L., Alvarez F. (2008). Hepatopancreas alteration of the blue crab Callinectes sapidus by the rhizocephalan barnacle Loxothylacus texanus. J. Invertebr. Pathol. 99, 354–356. doi: 10.1016/j.jip.2008.08.004
Chang C. C., Tsai T. W., Hsiao N. W., Chang C. Y., Lin C. L., Watson R. D., et al. (2010). Structural and functional comparisons and production of recombinant crustacean hyperglycemic hormone (CHH) and CHH-like peptides from the mud crab Scylla olivacea. Gen. Comp. Endocrinol. 167, 68–76. doi: 10.1016/j.ygcen.2010.02.013
Charniaux-Cotton H., Payen G. (1985). “Sexual differentiation,” in The biology of Crustacea, vol. pp . Eds. Bliss D. E., Mantel L. H. (New York, NY: Academic Press), 217–299.
Chen H. Y., Toullec J., Lee C. Y. (2020). The crustacean hyperglycemic hormone superfamily: progress made in the past decade. Front. Endocrinol. 11, 578958. doi: 10.3389/fendo.2020.578958
Chen H. Y., Watson R. D., Liu H. F., Chen J. C., Lee C. Y. (2007). Molecular characterization and gene expression pattern of two molt-inhibiting hormone-like peptides from Litopenaeus vannamei. Gen. Comp. Endocrinol. 151, 72–81. doi: 10.1016/j.ygcen.2006.11.016
Fazhan H., Waiho K., Glenner H., Moh J. H. Z., Hassan M., Ikhwanuddin M. (2020). Gonadal degeneration and hepatopancreas alteration in orange mud crab Scylla olivacea infected with Sacculina beauforti (Crustacea; rhizocephala; sacculinidae). Front. Mar. Sci. 7, 534443. doi: 10.3389/fmars.2020.534443
Ginzinger D. G. (2002). Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp. Hematol. 30, 503–512. doi: 10.1016/S0301-472X(02)00806-8
Glenner H. (2001). Cypris metamorphosis, injection and earliest internal development of the rhizocephalan Loxothylacus panopaei (Gissler). Crustacea: Cirripedia: Rhizocephala: Sacculinidae. J. Morphol. 249, 43–75. doi: 10.1002/jmor.1040
Glenner H., Høeg J. T. (1995). A new motile, multicellular stage involved in host invasion by parasitic barnacles (Rhizocephala). Nature 377, 147–150. doi: 10.1038/377147a0
Glenner H., Høeg J. T., O'Brien J. J., Sherman T. D. (2000). Invasive vermigon stage in the parasitic barnacles Loxothylacus texanus and L. panopaei (Sacculinidae): closing of the rhizocephalan life-cycle. Mar. Biol. 136, 249–257. doi: 10.1007/s002270050683
Glenner H., Lützen J., Takahashi T. (2003). Molecular and morphological evidence for a monophyletic clade of asexually reproducing rhizocephala: Polyascus, new genus (Cirripedia). J. Crust Biol. 23, 548–557. doi: 10.1651/C-2361
Golubinskaya D. D., Korn O. M., Sharina S. N., Selin N. I. (2021). Sympatric two-species infestation by rhizocephalan barnacle parasites in the spider crab Pugettia aff. ferox ohtsuchi & kawamura 2019 from Peter the great bay (Northwestern Sea of Japan). Zool Stud. 60, e54. doi: 10.6620/ZS.2021.60-54
Høeg J. T. (1987). The relation between cypris ultrastructure and metamorphosis in male and female Sacculina carcini (Crustacea, cirripedia). Zoomorphology 107, 299–311. doi: 10.1007/BF00312176
Høeg J. T. (1995). The biology and life cycle of the rhizocephala (Cirripedia). J. Mar. Biol. Ass UK 75, 517–550. doi: 10.1017/S0025315400038996
Høeg J. T., Lützen J. (1995). Life cycle and reproduction in the cirripedia, rhizocephala. Oceanography Mar. Biology: Annu. Rev. 33, 427–485.
Høeg J. T., Noever C., Rees D. A., Crandall K. A., Glenner H. (2020). A new molecular phylogeny-based taxonomy of parasitic barnacles (Crustacea: Cirripedia: Rhizocephala). Zoological J. Linn. Soc. 190 (2), 632–653. doi: 10.1093/zoolinnean/zlz140
Hames B. D. (1990). “Onedimensional polyacrylamide gel electrophoresis,” in Gel electrophoresis of proteins, 2nd Edition. Eds. Hames B. D., Rickwood D. (New York: Oxford University Press), 382.
Hsiao C. J., Wu Y. I., Tung T. A., Wang G. Y., Toullec J. Y., Liu S. T., et al. (2016). Effects of parasitization by the barnacle Polyascus plana (Cirripedia: Rhizocephala) on a grapsid host Metopograpsus thukuhar: changes in metabolic profile and hemocyte counts. Dis. Aquat. Organisms 119, 199–206. doi: 10.3354/dao03000
Huang J. F., Lutzen J. (1998). Rhizocephalans (Crustacea: Cirripedia) from Taiwan. J. Nat. Hist 32, 1319–1337. doi: 10.1080/00222939800770661
Jayasankar V., Tomy S., Wilder M. N. (2020). Insights on molecular mechanisms of ovarian development in decapod Crustacea: Focus on vitellogenesis-stimulating factors and pathways. Front. Endocrinol. (Lausanne). 11:577925. doi: 10.3389/fendo.2020.577925
Korn O. M., Shukalyuk A. I., Trofimova A. V., Isaeva V. V. (2004). Reproductive stage of the life cycle in the rhizocephalan barnacle Polyascus polygenea (Crustacea: Cirripedia). Russian J. Mar. Biol. 30), 328–340. doi: 10.1023/B:RUMB.0000046552.07712.02
Lange S. (2002). The colleteric glands in sacculinidae (Crustacea, cirripedia, rhizocephala): an ultrastructural study of ovisac secretion. Contributions to Zoology 70 (4), 229–242. doi: 10.1163/18759866-07004004
Levy T., Tamone S. L., Manor R., Aflalo E. D., Sklarz M. Y., Chalifa-Caspi V., et al. (2020). The IAG-switch and further transcriptomic insights into sexual differentiation of a protandric shrimp. Front. Mar. Sci. 7, 587454. doi: 10.3389/fmars.2020.587454
Liu H. C., Lützen J. (2000). Asexual reproduction in Sacculina plana (Cirripedia: Rhizocephala), a parasite of six species of grapsid crabs from Taiwan. Zool Anz 239, 277–287.
Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25 (4), 402–408. doi: 10.1006/meth.2001.1262
Meusy J. J. (1980). Vitellogenin, the extraovarian precursor of the protein yolk in Crustacea : A review. Reprod. Nutr. Dev. 20 (1A), 1–21. doi: 10.1051/rnd:19800101
Miroliubov A., Borisenko I., Nesterenko M., Lianguzova A., Ilyutkin S., Lapshin N., et al. (2020). Specialized structures on the border between rhizocephalan parasites and their host’s nervous system reveal potential sites for host-parasite interactions. Sci. Rep. 10, 1128. doi: 10.1038/s41598-020-58175-4
Mouritsen K. N., Geyti S. N. S., Lützen J., Høeg J. T., Glenner. H. (2018). Population dynamics and development of the rhizocephalan Sacculina carcini, parasitic on the shore crab Carcinus maenas. Dis. Aquat. Organ. 131 (3), 199–211. doi: 10.3354/dao03290
Nagaraju G. P. (2011). Reproductive regulators in decapod crustaceans: an overview. J. Exp. Biol. 214, 3–16. doi: 10.1242/jeb.047183
Nielsen S. O. (1970). The effects of the rhizocephalan parasites peltogaster paguri rathke and gemmosaccus sulcatus (Lilljeborg) on five species of paguridan hosts (Crustacea decapoda). Sarsia 42, 17–32. doi: 10.1080/00364827.1970.10411160
Nour Eldeen M. F., El Gamal M. M., Abdelsalam K. M., Shoukr F. A., Mona M. H. (2019). Histomorphological investigation of the mature externa of Heterosaccus dollfusi (Crustacea, rhizocephala). Zoomorphology 138, 463–473. doi: 10.1007/s00435-019-00453-5
Okumura T., Hara M. (2004). Androgenic gland cell structure and spermatogenesis during the molt cycle and correlation to morphotypic differentiation in the giant freshwater prawn, Macrobrachium rosenbergii. Zoolog Sci. 21 (6), 621–628. doi: 10.2108/zsj.21.621
Owen R. (1839). In: Beechey F. W., The Zoology of Captain Beechey's Voyage; Compiled from the Collections and Notes Made by Captain Beechey, the Officers and Naturalists of the Expedition, during a Voyage to the Pacific and Behring Straits Performed in His Majesty's Ship Blossom, under the Command of Captain F.W. Beechey, R.N., F.R.S, &c, in the years 1825, 26, 27 and 28: 77–97, pls. 24–28. London.
Pantin C. F. A. (1934). On the excitation of crustacean muscle. J. Exp. Biol. 11, 11–27. doi: 10.1242/jeb.11.1.11
Poulin R. (2011). The many roads to parasitism: a tale of convergence. Adv. Parasitol. 74, 1–40. doi: 10.1016/B978-0-12-385897-9.00001-X
Poulin R., Randhawa H. S. (2015). Evolution of parasitism along convergent lines: from ecology to genomics. Parasitology 142 (Suppl 1), S6–S15. doi: 10.1017/S0031182013001674
Rowley A. F., Davies C. E., Malkin S. H., Bryan C. C., Thomas J. E., Batista F. M., et al. (2020). Prevalence and histopathology of the parasitic barnacle, Sacculina carcini in shore crabs, Carcinus maenas. J. Invertebr. Pathol. 171, 107338. doi: 10.1016/j.jip.2020.107338
Rubiliani-Durozoi M., Rubiliani C., Payen G. G. (1980). Deroulement des gametogeneses chez les crabes Carcinus maenas (L.) et c. mediterraneus czerniavsky parasites par la sacculine. Int. J. Invertebr. Reprod. Dev. 2, 107–120. doi: 10.1080/01651269.1980.10553346
Rubiliani C., Rubiliani-Durozoi M., Payen G. G. (1980). Effets de la sacculine sur les gonades, les glandes androgenes et le systeme nerveux central des crabes Carcinus maenas (L.) et C. mediterraneus czerniavsky. Bull. Soc Zool. Fr. 105, 95–100.
Schmittgen T. D., Livak K. J. (2008). Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3 (6), 1101–1108. doi: 10.1038/nprot.2008.73
Thomas F., Adamo S., Moore J. (2005). Parasitic manipulation: where are we and where should we go? Behav. Processes. 68 (3), 185–199. doi: 10.1016/j.beproc.2004.06.010
Tu T. H., Chan B. K. K., Jeng M. S. (2009). Larval development and sex ratio variation of Polyascus plana (Cirripedia: Rhizocephala), a parasite of the crab Grapsus albolineatus, in Taiwan. Bull. Mar. Sci. 84, 331–349.
Veillet A. (1945). Recherches sur le parasitisme des crabes at des galath^es par les rhizoce'phales etles epicarides. Annales de I'Institut Oceanographique, Monaco, 22, 193–341.
Veillet A., Graf F. (1959). Degenerescence de la glande androgene des crustaces decapodes parasites parles rhizocephales. Bull. Soc Sci. Nancy 18, 123–128.
Ventura T., Manor R., Aflalo E. D., Weil S., Raviv S., Glazer L., et al. (2009). Temporal silencing of an androgenic gland-specific insulin-like gene affecting phenotypical gender differences and spermatogenesis. Endocrinology 150, 1278–1286. doi: 10.1210/en.2008-0906
Ventura T., Rosen O., Sagi A. (2011). From the discovery of the crustacean androgenic gland to the insulin-like hormone in six decades. Gen. Comp. Endocrinol. 173, 381–388. doi: 10.1016/j.ygcen.2011.05.018
Waiho K., Glenner H., Miroliubove A., Noeverc C., Hassana ,. M., Ikhwanuddina M., et al. (2021). Rhizocephalans and their potential impact on crustacean aquaculture. Aquaculture 531, 735876. doi: 10.1016/j.aquaculture.2020.735876
Walker G. (2001). Introduction to the rhizocephala (Crustacea: Cirripedia). J. Morphol. 249, 1–8. doi: 10.1002/jmor.1038
WoRMS (2022) Rhizocephala. Available at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=1109 (Accessed 2022-07-24).
Keywords: parasitic barnacle, host control, externa, reproduction, vitellogenesis
Citation: Chen H-Y, Lee C-H, Tsai C-C, Liu H-C, Yang S-L and Lee C-Y (2022) Interaction between the parasitic barnacle Polyascus planus (Cirripedia: Rhizocephala) and its brachyuran host Metopograpsus thukuhar during the development of the externa of the parasite: Control of the gonadal development and vitellogenin synthesis of the host. Front. Mar. Sci. 9:1073459. doi: 10.3389/fmars.2022.1073459
Received: 18 October 2022; Accepted: 17 November 2022;
Published: 20 December 2022.
Edited by:
Hongyu Ma, Shantou University, ChinaReviewed by:
Khor Waiho, University of Malaysia Terengganu, MalaysiaWeiWei Li, East China Normal University, China
Copyright © 2022 Chen, Lee, Tsai, Liu, Yang and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chi-Ying Lee, YmljeWxlZUBjYy5uY3VlLmVkdS50dw==
†These authors have contributed equally to this work
 Hsiang-Yin Chen1†
Hsiang-Yin Chen1† Chi-Ying Lee
Chi-Ying Lee