
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 30 January 2023
Sec. Marine Megafauna
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.1067660
 Carol Palmer1*
Carol Palmer1* Karen K. Martien2
Karen K. Martien2 Holly Raudino3
Holly Raudino3 Kelly M. Robertson2
Kelly M. Robertson2 Alan Withers4
Alan Withers4 Emma Withers4
Emma Withers4 Robert Risk4
Robert Risk4 Dylan Cooper4
Dylan Cooper4 Ellen D’Cruz4
Ellen D’Cruz4 Edmund Jungine3,5
Edmund Jungine3,5 Daniel Barrow4
Daniel Barrow4 Nick Cuff6
Nick Cuff6 Adrian Lane4,5
Adrian Lane4,5 Daniel Keynes7
Daniel Keynes7 Kelly Waples3
Kelly Waples3 Allyson Malpartida1
Allyson Malpartida1 Sam Banks1
Sam Banks1To help evaluate the distribution, residency, population size and structuring (and hence conservation status) of the poorly known false killer whale Pseudorca crassidens in northern Australian waters, we undertook studies of sightings, movement patterns based on satellite telemetry, and genetics. Sighting data indicates that false killer whales are regular, year-round inhabitants of coastal areas of northern Australia. Satellite-tagged animals spent extended periods of time in shallow coastal waters, with no tagged animals leaving the continental shelf. The lack of spatial overlap in the areas visited by individuals tagged in the Arafura/Timor Seas compared to those tagged in the Gulf of Carpentaria suggests that there may be more than one population in northern Australia coastal waters. All 14 genetic samples collected across 1600 km of coastline possessed the same newly identified mitochondrial control region haplotype, designated haplotype 45. Notably, haplotype 45 is distinct from all previously published false killer whale haplotypes globally and is most similar to the two haplotypes that typify the endangered main Hawaiian Islands insular false killer whale population. Based on these results and evidence from recent movement records of those tagged, false killer whales in northern Australia are apparently demographically independent from the offshore population(s). Further assessment of the population conservation status is now required.
There is limited knowledge of the population size, distribution, and genetic structuring of many cetacean species in Australian coastal waters, and such knowledge gaps hinder assessment of their conservation status and the implementing of conservation management (Woinarski et al., 2014). In this paper, we report on recent assessments of the genetic structuring and movement patterns of one species, the false killer whale, Pseudorca crassidens, whose status in Australian waters is poorly understood.
The false killer whale is a tropical and warm temperate dolphin species that is broadly distributed, but naturally rare throughout its range, and is recognised as Near Threatened by the IUCN (Baird, 2018). It is generally considered to be an oceanic species, with sightings close to land mostly in places where the continental shelf is narrow (Stacey and Baird, 1994). The previously reported exceptions to this general pattern are of resident population(s) around some isolated oceanic islands with precipitous slopes and deep water nearshore (Acevedo-Gutierrez et al., 1997; Baird et al., 2008; Baird et al., 2010; Zaeschmar et al., 2014).
In the Hawaiian Archipelago, two island-associated resident population(s) of false killer whales have been identified, one in the main Hawaiian Islands - MHI (Baird et al., 2008; Baird et al., 2010) - and one in the northwest Hawaiian Islands - NWHI (Baird et al., 2013). The two insular population(s) are genetically differentiated from each other and from the neighboring offshore population, with nearly all individuals possessing one of three closely-related haplotypes (haplotypes 1, 2 and 31) that are not found anywhere else (Martien et al., 2014). The one exception was an individual (labID 49052) sampled in the MHI whose haplotype (haplotype 5) is shared with three free swimming animals sampled in Garig Gunak Barlu National Park, Northern Territory (NT) (Martien et al., 2014). The NWHI resident population appears to have a higher rate of ongoing gene flow with the offshore population than the MHI population does (Martien et al., 2014). Martien et al. (2014) hypothesized that the lack of gene flow between the MHI and offshore population(s) of false killer whales likely results from a strong and temporally stable habitat discontinuity between the insular and offshore environments around the MHI.
Until recently, records of false killer whales from Australian waters have been scattered, irregular and infrequent, and records were mainly from strandings. Based on these records the species was assumed to be pelagic in Australian waters and generally not considered as a coastal species (Bannister et al., 1996). Recognising the sparse evidence base for its status in Australian waters, it was considered Data Deficient in a review of the conservation status of Australian mammals (Woinarski et al., 2014), and it is not listed as threatened under Australian legislation.
However, recent research provided more evidence of the false killer whale status in northern coastal Australian waters. During a 3-year study of coastal dolphins in Port Essington Harbour in Cobourg Marine Park, groups of false killer whales were recorded on several occasions, prompting a collation of records from NT coastal waters. The resulting review (Palmer et al., 2009) indicated that the species occupies these shallow waters more frequently than previously recognised. Palmer et al. (2009) reported regular sightings of false killer whales during the wet season (December - April) within the semi-enclosed Port Essington and Darwin Harbour between 2003 and 2009, indicating the presence of a potential resident population in these estuarine habitats.
During late March/April in 2014, Palmer et al. (2017) used satellite telemetry to describe movement patterns of four individuals tagged in Cobourg Marine Park, with such evidence showing that false killer whales use coastal and pelagic waters across much of the shallow Arafura and Timor Seas within the Australian Exclusive Economic Zone (EEZ). The tagged individuals, which were all part of the same group when the tags were deployed, exhibited similar movements through space and time, with all pairs of individuals regularly being recorded within 1 km of each other, indicating some degree of group cohesion over the duration of the tag transmission periods (64 to 104 days) (Palmer et al., 2017).
Here, we describe additional movement data for false killer whales from another site in the NT waters, collate additional sighting and stranding records, and complement this information with analysis of genetic data of false killer whales from the NT and from another north Australian site, off the Kimberley region of Western Australia (WA). Our objectives are to: (i) expand knowledge of the movement patterns for this species in northern Australian waters, and (ii) assess the extent of genetic structuring within these waters, and the genetic distinctiveness of this population relative to other global populations. Based on this information, we then discuss some implications for the conservation status of the species in Australian waters.
Data from a variety of sources were used to document the occurrence of false killer whales in Australian coastal waters. Opportunistic sightings from both research activities and crowd-sourced from open-access online biodiversity portals (e.g., Atlas of Living Australia), citizen science mobile applications and programs (Marine WildWatch, DolphinWatch and Marine Fauna Sightings), and government archives (Naturemap) were used. Anecdotal community reports from members of the public (e.g., wildlife tour operators, fishers etc.) were sourced through social media posts. Sightings from online portals and social media were all supplemented with photographs or videos to validate species identification; however, we note that most imagery was not of a high enough quality to be used for photo-identification of individuals.
Three stranding records within the NT were previously reported by Chatto and Warneke (2000). In WA, false killer whale stranding records were collated through the Western Australian Cetacean Stranding Database, established in 1982 (Groom and Coughran, 2012). This separate database was curated by a single custodian between 1982-2017 and contains cetacean stranding events that include both live-stranded and dead, beach-cast cetaceans. Reports of stranded cetaceans in WA were investigated and validated (most with accompanying photos) by a government officer before being entered. Records of beach-cast false killer whales were collated from 2005-2021 for the purpose of this paper. In WA this included seven strandings, four, of which were live strandings. In the NT, the only recorded live stranding of a false killer whale was of an animal rescued by the Anindilyakwa Land and Sea Rangers and Traditional Owners on Groote Eylandt in 2017.
In April 2018, a group of approximately 20 false killer whales were encountered in the Groote Archipelago in the western Gulf of Carpentaria (NT) (Figure 1). ARGOS-linked SPOT-5 satellite tags (AM-S240C, Wildlife Computers, Redmond, Washington, USA) were deployed on two individuals from the group following the tag deployment and attachment methods and duty cycles described in Palmer et al. (2017), which were adapted from Baird et al. (2010). The tags transmitted data for 9 hours every day during the first 50 days, then every other day for 10 days, then every fifth day for the remainder of the tag deployment. Processing and analysis of the location data were as described in Palmer et al. (2017). In brief, we used a maximum redundant distance of 3 km, maximum travel speed of 20 km h-1, and the default Ratecoeff of 25. LC2 and LC3 locations were automatically retained, while LC1, LC0, LCA, and LCB locations were only retained if they passed the Douglas Argos-filter. ArcGIS vers. 10.2 (Environmental Systems Research Institute, Redlands, CA, USA) was used to calculate water depth and distance from nearest coastline for every record, as well as total distance travelled and minimum convex polygons across the lifetime of the tagging for both individuals. See Palmer et al. (2017) for details.

Figure 1 False killer whale satellite tracking data across Top End of the Northern Territory (four tagged Cobourg Marine Park) and two tagged Groote Archipelago (Gulf of Carpentaria).
Our sample set comprised 12 biopsies from live individuals and two tissue samples from stranded individuals (Table S1). Five of the biopsy samples were collected using a PAXARMS (a modified rifle) (Krützen et al., 2002) or a biopsy pole (Bilgmann et al., 2006) during a single encounter of approximately 20 individuals in 2020 in Lalang-gaddam Marine Park, WA. Four of the five individuals were photographically identified at the time of sampling and confirmed to be distinct. The fifth individual that was sampled via the biopsy pole was not photographed (due to bow riding and being submerged) but was determined at the time, based on unique markings, to be a different individual than the other four, and therefore targeted. The remaining seven biopsy samples were collected using a crossbow during a single encounter of approximately 20 animals in Cobourg Marine Park, in 2021. The sampled false killer whales were individually identified via video footage taken by a drone during sampling and confirmed to be distinct. The samples from stranded individuals were skin tissue collected in 2015 in Darwin Harbor and 2017 near Eighty Mile Beach, WA.
The two samples from stranded individuals and the five Lalang-gaddam Marine Park biopsy samples were accessioned into the Southwest Fisheries Science Centre’s Marine Mammal and Sea Turtle Research (MMASTR) sample collection (https://swfsc.noaa.gov/MMTD-TissueCollection/), where they were preserved in 100% ETOH and stored at -20 °C. These samples were genetically sexed using the ZFX and ZFY genes and sequenced at a 947 bp portion of the mitochondrial control region using laboratory methods described in Martien et al. (2014).
The seven samples from Cobourg Marine Park were digested overnight in ATL buffer and Proteinase K at 37°C, then extracted using a Qiagen DNeasy Blood and Tissue Kit (Qiagen, Netherlands). The PCR cycling profile for these samples consisted of 95°C for 5 min, followed by 35 cycles of 95°C for 30 s, an annealing temperature of 60°C for 30 s, 72°C for 1 min, then a final extension for 72°C for 7 min. PCR products were purified using Millipore plate MSNU030 (Millipore SAS, Molsheim, France). The control region sequence was generated in two parts, each of which was sequenced in both directions with the BigDye terminator v3.1 sequencing kit and a 3730xl automated sequencer (Applied Biosystems, Foster City, CA) at the Macrogen sequencing facility (Macrogen Inc., Seoul, Korea). The 5’ primers for these samples were the same as in Martien et al. (2014) (H16498, Rosel et al., 1994), (L15829, Martien et al., 2014). Novel 3’ primers were designed using Primer 3 (Geneious v2022.0.1), which were false killer whale_3F (5’-CTCGTGGGGGTAGCTAATAATGA-3’) and false killer whale_3R (5’-TGCCCATCTAGACATTTTCAGTGT-3’). The resulting 3’ segments had an 11-bp overlap with the 5’ sequences and were trimmed to the same 947 bp as reported by Martien et al. (2014).
The sequences for all 14 samples were compared to a published global database of false killer whale sequences from Martien et al. (2014) and Crofts et al. (2019). A median joining network (MJN; Bandelt et al., 1999) of all haplotypes was generated in the program PopArt (Leigh and Bryant, 2015) with epsilon set to zero.
We collated 179 sightings of free-swimming false killer whales (Table S2) and six false killer whale strandings (Table S3) in Australian waters between 2005 and 2021. Group sizes ranged from single individuals to one group of 200, though the majority had group sizes of 20 or fewer (group size was not recorded for 64 sightings) (Figure 2).
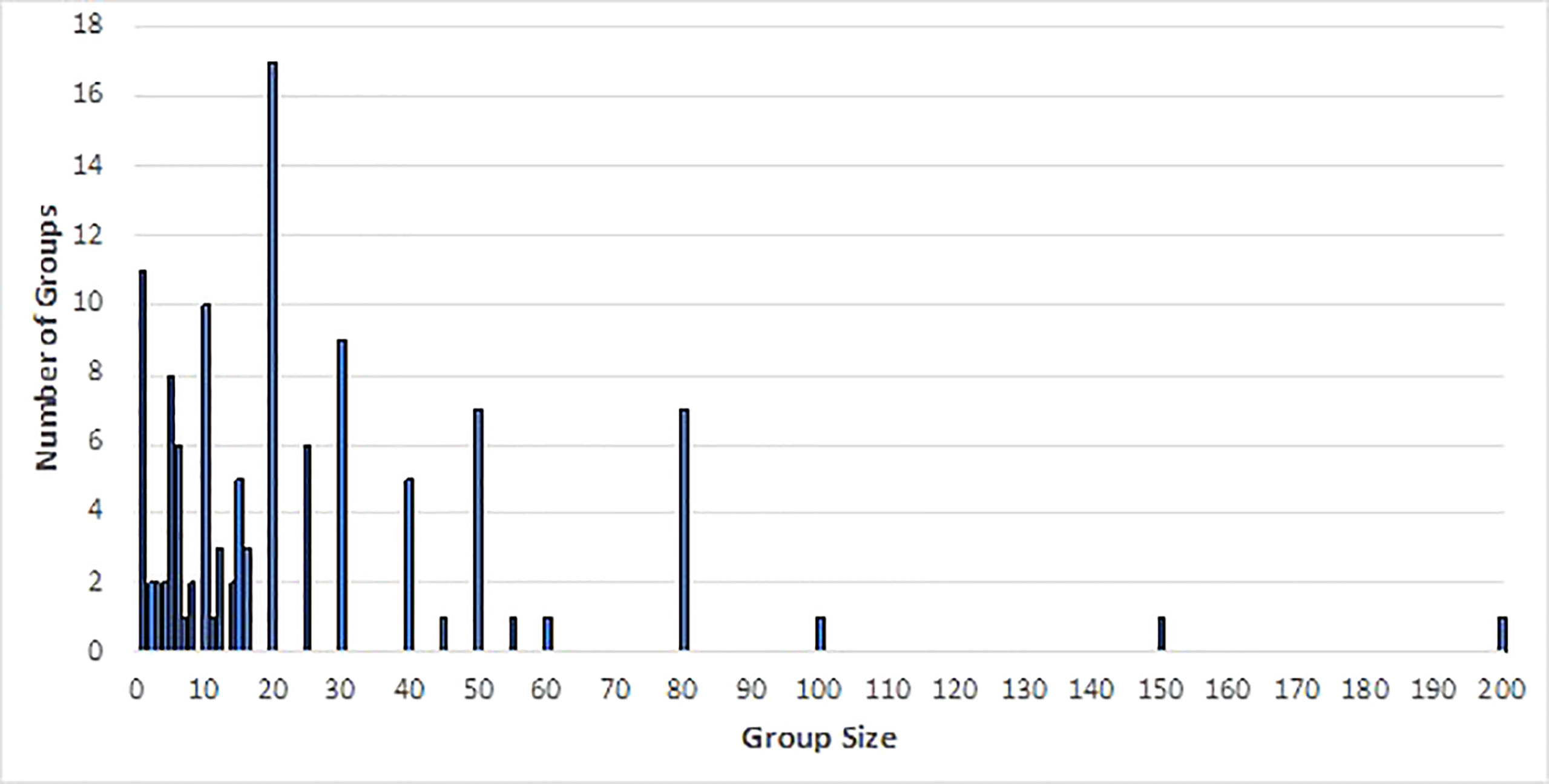
Figure 2 Group sizes of free-swimming false killer whale groups sighted 2005 to 2021 (based on all data sources).
Sightings of false killer whales have been recorded in every month with the most sightings in April and Sep, Oct and Nov (Figure 3). The majority of sightings (80%) were recorded in NT coastal waters, followed by records from northern WA (20%). Many WA sightings were from coastal locations where water depth is <10 metres (e.g., Gantheaume Point, Roebuck Bay in Broome) and this also applies to the sightings recorded in the NT (Figure 4). Gaps in the distribution of sightings and strandings likely represent coastal areas that are very sparsely populated, and we recognise that our records are likely to be biased towards sites populated by more people.
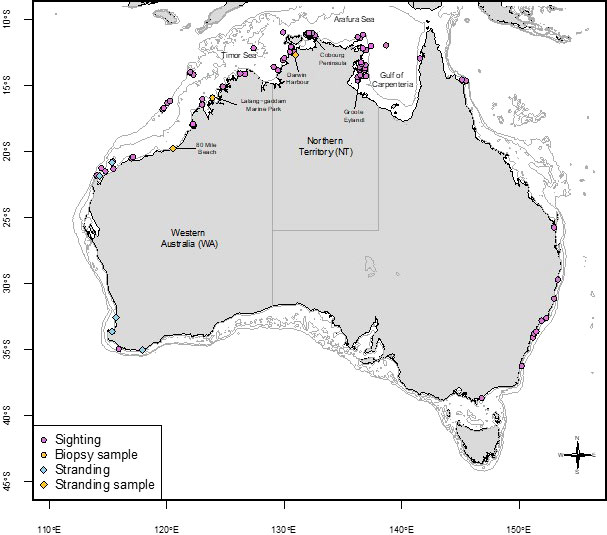
Figure 4 False killer whale records including sightings (2005 to 2021), strandings, and genetic sample locations. The 10m, 100m, and 500m depth contours are shown in light grey.
Results from tracking two false killer whales tagged around Groote Eylandt in 2018 are provided in Table 1 and Figure 1, along with summary results derived from Palmer et al. (2017) on four false killer whales tagged in Cobourg Marine Park in 2014.
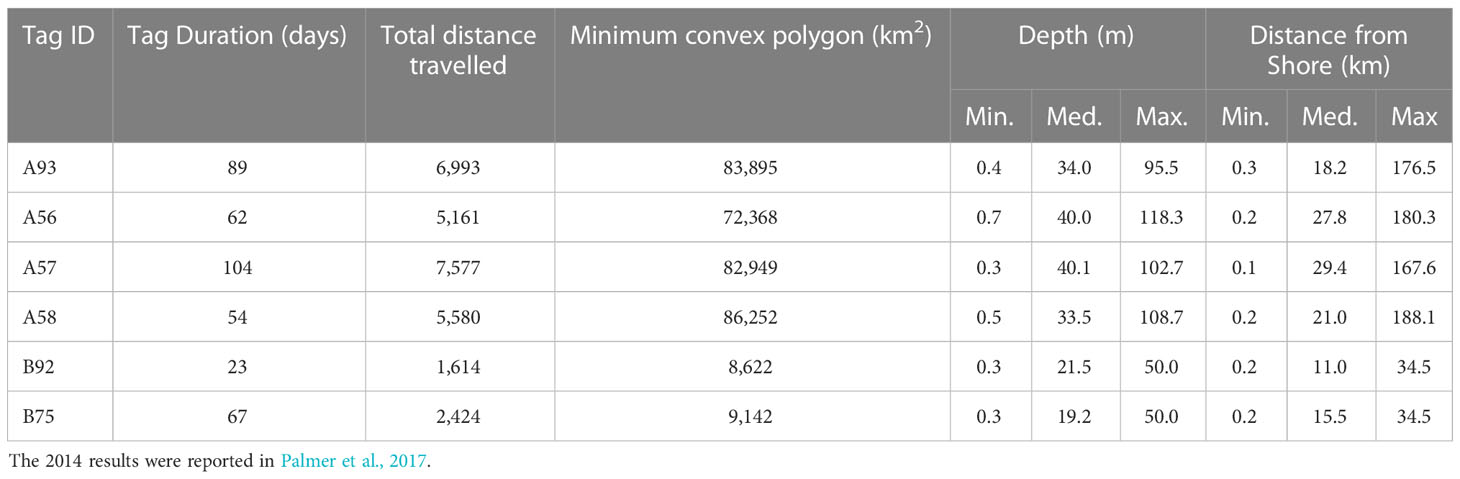
Table 1 Summary details on satellite-tag deployments (A = 2014 Garik Gunak Barlu & B = 2018 Groote Eylandt) with mean distances and depths.
The two tagged Groote Eylandt false killer whales exhibited similar movements. The total distance travelled was 1,614 km over a 23-day period and 2,424 km over 67 days. Distance from land varied from 0.2 up to 40 km. Over the tracking period, the total minimum convex polygons (MCP) for the individuals were 9,142 km2 and 8,622 km2, much smaller than those reported by Palmer et al. (2017) for animals tagged in Cobourg Marine Park (Table 1).
All 14 samples sequenced as part of this study possessed the same haplotype (Table S1), which is distinct from all previously published haplotypes. The new haplotype, designated haplotype 45, is most similar to the two haplotypes that typify the Hawaiian Island resident population(s), haplotypes 1 and 2 (Martien et al., 2014). While haplotypes 1 and 2 differ from each other by two substitutions, haplotype 45 differs from each of them by only one substitution (Figure 5).
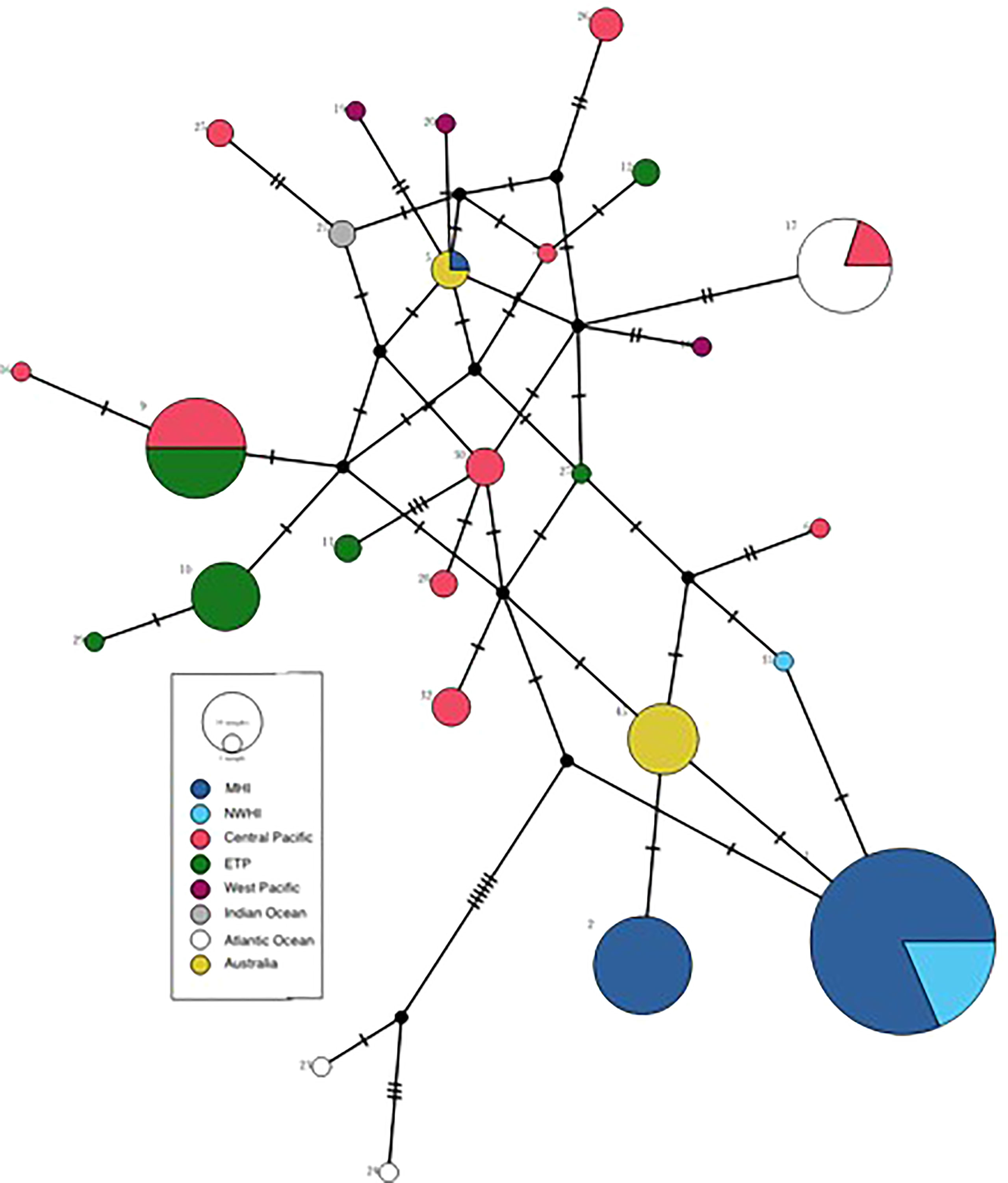
Figure 5 Median joining network of false killer whale haplotypes from this study (haplotype 45) and those published by Martien et al. (2014) and Crofts et al. (2019). Node size is proportional to the number of samples possessing that haplotype, while colours indicate the geographic regions in which the haplotype has been identified. Black nodes represent undetected haplotypes. Hash marks indicate the number of substitutions between haplotypes. Strata are as defined in Figure 1 of Martien et al. (2014).
There were four females and one male that were genetically identified from the five samples collected in WA from a pod of 20 individuals (Table S1). The animal that was stranded in 2017 was male, while the 2015 stranded sample could not be genetically sexed.
The data and analyses reported here provide further evidence that the northern Australian coastal waters host one or more resident populations of false killer whales that are demographically distinct from oceanic populations in surrounding waters. This builds on the recent research by Palmer et al. (2009); Palmer et al., 2017) reporting that false killer whales occur in shallow coastal waters in the Arafura and Timor Seas, ranging up to depths of 118 m in the Top End of the NT (Palmer et al., 2009; Palmer et al., 2017).
The collated sighting and stranding data presented here show that false killer whales are regular, year-round inhabitants of coastal areas of NT and WA (Figure 4). Because the sighting data were collected opportunistically, they are heavily biased by effort and therefore cannot be used to draw conclusions regarding density. Similarly, geographic gaps in sightings do not necessarily indicate distributional gaps. Our data show seasonal variation in sightings rates (Figure 4), which again could be the result of variation in effort. Northern Australia is defined by a very strongly seasonal (monsoonal) climate, which is manifested in coastal waters by extreme seasonal variation in discharge of rivers and marked seasonal variation in sea surface temperature, turbidity, and prevailing winds (Palmer, 2014). These seasonal environmental variations could result in seasonal differences in effort and/or detectability of false killer whales. It is also likely that these seasonal environmental variations impose marked variations in food resource availability for coastal delphinids, which may influence their patterns of habitat use, movement, and breeding activity (Palmer, 2014).
Sighting and stranding records extend beyond NT and WA in Australia (Figure 4) and it is unknown whether these represent more populations in other coastal areas or there may be links to oceanic populations that occasionally frequent coastal waters in other locations. The southwest of WA is an example of a site where there are multiple stranding records and few live sightings at sea reported and it is unknown whether this is because the population uses oceanic waters more and coastal waters less or they are under reported in this area. Further investigation is required to understand if there is any contemporary genetic connectivity or links between false killer whales in northern and southern Australia.
It is nonetheless noteworthy that a high proportion of the sighting records have been recorded in conservation reserves: Coburg Marine Park, Groote Archipelago Indigenous Protected Area (IPA), Yawuru Nagulagun Roebuck Bay Marine Park, and in Lalang-gaddam Marine Park. Many marine protected areas are known to have greater abundance and biomass of fish when compared to adjacent areas (Goetze et al., 2021). Systematic surveys of coastal waters of the NT and WA would be valuable in assessing the importance of protected areas for false killer whales in this region.
Location data obtained from the Groote Archipelago represent only the second description of movement patterns of false killer whales in the Southern Hemisphere. They are consistent with previously published satellite tag data from Cobourg (Palmer et al., 2017), showing that false killer whales spend extended periods of time in shallow coastal waters. There was also a marked degree of overlap of the two individuals in their ranging patterns, suggesting some group cohesion was maintained, similar to the level of cohesion that was apparent in the tagging data from Cobourg (Palmer et al., 2017) and that reported by Baird et al. (2010); Baird et al. (2012) for the main Hawaiian Islands insular population.
The minimum convex polygon (MCP) areas reported by Palmer et al. (2017) for false killer whales tagged near Cobourg (72,368 to 86,252 km2; Table 1) were comparable to the MCP area calculated by Baird et al. (2012) for all MHI insular false killer whales (82,800 km2), suggesting that the range size for the Cobourg animals is similar to that of the MHI insular population. However, the false killer whales from Groote Archipelago travelled much shorter distances and had nearly 10-fold smaller MCPs (8,622 and 9,142 km2) than the Cobourg animals and MHI false killer whales (Table 1). Though one of the Groote Archipelago animals (Tag ID E) had a relatively short tag duration of only 23 days which could influence the distance travelled and the MCP, the other (Tag ID F) had a tag duration (67 days) comparable to those for the animals tagged at Cobourg and in Hawai’i. Furthermore, the monthly MCPs for Cobourg animals (Figure 2 of Palmer et al., 2017) shows that they covered most of their overall MCP during the first month after tagging. Thus, the smaller ranges covered by animals tagged at Groote Archipelago is likely not a result of tag duration.
The notable difference in movement patterns between the individuals tagged near Groote and those tagged at Cobourg, and the lack of spatial overlap in the areas visited by the two groups, indicates that there may be separate populations in the Arafura/Timor Seas and the Gulf of Carpentaria. Photo identification has commenced, with catalogues being developed for the Kimberley, WA and Cobourg and Groote Archipelago, NT. To date, there have not been any photo-identification matches of individual dorsal fins between these locations (unpublished data). Additional satellite tagging, and photo-identification work is needed to determine whether these patterns hold with larger sample sizes. The WA catalogue currently is limited to 23 individuals and the NT catalogues now includes 101 individuals. Future research will focus on the systematic collection of photo identification data and a comparison between locations. The aim will be to collect data suitable for mark-resight abundance estimation.
Our genetic results provide strong evidence that false killer whales in the coastal waters around northern Australia are demographically distinct from oceanic populations. The fact that there is a single, previously undetected haplotype amongst all samples in our study indicates that there is little to no immigration into the north Australian coastal population(s) from the more genetically diverse oceanic populations documented by Martien et al. (2014), as immigration of females would result in shared haplotypes. Our genetic sample size is small and represents only two strandings and two live encounters. False killer whales have strong social structure such that most groups contain only one or two haplotypes (Martien et al., 2014; Martien et al., 2019). However, the fact our samples come from four sampling events spanning 6 years and across nearly 1,600 km of coastline indicates the lack of haplotypic diversity we documented does not result from sampling a single social group.
Because mitochondrial genomes are traditionally considered to be maternally inherited, we cannot rule out the possibility of ongoing gene flow between the north Australian coastal populations and neighboring oceanic animals. In fact, Martien et al. (2014) documented ongoing gene flow in the absence of shared haplotypes when comparing the NWHI resident population and the surrounding oceanic population. Nonetheless, the absence of dispersal (i.e., the permanent movement of animals between populations) renders populations demographically independent, regardless of whether they exchange genes through occasional inter-population mating (Waples and Gaggiotti, 2006; Palsbøll et al., 2007; Martien et al., 2012). Thus, the data presented here are sufficient to conclude that the coastal waters of northern Australia likely harbor one or more demographically independent resident populations, similar to the resident insular populations in the Main and Northwest Hawaiian Islands. Additional genetic studies, including a greatly expanded sample set and data from nuclear loci, are needed to further clarify the degree of connectivity between the Australian resident population(s) and investigate possible population structure within north Australian coastal waters.
Our genetic data, combined with that of Martien et al. (2014), suggest a possible evolutionary link between the MHI resident false killer whale population and northern Australia. Haplotype 45, possessed by all individuals in our current sample set, is intermediate between the two haplotypes (haplotypes 1 and 2) that typify the MHI resident population (Figure 5), suggesting a recent evolutionary divergence of the MHI resident population from the Australia resident population. Martien et al. (2014) found that the only individual (#49052) from the MHI resident population that does not possess haplotype 1 or 2 is a male whose haplotype 5 is shared with three free swimming false killer whales sampled in Garig Gunak Barlu National Park near the Cobourg Peninsula, NT, very close to the location where Palmer et al. (2017) tagged false killer whales in 2014. Based on its long sighting history in the MHI resident population and genetic data indicating that it has fathered at least one offspring in the population, Martien et al. (2019) concluded that 49052 was an immigrant into the MHI resident population. The evolutionary relationship between the MHI and Australia resident populations should be further investigated in future genetic studies. Additional samples collected from both Australian continental shelf and the largely-unsampled South and West Pacific could help to elucidate the mechanism by which Hawaiian and Australian resident populations came to share evolutionarily distinct haplotypes.
Resource specialization in different habitats has been proposed as an important mechanism driving population structure in many cetacean species (Hoelzel et al., 1998; Natoli et al., 2005; Möller et al., 2007; Martien et al., 2012), including false killer whales (Baird et al., 2013; Martien et al., 2014). Strong habitat differences between the extensive shallow waters of the Timor and Arafura Seas and the surrounding deep ocean have likely contributed to the evolution of the Australia resident populations. Differences in foraging strategies required to efficiently hunt in these very different environments can lead to divergence, especially in a species like the false killer whale, which is highly social and engages in cooperative hunting (Baird et al., 2008; Baird, 2016). Northern Australia is characterised by a very strongly seasonal (monsoonal) climate that manifests in coastal waters by extreme variation in discharge of rivers (Godfrey and Mansbridge, 2000), and some marked seasonal variation in sea surface temperature, turbidity, and prevailing winds (Condie, 2011). These characteristics may impose seasonal variations in food resource availability for coastal delphinids, which could further drive population divergence via habitat specialization.
For false killer whales in Australian waters, the assessment of population size and trends for conservation status – and hence the framing and implementation of conservation management responses – has been hampered by a lack of critical information. Though previous studies focussing on the smaller coastal dolphins have provided noteworthy and substantial advances in knowledge about their ecology and status (including two species endemic to northern Australia; snubfin Orcaella heinsohni and humpback dolphin Sousa sahulensis) (Corkeron et al., 1997; Parra et al., 2006a; Parra, 2006b; Cagnazzi et al., 2011; Allen et al., 2012; Brown et al., 2012; Palmer, 2014; Palmer et al., 2014a; Palmer et al., 2014b; Brown et al., 2014; Parra and Cagnazzi, 2016; Brooks et al., 2017; Bouchet et al., 2021), false killer whales have received little attention and research effort in comparison (Palmer et al., 2009; Palmer, 2014; Palmer et al., 2017). The only two conservation assessments of the status of the species in Australia over the last 25 years called for research to clarify critical parameters, such as population size, recruitment, mortality, and survival rates (Bannister et al., 1996; Ross, 2006; Woinarski et al., 2014), but the information base at a national level remains inadequate.
Based on the current results of sightings, movements, and genetic data presented here and in previous publications, the false killer whales in northern Australia likely constitute one or more resident populations that experience very little demographic exchange with widespread oceanic populations. The broad distribution (>1600 km of linear distance between the genetic sampling) of the resident population(s) within coastal waters and their apparent low density makes them more vulnerable to anthropogenic impacts than previously thought. Research on the species should be prioritised to improve our understanding of their population structure, distribution and movement patterns, and interactions with fisheries in order to better inform conservation management across their range.
The original contributions presented in the study are publicly available. This data can be found here: collection data for all genetic samples and the sighting and stranding data sets are available as Supplementary Materials, full sequence of haplotype 45 is available from GenBank (OP594724).
The animal study was reviewed and approved by Satellite tagging in 2018 was under the Charles Darwin University Animal Ethics Approval A17013. Biopsy sampling in the Northern Territory is under the Charles Darwin University Animal Ethics Project Permit Approval A21008 and Northern Territory Government Permit Approval 69214. Biopsy samples in Western Australia were collected under Animal Ethics Committee licence DPIRD U 10 2020-2022 and Scientific licence DBCA FO25000197. Genetic samples were imported into the USA under CITES permit 13US774223/9 and NMFS permit 14097.
CP: Conceptualization, data collection and curation, formal analysis, writing – original draft, review and editing. KM Conceptualization, data curation, formal analysis, writing – original draft, review and editing. HR: Data collection and curation, formal analysis, writing – original draft, reviewing and editing. AW: Data collection, biopsy samples. EW: Data collection, biopsy samples. RR: Data collection, biopsy samples. DC: Data collection, biopsy samples. ED’C: Data collection, biopsy samples, review and editing. EJ: Data collection, biopsy samples and review. NC: Data review, mapping and review. DB: Data collection, biopsy samples and review. AL: data collection, biopsy samples and review. KW: Review and editing. KR: Data collection and curation, writing – review and editing. AM: DNA analysis (NT). SM: Review and editing. All authors contributed to the article and approved the submitted version.
The satellite tagging of two false killer whales around the Groote Archipelago in 2018 was funded though the Anindilyakwa Land and Sea Rangers, Anindilyakwa Land Council and Flora and Fauna Division, Northern Territory Government. The current biopsy samples and the composing of this paper is under the recently funded ARC Marine Megafauna Project (LP200100222) (Charles Darwin University). The genetic analyses were funded by NOAA Fisheries’ Southwest Fisheries Science Center.
We thank the Traditional Owners of the Garik Gunak Barlu National Park (Cobourg Marine Park) and Groote Archipelago in the NT. In Western Australia, we thank Augustine Badal and Nathan Kay Traditional Owners and Nyangumarta Rangers caring for their sea country and Adrian Ferguson who sampled and reported the stranding in Eighty Mile Beach Marine Park, Dambeemangardee Rangers and Traditional Owners for their work in the Lalang-gaddam Marine Park and Yawuru Sea Country (Kimberley), where much of the sighting data were collected. We particularly thank elder Janet Oobagooma, who was present during the 2020 voyage where biopsies were taken in Lalang-Gaddam Marine Park. Jenelle Ritchie at the Western Australian Museum Boola Bardip, facilitated the samples being sent from Western Australia. Professor John Woinarski and Dr. Robin Baird provided expertise and support for this research, and Dr. Aimée Lang and Dr Julian Tyne provided valuable comments on the manuscript. Thank you also to the many recreational fishers, citizen scientists, Cygnet Bay Pearl Farm, and various departments the Northern Territory and Western Australian Governments who collected and curated sighting and stranding data.
Author EJ and AL were employed by Dambimangari Aboriginal Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.1067660/full#supplementary-material
Acevedo-Gutierrez A., Brennan B., Rodriguez P., Thomas M. (1997). Resighting’s and behaviour of false killer whales (Pseudorca crassidens) in Costa Rica. Mar. Mammal Sci. 13, 307–314. doi: 10.1111/j.1748-7692.1997.tb00634.x
Allen S. J., Cagnazzi D. D. B., Hodgson A. J., Loneragan N. R., Bejder L. (2012). Tropical inshore dolphins of north-western Australia: Unknown populations in a rapidly changing region. Pacific Conserv. Biol. 18, 56–63. doi: 10.1071/PC120056
Baird R. W. (2016). The lives of hawai’i's dolphins and whales: Natural history and conservation (Honolulu:University of Hawai’i Press).
Baird R. W. (2018). “False killer whale: Pseudorca crassidens,” in Encyclopedia of marine mammals, 3rd edition. Eds. Würsig B., Thewissen J. G. M., Kovacs K. M. (San Diego, CA: Academic Press), 347–349.
Baird R. W. (2018). “Pseudorca crassidens (errata version published in 2019),” in The IUCN red list of threatened species 2018 (IUCN).
Baird R. W., Gorgone A. M., McSweeney D. J., Webster D. L., Salden D. R., Deakos M. H., et al. (2008). False killer whales (Pseudorca crassidens) around the main Hawaiian islands: Long-term site fidelity, inter-island movements, and association patterns. Mar. Mammal Sci. 24, 591–612. doi: 10.1111/j.1748-7692.2008.00200.x
Baird R. W., Hanson M. B., Schorr G. S., Webster D. L., McSweeney D. J., Gorgone A. M., et al. (2012). Range and primary habitats of Hawaiian insular false killer whales: Informing determination of critical habitat. Endangered Species Res. 18, 47–61. doi: 10.3354/esr00435
Baird R. W., Oleson E. M., Barlow J., Ligon A. D., Gorgone A. M., Mahaffy S. D. (2013). Evidence of an island-associated population of false killer whales (Pseudorca crassidens) in the north-western Hawaiian islands. Pacific Sci. 67, 513–521. doi: 10.2984/67.4.2
Baird R. W., Schorr G. S., Webster D. L., McSweeney D. J., Hanson M. B., Andrews R. D. (2010). Movements of satellite-tagged false killer whales around the main Hawaiian islands. Endangered Species Res. 10, 107–121. doi: 10.3354/esr00258
Bandelt H.-J., Forster P., Rohl A. (1999). Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 16, 37–48. doi: 10.1093/oxfordjournals.molbev.a026036
Bannister J. L., Kemper C. M., Warneke R. M. (1996). The action plan for Australian cetaceans (Canberra, ACT, Australia: Australian Nature Conservation Agency).
Bilgmann K., Griffiths O. J., Allen S. J., Moller L. M. (2006). A biopsy pole system for bow-riding dolphins: Sampling success, behavioural responses, and test for sampling bias. Mar. Mammal Sci. 23, 218–225. doi: 10.1111/j.1748-7692.2006.00099.x
Bouchet P. J., Thiele D., Marley S. A., Waples K., Weisenberger F., Rangers B., et al. (2021). Regional assessment of the conservation status of snubfin dolphins (Orcaella heinsohni) in the Kimberley region. Front. Mar. Sci. (Western Australia) 7. doi: 10.3389/fmars.2020.614852
Brooks L., Palmer C., Griffiths A. D., Pollock K. H. (2017). Monitoring variation in small coastal dolphin populations: An example from Darwin, northern territory, Australia. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00094
Brown A., Bejder L., Cagnazzi D., Parra G. J., Allen S. (2012). The Northwest cape, Western Australia: A hotspot for indo-pacific humpback dolphins Sousa chinensis. Pacific Conserv. Biol. 18, 240–246. doi: 10.1071/PC120240
Brown A. M., Kopps A. M., Allen S. J., Bejder L., Littleford-Colquhoun B., Parra G. J., et al. (2014). Population differentiation and hybridisation of Australian snubfin (Orcaella heinsohni) and indo-pacific humpback (Sousa chinensis) dolphins in north-western Australia. PloS One 9, e101427. doi: 10.1371/journal.pone.0101427
Cagnazzi D. D. B., Harrison P. L., Ross G. J. B., Lynch P. (2011). Abundance and site fidelity of indo-pacific humpback dolphins in the great sandy strait, Queensland, Australia. Mar. Mammal Sci. 27, 255–281. doi: 10.1111/j.1748-7692.2009.00296.x
Chatto R., Warneke R. M. (2000). Records of cetacean strandings in the northern territory of Australia. Beagle 16, 163–175. doi: 10.5962/p.254544
Condie S. A. (2011). Modelling seasonal circulation, upwelling and tidal mixing in the arafura and timor seas. Continental Shelf Res. 31, 1427–1436. doi: 10.1016/J.CSR.2011.06.005
Corkeron P. J., Morissette N. M., Porter L., Marsh H. (1997). Distribution and status of humpbacked dolphins Sousa chinensis, in Australian waters. Asian Mar. Biol. 14, 49–57.
Crofts S., Martien K. K., Robertson K. M., Stanworth A., Massam S., Weir C. R. (2019). First record of false killer whales (Pseudorca crassidens) in the Falkland islands (Malvinas). Polar Biol. 42, 1923–1929. doi: 10.1007/s00300-019-02554-9
Godfrey J. S., Mansbridge J. V. (2000). Ekman transports, tidal mixing, and the control of temperature structure in australia’s northwest waters. J. Geophys. Res. 105, 24021–24044. doi: 10.1029/2000JC900104
Goetze J. S., Wilson S., Radford B., Fisher R., Langlois T. J., Monk J., et al. (2021). Increased connectivity and depth improve the effectiveness of marine reserves. Global Change Biol. 27, 3432–3447. doi: 10.1111/gcb.15635
Groom C. J., Coughran D. K. (2012). Three decades of cetacean strandings in Western Australia: 1981 to 2010. J. R. Soc. Western Aust. 95, 63–76.
Hoelzel A. R., Dahlheim M., Stern S. J. (1998). Low genetic variation among killer whales (Orcinus orca) in the Eastern north pacific and genetic differentiation between foraging specialists. J. Heredity 89, 121–128. doi: 10.1093/jhered/89.2.121
Krützen M., Barré L. M., Möller L. M., Heithaus M. R., Simms C., Sherwin W. B. (2002). A biopsy system for small cetaceans: Darting success and wound healing in tursiops spp. Mar. Mammal Sci. 18, 863–878. doi: 10.1111/j.1748-7692.2002.tb01078.x
Leigh J., Bryant D. (2015). PopART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 6, 1110–1116. doi: 10.1111/2041-210X.12410
Martien K. K., Baird R. W., Hedrick N. M., Gorgone A. M., Thieleking J. L., McSweeney D. J., et al. (2012). Population structure of island-associated dolphins: evidence from mitochondrial and microsatellite markers for common bottlenose dolphins (Tursiops truncatus) around the main Hawaiian islands. Mar. Mammal Sci. 28, E208–E232. doi: 10.1111/j.1748-7692.2011.00506.x
Martien K. K., Chivers S. J., Baird R. W., Archer F. I., Gorgone A. M., Hancock-Hanser B. L., et al. (2014). Nuclear and mitochondrial patterns of population structure in north pacific false killer whales (Pseudorca crassidens). J. Heredity 105, 611–626. doi: 10.1093/jhered/esu029
Martien K. K., Taylor B. L., Chivers S. J., Mahaffy S. D., Gorgone A. M., Baird R. W. (2019). Fidelity to natal social groups and mating within and between social groups in an endangered false killer whale population. Endangered Species Res. 40, 219–230. doi: 10.3354/esr0 0995
Möller L. M., Wiszniewski J., Allen S. J., Beheregaray L. B. (2007). Habitat type promotes rapid and extremely localised genetic differentiation in dolphins. Mar. Freshw. Res. 58, 640–648. doi: 10.1071/MF06218
Natoli A., Birkun A., Aguilar A., Lopez A., Hoelzel A. R. (2005). Habitat structure and the dispersal of male and female bottlenose dolphins (Tursiops truncatus). Proc. R. Soc. B-Biol. Sci. 272, 1217–1226. doi: 10.1098/rspb.2005.3076
Palmer C. (2014). “Conservation biology of dolphins in coastal waters of the northern territory, Australia,” in Doctor of philosophy (Darwin: Charles Darwin University).
Palmer C., Baird R. W., Webster D. L., Patterson R., Edwards A., Withers. A., et al. (2017). “A preliminary study of the movement patterns of false killer whales pseudorca crassidens in coastal waters of the northern territory, Australia,” in Marine and freshwater research. 68 (9), 1726–1733 doi: 10.1071/MF16296
Palmer C., Brooks L., Parra G. J., Rogers T., Glasgow D., Woinarski J. C. Z. (2014b). Estimates of abundance and apparent survival of coastal dolphins in port essington harbour, northern territory, Australia. Wildlife Res. 41, 35–45.
Palmer C., Fitzgerald P., Wood A., Harley S., McKenzie A. (2009). “). false killer whales pseudorca crassidens: Regular visitors to port essington and Darwin harbour in the northern territory, Australia,” in Northern territory naturalist, vol. 21. , 49–53.
Palmer C., Parra G. J., Rogers T., Woinarski J. (2014a). Collation and review of sightings and distribution of three coastal dolphin species in waters of the northern territory, Australia. Pacific Conserv. Biol. 20, 116–125.
Palsbøll P. J., Bérubé M., Allendorf F. W. (2007). Identification of management units using population genetic data. Trends Ecol. Evol. 22, 11–16. doi: 10.1016/j.tree.2006.09.003
Parra G. J. (2006b). Resource partitioning in sympatric delphinids: Space use and habitat preferences of Australian snubfin and indo-pacific humpback dolphins. J. Anim. Ecol. 75, 862–874. doi: 10.1111/j.1365-2656.2006.01104.x
Parra G. J., Cagnazzi D. (2016). Conservation status of the Australian humpback dolphin (Sousa sahulensis) using the IUCN red list criteria. Adv. Mar. Biol. 73, 157–192. doi: 10.1016/bs.amb.2015.07.006
Parra G. J., Corkeron P. J., Marsh H. (2006a). Population sizes, site fidelity and residence patterns of Australian snubfin and indo-pacific humpback dolphins: Implications for conservation. Biol. Conserv. 129, 167–180. doi: 10.1016/j.biocon.2005.10.031
Rosel P. E., Dizon A. E., Heyning J. E. (1994). Genetic analysis of sympatric morphotypes of common dolphins (genus delphinus). Mar. Biol. 119, 159–167.
Ross G. J. B. (2006). Review of the conservation status of australia's smaller whales, and dolphins (Canberra: Australian Government).
Stacey P. J., Baird R. W. (1994). Status of the false killer whale, pseudorca crassidens, in Canada. Can. Field Nat. 105, 189–197.
Waples R. S., Gaggiotti O. (2006). What is a population? an empirical evaluation of some genetic methods for identifying the number of gene pools and their degree of connectivity. Mol. Ecol. 15, 1419–1439. doi: 10.1111/j.1365-294X.2006.02890
Woinarski J. C. Z., Burbidge A. A., Harrison P. L. (2014). “The action plan for Australian mammals 2012,” (Melbourne: CSIRO Publishing).
Zaeschmar J. R., Visser I. N., Fertl D., Dwyer S. L., Meissner A. M., Halliday J., et al. (2014). Occurrence of false killer whales (Pseudorca crassidens) and their association with common bottlenose dolphins (Tursiops truncatus) off northeastern new Zealand. Marine Mammal Science 30 (2), 594–608. doi: 10.1111/mms.12065
Keywords: residency, population structure, movement, mitochondrial DNA, Indo-Pacific, satellite telemetry, conservation, management
Citation: Palmer C, Martien KK, Raudino H, Robertson KM, Withers A, Withers E, Risk R, Cooper D, D’Cruz E, Jungine E, Barrow D, Cuff N, Lane A, Keynes D, Waples K, Malpartida A and Banks S (2023) Evidence of resident coastal population(s) of false killer whales (Pseudorca crassidens) in northern Australian waters. Front. Mar. Sci. 9:1067660. doi: 10.3389/fmars.2022.1067660
Received: 12 October 2022; Accepted: 14 December 2022;
Published: 30 January 2023.
Edited by:
Jeremy Kiszka, Florida International University, United StatesReviewed by:
Jessica M. Aschettino, Independent Researcher, Virginia Beach, VA, United StatesCopyright © 2023 Palmer, Martien, Raudino, Robertson, Withers, Withers, Risk, Cooper, D’Cruz, Jungine, Barrow, Cuff, Lane, Keynes, Waples, Malpartida and Banks. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carol Palmer, Y2Fyb2wucGFsbWVyMkBjZHUuZWR1LmF1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.