
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 05 January 2023
Sec. Coral Reef Research
Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.1067449
 Alwin Hylkema1,2*
Alwin Hylkema1,2* Kimani Kitson-Walters3,4
Kimani Kitson-Walters3,4 Patricia R. Kramer5
Patricia R. Kramer5 Joshua T. Patterson6
Joshua T. Patterson6 Lynnette Roth5
Lynnette Roth5 Moriah L. B. Sevier7
Moriah L. B. Sevier7 Maria Vega-Rodriguez8
Maria Vega-Rodriguez8 Matthew M. Warham9
Matthew M. Warham9 Stacey M. Williams10
Stacey M. Williams10 Judith C. Lang5
Judith C. Lang5The 1983-1984 die-off of the long-spined sea urchin Diadema antillarum stands out as a catastrophic marine event because of its detrimental effects on Caribbean coral reefs. Without the grazing activities of this key herbivore, turf and macroalgae became the dominant benthic group, inhibiting coral recruitment and compromising coral reef recovery from other disturbances. In the decades that followed, recovery of D. antillarum populations was slow to non-existent. In late January 2022, a new mass mortality of D. antillarum was first observed in the U.S. Virgin Islands. We documented the spread and extent of this new die-off using an online survey. Infected individuals were closely monitored in the lab to record signs of illness, while a large population on Saba, Dutch Caribbean, was surveyed weekly before and during mortality to determine the lethality of this event. Within four months the die-off was distributed over 1,300 km from north to south and 2,500 km east to west. Whereas the 1983-1984 die-off advanced mostly with the currents, the 2022 event has appeared far more quickly in geographically distant areas. First die-off observations in each jurisdiction were often close to harbor areas, which, together with their rapid appearance, suggests that anthropogenic factors may have contributed to the spread of the causative agent. The signs of illness in sick D. antillarum were very similar to those recorded during the 1983-1984 die-off: lack of tube feet control, slow spine reaction followed by their loss, and necrosis of the epidermis were observed in both lab and wild urchins. Affected populations succumbed fast; within a month of the first signs of illness, a closely monitored population at Saba, Dutch Caribbean, had decreased from 4.05 individuals per m2 to 0.05 individuals per m2. Lethality can therefore be as high as 99%. The full extent of the 2022 D. antillarum die-off event is not currently known. The slower spread in the summer of 2022 might indicate that the die-off is coming to a (temporary) standstill. If this is the case, some populations will remain unaffected and potentially supply larvae to downstream areas and augment natural recovery processes. In addition, several D. antillarum rehabilitation approaches have been developed in the past decade and some are ready for large scale implementation. However, active conservation and restoration should not distract from the primary goal of identifying a cause and, if possible, implementing actions to decrease the likelihood of future D. antillarum die-off events.
Diadema antillarum is a conspicuous sea urchin native to the western Atlantic between Bermuda and Brazil. It inhabits coral reefs, hard bottoms, rubble, rocks, seagrass meadows, mangrove prop roots and tide pools, as well as seawalls and other human structures. D. antillarum is a nocturnally active herbivore, with a diet that varies with habitat and includes diverse algae (turfs, endoliths, crustose corallines, peyssonnelids, erect macroalgae), seagrasses and silty detritus (Randall et al., 1964; Bak et al., 1984; Williams, 2022). Known as a key consumer of the benthic algae that compete with corals for space on reefs (Sammarco, 1980), D. antillarum is also a facultative carnivore that can ingest coral tissues and other sessile reef invertebrates (Bak and van Eys, 1975). Grazing by D. antillarum on juvenile stony corals affects the diversity and abundance of stony corals on shallow reefs (Sammarco, 1980), while the bioerosion caused by its scraping of the substratum when feeding can exceed local rates of reef carbonate production (Ogden, 1976; Bak et al., 1984).
Before 1983, D. antillarum was the most abundant herbivore on Caribbean coral reefs, as herbivorous fish were already severely overfished in many locations (Jackson et al., 2001; Pandolfi et al., 2003). The species’ importance in controlling algal growth became clear after a Caribbean wide die-off in 1983 and 1984. This mass mortality was one of the most extensive and severe die-offs ever recorded for a marine invertebrate (Lessios, 1995) and it is assumed that no D. antillarum population escaped its effects (Lessios, 2016). The lethality of the die-off in the 1980s was drastic, as populations were reduced, on average, by 98% (Lessios, 2016). Within five days after D. antillarum populations succumbed, turf algal communities increased 20% in biomass and their primary productivity plummeted (Carpenter, 1988). In the years that followed, stony coral and crustose coralline algal cover started to decrease, while filamentous algal turfs and macroalgae became the dominant benthic groups on many reefs (Carpenter, 1986; de Ruyter van Steveninck and Bak, 1986; Liddell and Ohlhorst, 1986; Hughes et al., 1987; Carpenter, 1990) and inhibited coral recruitment (McCook et al., 2001; Box and Mumby, 2007; Arnold et al., 2010). Coral recruitment failure reduced the ability of Caribbean coral reefs to recover from other disturbances (Mumby et al., 2007), such as coral diseases, bleaching events and hurricanes, which led to their stepwise degradation (Hughes, 1994; Jackson et al., 2014). The catastrophic effects of the D. antillarum die-off were summarized by Lessios (2016): “The 1983–1984 mass mortality of the long-spined black sea urchin D. antillarum in the western Atlantic stands out for its sudden onset, intensity, geographic extent, and impact of the loss of a single species on entire ecological communities.”
The D. antillarum mass mortality was believed to have originated at Punta Galeta, Panama during the middle of January 1983 (Lessios et al., 1984a). Over the next 13 months, the mass mortality spread to all parts of the tropical western Atlantic. By February 1984, an area of 3.5 million square kilometers had been affected (Lessios, 1988). Given the pattern of spread, it was theorized that the causative agent of the die-offs was mainly transported by surface currents (Lessios et al., 1984a). After reanalyzing satellite data it became clear that not all dispersal could be attributed to surface currents and that boat traffic probably had also played a role in the spread of the mass mortalities (Phinney et al., 2002).
The first visible sign of D. antillarum behaving abnormally was the presence and accumulation of sediments or mucoid material on their spines (Bak et al., 1984; Lessios et al., 1984b; Hughes et al., 1985), followed by disappearance of epidermis and loss of spines (Bak et al., 1984; Lessios et al., 1984b; Hughes et al., 1985) and loss of tube feet control (Lessios et al., 1984b). The tissue on the spines became a dull color, almost opaque and would easily slough off if disturbed. Tissue degradation on the test would usually occur first on the ventral side of the sea urchin and progress dorsally giving a look of white patches or lesions (Personal communication N. Ogden). Individuals succumbed within four days after showing first signs of illness (Bak et al., 1984; Hughes et al., 1985).
The cause of the 1983-1984 die-off was never confirmed (Lessios, 2005; DeFilippo et al., 2018). Bauer and Agerter (1987) caused lethal infection in apparently healthy D. antillarum by using two species of Clostridium (anaerobic spore-forming bacteria) that were isolated and cultured from dead urchins that may have succumbed to the 1983-1984 pathogen. Later, Beck et al. (2014) found that D. antillarum may have humoral immune response deficiencies against gram-negative bacteria relative to other Caribbean urchin species, which remained unaffected by the 1983-84 mortality event. The authors of both studies cautioned against drawing conclusions regarding the causative agent of mass mortality. Several smaller D. antillarum die-offs were reported after the 1983-1984 mass mortality, but their extent remained limited to Panama, and St. Croix (Lessios, 1988) and subsequently to Florida (Forcucci, 1994) and provided no further insight about the causative agent of these die-offs. In the decades after the mass mortality, D. antillarum recovered up to 12% of their previous densities (Lessios, 2016) and started to refill their important ecological roles on several mostly shallow (<7m depth) reefs (Edmunds and Carpenter, 2001; Carpenter & Edmunds, 2006; Myhre and Acevedo-Gutiérrez, 2007; Idjadi et al., 2010).
However, in mid-February 2022 a local diver in St. Thomas, U.S. Virgin Islands (USVI) posted images on social media of dozens of D. antillarum dying at a popular dive site. At first, this die-off was thought to be a local event, but informal reports of dead and dying D. antillarum from geographically separated Caribbean islands began to circulate in mid-March. To help coordinate efforts to investigate and disseminate information about this troubling new die-off, a group of Caribbean-focused researchers and managers created an open, inclusive Diadema Response Network, which launched on 30 March 2022. In this first study by the Response Network, we describe the spread, extent, signs of illness, and lethality of the 2022 D. antillarum die-off and discuss similarities and dissimilarities with the 1983-84 die-off, as well as possible consequences for Caribbean coral reefs.
Affected D. antillarum were first observed in the USVI in late January 2022. However, this initial observation was not shared with the public until 16 February 2022, when a local diver posted his images from 8 February on social media. Upon seeing the die-off images, the Virgin Islands Coral Reef Advisory Group decided to use an existing citizen science reporting tool that had been developed to monitor the occurrence of Stony Coral Tissue Loss Disease (SCTLD) to launch a territory-wide Diadema tracking effort (https://www.vicoraldisease.org/sctld-disease-tracking). Through use of social media and newspaper articles the USVI public was informed of the die-off and urged to make reports to the online database of healthy, sick, or dying urchins. Observations from local community members, recreational divers, and the research community, some extending back to early February, were collected in the online database and reviewed to track the die-off spread in real time. All observations were verified via email or via submitted photos. Together with entries in the Diadema Health Tracking database (see below) this resulted in 209 observations of D. antillarum mass mortalities in St John and St Thomas. For the purposes of this publication local spread at these islands was documented through the end of May 2022.
To determine the regional spread and spatial distribution of the recent die-offs in the Caribbean, we developed an online ArcGIS 123 Survey Form on 30 March 2022, allowing surveyors to report observations of healthy, sick, or dead D. antillarum and, whenever possible, quantitative data, descriptions, photographs, and videos. In addition, the Atlantic and Gulf Rapid Reef Assessment (AGRRA) program developed an interactive map to track the health of D. antillarum (https://www.agrra.org/sea-urchin-die-off/), which was modeled after the program’s previous efforts to trace the movement of SCTLD around the Caribbean. Once a report was submitted, it appeared on the Diadema Tracking Map as a purple marker to be reviewed by experienced members of the Diadema Response Network. Criteria used to determine the health of urchins were based on behavioral assessment categories developed by Francis-Floyd (2020) including Category One behaviors (e.g., attachment to substrate and assessment of spine position) and Category Two behaviors (e.g., righting response, touch and defense responses, and inferred tube feet activity), as well obvious signs of epidermal lesions, detached spines, or mortality. Reports that lacked quantitative observations of more than several dead urchins and/or imagery depicting mass mortality were further assessed by requesting additional information from the contributors and, for any who were not already established reef observers, from local researchers or governmental representatives. Once surveys were reviewed, the markers were coded green for healthy urchins, red for evidence of die-off, or remained purple for any reports with an unknown status. Sites in which previously known D. antillarum populations had recently disappeared were coded white. All site markers can be clicked to open a pop-up table of additional information and data, with links to photos when available. The first record of D. antillarum mass mortality in each jurisdiction (country or territory) is a proxy for its first occurrence in these locations. As of 31 August 2022, 306 respondents in 34 jurisdictions (countries/territories) had submitted 582 reports and 426 photographs to the Diadema Health Tracking database, along with several videos. The Measure tool of ArcMap 10.8.1 was used to measure the distance between the first D. antillarum die-off at each jurisdiction and the nearest port, harbor, jetty, or other infrastructure for boats. Entries in the database through 31 August 2022 were included in this paper.
To determine the effect of the die-off on D. antillarum populations, pre- and post-die-off-D. antillarum densities were determined at dive site Diadema City in Saba, Dutch Caribbean (Figure 1). The dive site is a former breakwater, which was destroyed by hurricane Hugo in 1989 and is now fully submerged with a depth range between 5 and 10 m. Diadema City is 100 m long and 10-20 m wide and contained the largest population of D. antillarum around Saba. After D. antillarum had been observed dying in Saba’s Fort Bay Harbor, three permanent transects of 30 m each were established at Diadema City, which is located 200 m upstream of the harbor and where no D. antillarum had yet shown any sign of illness. A base-line survey was conducted on 17 March 2022. All D. antillarum within 1 m of the transect lines were inspected for any signs of illness and categorized as healthy or sick. Healthy individuals lacked all signs of illness and sick individuals had at least one of the signs described in more detail in the Results section of this study: lack of tube feet control, slow spine reaction, loss of spines and necrosis of the epidermis. The population was inspected for sick or dying D. antillarum twice a week and quantitatively monitored weekly for six weeks after the first signs of illness were observed, following the above-described method.
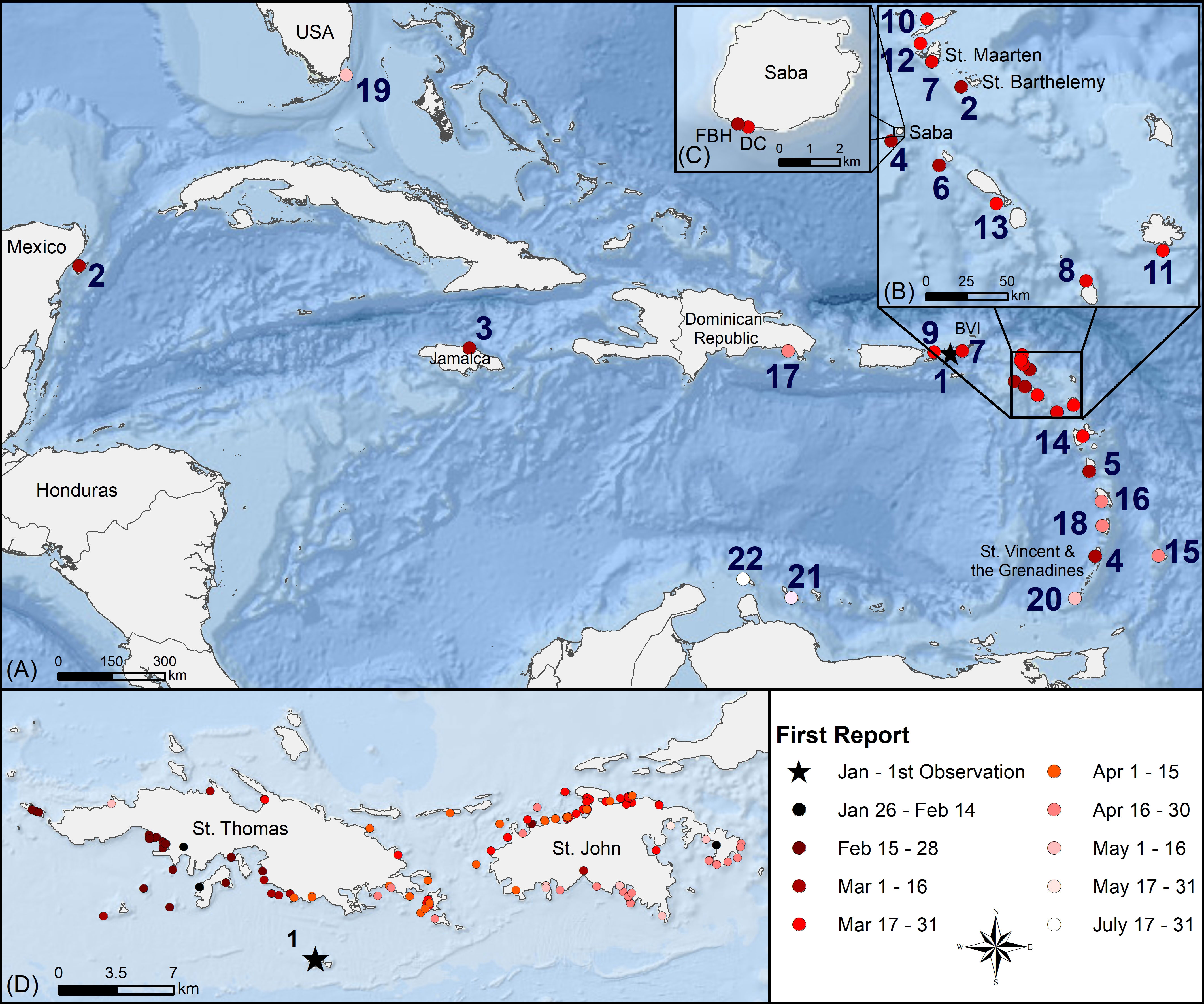
Figure 1 Progression of the 2022 D. antillarum die-off in the Caribbean. (A) Regional map shows the chronology of the die-off colored in bimonthly intervals from January to 31 August 2022, with the (B) northeastern Caribbean islands enhanced for clarity. Map numbers in (A, B) represent the first observed outbreak date for each affected jurisdiction (country or territory) in sequential order. Ties (duplicate numbers) for identical first observed outbreak dates in two jurisdictions. (C) Saba and research locations Fort Bay Harbor (FBH) and Diadema City (DC). (D) Local map shows the bimonthly die-off spread in the U.S. Virgin Islands from January to May. Esri Oceans Basemap Credits: Esri, Garmin, GEBCO, NOAA NGDC, and other contributors.
To determine the effect of the die-off on other sea urchin species, we recorded the post-die-off densities of D. antillarum and three other sea urchin species in Saba’s Fort Bay Harbor. A shallow breakwater (<2 m) of approximately 60 m2 contained a large D. antillarum population, along with individuals of the species Echinometra lucunter, Tripneustes ventricosus and Eucidaris tribuloides. The breakwater was surveyed for the first time on 18 March 2022, six days after local dive shop staff reported that urchins were dying in the harbor and three days after we confirmed this was the case, but large swells prevented an earlier survey. We categorized all observed sea urchins as healthy, sick or dead per species. Sick individuals had at least one of the signs of illness described above. Dead D. antillarum were only recorded during the first survey, as swells and currents quickly washed away the skeletons. The survey was repeated weekly for nine weeks.
Field observations were made of the dying D. antillarum in Fort Bay Harbor, Saba and in Oranje Bay, on the neighboring island of St. Eustatius on 15 March 2022. At that time, 96 D. antillarum juveniles (test size 12-37 mm) were grown-out in 12 static 70 L tanks in the Saba Research Center. The tanks were equipped with aeration and air driven sponge filters, receiving weekly water changes of 15 L with natural seawater. On 15 March 2022, a water replacement was conducted with water from the harbor, which was taken in close to the wild D. antillarum population. Only later that day it was discovered that half the D. antillarum in the harbor were dead or dying. The first four lab D. antillarum individuals that showed abnormal behavior were closely inspected twice a day and signs of illness were recorded in the order they occurred.
A linear mixed model (LMM) was used to test the effect of monitoring day (fixed effect) on D. antillarum density at Diadema City. To correct for the repeated counts at the same transects, transect ID was included as a random factor. The model was composed using the lmer function in the R package “lme4” (Bates et al., 2015) and model validation was performed according to Zuur et al. (2009). Pairwise comparisons between monitoring days were performed using the package “emmeans” (Lenth and Herve, 2019). Statistical analyses were performed with R (R Core Team, 2022). No statistical interference could be performed on the Fort Bay Harbor counts, as this survey contained a single transect. Reported values are means ± standard deviation, unless otherwise indicated.
During the first two weeks after initial observation of the D. antillarum die-off in late January, the spread seemed to be localized to southwestern St. Thomas but, by mid-February, reports of affected urchins were extending along the southeastern coast (Figure 1D). Mortality in northern St. Thomas was first reported in early March, at the same time as the die-off reached the neighboring island of St. John, more than 16 km away from the initial die-off site. Once arriving in St. John, the die-off quickly spread across the north coast and by early May it had enveloped the island.
Major die-offs of D. antillarum were verified in 25 jurisdictions, as shown in chronological order with ties for duplicate entries on the same day in Figure 1 and Table 1; all individuals in another nine appeared healthy. The earliest record of dying urchins came from January 26 in the USVI. By mid-March, reports of D. antillarum die-offs extended across the Caribbean from St. Barthélemy in the east, to the centrally located island of Jamaica and west to Cozumel, Mexico, while also impacting the Eastern Caribbean islands of St. Vincent and the Grenadines, Saba, Dominica, and St. Eustatius. The British Virgin Islands, St. Maarten, and Montserrat followed in the second half of March. There were die-off reports for Puerto Rico and the Eastern Caribbean islands of Anguilla, Antigua, St. Martin, St. Kitts and Nevis, and Guadeloupe in the first half of April. By the end of April, the islands of Barbados, Martinique, the Dominican Republic and St. Lucia had reported mass die-offs of D. antillarum. In early May, sick and dying D. antillarum were found at a few sites in Florida, U.S.A., and Grenada. The first observations in the Southern Caribbean occurred in Curaçao before the end of May. Thus, in a little more than 4 months, the 2022 mortality event had spanned distances of over 1,300 km from north to south and 2,500 km from east to west. Two months later, on 31 July 2022, dying urchins were reported in Aruba. Jurisdictions in which, as of 31 August 2022, only healthy urchin populations have been noted include Bonaire, Belize, Bermuda, Colombia, Cayman Islands, Honduras, Panama, Trinidad and Tobago, and the Turks and Caicos Islands. Excepting St. Barthélemy, where newly deceased D. antillarum on the windward side of the island were observed from 10-23 June 2022 next to a patch of Acropora palmata that had been monitored weekly since April, no reports of ongoing mortalities have been received from the Eastern Caribbean since May 2022. Occasionally new observations of disease were noted off the north coast of Puerto Rico, most recently on August 18, and off the northern mainland of the Mexican Caribbean, on August 23. The first occurrence in each jurisdiction was, on average, 1.9 km from the nearest harbor or other infrastructure for boats (Table 1). Eleven of the 25 first occurrences were within 1 km of the nearest harbor and 18 of 25 were within 3 km of the nearest harbor.
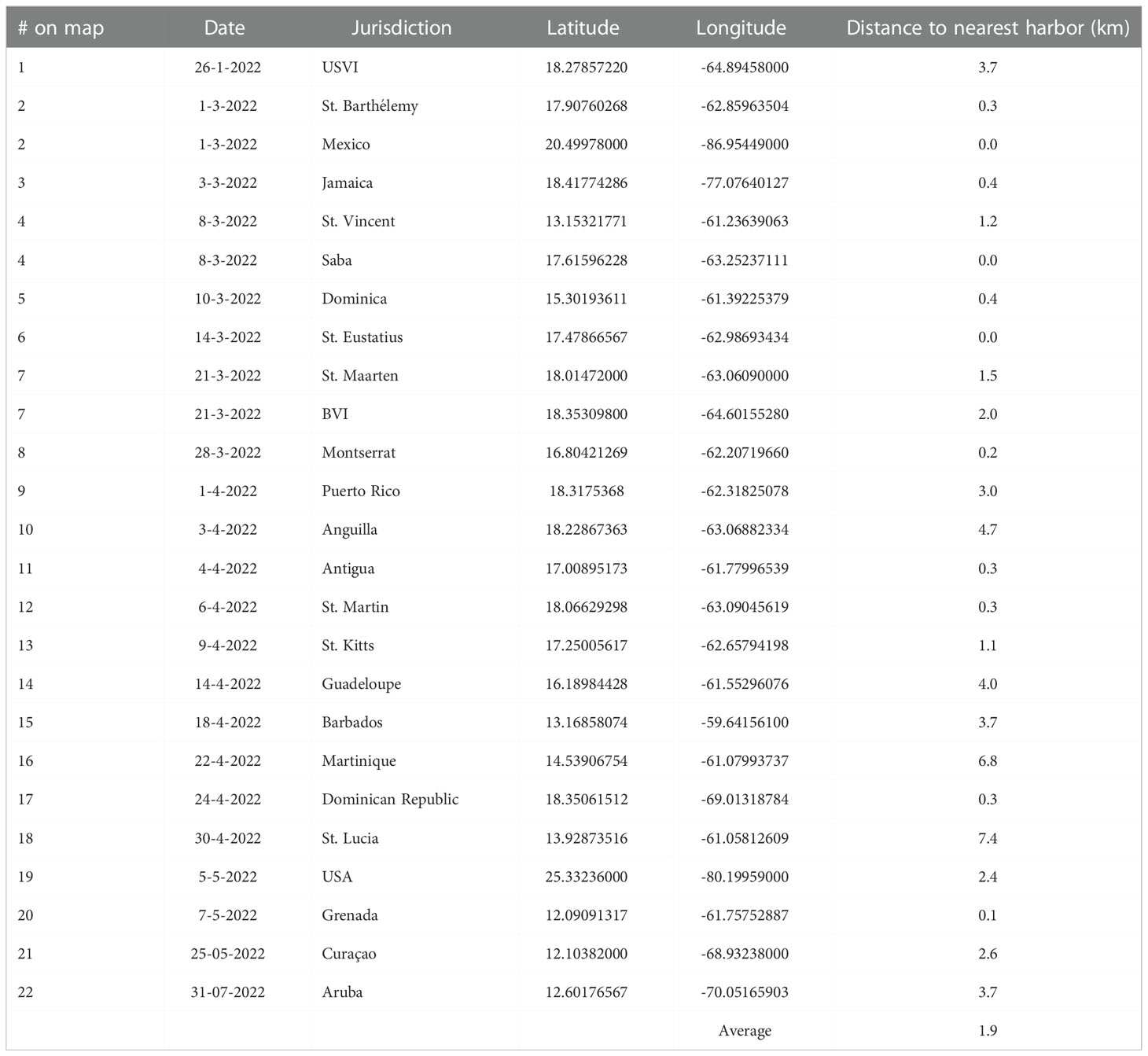
Table 1 # on map (in chronological order, with duplicate numbers for ties), date of occurrence (as day-month-year), jurisdiction, GPS location and distance to the nearest harbor of the first reported D. antillarum die-off per jurisdiction.
Before the die-off the average D. antillarum population density at Diadema City was 4.05 ± 0.68 D. antillarum per m2 (Figure 2). During a biweekly inspection of the dive site, the first individuals with signs of illness were observed on 29 March 2022. Time after first observed signs of illness had a significant effect on the D. antillarum density (F=80.67, df=6, P<0.001). Nine days after the first signs of illness were recorded, the D. antillarum density was reduced to 1.76 ± 0.99 individuals per m2, with 1.18 ± 0.53 healthy and 0.58 ± 0.47 sick individuals per m2. By 22 days after the first observed signs of illness, the D. antillarum density was further reduced to 0.09 ± 0.03 healthy and 0.02 ± 0.02 sick individuals per m2. No sick individuals were observed during concurrent monitoring and the remaining D. antillarum population density fluctuated between 0.03 and 0.05 individuals per m2 for the remainder of the monitoring period, which was significantly less than the pre-die-off density (P<0.001). Using the higher post-die-off population estimate of 0.05, this equates to a 99% mortality rate for the D. antillarum population at Diadema City.
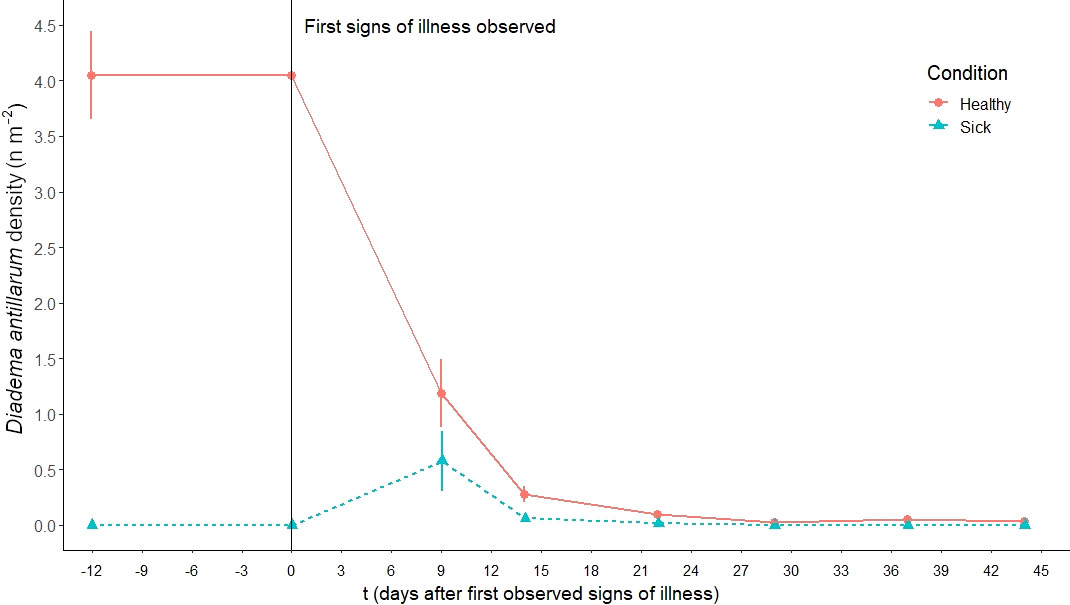
Figure 2 D. antillarum densities (mean ± standard error) at dive site Diadema City before and during the 2022 die-off.
The D. antillarum population on a small breakwater in the harbor was surveyed for the first time on 18 March 2022 after large swells had prevented monitoring for six days after the first dead urchins were reported. D. antillarum densities were 1.32 healthy per m2, 0.18 sick per m2 and 1.38 dead per m2 (Table 2), so the pre-die-off density was at least 2.88 D. antillarum per m2. E. lucunter, T. ventricosus, and E. tribuloides were also observed on the breakwater and all appeared healthy, although population densities of non-Diadema urchin species were not recorded on this initial survey date. One week later, the D. antillarum density was reduced to 0.18 healthy and 0.07 sick individuals per m2, while other sea urchin species all appeared healthy. Throughout the monitoring period, E. lucunter densities fluctuated between 0.33 and 0.9 individuals per m2, T. ventricosus densities between 0.03 and 0.05 individuals per m2, and E. tribuloides densities between 0.00 and 0.07 individuals per m2. Apart from D. antillarum, no sick or dying urchins were observed during the surveys in the Fort Bay Harbor.
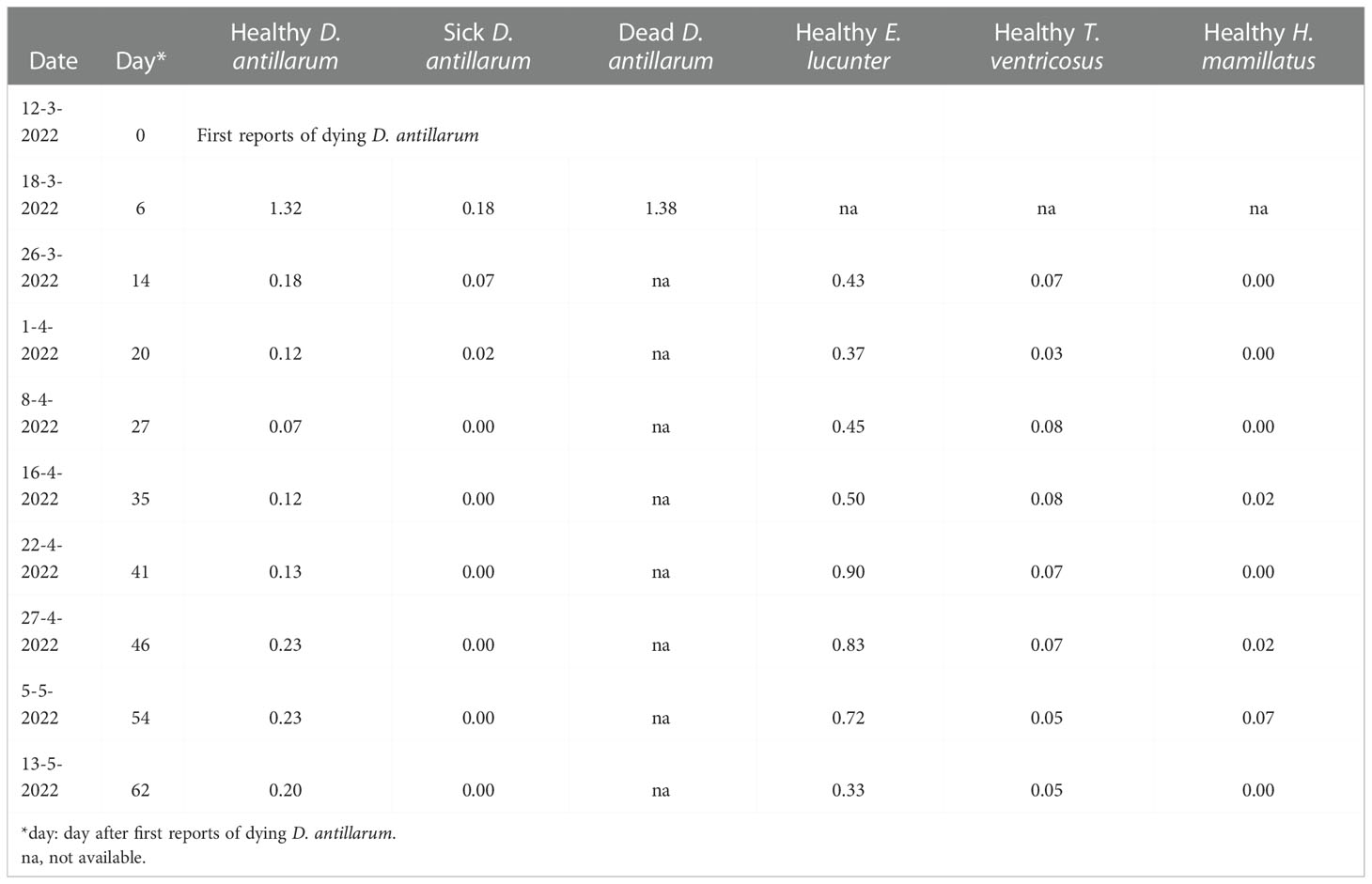
Table 2 Healthy and sick sea urchins (individuals per m2) in the Fort Bay Harbor, Saba, during the D. antillarum die-off.
When field observations were made of abnormal D. antillarum individuals in Fort Bay and Oranje Bay on 15 March 2022, live individuals without visible signs of illness were found detached from the substrate and were swaying back and forth in the surge. Others had fallen from nearby vertical surfaces and were lying on their sides, apparently unable to stand upright. Spine reaction of affected individuals was slower than usual and many individuals only reacted to physical stimuli after 2-3 attempts. A few individuals had epidermal lesions that exposed the underlying skeleton. In most cases, these individuals did not exhibit spine movement. Numerous white D. antillarum skeletons with and without mouth parts were found surrounded by detached spines. We suspect that those without mouth parts were due to opportunistic predation, as multiple fish species were observed attacking the impaired D. antillarum.
On 19 March 2022 at 10 am, four days after the last water replacement that possibly contained a pathogen, four D. antillarum from two different tanks in the Saba Research Center showed signs of illness. It was obvious that their water-vascular system had been affected. The abnormal D. antillarum were standing on their spines (Figure 3A). Initially, these individuals were still able to move their tube feet, but they appeared to lack suction power and were unable to use them to grip the bottom of the tanks (Figure 3B). At 4 pm, the tube feet of one of the affected individuals were totally flaccid, hanging from the ventral surface of the animal. Necrosis of the epidermis around the base of the spines was visible (Figure 3C) and some spines fell off. Spines also appeared to be very brittle at this stage and were easily broken when the animal was handled. The tissue around broken spines immediately sloughed away creating rings around the broken spine (Figure 3D). At this stage, the abnormal individual had a very slow spine reaction when stimulated. The other three individuals were still able to move their tube feet and had a normal spine reaction in the afternoon. One day later, by 10 am on 20 March 2022, the sickest individual had died. By then, the disease had progressed in the other three individuals, who were not able to move their tube feet anymore, while spine reaction was very slow. On 21 March 2022, 48 hours after the first observed signs of illness, all abnormal individuals had died. In the following weeks, many individuals showed signs of illness and subsequently died. Two weeks after the first signs of illness appeared in the lab, 63 of the 96 D. antillarum were still alive. After four weeks, 55 individuals were still alive.
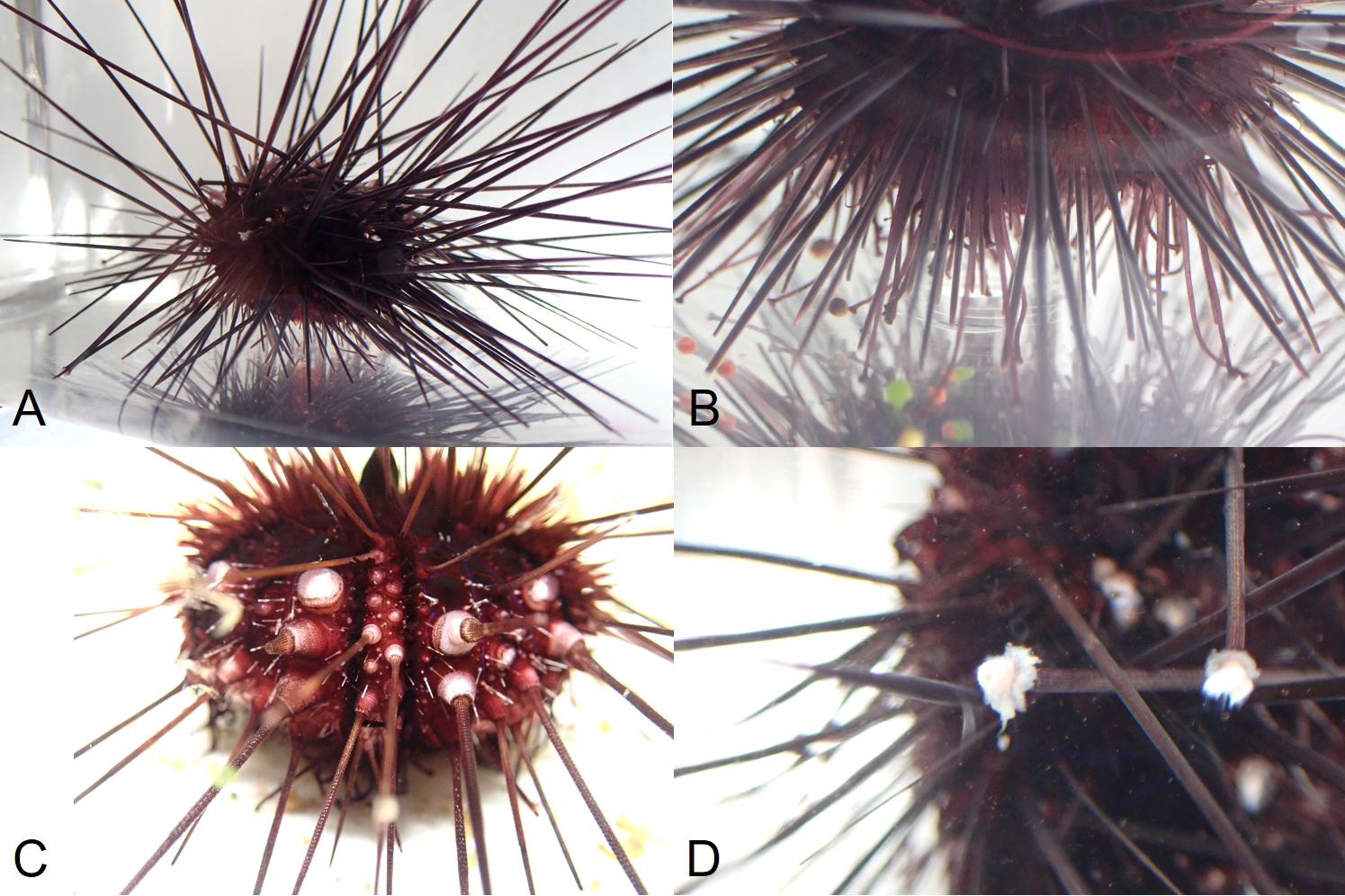
Figure 3 Signs of illness of D. antillarum during the 2022 die-off as reported in this study. (A) Standing on spines, tube feet lack suction power. (B) Standing on spines not being able to use tube feet. (C) Necrosis of epidermis and spine loss. (D) Tissue around broken spines sloughs off.
The full extent of the D. antillarum die-off event that began in January 2022 is not currently known. Relative to the 1983-1984 event, features of spread, signs of illness, and lethality span the spectrum of similar to dissimilar. What greatly differs are the location of the first occurrence and the pattern and speed of initial spread. The visible signs of disease and population density losses appear to be more similar.
The 1983-1984 die-off event was first observed near the Atlantic opening of the Panama Canal (Lessios et al., 1984a). Within a year, mortality fronts had advanced the ~2000 km distance from Panama east to Tobago (with an anomalous early appearance in Barbados, ~350 km northeast of Tobago) and ~4000 km north to Bermuda, after infecting populations on the Atlantic coast of Central America and Mexico, Cayman Islands, Jamaica, and Florida (Lessios, 1988). Countercurrents and probably boat traffic transported the disease eastward from Florida to The Bahamas, Puerto Rico, Virgin Islands (where it reached St. Thomas in December), and the Southern and Eastern Caribbean (Lessios et al., 1984b; Phinney et al., 2002). The freshwater locks and lakes of the Panama Canal serve as a barrier to the migration of most stenohaline marine organisms (Jones and Dawson, 1973). However, multiple Atlantic-Pacific biotic exchanges have been recorded (Davidson et al., 2008; Schloder et al., 2013; Castellanos-Galindo et al., 2020) and marine species migrate through the freshwater canal unaided or associated with ship fouling or in ballast water. While it is highly speculative to assume a Pacific origin for the unknown causative agent of the 1983-1984 die-off, its initial location certainly increases the probability that this was the case. Regardless of geographic origin, the very high levels of ship traffic through the Panama Canal increase the probability that the causative agent could have been initially introduced via an anthropogenic vector. However, the speed at which it traveled and the routes which it took once within the Caribbean, suggest that the causative agent of the 1983-1984 mass mortality was mainly driven by features of the natural environment (Lessios et al., 1984b).
By contrast, the 2022 die-off was first observed in the opposite quadrant of the Caribbean, on the southern shores of St. Thomas USVI, in January 2022. About five weeks later, mass D. antillarum mortality appeared in Jamaica (~1300 km to the west), Cozumel, Mexico (over 2300 km to the west), and St. Vincent (~700 km to the south). The near-simultaneous occurrence of dying urchins in such geographically separate areas suggests that the primary dispersal mechanism in the present event might be anthropogenic rather than current-driven. This interpretation is strengthened by the estimated distance of the first occurrence in each jurisdiction to the nearest harbor. Of the 25 affected jurisdictions, 18 were within 3 km of a harbor and 11 were within 1 km. The close proximity of the first observed mass mortalities to harbors for most jurisdictions reduces the chances of an alternative explanation for the near simultaneous outbreaks in different parts of the Caribbean, in which a causative agent was already widely present throughout the area in spring 2022. If that would be the case, local environmental changes, occurring at similar times in different jurisdictions, could have produced the distributional pattern that was observed. Nevertheless, locally, the causative agent of the die-off appeared to spread predominantly with surface currents.
The five week hiatus after the start of the D. antillarum die-off at St. Thomas in late January 2022 ended in early March when similar mass mortalities were observed weekly for several months in new jurisdictions, totaling 10 each for both March and April 2022. But as only three new reports were received in May, one in late July and none in both June and August, perhaps the speed at which the causative agent was traveling decreased, its presence decreased or it may have become less lethal. This reduced dispersal activity differs from that of the 1983-1984 event, in which the mass mortalities continued throughout 1983 (Lessios et al., 1984b). It is possible that the remaining D. antillarum populations will be spared or at least persist for longer than the speed of the initial outbreak would have suggested. However, as long as the causative and dispersal agents of the mass mortality events remain unknown, it is impossible to predict the trajectory of the current or future D. antillarum die-offs.
The speed at which a population, once affected, succumbs was similar to that reported for the 1983-1984 die-off (Bak et al., 1984; Lessios et al., 1984b) and population density losses appear similar as well. Within one month after the first signs of illness were observed, Saba’s Diadema City location lost 99% of its D. antillarum population. This is a very similar percentage to the Caribbean-wide estimate of the lethality of the 1983-1984 die-off, which was 98% (Lessios, 2016). Intriguingly, the lethality of the 1983-1984 and 2022 die-offs was much higher than that reported from local D. antillarum die-offs in Panama and St. Croix in 1985, which was less than 1% (Lessios, 1988), but very similar to the 97% lethality reported for a local D. antillarum die-off around Florida in 1991 (Forcucci, 1994). It could be that during the 1985 die-offs in Panama and St. Croix, most D. antillarum were direct survivors of the 1983-1984 die-off and had immunity against or insusceptibility to the original causative agent.
We suspect that the lower lethality rate (~90%) observed at the Saba Fort Bay Harbor population compared to Diadema City was the result of removal of many skeletons by the large swells that prevented an earlier survey. The Fort Bay Harbor population data are very useful to estimate the effect on three other reef and rock dwelling sea urchin species (E. lucunter, T. ventricosus and E. tribuloides) which remained healthy in between the dead and dying D. antillarum at the time of the die-off. Whatever caused the D. antillarum die-off did not have any effect on the other sea urchin species, as no sick or dead individuals of these species were observed for at least two months after the D. antillarum started to die. This is, again, very similar to the 1983-1984 die-off, which also did not visibly affect other sea urchin species (Lessios et al., 1984b; Bak et al., 1984). At the moment of writing this manuscript (September 2022) the small breakwater in Fort Bay Harbor still harbors healthy populations of E. lucunter, T. ventricosus and E. tribuloides (Personal observation A. Hylkema).
During the 1983-1984 die-off, D. antillarum held in land-based systems, with sea water inflow, exhibited similar signs of illness and mortality at the same time as natural populations on nearby reefs were affected, suggesting a water-borne mode of transportation of the causative agent (Lessios, 1988). As described here, this phenomenon has also been observed during the current die-off, as D. antillarum in the Saba Research Center started to show signs of illness around the same time as their conspecifics in Fort Bay Harbor were dying. Around 43% of the D. antillarum in the Saba Research Center died within a month after the first signs of illness were observed. This lower lethality can possibly be explained by certain measures that could have increased survival. Tanks were heavily aerated, dying individuals were removed daily and weekly water changes were performed with artificial seawater, all possibly reducing exposure to the causative agent. Moreover, multiple D. antillarum larvae cultures were running in the Saba Research Center at the time of the die-offs. Water of these larvae cultures was also replaced with unfiltered harbor water, which was taken in next to the dead and dying D. antillarum in the harbor before the die-off was discovered on 15 March 2022. However, no abnormalities were observed in the larvae culture and most of the larvae metamorphosed into healthy settlers one month later, indicating that the causative agent for the 2022 die-off had no discernable effect on D. antillarum larvae. These observations are similar to those during the 1983-1984 die-off, when D. antillarum recruitment continued as normal for several months after the 1983-1984 die-off, suggesting the causative agent for the die-off did not affect the pelagic D. antillarum larvae (Bak, 1985).
The observed signs of illness in the present study were very similar to those reported for the 1983-1984 D. antillarum die-off (Bak et al., 1984; Lessios et al., 1984b; Hughes et al., 1985). Except for the accumulation of detritus on spines, described by Bak et al. (1984), all signs were observed in the present study. However, on Saba in 2022, necrosis of the epidermis and spine loss were usually observed after, rather than before, the water-vascular system had stopped working and where D. antillarum were standing or lying on their spines. The order in which signs of illness became clearly visible might have to do with swell and wave action, as this requires a higher capacity of D. antillarum to cling to the substrate. As the 2022 die-off on Saba started in weeks with high swell, it could be that the loss of tube feet control became visible in an earlier stage compared to Curaçao (Bak et al., 1984) and Panama (Lessios et al., 1984b) in 1983. The observed signs of illness in 2022 were also similar to those described by Forcucci (1994) for D. antillarum that were dying off around Florida in 1991. The similarity in signs of illness could either be an indication that the causative agent for the die-offs was the same or perhaps that echinoids have a limited repertoire of visible signs of illness.
Due to its ~30 species with documented, sometimes recurrent, large fluctuations in population density, the Echinodermata has been characterized as a “boom and bust” phylum (Uthicke et al., 2009). Identification of causative agent(s) and/or putative pathogens is essential for the management of our interactions with marine resources, but the cause for the 1983-1984 D. antillarum die-off has never been confirmed (Lessios, 2005; DeFilippo et al., 2018). When mass mortalities of the closely related D. africanum were observed in the Canary Islands in 2010, Clemente et al. (2014) identified the bacterium Vibrio alginolyticus as a putative pathogen responsible for this event. However, Hernández et al. (2020) investigated a subsequent die-off at the same locations in 2018 and suggested primary infection by the eukaryotic Paramoeba brachiphila, with a potential secondary role for V. alginolyticus. An anomalous meteorological event that Hernández et al. (2020) proposed as a trigger for die-off conditions in both years supported Scheibling et al. (2010) “killer-storm” hypothesis regarding recurrent die-offs of the sea urchin Strongylocentrotus drobachiensis in the northwest Atlantic from infection by the closely related Paramoeba invadens (Jones and Scheibling, 1985; Feehan et al., 2013). Analysis of multiple rounds of emergence and transmission of this pathogen have suggested that large-scale oceanographic and meteorological conditions may be important in transport of P. invadens and in creating temperature-dependent environmental conditions favorable for disease propagation (Scheibling and Hennigar, 1997; Scheibling et al., 2010; Feehan et al., 2012). Oceanographic and meteorological conditions may also have initiated the 2022 die-off in some locations, as heavy rainfall was observed prior to the first mortalities at least in Aruba Puerto Rico, Saba and St. Eustatius. However, ambient weather conditions were reported to have been normal in the Dominican Republic, Jamaica, and Mexico when the urchins died and possible environmental correlates of the 2022 D. antillarum die-off warrant further investigation in follow-up studies.
Differences between the 1983-1984 and 2022 mass mortality events included the location where each was first observed, their geographical spread and presumed primary dispersal modes. The rapidity with which affected populations succumb, the lethality, effects on other urchin species and signs of illness were very similar, although the latter could be explained by a limited repertoire of visible illness responses. The different geographic spread might be explained by the increase in boat traffic in 2022 compared to 1983. The importance of boat traffic in the spread is emphasized by the close proximity to harbors of the first mass mortality in most jurisdictions. The initial speed of spread and broad geographic range of the 2022 D. antillarum die-off event indicated that, like the 1983-1984 event, it would eventually reach all areas of the Caribbean. However, few new jurisdictions have been affected since early May 2022, meaning some D. antillarum populations may remain unimpacted by this mortality event. Nevertheless, due to the high lethality rate observed during the current die-off, D. antillarum may once again be functionally extinct on many Caribbean islands. There are at least three major differences between the aftermath of the 2022 and the 1983-1984 mass mortalities:
- Assuming the spread and/or lethality of the current die-off is reduced and certain parts of the Caribbean remain unaffected, there should be larger numbers of D. antillarum adults to contribute larvae to downstream areas. Thus, it is possible any natural recovery could occur faster than following the previous mass mortality event, when it took more than a decade before natural recovery was seen on most reefs (Lessios, 1995). Preliminary quantitative data from Saba and Puerto Rico (A. Hylkema and S. Williams, unpublished data) and qualitative observations elsewhere in the region confirm that substantial settlement has occurred since the 2022 mass mortality event.
- The stressors impacting coral reefs have progressively increased in magnitude since the 1980s. Over the past four decades, coral cover has decreased, while macroalgal cover has increased (Jackson et al., 2014) and structural complexity has been reduced (Alvarez-Filip et al., 2009). At reef sites with recovered D. antillarum populations, macroalgae have been reduced, stimulating coral recruitment, growth and survival (Edmunds and Carpenter, 2001; Carpenter and Edmunds, 2006; Myhre and Acevedo-Gutiérrez, 2007; Idjadi et al., 2010). Such improvements are likely to be lost in areas that were impacted by the 2022 die-off. In the few short months since St. John, USVI, lost its D. antillarum, those sites with previous densities of one per meter or more are now overgrown with turf algae (Personal observation M. Sevier). The impacts of the 2022 D. antillarum die-off on reef structure and functionality will be exacerbated by increases in other stressors, for example the recent loss of corals due to SCTLD.
- Efforts to restore wild populations have advanced significantly since the 1983 die-off. New and developing technologies and techniques for D. antillarum rehabilitation include in-situ collection of settlers followed by head starting the settlers in the lab and restocking them on the reef as juveniles (Williams et al., 2017; Williams, 2022), new or improved culture methods (Pilnick et al., 2021; Pilnick et al., 2022; Wijers et al., 2023) and assisting natural recovery by providing suitable settlement substrate (Hylkema et al., 2022a). Except for culture, all approaches rely on natural larval supply, which may be severely reduced if upstream populations were eradicated by the 2022 die-off. On the other hand, potential settlement hotspots have already been identified for certain islands (Williams et al., 2010; Hylkema et al., 2022b) and a similar approach can be used in different areas, lending to a more optimistic outlook than after the 1983-1984 die-off.
As a causative agent responsible for the 1983-1984 die-off was never identified, the primary research priority for the 2022 die-off should be identifying its cause. Many countries, institutions, and individuals, including the authors of this paper, have contributed to this effort. Advances in technology and communication provide us with hope that this objective may be achieved. Once a cause is identified, the next steps will be identifying and implementing actions to reduce the risk of similar events occurring in the future. The scale at which population rehabilitation will be required presents a massive challenge, but at least several rehabilitation approaches are already under development. Ultimately, we encourage proactive conservation and restoration efforts to preserve and enhance remaining populations, while continuing efforts to identify the cause of the 2022 mortality event and implementing actions to decrease the likelihood of future D. antillarum die-offs.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
AH: Methodology, investigation, writing original draft, formal analysis, writing - review & editing, coordination. KK-W: Methodology, investigation, writing - review & editing. PK: Methodology, investigation, formal analysis. JP: Methodology, writing original draft, writing - review & editing. LR: Investigation, formal analysis. MS: Investigation, formal analysis, writing - review & editing. MV-R: Methodology, writing - review & editing. MW: Methodology, formal analyis, writing - review & editing. SW: Methodology, writing original draft, writing, review & editing. JL: Methodology, investigation, writing original draft, formal analysis, writing - review & editing, guidance. All authors contributed to the article and approved the submitted version.
The Ocean Research and Education Foundation (ORE) funded the development and maintenance of the Diadema Dieoff Tracking Map and data platform. The fieldwork conducted on Saba was partly funded by SIA, part of the Dutch Research Council (NWO) in the context of the RAAK PRO Diadema project (project#RAAK.PRO03.005). MV-R likes to acknowledge Consolidated Safety Services and the Puerto Rico Department of Natural and Environmental Resources for supporting her.
We thank the many contributors who voluntarily submitted observations and photos to the tracking map. Their efforts have contributed to a greater collective understanding of the 2022 mortality event. We thank ORE’s Shirley Gun for her assistance to the Diadema Response Network. We want to thank the Saba Conservation Foundation staff, especially Marijn van der Laan, for providing logistical support. We also want to thank Tom Wijers, Marit Pistor, Tomas Cornwell, Oliver Klokman, Bob Sesink Clee and Michael Nijlunsing for their help with conducting the sea urchin surveys. Finally, we appreciate the valuable comments and suggestions from the editor and two reviewers, which helped us in improving the quality of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alvarez-Filip L., Dulvy N. K., Gill J. A., Côté I. M., Watkinson A. R. (2009). Flattening of Caribbean coral reefs: Region-wide declines in architectural complexity. Proc. R. Soc. B: Biol. Sci. 276 (1669), 3019–3025. doi: 10.1098/rspb.2009.0339
Arnold S. N., Steneck R. S., Mumby P. J. (2010). Running the gauntlet: inhibitory effects of algal turfs on the processes of coral recruitment. Mar. Ecol. Prog. Ser. 414, 91–105. doi: 10.3354/meps08724
Bak R. P. M. (1985). “Recruitment patterns and mass mortalities in the sea urchin diadema antillarum,” in Proc. 5th int. coral reef congress, Vol. 5. (Tahiti), 267–272.
Bak R. P. M., Carpay M. J. E., De Ruyter van Steveninck E. D. (1984). Densities of the sea urchin diadema antillarum before and after mass mortalities on the coral reefs on curacao. Mar. Ecol. Prog. series Oldendorf 17 (1), 105–108. doi: 10.3354/meps017105
Bak R. P. M., van Eys G. (1975). Predation of the sea urchin Diadema antillarum philippi on living coral. Oecologia 20, 111–115. doi: 10.1007/BF00369023
Bates D., Mächler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Software 67 (1), 1–48. doi: 10.18637/jss.v067.i01
Bauer J. C., Agerter C. J. (1987). Isolation of bacteria pathogenic for the sea urchin diadema antillarum (Echinodermata: Echinoidea). Bull. Mar. Sci. 40 (1), 161–165.
Beck G., Miller R., Ebersole J. (2014). Mass mortality and slow recovery of Diadema antillarum: Could compromised immunity be a factor? Mar. Biol. 161 (5), 1001–1013. doi: 10.1007/s00227-013-2382-6
Box S. J., Mumby P. J. (2007). Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar. Ecol. Prog. Ser. 342, 139–149. doi: 10.3354/meps342139
Carpenter R. C. (1986). Partitioning herbivory and its effects on coral reef algal communities. Ecol. Monogr. 56 (4), 345–364. doi: 10.2307/1942551
Carpenter R. C. (1988). Mass mortality of a Caribbean sea urchin: immediate effects on community metabolism and other herbivores. Proc. Natl. Acad. Sci. 85 (2), 511–514. doi: 10.1073/pnas.85.2.511
Carpenter R. C. (1990). Mass mortality of Diadema antillarum. Mar. Biol. 104 (1), 67–77. doi: 10.1007/BF01313159
Carpenter R. C., Edmunds P. J. (2006). Local and regional scale recovery of diadema promotes recruitment of scleractinian corals. Ecol. Lett. 9 (3), 271–280. doi: 10.1111/j.1461-0248.2005.00866.x
Castellanos-Galindo G. A., Robertson D. R., Torchin M. E. (2020). A new wave of marine fish invasions through the Panama and Suez canals. Nat. Ecol. Evol. 4 (11), 1444–1446. doi: 10.1038/s41559-020-01301-2
Clemente S., Lorenzo-Morales J., Mendoza J. C., López C., Sangil C., Alves F., et al. (2014). Sea Urchin diadema africanum mass mortality in the subtropical eastern Atlantic: role of waterborne bacteria in a warming ocean. Mar. Ecol. Prog. Ser. 506, 1–14. doi: 10.3354/meps10829
Davidson I. C., McCann L. D., Fofonoff P. W., Sytsma M. D., Ruiz G. M. (2008). The potential for hull-mediated species transfers by obsolete ships on their final voyages. Diversity Distributions 14 (3), 518–529. doi: 10.1111/j.1472-4642.2008.00465.x
DeFilippo J., Ebersole J., Beck G. (2018). Comparison of phagocytosis in three Caribbean Sea urchins. Dev. Comp. Immunol. 78, 14–25. doi: 10.1016/j.dci.2017.09.007
de Ruyter van Steveninck E. D., Bak R. P. M. (1986). Changes in abundance of coral-reef bottom components related to mass mortality of the sea urchin diadema antillarum. Mar. Ecol. Prog. Ser. 34, 87–94. doi: 10.3354/meps034087
Edmunds P. J., Carpenter R. C. (2001). Recovery of diadema antillarum reduces macroalgal cover and increases abundance of juvenile corals on a Caribbean reef. Proc. Natl. Acad. Sci. 98 (9), 5067–5071. doi: 10.1073/pnas.071524598
Feehan C. J., Johnson-Mackinnon J., Scheibling R. E., Lauzon-Guay J. S., Simpson A. G. (2013). Validating the identity of paramoeba invadens, the causative agent of recurrent mass mortality of sea urchins in Nova Scotia, Canada. Dis. Aquat. organisms 103 (3), 209–227. doi: 10.3354/dao02577
Feehan C. J., Scheibling R. E., Lauzon-Guay J. S. (2012). An outbreak of sea urchin disease associated with a recent hurricane: Support for the “killer storm hypothesis” on a local scale. J. Exp. Mar. Biol. Ecol. 413, 159–168. doi: 10.1016/j.jembe.2011.12.003
Forcucci D. (1994). Population density, recruitment and 1991 mortality event of diadema antillarum in the Florida keys. Bull. Mar. Sci. 54 (3), 917–928.
Francis-Floyd R. (2020). Diagnostic methods for the comprehensive health assessment of the long-spined Sea urchin, Diadema antillarum: VM244/VM244, 05/2020. EDIS 2020 (3), 55. doi: 10.32473/edis-vm244-2020
Hernández J. C., Sangil C., Lorenzo-Morales J. (2020). Uncommon southwest swells trigger sea urchin disease outbreaks in Eastern Atlantic archipelagos. Ecol. Evol. 10 (15), 7963–7970. doi: 10.1002/ece3.6260
Hughes T. P. (1994). Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265 (5178), 1547–1551.
Hughes T. P., Keller B. D., Jackson J. B. C., Boyle M. J. (1985). Mass mortality of the echinoid diadema antillarum philippi in Jamaica. Bull. Mar. Sci. 36 (2), 377–384.
Hughes T. P., Reed D. C., Boyle M. J. (1987). Herbivory on coral reefs: community structure following mass mortalities of sea urchins. J. Exp. Mar. Biol. Ecol. 113 (1), 39–59.
Hylkema A., Debrot A. O., Pistor M., Postma E., Williams S. M., Kitson-Walters K. (2022b). High peak settlement of diadema antillarum on different artificial collectors in the Eastern Caribbean. J. Exp. Mar. Biol. Ecol. 549, 151693. doi: 10.1016/j.jembe.2022.151693
Hylkema A., Debrot A. O., van de Pas E. E., Osinga R., Murk A. J. (2022a). Assisted natural recovery: A novel approach to enhance diadema antillarum recruitment. Front. Mar. Sci. 9, 929355. doi: 10.3389/fmars.2022.929355
Idjadi J. A., Haring R. N., Precht W. F. (2010). Recovery of the sea urchin diadema antillarum promotes scleractinian coral growth and survivorship on shallow Jamaican reefs. Mar. Ecol. Prog. Ser. 403, 91–100. doi: 10.3354/meps08463
Jackson J. B. C., Donovan M. K., Cramer K. L., Lam V. V. (2014). Status and trends of Caribbean coral reefs (Gland, Switzerland: Global coral reef monitoring network, IUCN), 1970–2012. doi: 10.1126/science.1059199
Jackson J. B. C., Kirby M. X., Berger W. H., Bjorndal K. A., Botsford L. W., Bourque B. J., et al. (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science 293 (5530), 629–637. doi: 10.1126/science.1059199
Jones M. L., Dawson C. E. (1973). Salinity-temperature profiles in the Panama canal locks. Mar. Biol. 21 (2), 86–90. doi: 10.1007/BF00354602
Jones G. M., Scheibling R. E. (1985). Paramoeba sp.(Amoebida, paramoebidae) as the possible causative agent of sea urchin mass mortality in Nova Scotia. J. Parasitol. 71, 559–565.
Lenth R., Herve M. (2019). “Emmeans: Estimated marginal means, aka least-square means,” in R package version 1.1, vol. 2. [computer software]
Lessios H. A. (1988). Mass mortality of diadema antillarum in the Caribbean: What have we learned? Annu. Rev. Ecol. systematics 19 (1), 371–393. doi: 10.1146/annurev.es.19.110188.002103
Lessios H. A. (1995). Diadema antillarum 10 years after mass mortality: still rare, despite help from a competitor. Proc. R. Soc. London Ser. B: Biol. Sci. 259 (1356), 331–337.
Lessios H. A. (2005). Diadema antillarum populations in Panama twenty years following mass mortality. Coral Reefs 24 (1), 125–127. doi: 10.1007/s00338-004-0443-5
Lessios H. A. (2016). The great diadema antillarum die-off: 30 years later. Annu. Rev. Mar. Sci. 8, 267–283. doi: 10.1146/annurev-marine-122414-033857
Lessios H. A., Cubit J. D., Robertson D. R., Shulman M. J., Parker M. R., Garrity S. D., et al. (1984a). Mass mortality of diadema antillarum on the Caribbean coast of Panama. Coral Reefs 3 (4), 173–182.
Lessios H. A., Robertson D. R., Cubit J. D. (1984b). Spread of diadema mass mortality through the Caribbean. Science 226 (4672), 335–337.
Liddell W. D., Ohlhorst S. L. (1986). Changes in benthic community composition following the mass mortality of diadema at Jamaica. J. Exp. Mar. Biol. Ecol. 95 (3), 271–278.
McCook L., Jompa J., Diaz-Pulido G. (2001). Competition between corals and algae on coral reefs: A review of evidence and mechanisms. Coral reefs 19 (4), 400–417. doi: 10.1007/s003380000129
Mumby P. J., Hastings A., Edwards H. J. (2007). Thresholds and the resilience of Caribbean coral reefs. Nature 450 (7166), 98–101. doi: 10.1038/nature06252
Myhre S., Acevedo-Gutiérrez A. (2007). Recovery of sea urchin diadema antillarum populations is correlated to increased coral and reduced macroalgal cover. Mar. Ecol. Prog. Ser. 329, 205–210. doi: 10.3354/meps329205
Ogden J. C. (1976). Some aspects of herbivore-plant relationships on Caribbean reefs and seagrass beds. Aquat. Bot. 2, 103–116. doi: 10.1016/0304-3770(76)90013-9
Pandolfi J. M., Bradbury R. H., Sala E., Hughes T. P., Bjorndal K. A., Cooke R. G., et al. (2003). Global trajectories of the long-term decline of coral reef ecosystems. Science 301 (5635), 955–958. doi: 10.1126/science.1085706
Phinney J. T., Muller-Karger F., Dustan P., Sobel J. (2002). Using remote sensing to reassess the mass mortality of diadema antillarum 1983–1984. Conserv. Biol. 15 (4), 885–891. doi: 10.1046/j.1523-1739.2001.015004885.x
Pilnick A., O’Neil K., DiMaggio M., Patterson J. (2022). Development of larviculture protocols for the long-spined sea urchin (Diadema antillarum) and enhanced performance with diets containing the cryptophyte rhodomonas lens. Aquacult Int. 30(6), 3017–3034. doi: 10.1007/s10499-022-00945-0
Pilnick A. R., O’Neil K. L., Moe M., Patterson J. T. (2021). A novel system for intensive diadema antillarum propagation as a step towards population enhancement. Sci. Rep. 11 (1), 1–13. doi: 10.1038/s41598-021-90564-1
Randall J. E., Schroeder R. E., Strack W. A. (1964). Notes on the biology of the echinoid Diadema-antillarum. Carib. J. Sci. 4 (2&3), 421–433.
R Core Team (2022). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
Sammarco P. W. (1980). Diadema and its relationship to coral spat mortality: Grazing, competition, and biological disturbance. J. Exp. Mar. Biol. Ecol. 45, 245–272. doi: 10.1016/0022-0981(80)90061-1
Scheibling R. E., Feehan C., Lauzon-Guay J. S. (2010). Disease outbreaks associated with recent hurricanes cause mass mortality of sea urchins in Nova Scotia. Mar. Ecol. Prog. Ser. 408, 109–116. doi: 10.3354/meps08579
Scheibling R. E., Hennigar A. W. (1997). Recurrent outbreaks of disease in sea urchins Strongylocentrotus droebachiensis in Nova Scotia: evidence for a link with large-scale meteorologic and oceanographic events. Mar. Ecol. Prog. Ser. 152, 155–165. doi: 10.3354/meps152155
Schloder C., Canning-Clode J., Saltonstall K., Strong E. E., Ruiz G. M., Torchin M. E. (2013). The pacific bivalve Anomia peruviana in the Atlantic: A recent invasion across the Panama canal? Aquatic Invasions 8(4), 443–448. doi: 10.3391/ai.2013.8.4.08
Uthicke S., Schaffelke B., Byrne M. (2009). A boom–bust phylum? ecological and evolutionary consequences of density variations in echinoderms. Ecol. Monogr. 79 (1), 3–24. doi: 10.1890/07-2136.1
Wijers T., Hylkema A., Pilnick A. R., Murk A. J., Patterson J. T. (2023). Novel shaker bottle cultivation method for the long spined sea urchin (Diadema antillarum; philippi 1845) results in high larval survival and settlement rates. Aquaculture 562, 738855. doi: 10.1016/j.aquaculture.2022.738855
Williams S. M. (2022). The reduction of harmful algae on Caribbean coral reefs through the reintroduction of a keystone herbivore, the long-spined sea urchin diadema antillarum. Restor. Ecol. 30 (1), e13475. doi: 10.1111/rec.13475
Williams S. M., Sánchez-Godínez C., Newman S. P., Cortés J. (2017). Ecological assessments of the coral reef communities in the Eastern Caribbean and the effects of herbivory in influencing coral juvenile density and algal cover. Mar. Ecol. 38 (2), e12395. doi: 10.1111/maec.12395
Williams S. M., Yoshioka P. M., García Sais J. R. (2010). Recruitment pattern of diadema antillarum in la parguera, Puerto Rico. Coral Reefs 29 (3), 809–812. doi: 10.1007/s00338-010-0633-2
Keywords: urchin, disease, mass mortality, Caribbean, echinoid, coral reef
Citation: Hylkema A, Kitson-Walters K, Kramer PR, Patterson JT, Roth L, Sevier MLB, Vega-Rodriguez M, Warham MM, Williams SM and Lang JC (2023) The 2022 Diadema antillarum die-off event: Comparisons with the 1983-1984 mass mortality. Front. Mar. Sci. 9:1067449. doi: 10.3389/fmars.2022.1067449
Received: 20 October 2022; Accepted: 05 December 2022;
Published: 05 January 2023.
Edited by:
Thanos Dailianis, Hellenic Centre for Marine Research, GreeceReviewed by:
Carlos Renato Ventura, Museu Nacional, BrazilCopyright © 2023 Hylkema, Kitson-Walters, Kramer, Patterson, Roth, Sevier, Vega-Rodriguez, Warham, Williams and Lang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alwin Hylkema, YWx3aW4uaHlsa2VtYUBodmhsLm5s
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.