- 1Key Laboratory of Sustainable Utilization of Technology Research for Fishery Resources of Zhejiang Province, Zhejiang Marine Fisheries Research Institute, Scientific Observing and Experimental Station of Fishery Resources for Key Fishing Grounds, Ministry of Agriculture and Rural Affairs, Zhoushan, China
- 2Marine and Fishery Institute of Zhejiang Ocean University, Zhoushan, China
- 3Key Lab of East China Sea & Oceanic Fishery Resources Exploitation and Utilization, Ministry of Agriculture East China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Shanghai, China
Fishery resources assessment is the basis of scientific management and sustainable development of fisheries. Trichiurus lepturus, one of the major commercial fishes in the East China Sea, is of great significance to study its stocks status. Based on length frequency data of T. lepturus collected in the East China Sea from 2016 to 2020, we estimated asymptotic length, optimal length at first capture, relative mortality, and relative biomass of the stock using length–based Bayesian biomass estimation (LBB). The analysis shows a high exploitation rate and low biomass suggesting that the stock of T. lepturus has been overfished and is currently under heavy fishing pressure. Although the number of fishing vessels decreased by 29% from 2016 to 2020, the fishing horsepower decreased by only 9%, indicating that the fishing pressure on fishery resources is still high. To recover the stock, we consider the reduction of fishing intensity and enforcing of size–at–first–capture regulations to be imperative. In addition to reducing fishing boats and horsepower, it is essential to increase the escape proportion of juvenile fish by increasing the mesh size, and reduce the proportion of juvenile fish in the catch. The result in this study can provide informative reference for fishery stock assessment T. lepturus in the East China Sea under the data–limited conditions.
1. Introduction
Marine fishing is the primary means of obtaining marine aquatic products (Moffitt and Cajas-Cano, 2014). Although the catch in China Seas remains high, with the continuous increase in fishing efforts, changes in the marine climate, and environmental degradation, more and more commercial fish species in China’s waters have been over–exploited, causing a decline in resources, which seriously hinders the sustainable utilization of fishery resources (Shi and Chen, 2019). As an important basis for fishery resource management, stock assessment can accurately evaluate the biological reference points such as fisheries biomass, allowable catch, and maximum sustainable yield using mathematical statistical methods based on life history patterns and the quantification of fishery resources and commercial fishing (Hilborn and Walters, 1992; Quinn and Deriso, 1999). Traditional fish stock assessment methods require many parameters. In addition to statistical data, they need more complete survey data, such as long–term reliable catch yield data, density indices of resources, age/length structure data, and even tagging and releasing data (Methot and Wetzel, 2013). Obtaining this information requires technical know–how, skilled workers and financial support, and is therefore inconvenient when evaluating data–limited fisheries–as is the case for more than 80% of the world’s stocks, with only 1% of fish species having been systematically assessed (Costello et al., 2012). Due to the belated onset of fishery resource surveys in the China sea, the paucity of recorded survey data for most fisheries makes it difficult to evaluate them with traditional fish stock assessment methods. Stock assessments based on the existing data require the application of stock assessment methods applicable to data–limited fisheries to carry out fisheries stock assessment in China’s marine areas.
The East China Sea lies to the east of the Chinese mainland and is the marginal sea of the Western Pacific Ocean. Marine capture yield in the East China Sea accounts for more than 40% of the total output of China’s ocean fishing industry (Fishery Management Bureau of Ministry of Agriculture and Rural Affair (FMBMARA), (2000–2021)).
Trichiurus lepturus, distributed in the Western Pacific and Indian Ocean, belongs to Perciformes, Trichiuridae family, and the Trichiurus genus. Three Trichiurus species, T. lepturus, Trichiurus nanhaiensis and Trichiurus brevis, are distributed in China Seas (Hou et al., 2021; Hsu et al., 2022). Among them, Trichiurus in the East China Sea is mainly T. lepturus (Hsu et al., 2022).There are two migration routes for T. lepturus, most specimens migrated southward from the Yellow Sea to the ECS and South China Sea, and some specimens migrated northward from the South China Sea to the Taiwan Strait (He, 2019; Hsu et al., 2022). It is a commercially important species in China’s fishery in the East China Sea, harvested by bottom trawl, longline fishing, bag seine, and tow net operations. The catch of T. lepturus in the East China Sea showed an upward trend from the early 1950s to 1974, after which its downward trend reached its lowest level in 1988. After the protection of breeding individuals and young fish due to the establishment of the fishing system in the summer season in 1995, the annual catch of T. lepturus increased significantly, and the catch has continued to exceed 800, 000 tons since 1998. Since 2006, because the fishing effort has exceeded the bearing capacity of the resources, the catch of T. lepturus has shown a downward trend, with catch in 2020 of less than 600, 000 tons (Zheng et al., 2013; Fishery Management Bureau of Ministry of Agriculture and Rural Affair (FMBMARA), (2000–2021)). Studies on T. lepturus in the ECS included its life history characteristics (Panhwar et al., 2018), migrations (Sun et al., 2020), population genetics (Hsu et al., 2022), and reproductive protection (Chiang et al., 2002), with the studies of its resource status obtained using mostly the Beverton–Holt model (Ling et al., 2008), yield per recruit model (Cao and Liu, 2007), and surplus production model (Wang and Liu, 2013). T. lepturus is one of the most abundant species of marine fish in China, but its CPUE data is relatively lacking, which leads to some flaws in the evaluation results obtained using traditional fishery resource evaluation models. It is therefore crucial to carry out appropriately effective, reasonable, and accurate fishery resource assessment for T. lepturus. However, in the ECS the stocks had not been assessed based on data limited methods for the species.
Body length data can be used to fit and calculate biological parameters such as growth rate, mortality, and population size, and then evaluate the resource status of its population (Petrakis and Stergiou, 1995; Stergiou and Moutopoulos, 2001). The resource assessment models based on body length data include mainly LBSPR (Hordyk et al., 2015), LIME1 (Rudd and Thorson, 2018), LBB (Froese et al., 2018) and LBRA (Ault et al., 2019) models. Among them, The LBB model, which does not need to input data other than body length frequency data, has the advantage of lower input data requirements. Based on the survey data of trawling nets, we used the LBB method to analyze length frequency data from 2016 to 2020, and calculate the population evaluation indices such as asymptotic body length, growth, and death coefficients of T. lepturus in the East China Sea, in order to provide an evidential basis for the resource assessment of T. lepturus in the East China Sea, and to promote the management of fishery resources at the level of sustainable utilization in this area.
2. Material and methods
2.1. Study area and sampling
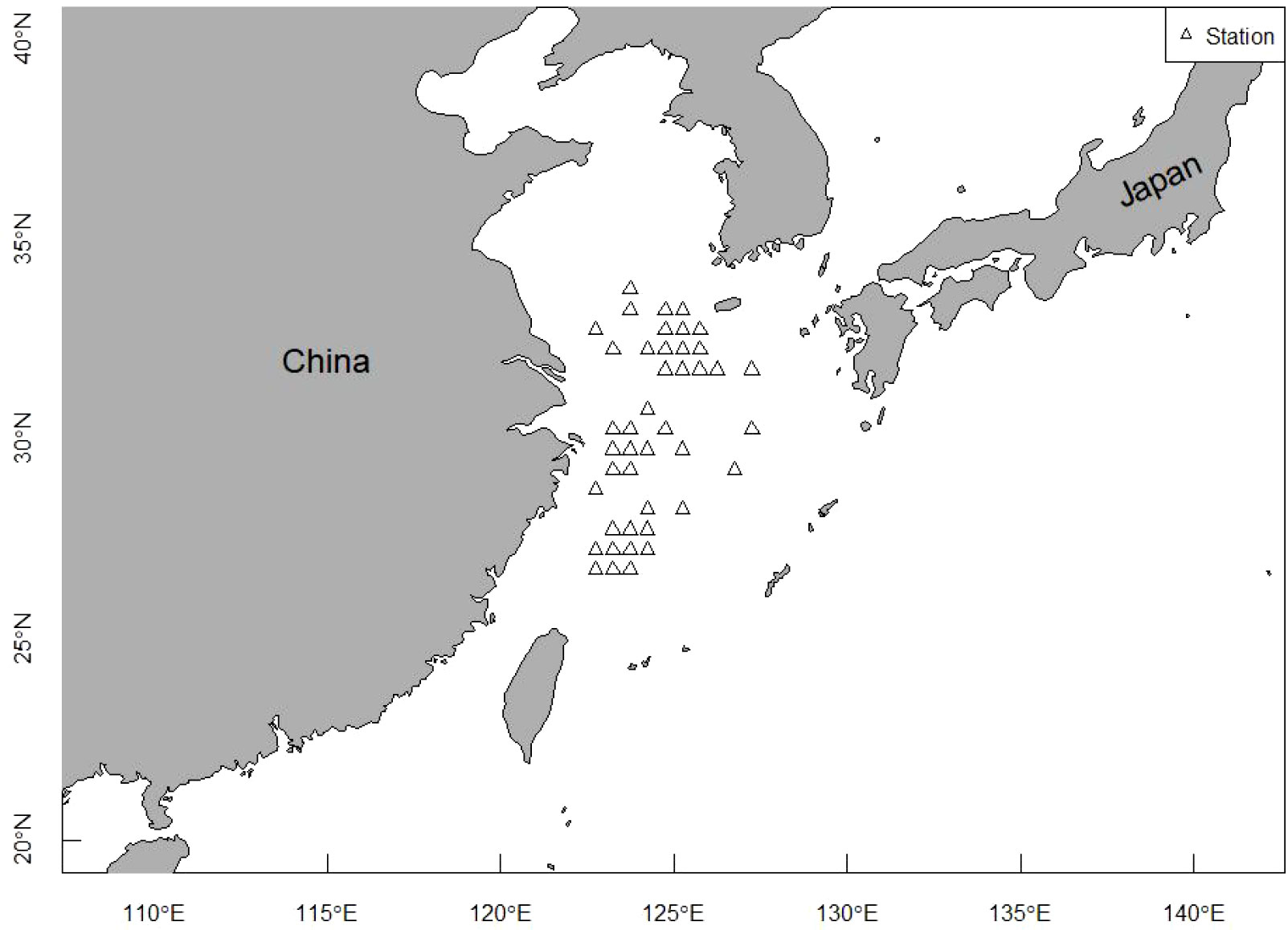
Length frequency data from the East China Sea was used to assess the population status of T. lepturus. The sampling area ranged from 26°N to 34°N and 122°E to 127°E (Figure 1). We collected a total of 1, 973 T. lepturus from 41 fishing ports in the ECS during 2016 to 2020, utilizing fishermen with trawl nets. During the sample collection, we collected 50 specimens randomly for measurement when > 30 T. lepturus were caught in any trawl; otherwise, we retained all specimens for measurement. We immediately froze and stored the specimens, thereafter thawing and measuring them in the laboratory. We measured the anal length and body weight to the nearest 0.1 cm and 0.1 g, respectively. In Table 1 we show the basic information for T. lepturus used in this study (Figure 2).
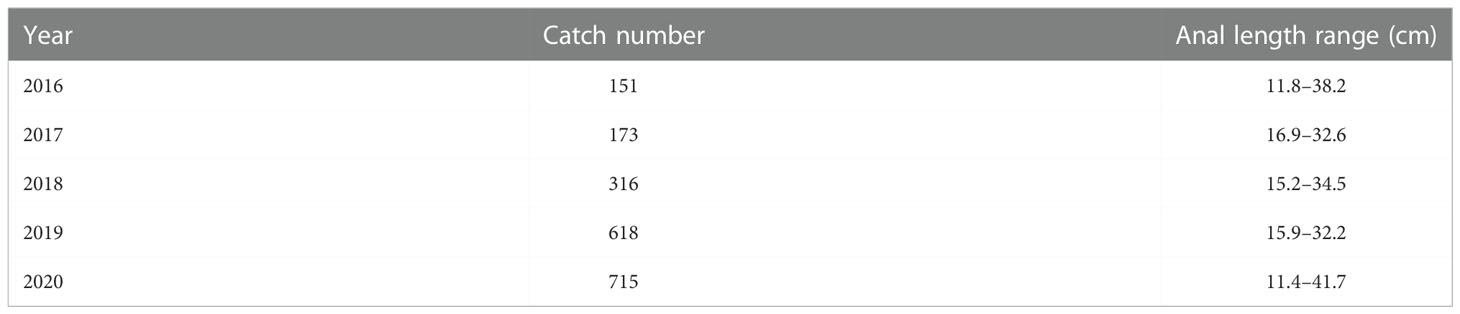
Table 1 Summary of year, catch number and anal length range of individuals measured of T. lepturus in the East China Sea.
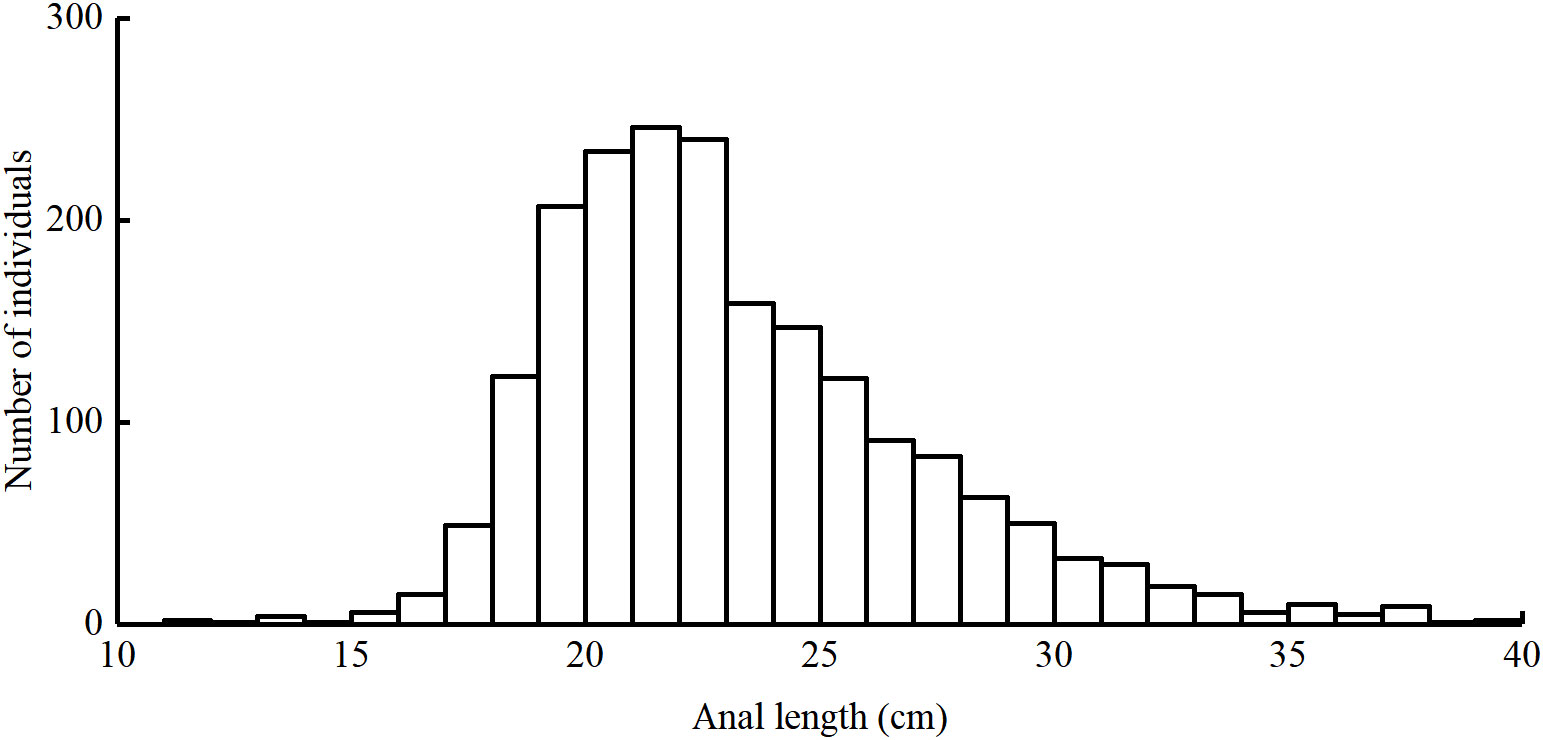
Figure 2 Length frequencies of T. lepturus in the the East China Sea specimens captured between 2016 and 2020.
2.2. The LBB model
We used the LBB model to analyze the length frequency data of T. lepturus with an average interval of 10 mm. The LBB model, which can estimate asymptotic length (Linf), mean length at first capture (Lc), optimal length at first capture (Lc_opt), the size at which cohort biomass is at maximum (Lc_opt), relative natural mortality (M/K), and relative fishing mortality (F/M) according to the body length frequency data of the evaluation object (Froese et al., 2018; Froese et al., 2019), and can also provide the prior distribution of current relative resources (B/B0), is proposed by Froese et al. (2018). This model can estimate the derivation of all relevant parameters adopts the Markov Chain Monte Carlo method, and details of formulas of LBB are given in Froese et al. (2018). Using these parameters, we can calculate the current relative biomass (B/BMSY), which is used to convert into a representative statement of the state of fishery resources (Palomares et al., 2018). In this study, the prior of Linf was set to 42 cm. The results of the LBB model can directly be used in the provisional management of data–limited fisheries populations based on two basic and simple rules: if the relative stock size B/B0 < BMSY/B0, catches of this species should be reduced; and if Lc < Lc_opt, the minimum fishing size should rather be increased. We performed data analyses in this study using the LBB software which is installed in the R–core environment.
3. Results
3.1. Population parameters
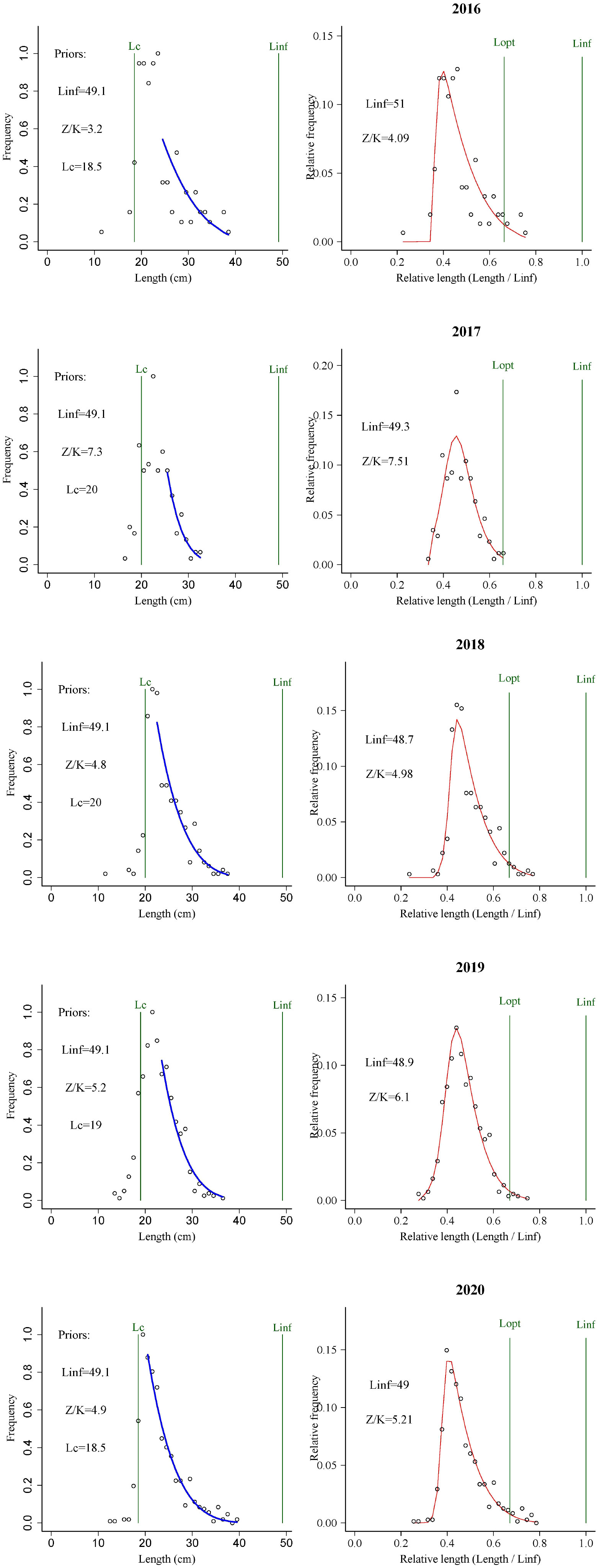
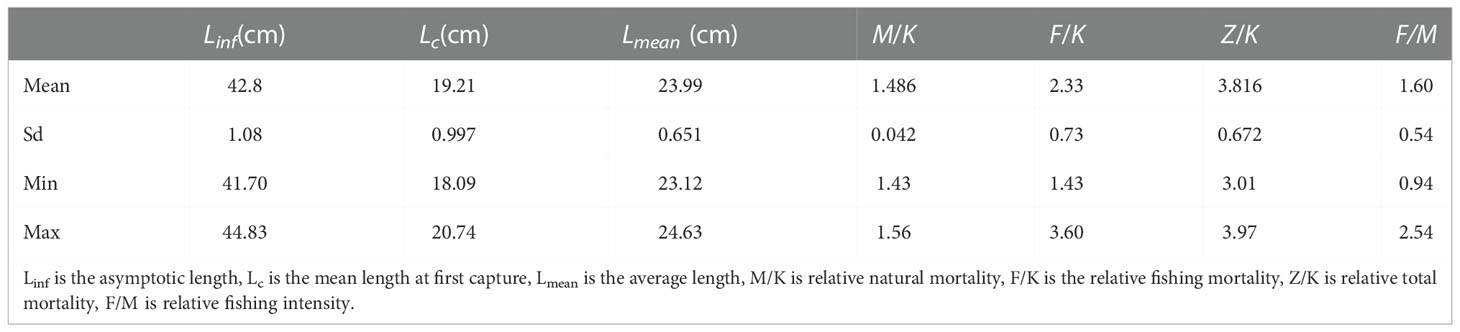
In Figure 3 we show the stock assessment results for T. lepturus using the LBB method, and in Table 2 we show the population parameters of T. lepturus in the East China Sea from 2016 to 2020 estimated based on LBB. From 2016 to 2020, the Linf and Lc of T. lepturus in the East China Sea showed a fluctuating trend, in which the asymptotic body length was the highest in 2016, the lowest in 2019, and the mean length at first capture was highest in 2017 and the lowest in 2016. The average length of T. lepturus and the length at the first capture were small and Lmean/Lopt (0.83) and Lc/Lc_opt (0.77) were lower than the standard value (1.0), which means that the proportion of large individuals in the population was very low.
3.3. Stock utilization
The B/B0 of T. lepturus in the ECS was 0.23 ± 0.06, and its distribution range was 0.15–0.33, of which the lowest in 2017 and the highest in 2016 were less than 0.5 in each year, indicating that its current biomass was very low. B/B0 showed interannual variation, and its fluctuation range tended to increase with the progression of years, indicating that the T. lepturus resources in the East China Sea have been relatively fragile, an observation supported by exploitation rate (E) and the ratio F/K. The fluctuation trend of E and the ratio F/K increased, reaching a maximum in 2017 of 0.72 and 3.60, respectively. Although the number of fishing vessels in the East China Sea decreased by 29% from 2016 to 2020, the fishing horsepower decreased by only 9% (Figure 4). According to statistics, the catch of T. lepturus in the ECS has decreased by 17%, which is twice the decline in fishing horsepower (Figure 4).
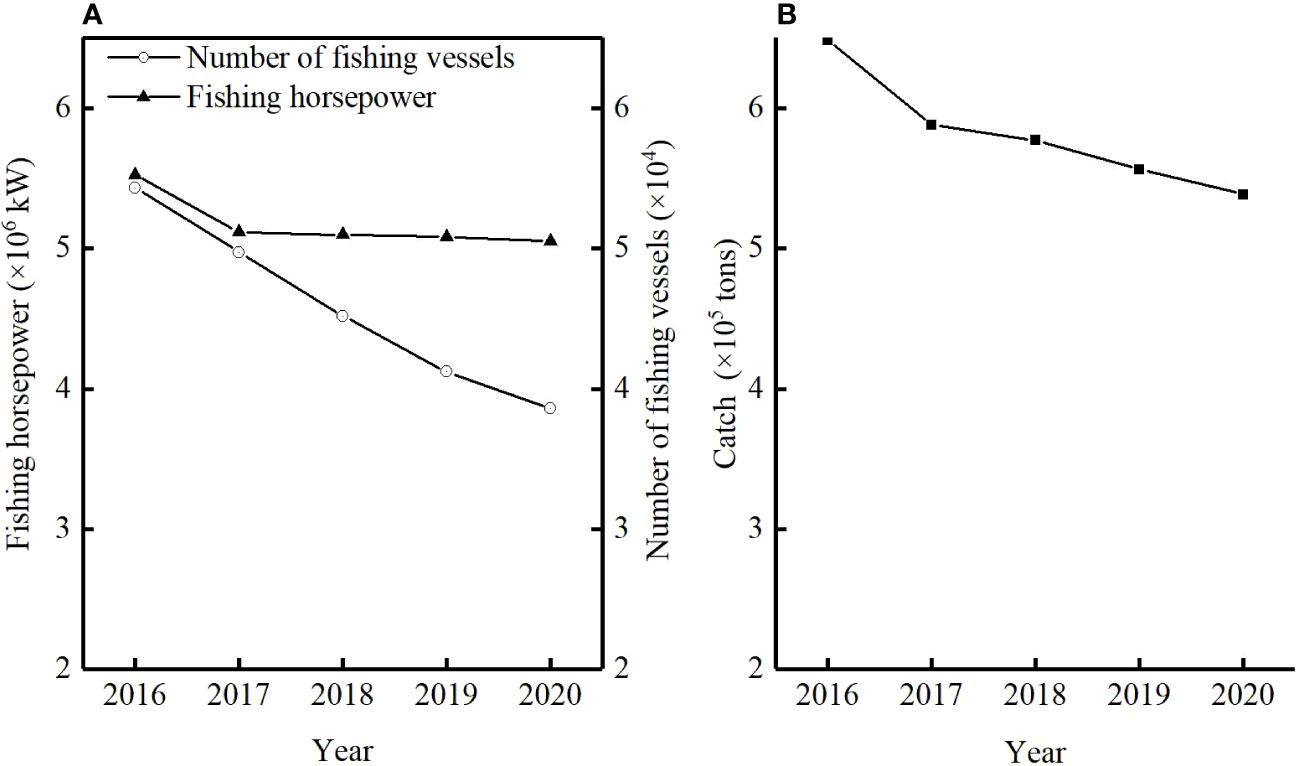
Figure 4 Statistics of fishing horsepower, number of fishing vessels (A) and catch (B) for T. lepturus in the East China Sea from the Zhejiang, Jiangsu, Shanghai and Fujian province from 2016 to 2020 {data from China Fishery Statistical Yearbook [Fishery Management Bureau of Ministry of Agriculture and Rural Affair (FMBMARA), (2000–2021)]}.
4. Discussion
The estimated Linfdistribution range of T. lepturus in the East China Sea over the years was 49.3-76.9 cm (Table 3), which was higher than the average of 42.8 (41.7-44.8) cm (Table 2) estimated in this study based on LBB from 2016 to 2020, indicating that T. lepturus in ECS comprises mainly small individuals smaller than in the past. This may be the response of of T. lepturus to continuous high–intensity fishing, which was caused mainly by the selectivity of the nets to the species during the fishing operation and the different methods used for research. On the one hand, due to high–intensity fishing activities, a large number of larger individuals will be removed (Enberg et al., 2012), which has changed the population structure of T. lepturus, and hence the proportion of small individual in the population is increasing. In the study of Trichiurus in the South China Sea, due to long–term high–intensity fishing, the Linf decreased from 62.2 cm in the 1980s to 58.5 cm in 2014–2015 but the average anal length showed signs of recovery after the fishing pressure was eased (Shi et al., 2020). This positive effect on the biological characteristics of fish due to factors such as fisheries policy also exists for other fish species, such as Evynnis cardinalis (Zhang et al., 2020) in the Beibu Gulf of the South China Sea, and Oncorhynchus keta (Fukuwaka and Morita, 2008) in Hokkaido, Japan. The Linfof T. lepturus varied greatly among different sea areas and there was no obvious law, which was confirmed by the results of Zhang et al. (2022) and Amador and Aggrey-Fynn (2020). On the other hand, the Linf estimated based on LBB depends on the length distribution of the population represented by the length frequency data, which was different from the results estimated by other methods (Wang et al., 2018). If the proportion of supplementary population in the population is high, the estimated Linf of LBB will be reduced (Froese et al., 2018). In recent years, the catch of T. lepturus in the East China Sea had a large proportion of small individuals (Zhou et al., 2002), leading to fluctuations in the estimated Linf in different years. However, it was obvious that the Linfof T. lepturus in this study was significantly smaller than that in other sea areas of China. Similarly, there were differences in Linfof T. lepturus in different sea areas of India.The habitat and fishing intensity in different sea areas are also the main factors causing the difference of T. lepturus individual size (Bernatchez, 2016).
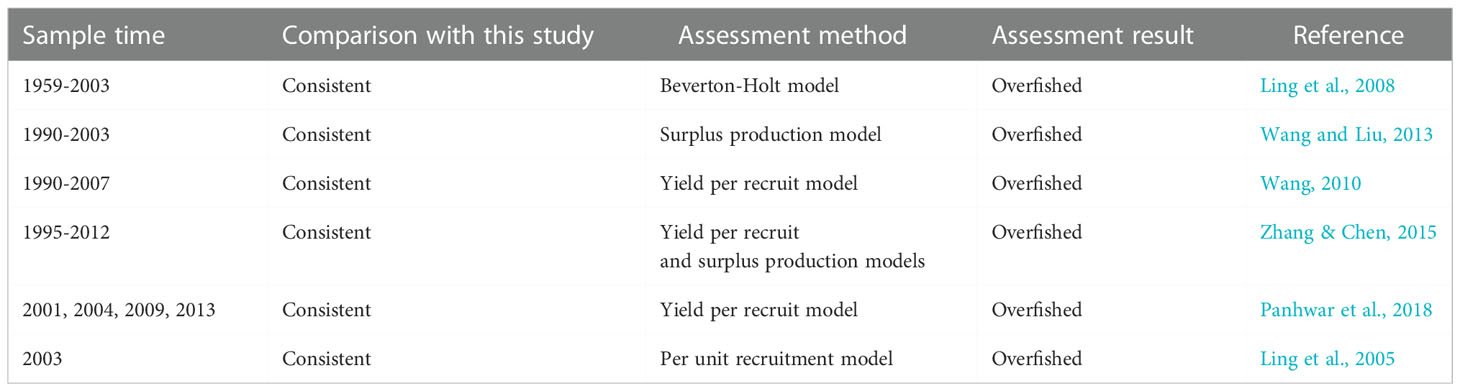
In this study, the estimates of F/M > 1 and E > 0.5 suggested that the stock was overfished, while the estimates of B/Bo < 0.5 showed that the current biomass was very low. This result was compared with other studies (Table 4). Our result was confirmed by the studies published by other researchers (Ling et al., 2005; Ling et al., 2008; Wang, 2010; Wang and Liu, 2013; Zhang & Chen, 2015; Panhwar et al., 2018), where Beverton–Holt, yield per recruit, surplus production and per unit recruitment models were used to assess the resource of T. lepturus in ECS, with all results showing the stock was suffering from overfishing (Table 4). Compared with the LBB model, other models have the disadvantages of low accuracy of assessment results and difficulty in obtaining the required data for data-limited fisheries. For example, the surplus yield model does not consider the biological characteristics of the research object, resulting in the lack of corresponding biological interpretation of the evaluation results, and cannot reflect the impact of environmental factors on fish population dynamics (Hilborn & Walters, 1992; Zhan, 1995). In addition, because most of the fisheries in China’s offshore waters are mixed fisheries, the catches and fishing methods are diversified, and the statistical yield data may be higher than the real value (Watson and Pauly, 2001; Xu et al., 2003), which will also lead to the evaluation results of MSY and BMSY.
Although T. lepturus has been overfished, its resources have not decreased as sharply as those of the the large yellow croaker (Liu and Mitcheson, 2008), and its catch has remained at a high level, which may be related to three factors. First, the reproductive characteristics of T. lepturus make it possible to generate more supplementary populations under overfishing. Studies have shown that T. lepturus has a long spawning period and a wide distribution of spawning individuals, and can reach sexual maturity at only one year (Tang et al., 2006; Chen et al., 2013), which provides a certain resilience. Second, the seasonal fishing moratorium and the protection measures for T. lepturus spawning grounds implemented in the ECS provide a guarantee for the growth and reproduction of T. lepturus. A new summer fishing moratorium policy has been implemented in the ECS since 1995. The fishing moratorium from May to June stipulated by the policy coincides with the peak spawning period of T. lepturus (Zhang et al., 2017b), and many types of fishing are not allowed to operate during this period, which allows for substantial increases in the supplementation of T. lepturus. Third, there may be hidden increased effort due to electronics and increasing experience of fishers. Fishing boats in the ECS have greater power and are equipped with more advanced technology than before. Such effort creep will capture an increased proportion of the population, thereby keeping catches stable while the stock is declining.
The novel aspect of this study is that few studies have applied data–limited method based on length frequency data to the resource assessment of T. lepturus in the East China Sea. In this study, B/B0 and Lc were lower than BMSY/B0 and Lc_opt, respectively, indicating that the catch should be reduced and fishing should start at larger size. Faced with the situation of overfishing of T. lepturus in the ECS, we should first reduce the fishing intensity and the catch of T. lepturus. However, the decline in fishing horsepower was low despite the significant decrease in the number of fishing vessels, indicating that the fishing pressure on fishery resources remained high (Figure 4). The large number of fishermen in the ECS and other factors mean that fishing efforts cannot quickly be reduced to a reasonable level, so that the effect of the recent measures to reduce the fishing horsepower is not obvious. Second, protecting juvenile fishes can effectively improve the resource level of T. lepturus in the ECS. The mesh size of fishing gear used by fishing vessels–especially the two–stick swingnet–is too small (3.5 cm, the minimum mesh size of two–stick swingnet should be 8.8 cm (Zhuang et al., 2022), which prevents young fish in the catch from being released; they are consequently caught in large quantities and the population structure tends to be miniaturized and younger, which limits the spawning stock biomass per recruit (Zhang et al., 2017a). While continuing to implement the fishing moratorium policy, by increasing the mesh size of the nets, it is also possible to increase the escape rate of juveniles; strict adherence to the catchable specifications will also reduce the capture of juveniles, thus achieving the conservation of juveniles in the ECS.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical review and approval was not required for the animal study because the samples we studied were all from frozen fishes caught in commercial fishing activities.
Author contributions
KZ: Conception and design of this study, Acquisition of data, Visualization, Software, Writing–original draft. WZ: Writing–review & editing. YS: Writing–review & editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China (2019YFD0901505, 2020YFD0901203), the Key R&D Program of Zhejiang Province (2018C02056), and the Key R&D Program of Zhejiang Province (2019C02056), Zhejiang Provincial Natural Science Foundation of China under Grant (LGN21C190005), the Key Technology and System Exploration of Quota Fishing, Ministry of Agriculture and Rural Affairs Agricultural Finance of China (36, 2017) and the Science and Technology Program of Marine Fisheries Research Institute of Zhejiang Province
Acknowledgments
We thank the staff members of the Scientific Observing and Experimental Station of Fishery Resources for Key Fishing Grounds, Ministry of Agriculture, Marine Fisheries Research Institute of Zhejiang for providing assistance at laboratory. We would like to thank the developer of the LBB method, Rainer Froese. We are also grateful to all the reviewers for their valuable comments and advices.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amador E., Aggrey-Fynn J. (2020). Growth, mortality, sexual maturity and exploitation of the ribbonfish (Linnaeus 1758) (Pisces: Trichiuridae) in ghanaian waters. Int. J. Fish Aquat Stud. 8 (6), 96–104. doi: 10.22271/fish.2020.v8.i6b.2366
Ault J. S., Smith S. G., Bohnsack J. A., Luo J. G., Stevens M. H., DiNardo G. T., et al. (2019). Length–based risk analysis for assessing sustainability of data–limited tropical reef fisheries. ICES J. Mar. Sci. 76 (1), 165–180. doi: 10.1093/icesjms/fsy123
Bernatchez L. (2016). On the maintenance of genetic variation and adaptation to environmental change: considerations from population genomics in fishes. J. Fish Biol. 89 (6), 2519–2556. doi: 10.1111/jfb.13145
Cao S. P., Liu Q. (2007). Stock assessment of the hairtail (Trichiurus haumela) fishery in the East China Sea by incorporating uncertainty into the estimation of the biological reference points f 0.1 and f max. S. China. Fish. Sci. 3 (2), 42–48. doi: 10.3969/j.issn.2095-0780.2007.02.008
Chen Y. L., Shan X. J., Dai F. Q., Jin X. S. (2013). Relative stock density and distribution of hairtail Trichiurus lepturus and its spawning stock structure in coastal waters of the East China Sea. Prog. Fish. Sci. 34 (04), 8–15. doi: 10.3969/j.issn.1000-7075.2013.04.002
Chiang K. P., Kuo M. C., Chang J., Wang R. H., Gong G. C. (2002). Spatial and temporal variation of the synechococcus population in the East China Sea and its contribution to phytoplankton biomass. Cont. Shelf. Res. 22, 3–13. doi: 10.1016/S0278–4343(01)00067–X
Costello C., Ovando D., Hilborn R., Gaines S. D., Deschenes O., Lester S. E. (2012). Status and solutions for the world’s unassessed fisheries. Science 338 (6106), 517–520. doi: 10.1126/science.1223389
Enberg K., Jørgensen C., Dunlop E. S., Varpe Ø., Boukal D. S., Baulier L., et al. (2012). Fishing–induced evolution of growth: Concepts, mechanisms and the empirical evidence. Mar. Ecol. 33 (1), 1–25. doi: 10.1111/j.1439–0485.2011.00460.x
Fishery Management Bureau of Ministry of Agriculture and Rural Affair (FMBMARA) (2000–2021). Fishery statistical yearbook of China (Beijing: FMBMARA).
Froese R., Winker H., Coro G., Demirel N., Tsikliras A. C., Dimarchopoulou D., et al. (2018). A new approach for estimating stock status from length frequency data. ICES J. Mar. Sci. 75 (6), 2004–2015. doi: 10.1093/icesjms/fsy078
Froese R., Winker H., Coro G., Demirel N., Tsikliras A. C., Dimarchopoulou D., et al. (2019). On the pile–up effect and priors for linf and M/K: response to a comment by hordyk et al. on “A new approach for estimating stock status from length–frequency data. ICES J. Mar. Sci. 76, 461–465. doi: 10.1093/icesjms/fsy199
Fukuwaka M. A., Morita K. (2008). Increase in maturation size after the closure of a high seas gillnet fishery on hatchery–reared chum salmon Oncorhynchus keta. Evol. App. 1 (2), 376–387. doi: 10.1111/j.1752–4571.2008.00029.x
He X. B. (2019). Spatial Distribution,Population structure and trophic ecology of common trichiuridae species in the coastal waters of China (China: PhD Thesis. Jimei University).
Hilborn R., Walters C. J. (1992). Quantitative fisheries stock assessment: Choice, dynamics, and uncertainty (London, United Kingdom: Chapman and Hall), 570.
Hordyk A., Ono K., Valencia S., Loneragan N., Prince J. (2015). A novel length–based empirical estimation method of spawning potential ratio (SPR), and tests of its performance, for small–scale, data–poor fisheries. ICES J. Mar. Sci. 72 (1), 217–231. doi: 10.1093/icesjms/fsu004
Hou G., Xu Y., Chen Z., Zhang K., Huang W., Wang J., et al. (2021). Identification of eggs and spawning zones of hairtail fishes trichiurus (Pisces: Trichiuridae) in northern south China Sea, using DNA barcoding. Front. Environ. Sci. 9. doi: 10.3389/fenvs.2021.703029
Hsu K. C., Yi M. R., Gu S., He X. B., Luo Z. S., Kang B., et al. (2022). Composition, demographic history, and population structures of trichiurus. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.875042
Ling J. Z., Li S. F., Yan L. P., Cheng J. H. (2008). Utilization and management of Trichiurus japonicus resources in East China Sea based on beverton–Holt model. Chin. J. Appl. Ecol. 19 (1), 178–182. doi: 10.13287/j.1001-9332.2008.0038
Ling J. Z., Yan L. P., Lin L. S., Li J. S., Cheng J. H. (2005). Reasonable utilization of hairtail Trichiurus japonicus resource in the East China Sea based on its fecundity. J. Fish. Sci. China 12 (6), 726–730. doi: 10.3321/j.issn:1005-8737.2005.06.010
Liu M., Mitcheson Y. (2008). Profile of a fishery collapse: Why mariculture failed to save the large yellow croaker. Fish. Fish. 9 (3), 219–242. doi: 10.1111/j.1467–2979.2008.00278.x
Methot R. D. J., Wetzel C. R. J. (2013). Stock synthesis: A biological and statistical framework for fish stock assessment and fishery management. Fish. Res. 142, 86–99. doi: 10.1016/j.fishres.2012.10.012
Moffitt C. M., Cajas-Cano L. (2014). Blue growth: The 2014 FAO state of world fisheries and aquaculture. Fish 39 (11), 552–553. doi: 10.1080/03632415.2014.966265
Palomares M. L. D., Froese R., Derrick B., Nöel S. L., Tsui G., Woroniak J., et al. (2018). “A preliminary global assessment of the status of exploited marine fish and invertebrate populations,” in A report prepared by the Sea around us for OCEANA, vol. 64. (Washington, DC: OCEANA).
Panhwar S. K., Zhou Y. D., Gao T. X., Wang P., Han Z. Q., Wang Z. M., et al. (2018). Decadal population traits and fishery of largehead hairtail, Trichiurus lepturus (Linnaeus 1758) in the East China Sea. Pak. J. Zool. 50 (1), 1–5. doi: 10.17582/journal.pjz/2018.50.1.1.5
Petrakis G., Stergiou K. I. (1995). Weight–length relationships for 33 fish species in Greek waters. Fish. Res. 21, 465–469. doi: 10.1016/0165–7836(94)00294–7
Rudd M. B., Thorson J. T. (2018). Accounting for variable recruitment and fishing mortality in length–based stock assessments for data–limited fisheries. Can. J. Fish. Aquat. Sci. 75, 1019–1035. doi: 10.1139/cjfas–2017–0143
Shi Y. C., Chen X. J. (2019). A review of stock assessment methods on small pelagic fish. Mar. Fish. 41 (1), 118–128. doi: 10.3969/j.issn.1004-2490.2019.01.014
Shi D. F., Zhang K., Cai Y. C., Geng P., Xu Y. W., Sun M. S., et al. (2020). Population structure of Trichiurus japonicus in northern south China Sea and parameters of its growth, mortality and maturity. S. China. Fish. Sci. 16 (05), 51–59. doi: 10.12131/20200055
Stergiou K. I., Moutopoulos D. K. (2001). A review of length–weight relationships of fishes from Greek marine waters. Naga ICLARM Quarterly. 24, 23–39.
Sun P., Chen Q., Fu C. H., Xu Y., Sun R. L., Li J. C., et al. (2020). Latitudinal differences in early growth of Largehead hairtail (Trichiurus japonicus) in relation to environmental variables. Fish. Oceanogr. 29, 470–483. doi: 10.1111/fog.12490
Tang Q. S., Jia. X. P., Zheng. Y. J., Meng. T. X. (2006). Marine living resources and habitat in china's exclusive economic zone (Beijing: Science Press), 663–680.
Wang Y. (2010). The resource evaluation of trichiurus japonicus on East China Sea in summer close season (China: M.D Thesis. Zhejiang Ocean University).
Wang Y., Liu Q. (2013). Applications of CEDA and ASPIC computer packages to the hairtail (Trichiurus japonicus) fishery in the East China Sea. Chin. J. Oceanol. Limnol. 31 (1), 92–96. doi: 10.1007/s00343–013–2073–7
Wang X. H., Qiu Y. S., Du F. Y., Sun D. R., Wang Y. Z. (2018). Using length-based Bayesian biomass method to estimate Parargyrops edita population parameters in the beibu gulf, south China Sea. J. Fish. Sci. China 44 (10), 1654–1662. doi: 10.11964/20191012025
Watson R., Pauly D. (2001). Systematic distortions in world fisheries catch trends. Nature 414 (29), 534–536. doi: 10.1038/35107050
Wu J. Z. (1985). Age and growth of Trichiurus lepturus on the off–shore fishing ground of zhejiang province. J. Zhejiang. college. Fish. 4 (1), 9–23.
Wu H. Z., Cheng S. G., Zhou J. K., Wang J. F. (1985). Study on the growth of the hairtails, Trichiurus haumela (Forskal). Oceanol. Limnol. Sin. 162, 156–168.
Xu H. X., Liu Z. F., Zhou Y. D. (2003). A elementary study on quota catch of hairtail, Trichiurus haumela in the East China Sea. J. Zhejiang. Ocean. U. 22 (1), 1–6. doi: 10.3969/j.issn.1008-830X.2003.01.001
Yan L. P., Hu F., Li J. S., Liu Y., Cheng J. H. (2005). Age and growth of Trichiurus haumela in the East China Sea. Mar. Fish. 02), 139–142.
Zhang K., Cai Y. C., Liao B., Jiang Y. E., Sun M. S., Su L., et al. (2020). Population dynamics of threadfin porgy Evynnis cardinalis, an endangered species on IUCN red list in the beibu gulf, south China Sea. J. Fish. Biol. 97 (2), 14398. doi: 10.1111/jfb.14398
Zhang K., Chen Z. Z. (2015). Using Bayesian state-space modelling to assess Trichiurus japonicus stock in the East China Sea. J. Fishery Sci. China 22 (5), 1015–1026. doi: 10.3724/SP.J.1118.2015.14536
Zhang Q. H., Cheng J. H., Xu H. X., Shen X. Q., Yu G. P., Zheng Y. J. (2007). Fishery resources and their sustainable utilization in the East China Sea (Shanghai: Fudan University Press).
Zhang Q. Y., Hong W. S., Chen S. X. (2017b). Stock changes and resource protection of the large yellow croaker (Larimichthys crocea) and ribbon fish (Trichiurus japonicus) in coastal waters of China. J. Appl. Oceanogr. 36 (03), 438–445. doi: 10.3969/J.ISSN.2095-4972.2017.03.018
Zhang J., Jiang R., Fang Y. H. (2017a). Study on the fish size selectivity of codends of canvas stow net in the East China Sea. Mar. Fish. 399 (3), 340–350. doi: 10.13233/j.cnki.mar.fish.2017.03.012
Zhang M., Wang X. H., Wang M. D., Du F. Y., Sun D. R., Wang L. G., et al. (2022). Assessment of trichiurus haumela stocks in the beibu gulf based on length-based Bayesian biomass estimation method. Haiyang Xuebao 44 (1), 11–21. doi: 10.12284/hyxb2022002
Zheng Y. J., Hong W. S., Zhang Q. Y. (2013). Review and prospects for resource biology of main marine demersal food fishes along the coastal waters of China. J. Fish. Sci. China. 37 (1), 151–160. doi: 10.3724/SP.J.1231.2013.38253
Zhou Y. D., Xu H. X., Liu Z. F., Xue L. J. (2002). A study on variation of stock structure of hairtall, trichiurus haumela in the East China Sea. J. Zhejiang. Ocean. U 21 (4), 314–320. doi: 10.3969/j.issn.1008-830X.2002.04.002
Keywords: Trichiurus lepturus, LBB model, data–limited method, stock assessment, East China Sea
Citation: Zhu K, Zhu W and Shi Y (2023) Stock assessment using length–based Bayesian evaluation method for Trichiurus lepturus in the East China Sea. Front. Mar. Sci. 9:1065954. doi: 10.3389/fmars.2022.1065954
Received: 10 October 2022; Accepted: 05 December 2022;
Published: 04 January 2023.
Edited by:
Mhd Ikhwanuddin, University of Malaysia Terengganu, MalaysiaReviewed by:
Jorge Paramo, University of Magdalena, ColombiaAna Laura Ibañez, Autonomous Metropolitan University, Mexico
Copyright © 2023 Zhu, Zhu and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenbin Zhu, emh1d2VuYmluMjAyMUAxMjYuY29t; Yongchuang Shi, c3ljMTMwNTIzMjYwOTFAMTYzLmNvbQ==
 Kai Zhu
Kai Zhu Wenbin Zhu1,2*
Wenbin Zhu1,2* Yongchuang Shi
Yongchuang Shi