- 1Animal and Fish Production Department, College of Agricultural and Food Sciences, King Faisal University, Al-Ahsa, Saudi Arabia
- 2Fish and Animal Production Department, Faculty of Agriculture (Saba Basha), Alexandria University, Alexandria, Egypt
- 3Aquatic Microbiology, Faculty of Aquatic and Fisheries Sciences, Kafrelsheikh University, Kafrelsheikh, Egypt
- 4Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, Alexandria University, Alexandria, Egypt
- 5Department of Fish Health and Diseases, Faculty of Fish Resources, Suez University, Suez, Egypt
- 6Department of Veterinary Pharmacology, Faculty of Veterinary Medicine, Alexandria University, Alexandria, Egypt
- 7Department of Physiology, Faculty of Veterinary Medicine, Cairo University, Cairo, Egypt
- 8Department of Aquatic Animal Medicine and Management, Faculty of Veterinary Medicine, Cairo University, Cairo, Egypt
The use of phytotherapy in aquaculture is rapidly increasing for more environmentally sustainable measures. The present work aimed to investigate the effects of different dietary levels of sweet basil, Ocimum basilicum, leaves ethanol (BEE) or aqueous (BAE) extracts (0, 200, 300, and 500 mg/kg) on Oreochromis niloticus growth, digestive enzyme activities, hemato-biochemical profile, antioxidant status, immune responses, and resistance against Streptococcus agalactiae. Oreochromis niloticus fingerlings (40.00 ± 1.00 g/fish, n = 210) were randomly divided into seven triplicated groups (control, BEE200, BEE300, BEE500, BAE200, BAE300, and BAE500) and fed the experimental diets for 8 weeks. The results revealed that dietary inclusion of BEE and BAE significantly improved final weight, weight gain, survival, and digestive enzyme activities. The growth response revealed a dose-dependent in favor of fish fed BEE. The hemato-biochemical biomarkers showed a significant improvement in RBCs, WBCs, hemoglobin, and lymphocyte, and a significant decrease in aminotransferases, creatinine, and cholesterol levels with dietary basil extracts. The cellular and humoral immune responses (phagocytic activity, phagocytic index, lysozyme activity, immunoglobulin) were significantly improved with increasing BEE and BAE in a dose-dependent manner. The expression of IL-1β and TNF-α genes were increased, while TGF-β was decreased in a dose-dependent manner and BEE500 have the highest expression. The antioxidant balance was improved with increasing basil extracts supplementation, and the BEE500 group showed the best antioxidant status. Dietary BEE and BAE increased Nile tilapia resistance to S. agalactiae. In conclusion, the dietary supplementation of both ethanolic and aqueous extracts could improve the growth performance and physiological, and immune-antioxidant status of Nile tilapia.
Introduction
The aquaculture industry recorded the highest annual growth among different animal production sectors during the last few decades. Aquaculture can extend aquatic products with reasonable prices to countries or regions that have limited access to natural fisheries and improve nutrition and food security (Stead, 2019). Nile tilapia, Oreochromis niloticus, is considered one of the predominant farmed freshwater fish species in the world (Diab et al., 2022; Mansour et al., 2022a). It accounted for 8% of total finfish produced globally in 2018 (FAO, 2020). Due to the fast-growing rates, efficient feed utilization, disease resistance, robustness, and adaptability to different environmental conditions, and systems, tilapia could lead freshwater fish farming (El-Sayed, 2019). Nevertheless, in recent years, high mortalities have been reported among tilapia farms due to the spreading of different infectious diseases that threaten the global tilapia industry (Behera et al., 2018; Legario et al., 2020). Accordingly, improving fish health, immunity, and/or pathogen resistance by dietary supplementation, especially with eco-friendly and natural additives, is a very attractive research strategy.
Due to their numerous vital properties, such as immune stimulation, antimicrobial, growth promotion, and anti-stress, many studies have suggested using herbal extracts in aquaculture as a safer, more effective, and less expensive alternative to chemotherapeutic agents (Sallam et al., 2017; Mansour A. et al., 2018; Mansour A. T. et al., 2018; Mansour et al., 2020; Mansour et al., 2021; Mansour et al., 2022b). Sweet basil (Ocimum basilicum) is a very common, annual aromatic plant (herb) worldwide and belongs to the family Lamiaceae (Chung et al., 2020). Basil grows in tropical, sub-tropical, and Mediterranean regions (Sipos et al., 2021). Basil has a long history of use in traditional medicine as an antimicrobial, anti-inflammatory, anti-aging, antiviral, antioxidant, and anticancer (Shahrajabian et al., 2020). The therapeutic activities of O. basilicum are related to its bioactive compounds, including alkaloids, flavonoids, saponins, essential oils, phytosterols, phenolic compounds, terpenoids, anthraquinones, and tannins (Sakr and Al-Amoudi, 2012; Andayani et al., 2020). In addition, basil contains several volatile components, including linalool, methyl chavicol, eugenol, bergamotene, and methyl cinnamate (Klimankova et al., 2008; Sonmezdag et al., 2018).
The leaves and seeds of basil have several applications in the animal diet. Basil leaves can be used as a feed ingredient (Tolay, 2021). Basil leaf mucilage extract, as a natural polymer, was used as a binder in the diets of Cyprinus carpio fingerlings and improved the physical properties of the diet, palatability, and enhanced growth performance (Al-Hamdani et al., 2021). In addition, basil essential oil was used as an anesthetic for several fish species, tambaqui (Colossoma macropomum) and silver catfish (Rhamdia quelen) (Silva et al., 2012; de Lima Boijink et al., 2016). In addition, basil essential oils and extracts revealed several beneficial effects as dietary supplement. It could improve the growth performance, feed utilization, immune responses, and antioxidant status of hybrid Nile tilapia (El-Dakar et al., 2008) gilthead seabream (Sparus aurata) (El-Dakar et al., 2015), common carp (C. carpio) (Amirkhani and Firouzbakhsh, 2015) pirarucu (Arapaima gigas) juveniles (Chung et al., 2020), Indian shrimp (Penaeus indicus) (Abdel-Tawwab et al., 2021), and rainbow trout (Oncorhynchus mykiss) (Magara et al., 2022).
Streptococcus agalactiae is a gram-positive spherical or ovoid-shaped bacteria, arranged in chains or pairs, and a non-motile, non-spore former (Delfani et al., 2017). S. agalactiae is one of the fish-streptococcosis main causative agents, which is characterized clinically by high morbidity, severe mortality, erratic swimming, low growth rate, exophthalmia, and abdominal distension (Evans et al., 2006; Bowater et al., 2012). Streptococcosis is a dangerous veterinary disease as well as a zoonotic disease that causes significant economic losses worldwide (Pereira et al., 2010; Diab et al., 2019). The use of herbs and their natural extracts for the treatment of various fish diseases or as immunostimulants has been commonly used (Delfani et al., 2017). Therefore, the present study aimed to evaluate the use of sweet basil, O. basilicum, ethanolic and aqueous extracts as health-improving and immune stimulant feed supplements in O. niloticus diet by investigating their effects on growth performance, digestive enzyme activities, hemato-biochemical profile, immune and antioxidant status, some immune-related genes expressions, and resistance against S. agalactiae challenge.
Materials and methods
Basil extract preparation
The basil, O. basilicum, leaves were obtained from a local market in Alexandria governorate, Egypt. Basil leaves were washed, dried, and ground into a fine powder. Basil ethanol extract (BEE) was prepared according to Zhang et al. (2018). Briefly, the basil leaves fine powder was immersed in 70% ethanol solution at a ratio of (1:1) for 48 h. The basil leaves residue was filtered using filter paper and the obtained extract was concentrated at 40°C using a rotatory evaporator. The obtained BEE extract was kept at 4°C until use. Basil aqueous extract (BAE) was conducted according to Zhang et al. (2018). Briefly, samples of 50 g of the dried fine-ground basil leaves powder were immersed in 500 ml sterile distilled water and shaken at room temperature for 4 h. The BAE was filtered twice through a 100-μm pore size nylon net, then concentrated using a rotatory evaporator and stored at -20°C until it was used.
Experimental fish and rearing conditions
A total number of 210 apparently healthy O. niloticus (40.0 ± 1.0 g/fish) were obtained from a private farm, Kafr El-Sheikh, Egypt. The fish was transferred to the wet laboratory, Faculty of Veterinary Medicine, Alexandria University, Egypt, and randomly allocated into 21 glass aquaria (90 × 50 × 35 cm, 10 fish per aquarium). Each aquarium has continuous aeration by using an electric air-pumping compressor. Wastes were daily siphoned with water exchange at a daily rate of 30% using dechlorinated tap water. The water temperature was kept at 27°C ± 2°C. Fish were adapted for 2 weeks in the laboratory conditions before the start of the experiment. Fish were manually fed twice a day at 9:00 and 15:00 on 32% crude protein commercial tilapia diet (Aller-Aqua Egypt Co, 6th of October, Giza, Egypt). The feeding rate was 3% of the total fish body weight. The actual feed intake was adjusted bi-weekly according to the change in fish body weight.
Experimental design
The fish were randomly divided into seven groups (three replicates each). The control group was fed basal diets free of BEE and BAE extracts. The second, third, and fourth groups were fed diets containing basil ethanol extract (BEE) at 200, 300, and 500 mg/kg of feed (BEE200, BEE300, and BEE500). The fifth, sixth, and seventh groups were fed diets that contained basil aqueous extract (BAE) at 200, 300, and 500 mg/kg of feed, respectively (BAE200, BAE300, and BAE500) (Table 1). The BEE or BAE powder was separately dissolved in distal water to have a concentration of 10% w/v and was sprayed on the diet of the respected treatment and then dried in an air-force oven at a temperature of 45–50°C (Motlagh et al., 2020; Zenhom and Ibrahim, 2020). The selected doses of basil extract were done according to the study of (Amirkhani and Firouzbakhsh, 2015).
The fish diet was kept at 4°C until use. Fish were fed the experimental diets for 8 weeks before the bacterial challenge, followed by 2 weeks of the observation period after the challenge by S. agalactiae for recording the mortality.
Fish growth performance
At the end of the eighth week, the fish from each replicate were anaesthetized with tricaine methane sulfonate (MS222, 25 mg/L, Argent Laboratories, Redmond, WA, USA) to get fish weight (individually). Fish growth performance was evaluated, including final weight (g), weight gain (%; [100 × initial weight – final weight/initial weight), and survival (%).
Blood and serum collection
After 8 weeks of dietary supplementation of BEE or BAE, blood samples (15 fish/treatment) were collected from the caudal vein by a disposable plastic syringe. Blood samples were divided into two parts; one part was added to heparinized tubes, while the other part of blood samples was added to plain tubes without anticoagulants for serum collection after centrifugation at 3000 rpm for 15 minutes at 4°C.
Haemato-biochemical analysis
The red and white blood cells (RBCs and WBCs) count, the differential leukocyte count, packed cell volume (PCV), and hemoglobin content, were determined as described by Mansour and Esteban (2017). The activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT) (Reitman and Frankel, 1957), in addition to the serum total proteins (Gornall et al., 1949), albumin (Doumas et al., 1971), globulins, and creatinine levels (Henry, 1964) were estimated using commercial kits (Biodiagnostic Co., Cairo, Egypt). Cholesterol and triglyceride were determined according to Reitman (1957).
Immune and oxidative stress responses
The cellular immune response was determined, including blood respiratory burst activity, which was examined by the nitroblue tetrazolium (NBT) assay (Secombes, 1990) and phagocytic cells activity and index (Kawahara et al., 1991). The humoral immune response was determined, including serum lysozyme activity, which was determined by a turbidimetric assay according to Ellis (1990). Bactericidal activity was detected as described by Rainger and Rowley (1993). Immunoglobulin M (IgM) was estimated by ELISA using a commercial kit (Bio Diagnostic Co., Cairo, Egypt). (Siwicki and Anderson, 1993).
The antioxidant status parameters include catalase (CAT) (Aebi, 1984), superoxide dismutase (SOD) (Nishikimi et al., 1972), total antioxidant capacity (TAC) (Bartosz, 2003), and malondialdehyde (MDA) (Uchiyama and Mihara, 1978) were determined calorimetrically (Bio diagnostic Co., Cairo, Egypt).
Some immune genes examination
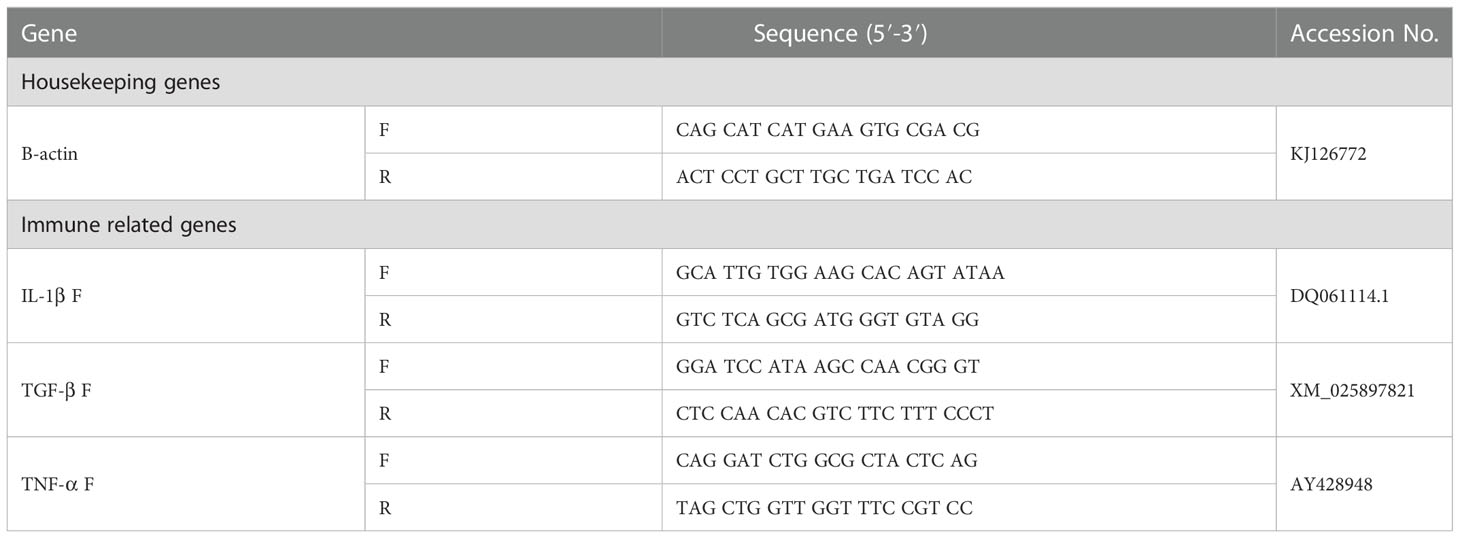
Interleukin 1 beta (IL-1β), transforming growth factor-beta 1 (TGF-β), tumor necrosis factor (TNF), and β-actin expression in the head kidney of O. niloticus were quantitatively examined by real-time PCR amplifications. The PCR primer sequences of the studied genes are presented in (Table 1). Briefly, total RNA was extracted from the head kidney tissue after 8 weeks of treatment. The concentration and purity of the extracted RNA were determined via spectrophotometry with absorption at 260 and 280 nm. The following thermal cycling conditions were used for the expressed genes: initial denaturation at 95°C for 5 min, 40 cycles of amplification (DNA denaturation at 95°C for 15 s, annealing at 60°C for 15 s, extension at 72°C or 15 s), and final extension at 72°C for 5 min. The comparative CT method (2−ΔΔCt) (Livak and Schmittgen, 2001) was used to calculate the gene expression values using β-actin as a housekeeping gene.
Activities of some digestive enzymes
The intestinal samples were collected from the anesthetized fish after blood collection then washed with PBS, then the intestinal samples were homogenized in PBS (1 g/10 ml), centrifuged (5000 rpm/10 min), and the supernatant was stored at 4°C until it was used for determination of the activities of lipase, amylase, and protease enzymes as reported in by Dawood et al. (2019).
Bacterial challenge
At the end of the experiment, fish were bacteriologically examined to be free from bacterial infection. Twenty fish from each treatment were challenged by S. agalactiae (Kindly provided by Prof. Dr./Khalil H.R., Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, Alexandria University, Alexandria, Egypt). Fish was intra-peritoneally injected with 0.2 ml of a suspension containing 2 × 107 CFU/ml of S. agalactiae (Moustafa et al., 2020). During 14 days of the observation period, the mortality was recorded and death specificity was estimated by S. agalactiae re-isolation from freshly dead fish (Zahran et al., 2018). During the observation period, fish was fed their corresponding diets according to their groups. The cumulative mortality was reported and the relative level of protection (RLP) among the challenged fish was determined according to Ruangroupan et al. (1986):
Statistical analysis
The data was expressed as means ± pooled standard error. The results were checked for homogeneity and normal distribution before conducting the parametric analysis of the two-way ANOVA test using SPSS 21 (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.). The post hoc Tukey test was used to identify the significant differences among means at significant levels of 0.05. Some selected parameters were used to identify the fit regression trend of increasing supplementation levels of basil extracts and fish response to compare the dose-response of each extract.in addition to comparing the significant difference between slopes of the two extracts using GraphPad Prism 7.0 (GraphPad Software, Inc., San Diego, CA, USA).
Results
Growth performance
The growth performance of fish-fed ethanolic and aqueous basil extract-supplemented diets revealed significant differences among treatments. The FBW, WG, and survival were improved with increasing supplementation levels of BEE and BAE with the superiority of groups supplemented with BEE (Table 2). The two-way ANOVA analysis revealed significant effects of extract and dose on the growth response of treated fish.
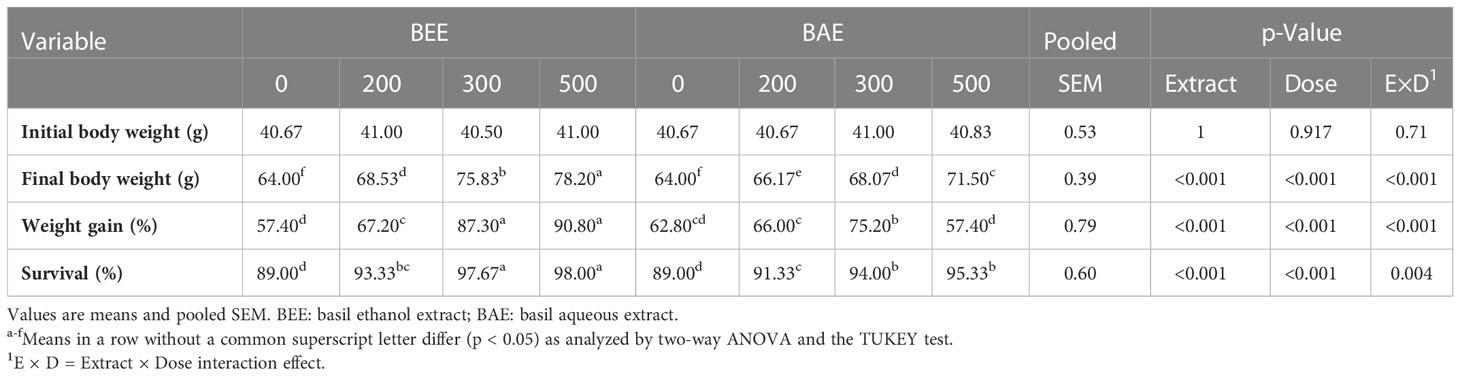
Table 2 Effect of dietary supplementation of basil ethanol and aqueous extracts (mg kg-1 of feed) on growth performance and survival of Nile tilapia, Oreochromis niloticus.
The regression analysis of FBW and survival with increasing dietary supplementation of BEE and BAE showed a fit linear regression model and the difference between the slopes of both extracts is highly significant in favor of BEE (Figure 1).
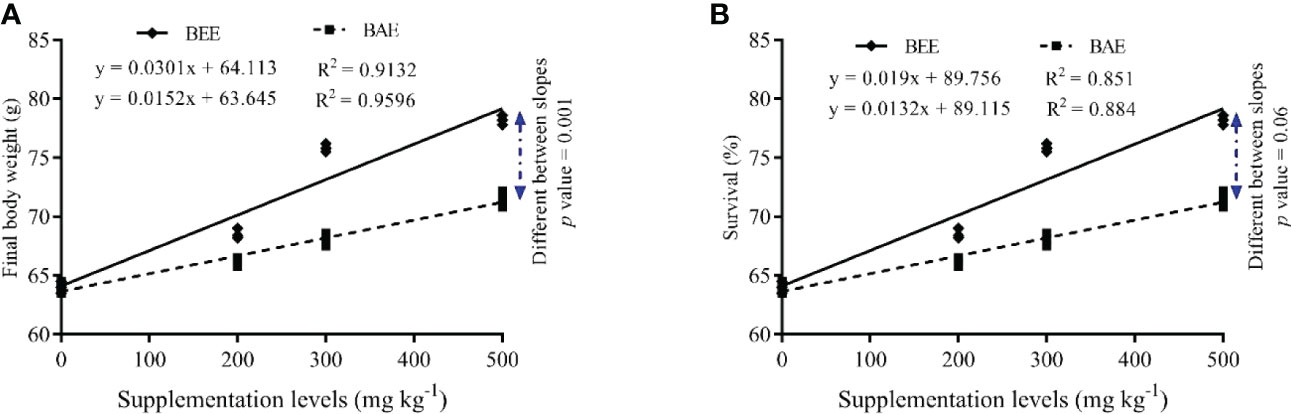
Figure 1 The fit regression model and slope differences between dietary supplementation of basil ethanol (BEE) and aqueous extracts (BAE) on final body weight (A) and survival (B) of Nile tilapia, Oreochromis niloticus.
Activities of some digestive enzymes
The protease, amylase, and lipase results revealed significant differences in their activities in relation to extracts, dose of supplementation and their interaction. The highest protease and amylase activities were observed in BEE500, while the best lipase activity was detected in BAE500 (Table 3).
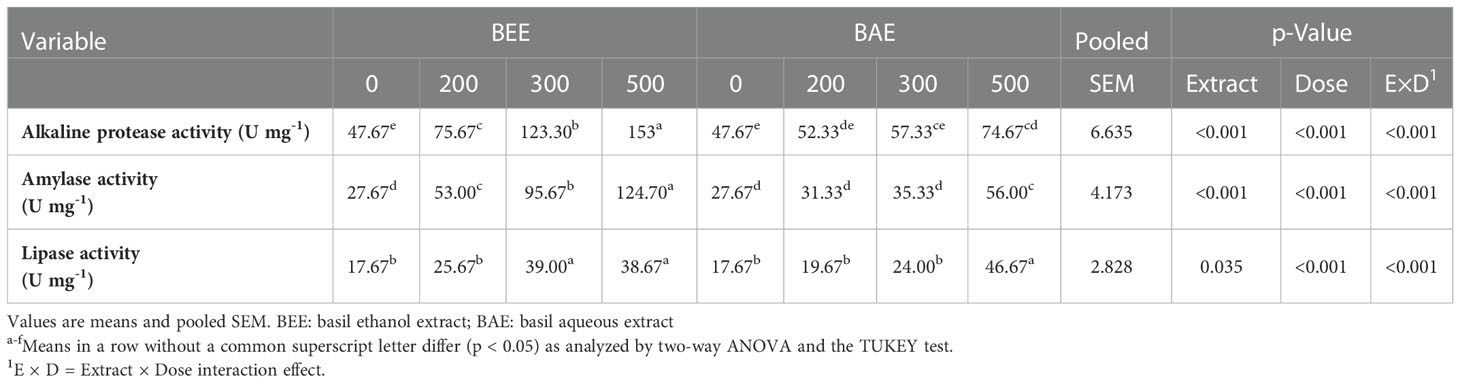
Table 3 Effect of dietary supplementation of basil ethanol and aqueous extracts (mg kg-1 of feed) on digestive enzyme activities of Nile tilapia, O. niloticus.
Hemato-biochemical examination
Hematological results were tabulated in Table 4. All dietary-supplemented groups had significantly higher RBCs, WBCs, hemoglobin (Hb), and PCV values compared to the control. The hematological status of groups fed BEE-supplemented diets was better than BAE-treated groups, except for BAE500, which had non-significant results compared to BEE200. The lymphocytes increased significantly with all supplementation treatments compared to the control and the highest levels were reported in the group BEE500. No significant differences were recorded in monocytes, eosinophil, basophil, and neutrophil counts neither for extracts nor doses among the experimental groups.
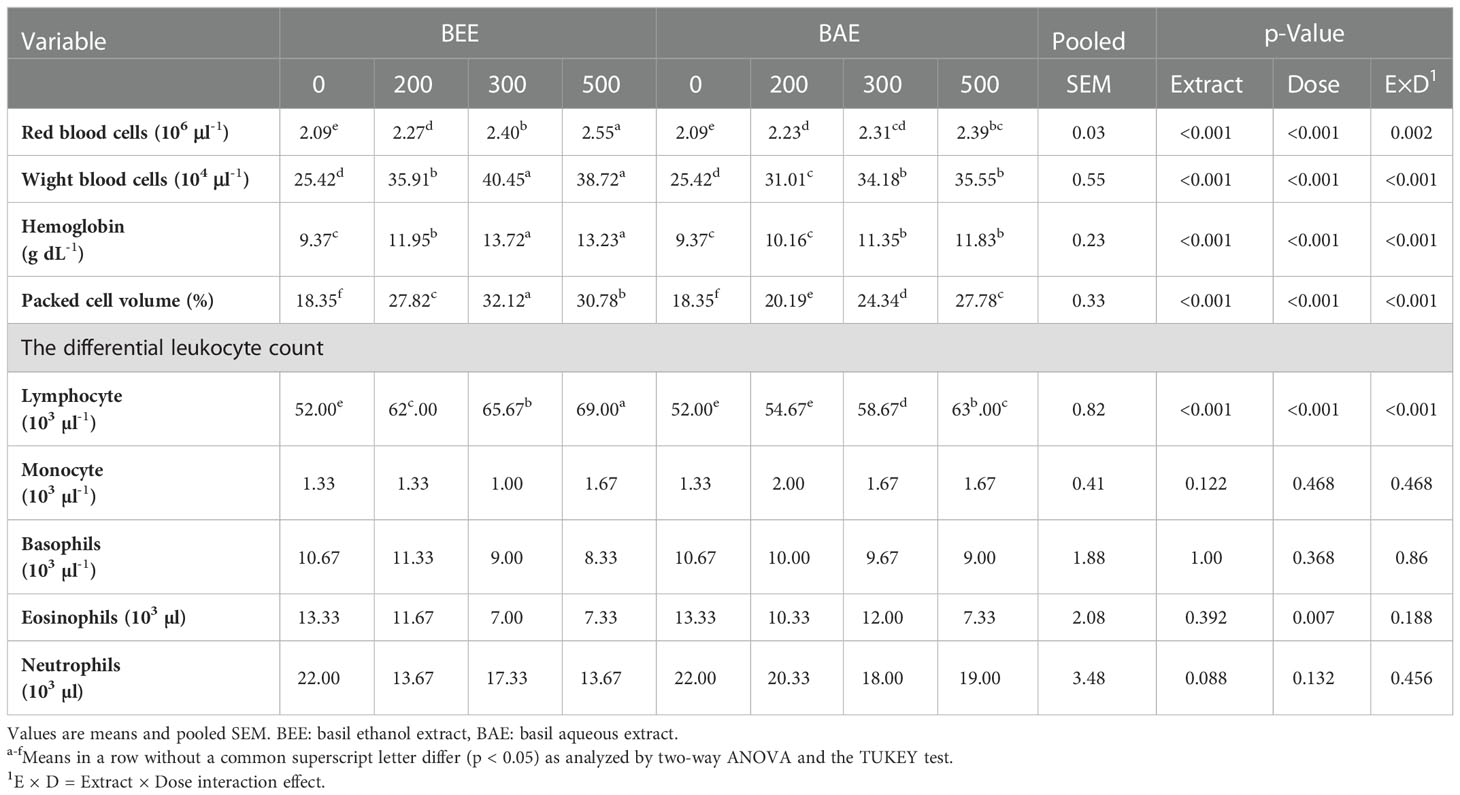
Table 4 Effect of dietary supplementation of basil ethanol and aqueous extracts (mg kg-1 of feed) on hematological parameters of Nile tilapia, O. niloticus.
Different biochemical parameters of Nile tilapia fed experimental diets were evaluated (Table 5). Total protein and globulin were improved significantly with both BEE- and BAE-supplemented groups compared to the control, and the best results were recorded in the BEE500 group. Meanwhile, the transaminase activities (ALT and AST), creatinine, cholesterol, and triglyceride levels were significantly decreased in BEE- and BAE-supplemented groups than in the control.
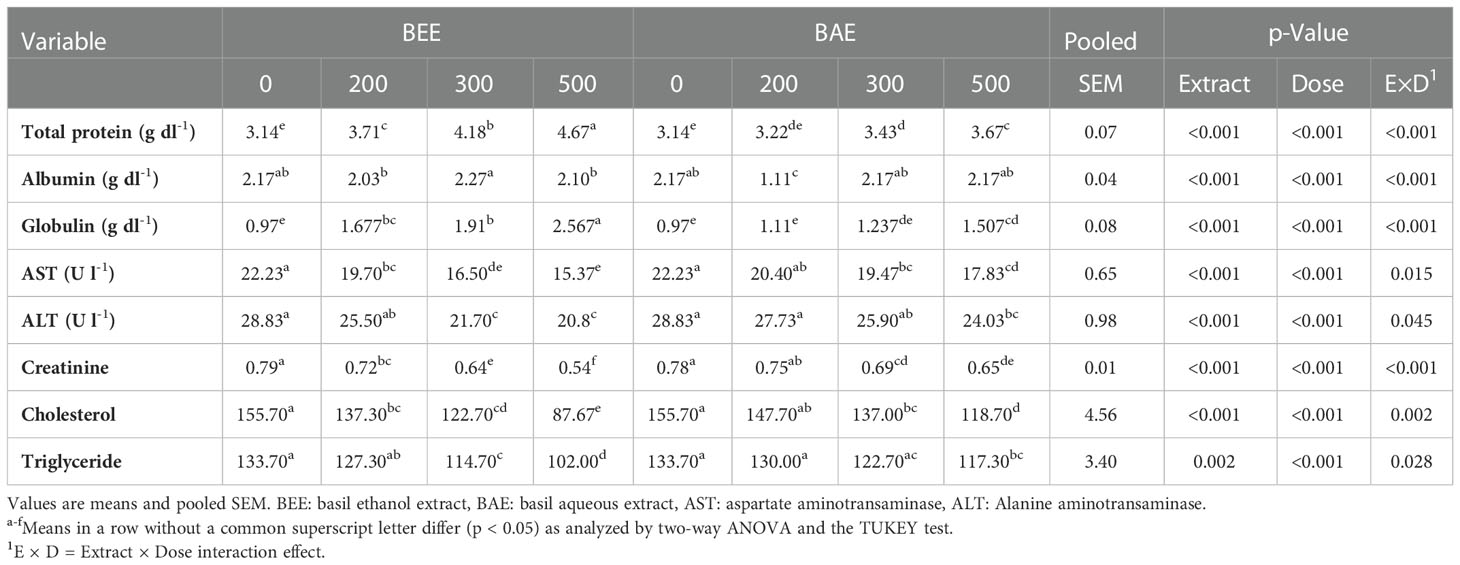
Table 5 Effect of dietary supplementation of basil ethanol and aqueous extracts (mg kg-1 of feed) on serum biochemical examination of Nile tilapia, O. niloticus.
Innate immune parameters
The cellular immune response showed a significant improvement in the NBT, phagocytic activity, and index in all BEE and BAE supplemented groups in a dose-dependent manner (Table 6). The lyzosome activity and IgM as non-specific humoral immune indicators showed significant improvement with different basil extracts and the best results with BEE500. The bactericidal activity affected significantly with both basil extracts and dietary doses of application.
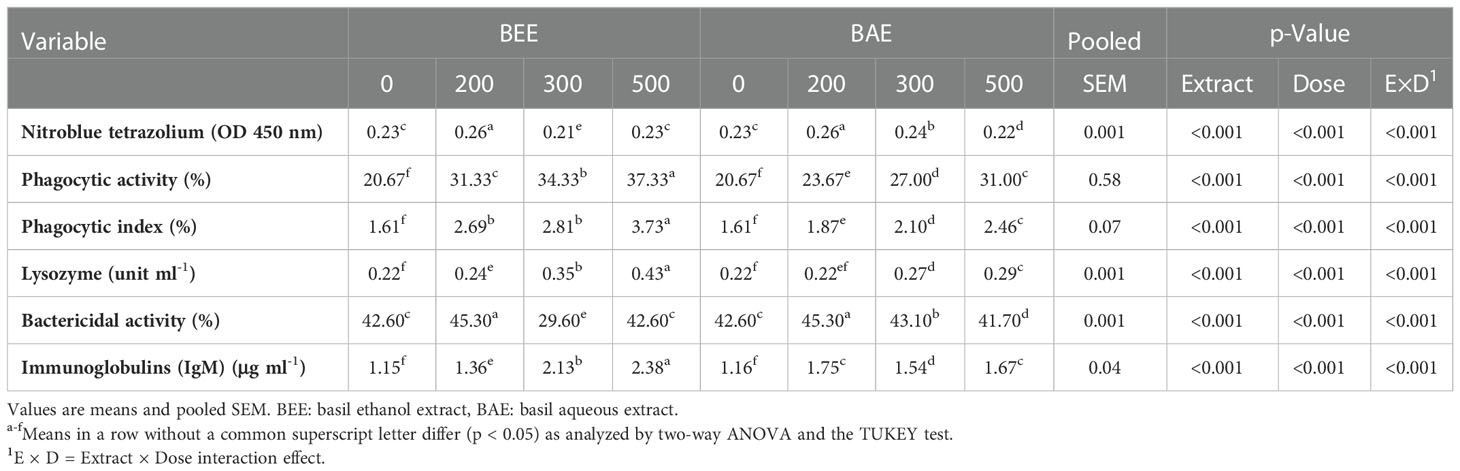
Table 6 Effect of dietary supplementation of basil ethanol and aqueous extracts (mg kg-1 of feed) on cellular and humoral immune parameters, and antioxidant status of Nile tilapia, O. niloticus.
The dose response study showed a dose-dependent increase in phagocytosis activity and lysozyme with increasing dietary supplementation of both basil extracts. The difference between the effects of both extracts on immune response is highly significant, with the highest response in groups feed BEE (Figure 2).
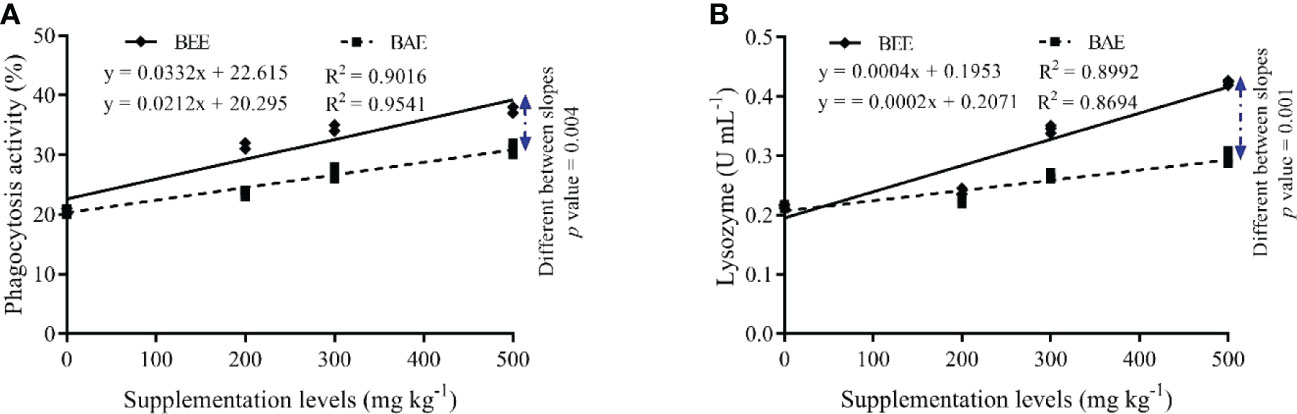
Figure 2 The fit regression model and slope differences between dietary supplementation of basil ethanol (BEE) and aqueous extracts (BAE) on phagocytosis activity (A) and lysozyme activity (B) of Nile tilapia, Oreochromis niloticus.
Immune related genes expression
The two-way analysis of variance of immune-related gene expressions in the head kidneys of O. niloticus fed different dietary levels of BEE or BAE showed significant effects of both basil extracts and dietary doses (Table 7). The expression of IL-1β and TNF-α genes increased in a dose-dependent manner and the highest expression was recorded in BEE500. Meanwhile, the expression of TGF-β was down-regulated with increasing different extract supplementation levels.
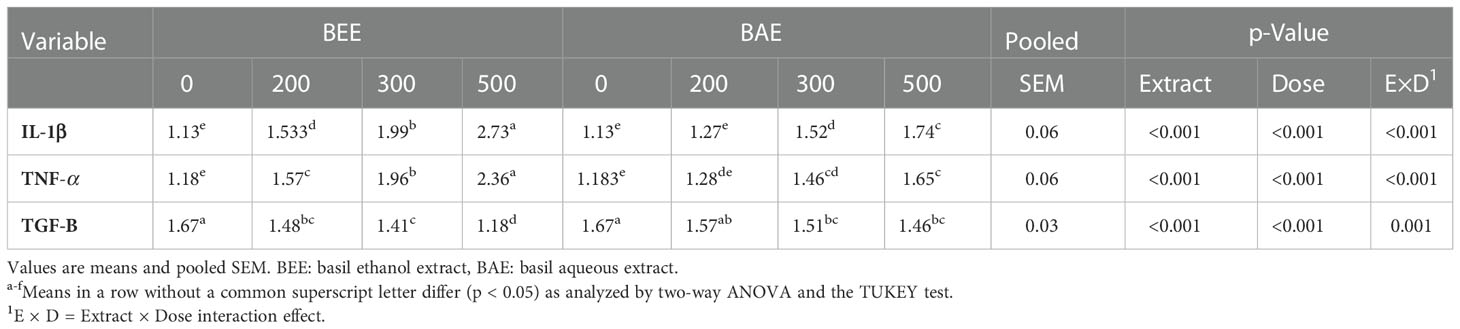
Table 7 Effect of dietary supplementation of basil ethanol and aqueous extracts (mg kg-1 of feed) on relative expression of some immune related genes in head kidney of Nile tilapia, O. niloticus.
Antioxidant status
The antioxidant status of fish that received different basil extracts showed an improvement in oxidant/antioxidant balance via significant improvement of SOD and CAT activities associated with a significant reduction of MDA levels (Table 8). The significant differences were reported among different extracts, dietary levels, and interaction. The BEE500 group showed the best antioxidant status among studied groups. The fit regression trend was positive linear regression with total antioxidant capacity and negative linear regression with MDA levels in favor of increasing BEE supplementation levels (Figure 3).
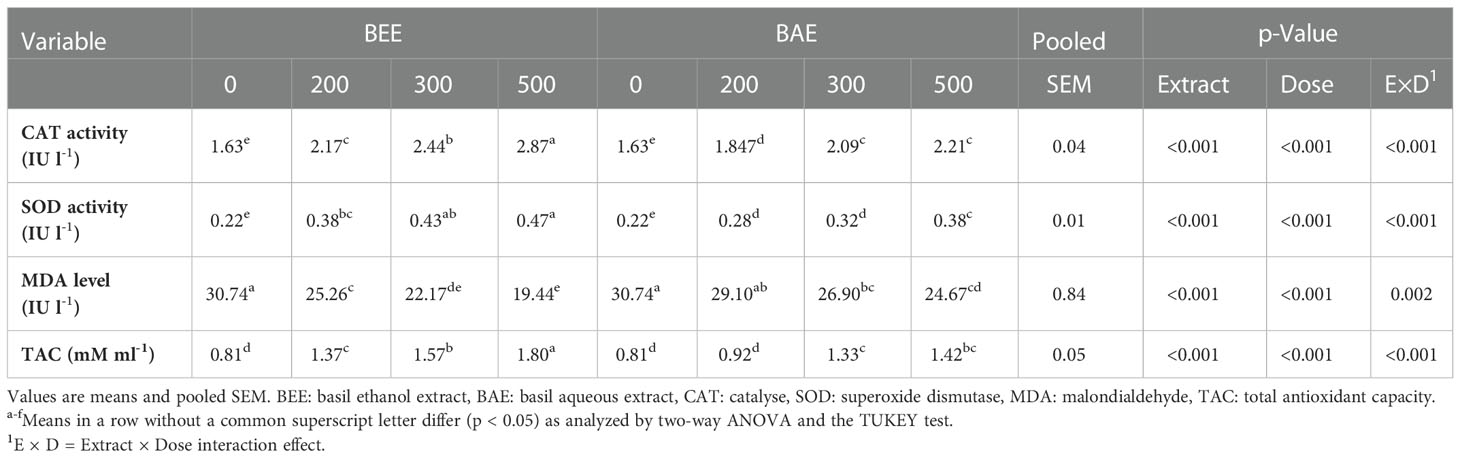
Table 8 Effect of dietary supplementation of basil ethanol and aqueous extracts (mg kg-1 of feed) on serum antioxidant status of Nile tilapia, O. niloticus.
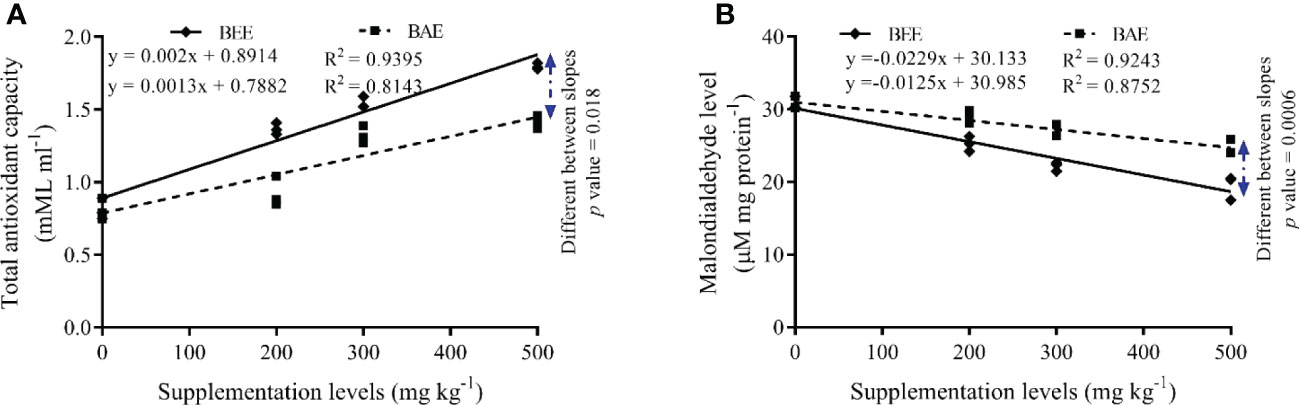
Figure 3 The fit regression model and slope differences between dietary supplementation of basil ethanol (BEE) and aqueous extracts (BAE) on total antioxidant capacity (A) and malondialdehyde levels (B) of Nile tilapia, Oreochromis niloticus.
Bacterial challenge
After bacterial challenge, the cumulative mortality and RPL in different groups of fish were recorded (Figure 4). The RPL of O. niloticus challenged with S. agalactiae was improved in all basil extract groups than the control group. In addition, the BEE500 recorded the lowest mortality (20%); meanwhile the control recorded the highest cumulative mortality (93%).
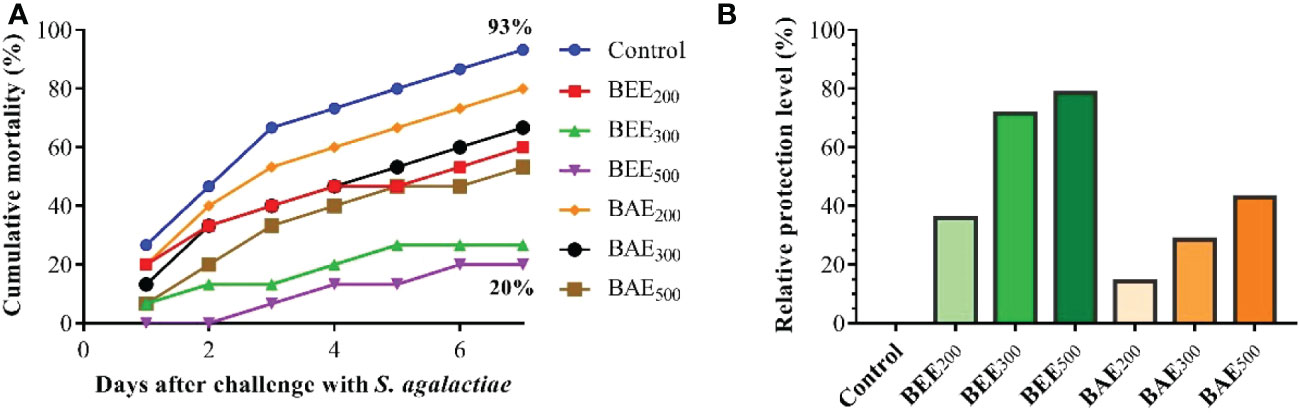
Figure 4 Cumulative mortality curve (A) and relative protection level (B) of Nile tilapia, Oreochromis niloticus, fed different dietary supplementation of basil ethanol (BEE) and aqueous extracts (BAE) and challenged with S. agalactiae.
Discussion
In aquaculture, herbal extracts revealed the potential to be used as cheap, safe, non-toxic, biocompatible, and biodegradable immunostimulants and/or antimicrobial agents (Amirkhani and Firouzbakhsh, 2015; García-Beltrán et al., 2020). Sweet basil is one of the most common medicinal plants worldwide due to its rich phytochemicals and essential oil constituents with multiple purposes (Shahrajabian et al., 2020). Basil is rich in substances that could improve fish growth and health as natural antioxidants, including flavonoids (quercetin, rutin, and kaempferol), phenolic acids (caftaric acid, caffeic acid, and p-coumaric acid), steroids, and vitamins A, E, C, and K (Amirkhani and Firouzbakhsh, 2015; Chung et al., 2020).
The present findings revealed a significant improvement in Nile tilapia growth performance with dietary supplementation of either BEE or BAE compared to the control group, with the superiority of fish-fed BEE-supplemented diets. In accordance, dietary dried basil leaves at 20 g kg-1 diet improved the growth performance of hybrid tilapia fingerling over the control (El-Dakar et al., 2008). Common carp, C. carpio, fed BEE-supplemented diets experienced higher growth performance than the non-supplemented group (Amirkhani and Firouzbakhsh, 2015). In addition, the use of 2.0 ml of basil essential oil/kg diet improved the FBW, WG, and feed conversion ratio of pirarucu, A. gigas, juveniles (Chung et al., 2020). Dietary supplementation of basil oil at levels of 1–5 g/kg significantly improved FBW, WG (%), and feed intake of P. indicus in a dose-dependent manner (Abdel-Tawwab et al., 2021). The growth-stimulating effects of basil extracts could be attributed to the aromatic flavor, which enhances the olfactory and palatability of the experimental diets (El-Dakar et al., 2015). The stimulation of fish appetite could improve voluntary feed intake, and consequently, provide enough nutrients to improve fish weight (Sallam et al., 2017). In addition, basil extract has been shown to improve digestive system functions and enhance feed utilization in fish (de Souza et al., 2019) as well as modulate intestinal microbiota (Reverter et al., 2014).
Regarding the difference in Nile tilapia response to both basil extracts (BAE and BEE) in the present study, fish fed BEE gained higher final weight than those fed BAE supplemented diet. Nguyen et al. (2021) reported that the extraction techniques could affect the phytochemical constituents of the extract and consequently the biological properties. Basil ethanolic and aqueous extracts have several compounds as alkaloids, flavonoids, saponins, coumarins, reducing sugar, terpenoids, and tannins (Nguyen et al., 2021). However, reducing sugar and saponin were not detected in BEE as well as alkaloids were not present in BAE, which could interpret the difference in fish responses with both extracts.
In addition, in the present study, the inclusion of BEE, as well as BAE to O. niloticus diets relatively improved protease, amylase, and lipase activities compared to the control. These findings were in coordination with El-Dakar et al. (2015), where dietary supplementation of basil in gilthead seabream, S. aurata, diet increased the concentrations of both lipase and amylase. Moreover, the use of 1 ml of basil essential oil/kg diet of Nile tilapia improved digestive enzyme activities, including protease, amylase, and lipase (de Souza et al., 2019). Also, several medicinal plant supplements to fish feed improve digestion by increasing digestive enzyme secretion and activity (Bilen et al., 2018; Mohamed et al., 2018). The enhancement of digestive systems reported herein could participate in the improvement of fish growth.
Hematological and biochemical evaluations are an important analyses for assessing the health status of the animal in relation to nutritional and cultural conditions (Bicudo et al., 2009). In the present study, both BEE and BAE improved RBCs, WBCs, Hb, PCV, and lymphocytes of Nile tilapia in a dose-dependent manner. The increase in RBCs and Hb in fish fed with basil extract reflects providing a higher oxygen supply for fish growth. The significant increase in lymphocyte count may be related to the increase of white blood cells synthesis, which may be due to the immunostimulating effect of O. basilicum extracts which matches the observed results of Flores et al. (2008) in O. basilicum aqueous extract. Also, Nile tilapia-fed basil essential oils reported higher hematological indices than the control (de Souza et al., 2019).
In addition, plasma total proteins reveal the status of nitrogen synthesis and degradation in the fish body, whereas good nutritional status increases plasma proteins and promotes protein deposition (Higuchi et al., 2011). The current findings revealed a significant increase in total protein, albumin, and globulin in BEE and BAE-supplemented groups. This improvement was associated also with higher growth performance. In line with this, high plasma total protein concentrations were linked to rainbow trout growth performance and protein utilization (Rumsey et al., 1994). Moreover, increasing dietary concentrations of basil essential oils in the diet increased plasma total proteins and reduced triglycerides, and ALT levels of Nile tilapia (de Souza et al., 2019) and pirarucu (A. gigas) (Chung et al., 2020). Total protein, albumin, and globulin levels were significantly increased but AST, ALT, creatinine, and urea were significantly decreased in P. indicus fed 2.5–5.0 g basil oil/kg diet (Abdel-Tawwab et al., 2021).
The serum level of transaminases revealed liver integrity status (Xia et al., 2022). The serum AST and ALT levels of Nile tilapia-fed basil extracts supplemented diet decreased in the current study, indicating the safety of basil ethanolic and aqueous extracts up to 500 mg/kg diet. The hepato-protective activity of BEE or BAE was due to their antioxidant effects, which are related to BEE or BAE phenolic compounds high contents, which act as free radical scavengers, and reducing agents, metal chelators (Jayasinghe et al., 2003).
Furthermore, the effect of dietary supplementation of BEE and BAE on kidney filtration activities showed a decrease in creatinine levels with increasing supplementation levels. In accordance, dietary basil extract decreased creatinine levels in the serum of rainbow trout after 15 days of feeding (Pastorino et al., 2022). In addition, plasma urea levels decreased with 2.0 ml basil essential oil/kg (Chung et al., 2020). This could indicate better kidney functions, together with decreasing urea levels (Ajeniyi and Solomon, 2014).
The oxidant/antioxidant balance is a normal biological process among the continuously produced free radicals and antioxidant defense systems (Di Giulio et al., 1989). However, under stressful conditions, including farming practices or during competing for infectious diseases, this balance could be interrupted, leading to lipid peroxidation and several oxidative stress implications (Ji, 1995; Guo et al., 2015). The present findings revealed an increase in CAT and SOD activities and decreasing in MDA levels with increasing both BEE and BAE concentrations in O. niloticus diets compared to the control. Similarly, the oxidant/antioxidant balance was improved in terms of high SOD, CAT, and glutathione peroxidase activities, and low MDA and nitric oxide levels with increasing dietary levels of basil oils. (Abdel-Tawwab et al., 2021). Rainbow trout, O. mykiss, fed a diet supplemented with a basil supercritical extract at a level of 0.5% experienced higher SOD, CAT, and glutathione activities, and reduced the MDA levels in liver and kidney of supplemented fish (Magara et al., 2022). Basil extract’s showed high antioxidant properties, likely due to the presence of polyphenols recognized as antioxidant molecules (Perron and Brumaghim, 2009). The anti-oxidative activity of basil extract may be related to the high content of some bioactive compounds, such as rutin, epicatechin (Rezzoug et al., 2019), flavonoid content of BEE or BAE (Nguyen et al., 2021), and their antioxidant ability (Chin and Lindsay, 1994).
The innate immune response is the first defense line of fish against pathogens. In the present study, dietary supplementation of both BEE and BAE stimulated cellular and humoral immune responses of Nile tilapia, whereas phagocytosis activity and index, lysozyme, and Ig significantly improved compared to the control. In the same line, various herbal compounds triggered the increase of plasma lysozyme activity (Abdel-Tawwab et al., 2021; Mansour et al., 2022b) as an important component of the innate immune system, as lysozyme is a mucolytic enzyme excreted by leukocytes and found in most body fluids and mucus (Motlagh et al., 2020). It increases in the present study associated with increased lymphocyte count. In addition, dietary basil oil up to 1% increased respiratory burst activity, serum lysozyme, and serum bactericidal activity of Nile tilapia after 42 days of the experiment (El-Ashram et al., 2017). Basil oil supplementation stimulated the humoral innate immune response, including lysozyme and phenol oxide activities in P. indicus (Abdel-Tawwab et al., 2021). Furthermore, basil aqueous extract is a powerful natural immunomodulatory substance and could directly affect the lymphocytes and modulate several immune-related genes expression (Jaw-Ming, 2011). Also, the O. basilicum immunostimulant effect could be due to the high flavonoid content (El-Ashram et al., 2017), a phenolic compound content that enhances both non-specific and specific immune response (Nahak and Sahu, 2014).
In addition, the balanced cytokine secretion orchestrated the immune response, including IL-1β and TNF-α as pro-inflammatory and TGF- β as an anti-inflammatory cytokine, which acts on enhancing the defense against pathogens and regulates the balanced inflammatory response (Goldstein et al., 2006). In the present study, supplementation with BEE or BAE up-regulated IL-1β and TNF-α and down-regulated TGF-β in a concentration-dependent manner in both BEE and BAE-treated groups compared to the control group. In accordance, oregano essential oil increased the transcription levels of IL-1 and IL-10 and downregulated TNF and TGF (Abdel-Latif et al., 2020). Meanwhile, Güez et al. (2017) did not report any difference in pro-inflammatory cytokines (TNF-α and IL-6) in response to O. basilicum hydro-alcoholic.
The wide use of antimicrobial agents in modern food animal production has led to the emergence of antimicrobial resistance worldwide (Park et al., 2012; Behera et al., 2018; Legario et al., 2020). It is worth noting that in the present study dietary basil extract improved the resistance and decreased mortality of Nile tilapia against S. agalactiae infection. O. basilicum was effective against S. agalactiae and Pseudomonas fluorescens pathogens of particular concern for farmed fish (El-Ekiaby, 2019). In the same line, the mortality rate of Nile tilapia was decreased significantly with dietary basil essential oils (El-Ashram et al., 2017). The relative percentage of survival was improved in P. indicus fed basil oil and challenged with Vibrio parahaemolyticus (Abdel‐Tawwab et al., 2021). Also, Mozambique tilapia, O. mossambicus, fed with diets supplemented with citrus limon peel extract experienced improved non-specific immune parameters and decreased mortality (Baba et al., 2016). Amirkhani and Firouzbakhsh (2015) found that BEE improved C. carpio resistance against A. hydrophila. These results might be attributed to the antibacterial activities of basil flavonoids (Rezzoug et al., 2019), and BAE was reported to have enhancement effects on the immune responses (Nahak and Sahu, 2014). Moreover, dietary basil oil revealed stress releasing effect against high stocking density (Chung et al., 2020).
Conclusion
The dietary supplementation of both ethanolic and aqueous extracts of sweet basil (Ocimum basilicum) significantly improved the growth performance, digestive enzyme activities, and physiological responses of Nile tilapia after eight weeks of treatment. The cellular and humoral immune responses, resistance against Streptococcus agalactiae, and antioxidant balance were improved with increasing dietary supplementation of basil extracts. The dose-response study revealed a significant superiority of ethanolic over aqueous extract in most of the investigated parameters. Accordingly, the use of 500 mg ethanol extract/kg diet of Nile tilapia could promote growth performance, improve antioxidant status, and stimulate the immune response. However, higher levels of the ethanolic extract could be evaluated to determine the optimum dietary levels of basil extract.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Ethics Committee of Animal Use in Research Committee, Faculty of Veterinary Medicine, Alexandria University.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
The authors would like to express their gratitude to Ahmed Nassar of Egypt. Also, this work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [GRANT2183].
Acknowledgments
The authors would like to express their gratitude to Master/Ahmed Nassar of Egypt.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Latif H. M., Abdel-Tawwab M., Khafaga A. F., Dawood M. A. (2020). Dietary origanum essential oil improved antioxidative status, immune-related genes, and resistance of common carp (Cyprinus carpio L.) to Aeromonas hydrophila infection. Fish Shellfish Immunol. 104, 1–7. doi: 10.1016/j.fsi.2020.05.056
Abdel-Tawwab M., El-Ashram A. M., Tahoun A. A., Abdel-Razek N., Awad S. M. (2021). Effects of dietary sweet basil (Ocimum basilicum) oil on the performance, antioxidants and immunity welfare, and resistance of Indian shrimp (Penaeus indicus) against vibrio parahaemolyticus infection. Aquacult. Nutr. 27 (4), 1244–1254. doi: 10.1111/anu.13265
Aebi H. (1984). Catalase in vitro. Methods Enzymol. 105, 121–126. doi: 10.1016/S0076-6879(84)05016-3
Ajeniyi S. A., Solomon R. J. (2014). Urea and creatinine of Clarias gariepinus in three different commercial ponds. Nat. Sci. 12, 124–138.
Al-Hamdani Q. H., Al-Dubakel A. Y., Muhammad A. A. (2021). Mucilage extraction from basil (Ocimum basilicum) and its applications in the diets of Cyprinus carpio fingerlings. Biol. Appl. Environ. Res. 5 (1), 86–97. doi: 10.51304/baer.2021.5.1.86
Amirkhani N., Firouzbakhsh F. (2015). Protective effects of basil (Ocimum basilicum) ethanolic extract supplementation diets against experimental Aeromonas hydrophila infection in common carp (Cyprinus carpio). Aquacult. Res. 46 (3), 716–724. doi: 10.1111/are.12217
Andayani S. R. I., Dadiono M. S., Elwira W. T., Setyawan F. H. (2020). Potency of aloe extract as immunostimulant for carp (Cyprinus carpio) against Aeromonas salmonicida. Biodiversitas 21 (3), 860–864. doi: 10.13057/biodiv/d210302
Baba E., Acar Ü., Önta¸ S. C., Kesbiç O. S., Yılmaz S. (2016). Evaluation of citrus limon peels essential oil on growth performance, immune response of mozambique tilapia Oreochromis mossambicus challenged with Edwardsiella tarda. Aquaculture 465, 13–18. doi: 10.1016/j.aquaculture.2016.08.023
Bartosz G. (2003). Total antioxidant capacity. Adv. Clin. Chem. 37, 219–292. doi: 10.1016/S0065-2423(03)37010-6
Behera B., Pradhan P., Swaminathan T., Sood N., Paria P., Das A., et al. (2018). Emergence of tilapia lake virus associated with mortalities of farmed Nile tilapia Oreochromis niloticus (Linnaeus 1758) in India. Aquaculture 484, 168–174. doi: 10.1016/j.aquaculture.2017.11.025
Bicudo I. J., Sado R. Y., Cyrino J. E. P. (2009). Growth and haematology of pacu Piaractus mesopotamicus, fed diets with varying protein to energy ratio. Aquac. Res. 40, 486–495. doi: 10.1111/j.1365-2109.2008.02120.x
Bilen S., Özkan O., Alagöz K., Özdemir K. Y. (2018). Effect of dill (Anethum graveolens) and garden cress (Lepidium sativum) dietary supplementation on growth performance, digestive enzyme activities and immune responses of juvenile common carp (Cyprinus carpio). Aquaculture 495, 611–616. doi: 10.1016/j.aquaculture.2018.06.037
Bowater R., Forbes-Faulkner J., Anderson I., Condon K., Robinson B., Kong F., et al. (2012). Natural outbreak of Streptococcus agalactiae (GBS) infection in wild giant Queensland grouper, Epinephelus lanceolatus (Bloch), and other wild fish in northern Queensland, Australia. J. Fish Dis. 35 (3), 173–186. doi: 10.1111/j.1365-2761.2011.01332.x
Chin H.-W., Lindsay R. C. (1994). Ascorbate and transition-metal mediation of methanethiol oxidation to dimethyl disulfide and dimethyl trisulfide. Food Chem. 49 (4), 387–392. doi: 10.1016/0308-8146(94)90009-4
Chung S., Lemos C., Teixeira D., Fortes-Silva R., Copatti C. E. (2020). Essential oil from Ocimum basilicum improves growth performance and does not alter biochemical variables related to stress in pirarucu (Arapaima gigas). Acad. Bras. Cienc. 92, e20181374. doi: 10.159/0001-3765202020181374
Dawood M. A., Shukry M., Zayed M. M., Omar A. A., Zaineldin A. I., ElBasuini M. F. (2019). Digestive enzymes, immunity and oxidative status of Nile tilapia (Oreochromis niloticus) reared in intensive conditions. Slov. Vet. Res. 56 (22), 99–108. doi: 10.26873/SVR-747-2019
Delfani S., BahMani M., MohaMMaDRezaei-KhoRRaMaBaDi R., Rafieian-Kopaei M. (2017). Phytotherapy in Streptococcus agalactiae: An overview of the medicinal plants effective against Streptococcus agalactiae. J. Clin. Diagn. Res.: JCDR 11 (6), DE01–DE02. doi: 10.7860/JCDR/2017/25530.9988
de Lima Boijink C., Queiroz C. A., Chagas E. C., Chaves F. C. M., Inoue L. A. K. A. (2016). Anesthetic and anthelminthic effects of clove basil (Ocimum gratissimum) essential oil for tambaqui (Colossoma macropomum). Aquaculture 457, 24–28. doi: 10.1016/j.aquaculture.2016.02.010
de Souza E. M., de Souza R. C., Melo J. F., da Costa M. M., de Souza A. M., Copatti C. E. (2019). Evaluation of the effects of Ocimum basilicum essential oil in Nile tilapia diet: Growth, biochemical, intestinal enzymes, haematology, lysozyme and antimicrobial challenges. Aquaculture 504, 7–12. doi: 10.1016/j.aquaculture.2019.01.052
Diab A. M., Khalil R. H., Khallaf M. (2019). Autogenous bacterins cross-protection as a trial for streptococcosis control in Oreochromis niloticus. Aquacult. Int. 27 (6), 1787–1800. doi: 10.1007/s10499-019-00432-z
Diab A. M., Shokr B. T., Shukry M., Farrag F. A., Mohamed R. A. (2022). Effects of dietary supplementation with green−synthesized zinc oxide nanoparticles for candidiasis control in Oreochromis niloticus. Biol. Trace Element Res. 200, 4126–4141. doi: 10.1007/s12011-021-02985-8
Di Giulio R. T., Washburn P. C., Wenning R. J., Winston G. W., Jewell C. S. (1989). Biochemical responses in aquatic animals: A review of determinants of oxidative stress. Environ. Toxicol. Chem. Int. J. 8, 1103–1123. doi: 10.1002/etc.5620081203
Doumas B. T., Watson W. A., Biggs H. G. (1971). Albumin standards and the measurement of serum albumin with bromcresol green. Clinica Chimica Acta 31 (1), 87–96. doi: 10.1016/0009-8981(71)90365-2
El-Ashram A., Afifi A., Sakr S. F. (2017). Effect of basil oil (Ocimum basilicum) on nonspecific immune response of Nile-tilapia (Oreochromis niloticus). Egyptian J. Aquacult. 7 (2), 15–31. doi: 10.21608/eja.2017.31475
El-Dakar A. Y., Hassanien G. D., Gad S. S., Sakr S. E. (2008). Use of dried basil leaves as a feeding attractant for hybrid tilapia, Oreochromis niloticus x Oreochromis aureus, fingerlings. Mediterr. Aquacult. J. 1 (1), 35–44. doi: 10.21608/maj.2008.2662
El-Dakar A. Y., Shalaby S. M., Nemetallah B. R., Saleh N. E., Sakr E. M., Toutou M. M. (2015). Possibility of using basil (Ocimum basilicum) supplementation in gilthead sea bream (Sparus aurata) diet. Egypt J. Aquat. Res. 41, 203–210. doi: 10.1016/j.ejar.2015.03.001
El-Ekiaby W. T. (2019). Basil oil nanoemulsion formulation and its antimicrobial activity against fish pathogen and enhance disease resistance against Aeromonas hydrophila in cultured Nile tilapia. Egypt J. Aquac. 9, 13–33. doi: 10.21608/eja.2019.18567.1007
Ellis A. E. (1990). "Lysozyme assay," in techniques in fish immunology, eds. Eds. Stolen J. S., Fletcher T. C., Anderson D. P., Roberson B. S., Van W. B., Winkel M. (Fair Haven, NJ, USA: SOS Publications), 101–103.
Evans J., Klesius P., Shoemaker C. (2006). An overview of streptococcus in warmwater fish. Aquacult. Health Int. 7, 10–14.
FAO (2020). The state of world fisheries and aquaculture 2020. sustainability in action, Food and Agriculture Organization (Rome, Italy).
Flores G. A., Rodriguez V. L., Licea Q. R., Guerra T. P., Padilla R. C. (2008). In vitro lymphocyte proliferation induced by Ocimum basilicum, persea americana, plantago virginica and Rosa spp. extracts. J. Medicinal Plants Res. 2 (1), 005–010.
García-Beltrán J. M., Mansour A. T., Alsaqufi A. S., Ali H. M., Esteban M. Á. (2020). Effects of aqueous and ethanolic leaf extracts from drumstick tree (Moringa oleifera) on gilthead seabream (Sparus aurata l.) leucocytes, and their cytotoxic, antitumor, bactericidal and antioxidant activities. Fish Shellfish Immunol. 106, 44–55. doi: 10.1016/j.fsi.2020.06.054
Goldstein S. L., Leung J. C., Silverstein D. M. (2006). Pro-and anti-inflammatory cytokines in chronic pediatric dialysis patients: effect of aspirin. Clin. J. Am. Soc. Nephrol. 1 (5), 979–986. doi: 10.2215/CJN.02291205
Gornall A. G., Bardawill C. J., David M. M. (1949). Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 177 (2), 751–766. doi: 10.1016/S0021-9258(18)57021-6
Güez C. M., Souza R. O. D., Fischer P., Leão M. F. D. M., Duarte J. A., Boligon A. A., et al. (2017). Evaluation of basil extract (Ocimum basilicum l.) on oxidative, anti-genotoxic and anti-inflammatory effects in human leukocytes cell cultures exposed to challenging agents. Braz. J. Pharm. Sci. 53, e15098. doi: 10.1590/s2175-97902017000115098
Guo X., He Y., Zhang L., Lelong C., Jouaux A. (2015). Immune and stress responses in oysters with insights on adaptation. Fish Shellfish Immunol. 46, 107–119. doi: 10.1016/j.fsi.2015.05.018
Henry R. J. (1964). Clinical chemistry, principles and technics (New York, USA: Harper and Row Publishers).
Higuchi L. H., Feiden A., Maluf M. L. F., Dallagnol J. M., Zaminhan M., Boscolo W. R. (2011). Avaliação eritrocitária e bioquímica de jundiás (Rhamdia quelen) submetidos à dieta com diferentes níveis protéicos e energéticos. Ciec. Anim. Bras. 12 (1), 70–75. doi: 10.5216/cab.v12i1.8986
Jaw-Ming C. (2011). Immunomodulatory effects of aqueous extract of Ocimum basilicum (Linn.) and some of its constituents on human immune cells. J. Medicinal Plants Res. 5 (10), 1873–1883.
Jayasinghe C., Gotoh N., Aoki T., Wada S. (2003). Phenolics composition and antioxidant activity of sweet basil (Ocimum basilicum l.). J. Agric. Food Chem. J. Agric. Food Chem. 51, 4442–4449. doi: 10.1021/jf034269o
Ji L. L. (1995). Exercise and oxidative stress: Role of the cellular antioxidant systems. Exerc. Sport Sci. Rev. 23, 135–166. doi: 10.1249/00003677-199500230-00007
Kawahara E., Ueda T., Nomura S. (1991). In vitro phagocytic activity of white-spotted char blood cells after injection with Aeromonas salmonicida extracellular products. Fish. Pathol. 26 (4), 213–214. doi: 10.3147/jsfp.26.213
Klimankova E., Holadová K., Hajšlová J., Čajka T., Poustka J., Koudela M. (2008). Aroma profiles of five basil (Ocimum basilicum l.) cultivars grown under conventional and organic conditions. Food Chem. 107 (1), 464–472. doi: 10.1016/j.foodchem.2007.07.062
Legario F. S., Choresca C. H. Jr., Turnbull J. F., Crumlish M. (2020). Isolation and molecular characterization of streptococcal species recovered from clinical infections in farmed Nile tilapia (Oreochromis niloticus) in the Philippines. J. Fish Dis. 43 (11), 1431–1442. doi: 10.1111/jfd.13247
Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. methods 25 (4), 402–408. doi: 10.1006/meth.2001.1262
Magara G., Prearo M., Vercelli C., Barbero R., Micera M., Botto A., et al. (2022). Modulation of antioxidant defense in farmed rainbow trout (Oncorhynchus mykiss) fed with a diet supplemented by the waste derived from the supercritical fluid extraction of basil (Ocimum basilicum). Antioxidants 11 (2), 415. doi: 10.3390/antiox11020415
Mansour A. T., Alsaqufi A. S., Alkhamis, Yousef A., Al-Gazar F. F., Zaki M. A., Nour A. A. M., et al. (2021). The evaluation of Arthrospira platensis bioactivity and their dietary supplementation to Nile tilapia vegetarian diet on growth performance, feed utilization, body composition and hemato-biochemical parameters. Ann. Anim. Sci. 21 (3), 1061–1080. doi: 10.2478/aoas-2021-0003
Mansour A. T., Ashour M., Alprol A. E., Alsaqufi A. S. (2022a). Aquatic plants and aquatic animals in the context of sustainability: Cultivation techniques, integration, and blue revolution. Sustainability 14, 3257. doi: 10.3390/su14063257
Mansour A. T., Espinosa C., García-Beltrán J. M., Miao L., Francisco D. C. C., Alsaqufi A. S., et al. (2020). Dietary supplementation of drumstick tree, Moringa oleifera, improves mucosal immune response in skin and gills of seabream, Sparus aurata, and attenuates the effect of hydrogen peroxide exposure. Fish. Physiol. Bioch. 46 (3), 981–996. doi: 10.1007/s10695-020-00763-2
Mansour A. T., Esteban M. Á. (2017). Effects of carbon sources and plant protein levels in a biofloc system on growth performance, and the immune and antioxidant status of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 64, 202–209. doi: 10.1016/j.fsi.2017.03.025
Mansour A. T., Hamed H. S., El-Beltagi H. S., Mohamed W. F. (2022b). Modulatory effect of papaya extract against chlorpyrifos-induced oxidative stress, immune suppression, endocrine disruption, and DNA damage in female Clarias gariepinus. Int. J. Environ. Res. Public 19, 4640. doi: 10.3390/ijerph19084640
Mansour A. T., Miao L., Espinosa C., García-Beltrán J. M., Francisco D. C. C., Esteban M. Á. (2018). Effects of dietary inclusion of Moringa oleifera leaves on growth and some systemic and mucosal immune parameters of seabream. Fish Physiol. Bioch. 44 (4), 1223–1240. doi: 10.1007/s10695-018-0515-z
Mansour A., Omar E., Srour T., Yousef M. (2018). Effect of three natural phytochemicals supplementation on growth performance, testosterone level and feed utilization of Nile tilapia (Oreochromis niloticus). Aquacult. Nutr. 24 (1), 408–415. doi: 10.1111/anu.12572
Mohamed G. A., Amhamed I. D., Almabrok A. A., Barka A. B. A., Bilen S., Elbeshti R. T. (2018). Effect of celery (Apium graveolens) extract on the growth, haematology, immune response and digestive enzyme activity of common carp (Cyprinus carpio). Mar. Sci. Technol. Bull. 7 (2), 51–59. doi: 10.33714/masteb.457721
Motlagh H. A., Safari O., Selahvarzi Y., Baghalian A., Kia E. (2020). Non-specific immunity promotion in response to garlic extract supplemented diets in female guppy (Poecilia reticulata). Fish Shellfish Immunol. 97, 96–99. doi: 10.1016/j.fsi.2019.12.007
Moustafa E. M., Dawood M. A., Assar D. H., Omar A. A., Elbialy Z. I., Farrag F. A., et al. (2020). Modulatory effects of fenugreek seeds powder on the histopathology, oxidative status, and immune related gene expression in Nile tilapia (Oreochromis niloticus) infected with aeromonas hydrophila. Aquaculture 515, 734589. doi: 10.1016/j.aquaculture.2019.734589
Nahak G., Sahu R. K. (2014). Immunomodulatory activity of aqueous leaf extract of Ocimum basilicum Linn in Clarias batrachus. Int. J. Pharm. Pharm. Sci. 6, 433–440.
Nguyen V., Nguyen N., Thi N., Thi C., Truc T., Nghi P. (2021). Studies on chemical, polyphenol content, flavonoid content, and antioxidant activity of sweet basil leaves (Ocimum basilicum l.), in IOP conference series: Mater. Sci. Eng. 1092(1):012083. doi: 10.1088/1757-899X/1092/1/012083
Nishikimi M., Rao N. A., Yagi K. (1972). The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 46 (2), 849–854. doi: 10.1016/S0006-291X(72)80218-3
Park Y. H., Hwang S. Y., Hong M. K., Kwon K. H. (2012). Use of antimicrobial agents in aquaculture. Rev. Sci. Tech. (Int. Off. Epizoot.) 31, 189–197. doi: 10.20506/rst.31.1.2105
Pastorino P., Bergagna S., Vercelli C., Pagliasso G., Dellepiane L., Renzi M., et al. (2022). Changes in serum blood parameters in farmed rainbow trout (Oncorhynchus mykiss) fed with diets supplemented with waste derived from supercritical fluid extraction of sweet basil (Ocimum basilicum). Fishes 7 (2), 89. doi: 10.3390/fishes7020089
Pereira U., Mian G., Oliveira I., Benchetrit L., Costa G., Figueiredo H. (2010). Genotyping of Streptococcus agalactiae strains isolated from fish, human and cattle and their virulence potential in Nile tilapia. Vet. Microbiol. 140 (1-2), 186–192. doi: 10.1016/j.vetmic.2009.07.025
Perron N. R., Brumaghim J. L. (2009). A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 53, 75–100. doi: 10.1007/s12013-009-9043-x
Rainger G. E., Rowley A. F. (1993). Antibacterial activity in the serum and mucus of rainbow trout, Oncorhynchus mykiss, following immunization with Aeromonas salmonicida. Fish Shellfish Immunol. 3, 475–482. doi: 10.1006/fsim.1993.1046
Reitman S. (1957). Colorimetric determination of GPT activity according to the reitman and Frankel method. Am. J. Clim. Path 28, 56–63.
Reitman S., Frankel S. (1957). Colorimetric determination of GOT and GPT activity. Am. J. Clin. Path 28, 56. doi: 10.1093/ajcp/28.1.56
Reverter M., Bontemps N., Lecchini D., Banaigs B., Sasal P. (2014). Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture 433, 50–61. doi: 10.1016/j.aquaculture.2014.05.048
Rezzoug M., Bakchiche B., Gherib A., Roberta A., Kilinçarslan Ö., Mammadov R., et al. (2019). Chemical composition and bioactivity of essential oils and ethanolic extracts of Ocimum basilicum l. and Thymus algeriensis boiss. & reut. from the Algerian Saharan atlas. BMC Complement. Altern. Med. 19 (1), 1–10. doi: 10.1186/s12906-019-2556-y
Ruangroupan L., Kitao T., Yoshida T. (1986). Protective efficacy of aeromonas hydrophila vaccines in Nile tilapia. Vet. Immunol. Immunopathol. 12, 345–350. doi: 10.1016/0165-2427(86)90139-X
Rumsey G. L., Siwicki A. K., Anderson D. P., Bowser P. R. (1994). Effect of soybean protein on serological response, non-specific defense mechanisms, growth, and protein utilization in rainbow trout. Vet. Immunol. immunopathol. 41 (3-4), 323–339. doi: 10.1016/0165-2427(94)90105-8
Sakr S. A., Al-Amoudi W. M. (2012). Effect of leave extract of Ocimum basilicum on deltamethrin induced nephrotoxicity and oxidative stress in albino rats. J. Appl. Pharm. Sci. 2 (5), 22–27. doi: 10.7324/JAPS.2012.2507
Sallam A. E., Mansour A. T., Srour T. M., Goda A. M. A. (2017). Effects of different carotenoid supplementation sources with or without sodium taurocholate on growth, feed utilization, carotenoid content and antioxidant status in fry of the European seabass, Dicentrarchus labrax. Aquacult. Res. 48, 848–3858. doi: 10.1111/are.13212
Secombes C. J. (1990). “Isolation of salmonid macrophages and analysis of their killing ability,” in Techniques in fish immunology. Eds. Stolen J. S., Fletcher T. C., Anderson D. P., Roberson B. S., Van W. B., Winkel M. (Fair Haven, NJ: SOS Publications), 137–152.
Shahrajabian M. H., Sun W., Cheng Q. (2020). Chemical components and pharmacological benefits of basil (Ocimum basilicum): A review. Int. J. Food Properties 23 (1), 1961–1970. doi: 10.1080/10942912.2020.1828456
Silva L. D. L., Parodi T. V., Reckziegel P., Garcia V. D. O., Bürger M. E., Baldisserotto B., et al. (2012). Essential oil of Ocimum gratissimum l.: Anesthetic effects, mechanism of action and tolerance in silver catfish, Rhamdia quelen. Aquaculture, 350–353. doi: 10.1016/j.aquaculture.2012.04.012
Sipos L., Balázs L., Székely G., Jung A., Sárosi S., Radácsi P., et al. (2021). Optimization of basil (Ocimum basilicum l.) production in LED light environments–a review. Scientia Hortic. 289, 110486.
Siwicki A. K., Anderson D. P. (1993). Nonspecific defense mechanisms assay in fish: II. potential kill. activity neutrophils macrophages, lysozyme activity serum organs total immunoglobulin level serum, FAO project GCP/INT/JPA, IFI, olsztyn (Poland), 105–112.
Sonmezdag A. S., Amanpour A., Kelebek H., Selli S. (2018). The most aroma-active compounds in shade-dried aerial parts of basil obtained from Iran and Turkey. Ind. Crops Products 124, 692–698. doi: 10.1016/j.indcrop.2018.08.053
Stead S. M. (2019). Using systems thinking and open innovation to strengthen aquaculture policy for the united nations sustainable development goals. J. Fish Biol. 94 (6), 837–844. doi: 10.1111/jfb.13970
Tolay I. (2021). The impact of different zinc (Zn) levels on growth and nutrient uptake of basil (Ocimum basilicum l.) grown under salinity stress. PloS One 16 (2), e0246493. doi: 10.1371/journal.pone.0246493
Uchiyama M., Mihara M. (1978). Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 86 (1), 271–278. doi: 10.1016/0003-2697(78)90342-1
Xia Y., Wang Y., Xie J., Yu E., Tian J., Li Z., et al. (2022). Effects of BBR on growth performance, serum and hepatic biochemistry parameters, hepatic morphology and gene expression levels related to glucose metabolism in largemouth bass, Micropterus salmoides. Aquacult. Res. 53, 3807–3817. doi: 10.1111/are.15887
Zahran E., Abd El-Gawad E. A., Risha E. (2018). Dietary withania sominefera root confers protective and immunotherapeutic effects against aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 80, 641–650. doi: 10.1016/j.fsi.2018.06.009
Zenhom M., Ibrahim I. H. (2020). Effect of fenugreek seeds by-produced meal on growth performance, feed utilization, body composition and some physiological traits for common carp (Cyprinus carpio). Egyptian J. Aquacult. 10 (3), 81–95. doi: 10.21608/eja.2020.44476.1037
Keywords: sweet basil, phytotherapy, Nile tilapia, physiological performance, bacterial challenge
Citation: Mansour AT, Diab AM, Khalil RH, Eldessouki EA, El-Sabbagh N, Elsamannoudy SI and Younis NA (2023) Physiological and immunological responses of Nile tilapia fed dietary supplementation of sweet basil ethanolic and aqueous extracts. Front. Mar. Sci. 9:1064455. doi: 10.3389/fmars.2022.1064455
Received: 08 October 2022; Accepted: 30 December 2022;
Published: 19 January 2023.
Edited by:
Alaa El-Din Hamid Sayed, Assiut University, EgyptCopyright © 2023 Mansour, Diab, Khalil, Eldessouki, El-Sabbagh, Elsamannoudy and Younis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdallah Tageldein Mansour, YW1hbnNvdXJAa2Z1LmVkdS5zYQ==
†ORCID: Abdallah Tageldein Mansour, orcid.org/0000-0002-5963-5276
 Abdallah Tageldein Mansour
Abdallah Tageldein Mansour Amany M. Diab3
Amany M. Diab3